- 1Institute of Herbgenomics, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2School of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Department of Nephrology, First Affiliated Hospital of Chengdu Medical College, Chengdu, China
Renal fibrosis (RF) represents the pathognomonic end-stage phenotype of progressive nephropathies, pathologically characterized by excessive deposition of fibrillar extracellular matrix (ECM) and irreversible obliteration of parenchymal architecture. G protein-coupled receptors (GPCRs)—members of the heptahelical transmembrane receptor superfamily—function as master regulators orchestrating both physiological renal homeostasis and maladaptive fibrotic reprogramming in response to injury. Despite robust clinical evidence validating the therapeutic tractability of GPCR-targeted interventions for chronic kidney disease (CKD), no approved agents specifically antagonize the core pathogenic drivers of RF. Consequently, this review systematically delineates GPCRs exhibiting mechanistic primacy in RF pathobiology and translational promise, with focused interrogation of endothelin receptors, angiotensin receptors, chemokine receptors, and adenosine receptors. Beyond canonical modulation of inflammatory leukocyte infiltration and pro-fibrotic phenotypic transitions, emerging paradigms highlight GPCR governance over metabolomic reprogramming and mechanotransductive signaling during fibrogenesis. Notwithstanding these mechanistic advances, clinical translation of GPCR-directed anti-fibrotic therapeutics remains nascent, constrained by target pleiotropy, biodistribution barriers, and species-divergent pathophysiology. Collectively, GPCRs constitute high-value molecular targets for intercepting the progression of RF at its mechanistic nexus.
1 Global challenge of renal fibrosis
Since 1990, the global burden of chronic kidney disease (CKD) has escalated markedly, with prevalence increasing by 29.3%, and mortality rising by 41.5%, constituting a major public health challenge (GBD Chronic Kidney Disease, 2020). However, early-to-moderate stage CKD is highly preventable and potentially reversible (Shlipak et al., 2021). Regrettably, the clinically silent nature of incipient CKD precludes timely intervention, frequently permitting inexorable progression to end-stage renal disease (ESRD). This trajectory is evidenced by a doubling of ESRD prevalence over the past 2 decades (Kuehn, 2022). Renal fibrosis (RF) represents the terminal pathological convergence in CKD progression, morphologically characterized by glomerulosclerosis, tubular atrophy, vascular rarefaction, and interstitial fibrosis, culminating in excessive extracellular matrix (ECM) deposition and scar formation (Huang et al., 2023; Li L. et al., 2022). Conventionally, RF pathogenesis was attributed to aberrant cellular phenotypic plasticity, encompassing mesenchymal transformation of renal epithelial and endothelial cells (EMT/EndMT) and pathological activation of matrix-producing myofibroblasts (Yamashita and Kramann, 2024). However, contemporary research has elucidated previously unrecognized regulatory axes governing fibrogenic commitment, including non-coding RNA networks (Van der Hauwaert et al., 2019), epigenetic modifications (Li X. et al., 2022), metabolic reprogramming (Zhu et al., 2021), and extracellular vesicles-mediated signaling (Kosanović et al., 2021). These mechanisms present promising direction for modulating—and potentially reversing—established fibrosis. Despite these mechanistic advances, no therapeutics directly and selectively targeting RF pathogenesis are clinically available (Huang et al., 2024). Consequently, current clinical practice relies on agents developed for broader CKD management—angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), mineralocorticoid receptor antagonists (MRAs), and sodium-glucose co-transporter 2 inhibitors (SGLT2i) —to indirectly attenuate fibrotic progression (Wang and Zhang, 2024). Nevertheless, their efficacy remains suboptimal and variable, while safety profiles are constrained by underlying etiological heterogeneity, disease stage disparities, and diverse environmental determinants (Reiss et al., 2024).
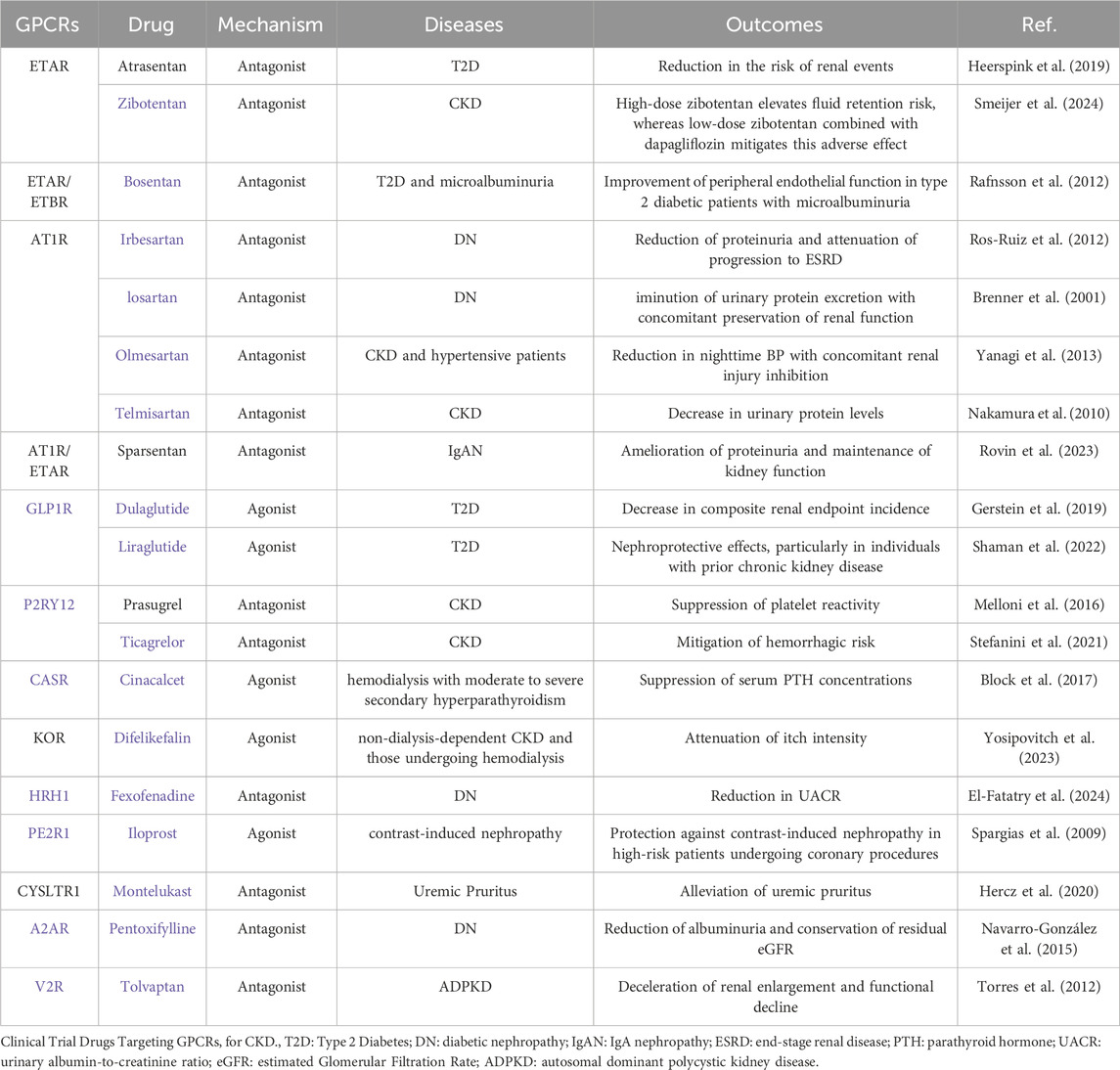
Consequently, our focus centers on the G protein-coupled receptors (GPCRs) superfamily, Representing the largest cohort of human membrane proteins and historically constituting the most therapeutically exploited target class, GPCRs hold profound significance (Zhang et al., 2024). Within nephrology, GPCR-directed pharmacotherapies have established pivotal clinical utility (Lv et al., 2024; Tang et al., 2025). The most substantiated classes encompass AT1R antagonists (Rianto et al., 2021), GLP-1R agonists (Rossing et al., 2023), ETR antagonists (Martínez-Díaz et al., 2023) and dual angiotensin/endothelin receptor antagonists (Kohan et al., 2024), collectively demonstrating immense promise for innovative renal disease drug development (Table 1). Critically, GPCR-targeted agents constitute the predominant share of receptor-focused therapeutic candidates in current clinical trials for RF (Abbad et al., 2025). Moreover, we emphasize that GPCR signal transduction and functionality are intimately implicated in the initiation and modulation of RF (Tang et al., 2025). Consequently, despite the formidable global challenge of developing effective clinical interventions for RF, the therapeutic promise of targeting GPCRs—leveraging their well-defined pathophysiological roles and notable inherent druggability—is increasingly commanding significant scientific and clinical attention.
2 GPCR signaling transduction paradigms and targeted modulation strategies
GPCRs belong to the family of seven-transmembrane proteins. The human genome encodes approximately 800 GPCRs that orchestrate diverse physiological and pathophysiological processes across multiple organ systems (Congreve et al., 2020). The transmembrane helix structure comprises the extracellular N-terminus, three extracellular loops, an intracellular C-terminus, and three intracellular loops. Heterotrimeric G proteins, consisting of α, β, and γ subunits, serve as primary signaling partners. In the basal state, Gα subunits remain guanosine diphosphate (GDP)-bound and conformationally constrained. Ligand engagement induces allosteric transitions within the receptor’s transmembrane core, catalyzing GDP-guanosine triphosphate (GTP) exchange on the Gα subunit, This nucleotide switch triggers dissociation of the GTP-bound Gα subunit from the Gβγ dimer (Ballante et al., 2021) (Figure 1). The liberated Gα-GTP complex and Gβγ heterodimer regulate distinct downstream effectors. Gα subunits are phylogenetically categorized into four classes: Gαs, Gαi/o, Gαq/11, and Gα12/13. For example, Gαs primarily activates adenylate cyclase (AC), promoting the production of cAMP. Conversely, Gαi/o inhibits AC and cAMP activity; Gαq/11 binds with phospholipase C-β (PLCβ) to promote the hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), which further activates downstream protein kinase C (PKC) and triggers Ca2+ release. The downstream signaling of Gα12/13 primarily involves Rho GTPase, with a more complex and diverse regulatory pattern (Rasheed et al., 2022; Jiang et al., 2022). The Gβγ complex independently regulates ion channels, kinases, and secondary messenger systems (Senarath et al., 2018). Signal termination is mediated by regulator of G protein signaling (RGS) domains, which accelerate GTP hydrolysis via intrinsic GTPase-activating protein (GAP) activity. Gα-GDP subsequently reassociates with Gβγ, reconstituting the inactive heterotrimer and completing the catalytic cycle (Masuho et al., 2023). Additionally, GPCR activation is partially independent of G proteins. For example, phosphorylated GPCRs recruit β-arrestins, which prevent G protein signaling and promote receptor internalization, initiating new signaling pathways (Asher et al., 2022). Furthermore, most adhesion GPCRs (aGPCRs) contain a special domain with a hydrolysis site. Their self-proteolysis leads to aGPCR autoactivation, causing the separation of Gα from Gβγ and initiating downstream signaling (Zhu X. et al., 2022).
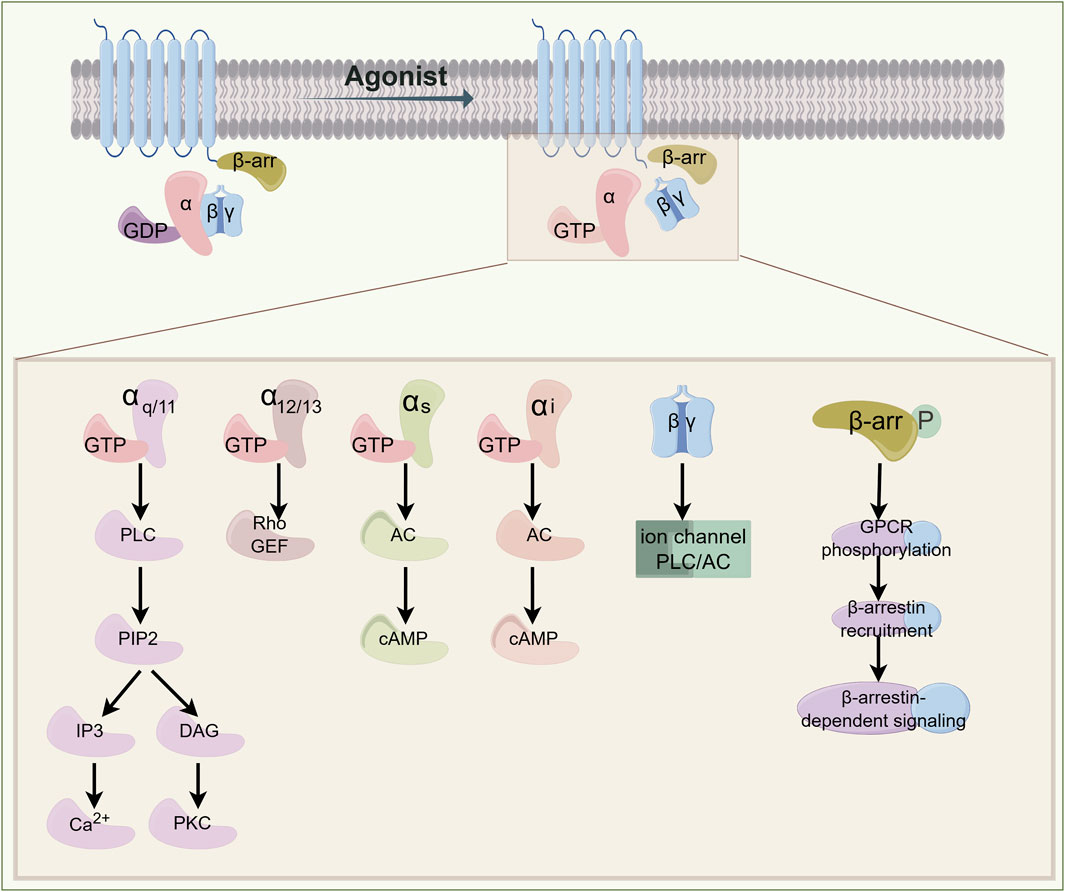
Figure 1. Signal Transduction Paradigm of GPCR. Ligand-receptor binding induces conformational changes in G proteins, triggering receptor activation. Different G protein subunits regulate distinct effector enzymes or ion channels, generating second messengers such as Ca2+, cAMP, and IP3. These signaling cascades initiate cellular responses, whereas receptor desensitization leads to signal termination. β-arrestin signaling is involved in receptor desensitization and endocytosis. GPCR: G-protein-coupled receptor; GTP: guanosine triphosphate; GDP: guanosine diphosphate; PLC: phospholipase C; PIP2: phosphatidylinositol 4,5-bisphosphate; IP3: inositol trisphosphate; DAG: diacylglycerol; PKC: protein kinase C; RhoGEF: Rho guanine nucleotide exchange factor; AC: adenylyl cyclase; cAMP: cyclic adenosine monophosphate.
