- 1Department of Pharmacy and Institutes for Systems Genetics, Center for High Altitude Medicine, Frontiers Science Center for Disease-related Molecular Network, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2iGlobal Research and Publishing Foundation, New Delhi, India
- 3Department of Pathology, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States
- 4Department of Pharmacology, Faculty of Pharmacy, Karpagam Academy of Higher Education, Coimbatore, Tamil Nadu, India
- 5School of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab, India
- 6Faculty of Health, Australian Research Centre in Complementary and Integrative Medicine, University of Technology Sydney, Sydney, NSW, Australia
- 7Department of Medical Laboratory Sciences, Lovely Professional University, Phagwara, India
- 8Department of Pharmaceutics and Pharmaceutical Technology, L M College of Pharmacy, Ahmedabad, Gujarat, India
- 9ER stress and mucosal immunology lab, School of Health Sciences, University of Tasmania, Launceston, TAS, Australia
- 10School of Medicine, University of California, San Francisco, San Francisco, CA, United States
- 11Department of Pharmaceutical Engineering, B V Raju Institute of Technology, Medak, Telangana, India
- 12Delhi Institute of Pharmaceutical Sciences and Research, New Delhi, India
- 13IES Institute of Pharmacy, IES University, Bhopal, Madhya Pradesh, India
- 14Amity Institute of Pharmacy, Amity University Uttar Pradesh, Noida, India
- 15MM College of Pharmacy, Maharishi Markandeshwar (Deemed to be) University, Mullana-Ambala, Haryana, India
- 16Department of Medical Biochemistry, Faculty of Basic Medical Sciences, College of Health Sciences, University of Abuja, Abuja, Nigeria
Background: Flavonoids that are widely distributed across various plant species exhibit significant anticancer activity in various preclinical and clinical studies, thus offering promising therapeutic prospects. However, a thorough understanding of their pharmacokinetic properties, drug-likeness characteristics, and safety profile is essential for the translational applicability of these molecules into clinical settings.
Methods: A systematic search was carried out using various electronic databases such as PubMed Central, ScienceDirect, Clinical Registry, and Google Scholar, using different keywords like “flavonoids”, “cancer”, “pharmacokinetics”, “toxicity”, “tumor”, and their combinations. Non-English literature was excluded due to language barriers, limited accessibility, non-indexing, and the risk of misinterpreting methods or results, which could compromise the accuracy and reliability of the review.
Results and discussion: This review provides an in-depth overview of various mechanistic pathways, such as oxidative stress-mediated and immunomodulatory pathways, that are considered to be responsible for the anti-cancer potential of flavonoids. In addition, the pharmacokinetic properties and toxicity profile of flavonoids have been discussed, which are the crucial factors in their clinical translation. Lastly, the review briefly explores various strategies that can be adopted to improve the effectiveness of flavonoids in the treatment of cancer.
Conclusion: This investigation enhances our understanding of the translational potential of flavonoid-based therapies by highlighting these essential elements, bringing us one step closer to the development of effective and safe cancer treatments.
1 Introduction
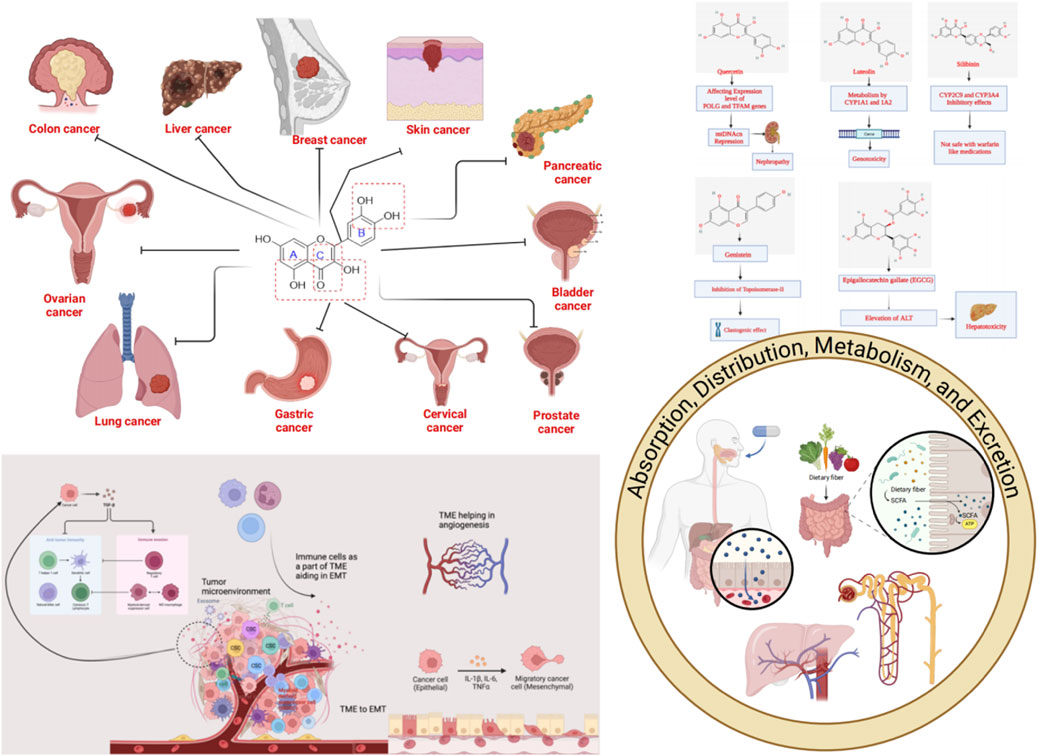
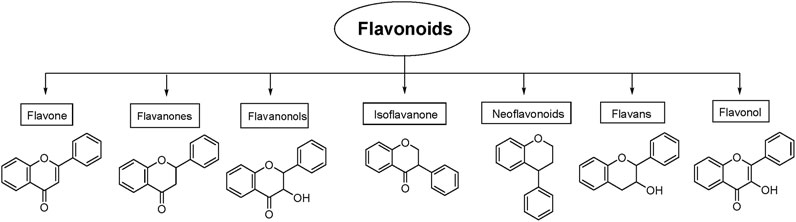
Flavonoids are naturally occurring secondary metabolites that can be obtained from various plant parts such as fruits, vegetables, roots, stems, flowers (Zheng et al., 2025). They function in plants as light filters, visual attractants, feeding repellents, antioxidants, antimicrobials, and photoreceptors (Pietta, 2000). These properties underpin their wide applicability in cosmetics and medicine (Kurek-Gorecka et al., 2020). Flavonoids also have diverse therapeutic applications in humans due to their antioxidant, anticancer, antiviral, anti-inflammatory, and anti-allergic properties (Pietta, 2000; Chavda et al., 2022). Chemically, flavonoids are the polyphenolic compounds that can be classified as flavanols, isoflavonoids, anthocyanidins, flavones, and flavonols (Figure 1) (Panche et al., 2016).
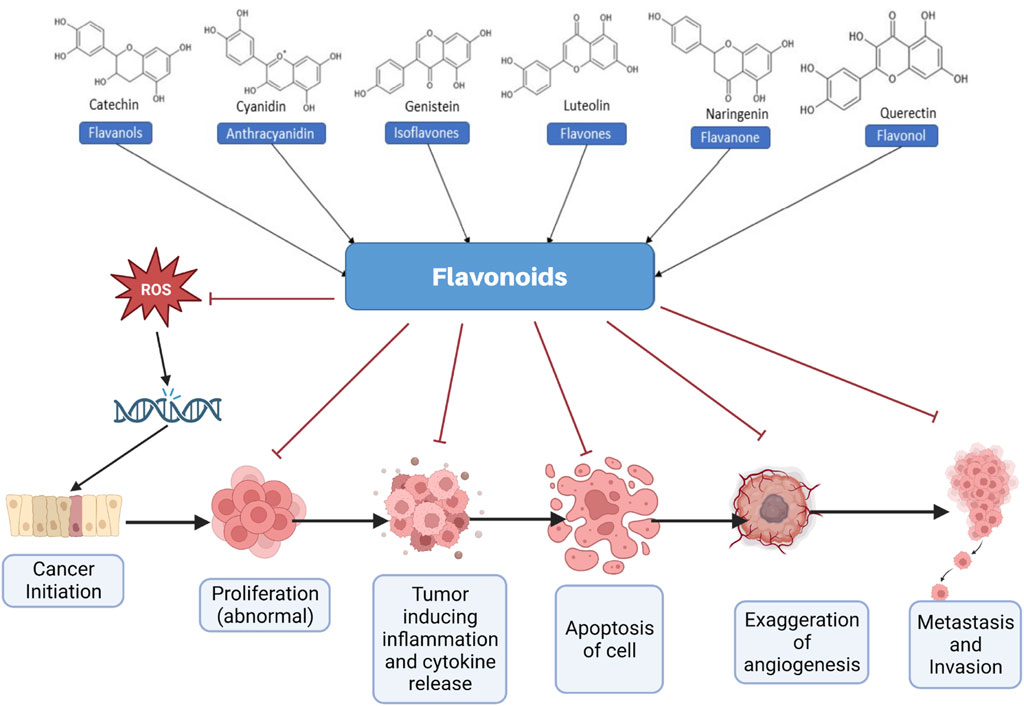
Figure 1. Different types of flavonoids and their role in effectively managing cancer. ROS, Reactive oxygen species.
Flavonoids are widely being recognized as potent anticancer agents that exert their effects through various mechanisms, including scavenging of reactive oxygen species (ROS), promoting apoptosis, inducing cell cycle arrest, anti-angiogenesis, and inhibiting the proliferation and invasion of cancer cells (Pietta, 2000; Rana and Mumtaz, 2025). The hydroxyl groups present in flavonoids are primarily responsible for their therapeutic activities, particularly their antioxidant and ROS-scavenging properties (Vo et al., 2019). Consumption of a flavonoid-rich diet is reported to decrease the cancer risk in various studies (Rodriguez-Garcia et al., 2019; Chavda et al., 2021; Khan et al., 2021; Parmenter et al., 2025). Moreover, various flavonoids, including quercetin, kaempferol, and morin, effectively modulate cancer cell chemoresistance by inhibiting efflux transporters such as P-glycoprotein (P-gp) and multidrug resistance-associated proteins (MRPs), thereby enhancing intracellular drug accumulation and restoring drug sensitivity (Liskova et al., 2021). Additionally, flavonoids modulate resistance-associated signaling pathways, including nuclear factor-κB (NF-κB), phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), mitogen-activated protein kinase (MAPK), and signal transducer and activator of transcription 3 (STAT3), thereby influencing cell survival, apoptosis, and DNA repair mechanisms (Bukowski et al., 2020; Tian et al., 2023; Mir et al., 2024). Figure 1 briefly captures various inhibitory anticancer mechanisms elicited by various flavonoids, in general.
Despite immense therapeutic potential, the unfavorable pharmacokinetic properties of flavonoids limit their use in clinical settings (Hussain et al., 2025). Most flavonoids exhibit poor water solubility, low oral absorption, and undergo extensive first-pass metabolism, resulting in limited systemic bioavailability (Halevas et al., 2022). These pharmacokinetic challenges necessitate the development of innovative delivery systems such as nanoparticles, liposomes, and solid lipid carriers to enhance their stability, absorption, and targeted delivery of flavonoids (Billowria et al., 2024; Hu et al., 2025). Nanoparticle system loaded with flavonoids is reported to increase the drug retention time, thereby enhancing their circulation time and stability at a lower drug dose (Sharma et al., 2022; Abdi Syahputra et al., 2024; Rana and Mumtaz, 2025). Furthermore, structural modification using a prodrug strategy has been explored to introduce favorable physicochemical properties that enhances chemical stability and target specificity of flavonoids (Zhao et al., 2019; Dehelean et al., 2022). Recently, an apigenin prodrug has been synthesized, which exhibited increased stability and cytotoxic potential as compared to the parent compound apigenin (Diamantis et al., 2023). In another study, conjugation of quercetin with amino acids is reported to increase the solubility, stability, cellular permeability, as well as anticancer activity (Dobrydnev et al., 2020). Overall, the expanding field of flavonoid pharmacology now bridges traditional phytomedicine with modern drug development tools, paving the way for clinically translatable anticancer therapeutics (Abdi Syahputra et al., 2024). This article emphasizes the exploration of the aforementioned parameters to ensure the therapeutic efficacy and safety of flavonoids in cancer treatment and management. Flavonoids are highlighted as potent natural anticancer agents with diverse mechanisms but limited clinical applications due to poor pharmacokinetics. Emerging drug delivery systems and prodrug approach aim to bridge the gap.
Rather than a catalog, this review is organized around translation bottlenecks (exposure, selectivity, and safety) and how modern solutions shrink them. Nanoparticle and nanogel carriers consistently raise bioavailability and tumor deposition while reducing off-target exposure; prodrugs and methyl/glycosyl edits rebalance permeability-metabolism tradeoffs; and drug-drug interactions (DDI)-aware scheduling mitigates CYP3A4-linked risks. We map each mechanistic promise (for example, tumor microenvironment (TME) reprogramming, efflux modulation) to an ADME (absorption, distribution, metabolism, and excretion)-compatible formulation and a clinical next step (PK/PD (pharmacokinetics/pharmacodynamics) markers, combo logic), emphasizing 2024–2025 advances (Dewanjee et al., 2023; Abdi Syahputra et al., 2024; Kondža et al., 2024; Hu et al., 2025; Liga and Paul, 2025).
2 Cancer and drug resistance
Cancer remains one of the leading causes of chronic disease-related deaths worldwide, accounting for nearly 10 million deaths in 2020, according to the World Health Organization (WHO). Cancer is characterized by the uncontrolled growth of cells, and can be cured if diagnosed at an early stage (Mathur et al., 2015). Currently, cancer is treated using chemotherapy, surgery, radiation therapy, immunotherapy, endocrine therapy, and targeted therapy. Among these, immunotherapy remains a promising but challenging approach. Side effects associated with cancer chemotherapy, such as immunosuppression, drug resistance, and systemic toxicities, limit the scope of chemotherapeutic agents (Aslam et al., 2014). Surgery and radiation therapy are used to treat a specific tumor or an area of the body (Baskar et al., 2012). Surgical resection causes physical pain to the patients as well as impairs the normal functioning of surrounding tissues (Jain et al., 2014; Chen et al., 2022).
In clinical practice, surgery and chemotherapy are the most commonly used treatments for cancer; however, cancer cells often develop resistance to anticancer drugs over time. Drug resistance is responsible for 90% of chemotherapy failures (Davodabadi et al., 2023). Cancer cells develop resistance to cytotoxic drugs through multiple complex mechanisms, which can be intrinsic or acquired. Intrinsic resistance refers to the inherent ability of cancer cells to withstand therapeutic interventions even before treatment begins. This resistance is heterogeneous and may arise due to inherited genetic mutations that render tumor cells less responsive to chemotherapeutic agents, pre-existence of unresponsive cancer stem cells or activation of intrinsic pathways that can remove or decrease the intracellular concentration of drugs. On the other hand, acquired resistance emerges during treatment and is typically attributed to the development of drug-resistant cancer cell populations that often contain secondary genetic alterations, ultimately leading to therapy failure. Multiple mechanisms have been implicated in acquired resistance, including modulation of TME, alteration in DNA repair mechanisms, dysregulation of transmembrane transporters that reduced intracellular drug concentrations, epigenetic and epistatic modifications, inactivation of drug due to metabolism, and mutations in drug targets (Michael and Doherty, 2005; Eslami et al., 2024; Khan et al., 2024). This critical issue can ultimately result in disease recurrence, treatment failure, and increased mortality. Figure 2 illustrates the diverse factors contributing to drug resistance in cancer. Cancer drug resistance arises from both intrinsic and acquired mechanisms, complicating treatment and contributing to therapy failure. This underscores the need for alternative or adjunctive approaches.
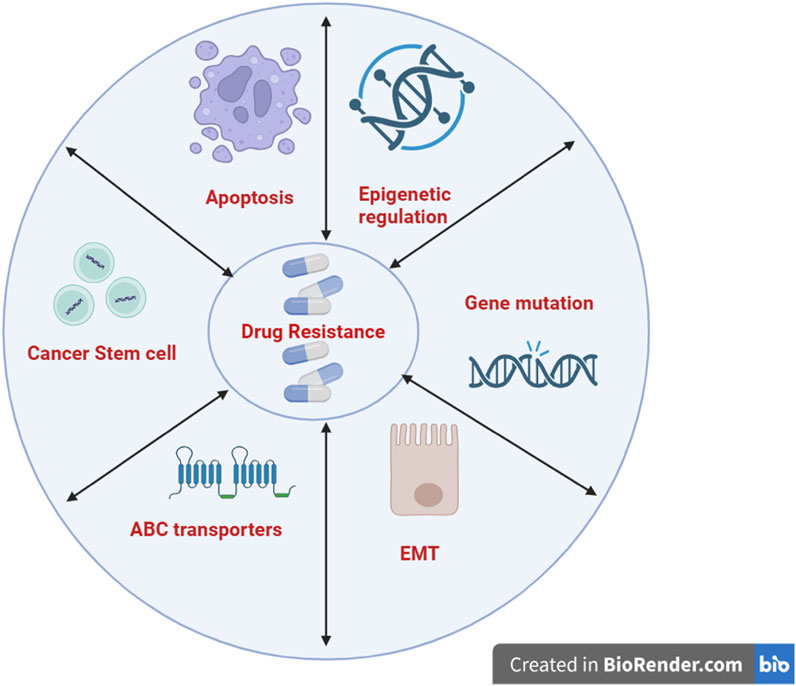
Figure 2. Drug resistance and cancer. Molecular mechanisms like apoptosis, epigenetic regulation, gene mutation, EMT, and ABC transporters are involved. (Created with Biorender.com). EMT, Epithelial-mesenchymal transition; ABC, ATP-binding cassette.
3 Tumor microenvironment- an update
The TME comprises a complex and dynamic network of non-malignant cells, blood and lymphatic vessels, lymphoid organs, and metabolites. TME plays a critical role in the recruitment of non-transformed cells, leading to intercellular communication via the release of signaling molecules such as cytokines (Figure 3). Wound healing is another critical process in TME that contributes to oncogenic signaling and promotes tumorigenesis (Jin and Jin, 2020). The TME is a complex ecosystem, particularly enriched with various immune components, making it a critical target for cancer therapy. For example, T-cells have been central to the development of immune checkpoint inhibitors and chimeric antigen receptor T-cell (CAR-T) therapies. Additionally, the TME hosts multiple innate immune cells, including myeloid-derived suppressor cells (MDSCs) and natural killer (NK) cells, which are modulated by cytokine signaling and can either support immune surveillance or contribute to immune dysfunction, ultimately leading to tumor progression. Given this complexity, a thorough understanding of the immune landscape of the TME is essential for the development of effective immunotherapeutic strategies (Hinshaw and Shevde, 2019).
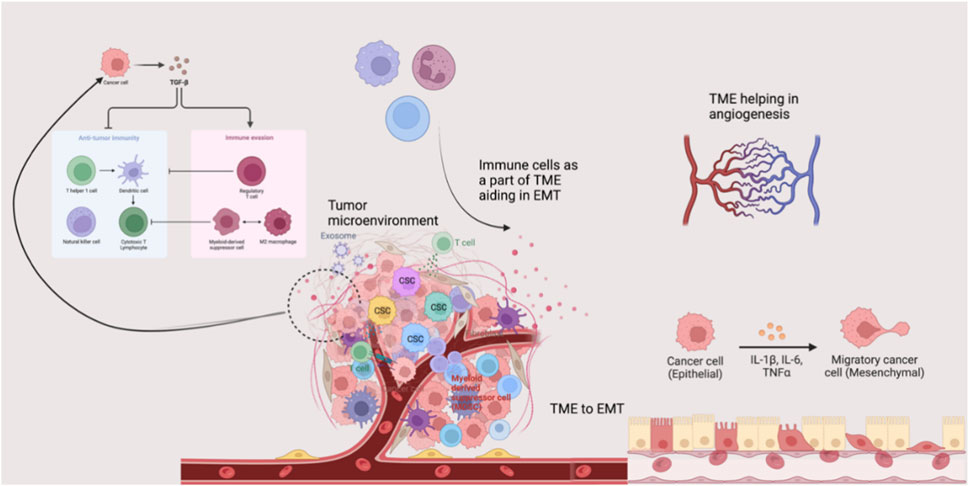
Figure 3. Tumor microenvironment and disease progression. The tumor microenvironment possesses several components that can orchestrate events such as angiogenesis, EMT, and aggressive cancer invasion. TME, Tissue microenvironment; EMT, Epithelial-mesenchymal transition; TGF-β, Transforming growth factor-beta; IL, Interleukin.
