- 1Institute of Islam Hadhari, Universiti Kebangsaan Malaysia, Bangi, Selangor, Malaysia
- 2Department of Physiology, Faculty of Medicine, Universiti Kebangsaan Malaysia, Cheras, Kuala Lumpur, Malaysia
- 3Centre of Shariah, Faculty of Islamic Studies, Universiti Kebangsaan Malaysia, Bangi, Selangor, Malaysia
Diabetes mellitus (DM) is a chronic metabolic disease that affects around 10.5% of adults worldwide. It leads to significant complications, including nephropathy, retinopathy and cardiovascular disease. Conventional treatments for DM often involve the long-term use of pharmacological agents, which can be costly and are associated with various side effects. Due to these challenges, there is growing interest in complementary treatments, particularly those derived from botanical drugs, to explore their potential antidiabetic properties. Gynura procumbens (Lour.) Merr. (GP) has been scientifically studied and shown to possess antioxidant properties that lead to a significant reduction in blood glucose levels and an improvement in lipid profile. The aim of this review is therefore to provide a detailed overview of the current state of knowledge on the antidiabetic potential of GP based on four in vitro studies and 12 in vivo studies. GP extract in concentrations between 50 mg and 3,000 mg shows promising potential as an antidiabetic agent, with some studies suggesting comparable efficacy to metformin in the treatment of diabetes. In addition, phytochemical studies of GP have revealed a diverse phytochemical metabolite, with a predominance of polyphenolic metabolites, especially phenolic acids and flavonoids, extracted from various solvents. However, the evidence remains mixed, as other studies have presented varying results on the efficacy of GP in the treatment of diabetes. This could be due to the lack of standardisation of the extract preparation, insufficient information on the bioactive metabolite responsible for the observed effects and the lack of clinical studies. Therefore, more comprehensive studies including clinical trials are needed to clarify the discrepancies in the findings and provide a clearer effect of GP in alleviating DM. With these improvements, GP could complement standard DM treatments and offer patients a safer, more holistic approach.
1 Introduction
Diabetes mellitus (DM) is a chronic metabolic disease characterised by persistent hyperglycaemia due to impaired insulin secretion, insulin action or both (Kharroubi and Darwish, 2015; Artasensi et al., 2020). It can be divided into type 1 DM (T1DM) and type 2 DM (T2DM). T1DM is characterised by an absolute insulin deficiency, which is often accompanied by symptoms such as thirst, weight loss and polyuria and accounts for 10% of DM cases (Sun et al., 2021). T2DM, on the other hand, is characterised by insulin resistance in the target tissue, relatively insufficient insulin secretion and resulting dysfunction of the β-cells, which often causes no symptoms (Nyenwe et al., 2011). The DM condition leads to long-term complications affecting various organs, including the eyes, kidneys, nerves, heart and blood vessels. There are several factors that can lead to DM, such as genetic inheritance, viral infections, unhealthy lifestyle and other physical or chemical damage that leads to the destruction of β-cells (Roglic, 2016).
The global prevalence of DM is increasing at an alarming rate and poses major challenges for healthcare systems worldwide (Hossain et al., 2024). A recent study highlights that the global prevalence of DM is estimated to reach 700 million people by 2045 if current trends continue, emphasising the urgent need for effective prevention and management strategies (Sun et al., 2022). Another study notes that the prevalence of DM is particularly high in low- and middle-income countries, where health systems are often less well equipped to cope with the disease burden (Dendup et al., 2018).
Currently, conventional treatments for DM mainly focus on controlling blood glucose levels through the use of medications such as insulin, metformin and other antidiabetic drugs (Weinberg Sibony et al., 2023). However, they often have side effects and do not address the multifactorial nature of the disease. For example, long-term use of certain medications can lead to gastrointestinal problems, weight gain and an increased risk of cardiovascular events (Shurrab and Arafa, 2020). In addition, these treatments do not adequately target the underlying causes of DM, such as insulin resistance and beta-cell dysfunction.
As a result, there is growing interest in alternative and complementary therapies, including herbal medicines, for the treatment of diabetes. Botanical drugs offer a holistic approach by targeting multiple metabolic pathways involved in the development of DM (Pang et al., 2019). They are often considered to have few side effects and may offer additional benefits such as antioxidant and anti-inflammatory effects (Adam et al., 2022). In addition, conventional antidiabetic drugs may be costly or insufficiently available in low- and middle-income countries. Botanical drug treatments, especially those grown locally, are often considered more accessible and affordable (Mohan et al., 2020; Chaachouay and Zidane, 2024). Furthermore, integrative medicine, which combines evidence-based complementary practises with conventional treatments, is becoming increasingly important in modern healthcare systems. This has led to increased research into the antidiabetic properties of various botanical drugs, which have shown promising results in both preclinical and clinical studies (Vivó-Barrachina et al., 2022).
Botanical drugs represent a vast and invaluable reservoir of bioactive metabolites, many of which have a wide range of pharmacological properties. Due to their diverse mechanisms of action and generally lower risk of adverse effects, they represent a promising avenue for research and hold significant potential for improving the comprehensive treatment of DM (Yedjou et al., 2023). Gynura procumbens (Lour.) Merr. (GP) is an herbal plant rich in bioactive metabolites that may provide therapeutic benefits for a number of diseases. It is a perennial evergreen shrub belonging to the Asteraceae family (Tan et al., 2016). It is called ‘Sambung Nyawa’ or Sabungai in Malay (Jobaer et al., 2023), ‘bai bing cha’ by Chinese and Malay communities (Murugaiyah et al., 2018), and ‘pyar-hmee’ in Myanmar (Aung et al., 2021). It is also known as ‘longevity spinach’ (Jobaer et al., 2023). This green vegetable is typically small-growing and reaches a height of around 1–3 m (Tan et al., 2016). It also has yellow, slender and panicle-shaped flower heads that are 1–1.5 cm long and ovate-elliptical or lanceolate leaves that are 3.5–8 cm long and 0.8–3.5 cm wide. This edible herbaceous plant is widely distributed in Malaysia, Indonesia, Thailand, Vietnam and China. In Thailand, the GP leaves are used as a popular vegetable for a variety of culinary creations and play an important role in dishes such as chilli paste, curries, salads and soups and can be enjoyed both raw and cooked (Kaewseejan et al., 2015). In Malaysia, it is often eaten uncooked with rice and used as an ingredient in salads and ulam (Hew and Gam, 2011).
This plant has long been used in traditional medicine for the treatment of diabetes, hyperlipidaemia, kidney disease, hypertension, fever, constipation, skin irritation, migraine, rheumatism, haemorrhoids and colon cancer (Zhang and Tan, 2000; Rosidah et al., 2009; Hassan et al., 2010; Mohamed et al., 2023). In addition, this plant has a variety of therapeutic properties, such as anti-hyperlipidaemic, anti-inflammatory, antibacterial, antifungal, antihypertensive, antioxidant and anticancer properties (Iskander et al., 2002; Kim et al., 2011; Algariri et al., 2013; Hew et al., 2013; Jarikasem et al., 2013; Jeon and Kwon, 2016). Since it is a natural-based product, it is a better option with minimal side effects compared to the commercially available synthetic drugs. The availability of GP at a reasonable price and easy accessibility makes it a significant alternative to modern medicine, which is important specifically for an underprivileged community. Furthermore, the increasing use of GP as a natural remedy has led to this plant having potential for commercial cultivation and processing of organic products due to the growing markets for herbal raw materials and processed products.
Therefore, this review aims to summarise the current information on the potential health benefits of GP in DM and its complications from animal models and in vitro studies. The traditional use of this plant in folk medicine combined with scientific validation emphasises its potential as a complementary therapy for the treatment of DM.
2 Nutritional and Phytochemistry of Gynura procumbens (Lour.) Merr
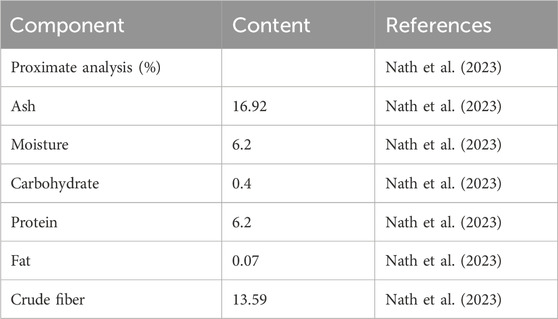
The nutritional analysis of GP leaves revealed that they contain 13.59% crude fibre and 16.92% ash. Its nutritional properties, especially the high fibre content and low glycaemic profile, increase the antidiabetic potential of GP and make it a promising candidate for dietary intervention for the prevention or treatment of T2DM (Nath et al., 2023). In addition, GP leaves contain a low composition of protein (6.2%), moisture (6.2%), carbohydrate (0.4%), and fat (0.07%) (Nath et al., 2023). This is important as it can help reduce the risk of various chronic diseases, such as type 2 diabetes by improving insulin sensitivity, regulating blood glucose levels, and reducing the risk of developing diabetes (Jobaer et al., 2023). The nutritional contents of the GP extract are listed in Table 1.
