Abstract
Background:
The present study aimed to evaluate the cost-effectiveness of pembrolizumab combined with chemotherapy versus placebo plus chemotherapy for patients with previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer from the perspective of the Chinese healthcare system.
Methods:
A Markov model was developed to track patients’ transitions over 3-week cycles and evaluate the health and economic outcomes over a 10-year horizon for the two competing treatments. The survival data were gathered from the KEYNOTE-355 trial, and cost and utility values were obtained from the published studies. Total costs, life-years, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio (ICER) were the model outcomes. We conducted analysis based on patients’ programmed death-ligand 1 (PD-L1) combined positive score (CPS), including subgroups with CPS≥10, CPS≥1, and the intention-to-treat population. One-way sensitivity analysis, probabilistic sensitivity analysis and scenario analysis were performed to examine the robustness of the model results.
Results:
In the base case analysis for patients highly expressing PD-L1 (CPS≥10), pembrolizumab plus chemotherapy yielded a marginal cost of $85,838.75 and an additional 0.47 QALYs, resulting in an ICER of $184,030.56 per additional QALY gained, which exceeded the willingness-to-pay (WTP) threshold of $38,224 per QALY in China. And the ICERs were $319,506.90/QALY for patients lowly expressing PD-L1 (CPS≥1) and $776,786.75/QALY for the intention-to-treat population. Sensitivity analyses confirmed the robustness of the model outcomes. Scenario analysis demonstrated that price reductions for pembrolizumab could enhance its likelihood of achieving cost-effectiveness.
Conclusion:
The findings of this cost-effectiveness analysis suggest that pembrolizumab plus chemotherapy was not a cost-effective treatment for patients with previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer in China.
1 Introduction
Breast cancer is the most prevalent malignancy among women globally, with 2,308,897 new cases worldwide in 2022, making it the leading cause of cancer-related deaths among women (Bray et al., 2024). Triple-negative breast cancer (TNBC), a clinically aggressive subtype, is characterized by the absence of key receptors—specifically estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) (Patel et al., 2024). TNBC is notorious for its aggressive clinical behavior, characterized by high invasiveness, a pronounced propensity for early metastasis, elevated recurrence rates following treatment, and ultimately, a dismal prognosis with limited therapeutic options (Cho et al., 2021). Due to its poor prognosis and lack of actionable targets for targeted therapies, chemotherapy has long been the primary treatment option for TNBC (Ye et al., 2023). However, its limited efficacy and significant toxicity profile underscore the critical unmet need for novel therapeutic strategies. In recent years, immunotherapy has made great progress in patients with TNBC, particularly for PD-1-positive patients. Immune checkpoint inhibitors (ICIs) have brought more survival benefits to patients, which would enhance the anti-tumor immune function of T cells by inhibiting the immune checkpoint and ligand binding on the surface of patients’ immune cells, thus playing a role in killing tumor cells (Zhang et al., 2021). It is a novel treatment model for patients with previously untreated locally recurrent inoperable or metastatic TNBC. Pembrolizumab is a humanized IgG4 monoclonal antibody with high specificity of binding to the programmed cell death-protein 1 (PD-1), effectively blocking its immunosuppressive signaling pathway and enabling enhanced antitumor immune responses (Kwok et al., 2016).
Recently, Javier Cortes et al. conducted a randomized, double-blind phase three trial, KEYNOTE-355, to assess the efficacy and safety of pembrolizumab combined with chemotherapy in comparison to placebo alongside chemotherapy for patients diagnosed with previously untreated locally recurrent inoperable or metastatic TNBC (Cortes et al., 2020). Compared to the group receiving placebo and chemotherapy, participants receiving pembrolizumab plus chemotherapy represented improvements in terms of overall survival (OS) and progression-free survival (PFS). For patients with combined positive score (CPS)≥10, pembrolizumab plus chemotherapy could significantly improve median OS (23.0 months vs. 16.1 months, hazard ratio [HR] = 0.73, 95% confidence interval [CI]: 0.55–0.95]) and median PFS (9.7 months vs. 5.6 months, HR = 0.65, 95% CI: 0.49–0.86]) in comparison with placebo and chemotherapy. For patients with CPS≥1 and the intention-to-treat population, the median PFS was 7.6 months vs. 5.6 months (HR = 0.74, 95% CI: 0.61–0.90) and 7.5 months vs. 5.6 months (HR = 0.82, 95% CI: 0.69–0.97), respectively. Despite this, no substantial enhancement in OS was observed. Meanwhile, pembrolizumab plus chemotherapy exhibited a manageable safety profile in patients with TNBC. However, there is a lack of comprehensive evaluation regarding the cost-effectiveness of pembrolizumab in combination with chemotherapy across the three subgroups. Cost-effectiveness analysis will not only offer valuable insights for clinicians and policymakers but also play an important role in the decision to reimburse healthcare providers for pharmaceuticals worldwide. The objective of our current study was to assess the cost-effectiveness of pembrolizumab plus chemotherapy compared with placebo plus chemotherapy for patients with previously untreated locally recurrent inoperable or metastatic TNBC from the perspective of Chinese healthcare system.
2 Methods
2.1 Analytical overview and model structure
A Markov model including three mutually exclusive health states named progression-free survival (PFS), progressed disease (PD) and death was established to analyze the costs and clinical outcomes. In the model, patients received two competing first-line treatments: pembrolizumab plus chemotherapy or placebo plus chemotherapy once every 3 weeks. And the time horizon of the model was set to 10 years, in order to capture most future outcomes and costs associated with the treatment strategies. All patients obtained from the KEYNOTE-355 trial were at the PFS state when entered the model, as the disease continued to progress, patients may remain in the PFS state or enter the state of PD or Death (Figure 1). The primary outcomes measured were total costs, quality-adjusted life-years (QALYs) and the incremental cost-effectiveness ratio (ICER). An annual discount rate of 5% was used to account for the costs and benefits (Liu et al., 2020). All the costs were converted to 2024 US dollars (US $1 = CNY 7.309). The willingness-to-pay (WTP) threshold was set as three times of per capita gross domestic product (GDP) of China in 2023 ($38,224/QALY) based on the WHO recommendations (Thokala et al., 2018; Eichler et al., 2004).
FIGURE 1
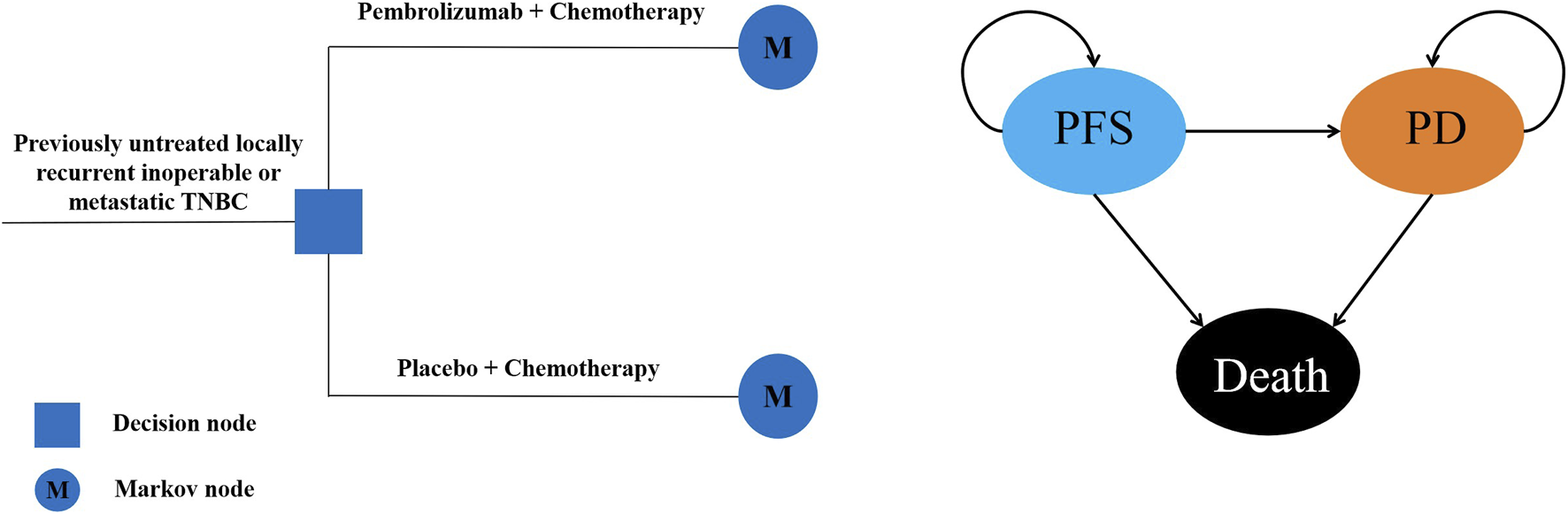
Structure of Markov model PFS, progression-free survival; PD, progressed disease.
2.2 Clinical data and transition probabilities
The survival data used in the model were obtained from the KEYNOTE-355 trial, which specifically enrolled patients with previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer, identical to our target population. The trial stratified patients by programmed death-ligand 1 (PD-L1) CPS, with efficacy analysis conducted in three populations: the CPS≥10 subgroup, the CPS≥1 subgroup (which includes all CPS≥10 patients) and the intention-to-treat population. Long-term survival data were obtained by fitting parametric distributions and extrapolating the PFS curve and OS curves from this clinical trial. In the Markov model, time-dependent transition probabilities between states were then calculated using the parameters derived from these survival curves.
