- 1Neck-Shoulder and Lumbocrural Pain Hospital of Shandong First Medical University, Shandong First Medical University & Shandong Academy of Medical Sciences, Jinan, China
- 2Qingdao Traditional Chinese Medicine Hospital, Qingdao Hiser Hospital Affiliated of Qingdao University, Qingdao, China
- 3Qingdao Haici Traditional Chinese Medicine Medical Group North Campus(Qingdao Hongdao People’s Hospital), Qingdao, China
- 4Institute of Brain Science and Brain-Inspired Research, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, China
Panax ginseng C.A. Mey. (PG), a well-documented medicinal food plant with generally recognized as safe status, exhibits therapeutic potential for managing metabolic disorders (type 2 diabetes mellitus (T2DM), obesity), inflammatory bowel diseases (IBD), and neurodegenerative conditions via modulation of the gut microbiota (GM). This systematic review of 102 studies reveals that ginsenosides (Rb1, Rg1, and Rg3) undergo biotransformation mediated by the GM into bioactive metabolites (e.g., compound K), enhancing their bioavailability by 3- to 5-fold (p < 0.01). Three core mechanisms were identified: 1) inhibition of the TLR4/NF-κB pathway reduces pro-inflammatory cytokine levels by 40%–60%; 2) upregulation of tight junction proteins (ZO-1/claudin-4) strengthens intestinal barrier function by 2.3-fold; and 3) selective GM modulation increases the relative abundance of probiotics (Lactobacillus ↑2.1-fold, Bifidobacterium ↑1.8-fold) while decreasing pathogenic bacteria (Clostridium ↓65%), collectively increasing short-chain fatty acid (SCFA) production by 3.2-fold, and activating AMPK/SIRT1 signaling. Clinical evidence supports PG’s efficacy: 15.2% reduction in fasting blood glucose levels in T2DM, 28.5% decrease in diamine oxidase (DAO) activity in IBD, and improvements in cognitive function scores (Mini-Mental State Examination scores increased by 2.4 points) in mild cognitive impairment. Emerging research further reveals a “microbiota-gut-brain axis” mediated by GM-derived metabolites acting via G protein-coupled receptors (GPCRs) and vagal pathways.
1 Introduction
Chronic diseases impose a substantial global health burden and cause high mortality rates (Cifuentes et al., 2025). The conventional “one molecule, one target” pharmacological paradigm, rooted in reductionist principles, exhibits intrinsic limitations in addressing the multifactorial pathogenesis of these complex disorders, underscoring the urgent need for innovative therapeutic strategies.
Panax ginseng C. A. Mey. (PG), a widely cultivated medicinal food plant (classified as a GRAS resource), is primarily harvested in autumn, washed, and processed via sun-drying or mechanical drying. As a cornerstone of traditional and modern integrative medicine, PG exerts pleiotropic therapeutic effects against chronic diseases through synergistic interactions with herbal preparations and dietary supplements. Its pharmacological activities, including glycemic control (Chen Q. et al., 2017), immune modulation (Qu et al., 2021), and antitumor efficacy (Wang et al., 2023), are primarily attributed to bioactive metabolites such as ginsenosides (e.g., Rb1, Rg1, Rg3, and Rh1) and polysaccharides. Recent studies have underscored the critical role of the GM in mediating the therapeutic effects of PG. Central to this mechanism is the GM’s facilitation of phytochemical biotransformation: it converts ginsenosides (e.g., Rb1→compound K (CK)), catechins, and quercetin into pharmacologically active metabolites, thereby enhancing both intestinal absorption and systemic bioavailability of PG-derived compounds (Santangelo et al., 2019; Furman et al., 2019). Comparative analyses of Asian and American ginseng chemotypes—defined by distinct chemical profiles—reveal variations in secondary metabolite composition (Liang et al., 2015) and tissue-specific accumulation patterns (Chen et al., 2015); these differences not only establish evidence-based quality control benchmarks for herbal preparations but also provide scientific rationale for their differential clinical applications (e.g., preferential use of Asian ginseng in metabolic disorders versus American ginseng in immune modulation). Notably, current evidence indicates that age-related dysbiosis (e.g., reduced microbial diversity, altered Firmicutes/Bacteroidetes ratios) or antibiotic-induced disruptions in GM homeostasis may significantly impair PG’s biotransformation efficiency and subsequent bioactivity (Chen Y. et al., 2017). This review aims to systematically synthesize PG’s multicomponent metabolites and their multitarget mechanisms in chronic disease management, highlight the GM’s role as a key determinant of PG’s bioactivity, evaluate its safety profile, and assess its therapeutic potential within integrative medicine frameworks.
The therapeutic effects of PG across diverse disease contexts are linked to GM modulation, though the robustness of supporting evidence varies notably. A comprehensive systematic review of the literature stratifies the mechanisms of ginsenosides into three evidence tiers: In diabetes management, for example, ginsenoside Rb1 enhances insulin sensitivity via GM-mediated metabolic transformation—a pathway validated by multi-omics profiling (Chen et al., 2022). This process involves augmenting probiotic abundance (e.g., Lactobacillus species), promoting SCFA biosynthesis, and suppressing the pro-inflammatory TLR4/NF-κB signaling axis. Beyond this well-supported mechanism, emerging evidence in antitumor research suggests ginsenosides Rg3 and CK may increase PD-1 inhibitor efficacy through GM modulation (Huang et al., 2022). However, these findings are currently limited to mouse models and lack clinical validation. Further complicating the landscape, speculative associations, such as the proposed “gut-brain axis” regulation in neuroprotection (Li et al., 2024), rely on indirect evidence where the GM composition changes correlate with behavioral improvement, but causal relationships remain unclear. Collectively, these findings underscore the need for further investigation to advance research in this field.
2 Methods and materials
Panax ginseng C.A. Mey. (Renshen; Ginseng Radix et Rhizoma) refers to the dried roots and rhizomes of plants in the Panax genus (family Araliaceae). Quality control adheres to HPLC analysis as specified in General Principle 0512 of the Chinese Pharmacopoeia. Each batch, when calculated on a dry weight basis, must contain no less than 0.30% combined ginsenosides Rg1 (C42H72O14) and Re (C48H82O18), and no less than 0.20% ginsenoside Rb1 (C54H92O23). Pharmacologically standardized batches further require ≥0.27% combined Rg1 and Re, and ≥0.18% Rb1 (Chinese Pharmacopoeia, 2025).
Physical characteristics of the product include round or nearly round thin slices prepared via moistening, cutting, and drying. Key morphological features are a grayish-yellow outer skin; pale yellowish-white to nearly white, powdery cross-sections with brownish-yellow vascular cambium rings; and a periderm displaying brown, dot-like resin ducts and radiating fissures. The slices have a lightweight and brittle texture, with a distinctive aroma and mildly bittersweet taste.
Compliance of PG (particularly wild or protected populations) with collection, processing, and trade regulations, including the Nagoya Protocol, the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES), and plant quarantine requirements, requires verification. Product categories include: Raw materials/primary processed products: Dried ginseng roots (sliced or whole), ginseng powder, ginseng extracts (e.g., red ginseng extract). Finished products: Pharmaceuticals (e.g., ginseng capsules, tablets, and oral liquids); health supplements (e.g., ginseng tonics and energy drink additives); cosmetics (e.g., ginseng creams and essences).
Within our institution, a batch of herbal medicine sourced from Jilin Province is retained, bearing the batch number 2305210072.
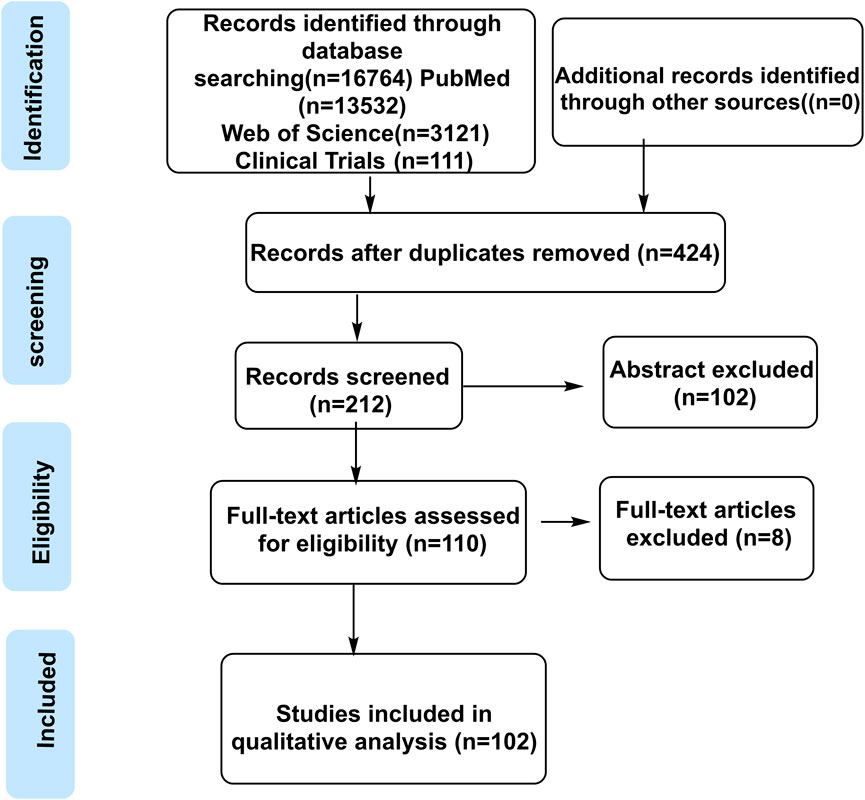
This study employed a systematic and transparent literature search strategy aligned with the Population, Intervention, Comparison, Outcome, Study Design (PICOS) framework to ensure methodological rigor. The search targeted the following: 1) Population (chronic disease models/patients); 2) Intervention (ginseng/active plant metabolites); 3) Study Design (controlled settings); 4) Outcome (gut microbiota parameters); and 5) Study Types (clinical/preclinical research). Databases including PubMed, Web of Science, Embase, Cochrane Library, CNKI, Wanfang, and ClinicalTrials.gov were queried using a combination of keywords: interventions (“Panax ginseng,” “ginsenoside”), mechanisms (“gut microbiota,” “intestinal flora”), and diseases (“chronic disease,” “metabolic disorder”).
After removing duplicate articles with Zotero, two independent reviewers conducted title/abstract screening and full-text assessment based on predefined inclusion criteria (clinical or preclinical design, documented ginseng intervention, microbiota analysis, data integrity) and exclusion criteria (reviews, case reports, small sample sizes, and methodological limitations). Discrepancies in screening decisions were resolved through discussion with a third reviewer, with final judgments documented using a standardized form. Quality assessment of included studies was performed using the Cochrane Risk of Bias tool (for clinical studies) and SYRCLE’s Risk of Bias tool (for animal studies) to ensure the reliability of the evidence base.
A total of 102 references were screened (final inclusion number to be updated by 2025). Panax ginseng C.A. Mey. (Latin name: P. ginseng C.A. Mey.), a perennial medicinal and edible plant, belongs to the genus Panax within the family Araliaceae. Taxonomic nomenclature and species illustrations were validated against authoritative sources, including Flora of China (1994) and The World Flora Online (http://www.worldfloraonline.org, accessed 10 March 2025), with chemical structure data sourced from PubChem and visualized using ChemDraw software. The literature retrieval flowchart is presented in Figure 1.
Methodological limitations of the current study are acknowledged as follows: 1) Most included studies did not explicitly describe the randomization procedure; 2) Only a minority adopted double-blind study designs; 3) Statistical power calculation was prespecified in few investigations; 4) Metagenomic sequencing was utilized in only a limited number of studies.
3 Gut microbiota and metabolism of plant metabolites of PG
PG, as a medicinal food plant, represents a highly dynamic research field. The gut microbiota, recognized for its pivotal role in host physiology, has been extensively acknowledged—particularly its function as a “superorganism” in conjunction with the host, mediating critical processes such as drug metabolism and other physiological functions (Zinöcker and Lindseth, 2018).
Long-term administration of PG extract (PE) induces significant modulation of GM composition: probiotics, including Bifidobacterium, Lactobacillus, Allobaculum, and Clostridium, are markedly upregulated, whereas pathogenic bacteria, such as Butyricimonas, Parabacteroides, Helicobacter, and Alistipes, are significantly downregulated (Qu et al., 2021). These findings demonstrate PE’s capacity to promote probiotic proliferation while suppressing pathogen growth, thereby providing foundational evidence for its regulatory effects on the GM structure in rat models (Sun et al., 2018).
The bioactivities of key PG-derived metabolites, including CK, ginsenosides Rg3, Rh2, 20(S)-protopanaxatriol (20(S)-PPT), and 20(S)-protopanaxadiol (20(S)-PPD), are critically dependent on GM-mediated metabolism. Ginsenosides generally exhibit poor gastrointestinal bioavailability but undergo extensive biotransformation mediated by the GM, with in vivo metabolic profiles aligning closely with in vitro GM fermentation patterns (Wang et al., 2023). Deglycosylated ginsenoside metabolites produced by the GM are then absorbed into the systemic circulation.
Notably, interindividual variability in GM composition results in divergent metabolic phenotypes. For example, ginsenoside Rb1 undergoes specific biotransformation by Bifidobacterium spp. within the GM to generate compound Rd (Dong et al., 2017). Oral administration of Rb1 and Re follows distinct metabolic pathways: Rb1/Re are metabolized via the GM through Rd to form 20(S)-PPD or CK, whereas Rg1 is processed through a distinct route involving retention of the Rg1 aglycone to generate 20(S)-PPT or Rh1 (Oh H. A. et al., 2015). PG plant metabolites further differentially regulate the GM across multiple taxonomic levels: At the phylum level, significant reductions in Escherichia abundance are observed; at the genus level, treatment with Rb1/Rb2/Rb3/Rc leads to decreased relative abundances of Dorea, Prevotella, and Megasphaera, concurrent with increased Escherichia (Zheng et al., 2021).
These structural and compositional changes enhance GM integrity and metabolic diversity. For example, GM hydrolysis plays a critical role in the appearance of CK in rat plasma following Rb1 administration (Akao et al., 1998). PE-induced modifications in GM composition, specifically the enrichment of Peptococcaceae, Rikenellaceae, and Hungarian legume-associated taxa, exhibit a strong positive correlation with increased absorption of ginsenosides (e.g., Rd and Rg3) (Kim et al., 2019). Exposure to stress models, including antimicrobial agents, fatigue, and acute cold, has been shown to suppress the production of deglycosylated ginsenoside Re metabolites, a phenomenon attributed to altered glycosidase activity in Proteus, Lactobacillus, and Bacteroides populations (Zhang et al., 2021). Ultra-high-performance liquid chromatography coupled with ion mobility-quadrupole time-of-flight mass spectrometry (UHPLC/IM-QTOF-MS) analysis of six biotransformation samples identified 248 ginsenoside/metabolite features, with desaccharification emerging as the dominant metabolic pathway. Notably, protopanaxatriol-type and oleanolic acid ginsenosides demonstrate heightened metabolic susceptibility (Mi et al., 2023). Probiotic supplementation synergizes with PG to optimize GM composition. In male Sprague–Dawley (SD) rats, probiotic-enriched feed increases the Rb1→CK conversion and gastrointestinal absorption of ginsenosides (Kim et al., 2019). Additionally, the PPD (protopanaxadiol) treatment group exhibits significant fecal microbiota alterations: increased relative abundances of Proteus_9, Fusarium, and Monomonas at the genus level and decreased Bacteroides and Proteus at the phylum level (Zhang et al., 2021).
