- 1School of Pharmaceutical Education and Research (SPER), Jamia Hamdard University, New Delhi, India
- 2Metro College of Health Sciences and Research, Greater Noida, Uttar Pradesh, India
- 3Department of Pharmaceutical Technology, Faculty of Pharmacy, Universiti Malaya, Kuala Lumpur, Malaysia
- 4Department of Pharmaceutical Life Sciences, Faculty of Pharmacy, Universiti Malaya, Kuala Lumpur, Malaysia
- 5School of Pharmacy, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong SAR, China
- 6Faculty of Medicine, Universiti Malaya Research Centre for Biopharmaceuticals and Advanced Therapeutics (UBAT), Universiti Malaya, Kuala Lumpur, Malaysia
- 7Centre of Advanced Materials (CAM), Faculty of Engineering, Universiti Malaya, Kuala Lumpur, Malaysia
- 8Faculty of Pharmaceutical Sciences, Chulalongkorn University, Bangkok, Thailand
Background: CD4+ T-cells play a pivotal role in cancer immunology, functioning as both tumor-suppressing and tumor-promoting agents depending on their differentiation and cytokine profiles. Targeting CD4+ T-cells with novel drug delivery systems, particularly nanoparticle-based formulations, offers a promising approach to enhancing antitumor immune responses while minimizing systemic toxicity.
Objective: This review aims to explore the immunological significance of CD4+ T-cells in cancer and their modulation using novel drug delivery systems. The focus is on understanding CD4+ T-cell subtypes, their functional roles in tumor progression and suppression, and the application of novel drug delivery systems to selectively regulate these cells.
Methods: A comprehensive analysis of CD4+ T-cell subsets, including Th1, Th2, Th17, Tregs, and Tfh, was conducted, along with their immunological roles in cancer. Various nanoparticle platforms, including liposomes, polymeric nanoparticles, dendrimers, gold, silver, and mesoporous silica, were evaluated for their ability to target CD4+ T-cells.
Results: Novel drug delivery systems demonstrate significant potential in selectively modulating CD4+ T-cell responses. Liposomes and polymeric nanoparticles efficiently transport cytokines, antigens, as well as immunological modulators to CD4+ T-cells, enhancing antitumor immunity. Notably, MHC II-coated nanoparticles expanded antigen-specific CD4+ T-cells, while mRNA nano vaccines activated CD4+ and CD8+ responses.
Conclusion: Novel drug delivery systems provide a versatile platform for precise CD4+ T-cell modulation in cancer therapy, enhancing antitumor responses while reducing toxicity. Future advancements should focus on overcoming biological barriers, improving targeting, and optimizing clinical translation.
1 Introduction
Cancer immunology has become a foundation of modern oncology, highlighting the complex relationships between the host immune system and cancerous cells (Finn, 2012). The concept of immune surveillance, by which immune cells identify and eradicate transformed cells, is crucial for tumour prevention (Kim, 2007). However, cancer cells frequently develop defenses against immune system recognition and elimination, leading to a phenomenon known as immunoediting, which promotes tumour progression (Kim, 2007; Ribatti, 2017). In recent years, immune checkpoint inhibitors and other immunotherapeutic approaches, adoptive T-cell transfer, and cancer vaccines have revolutionized treatment strategies by reactivating and enhancing anti-tumour immune responses (Parsonidis and Papasotiriou, 2022; Perica et al., 2015). Despite their success, these therapies are not universally effective, and a large number of patients either show no response at all or eventually become resistant, underscoring the need for more targeted treatments and a deeper understanding of immune regulation (Kazemi et al., 2016; Klener et al., 2015). Among the key players in tumour immunology, Traditionally, CD4+ T-cells have been acknowledged for their role as helper cells that support cytotoxic CD8+ T-cell responses and B-cell activation (Alberts et al., 2002; Bevan, 2004). However, emerging evidence reveals that CD4+ T-cells are far more versatile. Beyond their helper function, certain CD4+ T-cell subsets possess direct cytotoxic activity and perform important functions in controlling other immune cells and modifying the tumor microenvironment (TME) by secreting cytokines. For example, Th1 and Th17 subsets are associated with potent anti-tumour effects, while regulatory T-cells (Tregs) often contribute to immune suppression and tumour tolerance (Accogli et al., 2021; Li et al., 2020). The dynamic balance between these subsets can significantly influence the ability of the immune system to control tumour growth and metastasis. Alongside these developments, nanotechnology has emerged as a valuable tool to enhance the efficacy and precision of cancer immunotherapies. Nanoformulations offer multiple advantages, including improved solubility and stability of therapeutic agents, targeted delivery, and controlled release kinetics (Jeevanandam et al., 2016) (Han and Park, 2023). These properties enable more effective modulation of the immune system with reduced systemic toxicity. Specifically, Tumor antigens, adjuvants, immune checkpoint inhibitors, or cytokines can all be delivered using nanoparticles. Directly to antigen-presenting cells or T-lymphocyte subsets, including CD4+ T-cells (Goldberg, 2019; Gao et al., 2019). Innovations such as ligand-functionalized nanoparticles, pH-responsive carriers, and biomimetic nanocarriers further enhance the ability to selectively modulate immune responses within the tumour microenvironment, thereby potentiating CD4+ T-cell activation and function (Niculescu and Grumezescu, 2022; Gowd et al., 2022).
In parallel with advancements in immunology, the evolution of Novel Drug Delivery Systems (NDDS) has significantly improved the accuracy and effectiveness of cancer treatments. By facilitating the regulated and site-specific distribution of therapeutic drugs, NDDS plays a crucial role in targeted therapy by reducing systemic toxicity and optimising drug accumulation at the tumour site (Theivendren et al., 2024). Liposomes, dendrimers, polymeric nanoparticles, and lipid-based nanoparticles have all demonstrated considerable potential among the many NDDS platforms (Hassanzadeh-Khanmiri et al., 2025). Their use in immunotherapy has been transformed by surface modification techniques, such as PEGylation for longer circulation time, ligand conjugation (e.g., antibodies, peptides, or aptamers) for receptor-mediated targeting, and pH- or enzyme-sensitive coatings for tumour microenvironment responsiveness (Maurya and Tyagi, 2024). These modified nanocarriers can be made to target certain immune cells, such as CD4+ T-cells, or elements of the cancer microenvironment, increasing the therapeutic index of checkpoint inhibitors, cytokines, and immunomodulators (Saeed et al., 2019). Among these methods, ligand-functionalized polymeric nanoparticles have become especially well-known because of their biocompatibility and adaptable surface chemistry, which enable improved tumour penetration and precise engagement with immunological targets (Anwar et al., 2024).
This review offers a unique perspective by integrating the immunological complexity of CD4+ T-cell subsets with the latest advancements in NDDS. While prior reviews have extensively discussed CD4+ T-cell biology, PEGylation, and checkpoint inhibitors separately, this article distinguishes itself by focusing on how nanocarriers such as liposomes, polymeric nanoparticles, dendrimers, and inorganic nanoparticles can be specifically designed to modulate subsets of CD4+ T-cells (e.g., Th1, Th17, Tregs) within the TME. The review also emphasizes novel approaches like MHC II-coated nanoparticles for ex vivo CD4+ T-cell expansion, dual CD4+/CD8+ activation through mRNA nanovaccines, and functionalized nanocarriers for the targeted delivery of cytokines or checkpoint inhibitors (Lee et al., 2021). By combining cutting-edge nanotechnology with immunological insights, this work highlights the transformative potential of nano-immunotherapy for targeting CD4+ T-cells in cancer treatment. It provides a comprehensive overview of CD4+ T-cells’ diverse roles in tumor suppression and promotion, as well as innovative NDDS-based strategies to enhance therapeutic outcomes. Moreover, the review explores challenges and opportunities for the clinical application of these novel strategies, filling a critical gap in existing literature that typically treats these fields separately (Shen et al., 2024).
2 CD4+ T-Cells in cancer immunology: a comprehensive exploration
2.1 Overview of CD4+ T-cells in the immune system and cancer microenvironment
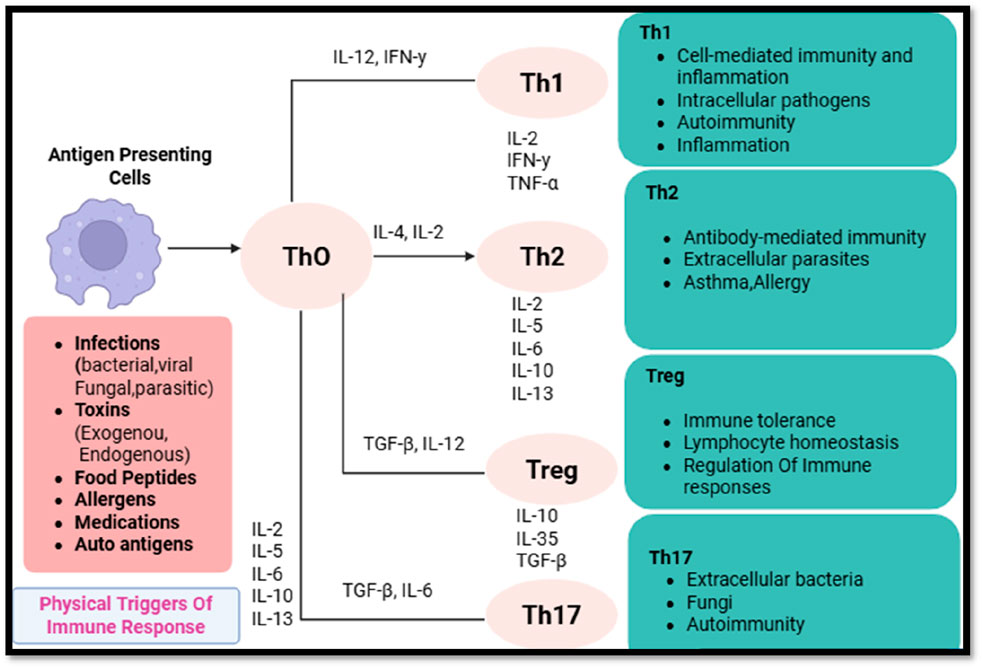
Helper T-cells are a major class of T-lymphocytes and play an important role in orchestrating adaptive immune responses. They determine the antigenic peptides linked with the major histocompatibility complex class II (MHC-II) molecules on dendritic cells, macrophages and B-cells, which are referred to as antigen-presenting cells (APCs),2 whose main role is to present these peptides to the major histocompatibility complex class II (MHC-II) molecules. These cells are then activated and differentiated into a variety of functional subtypes, each with specialized immune modulating activities (Borst et al., 2018; Speiser et al., 2023). CD4+ T-cells play a dual role in cancer immunology, either supporting immune evasion or enhancing anti-tumor immunity, depending on the surrounding cytokine milieu and cellular interactions within the TME. Their distinct functional differences, from driving cytotoxic responses to establishing an immunosuppressive niche, highlight their significance as both therapeutic targets and prognostic biomarkers in oncology.
2.2 Subtypes of CD4+ T-cells and their functional implications in cancer immunity
The differentiation of CD4+ T-cells into specialized subsets is governed by specific transcription factors, cytokine secretion patterns, and epigenetic modifications (Speiser et al., 2023). These subsets include helper T-cells (Th1, Th2, Th17, and Tfh), regulatory T-cells (Tregs), and effector/memory T-cells, each of which modulates tumor immunity in unique ways (Chatzileontiadou et al., 2021). CD4+ T-cell subset distinction and functions in reaction to antigens, depicted in Figure 1.
2.2.1 Helper T-cells (Th1, Th2, Th17, Tfh) and their contrasting roles
Th1 Cells: Th1 cells, A hallmark of these cells is the T-bet transcription factor, which mediates the secretion of interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), that, in turn, augments the cytolytic function of CD8+ T-cells and localizes natural killer (NK) cells. NK cells can recognize and kill tumor cells independent of antigen, and they secrete IFN-γ.26-28. In addition, IFN-γ promotes expression of MHC-I on tumor cells, thereby enhancing antigen presentation and cross-presentation of proinflammatory recognition (Haabeth et al., 2011). A strong Th1 response in the TME is correlated with good clinical outcomes because of the persisting inflammatory microenvironment that prevents tumors from spreading.
Th2 Cells: In contrast, Th2 cells, under the influence of the transcription factor GATA3, secrete IL-4, IL-5, IL-10, and IL-13, which skew immune responses towards humoral immunity and eosinophil activation (Jacenik et al., 2023). Th2-mediated cytokine profiles have been implicated in immune evasion by fostering an anti-inflammatory microenvironment that suppresses CD8+ T-cell cytotoxicity and promotes macrophage polarization towards the tumor-supportive M2 phenotype. Consequently, Th2-dominated immune responses are frequently correlated with poor prognostic outcomes across various malignancies.
Th17 Cells: Governed by the transcription factor RORγt, Th17 cells produce IL-17, IL-21, and IL-22, cytokines that exhibit a dual role in cancer biology. Acute IL-17 signaling can enhance anti-tumor immunity by recruiting neutrophils and promoting inflammation, yet chronic IL-17 exposure fosters immune tolerance, angiogenesis, and stromal remodeling, ultimately contributing to tumor progression and metastasis.
Tfh Cells: Follicular helper T-cells (Tfh), defined by Bcl-6 expression, primarily reside within germinal centers, where they assist in B-cell maturation and antibody production. Although their role in cancer immunity is less well-characterized, emerging evidence suggests that tumor-infiltrating Tfh cells may influence anti-tumor humoral responses, potentially affecting immunotherapeutic efficacy.
