Abstract
Background:
Dexmedetomidine has been demonstrated to have cardioprotective effects in previous studies, prompting our investigation into its potential to improve survival outcomes in patients with cardiogenic shock (CS).
Methods:
This retrospective cohort study analyzed data from the Medical Information Mart for Intensive Care (MIMIC) IV database, focusing on patients with CS. Exposure was defined as intravenous dexmedetomidine administration during intensive care unit (ICU) stay. The primary endpoints included 7-day and 30-day all-cause mortality. External validation was conducted using the eICU 2.0 database.
Results:
The pre-matched and propensity score matched cohorts comprised 2,341 and 1,038 patients, respectively. Multivariable Cox regression analysis of the overall cohort revealed that dexmedetomidine administration was significantly associated with reduced risk of both 7-day (hazard ratio (HR) = 0.473, 95% confidence interval (CI): 0.359–0.624, p < 0.001) and 30-day all-cause mortality (HR = 0.606, 95% CI: 0.500–0.735, p < 0.001). This protective association persisted after propensity score matching (PSM) for 7-day (HR = 0.418, 95% CI: 0.317–0.552, p < 0.001) and 30-day mortality (HR = 0.579, 95% CI: 0.475–0.705, p < 0.001). Subgroup analyses demonstrated that patients older than 75 years, those with chronic pulmonary disease, or those with lower systolic blood pressure may not benefit from dexmedetomidine. External validation using 1411 CS patients from the eICU 2.0 database confirmed these findings, with PSM-adjusted analyses showing reduced in-hospital (HR = 0.597; 95% CI: 0.395–0.901; p = 0.014) and in-ICU mortality (HR = 0.425; 95% CI: 0.262–0.689; p < 0.001) among dexmedetomidine treated patients.
Conclusion:
Dexmedetomidine administration was associated with reduced risk of 7-day and 30-day all-cause mortality in CS patients, though this protective effect may not be significant in patients over 75 years, those with chronic pulmonary disease, or those with lower systolic blood pressure. Prospective studies are required to validate these findings.
1 Introduction
Cardiogenic shock (CS) is a life-threatening systemic hypoperfusion syndrome resulting from severe cardiac dysfunction. Without prompt intervention, it rapidly progresses to irreversible multiorgan failure and carries substantial mortality (van Diepen et al., 2017; Chioncel et al., 2020). Recent data indicate that in-hospital mortality for CS in the United States approaches 37% (Osman et al., 2021). European studies report comparable mortality rates, ranging from 30% to 60% (Chioncel et al., 2020). Current therapeutic interventions with proven efficacy in improving outcomes for CS patients remain limited. Developing novel treatment strategies for CS is therefore imperative (van Diepen et al., 2017).
Dexmedetomidine, a highly selective α2-adrenergic receptor agonist, exhibits sedative, analgesic, and anxiolytic properties (Keating, 2015; Bilotta and Pugliese, 2020; Homberg et al., 2023). This agent is frequently administered to perioperative and intensive care patients (Bilotta and Pugliese, 2020). Recent studies indicate that dexmedetomidine exerts cardioprotective effects (Takahashi et al., 2023) in the setting of cardiac surgery (Ji et al., 2013; Peng et al., 2021; Chen et al., 2022) and acute myocardial infarction (AMI) (Liu et al., 2024) by modulating inflammatory responses (Yang et al., 2017), alleviating microcirculatory dysfunction (Lawrence et al., 1996), attenuating oxidative stress (Han et al., 2019), improving cardiac function and protecting against maladaptive remodeling (Han et al., 2019). These pharmacological effects may collectively improve outcomes in CS patients. However, limited clinical evidence exists on dexmedetomidine’s effects on clinical outcomes in CS patients. Using the Medical Information Mart in Intensive Care-IV (MIMIC-IV) database, this propensity score-matched cohort study investigated dexmedetomidine’s potential therapeutic role in CS, with external validation through an independent dataset.
2 Methods
2.1 Study design and participants
This retrospective observational cohort study investigated the potential survival benefit of dexmedetomidine in CS patients. We extracted clinical data from MIMIC-IV version 3.0 (Johnson et al., 2023), a publicly accessible database containing 94,458 intensive care unit (ICU) admissions at Beth Israel Deaconess Medical Center between 2008 and 2022. The use of anonymized patient records waived the requirement for individual informed consent. Author L.F.X obtained database access after completing the Collaborative Institutional Training Initiative program (Certification number: 57983166). The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University and complied with the Declaration of Helsinki.
The study enrolled patients diagnosed with CS according to the International Classification of Diseases (9th and 10th Revisions). We excluded individuals with multiple ICU admissions for CS, retaining only data from their first admission, those under 18 years of age, patients with ICU stays shorter than 24 h, patients with heart rate less than 60 per minute, and patients with respiratory rate less than 12 per minute. Figure 1 presents the study flowchart.
FIGURE 1
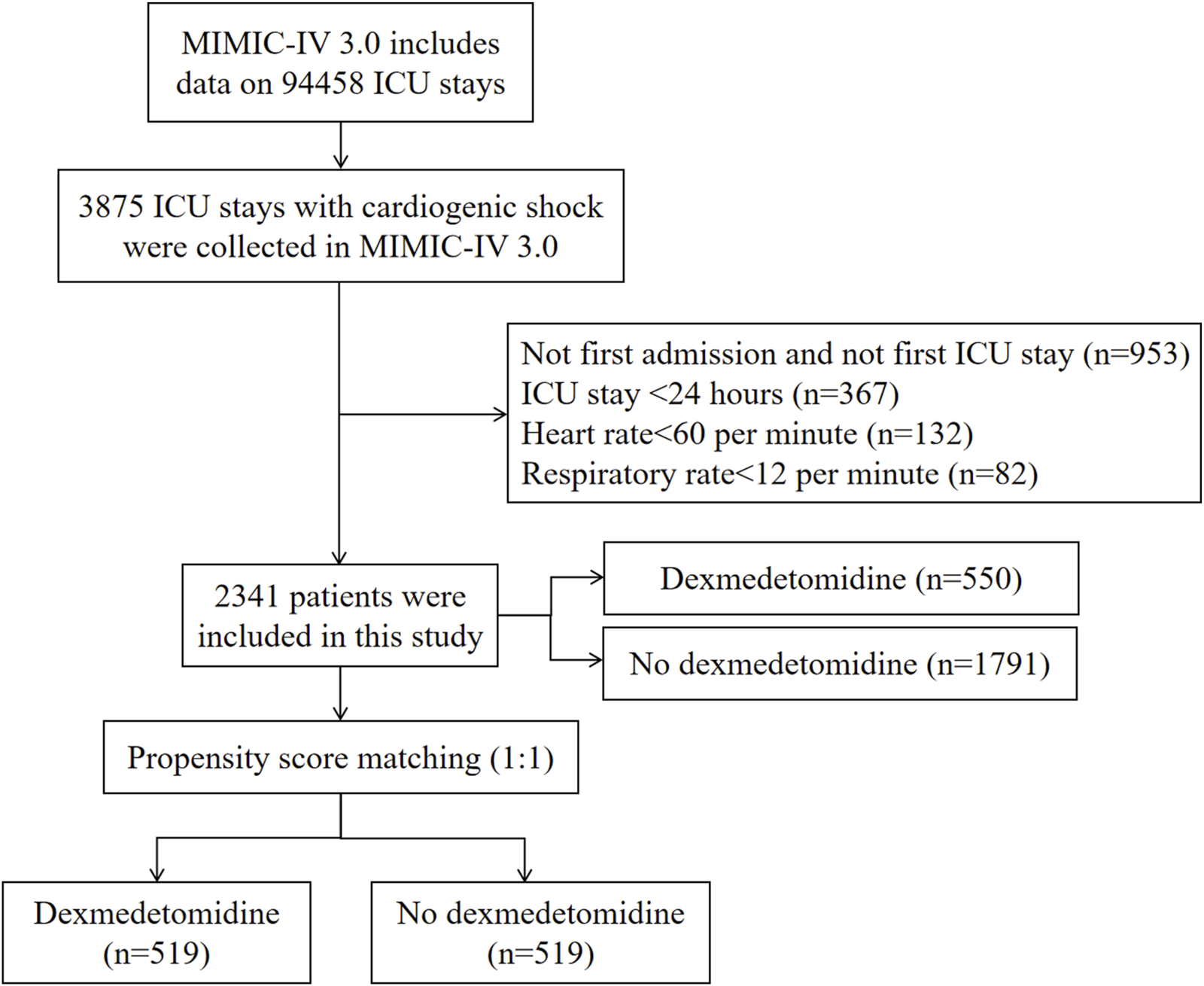
Flowchart of study participants in MIMIC-IV database. ICU, intensive care unit; MIMIC, Medical Information Mart for Intensive Care.
