Abstract
Traditional Chinese Medicine (TCM) possesses a well-documented historical legacy and substantial clinical experience in treating burn injuries and diverse wound conditions. Grounded in TCM theory, therapeutic strategies incorporate herbal medicine and its external preparations, as well as TCM auxiliary treatment, forming a comprehensive treatment framework. A systematic evaluation of burn management, particularly recent advancements in TCM research, carries significant implications for both theoretical and clinical applications. This paper synthesizes information from a plethora of online resources to explicate the mechanisms of TCM in burn treatment from multifaceted perspectives. Specifically, a comprehensive collection of literature pertaining to TCM burn treatment from the past three decades was amassed from electronic databases including PubMed, CNKI, and Web of Science. A meticulous keyword information statistical analysis was performed on this corpus. The search strategy employed keyword clusters such as “traditional Chinese medicine, phytochemistry, or herbs” combined with “burn, scald, or skin wound”. The scientific nomenclature of plants was verified using “The Plant List” (www.plantsoftheworldonline.org). This review encapsulates the methodologies of burn treatment within TCM and underscores a multitude of herbs with burn-treating capabilities, including Arnebia euchroma (Royle ex Benth.) I.M.Johnst., Rheum palmatum L., Coptis chinensis Franch., Phellodendron chinense C.K.Schneid., Sanguisorba officinalis L., and Angelica sinensis (Oliv.) Diels, as well as natural borneol (from Dryobalanops aromatica C.F.Gaertn.), Frankincense (from Boswellia sacra Flück.), and Myrrh (from Commiphora myrrha (T.Nees) Engl.). The principal active ingredients identified are shikonin, emodin, berberine, ferulic acid, and curcumin; however, their mechanisms warrant further in-depth investigation. Notable strides have been made in the innovation and research of TCM in burn treatment. Beyond traditional external formulations, hydrogel, liposome, microsphere, and nanofibers have emerged as pivotal elements in burn management. These advanced materials have introduced an innovative drug delivery system by integrating the active components, thereby enhancing the efficacy of burn treatment.
Graphical Abstract
Introduction
Burns represent a prevalent form of accidental injury in clinical practice, with an estimated 70 million cases occurring globally each year. These injuries result in approximately 18 million disabilities and over 20,000 deaths annually (Peck, 2011; 2012). Burns are caused by thermal, electrical, radiation, chemical (acids, alkalis, irritants, and corrosive substances), or other physical and chemical factors, leading to damage and necrosis of superficial and subcutaneous tissues, accompanied by a cascade of pathological changes (Jeschke et al., 2020). The efficacy of burn treatment depends on the extent and severity of the injury, as well as the timeliness and precision of therapeutic interventions (Radzikowska-Büchner et al., 2023). Conventional treatments often include antibiotics, energy supplements, micronutrients, immune-modulating agents, and topical growth factors or recombinant human growth hormone therapy. However, these treatments may carry potential side effects, such as disruptions to metabolic processes, induction of secondary diseases, or drug dependence (Roshangar et al., 2019; Markiewicz-Gospodarek et al., 2022).
Traditional Chinese Medicine (TCM) has a long history of treating burns, with the earliest records dating back to the Fifty-Two Diseases Prescriptions from the pre-Qin period in recent years, TCM has gained increasing recognition in burn management due to its notable efficacy, low toxicity, and diverse formulation types (Zhou et al., 2024). Despite this, there is a notable lack of comprehensive reviews on TCM approaches to burn treatment. Despite this, there is a notable lack of comprehensive reviews on TCM approaches to burn treatment. The existing literature on this topic often remains at the overview level (Kopp et al., 2003; Herman and Herman, 2020; Mrabti et al., 2022), and more detailed studies exploring the underlying mechanisms are still needed. Furthermore, reviews that integrate modern medical methodologies with TCM strategies or explore burn treatment from a contemporary TCM perspective are currently limited.
To address this research gap, a systematic and comprehensive literature review was conducted to evaluate the current application of TCM in burn treatment. The literature search was performed across multiple electronic databases, including PubMed, CNKI, and Web of Science. The search strategy utilized the following keyword clusters: (“traditional Chinese medicine” OR “phytochemistry” OR “herbs”) AND (“burn” OR “scald” OR “skin wound”). The scientific nomenclature of medicinal plants was verified using “The Plant List” (www.plantsoftheworldonline.org). The initial search yielded 92 records from PubMed, 256,153 from CNKI, and an unspecified number from Web of Science. After importing all records into EndNote literature management software (https://endnote.com/), 75 duplicates were removed. The remaining articles underwent a two-stage screening process based on the following criteria: Inclusion Criteria: Studies focused on the use of TCM compounds, single herbs, or active ingredients in the treatment of burns, scalds, or skin wounds. Articles published in peer-reviewed journals within the past 30 years. Reports available in either English or Chinese. Studies involving in vitro, in vivo, or clinical evaluations. Exclusion Criteria: Publications not relevant to TCM or burn treatment (e.g., veterinary use, non-therapeutic research). Non-original research articles such as editorials, commentaries, or conference abstracts without full text.
After title and abstract screening, irrelevant studies were excluded manually. Ultimately, 109 articles met the inclusion criteria and were selected for in-depth analysis. Additionally, 20 supplementary literatures were included to provide foundational context: 8 pertaining to general burn overviews, 6 related to wound healing mechanisms, and 6 focused on TCM auxiliary therapies. The literature selection process is summarized in Figure 1.
FIGURE 1
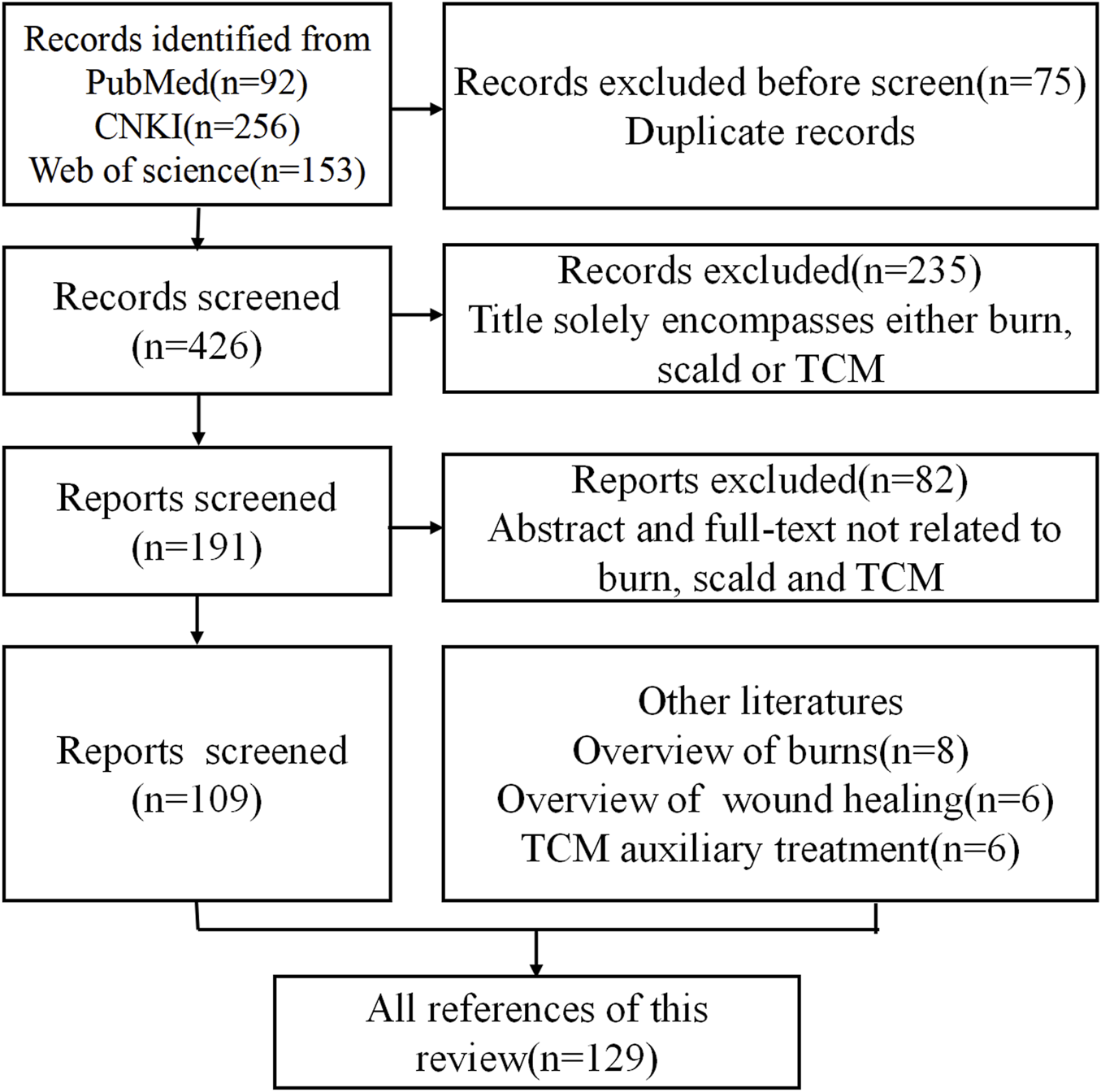
Flowchart for inclusion and exclusion of literature in this review.
This paper summarizes the understanding of burns in TCM and modern medicine, particularly focused on underlying mechanisms, therapeutic approaches, innovative external preparations, and TCM auxiliary therapies, aims to provide valuable insights and research directions regarding therapeutic drugs for burn management.
Burn pathology in modern medicine
Burn injuries are classified into four degrees (I–IV) based on the depth and extent of tissue damage (Warby and Maani, 2023). Superficial burns (first-degree or superficial second-degree) affect only the epidermis, typically healing with minimal scarring. Deep second-degree burns involve the deep dermal layer, presenting with blisters, a red-white base, and increased exudate, often leading to hypertrophic scarring. Third-degree burns extend through the entire dermis, usually requiring surgical intervention, while fourth-degree burns involve deeper structures such as muscle or bone, resulting in significant functional impairment (Żwierełło et al., 2023).
Wound healing aims to restore tissue integrity and homeostasis through three overlapping phases: inflammation, proliferation, and remodeling. Disruptions in these phases can lead to delayed healing or chronic wounds (Wallace et al., 2023; Pena and Martin, 2024). Inflammatory Phase initiated by immune responses to remove damaged tissue and pathogens, this phase involves vascular and cellular responses. Cytokines such as transforming growth factor (TGF-β), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), and interleukin-8 (IL-8) are released, recruiting neutrophils and macrophages for pathogen clearance, inflammation resolution, and tissue repair (Childs and Murthy, 2017; Wilkinson and Hardman, 2020). The proliferative phase occurs 2–10 days post-injury, this phase is driven by macrophages, which release growth factors to recruit fibroblasts and keratinocytes. Angiogenesis, mediated by vascular endothelial growth factor (VEGF) and FGF, is initiated, and fibroblasts synthesize extracellular matrix (ECM) proteins to form granulation tissue (G El Baassiri et al., 2023). Finally, remodeling phase begins 2–3 weeks post-injury and lasting up to 2 years, this phase involves the maturation of the scar. Fibroblasts, macrophages, and endothelial cells secrete matrix metalloproteinases (MMPs) to degrade type III collagen, which is replaced by type I collagen organized into parallel fibrils. Apoptosis of excess cells and remodeling of the epidermis, vasculature, and nerves occur during this stage (Mathew-Steiner et al., 2021). Effective wound healing relies on growth factors, nutrient supply, cell-to-cell interactions, and oxygen availability. Disruptions due to infection, malnutrition, chronic diseases, or diabetes can impair healing, leading to chronic wounds. Understanding these mechanisms is critical for optimizing burn treatment strategies (Figure 2).
FIGURE 2
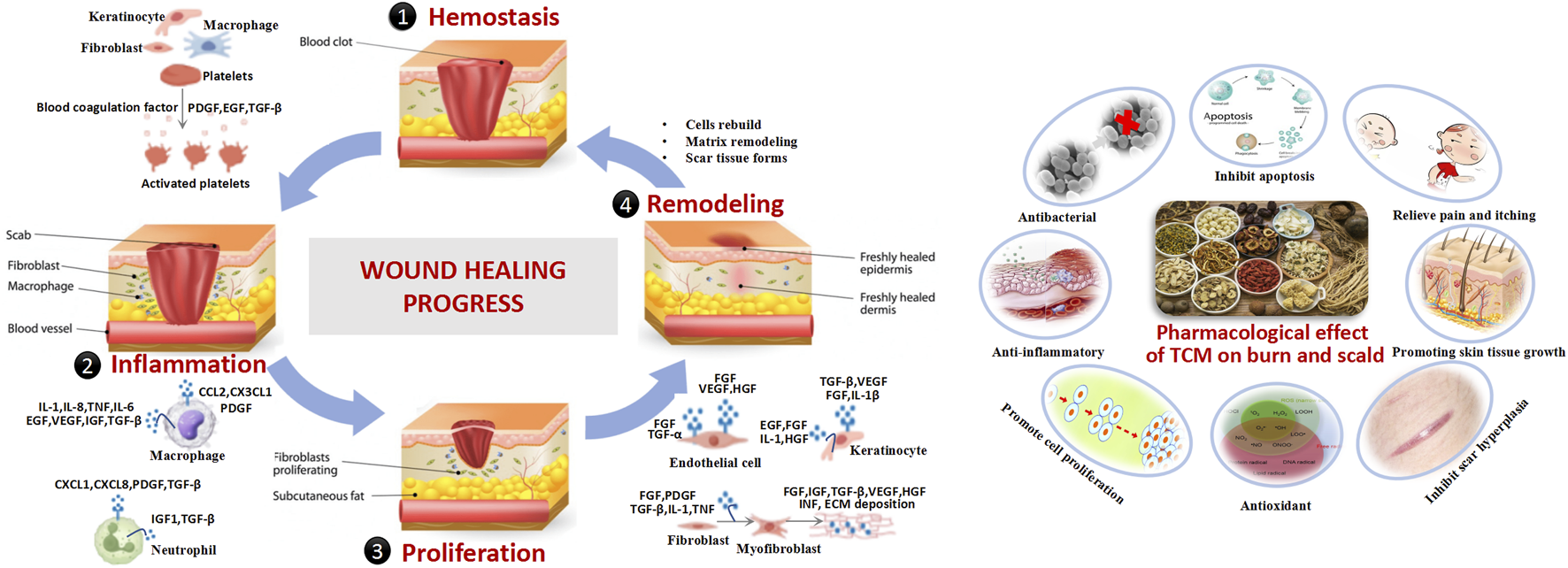
Burn pathology in modern medicine.
