- 1Department of Microbiology and Immunology, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 2Center of Infectious Disease and Signaling Research, National Cheng Kung University, Tainan, Taiwan
- 3Institute of Basic Medical Sciences, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 4Center for Infection Control, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 5School of Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan
Enterovirus 71 (EV71) infection gave a hard hit on young children because of fatal complication, brainstem encephalitis with pulmonary edema. The occurrence of severe EV71 infections highlight the urgent need for the development and repurposing of novel antivirals for medical use. Drugs target specific steps in the cycle of viral replication, and the modification of existing factors in EV71 immunopathogenesis deciphers the current approaches for developing antivirals. In addition to identifying chemical compounds, we highlight active constituents and explore underlying mechanisms of action of antimicrobial peptides and natural products that may be active against EV71 and provide pharmacological benefits.
1 Introduction
International travel, economics, cultural interactions, and climate change have moved much of the world into a becoming a global village. Viruses, which are potential silent reservoirs for emerging and re-emerging diseases that may lead to global outbreaks, have political, economic, and social impacts. For example, the pandemic of coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), presented an unprecedented challenge to managing a global contagion (Weston et al., 2020). Enteroviruses infections can cause systemic dissemination and central nervous system (CNS) diseases, become emerging or re-emerging epidemics and pose a heavy societal burden. The initial outbreak of enterovirus 71 (EV71) infections in Taiwan was in 1998, with about 1.5 million infected children. The prominent complication of CNS in EV71 infection is brainstem encephalitis (BE) (Wang, 2016). The EV71 BE is reported to be the most serious neurological manifestation of enterovirus infections, may progress to autonomic nervous system (ANS) dysregulation, neurogenic pulmonary edema (NPE) and cardiopulmonary failure (Wang, 2016). With regard to the previous pioneer and novel findings on EV71 BE and severe complications, further investigation for the potential effectiveness and efficacy of treatments of EV71 infections is life-critical.
2 Clinical manifestations
Enterovirus 71 (EV71) belongs to the genus Enterovirus which causes multiple mild-to-severe clinical illnesses, including herpangina, hand, foot, and mouth disease, meningitis, brainstem encephalitis, autonomous nervous system (ANS) dysregulation, and pulmonary edema in young children (Wang, 2016). EV71 consists of a non-enveloped, single-stranded, positive-sense RNA within the family of Piconaviridae. EV71 is divided into four genotypes, A, B, C and D. The capsid contains four coat proteins, including VP1, VP2, VP3, and VP4. Variations within capsid proteins VP1 to VP3 are responsible for the antigenic diversity, and neutralization sites are most clustered on VP1 (Liu and Long, 2025). EV71 is similar to many emerging and re-emerging viruses with specific neuro-invasive targets, such as the brainstem. Since 1997, EV71 has emerged as a serious childhood infection in the Asia-Pacific region (Modlin, 2007). Based on clinical, laboratory, radiological, and pathological evidence, EV71 was the cardinal pathogen of brainstem encephalitis during the first outbreak in 1998, in Taiwan (Wang, 2016).
3 Immunopathogenesis
The immunopathogenesis of EV71 has been studied in terms of its width and depth for the diagnosis and clinical treatment during the past 25 years (Wang et al., 2003). Humoral mediators, including cytokines, play key roles in the pathophysiology of critical diseases caused by microbiological pathogens. Cytokine storm surges occur and persist in the condition known as hypercytokinemia, resulting in progression to multiple organ failure. Previous studies have clearly demonstrated that cytokines and chemokines play important roles in the pathogenesis of EV71 severe complications. Both CNS (IL-1β, IL-6, IFN-γ, IP-10, MIG) and systemic inflammatory responses (IL-6, IL-8, IL-10, IL-13, IP-10, MCP-1, MIG) to infection play leading, but distinctly different, roles in the pathogenesis of EV71 pulmonary edema (Wang et al., 2003; Wang et al., 2007). Cellular immunity also contributes to the pathogenesis of complications arising during EV71 infection. EV71-infected patients with pulmonary edema have fewer circulating CD4+ T cells, CD8+ T cells, and natural killer cells (Wang et al., 2003). Furthermore, CD19+ and CD20+ B cell expression frequency and absolute cell number did not change significantly during EV71 infection according to disease severity (Wang, unpublished data).
4 Therapeutics options, past and current perspectives
Optional therapeutic measures for viral diseases include the use of antiviral agents, neutralizing antibodies, and vaccines to reduce disease severity and mortality. Although EV71 vaccination alleviated the severity, incidence and altered epidemiological trends. Whereas, a limitation of EV71 vaccines is lack of geographic scope, to generalize the efficacy and effectiveness of vaccines against EV71 to every epidemical regions or countries (Swain et al., 2022).