2.2 Data extraction
L.F.X extracted the data using PostgreSQL (version 13.7.2), Navicat Premium (version 16), and structured query language (SQL). Clinical variables encompassed demographic information, vital signs, initial laboratory parameters post-ICU admission, comorbidities, and treatments. The Sequential Organ Failure Assessment (SOFA) score was also recorded. Dexmedetomidine usage data originated from the prescriptions table in the MIMIC-IV database.
2.3 Exposure and study endpoint
Exposure was defined as intravenous dexmedetomidine administration during ICU stay without dosage restrictions. The primary endpoints included 7-day and 30-day all-cause mortality. For those treated with dexmedetomidine, the time of dexmedetomidine administration was taken as the start time of follow-up.
2.4 External validation in eICU 2.0 database
The external validation cohort sourced from the publicly available eICU Database (version 2.0), comprised 139,367 patients with 200,859 ICU admissions from 208 U.S. hospitals between 2014 and 2015. We extracted demographic characteristics, laboratory results, treatment records, and mortality outcomes for patients diagnosed with CS.
2.5 Statistical analysis
Continuous variables conforming to a normal distribution were expressed as mean ± standard deviation (SD) and compared using independent sample t-tests, whereas non-normally distributed variables were reported as median (25th-75th percentiles) and analyzed with the Mann-Whitney U test. Categorical variables were presented as absolute numbers (percentages) and assessed with either chi-square or Fisher’s exact tests.
To account for potential confounding variables and strengthen the validity of the results, we performed propensity score matching (PSM) following Lonjon et al.'s methodology (Lonjon et al., 2017). Using 1:1 nearest neighbor matching based on propensity scores, we matched patients in the dexmedetomidine and non-dexmedetomidine groups. Standardized mean differences (SMD) below 0.10 confirmed adequate covariate balance before and after PSM (Austin, 2009).
We compared 7-day and 30-day all-cause mortality between the two groups, analyzing cumulative survival probabilities using Kaplan-Meier curves with log-rank tests. Cox proportional hazards models assessed dexmedetomidine’s independent effect on mortality before and after PSM. Three pre-PSM models were constructed: Model 1 remained unadjusted; Model 2 adjusted for demographic and vital signs (age, gender, race, systolic blood pressure, heart rate, respiratory rate); Model 3 adjusted for demographic, vital signs, laboratory values, comorbidities, and interventions (including age, gender, race, systolic blood pressure, heart rate, respiratory rate, temperature, SPO2, hematocrit, hemoglobin, platelet, white blood cell, red blood cell distribution width, anion gap, bicarbonate, blood urea nitrogen, calcium, chlorine, creatinine, glucose, sodium, potassium, SOFA score, AMI, heart failure, cerebrovascular disease, chronic pulmonary disease, liver disease, diabetes, chronic kidney disease, cancer, hypertension, corona virus disease 2019, dobutamine, dopamine, adrenaline, norepinephrine, vasopressin, milrinone, midazolam, diazepam, propofol, fentanyl, invasive ventilation, extracorporeal membrane oxygenation, intraaortic balloon pump, and impella devices). Subgroup analyses evaluated mortality differences across strata defined by age, gender, race, key comorbidities (AMI, heart failure, chronic pulmonary disease, and cerebrovascular disease), and vital signs (heart rate, systolic blood pressure, and respiratory rate). Subgroup analysis based on the timing for dexmedetomidine initiation, duration, and cumulative dose was also conducted. In this study, a 2-tailed p-value of <0.05 was considered statistically significant and all statistical analyses were carried out using SPSS statistical software, version 25.0 (IBM, United States), GraphPad Prism 8.4.3, and R version 4.1.2 (R Foundation).
3 Results
3.1 The baseline characteristics
The study enrolled 2,341 eligible CS patients (Figure 1), with 550 receiving dexmedetomidine and 1791 not receiving it during ICU admission. The cohort had a mean age of 69.83 years (SD 14.43), and 60.66% (n = 1,420) were male. PSM yielded 1038 CS patients for final analysis.
Table 1 presents the baseline characteristics of CS patients treated with and without dexmedetomidine. Prior to PSM, significant imbalances existed between the dexmedetomidine and control group in age, gender, systolic blood pressure, prevalence of AMI, chronic kidney disease, cerebrovascular disease, and cancer, along with laboratory values including white blood cell count, anion gap, chlorine, sodium, and blood urea nitrogen. Treatment differences were also observed in the use of vasopressors (dopamine, epinephrine, norepinephrine, vasopressin), other sedative/analgesic medications (midazolam, propofol, fentanyl), invasive ventilation, and intra-aortic balloon pump support (all SMD >0.1). Following PSM, these baseline characteristics achieved balance between the two groups (all SMD<0.1; Table 1; Figure 2).
TABLE 1
| Variables | Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 2,341) | No dexmedetomidine (n = 1791) | Dexmedetomidine (n = 550) | P Value | SMD | Total (n = 1,038) | No dexmedetomidine (n = 519) | Dexmedetomidine (n = 519) | SMD | |
| Demographic characteristic | |||||||||
| Age, years, Mean ± SD | 69.83 ± 14.43 | 70.84 ± 14.29 | 66.54 ± 14.39 | <0.001 | 0.299 | 67.23 ± 14.47 | 67.63 ± 14.70 | 66.84 ± 14.24 | 0.056 |
| Gender, male, n (%) | 1,420 (60.66) | 1,061 (59.24) | 359 (65.27) | 0.011 | 0.127 | 662 (63.78) | 330 (63.58) | 332 (63.97) | 0.008 |
| Race, white, n (%) | 1,460 (62.37) | 1,126 (62.87) | 334 (60.73) | 0.364 | 0.044 | 633 (60.98) | 317 (61.08) | 316 (60.89) | 0.004 |
| Comorbidities | |||||||||
