- Department of Rheumatology and Immunology, Daping Hospital, Army Medical University, Chongqing, China
Background: Interstitial lung disease (ILD) is the most common complication and the major cause of mortality among patients with idiopathic inflammatory myopathies (IIMs). Currently, no recommended standard treatment for IIM-associated ILD. In this observational study, we evaluated the efficacy and safety of telitacicept in treating IIM-associated ILD.
Methods: We included 10 patients with IIM-associated ILD; of them, seven had antisynthetase syndrome-associated ILD, one had anti-MDA5 antibody-positive dermatomyositis (DM)-associated ILD, and 2 had DM-associated ILD. Four patients with severe ILD were treated with a combination of rituximab (RTX) (375 mg/m2/week for 4 weeks) and telitacicept (160 mg/week). Six patients had refractory IIM-associated ILD; of them, two received RTX (375 mg/m2/week for 4 weeks) in combination with telitacicept (160 mg/week), and four were treated with telitacicept (160 mg/week) alone because they had an increased infection risk.
Result: Over the 24-week follow-up, glucocorticoid dosage was reduced to 5–10 mg/day and that of telitacicept treatment was increased to 160 mg every 2 weeks in all patients. These patients exhibited alleviation of rash, joint swelling and pain, muscle pain and weakness, and dyspnea. Compared with before treatment, the Manual Muscle Testing 8 score and PaO2/FiO2 ratio increased by 25.1% and 28.2% after treatment, respectively. Lung function also exhibited considerable improvements, with percentages of forced vital capacity and diffusing capacity of the lungs for carbon monoxide increasing by 20.4% and 30.2%, respectively. Posttreatment chest high-resolution computed tomography revealed significant improvements compared with baseline. Only one patient experienced a mild lung infection, and no further infections occurred after telitacicept dose was reduced. One patient was administered additional nintedanib for pulmonary fibrosis due to insufficient improvement in lung function.
Conclusion: Telitacicept demonstrates substantial clinical efficacy in the treatment of IIM-associated ILD, accompanied by a low infection rate and a favorable safety profile.
1 Introduction
Idiopathic inflammatory myopathies (IIMs) are a group of autoimmune diseases primarily involving skeletal muscles of the limbs and the skin (Lundberg et al., 2017). The clinical manifestations of IIMs are diverse and highly heterogeneous. They can be classified into several subtypes, including dermatomyositis (DM), antisynthetase syndrome (ASS), immune-mediated necrotizing myopathy (IMNM), polymyositis (PM), and sporadic inclusion body myositis (sIBM); of them, DM, ASS, and IMNM are the most prevalent (Lundberg et al., 2017; Tanboon and Nishino, 2019).
Interstitial lung disease (ILD) is the most prevalent complication associated with IIMs and the leading cause of hospitalization and mortality in patients with IIMs. Based on the onset pattern of ILD, ILD can be categorized into acute/subacute and chronic types. Patients with acute/subacute ILD exhibit a lower survival rate than those with chronic ILD (Fujisawa, 2021; Vu et al., 2023).
Thus far, no standard treatment has been recommended for IIM-associated ILD (IIM-ILD). Research on supporting optimal treatment options for IIM-ILD has been limited; most relevant studies have used a retrospective nonrandomized observational or case series design. Treatment approaches primarily include glucocorticoids (GCs), immunosuppressants [e.g., cyclophosphamide (CTX), mycophenolate mofetil (MMF), calcineurin inhibitors (CNIs), and azathioprine], rituximab (RTX), tofacitinib, and plasma exchange (Fujisawa, 2021; Thong et al., 2023). In this study, we assessed the outcomes of telitacicept treatment in 10 IIM-ILD cases. Our results may offer a novel therapeutic option for IIM-ILD management.
2 Materials and methods
2.1 Study design
This was a retrospective observational study evaluating the efficacy of telitacicept, alone or in combination with RTX, in IIM-ILD treatment. This study spanned 24 weeks and included a cohort of 10 patients.
The study design, methods, objectives, and plans were thoroughly reviewed and approved by our institutional ethics committee to ensure ethical integrity. In accordance with ethical research standards, all participants were asked to provide informed consent after we offered a comprehensive explanation of the study’s purpose, methodology, and potential implications for patients.
2.2 Inclusion and exclusion criteria
We included patients who were diagnosed as having IIMs according to the 2020 European Neuromuscular Center (ENMC) diagnostic criteria for DM or the 2011 Solomon classification criteria for ASS, had a chest high-resolution computed tomography (HRCT) scan indicating the presence of ILD, and developed progressive ILD after traditional treatments (e.g., GCs and immunosuppressants). If a patient was newly diagnosed, ILD was required to have presented severely, characterized by a PaO2/FiO2 ratio of <300 or the presence of respiratory failure.
We excluded patients who had severe infections, drug allergies, or experienced serious adverse reactions, or patients who had not signed the informed consent form.
