- 1Myriad Genetics, Salt Lake City, UT, United States
- 2Mental Illness Research, Education and Clinical Center, Corporal Michael J Crescenz VA Medical Center, Philadelphia, PA, United States
- 3Department of Psychiatry, University of Pennsylvania, Philadelphia, PA, United States
Background: In patients with major depressive disorder (MDD), achieving remission and/or response may take many months because of the lengthy trial-and-error process often needed to identify effective medication. Pharmacogenomic testing is a prescribing tool that has been shown to improve remission and response rates for MDD patients, but data describing its impact over time is limited. The objective of this study was to determine whether pharmacogenomic-guided treatment increases the rate of remission and response over time in patients with MDD, and if so, to assess the persistence of that effect.
Methods: This study was a prespecified post hoc analysis of the PRIME Care (Precision Medicine in Mental Healthcare) randomized clinical trial, a pragmatic trial that compared pharmacogenomic-guided treatment with usual care among veterans with depression. Participants were recruited at 22 Department of Veterans Affairs medical centers by 676 clinicians and were randomized to the pharmacogenomic-guided arm or the usual care arm. Multivariate Cox proportional hazards models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for associations between study arm (pharmacogenomic-guided treatment or usual care) and the first instance of response or remission as assessed by the Patient Health Questionnaire-9 (PHQ-9) scale.
Results: 1,764 (90.7%) of the 1,944 veterans who participated in the PRIME Care trial had sufficient visit data to be included in this analysis. Patients who received pharmacogenomic-guided treatment had higher rates of remission (HR [95% CI] = 1.27 [1.05, 1.53]; p = 0.015) and response (HR [95% CI] = 1.21 [1.05, 1.40]; p = 0.010) at any time relative to patients receiving usual care. Schoenfeld residuals tests were not statistically significant for remission (p = 0.931) or response (p = 0.112), providing no evidence that the benefit due to pharmacogenomic-guided treatment changed over the 24-week period.
Conclusion: Pharmacogenomic-guided treatment led to faster initial remission and response in patients with MDD, and this benefit persisted over 6 months with no evidence of changing over time.
1 Introduction
Major depressive disorder (MDD) is a significant cause of morbidity, with a lifetime prevalence of 20% in the United States and 4% worldwide (Hasin et al., 2018; World Health Organization, 2023). Treatment of MDD often involves the use of psychotropic medications to achieve clinically significant response and ultimately symptom remission. However, fewer than 40% of individuals achieve remission after their first antidepressant medication, and the chances of remission diminish with each subsequent medication trial (Rush et al., 2006). Since antidepressants can take several weeks to show efficacy (Gelenberg, 2010; McQuaid et al., 2022), a patient may require multiple medication trials over the course of many months before reaching remission. Approximately half of MDD patients receive two or more different medications in the 3 years following diagnosis, while one-third receive three or more (Kern et al., 2020). This trial-and-error prescribing approach may prolong the burden of MDD for the patient and increase healthcare costs (Ionescu et al., 2015).
Pharmacogenomic (PGx) testing is a prescribing tool that can identify medications that may require dose adjustments, be less likely to work, or have a higher risk of side effects by analyzing genetic variants that impact medication pharmacokinetics or pharmacodynamics (Bousman et al., 2023a). Guidelines for the use of PGx test results in prescribing antidepressants are available (Hicks et al., 2017; Bousman et al., 2023b; Beunk et al., 2024; Lam et al., 2023), and FDA labeling for many antidepressants includes PGx information (FDA, 2022). Data from multiple randomized clinical trials have shown that PGx-guided treatment increases the overall proportion of patients achieving remission from MDD compared to usual care (Greden et al., 2019; Oslin et al., 2022). A meta-analysis of thirteen trials including 4,767 MDD patients concluded that PGx-guided treatment is associated with a 41% higher likelihood of remission compared to treatment as usual (Brown et al., 2022). Subsequent meta-analyses have replicated the observation of higher remission rates with PGx-guided treatment in MDD (Arnone et al., 2023; Bunka et al., 2023; Wang et al., 2023; Milosavljević et al., 2024; Santenna et al., 2024; Albers et al., 2025).
PGx testing may improve remission and response rates in MDD by reducing trial-and-error prescribing. However, evidence demonstrating the impact of PGx-guided treatment on time-to-remission and time-to-response, as well as persistence of such effects over time, is limited. The Genomics Used to Improve DEpression Decisions (GUIDED) trial found that likelihood of remission in the PGx-guided arm doubled from 8 weeks to 24 weeks, suggesting a persistent effect (Greden et al., 2019). However, comparison to the control arm was not possible in GUIDED after 8 weeks because clinicians could access PGx results for patients in the control arm at that time.
