Abstract
Ethnopharmacological relevance:
Mylabris (“斑蝥’’), derived from the dried bodies of the Chinese blister beetles Mylabris phalerata Pallas and Mylabris cichorii Linnaeus, which has the effect of breaking blood and chasing blood stasis (“破血逐瘀”), dispersing knots and eliminating symptoms (“散结消癥”), and attacking poison and eroding sores (“攻毒蚀疮”).
Aim:
This review provides the firstly comprehensive summary of mylabris, covering its biological characteristics, chemical composition, pharmacological, toxicology, pharmacokinetics, and clinical use.
Materials and methods:
A systematic literature search was conducted in databases (“Web of Science”, “PubMed”, “Google Scholar”, “CNKI”, and “WanFang”) using the following query (“Mylabris phalerata Pallas” OR “Mylabris cichorii Linnaeus” OR “Mylabris” OR “Banmao” OR “Cantharidin”) AND (“Pharmacology” OR “Toxicity” OR “Pharmacokinetics” OR “Marketed drugs”), to identify literature published between 2000 and 2025, focus on referring to 2015–2025. Articles with methodological defects (e.g., sample size less than 5 per group, no standardized purity detection method used), incomplete data (e.g., no access to the original literature, lack of key data values), and ethical problems (no declaration of ethical approval) were excluded. Online websites were also used, including https://ydz.chp.org.cn/#/main (Chinese Pharmacopoeia), https://www.nmpa.gov.cn/datasearch/home-index.html#category=yp (National Medical Products Administration), to obtain information on mylabris- or cantharidin-marketed drugs. Chemical structures in SMILES format were retrieved from the PubChem, and two-dimensional chemical structures were generated using ChemDraw 22.0.0.
Results:
The major components of mylabris include terpenoids, metallic elements, fatty acids, and peptides. Pharmacological research have demonstrated its anticancer, antithrombotic, and antiviral effects in preclinical study, as well as insecticidal and antifungal in agriculture. Cantharidin is considered to be the main active and toxic component, which can cause gastrointestinal, cardiovascular and respiratory toxicity if used improperly. Pharmacokinetic studies reveal that orally cantharidin predominantly accumulates in the liver and kidneys, exhibiting strong irritancy and low bioavailability. Given its therapeutic efficacy, researchers have also developed various mylabris and cantharidin-based drugs in clinical setting.
Conclusion:
Mylabris has been used in traditional Chinese medicine for millennia. Now, it treats various diseases and shows development potential. Future studies should focus on four key aspects: comprehensive characterization of active components, elucidation of pharmacological mechanisms, supplementation of pharmacokinetic data, and clarification of toxicological mechanisms. This paper reviews the research progress of mylabris, bridging traditional applications and modern investigations to advance contemporary research and evaluate its therapeutic potential for human diseases.

1 Introduction
Mylabris, a traditional animal-derived medicine, derived from the blister beetles Mylabris phalerata Pallas and Mylabris cichorii Linnaeus of the family Meloidae. It has been utilized in Chinese medicine since the Han Dynasty. Its therapeutic applications are documented in ancient pharmacopoeias such as Shennong Bencao jing and traditional medical texts of ethnic minorities, including Tibetan and Mongolian medicine (Li et al., 2024). As recorded in the Chinese pharmacopoeia (2020 edition), mylabris is characterized by pungent flavor, hot nature, extremely toxic and is attributed to the liver, stomach, and kidney meridians. It possesses potent effects in breaking blood stasis, dispersing hard accumulations, and counteracting toxins, making it clinically valuable for treating amenorrhea, abdominal masses, and stubborn dermatoses (Efferth et al., 2007). Modern research has revealed that mylabris contains terpenoids, fatty acids, amino acids and various metal elements. Cantharidin (CTD), the core active ingredient, is regarded as a key quality marker and exhibits antitumor (Li S. et al., 2023), antithrombotic, and antiviral properties, along with agricultural uses as a bioinsecticide and antifungal agent (Ratcliffe et al., 2011). Capitalizing on its pharmacological potential, mylabris have been formulated into various preparations, including the antitumor Aidi injection, and the topical cream Ycanth for treating molluscum contagiosum, highlighting its broad clinical prospects (Zhu et al., 2020).
However, the significant toxicity of mylabris severely limits its pharmaceutical development. Improper use may lead to severe damage to the digestive, circulatory, and reproductive systems (Fang and Du, 2018). The paucity of data regarding its absorption, distribution, metabolism, and excretion (ADME) properties presents a fundamental challenge to developing effective detoxification strategies and next-generation derivatives with improved safety profiles (Duan et al., 2021). Moreover, with the surge in market demand, wild mylabris populations are on the edge of exhaustion, while artificial breeding technology is not mature, resulting in supply-demand imbalance, the soaring price, and the proliferation of counterfeit products (Mo et al., 2014).
To address these challenges, this review provides, for the first time, a comprehensive synthesis of mylabris, encompassing its biological characteristics, chemical composition, pharmacological, toxicological, pharmacokinetics, and marketed drugs. We hope that this in-depth review could serve as a foundation for the rational development, safe clinical application, and future research on mylabris.
2 Biological characteristics
Mylabris belongs to the Meloidae family. There are more than 3,000 species in the 120 genera of Meloidae in the world wide, with more than 202 species in 26 genera reported in China alone (Wang, 2014). These species are distributed across most provinces and regions. Their distribution varies slightly. The vertical distribution ranges from low-altitude plains or hilly areas to high-altitude plateaus, for example, M. phalerata Pallas is mainly distributed in Jiangsu, Zhejiang, Hubei, Jiangxi, Fujian, Taiwan, Guangdong (Deng et al., 2017), and M. przewalskyi can be found at altitudes of up to 4,900 m. Additionally, the distribution of mylabris in a given area is positively correlated with the density of locusts and the types and quantities of legume crops (Mo et al., 2014). The medicinal species include M. phalerata Pallas and M. cichorii Linnaeus. Due to the high cost and limited availability, adulterated or counterfeit products are frequently encountered in the market.
Given the clinical relevance and toxicological concerns of mylabris, its accurate identification is a prerequisite for safe and effective application. The key identification methods, including morphological and microscopic, are summarized in Table 1.
TABLE 1
| Diagnostic feature | M. phalerata pallas | M. cichorii linnaeus |
|---|---|---|
| Body length | 1.5–3.0 cm long, 0.5–1 cm wide | 1.0–1.5 cm long, 0.5–0.7 cm wide |
| Elytra | Dorsal with leathery elytra pair, with 3 wavy yellow-brown to red-brown stripes | Dorsal with leathery elytra, with 3 pale yellow to brown yellow transverse stripes |
| Inner wing | More folds, veins brown red to brown black | Few folds, veins pale yellow to yellowish brown |
| Tentacle | Antennal terminal segment base markedly narrower than anterior segment | Antennal terminal segment base nearly as wide as anterior segment |
| Setae | Setae are very much, fine spiny, more straight | Setae are less, shorter, and more twisted |
| Body wall fragments | Yellowish-white to tan | Pale yellow and translucent |
| Elytra fragments | Round, button-shaped with prominent concentric rings, the hair follicle pits left after bristle shedding are inconspicuous | The circular button-shaped concentric ring pattern is relatively small, and the concave left after the bristles fall off is obvious |
| Inner wing fragment | The threaded catheter-like wing veins are thick | The threaded catheter-like wing veins are relatively slender |
Diagnostic characteristics of M. phalerata Pallas and M. cichorii Linnaeus.
This table is summarized from website “http://www.zhongyoo.com/jianding/3004.html” (Zhongyoo, 2012).
3 Chemical composition
The chemical composition of mylabris is complex and includes terpenoids, metallic elements, fatty acids, polypeptides and amino acids.
3.1 Terpenoids
Currently, 11 terpenoids have been found in mylabris (Figure 1). CTD, the principal bioactive terpenoid in mylabris, serves as both a quality control marker and the most extensively researched component of this medicinal insect. CTD contents in the head, foot, outer wing, and inner wing are 0.050%, 0.042%, 0.044%, and 0.024%, respectively, amounting to a total of 0.160% (Li et al., 2007; Qi et al., 2010). Conjugated CTD is the major storage form and also an important water-soluble component in mylabris. It can cause adverse clinical reactions due to its low bioavailability, high toxicity, and strong irritant. Based on the structure-activity relationship of its parent nucleus, researchers have derived a series of compounds, including sodium cantharidinate (C10H12O4Na2), demethylcantharidin (C8H8O4), and sodium demethylcantharidinate (C8H8O5Na2), which exhibit enhanced efficacy and less toxicity (Wang G. et al., 2018).
FIGURE 1
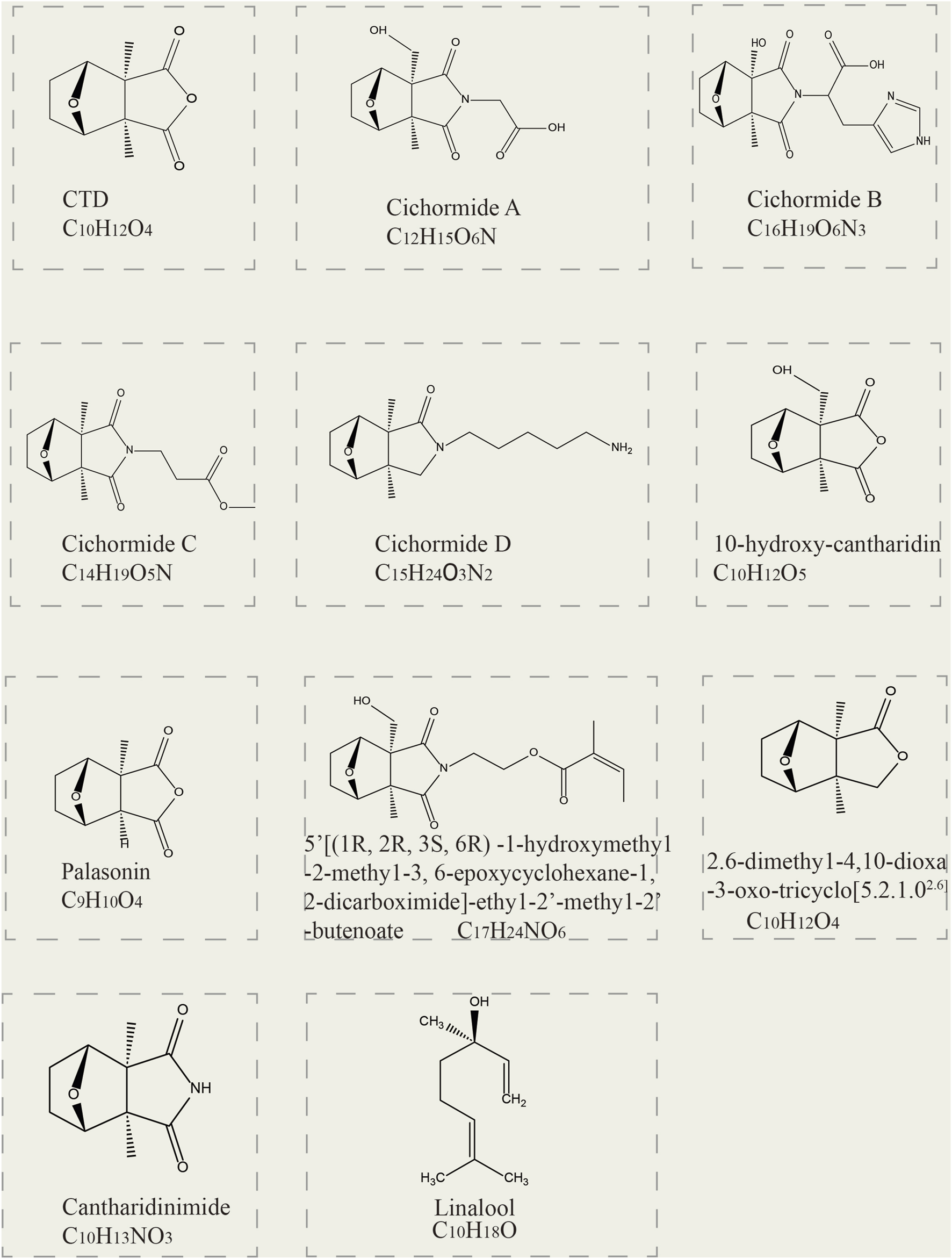
Two-dimensional structural formulas of 11 terpenoid constituents derived from mylabris.
3.2 Metallic elements
The synthesis of conjugated CTD in mylabris requires specific metal elements as raw materials. The content of the same metal elements varies across different breeds and regions. This variation can serve as an important indicator to identify regions and breeds (Lou et al., 2018). The metal elements in mylabris exhibit diverse functions. For example, magnesium (Mg), calcium (Ca), potassium (K), and natrium (Na) are the raw materials to synthesize conjugated CTD. Manganese (Mn), zinc (Zn), copper (Cu), and Mg are anticancer elements. However, mylabris also contains elements, such as chromium (Cr), hydrargyrum (Hg), lead (Pb), cadmium (Cd), beryllium (Be), tin (Sn), and nickel (Ni), which could be detrimental (Yin and Jin, 2010).
3.3 Fatty acids, polypeptides, and other compounds
Fatty acids are significant components in mylabris. Oleic acid, linoleic acid, and octadecenoic acid exhibit potential as therapeutic agents against hepatocellular carcinoma (HCC). Palmitic acid inhibits the proliferation and migration of HCC cells by modulating glucose metabolism and reducing the membrane fluidity of HCC cells (Chai, 2023). The other fatty acids identified in mylabris include stearic acid, palmitoleic acid, linolenic acid, arachidic acid, 6-octadecenoic acid, myristic acid, and palmitic acid (Díaz-Navarro et al., 2021), there are no reports regarding the pharmacological effects of the aforementioned components (Li K. M. et al., 2023).
Mylabris also contains various peptides, including ring-(L-proline-L-alanine), ring-(R-proline-R-leucine), ring-(S-proline-R-leucine), and ring-(D-proline-L-tyrosine); nucleosides such as uracil, uridine, and hypoxanthine; aromatic compounds such as indole-3-aldehyde, indole-acetic acid, 2-piperidone, 4-hydroxyphthalid (Zeng et al., 2016), 3-phenyl-4-aza-fluorene, phenylacetaldehyde (Liu et al., 2013), and p-hydroxybenzoic acid. With the advances in modern science and technology, more components of mylabris could be discovered and serve as valuable references for its quality control.