The dynamic interplay between PG plant metabolites and GM metabolism is summarized in Figure 2.
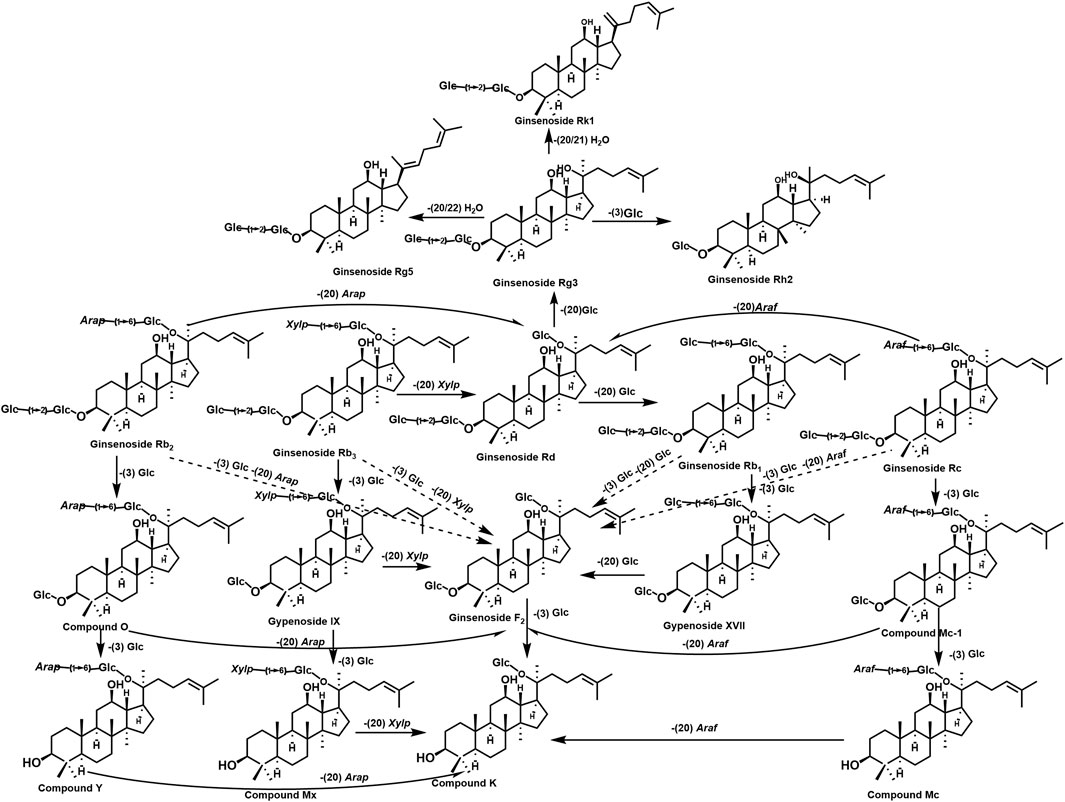
Figure 2. Plant metabolites in PG and their transformation in the human body. The figure shows the generation and conversion of different metabolites in PG, which are formed by the deglycosylation of sugar groups, including Rb1, Rb3, Rb2, Rc, and compound CK.
4 Pharmacodynamic material basis of PG in the treatment of diseases by regulating gut microbiota
4.1 The pharmacological effects of the plant metabolites in PG
PG, a highly valued traditional Chinese medicine (TCM), is often hailed as the “king of botanical drugs” due to its comprehensive pharmacological profile. Its primary plant-derived metabolites encompass three key categories: 1) Ginsenosides: Diverse analogs (e.g., Rb1, Rg1, Re); 2) Macronutrients: Carbohydrates and amino acids; 3) Micronutrients: Vitamins (B1, B2, C, etc.) and essential trace elements (potassium [K], sodium [Na], calcium [Ca], magnesium [Mg], etc.).
PG exerts multifaceted physiological effects, systematically summarized as follows: Immunomodulation: Ginsenosides and polysaccharides enhance immune competence by activating host defense mechanisms (Figure 2). Anti-fatigue and Anti-senescence: Improves physical performance and mitigates cellular aging processes. Endocrine Regulation: Stabilizes hormonal balance through systemic modulation of endocrine function. Neuroprotective Effects: Enhances cognitive function and memory retention via protection of the nervous system. Cardiovascular Protection: Improves myocardial function, increases cardiac output, and regulates glucose/lipid metabolism. Anticancer Activity: Inhibits carcinogenesis through metabolite-mediated cytostatic effects. Stress Adaptation: Enhances resilience to physical/psychological stressors while attenuating stress-induced damage.
The integrated pharmacological actions of PG are visually represented in Figure 3.
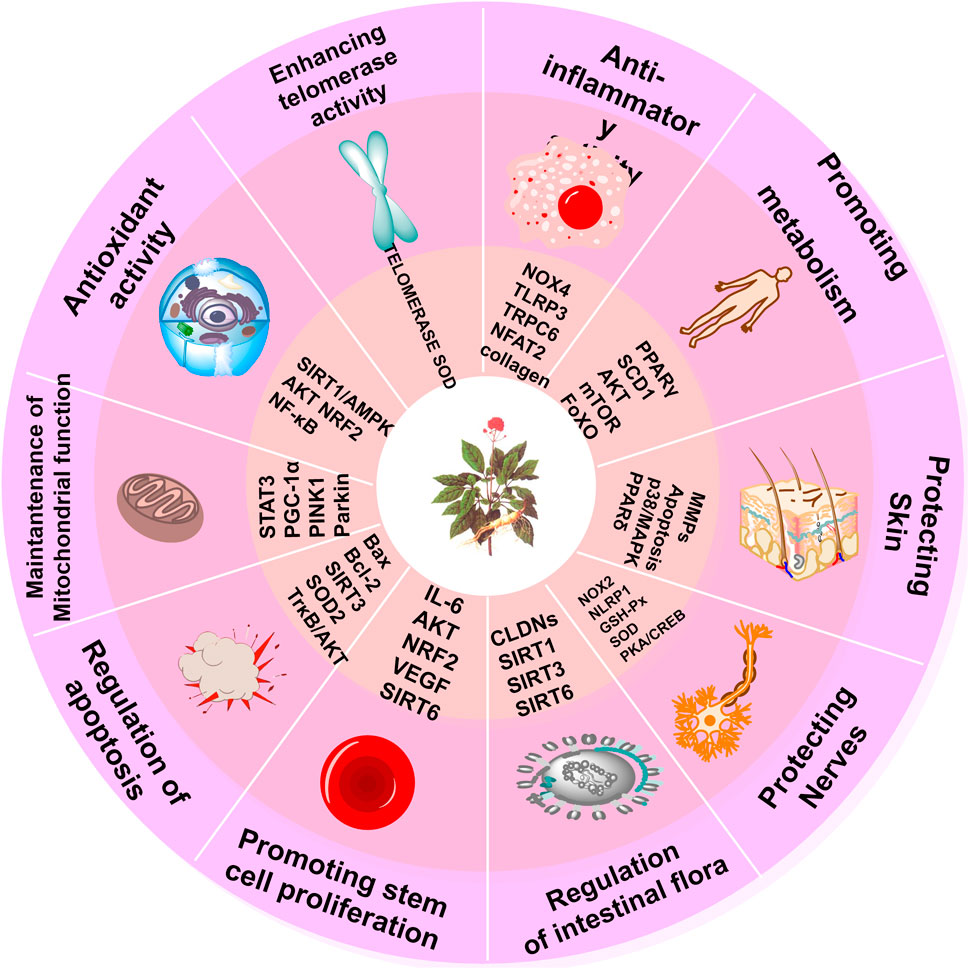
Figure 3. Pharmacological effects of PG. The figure shows the pharmacological mechanisms of action and targets of PG, including anti-inflammatory and antioxidant activity, promoting metabolism, protecting skin, protecting nerves, regulating GM, promoting stem cell proliferation, regulating apoptosis, maintaining mitochondrial function, and increasing telomerase activity.
4.2 Metabolic disorders
4.2.1 Diabetes
4.2.1.1 Pathogenesis
Type 2 diabetes mellitus (T2DM) is characterized by progressive insulin resistance and pancreatic β-cell dysfunction, which collectively impair insulin secretion and glucose homeostasis, necessitating lifelong pharmacological management (Feng et al., 2018; Lao et al., 2014; Sharma et al., 2017). Contemporary chemical and biological research methodologies have enabled the discovery of novel therapeutic strategies for diabetes prevention and management of complications (Liu et al., 2022; Zhou et al., 2022). The pathogenesis of T2DM involves multiple interconnected mechanisms, including increased circulating free fatty acids and adipokines contributing to dysregulated lipid metabolism, pancreatic lipase hyperactivity, impaired dietary cholesterol absorption, gut microbiota dysbiosis, central appetite dysregulation, nuclear receptor (e.g., PPAR and LXR) signaling abnormalities, and dysfunctional AMP-activated protein kinase (AMPK) pathways and adipose tissue homeostasis.
4.2.1.2 Plant metabolites for treating diabetes in PG
Ginsenoside Rb1 exerts multifaceted anti-diabetic effects through its potential prebiotic properties, which regulate specific intestinal microbiota and their metabolites, which are key contributors to diabetes-related metabolic disorders and insulin resistance. In diabetic Kkay mice, Rb1 significantly reduces blood glucose levels, improves oral glucose tolerance test (OGTT) performance, decreases serum insulin levels and homeostasis model assessment of insulin resistance (HOMA-IR) index, lowers hepatic index, alleviates pancreatic injury, reverses GM dysbiosis, and modulates fecal free fatty acid profiles (Zhou et al., 2023). Similarly, Rg1 demonstrates multifaceted anti-diabetic activities: in high-fat diet (HFD)- and streptozotocin (STZ)-induced T2DM rats, it significantly reduces blood glucose levels, improves insulin sensitivity, attenuates oxidative stress and inflammation, normalizes blood lipid profiles, and positively modulates intestinal microbial composition (Peng et al., 2022). As the primary circulating metabolite of PG in serum, CK not only confers renal protection but also modulates GM composition and downregulates the expression of TLR4 signaling pathway proteins induced by imidazole propionate (Chen et al., 2022). In male Wistar rats, ginsenoside Rb1 exhibits significant hypoglycemic activity via a specific metabolic pathway (Rb1→Rd→F2→CK), where the bioconversion rate of CK increases from 14.0% to 86.7% following 8 h of incubation. Ginseng pectin (GPS) synergizes with Rb1 to restore GM homeostasis and increase fecal D-glucosidase activity, thereby exerting potent anti-diabetic effects (Li et al., 2018). GPS treatment further ameliorates hyperglycemia, hyperlipidemia, and hepatic steatosis in T2DM rats by activating AMP-activated protein kinase (AMPK) and promoting the phosphorylation of acetyl-CoA carboxylase, which subsequently reduces the expression of sterol regulatory element binding protein-1c (SREBP-1c) and fatty acid synthase (FAS) (Ren et al., 2023a). Additionally, 25-hydroxy-protopanaxatriol (T19), a novel ginsenoside derivative derived from PG, inhibits α-glucosidase and protein tyrosine phosphatase 1B (PTP-1B) in vitro. These inhibitory effects, combined with T19’s ability to modulate glucose and lipid metabolism via beneficial bacterial interactions, effectively reduce blood glucose and lipid levels, increase insulin sensitivity, and ameliorate hepatic and pancreatic histopathology (Xu et al., 2020).
4.2.1.3 Therapeutic mechanism
The active plant metabolites of PG, including Rb1, Rg1, CK, and T19, exert anti-diabetic effects through multiple synergistic mechanisms: (1) modulation of the gut microbiota; (2) mitigation of metabolic disorders; (3) activation of the AMPK signaling pathway; (4) exertion of anti-inflammatory and antioxidant effects; and (5) protection of organ functions (e.g., pancreas and kidneys). Collectively, these effects are mediated via the gut microbiota–metabolism–immunity network. The anti-T2DM mechanism of PG is summarized in Figure 4.
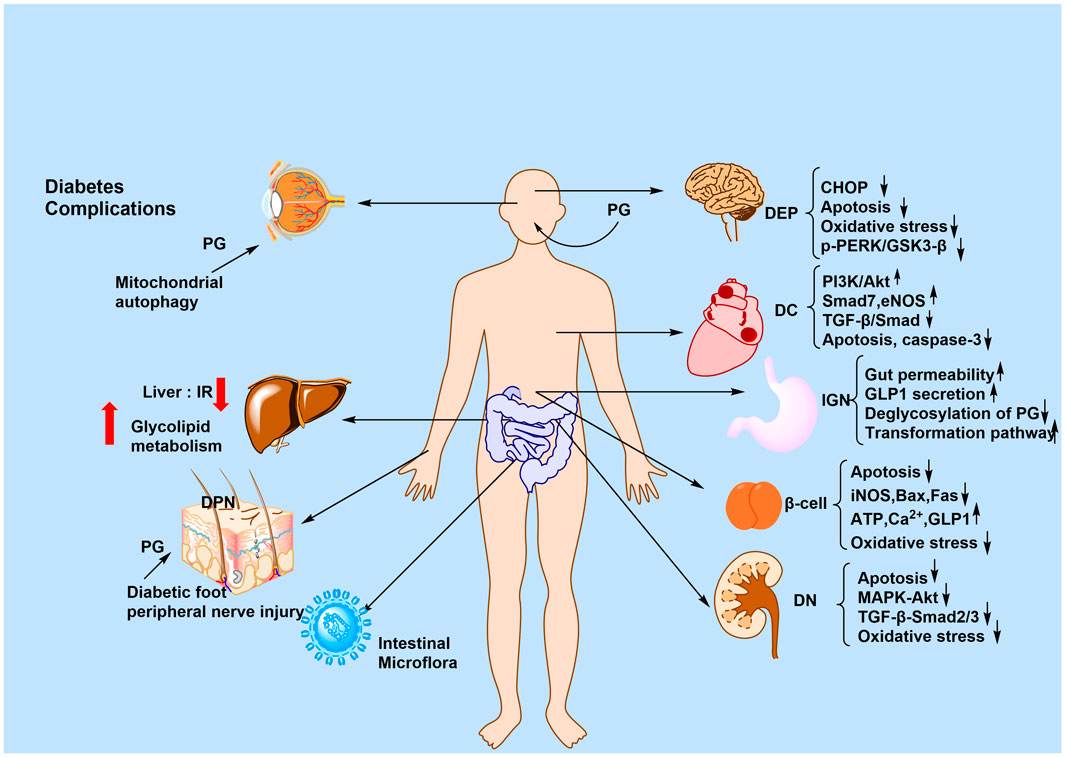
Figure 4. Anti-T2DM mechanism of PG. The figure shows the mechanism of action of PG for the treatment of T2DM and its complications, summarized in terms of the brain, heart, stomach, kidney, liver, skin, and intestines. The signaling pathways involved may include p-PERK/GSK3-β, PI3K/AKT, and MAPK/AKT.
4.2.2 Obesity
4.2.2.1 Pathogenesis
Obesity remains a major global public health concern (Safaei et al., 2021). Numerous obesity-associated chronic diseases, including T2DM, cardiovascular disease, and non-alcoholic fatty liver disease (NAFLD), represent leading causes of global mortality (Hu et al., 2017).
