Abstract
Patient-derived airway cell cultures are used in personalized medicine strategies for people with cystic fibrosis (pwCF) to predict potential clinical response to cystic fibrosis transmembrane conductance regulator (CFTR) modulator drugs. While bronchial epithelial cells from lung explants (HBEx) are the gold standard for CFTR functional measurements, nasal epithelial cells (HNE) are a more practical tissue source resulting in widespread use for preclinical functional platforms. HNE have so far not been rigorously validated against the gold standard for this purpose. In this study, we collected nasal and bronchial cells, and lung explants from pwCF undergoing lung transplantation as well as non-CF controls. Comparative studies in non-CF cells showed that while CFTR-mediated transepithelial currents in HNE underestimated those in HBEx, the magnitude of the CFTR modulator response was similar between CF HNE, brushed HBE (HBEb), and HBEx with significant correlation between matched HNE and HBEb from 16 pwCF. These findings confirm use of HNE as surrogate of bronchial airway for preclinical drug testing with report of drug responses in relation to the tissue-specific non-CF or baseline controls rather than as absolute results. Furthermore, CF centres offering HNE-based drug testing utilize different techniques, challenging the comparison of results between centres. We show how culture media, use of fresh or freeze-thawed cells as well as difference in Ussing technique impact the magnitude of measured CFTR function, which is why we suggest diligence in reporting of these factors when presenting CFTR modulator drug response results.
Introduction
Cystic fibrosis transmembrane conductance regulator (CFTR) modulator drugs improve CFTR function by targeting defective CFTR protein to improve cellular trafficking and stabilization of channel expression at the apical membrane (corrector drugs) (Van Goor et al., 2011; Veit et al., 2020) as well as promote CFTR channel opening (potentiator drugs) (Van Goor et al., 2009). CFTR modulator drugs have dramatically changed the trajectory of CF disease by significantly improving lung disease and nutrition, as well as reducing sweat chloride concentration as in vivo proof of restored CFTR function in people with CF (pwCF) (Ramsey et al., 2011; Wainwright et al., 2015; Keating et al., 2018; Heijerman et al., 2019). Heterogeneity in clinical response ranges from those who respond very well to those who do not respond. This can be explained by differences in CFTR genotypes but has also been observed among pwCF carrying the same CFTR genotypes which can be a result of other genetic factors, individual pharmacokinetics, drug adherence, etc. (Wainwright et al., 2015; Keating et al., 2018). Furthermore, a subset of pwCF are currently ineligible for CFTR modulator therapies as they either a) do not carry gene variants amenable to current CFTR modulator therapy or b) carry rare CFTR gene variants which were not included in clinical trials or secondary functional studies (Raraigh et al., 2022) and are therefore still excluded from these therapies. Addressing these challenges, preclinical platforms using patient-derived airway or intestinal tissues have been established worldwide to help predict potential clinical drug response and to guide/advocate for its clinical use at an individual level (Burgel et al., 2023).
One way to assess potential drug response is by comparing in vitro CFTR function at baseline and after drug treatment in patient-derived tissue where CFTR function can be measured as changes in transepithelial electrical currents with pharmacological activation and inhibition. So far, human bronchial epithelial cells derived from lung explants (HBEx) have constituted the gold standard for these measurements (Van Goor et al., 2006; 2009; Keating et al., 2018). Human nasal epithelial cells (HNE) derived from nasal brushes of pwCF are now commonly used as a HBEx/brushed HBE (HBEb) surrogate as they allow relatively non-invasive and repeatable collection. While HNE have been shown to be a good in vitro model to predict clinical CFTR modulator response for pwCF (Pranke et al., 2017; Brewington et al., 2018; Anderson et al., 2021; Noel et al., 2022), there is limited understanding of how they compare to the performance of HBE cells. Our group reported that cultured HNE and HBEb showed similar expression levels in over 90% of genes, including CFTR (He et al., 2022). However, there has been no direct comparison of CFTR function and drug response assessment between HBEx as gold standard airway model, HBEb, and HNE, leaving a knowledge gap regarding the extent with which CFTR-mediated electrical currents measured in HNE reflect those measured in HBE.
Furthermore, centres offering individual pre-clinical CF drug testing vary in cell culture and CFTR functional measurement techniques, while struggling to find ubiquitously valid cut-off levels of drug response to help guide a more global approach for CFTR modulator use in those with rare CFTR genotypes. Providing this guidance is important as some jurisdictions, including the United States (Durmowicz et al., 2018) and France (Burgel et al., 2023), have programs which allow patients to trial CFTR modulators when certain CFTR functional thresholds are met in vitro tests. We aimed to interrogate the quantity and quality of HNE-measured drug responses in comparison to HBEx to support and validate this global approach. Additionally, we evaluated the impact of different cell culturing and measurements techniques on the magnitude of CFTR function measurements to help interpretation of drug response results among different assessment centres.
Methods
Patients
PwCF post lung transplantation attending routine clinical follow-up visits for lung rejection surveillance using bronchoscopy were recruited for this study. The study was approved by the Research Ethics Boards of the Hospital for Sick Children (REB# 1000044783) and the Toronto General Hospital (REB#15–8936). All study subjects and guardians of study subjects with insufficient capacity signed consent forms.
Bronchial explant cultures
Bronchial tissue from non-CF patients and pwCF was collected during lung transplantation and sent to Phil Karp to the Cell Culture Facility at the University of Iowa, thanks to a collaboration between Dr. J Zabner (University of Iowa) and Dr. S Keshavjee (University of Toronto). Bronchial tissue was used to generate HBEx cell cultures, as previously described (Karp et al., 2002). Briefly, cells were expanded in growth media containing bronchial epithelial cell growth media and 2% Ultroser G (USG media). HBEx were seeded onto transwell inserts (Costar 3,470, 6.5 mm diameter, 0.4 μm pore size; Corning) for 2 weeks at air liquid interface (ALI) in Iowa after which they were sent to our lab at the Hospital for Sick Children. Once received, HBEx cultures were incubated in one of four ALI media for 10–14 days before electrophysiological studies: (1) USG media (Karp et al., 2002), (2) UNC media (Randell et al., 2011) (a gift from Scott Randell, UNC), (3) Pneumacult ALI (PC; StemCell Technologies, Vancouver, BC, CA), or (4) Vertex media + USG (VALI) media (Neuberger et al., 2011). The comparisons between HBEx, HBEb and HNE only included HBEx grown in PC ALI media, unless otherwise stated.
Nasal and bronchial brushing and culturing
At routine surveillance post-transplant bronchoscopy, a bronchoscopic cytology brush was used to brush the bronchial airway lumen proximal to the anastomosis by the attending Respirologist. In the same setting, nasal brushing was performed by a research nurse or Respirologist using a 3-mm diameter cytology brush.
Bronchial and nasal brushes were processed similarly, as described previously for nasal cells (Terrance et al., 2024). Briefly, cells were dissociated from the brush and seeded on a collagen-coated (PureCol, Advanced Biomatrix) flask (T25 for HNE and T75 for HBEb) in expansion media (Pneumacult Ex, StemCell Technologies) with antibiotics and rho kinase inhibitor (5 µM). At passage 2, cells were seeded on collagen-IV coated Transwell inserts (6.5 mm diameter, 0.4 µm pore size, Corning) at a density of 1x105 cells per insert. Once confluent, cells were cultured at an air liquid interface (ALI) with basal differentiation media (PneumaCult ALI, StemCell Technologies) for 14 days before electrophysiological studies.
For cryopreservation, after passaging cells were resuspended in a freezing media consisting of 70% expansion media, 20% FBS (Gibco), and 10% DMSO. Cryovials were transferred to a Nalgene freezing container (“Mr Frosty”) and placed in a −80°C freezer overnight then to liquid Nitrogen storage the following day for long-term storage.
Electrophysiological studies
Electrophysiological measurements were performed using a circulating Ussing chamber system (EM-CSYS-4; Physiologic Instruments) in open-circuit mode as previously described (Laselva et al., 2021) with symmetrical buffer solutions containing (in mM): 126 NaCl, 24 NaHCO3, 2.13 K2HPO4, 0.38 KH2PO4, 1 MgSO4, 1 CaCl2 and 10 glucose. Where indicated, short-circuit measurements were performed with a low chloride buffer in the apical side containing (in mM): 1.2 NaCl, 115 mM Na-gluconate, 1.24 K2HPO4, 2.4 KH2PO4, 1 MgCl2, 1 CaCl2 25 NaHCO3, 10 glucose. Following inhibition of the epithelial sodium channel (ENaC) with amiloride (100 µM, Spectrum Chemical, Gardena, CA), CFTR function was measured using forskolin stimulation (Fsk, 10 μM, Sigma-Aldrich, United States of America) and CFTRinh172 inhibition (CFTRinh, 10 μM, EMD Millipore Corp. United States of America). CFTR function is presented as difference in calculated transepithelial current where ΔIeq Fsk is indicative of CFTR activation in response to cAMP stimulation and ΔIeq CFTRinh is indictive of current directly attributed to CFTR in response to CFTR inhibition.