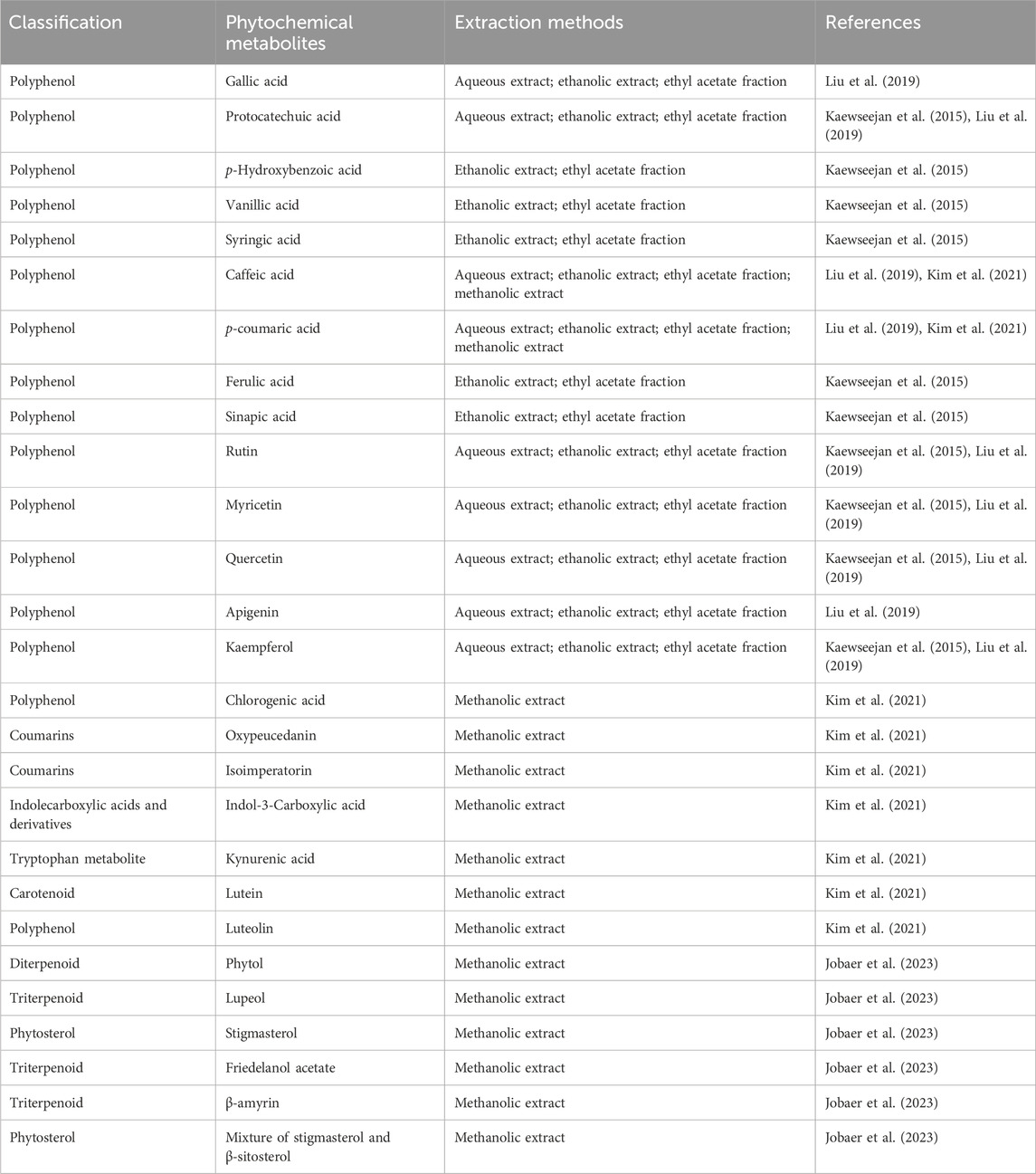
Meanwhile, phytochemical studies of GP have revealed a diverse phytochemical metabolite, with a predominance of polyphenolic metabolites, especially phenolic acids and flavonoids, extracted from various solvents. Of the phenolic acids, gallic acid, protocatechuic acid, p-hydroxybenzoic acid, vanillic acid and syringic acid, which belong to the hydroxybenzoic acid subclass were consistently detected. They can be detected in the aqueous, ethanolic and ethyl acetate fractions of the leaves (Kaewseejan et al., 2015; Liu et al., 2019). Caffeic acid, p-coumaric acid, ferulic acid and sinapic acid, which belong to the hydroxycinnamic acids, can be found in both polar (aqueous, methanol) and semi-polar (ethyl acetate) extracts, indicating their solubility in a range of solvents (Kaewseejan et al., 2015; Alam et al., 2016; Liu et al., 2019; Kim et al., 2021). In addition, phenolic acids have been shown to lower blood glucose levels and protect against chronic diseases caused by hyperglycaemia through antioxidant protection (Deka et al., 2022).
Important flavonoids such as rutin (a quercetin glycoside), quercetin, myrricetin, kaempferol, apigenin and luteolin have been found in several extracts, reflecting their richness and structural diversity within the plant matrix (Kaewseejan et al., 2015; Liu et al., 2019; Kim et al., 2021). These metabolites act on multiple diabetes targets and regulate key signalling pathways that improve both the symptoms and complications of T2DM (Dhanya, 2022). Furthermore, chlorogenic acid, a biologically active caffeoylquinic acid ester, was exclusively detected in the methanolic extract. This indicates its preferential solubility in more polar organic solvents (Kim et al., 2021).
In addition to polyphenols, the methanolic extract of GP also contained considerable amounts of non-phenolic bioactive metabolites, including coumarins such as oxypeucedanin and isoimperatorin, which are known for their anti-inflammatory and vasodilatory effects (Kim et al., 2021; Flores-Morales et al., 2023; Kubrak et al., 2025). Indole-derived compounds such as indole-3-carboxylic acid and kynurenic acid, a tryptophan metabolite with neuroprotective properties, have also been identified and broaden the pharmacological spectrum of the plant (Kim et al., 2021).
On the other hand, the methanolic extract of GP also contained numerous lipophilic metabolites. These include lutein, a potent antioxidant involved in chlorophyll biosynthesis and known for its antimicrobial properties. Several triterpenoids such as lupeol, β-amyrin and friedelanol acetate, which are associated with antidiabetic, anti-inflammatory, anticancer and hepatoprotective activities have also been detected in the methanolic extract of GP (Szakiel et al., 2012; Saha and Bandyopadhyay, 2020; Kim et al., 2021; Jobaer et al., 2023; Dalimunthe et al., 2024). In addition, phytosterols such as stigmasterol and a mixture of stigmasterol and β-sitosterol have also been identified, which are known for their cholesterol-lowering effect as well as their cytoprotective potential and reduced hyperglycaemic effects (Vezza et al., 2020; Jobaer et al., 2023). The antidiabetic effect of stigmasterol may be due to the regeneration of the pancreatic β-cells of Langerhans and thus the secretion of insulin, which controls blood glucose levels (Eidi et al., 2006; Nualkaew et al., 2015).
However, the non-specific bioactivity of natural products is often a hurdle in their bioassay evaluation, including GP (Baell, 2016). Some of its metabolites can behave as pan-assay interference compounds (PAINS), causing false positive signals in various assays and thus complicating their interpretation. For example, the pyrrolizidine alkaloids in GP are known to cause interference in a variety of bioassays due to their reactive nature and the formation of DNA adducts (Ji et al., 2019). Lupeol, stigmasterol and β-sitosterol can interfere with enzyme assays and receptor binding studies due to their structural similarity to endogenous steroids and thus bind non-selectively to other non-endogenous targets (Jobaer et al., 2023). In addition, polyphenolic metabolites such as chlorogenic acid, caffeic acid and various flavonoids could also interfere with redox-based assays (Kim et al., 2021). In addition, some pharmacological profiles of GP may also correspond to mechanisms typical of PAINS. For example, its anti-inflammatory activities have been shown to inhibit the NF-κB signalling pathway and downregulate the expression of pro-inflammatory cytokines such as IL-1β and TNF-α (Wong et al., 2015; Tan et al., 2022). These activities, which involve multiple signalling pathways, are often considered characteristic of PAINS.
