- 1Pharmacy Department, Yantaishan Hospital, Yantai, Shandong, China
- 2School of Pharmacy, Yantai University, Yantai, Shandong, China
As pivotal medicinal resources in the Gentiana genus (Gentianaceae), Gentianae Radix et Rhizoma exhibit remarkable chemical diversity and multi-target pharmacological activities. This review highlights G. scabra Bunge., Gentiana rhodantha Franch., Gentiana manshurica Kitag., Gentiana veitchiorum Hemsl., and other species, compiling 172 constituents from literature (2004–2024) and traditional sources: terpenoids (66 iridoids, 47 triterpenoids, and others), flavonoids, lignans, and alkaloids. Iridoids (e.g., gentiopicroside, swertiamarin) and triterpenoids are key bioactive agents. Pharmaco-logically, Gentiana extracts target NF-κB and MAPK pathways to suppress in-flammation and oxidative liver injury via Nrf2 activation, while inducing tumor cell apoptosis (Bax/Bcl-2) and S/G2-M phase arrest to inhibit lung/liver cancer proliferation. They enhance gastrointestinal repair, regulate motility, and mitigate chronic pain through central-peripheral analgesic synergy. Clinically, gentiopicroside demonstrates hepatoprotective, antiviral, and neuroprotective effects, with applications in herpes zoster, non-alcoholic fatty liver disease, and metabolic disorders. This review represents the first comprehensive integration of multidimensional chemi-cal-pharmacological-clinical data on Gentianae Radix et Rhizoma constituents, aiming to promote the expanded application of Gentianae Radix et Rhizoma in the pharma-ceutical field, provide a scientific basis for further development and utilization, and lay a foundation for subsequent research and industrial development.
1 Introduction
There are approximately 400 species of Gentiana, widely distributed in the temperate regions of the Northern Hemisphere and the alpine regions of the tropics, including Europe, Asia, the northern part of Australia and New Zealand, North America and along the Andes to Cape Horn, and the northern part of Africa. In China, there are about 247 species, found throughout the country, with most species concentrated in the mountainous areas of the southwest, primarily growing in alpine talus slopes, alpine meadows, and shrublands (Editorial Committee of Flora of China and Chinese Academy of Sciences, 1988; Wang et al., 2009; Che and Liang, 2008).
The Gentiana genus is composed of a diverse array of plants. Adhering to the principle of combining characteristics of vegetative and reproductive organs, the Gentiana genus is divided into 15 sections (Dong et al., 2017). The section Gentiana, represented by the traditional Chinese medicinal herb Gentianae Radix et Rhizoma, is known for its bitter taste and cold nature, which possess the effects of purging excess fire in the liver and gallbladder, as well as clearing damp-heat in the lower jiao. It is used in the treatment of diseases such as excessive heat in the liver meridian, convulsions, mania, jaundice, dysentery, Japanese encephalitis, sore throat, red eyes, scrotal swelling and pain, and damp itchiness in the genital area (Che and Liang, 2008; Jiao et al., 2024). Gentianae Radix et Rhizoma, derived from perennial herbs of the Gentianaceae family, was first recorded in the “Shen nong Bencaojing” during the Han Dynasty (202 B.C. – 220 C.D.) and is categorized as a moderate herb. It is known for its efficacy in treating cold and heat within the bones, convulsions, expelling noxious qi, healing severe injuries, stabilizing the functions of the five viscera, and detoxifying. Prolonged use is believed to enhance memory, invigorate the body, and delay aging (Liu, 2022). The 2020 edition of the “Chinese Pharmacopoeia” stipulates that Gentiana is the dried root and rhizome of Gentiana manshurica Kitag., G. scabra Bge., Gentiana triflora Pall., or G. rigescens Franch., which belong to the Gentianaceae family. These plants are perennial herbs, and their roots and rhizomes are used medicinally (Chinese Pharmacopoeia Commission, 2020).
As a traditional remedy for clearing heat and drying dampness, Gentianae Radix et Rhizoma demonstrates a variety of pharmacological actions in clinical settings. Modern pharmacological research indicates that Gentianae Radix et Rhizoma possesses hepatoprotective, choleretic, anti-inflammatory, analgesic, antimicrobial, antiviral, antiallergic, antitumor, neuroprotective, and stomachic activities. These diverse pharmacological effects are associated with its complex chemical structure, particularly with compounds such as gentiopicroside, swertiamarin, amarogentin, linarin, oleanolic acid, gentianine, and polysaccharides, highlighting the significant value of Gentianae Radix et Rhizoma (Jiao et al., 2024; Ning et al., 2017; Jiang et al., 2019; Xiao et al., 2019).
2 Chemical composition
For Gentianae Radix et Rhizoma are rich in diverse chemical constituents, primarily including terpenoids (iridoids and triterpenoids), flavonoids, lignans, and alkaloids. Among these, iridoids such as gentiopicroside, swertiamarin, and sweroside are not only the most abundant phytochemicals in Gentianae Radix et Rhizoma but also serve as key bioactive components responsible for their pharmacological activities (Song, 1986; Ye et al., 2023). Unless otherwise specified, all chemical constituents discussed in this section were isolated from roots/rhizomes, as defined in the Chinese Pharmacopoeia (2020). Compounds one to three were isolated from flowers of Gentiana rhodantha Franch. (non-pharmacopeial species), included for comparative chemical profiling.
2.1 Terpenoids
This article primarily describes 120 terpenoid compounds identified in G. rigescens, including 3 monoterpenes (1–3), 68 iridoids (4–71), 2 sesterterpenes (72–73), and 47 triterpenoids (74–120).
2.1.1 Monoterpenoid
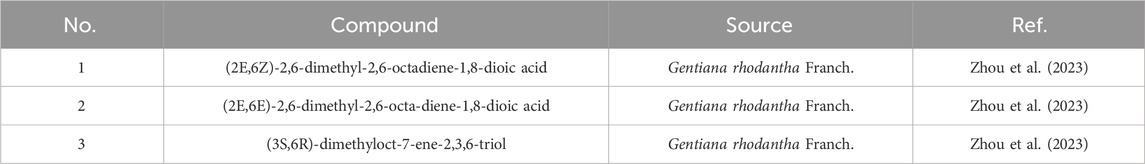
Monoterpenoid compounds in Gentianae Radix et Rhizoma have been relatively less reported. Nevertheless, recent studies have gradually unveiled some monoterpenoid compounds within it. For instance, three monoterpenoid compounds were isolated from the flowers of Gentiana rhodantha, among which compound 1 is a novel compound, while compounds 2 and 3 are new natural products. These findings suggest that there may be more undiscovered monoterpenoid compounds in Gentianae Radix et Rhizoma, warranting further investigation. The 1H NMR and 13C NMR data for these compounds have been assigned (Zhou et al., 2023). Monoterpenoid compounds from Gentianae Radix et Rhizoma are presented in Table 1, and their chemical structures are illustrated in Figure 1.
2.1.2 Iridoids
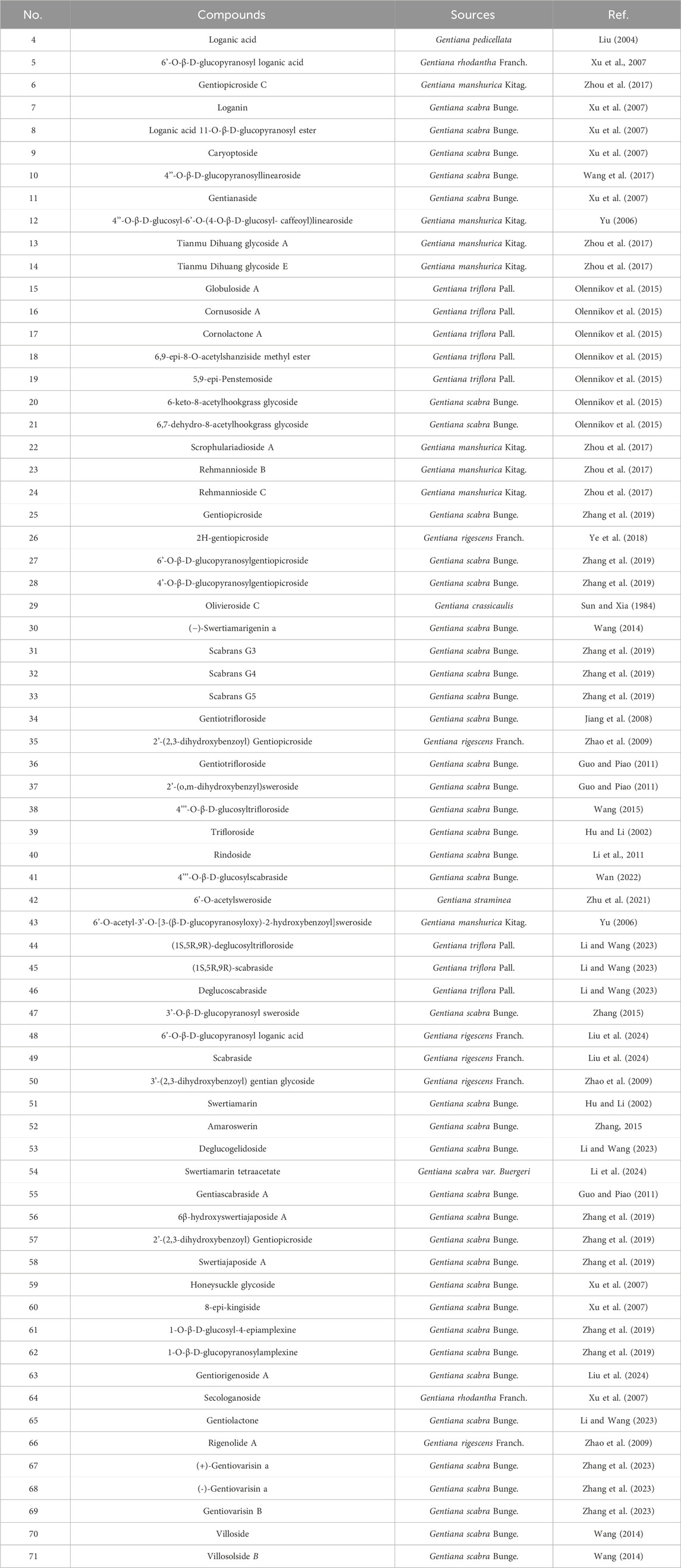
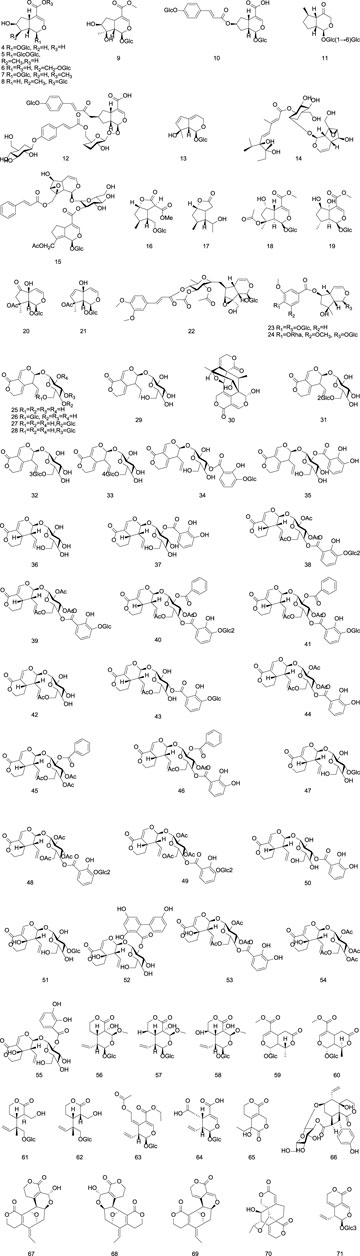
The majority of iridoid components in Gentianae Radix et Rhizoma are secoiridoids, which are commonly glycosylated at the C-1 position. The isolated iridoids are primarily classified into regular iridoids, 4-demethylated iridoids, and secoiridoids (Ye et al., 2023). The regular iridoids include loganic acid (4), 6′-O-β-D-glucopyranosyl loganic acid (5), and loganin (7); the 4-demethylated iridoids include Scrophulariadioside A (22), Rehmannioside B (23), Rehmannioside C (24); and the secoiridoids include gentiopicroside (25), Gentiotrifloroside (36), and Swertiamarin (51). For detailed information on the iridoid components in Gentianae Radix et Rhizoma, refer to Table 2 and Figure 2.
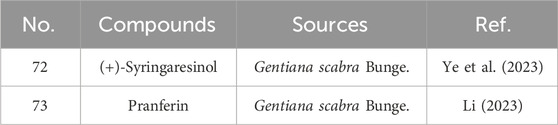
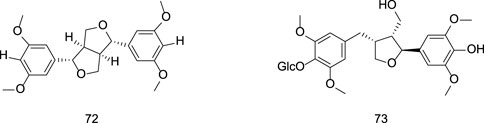
2.1.3 Sesterterpenoids
Sesterterpenoid compounds found in gentians are similar in scarcity to monoterpenoids. Liu et al. identified the sesterterpenoid pranferin from a methanol extract of G. scabra Bunge. (Liu, 2004). Specific information on sesterterpenoid constituents from Gentiana can be found in Table 3 and Figure 3.