2.2.2 Regulatory T-cells (Tregs) and their immunosuppressive influence in tumors
Tregs, identified by FoxP3 expression, constitute a specialized subset of CD4+ T-cells dedicated to maintaining immune homeostasis by suppressing excessive inflammatory responses (Togashi et al., 2019). However, within the TME, Tregs contribute to immune evasion through several mechanisms:
• Secretion of immunosuppressive cytokines such as IL-10 and transforming growth factor-beta (TGF-β), which dampen effector T-cell activation and promote tissue remodeling.
• The expression of immunological checkpoint molecules that suppress T-cell receptor (TCR) signaling and prevent effector T-cell proliferation, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1).
• Metabolic competition for IL-2, depriving effector T-cells of a critical survival factor, thereby limiting their expansion and functionality.
Increased infiltration of Tregs in solid tumors correlates with poor prognostic outcomes, as their suppressive activity facilitates immune escape and tumor progression. Targeting Tregs through selective depletion or functional modulation remains a promising strategy in cancer immunotherapy.
2.2.3 Effector vs. memory CD4+ T-cells: implications for long-term cancer immunity
Effector CD4+ T-cells are short-lived and primarily function by secreting cytokines that drive immediate immune responses (Barnaba, 2022). In contrast, memory CD4+ T-cells, which can be categorized into central memory (Tcm) and effector memory (Tem) subsets, play a pivotal role in long-term tumor surveillance:
• Tcm Cells: Residing in secondary lymphoid organs, Tcm cells exhibit rapid proliferative capacity upon antigen re-exposure and sustain long-lasting immune responses.
• Tem Cells: Circulating in peripheral tissues, Tem cells mediate immediate effector responses upon antigen recognition, thus playing a critical role in controlling tumor recurrence.
The presence of tumor-specific memory CD4+ T-cells has been linked to improved survival outcomes, highlighting their significance as potential targets for cancer vaccines and adoptive cell therapies.
2.3 CD4+ T-cells and the TME
2.3.1 Cellular interactions and immune modulation
CD4+ T-cells dynamically interact with various immune cells within the TME, shaping the overall immune landscape of tumors (Accogli et al., 2021). These interactions include:
CD8+ T-Cells: CD4+ T-cells enhance CD8+ T-cell priming and expansion through IL-2 and IFN-γ secretion. However, in immunosuppressive TMEs, exhausted CD4+ T-cells fail to provide adequate support, leading to diminished CD8+ cytotoxic responses.
Macrophages: Th1-driven signaling promotes M1 macrophage polarization, enhancing anti-tumor activity, whereas Th2 and Tregs contribute to M2 macrophage differentiation, facilitating immune evasion.
Dendritic Cells: CD4+ T-cells modulate dendritic cell function by influencing antigen presentation and cytokine secretion. In tumor settings, tolerogenic dendritic cells may limit effective T-cell activation, contributing to immune escape.
2.3.2 Cytokine and chemokine regulation of CD4+ T-cells in tumors
Cytokines such as IL-2 (promoting T-cell proliferation), IFN-γ (enhancing antigen presentation), and IL-10 (mediating immune suppression) critically regulate CD4+ T-cell function in cancer. Furthermore, chemokines such as CCL5 and CXCL9 dictate CD4+ T-cell infiltration patterns within tumors, ultimately influencing the strength and nature of the anti-tumor immune response (Zhang Y. et al., 2020).
2.3.3 Tumor immune evasion mechanisms targeting CD4+ T-cells
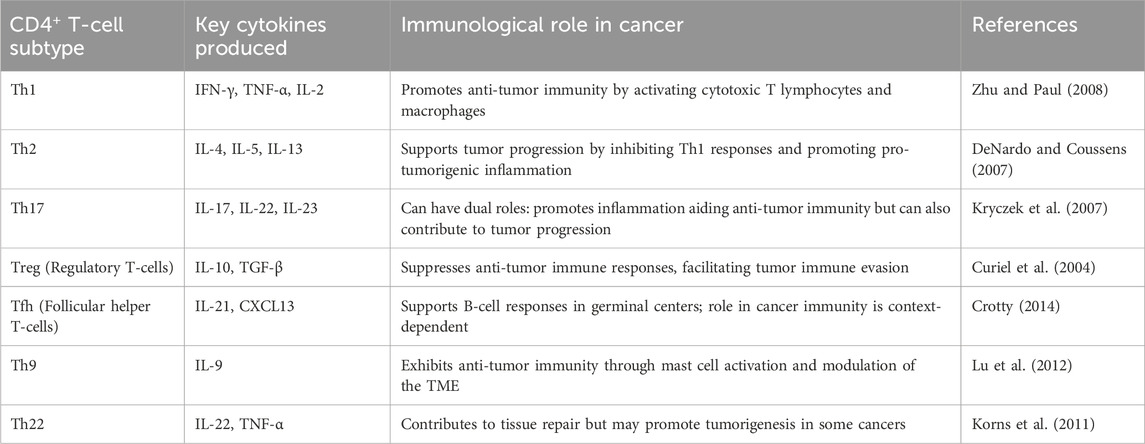
Tumors employ a variety of mechanisms to evade CD4+ T-cell-mediated immune responses. One key strategy involves the upregulation of PD-L1 and other immune checkpoint ligands, which suppress T-cell activation and function. Additionally, tumors secrete immunosuppressive cytokines such as TGF-β and IL-10, which influence CD4+ T-cell differentiation, favoring regulatory T cells (Tregs) and Th2 phenotypes that contribute to an immunosuppressive TME. Furthermore, tumors recruit immunosuppressive cell populations, including Tregs and myeloid-derived suppressor cells (MDSCs), which further dampen effective anti-tumor immunity. The various subtypes of CD4+ T cells and their immunological roles in cancer are summarized in Table 1 (Dobrzanski, 2013).
3 Mechanisms of CD4+ T-cells in tumor progression and suppression
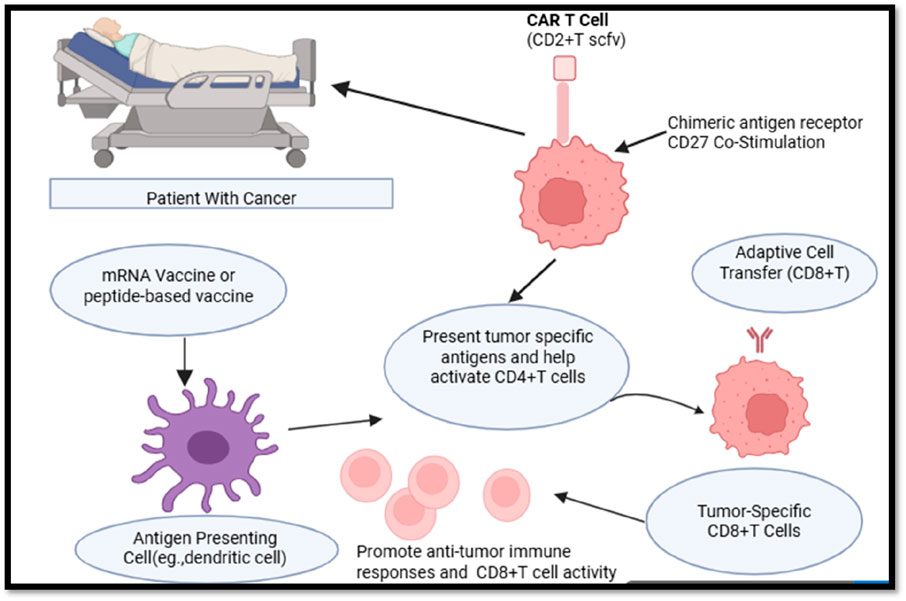
CD4+ T-cells play a dual role in cancer immunology, acting as both tumor-promoting and tumor-suppressing agents depending on their differentiation, cytokine profiles, and interactions with other immune components (Ostroumov et al., 2018). The majority of cancer immunotherapy techniques may benefit from including CD4+ activation techniques. T cell assistance or its byproducts. Peptides, or the DNA or mRNA that codes for them, are used in Both MHC class I and MHC class II epitopes should be included in therapeutic vaccinations to guarantee that CD4+ Activation of T cells in the answer. To overcome tumor-associated immune suppression, agonistic CD27 antibodies can be used to modulate the signaling pathways of CD4+ T-cells, effectively enhancing their helper functions and promoting robust T cell-mediated anti-tumor responses. These antibodies may also function in concert with PD1 inhibition. DC subsets that can prime CD4+ T cells and provide aid signals to cytotoxic T lymphocytes (CTLs) are essential for dendritic cell (DC)-based immunization. As an alternative, XC-chemokine receptor 1 (XCR1)+ may be the target of antigens and activation signals. DCs that migrate and stay in lymph nodes employing antibody-conjugates that are unique to this lineage of DC139. CD4+ should be included into adoptive cell treatment. T lymphocytes to aid at the location of the tumor or make advantage of preprogrammed assistance CD8+ T cells. When assistance signals are sent, transferred CTLs need to be increased. Cells that carry the signaling pattern of suitable co-stimulatory receptors may be used in chimeric antigen receptor (CAR) T cell therapy to replicate the delivery of assistance. The activation of conventional type 1 DCs (cDC1s) and their subsequent interaction with CD4+ T cells may be supported by treatments that elicit a type I interferon response, such as radiation or stimulator of interferon genes protein (STING) agonists (Figure 2).
3.1 Tumor-promoting CD4+ T Cells: Tregs and Th2 roles
Regulatory T-cells (Tregs) and T-helper 2 (Th2) cells are implicated in tumor progression due to their immunosuppressive functions. Tregs, identified by the expression of FOXP3, exert their immunosuppressive effects primarily through the secretion of inhibitory cytokines such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β) (Whiteside, 2012). These cytokines suppress effector T-cell proliferation, impair antigen-presenting cell (APC) function, and promote the development of an immune-privileged environment conducive to tumor growth. Furthermore, Tregs contribute to metabolic competition by consuming high levels of interleukin-2 (IL-2), thereby depriving effector T-cells of a critical survival signal.
Th2 cells promote a tumor-supportive milieu by secreting IL-4, IL-5, and IL-13, which facilitate alternative macrophage activation and suppress cytotoxic immune responses. The predominance of Th2-driven immunity is associated with poor prognosis in several malignancies, as it fosters an immunosuppressive microenvironment that inhibits effective tumor clearance.
3.1.1 Role of tregs in immune suppression
Tregs exert immunosuppressive effects through multiple mechanisms, including cytokine-mediated inhibition, metabolic disruption, and direct cell-cell interactions. The secretion of IL-10 and TGF-β not only suppresses CD8+ cytotoxic T lymphocytes (CTLs) but also inhibits the maturation and antigen-presenting capacity of dendritic cells (DCs) (Palomares et al., 2010). Tregs also engage in contact-dependent suppression via CTLA-4-mediated inhibition of co-stimulatory signaling on APCs, further dampening anti-tumor immune responses. Experimental depletion of Tregs in preclinical cancer models leads to enhanced T-cell infiltration and improved tumor control, highlighting their role as key mediators of immune evasion.
3.1.2 CD4+ T-cell exhaustion and dysfunction
Prolonged antigen exposure within the TME leads to the progressive dysfunction and exhaustion of CD4+ T-cells. Exhausted T-cells are characterized by the sustained upregulation of immune checkpoint receptors, including programmed cell death protein-1 (PD-1), T-cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), and lymphocyte activation gene-3 (LAG-3) (Miggelbrink et al., 2021; Xiao et al., 2022). T-cell exhaustion is characterized by the co-expression of inhibitory receptors on CD4+ T cells, including PD-1, CTLA-4, LAG-3, and TIM-3. A progressive loss of effector functions, such as decreased proliferative capacity, decreased production of important cytokines like IL-2, IFN-γ, and TNF-α, and impaired cytotoxic activity, are characteristics of this exhausted phenotype (Raziorrouh et al., 2014). Long-term antigenic stimulation in the TME causes these functional deficits, which are frequently exacerbated by immunosuppressive signals and metabolic stress. Consequently, worn-out CD4+ T cells are less able to coordinate efficient antitumor immunity or sustain cytotoxic CD8+ T-cell responses (Xiao et al., 2022). Especially in solid tumors where chronic antigen exposure is common, this dysfunction poses a significant challenge to cancer immunotherapy. Importantly, immune checkpoint blockade therapies, like anti-PD-1 and anti-CTLA-4 antibodies, have been developed as a result of the identification of this exhaustion program (Retnakumar et al., 2023). These therapies are intended to revitalize exhausted T cells and restore their functional potential. Although CD8+ T cells received most of the early attention from ICB, mounting data shows that CD4+ T-cell renewal is essential for mediating long-lasting and potent immunotherapy responses (Wang et al., 2022).
3.2 Tumor-suppressing CD4+ T-cells
3.2.1 Th1-mediated anti-tumor responses
Th1 cells play a pivotal role in anti-tumor immunity by orchestrating CD8+ T-cell responses and enhancing antigen presentation. The hallmark cytokines of Th1 cells, including interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), facilitate macrophage activation, upregulate major histocompatibility complex (MHC) expression on tumor cells, and potentiate CTL-mediated cytotoxicity (Ghiringhelli et al., 2004). The presence of Th1-skewed immunity is often associated with improved clinical outcomes in various cancers, as it promotes sustained anti-tumor immune responses and tumor cell apoptosis.
3.2.2 Th17 cells and immune activation
Th17 cells, characterized by the production of interleukin-17 (IL-17), contribute to immune activation by recruiting neutrophils and modulating inflammatory responses. However, their role in cancer is context-dependent (Kim et al., 2013). In some tumor settings, Th17 cells enhance immune surveillance and drive anti-tumor inflammation. Conversely, in certain malignancies, chronic Th17-driven inflammation can support tumor progression by fostering an immunosuppressive niche. The dualistic nature of Th17 responses underscores the complexity of CD4+ T-cell-mediated immune modulation in cancer.