Previous clinical experiences with EV71 infection treatment are discussed first. Intravenous immunoglobulin (IVIG) has been therapeutically used against EVs in the clinical settings of neonates and children, depending on the serotypes of infection (Rotbart, 2000). IVIG administration neutralizes specific EV, alters immunoregulatory molecules, and reduces inflammatory mediators to attenuate the disease course in EV71-infected patients with ANS dysregulation. IVIG consists of antigen-specific IgG antibodies that can move to a population with reduced sialic acid levels, which are capable of mediating antigen clearance and a protective inflammatory response through the engagement of subclass-specific FcγRs on effector cells (Kaneko et al., 2006). Milrinone is a subtype III cyclic nucleotide phosphodiesterase (PDE) inhibitor that increases intracellular cyclic adenosine monophosphate (cAMP) levels. Milrinone treatment has both inotropic and vasodilatory effects and is associated with decreased mortality through increased regulatory T cell populations, cAMP expression, and modulation of cytokine levels in EV71-infected patients (Wang, 2016). Furthermore, treatment with EV71-specific antibodies before or after infection considerably reduced the disease severity, mortality, and tissue viral load in B cell-deficient mice. Above treatments indicated that both lymphocyte and antibody responses protected mice from EV71 infection (Lin et al., 2009). Recently, Yang et al. discovered that the recruitment of CCL3-mediated neutrophils to the brain is critical in the fatal immunopathology of EV71 infection, suggesting that CCL3 is a potential therapeutic target that downregulates the detrimental roles of neutrophils during EV71 infection (Yang et al., 2024).
Clinically, no antiviral agents have been approved by the Food and Drug Administration (FDA) for the treatment of EVs. Sustained development of drugs for the treatment of picornaviral infections is essential. Research may focus mainly on the viral capsid, viral proteases, or replication inhibitors targeting other non-structural enteroviral proteins. Entry is the first step in viral infection and involves viral attachment, endocytosis, and uncoating. Binding of the virus to its receptor is critical for tropism of the virus. The design of novel antiviral agents that target specific steps in the viral replication cycle and modification of existing antiviral compounds are current approaches for developing antiviral drugs (Abzug et al., 2003).
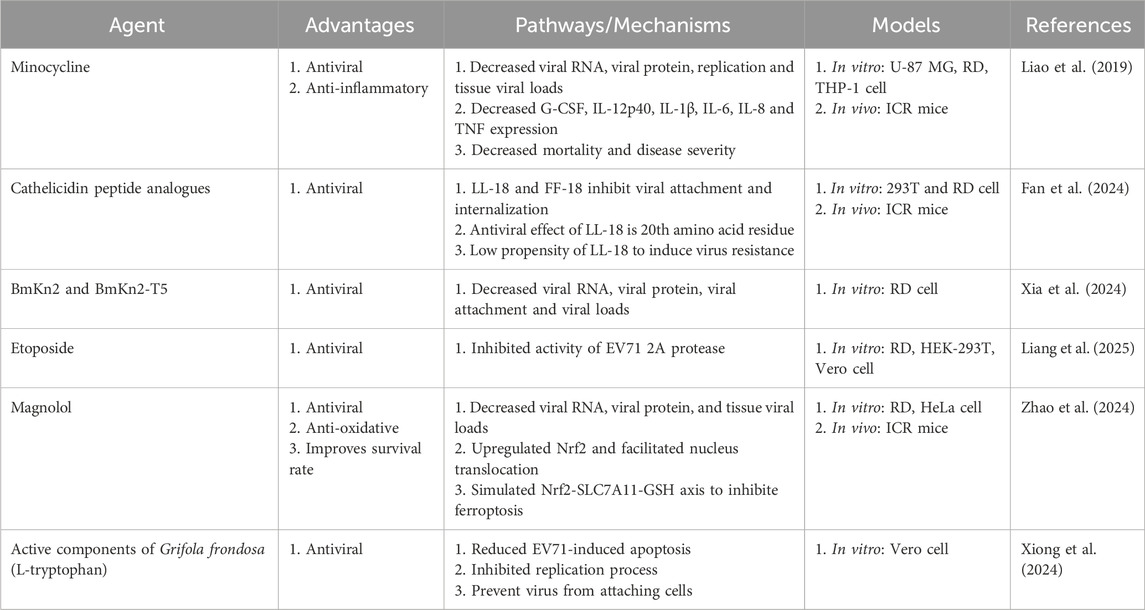
Pleconaril, a capsid function inhibitor, was the first compound to exhibit broad-spectrum anti-EV activity (Abzug et al., 2003). However, pleconaril treatment failed to show consistent efficacy in patients with EV meningitis or meningoencephalitis in a randomized double-blind placebo-controlled trial (Norder et al., 2011). Ribavirin is a nucleoside analog with broad-spectrum antiviral activity against various viruses. Ribavirin effectively reduced viral production in vitro and also reduced viral loads in tissues, as well as mortality, morbidity, and subsequent paralysis sequelae in an ICR mouse model (Li et al., 2008). Minocycline has neuroprotective effects through anti-inflammatory, anti-apoptotic, and immunomodulatory properties (Bastos et al., 2013). It possesses high lipophilicity and is easily transferred through the blood-brain barrier to accumulate in both cerebrospinal fluid and neurons. We clearly demonstrated that clinical scores, mortality rates, and viral titers in various brain tissues decreased after minocycline treatment in an MP4-infected animal model (Liao et al., 2019). Minocycline inhibited IL-6 and granulocyte colony-stimulating factor (G-CSF) in plasma and TNF in the cerebellum. Minocycline has characteristics and properties that function both as an antiviral and anti-inflammatory agent