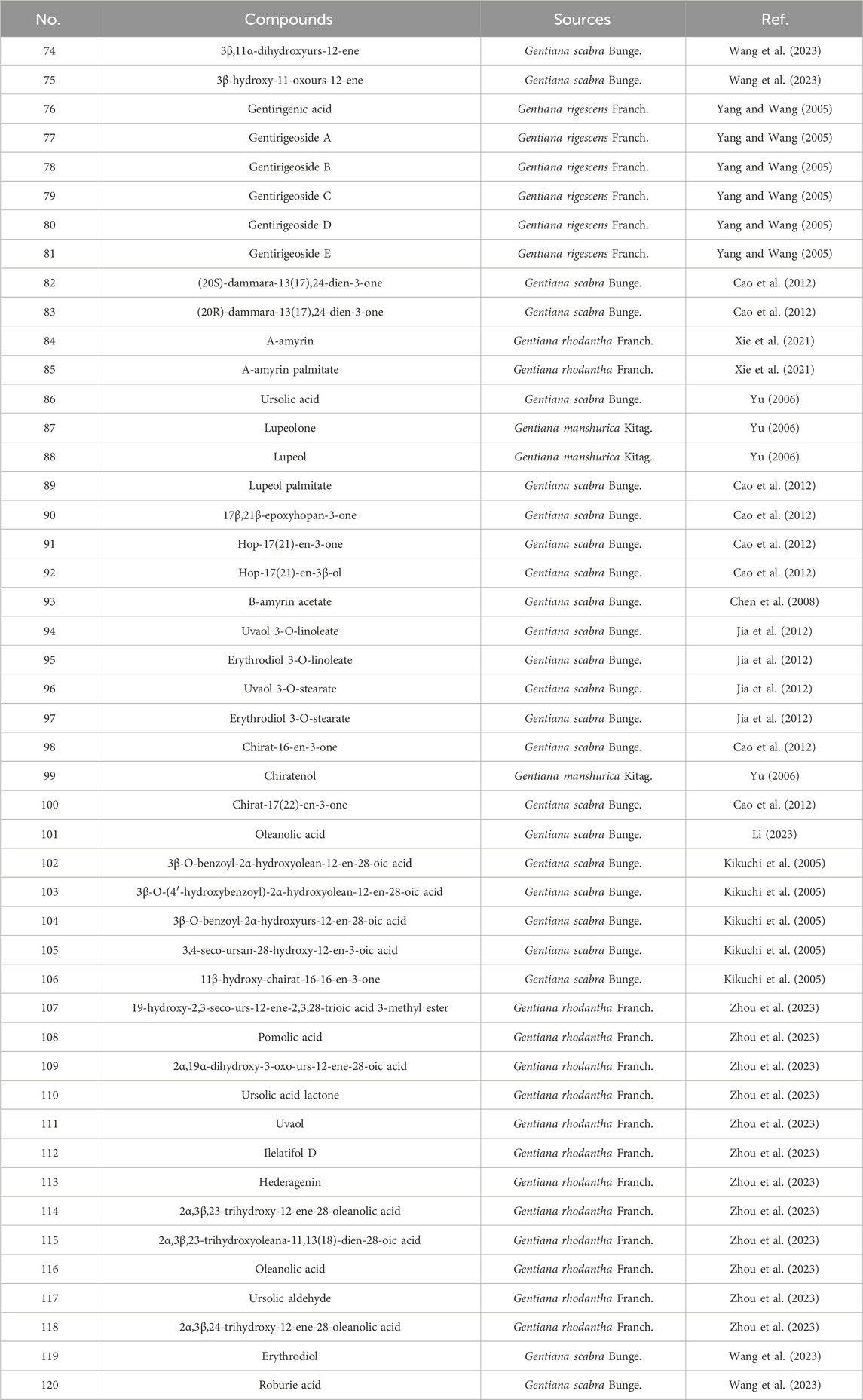
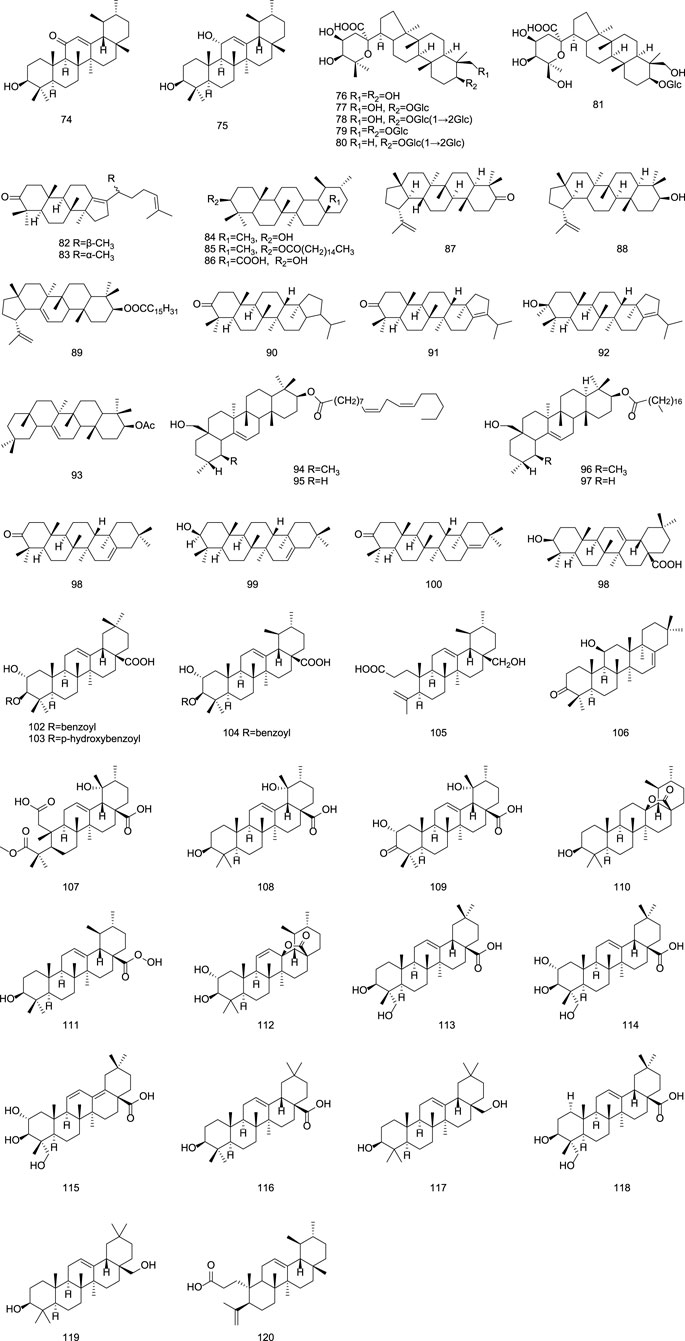
2.1.4 Triterpenoids
Triterpenoids isolated from Gentiana plants mainly include dammarane-type compounds, characterized by a β-configured angular methyl group at the C-8 position, such as gentirigenic acid (76), gentirigeoside A (77), and gentirigeoside B (78); ursane-type compounds, with A/B, B/C, and C/D rings in a trans configuration and D/E rings mostly in a cis configuration, such as α-amyrin (84), α-amyrin palmitate (85), and ursolic acid (86); and lupane-type compounds, featuring a five-membered E ring with isopropyl substitution, such as 17β,21β-epoxyhopan-3-one (90), hop-17 (21)-en-3-one (91), and hop-17 (21)-en-3β-ol (92). Specific information on triterpenoid constituents from Gentianae Radix et Rhizoma can be found in Table 4 and Figure 4. Additionally, purified dammarane-type triterpenoids from Gentiana rigescens exhibited in vitro antifungal activity against Glomerella cingulata (inhibition zones: 0.8–2.0 cm) in a disk diffusion assay at 1 mg/mL, with Carbendazim as a positive control after 72 h of incubation (Xu et al., 2007).
2.2 Flavonoid
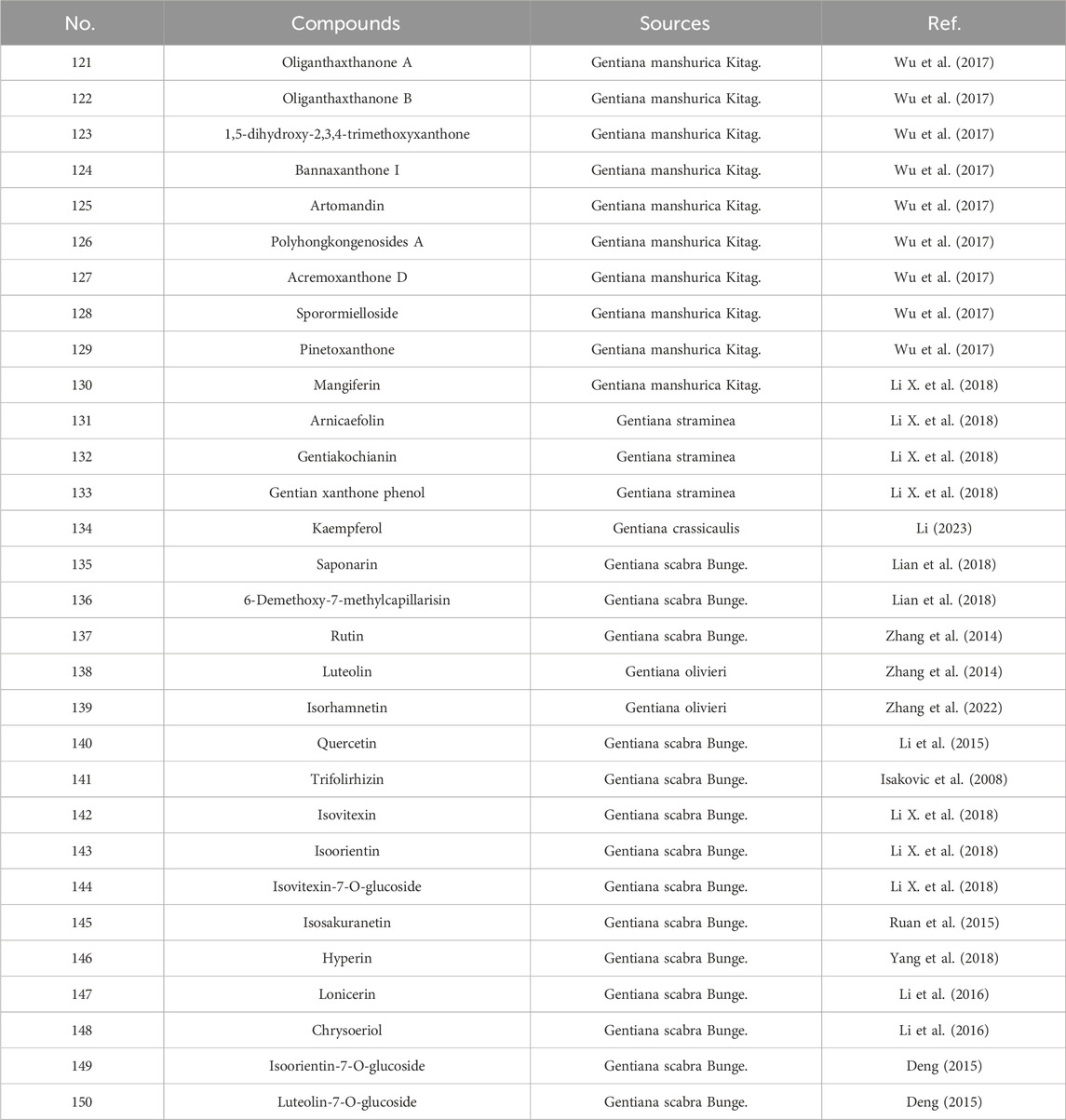
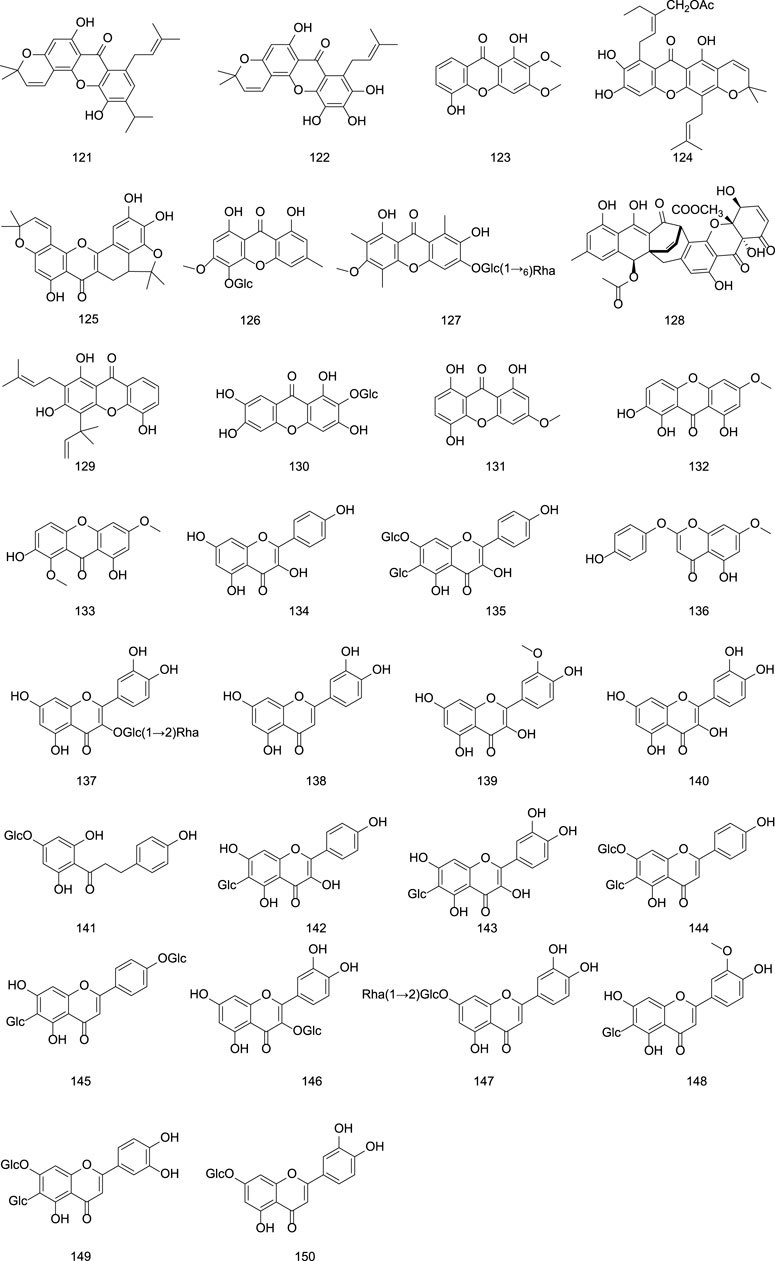
The flavonoid constituents in G. rigescens are primarily composed of benzochromones and flavonoid glycosides. Representative compounds include oliganthaxthanone A (121), pinetoxanthone (122), mangiferin (130), luteolin (138), kaempferol (134), quercetin (140), isoorientin (142), isovitexin (143), and their derivatives (Liu, 2004; Zhou et al., 2017; Wang, 2015; Yu, 2006; Olennikov et al., 2015). as shown in Table 5 and Figure 5.