The largest PGx trial conducted for depression, the Precision Medicine in Mental Healthcare (PRIME Care) study, evaluated 1,944 veterans with MDD and compared outcomes for PGx-guided versus usual care (Oslin et al., 2022). Compared to GUIDED, the PRIME Care study included a larger cohort size and longer duration for the primary outcome (over 24 weeks) and assessed depression outcomes at more time points after randomization (4, 8, 12, 18, 24 weeks). PRIME Care met both of its prespecified primary outcomes: patients in the PGx-guided arm were less likely to be prescribed an antidepressant medication with a significant gene-drug interaction and were 28% more likely to achieve remission across the 24-week duration of the trial compared to patients in the usual care arm. Additional analyses in the PRIME Care study showed that the proportion of remitters was significantly higher in the PGx-guided arm at 8 and 12 weeks but was not significantly different at 18 and 24 weeks. Although no interaction between time and study arm was observed in the analysis, the study concluded that provision of PGx test results had a nonpersistent effect on symptom remission; however, direct analysis was not performed to verify this statement (Oslin et al., 2022). Herein, we directly tested the hypothesis that PGx testing leads to persistently higher rates of MDD remission and response over time by assessing the impact of PGx testing on initial time-to-remission and time-to-response in the PRIME Care trial.
2 Materials and methods
2.1 Design and patient data
This study was a post hoc analysis, utilizing a prespecified analysis plan, of the PRIME Care study. Briefly, the PRIME Care trial was a randomized pragmatic clinical trial in which patients with MDD and at least one treatment failure were randomized to receive either PGx-guided treatment or usual care for a period of 24 weeks at the beginning of an antidepressant treatment episode. Baseline assessments were performed prior to randomization, with post-randomization follow-up visits scheduled at 4, 8, 12, 18, and 24 weeks. A full description of the trial, including eligibility and exclusion criteria, is available (Oslin et al., 2021; Oslin et al., 2022). This post hoc study was reviewed by the Advarra Institutional Review Board and determined to qualify as exempt research per 45 CFR 46.104(d)(4).
Relevant clinical and demographic information collected at the baseline or follow-up visits included age, race (Black/African American, White, Other/unspecified), Hispanic ethnicity, sex (female, male), smoking status (cigarettes per day over the prior 30 days), patient-reported psychotropic treatment history, reported visit date (days since randomization), and practice location (primary care, mental healthcare, or integrated care) (Table 1).
2.2 Clinical assessments and endpoints
Assessments relevant for this analysis, conducted at baseline and each follow-up visit, included the Patient Health Questionnaire-9 (PHQ-9) (Spitzer et al., 1999) and the Post Traumatic Stress Disorder (PTSD) Checklist for DSM-5 (PCL-5) (Weathers et al., 2013).
The primary endpoints of this analysis were the time of the first instance of remission (defined as PHQ-9 ≤5) and response (defined as ≥50% reduction from baseline PHQ-9 score). PHQ-9 scores range from 0–27 points.
2.3 Statistical analysis
Baseline characteristics were compared between study arms using chi-squared tests for categorical variables and Wilcoxon rank-sum test for baseline PHQ-9 score to assess significant differences, with a two-sided α level of 5%.
In the primary analysis, two separate multivariate Cox proportional hazards models (one for remission, one for response) were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between study arm (PGx-guided or usual care) and the first instance of either PHQ-9 remission or PHQ-9 response, respectively, measured at each follow-up visit (Klein and Moeschberger, 2003). Baseline PHQ-9 score was included as the only additional covariate in each model as an explanatory variable. The Efron method was selected to handle tied event times. The statistical significance of individual explanatory variables was determined using likelihood ratio tests. The proportional hazard assumption for the study arm variable was assessed using the Schoenfeld residuals test. Two-sided tests of significance were used, with a Bonferroni-adjusted α level of 0.025 (0.05/2) to account for testing two outcomes. Cox models used study week (4, 8, 12, 18, or 24 weeks) as the time scale in accordance with the timing of data collection in the PRIME Care study design (set prior to this post hoc analysis). Patients were right-censored at the first instance of a missing follow-up visit (defined as any visit without an associated PHQ-9 score). Patients were also right-censored at the first instance of a follow-up visit occurring outside of the expected time window based on reported visit date (14–42 days [week 4], 42–70 days [week 8], 70–105 days [week 12], 105–147 days [week 18], 147–189 days [week 24]). These windows were defined post hoc, based on the midpoints between the weeks when follow-up visits were scheduled to occur. Patients were excluded from the analysis if they only had baseline data remaining after right-censoring (Supplementary Figure S1). As a sensitivity analysis, models were re-analyzed without the right-censoring based on reported visit date. As an additional sensitivity analysis, models were re-analyzed with the inclusion of additional prespecified covariates (age [60+], race, Hispanic ethnicity, sex, presence of PTSD at baseline, history of treatment refractory depression, smoking status [≥1 cigarette per day over the prior 30 days], and practice location).