In addition to its cellular and molecular components, the TME plays a critical role in regulating epithelial-mesenchymal transition (EMT). During cancer progression and invasion, tumor cells interact with diverse components of TME that switch their phenotype while losing epithelial features. This process helps in the acquisition of mesenchymal and stem cell-like traits that significantly enhance invasiveness and metastatic potential. Evidence reports that TME assists EMT through various signaling pathways and cellular interactions (Suarez-Carmona et al., 2017). Among the various signaling molecules, transforming growth factor-beta (TGF-β) is a key EMT inducer in mediating this process (Yu et al., 2013; de Visser and Joyce, 2023).
Angiogenesis is another crucial TME-regulated mechanism required for advanced cancer progression and metastasis. Angiogenesis ensures a continuous supply of oxygen and nutrients to rapidly growing tumors, thereby facilitating tumor dissemination (Quail and Joyce, 2013). Immune cells present in TME actively contribute to angiogenesis by releasing pro-angiogenic factors that facilitate tumor vascularization. During intravasation and extravasation, macrophages play a significant role in promoting tumor vascularization and facilitating the penetration and traversal of tumor cells across vascular barriers. In systemic circulation, platelets protect tumor cells from immune recognition and channel them to the extravasation site. Additionally, MDSCs and NK cells accumulated in pre-metastatic and metastatic niches support tumor cell dissemination and colonization through modulation of immune responses (Quail and Joyce, 2013). Thus, the TME significantly influences cancer progression via EMT, angiogenesis, and immune modulation. Understanding its complexity is crucial for targeted therapies.
4 Chemistry of flavonoids
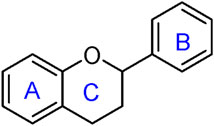
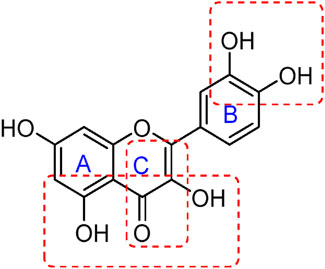
Flavonoids are a group of natural compounds with variable phenolic structures. Chemically, flavonoids are based upon a 15-carbon skeleton made up of two benzene rings, referred to as “ring A” and “ring B” respectively (Figure 4) (Sharma et al., 2019). Heterocyclic pyran “ring C” further connects these rings. Interestingly, “isoflavones” are flavonoids with “ring B” linked to “position 3” of “ring C”. The term “neoflavonoids” refers to phytocompounds where “ring B” is connected in the fourth position of “ring C”. Additionally, linking of “ring B” at “position 2” causes the classification of flavonoids into several groups, including flavones (such as luteolin and apigenin), flavonols (such as quercetin, myricetin, and kaempferol), flavanones (such as naringenin and hesperetin), and flavan-3-ols (such as catechin and epicatechin) (Ignat et al., 2011). Generally, the degree of oxidation and the pattern of “ring C” placement vary among distinct types of flavonoids (Figure 5). In contrast, the diversity of specific compounds within a given class of flavonoids is primarily determined by the substitution patterns on rings A and B (Panche et al., 2016). Thus, flavonoids share a common phenolic skeleton but differ structurally, influencing classification and biological activity. Structural diversity underpins their varied anticancer roles.
5 Flavonoids and their anticancer potential
Numerous chemotherapeutic agents are available in the market for the treatment of cancers, and several are in the development phase. However, the side effects associated with the non-selectivity towards tumor cells of clinically-used chemotherapeutic agenst have limited their widespread use. These limitations have encouraged research on natural products, which are considered much safer than synthetic agents and have traditionally been used to combat cancers for decades (Demain and Vaishnav, 2011; Khan et al., 2021). Several epidemiological studies documented the reduced risk of cancer by consuming a diet rich in fruits and vegetables (Key, 2011; Wu et al., 2019), which is primarily considered due to the presence of flavonoids. The anticancer potential of flavonoids is attributed to the modulation of enzymes and receptors involved in the signal transduction pathways related to cellular differentiation, proliferation, apoptosis, angiogenesis, metastasis, and reversal of multidrug resistance (Ravishankar et al., 2013). The beneficial effects of flavonoids in cancers are attributed to their antioxidant, immunomodulatory, and anti-inflammatory properties. Natural flavonoids shows anticancer effects by modulating signaling pathways, reducing oxidative stress, and reversing drug resistance. Their safety profile makes them attractive alternatives to synthetic agents.
5.1 Influence on tumor microenvironment
Flavonoids have been traditionally employed to carry out potent anticancer activity, incredibly successfully altering the TME. Compounds like epigallocatechin-3-gallate (EGCG) were used to impede TME activity by decreasing the infiltration of tumor-associated macrophages (TAM) in a mouse model of breast cancer (Kopustinskiene et al., 2020). Another flavonoid, anthocyanin, a typical pigment, was noted to increase the levels of anti-inflammatory cytokines such as interleukin-10 (IL-10), enhancing NK cell activation and eliminating tumor cells. Anthocyanins also suppress macrophage infiltration, which affects TME (Lin et al., 2017). Flavonoids possess potent anticancer and antioxidant activities, particularly through their ability to influence tumor regression. One such compound, apigenin, has been shown to profoundly impact the vascular aspects of the TME. Specifically, in a 2014 preclinical study by Mirzoeva et al., treatment with apigenin at 20 µM led to a significant decrease (∼45%, p < 0.01) in vascular endothelial growth factor (VEGF) expression in TGF-β-stimulated human breast cancer cells (MDA-MB-231). This anti-angiogenic effect was mechanistically linked to apigenin’s inhibition of Smad2/3 phosphorylation, as well as reduced activation of Src and downstream FAK/Akt signaling pathways. In vivo, mice bearing MDA-MB-231 xenografts treated with 50 mg/kg apigenin exhibited a ∼35% reduction in microvessel density, measured via CD31 immunostaining, corroborating the in vitro pathway modulation (Mirzoeva et al., 2014; Ou et al., 2024). Simultaneously, naringenin, a related flavonoid, demonstrated complementary TME-modulating effects by promoting anticancer immunity. Kaufman et al. observed that naringenin increased the number of CD169+ tumor-associated macrophages, which in turn led to the activation of cytotoxic T cells. It also inhibited lung cancer and melanoma metastasis through suppression of TGF-β/Smad-mediated MMP-2 expression (Kaufman et al., 1997; Liskova et al., 2020; Kawaguchi et al., 2022). These mechanistic and preclinical findings support the proposition that flavonoids such as apigenin and naringenin can effectively modulate TME vascularity and suppress metastatic pathways, reinforcing their promise as bioactive agents against cancer (Dobrzynska et al., 2020). Thus, the results support the translational promise for flavonoids like apigenin and naringenin.
5.2 Oxidative stress-mediated pathways
The ROS produced by many cellular organelles such as mitochondria, peroxisomes, and endoplasmic reticulum during cellular metabolism is one of the critical signaling molecules that play prominent roles in various diseases, including cancer (Aggarwal et al., 2019). The body’s defense mechanisms regulate the levels of ROS through the production of specific enzymes like superoxide dismutase, catalase, and glutathione peroxidase, and their nonenzymatic counterparts such as glutathione, ascorbic acid, and α-tocopherol. However, the imbalance between ROS production and antioxidant defense mechanisms results in oxidative stress. An abnormally high level of ROS activates several signaling pathways, ultimately leading to cell death. They react with various biomolecules, viz. proteins, lipids, and DNA, to initiate chain reactions, leading to the oxidation of lipids and proteins, DNA damage, and mitochondrial dysfunction (Kaushal et al., 2019).
Flavonoids have dual actions in ROS homeostasis, acting as antioxidants and pro-oxidants (Kopustinskiene et al., 2020). Due to their reducing nature, flavonoids exert an antioxidant effect and exhibit anticancer properties. The underlying mechanisms of the anti-oxidative potential of flavonoids are direct scavenging of ROS, inhibiting oxidases that are responsible for the production of superoxide anion, chelating trace metals, and activating antioxidant enzymes (Procházková et al., 2011; Slika et al., 2022). Interestingly, a study by Lotito and Frei concluded that after consumption of the flavonoid-rich foods, the increase in the plasma antioxidant capacity is not attributed to the flavonoids themselves, but to the consequent increase in uric acid levels (Lotito and Frei, 2006). Filipe and the team showed that the flavonoids inhibited the urate (uric acid) degradation, and it is positively correlated with the inhibition of lipid peroxidation. In a dose-dependent manner, urate inhibited copper-induced lipid peroxidation (Filipe et al., 2001). However, this is contradictory to the studies where flavonoids lead to a decrease in uric acid levels in the patients/subjects/treatment groups with conditions of hyperuricemia, via inhibition of xanthine oxidase (Lin S. et al., 2015). In a cross-sectional study by Li and the team, they demonstrated that a diet that is rich in anthocyanins and flavanones significantly lowers uric acid levels and also has a lower incidence of hyperuricemia (Li H. et al., 2023). Acute intake of flavonoid-rich foods like apples leads to an increase in uric acid levels, possibly because of fructose content. Chronic flavonoid intake, especially pure compounds like quercetin, leads to improved renal excretion of uric acid and also a decrease in uric acid synthesis via xanthine oxidase inhibition. Thus, flavonoids exhibited a dual role on the uric acid level, which is a context-dependent relationship and needs larger population-based studies for a clear understanding.
Importantly, elevated ROS levels are implicated in cancer drug resistance through various mechanisms such as activating pro-survival pathways (e.g., PI3K/Akt, MAPK, and NF-κB), enhancing DNA repair processes, and inducing the expression of efflux transporters (e.g., P-gp), thus limiting chemotherapy effectiveness. Flavonoids specifically counteract the ROS-mediated drug resistance by directly scavenging ROS, thereby attenuating oxidative stress-induced signaling pathways that promote chemoresistance. Additionally, flavonoids modulate key enzymes such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, xanthine oxidase, and cyclooxygenase-2, which are major sources of ROS involved in chemotherapy resistance (Passaniti et al., 2022; Li Y. et al., 2023; Attique et al., 2025). By inhibiting these ROS-producing enzymes, flavonoids effectively attenuate oxidative stress, thereby sensitizing cancer cells to conventional chemotherapeutics (Al-Khayri et al., 2022; Billowria et al., 2024). Recently, kaempferol has been reported to induce ROS-dependent apoptosis in pancreatic cancer cells via transglutaminase (TGM2)-mediated Akt/mTOR signaling. Hence, TGM2 is regarded as a promising prognostic biomarker for pancreatic cancer (Wang F. et al., 2021). In addition to oxidative stress-induced apoptosis, kaempferol enhanced the cytotoxic potential of doxorubicin by amplifying ROS toxicity and decreasing the efflux of doxorubicin (Sharma et al., 2007). At the molecular levels, kaempferol suppressed cancer cell proliferation by inhibiting the function of phosphorylated Akt, CyclinD1, CDK4, Bid, Mcl-1, and Bcl-xL, and promoting p-BRCA1, p-ATM, p53, p21, p38, Bax, and Bid expression that trigger apoptosis and S phase arrest (Wu et al., 2018). Similarly, quercetin is also reported to induce apoptosis and ROS-mediated cell death, exert cell cycle arrest at the S and G2/M phases, and display notable anti-metastatic properties (Tubtimsri et al., 2025). In a preclinical study, both quercetin and kaempferol significantly reduced tumor size, demonstrating anticancer activity consistent with their in vitro findings (Kusaczuk et al., 2024).
5.2.1 Scavenging of ROS
The unique structural features of flavonoids, such as hydroxy groups in ring B, 2,3-double bond in conjugation with a 4-oxo functionality in the C ring, and hydroxyl groups at positions 3 and 5 for hydrogen bonding to the oxo group, have empowered the flavonoids with ROS scavenging abilities (Figure 6). Flavonoids scavenge the highly oxidizing free radicals by directly donating hydrogen atoms and forming flavonoid radicals (o-semiquinone) (Rolt and Cox, 2020). These radicals, in turn, depending upon their structures, further undergo either coupling with another oxidizing radical or donate another hydrogen atom to form quinone or form a dimeric product by dimerization with another flavonoid radical (Figure 7) (Slika et al., 2022).
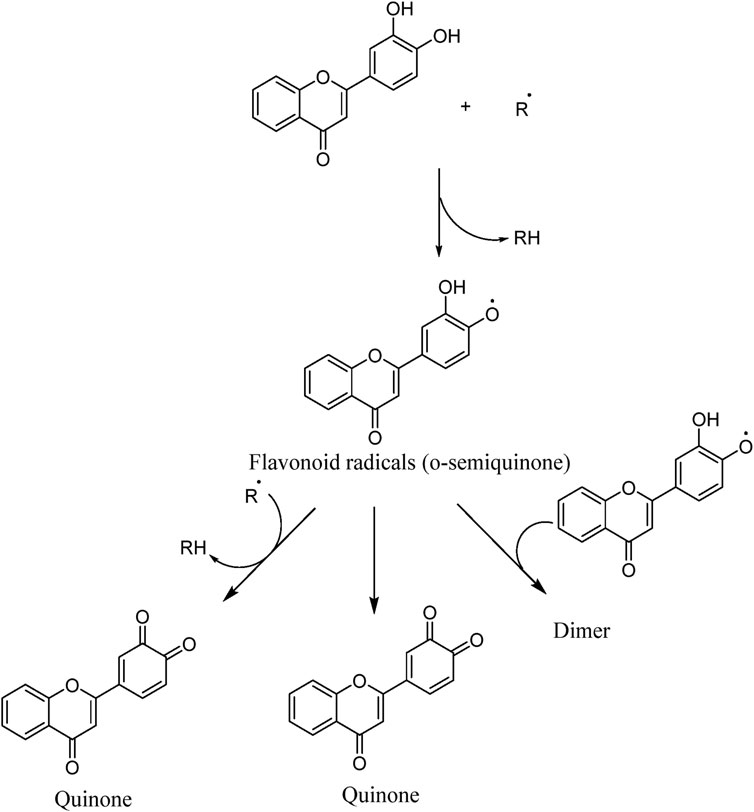
Figure 7. ROS scavenging mechanism of flavonoids (Slika et al., 2022).
5.2.2 Inhibition of ROS-producing enzymes
In addition to directly scavenging ROS species, inhibition of ROS-producing enzymes such as xanthine oxidase, cyclooxygenase, and NADPH oxidase is a significant attribute of the antioxidant ability of flavonoids. Xanthine oxidase is a critical enzyme that catalyzes the oxidative hydroxylation of hypoxanthine and xanthine to uric acid, which subsequently reacts with molecular oxygen, leading to the generation of ROS, primarily superoxide anion radical or hydrogen peroxide (Lin S. et al., 2015). Moreover, inhibition of xanthine oxidase increases the levels of hypoxanthine and xanthine, which in turn inhibit de novo purine biosynthesis via a feedback mechanism, an essential treatment pathway for some of the antineoplastic drugs (Rahaman et al., 2022). Several flavonoids such as quercetin, chrysin, luteolin, kaempferol, myricetin, and isorhamnetin are potent inhibitors of xanthine oxidase (Chang et al., 1993; Nagao et al., 2014), and therefore decrease the risk of oxidative stress-induced diseases. Among the various flavonoids, planar flavones and flavonols with a 7-hydroxyl group have been reported to possess potent xanthine oxidase inhibitor activity as compared to nonplanar flavonoids like isoflavones and anthocyanidins (Nagao et al., 2014). Hydroxyl groups at positions 5 and 7 in ring A are essential for binding to amino acid residues of the active site of the enzyme xanthine oxidase via hydrogen bonding. In addition, a double bond at the 2,3-position in ring C and the presence of keto functionality at the 4-position are required for inhibitory activity. Furthermore, pi-pi stacking between ring B and the amino acid residue of xanthine oxidase enhances the active site binding (Van Hoorn et al., 2002; Mathew et al., 2015). Blumeatin is a predominantly non-coplanar flavonoid, featuring an over-twisted torsion angle of approximately −94.6° between rings A and B. Contrary to expectations, this structural deviation correlates with enhanced radical scavenging activity, likely by influencing the accessibility and reactivity of its hydroxyl groups, although it may still reduce efficacy in some enzyme-binding contexts (Altunayar-Unsalan and Unsalan, 2024; Li et al., 2024).
Overexpression of cyclooxygenase (COX)-2 has been implicated in the development and progression of various human cancers and is associated with the resistance of cancer cells to conventional chemotherapy and radiotherapy (Shim et al., 2003; Pang et al., 2016). Expression of COX-2 is modulated by various vital regulators such as peroxisome proliferator-activated receptor gamma (PPAR-γ) (KnopfovÁ and ŠMarda, 2010), NK-κB (Lim et al., 2001), and TGF-β (Saha et al., 1999). The interplay between flavonoids and these key regulators suppresses the COX expression, showing beneficial effects in many diseases (Ramachandran et al., 2012; Li et al., 2020; Yan et al., 2020). Mutoh et al. reported quercetin as the most potent suppressor of COX-2 transcription, while catechin and epicatechin showed weak activity (Mutoh et al., 2000). The presence of the 4-oxo group in ring C, 3,4-dihyroxyl groups in ring B, and low electron density of the oxygen atom in the 7-hydroxyl group in ring A are considered to be important structural features in flavonoids for the suppression of COX-2 transcriptional activity (Mutoh et al., 2000; Hou et al., 2005). In several studies, flavonoids have been reported to show chemopreventive action via suppression of the COX enzyme (Sakata et al., 2003; Ye et al., 2004; Al-Fayez et al., 2006; Chen et al., 2008). In contrast to xanthine oxidase and other enzymes that generate ROS as byproducts, NADPH oxidases are the only enzymes dedicated to ROS generation as their primary product (Maraldi, 2013). Flavonoids have been identified as more potent NADPH oxidase inhibitors than ROS scavengers (Jimenez et al., 2015; Luo et al., 2019). Flavonoids having hydroxyl groups in ring B and saturated 2,3 bond in C ring exhibited NADPH oxidase inhibitor activity, which is potentiated by the presence of vicinal hydroxy-methoxy arrangement at an aromatic ring (Steffen et al., 2008).
5.2.3 Chelating trace metals
Metal ions such as Fe and Cu are reported to catalyze the production of ROS species, which have detrimental effects on many diseases. In the presence of hydrogen peroxide, iron, even in small traces, catalyzes hydroxyl radical formation via the Fenton reaction (Lyngsie et al., 2018). Copper reacts with hydrogen peroxide via a free radical mechanism to form superoxide and hydroxyl radicals (Angelé-Martínez et al., 2017). Oxidative stress-induced cellular redox imbalance has been implicated in various cancer cells. By their metal-chelating properties, flavonoids play a significant role in metal overload diseases. Quercetin, rutin, and catechin have more substantial complexation properties toward iron and zinc ions than copper ions (Chamani et al., 2016). In another study, flavones (apigenin, luteolin, kaempferol, quercetin, myricetin, and rutin), isoflavones (daidzein and genistein), flavanones (taxifolin, naringenin, and naringin), and a flavanol (catechin) exhibited higher reducing capacity for copper ions than for iron ions (Mira et al., 2009). Morin, combined with another flavonoid (naringin), exhibits synergistic activity in the chelation of lead ions (Adhikari et al., 2018).
Moreover, metal ion-flavonoid complexes, besides retaining the antioxidant activities, are much more effective free radical scavengers than the free flavonoids, which is assumed to be due to the acquisition of additional superoxide dismutating centers (de Souza and De Giovani, 2013). Structural features of flavonoids revealed that unsaturation at the 2,3-position, a hydroxyl group at the three-position, and a catechol group in ring B showed better iron-reducing activity (Mira et al., 2009). In flavonols and flavones, the most efficient copper chelation sites were the 3-hydroxy-4-keto group (ring C) and 5,6,7-trihydroxyl group (ring A), respectively, as the 3′,4′-dihydroxyl group (ring B) was associated only with weak activity (Ríha et al., 2014). Chelation of metal ions by flavonoids renders them inactive in generating radicals. Also, the generated radicals are intercepted by flavonoids themselves, making them an essential compound in managing metal-overload-induced oxidative stress-based diseases.