4 Traditional use and modern research
4.1 Traditional use
Mylabris has been documented in the material medica of different dynasties in China (Figure 2). Mylabris was originally recorded in the Shennong Materia Medica as an inferior-grade drug to treat tuberculous fistula, skin ulcers, and urolithiasis. It is also noted that mylabris should not be used in combination with Croton tiglium L. or Salvia miltiorrhiza Bunge. During the Wei and Jin dynasties, its use for activating blood circulation, promoting scab formation, and inducing abortion was documented in the Ming Yi Bie Lu. In the Tang dynasty, it was used for diuresis, as recorded in the Yao Xing Lun. In the Five dynasties period, processing methods such as the removal of wings, feet, and frying were documented in the Ri Hua Zi Ben Cao to prevent vomiting and diarrhea. In the Ming dynasty, mylabris was used to treat hydrophobia, furuncle, and neuralgia, and it was noted that this drug should not be used with Glycyrrhiza uralensis Fisch. To date, the efficacy and applications of mylabris have been detailed and revised in the China Pharmacopoeia, which details its appearance and shape, physicochemical characteristics, dosage and administration (Li and Chen, 2025).
FIGURE 2
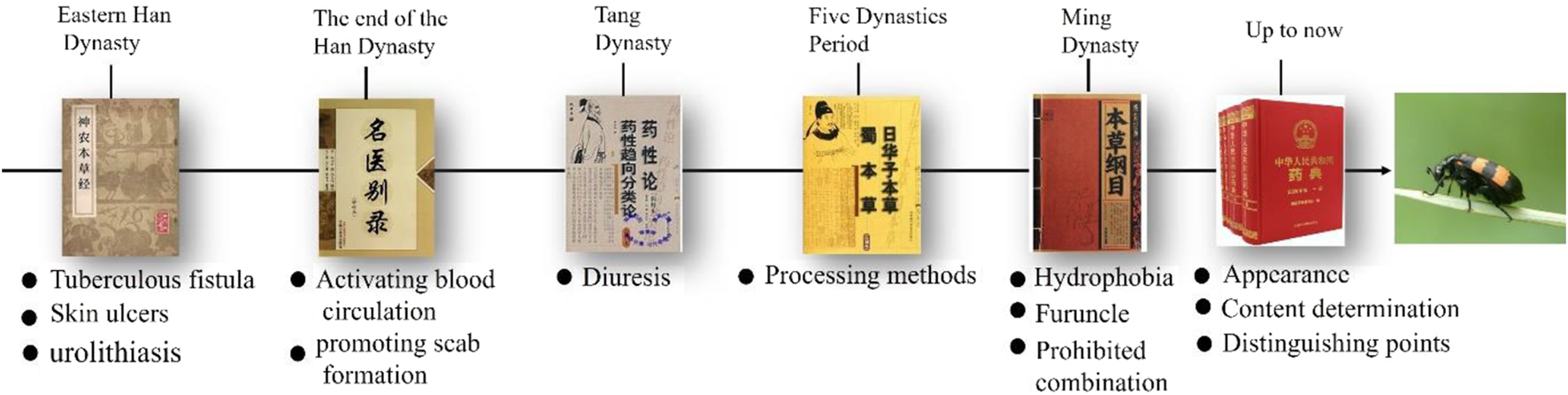
Ancient books pertaining the utilization of mylabris in China.
Ethnopharmacological applications of mylabris vary across different ethnic groups in China. Specifically, Tibetan practitioners use it to treat food stagnation, while the Li ethnic group employs it for facial hemiplegia and tonsillitis. In Uygur traditional medicine, it is prescribed for vitiligo, eczema, pruritus, rheumatism, and erectile dysfunction. Additionally, the Hmong people utilize it to manage lymphatic tuberculosis, scrofula, and rabies. The Manchu traditionally apply mylabris against malaria. Meanwhile, the Achang, Jingpo, and De’ang ethnic groups employ it therapeutically for managing bone fractures and hemorrhagic wouns (Liu et al., 2019).
Mylabris possess notable medicinal value and is widely used in traditional Chinese medicine (TCM) formulations for treating diverse diseases (Table 2). Due to its toxicity, it is often used topically in the form of a powder (mixed with honey or vinegar) to treat carbuncles, boils, rhinitis, cough, and asthma. For oral administration, it is commonly roasted (with or without the removal of the head, feet, and wings) by cleaning or stir-frying with rice to reduce its toxicity and irritation, when treating scrofula, amenorrhea, and rabies (Ren et al., 2020). Nowadays, several prescriptions containing mylabris have been used in the form of tablet, injection, and capsules. Some prescriptions, such as Kangsaidi capsules, Aidi injection, and Ycanth, have been widely used.
TABLE 2
| Preparation name | Type | Composition | Indications | References |
|---|---|---|---|---|
| Awei leiwan San | Powder | 1. Ferula sinkiangensis K.M.Shen., 2. Realgar, 3. Fluorite, 4. Cinnabar, 5. Talc, 6. Chalcanthite, 7. Cinnabar, 8. Arisaema heterophyllum Blume, 9. Paeonia lactiflora Pall, 10. Rhinoceros Horn, 11. Mylabris,12. Calculus bovis | Hansen’s disease | Qian jin yi |
| Bading Dan | Pills | 1. Olibanum, 2. Bufonis venenum, 3. Arsenic trioxide, 4. Caryophylli flos, 5. Draconis sanguis, 6. Moschus, 7. Mylabris, 8. Chalcanthite, 9. Realgar, 10. Magnetite, 11. Ricini semen | Boils, carbuncles, swelling, and pain | Jing yan fang |
| Badou Wan | Pills | 1. Crotonis fructus., 2. Mylabris | Deafness | Zhou hou fang |
| Badu San | Powder | 1. Mylabris, 2. Crotonis Fructus, 3. Olibanum, 4. Myrrha, 5. Peucedani radix, 6. Scrophularia ningpoensis Hemsl., 7. Bovis cornu, 8. Moschus, 9. Borneol | Localized swelling with hardness | Gu jin wai fang |
| Bafu Wan | Pills | 1. Aconiti Lateralis Radix Praeparata, 2. mylabris, 3. Crotonis fructus | Infantile Intestinal gas distension | Pu ji fang |
| Banji Wan | Pills | 1.Menthae haplocalycis herba, 2. Mylabris | Scrofula | Yi xue ru men |
| Banmao Ding | Water | Mylabris | Neurodermatitis | Zhong yi pi fu bing xue jian bian |
| Banmao Fen | Powder | 1. Mylabris, 2. Alumen, 3. Sulfur, 4. Plumbum 5. Oxidatum, 6. Trisulphur, 7.Synthetic borneol, 8. Arsenic trioxide, 9. Borax, 10. Moschus, 11. Glycerin | Tinea corporis | Zhong yi pi fu bing xue jian bian |
| Banmao Gao | Paste | 1. Mylabris, 2. Colophonium, 3. Crotonis Fructus | Scrofula | Sheng hui |
| Banmao San | Powder | 1. Mylabris, 2. Margarita, 3. Hydrargyrum | Multiple fistulas | Sheng ji zong lu |
| Banmao Wan | Pills | 1. Mylabris, 2. Moschus, 3. Cinnabaris, 4. Zingiberis rhizoma, 5. Glycyrrhiza uralensis Fisch. ex DC., 6. Rhinoceros horn, 7. Oryzae semen | Scrofula | Sheng hui |
| Baoming Wan | Pills | 1. Angelica sinensis (Oliv.) Diels., 2. Aconiti lateralis radix praeparata, 3. Paeonia lactiflora Pall., 4. Cinnamomi cortex, 5. Zingiberis rhizoma, 6. Rheum officinale Baill., 7. mylabris | Blood stasis, dyspepsia, gastrointestinal disturbance | Sheng ji zong lu |
| Baozhu Dan | Powder | 1. Moschus, 2. Camphora, 3. Caryophylli flos, 4. Crotonis fructus, 5. Mylabris, 6. Lycium barbarum L | Pausimenia | Chi shui xuan zhu |
| Bima San | Powder | 1. Abutilon theophrasti, 2. Agapanthus africanus, 3. Hippocampus, 4. Meloe coarctatus, 5. Viola yedoensis Makino, 6. Cuprum, 7. mylabris, 8. Realgar, 9. Arsenic trioxide | Furuncle | Yi fang lei ju |
| Binlang San | Powder | 1. Scorpio, 2. mylabris, 3. Crotonis fructus, 4. Arecae semen, 5. Oleum sesami, 6. Phellodendri cortex, 7. Sulfur, 8. Cnidii fructus, 9. Realgar, 10. Sepiae endoconcha, 11. Amyris, 12. Coptis chinensis Franch, 13. Armeniacae semen amarum, 14. Sublimed mercury | Chronic shank ulcer | Zhu shi ji yan fang |
| Bixiao San | Powder | 1. Hibiscus syriacus cortex, 2. mylabris, 3. Pinellia ternata (Thunb.) Makino, 4. Momordicae semen, 5. Arecae semen, 6. Realgar 7. Arsenic trioxide | Rheumatism, scabies, and long-standing stubborn tinea | Gu jin yi jian |
| Ezhang fengjinji | Powder | 1. Mylabris, 2. Scolopendra, 3. Arsenic trioxide, 4. Strychni semen, 5. Bletilla striata (Thunb.) Reichb. f. , 6. Rheum officinale Baill, 7. Strychnos nux-vomica semen | Tinea manuum | Zhong yi pi fu bing xue jian bian |
| Ezhang fengyaoshui | Water | 1. Pseudolaricis cortex, 2. Cnidii fructus, 3. Hydnocarpus anthelminticus, 4. Stemona sessilifolia (Miq.)Miq, 5. Saposhnikovia divaricata (Turcz.) Schischk. , 6. Angelica sinensis (Oliv.) Diels, 7. Impatiens balsamina, 8. Platycladi semen, 9. Evodia rutaecarpa (Juss.) Benth, 10. Zanthoxyli pericarpium, 11. Cicadae periostracum, 12. mylabris | Tinea manuum, onychomycosis, eczema, athlete’s foot | Pharmacopoeia of the People’s Republic of China |
Traditional use of mylabris to treat diseases recorded in ancient books.
4.2 Modern research
Extensive studies have been conducted to determine the biological activities and pharmacological effects of mylabris extracts, particularly CTD. The currently reported activities of CTD include anticancer (Deng et al., 2013), antithrombotic (Viallard and Larrivée, 2017), antiviral (Braue et al., 2005), insecticidal, and antifungal effects (Eisner et al., 1974). Next, the pharmacological effects of CTD are summarized in Figure 3.
FIGURE 3
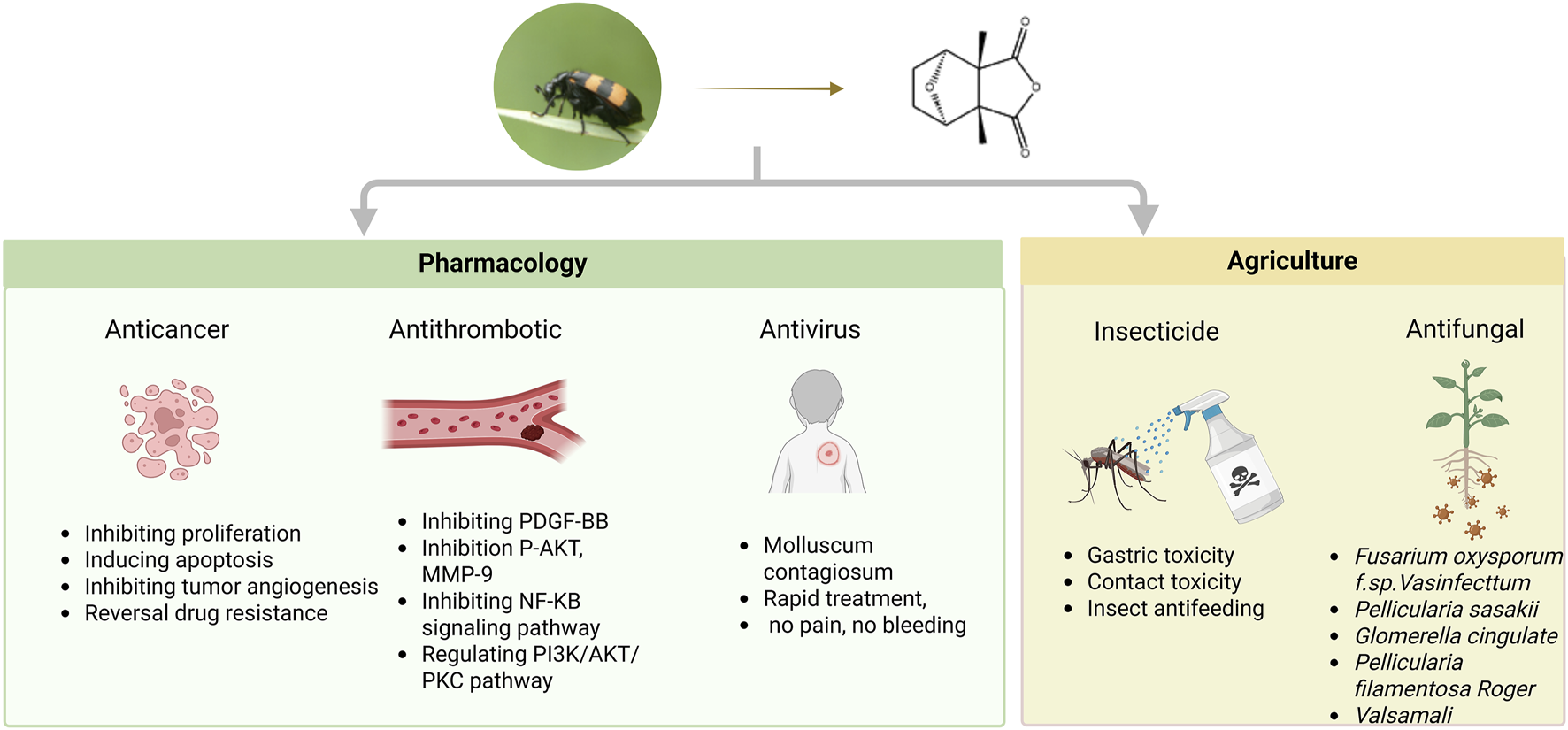
Multifunctional pharmacological properties of CTD.
4.2.1 Anticancer effects
The medicinal use of mylabris for cancer was first documented by Yang Shiying, a renowned physician of the Southern Song Dynasty (Safenraiter et al., 2024). In 1980, CTD was identified as an active compound against liver cancer, and subsequent preclinical studies have since demonstrated its efficacy against multiple tumor types. Research on CTD’s antitumor properties remains more extensive than its other pharmacological activities (Li et al., 2018). Table 3 summarizes in vivo and in vitro models, dosages, and mechanistic insights underlying CTD’s anticancer effects.
