- 1Department of Clinical Pharmacy, The First Hospital of Hebei Medical University, Shijiazhuang, China
- 2The Technology Innovation Center for Artificial Intelligence in Clinical Pharmacy of Hebei Province, The First Hospital of Hebei Medical University, Shijiazhuang, China
- 3Department of Clinical Pharmacy, Hebei Medical University, Shijiazhuang, China
- 4Medical Affairs Department, The First Hospital of Hebei Medical University, Shijiazhuang, China
Objective: Lower respiratory tract infections (LRTIs) are a leading cause of morbidity and mortality worldwide and contribute to excessive empirical antibiotic use due to diagnostic delays. Rapid and accurate pathogen identification is essential for guiding targeted antimicrobial therapy and improving drug outcomes.
Aim: This study aimed to evaluate the impact of pathogen-targeted quick multiplex PCR (pt-qPCR) compared to conventional microbiological testing on antimicrobial use and clinical outcomes in hospitalized patients with LRTIs.
Methods: In this retrospective cohort study conducted at a tertiary hospital in China (March 2023–March 2024), patients with LRTIs were assigned to either a conventional testing group or a pt-qPCR group. Outcomes included pathogen detection rate, length of hospital stay (LOS), antimicrobial days of therapy (DOT), antimicrobial duration before and after testing, time to targeted therapy, mortality, and ICU transfer rate.
Results: A total of 220 patients were enrolled (conventional: n = 112; pt-qPCR: n = 108). Baseline characteristics were comparable, except for higher chronic pulmonary disease prevalence (58.0% vs. 20.4%, P < 0.001) and lower IL-6 levels (133.22 vs. 171.28, P < 0.001) in the pt-qPCR group. Pathogen detection was significantly higher with pt-qPCR (94.4% vs. 53.6%, P < 0.001). Compared to conventional testing, the pt-qPCR group showed reduced LOS (16 vs. 16 days, P = 0.041), DOT (20 vs. 24 days, P = 0.013), post-test antimicrobial use (8 vs. 12 days, P < 0.001), and ICU transfer rate (31.5% vs. 49.2%, P = 0.006). Mortality did not differ significantly between groups. The pt-qPCR group had a higher rate of antimicrobial regimen adjustment (34.3% vs. 19.6%, P = 0.014) and fewer instances of escalation. A broader pathogen spectrum was detected using pt-qPCR, including 208 bacteria, 73 fungi, and 103 viruses, with 87 patients harboring multiple pathogens.
Conclusion: Pharmacist-interpreted pt-qPCR significantly improves pathogen detection and optimizes antimicrobial therapy in LRTI patients. Its clinical use may enhance antibiotic stewardship, reduce ICU burden, and support precision medicine in respiratory infections.
1 Introduction
Lower respiratory tract infections (LRTIs) remain among the most prevalent and deadly infectious diseases worldwide. In 2019, LRTIs were responsible for over 2.6 million deaths, ranking as the fourth leading cause of mortality globally (GBD, 2019 Mental Disorders Collaborators, 2022). Beyond their clinical burden, LRTIs impose substantial economic costs on healthcare systems and patients (Zhang et al., 2016; Zhao et al., 2016; Yan et al., 2018). Early identification of causative pathogens and timely initiation of targeted therapy are critical to improving patient outcomes and reducing inappropriate antibiotic use (Buehler et al., 2016). However, traditional diagnostic methods (e.g., culture, antigen detection, serology) have long turnaround times and low sensitivity. Conventional techniques identify pathogens in only 38% of adult community-acquired pneumonia cases (Zaas et al., 2014; Jain et al., 2015). Even with combined methods, 34%–57% of pediatric and 13%–62% of adult pneumonia cases remain without a definitive microbial diagnosis (Jain et al., 2015; Emr et al., 2018; Saboktakin et al., 2019; Mani, 2018; Gadsby et al., 2016). These diagnostic limitations frequently result in empirical antibiotic overuse, delayed de-escalation, and increased risk of antimicrobial resistance.
The utility of rapid molecular diagnostics in optimizing antimicrobial therapy for respiratory infections is further supported by recent studies in this field, which highlight the value of multiplex PCR in improving diagnostic efficiency and guiding targeted treatment (Zhang et al., 2025; Hua et al., 2024). These studies, consistent with our approach, emphasize the integration of molecular testing with clinical decision-making to enhance antimicrobial stewardship. The emergence of molecular diagnostics, particularly multiplex polymerase chain reaction (PCR), presents a promising strategy to enhance both diagnostic efficiency and antimicrobial stewardship. We employed a novel pathogen-targeted rapid multiplex PCR platform (pt-qPCR) capable of detecting 96 pathogens and 8 resistance genes within 4 hours. This platform offers rapid, accurate, and cost-effective results with high reproducibility, which may directly influence prescribing behavior and reduce unnecessary antibiotic exposure. Prior studies have shown that multiplex PCR can shorten time to appropriate treatment in infectious diseases (Porter et al., 2018). Furthermore, pharmacist-led antimicrobial stewardship interventions have independently demonstrated improvements in antibiotic selection, reduced therapy duration, and enhanced clinical outcomes (Kim et al., 2021).
