- 1Department of Neurology, Clinical Neuroscience Center, The 7th Affiliated Hospital, Sun Yat-Sen University, Shenzhen, China
- 2Department of Rehabilitation Medicine, The 7th Affiliated Hospital, Sun Yat-Sen University, Shenzhen, China
- 3Shenzhen Key Laboratory of Modern Toxicology, Shenzhen Medical Key Discipline of Health Toxicology (2020-2024), Shenzhen Center for Disease Control and Prevention, Shenzhen, China
Tetramethylpyrazine (TMP), a bioactive alkaloid isolated from the traditional Chinese medicine Ligusticum wallichii (Chuanxiong), has gained significant attention for its therapeutic potential in cerebrovascular diseases and cognitive impairment, mainly due to its antioxidant, anti-inflammatory, and anti-apoptotic properties. However, its clinical application is often limited by suboptimal pharmacokinetic characteristics and modest potency. This review highlights recent advancements in the structure-activity relationship (SAR) optimization of TMP, focusing on its derivatives’ neuroprotective efficacy and vascular benefits. We specifically emphasize the clinical translational potential of several TMP derivatives, such as T-006, TMP-nitrone hybrids (e.g., TN-2), TMP-piperazine derivatives, and TMP-phenolic acid hybrids (e.g., T-VA). These compounds exhibit markedly improved drug-like properties, including enhanced lipid solubility, oral bioavailability, blood-brain barrier (BBB) permeability, and multi-target neuroprotective actions. Additionally, we critically examine the challenges these TMP derivatives face in clinical translation, such as metabolic instability, hepatotoxicity, and formulation challenges, while discussing current strategies to address these issues. The review concludes by emphasizing the significant promise of these next-generation TMP derivatives as therapeutic candidates for cerebrovascular and neurodegenerative disorders, and their need for further preclinical and clinical exploration to fully realize their therapeutic potential.
1 Introduction
Cerebrovascular and cognitive disorders constitute a mounting global health crisis, contributing substantially to disability, mortality, and socioeconomic burden worldwide. The search for effective neurovascular therapeutics remains a pressing priority, especially given the limitations of existing pharmacological options in mitigating neuronal loss and vascular dysfunction. Among natural products, tetramethylpyrazine (TMP)––the principal active alkaloid extracted from Ligusticum chuanxiong Hort––has garnered considerable attention for its multifaceted neurovascular protective properties (Yuan et al., 2020; Li L. et al., 2022) (Figure 1).
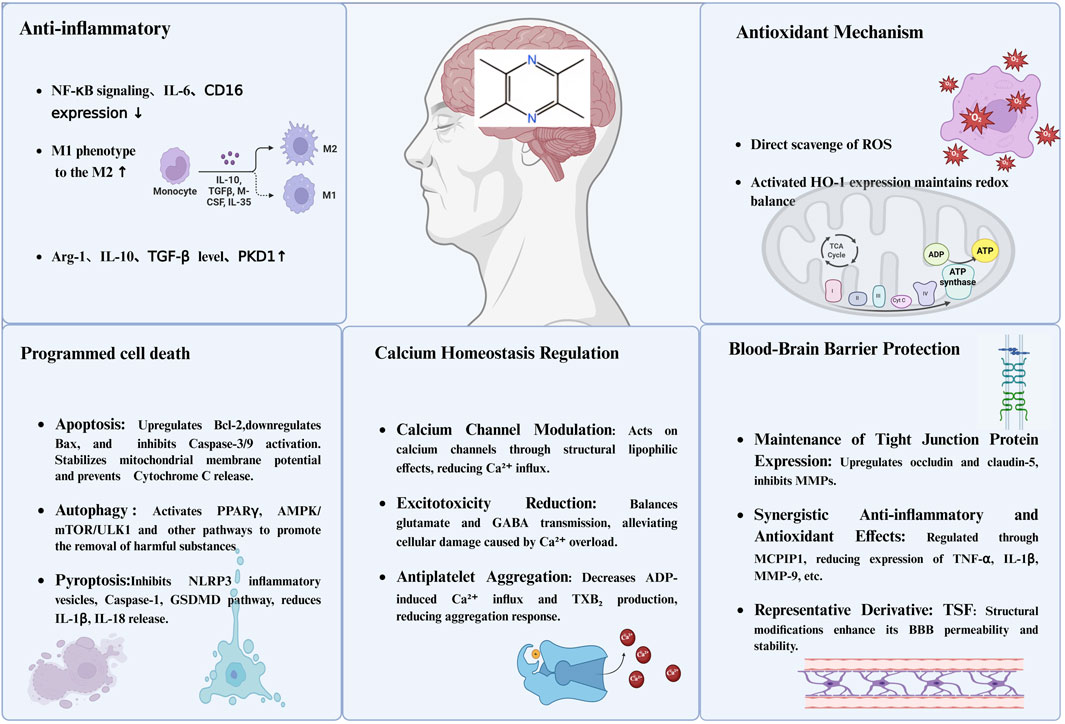
Figure 1. The primary neuroprotective mechanisms of TMP. Notes: This figure illustrates the multifaceted neuroprotective mechanisms of TMP in the central nervous system. (1) Anti-inflammatory effects: TMP downregulates NF-κB signaling, IL-6, and CD16 expression, promotes M1-to-M2 macrophage polarization, and increases levels of anti-inflammatory mediators such as IL-10, TGF-β, and PKD1. (2) Antioxidant mechanisms: It directly scavenges reactive oxygen species (ROS) and induces HO-1 expression to maintain redox homeostasis. (3) Programmed cell death inhibition: TMP modulates apoptosis by upregulating Bcl-2 and inhibiting caspase pathways, promotes autophagy via PPARγ and AMPK/mTOR/ULK1 signaling, and suppresses pyroptosis by inhibiting NLRP3 inflammasome and GSDMD activation. (4) Calcium homeostasis regulation: It modulates calcium channels to reduce Ca2+ influx, prevents excitotoxicity by balancing glutamate and GABA transmission, and reduces platelet aggregation by inhibiting TXB2 production. (5) BBB protection: TMP enhances tight junction protein expression (occludin and claudin-5), suppresses MMP activity, and exhibits synergistic anti-inflammatory and antioxidant effects, thereby improving BBB integrity and permeability.
Extensive preclinical and clinical studies have substantiated TMP’s capacity to ameliorate ischemic stroke, cognitive impairment, and various neurodegenerative conditions through anti-inflammatory, antioxidant, and vasodilatory mechanisms (Lian et al., 2023; Zhao et al., 2016; Lin et al., 2022; Zhang et al., 2021). However, its therapeutic translation is critically hampered by pharmacokinetic drawbacks—most notably, a rapid systemic clearance and low oral bioavailability (Ge et al., 2020)—which limit sustained brain exposure and clinical efficacy.
In response, significant advances in medicinal chemistry have focused on the rational design and structural modification of TMP. Contemporary efforts have yielded diverse TMP derivatives—including piperazine conjugates, cinnamic acid hybrids, and amide analogues (Zou et al., 2018)—that not only augment pharmacokinetic performance but also expand the therapeutic landscape through novel biological activities and mechanisms of action. These derivatives show promise in modulating cerebrovascular integrity, maintaining BBB function, attenuating neuroinflammation, and enhancing cognitive outcomes.
Despite these advances, it is an undeniable fact that the derivatives of TMP developed thus far still exhibit various shortcomings and remain far from achieving excellence. It is imperative to reflect on the future direction of our efforts by analyzing the gains and losses in the development of these derivatives, particularly in enhancing neurovascular protective functions—an area where the parent compound TMP itself has demonstrated significant potential. In a recent effort, Feng et al. (2024) try to outline TMP’s pharmacokinetic challenges and describe SAR-based strategies—such as phenolic acid conjugation and prodrug design—for creating enhanced derivatives with improved efficacy and reduced toxicity. However, there is a conspicuous lack of comprehensive systematic reviews that elucidate the SAR of TMP-based derivatives specifically in the context of cerebrovascular and cognitive function. Bridging this knowledge gap is critical for guiding the next-generation of TMP-inspired drug discovery and development.
Therefore, we systematically categorize derivatives by design strategy and critically dissect the advantages and disadvantages imparted by specific structural changes on critical ADME (absorption, distribution - particularly BBB penetration, metabolism, excretion) parameters. Furthermore, we integrate discussion of advanced strategies employed to overcome these ADME limitations–such as nanodelivery systems, prodrug approaches, and targeted structural optimization–and evaluate their effectiveness. By providing this integrated analysis linking chemical structure to biological activity, ADME properties, and delivery solutions, we intend to offer actionable insights for the rational design and accelerated development of innovative TMP-based therapeutic agents targeting cerebrovascular and cognitive impairment.
2 Molecular structure, pharmacological profile, and SAR principles of TMP
2.1 The parent scaffold
The unique molecular architecture of TMP is the foundation of its diverse pharmacological activities. Its structure consists of a pyrazine core—a six-membered aromatic ring with two nitrogen atoms in para-positions—and four methyl substituents at the 2, 3, 5, and 6 positions (Figure 2).
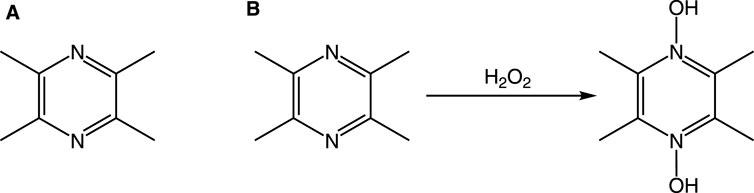
Figure 2. Chemical structure of TMP and its proposed interaction with H2O2. Notes: (A) The structure of TMP features a pyrazine ring with two nitrogen atoms at positions 1 and 4, and four methyl groups (-CH3) at positions 2, 3, 5, and 6. (B) In the presence of an oxidant like H2O2, the nitrogen atoms can be oxidized, for instance, by accepting an oxygen atom to form an N-oxide, without altering the methyl groups. This represents one of its potential antioxidant mechanisms.
This structure imparts a dual nature to the molecule. The pyrazine ring itself is considered the core pharmacophore, a relatively planar and electron-deficient system that underpins TMP’s broad therapeutic effects, including its vasodilatory, neuroprotective, anti-inflammatory, and antioxidant activities (Zheng et al., 2018; Jiang et al., 2020; Li et al., 2020; Ma et al., 2021; Li and Yang, 2022; Liu et al., 2022; Chen et al., 2023). Concurrently, the four symmetrically placed methyl groups increase the molecule’s lipophilicity. This enhancement is critical, as it facilitates TMP’s ability to cross the BBB and engage with a wide range of biological targets within the CNS, establishing its multi-target therapeutic potential (Wang and Wang, 2013).
However, these same structural features also introduce significant clinical limitations. The enhanced lipophilicity that aids BBB penetration also makes TMP susceptible to rapid metabolic clearance in the liver, resulting in a short biological half-life (t1/2 ≈ 2.89 h) and consequently, low bioavailability (Cai et al., 1989). This “double-edged sword” characteristic—whereby the structure enabling its therapeutic action also causes its rapid elimination—is the primary driver for the structural optimization of TMP derivatives.
2.2 Foundational principles of SAR-Driven optimization
The limitations of the parent TMP molecule provide a clear rationale for applying SAR principles to design superior derivatives. The goal is to retain or enhance the therapeutic efficacy of the core pyrazine pharmacophore while systematically modifying the structure to improve its pharmacokinetic profile (ADME) and potency. These research efforts are primarily guided by two integrated principles: (1) Deepening Structure-Mechanism Links: This involves understanding how a specific structural modification directly impacts a biological mechanism. For example, instead of just noting general anti-inflammatory effects, SAR analysis seeks to determine how adding a specific functional group (e.g., a phenolic acid) to the TMP scaffold creates a hybrid molecule that is a more potent inhibitor of a specific inflammatory target, such as the NF-κB signaling pathway. The goal is to translate a defined structural change into a predictable and enhanced mechanistic outcome. (2) Connecting Structure to Pharmacokinetics: This principle focuses on how structural changes directly affect the drug’s journey through the body (Wang and Wang, 2013). A key example is analyzing how replacing a methyl group with a different, bulkier moiety can create steric hindrance. This structural shield can protect the pyrazine ring from rapid metabolism by hepatic enzymes, thereby directly prolonging the molecule’s half-life and increasing its bioavailability. Here, the structural modification is rationally designed to overcome a specific pharmacokinetic barrier.
By applying these principles, researchers have developed a new generation of TMP-based compounds with enhanced activity and more favorable pharmacological profiles, providing a strong foundation for their potential clinical application in cerebrovascular and cognitive disorders.