2.3 Data collection
Before commencing treatment, we collected baseline clinical data of the included patients, including age, sex, body mass index, and history of previous treatments. Subsequently, at 0, 12, and 24 weeks after treatment initiation, we collected the following patient data: arterial blood gas analysis, 6-min walk test (6MWT), Manual Muscle Testing 8 (MMT-8) scores, lung function assessments, chest HRCT scans, and laboratory indicators [namely, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), ferritin, creatine kinase (CK), alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and immunoglobulin G (IgG)].
2.4 Observational outcomes
2.4.1 Efficacy outcomes
We evaluated improvements in IIM and ILD symptoms in the included patients after 24 weeks of treatment. Simultaneously, we compared the changes in the PaO2/FiO2 ratio, forced vital capacity percentage (FVC%), diffusing capacity of the lungs for carbon monoxide percentage (DLCO%), and MMT-8 score at baseline and 12 and 24 weeks after treatment initiation.
2.4.2 Safety outcomes
We evaluated the incidence rates of adverse drug reactions, including allergies, infections, hypogammaglobulinemia, leukopenia, gastrointestinal disorders, and neurological disorders.
2.5 Statistical analysis
All statistical analyses were performed using SPSS (version 26). We used t tests to compare pretreatment and posttreatment data. A p-value of <0.05 was considered to indicate statistical significance.
3 Results
Among the 10 patients included in this study, four were male and six were female; their ages ranged from 45 to 75 years. Of them, seven patients had ASS-associated ILD, one had anti-MDA5 antibody-positive DM-associated ILD, and two had DM-associated ILD. Table 1 presents the baseline data of all patients.
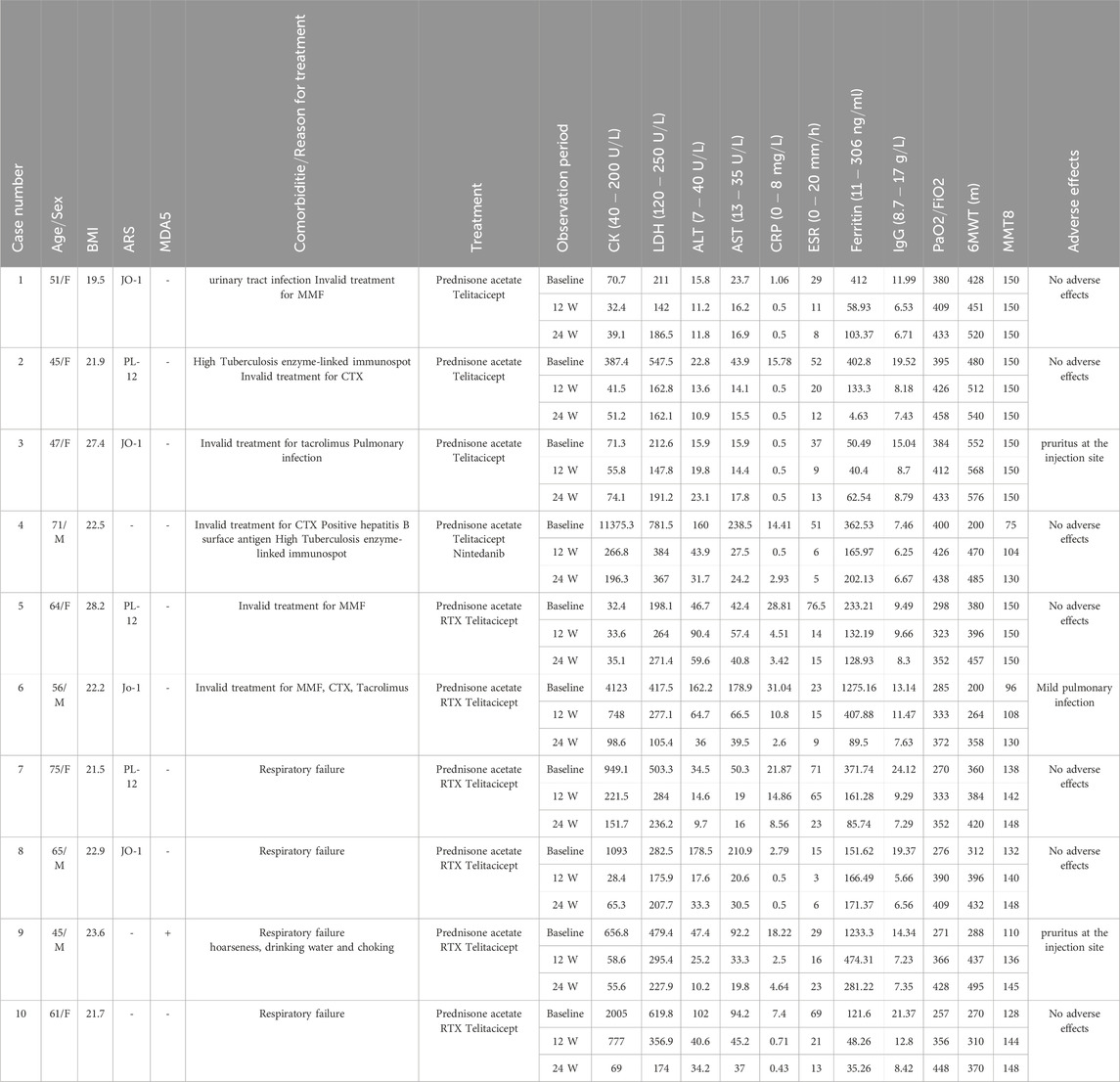
Table 1. Patient baseline data and laboratory indicators, PaO2/FiO2, 6MWT, and MMT8 before and after treatment. All laboratory indicators returned to normal after treatment.