5.2.4 Activation of antioxidant enzymes
Another possible mechanism by which flavonoids act is by activating antioxidant enzymes, suppressing pro-oxidant enzymes, and stimulating the production of antioxidant enzymes and phase II detoxification enzymes (Kopustinskiene et al., 2020). On exposure to ROS, various genes encoding detoxifying and antioxidative stress enzymes/proteins are coordinately induced, which is regulated by antioxidant-responsive element (ARE) or electrophile-responsive element (EpRE). The coordination of ARE/EpRE encodes various phase II detoxifying enzymes such as NAD(P)H-quinone oxidoreductase 1, glutathione-S-transferase, and UDP-glucuronosyl transferase (Itoh et al., 2004). Nuclear factor erythroid-derived 2-related factor 2 (Nrf2), sequestered in the cytoplasm by Kelch-like ECH-associated protein 1 (Keap1), regulates the transcription of ARE-driven genes. On exposure of cells to ARE inducers, Nrf2, after dissociation from Keap1, is translocated to the nucleus. Here, it heterodimerizes with a small Maf protein and binds to ARE, eventually leading to transcriptional regulation of target genes (Lee and Surh, 2005; Uruno and Motohashi, 2011). Flavonoids promote the translocation of Nrf2 to the nucleus, activating detoxifying enzymes that facilitate the detoxification of carcinogens (Moon et al., 2006). Genistein, a dietary flavonoid, has been reported to show antioxidant defense by induction of Nrf2 and phase II detoxification gene expression via extracellular signal-related protein kinases and its pathway (Zhai et al., 2013). Another class of flavonoids, anthocyanins, has been demonstrated to show antioxidant capacity in cell line studies by activating ARE upstream of genes involved in antioxidation and detoxification (Shih et al., 2007).
In summary, flavonoids regulate ROS homeostasis by scavenging radicals, inhibiting ROS-producing enzymes, chelating metals, and activating antioxidant defenses. This dual role contributes to cancer prevention and sensitization to therapy.
5.3 Anti-inflammatory and immunomodulatory-mediated pathways
Chronic inflammation plays a significant role in cancer initiation, promotion, and progression (Coussens and Werb, 2002). Inflammatory mediators like cytokines, ROS, and reactive nitrogen species derived from tumor-infiltrating immune cells induce epigenetic alterations in premalignant lesions and silence tumor suppressor genes in the initial phase of tumor development (Multhoff et al., 2012). Various oncogenic transcription factors, such as NK-κB and STAT3, are often activated by inflammatory mediators (primarily due to the elevated production of IKK-activating cytokines, including tumor necrosis factor (TNF) and IL-1). In contrast, oncogenes such as Ras and Myc can initiate inflammatory responses (Taniguchi and Karin, 2018). Tumor-associated inflammation transforms tumor-specific immune cells from anti-tumorigenic to pro-tumorigenic, suppressing the anti-tumor immune response (Grivennikov and Karin, 2010). During the promotion phase, various chemokines and cytokines secreted by immune cells act as survival and proliferation factors for malignant cells. During the tumor progression and metastasis, tumor and immune cells produce further cytokines and chemokines, increasing cell survival, motility, and invasiveness (Grivennikov and Karin, 2010; Multhoff et al., 2012). Alterations in many of the cell signaling pathways, particularly those in levels of kinases such as MAPK and protein kinases that regulate cell proliferation and differentiation, have been implicated in the inflammatory processes. Silencing or abnormal activation of these kinases or their downstream transcription factors can result in uncontrolled cell growth, leading to malignant transformation (Fresco et al., 2006; García-Lafuente et al., 2009).
Increasing scientific documentation regarding the anti-inflammatory potential of flavonoids (Wei et al., 2005; Feng et al., 2012) has led to their exploration of several diseases, including cancer. Mechanistically, the anti-inflammatory potential of flavonoids is attributed to their antioxidant and radical scavenging activities, regulation of immune cells, modulation of enzymes involved in the biosynthesis of inflammatory mediators, and regulation of proinflammatory gene expression (García-Lafuente et al., 2009) (Figure 8). Several in vitro and preclinical studies have demonstrated the potential role of flavonoids in preventing carcinogenesis. Some of these studies and their mechanism have been summarized in Table 1. Concludingly, flavonoids modulate immune and inflammatory pathways, suppressing tumor-promoting signals while enhancing chemoprevention. Their mechanisms span enzyme regulation, cytokine modulation, and signaling interference.
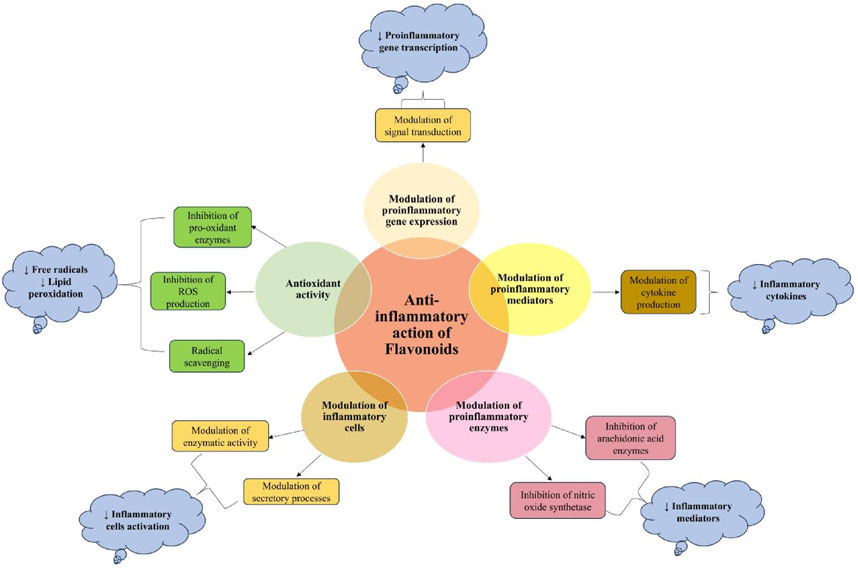
Figure 8. Mechanistic pathways of anti-inflammatory action of flavonoids (García-Lafuente et al., 2009).
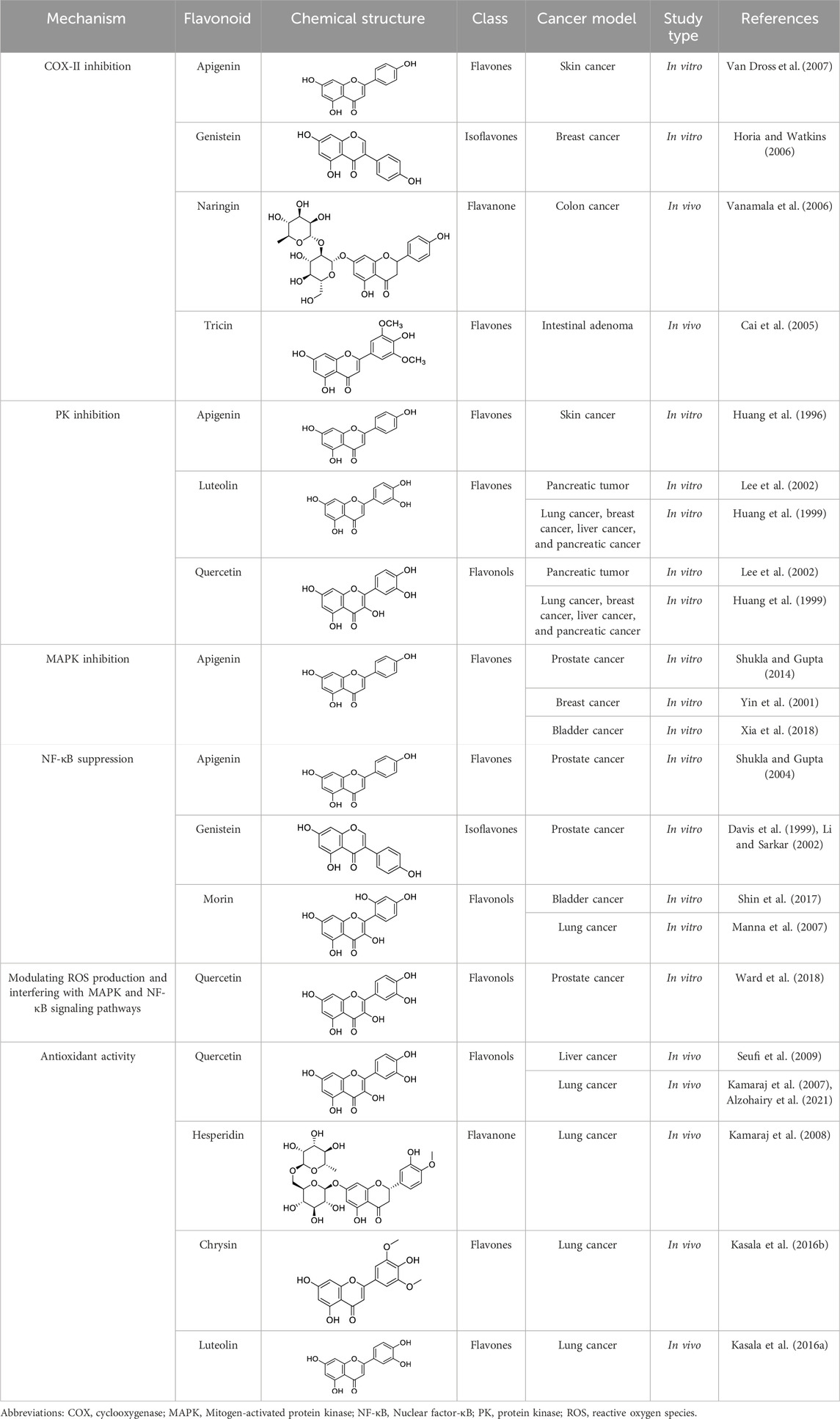
Table 1. In vitro and preclinical studies demonstrating anti-inflammatory mechanisms implicated in chemoprevention.
6 Emerging flavonoid subclasses with translational promise
While quercetin, apigenin, and kaempferol dominate historical discussion, several less-covered subclasses now show compelling anticancer signals together with formulation paths that address exposure limits. Below we highlight four such leads and outline mechanisms, ADME opportunities, and near-term study designs.
6.1 Isorhamnetin (flavonol; 3′-O-methylquercetin)
A 2025 state-of-the-art review comprehensively mentioned isorhamnetin’s multi-hallmark actions, including cell cycle arrest (G1/S, G2/M), apoptosis (mitochondrial/caspase), anti-angiogenesis, anti-metastasis, and immune/TME modulation, and collates nanoformulation-based approaches that improve delivery and bioavailability (Rana et al., 2025). These data support rational combinations (for example, with cytotoxic or targeted agents) and motivate PK-anchored designs that track target-pathway readouts (e.g., PI3K/AKT/mTOR, NF-κB) (Rana et al., 2025).
6.2 Prunin (naringenin-7-O-glucoside; flavone glycoside)
The first focused review in 2025 positions prunin as emerging anticancer candidate that modulates cell cycle and apoptosis pathways, with early evidence for combination use and nanoformulations to overcome solubility/bioavailability limits (Rana and Mumtaz, 2025). Given its glycosidic nature and gut microbiota conversion to prunetin, studies should integrate metabolite profiling with exposure-response endpoints (Rana and Mumtaz, 2025).
6.3 Taxifolin/dihydroquercetin (flavanonol)
A recent comprehensive review details direct antitumor activity (proliferation/migration inhibition, apoptosis, and anti-angiogenesis), chemosensitization (e.g., P-gp downregulation), immunomodulation, and a strong cytoprotective profile against several chemotherapy-based toxicities, while explicitly noting PK limitations and delivery/chemistry solutions (Sarg et al., 2024). These features make taxifolin a pragmatic adjunct in combination regimens, provided exposure is optimized (Sarg et al., 2024).
6.4 Dihydromyricetin/ampelopsin (dihydroflavonol)
A recent mechanistic review summarizes broad anticancer actions, including anti-proliferative, pro-apoptotic, anti-invasion, autophagy modulation, and redox regulation, across diverse array of cancers. This review further highlights instability/low bioavailability as translation bottlenecks that are being addressed with nano- and crystal-based systems (Xia and Zhu, 2024). Early combination data with standard chemotherapies further justify exposure-controlled and biomarker-rich trials (Xia and Zhu, 2024).
These subclasses broaden the field beyond “the usual suspects”, and critically arrive with formulation blueprints (such as nanoparticles, nanogels, and lipid carriers), that raise tumor deposition and reduce systemic peaks.
7 Clinical trials evaluating the anticancer potential of flavonoids
Numerous epidemiologic studies have demonstrated that certain flavonoids, as well as diets or foods rich in flavonoids, are beneficial in preventing cancer development and its progression. Moreover, flavonoids reduce the established high-risk indicators associated with cancer (Baby et al., 2021). However, additional clinical trial studies are required to assess the efficacy of flavonoids in people with a range of characteristics, including age, gender, disease severity and stage, prior treatment history, and comorbid medical conditions (Kikuchi et al., 2019).
Flavonoids, like those found in tea and coffee, are consumed worldwide, and several research studies have demonstrated their positive benefits on human wellbeing, including their ability to prevent cancer. According to an analysis by Yang and Hong of case-controlled and prospective cohort studies that were published in 2008, the consumption of green tea reduced the cancer risk in 39 cases of colon, breast, esophageal, kidney, lung, ovarian, pancreatic, stomach, and prostate cancers, whereas 46 cases indicated no risk reduction. These observations imply that green tea may help to avoid certain cancers (Yang and Hong, 2013; Suzuki et al., 2016; Hayakawa et al., 2020). Therapeutic potential of flavonoids significantly differs with various factors like age, gender, and disease state. Geriatric patients exhibit enhanced benefits due to increased oxidative stress patterns but decreased bioavailability from various metabolic changes with age-related factors. The flavonoid’s phyloestrogenic activity is associated with various gender differences. In the cases of various disease states like diabetes/inflammation may enhance the antioxidant effects of flavonoids by making a mediation in gut microbiota-mediated metabolism. Such factors as age, gender, and diseases need to be considered for flavonoid-based therapeutic approaches (Del Rio et al., 2013; Fraga et al., 2019; Favari et al., 2024; Hu et al., 2025).
To assess the effectiveness of flavonoids in cancer therapy, Bisol et al. looked at some clinical trial studies (Phases II and III). The U.S. Food and Drug Administration (FDA) states that phase II clinical studies are carried out to evaluate a medicine’s effectiveness in treating an illness, and phase III studies are carried out to confirm that a possible drug is superior to the gold standard treatment option. In all, 1 phase III and 22 phase II clinical trials that employed flavonoid compounds as a single entity or in combination with other therapies to treat lymphoid cancer that were published by January 2019 were chosen for study. It was shown that flavopiridol is the most frequently administered flavonoid compound (at a dose of 50 mg/m2 IV) for most of the cases. Flavonoids demonstrated sophisticated positive consequences for lymphoid and hematopoietic tissues than for solid tumors (4 patients with complete response (CR) and 21 with partial response (PR) among 525 patients in 12 trials), despite the relatively low rate of CR or partial response PR with any administration protocol (Bisol et al., 2020). Some of the flavonoids whose anticancer potential in human subjects has been evaluated or are currently under investigation have been summarized in Table 2. 60 trial entries spanning ∼17 flavonoid categories/combos are covered where the most studied agents are catechin, genistein, quercetin, and EGCG. While there was strong emphasis on prostate malignancies followed by multiple studies related to breast, lung, bladder, colon/colorectal, and skin cancer, there were trials related to ovarian, cervical, esophageal, kidney, head and neck premalignancy, glioma/glioblastoma, multiple myeloma, and follicular lymphoma. When considering the phase trials status, predominantly phase 2, with some phase 1/early phase 1, and only a handful of phase 3 studies. Further, studies include purified flavonoids, botanical preparations, and drug-flavonoid combinations, reflecting an adjunct/chemo-sensitization emphasis. While epidemiological studies and early-phase trials suggest promise, clinical outcomes remain variable and context-dependent. More large-scale, controlled studies are needed.
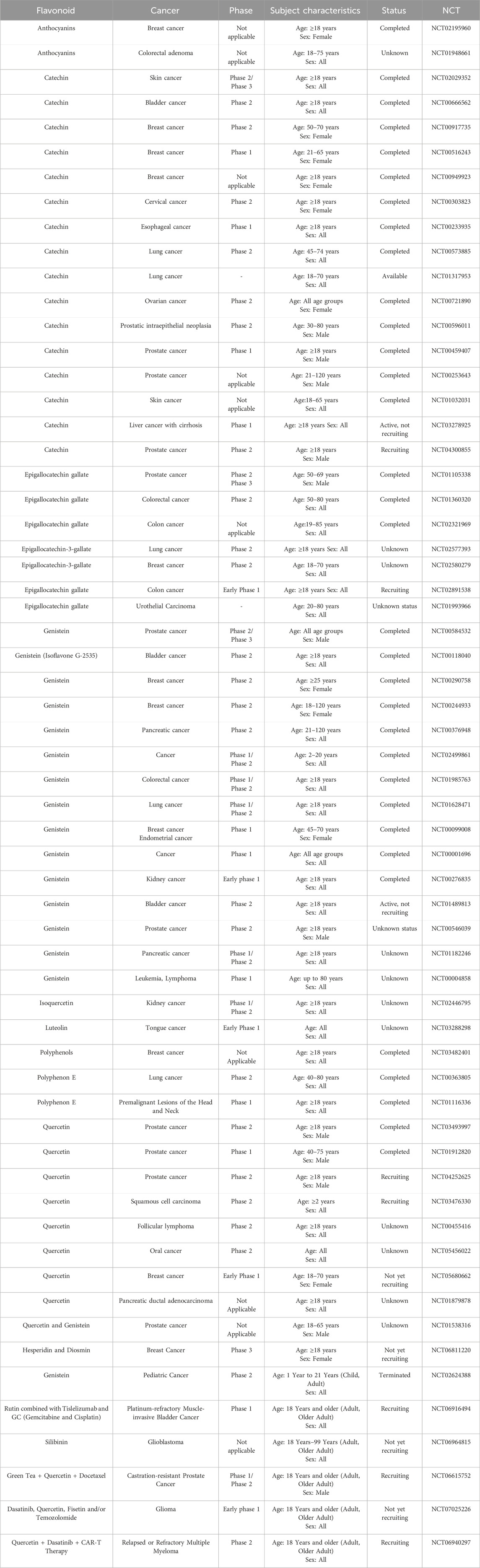
Table 2. Clinical studies on the anticancer potential of flavonoids [https://www.clinicaltrials.gov/(Accessed August, 2025)].
8 ADME properties of anticancer flavonoids
Here we summarize what currently limits oral/systemic exposure for flavonoids and which delivery/chemistry knobs reliably move the PK needle. Once flavonoids are taken orally, it becomes crucial to comprehend their pharmacodynamics. Additionally, it is beneficial to analyse the available structure-activity relationship (SAR) data to identify the pharmacological activities associated with flavonoids. In conjunction with SAR attributes, the pharmacokinetics, biotransformation, and metabolism of flavonoids play a significant role in determining their pharmacological effects. Further investigation is required to elucidate factors such as absorption rate, pharmacokinetics, metabolite characterization, and the physiological effects of these metabolites to establish a correlation between the SAR of flavonoids and their impact on human nutrition and medicine. Flavonoids are commonly found in food items in the form of O-glycosidic compounds, which are conjugated with glucose, glucorhamnose, arabinose, galactose, or rhamnose units (Hammerstone et al., 2000). When checked on PubMed using search terms including flavonoid, ADME, and cancer, it yielded 67 articles. We read the abstracts of each of these articles and then selected studies relevant to this theme.
The β-linkages in these sugars make them resistant to hydrolysis by pancreatic enzymes. However, the process of β-hydrolysis is carried out by specific intestinal microbiota. Such microbiota include Peptococcus, Peptostreptococcus, and Clostridia, which ferment these sugars in the colon (Cummings and Macfarlane, 1991). Specific enzymes like lactase, phlorizin hydrolase, classified as β-endoglycosidase, are known to deglycosylate flavonoids, creating sites for conjugation (Leese and Semenza, 1973; Day et al., 2000). Flavonoids such as quercetin-3-glucoside, luteolin-7-glucoside, and kaempferol-3-glucoside are absorbed and hydrolyzed in the small intestine through the action of β-glucosidases (Gopalan et al., 1992).