| AMI, n (%) | 780 (33.32) | 567 (31.66) | 213 (38.73) | 0.002 | 0.145 | 393 (37.86) | 194 (37.38) | 199 (38.34) | 0.020 |
| Heart failure, n (%) | 1853 (79.15) | 1,434 (80.07) | 419 (76.18) | 0.050 | 0.091 | 783 (75.43) | 387 (74.57) | 396 (76.30) | 0.041 |
| Hypertension, n (%) | 536 (22.9) | 412 (23.00) | 124 (22.55) | 0.823 | 0.011 | 227 (21.87) | 110 (21.19) | 117 (22.54) | 0.032 |
| Diabetes, n (%) | 920 (39.3) | 711 (39.70) | 209 (38.00) | 0.476 | 0.035 | 392 (37.76) | 198 (38.15) | 194 (37.38) | 0.016 |
| Cerebrovascular disease, n (%) | 307 (13.11) | 210 (11.73) | 97 (17.64) | <0.001 | 0.155 | 172 (16.57) | 84 (16.18) | 88 (16.96) | 0.021 |
| Chronic pulmonary disease, n (%) | 683 (29.18) | 523 (29.20) | 160 (29.09) | 0.960 | 0.002 | 302 (29.09) | 152 (29.29) | 150 (28.90) | 0.009 |
| Liver disease, n (%) | 316 (13.5) | 248 (13.85) | 68 (12.36) | 0.373 | 0.045 | 127 (12.24) | 60 (11.56) | 67 (12.91) | 0.040 |
| Chronic kidney disease, n (%) | 928 (39.64) | 736 (41.09) | 192 (34.91) | 0.009 | 0.130 | 361 (34.78) | 181 (34.87) | 180 (34.68) | 0.004 |
| Cancer, n (%) | 213 (9.1) | 180 (10.05) | 33 (6.00) | 0.004 | 0.171 | 71 (6.84) | 39 (7.51) | 32 (6.17) | 0.056 |
| COVID-19, n (%) | 88 (3.76) | 58 (3.24) | 30 (5.45) | 0.017 | 0.098 | 49 (4.72) | 23 (4.43) | 26 (5.01) | 0.026 |
| Vital signs | |||||||||
| SBP, mmHg, Mean ± SD | 112.39 ± 22.21 | 111.46 ± 22.01 | 115.39 ± 22.59 | <0.001 | 0.174 | 114.77 ± 22.44 | 114.28 ± 22.31 | 115.26 ± 22.57 | 0.044 |
| Heart rate, per minute, Mean ± SD | 94.30 ± 20.97 | 93.88 ± 20.89 | 95.66 ± 21.20 | 0.083 | 0.084 | 94.67 ± 21.56 | 94.24 ± 21.98 | 95.11 ± 21.16 | 0.041 |
| Respiratory rate, per minute, Mean ± SD | 21.48 ± 6.03 | 21.39 ± 5.95 | 21.80 ± 6.26 | 0.162 | 0.066 | 21.60 ± 6.28 | 21.43 ± 6.31 | 21.77 ± 6.25 | 0.054 |
| SPO2, %, Mean ± SD | 95.88 ± 5.21 | 95.87 ± 5.14 | 95.93 ± 5.41 | 0.787 | 0.013 | 95.89 ± 5.77 | 95.87 ± 6.03 | 95.91 ± 5.51 | 0.007 |
| Laboratory test | |||||||||
| Hemoglobin, g/dL, Mean ± SD | 11.19 ± 2.54 | 11.14 ± 2.48 | 11.33 ± 2.72 | 0.157 | 0.068 | 11.23 ± 2.73 | 11.17 ± 2.71 | 11.28 ± 2.75 | 0.041 |
| Platelet, K/uL, M (Q1, Q3) | 201.00 (145.00, 270.00) | 202.00 (146.00, 271.00) | 197.00 (142.00, 262.75) | 0.316 | 0.019 | 201.00 (143.00, 266.00) | 202.00 (144.00, 265.00) | 201.00 (142.50, 266.00) | 0.053 |
| WBC, K/uL, M (Q1, Q3) | 11.90 (8.50, 16.50) | 11.80 (8.30, 16.20) | 12.55 (9.50, 17.80) | <0.001 | 0.120 | 12.60 (9.20, 17.90) | 12.50 (9.00, 17.95) | 12.60 (9.45, 17.90) | 0.005 |
| Anion gap, mEq/L, Mean ± SD | 17.46 ± 5.59 | 17.64 ± 5.47 | 16.87 ± 5.90 | 0.005 | 0.130 | 17.08 ± 5.93 | 17.19 ± 5.91 | 16.97 ± 5.96 | 0.037 |
| Bicarbonate, mEq/L, Mean ± SD | 20.99 ± 5.40 | 21.08 ± 5.45 | 20.71 ± 5.23 | 0.165 | 0.070 | 20.60 ± 5.37 | 20.48 ± 5.48 | 20.71 ± 5.26 | 0.042 |
| Calcium, mg/dL, Mean ± SD | 8.52 ± 0.98 | 8.53 ± 0.94 | 8.47 ± 1.10 | 0.254 | 0.050 | 8.45 ± 1.06 | 8.43 ± 1.01 | 8.46 ± 1.10 | 0.027 |
| Chlorine, mEq/L, Mean ± SD | 100.45 ± 7.40 | 100.18 ± 7.43 | 101.33 ± 7.27 | 0.002 | 0.157 | 101.38 ± 7.26 | 101.47 ± 7.18 | 101.29 ± 7.35 | 0.024 |
| Potassium, mEq/L, Mean ± SD | 4.56 ± 0.99 | 4.54 ± 0.99 | 4.62 ± 1.00 | 0.103 | 0.079 | 4.58 ± 1.01 | 4.54 ± 1.00 | 4.61 ± 1.01 | 0.065 |
| Sodium, mEq/L, Mean ± SD | 136.65 ± 5.70 | 136.39 ± 5.70 | 137.49 ± 5.60 | <0.001 | 0.196 | 137.49 ± 5.47 | 137.51 ± 5.31 | 137.46 ± 5.64 | 0.009 |
| BUN, mg/dL, M (Q1, Q3) | 31.00 (20.00, 50.00) | 33.00 (20.00, 52.00) | 26.00 (17.00, 42.00) | <0.001 | 0.275 | 27.00 (18.00, 42.00) | 27.00 (18.00, 42.00) | 27.00 (18.00, 42.00) | 0.031 |
| Creatinine, mg/dL, M (Q1, Q3) | 1.50 (1.00, 2.30) | 1.50 (1.10, 2.40) | 1.40 (1.00, 2.10) | 0.001 | 0.074 | 1.40 (1.00, 2.08) | 1.40 (1.00, 2.00) | 1.40 (1.00, 2.10) | 0.004 |
| Glucose, mg/dL, M (Q1, Q3) | 147.0 (114.0, 207.0) | 146.0 (113.0, 205.8) | 152.0 (118.0, 212.0) | 0.085 | 0.005 | 150.0 (116.0, 213.0) | 147.0 (114.0, 214.0) | 152.0 (118.0, 207.0) | 0.058 |
| Treatment | |||||||||
| Dobutamine, n (%) | 514 (21.96) | 394 (22.00) | 120 (21.82) | 0.929 | 0.004 | 216 (20.81) | 104 (20.04) | 112 (21.58) | 0.037 |
| Dopamine, n (%) | 397 (16.96) | 347 (19.37) | 50 (9.09) | <0.001 | 0.358 | 105 (10.12) | 55 (10.60) | 50 (9.63) | 0.033 |
| Adrenaline, n (%) | 441 (18.84) | 288 (16.08) | 153 (27.82) | <0.001 | 0.262 | 287 (27.65) | 148 (28.52) | 139 (26.78) | 0.039 |
| Norepinephrine, n (%) | 1,255 (53.61) | 898 (50.14) | 357 (64.91) | <0.001 | 0.309 | 656 (63.2) | 328 (63.20) | 328 (63.20) | 0.000 |
| Vasopressin, n (%) | 604 (25.8) | 398 (22.22) | 206 (37.45) | <0.001 | 0.315 | 379 (36.51) | 190 (36.61) | 189 (36.42) | 0.004 |
| Milrinone, n (%) | 263 (11.23) | 196 (10.94) | 67 (12.18) | 0.421 | 0.038 | 131 (12.62) | 68 (13.10) | 63 (12.14) | 0.029 |
| Midazolam, n (%) | 818 (34.94) | 572 (31.94) | 246 (44.73) | <0.001 | 0.257 | 459 (44.22) | 226 (43.55) | 233 (44.89) | 0.027 |
| Diazepam, n (%) | 18 (0.77) | 10 (0.56) | 8 (1.45) | 0.068 | 0.075 | 9 (0.87) | 3 (0.58) | 6 (1.16) | 0.054 |
| Propofol, n (%) | 1,114 (47.59) | 650 (36.29) | 464 (84.36) | <0.001 | 1.324 | 865 (83.33) | 432 (83.24) | 433 (83.43) | 0.005 |
| Fentanyl, n (%) | 1,451 (61.98) | 949 (52.99) | 502 (91.27) | <0.001 | 1.357 | 940 (90.56) | 469 (90.37) | 471 (90.75) | 0.013 |
| Invasive ventilation, n (%) | 1,411 (60.27) | 914 (51.03) | 497 (90.36) | <0.001 | 1.333 | 939 (90.46) | 473 (91.14) | 466 (89.79) | 0.045 |
| ECMO, n (%) | 42 (1.79) | 31 (1.73) | 11 (2.00) | 0.677 | 0.019 | 22 (2.12) | 11 (2.12) | 11 (2.12) | 0.000 |
| IABP, n (%) | 215 (9.18) | 146 (8.15) | 69 (12.55) | 0.002 | 0.133 | 123 (11.85) | 57 (10.98) | 66 (12.72) | 0.052 |
| Impella devices, n (%) | 59 (2.52) | 38 (2.12) | 21 (3.82) | 0.026 | 0.089 | 39 (3.76) | 19 (3.66) | 20 (3.85) | 0.010 |
| SOFA score, M (Q1, Q3) | 2.00 (0.00, 4.00) | 2.00 (1.00, 4.00) | 2.00 (0.00, 5.00) | 0.362 | 0.081 | 2.00 (0.00, 5.00) | 2.00 (0.00, 5.00) | 2.00 (0.00, 5.00) | 0.034 |
The baseline characteristics in MIMICIV database.
