Abstract
Sirtuin-3 (SIRT3) is a mitochondrial deacetylase highly expressed in the nervous system, known to regulate mitochondrial homeostasis, energy metabolism, neuroinflammation, apoptosis, and oxidative stress, suggesting its potential neuroprotective role in central nervous system (CNS) disorders. Recent studies indicated that SIRT3 improves neuronal survival by reducing oxidative damage, alleviating neuroinflammation, and modulating autophagy. Therefore, it is imperative to conduct more in-depth and extensive investigations into the mechanisms underlying SIRT3 in central nervous system disorders. This review summarized current research advances on SIRT3, including its fundamental molecular structure, key downstream targets, and mechanisms of action in certain CNS diseases. It further analyzed the potential pharmacological mechanisms of several SIRT3 agonists and explored their therapeutic value in improving CNS disorders. Based on existing evidence, SIRT3 emerges as a promising therapeutic target, offering novel strategies for treating neurological diseases.
1 Introduction
In recent years, research on the pathological mechanisms of central nervous system (CNS) diseases has increasingly focused on the regulatory network of mitochondrial dysfunction and oxidative stress imbalance (Rakshe et al., 2024; Lautrup et al., 2019). The mitochondrial deacetylase Sirtuin-3 (SIRT3) has been identified as a critical regulator of metabolic systems, demonstrating its essential function in sustaining mitochondrial efficiency and coordinating cellular bioenergetics (Trinh et al., 2024). SIRT3 governs key physiological processes including mitochondrial quality control, oxidative stress resistance, energy metabolism, and apoptosis by mediating protein deacetylation (Meng et al., 2019). By activating superoxide dismutase 2 (SOD2), SIRT3 reduces reactive oxygen species (ROS) accumulation, thereby suppressing neuroinflammation and oxidative damage to enhance neuronal survival (Tyagi and Pugazhenthi, 2023). Furthermore, SIRT3 regulates mitophagy by activating downstream signaling pathways, including peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α), to promote mitochondrial biogenesis and maintain mitochondrial homeostasis (Li Y. et al., 2018). Additionally, SIRT3 modulates the NAD+/NADH ratio to enhance the tricarboxylic acid cycle and oxidative phosphorylation, ensuring neuronal energy supply and slowing disease progression (Cheng et al., 2021; Zhang et al., 2023). Notably, SIRT3 expression progressively decreases with advancing age, correlating with increased neurodegenerative disease susceptibility (Munteanu et al., 2024). Evidence from animal study demonstrated that exogenous upregulation of SIRT3 effectively ameliorated cognitive dysfunction in transgenic mouse models of Alzheimer’s disease (AD) (Yin et al., 2018a). These findings highlighted that SIRT3 emerged as a druggable target with significant clinical potential, while pharmacological activation of this mitochondrial deacetylase may represent a novel therapeutic avenue for treating CNS disorders. Therefore, further investigation of the upstream regulatory mechanisms of SIRT3 and its interactions with other cellular signaling pathways will enhance comprehension of the pathological mechanisms underlying CNS diseases while providing innovative conceptual frameworks for developing preventive and therapeutic strategies.
2 Physiological basis of SIRT3
2.1 Structure
SIRT3 is a mitochondrial NAD+-dependent deacetylase that plays critical roles in cellular energy metabolism, oxidative stress response, and apoptosis regulation (Shen et al., 2020). The gene of SIRT3 is located on human chromosome 11p15.5, encoding a 399-amino acid protein with a full-length molecular weight of approximately 44 kDa. Upon mitochondrial translocation, the mitochondrial targeting sequence (MTS) is cleaved, yielding mature SIRT3 with a molecular weight of about 28 kDa (Lescai et al., 2009; Donadini et al., 2013). The protein structure comprises a large domain and a small domain connected by a flexible loop region, forming a stable catalytic core. The large domain contains a Rossmann fold responsible for NAD+ binding and providing fundamental structural elements for catalytic activity, while the small domain stabilizes NAD+ binding and regulates substrate access to the catalytic pocket (Zhang J. et al., 2020). Additionally, the C-terminal region mediates protein-protein interactions to enhance substrate specificity or modulate enzymatic activity (Jin et al., 2009a). The intricate structural architecture of SIRT3 underpins its multifunctional roles.
2.2 Cellular localization and catalytic properties
SIRT3 primarily localizes to the mitochondrial matrix, and its sub-cell localization is tightly regulated by the MTS, transmembrane transport systems, post-translational modifications (PTMs), and intracellular signaling pathways (Zhang et al., 2015). The mRNA of SIRT3 is translated by free ribosomes in the cytoplasm, producing a precursor protein containing the MTS. This sequence, approximately 30 amino acids in length and enriched in arginine and lysine, confers mitochondrial affinity (Bao et al., 2010; Jin et al., 2009a). Besides, it can be recognized by the translocase of the outer mitochondrial membrane and translocase of the inner mitochondrial membrane complexes, mediating the transmembrane transport of SIRT3 (Zhang J. et al., 2020). Upon entering the mitochondrial matrix, the MTS of SIRT3 is cleaved by mitochondrial processing peptidase (MPP) at residues Arg31 and Ala32, forming a mature protein with intact catalytic activity (Ansari et al., 2017). The mature SIRT3 predominantly anchors to mitochondrial matrix protein complexes and stabilizes through interactions with mitochondrial matrix proteins such as heat shock protein 60 and heat shock protein 70 (Yang et al., 2011; Hu et al., 2022) (Figure 2b).
SIRT3 exhibited higher catalytic efficiency compared to Sirtuin-1 (SIRT1) and Sirtuin-2 (SIRT2). Firstly, mitochondria serve as the central hub for cellular energy metabolism and oxidative stress responses. Meanwhile, due to the hydrophobic pocket within the catalytic domain of SIRT3, SIRT3 can recognize complex groups (Zhang et al., 2019) (Figure 1), thereby enabling it to react with more substrates. Secondly, the catalytic activity of SIRT3 relies on NAD+ and involves multiple key amino acid residues, with His-248 serving as the core catalytic residue (Jin et al., 2009b). SIRT3 participates in physiological or pathological processes by mediating the deacetylation of diverse biological macromolecules (Lambona et al., 2024). Studies demonstrated that the enzymatic activity of SIRT3 is directly regulated by NAD + concentration and increasing proportionally with elevated NAD+/NADH ratios (Lambona et al., 2024; Feldman et al., 2015; Anderson et al., 2017).
FIGURE 1
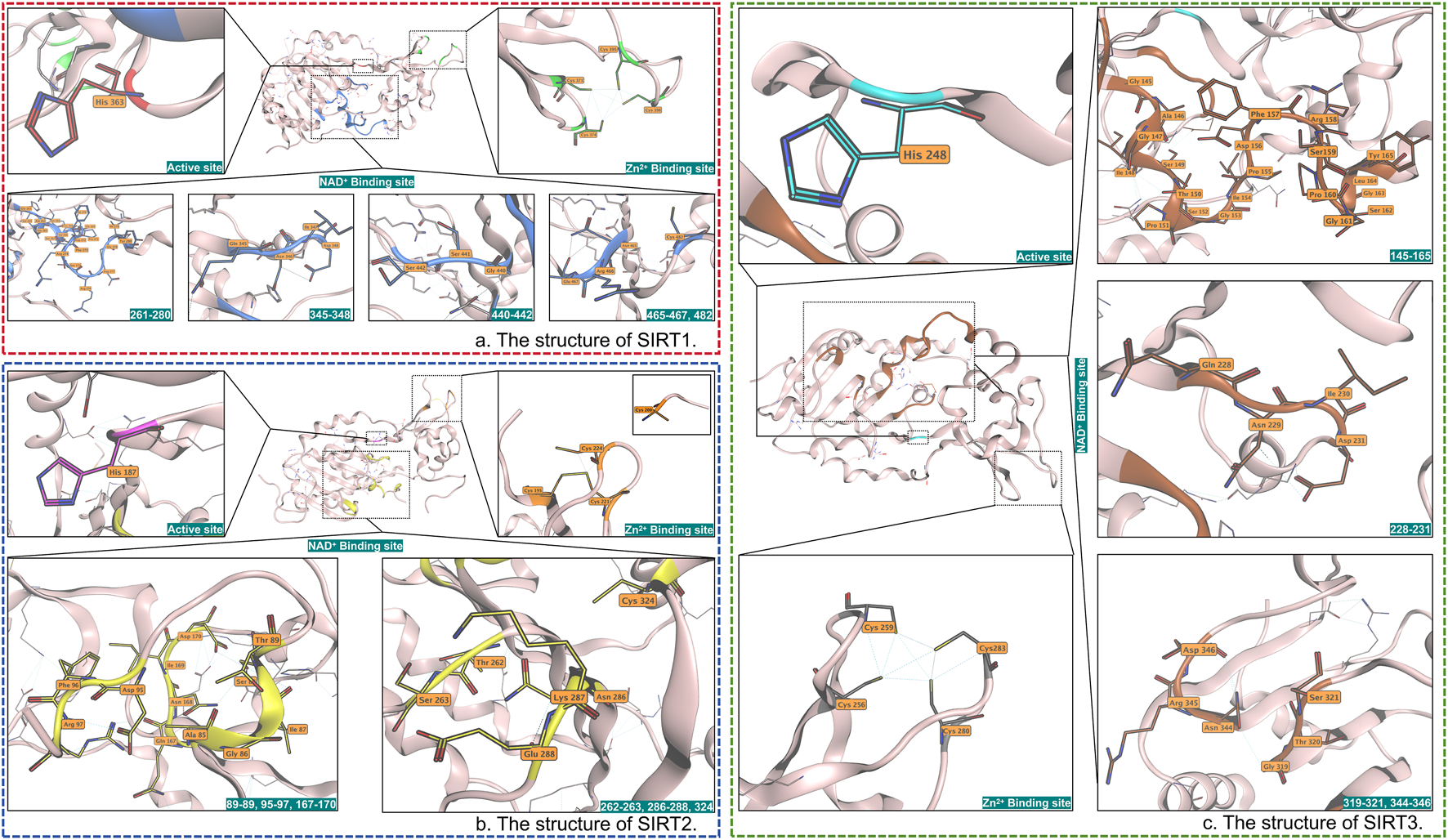
(a) The active site, Zn2+ binding site, and NAD+-binding site of SIRT1 are labeled red, green, and blue. (b) The active site, Zn2+ binding site, and NAD+ binding site of SIRT2 are labeled pink, orange, and yellow. (c) The active site, Zn2+ binding site, and NAD+ binding site of SIRT2 are labeled cyan, gray, and orange, respectively. SIRT3 specifically recognizes the β-hydroxy group and chiral center (preferring the S-configuration) of β-hydroxybutyrylation modification (Kbhb) through a hydrogen bond network in its active pocket, which is composed of residues H248, Q228, and V292. Meanwhile, the hydrophobic environment formed by F180, F294, I230, and V324 accommodates the acyl chain of Kbhb. Additionally, the hydrogen bond system consisting of E296, G295, and E325 forces the substrate peptide chain to adopt a β-sheet conformation, which, due to the entropic penalty effect, repels glycine flanking sites.
2.3 Expression
The expressions of SIRT3 are regulated at multiple levels, including gene transcription, mRNA stability, PTMs, protein stability, and enzymatic activity. These mechanisms work synergistically under diverse physiological and pathological conditions to maintain mitochondrial metabolic homeostasis and cellular energy balance. For example, the promoter region of the SIRT3 gene contains multiple transcription factor binding sites. It is positively regulated by nuclear factor erythroid 2-related factor 2 (Nrf2), while hypoxia-inducible factor 1alpha (HIF-1α) suppress its transcription under hypoxic or stress conditions (Loboda et al., 2009; Yao et al., 2022) (Figure 2a). Metabolic regulatory signals also influence the transcriptional level of SIRT3. Chronic exposure to biochemical stress or mitochondrial metabolic abnormalities can accumulate ROS intracellularly, generating excessive superoxide, hydrogen peroxide, and hydroxyl radicals (Albano, 2006). These sequentially activate the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) and PGC-1α, thereby enhancing SIRT3 transcription (Wang Y. et al., 2025; Wang et al., 2022). On the other hand, nuclear-localized SIRT1 also participates in the positive regulation of SIRT3 expression by deacetylating PGC-1α in the nucleus (Zhang et al., 2021; Rodgers et al., 2005) (Figure 2d).