2.3 Lignans
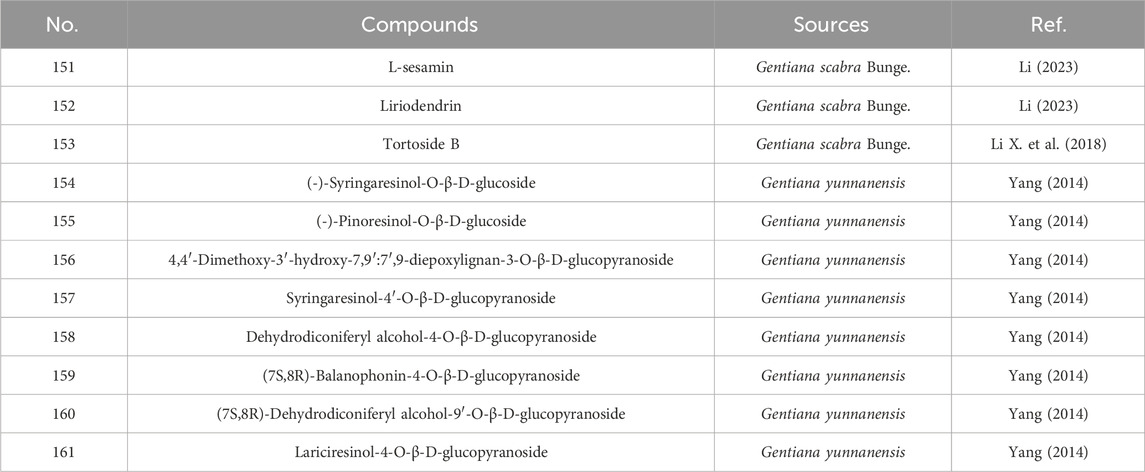
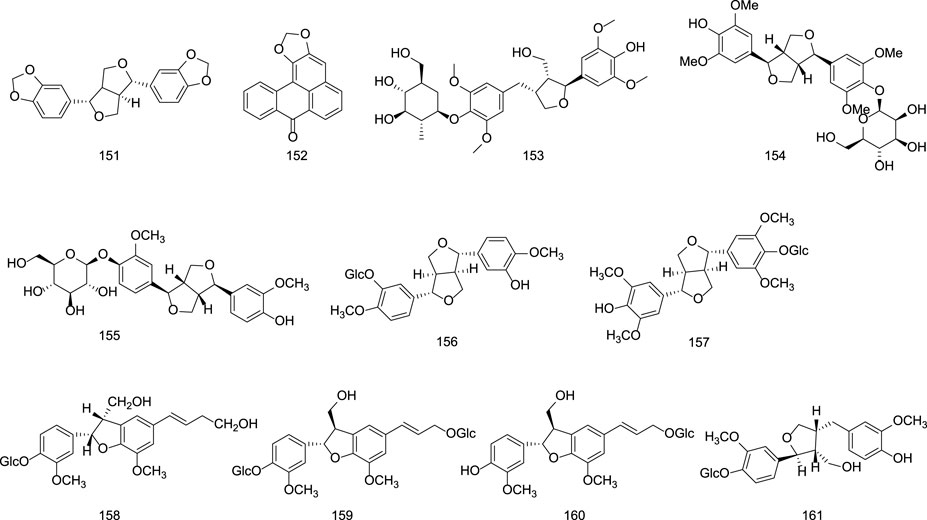
Representative lignans isolated from G. rigescens include L-sesamin (151), liriodendrin (152), tortoside B (153), and lignan glycosides such as (−)-syringaresinol-O-β-D-glucoside (154), (−)-pinoresinol-O-β-D-glucoside (155), syringaresinol-4′-O-β-D-glucopyranoside (157), and lariciresinol-4-O-β-D-glucopyranoside (161) (Liu, 2004; Zhang et al., 2019; Ye et al., 2018). Specific information on lignans constituents from Gentianae Radix et Rhizoma can be found in Table 6 and Figure 6.
2.4 Alkaloids
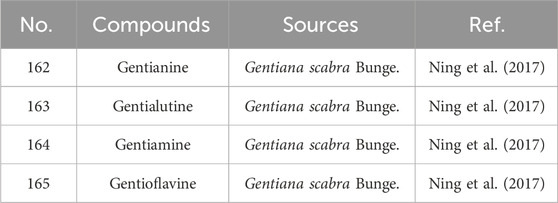
Since the 1950s, several alkaloids, including gentianine (162), gentialutine (163), gentiamine (164), and gentioflavine (165), have been isolated from G. rigescens (Sun and Xia, 1984). However, studies suggest that these alkaloids may not be naturally occurring in the plant but rather artifacts formed during extraction. For instance, gentianine and gentialutine are proposed to arise from the conversion of gentiopicroside in the presence of ammonia during processing. Such interconversions between constituents not only influence the diversity and abundance of alkaloids but also modulate their pharmacological profiles. For example, gentianine exhibits significant anti-inflammatory, sedative, and antibacterial activities, whereas its precursor gentiopicroside primarily demonstrates hepatoprotective, anti-inflammatory, and immunomodulatory effects (Ning et al., 2017). Specific information on alkaloids constituents from Gentianae Radix et Rhizoma can be found in Table 7 and Figure 7.
2.5 Other constituents
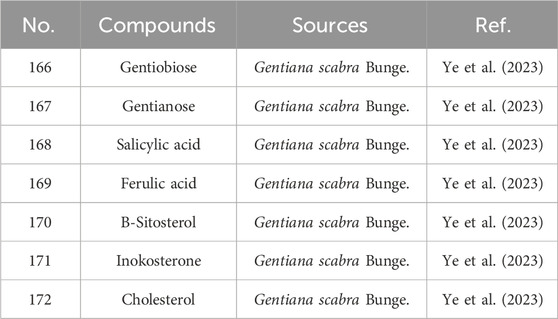
Beyond terpenoids, flavonoids, lignans, and alkaloids, Gentianae Radix et Rhizoma contain diverse secondary metabolites. Polysaccharides such as gentiobiose (166) have been isolated (Wang, 2014; Jiang et al., 2008). Phenolic acids, including salicylic acid (168) and ferulic acid (169), are also documented (Liu, 2004). Additionally, Gentiana contains amino acids (e.g., threonine, valine, methionine) and essential minerals such as calcium (Ca), copper (Cu), iron (Fe), zinc (Zn), and manganese (Mn) (Ye et al., 2023). Steroidal compounds, including β-sitosterol (170) and inokosterone (171), have been identified (Zhao et al., 2009; Guo and Piao, 2011). The specific information is presented in Table 8 and Figure 8.
The identified iridoids and flavonoids not only contribute to the pharmacological efficacy but also serve as pivotal quality markers (Q-markers) for the standardization of Gentianae Radix et Rhizoma. Extraction methodologies across cited studies predominantly utilized methanol or ethanol (60%–95%) via reflux/maceration. For instance: Iridoids (e.g., gentiopicroside): 70% ethanol reflux, 2 h; Triterpenoids: Methanol maceration (48 h) silica gel chromatography; Polysaccharides: Hot water extraction (90 °C, 3 h); Comparative data suggest methanol outperforms ethyl acetate for iridoid yield (Δ = 12–18%), while ultrasound-assisted extraction reduces processing time by 40%.
Key compounds such as gentiopicroside, swertiamarin, and luteolin are critical for species authentication and ensuring batch-to-batch consistency, with HPLC-UV analysis typically requiring a relative standard deviation (RSD) of less than 5% for these benchmarks (Hu and Li, 2002). The establishment of these Q-markers bridges the chemical profiles with the efficacy, laying a foundation for quality control in pharmaceutical applications.
3 Biological activities
Gentianae Radix et Rhizoma, a traditional Chinese medicinal herb commonly known as Longdan, has been utilized for millennia to clear heat, dry dampness, and purge liver-gallbladder fire. Its therapeutic applications were first documented in the Shennong Bencao Jing (ca. 200 CE), where it was classified as a middle-grade herb with bitter-cold properties, noted for treating bone-interstice disorders, convulsions, and parasitic toxins, while enhancing cognition and longevity with prolonged use (Liu, 2022). Subsequent dynastic texts expanded its pharmacological profile. The Mingyi Bielu (Liang Dynasty) highlighted its efficacy in resolving gastric heat, seasonal febrile diseases, and heat-type diarrhea (Li et al., 2011), establishing its foundational role in heat-clearing and damp-drying therapies.
During the Song-Yuan period, the Taiping Shenghui Fang introduced the prototype of Longdan Xiegan Tang (Gentian Liver-Draining Decoction), combining gentian with Bupleurum and Scutellaria to address liver-gallbladder fire excess syndromes (Wan, 2022). Kou Zongshi’s Bencao Yanyi emphasized its morphological distinction as a short-rooted herb with intense bitterness (Zhu et al., 2021). By the Ming-Qing era, Li Shizhen’s Bencao Gangmu systematized its uses, including throat pain, wind-heat night sweats, and ocular inflammation, while noting enhanced efficacy through wine-processing (Li and Wang, 2023). The Yaopin Huiyi further refined its meridian tropism, specifying its action on liver-gallbladder fire and associated disorders such as ocular pain and pediatric convulsions (Zhang, 2015).
Modern pharmacological studies validate its bioactive potential, demonstrating anti-inflammatory, analgesic, hepatoprotective, choleretic, antitumor, antioxidant, and digestive properties (Liu et al., 2024; Li et al., 2024; Zhang et al., 2023; Li, 2023; Wang et al., 2023). The 2020 Chinese Pharmacopoeia lists six gentian-containing formulas, including Longdan Xiegan Wan and Qingre Jiedu Koufuye, reflecting its clinical versatility. Clinically, it is widely employed in managing herpes zoster, hypertension, sudden deafness, and inflammatory conditions, with Longdan Xiegan Tang remaining a cornerstone therapy for damp-heat liver disorders. Globally, gentian is recognized as a bitter stomachic, underscoring its cross-cultural pharmacological relevance (Yang and Wang, 2005).
This integration of historical wisdom and contemporary science positions Gentianae Radix et Rhizoma as a multifaceted medicinal agent, bridging traditional applications with evidence-based therapeutic potential.
3.1 Anti-inflammatory and analgesic effects
As a pivotal herb in traditional heat-clearing and damp-drying therapies, Gentianae Radix et Rhizoma has garnered significant attention for its anti-inflammatory and analgesic properties. Modern studies reveal that these activities stem from multi-target and multi-pathway synergistic mechanisms, involving inflammation mediator regulation, signaling pathway modulation, and neurotransmitter adjustment, with demonstrated efficacy across diverse disease models.
