- 1School of Integrated Chinese and Western Medicine, Hunan University of Chinese Medicine, Changsha, Hunan, China
- 2Department of Anesthesiology, People's Hospital of Ningxiang City, Changsha, Hunan, China
- 3Department of Gastroenterology, The First Hospital of Hunan University of Chinese Medicine, Changsha, Hunan, China
Objective: Ciprofol is increasingly being used for sedation and induction of anesthesia in China. However, it remains unclear whether ciprofol is a more appropriate sedative than propofol in gastrointestinal endoscopy, especially in the elderly population. This study aimed to compare the safety of ciprofol with propofol in elderly patients undergoing gastrointestinal endoscopy.
Methods: Eight common databases were used to search the relevant literature up to 1 January 2025. Included studies were screened according to established criteria, and their basic characteristics, outcome data, and risk of bias were recorded. Subsequently, Review Manager 5.3 software was used to perform meta-analysis and Trial Sequential Analysis (TSA) 0.9.5.10 Beta software was used to perform TSA.
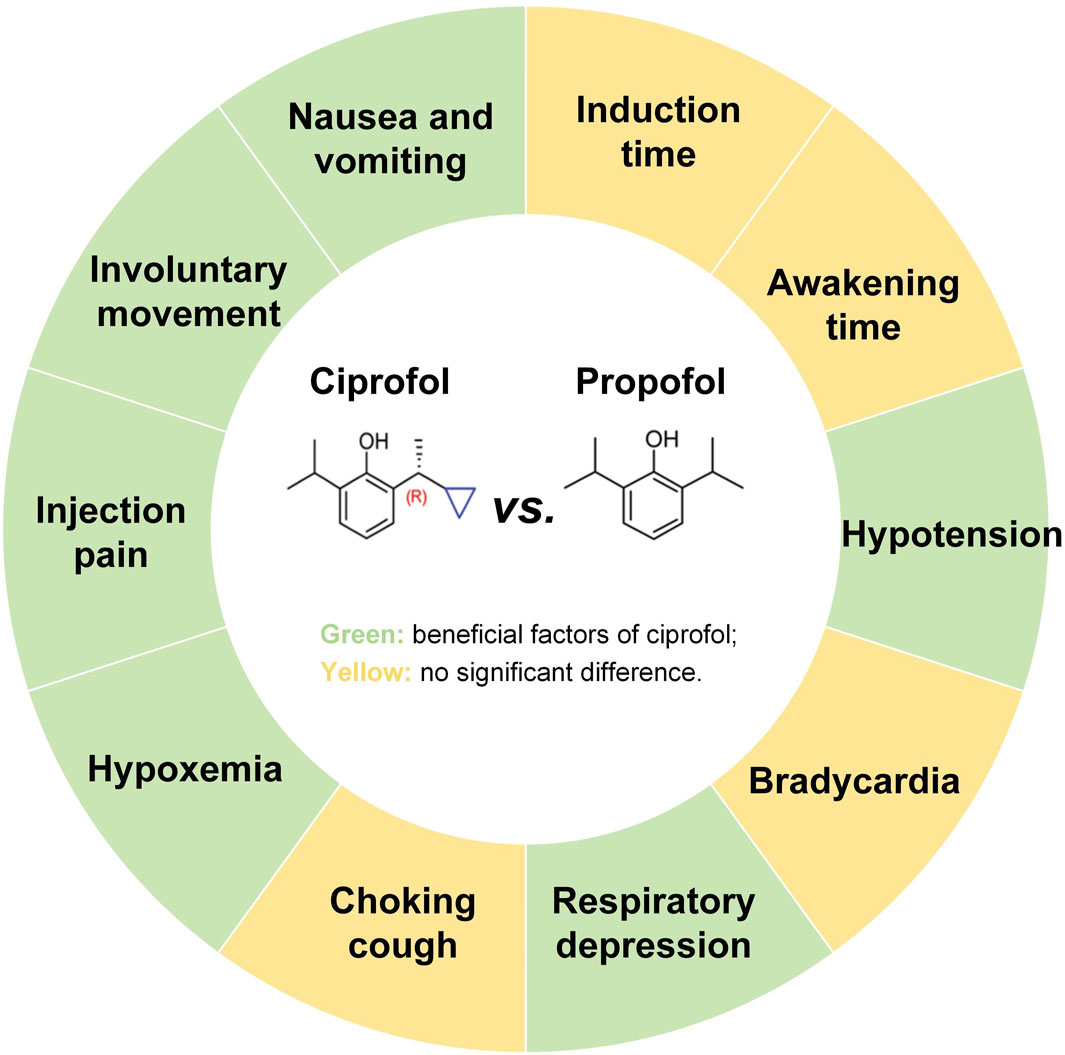
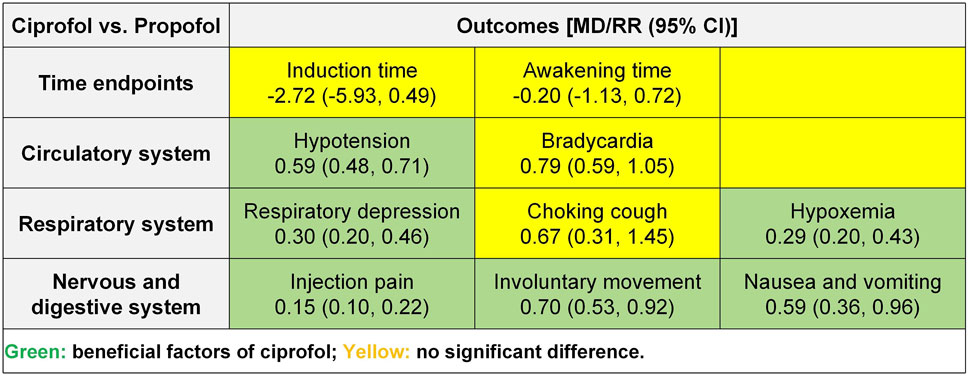
Results: Twelve randomized controlled trials and 1,653 participants were included in this study. Meta-analysis showed that compared to propofol, ciprofol reduced the incidence of hypotension (risk ratio [RR] 0.59, 95% confidence interval [CI] 0.48–0.71, P < 0.00001), respiratory depression (RR 0.30, 95% CI 0.20–0.46, P < 0.00001), hypoxemia (RR 0.29, 95% CI 0.20–0.43, P < 0.00001), injection pain (RR 0.15, 95% CI 0.10–0.22, P < 0.00001), involuntary movements (RR 0.70, 95% CI 0.53–0.92, P = 0.01) as well as nausea and vomiting (RR 0.59, 95% CI 0.36–0.96, P = 0.03), while there was no significant effect on induction time, awakening time, bradycardia, and choking cough (P > 0.05). The TSA revealed conclusive differences in hypotension, respiratory depression, hypoxemia, and injection pain. Regression analysis indicated no publication bias for any of the outcomes (P > 0.05) except awakening time (P = 0.007).
Conclusion: These findings suggest that in elderly patients undergoing gastrointestinal endoscopy, ciprofol has fewer cardiovascular, respiratory, and neurological adverse events than propofol, highlighting the potential of ciprofol as an alternative to propofol. However, the optimal dose of ciprofol for gastrointestinal endoscopic sedation in the elderly remains to be explored.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42025643465, identifier CRD42025643465.
1 Introduction
Population aging has become an irreversible global trend as the number and proportion of elderly people in various countries continue to rise (Partridge et al., 2018). According to the World Health Organization, the population aged 60 years or older is projected to increase from 1 billion in 2020 to 1.4 billion in 2030 and grow to 2.1 billion in 2050 (Aging and Health, 2025). With the intensification of global aging, the medical needs of the elderly are experiencing a rapid surge (Christensen et al., 2009). 85% of the elderly are reported to suffer from one or more chronic diseases, especially gastrointestinal diseases (Cristina and d’Alba, 2021). Gastrointestinal endoscopy is the gold standard for diagnosing digestive disorders and plays a crucial role in the screening of gastrointestinal disorders such as chronic atrophic gastritis, esophageal cancer, gastric cancer, and colorectal cancer, which are prevalent in the elderly (Finocchiaro et al., 2021). Nevertheless, gastrointestinal endoscopy is an invasive procedure that can lead to adverse events such as pain, nausea, vomiting, choking, and anxiety suffered by the patients examined (Smale et al., 2003). These not only cause pain and discomfort to the patient, but also reduces the accuracy and speed of the examination (Mitsonis et al., 2011).
Therefore, the guidelines for sedation and anesthesia for gastrointestinal endoscopy recommend the use of sedative in gastrointestinal endoscopy to reduce the discomfort of the patient and improve the success rate of the examination (Early et al., 2018). With the gradual popularization of procedural sedation in gastrointestinal endoscopy, both the acceptance and experience of the operation among patients have steadily improved (Sidhu et al., 2024; Benzoni and Cascella, 2025). As a classic short-acting intravenous sedative, propofol has long been recognized as the preferred sedative for gastrointestinal endoscopy (Ferreira and Cravo, 2015). However, propofol induces side effects of cardiovascular and respiratory depression when depressing the central nervous system, which is particularly prevalent in the elderly population (Short and Bufalari, 1999). Reports indicate that the incidence of hypotension, respiratory suppression, and hypoxemia in elderly patients undergoing gastrointestinal endoscopy with propofol is 69.6%, 10.0%, and 17.4%, respectively (Lu et al., 2022; Liu et al., 2023). Therefore, the potential risks of cardiovascular and respiratory depression associated with propofol in elderly patients undergoing gastrointestinal endoscopy are a cause for concern. This has prompted anesthesiologists to continually seek safer sedation strategies for this vulnerable patient group.
Ciprofol is a new type of short-acting intravenous anesthetic independently developed in China, which is a derivative of propofol. The mechanisms of action of ciprofol and propofol are similar, and both are characterized by rapid onset of action and rapid metabolism. However, the inhibitory effects of ciprofol on the cardiovascular and respiratory systems are milder than those of propofol (Task Force on Guidelines on Clinical Application of Ciprofol, 2023; Lu et al., 2023). Ciprofol was first approved for gastrointestinal endoscopic sedation by the National Medical Products Administration in 2021 and is under review by the Food and Drug Administration (The National Medical Products Administration, 2020; Huanbo, 2024). A previous meta-analysis showed that ciprofol reduced adverse events such as hypotension, bradycardia, respiratory depression, and hypoxemia in general anesthesia in the elderly compared to propofol, highlighting the value of ciprofol in the elderly population (Chen et al., 2025). A subsequent clinical study demonstrated that patients sedated with ciprofol exhibited more stable hemodynamics compared to those sedated with propofol, suggesting that ciprofol has potential as an alternative to propofol for gastrointestinal endoscopy (He et al., 2024). However, no relevant meta-analysis has reported differences between ciprofol and propofol in procedural sedation for the elderly, especially in gastrointestinal endoscopic sedation. Therefore, in this study, we employed both meta-analysis and trial sequential analysis (TSA) to comprehensively compare the safety of ciprofol and propofol in sedation for elderly patients undergoing gastrointestinal endoscopy, aiming to provide evidence for the clinical use of ciprofol. The main findings of this study are shown in Figure 1.