TABLE 3
| Cancer type | Cell lines/model | Dosage/administration/IC50 | Mechanism of action | References |
|---|---|---|---|---|
| Liver cancer | HepG2, MHCC-97H, Hep3B, MHCC-97L, SMMC-7721, Huh-7 cells | 0.5, 1, 1.5, 2 µM for 48 h | Downregulation of EphB4, blocking of the EphB4/PI3K/Akt signaling pathway, blocking of the EphB4/JAK2/STAT3 signaling pathway | Zhu et al. (2020) |
| HepG2 xenograft models | 0.1, 0.2, or 0.4 mg/kg, oral administration | |||
| HepG2 | 0, 2, 4, 5, 10, 15, 20 µM for 48 h | G2/M phase arrest, inducted of apoptosis | Le et al. (2016) | |
| HepG2 xenograft male BALB/c mice | 0.25, 0.5, 1 mg/kg, oral administration | Cell apoptosis, immune response | Yan et al. (2023) | |
| LO2 cells | 0, 6.25, 12.5, 24, 50, and 100 µM for 24 h | Inhibition of ERS (GRP78, ATF4, PERK, p-PERK, XBP1–1, and CHOP), induction autophagy (LC3, Beclin-1, Atg3, Atg4A, Atg4B, and Atg7), induction of apoptosis (Bax/Bcl-2 and caspase-3) | Liu et al. (2020) | |
| HEK293T cells, L02 cells, HepG2 cells | 0.5, 1, 2 ug/mL | Downregulation of MDR1 gene expression | Zheng et al. (2008) | |
| SMMC-7721, HepG2 cells | 0, 5, 10, 15 µM for 24 h | Induction of DNA damage, enhancement of chemotherapy sensitivity via KDM4A-dependent demethylation of histone H3K36 | Wei et al. (2022) | |
| HepG2 xenograft models | 1.34, 2.67 mg/kg, oral administration | ERS, autophagy, and apoptosis | Wu et al. (2015) | |
| Gastric cancer | MGC803, BGC823 | 0, 5, 10 µM for 0, 12, and 24 h | Downregulation of CCAT1, Akt, and MDM2, upregulation of the PI3K/Akt signaling pathway | Song et al. (2020) |
| SGC-7901, BGC-823 cells | 2.5, 5, 10, 20, 40, 80 µM for 24, 48, and 72 h | G2/M phase arrest (downregulation of cyclin A and B and CDK1, upregulation of p21); induction of apoptosis (upregulation of caspases-7, -8 and -9; activated caspase-3, PARP, and Bad, downregulation of Bcl-2 and Bid) | Zhang et al. (2014) | |
| Colorectal cancer | HCT116, SW620 | HCT116 24 h: IC50 = 12.4 ± 0.27 µM; HCT116 48 h: IC50 = 6.32 ± 0.2 µM; SW620 24 h: IC50 = 27.43 ± 1.6 µM; SW620 48 h: IC50 = 14.30 ± 0.44 µM | Inhibition of S100A4 and MACC1 | Schöpe et al. (2023) |
| Colo 205 cells | IC50 = 20.53 µM | G2/M phase arrest, downregulation of CDK1 activity, decrease Cyclin A, Cyclin B, CDK1, pro-caspase-8, pro-caspase-9, and Bcl-2, increase CHK1 and p21 protein levels, ROS production | Huang et al. (2011) | |
| HCT116 cells | 1, 5, 10, 30, and 50 µM for 24 h | Inhibition of HSP70 and BAG3, downregulation of BCL-2 family proteins | Kim et al. (2013) | |
| HCT116 cells | 0, 10, 20, and 30 µM or 48 h | Inhibition of proliferation and migration, promotion of apoptosis | Hou et al. (2024) | |
| Pancreatic cancer | PANC-1, CFPAC-1 | 0.1, 0.3, 1, 3, and 10 µM for 24 h | G2/M phase arrest, DNA damage, repression of JNK, ERK, PKC, P38, and NF-κB | Xu et al. (2018) |
| Lung cancer | A549 cells | 0, 1, 3, 10, 30, and 100 µM for 24 h | Downregulation of MMP-9 and MMP-2, inhibition of the PI3K/Akt signaling pathway | Kim et al. (2013) |
| H460 cells | 0, 5, 7.5, 10, 15, and 30 µM for 24 h and 48 h | Increased caspase-3 and -8, cytochrome c, Bax, AIF, calpain 2, and XBP-1 levels, inhibition of Bcl-xL and calpain 1 | Hsia et al. (2014) | |
| NCI-H460 cells | 0, 5, 10, 15, and 20 µM CTD for 24 h and 48 h | Reduction of BRCA-1, 14-3-3σ DNA-PK and MGMT | Hsia et al. (2015) | |
| H460 cells | 10 µM for 24 h | DNA damage, cell cycle progression, and apoptotic cell death | Hsia et al. (2015) | |
| A549 cells | 1 µM | Apoptosis (downregulation of Bcl-2, upregulation caspase-3 and Bax levels), autophagy (downregulation of p62, upregulation 1A/1B light chain 3B and Beclin-1) | Liu et al. (2018) | |
| NCI-H460 cells | 0, 1, 2.5, 5, 7.5, and 10 µM for 24 h and 48 h | Reduction in FAK, GRB2, Ras, TIMP2, TIMP1, ROCK1, PI3K, IRE1α, MKK7, p-AKT, p-JNK1/2, p-p38, p-ERK1/2, iNOS, COX-2, NF-κB p65, UPA, and MMP-1, -2, -9, 13, PI3K, AKT, UPA, p38, JNK and ERK | Hsia et al. (2016) | |
| A549, H460, and H358 cells | 2.5 μM for 24 h | Inhibition of PP5 induces apoptosis | Hsieh et al. (2017) | |
| H460 xenograft | 20 mg/kg, every 3 days, oral administration | |||
| Acute myeloid leukemia | HL-60 | IC50 = 6.21 mM (72 h) | G2/M phase arrest, downregulation of cyclin E, cyclin B1, and CDK2, upregulation of p27 and p53, induction of Nur77 | Yu et al. (2020b) |
| Kasumi-1 | IC50 = 8.00 mM (72 h) | |||
| OCI-AML3 | IC50 = 28.70 mM (72 h) | |||
| HUVECs (normal cell) | IC50 = 75.63 mM (72 h) | \ | ||
| K562 | IC50 = 28.23 µM (24 h), 27.63 µM (72 h) | Induction of mitotic arrest, DNA damage, downregulation of BCR-ABL | Sun et al. (2016) | |
| K562R | IC50 = 54.42 µM (24 h) IC50 = 1.34 µM (72 h) |
|||
| Prostate cancer | DU145, LNCaP | 0, 0.25, 0.50, and 1 μM for 18 h | Downregulation of c-FLIP and upregulation of DR-5 | Nazim et al. (2020) |
| PC-3 | 1, 2.5, 5, 10, 15, 20 μM for 24, 48 and 72 h | \ | Lou et al. (2014) | |
| Breast cancer | MDA-MB-231 | 0, 0.1, 0.5, 1, and 2 µM for 24 h | Downregulation of EGFR, GLUT1, MCT4, and MCT1 | Pan et al. (2019) |
| MCF-7 | ||||
| Xenograft model | Injected intraperitoneally, 0.2 mg/kg/day, 0.5 mg/kg/day | \ | ||
| MDA-MB-231 | 0, 5, 10, 15 μM | Inhibition of EGFR-mediated STAT3 and AKT, induction of apoptosis (caspase-3, caspase-8, and PARP1) | Chun et al. (2018) | |
| Breast cancer | MDA-MB-231 | 2.5, 5, 10, and 20 mM for 48 h | G2/M phase arrest, decrease in MEK, ERK, MAPK, JNK, MMP-9, and MMP-2 | Gu et al. (2017) |
| MDA-MB-231 cell xenograft model | 20 or 40 mg/kg daily for 3 weeks | \ | ||
| MCF-7 cells, MDA-MB-231 and HBL-100 | 0.8, 1.6, 3.2, 6.4, and 12.8 μg/mL | Inhibition of MCM7, E2F1, PTEN, p21 | H. Zhang and Yan (2015) |
Anticancer properties and mechanisms of CTD.
4.2.1.1 Inhibition of proliferation
CTD inhibits the proliferation of tumor cells by blocking different cell cycles. CTD blocks the G2/M phase in breast cancer cells by inhibiting the activation of mesenchymal epithelial transition factor (Met)/Sarcoma (Src)/protein kinase B (Akt 2) (Du, 2019) and reducing the expression of matrix metalloproteinase (MMP)-2 and MMP-9 (Gu et al., 2017). It also inhibits the proliferation of human gastric cancer cells SGC-7901 and BGC-823, blocks the G2/M phase. The mechanism may be related to activation of the caspase cascade and/or induction of apoptosis by regulating Bcl-2 family proteins (Zhang et al., 2014). Moreover, CTD can block human malignant melanoma A375 cells in the G0/G1 phase, likely by interfering with the extracellular regulated protein kinases (ERK) and AKT signaling pathways (Liu et al., 2018). The cell cycle also affects the radiosensitivity of tumor cells, with cells being most sensitive in the G2/M phase, less sensitive in the G0/G1 phase, and least sensitive in the late S phase. A study on pancreatic cancer indicated that CTD could enhance cellular radiosensitivity by driving cancer cells out of the quiescent G0/G1 phase and arresting the cell cycle in the G2/M phase (Xu et al., 2018). Similar results were obtained for acute myeloid leukemia (AML) (Yu Z. et al., 2020) and Lung cancer (Zhang, 2021). So, CTD exhibits broad-spectrum antitumor activity by arresting the cell cycle at different phases across multiple cancer types.
4.2.1.2 Induction of apoptosis
The Bcl-2 family and caspase family are key molecules in the cell apoptosis pathway (Huang et al., 2011; Le et al., 2016). The combination of CTD and pemetrexed can inhibit the growth-inhibition rate of HCT116 colon cancer cells, reduce the expression of pro-caspase-3, and increase the expression of cleaved-poly ADP-ribose polymerase (PARP) (Sui et al., 2018). CTD downregulates epidermal growth factor receptor (EGFR) through mir607, and blocks the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) and extracellular regulated protein kinases (ERK)/mitogen-activated protein kinase (MAPK) signaling pathways to inhibit the proliferation of BT474 and MDAMB-468 breast cancer cells (Yang et al., 2023). In liver cancer, CTD upregulates the mRNA expression of TP53, inhibits the activation of the PI3K/Akt signaling pathway (Wang et al., 2022), and enhances DNA damage and inhibits the proliferation of HepG2 and SMMC-7721 cells (Wei et al., 2022). In human bladder cancer, CTD induces the apoptosis of T24 and RT4 cells by increasing the expression of caspase-9/7/3, and regulates the ER stress pathway through the calcium/protein kinase C (PKC) pathway (Su et al., 2015). In oral cancer, CTD inhibits the proliferation of SAS, CAL-27, and SCC4 cells by increasing the expression of Bax, and Bid proteins, and decreasing Bcl-2 protein (Su et al., 2016). Additionally, CTD is used to inhibit the proliferation of HL-60 leukemia cell. The mechanism may be related to blocking the G2/M phase of the cell cycle and promoting the activation of caspase-8 and PARP (Yu Z. et al., 2020). To sum up, CTD exerts potent pro-apoptotic effects across multiple cancer types by modulating key apoptotic regulators and critical signaling pathways. Its ability to synergize with chemotherapeutics (e.g., pemetrexed) and induce DNA damage highlights its potential as a combinatorial antitumor agent.
4.2.1.3 Inhibition of tumor angiogenesis
In 2014, it was first confirmed that CTD could inhibit the proliferation, migration, and tube formation in human umbilical cord vein endothelial cells (HUVEC) in a dose-dependent manner (Wang et al., 2015). In breast cancer, upon tube formation and in the rat aortic ring assay, CTD could decrease the number of completely formed tubes and reduce the density and length of vascular sprouting. These findings show that CTD can inhibit breast cancer angiogenesis (Pan et al., 2019; Li et al., 2020).
Several studies have also indicated that CTD may exert a pro-angiogenic effect on tumors. In pancreatic cancer, a study demonstrated that CTD significantly increased the size of transplanted tumors in nude mice. Consistent findings have also been reported in xenograft models of lung and colon cancers, indicating that CTD might promote tumor growth by enhancing angiogenesis in these malignancies. CTD can increase the levels of angiogenic factors, including vascular endothelial growth factor (VEGF), IL-6, IL-8, and TNF-α, and upregulate the expression of angiogenesis-related genes, including IL-8/C-X-C Motif Chemokine Ligand 8 (CXCL8), CXCL1/growth-related oncogene (GRO) -α, VEGF/VEGFA, granulocyte-macrophage colony-stimulating (GM-CSF)/colony stimulating factor 2 (CSF2), and plasminogen activator urokinase receptor (PLAUR)/uPAR at both the mRNA and protein levels. These findings suggest that CTD can stimulate tumor growth and potentially tumor vascular remodeling (Xu, 2019). Therefore, the antitumor effect of CTD may be masked by its angiogenic effect. However, the proangiogenic effects of CTD can be antagonized by anti-angiogenic drugs and kinase pathway inhibitors, such as, Bevacizumab, and Endostar, which exhibit significant synergistic antitumor effects. Figure 4 summarizes the synergistic mechanism of CTD and anti-angiogenic drugs. These results indicate that the clinical use of CTD to treat tumors should be conducted in conjunction with antiangiogenic therapy (Xu, 2019).