Given these complementary advances, there is a growing need to evaluate integrated models that combine rapid diagnostic technologies with pharmacist interpretation to optimize drug-related outcomes in LRTIs. This study aimed to evaluate the clinical utility of pharmacist-interpreted pt-qPCR testing in the diagnosis and management of LRTIs. By assessing its impact on diagnostic yield, antibiotic usage patterns, and therapeutic adjustments, we aim to generate evidence to support precision prescribing practices and inform antimicrobial stewardship policies.
2 Materials and methods
2.1 Study design
This retrospective cohort study was conducted at a tertiary teaching hospital in China from March 2023 to March 2024. Hospitalized patients with LRTIs were enrolled if they met these criteria: (1) increased or purulent airway secretions; (2) white blood cell count≥10 × 109/L (neutrophils >70%); (3) fever; (4) moist rales on auscultation or inflammatory changes on chest CT (Wei et al., 2022). Inclusion also required receipt of either conventional microbiological testing or pt-qPCR during hospitalization. Exclusion criteria included: (1) age under 16 years; (2) incomplete clinical records; and (3) discharge within 48 h of admission. For patients who underwent multiple pathogen tests during a single hospitalization, only the first test result was included in the analysis. Based on the etiological diagnostic protocol implemented during hospitalization, patients were categorized into the following two groups: (1) Conventional detection group: Patients who underwent only conventional microbiological examinations; (2) pt-qPCR detection group: Patients who underwent pt-qPCR testing.
2.2 Clinical data collection
Clinical data were extracted from patients’ electronic medical records and included demographic characteristics such as age and gender, history of antimicrobial allergy, and comorbidities. Microbiological data included the results of all pathogen detection tests and antimicrobial susceptibility profiles conducted during hospitalization. Information on antimicrobial therapy, including drug names and the timing of initiation and discontinuation, was collected. Additional variables included length of hospital stay (LOS), in-hospital mortality, the initially suspected and confirmed sites of infection, and disease severity classification. Inflammatory biomarkers such as C-reactive protein (CRP), procalcitonin (PCT), and interleukin-6 (IL-6) were also recorded to assess the clinical status of each patient. These data were used to evaluate baseline comparability and infection-related outcomes.
2.3 Primary outcomes
The primary outcomes included the pathogen detection rate, LOS, antimicrobial days of therapy (DOT), duration of antimicrobial use before and after pathogen testing, time from test sampling to targeted therapy initiation, time from empirical therapy initiation to targeted therapy adjustment, and in-hospital mortality. DOT was defined as the total number of days a patient received any systemic antimicrobial agent; if more than one antibiotic was administered on the same day, each was counted as a separate DOT. The interval from empirical to targeted therapy was defined as the time between the initial administration of empirical antibiotics and the first administration of targeted therapy guided by microbiological testing. All time-related outcomes were measured in days. All reported medians for duration-related outcomes (including LOS, DOT, and duration of antimicrobial use before/after testing) were calculated per patient.
2.4 Secondary outcomes
Secondary outcomes included the number of antibiotic regimen adjustments, the rate of antimicrobial escalation and de-escalation, intensive care unit (ICU) admission rate, 30-day hospital readmission rate, and the appropriateness of initial empirical antibiotic therapy. Antimicrobial Adjustment: Refers to any modification made to the current antimicrobial therapy regimen based on etiological test results or clinical efficacy assessment. This encompasses changes in drug type, dosage adjustment, alteration of administration frequency, or modification of treatment duration. Escalation: The practice of switching the treatment regimen to a higher-tier (e.g., moving from the Access group to the Watch group or Reserve group within the WHO AWaRe classification) or broader-spectrum antimicrobial agent when resistant pathogens are detected or when clinical efficacy is suboptimal. Example: Changing from a second-generation cephalosporin to a carbapenem. De-escalation: The practice of narrowing the spectrum of antimicrobial therapy or stepping down to a lower-tier agent after establishing an etiological diagnosis and observing clinical improvement. Example: Switching from a combination of a broad-spectrum β-lactam and a fluoroquinolone to penicillin upon confirmation of streptococcal infection. These measures were used to assess the impact of pathogen detection methods on clinical decision-making and antimicrobial stewardship.