2.3 Core pharmacological mechanisms of the TMP scaffold
2.3.1 Linking structure to mechanism: anti-inflammatory effects
Chronic inflammation serves as a central pathogenic driver in cerebrovascular and cognitive disorders, orchestrated by pro-inflammatory cytokines including interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β). These mediators propagate oxidative stress, disrupt BBB integrity, and amplify neuroinflammation, culminating in neuronal damage and cognitive decline (Wang et al., 2020; Amanollahi et al., 2023). TMP demonstrates significant anti-inflammatory efficacy attributable to its unique molecular architecture, positioning it as a robust neuroprotective agent for ischemic stroke and Alzheimer’s disease (Li et al., 2018; Huang et al., 2021; Mu et al., 2023). The anti-inflammatory activity of TMP is structurally governed by its electron-deficient pyrazine ring and lipophilic methyl substituents, which collectively enable multi-pathway modulation. Specifically, the pyrazine pharmacophore directly inhibits the TLR4/NF-κB signaling cascade, suppressing nuclear translocation of transcription factors and downregulating synthesis of TNF-α, IL-1β, and IL-6, thereby attenuating inflammatory cell activation and endothelial dysfunction (Zhang et al., 2020; Jiang et al., 2022; Jiang et al., 2023; Alsfouk, 2024). Concurrently, the methyl groups at positions 2,3,5,6 enhance BBB penetration, facilitating interactions with CNS-resident microglia to promote polarization from pro-inflammatory M1 (↓IL-6, ↓CD16) to anti-inflammatory M2 phenotypes (↑Arg-1, ↑IL-10, ↑TGF-β1), effectively resolving neuroinflammation (Feng et al., 2023). The planar configuration of the pyrazine scaffold further enables crosstalk with oxidative pathways through upregulation of Sirt1/NF-κB and Nrf-2/HO-1 axes, enhancing cellular antioxidant capacity and mitigating ROS-driven inflammatory amplification (Li et al., 2019; Huo et al., 2025). Moreover, methyl group-mediated lipophilicity permits vascular compartment access, where TMP scavenges ROS and inhibits matrix metalloproteinase-9 (MMP-9), preserving tight junction proteins (occludin, claudin-5) and blocking leukocyte CNS infiltration (Tan et al., 2015). Strategic structural optimization of TMP further enhances anti-inflammatory potency, exemplified by Tetramethylpyrazine-2′-O-sodium ferulate (TSF), a hybrid derivative integrating TMP with sodium ferulate. This covalent linkage merges dual pharmacophores: the sodium ferulate moiety augments antioxidant capacity (direct ROS scavenging), while the TMP core maintains NF-κB suppression, yielding synergistic anti-inflammatory action. Critically, the polar ferulate group improves aqueous solubility and bioavailability, demonstrating concurrent pharmacokinetic/pharmacodynamic refinement (Xu et al., 2017; Zhou H. et al., 2019). Therapeutically, these structural attributes translate to neurovascular protection across disease models. In Alzheimer’s pathology, lipophilic methyl groups facilitate exosomal mRNA transfer (e.g., CDK5), while the planar pyrazine ring promotes STAT3 activation to enhance synaptic plasticity (Duan et al., 2020; Chen et al., 2024). Vascular homeostasis is restored through endothelial anti-inflammatory effects (↓IL-2/IL-6/IL-1β) (Yang et al., 2019; Ai and Zheng, 2025) and SIRT1/VEGFA-mediated reduction of ischemic infarct volume (Shu et al., 2024). Combinatorial regimens leverage TMP’s properties, such as in the Gardenia-Ligusticum Chuanxiong herb pair, where it regulates MAPK signaling and inhibits vascular apoptosis via FGFR3/AKT activation (Zhang et al., 2024b; Zhang et al., 2024a), while enhancing BMSC homing to ischemic regions through CXCR4 upregulation (Li et al., 2017). In summary, TMP’s anti-inflammatory efficacy is intrinsically governed by its pyrazine-methyI synergy, enabling concurrent targeting of cytokine networks, oxidative stress, and endothelial integrity to address core neurovascular pathology. Future development of structurally optimized derivatives and advanced delivery systems holds significant clinical promise.
2.3.2 Linking structure to mechanism: antioxidant effects
Oxidative stress, driven by excessive generation of reactive oxygen species (ROS) including superoxide anions (O2•-) and hydroxyl radicals (•OH), critically contributes to neuronal injury and vascular damage (Cheung and Vousden, 2022; Teleanu et al., 2022; Angelova and Abramov, 2018; Jomova et al., 2023). Within the CNS, dysregulated enzymes like NADPH oxidase (NOX) serve as primary ROS sources, compromising brain homeostasis and vascular integrity (Zilberter et al., 2023). TMP counteracts these pathological processes through a dual antioxidant mechanism intrinsically governed by its molecular architecture. The antioxidant properties of TMP derive directly from its pyrazine scaffold, operating via complementary pathways: direct radical scavenging and indirect endogenous pathway modulation. For direct scavenging, the electron-rich pyrazine core serves as a redox-active center where hyperconjugation effects from the four methyl groups enhance nitrogen electron-donating capacity, enabling neutralization of diverse ROS including •OH and DPPH• radicals (Donkor et al., 2016). Structurally, this allows oxidation by H2O2 to form TMP-dioxide, directly consuming oxidants and preserving mitochondrial function (Li et al., 2010). Concurrently, TMP’s planar configuration facilitates indirect antioxidant effects through Nrf2/HO-1 pathway activation, promoting nuclear translocation of Nrf2 and subsequent upregulation of cytoprotective enzymes (Wang et al., 2016), while its structural compatibility with endothelial signaling upregulates DDAHII to maintain nitric oxide bioavailability and attenuate ischemia-reperfusion injury (Zhang et al., 2022; Chang et al., 2023). SAR studies demonstrate how targeted modifications enhance antioxidant efficacy, exemplified by HTMP (2-hydroxymethyl-3,5,6-trimethylpyrazine), where strategic replacement of one methyl group with a hydroxyl moiety confers dual advantages (Jiang et al., 2025). The hydroxyl group acts as a potent hydrogen donor, augmenting radical scavenging capacity, while its polarity reduces metabolic susceptibility, extending biological half-life (Chen Xin, 1996; Xuemin et al., 1998). Therapeutically, TMP’s BBB-permeable antioxidant structure mitigates neurodegenerative pathology, reducing Aβ aggregation and tau hyperphosphorylation in Alzheimer’s models via inhibition of oxidative-stress-dependent kinases like GSK-3β (Lu et al., 2017; Feng et al., 2022), while vascular protection is achieved through ROS scavenging that enhances nitric oxide bioavailability (Chang et al., 2015) and mitochondrial stabilization that reduces electron transport chain ROS leakage during ischemia-reperfusion (Ding et al., 2019; Yang et al., 2019). These findings underscore TMP derivatives as potent therapeutic candidates for oxidative stress pathologies, with future research poised to optimize clinical translation through advanced delivery systems.
2.3.3 Linking structure to mechanism: regulation of programmed cell death (PCD)
Programmed cell death (PCD) pathways—including apoptosis, autophagy, and pyroptosis—play complex roles in neurodegenerative and vascular pathogenesis (Tower, 2015). While excessive apoptosis drives neuronal and endothelial loss in conditions like ischemic stroke, protective autophagy maintains cellular homeostasis by clearing damaged organelles (Sorice, 2022; Ekundayo et al., 2024). TMP and its derivatives precisely modulate these pathways through structure-dependent mechanisms (Dhaliwal et al., 2022). For apoptosis inhibition, TMP’s lipophilic methyl groups facilitate membrane penetration and mitochondrial accumulation, while its electron-rich pyrazine core scavenges ROS to prevent mitochondrial depolarization and cytochrome c release (Chang et al., 2023). Structurally, this preserves mitochondrial integrity, suppressing caspase-9/-3 activation while upregulating Bcl-2 and downregulating Bax (Cheng et al., 2009; Li et al., 2017; Tong et al., 2022). The derivative TSF exemplifies SAR-driven enhancement, where sodium ferulate conjugation improves aqueous solubility and introduces a negative surface charge that inhibits platelet aggregation, demonstrating pharmacokinetic and therapeutic dual optimization (Ji et al., 1996). Autophagy modulation is structurally augmented in derivatives like LPD, where a benzhydryl-hexahydropyrimidine side chain creates steric bulk that activates PPARγ, enhancing autophagic flux and mitochondrial energetics in Alzheimer’s models (Li et al., 2022). Pyroptosis inhibition leverages TMP’s redox-active pyrazine ring to disrupt ROS-dependent NLRP3 inflammasome activation, preventing caspase-1 cleavage and Gasdermin D pore formation that would release IL-1β/IL-18 (Zhang et al., 2022; Xu et al., 2025; Vasudevan et al., 2023). Therapeutically, these structural attributes enable multi-pathway neurovascular protection: neuronal differentiation via PI3K/Akt/Sp1 activation (Yan et al., 2015); atherosclerosis reduction through endothelial apoptosis/pyroptosis inhibition and VEGF-driven angiogenesis (Li et al., 2022; Shu et al., 2024); and derivative-enabled dual actions like T-006s concurrent apoptosis blockade and autophagy promotion in Parkinsonian models (Zhou P. et al., 2019). Thus, TMP’s molecular architecture provides a versatile scaffold for regulating cell death/survival balance in cognitive and vascular disorders.
2.3.4 Linking structure to mechanism: regulation of calcium homeostasis
Calcium ion (Ca2+) dysregulation fundamentally drives pathophysiology in neurovascular diseases including ischemic stroke and Alzheimer’s disease (Metwally et al., 2021; Webber et al., 2023), where excessive intracellular Ca2+ overload triggers mitochondrial dysfunction, apoptosis, and excitotoxicity culminating in neuronal death and vascular damage (Garbincius and Elrod, 2022; Rahi and Kaundal, 2024). TMP modulates aberrant Ca2+ signaling through structure-dependent mechanisms anchored in two key physicochemical properties: lipophilic methyl groups facilitating membrane partitioning for direct interaction with calcium channels, and the redox-active pyrazine core protecting these channels from oxidative damage during ischemia (Hu et al., 2017). Structurally, this enables dose-dependent inhibition of pathological Ca2+ influx through voltage-gated channels while balancing excitatory neurotransmitter release (Li et al., 2019; Yu et al., 2019; Jin et al., 2024), thereby preserving mitochondrial function and suppressing Ca2+-dependent inflammatory cascades like TLR4-NLRP3 (Fu et al., 2019). SAR-driven enhancement further optimizes calcium regulation through strategic molecular hybridization, exemplified by nitro-functionalized derivatives that introduce nitric oxide (NO) donation to modulate channels via cGMP pathways, and L-carnitine conjugates that bolster mitochondrial fatty acid metabolism to intrinsically enhance Ca2+ buffering capacity (Wang et al., 2017). Therapeutically, these structural attributes translate to neurovascular protection through attenuated vascular smooth muscle cell (VSMC) hypercontraction and potently inhibited platelet aggregation (Li and Yang, 2022), establishing calcium homeostasis regulation as a cornerstone of TMP’s therapeutic efficacy against cognitive and vascular pathologies.
2.3.5 Linking structure to mechanism: protection of BBB integrity
The BBB constitutes a highly selective endothelial border critical for cerebral homeostasis, whose breakdown through tight junction (TJ) disruption is a pathological hallmark of neurological disorders from ischemic stroke to Alzheimer’s disease (Gu et al., 2011). TMP exhibits dual structure-enabled functions: its small molecular dimensions and methyl group-conferred lipophilicity facilitate efficient BBB penetration, while the redox-active pyrazine core counteracts barrier-degrading processes through antioxidant and anti-inflammatory actions. Mechanistically, TMP preserves TJ proteins (occludin, claudin-5) by inhibiting matrix metalloproteinase (MMP)-mediated extracellular matrix degradation (Gong et al., 2019), while simultaneously suppressing inflammatory signaling cascades (e.g., JAK/STAT pathway) and cytokine-driven MMP upregulation that increase barrier permeability (Xu et al., 2017; Jin et al., 2021). Structural optimization is exemplified by TSF (tetramethylpyrazine-2′-O-sodium ferulate), where the TMP scaffold delivers conjugated ferulate antioxidant moieties across compromised barriers during ischemia-reperfusion, yielding enhanced local ROS scavenging, reduced BBB disruption, diminished infarct volume, and improved neurological outcomes (Xu et al., 2017). This targeted delivery paradigm demonstrates how rational molecular hybridization leverages TMP’s innate biodistribution properties to amplify therapeutic efficacy at pathological sites, reinforcing its multifunctional potential in neurovascular disorders where BBB integrity is compromised.
3 SAR-driven design of novel TMP derivatives for neurovascular protection
The inherent therapeutic potential of the TMP scaffold, combined with its significant pharmacokinetic limitations, has driven extensive research into the rational design of novel derivatives. The primary goal of these medicinal chemistry efforts is to retain or enhance the core pharmacophore’s beneficial activities while systematically overcoming its drawbacks, such as poor bioavailability and short half-life. This chapter reviews several key classes of TMP derivatives, organized by their distinct design strategies, to illustrate how SAR principles have been applied to create next-generation compounds with superior efficacy for cerebrovascular and cognitive disorders.
3.1 Strategy 1: Molecular hybridization for multi-target efficacy (T-006)
3.1.1 Design rationale
The design of T-006 is a prime example of a molecular hybridization strategy. The goal was to create a single chemical entity with multi-target capabilities by combining the structural features of two distinct pharmacophores: the neuroprotective scaffold of J147 and the vasoprotective TMP moiety. The specific aim was to replace a biologically inactive portion of the J147 molecule with TMP, thereby creating a novel compound with synergistic and enhanced neuroprotective and cognitive-enhancing effects.
