Abstract
Allergic rhinitis (AR), a common IgE-mediated inflammatory condition of the nasal mucosa, presents with nasal itching, episodic sneezing, and runny nose. Emerging evidence indicates that type 2 innate lymphoid cells (ILC2s) are key players in AR development. Epithelial-derived alarmins (IL-33, IL-25, TSLP) activate ILC2s, leading to Th2 cytokine production (IL-4, IL-5, IL-13) that enhances inflammation. Recent research shows that NOD-like receptor protein 3 (NLRP3) can function as a transcriptional regulator of interleukin-33 (IL-33), offering new mechanistic insights into ILC2s dysregulation. Based on analysis and pharmacological validation of various effective components against AR, three compounds—luteolin, calycosin, and formononetin—have been identified as key ingredients due to their notable anti-inflammatory properties. This review systematically explores how these compounds regulate the NLRP3/IL-33/ILC2s signaling pathway, laying the groundwork for developing targeted AR treatments.
1 Introduction
Allergic rhinitis (AR) is one of the most common diseases in otolaryngology, with the incidence rate continuing to rise due to various cultural, economic, and geographical factors. Persistent symptoms such as runny nose, sneezing, nasal congestion, and nasal itching significantly impair patients’ quality of life (Nathan et al., 2008). As a type I hypersensitivity reaction mediated by IgE, AR affects 10%–40% of the global population, placing a significant burden on society and the economy (Zhang et al., 2021a). Recent paradigm shifts emphasize type 2 innate lymphoid cells (ILC2s) as essential innate immune effectors driving AR pathophysiology (Kato, 2019; Luo et al., 2020). Upon allergen exposure, bronchial epithelium-derived alarmins interleukin-33 (IL-33), interleukin-25 (IL-25), and thymic stromal lymphocytin (TSLP) activate ILC2s via ST2 receptors, initiating Th2-polarized immune responses through phosphorylation-dependent activation of NF-κB and MAPK signaling pathways (Hong et al., 2020). The NLRP3 inflammasome, as a standard inflammatory pathway, has been confirmed to play a role in the pathogenesis of AR (Sang et al., 2022). Recent studies have shown that NLRP3 can function as a transcription factor to regulate downstream inflammatory pathways. It can also act as a transcription factor for IL-33 to control the abnormal activation of ILC2s. Furthermore, NLRP3 is involved in the development of AR independently of the NLRP3 inflammasome (Zheng et al., 2021). This review will incorporate the novel idea that NLRP3 acts as a transcriptional regulator in AR through a non-inflammasome pathway. After an initial analysis of the components proven effective against AR, we have discovered that luteolin (Rakariyatham et al., 2018; Hussain et al., 2023; Vajdi et al., 2023), calycosin (Peng et al., 2024), and formononetin (Zhang et al., 2024a; Aladaileh et al., 2019). These three compounds possess strong anti-inflammatory and other pharmacological activities. For example,in a study using an animal model of allergic asthma in mice, calycosin was found to significantly alleviate nasal mucosal inflammation and reduce the levels of Th2-type inflammatory cytokines, such as IL-4, IL-5, and IL-13 (Xue et al., 2021). In addition, luteolin and formononetin also have immunomodulatory effects in various inflammation and immune-related models. Studies have shown that luteolin can reduce the expression of inflammatory factors such as IL-6 and TNF-α by inhibiting the NF-κB and MAPK pathways (Che et al., 2020b). Luteolin has also been shown to alleviate allergic nasal inflammation and inhibit the production of IL-4 in mouse models and peripheral blood mononuclear cells from AR patients (Liang et al., 2020). Formononetin inhibits pro-inflammatory factors and enhances the expression of anti-inflammatory factor IL-10 by regulating the NF-κB and JAK2/STAT3 pathways (Li et al., 2018; Y et al., 2022). Although there is currently a lack of direct evidence of the combined effect of the three monomers in AR models, their independent mechanisms of action support their potential synergistic therapeutic value. Therefore, they can be considered candidate components for treating AR. This review uniquely separates the “nuclear transcriptional function” of NLRP3 from its “cytoplasmic inflammasome function” into two parallel pathways. It systematically compares how three natural compounds intervene selectively at different stages of these pathways, clarifying their complementary mechanisms in treating AR. Due to the side effects associated with hormonal therapy for AR, this review proposes a new therapeutic approach of “non-hormonal, barrier repair first,” encouraging the development of non-hormonal, barrier repair-focused treatments for allergic rhinitis.
In conclusion, this article will examine the potential of the three monomers to influence the NLRP3/IL-33/ILC2s signaling axis, offering a reference for future AR researchers.
2 NLRP3 and IL-33 regulatory network
NLRP3 is an important pattern recognition receptor. Its structural domain consists of a N-terminal pyrrole domain (PYD), a nucleotide-binding oligomeric domain (NOD), and a C-terminal leucine-rich repeat (LRR) domain that consists of 12 repeats (Sharif et al., 2019). NLRP3 can function as a transcription factor to activate downstream inflammatory pathways. It can also assemble with ASC and caspase-1 to form the NLRP3 inflammasome. Similar to most inflammasomes, the NLRP3 inflammasome consists of the adaptor protein ASC, PYD, and caspase-1. The ASC adaptor protein includes PYD and CARD domains. The PYD domain of ASC binds to the PYD domain of NLRP3, and the CARD domain of ASC binds to the CARD domain of caspase-1, thereby forming the NLRP3 inflammasome (Xu and Núñez, 2023). Activation of the NLRP3 inflammasome results in the release of pro-inflammatory cytokines, including IL-1β and IL-18, thereby promoting inflammation (He et al., 2016).
Research indicates that NLRP3 can act as a transcription factor to activate inflammatory pathways and influence TH2 differentiation. NLRP3 interacts with IRF4 and enhances the ability of IRF4 and IL4 to bind to and activate the promoter. This suggests that NLRP3 may act as a transcription factor for CD4 Th2 cells (Bruchard et al., 2015). In additionally, NLRP3 also has a regulatory effect on IL-33. Recent studies have shown that NLRP3, localized in the nucleus of epithelial cells, interacts with IRF4 and directly binds to the IL-33-specific promoter, thereby activating its transcription and increasing IL-33 expression. The release of IL-33 further activates downstream signaling pathways, triggering inflammatory responses (Zhou et al., 2024; Im and Ammit, 2014). Notably, a study by Hong et al. (Zheng et al., 2021) demonstrated that in the absence of NLRP3, the expression of IL-33 in airway epithelial cells was reduced by 72.3% (p < 0.001), with significant inhibition of ILC2s activation, leading to reduced production of Th2-type cytokines and emphasizing the critical role of this pathway in AR pathogenesis.