CFTR modulator drug treatment
For drug studies, airway cultures were incubated for 48 h prior to electrophysiological studies with VX-809 (CFTR corrector, Lumacaftor, 3µM, Selleckchem) and/or PTI-428 (CFTR amplifier, nesolicaftor, 10 µM, Proteostasis Therapeutics) or 0.1% DMSO. VX-770 (CFTR potentiator, Ivacaftor, 1 µM, Selleckchem) was added acutely during Ussing chamber experiments.
Statistical analysis
Individual experimental data are summarized as mean ± SD as they distribute normally. Statistical significance was determined using paired t-test, one-way or two-way ANOVA followed by Tukey’s post hoc test, using a significance level of 0.05. Pearson’s correlation was used for correlation analysis. All analyses were performed using GraphPad Prism 10.4.1.
Results
Sample description
HNE, HBEb, and HBEx were collected from 29 pwCF, 26 of which carried F508del/F508del. Table 1 lists the individual pwCF with successfully grown HNE, HBEb and HBEx cultures. Although we set out to compare CFTR modulator response in HNE, HBEb and HBEx from each participant, culture failures, initial differences in culture media, and time delays in HNE/HBEb collection of up to 2 years after transplant limited complete individual matching. Comparisons between HNE, HBEb, and HBEx were thus made in an unpaired fashion and included only HBEx differentiated in PneumaCult (PC) ALI media to match the media used for HNE and HBEb. However, for 16 pwCF we have paired data from HNE and HBEb.
TABLE 1
| Subject | Age at Tx | Gender | Genotype | Nasal cell | Bronchial brush | Bronchial explant |
|---|---|---|---|---|---|---|
| 1 | 19 | F | ∆F508/∆F508 | ✓ | ✓ (non-CF) | ✓ |
| 2 | 20 | M | ∆F508/∆F508 | ✓ | ✓ | |
| 3 | 19 | F | ∆F508/∆F508 | ✓ | ✓ | |
| 4 | 26 | M | ∆F508/∆F508 | ✓ | ✓ | ✓ |
| 5 | 25 | F | ∆F508/∆F508 | ✓ | ✓ | ✓ |
| 6 | 29 | F | ∆F508/c.2052dupA | ✓ | ✓ | ✓ |
| 7 | 40 | F | ∆F508/∆F508 | ✓ | ✓ (non-CF) | ✓ |
| 8 | 35 | M | ∆F508/∆F508 | ✓ | ✓ | ✓ |
| 9 | 18 | F | ∆F508/∆1507 | ✓ | ✓ | |
| 10 | 41 | M | ∆F508/∆F508 | ✓ | ✓ | |
| 11 | 27 | M | ∆F508/∆F508 | ✓ | ✓ | ✓ |
| 12 | 34 | M | ∆F508/∆F508 | ✓ | ✓ | |
| 13 | 28 | M | ∆F508/∆F508 | ✓ | ✓ | ✓ |
| 14 | 28 | M | ∆F508/∆F508 | ✓ | ||
| 15 | 24 | F | ∆F508/∆F508 | ✓ | ✓ | ✓ |
| 16 | 22 | M | ∆F508/∆F508 | ✓ | ✓ | ✓ |
| 17 | 38 | M | ∆F508/∆F508 | ✓ | ✓ | ✓ |
| 18 | 24 | M | ∆F508/∆F508 | ✓ | ✓ | ✓ |
| 19 | 31 | F | G85E/G85E | ✓ (non-CF) | ||
| 20 | 25 | F | ∆F508/∆F508 | ✓ | ✓ | ✓ |
| 21 | 23 | M | ∆F508/∆F508 | ✓ | ✓ | ✓ |
| 22 | 27 | M | ∆F508/∆F508 | ✓ | ✓ | ✓ |
| 23 | 36 | F | ∆F508/∆F508 | ✓ (non-CF) | ✓ | |
| 24 | 30 | M | ∆F508/∆F508 | ✓ | ||
| 25 | 25 | F | ∆F508/∆F508 | ✓ (non-CF) | ✓ | |
| 26 | 37 | M | ∆F508/∆F508 | ✓ | ✓ | |
| 27 | 24 | F | ∆F508/∆F508 | ✓ | ✓ | ✓ |
| 28 | 34 | F | ∆F508/∆F508 | ✓ | ✓ | |
| 29 | 14 | M | M1101K/M1101K | ✓ | ✓ |
Demographics of CF subjects.
HNE and HBEx were collected from 19 to 16 non-CF subjects, respectively. We received HBEx from non-CF lung transplants and HBEb from bronchoscopies of pwCF post-lung transplantation with accidental bronchial brushings distal to the bronchial anastomosis from the newly transplanted non-CF lung. There was no individual matching between collected non-CF tissues.
Ieq is reduced in HNE compared to HBEx in non-CF
Transepithelial electrophysiological properties were evaluated in non-CF airway cultures using open-circuit Ussing chamber studies. While the transepithelial resistance at baseline (Rte baseline, Ωcm2) was comparable between HNE, HBEb, and HBEx (Figure 1A), the baseline (Ieq baseline, µA/cm2) and amiloride-sensitive current (ΔIeq amiloride) were significantly lower in HNE compared to HBEx (p < 0.0001, Figures 1B,C). Similarly, HNE showed a 2.7- and 1.9-fold reduction in the magnitude of ΔIeq Fsk and ΔIeq CFTRinh as measure of CFTR function, respectively, when compared to HBEx (p < 0.0001 and p = 0.0012, respectively) and were reduced, but not statistically significantly different from HBEb (Figures 1D,E).