FIGURE 2
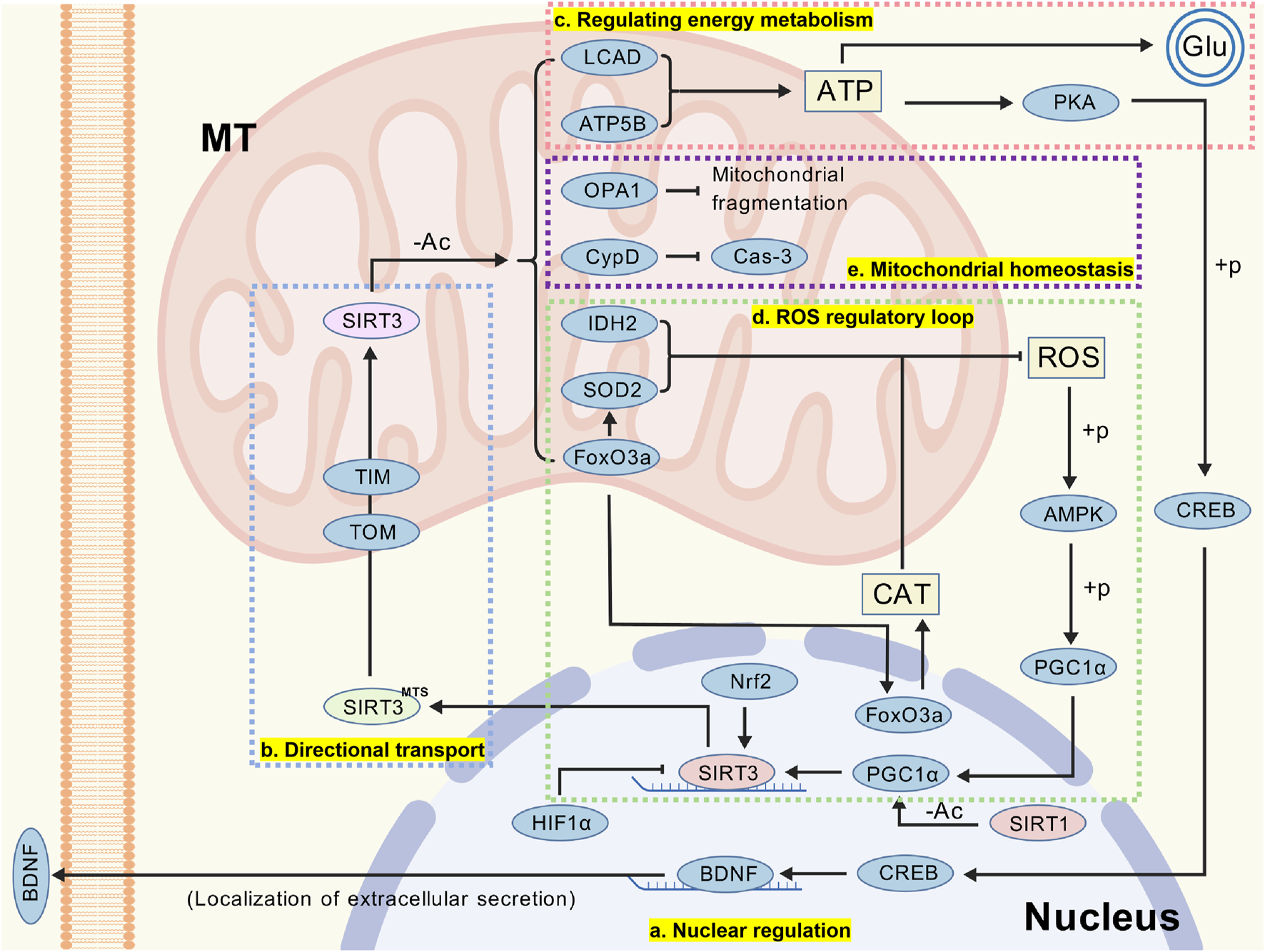
(a) SIRT3 expression and activity are regulated at multiple levels, including gene transcription, mRNA stability, post-translational modifications, protein stability, and enzyme activity regulation. (b) The MTS within the SIRT3 precursor is a critical fragment recognized and transported by TOM and TIM. After entering the mitochondria, the SIRT3 precursor undergoes cleavage to form mature SIRT3. (c) SIRT3 influences neuronal stability and information transmission by regulating ATP production. (d) SIRT3 plays a significant role in oxidative stress, and this article only illustrates one pathway through which SIRT3 modulates intracellular ROS homeostasis. (e) The cytoprotective effects of SIRT3 are also associated with its regulation of mitochondrial homeostasis. (Abbreviation: MT: mitochondria; SIRT3: Sirtuin-3; HIF-1α: hypoxia-inducible factor 1alpha; Nrf2: nuclear factor erythroid 2-related factor 2; MTS: mitochondrial targeting sequence; TOM: translocase of the outer mitochondrial membrane; TIM: translocase of the inner mitochondrial membrane; LCAD: long-chain acyl CoA dehydrogenase; ATP5B: ATP synthase β; Glu: glutamic acid; PKA: protein kinase A; CREB: cAMP-response element binding protein; BDNF: brain-derived neurotrophic factor; OPA1: optic atrophy 1; CypD: cyclophilin D; Cas-3: caspase-3; ROS: reactive oxygen species; SOD2: superoxide dismutase 2; IDH2: isocitrate dehydrogenase 2; FoxO3a: forkhead box O3; CAT: catalase; AMPK: adenosine monophosphate-activated protein kinase; PGC-1α: peroxisome proliferator-activated receptor γ coactivator 1 α).
2.4 Function and related pathways
SIRT3 modulates energy metabolism, antioxidant responses, and mitochondrial homeostasis by deacetylating specific sites on key metabolic enzymes (Table 1). SIRT3 enhances the activity of long-chain acyl CoA dehydrogenase (LCAD) through deacetylation, accelerating fatty acid β-oxidation and promoting the generation of more acetyl-CoA. Via the tricarboxylic acid cycle, this process boosts ATP production (Hirschey et al., 2010). For glutamatergic neurons, this step helps enhance synaptic transmission efficiency. On the other hand, deacetylation of ATP synthase β by SIRT3 strengthens its catalytic activity, improving the ATP synthesis efficiency at the terminal of the mitochondrial electron transport chain and increasing the availability of ATP in the cytoplasm to maintain synaptic vesicle cycling and neuronal electrical activity (Zhang et al., 2016). The increased ATP production also activates the protein kinase A/cAMP-response element binding protein (PKA/CREB) pathway, leading to CREB phosphorylation and promoting the expression of neurotrophic factors such as brain-derived neurotrophic factor (BDNF), which can enhance neuronal survival and plasticity (Mo et al., 2024) (Figure 2c).
TABLE 1
| Protein substrate | Deacetylation site | Function | References |
|---|---|---|---|
| LCAD | K42 | Promote fatty acid oxidation | Hirschey et al. (2010) |
| ATP5B | K485 | Synthesize ATP | Zhang et al. (2016) |
| SOD2 | K68, K122 | Enhance antioxidant stress ability | Dikalova et al. (2017) |
| IDH2 | K413 | Promote NADPH generation | Zou et al. (2017) |
| FoxO3a | K271, K290 | Regulate mitochondrial oxidative stress | Wang J. et al. (2025) |
| CypD | K166 | Regulate mPTP activity | Yan et al. (2022) |
| OPA1 | K926, K931 | Regulate mitochondrial fusion | Samant et al. (2014) |
Specific sites of key metabolic enzymes involved in deacetylation of SIRT3.
Abbreviation: SIRT3: Sirtuin-3; LCAD: long-chain acyl CoA dehydrogenase; ATP5B: ATP, synthase β; SOD2: superoxide dismutase 2; IDH2: isocitrate dehydrogenase 2; FoxO3a: forkhead box O3; CypD: cyclophilin D; OPA1: optic atrophy 1; NADPH: nicotinamide adenine dinucleotide phosphate; mPTP: mitochondrial permeability transition pore.
As previously mentioned, elevated ROS in the cytoplasm can increase SIRT3 expression by activating the AMPK/PGC-1α pathway. In turn, SIRT3 deacetylates SOD2 and isocitrate dehydrogenase 2 (IDH2), significantly enhancing their enzymatic activity to inhibit ROS (Dikalova et al., 2017; Zou et al., 2017). On the other hand, SIRT3-mediated deacetylation of forkhead box O3 (FoxO3a) in mitochondria activates FoxO3a-dependent gene expression (Jacobs et al., 2008). This not only regulates SOD2 to inhibit ROS but also enhances the activity of catalase, achieving clearance of hydrogen peroxide and reducing oxidative stress. Overall, SIRT3 plays a critical role in maintaining intracellular ROS homeostasis (Figure 2d).
Optic atrophy 1 (OPA1) is a critical regulatory protein involved in maintaining mitochondrial inner membrane fusion and the formation of mitochondrial cristae structures (von der Malsburg et al., 2023). The regulation of OPA1 by SIRT3 facilitates the repair and functional recovery of mitochondrial structures, preventing mitochondrial fragmentation (Chen et al., 2024b; Samant et al., 2014). The opening of the mitochondrial permeability transition pore (mPTP) is a hallmark of mitochondrial dysfunction, which is closely associated with excessive acetylation of cyclophilin D (CypD) (Dikalova et al., 2024). By deacetylating CypD, SIRT3 modulates mPTP activity to inhibit the release of cytochrome C, which suppresses the activation of Caspase-3 and ultimately inhibiting apoptosis (Yan et al., 2022; Poppe et al., 2001) (Figure 2e).
3 Role of SIRT3 in central neurons
SIRT3 is widely distributed across various types of central neurons, and its expression and function are cell specific. Current research primarily focuses on glutamatergic neurons, GABAergic neurons, dopaminergic neurons, and astrocytes (Figure 3).
FIGURE 3
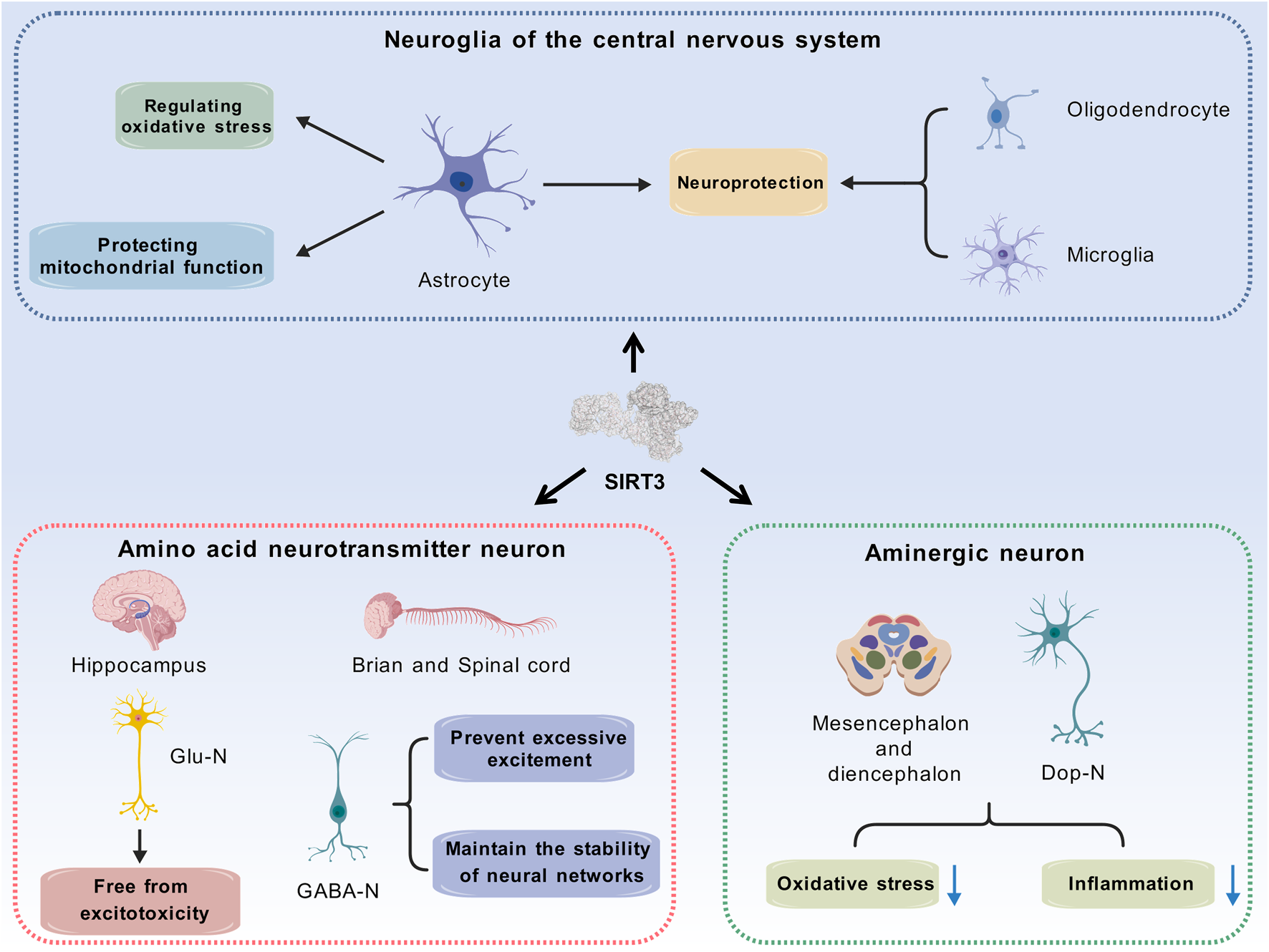
SIRT3 exerts distinct physiological functions in different types of cells. Glutamatergic neurons and GABAergic neurons serve as representative excitatory and inhibitory neurons, and the roles of SIRT3 in these 2 cell types contribute to maintaining excitatory-inhibitory homeostasis. Mitochondrial damage induced by reduced SIRT3 expression accelerates the degeneration of dopaminergic neurons, which may serve as a potential pathogenesis of Parkinson’s disease. As for neuroglia in the central nervous system, SIRT3 achieves neuroprotective effects through intracellular regulatory mechanisms. (Abbreviation: SIRT3: Sirtuin-3; Glu-N: Glutamatergic Neurons; GABA-N: GABAergic Neurons; Dop-N: Dopaminergic Neurons).