in EV71 infection (Liao et al., 2019). Antimicrobial peptides (Tripodi et al.) are important effectors of the innate immune system and are critical defense mechanisms against various pathogenic infections in humans (Huan et al., 2020). Cathelicidin peptide analogs were effective against EV71 infection, but could not inhibit echovirus or coxsackievirus, suggesting that these peptides are highly specific. LL-18 and FF-18, which belong to the cathelicidin family, are effective against EV71 infections in vitro and in vivo. These peptides bind directly to the viral particle surface and prevent the virus from entering the host cell, thereby blocking viral genome release (Fan et al., 2024). Scorpion venom antimicrobial peptide BmKn2, isolated from the scorpion M. martensii, and its derivative BmKn2-T5, have a typical amphiphilic α-helical structure and exhibit significant inhibitory activity against EV71 during early stages, especially in attachment and entry (Xia et al., 2024).
Numerous herbal compounds and their bioactive metabolites have become a significant focus of research because of their efficacy and cost-effectiveness (Bachar et al., 2021). The EV71 2A protease (2Apro), a cysteine protease produced by the virus, is essential for viral replication (Lin et al., 2019). It is commonly isolated from the roots and rhizomes of Podophyllum spp. Etoposide inhibited EV71 replication in a concentration-dependent manner in various cell lines with minimal cytotoxicity. Etoposide inhibits the activity of EV71 2A protease by binding mainly to two residues: Y89 and P107 (Liang et al., 2025). Magnolol, which is extracted from Magnolia officinalis, is known for its antitumor, anti-inflammatory, and antioxidant effects (Lee et al., 2011). Magnolol demonstrated significant dose-dependent inhibition of EV71 replication in vitro. It attenuated viral load in brain and limb tissues and mitigated tissue inflammation. It upregulated the overall expression of nuclear factor erythroid 2 - related factor 2 (Nrf2) and facilitated its nuclear translocation, resulting in increased expression of various ferroptosis inhibitory genes (Zhao et al., 2024). Grifola frondosa is an important medicinal and edible fungus rich in flavonoids, fatty acids, and other active substances (Tripodi et al., 2022). L-tryptophan has been used for the separation and purification of Grifola frondosa. Xiong et al. found that L-tryptophan has high anti-EV71 activity and reduced EV71-induced apoptosis. It significantly inhibited the replication process after viral adsorption by binding to the VP1 capsid protein, preventing the virus from attaching to and entering cells (Xiong et al., 2024). The information on various aspects of the promising compounds, including the functional pathways and mechanisms were summarized in Table 1. There is no clinical approved natural products currently available for the prevention and treatment of the EV71 infections. One of the technical block is to develop an amendable assay for high-throughput screening (Wang et al., 2015).
5 Conclusion
Drug repurposing is a way to discover at existing drugs function to extent if they could be used to treat other diseases. These agents may represent potential roles of new therapeutics for controlling or preventing EV71 complications. Further, evaluate the therapeutics for EV71 infection is undergoing in animal models. An extensive future investigation is also required with respect to the mechanisms of action at systemic, cellular, and molecular level to extend up to broad-spectrum clinical trials. The resurgence of EV71, particularly its association with complications, including brainstem encephalitis, autonomous nervous system (ANS) dysregulation, and pulmonary edema in patients, underscores the urgent need for effective antiviral strategies against virulent strains that may emerge, in addition to vaccines.