Concurrently, emerging research has revealed intimate connections between RF pathogenesis and GPCR signaling cascades. Therapeutic targeting of the cAMP/PKA pathway (Stokman et al., 2021), Gβγ-GRK2 interface (Rudomanova and Blaxall, 2017), and β-arrestin-dependent signaling (Gu et al., 2015) has emerged as a validated strategy for antagonizing RF progression. Furthermore, the intricate crosstalk among non-coding RNAs (ncRNAs), epigenetic modifications, and GPCR regulation constitutes a pivotal investigative frontier (Zhao et al., 2014; Alghamdi et al., 2018; Liu et al., 2019). Supporting this paradigm, transcriptomic profiling of proximal tubule-mediated RF identifies 143 differentially expressed lncRNAs and 91 dysregulated GPCRs (Wu H. et al., 2020), whereas CaSR signaling—primarily orchestrating Ca2+ and water transport—demonstrates extensive miRNA interactions (Ranieri, 2019). Critically, bidirectional regulatory crosstalk exists between GPCR signaling cascades and ncRNAs networks. GLP-1R not only governs the circ8411/miR-23a-5p axis to mitigate lipid toxicity and endothelial pyroptosis (Wu W. et al., 2024) but is reciprocally modulated by extracellular vesicle-encapsulated miR-192 to exert renoprotection (Jia et al., 2018). Moreover, In butyrate-mediated protection against diabetic nephropathy (DN), GPR41, GPR43, and GPR109A engage in crosstalk networks involving histone deacetylase (HDAC) inhibition, histone butyrylation, and miRNA repertoire alterations, collectively modulating DN-associated inflammatory and fibrotic pathologies (Cheng et al., 2022). Notably, GPR109a activation rectifies promoter region acetylation and methylation patterns, preserving glomerular basement membrane (GBM) integrity (Felizardo et al., 2019). These findings establish ncRNAs and epigenetic machinery as critical upstream regulators of GPCR functionality, thereby revealing their therapeutic potential as precision targets for renal fibrosis intervention.
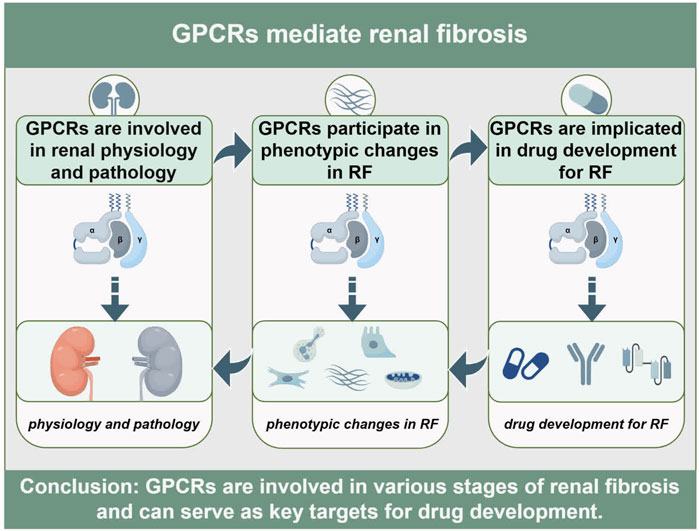
3 GPCRs are involved in regulating renal physiology and pathology
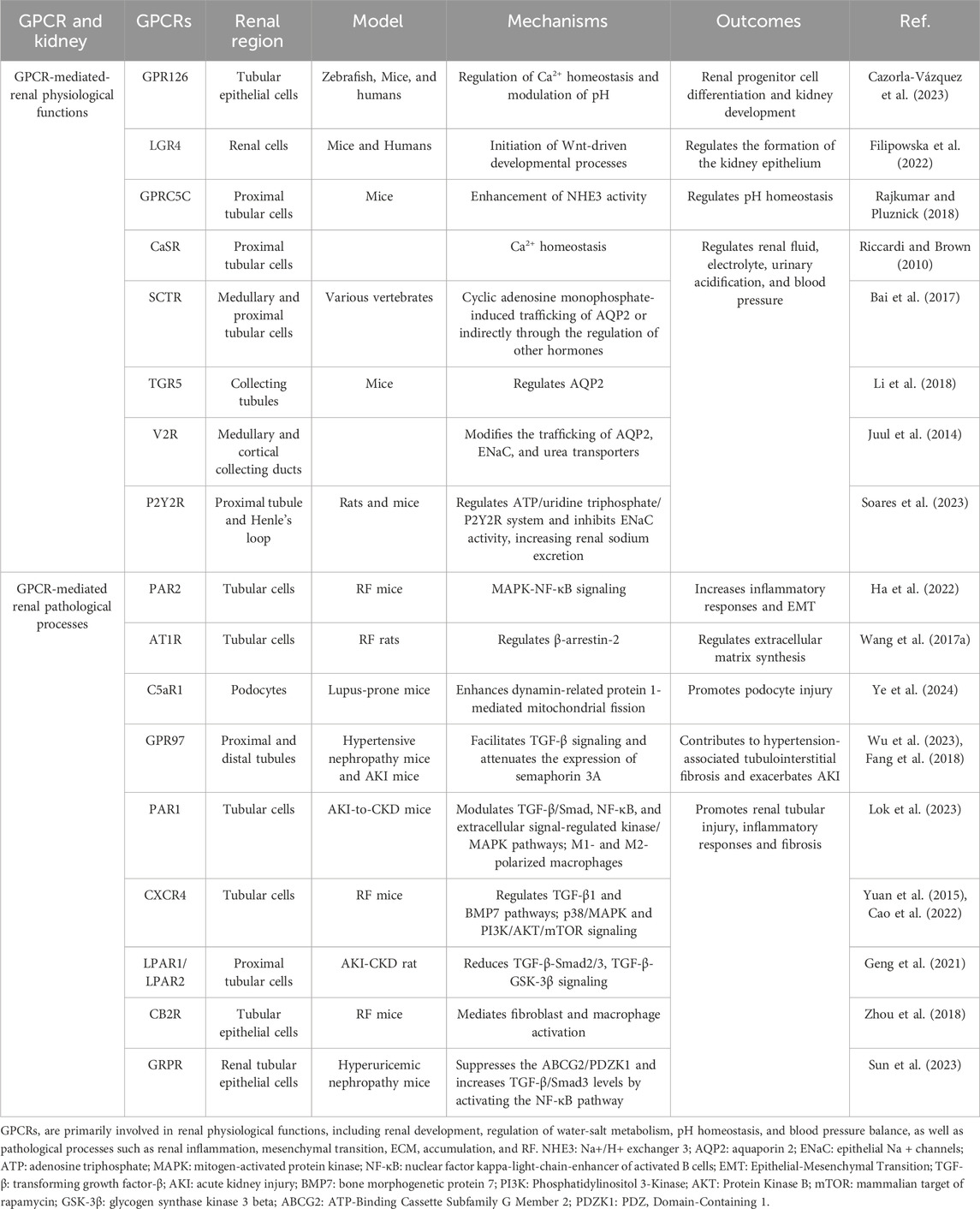
Within the kidneys, GPCRs exhibit ubiquitous expression and critically orchestrate essential physiological processes including renal development, fluid-electrolyte homeostasis, and blood pressure regulation (Table 2). Spatiotemporal mapping of GPCR distribution across nephron segments reveals prominent enrichment of aGPCRs, adrenergic receptors (ARs), and lysophosphatidic acid receptors (LPARs) along renal tubules (Poll et al., 2021). In alignment with prior evidence of olfactory receptors (ORs) participating in renal physiology (Kalbe et al., 2016), this profiling further identifies substantial enrichment of ORs along the nephron (Poll et al., 2021). In the renal vasculature and glomeruli, receptors including GPR91, GPR43, and apelin receptor (APJ) are functionally co-expressed and collectively participate in renal physiological regulation (Rajkumar and Pluznick, 2017). Moreover, Transcriptomic profiling identifies 56 GPCRs dysregulated in activated renal fibroblasts, underscoring their pathogenic involvement in fibrogenesis (Kaur et al., 2023). Developmental regulation is exemplified by GPR126, which exhibits progressive upregulation in ureteric buds and renal epithelia during murine nephrogenesis. Its persistent expression in mature tubular epithelium and collecting ducts implicates roles in progenitor cell differentiation and renal morphogenesis (Cazorla-Vázquez and Engel, 2018; Cazorla-Vázquez et al., 2023). Apically expressed GPR37L1 in renal tubular epithelial cells enhances Na+/H+ exchanger isoform 3 (NHE3) activity, thereby promoting natriuresis and diuresis. This regulation potentially involves cAMP dynamics and PI3K/AKT/mTOR signaling (Zheng et al., 2019; Armando et al., 2022). Notably, GPCR-NHE3 crosstalk establishes a novel paradigm for fluid-electrolyte homeostasis via coordinated intra- and extracellular pH/ion balance. For example, OGR1 inhibits NHE3 activity to mediate renal calcium excretion (Imenez Silva et al., 2020), whereas GPRC5C elevates its activity to regulate systemic pH (Rajkumar et al., 2018). Furthermore, renal perfusion-sodium excretion equilibrium crucially maintains blood pressure stability, with key contributions from Dopamine receptors (DRs) (Yang J. et al., 2021), prostaglandin receptors (EPRs) (Wang et al., 2022), and Angiotensin Receptors (ATRs) (Colafella et al., 2016).
Meanwhile, the GPCR superfamily orchestrates pivotal pathological processes in renal diseases, including inflammatory cascades, immune dysregulation, fluid-electrolyte imbalances, and RF (Lv et al., 2024). Inflammation serves as the primary instigator of renal injury, wherein complement C5aR activation drives pathogenesis in inflammatory nephropathies such as lupus nephritis (Ye et al., 2024), ANCA-associated vasculitis (Xiao et al., 2014), and acute pyelonephritis (Li et al., 2017). Autoimmune mechanisms further characterize renal pathology, with chemokine receptors (CCRs) orchestrating leukocyte trafficking and tissue infiltration (Hamdan and Robinson, 2021). Notably, CXCR3-dependent immune cell crosstalk represents an emerging therapeutic target (Yoshikawa et al., 2023). In contrast to normal homeostatic functions, AT1R (Dalman and Coleman, 2023), V2R (Bankir et al., 2010), and ETRs (Hunter et al., 2017) promotes sodium-water retention and hypertensive nephropathy (HN). RF, a hallmark pathological endpoint of progressive CKD, is orchestrated by GPCRs at multiple regulatory tiers. For instance, Prostaglandin E2 (EP2) engages four distinct EPR subtypes to stimulate diverse intracellular signaling cascades (Mutsaers and Nørregaard, 2022). LPA activates six GPCR subtypes that drive immune cell recruitment and sustain profibrotic mediator production (Park and Miller, 2017). Emerging evidence further elucidates the contributions of ORs (Motahharynia et al., 2022), GPCR-Gβγ complexes (Kamal et al., 2017), and GPCR-β-arrestin-biased pathways (Gu et al., 2015) in RF pathogenesis, collectively unveiling viable therapeutic strategies to reverse fibrosis. In summary, GPCRs exhibit profound dualistic involvement in renal physiology and pathobiology, positioning them as high-priority therapeutic targets for innovative renal disease interventions.
4 Key GPCRs in RF
4.1 Endothelin receptors
Accumulating evidence implicates ETRs are involved in the pathological changes of RF. Typically, endothelin-1 (ET-1) initiates the Gq/G11 signaling cascade to trigger downstream Ca2+ mobilization, thereby activating both ETAR and ETBR. Interestingly, ligand-stimulated ETAR and ETBR exhibit functionally antagonistic roles in renal pathophysiology (Mazzuca and Khalil, 2012). ETBR activation causes vasodilation and clears ET-1, conferring renoprotective effects, whereas ETAR activation primarily exerts vasoconstrictive effects (Martínez-Díaz et al., 2023). This vasoconstrictive response correlates with increased renal vascular resistance, cortical/medullary vasoconstriction, mesangial cell contraction, and stimulated ECM production (Neuhofer and Pittrow, 2006). Notably, compared to other organs, renal ETRs exhibit heightened sensitivity to ET-1. Critically, ETRs are expressed throughout the kidney, with particularly high levels of ET-1 and ETAR in podocytes and mesangial cells (Anguiano et al., 2015) – cell types recognized as major precursors of fibrogenic fibroblasts (Roccatello et al., 2024). Consequently, ETAR antagonism represents a strategic therapeutic target for RF suppression by effectively inhibiting renal fibroblast proliferation, reducing ECM deposition and antagonizing pro-fibrotic mediators such as ET-1, TGF-β, angiotensin II, and aldosterone (Kohan et al., 2023). While initial monotherapies revealed paradoxical fluid retention risks (Schinzari et al., 2024), contemporary regimens combining ETAR antagonists with ATR blockers or SGLT2i demonstrate optimized efficacy in reducing albuminuria while mitigating hydrostatic complications (Rovin et al., 2023; Heerspink et al., 2023). FDA-approved dual-targeting agents sparsentan (ETAR/AT1R antagonist) and aprocitentan(ETAR/ETBR antagonist) exemplify this synergistic therapeutic approa-ch (Smeijer et al., 2025). Collectively, ETRs signaling constitutes a mechanistically validated axis for targeted RF intervention.