4.2.2.2 The plant metabolites for treating obesity in PG
PG exerts anti-adipose effects by inhibiting peroxisome proliferator-activated receptors γ/α (PPAR-γ/PPAR-α). Additionally, PG modulates EBP-α, potentially augmenting lipid oxidation and energy expenditure. Pharmacological interventions utilizing PG have shown significant lipid-regulatory effects mediated by GM modulation. In HFD-induced obese rats, PG treatment alleviates obesity symptoms, increases fecal bile acid excretion, and increases gut microbiota (GM) diversity alongside the relative abundance of Prevotella_9 (Zhao et al., 2021). Mechanistic investigations in db/db mice further reveal that PG-derived gut enterococci (Enterococcus faecalis) convert dietary plant metabolites into unsaturated long-chain fatty acids (LCFAs), notably myristic acid (MA), via acyl-CoA thioesterase (ACOT)-mediated biosynthesis. Intriguingly, PG concurrently downregulates MA production through as-yet-uncharacterized mechanisms (Quan et al., 2020).
In dyslipidemic rat models, PE enriches the abundance of beneficial bacterial taxa (Akkermansia, Bifidobacterium, Bacteroides, and Proteus) and increases SCFA biosynthesis (acetate, propionate, butyrate, and valerate). These changes improve lipid metabolism through GM remodeling and activation of the AMPK signaling pathway (Ren et al., 2023b). Ginsenosides, including Rb1, Rb2, Rb3, Re, Rg1, Rg3, Rh2, Rh4, and F2, exert comprehensive cardiometabolic protective effects by increasing antioxidant enzyme activities, promoting fatty acid β-oxidation and autophagy, and modulating the GM to mitigate oxidative stress (Jin et al., 2023).
Notably, PPD maintains intestinal barrier integrity in leptin-deficient (ob/ob) mice exhibiting Parkinson’s disease-like pathologies. However, specific bacterial taxa (e.g., Lactobacillus spp.) that mediate this effect require further characterization (Lv et al., 2022).
4.2.2.3 Therapeutic mechanism
PG combats obesity through multitarget mechanisms, including 1) modulation of GM; 2) increasing lipid oxidation via PPAR-γ/PPAR-α inhibition and AMPK activation; 3) promotion of SCFA production (acetate, propionate, butyrate); 4) reduction of oxidative stress and inflammation; and 5) improvement of intestinal barrier integrity. Key plant metabolites, such as ginsenosides Rb1 and Rg3, further regulate bile acid excretion and inhibit myristic acid synthesis, exerting synergistic anti-obesity effects through the gut–liver–metabolic axis. The mechanisms by which PG regulates lipid metabolism via the GM are summarized in Figure 5.
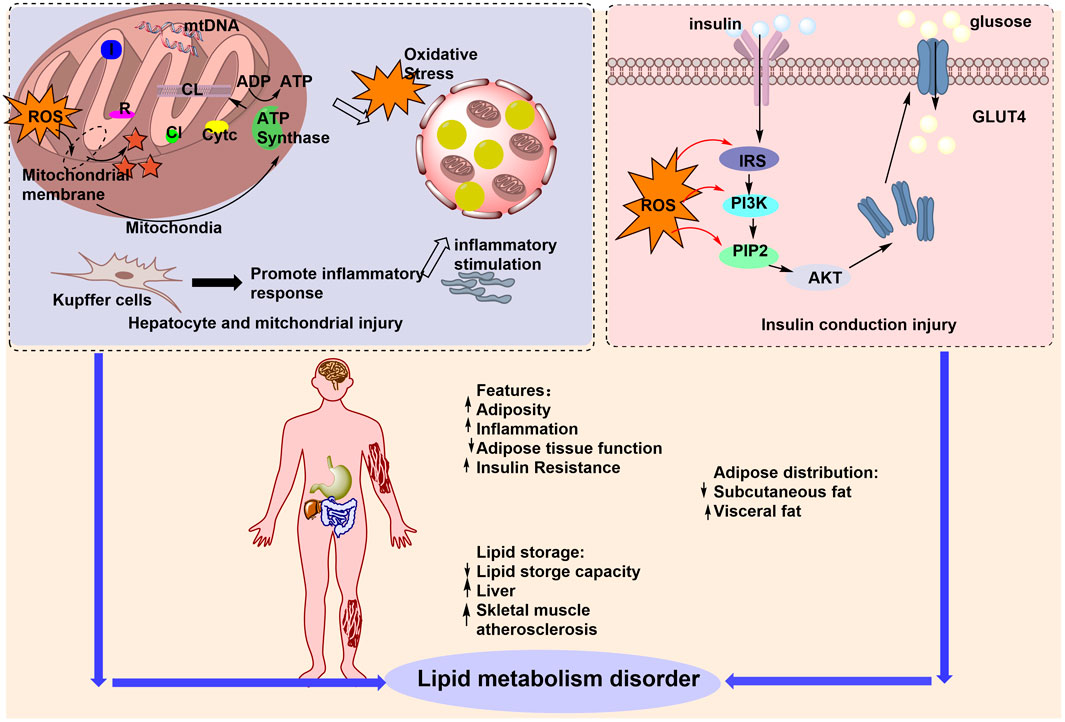
Figure 5. PG regulation of lipid metabolism by the GM for the treatment of obesity. The figure illustrates the multiple factors contributing to lipid metabolism disorders that lead to obesity, including increased adiposity, upregulation of inflammation, and increased lipid retention capacity. PG can improve lipid metabolism disorders by regulating ROS, affecting the GM, and treating obesity.
4.3 Gastrointestinal and liver diseases
4.3.1 Colitis
4.3.1.1 Pathogenesis
Colitis, a chronic inflammatory disorder that promotes a tumor-prone microenvironment and ranks as the third leading cause of cancer-related mortality globally (Bocchetti et al., 2021), is primarily driven by GM dysbiosis among multiple contributing factors. Such dysbiosis disrupts intestinal barrier integrity, impairs mucosal immune function, and triggers excessive production of pro-inflammatory cytokines, ultimately leading to mucosal erosion, ulceration, and the development of clinically relevant IBDs. The spectrum of IBDs encompasses not only IBD itself but also irritable bowel syndrome, intestinal ischemia–reperfusion injury, necrotizing enterocolitis, radiation proctitis, and NSAID-induced enteritis. Notably, ginsenosides counteract these pathological processes through multifaceted mechanisms: attenuating intestinal inflammation, promoting intestinal barrier repair, maintaining GM diversity, and reducing the risk of colitis-associated colon cancer.
4.3.1.2 Plant metabolites for treating colitis in PG
In Caco-2 cell models, co-treatment with colitis-derived intestinal microorganism citrate synthase (CS) and PG + UC (PG under ulcerative colitis conditions) significantly impaired P-glycoprotein (P-gp) function. Concurrently, PG directly disrupted zonula occludens-1 (ZO-1) localization and suppressed tight junction (TJ) protein expression (Yang et al., 2021). In dextran sulfate sodium salt (DSS)-induced colitis models, PG treatment partially reversed the DSS-induced reduction in β-glucosidase activity, alleviated colitis symptoms, and enhanced systemic Rb1 exposure through two mechanisms: promoting microbial deglycosylation of Rb1 and facilitating its absorption by intestinal epithelial cells (Shen et al., 2018).
Ginsenoside Rg1 exerts immunomodulatory effects by increasing secretory immunoglobulin A (SIgA) secretion and modulating the expression of interleukin (IL)-2, IL-4, IL-10, and interferon-γ (IFN-γ). Concurrently, Rg1 regulates GM composition. Metabolites generated by Rg1 through metabolism mediated by Lachnospiraceae family bacteria further increase the proliferation of T cells and regulatory T cells (Tregs), thereby ameliorating colonic inflammation and restoring mucosal barrier integrity (Yousuf et al., 2022).
Similarly, ginsenoside Rh4 alleviated antibiotic-induced pathophysiological changes, intestinal barrier disruption, and inflammation in mice, concomitant with beneficial shifts in GM diversity and composition, thereby underscoring its bidirectional interaction with host microbiota (Bai et al., 2021a). Rk3 treatment mitigated HFD-induced intestinal barrier dysfunction in mice by upregulating tight junction proteins (ZO-1, claudin, and occludin), reducing colitis-associated cytokines, oxidative stress, and macrophage infiltration, while concurrently inducing favorable GM modulation (Chen et al., 2021; Bai et al., 2021b).
Comparative in vitro studies demonstrated that red ginseng (RG) and betel nut exhibit prebiotic effects, with RG showing superior efficacy to betel nut in improving GM structure and alleviating UC symptoms in TNBS-induced UC rat models (Wan et al., 2024). Mechanistically, PG restores mTOR-dependent autophagy and modulates GM composition (Wang et al., 2021).
4.3.1.3 Therapeutic mechanism
PG and its bioactive metabolites, including Rg1, Rh4, Rk3, and red ginseng, exert anti-colitis effects through multiple synergistic mechanisms: 1) Enhancing intestinal barrier integrity by upregulating tight junction proteins (ZO-1, claudin, and occludin) and restoring β-glucosidase activity; 2) modulating GM composition by increasing the relative abundance of beneficial taxa (Lactobacillus and Bifidobacterium) and suppressing pathogenic bacteria; 3) Regulating immune homeostasis via increasing regulatory Tregs and SIgA, coupled with cytokine balancing (reducing pro-inflammatory IL-2, IL-4, IL-10, and IFN-γ); 4) Activating protective signaling pathways, such as mTOR-dependent autophagy; and 5) Promoting Rb1 bioavailability through microbial metabolism. Collectively, these coordinated actions underscore ginseng’s critical role in restoring intestinal homeostasis during colitis alleviation.
4.3.2 Liver diseases
4.3.2.1 Pathogenesis
The liver plays vital physiological roles in synthesizing plasma proteins, secreting bile acids, metabolizing xenobiotics, and regulating glucose and lipid homeostasis. Dysregulated metabolism of these critical substances can give rise to diverse hepatic disorders and their associated complications, contributing to significant global morbidity and mortality. These disruptions pose substantial public health challenges and economic burdens (Marchesi et al., 2016).
4.3.2.2 The plant metabolites for treating liver diseases in PG
Ginsenoside Rg1 enhances intestinal barrier function by upregulating tight junction protein levels and secretory SIgA, while mitigating alcohol-induced inflammatory responses through inhibition of the TLR4/NF-κB signaling pathway. Additionally, Rg1 modulates GM composition: Verrucomicrobia, Bacteroidetes, Akkermansia, Bacteroidae, the Lachnospiraceae_NK4A136_group, and Alloprevotella exhibit positive correlations with intestinal barrier indices and negative correlations with liver inflammation indices (Xia et al., 2022). Analyses of cecal microbial diversity revealed that alcohol exposure significantly increased the abundance of Bacteroides S24-7 and Proteus S24-7, while reducing the relative abundances of SCFA-producing bacteria, including Akkermansia (phylum Verrucomicrobia), Allobaculum, Luminococcus, and Adlercreutzia (phylum Actinobacteria). Notably, fermented ginseng (FG) effectively reverses these alcohol-induced alterations in gut microbiota composition (Fan et al., 2019).
PPD ameliorates dysregulated glucose and lipid metabolism while improving liver function. Strong correlations were observed between the GM and anti-inflammatory/antioxidant metabolites in mice. Furthermore, (20R)-PPD enhances the integrity of the intestinal wall. Concurrently, downregulation of TNF-α, malondialdehyde (MDA), and superoxide dismutase (SOD) expression confirms PPD’s antioxidant and anti-inflammatory effects (Fang et al., 2023; Feng et al., 2023).
4.3.2.3 Therapeutic mechanism
PG alleviates liver diseases through multifaceted mechanisms. First, via the gut–liver axis, ginsenoside Rg1 and FG restore intestinal barrier integrity and rebalance gut microbiota composition, thereby reducing endotoxin translocation. Second, PG exerts anti-inflammatory and antioxidant effects to mitigate hepatic damage. Third, metabolic regulation is achieved through PPD, which enhances glucose and lipid metabolism while improving liver function via mannose-derived metabolites such as SCFA. Fourth, FG promotes microbial detoxification by antagonizing alcohol-induced gut dysbiosis and enriching liver-protective bacterial populations. Collectively, these synergistic actions alleviate liver inflammation, oxidative stress, and metabolic dysfunction.
4.3.3 Diarrhea
Diarrhea, a leading global cause of morbidity and mortality, manifests as an acute infectious condition (Shankar and Rosenbaum, 2020) and can induce severe complications, including hypovolemia, electrolyte imbalance, malnutrition, and cutaneous damage, particularly when prolonged (Kyu et al., 2025). Its pathogenesis involves multifactorial drivers such as bile acid metabolism disorders, viral infections, and Escherichia coli colonization, with emerging evidence highlighting GM dysbiosis as a critical factor (Fan and Pedersen, 2021). Notably, restoring GM homeostasis has demonstrated both preventive and therapeutic potential against diarrhea (Gallo et al., 2016).
FG demonstrates notable therapeutic efficacy in antibiotic-induced GM dysbiosis models, as evidenced by its ability to restore GM structure in antibiotic-treated rats (with dose-dependent effects on microbial composition) (Qu et al., 2021). In male SD rats, FG alleviates diarrhea and colitis symptoms while normalizing colonic expression of immune factors (TNF-α, IL-1β, IL-6, and IL-10) (Li et al., 2019), and further downregulates pro-inflammatory cytokines to restore GM homeostasis. Comparative investigations with water-extracted ginseng (WGP) have revealed distinct mechanisms underlying antibiotic-associated diarrhea: WGP significantly modulates GM composition and diversity, particularly through the enrichment of Lactobacillus populations as key responders, and achieves GM rebalancing via dual mechanisms: structural restoration and modulation of metabolic processes (Li et al., 2019).
4.4 Cancer
4.4.1 The anticancer mechanism of PG
PG, a medicinal plant endemic to Asian countries with a millennia-long history of traditional use, has garnered significant attention for its pharmacological properties. Ginsenosides, a class of bioactive metabolites derived from PG, exhibit promising anticancer potential, with multifaceted effects on the tumor microenvironment (TME). For instance, Rg3, Rd, and Rk3 have been shown to inhibit tumor angiogenesis; Rg3, Rh2, and M4 modulate immune cell function; and Rg3, Rd, and Rg5 suppress the dormancy of tumor stem cells. GP, including acidic polysaccharides, and those extracted from PG fruits and leaves, principally regulate the TME through immune cell stimulation (Li et al., 2021). The antitumor effects of specific ginsenosides, including Rg3, Rh2, Rg5, and CK, are well-documented, with proposed mechanisms involving apoptosis induction, immune response enhancement, chemotherapy resistance reversal, and regulation of key signaling pathways such as MAPK, PI3K/Akt/mTOR, Wnt/β-catenin, NF-κB, ASK-1/JNK, AMPK, and EGFR/Akt/SOX2. Notably, the PI3K-AKT/NF-κB signaling pathway serves as a critical mediator through which ginsenoside Rb1 and CK exert their anti-gastric cancer effects (Wang S. et al., 2020). The role of PG-derived plant metabolites in regulating the TME via the GM is schematically illustrated in Figure 6.