2.5 Statistical methods
All statistical analyses were conducted using Microsoft Excel 2019 (Microsoft Corp., Redmond, WA, United States) and SPSS version 26.0 (IBM Corp., Armonk, NY, United States). Continuous variables with a normal distribution were reported as mean ± standard deviation (SD) and compared using independent samples t-tests or paired t-tests, as appropriate. For non-normally distributed data, results were expressed as medians with interquartile ranges (IQR) and analyzed using the Mann–Whitney U test or the Wilcoxon signed-rank test. Categorical variables were presented as absolute counts and percentages, with group comparisons performed using the Chi-squared test or Fisher’s exact test, depending on data distribution and sample size. All statistical tests were two-tailed, and a p-value less than 0.05 was considered statistically significant.
2.6 Pharmacist interpretation protocol
The interpretation of pt-qPCR results was performed by clinical pharmacists from the Department of Clinical Pharmacy, First Hospital of Hebei Medical University. All participating pharmacists possessed no less than 5 years of experience in antimicrobial stewardship. Interpretation reports were delivered to clinicians within 24 h after the pt-qPCR results were issued. Primary interpretive recommendations were based on relevant guidelines and expert consensus. For complex or challenging infections, clinical pharmacists initiated real-time multidisciplinary consultations to discuss treatment strategies.
3 Results
3.1 Sample and patient characteristics
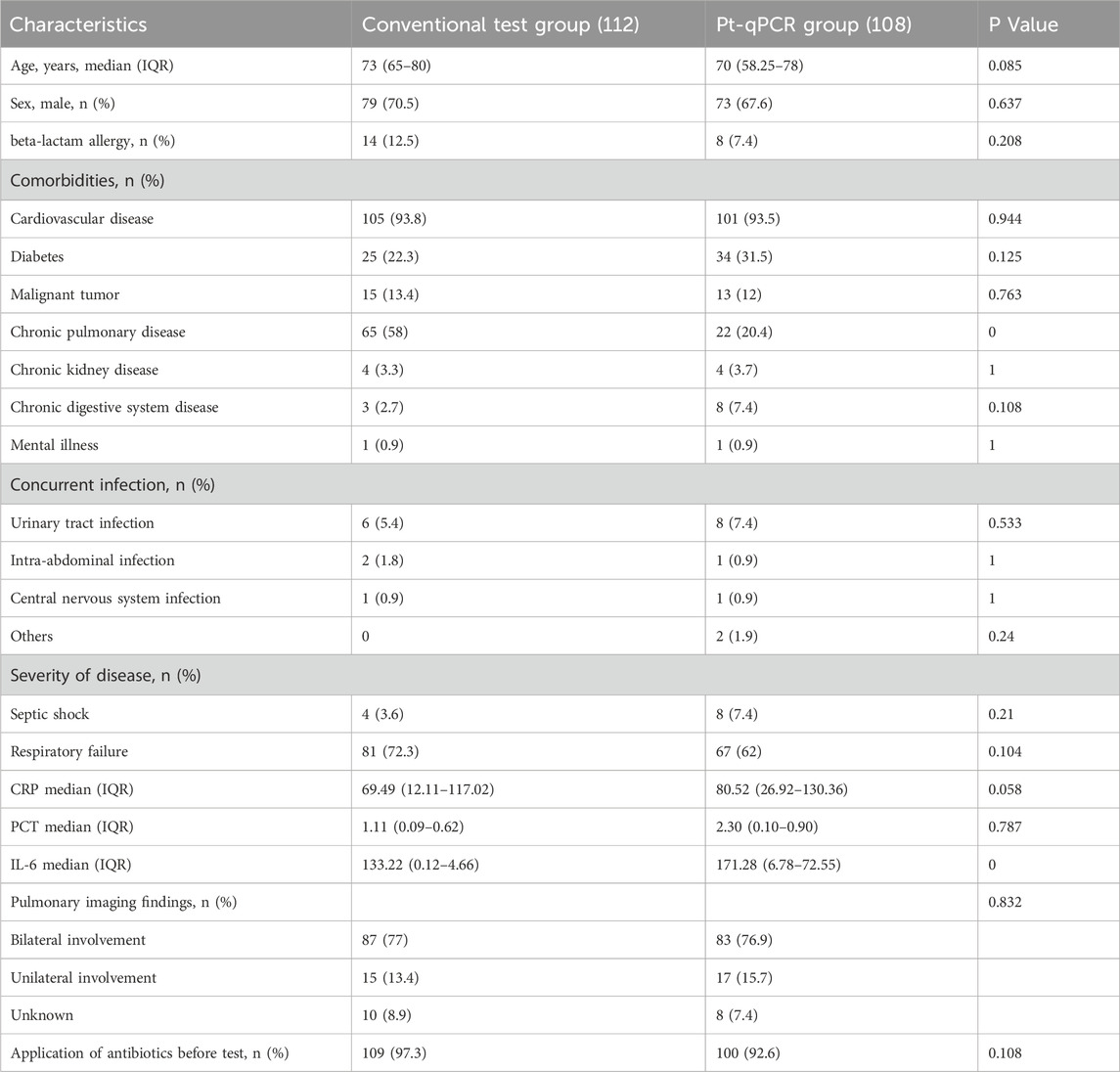
A total of 220 patients met the inclusion criteria, with 112 assigned to the conventional test group and 108 to the pt-qPCR group. Baseline demographic and clinical characteristics are summarized in Table 1. There were no statistically significant differences between the two groups in most baseline variables, indicating general comparability. However, notable exceptions included a higher prevalence of chronic lung disease in the conventional group compared to the pt-qPCR group (58.0% vs. 20.4%, P < 0.001) and a lower median IL-6 level in the conventional group (133.22 vs. 171.28, P < 0.001).