4.2 Angiotensin receptors
Recently, Renin-angiotensin-aldosterone system (RAAS) inhibitors now constitute the foundational pharmacotherapy for CKD. wherein ATR subtypes play pivotal roles and their anti-fibrotic properties have garnered increasing scientific attention (AlQudah et al., 2020). Angiotensin II stimulation diversely engages AT1R through Gq/11, Gi/o, G12/13, and β-arrestin pathways to orchestrate pro-fibrotic cascades (Tóth et al., 2018), while AT2R signals through Gi cascades to exert anti-fibrotic effects (Azushima et al., 2020). Mechanistically, AT1R activation promotes vasoconstriction, inflammatory responses, oxidative stress, and fibrogenesis, whereas AT2R activation partially antagonizes AT1R-mediated pathological processes (Forrester et al., 2018). This functional opposition is exemplified by β-arrestin-biased AT1R signaling, which elicits rapid intracellular Ca2+ transients in podocytes—accelerating podocyte detachment and glomerulosclerosis (Semenikhina et al., 2023). Conversely, AT2R activation confers renoprotection against fibrosis by modulating Ca2+ handling dynamics (Wang et al., 2017b). In summary, although clinical applications targeting angiotensin receptors are well-established, developing innovative ligands for dual receptor modulation and elucidating their spatiotemporal signaling dynamics constitute active investigative frontiers in nephrology (Chow et al., 2019).
4.3 Chemokine receptors
Chemokines represent a class of chemotactic cytokines classified into four structural subtypes: XCL, CXCL, CCL, and CX3CL. Their cognate receptors similarly comprise four families: XCR, CXCR, CCR, and CX3CR (Wu F. et al., 2020). These receptor systems critically regulate cellular migration, proliferation, and adhesion dynamics, thereby modulating renal disease progression and regression (Lai and Mueller, 2021). Typically, chemokine coupling to G proteins activates both Gi and Gq pathways, mobilizing secondary messengers including cAMP and Ca2+ that mediate heterogeneous biological outcomes. Distinct CCR mediate heterogeneous biological effects through these cascades (Legler and Thelen, 2018; Zweemer et al., 2014). After kidney injury, activated inflammatory cells release chemokines that bind specifically to cognate receptors on immune cells, and orchestrate inflammatory cell recruitment to injury sites, thereby accelerating RF (Yoshikawa et al., 2023; Wu F. et al., 2020). CCR2, a specific pro-fibrotic gene in CKD (Fu et al., 2024), recruits Vδ1 T cells infiltration into renal parenchyma, promoting interstitial fibrosis in IgA nephropathy (Deng et al., 2023). Notably, CCR2 also exerts fibrogenic effects in renal resident cells, including podocytes, independent of immune cell recruitment, indicating that cell-specific CCR2 targeting may offer improved therapeutic precision (You et al., 2017). Furthermore, substantial evidence demonstrates that chemokine axes—including CXCL12/CXCR4 (Chen et al., 2024), CCL20/CCR6 (Zhu et al., 2024), and CXCL5/CXCR2 (Chang et al., 2024)—drive RF progression. Conversely, atypical chemokine receptors (ACKRs) exert counter-regulatory effects in RF, ACKR2 attenuates fibrosis by scavenging CCL2, thereby limiting immune cell and fibroblast infiltration into the interstitium. ACKR2 deficiency, however, exacerbates RF (Eller and Rosenkranz, 2018; Lux et al., 2019). In summary, the CCR network represents a druggable target system for intercepting multifactorial fibrogenic pathways in renal disease.
4.4 Adenosine receptors
Extracellular adenosine accumulates pathognomonically during chronic inflammation and hypoxia, with sustained elevations stimulating downstream signaling through four GPCRs: A1AR; A2AR; A2BR; and A3AR. These GPCRs exhibit differential G-protein coupling, Typically, adenosine stimulation induces A1AR and A3AR preferentially engage Gi pathways, while A2AR and A2BR signal through Gs pathways, collectively mediating downstream cAMP signaling transduction (Borea et al., 2018). In RF, A1AR and A2AR activation attenuates EMT/EndMT and ECM accumulation, exerting renoprotective effects (Tian et al., 2021; Chen et al., 2019). Conversely, A2BR and A3AR activation drive profibrotic pathways to accelerate disease progression (Dai et al., 2011; Yu et al., 2019). Notably, receptor functions demonstrate anatomical and mechanistic specialization, A1AR modulates hemodynamic homeostasis through its association with afferent arteriolar vasoconstriction, whereas A3AR primarily underlies metabolic disorder-driven renal injury. Conversely, A2AR and A2BR exhibit stronger associations with direct profibrotic pathways—specifically mesenchymal transition and ECM dysregulation (Dorotea et al., 2018; Li et al., 2012; Roberts et al., 2014). Thus, the AR family exerts complex and context-dependent effects on RF pathogenesis, mediated through GPCR signaling pathways.
4.5 Other GPCRs
In addition to the aforementioned GPCRs involved in RF, multiple additional GPCR families—including LPARs (Lee et al., 2019), protease-activated receptors (PARs) (Bagang et al., 2023), cannabinoid receptors (CBRs) (Barutta et al., 2018), and prostaglandin E receptors (EPRs) (Nasrallah et al., 2014)—contribute to fibrogenesis through distinct pathological mechanisms. Significantly, orphan GPCRs (oGPCRs) —defined by unidentified endogenous ligands—have emerged as critical microenvironmental sensors (Rajkumar and Pluznick, 2017). Members of the retinoic acid-inducible GPRC5 subfamily exhibit cell-type-specific pathophysiological roles, Podocyte-localized GPRC5A attenuates fibrosis by suppressing TGF-β-mediated glomerular basement membrane thickening and mesangial hyperplasia (Ma et al., 2018); GPRC5B conversely exacerbates fibrogenesis via NF-κB-driven podocyte inflammation (Zambrano et al., 2019); Tubular GPRC5C primarily modulates acid-base homeostasis (Rajkumar et al., 2018). Additionally, orphan receptor GPR176 demonstrates fibroblast-specific enrichment where it promotes fibroblast activation through TGF-β-independent pathways (Okamoto et al., 2024), positioning orphan receptor as compelling therapeutic targets. Furthermore, Emerging evidence further implicates ectopically expressed ORs in renal pathology (Wu C. et al., 2024), with Olfr433 showing specific enrichment in injury-responsive renal macrophages—suggesting direct involvement in fibrotic cascades (Motahharynia et al., 2022). Collectively, these findings substantiate the multidimensional regulatory architecture of GPCRs networks in RF pathogenesis and reveal novel druggable nodes for anti-fibrotic intervention.
5 GPCRs are involved in the pathological phenotypic transition in renal fibrosis
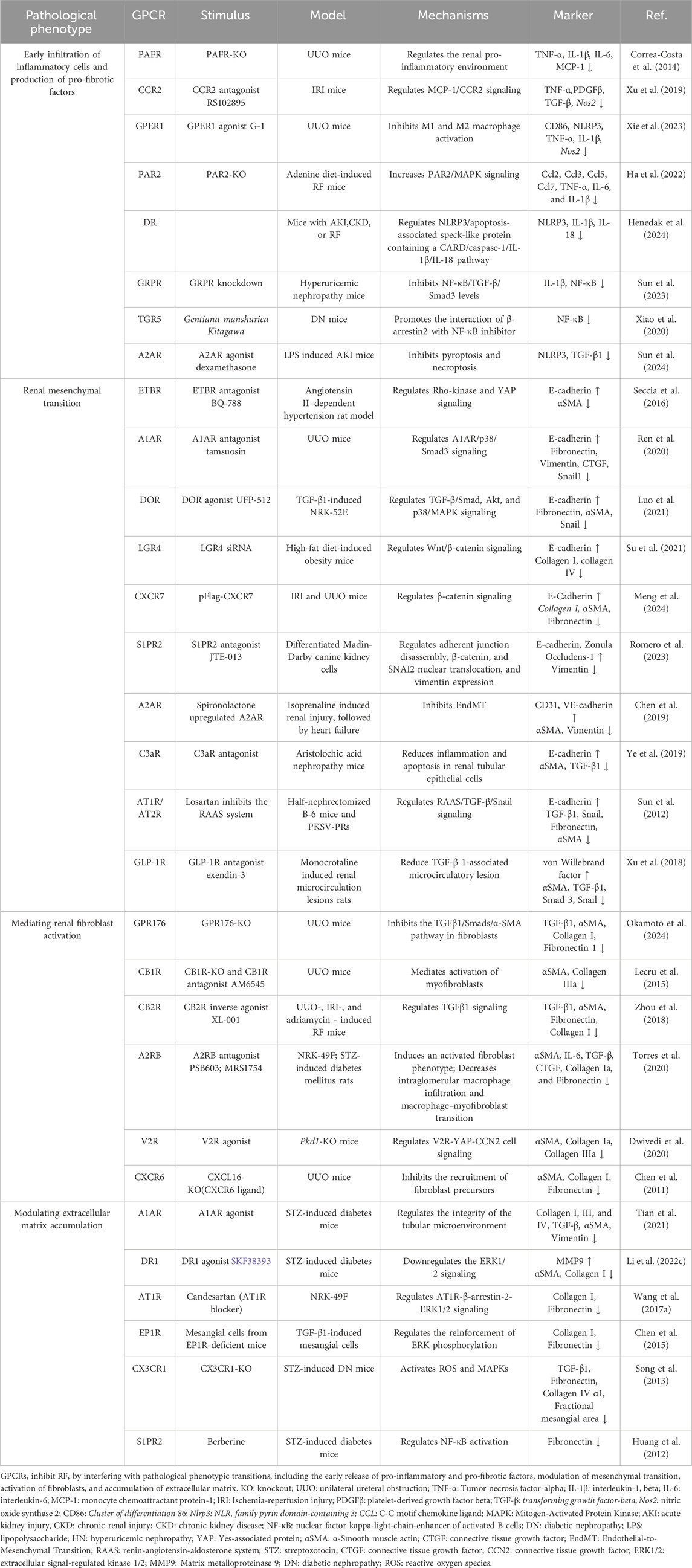
5.1 Early infiltration of inflammatory cells and production of pro-fibrotic factors
In the early stages of renal injury, GPCRs critically mediate inflammatory cell infiltration and pro-fibrotic factor release, serving as pivotal initiators of RF progression (Meng, 2019) (Figure 2) (Table 3). This pathogenic cascade is characterized by damage-associated molecular patterns (DAMPs) activating pattern recognition receptors post-injury, triggering immune cell recruitment and polarization that amplify fibrogenic signaling networks (Zhou et al., 2020; Anders and Schaefer, 2014). CCRs constitute essential molecular conduits in this process (Zhou et al., 2020): CXCL16 functions as a scavenger receptor binding oxidized LDL (oxLDL), exhibiting tubular epithelial upregulation that activates CXCR6+ fibroblasts to potentiate tubular injury (Korbecki et al., 2021); concurrently, CCL2 induces ACKR2 expression in renal interstitial lymphatic endothelial cells, attenuating CD4+ T-cell and mononuclear phagocyte infiltration while suppressing inflammatory cascades (Bideak et al., 2018). Additional receptors including CCR6 (Zhu et al., 2024), GPER1 (Xie et al., 2023), and PAR-1 (Lok et al., 2023) regulate macrophage infiltration and M0-to-M1/M2 phenotypic polarization. Critically, GPR120 agonism in in vitro-programmed peritoneal macrophages sustains the M2 phenotype, thereby inhibiting EMT and conferring renoprotection (Wang et al., 2019). These findings collectively indicate that early-phase reprogramming of inflammatory cells represents a strategic intervention to decelerate inflammation-fibrosis transition.
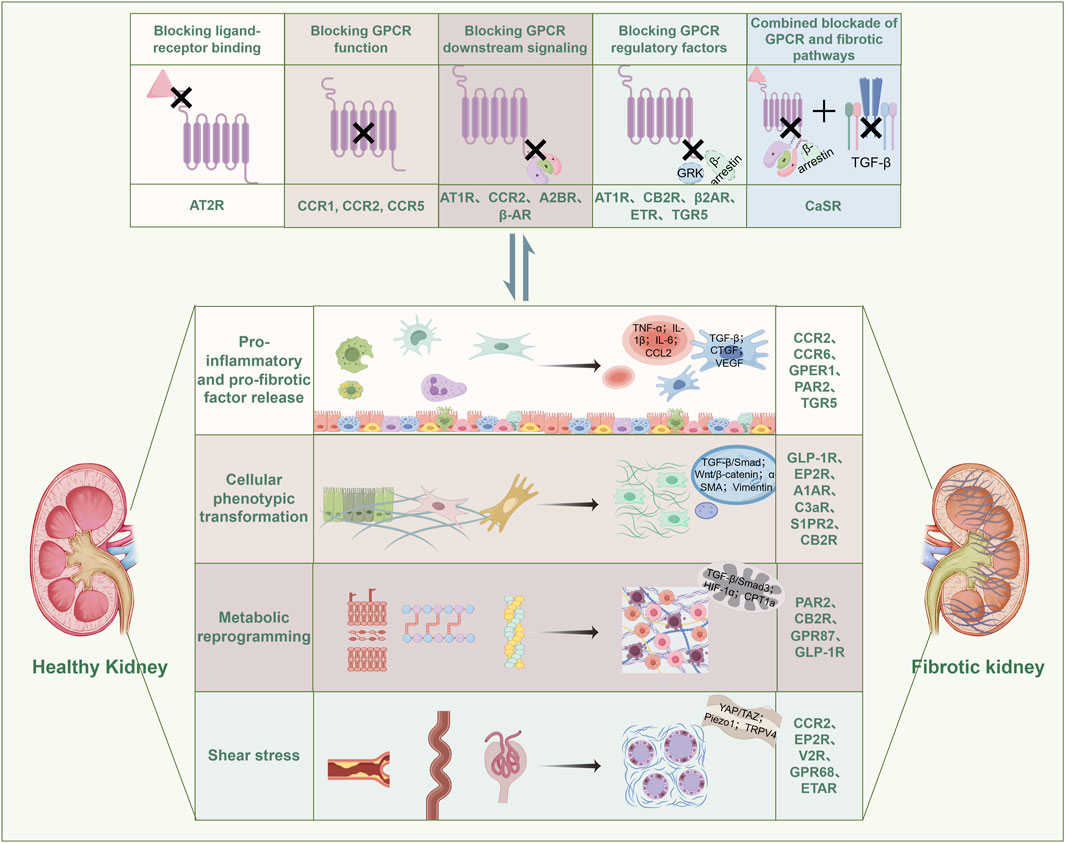
Figure 2. GPCR-mediated signal transduction and pathological alterations in RF engage in a reciprocal interplay. Specifically, GPCRs are involved in the release of pro-inflammatory and pro-fibrotic factors, cellular phenotypic transitions, metabolic reprogramming, and mechanical stress-induced injury during RF progression. Conversely, the activity of GPCRs is also modulated by the pathological changes inherent to RF. GRK: G protein-Coupled Receptor Kinase; TGF-β: Transforming Growth Factor-β; TNF-α: Tumor Necrosis Factor-α; IL-1β: Interleukin-1β; IL-6: Interleukin-6; CCL2: C-C Motif Chemokine Ligand 2; CTGF: Connective Tissue Growth Factor; VEGF: Vascular Endothelial Growth Factor; α-SMA: α-Smooth Muscle Actin; HIF-1α: Hypoxia-Inducible Factor-1α; YAP/TAZ: Yes-Associated Protein/Transcriptional Co-Activator with PDZ-Binding Motif; Piezo1: Piezo Type Mechanosensitive Ion Channel Component 1; TRPV4: Transient Receptor Potential Vanilloid 4.