FIGURE 1
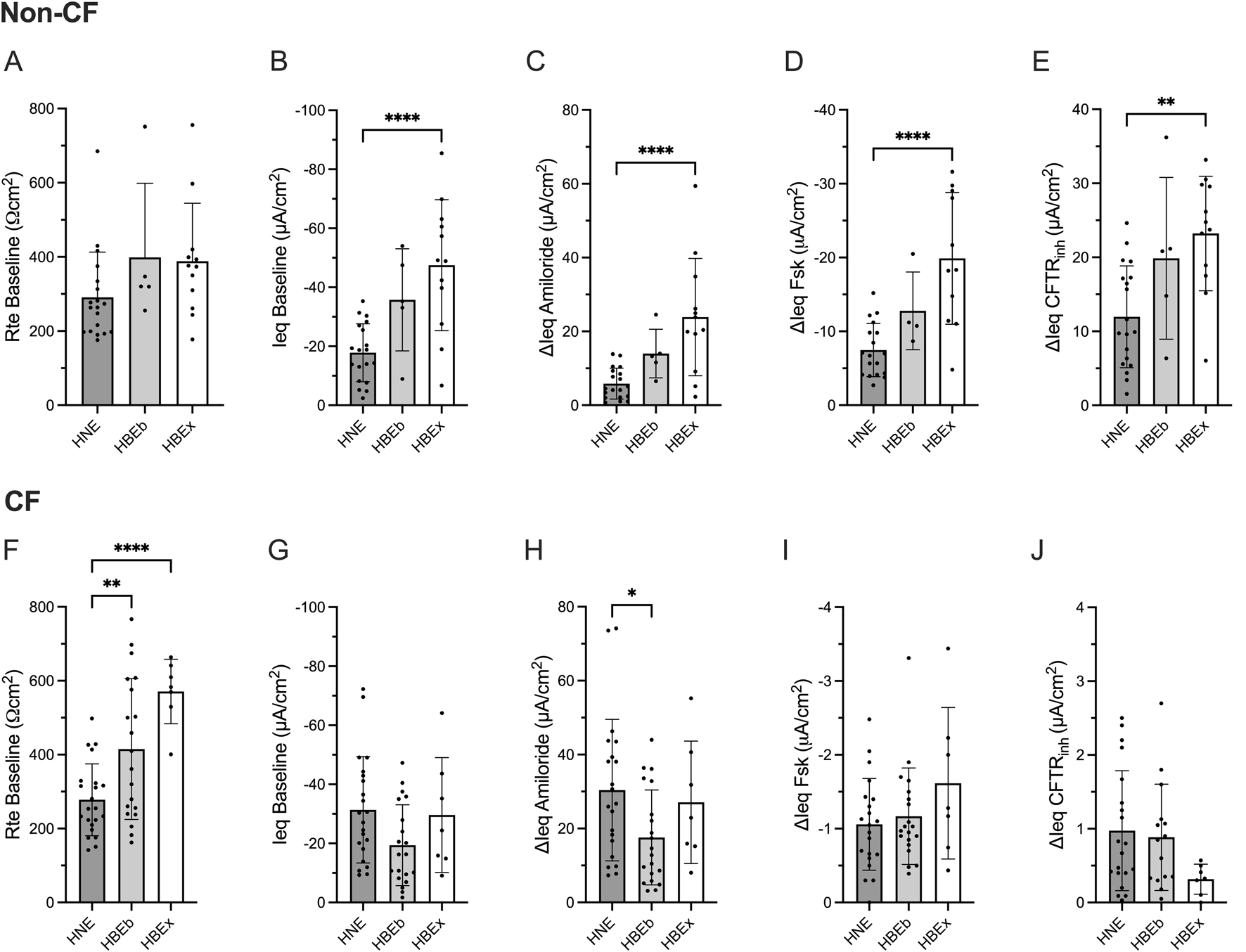
Compared to HBE, non-CF HNE have reduced bioelectric properties, while CF HNE have decreased baseline resistance and increased ENaC activity. Bar graphs with mean ± SD summarizes the transepithelial electrical results from measurements performed in cultures derived from nasal brush (HNE; non-CF, n = 19; CF, n = 22), bronchial brush (HBEb; non-CF, n = 5; CF, n = 20), and bronchial explant (HBEx; non-CF, n = 12; CF, n = 7) grown in Pneumacult ALI media. Each dot represents a single experiment from a single subject, n values provided are indicative of biological replicates. (A,F) Baseline transepithelial resistance (Rte baseline, Ωcm2), (B,G) Baseline transepithelial current (Ieq baseline, µA/cm2), and change in transepithelial current with (C,H) Amiloride (ΔIeq amiloride, µA/cm2), (D,I) Forskolin (ΔIeq Fsk, µA/cm2) and (E,J) CFTRinh172 response (ΔIeq CFTRinh, µA/cm2). Statistical analysis by one way ANOVA: Tukey’s multiple comparison test, *P < 0.05, **P < 0.01, ****P < 0.0001.
Rte baseline and Ieq amiloride is reduced in HNE compared to HBEx in CF
We next examined transepithelial electrophysiological properties in cultures derived from pwCF. CF HNE showed significantly lower Rte baseline compared to HBEb (p = 0.009) and HBEx (p < 0.0001) (Figure 1F). Additionally, ΔIeq amiloride was significantly greater in HNE compared to HBEb (p = 0.042) but not significantly different from HBEx (Figure 1H), and baseline Ieq was similar between airway cultures (Figure 1G). CFTR activity, assessed as ΔIeq Fsk and ΔIeq CFTRinh was minimal, as expected in CF cells, and similar between airway cultures (Figures 1E,J).
These differences were also observed when including HBEx cultures grown with different ALI media (Supplementary Figure S1).
The magnitude of responses to CFTR modulator drugs in CF cells is comparable between nasal and bronchial cells
Next, we assessed the response to CFTR modulator drugs in HNE, HBEb, and HBEx collected from pwCF. Cells were treated with increasingly potent CFTR modulator combinations (1) VX-809 (Lumacaftor, a CFTR corrector), (2) VX-809 and VX-770 (Ivacaftor, CFTR potentiator), or (3) VX-809, VX-770 and PTI-428 (nesolicaftor, CFTR amplifier). Increasingly efficacious CFTR modulator drug combinations produced stepwise increases in CFTR function measured as ΔIeq Fsk and ΔIeq CFTRinh. The functional response to VX-809 alone treatment was only minimal, though associates with increased CFTR protein, as shown previously (Molinski et al., 2017). We observed higher response magnitudes with VX-809+VX-770 and then VX-809+PTI-428+VX-770 (Figures 2A,B). The same patterns were seen when including HBEx grown in different ALI media (Supplementary Figure S2). Importantly, the magnitude of the CFTR response for each drug condition was comparable between HNE, HBEb, and HBEx.
FIGURE 2
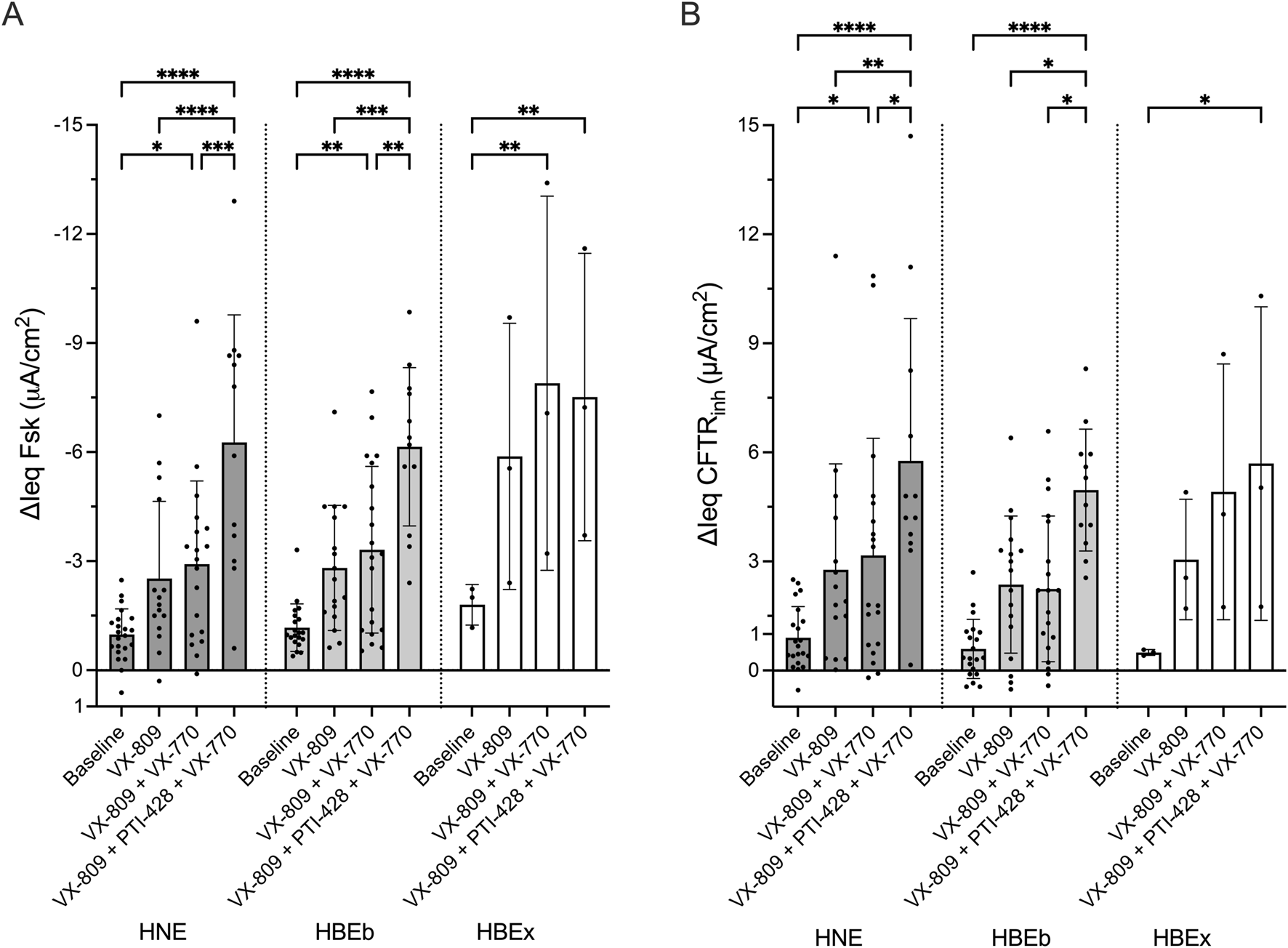
In CF cells, CFTR modulator drug response in HNE is comparable to HBEb and HBEx. Bar graphs with mean ± SD summarizes changes in transepithelial current with (A) forskolin-stimulation (ΔIeq Fsk, µA/cm2) and (B) CFTRinh-172 inhibition (ΔIeq CFTRinh, µA/cm2) in CF cells derived from nasal brush (HNE, n = 12–19), bronchial brush (HBEb, n = 12–20), and bronchial explant (HBEx, n = 3) cells grown in Pneumacult ALI media. Cells were untreated (baseline) or received 48-h incubation with VX-809, VX-809 with acute VX-770 (VX-809 + VX-770) or VX-809 and PTI-428 with acute VX-770 (VX-809 + PTI-428 + VX-770). Each dot represents a single experiment from a single subject, n values provided are indicative of biological replicates. Statistical analysis by two-way ANOVA: Tukey’s multiple comparison test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Interindividual differences in CFTR modulator drug response is similar between nasal and bronchial cells
We were also interested in examining whether CFTR modulator drug response in HNE identifies drug responders and non-responders in a similar fashion as HBEb or HBEx. Figures 3A and B show drug responses to the CFTR drug modulator combinations, listed above, in up to 7 individual pwCF using HBEx cultured in any ALI media. Visually we observed good agreement in responses to CFTR modulator drugs between individual pwCF and different airway cells. This agreement suggests that HNE allow for similar interindividual stratification into good and poor response compared to the gold standard HBEx. We observed greater heterogeneity between airway cultures in ΔIeq Fsk compared to ΔIeq CFTRinh (Figures 3A,B). This is likely due to differences cAMP levels prior to forskolin stimulation and thus associated differences in the constitutive currents of the culture. In contrast CFTRinh is applied after maximal CFTR activation and thus captures the sum of constitutive and stimulated CFTR-mediated currents. (Supplementary Figure S3). Lastly, we saw good correlation in both ΔIeq Fsk (r = 0.57, p < 0.0001) and ΔIeq CFTRinh (r = 0.64, p < 0.0001), in CFTR modulator treated matched HNE and HBEb from 16 pwCF (Figures 3C,D). Matched data from HNE and HBEx cultures were only available for six individuals, five of which were cultured in differing media, which likely explains that CFTR function did not correlate between these (data not shown).