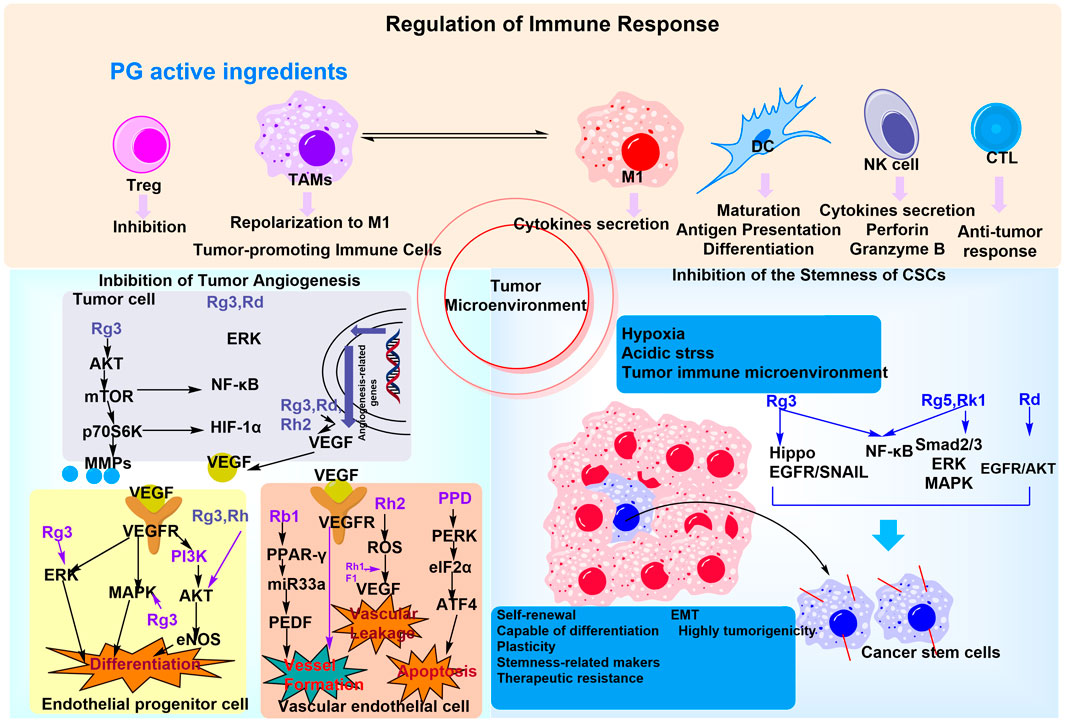
Figure 6. The mechanism of PG plant metabolites regulating the tumor microenvironment and anti-inflammation and immune regulation through the GM. PG may modulate immune cells to improve the GM by regulating the tumor immune microenvironment, in addition to involving multiple signaling pathways to promote tumor cell apoptosis.
4.4.2 The plant metabolites in PG for treating cancer
CK, but not Rb1, significantly inhibited the proliferation of HGC-27 gastric cancer cells by downregulating cyclins B1 and D1, suppressing the anti-apoptotic protein Bcl-2, and activating pro-apoptotic proteins Bax and Caspase-3 through inhibition of the PI3K/AKT/NF-κB signaling pathway (Wan et al., 2022). This differential effect underscores the role of GM-mediated regulation of PG metabolites in clinical applications.
Combination therapy with GPs and αPD-1 monoclonal antibody potentiated antitumor responses by increasing valeric acid production and reducing L-kynurenine levels, thereby decreasing the kynurenine/tryptophan (Kyn/Trp) ratio to suppress regulatory Tregs and promote expansion of effector T cells (Huang et al., 2022). Mechanistically, GPs modulated GM composition and regulated tryptophan metabolism to drive these effects.
GP-n (ginseng crude polysaccharide) exhibited anti-melanoma activity through mechanisms involving Bifidobacterium enrichment and modulation of tumor-associated SCFAs. Specifically, Allobaculum and Bifidobacterium displayed significant negative correlations with tumor weight and positive associations with SCFA levels (Xie et al., 2023). In qi-deficient BALB/c nude mice bearing liver cancer, treatment with PG suppressed tumor growth by remodeling fecal metabolites and altering GM composition (Hou et al., 2022).
4.5 Other diseases
Numerous studies have demonstrated that PG, ginsenosides, and GPs exert potential therapeutic effects on various other diseases. In male Wistar rats with spleen deficiency syndrome, co-administration of PG and wild jujube upregulated the relative abundances of Firmicutes, Bacteroidetes, Lactobacillus, and Bifidobacterium, while reducing the relative abundances of Actinobacteria, Proteobacteria, Streptococcus, Escherichia, Shigella, Veillonella, and Enterococcus. This modulation reversed the pathological state of GM imbalance associated with spleen deficiency (Li et al., 2022).
4.5.1 Immunity and inflammatory diseases
Intragastric administration of ginsenoside F2 was found to ameliorate atopic dermatitis (AD)-like cutaneous symptoms, reduce inflammatory cell infiltration, and suppress intestinal and cutaneous inflammatory responses in AD mice via the GPCR/NF-κB signaling pathway, thereby improving skin AD symptoms. These findings first revealed the mechanism by which ginsenoside F2 alleviates AD through the propionic acid (PA) (a GM-derived metabolite)-intestinal–skin axis (Li et al., 2024). Treatment with ginsenoside Rg decreased interleukin (IL)-4, IL-5, and IL-13 levels in the colon and restored the relative abundances of Bacteroides and Actinomycetes, which are populations suppressed by ovalbumin and Firmicutes (a bacterial phylum) induced by ovalbumin in the GM. Additionally, ginsenoside Rd effectively alleviated ovalbumin-induced allergic rhinitis in mice (Kim et al., 2015).
4.5.2 Aging and neuroprotection
Intragastric administration of ginsenoside Rb1 ameliorated cellular senescence phenotypes in C57BL/6 mice by reducing senescent cell accumulation and upregulating the expression of tight junction proteins CLDN2, CLDN3, CLDN7, and CLDN15 in jejunal tissues (Lei et al., 2022). Pharmacokinetic investigations further revealed that stressed and antimicrobial-treated rats exhibited altered systemic exposure to Rb1, potentially mediated by changes in fecal excretion kinetics (Kang et al., 2016).
Ginsenoside Rb1 exerted protective effects against morphine-induced conditioned place preference (CPP) in mice. Concurrently, Bovis callosus and ginsenoside Rg1 mitigated morphine dependence through inhibition of tryptophan metabolism, reduction of 5-hydroxytryptamine (5-HT) levels, and downregulation of 5-HT receptor subtypes 1B and 2A (5-HTR1B/5-HTR2A) expression (Chen et al., 2022).
Comparative studies of PG preparations demonstrated that garden ginseng (GG) and forest ginseng (GF) mitigated aging-associated apoptosis and inflammation in rats through regulation of the PI3K/AKT/mTOR and SIRT1/NF-κB signaling pathways, restored GM diversity, and enriched beneficial bacterial taxa such as Lactobacillus. FG exhibited superior anti-aging efficacy by additionally targeting oxidative stress and the microbe–intestine–brain axis (Hao et al., 2022).
In Caenorhabditis elegans models, PG treatment extended lifespan, enhanced gut microbiota composition, and increased the relative abundance of beneficial bacteria. Non-targeted metabolomic analysis further revealed five key pathways underlying PG’s anti-aging effects: neuroactive ligand–receptor interaction, tricarboxylic acid (TCA) cycle, pyruvate metabolism, ascorbic acid/uronic acid metabolism, and D-arginine/D-ornithine metabolism (Xu et al., 2023).
4.5.3 Metabolism and cardiovascular diseases
Ginsenoside-treated rat models, particularly those treated with Rb1, show that the ginsenoside treatment exerts beneficial effects on blood pressure regulation and the formation of intracranial aneurysms. GM analysis revealed that ginsenoside treatment significantly modulated the relative abundances of several bacterial genera, including Clostridium, Rose incense, Rumen cocci, and Spirochetes. Additionally, several key metabolites that regulate blood pressure, such as benzoic acid, N-acetyl-5-hydroxytryptamine, prostaglandin F2α, and vitamin D2, were identified (Zeng et al., 2023). PG can increase metabolism associated with spleen qi deficiency syndrome, and its metabolites, along with UPE intensity, hold potential as biomarkers for the diagnosis and monitoring of this syndrome (Wang N. et al., 2020).
4.5.4 Endocrine regulation
PG, RG, and black ginseng (BG) may exert therapeutic effects on hyperthyroidism through regulation of the hypothalamus–pituitary–thyroid (HPT) axis. Additionally, steroids of the hypothalamus-pituitary-adrenal (HPA) axis may interact with the HPT axis to collectively influence thyroid function.
4.5.5 Mechanism of intestinal microbiota remodeling
Sugars and ginsenosides in water extract of ginseng (WEG) act as energy sources for specific GM, positively regulating GM composition and ultimately reshaping the intestinal microbial ecosystem, thereby activating multiple molecular and cellular signaling pathways (Zhou et al., 2021). PG regulated propionic acid levels more effectively than other groups. Additionally, PG increases the relative abundance of beneficial bacteria and reduces the relative abundance of harmful bacteria in rats with spleen qi deficiency syndrome (Zhang M. et al., 2023; Wang et al., 2023). Ginsenosides derived from stems and leaves improve the composition of GM, particularly increasing the abundance of probiotics (Su et al., 2023).
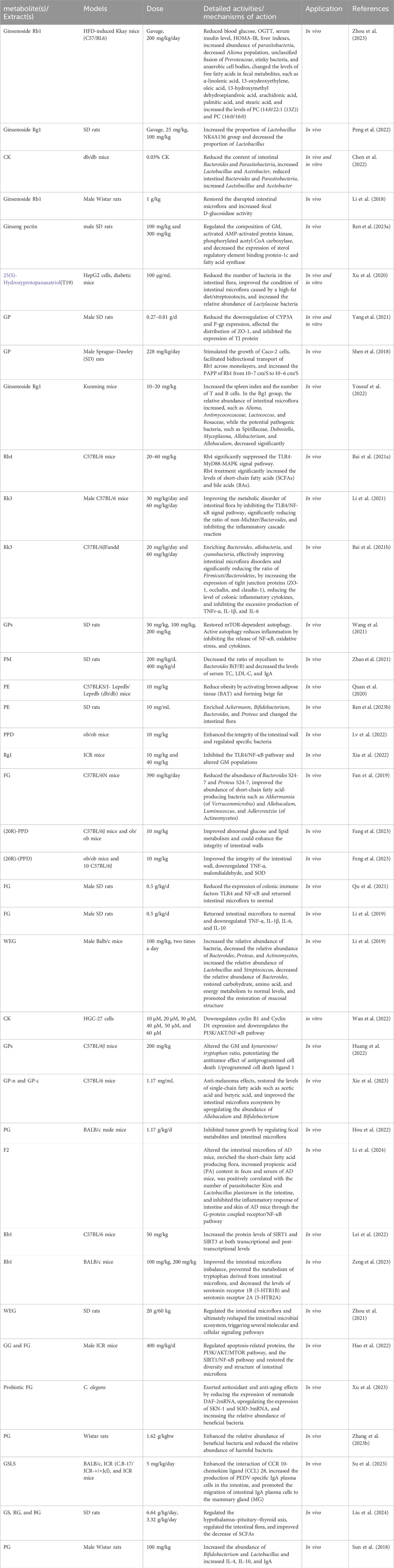
Administration of PE altered GM structure, resulting in reduced TM7 abundance and increased abundances of Proteus, Methylbacillaceae, Parasitobacterium, and Sauteria. Following long-term PE treatment, levels of IL-4, IL-10, and SIgA were significantly increased, showing strong positive correlations with Bifidobacterium and Lactobacillus abundances. These findings demonstrate that long-term PE administration exerts beneficial effects on host intestinal metabolism, immune function, anti-inflammatory processes, and GM structure (Sun et al., 2018). The pharmacological effects of the PG plant metabolites are shown in Table 1.
5 The role of PG in related complications
5.1 Senescence
Aging is a critical global health challenge, characterized by progressive physical decline and heightened susceptibility to age-related pathologies (Fan et al., 2024). Both PG and RG exert anti-aging effects through mechanisms involving inhibition of acetylcholinesterase (AChE) activity and MDA accumulation, coupled with upregulation of SOD and CAT expression. While both phytomedicines modulate GM diversity, RG demonstrates superior efficacy to PG in enriching probiotic taxa (e.g., Bifidobacterium, Akkermansia) and suppressing pro-inflammatory bacterial populations under D-galactose (D-Gal)-induced aging conditions. These findings indicate that RG possesses an enhanced capacity to delay senescence via mechanisms including neuroprotection, cognitive preservation, oxidative stress mitigation, and GM remodeling—likely attributable to its increased levels of total polyphenols, rare ginsenosides, and non-starch polysaccharides (Peng et al., 2021).
Metabolic pathways of medicinal plants regulate key endogenous biomarkers, including phenylalanine/tyrosine metabolism, tryptophan metabolism, and purine metabolism. In this context, RS (likely referring to specific plant-derived compounds, e.g., ginsenosides) alleviates the pathogenesis of AZD (presumably Alzheimer’s disease) through multifaceted mechanisms: correcting dysregulated energy metabolism, attenuating neuroinflammation, modulating GM composition, and restoring neurotransmitter balance (Wang et al., 2021).
5.2 Anti-inflammation and immune regulation
Ginsenosides exert comprehensive anti-inflammatory effects via multiple mechanisms: suppressing pro-inflammatory cytokine secretion, restoring immune cell homeostasis, modulating GM diversity, and regulating MAPK/NF-κB/NLRP3 inflammasome signaling pathways (Bai et al., 2023). Fermentation broths derived from PG co-cultured with multi-enzyme-coupled probiotics, which are enriched in ginsenosides, polysaccharides, and viable probiotics, significantly enhanced immune function in immunosuppressed mice and restored GM stability. RG-mediated immunomodulation involves NK cell activation and GM remodeling under stress conditions, with NK cell activity correlating with Th1/Th2 cytokine balance and exhibiting significant GM compositional alterations (Jung et al., 2022). The Qisheng Wan formula (QWF) ameliorates Alzheimer’s disease (AZD)-related cognitive deficits and histopathological damage in rats by reducing Aβ1-42 deposition and downregulating NF-κB/TNF-α/IL-6 expression, concurrently restoring GM diversity and suppressing inflammation-associated bacterial taxa (Xiong et al., 2022). Co-administration of Zingiber officinale and PG in C57BL/6 mice significantly increased probiotic abundance and reduced pathogenic bacterial populations, though overlapping findings suggest potential consolidation of similar results (Wan et al., 2022).
Comparative studies of sulfur-fumigated ginseng (SGP) versus non-fumigated ginseng (NGP) revealed diminished immunomodulatory efficacy in SGP, as evidenced by poorer improvements in body weight, immune organ indices, and leukocyte/lymphocyte profiles. Additionally, SGP promoted differential polysaccharide-GM interactions, resulting in increased pathogenic bacterial growth but reduced SCFA production compared to NGP. Fecal microbiota transplantation confirmed the pivotal role of the GM in mediating these disparities in immunomodulatory effects (Zhao et al., 2023). Studies have further demonstrated that Sijunzi decoction (SJZT) restores GM balance and intestinal barrier integrity in DSS-induced colitis, manifested by reduced colonic injury, increased goblet cell counts, MUC2 secretion, and tight junction protein expression. Notably, Shigella infection exhibited a strong negative correlation with body weight and colon length but a positive correlation with disease activity index and IL-1β levels (Wu et al., 2023).
