- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 2College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 3Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan
- 4Division of Nephrology, Department of Internal Medicine, Chi Mei Medical Center, Tainan, Taiwan
- 5Department of Health and Nutrition, Chia Nan University of Pharmacy and Science, Tainan, Taiwan
- 6Division of Endocrinology and Metabolism, Department of Internal Medicine, New Taipei Municipal TuCheng Hospital (built and operated by Chang Gung Medical Foundation), New Taipei City, Taiwan
Background: Renin-angiotensin system inhibitors (RASi), comprising angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB) are known for cardio- and renoprotection. However, there is uncertainty regarding the continuation of ACEi or ARB treatment in patients with chronic kidney disease (CKD) stages 3–5.
Methods: In this meta-analysis, we systematically searched all relevant studies published in PubMed, Embase, and the Cochrane Library up to 30 May 2024. Our objective was to assess the impacts of continuation or discontinuation of RASi in patients with CKD stages 3–5 on all-cause mortality, end-stage kidney disease, major adverse cardiovascular events (MACE), and hyperkalemia. We rated the certainty of the evidence using the Cochrane methods and the GRADE approach.
Results: The search identified 520 studies, of which 8 studies, encompassing a total of 243,775 patients, were included in the analysis. The incidence of all-cause mortality was 40.3% (29,993 out of 74,447 patients), while ESKD occurred in 27.9% (8,992 out of 32,191 patients), MACE in 37.3% (11,225 out of 30,059 patients), and hyperkalemia in 39.4% (8,533 out of 21,642 patients). Pooled analysis revealed that patients who discontinued RASi therapy had a higher risk of developing ESKD compared to those who continued treatment [Hazard ratio (HR): 1.40, 95% confidence interval (CI): 1.19–1.65, P < 0.001], but a lower risk of hyperkalemia [Odds ratio (OR): 0.68, 95% CI: 0.60–0.77, P < 0.001]. There were no significant differences between the groups in all-cause mortality (HR: 1.34, 95% CI: 0.91–1.95, P = 0.135) and MACE (OR: 1.27, 95% CI: 0.93–1.73, P = 0.138).
Conclusion: Patients who discontinued RASi therapy exhibited a higher risk of developing ESKD but a reduced risk of hyperkalemia compared to those who continued RASi treatment. However, there were no significant differences in all-cause mortality and MACE between the two groups.
Systematic Review Registration: identifer, PROSPERO (CRD42023494698).
Introduction
The renin-angiotensin system (RAS) promotes inflammation and fibrosis, enhances sympathetic nervous system activity, increases sodium and chloride reabsorption in the tubules, elevates aldosterone release, induces arteriolar constriction, and stimulates anti-diuretic hormone (ADH) secretion (Sawaf et al., 2022). Angiotensin-converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) can mitigate these effects to reduce myocyte hypertrophy, arrhythmogenic effects, and fibrosis in both the heart and kidneys (Mukoyama and Kuwabara, 2022).
For chronic kidney disease (CKD) patients, the use of ACEi/ARB is associated with a lower risk of major cardiovascular events (Xie et al., 2016) and mortality (Wright et al., 2002). Additionally, ACEi/ARB users have a reduced risk of doubling of serum creatinine levels (Wright et al., 2002), and a decreased incidence of end-stage kidney disease (ESKD) (Deng et al., 2022). Therefore, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines suggests the substantial benefits of ACEi and ARB in managing CKD. These guidelines strongly advocate for the use of ACEi or ARB in individuals with CKD (Kidney Disease: Improving Global Outcomes Blood Pressure Work, 2021; Kidney Disease: Improving Global Outcomes Diabetes Work, 2022).
However, the administration of RAS inhibitors (RASi) may lead to a temporary decrease in estimated glomerular filtration rate (eGFR). This effect is attributed to the reduction in systemic blood pressure and the vasodilatory impact on efferent arterioles, leading to a consequent decrease in intraglomerular pressure (Burnier, 2020). Besides, the use of RASi is associated with an increased risk of hyperkalemia, primarily due to their role in inhibiting aldosterone secretion, which in turn impairs the kidneys’ ability to excrete potassium. This risk is particularly elevated in patients with deteriorating renal function (Raebel, 2012; Bandak et al., 2017).
Multiple clinical trials have demonstrated that blockade of the renin–angiotensin system is reno-protective and effectively reduces CKD progression. However, most trials excluded participants with advanced CKD, especially stage 4 and 5 (Weir et al., 2018). Therefore there is no definitive conclusion on the impact of continued RASi use on kidney function and the risk of hyperkalemia in these patients. We undertook a meta-analysis to evaluate the impact of discontinuing versus continuing RASi on clinical outcomes among patients with CKD stage 3–5.
Methods
Methodology
This meta-analysis adhered to the principles outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Higgins et al., 2011) (Supplementary Material 1).
Search strategy
Three investigators (I.W. Chen, Y.H. Lin, and M.H. Wu) conducted electronic database searches in PubMed, EMBASE, and the Cochrane Library for relevant studies published from the inception of 30 May 2024. We utilized the subsequent sets of keywords and their combinations, (1) “chronic kidney disease” and (2) “Renin angiotensin system inhibitor” or “Angiotensin converting enzyme inhibitor” or “Angiotensin receptor antagonist” and (3) “Discontinue” or “continue” (Supplementary Material 2).
We also used Medical Subject Headings (MeSH) terms to improve the search’s sensitivity and identify additional pertinent studies. Additionally, we conducted a manual review of the reference lists of the included articles to identify potentially suitable studies. After eliminating duplicate entries, the three authors (I.W. Chen, Y.H. Lin, M.H. Wu) individually carried out an initial assessment of the studies by evaluating their titles and abstracts. Subsequently, they conducted a comprehensive examination of eligible studies by reviewing their complete texts. Discrepancies that arose during the search and study selection process were resolved through discussion. We submitted the protocol for our systematic review to PROSPERO for prospective registration (CRD42023494698).