FIGURE 4
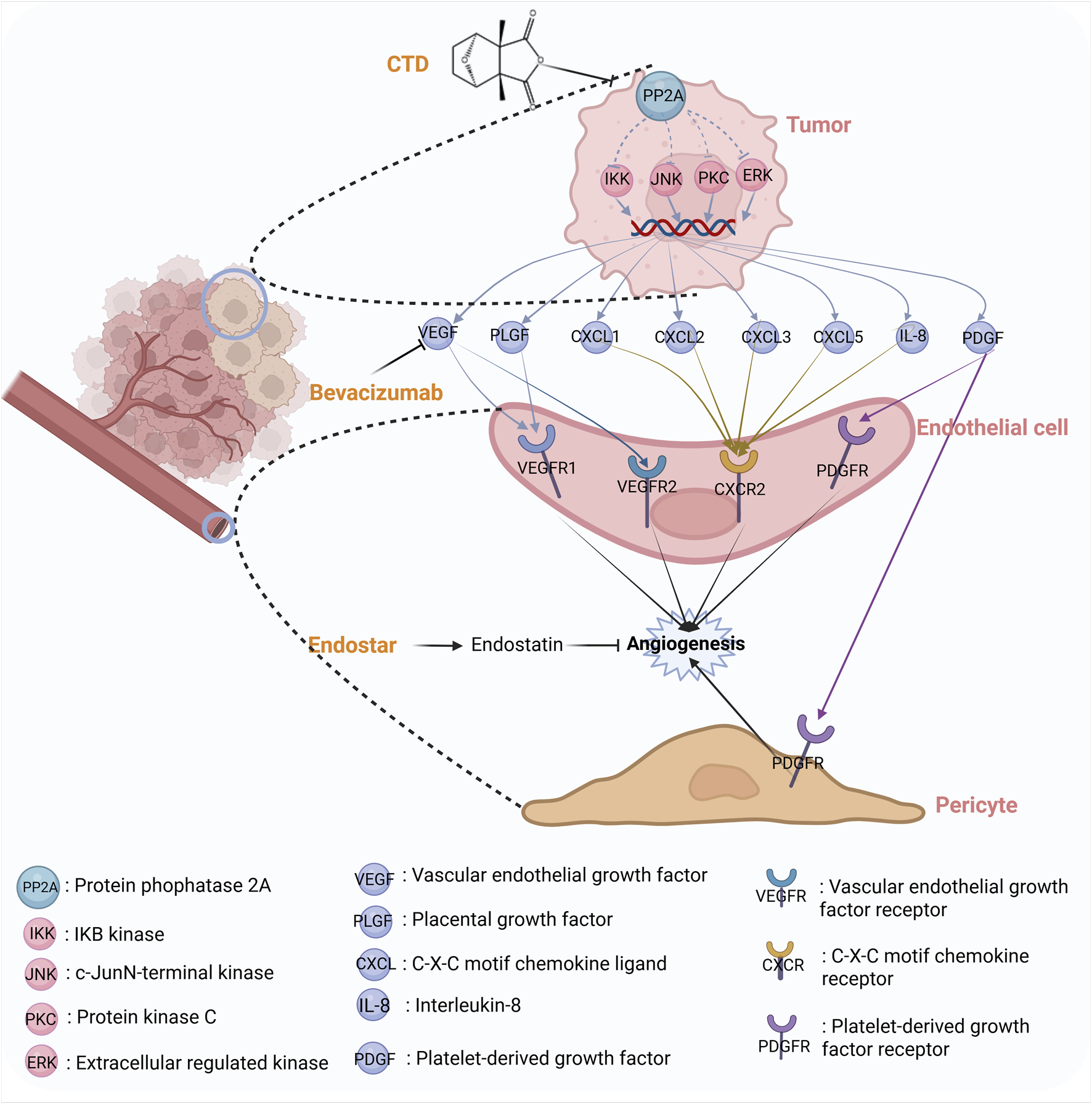
The synergistic mechanism between CTD and antiangiogenic drugs.
4.2.1.4 Reversal of drug resistance
Imatinib is a highly specific and efficacious tyrosine kinase inhibitor that is used to treat chronic myeloid leukemia. However, imatinib resistance poses a significant challenge, limiting its clinical use. CTD can arrest cell cycle at the mitotic phase, trigger DNA damage, and downregulate B-cell receptor (BCR)-ABL protein expression, thereby overcoming imatinib resistance (Sun et al., 2016). Similarly, multidrug resistance (MDR) is a major obstacle in treating HCC. A study have demonstrated that CTD can effectively inhibit P-glycoprotein expression, mRNA transcription, and MDR1 promoter activity, suggesting the role of CTD as a novel and potent agent that can reverse MDR (Zheng et al., 2008).
Norcantharidin (NCTD), 7-oxabicyclo [2.2.1] heptane-2,3-dicarboxylic anhydride, is a demethylated analog of CTD that can reverse resistance and enhance the sensitivity to antitumor agents in various human cancers (Chen et al., 2009; Hsieh et al., 2013). NCTD exhibits enhanced anticancer potential and fewer side effects compared with CTD (Pan et al., 2020). Preclinical studies have demonstrated that NCTD can reverse chemotherapeutic resistance in cancer through multiple molecular mechanisms, including the induction of apoptosis, impairment of cancer cell stemness, and blocking of mitotic (Zeng et al., 2024).
Synthesis of the available evidence suggests that CTD and NCTD exhibit potential in reversing drug resistance. However, the scarcity of clinical trials, combined with the lack of established safe dosages, optimal timing, and well-defined combination strategies, indicates that further research is needed before this approach can be translated into clinical practice.
4.2.1.5 Increasing leukocyte
CTD exerts antitumor effects without causing significant damage to the immune organs or hematopoietic factors in mice. It activates the JAK2-STAT5 signaling pathway in the bone marrow cells of mouse, thereby promoting their proliferation and differentiation while upregulating peripheral blood cell levels and decreasing myelosuppression (Li, 2023).
NCTD is the only anticancer drug that can increase white blood cell counts in a clinical setting (Mo et al., 2018). In the cyclophosphamide-induced model of leukopenia, NCTD restored hematopoietic function by promoting the G0/G1 phase of bone marrow cells to enter the S phase and G2/M phase, leading to recovery from DNA damage. At the molecular level, NCTD can downregulate the expression of BAX protein, upregulate the expression of BCL-2 protein, and decrease the ratio of BAX/BCL-2 (Zheng et al., 2015). In a clinical setting, a patient took NCTD tablets during chemotherapy, this decreased incidence of adverse effects including leukopenia, granulocytopenia, and emesis (Shen et al., 2013; Xiao et al., 2016). This study suggests that an optimal anti-cancer strategy should evolve from the conventional “fighting fire with fire” approach to a dual action paradigm that combines tumor suppression with system restoration.
4.2.2 Antithrombotic effects
Mylabris contains abundant trace elements, and its fibrinolytic proteins demonstrate thrombolytic activity with dose-dependent in vitro. Furthermore, CTD demonstrates inhibitory effects on platelet aggregation, release, and spreading, potentially mediated through modulation of the PI3K/Akt/PKC signaling pathway (Guo et al., 2023). Further studies revealed that CTD could inhibit the platelet-derived growth factor-BB (PDGF-BB)-induced proliferation and migration of rat thoracic aortic vascular smooth cells (VSMCs), attenuating Lipopolysaccharide (LPS)-induced vascular inflammation (Qiu et al., 2019a; Qiu et al., 2019b). Given the close interplay between thrombosis and inflammation (e.g., in atherosclerosis), CTD’s dual capacity to suppress vascular inflammation and platelet activation may offer distinct therapeutic advantages against atherothrombosis.
4.2.3 Antiviral effects
The antiviral effects of mylabris are attributed to its high toxicity, which aligns with the TCM theory of “fight poison with poison” (Pan et al., 2019). Molluscum contagiosum (MC) is an increasingly common skin infection caused by MC virus, a member of the poxvirus family. Its prevalence ranges from 2% to 10% in children (Braue et al., 2005). The use of CTD in the treatment of MC has been documented since the 1950s (Moed et al., 2001). The advantages of CTD over other treatments include a rapid treatment time, minimal pain at the time of application, and no bleeding. CTD is a commonly used and efficacious therapy for MC, which is generally well tolerated and associated with high rates of parental satisfaction (Moye et al., 2013). Subsequent studies have demonstrated that the combination of CTD with podophyllotoxin and salicylic acid, typically following, is highly effective removing plantar warts, with 100% success rate (Vakharia et al., 2018). This finding demonstrates the potential efficacy of the combination therapy in treating plantar warts. However, the safety and efficacy of combination therapies in younger children have not been fully described. Therefore, well-designed, randomized controlled trials with adequate blinding, control groups, and follow-up periods, as well as valid and reliable outcomes, are necessary to determine optimal protocols for CTD-based treatments (Vakharia et al., 2018).
4.2.4 Agricultural application
Bioinsecticides utilize naturally derived organisms or their metabolites for pest control, exhibiting superior target specificity, low non-target toxicity, and environmental compatibility. Growing concerns over chemical pesticide safety have positioned mylabris and other bioactive natural compounds as promising candidates for next-generation bioinsecticide development.
Mylabris has insecticide effects as documented in ancient texts. However, the methodology of its application has not been documented. In 1937, Goernitz reported that microgram quantities of CTD could kill Phyllopertha horticola, Lymantyia mornachl, and Pyrrhocoris apterus among other insects. In 1974, Carrel and Eisner discovered that ants exhibited a significant antifeedant response to CTD (Eisner et al., 1974). In 1992, Frenzel reported that CTD had trapping effect on dipteran Ceratopgonidae, Anthomyiidae and other insects. In 2008, studies have demonstrated that contact-killing effect of CTD on agricultural pests, including the brown planthopper and diamondback moth. On Lepidopteran insects such as Plutella xylostella (Huang et al., 2015), CTD exerts strong stomach toxicity, and antifeeding effects upon contact (Zhang et al., 2000). Subsequent studies have shown the toxic effects of CTD exerts on pests, including Agrotis ipsilon, Nilaparvata lugens Stal, Sogatella furcifera, and Bambusiphaga furca. Mukaria pallipes was found to be the most sensitive and A. grotis to be the least sensitive to CTD (Eisner et al., 1974). Generally, CTD exhibits toxic effects on numerous pests, mainly through gastric toxicity, contact action, and antifeeding effect (Eisner et al., 1974; Pradhan et al., 2024).
CTD also exerts potent antifungal effects against several plant pathogens, including Fasarium oxysporum f. sp.vasinfecttum, Pellicularia sasakii, Sclerotinia sclertiorum, Glomerella cingulate, Pellicularia filamentosa Roger, and Valsamali (Cao et al., 2008). However, these findings have been reported based on basic experimental research, and no pesticides are being sold commercially.
The safety of biological pesticides cannot be ignored. CTD is a pesticide that has weak mobility in soil. When it reaches equilibrium in the soil-water system, it is mainly adsorbed by the soil phase, which will not pollute to the surrounding environment due to rapid degradation. Therefore, CTD is an ideal pesticide with low residue, making it environmentally friendly (Cui et al., 2009).
Fundamental studies have identified CTD as a broad-spectrum, highly efficient, and environmentally friendly bioinsecticide with significant potential for green agriculture. However, translating laboratory findings to large-scale agricultural applications requires further evaluation of its biosafety, target specificity, and environmental stability. Future research should integrate multidisciplinary approaches, including biotechnology, nanotechnology, and ecotoxicology to facilitate its industrial development.
5 Toxicology
In the Chinese Pharmacopoeia, mylabris has been recorded to be extremely poisonous. Due to the lack of knowledge about its pharmacological effects, dosage, and toxicity, and lax drug management among users, there have been cases of toxicity due to multiple doses and accidental ingestion. The different administration routes and dosage forms of mylabris will affect the occurrence of toxic reactions. Mylabris poisoning mostly results from oral administration. There are differences in toxic reactions when decoctions and powders are used. The average toxic dose is 3.91 g in decoctions, with the minimum dose being 0.1 g and the maximum dose being 60 g. Mylabris toxicity generally occurs immediately after ingestion of the oral decoction. Sometimes, it occurred at 1 h after ingestion and led to death within 6 days. However, the intensity of toxicity is higher in the powder form, and is speculated to be related to the sublimation of CTD when exposed to high temperatures during decoction. Poisoning has also occurred in individuals coming in contact with external applications and during processing. Therefore, adequate protection must be ensure during preparation and use to avoid episodes of poisoning. In-depth studies have reported CTD to be the toxic component of mylabris. The characteristics of CTD poisoning are summarized in Figure 5 (Zhang et al., 2020a; Zhang, 2022).
FIGURE 5
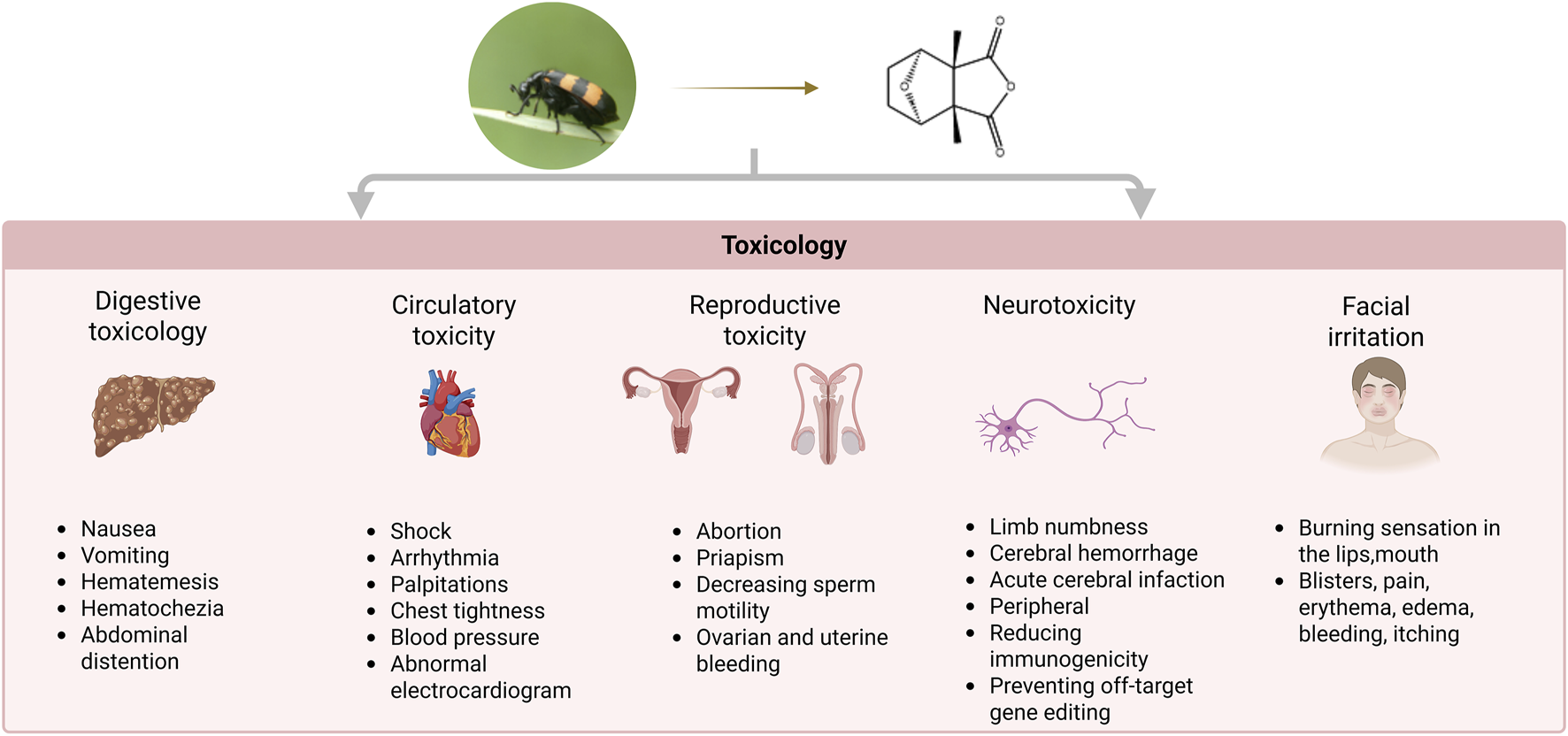
Comprehensive toxicological profile of CTD: multiorgan adverse effects.