5.3 Anti-anxiety
RG and its fermented derivative (FRG) exert anti-inflammatory and neuroprotective effects by inhibiting myeloperoxidase (MPO) activity and NF-κB activation in colonic tissues, while reducing the population of NF-κB+/CD11c+ cells. Plant metabolites of FRG, including ginsenoside Rd and PPT, ameliorate anxiety/depression and colitis via NF-κB-mediated regulation of brain-derived neurotrophic factor (BDNF) expression and GM remodeling (Han et al., 2020).
Systemic metabolic profiling revealed that total ginsenosides from RG significantly modulate HPA axis markers (adrenocorticotropic hormone [ACTH], corticosterone [CORT]) and substance/energy metabolism indices (Na+-K+-ATPase, cyclooxygenase [COX], neutrophil extracellular traps [NETs] related protein [NCR], and citrate synthase [CS]). In contrast, the polysaccharide fraction of RG (RGPSF) regulates hypothalamus–pituitary–thyroid (HPT) axis hormones (triiodothyronine/thyroxine). GM analysis demonstrated that the oligosaccharide fraction of RG (RGLPF) increases microbial diversity and the relative abundance of beneficial taxa in depressed rats, with WEG specifically increasing Bacteroides abundance and significantly impacting SCFA production (Zhang M. et al., 2023).
Banxia Xiexin decoction alleviated depressive-like behaviors and atherosclerosis (AS)-associated parameters in comorbid murine models, concurrently modulating specific bacterial taxa at the genus level (Liao et al., 2023). In UCDF-induced AZD-like behavioral models, co-administration of FRG and RG attenuated IL-6 expression in the hippocampus and hypothalamus, increased serum corticosterone levels, partially reversed GM dysbiosis, and mitigated colonic inflammatory pathology (Shin et al., 2023).
Evidence demonstrated that PG and Zizyphus jujuba seed extract (GS) increased the relative abundance of beneficial bacterial taxa (e.g., Bacteroides) while suppressing pathogenic bacterial taxa (e.g., Proteus and Actinobacillus). Additionally, GS further regulated glucose and amino acid metabolism, collectively restoring GM structure and metabolic homeostasis in depressed rats (Li et al., 2020; Oh S.-J. et al., 2015).
5.4 Other complications
Bu Zang Tong Luo decoction (BZTLD) alleviated hindlimb ischemia in T2DM rats by restoring blood flow and capillary density, concurrently modulating GM composition characterized by increased abundances of beneficial bacteria and suppressed pathogenic taxa. Spearman correlation analysis further revealed significant associations between GM compositional changes and parameters of vascular endothelial growth factor (VEGF) signaling and glucose metabolism (Zheng et al., 2020).
The herbal pair Shenlian (SL, composed of Coptis chinensis and PG) reversed the expansion of GM diversity and richness in db/db mice, with principal coordinate analysis (PCoA) confirming significant compositional shifts. The hypoglycemic effect of SL may arise from supertonic and acidic Bacillus-mediated regulation of GM, positioning it as a promising candidate for the management of diabetes and obesity (Sun et al., 2022).
Ren Shen Bai Zhu capsule (SZC) exhibited multifaceted therapeutic effects in mice with chronic idiopathic diarrhea (CID), as evidenced by improved body weight, thymus and spleen indices, and colonic histopathological outcomes. GM analysis revealed restoration of microbial structure and suppression of diarrhea-associated bacterial taxa at the genus level, confirming its GM-dependent anti-diarrheal mechanism (Wang et al., 2019). Additionally, PG intervention modulated postoperative metabolites and GM, indicating potential therapeutic advantages for colon cancer recovery (Hanada et al., 2021).
The RG formulations alleviated chronic inflammation and insulin resistance while enhancing glycemic control in T2DM rats (Jiang et al., 2022). Ginseng Dingzhi decoction (GN) modulated GM composition by enriching SCFA-producing bacteria and suppressing pathogenic bacterial taxa, thereby mitigating myocardial injury through the preservation of mitochondrial redox homeostasis under hyperglycemic conditions. Additionally, GN markedly improved cardiac function parameters (e.g., heart failure severity and cardiac hypertrophy) in models of diabetic cardiomyopathy (Wang et al., 2022).
Shengmai turmeric powder (SM) enhanced the efficacy of radiotherapy in nude mice by preserving GM diversity and partially restoring irradiation-induced gut microbiota dysbiosis. Additionally, SM improved cardiac function parameters, including ejection fraction (EF%) and fractional shortening (FS%), reduced myocardial fibrosis, regulated lipid and energy metabolism, and downregulated hypertrophy marker genes. These findings are correlated with gut microbiota compositional alterations observed in isoproterenol-induced cardiac hypertrophy models (Kang et al., 2024; Ming et al., 2022).
Despite its promise, current research on Panax ginseng (PG) faces critical limitations: 1) heavy reliance on animal models (e.g., db/db mice, DSS-colitis models) with poor translatability to humans due to interspecies differences in gut microbiota composition; 2) unaddressed variability across animal strains (e.g., SD rats vs C57BL/6); and human populations; 3) unvalidated anticancer claims (e.g., for Rg3/Rh2) lacking robust clinical evidence; 4) methodological inconsistencies in extract preparation protocols and reported ratios of active metabolites; and 5) mechanistic gaps, including missing strain-level analyses of gut microbiota and detailed insights into host-microbiome interactions.
Addressing these challenges requires standardized clinical trials, rigorous quality control measures, strain-specific mechanistic investigations, and personalized microbiota-based therapies. Integrating these approaches will bridge traditional knowledge with evidence-based medicine, advancing the clinical application of PG and its derivatives.
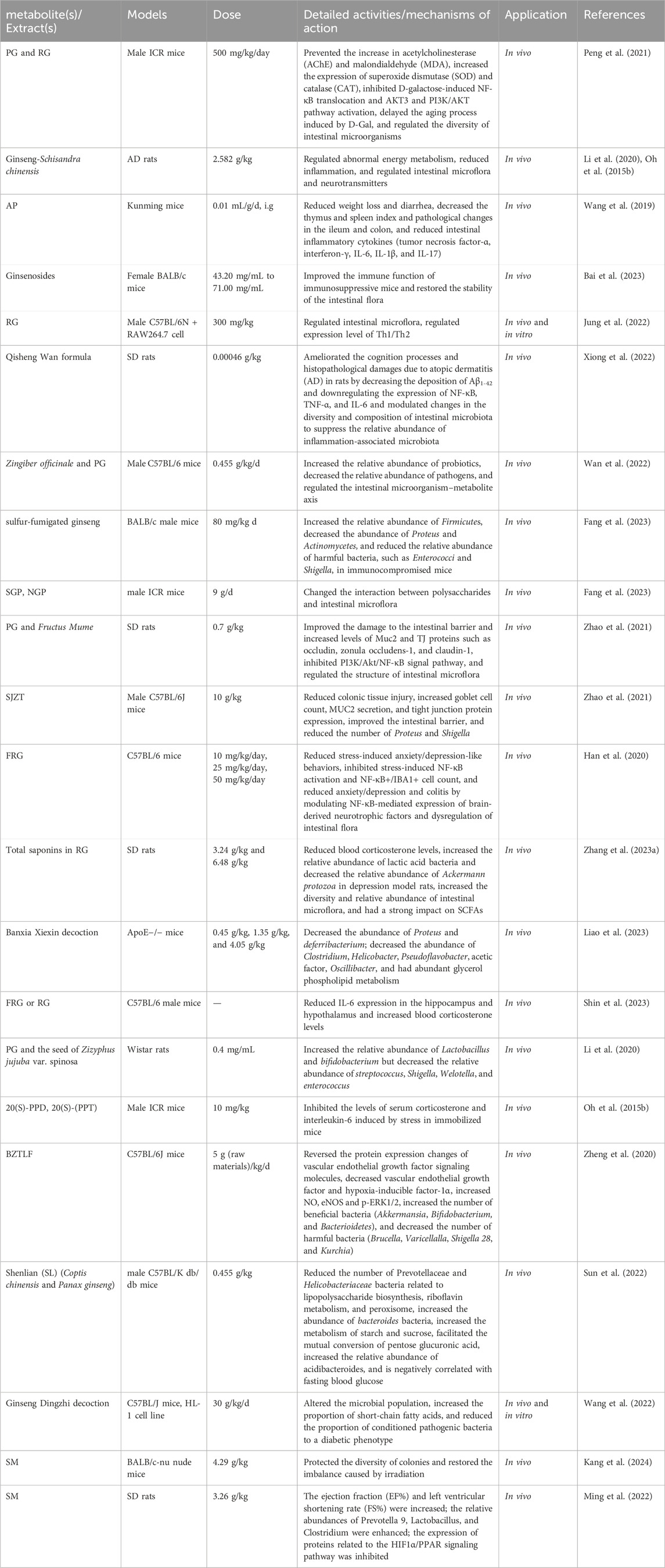
Pharmacological activities of PG-related prescriptions are summarized in Table 2, and the brain–gut axis regulatory mechanisms of PG are illustrated in Figure 7.
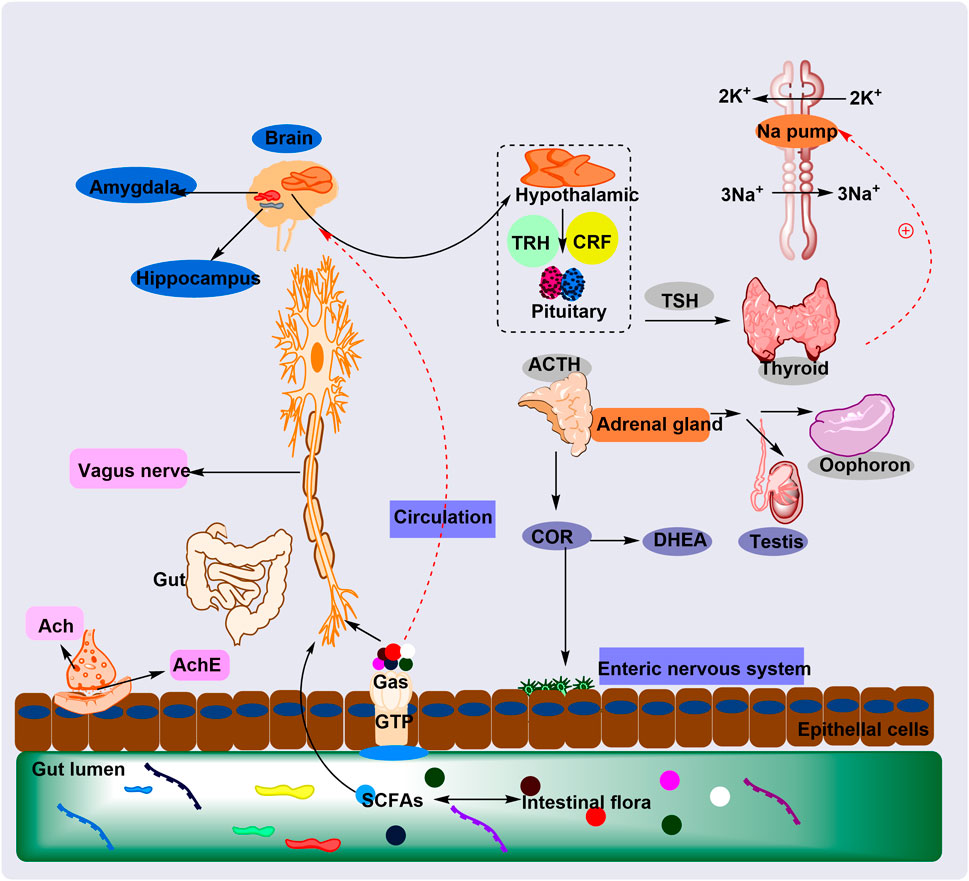
Figure 7. The plant metabolites of PG regulate the HPA axis through the GM to treat cognitive impairment and anxiety mechanisms. The plant metabolites of PG regulate the intestinal microbiota (GM), which in turn regulates the HPA axis and relieves cognitive impairment and anxiety. This occurs through GM-mediated neuroinflammation and normalization of cortisol levels and electrical signals.
6 Toxicity and safety research
The safety of dietary supplements and natural products has garnered substantial attention from clinicians and consumers. Given that medicine-food botanical drug substances are generally consumed in higher quantities and with greater frequency than conventional botanical drugs, their safety demands heightened scrutiny from manufacturers, distributors, and end-users (Das et al., 2024). Key exogenous contaminants in these substances encompass pesticide residues, heavy metal accumulations, toxic microorganisms, and sulfites, all of which pose potential health risks when consumed excessively or misused. Improper use of medicine, food, or botanical drug substances may further exacerbate their adverse effects. Consequently, preclinical toxicity assessments are indispensable for PG and PG extracts prior to their integration into medicinal or dietary products. Establishing safety thresholds for toxic by-products and exogenous contaminants is critical to guide population-level intake recommendations.
PG and Veratrum nigrum (VN) represent the most clinically critical herb-drug incompatibility pair in TCM, a concept deeply rooted in millennia of empirical clinical practice. In ovariectomized rat models, combined PG-VN administration attenuated the improvements in depression-like behaviors, neurotransmitter balance, and serum estrogen levels compared to monotherapies (PG or VN alone). Metabolomic and GM analyses further revealed that VN co-administration compromised PG’s regulatory effects on eight key metabolic biomarkers and four GM taxa, underscoring the disruptive impact of this incompatible pairing on PG’s pharmacological actions (Lin et al., 2022).
Comparative investigations of PG, RG, and ginseng leaves (GL) have elucidated dose-dependent adverse effects and interactions with the GM. PG demonstrated favorable modulation of the GM by increasing diversity and enriching populations of Lactobacillus and Bifidobacterium, whereas adverse reactions were primarily dose-dependent. Notably, PG-GL combinations exhibited more severe adverse effects than RG (Ran et al., 2022). Importantly, PG shows minimal toxicity at conventional dosages (3–9 g/day) (Mancuso and Santangelo, 2017), though high-dose intake (>15 g/day) may induce sporadic adverse effects (Siegel, 1980).