Of all patients, one experienced hoarseness, difficulty drinking water, and choking, in addition to involvement of the skin, muscles, joints, and lungs. Four patients with severe ILD and PaO2/FiO2 ratio <300 were treated with GCs and RTX, followed by telitacicept. Six patients with a poor response to conventional immunosuppressants were classified as having refractory IIM-ILD. Of them, two patients received treatment with RTX combined with telitacicept, whereas four were treated only with telitacicept because they had an increased risk of infection.
During treatment, all patients were monitored for 24 weeks. Over the course of this treatment, prednisone acetate dosage was gradually reduced to 5–7.5 mg/day, whereas that of telitacicept was increased to 160 mg every 2 weeks. No patient experienced muscle pain, weakness, rash, or joint pain, and all reported considerable relief from dyspnea. Laboratory indicators, including CK, LDH, ALT, AST, ferritin, ESR, and CRP, returned to normal levels (Table 1). The PaO2/FiO2 ratio improved by 28.2% compared with baseline (Figure 1A), whereas the 6MWT outcomes improved by 34% (Figure 1C). In all patients with muscle involvement, the MMT-8 score increased by 25.1% (Figure 1B). All patients’ chest HRCT scans revealed varying degrees of reduction in bilateral lung lesions (Figure 2), and lung function demonstrated considerable improvements (Figure 3); in particular, compared with baseline, FVC% and DLCO% increased by 20.4% and 30.2%, respectively (Figure 4). Only one patient received additional treatment with nintedanib for pulmonary fibrosis due to insufficient improvement in lung function.
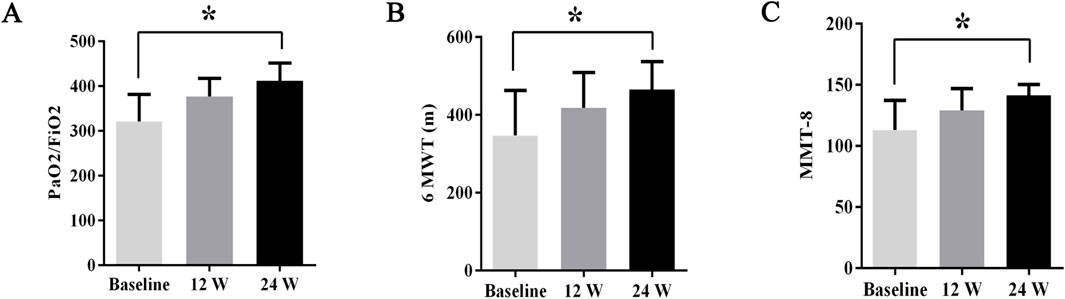
Figure 1. The changes in PaO2/FiO2 ratios, 6MWT outcomes, and MMT8 scores before and after treatment. Post-treatment results demonstrated significant improvements: (A) PaO2/FiO2 ratios increased by 28.2%, (B) 6MWT outcomes improved by 34%, and (C) MMT8 scores increased by 25.1%. All data are presented as means ± standard errors of the means, with *p <0.05 indicating statistical significance compared to pretreatment data.