5.1 Digestive toxicity
The digestive toxicity of CTD is primarily characterized by digestive tract damage and liver failure (He et al., 2022). The principal symptoms include nausea, vomiting, hematemesis, abdominal distention, and hematochezia. Although the symptoms are commonly assumed to be linked to acute stress, the precise mechanism remains unclear.
The liver is the primary target organ in CTD-induced digestive toxicity, with inflammatory cell infiltration and focal necrosis being the predominant pathological manifestations of CTD-induced hepatotoxicity (Yu Y. L. et al., 2020). Biochemical markers, including aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase, are significantly elevated in animal models, indicating liver injury (Cheng et al., 2022). CTD-induced hepatotoxicity is associated with following 3 metabolic pathways (Figure 6): lipid metabolism, glycerophospholipid metabolism, and glyceride metabolism (Li et al., 2024). Further studies have revealed that liver injury following CTD is associated with ER stress, autophagy, apoptosis, and metabolism (He et al., 2024).
FIGURE 6
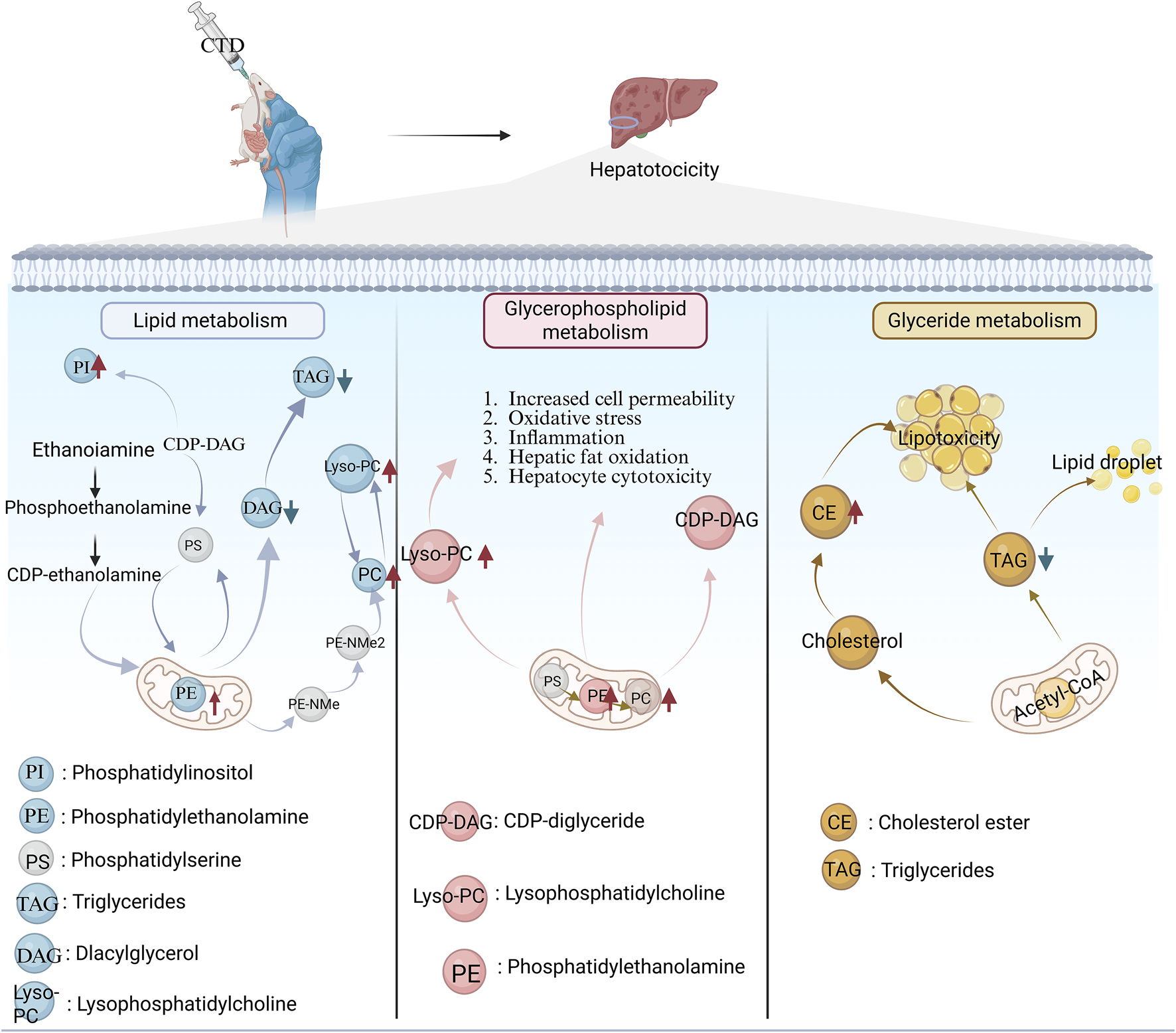
Three metabolic pathways of CTD-induced liver injury. Red upward arrows represent metabolite upregulation; Blue down arrows represent metabolite downregulation.
5.2 Cardiotoxicity
The heart is the primary target organ in circulatory system-related toxicity caused by CTD. With sinus tachycardia being the most common symptom (Wang G. et al., 2018). Pathological changes, including myofibrillar aggregation, fibroinflammatory response, and subepicardial hemorrhage have been confirmed in the tissue samples of the heart exposed to CTD. The mechanism of myocardial injury may be related to the inhibition of VEGF by CTD, based on changes in protein expression after poisoning (Zhang et al., 2020b). Further studies have revealed that CTD may inhibit the PI3K signaling pathway, leading to a reduced neovascularization and induction of myocardial injury (Zhang et al., 2020c).
5.3 Reproductive toxicity
CTD, as an “aphrodisiac”, has a long history of use in Europe (Moed et al., 2001). Due to its excitatory effects on reproductive organs, it often causes ovarian and uterine bleeding or abortion in women, as well as priapism and reduced sperm motility in men during poisoning (West and Krychman, 2015). CTD-induced testicular injury involves multiple targets. CTD may inhibited expression of PI3K, AKT and BCL-2, while promoting expression of the proapoptotic proteins Bax and Caspase 3 in testicular injury (Liu R. et al., 2024). Further studies have revealed that CTD may upregulate LC3 and Beclin1 expression, while downregulating P62 and mTOR/pmTOR in the testicular tissue of mouse, leading to excessive autophagy. Furthermore, CTD can markedly diminish ZO-1, CX-43, and testosterone, indicating that it impairs the blood-testis barrier in mouse (Xiao et al., 2024).
5.4 Neurotoxicity
There is a paucity of reports related to poisoning of the nervous system by CTD. The primary causes of neurological impairment are acute ischemia and hypoxia. Bederson method has been used to evaluate the neurological deficit score of rats exposed to CTD, and the results demonstrate a positive correlation between the neurological deficit score and CTD dose. Previous studies have demonstrated that CTD poisoning can result in acute cerebral infarction, cerebral hemorrhage, and other focal neurological deficits, as well as peripheral neuropathy, including peripheral facial paralysis and limb numbness (Zhang et al., 2020a). Future investigations should focus on constructing complete dose-toxicity profiles to establish precise safety thresholds, thereby generating toxicology data to guide clinical practice - especially for optimizing long-term treatment safety.
5.5 Facial irritation
After the ingestion of CTD, a burning sensation in the lips, mouth, and pharynx occurs within minutes. Subsequently, blisters form, causing difficulty in swallowing, and abdominal cramps, hematemesis, and vomiting have also been noted. Blisters formation, pain, erythema, edema, bleeding, itching, and post-inflammatory hyperpigmentation occur in 6%–46% of patients (Zhang et al., 2018; Pradhan et al., 2024).
5.6 Detoxification
The management of CTD poisoning is primarily supportive, as no specific antidotes are currently available (Li et al., 2022). If accidentally exposed to CTD or ingested, the following measures may help mitigate its toxic effects: for local exposure, the affected area should be washed immediately with acetone, ether, soap, or alcohol to dissolve and dilute the toxin. The affected area should subsequently be washed thoroughly with soap and water. Topical steroids may be applied to intact skin if symptoms are present (Karras et al., 1996). Several supportive measures may be used. If CTD is taken orally, patients should drink an adequate quantity of water, while avoiding foods that high in fat (such as milk), as these can increase CTD absorption. Vomiting is not recommended as it can re-expose CTD to the mouth and trachea (Moed et al., 2001).
It is crucial to deepen toxicological and pathological studies on CTD to elucidate its mechanisms of toxicity. Such research will provide valuable insights for guiding its safe clinical use, developing antidotes for poisoning, and improving postmortem identification.
6 Pharmacokinetics
In order to improve the pharmacokinetic study of mylabris, research have used a variety of methods. The pharmacokinetic parameters of mylabris’ ethanol extract have been determined using multipoint dynamic measurement combined with assessments of animal mortality and drug accumulation. The intraperitoneal injection of mylabris ethanolic extract (75% ethanol) was a two-compartment model with first-order kinetic elimination, and the median lethal dose (LD50) was 344 mg/kg (Song, 2014). However, this pharmacokinetic study utilized acute mortality as an indicator in animal models. It is crucial to not only consider the differences in physiology and pathology between humans and animals, but also the significant differences between the oral and intraperitoneal routes of drug administration. Objectively, the pharmacokinetic parameters derived from the toxicodynamic method merely serve as a general estimate, providing only a reference point for drug application.
The WinNonlin 6.4 software was used to fit the concentrations and time of CTD in rat plasma following the administration of different doses of the aqueous extract of the mylabris compound (AEMC, 20 g of Mylabris powder was refluxed with 400 mL 75% ethanol for 1.5 h twice and was afterwards filtered). The time to peak concentration was determined to be 0.17 h for all tested doses of AEMC, indicating the rapid absorption of CTD into the bloodstream following its oral administration. Additionally, the volume of distribution (Vz/F) was greater than 1 L/kg, indicating that CTD from AEMC is rapidly and widely distributed in rat tissues after oral administration. Studies have also found that CTD from AEMC is mainly distributed in the liver and kidneys after oral administration. CTD is characterized in rat by its rapid distribution, and elimination in a short time for hepatic metabolism. This rapid hepatic uptake may be one of the reasons why the liver is a primary target organ for CTD toxicity (Duan et al., 2021).
Studies have shown that both oral and injectable preparations of CTD follow one-compartment model. The rapid t½e of CTD is 0.63 h in vivo, which readily leads to peak and trough fluctuations in blood concentration when used clinically. This may be one of the reasons underlying the irritant nature of CTD. Additionally, the oral bioavailability of CTD in beagles has been reported to be low at 6.7%, likely due to its low solubility (Dang and Zhu, 2009).
Pharmacokinetic studies have provided basic data for the clinical safety of CTD, but there are still areas for improvement, such as insufficient assessment of species differences, dosage for special populations, and vigilance against data accuracy caused by errors in analytical methods.
7 Marketed drugs
Toxic mylabris has been combined with tonic drugs based on modern scientific techniques to develop, oral capsules (Kangsaidi capsules, China Food and Drug Administration approval number Z52020238) and injections (Aidi injection, China Food and Drug Administration approval number Z52020236). It represents a model for the application of contemporary preparations of mylabris and exemplifies the principle of “supporting the positive and dispelling the evil” in treating malignant tumors while using the TCM framework.
CTD is highly toxic and mostly used externally in the clinic settings. Based on the structure-activity relationship, a series of CTD derivatives, such as sodium cantharidinate and NCTD, have been developed to enhance the potency and attenuate the toxicity (Wang et al., 2019; Xiao et al., 2019; Zhu et al., 2020; Wu et al., 2021). These compounds have been approved by the China Food and Drug Administration for clinical use, and clinical studies have demonstrated their efficacy in patients. Table 4 summarizes the drug names, components/constituents, efficacy, and clinical uses of these marketed preparations. In addition, a summary of meta-analysis of Aidi injection and sodium cantharidinate Vitamin B6 Injection (DCVB6 injection) can lead to a more comprehensive and objective basis for the assessment of the therapeutic efficacy and safety of these drugs.