TCM perspective on burn
In TCM, burn injury is collectively referred to as “water and fire burns”. It occurs when the skin and deeper tissues are exposed to high temperatures, leading to Qi and blood stagnation and an imbalance between defensive (Wei) Qi and nourishing (Ying) Qi. The pathogenesis involves heat acting on the superficial muscles, causing local Qi stagnation and meridian obstruction. This weakens the Wei Qi, the body’s first line of defense, reducing its protective function and resulting in fluid leakage, blister formation, and exudate (Que et al., 2005). Excessive blistering may deplete Yin fluids, eventually leading to Yin deficiency, Yang collapse, and an imbalance between Yin and Yang (Ripszky Totan et al., 2022). Furthermore, the invasion of fire toxins can impair the functions of the spleen, kidney, and heart, exacerbating Qi stagnation. Sleep disorders in burn patients are closely associated with Yin-Yang imbalances and internal organ dysfunctions (Liang et al., 2021; Liu et al., 2023). In TCM, normal sleep relies on the harmonious coordination of Yin-Yang and mental tranquility. Disruptions in these factors or mental unrest can lead to sleep disturbances (O'Brien and Weber, 2016). Burn patients often experience severe nocturnal skin itching, which disrupts sleep and may result in neurasthenia or other health complications (Chung et al., 2020) (Figure 3).
FIGURE 3
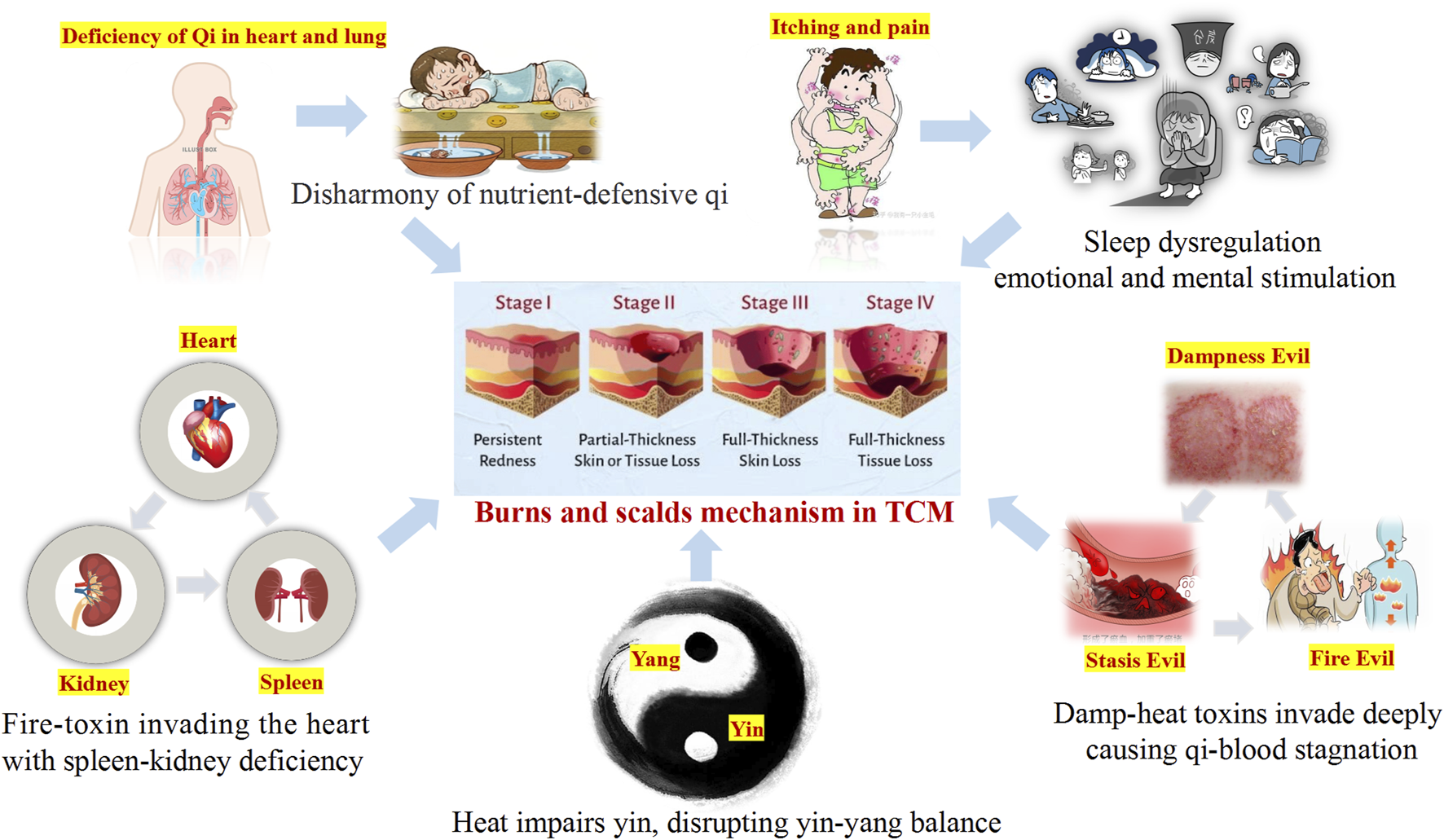
TCM perspective on burn injury.
Based on the fundamental tenets of TCM, burn injuries can be classified into three distinct stages according to their typical symptomatic manifestations: the stage of excessive heat - toxin accumulation, the stage of Yin - fluid depletion, and the stage of Yin deficiency. These stages often exhibit overlapping pathological features. In the early stage, treatment predominantly focuses on heat-clearing and detoxification strategies to prevent the intrusion of toxins into the body. In the middle stage, the emphasis lies in nourishing Yin, promoting blood generation, clearing heat toxins, and facilitating tissue regeneration. In the late stage, the focus is on Qi supplementation, blood nourishing, and regulating the balance of Qi and blood.
Treatment of burn with Chinese herbal medicine
With the advancement of modern TCM, there is a growing body of theoretical and practical evidence supporting its use in burn treatment. TCM employs a holistic approach, utilizing single herbs (e.g., Rheum palmatum L.), Coptis chinensis Franch., Angelica sinensis (Oliv.) Diels) and compound prescriptions to address both systemic and local symptoms of burn injuries. Therapeutic methods include internal administration, which regulates systemic conditions and enhances the body’s resistance to pathogens, and external application, which directly targets the burn site to promote wound healing and prevent complications such as tissue necrosis, vascular occlusion, and infection. External medications, including ointments, sprays, and hydrogels, play a critical role in addressing local blood circulation disorders and controlling wound infections. Additionally, auxiliary TCM therapies such as acupuncture, cupping, guasha, tuina, and aromatherapy provide complementary benefits by improving blood flow, reducing pain, and enhancing overall recovery (Figure 4).
FIGURE 4
Treatment of burn injury with TCM and modern medical.
Chinese herbal medicine and natural products are widely recognized for their efficacy, low side effects, and minimal resistance development in treating burns. The healing process of burn wounds is highly complex, involving growth factors, inflammatory mediators, and ECM remodeling. TCM exerts its therapeutic effects through multi-component, multi-target, and multi-pathway mechanisms. Experimental studies have demonstrated that products used in TCM can enhance the production and secretion of growth factors, including VEGF and EGF. Additionally, they have been shown to reduce the expression of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), inhibit bacterial proliferation, improve immune function, and regulate collagen synthesis along with ECM remodeling. Furthermore, TCM-based treatments exhibit antioxidant properties, eliminate free radicals, facilitate cell proliferation, inhibit apoptosis, and provide analgesic and antipruritic effects.
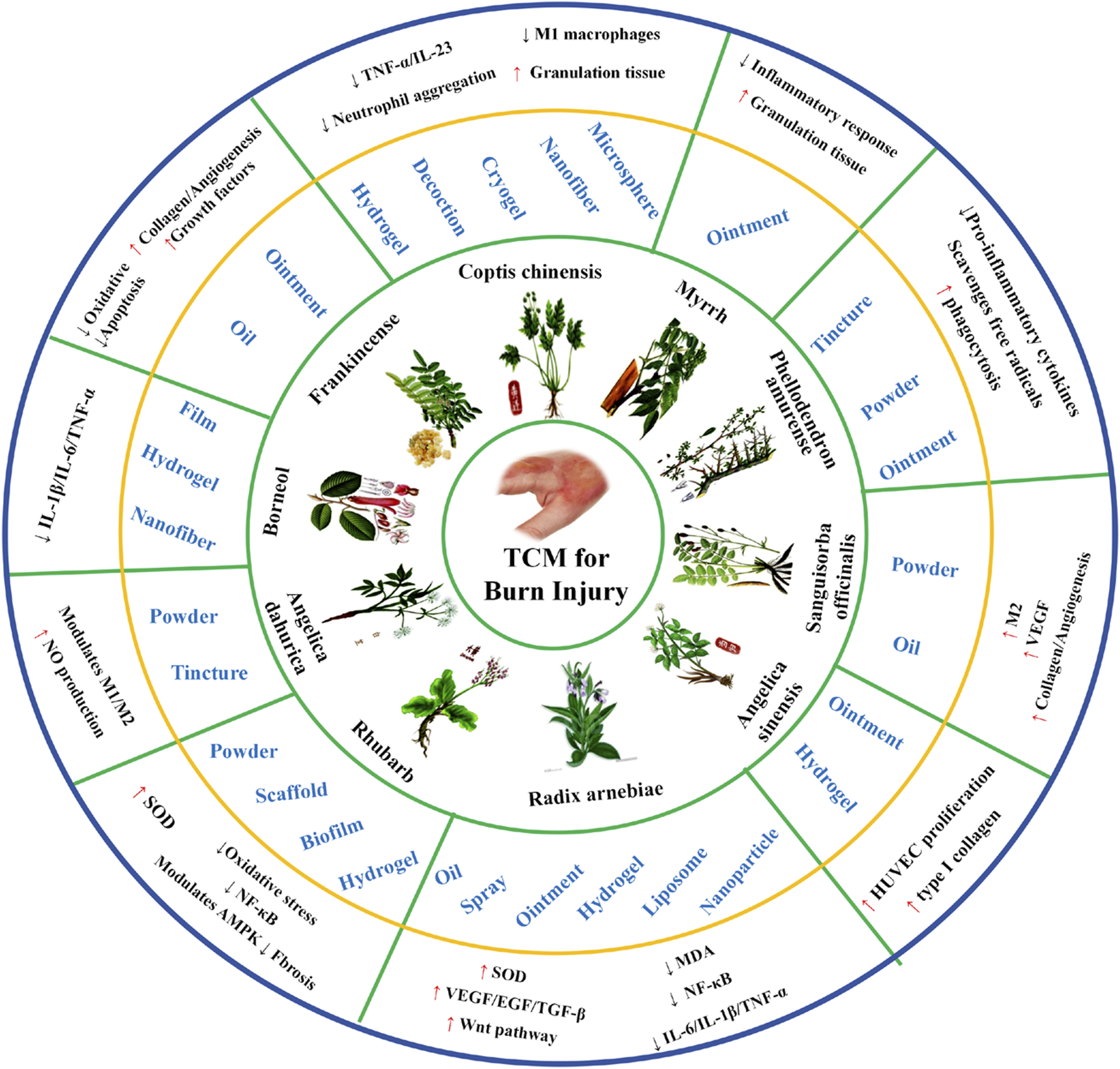
This review highlights the active ingredients, traditional uses, pharmacological actions, target functions, and mechanisms of commonly used Chinese herbal medicine, underscoring their potential in modern burn treatment strategies (Table 1).