Nonetheless, the potential for interference by PAINS does not undermine the medical value of GP. Evidence from a wide range of in vitro and in vivo studies, as well as from thousands of years of traditional use, has provided a solid basis for its efficacy and safety. This evaluation can be further enhanced by modern analytical and computational techniques. High-resolution techniques, in particular UHPLC-QTOF-MS/MS and HPLC-MS, and the use of computational tools such as molecular docking and molecular dynamics simulations enable the prediction of binding affinities and target interactions. These strategies are particularly useful for the identification of compounds with high promiscuity and thus potential PAINS properties (Tithi et al., 2023). By integrating such approaches, researchers can minimise concerns about assay interference and detect consistent, target-specific biological effects across multiple experimental platforms.
Table 2 provides an overview of the phytochemical metabolite found in GP.
3 Pharmacokinetics of Gynura procumbens (Lour.) Merr
The pharmacokinetics of GP have significant implications for its potential therapeutic use; however, they are far from clear. The antidiabetic effect of GP, which is attributed to increased glucose uptake and improved insulin sensitivity, may also be associated with metabolic interactions (Hassan et al., 2010; Guo W. et al., 2021). There are also scants in vivo reports on the absorption, distribution, metabolism and excretion profiles of GP and its active fractions. However, useful information can be extrapolated from the known phytochemical profile of this plant and pharmacokinetic studies on structurally related metabolites.
It has been hypothesised that the absorption of polyphenolic metabolites extracted from GP may be poor. The flavonoids and phenolic acids generally have low oral bioavailability due to a high degree of first-pass metabolism (Xin et al., 2011; Ye et al., 2023). However, there is evidence that the presence of other molecules in GP can increase its absorption. For example, chlorogenic acid (CA), a predominant phenolic metabolite of GP, was found to increase intestinal absorption and improve bioavailability when combined with other phytochemicals in plants, with the improvement increasing from 6.7% (CA alone) to 16.0% (Wang et al., 2022). Pharmacokinetic analyses of the total extract are also not available, and the distribution of GP metabolites in the body remains unknown. However, similar flavonoids and phenolic acids have been found to accumulate in metabolically active organs such as adipose tissue, intestine, liver, kidneys and lungs (Ye et al., 2023). These results are in line with previous studies that have shown that GP molecules are widely distributed in numerous tissues, possibly leading to hepatoprotective and nephroprotective effects (Tahsin et al., 2022).
In addition, the metabolites of GP are generally expected to undergo phase I and phase II metabolism, namely, oxidative, reductive, hydrolytic, glucuronidated and sulphated, and methyl-mediated metabolic reactions, which mainly occur in the liver and gastrointestinal tract (Xin et al., 2011; Wang et al., 2022; Ye et al., 2023). Flavonoids and phenolic acids from GP are also likely to be metabolised via these pathways, leading to the formation of conjugated metabolites that can be detected in plasma after oral administration. Such modulating effects may also contribute to its antidiabetic effect. Although the excretion patterns of GP metabolites are not well studied, it is assumed that, like other plant polyphenols, they are mainly excreted via the kidneys and bile (Ye et al., 2023).
Overall, the data available to date indicate that the biological effects of GP are significantly influenced by its low bioavailability, extensive metabolism and wide tissue distribution. Further studies are needed to clarify this involvement and to translate the molecular understanding to the clinical level of GP-targeted therapy.
4 Effects of Gynura procumbens (Lour.) Merr. on diabetes mellitus
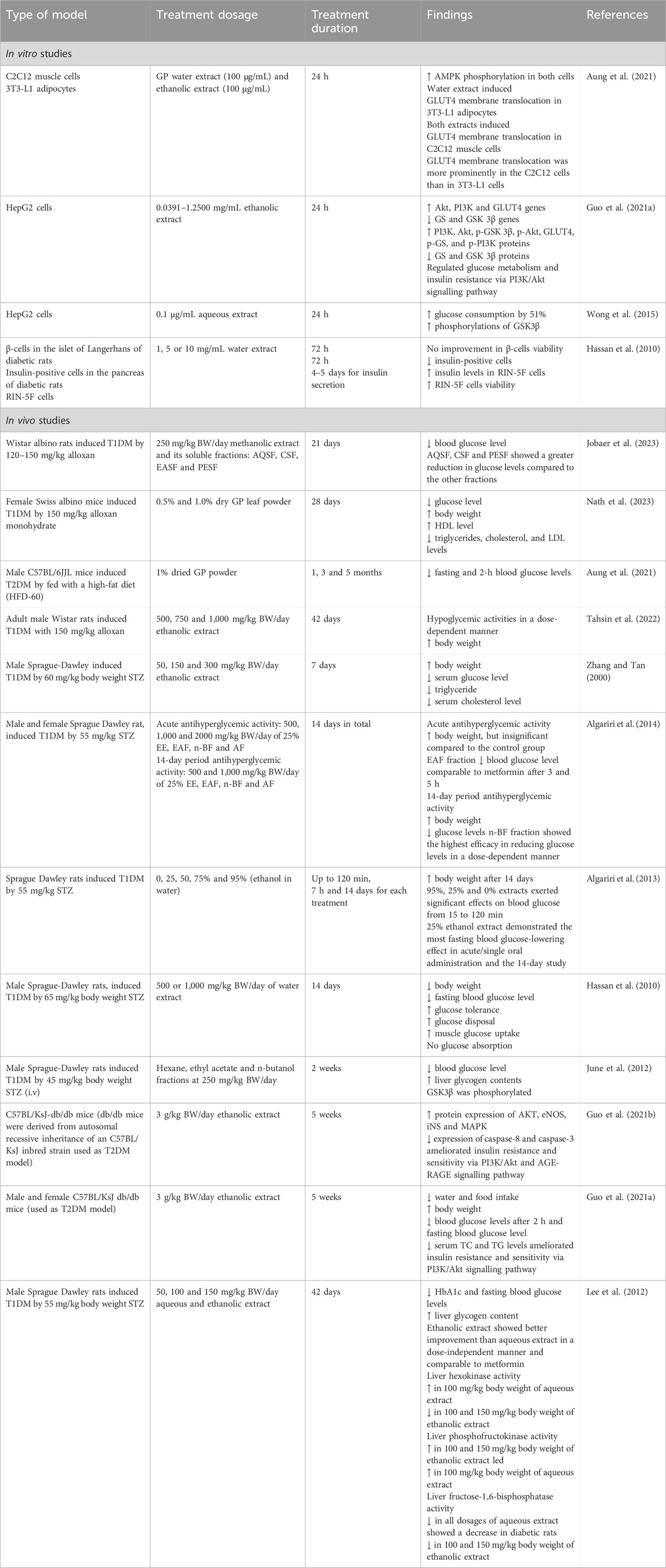
Previous studies have documented the potential antidiabetic effect of GP, which is characterised by a reduction in blood glucose levels and improved glucose tolerance in DM. A total of 16 studies were found including four in vitro studies and 12 in vivo studies.
4.1 In vitro studies
In vitro studies have elucidated the mechanisms underlying the antidiabetic properties of GP. The in vitro experiments revealed the mechanisms and signalling pathways at the molecular level involving a variety of cell types. For example, one study reported that the aqueous extract of GP triggered translocation of GLUT4 membrane into 3T3-L1 adipocytes, while both the aqueous and ethanolic extracts triggered translocation of GLUT4 membrane into C2C12 muscle cells, which is a critical site for insulin-stimulated glucose uptake (Merz and Thurmond, 2020; Aung et al., 2021). Interestingly, GP had a stronger effect on GLUT4 membrane translocation in C2C12 muscle cells compared to 3T3-L1 adipocyte cells. The observed effect could be due to the tissue-specific difference in sensitivity or responsiveness to the plant extract. The phosphorylation of AMP-activated protein kinase (AMPK), was also increased in both cells. AMPK is an important regulator of cellular energy homeostasis and a proven therapeutic target for metabolic disorders such as type 2 diabetes (Kim et al., 2016; Aydin et al., 2025). Activation of AMPK stimulates glucose uptake and fatty acid oxidation while inhibiting lipogenesis and gluconeogenesis, which is similar to the mechanism of action of metformin (Ke et al., 2018; O'Neill, 2013; Hasanvand, 2022).