PSM, propensity score matching; AMI, acute myocardial infarction; COVID-19, corona virus disease 2019; SBP, systolic blood pressure; WBC, white blood cell; BUN, blood urea nitrogen; ECMO, extracorporeal membrane oxygenation; IABP, intraaortic balloon pump; SMD, standardized mean differences.
FIGURE 2
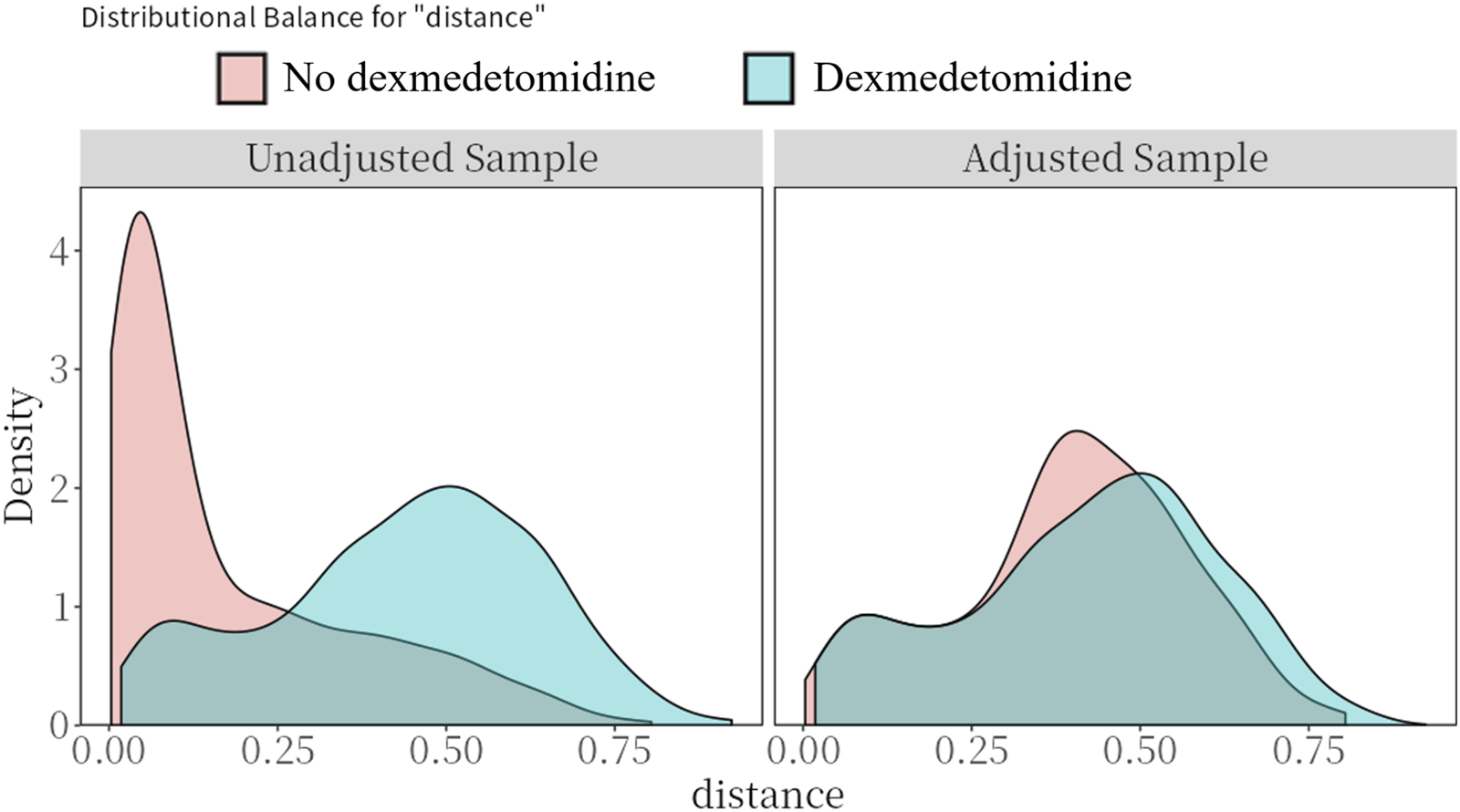
Preference score distributions. Greater overlap indicates that patients in the target and comparator populations are more similar in their likelihood of receiving the target treatment.
3.2 Outcomes
Figure 3 presents the 7-day and 30-day all-cause mortality rates. Patients treated with dexmedetomidine exhibited significantly lower 7-day mortality (14.0% vs. 22.8% before PSM, p < 0.001; 14.3% vs. 30.4% after PSM, p < 0.001) and 30-day mortality (31.3% vs. 41.0% before PSM, p < 0.001; 32.0% vs. 46.4% after PSM, p < 0.001).
FIGURE 3
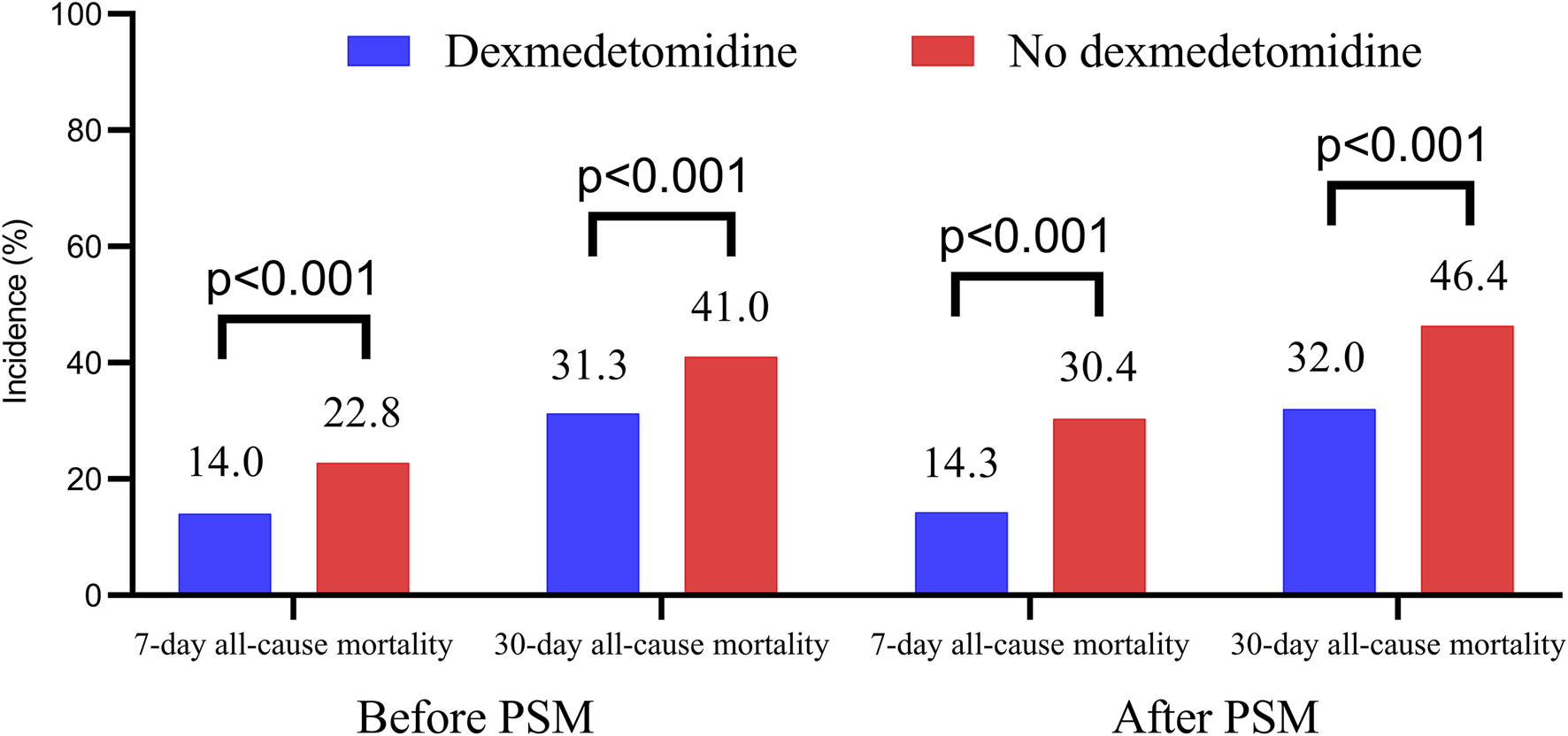
The 7-day and 30-day all-cause mortality in dexmedetomidine and no dexmedetomidine group. PSM, propensity score matching.