3.1.2 Structural modification and SAR
T-006 was synthesized by covalently linking TMP to the core structure of J147. Specifically, the methoxyphenyl group on J147, which was found to be non-essential for its activity, was replaced with a TMP molecule via a stable C-C bond (Figure 3). This strategic modification resulted in a hybrid compound with a significantly different physicochemical profile. The introduction of polar functional groups within the core scaffold optimizes the lipid-water partition coefficient, enhancing its ability to penetrate the BBB while improving its overall bioavailability compared to the parent TMP (Wu et al., 2019; Jiehong et al., 2021).
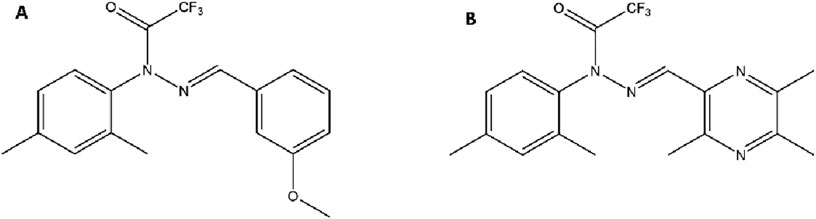
Figure 3. Chemical Structures of the Parent Compound J147 and the Hybrid Derivative T-006. Notes: (A) J147 features a central pyrazole ring linked to a bis(4-methoxyphenyl) group on one side and a phenyl ring with a trifluoroacetamide group on the other. (B) In T-006, one of the methoxyphenyl groups of J147 is strategically replaced with a TMP moiety, connected via a C-C single bond to the pyrazole core.
3.1.3 Pharmacological superiority and mechanisms
This structure-guided hybridization translates into markedly superior pharmacological activity. T-006 exhibits significantly enhanced neuroprotection (EC50 = 59.4 nM) compared to its parent compounds (Xu et al., 2016). This potency is quantified primarily by determining the half-maximal effective concentration (EC50) or half-maximal inhibitory concentration (IC50). EC50measures the concentration of an agonist required to elicit 50% of the maximal response, whereas IC50 measures the concentration of an inhibitor needed to suppress 50% of a biological process (Li et al., 2015). When the EC50/IC50 values of a derivative drop by several-fold or more, the compound is considered a promising candidate for further development (Zhang et al., 1999). Its multi-target mechanism includes: (1) Enhanced Mitochondrial Function: T-006 activates the PKA/Akt/GSK-3β and CREB/PGC-1α signaling networks, promoting mitochondrial biogenesis and restoring cellular energy balance (Zhou H. et al., 2019). (2) Potent Antioxidant and Anti-inflammatory Effects: It powerfully regulates both the mTOR and Nrf2/HO-1 pathways, providing more robust protection against oxidative stress and neuroinflammation than TMP alone (Zhang G. et al., 2024). (3) Synaptic Repair and Neuroregeneration: T-006 upregulates key synaptic proteins like BDNF and synaptophysin, promoting synaptic plasticity and long-term cognitive recovery in models of Alzheimer’s and Parkinson’s disease (Chen et al., 2020).
Despite its advantages, the increased lipophilicity of T-006 compared to TMP raises concerns about potential hepatic accumulation and long-term hepatotoxicity (Qi et al., 2016). Addressing this challenge requires further SAR-driven refinement. A logical next step would be to introduce controlled polarity into the T-006 scaffold—for example, by adding small, hydrophilic groups (e.g., hydroxyl or carboxyl moieties) at non-critical positions. Such modifications could reduce overall lipophilicity and mitigate liver accumulation without compromising BBB permeability or therapeutic efficacy. This represents a classic medicinal chemistry approach to “fine-tuning” a lead compound for a better safety profile.
3.2 Strategy 2: Incorporating a bioactive moiety for enhanced radical scavenging (TBN and TN-2)
3.2.1 Design rationale
This strategy focuses on incorporating a well-known bioactive functional group—a nitrone—directly onto the TMP scaffold. Nitrones are potent “spin traps,” capable of scavenging highly reactive and damaging free radicals. However, standalone nitrone drugs have historically been limited by poor BBB penetration. The design rationale for Tetramethylpyrazine Nitrone (TBN) was to create a hybrid molecule that uses the BBB-permeable TMP scaffold as a “delivery vehicle” to transport the highly effective nitrone trap into the CNS (Cancela et al., 2020; Zhou et al., 2023).
3.2.2 Structural modification and SAR
TBN is created by modifying one of the methyl groups on the TMP pyrazine ring to form a nitrone functional group (Figure 4A). This modification masterfully combines the properties (Figure 4B) of both parent structures. The resulting hybrid molecule, TBN, retains the lipophilicity and small size necessary to readily cross the BBB, achieving therapeutic concentrations in brain tissue that are approximately 68% of plasma levels—a significant improvement over traditional nitrones (Zhang et al., 2016). For TMP derivatives targeting the central nervous system (CNS), adequate blood–BBB permeability is essential. Assessment typically involves the following approaches: in silico models based on physicochemical properties (e.g., logP, molecular weight, polar surface area) can predict BBB penetration (Morales et al., 2017).
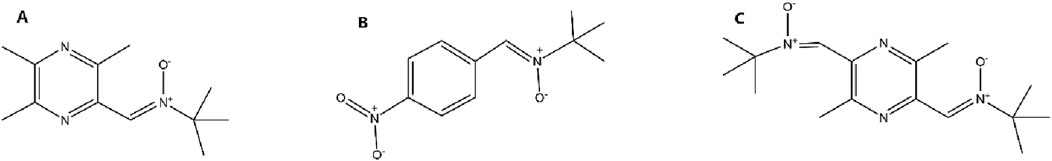
Figure 4. Chemical Structures of a Nitrone Spin Trap, TBN, and its Dual-Nitrone Derivative TN-2. Notes: (A) In TBN, the methyl group at position 3 of the TMP ring is oxidized to form a nitrone functional group. (B) A generic nitrone structure, which acts as a free radical “spin trap.” (C) In TN-2, the methyl groups at both the 3 and 6 positions of the TMP ring are converted into nitrone functional groups, creating a dual-trap molecule.
Building on this success, a second-generation derivative, TN-2, was developed (Figure 5C). The SAR logic here was straightforward: if one nitrone trap is effective, two may be even better. TN-2 incorporates two nitrone groups at positions 3 and 6 of the pyrazine ring (Figure 4C). This modification was designed to increase the molecule’s stoichiometric capacity for scavenging cytotoxic free radicals like hydroxyl radicals, superoxide, and peroxynitrite (Xu et al., 2014).
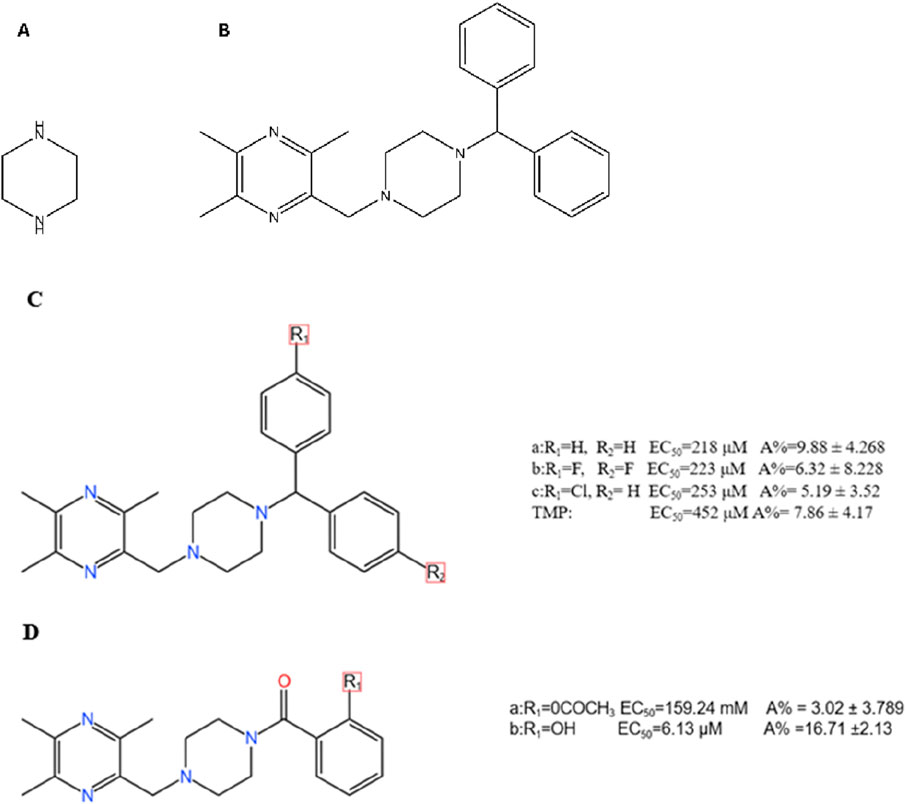
Figure 5. Chemical Structures of the Piperazine Scaffold and Representative TMP-Piperazine Derivatives. Notes: (A) The piperazine scaffold, a six-membered ring with two modifiable nitrogen atoms. (B) A TMP-piperazine derivative where the piperazine ring links the TMP moiety to a biphenylmethyl group. (C,D) Other examples of TMP-piperazine derivatives where various substituents are attached to the piperazine ring to modulate biological activity.
3.2.3 Pharmacological superiority and mechanisms
The incorporation of the nitrone moiety endows TBN with powerful, multi-faceted neuroprotective effects that surpass those of TMP alone: (1) Potent ROS Scavenging: The nitrogen-oxygen bond of the nitrone is highly effective at neutralizing ROS, directly reducing oxidative stress-induced neuronal damage (Sun et al., 2008). (2) Comprehensive Neuroprotection: TBN modulates Ca2+ influx, balances the Bax/Bcl-2 ratio to prevent apoptosis, and activates key pro-survival signaling pathways, including Nrf2/HO-1 and PI3K/Akt (Zhang et al., 2016; Zhang et al., 2018). (3) Enhanced Autophagy and Aβ Clearance: In AD models, TBN activates the AMPK/mTOR/ULK1 autophagy pathway, which is critical for clearing toxic protein aggregates like Aβ (Zhou et al., 2024). (4) Mitochondrial Protection: TBN modulates the AMPK/mTORC1 signaling axis to optimize mitochondrial biogenesis and improve cellular energy metabolism, contributing to its anti-aging effects (Nie et al., 2024; Zhang T. et al., 2024).
3.2.4 Clinical potential and future directions
TBN represents an innovative and successful molecular design. Its excellent BBB penetration, potent multi-target neuroprotective actions, and favorable safety and tolerability profile in early clinical studies make it a highly promising candidate for acute ischemic stroke and chronic neurodegenerative diseases (Cucchiara et al., 2009; Zhu et al., 2024). The development of dual-nitrone derivatives like TN-2 further demonstrates the potential for SAR-guided optimization to enhance radical-scavenging potency. Continued clinical validation is essential to fully realize the therapeutic potential of this class of compounds.
3.3 Strategy 3: Using a linker scaffold for pharmacokinetic and pharmacodynamic optimization (piperazine derivatives)
3.3.1 Design rationale
This strategy employs piperazine, a versatile six-membered heterocyclic ring, as a central linker or scaffold. The rationale is to use piperazine’s unique properties to systematically optimize the parent TMP molecule. The two nitrogen atoms of the piperazine ring offer distinct, modifiable sites: one can anchor the TMP moiety, while the other provides a handle to attach various other pharmacophores (Figure 5A). This design allows medicinal chemists to fine-tune key properties like solubility, basicity, and receptor affinity, thereby improving both the pharmacokinetic profile and the biological activity of the resulting derivatives (Clark et al., 2014; Jain et al., 2021; Brito et al., 2019). The goal is to create novel molecules where the piperazine linker bridges TMP with another functional group to achieve synergistic or enhanced therapeutic effects.
3.3.2 Structural modification and SAR
Early work revealed that the pharmacological effects of TMP were dependent on the pyrazine core, while its pharmacokinetics and toxicity were linked to its substituents (Guohui et al., 1996). This insight spurred the development of piperazine-linked derivatives (Figure 5C). (1) Introducing Benzoyl Groups for Antiplatelet Activity: In one series of compounds, various substituted benzoyl groups were attached to the piperazine linker (Figure 5D). SAR studies showed that this modification dramatically enhanced antiplatelet activity. Compound 5D(b), for example, emerged as a highly potent inhibitor (EC50 = 6.13 μM), far exceeding the efficacy of TMP itself. This demonstrates that using the piperazine linker to introduce an aromatic benzoyl moiety creates a new pharmacophore with strong anti-aggregating properties (Cheng et al., 2007). (2) Introducing Biphenylmethyl Groups for PPARγ Agonism: In a different approach, a bulky biphenylmethyl group was attached to the piperazine linker (Figure 5B). This structural design was intended to create a molecule capable of interacting with the nuclear receptor PPARγ, a key regulator of glucose metabolism with known neuroprotective roles (Prashantha Kumar et al., 2020). This strategy was highly successful, yielding novel PPARγ agonists that are not present in the parent TMP molecule (Li S. et al., 2022).