5.2 GPCR is involved in cellular crosstalk and phenotypic transformation in RF
Persistent research has established that cellular phenotypic transitions following renal injury constitute a central mechanism in RF pathogenesis (Liu, 2011). Within this process, apoptosis/necrosis of renal tubular epithelial cells, endothelial cell injury, and immune cell infiltration converge to activate matrix-producing myofibroblasts, which directly drives ECM accumulation and fibrotic phenotypic remodeling (Huang et al., 2023). Substantial evidence implicates GPCRs in orchestrating multiple phenotypic transitions during RF (Tang et al., 2025). For instance, during early disease stages, CCR2-expressing monocytes exhibit heightened differentiation into pro-inflammatory macrophages. subsequently driving macrophage-to-myofibroblast transdifferentiation that accelerates fibrogenesis (Xu et al., 2019; Braga et al., 2018). Concurrently, epithelial and endothelial cells undergo loss of polarity, transitioning from tightly adherent, organized morphologies to detached spindle-shaped structures that promote mesenchymal transition and fibrogenesis (Jacobs et al., 2024; Fintha et al., 2019). Pharmacological blockade of PAR-1 (Saifi et al., 2021) and A1AR (Ren et al., 2020) or EP2R (Jensen et al., 2019) activation effectively downregulates mesenchymal markers to attenuate RF. Notably, GPCR expression profiling in renal fibroblasts reveals significant enrichment of S1PR3 and A2AR/A2BR subtypes (Kaur et al., 2023), with sphingosine-1-phosphate (S1P) or its analogs directly stimulating fibroblast activation (Shiohira et al., 2013), while A2BR activation has been definitively demonstrated to drive macrophage-to-myofibroblast conversion, further amplifying fibrotic cascades (Torres et al., 2020). Thus, GPCR-mediated control over cellular phenotypic transitions constitutes a defining pathomechanism in RF, positioning these receptors as privileged therapeutic targets for intercepting fibrotic progression.
5.3 GPCR is involved in metabolic reprogramming in RF
Metabolic reprogramming—manifested by pathological remodeling of fatty acid β-oxidation (FAO), dysregulated aerobic glycolysis, mitochondrial insufficiency, and inflammatory infiltration, This reprogramming sustains heightened bioenergetic demands during fibrogenesis through altered substrate utilization (Zhu et al., 2021; Miguel et al., 2025; Zhu Z. et al., 2022). Substantial evidence establishes GPCRs as master regulators of metabolic flux in RF, particularly via the Gα12/13 signaling (Yang et al., 2020); for instance, PAR2 and CB2R activation induce tubular epithelial cell senescence and lipid droplet accumulation, impairing mitochondrial β-oxidation capacity (Ha et al., 2024; Zhou et al., 2024), while GPR87 accelerates glycolysis and mitochondrial damage, promoting ECM deposition (Cui et al., 2022). Beyond direct metabolic regulation, GLP-1R agonists normalizes lipidomic profiles and mitochondrial metabolites (acyl-carnitines, cholesterol, succinate), conferring renoprotection (Wang et al., 2018). Conversely, microbiota-derived metabolites serve as endogenous GPCR ligands (Rhee, 2018), exemplified by butyrate—GPR109a axis activation preserving podocyte integrity against glomerular basement membrane injury (Felizardo et al., 2019). Additionally, injured renal cells exhibit secretory dysfunction, the secretome of renal vascular endothelial cells serves as pivotal regulators of fibroblast activation (Lipphardt et al., 2017), exemplified by α2A-AR-driven β-arrestin2 signaling that promotes tubular senescence and pro-inflammatory cytokine secretion, thereby driving fibroblast activation and propagating RF (Li et al., 2022). Collectively, GPCR-mediated governance of metabolic reprogramming pathways represents a frontier in contemporary RF pathobiology research.
5.4 GPCRs orchestrate shear stress-induced injury in RF
Contemporary research has delineated shear stress—a fundamental biomechanical force—as a key driver of fibrotic pathogenesis through mechanosensation-signal transduction-epigenetic remodeling cascades (Long et al., 2022), with GPCRs serving as primary mechanosensors and signaling hubs that represent promising therapeutic targets for RF induced by tubular dilation, obstruction, and hyperfiltration (Xiao et al., 2023). The pathophysiological impact manifests through mechanosensitive injury across multiple renal cell types, exemplified by the shear-sensitive ion channel Piezo1 modulating CCR2-mediated macrophage inflammation to suppress mesenchymal transition and RF progression (He et al., 2022), while Yes - associated protein (YAP) —a transcriptional co-activator central to mechanotransduction (Panciera et al., 2017)—participates in myofibroblast activation via the V2R-YAP signaling axis (Jamadar et al., 2022), and EP2R functions as a pathological shear stress sensor in podocytes, directly driving cytoskeletal destabilization and detachment (Srivastava et al., 2018). Furthermore, multiple Gq/11-coupled GPCRs, including GPR68, ETAR, V1AR, and S1PR, demonstrate mechanosensory capabilities, though their precise mechanistic underpinnings warrant further investigation (Xiao et al., 2023). Collectively, GPCRs constitute pivotal mechanotransductive regulators of shear stress-induced renal parenchymal damage, presenting profound pathobiological significance and compelling therapeutic relevance for targeted intervention.
6 Challenges and prospects of GPCR target development in RF
Notwithstanding the preeminent status of GPCRs as the most therapeutically exploited target class, their translational deployment against fibrotic disorders remains incipient (Tang et al., 2025; Rieder et al., 2025). This therapeutic inertia predominantly arises from the intricately orchestrated, multifactorial pathoetiology of organ fibrosis, characterized by dynamic oscillations between inflammatory and profibrotic signaling cascades (Abbad et al., 2025). Mononodal pharmacotherapeutic interventions targeting singular nodal points are frequently subverted by compensatory pathway rewiring—a phenomenon starkly evidenced by terminated clinical trials targeting canonical profibrotic networks (e.g., TGF-β, PI3K/mTOR, JAK/STAT) (Di et al., 2025; Zhao et al., 2022). Concomitantly, extant in vitro and in vivo fibrosis models exhibit limited recapitulation of the human pathophysiological niche, thereby compromising translational fidelity (Addario et al., 2025). Furthermore, the pathologically remodeled ECM in RF imposes steric hindrance that severely restricts lesional drug bioavailability (Xu et al., 2021). Therefore, overcoming the bottlenecks in targeted GPCR intervention for organ fibrosis is of crucial importance.
Despite these challenges, combining computational and experimental tools is driving significant progress. Innovations in 3D microphysiological systems—encompassing organ-on-chip platforms with multicellular co-cultures, vascularized bioprinted constructs, and patient-derived organoids—are progressively standardizing human-relevant fibrotic pathomimetics (Addario et al., 2025; Sacchi et al., 2020; Miyoshi et al., 2020). Parallel breakthroughs in nanotherapeutic delivery—including lipid-encapsulated GPCR ligands, renal-compartment-specific targeting moieties, and pathology-responsive nanovehicles—are circumventing biodistribution barriers (Oroojalian et al., 2020). In GPCR drug discovery, AI-driven compound design and biased ligand development are reaching maturity (Zhang et al., 2024; Yang D. et al., 2021), GPCR-targeted candidates now make up over 60% of receptor-focused clinical pipelines for fibrosis. Key examples include clinical trials targeting S1PR (e.g., Fingolimod), CCR2 (e.g., DMX-200), and GLP-1R (e.g., Exenatide) epitomize this mechanistic momentum (Abbad et al., 2025). Collectively, the precision targeting of GPCR signaling nodes harbors exceptional potential for intercepting the fibrotic cascade at its evolutionary nexus.
7 Conclusion
Given the persistent high disease burden and suboptimal therapeutic outcomes in RF, convergent preclinical and clinical evidence has validated the therapeutic tractability of GPCRs. This review delineates the pathophysiological primacy of key GPCR families—notably endothelin, angiotensin, chemokine, and adenosine receptors—in orchestrating RF progression through multimodal regulation spanning inflammatory/fibrogenic cascade initiation, maladaptive cellular phenotypic transitions, metabolomic reprogramming, and mechanotransductive injury responses. Collectively, GPCRs emerge as supramolecular signaling hubs whose precision modulation holds exceptional promise for next-generation anti-fibrotic therapeutics.
Author contributions
HW: Conceptualization, Data curation, Writing – original draft, Writing – review and editing. MY: Writing – original draft, Writing – review and editing. XL: Writing – original draft, Writing – review and editing. JF: Conceptualization, Supervision, Writing – review and editing. CW: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by “Major Science and Technology Programs in Sichuan Province [grant number 2024ZDZX0019]” and “Chengdu University of Traditional Chinese Medicine Research Start-up Funds for Introducing Talents [grant numbers 30040015, 030040017]”.
Acknowledgments
The authors are profoundly grateful to the “Figdraw” platform for providing drawing support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbad, L., Esteve, E., and Chatziantoniou, C. (2025). Advances and challenges in kidney fibrosis therapeutics. Nat. Rev. Nephrol. 21 (5), 314–329. doi:10.1038/s41581-025-00934-5
Addario, G., Moroni, L., and Mota, C. (2025). Kidney fibrosis in vitro and in vivo models: path toward physiologically relevant humanized models. Adv. Healthc. Mater. 14 (9), e2403230. doi:10.1002/adhm.202403230
Alghamdi, T. A., Batchu, S. N., Hadden, M. J., Yerra, V. G., Liu, Y., Bowskill, B. B., et al. (2018). Histone H3 serine 10 phosphorylation facilitates endothelial activation in diabetic kidney disease. Diabetes 67 (12), 2668–2681. doi:10.2337/db18-0124
AlQudah, M., Hale, T. M., and Czubryt, M. P. (2020). Targeting the renin-angiotensin-aldosterone system in fibrosis. Matrix Biol. J. Int. Soc. Matrix Biol. 91-92, 92–108. doi:10.1016/j.matbio.2020.04.005
Anders, H. J., and Schaefer, L. (2014). Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J. Am. Soc. Nephrol. JASN 25 (7), 1387–1400. doi:10.1681/asn.2014010117
Anguiano, L., Riera, M., Pascual, J., and Soler, M. J. (2015). Endothelin blockade in diabetic kidney disease. J. Clin. Med. 4 (6), 1171–1192. doi:10.3390/jcm4061171
Armando, I., Cuevas, S., Fan, C., Kumar, M., Izzi, Z., Jose, P. A., et al. (2022). G protein-coupled receptor 37L1 modulates epigenetic changes in human renal proximal tubule cells. Int. J. Mol. Sci. 23 (22), 14456. doi:10.3390/ijms232214456
Asher, W. B., Terry, D. S., Gregorio, G. G. A., Kahsai, A. W., Borgia, A., Xie, B., et al. (2022). GPCR-Mediated β-arrestin activation deconvoluted with single-molecule precision. Cell 185 (10), 1661–75.e16. doi:10.1016/j.cell.2022.03.042
Azushima, K., Morisawa, N., Tamura, K., and Nishiyama, A. (2020). Recent research advances in renin-angiotensin-aldosterone system receptors. Curr. Hypertens. Rep. 22 (3), 22. doi:10.1007/s11906-020-1028-6
Bagang, N., Gupta, K., Singh, G., Kanuri, S. H., and Mehan, S. (2023). Protease-activated receptors in kidney diseases: a comprehensive review of pathological roles, therapeutic outcomes and challenges. Chemico-biological Interact. 377, 110470. doi:10.1016/j.cbi.2023.110470
Bai, J. J., Tan, C. D., and Secretin, C. B. K. C. (2017). At the hub of water-salt homeostasis. Am. J. physiology Ren. physiology 312 (5), F852–F860. doi:10.1152/ajprenal.00191.2015
Ballante, F., Kooistra, A. J., Kampen, S., de Graaf, C., and Carlsson, J. (2021). Structure-Based virtual screening for ligands of G protein-coupled receptors: what can molecular docking Do for you? Pharmacol. Rev. 73 (4), 527–565. doi:10.1124/pharmrev.120.000246