Similarly, activation of the PI3K/Akt signalling pathway, a central pathway in the regulation of glucose homeostasis by the ethanolic extract of GP attenuates insulin resistance and restores glucose uptake (Guo S. et al., 2021; Fontana et al., 2024). Gene expression analysis revealed an upregulation of the Akt, PI3K and GLUT4 genes and a significant downregulation of the GS and GSK 3β genes. These gene findings were supported by protein analysis, which revealed higher levels of PI3K, Akt, p-GSK 3β, p-Akt, GLUT4, p-GS and p-PI3K in the GP-treated group compared to the control group. Conversely, GS and GSK 3β protein expression showed remarkable downregulation. The study showed a significant improvement in glucose content and glucose uptake after treatment with different concentrations of GP. This dose-dependent improvement emphasises the therapeutic potential of GP in improving insulin resistance and promoting effective glucose utilisation. Overall, these molecular and biochemical findings highlight the ability of the ethanolic extract of GP to modulate the PI3K/Akt signalling pathway and associated downstream targets, underpinning its role as a natural candidate for the treatment of insulin resistance and hyperglycaemia (Feng et al., 2024).
In addition, previous studies have shown that the antihyperglycaemic effects of GP may be mediated by the modulation of key enzymes involved in insulin signalling pathways, in particular glycogen synthase kinase three beta (GSK3β). Kaempferol, a bioactive flavonoid metabolites of GP, is thought to be involved in this mechanism and exerts an inhibitory effect on GSK3β activity (Wong et al., 2015; Yang et al., 2022). The study showed that HepG2 cells treated with the aqueous extract of GP exhibited increased phosphorylation of GSK3β (Ser9), which in turn led to an increase in glucose, while dysregulation of GSK3 activity is associated with insulin resistance and hyperglycaemia (Henriksen, 2010; Liu and Yao, 2016). GP was also found to increase glucose uptake in hyperglycaemic HepG2 cells in a concentration-dependent manner, highlighting its potential as a natural therapeutic agent to control elevated blood glucose levels.
In contrast, a study conducted with β-cells in the islets of Langerhans of T1DM rats showed no improvement in cell viability after administration of GP water extract (Hassan et al., 2010). However, remarkable changes were observed in the distribution pattern of insulin-positive cells in the pancreatic tissue of T1DM rats, with a significant decrease in the number of insulin-positive cells. This decrease is consistent with the autoimmune destruction of β-cells that characterises T1DM and suggests that GP extract has no protective or regenerative effects on pancreatic insulin-secreting cells (Roep et al., 2021; Atkinson and Mirmira, 2023). Treatment of RIN-5F cells, the cloned pancreatic β-cells, with different concentrations of GP water extract also did not lead to a significant increase in insulin levels or an improvement in cell viability. This indicates that the hypoglycaemic effect of the extract is not dependent on insulin secretion. The study concluded that the ability of GP to enhance or mimic insulin action at the cellular level may be related to the insulin-like properties of the active ingredient contained in the extract. Therefore, further studies are needed to identify the active ingredient in GP extract that may play a role in these insulin-like properties which could contribute to the development of new therapies to combat insulin resistance.
4.2 In vivo studies
Several in vivo studies have demonstrated the beneficial blood glucose-lowering effect of DM, which is consistent with traditional claims of its therapeutic use and indicates its potential for diabetes management. For example, repeated oral administration of 250 mg/kg body weight/day of the methanolic extract and its various soluble fractions (SF), including aqueous (AQSF), chloroform (CSF), ethyl acetate (EASF), and petroleum ether (PESF), was found to lower blood glucose levels in T1DM rats for 21 days. AQSF, CSF and PESF showed a greater decrease in blood glucose levels than the other fractions (Jobaer et al., 2023). Similarly, in a 28-day dietary experiment in T1DM rats, administration of dry GP leaf powder was shown to decrease blood glucose levels, increase body weight, decrease triglycerides, cholesterol and low-density lipoproteins (LDL), and increase high-density lipoproteins (HDL) (Nath et al., 2023). These changes reflect the lipid-lowering and cardioprotective potential of GP, which is particularly beneficial for diabetics, who are at increased risk of cardiovascular complications. Furthermore, in a T2DM model using eight-week-old male C57BL/6JJL mice fed a high-fat diet (HFD), administration of dried GP powder resulted in a significant reduction in fasting and 2-h blood glucose levels in HFD mice after 3 months and maintained this effect for up to 5 months (Aung et al., 2021).
A previous study has shown that administration of the ethanolic extract of GP at different doses (500, 750 and 1,000 mg/kg) over a treatment period of approximately 42 days led to an increase in body weight in rats with T1DM and showed dose-dependent hypoglycaemic effects (Tahsin et al., 2022). In rats receiving higher doses, the reduction in blood glucose levels was more pronounced, suggesting that the antihyperglycaemic effect of GP is concentration-dependent and possibly related to the better bioavailability of the active phytochemicals at higher doses (Alfahel et al., 2023). Similarly, repeated administration of 50, 150 and 300 mg/kg body weight of an ethanolic GP extract to T1DM rats over a 7-day period resulted in an increase in body weight, a decrease in serum total cholesterol levels and a decrease in triglycerides in these rats. This indicates that GP is able to improve glucose tolerance in STZ-induced T1DM rats but not in normal rats (Zhang and Tan, 2000). The improvement in glucose tolerance could be due to an improvement in insulin sensitivity or modulation of hepatic glucose production and the antidiabetic activity of GP could be more strongly activated under hyperglycaemic conditions. (Li et al., 2022).
The study on the effect of GP also shows acute antihyperglycaemic activity and 14-day antihyperglycaemic activity in T1DM rats. This study showed a significant reduction in blood glucose levels of 25% ethanol extract (EE) and all GP fractions (ethyl acetate fraction (EAF), n-butanol fraction (n-BF) and aqueous fraction (AF)), with the EAF significantly lowering blood glucose levels at 3 and 5 h (Algariri et al., 2014). Further analysis showed that the n-BF and AF fractions of GP exhibited antihyperglycaemic effects comparable to those of metformin, suggesting that it could affect glucose metabolism via similar pathways such as modulating AMPK activity, altering insulin signalling or increasing the expression of glucose transporters (Entezari et al., 2022; Lee et al., 2022). In addition, the 14-day antihyperglycaemic activity showed a significant reduction in blood glucose levels, with the n-BF fraction showing the most potent effect. The efficacy of this fraction could be related to the presence of flavonoids and other phytoconstituents known for their insulin-mimetic or insulin-sensitising properties (Vinayagam and Xu, 2015; Zanzabil et al., 2023).
Meanwhile, Hassan et al. (2010) showed a significant reduction in body weight and fasting blood glucose levels as well as improved glucose tolerance and marked improvement in glucose utilisation 15–120 min after glucose loading in T1DM rats after 14-day of administration of 500 or 1,000 mg/kg GP water extract (Hassan et al., 2010). In addition, the isolated abdominal muscle of T1DM rats showed a significant increase in glucose uptake. This finding suggests a peripheral mechanism of action likely mediated by increased glucose transporter activity or enhanced intracellular glucose metabolism in skeletal muscle, which plays an important role in glucose utilisation (Chang et al., 2004; Chadt and Al-Hasani, 2020). The same study also concluded that GP inhibits endogenous insulin production and does not stimulate insulin secretion. In another study using different fractions of GP (n-butanol, hexane and ethyl acetate), a significant decrease in blood glucose levels was observed in T1DM rats, with the ethyl acetate fraction showing the most significant effect compared to the other fractions (June et al., 2012). The study also found that GP inhibits GSK3β, suggesting that the hypoglycaemic effect of the GP fractions may be due to direct or indirect effects on the activities of one or more components upstream of the insulin signalling pathway.
GP has also been reported to have an insulinomimetic effect due to its high content of flavonoids and glycosides. GP has been found to inhibit gluconeogenesis in the liver, stimulate glycogenesis, stimulate hepatic glucose and lower hepatic endogenous glucose (Lee et al., 2012; Sok Yen et al., 2021). This contributes to better glycaemic control. In a study on the T1DM rat model, administration of ethanolic and aqueous extracts of GP led to an increase in liver glycogen content, but not to an increase in plasma insulin concentration (Lee et al., 2012). Interestingly, there was a decrease in fasting blood glucose and HbA1c levels. These findings suggest that the glucose-lowering and glycogen-promoting effect of GP is not mediated by increased insulin secretion, but may be due to insulin-independent mechanisms (Panahi et al., 2020). Thus, the ethanolic extract has the potential as an adjunct treatment in the treatment of DM due to its antidiabetic effect, which is comparable to that of metformin. However, further studies are needed to investigate the mechanism of action, long-term efficacy and safety profile.