3.3.3 Pharmacological superiority and mechanisms
The use of the piperazine linker has successfully generated derivatives with novel and enhanced mechanisms of action: (1) Enhanced Antiplatelet and Vasoprotective Effects: The benzoyl-piperazine derivatives achieve their potent antiplatelet effects by effectively inhibiting pathways involved in platelet aggregation, demonstrating a clear gain of function compared to TMP (Cheng et al., 2007). Other derivatives have shown strong cytoprotective effects against H2O2-induced endothelial cell damage (Cheng et al., 2009). (2) Novel PPARγ Agonism for Neuroprotection: The biphenylmethyl-piperazine derivatives act as novel PPARγ agonists. By activating this pathway, they improve mitochondrial autophagy and glucose metabolism in the hippocampus, leading to the alleviation of cognitive deficits in AD models (Li Z. et al., 2022). This represents the successful introduction of an entirely new mechanism of action through rational drug design.
3.3.4 Limitations and future directions
While versatile, the piperazine scaffold has known liabilities. Its inherent basicity can lead to lysosomotropism (accumulation in lysosomes), which can alter intracellular drug distribution and potentially cause off-target effects. Furthermore, achieving an optimal balance between BBB permeability, target engagement, and a long plasma half-life remains a significant challenge. Future research must focus on fine-tuning the physicochemical properties of these derivatives—perhaps by modifying the piperazine ring itself or by exploring bioisosteric replacements—to mitigate these risks while retaining the impressive therapeutic gains.
3.4 Strategy 4: Hybridization with phenolic acids for synergistic antioxidant and anti-inflammatory effects
3.4.1 Design rationale
This strategy involves creating hybrid molecules by linking the TMP scaffold to various naturally occurring phenolic acids, such as vanillic acid, ferulic acid, or salicylic acid. These phenolic acids are known to possess intrinsic neuroprotective, antioxidant, and anti-inflammatory properties (Krzysztoforska et al., 2019; Siddiqui et al., 2019; Ahmad Bainmahfouz et al., 2021). The design rationale is to create a single, synergistic molecule that combines the BBB-penetrating and vasoprotective effects of TMP with the potent antioxidant and anti-inflammatory activities of a phenolic acid. The goal is to produce derivatives with enhanced, multi-faceted neuroprotective efficacy and improved pharmacokinetic profiles compared to administering either compound alone.
3.4.2 Structural modification and SAR
These hybrids are typically formed by connecting TMP to the phenolic acid via an ester, ether, or amide linkage. Linker and Substituent Position: SAR studies have revealed critical structural rules for maximizing efficacy. First, ether-linked derivatives consistently demonstrate superior neuroprotective effects and metabolic stability compared to their ester-linked counterparts. Second, the position of the substituent on the phenolic acid’s benzene ring is crucial; para-substituted compounds exhibit stronger neuroprotective activity than those with ortho- or meta-substitutions (Wang et al., 2013). Example 1: T-VA: The derivative T-VA is a dimeric compound where two TMP-piperazine units are linked to a central vanillic acid molecule (Figure 6A). It exhibits exceptionally strong protective effects on damaged neuronal cells (EC50 = 4.249 μM), significantly outperforming TMP (Zhang et al., 2017). Example 2: TSF: The derivative TSF (tetramethylpyrazine-2′-O-sodium ferulate) links TMP with ferulic acid. This modification not only combines antioxidant effects but also alters the molecule’s electrostatic properties, which significantly inhibits platelet aggregation—a key benefit for treating ischemic conditions (Zhou P. et al., 2019; Wang et al., 2023).
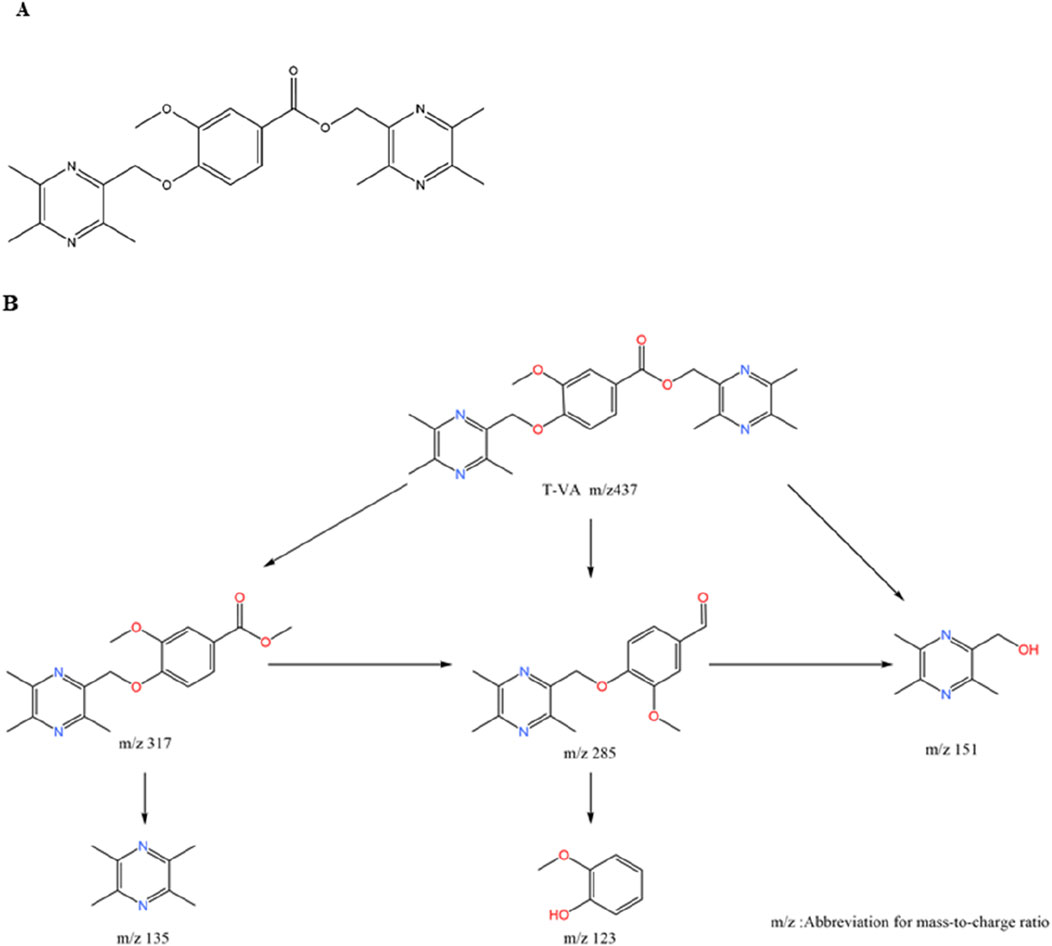
Figure 6. Chemical Structure of T-VA and its Major Metabolite. Notes: (A) T-VA is a dimeric structure where a central vanillic acid molecule is linked via its carboxyl group to two separate TMP-piperazine units. (B) The major metabolite of T-VA is formed by the metabolic cleavage of one of the TMP-piperazine units, leaving a single unit attached to the vanillic acid core.
3.4.3 Pharmacological superiority and mechanisms
The phenolic acid hybrids demonstrate enhanced and synergistic mechanisms of action: (1) Enhanced Neuroprotection via Anti-inflammation: T-VA and related derivatives exert their potent neuroprotective effects by downregulating key inflammatory pathways, including the expression of NF-κB and cyclooxygenase-2 (COX-2) (Wang et al., 2013). Similarly, TSF has been shown to inhibit the TLR4/NF-κB signaling pathway, reducing neuroinflammation and brain edema in stroke models (Zhou H. et al., 2019). (2) Metabolite Activity (Prodrug Effect): T-VA itself is active, but it also functions as a prodrug. In vivo, it is metabolized to a major metabolite that retains significant neuroprotective activity (Chenze et al., 2017) (Figure 6B). This extends the therapeutic duration of action, as the active metabolite continues to provide protection after the parent drug is cleared (Zhang et al., 2017). (3) Synergistic Vasoprotection: Hybrids like T-VA have been shown to upregulate VEGF expression and Ca2+-Mg2+ ATPase activity, promoting angiogenesis and restoring ionic balance, while TSF provides a direct and potent antiplatelet effect (Li et al., 2015).
While highly effective, the metabolic stability of the linker—particularly the ester linkage—remains a key challenge for some of these derivatives. The observation that ether-linked compounds are more stable provides a clear direction for future optimization. Future research should focus on designing phenolic acid hybrids with more robust linkers or exploring advanced prodrug strategies to ensure controlled release of the active moieties in vivo. Fine-tuning the balance between hydrophilicity (for solubility) and lipophilicity (for BBB penetration) will also be critical to maximizing the clinical potential of this promising class of compounds.
4 Advanced drug delivery systems to overcome pharmacokinetic hurdles
While the rational design of derivatives described in Chapter 3 can significantly enhance the therapeutic efficacy of the TMP scaffold, many of these new chemical entities still face pharmacokinetic challenges. Issues such as short half-life, poor aqueous solubility, or potential off-target toxicities (e.g., the risk of hepatic accumulation with highly lipophilic compounds like T-006) can limit their clinical translation. To overcome these hurdles, researchers are developing advanced drug delivery systems designed to control the release, improve the stability, and precisely target the delivery of TMP and its derivatives, thereby maximizing their therapeutic potential while minimizing systemic side effects.
The SAR-driven design of novel TMP derivatives for neurovascular protection systematically optimizes the core scaffold through four key strategies: (1) Molecular hybridization (e.g., T-006 fuses TMP with J147 via C-C bond, replacing J147s non-essential methoxyphenyl to enhance neuroprotection,but requiring polarity adjustments to mitigate hepatotoxicity); (2) Bioactive moiety incorporation (e.g., TBN/TN-2 add nitrone groups at pyrazine C3/C6, boosting ROS scavenging and BBB penetration); (3) Linker scaffold engineering (e.g., piperazine-linked derivatives attach benzoyl/biphenylmethyl groups to enable antiplatelet activity or PPARγ agonism); (4) Phenolic acid hybridization (e.g., T-VA uses ether-linked vanillic acid for synergistic anti-inflammation, with para-substitution critical for efficacy. Advanced delivery systems (ROS-responsive nanoparticles, conductive hydrogels, focused ultrasound) address residual PK challenges (e.g., hepatic accumulation, short half-life) by enabling targeted CNS release and sustained local action, collectively overcoming TMP’s limitations while amplifying its neurovascular benefits (Table 1).
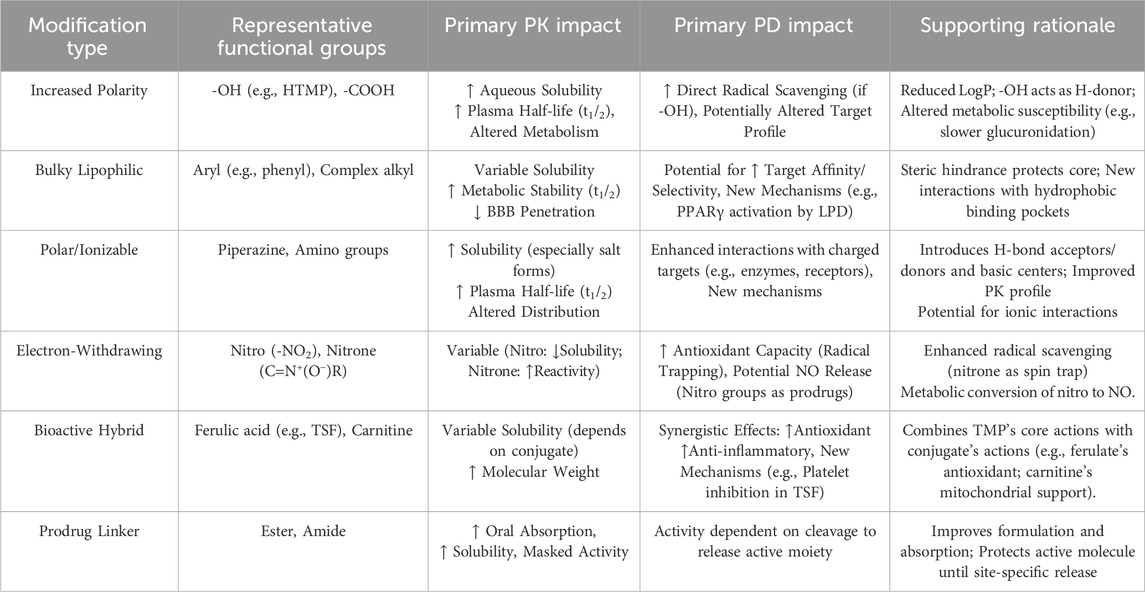
Table 1. Impact of key structural modifications on TMP derivatives’ pharmacokinetics (PK) and pharmacodynamics (PD).