Bankir, L., Bichet, D. G., and Bouby, N. (2010). Vasopressin V2 receptors, ENaC, and sodium reabsorption: a risk factor for hypertension? Am. J. physiology Ren. physiology 299 (5), F917–F928. doi:10.1152/ajprenal.00413.2010
Barutta, F., Bruno, G., Mastrocola, R., Bellini, S., and Gruden, G. (2018). The role of cannabinoid signaling in acute and chronic kidney diseases. Kidney Int. 94 (2), 252–258. doi:10.1016/j.kint.2018.01.024
Bideak, A., Blaut, A., Hoppe, J. M., Müller, M. B., Federico, G., Eltrich, N., et al. (2018). The atypical chemokine receptor 2 limits renal inflammation and fibrosis in murine progressive immune complex glomerulonephritis. Kidney Int. 93 (4), 826–841. doi:10.1016/j.kint.2017.11.013
Block, G. A., Bushinsky, D. A., Cheng, S., Cunningham, J., Dehmel, B., Drueke, T. B., et al. (2017). Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. Jama 317 (2), 156–164. doi:10.1001/jama.2016.19468
Borea, P. A., Gessi, S., Merighi, S., Vincenzi, F., and Varani, K. (2018). Pharmacology of adenosine receptors: the state of the art. Physiol. Rev. 98 (3), 1591–1625. doi:10.1152/physrev.00049.2017
Braga, T. T., Correa-Costa, M., Silva, R. C., Cruz, M. C., Hiyane, M. I., da Silva, J. S., et al. (2018). CCR2 contributes to the recruitment of monocytes and leads to kidney inflammation and fibrosis development. Inflammopharmacology 26 (2), 403–411. doi:10.1007/s10787-017-0317-4
Brenner, B. M., Cooper, M. E., de Zeeuw, D., Keane, W. F., Mitch, W. E., Parving, H. H., et al. (2001). Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345 (12), 861–869. doi:10.1056/NEJMoa011161
Cao, Q., Huang, C., Yi, H., Gill, A. J., Chou, A., Foley, M., et al. (2022). A single-domain i-body, AD-114, attenuates renal fibrosis through blockade of CXCR4. JCI insight 7 (4), e143018. doi:10.1172/jci.insight.143018
Cazorla-Vázquez, S., and Engel, F. B. (2018). Adhesion GPCRs in kidney development and disease. Front. cell Dev. Biol. 6, 9. doi:10.3389/fcell.2018.00009
Cazorla-Vázquez, S., Kösters, P., Bertz, S., Pfister, F., Daniel, C., Dedden, M., et al. (2023). Adhesion GPCR Gpr126 (Adgrg6) expression profiling in zebrafish, mouse, and human kidney. Cells 12 (15), 1988. doi:10.3390/cells12151988
Chang, T. T., Li, S. Y., Tsai, M. T., Chiang, C. H., Chen, C., and Chen, J. W. (2024). CXCL5 inhibition ameliorates acute kidney injury and prevents the progression from acute kidney injury to chronic kidney disease. Clin. Sci. Lond. Engl. 1979 138 (22), 1451–1466. doi:10.1042/cs20241713
Chen, G., Lin, S. C., Chen, J., He, L., Dong, F., Xu, J., et al. (2011). CXCL16 recruits bone marrow-derived fibroblast precursors in renal fibrosis. J. Am. Soc. Nephrol. JASN 22 (10), 1876–1886. doi:10.1681/asn.2010080881
Chen, X., Jiang, D., Wang, J., Xu, X., Xi, P., et al. (2015). Prostaglandin E2 EP1 receptor enhances TGF-β1-induced mesangial cell injury. Int. J. Mol. Med. 35 (1), 285–293. doi:10.3892/ijmm.2014.1979
Chen, X., Ge, W., Dong, T., Hu, J., Chen, L., Fan, X., et al. (2019). Spironolactone inhibits endothelial-mesenchymal transition via the adenosine A2A receptor to reduce cardiorenal fibrosis in rats. Life Sci. 224, 177–186. doi:10.1016/j.lfs.2019.01.017
Chen, X., Wang, T., Chen, L., Zhao, Y., Deng, Y., Shen, W., et al. (2024). Cross-species single-cell analysis uncovers the immunopathological mechanisms associated with IgA nephropathy progression. JCI insight 9 (9), e173651. doi:10.1172/jci.insight.173651
Cheng, X., Zhou, T., He, Y., Xie, Y., Xu, Y., and Huang, W. (2022). The role and mechanism of butyrate in the prevention and treatment of diabetic kidney disease. Front. Microbiol. 13, 961536. doi:10.3389/fmicb.2022.961536
Chow, B. S. M., Kocan, M., Shen, M., Wang, Y., Han, L., Chew, J. Y., et al. (2019). AT1R-AT2R-RXFP1 functional crosstalk in myofibroblasts: impact on the therapeutic targeting of renal and cardiac fibrosis. J. Am. Soc. Nephrol. JASN 30 (11), 2191–2207. doi:10.1681/asn.2019060597
Colafella, K. M., Hilliard, L. M., and Denton, K. M. (2016). Epochs in the depressor/pressor balance of the renin-angiotensin system. Clin. Sci. Lond. Engl. 1979 130 (10), 761–771. doi:10.1042/cs20150939
Congreve, M., de Graaf, C., Swain, N. A., and Tate, C. G. (2020). Impact of GPCR structures on drug discovery. Cell 181 (1), 81–91. doi:10.1016/j.cell.2020.03.003
Correa-Costa, M., Andrade-Oliveira, V., Braga, T. T., Castoldi, A., Aguiar, C. F., Origassa, C. S. T., et al. (2014). Activation of platelet-activating factor receptor exacerbates renal inflammation and promotes fibrosis. Laboratory investigation; a J. Tech. methods pathology 94 (4), 455–466. doi:10.1038/labinvest.2013.155
Cui, X., Shi, E., Li, J., Li, Y., Qiao, Z., Wang, Z., et al. (2022). GPR87 promotes renal tubulointerstitial fibrosis by accelerating glycolysis and mitochondrial injury. Free Radic. Biol. and Med. 189, 58–70. doi:10.1016/j.freeradbiomed.2022.07.004
Dai, Y., Zhang, W., Wen, J., Zhang, Y., Kellems, R. E., and Xia, Y. (2011). A2B adenosine receptor-mediated induction of IL-6 promotes CKD. J. Am. Soc. Nephrol. 22 (5), 890–901. doi:10.1681/ASN.2010080890
Dalman, J., and Coleman, D. M. (2023). Nonatherosclerotic renovascular hypertension. Surg. Clin. N. Am. 103 (4), 733–743. doi:10.1016/j.suc.2023.05.007
Deng, S., Zhou, F., Wang, F., Jiang, Y., Tang, J., Hu, X., et al. (2023). C5a enhances Vδ1 T cells recruitment via the CCL2-CCR2 axis in IgA nephropathy. Int. Immunopharmacol. 125 (Pt A), 111065. doi:10.1016/j.intimp.2023.111065
Di, X., Li, Y., Wei, J., Li, T., and Liao, B. (2025). Targeting fibrosis: from molecular mechanisms to advanced therapies. Adv. Sci. Weinheim, Baden-Wurttemberg, Ger. 12 (3), e2410416. doi:10.1002/advs.202410416
Dorotea, D., Cho, A., Lee, G., Kwon, G., Lee, J., Sahu, P. K., et al. (2018). Orally active, species-independent novel A(3) adenosine receptor antagonist protects against kidney injury in Db/Db mice. Exp. and Mol. Med. 50 (4), 38–14. doi:10.1038/s12276-018-0053-x
Dwivedi, N., Tao, S., Jamadar, A., Sinha, S., Howard, C., Wallace, D. P., et al. (2020). Epithelial vasopressin Type-2 receptors regulate myofibroblasts by a YAP-CCN2-Dependent mechanism in polycystic kidney disease. J. Am. Soc. Nephrol. JASN 31 (8), 1697–1710. doi:10.1681/asn.2020020190
El-Fatatry, B. M., El-Haggar, S. M., Ibrahim, O. M., and Shalaby, K. H. (2024). Repurposing fexofenadine as a promising candidate for diabetic kidney disease: randomized clinical trial. Int. urology Nephrol. 56 (4), 1395–1402. doi:10.1007/s11255-023-03804-w
Eller, K., and Rosenkranz, A. R. (2018). Atypical chemokine receptors-chemokine PACMANs as new therapeutic targets in glomerulonephritis. Kidney Int. 93 (4), 774–775. doi:10.1016/j.kint.2017.12.021
Fang, W., Wang, Z., Li, Q., Wang, X., Zhang, Y., Sun, Y., et al. (2018). Gpr97 exacerbates AKI by mediating Sema3A signaling. J. Am. Soc. Nephrol. JASN 29 (5), 1475–1489. doi:10.1681/asn.2017080932
Felizardo, R. J. F., de Almeida, D. C., Pereira, R. L., Watanabe, I. K. M., Doimo, N. T. S., Ribeiro, W. R., et al. (2019). Gut microbial metabolite butyrate protects against proteinuric kidney disease through epigenetic- and GPR109a-mediated mechanisms. Faseb J. 33 (11), 11894–11908. doi:10.1096/fj.201901080R
Filipowska, J., Kondegowda, N. G., Leon-Rivera, N., Dhawan, S., and Vasavada, R. C. (2022). LGR4, a G protein-coupled receptor with a systemic role: from development to metabolic regulation. Front. Endocrinol. 13, 867001. doi:10.3389/fendo.2022.867001
Fintha, A., Gasparics, Á., Rosivall, L., and Sebe, A. (2019). Therapeutic targeting of fibrotic epithelial-mesenchymal Transition-An outstanding challenge. Front. Pharmacol. 10, 388. doi:10.3389/fphar.2019.00388
Forrester, S. J., Booz, G. W., Sigmund, C. D., Coffman, T. M., Kawai, T., Rizzo, V., et al. (2018). Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev. 98 (3), 1627–1738. doi:10.1152/physrev.00038.2017
Fu, Z., Geng, X., Liu, C., Shen, W., Dong, Z., Sun, G., et al. (2024). Identification of common and specific fibrosis-related genes in three common chronic kidney diseases. Ren. Fail. 46 (1), 2295431. doi:10.1080/0886022x.2023.2295431
Geng, H., Lan, R., Liu, Y., Chen, W., Wu, M., Saikumar, P., et al. (2021). Proximal tubule LPA1 and LPA2 receptors use divergent signaling pathways to additively increase profibrotic cytokine secretion. Am. J. physiology Ren. physiology 320 (3), F359–F374. doi:10.1152/ajprenal.00494.2020
Gerstein, H. C., Colhoun, H. M., Dagenais, G. R., Diaz, R., Lakshmanan, M., Pais, P., et al. (2019). Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet London, Engl. 394 (10193), 131–138. doi:10.1016/s0140-6736(19)31150-x
GBD Chronic Kidney Disease (2020). Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of Disease Study 2017. Lancet London, Engl. 395 (10225), 709–733. doi:10.1016/s0140-6736(20)30045-3
Gu, Y. J., Sun, W. Y., Zhang, S., Wu, J. j., and Wei, W. (2015). The emerging roles of β-arrestins in fibrotic diseases. Acta Pharmacol. Sin. 36 (11), 1277–1287. doi:10.1038/aps.2015.74
Ha, S., Chung, K. W., Lee, J., and Moon, H. R. (2022). Renal tubular PAR2 promotes interstitial fibrosis by increasing inflammatory responses and EMT process. Archives pharmacal Res. 45 (3), 159–173. doi:10.1007/s12272-022-01375-5
Ha, S., Kim, H. W., Kim, K. M., Kim, B. M., Kim, J., Son, M., et al. (2024). PAR2-mediated cellular senescence promotes inflammation and fibrosis in aging and chronic kidney disease. Aging cell 23 (8), e14184. doi:10.1111/acel.14184
Hamdan, D., and Robinson, L. A. (2021). Role of the CX(3)CL1-CX(3)CR1 axis in renal disease. Am. J. physiology Ren. physiology 321 (2), F121–F134. doi:10.1152/ajprenal.00059.2021
He, Y., Deng, B., Liu, S., Luo, S., Ning, Y., Pan, X., et al. (2022). Myeloid Piezo1 deletion protects renal fibrosis by restraining macrophage infiltration and activation. Hypertens. Dallas, Tex. 79 (5), 918–931. doi:10.1161/hypertensionaha.121.18750
Heerspink, H. J. L., Parving, H. H., Andress, D. L., Bakris, G., Correa-Rotter, R., Hou, F. F., et al. (2019). Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet London, Engl. 393 (10184), 1937–1947. doi:10.1016/s0140-6736(19)30772-x
Heerspink, H. J. L., Kiyosue, A., Wheeler, D. C., Lin, M., Wijkmark, E., Carlson, G., et al. (2023). Zibotentan in combination with dapagliflozin compared with dapagliflozin in patients with chronic kidney disease (ZENITH-CKD): a multicentre, randomised, active-controlled, phase 2b, clinical trial. Lancet London, Engl. 402 (10416), 2004–2017. doi:10.1016/s0140-6736(23)02230-4
Hercz, D., Jiang, S. H., and Webster, A. C. (2020). Interventions for itch in people with advanced chronic kidney disease. Cochrane Database Syst. Rev. 12 (12), CD011393. doi:10.1002/14651858.CD011393.pub2
Henedak, N. T., El-Abhar, H. S., Soubh, A. A., and Abdallah, D. M. (2024). NLRP3 inflammasome: a central player in renal pathologies and nephropathy. Life Sci. 351, 122813. doi:10.1016/j.lfs.2024.122813
Huang, K., Liu, W., Lan, T., Xie, X., Peng, J., Huang, J., et al. (2012). Berberine reduces fibronectin expression by suppressing the S1P-S1P2 receptor pathway in experimental diabetic nephropathy models. PloS one 7 (8), e43874. doi:10.1371/journal.pone.0043874
Huang, R., Fu, P., and Ma, L. (2023). Kidney fibrosis: from mechanisms to therapeutic medicines. Signal Transduct. Target. Ther. 8 (1), 129. doi:10.1038/s41392-023-01379-7
Huang, H., Peng, Z., and Yuan, Q. (2024). Research progress in anti-renal fibrosis drugs. Zhong nan da xue xue bao Yi xue ban = J. Central South Univ. Med. Sci. 49 (8), 1353–1362. doi:10.11817/j.issn.1672-7347.2024.240284
Hunter, R. W., Moorhouse, R., Farrah, T. E., MacIntyre, I. M., Asai, T., Gallacher, P. J., et al. (2017). First-in-Man demonstration of direct endothelin-mediated natriuresis and diuresis. Hypertens. Dallas, Tex 70 (1), 192–200. doi:10.1161/hypertensionaha.116.08832