3.1 Glutamatergic neurons
Glutamate is the main excitatory neurotransmitter in the vertebrate brain. Its excessive release triggers membrane depolarization through receptor-mediated Na+ and Ca2+ influx, increasing neuronal mitochondrial oxidative phosphorylation and superoxide production (Rueda et al., 2016). A study on cortical mitochondria of SIRT3 knockout (KO) mice showed that the lack of SIRT3 affected the subcellular regulation of Ca2+ after glutamate induced Ca2+ influx and confirmed through neuronal cell and mouse running experiments that glutamatergic signaling mediates the upregulation of SIRT3. The bidirectional regulatory effect between SIRT3 and glutamatergic neurons enhanced the resistance to degeneration in hippocampal and cortical neurons (Cheng et al., 2016). The reduced incorporation of [1,6–13C]glucose-derived carbon into all isotopomers of glutamate, glutamine, γ-aminobutyric acid (GABA), and aspartate in SIRT3 knockout brains demonstrated diminished mitochondrial metabolic activity and tricarboxylic acid cycle flux in both neuronal and astrocytic compartments (Kristian et al., 2021). Continuous high levels of Ca2+ activated protein phosphatase 4, leading to high doses of glutamate inhibiting the AMPK/PGC-1α/SIRT3 pathway, while brief increased in Ca2+ levels could activate this pathway, providing important insights for precise regulation of glutamate and SIRT3 levels to alleviate brain related diseases (Gu et al., 2023). There are gender differences between glutamate and SIRT3. It was reported that the lack of SIRT3 significantly increased the expression of N-methyl-D-aspartate (NMDA) receptor in the hippocampus of female SIRT3 KO mice only. Excessive upregulation of NMDA receptor 2B could enhance glutamatergic excitotoxicity, affecting primary neural development and synaptic plasticity (Allen et al., 2023). Based on the complex relationship between glutamate and SIRT3, further exploration of strategies to balance their expression may provide novel therapeutic directions for neurological diseases.
3.2 GABAergic neurons
GABA is an inhibitory neurotransmitter that has effects on learning, sleep, memory, and muscle movement. Dysfunction and degeneration of GABAergic neurons lead to abnormal hyperexcitability of neural circuits, also causing degeneration of glutamatergic neurons (Palop and Mucke, 2016). After treatment with diazepam, the activation of GABA receptors significantly reduced seizures induced by kainic acid in SIRT3+/− AppPs1 mice. The experiment also showed that the loss of GABAergic neurons and the exacerbation of neural network overexcitation were caused by a decrease in SIRT3 (Cheng et al., 2020). Intermittent fasting improves multiple health indicators and can slow down the progression of diabetes, vascular disorders, and AD (Mattson et al., 2018). Experiment on SIRT3 KO mice demonstrated that SIRT3 was essential for enhancing GABAergic synaptic transmission adaptability and counteracted anxiety during intermittent fasting (Liu et al., 2019). In rats with middle cerebral artery occlusion, the neuroprotective effects of wogonoside in cerebral ischemia-reperfusion injury were mediated through regulating GABAergic amino acid metabolism, mitochondrial bioenergetics, and glutathione biosynthesis pathways, which collectively preserved redox equilibrium while suppressing oxidative damage through attenuation of reactive oxygen species overproduction (Xu et al., 2024). In summary, GABAergic neurons not only ameliorate neurological disorders through interactions with SIRT3 but also exhibit complex interplay with glutamatergic neurons.
3.3 Dopaminergic neurons
Midbrain dopaminergic neurons have been a focal point of intensive research. The nigrostriatal dopamine system is crucial for coordinating fine motor skills and maintaining movement balance. The degeneration of dopaminergic neurons in the substantia nigra is an important pathological mechanism of Parkinson’s disease (PD) (Arenas et al., 2015). It was reported that SIRT3 KO mice exhibited significantly elevated acetylation levels of manganese superoxide dismutase (MnSOD) on lysine 68 in substantia nigra pars compacta dopaminergic neurons compared to wild-type (WT) mice, alongside its mitochondrial MnSOD activity was significantly lower than that of WT mice. This proves that in mouse substantia nigra pars compacta dopaminergic neurons, SIRT3 deacetylates MnSOD on lysine 68 to increase its activity and reduce mitochondrial oxidative stress (Shi et al., 2017). In vitro experiments using MN9D cells revealed that SIRT3 promoted mitochondrial autophagy while inhibiting activation of the nucleotide-binding oligomerization domain-like receptor protein 3 (NLRP3) inflammasome in dopaminergic neurons (Jiang et al., 2022). Additionally, SIRT3 also mitigated oxidative stress-induced neurotoxicity in dopaminergic neurons (Lee et al., 2021). For instance, SIRT3 directly deacetylated SOD2 and adenosine triphosphate (ATP) synthase β in dopaminergic neurons to prevent cell death (Zhang et al., 2016). In the rat model of subarachnoid hemorrhage, dopamine-D2-agonists were shown to inhibit mitochondrial fission mediated by dynamin-related protein 1, via activating mitofusin 2 and optic atrophy 1. On the other hand, dopamine-D2-agonists regulated PGC-1α/SIRT3 pathway by restricting cytochrome C in mitochondria, which could improve mitochondrial dysfunction and exert neuroprotective effects (Rehman et al., 2025). To summarize, these findings highlighted the bidirectional regulatory interactions between SIRT3 and dopaminergic neurons, which collectively maintain neuronal homeostasis.
3.4 Astrocytes
Astrocytes are widely distributed in the mammalian brain, and involve in maintaining the neurovascular unit, facilitating synaptic network formation, regulating ionic balance, regulating synaptic neurotransmitter concentrations, and synthesizing bioactive molecules that influence neuronal activity (Khakh and Deneen, 2019). Cell experiments showed that hyperglycemic conditions significantly enhanced the susceptibility of SIRT3 to recurrent low glucose, inducing mitochondrial structural abnormalities in astrocytes. However, overexpression of SIRT3 demonstrated preservation of mitochondrial bioenergetics while decreasing oxidative damage biomarkers induced by recurrent low glucose. Meanwhile, SIRT3 inhibited the transformation of astrocytes into neuroinflammatory A1 like reactive phenotype (Gao R. et al., 2022). It was also reported that SIRT3 could modify astrocyte activation by regulating the Notch1/NF-κB pathway, alleviating inflammatory responses following status epilepticus (Zhu et al., 2024). As for ischemic stroke, SIRT3 exerted a protective effect by regulating the HIF-1α/VEGF pathway in astrocytes (Yang X. et al., 2021). Furthermore, SIRT3 prevents astrocyte A1 polarization and associated neurotoxicity under chronic hypoxia by inhibiting phosphorylation and nuclear translocation of the transcription factor signal transducer and activator of transcription 3 (STAT3) (Hu et al., 2023). Research indicated that caffeine improved astrocyte-mediated protein Tau (Tau) neurotoxicity via modulation of the EGR1/SIRT3 pathway (Gao et al., 2024). In total, the roles of SIRT3 in astrocytes are complex and critical, demonstrating its potential to mitigate astrocyte injury and protect CNS targets.
3.5 Other neuron types
Astrocytes, oligodendrocytes, and microglia are the main glial cells in CNS. The main functions of oligodendrocytes include wrapping around axons, forming myelin sheaths, assisting in the efficient transmission of biological electrical signals, and maintaining the normal function of neurons (Emery and Wood, 2024). Microglia, characterized by their multipolar morphology and plasticity, are immune effector cells in CNS and play critical roles in physiological processes (Colonna and Butovsky, 2017). Studies demonstrated enhanced expression of SIRT3 in astrocytes, oligodendrocytes, and microglia within the white matter of hypoxic newborn rats. Meanwhile, early hypoxia induced intense SIRT3 expression in microglia (Li X. et al., 2018). Quantitative PCR analyses across neuron subtypes revealed that SIRT3 exhibits the highest expression in primary neurons, followed by astrocytes, while oligodendrocytes and microglia show the lowest levels. PGC-1α altered the expression level of SIRT3 in neurons. Among different types of cells in PGC-1α KO mice, the expression level of SIRT3 was highest in astrocytes but lower than in WT mice (Buck et al., 2017).
4 SIRT3 in central nervous system diseases
4.1 Ischemic stroke
Ischemic stroke is primarily caused by cerebral blood flow interruption due to large-vessel atherosclerosis, small-vessel lacunar infarction, or cardioembolism (Sommer, 2017), which makes the brain starve of oxygen and glucose, leading to neuronal energy metabolism failure and secondary cell damage (Qin et al., 2022). Recent studies demonstrated that SIRT3 could repair mitochondrial ultrastructure and membrane composition, promote mitochondrial biogenesis, and alleviate mitochondrial dysfunction by upregulating the expression and activity of optic atrophy 1 (Chen et al., 2024b). Besides, SIRT3 also inhibited the expression of voltage-dependent anion channel 1 and adenine nucleotide translocase 1, preventing abnormal opening of the mitochondrial permeability transition pore and reducing mitochondrial apoptosis during ischemic injury (Yang Y. et al., 2021). Metabolomic analysis showed a significant increase in GABA and glutathione levels in the brain after wogonoside treatment, confirming that SIRT3 not only had ability to mitigate oxidative stress but also alleviated excitotoxicity caused by excessive glutamate release (Xu et al., 2024). Also, SIRT3 protects the integrity of the blood-brain barrier (BBB) in ischemic stroke mice by regulating the HIF-1α/VEGF pathway in astrocytes, reducing inflammatory responses and neuronal apoptosis (Yang X. et al., 2021). SIRT3 can also promote the migration of microglia in ischemic stroke by increasing the expression of CX3C chemokine receptor 1 (Cao et al., 2019). Furthermore, SIRT3 promoted PINK1/Parkin mediated mitochondrial autophagy, increased microvascular density and the expression of VEGF A, and reduced neuronal apoptosis in cerebral ischemia-reperfusion model rats (Wei et al., 2023). On the other hand, overexpression of SIRT1 can improve mitochondrial respiratory chain dysfunction by enhancing the deacetylation activity of SIRT3, reflecting the synergistic effect of the two in restoring mitochondrial structure and function (Chen et al., 2024c). However, the direct molecular interaction between SIRT1 and SIRT3 is not yet clear, and the synergistic effect lacks causal validation through gene knockout or dual intervention experiments. In general, intervention studies validated the therapeutic potential of SIRT3 for ischemic stroke (Table 2), but it is still necessary to explore its targeting ability and provide new directions for clinical treatment.
TABLE 2
| Activator | Mechanism | References |
|---|---|---|
| Silbene glycoside | Modulate SIRT3/AMPK pathway | Li et al. (2021) |
| Trilobatin | Modulate SIRT3/TLR4/Nrf2 pathway | Gao et al. (2020) |
| LanCL1 | Modulate Akt-PGC-1α-SIRT3 pathway | Xie et al. (2018) |
| Icariside II | Modulate Nrf2/SIRT3 pathway | Feng et al. (2018) |
| Luteolin | Modulate SIRT3/AMPK/mTOR pathway | Liu et al. (2020) |
| Notoginseng Leaf Triterpenes | Modulate SIRT1/2/3-FoxO3a-MnSOD/PGC-1α pathway | Xie et al. (2020) |
| Genipin | Modulate UCP2-SIRT3 pathway | Zhao et al. (2019) |
| 4′-O-methylbavachalcone | Modulate SIRT3-PARP-1 pathway | Chen et al. (2024d) |
| Honokiol | Modulate SIRT3/Drp1 pathway | Zheng et al. (2023) |
| AFPR | Modulate SIRT3/Pink1/Parkin pathway | Wei et al. (2023) |
SIRT3 activators that could improve ischemic stroke found in nearly a decade.
Abbreviation: SIRT3: Sirtuin-3; AMPK: Adenosine 5′-monophosphate (AMP)-activated protein kinase; TLR4: Toll-like receptor 4; Nrf2: nuclear factor erythroid 2-related factor 2; LanCL1: lanthionine synthetase C-like protein 1; Akt: protein kinase B; PGC-1α: proliferator-activated receptor γ coactivator-1α; mTOR: mammalian target of rapamycin; FoxO3a: forkhead box O3; MnSOD: superoxide dismutase; UCP2: uncoupling protein 2; PARP-1: Poly (ADP-ribose) polymerase-1; Drp1: dynamin-related protein 1; AFPR: active fraction of Polyrhachis vicina (Roger); PINK1: PTEN, induced putative kinase 1; Parkin: Parkin protein.
Diabetic cerebral ischemia-reperfusion injury (CIRI) refers to the secondary brain tissue damage caused by the restoration of cerebral blood flow after ischemic interruption in diabetic patients, leading to more severe neurological dysfunction (Zhou et al., 2025). Its main pathological mechanisms include enhanced inflammatory responses, exacerbated oxidative stress, and mitochondrial dysfunction (Przykaza, 2021). It was found that in diabetic CIRI rats, the levels of SIRT1/SIRT3 are significantly reduced, accompanied by decreased levels of mitochondria-regenerating proteins such as PGC-1α, nuclear respiratory factor 1 (NRF1), and transcription factor A (TFAM). This suggests that diabetes may hinder mitochondrial regeneration by inhibiting the SIRT1/SIRT3-PGC-1α-NRF1-TFAM signaling pathway, thereby exacerbating cerebral ischemia-reperfusion injury in rats (Xin et al., 2025). Although research on this topic is limited, existing data still confirms the critical protective role of SIRT3 in CIRI. For example, melatonin can alleviate CIRI in diabetic mice by activating the protein kinase B (Akt)/SIRT3/SOD2 pathway and improving mitochondrial damage. However, when SIRT3 upregulation is suppressed by 3-TYP, these protective effects are attenuated, confirming that SIRT3 plays a key role in diabetic cerebral ischemia-reperfusion injury (Liu L. et al., 2021). Further studies have demonstrated that rapamycin can maintain mitochondrial dynamic balance by regulating the SIRT3-dynamin-related protein 1 (DRP1)/OPA1 signaling pathway, thereby improving CIRI in diabetic rats (Hei et al., 2023). Given all that, exploring the synergistic interactions of SIRT3 and its differential expression in various neuron types will provide strong support for precise treatment of CIRI. Additionally, as a comorbidity of diabetes, clarifying the mechanism of SIRT3 in diabetes is of great significance for halting the further progression of neurological damage.