All analyses were conducted using R software version 4.4.1 (R Foundation for Statistical Computing, 2021).
3 Results
3.1 Baseline characteristics
Out of the 1,944 patients in the PRIME Care study, 180 (9.3%) were excluded due to having only baseline data remaining after right censoring, leaving 1,764 patients available for analysis (90.7%; n = 884 in the PGx-guided arm and n = 880 in the usual care arm), with the majority being under 60 years of age (73% in the PGx-guided arm, 73% in the usual care arm) and male (77% in the PGx-guided arm, 73% in the usual care arm) (Table 1; Supplementary Figure S1). This cohort was also mostly White (67% in the PGx-guided arm, 70% in the usual care arm), non-Hispanic (88% in the PGx-guided arm, 89% in the usual care arm), and non-smoking (82% in the PGx-guided arm, 81% in the usual care arm). Both arms had equal proportions of individuals with moderately severe depression (mean PHQ-9 values of 17.5 and 17.4, respectively), treatment refractory depression (31%), and comorbid PTSD (58%).
Baseline patient characteristics were compared by study arm (Table 1). No statistically significant differences were observed across study arms for any baseline characteristic.
3.2 Time-to-event analysis
Cox proportional hazards models and likelihood ratio tests revealed that patients who received PGx-guided treatment had higher rates of remission (HR [95% CI] = 1.27 [1.05, 1.53]; p = 0.015) and response (HR [95% CI] = 1.21 [1.05, 1.40]; p = 0.010) at any time relative to patients receiving usual care. Kaplan-Meier plots, using reported visit date as the timescale, show that the cumulative incidence of remission and response appeared higher in the PGx-guided arm starting early during the study period and continued through the end of the study period (Figure 1).
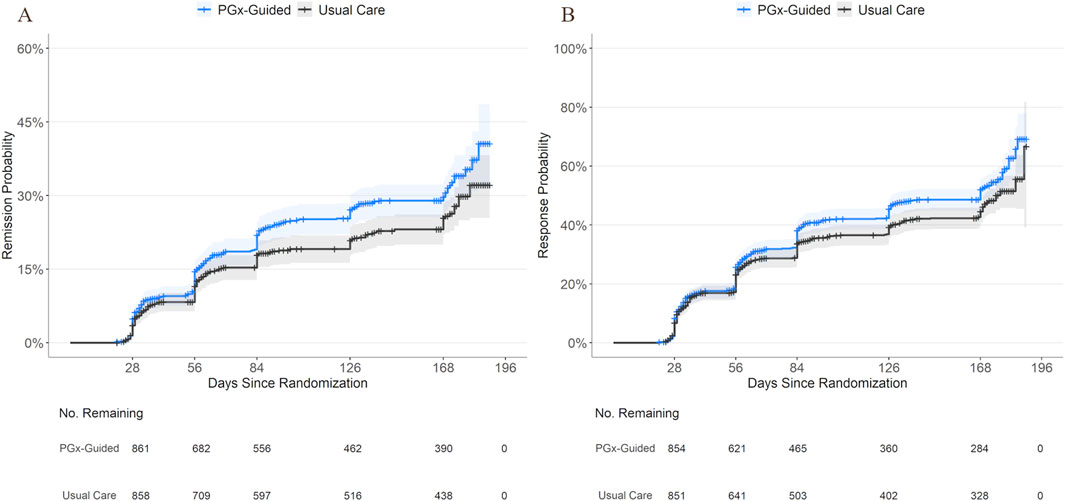
Figure 1. Cumulative Incidence of Remission and Response by Study Arm. Kaplan-Meier curves showing cumulative incidence of remission (A) and response (B) over time for patients receiving PGx-guided treatment or usual care using reported visit date (days since randomization) as the timescale for clarity. Shaded areas indicate 95% confidence intervals. The number of individuals remaining event-free per treatment group are shown below the x-axis. Censoring is indicated by cross tick marks on each curve. Note that the primary analysis used study week as the timescale and group differences were not evaluated using reported visit date.
The Schoenfeld residuals test of the study arm variable was not statistically significant for remission (p = 0.931) or response (p = 0.112), indicating no evidence of time-dependent effects of study arm, consistent with the proportional-hazards assumption. Schoenfeld residual plots did not show any significant fluctuation of the remission or response hazard ratios over time (Figure 2). These results indicate that the benefit due to PGx-guided treatment did not change significantly over the 24-week period.