Eligibility criteria
The inclusion criteria were as follows: (a) individuals aged 18 years or older; (b) CKD stage 3–5 defined by a GFR less than 60 mL/min per 1.73 m2; (c) the administration of RASi, including either ACEi or ARB; (d) the reporting at least one of the following outcomes, such as all-cause mortality, ESKD and cardiovascular events. Exclusion criteria included: (a) Studies including animal or healthy human subjects; (b) Studies including pregnant or lactating patients; (c) Comparison with RASi with other anti-hypertensive drugs; (d) case reports, editorials, and reviews; (e) no control group for comparing the effects of continuing versus discontinuing ACEi or ARB therapy were all excluded.
Data extraction and quality assessment of the included studies
The characteristics of these studies encompassed various details, including the first author’s name, year of publication, study design, data source, study groups, sample size, duration of follow-up, and the stage of CKD. The outcomes evaluated in this meta-analysis encompassed all-cause mortality (Qiao et al., 2020; Walther et al., 2021; Bhandari et al., 2022; Yang et al., 2023; Leon et al., 2022) as well as ESKD (Qiao et al., 2020; Walther et al., 2021; Bhandari et al., 2022; Nakayama et al., 2022; Yang et al., 2023; Leon et al., 2022), major adverse cardiovascular events (MACE) (Qiao et al., 2020; Fu et al., 2021a; Yang et al., 2023) and hyperkalemia (Hou et al., 2006; Qiao et al., 2020; Yang et al., 2023). ESKD was defined as initiation of dialysis or kidney transplantation. Cardiovascular events were defined as non-fatal stroke, non-fatal myocardial infarction, heart failure, percutaneous coronary intervention, or coronary artery bypass and cardiovascular death. Serum potassium level greater than 5.5 mEq/L was ascertained as hyperkalemia. The definitions of MACE and hyperkalemia varied across included studies and are summarized in Supplementary Material 5.
We conducted subgroup analyses based on randomized controlled trials (RCTs) versus non-RCTs. To assess the quality of the studies, we employed the Newcastle-Ottawa Scale (NOS) for cohort studies and Version 2.0 of the Cochrane risk of bias tool for randomized trials (RoB 2.0) in the case of RCTs. In this meta-analysis, the GRADE (Grading of Recommendations, Assessment, Development and Evaluations) system was used to assess the quality of evidence and the strength of recommendations (Supplementary Material 6).
Statistical analysis
To determine the magnitude of the effect for outcomes in this meta-analysis, we employed a hazard ratio (HR) in conjunction with a 95% confidence interval (CI). For our meta-analysis of outcomes, we applied random effects methods, utilizing the DerSimonian-Laird estimator for variance. This approach was chosen to compute the combined effect size for each outcome, considering the recognized clinical and methodological diversity among the studies (Veroniki et al., 2016). All statistical analyses were conducted using Comprehensive Meta-Analysis (Version 3.3.070, dated 20 November 2014).
Trial sequential analysis
Trial sequential analysis (TSA) in this meta-analysis was applied to minimize the risk of false-positive or false-negative results (Brok et al., 2008; Kang, 2021). This method determined the adequacy of evidence when the cumulative Z-curve either crossed the trial sequential monitoring boundary or reached the futility area, eliminating the need for further studies. If the Z-curve did not achieve these thresholds and the required information size (RIS) was not fulfilled, it suggested that the current evidence was insufficient, calling for additional research for verification (Liu et al., 2016). The RIS in our TSA was based on a projected 10% reduction in relative risk (RR). We maintained the type I error (α) at 0.05 (two-sided) and employed a power (1-β) of 0.90 to calculate the RIS. The proportion of control events was calculated using data from the comparator group (Liu et al., 2019). The TSA was executed using TSA software Version 0.9.5.10 Beta.
Results
Study search outcomes and included patients
As shown in Figure 1, a total of 520 articles were identified from PubMed, Embase, and Cochrane databases, respectively. Out of these, 143 articles were excluded due to duplication. Subsequently, 377 articles underwent screened based on their titles and abstracts. Following this initial screening, 11 articles were evaluated for full eligibility, leading to the exclusion of three articles (two lacked control group (Ahmed et al., 2010; Leon, 2020), and one used other agents as a control group (Fu et al., 2021a)). Finally, eight articles, including 243,775 patients with complete data and outcomes of interest, were enrolled for the final meta-analysis (Figure 1).
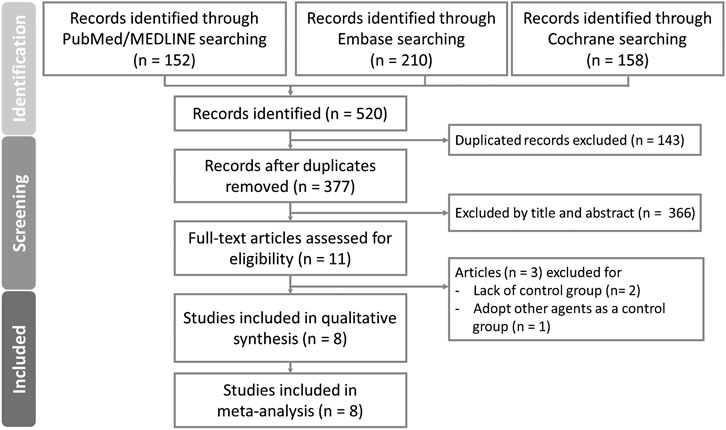
Figure 1. PRISMA flow diagram for systematic reviews which included searches of databases and registers.