4.2 Dementia
4.2.1 Vascular dementia
Vascular dementia (VD) is characterized by ischemic or hemorrhagic damage to brain tissue caused by cerebrovascular lesions, manifesting as multiple lesions, white matter lesions, neuronal loss, and disrupted neural networks (Kuang et al., 2021). In central neurons, SIRT3 alleviates neuroinflammation and mitochondrial dysfunction following ischemic hypoxic brain injury by inhibiting the activation of pro-inflammatory microglia (Yan et al., 2025). Gastrodin, an active component extracted from the root of Gastrodia elata Bl., has the ability to ameliorate brain tissue injury through multiple pathways (Xiao et al., 2023). It was shown that gastrodin increased SIRT3 expression in VD model rats and deacetylated mitochondrial TFAM at K5, K7, and K8 site, reversing mitochondrial dysfunction, alleviating oxidative stress, and reducing aging (Chen et al., 2024e). Additionally, gastrodin enhanced ATP production, superoxide dismutase activity, and glutathione levels via SIRT3 regulation, further mitigating mitochondrial dysfunction in VD (Shi et al., 2024). As mentioned earlier, SIRT3 inhibited A1 polarization in astrocyte by regulating STAT3 to reduce oxidative stress and subsequent synaptic damage (Hu et al., 2023). Autophagy plays an important role in the progression of VD. DL-3-n-butylphthalide improved learning and cognitive impairment in VD mice by inhibiting the Nrf2/SIRT3 pathway, which reduced autophagy and apoptosis (Gao L. et al., 2022). SIRT3 also reduced neuronal apoptosis by regulating BDNF expression and synaptic plasticity (Guo et al., 2021).
Future studies may integrate blood or cerebrospinal fluid biomarkers to monitor SIRT3 activity and downstream molecular changes. Exploring the expression characteristics of SIRT3 in different brain cells such as neurons, astrocytes, and endothelial cells using single cell sequencing technology, and clarifying the feasibility of SIRT3 as a therapeutic target in VD may be also a potential research direction. Notably, cerebral small vessel disease (CSVD), recognized for its unique clinical and imaging features, progresses to vascular cognitive impairment or coexists with AD. The pathological feature of CSVD includes BBB disruption (Duering et al., 2023). Recent studies revealed that SIRT3, as a core effector molecule in the NAD+/SIRT3 axis, maintains BBB integrity by regulating mitochondrial metabolism, autophagy, and antioxidant defense (Zhan et al., 2025). Therefore, further research into potential pathways of SIRT3 in BBB regulation hold significant clinical value for developing targeted therapies against CSVD.
4.2.2 Alzheimer’s disease
The core pathological features of AD include deposition of β-amyloid protein (Aβ), abnormal phosphorylation of microtubule associated Tau, and synaptic dysfunction (Spires-Jones and Hyman, 2014). SIRT3 regulates electron transport chain activity, stabilizes mitochondrial membrane potential, and enhances antioxidant capacity. Reduced SIRT3 expression may accelerate mitochondrial metabolic dysregulation, making neurons more susceptible to the effects of Aβ and Tau, finally accelerating neuronal apoptosis (Yin et al., 2018b). SIRT3 deficiency in GABAergic neurons exacerbates cell loss, leading to excessive hyperexcitability of neural networks and epileptiform activity. That caused the increasing mortality of AD model mice (Cheng et al., 2020). A study on APP/PS1/SIRT3−/− mice revealed a significant decrease in insulin-degrading enzyme (IDE) levels in the brain compared to APP/PS1 mice. However, activation of SIRT3 by nicotinamide riboside upregulated IDE expression in normal mice, which was facilitating Aβ degradation. This study suggested SIRT3 may be a potential target for treating AD (Tyagi et al., 2022). Furthermore, SIRT3 activated the FoxO3a-SOD2 axis to mediate mitochondrial antioxidant defense system. It could reduce oxidative stress damage induced by Aβ. In AD patients, the dysfunction of this pathway may exacerbate neurodegenerative changes (Jęśko et al., 2017). In SIRT3 KO mice, the mitochondrial membrane potential of hippocampal neurons and synaptic density both decreased, and learning and memory abilities of mice were impaired. However, overexpression of SIRT3 could reduce Aβ deposition, enhance synaptic plasticity, and improve cognitive function (Yao et al., 2022). Also, SIRT3 improved neural stem cell neurogenesis via regulation of the DVL/GSK3/ISL axis, providing a theoretical basis for its clinical application (Dai et al., 2024). In chronic unpredictable mild stress mice, SIRT1 improves mitochondrial disorder and GABAergic function via SIRT1/PGC-1α/SIRT3 pathway, demonstrating their parallel contributions to neuroprotection against brain diseases (Tabassum et al., 2023).
Clinical studies confirmed that NAD+ precursors such as nicotinamide riboside could enhance mitochondrial metabolism by restoring SIRT3 activity, partially improving cognitive function in AD patients (Wang et al., 2021). In recent years, multiple drugs and compounds were proved to mitigate AD pathology by targeting SIRT3 (Table 3). Curcumin is a bioactive polyphenolic compound extracted from the rhizome of Curcuma Longa L. It could significantly improve cognitive impairment in APPTG mice and alleviate neuronal metabolic dysfunction induced by Aβ42 though regulating the NAD+/NADH ratio and activating SIRT3 (Zia et al., 2021; Liu M. et al., 2021). Resveratrol, a non-flavonoid polyphenol, which not only can be extracted from the rhizome and root of Polygonum cuspidatum Sieb. et Zucc., but also can be synthesized in grape leaves and skins, were widely reported to improve AD by activating the SIRT1 pathway (Surya et al., 2023). However, whether resveratrol slows down AD progression via SIRT3 activation remains to be explored. Although the important role of SIRT3 in the progression of AD has been widely recognized, targeted therapies based on SIRT3 are still in the exploratory stage. Future research should focus on discovering SIRT3 regulatory mechanisms and developing precise interventions to target its activity, offering new therapies for AD patients (Lee et al., 2018).
TABLE 3
| Activator | Mechanism | References |
|---|---|---|
| Honokiol | Enhance mitochondrial SIRT3 expression and activity | Li H. et al. (2018) |
| Trilobatin | Modulate SIRT3/SOD2 pathway | Gao J. et al. (2022) |
| Salidroside | Modulate Nrf2/SIRT3 pathway | Yao et al. (2022) |
| Kai-Xin-San | Modulate SIRT3/NLRP3 pathway | Su et al. (2023) |
| ESP | Modulate Mst1/Nrf2/SIRT3 pathway | Yang et al. (2025) |
| PL171 | Activate SIRT3 against Aβ42O | Li et al. (2020) |
| 3,14,19-Triacetylandrographolide | Modulate SIRT3/FoxO3a pathway | Zhou et al. (2024) |
SIRT3 activators that could improve Alzheimer’s disease found in nearly a decade.
Abbreviation: SIRT3: Sirtuin-3; SOD2: superoxide dismutase 2; Nrf2: nuclear factor erythroid 2-related factor 2; NLRP3: nucleotide-binding oligomerization domain-like receptor-related protein 3; ESP: the phenylpropanoid components of Eleutherococcus senticosus (Rupr. and maxim.) maxim; Mst1: mammalian sterile 20-like kinase 1; Aβ42O: amyloid-β42, oligomers; FoxO3a: forkhead box O3.
4.3 Movement disorders
4.3.1 Parkinson’s disease
The main symptoms of PD include tremors, bradykinesia, and muscle rigidity. Degeneration of dopaminergic neurons in the substantia nigra, abnormal aggregation of α-synuclein (α-Syn), mitochondrial dysfunction, and neuroinflammation are pathological features of the disease (Trinh et al., 2023). Reduced SIRT3 expression in nigral neurons of PD patients disrupted mitochondrial function and autophagy regulation, impairing the clearance of damaged mitochondria. This resulted in α-Syn accumulation and exacerbated oxidative stress damage (Trinh et al., 2023). The abnormal expressions of SIRT3, PINK1, and TFAM in PD patients prevented neurons from mitochondria turnover and maintaining mitochondrial protein. That would aggravate oxidative phosphorylation defects and age-related oxidative stress, leading to neuronal degeneration (Chen et al., 2023). Furthermore, low SIRT3 expression cause mitophagy via PINK1/Parkin pathway, which could accelerate neuronal death, and worse PD symptoms (Gleave et al., 2017). SIRT3 knockout in 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine (MPTP)-treated PD mice exacerbated nigral neuronal degeneration and significantly reduced the expression of tyrosine hydroxylase. This process affected the synthesis of dopamine and exacerbated the movement disorders of PD (Zhang et al., 2016). Conversely, SIRT3 overexpression enhanced nigral neuronal survival, restored dopamine levels, and improved movement function (Gleave et al., 2017). It was reported that NAD+ metabolism regulation strategies could improve mitochondrial function and alleviate movement symptoms in PD patients (Radenkovic et al., 2020). The research on SIRT3 activators for improving PD was listed in Table 4.
TABLE 4
| Activator | Mechanism | References |
|---|---|---|
| s-viniferin | Modulate SIRT3/FoxO3 pathway | Zhang S. et al. (2020) |
| Theacrine | Direct activation of the SIRT3/SOD2 pathway | Duan et al. (2020) |
| Icarim | Enhanced SIRT3 activity | Zeng et al. (2019) |
| P7C3 | Modulate Nrf2/Sirt3 pathway | Chen et al. (2024f) |
| Canagliflozin | Modulate PGC-1α/SIRT3 pathway | Abdelaziz et al. (2025) |
| Ginsenoside Rk1 | Modulate SIRT3/Nrf2/HO-1 pathway | Ren et al. (2023) |
SIRT3 activators that could improve Parkinson’s disease found in nearly a decade.
Abbreviation: SIRT3: Sirtuin-3; FoxO3a: forkhead box O3; SOD2: superoxide dismutase 2; Nrf2: nuclear factor erythroid 2-related factor 2; PGC-1α: proliferator-activated receptor γ coactivator-1α; HO-1: heme oxygenase-1.
Electroacupuncture was commonly used in traditional Chinese medicine as a complementary and alternative medicine therapy for neurodegenerative diseases with minimal side effects (Zheng et al., 2021). It was shown that electroacupuncture could repair neuronal damage in PD model rats by regulating the SIRT3/NLRP3/GSDMD pathway, mitigating mitochondrial damage by clearing abnormal α-Syn accumulation in the substantia nigra (Wang et al., 2024). Additionally, electroacupuncture could activate the SIRT3/PINK1/Parkin pathway to enhance tyrosine hydroxylase expression. The aggregation of α-Syn was reduced in the MPTP-treated PD mice, and their exercise ability was improved (Zhang et al., 2024).
Notably, multiple system atrophy-parkinsonian type exhibits parkinsonian symptoms, while late-stage patients may develop cerebellar ataxia and cognitive dysfunction, with pathological features including α-Syn aggregation (Laferrière et al., 2022). However, no literature was retrieved on the relationship between SIRT3 and multiple system atrophy (MSA). Further exploration is warranted to clarify whether SIRT3 plays a role in MSA pathogenesis.
4.3.2 Huntington’s disease
Huntington’s disease (HD) is a hereditary neurodegenerative disorder caused by mutations in the huntingtin protein gene. Degeneration of medium spiny neurons, synaptic dysfunction, and increased glutamate excitotoxicity are the main pathological changes, leading to choreiform movements, cognitive impairment, and psychiatric symptoms (Bates et al., 2015). Studies showed that SIRT3 expression could significantly decrease in the brains of HD patients, which correlates with overactivation of NMDA receptors mediated by NMDA receptor 2B, mitochondrial dysfunction, and impaired synaptic plasticity (Buck et al., 2017; Someya et al., 2010). Low expression of SIRT3 imbalanced excitatory synaptic, accelerating synaptic degeneration and neuronal death (Kim et al., 2019). It was reported that in models relevant to HD, SIRT3-deficient mice got worse movement dysfunction. The synaptic density of the mice decreased, while excessive activation of NMDA receptors significantly increased glutamate excitotoxicity (Cheng et al., 2016). Additionally, in HD cell models treated with the SIRT3 activator viniferin, the acetylation of SOD2 was reduced and mitochondrial function and antioxidant capacity was enhanced by activating the AMPK pathway (Fu et al., 2012). Clinical research also focuses on exploring SIRT3 as a potential therapeutic target for HD. The NAD+ metabolic regulation strategy was proved to improve the energy metabolism level and enhance the movement and cognitive functions of HD patients (Naia et al., 2021; Reiten et al., 2021). Furthermore, SIRT1 could active BDNF in HD striatal-like neurons, which could improve neurodegeneration and neuronal dysfunction (Duan, 2013). Thus, the synergistic roles of SIRT3 and SIRT1 in HD pathogenesis may be valuable to discover.