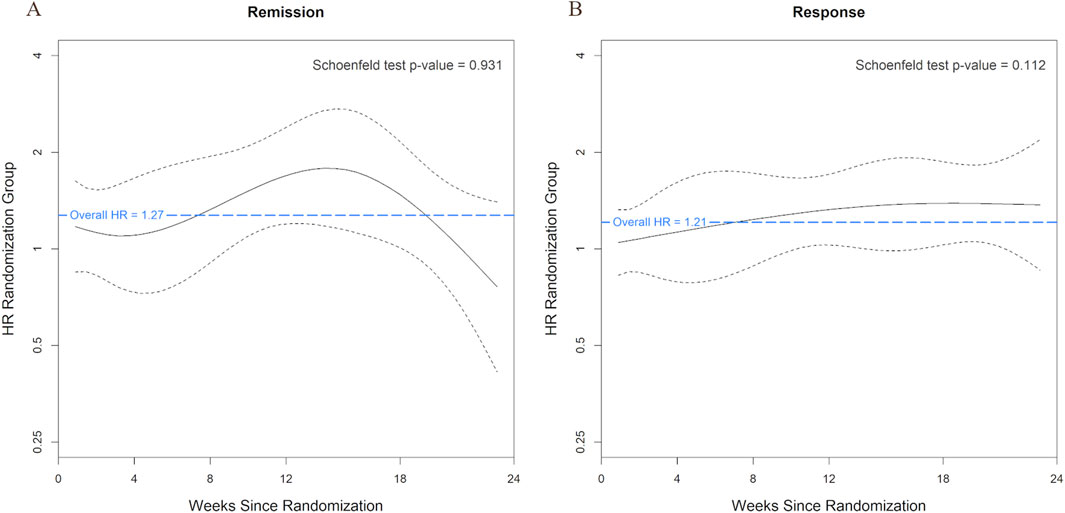
Figure 2. Time-Varying Effects of Study Arm on Remission and Response on the Log Hazard Ratio Scale. Smoothed trend lines of scaled Schoenfeld residuals for the effect of study arm on remission (A) and response (B) using study week as the timescale. A blue horizontal dashed line indicates the overall hazard ratio estimated from the Cox model. Black dashed lines represent the 95% confidence band around the smoothed effect estimate over time. Systematic deviation from the confidence band may indicate violation of the proportional hazards assumption.
3.3 Sensitivity analyses
As a sensitivity analysis, models were re-analyzed without right-censoring based on reported visit date, resulting in fewer (n = 125 [6.4%]) patients excluded. Among patients included in the sensitivity analysis (n = 1819 [93.6%]), the associations between study arm and time-to-remission (HR [95% CI] = 1.24 [1.03, 1.49]; p = 0.022) or response (HR [95% CI] = 1.20 [1.05, 1.38]; p = 0.010), as well as Schoenfeld residuals test results for study arm and remission (p = 0.699) or response (p = 0.227), were similar to those seen in the primary analysis. Models were also re-analyzed with the inclusion of additional prespecified covariates (age [60+], race, Hispanic ethnicity, sex, presence of PTSD at baseline, history of treatment refractory depression, smoking status [≥1 cigarette per day over the prior 30 days], and practice location). After adjusting for these prespecified covariates, associations between study arm and time-to-remission (HR [95% CI] = 1.30 [1.07, 1.57]; p = 0.008) or response (HR [95% CI] = 1.23 [1.07, 1.43]; p = 0.004), as well as Schoenfeld residuals test results for study arm and remission (p = 0.990) or response (p = 0.092), were similar to those seen in the primary analysis. Primary analysis findings were robust to changes in right-censoring and to the inclusion of additional covariates.
4 Discussion
This is the first study to demonstrate that for patients with MDD, PGx-guided treatment is associated with higher rates of initial remission and response that persist for 6 months after PGx testing, with no evidence of the effect changing over time. These findings build upon previous studies showing that PGx testing increases the overall proportion of MDD patients achieving remission and response compared to usual care (Greden et al., 2019; Brown et al., 2022; Oslin et al., 2022; Arnone et al., 2023; Bunka et al., 2023; Wang et al., 2023; Milosavljević et al., 2024; Santenna et al., 2024).