3 IL-33/ILC2s/Th2 cascade reaction mechanism
AR is a common allergic disease whose development has long been linked to an imbalance between Th1 and Th2 immunity (Zhang et al., 2021a). However, recent studies have shown that the traditional Th1/Th2 imbalance theory only partially explains the complex immunopathological processes underlying AR. With a deeper understanding of the innate immune system, ILC2s have emerged as key effector cells that initiate early responses in AR (Kato, 2019). Additionally, the essential role of epithelial barrier dysfunction in the development of AR has become increasingly evident (Hellings and Steelant, 2020). Epithelial barrier dysfunction is a key factor in the development of AR. A healthy epithelial barrier can effectively prevent allergens from entering the submucosa of the nasal mucosa, while a damaged barrier allows allergens to penetrate, triggering immune responses and leading to AR. As AR becomes chronic, repeated inflammation continues to damage the epithelial barrier, creating a vicious cycle that results in persistent worsening and chronicity of the condition. Additionally, long-term inflammation can cause apoptosis, necrosis, and other forms of programmed cell death in epithelial cells, further weakening the barrier’s integrity and intensifying the inflammatory response AR (Yuan et al., 2025; Huang et al., 2024). According to the conventional mechanism, when the body is exposed to allergens such as mites, pollen, and dust, the epithelial mucosal barrier is disrupted. This breach greatly increases the likelihood of allergen contact with antigen-presenting cells (APCs), such as dendritic cells, thus amplifying local allergic reactions. Naive T cells, upon recognizing specific antigenic peptides in complex with major histocompatibility complex (MHC) molecules presented by APCs, differentiate into Th2 cells (Breiteneder et al., 2019). These Th2 cells secrete a range of cytokines (IL-4, IL-5, IL-13), which further stimulate B cells to produce allergen-specific IgE. The resulting IgE antibodies then bind to high-affinity IgE receptors (FcεRI) on the surfaces of mast cells and basophils (Chen et al., 2025). Upon re-exposure to the same allergen, it binds to IgE, activating mast cells and basophils (El Ansari et al., 2022). This activation triggers degranulation, releasing histamine, leukotrienes, and other inflammatory mediators that cause allergic symptoms (Figure 1). However, as research deepens, increasing evidence shows that the development of AR can occur independently of IgE (Luo et al., 2020; Hong et al., 2020). Dysfunction of the epithelial barrier directly causes the release of inflammatory cytokines (IL-33, IL-25, TSLP). These cytokines can activate innate immune cells, such as mast cells and basophils, in an IgE-independent manner (Hellings and Steelant, 2020; Kortekaas et al., 2020; Nur Husna et al., 2021; Steelant et al., 2016). IL-33 plays a crucial role in initiating type 2 immunity. The response of ILC2s to IL-33 is significantly more severe compared to that of IL-25. Studies comparing IL-33−/− and IL-25−/− gene mice in airway hyperresponsiveness and acetylcholine-induced airway contraction have shown that diseases stimulated by IL-33 are more severe, with clear activation of ILC2s. This indicates that IL-33 is vital in regulating immune-mediated respiratory responses diseases (Barlow et al., 2013). When allergens breach the epithelial barrier, the release of alarmins like IL-33 activates ILC2s by binding to the ST2 receptor (IL-1RL1) on these cells. This triggers downstream NF-κB and MAPK signaling pathways, leading to high expression of GATA3 and promoting the secretion of Th2-type cytokines ILC2s (Dwyer et al., 2022). Importantly, IL-33 works together with IL-25 and TSLP to boost the response: TSLP increases IL-33 sensitivity by raising ST2 receptor levels on ILC2s, while IL-25 extends ILC2s survival through activation of the STAT5 pathway, creating a positive feedback loop. Activated ILC2s not only release IL-5 and IL-13 directly but also promote Th2 cell polarization via MHC II antigen presentation, enabling communication between adaptive and innate immunity (Hong et al., 2020; Kitano et al., 2022) (Figure 2).
FIGURE 1
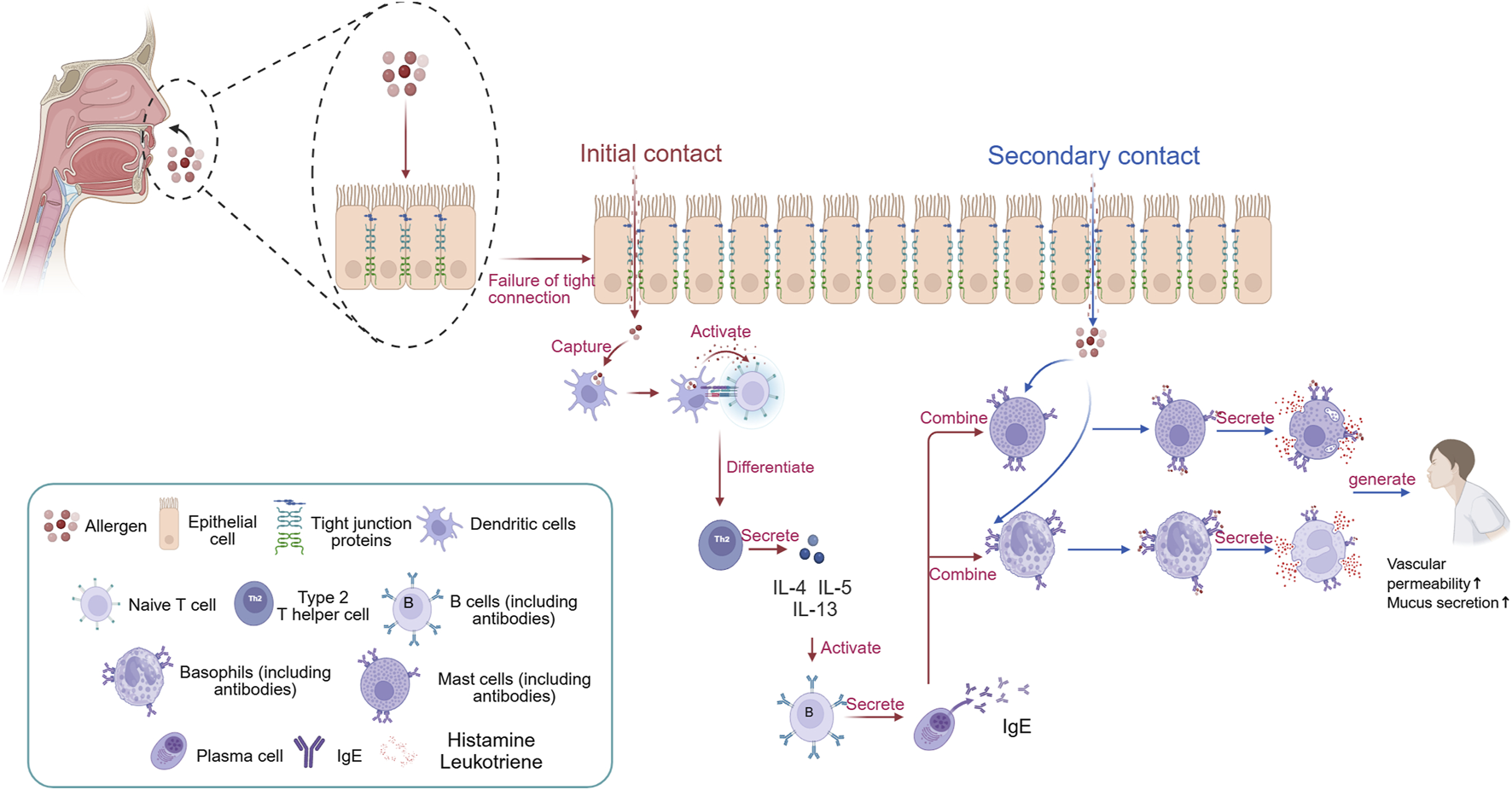
The traditional pathogenesis of AR. When the body first encounters allergens, they disrupt the tight junction proteins of epithelial cells, allowing them to enter the body. At this point, dendritic cells engulf the allergen and degrade it into peptide fragments. Subsequently, dendritic cells transmit signals to naive T cells, causing them to differentiate into Th2 cells. The cytokines produced by Th2 cells (IL-4, IL-5, and IL-13) stimulate B cells to differentiate into plasma cells, which then secrete IgE antibodies. The constant region of IgE antibodies in the body targets mast cells and eosinophils, where they reside in a latent state. When the body is re-exposed to the same allergen, the allergen can specifically bind to the variable region of IgE antibodies on the surface of mast cells and eosinophils. Stimulated mast cells and eosinophils then degranulate, releasing inflammatory mediators such as histamine and leukotrienes, leading to the occurrence of AR. Created in BioRender. xi, l. (2025) https://BioRender.com/pch6h63.