TABLE 1
| Chinese herbal medicine | Origin and medicinal parts | Active ingredients | Traditional use | Pharmacological action | Target function | Mechanism | References |
|---|---|---|---|---|---|---|---|
| Radix arnebiae (Zi Cao) |
 Arnebia euchroma (Royle) Johnst Rhizoma
Arnebia euchroma (Royle) Johnst Rhizoma |
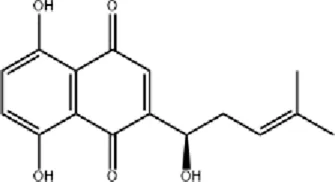 Shikonin Shikonin |
Cool blood and promote blood circulation Clear internal heat Neutralize toxins |
Anti-inflammatory Wound healing Promotes epithelial regeneration |
TGF-β1/PI3K/Akt NF-κB Wnt4 Bax/Bcl-2 |
↑SOD activity ↓MDA ↓IL-6/IL-1β/TNF-α ↑VEGF/EGF/TGF-β Activates Wnt pathway Inhibits NF-κB |
Shu et al. (2022), Sun et al. (2022), Wu T. et al. (2022), Gao et al. (2023) |
| Rhubarb (Da Huang) |
 Rheum palmatum L. Rhizoma
Rheum palmatum L. Rhizoma |
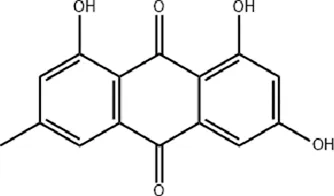 Emodin Rhubarb-derived characoal Emodin Rhubarb-derived characoal |
Eliminate heat toxins Remove accumulations and stagnations Promote circulation of blood stasis |
Anti-inflammatory Antimicrobial Promotes collagen synthesis |
TLR4/NF-κB AMPK/mTOR Notch/TGF-β |
↑SOD ↓oxidative stress Inhibits NF-κB Modulates AMPK pathway to reduce fibrosis |
Tang et al. (2007), Sánchez et al. (2020), Wang et al. (2023), Tan et al. (2024) |
| Angelica sinensis (Dang Gui) |
 Angelica sinensis (Oliv.) Diels Root
Angelica sinensis (Oliv.) Diels Root |
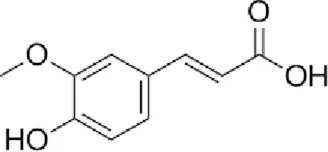 Ferulic acid Polysaccharides Ferulic acid Polysaccharides |
Blood-nourishing and circulation-activating Menstruation-regulating and pain-relieving Intestine-moistening and laxative |
Anti-inflammatory Angiogenesis Collagen synthesis Promotes re-epithelialization |
p38/JNK1/2 VEGF |
↑HUVEC proliferation ↑type I collagen |
Lam et al. (2008), Zhao et al. (2012), Tsai et al. (2016), Li et al. (2021) |
| Coptis chinensis (Huang Lian) |
 Coptis chinensis Franch Rhizoma
Coptis chinensis Franch Rhizoma |
 Berberine Berberine |
Dispel pathogenic fire Remove dampness Neutralize toxins |
Antibacterial Anti-inflammatory |
NF-κB S100B/caspase-8/ β-catenin |
↓TNF-α/IL-23 ↓neutrophil aggregation Enhance granulation tissue Inhibit M1 macrophages |
Habtemariam (2020), Feng et al. (2024), Wang S. et al. (2024) |
| Phellodendron amurense (Huang Bo) |
 Phellodendron chinense Schneid. Bark
Phellodendron chinense Schneid. Bark |
 Phellamurin PhellamurinBerberine |
Clearing internal heat Removing dampness Reducing pathogenic fire Detoxification |
Immunomodulation Antimicrobial | INF-γ IL-1 TNF-α IL-2 |
↓Pro-inflammatory cytokines Scavenges free radicals Enhances phagocytosis |
Xian et al. (2011), Liu et al. (2022) |
| Sanguisorba officinalis (Di Yu) |
 Sanguisorba officinalis L. Rhizoma
Sanguisorba officinalis L. Rhizoma |
 Quercetin QuercetinTannins Polysaccharides |
Cool blood for hemostasis Polysaccharides Clear heat and remove toxins Reduce swelling Promote ulcer healing. |
Antibacterial Anti-inflammatory |
NF-κB/NLRP3 VEGF IL-1β |
↓S. aureus/P. aeruginosa ↑collagen/angiogenesis Promotes M2 macrophage polarization |
Zhang et al. (2018), Song et al. (2023) |
| Angelica dahurica (Bai Zhi) |
 Angelica dahurica (Fisch.ex Hoffm.)Benth. et Hook.f. Rhizoma
Angelica dahurica (Fisch.ex Hoffm.)Benth. et Hook.f. Rhizoma |
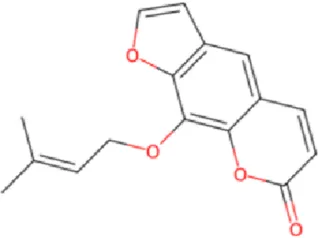 Imperatorin ImperatorinIsorhamnetin |
Dispel pathogenic wind Eliminate dampness Subside swelling Relieve pain |
Antibacterial Angiogenesis Anti-inflammatory |
HIF-1α/PDGF-β ERK1/2/Akt/eNOS |
Modulates M1/M2 macrophages ↑NO production |
Zhang et al. (2017), Guo et al. (2020), Yang et al. (2020), Hu et al. (2021) |
| Natural Borneol (Long Nao) |
 Dryobalanops aromatica C.F.Gaertn.
Dryobalanops aromatica C.F.Gaertn.Natural crystalline |
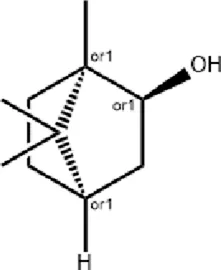 Borneol Borneol |
Revives consciousness Reduces fever and pain Enhances vision and clears eye opacity |
Antioxidant Anti-inflammatory Enhance collagen density |
HIF-1α/NF-κB | ↓IL-1β/IL-6/TNF-α | Barreto et al. (2016), Lv et al. (2022), Chen et al. (2024) |
| Frankincense (Ru Xiang) |
 Boswellia carterii Birdw. Resin
Boswellia carterii Birdw. Resin |
 Boswellic acid Boswellic acid |
Regulate qi and promote blood circulation Relieve pain Eliminate toxins |
Anti-inflammatory Tissue regeneration |
β-catenin Dlk1 COX-2 |
↓Oxidative stress ↓Apoptosis ↑collagen/angiogenesis ↑Growth factors |
Pengzong et al. (2019), Yin et al. (2022) |
| Myrrh (Mo Yao) |
 Commiphora myrrha
Commiphora myrrha
(T.Nees) Engl. Resin |
 Bisacurone Bisacurone |
Disperse and eliminate blood stasis Subside swelling Relieve pain | Angiogenesis Anti-inflammatory |
Oxidative stress markers pro-inflammatory cytokines | ↓Inflammatory response ↑granulation tissue |
Soliman et al. (2019), Batiha et al. (2023), Yan et al. (2023) |
Commonly used Chinese herbal medicine for treating burns.
Radix arnebiae
Radix Arnebiae (Zi Cao), derived from the dried roots of Arnebia euchroma (Royle) Johnst. or Arnebia guttata Bunge as specified in the Chinese Pharmacopoeia, is widely used in TCM to clear heat, cool blood, promote circulation, detoxify, and eliminate rashes (Zhan et al., 2015). Clinically, it is often combined with herbs such as Phellodendron amurense Rupr., Saposhnikovia divaricata (Turcz. ex Ledeb.) Schischk., Angelica dahurica (Hoffm.) Benth. & Hook.f., Angelica sinensis (Oliv.) Diels, and borneol to accelerate wound healing and reduce treatment time for burns (Guo et al., 2019). Radix arnebiae oil (RAO), a common TCM formulation, has shown significant therapeutic efficacy in burn treatment. In a rat burn model, topical application of RAO at a dose of 0.3 g twice daily was initiated on day 1 post-burn. This treatment regimen significantly enhanced superoxide dismutase activity, reduces malondialdehyde production, downregulates pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α), and promotes the secretion of growth factors (VEGF, EGF, and TGF-β), thereby accelerating epithelial regeneration and scar repair. Its mechanism may involve activation of the TGF-β1/PI3K/Akt pathway (Gao et al., 2023).
Shikonin (SNK), the principal active component of Arnebia euchroma (Royle) Johnst., exhibits anti-inflammatory, antibacterial, and wound-healing properties. SNK suppresses inflammation by inhibiting the NF-κB signaling pathway, reducing the expression of Bax, p-p65, and p-p38, while upregulating Bcl-2. It also activates the Wnt signaling pathway through the upregulation of Wnt4, promoting cell proliferation and epithelial tissue regeneration (Sun et al., 2022). Topical SNK ointment enhances wound healing by activating the PI3K/Akt pathway and protecting deep hair follicles (Wu T. et al., 2022).
Hypertrophic scar formation remains a significant clinical challenge post-burn. SNK regulates the AMPK/mTOR signaling pathway, promoting autophagy and apoptosis in hypertrophic scar-derived fibroblasts (HSFs) (Xie et al., 2015; Zhang et al., 2023). Animal studies demonstrate that sprayed 1 mL of 1.0 μg/mL SNK onto the surface of hypertrophic scars every 2 days improves scar repair. This improvement is mediated by the suppression of p63, keratin 10, α-smooth muscle actin, TGF-β, and type I collagen (Deng et al., 2018). A recent innovation involves a temperature-sensitive hydrogel composed of chitosan-β-glycerophosphate, mesoporous carbon nanospheres, nitric oxide (NO) donor sodium nitroprusside, and SNK (loaded at 800 μg/mL). This hydrogel exhibits broad-spectrum antibacterial activity, releases NO under near-infrared (NIR) laser irradiation to promote angiogenesis, inhibits fibroblast overproliferation, and effectively reduces scars in deep second-degree burns, highlighting its potential as a novel clinical product for scar treatment (Bai et al., 2024).
Burn wounds are prone to bacterial infections, including methicillin-resistant Staphylococcus aureus (MRSA), which often form drug-resistant biofilms. SNK-liposomes (SNK concentration 4.6% ± 0.17%), prepared using a film formation method, exhibit sustained release and strong antibacterial activity by disrupting bacterial cell walls and membranes. These liposomes modulate the IκBα/NFκB-p65 signaling pathway, alleviating inflammation and promoting healing in MRSA-infected burn wounds (Shu et al., 2022). Additionally, bio-adhesive nanoparticles (BNP) based on polylactic acid-hyperbranched polyglycerol (PLA-HPG) enhance SNK’s (loaded at 3.6% ± 0.1%) anti-biofilm and wound-healing properties, making SNK/BNP a promising treatment for infected burn wounds (Han et al., 2023).
Rhubarb
Rhubarb (Da Huang), the dried rhizome and root of Rheum palmatum L. is widely used in TCM to eliminate heat-toxins, clear accumulations, promote blood circulation, and facilitate tissue regeneration. Standardized extracts (typically containing 2%–4% total anthraquinones) is frequently employed in burn treatment. Animal studies demonstrate that rhubarb extract (administered at 50 mg/kg/day) enhances the activity of cytochrome oxidase and superoxide dismutase (SOD) in intestinal mucosal epithelial cells of burned rats, reducing mitochondrial oxygen free radical leakage. It also decreases immunoglobulin A (IgA) content in intestinal fluid and alleviates trauma or burn-induced intestinal mucosal damage (Chen et al., 2000). A clinical trial involving 30 severely burned patients revealed that rhubarb (at 30g/day) increases gastrointestinal hormone secretion, restores gastrointestinal motility, and protects the intestinal mucosal barrier (Meng et al., 2011). Furthermore, rhubarb (at 50 mg/kg) mitigates antibiotic-induced dysbiosis by reducing the bactericidal effect on symbiotic bacteria in early sepsis and exerts anti-inflammatory and immune-regulating effects during burn-induced sepsis (Chen et al., 2009; Liu et al., 2019). Recent studies highlight a scaffold composed of cross-linked chitosan and rhubarb-derived charcoal (RCS/SF), which exhibits rapid hemostasis, antibacterial activity, and efficient drug release (rhubarb extract loaded at 20 or 100 mg). This scaffold promotes diabetic wound healing in db/db mice by enhancing neovascularization, collagen deposition, and re-epithelialization within 2 weeks. Additionally, it modulates the AMPK signaling pathway, reducing hepatic lipid accumulation, inflammation, and oxidative stress, underscoring its systemic regulatory role (Wang et al., 2023; Tan et al., 2024).
Emodin, a primary active compound in rhubarb (standardized to >90% purity), enhances fibroblast fibrinolytic activity and migration at concentrations of 30 or 50 μM, facilitating wound healing (Radha et al., 2008). It also promotes type I collagen synthesis in dermal fibroblasts at 1 μM (Song et al., 2021). In animal studies, emodin (applied at (400 μg/mL) accelerates excisional wound healing by stimulating epidermal cell proliferation, capillary generation, and microcirculation, while reducing inflammation via inhibition of the TLR4/NF-κB signaling pathway (Tang et al., 2007). Emodin also alleviates hypertrophic scar formation by inhibiting macrophage polarization (at 10–40 μM), potentially through suppression of the Notch and TGF-β pathways (Sánchez et al., 2020). Additionally, emodin shows therapeutic potential for corneal alkali burns (at 10–20 μM) by suppressing inflammatory cell infiltration and angiogenesis (Kitano et al., 2007; Xueying et al., 2024). To address bacterial infections, a critical factor in wound healing, nano-emodin (N-EMO)-mediated photodynamic therapy (at 40 μg/mL) effectively targets multi-species bacterial biofilms, reducing biofilm formation and virulence factors (Pourhajibagher et al., 2021). A recent study developed a double-network hydrogel incorporating emodin (at a concentration of 0.03%) and chitosan, which significantly promotes blood vessel and collagen regeneration, accelerating wound healing in animal models (Wan et al., 2023).
Angelica sinensis
Angelica sinensis, the dried root of Angelica sinensis (Oliv.) Diels (known as Dang Gui), is widely used in TCM to promote blood circulation, regulate menstruation, alleviate pain, and relieve constipation (Nai et al., 2021). Standardized extracts (often containing ligustilide >0.5%) are clinically employed to treat various skin wounds and accelerate wound healing. Research has shown that Angelica sinensis extract (at 100 μg/mL) promotes the proliferation of human umbilical vein endothelial cells (HUVECs) by modulating the phosphorylation of p38 and JNK 1/2, upregulating VEGF expression, and facilitating angiogenesis (Lam et al., 2008). Additionally, it enhances the proliferation of human dermal fibroblasts and the production of type I collagen, significantly accelerating wound healing in mice (topical application of 2% gel) (Zhao et al., 2012).
The main bioactive constituents of Angelica sinensis (Oliv.) Diels include Angelica sinensis polysaccharides, ligustilide, and ferulic acid (FA). FA (typically used at 10–100 μM in vitro or 5–100 mg/kg in vivo), in particular, exhibits multifunctional properties such as anti-inflammatory, antibacterial, collagen-promoting, angiogenic, and re-epithelialization effects, making it a promising candidate for burn-related wound healing (Li et al., 2021). Yuhong Ointment (YHO), a traditional formulation used for over 600 years to treat skin diseases, contains active constituents such as FA, L-hydroxyproline, chlorogenic acid, and sermanine. These components exert anti-inflammatory and tissue-regenerative effects, demonstrating significant therapeutic efficacy in burns and scalds (Yu et al., 2023).