4.4 Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease affecting spinal α-motor neurons, characterized by muscle atrophy, loss of movement function, and respiratory failure. Pathological mechanisms of ALS include mitochondrial dysfunction, ROS accumulation, and protein misfolding (Suk and Rousseaux, 2020). The decrease in SIRT3 expression affected mitochondrial electron transport chain function in anterior horn motor neurons of ALS patients. This leads to insufficient energy supply to neurons, exacerbating oxidative stress damage (Hor et al., 2021). Low SIRT3 expression reduced neuronal tolerance to metabolic stress and accelerated neuronal death and muscle atrophy though AMPK/PGC-1α axis (Kuczynska et al., 2021). SIRT3 KO ALS mice showed decreased muscle strength, decreased survival rate of motor neurons, significant axonal atrophy, and myelin sheath degeneration, while the overexpression of SIRT3 attenuated neuronal damage and delayed muscle atrophy progression (Buck et al., 2017). Additionally, NAM could improve movement function and antioxidant capacity in ALS animal models (Hor et al., 2021). It was also shown that NAD+ precursors enhance mitochondrial bioenergetics in ALS patients and improve muscle control and movement function (Obrador et al., 2021). Although previous studies suggested that the activation of SIRT3 could reverse metabolic defects in motor neurons and alleviate symptoms, no clinical trials targeting SIRT3 were conducted in human patients to date. Future studies should advance clinical trials to explore SIRT3-targeted therapies in ALS patients.
4.5 Multiple sclerosis
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system. The neuropathology comprises inflammatory demyelination, axonal transection, and progressive neurodegeneration. Clinically, it manifests as movement disorders, sensory disturbances, visual impairment, and progressive cognitive decline (Woo et al., 2024). It was found that SIRT3 plays a critical role in maintaining energy metabolism and antioxidant defense in oligodendrocytes. Low SIRT3 expression decreased the activity of electron transport chain, promoted ROS accumulation, exacerbated neuroinflammation, and impaired remyelination capacity (Singh et al., 2018; Khodaei et al., 2019). For MS mice treated with ellagic acid, both myelin regeneration ability and movement function were improved, suggesting that SIRT3 may slow down MS progression by enhancing mitochondrial function and antioxidant capacity (Khodaei et al., 2019). Furthermore, SIRT3 regulated myelin homeostasis via the Nrf2-mediated antioxidant pathway. For MS patients, decreased expression of SIRT3 could impair myelin regeneration ability, weaken axonal protection mechanisms, and lead to disease progression (Theodosis-Nobelos and Rekka, 2022). As a potential therapeutic target for MS, intervention in its regulation strategy is expected to provide new treatment ideas for MS patients. This is beneficial for improving remyelination, slowing down disease progression, and improving patients’ quality of life.
4.6 Epilepsy
Epilepsy, a chronic brain disorder, arises from excessive synchronous neuronal activity in the central nervous system, accompanied by mitochondrial impairment, disrupted neurotransmitter homeostasis, and inflammatory cascades as core pathophysiological hallmarks (Milligan, 2021). A prospective observational study reported that SIRT3 levels were significantly decreased in epilepsy patients, and even lower in drug-resistant epilepsy cases (Hu et al., 2024). Research showed that blocking MciroRNA-134–5p activity preserved neuronal integrity against kainic acid neurotoxicity via SIRT3-dependent mechanisms that maintain mitochondrial homeostasis (Lin et al., 2021). Additionally, SIRT3 regulated astrocyte activation via the Notch1/NF-κB pathway, which helps alleviate the inflammatory response after epilepsy (Zhu et al., 2024). And the regulation of the NLRP3/BDNF/SIRT3 axis could reduce inflammation and oxidative stress during seizures, improving cognitive impairment caused by seizure (Fawzy et al., 2025). On the other hand, citric acid treatment in epileptic rats increased SIRT3 expression, which promoted mitochondrial autophagy and reduced hippocampal oxidative stress and apoptosis (Wu et al., 2020). It is worth noting that SIRT3 could enhance autophagy by regulating the AMPK/mTOR pathway to exert a protective effect against epilepsy induced brain damage (Chen et al. 2024a). The probability of epilepsy in patients with diabetes is significantly higher than that in normal people. Insulin could activate the SIRT1/PGC-1α/SIRT3 pathway, prolonging seizure latency, reducing seizure severity, reversing mitochondrial dysfunction, and lowering oxidative stress levels (Cheng et al., 2021). The discovery of anticonvulsant effects mediated by insulin revealed innovative strategies for health management in diabetic populations (Chou et al., 2016).
5 Conclusion
CNS diseases often lead to irreversible cognitive, motor, and functional impairments. Currently, these diseases generally lack effective curative therapies, making the exploration of novel neuroprotective strategies a critical direction in current research. We reviewed the core roles of SIRT3 in CNS neurons and diseases, including its critical contributions to energy metabolism, anti-oxidative stress, mitophagy, and neuroinflammation regulation. Existing studies have confirmed that the deacetylation activity of SIRT3 plays a key role in maintaining cellular homeostasis. The functions of SIRT3 may exhibit certain preferences across different types of neurons, and reduced SIRT3 activity has been observed in various CNS diseases. Therefore, it is necessary to develop SIRT3-targeted therapeutics for CNS diseases by targeting relevant pathways. However, we did not identify any clinically applicable SIRT3-targeted treatment, which may be related to the challenges in the development of BBB-penetrating drug carriers. Secondly, SIRT3 exhibits higher catalytic efficiency. Further exploring of the catalytic properties of SIRT3 is also significant. Thirdly, most current studies still focus on in vitro models and animal studies, where SIRT3 is activated via genetic overexpression or pharmacological activation. The clinical applicability of these approaches remains to be further validated. Notably, studies on intermittent fasting and electroacupuncture activating SIRT3 inspire us that non-pharmacological therapies may also serve as highly promising complementary alternative treatments for CNS diseases. Furthermore, since SIRT3 is expressed in major organs such as the brain, heart, and kidneys, research on SIRT3 may provide insights into the mechanisms and treatment of comorbidities such as stroke with diabetes and stroke combined with coronary heart disease. In the future, interventions targeting SIRT3, including NAD+ supplements, small-molecule activators, and gene modulation strategies, require systematic preclinical investigations, and their therapeutic potential in CNS diseases must be evaluated through rigorous clinical trials. In the future, interventions for SIRT3, including NAD+ supplements, small molecule activators, and gene regulation strategies, followed by restrictedly and scientifically designed clinical trials to evaluate their therapeutic potential in CNS.
Statements
Author contributions
CH: Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft, Writing – review and editing. YpW: Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft, Writing – review and editing. YcW: Investigation, Writing – review and editing. GL: Writing – review and editing. XD: Conceptualization, Methodology, Supervision, Validation, Writing – review and editing. XH: Conceptualization, Methodology, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Mechanism of Oviductus Ranae in remodeling microbiota metabolites to promote HPS90/GR-GC nuclear translocation in the treatment of depression, which belonged to Science and Technology Development Project of Jilin province (YDZJ202501ZYTS770).
Acknowledgments
The authors acknowledge contributions from all the included studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- CNS
central nervous system
- SIRT3
Sirtuin-3
- SOD2
superoxide dismutase 2
- ROS
reactive oxygen species
- PGC-1α
proliferator-activated receptor γ coactivator-1α
- NAD+
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- AD
Alzheimer’s disease
- MTS
mitochondrial targeting sequence
- PTM
post-translational modificationsFoxO3a forkhead box O3
- Nrf2
nuclear factor erythroid 2-related factor 2
- AMPK
Adenosine 5′-monophosphate (AMP)-activated protein kinase
- FoxO1
forkhead box O1
- KO
knockout
- GABA
γ-aminobutyric acid
- NMDA
N-methyl-D-aspartate
- PD
Parkinson’s disease
- MnSOD
superoxide dismutase
- WT
wild-type
- NLRP3
nucleotide-binding oligomerization domain-like receptor protein 3
- ATP
adenosine triphosphate
- NF-κB
nuclear factor-κB
- HIF-1α
hypoxia inducible factor-1α
- VEGF
vascular endothelial growth factor
- STAT3
signal transducer and activator of transcription 3
- Tau
protein Tau
- EGR1
early growth response 1
- PINK1
PTEN induced putative kinase 1
- Parkin
Parkin protein
- VD
vascular dementia
- TFAM
transcription factor A
- BDNF
brain-derived neurotrophic factor
- CSVD
cerebral small vessel disease
- BBB
blood-brain barrier
- Aβ
β-amyloid protein
- IDE
insulin-degrading enzyme
- α-Syn
α-synuclein
- mPTP
mitochondrial permeability transition pore
- MPTP
1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine
- GSDMD
Gasdermin D
- MSA
multiple system atrophy
- HD
Huntington’s disease
- MS
Multiple sclerosis
- mTOR
mammalian target of rapamycin
References
1
Abdelaziz A. M. Rasheed N. O. A. Zaki H. F. Salem H. A. El-Sayed R. M. (2025). Canagliflozin attenuates neurodegeneration and ameliorates dyskinesia through targeting the NLRP3/Nurr1/GSK-3β/SIRT3 pathway and autophagy modulation in rotenone-lesioned rats. Int. Immunopharmacol.146, 113839. 10.1016/j.intimp.2024.113839
2
Albano E. (2006). Alcohol, oxidative stress and free radical damage. Proc. Nutr. Soc.65 (3), 278–290. 10.1079/pns2006496
3
Allen A. R. Jones A. V. Lobianco F. V. Krager K. J. Aykin-Burns N. (2023). Effect of Sirt3 on hippocampal MnSOD activity, mitochondrial function, physiology, and cognition in an aged murine model. Behav. Brain Res.444, 114335. 10.1016/j.bbr.2023.114335
4
Anderson K. A. Madsen A. S. Olsen C. A. Hirschey M. D. (2017). Metabolic control by sirtuins and other enzymes that sense NAD(+), NADH, or their ratio. Biochimica Biophysica Acta. Bioenergetics1858 (12), 991–998. 10.1016/j.bbabio.2017.09.005
5
Ansari A. Rahman M. S. Saha S. K. Saikot F. K. Deep A. Kim K. (2017). Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease. Aging Cell16 (1), 4–16. 10.1111/acel.12538
6
Arenas E. Denham M. Villaescusa J. C. (2015). How to make a midbrain dopaminergic neuron. Dev. Camb. Engl.142 (11), 1918–1936. 10.1242/dev.097394
7
Bao J. Lu Z. Joseph J. J. Carabenciov D. Dimond C. C. Pang L. et al (2010). Characterization of the murine SIRT3 mitochondrial localization sequence and comparison of mitochondrial enrichment and deacetylase activity of long and short SIRT3 isoforms. J. Cell. Biochem.110 (1), 238–247. 10.1002/jcb.22531
8
Bates G. P. Dorsey R. Gusella J. F. Hayden M. R. Kay C. Leavitt B. R. et al (2015). Huntington disease. Nat. Rev. Dis. Prim.1, 15005. 10.1038/nrdp.2015.5