Imenez Silva, P. H., Katamesh-Benabbas, C., Chan, K., Pastor Arroyo, E. M., Knöpfel, T., Bettoni, C., et al. (2020). The proton-activated ovarian cancer G protein-coupled receptor 1 (OGR1) is responsible for renal calcium loss during acidosis. Kidney Int. 97 (5), 920–933. doi:10.1016/j.kint.2019.12.006
Jacobs, M. E., de Vries, D. K., Engelse, M. A., Dumas, S. J., and Rabelink, T. J. (2024). Endothelial to mesenchymal transition in kidney fibrosis. Nephrol. Dial. Transplant. 39 (5), 752–760. Official publication of the European Dialysis and Transplant Association - European Renal Association, 2024. doi:10.1093/ndt/gfad238
Jamadar, A., Dwivedi, N., Mathew, S., Calvet, J. P., Thomas, S. M., and Rao, R. (2022). Vasopressin receptor Type-2 mediated signaling in renal cell carcinoma stimulates stromal fibroblast activation. Int. J. Mol. Sci. 23 (14), 7601. doi:10.3390/ijms23147601
Jensen, M. S., Mutsaers, H. A. M., Tingskov, S. J., Christensen, M., Madsen, M. G., Olinga, P., et al. (2019). Activation of the prostaglandin E(2) EP(2) receptor attenuates renal fibrosis in unilateral ureteral obstructed mice and human kidney slices. Acta physiol. Oxf. Engl. 227 (1), e13291. doi:10.1111/apha.13291
Jia, Y., Zheng, Z., Guan, M., Zhang, Q., Li, Y., Wang, L., et al. (2018). Exendin-4 ameliorates high glucose-induced fibrosis by inhibiting the secretion of miR-192 from injured renal tubular epithelial cells. Exp. and Mol. Med. 50 (5), 1–13. doi:10.1038/s12276-018-0084-3
Jiang, H., Galtes, D., Wang, J., and Rockman, H. A. (2022). G protein-coupled receptor signaling: transducers and effectors. Am. J. physiology Cell physiology 323 (3), C731–C748. doi:10.1152/ajpcell.00210.2022
Juul, K. V., Bichet, D. G., Nielsen, S., and Nørgaard, J. P. (2014). The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am. J. physiology Ren. physiology 306 (9), F931–F940. doi:10.1152/ajprenal.00604.2013
Kalbe, B., Schlimm, M., Wojcik, S., Philippou, S., Maßberg, D., Jansen, F., et al. (2016). Olfactory signaling components and olfactory receptors are expressed in tubule cells of the human kidney. Archives Biochem. biophysics 610, 8–15. doi:10.1016/j.abb.2016.09.017
Kamal, F. A., Travers, J. G., Schafer, A. E., Ma, Q., Devarajan, P., and Blaxall, B. C. (2017). G protein-coupled Receptor-G-Protein βγ-Subunit signaling mediates renal dysfunction and fibrosis in heart failure. J. Am. Soc. Nephrol. JASN 28 (1), 197–208. doi:10.1681/asn.2015080852
Kaur, H., Yerra, V. G., Batchu, S. N., Tran, D. T., Kabir, M. D. G., Liu, Y., et al. (2023). Single cell G-protein coupled receptor profiling of activated kidney fibroblasts expressing transcription factor 21. Br. J. Pharmacol. 180 (22), 2898–2915. doi:10.1111/bph.16101
Kohan, D. E., Barratt, J., Heerspink, H. J. L., Campbell, K. N., Camargo, M., Ogbaa, I., et al. (2023). Targeting the endothelin A receptor in IgA nephropathy. Kidney Int. Rep. 8 (11), 2198–2210. doi:10.1016/j.ekir.2023.07.023
Kohan, D. E., Bedard, P. W., Jenkinson, C., Hendry, B., and Komers, R. (2024). Mechanism of protective actions of sparsentan in the kidney: lessons from studies in models of chronic kidney disease. Clin. Sci. Lond. Engl. 1979 138 (11), 645–662. doi:10.1042/cs20240249
Korbecki, J., Bajdak-Rusinek, K., Kupnicka, P., Kapczuk, P., Simińska, D., Chlubek, D., et al. (2021). The role of CXCL16 in the pathogenesis of cancer and other diseases. Int. J. Mol. Sci. 22 (7), 3490. doi:10.3390/ijms22073490
Kosanović, M., Llorente, A., Glamočlija, S., Valdivielso, J. M., and Bozic, M. (2021). Extracellular vesicles and renal fibrosis: an odyssey toward a new therapeutic approach. Int. J. Mol. Sci. 22 (8), 3887. doi:10.3390/ijms22083887
Kuehn, B. M. (2022). End-stage kidney disease doubles. Jama 327 (16), 1540. doi:10.1001/jama.2022.5342
Lai, W. Y., and Mueller, A. (2021). Latest update on chemokine receptors as therapeutic targets. Biochem. Soc. Trans. 49 (3), 1385–1395. doi:10.1042/bst20201114
Lecru, L., Desterke, C., Grassin-Delyle, S., Chatziantoniou, C., Vandermeersch, S., Devocelle, A., et al. (2015). Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney Int. 88 (1), 72–84. doi:10.1038/ki.2015.63
Lee, J. H., Kim, D., Oh, Y. S., and Jun, H. S. (2019). Lysophosphatidic acid signaling in diabetic nephropathy. Int. J. Mol. Sci. 20 (11), 2850. doi:10.3390/ijms20112850
Legler, D. F., and Thelen, M. (2018). New insights in chemokine signaling. F1000Res., 7, 95. doi:10.12688/f1000research.13130.1
Li, Q., Deng, Y., Liu, L., Zhang, C., Cai, Y., Zhang, T., et al. (2022). Sympathetic denervation ameliorates renal fibrosis via inhibition of cellular senescence. Front. Immunol. 12, 823935. doi:10.3389/fimmu.2021.823935
Li, L., Lai, E. Y., Huang, Y., Eisner, C., Mizel, D., Wilcox, C. S., et al. (2012). Renal afferent arteriolar and tubuloglomerular feedback reactivity in mice with conditional deletions of adenosine 1 receptors. Am. J. physiology Ren. physiology 303 (8), F1166–F1175. doi:10.1152/ajprenal.00222.2012
Li, K., Wu, K. Y., Wu, W., Wang, N., Zhang, T., Choudhry, N., et al. (2017). C5aR1 promotes acute pyelonephritis induced by uropathogenic E. coli. JCI insight 2 (24), e97626. doi:10.1172/jci.insight.97626
Li, S., Qiu, M., Kong, Y., Zhao, X., Choi, H. J., Reich, M., et al. (2018). Bile acid G protein-coupled membrane receptor TGR5 modulates aquaporin 2-Mediated water homeostasis. J. Am. Soc. Nephrol. JASN 29 (11), 2658–2670. doi:10.1681/asn.2018030271
Li, L., Fu, H., and Liu, Y. (2022a). The fibrogenic niche in kidney fibrosis: components and mechanisms. Nat. Rev. Nephrol. 18 (9), 545–557. doi:10.1038/s41581-022-00590-z
Li, X., Lu, L., Hou, W., Huang, T., Chen, X., Qi, J., et al. (2022b). Epigenetics in the pathogenesis of diabetic nephropathy. Acta biochimica biophysica Sinica 54 (2), 163–172. doi:10.3724/abbs.2021016
Li, H., Sun, F., Bai, S., Chang, G., Wu, R., Wei, Y., et al. (2022c). The DR1-CSE/H(2)S system inhibits renal fibrosis by downregulating the ERK1/2 signaling pathway in diabetic mice. Int. J. Mol. Med. 49 (1), 7. doi:10.3892/ijmm.2021.5062
Lipphardt, M., Song, J. W., Matsumoto, K., Dadafarin, S., Dihazi, H., Müller, G., et al. (2017). The third path of tubulointerstitial fibrosis: aberrant endothelial secretome. Kidney Int. 92 (3), 558–568. doi:10.1016/j.kint.2017.02.033
Liu, Y. (2011). Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 7 (12), 684–696. doi:10.1038/nrneph.2011.149
Liu, Z., Wang, Y., Shu, S., Cai, J., Tang, C., and Dong, Z. (2019). Non-coding RNAs in kidney injury and repair. Am. J. physiology Cell physiology 317 (2), C177–C188. doi:10.1152/ajpcell.00048.2019
Lok, S. W. Y., Yiu, W. H., Zou, Y., Xue, R., Li, H., Ma, J., et al. (2023). Tubulovascular protection from protease-activated receptor-1 depletion during AKI-to-CKD transition. Nephrol. Dial. Transplant. 38 (10), 2232–2247. Official publication of the European Dialysis and Transplant Association - European Renal Association. doi:10.1093/ndt/gfad051
Long, Y., Niu, Y., Liang, K., and Du, Y. (2022). Mechanical communication in fibrosis progression. Trends cell Biol. 32 (1), 70–90. doi:10.1016/j.tcb.2021.10.002
Luo, F., Xu, R., Song, G., Xue, D., He, X., and Xia, Y. (2021). Alleviation of TGF-β1 induced tubular epithelial-mesenchymal transition via the δ-opioid receptor. FEBS J. 288 (4), 1243–1258. doi:10.1111/febs.15459
Lux, M., Blaut, A., Eltrich, N., Bideak, A., Müller, M. B., Hoppe, J. M., et al. (2019). The atypical chemokine receptor 2 limits progressive fibrosis after Acute Ischemic Kidney injury. Am. J. pathology 189 (2), 231–247. doi:10.1016/j.ajpath.2018.09.016
Lv, L., Liu, Y., Xiong, J., Wang, S., Li, Y., Zhang, B., et al. (2024). Role of G protein coupled receptors in acute kidney injury. Cell Commun. Signal. CCS 22 (1), 423. doi:10.1186/s12964-024-01802-8
Ma, X., Schwarz, A., Sevilla, S. Z., Levin, A., Hultenby, K., Wernerson, A., et al. (2018). Depletion of Gprc5a promotes development of diabetic nephropathy. J. Am. Soc. Nephrol. JASN 29 (6), 1679–1689. doi:10.1681/asn.2017101135
Martínez-Díaz, I., Martos, N., Llorens-Cebrià, C., Álvarez, F. J., Bedard, P. W., Vergara, A., et al. (2023). Endothelin receptor antagonists in kidney disease. Int. J. Mol. Sci. 24 (4), 3427. doi:10.3390/ijms24043427
Masuho, I., Kise, R., Gainza, P., Von Moo, E., Li, X., Tany, R., et al. (2023). Rules and mechanisms governing G protein coupling selectivity of GPCRs. Cell Rep. 42 (10), 113173. doi:10.1016/j.celrep.2023.113173
Mazzuca, M. Q., and Khalil, R. A. (2012). Vascular endothelin receptor type B: structure, function and dysregulation in vascular disease. Biochem. Pharmacol. 84 (2), 147–162. doi:10.1016/j.bcp.2012.03.020
Melloni, C., Cornel, J. H., Hafley, G., Neely, M. L., Clemmensen, P., Zamoryakhin, D., et al. (2016). Impact of chronic kidney disease on long-term ischemic and bleeding outcomes in medically managed patients with acute coronary syndromes: insights from the TRILOGY ACS trial. Eur. heart J. Acute Cardiovasc. care 5 (6), 443–454. doi:10.1177/2048872615598631
Meng, X. M. (2019). Inflammatory mediators and renal fibrosis. Adv. Exp. Med. Biol. 1165, 381–406. doi:10.1007/978-981-13-8871-2_18
Meng, P., Liu, C., Li, J., Fang, P., Yang, B., Sun, W., et al. (2024). CXC chemokine receptor 7 ameliorates renal fibrosis by inhibiting β-catenin signaling and epithelial-to-mesenchymal transition in tubular epithelial cells. Ren. Fail. 46 (1), 2300727. doi:10.1080/0886022x.2023.2300727
Miguel, V., Shaw, I. W., and Kramann, R. (2025). Metabolism at the crossroads of inflammation and fibrosis in chronic kidney disease. Nat. Rev. Nephrol. 21 (1), 39–56. doi:10.1038/s41581-024-00889-z
Miyoshi, T., Hiratsuka, K., Saiz, E. G., and Morizane, R. (2020). Kidney organoids in translational medicine: disease modeling and regenerative medicine. Dev. Dyn. official Publ. Am. Assoc. Anatomists 249 (1), 34–45. doi:10.1002/dvdy.22
Motahharynia, A., Moein, S., Kiyanpour, F., Moradzadeh, K., Yaqubi, M., and Gheisari, Y. (2022). Olfactory receptors contribute to progression of kidney fibrosis. NPJ Syst. Biol. Appl. 8 (1), 8. doi:10.1038/s41540-022-00217-w
Mutsaers, H. A. M., and Nørregaard, R. (2022). Prostaglandin E2 receptors as therapeutic targets in renal fibrosis. Kidney Res. Clin. Pract. 41 (1), 4–13. doi:10.23876/j.krcp.21.222
Nakamura, T., Fujiwara, N., Kawagoe, Y., Sugaya, T., Ueda, Y., and Koide, H. (2010). Effects of telmisartan and enalapril on renoprotection in patients with mild to moderate chronic kidney disease. Eur. J. Clin. investigation 40 (9), 790–796. doi:10.1111/j.1365-2362.2010.02319.x
Nasrallah, R., Hassouneh, R., and Hébert, R. L. (2014). Chronic kidney disease: targeting prostaglandin E2 receptors. Am. J. physiology Ren. physiology 307 (3), F243–F250. doi:10.1152/ajprenal.00224.2014
Navarro-González, J. F., Mora-Fernández, C., Muros de Fuentes, M., Chahin, J., Méndez, M. L., Gallego, E., et al. (2015). Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J. Am. Soc. Nephrol. JASN 26 (1), 220–229. doi:10.1681/asn.2014010012
Neuhofer, W., and Pittrow, D. (2006). Role of endothelin and endothelin receptor antagonists in renal disease. Eur. J. Clin. investigation 36 (Suppl. 3), 78–88. doi:10.1111/j.1365-2362.2006.01689.x
Okamoto, Y., Kitakaze, K., Takenouchi, Y., Matsui, R., Koga, D., Miyashima, R., et al. (2024). GPR176 promotes fibroblast-to-myofibroblast transition in organ fibrosis progression. Biochimica biophysica acta Mol. cell Res. 1871 (7), 119798. doi:10.1016/j.bbamcr.2024.119798
Oroojalian, F., Charbgoo, F., Hashemi, M., Amani, A., Yazdian-Robati, R., Mokhtarzadeh, A., et al. (2020). Recent advances in nanotechnology-based drug delivery systems for the kidney. J. Control. release official J. Control. Release Soc. 321, 442–462. doi:10.1016/j.jconrel.2020.02.027
Panciera, T., Azzolin, L., Cordenonsi, M., and Piccolo, S. (2017). Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. cell Biol. 18 (12), 758–770. doi:10.1038/nrm.2017.87