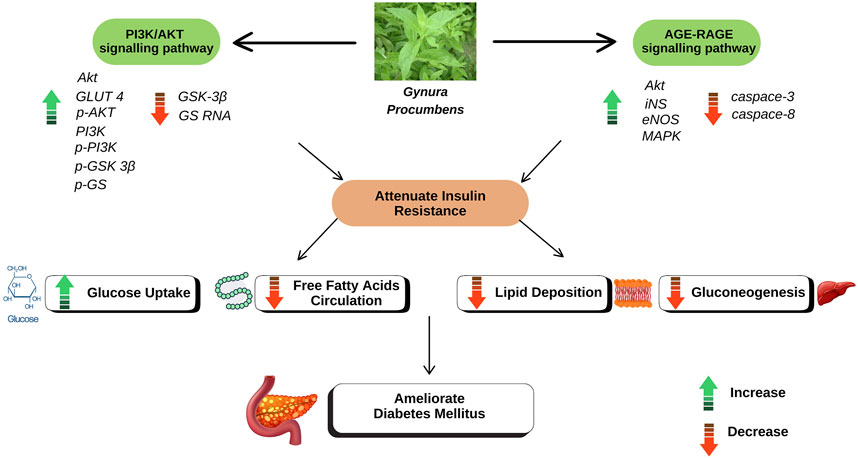
On the other hand, in-depth studies at the molecular level revealed several potential signalling pathways associated with the role of GP in alleviating T2DM. The ethanolic extract of GP was found to strongly affect key proteins and markers in the two disease-related protein signalling pathways, such as phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) and receptor for advanced glycation end products (AGE-RAGE) (Guo W. et al., 2021). Genetic analysis of the study using the T2DM rat model revealed that the genes for eNOS, AKT, MAPK and iNS were significantly upregulated in the GP group compared to the model group, while the genes for caspase-8 and caspase-3 were downregulated. This study also showed the involvement of two other metabolic pathways: retinol metabolism and glycerol phosphate metabolism. Further genetic and protein analyses in the T2DM rat model also showed the effect of the ethanolic extract of GP in upregulating GLUT4, Akt and PI3K genes by GP, along with downregulation of GS and GSK 3β genes (Guo S. et al., 2021). This suggest that GP promotes glycogen synthesis and facilitates glucose uptake via insulin-mimetic or insulin-sensitising mechanisms (El-Ashmawy et al., 2022).
In particular, RAGE, a molecule from the immunoglobulin superfamily, acts as a receptor for advanced glycation end products (AGEs) (Ong et al., 2018). AGEs are formed by non-enzymatic glycation and protein oxidation, especially in the presence of elevated blood glucose levels (Twarda-Clapa et al., 2022). Consequently, RAGE is considered a key player in the accumulation of various ligands in diabetic tissues (Ramasamy et al., 2011). In addition, PI3K/Akt also plays an important role in cell physiology by regulating the transmission of growth factor signals during important cellular processes and organismal growth, including glucose homeostasis and metabolism (Huang et al., 2018; Savova et al., 2023). The PI3K/AKT signalling pathway controls lipid and glucose metabolism under the guidance of insulin. Under normal circumstances, insulin is released immediately after food intake, which triggers activation of the PI3K/AKT signalling pathway. In T2DM, however, there is a reduced response to insulin which disrupts the activity of the PI3K/AKT signalling pathway. This can lead to reduced insulin secretion by the pancreas, impaired glucose utilisation, increased release of free fatty acids in adipose tissue, decreased lipid accumulation in the body, increased gluconeogenesis in the liver and muscles, and a loss of fine regulation of lipid and glucose metabolism (Huang et al., 2018; Petersen and Shulman, 2018; Feng et al., 2024). A summary of the antidiabetic properties of GP can be found in Table 3 and the proposed mechanism of action of GP is shown in Figure 1.
5 Safety of Gynura procumbens (Lour.) Merr
Preclinical studies have generally shown that GP is safe for consumption at various doses. For example, the study by Algariri et al. (2014) showed that the maximum dose of the extract of 2000 mg/kg in an acute toxicity test did not result in any treatment-related mortality during the 14-day observation period (Algariri et al., 2014). In addition, the acceptable daily intake (ADI) was set at 700 mg/kg/day. At the same time, the growth rate and indices of liver, kidney and haematopoietic function were also unaffected, indicating that the extract is safe and no acute toxicity was observed, as the LD50 for female rats is above 2000 mg/kg.
Furthermore, administration of 2 and 4 g/kg GP to rats in a 14-day toxicity trial did not cause any abnormalities in serum biochemical parameters (liver and kidney) or the structure of their organ tissues, with a zero-mortality rate even after the experimental period (Jabbar et al., 2023). The possibility of safe therapeutic use is supported by the absence of adverse effects on organ function, biochemical parameters and histological structures. These results may indicate that GP is a safe candidate of complementary therapy for the treatment of DM, as it has a wide safety margin. Despite the encouraging preclinical results, long-term toxicity, reproductive safety and human clinical studies are still scarce. To ensure the safety of the extract for long-term use in DM, these factors still need to be thoroughly investigated.
6 Future direction
Despite these encouraging data on the potential antidiabetic properties of GP, the evidence for its efficacy in the treatment of DM is still inconsistent. Future experimental research should focus on the testing of plant extracts for which comprehensive phytochemical fingerprints should be established according to the ConPhyMP guidelines to improve reproducibility and transparency in phytochemical pharmacology (Heinrich et al., 2022). This includes comprehensive taxonomic authentication using voucher specimens, comprehensive reporting of collection and extraction conditions, and the use of more than one orthogonal analytical technique, such as UHPLC-QTOF-MS/MS, HPLCMS, GCMS and HPTLC, to generate robust chemical fingerprints.
In addition, the lack of standardisation of the extract preparation can lead to different concentrations of the bioactive metabolites, which poses a major challenge for reproducibility and efficacy. For example, aqueous extracts can have different pharmacological effects compared to ethanolic extracts due to differences in solubility and stability of the active metabolites. Thus, standardising the extraction method and ensuring a consistent phytochemical profile is crucial to improve the reproducibility of results and demonstrate the clinical utility of GP in the treatment of DM.
In addition, the bioactive compound responsible for the observed effects was not clearly identified and quantified in some studies. The different study designs and the lack of clinical trials may also contribute to some inconsistencies and limit the generalisability of the results. To clarify these issues, more comprehensive studies are needed to identify the bioactive compounds and molecular targets as well as the mechanisms of action and to assess long-term safety. Potential risks that may be associated with prolonged use of GP, such as hepatotoxicity, nephrotoxicity and other systemic effects, also need to be carefully assessed. An understanding of the pharmacokinetics and pharmacodynamics of GP will also support its integration into current treatment regimens.
However, studies on the safety and efficacy, dose and long-term effects of GP in humans in clinical trials are very limited. Most current studies are conducted to investigate the underlying mechanisms and pharmacodynamics in controlled laboratory settings that have not yet been translated to the clinical. Accordingly, the lack of comprehensive clinical data on this product has greatly hindered its inclusion in evidence-based medical systems and practises. Therefore, clinical studies need to be conducted to assess safety, tolerability and dose in healthy humans. This will allow the identification of relevant biomarkers that should be useful not only for monitoring the efficacy of the therapy, but also for the early detection of signs of toxicity. These studies should also include long-term use to detect any chronic toxicities or late-onset adverse effects.
7 Conclusion
GP shows promising potential as an antidiabetic agent and has an effect comparable to metformin. By targeting the AGE-RAGE and PI3K/AKT signalling pathways, GP could help to reduce insulin resistance and increase insulin production. However, the evidence remains mixed, as other studies have presented varying results on the efficacy of GP in the treatment of diabetes. This could be due to the lack of standardisation of the extract preparation, insufficient information on the bioactive substance responsible for the observed effects and the lack of clinical studies. Therefore, more comprehensive studies including clinical trials are needed to clarify the discrepancies in the findings and provide a clearer effect of GP in alleviating DM. With these improvements, GP could complement standard DM treatments and offer patients a safer, more holistic approach.