3.3 Signaling pathways in CD4+ T-cell-mediated cancer immunity
3.3.1 PI3K/AKT, JAK/STAT, NF-κB pathway
The differentiation, survival, and effector activity of CD4+ T-cells are regulated by a variety of signaling pathways (Hwang et al., 2020). The phosphoinositide 3-kinase (PI3K)/AKT signaling axis is essential for the regulation of T-cell metabolism, proliferation, and survival (Mayer and Arteaga, 2016). In the context of cancer, PI3K activation enhances Treg function and promotes immunosuppression, whereas inactivating this pathway can restore effector T-cell responses and enhance anti-tumor immunity.
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway is integral to the differentiation of CD4+ T-cells and the signaling of cytokines (Leonard and Lin, 2000). STAT1 activation induces Th1 differentiation and IFN-γ production, whereas STAT3 signaling induces Th17 differentiation and IL-17 production. An disparity between” pro- and anti-tumor CD4+ T-cell subsets can be the consequence of dysregulation of this pathway, which can affect the progression of cancer and the effectiveness of therapy.
Inflammation and T-cell activation are both dependent on the nuclear factor kappa B (NF-κB) pathway. It facilitates the transcription of genes that are involved in the regulation of the immune system, cytokine production, and T-cell survival (Lawrence, 2009). Depending on the context, the activation of NF-κB within the TME can either enhance anti-tumor immunity or facilitate immune evasion.
3.3.2 Checkpoint regulation and CD4+ T-Cell exhaustion
The inhibitory checkpoint receptors PD-1, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and LAG-3 serve as critical regulators of CD4+ T-cell exhaustion (Fu et al., 2020). These molecules limit excessive immune activation but also contribute to immune suppression in cancer. Therapeutic blockade of PD-1 and CTLA-4 has demonstrated significant efficacy in reinvigorating exhausted T-cells and enhancing anti-tumor responses. Combining checkpoint inhibitors with strategies that promote CD4+ T-cell differentiation into effector subsets holds promise for improving the efficacy of cancer immunotherapies.
4 Nanoformulation-based approaches for targeting CD4+ T-cells in cancer therapy
4.1 Rationale for nanoformulation-based CD4+ T-cell therapy
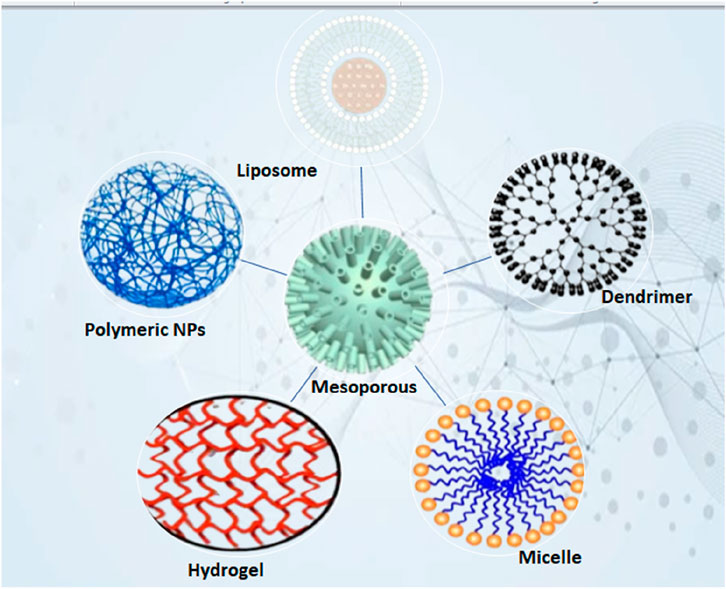
Nanoformulations provide an effective and well-regulated means of adjusting CD4+ T-cell responses in cancer immunotherapy. Nanocarriers enhance the bioavailability of drugs, shield therapeutic agents from degradation, and facilitate targeted delivery to CD4+ T cells, thereby minimizing off-target effects (Peer et al., 2007). Nanocarriers shield immunomodulators from enzymatic breakdown in the blood Figure 3. Functionalized nanoparticles provide selective uptake by CD4+ T cells, enhancing therapeutic efficacy. Nanoformulations have the capability to modulate Th1, Th17, and Treg responses for better tumor control (Park et al., 2013).

Figure 3. Schematic representation of a nanoformulation-based drug delivery system targeting CD4+ T-cells.
4.2 Types of nanocarriers for CD4+ T-cell modulation
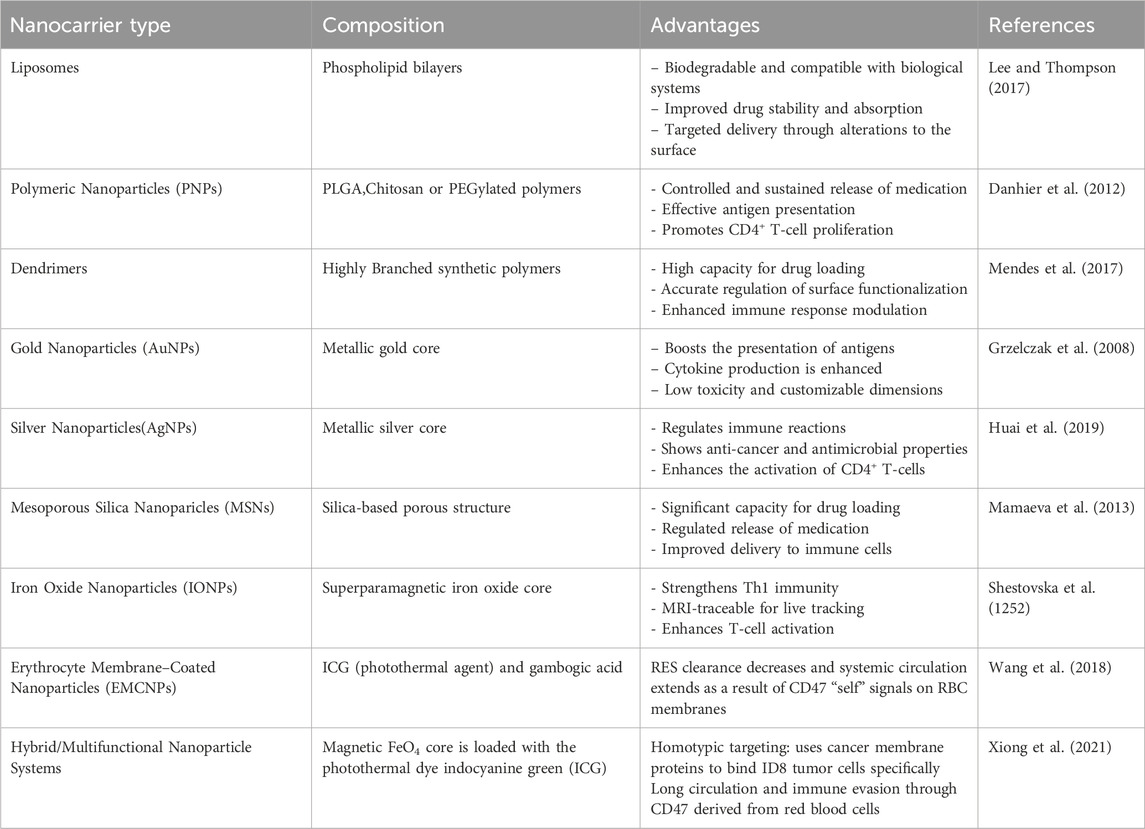
4.2.1 Liposomes
Phospholipid-based vesicles known as liposomes can encapsulate both hydrophilic and hydrophobic therapeutic agents, safeguarding them from enzymatic degradation and improving their targeted delivery to CD4+ T cells. Due to their structural flexibility and capacity to emulate biological membranes, they serve as excellent carriers for immunomodulatory treatments such as cancer immunotherapy, autoimmune disease management, and vaccine creation (Allen and Cullis, 2013). Liposomes safeguard enclosed medications or antigens from being broken down in the blood, thereby improving their therapeutic half-life (Figure 4). The use of functionalized liposomes enhances the uptake by antigen-presenting cells (APCs), which leads to a more efficient activation of CD4+ T cells. Liposomes can be designed for drug release that is sensitive to pH, temperature, or enzymes, enabling exact adjustment of CD4+ T-cell activation (Lee and Thompson, 2017).
4.2.2 Polymeric nanoparticles
Due to their low toxicity, biodegradability, and FDA approval, poly(lactic-co-glycolic acid) (PLGA) nanoparticles are among the most widely used polymeric carriers for targeted immune modulation. Research indicates that PLGA nanoparticles containing tumor antigens or immune adjuvants can foster antigen-specific CD4+ T-cell proliferation and cytokine release, thereby intensifying the Th1-mediated anti-tumor response. With regard to a slow and sustained release of IL-12 or IFN-γ, PLGA nanoparticles can be designed in such a way that they promote long-term activation of CD4+ T cells within TME. PLGA nanoparticles that carry both immune checkpoint inhibitors (such as anti-PD-L1) and cytokines enhance tumor regression mediated by CD4+ T cells (Danhier et al., 2012).
4.2.3 Dendrimers
Dendrimers, which are highly branched polymers at the nanoscale with precise size, shape, and surface characteristics, serve as outstanding carriers for targeted drug delivery, vaccine formulations, and immunomodulatory therapy Due to their high drug-loading capacity and tunable surface chemistry, Poly(amidoamine) (PAMAM) dendrimers have garnered interest in T-cell-targeted immunotherapies (Mendes et al., 2017). PAMAM dendrimers linked to TLR agonists or IL-12 greatly enhance Th1 polarization, resulting in elevated levels of TNF-α and IFN-γ.By enhancing the activation of CD4+ T-cells, tumor clearance is improved with the use of functionalized PAMAM dendrimers that encapsulate immune checkpoint inhibitors (such as anti-PD-1 and anti-CTLA-4) (Huai et al., 2019).
4.2.4 Gold and silver nanoparticles
Gold nanoparticles (AuNPs) and silver nanoparticles (AgNPs) are commonly utilized because of their distinctive immunomodulatory characteristics, compatibility with biological systems, and capacity to improve antigen presentation. The activation of dendritic cells is boosted by the conjugation of tumor antigens with gold nanoparticles, resulting in more robust priming of CD4+ T cells. AuNPs that are functionalized with CpG oligonucleotides enhance Th1 cytokine responses, thereby improving anti-tumor immunity (Grzelczak et al., 2008). Silver nanoparticles promote IL-12 secretion in dendritic cells, resulting in improved Th1 differentiation. AgNPs show selective cytotoxic effects on tumor cells, diminishing immunosuppressive factors and improving CD4+ T-cell function (Huai et al., 2019).
4.2.5 Inorganic nanoparticles (mesoporous silica, iron oxide)
Due to their large surface area, adjustable pore size, and ability for controlled drug release, mesoporous silica nanoparticles (MSNs) are valuable carriers for immunotherapies. Immune-stimulatory cytokines (IL-2, IL-15) loaded in MSNs facilitate targeted activation of CD4+ T-cells within the TME. MSNs can be engineered for the simultaneous delivery of tumor antigens and immune adjuvants, which boosts CD4+ T-cell proliferation (Mamaeva et al., 2013). Iron Oxide Nanoparticles (IONPs) for Th1 Immunity and MRI Tracking In the realm of cancer immunotherapy, iron oxide nanoparticles (IONPs) offer both therapeutic and diagnostic benefits. IONPs encourage the polarization of macrophages towards an M1 phenotype, enhancing CD4+ T-cell activation. IONPs enable the real-time monitoring of immune cell movement during T-cell therapy (Shestovska et al., 1252).
4.3 Functionalization of nanoparticles for targeted CD4+ T-Cell therapy
4.3.1 Surface modifications for CD4+ targeting
Nanoparticles linked with certain monoclonal antibodies (mAbs) boost their selectivity for CD4+ T cells. Anti-CD4-functionalized nanoparticles exhibit selective binding to CD4+ T cells, which enhances antigen-specific activation and drug delivery. T-cell receptor (TCR) activation is brought on by anti-CD3-coated nanoparticles, which enhances immunological responses and proliferation (Lo et al., 2013). Bispecific antibody-conjugated nanoparticles that target both CD4+ and tumor-associated antigens enhance immune synapse formation and amplify T-cell cytotoxicity (Qin et al., 2024). Immuno stimulatory cytokines can be added to nanoparticles to modify their function and development of CD4+T cells. IL-2-functionalized nanoparticles promote T-cell expansion and survival, thereby extending their anti-tumor activity. IL-2 attached to polymeric nanoparticles preferentially encourages the growth of effector T-cells over regulatory T-cells, enhancing Th1 responses (Raker et al., 2020).
4.3.2 PEGylation and ligand-based targeting
Opsonization and the mononuclear phagocyte system’s premature clearance are minimized through the use of PEGylated liposomes and polymeric nanoparticles. PEGylation enhances the biodistribution of nanoparticles, leading to increased tumor accumulation and extended immune activation. Excessive PEGylation over a long duration may hinder immune recognition; approaches such as reversible PEG coatings (e.g., cleavable PEG linkers) are under investigation to achieve a balance between stability and immunogenicity (Blanco et al., 2015). Targeting Based on Ligands By attaching to particular receptors found on CD4+ T cells, nanoparticles that are functionalized with ligands enhance selectivity. Folic acid-decorated nanoparticles preferentially attach to activated CD4+ T cells that express folate receptors, thereby improving targeted delivery (Table 2). Transferrin-coated nanoparticles enhance the uptake of CD4+ T-cells via receptor-mediated endocytosis, thus promoting effective drug delivery (Soe et al., 2019).