3.2 Primary outcomes
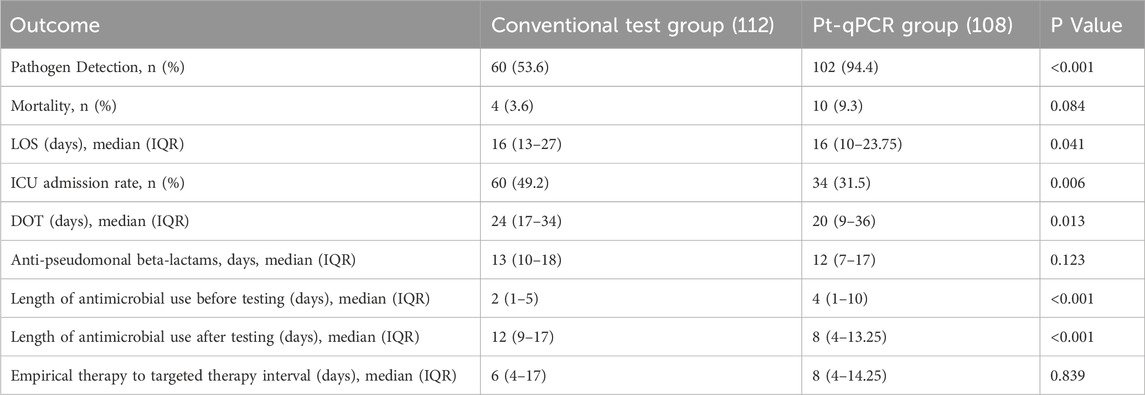
Of the 220 patients included, 162 had positive pathogen detection results, 60 in the conventional test group and 102 in the pt-qPCR group. The pathogen detection rate was significantly higher in the pt-qPCR group compared to the conventional group (94.4% vs. 53.6%, P < 0.001), indicating a marked advantage of the pt-qPCR platform in improving diagnostic yield.
In addition to diagnostic performance, several clinical outcomes favored the pt-qPCR group. LOS, ICU admission rate, DOT, and the duration of antimicrobial use after pathogen testing were all significantly reduced in the pt-qPCR group (Table 2). Specifically, the average DOT was 20 days in the pt-qPCR group, 4 days fewer than in the conventional group (24 days, P = 0.013). The duration of antimicrobial therapy following pathogen testing was also significantly shorter in the pt-qPCR group (8 days vs. 12 days, P < 0.001). However, no significant differences were observed between the two groups regarding in-hospital mortality or the interval from empirical to targeted antibiotic therapy.
3.3 Secondary outcomes
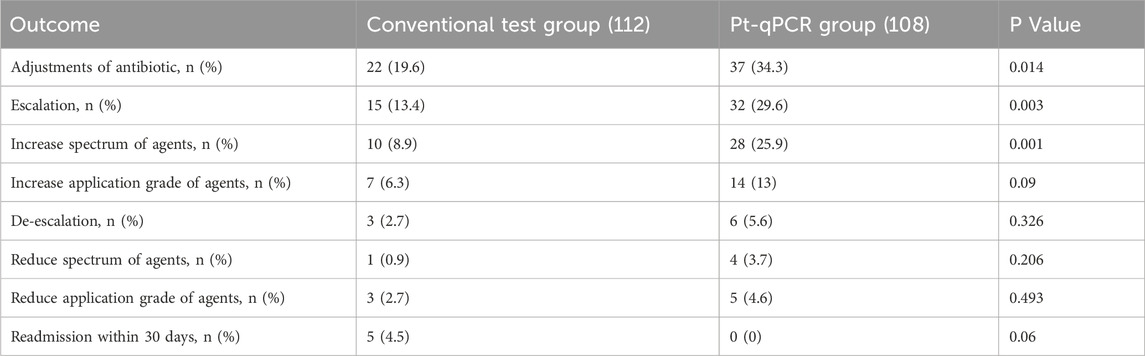
The application of pt-qPCR technology significantly improved the rate of antimicrobial regimen adjustments among patients with LRTIs, increasing from 19.6% in the conventional test group to 34.3% in the pt-qPCR group (P = 0.014). The number of antimicrobial escalation events was also significantly reduced in the pt-qPCR group, with 15 escalation instances compared to 32 in the conventional test group (P = 0.003). Furthermore, the use of broader-spectrum agents in initial empirical therapy was more effectively refined with pt-qPCR guidance, with the number of spectrum modifications increasing from 10 to 28 instances (P = 0.001). These findings highlight the utility of pt-qPCR in supporting precision antimicrobial management. However, no significant differences were observed between the two groups in terms of antimicrobial de-escalation rates or 30-day hospital readmission rates (Table 3).