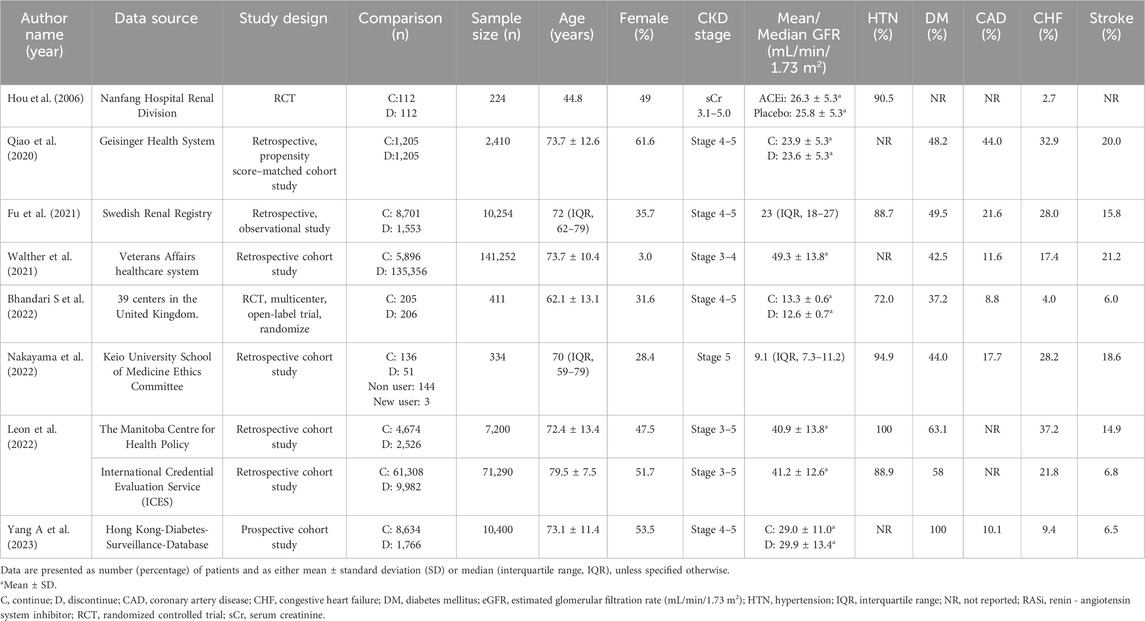
Five studies were retrospective studies (Qiao et al., 2020; Walther et al., 2021; Fu et al., 2021b; Nakayama et al., 2022; Leon et al., 2022), while one was a prospective cohort study (Yang et al., 2023). The remaining two studies were randomized control studies (Bhandari et al., 2022; Hou et al., 2006). Baseline characteristics and outcomes for the included studies are presented on Tables 1 and 2, respectively.
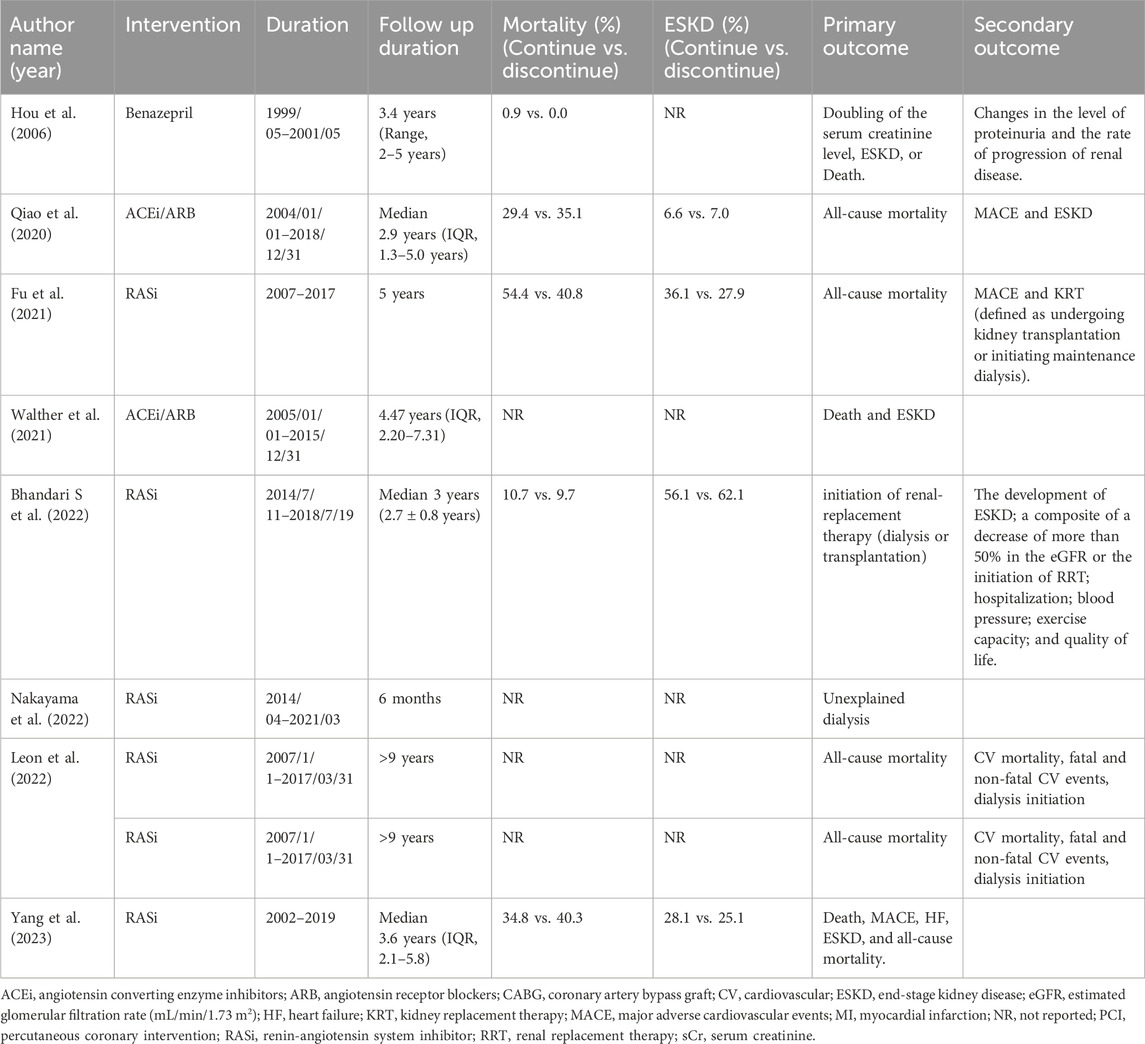
The follow-up durations in the eight articles ranged from 6 months to up to 9 years. Moreover, the range of mean/median baseline eGFR was 9.1–49.3 mL/min/1.73 m2. Among the included articles, the primary outcome was defined as patient mortality in six articles (Hou et al., 2006; Qiao et al., 2020; Fu et al., 2021b; Walther et al., 2021; Leon et al., 2022; Yang et al., 2023), while ESKD served as the primary outcome in three articles (Hou et al., 2006; Walther et al., 2021; Yang et al., 2023). ESKD was defined as secondary outcomes in another article (Bhandari et al., 2022). One article focused on the incidence of unplanned dialysis initiation as its primary outcome (Nakayama et al., 2022).