Author contributions
S-YY: Investigation, Writing – original draft. S-HC: Conceptualization, Writing – review and editing. S-MW: Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was partially funded by the National Science and Technology Council (NSTC 111-2327-B-006-010, 112-2314-B-006-048-MY3, 113-2327-B-006-003 and 114-2327-B-006-004) and National Cheng Kung University Hospital (NCKUH-11404037).
Acknowledgments
We are indebted to Dr. Yin-Ping Teresa Teng, Mr. Jen-Wei Wang and Mr. Jen-Yi Wang who provided invaluable suggestions during preparation of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abzug, M. J., Cloud, G., Bradley, J., Sanchez, P. J., Romero, J., Powell, D., et al. (2003). Double blind placebo-controlled trial of pleconaril in infants with enterovirus meningitis. Pediatr. Infect. Dis. J. 22 (4), 335–341. doi:10.1097/01.inf.0000059765.92623.70
Bachar, S. C., Mazumder, K., Bachar, R., Aktar, A., and Al Mahtab, M. (2021). A review of medicinal plants with antiviral activity available in Bangladesh and mechanistic insight into their bioactive metabolites on SARS-CoV-2, HIV and HBV. Front. Pharmacol. 12, 732891. doi:10.3389/fphar.2021.732891
Bastos, L. F., Godin, A. M., Zhang, Y., Jarussophon, S., Ferreira, B. C., Machado, R. R., et al. (2013). A minocycline derivative reduces nerve injury-induced allodynia, LPS-Induced prostaglandin E2 microglial production and signaling via toll-like receptors 2 and 4. Neurosci. Lett. 543, 157–162. doi:10.1016/j.neulet.2013.03.014
Fan, T., Liu, B., Yao, H., Chen, X., Yang, H., Guo, S., et al. (2024). Cathelicidin peptide analogues inhibit EV71 infection through blocking viral entry and uncoating. PLoS Pathog. 20 (1), e1011967. doi:10.1371/journal.ppat.1011967
Huan, Y., Kong, Q., Mou, H., and Yi, H. (2020). Antimicrobial peptides: classification, design, application and research progress in multiple fields. Front. Microbiol. 11, 582779. doi:10.3389/fmicb.2020.582779
Kaneko, Y., Nimmerjahn, F., and Ravetch, J. V. (2006). Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313 (5787), 670–673. doi:10.1126/science.1129594
Lee, Y. J., Lee, Y. M., Lee, C. K., Jung, J. K., Han, S. B., and Hong, J. T. (2011). Therapeutic applications of compounds in the magnolia family. Pharmacol. Ther. 130 (2), 157–176. doi:10.1016/j.pharmthera.2011.01.010
Li, Z. H., Li, C. M., Ling, P., Shen, F. H., Chen, S. H., Liu, C. C., et al. (2008). Ribavirin reduces mortality in enterovirus 71-infected mice by decreasing viral replication. J. Infect. Dis. 197 (6), 854–857. doi:10.1086/527326
Liang, Q., Shi, S., Zhang, Q., Wang, Y., Ye, S., and Xu, B. (2025). Etoposide targets 2A protease to inhibit enterovirus 71 replication. Microbiol. Spectr. 13 (1), e0220024. doi:10.1128/spectrum.02200-24
Liao, Y. T., Wang, S. M., and Chen, S. H. (2019). Anti-inflammatory and antiviral effects of minocycline in enterovirus 71 infections. Biomed. Pharmacother. 118, 109271. doi:10.1016/j.biopha.2019.109271
Lin, Y. W., Chang, K. C., Kao, C. M., Chang, S. P., Tung, Y. Y., and Chen, S. H. (2009). Lymphocyte and antibody responses reduce enterovirus 71 lethality in mice by decreasing tissue viral loads. J. Virol. 83 (13), 6477–6483. doi:10.1128/JVI.00434-09
Lin, J. Y., Kung, Y. A., and Shih, S. R. (2019). Antivirals and vaccines for enterovirus A71. J. Biomed. Sci. 26 (1), 65. doi:10.1186/s12929-019-0560-7
Liu, Q., and Long, J. E. (2025). Insight into the life cycle of Enterovirus-A71. Viruses 17 (2), 181. doi:10.3390/v17020181