TABLE 4
| Drug | Components/constitutes | Efficacy | Clinical application |
|---|---|---|---|
| Kangsaidi capsules | 1. Mylabris, 2. Panax ginseng C.A. Mey., 3. Astragalus membranaceus (Fisch.) Bunge., 4. Acanthopanax senticosus (Rupr. et Maxim.) Harms., 5. Curcuma zedoaria., 6. Scutellaria barbata D. Don., 7. Curcuma aerugionosa., 8. Cornus officinalis Sieb. et Zucc., 9. Ursodeoxycholic acid., 10. Glycyrrhiza uralensis Fisch. ex DC. | Removing blood stasis, resolving static blood, attacking toxicity and corroding sores | Primary liver cancer, lung cancer, colorectal cancer, malignant lymphoma, gynecological malignancies |
| Aidi injection | 1. Mylabris, 2. Panax ginseng C.A. Mey., 3. Astragalus membranaceus., 4. Eleutherococcus senticosus (Rupr. and Maxim.) Maxim | Clearing heat and detoxifying, eliminating mass, and relieving swelling | Primary liver cancer, lung cancer, colorectal cancer, malignant lymphoma, gynecological malignancies |
| XuanShi YaoShui | 1. Mylabris, 2. Cortex cercidis., 3. Zanthoxylum bungeanum Maxim.,4. Stemona tuberosa Lour., 5. Saposhnikovia divaricata (Turcz.) Schischk | Expelling wind and removing dampness, killing parasites, and relieving itching, antibacterial and anti-inflammatory effects | Dermatophytosis |
| Hupo Zhitong Gao | 1. Kaempferia galanga L, 2. Acorus calamus var. angustatus Besser., 3. Coptis chinensis Franch., 4. Strychni Semen., 5. Mylabris, 6. Clematis chinensis Osbeck., 7. Pinellia ternata (Thunb.) Makino., 8. Venenum Bufonis., 9. Amber oil., 10. Clove basil oil., 11. Peppermint oil., 12. Star anise oil., 13. Cassia oil., 14. Borneolum syntheticum., 15. Camphorae | Activating blood circulation and resolving phlegm, reducing swelling, and dispersing nodules, promoting the flow of Qi and blood through the meridians, and relieving pain | Cancer pain, neuropathic pain, rheumatic pain, traumatic pain |
| Gan Ning tablets | 1. Mylabris, 2. Oryza sativa., 3. Lithospermum erythrorhizon Sieb. et Zucc | Clearing heat and detoxification, removing dampness, resolving blood stasis, and dispersing nodules | Acute and chronic hepatitis, prevention of hepatitis B related liver lesions, abnormal liver function |
| Cantharidine cream | Cantharidin | Antiviral | Verruca acuminata |
| Ycanth | Cantharidin | Antiviral | Molluscum contagiosum |
| Disodium cantharidinate and Vitamin B6 Injection | 1. Disodium cantharidinate, 2. Vitamin B6 | Antitumor | Advanced hepatocellular carcinoma, advanced lung cancer |
| Disodium cantharidinate injection | Disodium Cantharidinate | Antitumor | Primary liver cancer |
| Sodium demethylcantharidate injection | Sodium demethylcantharidate | Antitumor | Hepatocellular carcinoma, esophageal cancer, gastric and cardic cancer, lung cancer., as well as leukopenia, hepatitis, liver cirrhosis, and hepatitis B virus carriers |
| Demethylcantharidin Tablets | Norcantharidin | Antitumor | Hepatocellular carcinoma, esophageal cancer, gastric and cardiac cancer, leukopenia, hepatitis, liver cirrhosis and hepatitis B virus carriers |
Marketed products containing Mylabris or its related bioactive ingredients.
7.1 Aidi injection
Aidi injection is Chinese herbal injections belonging to the anti-cancer drugs categorys (CZ01) covered by the national basic medical insurance program for Chinese patent medicine in China (2004, 2009, 2017, 2019, 2023 version). It is the most competitive product in the field of cancer care in China in the Report Science and Technology Competitiveness of Large Varieties of Chinese Medicine. A meta-analysis of 3,300 patients revealed that Aidi injection is primarily used to treat lung, liver, and colon cancer. Its principal function is to enhance the survival of cancer patients, improve life quality and alleviate the adverse effects of chemotherapy and radiotherapy. This may be a contributing factor for the recommendation of Aidi injection as an adjuvant therapy to chemotherapy/radiotherapy in almost all of the included systematic reviews (Wang J. et al., 2018; Lan et al., 2021; Yang et al., 2022; Lu et al., 2024), in accordance with the national comprehensive cancer network guidelines.
7.2 DCVB6 injection
DCVB6 injection (China Food and Drug Administration approval number H20053,862) is a combination of sodium cantharidinate and vitamin B6 that is mainly used to treat HCC and non-small-cell lung cancer (NSCLC) (Zhu et al., 2020). A study evaluated the efficacy and safety of DCVB6 Injection in 104 patients with HCC and reported improvements in liver function, changes in tumor morphology and patient’s overall conditions (Shao et al., 2014). Furthermore, chemoradiotherapy regimens that are commonly used to treat HCC can often cause serious adverse effects. A meta-analysis evaluated its the feasibility as an alternative therapy to chemotherapy to evaluated survival, liver function, immune function, and quality of life (Zhu et al., 2020). A meta-analysis (19 trials, 1,428 patients with NSCLC) evaluated the combination of DCVB6 injection with chemotherapy versus chemotherapy alone and highlighted a reduction chemotherapy-induced side effects and improvement in clinical symptoms after using the combination (Wang et al., 2019). Another clinic trial (86 patients) has evaluated the improvement in the quality, and reduction of the side effects of chemotherapy in patients treated with DCVB6 compared with chemotherapy (Wang and Cui, 2014), providing strong clinical evidence for the use of DCVB6 in treating patients with neoplasms.
However, the clinical use of mylabris and its derivatives requires more high-quality clinical studies to be conducted and analyzed in line with the requirements for evidence-based medicine. Moreover, elucidation of its mechanism of action using modern molecular biology techniques is required, to better support its rational clinical application.
8 Conclusions and future prospects
Animal-derived medicines constitute a critical component of TCM, with documented therapeutic applications spanning millennia. The compendium of materia medica (Ben Cao Gang Mu), compiled by Li Shizhen during the Ming Dynasty, catalogs 1,892 medicinal substances, of which 444 agents are derived from animal sources. Similarly, the Encyclopedia of Chinese Herbal Medicine documents 5,767 TCM, including 740 animal-derived medicines, underscoring their enduring pharmacological and clinical relevance (Liu Y. et al., 2024). Contemporary studies have elucidated the mechanistic basis for their efficacy, particularly in antitumor, anti-inflammatory, and immunoregulation.
As an animal-derived TCM, mylabris holds significant medicinal value, yet its research and application remain inadequate. Regarding resources, the World Health Organization, in its Traditional Medicine Strategy (2014–2023), also emphasized that the development of TCM should be balanced with biodiversity conservation. The scarcity of mylabris and the overexploitation of wild populations pose a serious threat to its sustainable utilization. Regarding chemical composition, only a limited number of compounds have been isolated and characterized, and systematic investigations into the complex chemical basis of mylabris remain insufficient. Pharmacological studies have mainly focused on antitumor, and developed a series of clinical preparations, such as Aidi injection and DCVB6 Injection. However, the potential therapeutic effects recorded in ancient books on skin diseases, reproductive dysfunction, and digestive diseases have not yet been fully verified. Meanwhile, in agriculture, CTD exerts its broad spectrum insecticidal effects through gastric toxicity, contact effects, and anti-ingestion effects. It also has the potential to be a low residue, environmentally friendly insecticide, but is still in its early stages of development. In terms of toxicology, traditional records have suggested that it has contraindicated combination, such as its incompatibility with Croton tiglium L. and Salvia miltiorrhiza Bunge. Modern research has also confirmed that it can cause damage to multiple organs including the liver and kidneys, highlighting its safety risks. In terms of pharmacokinetics, preclinical studies have shown that CTD is primarily distributed in the liver and kidneys and exhibits low bioavailability. However, due to species differences and methodological limitations, there is a lack of clinical validation, which hinders the evaluation of safety and efficacy.
In the future, mylabris should be studied and developed from different levels. (1) artificial breeding and resource conservation strategies should be established to ensure the sustainable utilization of this medicinal material. (2) only a limited number of compounds have been identified, and a comprehensive analysis of the complex chemical composition, including small molecules, polysaccharides, lipids and other potential bioactive constituents, is still needed. Additionally, chemical fingerprint and quality control standards for mylabris should be established, along with quantitative methods for key active ingredients, to provide a foundation for clinical applications and international promotion. (3) by integrating traditional processing knowledge with modern pharmaceutical technologies, toxicity reduction and efficacy enhancement can be achieved through approaches such as structural modification, nanocarriers, liposomes, and other novel delivery systems (Xu et al., 2022). (4) Future pharmacological research on mylabris could extend beyond antitumor activity to explore other therapeutic areas, such as dermatological disorders or reproductive dysfunction, among others, guided by traditional records. In agriculture, mylabris also shows potential as an environmentally friendly biopesticide, suggesting promising directions for both medical and agricultural applications. (5) clinical pharmacokinetic studies and evidence based research should be strengthened to provide solid data support for rational drug use, safety testing, and international promotion. So, by integrating traditional wisdom with modern technology, mylabris is expected to realize safe and sustainable development.
Statements
Author contributions
QC: Writing – review and editing, Writing – original draft. JY: Writing – review and editing, Visualization. XL: Investigation, Writing – review and editing. LH: Writing – review and editing, Project administration. SX: Visualization, Writing – review and editing, Investigation. YY: Conceptualization, Investigation, Writing – review and editing. HW: Conceptualization, Supervision, Writing – review and editing. FZ: Supervision, Writing – review and editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from National Natural Science Foundation of China (No: 81973711).
Conflict of interest
Authors LH, SX, and YY were employed by Guizhou Shenqi Pharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- AKT
Protein kinase B
- ATG3
Autophagy-related 3
- ATF4
Activating Transcription Factor 4
- AUC
Area under the curve
- BAG3
BCL2-associated athanogene-3
- Bcl-2
B-cell lymphoma-2
- Be
Beryllium
- BRCA-1
Breast cancer 1
- Ca
Calcium
- CCAT1
Colon Cancer Associated Transcript 1
- CDK1
Cyclin-dependent kinases 1
- CDK2
Cyclin-dependent kinases 2
- Cd
Cadmium
- CHK1
Checkpoint Kinase 1
- Cu
Copper
- CTD
Cantharidin
- COX
Cytochrome c oxidase
- Cr
Chromium
- Crb2
Growth factor receptor-bound protein 2
- CSF2
Colony stimulating factor 2
- CXCL8
C-X-C Motif Chemokine Ligand 8
- DCVB6 injection
Sodium cantharidinate Vitamin B6 Injection
- EGFR
Epidermal growth factor receptor
- EphB4
PH Receptor B4
- ER
Endoplasmic reticulum
- ERK
Extracellular regulated protein kinases
- FAK
Focal adhesion kinase
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- Glut1
Glucose transporter type 1
- GRO
Growth-related oncogene
- GRP78
Glucose regulatory protein 78
- HCC
Hepatocellular carcinoma
- Hg
Mercury
- HSP70
Heat Shock Protein 70
- IRE1α
Inositol-requiring enzyme 1α
- JNK
C-Jun N-terminal kinase
- KDM4A
Lysine Demethylase 4A
- Ka
Absorption rate constant
- Ke
Elimination rate constant
- LC3
Microtubule-associated proteins light chain 3
- LD50
Median lethal dose
- LPS
Lipopolysaccharide
- MACC1
Metastasis-associated in colon cancer 1
- MAPK
Mitogen-activated protein kinase
- MC
Molluscum contagiosum
- MCT4
Monocarboxylate transporter 4
- MDR
Multidrug resistance
- MDM2
Mouse doubleminute 2 homolog
- MEK
Mitogen-activated protein
- Mg
Magnesium
- MKK7
Mitogen-activated protein kinase 7
- MMP
Matrix metalloproteinase
- MMAC1
Mutated in multiple advanced cancer 1
- Mn
Manganese
- mTOR
Mammalian target of rapamycin
- Na
Natrium
- NCTD
Norcantharidin
- Ni
Nickel
- PARP
Poly ADP-ribose polymerase
- Pb
Lead
- PERK
Protein kinase R-like endoplasmic reticulum kinase
- PI3K
Phosphatidylinositol 3-kinase
- PKC
Protein kinase C
- PTGS2
Prostaglandin-endoperoxide synthase 2
- ROCK1
Rho associated coiled-coil containing protein kinase 1
- Sn
Tin
- TIMP2
Tissue inhibitor of metalloproteinase 2
- t½e
Apparent elimination half-life
- t½a
Half-life of apparent distribution
- uPA
Urokinase-type plasminogen activator
- VEGF
Vascular endothelial growth factor
- Vz/F
Volume of distribution
- WHO
World health organization
- XBP-1
X-box binding protein 1
- Zn
Zinc
References
1
Braue A. Ross G. Varigos G. Kelly H. (2005). Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatr. Dermatol.22 (4), 287–294. 10.1111/j.1525-1470.2005.22401.x
2
Cao W. D. Zhang Z. Y. Yang B. D. Zhang M. Z. Sun S. L. (2008). Inhibition of cantharidin and demethylcantharidin to seven phytopathogenic fungi. Acta. Phytophy Sin. (01), 63–68. 10.13802/j.cnki.zwbhxb.2008.01.008
3
Chai J. (2023). Study on the potential active components and mechanism of aidi injection in the treatment of primary hepatic carcinoma based on network pharmacology. Shandong: Shandong University of Traditional Chinese Medicine. master.
4
Chen Y. J. Tsai Y. M. Kuo C. D. Ku K. L. Shie H. S. Liao H. F. (2009). Norcantharidin is a small-molecule synthetic compound with anti-angiogenesis effect. Life. Sci.85, 642–651. 10.1016/j.lfs.2009.09.003
5
Cheng W. Wang Y. Liu J. Li X. Yu M. Duan C. et al (2022). Hepatotoxicity of cantharidin is associated with the altered bile acid metabolism. J. Appl. Toxicol.42, 970–980. 10.1002/jat.4267
6
Chun J. Park M. K. Ko H. Lee K. Kim Y. S. (2018). Bioassay-guided isolation of cantharidin from blister beetles and its anticancer activity through inhibition of epidermal growth factor receptor-mediated STAT3 and Akt pathways. J. Nat. Med.72, 937–945. 10.1007/s11418-018-1226-6
7
Cui F. L. Li X. Ma Z. Q. Zhang Y. L. (2009). Safety evaluation of animal-origin pesticide cantharidin against some non-target organisms. J. Environ. Entomol.31, 143–149. 10.3969/j.issn.1674-0858.2009.02.008
8
Dang Y. J. Zhu C. Y. (2009). Pharmacokinetics and bioavailability of cantharidin in beagle dogs. Zhongguo Zhong Yao Za Zhi34, 2088–2091. 10.3321/j.issn:1001-5302.2009.16.021
9
Deng L. P. Dong J. Cai H. Wang W. (2013). Cantharidin as an Antitumor agent: a retrospective review. Curr. Med. Chem.20 (2), 159–166. 10.2174/092986713804806711
10
Deng Y. Y. Zhang W. Li N. P. Lei X. P. Gong X. Y. Zhang D. M. et al (2017). Cantharidin derivatives from the medicinal insect Mylabris phalerata. Tetrahedron73, 5932–5939. 10.1016/j.tet.2017.08.034
11
Díaz-Navarro M. Bolívar P. Andrés M. F. Gómez-Muñoz M. T. Martínez-Díaz R. A. Valcárcel F. et al (2021). Antiparasitic effects of potentially toxic beetles (Tenebrionidae and Meloidae) from steppe zones. Toxins13, 489. 10.3390/toxins13070489
12
Du Y. F. (2019). The mechanism of cantharidin induced apoptosis in breast cancer cells. Nanjing: China Medical University. master.