Author contributions
NM: Writing – original draft, Visualization, Methodology, Conceptualization, Writing – review and editing. FJ: Writing – review and editing, Writing – original draft, Validation. MK: Validation, Writing – review and editing, Writing – original draft. MM: Visualization, Validation, Methodology, Project administration, Conceptualization, Writing – review and editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by an institutional grant from Universiti Kebangsaan Malaysia (UKM; FF-2023-061).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adam, S. H., Mohd Nasri, N., Kashim, M., Abd Latib, E. H., Ahmad Juhari, M. a.A., and Mokhtar, M. H. (2022). Potential health benefits of Nigella sativa on diabetes mellitus and its complications: a review from laboratory studies to clinical trials. Front. Nutr. 9, 1057825. doi:10.3389/fnut.2022.1057825
Alam, M. A., Subhan, N., Hossain, H., Hossain, M., Reza, H. M., Rahman, M. M., et al. (2016). Hydroxycinnamic acid derivatives: a potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. and Metabolism 13, 27. doi:10.1186/s12986-016-0080-3
Alfahel, R., Sawicki, T., Jabłońska, M., and Przybyłowicz, K. E. (2023). Anti-Hyperglycemic effects of bioactive compounds in the context of the prevention of diet-related diseases. Foods 12, 3698. doi:10.3390/foods12193698
Algariri, K., Meng, K. Y., Atangwho, I. J., Asmawi, M. Z., Sadikun, A., Murugaiyah, V., et al. (2013). Hypoglycemic and anti-hyperglycemic study of Gynura procumbens leaf extracts. Asian Pac J. Trop. Biomed. 3, 358–366. doi:10.1016/S2221-1691(13)60077-5
Algariri, K., Atangwho, I. J., Meng, K. Y., Asmawi, M. Z., Sadikun, A., and Murugaiyah, V. (2014). Antihyperglycaemic and toxicological evaluations of extract and fractions of Gynura procumbens leaves. Trop. Life Sci. Res. 25, 75–93.
Artasensi, A., Pedretti, A., Vistoli, G., and Fumagalli, L. (2020). Type 2 diabetes mellitus: a review of Multi-target drugs. Molecules 25, 1987. doi:10.3390/molecules25081987
Atkinson, M. A., and Mirmira, R. G. (2023). The pathogenic “symphony” in type 1 diabetes: a disorder of the immune system, β cells, and exocrine pancreas. Cell Metab. 35, 1500–1518. doi:10.1016/j.cmet.2023.06.018
Aung, C. L., Kawakami, F., Imai, M., Lwin, T.-T., Kanzaki, M., Mar, O., et al. (2021). Blood glucose-lowering effect of water and ethanolic extracts of Gynura procumbens (Lour.) Merr. Traditional and Kampo Med. 8, 138–147. doi:10.1002/tkm2.1277
Aydin, S., Tekinalp, S. G., Tuzcu, B., Cam, F., Sevik, M. O., Tatar, E., et al. (2025). The role of AMP-activated protein kinase activators on energy balance and cellular metabolism in type 2 diabetes mellitus. Obes. Med. 53, 100577. doi:10.1016/j.obmed.2024.100577
Baell, J. B. (2016). Feeling Nature's PAINS: natural products, natural product drugs, and Pan assay interference compounds (PAINS). J. Nat. Prod. 79, 616–628. doi:10.1021/acs.jnatprod.5b00947
Chaachouay, N., and Zidane, L. (2024). Plant-derived natural products: a source for drug Discovery and development. Drugs Drug Candidates 3, 184–207. doi:10.3390/ddc3010011
Chadt, A., and Al-Hasani, H. (2020). Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflügers Archiv - Eur. J. Physiology 472, 1273–1298. doi:10.1007/s00424-020-02417-x
Chang, L., Chiang, S.-H., and Saltiel, A. R. (2004). Insulin signaling and the regulation of glucose Transport. Mol. Med. 10, 65–71. doi:10.2119/2005-00029.Saltiel
Dalimunthe, A., Carensia Gunawan, M., Dhiya Utari, Z., Dinata, M. R., Halim, P., Estherina, S. P. N., et al. (2024). In-depth analysis of lupeol: delving into the diverse pharmacological profile. Front. Pharmacol. 15, 1461478. doi:10.3389/fphar.2024.1461478
Deka, H., Choudhury, A., and Dey, B. K. (2022). An overview on plant derived phenolic compounds and their role in treatment and management of diabetes. J. Pharmacopuncture 25, 199–208. doi:10.3831/KPI.2022.25.3.199
Dendup, T., Feng, X., Clingan, S., and Astell-Burt, T. (2018). Environmental risk factors for developing type 2 diabetes mellitus: a Systematic review. Int. J. Environ. Res. Public Health 15, 78. doi:10.3390/ijerph15010078
Dhanya, R. (2022). Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. and Pharmacother. 146, 112560. doi:10.1016/j.biopha.2021.112560
Eidi, A., Eidi, M., and Esmaeili, E. (2006). Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine 13, 624–629. doi:10.1016/j.phymed.2005.09.010
El-Ashmawy, N. E., Khedr, E. G., Alfeky, N. H., and Ibrahim, A. O. (2022). Upregulation of GLUT4 and PI3K, and downregulation of GSK3 mediate the anti-hyperglycemic effects of proanthocyanidins. Med. Int. (Lond) 2, 14. doi:10.3892/mi.2022.39
Entezari, M., Hashemi, D., Taheriazam, A., Zabolian, A., Mohammadi, S., Fakhri, F., et al. (2022). AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: a pre-clinical and clinical investigation. Biomed. and Pharmacother. 146, 112563. doi:10.1016/j.biopha.2021.112563
Feng, Y., Ren, Y., Zhang, X., Yang, S., Jiao, Q., Li, Q., et al. (2024). Metabolites of traditional Chinese medicine targeting PI3K/AKT signaling pathway for hypoglycemic effect in type 2 diabetes. Front. Pharmacol. 15, 1373711. doi:10.3389/fphar.2024.1373711
Flores-Morales, V., Villasana-Ruíz, A. P., Garza-Veloz, I., González-Delgado, S., and Martinez-Fierro, M. L. (2023). Therapeutic effects of coumarins with different Substitution patterns. Molecules 28, 2413. doi:10.3390/molecules28052413
Fontana, F., Giannitti, G., Marchesi, S., and Limonta, P. (2024). The PI3K/Akt pathway and glucose metabolism: a Dangerous Liaison in cancer. Int. J. Biol. Sci. 20, 3113–3125. doi:10.7150/ijbs.89942
Guo, S., Ouyang, H., Du, W., Li, J., Liu, M., Yang, S., et al. (2021a). Exploring the protective effect of Gynura procumbens against type 2 diabetes mellitus by network pharmacology and validation in C57BL/KsJ db/db mice. Food Funct. 12, 1732–1744. doi:10.1039/d0fo01188f
Guo, W., Ouyang, H., Liu, M., Wu, J., He, X., Yang, S., et al. (2021b). Based on plasma Metabonomics and network pharmacology exploring the therapeutic mechanism of Gynura procumbens on type 2 diabetes. Front. Pharmacol. 12, 674379. doi:10.3389/fphar.2021.674379
Hasanvand, A. (2022). The role of AMPK-dependent pathways in cellular and molecular mechanisms of metformin: a new perspective for treatment and prevention of diseases. Inflammopharmacology 30, 775–788. doi:10.1007/s10787-022-00980-6
Hassan, Z., Yam, M. F., Ahmad, M., and Yusof, A. P. (2010). Antidiabetic properties and mechanism of action of Gynura procumbens water extract in streptozotocin-induced diabetic rats. Molecules 15, 9008–9023. doi:10.3390/molecules15129008
Heinrich, M., Jalil, B., Abdel-Tawab, M., Echeverria, J., Kulić, Ž., Mcgaw, L. J., et al. (2022). Best Practice in the chemical characterisation of extracts used in pharmacological and toxicological research-The ConPhyMP-Guidelines. Front. Pharmacol. 13, 953205. doi:10.3389/fphar.2022.953205
Henriksen, E. J. (2010). Dysregulation of glycogen synthase kinase-3 in skeletal muscle and the etiology of insulin resistance and type 2 diabetes. Curr. Diabetes Rev. 6, 285–293. doi:10.2174/157339910793360888
Hew, C. S., and Gam, L. H. (2011). Proteome analysis of abundant proteins extracted from the leaf of Gynura procumbens (Lour.) Merr. Appl. Biochem. Biotechnol. 165, 1577–1586. doi:10.1007/s12010-011-9377-x
Hew, C. S., Khoo, B. Y., and Gam, L. H. (2013). The anti-cancer property of proteins extracted from Gynura procumbens (Lour.) Merr. PLoS One 8, e68524. doi:10.1371/journal.pone.0068524
Hossain, M. J., Al-Mamun, M., and Islam, M. R. (2024). Diabetes mellitus, the fastest growing global public health concern: early detection should be focused. Health Sci. Rep. 7, e2004. doi:10.1002/hsr2.2004
Huang, X., Liu, G., Guo, J., and Su, Z. (2018). The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 14, 1483–1496. doi:10.7150/ijbs.27173
Iskander, M. N., Song, Y., Coupar, I. M., and Jiratchariyakul, W. (2002). Antiinflammatory screening of the medicinal plant Gynura procumbens. Plant Foods Hum. Nutr. 57, 233–244. doi:10.1023/a:1021851230890
Jabbar, A. a.J., Alamri, Z. Z., Abdulla, M. A., Salehen, N. A., Salim Amur Al Sinawi, Z., and Alfaifi, S. M. (2023). Hepatoprotective effects of Gynura procumbens against thioacetamide-induced cirrhosis in rats: targeting inflammatory and oxidative stress signalling pathways. Heliyon 9, e19418. doi:10.1016/j.heliyon.2023.e19418
Jarikasem, S., Charuwichitratana, S., Siritantikorn, S., Chantratita, W., Iskander, M., Frahm, A. W., et al. (2013). Antiherpetic effects of Gynura procumbens. Evid. Based Complement. Altern. Med. 2013, 394865. doi:10.1155/2013/394865
Jeon, H.-J., and Kwon, H.-J. (2016). Anti-inflammation effect of Gynura procumbens extract. J. Digital Convergence 14, 515–520. doi:10.14400/jdc.2016.14.10.515
Ji, Y.-B., Wang, Y.-S., Fu, T.-T., Ma, S.-Q., Qi, Y.-D., Si, J.-Y., et al. (2019). Quantitative analysis of pyrrolizidine alkaloids in Gynura procumbens by liquid chromatography–tandem quadrupole mass spectrometry after enrichment by PCX solid-phase extraction. Int. J. Environ. Anal. Chem. 99, 1090–1102. doi:10.1080/03067319.2019.1616705
Jobaer, M. A., Ashrafi, S., Ahsan, M., Hasan, C. M., Rashid, M. A., Islam, S. N., et al. (2023). Phytochemical and biological investigation of an Indigenous plant of Bangladesh, Gynura procumbens (Lour.) Merr.: drug Discovery from nature. Molecules 28, 4186. doi:10.3390/molecules28104186
June, C., Lee, H. W., Sani, H., Latip, J., Jualang Azlan, G., Lee, P.-C., et al. (2012). Hypoglycemic effects of Gynura procumbens fractions on streptozotocin-induced diabetic rats involved phosphorylation of GSK3b (Ser-9) in liver. Sains Malays. 41, 969–975.