FIGURE 3
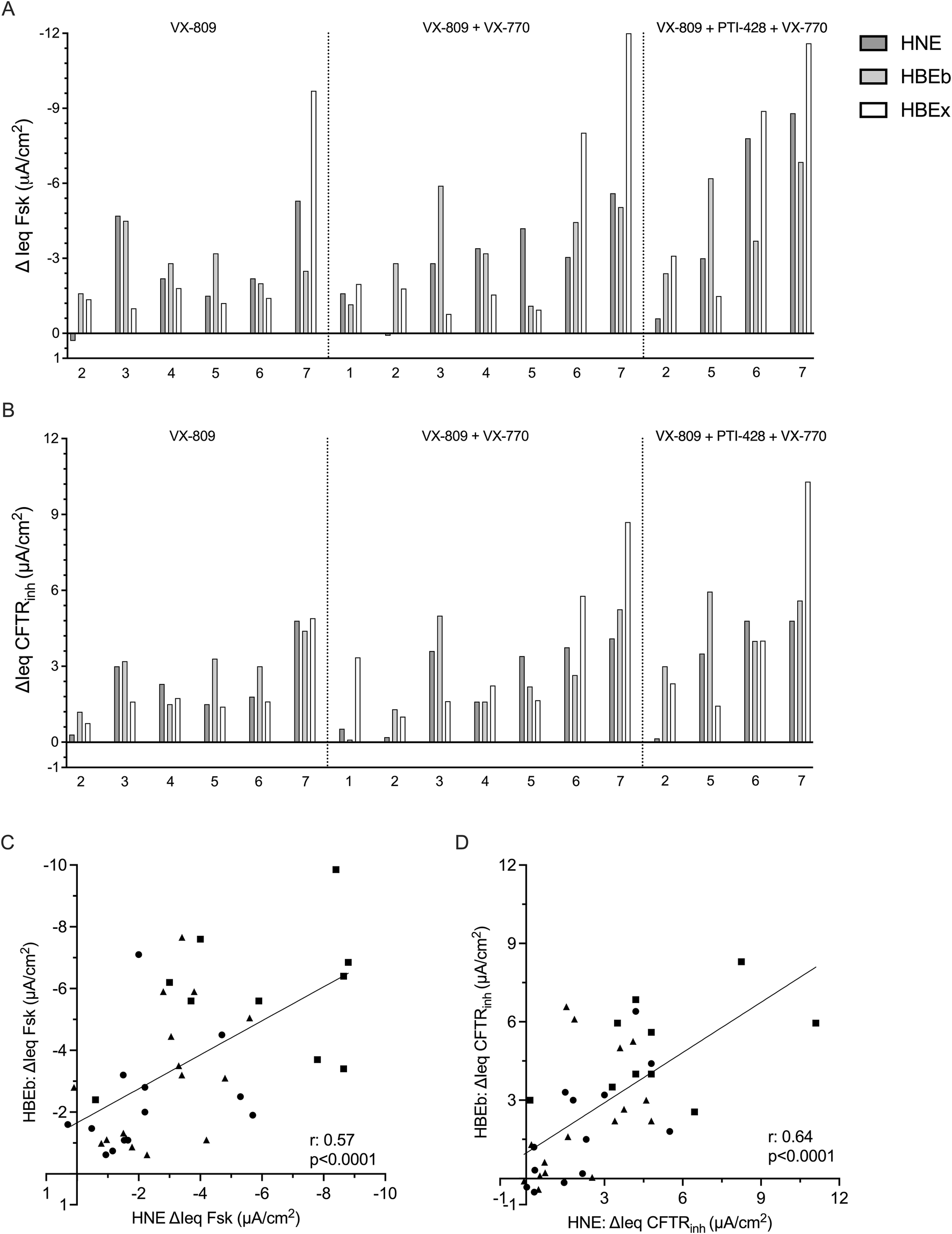
Interindividual variability in response to CFTR modulator drugs is comparable between HNE and HBE. Bar graphs showing the change in transepithelial current with (A) forskolin-stimulation (ΔIeq Fsk, µA/cm2) and (B) CFTRinh-172 inhibition (ΔIeq CFTRinh, µA/cm2) HNE, HBEb, and HBEx with CFTR modulator drug treatment. Cells received 48-h incubation with VX-809, VX-809 with acute VX-770 (VX-809 + VX-770) or VX-809 and PTI-428 with acute VX-770 (VX-809 + PTI-428 + VX-770). Each bar represents HNE, HBEb, or HBEx from a single individual. Graphs showing correlation of (C) ΔIeq Fsk and (D) ΔIeq CFTRinh between HBEb (y-axis) and HNE (x-axis) cultures with CFTR modulator treatment (n = 16). Circles: VX-809; Triangles: VX-809 + VX-770; Squares: VX-809 + PTI-428 + VX-770. Statistical analysis by simple linear regression and Pearson’s correlation.
Technical aspects of cell culturing impact CFTR functional read-outs
Since pre-clinical HNE drug testing protocols differ among CF centres worldwide, we wanted to interrogate the impact of some key technical differences on the magnitude of the measured and reported CFTR function.
First, we evaluated the impact of ALI differentiation media on the CFTR functional read-out. We exposed HBEx cultures derived from non-CF and CF lung explants to commonly used ALI differentiation media including Ultroser G (USG), UNC, Pneumacult (PC) and VALI for 10-14 days prior to electrophysiology studies.
In non-CF HBEx, transepithelial electrophysiological properties significantly differed between ALI media (Figures 4A–E). Non-CF HBEx grown with USG and UNC ALI media showed higher Rte baseline than PC and VALI HBEx cultures while ΔIeq Amiloride was greatest in VALI followed by USG compared to UNC and PC HBEx cultures. Measured CFTR-mediated currents were significantly larger in PC and VALI HBEx cultures (ΔIeq Fsk of −22.20 ± 7.87 and −22.19 ± 7.93 μA/cm2, respectively) compared to UNC (−12.72 ± 8.73 μA/cm2, vs. PC p = 0.0067 and vs. VALI p = 0.03) and USG (−3.22 ± 2.02 μA/cm2, vs. PC p < 0.0001 and vs. VALI p < 0.0001). Interestingly, HBEx grown in UNC and USG showed greater ΔIeq CFTRinh than ΔIeq Fsk, a consequence of larger constitutive CFTR-mediated currents in comparison to PC and VALI grown cultures. This illustrates that comparison of CFTR function relying solely on ΔIeq Fsk may underestimate total CFTR function depending on ALI media. In HBEx from pwCF, ALI media impacted Rte baseline and ΔIeqs in the same way as non-CF HBEx. Residual measured CFTR currents, though overall low, were also greatest in PC and VALI cultures.