Modlin, J. F. (2007). Enterovirus deja vu. N. Engl. J. Med. 356 (12), 1204–1205. doi:10.1056/NEJMp078016
Norder, H., De Palma, A. M., Selisko, B., Costenaro, L., Papageorgiou, N., Arnan, C., et al. (2011). Picornavirus non-structural proteins as targets for new anti-virals with broad activity. Antivir. Res. 89 (3), 204–218. doi:10.1016/j.antiviral.2010.12.007
Rotbart, H. A. (2000). Antiviral therapy for enteroviruses and rhinoviruses. Antivir. Chem. Chemother. 11 (4), 261–271. doi:10.1177/095632020001100402
Swain, S. K., Gadnayak, A., Mohanty, J. N., Sarangi, R., and Das, J. (2022). Does enterovirus 71 urge for effective vaccine control strategies? Challenges and current opinion. Rev. Med. Virol. 32 (4), e2322. doi:10.1002/rmv.2322
Tripodi, F., Falletta, E., Leri, M., Angeloni, C., Beghelli, D., Giusti, L., et al. (2022). Anti-aging and neuroprotective properties of Grifola frondosa and Hericium erinaceus extracts. Nutrients 14 (20), 4368. doi:10.3390/nu14204368
Wang, S. M. (2016). Milrinone in enterovirus 71 brain stem encephalitis. Front. Pharmacol. 7, 82. doi:10.3389/fphar.2016.00082
Wang, S. M., Lei, H. Y., Huang, K. J., Wu, J. M., Wang, J. R., Yu, C. K., et al. (2003). Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J. Infect. Dis. 188 (4), 564–570. doi:10.1086/376998
Wang, S. M., Lei, H. Y., Su, L. Y., Wu, J. M., Yu, C. K., Wang, J. R., et al. (2007). Cerebrospinal fluid cytokines in enterovirus 71 brain stem encephalitis and echovirus meningitis infections of varying severity. Clin. Microbiol. Infect. 13 (7), 677–682. doi:10.1111/j.1469-0691.2007.01729.x
Wang, L., Wang, J., Wang, L., Ma, S., and Liu, Y. (2015). Anti-enterovirus 71 agents of natural products. Molecules 20 (9), 16320–16333. doi:10.3390/molecules200916320
Weston, S., Coleman, C. M., Haupt, R., Logue, J., Matthews, K., Li, Y., et al. (2020). Broad anti-coronavirus activity of food and drug administration-approved drugs against SARS-CoV-2 in vitro and SARS-CoV in vivo. J. Virol. 94 (21), e01218-20. doi:10.1128/JVI.01218-20
Xia, Z., Wang, H., Chen, W., Wang, A., and Cao, Z. (2024). Scorpion venom antimicrobial peptide derivative BmKn2-T5 inhibits enterovirus 71 in the early stages of the viral life cycle in vitro. Biomolecules 14 (5), 545. doi:10.3390/biom14050545
Xiong, W., Jiang, X., He, J., Zhong, Y., Ge, X., Liu, B., et al. (2024). Isolation and identification of active components from Grifola frondosa and its anti-EV71 virus effect. J. Sci. Food Agric. 104 (7), 4453–4464. doi:10.1002/jsfa.13332
Yang, W., Li, L., Li, G., Li, X., Liu, H., Han, X., et al. (2024). Blocking CCL3-mediated neutrophil recruitment into the brain alleviates immunopathology following severe enterovirus 71 infection. iScience 27 (12), 111388. doi:10.1016/j.isci.2024.111388
Keywords: enterovirus 71, brainstem encephalitis, pulmonary edema, antiviral, entrovirus
Citation: You S-Y, Chen S-H and Wang S-M (2025) Promising therapeutics of enterovirus 71 infection: sunshine behind cloud. Front. Pharmacol. 16:1652159. doi: 10.3389/fphar.2025.1652159
Received: 23 June 2025; Accepted: 29 August 2025;
Published: 16 September 2025.
Edited by:
Shaoyong Ke, Hubei Academy of Agricultural Sciences, ChinaReviewed by:
Abid Qureshi, University of Kashmir, IndiaOluwatayo Olasunkanmi, Harbin Medical University, China
Yunqi Ma, Binzhou Medical University, China
Copyright © 2025 You, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shih-Min Wang, cGVkd2FuZ0BtYWlsLm5ja3UuZWR1LnR3, cGVkd2FuZ3NtQGdtYWlsLmNvbQ==
 Sheng-Yu You1,2
Sheng-Yu You1,2 Shih-Min Wang
Shih-Min Wang