Kaplan-Meier survival analysis revealed significantly higher 7-day and 30-day survival probabilities in patients receiving dexmedetomidine (Log-rank p < 0.001; Figure 4). Cox regression models adjusted for multiple confounders showed dexmedetomidine was associated with significantly reduced 7-day (HR = 0.473, 95% CI: 0.359–0.624, p < 0.001) and 30-day (HR = 0.606, 95% CI: 0.500–0.735, p < 0.001) all-cause mortality. PSM confirmed this protective effect for both 7-day (HR = 0.418, 95% CI: 0.317–0.552, p < 0.001) and 30-day (HR = 0.579, 95% CI: 0.475–0.705, p < 0.001) all-cause mortality (Table 2).
FIGURE 4
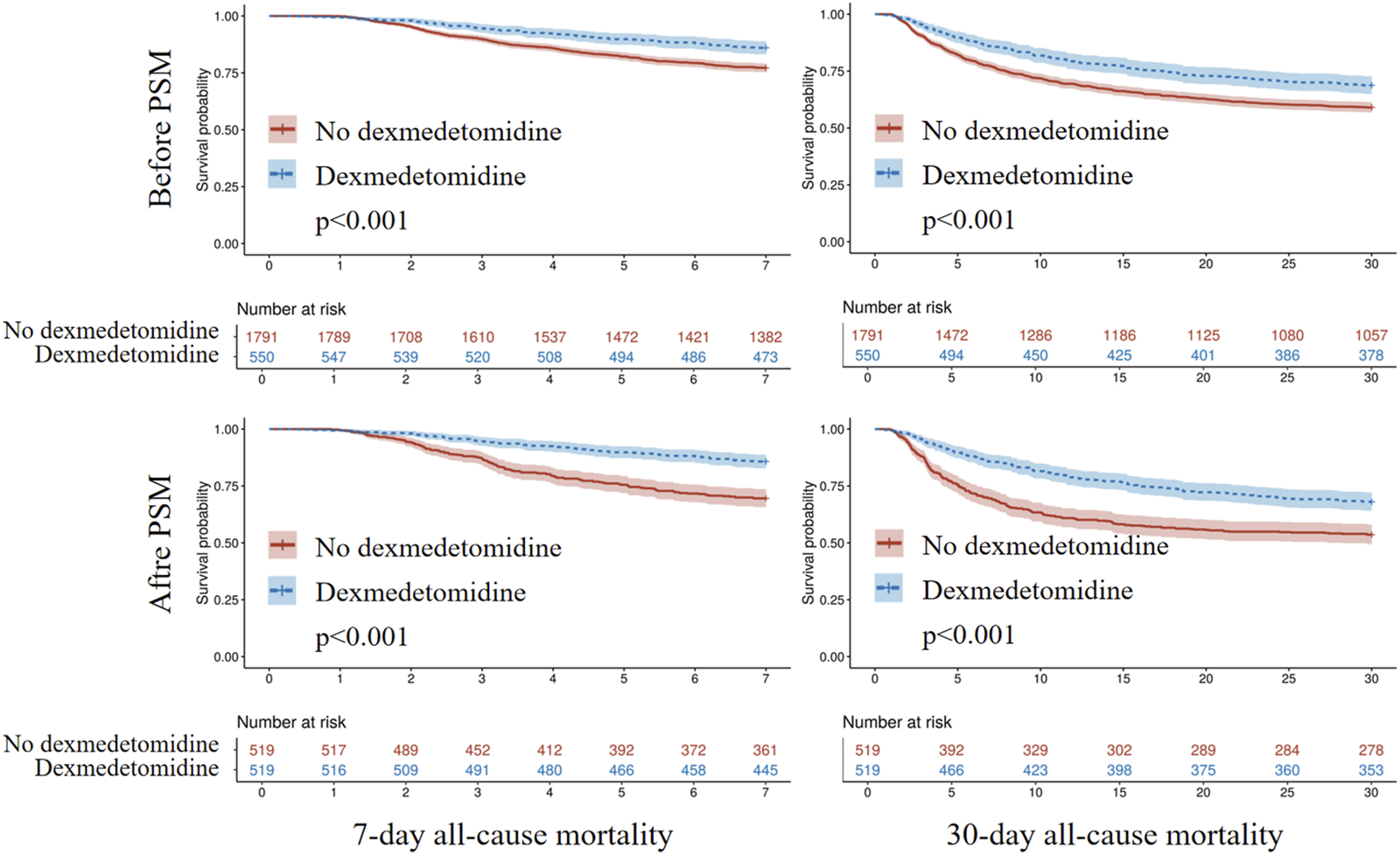
Kaplan-Meier survival analysis curves for all-cause mortality. PSM, propensity score matching.
TABLE 2
| Models | 7-day all-cause mortality | 30-day all-cause mortality | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Before PSM | ||||||
| Model 1 | 0.578 | 0.453, 0.737 | <0.001 | 0.692 | 0.586, 0.817 | <0.001 |
| Model 2 | 0.617 | 0.482, 0.789 | <0.001 | 0.762 | 0.644, 0.902 | 0.002 |
| Model 3 | 0.473 | 0.359, 0.624 | <0.001 | 0.606 | 0.500, 0.735 | <0.001 |
| After PSM | 0.418 | 0.317, 0.552 | <0.001 | 0.579 | 0.475, 0.705 | <0.001 |
Association of dexmedetomidine with all-cause mortality in MIMIC-IV database.
Model 1, unadjusted; Model 2, adjusted by age, gender, race, systolic blood pressure, heart rate, respiratory rate; Model 3, adjusted by age, gender, race, systolic blood pressure, heart rate, respiratory rate, temperature, SPO2, hematocrit, hemoglobin, platelet, white blood cell, red blood cell distribution width, anion gap, bicarbonate, blood urea nitrogen, calcium, chlorine, creatinine, glucose, sodium, potassium, SOFA, score, acute myocardial infarction, heart failure, cerebrovascular disease, chronic pulmonary disease, liver disease, diabetes, chronic kidney disease, cancer, hypertension, corona virus disease 2019, dobutamine, dopamine, adrenaline, norepinephrine, vasopressin, milrinone, midazolam, diazepam, propofol, fentanyl, invasive ventilation, extracorporeal membrane oxygenation, intraaortic balloon pump, and impella devices. HR, hazard ratio; CI, confidence interval; PSM, propensity score matching.