Time-to-event analyses in this study indicated that PGx-guided treatment was associated with a 27% higher rate of remission and a 21% higher rate of response at any time between 4–24 weeks after randomization compared to usual care. The original PRIME Care analysis found that the overall proportion of remitters was not significantly different at the 18 weeks (16% in the PGx-guided arm, 14% in the usual care arm) or 24 weeks (17% in PGx-guided arm, 16% in the usual care arm) time points (Oslin et al., 2022). However, in the original analysis, all remission and response events were analyzed, while in this study, only the first remission or response event after randomization was evaluated. As such, the original analysis (Oslin et al., 2022) may have included patients who had relapsed and had a subsequent response or remission. Relapse, defined as a return of depressive symptoms, is a common event affecting about one in five MDD patients within a year of achieving remission (Geddes et al., 2003), and is likely to have occurred in the dataset. The higher rates of remission and response observed in this study indicate that patients in the PGx-guided arm achieved remission and response faster than those in the usual care arm. These findings are consistent with a recent study showing that PGx-guided dosing of tricyclic antidepressants led to faster attainment of therapeutic plasma concentrations compared to usual treatment (Vos et al., 2023). Achieving remission sooner in a patient’s treatment trajectory may have long-term clinical benefits. Analyses of the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial (Rush et al., 2006) have shown that patients with earlier remission had both lower rates of relapse and a longer time to relapse in the year following remission (Rush et al., 2006; Kubo et al., 2023). Similarly, in another study, those who achieved response or remission within the first 6 weeks of antidepressant treatment were more likely than later remitters to still be in remission at 1 year after starting treatment (Ciudad et al., 2012). Additional clinical benefits of faster remission and response may include reduced risk of treatment-resistant depression (Nie et al., 2018; Arnaud et al., 2023). Furthermore, faster remission may also reduce residual symptoms, such as reactivity of mood, feeling sad, and pleasure/enjoyment. In a study of 1,595 depressed patients, those who achieved remission after 6–8 weeks of treatment had fewer and less severe residual symptoms compared to those who achieved remission after 16–20 weeks of treatment (Roca et al., 2011).
Faster remission and response may also have economic benefits. In the United States, the total economic burden of MDD has been estimated at $333.7 billion in 2019, including direct healthcare costs as well as indirect work-related costs (e.g., unemployment, productivity loss, absenteeism) (Greenberg et al., 2023). In one simulation, a novel therapy with faster time-to-response had an estimated cost savings of $25 billion per year (Greenberg et al., 2023). Reduction in relapse and incidence of treatment-resistant depression, driven by faster remission, is also predicted to reduce healthcare costs (Gauthier et al., 2019; Li et al., 2020; Touya et al., 2022).
The persistent impact of PGx on remission and response rates over time demonstrated in this study may explain new findings that PGx-guided care reduces healthcare resource utilization, including psychiatric hospitalizations (Del Tredici et al., 2025). Indeed, we hypothesize that the benefit of PGx-guided treatment may well extend beyond the 6-month timeframe of this study. Sustained remission is the ultimate treatment goal for patients with MDD (Trivedi and Daly, 2008), and PGx-guided treatment with conventional antidepressants could be more effective over time than newer rapidly acting agents, whose durability is unproven (Schatzberg and Mathew, 2024).
4.1 Strengths and limitations
The PRIME Care study, on which this analysis was based, was conducted on a large cohort of depressed patients and the design was pragmatic to represent real-world clinical practice (Oslin et al., 2022). The PRIME Care study also followed a prespecified analysis plan, and included multiple time points to assess depression outcomes between the PGx-guided and usual care arms over 24 weeks after randomization (Oslin et al., 2022). Building upon the original study, which assessed outcome differences at each time point individually, the current analysis was able to directly assess timing of initial response and remission across the entire study duration. Moreover, this analysis expands upon the non-significant interaction between arm and time reported in Oslin et al. (2022) by using statistical methods that directly assess time-to-event, providing a more statistically principled and clinically interpretable framework for evaluating the persistence of study arm effects.
While the PRIME Care study was the largest randomized clinical trial evaluating depression outcomes with and without PGx testing, the study design did not include time points after 24 weeks. As such, this analysis could not evaluate the persistence of the impact of PGx-guided treatment for longer than 24 weeks. Moreover, only time-to-first response or remission was evaluated, and any response/remission events occurring after relapse were not included in the analysis. Additionally, the outcomes observed in this study were limited to a specific PGx test and may not apply to all PGx tests. Finally, this study was conducted in a cohort of veterans, which may not be representative of the MDD patient population in the United States or worldwide; for example, the frequencies of PTSD and male sex were higher than in other large US depression cohorts.
5 Conclusion
Pharmacogenomic testing led to faster initial remission and response in patients with MDD. This effect persisted over 6 months without any evidence of changing over time. Future directions may include evaluating the economic impact of higher remission and response rates due to PGx testing, studying the effect of PGx testing on relapse, and analyzing other depression cohorts to understand the generalizability of these findings.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: Deidentified participant data is available. Contact ZGF2ZS5vc2xpbkB2YS5nb3Y= to propose secondary study analyses. If proposal is approved, a data dictionary and supporting documentation will be available via file transfer.
Ethics statement
The studies involving humans were approved by VA Central Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DH: Data curation, Software, Visualization, Conceptualization, Methodology, Writing – review and editing, Writing – original draft, Formal Analysis. ADT: Project administration, Writing – original draft, Conceptualization, Writing – review and editing. RG: Writing – review and editing, Conceptualization, Writing – original draft. RL: Supervision, Writing – review and editing, Methodology. BM: Writing – review and editing, Methodology, Validation. HJ: Writing – review and editing, Conceptualization. KJT: Supervision, Conceptualization, Writing – review and editing, Resources. KL: Conceptualization, Writing – review and editing, Methodology. AG: Writing – review and editing, Conceptualization, Supervision, Methodology, Resources. DO: Writing – review and editing, Conceptualization, Methodology, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
Authors DH, AD, RG, RL, BM, HJ, KJ, and AG were employed by Myriad Genetics.