FIGURE 2
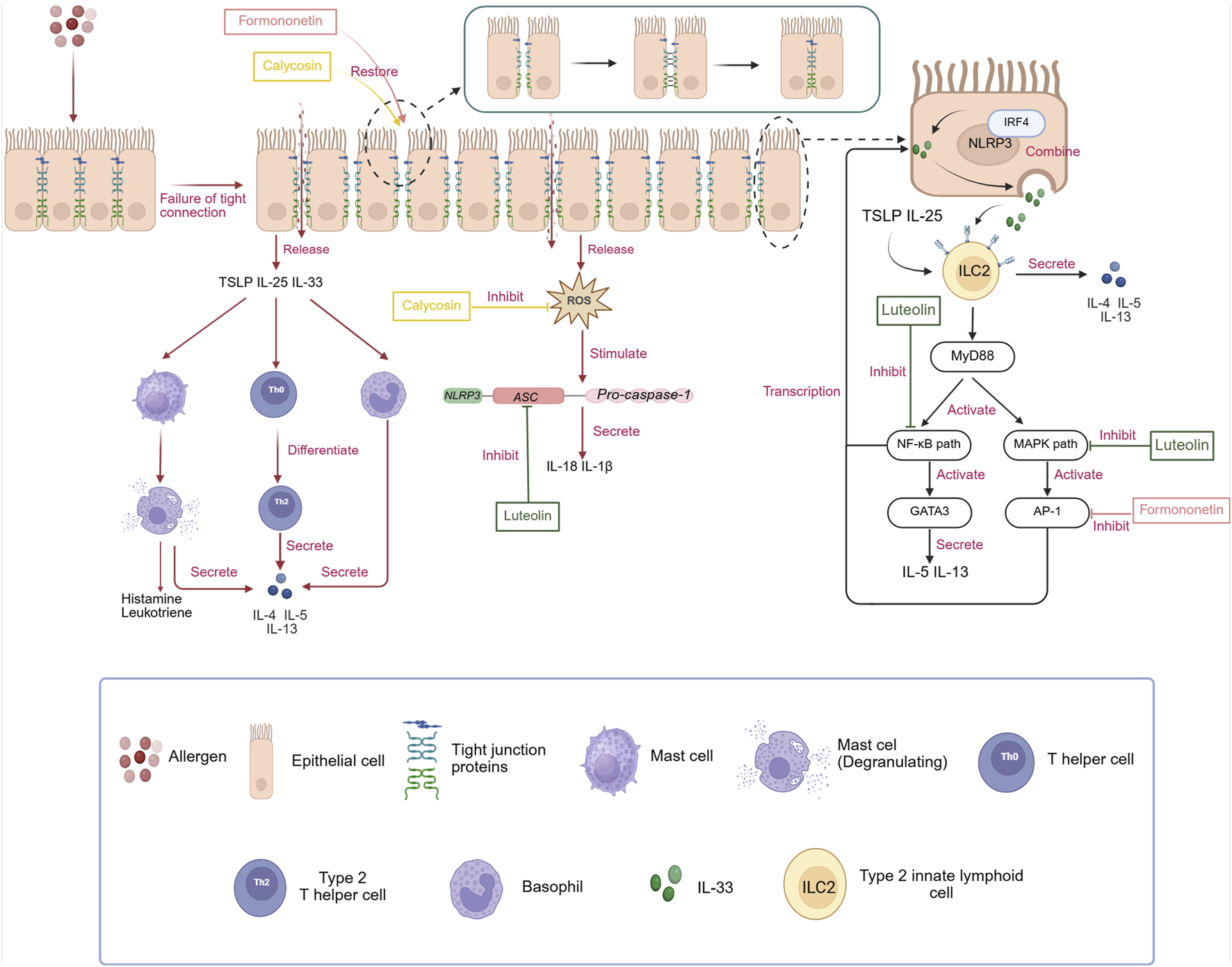
The pathogenesis of AR independent of IgE and the regulatory effects of three monomeric compounds (luteolin, calycosin, and formononetin)on the NLRP3/IL-33/ILC2s pathway in treating AR. i: Unlike the traditional pathogenesis of AR, allergens, after disrupting the tight junction proteins of epithelial cells, cause the release of alarmins, such as TSLP, IL-25, and IL-33, as well as inflammatory substances like ROS. These alarmins directly stimulate the differentiation of Th0 cells into Th2 cells and also directly trigger the secretion of Th2-type cytokines from mast cells and basophils, creating an inflammatory environment. ROS, in turn, directly promote the assembly of downstream NLRP3 inflammasomes, leading to the release of IL-18 and IL-1β, which exacerbate the symptoms of AR. In addition, NLRP3 exists independently of the inflammasome in the nucleus of epithelial cells, interacts with IRF4, binds directly to the IL-33-specific promoter, and activates the transcription of IL-33, increasing its expression. IL-33 released from epithelial cells can also synergistically promote the activation of ILC2 cells with alarmins such as IL-25 and TSLP, leading to a series of subsequent inflammatory reactions. ii: Luteolin can inhibit the activation of the NLRP3 inflammasome by preventing ASC oligomerization, thereby reducing the expression of IL-18 and IL-1β. Furthermore, it can block the MAPK and NF-κB pathways to decrease the transcription of IL-33, thereby regulating the NLRP3/IL-33/ILC2s pathway to treat AR. Calycosin can directly inhibit the generation of ROS, interrupting the signaling for the assembly of the NLRP3 inflammasome. Formononetin can downregulate the expression of the IL-33 transcription factor AP-1 to treat AR. It is worth noting that both calycosin and formononetin can promote the repair of the epithelial cell barrier, fundamentally reducing the stimulation of allergens on epithelial cells and the release of inflammatory factors. Created in BioRender. xi, l. (2025) https://BioRender.com/yxemxgb.
In summary, blocking the activation of ILC2s and their release of Th2-type cytokines is a key strategy for treating AR. Although traditional Chinese medicine (TCM) formulas have proved effective for AR, their complex makeup makes it hard to pinpoint the specific components responsible for their healing effects. This review will, therefore, select effective monomers from TCM formulas used to treat AR based on formula analysis and previous research, to clarify their therapeutic mechanisms.
4 Modulation of the NLRP3/IL-33/ILC2s signaling pathway by monomeric compounds
Recent studies have shown that respiratory symptoms such as bronchial asthma, chronic pharyngitis, and allergic rhinitis, which cause coughing, expectoration, and wheezing, are linked to local inflammation infiltration (Eifan and Durham, 2016; Poletti et al., 2006). The main treatment approach for these conditions involves eliminating chronic airway inflammation. Corticosteroids are commonly used in Western medicine to reduce airway inflammation, but their numerous side effects limit their long-term use. According to the literature, 38.3% of patients with allergic rhinitis are refractory to corticosteroids treatment (Sun et al., 2023). In addition, long-term use of corticosteroids may cause weight gain or glaucoma patients (Santiago and da, 2014). Currently, medications are being developed to target type 2 immune responses in AR. Only a few biological agents targeting cytokines such as IL-33 are under development, with most still in phase I or II clinical trials (Hong et al., 2020). Therefore, given the serious treatment situation for AR, it is urgent to find a component with higher safety and fewer side effects to replace it corticosteroids.
Based on statistical analysis, we found that among the TCM with fewer side effects in treating AR, monomeric components such as luteolin, calycosin, and formononetin are particularly significant. The statistical results are as follows (Table 1; Table 2).
TABLE 1
| Monomer | TCM containing luteolin | Content level (ug/g) | Key compositional herbs | Chinese medicine combined with Chinese patent medicine to treat AR | Pharmacokinetics |
|---|---|---|---|---|---|
| Luteolin | Lonicerae Japonicae Flos (Zhang et al., 2016; Kang et al., 2010; Yang et al., 2025) | 19.93–239.49 (Zhang et al., 2024b) | Xanthii Fructus, Magnoliae Flos, Rubiae Radix et Rhizoma, Chrysanthemi Indici Flos | Biyuan Pian | The bioavailability of luteolin in the human body is approximately 25%, with a half-life of 4.5 h. It is mainly metabolized in the liver and excreted through urine (Lee et al., 2021) |
| Pogostemonis Herba, Angelicae Dahuricae Radix, Xanthii Fructus, Schizonepetae Herba, et al. | Dare Fragrance Rhinitis Tablets | ||||
| Astragali Radix (Zhang et al., 2022a) | 55 (Güven et al., 2023) | Saposhnikoviae Radix, Atractylodis Macrocephalae Rhizoma (fried) | Yu Ping Feng Granule | ||
| Xanthii Fructus (fried), Saposhnikoviae Radix, Angelicae Dahuricae Radix, Magnoliae Flos et al. | Tongqiao Biyan Capsules | ||||
| Chrysanthemi Flos (Tian et al., 2020; Yang et al., 2024) | 205.0–1787 (Wu et al., 2017) | Pogostemonis Herba, Scutellariae Radix, Xanthii Fructus, Ephedrae Herba, et al. | Biyan Kang Tablets | ||
| Scutellariae Radix (Song et al., 2023; Sonoda et al., 2004) | 113.2–17798.8 (Guo et al., 2020) | Asari Radix et Rhizoma, Schizonepetae Herba, Saposhnikoviae Radix, Angelicae Dahuricae Radix, et al. | Xin Qin Granules |
TCM formulas containing luteolin for the treatment of AR.
The exact proportions of herbal components in the TCM formulas listed in the table are typically not disclosed in academic literature and are often protected as proprietary intellectual property. The herbal combinations presented under “Key compositional herbs” are all based on their publicly available package inserts, reflecting their main constituent herbs.