4.1 Nanoparticle-based targeted delivery
Nano-drug delivery systems (NDDS) utilize carriers on the nanometer scale to encapsulate therapeutic agents. This strategy offers several key advantages for TMP-based compounds: it can protect the drug from premature degradation, improve the solubility of poorly soluble derivatives, and, most importantly, achieve targeted delivery to specific tissues. Mechanism and Application:
In a state-of-the-art example, researchers developed nanoparticles with a shell made of a polymer containing reactive oxygen species (ROS)-responsive linkers to encapsulate TMP. The nanoparticle surface was then decorated with a peptide (CFLFLF) that specifically binds to formyl peptide receptors (FPR) on neutrophils. In the inflammatory environment of ischemia-reperfusion injury, these nanoparticles are carried by neutrophils directly to the site of damage. The high local concentration of ROS then breaks the nanoparticle shell, releasing the TMP payload precisely where it is needed. This “smart” delivery system dramatically improves drug accumulation at the target site (Mu et al., 2023). Addressing Derivative Limitations: This approach is particularly promising for potent but potentially toxic derivatives like T-006. By encapsulating the lipophilic drug, nanoparticles can prevent its accumulation in the liver and ensure it is released primarily in the brain, significantly improving its safety profile.
4.2 Conductive hydrogels for sustained local release
For site-specific injuries, such as ischemic stroke or spinal cord injury, maintaining a high local concentration of a therapeutic agent over an extended period is crucial for promoting tissue repair. Conductive hydrogels have emerged as ideal carriers for this purpose. Mechanism and Application: These hydrogels have a three-dimensional network structure and biomechanical properties similar to natural neural tissue (Xu et al., 2020). A gelatin methacrylate-polypyrrole-TMP (GMPT) conductive hydrogel has been developed that can be implanted at the site of injury. The hydrogel maintains the structural integrity of TMP while providing slow, sustained release over time. In spinal cord injury models, this system demonstrated significant efficacy in promoting neural function recovery (Deng et al., 2024). Addressing Derivative Limitations: This technology is perfectly suited for delivering derivatives designed for neuroregeneration. By providing a continuous, localized supply of the drug, it can support long-term processes like neurite outgrowth and synaptic repair without requiring frequent systemic administration.
4.3 Focused ultrasound for on-demand, precise delivery
Focused ultrasound (FUS) offers unparalleled spatiotemporal control over drug delivery. By non-invasively focusing ultrasound waves on a specific region of the brain, it can transiently and safely open the BB or create microchannels in cell membranes (sonoporation). Mechanism and Application: When combined with systemically administered microbubbles, FUS can temporarily increase the permeability of the BBB, allowing drugs like TMP or its derivatives to enter the brain in much higher concentrations, but only at the targeted location. Studies have shown that this ultrasound-mediated delivery enhances TMP’s protective effects against oxidative stress (Zhang et al., 2014; Zhang et al., 2018). Addressing Derivative Limitations: This on-demand, reversible delivery method is a powerful tool for maximizing efficacy while minimizing systemic exposure. It allows clinicians to deliver a potent drug precisely when and where it is needed, overcoming the general challenge of getting drugs into the CNS and reducing the risk of side effects associated with high systemic doses.
5 Translational challenges and future directions
TMP and its derivatives exhibit significant neuroprotective effects and have shown promising efficacy in multiple models of neurodegenerative diseases, including PD and AD (Feng et al., 2024). However, despite their favorable in vitro and animal-model performance, clinical translation still faces numerous challenges, chief among them metabolic instability, hepatotoxicity, and a lack of readiness for human trials (Liu et al., 2025). Moreover, critical issues such as off-target toxicity, interspecies pharmacokinetic differences, and formulation challenges have not been fully addressed, potentially hindering future clinical application.
Metabolic instability represents a major barrier to clinical translation. Many compounds that demonstrate robust efficacy in animal models fail in humans due to rapid or aberrant metabolism leading to loss of activity or toxic metabolites. For example, T-006 may be metabolized in vivo by cytochrome P450 enzymes, whose activity can vary markedly between species, potentially resulting in altered clearance rates and safety profiles in humans compared to preclinical models (Guengerich, 2006). Indeed, the short in vivo half-life of TBN limits its sustained action in vivo (Zhao et al., 2016). Although definitive evidence of T-006–induced hepatotoxicity is lacking, its reliance on hepatic cytochrome P450 metabolism underscores the need to investigate its metabolic fate and potential impact on liver function.
Off-target toxicity is another critical concern in drug development. Prior failures of anticancer agents in clinical trials often stemmed from unintended interactions with proteins other than the intended target, leading to unacceptable toxicity (Lin et al., 2019). While TMP derivatives display strong neuroprotective activity, it remains to be determined whether they provoke toxic effects in non-neural tissues. In particular, T-006s activation of PI3K/AKT/mTOR and Nrf2 pathways—key regulators of cell survival, metabolism, and stress responses—could perturb physiological processes in other organs, increasing the risk of adverse events (Zhang G. et al., 2024).
Interspecies pharmacokinetic differences further complicate the transition from animal models to human subjects. Variations in absorption, distribution, metabolism, and excretion between species can lead to discrepancies in bioavailability and clearance. For instance, although T-006 may exhibit adequate bioavailability and half-life in rodent models, accelerated metabolism in humans could result in rapid clearance and reduced therapeutic efficacy. Such differences may also be influenced by the formulation type—whether oral, controlled-release, or other delivery systems—which affects absorption kinetics and tissue distribution (Bae et al., 2005).
Formulation challenges are likewise pivotal. Researchers have explored several advanced delivery strategies—such as porous osmotic pumps, microemulsions, and transdermal patches—to enhance TMP derivative stability and bioavailability, yet clinical evidence remains sparse (Mei et al., 2008; Wei et al., 2012; Tang et al., 2013). Poor aqueous solubility of T-006, for example, may necessitate nanotechnology-based formulations or liposomal encapsulation to improve systemic exposure. The chosen formulation can profoundly affect both pharmacokinetics and safety, underscoring the importance of appropriate drug-delivery design for successful clinical outcomes.
Despite the compelling neuroprotective data in preclinical systems, the clinical-trial readiness of TMP derivatives warrants further evaluation. Optimal trial design—including dose selection, safety monitoring, and patient stratification—relies on comprehensive preclinical data. Factors such as age, sex, and genetic background can influence pharmacokinetics and pharmacodynamics in human populations and must be considered in trial protocols. Ultimately, rigorous investigation of metabolic pathways, off-target profiles, pharmacokinetic behavior, and formulation strategies, followed by well-controlled clinical studies, is essential to establish the safety and efficacy of TMP and its derivatives. Only by addressing these challenges can these compounds realize their potential as effective neuroprotective agents, offering new hope for the treatment of cognitive and neurovascular disorders.
6 Summary and prospects
This review has chronicled the remarkable evolution of TMP from a single active monomer into a versatile and highly adaptable pharmacophore for treating complex neurovascular and cognitive disorders. While the parent TMP molecule possesses intrinsic multi-target protective effects—including anti-inflammatory, antioxidant, and calcium-regulating activities—its clinical utility is hampered by poor pharmacokinetics. The core theme of this review is the power of medicinal chemistry and SAR principles to systematically overcome these limitations.
Through strategic molecular design, researchers have developed next-generation derivatives with vastly superior properties. Key successful strategies include: (1) Molecular Hybridization: Creating multi-target agents like T-006, which combines distinct pharmacophores to achieve synergistic efficacy. (2) Bioactive Moiety Incorporation: Developing potent radical scavengers like TBN, which leverages the TMP scaffold as a brain-penetrant delivery vehicle for a nitrone “trap.” (3) Synergistic Conjugation: Designing hybrids like TSF (TMP-ferulic acid), which couple TMP with phenolic acids to enhance both vasoprotective and antioxidant effects. Furthermore, the development of advanced drug delivery systems—such as targeted nanoparticles and localized hydrogels—provides a parallel and complementary approach to enhance the therapeutic index of these compounds, ensuring they reach their target site in effective concentrations while minimizing systemic exposure.
The journey of TMP is far from over. To translate the impressive preclinical success of its derivatives into clinical reality, future research should focus on several critical and innovative directions: (1) Next-Generation Medicinal Chemistry: The field should move beyond simple conjugation towards more sophisticated chemical biology approaches. This includes designing covalent or allosteric modulators of specific enzymes or receptors to achieve greater target selectivity and potency. Exploring novel modalities like proteolysis-targeting chimeras (PROTACs) that use the TMP scaffold to recruit E3 ligases for the targeted degradation of pathogenic proteins (e.g., misfolded tau) represents a truly next-generation strategy. (2) Exploring Novel Biological Targets: While current derivatives effectively manage downstream effects like oxidative stress and inflammation, future work should aim to modulate more fundamental upstream drivers of neurodegeneration. Promising targets include the glymphatic system (the brain’s waste clearance pathway) and the accumulation of senescent cells within the neurovascular unit. Designing TMP derivatives that can enhance glymphatic clearance or selectively eliminate senescent cells could offer disease-modifying, rather than merely symptomatic, benefits. (3) Overcoming Hurdles in Clinical Translation: The path to clinical approval requires addressing several key challenges. A top priority is the identification and validation of predictive biomarkers that can identify patient populations most likely to respond to these multi-target agents. Clinical trial designs must also evolve, perhaps employing genetic stratification or biomarker-based enrichment strategies. Finally, for complex derivatives or drug-device combinations (e.g., TSF, GMPT hydrogels), ensuring the scalability and cost-effectiveness of manufacturing will be paramount for successful commercialization and patient access.
By pursuing these advanced strategies, the scientific community can build upon the solid foundation of TMP chemistry to develop truly transformative therapies for Alzheimer’s disease, stroke, and other devastating neurovascular disorders.
Author contributions
XW: Writing – review and editing, Supervision, Writing – original draft, Software. MC: Supervision, Writing – review and editing, Validation. YX: Supervision, Conceptualization, Validation, Writing – review and editing. XY: Supervision, Conceptualization, Writing – review and editing. QH: Conceptualization, Funding acquisition, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Free Exploration Project of Shenzhen Science and Technology Innovation Committee (No. JCYJ20180307150610733), the Provincial Science and Technology Project of Guangdong Province (2021A1515010111), Basic Research Project of Shenzhen Science and Technology Innovation Commission (No. JCYJ20210324121804013), and the Seventh Affiliated Hospital, Sun Yat-sen University Clinical Research 735 Program (ZSQY202373520).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad Bainmahfouz, F. R., Ali, S. S., Al-Shali, R. A., and El-Shitany, N. A. E. A. (2021). Vitamin E and 5-amino salicylic acid ameliorates acrylamide-induced peripheral neuropathy by inhibiting caspase-3 and inducible nitric oxide synthase immunoexpression. J. Chem. Neuroanat. 113, 101935. doi:10.1016/j.jchemneu.2021.101935
Ai, J., and Zheng, J. (2025). Tetramethylpyrazine promotes osteo-angiogenesis during bone fracture repair. J. Orthop. Surg. Res. 20 (1), 58. doi:10.1186/s13018-024-05371-x
Alsfouk, A. (2024). Pyrazine-based small molecule kinase inhibitors: clinical applications and patent review (2019-2023). Future Med. Chem. 16 (18), 1899–1921. doi:10.1080/17568919.2024.2385293
Amanollahi, M., Jameie, M., Heidari, A., and Rezaei, N. (2023). The dialogue between neuroinflammation and adult neurogenesis: mechanisms involved and alterations in neurological diseases. Mol. Neurobiol. 60 (2), 923–959. doi:10.1007/s12035-022-03102-z
Angelova, P. R., and Abramov, A. Y. (2018). Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 592 (5), 692–702. doi:10.1002/1873-3468.12964
Bae, S. K., Lee, S. J., Kim, Y. G., Kim, S. H., Kim, J. W., Kim, T., et al. (2005). Interspecies pharmacokinetic scaling of oltipraz in mice, rats, rabbits and dogs, and prediction of human pharmacokinetics. Biopharm. Drug Dispos. 26 (3), 99–115. doi:10.1002/bdd.437
Brito, A. F., Moreira, L. K. S., Menegatti, R., and Costa, E. A. (2019). Piperazine derivatives with central pharmacological activity used as therapeutic tools. Fundam. Clin. Pharmacol. 33 (1), 13–24. doi:10.1111/fcp.12408
Cai, W., Dong, S., and Lou, Y. Q. (1989). Pharmacokinetic study of orally administered tetramethylpyrazine phosphate in healthy subjects. Acta Pharm. Sin. B 24 (12), 881–886. doi:10.16438/j.0513-4870.1989.12.001
Cancela, S., Canclini, L., Mourglia-Ettlin, G., Hernández, P., and Merlino, A. (2020). Neuroprotective effects of novel nitrones: in vitro and in silico studies. Eur. J. Pharmacol. 871, 172926. doi:10.1016/j.ejphar.2020.172926
Chang, C., Kao, T., Chen, W. Y., Ou, Y. C., Li, J. R., Liao, S. L., et al. (2015). Tetramethylpyrazine inhibits neutrophil activation following permanent cerebral ischemia in rats. Biochem. Biophys. Res. Commun. 463 (3), 421–427. doi:10.1016/j.bbrc.2015.05.088
Chang, C., Wu, C., Pan, P. H., Wang, Y. Y., Lin, S. Y., Liao, S. L., et al. (2023). Tetramethylpyrazine alleviates mitochondrial abnormality in models of cerebral ischemia and oxygen/glucose deprivation reoxygenation. Exp. Neurol. 367, 114468. doi:10.1016/j.expneurol.2023.114468
Chen, H., Cao, J., Zha, L., Wang, P., Liu, Z., Guo, B., et al. (2020). Neuroprotective and neurogenic effects of novel tetramethylpyrazine derivative T-006 in parkinson's disease models through activating the MEF2-PGC1α and BDNF/CREB pathways. Aging (Albany NY) 12 (14), 14897–14917. doi:10.18632/aging.103551
Chen, G., Guo, H., Quan, Z. S., Shen, Q. K., Li, X., and Luan, T. (2023). Natural products-pyrazine hybrids: a review of developments in medicinal chemistry. Molecules 28 (21), 7440. doi:10.3390/molecules28217440
Chen, H., Deng, C., Meng, Z., Zhu, M., Yang, R., Yuan, J., et al. (2024). Combined catalpol and tetramethylpyrazine promote axonal plasticity in Alzheimer's Disease by inducing astrocytes to secrete exosomes carrying CDK5 mRNA and regulating STAT3 phosphorylation. Mol. Neurobiol. 61 (12), 10770–10791. doi:10.1007/s12035-024-04251-z
Chen Xin, D. S. (1996). In vivo metabolite profiling of tetramethylpyrazine in rabbit models. Acta Pharm. Sin. B (08), 59–63. doi:10.16438/j.0513-4870.1996.08.010
Cheng, X., Liu, X., Xu, W. F., Guo, X. L., and Ou, Y. (2007). Design, synthesis, and biological activities of novel Ligustrazine derivatives. Bioorg. Med. Chem. 15 (10), 3315–3320. doi:10.1016/j.bmc.2007.03.033
Cheng, X., Liu, X., Xu, W. F., Guo, X. L., Zhang, N., and Song, Y. N. (2009). Ligustrazine derivatives. Part 3: design, synthesis and evaluation of novel acylpiperazinyl derivatives as potential cerebrocardiac vascular agents. Bioorg. Med. Chem. 17 (8), 3018–3024. doi:10.1016/j.bmc.2009.03.012
Chenze, Z., Mengmeng, Y., Bing, X., Hongying, L., Wenqiang, Y., Shuai, L., et al. (2017). Study on the mass spectrometry fragmentation pattern and metabolic products of the candidate drug T-VA in rats. J. Mass Spectrom. 38 (01), 67–74.