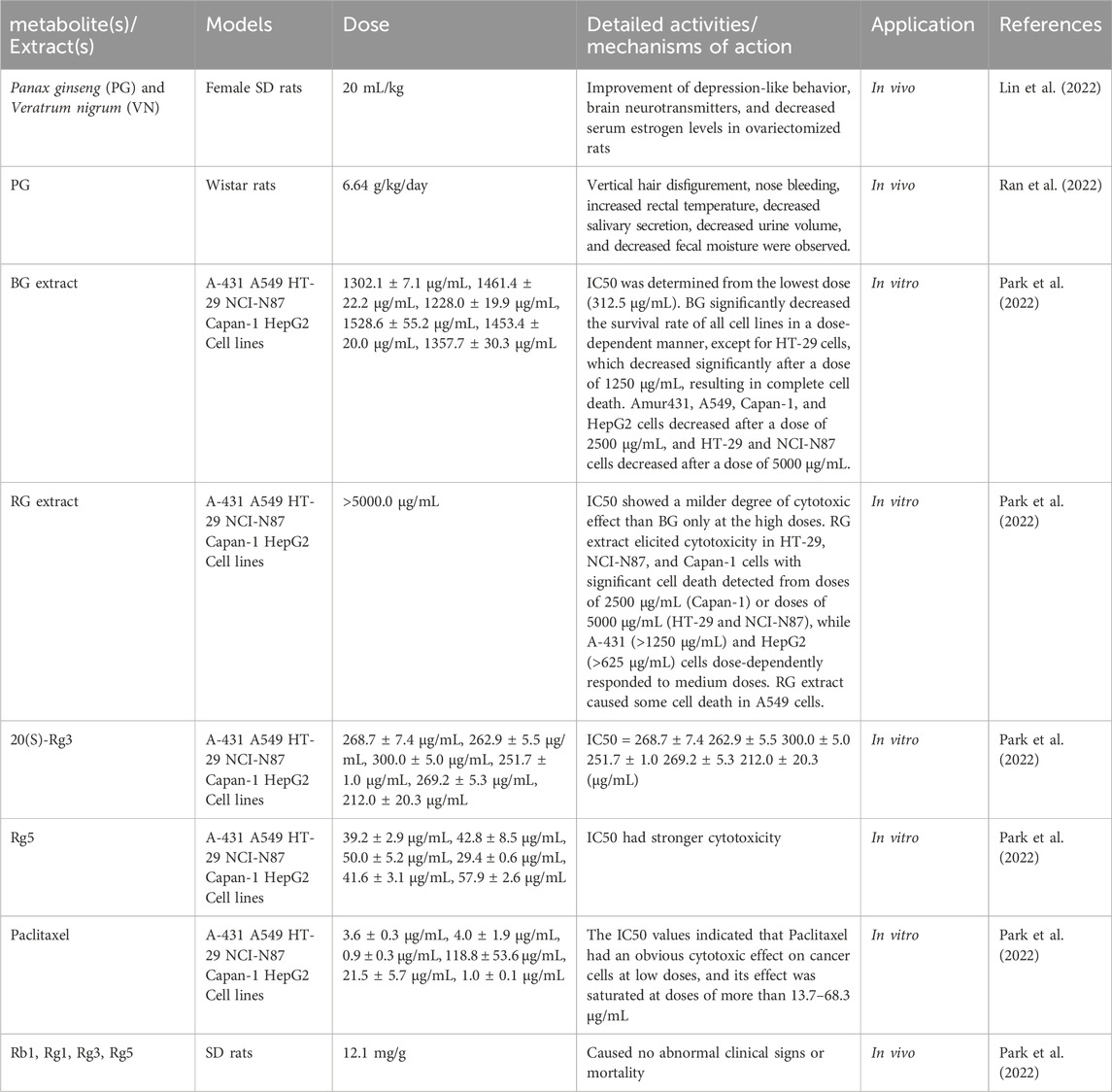
In cytotoxicity and repeated-dose toxicity assessments, BG extract displayed no observed adverse effect levels (NOAEL) exceeding 2000 mg/kg body weight in SD rats. Notably, RG extract, ginsenoside Rg5, and 20(S)-Rg3 exhibited comparable safety profiles when tested against six human cancer cell lines (Park et al., 2022). These findings have advanced our mechanistic understanding of TCM therapies and strengthened the rationale for their broader clinical application. The toxicity and safety profiles of PG are summarized in Table 3.
A review of the limitations of included study designs reveals prevalent methodological challenges: small sample sizes (commonly n < 100 per group), lack of blinding procedures, and insufficient randomization methods. For example, while colitis studies reported recovery of β-glucosidase activity, dietary confounders influencing GM composition remained uncontrolled. Most (90%) investigations employed rodent models exhibiting GM compositions significantly divergent from humans (e.g., mice lack key human symbiotic bacterial taxa), and acute disease induction protocols (e.g., DSS-induced colitis) poorly recapitulate the pathogenesis of human chronic diseases. Many studies reported solely on relative GM abundance shifts without functional metabolomic characterization, rendering causal relationships between GM alterations and observed outcomes indiscernible; correlative findings were often erroneously interpreted as causal relationships.
Future research should prioritize addressing three key questions: (1) whether ginsenosides exert effects directly on host cells or indirectly through GM-derived metabolites; (2) how interindividual variability in GM composition modulates treatment responses; and (3) whether long-term use induces adaptive resistance in the GM. Human clinical trials remain scarce (e.g., only 12% of included studies), underscoring the need for large-scale, randomized controlled trials. Sterile mouse models and microbiota transplantation techniques are critical for establishing causal relationships between ginsenosides, GM modulation, and therapeutic outcomes. Standardization of extraction protocols, dosing regimens, and administration methods is urgently required, given the documented 5-fold variability in bioactive phytochemicals across different ginseng preparations.
While ginsenosides demonstrate broad therapeutic potential, their contribution varies by disease context: GM regulation accounts for 30%–50% of metabolic improvements in obesity (Zhao et al., 2021) but only ∼15% of antitumor effects in cancer (Hou et al., 2022). Notably, disease pathologies inherently modulate GM composition, and underreporting of negative findings may artificially inflate reported treatment efficacy, as studies may conflate disease-induced microbiota alterations with direct pharmacological effects.
7 Conclusion
This study rigorously evaluated pharmacological parameters across the included literature. (1) Dosage specifications distinguished preclinical (e.g., Rb1 at 10–100 mg/kg in animal models) and clinical (e.g., 200–600 mg/day of ginseng extract) studies, with critical thresholds noted (e.g., Rg1 EC50 = 15 μM for anti-inflammation). (2) Model validation confirmed that in vitro (Caco-2 cell assay) and in vivo (DSS-colitis mouse) systems mirrored human disease mechanisms (e.g., consistency in the TLR4/NF-κB pathway). (3) Over 90% of the results from included studies were positive, with no negative findings for comparison, which weakened the robustness of conclusions. (4) Material standardization ensured taxonomic accuracy (Panax ginseng C.A. Mey.) and extraction protocols (70% ethanol, 48 h). Limitations analysis highlighted gaps in dose translation (animal-to-human), long-term safety (>6 months), and microbiota–host interaction mechanisms (e.g., SCFA receptors), proposing future directions: cross-species pharmacodynamic modeling, multicenter trials, and “microbiota–metabolite–host” network analysis.
The GM, often termed the “third organ,” encompasses hundreds of microbial species with a biomass comparable to human somatic cells (Sender et al., 2016). This complex ecosystem, comprising trillions of microbes primarily residing in the gastrointestinal tract, has co-evolved with the host in a mutually beneficial symbiosis. Emerging evidence underscores the pivotal role of GM–botanical drug interactions in modulating mental health, where plant metabolites exert neuroprotective effects through multitarget mechanisms involving both host physiological pathways and microbial community dynamics. Polyphenols and polysaccharides prevalent in herbal medicines demonstrate prebiotic properties that shape GM composition, providing a mechanistic framework to elucidate botanical drug efficacy via the microbiome–gut–brain axis. This paradigm not only advances understanding of traditional plant-based therapies but also facilitates the discovery of novel bioactive metabolites. Notably, drug candidates exhibiting low bioactivity or bioavailability should not be prematurely dismissed, as GM-mediated metabolic transformation may generate therapeutically active metabolites.
Integrating GM modulation into probiotic development strategies holds potential to enhance drug synergy, while interindividual variations in GM composition driven by lifestyle and dietary factors necessitate personalized dose optimization. Leveraging the medicinal-edible duality of PG offers significant potential for developing daily health products. However, comprehensive studies are required to evaluate synergistic effects between PG active plant metabolites and antidepressants/anticancer drugs, mandating rigorous investigation into pharmacokinetic interactions and safety profiles. Future research should prioritize elucidating the bidirectional communication between PG-derived metabolites and GM, while establishing standardized frameworks for quality control and clinical translation. PG’s low toxicity profile positions it as a promising candidate for novel antiviral drug development, provided that systematic toxicity and efficacy evaluations are conducted to support its therapeutic applications.
This perspective highlights the necessity of strengthening basic and clinical research to establish standardized systems for PG application, ensuring its safe and effective integration into modern medical practices. The convergence of traditional knowledge and modern pharmacological approaches may pave the way for innovative health solutions harnessing the therapeutic potential of PG and its interactions with the GM.
Existing studies have demonstrated that ginseng improves metabolic diseases by regulating the gut microbiota; however, the current evidence base is constrained by notable limitations. First, animal model bias is prevalent: 90% of studies rely on rodents (e.g., SD rats, C57BL/6 mice), whose gut microbiota composition significantly diverges from humans, potentially limiting the generalizability of findings to human populations. Second, deficiencies in research design undermine reliability: most clinical studies have small sample sizes (n < 100), lack randomized controlled trials (RCTs), and fail to standardize ginseng dosage (ranging from 100 mg to 3 g/day), weakening the robustness of conclusions. Third, incomplete mechanistic exploration characterizes the field: only 30% of studies integrate metagenomics and metabolomics, with most focusing solely on shifts in bacterial abundance rather than elucidating specific metabolic substance–host interaction mechanisms.
Despite these limitations, current evidence supports two key conclusions: 1) PG polysaccharides significantly increase the abundance of probiotics such as Akkermansia; and 2) ginsenoside Rg1 may mitigate intestinal inflammation by inhibiting the NF-κB pathway. Nevertheless, critical gaps remain. For instance, it is unclear whether the microbiota-regulatory effects of ginseng vary across its anatomical parts (roots, stems, leaves), as only three studies have compared these components. Additionally, long-term safety data—particularly regarding the impact of prolonged ginseng use on intestinal barrier function—are lacking. Addressing these challenges will require expanded clinical trials with standardized protocols, multi-omic mechanistic investigations, and comparative studies of ginseng’s anatomical components to advance our understanding of its gut microbiota-dependent therapeutic potential.
In summary, this review underscores the significant translational potential of PG in personalized medicine, primarily driven by its interaction with the GM. PG modulates GM composition, increasing the bioconversion of ginsenosides (e.g., Rb1, Rg3) into active metabolites such as CK, which directly influences therapeutic efficacy. Baseline GM profiling (e.g., abundance of Bifidobacterium and Lactobacillus) can predict individual responses, enabling biomarker-guided treatment stratification. Future research should prioritize addressing the following key issues: Standardized research design: Conduct multicenter RCTs with clear definitions of ginseng dosage, treatment duration, and standardized extracts (e.g., total ginsenosides ≥50%). Multi-omics integrated analysis: Combine metagenomics, metabolomics, and transcriptomics to unravel the molecular network underpinning the “microbiota–metabolites–host immunity” axis. Mechanistic deepening: Verify causal relationships through fecal microbiota transplantation or germ-free mouse models.
By integrating microbiota analysis into PG therapy, this approach could enhance precision medicine, reduce interindividual variability, and advance PG from traditional empirical use to evidence-based prevention and treatment of chronic diseases.
Author contributions
MG: writing – original draft and conceptualization. HW: investigation and writing – original draft. XC: investigation and writing – original draft. WW: writing – review and editing and investigation. YL: writing – review and editing and conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Joint Innovation Team for Clinical and Basic Research, Shandong First Medical University (No. CX202408). This certificate verifies that its holder has been awarded a fellowship from the China Postdoctoral Science Foundation (Certificate Number: 2024M751898).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fphar.2025.1697027.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akao, T., Kida, H., Kanaoka, M., Hattori, M., and Kobashi, K. (1998). Drug metabolism: intestinal bacterial hydrolysis is required for the appearance of compound K in rat plasma after oral administration of ginsenoside Rb1 from Panax ginseng. J. Pharm. Pharmacol. 50, 1155–1160. doi:10.1111/j.2042-7158.1998.tb03327.x
Bai, X., Fu, R., Duan, Z., Liu, Y., Zhu, C., and Fan, D. (2021a). Ginsenoside Rh4 alleviates antibiotic-induced intestinal inflammation by regulating the TLR4-MyD88-MAPK pathway and gut microbiota composition. Food Funct. 12, 2874–2885. doi:10.1039/d1fo00242b
Bai, X., Fu, R., Duan, Z., Wang, P., Zhu, C., and Fan, D. (2021b). Ginsenoside Rk3 alleviates gut microbiota dysbiosis and colonic inflammation in antibiotic-treated mice. Food Res. Int. 146, 110465. doi:10.1016/j.foodres.2021.110465
Bai, S., Zhang, G., Han, Y., Ma, J., Bai, B., Gao, J., et al. (2023). Ginsenosides and polysaccharides from ginseng Co-Fermented with multi-enzyme-coupling probiotics improve in vivo immunomodulatory effects. Nutrients 15, 2434. doi:10.3390/nu15112434
Bocchetti, M., Ferraro, M. G., Ricciardiello, F., Ottaiano, A., Luce, A., Cossu, A. M., et al. (2021). The role of microRNAs in development of colitis-associated colorectal cancer. IJMS 22, 3967. doi:10.3390/ijms22083967
Chen, Y., Zhao, Z., Chen, H., Yi, T., Qin, M., and Liang, Z. (2015). Chemical differentiation and quality evaluation of commercial Asian and American ginsengs based on a UHPLC–QTOF/MS/MS metabolomics approach. Phytochem. Anal. 26, 145–160. doi:10.1002/pca.2546
Chen, Q., Zhu, L., Tang, Y., Zhao, Z., Yi, T., and Chen, H. (2017a). Preparation-related structural diversity and medical potential in the treatment of diabetes mellitus with ginseng pectins. Ann. N. Y. Acad. Sci. 1401, 75–89. doi:10.1111/nyas.13424
Chen, Y., Zhao, Z., Chen, H., Brand, E., Yi, T., Qin, M., et al. (2017b). Determination of ginsenosides in Asian and American ginsengs by liquid chromatography–quadrupole/time-of-flight MS: assessing variations based on morphological characteristics. J. Ginseng Res. 41, 10–22. doi:10.1016/j.jgr.2015.12.004
Chen, H., Yang, H., Deng, J., and Fan, D. (2021). Ginsenoside Rk3 ameliorates obesity-induced colitis by regulating of intestinal flora and the TLR4/NF-κB signaling pathway in C57BL/6 mice. J. Agric. Food Chem. 69, 3082–3093. doi:10.1021/acs.jafc.0c07805