We gathered the time and survival rate from the original K-M curves by using the GetData Graph Digitizer V2.26 software. After that, we reconstructed the individual patient data (IPD) and conducted parametric distribution fitting for the survival curves by using the R language 4.4.2 software. The parametric survival model includes exponential, Gamma, Weibull, Log-normal, Log-logistic, Gompertz and Royston/Parmar spline model (Andersson et al., 2011; Royston and Parmar, 2002). To address uncertainty in model selection, we employed a systematic approach. The optimal model was selected based on the Akaike information criterion (AIC), Bayesian Information Criterion (BIC), and visual inspection between the fitting model and K-M curves (Williams et al., 2017). The smaller the AIC and BIC values, the better of the model fitting (Ishak et al., 2013). The AIC and BIC values of the all parametric survival models were shown in Supplementary Table S1. The distribution parameters of the each curve were shown in Table 1. Log-normal survival function S(t) = 1−Φ[(lnt−μ)/σ], log-logistic survival function S(t) = 1/(1+λtγ) and generalized gamma survival function were used to calculate the time-dependency transition probabilities (Djalalov et al., 2019).
TABLE 1
| Patients with CPS≥10 | PFS | OS | ||
|---|---|---|---|---|
| Model | Parameters | Model | Parameters | |
| Pembrolizumab plus chemotherapy | Log-normal | Meanlog = 2.3487 Sdlog = 1.2258 |
Log-normal | Meanlog = 3.1454 Sdlog = 1.1058 |
| Placebo plus chemotherapy | Log-logistic | Shape = 1.763 Scale = 6.618 |
Log-logistic | Shape = 1.702 Scale = 16.631 |
| Patients with CPS≥1 | PFS | OS | ||
|---|---|---|---|---|
| Model | Parameters | Model | Parameters | |
| Pembrolizumab plus chemotherapy | Generalized gamma | Mu = 1.8691 Sigma = 1.1493 |
Log-logistic | Shape = 1.641 Scale = 17.819 |
| Placebo plus chemotherapy | Log-logistic | Shape = 1.922 Scale = 6.190 |
Log-logistic | Shape = 1.902 Scale = 16.164 |
| Intention-to-treat patients | PFS | OS | ||
|---|---|---|---|---|
| Model | Parameters | Model | Parameters | |
| Pembrolizumab plus chemotherapy | Generalized gamma | Mu = 1.8910 Sigma = 1.1408 |
Log-logistic | Shape = 1.7151 Scale = 17.2814 |
| Placebo plus chemotherapy | Log-logistic | Shape = 1.816 Scale = 6.068 |
Log-logistic | Shape = 1.870 Scale = 15.734 |
Survival model parameters fitting to the PFS and OS data from the KEYNOTE-355 trial.
PFS: progression-free survival, OS: overall survival.
CPS≥1: PD-L1 CPS, of 1 or higher, CPS≥10: PD-L1 CPS, of 10 or higher.
We assumed that the transition probability of PFS state to Death (pFTD) was equal to the mortality rate of the Chinese general population. The probability of remaining in the PFS state in the next cycle (pFTF) was calculated using the formula: pFTF = S(t)/S (t-1), where S(t) represents the survival probability at time t. Consequently, the transition probability from PFS to PD, denoted as pFTP, was derived as: pFTP = 1- pFTD - pFTF. Similarly, the transition probability from overall survival (including patients in both PFS and PD states) to survival (pSTS) was estimated. Based on this, the transition probability from PD to PD (pPTP) was calculated using the following formula: pPTP = [(nPFS + nPD)*pSTS - (nPFS*pFTF) - (nPFS*pFTP)]/nPD, where nPFS and nPD represent the number of patients in the PFS and PD states, respectively, during the previous Markov cycle. The transition probability from PD to death (pPTD) was then determined as: pPTD = 1 - pPTP.
2.3 Cost and utility value
The study was conducted from the perspective of the Chinese healthcare system. Therefore, only direct medical costs were involved, including the drug costs of the pembrolizumab and first-line chemotherapy, supportive treatment, routine follow-up, palliative treatment, and management of treatment-related serious adverse events (SAEs, grade≥3). Grade ≥3 adverse event (AE) incidence rates were extracted directly from the KEYNOTE-355 trial protocol. In our study, it was assumed that the body surface area of patients was 1.72 m2, the body weight was 65 kg, and the height was 1.64 m, which were used to calculate the drug dosages and estimate drug costs (Zhu et al., 2018). The prices of therapeutic drugs were obtained from the YAOZHI database as the average of the bid prices for drug procurement around the country (Yaozhi, 2025). The other relevant cost data were from published literature, and costs were adjusted according to the consumer price index (CPI) (National Bureau of Statistics, 2025). Health-state utilities, measured on a scale from 0 (death) to 1 (perfect health), were assigned to approximate QALYs. The utility values of health states were derived from published literature, and the utility values of PFS, PD, and Death were 0.76, 0.55, and 0, respectively (Cai et al., 2024). The specific parameters and their distributions are shown in Table 2.
TABLE 2
| Parameter | Range | ||||
|---|---|---|---|---|---|
| Baseline value | Lower bound | Upper bound | Distribution | References | |
| Cost values (US $) | |||||
| Pembrolizumab per 100 mg | 2,451.60 | 1,838.70 | 3,064.50 | Gamma | Yaozhi (2025) |
| Gemcitabine per 100 mg | 18.64 | 13.98 | 23.30 | Gamma | Yaozhi (2025) |
| Carboplatin per 100 mg | 7.06 | 5.29 | 8.82 | Gamma | Yaozhi (2025) |
| Nab-paclitaxel per 100 mg | 20.25 | 15.19 | 25.31 | Gamma | Yaozhi (2025) |
| Paclitaxel per 100 mg | 24.75 | 18.56 | 30.94 | Gamma | Yaozhi (2025) |
| Anemia per event | 607.06 | 455.3 | 758.83 | Gamma | Zheng et al. (2024) |
| Neutropenia per event | 547.5 | 410.63 | 684.38 | Gamma | Zheng et al. (2024) |
| Neutrophil count decreased per event | 104.95 | 78.71 | 131.19 | Gamma | Zhu et al. (2023) |
| Supportive treatment per cycle | 359 | 269.25 | 448.75 | Gamma | Zheng et al. (2024) |
| Routine follow-up per cycle | 170 | 127.5 | 212.5 | Gamma | Zhu et al. (2022) |
| Terminal care | 2,325.75 | 1,744.34 | 2,907.24 | Gamma | Liao and Kang (2024) |
| Utility values | |||||
| PFS | 0.76 | 0.57 | 0.95 | Beta | Cai et al. (2024) |
| PD | 0.55 | 0.41 | 0.69 | Beta | Cai et al. (2024) |
| Disutility of toxicities | |||||
| Anemia | −0.029 | −0.022 | −0.036 | Beta | Liu et al. (2021) |
| Neutropenia | −0.012 | −0.050 | −0.083 | Beta | Mistry et al. (2018) |
| Neutrophil count decreased | 0.2 | 0.149 | 0.498 | Beta | Gong et al. (2022) |
| Risk of SAEs in pembrolizumab plus chemotherapy group | |||||
| Anemia | 0.165 | 0.134 | 0.196 | Beta | Cortes et al. (2020) |
| Neutropenia | 0.297 | 0.259 | 0.335 | Beta | Cortes et al. (2020) |
| Neutrophil count decreased | 0.174 | 0.142 | 0.206 | Beta | Cortes et al. (2020) |
| Risk of SAEs in placebo plus chemotherapy group | |||||
| Anemia | 0.146 | 0.104 | 0.188 | Beta | Cortes et al. (2020) |
| Neutropenia | 0.299 | 0.245 | 0.353 | Beta | Cortes et al. (2020) |
| Neutrophil count decreased | 0.203 | 0.156 | 0.251 | Beta | Cortes et al. (2020) |
| Others | |||||
| Discount rate | 0.05 | 0 | 0.08 | Beta | Liu et al. (2020) |
| Body area surface/m2 | 1.72 | 1.29 | 2.15 | Beta | Yue et al. (2021) |
Model inputs: baseline values and ranges for sensitivity analyses.
PFS: progression-free survival, PD: progressed disease.
2.4 Sensitivity analyses and scenario analysis
One-way sensitivity analyses and probabilistic sensitivity analyses (PSA) were conducted to examine the robustness of model results when parameters changed. In the one-way sensitivity analysis, the impact of a single variable change on the ICER value was calculated one by one according to the upper and lower limit ranges of the variables. The reasonable range for each parameter was determined based on the 95% confidence intervals derived from published studies or by assuming ±25% of the base-case values when the relevant data were not available, and the results of one-way sensitivity analysis were shown in the Tornado diagram (Zhang et al., 2012). To further assess uncertainty, we conducted PSA using Monte Carlo simulations with 1,000 replications. Key model parameters were jointly sampled from the pre-specified statistical distribution: Gamma distributions for cost data, and Beta distributions for utility values and incidence rates. This approach allowed us to evaluate the robustness of extrapolated survival outcomes, with results visualized in cost-effectiveness scatter plots and acceptability curves. Recently, the average price reduction for drugs included in the National Reimbursement Drug List (NRDL) demonstrated a significant upward trend, especially the price discount of some PD-1/PD-L1 inhibitors. We systematically assessed the impact of varying pembrolizumab price discount scenarios on ICERs to provide valuable information for policymakers.