Cheung, E. C., and Vousden, K. H. (2022). The role of ROS in tumour development and progression. Nat. Rev. Cancer 22 (5), 280–297. doi:10.1038/s41568-021-00435-0
Clark, R. B., Lamppu, D., Libertine, L., McDonough, A., Kumar, A., LaRosa, G., et al. (2014). Discovery of novel 2-((pyridin-3-yloxy)methyl)piperazines as α7 nicotinic acetylcholine receptor modulators for the treatment of inflammatory disorders. J. Med. Chem. 57 (10), 3966–3983. doi:10.1021/jm5004599
Cucchiara, B., Kasner, S. E., Tanne, D., Levine, S. R., Demchuk, A., Messe, S. R., et al. (2009). Factors associated with intracerebral hemorrhage after thrombolytic therapy for ischemic stroke: pooled analysis of placebo data from the Stroke-Acute Ischemic NXY Treatment (SAINT) I and SAINT II Trials. Stroke 40 (9), 3067–3072. doi:10.1161/STROKEAHA.109.554386
Deng, B., Jiang, S., Liu, G., Li, X., Zhao, Y., Fan, X., et al. (2024). Tetramethylpyrazine-loaded electroconductive hydrogels promote tissue repair after spinal cord injury by protecting the blood-spinal cord barrier and neurons. J. Mater. Chem. B 12 (18), 4409–4426. doi:10.1039/d3tb02160b
Dhaliwal, J., Dhaliwal, N., Akhtar, A., Kuhad, A., and Chopra, K. (2022). Tetramethylpyrazine Attenuates cognitive impairment via suppressing oxidative stress, neuroinflammation, and apoptosis in type 2 diabetic rats. Neurochem. Res. 47 (8), 2431–2444. doi:10.1007/s11064-022-03640-x
Ding, Y., Du, J., Cui, F., Chen, L., and Li, K. (2019). The protective effect of ligustrazine on rats with cerebral ischemia-reperfusion injury via activating PI3K/Akt pathway. Hum. Exp. Toxicol. 38 (10), 1168–1177. doi:10.1177/0960327119851260
Donkor, P. O., Chen, Y., Ding, L., and Qiu, F. (2016). Locally and traditionally used Ligusticum species - a review of their phytochemistry, pharmacology and pharmacokinetics. J. Ethnopharmacol. 194, 530–548. doi:10.1016/j.jep.2016.10.012
Duan, Y., An, W., Wu, Y., and Wang, J. (2020). Tetramethylpyrazine reduces inflammation levels and the apoptosis of LPS-stimulated human periodontal ligament cells via the downregulation of miR-302b. Int. J. Mol. Med. 45 (6), 1918–1926. doi:10.3892/ijmm.2020.4554
Ekundayo, B. E., Obafemi, T. O., Adewale, O. B., Obafemi, B. A., Oyinloye, B. E., and Ekundayo, S. K. (2024). Oxidative stress, endoplasmic reticulum stress and apoptosis in the pathology of Alzheimer's Disease. Cell Biochem. Biophys. 82 (2), 457–477. doi:10.1007/s12013-024-01248-2
Feng, Y., Wang, K., Wang, N., Jia, P., Zhang, L., Yuan, H., et al. (2022). Tetramethylpyrazine protects neural stem cells against sevoflurane-induced toxicity through Akt/GSK-3β pathway. Metab. Brain Dis. 37 (7), 2457–2466. doi:10.1007/s11011-022-01008-2
Feng, X., Li, M., Lin, Z., Lu, Y., Zhuang, Y., Lei, J., et al. (2023). Tetramethylpyrazine promotes axonal remodeling and modulates microglial polarization via JAK2-STAT1/3 and GSK3-NFκB pathways in ischemic stroke. Neurochem. Int. 170, 105607. doi:10.1016/j.neuint.2023.105607
Feng, F., Xu, D., Yue, S. J., Chen, Y. Y., and Tang, Y. P. (2024). Neuroprotection by tetramethylpyrazine and its synthesized analogues for central nervous system diseases: a review. Mol. Biol. Rep. 51 (1), 159. doi:10.1007/s11033-023-09068-y
Fu, S., Wang, J., Hao, C., Dang, H., and Jiang, S. (2019). Tetramethylpyrazine ameliorates depression by inhibiting TLR4-NLRP3 inflammasome signal pathway in mice. Psychopharmacol. Berl. 236 (7), 2173–2185. doi:10.1007/s00213-019-05210-6
Garbincius, J. F., and Elrod, J. W. (2022). Mitochondrial calcium exchange in physiology and disease. Physiol. Rev. 102 (2), 893–992. doi:10.1152/physrev.00041.2020
Ge, H., Lin, P., Luo, T., Yan, Z., Xiao, J., Miao, S., et al. (2020). Fabrication of Ligusticum chuanxiong polylactic acid microspheres: a promising way to enhance the hepatoprotective effect on bioactive ingredients. Food Chem. 317, 126377. doi:10.1016/j.foodchem.2020.126377
Gong, P., Zhang, Z., Zou, Y., Tian, Q., Han, S., Xu, Z., et al. (2019). Tetramethylpyrazine attenuates blood-brain barrier disruption in ischemia/reperfusion injury through the JAK/STAT signaling pathway. Eur. J. Pharmacol. 854, 289–297. doi:10.1016/j.ejphar.2019.04.028
Gu, Y., Dee, C. M., and Shen, J. (2011). Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Front. Biosci. Sch. Ed. 3 (4), 1216–1231. doi:10.2741/222
Guengerich, F. P. (2006). Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J. 8 (1), E101–E111. doi:10.1208/aapsj080112
Guohui, J., Shizhen, W., Ji, J., Jun, L., and Qiang, T. (1996). Comparative Study of the pharmacological effects of deuterated chuanxiongzine and chuanxiongzine (I): antithrombotic and antiplatelet effects. Chin. Pharmacol. Bull. (02), 133–137.
Hu, N., Chang, H., Du, B., Zhang, Q. W., Arfat, Y., Dang, K., et al. (2017). Tetramethylpyrazine ameliorated disuse-induced gastrocnemius muscle atrophy in hindlimb unloading rats through suppression of Ca(2+)/ROS-mediated apoptosis. Appl. Physiol. Nutr. Metab. 42 (2), 117–127. doi:10.1139/apnm-2016-0363
Huang, X., Yang, J., Huang, X., Zhang, Z., Liu, J., Zou, L., et al. (2021). Tetramethylpyrazine improves cognitive impairment and modifies the hippocampal proteome in two mouse models of Alzheimer's Disease. Front. Cell Dev. Biol. 9, 632843. doi:10.3389/fcell.2021.632843
Huo, L., Zhao, Y., Bai, H., Liu, G., Yang, X., Li, X., et al. (2025). Tetramethylpyrazine exerts a neuroprotective effect in acute spinal cord injury by mitigating oxidative stress through PKD1: Multi-omics analysis and experimental validation. Eur. J. Pharmacol. 998, 177514. doi:10.1016/j.ejphar.2025.177514
Jain, A., Chaudhary, J., Khaira, H., Chopra, B., and Dhingra, A. (2021). Piperazine: a promising scaffold with analgesic and anti-inflammatory potential. Drug Res. (Stuttg) 71 (2), 62–72. doi:10.1055/a-1323-2813
Ji, J., Yunpeng, Y., and Shizhen, W. (1996). Study on the metabolic products of chuanxiongzine in human urine. J. Chin. Acad. Med. Sci. (04), 288–291.