A recent study developed a biodegradable, multifunctional spray hydrogel containing FA (at 1.0 wt%), which significantly enhances fibroblast proliferation, accelerates infected wound healing, and prevents secondary injuries, highlighting its potential for clinical application (Zhong et al., 2024). In a rabbit corneal alkali burn model, a thermosensitive chitosan-based hydrogel containing FA (1 mg/mL) significantly reduced inflammatory factors and suppressed cell apoptosis, promoting corneal wound healing (Tsai et al., 2016). Furthermore, a multifunctional hydrogel containing FA (at 2% (w/v)) has been shown to inhibit MRSA infection, reduce excessive inflammation, promote angiogenesis, and accelerate wound healing and skin tissue regeneration (Li et al., 2024). A nano-hydrogel composed of FA-grafted chitosan exhibits enhanced antioxidant activity by scavenging ABTS and DPPH free radicals, while effectively inhibiting Bacillus subtilis, MRSA, Escherichia coli, and Pseudomonas aeruginosa, facilitating the healing of infected wounds (Prasathkumar et al., 2024).
Coptis chinensis
Coptis chinensis (Huang Lian), the dried rhizome of Coptis chinensis Franch., is widely used in TCM to clear heat and dampness, purge fire, and detoxify (Wang et al., 2019). Its standardized extracts, which typically contain total alkaloids >10% (including berberine >5%), are commonly incorporated into modern formulations. Huang Lian Jie Du Decoction, a classic TCM formula, exhibits anti-inflammatory, antibacterial, and microcirculation-improving effects, making it effective for treating burns and febrile diseases (Qi et al., 2019). Berberine (BBR), the main active ingredient of Coptis chinensis Franch. (standardized to >97% purity), demonstrates potent antibacterial and anti-inflammatory properties (Kong et al., 2022). BBR inhibits the secretion of pro-inflammatory cytokines (e.g., TNF-α and IL-23) by suppressing NF-κB activity, modulating neutrophil migration, and reducing neutrophil aggregation in inflammatory regions, thereby mitigating inflammatory responses (Habtemariam, 2020). Recent studies indicate that BBR (at 100 mg/kg/d) reduces burn-induced gut vascular barrier hyperpermeability by modulating the S100B/caspase-8/β-catenin pathway, potentially involving enteric glial cells (Feng et al., 2024).
To address antibiotic resistance, Sun S. et al. developed an antibiotic-free polysaccharide-based hydrogel dressing (ATB) containing BBR hydrochloride (loaded at 1 mg/mL). This dressing synergistically eliminates bacteria and accelerates wound healing in both in vitro and in vivo experiments, offering a solution to the overuse of antibiotics (Sun et al., 2024). Additionally, silk fibroin microspheres loaded with berberine (Ber@MPs, BBR loading 40 mg) exhibit strong antibacterial effects against Staphylococcus aureus and Staphylococcus epidermidis, reduce inflammation, promote fibroblast migration and endothelial cell neovascularization, and significantly accelerate infected wound healing (Sang et al., 2023; Zhang et al., 2024).
Wound healing, a complex biological process critical for tissue repair, is enhanced by BBR-containing cryogels (loading at 2.08%–5.88%), which accelerate granulation tissue formation, epithelial regeneration, and collagen deposition (Dar et al., 2024). Furthermore, nanofiber dressing patches containing BBR (loading at 4.82%–13.69%) inhibit pro-inflammatory factor secretion by M1 macrophages, promote fibroblast proliferation, and exhibit broad-spectrum antimicrobial activity. Animal studies demonstrate that these dressings (applied at 1–2 mg/cm2) accelerate full-thickness skin wound healing, shorten healing time, and improve healing quality, highlighting their potential for treating chronic and difficult-to-heal wounds (Wang Q. et al., 2024).
Phellodendron amurense
Phellodendri Cortex (Huang Bo), the dried bark of Phellodendron chinense Schneid. or Phellodendron amurense Rupr. as specified in the Chinese Pharmacopoeia, is widely used in TCM to clear heat, remove dampness, purge fire, and detoxify (Sun et al., 2019). Standardized extracts (typically containing berberine >3%, total alkaloids >5%) are commonly used. Research demonstrates that Phellodendron chinense Schneid. extract (at 100–500 mg) exerts immunomodulatory effects by inhibiting the production and secretion of key cytokines such as interferon-γ (INF-γ), IL-1, TNF-α, and IL-2, thereby alleviating inflammatory damage (Xian et al., 2011). It also exhibits antioxidant effects by scavenging free radicals and enhances the phagocytic function of monocytes/macrophages, improving nonspecific immunity (Liu et al., 2022). The main bioactive constituents, including phellodendri, berberine, and other alkaloids, display significant antimicrobial activity against pathogens such as Staphylococcus aureus, Streptococcus albus, Streptococcus pneumoniae, Bacillus subtilis, and Pseudomonas aeruginosa (Chen et al., 2010).
Sanguisorba officinalis
Sanguisorba officinalis (Di Yu), the dried rhizome of Sanguisorba officinalis L., is widely used in TCM to cool blood, stop bleeding, clear heat, detoxify, reduce swelling, and promote wound healing. Standardized extracts (often containing tannins >10%, total flavonoids >2%) are typically employed. Modern research has identified a rich variety of bioactive compounds in Sanguisorba officinalis, including tannins, triterpenoids, flavonoids, and polysaccharides. Pharmacological studies have demonstrated its diverse functions, such as hemostatic, antibacterial, anti-tumor, anti-allergic, anti-inflammatory, and anti-edema effects (Zhou et al., 2021). The antibacterial activity of Sanguisorba officinalis L. is primarily attributed to its tannin components, which exhibit inhibitory effects against pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter baumannii, and Streptococcus pneumoniae (Zhao et al., 2017). Quercetin, a flavonoid present in Sanguisorba officinalis, has been identified as a key contributor to its antioxidant effects, which are beneficial for promoting wound healing (Jang et al., 2018). Additionally, the ethanol extract of Sanguisorba officinalis extracts (at 2.5–10 g/kg/d) accelerates diabetic wound healing by inhibiting the NF-κB/NLRP3 signaling pathway and facilitating macrophage polarization from the M1 to the M2 phenotype (Song et al., 2023). A purified polysaccharide (SOP) extracted from Sanguisorba officinalis L. has shown remarkable efficacy in mouse burn models, significantly accelerating wound contraction and reducing epithelialization time. SOP administration increases levels of IL-1β and VEGF, promoting granulation tissue formation, collagen synthesis, and angiogenesis, thereby expediting wound repair (Zhang et al., 2018).
Angelica dahurica
Angelica dahurica (Bai Zhi), the dried root of Angelica dahurica (Fisch. ex Hoffm.) Benth. & Hook.f., is traditionally used in TCM to dispel wind, eliminate dampness, reduce swelling, and relieve pain. Standardized extracts (typically containing imperatorin >0.5%, total coumarins >2%) are used. Modern pharmacological studies reveal its anti-inflammatory, analgesic, antispasmodic, and antibacterial properties (Zhao et al., 2022). Animal experiments demonstrate that Angelica dahurica (Fisch. ex Hoffm.) Benth. & Hook.f. water extract (at 6g//kg/d) modulates macrophage polarization (M1/M2), exerting anti-inflammatory effects and promoting wound healing (Hu et al., 2021). Its combination with Angelica sinensis (Oliv.) Diels and Rheum officinale Baill. enhances wound healing during inflammatory and proliferative phases (Yang et al., 2017; Chao et al., 2021). Additionally, Angelica dahurica (Fisch. ex Hoffm.) Benth. & Hook.f. promotes angiogenesis in HUVECs by upregulating the HIF-1α/PDGF-β pathway and enhancing angiogenic signals such as ERK1/2, Akt, eNOS, and NO production, suggesting its potential for vascular injury-related wounds (Zhang et al., 2017; Guo et al., 2020). Key bioactive compounds include imperatorin, isoimperatorin, and psoralen. Recent studies highlight isorhamnetin (applied at 0.1%–0.5% topically), which alleviates chronic inflammation, promotes epithelial regeneration, and accelerates Staphylococcus aureus infected wound healing through its anti-inflammatory, proliferative, and antibacterial properties (Yang et al., 2020; Lakshmanan et al., 2024).
Natural borneol
Natural borneol, which is derived from the tree Dryobalanops aromatica C.F.Gaertn. and standardized to a purity of >96%, is used in TCM for opening the orifices, improving mental alertness, clearing heat, and relieving pain. Research shows that borneol promotes wound healing by mitigating oxidative stress, facilitating neutrophil recruitment, and suppressing inflammatory cytokines (IL-1β, IL-6, and TNF-α) via inhibition of the HIF-1α/NF-κB pathway (Chen et al., 2024). A chitosan-based film containing 1% borneol (QUIBO1) accelerates wound contraction, enhances granulation tissue formation, and improves collagen density (Barreto et al., 2016). Nanofibers incorporating alum and borneol, fabricated via coaxial electrospinning, increase borneol dissolution and wound healing efficacy (Lv et al., 2022). Additionally, a natural antibacterial hydrogel, synthesized through Schiff base cross-linking of carboxymethyl chitosan and dialdehyde dextran grafted with borneol, exhibits strong antibacterial activity against Escherichia coli and Staphylococcus aureus, excellent cytocompatibility, and targeted delivery potential for localized wound infections (Zhao et al., 2024).
Frankincense
Frankincense (Chinese name: Ru Xiang), the resin obtained from Boswellia sacra Flück. and related species and standardized to contain boswellic acids (>30%), is traditionally used in TCM to promote blood circulation, alleviate pain, and eliminate toxins. Its bioactive constituents, primarily pentacyclic triterpenes (e.g., boswellic acids) and volatile oils, have been demonstrated to possess anti-inflammatory, anti-proliferative, analgesic, antioxidant, and antibacterial properties (Morikawa et al., 2017). Boswellic acids (effective at 5–50 μM) promote wound healing by inhibiting oxidative inflammatory markers, enhancing collagen synthesis and angiogenesis, promoting growth factors, and suppressing apoptosis (Pengzong et al., 2019). Clinical studies confirm the efficacy of myrrh and frankincense-based sitz baths in post-episiotomy wound healing (Faraji et al., 2021). Incorporating essential oils (e.g., clove, cinnamon, frankincense) into biodegradable polymer membranes enhances biological activity and protects against degradation, offering a novel approach to wound healing dressings (Borges et al., 2024). ShengFu Oil, a topical TCM formulation, contains standardized extracts of Scutellaria baicalensis Georgi, Boswellia carterii Birdw., and Rheum palmatum L. It has been demonstrated to possess anti-inflammatory, analgesic, and antibacterial properties. It facilitates burn wound healing through the regulation of key biomarkers (β-catenin, Dlk1, COX-2) and concurrent modulation of the inflammatory microenvironment, playing a vital role in the prevention and treatment of oral chemical burns (Han et al., 2017; Yin et al., 2022).
Myrrh
Myrrh (Chinese name: Mo Yao), the resin obtained from Commiphora myrrha (T.Nees) Engl. and standardized to contain volatile oils (>5%), is traditionally used in TCM to disperse blood stasis, alleviate pain, reduce swelling, and promote tissue regeneration.
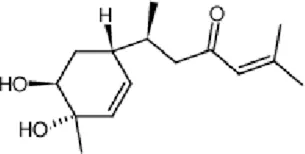
The therapeutic efficacy and indications of myrrh are highly similar to those of frankincense, often resulting in their combined application in clinical practice. Research results have shown that the topical application of perilla-frankincense-myrrh volatile oil can attenuate the inflammatory response in the early stage of wounds and expedite wound healing in mice (Batiha et al., 2023). Jinchuang ointment, a TCM compound composed of borneol, catechu, frankincense, and myrrh, displays the functions of promoting angiogenesis, cell proliferation, and migration activity, which is conducive to the enhancement of the wound healing process (Ho et al., 2016). Bisacurone is one of the main bioactive compounds in myrrh. Recent research has demonstrated that the topical application of bisacurone gel (0.5%–2% concentration) can effectively diminish oxidative stress and pro-inflammatory cytokines, promote angiogenesis and granulation tissue formation, and remarkably accelerate the healing of wounds in second-degree burn rats (Yan et al., 2023).
Treatment of burn with TCM preparations
TCM external treatments are a cornerstone of burn management, offering remarkable therapeutic efficacy with minimal side effects. With advancements in modern TCM pharmacology, a variety of external preparations, such as ointments, sprays, powders, tinctures, hydrogels, and nanofibers, have been developed and widely used in clinical practice. These formulations, combined with innovations in biomaterials and tissue engineering, provide effective solutions for wound management. This review systematically summarizes the application of TCM preparations in burn treatment, with a focus on their active ingredients, therapeutic effects and mechanisms (Table 2).