3 Results
3.1 Base case analysis
From the Chinese healthcare system perspective, in 10-year time horizon, for patients with previously untreated locally recurrent inoperable or metastatic TNBC, pembrolizumab plus chemotherapy could bring more clinical benefit and cost compared with placebo plus chemotherapy. For patients with CPS≥10, pembrolizumab plus chemotherapy yielded a marginal cost of $85,838.75 and an additional 0.47 QALYs, resulting in an ICER of $184,030.56 per additional QALY gained, which was higher than the WTP threshold of $38,224/QALY in China. For patients with CPS≥1, pembrolizumab plus chemotherapy could bring 0.24 QALYs with the incremental cost of $76,234, resulting in the ICER was $319,506.90/QALY. And for the intention-to-treat population, pembrolizumab plus chemotherapy could bring 0.20 QALYs with the incremental cost of $155,263, resulting in the ICER was $776,786.75/QALY. The ICERs obtained by the three subgroups all exceeded the WTP threshold of $38,224/QALY in China, and the base-case results were shown in Table 3. The base-case results revealed that adding pembrolizumab to chemotherapy could not be considered the cost-effective option in China.
TABLE 3
| Subgroup | Treatment | Incremental cost ($) | Incremental QALY | ICER($/QALY) |
|---|---|---|---|---|
| Patients with CPS≥10 | Pembrolizumab group | 85,838.75 | 0.47 | 184,030.56 |
| Placebo group | NA | NA | NA | |
| Patients with CPS≥1 | Pembrolizumab group | 76,234 | 0.24 | 319,506.90 |
| Placebo group | NA | NA | NA | |
| Intention-to-treat patients | Pembrolizumab group | 155,263 | 0.20 | 776,786.75 |
| Placebo group | NA | NA | NA |
The results of base-case analyses.
QALYs: quality-adjusted life-years, ICER: incremental cost-effectiveness ratio, CPS≥1: PD-L1 CPS, of 1 or higher, CPS≥10: PD-L1 CPS, of 10 or higher, NA: not applicable.
3.2 Sensitivity analysis
In the one-way sensitivity analyses, results were visualized in a tornado diagram, which highlighted that PFS utility values, PD utility values and the cost of pembrolizumab exerted the greatest influence on the ICER. Parameter variations demonstrated the robustness of model outcomes (Figures 2–4). Probabilistic sensitivity analysis showed a 0% probability that pembrolizumab plus chemotherapy was cost-effective across all three subgroups at China’s WTP threshold of $38,224/QALY, confirming the model’s robustness (Figures 5–10). In the CPS≥10 subgroup, the probability of pembrolizumab being cost-effective increased to 55.4% when the WTP threshold was $200,000/QALY. In the CPS≥1 subgroup, at a WTP threshold of $360,000/QALY, this probability reached 58.0%. And the probability increased to 50.2% when the WTP threshold was $750,000/QALY for the intention-to-treat population. As the WTP threshold increased, the probability of pembrolizumab being economically rose accordingly.
FIGURE 2
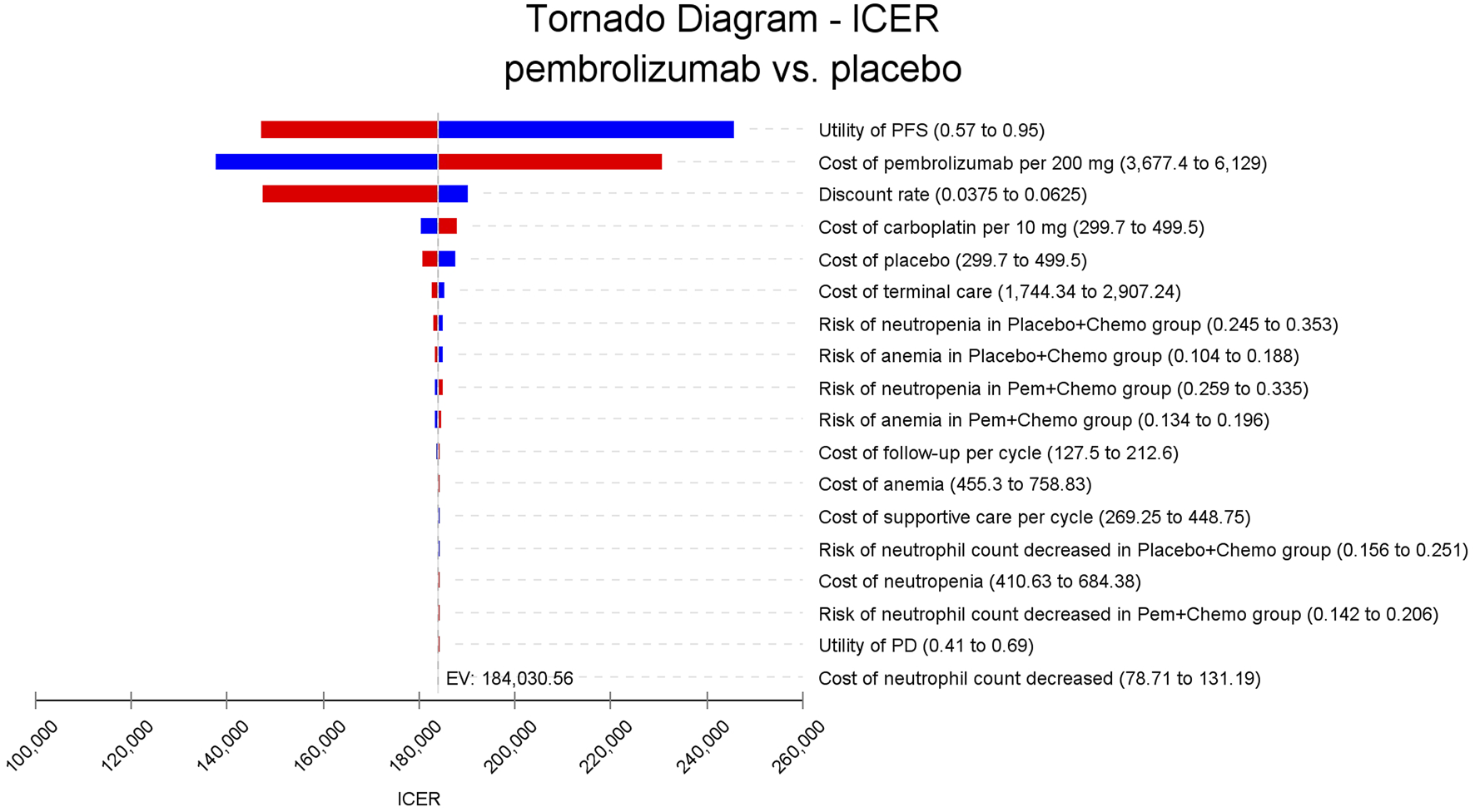
Tornado diagram of one-way sensitivity analyses for patients with CPS≥10. PFS, progression-free survival; PD, progressed disease; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; CPS≥1, PD-L1 CPS of 1 or higher; CPS≥10, PD-L1 CPS of 10 or higher.
FIGURE 3
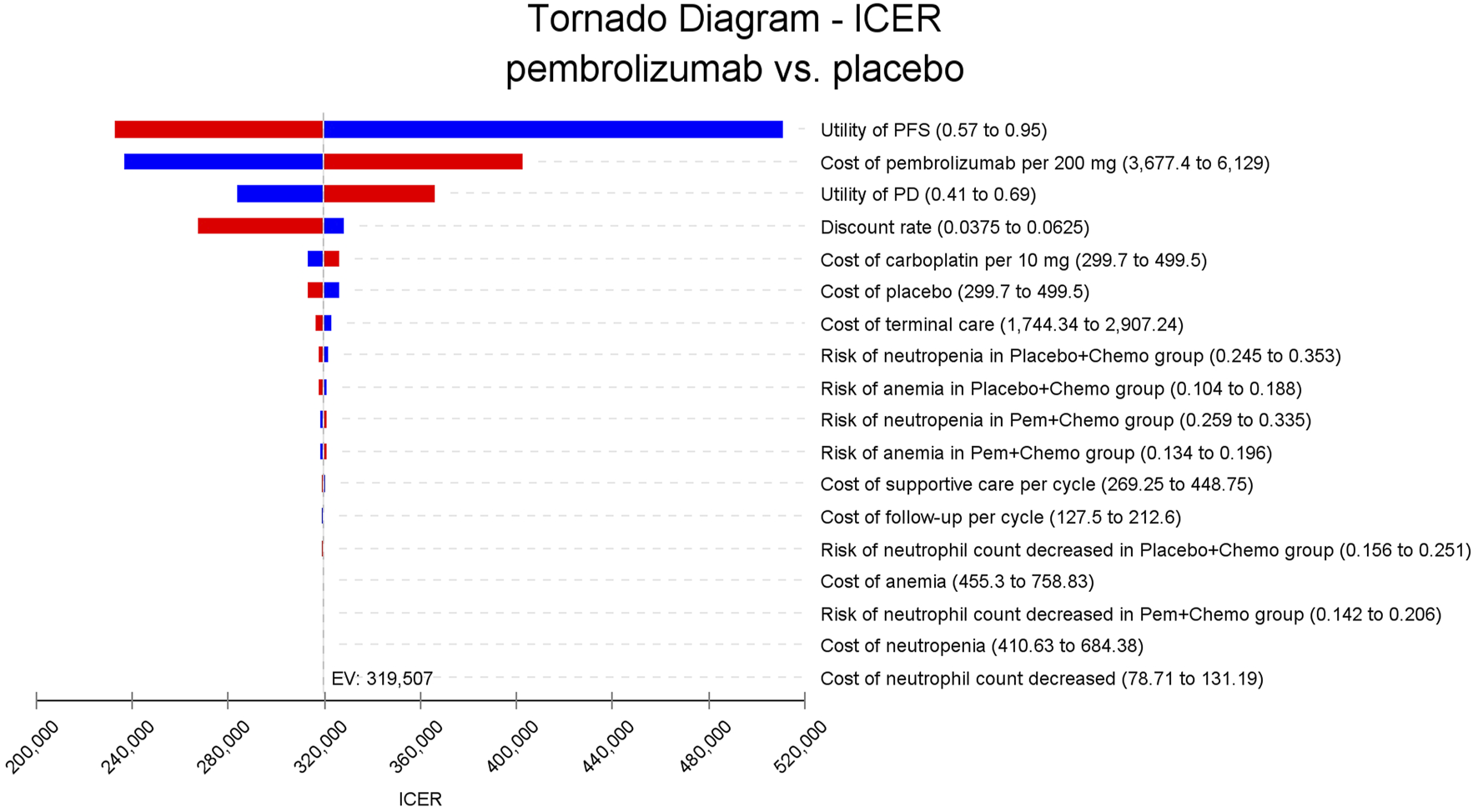
Tornado diagram of one-way sensitivity analyses for patients with CPS≥1.