3.4 Pathogenetic detection
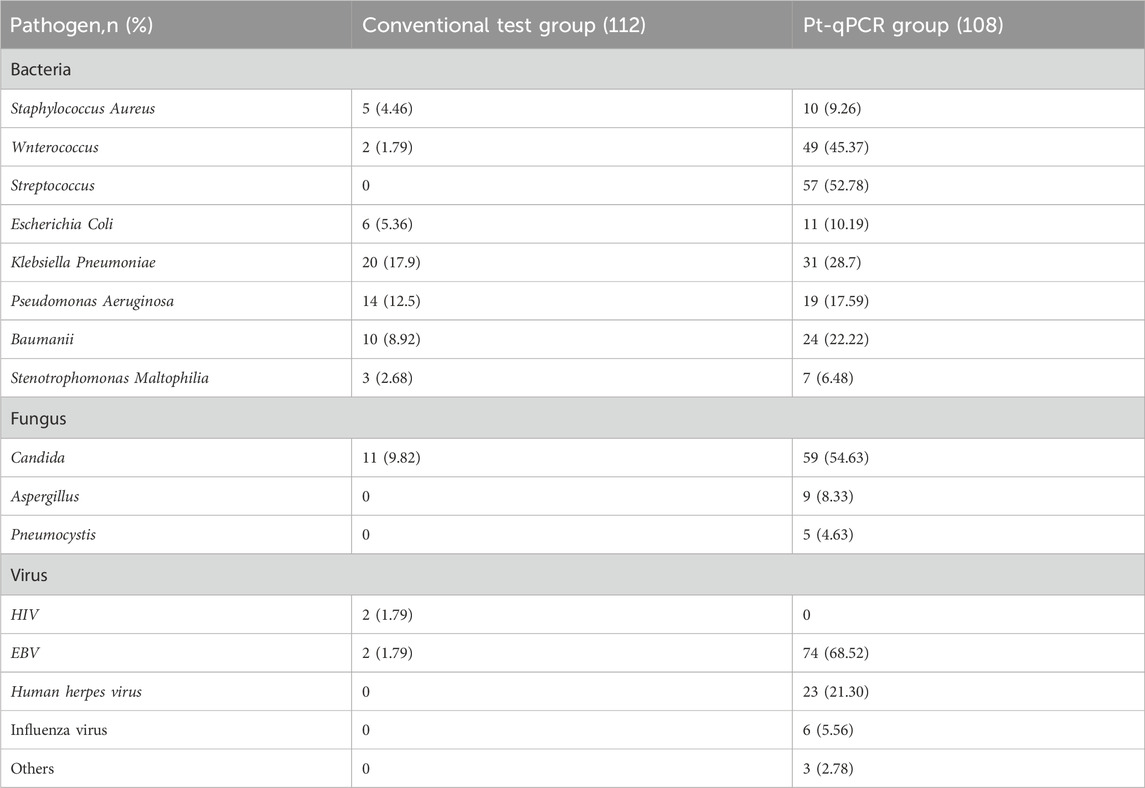
The spectrum and quantity of pathogens detected differed substantially between the pt-qPCR and conventional testing methods (Table 4). Using pt-qPCR, 384 pathogens were identified, comprising 208 bacteria (including 49 Enterococcus spp. And 57 Streptococcus pneumoniae), 73 fungi (predominantly 59 Candida spp. And 9 Aspergillus spp.), and 103 viruses, with Epstein–Barr virus (EBV) being the most common (74 detections). In contrast, conventional testing identified only 75 pathogens in the 60 positive cases, including 60 bacteria, 11 fungi, and 4 viruses. The most frequently isolated organisms in the conventional group were Klebsiella pneumoniae (20 isolates) and Pseudomonas aeruginosa (14 isolates). Notably, co-infection was common in the pt-qPCR group, with 87 patients presenting with two or more pathogens detected simultaneously, highlighting the enhanced sensitivity and breadth of the pt-qPCR platform.