Quality of enrolled trials
The studies spanned various years (2006–2022) and exhibited substantial variation in sample sizes from 104 to 141,252 patients. Besides, the data were collected from different sources by the authors, including population databases, health insurance systems, and/or multiple hospitals. Patients from all enrolled studies were categorized into either the discontinued-RASi and continued-RASi groups, and outcomes were compared between the two groups (Tables 1 and 2).
The results of quality assessment, based on the NOS for included the six cohort studies (Qiao et al., 2020; Fu et al., 2021b; Walther et al., 2021; Nakayama et al., 2022; Yang et al., 2023; Leon et al., 2022), ranged from 7 to 8 (Supplementary Table S1A). This indicated that six studies demonstrated good methodological quality. In the studies of Qiao et al., Nakayama et al., Yang A et al., and Silva J. Leon et al., there were patients who expired or progressed to ESKD at the beginning of follow-up period (Qiao et al., 2020; Nakayama et al., 2022; Yang et al., 2023; Leon et al., 2022). Besides, the study conducted by Walther et al., no additional confounders was adjusted in the analysis (Walther et al., 2021). Therefore, four of six cohort studies scored 7 points by NOS. The RoB 2.0 was adopted for two randomized control studies. Low risk was assessed in the study of Bhandari et al. (2022). However, moderate risk was assessed in Hou et al., which was unable to discern differences in each component of the kidney composite outcome (Hou et al., 2006). Thus some concerns were judged in the domain “Bias in selection of the reported result” (Supplementary Table S1B).
All-cause mortality
The main outcome of interest assessed in five studies encompassing 29,993 patients and 12,096 deaths with overall all-cause mortality rate of 40.3% (Qiao et al., 2020; Fu et al., 2021b; Bhandari et al., 2022; Yang et al., 2023; Hou et al., 2006; Leon et al., 2022). The risk of all-cause mortality did not demonstrate statistical difference between the discontinued-RASi users and continued-RASi users [OR: 0.94, 95% CI: 0.56–1.58, P = 0.816, certainty of evidence (COE): moderate] (Supplementary Figure S1A) and the funnel plot showed symmetrical distributions (Supplementary Figure S2A). Additionally, HR for mortality was reported in five articles (Qiao et al., 2020; Walther et al., 2021; Bhandari et al., 2022; Yang et al., 2023; Leon et al., 2022) and no statistically significant difference was observed between discontinued-RASi users and continued-RASi users (HR: 1.34, 95% CI: 0.91–1.95, P = 0.135, I2 = 98.91%) with considerable heterogeneity among the study results (I2 = 98.91%) (Figure 2A).
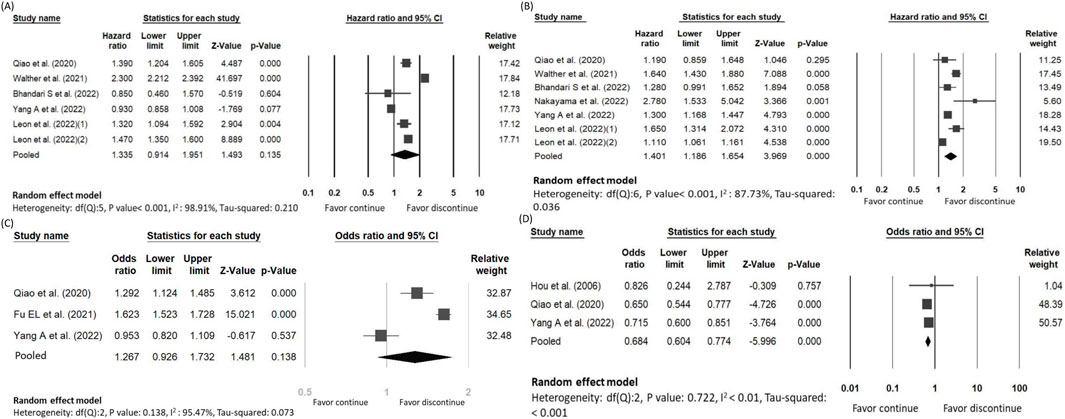
Figure 2. Forest plots showing the pooled risk of (A) all-cause mortality, (B) ESKD, (C) MACE and (D) hyperkalemia between continuing and discontinuing ACEi/ARB groups.
The risk of ESKD
Our secondary outcome of interest was the occurrence of ESKD in 8,992 out of 32,191 patients (27.9%) across the six studies, with a mean follow-up of 2.92 years (Qiao et al., 2020; Bhandari et al., 2022; Fu et al., 2021b; Yang et al., 2023; Nakayama et al., 2022). There was no statistical significance about the difference of ESKD between the discontinued-RASi users and continued-RASi users (OR: 1.05, 95% CI: 0.80 to 1.39, P = 0.708, COE: very low) (Supplementary Figure S1B) and the funnel plot showed symmetrical distributions (Supplementary Figure S2B). In six articles reporting HR for ESKD (Qiao et al., 2020; Walther et al., 2021; Bhandari et al., 2022; Yang et al., 2023; Nakayama et al., 2022; Leon et al., 2022), pooled results indicated a higher risk of ESKD in patients in the discontinued-RASi users compared to the continued-RASi users (HR: 1.40, 95% CI: 1.19–1.65, P < 0.001, I2 = 87.73%) (Figure 2B). For the incidence of ESKD, the TSA indicated the accrued information size was 59,717. The cumulative Z-curve crossed the conventional boundary and even the monitoring boundary, reached the superiority zone, indicating that discontinued-RASi users has higher risk of ESKD than the continued-RASi users (Figure 3). However, the cumulative z-curve did not reach the line of required information size.