FIGURE 4
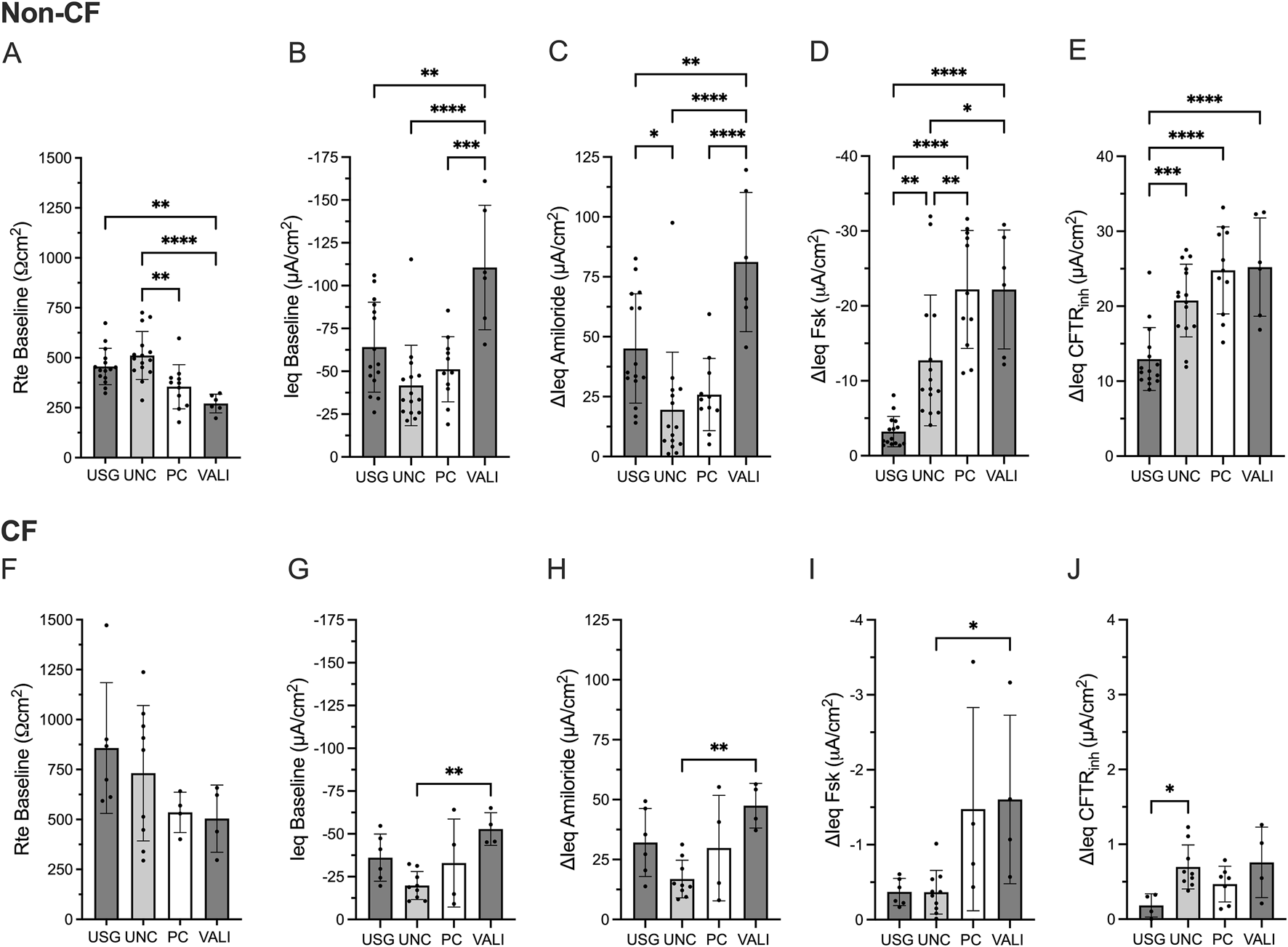
Transepithelial electrical properties in non-CF and CF bronchial explant cultures differ based on ALI media. Bar graphs with mean ± SD summarizes the transepithelial electrical results from measurements performed in non-CF (A–E) HBEx in USG (n = 15), UNC (n = 15), PC (n = 11) or VALI (n = 6) ALI media and (F–J) CF HBEx in USG (n = 6), UNC (n = 9), PC (n = 4) or VALI (n = 4) ALI media. Each dot represents a single experiment from a single subject, n values provided are indicative of biological replicates. (A,F) Baseline transepithelial resistance (Rte baseline, Ωcm2), (B,G) baseline transepithelial current (Ieq baseline, µA/cm2), and change in transepithelial current with (C,H) Amiloride (ΔIeq amiloride, µA/cm2), (D,I) Forskolin (ΔIeq Fsk, µA/cm2) and (E,J) CFTRinh172 response (ΔIeq CFTRinh, µA/cm2). Statistical analysis by one way ANOVA: Tukey’s multiple comparison test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We also examined the effect of cell cryopreservation on CFTR functional measurements in non-CF HNE. In cryopreserved HNE, ΔIeq Fsk reduced by 50% (p = 0.003), and ΔIeq CFTRinh reduced by 20% (p = 0.064) compared to fresh HNE (Figures 5A,B).
FIGURE 5
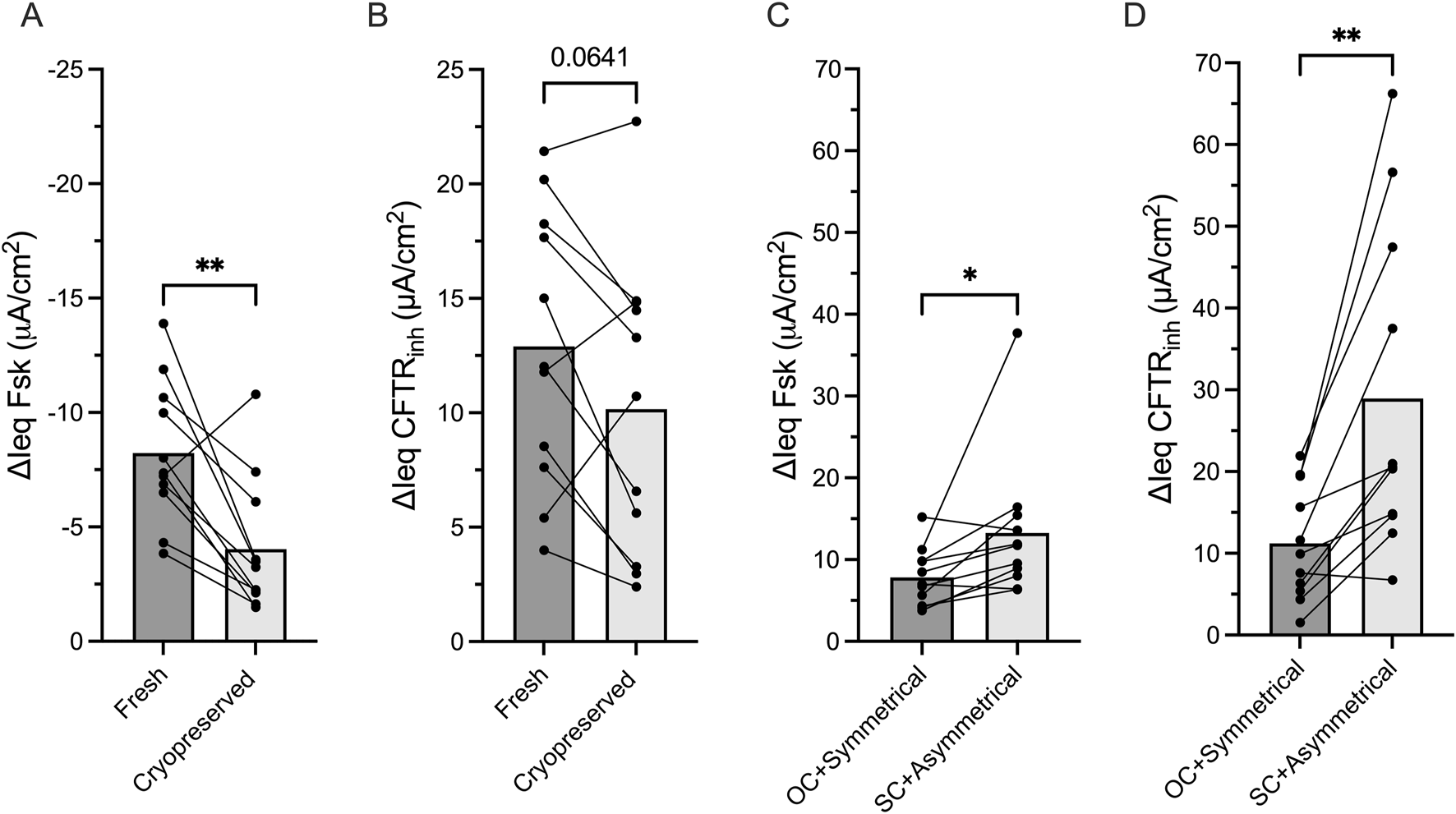
Experimental conditions impact CFTR function measurements Bar graphs with mean ± SD showing the change in transepithelial current with (A) forskolin stimulation (ΔIeq Fsk, μA/cm2) current and (B) CFTRinh-172 inhibition (ΔIeq CFTRinh, μA/cm2) in paired non-CF nasal cells (HNE) that had never been frozen (fresh) or had been thawed after cryopreservation. (C) ΔIeq Fsk and (D) ΔIeq CFTRinh in paired non-CF HNE comparing open-circuit and short-circuit Ussing chamber studies. Each dot represents a single experiment from a single subject, n values provided are indicative of biological replicates. Statistical analysis by paired students t-test, *P < 0.05, **P < 0.01.