13
Duan C. Cheng W. Chen Q. Li X. Zhang J. (2021). Pharmacokinetics and tissue distribution of cantharidin after oral administration of aqueous extracts from mylabris in rats. Biomed. Chromatogr.35, e5172. 10.1002/bmc.5172
14
Efferth T. Li P. C. H. Konkimalla V. S. B. Kaina B. (2007). From traditional Chinese medicine to rational cancer therapy. Trends. Mol. Med.13, 353–361. 10.1016/j.molmed.2007.07.001
15
Eisner T. Johnessee J. S. Carrel J. Hendry L. B. Meinwald J. (1974). Defensive use by an insect of a plant resin. Science184, 996–999. 10.1126/science.184.4140.996
16
Fang L. Du G. (2018). The historical cognition and evaluation of mylabris toxicity. Pharmacol. Clin. Chin. Mater. Medica.34, 150–152. 10.13412/j.cnki.zyyl.2018.05.037
17
Gu X. D. Xu L. L. Zhao H. Gu J. Z. Xie X. H. (2017). Cantharidin suppressed breast cancer MDA-MB-231 cell growth and migration by inhibiting MAPK signaling pathway. Braz. J. Med. Biol. Res.50, e5920. 10.1590/1414-431x20175920
18
Guo F. Tian X. Y. Xiong X. Q. Yuan Z. W. Zhang L. Yuan Y. J. et al (2023). Regulation of platelet function by cantharidin via PI3K/Akt/PKC pathway. Chin. Pharmacol. Bull.39, 1248–1255. 10.12360/CPB202209015
19
He T. M. Zhang J. Y. Liu L. Li X. F. (2022). Research advances of mylabris-induced hepatorenal toxicity in recent years. Chin. J. Mod. Appl. Pharm.39, 3310–3315. 10.13748/j.cnki.issn1007-7693.2022.24.020
20
He T. M. Chen K. Xiong L. J. Lin K. X. Lu D. Y. Li X. F. et al (2024). Liver injury induced by cantharidin through endoplasmic reticulum stress, autophagy, and apoptosis in rat. Chin. J. Mod. Appl. Pharm.41, 156–165. 10.13748/j.cnki.issn1007-7693.20232191
21
Hou B. C. Wang X. W. He Z. J. Liu H. Y. (2024). Integrative approach using network pharmacology, bioinformatics, and experimental methods to explore the mechanism of cantharidin in treating colorectal cancer. Naunyn Schmiedeb. Arch. Pharmacol.397, 6745–6761. 10.1007/s00210-024-03041-7
22
Hsia T. C. Yu C. C. Hsu S. C. Tang N. Y. Lu H. F. Huang Y. P. et al (2014). Cantharidin induces apoptosis of H460 human lung cancer cells through mitochondria-dependent pathways. Int. J. Oncol.45, 245–254. 10.3892/ijo.2014.2428
23
Hsia T. C. Lin J. H. Hsu S. C. Tang N. Y. Lu H. F. Wu S. H. et al (2015). Cantharidin induces DNA damage and inhibits DNA repair-associated protein levels in NCI-H460 human lung cancer cells. Environ. Toxicol.30, 1135–1143. 10.1002/tox.21986
24
Hsia T. C. Yu C. C. Hsiao Y. T. Wu S. H. Bau D. T. Lu H. F. et al (2016). Cantharidin impairs cell migration and invasion of human lung cancer NCI-H460 cells via UPA and MAPK signaling pathways. Anticancer Res.36, 5989–5997. 10.21873/anticanres.11187
25
Hsieh C. H. Chao K. S. C. Liao H. F. Chen Y. J. (2013). Norcantharidin, derivative of cantharidin, for cancer stem cells. Evid. Based. Complement. Altern. Med.2013, 1–11. 10.1155/2013/838651
26
Hsieh F. S. Hung M. H. Wang C. Y. Chen Y. L. Hsiao Y. J. Tsai M. H. et al (2017). Inhibition of protein phosphatase 5 suppresses non-small cell lung cancer through AMP-Activated kinase activation. Lung Cancer112, 81–89. 10.1016/j.lungcan.2017.07.040
27
Huang W. W. Ko S. W. Tsai H. Y. Chung J. G. Chiang J. H. Chen K. T. et al (2011). Cantharidin induces G2/M phase arrest and apoptosis in human colorectal cancer colo 205 cells through inhibition of CDK1 activity and caspase-dependent signaling pathways. Int. J. Oncol.38, 1067–1073. 10.3892/ijo.2011.922
28
Huang Z. Wang Y. Zhang Y. (2015). Lethal and sublethal effects of cantharidin on development and reproduction of Plutella xylostella (lepidoptera: plutellidae). J. Econ. Entomol.108 (3), 1054–1064. 10.1093/jee/tov057
29
Karras D. J. Farrell S. E. Harrigan R. A. Henretig F. M. Gealt L. (1996). Poisoning from “Spanish fly” (Cantharidin). Am. J. Emerg. Med.14, 478–483. 10.1016/S0735-6757(96)90158-8
30
Kim J. A. Kim Y. Kwon B. M. Han D. C. (2013). The natural compound cantharidin induces cancer cell death through inhibition of heat shock protein 70 (HSP70) and Bcl-2-associated athanogene domain 3 (BAG3) expression by blocking heat shock factor 1 (HSF1) binding to promoters. J. Biol. Chem.288, 28713–28726. 10.1074/jbc.M113.488346
31
Lan H. Y. An P. Liu Q. P. Chen Y. Y. Yu Y. Y. Luan X. et al (2021). Aidi injection induces apoptosis of hepatocellular carcinoma cells through the mitochondrial pathway. J. Ethnopharmacol.274, 114073. 10.1016/j.jep.2021.114073
32
Le A. P. Zhang L. L. Liu W. Shi Y. F. (2016). Cantharidin inhibits cell proliferation and induces apoptosis through G2/M phase cell cycle arrest in hepatocellular carcinoma stem cells. Oncol. Rep.35, 2970–2976. 10.3892/or.2016.4684
33
Li J. (2023). The effect of cantharides on bone marrow cells in mouse and its regulatory mechanism. Henan: Henan University. master. 10.27114/d.cnki.ghnau.2021.000858
34
Li Y. K. Chen Z. (2025). Research progress on processing history evolution, chemical constituents and pharmacological action of Chinese blister beetle. Chin. Arch. Tradit. Chin. Med.43 (05), 202–209. 10.13193/j.issn.1673-7717.2025.05.037
35
Li X. F. Chen X. S. Wang X. M. Hou X. H. (2007). Contents and existing forms of cantharidin in Meloidae(Coleoptera). Acta Entomol. Sin., 750–754. 10.16380/j.kcxb.2007.07.001
36
Li T. Kang G. Wang T. Huang H. (2018). Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol. Lett.16, 687–702. 10.3892/ol.2018.8733
37
Li W. J. Xia H. Jiang H. Xu C. W. Qiu L. (2020). Effect of cantharidin on neointimal hyperplasia after carotid balloon injury in rats. Chin. Circ. J.35, 293–298. 10.3969/j.issn.1000-3614.2020.03.012
38
Li N. Miao M. S. Bai L. (2022). Clinical toxicity mechanism and rescue measures of highly toxic traditional Chinese medicine. Chin. Arch. Tradit. Chin. Med.37, 659–664.
39
Li S. Duan X. Zhang Y. Zhao C. Yu M. Li X. et al (2024). Lipidomics reveals serum lipid metabolism disorders in CTD-induced liver injury. Bmc. Pharmacol. Toxicol.25 (1), 10. 10.1186/s40360-024-00732-y
40
Li. K. M. Li J. J. Wan L. Cheng Y. X. (2023). Five new cantharidin derivatives from the insect Mylabris cichorii L. and their potential against kidney fibrosis in vitro. Basel Switz.28 (6), 2822. 10.3390/molecules28062822
41
Li S. Wu X. Fan G. Du K. Deng L. (2023). Exploring cantharidin and its analogues as anticancer agents: a review. Curr. Med. Chem.30, 2006–2019. 10.2174/0929867330666221103151537
42
Liu X. S. Zhang Y. F. Guo Z. M. Wang Y. (2013). Chemical constituents of Ethyl acetate fraction of Mylabris phalerata. J. Mt. Agric. Biol.32, 187–188. 10.15958/j.cnki.sdnyswxb.2013.02.001
43
Liu T. T. Wang J. Q. Zhao L. J. (2018). Influence of cantharidin on proliferation and apoptosis of malignant melanoma A375 cells and its mechanism. J. QIqihar. Med. Univ.39, 874–878. 10.3969/j.issn.1002-1256.2018.08.002
44
Liu H. B. Shi Y. C. Ren Y. Wan D. G. (2019). Overview of the research on the national medicinal research of cantharides and its related species. Chin. Tradit. Pat. Med.41, 691–694. 10.3969/j.issn.1001-1528.2019.03.046
45
Liu F. Duan C. Zhang J. Li X. (2020). Cantharidin-induced LO2 cell autophagy and apoptosis via endoplasmic reticulum stress pathway in vitro. J. Appl. Toxicol.40, 1622–1635. 10.1002/jat.4022
46
Liu R. Yang C. Yang X. Yu J. Tang W. (2024). Network toxicology, molecular docking technology, and experimental verification revealed the mechanism of cantharidin-induced testicular injury in mice. Toxicol. Appl. Pharmacol.486, 116921. 10.1016/j.taap.2024.116921
47
Liu Y. Song Y. G. Zhao R. S. Miao M. S. (2024). Characteristics and analysis of animal-derived drugs in 2020 edition of Chinese pharmacopoeia. Chin. J. Exp. Tradit. Med. From.30, 218–224. 10.13422/j.cnki.syfjx.20231712
48
Lou T. T. Du J. Chen X. S. Li S. W. (2014). Inhibitory effect of cantharidin on proliferation of prostate cancer PC- 3 cells. J. Mt. Agric. Biol.33, 61–63+68. 10.15958/j.cnki.sdnyswxb.2014.02.018
49
Lou F. M. Li X. F. Liu Y. (2018). Study on the differences of metal elements in cantharides from different regions and varieties. J. South China Normal Univ. Sci. Ed.50 (04), 33–36. 10.6054/j.jscnun.2018061
50
Lu S. Huang J. Zhang J. Wu C. Huang Z. Tao X. et al (2024). The anti-hepatocellular carcinoma effect of aidi injection was related to the synergistic action of cantharidin, formononetin, and isofraxidin through BIRC5, FEN1, and EGFR. J. Ethnopharmacol.319, 117209. 10.1016/j.jep.2023.117209
51
Mo R. Y. Sun N. N. Peng R. (2014). Study on preferred food of adult Mylabris phalerata in different geographical populations. China J. Chin. Mater. Medica.39, 4293–4296.
52
Mo L. Zhang X. Shi X. Wei L. Zheng D. Li H. et al (2018). Norcantharidin enhances antitumor immunity of GM ‐ CSF prostate cancer cells vaccine by inducing apoptosis of regulatory T cells. Cancer. Sci.109, 2109–2118. 10.1111/cas.13639
53
Moed L. Shwayder T. A. Chang M. W. (2001). Cantharidin revisited: a blistering defense of an ancient medicine. Arch. Dermatol.137, 1357–1360. 10.1001/archderm.137.10.1357
54
Moye V. Cathcart S. Burkhart C. N. Morrell D. S. (2013). Beetle juice: a guide for the use of cantharidin in the treatment of molluscum contagiosum. Dermatol. Ther.26, 445–451. 10.1111/dth.12105
55
Nazim U. M. Yin H. Park S. Y. (2020). Downregulation of c FLIP and upregulation of DR 5 by cantharidin sensitizes TRAIL mediated apoptosis in prostate cancer cells via autophagy flux. Int. J. Mol. Med.46, 280–288. 10.3892/ijmm.2020.4566
56
Pan Y. Zheng Q. Ni W. Wei Z. Yu S. Jia Q. et al (2019). Breaking glucose transporter 1/Pyruvate kinase M2 glycolytic loop is required for cantharidin inhibition of metastasis in highly metastatic breast cancer. Front. Pharmacol.10, 590. 10.3389/fphar.2019.00590
57
Pan M. S. Cao J. Fan Y. Z. (2020). Insight into norcantharidin, a small-molecule synthetic compound with potential multi-target anticancer activities. Chin. Med.15, 55. 10.1186/s13020-020-00338-6
58
Pradhan R. N. Shrestha B. Lee Y. (2024). Avoiding cantharidin through ionotropic receptors. J. Hazard. Mater.466, 133497. 10.1016/j.jhazmat.2024.133497
59
Qi L. W. Zhou J. L. Hao H. P. Li H. J. Wen X. D. Chen J. et al (2010). Biological-chemical profiling of in vitro and in vivo bioactive compounds from traditional Chinese medicines with holistic views. J. China. Pharm. Univ.41, 195–202.
60
Qiu L. Q. Xia H. Jiang H. Li W. J. Wu G. Luo Q. et al (2019a). Inhibitory effect of cantharidin on rat vascular smooth muscle cells proliferation and migration by blocking NF-κB signaling pathway. Chin. Circ. J.34, 503–510. 10.3969/j.issn.1000-3614.2019.05.015
61
Qiu L. Q. Xu C. W. Li W. J. Jiang H. Wu G. Luo Q. et al (2019b). Mechanism of cantharidin underlying proliferation and migration of vascular smooth muscle cells induced by PDGF-BB. Chin. J. Geriatr. Heart. Brain. Vessel. Dis.21 (01), 58–62. 10.3969/j.issn.1009-0126.2019.01.015
62
Ratcliffe N. A. Mello C. B. Garcia E. S. Butt T. M. Azambuja P. (2011). Insect natural products and processes: new treatments for human disease. Insect biochem. Mol. Biol.41, 747–769. 10.1016/j.ibmb.2011.05.007
63
Ren Y. Liu H. B. Deng D. Mo R. Y. Wu F. M. (2020). Historical evolution in processing and some problems in modern research of mylabris. Chin. Tradit. Herb. Drugs.51, 4082–4091. 10.7501/j.issn.0253-2670.2020.15.029
64
Safenraiter M. E. Soldini M. P. C. Río M. G. (2024). Cantharidin: a multiporpuse beetlejuice. Neotrop. Entomol.53, 964–971. 10.1007/s13744-024-01164-3
65
Schöpe P. C. Zinnow V. Ishfaq M. A. Smith J. Herrmann P. Shoemaker R. H. et al (2023). Cantharidin and its analogue norcantharidin inhibit metastasis-inducing genes S100A4 and MACC1. Int. J. Mol. Sci.24, 1179. 10.3390/ijms24021179
66
Shao H. Hong G. Luo X. (2014). Evaluation of sodium cantharidinate/vitamin B6 in the treatment of primary liver cancer. J. Cancer Res. Ther.10, 75–78. 10.4103/0973-1482.139770
67
Shen B. He P. J. Shao C. L. (2013). Norcantharidin induced DU145 cell apoptosis through ROS-mediated mitochondrial dysfunction and energy depletion. PLOS. ONE.8, e84610. 10.1371/journal.pone.0084610
68
Song X. X. (2014). Study on quality control methods and toxicokinetics of mylabris and its preparations. Shenyang: Shenyang Pharmaceutical University. master.