FIGURE 4
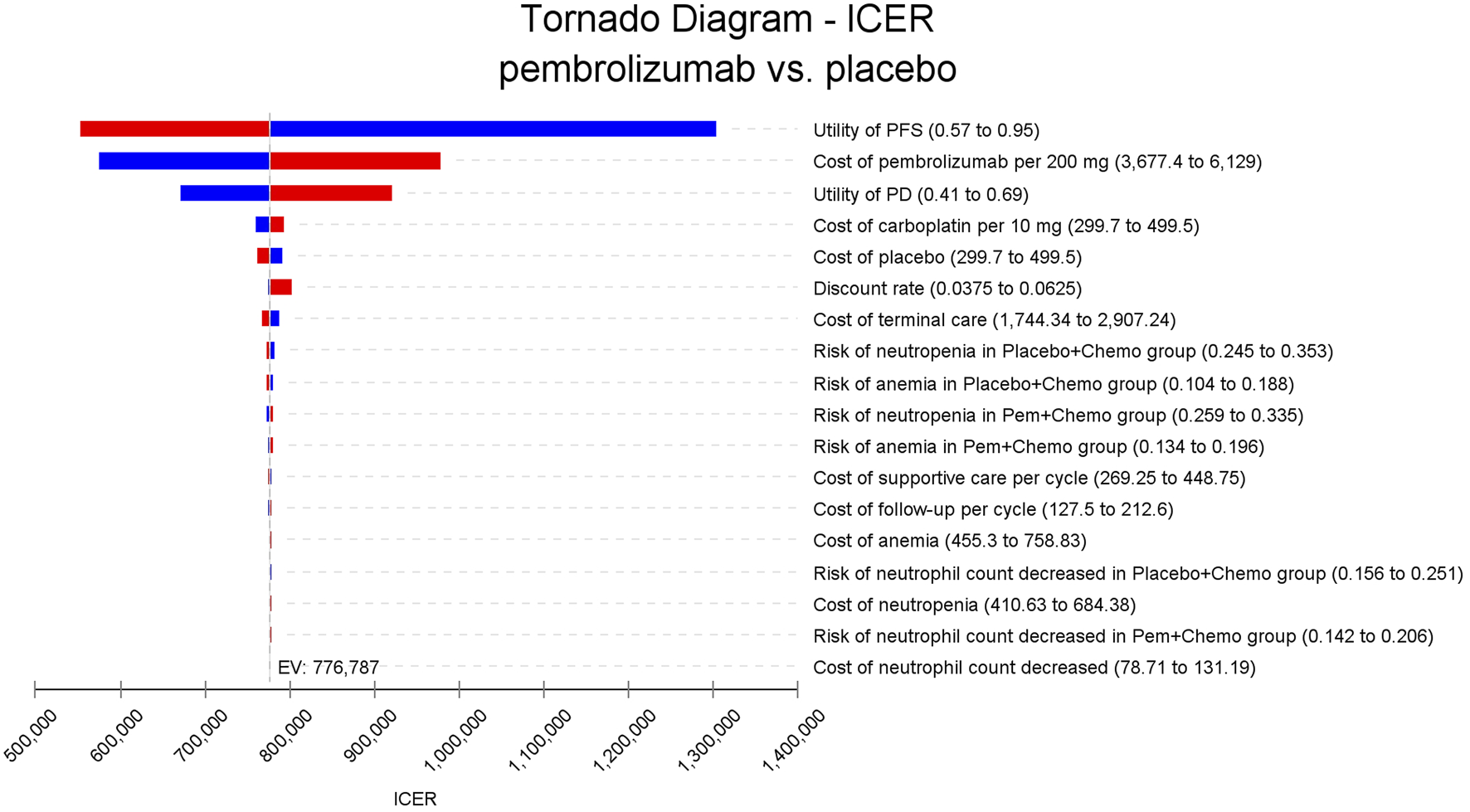
Tornado diagram of one-way sensitivity analyses for the intention-to-treat population.
FIGURE 5
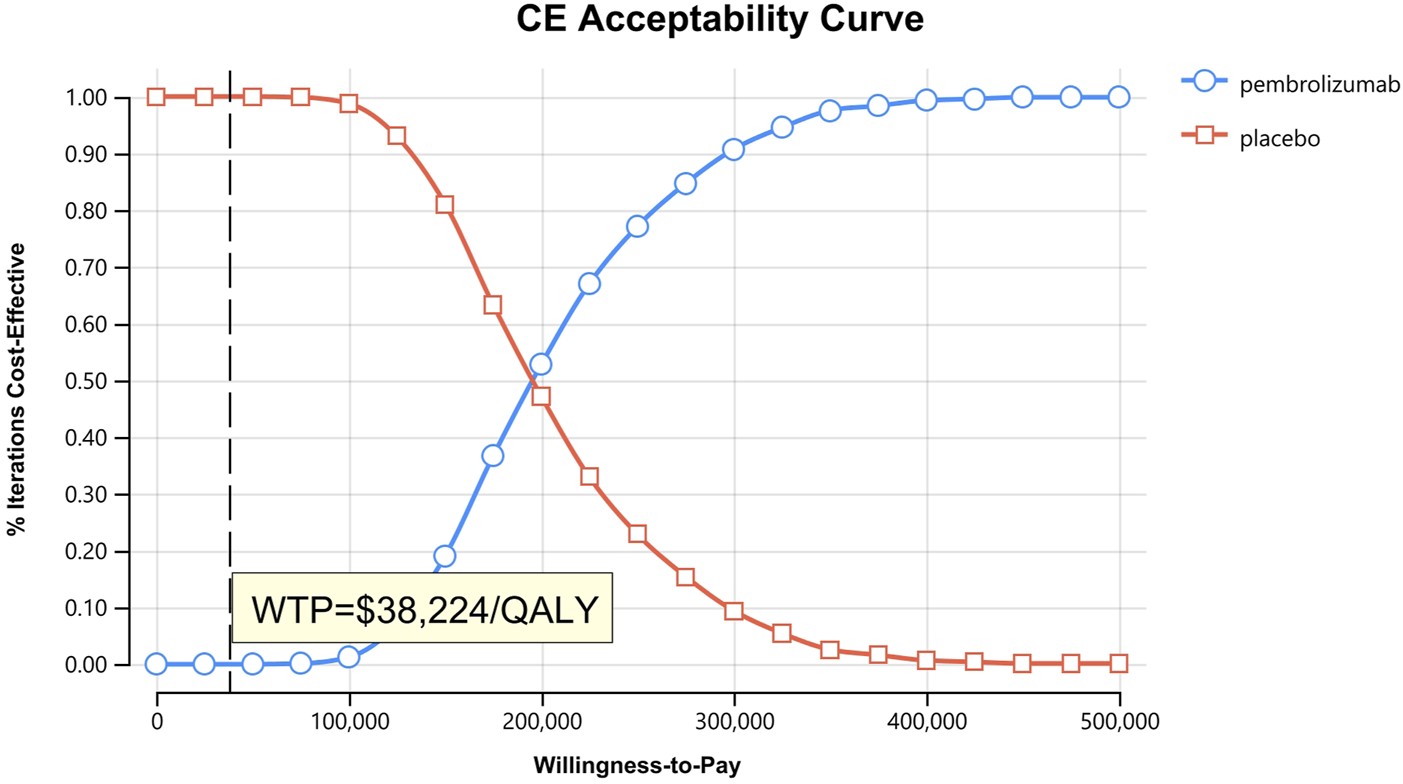
Cost-effectiveness acceptability curves for patients with CPS≥10.