Lastly, we evaluated the impact of commonly used electrophysiological techniques on the magnitude of CFTR functional measurements as most centres measure CFTR function under short-circuit conditions with the application of a basolateral to apical chloride gradient, while a few others use symmetrical chloride and open-circuit conditions. In paired analysis of non-CF HNE, we demonstrated that ΔIeq Fsk and ΔIeq CFTRinh were significant larger under short-circuit conditions with a chloride gradient compared to open-circuit condition with symmetrical chloride (p = 0.04 and p = 0.002, respectively, Figures 5C,D).
Discussion
In this study, we demonstrate that HNE discriminated between different levels of CFTR modulator responses on an individual and interindividual level in pwCF and performed similarly to HBE. In non-CF cultures, HNE underestimated CFTR function compared to HBE. We also highlight that care needs to be taken when comparing CFTR functional read outs due to significant impact of cell culturing and measurement techniques.
HBEx have laid the foundation for in vitro CFTR functional measurements and have been instrumental to assess the efficacy of CFTR modulator drugs (Van Goor et al., 2009; Pranke et al., 2017; Brewington et al., 2018; Keating et al., 2018; Anderson et al., 2021). Since these cells do not suit personalized medicine approaches, HNE are being widely utilized for preclinical drug testing platform building. It has been shown that HNE have the capability to link with in vivo outcomes, but there are only few studies directly comparing their performance to HBE. In a comparison study of matched HNE and HBEb, but not HBEx, from ten pwCF with a wide variety in CFTR gene variants, Brewington et al. reported comparable magnitudes of CFTR function when these were treated with VX-809+VX-770 and showed that CFTR function correlated between these two cell types (Brewington et al., 2018). Pranke et al. showed similar VX-809+VX-770-induced changes in CFTR function between HNE and HBEx sampled from 22 pwCF (unmatched) with various CFTR gene variants (Pranke et al., 2017). In this study, we took the comparison one step further trying to overcome limitations of the previous studies. We showed comparable CFTR modulator responses between HNE, HBEb, and HBEx collected from a cohort of 28 pwCF with mainly F508del/F508del CFTR variants. While VX-809 produces minimal levels of CFTR rescue, it was the only CFTR corrector available at the time of study. We report small changes in magnitude of CFTR function with VX-809 alone, which are comparable to those measured by Farinha et al. (Farinha et al., 2015). While we showed only changes in functional expression in this manuscript, our group has previously shown that the changes in CFTR function are associated to increase in mature protein expression in Western blot analysis (Molinski et al., 2017). More robust increases in CFTR function were measured with addition of PTI-428, a CFTR amplifier (Giuliano et al., 2018; Dukovski et al., 2020). Additionally we report correlation of CFTR function between 16 subject-matched HNE and HBEb at an individual level. Importantly, in addition to comparing baseline to rescue level, layering of CFTR modulator combinations allowed us to demonstrate that HNE, similarly to HBEb and HBEx, not only discriminated different response levels between individual pwCF (responders and non-responders), but also between different levels of rescued CFTR function within each individual pwCF. This is the prerequisite for using HNE-based pre-clinical platforms to allow ranking drug responses rather than just assessing for yes or no response.
While HNE and HBE cultures have the structural qualities of a mature respiratory epithelia including pseudocolumnar structure, ciliated cells, and mucus-secreting cells following differentiation (Pranke et al., 2017; Brewington et al., 2018) they are distinct tissues with intrinsic differences from function to the transcriptome (Imkamp et al., 2019; Vieira Braga et al., 2019; Deprez et al., 2020; Cho et al., 2021) that can remain even with cell culturing (Rodenburg et al., 2023). In CF HNE and HBE, cell composition was found to differ despite being grown under the same conditions with CF HNE having greater prevalence of MUC5AC positive cells and HBE having more ciliated (β-tubulin-positive) cells (Rodenburg et al., 2023). In RNAseq analysis of these cultures, numerous differentially expressed genes were identified many of which being associated with tissue-specific pathways, i.e., genes in pathways involved in lung development were higher in HBE than HNE (Rodenburg et al., 2023). HNE had reduced expression of ZO-1, E-cadherin, and tight-junction associated claudins explaining the reduced baseline transepithelial resistance (Rte) compared to HBE found reported here and by others (Brewington et al., 2018; Rodenburg et al., 2023). Importantly, no significant differences in expression of CFTR or other apical ion transporters including ANO1, SLC26A9, and SLC26A4 were reported (Rodenburg et al., 2023), aligning with previous work from our group showing equivalent expression of CFTR and other CF lung disease modifier genes (Corvol et al., 2015) in paired fresh and cultured HNE and HBEx from pwCF (He et al., 2022). Thus, while differences remain, key features impacting CFTR functional measures and lung disease in pwCF are similarly captured in HNE and HBE cultures further validating the use of HNE as a HBE surrogate in this setting.
Non-CF CFTR functional measurements are used as a benchmark when examining CFTR modulator response in CF cultures whether by comparing absolute values or reporting response as a percentage of non-CF. However, there is scarce characterization of ion transport between non-CF HNE and HBE. Here we report HNE have reduced ion transport compared to HBEx in non-CF cultures. This aligns with Pranke et al. who showed reduced, albeit not significant, ΔIeq amiloride, Fsk, and CFTRinh in non-CF HNE compared to HBEx (Pranke et al., 2017). In contrast, the magnitude of CFTR modulator induced CFTR function in cells from pwCF was similar between HNE and HBEx. It remains to be seen whether the tissue specific difference in respect to magnitude of CFTR function may only be revealed at higher levels of CFTR function not captured with the CFTR modulators currently examined. However, at the very least with more potent CFTR functional rescue HNE may underestimate but not inflate the absolute CFTR current of HBE.