2 Methods
This study strictly followed Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) (Page et al., 2021) and was registered in PROSPERO (CRD42025643465).
2.1 Literature search
The articles were searched across English databases such as PubMed, Embase, the Cochrane Library, and Web of Science, as well as Chinese databases including China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (CSTJ), WanFang, and SinoMed. The search field was set to Title/Abstract, and the search formula was set to ((Ciprofol OR Cyclopropanes) AND (Endoscopy OR Endoscope OR Endoscopies OR Endoscopic OR gastroscope OR gastroscopy OR colonoscope OR colonoscopy OR sigmoidoscopy)). The search was conducted up until 1 January 2025, with no restrictions on language or others.
2.2 Inclusion and exclusion criteria
Inclusion criteria: (1) Study design: randomized controlled trials (RCTs); (2) Participants: elderly individuals undergoing upper gastrointestinal endoscopy or lower gastrointestinal endoscopy, with no significant abnormalities in coagulation or liver function. The gastrointestinal endoscopy procedures were conducted according to established protocols, including comprehensive steps for cleaning, disinfection, rinsing, and drying/storing. (3) Intervention: the experimental group received ciprofol sedation; (4) Comparison: the control group received propofol sedation; (5) Outcomes: time-related outcomes included induction time and recovery time; cardiovascular outcomes included hypotension and bradycardia; respiratory outcomes included respiratory depression, choking cough, and hypoxemia; neurological outcomes included injection pain and involuntary movements; gastrointestinal outcomes included nausea and vomiting.
Exclusion criteria: (1) Repeatedly published studies; (2) Studies with missing baseline data; (3) Studies with unavailable data; (4) Studies involving endoscopic treatments such as endoscopic retrograde cholangiopancreatography.
2.3 Literature screening
Initially, all retrieved studies were imported into Zotero 7.0, where duplicates were automatically removed using the software. Subsequently, the titles, authors, journals, volumes, issues, and DOIs of the remaining studies were manually verified, and any remaining duplicates were eliminated. Subsequently, by reviewing the title and abstract, further studies unrelated to the research topic were excluded. Finally, the full texts of the remaining studies were examined to confirm the final selection. Every step of the literature screening was independently conducted and cross-verified by YY and JD, with any disagreements resolved by XY.
2.4 Data collection
Excel 2010 was utilized to create statistical tables recording the basic characteristics and outcome data for included studies. The basic characteristics table included the first author, publication year, participant source, sample size, male-to-female ratio, average age, body mass index (BMI), ASA classification, anesthesia induction, and operation type. Any data related to outcomes were recorded in the data statistics table. Each step was independently performed and cross-verified by YY and JD, with any disagreements resolved by XY.
2.5 Risk assessment of bias
The bias risk assessment tool provided by the Cochrane Collaboration was employed to evaluate the methodological quality of the included studies. The tool encompasses aspects such as random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other biases. Each aspect was classified as low risk, unclear risk, or high risk. The bias risk assessment was independently carried out and cross-checked by YY and JD, with any disagreements settled by XY.
2.6 Statistical analysis
Review Manager 5.3 software was utilized to perform meta-analysis, subgroup and sensitivity analyses, as well as publication bias assessment. Firstly, the risk ratio (RR) was set as the effect size for dichotomous variable, whereas the mean difference (MD) was employed for continuous variables. If the measurement methods were identical, the weighted mean difference (WMD) was adopted; otherwise, the standardized mean difference (SMD) was used. The I2 test was conducted to evaluate the heterogeneity across the included studies. An I2 value of <50% indicated no significant heterogeneity, leading to the use of a fixed-effect model for the meta-analysis; when I2 was ≥50%, indicating moderate to high heterogeneity, a random-effects model was applied. Secondly, subgroup and sensitivity analyses were conducted to explore and identify sources of heterogeneity. Subgroup analysis was performed based on factors such as male-to-female ratio, average age, BMI, ASA I ratio, and propofol dose. Sensitivity analysis, conducted using the leave-one-out method, aimed to identify sources of heterogeneity. Thirdly, Egger and Harbord regression analyses were employed to assess publication bias for continuous and dichotomous variables, respectively. Egger regression analysis is a method to evaluate publication bias by weighted regression of effect estimates and their precision. Harbord regression analysis is a technique to correct Egger linear regression using score test statistics and their variance, particularly suited for publication bias assessment of dichotomous variables. A P value of ≥0.1 in the regression analyses indicated no potential publication bias.
TSA 0.9.5.10 Beta software was used to perform sequential analysis. A type I error rate of 5% and a type II error rate of 20% were set, and MD, standard deviation, and relative risk reduction were calculated based on the included studies to conduct a bidirectional trial sequential analysis. When the cumulative Z curve crossed the trial sequential monitoring boundary, the current data were deemed sufficient to draw a definitive conclusion, and further data collection was unnecessary.
The “Grading of Recommendations, Assessments, Development and Evaluations” (GRADE) guideline was used to evaluate the certainty of evidence. This method classifies the certainty of evidence into high, moderate, low, and very low categories based on factors such as risk of bias, inconsistency, indirectness, imprecision, and publication bias.
3 Results
3.1 Literature screening process
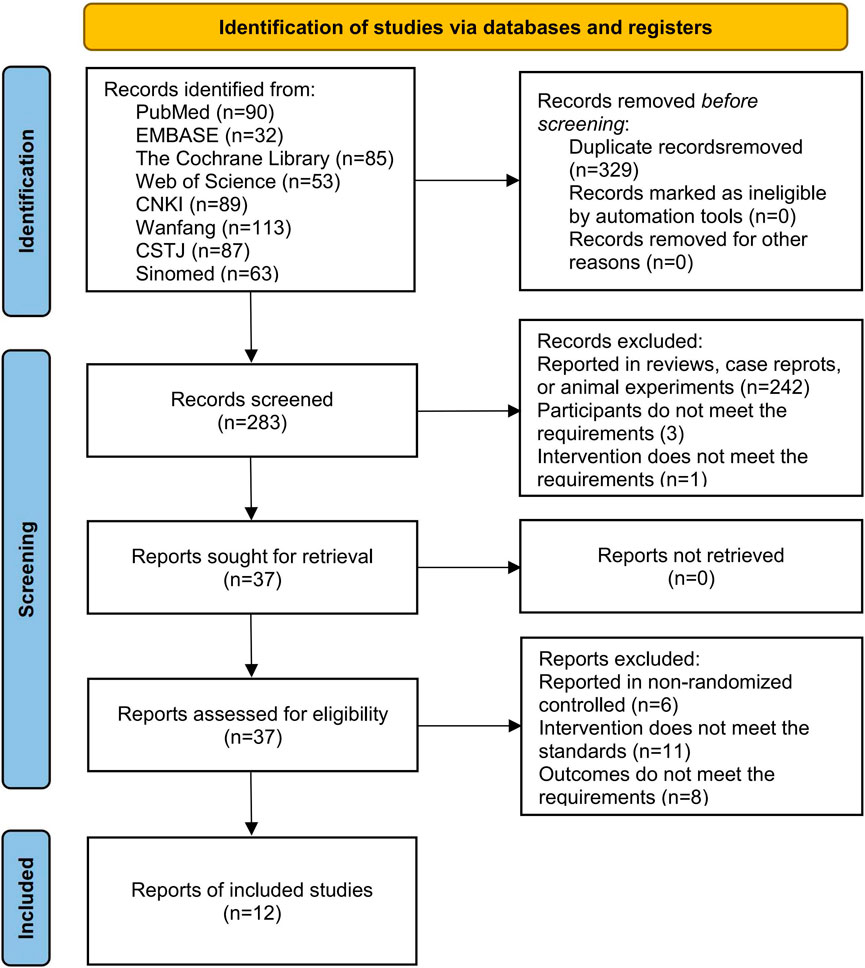
We obtained a total of 612 studies from eight databases, 90 from PubMed, 32 from EMBASE, 85 from the Cochrane Library, 53 from Web of Science, 89 from CNKI, 87 from CSTJ, 113 from Wanfang, and 63 from Sinomed. During the screening process, 329 studies were excluded due to duplicates, and 242 studies were excluded due to irrelevant topics. Subsequently, 29 studies were excluded due to not meeting the inclusion criteria when reviewing the full text. Among these, 6 studies reported non-RCT, 11 studies reported inconsistent interventions, and 8 studies reported unavailable outcomes. Finally, 12 studies (Gao et al., 2023; Huo and Dan, 2024; Liu Q. Z. et al., 2024; Ma and Huang, 2023; Wang et al., 2023; Xin, 2023; Xu et al., 2023; Yang et al., 2023; Yao et al., 2024; Yi et al., 2022; Yuan and Chen, 2024; Zhang and Zhu, 2023) were included in this analysis (Figure 2).
3.2 Basic characteristics of included studies
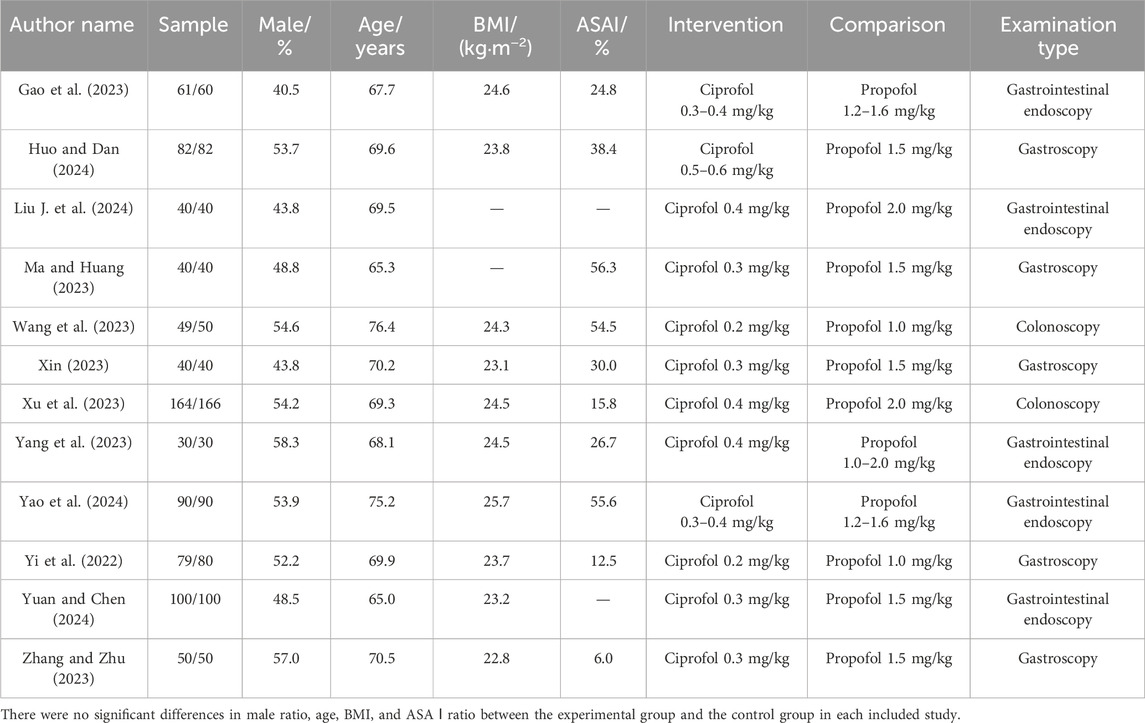
We included a total of 12 RCTs (Gao et al., 2023; Huo and Dan, 2024; Liu Q. Z. et al., 2024; Ma and Huang, 2023; Wang et al., 2023; Xin, 2023; Xu et al., 2023; Yang et al., 2023; Yao et al., 2024; Yi et al., 2022; Yuan and Chen, 2024; Zhang and Zhu, 2023) and 1,653 elderly individuals undergoing gastrointestinal endoscopy. Among them, 825 participants received ciprofol sedation and 828 participants received propofol sedation. The publication dates of the included studies were all in the last 3 years, the experimental centers were all in China, and the participants were all Chinese. The mean male ratio of the participants was 50.8%, the mean age was 69.70 years, the mean BMI was 24 kg/m2, the mean ASA I ratio was 32.05%, and the range of propofol anesthesia induction doses was 0.3–0.6 mg/kg (Table 1). In addition, none of the included studies reported comorbidity and their ratios.