9
Buck E. Bayer H. Lindenberg K. S. Hanselmann J. Pasquarelli N. Ludolph A. C. et al (2017). Comparison of sirtuin 3 levels in ALS and huntington's disease-differential effects in human tissue samples vs. transgenic mouse models. Front. Mol. Neurosci.10, 156. 10.3389/fnmol.2017.00156
10
Cao R. Li S. Yin J. Guo L. Shi J. (2019). Sirtuin 3 promotes microglia migration by upregulating CX3CR1. Cell Adhesion and Migr.13 (1), 229–235. 10.1080/19336918.2019.1629224
11
Chen C. Mcdonald D. Blain A. Mossman E. Atkin K. Marusich M. F. et al (2023). Parkinson's disease neurons exhibit alterations in mitochondrial quality control proteins. NPJ Parkinson's Dis.9 (1), 120. 10.1038/s41531-023-00564-3
12
Chen F. Liu J. Zhou D. (2024a). SIRT3 enhances the protective effect of xyloketal B on seizure-induced brain injury by regulating AMPK/mTOR signaling-mediated autophagy. Kaohsiung J. Med. Sci.40 (1), 74–85. 10.1002/kjm2.12765
13
Chen H. Liu J. Chen M. Wei Z. Yuan J. Wu W. et al (2024b). SIRT3 facilitates mitochondrial structural repair and functional recovery in rats after ischemic stroke by promoting OPA1 expression and activity. Clin. Nutr. Edinb. Scotl.43 (7), 1816–1831. 10.1016/j.clnu.2024.06.001
14
Chen H. Zhang Q. Zhang X. Zeng X. Xu J. Ling S. (2024d). 4'-O-methylbavachalcone alleviates ischemic stroke injury by inhibiting parthanatos and promoting SIRT3. Eur. J. Pharmacol.972, 176557. 10.1016/j.ejphar.2024.176557
15
Chen M. Liu J. Wu W. Guo T. Yuan J. Wu Z. et al (2024c). SIRT1 restores mitochondrial structure and function in rats by activating SIRT3 after cerebral ischemia/reperfusion injury. Cell Biol. Toxicol.40 (1), 31. 10.1007/s10565-024-09869-2
16
Chen Y. Yang H. Wang D. Chen T. Qi X. Tao L. et al (2024e). Gastrodin alleviates mitochondrial dysfunction by regulating SIRT3-mediated TFAM acetylation in vascular dementia. Phytomedicine128, 155369. 10.1016/j.phymed.2024.155369
17
Chen Y. Zhu Z. Yan Y. Sun H. Wang G. Du X. et al (2024f). P7C3 suppresses astrocytic senescence to protect dopaminergic neurons: implication in the mouse model of parkinson's disease. CNS Neurosci. and Ther.30 (7), e14819. 10.1111/cns.14819
18
Cheng A. Yang Y. Zhou Y. Maharana C. Lu D. Peng W. et al (2016). Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab.23 (1), 128–142. 10.1016/j.cmet.2015.10.013
19
Cheng A. Wang J. Ghena N. Zhao Q. Perone I. King T. M. et al (2020). SIRT3 haploinsufficiency aggravates loss of GABAergic interneurons and neuronal network hyperexcitability in an alzheimer's disease model. J. Neurosci.40 (3), 694–709. 10.1523/JNEUROSCI.1446-19.2019
20
Cheng Y. Zeng X. Mai Q. Bai X. Jiang Y. Li J. et al (2021). Insulin injections inhibits PTZ-Induced mitochondrial dysfunction, oxidative stress and neurological deficits via the SIRT1/PGC-1α/SIRT3 pathway. Biochimica Biophysica Acta. Mol. Basis Dis.1867 (6), 166124. 10.1016/j.bbadis.2021.166124
21
Chou I. Wang C. Lin W. Tsai F. Lin C. Kao C. (2016). Risk of epilepsy in type 1 diabetes mellitus: a population-based cohort study. Diabetologia59 (6), 1196–1203. 10.1007/s00125-016-3929-0
22
Colonna M. Butovsky O. (2017). Microglia function in the central nervous system during health and neurodegeneration. Annu. Rev. Immunol.35, 441–468. 10.1146/annurev-immunol-051116-052358
23
Dai N. Su X. Li A. Li J. Jiang D. Wang Y. (2024). DVL/GSK3/ISL1 pathway signaling: unraveling the mechanism of SIRT3 in neurogenesis and AD therapy. Stem Cell Res. and Ther.15 (1), 299. 10.1186/s13287-024-03925-8
24
Dikalova A. E. Itani H. A. Nazarewicz R. R. Mcmaster W. G. Flynn C. R. Uzhachenko R. et al (2017). Sirt3 impairment and SOD2 hyperacetylation in vascular oxidative stress and hypertension. Circulation Res.121 (5), 564–574. 10.1161/CIRCRESAHA.117.310933
25
Dikalova A. Fehrenbach D. Mayorov V. Panov A. Ao M. Lantier L. et al (2024). Mitochondrial CypD acetylation promotes endothelial dysfunction and hypertension. Circulation Res.134 (11), 1451–1464. 10.1161/CIRCRESAHA.123.323596
26
Donadini A. Rosano C. Felli L. Ponassi M. (2013). Human sirtuins: an overview of an emerging drug target in age-related diseases and cancer. Curr. Drug Targets14 (6), 653–661. 10.2174/1389450111314060006
27
Duan W. (2013). Targeting sirtuin-1 in huntington's disease: rationale and current status. CNS Drugs27 (5), 345–352. 10.1007/s40263-013-0055-0
28
Duan W. Liang L. Pan M. Lu D. Wang T. Li S. et al (2020). Theacrine, a purine alkaloid from kucha, protects against parkinson's disease through SIRT3 activation. Phytomedicine Int. J. Phytotherapy Phytopharm.77, 153281. 10.1016/j.phymed.2020.153281
29
Duering M. Biessels G. J. Brodtmann A. Chen C. Cordonnier C. de Leeuw F. et al (2023). Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet. Neurology22 (7), 602–618. 10.1016/S1474-4422(23)00131-X
30
Emery B. Wood T. L. (2024). Regulators of oligodendrocyte differentiation. Cold Spring Harb. Perspect. Biol.16 (6). 10.1101/cshperspect.a041358
31
Fawzy M. N. Abd El-Haleim E. A. Zaki H. F. Salem H. A. El-Sayed R. M. (2025). Mitigating seizure-induced cognitive deficits in mice induced with pentylenetetrazol by roflumilast through targeting the NLRP3 inflammasome/BDNF/SIRT3 pathway and regulating ferroptosis. Life Sci.366-367, 123488. 10.1016/j.lfs.2025.123488
32
Feldman J. L. Dittenhafer-Reed K. E. Kudo N. Thelen J. N. Ito A. Yoshida M. et al (2015). Kinetic and structural basis for acyl-group selectivity and NAD(+) dependence in sirtuin-catalyzed deacylation. Biochemistry54 (19), 3037–3050. 10.1021/acs.biochem.5b00150
33
Feng L. Gao J. Liu Y. Shi J. Gong Q. (2018). Icariside II alleviates oxygen-glucose deprivation and reoxygenation-induced PC12 cell oxidative injury by activating Nrf2/SIRT3 signaling pathway. Biomed. and Pharmacother. = Biomedecine and Pharmacother.103, 9–17. 10.1016/j.biopha.2018.04.005
34
Fu J. Jin J. Cichewicz R. H. Hageman S. A. Ellis T. K. Xiang L. et al (2012). trans-(-)-ε-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of huntington disease. J. Biol. Chem.287 (29), 24460–24472. 10.1074/jbc.M112.382226
35
Gao J. Chen N. Li N. Xu F. Wang W. Lei Y. et al (2020). Neuroprotective effects of trilobatin, a novel naturally occurring Sirt3 agonist from Lithocarpus polystachyus rehd., mitigate cerebral ischemia/reperfusion injury: Involvement of TLR4/NF-κB and Nrf2/Keap-1 signaling. Antioxidants and Redox Signal.33 (2), 117–143. 10.1089/ars.2019.7825
36
Gao L. Sun W. Zhang L. Liang C. Zhang D. (2024). Caffeine upregulates SIRT3 expression to ameliorate astrocytes-mediated HIV-1 tat neurotoxicity via suppression of EGR1 signaling pathway. J. Neurovirology30 (3), 286–302. 10.1007/s13365-024-01222-x
37
Gao J. Zhang X. Shu G. Chen N. Zhang J. Xu F. et al (2022). Trilobatin rescues cognitive impairment of alzheimer's disease by targeting HMGB1 through mediating SIRT3/SOD2 signaling pathway. Acta Pharmacol. Sin.43 (10), 2482–2494. 10.1038/s41401-022-00888-5
38
Gao L. Guo X. Liu S. Sun Q. Qin X. Lv P. et al (2022). DL-3-n-butylphthalide imparts neuroprotection via Nrf2/SIRT3 pathway in a mouse model of vascular dementia. Brain Res.1779, 147785. 10.1016/j.brainres.2022.147785
39
Gao R. Chen Z. Wu Y. Chen R. Zheng W. Qi L. et al (2022). SIRT3 alleviates mitochondrial dysfunction induced by recurrent low glucose and improves the supportive function of astrocytes to neurons. Free Radic. Biol. and Med.193 (Pt 1), 405–420. 10.1016/j.freeradbiomed.2022.10.313
40
Gleave J. A. Arathoon L. R. Trinh D. Lizal K. E. Giguère N. Barber J. H. M. et al (2017). Sirtuin 3 rescues neurons through the stabilisation of mitochondrial biogenetics in the virally-expressing mutant α-synuclein rat model of parkinsonism. Neurobiol. Dis.106, 133–146. 10.1016/j.nbd.2017.06.009
41
Gu C. Kong F. Zeng J. Geng X. Sun Y. Chen X. (2023). Remote ischemic preconditioning protects against spinal cord ischemia-reperfusion injury in mice by activating NMDAR/AMPK/PGC-1α/SIRT3 signaling. Cell and Biosci.13 (1), 57. 10.1186/s13578-023-00999-4
42
Guo X. Tian Y. Yang Y. Li S. Guo L. Shi J. (2021). Pituitary adenylate cyclase-activating polypeptide protects against cognitive impairment caused by chronic cerebral hypoperfusion. Mol. Neurobiol.58 (9), 4309–4322. 10.1007/s12035-021-02381-2
43
Hei C. Zhou Y. Zhang C. Gao F. Cao M. Yuan S. et al (2023). Rapamycin ameliorates brain damage and maintains mitochondrial dynamic balance in diabetic rats subjected to middle cerebral artery occlusion. Metab. Brain Dis.38 (2), 409–418. 10.1007/s11011-022-01020-6
44
Hirschey M. D. Shimazu T. Goetzman E. Jing E. Schwer B. Lombard D. B. et al (2010). SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature464 (7285), 121–125. 10.1038/nature08778
45
Hor J. Santosa M. M. Lim V. J. W. Ho B. X. Taylor A. Khong Z. J. et al (2021). ALS motor neurons exhibit hallmark metabolic defects that are rescued by SIRT3 activation. Cell Death Differ.28 (4), 1379–1397. 10.1038/s41418-020-00664-0
46
Hu B. Wang P. Zhang S. Liu W. Lv X. Shi D. et al (2022). HSP70 attenuates compression-induced apoptosis of nucleus pulposus cells by suppressing mitochondrial fission via upregulating the expression of SIRT3. Exp. and Mol. Med.54 (3), 309–323. 10.1038/s12276-022-00745-9
47
Hu Y. Zhang M. Liu B. Tang Y. Wang Z. Wang T. et al (2023). Honokiol prevents chronic cerebral hypoperfusion induced astrocyte A1 polarization to alleviate neurotoxicity by targeting SIRT3-STAT3 axis. Free Radic. Biol. and Med.202, 62–75. 10.1016/j.freeradbiomed.2023.03.018
48
Hu Y. Zhou T. Li Q. (2024). Serum SIRT3 levels in epilepsy patients and its association with clinical outcomes and severity: a prospective observational study. Open Med. Wars. Pol.19 (1), 20241011. 10.1515/med-2024-1011
49
Jacobs K. M. Pennington J. D. Bisht K. S. Aykin-Burns N. Kim H. Mishra M. et al (2008). SIRT3 interacts with the daf-16 homolog FOXO3a in the mitochondria, as well as increases FOXO3a dependent gene expression. Int. J. Biol. Sci.4 (5), 291–299. 10.7150/ijbs.4.291
50
Jęśko H. Wencel P. Strosznajder R. P. Strosznajder J. B. (2017). Sirtuins and their roles in brain aging and neurodegenerative disorders. Neurochem. Res.42 (3), 876–890. 10.1007/s11064-016-2110-y
51
Jiang D. Zang Q. Jiang L. Lu C. Zhao S. Xu L. (2022). SIRT3 expression alleviates microglia activation-induced dopaminergic neuron injury through the mitochondrial pathway. Exp. Ther. Med.24 (5), 662. 10.3892/etm.2022.11598
52
Jin L. Galonek H. Israelian K. Choy W. Morrison M. Xia Y. et al (2009b). Biochemical characterization, localization, and tissue distribution of the longer form of mouse SIRT3. Protein Sci. a Publ. Protein Soc.18 (3), 514–525. 10.1002/pro.50
53
Jin L. Wei W. Jiang Y. Peng H. Cai J. Mao C. et al (2009a). Crystal structures of human SIRT3 displaying substrate-induced conformational changes. J. Biol. Chem.284 (36), 24394–24405. 10.1074/jbc.M109.014928
54
Khakh B. S. Deneen B. (2019). The emerging nature of astrocyte diversity. Annu. Rev. Neurosci.42, 187–207. 10.1146/annurev-neuro-070918-050443
55
Khodaei F. Rashedinia M. Heidari R. Rezaei M. Khoshnoud M. J. (2019). Ellagic acid improves muscle dysfunction in cuprizone-induced demyelinated mice via mitochondrial Sirt3 regulation. Life Sci.237, 116954. 10.1016/j.lfs.2019.116954
56
Kim H. Kim S. Choi J. E. Han D. Koh S. M. Kim H. et al (2019). Decreased neuron number and synaptic plasticity in SIRT3-Knockout mice with poor remote memory. Neurochem. Res.44 (3), 676–682. 10.1007/s11064-017-2417-3