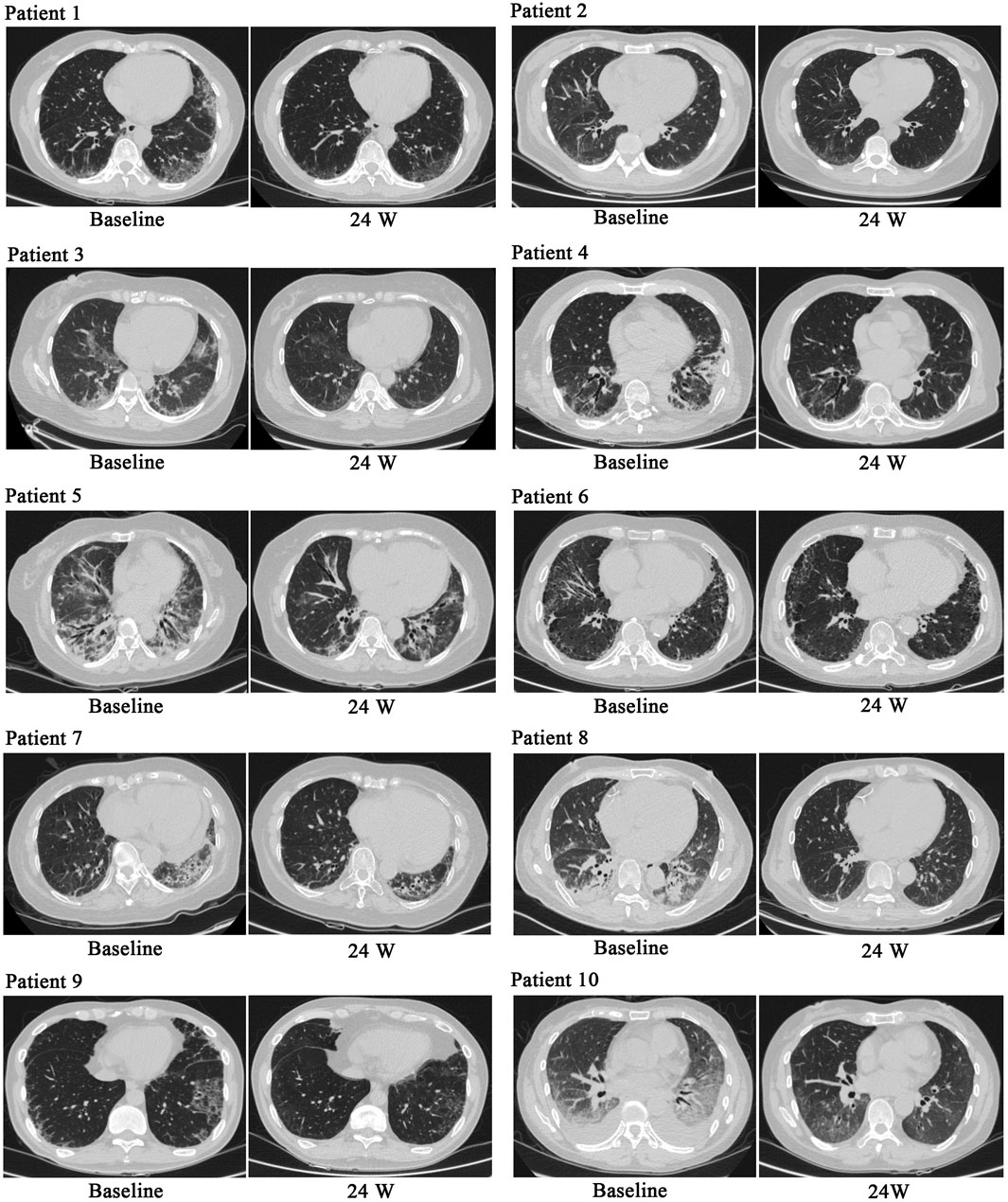
Figure 2. Chest HRCT before and after treatment. After treatment, chest HRCT scans demonstrated significant improvements compared with baseline.
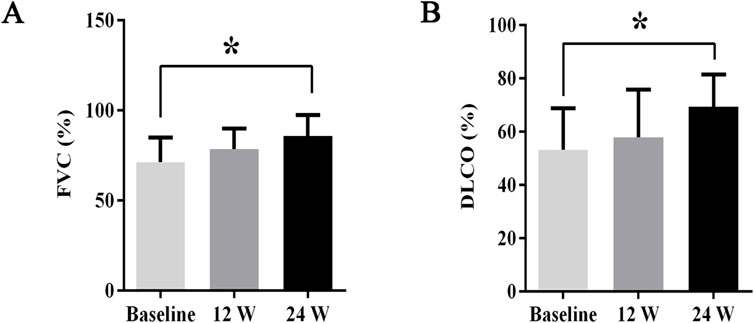
Figure 4. The changes in FVC% and DLCO% before and after treatment. Post-treatment, (A) FVC% increased by 20.4%, (B) DLCO% improved by 30.2%. All data are presented as means ± standard errors of the means, with *p < 0.05 indicating statistical significance compared to pretreatment data.
Regarding adverse events, one patient experienced a mild lung infection; nevertheless, no further infection occurred after telitacicept dosage was reduced. Two patients experienced pruritus at the injection site, lasting for 1–2 days, which resolved spontaneously. None of the patients exhibited leukopenia, gastrointestinal disorders, or neurological diseases; moreover, they demonstrated minimal IgG-related adverse reactions during treatment, indicating good safety.
4 Discussion
The current recommendations for IIM-ILD treatment have been primarily based on empirical research; moreover, definitive guidelines for the optimal treatment of IIM-ILD remain unavailable. Recommendations suggest that treatment decisions should be tailored to a patient’s specific form of ILD and myositis antibody profile (Fujisawa, 2021). For patients with acute and subacute ILD, including those with anti-MDA5 antibody-positive DM and ASS, high-dose GCs should be combined with CNIs (Fujisawa, 2021; Fujisawa et al., 2021; Chen et al., 2022). A triple therapy regimen comprising GCs, CNIs, and CTX may be administered to patients with rapid ILD progression and multiple adverse prognostic factors (Tsuji et al., 2020; Fujisawa, 2021). For patients with refractory ILD, options such as RTX, plasma exchange, and tofacitinib may be considered. Additionally, intravenous immunoglobulin has the functions of regulating immune cell activity, neutralizing autoantibodies, and downregulating chemotactic cytokines. Therefore, for patients with recurrent or refractory IIMs-ILD, intravenous immunoglobulin therapy can be administered (Gandiga et al., 2023). For those with chronic ILD, GCs and immunosuppressants, including CTX, azathioprine, MMF, and CNIs, may be administered (Mecoli and Christopher-Stine, 2018; Fujisawa, 2021; Moda et al., 2025).