Kaewseejan, N., Sutthikhum, V., and Siriamornpun, S. (2015). Potential of Gynura procumbens leaves as source of flavonoid-enriched fractions with enhanced antioxidant capacity. J. Funct. Foods 12, 120–128. doi:10.1016/j.jff.2014.11.001
Ke, R., Xu, Q., Li, C., Luo, L., and Huang, D. (2018). Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism. Cell Biol. Int. 42, 384–392. doi:10.1002/cbin.10915
Kharroubi, A. T., and Darwish, H. M. (2015). Diabetes mellitus: the epidemic of the century. World J. Diabetes 6, 850–867. doi:10.4239/wjd.v6.i6.850
Kim, J., Lee, C. W., Kim, E. K., Lee, S. J., Park, N. H., Kim, H. S., et al. (2011). Inhibition effect of Gynura procumbens extract on UV-B-induced matrix-metalloproteinase expression in human dermal fibroblasts. J. Ethnopharmacol. 137, 427–433. doi:10.1016/j.jep.2011.04.072
Kim, J., Yang, G., Kim, Y., Kim, J., and Ha, J. (2016). AMPK activators: mechanisms of action and physiological activities. Exp. and Mol. Med. 48, e224. doi:10.1038/emm.2016.16
Kim, H. H., Ha, S. E., Vetrivel, P., Bhosale, P. B., Kim, S. M., and Kim, G. S. (2021). Potential antioxidant and anti-inflammatory function of Gynura procumbens polyphenols ligand. Int. J. Mol. Sci. 22, 8716. doi:10.3390/ijms22168716
Kubrak, T. P., Makuch-Kocka, A., and Aebisher, D. (2025). Coumarins in anticancer therapy: mechanisms of action, potential Applications and research Perspectives. Pharmaceutics 17, 595. doi:10.3390/pharmaceutics17050595
Lee, H. W., Hakim, P., Rabu, A., and Sani, H. (2012). Antidiabetic effect of Gynura procumbens leaves extracts involve modulation of hepatic carbohydrate metabolism in streptozotocin-induced diabetic rats. J. Med. plant Res. 6, 796–812. doi:10.5897/jmpr11.1466
Lee, S. H., Park, S. Y., and Choi, C. S. (2022). Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab. J. 46, 15–37. doi:10.4093/dmj.2021.0280
Li, M., Chi, X., Wang, Y., Setrerrahmane, S., Xie, W., and Xu, H. (2022). Trends in insulin resistance: insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 7, 216. doi:10.1038/s41392-022-01073-0
Liu, X., and Yao, Z. (2016). Chronic over-nutrition and dysregulation of GSK3 in diseases. Nutr. and Metabolism 13, 49. doi:10.1186/s12986-016-0108-8
Liu, Y.-Y., You, J.-J., Xu, W., Zhai, T., Du, C.-Y., Chen, Y., et al. (2019). Gynura procumbens aqueous extract alleviates nonalcoholic steatohepatitis through CFLAR-JNK pathway in vivo and in vitro. Chin. Herb. Med. 11, 369–378. doi:10.1016/j.chmed.2019.09.005
Merz, K. E., and Thurmond, D. C. (2020). Role of skeletal muscle in insulin resistance and glucose uptake. Compr. Physiol. 10, 785–809. doi:10.1002/cphy.c190029
Mohamed, R., Lim, V., and Aziz, M. Y. (2023). RECENT UPDATE ON ANTI-CANCER ACTIVITY OF GYNURA PROCUMBENS (LOUR.) MERR. J. Health Transl. Med. sp2023, 66–73. doi:10.22452/jummec.sp2023no1.6
Mohan, V., Khunti, K., Chan, S. P., Filho, F. F., Tran, N. Q., Ramaiya, K., et al. (2020). Management of type 2 diabetes in developing countries: Balancing Optimal glycaemic control and Outcomes with Affordability and accessibility to treatment. Diabetes Ther. 11, 15–35. doi:10.1007/s13300-019-00733-9
Murugaiyah, V., Saeed, M., Yow Meng, K., Murugesu, K., Parasuraman, S., Abdullah, M., et al. (2018). Lipid-lowering effect of hydroalcoholic extracts of Gynura procumbens in Chemical-A nd High-fat diet-induced hyperlipidemic rats. Pharmacogn. Mag. 14, 184. doi:10.4103/pm.pm_451_17
Nath, M., Adhikary, K., Ahamed, M. T., Devnath, H., and Islam, M. M. (2023). Effects of Gynura procumbens leaf-based meal on glucose level, lipid profile and mineral content of alloxan-induced diabetic mice. Food Res. 7, 332–340. doi:10.26656/fr.2017.7(2).818
Nualkaew, S., Padee, P., and Chusri, T. (2015). Hypoglycemic activity in diabetic rats of stigmasterol and sitosterol-3-O--D-glucopyranoside isolated from Pseuderanthemum palatiferum (Nees) Radlk. leaf extract. J. Med. Plants Res. 9, 629–635. doi:10.5897/jmpr2014.5722
Nyenwe, E. A., Jerkins, T. W., Umpierrez, G. E., and Kitabchi, A. E. (2011). Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism 60, 1–23. doi:10.1016/j.metabol.2010.09.010
O'neill, H. M. (2013). AMPK and Exercise: glucose uptake and insulin sensitivity. Diabetes Metab. J. 37, 1–21. doi:10.4093/dmj.2013.37.1.1
Ong, S. B., Hernández-Reséndiz, S., Crespo-Avilan, G. E., Mukhametshina, R. T., Kwek, X. Y., Cabrera-Fuentes, H. A., et al. (2018). Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 186, 73–87. doi:10.1016/j.pharmthera.2018.01.001
Panahi, M., Rodriguez, P. R., Fereshtehnejad, S. M., Arafa, D., Bogdanovic, N., Winblad, B., et al. (2020). Insulin-independent and dependent glucose transporters in Brain Mural cells in CADASIL. Front. Genet. 11, 1022. doi:10.3389/fgene.2020.01022
Pang, G. M., Li, F. X., Yan, Y., Zhang, Y., Kong, L. L., Zhu, P., et al. (2019). Herbal medicine in the treatment of patients with type 2 diabetes mellitus. Chin. Med. J. Engl. 132, 78–85. doi:10.1097/CM9.0000000000000006
Petersen, M. C., and Shulman, G. I. (2018). Mechanisms of insulin action and insulin resistance. Physiol. Rev. 98, 2133–2223. doi:10.1152/physrev.00063.2017
Ramasamy, R., Yan, S. F., and Schmidt, A. M. (2011). Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann. N. Y. Acad. Sci. 1243, 88–102. doi:10.1111/j.1749-6632.2011.06320.x
Roep, B. O., Thomaidou, S., Van Tienhoven, R., and Zaldumbide, A. (2021). Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 17, 150–161. doi:10.1038/s41574-020-00443-4
Rosidah, M., Yam, M. F., Sadikun, A., Ahmad, M., Akowuah, G. A., and Asmawi, M. Z. (2009). Toxicology evaluation of standardized methanol extract of Gynura procumbens. J. Ethnopharmacol. 123, 244–249. doi:10.1016/j.jep.2009.03.011
Saha, M., and Bandyopadhyay, P. K. (2020). In vivo and in vitro antimicrobial activity of phytol, a diterpene molecule, isolated and characterized from Adhatoda vasica Nees. (Acanthaceae), to control severe bacterial disease of ornamental fish, Carassius auratus, caused by Bacillus licheniformis PKBMS16. Microb. Pathog. 141, 103977. doi:10.1016/j.micpath.2020.103977