3.3 Risk assessment of bias
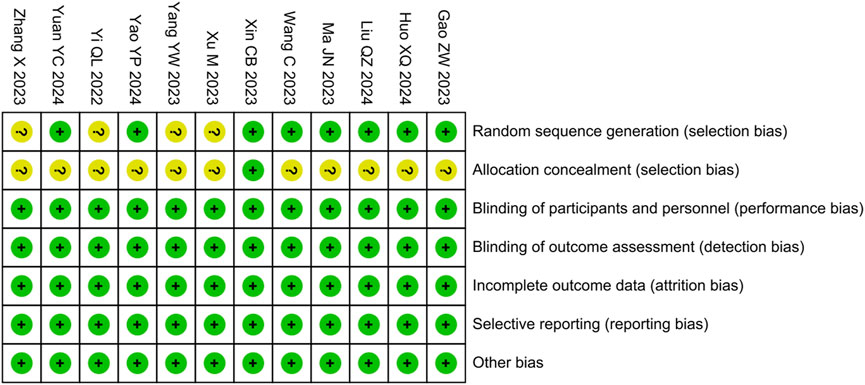
We assessed the risk of bias for included studies using the tool provided by the Cochrane Collaboration. Among them, four studies were evaluated as having an unclear risk of bias for random sequence generation due to not describing the randomization method, 11 studies were evaluated as having an unclear risk of bias for allocation concealment due to not describing the concealment method, whereas the rest of the domains were evaluated as having a low risk (Figure 3).
3.4 Meta analysis
We conducted meta-analyses on time-related outcomes, as well as cardiovascular, respiratory, neurological, and gastrointestinal outcomes, and the summary results is shown in Figure 4.
3.4.1 Time-related outcomes
3.4.1.1 Induction time
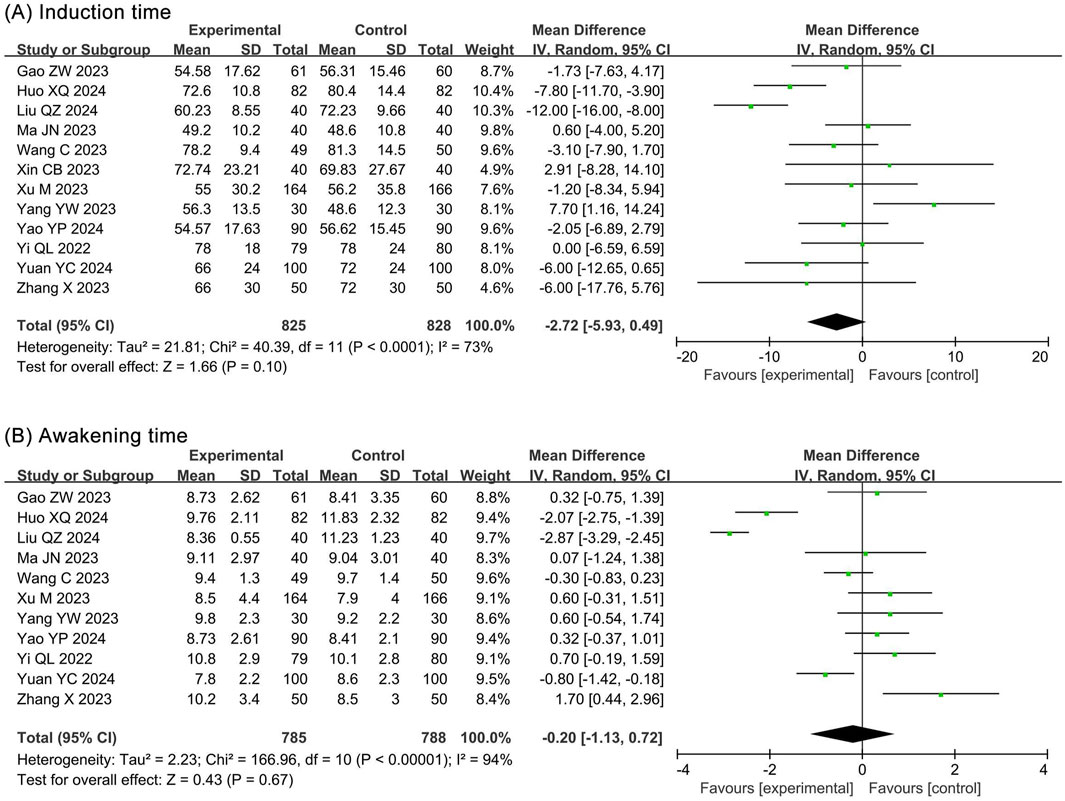
The meta-analysis of induction times included 12 RCTs (Gao et al., 2023; Huo and Dan, 2024; Liu Q. Z. et al., 2024; Ma and Huang, 2023; Wang et al., 2023; Xin, 2023; Xu et al., 2023; Yang et al., 2023; Yao et al., 2024; Yi et al., 2022; Yuan and Chen, 2024; Zhang and Zhu, 2023) and 1,653 participants. The results showed no statistical significance in induction time (MD −2.72, 95% CI −5.93 to 0.49, P = 0.10, I2 = 73%) between the ciprofol and propofol groups (Figure 5A).
3.4.1.2 Awakening time
The meta-analysis of awakening times included 11 RCTs (Gao et al., 2023; Huo and Dan, 2024; Liu Q. Z. et al., 2024; Ma and Huang, 2023; Wang et al., 2023; Xu et al., 2023; Yang et al., 2023; Yao et al., 2024; Yi et al., 2022; Yuan and Chen, 2024; Zhang and Zhu, 2023) and 1,573 participants. The results showed no statistical significance in awakening time (MD −0.20, 95% CI −1.13 to 0.72, P = 0.67, I2 = 94%) between the ciprofol and propofol groups (Figure 5B).
3.4.2 Cardiovascular system outcomes
3.4.2.1 Hypotension
The meta-analysis of hypotension included 9 RCTs (Huo and Dan, 2024; Liu Q. Z. et al., 2024; Ma and Huang, 2023; Wang et al., 2023; Xin, 2023; Xu et al., 2023; Yang et al., 2023; Yao et al., 2024; Yi et al., 2022) and 1,406 participants. The results showed a significantly lower incidence of hypotension in the ciprofol group (RR 0.59, 95% CI 0.48–0.71, P < 0.00001, I2 = 39%) compared to the propofol group (Figure 6A).
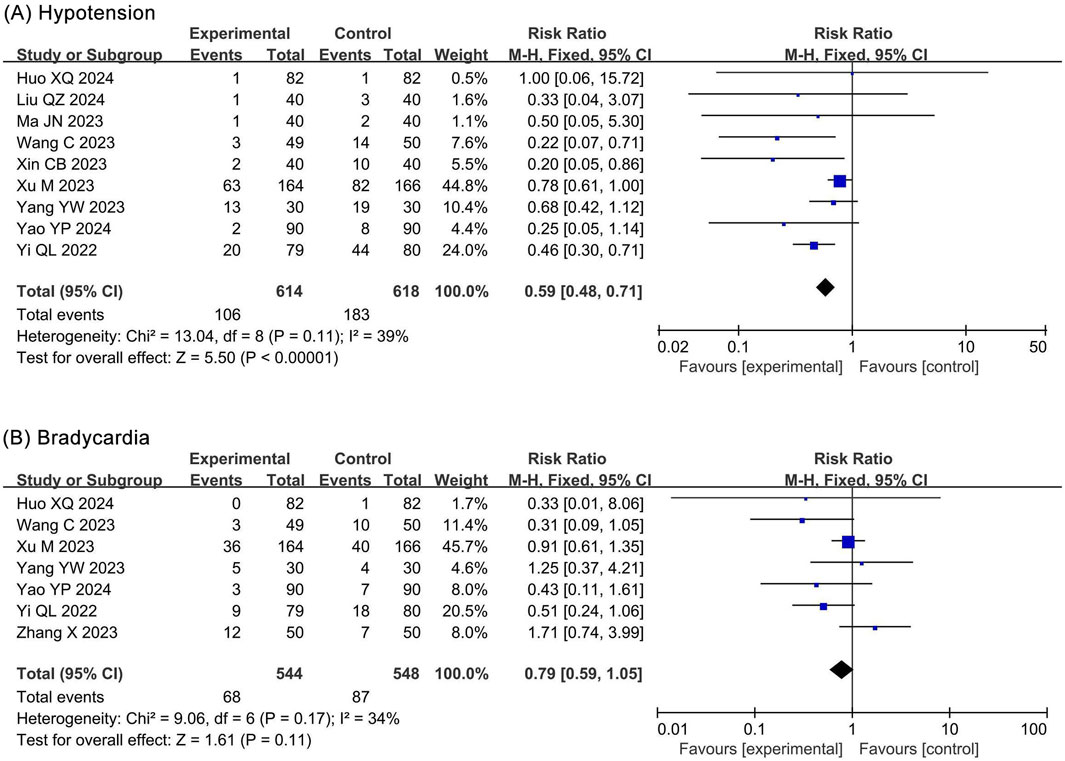
Figure 6. Forest plots of meta-analysis on circulatory system outcomes. (A) Hypotension; (B) Bradycardia.
3.4.2.2 Bradycardia
The meta-analysis of bradycardia included 7 RCTs (Huo and Dan, 2024; Wang et al., 2023; Xu et al., 2023; Yang et al., 2023; Yao et al., 2024; Yi et al., 2022; Zhang and Zhu, 2023) and 1,092 participants. The results showed no statistical significance in bradycardia (RR 0.79, 95% CI 0.59–1.05, P = 0.11, I2 = 34%) between the ciprofol and propofol groups (Figure 6B).