TABLE 2
| Herbs/Active ingredients | Dosage form | Preparations | Therapeutic effects | Mechanism | References |
|---|---|---|---|---|---|
| Radix arnebiae Shikonin (SNK) | Oil Spray Ointment Hydrogel Liposome Nanoparticle |
Radix arnebiae oil (RAO) Radix arnebiae spray Shikonin (SNK) ointment Temperature-sensitive hydrogel SNK-liposomes SNK/BNP nanoparticles |
Anti-inflammatory Antibacterial Promotes epithelial regeneration Promotes angiogenesis Wound healing Scar reduction |
Inhibit NF-κB Activate Wnt/PI3K/Akt Modulate TGF-β1/PI3K/Akt AMPK/mTOR pathways |
Shu et al. (2022), Wu T. et al. (2022), Han et al. (2023), Liu et al. (2024) |
| Rhubarb Emodin Rhubarb-derived charcoal |
Scaffold Biofilm Hydrogel |
Rhubarb charcoal-crosslinked chitosan/silk fibroin sponge scaffold Nano-emodin (N-EMO) biofilms Chitosan-emodin network hydrogel |
Antioxidant Anti-inflammatory Promote tissue regeneration Wound healing |
Enhance SOD activity Inhibit TLR4/NF-κB Modulate AMPK pathway |
Pourhajibagher et al. (2021), Wan et al. (2023), Wang et al. (2023), Tan et al. (2024) |
| Angelica sinensis Ferulic acid (FA) Angelica polysaccharides Ligustilide |
Ointment Hydrogel |
Yuhong Ointment (YHO) CSMA-FA/OBSP (CSOB-FA) hydrogel Thermosensitive chitosan-FA hydrogel Bioactive poly(FA) hydroge FA-grafted chitosan nano-hydrogel |
Angiogenesis Collagen synthesis Anti-inflammatory Anti-scarring |
Upregulate VEGF Modulate p38/JNK pathways Promote fibroblast proliferation |
Tsai et al. (2016), Yu et al. (2023), Li et al. (2024), Prasathkumar et al. (2024), Zhong et al. (2024) |
| Coptis chinensis Berberine (BBR) |
Decoction Hydrogel Cryogel Microsphere Nanofiber |
Huang Lian Jie Du Decoction Polysaccharide-based hydrogel with BBR BBR-containing cryogels Berberine-loaded silk fibroin microspheres BBR nanofiber dressing patches |
Antibacterial Anti-inflammatory Promote fibroblast migration Enhance neovascularization Wound healing |
Inhibit NF-κB, Modulates S100B/caspase-8/β-catenin ↑Granulation tissue formation, collagen deposition and epithelial regeneration |
Qi et al. (2019), Sang et al. (2023), Dar et al. (2024), Sun et al. (2024), Wang Q. et al. (2024) |
| Phellodendron amurense Coptis chinensis Scutellaria |
Ointment | Moist Exposed Burn Ointment (MEBO) | Antibacterial Analgesi Promote granulation tissue formation Activate epidermal stem cells |
↑VEGF/bFGF Activate PI3K-Akt-mTOR pathway Induce the autophagy process |
Mabvuure et al. (2020), Zheng et al. (2020) |
| Phellodendron amurense Coptis chinensis Rhubarb |
Powder | Sanhuang powder | Anti-inflammatory Heat-clearing Detoxifying |
↓IL-8/GM-CSF | Wu et al. (2021) |
| Phellodendron amurense Cinnabar, Safflower | Tincture | Qi Wei Anti-burn Tincture | Anti-inflammatory Antioxidant Liver protection |
↑TGF-β1, FGF-2 ↓TNF-α, IL-1β, IL-6 ↓ROS reduction |
(Wang S. et al., 2024b) |
| Borneol | Film Hydrogel Nanofiber |
Borneol-chitosan film Schiff base-crosslinked hydrogel Alum/borneol coaxial nanofibers |
Anti-inflammatory Antibacterial activity Wound healing |
Inhibit HIF-1α/NF-κB Promote granulation Improve collagen density |
Barreto et al. (2016), Lv et al. (2022), Zhao et al. (2024) |
| Aloe vera, Borneol Musk, Mint | Gel | Aloe vera gel | Reduce itching and pain Enhance re-epithelialization |
Stimulate fibroblast and keratinocyte proliferation |
Mahboub et al. (2021) |
| Scutellaria baicalensis Frankincense, Rhubarb |
Oil | ShengFu Oil | Anti-inflammatory Wound healing |
Regulate β-catenin/Dlk1/COX-2 | Yin et al. (2022) |
| Borneol, Catechu, Frankincense, Myrrh | Ointment | Jinchuang ointment | Stimulate angiogenesis Promote cell proliferation Enhance cell migration |
Angiogenic activity Wound healing promotion |
Ho et al. (2016) |
| Bisacurone | Gel | Chitosan-based bisacurone gel | Anti-inflammatory Oxidative stress reduction Enhance angiogenesis |
↓Pro-inflammatory cytokines ↓MDA, NO; ↑SOD, glutathione ↑Growth factors |
Yan et al. (2023) |
| Curcumin | Hydrogel Nanofiber |
Curcumin-loaded magnesium polyphenol network (Cur-Mg@PP) hydrogel mPEG-CUR loaded PVA/CS-g-PNVIS nanofibers |
Antimicrobial Antioxidant, Anti-inflammatory Analgesic, Angiogenesis Tissue regeneration |
Enhance biocompatibility Enable electrospinning process Structure mimics ECM Moist wound environment maintenance |
Gong et al. (2023); Shaabani et al. (2023) |
| Epigallocatechin gallate | Injectable hydrogel | GelMA/HA-E/Ag@MOF composite hydrogel | Antibacterial/Anti-inflammatory Accelerated wound closure Angiogenesis promotion |
Macrophage polarization (M1→M2) Activation Noncanonical Wnt pathway |
Xiong et al. (2022) |
| Asiaticoside | Injectable hydrogel | rColMA/QCSG/LIP@AS/Ag@MOF (RQLAg) hydrogel | Antibacterial Anti-inflammatory Accelerates wound healing |
Activate M1 macrophages Promote angiogenesis Enhance cell migration |
Feng et al. (2023) |
| Alginate | Core-shell nanofiber | Asiaticoside-loaded nanofibers | Antibacterial/Anti-inflammatory Angiogenesis promotion | ↑VEGF, CD31 expression ↓TNF-α, IL-6 Improve cell proliferation |
Zhu et al. (2016) |
| Lavender active compound | Electrospun nanofiber | Alginate-lavender essential oil nanofibers | Antibacterial,Anti-inflammatory UVB burn protection Prevent erythema formation Promote tissue regeneration |
Moist wound environment Biocompatibility Wound exudate management |
Hajiali et al. (2016) |
| Bakuchiol | Nanofibrous electrospun scaffold | Bakuchiol nanoemulsion-loaded gelatin scaffold | Antioxidant, Analgesic Enhanced wound healing Antibacterial, Anti-inflammatory |
Enhance BAK stability Controll drug release Uniform biomarker distribution |
Kaur et al. (2024) |
TCM preparations for burn treatment.
Ointments
Ointments are a common dosage form for burn treatment, offering excellent adhesiveness and direct application to wounds. They prevent external stimuli and bacterial infections while providing anti-inflammatory, analgesic, and tissue-repairing effects. Moist Exposed Burn Ointment (MEBO), a patented TCM formulation, accelerates wound healing, exhibits antibacterial properties, and alleviates pain (Mabvuure et al., 2020). MEBO enhances granulation tissue formation, promotes the production of VEGF and bFGF, and activates epidermal stem cells (El-Hadidy et al., 2014; Tang et al., 2014). It also facilitates diabetic ulcer healing through autophagy and the PI3K-Akt-mTOR signaling pathway (Zheng et al., 2020). Aloe vera burn cream stimulates fibroblast and keratinocyte proliferation, significantly improving re-epithelialization rates and outperforming 1% sulfadiazine silver cream in treating second-degree burns (Teplicki et al., 2018; Mahboub et al., 2021).
Sprays
Sprays offer a convenient application method, reducing pain during drug administration and making them ideal for large-area burns. They form a breathable, elastic film on the wound surface, promoting granulation tissue growth. A spray formulation containing the extracts of Arnebia euchroma (Royle) Johnst.), Taraxacum mongolicum Hand.-Mazz., Phellodendron chinense Schneid. and borneol rapidly forms a protective film within 3–5 min. This film effectively shields wounds from contamination and infection while accelerating eschar formation (Liu et al., 2024). Autologous cell spray grafting, an innovative approach, uses a suspension of the patient’s skin cells to treat deep burns, significantly enhancing re-epithelialization and wound healing (Esteban-Vives et al., 2016; Shree and Vagga, 2022).
Powders
Powders are simple to prepare and effectively absorb necrotic tissue from burn wounds. However, they may cause excessive crust formation and contamination risks. Sanhuang Powder, a classic TCM formula composed of Rheum palmatum L., Phellodendron chinense Schneid., and Coptis chinensis Franch., is used to clear heat and resolve toxins. Modern studies indicate that it also reduces the levels of pro-inflammatory cytokines, including IL-8 and GM-CSF (Wu et al., 2021). Jinhuang powder, a classic TCM surgical preparation, promotes fibroblast proliferation and migration via the Wnt/β-catenin signaling pathway, effectively treating diabetic foot wounds when combined with MEBO (Zhan et al., 2021; Wu M. et al., 2022).
Tinctures
Tinctures, which are herbal extracts dissolved in ethanol, facilitate easy monitoring of wounds; however, their irritant properties limit their application to first-degree burns. The Qi Wei Anti-burn Tincture, formulated with Phellodendron chinense C.K.Schneid., Melaleuca phoenicea (Lindl.) Craven and synthetic borneol, has been demonstrated to upregulate the expression of growth factors TGF-β1 and FGF-2, while downregulating the levels of pro-inflammatory mediators (TNF-α, IL-1β, IL-6) and reactive oxygen species in the livers of burned mice (Dinda et al., 2015; Wang S. et al., 2024).
Hydrogels
Hydrogels are highly promising for burn treatment due to their ability to adhere to uneven wound surfaces, inhibit bacterial growth, and reduce pain during dressing changes (Stoica et al., 2020). Multifunctional hydrogels derived from TCM active components offer antibacterial, anti-inflammatory, hemostatic, and tissue-regenerative properties (Shu et al., 2021). For example, a magnesium polyphenol network (Cur-Mg@PP) hydrogel loaded with curcumin demonstrates excellent therapeutic effects in pain relief, anti-inflammation, angiogenesis, and tissue regeneration (Gong et al., 2023). Another hydrogel loaded with epigallocatechin gallate exhibits dual antibacterial and anti-inflammatory properties, accelerating wound healing via the non-classical Wnt signaling pathway (Xiong et al., 2022). Additionally, a liposome-based hydrogel containing asiaticoside and superfine silver nanoparticles promotes cell migration, angiogenesis, and M1 macrophage polarization, effectively treating bacterial-infected burn wounds (Feng et al., 2023).
Nanofibers
Nanofibers loaded with bioactive compounds like curcumin and quercetin mimic the extracellular collagen matrix, supporting cell growth and accelerating burn wound healing (Shaabani et al., 2023). Asiaticoside-loaded nanofibers exhibit fast drug release and anti-inflammatory effects, significantly promoting healing in deep partial-thickness burns (Zhu et al., 2016). Alginate-lavender nanofibers possess antibacterial and anti-inflammatory properties, effectively treating burns by inhibiting Staphylococcus aureus growth and reducing inflammation in fibroblasts (Hajiali et al., 2016). Recent research shows that bakuchiol nanoemulsion-loaded gelatin scaffold exhibit significant analgesic, anti-inflammatory and wound healing promoting effects, and have potential application value in the treatment of burn wounds (Kaur et al., 2024).
Treatment of burn with TCM auxiliary therapies
In addition to TCM drugs and preparations, the TCM system includes unique therapies such as acupuncture, cupping, gua sha, tuina, and aromatherapy. These therapies complement conventional treatments, enhancing burn recovery and improving patients’ quality of life. Acupuncture, a cornerstone of TCM, plays a vital role in burn management. It alleviates pain, modulates inflammatory responses, promotes epithelialization and angiogenesis, and accelerates wound healing. A case study involving 1,008 burn patients demonstrated that acupuncture significantly improves wound healing outcomes in medical, economic, and biopsychosocial aspects (Loskotova and Loskotova, 2017). Acupoint stimulation therapy, a key form of acupuncture, modulates the neuroendocrine system by targeting specific acupoints such as Quchi, Hegu, Taichong, Xuehai, Sanyinjiao, and Zhiyang, reducing pain and inflammation. Electroacupuncture, which combines traditional acupuncture with electrical stimulation, enhances blood circulation, improves nerve conduction, reduces edema, and promotes wound healing. A randomized controlled trial showed that electroacupuncture at the bilateral Dingchuan acupoint improves lung function and diaphragm activity in patients with inhalation burns (Ali et al., 2022). Auricular therapy, targeting ear acupoints like Shenmen and Subcortical, effectively reduces pain, itching, and sleep disturbances in burn patients (Chen et al., 2021). Cupping induces local congestion through negative pressure, promoting blood circulation and lymphatic drainage. This process accelerates toxin elimination and generates anti-inflammatory, analgesic, and wound-healing effects. Before treatment, the burn area must be thoroughly cleaned, and key acupoints such as DU14 (Dazhui), LI11 (Quchi), and ST36 (Zusanli) are selected. Cupping can be performed using fire cups, air cups, or electric cups, followed by skin cleaning and medicinal cream application (Al-Bedah et al., 2019). Gua sha and tuina improve local blood circulation, enhance metabolic processes, relieve inflammation and edema, reduce pain, and promote wound healing. These manual therapies are particularly effective in managing burn-related discomfort and accelerating recovery (Xie et al., 2022). Aromatherapy utilizes plant essential oils extracted from flowers, leaves, and fruits to promote physical and mental wellbeing. It helps relax the nervous system, reduce stress, facilitate deep sleep, and alleviate the physical and mental stress caused by burns (Lee et al., 2021).
Combined treatment of burn with TCM and modern medicine
The integration of TCM and modern medicine represents a pivotal strategy in burn treatment, combining the strengths of both systems to optimize patient outcomes. Western medicine excels in rapid infection control, pain relief, and body temperature regulation, while TCM offers a holistic approach, minimal side effects, and favorable conditions for recovery. Together, they provide complementary benefits that address the multifaceted challenges of burn management. Modern medicine’s dry therapy facilitates convenient wound observation and rapid healing by drying the wound and promoting scabbing, often followed by surgical or other reparative interventions. In contrast, TCM’s moist therapy emphasizes moist wound repair, promotes physiological regeneration, and reduces scar formation. Additionally, traditional TCM therapies such as acupuncture and cupping play a distinctive role in alleviating pain, enhancing local blood circulation, and improving overall recovery. The research and development of TCM preparations further expand treatment options through the integration of TCM and modern medicine. However, several challenges remain. Firstly, the multifaceted components and diverse mechanisms of TCM make it difficult to fully elucidate its efficacy using modern medical standards. Secondly, inconsistent quality control standards in TCM may lead to batch-to-batch variations, affecting reproducibility and reliability. Finally, variations in treatment methods and medication practices between TCM and Western medicine necessitate enhanced communication and collaboration to bridge gaps and optimize integrated care.