4 Discussion
The selection and duration of empirical antimicrobial therapy for lower respiratory tract infections (LRTIs) remain a significant clinical challenge, particularly in low- and middle-income countries where multidrug-resistant (MDR) pathogens are prevalent. Although culture-based diagnostics are the current standard to guide definitive therapy, their long turnaround time often delays targeted treatment and contributes to inappropriate empirical antibiotic use and increased mortality (Dung et al., 2024; Jia et al., 2019). These challenges demonstrate the need for rapid, cost-effective, and clinically actionable diagnostic tools. However, as molecular platforms become more sensitive and detect a wider range of organisms, the lack of appropriate clinical interpretation may risk over-treatment and antimicrobial misuse (Zhuo et al., 2022a). In this study, the application of pathogen-targeted pt-qPCR combined with pharmacist-led interpretation provided a balanced approach, improving diagnostic accuracy while promoting rational antimicrobial use. Our findings confirmed that this integrated model significantly improved pathogen detection rates and contributed to reduced hospital stays.
The pt-qPCR platform demonstrated a clear advantage over conventional methods in detecting bacteria, fungi, and viruses, as well as identifying polymicrobial infections in respiratory samples. These findings are consistent with prior research on the clinical value of molecular diagnostics. For instance, Zhuo et al. used multiplex quantitative PCR (mqPCR) in 211 LRTI patients and reported a significant increase in the bacterial detection rate, from 29.9% with traditional culture to 64.5% with mqPCR, particularly in cases involving co-infections (Zhuo et al., 2022b). Similarly, Probst et al. demonstrated the effectiveness of combining real-time multiplex PCR with the carbapenem inactivation method (CIM) as a rapid and user-friendly alternative to whole-genome sequencing for surveillance of carbapenemase-producing Enterobacterales (Probst et al., 2021). In pediatric populations, Wang et al. found that GeXP-based PCR and multiplex qPCR had a significantly higher detection rate compared to routine diagnostics (76.09% vs. 36.13%, P = 0.004), suggesting that molecular tools can meaningfully enhance the diagnosis and treatment of LRTIs in children (Wang et al., 2021).
While total antimicrobial duration was shorter in the pt-qPCR group, pre-test therapy was longer. This likely reflects clinicians’ reluctance to use higher-cost diagnostics upfront, preferring empirical therapy or cheaper conventional tests. Nonetheless, the reduction in antimicrobial duration post-testing, combined with shorter LOS and reduced ICU utilization, suggests that pt-qPCR may offer long-term economic advantages (Darie et al., 2022). Furthermore, pt-qPCR significantly increased the rate of antimicrobial regimen adjustments. This technology enabled timely shifts to targeted therapy, improving infection control while minimizing unnecessary broad-spectrum antimicrobial use. Our findings align with prior research in this journal demonstrating that rapid pathogen detection technologies, when combined with structured clinical interpretation, can significantly reduce inappropriate antimicrobial use (Zhang et al., 2023). This consistency reinforces the methodological robustness of leveraging molecular diagnostics for LRTI management. These benefits are critical in the fight against antimicrobial resistance. Notably, while escalation rates differed significantly between groups, de-escalation rates did not. This may reflect a conservative approach in initial empirical therapy, where clinicians prioritize broad coverage in severely ill patients (Claeys et al., 2020).
These findings suggest that pt-qPCR testing, when integrated with pharmacist interpretation, contributes directly to improved drug outcomes by reducing unnecessary antimicrobial exposure and promoting early targeted therapy. This has the potential to reduce adverse drug reactions and slow the development of antimicrobial resistance in hospital settings. From a health policy perspective, this study supports the integration of pharmacist-led diagnostic interpretations into hospital antimicrobial stewardship programs. The adoption of rapid molecular testing as a routine clinical tool could be incentivized in formulary and reimbursement policies to promote evidence-based antibiotic prescribing. Although the upfront cost of pt-qPCR testing may be higher, the associated reductions in total antibiotic use, LOS, and ICU admissions suggest improved cost-effectiveness, particularly in resource-constrained settings where efficient drug use is critical.
Despite its promising findings, this study has several limitations. First, it was conducted at a single center with a modest sample size and limited duration, which may affect generalizability. Second, While pt-qPCR shows clinical value with pharmacist interpretation, its scalability in resource-limited settings faces challenges: limited pharmacists may delay or suboptimally interpret complex results (e.g., polymicrobial infections); underdeveloped AMS infrastructure hinders workflow integration; and upfront costs are prohibitive. To address these challenges, several strategies may enhance feasibility. Feasibility can be enhanced via algorithm-based decision tools to reduce pharmacist reliance, telepharmacy to address staffing shortages, phased implementation prioritizing high-risk patients, and policy support (e.g., subsidies, integration into national AMS frameworks) to adapt to resource constraints.