3.1.1 Molecular mechanisms of anti-inflammatory action and disease intervention
The anti-inflammatory effects of Gentianae Radix et Rhizoma primarily target the NF-κB (Nuclear Factor κB) signaling axis. Experimental evidence confirms that its active component, gentiopicroside, inhibits IκB kinase β (IKKβ) phosphorylation, blocks NF-κB nuclear translocation, and subsequently downregulates key inflammatory mediators such as COX-2 (Cyclooxygenase-2) and TNF-α (Tumor Necrosis Factor-α). Gentiopicroside directly inhibits the phosphorylation of IKKβ, which prevents the degradation of IκB and subsequent nuclear translocation of the NF-κB p65 subunit. This blockade leads to the downregulation of key inflammatory mediators, including COX-2, TNF-α, and IL-6. Concurrently, gentiopicroside suppresses the activation of JNK and p38 within the MAPK pathway, further reducing the production of pro-inflammatory cytokines. In rheumatoid arthritis models, this mechanism significantly alleviates synovial inflammation and joint destruction. Concurrently, it suppresses JNK (c-Jun N-terminal Kinase)/p38 activation in the MAPK (Mitogen-Activated Protein Kinase) pathway, reducing macrophage secretion of IL-6 (Interleukin-6) and IL-1β (Interleukin-1β), thereby mitigating inflammatory infiltration in alcoholic liver injury. An in vivo study demonstrated that the ethyl acetate crude extract of Gentiana striata Maxim alleviated paw edema in a rat model of rheumatoid arthritis after 28 days of oral administration at doses of 100 and 200 mg/kg. This protective effect was associated with decreased levels of PGE2 and NO and was superior to that achieved with 100 mg/kg prednisone, a common anti-inflammatory drug (Cao et al., 2012). Notably, Gentianae Radix et Rhizomacomponents also remodel the inflammatory microenvironment via epigenetic regulation: in ulcerative colitis models, they promote macrophage polarization toward the M2 anti-inflammatory phenotype and inhibit histone deacetylase activity, enhancing chromatin accessibility of anti-inflammatory genes (Xie et al., 2021).
3.1.2 Multi-dimensional regulation of analgesic effects
The analgesic mechanisms of Gentianae Radix et Rhizoma extend beyond conventional anti-inflammatory frameworks, exerting analgesic effects through a central-peripheral synergistic network. Centrally, gentiopicroside downregulates NR2B (N-Methyl-D-aspartate receptor subunit 2B) subunit expression of NMDAR (N-Methyl-D-Aspartate Receptor) in the anterior cingulate cortex, inhibiting glutamatergic synaptic transmission and blocking pain sensitization (Chen et al., 2008). Peripherally, it reduces substance P release in the spinal dorsal horn and activates the μ-opioid receptor pathway to stimulate endogenous analgesic substances such as β-endorphin (Jia et al., 2012). In neuropathic pain models, this multi-target approach significantly alleviates mechanical allodynia and thermal hyperalgesia without inducing tolerance typically associated with traditional opioids (Xie et al., 2021). Particularly in chronic inflammatory pain, Gentianae Radix et Rhizoma synergistically enhance anti-inflammatory and analgesic outcomes by inhibiting PGE2 (Prostaglandin E2) synthesis and TRPV1 (Transient Receptor Potential Vanilloid 1) channel activation, offering novel strategies for managing inflammatory bowel disease. The specific compounds with anti-inflammatory and analgesic effects, as well as their mechanisms of action, are presented in Table 9.
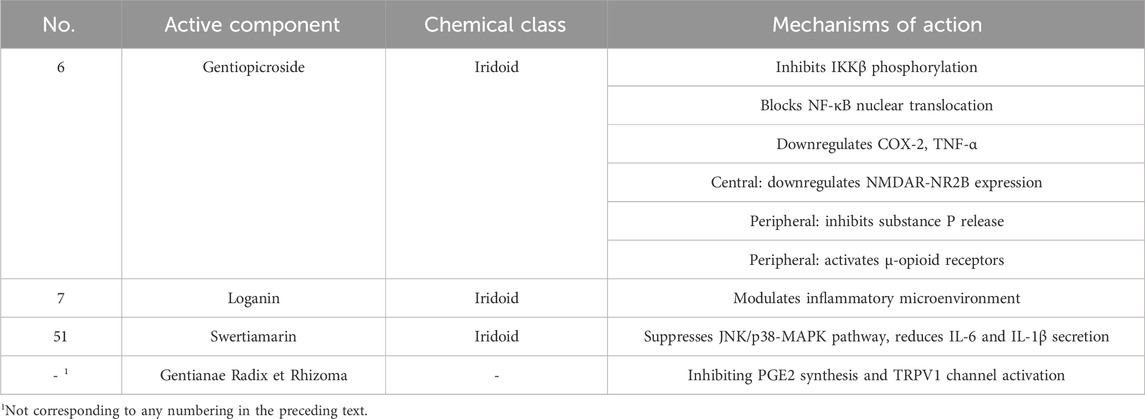
Table 9. Anti-inflammatory and analgesic compounds in Gentianae Radix et Rhizoma: Bioactive components and mechanisms of action.
3.2 Hepatoprotective and choleretic activities
Gentianae Radix et Rhizomaexerts hepatoprotective effects through a multidimensional “antioxidant-anti-inflammatory-metabolic modulation-anti-fibrotic” mechanism, while its choleretic activity is closely associated with bile acid transport regulation. Core constituents such as gentiopicroside and swertiamarin have emerged as pivotal lead compounds for liver disease drug development, particularly in alcohol-associated liver disease (ALD), metabolic dysfunction-associated steatotic liver disease, and hepatic fibrosis.
3.2.1 Hepatoprotective mechanisms
Crude Gentianae Radix et Rhizomaextracts and gentiopicroside significantly reduce ALT (Alanine Aminotransferase) and AST (Aspartate Aminotransferase) levels in acute liver injury models induced by CCl4 or D-galactosamine, while enhancing glutathione peroxidase and superoxide dismutase (SOD) activity, reducing malondialdehyde (MDA) accumulation, directly scavenging free radicals, and reinforcing hepatic antioxidant defenses (Kikuchi et al., 2005). Swertiamarin accelerates toxicant metabolism by activating cytochrome P450 family 3 subfamily A member 4 (CYP3A4) and cytochrome P450 family 2 subfamily E member 1 (CYP2E1) enzymes, thereby attenuating CCl4-induced hepatotoxicity. Oral swertiamarin (100–200 mg/kg, 8 weeks) alleviated CCl4-induced hepatotoxicity in rats by activating the Nrf2/HO-1 pathway, reducing oxidative stress and inflammation (Wu et al., 2017). Additionally, Gentianae Radix et Rhizomacomponents suppress NF-κB signaling, downregulating hepatic pro-inflammatory cytokines such as TNF-α and IL-6, thereby ameliorating lipopolysaccharide-and Bacillus Calmette-Guérin-induced liver injury. In ALD models, gentiopicroside targets the P2X purinoceptor 7 (P2X7) receptor/NOD-like receptor thermal protein domain-associated protein 3 (NLRP3) inflammasome axis, inhibiting inflammasome activation, reducing lipogenesis, and promoting lipid oxidation to alleviate alcoholic steatosis (Li X. et al., 2018). Swertiamarin exerts its hepatoprotective effects by activating the Nrf2 antioxidant pathway. Furthermore, gentiopicroside targets the P2X7 receptor, inhibiting NLRP3 inflammasome assembly and subsequent IL-1β maturation.
3.2.2 Anti-fibrotic interventions
Angiotensin system modulation: Swertiamarin inhibits AngII-AT1R signaling, blocking ERK (Extracellular Signal-Regulated Kinase)/c-Jun phosphorylation, thereby attenuating N-nitrosodimethylamine-induced hepatic stellate cell activation and fibrosis. The in vitro study using primary rat hepatic stellate cells showed that purified tetramethylpyrazine (5–20 μM, 12–24 h treatment) inhibited Ang II-induced activation, with DMSO as vehicle control and imatinib/rapamycin as positive controls (Zhang et al., 2014). This in vitro and in vivo study demonstrated that purified swertiamarin (Swe, 98% purity) at doses of 2.4–15 µM (in primary rat HSCs) and 15–20 mg/kg (in DMN-induced fibrotic rats) inhibited angiotensin II–induced activation and fibrosis, with losartan as a positive control and vehicle as negative control; treatment durations were 24 h (in vitro) and 2 weeks (in vivo), but no IC50/EC50 values were provided (Li et al., 2016). TGF-β1/Smad pathway regulation: G. rigescens suppresses TGF-β1/Smad2/3 signaling, downregulates connective tissue growth factor expression, reduces collagen deposition, and mitigates bleomycin-induced hepatic fibrosis (Li X. et al., 2018). Lipid metabolic balance: Gentiopicroside reduces triglyceride accumulation in ALD by modulating fatty acid synthase (FASN) and carnitine palmitoyltransferase 1 (CPT1) expression, restoring lipid oxidation-synthesis equilibrium. The study used acute and chronic alcoholic hepatosteatosis mouse models (40–80 mg/kg GPS) and ethanol-exposed HepG2 cells to show that purified genitopicroside (>99%) improved lipid metabolism via LKB1/AMPK activation and P2x7R–NLRP3 inflammasome inhibition. Metformin and A438079 served as positive controls, with treatments lasting 24 h (in vitro) to 10 days (in vivo); IC50/EC50 values were not provided (Lian et al., 2018).
3.2.3 Choleretic mechanisms
Swertiamarin enhances bile acid efflux by upregulating bile salt export pump (BSEP) and multidrug resistance-associated protein (MRP) expression, improving cholestasis. Crude Gentianae Radix et Rhizoma extracts increase hepatocyte membrane fluidity, enhance hepatic microcirculation, and alleviate biliary dysfunction in thioacetamide-induced models. In cecal ligation and puncture (CLP)-induced septic liver injury, gentiopicroside protects hepatocyte mitochondrial function by inhibiting inducible nitric oxide synthase activity and reducing excessive nitric oxide production (Zhang et al., 2022). The hepatoprotective and choleretic compounds in Gentianae Radix et Rhizoma, along with their bioactive components and mechanisms of action, are displayed in Table 10.
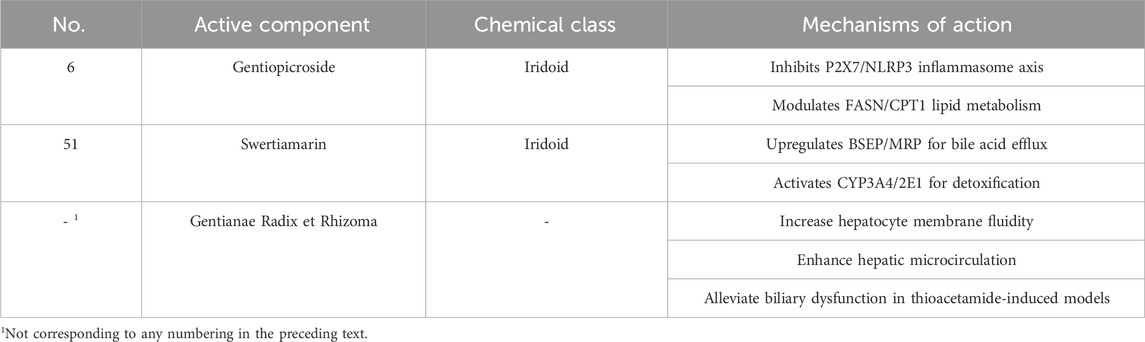
Table 10. Hepatoprotective and Choleretic compounds in Gentianae Radix et Rhizoma: Bioactive components and mechanisms of action.
3.3 Antitumor activity
Gentianae Radix et Rhizomaexhibits broad-spectrum antitumor activity through multi-component interactions (e.g., gentiopicroside, polysaccharides, and Luteolin), targeting cell cycle regulation, apoptosis induction, autophagy modulation, and critical signaling pathway inhibition.
3.3.1 Inhibition of tumor cell proliferation and cell cycle arrest
Gentiopicroside activates the p38/MAPK signaling pathway, upregulates pro-apoptotic B-cell lymphoma-2-associated X protein (Bax), downregulates anti-apoptotic B-cell lymphoma-2 (Bcl-2), and suppresses proliferation in human ovarian cancer HO8910 cells. It induces S-phase and G2/M-phase arrest in hepatocellular carcinoma HepG2 cells while increasing the G0/G1-phase population, effectively inhibiting proliferation. This compound also significantly reduces viability in human liver cancer SMMC-7721 and lung cancer A549 cells. Gentianae Radix et Rhizoma polysaccharides enhance thymic and splenic indices in tumor-bearing mice post-chemotherapy, boosting immune responses. Additionally, compounds such as gentiakochianin and gentiacaulein induce apoptosis in glioma U251 cells by reducing mitochondrial membrane potential and promoting reactive oxygen species (ROS) generation. Luteolin inhibits non-small cell lung cancer (NSCLC) proliferation and induces autophagy via suppression of the PI3K/Akt/mTOR/p70S6K signaling axis. Furthermore, the study demonstrated through in vitro assays that certain triterpenoids isolated from Gentiana scabra exhibit potent inhibitory activity against human indoleamine 2,3-dioxygenase (IDO), with the most active compounds showing IC50 values below 10 μM (Li et al., 2015).