5 Applications of nano formulations in CD4+ T-cell-based cancer therapy
5.1 Nanoparticle-based drug delivery for CD4+ T-cell activation and regulation
5.1.1 Delivery of cytokines and immune modulators
Nanoparticles provide a flexible means for the targeted transport of cytokines and immunomodulators to CD4+ T cells, facilitating accurate immune response adjustment with reduced systemic toxicity (Peer et al., 2007). For CD4+ T-cells to proliferate and survive, IL-2 is necessary, especially Th1 and Treg subsets. Nanoparticles loaded with IL-2 enhance Th1-biased CD4+ responses, boosting IFN-γ and IL-2 secretion while improving cytotoxic activity against tumor cells (Malek, 2008). IL-12 is a strong immunostimulatory cytokine that drives Th1 differentiation and boosts IFN-γ production. By boosting CD4+ T-cell activation and antigen presentation, nanoparticles loaded with IL-12 (such as liposomes or hydrogels) trigger a robust anti-tumor immune response (Trinchieri, 2003). IFN-γ operates downstream of IL-12 and IL-2 signaling, playing a crucial role in macrophage activation, MHC upregulation, and anti-tumor effects. IFN-γ-loaded nanoparticles can be administered either intratumorally or systemically to aid in tumor rejection by enhancing CD4+ T-cell function and facilitating CTL priming (Schroder et al., 2004). Nanoparticles that allow for the controlled or sustained release of cytokines provide significant immunological benefits. These include avoiding CD4+ T-cell exhaustion, sustaining therapeutic cytokine concentrations in the TME, achieving spatially targeted immune modulation, and minimizing the necessity for repeated dosing—all of which contribute to improved safety, efficacy, and patient compliance (Blanco et al., 2015).
5.1.2 Encapsulation of tumor antigens for vaccine development
Encapsulating tumor-associated antigens (TAAs) like TRP-1 or gp100 peptides within nanoparticles (NPs) represents a promising approach for eliciting antigen-specific CD4+ T-cell responses. These nanosystems safeguard the antigen against enzymatic degradation, boost its delivery to antigen-presenting cells (APCs), and enhance lymph node trafficking—essential for triggering adaptive immunity (Liu et al., 2014). Immunostimulatory adjuvants like CpG oligodeoxynucleotides (TLR9 agonists) or Poly I:C (TLR3 agonist) can be co-formulated with nanoparticle vaccines. These adjuvants enhance dendritic cell (DC) maturation and improve antigen processing and presentation through MHC-II pathways, which is essential for CD4+ T-cell priming and Th1 polarization (Takahashi et al., 2009).
5.2 Nano vaccines for enhancing CD4+ T-cell responses
5.2.1 Peptide-based nano vaccines
Nanoparticles loaded with peptides safeguard them against enzymatic breakdown while enhancing their lymphatic transport and delivery to antigen-presenting cells (APCs). These nanoparticles improve MHC class II presentation by dendritic cells (DCs), resulting in heightened activation of CD4+ T-cells and their differentiation into Th1 effector cells (Buss et al., 2020). Nano-vaccines that are based on peptides are frequently combined with TLR agonists like CpG (TLR9) or MPLA (TLR4). This combination skews responses towards Th1 immunity and boosts IFN-γ production. These vaccines also aid in the formation of immunological memory, thereby ensuring long-term protection against tumor recurrence through the promotion of CD4+ T-helper memory subsets (Chehelgerdi et al., 2023).
5.2.2 mRNA and DNA nanovaccines
mRNA nanovaccines, which are encapsulated in lipid nanoparticles (LNPs), carry the genetic instructions for tumor-associated antigens (TAAs) and immunostimulatory proteins. These are translated in situ to elicit responses from both CD4+ and CD8+ T-cells. LNPs shield mRNA from degradation, enhance efficient endosomal escape, and aid in its delivery to dendritic cells and lymphoid tissues (Pardi et al., 2018). DNA vaccines that utilize electroporation for delivery or are encapsulated in polymeric nanoparticles (like PLGA, PEI) encode for TAAs that are expressed endogenously, leading to persistent antigen presentation. These platforms enhance the CD4+ T-cell support for cytotoxic T lymphocytes (CTLs), resulting in improved tumor cell destruction (Xu et al., 2020).
5.3 Combination strategies: nano formulations with other therapies
5.3.1 Chemo-immunotherapy using nanoparticles
DNA vaccines that utilize electroporation for delivery or are encapsulated in polymeric nanoparticles (like PLGA, PEI) encode for TAAs that are expressed endogenously, leading to persistent antigen presentation. These platforms enhance the CD4+ T-cell support for cytotoxic T lymphocytes (CTLs), resulting in improved tumor cell destruction. These systems lead to Damage-associated molecular patterns (DAMPs) and tumor antigens are released as a result of immunogenic cell death (ICD), which promotes dendritic cell maturation and CD4+ T-cell priming (Lang et al., 2024). This method converts “cold” tumors (those without immune infiltration) into “hot” tumors characterized by increased T-cell recruitment, elevated MHC expression, and enhanced immune visibility. In addition, using nanoparticles for targeted chemotherapy delivery minimizes systemic toxicity and maintains the viability of immune cells, especially CD4+ T-cells, which are generally vulnerable to drug-induced apoptosis (Mizrahy et al., 2017).
5.3.2 Radiation therapy combined with nano-immunotherapy
RT (radiation therapy) brings about death of immunogenic cells, which emits antigens linked to tumors that are able to be captured by APCs. However, due to the immune response produced by RT alone is frequently insufficient in the immunosuppressive TME (Demaria et al., 2015). Immunomodulators like STING agonists, cytokines, or checkpoint inhibitors can be delivered directly to irradiated tumors using nanoparticles, thereby boosting immune activation (Min et al., 2015). A striking instance concerns the use of nanoparticles to deliver anti-CD47 antibodies alongside radiotherapy. This approach encourages macrophages to phagocytose irradiated tumor cells, enhances antigen cross-presentation, and ultimately boosts the infiltration and activity of CD8+ and CD4+ T-cells (Boone et al., 2022). Crucially, nanoparticles allow for the spatial and temporal coordination of immunotherapy delivery with RT-induced immune priming, a strategy that has proven to enhance therapeutic efficacy and diminish immune-related adverse events (Ngwa et al., 2018).
5.3.3 Checkpoint inhibitors and nanoparticles
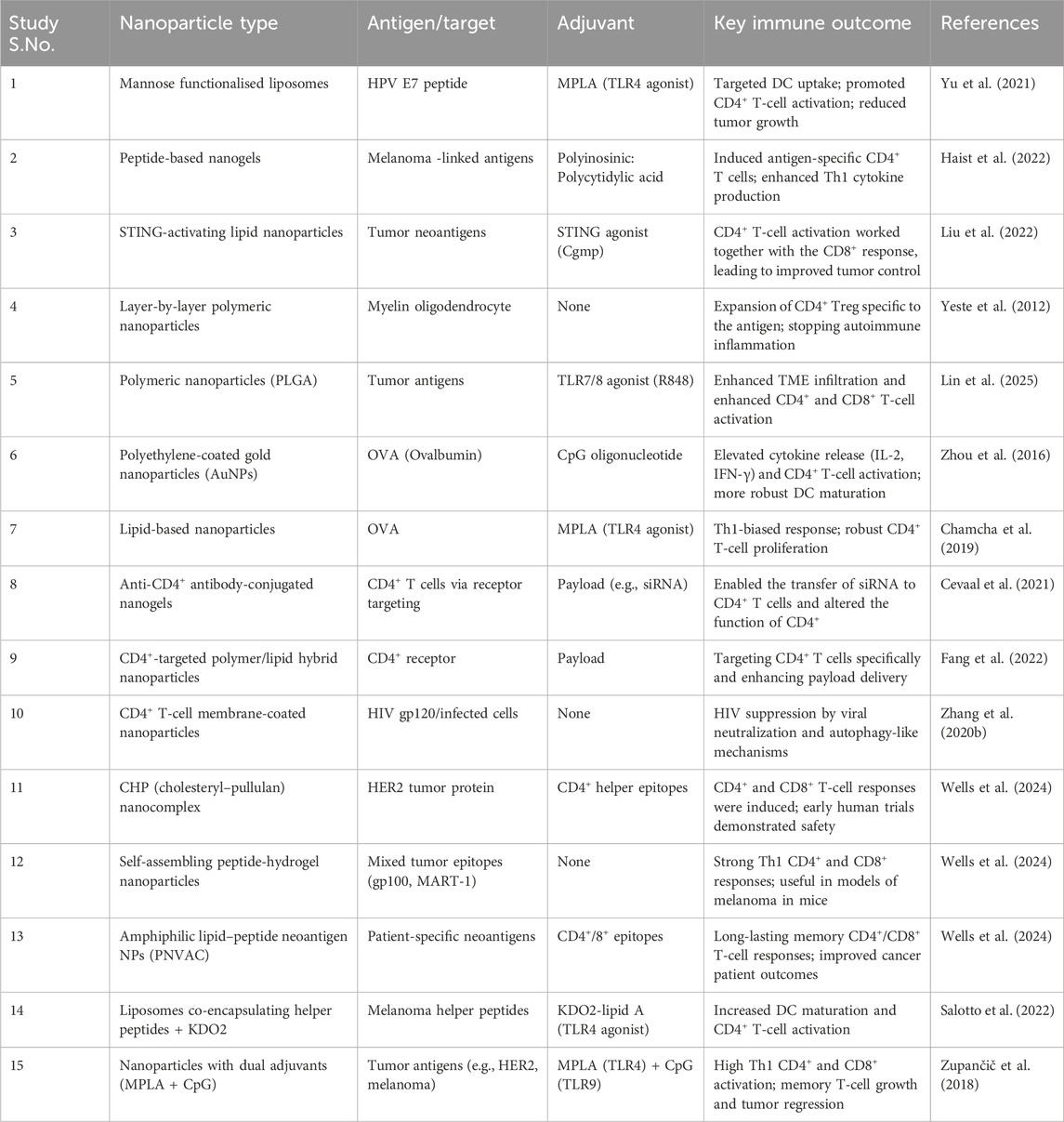
Immune checkpoint inhibitors (ICIs), including anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies, have revolutionized cancer immunotherapy by activating cytotoxic T lymphocytes against tumors (Topalian et al., 2015). Nevertheless, the systemic use of these agents is commonly associated with immune-related adverse effects (irAEs), including colitis., pneumonitis, and endocrinopathies, resulting from off-target immune activation. Furthermore, their effectiveness is restricted by their inadequate tumor penetration and brief half-life within the TME, particularly in poorly immunogenic or “cold” tumors (Postow et al., 2018). With the progression of research, nanotechnology is becoming recognized as a crucial facilitator of next-generation checkpoint immunotherapy (Table 3). It provides multifunctional platforms capable of enhancing drug delivery, adjusting the tumor immune landscape, and combining with additional therapies like radiotherapy and vaccinations (Irvine and Dane, 2020).
5.3.4 Surface modification strategies for enhanced targeting and biocompatibility
A key component of NDDS optimization for targeted therapy and enhanced biocompatibility is surface modification. These changes are essential for minimizing toxicity and off-target effects while guaranteeing that nanocarriers can interact with target cells (like CD4+ T-cells) in a selective manner (Anwar et al., 2024). PEGylation is a common surface modification technique that entails affixing polyethylene glycol (PEG) chains to the surface of nanoparticles. By decreasing recognition by the mononuclear phagocyte system (MPS), this alteration lengthens the circulation time, delaying rapid clearance and boosting the nanoparticles’ therapeutic efficacy (Pershina et al., 2023). Another approach is ligand-functionalization, in which nanoparticles are coupled with targeting ligands like aptamers, peptides, or antibodies to enable them to bind specifically to particular receptors on CD4+ T-cells or tumor cells. For instance, anti-CD4+ antibody-coated nanoparticles can enhance CD4+ T-cell targeting, increasing the accuracy of immunotherapeutic approaches. The controlled release of drugs within the TME can also be facilitated by the use of pH-sensitive or enzyme-responsive coatings (Wang Y. et al., 2021). These changes guarantee that the nanocarriers release their payload precisely where the local conditions (like a lower pH or particular enzymes) cause it to do so. These surface alterations can increase NDDS’s biocompatibility, improving therapeutic treatments’ safety profile and targeting effectiveness. To fully realize these strategies’ potential in maximizing cancer immunotherapy through CD4+ T-cell modulation, future discussions should go into greater detail (Richardson et al., 2021).