FIGURE 6
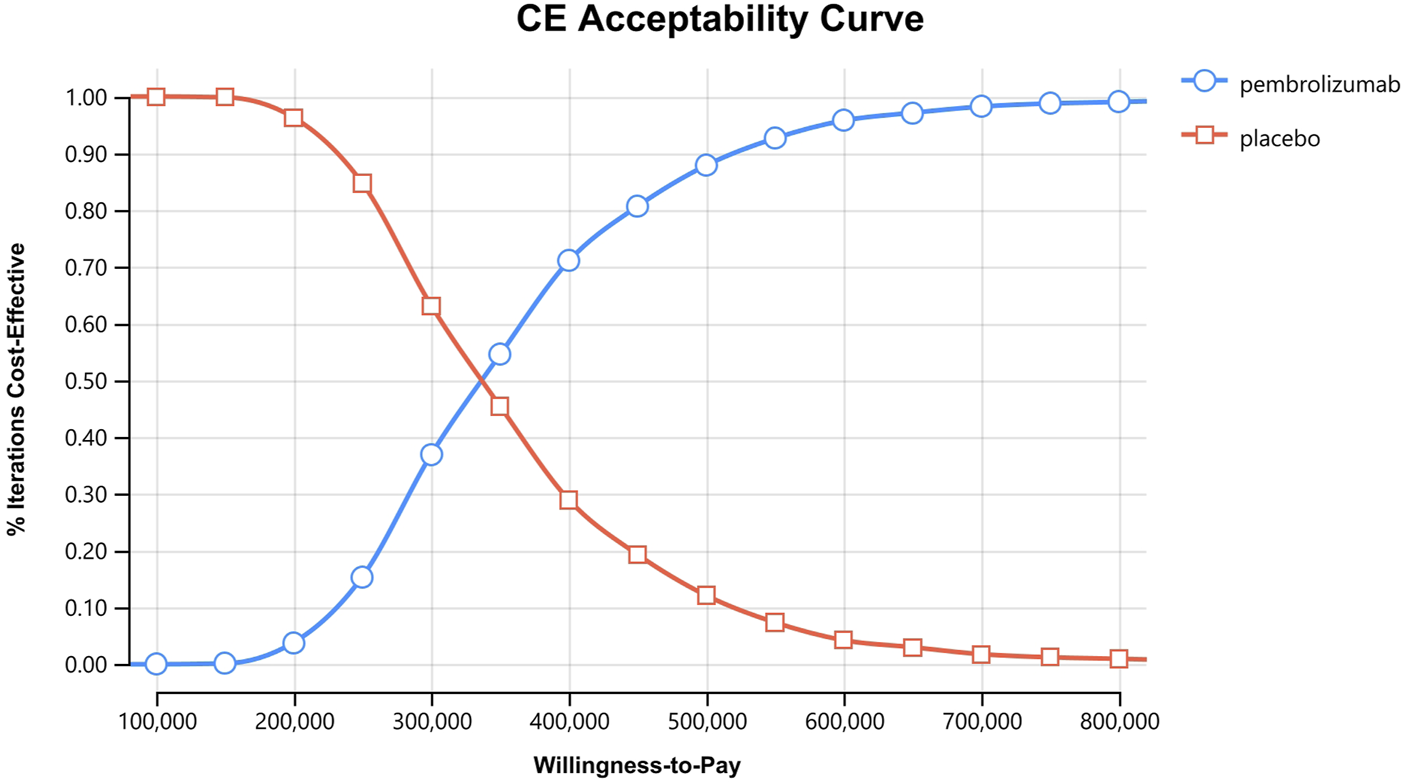
Cost-effectiveness acceptability curves for patients with CPS≥1.
FIGURE 7
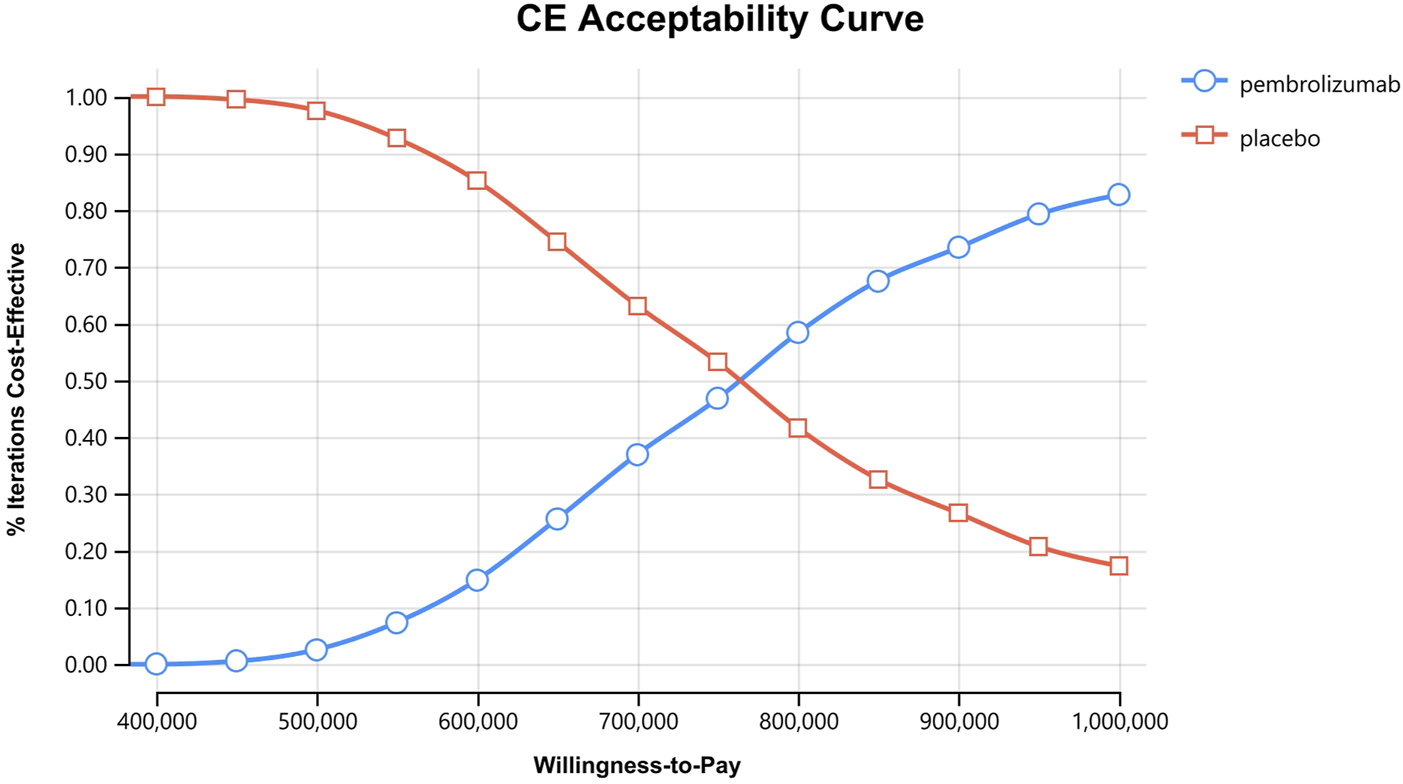
Cost-effectiveness acceptability curves for the intention-to-treat population.
FIGURE 8
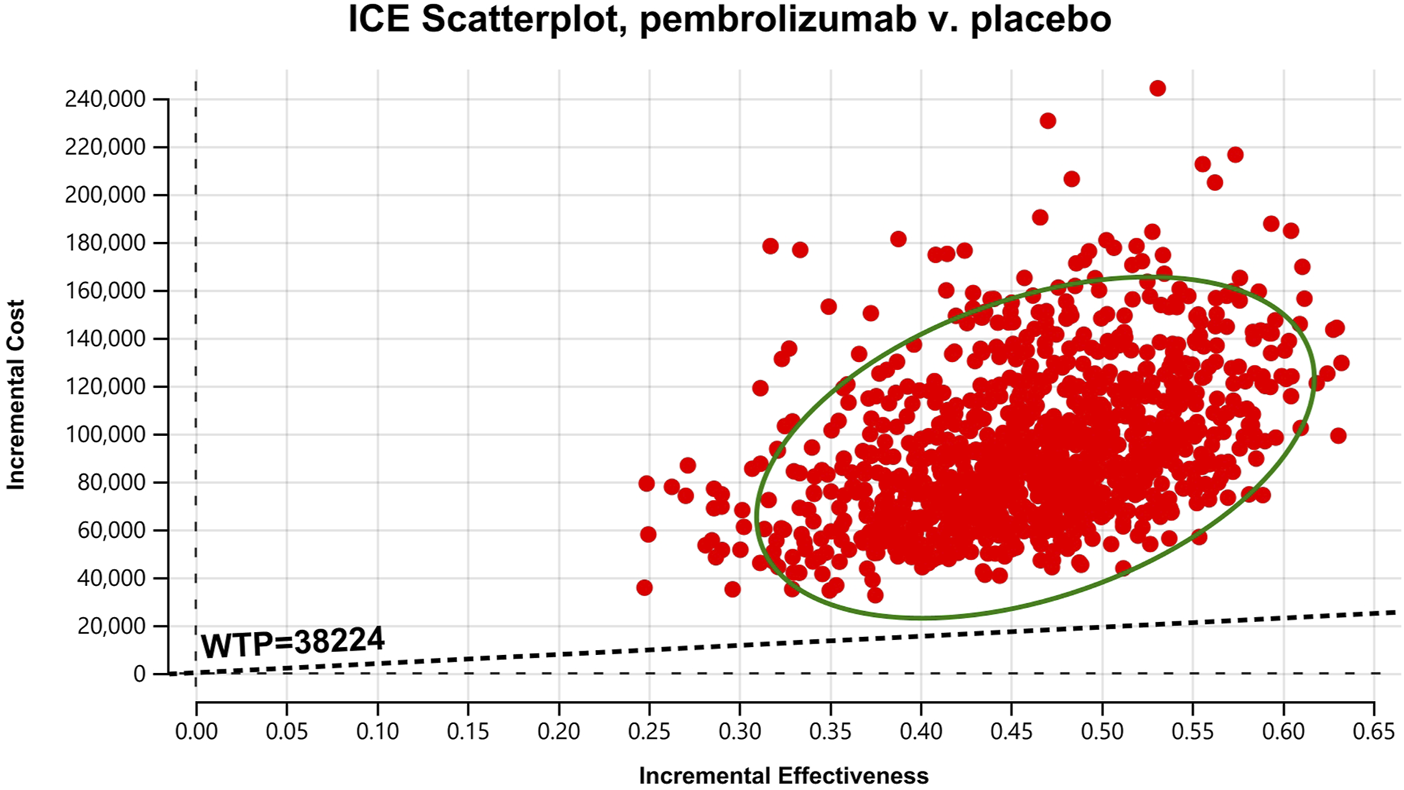
Cost-effectiveness scatter Chart for patients with CPS≥10.