57
Kristian T. Karimi A. J. Fearnow A. Waddell J. Mckenna M. C. (2021). Perturbed brain glucose metabolism caused by absent SIRT3 activity. Cells10 (9). 10.3390/cells10092348
58
Kuang H. Zhou Z. Zhu Y. Wan Z. Yang M. Hong F. et al (2021). Pharmacological treatment of vascular dementia: a molecular mechanism perspective. Aging Dis.12 (1), 308–326. 10.14336/AD.2020.0427
59
Kuczynska Z. Metin E. Liput M. Buzanska L. (2021). Covering the role of PGC-1α in the nervous system. Cells11 (1). 10.3390/cells11010111
60
Laferrière F. Claverol S. Bezard E. De Giorgi F. Ichas F. (2022). Similar neuronal imprint and no cross-seeded fibrils in α-synuclein aggregates from MSA and parkinson's disease. NPJ Parkinson's Dis.8 (1), 10. 10.1038/s41531-021-00264-w
61
Lambona C. Zwergel C. Valente S. Mai A. (2024). SIRT3 activation a promise in drug development? New insights into SIRT3 biology and its implications on the drug discovery process. J. Med. Chem.67 (3), 1662–1689. 10.1021/acs.jmedchem.3c01979
62
Lautrup S. Sinclair D. A. Mattson M. P. Fang E. F. (2019). NAD(+) in brain aging and neurodegenerative disorders. Cell Metab.30 (4), 630–655. 10.1016/j.cmet.2019.09.001
63
Lee J. Kim Y. Liu T. Hwang Y. J. Hyeon S. J. Im H. et al (2018). SIRT3 deregulation is linked to mitochondrial dysfunction in alzheimer's disease. Aging Cell17 (1). 10.1111/acel.12679
64
Lee S. Jeon Y. Jo M. Kim H. (2021). Overexpression of SIRT3 suppresses oxidative stress-induced neurotoxicity and mitochondrial dysfunction in dopaminergic neuronal cells. Exp. Neurobiol.30 (5), 341–355. 10.5607/en21021
65
Lescai F. Blanché H. Nebel A. Beekman M. Sahbatou M. Flachsbart F. et al (2009). Human longevity and 11p15.5: a study in 1321 centenarians. Eur. J. Hum. Genet. EJHG.17 (11), 1515–1519. 10.1038/ejhg.2009.54
66
Li Y. Lu J. Cao X. Zhao H. Gao L. Xia P. et al (2020). A newly synthesized rhamnoside derivative alleviates alzheimer's Amyloid-β-Induced oxidative stress, mitochondrial dysfunction, and cell senescence through upregulating SIRT3. Oxidative Med. Cell. Longev.2020, 7698560. 10.1155/2020/7698560
67
Li Y. Hu K. Liang M. Yan Q. Huang M. Jin L. et al (2021). Stilbene glycoside upregulates SIRT3/AMPK to promotes neuronal mitochondrial autophagy and inhibit apoptosis in ischemic stroke. Adv. Clin. Exp. Med. Official Organ Wroclaw Med. Univ.30 (2), 139–146. 10.17219/acem/130608
68
Li H. Jia J. Wang W. Hou T. Tian Y. Wu Q. et al (2018). Honokiol alleviates cognitive deficits of alzheimer's Disease (PS1V97L) transgenic mice by activating mitochondrial SIRT3. J. Alzheimer's Dis. JAD.64 (1), 291–302. 10.3233/JAD-180126
69
Li X. Liu S. Liu X. Zhao H. Yang M. Xu D. et al (2018). Expression of SIRT3 in various glial cell types in the periventricular white matter in the neonatal rat brain after hypoxia. Tissue and Cell52, 1–8. 10.1016/j.tice.2018.03.004
70
Li Y. Ma Y. Song L. Yu L. Zhang L. Zhang Y. et al (2018). SIRT3 deficiency exacerbates p53/Parkin-mediated mitophagy inhibition and promotes mitochondrial dysfunction: implication for aged hearts. Int. J. Mol. Med.41 (6), 3517–3526. 10.3892/ijmm.2018.3555
71
Lin W. Qian X. Yang L. Zhu J. Wang D. Hang C. et al (2021). Inhibition of miR-134-5p protects against kainic acid-induced excitotoxicity through Sirt3-mediated preservation of mitochondrial function. Epilepsy Res.176, 106722. 10.1016/j.eplepsyres.2021.106722
72
Liu Y. Cheng A. Li Y. Yang Y. Kishimoto Y. Zhang S. et al (2019). SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat. Commun.10 (1), 1886. 10.1038/s41467-019-09897-1
73
Liu S. Su Y. Sun B. Hao R. Pan S. Gao X. et al (2020). Luteolin protects against CIRI, potentially via regulation of the SIRT3/AMPK/mTOR signaling pathway. Neurochem. Res.45 (10), 2499–2515. 10.1007/s11064-020-03108-w
74
Liu L. Cao Q. Gao W. Li B. Xia Z. Zhao B. (2021). Melatonin protects against focal cerebral ischemia-reperfusion injury in diabetic mice by ameliorating mitochondrial impairments: involvement of the Akt-SIRT3-SOD2 signaling pathway. Aging13 (12), 16105–16123. 10.18632/aging.203137
75
Liu M. Zhang X. Wang Y. (2021). Curcumin alleviates Aβ(42)-Induced neuronal metabolic dysfunction via the Thrb/SIRT3 axis and improves cognition in APP(TG) mice. Neurochem. Res.46 (12), 3166–3178. 10.1007/s11064-021-03414-x
76
Loboda A. Stachurska A. Florczyk U. Rudnicka D. Jazwa A. Wegrzyn J. et al (2009). HIF-1 induction attenuates Nrf2-dependent IL-8 expression in human endothelial cells. Antioxidants and Redox Signal.11 (7), 1501–1517. 10.1089/ars.2008.2211
77
Mattson M. P. Moehl K. Ghena N. Schmaedick M. Cheng A. (2018). Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci.19 (2), 63–80. 10.1038/nrn.2017.156
78
Meng H. Yan W. Lei Y. Wan Z. Hou Y. Sun L. et al (2019). SIRT3 regulation of mitochondrial quality control in neurodegenerative diseases. Front. Aging Neurosci.11, 313. 10.3389/fnagi.2019.00313
79
Milligan T. A. (2021). Epilepsy: a clinical overview. Am. J. Med.134 (7), 840–847. 10.1016/j.amjmed.2021.01.038
80
Mo J. Liao W. Du J. Huang X. Li Y. Su A. et al (2024). Buyang huanwu decoction improves synaptic plasticity of ischemic stroke by regulating the cAMP/PKA/CREB pathway. J. Ethnopharmacol.335, 118636. 10.1016/j.jep.2024.118636
81
Munteanu C. Onose G. Poştaru M. Turnea M. Rotariu M. Galaction A. I. (2024). Hydrogen sulfide and gut microbiota: their synergistic role in modulating sirtuin activity and potential therapeutic implications for neurodegenerative diseases. Pharm. Basel. Switz.17 (11). 10.3390/ph17111480
82
Naia L. Carmo C. Campesan S. Fão L. Cotton V. E. Valero J. et al (2021). Mitochondrial SIRT3 confers neuroprotection in huntington's disease by regulation of oxidative challenges and mitochondrial dynamics. Free Radic. Biol. and Med.163, 163–179. 10.1016/j.freeradbiomed.2020.11.031
83
Obrador E. Salvador-Palmer R. López-Blanch R. Dellinger R. W. Estrela J. M. (2021). NAD(+) precursors and antioxidants for the treatment of amyotrophic lateral sclerosis. Biomedicines9 (8). 10.3390/biomedicines9081000
84
Palop J. J. Mucke L. (2016). Network abnormalities and interneuron dysfunction in alzheimer disease. Nat. Rev. Neurosci.17 (12), 777–792. 10.1038/nrn.2016.141
85
Poppe M. Reimertz C. Düssmann H. Krohn A. J. Luetjens C. M. Böckelmann D. et al (2001). Dissipation of potassium and proton gradients inhibits mitochondrial hyperpolarization and cytochrome c release during neural apoptosis. J. Neurosci.21 (13), 4551–4563. 10.1523/JNEUROSCI.21-13-04551.2001
86
Przykaza A. (2021). Understanding the connection between common stroke comorbidities, their associated inflammation, and the course of the cerebral ischemia/reperfusion Cascade. Front. Immunol.12, 782569. 10.3389/fimmu.2021.782569
87
Qin C. Yang S. Chu Y. Zhang H. Pang X. Chen L. et al (2022). Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther.7 (1), 215. 10.1038/s41392-022-01064-1
88
Radenkovic D. Reason Verdin E. (2020). Clinical evidence for targeting NAD therapeutically. Pharm. Basel. Switz.13 (9). 10.3390/ph13090247
89
Rakshe P. S. Dutta B. J. Chib S. Maurya N. Singh S. (2024). Unveiling the interplay of AMPK/SIRT1/PGC-1α axis in brain health: promising targets against aging and NDDs. Ageing Res. Rev.96, 102255. 10.1016/j.arr.2024.102255
90
Rehman A. S. Kumar P. Parvez S. (2025). Dopamine-D2-agonist targets mitochondrial dysfunction via diminishing Drp1 mediated fission and normalizing PGC1-α/SIRT3 pathways in a rodent model of subarachnoid haemorrhage. Neuroscience564, 60–78. 10.1016/j.neuroscience.2024.11.028
91
Reiten O. K. Wilvang M. A. Mitchell S. J. Hu Z. Fang E. F. (2021). Preclinical and clinical evidence of NAD(+) precursors in health, disease, and ageing. Mech. Ageing Dev.199, 111567. 10.1016/j.mad.2021.111567
92
Ren Y. Ye D. Ding Y. Wei N. (2023). Ginsenoside Rk1 prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinson's disease via activating silence information regulator 3-mediated Nrf2/HO-1 signaling pathway. Hum. and Exp. Toxicol.42, 9603271231220610. 10.1177/09603271231220610
93
Rodgers J. T. Lerin C. Haas W. Gygi S. P. Spiegelman B. M. Puigserver P. (2005). Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature434 (7029), 113–118. 10.1038/nature03354
94
Rueda C. B. Llorente-Folch I. Traba J. Amigo I. Gonzalez-Sanchez P. Contreras L. et al (2016). Glutamate excitotoxicity and Ca2+-regulation of respiration: role of the Ca2+ activated mitochondrial transporters (CaMCs). Biochimica Biophysica Acta1857 (8), 1158–1166. 10.1016/j.bbabio.2016.04.003
95
Samant S. A. Zhang H. J. Hong Z. Pillai V. B. Sundaresan N. R. Wolfgeher D. et al (2014). SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol. Cell. Biol.34 (5), 807–819. 10.1128/MCB.01483-13
96
Shen Y. Wu Q. Shi J. Zhou S. (2020). Regulation of SIRT3 on mitochondrial functions and oxidative stress in Parkinson's disease. Biomed. and Pharmacother. = Biomedecine and Pharmacother.132, 110928. 10.1016/j.biopha.2020.110928
97
Shi H. Deng H. Gius D. Schumacker P. T. Surmeier D. J. Ma Y. (2017). Sirt3 protects dopaminergic neurons from mitochondrial oxidative stress. Hum. Mol. Genet.26 (10), 1915–1926. 10.1093/hmg/ddx100
98
Shi Z. Zhang Y. Xiao Y. Shi Z. Wei X. Wang B. et al (2024). The protective effects of gastrodin on neurological disorders: an update and future perspectives. Front. Pharmacol.15, 1494277. 10.3389/fphar.2024.1494277
99
Singh C. K. Chhabra G. Ndiaye M. A. Garcia-Peterson L. M. Mack N. J. Ahmad N. (2018). The role of sirtuins in antioxidant and redox signaling. Antioxidants and Redox Signal.28 (8), 643–661. 10.1089/ars.2017.7290
100
Someya S. Yu W. Hallows W. C. Xu J. Vann J. M. Leeuwenburgh C. et al (2010). Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell143 (5), 802–812. 10.1016/j.cell.2010.10.002
101
Sommer C. J. (2017). Ischemic stroke: experimental models and reality. Acta Neuropathol.133 (2), 245–261. 10.1007/s00401-017-1667-0
102
Spires-Jones T. L. Hyman B. T. (2014). The intersection of amyloid beta and tau at synapses in alzheimer's disease. Neuron82 (4), 756–771. 10.1016/j.neuron.2014.05.004
103
Su S. Chen G. Gao M. Zhong G. Zhang Z. Wei D. et al (2023). Kai-xin-san protects against mitochondrial dysfunction in alzheimer's disease through SIRT3/NLRP3 pathway. Chin. Med.18 (1), 26. 10.1186/s13020-023-00722-y
104
Suk T. R. Rousseaux M. W. C. (2020). The role of TDP-43 mislocalization in amyotrophic lateral sclerosis. Mol. Neurodegener.15 (1), 45. 10.1186/s13024-020-00397-1
105
Surya K. Manickam N. Jayachandran K. S. Kandasamy M. Anusuyadevi M. (2023). Resveratrol mediated regulation of hippocampal neuroregenerative plasticity via SIRT1 pathway in synergy with wnt signaling: neurotherapeutic implications to mitigate memory loss in alzheimer's disease. J. Alzheimer's Dis.94 (s1), S125–S140. 10.3233/JAD-220559
106
Tabassum S. Misrani A. Huang H. Zhang Z. Li Q. Long C. (2023). Resveratrol attenuates chronic unpredictable mild stress-induced alterations in the SIRT1/PGC1α/SIRT3 pathway and associated mitochondrial dysfunction in mice. Mol. Neurobiol.60 (9), 5102–5116. 10.1007/s12035-023-03395-8
107
Theodosis-Nobelos P. Rekka E. A. (2022). The multiple sclerosis modulatory potential of natural multi-targeting antioxidants. Molecules27 (23). 10.3390/molecules27238402
108