5 Conclusion
Pharmacist-interpreted pt-qPCR significantly improved pathogen detection and reduced hospital stay, antimicrobial duration, and ICU admissions in patients with LRTIs. These findings support its role in promoting targeted antibiotic use and enhancing antimicrobial stewardship. pt-qPCR has strong potential for routine clinical integration and policy adoption to optimize drug outcomes and resource use.
Author contributions
ZW: Writing – original draft. JW: Writing – original draft. YL: Writing – original draft. YW: Writing – original draft. YZ: Writing – original draft. SY: Writing – original draft. SL: Writing – original draft. WY: Writing – review and editing. JY: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Medical Science Research Project of Hebei Provincial Health Commission (Grant Number: 20241797).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Buehler, S. S., Madison, B., Snyder, S. R., Derzon, J. H., Cornish, N. E., Saubolle, M. A., et al. (2016). Effectiveness of practices to increase timeliness of providing targeted therapy for inpatients with bloodstream infections: a laboratory medicine best practices systematic review and meta-analysis. Clin. Microbiol. Rev. 29 (1), 59–103. doi:10.1128/CMR.00053-14
Claeys, K. C., Heil, E. L., Hitchcock, S., Johnson, J. K., and Leekha, S. (2020). Management of gram-negative bloodstream infections in the era of rapid diagnostic testing: impact with and without antibiotic stewardship. Open Forum Infect. Dis. 7 (10), ofaa427. doi:10.1093/ofid/ofaa427
Darie, A. M., Khanna, N., Jahn, K., Osthoff, M., Bassetti, S., Osthoff, M., et al. (2022). Fast multiplex bacterial PCR of bronchoalveolar lavage for antibiotic stewardship in hospitalised patients with pneumonia at risk of Gram-negative bacterial infection (Flagship II): a multicentre, randomised controlled trial. Lancet Respir. Med. 10 (9), 877–887. doi:10.1016/S2213-2600(22)00086-8
Dung, T. T. N., Phat, V. V., Vinh, C., Lan, N. P. H., Phuong, N. L. N., Ngan, L. T. Q., et al. (2024). Development and validation of multiplex real-time PCR for simultaneous detection of six bacterial pathogens causing lower respiratory tract infections and antimicrobial resistance genes. BMC Infect. Dis. 24 (1), 164. doi:10.1186/s12879-024-09028-2
Emr, B. M., Alcamo, A. M., Carcillo, J. A., Aneja, R. K., and Mollen, K. P. (2018). Pediatric Sepsis update: how are children different? Surg. Infect. (Larchmt) 19 (2), 176–183. doi:10.1089/sur.2017.316
Gadsby, N. J., Russell, C. D., McHugh, M. P., Mark, H., Conway Morris, A., Laurenson, I. F., et al. (2016). Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin. Infect. Dis. 62 (7), 817–823. doi:10.1093/cid/civ1214
GBD 2019 Mental Disorders Collaborators (2022). Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 9 (2), 137–150. doi:10.1016/S2215-0366(21)00395-3
Hua, Y., Li, N., Lao, J., Chen, Z., Ma, S., and Li, X. (2024). Machine learning models for coagulation dysfunction risk in inpatients administered β-lactam antibiotics. Front. Pharmacol. 15, 1503713. doi:10.3389/fphar.2024.1503713
Jain, S., Self, W. H., Wunderink, R. G., Fakhran, S., Balk, R., Bramley, A. M., et al. (2015). Community-acquired pneumonia requiring hospitalization among U.S. Adults. N. Engl. J. Med. 373 (5), 415–427. doi:10.1056/NEJMoa1500245
Jia, X., Ren, H., and Nie, X., (2019). Research progress on detection methods for infectious pathogens in lower respiratory tract. Chin. J. Zoonoses 35 (03), 263–270.
Kim, S. H., Yoon, J. G., Park, H. J., Won, H., Ryoo, S. S., Choi, E., et al. (2021). Effects of a comprehensive antimicrobial stewardship program in a surgical intensive care unit. Int. J. Infect. Dis. 108, 237–243. doi:10.1016/j.ijid.2021.02.082
Mani, C. S. (2018). “Acute pneumonia and its complications,” in Principles and practice of pediatric infectious diseases, 238–249.e4.