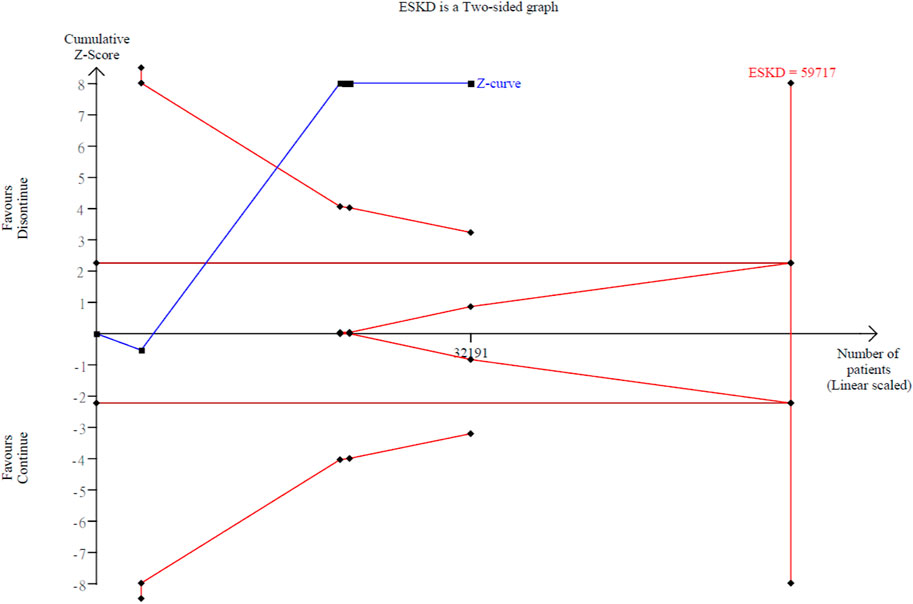
Figure 3. Trial sequential analysis over the ESKD events with continuing versus discontinuing of ACEi/ARB groups.
The risk of MACE
11,225 out of 30,059 patients had MACE (37.3%) according to three studies (Qiao et al., 2020; Fu et al., 2021b; Yang et al., 2023). The pooled findings indicated that discontinued-RASi users had a higher risk of developing MACE compared to continued-RASi users. However, this difference did not reach statistical significance (OR: 1.27, 95% CI: 0.93–1.73, P = 0.138, I2 = 95.47%, COE: very low) (Figure 2C).
The risk of hyperkalemia
We evaluated risk of hyperkalemia based on three included studies, involving 8,533 patients (Hou et al., 2006; Qiao et al., 2020; Yang et al., 2023). Among them, 3,362 individuals experienced hyperkalemia (39.40%). The risk of hyperkalemia was lower in patients in the discontinued-RASi users compared to the continued-RASi users (OR: 0.68, 95% CI: 0.60–0.77, P < 0.001, I2 < 1.00%, COE: low), as depicted in Figure 2D.
Subgroup analysis of RCTs versus non-RCTs
We conducted a subgroup analysis according to whether RCTs or non-RCTs in our included studies. For non-RCT trials, the pooled risk of all-cause mortality revealed no statistical significance between the two groups (HR: 1.42, 95% CI: 0.95–2.13, P = 0.088) (Supplementary Figure S3A). In addition, the pooled risk of ESKD was higher in discontinued-RASi users compared to the continued-RASi users (HR: 1.42, 95% CI: 1.18–1.72, P < 0.001) (Supplementary Figure S3B). The risk of hyperkalemia was lower in the discontinued-RASi users than the continued-RASi users (OR: 0.68, 95% CI: 0.60–0.77, P < 0.001) (Supplementary Figure S3C).
Subgroup analysis of the CKD stage
We conducted a subgroup analysis according to the CKD stage of patients in our included studies. The pooled risk of all-cause mortality revealed no statistical significance between the two groups whether CKD stage 3 or 4 (HR: 1.65, 95% CI: 0.79–3.42 in CKD stage 3; HR: 1.08, 95% CI: 0.57–2.05 in CKD stage 4) (Supplementary Figure S4A). However, the pooled risk of ESKD was higher in discontinued-RASi users compared to the continued-RASi users in CKD stage 4 (HR: 1.31, 95% CI: 1.06–1.62) but no significant difference in CKD stage 3 (HR: 1.43, 95% CI: 0.81–2.54) (Supplementary Figure S4B).
Discussion
This study incorporated trials encompassing patients with CKD stages 3–5 to evaluate the differential impacts of discontinuing versus continuing RASi therapy. Patients who discontinued RASi exhibited a higher risk of progression to ESKD compared to those who maintained the therapy. However, no significant difference in mortality risk was observed between the two cohorts. Furthermore, the discontinuation of RASi was associated with a reduced incidence of hyperkalemia in contrast to the continued use of RASi.
Across the included studies, definitions of MACE and hyperkalemia were heterogeneous. Some cohorts adopted conventional 3-point MACE (cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke), whereas others included additional endpoints such as revascularization, arrhythmia, or even all-cause mortality as part of the composite. Similarly, thresholds for hyperkalemia ranged from ≥5.5 to ≥6.0 mmol/L, with some studies relying on registry-based ascertainment and others reporting only trial-adjudicated events. Such variability may alter absolute event rates and bias comparative estimates, thereby limiting the interpretability of pooled results. In recognition of this limitation, we refrained from cross-study pooling of MACE and hyperkalemia, instead reporting them descriptively, and emphasized endpoints with consistent definitions (all-cause mortality and ESKD).
In interpreting observational evidence, it is important to note that the Geisinger Health System analysis (Qiao et al., 2020) was not a randomized trial but a propensity score–matched cohort study comparing patients who discontinued ACEi/ARB after eGFR fell <30 mL/min/1.73 m2 with matched continuers within the same health system. The matched sample comprised 1,205 pairs with baseline balance (all standardized mean differences <0.1), but—like all nonrandomized designs—susceptibility to unmeasured confounding remains. Notably, the investigators complemented the primary matched analysis with a target-trial emulation sensitivity analysis, which produced concordant estimates, lending robustness to the observed associations. Accordingly, in our synthesis we treat the Geisinger findings as arising from a matched comparator design (rather than a true ‘control’ group) and weigh them with appropriate caution.