FIGURE 9
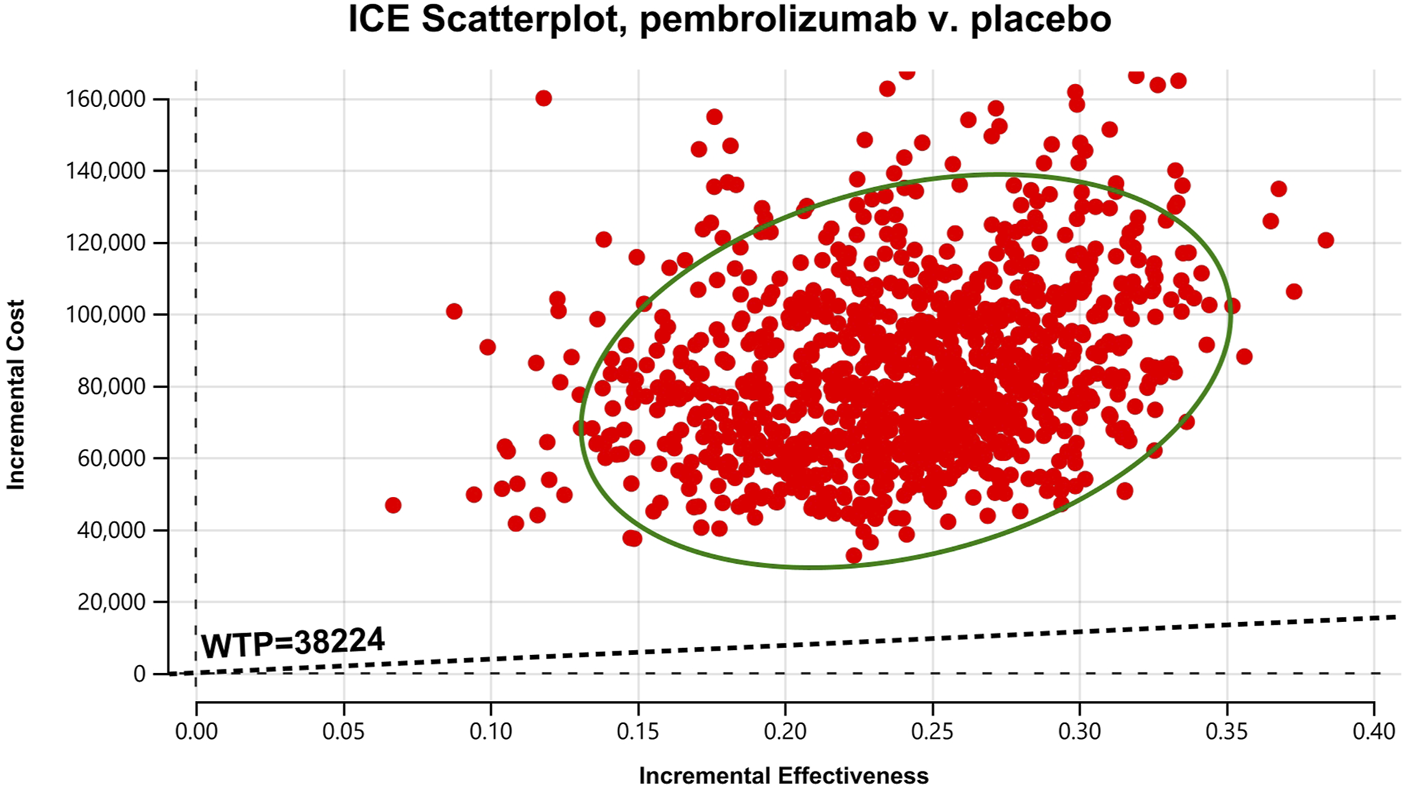
Cost-effectiveness scatter Chart for patients with CPS≥1.
FIGURE 10
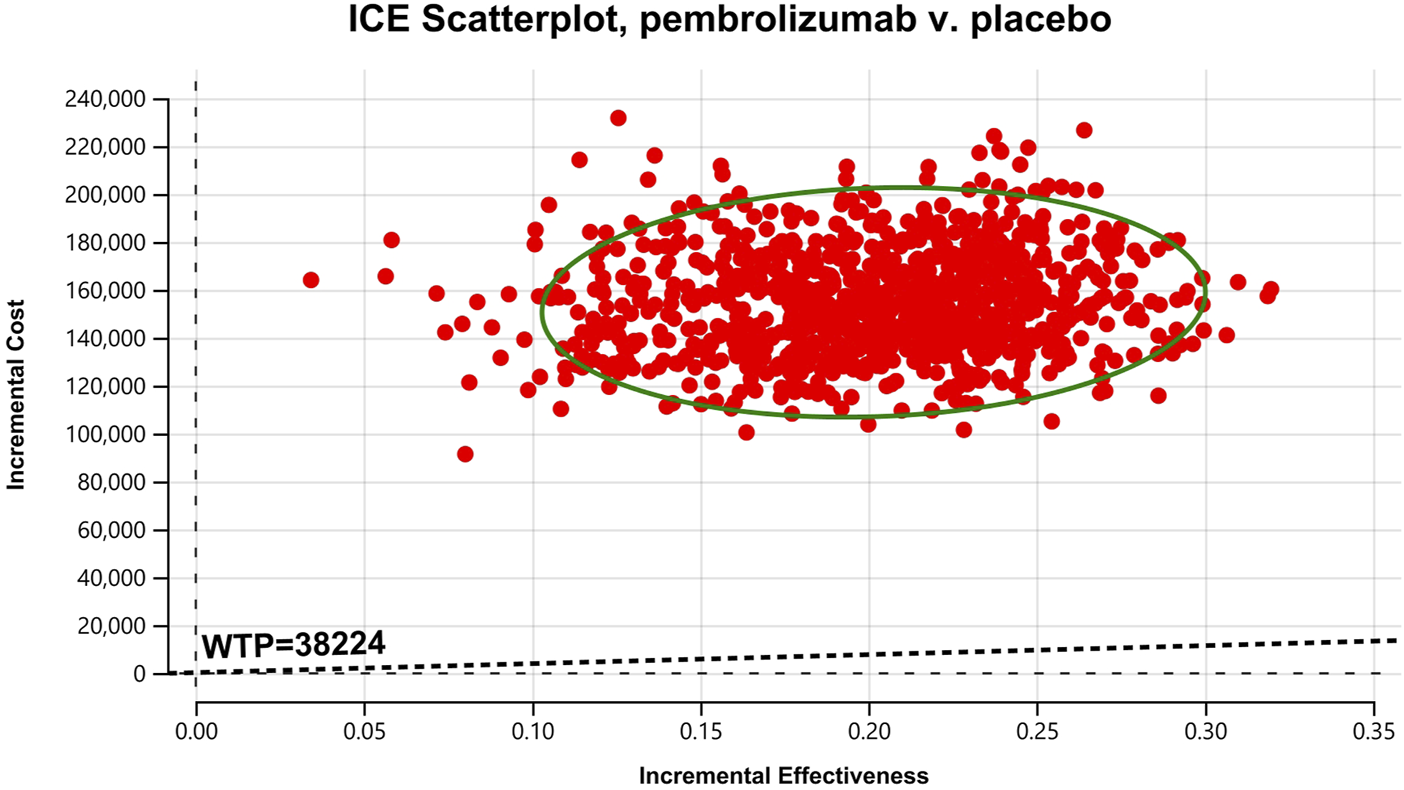
Cost-effectiveness scatter Chart for the intention-to-treat population.
3.3 Scenario analysis
We performed scenario analyses evaluating different price reductions for pembrolizumab (80%, 90%, and 95% discount) within the economic models of three subgroups. Our findings demonstrated that as the discount rate of pembrolizumab increased, the ICERs gradually decreased. For patients with CPS≥10, when the cost of pembrolizumab decreased by 80%, the ICER was $35,362/QALY, which was lower than the WTP threshold of $38,224/QALY in China. For patients with CPS≥1 and the intention-to-treat population, the pembrolizumab group was cost-effective compared to the placebo group when the price of pembrolizumab was reduced by 90% and 95%, respectively (Table 4).
TABLE 4
| Subgroup | Treatment | Incremental cost ($) | Incremental QALY | ICER($/QALY) | Discount Rate(%) |
|---|---|---|---|---|---|
| Patients with CPS≥10 | Pembrolizumab group | 16,494.37 | 0.47 | 35,362.45 | 80% |
| Placebo group | NA | NA | NA | ||
| Patients with CPS≥1 | Pembrolizumab group | 4,936 | 0.24 | 20,688.76 | 90% |
| Placebo group | NA | NA | NA | ||
| Intention-to-treat patients | Pembrolizumab group | 2,011 | 0.20 | 10,058.71 | 95% |
| Placebo group | NA | NA | NA |
The results of scenario analyses.
QALYs: quality-adjusted life-years, ICER: incremental cost-effectiveness ratio, CPS≥1: PD-L1 CPS, of 1 or higher, CPS≥10: PD-L1 CPS, of 10 or higher, NA: not applicable.