Porter, A. M., Bland, C. M., Young, H. N., Allen, D. R., Croft, S. R., Gayheart, R. E., et al. (2018). Comparison of pharmacist-directed management of multiplex PCR blood culture results with conventional Microbiology methods on effective and optimal therapy within a community hospital. Antimicrob. Agents Chemother. 63 (1), e01575-18. doi:10.1128/AAC.01575-18
Probst, K., Nurjadi, D., Heeg, K., Frede, A. M., Dalpke, A. H., and Boutin, S. (2021). Molecular detection of carbapenemases in Enterobacterales: a comparison of real-time multiplex PCR and whole-genome sequencing. Antibiot. (Basel) 10 (6), 726. doi:10.3390/antibiotics10060726
Saboktakin, L., Bilan, N., Ghalehgolab Behbahan, A., and Poorebrahim, S. (2019). Relationship between resistin levels and sepsis among children under 12 Years of age: a case control study. Front. Pediatr. 7, 355. doi:10.3389/fped.2019.00355
Wang, H., Gu, J., Li, X., van der Gaast-de Jongh, C. E., Wang, W., He, X., et al. (2021). Broad range detection of viral and bacterial pathogens in bronchoalveolar lavage fluid of children to identify the cause of lower respiratory tract infections. BMC Infect. Dis. 21 (1), 152. doi:10.1186/s12879-021-05834-0
Wei, J., Zhang, Z., and Shen, H., (2022). Clinical value of rapid detection of Acinetobacter baumannii in ICU patients with lower respiratory tract infection by qPCR. J. Med. Res. & Combat Trauma Care 35 (11), 1176–1179.
Yan, T., Li, Y., Sun, Y., Wang, H., Wang, J., Wang, W., et al. (2018). Hospital-acquired lower respiratory tract infections among high risk hospitalized patients in a tertiary care teaching hospital in China: an economic burden analysis. J. Infect. Public Health 11 (4), 507–513. doi:10.1016/j.jiph.2017.10.003
Zaas, A. K., Garner, B. H., Tsalik, E. L., Burke, T., Woods, C. W., and Ginsburg, G. S. (2014). The current epidemiology and clinical decisions surrounding acute respiratory infections. Trends Mol. Med. 20 (10), 579–588. doi:10.1016/j.molmed.2014.08.001
Zhang, Y., Zhang, J., Wei, D., Yang, Z., Wang, Y., and Yao, Z. (2016). Annual surveys for point-prevalence of healthcare-associated infection in a tertiary hospital in Beijing, China, 2012-2014. BMC Infect. Dis. 16, 161. doi:10.1186/s12879-016-1504-4
Zhang, R., Gao, L., Chen, P., Liu, W., Huang, X., and Li, X. (2023). Risk-factor analysis and predictive-model development of acute kidney injury in inpatients administered cefoperazone-sulbactam sodium and mezlocillin-sulbactam sodium: a single-center retrospective study. Front. Pharmacol. 14, 1170987. doi:10.3389/fphar.2023.1170987
Zhang, P., Chen, Q., Lao, J., Shi, J., Cao, J., Li, X., et al. (2025). Machine learning modeling for the risk of acute kidney injury in inpatients receiving amikacin and etimicin. Front. Pharmacol. 16, 1538074. doi:10.3389/fphar.2025.1538074
Zhao, X., Li, S., Sun, X., Liu, S., and Duan, F. (2016). Risk factors for hospital-acquired infection in cancer patients in a central Chinese hospital. Am. J. Infect. Control 44 (9), e163–e165. doi:10.1016/j.ajic.2016.02.026
Zhuo, X., Zhao, J., and Cao, B. (2022a). Comparison of the quantitative results of a multiplex quantitative PCR between positive and negative bacterial culture groups of respiratory pathogens. J. Cap. Med. Univ. 43 (05), 782–786.
Zhuo, X., Zhao, J., Wang, L., Sun, B., Sun, L., Wang, C., et al. (2022b). Development and evaluation of a multiplex quantitative polymerase chain reaction assay for detecting bacteria associated with lower respiratory tract infection. Int. J. Infect. Dis. 122, 202–211. doi:10.1016/j.ijid.2022.05.052
Keywords: pt-qPCR, antimicrobial stewardship, lower respiratory tract infections, rapid molecular diagnostics, real-world study, hospital-based study
Citation: Wang Z, Wang J, Liu Y, Wei Y, Zhao Y, Yuan S, Liu S, Yin W and Yu J (2025) Impact of pharmacist-interpreted pt-qPCR technology on antimicrobial use and clinical outcomes in patients with lower respiratory tract infections: a retrospective cohort study. Front. Pharmacol. 16:1656557. doi: 10.3389/fphar.2025.1656557
Received: 30 June 2025; Accepted: 20 August 2025;
Published: 08 September 2025.
Edited by:
Shusen Sun, Western New England University, United StatesReviewed by:
Xiao Li, The First Affiliated Hospital of Shandong First Medical University, ChinaDi Li, Chongqing Medical University, China
Copyright © 2025 Wang, Wang, Liu, Wei, Zhao, Yuan, Liu, Yin and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Yu, eXVqaW5nQGhlYm11LmVkdS5jbg==; Wanyi Yin, NTc5MDEzMjJAaGVibXUuZWR1
†These authors have contributed equally to this work and share first authorship
 Ziyi Wang1,2†
Ziyi Wang1,2† Yan Liu
Yan Liu Jing Yu
Jing Yu