3.3.2 Multi-pathway antitumor effects
Crude Gentianae Radix et Rhizomaextracts inhibit proliferation in diverse cancer cell lines in vitro, including lung A549, colon HCT-116, prostate PC-3, and breast MCF-7 cells. In vivo, these extracts significantly suppress growth of mouse sarcoma S180 solid tumors, with medium- and high-dose groups showing tumor inhibition rates exceeding 30%. Specific compounds, such as chirat-16-en-3-one and chiratenol, demonstrate antiproliferative effects against cervical cancer HeLa cells. Globuloside A, cornusoside A, cornolactone A, 6,9-epi-8-O-acetylshanziside methyl ester, and polar extracts (petroleum ether, ethyl acetate) from Gentiana manshurica exhibit potent inhibition of HepG2 cell viability (Isakovic et al., 2008). The antitumor components in Gentianae Radix et Rhizoma, including their bioactive components and mechanisms of action, are presented in Table 11.
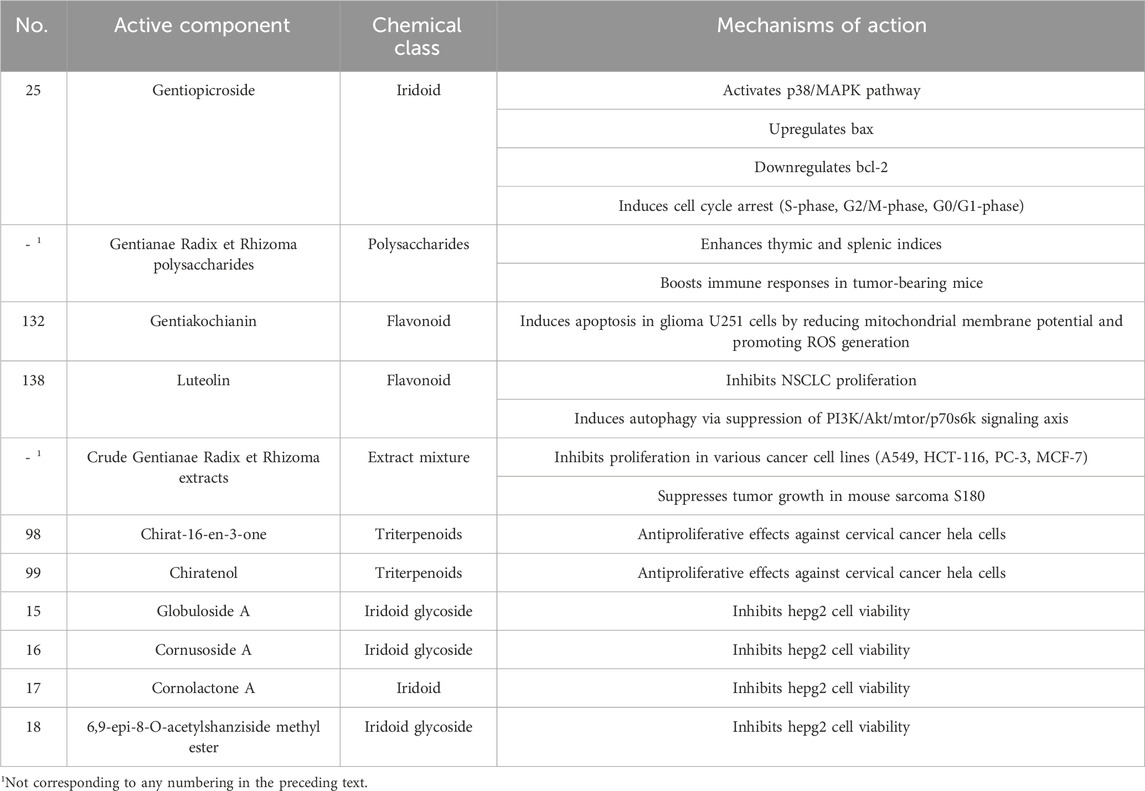
Table 11. Antitumor component in Gentianae Radix et Rhizoma: Bioactive components and mechanisms of action.
3.4 Regulation of gastrointestinal function
Gentiopicroside, a key bioactive component of G. scabra, improves gastrointestinal function through enhancing gastric motility, protecting gastric mucosa, and regulating gastrointestinal hormones.
3.4.1 Promotion of gastric motility
Gentiopicroside ameliorates stress-induced gastrointestinal dysmotility in rat models by upregulating motilin receptor expression and downregulating vasoactive intestinal peptide receptor 2 (VIPR2) levels, thereby enhancing gastric emptying and intestinal peristalsis (Ruan et al., 2015).
3.4.2 Gastric mucosal protection
In ethanol-induced gastric mucosal injury models, gentiopicroside upregulates heat shock protein-70 (HSP70), restores epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) levels, and promotes mucosal repair (Yang et al., 2018). The gastrointestinal-regulating bioactive compounds in Gentianae Radix et Rhizoma and their mechanisms of action are presented in Table 12.

Table 12. Gastrointestinal-Regulating Bioactive compounds in Gentianae Radix et Rhizoma: mechanisms of action
3.5 Regulation of the nervous system
The neuroregulatory properties of Gentianae Radix et Rhizomaare primarily mediated by its core constituent, gentiopicroside, which exerts neuroprotective, anti-neurodegenerative, and psychotherapeutic effects through multi-target synergy involving oxidative stress modulation, neurotransmitter regulation, and signaling pathway intervention.
3.5.1 Neuroprotective effects
Antioxidant Synergy and Cell Survival: Bellidifolin, gentisides A/B, amarogentin, and gentiopicroside enhance neuronal survival under oxidative stress (e.g., H2O2-induced PC12 cells) by activating insulin receptor downstream pathways, including PI3K/Akt and Ras/Raf/ERK. These compounds elevate superoxide dismutase (SOD, SOD2) activity, reduce ROS and MDA levels, and mitigate oxidative neuronal damage (Deng, 2015). Inokosterone further attenuates mitochondrial dysfunction-mediated apoptosis in oxidative stress models.
3.5.2 Cognitive enhancement
G. rigescens extracts enhance cognitive function in memory-impaired models by inhibiting acetylcholinesterase activity, thereby preserving acetylcholine levels, and by modulating the insulin-like growth factor 1 receptor and ERK signaling pathway (Ma, 2012).
3.5.3 Antidepressant and analgesic effects
Gentiopicroside alleviates pain-depression comorbidity in reserpine-induced models by downregulating glutamate NMDA receptor subunit 2B (GluN2B) subunit expression of NMDA receptors in the basolateral amygdala, reducing glutamatergic excitotoxicity. It also restores monoamine neurotransmitter balance (e.g., serotonin, dopamine), demonstrating antidepressant efficacy (Deng, 2015; Ma, 2012).
3.5.4 Anti-parkinsonian effects
In 6-hydroxydopamine-induced Parkinson’s disease models, gentiopicroside ameliorates motor deficits and tremors by inhibiting dopaminergic neuron degeneration in the nigrostriatal pathway, suppressing α-synuclein aggregation, and attenuating neuroinflammation (Yang, 2014).
3.5.5 Anti-addictive effects
Gentiopicroside reduces morphine-induced addictive behaviors by modulating opioid receptor signaling and dopamine reward pathways, reversing drug dependence-associated neuroplasticity (Ma, 2012).
3.5.6 Central nervous system stimulation
Early studies indicate that gentianine enhances central nervous system excitability in mice, potentially through γ-aminobutyric acid (GABA) ergic system modulation (Sun and Xia, 1984). The nervous system-regulating bioactive compounds in Gentianae Radix et Rhizoma and their mechanisms of action are presented in Table 13.
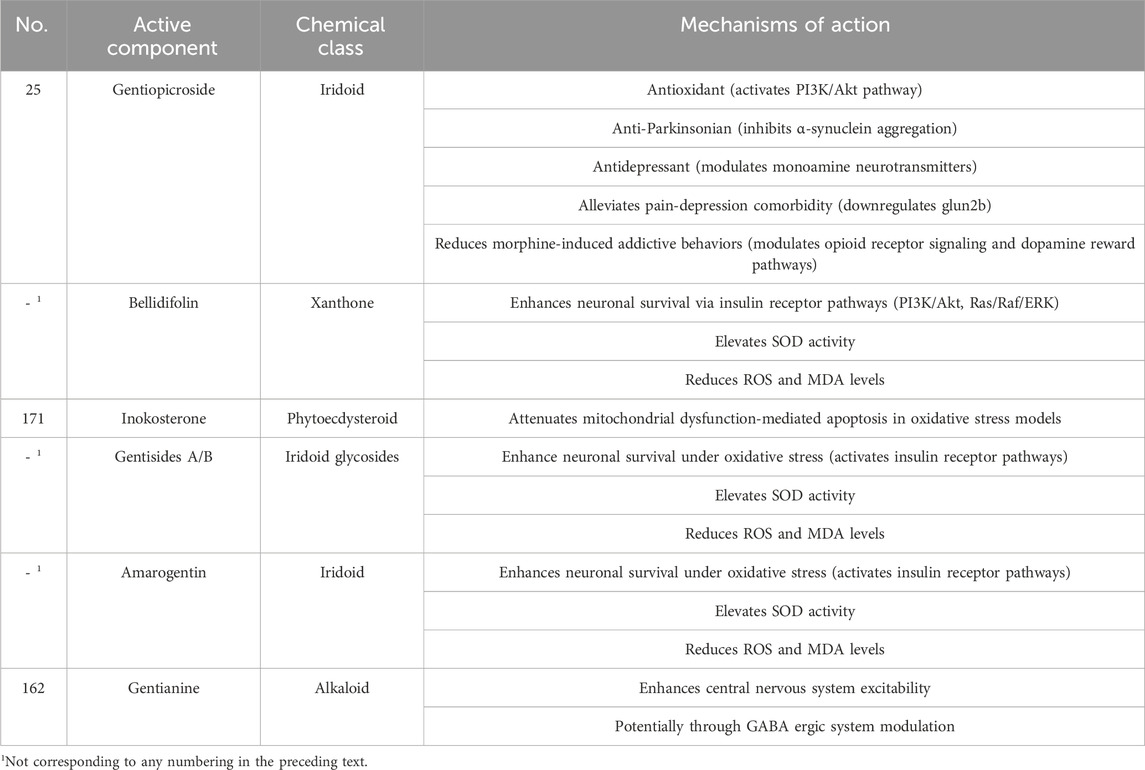
Table 13. Nervous system-Regulating Bioactive compounds in Gentianae Radix et Rhizoma: mechanisms of action.
3.6 Antioxidant activity
Reports in the literature indicate that flavonoids and iridoids in Gentiana possess antioxidant activity. Li Peiyuan et al. (Li PY. et al., 2018) used the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical to measure the antioxidant capacity of Gentiana extracts and found that flavonoid compounds extracted with different solvents can effectively scavenge DPPH radicals, with antioxidant activity being directly proportional to the total flavonoid content. Additionally, GSP-IIb, GSP-IIa and iridoid compounds isolated from the rhizomes of Gentiana can scavenge DPPH radicals, exhibiting stable antioxidant activity (Suyama et al., 2013). Gentirigeoside B exerts antioxidant effects by inhibiting the mTOR/Sch9/Rim15/Msn signaling pathway and enhancing autophagy (Xiang et al., 2022). Furthermore, in vitro assays of 60% methanol extracts from six Caucasian Gentiana species (herbs and roots) demonstrated antioxidant (DPPH, superoxide scavenging, lipid peroxidation inhibition) and digestive enzyme inhibitory (α-amylase/α-glucosidase) activities, with positive controls including trolox, quercetin, caffeic acid, and acarbose; activity strongly correlated with phenolic content, though no specific IC50 values or dose ranges for crude extracts were provided (Olennikov et al., 2019). The antioxidant bioactive compounds in Gentianae Radix et Rhizoma and their mechanisms of action are presented in Table 14.
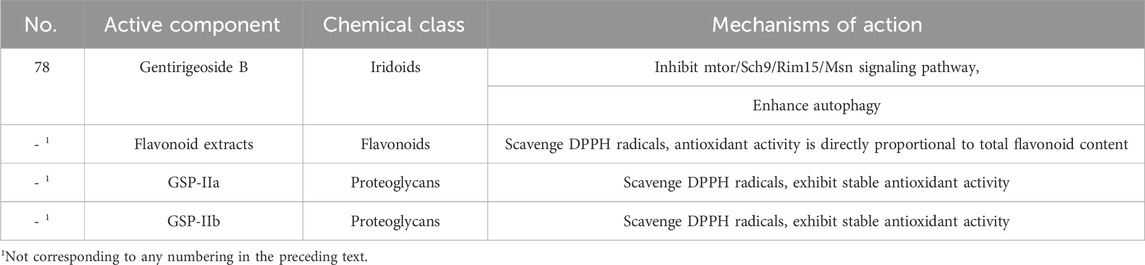
Table 14. Antioxidant component in Gentianae Radix et Rhizoma: Bioactive components and mechanisms of action.