Flavonoids, a diverse group of compounds known for their abundance and potency, have been extensively studied to gain insights into their absorption and metabolism (Hollman et al., 2009). The absorption kinetics of flavonoids can vary significantly due to different sugars and functional groups in the flavan nucleus. Several factors, including dosage, administration route, vehicle of administration, colon microbiota, diet, food matrix, and sex differences, contribute to the variability in flavonoid absorption (Erlund, 2001).
In addition to the hydrolysis of flavonoid glycosides, the cecal microflora also plays a role in breaking down monomeric flavonoids into monophenolic acids. For instance, intestinal bacteria can cleave the C3-C4 bond of the heterocyclic structure, resulting in the production of quercetin metabolites such as 3,4-dihydroxy-phenylacetic acid and phloroglucinol (Winter et al., 1991).
A previous study revealed that rats exposed to rutin produced specific compounds, including 3,4-dihydroxytoluene, phenylacetic acids, and 3-(m-hydroxyphenyl) propionic acid, in their urine (Baba et al., 1983). The hydrolysis of rutinosides (quercetin-3-rutinoside) by colonic microflora was found to be more significant compared to quercetin glycoside (quercetin-3-glucoside), explaining the lower bioavailability of rutin observed in human studies (Olthof et al., 2000). In human subjects, the absorption of quercetin in the small intestine was found to increase to 52% when a glucose unit was present in the molecular structure, compared to 24% for the aglycone unit and 17% for rutin-based compounds. This increase in absorption suggests that quercetin glucoside facilitates interaction with epithelial glucose transporters, leading to a more rapid uptake and improved bioavailability of glucosides following ingestion (Gee et al., 1998).
The biodegradation of larger flavone molecules into more minor, low molecular weight compounds is necessary to cross the intestinal epithelium. According to Déprez et al., dimers and trimers of procyanidin can move across the epithelium of the small intestine. Since these molecules typically consist of (+) catechin and (−) epicatechin subunits, catechins are likely the primary by-products of degradation (Déprez et al., 1999). The bacterial colony facilitates this degradation process in the cecum, and the lower pH in the stomach (Spencer et al., 2000; Groenewoud and Hundt, 2009). The hydrolysis of proanthocyanidin oligomers into catechin dimers and free catechins takes approximately 3.5 h in the gastric environment. After three catechin units, the degradation rate increases proportionally to the degree of polymerization. Although it has been suggested that catechins are responsible for the pharmacological effects of proanthocyanidins with high molecular weight, the duration of 3.5 h or more exceeds the average human gastric emptying rate of 30–90 min. Therefore, the influence of acid hydrolysis is undoubtedly less significant than subsequent metabolic processes (Winter et al., 1991).
In a study carried out by Doostdar et al., it was discovered that the flavones acacetin and diosmetin possess the ability to inhibit the ethoxy resorufin O-dealkylase (EROD) activity of two specific cytochrome P450 enzymes, namely CYP1B1 and CYP1A. The selectivity of these enzymes was primarily influenced by substitutions at the 3′ and 4′ positions of the flavonoid structures involving hydroxy and/or methoxy functional groups. Interestingly, other flavonoids such as naringenin, eriodictyol, and homoeriodictyol exhibited poor inhibitory effects on human CYP1A EROD activity (Doostdar et al., 2000).
Hesperetin and homoeriodictyol were found to perform O-demethylation of hesperetin to produce eriodictyol in both human CYP1A1 and CYP1B1. Furthermore, homoeriodictyol demonstrated the ability to inhibit human CYP1B1 selectively. It was observed that hesperetin could not be metabolized by human cytochromes CYP1A2 or CYP3A4. In vitro studies using human liver and intestinal microsomes indicated that luteolin primarily undergoes glucuronidation at the seventh position in liver cells. In contrast, in intestinal cells, it occurs at the third and fourth positions. The conjugation of luteolin with intestinal microsomes was approximately three times more prevalent than with liver microsomes. Individual testing of the enzymes revealed variations in the efficiency of glucuronidated luteolin, with certain forms displaying higher efficiency than others (Hostetler et al., 2017).
A recent study by Semwal et al. revealed that specific structural features of myricetin, such as the C2 and C3 double bond, the aromatic ring B at position C2, and the hydroxy groups in ring B, may contribute to its cytotoxic properties (Semwal et al., 2016). Previously, it was believed that quercetin was excreted in feces due to its inability to be absorbed in the intestine. However, another research by Murota et al. demonstrated that quercetin is absorbed in the intestine and converted into metabolites (Murota et al., 2002). The transportation of these quercetin metabolites involves the lymphatic system, as observed in a study (Terao et al., 2008).
Furthermore, a study by Moon et al. indicated that females’ regular consumption of onions leads to the accumulation of quercetin metabolites in the bloodstream and various tissues. Specifically, after 1 week of treatment, the total plasma concentration of these metabolites reached 0.6 μM. Rutin exhibits limited bioavailability, which hinders its biological effectiveness. Several drug delivery systems have been developed to overcome this challenge and enhance rutin’s bioavailability (Moon et al., 2000). These systems include nanoparticulate formulations, sulphonation and carboxylation of rutin, enzymatic oligomerization, and cyclodextrin complexation, which improve its aqueous solubility.
Additionally, phospholipid complexation, enzymatic and chemical acylation, and nanoparticulation techniques are employed to enhance the lipid solubility of rutin (Choi et al., 2021). Flavonoids, such as myricetin, kaempferol, quercetin, apigenin, and luteolin, are commonly found in plants and are widely consumed. On average, individuals consume approximately 23 mg/day of these antioxidant flavonoids, surpassing the intake of well-known antioxidants like β-carotene (2–3 mg/day) and vitamin E (7–10 mg/day). Additionally, this intake represents around one-third of the average intake of vitamin C (70–100 mg/day) (Hertog et al., 1993).
Among the various flavonoids, quercetin is the most significant contributor to the dietary consumption of flavonoids. It is predominantly obtained from apples and onions (Knekt et al., 2000; Gibellini et al., 2011). Both preclinical and clinical studies have indicated that quercetin glucosides, cinnamate conjugates, and flavonols are readily absorbed in the small intestine (Olthof et al., 2003; Cermak et al., 2007). However, quercetin galactosides, quercetin, rutin, and naringenin are not absorbed in the intestine. While the exact mechanism of absorption remains unclear, the transport of flavonoids across membranes plays a crucial role in their bioavailability in plants and animals. Further research suggests that ATP-dependent pumps and ATP-independent transporters contribute to this process (Passamonti et al., 2009).
Genistein, an isoflavone, is commonly found in high-soy diets. It has gained significant recognition due to its potential in preventing and treating different cancer types. Many research studies have demonstrated that genistein exhibits anticancer properties, specifically against ovarian cancer. However, its bioavailability, which refers to the amount of the compound that reaches the bloodstream and target tissues, is limited (Lee et al., 2012). Yang et al. conducted mechanistic studies to investigate the factors influencing the ADME of genistein. In terms of absorption, they found that genistein is efficiently absorbed in the small intestine but undergoes significant metabolism in the liver, resulting in lower levels reaching the systemic circulation. The absorption process is influenced by various factors, including the presence of food, the gut microbiota, and the transporters responsible for moving genistein across cell membranes. Regarding distribution, genistein has been detected in various tissues, indicating its ability to reach different target sites in the body. However, its distribution may be affected by factors like protein binding and tissue-specific uptake mechanisms (Yang et al., 2012).
Metabolism studies revealed that genistein undergoes extensive biotransformation in the liver, primarily through phase II enzymes. These enzymes attach other molecules to genistein, making it more water-soluble and facilitating its excretion from the body. Additionally, the gut microbiota also plays a role in the metabolism of genistein. It is primarily excreted through the bile into the feces, with a small portion eliminated in the urine. The researchers highlight the importance of understanding the factors affecting genistein’s ADME to optimize its therapeutic potential and develop effective strategies for its delivery. Understanding these mechanisms is crucial for harnessing the potential health benefits of genistein and developing strategies to enhance its therapeutic efficacy (Yang et al., 2012).
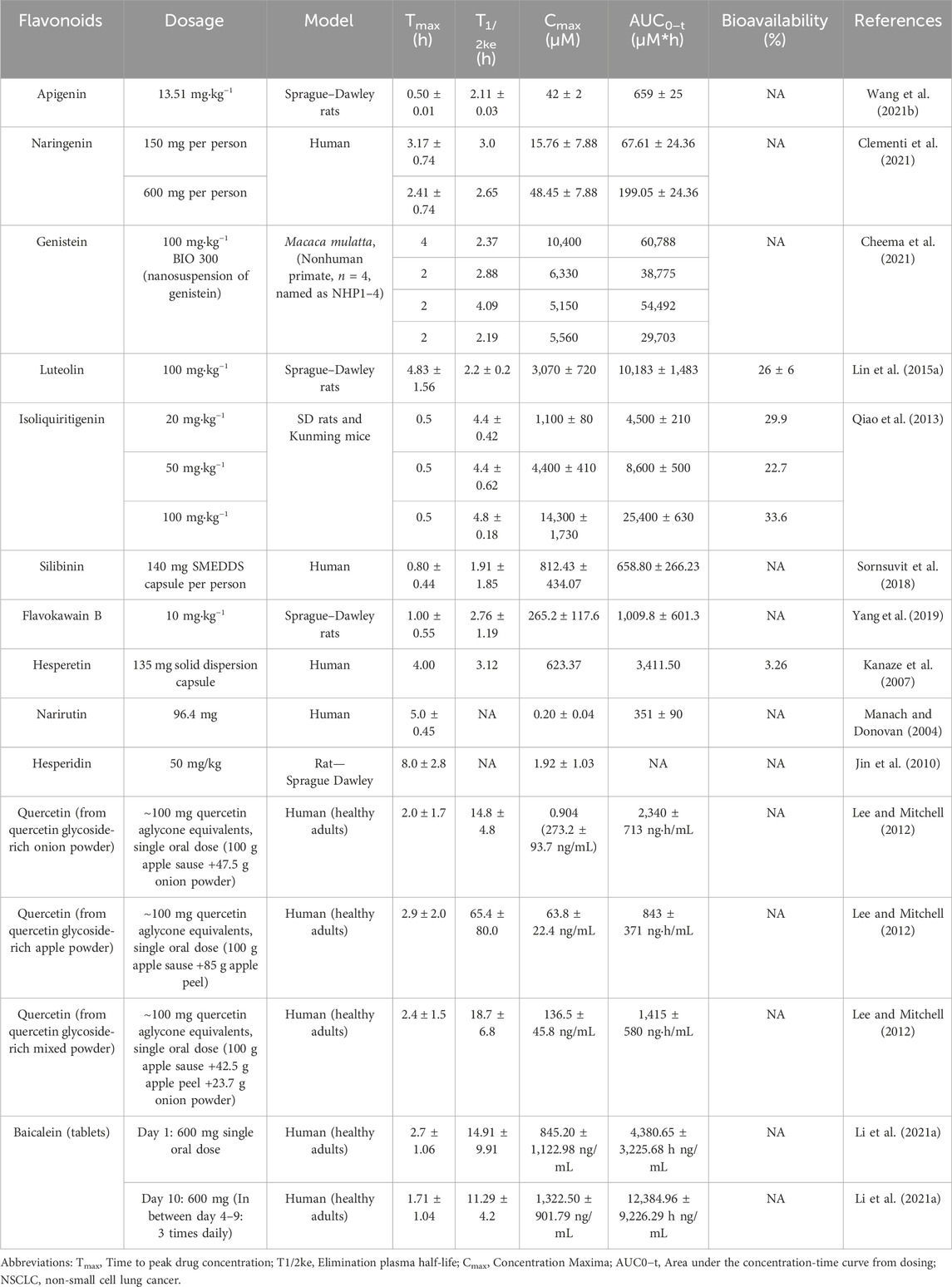
In laboratory studies, flavonoids have shown significant anticancer effects in refractory non-small cell lung cancer (NSCLC) models. However, their effectiveness in clinical applications depends on ADME factors within the body (Manach and Donovan, 2004). Consequently, researchers have recognized the importance of pharmacokinetics as a critical factor in the anticancer properties induced by flavonoids. Investigations have been conducted to assess the ingestion and excretion of flavonoids to understand this aspect (Gugler et al., 1975). The findings from these pharmacokinetic studies, summarized in Table 3, offer valuable insights for guiding effective flavonoid treatments in various types and stages of cancers. Flavonoid absorption, metabolism, and bioavailability are influenced by structural features, gut microbiota, and formulation strategies. Optimizing ADME is essential for clinical translation.
9 Undesired toxicity of anticancer flavonoids
We also outline actionable safety practices-DDI screens (especially CYP3A4, exposure-tuning via delivery, and dose/schedule choices to preserve efficacy while minimizing risk.
9.1 Quercetin
One of the most essential dietary phytoconstituents among bioflavonoids is quercetin. Its name comes from the Latin word “quercetum”, which means “oak forest”. Its formal name, 2-(3, 4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one, is known chemically (Miltonprabu, 2019; Magar and Sohng, 2020). It has a bitter flavor and is common in meals, drinks, and dietary supplements. When the enzymes phenylalanine ammonia-lyase, cinnamate-4-hydroxylase, and 4-coumaroyl-CoA ligase are present, phenylalanine is converted to 4-coumaroyl-CoA via the phenylpropanoid route to produce quercetin (Lesjak et al., 2018).
The enzymatic action of 7,2′-dihydroxy-4′-methoxyisoflavanol synthase further combines 4-coumaroyl-CoA to malonyl-CoA (3 molecules), which results in the production of tetrahydroxychalcone. Additionally, the enzyme chalcone isomerase changes tetrahydroxychalcone into naringenin, which is then changed back into eriodictyol by the enzyme flavonoid 3′-hydroxylase (F3′H). Furthermore, the enzymatic action of flavanol synthase transforms eriodictyol into dihydroquercetin, followed by creating quercetin (Lesjak et al., 2018). Due to its antioxidant and therapeutic properties, which include anticancer, anti-inflammatory, and anti-obesity actions, quercetin has attracted much interest from scientists recently (Devappa et al., 2015; Ezzati et al., 2020; Khazdair et al., 2021). Additionally, it has demonstrated its effectiveness against various conditions, including gout, pancreatic cancer, asthma, and disorders similar to Alzheimer’s disease (Ulusoy and Sanlier, 2019). Interestingly, quercetin has been linked to lower cataract risk due to dermatological problems and diabetes complications (Mishra and Rizvi, 2012).
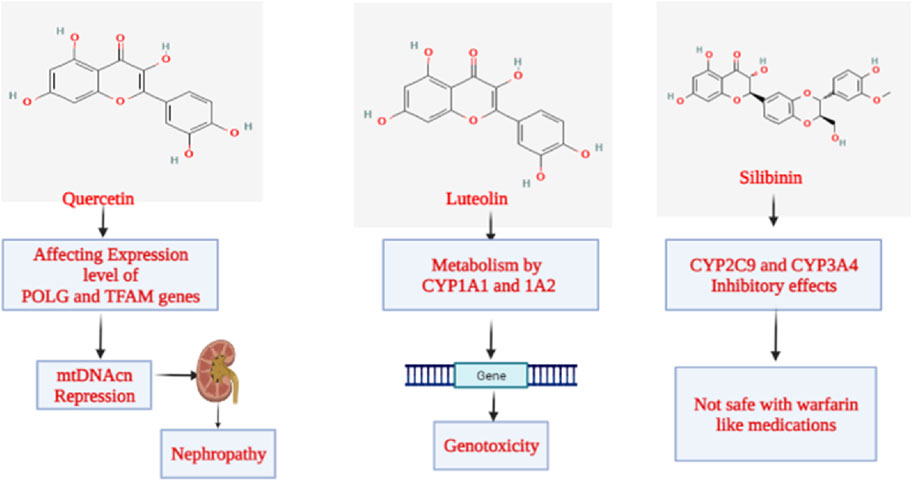
Regarding the toxicity parameters, specific points need to be discussed. Chen et al. surprisingly discovered using an ICR murine model that animals subjected to 7 Gy whole-body irradiation (TBI) demonstrated general in vivo toxicity following the administration of quercetin (100 mg/kg PO). However, this result was not seen in mice that received TBI alone. They employed a real-time qPCR to analyze the mitochondrial DNA copy number (mtDNAcn) by amplifying the MTRNR1 (12S rRNA) gene in the murine bone marrow to comprehend the role of changes in mitochondrial biogenesis. Reverse transcription-quantitative PCR was also used to quantify the levels of mRNA produced in the tissue from the polymerase gamma (POLG), polymerase gamma 2 (POLG2), and mammalian mitochondrial transcription factor A (TFAM) genes. Brought on by the suppression of POLG expression and the overexpression of TFAM, the findings indicate that the overall toxicity was partially linked to the reduction in mtDNAcn; POLG2 expression that was left intact did not appear to contribute to toxicity (Chen et al., 2014). According to the US National Toxicology Program, the high quercetin dosage from the 6-month intervention has already contributed to developing nephropathy. Based on the research mentioned above, the issue of whether large doses of quercetin ingestion in humans and animals might exacerbate the underlying kidney-damaging processes is raised (Figure 9). Although no significant detrimental effects of quercetin on kidney function have been shown in human intervention trials, quercetin use by individuals with renal failure should be interpreted cautiously (Andres et al., 2018).
9.2 Luteolin
The class “flavones” of flavonoids includes luteolin, often referred to as 3′,4′,5,7-tetrahydroxyflavone, another naturally occurring phytoconstituent that is extensively distributed. It has three rings with a carbon double bond at position 2-3 and a C6-C3-C6 structure. The 3rd, 5th, and 7th carbon locations of luteolin are known to have hydroxyl groups, respectively (Weng and Yen, 2012). Furthermore, it has been shown that the hydroxyl moieties and the double bond at positions 2nd and 3rd are necessary for luteolin’s bioactivities. Additionally, luteolin has been linked to glycosylation in plants, which leads to additional hydrolysis and the luteolin release (Aziz et al., 2018). Luteolin is a harmless heat-stable phytocompound. Several papers have mentioned its anti-carcinogenic, anti-inflammatory, and antioxidant activities (Choi et al., 2014; Tuorkey, 2016). Additionally, it has demonstrated the ability to prevent diabetes and cardiovascular disorders by triggering the immune system. It functions as a scavenger of nitrogen and oxygen species that may directly or indirectly impair cellular integrity (Bharti et al., 2014). Luteolin has anti-estrogenic properties that inhibit cell growth, making it a powerful antiproliferative agent (Bharti et al., 2014). Li et al. investigated the cytotoxicity and genotoxicity that luteolin causes and the part that several CYPs play in luteolin’s bioactivation. Human lymphoblastoid TK6 cells and TK6-derived cell lines (CYP1A1, 1A2, 1B1, 2A6, 2B6, 2C8, 2C19, 2D6, 2E1, 3A4, 3A5, and 3A7) that each stably express a single human cytochrome P450 were used in that study. Treatments with luteolin for 4–24 h caused concentration-dependent cytotoxicity, apoptosis, DNA damage, and chromosomal damage. Then, we discovered that TK6 cells transduced with CYP1A1 and 1A2 greatly boosted luteolin-induced cytotoxicity and genotoxicity as determined by the high-throughput micronucleus assay. In addition, CYP1A1-and 1A2-expressing cells showed a substantial increase in important DNA damage and apoptosis indicators, such as cleaved PARP-1, cleaved caspase-3, and phosphorylated histone 2AX (H2A.X), when compared to empty vector controls. After 24 h, TK6 cells bio-transformed the bulk of luteolin into diosmetin, a less toxic O-methylated flavone; the presence of CYP1A1 and 1A2 partly inhibited this process, according to LC-MS/MS analysis. These findings collectively show that CYP1A1 and 1A2 metabolism increased the toxicity of luteolin in vitro (Figure 9). These studies provide additional evidence for the effectiveness of TK6 cell system in identifying the precise CYPs accountable for chemical bio-activation and toxicity potential (Li X. et al., 2021).