3.4.3 Respiratory system outcomes
3.4.3.1 Respiratory depression
The meta-analysis of respiratory depression included 8 RCTs (Gao et al., 2023; Huo and Dan, 2024; Liu Q. Z. et al., 2024; Ma and Huang, 2023; Xin, 2023; Yao et al., 2024; Yi et al., 2022; Zhang and Zhu, 2023) and 964 participants. The results showed a significantly lower incidence of respiratory depression (RR 0.30, 95% CI 0.20–0.46, P < 0.00001, I2 = 0%) in the ciprofol group compared to the propofol group (Figure 7A).
![Forest plots from three separate meta-analyses show risk ratios for respiratory depression, choking cough, and hypoxemia, comparing experimental and control groups. (A) Respiratory depression shows a pooled risk ratio of 0.30 [0.20, 0.46]; (B) Choking cough, 0.67 [0.31, 1.45]; (C) Hypoxemia, 0.29 [0.20, 0.43]. Each plot exhibits study-specific data with squares indicating risk ratios, lines showing confidence intervals, and diamonds representing overall pooled estimates. Heterogeneity is low across all analyses.](https://www.frontiersin.org/files/Articles/1644182/fphar-16-1644182-HTML-r1/image_m/fphar-16-1644182-g007.jpg)
Figure 7. Forest plots of meta-analysis on respiratory system outcomes. (A) Respiratory depression; (B) Choking cough; (C) Hypoxemia.
3.4.3.2 Choking cough
The meta-analysis of choking cough included 2 RCTs (Yi et al., 2022; Yuan and Chen, 2024) and 359 participants. The results showed no statistical significance in choking cough (RR 0.67, 95% CI 0.31–1.45, P = 0.31, I2 = 0%) between the ciprofol and propofol groups (Figure 7B).
3.4.3.3 Hyoxemia
The meta-analysis of hypoxemia included 7 RCTs (Ma and Huang, 2023; Wang et al., 2023; Xin, 2023; Xu et al., 2023; Yang et al., 2023; Yuan and Chen, 2024; Zhang and Zhu, 2023) and 949 participants. The results showed a significantly lower incidence of hypoxemia (RR 0.29, 95% CI 0.20–0.43, P < 0.00001, I2 = 0%) in the ciprofol group compared with the propofol group (Figure 7C).
3.4.4 Neurological and gastrointestinal system outcomes
3.4.4.1 Injection pain
The meta-analysis of injection pain included 10 RCTs (Gao et al., 2023; Huo and Dan, 2024; Liu J. et al., 2024; Ma and Huang, 2023; Wang et al., 2023; Xin, 2023; Xu et al., 2023; Yang et al., 2023; Yao et al., 2024; Yi et al., 2022; Yuan and Chen, 2024) and 1,473 participants. The results showed a significantly lower incidence of injection pain (RR 0.15, 95% CI 0.10–0.22, P < 0.00001, I2 = 0%) in the ciprofol group compared to the propofol group (Figure 8A).
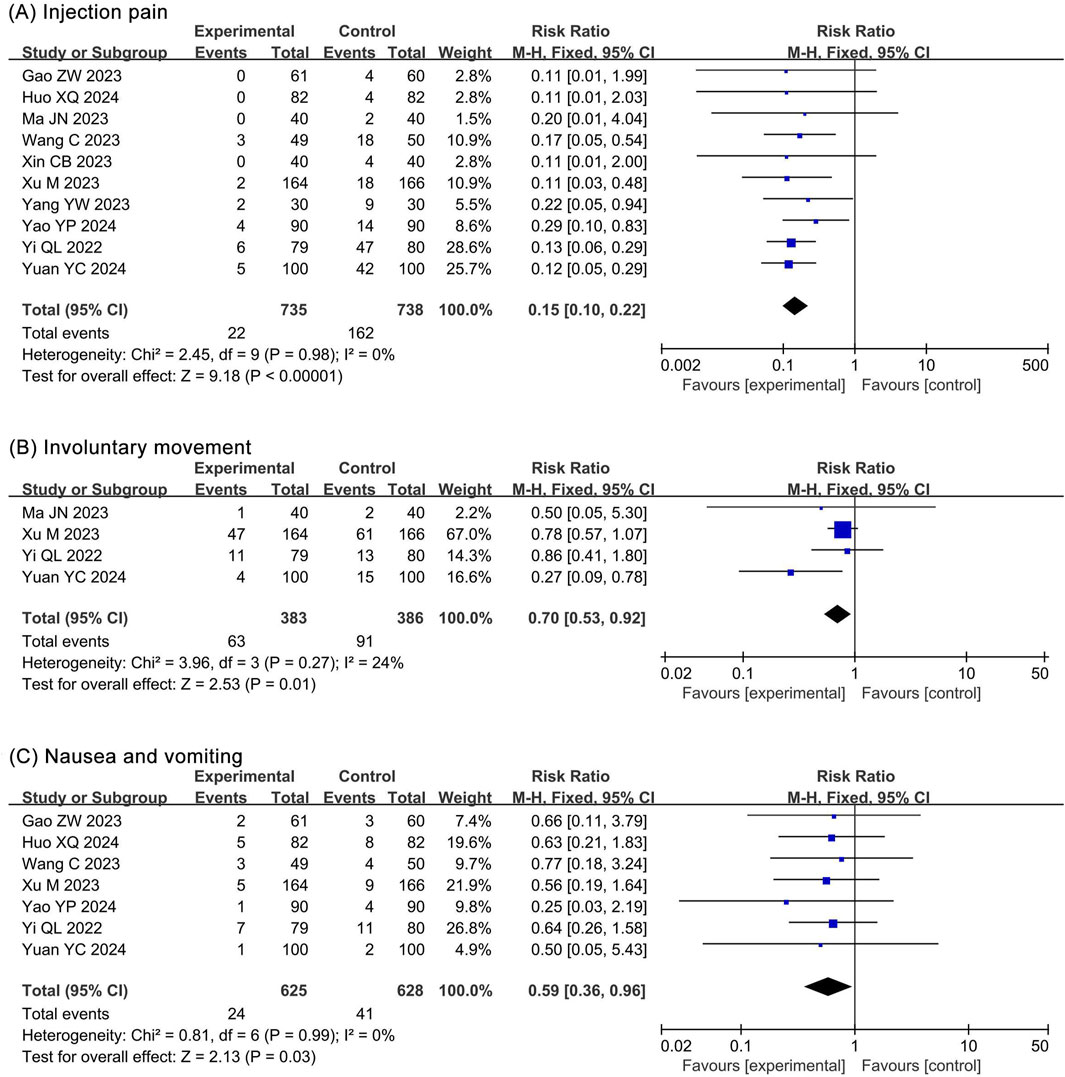
Figure 8. Forest plots of meta-analysis on nervous and digestive system outcomes. (A) Injection pain; (B) Involuntary movement; (C) Nausea and vomiting.
3.4.4.2 Involuntary movement
The meta-analysis of involuntary movements included 4 RCTs (Ma and Huang, 2023; Xu et al., 2023; Yi et al., 2022; Yuan and Chen, 2024) and 769 participants. The results showed a significantly lower incidence of involuntary movements (RR 0.70, 95% CI 0.53–0.92, P = 0.01, I2 = 24%) in the ciprofol group compared to the propofol group (Figure 8B).
3.4.4.3 Nausea and vomitting
The meta-analysis of nausea and vomiting included 7 RCTs (Gao et al., 2023; Huo and Dan, 2024; Wang et al., 2023; Xu et al., 2023; Yao et al., 2024; Yi et al., 2022; Yuan and Chen, 2024) and 1,253 participants. The results showed a significantly lower incidence of nausea and vomiting (RR 0.59, 95% CI 0.36–0.96, P = 0.03, I2 = 0%) in the ciprofol group compared to the propofol group (Figure 8C).
3.5 Subgroup analysis
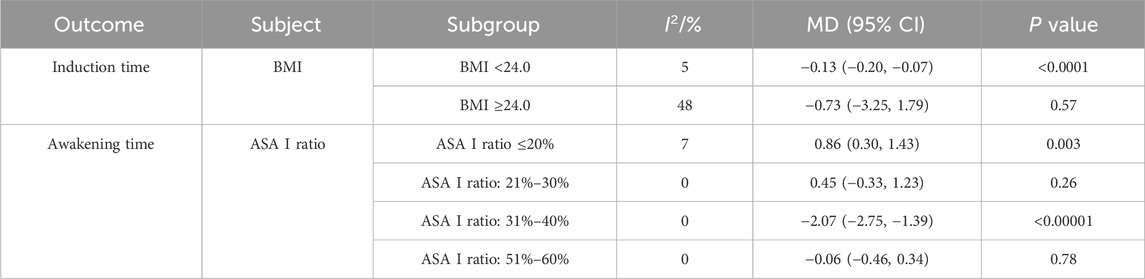
The meta-analyses showed significant heterogeneity in the results of induction time and awakening time. To explore the sources of heterogeneity, we performed subgroup analyses of induction time and awakening time based on male ratio, mean age, BMI, ASA I ratio, and the dose of ciprofol. The results of subgroup analyses that identified heterogeneous sources are shown in Table 2.
The subgroup analyses revealed that the heterogeneity of induction time was related to BMI. However, induction time was not statistically different between the two groups in the “BMI ≥24.0” subgroup (MD −0.73, 95% CI −3.25 to 1.79, P = 0.57, I2 = 48%), whereas it was significantly different in the “BMI <24.0” subgroup (MD −0.13, 95% CI −0.20 to −0.07, P < 0.0001, I2 = 5%). This suggests that the meta-analysis result of induction times were not robust and that ciprofol may have shortened induction times in elderly individuals with a BMI <24.0.
Moreover, the heterogeneity of awakening time was related to ASA I ratio. However, awakening time was significant in the ASA I ratio ≤20% (MD 0.86, 95% CI 0.30–1.43, P = 0.003, I2 = 7%) and ASA I ratio 31%–40% (MD −2.07, 95% CI −2.75 to −1.39, P < 0.00001, I2 = 0%) subgroups, whereas it was not significant in the ASA I ratio 21%–30% (MD 0.45, 95% CI −0.33 to 1.23, P = 0.26, I2 = 0%) and ASA I ratio 51%–60% (MD −0.06, 95% CI −0.46 to 0.34, P = 0.78, I2 = 0%) subgroups. This suggested that the meta-analysis result of awakening time was not robust.
3.6 Sensitivity analysis
Except for induction and awakening times, mild heterogeneity was observed in the results of hypotension and bradycardia. Therefore, we assessed the heterogeneity sources of the above outcomes using leave-one-out sensitivity analysis. The sensitivity analysis showed that the heterogeneity for bradycardia originated from the study by Zhang and Zhu (2023). However, removing the study by Zhang and Zhu (2023) resulted in a significant difference in bradycardia (RR 0.71, 95% CI 0.52–0.97, P = 0.03, I2 = 13%), suggesting that the meta-analysis result of bradycardia was not robust. Furthermore, the heterogeneity of hypotension was derived from the study by Xu et al. (2023). After removing this study, there was still a significant difference in hypotension (RR 0.43, 95% CI 0.32–0.58, P < 0.00001, I2 = 0%), suggesting that the results for hypotension were robust.