69
Song M. Wang X. Luo Y. Liu Z. Tan W. Ye P. et al (2020). Cantharidin suppresses gastric cancer cell migration/invasion by inhibiting the PI3K/Akt signaling pathway via CCAT1. Chem. Biol. Interact.1 (317), 108939. 10.1016/j.cbi.2020.108939
70
Su C. C. Liu S. H. Lee K. I. Huang K. T. Lu T. H. Fang K. M. et al (2015). Cantharidin induces apoptosis through the Calcium/PKC-Regulated endoplasmic reticulum stress pathway in human bladder cancer cells. Am. J. Chin. Med.43, 581–600. 10.1142/S0192415X15500366
71
Su C. C. Lee K. I. Chen M. K. Kuo C. Y. Tang C. H. Liu S. H. (2016). Cantharidin induced oral squamous cell carcinoma cell apoptosis via the JNK-regulated mitochondria and endoplasmic reticulum stress-related signaling pathways. PLOS. ONE.11, e0168095. 10.1371/journal.pone.0168095
72
Sui T. T. Tian L. C. Zhang X. Ma Q. X. Feng Y. Y. Hu X. W. et al (2018). Synergistic effect of cantharidin and pemetrexed on HCT116 colorectal cancer cells. Chin. J. Exp. Tradit. Med. Formulae24, 43–48. 10.13422/j.cnki.syfjx.20181618
73
Sun X. Cai X. Yang J. Chen J. Guo C. Cao P. (2016). Cantharidin overcomes imatinib resistance by depleting BCR-ABL in chronic myeloid leukemia. Mol. Cells.39, 869–876. 10.14348/molcells.2016.0023
74
Vakharia P. P. Chopra R. Silverberg N. B. Silverberg J. I. (2018). Efficacy and safety of topical cantharidin treatment for molluscum contagiosum and warts: a systematic review. Am. J. Clin. Dermatol.19, 791–803. 10.1007/s40257-018-0375-4
75
Viallard C. Larrivée B. (2017). Tumor angiogenesis and vascular normalization: alternative therapeutic targets. Angiogecnesis20, 409–426. 10.1007/s10456-017-9562-9
76
Wang Z. J. (2014). Research on insecticidal technology of the cantharidin and its derivatives. Shanxi: Northwest A&F University. master.
77
Wang B. Cui J. (2014). Treatment of mid-late stage NSCLC using sodium cantharidinate/vitamin B6/GP regimen in clinic. J. Cancer. Res. Ther.10, 79–C81. 10.4103/0973-1482.139771
78
Wang T. Liu J. Xiao X. Q. (2015). Cantharidin inhibits angiogenesis by suppressing VEGF-Induced JAK1/STAT3, ERK and AKT signaling pathways. Arch. Pharm. Res.38, 282–289. 10.1007/s12272-014-0383-8
79
Wang G. Dong J. Deng L. (2018a). Overview of cantharidin and its analogues. Curr. Med. Chem.25, 2034–2044. 10.2174/0929867324666170414165253
80
Wang J. Li G. Yu L. Mo T. Wu Q. Zhou Z. (2018b). Aidi injection plus platinum-based chemotherapy for stage IIIB/IV non-small cell lung cancer: a meta-analysis of 42 RCTs following the PRISMA guidelines. J. Ethnopharmacol.221, 137–150. 10.1016/j.jep.2018.04.013
81
Wang Z. Feng F. Wu Q. Jin Y. Gu C. Xu Y. et al (2019). Disodium cantharidinate and vitamin B6 injection adjunct with platinum-based chemotherapy for the treatment of advanced non-small-cell lung cancer: a meta-analysis. Evid. Based Complement. Altern. Med.2019, 9386273–14. 10.1155/2019/9386273
82
Wang J. Bao L. Du J. Y. Zhang L. F. Wang H. S. (2022). Action mechanism and experimental verification of WBGSF in treating DN based on network pharmacology. Inf. Tradit. Chin. Med.39, 21–26. 10.19656/j.cnki.1002-2406.20221205
83
Wei C. Deng X. Gao S. Wan X. Chen J. (2022). Cantharidin inhibits proliferation of liver cancer by inducing DNA damage via KDM4A-Dependent histone H3K36 demethylation. Evid. Based Complement. Altern. Med.2022, 2197071–2197079. 10.1155/2022/2197071
84
West E. Krychman M. (2015). Natural aphrodisiacs—A review of selected sexual enhancers. Sex. Med. Rev.3, 279–288. 10.1002/smrj.62
85
Wu W. Su M. Li T. Wu K. Wu X. Tang Z. (2015). Cantharidin-induced liver injuries in mice and the protective effect of vitamin C supplementation. Int. Immunopharmacol.28, 182–187. 10.1016/j.intimp.2015.06.003
86
Wu L. Deng C. Zhang H. Weng J. Wu Y. Zeng S. et al (2021). Efficacy and safety of docetaxel and sodium cantharidinate combination vs. either agent alone as second-line treatment for advanced/metastatic NSCLC with wild-type or unknown EGFR status: an open-Label, randomized controlled, prospective, multi-center phase III trial (Cando-L1). Front. Oncol.11, 769037. 10.3389/fonc.2021.769037
87
Xiao W. J. Dai B. Zhu Y. Ye D. W. (2016). Norcantharidin induces autophagy-related prostate cancer cell death through Beclin-1 upregulation by miR-129-5p suppression. Tumor Biol.37, 15643–15648. 10.1007/s13277-015-4488-6
88
Xiao Z. Wang C. Tan Z. Hu S. Chen Y. Zhou M. et al (2019). Clinical efficacy and safety of sodium cantharidinate plus chemotherapy in non-small-cell lung cancer: a systematic review and meta-analysis of 38 randomized controlled trials. J. Clin. Pharm. Ther.44, 23–38. 10.1111/jcpt.12761
89
Xiao Y. Liu R. Tang W. Yang C. (2024). Cantharidin-induced toxic injury, oxidative stress, and autophagy attenuated by Astragalus polysaccharides in mouse testis. Reprod. Toxicol.123, 108520. 10.1016/j.reprotox.2023.108520
90
Xu M. D. (2019). The combination of cantharidin and anti-angiogenic therapeutics presents synergistic antitumor effects against pancreatic cancer. Jiangsu: Soochow University. master.
91
Xu M. D. Liu S. L. Zheng B. B. Wu J. Wu M. Y. Zhang Y. et al (2018). The radiotherapy-sensitization effect of cantharidin: mechanisms involving cell cycle regulation, enhanced DNA damage, and inhibited DNA damage repair. Pancreatology18, 822–832. 10.1016/j.pan.2018.08.007
92
Xu Y. Wang M. Ning S. Yang Z. Zhou L. Xia X. (2022). Development of glycyrrhetinic acid and folate modified cantharidin loaded solid lipid nanoparticles for targeting hepatocellular carcinoma. Mol. Basel. Switz.27, 6786. 10.3390/molecules27206786
93
Yan J. Deng X. L. Ma S. Q. Li Y. H. Gao Y. M. Shi G. T. et al (2023). Cantharidin suppresses hepatocellular carcinoma development by regulating EZH2/H3K27me3-dependent cell cycle progression and antitumour immune response. BMC Complement. Med. Ther.18, 160. 10.1186/s12906-023-03975-0
94
Yang M. Shen C. Zhu S. Zhang Y. Jiang H. Bao Y. et al (2022). Chinese patent medicine aidi injection for cancer care: an overview of systematic reviews and meta-analyses. J. Ethnopharmacol.282, 114656. 10.1016/j.jep.2021.114656
95
Yang T. Yu R. Cheng C. Huo J. Gong Z. Cao H. et al (2023). Cantharidin induces apoptosis of human triple negative breast cancer cells through mir-607-mediated downregulation of EGFR. J. Transl. Med.21, 597. 10.1186/s12967-023-04483-y
96
Yin Y. Jin G. (2010). Biosynthesis transfer and biological function of cantharidin in blister beetles. Acta. entamologica. Sin.53, 1305–1313. 10.16380/j.kcxb.2010.11.010
97
Yu Y. L. Zhang Y. Y. Zhang J. Guan C. Liu L. Ren L. (2020a). Cantharidin‐induced acute hepatotoxicity: the role of TNF‐α, IKK‐α, Bcl‐2, bax and caspase3. J. Appl. Toxicol.40, 1526–1533. 10.1002/jat.4003
98
Yu Z. Li L. Wang C. He H. Liu G. Ma H. et al (2020b). Cantharidin induces apoptosis and promotes differentiation of AML cells through nuclear receptor Nur77-Mediated signaling pathway. Front. Pharmacol.11, 1321. 10.3389/fphar.2020.01321
99
Zeng Y. B. Liu X. L. Li C. J. Zhou X. Zhang X. M. Yang Y. et al (2016). Chemical constituents from Mylabris phalerata and their cytotoxic activity in vitro. China J. Chin. Mater. Medica.41, 859–863. 10.4268/cjcmm20160516
100
Zeng B. Chen X. Zhang L. Gao X. Gui Y. (2024). Norcantharidin in cancer therapy – a new approach to overcoming therapeutic resistance: a review. Med. Baltim.103, e37394. 10.1097/MD.0000000000037394
101
Zhang H. (2021). Effect of cantharidin on radiosensitivity of lung adenocarcinoma A-549 cells. Inner Mongolia: Inner Mongolia Medical University. master.
102
Zhang Y. Y. (2022). Experimental research on toxicological pathology and molecular biology in cardiotoxicity of acute cantharidin poisoning in sprague dawley rats. Zhejiang: Huazhong University of Science and Technology. master.
103
Zhang H. Yan X. (2015). Cantharidin modulates the E2F1/MCM7-miR-106b-93/p21-PTEN signaling axis in MCF-7 breast cancer cells. Oncol. Lett.10, 2849–2855. 10.3892/ol.2015.3681
104
Zhang Z. Y. Yuan F. Zhang X. (2000). Effect of catharidin on the digestive enymes and esterases of diamondback moth larva. Acta. phytophylacica. Sin., 355–358. 10.13802/j.cnki.zwbhxb.2000.04.013
105
Zhang C. Chen Z. Zhou X. Xu W. Wang G. Tang X. et al (2014). Cantharidin induces G2/M phase arrest and apoptosis in human gastric cancer SGC-7901 and BGC-823 cells. Oncol. Lett.8, 2721–2726. 10.3892/ol.2014.2611
106
Zhang Y. Zhou X. Zhang J. Guan C. Liu L. (2018). Cantharides poisoning: a retrospective analysis from 1996 to 2016 in China. Regul. Toxicol. Pharmacol.96, 142–145. 10.1016/j.yrtph.2018.05.007
107
Zhang Y. Yu Y. Zhang J. Guan C. Liu L. Ren L. (2020). Biomarkers of myocardial injury in rats after cantharidin poisoning: application for postmortem diagnosis and estimation of postmortem interval. Sci. Rep.10, 12069. 10.1038/s41598-020-69118-4
108
Zhang Y. Y. Yu Y. L. Zhang J. Guan C. H. Liang L. Liang R. (2020). Molecular biomarkers of cantharidin‐induced cardiotoxicity in sprague‐dawley rats: troponin T, vascular endothelial growth factor and hypoxia inducible factor‐1α. J. Appl. Toxicol.40, 1153–1161. 10.1002/jat.3974
109
Zhang Y. Y. Yu Y. L. Zhang J. Guan C. H. Ren L. Liu L. (2020). Research progress on multiple organ damage and mechanism of cantharidin poisoning. J. Forensic. Med.36, 545–548. 10.12116/j.issn.1004-5619.2020.04.021
110
Zheng L. H. Bao Y. L. Wu Y. Yu C. L. Meng X. Li Y. X. (2008). Cantharidin reverses multidrug resistance of human hepatoma HepG2/ADM cells via down-regulation of P-glycoprotein expression. Cancer. Lett.272, 102–109. 10.1016/j.canlet.2008.06.029
111
Zheng D. Sha Q. M. Wang J. Q. Liu Z. M. Wan X. F. Wang G. C. et al (2015). Effect of Norcantharidin on Hematopoietic Function in Leucopenia Model Rat Induced by Cyclophosphamide. J. Exp. Hematol.23, 826–831. 10.7534/j.issn.1009-2137.2015.03.043
112
Zhongyoo (2012). Jianding. Available online at: http://www.zhongyoo.com/jianding/3004.html (Accessed March 16, 2025).
113
Zhu M. Liu X. Zhou C. Li J. (2020). Effect of sodium cantharidinate/vitamin B6 injection on survival, liver function, immune function, and quality of life in patients with hepatocellular carcinoma: protocol for a meta-analysis. Med. Baltim.99, e21952. 10.1097/MD.0000000000021952
Summary
Keywords
mylabris, cantharidin, pharmacological, toxicity, pharmacokinetics
Citation
Cai Q, Yan J, Li X, He L, Xie S, Yang Y, Wu H and Zhang F (2025) Mylabris: a review of its biological characteristics, chemical composition, pharmacological, toxicology, pharmacokinetics, and marketed drugs. Front. Pharmacol. 16:1652857. doi: 10.3389/fphar.2025.1652857
Received
24 June 2025
Accepted
29 September 2025
Published
05 November 2025
Volume
16 - 2025
Edited by
Rajeev K. Singla, Sichuan University, China
Reviewed by
Aditya Singh Ranout, Institute of Himalayan Bioresource Technology (CSIR), India
Iedo Souza Santos, Universidade do Estado do Pará, Brazil
Updates
Copyright
© 2025 Cai, Yan, Li, He, Xie, Yang, Wu and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongwei Wu, whw9905012@163.com; Fangbo Zhang, fbzhang@icmm.ac.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.