Park, F., and Miller, D. D. (2017). Role of lysophosphatidic acid and its receptors in the kidney. Physiol. genomics 49 (11), 659–666. doi:10.1152/physiolgenomics.00070.2017
Poll, B. G., Chen, L., Chou, C. L., Raghuram, V., and Knepper, M. A. (2021). Landscape of GPCR expression along the mouse nephron. Am. J. physiology Ren. physiology 321 (1), F50–f68. doi:10.1152/ajprenal.00077.2021
Rafnsson, A., Böhm, F., Settergren, M., Gonon, A., Brismar, K., and Pernow, J. (2012). The endothelin receptor antagonist bosentan improves peripheral endothelial function in patients with type 2 diabetes mellitus and microalbuminuria: a randomised trial. Diabetologia 55 (3), 600–607. doi:10.1007/s00125-011-2415-y
Rajkumar, P., and Pluznick, J. L. (2017). Unsung renal receptors: Orphan G-protein-coupled receptors play essential roles in renal development and homeostasis. Acta physiol. Oxf. Engl. 220 (2), 189–200. doi:10.1111/apha.12813
Rajkumar, P., and Pluznick, J. L. (2018). Acid-base regulation in the renal proximal tubules: using novel pH sensors to maintain homeostasis. Am. J. physiology Ren. physiology 315 (5), F1187-F1190–f90. doi:10.1152/ajprenal.00185.2018
Rajkumar, P., Cha, B., Yin, J., Arend, L. J., Păunescu, T. G., Hirabayashi, Y., et al. (2018). Identifying the localization and exploring a functional role for Gprc5c in the kidney. Faseb J. 32 (4), 2046–2059. doi:10.1096/fj.201700610RR
Ranieri, M. (2019). Renal Ca(2+) and water handling in response to calcium sensing receptor signaling: physiopathological aspects and role of CaSR-Regulated microRNAs. Int. J. Mol. Sci. 20 (21), 5341. doi:10.3390/ijms20215341
Rasheed, S. A. K., Subramanyan, L. V., Lim, W. K., Udayappan, U. K., Wang, M., and Casey, P. J. (2022). The emerging roles of Gα12/13 proteins on the hallmarks of cancer in solid tumors. Oncogene 41 (2), 147–158. doi:10.1038/s41388-021-02069-w
Reiss, A. B., Jacob, B., Zubair, A., Srivastava, A., Johnson, M., and De Leon, J. (2024). Fibrosis in chronic kidney disease: pathophysiology and therapeutic targets. J. Clin. Med. 13 (7), 1881. doi:10.3390/jcm13071881
Ren, H., Zuo, S., Hou, Y., Shang, W., Liu, N., and Yin, Z. (2020). Inhibition of α1-adrenoceptor reduces TGF-β1-induced epithelial-to-mesenchymal transition and attenuates UUO-Induced renal fibrosis in mice. Faseb J. 34 (11), 14892–14904. doi:10.1096/fj.202000737RRR
Rhee, E. P. (2018). A systems-level view of renal metabolomics. Seminars Nephrol. 38 (2), 142–150. doi:10.1016/j.semnephrol.2018.01.005
Rianto, F., Hoang, T., Revoori, R., and Sparks, M. A. (2021). Angiotensin receptors in the kidney and vasculature in hypertension and kidney disease. Mol. Cell. Endocrinol. 529, 111259. doi:10.1016/j.mce.2021.111259
Riccardi, D., and Brown, E. M. (2010). Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am. J. physiology Ren. physiology 298 (3), F485–F499. doi:10.1152/ajprenal.00608.2009
Rieder, F., Nagy, L. E., Maher, T. M., Distler, J. H. W., Kramann, R., Hinz, B., et al. (2025). Fibrosis: cross-organ biology and pathways to development of innovative drugs. Nat. Rev. Drug Discov. 24 (7), 543–569. doi:10.1038/s41573-025-01158-9
Roberts, V. S., Cowan, P. J., Alexander, S. I., Robson, S. C., and Dwyer, K. M. (2014). The role of adenosine receptors A2A and A2B signaling in renal fibrosis. Kidney Int. 86 (4), 685–692. doi:10.1038/ki.2014.244
Roccatello, D., Lan, H. Y., Sciascia, S., Sethi, S., Fornoni, A., and Glassock, R. (2024). From inflammation to renal fibrosis: a one-way road in autoimmunity? Autoimmun. Rev. 23 (4), 103466. doi:10.1016/j.autrev.2023.103466
Romero, D. J., Pescio, L. G., Santacreu, B. J., Mosca, J. M., Sterin-Speziale, N. B., and Favale, N. O. (2023). Sphingosine-1-phosphate receptor 2 plays a dual role depending on the stage of cell differentiation in renal epithelial cells. Life Sci. 316, 121404. doi:10.1016/j.lfs.2023.121404
Ros-Ruiz, S., Aranda-Lara, P., Fernández, J. C., Martínez-Esteban, M. D., Jironda, C., Hidalgo, P., et al. (2012). High doses of irbesartan offer long-term kidney protection in cases of established diabetic nephropathy. Nefrol. publicacion Of. la Soc. Espanola Nefrol. 32 (2), 187–196. doi:10.3265/Nefrologia.pre2011.Nov.10962
Rossing, P., Agarwal, R., Anker, S. D., Filippatos, G., Pitt, B., Ruilope, L. M., et al. (2023). Finerenone in patients across the spectrum of chronic kidney disease and type 2 diabetes by glucagon-like peptide-1 receptor agonist use. Diabetes, Obes. and metabolism 25 (2), 407–416. doi:10.1111/dom.14883
Rovin, B. H., Barratt, J., Heerspink, H. J. L., Alpers, C. E., Bieler, S., Chae, D. W., et al. (2023). Efficacy and safety of sparsentan versus irbesartan in patients with IgA nephropathy (PROTECT): 2-Year results from a randomised, active-controlled, phase 3 trial. Lancet London, Engl. 402 (10417), 2077–2090. doi:10.1016/s0140-6736(23)02302-4
Rudomanova, V., and Blaxall, B. C. (2017). Targeting GPCR-Gβγ-GRK2 signaling as a novel strategy for treating cardiorenal pathologies. Biochimica biophysica acta Mol. basis Dis. 1863 (8), 1883–1892. doi:10.1016/j.bbadis.2017.01.020
Sacchi, M., Bansal, R., and Rouwkema, J. (2020). Bioengineered 3D models to recapitulate tissue fibrosis. Trends Biotechnol. 38 (6), 623–636. doi:10.1016/j.tibtech.2019.12.010
Saifi, M. A., Annaldas, S., and Godugu, C. (2021). A direct thrombin inhibitor, dabigatran etexilate protects from renal fibrosis by inhibiting protease activated receptor-1. Eur. J. Pharmacol. 893, 173838. doi:10.1016/j.ejphar.2020.173838
Schinzari, F., Tesauro, M., and Cardillo, C. (2024). Is endothelin targeting finally ready for prime time? Clin. Sci. Lond. Engl. 1979 138 (11), 635–644. doi:10.1042/cs20240607
Seccia, T. M., Caroccia, B., Gioco, F., Piazza, M., Buccella, V., Guidolin, D., et al. (2016). Endothelin-1 drives epithelial-mesenchymal transition in hypertensive nephroangiosclerosis. J. Am. Heart Assoc. 5 (7), e003888. doi:10.1161/jaha.116.003888
Semenikhina, M., Fedoriuk, M., Stefanenko, M., Klemens, C. A., Cherezova, A., Marshall, B., et al. (2023). β-Arrestin pathway activation by selective ATR1 agonism promotes calcium influx in podocytes, leading to glomerular damage. Clin. Sci. Lond. Engl. 1979 137 (24), 1789–1804. doi:10.1042/cs20230313
Senarath, K., Kankanamge, D., Samaradivakara, S., Ratnayake, K., Tennakoon, M., and Karunarathne, A. (2018). Regulation of G protein βγ signaling. Int. Rev. cell Mol. Biol. 339, 133–191. doi:10.1016/bs.ircmb.2018.02.008
Shaman, A. M., Bain, S. C., Bakris, G. L., Buse, J. B., Idorn, T., Mahaffey, K. W., et al. (2022). Effect of the glucagon-like Peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation 145 (8), 575–585. doi:10.1161/circulationaha.121.055459
Shiohira, S., Yoshida, T., Sugiura, H., Nishida, M., Nitta, K., and Tsuchiya, K. (2013). Sphingosine-1-phosphate acts as a key molecule in the direct mediation of renal fibrosis. Physiol. Rep. 1 (7), e00172. doi:10.1002/phy2.172
Shlipak, M. G., Tummalapalli, S. L., Boulware, L. E., Grams, M. E., Ix, J. H., Jha, V., et al. (2021). The case for early identification and intervention of chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 99 (1), 34–47. doi:10.1016/j.kint.2020.10.012
Smeijer, J. D., Wasehuus, V. S., Dhaun, N., Górriz, J. L., Soler, M. J., Åstrand, M., et al. (2024). Effects of zibotentan alone and in combination with dapagliflozin on fluid retention in patients with CKD. J. Am. Soc. Nephrol. JASN 35 (10), 1381–1390. doi:10.1681/asn.0000000000000436
Smeijer, J. D., Kohan, D. E., Dhaun, N., Noronha, I. L., Liew, A., and Heerspink, H. J. L. (2025). Endothelin receptor antagonists in chronic kidney disease. Nat. Rev. Nephrol. 21 (3), 175–188. doi:10.1038/s41581-024-00908-z
Soares, A. G., Contreras, J., Mironova, E., Archer, C. R., Stockand, J. D., and Abd El-Aziz, T. M. (2023). P2Y2 receptor decreases blood pressure by inhibiting ENaC. JCI insight 8 (14), e167704. doi:10.1172/jci.insight.167704
Song, K. H., Park, J., Park, J. H., Natarajan, R., and Ha, H. (2013). Fractalkine and its receptor mediate extracellular matrix accumulation in diabetic nephropathy in mice. Diabetologia 56 (7), 1661–1669. doi:10.1007/s00125-013-2907-z
Spargias, K., Adreanides, E., Demerouti, E., Gkouziouta, A., Manginas, A., Pavlides, G., et al. (2009). Iloprost prevents contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation 120 (18), 1793–1799. doi:10.1161/circulationaha.109.863159
Srivastava, T., Dai, H., Heruth, D. P., Alon, U. S., Garola, R. E., Zhou, J., et al. (2018). Mechanotransduction signaling in podocytes from fluid flow shear stress. Am. J. physiology Ren. physiology 314 (1), F22-F34–f34. doi:10.1152/ajprenal.00325.2017
Stefanini, G. G., Briguori, C., Cao, D., Baber, U., Sartori, S., Zhang, Z., et al. (2021). Ticagrelor monotherapy in patients with chronic kidney disease undergoing percutaneous coronary intervention: TWILIGHT-CKD. Eur. heart J. 42 (45), 4683–4693. doi:10.1093/eurheartj/ehab533
Stokman, M. F., Saunier, S., and Benmerah, A. (2021). Renal ciliopathies: sorting out therapeutic approaches for nephronophthisis. Front. cell Dev. Biol. 9, 653138. doi:10.3389/fcell.2021.653138
Su, X., Zhou, G., Tian, M., Wu, S., and Wang, Y. (2021). Silencing of RSPO1 mitigates obesity-related renal fibrosis in mice by deactivating Wnt/β-catenin pathway. Exp. cell Res. 405 (2), 112713. doi:10.1016/j.yexcr.2021.112713
Sun, C. Y., Chang, S. C., and Wu, M. S. (2012). Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PloS one 7 (3), e34026. doi:10.1371/journal.pone.0034026
Sun, H. L., Bian, H. G., Liu, X. M., Zhang, H., Ying, J., Yang, H., et al. (2023). GRP/GRPR signaling pathway aggravates hyperuricemia-induced renal inflammation and fibrosis via ABCG2-dependent mechanisms. Biochem. Pharmacol. 218, 115901. doi:10.1016/j.bcp.2023.115901
Sun, Q., Kamath, P., Sun, Y., Liang, M., Wu, L., et al. (2024). Dexmedetomidine attenuates lipopolysaccharide-induced renal cell fibrotic phenotypic changes by inhibiting necroinflammation via activating α(2)-adrenoceptor: a combined randomised animal and in vitro study. Biomed. Pharmacother. 174, 116462. doi:10.1016/j.biopha.2024.116462
Tang, H., Li, K., Shi, Z., and Wu, J. (2025). G-Protein-Coupled receptors in chronic kidney disease induced by hypertension and diabetes. Cells 14 (10), 729. doi:10.3390/cells14100729
Tian, D., Li, J., Zou, L., Lin, M., Shi, X., Hu, Y., et al. (2021). Adenosine A1 receptor deficiency aggravates extracellular matrix accumulation in diabetic nephropathy through disturbance of peritubular microenvironment. J. diabetes Res. 2021, 5584871. doi:10.1155/2021/5584871
Torres, V. E., Chapman, A. B., Devuyst, O., Gansevoort, R. T., Grantham, J. J., Higashihara, E., et al. (2012). Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 367 (25), 2407–2418. doi:10.1056/NEJMoa1205511
Torres, Á., Muñoz, K., Nahuelpán, Y., R Saez, A. P., Mendoza, P., Jara, C., et al. (2020). Intraglomerular monocyte/macrophage infiltration and macrophage-myofibroblast transition during diabetic nephropathy is regulated by the A(2B) adenosine receptor. Cells 9 (4), 1051. doi:10.3390/cells9041051
Tóth, A. D., Turu, G., Hunyady, L., and Balla, A. (2018). Novel mechanisms of G-protein-coupled receptors functions: AT(1) angiotensin receptor acts as a signaling hub and focal point of receptor cross-talk. Best Pract. and Res. Clin. Endocrinol. and metabolism 32 (2), 69–82. doi:10.1016/j.beem.2018.02.003
Van der Hauwaert, C., Glowacki, F., Pottier, N., and Cauffiez, C. (2019). Non-coding RNAs as new therapeutic targets in the context of renal fibrosis. Int. J. Mol. Sci. 20 (8), 1977. doi:10.3390/ijms20081977
Wang, N., and Zhang, C. (2024). Recent advances in the management of diabetic kidney disease: slowing progression. Int. J. Mol. Sci. 25 (6), 3086. doi:10.3390/ijms25063086
Wang, Y., Huang, J., Liu, X., Niu, Y., Zhao, L., Yu, Y., et al. (2017a). β-Arrestin-biased AT1R stimulation promotes extracellular matrix synthesis in renal fibrosis. Am. J. physiology Ren. physiology 313 (1), F1–F8. doi:10.1152/ajprenal.00588.2016