4 Discussion
Currently, ICIs, either as monotherapy or in combination with other regimens, have emerged as a key therapeutic option for TNBC. In the KEYNOTE-355 trial, pembrolizumab plus chemotherapy statistically extended PFS and OS in patients with previously untreated locally recurrent inoperable or metastatic TNBC compared with placebo plus chemotherapy. However, the financial burden imposed by immuno-oncology drugs for the majority of middle- and low-income patients and the consequent challenges to healthcare system sustainability represent a critical issue in contemporary medical practice. Therefore, it is an urgent agenda to conduct a cost-effectiveness analysis to evaluate their economic impact, particularly in resource-constrained countries like China. In this study, we developed a Markov model to explore the economic ramifications of using pembrolizumab as a primary treatment for Chinese patients with previously untreated locally recurrent inoperable or metastatic TNBC. The results showed that in 10-year horizon, pembrolizumab plus chemotherapy is not cost-effective compared to placebo plus chemotherapy. For patients with CPS≥10, pembrolizumab plus chemotherapy yielded a marginal cost of $85,838.75 and an additional 0.47 QALYs, resulting in an ICER of $184,030.56 per additional QALY gained. For patients with CPS≥1, pembrolizumab plus chemotherapy could bring 0.24 QALYs with the incremental cost of $76,234, resulting in the ICER was $319,506.90 per additional QALY gained. And for the intention-to-treat population, pembrolizumab plus chemotherapy could bring 0.20 QALYs with the incremental cost of $155,263, resulting in the ICER was $776,786.75 per additional QALY gained. The ICERs obtained by the three subgroups all exceeded the WTP threshold of $38,224 in China, indicating that they were not economically viable. Our analysis revealed a clear cost-effectiveness gradient across PD-L1 expression levels. The CPS≥10 subgroup demonstrated better economic outcomes compared to the CPS≥1 subgroup, with higher incremental QALYs and lower ICERs. The one-way sensitivity analysis and probabilistic sensitivity analysis demonstrated that the model outcomes were robustness. The results indicated that adding pembrolizumab to chemotherapy was not a cost-effective treatment for patients with different PD-L1 CPS expression and the intention-to-treat population. Scenario analysis demonstrated that price reductions for pembrolizumab could enhance its likelihood of achieving cost-effectiveness. And differential price reductions were required to achieve cost-effectiveness: an 80% reduction for CPS≥10 patients, 90% for CPS≥1 patients, and 95% for the intention-to-treat population.
Prior clinical trials have consistently demonstrated the therapeutic benefits of immune checkpoint inhibitors as a treatment regimen for TNBC (U.S. National Library of Medicine, 2025; Schmid et al., 2020; Jiang et al., 2024). Up to now, ICIs including pembrolizumab, atezolizumab and toripalimab are available for the treatment for patients with TNBC, and pharmacoeconomic evaluations for TNBC have been increasingly conducted. Based on the KEYNOTE-522 clinical trial, four studies showed that the addition of pembrolizumab in various combinations in patients with TNBC was likely to be cost-effective from the perspectives of different countries (Favre-Bulle et al., 2024; Huang et al., 2023; Kwong et al., 2024; Pöllinger et al., 2025). For the KEYNOTE-355 clinical trial, while two studies supported pembrolizumab’s cost-effectiveness in the US and Japan (Huang et al., 2022; Chisaki et al., 2025), another study questioned its economic value in China (Zhu et al., 2023). Notably, previous studies have not conducted subgroup analyses or evaluated outcome indicators across the three populations: different PD-L1 CPS expression and the intention-to-treat group (Zhu et al., 2023; Huang et al., 2022; Chisaki et al., 2025). Compared with other ICIs, atezolizumab has not shown better affordability in metastatic TNBC (Liu et al., 2021), whereas toripalimab, a domestically developed PD-1 inhibitor in China, may present moderate cost-effectiveness due to its lower price (Cai et al., 2024), though direct comparative studies remain scarce. The translation of clinical benefits into economic value poses particular challenges for China’s healthcare system amid expanding ICI applications, necessitating thorough subgroup economic evaluations to inform precision decision-making.
This study has several limitations that warrant consideration in healthcare decision-making. First, while the clinical data included in the model have been obtained from the KEYNOTE-355 trial, several factors—such as differences in clinical practice patterns, the cost structure of oncology care, patient characteristics could vary across different countries, may limit the generalizability of our findings to the Chinese healthcare setting. Second, utility values were derived from published literature and not locally validated, without distinguishing potential biases among treatment strategies, which may lead to an overestimation of QALY gains. Third, cost data were obtained from published sources rather than real-world settings, future studies should prioritize real-world cost evidence to enhance the generalizability and accuracy of cost estimates. The substantial urban-rural divide in China’s healthcare system merits particular attention, where urban centers typically exhibit 2–3 times higher per capita health expenditures compared to rural regions, potentially affecting medication affordability and reimbursement decision applicability across different socioeconomic strata (Tang et al., 2021). Fourth, our study only included the costs of management SAEs (Grade≥3), and ignored the costs of AEs below grade 3, although these cost values only had minimal influence of the model results revealed by one-way sensitivity analyses. Fifth, in this study, we employed a Markov model rather than the partitioned survival model. Although Markov model can better capture the progression of diseases based on their distinct characteristics, they require the calculation of transition probabilities and impose extremely high demands on clinical evidence, making it difficult to ensure accuracy and feasibility. Notably, a literature have demonstrated there was no significant difference in most outcome measures derived from the two models, both of them are recommended to assess model structure uncertainty (Rui et al., 2021). Finally, our evaluation focused solely on pembrolizumab, future studies should incorporate comparative analyses of other ICIs through network meta-analysis approaches. Despite the limitations of the study, the sensitivity analysis demonstrated the robustness of our model outcomes. Therefore, it might provide valuable reference for clinical treatment decisions and policymakers.
Our findings highlight the economic challenges of pembrolizumab adoption in China, with all ICER estimates exceeding the WTP thresholds. These results carry important implications for both clinical practice and health policy. For physicians, our study highlights the need to balance therapeutic benefits with economic burdens when selecting treatment regimens. For policymakers, we emphasizes the importance of considering regional economic variations and alternative ICIs when making reimbursement decisions, particularly in resource-constrained settings. In addition, price negotiation mechanisms should be strengthened for high-cost ICIs, particularly through volume-based procurement approaches that have successfully reduced drug prices in China’s national reimbursement drug list negotiations (Mingge et al., 2023). Future research should focus on comprehensive comparisons of available ICIs and develop region-specific cost-effectiveness thresholds to optimize resource allocation in China’s heterogeneous healthcare landscape.
5 Conclusion
In summary, from the Chinese healthcare system perspective, pembrolizumab in combination with chemotherapy has been shown a survival benefit in improving the survival of patients with previously untreated locally recurrent inoperable or metastatic TNBC and has no cost-effectiveness advantages in terms of economics in any subgroups. Price reduction of pembrolizumab may be a potential solution to make it cost-effective.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
YH: Writing – review and editing, Writing – original draft. SY: Writing – review and editing. XW: Writing – review and editing. SZ: Writing – review and editing. HL: Writing – review and editing, Writing – original draft. SK: Writing – review and editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1654177/full#supplementary-material
References
1
Andersson T. M. Dickman P. W. Eloranta S. Lambert P. C. (2011). Estimating and modelling cure in population-based cancer studies within the framework of flexible parametric survival models. BMC Med. Res. Methodol.11, 96. 10.1186/1471-2288-11-96
2
Bray F. Laversanne M. Sung H. Ferlay J. Siegel R. L. Soerjomataram I. et al (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.2024, 229–263. 10.3322/caac.21834
3
Cai H. Huang L. Zheng Z. (2024). Toripalimab plus chemotherapy in the treatment of metastatic triple-negative breast cancer: a cost-effectiveness analysis. Front. Public Health12, 1421826. 10.3389/fpubh.2024.1421826
4
Chisaki Y. Nakamura N. Komuro T. Nyuji H. Harano M. Kitada N. (2025). Cost-effectiveness analysis of pembrolizumab plus chemotherapy compared with chemotherapy as first-line treatment for advanced PD-L1-Positive triple-negative breast cancer from a Japanese healthcare perspective. Clin. Drug Investig.45, 317–326. 10.1007/s40261-025-01445-8
5
Cho B. Han Y. Lian M. Colditz G. A. Weber J. D. Ma C. et al (2021). Evaluation of racial/Ethnic differences in treatment and mortality among women with triple-negative breast cancer. JAMA Oncol.7 (7), 1016–1023. 10.1001/jamaoncol.2021.1254
6
Cortes J. Cescon D. W. Rugo H. S. Nowecki Z. Im S. A. Yusof M. M. et al (2020). Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet396 (10265), 1817–1828. 10.1016/S0140-6736(20)32531-9
7
Djalalov S. Beca J. Ewara E. M. Hoch J. S. (2019). A comparison of different analysis methods for reconstructed survival data to inform cost-effectiveness analysis. Pharmacoeconomics37 (12), 1525–1536. 10.1007/s40273-019-00830-4
8
Eichler H. G. Kong S. X. Gerth W. C. Mavros P. Jönsson B. (2004). Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge?Value Health7 (5), 518–528. 10.1111/j.1524-4733.2004.75003.x
9
Favre-Bulle A. Huang M. Haiderali A. Bhadhuri A. (2024). Cost-Effectiveness of neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab in patients with High-Risk, Early-Stage, triple-negative breast cancer in Switzerland. Pharmacoecon Open8 (1), 91–101. 10.1007/s41669-023-00445-8
10
Gong J. Su D. Shang J. Xu S. Tang L. Sun Z. et al (2022). Cost-Effectiveness of Tislelizumab versus docetaxel for previously treated advanced non-small-cell lung cancer in China. Front. Pharmacol.13, 830380. 10.3389/fphar.2022.830380
11
Huang M. Fasching P. Haiderali A. Pan W. Gray E. Zhou Z. Y. et al (2022). Cost-effectiveness of pembrolizumab plus chemotherapy as first-line treatment in PD-L1-positive metastatic triple-negative breast cancer. Immunotherapy14 (13), 1027–1041. 10.2217/imt-2022-0082
12
Huang M. A Fasching P. Haiderali A. Xue W. Yang C. Pan W. et al (2023). Cost-Effectiveness of neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant single-agent pembrolizumab for high-risk early-stage triple-negative breast cancer in the United States. Adv. Ther.40 (3), 1153–1170. 10.1007/s12325-022-02365-1
13
Ishak K. J. Kreif N. Benedict A. Muszbek N. (2013). Overview of parametric survival analysis for health-economic applications. Pharmacoeconomics31 (8), 663–675. 10.1007/s40273-013-0064-3
14
Jiang Z. Ouyang Q. Sun T. Zhang Q. Teng Y. Cui J. et al (2024). Toripalimab plus nabpaclitaxel in metastatic or recurrent triple-negative breast cancer: a randomized phase 3 trial. Nat. Med.30, 249–256. 10.1038/s41591-023-02677-x
15
Kwok G. Yau T. C. Chiu J. W. Tse E. Kwong Y. L. (2016). Pembrolizumab (Keytruda). Hum. Vaccin Immunother.12 (11), 2777–2789. 10.1080/21645515.2016.1199310
16
Kwong A. Leung R. Chan T. C. Khandelwal A. Mishra K. Huang M. (2024). Cost-effectiveness of pembrolizumab in combination with chemotherapy as neoadjuvant treatment and continued as a single agent adjuvant treatment for high-risk early-stage triple-negative breast cancer in Hong Kong. Oncol. Ther.12 (3), 525–547. 10.1007/s40487-024-00285-4
17
Liao M. Kang S. (2024). Economic evaluation of sintilimab versus docetaxel as second-line treatment for patients with advanced or metastatic squamous non-small-cell lung cancer in China: a model-based cost-effectiveness analysis. Expert Rev. Pharmacoecon Outcomes Res.24 (1), 161–166. 10.1080/14737167.2023.2267177
18
Liu G. Hu S. Wu J. Dong Z. Li H. Sang G. et al (2020). China guidelines for pharmacoeconomic evaluations. Beijing: China Market Press.