Upregulation of RAS contributes to the development of hypertension in CKD (Pugh et al., 2020). ACEi inhibits the conversion of angiotensin I (Ang I) to angiotensin II (Ang II), and ARB selectively prevents Ang II from binding to angiotensin II type I receptor (AT1R). Both drugs demonstrated a renoprotective effect due to their antihypertensive and antiproteinuric effects (Zheng et al., 2019). Proteinuria is strongly associated with the risk of CKD progression in both non-diabetic and diabetic patients. In non-diabetic CKD patients included the Ramipril Efficacy in Nephropathy (REIN) trial, urinary protein excretion was the only baseline variable that correlated with the rate of GFR decline and progression to ESKD (Ruggenenti et al., 1997). In according to two previous large RCTs on the effect of ARB in diabetic nephropathy, losartan and irbesatan, demonstrated ARB therapy was effective in protecting against the progression of nephropathy (Brenner et al., 2001). In a post hoc analysis of the REIN trial, the ramipril therapy was still beneficial for individuals with low eGFR, which decreased the rate of eGFR decline by 22% and the incidence of ESRD by 33% compared with the conventional group (non-ACEi treatment) (Ruggenenti et al., 2001). This post hoc analysis suggests that ACEi should not be withheld, even when eGFR approaches levels requiring replacement therapy.
Patients with CKD exhibit a pronounced risk for cardiovascular events. In a report, 50% of all patients with CKD stage 4–5 have cardiovascular disease (CVD) (Stevens et al., 2007). The traditional cardiovascular risk factors such as hypertension, dyslipidemia and insulin resistance are highly prevalent in patients with CKD (Roehm and Weiner, 2019; Zewinger et al., 2017). The hormones, enzymes, and cytokines in response to kidney injury or renal insufficiency lead to characteristic changes in the vasculature (Jankowski et al., 2021). ACEi reduce angiotensin II levels, thereby lowering blood pressure, but also prevent the breakdown of bradykinin to reduce both systemic and coronary resistance, thus providing additional cardioprotective effects (Roth et al., 2020). Although lack of individual trial or meta-analysis to prove ARB treatment on the incidence of cardiovascular evens, the European Society of Cardiology (ESC) guidelines still recommend ARBs in case of ACE inhibitor intolerance in patients at high cardiovascular risk (Knuuti et al., 2020). One network meta-analysis including 44 RCTs comprising 42,139 participants with non-dialysis CKD stage 3–5 found RAS blockade therapy increased the likelihood for hyperkalemia, hypotension, and cough. However it was still beneficial to protect kidney and cardiovascular functions (Zhang et al., 2020). Although the pool analysis in our study did not reach the significance (OR: 1.27, 95% CI: 0.93–1.73, P = 0.138), we could observe the trend of higher MACE risk when discontinuing ACEi/ARB in CKD stage 3–5 patients. This result might be limited by the limited number of included trials.
Hyperkalemia is a worrisome issue when continuing RASi in patients with impaired kidney function. Hsu et al. conducted a study enrolling 28,497 patients with serum creatinine levels more than 6 mg/dL. The result indicated that ACEi/ARB users (9.2%) had a higher risk of hyperkalemia-associated hospitalization than non-users (6.7%) (Hsu et al., 2014). In accordance with our results by enrolling three studies (Yang et al., 2023; Qiao et al., 2020; Hou et al., 2006), the pooled analysis showed lower incidence of hyperkalemia in discontinued group than the continued one (OR: 0.68, 95% CI: 0.60–0.77, P < 0.001).
Risk–benefit of RASi continuation. Discontinuation was associated with higher ESKD risk in our meta-analysis, whereas continuation increased hyperkalemia. In line with KDIGO 2024, we favor continuing ACEi/ARB with monitoring at 2–4 weeks and active hyperkalemia mitigation (dietary counseling, diuretics/sodium bicarbonate, potassium binders), reserving dose reduction/cessation for uncontrolled hyperkalemia. KDIGO’s algorithm explicitly sequences these steps and supports continuation even at eGFR <30 mL/min/1.73 m2, which frames the observed hyperkalemia as a manageable safety signal rather than a reason for routine discontinuation (Kidney Disease: Improving Global Outcomes, 2024).
To the best of our knowledge, this is the first systematic review and meta-analysis evaluating whether discontinuing RASi among CKD stage 3–5 patients is associated with poor outcome in terms of ESKD. Discontinuing RASi is associated with lower risk of hyperkalemia. Additionally, there is further evidence from the TSA, indicating that discontinued-RASi users has higher risk of ESKD than the continued-RASi users. However, this study had several limitations. First, Hou et al. conducted an RCT showing kidney benefits of benazepril in advanced CKD (Hou et al., 2006). However, because the primary outcome was a composite measure (doubling of serum creatinine, ESRD, or death), the individual outcomes for ESKD were not distinguishable, leading to the exclusion of this RCT from our meta-analysis. Second, our findings were predominantly extracted from observational studies. RCTs are anticipated to provide more solid strength. Third, the data extracted for the subgroup analyses from some enrolled studies lacked comprehensive information regarding the duration of CKD and the discontinuation of RASi, which could potentially bias our estimates. Forth, most included studies did not report RASi dose or exposure intensity in a standardized, comparable format, precluding a dose–response meta-analysis of hyperkalemia risk. Consequently, we cannot exclude residual confounding from clinical down-titration or temporary withholding of RASi in higher-risk patients, which may bias pooled estimates. Lastly, ACEi and ARB have different feedback in the RAAS; however we could not distinguish the two subgroups in our meta-analysis due to lack of data.
Conclusion
Among patients with CKD stages 3–5, discontinuation of RASi therapy was associated with an increased risk of progression to ESKD. Continued use of RASi in this population raises concerns about hyperkalemia. Further large-scale randomized controlled trials are necessary to confirm these findings and provide more definitive evidence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
I-WC: Writing – original draft, Data curation. Y-HL: Writing – original draft, Data curation. V-CW: Validation, Writing – review and editing, Supervision. J-YC: Writing – review and editing, Formal Analysis. M-HW: Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Chi-Mei Medical Center (CMOR11303).
Acknowledgments
We appreciate the assistance from Center for Big Data Analytics and Statistics at Chang Gung Memorial Hospital at Linkou, Taiwan.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1646969/full#supplementary-material
Abbreviations
ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; ADH, anti-diuretic hormone; Ang I, angiotensin I; Ang II, angiotensin II; AT1R, angiotensin II type I receptor; CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ESKD, End-Stage Kidney Disease; ESC, European Society of Cardiology; GRADE, Grading of Recommendations, Assessment, Development and Evaluations; HR, hazard ratio; KDIGO, Kidney Disease Improving Global Outcomes; MACE, major adverse cardiovascular events; MeSH, Medical Subject Headings; NOS, Newcastle-Ottawa Scale; OR, odds ratio; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RAS, renin-angiotensin system; RASi, renin-angiotensin system inhibitors; RCTs, randomized controlled trials (RCTs); RIS, required information size; RoB 2.0, Version 2.0 of the Cochrane risk of bias; RR, relative risk; TSA, Trial sequential analysis.