Conclusion
TCM has a long history and a well-established theoretical system for treating burn injuries, providing a robust foundation for modern scientific evaluation. As TCM modernization advances, the active ingredients and molecular mechanisms underlying its efficacy in burn treatment have been increasingly elucidated. This review explores the approaches and research progress in TCM for burn management, summarizing current TCM drugs, external preparations, adjunctive therapies, and their potential mechanisms. Regarding safety concerns, topical TCM applications for burn treatment generally demonstrate favorable safety profiles with minimal systemic side effects, owing to their localized administration and natural origins. However, vigilance remains essential as certain herbal components may cause local skin reactions, allergic responses, or interact with conventional therapies. High-dose applications of specific active ingredients, particularly alkaloids and anthraquinones, warrant careful consideration due to potential cytotoxic effects at elevated concentrations. Furthermore, the integration of TCM with modern burn therapies necessitates attention to potential pharmacological interactions, especially when combining herbal preparations with systemic medications.
Future research should prioritize comprehensive safety assessments, including long-term toxicity studies and drug interaction profiling, to establish evidence-based guidelines for safe clinical application. Most TCM burn treatments remain limited to animal models, highlighting the need for randomized, double-blind, placebo-controlled clinical trials in human patients to validate their efficacy and safety. Furthermore, the diversification of TCM external dosage forms, driven by advancements in pharmaceutical technology, presents both challenges and opportunities. Future research should focus on enhancing TCM preparation development, establishing systematic quality control systems, and improving therapeutic efficacy and safety. These efforts are essential for integrating TCM into mainstream burn treatment protocols and optimizing patient outcomes.
Statements
Author contributions
YL: Writing – original draft, Funding acquisition, Writing – review and editing, Conceptualization. CY: Writing – review and editing, Validation. TD: Writing – review and editing, Software. YS: Writing – review and editing. HL: Writing – review and editing, Validation. ML: Data curation, Writing – review and editing. WX: Writing – review and editing. WZ: Writing – review and editing. ZF: Writing – review and editing. MH: Supervision, Writing – review and editing. YZ: Writing – review and editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Macau Science and Technology Development fund (FDCT (0012/2021/AMJ, 003/2022/ALC, 0092/2022/A2,0144/2022/A3)). Shenzhen-Hong Kong-Macao Science and Technology Fund (Category C: SGDX20220530111203020). Guangdong Province’s Special Fund for Science and Technology Innovation Strategy (pdjh2024a523).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- TCM
Traditional Chinese Medicine
- TGF-β
transforming growth factor-β
- PDGF
platelet-derived growth factor
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- IL-8
interleukin-8
- IL-6
interleukin-6
- IL-1β
interleukin-8
- IL-2
interleukin-8
- IL-23
interleukin-8
- TNF-α
tumor necrosis factor-alpha
- VEGF
vascular endothelial growth factor
- ECM
extracellular matrix
- MMPs
matrix metalloproteinases
- RAO
Radix arnebiae oil
- SNK
shikonin
- HSFs
hypertrophic scar-derived fibroblasts
- MRSA
methicillin-resistant Staphylococcus aureus
- SOD
superoxide dismutase
- IgA
immunoglobulin A
- HUVECs
human umbilical vein endothelial cells
- FA
ferulic acid
- BBR
Berberine
- MEBO
Moist Exposed Burn Ointment
- NO
nitric oxide
- NIR
near-infrared
- BNP
bio-adhesive nanoparticles
- PLA-HPG
polylactic acid-hyperbranched polyglycerol
- RCS/SF
scaffold composed of cross-linked chitosan and rhubarb-derived charcoal
- N-EMO
nano-emodin
- SDT
photodynamic therapy
- YHO
Yuhong Ointment
- ABTS
2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid)
- DPPH
1,1-Diphenyl-2-picrylhydrazyl radical
- ATB
antibiotic-free polysaccharide-based hydrogel dressing
- Ber@MPs
silk fibroin microspheres loaded with berberine
- SOP
polysaccharide
- QUIBO1
chitosan-based film containing 1% borneol
- Cur-Mg@PP
magnesium polyphenol network
References
1
Al-Bedah A. M. N. Elsubai I. S. Qureshi N. A. Aboushanab T. S. Ali G. I. M. El-Olemy A. T. et al (2019). The medical perspective of cupping therapy: effects and mechanisms of action. J. Tradit. Complement. Med.9 (2), 90–97. 10.1016/j.jtcme.2018.03.003
2
Ali Z. A. Eladl H. M. Abdelbasset W. K. Eid M. M. Mosa H. E. Elsayeh S. M. (2022). Inhalation injury in adult males: evaluation of the short-term efficacy of transcutaneous electrical acupoint stimulation on pulmonary functions and diaphragmatic mobility after burn: a double-blind randomized controlled study. Burns48 (8), 1933–1939. 10.1016/j.burns.2022.01.015
3
Bai D. Cheng H. Mei J. Tian G. Wang Q. Yu S. et al (2024). Rapid formed temperature-sensitive hydrogel for the multi-effective wound healing of deep second-degree burn with shikonin based scar prevention. Biomater. Adv.160, 213851. (Electronic). 10.1016/j.bioadv.2024.213851
4
Barreto R. S. Quintans J. S. Barreto A. S. Albuquerque-Junior R. L. Galvao J. G. Gonsalves J. K. et al (2016). Improvement of wound tissue repair by chitosan films containing (-)-borneol, a bicyclic monoterpene alcohol, in rats. Int. Wound J.13 (5), 799–808. 10.1111/iwj.12385
5
Batiha G. E. Wasef L. Teibo J. O. Shaheen H. M. Zakariya A. M. Akinfe O. A. et al (2023). Commiphora myrrh: a phytochemical and pharmacological update. Naunyn Schmiedeb. Arch. Pharmacol.396 (3), 405–420. 10.1007/s00210-022-02325-0
6
Borges J. C. de Almeida Campos L. A. Kretzschmar E. A. M. Cavalcanti I. M. F. (2024). Incorporation of essential oils in polymeric films for biomedical applications. Int. J. Biol. Macromol.269 (Pt 1), 132108. 10.1016/j.ijbiomac.2024.132108
7
Chao Y. H. Yang W. T. Li M. C. Yang F. L. Lee R. P. (2021). Angelica dahurica and Rheum officinale facilitated diabetic wound healing by elevating vascular endothelial growth factor. Am. J. Chin. Med.49 (6), 1515–1533. 10.1142/S0192415X21500713
8
Chen D. Qiao L. Jing B. (2000). Effect of rhubarb on oxygen radicals leakage from mitochondria of intestinal mucosa in burned rats. Zhongguo Zhong Xi Yi Jie He Za Zhi20 (11), 849–852.
9
Chen D. C. Ma L. Q. Liu S. Z. (2009). Effects of rhubarb on intestinal flora and bacterial translocation in rats with sepsis. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue21 (1), 17–20.
10
Chen M. L. Xian Y. F. Ip S. P. Tsai S. H. Yang J. Y. Che C. T. (2010). Chemical and biological differentiation of cortex phellodendri chinensis and cortex phellodendri amurensis. Planta Med.76 (14), 1530–1535. 10.1055/s-0030-1249774
11
Chen C. C. Chen S. P. Lyu S. Y. Hsu C. H. (2021). Application of auriculotherapy for post-burn scar syndrome in young adults with major burns. J. Acupunct. Meridian Stud.14 (4), 127–136. 10.51507/j.jams.2021.14.4.127
12
Chen G. Yang J. Wang A. Deng J. Wang K. Ye M. et al (2024). L-Borneol promotes skin flap survival by regulating HIF-1α/NF-κB pathway. J. Ethnopharmacol.321, 117543. (Electronic). 10.1016/j.jep.2023.117543
13
Childs D. R. Murthy A. S. (2017). Overview of wound healing and management. Surg. Clin. North Am.97 (1), 189–207. 10.1016/j.suc.2016.08.013
14
Chung B. Y. Kim H. B. Jung M. J. Kang S.A.-O. Kwak I. S. Park C. W. et al (2020). Post-burn pruritus. Int. J. Mol. Sci.21, 3880. 10.3390/ijms21113880
15
Dar L. A. Manzoor T. Shafi S. Kumar A. Ahmad S. M. (2024). Fabrication and characterization of calcium peroxide and berberine loaded cryogels for enhanced wound healing. J. Mater. Chem. B12 (34), 8431–8443. 10.1039/d4tb00989d
16
Deng X. Chen Q. Qiang L. Chi M. Xie N. Wu Y. et al (2018). Development of a porcine full-thickness burn hypertrophic scar model and investigation of the effects of shikonin on hypertrophic scar remediation. Front. Pharmacol.9, 590. (Print). 10.3389/fphar.2018.00590
17
Dinda M. Dasgupta U. Singh N. Bhattacharyya D. Karmakar P. (2015). PI3K-mediated proliferation of fibroblasts by Calendula officinalis tincture: implication in wound healing. Phytother. Res.29 (4), 607–616. 10.1002/ptr.5293
18
El-Hadidy M. R. El-Hadidy A. R. Bhaa A. Asker S. A. Mazroa S. A. (2014). Role of epidermal stem cells in repair of partial-thickness burn injury after using moist exposed burn ointment (MEBO(®)) histological and immunohistochemical study. Tissue Cell, 1532–3072. (Electronic). 10.1016/j.tice.2014.01.002
19
Esteban-Vives R. Young M. T. Zhu T. Beiriger J. Pekor C. Ziembicki J. et al (2016). Calculations for reproducible autologous skin cell-spray grafting. Burns42 (8), 1756–1765. 10.1016/j.burns.2016.06.013
20
Faraji A. Aghdaki M. Hessami K. Hosseinkhani A. Roozmeh S. Asadi N. et al (2021). Episiotomy wound healing by commiphora myrrha (nees) Engl. and Boswellia carteri Birdw. in primiparous women: a randomized controlled trial. J. Ethnopharmacol.264, 113396–117573. (Electronic). 10.1016/j.jep.2020.113396
21
Feng L. Liu Y. Chen Y. Xiang Q. Huang Y. Liu Z. et al (2023). Injectable antibacterial hydrogel with asiaticoside-loaded liposomes and ultrafine silver nanosilver particles promotes healing of burn-infected wounds. Adv. Healthc. Mater12 (22), e2203201. 10.1002/adhm.202203201
22
Feng A. Su S. Li C. Kang Y. Qiu J. Zhou J. (2024). Berberine decreases S100B generation to regulate gut vascular barrier permeability in mice with burn injury. Pharm. Biol.62 (1), 53–61. 10.1080/13880209.2023.2291679
23
G. El Baassiri M. Dosh L. Haidar H. Gerges A. Baassiri S. Leone A. et al (2023). Nerve growth factor and burn wound healing: update of molecular interactions with skin cells. Burns49, 989–1002. (Electronic). 10.1016/j.burns.2022.11.001
24
Gao T. Zhao Y. Zhao Y. He Y. Huang Q. Yang J. et al (2023). Curative effect and mechanisms of Radix arnebiae oil on burn wound healing in rats. Planta Med.89 (7), 709–717. 10.1055/a-1997-5566
25
Gong Y. Wang P. Cao R. Wu J. Ji H. Wang M. et al (2023). Exudate absorbing and antimicrobial hydrogel integrated with multifunctional curcumin-loaded magnesium polyphenol network for facilitating burn wound healing. ACS Nano17 (22), 22355–22370. 10.1021/acsnano.3c04556
26
Guo C. He J. Song X. Tan L. Wang M. Jiang P. et al (2019). Pharmacological properties and derivatives of shikonin-A review in recent years. Pharmacol. Res.149, 104463–1186. (Electronic). 10.1016/j.phrs.2019.104463
27
Guo J. Hu Z. Yan F. Lei S. Li T. Li X. et al (2020). Angelica dahurica promoted angiogenesis and accelerated wound healing in db/db mice via the HIF-1α/PDGF-β signaling pathway. Free Radic. Biol. Med.160, 1873–4596. 10.1016/j.freeradbiomed.2020.08.015
28
Habtemariam S. (2020). Berberine pharmacology and the gut microbiota: a hidden therapeutic link. Pharmacol. Res.155, 104722–1186. (Electronic). 10.1016/j.phrs.2020.104722
29
Hajiali H. Summa M. Russo D. Armirotti A. Brunetti V. Bertorelli R. et al (2016). Alginate-lavender nanofibers with antibacterial and anti-inflammatory activity to effectively promote burn healing. J. Mater Chem. B4 (9), 1686–1695. 10.1039/c5tb02174j
30
Han X. Beaumont C. Stevens N. (2017). Chemical composition analysis and in vitro biological activities of ten essential oils in human skin cells. Biochim. Open5, 1–7. 10.1016/j.biopen.2017.04.001
31
Han H. Chen L. Liang S. Lu J. Wu Y. Wang X. et al (2023). PLA-HPG based coating enhanced anti-biofilm and wound healing of Shikonin in MRSA-infected burn wound. Front. Bioeng. Biotechnol.11, 1243525–1244185. (Print). 10.3389/fbioe.2023.1243525
32
Herman A. Herman A. P. (2020). Herbal products for treatment of burn wounds. J. Burn Care Res.41 (3), 457–465. 10.1093/jbcr/iraa010
33
Ho T. J. Jiang S. J. Lin G. H. Li T. S. Yiin L. M. Yang J. S. et al (2016). The in vitro and in vivo wound healing properties of the Chinese herbal medicine “Jinchuang Ointment”. Evid. Based Complement. Altern. Med., 1741–427X. (Print). 10.1155/2016/1654056
34
Hu Y. Lei S. Yan Z. Hu Z. Guo J. Guo H. et al (2021). Angelica dahurica regulated the polarization of macrophages and accelerated wound healing in diabetes: a network pharmacology study and in vivo experimental validation. Front. Pharmacol.12, 678713–679812. (Print). 10.3389/fphar.2021.678713
35