Trinh D. Israwi A. R. Brar H. Villafuerte J. E. A. Laylo R. Patel H. et al (2023). Parkinson's disease pathology is directly correlated to SIRT3 in human subjects and animal models: implications for AAV.SIRT3-myc as a disease-modifying therapy. Neurobiol. Dis.187, 106287. 10.1016/j.nbd.2023.106287
109
Trinh D. Al Halabi L. Brar H. Kametani M. Nash J. E. (2024). The role of SIRT3 in homeostasis and cellular health. Front. Cell. Neurosci.18, 1434459. 10.3389/fncel.2024.1434459
110
Tyagi A. Pugazhenthi S. (2023). A promising strategy to treat neurodegenerative diseases by SIRT3 activation. Int. J. Mol. Sci.24 (2). 10.3390/ijms24021615
111
Tyagi A. Musa M. Labeikovsky W. Pugazhenthi S. (2022). Sirt3 deficiency induced Down regulation of insulin degrading enzyme in comorbid alzheimer's disease with metabolic syndrome. Sci. Rep.12 (1), 19808. 10.1038/s41598-022-23652-5
112
von der Malsburg A. Sapp G. M. Zuccaro K. E. von Appen A. Moss F. R. R. Kalia R. et al (2023). Structural mechanism of mitochondrial membrane remodelling by human OPA1. Nature620 (7976), 1101–1108. 10.1038/s41586-023-06441-6
113
Wang X. He H. Xiong X. Zhou S. Wang W. Feng L. et al (2021). NAD(+) in alzheimer's disease: molecular mechanisms and systematic therapeutic evidence obtained in vivo. Front. Cell Dev. Biol.9, 668491. 10.3389/fcell.2021.668491
114
Wang D. Cao L. Zhou X. Wang G. Ma Y. Hao X. et al (2022). Mitigation of honokiol on fluoride-induced mitochondrial oxidative stress, mitochondrial dysfunction, and cognitive deficits through activating AMPK/PGC-1α/Sirt3. J. Hazard. Mater.437, 129381. 10.1016/j.jhazmat.2022.129381
115
Wang Y. Wang Y. Ma J. (2024). Effects of electroacupuncture on Sirt3/NLRP3/GSDMD signaling pathway in the substantia nigra of midbrain of rats with parkinson's disease. Zhen ci yan jiu49 (4), 384–390. 10.13702/j.1000-0607.20230024
116
Wang J. Yue H. Dong Y. Liu T. Li J. (2025). Effective compound combination of bufei yishen formula ameliorates PM2.5-induced COPD by inhibiting mitochondrial oxidative stress through SIRT3-mediated FOXO3 deacetylation. Phytomedicine140, 156568. 10.1016/j.phymed.2025.156568
117
Wang Y. Ge Y. Hua S. Shen C. Cai B. Zhao H. (2025). Aloe-emodin improves mitophagy in alzheimer's disease via activating the AMPK/PGC-1α/SIRT3 signaling pathway. CNS Neurosci. and Ther.31 (3), e70346. 10.1111/cns.70346
118
Wei J. Xie J. He J. Li D. Wei D. Li Y. et al (2023). Active fraction of polyrhachis vicina (roger) alleviated cerebral ischemia/reperfusion injury by targeting SIRT3-mediated mitophagy and angiogenesis. Phytomedicine121, 155104. 10.1016/j.phymed.2023.155104
119
Woo M. S. Engler J. B. Friese M. A. (2024). The neuropathobiology of multiple sclerosis. Nat. Rev. Neurosci.25 (7), 493–513. 10.1038/s41583-024-00823-z
120
Wu G. Liu J. Li S. Gao W. Qiu M. Yang C. et al (2020). Glycyrrhizic acid protects juvenile epileptic rats against hippocampal damage through activation of Sirtuin3. Brain Res. Bull.164, 98–106. 10.1016/j.brainresbull.2020.08.008
121
Xiao G. Tang R. Yang N. Chen Y. (2023). Review on pharmacological effects of gastrodin. Archives Pharmacal Res.46 (9-10), 744–770. 10.1007/s12272-023-01463-0
122
Xie Z. Cao B. Wang T. Lei Q. Kang T. Ge C. et al (2018). LanCL1 attenuates ischemia-induced oxidative stress by Sirt3-mediated preservation of mitochondrial function. Brain Res. Bull.142, 216–223. 10.1016/j.brainresbull.2018.07.017
123
Xie W. Zhu T. Zhou P. Xu H. Meng X. Ding T. et al (2020). Notoginseng leaf triterpenes ameliorates OGD/R-Induced neuronal injury via SIRT1/2/3-Foxo3a-MnSOD/PGC-1α signaling pathways mediated by the NAMPT-NAD pathway. Oxidative Med. Cell. Longev.2020, 7308386. 10.1155/2020/7308386
124
Xin C. Liu O. Sun M. Han P. Li X. Niu Q. et al (2025). Mechanisms of SIRT1/SIRT3-mediated reduction of mitochondrial regeneration and inflammatory response in diabetic cerebral ischemia-reperfusion injury. Metab. Brain Dis.40 (6), 236. 10.1007/s11011-025-01662-2
125
Xu D. Zhang L. Meng H. Zhao W. Hu Z. Wang J. (2024). Exploring the anti-ischemic stroke potential of wogonoside: insights from Nrf2/Sirt3 signaling pathway and UPLC-TripleTOF-MS/MS-based metabolomics. J. Pharm. Biomed. Analysis246, 116206. 10.1016/j.jpba.2024.116206
126
Yan B. Liu Q. Ding X. Lin Y. Jiao X. Wu Y. et al (2022). SIRT3-Mediated CypD-K166 deacetylation alleviates neuropathic pain by improving mitochondrial dysfunction and inhibiting oxidative stress. Oxidative Med. Cell. Longev.2022, 4722647. 10.1155/2022/4722647
127
Yan K. Bian J. He L. Song B. Shen L. Zhen Y. (2025). SIRT3 mitigates neuroinflammation and mitochondrial damage post-hypoxic-ischemic brain injury. Mol. Immunol.179, 18–28. 10.1016/j.molimm.2025.01.002
128
Yang Y. Chen K. Y. Tong Q. (2011). Murine Sirt3 protein isoforms have variable half-lives. Gene488 (1-2), 46–51. 10.1016/j.gene.2011.07.029
129
Yang R. Meng X. Zhao W. Xu S. Wang S. Li M. et al (2025). Phenylpropanoids of Eleutherococcus senticosus (rupr. and maxim.) maxim. Alleviate oxidative stress in alzheimer's disease in vitro and in vivo models by regulating Mst1 and affecting the Nrf2/Sirt3 pathway. Bioorg. Chem.159, 108347. 10.1016/j.bioorg.2025.108347
130
Yang X. Zhang Y. Geng K. Yang K. Shao J. Xia W. (2021). Sirt3 protects against ischemic stroke injury by regulating HIF-1α/VEGF signaling and blood-brain barrier integrity. Cell. Mol. Neurobiol.41 (6), 1203–1215. 10.1007/s10571-020-00889-0
131
Yang Y. Tian Y. Guo X. Li S. Wang W. Shi J. (2021). Ischemia injury induces mPTP opening by reducing Sirt3. Neuroscience468, 68–74. 10.1016/j.neuroscience.2021.06.003
132
Yao Y. Ren Z. Yang R. Mei Y. Dai Y. Cheng Q. et al (2022). Salidroside reduces neuropathology in alzheimer's disease models by targeting NRF2/SIRT3 pathway. Cell and Biosci.12 (1), 180. 10.1186/s13578-022-00918-z
133
Yin J. Han P. Song M. Nielsen M. Beach T. G. Serrano G. E. et al (2018b). Amyloid-β increases tau by mediating sirtuin 3 in alzheimer's disease. Mol. Neurobiol.55 (11), 8592–8601. 10.1007/s12035-018-0977-0
134
Yin J. Li S. Nielsen M. Carcione T. Liang W. S. Shi J. (2018a). Sirtuin 3 attenuates amyloid-β induced neuronal hypometabolism. Aging10 (10), 2874–2883. 10.18632/aging.101592
135
Zeng R. Wang X. Zhou Q. Fu X. Wu Q. Lu Y. et al (2019). Icariin protects rotenone-induced neurotoxicity through induction of SIRT3. Toxicol. Appl. Pharmacol.379, 114639. 10.1016/j.taap.2019.114639
136
Zhan R. Meng X. Tian D. Xu J. Cui H. Yang J. et al (2025). NAD(+) rescues aging-induced blood-brain barrier damage via the CX43-PARP1 axis. Neuron113 (9), 1460–1461. 10.1016/j.neuron.2025.04.002
137
Zhang Y. Bharathi S. S. Rardin M. J. Uppala R. Verdin E. Gibson B. W. et al (2015). SIRT3 and SIRT5 regulate the enzyme activity and cardiolipin binding of very long-chain acyl-CoA dehydrogenase. PloS One10 (3), e0122297. 10.1371/journal.pone.0122297
138
Zhang X. Ren X. Zhang Q. Li Z. Ma S. Bao J. et al (2016). PGC-1α/ERRα-Sirt3 pathway regulates DAergic neuronal death by directly deacetylating SOD2 and ATP synthase β. Antioxidants and Redox Signal.24 (6), 312–328. 10.1089/ars.2015.6403
139
Zhang X. Cao R. Niu J. Yang S. Ma H. Zhao S. et al (2019). Molecular basis for hierarchical histone de-β-hydroxybutyrylation by SIRT3. Cell Discov.5, 35. 10.1038/s41421-019-0103-0
140
Zhang T. Li Z. Qin Z. Cao Y. Shan T. Fang Y. et al (2021). Neuroprotection of chikusetsu saponin V on transient focal cerebral ischemia/reperfusion and the underlying mechanism. Phytomedicine84, 153516. 10.1016/j.phymed.2021.153516
141
Zhang H. Dai S. Yang Y. Wei J. Li X. Luo P. et al (2023). Role of sirtuin 3 in degenerative diseases of the central nervous system. Biomolecules13 (5). 10.3390/biom13050735
142
Zhang G. Wang Y. Li J. Ma J. Wang Y. (2024). Effects of electroacupuncture on mitophagy mediated by SIRT3/PINK1/Parkin pathway in parkinson's disease mice. Zhen ci yan jiu49 (3), 221–230. 10.13702/j.1000-0607.20230654
143
Zhang J. Xiang H. Liu J. Chen Y. He R. Liu B. (2020). Mitochondrial sirtuin 3: new emerging biological function and therapeutic target. Theranostics10 (18), 8315–8342. 10.7150/thno.45922
144
Zhang S. Ma Y. Feng J. (2020). Neuroprotective mechanisms of ε-viniferin in a rotenone-induced cell model of parkinson's disease: significance of SIRT3-mediated FOXO3 deacetylation. Neural Regen. Res.15 (11), 2143–2153. 10.4103/1673-5374.282264
145
Zhao B. Sun L. Jiang X. Zhang Y. Kang J. Meng H. et al (2019). Genipin protects against cerebral ischemia-reperfusion injury by regulating the UCP2-SIRT3 signaling pathway. Eur. J. Pharmacol.845, 56–64. 10.1016/j.ejphar.2018.12.028
146
Zheng X. Lin W. Jiang Y. Lu K. Wei W. Huo Q. et al (2021). Electroacupuncture ameliorates beta-amyloid pathology and cognitive impairment in alzheimer disease via a novel mechanism involving activation of TFEB (Transcription factor EB). Autophagy17 (11), 3833–3847. 10.1080/15548627.2021.1886720
147
Zheng X. Gao J. Zhao M. Han L. Zhang D. Wang K. et al (2023). Honokiol attenuates mitochondrial fission and cell apoptosis by activating Sirt3 in intracerebral hemorrhage. Chin. Med. J.136 (6), 719–731. 10.1097/CM9.0000000000002178
148
Zhou Y. Zhao Q. Zhang Y. Di L. Xue F. Xu W. et al (2024). A new andrographolide derivative ADA targeting SIRT3-FOXO3a signaling mitigates cognitive impairment by activating mitophagy and inhibiting neuroinflammation in Apoe4 mice. Phytomedicine124, 155298. 10.1016/j.phymed.2023.155298
149
Zhou W. Tan C. Xiong D. Chen C. Zhao Y. Xie Y. et al (2025). LncRNA-MEG3 mediated diabetic cerebral ischemia-reperfusion injury-induced apoptosis via modulating interaction between annexin A2 and akt in mitochondria. CNS Neurosci. and Ther.31 (2), e70242. 10.1111/cns.70242
150
Zhu J. Park S. Kim S. H. Kim C. H. Jeong K. H. Kim W. (2024). Sirtuin 3 regulates astrocyte activation by reducing Notch1 signaling after status epilepticus. Glia72 (6), 1136–1149. 10.1002/glia.24520
151
Zia A. Farkhondeh T. Pourbagher-Shahri A. M. Samarghandian S. (2021). The role of curcumin in aging and senescence: molecular mechanisms. Biomed. and Pharmacother. = Biomedecine and Pharmacother.134, 111119. 10.1016/j.biopha.2020.111119
152
Zou X. Zhu Y. Park S. Liu G. O'Brien J. Jiang H. et al (2017). SIRT3-Mediated dimerization of IDH2 directs cancer cell metabolism and tumor growth. Cancer Res.77 (15), 3990–3999. 10.1158/0008-5472.CAN-16-2393
Summary
Keywords
sirt3, silent information regulator 3, sirtuin-3, central nervous system diseases, mitochondrial function, therapeutic potential
Citation
Hong C, Wei Y, Wang Y, Lv G, Dong X and Huang X (2025) The mechanism and therapeutic potential of SIRT3 in central nervous system diseases: a review. Front. Pharmacol. 16:1652296. doi: 10.3389/fphar.2025.1652296
Received
23 June 2025
Accepted
18 August 2025
Published
03 September 2025
Volume
16 - 2025
Edited by
Giuseppe Di Giovanni, University of Magna Graecia, Italy
Reviewed by
Yijia Fangma, Zhejiang Chinese Medical University, China
Ines ElBini, Pasteur Institute of Tunis, Tunisia
Updates
Copyright
© 2025 Hong, Wei, Wang, Lv, Dong and Huang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinglu Dong, arthasdxl@163.com; Xiaowei Huang, huangxw@ccucm.edu.cn
‡These authors have contributed equally to this work
ORCID: Yupeng Wei, orcid.org/0009-0009-2541-3534; Xiaowei Huang, orcid.org/0009-0006-0268-759X; Xinglu Dong, orcid.org/0000-0003-3071-295X
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.