3.7 TSA
The TSA showed that the cumulative Z curves for hypotension, respiratory depression, hypoxemia, and injection pain crossed the trial sequential monitoring boundary, suggesting that these outcomes observed in the current sample size were conclusive. However, the cumulative Z curves for involuntary movement as well as nausea and vomiting did not reach the trial sequential monitoring boundary, suggesting that their results observed in the current sample size are not conclusive and need to be included in new studies for further assessment (Figure 9).
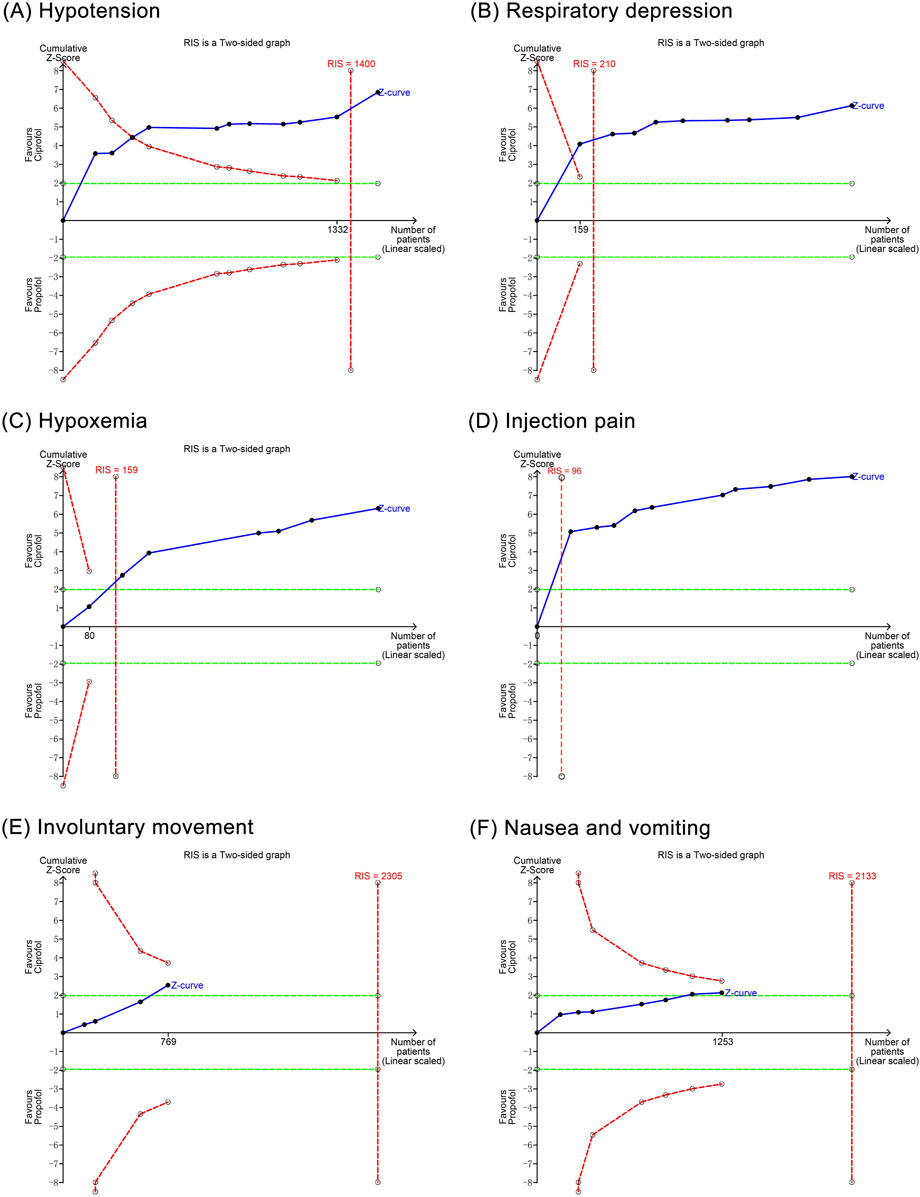
Figure 9. Trial sequential analysis results of positive outcomes. (A) Hypotension; (B) Respiratory depression; (C) Hypoxemia; (D) Injection pain; (E) Involuntary movements; (F) Nausea and vomiting.
3.8 Publication bias
Regression analyses showed that induction time (P = 0.122), hypotension (P = 0.485), bradycardia (P = 0.478), respiratory depression (P = 0.548), choking cough (P = 1.00), hypoxemia (P = 0.156), injection pain (P = 0.555), involuntary movement (P = 0.607), and nausea and vomiting (P = 0.475) did not have significant publication bias, whereas awakening time (P = 0.007) had a potential publication bias (Figure 10).
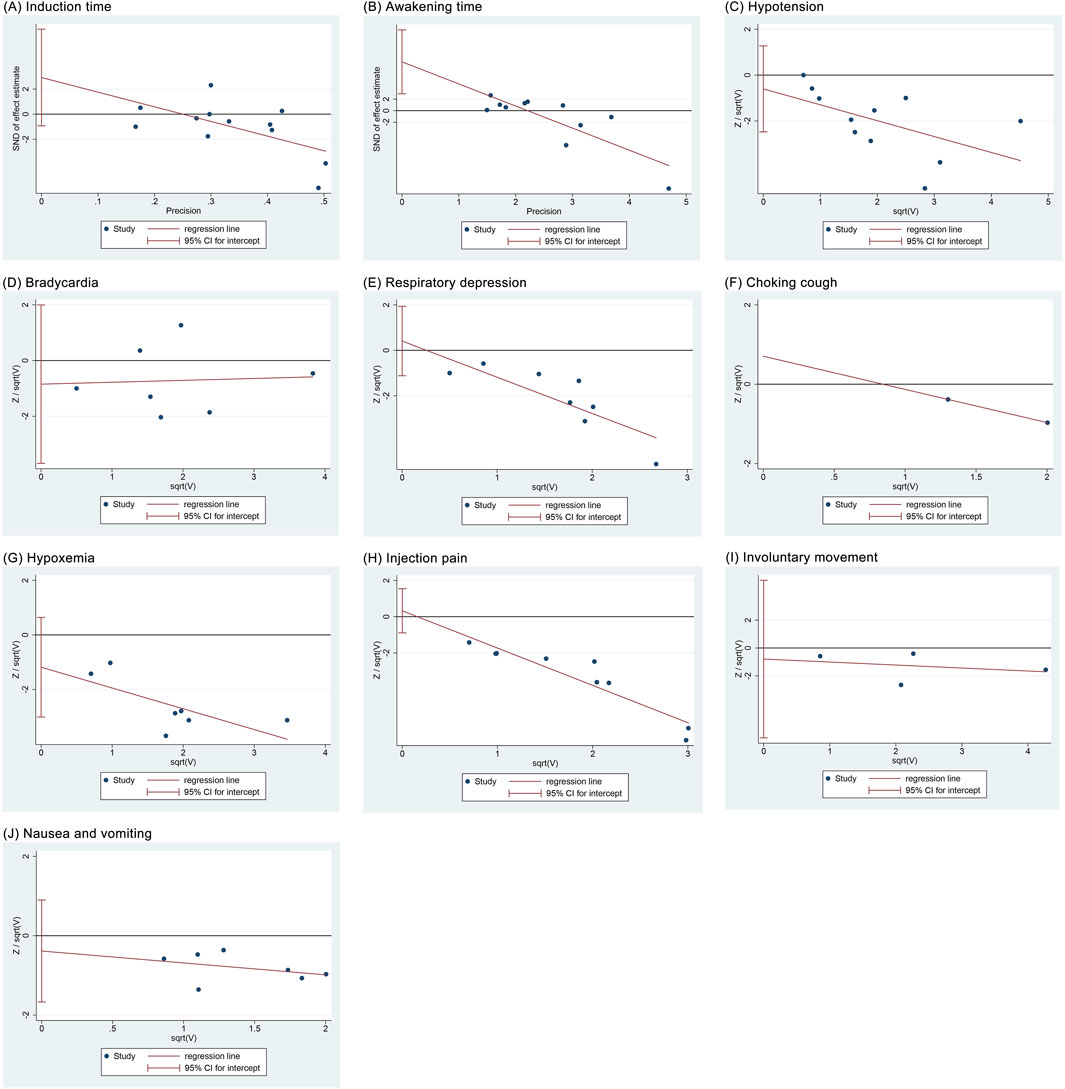
Figure 10. Regression analysis results of publication bias. (A) Induction time; (B) Recovery time; (C) Hypotension; (D) Bradycardia; (E) Respiratory depression; (F) Choking cough; (G) Hypoxemia; (H) Injection pain; (I) Involuntary movements; (J) Nausea and vomiting.
3.9 Certainty of evidence
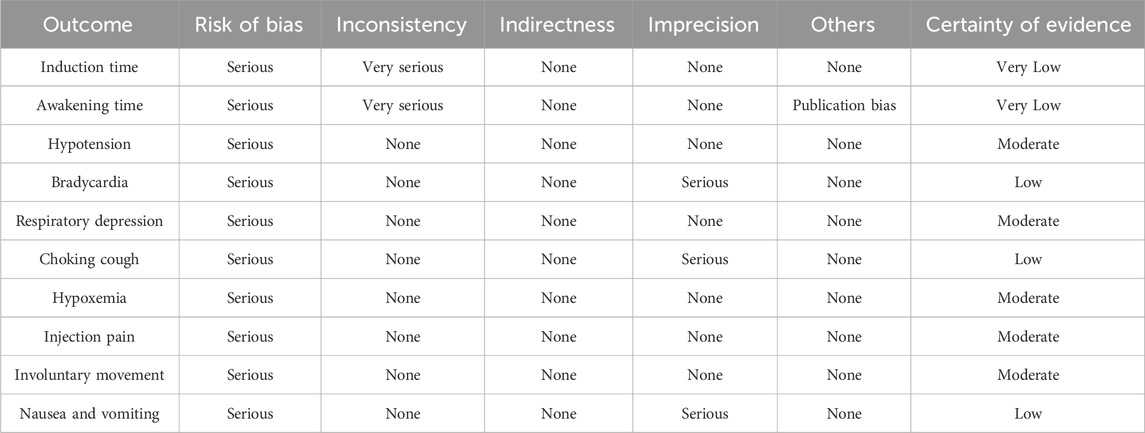
The GRADE showed that the qualities of evidence for hypotension, respiratory depression, hypoxemia, injection pain, and involuntary movement were moderate, those for bradycardia, choking cough, and nausea and vomiting were low, and those for induction time and awakening time were very low (Table 3).