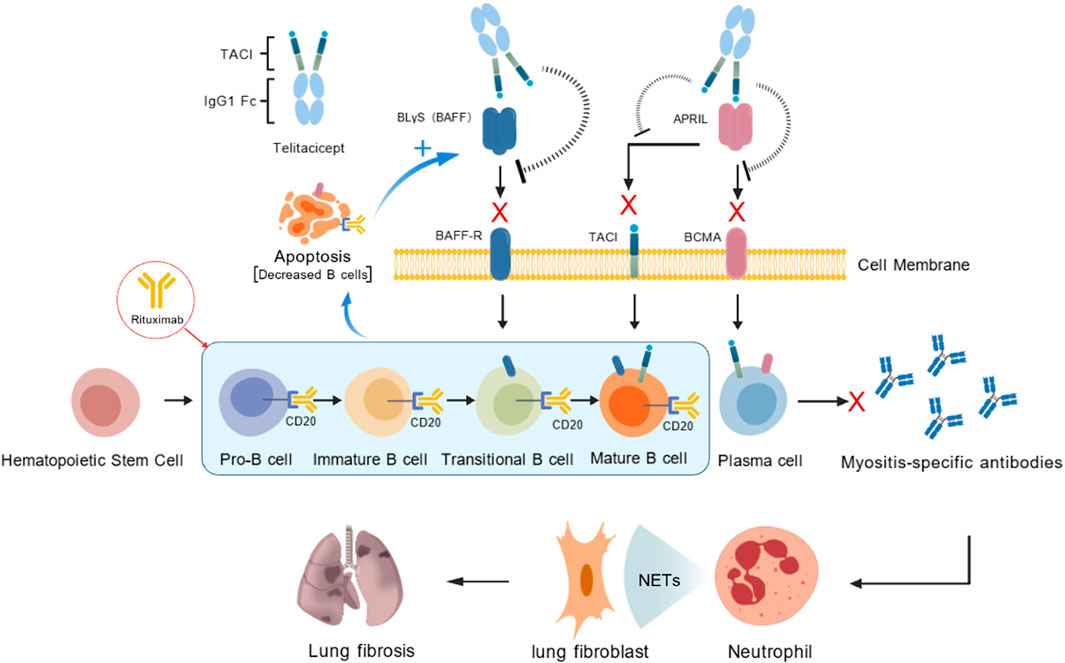
IIM-ILD pathogenesis remains unclear. Nevertheless, in locally inflamed tissue of patients with DM, B-cell infiltration occurs around blood vessels and the muscle endometrium (Saketkoo et al., 2010). Moreover, JO-1 antibodies have recently been isolated from the alveolar lavage fluid of patients with IIM-ILD (Galindo-Feria et al., 2020). Activated B cells differentiate into plasma cells, which produce various antibodies such as antisynthetase and MDA5 antibodies; these antibodies bind to lung autoantigens and trigger tissue damage (Moda et al., 2025). Furthermore, studies have reported that follicular dendritic, T, and B cells contribute to the formation of inducible bronchus-associated lymphoid tissue (iBALT) at pulmonary interstitial lesion sites. iBALT exhibits germinal center activity, facilitating the induction of plasma cells that produce high-affinity antibodies, release neutrophil extracellular traps, and contribute to pulmonary fibrosis development (Akiyama and Kaneko, 2022). These findings indicate that B cells and plasma cells may be pivotal in IIM-ILD pathogenesis.
RTX, a chimeric CD20 monoclonal antibody, targets B cells and induces B-cell depletion; it is thus used in salvage treatment for refractory ILD (Reff et al., 1994; Ogawa et al., 2017; Liossis and Bounia, 2022). RTX exhibits substantial efficacy in patients with refractory ASS-ILD, severe ASS-ILD, recurrent or progressive ASS-ILD, and clinically amyopathic DM characterized by rapidly progressive (RP) ILD, as well as in patients with MDA5 antibody-positive DM-ILD (Allenbach et al., 2015; Doyle et al., 2018; So et al., 2018; Ge et al., 2021). However, RTX treatment can increase B-lymphocyte stimulator (BLyS) levels, possibly promoting reemergence of autoreactive B cells and contributing to disease recurrence over time (Cambridge et al., 2008; Ehrenstein and Wing, 2016).