Wang, Y., Del Borgo, M., Lee, H. W., Baraldi, D., Hirmiz, B., Gaspari, T. A., et al. (2017b). Anti-fibrotic potential of AT(2) receptor agonists. Front. Pharmacol. 8, 564. doi:10.3389/fphar.2017.00564
Wang, C., Li, L., Liu, S., Liao, G., Chen, Y., et al. (2018). GLP-1 receptor agonist ameliorates obesity-induced chronic kidney injury via restoring renal metabolism homeostasis. PloS one 13 (3), e0193473. doi:10.1371/journal.pone.0193473
Wang, L., Ren, X., Tian, X. F., Cheng, X. L., Zhao, Y. Y., Li, Q. Y., et al. (2019). Protective effects of GPR120 agonist-programmed macrophages on renal interstitial fibrosis in unilateral ureteral obstruction (UUO) rats. Biomed. Pharmacother. 117, 109172. doi:10.1016/j.biopha.2019.109172
Wang, L., Wu, Y., Jia, Z., Yu, J., and Huang, S. (2022). Roles of EP receptors in the regulation of fluid balance and blood pressure. Front. Endocrinol. 13, 875425. doi:10.3389/fendo.2022.875425
Wu, H., Lai, C. F., Chang-Panesso, M., and Humphreys, B. D. (2020a). Proximal tubule translational profiling during kidney fibrosis reveals proinflammatory and long noncoding RNA expression patterns with sexual dimorphism. J. Am. Soc. Nephrol. JASN 31 (1), 23–38. doi:10.1681/asn.2019040337
Wu, F., Sun, C., and Lu, J. (2020b). The role of chemokine receptors in renal fibrosis. Rev. physiology, Biochem. Pharmacol. 177, 1–24. doi:10.1007/112_2020_21
Wu, J. C., Wang, X. J., Zhu, J. H., Huang, X. Y., Liu, M., Qiao, Z., et al. (2023). GPR97 deficiency ameliorates renal interstitial fibrosis in mouse hypertensive nephropathy. Acta Pharmacol. Sin. 44 (6), 1206–1216. doi:10.1038/s41401-022-01041-y
Wu, W., Wang, Y., Shao, X., Huang, S., Wang, J., Zhou, S., et al. (2024a). GLP-1RA improves diabetic renal injury by alleviating glomerular endothelial cells pyrotosis via RXRα/circ8411/miR-23a-5p/ABCA1 pathway. PloS one 19 (12), e0314628. doi:10.1371/journal.pone.0314628
Wu, C., Xu, M., Dong, J., Cui, W., and Yuan, S. (2024b). The structure and function of olfactory receptors. Trends Pharmacol. Sci. 45 (3), 268–280. doi:10.1016/j.tips.2024.01.004
Xiao, H., Dairaghi, D. J., Powers, J. P., Ertl, L. S., Baumgart, T., Wang, Y., et al. (2014). C5a receptor (CD88) blockade protects against MPO-ANCA GN. J. Am. Soc. Nephrol. JASN 25 (2), 225–231. doi:10.1681/asn.2013020143
Xiao, H., Sun, X., Liu, R., Chen, Z., Lin, Z., Yang, Y., et al. (2020). Gentiopicroside activates the bile acid receptor Gpbar1 (TGR5) to repress NF-kappaB pathway and ameliorate diabetic nephropathy. Pharmacol. Res. 151, 104559. doi:10.1016/j.phrs.2019.104559
Xiao, R., Liu, J., and Xu, X. Z. S. (2023). Mechanosensitive GPCRs and ion channels in shear stress sensing. Curr. Opin. cell Biol. 84, 102216. doi:10.1016/j.ceb.2023.102216
Xie, L., Cheng, Y., Du, W., Fu, L., Wei, Z., Guan, Y., et al. (2023). Activation of GPER1 in macrophages ameliorates UUO-Induced renal fibrosis. Cell Death Dis. 14 (12), 818. doi:10.1038/s41419-023-06338-2
Xu, J., Wang, J., Cheng, Y., Li, X., He, M., Zhu, J., et al. (2018). Glucagon-like Peptide-1 mediates the protective effect of the dipeptidyl peptidase IV inhibitor on renal fibrosis via reducing the phenotypic conversion of renal microvascular cells in monocrotaline-treated rats. BioMed Res. Int. 2018, 1864107. doi:10.1155/2018/1864107
Xu, L., Sharkey, D., and Cantley, L. G. (2019). Tubular GM-CSF promotes late MCP-1/CCR2-Mediated fibrosis and inflammation after ischemia/reperfusion injury. J. Am. Soc. Nephrol. JASN 30 (10), 1825–1840. doi:10.1681/asn.2019010068
Xu, H., Wu, T., and Huang, L. (2021). Therapeutic and delivery strategies of phytoconstituents for renal fibrosis. Adv. drug Deliv. Rev. 177, 113911. doi:10.1016/j.addr.2021.113911
Yanagi, M., Tamura, K., Fujikawa, T., Kanaoka, T., Ohsawa, T., Azushima, K., et al. (2013). The angiotensin II type 1 receptor blocker olmesartan preferentially improves nocturnal hypertension and proteinuria in chronic kidney disease. Hypertens Res. 36, 262–269. doi:10.1038/hr.2012.184
Yamashita, N., and Kramann, R. (2024). Mechanisms of kidney fibrosis and routes towards therapy. Trends Endocrinol. metabolism TEM 35 (1), 31–48. doi:10.1016/j.tem.2023.09.001
Yang, Y. M., Kuen, D. S., Chung, Y., Kurose, H., and Kim, S. G. (2020). Gα(12/13) signaling in metabolic diseases. Exp. and Mol. Med. 52 (6), 896–910. doi:10.1038/s12276-020-0454-5
Yang, J., Villar, V. A. M., Jose, P. A., and Zeng, C. (2021a). Renal dopamine receptors and oxidative stress: role in hypertension. Antioxidants and redox Signal. 34 (9), 716–735. doi:10.1089/ars.2020.8106
Yang, D., Zhou, Q., Labroska, V., Qin, S., Darbalaei, S., Wu, Y., et al. (2021b). G protein-coupled receptors: Structure- and function-based drug discovery. Signal Transduct. Target. Ther. 6 (1), 7. doi:10.1038/s41392-020-00435-w
Ye, J., Qian, Z., Xue, M., Liu, Y., Zhu, S., Li, Y., et al. (2019). Aristolochic acid I aggravates renal injury by activating the C3a/C3aR complement system. Toxicol. Lett. 312, 118–124. doi:10.1016/j.toxlet.2019.04.027
Ye, B., Chen, B., Guo, C., Xiong, N., Huang, Y., Li, M., et al. (2024). C5a-C5aR1 axis controls mitochondrial fission to promote podocyte injury in lupus nephritis. Mol. Ther. J. Am. Soc. Gene Ther. 32 (5), 1540–1560. doi:10.1016/j.ymthe.2024.03.003
Yoshikawa, T., Oguchi, A., Toriu, N., Sato, Y., Kobayashi, T., Ogawa, O., et al. (2023). Tertiary lymphoid tissues are microenvironments with intensive interactions between immune cells and proinflammatory parenchymal cells in aged kidneys. J. Am. Soc. Nephrol. JASN 34 (10), 1687–1708. doi:10.1681/asn.0000000000000202
Yosipovitch, G., Awad, A., Spencer, R. H., Munera, C., and Menzaghi, F. (2023). A phase 2 study of oral difelikefalin in subjects with chronic kidney disease and moderate-to-severe pruritus. J. Am. Acad. Dermatology 89 (2), 261–268. doi:10.1016/j.jaad.2023.03.051
You, H., Gao, T., Raup-Konsavage, W. M., Cooper, T. K., Bronson, S. K., Reeves, W. B., et al. (2017). Podocyte-specific chemokine (C-C motif) receptor 2 overexpression mediates diabetic renal injury in mice. Kidney Int. 91 (3), 671–682. doi:10.1016/j.kint.2016.09.042
Yu, J., Kim, G., Jarhad, D. B., Lee, H. W., Lee, J., Park, C. W., et al. (2019). Correlation study between A(3) adenosine receptor binding affinity and anti-renal interstitial fibrosis activity of truncated adenosine derivatives. Archives pharmacal Res. 42 (9), 773–779. doi:10.1007/s12272-018-1079-2
Yuan, A., Lee, Y., Choi, U., Moeckel, G., and Karihaloo, A. (2015). Chemokine receptor Cxcr4 contributes to kidney fibrosis via multiple effectors. Am. J. physiology Ren. physiology 308 (5), F459–F472. doi:10.1152/ajprenal.00146.2014
Zambrano, S., Möller-Hackbarth, K., Li, X., Rodriguez, P. Q., Charrin, E., Schwarz, A., et al. (2019). GPRC5b modulates inflammatory response in glomerular diseases via NF-κB pathway. J. Am. Soc. Nephrol. JASN 30 (9), 1573–1586. doi:10.1681/asn.2019010089
Zhang, M., Chen, T., Lu, X., Lan, X., Chen, Z., and Lu, S. (2024). G protein-coupled receptors (GPCRs): advances in structures, mechanisms, and drug discovery. Signal Transduct. Target. Ther. 9 (1), 88. doi:10.1038/s41392-024-01803-6
Zhao, M., Liu, S., Luo, S., Wu, H., Tang, M., Cheng, W., et al. (2014). DNA methylation and mRNA and microRNA expression of SLE CD4+ T cells correlate with disease phenotype. J. Autoimmun. 54, 127–136. doi:10.1016/j.jaut.2014.07.002
Zhao, M., Wang, L., Wang, M., Zhou, S., Lu, Y., Cui, H., et al. (2022). Targeting fibrosis, mechanisms and cilinical trials. Signal Transduct. Target. Ther. 7 (1), 206. doi:10.1038/s41392-022-01070-3
Zheng, X., Asico, L. D., Ma, X., and Konkalmatt, P. R. (2019). G protein-coupled receptor 37L1 regulates renal sodium transport and blood pressure. Am. J. physiology Ren. physiology 316 (3), F506–F516. doi:10.1152/ajprenal.00289.2018
Zhou, L., Zhou, S., Yang, P., Tian, Y., Feng, Z., Xie, X. Q., et al. (2018). Targeted inhibition of the type 2 cannabinoid receptor is a novel approach to reduce renal fibrosis. Kidney Int. 94 (4), 756–772. doi:10.1016/j.kint.2018.05.023
Zhou, Z. F., Jiang, L., Zhao, Q., Wang, Y., Zhou, J., Chen, Q. K., et al. (2020). Roles of pattern recognition receptors in diabetic nephropathy. J. Zhejiang Univ. Sci. B 21 (3), 192–203. doi:10.1631/jzus.B1900490
Zhou, S., Ling, X., Liang, Y., Feng, Q., Xie, C., Li, J., et al. (2024). Cannabinoid receptor 2 plays a key role in renal fibrosis through inhibiting lipid metabolism in renal tubular cells. Metabolism Clin. Exp. 159, 155978. doi:10.1016/j.metabol.2024.155978
Zhu, X., Jiang, L., Long, M., Wei, X., Hou, Y., and Du, Y. (2021). Metabolic reprogramming and renal fibrosis. Front. Med. 8, 746920. doi:10.3389/fmed.2021.746920
Zhu, X., Qian, Y., Li, X., Xu, Z., Xia, R., Wang, N., et al. (2022a). Structural basis of adhesion GPCR GPR110 activation by stalk peptide and G-proteins coupling. Nat. Commun. 13 (1), 5513. doi:10.1038/s41467-022-33173-4
Zhu, Z., Hu, J., Chen, Z., Feng, J., Yang, X., Liang, W., et al. (2022b). Transition of acute kidney injury to chronic kidney disease: role of metabolic reprogramming. Metabolism Clin. Exp. 131, 155194. doi:10.1016/j.metabol.2022.155194
Zhu, Y., Tan, J., Wang, Y., Gong, Y., Zhang, X., Yuan, Z., et al. (2024). Atg5 deficiency in macrophages protects against kidney fibrosis via the CCR6-CCL20 axis. Cell Commun. Signal. CCS 22 (1), 223. doi:10.1186/s12964-024-01600-2
Zweemer, A. J., Toraskar, J., Heitman, L. H., and Ijzerman, A. P. (2014). Bias in chemokine receptor signalling. Trends Immunol. 35 (6), 243–252. doi:10.1016/j.it.2014.02.004
Glossary
RF Renal fibrosis
GPCRs G protein-coupled receptors
CKD Chronic kidney disease
ESRD End-stage renal disease
EMT Epithelial - Mesenchymal Transition
EndMT Endothelial - Mesenchymal Transition
ECM Extracellular matrix
ACEIs Angiotensin-converting enzyme inhibitors
ARBs Angiotensin II receptor blockers
MRAs Mineralocorticoid receptor antagonists
SGLT-2i Sodium-glucose co-transporter 2 inhibitors
ETR Endothelin receptor
TGF-β Transforming growth factor-β
GDP Guanosine diphosphate
GTP Guanosine triphosphate
AC Adenylate cyclase
PLCβ Phospholipase C-β
PIP2 Phosphatidylinositol-4,5-bisphosphate
IP3 Inositol 1,4,5-trisphosphate
DAG Diacylglycerol
PKC Protein kinase C
RGS Regulator of G protein Signaling
GAP GTPase-activating protein
ncRNAs non-coding RNAs
aGPCRs Adhesion GPCRs
DN Diabetic nephropathy
GBM Glomerular basement membrane
ARs adrenergic receptors
LPARs lysophosphatidic acid receptors
ETRs Endothelin receptors
ORs olfactory receptors
NHE3 Na+/H+ exchanger isoform 3
DRs Dopamine receptors
EPRs Prostaglandin receptors
ATRs Angiotensin Receptors
CCRs chemokine receptors
HN hypertensive nephropathy
EP2 Prostaglandin E2
ET-1 Endothelin-1
RAAS Renin-angiotensin-aldosterone system
ACKRs Atypical chemokine receptors
PARs Protease-activated receptors
CBRs Cannabinoid receptors
DAMPs damage-associated molecular patterns
oGPCRs orphan GPCRs
oxLDL oxidized LDL
S1P sphingosine-1-phosphate
FAO fatty acid β-oxidation
YAP Yes - associated protein
Keywords: GPCRs, renal fibrosis, signal transduction, drug development, phenotypic transformation
Citation: Wang H, Yang M, Liu X, Fan J and Wang C (2025) G protein-coupled receptor-mediated renal fibrosis: a key focus on kidney disease drug development. Front. Pharmacol. 16:1645888. doi: 10.3389/fphar.2025.1645888
Received: 12 June 2025; Accepted: 18 August 2025;
Published: 04 September 2025.
Edited by:
Duuamene Nyimanu, University of Kansas Medical Center, United StatesReviewed by:
Qinghe Meng, Upstate Medical University, United StatesGanesh Lahane, Birla Institute of Technology and Science, India
Copyright © 2025 Wang, Yang, Liu, Fan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junming Fan, anVubWluZ2ZhbkAxNjMuY29t; Can Wang, d2FuZ2NhbkBjZHV0Y20uZWR1LmNu
 Hui Wang
Hui Wang Mengfan Yang
Mengfan Yang Xiongfeng Liu
Xiongfeng Liu Junming Fan
Junming Fan Can Wang
Can Wang