4 Discussion
4.1 Research background and findings
Gastrointestinal endoscopy is the mainstay of screening for gastrointestinal diseases such as esophageal, gastric, and colorectal cancers, which are prevalent among the elderly (Lambert, 2012). Although propofol is widely used for procedural sedation for gastrointestinal endoscopy, its side effects on the cardiovascular and respiratory systems remain a concern, especially in the elderly population (Guo et al., 2022). Ciprofol, a derivative of propofol, has recently been considered a potential alternative for procedural sedation in elderly population (He et al., 2024). However, this argument has not been supported by sufficient evidence due to the lack of relevant systematic reviews and meta-analyses. Therefore, we are the first to compare the effects of ciprofol and propofol on elderly patients undergoing gastrointestinal endoscopy using meta-analysis and TSA. Our findings showed that compared to propofol, ciprofol significantly reduced the incidence of hypotension, respiratory depression, hypoxemia, injection pain, involuntary movements, as well as nausea and vomiting, but had no significant effect on induction time, awakening time, bradycardia, and choking cough.
4.2 Effects on time related outcomes
The induction time is the duration from anesthetic drug administration to when the patient loses consciousness, reflecting the drug’s onset speed (Miller’s basics of anesthesia, 2024). In previous studies, an RCT by Ma and Huang (2023) reported that in elderly people undergoing gastrointestinal endoscopy, the induction time was almost the same in the ciprofol group and the propofol group (0.82 ± 0.17 min vs. 0.81 ± 0.18 min); and a meta-analysis by Chen et al. (2025) noted no significant difference in the induction time of ciprofol and propofol in general anesthesia for elderly people (SMD 0.11, 95% CI −0.39 to 0.61, P = 0.655), supporting our findings. Moreover, subgroup analyses revealed heterogeneity in induction time stemming from BMI and found that its significance was not consistent across subgroups. Specifically, the difference in induction time was significant in the BMI <24.0 subgroup (MD −0.13, 95% CI −0.20 to −0.07, P < 0.00001), whereas it was not significant in the BMI ≥24.0 subgroup (MD −0.73, 95% CI −3.25 to 1.79, P = 0.57), suggesting the meta-analysis result on induction time was not robust. This phenomenon could be related to obesity-associated alterations in cerebral hemodynamics, as increasing BMI has been shown to significantly decrease cerebral blood flow and flow velocity (Yang et al., 2011). Reduced cerebral blood flow can directly slow the delivery of ciprofol from the bloodstream to brain tissue, potentially prolonging its onset of action (Yang et al., 2011). However, ciprofol’s reduction of induction time was only 0.13 s in the BMI <24.0 subgroup, which is statistically significant but unlikely to be clinically meaningful. Therefore, although heterogeneity in induction time appears to be associated with BMI, it does not seem to influence clinical anesthetic decision-making.
The awakening time represents the duration from the end of anesthesia to the restoration of consciousness (Miller’s basics of anesthesia, 2024), reflecting the metabolic speed of the drug and the depression of the central neurological system (Miller’s basics of anesthesia, 2024). In a previous study, the RCT by Ma and Huang (2023) showed no significant difference in the awakening time between the ciprofol and propofol groups in gastrointestinal endoscopic sedation among the elderly population (9.11 ± 2.97 min vs. 9.04 ± 3.01 min), which supports our findings. However, a meta-analysis by Chen et al. (2025) found that elderly people who received general anesthesia with ciprofol had longer awakening times than those who received propofol (SMD 0.46, 95% CI 0.16–0.76); and a meta-analysis by Liu J. et al. (2024) also demonstrated that in endoscopic sedation, the awakening time was significantly longer in the ciprofol group than in the propofol group (MD 0.70, 95% CI 0.00–1.40). This contradiction was attributed to the participants, as both we and Ma and Huang (2023) focused on the elderly undergoing gastrointestinal endoscopy, whereas Liu J. et al. (2024) and Chen et al. (2025) included a wide range of patients undergoing endoscopic procedures and surgical procedures, respectively. Furthermore, subgroup analyses showed heterogeneity in awakening time stemming from ASA I ratio, suggesting that the results were not robust. Specifically, differences to awakening time in the ASA I ratio ≤20% subgroup (MD 0.86, 95% CI 0.30–1.43, P = 0.003) and the ASA I ratio 31%–40% subgroup (MD −2.07, 95% CI −2.75 to −1.39, P < 0.00001) were significant, whereas it was not significant in either the ASA I ratio 21%–30% subgroup (MD 0.45, 95% CI −0.33 to 1.23, P = 0.26) or ASA I ratio 51%–60% subgroup (MD −0.06, 95% CI −0.46 to 0.34, P = 0.78). However, upon reviewing the included studies, we found no clear clinical or methodological factors that could explain these discrepancies. We speculate that the heterogeneous findings might be due to the limited sample sizes within each subgroup, as only 164–589 participants were included. Consequently, the heterogeneity in awakening time related to ASA I ratio is unlikely to have significant clinical implications.
4.3 Effects on cardiovascular system
Our meta-analysis showed that ciprofol reduced the incidence of hypotension by 41% compared to propofol, but had no significant effect on the incidence of bradycardia. TSA suggested that the significant difference in hypotension between ciprofol and propofol was conclusive. In previous studies, Chen et al. (2025) reported a reduction in the risk of hypotension by 28% with ciprofol compared to propofol in general anesthesia (RR 0.72, 95% CI 0.58–0.94), and Liu J. et al. (2024) found that ciprofol reduced the risk of hypotension by 27% compared to propofol in endoscopic sedation (RR 0.73, 95% CI 0.58–0.92), supporting our findings. Although leave-one-out sensitivity analysis showed heterogeneity in hypotension related to the study by Xu et al. (2023), there was still a significant difference in hypotension after excluding this study (RR 0.43, 95% CI 0.32–0.58, P < 0.00001), indicating that the meta-analysis results were robust. The advantage of ciprofol over propofol in the risk of hypotension may be related to pharmacologic properties (Durai Samy and Taksande, 2024). Although both drugs act by activating GABA receptors, ciprofol exerts a weaker inhibitory effect on the cardiovascular system, thus avoiding drastic hemodynamic fluctuations (Petkar et al., 2024). In contrast, propofol has a multifaceted inhibitory effect on the cardiovascular system: First, propofol dilates peripheral vasculature and decreases resistance through direct action on vascular smooth muscle (Kuriyama et al., 2012; Carey et al., 2019); second, propofol decreases myocardial contractility and cardiac output through myocardial inhibition (Meng et al., 2016); and, third, propofol interferes with positive cardiovascular regulatory functions through inhibition of sympathetic nerve activity (Liu et al., 2019). These differences in pharmacological properties dictate that ciprofol possesses less cardiovascular depression and more stable hemodynamics than propofol.
Furthermore, a meta-analysis by Liu J. et al. (2024) showed no significant difference in the incidence of bradycardia between ciprofol and propofol in endoscopic sedation (RD 0.00, 95% CI −0.04 to 0.04), which supports our findings. However, a meta-analysis by Chen et al. (2025) reported a reduction in bradycardia of approximately 36% in the ciprofol group compared to the propofol group during general anesthesia surgery (RR 0.64, 95% CI 0.48–0.85), which is in contradiction to our results. This contradiction may be related to the difference in participants, as both we and Liu J. et al. (2024) included endoscopy subjects undergoing procedural sedation, whereas Chen et al. (2025) included surgical patients undergoing general anesthesia. However, the type of surgery determines the anesthetic dosage and duration, which in turn affects the incidence of bradycardia. Specifically, in endoscopic sedation, the dosage of anesthetic is relatively small and of short duration, resulting in a relatively limited effect on heart rate. Conversely, in general anesthesia procedures, the dosage of anesthetics is greater and of longer duration, which makes the patient more likely to experience bradycardia and thus sets the stage for highlighting the benefits of ciprofol in reducing bradycardia. Additionally, sensitivity analysis revealed heterogeneity in bradycardia associated with the study by Zhang and Zhu (2023), which was attributed to a low ASA I ratio. However, after excluding the Zhang and Zhu (2023) study, the difference in bradycardia incidence between the two drugs became significant (RR 0.71, 95% CI 0.52–0.97, P = 0.03, I2 = 13%). This suggests that the meta-analysis results regarding bradycardia are not robust and may be influenced by the ASA I ratio. The ASA classification is a crucial measure for assessing a patient’s overall physical status and potential risk (Sankar et al., 2014), with a lower ASA I ratio indicating poorer health and a higher burden of comorbidities among participants. This implies that ciprofol may be particularly beneficial for elderly patients with fewer comorbidities, as it demonstrated a reduction in bradycardia after excluding studies with a low ASA I ratio. Patients with more comorbidities may be more sensitive to the side effects of anesthetic drugs (Hendrix and Garmon, 2025). In individuals with a lower ASA I ratio, the heart may already be under stress due to pre-existing conditions, and anesthetic agents can further impair cardiac function, increasing the likelihood of bradycardia. Therefore, the effect of ciprofol in reducing bradycardia may not be significant in patients with higher ASA classifications, especially those with cardiovascular complications. This finding provides guidance for the clinical application of ciprofol, suggesting that it may further reduce the incidence of bradycardia in ASA I patients and supports the idea of making anesthesia decisions based on ASA classification.
4.4 Effects on respiratory system
This study demonstrated that, compared to the propofol group, the ciprofol group reduced the incidence of respiratory depression by 70% and hypoxemia by 71%, with no significant effect on the incidence of choking cough. The TSA confirmed that the significant differences in respiratory depression and hypoxemia between ciprofol and propofol were conclusive. In a previous meta-analysis, Chen et al. (2025) reported that ciprofol reduced the risk of respiratory depression by 71% (RR 0.29, 95% CI 0.19–0.43) and hypoxemia by 62% (RR 0.38, 95% CI 0.26–0.55) in the elderly population during general anesthesia surgery compared to propofol, supporting our findings. The differences in respiratory safety between the two anesthetics may be related to the GABA receptor subtypes. GABA receptors consist of eight subunits, including α, β, γ, ρ, δ, ε, π, and θ. Except for δ, ε, π and θ, which have only one subtype, the α subunit has six subtypes and the β, γ and ρ subunits have three subtypes. The combination of different subtypes of these subunits confers GABA receptor diversity and determines its function (Chen et al., 2024; Sallard et al., 2021). When binding to GABA receptors, ciprofol selectively binds the α1, β2, and γ2 subunits to exert a sedative effect (Liao et al., 2022). This selectivity allows ciprofol to achieve sedation while attenuating inhibition of the respiratory center (Liao et al., 2022). Conversely, propofol binds a wide range of different subtypes of the α, β, and γ subunits of the GABA receptor, including subtypes that are critical for respiratory regulation (Jiang et al., 2021). For example, the β3 subtype, a key target of propofol, binds to propofol and directly inhibits respiratory-related brain nuclei function, leading to an increased risk of respiratory depression and hypoxemia (Jiang et al., 2021). This evidence provides an explanation for the respiratory benefit of ciprofol relative to propofol. Additionally, our meta-analysis reported no significant difference in the incidence of choking cough between ciprofol and propofol for the first time, filling a gap in previous meta-analyses.