9.3 Silibinin
Silibinin, a flavonoid derived from the parent plant Silybum marianum (L.) Gaertn. (Milk thistle) is one of the significant flavonoids obtained from plants. A group of phytocompounds known as “flavonolignan” is generally present in milk thistle (Li X. et al., 2021). However, silybin, also known as silibinin, the first recognized component in this complex, combines two diastereomers: silybin A and silybin B. A semi-purified, commercially accessible portion of silibinin is Silymarin (Samu et al., 2004; Tahsin et al., 2019). It is interesting to note that the production of silibinin requires the coupling of two crucial substances, namely taxifolin and coniferyl alcohol. Numerous pharmacological effects have been reported, mainly concerning fatty liver conditions such as non-alcoholic fatty liver, steatohepatitis, and alcoholic liver cirrhosis (Shaker et al., 2010; Zhu et al., 2018). According to Soleiman et al., the Asteraceae family includes therapeutic plants like milk thistle [S. marianum (L.) Gaertn.]. The main component of milk thistle extract is Silymarin, a combination of flavonolignans such as silybin, which is Silymarin’s most potent component. It is mainly recognized for its hepatoprotective properties. Its safety is crucial since studies have revealed further therapeutic benefits, including those against cancer, Alzheimer’s disease, Parkinson’s disease, and diabetes. Animal tests show no significant toxicity. Salmonella typhimurium strains were mutagenic to Silymarin in the presence of metabolic enzymes. At a concentration of 100 μM, silybin, silydianin, and silychristin were not cytotoxic or genotoxic. Silymarin is well tolerated, even at high dosages of 700 mg three times per day for 24 weeks, and is safe in humans at therapeutic levels. There were some gastrointestinal discomforts, such as diarrhea and nausea. According to a clinical investigation, Silymarin is safe during pregnancy, and there were no malformations. As a result, prenatal care should be taken, and additional research, especially in humans, is required. Silymarin does significantly affect cytochromes P450 and has few DDIs. Studies have shown that Silymarin should be used cautiously with medications with a limited therapeutic window (Figure 9) (Soleimani et al., 2019).
9.4 Genistein
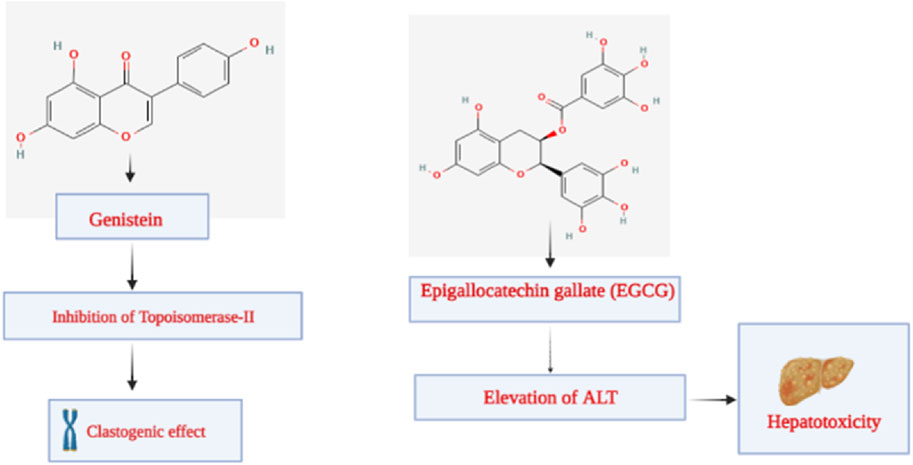
Genistein is a member of the isoflavone subclass. It is a phytoestrogen from plants in the Fabaceae family, including Vicia faba L., Glycine max (L.) Merr., Pueraria lobata (Wild.), Lupinus albus L., and others. According to its chemical name, 5,7-dihydroxy-3-(4-hydroxyphenyl) chromene-4-one, genistein has a structure comparable to mammalian estrogens (Dixon, 2002). It consists of 15 carbons, each of which has two aromatic rings, referred to as “ring A” and “ring B”, which are connected to “ring C”, a carbon pyran ring. In addition to the oxo group at the fourth position of ring C, this phytoconstituent’s carbon skeleton/structure also includes a double bond between the second and third locations. Recent investigations have demonstrated the therapeutic efficacy of genistein in preventing cancer, osteoporosis, cardiovascular disorders, and postmenopausal issues (Marini et al., 2007; Thangavel et al., 2019). As an anti-inflammatory agent, it has also demonstrated a crucial function in inflammatory disorders (Chen et al., 2020). According to recent research, genistein may play a significant role in neurodegenerative diseases through an autophagy-dependent mechanism (Chen et al., 2020). In the studies regarding the genetic toxicity of genistein conducted by McClain et al., it was revealed that genistein was found to be neither mutagenic nor clastogenic in vivo in the mouse and rat micronucleus test or in the S. typhimurium assay. Genistein increased the number of primarily tiny colonies in the mouse lymphoma experiment, showing clastogenic properties. This finding is consistent with research published on genistein’s inhibitory effect on topoisomerase II, which is known to cause chromosomal damage with a threshold dose response (Figure 10) (Michael McClain et al., 2006).
9.5 Epigallocatechin gallate
Epigallocatechin gallate (EGCG), also known as epigallocatechin-3-gallate, is an ester of epigallocatechin and gallic acid and is a member of the catechin family of polyphenols. It has been noted that catechins are responsible for the tea [Camellia sinensis (L.) Kuntze]’s ability to prevent cancer (Chu et al., 2017). The primary phytochemical in green tea, EGCG, is one of the several catechins. It is interesting to note that the dried leaves of green tea contain the most EGCG overall, followed by those of white tea and black tea, respectively (Jin et al., 2015).
Researchers have discovered a wide range of health benefits of drinking tea in recent years, many of which are attributable to the presence of EGCG. It has demonstrated favorable activity against various illnesses, including cancer, rheumatoid arthritis, cardiovascular disease, and inflammation (Lecumberri et al., 2013; Chourasia et al., 2021). Lambert et al. studied the hepatotoxicity of EGCG. They looked at how highly-dosed EGCG affected the livers of male CF-1 mice. EGCG elevated plasma alanine aminotransferase (ALT) by 138-fold and decreased survival by 85% after a single dosage (1,500 mg/kg, i.g.). The hepatotoxic reaction was exacerbated by EGCG administered once daily. Following two 750 mg/kg once-daily doses of EGCG, plasma ALT levels rose by 184 times. Following therapy with EGCG, moderate to severe hepatic necrosis was seen. The hepatotoxicity of EGCG was linked to oxidative stress, which included elevated plasma 8-isoprostane (9.5-fold rise), hepatic metallothionein, and gamma-histone 2AX protein expression. Hepatic lipid peroxidation was also enhanced (5-fold). Monocyte chemoattractant protein-1 and plasma IL-6 were likewise elevated by EGCG. According to those studies, mice’s livers are damaged by greater bolus dosages of EGCG. Given the growing popularity of green tea dietary supplements, which may provide significantly greater plasma and tissue concentrations of EGCG than tea drinks, further research is needed to understand the dose-dependent hepatotoxic effects of EGCG and the underlying processes (Figure 10) (Lambert et al., 2010).
In conclusion, high flavonoid intake may lead to the development of various adverse effects, which include primarily hepatotoxicity, disruption of the endocrine system, pro-oxidant activity, and other endocrine disruptions. Exclusively high doses of catechins from tea are associated with liver injury. Isoflavones act mainly as endocrine system disrupters. Flavonoids also produce their pharmacological action by blocking various cytochrome P450 enzymes and increasing the risk of toxicity. Various side effects such as gastrointestinal disturbances and renal damage were also reported in supplements with highly concentrated flavonoids. This implies the need for highly concentrated research focusing on the toxicity profile of flavonoids (Galati and O'Brien, 2004; Deng et al., 2025).
While flavonoids show therapeutic benefits, toxicity profiles vary across compounds and conditions, necessitating careful dose optimization and safety evaluation.
10 Future perspectives
Studying the anticancer flavonoids’ PK, drug-likeness, and toxicological properties offers considerable potential for developing new treatments. Because of their antioxidant, anti-inflammatory, and cell-signaling-modulating actions, flavonoids, a varied collection of naturally occurring substances found in plants, have been researched for their potential anticancer qualities. The following are some potential outcomes of studying these issues in the future.
10.1 Enhanced drug design and development
Researchers may find it helpful to better grasp the PK parameters of anticancer flavonoids to develop more accurate and successful pharmacological formulations. With this knowledge, flavonoid derivatives may be developed with a higher bioavailability and tissue-specific dispersion, boosting their therapeutic potential (Kopustinskiene et al., 2020).
10.2 Optimized dosing strategies
Pharmacokinetic investigations can guide the development of ideal dose regimens for anticancer flavonoids. This is essential for the body to remain at therapeutic levels while reducing possible toxicity and negative consequences (Liskova et al., 2021).
10.3 Prediction of drug-likeness
Evaluation of flavonoids’ chemical and physical characteristics is necessary to identify whether they can develop into new drugs. To prioritize flavonoids with a better chance of being effective in developing pharmaceuticals, mathematical and machine learning methods can be employed to forecast how well these molecules conform to the features of currently used medications (Dias et al., 2021).
10.4 Toxicological insights
It is crucial to investigate and ensure about the toxicological characteristics of anticancer flavonoids during clinical trials and ultimate clinical application. Establishing acceptable dosage ranges and identifying probable side effects/off-targets can be aided by thorough toxicological analyses (Choudhari et al., 2019).
10.5 Combination therapy
By understanding these compounds’ ADMET characteristics, researchers can investigate the potential of anticancer flavonoids in combination therapies with other cancer treatments, such as chemotherapy or targeted therapies. This might improve therapy effectiveness and possibly lower the dosages needed for conventional anticancer medications, reducing their adverse effects, and for reversing the anticancer drug resistance (Choudhari et al., 2019).
10.6 Personalized medicine
Researchers can work toward creating individualized/personalized therapy methods by examining the variation in PK and toxicological reactions to anticancer flavonoids among people. This could entail adjusting doses following a patient’s genetic composition and other aspects, resulting in more efficient and secure therapies (Hu et al., 2017).
10.7 Regulatory approval
Comprehensive PK and toxicological data are required to assess the safety and efficacy of novel anticancer treatments for compliance with regulatory organizations like the FDA. Clinical translation can be facilitated by thorough research in several fields, speeding up the approval process. Future research of flavonoids will focus on pharmacogenomics and the gut microbiome will personalize the treatment because the ethnographic variation in metabolism and biotransformation in the abdomen will influence the toxicity and efficacy. Moreover, we can suggest that combination therapies with existing chemo-medications will reduce the various adverse reactions and as well as drug resistance via different synergistic pharmacological mechanisms. In addition to that, advancing various PK understanding, proper toxicological evaluations will transform the flavonoids for developing novel nutraceutical-based approaches for anti-cancer drug therapy (de Sousa Silva et al., 2022; Farhan et al., 2023; Tomar et al., 2024).
Strategies such as improved drug design, optimized dosing, combination therapies, and personalized medicine approaches are critical for advancing flavonoid-based cancer treatment.
11 Conclusion
The study results on the pharmacokinetics, drug-likeness, and toxicological characteristics of anticancer flavonoids provide insight into their potential as attractive candidates for cancer treatment. Comprehensive studies have revealed that flavonoids, a group of polyphenolic chemicals abundantly found in various plant sources, have complex PK profiles that affect how quickly they are absorbed, distributed, metabolized, and excreted by the body. The benefits of flavonoids have been emphasized in several studies, including their capacity to regulate various drug-metabolizing enzymes, transporters, and signaling pathways. Because of their adaptability, they have the potential to be used as adjuvants to conventional chemotherapy, enhancing therapeutic efficacy and minimizing side effects through synergistic interactions.
Additionally, the drug-likeness evaluation highlights the structural variety of flavonoids, which have benefits and drawbacks. Although the intricacy of these compounds and their polyphenolic makeup can prevent them from being optimized as conventional small-molecule medicines, novel drug delivery methods and packaging techniques have been developed to overcome these drawbacks. These strategies seek to increase these drugs’ bioavailability, stability, and targeted delivery to tumor locations, increasing their clinical viability (Rosales and Fabi, 2023).
Extensive studies into the anticancer flavonoids’ safety profiles have shown their typically low toxicity and appropriate safety margins regarding toxicological characteristics. However, it is crucial to recognize that their toxicity profiles may be affected by the dose-response relationship, possible drug interactions, and individual variances in metabolism. Determine their long-term safety and potential side effects through rigorous preclinical and clinical testing (Zhang et al., 2020).
The potential flavonoids that have cytotoxic activity, which mainly consist of quercetin, kaempferol, genistein, and epigallocatechin gallate. These compounds possess a wide range of pharmacokinetic advantages when these molecules will be formulated as nanoformulations and also prodrugs that can increase bioavailability. These molecules possess a very favorable ADMET profile and will also be considered for further exploration in anticancer research. On considering the toxicological parameters, these compounds possess less systemic toxicity. Major developments in various drug delivery systems, the in silico ADMET profiling via various computational approaches can improve the safety and efficacy of anticancer flavonoids. On considering all the above facts, flavonoid molecules have a better clinical translational potential as effective anticancer drugs.
The core of the investigation into anticancer flavonoids is the confluence of PK insights, drug-likeness considerations, and toxicological evaluations. Despite the progress that has been achieved, there is still a need for pharmacologists, chemists, toxicologists, and clinicians to work together transdisciplinarily to bridge the gap between laboratory findings and clinical applications. Further studies should concentrate on improving the PK profiles of flavonoid compounds, enhancing their molecular mechanisms of action, and carrying out extensive clinical trials to determine their genuine therapeutic potential in cancer treatment.
In conclusion, the extensive investigation of the PK, drug-likeness, and toxicological characteristics of anticancer flavonoids reveals a terrain rife with prospects and difficulties. This study deepens our understanding of these substances and opens the door to novel therapeutic approaches that use their unique qualities to advance cancer treatment.
Author contributions
AD: Formal Analysis, Data curation, Writing – original draft, Visualization, Writing – review and editing. SC: Writing – review and editing, Data curation, Writing – original draft, Visualization, Formal Analysis. AG: Writing – review and editing, Formal Analysis, Writing – original draft, Data curation, Visualization. BK: Formal Analysis, Visualization, Data curation, Writing – review and editing. MG: Formal Analysis, Data curation, Writing – review and editing. PR: Data curation, Formal Analysis, Writing – review and editing. GS: Writing – review and editing, Formal Analysis, Data curation. VC: Writing – review and editing, Data curation, Formal Analysis, Visualization. RG: Data curation, Visualization, Writing – review and editing, Formal Analysis. HB: Data curation, Writing – review and editing, Formal Analysis, Visualization. RG: Data curation, Visualization, Writing – review and editing, Formal Analysis. RG: Formal Analysis, Data curation, Writing – review and editing, Visualization. MO: Formal Analysis, Data curation, Writing – review and editing. BS: Conceptualization, Resources, Project administration, Supervision, Funding acquisition, Writing – review and editing, Methodology, Formal Analysis. RS: Conceptualization, Investigation, Visualization, Funding acquisition, Validation, Formal Analysis, Methodology, Supervision, Data curation, Project administration, Writing – review and editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Sichuan Science and Technology Program (Grant Nos. 2025YFHZ0213 and 2024YFHZ0205) and the National Natural Science Foundation of China (Grant No. 32270690).
Acknowledgments
The figures were created using BioRender.com. We thank Caproslaxy Media (https://www.caproslaxymedia.com/) for linguistic assistance during the preparation of this manuscript. To maintain uniformity and to standardize as per international nomenclature, scientific terminologies for botanical sources have been adopted as per the information there on https://www.worldfloraonline.org/.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The author(s) declare that Generative AI like ChatGPT 4o/5 and DeepSeek-R1 have been used partially, to improve small sections of the manuscript and while seeking opinions for our queries. But the majority of the work is free from the involvement of the Gen AI role. After using these tools/services, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdi Syahputra, R., Dalimunthe, A., Utari, Z. D., Halim, P., Sukarno, M. A., Zainalabidin, S., et al. (2024). Nanotechnology and flavonoids: current research and future perspectives on cardiovascular health. J. Funct. Foods 120, 106355. doi:10.1016/j.jff.2024.106355
Adhikari, A., Darbar, S., Chatterjee, T., Das, M., Polley, N., Bhattacharyya, M., et al. (2018). Spectroscopic studies on dual role of natural flavonoids in detoxification of lead poisoning: bench-to-bedside preclinical trial. ACS Omega 3, 15975–15987. doi:10.1021/acsomega.8b02046
Aggarwal, V., Tuli, H., Varol, A., Thakral, F., Yerer, M., Sak, K., et al. (2019). Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. Biomolecules 9, 735. doi:10.3390/biom9110735
Al-Fayez, M., Cai, H., Tunstall, R., Steward, W. P., and Gescher, A. J. (2006). Differential modulation of cyclooxygenase-mediated prostaglandin production by the putative cancer chemopreventive flavonoids tricin, apigenin and quercetin. Cancer Chemother. Pharmacol. 58, 816–825. doi:10.1007/s00280-006-0228-3
Al-Khayri, J. M., Sahana, G. R., Nagella, P., Joseph, B. V., Alessa, F. M., and Al-Mssallem, M. Q. (2022). Flavonoids as potential anti-inflammatory molecules: a review. Molecules 27, 5965. doi:10.3390/molecules27185965
Altunayar-Unsalan, C., and Unsalan, O. (2024). Molecular structure, antioxidant potential, and pharmacokinetic properties of plant flavonoid blumeatin and investigating its inhibition mechanism on Xanthine oxidase for hyperuricemia by molecular modeling. ACS Omega 9, 13284–13297. doi:10.1021/acsomega.3c10083
Alzohairy, M. A., Khan, A. A., Ansari, M. A., Babiker, A. Y., Alsahli, M. A., Almatroodi, S. A., et al. (2021). Protective effect of Quercetin, a flavonol against Benzo(a)pyrene-induced lung injury via inflammation, oxidative stress, angiogenesis and cyclooxygenase-2 signalling molecule. Appl. Sci. 11, 8675. doi:10.3390/app11188675
Andres, S., Pevny, S., Ziegenhagen, R., Bakhiya, N., Schäfer, B., Hirsch-Ernst, K. I., et al. (2018). Safety aspects of the use of quercetin as a dietary supplement. Mol. Nutr. Food Res. 62, 1700447. doi:10.1002/mnfr.201700447
Angelé-Martínez, C., Nguyen, K. V. T., Ameer, F. S., Anker, J. N., and Brumaghim, J. L. (2017). Reactive oxygen species generation by copper(II) oxide nanoparticles determined by DNA damage assays and EPR spectroscopy. Nanotoxicology 11, 278–288. doi:10.1080/17435390.2017.1293750
Aslam, M. S., Naveed, S., Ahmed, A., Abbas, Z., Gull, I., and Athar, M. A. (2014). Side effects of chemotherapy in cancer patients and evaluation of patients opinion about starvation based differential chemotherapy. J. Cancer Ther. 05, 817–822. doi:10.4236/jct.2014.58089
Attique, I., Haider, Z., Khan, M., Hassan, S., Soliman, M. M., Ibrahim, W. N., et al. (2025). Reactive oxygen species: from tumorigenesis to therapeutic strategies in cancer. Cancer Med. 14, e70947. doi:10.1002/cam4.70947
Aziz, N., Kim, M.-Y., and Cho, J. Y. (2018). Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 225, 342–358. doi:10.1016/j.jep.2018.05.019
Baba, S., Furuta, T., Fujioka, M., and Goromaru, T. (1983). Studies on drug metabolism by use of isotopes XXVII: urinary metabolites of rutin in rats and the role of intestinal microflora in the metabolism of rutin. J. Pharm. Sci. 72, 1155–1158. doi:10.1002/jps.2600721011
Baby, J., Devan, A. R., Kumar, A. R., Gorantla, J. N., Nair, B., Aishwarya, T. S., et al. (2021). Cogent role of flavonoids as key orchestrators of chemoprevention of hepatocellular carcinoma: a review. J. Food Biochem. 45, e13761. doi:10.1111/jfbc.13761
Baskar, R., Lee, K. A., Yeo, R., and Yeoh, K.-W. (2012). Cancer and radiation therapy: current advances and future directions. Int. J. Med. Sci. 9, 193–199. doi:10.7150/ijms.3635
Bharti, S., Misro, M. M., and Rai, U. (2014). Quercetin supplementation restores testicular function and augments germ cell survival in the estrogenized rats. Mol. Cell. Endocrinol. 383, 10–20. doi:10.1016/j.mce.2013.11.021
Billowria, K., Ali, R., Rangra, N. K., Kumar, R., and Chawla, P. A. (2024). Bioactive flavonoids: a comprehensive review on pharmacokinetics and analytical aspects. Crit. Rev. Anal. Chem. 54, 1002–1016. doi:10.1080/10408347.2022.2105641
Bisol, A., De Campos, P. S., and Lamers, M. L. (2020). Flavonoids as anticancer therapies: a systematic review of clinical trials. Phytother. Res. 34, 568–582. doi:10.1002/ptr.6551
Bukowski, K., Kciuk, M., and Kontek, R. (2020). Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 21, 3233. doi:10.3390/ijms21093233
Cai, H., Al-Fayez, M., Tunstall, R. G., Platton, S., Greaves, P., Steward, W. P., et al. (2005). The rice bran constituent tricin potently inhibits cyclooxygenase enzymes and interferes with intestinal carcinogenesis in ApcMin mice. Mol. Cancer Ther. 4, 1287–1292. doi:10.1158/1535-7163.MCT-05-0165
Cermak, R., Landgraf, S., and Wolffram, S. (2007). Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br. J. Nutr. 91, 849–855. doi:10.1079/BJN20041128
Chamani, J., Cherrak, S. A., Mokhtari-Soulimane, N., Berroukeche, F., Bensenane, B., Cherbonnel, A., et al. (2016). In vitro antioxidant versus metal ion chelating properties of flavonoids: a structure-activity investigation. PLoS One 11, e0165575. doi:10.1371/journal.pone.0165575
Chang, W. S., Lee, Y. J., Lu, F. J., and Chiang, H. C. (1993). Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 13, 2165–2170. Available online at: https://pubmed.ncbi.nlm.nih.gov/8297130/.