3.3 Subgroup analysis
To examine the association between dexmedetomidine administration and 7-day or 30-day all-cause mortality across clinical subgroups, we conducted stratified analyses. Dexmedetomidine showed consistent effects on 7-day mortality in most subgroups (p-interaction>0.05). Although the interaction effects were observed in heart failure (heart failure vs. non-heart failure), the effect was only numerically different (Figure 5). Additionally, subgroup analysis suggested an interaction between systolic blood pressure (<100 vs. ≥100 mmHg) and the use of dexmedetomidine in relation to 7-day all-cause mortality (<100 mmHg, HR = 0.66, 95% CI: 0.40–1.09; ≥100 mmHg, HR = 0.35, 95% CI: 0.25–0.49, p-interaction = 0.046). Similarly, its effect on 30-day mortality remained homogeneous across most subgroups (p-interaction>0.05). Although the interaction effects were observed in heart failure (heart failure vs. non-heart failure), the effect was only numerically different (Figure 5). Additionally, subgroup analysis suggested an interaction between age (<75 vs.≥75 years) and the use of dexmedetomidine in relation to 30-day all-cause mortality (<75 years, HR = 0.45, 95% CI: 0.35–0.59; ≥75 years, HR = 0.89, 95% CI: 0.66–1.20, p-interaction<0.001). There was also a trend toward an interaction between the presence of chronic pulmonary disease and the use of dexmedetomidine in relation to 30-day all-cause mortality (with chronic pulmonary disease, HR = 0.74, 95% CI: 0.53–1.05; without chronic pulmonary disease, HR = 0.52, 95% CI: 0.41–0.66, p-interaction = 0.088). The further subgroup analysis based on the timing for dexmedetomidine initiation, duration, and cumulative dose was also conducted (Table 3), which showed that the effect of dexmedetomidine was consistent regardless of timing for dexmedetomidine initiation (within or after 48 h after ICU admission), duration (< or ≥1.25 days), and cumulative dose (< or ≥0.6 mg).
FIGURE 5
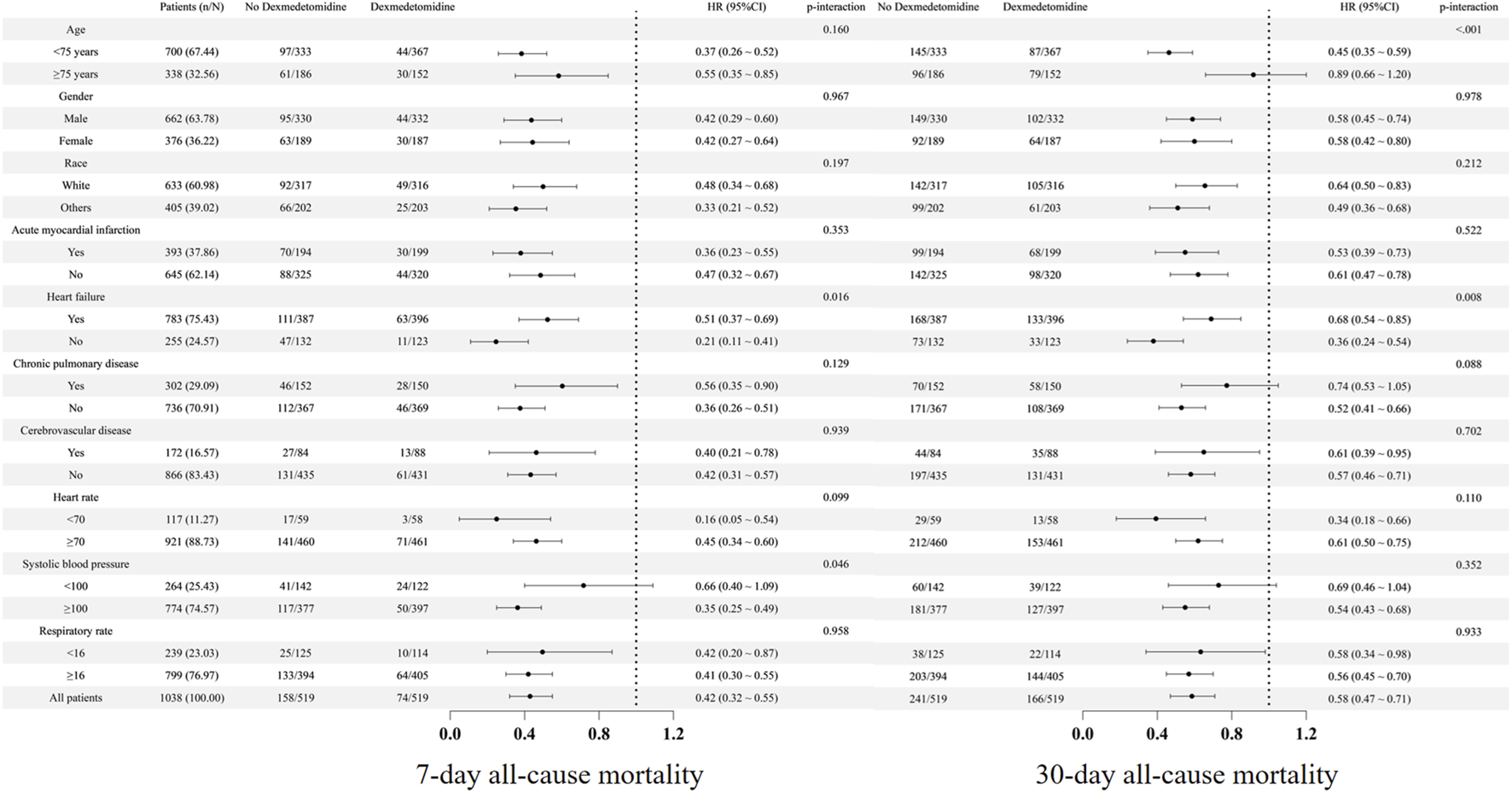
Subgroup analysis. HR, hazard ratio; CI, confidence interval.
TABLE 3
| Subgroups | n | 7-day all-cause mortality | 30-day all-cause mortality | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
| Duration | |||||||
| Duration<1.25 days | 263/519 | 0.408 | 0.284, 0.586 | <0.001 | 0.542 | 0.419, 0.699 | <0.001 |
| Duration≥1.25 days | 256/519 | 0.430 | 0.302, 0.613 | <0.001 | 0.623 | 0.488, 0.795 | <0.001 |
| Dosage | |||||||
| Dosage<0.6 mg | 371/519 | 0.418 | 0.306, 0.570 | <0.001 | 0.562 | 0.450, 0.702 | <0.001 |
| Dosage≥0.6 mg | 148/519 | 0.422 | 0.267, 0.665 | <0.001 | 0.630 | 0.465, 0.852 | 0.003 |
| Initiation time | |||||||
| Within 48 h after ICU admission | 265/519 | 0.406 | 0.284, 0.581 | <0.001 | 0.513 | 0.396, 0.663 | <0.001 |
| After 48 h after ICU admission | 354/519 | 0.433 | 0.303, 0.620 | <0.001 | 0.656 | 0.515, 0.836 | <0.001 |
Subgroup analysis based on the duration, dosage, and initiation time of dexmedetomidine in MIMICIV database.
HR, hazard ratio; CI, confidence interval; ICU, intensive care unit.
3.4 External validation with eICU 2.0 database
The external validation cohort comprised 1411 CS patients from the eICU 2.0 database (Supplementary Figure S1). Baseline characteristics of patients receiving dexmedetomidine versus those without showed balanced covariates after PSM (all SMD<0.1, Supplementary Table S1; Supplementary Figure S2). Multivariate Cox regression prior to PSM (Table 4 Model 3) demonstrated that dexmedetomidine administration significantly reduced both in-hospital (HR = 0.584, 95% CI: 0.400–0.855, p = 0.006) and in-ICU all-cause mortality (HR = 0.451, 95% CI: 0.288–0.706, p < 0.001). Post-PSM analysis (Table 4) confirmed these protective associations, with dexmedetomidine use linked to lower in-hospital (HR = 0.597; 95% CI: 0.395–0.901; p = 0.014) and in-ICU mortality (HR = 0.425; 95% CI: 0.262–0.689; p < 0.001).
TABLE 4
| Models | In-hospital all-cause mortality | In-ICU all-cause mortality | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Before PSM | ||||||
| Model 1 | 0.617 | 0.442, 0.862 | 0.005 | 0.468 | 0.312, 0.703 | <0.001 |
| Model 2 | 0.597 | 0.410, 0.870 | 0.007 | 0.466 | 0.299, 0.727 | <0.001 |
| Model 3 | 0.584 | 0.400, 0.855 | 0.006 | 0.451 | 0.288, 0.706 | <0.001 |
| After PSM | 0.597 | 0.395, 0.901 | 0.014 | 0.425 | 0.262, 0.689 | <0.001 |
Association of dexmedetomidine with all-cause mortality of CS patients in eICU 2.0 database.