Jiang, G., Xin, R., Yuan, W., Zhang, L., Meng, X., Sun, W., et al. (2020). Ligustrazine ameliorates acute kidney injury through downregulation of NOD2-mediated inflammation. Int. J. Mol. Med. 45 (3), 731–742. doi:10.3892/ijmm.2020.4464
Jiang, R., Xu, J., Zhang, Y., Liu, J., Wang, Y., Chen, M., et al. (2022). Ligustrazine alleviates psoriasis-like inflammation through inhibiting TRAF6/c-JUN/NFκB signaling pathway in keratinocyte. Biomed. Pharmacother. 150, 113010. doi:10.1016/j.biopha.2022.113010
Jiang, T., Wang, C., and Chen, Y. (2023). Tetramethylpyrazine inhibits keratitis and neovascularization induced by corneal alkali burn by suppressing the TLR4/NF-κB pathway activation and NLRP1/NLRP3 inflammasomes in rats. Exp. Eye Res. 237, 109704. doi:10.1016/j.exer.2023.109704
Jiang, X., Li, S., Wang, N., and Li, J. (2025). Ligustrazine as a multitarget scaffold in drug design and discovery. Bioorg. Med. Chem. 121, 118110. doi:10.1016/j.bmc.2025.118110
Jiehong, C., Guiliang, Z., Baojian, G., Yewei, S., Gaoxiao, Z., Yuqiang, W., et al. (2021). T-006 improves learning and memory function in APP/PS1/Tau transgenic mice and regulates the expression of synaptic-related proteins. J. Sun Yat-sen Univ. Med. Sci. Ed. 42 (05), 667–675. doi:10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2021.0504
Jin, Z., Liang, J., and Kolattukudy, P. E. (2021). Tetramethylpyrazine preserves the integrity of blood-brain barrier associated with upregulation of MCPIP1 in a murine model of focal ischemic stroke. Front. Pharmacol. 12, 710358. doi:10.3389/fphar.2021.710358
Jin, H., He, Y., Liu, T., Yang, T., Sun, X., Chen, Y., et al. (2024). Ligustrazine alleviates spinal cord injury-induced neuropathic pain by inhibiting the TLR4/NF-κB signaling pathway. Am. J. Transl. Res. 16 (8), 3557–3571. doi:10.62347/YXRQ5742
Jomova, K., Raptova, R., Alomar, S. Y., Alwasel, S. H., Nepovimova, E., Kuca, K., et al. (2023). Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol. 97 (10), 2499–2574. doi:10.1007/s00204-023-03562-9
Krzysztoforska, K., Mirowska-Guzel, D., and Widy-Tyszkiewicz, E. (2019). Pharmacological effects of protocatechuic acid and its therapeutic potential in neurodegenerative diseases: review on the basis of in vitro and in vivo studies in rodents and humans. Nutr. Neurosci. 22 (2), 72–82. doi:10.1080/1028415X.2017.1354543
Li, H., and Yang, M. (2022). Ligustrazine activates the PPAR-gamma pathway and plays a protective role in vascular calcification. Vascular 30 (6), 1224–1231. doi:10.1177/17085381211051477
Li, S., Jia, Y., Sun, W. G., Tang, Y., An, G. S., Ni, J. H., et al. (2010). Stabilization of mitochondrial function by tetramethylpyrazine protects against kainate-induced oxidative lesions in the rat hippocampus. Free Radic. Biol. Med. 48 (4), 597–608. doi:10.1016/j.freeradbiomed.2009.12.004
Li, G., Li, G., Tian, Y., Zhang, Y., Hong, Y., Hao, Y., et al. (2015). A novel ligustrazine derivative T-VA prevents neurotoxicity in differentiated PC12 cells and protects the brain against ischemia injury in MCAO rats. Int. J. Mol. Sci. 16 (9), 21759–21774. doi:10.3390/ijms160921759
Li, M., Chou, J., King, K. W., Jing, J., Wei, D., and Yang, L. (2015). ICECAP: an integrated, general-purpose, automation-assisted IC50/EC50 assay platform. J. Lab. Autom. 20 (1), 32–45. doi:10.1177/2211068214562449
Li, L., Chu, L., Fang, Y., Yang, Y., Qu, T., Zhang, J., et al. (2017). Preconditioning of bone marrow-derived mesenchymal stromal cells by tetramethylpyrazine enhances cell migration and improves functional recovery after focal cerebral ischemia in rats. Stem Cell Res. Ther. 8 (1), 112. doi:10.1186/s13287-017-0565-7
Li, S., Xiao, X., Ni, X., Ye, Z., Zhao, J., and Hang, C. (2017). Tetramethylpyrazine protects against early brain injury after experimental Subarachnoid hemorrhage by affecting mitochondrial-dependent Caspase-3 apoptotic pathway. Evid. Based Complement. Altern. Med. 2017, 3514914. doi:10.1155/2017/3514914
Li, G., Hong, G., Li, X., Zhang, Y., Xu, Z., Mao, L., et al. (2018). Synthesis and activity towards Alzheimer's disease in vitro: tacrine, phenolic acid and ligustrazine hybrids. Eur. J. Med. Chem. 148, 238–254. doi:10.1016/j.ejmech.2018.01.028
Li, L., Chen, H., Shen, A., Li, Q., Chen, Y., Chu, J., et al. (2019). Ligustrazine inhibits platelet activation via suppression of the Akt pathway. Int. J. Mol. Med. 43 (1), 575–582. doi:10.3892/ijmm.2018.3970
Li, Y., Zhu, Z., Zhang, T., and Zhou, Y. (2019). Ligustrazine attenuates inflammation and oxidative stress in a rat model of arthritis via the Sirt1/NF-κB and Nrf-2/HO-1 pathways. Arch. Pharm. Res. 42 (9), 824–831. doi:10.1007/s12272-018-1089-0
Li, G., Liu, S., Wang, H., Pan, R., Tang, H., Yan, X., et al. (2020). Ligustrazine ameliorates lipopolysaccharide-induced neurocognitive impairment by activating autophagy via the PI3K/AKT/mTOR pathway. Int. J. Mol. Med. 45 (6), 1711–1720. doi:10.3892/ijmm.2020.4548
Li, L., Zhang, D., Yao, W., Wu, Z., Cheng, J., Ji, Y., et al. (2022). Ligustrazine exerts neuroprotective effects via circ_0008146/miR-709/Cx3cr1 axis to inhibit cell apoptosis and inflammation after cerebral ischemia/reperfusion injury. Brain Res. Bull. 190, 244–255. doi:10.1016/j.brainresbull.2022.10.011
Li, S., Liu, P., Feng, X., Wang, Y., Du, M., and Wang, J. (2022). The role and mechanism of tetramethylpyrazine for atherosclerosis in animal models: a systematic review and meta-analysis. PLoS One 17 (5), e0267968. doi:10.1371/journal.pone.0267968
Li, Z., Meng, X., Liu, W., Li, W., and Cai, Q. (2022). Increasing brain glucose metabolism by ligustrazine piperazine ameliorates cognitive deficits through PPARγ-dependent enhancement of mitophagy in APP/PS1 mice. Alzheimer's Res. Ther. 14 (1), 150. doi:10.1186/s13195-022-01092-7
Lian, N., Tong, J., Zhu, W., Meng, Q., Jiang, M., Bian, M., et al. (2023). Ligustrazine and liguzinediol protect against doxorubicin-induced cardiomyocytes injury by inhibiting mitochondrial apoptosis and autophagy. Clin. Exp. Pharmacol. Physiol. 50 (11), 867–877. doi:10.1111/1440-1681.13811
Lin, A., Giuliano, C. J., Palladino, A., John, K. M., Abramowicz, C., Yuan, M. L., et al. (2019). Off-target toxicity is a common mechanism of action of cancer drugs undergoing clinical trials. Sci. Transl. Med. 11 (509), eaaw8412. doi:10.1126/scitranslmed.aaw8412
Lin, J., Wang, Q., Zhou, S., Xu, S., and Yao, K. (2022). Tetramethylpyrazine: a review on its mechanisms and functions. Biomed. Pharmacother. 150, 113005. doi:10.1016/j.biopha.2022.113005
Liu, Y., Yang, G., Cui, W., Zhang, Y., and Liang, X. (2022). Regulatory mechanisms of tetramethylpyrazine on central nervous system diseases: a review. Front. Pharmacol. 13, 948600. doi:10.3389/fphar.2022.948600
Liu, X., Shang, H., Wei, Q., Yao, X., Lian, L., Dang, J., et al. (2025). Tetramethylpyrazine nitrone in amyotrophic lateral sclerosis: a randomized clinical trial. JAMA Netw. Open 8 (2), e2461055. doi:10.1001/jamanetworkopen.2024.61055
Lu, F., Li, X., Li, W., Wei, K., Yao, Y., Zhang, Q., et al. (2017). Tetramethylpyrazine reverses intracerebroventricular streptozotocin-induced memory deficits by inhibiting GSK-3β. Acta Biochim. Biophys. Sin. (Shanghai) 49 (8), 722–728. doi:10.1093/abbs/gmx059
Ma, X., Ruan, Q., Ji, X., Yang, J., and Peng, H. (2021). Ligustrazine alleviates cyclophosphamide-induced hepatotoxicity via the inhibition of Txnip/Trx/NF-κB pathway. Life Sci. 274, 119331. doi:10.1016/j.lfs.2021.119331
Mei, D., Mao, S., Sun, W., Wang, Y., and Kissel, T. (2008). Effect of chitosan structure properties and molecular weight on the intranasal absorption of tetramethylpyrazine phosphate in rats. Eur. J. Pharm. Biopharm. 70 (3), 874–881. doi:10.1016/j.ejpb.2008.06.031
Metwally, E., Zhao, G., and Zhang, Y. Q. (2021). The calcium-dependent protease calpain in neuronal remodeling and neurodegeneration. Trends Neurosci. 44 (9), 741–752. doi:10.1016/j.tins.2021.07.003
Morales, J. F., Montoto, S. S., Fagiolino, P., and Ruiz, M. E. (2017). Current State and future perspectives in QSAR models to predict blood-brain barrier penetration in central nervous System drug R&D. Mini Rev. Med. Chem. 17 (3), 247–257. doi:10.2174/1389557516666161013110813
Mu, Q., Yao, K., Syeda, M. Z., Zhang, M., Cheng, Q., Zhang, Y., et al. (2023). Ligustrazine nanoparticle hitchhiking on neutrophils for enhanced therapy of cerebral ischemia-reperfusion injury. Adv. Sci. (Weinh) 10 (19), e2301348. doi:10.1002/advs.202301348
Nie, L., He, K., Qiu, C., Li, Q., Xiong, B., Gao, C., et al. (2024). Tetramethylpyrazine Nitrone alleviates D-galactose-induced murine skeletal muscle aging and motor deficits by activating the AMPK signaling pathway. Biomed. Pharmacother. 173, 116415. doi:10.1016/j.biopha.2024.116415
Prashantha Kumar, B. R., Kumar, A. P., Jose, J. A., Prabitha, P., Yuvaraj, S., Chipurupalli, S., et al. (2020). Minutes of PPAR-gamma agonism and neuroprotection. Neurochem. Int. 140, 104814. doi:10.1016/j.neuint.2020.104814
Qi, S., Qin, W., Weili, W., Min, S., Yu, Z., Zaijun, Z., et al. (2016). The improvement effect of new drug T-006 on learning and memory in model animals with scopolamine-induced memory impairment. Chin. Pharmacol. Bull. 32 (06), 812–817.
Rahi, V., and Kaundal, R. K. (2024). Exploring the intricacies of calcium dysregulation in ischemic stroke: insights into neuronal cell death and therapeutic strategies. Life Sci. 347, 122651. doi:10.1016/j.lfs.2024.122651
Shu, M., Dai, Y., Song, L. J., Ma, D., Liu, K. X., Miao, Z. Y., et al. (2024). Tetramethylpyrazine regulates angiogenesis of endothelial cells in cerebral ischemic stroke injury via SIRT1/VEGFA signaling pathway. Zhongguo Zhong Yao Za Zhi 49 (1), 162–174. doi:10.19540/j.cnki.cjcmm.20231116.303
Siddiqui, S., Kamal, A., Khan, F., Jamali, K. S., and Saify, Z. S. (2019). Gallic and vanillic acid suppress inflammation and promote myelination in an in vitro mouse model of neurodegeneration. Mol. Biol. Rep. 46 (1), 997–1011. doi:10.1007/s11033-018-4557-1
Sorice, M. (2022). Crosstalk of autophagy and apoptosis. Cells 11 (9), 1479. doi:10.3390/cells11091479
Sun, Y., Jiang, J., Zhang, Z., Yu, P., Wang, L., Xu, C., et al. (2008). Antioxidative and thrombolytic TMP nitrone for treatment of ischemic stroke. Bioorg. Med. Chem. 16 (19), 8868–8874. doi:10.1016/j.bmc.2008.08.075
Tan, F., Fu, W., Cheng, N., Meng, D. I., and Gu, Y. (2015). Ligustrazine reduces blood-brain barrier permeability in a rat model of focal cerebral ischemia and reperfusion. Exp. Ther. Med. 9 (5), 1757–1762. doi:10.3892/etm.2015.2365
Tang, Z., Wang, Q., Xu, H., and Zhang, W. (2013). Microdialysis sampling for investigations of tetramethylpyrazine following transdermal and intraperitoneal administration. Eur. J. Pharm. Sci. 50 (3-4), 454–458. doi:10.1016/j.ejps.2013.08.012
Teleanu, D. M., Niculescu, A., Roza, E., Vladâcenco, O., and Grumezescu, A. M. (2022). Neurotransmitters-Key factors in neurological and neurodegenerative disorders of the central nervous System. Int. J. Mol. Sci. 23 (11), 5954. doi:10.3390/ijms23115954
Tong, H., Wang, K., Wang, X., and Lu, T. (2022). Molecular mechanism of tetramethylpyrazine ameliorating neuroexcitotoxicity through activating the PKA/CREB signaling pathway. Biomed. Res. Int. 2022, 2812839. doi:10.1155/2022/2812839
Tower, J. (2015). Programmed cell death in aging. Ageing Res. Rev. 23 (Pt A), 90–100. doi:10.1016/j.arr.2015.04.002
Vasudevan, S. O., Behl, B., and Rathinam, V. A. (2023). Pyroptosis-induced inflammation and tissue damage. Semin. Immunol. 69, 101781. doi:10.1016/j.smim.2023.101781
Wang, L., and Wang, P. (2013). Structural-Activity relationship advances in tetramethylpyrazine derivatives: a therapeutic perspective. Chin. Herb. Med. 44 (19), 2766–2771.