Chavda, V. P., Ertas, Y. N., Walhekar, V., Modh, D., Doshi, A., Shah, N., et al. (2021). Advanced computational methodologies used in the discovery of new natural anticancer compounds. Front. Pharmacol. 12, 702611. doi:10.3389/fphar.2021.702611
Chavda, V. P., Patel, A. B., Mistry, K. J., Suthar, S. F., Wu, Z.-X., Chen, Z.-S., et al. (2022). Nano-drug delivery systems entrapping natural bioactive compounds for cancer: recent progress and future challenges. Front. Oncol. 12, 867655. doi:10.3389/fonc.2022.867655
Cheema, A. K., Li, Y., Singh, J., Johnson, R., Girgis, M., Wise, S. Y., et al. (2021). Microbiome study in irradiated mice treated with BIO 300, a promising radiation countermeasure. Anim. Microbiome 3, 71. doi:10.1186/s42523-021-00132-1
Chen, L.-G., Hung, L.-Y., Tsai, K.-W., Pan, Y.-S., Tsai, Y.-D., Li, Y.-Z., et al. (2008). Wogonin, a bioactive flavonoid in herbal tea, inhibits inflammatory cyclooxygenase-2 gene expression in human lung epithelial cancer cells. Mol. Nutr. Food Res. 52, 1349–1357. doi:10.1002/mnfr.200700329
Chen, R., Lin, J., Hong, J., Han, D., Zhang, A. D., Lan, R., et al. (2014). Potential toxicity of quercetin: the repression of mitochondrial copy number via decreased POLG expression and excessive TFAM expression in irradiated murine bone marrow. Toxicol. Rep. 1, 450–458. doi:10.1016/j.toxrep.2014.07.014
Chen, X., Wu, Y., Gu, J., Liang, P., Shen, M., Xi, J., et al. (2020). Anti-invasive effect and pharmacological mechanism of genistein against colorectal cancer. BioFactors 46, 620–628. doi:10.1002/biof.1627
Chen, X., Yao, J., Xin, Y., Ma, G., Yu, Y., Yang, Y., et al. (2022). Postoperative pain in patients undergoing cancer surgery and intravenous patient-controlled analgesia use: the first and second 24 h experiences. Pain Ther. 12, 275–292. doi:10.1007/s40122-022-00459-w
Choi, J. S., Islam, M. N., Ali, M. Y., Kim, Y. M., Park, H. J., Sohn, H. S., et al. (2014). The effects of C-glycosylation of luteolin on its antioxidant, anti-alzheimer’s disease, anti-diabetic, and anti-inflammatory activities. Archives Pharmacal Res. 37, 1354–1363. doi:10.1007/s12272-014-0351-3
Choi, S.-S., Park, H.-R., and Lee, K.-A. (2021). A comparative study of rutin and rutin glycoside: Antioxidant activity, anti-inflammatory effect, effect on platelet aggregation and blood coagulation. Antioxidants 10, 1696. doi:10.3390/antiox10111696
Choudhari, A. S., Mandave, P. C., Deshpande, M., Ranjekar, P., and Prakash, O. (2019). Phytochemicals in cancer treatment: from preclinical studies to clinical practice. Front. Pharmacol. 10, 1614. doi:10.3389/fphar.2019.01614
Chourasia, M., Koppula, P., Battu, A., Ouseph, M., and Singh, A. (2021). EGCG, a green tea catechin, as a potential therapeutic agent for symptomatic and asymptomatic SARS-CoV-2 infection. Molecules 26, 1200. doi:10.3390/molecules26051200
Chu, C., Deng, J., Man, Y., and Qu, Y. (2017). Green tea extracts Epigallocatechin-3-gallate for different treatments. BioMed Res. Int. 2017, 5615647–9. doi:10.1155/2017/5615647
Clementi, N., Scagnolari, C., D’amore, A., Palombi, F., Criscuolo, E., Frasca, F., et al. (2021). Naringenin is a powerful inhibitor of SARS-CoV-2 infection in vitro. Pharmacol. Res. 163, 105255. doi:10.1016/j.phrs.2020.105255
Coussens, L. M., and Werb, Z. (2002). Inflammation and cancer. Nature 420, 860–867. doi:10.1038/nature01322
Cummings, J. H., and Macfarlane, G. T. (1991). The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 70, 443–459. doi:10.1111/j.1365-2672.1991.tb02739.x
Davis, J. N., Kucuk, O., and Sarkar, F. H. (1999). Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr. Cancer 35, 167–174. doi:10.1207/S15327914NC352_11
Davodabadi, F., Sajjadi, S. F., Sarhadi, M., Mirghasemi, S., Nadali Hezaveh, M., Khosravi, S., et al. (2023). Cancer chemotherapy resistance: mechanisms and recent breakthrough in targeted drug delivery. Eur. J. Pharmacol. 958, 176013. doi:10.1016/j.ejphar.2023.176013
Day, A. J., Cañada, F. J., Dı́Az, J. C., Kroon, P. A., Mclauchlan, R., Faulds, C. B., et al. (2000). Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 468, 166–170. doi:10.1016/s0014-5793(00)01211-4
De Sousa Silva, G. V., Lopes, A. L. V. F. G., Viali, I. C., Lima, L. Z. M., Bizuti, M. R., Haag, F. B., et al. (2022). Therapeutic properties of flavonoids in treatment of cancer through autophagic modulation: a systematic review. Chin. J. Integr. Med. 29, 268–279. doi:10.1007/s11655-022-3674-9
De Souza, R. F. V., and De Giovani, W. F. (2013). Antioxidant properties of complexes of flavonoids with metal ions. Redox Rep. 9, 97–104. doi:10.1179/135100004225003897
De Visser, K. E., and Joyce, J. A. (2023). The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell 41, 374–403. doi:10.1016/j.ccell.2023.02.016
Dehelean, C. A., Coricovac, D., Pinzaru, I., Marcovici, I., Macasoi, I. G., Semenescu, A., et al. (2022). Rutin bioconjugates as potential nutraceutical prodrugs: an in vitro and in ovo toxicological screening. Front. Pharmacol. 13, 1000608. doi:10.3389/fphar.2022.1000608
Del Rio, D., Rodriguez-Mateos, A., Spencer, J. P. E., Tognolini, M., Borges, G., and Crozier, A. (2013). Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxidants Redox Signal. 18, 1818–1892. doi:10.1089/ars.2012.4581
Demain, A. L., and Vaishnav, P. (2011). Natural products for cancer chemotherapy. Microb. Biotechnol. 4, 687–699. doi:10.1111/j.1751-7915.2010.00221.x
Deng, H., Zhao, Y., He, Y., Teng, H., and Chen, L. (2025). Unveiling the dark side of flavonoid: rutin provokes hepatotoxicity in low-dose 2-Amino-3-methylimidazo [4,5-f] quinoline-exposed mice via regulating gut microbiota and liver metabolism. J. Agric. Food Chem. 73, 4253–4269. doi:10.1021/acs.jafc.4c07330
Déprez, S., Mila, I., and Scalbert, A. (1999). Carbon-14 biolabeling of (+)-Catechin and proanthocyanidin oligomers in willow tree cuttings. J. Agric. Food Chem. 47, 4219–4230. doi:10.1021/jf981380z
Devappa, R. K., Rakshit, S. K., and Dekker, R. F. H. (2015). Forest biorefinery: potential of poplar phytochemicals as value-added co-products. Biotechnol. Adv. 33, 681–716. doi:10.1016/j.biotechadv.2015.02.012
Dewanjee, S., Chakraborty, P., Bhattacharya, H., Singh, S. K., Dua, K., Dey, A., et al. (2023). Recent advances in flavonoid-based nanocarriers as an emerging drug delivery approach for cancer chemotherapy. Drug Discov. Today 28, 103409. doi:10.1016/j.drudis.2022.103409
Diamantis, D., Tsiailanis, A. D., Papaemmanouil, C., Nika, M.-C., Kanaki, Z., Golic Grdadolnik, S., et al. (2023). Development of a novel apigenin prodrug programmed for alkaline-phosphatase instructed self-inhibition to combat cancer. J. Biomol. Struct. Dyn. 42, 8638–8659. doi:10.1080/07391102.2023.2247083
Dias, M. C., Pinto, D., and Silva, A. M. S. (2021). Plant flavonoids: Chemical characteristics and biological activity. Molecules 26, 5377. doi:10.3390/molecules26175377
Dixon, R., and Ferreira, D. (2002). Genistein. Phytochemistry 60, 205–211. doi:10.1016/s0031-9422(02)00116-4
Dobrydnev, A. V., Tkachuk, T. M., Atamaniuk, V. P., and Popova, M. V. (2020). Quercetin-amino acid conjugates are promising anti-cancer agents in drug discovery projects. Mini-Reviews Med. Chem. 20, 107–122. doi:10.2174/1389557519666191009152007
Dobrzynska, M., Napierala, M., and Florek, E. (2020). Flavonoid nanoparticles: a promising approach for cancer therapy. Biomolecules 10, 1268. doi:10.3390/biom10091268
Doostdar, H., Burke, M. D., and Mayer, R. T. (2000). Bioflavonoids: selective substrates and inhibitors for cytochrome P450 CYP1A and CYP1B1. Toxicology 144, 31–38. doi:10.1016/s0300-483x(99)00215-2
Erlund, I., Alfthan, G., Mäenpää, J., and Aro, A. (2001). Tea and coronary heart disease: the flavonoid quercetin is more bioavailable from rutin in women than in men. Archives Intern. Med. 161, 1919–1920. doi:10.1001/archinte.161.15.1919
Eslami, M., Memarsadeghi, O., Davarpanah, A., Arti, A., Nayernia, K., and Behnam, B. (2024). Overcoming chemotherapy resistance in metastatic cancer: a comprehensive review. Biomedicines 12, 183. doi:10.3390/biomedicines12010183
Ezzati, M., Yousefi, B., Velaei, K., and Safa, A. (2020). A review on anti-cancer properties of quercetin in breast cancer. Life Sci. 248, 117463. doi:10.1016/j.lfs.2020.117463
Farhan, M., Rizvi, A., Aatif, M., and Ahmad, A. (2023). Current understanding of flavonoids in cancer therapy and prevention. Metabolites 13, 481. doi:10.3390/metabo13040481
Favari, C., Rinaldi De Alvarenga, J. F., Sánchez-Martínez, L., Tosi, N., Mignogna, C., Cremonini, E., et al. (2024). Factors driving the inter-individual variability in the metabolism and bioavailability of (poly)phenolic metabolites: a systematic review of human studies. Redox Biol. 71, 103095. doi:10.1016/j.redox.2024.103095
Feng, R., Guo, Z. K., Yan, C. M., Li, E. G., Tan, R. X., and Ge, H. M. (2012). Anti-inflammatory flavonoids from Cryptocarya chingii. Phytochemistry 76, 98–105. doi:10.1016/j.phytochem.2012.01.007
Filipe, P., Lança, V., Nuno Silva, J., Morlière, P., Santus, R., and Fernandes, A. (2001). Flavonoids and urate antioxidant interplay in plasma oxidative stress. Mol. Cell. Biochem. 221, 79–87. doi:10.1023/a:1010944919952
Fraga, C. G., Croft, K. D., Kennedy, D. O., and Tomás-Barberán, F. A. (2019). The effects of polyphenols and other bioactives on human health. Food Funct. 10, 514–528. doi:10.1039/c8fo01997e
Fresco, P., Borges, F., Diniz, C., and Marques, M. P. M. (2006). New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 26, 747–766. doi:10.1002/med.20060
Galati, G., and O'brien, P. J. (2004). Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 37, 287–303. doi:10.1016/j.freeradbiomed.2004.04.034
García-Lafuente, A., Guillamón, E., Villares, A., Rostagno, M. A., and Martínez, J. A. (2009). Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflamm. Res. 58, 537–552. doi:10.1007/s00011-009-0037-3
Gee, J. M., Dupont, M. S., Rhodes, M. J. C., and Johnson, I. T. (1998). Quercetin glucosides interact with the intestinal glucose transport pathway. Free Radic. Biol. Med. 25, 19–25. doi:10.1016/s0891-5849(98)00020-3
Gibellini, L., Pinti, M., Nasi, M., Montagna, J. P., De Biasi, S., Roat, E., et al. (2011). Quercetin and cancer chemoprevention. Evidence-Based Complementary Altern. Med. 2011, 591356–15. doi:10.1093/ecam/neq053
Gopalan, V., Pastuszyn, A., Galey, W. R., and Glew, R. H. (1992). Exolytic hydrolysis of toxic plant glucosides by Guinea pig liver cytosolic beta-glucosidase. J. Biol. Chem. 267, 14027–14032. doi:10.1016/s0021-9258(19)49673-7
Grivennikov, S. I., and Karin, M. (2010). Inflammation and oncogenesis: a vicious connection. Curr. Opin. Genet. Dev. 20, 65–71. doi:10.1016/j.gde.2009.11.004
Groenewoud, G., and Hundt, H. K. L. (2009). The microbial metabolism of condensed (+)-catechins by rat-caecal microflora. Xenobiotica 16, 99–107. doi:10.3109/00498258609043512
Gugler, R., Leschik, M., and Dengler, H. J. (1975). Disposition of quercetin in man after single oral and intravenous doses. Eur. J. Clin. Pharmacol. 9, 229–234. doi:10.1007/BF00614022
Halevas, E. G., Avgoulas, D. I., Katsipis, G., and Pantazaki, A. A. (2022). Flavonoid-liposomes formulations: physico-chemical characteristics, biological activities and therapeutic applications. Eur. J. Med. Chem. Rep. 5, 100059. doi:10.1016/j.ejmcr.2022.100059
Hammerstone, J. F., Lazarus, S. A., and Schmitz, H. H. (2000). Procyanidin content and variation in some commonly consumed foods. J. Nutr. 130, 2086S-92S–2092S. doi:10.1093/jn/130.8.2086S
Hayakawa, S., Ohishi, T., Miyoshi, N., Oishi, Y., Nakamura, Y., and Isemura, M. (2020). Anti-cancer effects of green tea Epigallocatchin-3-Gallate and coffee chlorogenic acid. Molecules 25, 4553. doi:10.3390/molecules25194553
Hertog, M. G. L., Hollman, P. C. H., Katan, M. B., and Kromhout, D. (1993). Intake of potentially anticarcinogenic flavonoids and their determinants in adults in the Netherlands. Nutr. Cancer 20, 21–29. doi:10.1080/01635589309514267
Hinshaw, D. C., and Shevde, L. A. (2019). The tumor microenvironment innately modulates cancer progression. Cancer Res. 79, 4557–4566. doi:10.1158/0008-5472.CAN-18-3962
Hollman, P. C. H., Bijsman, M. N. C. P., Van Gameren, Y., Cnossen, E. P. J., De Vries, J. H. M., and Katan, M. B. (2009). The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 31, 569–573. doi:10.1080/10715769900301141
Horia, E., and Watkins, B. A. (2006). Complementary actions of docosahexaenoic acid and genistein on COX-2, PGE2 and invasiveness in MDA-MB-231 breast cancer cells. Carcinogenesis 28, 809–815. doi:10.1093/carcin/bgl183
Hostetler, G. L., Ralston, R. A., and Schwartz, S. J. (2017). Flavones: food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 8, 423–435. doi:10.3945/an.116.012948
Hou, D.-X., Yanagita, T., Uto, T., Masuzaki, S., and Fujii, M. (2005). Anthocyanidins inhibit cyclooxygenase-2 expression in LPS-evoked macrophages: structure–activity relationship and molecular mechanisms involved. Biochem. Pharmacol. 70, 417–425. doi:10.1016/j.bcp.2005.05.003
Hu, M., Wu, B., and Liu, Z. (2017). Bioavailability of polyphenols and flavonoids in the era of precision medicine. Mol. Pharm. 14, 2861–2863. doi:10.1021/acs.molpharmaceut.7b00545
Hu, L., Luo, Y., Yang, J., and Cheng, C. (2025). Botanical flavonoids: efficacy, absorption, metabolism and advanced pharmaceutical technology for improving bioavailability. Molecules 30, 1184. doi:10.3390/molecules30051184
Huang, Y.-T., Kuo, M.-L., Liu, J.-Y., Huang, S.-Y., and Lin, J.-K. (1996). Inhibitions of protein kinase C and proto-oncogene expressions in NIH 3t3 cells by apigenin. Eur. J. Cancer 32, 146–151. doi:10.1016/0959-8049(95)00540-4
Huang, Y. T., Hwang, J. J., Lee, P. P., Ke, F. C., Huang, J. H., Huang, C. J., et al. (1999). Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br. J. Pharmacol. 128, 999–1010. doi:10.1038/sj.bjp.0702879
Hussain, N., Dubey, S. K., Katiyar, C. K., P, R., Agrawal, M., and Alexander, A. (2025). Harnessing potential of liposomal drug carriers for enhanced pharmacokinetic profile of flavonoids. J. Drug Deliv. Sci. Technol. 109, 106978. doi:10.1016/j.jddst.2025.106978
Ignat, I., Volf, I., and Popa, V. I. (2011). A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 126, 1821–1835. doi:10.1016/j.foodchem.2010.12.026
Itoh, K., Tong, K. I., and Yamamoto, M. (2004). Molecular mechanism activating nrf2–keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 36, 1208–1213. doi:10.1016/j.freeradbiomed.2004.02.075
Jain, P. N., Padole, D., and Bakshi, S. (2014). Prevalence of acute neuropathic pain after cancer surgery: a prospective study. Indian J. Anaesth. 58, 36–42. doi:10.4103/0019-5049.126788
Jimenez, R., Lopez-Sepulveda, R., Romero, M., Toral, M., Cogolludo, A., Perez-Vizcaino, F., et al. (2015). Quercetin and its metabolites inhibit the membrane NADPH oxidase activity in vascular smooth muscle cells from normotensive and spontaneously hypertensive rats. Food Funct. 6, 409–414. doi:10.1039/c4fo00818a
Jin, M.-Z., and Jin, W.-L. (2020). The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 5, 166. doi:10.1038/s41392-020-00280-x
Jin, M. J., Kim, U., Kim, I. S., Kim, Y., Kim, D. H., Han, S. B., et al. (2010). Effects of gut microflora on pharmacokinetics of hesperidin: a study on non-antibiotic and pseudo-germ-free rats. J. Toxicol. Environ. Health A 73, 1441–1450. doi:10.1080/15287394.2010.511549
Jin, P. a.N., Li, M., Xu, G., Zhang, K. U. N., Zheng, L. I., and Zhao, J. (2015). Role of (-)-epigallocatechin-3-gallate in the osteogenic differentiation of human bone marrow mesenchymal stem cells: an enhancer or an inducer? Exp. Ther. Med. 10, 828–834. doi:10.3892/etm.2015.2579
Kamaraj, S., Vinodhkumar, R., Anandakumar, P., Jagan, S., Ramakrishnan, G., and Devaki, T. (2007). The effects of quercetin on antioxidant status and tumor markers in the lung and serum of mice treated with benzo(a)pyrene. Biol. Pharm. Bull. 30, 2268–2273. doi:10.1248/bpb.30.2268
Kamaraj, S., Ramakrishnan, G., Anandakumar, P., Jagan, S., and Devaki, T. (2008). Antioxidant and anticancer efficacy of hesperidin in benzo(a)pyrene induced lung carcinogenesis in mice. Investig. New Drugs 27, 214–222. doi:10.1007/s10637-008-9159-7
Kanaze, F. I., Bounartzi, M. I., Georgarakis, M., and Niopas, I. (2007). Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 61, 472–477. doi:10.1038/sj.ejcn.1602543
Kasala, E. R., Bodduluru, L. N., Barua, C. C., and Gogoi, R. (2016a). Antioxidant and antitumor efficacy of Luteolin, a dietary flavone on benzo(a)pyrene-induced experimental lung carcinogenesis. Biomed. Pharmacother. 82, 568–577. doi:10.1016/j.biopha.2016.05.042
Kasala, E. R., Bodduluru, L. N., Barua, C. C., Madhana, R. M., Dahiya, V., Budhani, M. K., et al. (2016b). Chemopreventive effect of chrysin, a dietary flavone against benzo(a)pyrene induced lung carcinogenesis in Swiss albino mice. Pharmacol. Rep. 68, 310–318. doi:10.1016/j.pharep.2015.08.014
Kaufman, P. B., Duke, J. A., Brielmann, H., Boik, J., and Hoyt, J. E. (1997). A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: implications for human nutrition and health. J. Altern. Complementary Med. 3, 7–12. doi:10.1089/acm.1997.3.7
Kaushal, G. P., Chandrashekar, K., and Juncos, L. A. (2019). Molecular interactions between reactive oxygen species and autophagy in kidney disease. Int. J. Mol. Sci. 20, 3791. doi:10.3390/ijms20153791
Kawaguchi, S., Kawahara, K., Fujiwara, Y., Ohnishi, K., Pan, C., Yano, H., et al. (2022). Naringenin potentiates anti-tumor immunity against oral cancer by inducing lymph node CD169-positive macrophage activation and cytotoxic T cell infiltration. Cancer Immunol. Immunother. 71, 2127–2139. doi:10.1007/s00262-022-03149-w
Key, T. (2011). Fruit and vegetables and cancer risk. Br. J. Cancer. 104, 6–11. doi:10.1038/sj.bjc.6606032
Khan, A. U., Dagur, H. S., Khan, M., Malik, N., Alam, M., and Mushtaque, M. (2021). Therapeutic role of flavonoids and flavones in cancer prevention: current trends and future perspectives. Eur. J. Med. Chem. Rep. 3, 100010. doi:10.1016/j.ejmcr.2021.100010
Khan, S. U., Fatima, K., Aisha, S., and Malik, F. (2024). Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal. 22, 109. doi:10.1186/s12964-023-01302-1
Khazdair, M., Anaeigoudari, A., and Agbor, G. (2021). Anti-viral and anti-inflammatory effects of kaempferol and quercetin and COVID-2019: a scoping review. Asian Pac. J. Trop. Biomed. 11, 327–334. doi:10.4103/2221-1691.319567
Kikuchi, H., Yuan, B., Hu, X., and Okazaki, M. (2019). Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am. J. Cancer Res. 9, 1517–1535. Available online at: https://pmc.ncbi.nlm.nih.gov/articles/PMC6726994/
Knekt, P., Isotupa, S., Rissanen, H., Heliövaara, M., Järvinen, R., Häkkinen, S., et al. (2000). Quercetin intake and the incidence of cerebrovascular disease. Eur. J. Clin. Nutr. 54, 415–417. doi:10.1038/sj.ejcn.1600974
Knopfová, L., and Šmarda, J. a.N. (2010). The use of Cox-2 and PPARγ signaling in anti-cancer therapies. Exp. Ther. Med. 1, 257–264. doi:10.3892/etm_00000040
Kondža, M., Brizić, I., and Jokić, S. (2024). Flavonoids as CYP3A4 inhibitors in vitro. Biomedicines 12, 644. doi:10.3390/biomedicines12030644
Kopustinskiene, D. M., Jakstas, V., Savickas, A., and Bernatoniene, J. (2020). Flavonoids as anticancer agents. Nutrients 12, 457. doi:10.3390/nu12020457
Kurek-Gorecka, A., Gorecki, M., Rzepecka-Stojko, A., Balwierz, R., and Stojko, J. (2020). Bee products in dermatology and skin care. Molecules 25, 556. doi:10.3390/molecules25030556
Kusaczuk, M., Tovar-Ambel, E., Martín-Cabrera, P., Lorente, M., Salvador-Tormo, N., Mikłosz, A., et al. (2024). Cytotoxicity, proapoptotic activity and drug-like potential of quercetin and kaempferol in glioblastoma cells: preclinical insights. Int. J. Mol. Sci. 25, 10740. doi:10.3390/ijms251910740
Lambert, J. D., Kennett, M. J., Sang, S., Reuhl, K. R., Ju, J., and Yang, C. S. (2010). Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 48, 409–416. doi:10.1016/j.fct.2009.10.030
Lecumberri, E., Dupertuis, Y. M., Miralbell, R., and Pichard, C. (2013). Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin. Nutr. 32, 894–903. doi:10.1016/j.clnu.2013.03.008
Lee, J., and Mitchell, A. E. (2012). Pharmacokinetics of quercetin absorption from apples and onions in healthy humans. J. Agric. Food Chem. 60, 3874–3881. doi:10.1021/jf3001857
Lee, J.-S., and Surh, Y.-J. (2005). Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 224, 171–184. doi:10.1016/j.canlet.2004.09.042
Lee, L. T., Huang, Y. T., Hwang, J. J., Lee, P. P., Ke, F. C., Nair, M. P., et al. (2002). Blockade of the epidermal growth factor receptor tyrosine kinase activity by quercetin and luteolin leads to growth inhibition and apoptosis of pancreatic tumor cells. Anticancer Res. 22, 1615–1627. Available online at: https://pubmed.ncbi.nlm.nih.gov/12168845/.