6 Recent advances in nanoformulations for CD4+ T-cell immunotherapy
6.1 MHC II-coated nanoparticles for CD4+ T-cell expansion
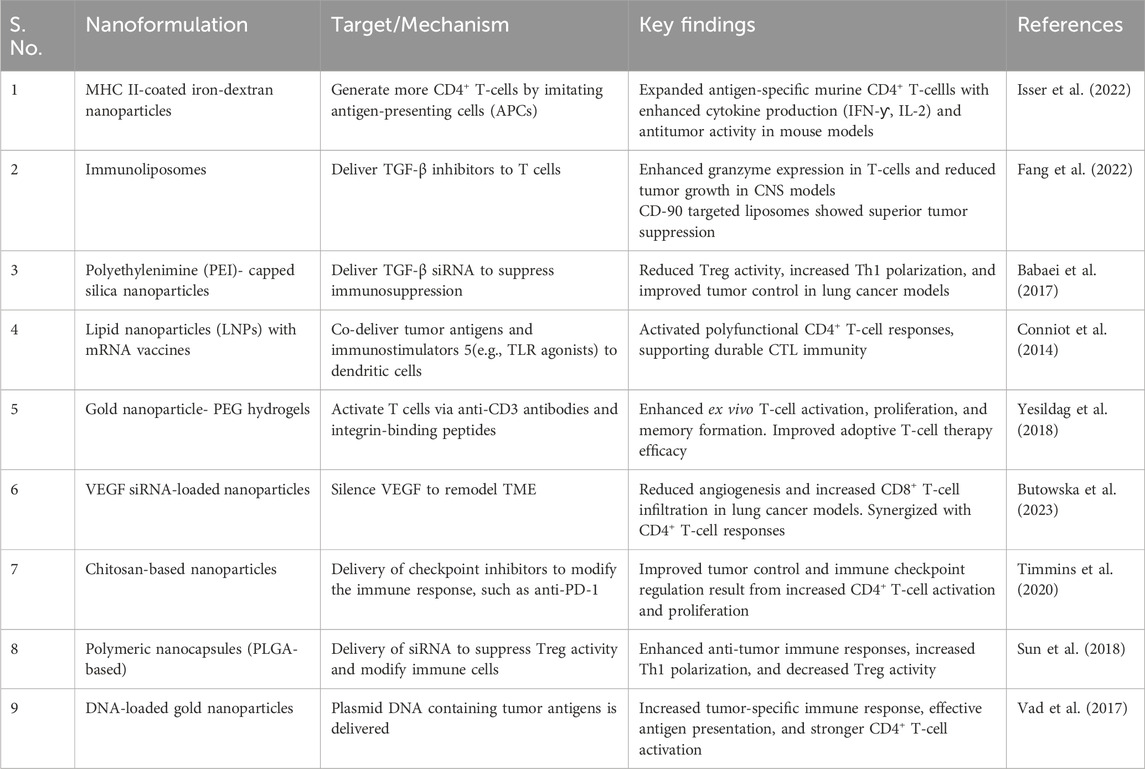
Iron-dextran nanoparticles functionalized with MHC II molecules and co-stimulatory proteins (e.g., CD80, CD86) mimic antigen-presenting cells (APCs) to expand antigen-specific CD4+ T cells ex vivo. These artificial APCs (aAPCs) enhance CD4+ T-cell effector functions, including cytokine production (IFN-γ, IL-2) and cytotoxic activity against tumors. In murine models, these aAPCs improved CD8+ T-cell responses by promoting memory formation and tumor infiltration, leading to sustained antitumor immunity (Isser et al., 2022).
6.2 mRNA nanoformulations for dual CD4+/CD8+ T-cell priming
Lipid nanoparticles (LNPs) encapsulating mRNA vaccines (e.g., encoding tumor-associated antigens) activate dendritic cells (DCs) in lymphoid organs (Table 4). This approach primes the CD8+ cytotoxic T lymphocytes (CTLs) and CD4+ T-helper cells at the same time. Preclinical studies show that LNPs co-delivering antigen mRNA and immunostimulatory molecules (e.g., TLR agonists) enhance cross-presentation by DCs, fostering polyfunctional CD4+ T-cell responses critical for durable antitumor immunity (Conniot et al., 2014).
6.3 Nanoparticle-mediated TGF-β suppression
Polyethylenimine (PEI)-capped silica nanoparticles loaded with TGF-β siRNA reduce immunosuppression in the TME. By silencing TGF-β, these nanoparticles decrease regulatory T-cell (Treg) activity and enhance CD4+ Th1 polarization, improving tumor control in lung cancer models (Khelil et al., 2022).
6.4 Immunoliposomes for T-cell activation
CD45- or CD90-targeted immunoliposomes encapsulating TGF-β inhibitors enhance adoptive T-cell therapy. Pre-incubation of T cells with these nanoparticles boosts granzyme expression and in vivo antitumor activity, particularly in central nervous system (CNS) tumor models (Conniot et al., 2014).
6.5 Clinical developments and clinical trails with NDDS
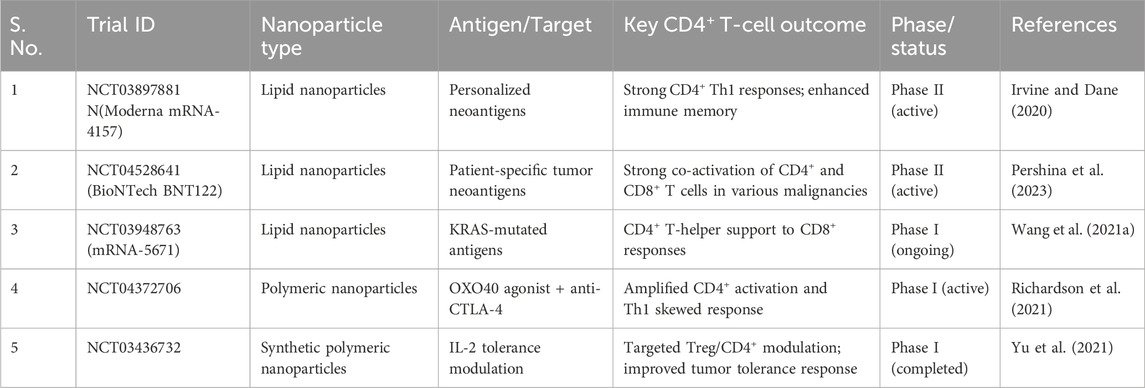
The table No. summarizes key clinical trials investigating nanoformulation -based drug delivery system concentrating on CD4+ T-cell reactions. Each trial highlights the use of different nanoparticle platforms designed to deliver specific tumor antigens or targets, aiming to enhance CD4+ T-cell activation and immune modulation (Lenders et al., 2020). The outcomes reported include improved helper T-cell responses, cytokine production, and overall immune support for anti-tumor activity (Table 5).
6.5.1 Adoptive transfer of nano-expanded CD4+ T cells
Under GMP settings, clinical experiments employing MHC II aAPCs show that tumor-specific CD4+ T cells can grow safely. In melanoma and lymphoma patients, these cells exhibit improved persistence and synergy with indigenous CD8+ T cells when co-administered with checkpoint inhibitors (e.g., anti-PD-1) (Yesildag et al., 2018).
6.5.2 Neoantigen-targeted CD4+ T-cell vaccines
Personalized mRNA vaccines (e.g., Moderna’s mRNA-4157) encoding patient-specific neoantigens induce polyfunctional CD4+ T-cell responses. In early-phase trials, vaccinated patients exhibited durable clinical responses, with CD4+ T cells recognizing neoantigens absent in healthy tissues (Yu et al., 2022).
6.5.3 Combination nano-immunotherapy
Trials combining nanoparticle-delivered OX40 agonists (CD4+ T-cell costimulators) with anti-CTLA-4 antibodies show improved tumor regression in advanced cancers. Nanoparticles localize OX40 agonists to tumor-draining lymph nodes, amplifying Th1 responses while minimizing systemic toxicity (Zhu and Li, 2023).
7 Challenges and limitations of nanoformulation-based CD4+ T-Cell therapy in cancer
Nanoformulation-based CD4+ T-cell therapies hold immense promise for cancer treatment by enhancing antitumor immune responses. However, several challenges limit their effectiveness and clinical translation. These challenges span biological barriers, stability issues, immunogenicity, and economic feasibility.
One of the critical hurdles in cancer immunotherapy is the ability to effectively deliver therapeutic agents to CD4+ T cells within the TME, which is highly immunosuppressive. Additionally, ensuring the stability and biocompatibility of nanoformulations while avoiding unintended immune responses remains a significant challenge. Finally, the high cost and scalability issues of these advanced therapies hinder their widespread adoption (Zhu and Li, 2023).
7.1 Biological barriers in CD4+ T-cell targeting
The TME presents formidable physical and immunological barriers that impede effective nanoparticle delivery to CD4+ T cells. Dense extracellular matrix (ECM), poor vascularization, and hypoxic conditions restrict nanoparticle penetration into tumors. For example, although MHC II-coated artificial antigen-presenting cells (aAPCs) have demonstrated efficacy in ex vivo expansion of antigen-specific CD4+ T cells, these obstacles restrict their in vivo delivery. Furthermore, when drug delivery systems enter the body, they can be unintentionally taken up by immune cells like macrophages. This process, known as nonspecific uptake, diverts the drug away from its intended target, such as tumor cells. As a result, the amount of drug reaching the tumor site is reduced. This not only lowers the treatment’s effectiveness but may also increase side effects in healthy tissues. Overcoming this challenge is crucial for improving the precision and success of targeted therapies. (Yu et al., 2022).
In CNS tumors, the blood-brain barrier (BBB) further complicates nanoparticle delivery. Immunoliposomes targeting CD45 or CD90 have shown promise in overcoming this issue by enhancing T-cell activation in CNS malignancies2. However, strategies like surface functionalization with tumor-specific ligands or size optimization (20–100 nm) are still required to improve lymph node trafficking and tumor infiltration.
7.2 Stability and biocompatibility of nanoformulations
Nanoformulations often face challenges related to stability during storage and circulation. Aggregation due to van der Waals forces or hydrophobic interactions alters nanoparticle size and distribution, leading to reduced efficacy. Protein corona formation—where serum proteins adsorb onto nanoparticle surfaces—can further alter their biological identity, leading to rapid clearance by the mononuclear phagocyte system (MPS) (Gavas et al., 2021).
Material toxicity is another concern. Inorganic nanoparticles like silica may induce oxidative stress, while polymeric carriers such as polyethylenimine (PEI) can cause cytotoxicity. To address these issues, surface modifications like PEGylation have been employed to improve stability and reduce immunogenicity. For example, PEG-functionalized carbon clusters have shown improved biocompatibility while targeting regulatory T cells (Tregs) in cancer models.
7.3 Potential side effects and immunogenicity of nanoparticles
Nanoparticles can elicit unintended immune responses that compromise their therapeutic potential. For instance, cytokine release syndrome (CRS) is a significant risk associated with overactivation of CD4+ T cells by MHC II aAPCs. Similarly, off-target effects may lead to autoimmunity or systemic inflammation when nanoparticles interact with non-cancerous tissues. Immunogenicity can also arise from the materials used in nanoparticle construction. Antibody-conjugated nanogels targeting CD4+ T cells may provoke anti-drug antibodies, reducing therapeutic efficacy over time5. Strategies such as using biodegradable materials or “stealth” coatings like polysaccharides are being explored to mitigate these risks (Zhu and Li, 2023; Lin et al., 2021).
7.4 Cost and scalability issues in clinical translation
One major obstacle to clinical translation is the expensive expense of producing nanoformulations under Good Manufacturing Practice (GMP) guidelines. Producing MHC II aAPCs or other complex nanoparticles involves rigorous quality control and specialized equipment, increasing costs by ∼30% compared to conventional therapies. Scalability is another major challenge. While modular platforms like plug-and-play aAPCs simplify production processes, reproducibility remains an issue for complex formulations such as cell-membrane-coated nanoparticles6. Microfluidic systems are being investigated for large-scale production but require further optimization for clinical use (Gavas et al., 2021).
7.5 Challenges and limitations: negative findings, off-target effects, and clinical trial failures
Although NDDS has great potential for cancer immunotherapy, expectations should be moderated due to a number of obstacles and contradictory findings in preclinical and clinical settings (Becht et al., 2024). The possibility of off-target effects, in which nanoparticles build up in tissues they are not intended to reach and may become toxic, is an important concern. For example, some nanoparticles have demonstrated a propensity to unintentionally activate immune cells that are not their intended target, like macrophages (Sousa-Ju et al., 2022). This can lead to inflammatory reactions or unintentional suppression of the intended immune activation. Furthermore, because nanoparticles are highly biocompatible and simple to surface functionalize, they occasionally cause unexpected interactions in the TME that alter immune responses and reduce the efficacy of treatment (Wang et al., 2023). Additionally, although a number of preclinical investigations have demonstrated encouraging outcomes in terms of modifying CD4+ T-cell responses with NDDS, it has proven more difficult to convert these results into fruitful clinical outcomes (Turtle, 2017). Results from clinical trials using checkpoint inhibitors and mRNA nanovaccines administered via nanoparticles have been inconsistent; some have shown insufficient immune response activation or limited tumor regression (Hongxia et al., 2019). The inability to accurately target all CD4+ T-cell subsets, the complexity of the TME, and the possibility of quick immune system clearance of nanoparticles continue to be major obstacles. These elements emphasize the necessity of more thorough investigation into the long-term safety and effectiveness of nanoparticle formulations in human trials, as well as more rigorous optimization of those formulations (Xiang et al., 2023).
7.6 NDDS and their manufacturing cost-benefit analysis with manufacturing challenges
A critical cost-benefit analysis of NDDS is necessary to assess the clinical translation of these systems in addition to their promising therapeutic benefits. Significant difficulties arise from the high cost of producing complex nanoformulations and the difficulty of manufacturing them under GMP guidelines (Su et al., 2022). Additionally, the commercial viability of customized nanocarriers or those that require complex surface modifications may be limited by the scale at which they can be produced. Making NDDS-based treatments more widely available in clinical settings may depend on resolving these problems through cost-cutting measures or more effective manufacturing techniques (Anwar et al., 2024).