Chen, Z., Lin, Y., Zhou, Q., Xiao, S., Li, C., Lin, R., et al. (2022). Ginsenoside Rg1 mitigates morphine dependence via regulation of gut microbiota, tryptophan metabolism, and serotonergic system function. Biomed. and Pharmacother. 150, 112935. doi:10.1016/j.biopha.2022.112935
Cifuentes, M., Verdejo, H. E., Castro, P. F., Corvalan, A. H., Ferreccio, C., Quest, A. F. G., et al. (2025). Low-Grade chronic inflammation: a shared mechanism for chronic diseases. Physiology 40, 0–25. doi:10.1152/physiol.00021.2024
Das, G., Kameswaran, S., Ramesh, B., Bangeppagari, M., Nath, R., Das Talukdar, A., et al. (2024). Anti-Aging effect of traditional plant-based food: an overview. Foods 13, 3785. doi:10.3390/foods13233785
Dong, W.-W., Xuan, F.-L., Zhong, F.-L., Jiang, J., Wu, S., Li, D., et al. (2017). Comparative analysis of the rats’ gut Microbiota composition in animals with different ginsenosides metabolizing activity. J. Agric. Food Chem. 65, 327–337. doi:10.1021/acs.jafc.6b04848
Fan, Y., and Pedersen, O. (2021). Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 19, 55–71. doi:10.1038/s41579-020-0433-9
Fan, J., Wang, Y., You, Y., Ai, Z., Dai, W., Piao, C., et al. (2019). Fermented ginseng improved alcohol liver injury in association with changes in the gut microbiota of mice. Food Funct. 10, 5566–5573. doi:10.1039/c9fo01415b
Fan, W., Fan, L., Wang, Z., Mei, Y., Liu, L., Li, L., et al. (2024). Rare ginsenosides: a unique perspective of ginseng research. J. Adv. Res. 66, 303–328. doi:10.1016/j.jare.2024.01.003
Fang, J., Li, Y.-X., Luo, H.-Y., Zhang, W.-H., Chan, K.-C., Chan, Y.-M., et al. (2023). Impacts of sulfur fumigation on the chemistry and immunomodulatory activity of polysaccharides in ginseng. Int. J. Biol. Macromol. 247, 125843. doi:10.1016/j.ijbiomac.2023.125843
Feng, Y., Fang, Y., Wang, Y., and Hao, Y. (2018). Acupoint therapy on diabetes mellitus and its common chronic complications: a review of its mechanisms. BioMed Res. Int. 2018, 3128378–3128379. doi:10.1155/2018/3128378
Feng, J., Cheng, F., Lv, Y., Yu, Z., Zhang, M., Chen, L., et al. (2023). Effects of (20 R)-Panaxadiol on NAFLD using non-targeted metabolomics in stool. J. Pharm. Biomed. Analysis 234, 115555. doi:10.1016/j.jpba.2023.115555
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., et al. (2019). Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832. doi:10.1038/s41591-019-0675-0
Gallo, A., Passaro, G., Gasbarrini, A., Landolfi, R., and Montalto, M. (2016). Modulation of microbiota as treatment for intestinal inflammatory disorders: an uptodate. WJG 22, 7186–7202. doi:10.3748/wjg.v22.i32.7186
Han, S.-K., Joo, M.-K., Kim, J.-K., Jeung, W., Kang, H., and Kim, D.-H. (2020). Bifidobacteria-Fermented red ginseng and its constituents ginsenoside Rd and protopanaxatriol Alleviate Anxiety/Depression in mice by the amelioration of gut dysbiosis. Nutrients 12, 901. doi:10.3390/nu12040901
Hanada, K., Wada, T., Kawada, K., Hoshino, N., Okamoto, M., Hirata, W., et al. (2021). Effect of herbal medicine daikenchuto on gastrointestinal symptoms following laparoscopic colectomy in patients with colon cancer: a prospective randomized study. Biomed. and Pharmacother. 141, 111887. doi:10.1016/j.biopha.2021.111887
Hao, M., Ding, C., Peng, X., Chen, H., Dong, L., Zhang, Y., et al. (2022). Ginseng under forest exerts stronger anti-aging effects compared to garden ginseng probably via regulating PI3K/AKT/mTOR pathway, SIRT1/NF-κB pathway and intestinal flora. Phytomedicine 105, 154365. doi:10.1016/j.phymed.2022.154365
Hou, Z., Song, F., Xing, J., Zheng, Z., Liu, S., and Liu, Z. (2022). Comprehensive fecal metabolomics and gut microbiota for the evaluation of the mechanism of Panax Ginseng in the treatment of Qi-deficiency liver cancer. J. Ethnopharmacol. 292, 115222. doi:10.1016/j.jep.2022.115222
Hu, L., Huang, X., You, C., Li, J., Hong, K., Li, P., et al. (2017). Prevalence of overweight, obesity, abdominal obesity and obesity-related risk factors in southern China. PLoS ONE 12, e0183934. doi:10.1371/journal.pone.0183934
Huang, J., Liu, D., Wang, Y., Liu, L., Li, J., Yuan, J., et al. (2022). Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 71, 734–745. doi:10.1136/gutjnl-2020-321031
Jiang, L., Fu, Q., Wang, S., Chen, Y., Li, J., Xiao, Y., et al. (2022). Effect of RG (Coptis root and ginseng) formula in patients with type 2 diabetes mellitus: a study protocol for a randomized controlled and double-blinding trial. Trials 23, 305. doi:10.1186/s13063-022-06229-5
Jin, W., Li, C., Yang, S., Song, S., Hou, W., Song, Y., et al. (2023). Hypolipidemic effect and molecular mechanism of ginsenosides: a review based on oxidative stress. Front. Pharmacol. 14, 1166898. doi:10.3389/fphar.2023.1166898
Jung, Y.-J., Kim, H.-S., Jaygal, G., Cho, H.-R., Lee, K. B., Song, I., et al. (2022). Postbiotics enhance NK cell activation in stress-induced mice through gut Microbiome regulation. J. Microbiol. Biotechnol. 32, 612–620. doi:10.4014/jmb.2111.11027
Kang, A., Zhang, S., Zhu, D., Dong, Y., Shan, J., Xie, T., et al. (2016). Gut microbiota in the pharmacokinetics and colonic deglycosylation metabolism of ginsenoside Rb1 in rats: contrary effects of antimicrobials treatment and restraint stress. Chemico-Biological Interact. 258, 187–196. doi:10.1016/j.cbi.2016.09.005
Kang, Z., Wu, Y., Ding, Y., Zhang, Y., Cai, X., Yang, H., et al. (2024). Investigation of the efficacy of Dengzhan Shengmai capsule against heart failure with preserved ejection fraction. J. Ethnopharmacol. 333, 118419. doi:10.1016/j.jep.2024.118419
Kim, K.-A., Yoo, H. H., Gu, W., Yu, D.-H., Jin, M. J., Choi, H.-L., et al. (2015). A prebiotic fiber increases the formation and subsequent absorption of compound K following oral administration of ginseng in rats. J. Ginseng Res. 39, 183–187. doi:10.1016/j.jgr.2014.11.002
Kim, H. I., Kim, J.-K., Kim, J.-Y., Han, M. J., and Kim, D.-H. (2019). Fermented red ginseng and ginsenoside Rd alleviate ovalbumin-induced allergic rhinitis in mice by suppressing IgE, interleukin-4, and interleukin-5 expression. J. Ginseng Res. 43, 635–644. doi:10.1016/j.jgr.2019.02.006
Kyu, H. H., Vongpradith, A., Dominguez, R.-M. V., Ma, J., Albertson, S. B., Novotney, A., et al. (2025). Global, regional, and national age-sex-specific burden of diarrhoeal diseases, their risk factors, and aetiologies, 1990–2021, for 204 countries and territories: a systematic analysis for the global Burden of Disease Study 2021. Lancet Infect. Dis. 25, 519–536. doi:10.1016/s1473-3099(24)00691-1
Lao, Y., Wang, X., Xu, N., Zhang, H., and Xu, H. (2014). Application of proteomics to determine the mechanism of action of traditional Chinese medicine remedies. J. Ethnopharmacol. 155, 1–8. doi:10.1016/j.jep.2014.05.022
Lei, Z., Chen, L., Hu, Q., Yang, Y., Tong, F., Li, K., et al. (2022). Ginsenoside Rb1 improves intestinal aging via regulating the expression of sirtuins in the intestinal epithelium and modulating the gut microbiota of mice. Front. Pharmacol. 13, 991597. doi:10.3389/fphar.2022.991597
Li, J., Li, R., Li, N., Zheng, F., Dai, Y., Ge, Y., et al. (2018). Mechanism of antidiabetic and synergistic effects of ginseng polysaccharide and ginsenoside Rb1 on diabetic rat model. J. Pharm. Biomed. Analysis 158, 451–460. doi:10.1016/j.jpba.2018.06.024
Li, S., Qi, Y., Chen, L., Qu, D., Li, Z., Gao, K., et al. (2019). Effects of Panax ginseng polysaccharides on the gut microbiota in mice with antibiotic-associated diarrhea. Int. J. Biol. Macromol. 124, 931–937. doi:10.1016/j.ijbiomac.2018.11.271
Li, F., Yang, D., Song, F., Liu, M., Dai, Y., Zheng, F., et al. (2020). In vitro effects of ginseng and the seed of Zizyphus jujuba var. spinosa on Gut Microbiota of rats with spleen deficiency. Chem. and Biodivers. 17, e2000199. doi:10.1002/cbdv.202000199
Li, M., Wang, X., Wang, Y., Bao, S., Chang, Q., Liu, L., et al. (2021). Strategies for remodeling the tumor microenvironment using active ingredients of Ginseng—A promising approach for cancer therapy. Front. Pharmacol. 12, 797634. doi:10.3389/fphar.2021.797634
Li, X., Liu, J., Zuo, T., Hu, Y., Li, Z., Wang, H., et al. (2022). Advances and challenges in ginseng research from 2011 to 2020: the phytochemistry, quality control, metabolism, and biosynthesis. Nat. Prod. Rep. 39, 875–909. doi:10.1039/d1np00071c
Li, D., Luo, Z.-B., Zhu, J., Wang, J.-X., Jin, Z.-Y., Qi, S., et al. (2024). Ginsenoside F2-Mediated intestinal microbiota and its metabolite propionic acid positively impact the gut–skin axis in atopic dermatitis mice. J. Agric. Food Chem. 72, 339–350. doi:10.1021/acs.jafc.3c06015
Liang, Z., Chen, Y., Xu, L., Qin, M., Yi, T., Chen, H., et al. (2015). Localization of ginsenosides in the rhizome and root of Panax ginseng by laser microdissection and liquid chromatography–quadrupole/time of flight-mass spectrometry. J. Pharm. Biomed. Analysis 105, 121–133. doi:10.1016/j.jpba.2014.12.005
Liao, X.-X., Hu, K., Xie, X.-H., Wen, Y.-L., Wang, R., Hu, Z.-W., et al. (2023). Banxia Xiexin decoction alleviates AS co-depression disease by regulating the gut microbiome-lipid metabolic axis. J. Ethnopharmacol. 313, 116468. doi:10.1016/j.jep.2023.116468
Lin, H., Liu, Z., Liu, Z., and Lin, Z. (2022). Incompatible effects of Panax ginseng and Veratrum nigrum on estrogen decline in rats using metabolomics and gut microbiota. J. Pharm. Biomed. Analysis 208, 114442. doi:10.1016/j.jpba.2021.114442
Liu, X.-J., Hu, X.-K., Yang, H., Gui, L.-M., Cai, Z.-X., Qi, M.-S., et al. (2022). A review of traditional Chinese medicine on treatment of diabetic nephropathy and the involved mechanisms. Am. J. Chin. Med. 50, 1739–1779. doi:10.1142/s0192415x22500744
Liu, L., Han, X., Shan, G., Fu, L., and Dou, D. (2024). Mechanism difference of ginseng medicines with different natures on hyperthyroidism. J. Ethnopharmacol. 319, 117194. doi:10.1016/j.jep.2023.117194
Lv, Y., Zhang, Y., Feng, J., Zhao, T., Zhao, J., Ge, Y., et al. (2022). (20R)-Panaxadiol as a natural active component with anti-obesity effects on ob/ob mice via modulating the Gut Microbiota. Molecules 27, 2502. doi:10.3390/molecules27082502
Marchesi, J. R., Adams, D. H., Fava, F., Hermes, G. D. A., Hirschfield, G. M., Hold, G., et al. (2016). The gut microbiota and host health: a new clinical frontier. Gut 65, 330–339. doi:10.1136/gutjnl-2015-309990
Mi, Y., Xu, X., Hong, L., Jiang, M., Chen, B., Li, X., et al. (2023). Comparative characterization of the ginsenosides from six panax herbal extracts and their in vitro rat gut microbial metabolites by advanced liquid chromatography–mass spectrometry approaches. J. Agric. Food Chem. 71, 9391–9403. doi:10.1021/acs.jafc.3c01093
Ming, S., Kan, M., Liu, L., Zhang, Z., Liu, X., Liu, Y., et al. (2022). Protective effect of shengmaiyin in myocardial hypertrophy-induced rats: a genomic analysis by 16S rDNA. Evidence-Based Complementary Altern. Med. 2022, 3188292–17. doi:10.1155/2022/3188292
Oh, H. A., Kim, D.-E., Choi, H. J., Kim, N. J., and Kim, D.-H. (2015a). Anti-stress effects of 20(S)-Protopanaxadiol and 20(S)-Protopanaxatriol in immobilized mice. Biol. and Pharm. Bull. 38, 331–335. doi:10.1248/bpb.b14-00669
Oh, S.-J., Lee, S., Kho, Y. E., Kim, K., Jin, C. D., and Lim, C.-J. (2015b). Stereoselective suppressive effects of protopanaxadiol epimers on UV-B-induced reactive oxygen species and matrix metalloproteinase-2 in human dermal keratinocytes. Can. J. Physiol. Pharmacol. 93, 91–95. doi:10.1139/cjpp-2014-0273
Park, J.-S., Kim, S.-H., Han, K.-M., Kim, Y.-S., Kwon, E., Paek, S.-H., et al. (2022). Efficacy and safety evaluation of black ginseng (Panax ginseng C.A. Mey.) extract (CJ EnerG): broad spectrum cytotoxic activity in human cancer cell lines and 28-day repeated oral toxicity study in Sprague-Dawley rats. BMC Complement. Med. Ther. 22, 44. doi:10.1186/s12906-022-03522-3
Peng, X., Hao, M., Zhao, Y., Cai, Y., Chen, X., Chen, H., et al. (2021). Red ginseng has stronger anti-aging effects compared to ginseng possibly due to its regulation of oxidative stress and the gut microbiota. Phytomedicine 93, 153772. doi:10.1016/j.phymed.2021.153772
Peng, M., Wang, L., Su, H., Zhang, L., Yang, Y., Sun, L., et al. (2022). Ginsenoside Rg1 improved diabetes through regulating the intestinal microbiota in high-fat diet and streptozotocin-induced type 2 diabetes rats. J. Food Biochem. 46, e14321. doi:10.1111/jfbc.14321
Qu, Q., Yang, F., Zhao, C., Liu, X., Yang, P., Li, Z., et al. (2021). Effects of fermented ginseng on the gut microbiota and immunity of rats with antibiotic-associated diarrhea. J. Ethnopharmacol. 267, 113594. doi:10.1016/j.jep.2020.113594
Quan, L.-H., Zhang, C., Dong, M., Jiang, J., Xu, H., Yan, C., et al. (2020). Myristoleic acid produced by enterococci reduces obesity through brown adipose tissue activation. Gut 69, 1239–1247. doi:10.1136/gutjnl-2019-319114
Ran, X., Dou, D., Chen, H., and Ren, G. (2022). The correlations of adverse effect and tonifying effect of ginseng medicines. J. Ethnopharmacol. 291, 115113. doi:10.1016/j.jep.2022.115113