3.7 Other effects
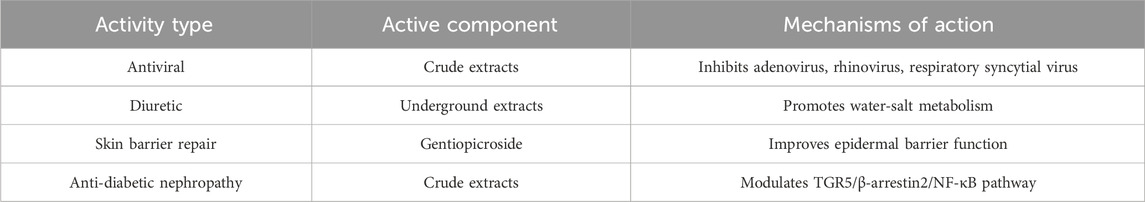
In vivo studies in normal, glucose-hyperglycemic, and streptozotocin-induced diabetic rats demonstrated that the ethyl acetate fraction of a methanol extract of Gentiana olivieri aerial parts, and its isolated active constituent isoorientin (doses: 7.5–30 mg/kg), significantly reduced blood glucose levels, with a minimal effective dose of 15 mg/kg; positive controls included tolbutamide, negative controls received vehicle, and treatment durations ranged from acute (four to six h) to subacute (15 days) administration (Sezik et al., 2005). Gentianae Radix et Rhizoma extracts and have demonstrated the ability to reduce the activity of hepatic enzymes such as alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase, as well as modulate the bile acid receptor G protein-coupled bile acid receptor 1 (TGR5)/β-arrestin2/NF-κB signaling pathway, thereby delaying the progression of diabetic nephropathy in mice fed a high-fat diet (Ghazanfar et al., 2017; Xi et al., 2020). Gentiopicroside can be utilized to improve skin conditions with impaired epidermal barriers (Wölfle et al., 2017); it also inhibits adipogenesis by modulating the 3T3-L1 pathway (Choi et al., 2019). Gentianae Radix et RhizomaBunge. Exhibits significant activity against adenovirus type 5 (C type), human rhinovirus type B (subtype 14), and respiratory syncytial virus; the underground parts of Gentianae Radix et Rhizoma possess diuretic effects (You, 2017). The other bioactivities of Gentianae Radix et Rhizoma are presented in Table 15.
Notwithstanding its wide spectrum of pharmacological activities, it is crucial to consider its safety profile; traditional wisdom cautions against its use in cases of spleen-stomach deficiency with cold symptoms due to its potent bitter and cold nature (Zhu et al., 2021), while modern toxicological assessments indicate a relatively low acute toxicity (LD50 > 5 g/kg in mice) and an absence of genotoxic concern as per OECD guidelines.
4 Clinical applications
As a classic herb in traditional Chinese medicine (TCM) for clearing heat, drying dampness, and purging liver-gallbladder fire, Gentianae Radix et Rhizoma has demonstrated enduring clinical value through centuries of practice. Modern research on its compound formulations and bioactive constituents has expanded its applications beyond traditional liver-gallbladder damp-heat syndromes to dermatological, cardiovascular, gynecological, otolaryngological, and systemic disorders, establishing a modern therapeutic framework integrating “disease-pattern differentiation, formula compatibility, and mechanistic clarity”.
4.1 Dermatological disorders: herpes zoster and postherpetic neuralgia
Herpes zoster, caused by reactivation of varicella-zoster virus (VZV), is attributed to liver fire hyperactivity and damp-heat toxin accumulation in TCM. G. scabra-based formulas exert antiviral, immunomodulatory, and analgesic effects (Sun and Sun, 2007; Liu et al., 2021).
4.1.1 Acute phase treatment
Longdan Xiegan Tang (Gentian Liver-Draining Decoction) (Cao, 2022; Wu and Chen, 2022; Zhang and Zhang, 2018): Combining Gentianae Radix et Rhizoma (as the sovereign herb) with Scutellaria and Gardenia, this formula alleviates herpetic pain and accelerates crust formation.
4.1.2 Inhibition of VZV replication and viral load reduction
Downregulation of serum TNF-α and IL-6, upregulation of IL-12 and CD3+/CD4+ T cells to enhance Th1 immune responses. Modulation of neuropeptides (e.g., substance P, β-endorphin) to reduce neurogenic inflammation.Longdan Jiedu Tang (Gentian Toxin-Resolving Decoction) (Sun and Sun, 2007): Enhanced with Isatis root and Clerodendrum, this formula shows superior efficacy in bacterial co-infections by reducing IgE levels, suppressing mast cell degranulation, and inhibiting histamine release to alleviate pruritus.
4.1.3 Postherpetic neuralgia management
Longdan Shenmai Zhijing Tang (Gentian-Ginseng Spasm-Relieving Decoction) (Shao et al., 2022): Targets residual damp-heat and qi-yin deficiency by inhibiting spinal dorsal horn glial cell activation, reducing IL-1β/TNF-α-mediated neuro-sensitization, and upregulating neurotrophic factors for nerve repair. Bloodletting Acupuncture Combined Therapy: Adjunctive use with modified Longdan Xiegan Tang enhances CD8+ T cell activity and cytotoxic T lymphocyte responses to eliminate pathogens and restore microcirculation.
4.1.4 Special localizations
Ramsay Hunt Syndrome: Longdan Dai Xie Xiao Zhen Tang (Gentian-Indigo-Scorpion Rash-Resolving Decoction) achieves an 89.3% efficacy rate by suppressing VZV reactivation in trigeminal ganglia and reducing vestibular nerve edema, improving otalgia and facial paralysis (Zhang, 2009).
4.2 Cardiovascular disorders: Hypertension and complications
Longdan Xiegan Tang exemplifies integrated TCM-Western therapy for liver fire-induced hypertension, targeting mechanisms such as: Blood Pressure Regulation: Suppression of angiotensin II/AT1 receptor signaling to counteract vasoconstriction and myocardial fibrosis. Activation of eNOS/NO pathway and inhibition of endothelin-1 to restore endothelial function. Metabolic Syndrome Management: Reduces total cholesterol, low-density lipoprotein, and blood pressure variability through HMG-CoA reductase inhibition, bile acid excretion, and modulation of the autonomic nervous system, particularly by enhancing vagal tone (Li, 2023; Li, 2014; Li, 1998; Yang et al., 2022; Zheng and RUAN, 2018; Zuo et al., 2020).
4.3 Hepatobiliary disorders: chronic hepatitis B and fatty liver
4.3.1 Chronic hepatitis B
Fufang Xiongdan Yigan Jiaonang (Compound Bear Bile Hepatitis Capsule) (Hu and Li, 2002): Inhibits Hepatitis B virus DNA replication and promotes HBeAg seroconversion by blocking viral transcription and enhancing interferon-γ responses. Modified Longdan Xiegan Tang: Reduces liver stiffness and fibrosis via TGF-β1/Smad3 pathway inhibition and matrix metalloproteinase activation (Liu et al., 2017; Lyu and Lin, 2018; Wang et al., 2017).
4.3.2 Non-alcoholic fatty liver disease
Gentiopicroside activates PPARα/CPT1 to enhance fatty acid β-oxidation and suppresses SREBP-1c-mediated lipogenesis. Combined with lifestyle intervention, Longdan Xiegan Tang reduces hepatic steatosis and serum free fatty acids (Ge et al., 2022).
4.4 Gynecological inflammation: Cervicitis, vaginitis, and HPV infection
Cervicitis with HPV: Longdan Xiegan Tang downregulates cervical IL-8, TNF-α, and HPV E6/E7 oncoprotein expression while enhancing secretory IgA and dendritic cell antigen presentation (Dai et al., 2023; Zhou, 2012; Zhou et al., 2013a; Zhou et al., 2013b; Jiang, 2018). Bacterial Vaginosis: Modified formulations disrupt biofilms, inhibit bacterial adhesins, and restore vaginal microbiota (lactobacilli increased by three to four log units; pH < 4.5) (Liu et al., 2017; Lyu and Lin, 2018; Wang et al., 2017).
4.5 Otolaryngological and ophthalmic disorders
Otitis Media: Longdan Xiegan Capsules with tympanocentesis reduce middle ear effusion and TGF-β1/β2 levels via TLR4/MyD88/NF-κB pathway inhibition (Su and Mou, 2022). Chronic Rhinosinusitis: Corrects Th17/Treg imbalance by suppressing IL-17A/IL-22 and improving Lund-Kennedy endoscopic scores (Li, 2020). Herpetic Keratitis: Longdan Mingmu Tang (Gentian Vision-Clearing Decoction) with ganciclovir inhibits HSV UL54 gene expression and enhances corneal repair (Huo and Guo, 2000; Qu, 2022). Acupuncture adjunct therapy modulates trigeminal ganglion microRNAs (miR-155, miR-146a) to reduce recurrence (Lu and Su, 2018).
4.6 Emerging applications
Diabetic Nephropathy: Gentiana extracts attenuate renal fibrosis via TGR5 receptor activation, inhibiting tubular epithelial-mesenchymal transition (EMT) and NF-κB-driven inflammation (Jiang et al., 2019). Chronic Kidney Disease: Diuretic effects of Gentiana roots match furosemide in sodium excretion but with reduced potassium loss, offering safer edema management (Huang, 2020).
The clinical applications and mechanisms of Gentianae Radix et Rhizoma and its compound formulations are illustrated in Figure 9.
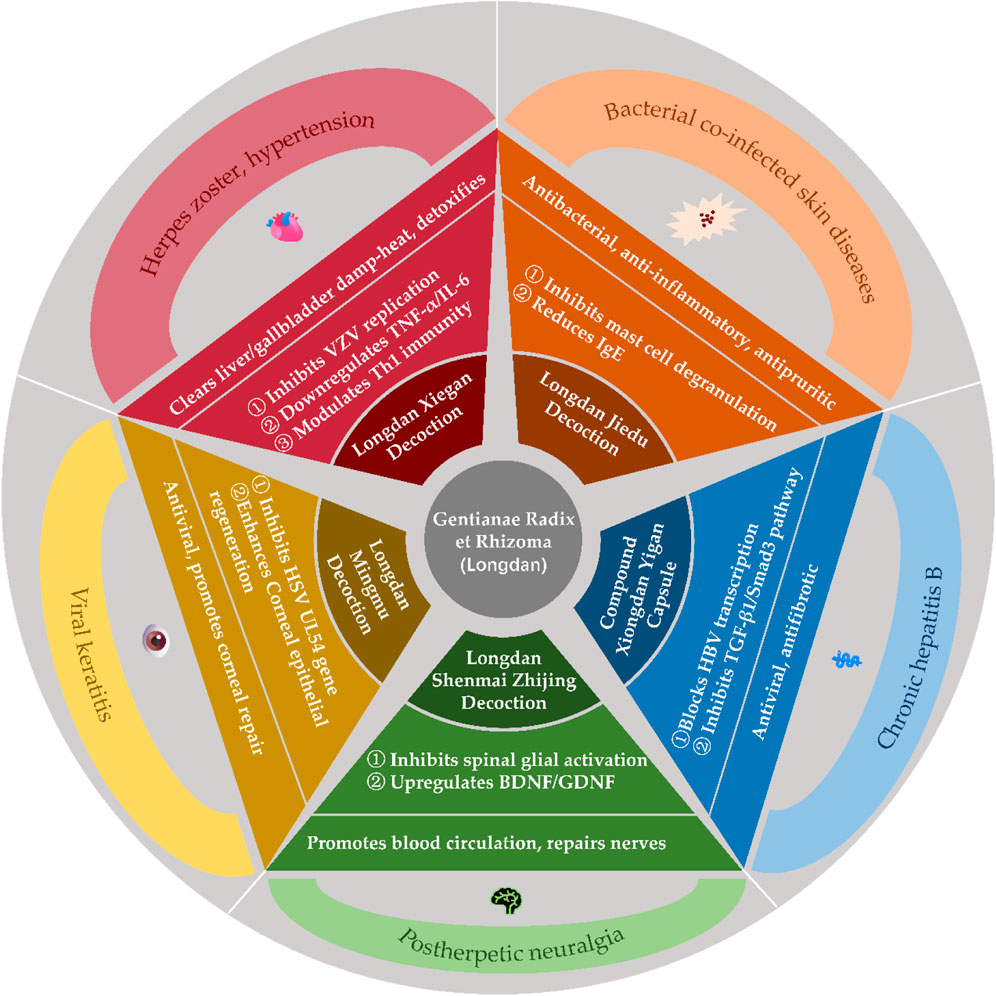
Figure 9. Clinical applications and Mechanisms of Gentianae Radix et Rhizoma and Its compound formulations.