4.5 Effects on neurological and gastrointestinal system
Our meta-analysis revealed that, compared to the propofol group, the ciprofol group significantly reduced the incidence of injection pain by 85% and involuntary movement by 30%. The TSA suggested that the significant difference between ciprofol and propofol in injection pain was conclusive, whereas the significant difference in involuntary movements needs to be validated by more studies. In previous studies, Chen et al. (2025) reported a reduction in injection pain by 87% with ciprofol compared to propofol in general anesthesia (RR 0.13, 95% CI 0.09–0.20), and Liu J. et al. (2024) noted a reduction in the incidence of injection pain with ciprofol compared to propofol by 34% in endoscopic sedation (RD −0.34, 95% CI −0.48 to −0.19), supporting our findings. The benefit of ciprofol in injection pain is closely related to its chemical structure (Usach et al., 2019). As a derivative of propofol, ciprofol replaces the methyl group of propofol with a cyclopropyl group, resulting in higher lipid solubility (Guo, 2023). This increases the solubility of propofol in the oil phase and decreases its aqueous-phase drug concentration (Doenicke et al., 1996; Sawant et al., 2021), thereby reducing irritation of the vascular intima (Doenicke et al., 1996). In contrast, propofol has a higher aqueous-phase drug concentration and thus is more likely to irritate the endothelium and local nerve endings to induce injection pain (Sim et al., 2009; Desousa, 2016).
Interestingly, the meta-analysis by Chen et al. (2025) supported the benefit of ciprofol on involuntary movements (RR 0.73, 95% CI 0.56–0.96) as we did, whereas the meta-analysis by Liu J. et al. (2024) denied this difference (RR 0.84, 95% CI 0.64–1.10). We hypothesized that this contradiction might be mediated by statistical heterogeneity and random effects models. The involuntary movements reported by us and Chen et al. (2025) were based on low heterogeneity and fixed-effects models, whereas the results of Liu J. et al. (2024) were based on random-effects models and had substantial heterogeneity. However, random effects models may amplify estimates of negative outcomes, which makes small differences difficult to detect. Additionally, while the meta-analysis supports that ciprofol reduces involuntary movements, the TSA recommends that the conclusiveness of this finding be tested by more studies of the same type.
Furthermore, our study reveals a significant reduction in nausea and vomiting by 41% in the ciprofol group compared to propofol. However, in previous meta-analyses, Chen et al. (2025) reported that ciprofol did not reduce nausea and vomiting in general anesthesia procedures (RR 0.69, 95% CI 0.43–1.11), and Liu J. et al. (2024) concluded that the incidence of nausea and vomiting was comparable between ciprofol and propofol in endoscopic sedation (RD −0.02, 95% CI −0.06 to 0.02). This contradiction may be related to the type of surgery, as Chen et al. (2025) included patients undergoing general anesthesia, and Liu J. et al. (2024) included patients undergoing a variety of endoscopic surgery including bronchoscopy, hysteroscopy, and endoscopic retrograde cholangiopancreatography. In contrast, our meta-analysis included only elderly patients undergoing gastrointestinal endoscopy. Compared to other endoscopic procedures and surgeries, gastroscopy is more prone to nausea and vomiting because it directly stimulates the pharynx and esophagus, especially the glossopharyngeal nerve in the pharynx. In this context, the stronger central inhibition of ciprofol gives it a relative advantage in reducing the incidence of nausea and vomiting. However, TSA indicated that the significant difference between ciprofol and propofol regarding nausea and vomiting lacks conclusive evidence, suggesting that this benefit needs further validation in future studies.
4.6 Discovery and inspiration
This meta-analysis with TSA compares the efficacy and safety of ciprofol and propofol in gastrointestinal endoscopic sedation for elderly patients, providing guidance for sedative selection in this context. Our findings indicate that in elderly patients undergoing gastrointestinal endoscopy, ciprofol significantly reduced hypotension, respiratory depression, hypoxemia, and injection pain compared to propofol, thereby benefiting the cardiovascular, respiratory, and nervous systems. Although the meta-analysis reported benefits of ciprofol regarding involuntary movements and nausea/vomiting, TSA indicated that these findings require further validation. Therefore, this study supports the conclusion that ciprofol reduces adverse cardiovascular and respiratory events, as well as injection pain, in elderly patients undergoing gastrointestinal endoscopy. However, the impact on the incidence of involuntary movements and nausea/vomiting warrants further discussion. In summary, for elderly patients undergoing gastrointestinal endoscopy, ciprofol may be a safer sedative than propofol, particularly for those with low pain thresholds and comorbid cardiovascular or respiratory disorders. Consequently, we recommend the use of ciprofol for sedation in gastrointestinal endoscopy to enhance comfort and anesthetic safety in elderly patients undergoing the procedure.
5 Limitations and prospects
Although our study provides evidence for the use of ciprofol in elderly patients undergoing gastrointestinal endoscopy, some limitations need to be recognized. First, four included studies did not report randomization methods and eleven studies did not report allocation concealment, which increases the risk of selectivity bias. Second, as ciprofol is currently approved and marketed only in China, the experimental centers of the included studies were all in China. This means that our findings may only apply to ethnic Chinese and need to be interpreted with caution in other races. Third, although the meta-analysis supported the benefits of ciprofol on involuntary movements as well as nausea and vomiting, the TSA suggests that these results require further validation from similar studies. Fourth, some of the included studies did not report the type and proportion of comorbidities, which prevented us from analyzing the effects of ciprofol and propofol on elderly populations with different comorbidities. Additionally, the role of ciprofol in elderly people with different comorbidities and the optimal dose range of ciprofol need to be assessed by block group randomization.
6 Conclusion
Our findings indicate that for elderly patients undergoing gastrointestinal endoscopy, ciprofol is associated with fewer adverse events in the cardiovascular, respiratory, and neurological systems compared to propofol. This highlights that ciprofol may be a more appropriate sedative for gastrointestinal endoscopy in the elderly. However, the optimal dose of ciprofol in gastrointestinal endoscopic sedation in the elderly remains to be explored.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YnY: Data curation, Formal analysis, Writing – original draft. ML: Writing – review and editing. JD: Conceptualization, Data curation, Methodology, Writing – original draft. XY: Data curation, Writing – original draft. YmY: Writing – original draft. ZL: Conceptualization, Supervision, Writing – review and editing. GZ: Writing – review and editing, Methodology, Data curation, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Hunan Province Natural Science Foundation Project (2025JJ90014).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AA, average age; BMI, body mass index; CI, confidence interval; CNKI, China National Knowledge Infrastructure; CSTJ, China Science and Technology Journal Database; MD, mean difference; RCT, randomized controlled trial; RR, risk ratio; SMD, standardized mean difference; TSA, trial sequential analysis; WMD, weighted mean difference.
References
Aging and Health (2025). Available online at: https://www.who.int/zh/news-room/fact-sheets/detail/ageing-and-health (Accessed January 8, 2025).
Benzoni, T., and Cascella, M. (2025). “Procedural sedation,” in StatPearls. Treasure Island, FL. StatPearls Publishing. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK551685/ (Accessed January 14, 2025).
Carey, J. J., Abalo, M., and Fabbro, M. (2019). Propofol-induced vasodilation: the cart before the horse? J. Cardiothorac. Vasc. Anesth. 33, 2192–2194. doi:10.1053/j.jvca.2019.02.037
Chen, W., Xu, Y., Zeng, Y., and Xing, G. (2025). A meta-analysis and systematic review based on perioperative management of elderly patients: is ciprofol an alternative to propofol? Eur. J. Clin. Pharmacol. 81, 111–121. doi:10.1007/s00228-024-03782-7
Chen, Z. W., Chintala, S. M., Bracamontes, J., Sugasawa, Y., Pierce, S. R., Varga, B. R., et al. (2024). Three classes of propofol binding sites on GABAA receptors. J. Biol. Chem. 300, 107778. doi:10.1016/j.jbc.2024.107778
Christensen, K., Doblhammer, G., Rau, R., and Vaupel, J. W. (2009). Ageing populations: the challenges ahead. Lancet 374, 1196–1208. doi:10.1016/S0140-6736(09)61460-4
Cristina, N. M., and d’Alba, L. (2021). Nutrition and healthy aging: prevention and treatment of gastrointestinal diseases. Nutrients 13, 4337. doi:10.3390/nu13124337
Desousa, K. A. (2016). Pain on propofol injection: causes and remedies. Indian J. Pharmacol. 48, 617–623. doi:10.4103/0253-7613.194845
Doenicke, A. W., Roizen, M. F., Rau, J., Kellermann, W., and Babl, J. (1996). Reducing pain during propofol injection: the role of the solvent. Anesth. Analg. 82, 472–474. doi:10.1097/00000539-199603000-00007
Durai Samy, N. K., and Taksande, K. (2024). Exploring ciprofol alternatives: a comprehensive review of intravenous anesthesia options. Cureus 16, e57581. doi:10.7759/cureus.57581
Early, D. S., Lightdale, J. R., Vargo, J. J., Acosta, R. D., Chandrasekhara, V., Chathadi, K. V., et al. (2018). Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest. Endosc. 87, 327–337. doi:10.1016/j.gie.2017.07.018
Ferreira, A. O., and Cravo, M. (2015). Sedation in gastrointestinal endoscopy: where are we at in 2014? World J. Gastrointest. Endosc. 7, 102–109. doi:10.4253/wjge.v7.i2.102
Finocchiaro, M., Cortegoso Valdivia, P., Hernansanz, A., Marino, N., Amram, D., Casals, A., et al. (2021). Training simulators for gastrointestinal endoscopy: current and future perspectives. Cancers (Basel) 13, 1427. doi:10.3390/cancers13061427
Gao, Z. W., Zhou, R. X., Li, J. X., and Wang, N. (2023). Applicability of ciprofol combined with nalbuphine in painless gastroenteroscopy on elderly patients. Med. J. Chin. People’s Armed Police Force 34, 330–334. doi:10.3969/j.issn.1004-3594.2023.04.012
Guo, J., Qian, Y., Zhang, X., Han, S., Shi, Q., and Xu, J. (2022). Remimazolam tosilate compared with propofol for gastrointestinal endoscopy in elderly patients: a prospective, randomized and controlled study. BMC Anesthesiol. 22, 180. doi:10.1186/s12871-022-01713-6
Guo, M. (2023). Analysis of the mechanism of action of cyclopol in clinical application. ACM 13, 14569–14573. doi:10.12677/ACM.2023.1392037
He, K. Q., Huang, T. T., Tan, M. Y., Gao, C., and Wang, S. (2024). Efficacy and safety of ciprofol versus propofol as anesthetic for patients undergoing painless colonoscopy. Pain Ther. 13, 1633–1644. doi:10.1007/s40122-024-00662-x
Hendrix, J. M., and Garmon, E. H. (2025). “American society of anesthesiologists physical status classification system,” in StatPearls. Treasure Island, FL. StatPearls Publishing. Available online at: http://www.ncbi.nlm.nih.gov/books/NBK441940/ (Accessed July 5, 2025).