19
Liu X. Lang Y. Liao Y. Zhu Y. (2021). Atezolizumab plus chemotherapy vs. chemotherapy in advanced or metastatic triple-negative breast cancer: a cost-effectiveness analysis. Front. Public Health9, 756899. 10.3389/fpubh.2021.756899
20
Mingge X. Jingyu W. Qi L. Zhe Z. Qing R. (2023). Promoting access to innovative anticancer medicines: a review of drug price and national reimbursement negotiation in China. Inquiry60, 469580231170729. 10.1177/00469580231170729
21
Mistry R. May J. R. Suri G. Young K. Brixner D. Oderda G. et al (2018). Cost-effectiveness of ribociclib plus Letrozole versus palbociclib plus Letrozole and Letrozole monotherapy in the first-line treatment of postmenopausal women with HR+/HER2- advanced or metastatic breast cancer: a U.S. payer perspective. J. Manag. Care Spec. Pharm.24 (6), 514–523. 10.18553/jmcp.2018.24.6.514
22
National Bureau of Statistics (2025). National data. Available online at: https://data.stats.gov.cn/easyquery.htm?Cn=C01 (Accessed April 06, 2025).
23
Patel G. Prince A. Harries M. (2024). Advanced triple-negative breast cancer. Semin. Oncol. Nurs.40, 151548. 10.1016/j.soncn.2023.151548
24
Pöllinger B. Haiderali A. Huang M. Akyol Ersoy B. Abdelaziz A. H. Kassem L. et al (2025). The cost-effectiveness of treatment for high-risk, early-stage, triple-negative breast cancer in Egypt: an analysis of neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant single-agent pembrolizumab. J. Med. Econ.28 (1), 105–113. 10.1080/13696998.2024.2441073
25
Royston P. Parmar M. K. (2002). Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat. Med.21 (15), 2175–2197. 10.1002/sim.1203
26
Rui M. Wang Y. Fei Z. Zhang X. Shang Y. Li H. (2021). Will the Markov model and partitioned survival model lead to different results? A review of recent economic evidence of cancer treatments. Expert Rev. Pharmacoecon Outcomes Res.21 (3), 373–380. 10.1080/14737167.2021.1893167
27
Schmid P. Rugo H. S. Adams S. Schneeweiss A. Barrios C. H. Iwata H. et al (2020). Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.21 (1), 44–59. 10.1016/S1470-2045(19)30689-8
28
Tang S. Yao L. Ye C. Li Z. Yuan J. Tang K. et al (2021). Can health service equity alleviate the health expenditure poverty of Chinese patients? Evidence from the CFPS and China health statistics yearbook. BMC Health Serv. Res.21 (1), 718. 10.1186/s12913-021-06675-y
29
Thokala P. Ochalek J. Leech A. A. Tong T. (2018). Cost-effectiveness thresholds: the past, the present and the future. Pharmacoeconomics36 (5), 509–522. 10.1007/s40273-017-0606-1
30
U.S. National Library of Medicine (2025). Study of pembrolizumab (MK-3475) plus chemotherapy vs placebo plus chemotherapy as neoadjuvant therapy and pembrolizumab vs placebo as adjuvant therapy in participants with triple negative breast cancer (TNBC) (MK-3475-522/KEYNOTE-522). Bethesda, MD: ClinicalTrials.gov.
31
Williams C. Lewsey J. D. Mackay D. F. Briggs A. H. (2017). Estimation of survival probabilities for use in cost-effectiveness analyses: a comparison of a multi-state modeling survival analysis approach with partitioned survival and Markov decision-analytic modeling. Med. Decis. Mak.37 (4), 427–439. 10.1177/0272989X16670617
32
Yaozhi (2025). The big data service platform for china’s health industry: information query of drug bid winning. Available online at: https://db.yaozh.com/ (Accessed April 06, 2025).
33
Ye F. Dewanjee S. Li Y. Jha N. K. Chen Z. S. Kumar A. et al (2023). Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer. Mol. Cancer22, 105. 10.1186/s12943-023-01805-y
34
Yue X. Li Y. Wu J. Guo J. J. (2021). Current development and practice of pharmacoeconomic evaluation guidelines for universal health coverage in China. Value Health Reg. Issues24, 1–5. 10.1016/j.vhri.2020.07.580
35
Zhang Y. Baik S. H. Fendrick A. M. Baicker K. (2012). Comparing local and regional variation in health care spending. N. Engl. J. Med.367 (18), 1724–1731. 10.1056/NEJMsa1203980
36
Zhang L. Zhao Y. Tu Q. Xue X. Zhu X. Zhao K. N. (2021). The roles of programmed cell death Ligand-1/programmed cell Death-1 (PD-L1/PD-1) in HPV-induced cervical cancer and potential for their use in blockade therapy. Curr. Med. Chem.28 (5), 893–909. 10.2174/0929867327666200128105459
37
Zheng Z. Chen H. Cai H. Xu S. (2024). Trastuzumab deruxtecan versus chemotherapy treated in patients with HER2-positive metastatic breast cancer: a cost-effectiveness analysis based on the DESTINY-Breast02 trial. Expert Rev. Pharmacoecon Outcomes Res.24 (3), 387–395. 10.1080/14737167.2023.2291157
38
Zhu J. He W. Ye M. Fu J. Chu Y. B. Zhao Y. Y. et al (2018). Cost-effectiveness of afatinib and erlotinib as second-line treatments for advanced squamous cell carcinoma of the lung. Future Oncol.14 (27), 2833–2840. 10.2217/fon2018-0321
39
Zhu Y. Liu K. Wang M. Zhu H. (2022). Trastuzumab deruxtecan versus trastuzumab emtansine for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: a cost-effectiveness analysis. Breast66, 191–198. 10.1016/j.breast.2022.10.010
40
Zhu W. Hong W. Zheng M. Ma G. Shen A. (2023). Combination of pembrolizumab and chemotherapy as first-line treatment in advanced triple-negative breast cancer: a cost-effectiveness analysis. J. Chin. Pharm. Sci.32 (7), 587–597. 10.5246/jcps.2023.07.049
Summary
Keywords
triple-negative breast cancer, cost-effectiveness, pembrolizumab, placebo, markov model
Citation
Hou Y, Yang S, Wang X, Zhao S, Liu H and Kang S (2025) Cost-effectiveness analysis of pembrolizumab plus chemotherapy versus placebo plus chemotherapy for patients with previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer in China. Front. Pharmacol. 16:1654177. doi: 10.3389/fphar.2025.1654177
Received
26 June 2025
Accepted
05 August 2025
Published
22 August 2025
Volume
16 - 2025
Edited by
Anick Bérard, Montreal University, Canada
Reviewed by
George Gourzoulidis, Health Through Evidence, Greece
Xiangzhong Xue, Auburn University, United States
Jie Zhao, First Affiliated Hospital of Zhengzhou University, China
Updates
Copyright
© 2025 Hou, Yang, Wang, Zhao, Liu and Kang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huanlong Liu, lhlong1026@hebmu.edu.cn; Shuo Kang, shuok628@hebmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.