5 Discussion and prospects
Gentianae Radix et Rhizoma, as a cornerstone of traditional and modern medicine, exhibits multifaceted therapeutic potential owing to its diverse chemical constituents and broad pharmacological activities. Despite significant advancements in identifying over 170 compounds, including terpenoids, flavonoids, and alkaloids, gaps remain in fully elucidating its chemical diversity. However, there is significant variation in efficacy between different species of Gentiana, such as G. scabra and G. rigescens. These species differ in their chemical profiles, which may affect their pharmacological actions. For instance, G. scabra is known for higher concentrations of certain iridoids like gentiopicroside, which contributes to its prominent anti-inflammatory and hepatoprotective properties. On the other hand, G. rigescens contains higher levels of specific triterpenoids, which may play a more substantial role in its antitumor and neuroprotective effects. These variations in chemical composition highlight the importance of considering species-specific differences when evaluating the therapeutic potential of Gentiana-based formulations. Advanced techniques such as metabolomics and AI-driven structural prediction could further uncover novel bioactive molecules, particularly minor or unstable constituents overlooked in conventional studies.
While the anti-inflammatory, hepatoprotective, and antitumor effects of key components like gentiopicroside and swertiamarin are well-documented, their molecular mechanisms—especially in modulating signaling pathways (e.g., TLR4/NF-κB, PI3K/Akt)—require deeper exploration. For instance, the interplay between gut microbiota and gentiopicroside’s neuroprotective effects or the epigenetic regulation of triterpenoids in fibrosis warrants systematic investigation.
Clinically, expanding applications beyond liver-gallbladder disorders to metabolic syndromes (e.g., diabetes, obesity) and neurodegenerative diseases (e.g., Parkinson’s, Alzheimer’s) represents a promising frontier. However, this necessitates rigorous clinical trials to validate efficacy and safety. Quality control remains a challenge; standardized protocols for active compound quantification, coupled with genomic and metabolomic fingerprinting, are critical to ensure batch consistency and minimize toxicity risks.
Furthermore, synergistic or antagonistic interactions between Gentiana extracts and conventional drugs (e.g., chemotherapeutics, antihypertensives) must be evaluated to optimize integrated therapies. Long-term toxicity studies and eco-friendly extraction methods should also be prioritized to align with sustainable pharmaceutical practices.
In summary, interdisciplinary collaboration—bridging phytochemistry, pharmacology, and clinical research—will unlock the full potential of Gentianae Radix et Rhizoma, transforming traditional wisdom into evidence-based solutions for global healthcare challenges.
Author contributions
HL: Conceptualization, Investigation, Writing – original draft. XL: Formal Analysis, Writing – original draft, Data curation. SL: Funding acquisition, Project administration, Conceptualization, Writing – review and editing. FZ: Supervision, Writing – review and editing, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by the Natural Science Foundation Project of Shandong Province (ZR2023QH088).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Cao, Y. H. (2022). Clinical study on longdan xiegan decoction combined with Western medicine for Herpes zoster. New Chin. Med. 54 (17), 47–50. doi:10.13457/j.cnki.jncm.2022.17.008
Cao, F., Shao, H., Li, Q., Li, J., Li, W., and Li, C. (2012). Anti-inflammatory activity of Gentiana striata maxim. Nat. Prod. Res. 26 (11), 1038–1044. doi:10.1080/14786419.2010.551643
Che, Y. Y., and Liang, J. Y. (2008). Research progress on gentiana plants. Strait Pharm. J. 20 (3), 1–4. doi:10.3969/j.issn.1006-3765.2008.03.001
Chen, L., Liu, J. C., Zhang, X. N., Guo, Y. y., Xu, Z. h., Cao, W., et al. (2008). Down-regulation of NR2B receptors partially contributes to analgesic effects of gentiopicroside in persistent inflammatory pain. Neuropharmacology 54 (8), 1175–1181. doi:10.1016/j.neuropharm.2008.03.007
Chinese Pharmacopoeia Commission (2020). Pharmacopoeia of the People's Republic of China. Beijing: China Medical Science Press.
Choi, R. Y., Nam, S. J., Lee, H. I., Leutou, A. S., Ri Ham, J., et al. (2019). Gentiopicroside isolated from Gentiana scabra bge. Inhibits adipogenesis in 3T3-L1 cells and reduces body weight in diet-induced obese mice. Bioorg. Med. Chem. Lett. 29 (14), 1699–1704. doi:10.1016/j.bmcl.2019.05.038
Dai, G. L., Fang, X. Y., and Xiao, G. Q. (2023). Analysis of clinical effect of longdan xiegan decoction combined with Western medicine on patients with chronic rhinosinusitis without nasal polyps. Chin. Med. Dig. Otorhinolaryngol. 38 (02), 53–55+52. doi:10.19617/j.issn1001-1307.2023.02.53
Deng, Y. T. (2015). Study on the central effects and mechanisms of gentiopicroside on analgesia and antidepressant.
Dong, L. P., Ni, L. H., and Zhao, Z. L. (2017). Research progress on iridoid chemical constituents from gentiana species. Chin. Traditional Herb. Drugs 48 (10), 2116–2128.
Editorial Committee of Flora of China, Chinese Academy of Sciences (1988). Beijing, China: Science Press.
Ge, S., Wei, Y., and Wu, C. (2022). Study on the anti-inflammatory mechanism of gentiopicroside and magnoflorine, the main components of Gentiana macrophylla and Clematis chinensis, in RA model rats. Pharmacol. Clin. Chin. Materia Medica 38 (04), 62–67. doi:10.13412/j.cnki.zyyl.2022.04.012
Ghazanfar, K., Mubashir, K., Dar, S. A., Nazir, T., Hameed, I., Ganai, B. A., et al. (2017). Gentiana kurroo royle attenuates the metabolic aberrations in diabetic rats; swertiamarin, swertisin and lupeol being the possible bioactive principles. J. Complement. Integr. Med. 14 (3), 20170002. doi:10.1515/jcim-2017-0002
Guo, H. F., and Piao, H. S. (2011). Study on antitumor active constituents of Gentiana scabra. West China J. Pharm. Sci. 26 (03), 204–207. doi:10.13375/j.cnki.wcjps.2011.03.005
Hu, X. W., and Li, Y. F. (2002). Determination of gentiopicroside in compound xiongdan yigan capsules by RP-HPLC. China Pharm. 2002 (01), 72–73. doi:10.3969/j.issn.1000-7369.2000.11.005
Huang, Q. P. (2020). Study on chemical constituents and biological activities of two medicinal plants. Beijing, China: University of Chinese Academy of Sciences.
Huo, R. L. Y., and Guo, W. M. (2000). Self-prescribed longdan Mingmu Decoction for 49 cases of viral keratitis. Shaanxi J. Traditional Chin. Med. (11), 485.
Isakovic, A., Jankovic, T., Harhaji, L., Kostic-Rajacic, S., Nikolic, Z., Vajs, V., et al. (2008). Antiglioma action of xanthones from gentiana kochiana: mechanistic and structure-activity requirements. Bioorg. Med. Chem. 16 (10), 5683–5694. doi:10.1016/j.bmc.2008.03.069
Jia, N., Li, Y., Wu, Y., Hur, G., Zhang, X., et al. (2012). Comparison of the anti-inflammatory and analgesic effects of Gentiana macrophylla pall. And Gentiana straminea maxim., and identification of their active constituents. J. Ethnopharmacol. 144 (3), 638–645. doi:10.1016/j.jep.2012.10.004
Jiang, X. T. (2018). Observation on clinical efficacy of longdan xiegan decoction for chronic rhinosinusitis with gallbladder fu stagnation-heat syndrome.
Jiang, W. X., Jiang, P., and Zhang, X. Y. (2008). Study on in vivo antitumor effect of gentiana polysaccharide. Chin. Tradit. Pat. Med. (10), 1530–1532.
Jiang, L. L., Ren, P. Y., and Wang, Y. Z. (2019). Research progress on pharmacological effects of gentiana. Technol. Innov. (36), 43–44.
Jiao, W. X., Li, F. Y., and Nan, L. L. (2024). Research progress on medicinal resources of gentiana. J. Gansu Univ. Chin. Med. 41 (03), 80–83. doi:10.16841/j.issn1003-8450.2024.03.15
Kikuchi, M., Kakuda, R., Kikuchi, M., and Yaoita, Y. (2005). Secoiridoid glycosides from Gentiana scabra. J. Nat. Prod. 68 (5), 751–753. doi:10.1021/np058017o
Li, Y. C. (1998). Longdan xiegan decoction for 136 cases of hypertension. Hunan J. Traditional Chin. Med. (03), 49. doi:10.16808/j.cnki.issn1003-7705.1998.03.047
Li, A. (2014). Clinical efficacy analysis of longdan xiegan Decoction in treating hypertension. Shenzhen J. Integr. Traditional Chin. West. Med. 24 (05), 163–164. doi:10.16458/j.cnki.1007-0893.2014.05.008
Li, X. F. (2020). Clinical efficacy of modified longdan xiegan decoction on cervicitis with human papillomavirus infection and its effect on serum inflammatory factors. Chin. J. Hum. Sex. 29 (08), 111–114. doi:10.3969/j.issn.1672-1993.2020.08.035
Li, Y. (2023). Intervention effect of longdan xiegan decoction on hypertension with liver fire flaming upward type. China Urban Rural Enterp. Health 38 (03), 119–121. doi:10.16286/j.1003-5052.2023.03.045
Li, J. T., Liang, Y. L., and Yang, Z. J. (2011). Comprehensive research on dunhuang medicine, 1. Beijing, China: China Press of Traditional Chinese Medicine.
Li, W., Li, L. Y., Zhou, W., Hwang, I., Ma, J. Y., and Kim, Y. H. (2015). Triterpenoids isolated from the rhizomes and roots of Gentiana scabra and their inhibition of indoleamine 2,3-dioxygenase. Arch. Pharm. Res. 38 (12), 2124–2130. doi:10.1007/s12272-015-0631-6
Li, S., Wang, Q., Tao, Y., and Liu, C. (2016). Swertiamarin attenuates experimental rat hepatic fibrosis by suppressing angiotensin II-Angiotensin type 1 receptor-extracellular signal-regulated kinase signaling. J. Pharmacol. Exp. Ther. 359 (2), 247–255. doi:10.1124/jpet.116.234179
Li, X., Zhang, Y., Jin, Q., Xia, K. L., Jiang, M., Cui, B. W., et al. (2018a). Liver kinase B1/AMP-activated protein kinase-mediated regulation by gentiopicroside ameliorates P2X7 receptor-dependent alcoholic hepatosteatosis. Br. J. Pharmacol. 175 (9), 1451–1470. doi:10.1111/bph.14145
Li, P. Y., Huo, L. N., and Jia, Z. R. (2018b). Study on antioxidant activity and total flavonoid content determination of Gentiana scabra. J. Anhui Agric. Sci. 46 (31), 182–183.
Li, Y. X., Lv, L., Li, S. L., and Qian, H. H. (2024). Gentianine alleviates dextran sulfate sodium-induced ulcerative colitis via inhibition of TLR4/NLRP3-mediated pyroptosis. Int. Immunopharmacol. 126, 111214. doi:10.1016/j.intimp.2023.111214
Lian, L. H., Zhang, Y., and Li, X. (2018). “LKB1/AMPK-Mediated regulation by gentiopicroside ameliorates P2x7R-Dependent alcoholic hepatosteatosis,” in Proceedings for annual meeting of the Japanese pharmacological society, PO4-8–24.
Liu, F. Y. L., Lin, W. H., and Tian, L. (2017). Comparison of efficacy between longdan xiegan decoction granules and decoction in treating chronic hepatitis B. Chin. Mod. Distance Educ. Traditional Chin. Med. 15 (06), 80–82. doi:10.3969/j.issn.1672-2779.2017.06.036
Liu, Y., Liu, J. Y., and Zhang, N. (2021). Clinical study on modified longdan xiegan decoction combined with bloodletting puncture therapy for Herpes zoster. New Chin. Med. 53 (24), 27–30. doi:10.13457/j.cnki.jncm.2021.24.008
Liu, B., Pang, F., Bi, H., and Guo, D. (2024). Regulatory mechanisms of gentiopicroside on human diseases: a brief review. Naunyn Schmiedeb. Arch. Pharmacol. 397 (2), 725–750. doi:10.1007/s00210-023-02672-6
Lu, S. Y., and Su, Y. (2018). Modified longdan xiegan decoction combined with acupuncture for Herpes simplex viral keratitis with liver fire excess syndrome. Chin. J. Exp. Traditional Med. Formulae 24 (15), 216–221. doi:10.13422/j.cnki.syfjx.20181537
Lyu, Q. F., and Lin, S. Q. (2018). Efficacy observation of modified longdan xiegan decoction combined with xiaoye jinhuacao in treating hepatitis B. Jilin Med. J. 39 (01), 15–17.
Ma, L. I. (2012). Experimental study on BKCa channel involvement in nicotine addiction; II: experimental study on gentiopicroside downregulating GluN2B receptor in nucleus accumbens to inhibit morphine addiction.