Jang E. Inn K. S. Jang Y. P. Lee K. T. Lee J. H. (2018). Phytotherapeutic activities of Sanguisorba officinalis and its chemical constituents: a review. Am. J. Chin. Med.46 (2), 299–318. 10.1142/S0192415X18500155
36
Jeschke M. G. van Baar M. E. Choudhry M. A. Chung K. K. Gibran N. S. Logsetty S. (2020). Burn injury. Nat. Rev. Dis. Prim.6, 11. (Electronic)). 10.1038/s41572-020-0145-5
37
Kaur K. Kant S. Chaudary T. K. Mehra A. Singh A. Attri S. et al (2024). Bakuchiol nanoemulsion loaded electrospun nanofibers for the treatment of burn wounds. Naunyn Schmiedeb. Arch. Pharmacol.397 (8), 6075–6091. 10.1007/s00210-024-03011-z
38
Kitano A. Saika S. Yamanaka O. Ikeda K. Okada Y. Shirai K. et al (2007). Emodin suppression of ocular surface inflammatory reaction. Invest Ophthalmol. Vis. Sci.48 (11), 5013–5022. 10.1167/iovs.07-0393
39
Kong Y. Li L. Zhao L. G. Yu P. Li D. D. (2022). A patent review of berberine and its derivatives with various pharmacological activities (2016-2020). Expert Opin. Ther. Pat., 1744–7674. (Electronic). 10.1080/13543776.2021.1974001
40
Kopp J. Wang G. Y. Horch R. E. Pallua N. Ge S. D. (2003). Ancient traditional Chinese medicine in burn treatment: a historical review. Burns. 2003 Aug29 (5), 473–478. 10.1016/s0305-4179(03)00053-6
41
Lakshmanan G. Altemimi A. B. Sivaraj C. Selvakumari J. Karthik L. Saravanan K. et al (2024). Imperatorin from the aerial parts of Cleome viscosa L.: a characterization study and evaluation of the antibacterial activity. Nat. Prod. Res.38 (5), 848–855. 10.1080/14786419.2023.2190116
42
Lam H. W. Lin H. C. Lao S. C. Gao J. L. Hong S. J. Leong C. W. et al (2008). The angiogenic effects of Angelica sinensis extract on HUVEC in vitro and zebrafish in vivo. J. Cell Biochem.103 (1), 195–211. 10.1002/jcb.21403
43
Lee H.A.-O. Ang L.A.-O. Kim J. T. Lee M.A.-O. (2021). Aromatherapy for symptom relief in patients with burn: a systematic review and meta-analysis. Med. Kaunas.58, 1. 10.3390/medicina58010001
44
Li D. Rui Y. X. Guo S. D. Luan F. Liu R. Zeng N. (2021). Ferulic acid: a review of its pharmacology, pharmacokinetics and derivatives. Life Sci.284, 119921. (Electronic). 10.1016/j.lfs.2021.119921
45
Li S. Ma J. Li J. Qu X. Lei B. (2024). Sprayable self-assembly multifunctional bioactive poly(ferulic acid) hydrogel for rapid MRSA infected wound repair. J. Biomed. Mater Res. A112 (3), 390–401. 10.1002/jbm.a.37636
46
Liang C. Y. Chen C. C. Wang K. Y. Chung C. H. Chang N. W. Chien W. C. (2021). Increased risk for sleep disorders in burn patients: a 14-year nationwide, population-based cohort study. Burns47 (6), 1408–1415. 10.1016/j.burns.2020.11.012
47
Liu J. Li G. Chen Y. Z. Zhang L. D. Wang T. Wen Z. L. et al (2019). Effects of rhubarb on the expression of glucocorticoids receptor and regulation of cellular immunity in burn-induced septic rats. Chin. Med. J. Engl.132 (10), 1188–1193. 10.1097/CM9.0000000000000201
48
Liu C. S. Hu Y. N. Luo Z. Y. Xia T. Chen F. L. Tang Q. F. et al (2022). Comparative pharmacokinetics, intestinal absorption and urinary excretion of six alkaloids from herb pair phellodendri chinensis cortex-Atractylodis Rhizoma. Biomed. Chromatogr.36 (1), e5254. 10.1002/bmc.5254
49
Liu H. Shu F. Ji C. Xu H. Zhou Z. Wang Y. et al (2023). Clarifying sleep characteristics and analyzing risk factors of sleep disorders to promote a predictive, preventive, and personalized medicine in patients with burn scars. EPMA J.14 (1), 131–142. 10.1007/s13167-022-00309-x
50
Liu H. Luo X. J. He Y. Zhang Y. Xie Y. Rao X. Y. (2024). Effects of maltodextrin on water adsorption and thermodynamic properties of codonopsis radix spray-dried powder. Zhongguo Zhong Yao Za Zhi49 (6), 1540–1548. 10.19540/j.cnki.cjcmm.20231211.302
51
Loskotova A. Loskotova J. (2017). The use of acupuncture in first aid of burns-clinical report. Burns43 (8), 1782–1791. 10.1016/j.burns.2017.04.025
52
Lv Y. Han Y. Yu Z. Chen J. Li C. Wang C. et al (2022). Core-shell alum-borneol fiber for high bioavailability. Prog. Biomater.11 (3), 253–261. 10.1007/s40204-022-00192-9
53
Mabvuure N. T. Brewer C. F. Gervin K. Duffy S. (2020). The use of moist exposed burn ointment (MEBO) for the treatment of burn wounds: a systematic review. J. Plast. Surg. Hand Surg.54 (6), 337–343. 10.1080/2000656X.2020.1813148
54
Mahboub M. Aghazadeh Attari A. M. Sheikhalipour Z. Mirza Aghazadeh Attari M. Davami B. Amidfar A. et al (2021). A comparative study of the impacts of Aloe vera gel and silver sulfadiazine cream 1% on healing, itching and pain of burn wounds: a randomized clinical trial. J. Caring Sci.11 (3), 132–138. 10.34172/jcs.2021.036
55
Markiewicz-Gospodarek A. Kozioł M.A.-O. Tobiasz M. Baj J.A.-O. Radzikowska-Büchner E. Przekora A.A.-O. (2022). Burn wound healing: clinical complications, medical care, treatment, and dressing types: the current state of knowledge for clinical practice. Int. J. Environ. Res. Public Health19, 1338. 10.3390/ijerph19031338
56
Mathew-Steiner S. S. Roy S. Sen C. K. (2021). Collagen in wound healing. Bioeng. (Basel)8, 63. 10.3390/bioengineering8050063
57
Meng Y. B. Lei J. Hao Z. M. Cao R. L. (2011). Influence of rhubarb on gastrointestinal motility and intestinal mucosal barrier in patients with severe burn. Zhonghua Shao Shang Za Zhi27 (5), 337–340.
58
Morikawa T. Matsuda H. Yoshikawa M. (2017). A review of anti-inflammatory terpenoids from the incense gum resins frankincense and myrrh. J. Oleo Sci.66 (8), 805–814. 10.5650/jos.ess16149
59
Mrabti H. N. Doudach L. Mekkaoui M. Khalil Z. Harraqui K. Fozia F. et al (2022). Profile of medicinal plants traditionally used for the treatment of skin burns. Evid. Based Complement. Altern. Med.2022, 3436665. 10.1155/2022/3436665
60
Nai J. Zhang C. Shao H. Li B. Li H. Gao L. et al (2021). Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. Int. J. Biol. Macromol.183, 2337–2353. 10.1016/j.ijbiomac.2021.05.213
61
O'Brien K. Weber D. (2016). Insomnia in Chinese medicine: the heart of the matter. J. Altern. Complement. Med.22 (9), 684–694. 10.1089/acm.2016.0044
62
Peck M. D. (2011). Epidemiology of burns throughout the world. Part I: distribution and risk factors. Burns37 (7), 1087–1100. 10.1016/j.burns.2011.06.005
63
Peck M. D. (2012). Epidemiology of burns throughout the world. Part II: intentional burns in adults. Burns38 (5), 630–637. 10.1016/j.burns.2011.12.028
64
Pena O. A. Martin P. (2024). Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol.25 (8), 599–616. 10.1038/s41580-024-00715-1
65
Pengzong Z. Yuanmin L. Xiaoming X. Shang D. Wei X. Zhigang L. et al (2019). Wound healing potential of the standardized extract of Boswellia serrata on experimental diabetic foot ulcer via inhibition of inflammatory, angiogenetic and apoptotic markers. Planta Med.85 (8), 657–669. 10.1055/a-0881-3000
66
Pourhajibagher M. Rahimi-Esboei B. Ahmadi H. Bahador A. (2021). The anti-biofilm capability of nano-emodin-mediated sonodynamic therapy on multi-species biofilms produced by burn wound bacterial strains. Photodiagnosis Photodyn. Ther.34, 102288–1597. (Electronic). 10.1016/j.pdpdt.2021.102288
67
Prasathkumar M. George A. Sadhasivam S. (2024). Influence of chitosan and hydroxyethyl cellulose modifications towards the design of cross-linked double networks hydrogel for diabetic wound healing. Int. J. Biol. Macromol.265 (Pt 1), 130851. 10.1016/j.ijbiomac.2024.130851
68
Qi Y. Zhang Q. Zhu H. (2019). Huang-lian Jie-Du decoction: a review on phytochemical, pharmacological and pharmacokinetic investigations. Chin. Med.14, 57–8546. (Print). 10.1186/s13020-019-0277-2
69
Que H. F. Tang H. J. Wang L. Y. Dai H. Y. Zhang S. Y. Qin H. G. et al (2005). Study on wound healing mechanism of replenishing qi and dissipating stagnation therapy for chronic skin ulcers. Zhong Xi Yi Jie He Xue Bao3 (3), 243–246. 10.3736/jcim20050324
70
Radha K. S. Madhyastha H. K. Nakajima Y. Omura S. Maruyama M. (2008). Emodin upregulates urokinase plasminogen activator, plasminogen activator inhibitor-1 and promotes wound healing in human fibroblasts. Vasc. Pharmacol.48 (4-6), 184–190. 10.1016/j.vph.2008.02.002
71
Radzikowska-Büchner E.A.-O. Łopuszyńska I. Flieger W.A.-O. X. Tobiasz M. Maciejewski R.A.-O. Flieger J.A.-O. (2023). An overview of recent developments in the management of burn injuries. Int. J. Mol. Sci.10.3390/ijms242216357
72
Ripszky Totan A. Greabu M. Stanescu S. I. I. Imre M. Spinu T. C. Miricescu D. et al (2022). The Yin and Yang dualistic features of autophagy in thermal burn wound healing. Int. J. Immunopathol. Pharmacol.36, 2058–7384. (Electronic). 10.1177/03946320221125090
73
Roshangar L. Soleimani Rad J. Kheirjou R. Reza Ranjkesh M. Ferdowsi Khosroshahi A. (2019). Skin burns: review of molecular mechanisms and therapeutic approaches. Wounds31 (12), 308–315.
74
Sánchez M. González-Burgos E. Iglesias I. Gómez-Serranillos M. P. (2020). Pharmacological update properties of Aloe vera and its major active constituents. Molecules25, 1324. 10.3390/molecules25061324
75
Sang S.A.-O. Wang S. Wu J.A.-O. Zhang X.A.-O. (2023). Sprayable berberine-silk fibroin microspheres with extracellular matrix anchoring function accelerate infected wound healing through antibacterial and anti-inflammatory effects. ACS Biomater. Sci. Eng.9, 3643–3659. 10.1021/acsbiomaterials.3c00030
76
Shaabani A. Bizari D. Khoshmohabat H. (2023). PEGylated curcumin-loaded poly(vinyl alcohol)/Zwitterionic poly(sulfobetaine vinylimidazole)-grafted chitosan nanofiber as a second-degree burn wound dressing. Carbohydr. Polym.321, 121307. 10.1016/j.carbpol.2023.121307
77
Shree A. Vagga A. A. (2022). Methodologies of autologous skin cell spray graft. Cureus14 (11), e31353. 10.7759/cureus.31353
78
Shu W. Wang Y. Zhang X. Li C. Le H. Chang F. (2021). Functional hydrogel dressings for treatment of burn wounds. Front. Bioeng. Biotechnol.9, 788461. (Print). 10.3389/fbioe.2021.788461
79
Shu G. Xu D. Zhang W. Zhao X. Li H. Xu F. et al (2022). Preparation of shikonin liposome and evaluation of its in vitro antibacterial and in vivo infected wound healing activity. Phytomedicine99, 154035. (Electronic). 10.1016/j.phymed.2022.154035
80
Song P. Jo H. S. Shim W. S. Kwon Y. W. Bae S. Kwon Y. et al (2021). Emodin induces collagen type I synthesis in Hs27 human dermal fibroblasts. Exp. Ther. Med.21 (5), 420. 10.3892/etm.2021.9864
81
Song J. Zeng J. Zheng S. Jiang N. Wu A.A.-O. Guo S. et al (2023). Sanguisorba officinalis L. promotes diabetic wound healing in rats through inflammation response mediated by macrophage. Phytother. Res.37, 4265–4281. 10.1002/ptr.7906
82
Soliman A. M. Teoh S. L. Ghafar N. A. Das S. (2019). Molecular concept of diabetic wound healing: effective role of herbal remedies. (1875-5607 (Electronic)). 10.2174/1389557518666181025155204
83
Stoica A. E. Chircov C. Grumezescu A. M. (2020). Hydrogel dressings for the treatment of burn wounds: an up-to-date overview. Mater. (Basel)13, 2853. 10.3390/ma13122853
84
Sun Y. Lenon G. B. Yang A. W. H. (2019). Phellodendri cortex: a phytochemical, pharmacological, and pharmacokinetic review. Evid. Based Complement. Altern. Med.2022, 3436665. 10.1155/2019/7621929
85
Sun Q. Gong T. Liu M. Ren S. Yang H. Zeng S. et al (2022). Shikonin, a naphthalene ingredient: therapeutic actions, pharmacokinetics, toxicology, clinical trials and pharmaceutical researches. Phytomedicine94, 153805. 10.1016/j.phymed.2021.153805
86
Sun S. Lin W. Yang L. Zhang C. Kan H. Xu C. et al (2024). Near-infrared light-actuated on-demand botanicals release and hyperthermia by an antibiotic-free polysaccharide-based hydrogel dressing for the synergistic treatment of wound infections. J. Mater Chem. B12 (5), 1307–1316. 10.1039/d3tb02714g
87
Tan Q. He Q. Peng Z. Zeng X. Liu Y. Li D. et al (2024). Topical rhubarb charcoal-crosslinked chitosan/silk fibroin sponge scaffold for the repair of diabetic ulcers improves hepatic lipid deposition in db/db mice via the AMPK signalling pathway. Lipids Health Dis.23 (1), 52. 10.1186/s12944-024-02041-z
88
Tang T. Yin L. Yang J. Shan G. (2007). Emodin, an anthraquinone derivative from Rheum officinale baill, enhances cutaneous wound healing in rats. Eur. J. Pharmacol.567 (3), 177–185. 10.1016/j.ejphar.2007.02.033
89
Tang Q. L. Han S. S. Feng J. Di J. Q. Qin W. X. Fu J. et al (2014). Moist exposed burn ointment promotes cutaneous excisional wound healing in rats involving VEGF and bFGF. Mol. Med. Rep.9 (4), 1277–1282. 10.3892/mmr.2014.1921
90
Teplicki E. Ma Q. Castillo D. E. Zarei M. Hustad A. P. Chen J. et al (2018). The effects of Aloe vera on wound healing in cell proliferation, migration, and viability. Wounds30 (9), 263–268.