TABLE 2
| Monomer | TCM containing calycosin and formononetin | Content level (ug/g) | Key compositional herbs | Chinese medicine combined with Chinese patent medicine to treat AR | Pharmacokinetics |
|---|---|---|---|---|---|
| Calycosin formononetin | Astragali Radix (Cao et al., 2025; Ding et al., 2024; Quan et al., 2015) | Calycosin 35.8–98.5 (Wang et al., 2016) | Saposhnikoviae Radix, Atractylodis Macrocephalae Rhizoma (fried) | Yu Ping Feng Granules | The oral bioavailability of isoflavones is approximately 30%, with a half-life of 5.2 h. Their metabolism mainly involves gluconaldehyde acidification and sulfation reactions (Yuan et al., 2020) |
| Xanthii Fructus (fried), Saposhnikoviae Radix, Angelicae Dahuricae Radix, Magnoliae Flos et al. | Tongqiao Biyan Capsules | ||||
| Prunellae Spica, Chrysanthemi Indici Flos, Magnoliae Flos, Saposhnikoviae Radix, et al. | Xiangju Capsules | ||||
| Formononetin 20.32–364.65 (Kim et al., 2007) | Bupleuri Radix, Saposhnikoviae Radix, Rehmanniae Radix (Dried), Mume Fructus, et al. | Jie Min Tang | |||
| Glycyrrhizae Radix et Rhizoma (Jiang et al., 2022; Li et al., 2024; Wu et al., 2016) | Calycosin 5.93–209.29 (Bo et al., 2002) | Ephedrae Herba, Cinnamomi Ramulus, Paeoniae Radix Alba, Zingiberis Rhizoma, et al. | Xiaoqinglong Granules | The bioavailability of Morinda officinalis is approximately 20%, with a half-life of 3.8 h. The main products after metabolism are excreted through feces (Y et al., 2022) | |
| Angelicae Dahuricae Radix, Notopterygii Rhizoma et Radix, Asari Radix et Rhizoma, Saposhnikoviae Radix, et al. | Chuan Xiong Cha Tiao Wan | ||||
| Formononetin 110.3–583.68 (Tian et al., 2007) | Magnoliae Flos, Xanthii Fructus (fried), Ephedrae Herba, Angelicae Dahuricae Radix, et al. | Biyan Ning Granules |
TCM formulas containing calycosin and formononetin for the treatment of AR.
The exact proportions of herbal components in the TCM formulas listed in the table are typically not disclosed in academic literature and are often protected as proprietary intellectual property. The herbal combinations presented under “Key compositional herbs” are all based on their publicly available package inserts, reflecting their main constituent herbs.
After conducting a statistical analysis of the effectiveness of TCM components in treating AR, we identified the usage frequencies of three monomers—luteolin, calycosin, and formononetin—in AR research. It is important to note that the analysis of the prescription serves only as a preliminary indication, and final confirmation of the active ingredients still requires further experimental validation. To better understand the therapeutic potential of these TCM monomers, we performed a detailed comparison with conventional drugs (Table 3). The results showed that these TCM monomers have notable advantages in reducing inflammation, repairing nasal mucosal barriers, and lowering recurrence rates, while also exhibiting lower toxicity and fewer side effects. This comparative analysis enhances our overall understanding of the potential of TCM monomers in AR treatment and provides valuable references for future research and clinical application.
TABLE 3
| Category | Inhibition rate of IL-33 | Nasal mucosal barrier repair | Recurrence rate within half a year | Risk of liver toxicity |
|---|---|---|---|---|
| Corticosteroid | 75% (Petersen et al., 2021) | No | 42% (Szaleniec et al., 2024) | High (Abdelaziz et al., 2025) |
| Anti-IgE antibody | 28% (McGowa et al., 2023) | No | 38% (Hayashi et al., 2025) | Low (Corren et al., 2009) |
| Luteolin combination | 67% (Liang et al., 2020; Hellings and Steelant, 2020) | Significant (Li et al., 2018; Bradding et al., 2024; Yuan et al., 2020) | 19% (Xue et al., 2021) | Middle (Aladaileh et al., 2019) |
AR treatment comparison table: Analysis of the efficacy and safety performance of traditional drugs and Chinese herbal monomers.
4.1 Luteolin: dual inhibition of NLRP3 inflammasome and nuclear transcriptional activation
Luteolin is a natural flavone compound found in various plants. It is generally very safe, with an intraperitoneal LD50 of 411 mg/kg and an oral LD50 of 5,000 mg/kg rats (Xiong et al., 2017). Additionally, it has various pharmacological effects, such as anti-inflammatory, anti-allergic, uric acid-lowering, anti-tumor, antibacterial, and antiviral activities effects (Gendrisch et al., 2021). Studies have demonstrated that luteolin can modulate the NLRP3 inflammasome (Lee et al., 2021), IL-33 and Th2-type cytokines may help treat various diseases. In the progression of AR, damaged epithelial cells release IL-33, which binds to the ST2 receptor on ILC2s, activating them and leading to the secretion of Th2-type cytokines that worsen AR symptoms (Dwyer et al., 2022; Bradding et al., 2024). Research has shown that the release of IL-33 involves complex pathways and substances, with the phosphorylation of AP-1 and NF-κB promoting IL-33 transcription and subsequent release. Luteolin can block the activation of MAPK, NF-κB, and AP-1 pathways (Chen et al., 2024; Zhang et al., 2021b), reducing IL-33 expression and subsequent inflammatory cascades. In allergic diseases, excessive secretion of Th2-type cytokines is viewed as a primary cause. IL-4 is a crucial factor in Th2 cell differentiation, as STAT6 binds to the IL-4 gene promoter to promote its expression and works with GATA3 to enhance Th2 cell differentiation. Luteolin can block the differentiation of Th2 cells by suppressing the IL-4/STAT6/GATA3 signaling pathway, thereby decreasing the secretion of Th2-type cytokines (IL-4, IL-5, and IL-13) and easing allergic symptoms diseases (Chen et al., 2024). Additionally, studies have shown that luteolin can influence the polarization of macrophages from the M1 to the M2 phenotype by downregulating STAT3 and upregulating STAT6, thereby exerting anti-inflammatory effects (Liu et al., 2022). Luteolin not only has strong anti-inflammatory effects but also shows antioxidant activity. In models of myocardial cell inflammatory injury, luteolin lowers reactive oxygen species (ROS) levels and inhibits the activity of the NLRP3 inflammasome (Zhang et al., 2021b). Regarding the assembly of the NLRP3 inflammasome, Lee et al. (2021) demonstrated via cryo-electron microscopy that the ASC oligomer level in the group treated with luteolin was reduced by 67% (p < 0.001). Co-immunoprecipitation (Co-IP) showed that luteolin reduced the binding of TXNIP to NLRP3 (binding ↓62%, p < 0.01). After dissociating from thioredoxin, TXNIP translocates to the mitochondria and other locations where it binds to NLRP3. ASC, an essential component of the NLRP3 inflammasome, decreased along with the TXNIP-NLRP3 binding, confirming that luteolin reduces the overall level of the NLRP3 inflammasome in the organism. Due to the complexity of biological systems, the interaction between antioxidant and anti-inflammatory mechanisms is complex and bidirectional. The varied pharmacological effects of luteolin provide a strong theoretical basis for developing new therapeutic agents.