We were surprised by the extent to which ALI media and Ussing techniques impact the magnitude of measured CFTR function. Nonetheless, our results are consistent with previous works reporting high CFTR function measurements with PC media (Saint-Criq et al., 2020; Scudieri et al., 2020) which has been explained by greater CFTR RNA and protein expression, specifically when compared to human airway cells grown in UNC ALI media (Saint-Criq et al., 2020). There are several studies demonstrating the impact of ALI media on various airway epithelia properties including prevalence of specific cell types such as ciliated cells (Leung et al., 2020; Luengen et al., 2020; Awatade et al., 2023) or ionocytes (Scudieri et al., 2020), epithelial thickness (Leung et al., 2020), viral response (Awatade et al., 2023; Redman et al., 2024), etc. It has yet to be determined which conditions provide the best representation of in vivo CFTR function and CFTR modulator drug response. Banking patient tissues using cryopreservation is a valuable technique allowing for repeat functional studies or testing of new CFTR modulators without resampling. Here we showed that cryopreservation decreases the magnitude of CFTR function in non-CF HNE. This supports previous work by Martinovich et al. who showed decreased CFTR functional measurements in non-CF HBEb with cryopreservation (Martinovich et al., 2017), but challenges findings reporting unchanged CFTR after cryopreservation in CF HNE and HBEb (Martinovich et al., 2017; Kelly et al., 2022). Finally, there have been decades of debate on techniques used in Ussing chamber studies of transepithelial ion transport which may change depending on the research question at hand. While short-circuit with asymmetrical chloride concentrations drive maximal chloride currents across the epithelium (including paracellular spaces), open-circuit with symmetrical chloride concentrations is believed to better reflect the physiological in vivo setting. Both techniques are commonly used to report CFTR functional measurements. Here we demonstrate that the magnitude of CFTR function is increased with an apical chloride gradient under short-circuit measurements when compared to symmetrical chloride and open-circuit measurements in matched non-CF HNE. This corresponds with previous work demonstrating the magnitude of CFTR function increased with presence of a chloride gradient in short-circuit (Bratcher et al., 2020). However, chloride and voltage mode may both independently impact CFTR functional measurements and remains to be examined. Cumulatively, with the use of different cell culture and measuring techniques, care need to be taken when comparing CFTR modulator results between different centres.
The main limitation of this study was the unavailability of elexacaftor, texacaftor and ivacaftor (ETI) during the study period. ETI is known as a highly efficient CFTR modulator combination and is widely implemented in clinical therapy. This level of functional correction may have impacted the magnitudes of CFTR drug response in our different cell cultures, widening the difference between HNE and HBEx, as seen in non-CF cultures. However, the window for such experiments was small, and with the introduction of this drug combination, the number of lung transplantation and thus availability of HBEx, has dropped. Additionally, while we set out to compare between HNE, HBEb, and HBEx on an individual level and we successfully cultured all 3 cell types from 7 patients, most HBEx were grown in USG media and given the significant impact of differences in ALI media on CFTR function, we decided against a direct parallel comparison.
In summary, while HNE may underestimate HBE at high levels of CFTR function seen in non-CF, the magnitude of CFTR modulator response in CF HNE correlates to matched HBEb and can discriminate between increments of CFTR rescue as well as stratify good and poor response equal to HBEb and HBEx cultures. Thus, HNE is a reliable and feasible tool for preclinical prediction platforms. In our global endeavor to assign all pwCF effective drug treatments, including those with rare CFTR mutations, collaboration between centres providing these preclinical functional platforms need to be fostered. We suggest that CFTR function should be reported as absolute as well as percent of baseline or non-CF controls grown in the same conditions and details about culture passage, fresh/frozen, media and Ussing conditions should be shared to allow accurate data comparisons and interpretation of results.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research Ethics Boards of the Hospital for Sick Children (REB# 1000044783) and the Toronto General Hospital (REB#15–8936). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was provided by study participants and legal guardians of participants with insufficient capacity.
Author contributions
MB: Writing – original draft, Formal Analysis, Writing – review and editing. JA: Data curation, Writing – review and editing. SB: Writing – review and editing, Data curation. ClB: Formal Analysis, Writing – original draft, Data curation, Writing – review and editing, Methodology. WI: Formal Analysis, Methodology, Data curation, Writing – review and editing. HO: Data curation, Methodology, Formal Analysis, Writing – review and editing. WD: Formal Analysis, Data curation, Methodology, Writing – review and editing. JZ: Resources, Writing – review and editing. SK: Resources, Writing – review and editing, Conceptualization. ChB: Conceptualization, Writing – review and editing, Funding acquisition, Resources. TJM: Supervision, Writing – original draft, Conceptualization, Writing – review and editing, Investigation. TG: Writing – review and editing, Supervision, Writing – original draft, Conceptualization, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Cystic Fibrosis Canada/The Program for Individualized Cystic Fibrosis Therapy (CFIT). Additional support was provided by Canadian Institutes of Health Research (CIHR) (Grant #: 175230). MB received salary support from a CIHR Doctoral Research Award.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1651122/full#supplementary-material
References
1
Anderson J. D. Liu Z. Odom L. V. Kersh L. Guimbellot J. S. (2021). CFTR function and clinical response to modulators parallel nasal epithelial organoid swelling. Am. J. Physiol. Lung Cell Mol. Physiol.321, L119–L129. 10.1152/ajplung.00639.2020
2
Awatade N. T. Reid A. T. Nichol K. S. Budden K. F. Veerati P. C. Pathinayake P. S. et al (2023). Comparison of commercially available differentiation media on cell morphology, function, and anti-viral responses in conditionally reprogrammed human bronchial epithelial cells. Sci. Rep.13, 11200. 10.1038/s41598-023-37828-0
3
Bratcher P. E. Yadav S. Shaughnessy C. A. Thornell I. M. Zeitlin P. L. (2020). Effect of apical chloride concentration on the measurement of responses to CFTR modulation in airway epithelia cultured from nasal brushings. Physiol. Rep.8, e14603. 10.14814/PHY2.14603
4
Brewington J. J. Filbrandt E. T. LaRosa F. J. Moncivaiz J. D. Ostmann A. J. Strecker L. M. et al (2018). Brushed nasal epithelial cells are a surrogate for bronchial epithelial CFTR studies. JCI Insight3, e99385. 10.1172/JCI.INSIGHT.99385
5
Burgel P.-R. Sermet-Gaudelus I. Durieu I. Kanaan R. Macey J. Grenet D. et al (2023). The French compassionate program of elexacaftor-tezacaftor-ivacaftor in people with cystic fibrosis with advanced lung disease and no F508del CFTR variant. Eur. Respir. J.61, 2202437. 10.1183/13993003.02437-2022
6
Cho H.-J. Ha J. G. Lee S. N. Kim C.-H. Wang D.-Y. Yoon J.-H. (2021). Differences and similarities between the upper and lower airway: focusing on innate immunity. Rhinology J.59 (5), 441–450. 10.4193/Rhin21.046
7
Corvol H. Blackman S. M. Boëlle P.-Y. Gallins P. J. Pace R. G. Stonebraker J. R. et al (2015). Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat. Commun.6, 8382. 10.1038/ncomms9382
8
Deprez M. Zaragosi L.-E. Truchi M. Becavin C. Ruiz García S. Arguel M.-J. et al (2020). A single-cell atlas of the human healthy airways. Am. J. Respir. Crit. Care Med.202, 1636–1645. 10.1164/rccm.201911-2199OC
9
Dukovski D. Villella A. Bastos C. King R. Finley D. Kelly J. W. et al (2020). Amplifiers co-translationally enhance CFTR biosynthesis via PCBP1-mediated regulation of CFTR mRNA. J. Cyst. Fibros.19, 733–741. 10.1016/j.jcf.2020.02.006
10
Durmowicz A. G. Lim R. Rogers H. Rosebraugh C. J. Chowdhury B. A. (2018). The U.S. food and drug administration’s experience with ivacaftor in cystic fibrosis. Establishing efficacy using in vitro data in lieu of a clinical trial. Ann. Am. Thorac. Soc.15, 1–2. 10.1513/AnnalsATS.201708-668PS