BLyS, a type II membrane-bound protein from the tumor necrosis factor family, is mainly secreted by monocytes and macrophages (Mockel et al., 2021). BLyS is essential for B-cell survival, maturation, and class-switching, as well as plasma cell survival (Vincent et al., 2013). It also plays a major role in the pathogeneses of various autoimmune diseases. Patients with DM exhibiting elevated BLyS levels have an increased ILD risk (Matsushita et al., 2019). Furthermore, serum BLyS levels are correlated with ILD severity (Hamada et al., 2015). Notably, serum BLyS levels are positively associated with rapidly progressive ILD (RP-ILD) occurrence in patients with MDA5-positive DM (Shi et al., 2024). Therefore, BLyS may be strongly associated with IIM-ILD pathogenesis.
Telitacicept is a novel recombinant fusion protein, comprising the ligand-binding domain of the TACI receptor and the Fc segment of human IgG. It can effectively bind to and inhibit BLyS and proliferation-inducing ligand (APRIL) activities, thereby suppressing the development and survival of plasma cells and mature B cells (Dhillon, 2021; Shi et al., 2021; Fan et al., 2022). This drug has recently demonstrated favorable clinical efficacy and safety in Sjögren’s syndrome (Xu et al., 2024), systemic lupus erythematosus (SLE) (Jin et al., 2023; Wu et al., 2024), and rheumatoid arthritis (RA) (Dhillon, 2021). However, its therapeutic effect on IIM-ILD has not been reported thus far.
A combination of RTX and telitacicept can effectively neutralize elevations in BLyS levels, thereby delaying B-cell reactivation, improving remission rates, and reducing recurrence rates in patients with refractory lupus nephritis (Chen et al., 2025a; 2025b). Furthermore, sequential treatment with RTX and telitacicept can significantly reduce recurrence rates in patients with refractory anti-NMDA receptor encephalitis and MOG-associated demyelination (Zhang et al., 2025). However, the therapeutic effects of this combination in IIM-ILD have not yet been reported.
In the current study, we included 10 IIM-ILD cases; of them, four, who were newly diagnosed as having severe ILD, were treated with a combination of RTX and telitacicept. Six patients exhibited refractory IIM-ILD. Of them, four were treated with telitacicept because they had a high risk of infection associated with RTX; the remaining two patients were administered a combination of RTX and telitacicept. All 10 patients demonstrated symptom relief and considerable improvements in laboratory and imaging indicators, thereby providing the dual treatment effects for idiopathic inflammatory IIM-ILD. Telitacicept demonstrated favorable clinical efficacy and safety in our patients with anti-MDA5 antibody-positive DM-associated ILD and ASS-ILD.
Our results indicated that telitacicept can be used as a novel therapeutic for IIM-ILD treatment. However, because we did not include a control group, our results and conclusions may be constrained. Future studies should employ a prospective, controlled design to further validate the efficacy of telitacicept, both alone and in combination with RTX, in managing IIM-ILD. In addition, further extensive multicenter clinical studies are necessary to confirm the safety and efficacy of telitacicept in patients with connective tissue diseases and interstitial lung lesions, such as Sjögren’s syndrome, systemic sclerosis, and antineutrophil cytoplasmic antibody–associated vasculitis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Daping Hospital, Army Military Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XC: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review and editing, Investigation, Validation. ML: Writing – review and editing. MS: Writing – review and editing. XX: Formal Analysis, Writing – review and editing. XZ: Writing – review and editing. YC: Writing – review and editing. HD: Data curation, Formal Analysis, Investigation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation Project of Chongqing (Key Projects) [grant number CSTB2024NSCQ-KJFZZDX0049].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akiyama, M., and Kaneko, Y. (2022). Pathogenesis, clinical features, and treatment strategy for rheumatoid arthritis-associated interstitial lung disease. Autoimmun. Rev. 21, 103056. doi:10.1016/j.autrev.2022.103056
Allenbach, Y., Guiguet, M., Rigolet, A., Marie, I., Hachulla, E., Drouot, L., et al. (2015). Efficacy of rituximab in refractory inflammatory myopathies associated with Anti- synthetase auto-antibodies: an open-label, phase II trial. PLoS One 10, e0133702. doi:10.1371/journal.pone.0133702