7.7 Toxicity considerations in NDDS-based CD4+ T-cell immunotherapy
Since these systems interact with the immune system and other body tissues, a critical evaluation of toxicity is in fact necessary when evaluating NDDS for therapeutic purposes. NDDS have the benefit of targeted delivery, but they may also carry some toxicity risks, especially when it comes to accumulation in non-target organs, immune system activation, and off-target effects (Milling et al., 2017). Some nanocarriers, like liposomes and polymeric nanoparticles, can cause cytotoxicity or trigger inflammatory reactions, especially if they build up in vital organs like the kidneys or liver. Moreover, PEGylation and other surface alterations that improve targeting may also trigger immunological reactions that jeopardize the effectiveness of treatment (Li et al., 2022; Estapé et al., 2022). Furthermore, there are still questions about the long-term safety of NDDS due to the possibility of toxic byproducts being released from the degradation of nanomaterials and chronic exposure (Fadeel et al., 2017). Additionally, the immune system may become resistant to repeated nanoparticle dosages, decreasing their efficacy and possibly leading to allergic or hypersensitive reactions. To guarantee the safety of NDDS in CD4+ T-cell-based immunotherapy, future research should concentrate on enhancing the biocompatibility of nanocarriers, refining dosage schedules, and closely observing toxicological profiles in preclinical and clinical trials (El-Sayed et al., 2015; Mohammapdour and Ghandehari, 2022).
8 Future directions and perspectives in nanoformulation-based cancer immunotherapy
Nanoformulations are revolutionizing cancer immunotherapy by enabling precision targeting, enhancing delivery mechanisms, and integrating computational tools for therapy optimization. Emerging strategies focus on personalized approaches, smart nanocarriers, and artificial intelligence (AI)-driven advancements (Gao et al., 2021).
8.1 Personalized nanoformulation strategies for cancer immunotherapy
Personalized cancer immunotherapy leverages nanotechnology to tailor treatments based on individual tumor profiles. Lipid nanoparticles (LNPs) encapsulating mRNA encoding patient-specific neoantigens have shown promise in activating CD4+ helper T cells and CD8+ cytotoxic T lymphocytes (CTLs). These vaccines target unique tumor antigens identified through genomic sequencing, inducing robust immune responses. Additionally, metal-organic frameworks (MOFs) combine tumor-killing agents with immune stimulators, addressing tumor heterogeneity while boosting immune recognition.
Dendritic cell-based vaccines also exemplify personalized strategies, where patient-derived dendritic cells are loaded ex vivo with tumor antigens to stimulate adaptive immunity. Provenge®, A prostate cancer vaccine approved by the FDA, demonstrates the feasibility of such approaches despite challenges like labor-intensive manufacturing and high costs (Thirumalai et al., 2024).
8.2 Smart nanocarriers with controlled release mechanism
Smart nanocarriers improve drug bioavailability and minimize systemic toxicity by releasing therapeutic drugs in reaction to particular stimuli, including pH shifts or hypoxia, within the TME. Polymeric nanoparticles with pH-sensitive coatings release immunomodulators only in acidic environments typical of tumors. Self-assembling micelles and scaffolds have been developed for localized delivery of immune checkpoint inhibitors (e.g., anti-PD-1/PD-L1 antibodies), enhancing efficacy while reducing off-target effects.
Dual-release mechanisms in nanocarriers enable combination therapies by co-delivering immune checkpoint inhibitors and tumor antigens, synergistically reversing immune evasion and activating T cells (Wang J. et al., 2021).
8.3 Role of artificial intelligence in nanomedicine development
AI and machine learning (ML) optimize nanoformulations by predicting nanoparticle interactions with immune cells and the TME. These technologies accelerate development by simulating biodistribution, stability profiles, and therapeutic efficacy under varying physiological conditions. AI-driven platforms identify optimal combinations of nanomaterials, payloads, and targeting ligands for specific cancer types while facilitating patient stratification based on biomarkers indicating responsiveness to nano-immunotherapy.
AI also enhances scalability by optimizing manufacturing processes for consistent production under GMP conditions, reducing costs while ensuring quality (Skepu et al., 2023).
9 Conclusion
This review highlights the critical role of CD4+ T-cells in cancer immunology, demonstrating their dual function as both tumor-suppressing and tumor-promoting agents. The dynamic interplay of CD4+ T-cell subsets, including Th1, Th2, Th17, Tregs, and Tfh, significantly influences the TME and patient outcomes. Targeting CD4+ T-cells with NDDS has become a viable tactic to improve treatment efficacy as immunotherapy advances. Nanoparticle-based formulations, including liposomes, polymeric nanoparticles, dendrimers, and metallic nanoparticles, have shown remarkable potential in modulating CD4+ T-cell responses, providing targeted delivery of cytokines, antigens, and immune checkpoint inhibitors. The advantages of NDDS include improved bioavailability, controlled release, reduced systemic toxicity, and enhanced targeting in the TME of CD4+ T-cells. Specific applications, such as MHC II-coated nanoparticles for CD4+ T-cell expansion, mRNA vaccines for dual CD4+/CD8+ activation, and TGF-β suppression with silica nanoparticles, demonstrate the versatility of NDDS in enhancing antitumor immunity. However, challenges persist, including biological barriers, stability, immunogenicity, and scalability issues, which limit the clinical translation of NDDS-based CD4+ T-cell therapies.
Future research should prioritize overcoming these challenges by optimizing nanoparticle design, improving targeting specificity, and integrating personalized medicine approaches. Additionally, leveraging artificial intelligence (AI) for nanoparticle optimization and combining NDDS with conventional therapies may further enhance therapeutic outcomes. By bridging the gap between nanotechnology and immunology, NDDS-based CD4+ T-cell
Author contributions
NS: Writing – original draft. AA: Writing – original draft. AR: Writing – original draft. FH: Writing – original draft. MA: Writing – review and editing, Conceptualization, Project administration. ZS: Writing – review and editing. NS: Writing – review and editing. KT: Writing – review and editing. SM: Supervision, Conceptualization, Writing – review and editing, Funding acquisition.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors sincerely thank Universiti Malaya for their support and for providing a research grant under the University Grant Research Program - Research Cluster No: CORG002-2025.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Accogli, T., Bruchard, M., and Végran, F. (2021). Modulation of CD4 T cell response according to tumor cytokine microenvironment. Cancers 13, 373. doi:10.3390/cancers13030373
Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K., and Walter, P. (2002). “Helper T cells and lymphocyte activation,” in Molecular biology of the cell. 4th edition (Garland Science).
Allen, T. M., and Cullis, P. R. (2013). Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 65, 36–48. doi:10.1016/j.addr.2012.09.037
Anwar, D. M., Hedeya, H. Y., Ghozlan, S. H., Ewas, B. M., and Khattab, S. N. (2024). Surface-modified lipid-based nanocarriers as a pivotal delivery approach for cancer therapy: application and recent advances in targeted cancer treatment. Beni-Suef Univ. J. Basic Appl. Sci.;13(1):106. doi:10.1186/s43088-024-00566-x
Babaei, M., Eshghi, H., Abnous, K., Rahimizadeh, M., and Ramezani, M. (2017). Promising gene delivery system based on polyethylenimine-modified silica nanoparticles. Cancer Gene Ther. 24 (4), 156–164. doi:10.1038/cgt.2016.73
Barnaba, V. T. (2022). T cell memory in infection, cancer, and autoimmunity. Front. Immunol. 12, 811968. doi:10.3389/fimmu.2021.811968
Becht, R., Kiełbowski, K., and Wasilewicz, M. P. (2024). New opportunities in the systemic treatment of hepatocellular carcinoma—today and tomorrow. Int. J. Mol. Sci. 25, 1456. doi:10.3390/ijms25031456
Bevan, M. J. (2004). Helping the CD8(+) T-cell response. Nat. Rev. Immunol. 4, 595–602. doi:10.1038/nri1413
Blanco, E., Shen, H., and Ferrari, M. (2015). Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951. doi:10.1038/nbt.3330
Boone, C. E., Wang, L., Gautam, A., Newton, I. G., and Steinmetz, N. F. (2022). Combining nanomedicine and immune checkpoint therapy for cancer immunotherapy. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology. 14, e1739. doi:10.1002/wnan.1739
Borst, J., Ahrends, T., Bąbała, N., Melief, C. J. M., and Kastenmüller, W. (2018). CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 18, 635–647. doi:10.1038/s41577-018-0044-0
Buss, C. G., Bhatia, S. N., Bashir, R., and Heller, D. A. (2020). Nanoparticle delivery of immunostimulatory oligonucleotides enhances response to checkpoint inhibitor therapeutics. Proc Natl Acad Sci U S A. doi:10.1073/pnas.2001569117
Butowska, K., Han, X., Gong, N., El-Mayta, R., Haley, R. M., Xue, L., et al. (2023). Doxorubicin-conjugated siRNA lipid nanoparticles for combination cancer therapy. Acta Pharm. Sin. B 13 (4), 1429–1437. doi:10.1016/j.apsb.2022.07.011
Cevaal, P. M., Ali, A., Czuba-Wojnilowicz, E., Symons, J., Lewin, S. R., Cortez-Jugo, C., et al. (2021). In vivo T cell-targeting nanoparticle drug delivery systems: considerations for rational design. ACS Nano. 15, 3736, 3753. doi:10.1021/acsnano.0c09514
Chamcha, V., Reddy, P. B. J., Kannanganat, S., Wilkins, C., Gangadhara, S., Velu, V., et al. (2019). Strong TH1-biased CD4 T cell responses are associated with diminished SIV vaccine efficacy. Sci. Transl. Med. 11 (519), eaav1800. doi:10.1126/scitranslmed.aav1800
Chatzileontiadou, D. S. M., Sloane, H., Nguyen, A. T., Gras, S., and Grant, E. J. (2021). The many faces of CD4+ T cells: immunological and structural characteristics. Int. J. Mol. Sci. 22, 73. doi:10.3390/ijms22010073
Chehelgerdi, M., Chehelgerdi, M., Kabiri, H., Salehian-Dehkordi, H., and Abdolvand, M. (2023). Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy. Mol. Cancer 22, 189. doi:10.1186/s12943-023-01873-0
Conniot, J., Silva, J. M., Fernandes, J. G., Silva, L. C., Gaspar, R., Brocchini, S., et al. (2014). Cancer immunotherapy: nanodelivery approaches for immune cell targeting and tracking. Front. Chem. 2, 105. doi:10.3389/fchem.2014.00105
Crotty, S. (2014). T follicular helper cell differentiation, function, and roles in disease. Immunity 41, 529–542. doi:10.1016/j.immuni.2014.10.004
Curiel, T. J., Coukos, G., Zou, L., Alvarez, X., Cheng, P., Mottram, P., et al. (2004). Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 10 (9), 942–949. doi:10.1038/nm1093
Danhier, F., Ansorena, E., Silva, J. M., Coco, R., Le Breton, A., and Préat, V. (2012). PLGA-based nanoparticles: an overview of biomedical applications. J. Control Release 161 (2), 505–522. doi:10.1016/j.jconrel.2012.01.043
Demaria, S., Golden, E. B., and Formenti, S. C. (2015). Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 1, 1325–1332. doi:10.1001/jamaoncol.2015.2756
DeNardo, D. G., and Coussens, L. M. (2007). Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 9, 212. doi:10.1186/bcr1746
Dobrzanski, M. J. (2013). Expanding roles for CD4 T cells and their subpopulations in tumor immunity and therapy. Front. Oncol. 3 (MAR), 63. doi:10.3389/fonc.2013.00063
El-Sayed, Y. S., Shimizu, R., Onoda, A., Takeda, K., and Umezawa, M. (2015). Carbon black nanoparticle exposure during middle and late fetal development induces immune activation in male offspring mice. Toxicology 327, 53–61. doi:10.1016/j.tox.2014.11.005
Estapé, S. M., de Jongh, C. A., Dijkxhoorn, K., Verhoef, J. J. F., Szebeni, J., Storm, G., et al. (2022). Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J. Control Release 341, 475–486. doi:10.1016/j.jconrel.2021.11.042
Fadeel, B., Pietroiusti, A., and Shvedova, A. A. (2017). Adverse effects of engineered nanomaterials: exposure, toxicology, and impact on human health. Second Edition.
Fang, P., Han, L., Liu, C., Deng, S., Zhang, E., Gong, P., et al. (2022). Dual-regulated functionalized liposome-nanoparticle hybrids loaded with dexamethasone/TGFβ1-siRNA for targeted therapy of glomerulonephritis. ACS Appl. Mater Interfaces 14 (1), 307–323. doi:10.1021/acsami.1c20053
Finn, O. J. (2012). Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann. Oncol. 23, viii6–viii9. doi:10.1093/annonc/mds256
Fu, J., Yu, A., Xiao, X., Tang, J., Zu, X., Chen, W., et al. (2020). CD4+ T cell exhaustion leads to adoptive transfer therapy failure which can be prevented by immune checkpoint blockade. Am. J. Cancer Res. 10 (12), 4234–4250.