Ning, Y., Cheng, Y. P., and Ma, A. P. (2017). Research progress on pharmacological effects of gentiana. Chem. Eng. 31 (06), 47–49. doi:10.7501/j.issn.0253-2670.2017.03.027
Olennikov, D. N., Kashchenko, N. I., Chirikova, N. K., and Tankhaeva, L. M. (2015). Iridoids and flavonoids of four Siberian gentians: chemical profile and gastric stimulatory effect. Molecules 20 (10), 19172–19188. doi:10.3390/molecules201019172
Olennikov, D. N., Gadimli, A. I., Isaev, J. I., Kashchenko, N. I., Prokopyev, A. S., Kataeva, T. N., et al. (2019). Caucasian gentiana species: untargeted LC-MS metabolic profiling, antioxidant and digestive enzyme inhibiting activity of six plants. Metabolites 9 (11), 271. doi:10.3390/metabo9110271
Qu, L. (2022). Clinical observation on longdan mingmu decoction combined with ganciclovir eye drops for viral keratitis. Guangming J. Chin. Med. 37 (12), 2214–2216. doi:10.3969/j.issn.1003-8914.2022.12.044
Ruan, M., Yu, B., Xu, L., Zhang, L., Long, J., and Shen, X. (2015). Attenuation of stress-induced gastrointestinal motility disorder by gentiopicroside, from Gentiana macrophylla pall. Fitoterapia 103, 265–276. doi:10.1016/j.fitote.2015.04.015
Sezik, E., Aslan, M., Yesilada, E., and Ito, S. (2005). Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci. 76 (11), 1223–1238. doi:10.1016/j.lfs.2004.07.024
Shao, J. W., Gong, M. J., and Ye, Y. Y. (2022). Qinggan xiere acupuncture combined with tongqiao erlong pills for sudden deafness with liver-gallbladder fire excess type. Jilin J. Traditional Chin. Med. 42 (10), 1219–1223. doi:10.13463/j.cnki.jlzyy.2022.10.025
Song, W. Z. (1986). General survey on medicinal plants of the Gentianaceae family in China. Bull. Chin. Materia Medica 11 (11), 3–7.
Su, Y. M., and Mou, J. W. (2022). Effect of longdan xiegan capsules as adjuvant therapy for secretory otitis media and its influence on levels of TNF-α, IL-8, TGF-β1 and TGF-β2 in middle ear effusion. Med. Innovation China 19 (31), 105–108. doi:10.3969/j.issn.1674-4985.2022.31.025
Sun, J. L., and Sun, X. Y. (2007). Self-prescribed longdan jiedu decoction combined with red light therapy apparatus in treating 102 cases of Herpes zoster. Mod. Tradit. Chin. Med. (03), 36–37. doi:10.3969/j.issn.1672-0571.2007.03.020
Sun, N. J., and Xia, C. F. (1984). Study on chemical constituents of Gentiana rigescens. Bull. Chin. Materia Medica (01), 33–34.
Suyama, Y., Kurimoto, S. I., Kawazoe, K., Murakami, K., Sun, H. D., Li, S. L., et al. (2013). Rigenolide A, a new secoiridoid glucoside with a cyclobutane skeleton, and three new acylated secoiridoid glucosides from Gentiana rigescens franch. Fitoterapia 91, 166–172. doi:10.1016/j.fitote.2013.08.006
Wang, Y. M. (2015). Study on enrichment process and quality control of flavonoids from aerial parts of Gentiana scabra. Harbin, Heilongjiang: Heilongjiang University of Chinese Medicine.
Wang, C. J., Wang, Z. M., and Wang, W. H. (2009). Research progress on chemical constituents and pharmacological activities of gentiana species. China J. Chin. Materia Medica 34 (23), 2987–2994.
Wang, D. D., Chen, Y. Q., and Lyu, J. L. (2017). Clinical study on longdan xiegan decoction for chronic hepatitis B with liver-gallbladder dampness-heat syndrome. Popular Sci. and Technol. 19 (05), 93–95+120.
Wang, L., Jiang, Y., Yu, Q., Xiao, C., Sun, J., Weng, L., et al. (2023). Gentiana scabra bge extract (GSE) protects against alcoholic liver disease by regulating the TLR4/NF-κB pathway in mice. Front. Biosci. Landmark Ed. 28 (11), 309. doi:10.31083/j.fbl2811309
Wölfle, U., Haarhaus, B., Seiwerth, J., Cawelius, A., Schwabe, K., Quirin, K. W., et al. (2017). The herbal bitter drug Gentiana lutea modulates lipid synthesis in human keratinocytes in vitro and in vivo. Int. J. Mol. Sci. 18 (8), 1814. doi:10.3390/ijms18081814
Wu, Q., and Chen, J. P. (2022). Case report on southern shauna chiropractic combined with longdan Xiegan Decoction for Herpes zoster. Clin. J. Chin. Med. 14 (25), 89–91. doi:10.3969/j.issn.1674-7860.2022.25.025
Wu, T., Li, J., Li, Y., and Song, H. (2017). Antioxidant and hepatoprotective effect of swertiamarin on carbon tetrachloride-induced hepatotoxicity via the Nrf2/HO-1 pathway. Cell Physiol. Biochem. 41 (6), 2242–2254. doi:10.1159/000475639
Xiao, H., Sun, X., Liu, R., Chen, Z., Lin, Z., Yang, Y., et al. (2020). Gentiopicroside activates the bile acid receptor Gpbar1 (TGR5) to repress NF-kappaB pathway and ameliorate diabetic nephropathy. Pharmacol. Res. 151, 104559. doi:10.1016/j.phrs.2019.104559
Xiang, L., Disasa, D., Liu, Y., Fujii, R., Yang, M., Wu, E., et al. (2022). Gentirigeoside B from Gentiana rigescens franch prolongs yeast lifespan via inhibition of TORC1/Sch9/Rim15/Msn signaling pathway and modification of oxidative stress and autophagy. Antioxidants (Basel) 11 (12), 2373. doi:10.3390/antiox11122373
Xiao, K. M., Kong, C. F., and Yao, S. K. (2019). Protective effect of gentiopicroside on oxidative stress injury induced by hydrogen peroxide in HepG2 cells. World J. Integr. Traditional West. Med. 14 (11), 1530–1534. doi:10.13935/j.cnki.sjzx.191112
Xie, Y. Y., Shao, Y. Y., and Han, W. C. (2021). Mechanism of gentiopicroside regulating macrophage polarization in ulcerative colitis mice via JAK2/STAT3 pathway. Mod. Chin. Med. 23 (12), 2107–2114. doi:10.13313/j.issn.1673-4890.20210108007
Xu, M., Wang, D., Zhang, Y. J., and Yang, C. R. (2007). Dammarane triterpenoids from the roots of Gentiana rigescens. J. Nat. Prod. 70 (5), 880–883. doi:10.1021/np070012z
Yang, Q. Q. (2014). Exploration of behavioral evaluation methods in 6-OHDA-induced parkinson's disease rat model and study on the neuroprotective effect of gentiopicroside on this model.
Yang, S. B., and Wang, C. (2005). Research progress on chemical constituents and pharmacological effects of gentiana. Acta Chin. Med. Pharmacol. (06), 54–56.
Yang, Y., Wang, Z., Zhang, L., Yin, B., Lv, L., He, J., et al. (2018). Protective effect of gentiopicroside from Gentiana macrophylla pall. In ethanol-induced gastric mucosal injury in mice. Phytother. Res. 32 (2), 259–266. doi:10.1002/ptr.5965
Yang, L. J., Kong, L. T., and Wang, P. P. (2022). Modified longdan xiegan decoction combined with auricular point pressing with beans as an adjunct to conventional Western medicine in the clinical study of insomnia complicated with primary hypertension. New Chin. Med. 54 (23), 198–202. doi:10.13457/j.cnki.jncm.2022.23.041
Ye, Z. Y., Wang, F. S., and Yang, H. Y. (2018). Study on lignan glycosides from Gentiana yunnanensis. J. Jinggangshan Univ. Nat. Sci. Ed. 39 (02), 78–81. doi:10.3969/j.issn.1674-8085.2018.02.014
Ye, F. Q., Sun, Y. J., and Fan, C. Y. (2023). Research progress on chemical constituents, biological activities and clinical applications of gentiana. Chin. Traditional Herb. Drugs 54 (19), 6497–6510. doi:10.7501/j.issn.0253-2670.2023.19.030
You, W. L. (2017). Comparison of pharmacological effects between aerial and underground parts of gentiana. Inn. Mong. J. Traditional Chin. Med. 36 (08), 135–136. doi:10.16040/j.cnki.cn15-1101.2017.08.133
Yu, Z. T. (2006). Determination of flavonoid compounds in gentiana. Heilongjiang Med. J. (06), 430–432. doi:10.3969/j.issn.1006-2882.2006.06.002
Zhang, Y. (2009). Longdan dai xie xiao zhen decoction for 50 cases of otic Herpes zoster. Chin. J. Emerg. Traditional Chin. Med. 18 (08), 1351.
Zhang, M. Z. (2015). Interpretation of famous traditional Chinese medicine book Titles—Yao pin hua yi. Res. Traditional Chin. Med. 28 (04), 25+70+80.
Zhang, Z. S., and Zhang, S. (2018). Effects of longdan xiegan decoction on clinical efficacy and serum levels of CGRP, 5-HT, and ICAM-1 in patients with Herpes zoster. Exp. Laboratory Med. 36 (01), 85–87+108. doi:10.3969/j.issn.1674-1129.2018.01.028
Zhang, X., Zhang, F., Kong, D., Wu, X., Lian, N., Chen, L., et al. (2014). Tetramethylpyrazine inhibits angiotensin II-induced activation of hepatic stellate cells associated with interference of platelet-derived growth factor β receptor pathways. Febs J. 281 (12), 2754–2768. doi:10.1111/febs.12818
Zhang, Y. Z., Liu, J. L., and Du, Y. M. (2019). Research progress on chemical constituents and pharmacological effects of Gentiana rigescens. Shandong Chem. Ind. 48 (16), 71–74+76. doi:10.19319/j.cnki.issn.1008-021x.2019.16.031
Zhang, Q. L., Xia, P. F., Peng, X. J., Wu, X. Y., Jin, H., Zhang, J., et al. (2022). Synthesis, and anti-inflammatory activities of gentiopicroside derivatives. Chin. J. Nat. Med. 20 (4), 309–320. doi:10.1016/S1875-5364(22)60187-0
Zhang, R., Yan, C., Yang, X., Hu, K., Hao, F., Yang, S., et al. (2023). Determination of lead in Gentiana rigescens and evaluation of the effect of lead exposure on the liver protection of the natural medicine. Anal. Chim. Acta 1251, 340992. doi:10.1016/j.aca.2023.340992
Zhao, L., Li, Z. M., and Bai, Y. T. (2009). Study on chemical constituents from aerial parts of Gentiana rigescens. J. Yunnan Univ. Traditional Chin. Med. 32 (02), 27–31. doi:10.19288/j.cnki.issn.1000-2723.2009.02.010
Zheng, W. H., and Ruan, S. J. (2018). Observation on the efficacy of longdan xiegan decoction in treating elderly patients with hypertension of liver-yang hyperactivity type. China Health Stand. Manag. 9 (12), 125–127. doi:10.3969/j.issn.1674-9316.2018.12.062
Zhou, L. (2012). A randomized controlled study on longdan xiegan decoction for chronic rhinosinusitis of gallbladder Fu stagnation-heat type.
Zhou, L., Tao, Z., and Xiong, D. J. (2013a). Observation on quality of life of longdan xiegan decoction for chronic rhinosinusitis of gallbladder Fu stagnation-heat type. Sichuan J. Traditional Chin. Med. 31 (04), 75–77.
Zhou, L., Tao, Z., and Xiond, D. J. (2013b). Clinical study on longdan xiegan decoction for chronic rhinosinusitis of gallbladder Fu stagnation-heat type. J. Chengdu Univ. Traditional Chin. Med. 36 (02), 39–41.
Zhou, Y. L., Zhang, Y., and Li, Y. Q. (2017). Study on xanthone chemical constituents from roots and rhizomes of Gentiana manshurica. Mod. Chin. Med. 19 (07), 960–964. doi:10.13313/j.issn.1673-4890.2017.7.011
Zhou, Y., Wang, M. M., and Liu, H. F. (2023). Study on terpenoid chemical constituents from Gentiana rhodantha. Chin. Pharm. J. 58 (17), 1540–1546.
Zhu, Z. H., Ding, L. W., and Zhu, J. P. (2021). 1. Beijing, China: China Press of Traditional Chinese Medicine.
Keywords: gentianae radix et rhizoma, chemical structure, pharmacological activities, clinical applications, active constituents
Citation: Liu H, Liu X, Liu S and Zhao F (2025) The chemical structure, pharmacological activity, and clinical progress of Gentianae Radix et Rhizoma. Front. Pharmacol. 16:1656493. doi: 10.3389/fphar.2025.1656493
Received: 04 July 2025; Accepted: 22 September 2025;
Published: 21 October 2025.
Edited by:
Javier Echeverria, University of Santiago, ChileReviewed by:
Baixin Kou, Changchun University of Chinese Medicine, ChinaDongxuan Zheng, Xinjiang Medical University, China
Priya Shah, Lok Jagruti Kendra, India
Copyright © 2025 Liu, Liu, Liu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng Liu, bGl1c2hlbmc4N0AxMjYuY29t; Feng Zhao, eXR1emhhb2ZlbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Hongfang Liu1†
Hongfang Liu1† Xiao Liu
Xiao Liu Feng Zhao
Feng Zhao