Cambridge, G., Isenberg, D. A., Edwards, J. C., Leandro, M. J., Migone, T. S., Teodorescu, M., et al. (2008). B cell depletion therapy in systemic lupus erythematosus: relationships among serum B lymphocyte stimulator levels, autoantibody profile and clinical response. Ann. Rheum. Dis. 67, 1011–1016. doi:10.1136/ard.2007.079418
Chen, Y., Bai, Z., Zhang, Z., Hu, Q., Zhong, J., and Dong, L. (2022). The efficacy and safety of tacrolimus on top of glucocorticoids in the management of IIM-ILD: a retrospective and prospective study. Front. Immunol. 13, 978429. doi:10.3389/fimmu.2022.978429
Chen, Y., Lei, X., Xu, J., Chen, X., Pan, H., Zhang, Q., et al. (2025a). Belimumab versus telitacicept in sequential treatment after rituximab for refractory lupus nephritis: a real-world multicentre study. Lupus Sci. Med. 12, e001296. doi:10.1136/lupus-2024-001296
Chen, Y., Shi, N., Lei, X., Ren, P., Lan, L., Chen, L., et al. (2025b). The efficacy of rituximab plus belimumab or telitacicept in refractory lupus nephritis. Rheumatol. Oxf. 64, 221–227. doi:10.1093/rheumatology/kead674
Dhillon, S. (2021). Telitacicept: first approval. Drugs 81, 1671–1675. doi:10.1007/s40265-021-01591-1
Doyle, T. J., Dhillon, N., Madan, R., Cabral, F., Fletcher, E. A., Koontz, D. C., et al. (2018). Rituximab in the treatment of interstitial lung disease associated with antisynthetase syndrome: a multicenter retrospective case review. J. Rheumatol. 45, 841–850. doi:10.3899/jrheum.170541
Ehrenstein, M. R., and Wing, C. (2016). The BAFFling effects of rituximab in lupus: danger ahead? Nat. Rev. Rheumatol. 12, 367–372. doi:10.1038/nrrheum.2016.18
Fan, Y., Gao, D., and Zhang, Z. (2022). Telitacicept, a novel humanized, recombinant TACI-Fc fusion protein, for the treatment of systemic lupus erythematosus. Drugs Today (Barc) 58, 23–32. doi:10.1358/dot.2022.58.1.3352743
Fujisawa, T. (2021). Management of myositis-associated interstitial lung disease. Med. Kaunas. 57, 347. doi:10.3390/medicina57040347
Fujisawa, T., Hozumi, H., Kamiya, Y., Kaida, Y., Akamatsu, T., Kusagaya, H., et al. (2021). Prednisolone and tacrolimus versus prednisolone and cyclosporin A to treat polymyositis/dermatomyositis-associated ILD: a randomized, open-label trial. Respirology 26, 370–377. doi:10.1111/resp.13978
Galindo-Feria, A. S., Albrecht, I., Fernandes-Cerqueira, C., Notarnicola, A., James, E. A., Herrath, J., et al. (2020). Proinflammatory histidyl-transfer RNA synthetase-specific CD4+ T cells in the blood and lungs of patients with idiopathic inflammatory myopathies. Arthritis Rheumatol. 72, 179–191. doi:10.1002/art.41075
Gandiga, P. C., Ghetie, D., Anderson, E., and Aggrawal, R. (2023). Intravenous immunoglobulin in idiopathic inflammatory myopathies: a practical guide for clinical use. Curr. Rheumatol. Rep. 25, 152–168. doi:10.1007/s11926-023-01105-w
Ge, Y., Li, S., Tian, X., He, L., Lu, X., and Wang, G. (2021). Anti-melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis responds to rituximab therapy. Clin. Rheumatol. 40, 2311–2317. doi:10.1007/s10067-020-05530-5
Hamada, T., Samukawa, T., Kumamoto, T., Hatanaka, K., Tsukuya, G., Yamamoto, M., et al. (2015). Serum B cell-activating factor (BAFF) level in connective tissue disease associated interstitial lung disease. BMC Pulm. Med. 15, 110. doi:10.1186/s12890-015-0105-0
Jin, H. Z., Li, Y. J., Wang, X., Li, Z., Ma, B., Niu, L., et al. (2023). Efficacy and safety of telitacicept in patients with systemic lupus erythematosus: a multicentre, retrospective, real-world study. Lupus Sci. Med. 10, e001074. doi:10.1136/lupus-2023-001074
Liossis, S. C., and Bounia, C. A. (2022). Treating autoimmune-related interstitial lung disease with B cell depletion. Front. Med. (Lausanne) 9, 937561. doi:10.3389/fmed.2022.937561
Lundberg, I. E., Tjarnlund, A., Bottai, M., Werth, V. P., Pilkington, C., De Visser, M., et al. (2017). 2017 european league against rheumatism/american college of rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol. 69, 2271–2282. doi:10.1002/art.40320
Matsushita, T., Kobayashi, T., Kano, M., Hamaguchi, Y., and Takehara, K. (2019). Elevated serum B-cell activating factor levels in patients with dermatomyositis: association with interstitial lung disease. J. Dermatol 46, 1190–1196. doi:10.1111/1346-8138.15117
Mecoli, C. A., and Christopher-Stine, L. (2018). Management of interstitial lung disease in patients with myositis specific autoantibodies. Curr. Rheumatol. Rep. 20, 27. doi:10.1007/s11926-018-0731-7