91
Tsai C. Y. Woung L. C. Yen J. C. Tseng P. C. Chiou S. H. Sung Y. J. et al (2016). Thermosensitive chitosan-based hydrogels for sustained release of ferulic acid on corneal wound healing. Carbohydr. Polym.135, 308–315. 10.1016/j.carbpol.2015.08.098
92
Wallace H. A. Basehore B. M. Zito P. M. (2023). Wound healing phases. BTI - StatPearls.
93
Wan J. Liang Y. Wei X. Liang H. Chen X. L. (2023). Chitosan-based double network hydrogel loading herbal small molecule for accelerating wound healing. Int. J. Biol. Macromol.246, 125610. (Electronic). 10.1016/j.ijbiomac.2023.125610
94
Wang J. Wang L. Lou G. H. Zeng H. R. Hu J. Huang Q. W. et al (2019). Coptidis Rhizoma: a comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm. Biol.57 (1), 193–225. 10.1080/13880209.2019.1577466
95
Wang S. Zhang Y. Shi Y. He Q. Tan Q. Peng Z. et al (2023). Rhubarb charcoal-crosslinked chitosan/silk fibroin sponge scaffold with efficient hemostasis, inflammation, and angiogenesis for promoting diabetic wound healing. Int. J. Biol. Macromol.253 (Pt 2), 126796. 10.1016/j.ijbiomac.2023.126796
96
Wang Q. Zhang S. Jiang J. Chen S. Ramakrishna S. Zhao W. et al (2024). Electrospun radially oriented berberine-PHBV nanofiber dressing patches for accelerating diabetic wound healing. Regen. Biomater.11, rbae063. (Print). 10.1093/rb/rbae063
97
Wang S. Zhou H. Cui W. Zhang J. Wu D. Zhang N. et al (2024). Qi Wei anti-Burn tincture remodels liver metabolic pathways and treats burn wounds efficiently. J. Burn Care Res.45 (4), 916–925. 10.1093/jbcr/irac175
98
Warby R. Maani C. V. (2023). Burn classification.
99
Wilkinson H. N. Hardman M. J. (2020). Wound healing: cellular mechanisms and pathological outcomes. Open Biol.10 (9), 200223. 10.1098/rsob.200223
100
Wu J. R. Lu Y. C. Hung S. J. Lin J. H. Chang K. C. Chen J. K. et al (2021). Antimicrobial and immunomodulatory activity of herb extracts used in burn wound healing: “San Huang Powder”. Evid. Based Complement. Altern. Med.2021, 2900060. 10.1155/2021/2900060
101
Wu M. Huang J. Shi J. Shi L. Zeng Q. Wang H. (2022). Ruyi Jinhuang powder accelerated diabetic ulcer wound healing by regulating Wnt/β-catenin signaling pathway of fibroblasts in vivo and in vitro. J. Ethnopharmacol.293, 115321. (Electronic). 10.1016/j.jep.2022.115321
102
Wu T. Zhang Y. M. Krishnan S. Jaisankar A. Wan Y. Gong S. J. et al (2022). Bioactive small molecule enhances skin burn wound healing and hair follicle regeneration by activating PI3K/AKT signaling pathway: a preclinical evaluation in animal model. J. Biomed. Nanotechnol.18 (2), 463–473. 10.1166/jbn.2022.3251
103
Xian Y. F. Mao Q. Q. Ip S. P. Lin Z. X. Che C. T. (2011). Comparison on the anti-inflammatory effect of cortex phellodendri chinensis and cortex phellodendri amurensis in 12-O-tetradecanoyl-phorbol-13-acetate-induced ear edema in mice. J. Ethnopharmacol.137 (3), 1425–1430. 10.1016/j.jep.2011.08.014
104
Xie Y. Fan C. Dong Y. Lynam E. Leavesley D. I. Li K. et al (2015). Functional and mechanistic investigation of shikonin in scarring. Chem. Biol. Interact.228 (1872-7786 (Electronic)), 18–27. 10.1016/j.cbi.2014.12.037
105
Xie M. D. Lei Y. Yang H. R. You Y. Gao J. J. Wang Z. et al (2022). Analysis on “the essence of tuina, arrival of qi ensuring curative effect”. Zhongguo Zhen Jiu. 10.13703/j.0255-2930.20201208-0002
106
Xiong Y. Xu Y. Zhou F. Hu Y. Zhao J. Liu Z. et al (2022). Bio-functional hydrogel with antibacterial and anti-inflammatory dual properties to combat with burn wound infection. Bioeng. Transl. Med.8 (1), e10373. 10.1002/btm2.10373
107
Xueying Z. Liang G. Siyi L. Fengyue L. I. Mingli L. Wanting L. et al (2024). Emodin suppresses alkali burn-induced corneal inflammation and neovascularization by the vascular endothelial growth factor receptor 2 signaling pathway. J. Tradit. Chin. Med.44 (2), 268–276. 10.19852/j.cnki.jtcm.20240203.005
108
Yan Z. Li S. Gong Z. (2023). Bisacurone gel ameliorated burn wounds in experimental rats via its anti-inflammatory, antioxidant, and angiogenic properties. Acta Cir. Bras.38, e382423. 10.1590/acb382423
109
Yang W. T. Ke C. Y. Wu W. T. Harn H. J. Tseng Y. H. Lee R. P. (2017). Effects of Angelica dahurica and Rheum officinale extracts on excisional wound healing in rats. Evid. Based Complement. Altern. Med.2017, 1583031. 10.1155/2017/1583031
110
Yang W. T. Ke C. Y. Wu W. T. Tseng Y. H. Lee R. P. (2020). Antimicrobial and anti-inflammatory potential of Angelica dahurica and Rheum officinale extract accelerates wound healing in staphylococcus aureus-infected wounds. Sci. Rep.10 (1), 5596. 10.1038/s41598-020-62581-z
111
Yin X. Hong J. Tang H. B. Liu M. Li Y. S. (2022). Enhanced healing of oral chemical burn by inhibiting inflammatory factors with an oral administration of shengFu oil. Front. Pharmacol.13, 913098. 10.3389/fphar.2022.913098
112
Yu Q. Shen Y. Xiao F. Zhao Y. Piao S. Li G. et al (2023). Yuhong ointment ameliorates inflammatory responses and wound healing in scalded mice. J. Ethnopharmacol.306, 116118. 10.1016/j.jep.2022.116118
113
Zhan Z. L. Hu J. Liu T. (2015). Advances in studies on chemical compositions and pharmacological activities of arnebiae radix. Zhongguo Zhong Yao Za Zhi.
114
Zhan H. B. Sun Q. Q. Yan L. Cai J. (2021). Clinical study of MEBO combined with Jinhuang powder for diabetic foot with infection. Evid. Based Complement. Altern. Med.2021, 5531988. 10.1155/2021/5531988
115
Zhang X. N. Ma Z. J. Wang Y. Sun B. Guo X. Pan C. Q. et al (2017). Angelica dahurica ethanolic extract improves impaired wound healing by activating angiogenesis in diabetes. PLoS One12 (5), e0177862. 10.1371/journal.pone.0177862
116
Zhang H. Chen J. Cen Y. (2018). Burn wound healing potential of a polysaccharide from Sanguisorba officinalis L. in mice. Int. J. Biol. Macromol.112, 862–867. 10.1016/j.ijbiomac.2018.01.214
117
Zhang Q. Wang M. Deng X. Zhao D. Zhao F. Xiao J. et al (2023). Shikonin promotes hypertrophic scar repair by autophagy of hypertrophic scar-derived fibroblasts. Acta Cir. Bras.38, e384623. (Electronic). 10.1590/acb384623
118
Zhang J. Li W. Tao Z. Zhou X. Chen X. Zhou J. et al (2024). Endogenous glucose-driven cascade reaction of nano-drug delivery for boosting multidrug-resistant bacteria-infected diabetic wound healing. J. Colloid Interface Sci.672, 63–74. (Electronic). 10.1016/j.jcis.2024.05.204
119
Zhao H. Deneau J. Che G. O. Li S. Vagnini F. Azadi P. et al (2012). Angelica sinensis isolate SBD.4: composition, gene expression profiling, mechanism of action and effect on wounds, in rats and humans. Eur. J. Dermatol22 (1), 58–67. 10.1684/ejd.2011.1599
120
Zhao Z. He X. Zhang Q. Wei X. Huang L. Fang J. C. et al (2017). Traditional uses, chemical constituents and biological activities of plants from the genus Sanguisorba L. Am. J. Chin. Med.45 (2), 199–224. 10.1142/S0192415X17500136
121
Zhao H. Feng Y. L. Wang M. Wang J. J. Liu T. Yu J. (2022). The angelica dahurica: a review of traditional uses, phytochemistry and pharmacology. Front. Pharmacol.13, 896637. 10.3389/fphar.2022.896637
122
Zhao Z. Fan X. Li X. Qiu Y. Yi Y. Wei Y. et al (2024). All-natural injectable antibacterial hydrogel enabled by chitosan and borneol. Biomacromolecules25 (1), 134–142. 10.1021/acs.biomac.3c00874
123
Zheng A. Ma H. Liu X. Huang Q. Li Z. Wang L. et al (2020). Effects of moist exposed burn therapy and ointment (MEBT/MEBO) on the autophagy mTOR signalling pathway in diabetic ulcer wounds. Pharm. Biol.58 (1), 124–130. 10.1080/13880209.2019.1711430
124
Zhong G. Lei P. Guo P. Yang Q. Duan Y. Zhang J. et al (2024). A photo-induced cross-linking enhanced A and B combined multi-functional spray hydrogel instantly protects and promotes of irregular dynamic wound healing. Small20 (23), e2309568. 10.1002/smll.202309568
125
Zhou P. Li J. Chen Q. Wang L. Yang J. Wu A. et al (2021). A comprehensive review of genus sanguisorba: traditional uses, chemical constituents and medical applications. Front. Pharmacol.12, 750165. (Print). 10.3389/fphar.2021.750165
126
Zhou T. Zhang C. Wang X. Lin J. Yu J. Liang Y. et al (2024). Research on traditional Chinese medicine as an effective drug for promoting wound healing. J. Ethnopharmacol.332, 118358. 10.1016/j.jep.2024.118358
127
Zhu L. Liu X. Du L. Jin Y. (2016). Preparation of asiaticoside-loaded coaxially electrospinning nanofibers and their effect on deep partial-thickness burn injury. Biomed. Pharmacother.83, 33–40. 10.1016/j.biopha.2016.06.016
128
Żwierełło W.A.-O. Piorun K. Skórka-Majewicz M. Maruszewska A.A.-O. Antoniewski J. Gutowska I. (2023). Burns: classification, pathophysiology, and treatment: a review. Int. J. Mol. Sci.10.3390/ijms24043749
Summary
Keywords
traditional Chinese medicine, burn injury, active ingredients, mechanism, preparation
Citation
Liu Y, Yu C, Dou T, Shen Y, Li H, Liu M, Xie W, Zeng W, Feng Z, Huang M and Zhu Y (2025) Advances in traditional Chinese medicine for burn treatment: mechanisms, therapeutic approaches, and innovative preparations. Front. Pharmacol. 16:1651219. doi: 10.3389/fphar.2025.1651219
Received
21 June 2025
Revised
09 October 2025
Accepted
10 October 2025
Published
17 November 2025
Volume
16 - 2025
Edited by
Mariangela Marrelli, University of Calabria, Italy
Reviewed by
Francisco Cruz-Sosa, Universidad Autónoma Metropolitana, Mexico
Maria Rosaria Perri, National Research Council of Italy, Italy
Updates
Copyright
© 2025 Liu, Yu, Dou, Shen, Li, Liu, Xie, Zeng, Feng, Huang and Zhu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Liu, yingliu@xhsysu.cn; Yizhun Zhu, yzzhu@must.edu.mo
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.