Huanbo. (2024). Huanbo phenol is steadily increasing in volume, and differentiated innovative drugs are being increasingly realized.
Huo, Q. X., and Dan, J. P. (2024). Comparison and safety analysis of sedative effects of ciprofol and propofol in elderly painless gastroscopy examination. Cap. Food Med. 31, 68–70. doi:10.3969/j.issn.1005-8257.2024.15.024
Jiang, J., Jiao, Y., Gao, P. o., Yin, W., Zhou, W., Zhang, Y., et al. (2021). Propofol differentially induces unconsciousness and respiratory depression through distinct interactions between GABAA receptor and GABAergic neuron in corresponding nuclei. ABBS 53, 1076–1087. doi:10.1093/abbs/gmab084
Kuriyama, T., Tokinaga, Y., Tange, K., Kimoto, Y., and Ogawa, K. (2012). Propofol attenuates angiotensin II-induced vasoconstriction by inhibiting Ca2+-dependent and PKC-mediated ca 2+ sensitization mechanisms. J. Anesth. 26, 682–688. doi:10.1007/s00540-012-1415-5
Lambert, R. (2012). Endoscopy in screening for digestive cancer. World J. Gastrointest. Endosc. 4, 518–525. doi:10.4253/wjge.v4.i12.518
Liao, J., Li, M., Huang, C., Yu, Y., Chen, Y., Gan, J., et al. (2022). Pharmacodynamics and pharmacokinetics of HSK3486, a novel 2,6-disubstituted phenol derivative as a general anesthetic. Front. Pharmacol. 13, 830791. doi:10.3389/fphar.2022.830791
Liu, F., Cheng, X., Wang, Y., Li, K., Peng, T., Fang, N., et al. (2023). Effect of remimazolam tosilate on the incidence of hypoxemia in elderly patients undergoing gastrointestinal endoscopy: a bi-center, prospective, randomized controlled study. Front. Pharmacol. 14, 1131391. doi:10.3389/fphar.2023.1131391
Liu, J., Hong, A., Zeng, J., and Liang, X. (2024). The efficacy of ciprofol versus propofol on anesthesia in patients undergoing endoscopy: a systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. 24, 359. doi:10.1186/s12871-024-02721-4
Liu, Q. Z., Lin, F. F., and Xu, T. M. (2024). Anesthetic effect of cyclopol combined with low dose sufentanil in painless gastrointestinal mirror diagnosis and treatment of elderly patients. Guide China Med. 22, 27–29. doi:10.15912/j.issn.1671-8194.2024.23.008
Liu, X., Rabin, P. L., Yuan, Y., Kumar, A., Vasallo, P., Wong, J., et al. (2019). Effects of anesthetic and sedative agents on sympathetic nerve activity. Heart Rhythm. 16, 1875–1882. doi:10.1016/j.hrthm.2019.06.017
Lu, K., Wei, S., Ling, W., Wei, Y., Ran, X., Huang, H., et al. (2022). Remimazolam versus propofol for deep sedation/anaesthesia in upper gastrointestinal endoscopy in elderly patients: a multicenter, randomized controlled trial. J. Clin. Pharm. Ther. 47, 2230–2236. doi:10.1111/jcpt.13797
Lu, M., Liu, J., Wu, X., and Zhang, Z. (2023). Ciprofol: a novel alternative to propofol in clinical intravenous anesthesia? Biomed. Res. Int. 2023, 7443226. doi:10.1155/2023/7443226
Ma, J. L., and Huang, Z. C. (2023). Treatment effect of cyclophenol in painless gastroscopy in elderly patients and analysis of adverse reactions to patients. World Healthy Living. doi:10.3969/j.issn.1671-2269
Meng, T., Bu, W., Ren, X., Chen, X., Yu, J., Eckenhoff, R. G., et al. (2016). Molecular mechanism of anesthetic-induced depression of myocardial contraction. FASEB J. Official Publ. Fed. Am. Soc. Exp. Biol. 30, 2915–2925. doi:10.1096/fj.201600290RR
Miller’s basics of anesthesia (2024). Eur. J. Anaesthesiol. 41, 153–154. doi:10.1097/EJA.0000000000001943
Mitsonis, C., Dimopoulos, N., Zavrou, M., Psarra, V., Giofkos, C., Fiorakis, C., et al. (2011). Panic attack during elective gastrointestinal endoscopy. Gastroenterol. Res. Pract. 2011, 162574. doi:10.1155/2011/162574
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 88, 105906. doi:10.1016/j.ijsu.2021.105906
Partridge, L., Deelen, J., and Slagboom, P. E. (2018). Facing up to the global challenges of ageing. Nature 561, 45–56. doi:10.1038/s41586-018-0457-8
Petkar, S., Bele, A., Priya, V., and Bawiskar, D. (2024). Pharmacological insights and clinical applications of ciprofol: a narrative review. Cureus 16, e68034. doi:10.7759/cureus.68034
Sallard, E., Letourneur, D., and Legendre, P. (2021). Electrophysiology of ionotropic GABA receptors. Cell Mol. Life Sci. 78, 5341–5370. doi:10.1007/s00018-021-03846-2
Sankar, A., Johnson, S. R., Beattie, W. S., Tait, G., and Wijeysundera, D. N. (2014). Reliability of the American society of anesthesiologists physical status scale in clinical practice. Br. J. Anaesth. 113, 424–432. doi:10.1093/bja/aeu100
Sawant, A., Kamath, S., Kg, H., and Kulyadi, G. P. (2021). Solid-in-oil-in-water emulsion: an innovative paradigm to improve drug stability and biological activity. AAPS PharmSciTech 22, 199. doi:10.1208/s12249-021-02074-y
Short, C. E., and Bufalari, A. (1999). Propofol anesthesia. Vet. Clin. North Am. Small Anim. Pract. 29, 747–778. doi:10.1016/s0195-5616(99)50059-4
Sidhu, R., Turnbull, D., Haboubi, H., Leeds, J. S., Healey, C., Hebbar, S., et al. (2024). British society of gastroenterology guidelines on sedation in gastrointestinal endoscopy. Gut 73, 219–245. doi:10.1136/gutjnl-2023-330396
Sim, J. Y., Lee, S. H., Park, D. Y., Jung, J. A., Ki, K. H., Lee, D. H., et al. (2009). Pain on injection with microemulsion propofol. Br. J. Clin. Pharmacol. 67, 316–325. doi:10.1111/j.1365-2125.2008.03358.x
Smale, S., Bjarnason, I., Forgacs, I., Prasad, P., Mukhood, M., Wong, M., et al. (2003). Upper gastrointestinal endoscopy performed by nurses: scope for the future? Gut 52, 1090–1094. doi:10.1136/gut.52.8.1090
Task Force on Guidelines on Clinical Application of Ciprofol (2023). Guidelines on clinical application of ciprofol. Chin. J. Crit. Care and Intensive Care Med. 43, 769–772. doi:10.3760/cma.j.cn131073.20230711.00701
The National Medical Products Administration (2020). The national medical products administration (NMPA) has approved the market launch of Huanbo phenol injection. Available online at: https://www.nmpa.gov.cn/zhuanti/cxylqx/cxypxx/20201214100345170.html (Accessed December 30, 2024).
Usach, I., Martinez, R., Festini, T., and Peris, J. E. (2019). Subcutaneous injection of drugs: literature review of factors influencing pain sensation at the injection site. Adv. Ther. 36, 2986–2996. doi:10.1007/s12325-019-01101-6
Wang, C., Dong, X., and Zhao, K. F. (2023). Effectiveness of cyproterone combined with alfentanil for colonoscopy in elderly debilitated patients. J. Clin. Anesthesiol. 39, 550–552. doi:10.12089/jca.2023.05.021
Xin, C. B. (2023). “Safety and efficacy of ciprofol and propofolduring painless gastroscopie anesthesia in elderly patients,”. Jilin University. [Master’s Thesis]. doi:10.27162/d.cnki.gjlin.2023.006791
Xu, M., Wang, Y. G., Song, D. D., and Diao, Y. G. (2023). Comparison of sedative effect of ciprofol and propofol in elder patients undergoing fibrocolonoscopy treatment. J. Clin. Anesth. 39, 705–708. doi:10.12089/jca.2023.07.006
Yang, Y. J., Guo, J. P., Huang, J. Y., Cao, Y. F., and Wang, Y. (2011). The relationship between body mass index and cerebrovascular hemodynamics and stroke. Chin. J. Integr. Med. Cardio/Cerebrovascular Dis. 9, 551–552. doi:10.3969/j.issn.1672-1349.2011.05.023
Yang, Y. W., and Jian, L. (2023). Analysis of the anesthetic effect of cyclobenzaprine combined with low-dose alfentanil hydrochloride in painless gastroenteroscopy in elderly patients. Yi Shou Bao Dian, 28–30.
Yao, Y. P., Zhu, X., and You, Z. Y. (2024). Application effect of nalbuphine combined with ciprofol or propofol in painless gastroscopy in elderly patients. Guangzhou Med. J. 55, 1152–1158. doi:10.20223/j.cnki.1000-8535.2024.10.009
Yi, Q. L., Mo, H. Z., Hu, H., and Xiang, M. (2022). Comparison of ciprofol and propofol in elderly patients undergoing gastroscopy. J. Clin. Anesthesiol. 38, 712–715. doi:10.12089/jca.2022.07.008
Yuan, Y. C., and Chen, L. (2024). Anesthetic effect of cyclopofol combined with dexmedetomidine for painless gastroenteroscopy in elderly patients. China J. Pharm. Econ. 19, 57–60. doi:10.12010/j.issn.1673-5846.2024.09.011
Keywords: ciprofol, propofol, elderly patients, gastrointestinal endoscopy, meta-analysis
Citation: Yu Y, Liao M, Deng J, Yang X, Yin Y, Liu Z and Zhang G (2025) Potential of ciprofol as an alternative to propofol in elderly patients undergoing gastrointestinal endoscopy: a meta-analysis and trial sequential analysis. Front. Pharmacol. 16:1644182. doi: 10.3389/fphar.2025.1644182
Received: 10 June 2025; Accepted: 11 July 2025;
Published: 11 August 2025.
Edited by:
Ralf Weiskirchen, RWTH Aachen University, GermanyReviewed by:
Enas Noseer, Aswan University, EgyptYangzi Zhu, Xuzhou Central Hospital, China
Marcelo Adrian Estrin, Interamerican Open University, Argentina
Copyright © 2025 Yu, Liao, Deng, Yang, Yin, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guomin Zhang, ODM0MDk1NzczQHFxLmNvbQ==; Zhenjie Liu, bGl1emhlbmppZTkyNkAxNjMuY29t
†These authors have contributed equally to this work
 Yunfeng Yu
Yunfeng Yu Min Liao
Min Liao Juan Deng
Juan Deng Xinyu Yang
Xinyu Yang Yuman Yin3
Yuman Yin3