4.2 Calycosin: modulation of the NLRP3/IL-33/ILC2s signaling pathway
Calycosin, a natural isoflavone compound, shows significant anti-inflammatory and immunomodulatory effects (Deng et al., 2021). In relevant studies, it has been shown to modulate the NLRP3/IL-33/ILC2s signaling pathway (Tian et al., 2024; Liao et al., 2024) and the assembly of the NLRP3 inflammasome (Xia et al., 2021), thereby exerting therapeutic effects. Some studies have shown that in treating intestinal fibrosis, calycosin decreases the mRNA levels of NLRP3 and its downstream caspase-1 and IL-1β in IBD mice, inhibiting the expression of inflammasome-related factors. Additionally, in NLRP3-knockout MODE-K cells, IL-33 signaling is significantly diminished. Therefore, calycosin may have therapeutic potential in intestinal fibrosis by regulating NLRP3 activation and its downstream IL-33/ST2 signaling (Liao et al., 2024). This finding aligns with the idea that NLRP3 can work independently of the NLRP3 inflammasome, laying the groundwork for future research. In cases of ovalbumin-induced allergic asthma, calycosin exerts its therapeutic effect by reducing IL-33/ST2 expression, which inhibits the polarization of ILC2s and M2 macrophages (Tian et al., 2024). Additionally, calycosin plays a role in the assembly and activation of the NLRP3 inflammasome. Xia Y et al. demonstrated that using the DCFH-DA method, calycosin at a concentration of just 20 μM can reduce ROS levels by 78.3% in a sepsis model. Moreover, calycosin dose-dependently inhibits ROS expression, further blocking the assembly of NLRP3, ASC, and pro-caspase-1, reducing the secretion of caspase-1, IL-1β, and IL-18, and thus attenuating the associated inflammatory responses (Xia et al., 2021; Chen et al., 2021). As previously mentioned, during the progression of AR, damaged epithelial cells release IL-33, IL-25, and TSLP, which promote the secretion of Th2-type cytokines by ILC2s, further amplifying the inflammatory response. Calycosin can inhibit the NF-κB pathway, repair epithelial tight junctions, and maintain the integrity of the epithelium barrier (Tao et al., 2017; Yuan et al., 2020), reducing the secretion of IL-33, TSLP, and IL-25 due to epithelial barrier damage, thereby reducing subsequent inflammatory responses. Jia Z et al. (Yuan et al., 2020) demonstrated through Western blot (WB) analysis, that the expression of Occludin protein in HaCaT cells treated with calycosin increased by 2.1-fold (p < 0.01). Occludin protein, a crucial component of tight junctions, when missing or impaired, can lead to disruption of intercellular connections, increasing tissue permeability and allowing harmful substances to enter the body through the barrier, potentially triggering diseases. In addition to WB validation showing that calycosin can enhance Occludin protein levels and strengthen epithelial barrier tightness, immunofluorescence (IF) results also indicated that, compared with the modeling group, the tight junctions between cells in the calycosin-treated group were restored in terms of continuity. In allergic asthma models similar to AR, studies have found that calycosin can modulate the activation of ILC2 and M2 macrophage polarization, thereby reducing the secretion of Th2-type cytokines in tissues and achieving therapeutic effects (Tian et al., 2024). From the analysis above, we see that calycosin has unique advantages in the anti-inflammatory process, especially its ability to repair damaged epithelial barriers, maintain barrier function, and reduce the release of related inflammatory factors. This provides a foundation for further exploring calycosin’s pharmacological mechanisms.
4.3 Formononetin: modulation of the NLRP3/IL-33/ILC2s signaling pathway
Formononetin, an isoflavone compound found in plants such as Astragalus and Millettia, shows anti-inflammatory and antioxidant activities. Like luteolin and calycosin, formononetin can modulate the expression of the NLRP3 inflammasome, IL-33, and Th2-type responses cytokines (Cho et al., 2019; Jia et al., 2022). In the context of allergic diseases, formononetin has shown significant therapeutic potential. Research indicates that formononetin can downregulate the expression of TSLP and IL-33, thereby helping to restore the epithelial barrier to treat atopic conditions dermatitis (Li et al., 2018) In a middle cerebral artery occlusion model, formononetin inhibits NLRP3 inflammasome activation by suppressing the NF-κB and JAK2/STAT3 signaling pathways, thereby decreasing the secretion of IL-1β and IL-18 (Y et al., 2022). Additionally, Yi et al. (2020) demonstrated via WB that formononetin inhibits JNK phosphorylation (p < 0.01). Immunofluorescence colocalization results showed a 63% decrease in nuclear c-Jun content. JNK, as a key MAPK, depends on its phosphorylation for activity, and the nuclear translocation of c-Jun is a crucial step in its function. Formononetin inhibits JNK phosphorylation, thereby decreasing AP-1 activity and preventing its entry into the nucleus, which in turn promotes IL-33 transcription and reduces IL-33 expression. (Yi et al., 2020). Under conditions of epithelial cell damage, formononetin can work synergistically with calycosin to promote cell proliferation and migration, thereby aiding in the repair of the epithelial barrier (Che et al., 2020b). and decreasing the secretion of IL-33, IL-25, and TSLP. In asthma models, groups treated with formononetin showed significantly lower secretion of Th2-type cytokines (IL-4, IL-5, IL-13), demonstrating its strong therapeutic potential in treating allergic diseases. However, the specific pharmacological mechanisms of formononetin need further investigation.
5 Discussion and future perspectives
Luteolin exerts strong anti-inflammatory effects by blocking the activation of the NLRP3/IL-33/ILC2s signaling pathway through multiple mechanisms, thereby decreasing the release of inflammatory substances cytokines (Che et al., 2020a; Che et al., 2020b). Its potential therapeutic value in AR has been preliminarily confirmed. Studies have demonstrated that luteolin exerts anti-inflammatory effects by inhibiting the PI3K-Akt signaling pathway (Gao et al., 2023; Chai et al., 2024). However, this pathway is vital for cell growth, proliferation, and survival, and excessive inhibition may cause cell apoptosis and other negative effects. The PI3K-Akt signaling pathway is closely linked to various diseases and is essential for maintaining normal cellular functions. When luteolin inhibits this pathway, it can disrupt the existing signaling balance within cells, leading to halted cell growth and increased apoptosis. Further clinical trials are needed to assess the safety and effectiveness of luteolin. Calycosin, which influences the activation of NLRP3 and its downstream signaling pathways (Zhang et al., 2022b; Chen et al., 2021), suppresses the secretion of related inflammatory cytokines and maintains the integrity of the epithelial barrier (Jia et al., 2018; Yuan et al., 2020), demonstrating significant anti-inflammatory and immunomodulatory effects. Formononetin, like calycosin, promotes the repair of the epithelial barrier (Yuan et al., 2020) and reduces the production of upstream cytokines in AR, thereby alleviating downstream inflammatory responses (Figure 2). The bioavailability of calycosin and formononetin in vivo has been shown to be low through chemical analysis and other methods (Lu et al., 2025; Guo et al., 2023; Xiang et al., 2025), which greatly limits their potential for use in treating AR. The absorption efficiency of drugs in the gastrointestinal tract and the strength of the first-pass effect can both cause low effective concentrations in the body, thereby restricting therapeutic effectiveness. To tackle this problem, future research could use nanocarrier delivery (Zou et al., 2024) to enhance the solubility and stability of the drugs, protect them from the harsh gastrointestinal environment, and promote their efficient release at target tissues, thereby improving overall bioavailability and achieving better therapeutic outcomes. After an in-depth investigation into the therapeutic effects of the three monomers on AR, it has been found that these three monomeric compounds exhibit multidimensional synergistic effects in AR treatment: luteolin focuses on inhibiting the signaling pathway (Liang et al., 2020), calycosin enhances barrier function (Jia et al., 2018; Yuan et al., 2020), and formononetin has the advantage of epigenetic regulation. Notably, all three compounds can downregulate IL-33 through different mechanisms (Li et al., 2018; Che et al., 2020b; Elsherbiny et al., 2020). However, their specific sites of action differ, indicating that combination therapy may produce additive effects. Future studies should explore whether the combined use of these compounds can improve therapeutic outcomes. To better illustrate the individual effects of these three monomers in AR treatment, the following table (Table 4) provides a detailed overview of their core structures, binding energies with NLRP3, inhibitory rates on IL-33, and barrier repair efficiencies As illustrated in Table 4, luteolin has the highest binding energy (a measure of interaction strength) with NLRP3 (−8.2 kcal mol−1), This pronounced interaction physically occludes the ASC-binding on NLRP3, thereby aborting inflammasome assembly (Lee et al., 2021). This is consistent with its strong inhibitory effect on IL-33 expression (inhibition by 68%). Calycosin, although displaying an intermediate binding energy (−7.5 kcal mol−1), demonstrates superior efficacy in restoring epithelial barrier integrity, intercepting the ‘inflammasome–barrier damage–alarmin release’ feed-forward loop at an intermediate step, consequently diminishing IL-33 secretion and indirectly restraining ILC2 activation. Formononetin, possessing the lowest NLRP3-binding energy among the three compounds (−6.9 kcal mol−1), appears to operate via an allosteric mode—interfering with AP-1/NF-κB-mediated transcriptional elongation of IL-33 rather than direct NLRP3 engagement—thus exemplifying a ‘weak binding–strong transcriptional repression’ paradigm.
TABLE 4
| Monomer | Core structure | NLRP3 binding energy (kcal/mol) | Inhibition rate of IL-33 (%) | Barrier repair efficiency |
|---|---|---|---|---|
| Luteolin | (C2 = C3) (Gendrisch et al., 2021) | −8.2 (Lee et al., 2021) | 68% (Che et al., 2020b) | Weak (Bradding et al., 2024) |
| Calycosin | (C3 = O) (Deng et al., 2021) | −7.5 (Zhang et al., 2022b) | 52% (Tian et al., 2024) | Strong (Jia et al., 2018) |
| Formononetin | (7-OH) (Aladaileh et al., 2019) | −6.9 (Jia et al., 2022) | 61% (Yi et al., 2020) | Middle (Li et al., 2018) |
Title:Basic data table of the performance of three monomers in the treatment of AR.