The author DO has received nonfinancial support from Myriad Genetics for the conduct of the trial.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1658616/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Patient Flow Diagram. 1A post hoc sensitivity analysis was performed including these patients. Flow diagram showing which patients were excluded from analysis due to missing the initial follow-up visit (week 4) or having the initial follow-up visit occur outside the expected timeframe.
References
Albers, R. E., Dyer, M. P., Kucera, M., Hain, D., Gutin, A., Del Tredici, A. L., et al. (2025). Meta-analysis of response and remission outcomes with a weighted multi-gene pharmacogenomic test for adults with depression. J. Clin. Psychopharmacol. doi:10.1097/JCP.0000000000002061
Arnaud, A., Benner, J., Suthoff, E., Werneburg, B., Reinhart, M., Sussman, M., et al. (2023). The impact of early remission on disease trajectory and patient outcomes in major depression disorder (MDD): a targeted literature review and microsimulation modeling approach based on the sequenced treatment alternatives to relieve depression (STAR*D) study. J. Affect Disord. 325, 264–272. doi:10.1016/j.jad.2022.12.147
Arnone, D., Omar, O., Arora, T., Östlundh, L., Ramaraj, R., Javaid, S., et al. (2023). Effectiveness of pharmacogenomic tests including CYP2D6 and CYP2C19 genomic variants for guiding the treatment of depressive disorders: systematic review and meta-analysis of randomised controlled trials. Neurosci. Biobehav Rev. 144, 104965. doi:10.1016/j.neubiorev.2022.104965
Beunk, L., Nijenhuis, M., Soree, B., Boer-Veger, N. J. d., Buunk, A.-M., Guchelaar, H.-J., et al. (2024). Dutch pharmacogenetics working group (DPWG) guideline for the gene-drug interaction between CYP2D6, CYP2C19 and Non-SSRI/Non-TCA antidepressants. Eur. J. Hum. Genet. 32, 1371–1377. doi:10.1038/s41431-024-01648-1
Bousman, C. A., Maruf, A. A., Marques, D. F., Brown, L. C., and Müller, D. J. (2023a). The emergence, implementation, and future growth of pharmacogenomics in psychiatry: a narrative review. Psychol. Med. 53, 7983–7993. doi:10.1017/S0033291723002817
Bousman, C. A., Stevenson, J. M., Ramsey, L. B., Sangkuhl, K., Hicks, J. K., Strawn, J. R., et al. (2023b). Clinical pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6, CYP2C19, CYP2B6, SLC6A4, and HTR2A genotypes and serotonin reuptake inhibitor antidepressants. Clin. Pharmacol. Ther. 114, 51–68. doi:10.1002/cpt.2903
Brown, L. C., Stanton, J. D., Bharthi, K., Maruf, A. A., Müller, D. J., and Bousman, C. A. (2022). Pharmacogenomic testing and depressive symptom remission: a systematic review and meta-analysis of prospective, controlled clinical trials. Clin. Pharmacol. Ther. 112, 1303–1317. doi:10.1002/cpt.2748
Bunka, M., Wong, G., Kim, D., Edwards, L., Austin, J., Doyle-Waters, M. M., et al. (2023). Evaluating treatment outcomes in pharmacogenomic-guided care for major depression: a rapid review and meta-analysis. Psychiatry Res. 321, 115102. doi:10.1016/j.psychres.2023.115102
Ciudad, A., Álvarez, E., Roca, M., Baca, E., Caballero, L., Polavieja, P. G. d., et al. (2012). Early response and remission as predictors of a good outcome of a major depressive episode at 12-month follow-up: a prospective, longitudinal, observational study. J. Clin. Psychiatry 73, 185–191. doi:10.4088/JCP.10m06314
Del Tredici, A. L., Johnson, H. L., DeHart, B., Gutin, A., Morin, P., Kasten, C. R., et al. (2025). Real-world impact of pharmacogenomic testing on medication use and healthcare resource utilization in patients with major depressive disorder. J. Clin. Psychopharmacol. 45, 320–328. doi:10.1097/JCP.0000000000001999
FDA (2022). FDA table of pharmacogenetic associations. Available online at: https://www.fda.gov/medical-devices/precision-medicine/table-pharmacogenetic-associations (Accessed May 15, 2025).