Gao, S., Yang, D., Fang, Y., Lin, X., Jin, X., Wang, Q., et al. (2019). Engineering nanoparticles for targeted remodeling of the tumor microenvironment to improve cancer immunotherapy. Theranostics 9 (1), 126–151. doi:10.7150/thno.29431
Gao, Y., Shen, M., and Shi, X. (2021). Interaction of dendrimers with the immune system: an insight into cancer nanotheranostics. View (Beijing). 2 (VIEW). doi:10.1002/viw.20200120
Gavas, S., Quazi, S., and Karpiński, T. M. (2021). Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res. Lett. 16, 173. doi:10.1186/s11671-021-03628-6
Ghiringhelli, F., Larmonier, N., Schmitt, E., Parcellier, A., Cathelin, D., Garrido, C., et al. (2004). CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 34 (2), 336–344. doi:10.1002/eji.200324181
Goldberg, M. S. (2019). Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 19, 587–602. doi:10.1038/s41568-019-0186-9
Gowd, V., Ahmad, A., Tarique, M., Suhail, M., Zughaibi, T. A., Tabrez, S., et al. (2022). Advancement of cancer immunotherapy using nanoparticles-based nanomedicine. Seminars Cancer Biol. 86, 624–644. doi:10.1016/j.semcancer.2022.03.026
Grzelczak, M., Pérez-Juste, J., Mulvaney, P., and Liz-Marzán, L. M. (2008). Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 37 (9), 1783–1791. doi:10.1039/b711490g
Haabeth, O. A. W., Lorvik, K. B., Hammarström, C., Donaldson, I. M., Haraldsen, G., Bogen, B., et al. (2011). Inflammation driven by tumour-specific Th1 cells protects against B-cell cancer. Nat. Commun. 2 (1), 240. doi:10.1038/ncomms1239
Haist, M., Mailänder, V., and Bros, M. (2022). Nanodrugs targeting T cells in tumor therapy. Front. Immunol. 13, 912594. doi:10.3389/fimmu.2022.912594
Han, J., and Park, J. H. (2023). Modulation of immune cells with mRNA nanoformulations for cancer immunotherapy. Curr. Opin. Biotechnol. 84, 103014. doi:10.1016/j.copbio.2023.103014
Hassanzadeh-Khanmiri, M., Moshari, A., Kheradmand, R., Haghgouei, T., Homaei, M., Charsouei, S., et al. (2025). Nanomedicine: a cost-effective and powerful platform for managing neurodegenerative diseases. Metab. Brain Dis. 40 (3), 142. doi:10.1007/s11011-025-01564-3
Hongxia, Z., Xinru, Y., Xiaojuan, W., Zining, W., Feifei, X., Jun, W., et al. (2019). Abstract LB-205: a lipoplex-based mRNA nanovaccine for cancer immunotherapy. Cancer Res. 79 (13_Suppl. ment), LB-205. doi:10.1158/1538-7445.am2019-lb-205
Huai, Y., Hossen, M. N., Wilhelm, S., Bhattacharya, R., and Mukherjee, P. (2019). Nanoparticle interactions with the tumor microenvironment. Bioconjugate Chem. 30, 2247–2263. doi:10.1021/acs.bioconjchem.9b00448
Hwang, J. R., Byeon, Y., Kim, D., and Park, S. G. (2020). Recent insights of T cell receptor-mediated signaling pathways for T cell activation and development. Exp. Mol. Med. 52, 750–761. doi:10.1038/s12276-020-0435-8
Irvine, D. J., and Dane, E. L. (2020). Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 20, 321–334. doi:10.1038/s41577-019-0269-6
Isser, A., Silver, A. B., Pruitt, H. C., Mass, M., Elias, E. H., Aihara, G., et al. (2022). Nanoparticle-based modulation of CD4+ T cell effector and helper functions enhances adoptive immunotherapy. Nat. Commun. 13 (1), 6086. doi:10.1038/s41467-022-33597-y
Jacenik, D., Karagiannidis, I., and Beswick, E. J. (2023). Th2 cells inhibit growth of colon and pancreas cancers by promoting anti-tumorigenic responses from macrophages and eosinophils. Br. J. Cancer 128 (2), 387–397. doi:10.1038/s41416-022-02056-2
Jeevanandam, J., Chan, Y. S., and Danquah, M. K. (2016). Nano-formulations of drugs: recent developments, impact and challenges. Biochimie, 128–129. doi:10.1016/j.biochi.2016.07.008
Kazemi, T., Younesi, V., Jadidi-Niaragh, F., and Yousefi, M. (2016). Immunotherapeutic approaches for cancer therapy: an updated review. Vol. 44, Artificial Cells. Nanomedicine Biotechnol. doi:10.3109/21691401.2015.1019669
Khelil, M. B., Godet, Y., Abdeljaoued, S., Borg, C., Adotévi, O., and Loyon, R. (2022). Harnessing antitumor CD4+ T cells for cancer immunotherapy. Cancers 14, 260. doi:10.3390/cancers14010260
Kim, R. (2007). “Cancer immunoediting: from immune surveillance to immune escape,” in Cancer immunotherapy: immune suppression and tumor growth.
Kim, C. J., McKinnon, L. R., Kovacs, C., Kandel, G., Huibner, S., Chege, D., et al. (2013). Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J. Immunol. 191 (5), 2164–2173. doi:10.4049/jimmunol.1300829
Klener, P., Otahal, P., Lateckova, L., and Klener, P. (2015). Immunotherapy approaches in cancer treatment. Curr. Pharm. Biotechnol. 16 (9), 771–781. doi:10.2174/1389201016666150619114554
Korns, D., Frasch, S. C., Fernandez-Boyanapalli, R., Henson, P. M., and Bratton, D. L. (2011). Modulation of macrophage efferocytosis in inflammation. Front. Immunol. 2 (NOV), 57–10. doi:10.3389/fimmu.2011.00057
Kryczek, I., Wei, S., Vatan, L., Escara-Wilke, J., Szeliga, W., Keller, E. T., et al. (2007). Cutting edge: opposite effects of IL-1 and IL-2 on the regulation of IL-17+ T cell pool IL-1 subverts IL-2-mediated suppression. J. Immunol. 179 (3), 1423–1426. doi:10.4049/jimmunol.179.3.1423
Lang, X., Wang, X., Han, M., and Guo, Y. (2024). Nanoparticle-mediated synergistic chemoimmunotherapy for cancer treatment. Int. J. Nanomedicine 19, 4533–4568. doi:10.2147/IJN.S455213
Lawrence, T. (2009). The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1, a001651. doi:10.1101/cshperspect.a001651
Lee, Y., and Thompson, D. H. (2017). Stimuli-responsive liposomes for drug delivery. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology. 9. doi:10.1002/wnan.1450
Lee, N. K., Kim, S. N., and Park, C. G. (2021). Immune cell targeting nanoparticles: a review. Biomaterials Res. 25, 44. doi:10.1186/s40824-021-00246-2
Lenders, V., Koutsoumpou, X., Sargsian, A., and Manshian, B. B. (2020). Biomedical nanomaterials for immunological applications: ongoing research and clinical trials. Nanoscale Adv. 2, 5046–5089. doi:10.1039/d0na00478b
Leonard, W. J., and Lin, J. X. (2000). Cytokine receptor signaling pathways. J. Allergy Clin. Immunol. 105 (5), 877–888. doi:10.1067/mai.2000.106899
Li, C., Jiang, P., Wei, S., Xu, X., and Wang, J. (2020). Regulatory T cells inTME: new mechanisms, potential therapeutic strategies and future prospects, 19. Molecular Cancer.
Li, Y., Lofchy, L., Wang, G., Gaikwad, H., Fujita, M., and Simberg, D. (2022). PEGylated liposomes accumulate in the areas relevant to skin toxicities via passive extravasation across “leaky” endothelium. ACS Nano. 16 (4), 6349–6358. doi:10.1021/acsnano.2c00423
Lin, X., Wang, X., Gu, Q., Lei, D., Liu, X., and Yao, C. (2021). Emerging nanotechnological strategies to reshape tumor microenvironment for enhanced therapeutic outcomes of cancer immunotherapy. Biomed. Mater 16 (4), 042001. doi:10.1088/1748-605X/abe7b3
Lin, Y., Lin, P., Xu, R., Chen, X., Lu, Y., Zheng, J., et al. (2025). Nanovaccines empowering CD8+ T cells: a precision strategy to enhance cancer immunotherapy. Theranostics. 15 (7), 3098–3121. doi:10.7150/thno.107856
Liu, H., Moynihan, K. D., Zheng, Y., Szeto, G. L., Li, A. V., Huang, B., et al. (2014). Structure-based programming of lymph-node targeting in molecular vaccines. Nat 507 (7493), 519–522. doi:10.1038/nature12978
Liu, W., Alameh, M. G., Yang, J. F., Xu, J. R., Lin, P. J. C., Tam, Y. K., et al. (2022). Lipid nanoparticles delivering constitutively active STING mRNA to stimulate antitumor immunity. Int. J. Mol. Sci. 23 (23), 14504. doi:10.3390/ijms232314504
Lo, Y. C., Edidin, M. A., and Powell, J. D. (2013). Selective activation of antigen-experienced T cells by anti-CD3 constrained on nanoparticles. J. Immunol. 191 (10), 5107–5114. doi:10.4049/jimmunol.1301433
Lu, Y., Hong, S., Li, H., Park, J., Hong, B., Wang, L., et al. (2012). Th9 cells promote antitumor immune responses in vivo. J. Clin. Invest 122 (11), 4160–4171. doi:10.1172/JCI65459
Malek, T. R. (2008). The biology of interleukin-2. Annu. Rev. Immunol. 26, 453–479. doi:10.1146/annurev.immunol.26.021607.090357
Mamaeva, V., Sahlgren, C., and Lindén, M. (2013). Mesoporous silica nanoparticles in medicine—recent advances. Adv. Drug Deliv. Rev. 65 (5), 689–702. doi:10.1016/j.addr.2012.07.018
Maurya, A., and Tyagi, S. (2024). Recent advances in nanoparticle-based drug delivery systems. J. Drug Discov. Heal Sci. 1 (04), 201–211. doi:10.21590/jddhs.01.04.03
Mayer, I. A., and Arteaga, C. L. (2016). The PI3K/AKT pathway as a target for cancer treatment. Annu. Rev. Med. 67, 11–28. doi:10.1146/annurev-med-062913-051343
Mendes, L. P., Pan, J., and Torchilin, V. P. (2017). Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 22. doi:10.3390/molecules22091401
Miggelbrink, A. M., Jackson, J. D., Lorrey, S. J., Srinivasan, E. S., Waibl-Polania, J., Wilkinson, D. S., et al. (2021). CD4 T-Cell exhaustion: does it exist and what are its roles in cancer? Clin. Cancer Res. 27, 5742–5752. doi:10.1158/1078-0432.CCR-21-0206
Milling, L., Zhang, Y., and Irvine, D. J. (2017). Delivering safer immunotherapies for cancer. Adv. Drug Deliv. Rev. 114, 79–101. doi:10.1016/j.addr.2017.05.011
Min, Y., Caster, J. M., Eblan, M. J., and Wang, A. Z. (2015). Clinical translation of nanomedicine. Chem. Rev. 115, 11147–11190. doi:10.1021/acs.chemrev.5b00116
Mizrahy, S., Hazan-Halevy, I., Landesman-Milo, D., Ng, B. D., and Peer, D. (2017). Advanced strategies in immune modulation of cancer using lipid-based nanoparticles. Front. Immunol. 8 (FEB), 69. doi:10.3389/fimmu.2017.00069
Mohammapdour, R., and Ghandehari, H. (2022). Mechanisms of immune response to inorganic nanoparticles and their degradation products. Adv. Drug Deliv. Rev. 180, 114022. doi:10.1016/j.addr.2021.114022
Ngwa, W., Irabor, O. C., Schoenfeld, J. D., Hesser, J., Demaria, S., and Formenti, S. C. (2018). Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 18, 313–322. doi:10.1038/nrc.2018.6
Niculescu, A. G., and Grumezescu, A. M. (2022). Novel tumor-targeting nanoparticles for cancer treatment—a review. Int. J. Mol. Sci. 23 (9), 5253. doi:10.3390/ijms23095253
Ostroumov, D., Fekete-Drimusz, N., Saborowski, M., Kühnel, F., and Woller, N. (2018). CD4 and CD8 T lymphocyte interplay in controlling tumor growth. Cell. Mol. Life Sci. 75, 689–713. doi:10.1007/s00018-017-2686-7
Palomares, O., Yaman, G., Azkur, A. K., Akkoc, T., Akdis, M., and Akdis, C. A. (2010). Role of Treg in immune regulation of allergic diseases. Eur. J. Immunol. 40, 1232–1240. doi:10.1002/eji.200940045
Pardi, N., Hogan, M. J., Porter, F. W., and Weissman, D. (2018). mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 17 (4), 261–279. doi:10.1038/nrd.2017.243
Park, Y. M., Lee, S. J., Kim, Y. S., Lee, M. H., Cha, G. S., Jung, I. D., et al. (2013). Nanoparticle-based vaccine delivery for cancer immunotherapy. Immune Netw. 13 (5), 177–183. doi:10.4110/in.2013.13.5.177
Parsonidis, P., and Papasotiriou, I. (2022). Adoptive cellular transfer immunotherapies for cancer. Cancer Treat. Res. Commun. 32, 100575. doi:10.1016/j.ctarc.2022.100575
Peer, D., Karp, J. M., Hong, S., Farokhzad, O. C., Margalit, R., and Langer, R. (2007). Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760. doi:10.1038/nnano.2007.387
Perica, K., Varela, J. C., Oelke, M., and Schneck, J. (2015). Adoptive T cell immunotherapy for cancer. Rambam Maimonides Med. J. 6 (1), e0004. doi:10.5041/RMMJ.10179
Pershina, A. G., Demin, A. M., Perekucha, N. A., Brikunova, O. Y., Efimova, L. V., Nevskaya, K. V., et al. (2023). Peptide ligands on the PEGylated nanoparticle surface and human serum composition are key factors for the interaction between immune cells and nanoparticles. Colloids Surfaces B Biointerfaces 221, 112981. doi:10.1016/j.colsurfb.2022.112981
Postow, M. A., Sidlow, R., and Hellmann, M. D. (2018). Adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378 (2), 1165. doi:10.1056/NEJMc1801663
Qin, X., Ning, W., Liu, H., Liu, X., Luo, W., and Xia, N. (2024). Stepping forward: T-cell redirecting bispecific antibodies in cancer therapy. Acta Pharm. Sin. B 14 (6), 2361–2377. doi:10.1016/j.apsb.2024.03.027
Raker, V. K., Becker, C., Landfester, K., and Steinbrink, K. (2020). Targeted activation of T cells with IL-2-coupled nanoparticles. Cells 9, 2063. doi:10.3390/cells9092063
Raziorrouh, B., Heeg, M., Kurktschiev, P., Schraut, W., Zachoval, R., Wendtner, C., et al. (2014). Inhibitory phenotype of HBV-specific CD4+ T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS One 9 (8), e105703. doi:10.1371/journal.pone.0105703
Retnakumar, S. V., Chauvin, C., and Bayry, J. (2023). The implication of anti-PD-1 therapy in cancer patients for the vaccination against viral and other infectious diseases. Pharmacol. Ther. 245, 108399. doi:10.1016/j.pharmthera.2023.108399
Ribatti, D. (2017). The concept of immune surveillance against tumors. The first theories. Oncotarget 8, 7175–7180. doi:10.18632/oncotarget.12739
Richardson, J. R., Schöllhorn, A., Gouttefangeas, C., and Schuhmacher, J. (2021). CD4+ T cells: multitasking cells in the duty of cancer immunotherapy. Cancers 13, 596. doi:10.3390/cancers13040596
Saeed, M., Gao, J., Shi, Y., Lammers, T., and Yu, H. (2019). Engineering nanoparticles to reprogram the tumor immune microenvironment for improved cancer immunotherapy. Theranostics 9 (26), 7981–8000. doi:10.7150/thno.37568
Salotto, K. E., Olson, W. C., Pollack, K. E., Illendula, A., Michel, E., Henriques, S., et al. (2022). A nano-enhanced vaccine for metastatic melanoma immunotherapy. Cancer Drug Resist 5 (3), 829–845. doi:10.20517/cdr.2021.132
Schroder, K., Hertzog, P. J., Ravasi, T., and Hume, D. A. (2004). Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75 (2), 163–189. doi:10.1189/jlb.0603252
Shen, H. H., Peng, J. F., Wang, R. R., Wang, P. Y., Zhang, J. X., Sun, H. F., et al. (2024). IL-12-Overexpressed nanoparticles suppress the proliferation of melanoma through inducing ICD and activating DC, CD8+ T, and CD4+ T cells. Int. J. Nanomedicine. 19, 2755–2772. doi:10.2147/IJN.S442446
Shestovskaya, M. V., Luss, A. L., Bezborodova, O. A., Makarov, V. V., and Keskinov, A. A.Moscow Oncology Research Institute of the National Medical Research Radiological Centre (1252). Ministry of Health of the Russian Federation.