Ren, T., Liu, F., Wang, D., Li, B., Jiang, P., Li, J., et al. (2023a). Rhamnogalacturonan-I enriched pectin from steamed ginseng ameliorates lipid metabolism in type 2 diabetic rats via gut microbiota and AMPK pathway. J. Ethnopharmacol. 301, 115862. doi:10.1016/j.jep.2022.115862
Ren, T., Xu, M., Zhou, S., Ren, J., Li, B., Jiang, P., et al. (2023b). Structural characteristics of mixed pectin from ginseng berry and its anti-obesity effects by regulating the intestinal flora. Int. J. Biol. Macromol. 242, 124687. doi:10.1016/j.ijbiomac.2023.124687
Safaei, M., Sundararajan, E. A., Driss, M., Boulila, W., and Shapi’i, A. (2021). A systematic literature review on obesity: understanding the causes and consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 136, 104754. doi:10.1016/j.compbiomed.2021.104754
Santangelo, R., Silvestrini, A., and Mancuso, C. (2019). Ginsenosides, catechins, quercetin and gut microbiota: current evidence of challenging interactions. Food Chem. Toxicol. 123, 42–49. doi:10.1016/j.fct.2018.10.042
Sender, R., Fuchs, S., and Milo, R. (2016). Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14, e1002533. doi:10.1371/journal.pbio.1002533
Shankar, S., and Rosenbaum, J. (2020). Chronic diarrhoea in children: a practical algorithm-based approach. J. Paediatr. Child. Health 56, 1029–1038. doi:10.1111/jpc.14986
Sharma, B. R., Gautam, L. N. S., Adhikari, D., and Karki, R. (2017). A comprehensive review on chemical profiling of Nelumbo Nucifera: potential for drug development. Phytotherapy Res. 31, 3–26. doi:10.1002/ptr.5732
Shen, H., Gao, X.-J., Li, T., Jing, W.-H., Han, B.-L., Jia, Y.-M., et al. (2018). Ginseng polysaccharides enhanced ginsenoside Rb1 and microbial metabolites exposure through enhancing intestinal absorption and affecting gut microbial metabolism. J. Ethnopharmacol. 216, 47–56. doi:10.1016/j.jep.2018.01.021
Shin, Y.-J., Lee, D.-Y., Kim, J. Y., Heo, K., Shim, J.-J., Lee, J.-L., et al. (2023). Effect of fermented red ginseng on gut microbiota dysbiosis- or immobilization stress-induced anxiety, depression, and colitis in mice. J. Ginseng Res. 47, 255–264. doi:10.1016/j.jgr.2022.08.004
Su, F., Li, J., Xue, Y., Yu, B., Ye, S., Xu, L., et al. (2023). Early oral administration of Ginseng stem-leaf saponins enhances the peyer’s patch-dependent maternal IgA antibody response to a PEDV inactivated vaccine in mice, with Gut Microbiota involvement. Vaccines 11, 830. doi:10.3390/vaccines11040830
Sun, Y., Chen, S., Wei, R., Xie, X., Wang, C., Fan, S., et al. (2018). Metabolome and gut microbiota variation with long-term intake of Panax ginseng extracts on rats. Food Funct. 9, 3547–3556. doi:10.1039/c8fo00025e
Sun, R., Huang, W., Xiao, Y., Wang, D., Mu, G., Nan, H., et al. (2022). Shenlian (SL) decoction, a traditional Chinese medicine compound, May ameliorate blood glucose via mediating the gut Microbiota in db/db mice. J. Diabetes Res. 2022, 7802107–7802120. doi:10.1155/2022/7802107
Wan, Y., Yang, L., Li, H., Ren, H., Zhu, K., Dong, Z., et al. (2022). Zingiber officinale and Panax ginseng ameliorate ulcerative colitis in mice via modulating gut microbiota and its metabolites. J. Chromatogr. B 1203, 123313. doi:10.1016/j.jchromb.2022.123313
Wan, L., Qian, C., Yang, C., Peng, S., Dong, G., Cheng, P., et al. (2024). Ginseng polysaccharides ameliorate ulcerative colitis via regulating gut microbiota and tryptophan metabolism. Int. J. Biol. Macromol. 265, 130822. doi:10.1016/j.ijbiomac.2024.130822
Wang, J., Feng, W., Zhang, S., Chen, L., Tang, F., Sheng, Y., et al. (2019). Gut microbial modulation in the treatment of chemotherapy-induced diarrhea with Shenzhu Capsule. BMC Complement. Altern. Med. 19, 126. doi:10.1186/s12906-019-2548-y
Wang, N., Huang, X., Li, T., Wang, M., Yue, H., Chen, C., et al. (2020a). Application of RRLC-QTOF-MS-based metabonomics and UPE for investigating Spleen-Qi deficiency syndrome with Panax ginseng treatment. J. Ethnopharmacol. 256, 112822. doi:10.1016/j.jep.2020.112822
Wang, S., Long, S., Deng, Z., and Wu, W. (2020b). Positive role of Chinese herbal medicine in cancer immune regulation. Am. J. Chin. Med. 48, 1577–1592. doi:10.1142/s0192415x20500780
Wang, A., Pi, Z., Liu, S., Zheng, Z., Liu, Z., and Song, F. (2021). Mass spectrometry-based urinary metabolomics for exploring the treatment effects of Radix ginseng-schisandra chinensis herb pair on Alzheimer’s disease in rats. J Sep. Sci. 44, 3158–3166. doi:10.1002/jssc.202100061
Wang, J., Chen, P., Cao, Q., Wang, W., and Chang, X. (2022). Traditional Chinese medicine Ginseng Dingzhi Decoction ameliorates myocardial fibrosis and high glucose-induced cardiomyocyte injury by regulating intestinal flora and mitochondrial dysfunction. Oxidative Med. Cell. Longev. 2022, 9205908. doi:10.1155/2022/9205908
Wang, Y., Han, Q., Zhang, S., Xing, X., and Sun, X. (2023). New perspective on the immunomodulatory activity of ginsenosides: focus on effective therapies for post-COVID-19. Biomed. and Pharmacother. 165, 115154. doi:10.1016/j.biopha.2023.115154
Wu, Y., Zheng, Y., Wang, X., Tang, P., Guo, W., Ma, H., et al. (2023). Ginseng-Containing sijunzi decoction ameliorates ulcerative colitis by orchestrating gut homeostasis in microbial modulation and intestinal barrier integrity. Am. J. Chin. Med. 51, 677–699. doi:10.1142/s0192415x23500325
Xia, T., Fang, B., Kang, C., Zhao, Y., Qiang, X., Zhang, X., et al. (2022). Hepatoprotective mechanism of ginsenoside Rg1 against alcoholic liver damage based on Gut Microbiota and network pharmacology. Oxidative Med. Cell. Longev. 2022, 5025237. doi:10.1155/2022/5025237
Xie, N.-N., Wu, C.-Y., Ge, Q., Zhou, J., Long, F., Mao, Q., et al. (2023). Structure-specific antitumor effects and potential gut microbiota-involved mechanisms of ginseng polysaccharides on B16F10 melanoma-bearing mice. Food Funct. 14, 796–809. doi:10.1039/d2fo03383f
Xiong, W., Zhao, X., Xu, Q., Wei, G., Zhang, L., Fan, Y., et al. (2022). Qisheng Wan formula ameliorates cognitive impairment of Alzheimer’s disease rat via inflammation inhibition and intestinal microbiota regulation. J. Ethnopharmacol. 282, 114598. doi:10.1016/j.jep.2021.114598
Xu, J., Li, T., Xia, X., Fu, C., Wang, X., and Zhao, Y. (2020). Dietary ginsenoside T19 supplementation regulates glucose and lipid metabolism via AMPK and PI3K pathways and its effect on intestinal microbiota. J. Agric. Food Chem. 68, 14452–14462. doi:10.1021/acs.jafc.0c04429
Xu, H.-Y., Li, Q.-C., Zhou, W.-J., Zhang, H.-B., Chen, Z.-X., Peng, N., et al. (2023). Anti-Oxidative and anti-aging effects of probiotic fermented ginseng by modulating Gut Microbiota and metabolites in Caenorhabditis elegans. Plant Foods Hum. Nutr. 78, 320–328. doi:10.1007/s11130-023-01055-9
Yang, Y., Hu, N., Gao, X.-J., Li, T., Yan, Z.-X., Wang, P.-P., et al. (2021). Dextran sulfate sodium-induced colitis and ginseng intervention altered oral pharmacokinetics of cyclosporine A in rats. J. Ethnopharmacol. 265, 113251. doi:10.1016/j.jep.2020.113251
Yousuf, S., Liu, H., Yingshu, Z., Zahid, D., Ghayas, H., Li, M., et al. (2022). Ginsenoside Rg1 modulates intestinal microbiota and supports re-generation of immune cells in dexamethasone-treated mice. AMicr 69, 259–269. doi:10.1556/030.2022.01881
Zeng, Z., Wang, H., Yi, R., Lou, J., Wen, S., and Hu, Z. (2023). Gut microbiome and metabolome in aneurysm rat with hypertension after ginsenoside Rb1 treatment. Front. Pharmacol. 14, 1287711. doi:10.3389/fphar.2023.1287711
Zhang, M., Wang, Y., Wu, Y., Li, F., Han, M., Dai, Y., et al. (2021). In vitro transformation of protopanaxadiol saponins in human intestinal flora and its effect on intestinal flora. Evidence-Based Complementary Altern. Med. 2021, 1735803–1735810. doi:10.1155/2021/1735803
Zhang, M., Pi, Y., Ma, L., Li, F., Luo, J., Cai, Y., et al. (2023a). Effects of ginseng on short-chain fatty acids and intestinal microbiota in rats with spleen-qi deficiency based on gas chromatography–mass spectrometry and 16s rRNA technology. Rapid Comm. Mass Spectrom. 37, e9640. doi:10.1002/rcm.9640
Zhang, X., Wang, X., Shi, R., Ran, X., He, X., and Dou, D. (2023b). Effective substances and mechanism of red ginseng on rats with spleen-deficiency syndrome based on the substance and energy metabolism as well as the “brain-gut” axis. J. Ethnopharmacol. 311, 116438. doi:10.1016/j.jep.2023.116438
Zhao, C., Qu, Q., Yang, F., Li, Z., Yang, P., Han, L., et al. (2021). Monascus ruber fermented Panax ginseng ameliorates lipid metabolism disorders and modulate gut microbiota in rats fed a high-fat diet. J. Ethnopharmacol. 278, 114300. doi:10.1016/j.jep.2021.114300
Zhao, M., Zhao, Q., Guan, Z., Liu, Q., Zhou, H., Huang, Q., et al. (2023). Effect of Panax ginseng and Fructus Mume on intestinal barrier and gut Microbiota in rats with diarrhea. J. Med. Food 26, 165–175. doi:10.1089/jmf.2022.k.0069
Zheng, J., Chen, M., Ye, C., Sun, X., Jiang, N., Zou, X., et al. (2020). BuZangTongLuo decoction improved hindlimb ischemia by activating angiogenesis and regulating gut microbiota in diabetic mice. J. Ethnopharmacol. 248, 112330. doi:10.1016/j.jep.2019.112330
Zheng, F., Zhang, M., Wu, Y., Wang, Y., Li, F., Han, M., et al. (2021). Biotransformation of ginsenosides (Rb1, Rb2, Rb3, Rc) in human intestinal bacteria and its effect on intestinal flora. Chem. and Biodivers. 18, e2100296. doi:10.1002/cbdv.202100296
Zhou, S.-S., Zhou, J., Xu, J.-D., Shen, H., Kong, M., Yip, K.-M., et al. (2021). Ginseng ameliorates exercise-induced fatigue potentially by regulating the gut microbiota. Food Funct. 12, 3954–3964. doi:10.1039/d0fo03384g
Zhou, X., Guo, Y., Yang, K., Liu, P., and Wang, J. (2022). The signaling pathways of traditional Chinese medicine in promoting diabetic wound healing. J. Ethnopharmacol. 282, 114662. doi:10.1016/j.jep.2021.114662
Zhou, R., He, D., Zhang, H., Xie, J., Zhang, S., Tian, X., et al. (2023). Ginsenoside Rb1 protects against diabetes-associated metabolic disorders in Kkay mice by reshaping gut microbiota and fecal metabolic profiles. J. Ethnopharmacol. 303, 115997. doi:10.1016/j.jep.2022.115997
Zinöcker, M., and Lindseth, I. (2018). The Western Diet–Microbiome-Host interaction and its role in Metabolic disease. Nutrients 10, 365. doi:10.3390/nu10030365
Glossary
AAD antibiotic-associated diarrhea
AChE acetylcholinesterase
AD atopic dermatitis
AGM combination of freeze-dried aronia
AKI acute kidney injury
AMO Atractylodes macrocephala
AMP adenosine monophosphate
AS atherosclerosis
ASD Alzheimer’s disease
BG black ginseng
BHRS Baihu Renshen decoction
BZTLD Bu Zang Tong Luo decoction
CAT catalase
CK compound K
DJZT Dajianzhong decoction
DSS dextran sulfate sodium salt
FAD fatty acid diet
FG fermented ginseng
FRG fermented red ginseng
FST forced swimming
GABAA gamma-aminobutyrate A
GG garden ginseng
GF ginseng under forest
GL ginseng leaves
GM gut microbiota
GN Ginseng Dingzhi decoction
GPS Ginseng pectin
GSLS ginsenosides from stems and leaves
HPA hypothalamus–pituitary–adrenal
HPLC-TQ-MS high-performance liquid chromatography coupled with triple quadrupole mass spectrometry
IBD inflammatory bowel disease
IG immunoglobulin
IL interleukin
LY Liangyi paste
MA myristic acid
MDA malondialdehyde
MG mammary gland
MGBA microbiome–gut–brain axis
NGP non-fumigated ginseng
PD-1/PD-L1 potentiating the antitumor effect of antiprogrammed cell death 1/programmed cell death ligand 1
PE ginseng extract
PF Panax ginseng and Fructus Mume
PG Panax ginseng C. A. Mey.
PPD protopanaxadiol, precancerous lesions of gastric cancer
PPS Peyer’s plaques
PPT protopanaxatriol
PTP-1B protein tyrosine phosphatase 1B
QYG Qiong-Yu-Gao
RG red ginseng
RS Radix ginseng-Schisandra chinensis
RSGB Renshen Guben prescription
SCFA short-chain fatty acid
SGP sulfur-fumigated ginseng
SJZT Sijunzi decoction
SL Coptis chinensis and Panax ginseng
SOD superoxide dismutase
STZ streptozotocin
SZC RenShenbaizhu capsule
TCM traditional Chinese medicine
T2DM type 2 diabetic
TLR4 toll-like receptor 4
TNF-α tumor necrosis factor-α
TST tail hanging tasks
UC ulcerative colitis
UCDF ulcerative colitis and depression
VN Veratrum nigrum
WEG water extract of ginseng
WFC Weifuchun capsule
5-HT 5-hydroxytryptamine
Keywords: medicinal food plant, Panax ginseng C. A. Mey., gut microbiota, pharmacology, plant metabolites, toxicology
Citation: Gao M, Wang H, Chen X, Wang W and Liu Y (2025) The potential of medicinal food plant Panax ginseng C. A. Mey. in managing chronic diseases via gut microbiota regulation: a systematic review of mechanisms and evidence. Front. Pharmacol. 16:1650565. doi: 10.3389/fphar.2025.1650565
Received: 20 June 2025; Accepted: 30 July 2025;
Published: 28 August 2025; Corrected: 17 September 2025.
Edited by:
Claudio Ferrante, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Tao Yi, Hong Kong Baptist University, Hong Kong SAR, ChinaMaria Loreta Libero, University “G. d’Annunzio” of Chieti-Pescara, Italy
Copyright © 2025 Gao, Wang, Chen, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongmei Liu, bHltZWkxNjhAMTI2LmNvbQ==; Wensheng Wang, MTE2MzYxNDgwMEBxcS5jb20=
 Meng Gao
Meng Gao Haijing Wang2
Haijing Wang2