The three monomers have complementary characteristics: ‘luteolin targeting intracellular signaling, calycosin strengthening barrier function, and formononetin offering a balanced approach’. These data not only provide experimental evidence for the synergistic effects of the three monomers but also establish a foundation for future research on the potential benefits of their combined use. By examining these specific indicators, we can better understand the unique strengths of each monomer and their potential value in AR treatment.
6 Conclusion
The NLRP3/IL-33/ILC2s signaling pathway plays a crucial role in the pathogenesis of AR by activating ILC2s via NLRP3 and cytokines secreted by damaged epithelial cells, thereby promoting the secretion of Th2-type cytokines and exacerbating inflammatory responses. The identification of the NLRP3/IL-33/ILC2s signaling axis provides a novel target for AR treatment. Luteolin, calycosin, and formononetin exert synergistic effects through multi-target and multi-level mechanisms, effectively interrupting the pathological cycle of ‘epithelial damage-alarmin release-ILC2s activation-Th2 polarization’. Compared with traditional corticosteroid therapy, this multi-target regulatory regimen based on natural plant monomers shows significant safety advantages. In light of the limitations of the aforementioned monomers, future research will focus on the development of nanoparticles and nasal spray formulations (Shrestha et al., 2020) to achieve more precise and convenient drug delivery. Meanwhile, on the basis of establishing the synergistic effects of the three monomers, a determination of the synergistic index of the three components will be carried out, and the synergistic effect of combined medication will be verified by the Chou-Talalay method. This will further optimize their ratios and dosing regimens, enabling the drug combination to exert remarkable therapeutic effects at lower doses, thereby enhancing the safety and efficacy of drug therapy as a whole and bringing breakthrough progress to the field of AR treatment.
Statements
Author contributions
MJ: Writing – original draft. XL: Writing – review and editing. FJ: Writing – review and editing. DL: Funding acquisition, Resources, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was generously supported by the National Natural Science Foundation of China (grant number 82374526), Science and Technology Project of Guangzhou (grant number 2024A04J4334), Elite Talent Program of the First Affiliated Hospital of Guangzhou University of Chinese Medicine by 2023 years and Guangdong Province lingnan Characteristic Hospital Preparation Transformation Engineering Technology Research Center (2023A170).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AR, Allergic rhinitis; ILC2s, Type 2 innate lymphoid cells; NLRP3, NOD-like receptor protein 3; IL-33, Interleukin-33; IL-25, Interleukin-25; TSLP, Thymic stromal lymphopoietin; PYD, Pyrin domain; NOD, Nucleotide-binding oligomeric domain; LRR, Leucine-rich repeat; APCs, Antigen-presenting cells; MHC, Major histocompatibility complex; FcεRI, High-affinity IgE receptors; TCM, Traditional Chinese medicine; ROS, Reactive oxygen species; WB, Western blot; IF, Immunofluorescence.
References
1
Abdelaziz H. M. Abdelmageed M. E. Suddek G. M. (2025). Trimetazidine improves dexamethasone-induced insulin resistance and associated hepatic abnormalities in rats. Life Sci.375, 123747. 10.1016/j.lfs.2025.123747
2
Aladaileh S. H. Hussein O. E. Abukhalil M. H. Saghir S. A. M. Bin-Jumah M. Alfwuaires M. A. et al (2019). Formononetin upregulates Nrf2/HO-1 signaling and prevents oxidative stress, inflammation, and kidney injury in methotrexate-induced rats. Antioxidants (Basel)8 (10), 430. 10.3390/antiox8100430
3
Barlow J. L. Peel S. Fox J. Panova V. Hardman C. S. Camelo A. et al (2013). IL-33 is more potent than IL-25 in provoking IL-13–producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J. Allergy Clin. Immunol.132 (4), 933–941. 10.1016/j.jaci.2013.05.012
4
Bo T. Li K. A. Liu H. (2002). Fast determination of flavonoids in glycyrrhizae radix by capillary zone electrophoresis. Anal. Chim. Acta458 (2), 345–354. 10.1016/s0003-2670(02)00075-2
5
Bradding P. Porsbjerg C. Côté A. Dahlén S. E. Hallstrand T. S. Brightling C. E. (2024). Airway hyperresponsiveness in asthma: the role of the epithelium. J. Allergy Clin. Immunol.153 (5), 1181–1193. 10.1016/j.jaci.2024.02.011
6
Breiteneder H. Diamant Z. Eiwegger T. Fokkens W. J. Traidl-Hoffmann C. Nadeau K. et al (2019). Future research trends in understanding the mechanisms underlying allergic diseases for improved patient care. Allergy74 (12), 2293–2311. 10.1111/all.13851
7
Bruchard M. Rebé C. Derangère V. Togbé D. Ryffel B. Boidot R. et al (2015). The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat. Immunol.16 (8), 859–870. 10.1038/ni.3202
8
Cao W. Huang L. Yu H. Qian Y. Liu L. Xu M. et al (2025). Calycosin extracted from astragali radix reduces NETs formation to improve renal fibrosis via TLR4/NF-κB pathway. J. Ethnopharmacol.342, 119391. 10.1016/j.jep.2025.119391
9
Chai S. Yang Y. Wei L. Cao Y. Ma J. Zheng X. et al (2024). Luteolin rescues postmenopausal osteoporosis elicited by OVX through alleviating osteoblast pyroptosis via activating PI3K-AKT signaling. Phytomedicine128, 155516. 10.1016/j.phymed.2024.155516
10
Che D. N. Cho B. O. Kim J. S. Shin J. Y. Kang H. J. Jang S. I. (2020a). Effect of luteolin and apigenin on the production of Il-31 and Il-33 in lipopolysaccharides-activated microglia cells and their mechanism of action. Nutrients12 (3), 811. 10.3390/nu12030811
11
Che D. N. Shin J. Y. Kang H. J. Cho B. O. Kim Y. S. Jang S. I. (2020b). Luteolin suppresses IL-31 production in IL-33-stimulated mast cells through MAPK and NF-κB signaling pathways. Int. Immunopharmacol.83, 106403. 10.1016/j.intimp.2020.106403
12
Chen G. Hou Y. Li X. Pan R. Zhao D. (2021). Sepsis-induced acute lung injury in young rats is relieved by calycosin through inactivating the HMGB1/MyD88/NF-κB pathway and NLRP3 inflammasome. Int. Immunopharmacol.96, 107623. 10.1016/j.intimp.2021.107623
13
Chen D. Shen F. Liu J. Tang H. Teng X. Yang F. et al (2024). Luteolin enhanced antioxidant capability and induced pyroptosis through NF-κB/NLRP3/Caspase-1 in splenic lymphocytes exposure to ammonia. Sci. Total Environ.919, 170699. 10.1016/j.scitotenv.2024.170699
14
Chen M. Su Q. Shi Y. (2025). Molecular mechanism of IgE-mediated FcεRI activation. Nature637 (8045), 453–460. 10.1038/s41586-024-08229-8
15
Cho I. A. Kim T. H. Lim H. Park J. H. Kang K. R. Lee S. Y. et al (2019). Formononetin antagonizes the Interleukin-1β-Induced catabolic effects through suppressing inflammation in primary rat chondrocytes. Inflammation42 (4), 1426–1440. 10.1007/s10753-019-01005-1
16
Corren J. Casale T. B. Lanier B. Buhl R. Holgate S. Jimenez P. (2009). Safety and tolerability of omalizumab. Clin. and Exp. Allergy39 (6), 788–797. 10.1111/j.1365-2222.2009.03214.x
17
Deng M. Chen H. Long J. Song J. Xie L. Li X. (2021). Calycosin: a review of its pharmacological effects and application prospects. Expert Rev. Anti Infect. Ther.19 (7), 911–925. 10.1080/14787210.2021.1863145
18
Ding M. Bao Y. Liang H. Zhang X. Li B. Yang R. et al (2024). Potential mechanisms of formononetin against inflammation and oxidative stress: a review. Front. Pharmacol.15, 1368765. 10.3389/fphar.2024.1368765
19
Dwyer G. K. D’Cruz L. M. Turnquist H. R. (2022). Emerging functions of IL-33 in homeostasis and immunity. Annu. Rev. Immunol.40, 15–43. 10.1146/annurev-immunol-101320-124243
20
Eifan A. O. Durham S. R. (2016). Pathogenesis of rhinitis. Clin. Exp. Allergy.46 (9), 1139–1151. 10.1111/cea.12780
21
El Ansari Y. S. Kanagaratham C. Burton O. T. Santos J. V. Hollister B. M. A. Lewis O. L. et al (2022). Allergen-specific IgA antibodies block IgE-Mediated activation of mast cells and basophils. Front. Immunol.13, 881655. 10.3389/fimmu.2022.881655
22
Elsherbiny N. M. Said E. Atef H. Zaitone S. A. (2020). Renoprotective effect of calycosin in high fat diet-fed/STZ injected rats: effect on IL-33/ST2 signaling, oxidative stress and fibrosis suppression. Chem. Biol. Interact.315, 108897. 10.1016/j.cbi.2019.108897
23
Gao H. L. Yu X. J. Feng Y. Q. Yang Y. Hu H. B. Zhao Y. Y. et al (2023). Luteolin attenuates hypertension via inhibiting NF-κB-Mediated inflammation and PI3K/Akt signaling pathway in the hypothalamic paraventricular nucleus. Nutrients15 (3), 502. 10.3390/nu15030502
24
Gendrisch F. Esser P. R. Schempp C. M. Wölfle U. (2021). Luteolin as a modulator of skin aging and inflammation. Biofactors47 (2), 170–180. 10.1002/biof.1699
25
Guo M. An Q. Sheng Y. Gao W. Guo L. Liu H. et al (2020). Evaluation of the optimal harvest period for Scutellaria baicalensis stem and leaves based on HPLC combined with chemometrics. J. Pharm. Analysis40 (12), 2099–2108.