References
Ahmed, A. K., Kamath, N. S., El Kossi, M., and El Nahas, A. M. (2010). The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol. Dial. Transpl. 25, 3977–3982. doi:10.1093/ndt/gfp511
Bandak, G., Sang, Y., Gasparini, A., Chang, A. R., Ballew, S. H., Evans, M., et al. (2017). Hyperkalemia after initiating renin-angiotensin system blockade: the stockholm Creatinine measurements (SCREAM) project. J. Am. Heart Assoc. 6, e005428. doi:10.1161/JAHA.116.005428
Bhandari, S., Mehta, S., Khwaja, A., Cleland, J. G. F., Ives, N., Brettell, E., et al. (2022). Renin-angiotensin system inhibition in advanced chronic kidney disease. N. Engl. J. Med. 387, 2021–2032. doi:10.1056/NEJMoa2210639
Brenner, B. M., Cooper, M. E., De Zeeuw, D., Keane, W. F., Mitch, W. E., Parving, H.-H., et al. (2001). Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345, 861–869. doi:10.1056/NEJMoa011161
Brok, J., Thorlund, K., Gluud, C., and Wetterslev, J. (2008). Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J. Clin. Epidemiol. 61, 763–769. doi:10.1016/j.jclinepi.2007.10.007
Burnier, M. (2020). Renin-angiotensin system blockade in advanced kidney disease: stop or continue? Kidney Med. 2, 231–234. doi:10.1016/j.xkme.2020.04.002
Deng, X., Li, D., Tang, Q., and Chen, Y. (2022). ACEI and ARB lower the incidence of end-stage renal disease among patients with diabetic nephropathy: a meta-analysis. Comput. Math. Methods Med. 2022, 6962654. doi:10.1155/2022/6962654
Fu, E. L., Clase, C. M., Evans, M., Lindholm, B., Rotmans, J. I., Dekker, F. W., et al. (2021a). Comparative effectiveness of renin-angiotensin system inhibitors and calcium channel blockers in individuals with advanced CKD: a nationwide observational cohort study. Am. J. Kidney Dis. 77, 719–729 e1. doi:10.1053/j.ajkd.2020.10.006
Fu, E. L., Evans, M., Clase, C. M., Tomlinson, L. A., Van Diepen, M., Dekker, F. W., et al. (2021b). Stopping renin-angiotensin system inhibitors in patients with advanced CKD and risk of adverse outcomes: a nationwide study. J. Am. Soc. Nephrol. 32, 424–435. doi:10.1681/ASN.2020050682
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hou, F. F., Zhang, X., Zhang, G. H., Xie, D., Chen, P. Y., Zhang, W. R., et al. (2006). Efficacy and safety of benazepril for advanced chronic renal insufficiency. N. Engl. J. Med. 354, 131–140. doi:10.1056/NEJMoa053107
Hsu, T. W., Liu, J. S., Hung, S. C., Kuo, K. L., Chang, Y. K., Chen, Y. C., et al. (2014). Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med. 174, 347–354. doi:10.1001/jamainternmed.2013.12700
Jankowski, J., Floege, J., Fliser, D., Bohm, M., and Marx, N. (2021). Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation 143, 1157–1172. doi:10.1161/CIRCULATIONAHA.120.050686
Kang, H. (2021). Trial sequential analysis: novel approach for meta-analysis. Anesth. Pain Med. Seoul. 16, 138–150. doi:10.17085/apm.21038
Kidney Disease: Improving Global Outcomes (2024). KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 105, S117–S314. doi:10.1016/j.kint.2023.10.018
Kidney Disease: Improving Global Outcomes Diabetes Work, G. (2022). KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 102, S1–S127. doi:10.1016/j.kint.2022.06.008
Kidney Disease: Improving Global Outcomes Blood Pressure Work, G (2021). KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 99, S1–S87. doi:10.1016/j.kint.2020.11.003
Knuuti, J., Wijns, W., Saraste, A., Capodanno, D., Barbato, E., Funck-Brentano, C., et al. (2020). 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41, 407–477. doi:10.1093/eurheartj/ehz425
Leon, S. J. (2020). Impact of ACEi/ARB discontinuation after an episode of hyperkalemia in patients with chronic kidney disease. A Population-Based Cohort Study.
Leon, S. J., Whitlock, R., Rigatto, C., Komenda, P., Bohm, C., Sucha, E., et al. (2022). Hyperkalemia-related discontinuation of renin-angiotensin-aldosterone system inhibitors and clinical outcomes in CKD: a population-based cohort study. Am. J. Kidney Dis. 80, 164–173 e1. doi:10.1053/j.ajkd.2022.01.002
Liu, C., Mao, Z., Kang, H., Hu, J., and Zhou, F. (2016). Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Crit. Care 20, 144. doi:10.1186/s13054-016-1299-0
Liu, C., Lu, G., Wang, D., Lei, Y., Mao, Z., Hu, P., et al. (2019). Balanced crystalloids versus normal saline for fluid resuscitation in critically ill patients: a systematic review and meta-analysis with trial sequential analysis. Am. J. Emerg. Med. 37, 2072–2078. doi:10.1016/j.ajem.2019.02.045
Mukoyama, M., and Kuwabara, T. (2022). Role of renin-angiotensin system blockade in advanced CKD: to use or not to use? Hypertens. Res. 45, 1072–1075. doi:10.1038/s41440-022-00902-7
Nakayama, T., Morimoto, K., Uchiyama, K., Kusahana, E., Washida, N., Azegami, T., et al. (2022). Effects of renin-angiotensin system inhibitors on the incidence of unplanned dialysis. Hypertens. Res. 45, 1018–1027. doi:10.1038/s41440-022-00877-5
Pugh, D., Gallacher, P. J., and Dhaun, N. (2020). Correction to: management of hypertension in chronic kidney disease. Drugs 80, 1381. doi:10.1007/s40265-020-01388-8
Qiao, Y., Shin, J. I., Chen, T. K., Inker, L. A., Coresh, J., Alexander, G. C., et al. (2020). Association between renin-angiotensin system blockade discontinuation and all-cause mortality among persons with low estimated glomerular filtration rate. JAMA Intern Med. 180, 718–726. doi:10.1001/jamainternmed.2020.0193
Raebel, M. A. (2012). Hyperkalemia associated with use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 30, e156–e166. doi:10.1111/j.1755-5922.2010.00258.x
Roehm, B., and Weiner, D. E. (2019). Blood pressure targets and kidney and cardiovascular disease: same data but discordant guidelines. Curr. Opin. Nephrol. Hypertens. 28, 245–250. doi:10.1097/MNH.0000000000000492
Roth, G. A., Mensah, G. A., Johnson, C. O., Addolorato, G., Ammirati, E., Baddour, L. M., et al. (2020). Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol. 76, 2982–3021. doi:10.1016/j.jacc.2020.11.010
Ruggenenti, P., Perna, A., Mosconi, L., Matalone, M., Pisoni, R., Gaspari, F., et al. (1997). Proteinuria predicts end-stage renal failure in non-diabetic chronic nephropathies. The “Gruppo Italiano di Studi Epidemiologici in Nefrologia” (GISEN). Kidney Int. Suppl. 63, S54–S57.