Lee, J. Y., Kim, H. S., and Song, Y. S. (2012). Genistein as a potential anticancer agent against ovarian cancer. J. Tradit. Complement. Med. 2, 96–104. doi:10.1016/s2225-4110(16)30082-7
Leese, H. J., and Semenza, G. (1973). On the identity between the small intestinal enzymes phlorizin hydrolase and glycosylceramidase. J. Biol. Chem. 248, 8170–8173. doi:10.1016/s0021-9258(19)43209-2
Lesjak, M., Beara, I., Simin, N., Pintać, D., Majkić, T., Bekvalac, K., et al. (2018). Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 40, 68–75. doi:10.1016/j.jff.2017.10.047
Li, Y., and Sarkar, F. H. (2002). Inhibition of nuclear factor kappaB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin. Cancer Res. 8, 2369–2377. Available online at: https://pubmed.ncbi.nlm.nih.gov/12114442/.
Li, G., Ding, K., Qiao, Y., Zhang, L., Zheng, L., Pan, T., et al. (2020). Flavonoids regulate inflammation and oxidative stress in cancer. Molecules 25, 5628. doi:10.3390/molecules25235628
Li, L., Gao, H., Lou, K., Luo, H., Hao, S., Yuan, J., et al. (2021a). Safety, tolerability, and pharmacokinetics of oral baicalein tablets in healthy Chinese subjects: a single-center, randomized, double-blind, placebo-controlled multiple-ascending-dose study. Clin. Transl. Sci. 14, 2017–2024. doi:10.1111/cts.13063
Li, X., He, X., Chen, S., Le, Y., Bryant, M. S., Guo, L., et al. (2021b). The genotoxicity potential of luteolin is enhanced by CYP1A1 and CYP1A2 in human lymphoblastoid TK6 cells. Toxicol. Lett. 344, 58–68. doi:10.1016/j.toxlet.2021.03.006
Li, H., Shi, L., Chen, X., and Wang, M. (2023a). Association between dietary intake of flavonoids and hyperuricemia: a cross-sectional study. BMC Public Health 23, 1227. doi:10.1186/s12889-023-16134-4
Li, Y., Zhang, X., Wang, Z., Li, B., and Zhu, H. (2023b). Modulation of redox homeostasis: a strategy to overcome cancer drug resistance. Front. Pharmacol. 14, 1156538. doi:10.3389/fphar.2023.1156538
Li, K., Wang, Y., Liu, W., Zhang, C., Xi, Y., Zhou, Y., et al. (2024). Structure–activity relationships and changes in the inhibition of Xanthine oxidase by polyphenols: a review. Foods 13, 2365. doi:10.3390/foods13152365
Liga, S., and Paul, C. (2025). Flavonoid-based nanogels: a comprehensive overview. Gels 11, 267. doi:10.3390/gels11040267
Lim, J. W., Kim, H., and Kim, K. H. (2001). Nuclear factor-kappaB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells, Lab. Invest. 81, 349–360. doi:10.1038/labinvest.3780243
Lin, L.-C., Pai, Y.-F., and Tsai, T.-H. (2015a). Isolation of luteolin and Luteolin-7-O-glucoside from Dendranthema morifolium ramat tzvel and their pharmacokinetics in rats. J. Agric. Food Chem. 63, 7700–7706. doi:10.1021/jf505848z
Lin, S., Zhang, G., Liao, Y., Pan, J., and Gong, D. (2015b). Dietary flavonoids as xanthine oxidase inhibitors: structure–affinity and structure–activity relationships. J. Agric. Food Chem. 63, 7784–7794. doi:10.1021/acs.jafc.5b03386
Lin, B.-W., Gong, C.-C., Song, H.-F., and Cui, Y.-Y. (2017). Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 174, 1226–1243. doi:10.1111/bph.13627
Liskova, A., Koklesova, L., Samec, M., Smejkal, K., Samuel, S. M., Varghese, E., et al. (2020). Flavonoids in cancer metastasis. Cancers (Basel) 12, 1498. doi:10.3390/cancers12061498
Liskova, A., Samec, M., Koklesova, L., Brockmueller, A., Zhai, K., Abdellatif, B., et al. (2021). Flavonoids as an effective sensitizer for anti-cancer therapy: insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 12, 155–176. doi:10.1007/s13167-021-00242-5
Lotito, S., and Frei, B. (2006). Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 41, 1727–1746. doi:10.1016/j.freeradbiomed.2006.04.033
Luo, M., Tian, R., Yang, Z., Peng, Y.-Y., and Lu, N. (2019). Quercetin suppressed NADPH oxidase-derived oxidative stress via heme oxygenase-1 induction in macrophages. Archives Biochem. Biophysics 671, 69–76. doi:10.1016/j.abb.2019.06.007
Lyngsie, G., Krumina, L., Tunlid, A., and Persson, P. (2018). Generation of hydroxyl radicals from reactions between a dimethoxyhydroquinone and iron oxide nanoparticles. Sci. Rep. 8, 10834. doi:10.1038/s41598-018-29075-5
Magar, R. T., and Sohng, J. K. (2020). A review on structure, modifications and structure-activity relation of quercetin and its derivatives. J. Microbiol. Biotechnol. 30, 11–20. doi:10.4014/jmb.1907.07003
Manach, C., and Donovan, J. L. (2004). Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic. Res. 38, 771–785. doi:10.1080/10715760410001727858
Manna, S. K., Aggarwal, R. S., Sethi, G., Aggarwal, B. B., and Ramesh, G. T. (2007). Morin (3,5,7,2',4'-Pentahydroxyflavone) abolishes nuclear factor-kappaB activation induced by various carcinogens and inflammatory stimuli, leading to suppression of nuclear factor-kappab-regulated gene expression and up-regulation of apoptosis. Clin. Cancer Res. 13, 2290–2297. doi:10.1158/1078-0432.CCR-06-2394
Maraldi, T. (2013). Natural compounds as modulators of NADPH oxidases. Oxidative Med. Cell. Longev. 2013, 271602–10. doi:10.1155/2013/271602
Marini, H., Minutoli, L., Polito, F., Bitto, A., Altavilla, D., Atteritano, M., et al. (2007). Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann. Intern. Med. 146, 839–847. doi:10.7326/0003-4819-146-12-200706190-00005
Mathew, B., Suresh, J., Mathew, G., Rasheed, S., Vilapurathu, J., and Jayaraj, P. (2015). Flavonoids: an outstanding structural core for the inhibition of xanthine oxidase enzyme. Curr. Enzyme Inhib. 11, 108–115. doi:10.2174/1573408011666150730204108
Mathur, G., Nain, S., and Sharma, P. K. (2015). Cancer: an overview. Acad. J. Cancer Res. 8, 01–09. doi:10.5829/idosi.ajcr.2015.8.1.9336
Michael, M., and Doherty, M. M. (2005). Tumoral drug metabolism: overview and its implications for cancer therapy. J. Clin. Oncol. 23, 205–229. doi:10.1200/JCO.2005.02.120
Michael Mcclain, R., Wolz, E., Davidovich, A., and Bausch, J. (2006). Genetic toxicity studies with genistein. Food Chem. Toxicol. 44, 42–55. doi:10.1016/j.fct.2005.06.004
Miltonprabu, S. (2019). “Quercetin: a flavonol with versatile therapeutic applications and its interactions with other drugs”, in Nonvitamin and nonmineral nutritional supplements. (Academic Press), 75–83. Available online at: https://www.sciencedirect.com/book/9780128124918/nonvitamin-and-nonmineral-nutritional-supplements#book-info.
Mir, S. A., Dar, A., Hamid, L., Nisar, N., Malik, J. A., Ali, T., et al. (2024). Flavonoids as promising molecules in the cancer therapy: an insight. Curr. Res. Pharmacol. Drug Discov. 6, 100167. doi:10.1016/j.crphar.2023.100167
Mira, L., Tereza Fernandez, M., Santos, M., Rocha, R., Helena Florêncio, M., and Jennings, K. R. (2009). Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic. Res. 36, 1199–1208. doi:10.1080/1071576021000016463
Mirzoeva, S., Franzen, C. A., and Pelling, J. C. (2014). Apigenin inhibits TGF-β-induced VEGF expression in human prostate carcinoma cells via a Smad2/3- and src-dependent mechanism. Mol. Carcinog. 53, 598–609. doi:10.1002/mc.22005
Mishra, N., and Rizvi, S. I. (2012). Quercetin modulates Na(+)/K(+) ATPase and sodium hydrogen exchanger in type 2 diabetic erythrocytes. Cell Mol. Biol. (Noisy-le-Grand) 58, 148–152. Available online at: https://pubmed.ncbi.nlm.nih.gov/23273205/.
Moon, J.-H., Nakata, R., Oshima, S., Inakuma, T., and Terao, J. (2000). Accumulation of quercetin conjugates in blood plasma after the short-term ingestion of onion by women. Am. J. Physiology-Regulatory, Integr. Comp. Physiology 279, R461–R467. doi:10.1152/ajpregu.2000.279.2.R461
Moon, Y. J., Wang, X., and Morris, M. E. (2006). Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol. Vitro 20, 187–210. doi:10.1016/j.tiv.2005.06.048
Multhoff, G., Molls, M., and Radons, J. (2012). Chronic inflammation in cancer development. Front. Immunol. 2, 98. doi:10.3389/fimmu.2011.00098
Murota, K., Shimizu, S., Miyamoto, S., Terao, J., Izumi, T., Obata, A., et al. (2002). Unique uptake and transport of isoflavone aglycones by human intestinal Caco-2 cells: comparison of isoflavonoids and flavonoids. J. Nutr. 132, 1956–1961. doi:10.1093/jn/132.7.1956
Mutoh, M., Takahashi, M., Fukuda, K., Komatsu, H., Enya, T., Matsushima-Hibiya, Y., et al. (2000). Suppression by flavonoids of Cyclooxygenase-2 promoter-dependent transcriptional activity in Colon cancer cells: structure-activity relationship. Jpn. J. Cancer Res. 91, 686–691. doi:10.1111/j.1349-7006.2000.tb01000.x
Nagao, A., Seki, M., and Kobayashi, H. (2014). Inhibition of xanthine oxidase by flavonoids. Biosci. Biotechnol. Biochem. 63, 1787–1790. doi:10.1271/bbb.63.1787
Olthof, M. R., Hollman, P. C. H., Vree, T. B., and Katan, M. B. (2000). Bioavailabilities of quercetin-3-glucoside and quercetin-4′-glucoside do not differ in humans. J. Nutr. 130, 1200–1203. doi:10.1093/jn/130.5.1200
Olthof, M. R., Hollman, P. C. H., Buijsman, M. N. C. P., Van Amelsvoort, J. M. M., and Katan, M. B. (2003). Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 133, 1806–1814. doi:10.1093/jn/133.6.1806
Ou, M., Deng, Z., Shi, Y., He, J., Ye, Z., Guo, M., et al. (2024). Mechanism of apigenin against breast cancer stem cells: network pharmacology and experimental validation. Front. Pharmacol. 15, 1496664. doi:10.3389/fphar.2024.1496664
Panche, A. N., Diwan, A. D., and Chandra, S. R. (2016). Flavonoids: an overview. J. Nutr. Sci. 5, e47. doi:10.1017/jns.2016.41
Pang, L. Y., Hurst, E. A., and Argyle, D. J. (2016). Cyclooxygenase-2: a role in cancer stem cell survival and repopulation of cancer cells during therapy. Stem Cells Int. 2016, 2048731–11. doi:10.1155/2016/2048731
Parmenter, B. H., Thompson, A. S., Bondonno, N. P., Jennings, A., Murray, K., Perez-Cornago, A., et al. (2025). High diversity of dietary flavonoid intake is associated with a lower risk of all-cause mortality and major chronic diseases. Nat. Food 6, 668–680. doi:10.1038/s43016-025-01176-1
Passamonti, S., Terdoslavich, M., Franca, R., Vanzo, A., Tramer, F., Braidot, E., et al. (2009). Bioavailability of flavonoids: a review of their membrane transport and the function of bilitranslocase in animal and plant organisms. Curr. Drug Metab. 10, 369–394. doi:10.2174/138920009788498950
Passaniti, A., Kim, M. S., Polster, B. M., and Shapiro, P. (2022). Targeting mitochondrial metabolism for metastatic cancer therapy. Mol. Carcinog. 61, 827–838. doi:10.1002/mc.23436
Pietta, P.-G. (2000). Flavonoids as antioxidants. J. Nat. Prod. 63, 1035–1042. doi:10.1021/np9904509
Procházková, D., Boušová, I., and Wilhelmová, N. (2011). Antioxidant and prooxidant properties of flavonoids. Fitoterapia 82, 513–523. doi:10.1016/j.fitote.2011.01.018
Qiao, H., Zhang, X., Wang, T., Liang, L., Chang, W., and Xia, H. (2013). Pharmacokinetics, biodistribution and bioavailability of isoliquiritigenin after intravenous and oral administration. Pharm. Biol. 52, 228–236. doi:10.3109/13880209.2013.832334
Quail, D. F., and Joyce, J. A. (2013). Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437. doi:10.1038/nm.3394
Rahaman, M. S., Siraj, M. A., Islam, M. A., Shanto, P. C., Islam, O., Islam, M. A., et al. (2022). Crosstalk between xanthine oxidase (XO) inhibiting and cancer chemotherapeutic properties of comestible flavonoids-a comprehensive update. J. Nutr. Biochem. 110, 109147. doi:10.1016/j.jnutbio.2022.109147
Ramachandran, L., Manu, K. A., Shanmugam, M. K., Li, F., Siveen, K. S., Vali, S., et al. (2012). Isorhamnetin inhibits proliferation and invasion and induces apoptosis through the modulation of peroxisome proliferator-activated receptor γ activation pathway in gastric cancer. J. Biol. Chem. 287, 38028–38040. doi:10.1074/jbc.M112.388702
Rana, J. N., and Mumtaz, S. (2025). Prunin: an emerging anticancer flavonoid. Int. J. Mol. Sci. 26, 2678. doi:10.3390/ijms26062678
Rana, J. N., Gul, K., and Mumtaz, S. (2025). Isorhamnetin: reviewing recent developments in anticancer mechanisms and nanoformulation-driven delivery. Int. J. Mol. Sci. 26, 7381. doi:10.3390/ijms26157381
Ravishankar, D., Rajora, A. K., Greco, F., and Osborn, H. M. I. (2013). Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 45, 2821–2831. doi:10.1016/j.biocel.2013.10.004
Ríha, M., Karlícková, J., Filipský, T., Jahodár, L., Hrdina, R., and Mladenka, P. (2014). In vitro copper-chelating properties of flavonoids. Free Radic. Biol. Med. 75, S46. doi:10.1016/j.freeradbiomed.2014.10.807
Rodriguez-Garcia, C., Sanchez-Quesada, C., and J, J. G. (2019). Dietary flavonoids as cancer chemopreventive agents: an updated review of human studies. Antioxidants (Basel) 8, 137. doi:10.3390/antiox8050137
Rolt, A., and Cox, L. S. (2020). Structural basis of the anti-ageing effects of polyphenolics: mitigation of oxidative stress. BMC Chem. 14, 50. doi:10.1186/s13065-020-00696-0
Rosales, T. K. O., and Fabi, J. P. (2023). Valorization of polyphenolic compounds from food industry by-products for application in polysaccharide-based nanoparticles. Front. Nutr. 10, 1144677. doi:10.3389/fnut.2023.1144677
Saha, D., Datta, P. K., Sheng, H., Morrow, J. D., Wada, M., Moses, H. L., et al. (1999). Synergistic induction of cyclooxygenase-2 by transforming growth factor-beta1 and epidermal growth factor inhibits apoptosis in epithelial cells. Neoplasia 1, 508–517. doi:10.1038/sj.neo.7900051
Sakata, K., Hirose, Y., Qiao, Z., Tanaka, T., and Mori, H. (2003). Inhibition of inducible isoforms of cyclooxygenase and nitric oxide synthase by flavonoid hesperidin in mouse macrophage cell line. Cancer Lett. 199, 139–145. doi:10.1016/s0304-3835(03)00386-0
Samu, Z., Nyiredy, S., Baitz-Gács, E., Varga, Z., Kurtán, T., Dinya, Z., et al. (2004). Structure elucidation and antioxidant activity of (−)-isosilandrin isolated from Silybum marianum L. Chem. Biodivers. 1, 1668–1677. doi:10.1002/cbdv.200490125
Sarg, N. H., Hersi, F. H., Zaher, D. M., Hamouda, A. O., Ibrahim, S. I., El-Seedi, H. R., et al. (2024). Unveiling the therapeutic potential of taxifolin in cancer: from molecular mechanisms to immune modulation and synergistic combinations. Phytomedicine 133, 155934. doi:10.1016/j.phymed.2024.155934
Semwal, D., Semwal, R., Combrinck, S., and Viljoen, A. (2016). Myricetin: a dietary molecule with diverse biological activities. Nutrients 8, 90. doi:10.3390/nu8020090
Seufi, A. M., Ibrahim, S. S., Elmaghraby, T. K., and Hafez, E. E. (2009). Preventive effect of the flavonoid, quercetin, on hepatic cancer in rats via oxidant/antioxidant activity: molecular and histological evidences. J. Exp. Clin. Cancer Res. 28, 80. doi:10.1186/1756-9966-28-80
Shaker, E., Mahmoud, H., and Mnaa, S. (2010). Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem. Toxicol. 48, 803–806. doi:10.1016/j.fct.2009.12.011
Sharma, V., Joseph, C., Ghosh, S., Agarwal, A., Mishra, M. K., and Sen, E. (2007). Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol. Cancer Ther. 6, 2544–2553. doi:10.1158/1535-7163.MCT-06-0788
Sharma, A., Singh Tuli, H., and Sharma, A. K. (2019). “Chemistry and synthetic overview of flavonoids”, in Current aspects of flavonoids: their role in cancer treatment. (Singapore: Springer Singapore), 23–38. Available online at: https://link.springer.com/book/10.1007/978-981-13-5874-6#about-this-book.