11
Farinha C. M. Sousa M. Canato S. Schmidt A. Uliyakina I. Amaral M. D. (2015). Increased efficacy ofVX‐809 in different cellular systems results from an early stabilization effect of F508del‐CFTR. Pharmacol. Res. Perspect.3, e00152. 10.1002/prp2.152
12
Giuliano K. A. Wachi S. Drew L. Dukovski D. Green O. Bastos C. et al (2018). Use of a high-throughput phenotypic screening strategy to identify amplifiers, a novel pharmacological class of small molecules that exhibit functional synergy with potentiators and correctors. SLAS Discov.23, 111–121. 10.1177/2472555217729790
13
He G. Panjwani N. Avolio J. Ouyang H. Keshavjee S. Rommens J. M. et al (2022). Expression of cystic fibrosis lung disease modifier genes in human airway models. J. Cyst. Fibros.21, 616–622. 10.1016/J.JCF.2022.02.007
14
Heijerman H. G. M. McKone E. F. Downey D. G. Van Braeckel E. Rowe S. M. Tullis E. et al (2019). Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet394, 1940–1948. 10.1016/S0140-6736(19)32597-8
15
Imkamp K. Bernal V. Grzegorzcyk M. Horvatovich P. Vermeulen C. J. Heijink I. H. et al (2019). Gene network approach reveals co-expression patterns in nasal and bronchial epithelium. Sci. Rep.9, 15835. 10.1038/s41598-019-50963-x
16
Karp P. H. Moninger T. O. Weber S. P. Nesselhauf T. S. Launspach J. L. Zabner J. et al (2002). “An in vitro model of differentiated human airway epithelia: methods for establishing primary cultures,” in Epithelial cell culture protocols (New Jersey: Humana Press), 115–137. 10.1385/1-59259-185-X:115
17
Keating D. Marigowda G. Burr L. Daines C. Mall M. A. McKone E. F. et al (2018). VX-445–Tezacaftor–Ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N. Engl. J. Med.379, 1612–1620. 10.1056/NEJMoa1807120
18
Kelly M. Dreano E. Hatton A. Lepissier A. Golec A. Sermet-Gaudelus I. et al (2022). Primary human nasal epithelial cells: biobanking in the context of precision medicine. J. Vis. Exp.2022. 10.3791/63409
19
Laselva O. Bartlett C. Gunawardena T. N. A. Ouyang H. Eckford P. D. W. Moraes T. J. et al (2021). Rescue of multiple class II CFTR mutations by elexacaftor+tezacaftor+ivacaftor mediated in part by the dual activities of elexacaftor as both corrector and potentiator. Eur. Respir. J.57, 2002774. 10.1183/13993003.02774-2020
20
Leung C. Wadsworth S. J. Yang S. J. Dorscheid D. R. (2020). Structural and functional variations in human bronchial epithelial cells cultured in air-liquid interface using different growth media. Am. J. Physiology-Lung Cell. Mol. Physiology318, L1063–L1073. 10.1152/ajplung.00190.2019
21
Luengen A. E. Kniebs C. Buhl E. M. Cornelissen C. G. Schmitz-Rode T. Jockenhoevel S. et al (2020). Choosing the right differentiation medium to develop mucociliary phenotype of primary nasal epithelial cells in vitro. Sci. Rep.10, 6963. 10.1038/S41598-020-63922-8
22
Martinovich K. M. Iosifidis T. Buckley A. G. Looi K. Ling K.-M. Sutanto E. N. et al (2017). Conditionally reprogrammed primary airway epithelial cells maintain morphology, lineage and disease specific functional characteristics. Sci. Rep.7, 17971. 10.1038/s41598-017-17952-4
23
Molinski S. V. Ahmadi S. Ip W. Ouyang H. Villella A. Miller J. P. et al (2017). Orkambi® and amplifier co‐therapy improves function from a rare CFTR mutation in gene‐edited cells and patient tissue. EMBO Mol. Med.9, 1224–1243. 10.15252/emmm.201607137
24
Neuberger T. Burton B. Clark H. Van Goor F. (2011). Use of primary cultures of human bronchial epithelial cells isolated from cystic fibrosis patients for the pre-clinical testing of CFTR modulators. Methods Mol. Biol.741, 39–54. 10.1007/978-1-61779-117-8_4
25
Noel S. Servel N. Hatton A. Golec A. Rodrat M. Ng D. R. S. et al (2022). Correlating genotype with phenotype using CFTR-Mediated whole-cell cl− currents in human nasal epithelial cells. J. Physiol.600, 1515–1531. 10.1113/JP282143
26
Pranke I. M. Hatton A. Simonin J. Jais J. P. Le Pimpec-Barthes F. Carsin A. et al (2017). Correction of CFTR function in nasal epithelial cells from cystic fibrosis patients predicts improvement of respiratory function by CFTR modulators. Sci. Rep.7, 7375–11. 10.1038/s41598-017-07504-1
27
Ramsey B. W. Davies J. McElvaney N. G. Tullis E. Bell S. C. Dřevínek P. et al (2011). A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med.365, 1663–1672. 10.1056/NEJMOA1105185
28
Randell S. H. Fulcher M. L. O’Neal W. Olsen J. C. (2011). Primary epithelial cell models for cystic fibrosis research. Methods Mol. Biol.742, 285–310. 10.1007/978-1-61779-120-8_18
29
Raraigh K. S. Lewis M. H. Collaco J. M. Corey M. Penland C. M. Stephenson A. L. et al (2022). Caution advised in the use of CFTR modulator treatment for individuals harboring specific CFTR variants. J. Cyst. Fibros.21, 856–860. 10.1016/j.jcf.2022.04.019
30
Redman E. Fierville M. Cavard A. Plaisant M. Arguel M.-J. Ruiz Garcia S. et al (2024). Cell culture differentiation and proliferation conditions influence the in vitro regeneration of the human airway epithelium. Am. J. Respir. Cell Mol. Biol.71, 267–281. 10.1165/rcmb.2023-0356MA
31
Rodenburg L. W. Metzemaekers M. van der Windt I. S. Smits S. M. A. den Hertog-Oosterhoff L. A. Kruisselbrink E. et al (2023). Exploring intrinsic variability between cultured nasal and bronchial epithelia in cystic fibrosis. Sci. Rep.13, 18573. 10.1038/s41598-023-45201-4
32
Saint-Criq V. Delpiano L. Casement J. Onuora J. C. Lin J. Gray M. A. (2020). Choice of differentiation media significantly impacts cell lineage and response to CFTR modulators in fully differentiated primary cultures of cystic fibrosis human airway epithelial cells. Cells9, 2137. 10.3390/cells9092137
33
Scudieri P. Musante I. Venturini A. Guidone D. Genovese M. Cresta F. et al (2020). Ionocytes and CFTR chloride channel expression in normal and cystic fibrosis nasal and bronchial epithelial cells. Cells9, 2090. 10.3390/cells9092090
34
Terrance M. Gunawardena T. Ouyang H. Avolio J. Duan W. Thanikachalam S. et al (2024). Primary human nasal epithelial cell culture. Methods Mol. Biol.2725, 213–223. 10.1007/978-1-0716-3507-0_13
35
Van Goor F. Straley K. S. Cao D. González J. Hadida S. Hazlewood A. et al (2006). Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am. J. Physiology-Lung Cell. Mol. Physiology290, L1117–L1130. 10.1152/ajplung.00169.2005
36
Van Goor F. Hadida S. Grootenhuis P. D. J. Burton B. Cao D. Neuberger T. et al (2009). Rescue of cf airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc. Natl. Acad. Sci.106, 18825–18830. 10.1073/pnas.0904709106
37
Van Goor F. Hadida S. Grootenhuis P. D. J. Burton B. Stack J. H. Straley K. S. et al (2011). Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc. Natl. Acad. Sci.108, 18843–18848. 10.1073/pnas.1105787108
38
Veit G. Roldan A. Hancock M. A. da Fonte D. F. Xu H. Hussein M. et al (2020). Allosteric folding correction of F508del and rare CFTR mutants by elexacaftor-tezacaftor-ivacaftor (trikafta) combination. JCI Insight5, e139983. 10.1172/JCI.INSIGHT.139983
39
Vieira Braga F. A. Kar G. Berg M. Carpaij O. A. Polanski K. Simon L. M. et al (2019). A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med.25, 1153–1163. 10.1038/s41591-019-0468-5
40
Wainwright C. E. Elborn J. S. Ramsey B. W. Marigowda G. Huang X. Cipolli M. et al (2015). Lumacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N. Engl. J. Med.373, 220–231. 10.1056/NEJMoa1409547
Summary
Keywords
nasal cells, cystic fibrosis, CFTR modulator, electrophyiology, in vitro model
Citation
Barillaro M, Avolio J, Balachandran S, Bartlett C, Ip W, Ouyang H, Duan W, Zabner J, Keshavjee S, Bear C, Moraes TJ and Gonska T (2025) Nasal cells as a bronchial cell surrogate for pre-clinical assessment of drug response in cystic fibrosis. Front. Pharmacol. 16:1651122. doi: 10.3389/fphar.2025.1651122
Received
20 June 2025
Accepted
14 August 2025
Published
05 September 2025
Volume
16 - 2025
Edited by
Domenico Mattoscio, University of Studies G.d’Annunzio Chieti and Pescara, Italy
Reviewed by
Neeraj Sharma, Johns Hopkins University, United States
Debora Baroni, National Research Council (CNR), Italy
Updates
Copyright
© 2025 Barillaro, Avolio, Balachandran, Bartlett, Ip, Ouyang, Duan, Zabner, Keshavjee, Bear, Moraes and Gonska.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tanja Gonska, tanja.gonska@sickkids.ca; Theo J. Moraes, theo.moraes@sickkids.ca
‡These authors share senior authorship
ORCID: Shaf Keshavjee, orcid.org/0000-0003-4547-8094
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.