Gauthier, G., Mucha, L., Shi, S., and Guerin, A. (2019). Economic burden of relapse/recurrence in patients with major depressive disorder. J. Drug Assess. 8, 97–103. doi:10.1080/21556660.2019.1612410
Geddes, J. R., Carney, S. M., Davies, C., Furukawa, T. A., Kupfer, D. J., Frank, E., et al. (2003). Relapse prevention with antidepressant drug treatment in depressive disorders: a systematic review. Lancet 361, 653–661. doi:10.1016/S0140-6736(03)12599-8
Gelenberg, A. J. (2010). A review of the current guidelines for depression treatment. J. Clin. Psychiatry 71, e15. doi:10.4088/JCP.9078tx1c
Greden, J. F., Parikh, S. V., Rothschild, A. J., Thase, M. E., Dunlop, B. W., DeBattista, C., et al. (2019). Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, Patient- and rater-blinded, randomized, controlled study. J. Psychiatr. Res. 111, 59–67. doi:10.1016/j.jpsychires.2019.01.003
Greenberg, P., Chitnis, A., Louie, D., Suthoff, E., Chen, S.-Y., Maitland, J., et al. (2023). The economic burden of adults with major depressive disorder in the United States (2019). Adv. Ther. 40, 4460–4479. doi:10.1007/s12325-023-02622-x
Hasin, D. S., Sarvet, A. L., Meyers, J. L., Saha, T. D., Ruan, W. J., Stohl, M., et al. (2018). Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 75, 336–346. doi:10.1001/jamapsychiatry.2017.4602
Hicks, J., Sangkuhl, K., Swen, J., Ellingrod, V., Müller, D., Shimoda, K., et al. (2017). Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin. Pharmacol. Ther. 102, 37–44. doi:10.1002/cpt.597
Ionescu, D. F., Rosenbaum, J. F., and Alpert, J. E. (2015). Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin. Neurosci. 17, 111–126. doi:10.31887/DCNS.2015.17.2/dionescu
Kern, D. M., Cepeda, M. S., Defalco, F., and Etropolski, M. (2020). Treatment patterns and sequences of pharmacotherapy for patients diagnosed with depression in the United States: 2014 through 2019. BMC Psychiatry 20, 4. doi:10.1186/s12888-019-2418-7
Klein, J. P., and Moeschberger, M. L. (2003). Survival analysis: techniques for censored and truncated data. 2 ed. New York, NY: Springer. doi:10.1007/b97377
Kubo, K., Sakurai, H., Tani, H., Watanabe, K., Mimura, M., and Uchida, H. (2023). Predicting relapse from the time to remission during the acute treatment of depression: a Re-Analysis of the STAR*D data. J. Affect Disord. 320, 710–715. doi:10.1016/j.jad.2022.09.162
Lam, R. W., Kennedy, S. H., Adams, C., Bahji, A., Beaulieu, S., Bhat, V., et al. (2023). Canadian Network for Mood and Anxiety Treatments (CANMAT) 2023 Update on Clinical Guidelines for Management of Major Depressive Disorder in Adults: réseau canadien pour les traitements de l'humeur et de l'anxiété (CANMAT) 2023: mise à jour des lignes directrices cliniques pour la prise en charge du trouble dépressif majeur chez les adultes. Can. J. Psychiatry 69 (9), 641–687. doi:10.1177/07067437241245384
Li, G., Zhang, L., DiBernardo, A., Wang, G., Sheehan, J. J., Lee, K., et al. (2020). A retrospective analysis to estimate the healthcare resource utilization and cost associated with treatment-resistant depression in commercially insured US patients. PLoS One 15, e0238843. doi:10.1371/journal.pone.0238843
McQuaid, J. R., Buelt, A., Capaldi, V., Fuller, M., Issa, F., Lang, A. E., et al. (2022). The management of major depressive disorder: synopsis of the 2022 U.S. department of veterans affairs and U.S. department of defense clinical practice guideline. ACP J. Club 175, 1440–1451. doi:10.7326/M22-1603
Milosavljević, F., Molden, P. E., Ingelman-Sundberg, P. M., and Jukić, A. P. M. M. (2024). Current level of evidence for improvement of antidepressant efficacy and tolerability by pharmacogenomic-guided treatment: a systematic review and meta-analysis of randomized controlled clinical trials. Eur. Neuropsychopharmacol. 81, 43–52. doi:10.1016/j.euroneuro.2024.01.005
Nie, Z., Vairavan, S., Narayan, V. A., Ye, J., and Li, Q. S. (2018). Predictive modeling of treatment resistant depression using data from STAR*D and an independent clinical study. PLoS One 13, e0197268. doi:10.1371/journal.pone.0197268
Oslin, D. W., Chapman, S., Duvall, S. L., Gelernter, J., Ingram, E. P., Kranzler, H. R., et al. (2021). Study design and implementation of the PRecision medicine in MEntal health care (PRIME care) trial. Contemp. Clin. Trials 101, 106247. doi:10.1016/j.cct.2020.106247
Oslin, D. W., Lynch, K. G., Shih, M.-C., Ingram, E. P., Wray, L. O., Chapman, S. R., et al. (2022). Effect of pharmacogenomic testing for drug-gene interactions on medication selection and remission of symptoms in major depressive disorder: the PRIME care randomized clinical trial. JAMA 328, 151–161. doi:10.1001/jama.2022.9805
R Foundation for Statistical Computing (2021). R: a language and environment for statistical computing. Available online at: https://www.R-project.org/(Accessed May 15, 2025).