Skepu, A., Phakathi, B., Makgoka, M., Mbita, Z., Damane, B. P., Demetriou, D., et al. (2023). “AI and nanomedicine in realizing the goal of precision medicine: tailoring the best treatment for personalized cancer treatment,” in Artificial intelligence and precision oncology: bridging cancer research and clinical decision support.
Soe, Z. C., Kwon, J. B., Thapa, R. K., Ou, W., Nguyen, H. T., Gautam, M., et al. (2019). Transferrin-conjugated polymeric nanoparticle for receptor-mediated delivery of doxorubicin in doxorubicin-resistant breast cancer cells. Pharmaceutics 11 (2), 63. doi:10.3390/pharmaceutics11020063
Sousa-Junior, A., Yang, C. T., Korangath, P., Ivkov, R., and Bakuzis, A. (2022). A predictive pharmacokinetic model for immune cell-mediated uptake and retention of nanoparticles in tumors. Int. J. Mol. Sci. 23 (24), 15664. doi:10.3390/ijms232415664
Speiser, D. E., Chijioke, O., Schaeuble, K., and Münz, C. (2023). CD4+ T cells in cancer. Nat. Cancer 4, 317–329. doi:10.1038/s43018-023-00521-2
Su, Y., Zhou, X., Meng, H., Xia, T., Liu, H., Rolshausen, P., et al. (2022). Cost–benefit analysis of nanofertilizers and nanopesticides emphasizes the need to improve the efficiency of nanoformulations for widescale adoption. Nat. Food 3 (12), 1020–1030. doi:10.1038/s43016-022-00647-z
Sun, S. S., Fu, P. C., Cheng, Y. W., Zhou, X. J., and Han, J. M. (2018). Characterization and transferability of microsatellites for Gentiana lawrencei var. farreri (Gentianaceae). Appl. Plant Sci. 6 (1), e1015. doi:10.1002/aps3.1015
Takahashi, Y., Nishikawa, M., and Takakura, Y. (2009). Nonviral vector-mediated RNA interference: its gene silencing characteristics and important factors to achieve RNAi-based gene therapy. Adv. Drug Deliv. Rev. 61, 760–766. doi:10.1016/j.addr.2009.04.006
Theivendren, P., Kunjiappan, S., Pavadai, P., Ravi, K., Murugavel, A., Dayalan, A., et al. (2024). Revolutionizing cancer immunotherapy: emerging nanotechnology-driven drug delivery systems for enhanced therapeutic efficacy. ACS Meas. Sci. Au 5 (1), 31–55. doi:10.1021/acsmeasuresciau.4c00062
Thirumalai, A., Harini, K., Pallavi, P., Gowtham, P., Girigoswami, K., and Girigoswami, A. (2024). Bile salt-mediated surface-engineered bilosome-nanocarriers for delivering therapeutics. Nanomedicine J. 11. doi:10.22038/nmj.2023.71268.1763
Timmins, I. R., Zaccardi, F., Nelson, C. P., Franks, P., Yates, T., and Dudbridge, F. (2020). Author Correction: genome-wide association study of self-reported walking pace suggests beneficial effects of brisk walking on health and survival. Commun. Biol. 3 (1), 679. doi:10.1038/s42003-020-01447-6
Togashi, Y., Shitara, K., and Nishikawa, H. (2019). Regulatory T cells in cancer immunosuppression — implications for anticancer therapy. Nat. Rev. Clin. Oncol. 16, 356–371. doi:10.1038/s41571-019-0175-7
Topalian, S. L., Drake, C. G., and Pardoll, D. M. (2015). Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461. doi:10.1016/j.ccell.2015.03.001
Trinchieri, G. (2003). Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3 (2), 133–146. doi:10.1038/nri1001
Turtle, C. J. (2017). Understanding outcomes of CD19-targeted CAR T cell immunotherapy. Blood 130 (Suppl. l_1), SCI-14. doi:10.1182/blood.v130.suppl_1.sci-14.sci-14
Vad, J., Orejas, C., Moreno-Navas, J., Findlay, H. S., and Roberts, J. M. (2017). Assessing the living and dead proportions of cold-water coral colonies: implications for deep-water Marine Protected Area monitoring in a changing ocean. PeerJ 2017 (10). doi:10.7717/peerj.3705
Wang, D., Dong, H., Li, M., Cao, Y., Yang, F., Zhang, K., et al. (2018). Erythrocyte-cancer hybrid membrane camouflaged hollow copper sulfide nanoparticles for prolonged circulation life and homotypic-targeting photothermal/chemotherapy of melanoma. ACS Nano. 12 (6), 5241–5252. doi:10.1021/acsnano.7b08355
Wang, Y., Wang, J., Gou, K., Kang, W., Guo, X., Zhu, K., et al. (2021a). PH/H2O2Dual-Responsive chiral mesoporous silica nanorods coated with a biocompatible active targeting ligand for cancer therapy. ACS Appl. Mater Interfaces 13 (30), 35397–35409. doi:10.1021/acsami.1c08532
Wang, J., Nie, G., Zhao, Y., and Chen, X. (2021b). Precisely designed nanotherapeutics for microenvironmenttargeted cancer therapy. Kexue Tongbao/Chinese Sci. Bull. 66 (36), 4608–4618. doi:10.1360/tb-2021-0340
Wang, P. H., Washburn, R., Maniar, R., Mu, M., Ringham, O., Kratchmarov, R., et al. (2022). Cutting edge: promoting T cell factor 1+ T cell self-renewal to improve programmed cell death protein 1 blockade. J. Immunol. 209 (4), 660–664. doi:10.4049/jimmunol.2200317
Wang, R., Hua, Y., Wu, H., Wang, J., Xiao, Y., Chen, X., et al. (2023). Hydroxyapatite nanoparticles promote TLR4 agonist-mediated anti-tumor immunity through synergically enhanced macrophage polarization. Acta Biomater., 164. doi:10.1016/j.actbio.2023.04.027
Wells, K., Liu, T., Zhu, L., and Yang, L. (2024). Immunomodulatory nanoparticles activate cytotoxic T cells for enhancement of the effect of cancer immunotherapy. Nanoscale 16 (38), 17699–17722. doi:10.1039/d4nr01780c
Whiteside, T. L. (2012). What are regulatory T cells (Treg) regulating in cancer and why? Seminars Cancer Biol. 22, 327–334. doi:10.1016/j.semcancer.2012.03.004
Xiang, Y., Tian, M., Huang, J., Li, Y., Li, G., Li, X., et al. (2023). LMP2-mRNA lipid nanoparticle sensitizes EBV-related tumors to anti-PD-1 therapy by reversing T cell exhaustion. J. Nanobiotechnology 21 (1), 324. doi:10.1186/s12951-023-02069-w
Xiao, M., Xie, L., Cao, G., Lei, S., Wang, P., Wei, Z., et al. (2022). CD4+T-cell epitope-based heterologous prime-boost vaccination potentiates anti-tumor immunity and PD-1/PD-L1 immunotherapy. J. Immunother. Cancer 10 (5), e004022. doi:10.1136/jitc-2021-004022
Xiong, J., Wu, M., Chen, J., Liu, Y., Chen, Y., Fan, G., et al. (2021). Cancer-erythrocyte hybrid membrane-camouflaged magnetic nanoparticles with enhanced photothermal-immunotherapy for ovarian cancer. ACS Nano 15 (12), 19756–19770. doi:10.1021/acsnano.1c07180
Xu, Z., Chokkalingam, N., Tello-Ruiz, E., Wise, M. C., Bah, M. A., Walker, S., et al. (2020). A DNA-launched nanoparticle vaccine elicits CD8+ T-cell immunity to promote in vivo tumor control. Cancer Immunol. Res. 8 (11), 1354–1364. doi:10.1158/2326-6066.CIR-20-0061
Yesildag, C., Zhang, Z., Ren, F., Vicente, G., and Lensen, M. C. (2018). “Nano- and micro-patterning of gold nanoparticles on PEG- based hydrogels for controlling cell adhesion,” in Noble and precious metals - properties, nanoscale effects and applications.
Yeste, A., Nadeau, M., Burns, E. J., Weiner, H. L., and Quintana, F. J. (2012). Nanoparticle-mediated codelivery of myelin antigen and a tolerogenic small molecule suppresses experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 109 (28), 11270–11275. doi:10.1073/pnas.1120611109
Yu, J., Wang, S., Qi, J., Yu, Z., Xian, Y., Liu, W., et al. (2021). Mannose-modified liposome designed for epitope peptide drug delivery in cancer immunotherapy. Int. Immunopharmacol. 101, 108148. doi:10.1016/j.intimp.2021.108148
Yu, M., Yang, W., Yue, W., and Chen, Y. (2022). Targeted cancer immunotherapy: nanoformulation engineering and clinical translation. Adv. Sci. 9, e2204335. doi:10.1002/advs.202204335
Zhang, Y., Guan, X. Y., and Jiang, P. (2020a). Cytokine and chemokine signals of T-cell exclusion in tumors. Front. Immunol. 11, 594609. doi:10.3389/fimmu.2020.594609
Zhang, G., Campbell, G. R., Zhang, Q., Maule, E., Hanna, J., Gao, W., et al. (2020b). CD4+ t cell-mimicking nanoparticles broadly neutralize hiv-1 and suppress viral replication through autophagy. MBio 11 (5), e00903-20. doi:10.1128/mBio.00903-20
Zhou, Q., Zhang, Y., Du, J., Li, Y., Zhou, Y., Fu, Q., et al. (2016). Different-sized gold nanoparticle activator/antigen increases dendritic cells accumulation in liver-draining lymph nodes and CD8+ T cell responses. ACS Nano 10 (2), 2678–2692. doi:10.1021/acsnano.5b07716
Zhu, X., and Li, S. (2023). Nanomaterials in tumor immunotherapy: new strategies and challenges. Mol. Cancer 22, 94. doi:10.1186/s12943-023-01797-9
Zhu, J., and Paul, W. E. (2008). CD4 T cells: fates, functions, and faults. Blood 112 (5), 1557–1569. doi:10.1182/blood-2008-05-078154
Keywords: CD4+ T-cells, cancer immunology, nanoformulation, novel drug delivery systems, tumor microenvironment
Citation: Shahreen N, Agnihotri A, Rizwan A, Hasan F, Ansari MD, Sofian ZM, Said NABM, To KKW and Mahmood S (2025) Nanoengineered-based delivery systems to modulate CD4+ T cell responses in cancer: emerging paradigms in cancer immunotherapy. Front. Pharmacol. 16:1643791. doi: 10.3389/fphar.2025.1643791
Received: 09 June 2025; Accepted: 28 July 2025;
Published: 11 August 2025.
Edited by:
Monica Baiula, University of Bologna, ItalyReviewed by:
Muhammad Asim Farooq, Monash University, AustraliaJaimin R. Shah, University of California, San Diego, United States
Copyright © 2025 Shahreen, Agnihotri, Rizwan, Hasan, Ansari, Sofian, Said, To and Mahmood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohd Danish Ansari, ZHJtb2hkZGFuaXNoQHVtLmVkdS5teQ==; Syed Mahmood, c3llZG1haG1vb2RAdW0uZWR1Lm15
 Nekhat Shahreen1
Nekhat Shahreen1 Mohd Danish Ansari
Mohd Danish Ansari Nur Akmarina B. M. Said
Nur Akmarina B. M. Said Kenneth K. W. To
Kenneth K. W. To Syed Mahmood
Syed Mahmood