Mockel, T., Basta, F., Weinmann-Menke, J., and Schwarting, A. (2021). B cell activating factor (BAFF): structure, functions, autoimmunity and clinical implications in systemic lupus erythematosus (SLE). Autoimmun. Rev. 20, 102736. doi:10.1016/j.autrev.2020.102736
Moda, M., Yanagihara, T., Nakashima, R., Sumikawa, H., Shimizu, S., Arai, T., et al. (2025). Idiopathic inflammatory myopathies-associated interstitial lung disease in adults. Tuberc. Respir. Dis. Seoul. 88, 26–44. doi:10.4046/trd.2024.0072
Ogawa, Y., Kishida, D., Shimojima, Y., Hayashi, K., and Sekijima, Y. (2017). Effective administration of rituximab in Anti-MDA5 antibody-positive dermatomyositis with rapidly progressive interstitial lung disease and refractory cutaneous involvement: a case report and literature review. Case Rep. Rheumatol. 2017, 5386797. doi:10.1155/2017/5386797
Reff, M. E., Carner, K., Chambers, K. S., Chinn, P. C., Leonard, J. E., Raab, R., et al. (1994). Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 83, 435–445. doi:10.1182/blood.v83.2.435.bloodjournal832435
Saketkoo, L. A., Ascherman, D. P., Cottin, V., Christopher-Stine, L., Danoff, S. K., and Oddis, C. V. (2010). Interstitial lung disease in idiopathic inflammatory myopathy. Curr. Rheumatol. Rev. 6, 108–119. doi:10.2174/157339710791330740
Shi, F., Xue, R., Zhou, X., Shen, P., Wang, S., and Yang, Y. (2021). Telitacicept as a BLyS/APRIL dual inhibitor for autoimmune disease. Immunopharmacol. Immunotoxicol. 43, 666–673. doi:10.1080/08923973.2021.1973493
Shi, Y., You, H., Liu, C., Qiu, Y., Lv, C., Zhu, Y., et al. (2024). Elevated serum B-cell activator factor levels predict rapid progressive interstitial lung disease in anti-melanoma differentiation associated protein 5 antibody positive dermatomyositis. Orphanet J. Rare Dis. 19, 170. doi:10.1186/s13023-024-03153-6
So, H., Wong, V. T. L., Lao, V. W. N., Pang, H. T., and Yip, R. M. L. (2018). Rituximab for refractory rapidly progressive interstitial lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis. Clin. Rheumatol. 37, 1983–1989. doi:10.1007/s10067-018-4122-2
Tanboon, J., and Nishino, I. (2019). Classification of idiopathic inflammatory myopathies: pathology perspectives. Curr. Opin. Neurol. 32, 704–714. doi:10.1097/WCO.0000000000000740
Thong, L., Chawke, L. J., Murphy, G., and Henry, M. T. (2023). Management of myositis associated interstitial lung disease. Rheumatol. Int. 43, 1209–1220. doi:10.1007/s00296-023-05336-z
Tsuji, H., Nakashima, R., Hosono, Y., Imura, Y., Yagita, M., Yoshifuji, H., et al. (2020). Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-Positive dermatomyositis. Arthritis Rheumatol. 72, 488–498. doi:10.1002/art.41105
Vincent, F. B., Saulep-Easton, D., Figgett, W. A., Fairfax, K. A., and Mackay, F. (2013). The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev. 24, 203–215. doi:10.1016/j.cytogfr.2013.04.003
Vu, T. T. T., Brown, K. K., and Solomon, J. J. (2023). Myositis-associated interstitial lung disease. Curr. Opin. Pulm. Med. 29, 427–435. doi:10.1097/MCP.0000000000001000
Wu, D., Li, J., Xu, D., Merrill, J. T., Van Vollenhoven, R. F., Liu, Y., et al. (2024). Telitacicept in patients with active systemic lupus erythematosus: results of a phase 2b, randomised, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 83, 475–487. doi:10.1136/ard-2023-224854
Xu, D., Fang, J., Zhang, S., Huang, C., Huang, C., Qin, L., et al. (2024). Efficacy and safety of telitacicept in primary sjögren's syndrome: a randomized, double-blind, placebo-controlled, phase 2 trial. Rheumatol. Oxf. 63, 698–705. doi:10.1093/rheumatology/kead265
Keywords: idiopathic inflammatory myopathies, interstitial lung disease, B-lymphocyte stimulator, rituximab, telitacicept
Citation: Chen X, Li M, Sun M, Xie X, Zhao X, Chen Y and Dai H (2025) Case Report: Telitacicept for interstitial lung disease associated with idiopathic inflammatory myopathies: an observational study. Front. Pharmacol. 16:1647543. doi: 10.3389/fphar.2025.1647543
Received: 21 June 2025; Accepted: 21 October 2025;
Published: 14 November 2025.
Edited by:
Narasaiah Kolliputi, University of South Florida, United StatesReviewed by:
Ioannis Tassiulas, Icahn School of Medicine at Mount Sinai, United StatesVinod Kumar Yata, Malla Reddy University, India
Copyright © 2025 Chen, Li, Sun, Xie, Zhao, Chen and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: HuanZi Dai, ZGFpaHVhbnppQHRtbXUuZWR1LmNu
 Xue Chen
Xue Chen Mengshan Li
Mengshan Li HuanZi Dai
HuanZi Dai