Roca, M., García-Toro, M., García-Campayo, J., Vives, M., Armengol, S., García-García, M., et al. (2011). Clinical differences between early and late remission in depressive patients. J. Affect Disord. 134, 235–241. doi:10.1016/j.jad.2011.05.051
Rush, A. J., Trivedi, M. H., Wisniewski, S. R., Nierenberg, A. A., Stewart, J. W., Warden, D., et al. (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry 163, 1905–1917. doi:10.1176/ajp.2006.163.11.1905
Santenna, C., Shubham, A., Ratinder, J., Abhijit, R., Tamonud, M., Jitendra, S., et al. (2024). Drug metabolizing enzymes pharmacogenetic variation-informed antidepressant therapy approach for common mental disorders: a systematic review and meta-analysis. J. Affect Disord. 367, 832–844. doi:10.1016/j.jad.2024.09.041
Schatzberg, A. F., and Mathew, S. J. (2024). The why, when, where, how, and so what of so-called rapidly acting antidepressants. Neuropsychopharmacology 49, 189–196. doi:10.1038/s41386-023-01647-8
Spitzer, R. L., Williams, J. B. W., and Kroenke, K. (1999). Patient health Questionnaire-9 (PHQ-9). Available online at: https://www.apa.org/depression-guideline/assessment (Accessed May 15, 2025).
Touya, M., Lawrence, D. F., Kangethe, A., Chrones, L., Evangelatos, T., and Polson, M. (2022). Incremental burden of relapse in patients with major depressive disorder: a real-world, retrospective cohort study using claims data. BMC Psychiatry 22, 152. doi:10.1186/s12888-022-03793-7
Trivedi, M. H., and Daly, E. J. (2008). Treatment strategies to improve and sustain remission in major depressive disorder. Dialogues Clin. Neurosci. 10, 377–384. doi:10.31887/DCNS.2008.10.4/mhtrivedi
Vos, C. F., Hark, S. E. T., Schellekens, A. F. A., Spijker, J., Meij, A. v. d., Grotenhuis, A. J., et al. (2023). Effectiveness of genotype-specific tricyclic antidepressant dosing in patients with major depressive disorder. JAMA Netw. Open 6, e2312443. doi:10.1001/jamanetworkopen.2023.12443
Wang, X., Wang, C., Zhang, Y., and An, Z. (2023). Effect of pharmacogenomics testing guiding on clinical outcomes in major depressive disorder: a systematic review and meta-analysis of RCT. BMC Psychiatry 23, 334. doi:10.1186/s12888-023-04756-2
Weathers, F. W., Litz, B. T., Keane, T. M., Palmieri, P. A., Marx, B. P., and Schnurr, P. P. (2013). The PTSD checklist for DSM-5 (PCL-5). Available online at: https://www.ptsd.va.gov/professional/assessment/adult-sr/ptsd-checklist.asp (Accessed May 15, 2025).
World Health Organization (2023). Depressive disorders (depression). Available online at: https://www.who.int/news-room/fact-sheets/detail/depression (Accessed May 15, 2025).
Keywords: pharmacogenetics, drug response, antidepressive agents, precision medicine, depression, pharmacogenomics
Citation: Hain D, Del Tredici AL, Griggs RB, Law R, Mabey B, Johnson HL, Johansen Taber K, Lynch KG, Gutin A and Oslin DW (2025) Persistent benefit of pharmacogenomic testing on initial remission and response rates in patients with major depressive disorder. Front. Pharmacol. 16:1658616. doi: 10.3389/fphar.2025.1658616
Received: 02 July 2025; Accepted: 29 September 2025;
Published: 30 October 2025.
Edited by:
Youssef Daali, University of Geneva, SwitzerlandReviewed by:
Julio Duarte, University of Florida, United StatesJosé A. G. Agúndez, University of Extremadura, Spain
Copyright © 2025 Hain, Del Tredici, Griggs, Law, Mabey, Johnson, Johansen Taber, Lynch, Gutin and Oslin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andria L. Del Tredici, cHVic2NvcnJlc3BvbmRlbmNlQG15cmlhZC5jb20=, YW5kcmlhLmRlbHRyZWRpY2lAbXlyaWFkLmNvbQ==
 Daniel Hain1
Daniel Hain1 Andria L. Del Tredici
Andria L. Del Tredici Ryan B. Griggs
Ryan B. Griggs Rebecca Law
Rebecca Law Katherine Johansen Taber
Katherine Johansen Taber