Savova, M. S., Mihaylova, L. V., Tews, D., Wabitsch, M., and Georgiev, M. I. (2023). Targeting PI3K/AKT signaling pathway in obesity. Biomed. and Pharmacother. 159, 114244. doi:10.1016/j.biopha.2023.114244
Shurrab, N. T., and Arafa, E.-S. A. (2020). Metformin: a review of its therapeutic efficacy and adverse effects. Obes. Med. 17, 100186. doi:10.1016/j.obmed.2020.100186
Sok Yen, F., Shu Qin, C., Tan Shi Xuan, S., Jia Ying, P., Yi Le, H., Darmarajan, T., et al. (2021). Hypoglycemic effects of plant flavonoids: a review. Evidence-Based Complementary Altern. Med. 2021, 2057333. doi:10.1155/2021/2057333
Sun, Y., Tao, Q., Wu, X., Zhang, L., Liu, Q., and Wang, L. (2021). The utility of Exosomes in Diagnosis and therapy of diabetes mellitus and associated complications. Front. Endocrinol. (Lausanne) 12, 756581. doi:10.3389/fendo.2021.756581
Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., et al. (2022). IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. doi:10.1016/j.diabres.2021.109119
Szakiel, A., Pączkowski, C., Pensec, F., and Bertsch, C. (2012). Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem. Rev. 11, 263–284. doi:10.1007/s11101-012-9241-9
Tahsin, M. R., Tithi, T. I., Mim, S. R., Haque, E., Sultana, A., Bahar, N. B., et al. (2022). In vivo and in silico Assessment of diabetes ameliorating Potentiality and safety profile of Gynura procumbens leaves. Evidence-Based Complementary Altern. Med. 2022, 9095504. doi:10.1155/2022/9095504
Tan, H. L., Chan, K. G., Pusparajah, P., Lee, L. H., and Goh, B. H. (2016). Gynura procumbens: an overview of the biological activities. Front. Pharmacol. 7, 52. doi:10.3389/fphar.2016.00052
Tan, J. N., Husain, K., Jubri, Z., Chan, K. M., Jantan, I., and Mohd Fauzi, N. (2022). Gynura procumbens (Lour.) Merr. extract attenuates monocyte adherence to endothelial cells through suppression of the NF-κB signaling pathway. J. Ethnopharmacol. 294, 115391. doi:10.1016/j.jep.2022.115391
Tithi, T. I., Tahsin, M. R., Anjum, J., Zaman, T. S., Aktar, F., Bahar, N. B., et al. (2023). An in vivo and in silico evaluation of the hepatoprotective potential of Gynura procumbens: a promising agent for combating hepatotoxicity. PLoS One 18, e0291125. doi:10.1371/journal.pone.0291125
Twarda-Clapa, A., Olczak, A., Białkowska, A. M., and Koziołkiewicz, M. (2022). Advanced glycation end-products (AGEs): formation, Chemistry, Classification, receptors, and diseases related to AGEs. Cells 11, 1312. doi:10.3390/cells11081312
Vezza, T., Canet, F., De Marañón, A. M., Bañuls, C., Rocha, M., and Víctor, V. M. (2020). Microbiota-mitochondria Inter-Talk: a potential therapeutic strategy in obesity and type 2 diabetes. Antioxidants (Basel) 9, 848. doi:10.3390/antiox9090848
Vinayagam, R., and Xu, B. (2015). Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr. and Metabolism 12, 60. doi:10.1186/s12986-015-0057-7
Vivó-Barrachina, L., Rojas-Chacón, M. J., Navarro-Salazar, R., Belda-Sanchis, V., Pérez-Murillo, J., Peiró-Puig, A., et al. (2022). The role of natural products on diabetes mellitus treatment: a Systematic review of Randomized controlled trials. Pharmaceutics 14, 101. doi:10.3390/pharmaceutics14010101
Wang, L., Li, J., and Di, L.-J. (2022). Glycogen synthesis and beyond, a comprehensive review of GSK3 as a key regulator of metabolic pathways and a therapeutic target for treating metabolic diseases. Med. Res. Rev. 42, 946–982. doi:10.1002/med.21867
Weinberg Sibony, R., Segev, O., Dor, S., and Raz, I. (2023). Drug therapies for diabetes. Int. J. Mol. Sci. 24, 17147. doi:10.3390/ijms242417147
Wong, S. K., Hassan, W., Sudi, S., Lee, P.-C., Embi, N., Sidek, H., et al. (2015). Anti-malarial and anti-inflammatory effects of Gynura procumbens are mediated by kaempferol via inhibition of glycogen synthase kinase-3β (GSK3β). Sains Malays. 44, 1489–1500. doi:10.17576/jsm-2015-4410-15
Xin, G. Z., Qi, L. W., Shi, Z. Q., Li, P., Hao, H. P., Wang, G. J., et al. (2011). Strategies for integral metabolism profile of multiple compounds in herbal medicines: pharmacokinetics, metabolites characterization and metabolic interactions. Curr. Drug Metab. 12, 809–817. doi:10.2174/138920011797470164
Yang, Y., Chen, Z., Zhao, X., Xie, H., Du, L., Gao, H., et al. (2022). Mechanisms of Kaempferol in the treatment of diabetes: a comprehensive and latest review. Front. Endocrinol. (Lausanne) 13, 990299. doi:10.3389/fendo.2022.990299
Ye, L., Cheng, L., Deng, Y., Wang, S., Wu, X., Ou, S., et al. (2023). Absorption, tissue distribution, and excretion of glycycoumarin, a major bioactive coumarin from Chinese licorice (Glycyrrhiza uralensis Fisch). Front. Pharmacol. 14, 1216985. doi:10.3389/fphar.2023.1216985
Yedjou, C. G., Grigsby, J., Mbemi, A., Nelson, D., Mildort, B., Latinwo, L., et al. (2023). The management of diabetes mellitus using medicinal plants and Vitamins. Int. J. Mol. Sci. 24, 9085. doi:10.3390/ijms24109085
Zanzabil, K. Z., Hossain, M. S., and Hasan, M. K. (2023). Diabetes mellitus management: an extensive review of 37 medicinal plants. Diabetology 4, 186–234. doi:10.3390/diabetology4020019
Keywords: diabetes mellitus, antidiabetic plant, Gynura procumbens, hypoglycaemic, antioxidative, phytochemical
Citation: Mohd Nor NH, Jaffar FHF, Kashim MIAM and Mokhtar MH (2025) Antidiabetic potential of Gynura procumbens (Lour.) Merr.: a review of in vitro and in vivo studies. Front. Pharmacol. 16:1646591. doi: 10.3389/fphar.2025.1646591
Received: 13 June 2025; Accepted: 04 August 2025;
Published: 14 August 2025.
Edited by:
Michael Heinrich, University College London, United KingdomReviewed by:
Lucia Guerrero-Becerra, Autonomous University of Queretaro, MexicoMohamed Elbakry, Tanta University, Egypt
Copyright © 2025 Mohd Nor, Jaffar, Kashim and Mokhtar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohd Helmy Mokhtar, aGVsbXlAdWttLmVkdS5teQ==
 Nurul Hafizah Mohd Nor
Nurul Hafizah Mohd Nor Farah Hanan Fathihah Jaffar
Farah Hanan Fathihah Jaffar Mohd Izhar Ariff Mohd Kashim
Mohd Izhar Ariff Mohd Kashim Mohd Helmy Mokhtar
Mohd Helmy Mokhtar