26
Guo Z. Liu Y. Xiang J. Liang X. (2023). Mechanochemical preparation of red clover extract/β-cyclodextrin dispersion: enhanced water solubility and activities in alleviating high-fat diet-induced lipid accumulation and gut microbiota dysbiosis in mice. Food Chem.15 (420), 136084. 10.1016/j.foodchem.2023.136084
27
Güven L. Erturk A. Miloğlu F. D. Alwasel S. Gulcin İ. (2023). Screening of antiglaucoma, antidiabetic, anti-alzheimer, and antioxidant activities of astragalus alopecurus Pall—Analysis of phenolics profiles by LC-MS/MS. Pharmaceuticals16 (5), 659. 10.3390/ph16050659
28
Hayashi H. Ishii M. Hasegawa Y. Taniguchi M. (2025). Critical pathomechanisms of NSAID-Exacerbated respiratory disease (N-ERD) clarified by treatment with omalizumab, an anti-IgE antibody. Allergol. Int.74 (1), 51–65. 10.1016/j.alit.2024.08.008
29
He Y. Hara H. Núñez G. (2016). Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci.41 (12), 1012–1021. 10.1016/j.tibs.2016.09.002
30
Hellings P. W. Steelant B. (2020). Epithelial barriers in allergy and asthma. J. Allergy Clin. Immunol.145 (6), 1499–1509. 10.1016/j.jaci.2020.04.010
31
Hong H. Liao S. Chen F. Yang Q. Wang D. Y. (2020). Role of IL-25, IL-33, and TSLP in triggering united airway diseases toward type 2 inflammation. Allergy75 (11), 2794–2804. 10.1111/all.14526
32
Huang Z. Q. Liu J. Sun L. Y. Ong H. H. Ye J. Xu Y. et al (2024). Updated epithelial barrier dysfunction in chronic rhinosinusitis: targeting pathophysiology and treatment response of tight junctions. Allergy79 (5), 1146–1165. 10.1111/all.16064
33
Hussain M. S. Gupta G. Goyal A. Thapa R. Almalki W. H. Kazmi I. et al (2023). From nature to therapy: luteolin’s potential as an immune system modulator in inflammatory disorders. J. Biochem. Mol. Toxicol.37 (11), e23482. 10.1002/jbt.23482
34
Im H. Ammit A. J. (2014). The NLRP3 inflammasome: role in airway inflammation. Clin. Exp. Allergy44 (2), 160–172. 10.1111/cea.12206
35
Jia Z. Wang X. Wang X. Wei P. Li L. Wu P. et al (2018). Calycosin alleviates allergic contact dermatitis by repairing epithelial tight junctions via down-regulating HIF-1α. J. Cell Mol. Med.22 (9), 4507–4521. 10.1111/jcmm.13763
36
Jia C. Hu F. Lu D. Jin H. Lu H. Xue E. et al (2022). Formononetin inhibits IL-1β-induced inflammation in human chondrocytes and slows the progression of osteoarthritis in rat model via the regulation of PTEN/AKT/NF-κB pathway. Int. Immunopharmacol.113 (Pt A), 109309. 10.1016/j.intimp.2022.109309
37
Jiang X. Lin Y. Wu Y. Yuan C. Lang X. Chen J. et al (2022). Identification of potential anti-pneumonia pharmacological components of Glycyrrhizae Radix et Rhizoma after the treatment with Gan An he Ji oral liquid. J. Pharm. Anal.12 (6), 839–851. 10.1016/j.jpha.2022.07.004
38
Kang O. H. Choi J. G. Lee J. H. Kwon D. Y. (2010). Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-kappaB and MAPKs activation pathways in HMC-1 cells. Molecules15 (1), 385–398. 10.3390/molecules15010385
39
Kato A. (2019). Group 2 innate lymphoid cells in airway diseases. Chest156 (1), 141–149. 10.1016/j.chest.2019.04.101
40
Kim J. H. Park S. Y. Lim H. K. Park A. Y. Kim J. S. Kang S. S. et al (2007). Quantitative evaluation of Radix astragali through the simultaneous determination of bioactive isoflavonoids and saponins by HPLC/UV and LC-ESI-MS/MS. Bull. Korean Chem. Soc.28 (7), 1187–1194.
41
Kitano M. Fukuoka S. Adachi N. Hisamitsu T. Sunagawa M. (2022). Shoseiryuto ameliorated TDI-induced allergic rhinitis by suppressing IL-33 release from nasal epithelial cells. Pharmaceutics14 (10), 2083. 10.3390/pharmaceutics14102083
42
Kortekaas K. I. Seys S. F. Lund G. Jonckheere A. C. Dierckx de Casterlé I. Ceuppens J. L. et al (2020). Nasal epithelial barrier dysfunction increases sensitization and mast cell degranulation in the absence of allergic inflammation. Allergy75 (5), 1155–1164. 10.1111/all.14132
43
Lee M. N. Lee Y. Wu D. Pae M. (2021). Luteolin inhibits NLRP3 inflammasome activation via blocking ASC oligomerization. J. Nutr. Biochem.92, 108614. 10.1016/j.jnutbio.2021.108614
44
Li L. Wang Y. Wang X. Tao Y. Bao K. Hua Y. et al (2018). Formononetin attenuated allergic diseases through inhibition of epithelial-derived cytokines by regulating E-cadherin. Clin. Immunol.195, 67–76. 10.1016/j.clim.2018.07.018
45
Li Z. Yuan J. Dai Y. Xia Y. (2024). Integration of serum pharmacochemistry and metabolomics to reveal the underlying mechanism of shaoyao-gancao-fuzi decoction to ameliorate rheumatoid arthritis. J. Ethnopharmacol.326, 117910. 10.1016/j.jep.2024.117910
46
Liang K. L. Yu S. J. Huang W. C. Yen H. R. (2020). Luteolin attenuates allergic nasal inflammation via inhibition of Interleukin-4 in an allergic rhinitis mouse model and peripheral blood from human subjects with allergic rhinitis. Front. Pharmacol.11, 291. 10.3389/fphar.2020.00291
47
Liao X. Xie H. Yu S. (2024). Calycosin prevents NLRP3-induced gut fibrosis by regulating IL-33/ST2 axis. Heliyon10 (9), e30240. 10.1016/j.heliyon.2024.e30240
48
Liu Z. Gao S. Bu Y. Zheng X. (2022). Luteolin protects cardiomyocytes cells against lipopolysaccharide-induced apoptosis and inflammatory damage by modulating Nlrp3. Yonsei Med. J.63 (3), 220–228. 10.3349/ymj.2022.63.3.220
49
Lu B. Zhang K. Dong Q. Wei L. Shi J. Liu F. et al (2025). Selenium nanoparticles combined with calycosin treated sepsis through synergistic anti-inflammatory and antioxidant effects. Nanoscale17 (26), 15866–15878. 10.1039/d5nr01444a
50
Luo X. Zeng Q. Yan S. Liu W. Luo R. (2020). MicroRNA-375-mediated regulation of ILC2 cells through TSLP in allergic rhinitis. World Allergy Organ J.13 (8), 100451. 10.1016/j.waojou.2020.100451
51
McGowan E. C. Medernach J. Keshavarz B. Workman L. J. Li R. C. Barnes B. H. et al (2023). Food antigen consumption and disease activity affect food-specific IgG4 levels in patients with eosinophilic esophagitis (EoE). Clin. Exp. Allergy53 (3), 307–315. 10.1111/cea.14215
52
Nathan R. A. Meltzer E. O. Derebery J. Campbell U. B. Stang P. E. Corrao M. A. et al (2008). The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc.29 (6), 600–608. 10.2500/aap.2008.29.3179
53