Wang, P., Zhang, H., Chu, F., Xu, X., Lin, J., Chen, C., et al. (2013). Synthesis and protective effect of new ligustrazine-benzoic acid derivatives against CoCl2-induced neurotoxicity in differentiated PC12 cells. Molecules 18 (10), 13027–13042. doi:10.3390/molecules181013027
Wang, C., Wang, P., Zeng, W., and Li, W. (2016). Tetramethylpyrazine improves the recovery of spinal cord injury via Akt/Nrf2/HO-1 pathway. Bioorg. Med. Chem. Lett. 26 (4), 1287–1291. doi:10.1016/j.bmcl.2016.01.015
Wang, Z., Zhou, Z., Wei, X., Wang, M., Wang, B. O., Zhang, Y., et al. (2017). Therapeutic potential of novel twin compounds containing tetramethylpyrazine and carnitine substructures in experimental ischemic stroke. Oxid. Med. Cell Longev. 2017, 7191856. doi:10.1155/2017/7191856
Wang, X., Zhang, B., Xia, R., and Jia, Q. Y. (2020). Inflammation, apoptosis and autophagy as critical players in vascular dementia. Eur. Rev. Med. Pharmacol. Sci. 24 (18), 9601–9614. doi:10.26355/eurrev_202009_23048
Wang, G., Guo, J., Ma, Y., Xin, Y., Ji, X., Sun, Y., et al. (2023). Ferulic acid alleviates carp brain damage and growth inhibition caused by avermectin by modulating the Nrf2/Keap1 and NF-κB signaling pathways. Pestic. Biochem. Physiol. 196, 105590. doi:10.1016/j.pestbp.2023.105590
Webber, E. K., Fivaz, M., Stutzmann, G. E., and Griffioen, G. (2023). Cytosolic calcium: judge, jury and executioner of neurodegeneration in Alzheimer's disease and beyond. Alzheimer's Dement. 19 (8), 3701–3717. doi:10.1002/alz.13065
Wei, L., Marasini, N., Yong, C. S., Kim, J. O., and Quan, Q. (2012). Development of ligustrazine-loaded lipid emulsion: formulation optimization, characterization and biodistribution. Int. J. Pharm. 437 (1-2), 203–212. doi:10.1016/j.ijpharm.2012.08.027
Wu, L., Cao, J., Li, N., Chen, H., Liu, W., Zhang, G., et al. (2019). Novel neuroprotective tetramethylpyrazine analog T-006 promotes neurogenesis and neurological restoration in a rat model of stroke. Neuroreport 30 (9), 658–663. doi:10.1097/WNR.0000000000001256
Xu, J., Tsai, Y.-L., and Hsu, S.-H. (2020). Synthetic methods of phosphonopeptides. Molecules 25 (22), 5894. doi:10.3390/molecules25245894
Xu, D., Zhang, K., Zhang, Z. J., Sun, Y. W., Guo, B. J., Wang, Y. Q., et al. (2014). A novel tetramethylpyrazine bis-nitrone (TN-2) protects against 6-hydroxyldopamine-induced neurotoxicity via modulation of the NF-κB and the PKCα/PI3-K/Akt pathways. Neurochem. Int. 78, 76–85. doi:10.1016/j.neuint.2014.09.001
Xu, D., Chen, H., Mak, S., Hu, S., Tsim, K. W. K., Hu, Y., et al. (2016). Neuroprotection against glutamate-induced excitotoxicity and induction of neurite outgrowth by T-006, a novel multifunctional derivative of tetramethylpyrazine in neuronal cell models. Neurochem. Int. 99, 194–205. doi:10.1016/j.neuint.2016.07.006
Xu, S., Yin, M., Liu, B., Chen, M. L., He, G. W., Zhou, P. P., et al. (2017). Tetramethylpyrazine-2'-O-sodium ferulate attenuates blood-brain barrier disruption and brain oedema after cerebral ischemia/reperfusion. Hum. Exp. Toxicol. 36 (7), 670–680. doi:10.1177/0960327116657401
Xu, K., Zhang, M., Zou, X., and Wang, M. (2025). Tetramethylpyrazine confers protection against oxidative stress and NLRP3-Dependent pyroptosis in rats with endometriosis. Organogenesis 21 (1), 2460261. doi:10.1080/15476278.2025.2460261
Xuemin, C., Yongxiao, C., Guanwu, L., Zhanquan, L., and Xuefei, L. (1998). Pharmacological effects of 2-Hydroxymethyl-3,5,6-Trimethylpyrazine on the cardiovascular System. China Pharm. Industry J. (06), 18–20. doi:10.16522/j.cnki.cjph.1998.06.007
Yan, Y., Zhao, J., Han, S., Zhou, N. J., Jia, Z. Q., Yao, S. J., et al. (2015). Tetramethylpyrazine induces SH-SY5Y cell differentiation toward the neuronal phenotype through activation of the PI3K/Akt/Sp1/TopoIIβ pathway. Eur. J. Cell Biol. 94 (12), 626–641. doi:10.1016/j.ejcb.2015.09.001
Yang, B., Li, H., Qiao, Y., Zhou, Q., Chen, S., Yin, D., et al. (2019). Tetramethylpyrazine Attenuates the endotheliotoxicity and the mitochondrial dysfunction by Doxorubicin via 14-3-3γ/Bcl-2. Oxid. Med. Cell Longev. 2019, 5820415. doi:10.1155/2019/5820415
Yu, B., Zhong, F., Yao, Y., Deng, S. Q., Xu, H. Q., Lu, J. F., et al. (2019). Synergistic protection of tetramethylpyrazine phosphate and borneol on brain microvascular endothelium cells injured by hypoxia. Am. J. Transl. Res. 11 (4), 2168–2180.
Yuan, X., Han, B., Feng, Z. M., Jiang, J. S., Yang, Y. N., and Zhang, P. C. (2020). Three new compounds from the rhizome of Ligusticum chuanxiong and their anti-inflammation activities. J. Asian Nat. Prod. Res. 22 (10), 920–926. doi:10.1080/10286020.2020.1803291
Zhang, J. H., Chung, T. D., and Oldenburg, K. (1999). A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen 4 (2), 67–73. doi:10.1177/108705719900400206
Zhang, C., Teng, F., Tu, J., and Zhang, D. (2014). Ultrasound-enhanced protective effect of tetramethylpyrazine against cerebral ischemia/reperfusion injury. PLoS One 9 (11), e113673. doi:10.1371/journal.pone.0113673
Zhang, Z., Zhang, G., Sun, Y., Szeto, S. S. W., Law, H. C. H., Quan, Q., et al. (2016). Tetramethylpyrazine nitrone, a multifunctional neuroprotective agent for ischemic stroke therapy. Sci. Rep. 6, 37148. doi:10.1038/srep37148
Zhang, C., Yan, W., Zhao, R., Xu, B., Fang, X., Yan, M., et al. (2017). Design, synthesis and evaluation of new ligustrazine derivatives as potential plasma-stable neuroprotective agents. Medchemcomm 8 (3), 652–656. doi:10.1039/c7md00003k
Zhang, C., Shen, M., Teng, F., Li, P., Gao, F., Tu, J., et al. (2018). Ultrasound-Enhanced protective effect of tetramethylpyrazine via the ROS/HIF-1A signaling pathway in an in vitro Cerebral Ischemia/Reperfusion Injury model. Ultrasound Med. Biol. 44 (8), 1786–1798. doi:10.1016/j.ultrasmedbio.2018.04.005
Zhang, G., Zhang, T., Wu, L., Zhou, X., Gu, J., Li, C., et al. (2018). Neuroprotective effect and mechanism of action of tetramethylpyrazine nitrone for ischemic stroke therapy. Neuromolecular Med. 20 (1), 97–111. doi:10.1007/s12017-018-8478-x
Zhang, H., Tang, W., Wang, S., Zhang, J., and Fan, X. (2020). Tetramethylpyrazine inhibits platelet adhesion and inflammatory response in vascular endothelial cells by inhibiting P38 MAPK and NF-κB signaling pathways. Inflammation 43 (1), 286–297. doi:10.1007/s10753-019-01119-6
Zhang, Q., Wang, J., Zhu, L., Jiang, S. J., Liu, J., Wang, L. X., et al. (2021). Ligustrazine attenuates hyperhomocysteinemia-induced alzheimer-like pathologies in rats. Curr. Med. Sci. 41 (3), 548–554. doi:10.1007/s11596-021-2379-1
Zhang, H., Chen, H., Wu, X., Sun, T., Fan, M., Tong, H., et al. (2022). Tetramethylpyrazine alleviates diabetes-induced high platelet response and endothelial adhesion via inhibiting NLRP3 inflammasome activation. Phytomedicine 96, 153860. doi:10.1016/j.phymed.2021.153860
Zhang, Y., Ma, C., He, L., Liao, L., Guo, C., Wang, C., et al. (2022). Tetramethylpyrazine protects endothelial injury and antithrombosis via antioxidant and antiapoptosis in HUVECs and zebrafish. Oxid. Med. Cell Longev. 2022, 2232365. doi:10.1155/2022/2232365
Zhang, Y., Li, D., Jia, Z., Mei, J., and Wang, Y. (2024). Zhizi-chuanxiong herb pair alleviates atherosclerosis progression in ApoE(-/-) mice by promoting the methylation of FGFR3 to inhibit MAPK/ERK-mediated apoptosis. J. Ethnopharmacol. 319 (Pt 1), 117188. doi:10.1016/j.jep.2023.117188
Zhang, Y., Qi, Y., Jia, Z., Li, Y., Wu, L., Zhou, Q., et al. (2024). Effects and mechanisms of Zhizi chuanxiong herb pair against atherosclerosis: an integration of network pharmacology, molecular docking, and experimental validation. Chin. Med. 19 (1), 8. doi:10.1186/s13020-023-00874-x
Zhang, G., Liang, Z., Wang, Y., Zhang, Z., and Hoi, P. M. (2024). Tetramethylpyrazine analogue T-006 protects neuronal and endothelial cells against oxidative stress via PI3K/AKT/mTOR and Nrf2 signaling. Antioxidants (Basel) 13 (10), 1272. doi:10.3390/antiox13101272
Zhang, T., Jing, M., Fei, L., Zhang, Z., Yi, P., Sun, Y., et al. (2024). Tetramethylpyrazine nitrone delays the aging process of C. elegans by improving mitochondrial function through the AMPK/mTORC1 signaling pathway. Biochem. Biophys. Res. Commun. 723, 150220. doi:10.1016/j.bbrc.2024.150220
Zhao, Y., Liu, Y., and Chen, K. (2016). Mechanisms and clinical application of tetramethylpyrazine (an interesting natural compound isolated from ligusticum wallichii): current status and perspective. Oxid. Med. Cell Longev. 2016, 2124638. doi:10.1155/2016/2124638
Zheng, Q., Huang, Y., Zhu, P. C., Tong, Q., Bao, X. Y., Zhang, Q. H., et al. (2018). Ligustrazine exerts cardioprotection in animal models of myocardial Ischemia/Reperfusion Injury: Preclinical evidence and possible mechanisms. Front. Pharmacol. 9, 729. doi:10.3389/fphar.2018.00729
Zhou, X., Huang, K., Wang, Y., Zhang, Z., Liu, Y., Hou, Q., et al. (2023). Evaluation of therapeutic effects of tetramethylpyrazine nitrone in Alzheimer's disease mouse model and proteomics analysis. Front. Pharmacol. 14, 1082602. doi:10.3389/fphar.2023.1082602
Zhou, X., Zhu, Z., Kuang, S., Huang, K., Li, Y., Wang, Y., et al. (2024). Tetramethylpyrazine nitrone (TBN) reduces amyloid beta deposition in Alzheimer's Disease models by Modulating APP Expression, BACE1 activity, and autophagy pathways. Pharm. (Basel) 17 (8), 1005. doi:10.3390/ph17081005
Zhou, H., Shao, M., Yang, X., Li, C., Cui, G., Gao, C., et al. (2019). Tetramethylpyrazine analogue T-006 exerts neuroprotective effects against 6-Hydroxydopamine-Induced Parkinson's Disease in vitro and in vivo. Oxid. Med. Cell Longev. 2019, 8169125. doi:10.1155/2019/8169125
Zhou, P., Du, S., Zhou, L., Sun, Z., Zhuo, L. H., He, G., et al. (2019). Tetramethylpyrazine‑2'O‑sodium ferulate provides neuroprotection against neuroinflammation and brain injury in MCAO/R rats by suppressing TLR-4/NF-κB signaling pathway. Pharmacol. Biochem. Behav. 176, 33–42. doi:10.1016/j.pbb.2018.08.010
Zhu, G., Wang, L., Zhong, S., Han, S., Peng, H., Tong, M., et al. (2024). Pharmacokinetics, safety profile, and tolerability of tetramethylpyrazine nitrone tablets after single and multiple ascending doses in healthy Chinese volunteers. Eur. J. Drug Metab. Pharmacokinet. 49 (2), 207–217. doi:10.1007/s13318-024-00877-5
Zilberter, Y., Tabuena, D. R., and Zilberter, M. (2023). NOX-induced oxidative stress is a primary trigger of major neurodegenerative disorders. Prog. Neurobiol. 231, 102539. doi:10.1016/j.pneurobio.2023.102539
Keywords: tetramethylpyrazine, pharmacology, pharmacodynamics, structure-activity relationships, cerebrovascular function, cognition
Citation: Wang X, Cao M, Xu Y, Yang X and Hou Q (2025) From a traditional medicine monomer to a modern neurotherapeutic scaffold: a review of SAR-Driven tetramethylpyrazine derivatives for cerebrovascular and cognitive health. Front. Pharmacol. 16:1653056. doi: 10.3389/fphar.2025.1653056
Received: 24 June 2025; Accepted: 31 July 2025;
Published: 21 August 2025.
Edited by:
Xiao-Yuan Mao, Xiangya Hospital Central South University, ChinaReviewed by:
Zhonghao Li, Beijing University of Chinese Medicine, ChinaIlhem Selatnia, University of Oum El Bouaghi, Algeria
Satsawat Visansirikul, Mahidol University Faculty of Pharmacy, Thailand
Copyright © 2025 Wang, Cao, Xu, Yang and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinghua Hou, aG91cWluZ2h1YUBzeXN1c2guY29t; Xifei Yang, eGlmZWl5YW5nQGdtYWlsLmNvbQ==; Yi Xu, eHV5aUBzeXN1c2guY29t
 Xiaodi Wang
Xiaodi Wang Muhan Cao
Muhan Cao Yi Xu
Yi Xu Xifei Yang
Xifei Yang Qinghua Hou
Qinghua Hou