Sharma, T., Singh, D., Mahapatra, A., Mohapatra, P., Sahoo, S., and Sahoo, S. K. (2022). Advancements in clinical translation of flavonoid nanoparticles for cancer treatment. OpenNano 8, 100074. doi:10.1016/j.onano.2022.100074
Shih, P.-H., Yeh, C.-T., and Yen, G.-C. (2007). Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J. Agric. Food Chem. 55, 9427–9435. doi:10.1021/jf071933i
Shim, J. Y., An, H. J., Lee, Y. H., Kim, S. K., Lee, K. P., and Lee, K. S. (2003). Overexpression of cyclooxygenase-2 is associated with breast carcinoma and its poor prognostic factors. Mod. Pathol. 16, 1199–1204. doi:10.1097/01.MP.0000097372.73582.CB
Shin, S.-S., Won, S. Y., Noh, D.-H., Hwang, B., Kim, W.-J., and Moon, S.-K. (2017). Morin inhibits proliferation, migration, and invasion of bladder cancer EJ cells via modulation of signaling pathways, cell cycle regulators, and transcription factor-mediated MMP-9 expression. Drug Dev. Res. 78, 81–90. doi:10.1002/ddr.21377
Shukla, S., and Gupta, S. (2004). Suppression of constitutive and tumor necrosis factor alpha-induced nuclear factor (NF)-kappaB activation and induction of apoptosis by apigenin in human prostate carcinoma PC-3 cells: correlation with down-regulation of NF-kappaB-responsive genes. Clin. Cancer Res. 10, 3169–3178. doi:10.1158/1078-0432.ccr-03-0586
Shukla, S., and Gupta, S. (2014). Apigenin-induced cell cycle arrest is mediated by modulation of MAPK, PI3K-Akt, and loss of cyclin D1 associated retinoblastoma dephosphorylation in human prostate cancer cells. Cell Cycle 6, 1102–1114. doi:10.4161/cc.6.9.4146
Slika, H., Mansour, H., Wehbe, N., Nasser, S. A., Iratni, R., Nasrallah, G., et al. (2022). Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 146, 112442. doi:10.1016/j.biopha.2021.112442
Soleimani, V., Delghandi, P. S., Moallem, S. A., and Karimi, G. (2019). Safety and toxicity of silymarin, the major constituent of milk thistle extract: an updated review. Phytotherapy Res. 33, 1627–1638. doi:10.1002/ptr.6361
Sornsuvit, C., Hongwiset, D., Yotsawimonwat, S., Toonkum, M., Thongsawat, S., and Taesotikul, W. (2018). The bioavailability and pharmacokinetics of silymarin SMEDDS formulation study in healthy Thai volunteers. Evidence-Based Complementary Altern. Med. 2018, 1507834–7. doi:10.1155/2018/1507834
Spencer, J. P. E., Chaudry, F., Pannala, A. S., Srai, S. K., Debnam, E., and Rice-Evans, C. (2000). Decomposition of cocoa procyanidins in the gastric milieu. Biochem. Biophysical Res. Commun. 272, 236–241. doi:10.1006/bbrc.2000.2749
Steffen, Y., Gruber, C., Schewe, T., and Sies, H. (2008). Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Archives Biochem. Biophysics 469, 209–219. doi:10.1016/j.abb.2007.10.012
Suarez-Carmona, M., Lesage, J., Cataldo, D., and Gilles, C. (2017). EMT and inflammation: inseparable actors of cancer progression. Mol. Oncol. 11, 805–823. doi:10.1002/1878-0261.12095
Suzuki, T., Miyoshi, N., Hayakawa, S., Imai, S., Isemura, M., and Nakamura, Y. (2016). “Beverage impacts on health and nutrition”, in Health benefits of tea consumption (Cham, Switzerland: Springer International Publishing), 49–67.
Tahsin, A., Ahamad, J., Uthirapathy, S., Anwer, E. T., Mohammed Ameen, M. S., and Porwal, O. (2019). Silybum marianum (Milk Thistle): review on its chemistry, morphology, ethno medical uses, phytochemistry and pharmacological activities. J. Drug Deliv. Ther. 9, 199–206. doi:10.22270/jddt.v9i5.3666
Taniguchi, K., and Karin, M. (2018). NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 18, 309–324. doi:10.1038/nri.2017.142
Terao, J., Kawai, Y., and Murota, K. (2008). Vegetable flavonoids and cardiovascular disease. Asia Pac J. Clin. Nutr. 17 (Suppl. 1), 291–293. Available online at: https://pubmed.ncbi.nlm.nih.gov/18296359/.
Thangavel, P., Puga-Olguín, A., Rodríguez-Landa, J. F., and Zepeda, R. C. (2019). Genistein as potential therapeutic candidate for menopausal symptoms and other related diseases. Molecules 24, 3892. doi:10.3390/molecules24213892
Tian, Y., Lei, Y., Wang, Y., Lai, J., Wang, J., and Xia, F. (2023). Mechanism of multidrug resistance to chemotherapy mediated by P‑glycoprotein (review). Int. J. Oncol. 63, 119. doi:10.3892/ijo.2023.5567
Tomar, R., Das, S. S., Balaga, V. K. R., Tambe, S., Sahoo, J., Rath, S. K., et al. (2024). Therapeutic implications of dietary polyphenols-loaded nanoemulsions in cancer therapy. ACS Appl. Bio Mater. 7, 2036–2053. doi:10.1021/acsabm.3c01205
Tubtimsri, S., Chuenbarn, T., and Manmuan, S. (2025). Quercetin triggers cell apoptosis-associated ROS-mediated cell death and induces S and G2/M-phase cell cycle arrest in KON oral cancer cells. BMC Complement. Med. Ther. 25, 34. doi:10.1186/s12906-025-04782-5
Tuorkey, M. J. (2016). Molecular targets of luteolin in cancer. Eur. J. Cancer Prev. 25, 65–76. doi:10.1097/CEJ.0000000000000128
Ulusoy, H. G., and Sanlier, N. (2019). A minireview of quercetin: from its metabolism to possible mechanisms of its biological activities. Crit. Rev. Food Sci. Nutr. 60, 3290–3303. doi:10.1080/10408398.2019.1683810
Uruno, A., and Motohashi, H. (2011). The Keap1–Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide 25, 153–160. doi:10.1016/j.niox.2011.02.007
Van Dross, R. T., Hong, X., Essengue, S., Fischer, S. M., and Pelling, J. C. (2007). Modulation of UVB-induced and basal cyclooxygenase-2 (COX-2) expression by apigenin in mouse keratinocytes: role of USF transcription factors. Mol. Carcinog. 46, 303–314. doi:10.1002/mc.20281
Van Hoorn, D. E. C., Nijveldt, R. J., Van Leeuwen, P. a.M., Hofman, Z., M'rabet, L., De Bont, D. B. A., et al. (2002). Accurate prediction of xanthine oxidase inhibition based on the structure of flavonoids. Eur. J. Pharmacol. 451, 111–118. doi:10.1016/s0014-2999(02)02192-1
Vanamala, J., Leonardi, T., Patil, B. S., Taddeo, S. S., Murphy, M. E., Pike, L. M., et al. (2006). Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis 27, 1257–1265. doi:10.1093/carcin/bgi318
Vo, Q. V., Nam, P. C., Thong, N. M., Trung, N. T., Phan, C. D., and Mechler, A. (2019). Antioxidant motifs in flavonoids: O-H versus C-H bond dissociation. ACS Omega 4, 8935–8942. doi:10.1021/acsomega.9b00677
Wang, F., Wang, L., Qu, C., Chen, L., Geng, Y., Cheng, C., et al. (2021a). Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer 21, 396. doi:10.1186/s12885-021-08158-z
Wang, X., Chen, B., Xu, D., Li, Z., Liu, H., Huang, Z., et al. (2021b). Molecular mechanism and pharmacokinetics of flavonoids in the treatment of resistant EGF receptor-mutated non-small-cell lung cancer: a narrative review. Br. J. Pharmacol. 178, 1388–1406. doi:10.1111/bph.15360
Ward, A. B., Mir, H., Kapur, N., Gales, D. N., Carriere, P. P., and Singh, S. (2018). Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 16, 108. doi:10.1186/s12957-018-1400-z
Wei, B.-L., Weng, J.-R., Chiu, P.-H., Hung, C.-F., Wang, J.-P., and Lin, C.-N. (2005). Antiinflammatory flavonoids from Artocarpus heterophyllus and Artocarpus communis. J. Agric. Food Chem. 53, 3867–3871. doi:10.1021/jf047873n
Weng, C.-J., and Yen, G.-C. (2012). Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 31, 323–351. doi:10.1007/s10555-012-9347-y
Winter, J., Popoff, M. R., Grimont, P., and Bokkenheuser, V. D. (1991). Clostridium orbiscindens sp. Nov., a human intestinal bacterium capable of cleaving the flavonoid C-Ring. Int. J. Syst. Bacteriol. 41, 355–357. doi:10.1099/00207713-41-3-355
Wu, S., Fisher-Hoch, S. P., Reininger, B. M., Lee, M., and McCormick, J. B. (2019). Fruit and vegetable intake is inversely associated with cancer risk in Mexican-Americans. Nutr. Cancer 71 (8), 1254–1262. doi:10.1080/01635581.2019.1603315
Wu, P., Meng, X., Zheng, H., Zeng, Q., Chen, T., Wang, W., et al. (2018). Kaempferol attenuates ROS-induced hemolysis and the molecular mechanism of its induction of apoptosis on bladder cancer. Molecules 23, 2592. doi:10.3390/molecules23102592
Xia, T., and Zhu, R. (2024). Multiple molecular and cellular mechanisms of the antitumour effect of dihydromyricetin (review). Biomed. Rep. 20, 82. doi:10.3892/br.2024.1769
Xia, Y., Yuan, M., Li, S., Thuan, U. T., Nguyen, T. T., Kang, T. W., et al. (2018). Apigenin suppresses the IL-1β-Induced expression of the urokinase-type plasminogen activator receptor by inhibiting MAPK-mediated AP-1 and NF-κB signaling in human bladder cancer T24 cells. J. Agric. Food Chem. 66, 7663–7673. doi:10.1021/acs.jafc.8b02351
Yan, Y., Liu, X., Gao, J., Wu, Y., and Li, Y. (2020). Inhibition of TGF-β signaling in gliomas by the flavonoid diosmetin isolated from Dracocephalum peregrinum L. Molecules 25, 192. doi:10.3390/molecules25010192
Yang, C. S., and Hong, J. (2013). Prevention of chronic diseases by tea: possible mechanisms and human relevance. Annu. Rev. Nutr. 33, 161–181. doi:10.1146/annurev-nutr-071811-150717
Yang, Z., Kulkarni, K., Zhu, W., and Hu, M. (2012). Bioavailability and pharmacokinetics of genistein: mechanistic studies on its ADME. Anti-Cancer Agents Med. Chem. 12, 1264–1280. doi:10.2174/187152012803833107
Yang, X., Zan, T., Yan, H., and Liu, B. (2019). UPLC-MS/MS determination of flavokawain B, a novel anti-tumor chemotherapeutic agent in rat plasma and its application to a pharmacokinetic study in rats. Biomed. Chromatogr. 33, e4391. doi:10.1002/bmc.4391
Ye, F., Wu, J., Dunn, T., Yi, J., Tong, X., and Zhang, D. (2004). Inhibition of cyclooxygenase-2 activity in head and neck cancer cells by genistein. Cancer Lett. 211, 39–46. doi:10.1016/j.canlet.2004.03.043
Yin, F., Giuliano, A. E., Law, R. E., and Van Herle, A. J. (2001). Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 21, 413–420. Available online at: https://pubmed.ncbi.nlm.nih.gov/11299771/.
Yu, Y., Xiao, C. H., Tan, L. D., Wang, Q. S., Li, X. Q., and Feng, Y. M. (2013). Cancer-associated fibroblasts induce epithelial–mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer 110, 724–732. doi:10.1038/bjc.2013.768
Zhai, X., Lin, M., Zhang, F., Hu, Y., Xu, X., Li, Y., et al. (2013). Dietary flavonoid genistein induces Nrf2 and phase II detoxification gene expression via ERKs and PKC pathways and protects against oxidative stress in Caco-2 cells. Mol. Nutr. Food Res. 57, 249–259. doi:10.1002/mnfr.201200536
Zhang, W., Lian, Y., Li, Q., Sun, L., Chen, R., Lai, X., et al. (2020). Preventative and therapeutic potential of flavonoids in peptic ulcers. Molecules 25, 4626. doi:10.3390/molecules25204626
Zhao, J., Yang, J., and Xie, Y. (2019). Improvement strategies for the oral bioavailability of poorly water-soluble flavonoids: an overview. Int. J. Pharm. 570, 118642. doi:10.1016/j.ijpharm.2019.118642
Zheng, X., Zhang, X., and Zeng, F. (2025). Biological functions and health benefits of flavonoids in fruits and vegetables: a contemporary review. Foods 14, 155. doi:10.3390/foods14020155
Zhu, S. Y., Jiang, N., Yang, J., Tu, J., Zhou, Y., Xiao, X., et al. (2018). Silybum marianum oil attenuates hepatic steatosis and oxidative stress in high fat diet-fed mice. Biomed. Pharmacother. 100, 191–197. doi:10.1016/j.biopha.2018.01.144
Glossary
ADMET Absorption, distribution, metabolism, excretion, and toxicity
ADME Absorption, distribution, metabolism, and excretion
ARE Antioxidant-responsive element
AUC0−t Area under the concentration-time curve from dosing
ALT Alanine aminotransferase
CYPs Cytochromes P450s
COX Cyclooxygenase
CR Complete response
Cmax Concentration Maxima
EMT Epithelial-mesenchymal transition
EpRE Electrophile-responsive element
EGCG Epigallocatechin gallate
FDA Food and Drug Administration
F3′H Flavonoid 3′-hydroxylase
Keap1 Kelch-like ECH-associated protein 1
LD50 Lethal dose that kills 50% of the test organisms
MAPK Mitogen-activated protein kinase
MDR Multidrug resistance
MDSCs Myeloid-derived suppressor cells
mtDNAcn Mitochondrial DNA copy number
TFAM Mitochondrial transcription factor A
NADPH Nicotinamide adenine dinucleotide phosphate
NF-κB Nuclear factor-κB
NSCLC Non-small cell lung cancer
PK Protein kinases
PR Partial response
POLG Polymerase gamma
POLG2 Polymerase gamma 2
ROS Reactive oxygen species
SAR Structure-activity relationship
STAT3 Signal transducer and activator of transcription-3
TAM Tumor-associated macrophages
TME Tumor microenvironment
TNF Tumor necrosis factor
Tmax Time to peak drug concentration
T1/2ke Elimination plasma half-life
Keywords: flavonoids, anticancer agents, pharmacokinetics, admet, drug toxicity
Citation: Dubey AK, Chandragiri SS, Geevarghese AV, Kapoor B, Gulati M, Rani P, Singh G, Chavda VP, Gundamaraju R, Bansal H, Gautam RK, Goyal R, Okoh MP, Shen B and Singla RK (2025) Exploring the pharmacokinetics, drug-likeness, and toxicological features of anticancer flavonoids: a Boulevard to explore their clinical translational potential. Front. Pharmacol. 16:1648395. doi: 10.3389/fphar.2025.1648395
Received: 17 June 2025; Accepted: 08 September 2025;
Published: 03 October 2025.
Edited by:
Nunziatina De Tommasi, University of Salerno, ItalyReviewed by:
Sohail Mumtaz, Gachon University, Republic of KoreaLei Hu, Lushan Botanical Garden (CAS), China
Copyright © 2025 Dubey, Chandragiri, Geevarghese, Kapoor, Gulati, Rani, Singh, Chavda, Gundamaraju, Bansal, Gautam, Goyal, Okoh, Shen and Singla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajeev K. Singla, cmFqZWV2c2luZ2xhMjZAZ21haWwuY29t; cmFqZWV2a3VtYXJAc2N1LmVkdS5jbg==; Bairong Shen, YmFpcm9uZy5zaGVuQHNjdS5lZHUuY24=
†These authors have contributed equally and thus should be considered as the first authors.
‡ORCIDs: Rajeev K. Singla, orcid.org/0000-0002-3353-7897; Bairong Shen, orcid.org/0000-0003-2899-1531
 Ankit Kumar Dubey
Ankit Kumar Dubey Siva Sai Chandragiri
Siva Sai Chandragiri Abin V. Geevarghese
Abin V. Geevarghese Bhupinder Kapoor
Bhupinder Kapoor Monica Gulati
Monica Gulati Pooja Rani5
Pooja Rani5 Vivek P. Chavda
Vivek P. Chavda Rohit Gundamaraju
Rohit Gundamaraju Himangini Bansal
Himangini Bansal Rupesh K. Gautam
Rupesh K. Gautam Rajat Goyal
Rajat Goyal Michael P. Okoh
Michael P. Okoh Bairong Shen
Bairong Shen Rajeev K. Singla
Rajeev K. Singla