Model 1, unadjusted; Model 2, adjusted by age, gender, race, systolic blood pressure, heart rate, respiratory rate; Model 3, age, gender, race, systolic blood pressure, heart rate, respiratory rate, hemoglobin, platelet, white blood cell, blood urea nitrogen, calcium, chloride, creatinine, sodium, potassium, hypertension, diabetes, acute myocardial infarction. HR, hazard ratio; CI, confidence interval; PSM, propensity score matching; ICU, intensive care unit.
4 Discussion
To our knowledge, this is the first study examining dexmedetomidine’s impact on short-term outcomes in CS patients. Our findings demonstrate that dexmedetomidine administration was associated with significantly reduced risk of 7-day and 30-day all-cause mortality in this population, supporting its clinical use for CS patients if there are indications and no contraindications.
Dexmedetomidine exhibits cardioprotective properties, as demonstrated in prior research. A meta-analysis of 48 trials involving 6,273 cardiac surgery patients revealed that dexmedetomidine shortened intensive care stays by approximately 5 h and reduced tracheal intubation duration by 1.6 h (Poon et al., 2023). Additionally, dexmedetomidine also significantly lowered the relative risk of postoperative delirium (HR = 0.58, 95% CI 0.43–0.78, p = 0.001), atrial fibrillation (HR = 0.76, 95% CI 0.61–0.95, p = 0.015), and short-term mortality (HR = 0.49, 95% CI 0.25–0.97, p = 0.041) (Poon et al., 2023). Another separate PSM analysis of dexmedetomidine in AMI patients revealed significantly lower in-hospital mortality rates among treated individuals (HR = 0.49, 95% CI 0.31–0.77, p = 0.0022) (Liu et al., 2024). These results align with our findings, indicating that dexmedetomidine administration was associated with improved short-term survival outcomes in CS patients.
The mechanisms underlying dexmedetomidine’s survival benefits in CS remain incompletely understood, though several pathways may contribute. First, dexmedetomidine exhibits anti-inflammatory properties by suppressing TNF-α and IL-6 production through inhibition of high mobility group box 1‐toll‐like receptor 4-nuclear factor κB (HMGB1-TLR4-NF-κB) signaling axis (Yang et al., 2017). A meta-analysis of 67 studies involving 4,842 patients demonstrated significantly lower TNF-α and IL-6 levels in patients receiving dexmedetomidine than in controls (Wang et al., 2019). Liu et al. (2024) also demonstrated that 13.7% of dexmedetomidine’s mortality-reducing benefit in AMI patients was mediated through lowered white blood cell counts. Inflammatory responses were significantly activated in CS, leading to impaired cardiac function, circulatory collapse, and adverse clinical outcomes (Shpektor, 2010). Dexmedetomidine demonstrates potential to enhance cardiac function and clinical outcomes in patients with CS by attenuating systemic inflammation. Second, dexmedetomidine significantly reduces myocardial oxygen consumption and cardiac output by decreasing heart rate and blood pressure. Consequently, coronary blood flow decreases physiologically to match the reduced metabolic demand. However, the reduction in heart rate prolongs diastolic perfusion time, which is also beneficial for coronary blood flow. Overall, dexmedetomidine reduces both myocardial oxygen demand and supply, but the reduction in oxygen demand is more pronounced. As a result, dexmedetomidine helps maintain or even improve the myocardial oxygen supply–demand balance (Lee, 2019; Talke et al., 2000; Flacke et al., 1993; Ebert et al., 2000; Weerink et al., 2017). Third, dexmedetomidine markedly redistributes cardiac output, primarily reducing blood flow to less critical tissues such as the skin, spleen, and arteriovenous shunts while enhancing perfusion to vital organs including the heart, brain, and kidneys (Lawrence et al., 1996). Fourth, dexmedetomidine suppressed excessive nicotinamide adenine inucleotide phosphate oxidase-derived oxidative stress, reducing myocardial apoptosis and attenuating subsequent adverse cardiac remodeling (Han et al., 2019). Additionally, dexmedetomidine also attenuates the stress response by suppressing epinephrine, norepinephrine, and cortisol release (Wang et al., 2019). Furthermore, it appears to enhance immune function by elevating natural killer cells, B cells, and CD4+ T cell counts while modulating the CD4+:CD8+ and Th1:Th2 ratios (Wang et al., 2019). Given these mechanisms, dexmedetomidine likely confers a survival benefit in CS patients, and our findings confirm its association with reduced short-term mortality.
Although our study suggests that dexmedetomidine administration was associated with improved outcomes in patients with CS, this does not imply that dexmedetomidine should be routinely used in such patients. Instead, the patient’s condition should be comprehensively evaluated and individualized treatment should be administered after assessment by experienced clinicians. Moreover, subgroup analysis revealed an interaction between age and the effect of dexmedetomidine (p < 0.001). In patients older than 75 years, dexmedetomidine use was not significantly associated with prognosis. There was also a trend toward an interaction between chronic lung disease and the effect of dexmedetomidine (p = 0.088); in patients with chronic lung disease, dexmedetomidine use was not significantly associated with prognosis. Moreover, subgroup analysis revealed an interaction between systolic blood pressure and the effect of dexmedetomidine (p = 0.046). In patients with systolic blood pressure less than 100 mmHg, dexmedetomidine use was not significantly associated with prognosis. Older patients often have more comorbidities and markedly reduced dexmedetomidine clearance. Consequently, the higher incidence of adverse reactions, such as hypotension, oversedation, and bradycardia, may offset the potential survival benefits of dexmedetomidine in this population (Weerink et al., 2017). In patients with chronic pulmonary disease, dexmedetomidine’s lack of survival advantage may stem from its respiratory depressant effects (Weerink et al., 2017). In patients with lower systolic blood pressure, dexmedetomidine’s lack of survival advantage may because of the adverse reaction, especially hypotension (Weerink et al., 2017). These findings underscore the need for careful risk-benefit assessment when considering dexmedetomidine in elderly populations, individuals with compromised pulmonary function or lower systolic blood pressure. However, these subgroup findings should be viewed as hypothesis-generating rather than definitive and future prospective studies are needed to confirm these observations and to provide more tailored guidance for clinical practice.
This study has several limitations. First, this is a retrospective observational study, and due to the limitations of its design, it can only establish associations rather than causal relationships. Second, although we employed methods such as propensity score analysis and multivariable regression adjustment, unmeasured confoundings such as physician’s prescribing preferences, subtle clinical indicators, or other unrecorded patient characteristics, etc. Still exist. These unmeasured factors may have influenced both the decision to initiate dexmedetomidine and patient outcomes. Third, while we demonstrated survival benefits of dexmedetomidine in CS patients, its safety profile including hypotension, bradycardia, excessive sedation, and arrhythmias in this population remains unclear due to the limitation of MIMIC-IV database itself, though prior studies demonstrated its safety in cardiac surgery (Poon et al., 2023) and AMI patients (Liu et al., 2024). Fourth, as a sedative, dexmedetomidine is not routinely used in clinical practice and has specific indications. Specifically, dexmedetomidine is often used as an adjunctive agent to facilitate liberation of mechanical ventilation and is an agent to treat delirium in awake patients. Therefore, patients that are improving, awake and able to liberated from mechanical ventilation are more likely to receive dexmedetomidine compared to patients that are not; however, the MIMIC-IV database does not include information on the clinical indications for which the drug was prescribed, and the lack of such data may lead to indication bias. Therefore, caution is needed when interpreting and generalizing the study findings. In addition, the endpoint assessed only all-cause mortality, while cardiovascular mortality, major cardiovascular events, and rehospitalization were unavailable, limiting the comprehensive evaluation of therapeutic efficacy and safety. Therefore, findings from this study needs to be validated by prospective studies, ideally large-scale randomized trials.