Ruggenenti, P., Perna, A., and Remuzzi, G. (2001). ACE inhibitors to prevent end-stage renal disease: when to start and why possibly never to stop: a post hoc analysis of the REIN trial results. Ramipril efficacy in nephropathy. J. Am. Soc. Nephrol. 12, 2832–2837. doi:10.1681/ASN.V12122832
Sawaf, H., Thomas, G., Taliercio, J. J., Nakhoul, G., Vachharajani, T. J., and Mehdi, A. (2022). Therapeutic advances in diabetic nephropathy. J. Clin. Med. 11, 378. doi:10.3390/jcm11020378
Stevens, P. E., O'Donoghue, D. J., De Lusignan, S., Van Vlymen, J., Klebe, B., Middleton, R., et al. (2007). Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 72, 92–99. doi:10.1038/sj.ki.5002273
Veroniki, A. A., Jackson, D., Viechtbauer, W., Bender, R., Bowden, J., Knapp, G., et al. (2016). Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res. Synth. Methods 7, 55–79. doi:10.1002/jrsm.1164
Walther, C. P., Winkelmayer, W. C., Richardson, P. A., Virani, S. S., and Navaneethan, S. D. (2021). Renin-angiotensin system blocker discontinuation and adverse outcomes in chronic kidney disease. Nephrol. Dial. Transpl. 36, 1893–1899. doi:10.1093/ndt/gfaa300
Weir, M. R., Lakkis, J. I., Jaar, B., Rocco, M. V., Choi, M. J., Kramer, H. J., et al. (2018). Use of renin-angiotensin system blockade in advanced CKD: an NKF-KDOQI controversies report. Am. J. Kidney Dis. 72, 873–884. doi:10.1053/j.ajkd.2018.06.010
Wright, J. T., Bakris, G., Greene, T., Agodoa, L. Y., Appel, L. J., Charleston, J., et al. (2002). Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA 288, 2421–2431. doi:10.1001/jama.288.19.2421
Xie, X., Liu, Y., Perkovic, V., Li, X., Ninomiya, T., Hou, W., et al. (2016). Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a bayesian network meta-analysis of randomized clinical trials. Am. J. Kidney Dis. 67, 728–741. doi:10.1053/j.ajkd.2015.10.011
Yang, A., Shi, M., Lau, E. S. H., Wu, H., Zhang, X., Fan, B., et al. (2023). Clinical outcomes following discontinuation of renin-angiotensin-system inhibitors in patients with type 2 diabetes and advanced chronic kidney disease: a prospective cohort study. EClinicalMedicine 55, 101751. doi:10.1016/j.eclinm.2022.101751
Zewinger, S., Kleber, M. E., Rohrer, L., Lehmann, M., Triem, S., Jennings, R. T., et al. (2017). Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur. Heart J. 38, 1597–1607. doi:10.1093/eurheartj/ehx118
Zhang, Y., He, D., Zhang, W., Xing, Y., Guo, Y., Wang, F., et al. (2020). ACE inhibitor benefit to kidney and cardiovascular outcomes for patients with non-dialysis chronic kidney disease stages 3-5: a network meta-analysis of randomised clinical trials. Drugs 80, 797–811. doi:10.1007/s40265-020-01290-3
Zheng, C. M., Wang, J. Y., Chen, T. T., Wu, Y. C., Wu, Y. L., Lin, H. T., et al. (2019). Angiotensin-converting enzyme inhibitors or angiotensin receptor blocker monotherapy retard deterioration of renal function in Taiwanese chronic kidney disease population. Sci. Rep. 9, 2694. doi:10.1038/s41598-019-38991-z
Keywords: angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, chronic kidney disease, continuation, discontinuation
Citation: Chen I-W, Lin Y-H, Wu V-C, Chen J-Y and Wu M-H (2025) Discontinuation versus continuation of renin–angiotensin system inhibitors in chronic kidney disease stage 3–5 patients: a systematic review and meta-analysis. Front. Pharmacol. 16:1646969. doi: 10.3389/fphar.2025.1646969
Received: 14 June 2025; Accepted: 11 September 2025;
Published: 26 September 2025.
Edited by:
Jing Miao, Mayo Clinic, United StatesReviewed by:
Nathan Andrew Holland, Texas Tech University Health Sciences Center El Paso, United StatesGopalakrishnan Natarajan, Madras Medical College, India
Copyright © 2025 Chen, Lin, Wu, Chen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jui-Yi Chen, a3d1aWx1czAxMDFAZ21haWwuY29t; Ming-Hsien Wu, Yjk1MDIwMTNAY2dtaC5vcmcudHc=
†These authors have contributed equally to this work
 I-Wen Chen
I-Wen Chen Yi-Hsuan Lin
Yi-Hsuan Lin Vin-Cent Wu
Vin-Cent Wu Jui-Yi Chen
Jui-Yi Chen Ming-Hsien Wu
Ming-Hsien Wu