5 Conclusion
Dexmedetomidine administration was associated with reduced risk of 7-day and 30-day all-cause mortality in CS patients, though this protective effect may not be significant in patients over 75 years or those with chronic pulmonary disease or lower blood pressure. Prospective studies are required to validate these findings.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
LX: Methodology, Data curation, Software, Writing – review and editing, Writing – original draft, Supervision, Investigation, Visualization, Resources, Conceptualization, Project administration, Formal Analysis, Validation. JC: Visualization, Methodology, Data curation, Investigation, Validation, Conceptualization, Project administration, Writing – review and editing, Resources, Supervision, Formal Analysis, Writing – original draft, Software. BS: Conceptualization, Methodology, Writing – original draft, Formal Analysis, Investigation, Software. YL: Writing – original draft, Data curation, Formal Analysis, Methodology. SL: Conceptualization, Investigation, Validation, Writing – review and editing, Software, Formal Analysis, Supervision, Writing – original draft, Data curation, Resources, Methodology, Visualization, Project administration. BH: Supervision, Data curation, Conceptualization, Methodology, Writing – review and editing, Software, Writing – original draft, Investigation, Project administration, Visualization, Formal Analysis, Validation, Resources.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1644635/full#supplementary-material
References
1
Austin P. C. (2009). Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med.28 (25), 3083–3107. 10.1002/sim.3697
2
Bilotta F. Pugliese F. (2020). The evolving clinical use of dexmedetomidine. Lancet396 (10245), 145–147. 10.1016/S0140-6736(20)30902-8
3
Chen M. Li X. Mu G. (2022). Myocardial protective and anti-inflammatory effects of dexmedetomidine in patients undergoing cardiovascular surgery with cardiopulmonary bypass: a systematic review and meta-analysis. J. Anesth.36 (1), 5–16. 10.1007/s00540-021-02982-0
4
Chioncel O. Parissis J. Mebazaa A. Thiele H. Desch S. Bauersachs J. et al (2020). Epidemiology, pathophysiology and contemporary management of cardiogenic shock - a position statement from the heart failure association of the european society of cardiology. Eur. J. Heart Fail22 (8), 1315–1341. 10.1002/ejhf.1922
5
Ebert T. J. Hall J. E. Barney J. A. Uhrich T. D. Colinco M. D. (2000). The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology93 (2), 382–394. 10.1097/00000542-200008000-00016
6
Flacke W. E. Flacke J. W. Bloor B. C. Mcintee D. F. Sagan M. (1993). Effects of dexmedetomidine on systemic and coronary hemodynamics in the anesthetized dog. J. Cardiothorac. Vasc. Anesth.7 (1), 41–49. 10.1016/1053-0770(93)90117-4
7
Han H. Dai D. Hu J. Zhu J. Lu L. Tao G. et al (2019). Dexmedetomidine improves cardiac function and protects against maladaptive remodeling following myocardial infarction. Mol. Med. Rep.20 (6), 5183–5189. 10.3892/mmr.2019.10774
8
Homberg M. C. Bouman E. Joosten B. (2023). Optimization of procedural sedation and analgesia during atrial fibrillation ablation. Curr. Opin. Anaesthesiol.36 (3), 354–360. 10.1097/ACO.0000000000001263
9
Ji F. Li Z. Nguyen H. Young N. Shi P. Fleming N. et al (2013). Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation127 (15), 1576–1584. 10.1161/CIRCULATIONAHA.112.000936
10
Johnson a bulgarelli l shen l Gayles A. Shammout A. Horng S. et al (2023). Mimic-iv, a freely accessible electronic health record dataset. Sci. data10 (1), 1. 10.1038/s41597-022-01899-x
11
Keating G. M. (2015). Dexmedetomidine: a review of its use for sedation in the intensive care setting. Drugs75 (10), 1119–1130. 10.1007/s40265-015-0419-5
12
Lawrence C. J. Prinzen F. W. de Lange S. (1996). The effect of dexmedetomidine on nutrient organ blood flow. Anesth. Analg.83 (6), 1160–1165. 10.1097/00000539-199612000-00005
13
Lee S. (2019). Dexmedetomidine: present and future directions. Korean J. Anesthesiol.72 (4), 323–330. 10.4097/kja.19259
14
Liu Y. Chen Q. Hu T. Deng C. Huang J. (2024). Dexmedetomidine administration is associated with improved outcomes in critically ill patients with acute myocardial infarction partly through its anti-inflammatory activity. Front. Pharmacol.15, 1428210. 10.3389/fphar.2024.1428210
15
Lonjon G. Porcher R. Ergina P. Fouet M. Boutron I. (2017). Potential pitfalls of reporting and bias in observational studies with propensity score analysis assessing a surgical procedure: a methodological systematic review. Ann. Surg.265 (5), 901–909. 10.1097/SLA.0000000000001797
16
Osman M. Syed M. Patibandla S. Sulaiman S. Kheiri B. Shah M. K. et al (2021). Fifteen-year trends in incidence of cardiogenic shock hospitalization and in-hospital mortality in the United States. J. Am. Heart Assoc.10 (15), e021061. 10.1161/JAHA.121.021061
17
Peng K. Shen Y. P. Ying Y. Y. Kiaii B. Rodriguez V. Boyd D. et al (2021). Perioperative dexmedetomidine and 5-year survival in patients undergoing cardiac surgery. Br. J. Anaesth.127 (2), 215–223. 10.1016/j.bja.2021.03.040
18
Poon W. H. Ling R. R. Yang I. X. Luo H. Kofidis T. Maclaren G. et al (2023). Dexmedetomidine for adult cardiac surgery: a systematic review, meta-analysis and trial sequential analysis. Anaesthesia78 (3), 371–380. 10.1111/anae.15947
19
Shpektor A. (2010). Cardiogenic shock: the role of inflammation. Acute Card. Care12 (4), 115–118. 10.3109/17482941.2010.523705
20
Takahashi K. Yoshikawa Y. Kanda M. Hirata N. Yamakage M. (2023). Dexmedetomidine as a cardioprotective drug: a narrative review. J. Anesth.37 (6), 961–970. 10.1007/s00540-023-03261-w
21
Talke P. Chen R. Thomas B. Aggarwall A. Gottlieb A. Thorborg P. et al (2000). The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth. Analg.90 (4), 834–839. 10.1097/00000539-200004000-00011
22
van Diepen S. Katz J. N. Albert N. M. Henry T. D. Jacobs A. K. Kapur N. K. et al (2017). Contemporary management of cardiogenic shock: a scientific statement from the american heart association. Circulation136 (16), e232–e268. 10.1161/CIR.0000000000000525
23
Wang K. Wu M. Xu J. Wu C. Zhang B. Wang G. et al (2019). Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta-analysis. Br. J. Anaesth.123 (6), 777–794. 10.1016/j.bja.2019.07.027
24
Weerink M. Struys M. Hannivoort L. N. Barends C. Absalom A. R. Colin P. (2017). Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin. Pharmacokinet.56 (8), 893–913. 10.1007/s40262-017-0507-7
25
Yang Y. F. Peng K. Liu H. Meng X. W. Zhang J. J. Ji F. H. (2017). Dexmedetomidine preconditioning for myocardial protection in ischaemia-reperfusion injury in rats by downregulation of the high mobility group box 1-toll-like receptor 4-nuclear factor κB signalling pathway. Clin. Exp. Pharmacol. Physiol.44 (3), 353–361. 10.1111/1440-1681.12711
Summary
Keywords
cardiogenic shock, dexmedetomidine, prognosis, mortality, MIMIC-IV
Citation
Xie L, Chen J, Sasmita BR, Li Y, Luo S and Huang B (2025) Association of dexmedetomidine with short-term outcome in patients with cardiogenic shock: a retrospective propensity score-matched cohort study from MIMIC-IV. Front. Pharmacol. 16:1644635. doi: 10.3389/fphar.2025.1644635
Received
12 June 2025
Accepted
04 September 2025
Published
15 September 2025
Volume
16 - 2025
Edited by
Tzu-Hurng Cheng, China Medical University, Taiwan
Reviewed by
Zoran Todorovic, University of Belgrade, Serbia
Shubhadarshini Pawar, Cedars Sinai Medical Center, United States
Updates
Copyright
© 2025 Xie, Chen, Sasmita, Li, Luo and Huang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bi Huang, huangbi120@163.com; Suxin Luo, luosuxin0204@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.