Nur Husna S. M. Tan H. T. T. Md Shukri N. Mohd Ashari N. S. Wong K. K. (2021). Nasal epithelial barrier integrity and tight junctions disruption in allergic rhinitis: overview and pathogenic insights. Front. Immunol.12, 663626. 10.3389/fimmu.2021.663626
54
Peng M. Yao Z. Zhang J. Lin Y. Xu L. Zhang Q. et al (2024). Discovery and validation of anti-arthritic ingredients and mechanisms of qingfu juanbi tang, a Chinese herbal formulation, on rheumatoid arthritis. J. Ethnopharmacol.329, 118140. 10.1016/j.jep.2024.118140
55
Petersen A. Ø. Jokinen M. Plichta D. R. Liebisch G. Gronwald W. Dettmer K. et al (2021). Cytokine-specific autoantibodies shape the gut microbiome in autoimmune polyendocrine syndrome type 1. J. Allergy Clin. Immunol.148 (3), 876–888. 10.1016/j.jaci.2021.03.025
56
Poletti V. Casoni G. Chilosi M. Zompatori M. (2006). Diffuse panbronchiolitis. Eur. Respir. J.28 (4), 862–871. 10.1183/09031936.06.00131805
57
Quan G. H. Wang H. Cao J. Zhang Y. Wu D. Peng Q. et al (2015). Calycosin suppresses RANKL-mediated osteoclastogenesis through inhibition of MAPKs and NF-κB. Int. J. Mol. Sci.16 (12), 29496–29507. 10.3390/ijms161226179
58
Rakariyatham K. Wu X. Tang Z. Han Y. Wang Q. Xiao H. (2018). Synergism between luteolin and sulforaphane in anti-inflammation. Food Funct.9 (10), 5115–5123. 10.1039/c8fo01352g
59
Sang W. Chen S. Lin L. Wang N. Kong X. Ye J. (2022). Antioxidant mitoquinone ameliorates EtOH-LPS induced lung injury by inhibiting mitophagy and NLRP3 inflammasome activation. Front. Immunol.13, 973108. 10.3389/fimmu.2022.973108
60
Santiago T. da S. J. A. P. (2014). Safety of low-to medium-dose glucocorticoid treatment in rheumatoid arthritis: myths and reality over the years. Ann. N. Y. Acad. Sci.1318 (1), 41–49. 10.1111/nyas.12428
61
Sharif H. Wang L. Wang W. L. Magupalli V. G. Andreeva L. Qiao Q. et al (2019). Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature570 (7761), 338–343. 10.1038/s41586-019-1295-z
62
Shrestha K. Van Strien J. Singh N. Inthavong K. (2020). Primary break-up and atomization characteristics of a nasal spray. PLoS One15 (8), e0236063. 10.1371/journal.pone.0236063
63
Song J. B. Zhao L. Q. Wen H. P. Li Y. P. (2023). Herbal combinations against COVID-19: a network pharmacology, molecular docking and dynamics study. J. Integr. Med.21 (6), 593–604. 10.1016/j.joim.2023.09.001
64
Sonoda M. Nishiyama T. Matsukawa Y. Moriyasu M. (2004). Cytotoxic activities of flavonoids from two scutellaria plants in Chinese medicine. J. Ethnopharmacol.91 (1), 65–68. 10.1016/j.jep.2003.11.014
65
Steelant B. Seys S. F. Boeckxstaens G. Akdis C. A. Ceuppens J. L. Hellings P. W. (2016). Restoring airway epithelial barrier dysfunction: a new therapeutic challenge in allergic airway disease. Rhinology.54 (3), 195–205. 10.4193/Rhino15.376
66
Sun D. Liu L. Yan Y. (2023). Development and evaluation of a nomogram for INCS insensitivity in Chinese adults with allergic rhinitis. Int. J. Clin. Pract.2023 (1), 3027092. 10.1155/2023/3027092
67
Szaleniec J. Bezshapkin V. Krawczyk A. Kopera K. Zapala B. Gosiewski T. et al (2024). Determinants of the microbiome spatial variability in chronic rhinosinusitis. Rhinology62 (1), 119–126. 10.4193/Rhin22.423
68
Tao Y. Wang Y. Wang X. Wang C. Bao K. Ji L. et al (2017). Calycosin suppresses epithelial derived initiative key factors and maintains epithelial barrier in allergic inflammation via TLR4 mediated NF-κB pathway. Cell Physiol. Biochem.44 (3), 1106–1119. 10.1159/000485416
69
Tian J. Liu H. Bai X. Jia T. (2007). Determination of the content of mangiferin in glycyrrhizin flavonoids by high performance liquid chromatography. Chin. Tradit. Pat. Med.08, 1232–1233.
70
Tian D. Yang Y. Yu M. Han Z. Z. Wei M. Zhang H. W. et al (2020). Anti-inflammatory chemical constituents of Flos chrysanthemi indici determined by UPLC-MS/MS integrated with network pharmacology. Food Funct.11 (7), 6340–6351. 10.1039/d0fo01000f
71
Tian C. Liu Q. Zhang X. Li Z. (2024). Blocking group 2 innate lymphoid cell activation and macrophage M2 polarization: potential therapeutic mechanisms in ovalbumin-induced allergic asthma by calycosin. BMC Pharmacol. Toxicol.25 (1), 30. 10.1186/s40360-024-00751-9
72
Vajdi M. Karimi A. Karimi M. Abbasalizad Farhangi M. Askari G. (2023). Effects of luteolin on sepsis: a comprehensive systematic review. Phytomedicine113, 154734. 10.1016/j.phymed.2023.154734
73
Wang Y. ling zeng L. Y. Zhang J. liang F. X. sheng Ge C. fang H. L. (2016). Simultaneous separation and determination of four main isoflavonoids in astragali radix by an isocratic LC/ESI-MS method. J. Cent. South Univ.23 (2), 303–309. 10.1007/s11771-016-3074-4
74
Wu J. Xie Y. Xiang Z. Wang C. Zhou H. Liu L. (2016). Simultaneous determination of multiple components in guanjiekang in rat plasma via the UPLC-MS/MS method and its application in pharmacokinetic study. Molecules21 (12), 1732. 10.3390/molecules21121732
75
Wu T. Yu C. Li R. (2017). Determination of flavonoids in Flos chrysanthemi and Flos chrysanthemi indici by capillary electrophoresis. Instrum. Sci. and Technol.45 (4), 412–422. 10.1080/10739149.2016.1258572
76
Xia Y. Cao Y. Sun Y. Hong X. Tang Y. Yu J. et al (2021). Calycosin alleviates sepsis-induced acute lung injury via the inhibition of mitochondrial ROS-mediated inflammasome activation. Front. Pharmacol.12, 690549. 10.3389/fphar.2021.690549
77
Xiang L. H. Feng M. G. Guo X. Y. Li Y. Zhao Z. G. Liu G. X. et al (2025). Studying the effects of saposhnikoviae radix on the pharmacokinetic profiles of 10 bioactive compounds originating from astragali radix in rat plasma by UHPLC-QTRAP-MS/MS. J. Ethnopharmacol.337 (Pt 1), 118813. 10.1016/j.jep.2024.118813
78
Xiong J. Wang K. Yuan C. Xing R. Ni J. Hu G. et al (2017). Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int. J. Mol. Med.39 (1), 113–125. 10.3892/ijmm.2016.2809
79
Xu J. Núñez G. (2023). The NLRP3 inflammasome: activation and regulation. Trends Biochem. Sci.48 (4), 331–344. 10.1016/j.tibs.2022.10.002
80
Xue L. Li C. Ge G. Zhang S. Tian L. Wang Y. et al (2021). Jia-wei-yu-ping-feng-san attenuates group 2 innate lymphoid cell-mediated airway inflammation in allergic asthma. Front. Pharmacol.12, 703724. 10.3389/fphar.2021.703724
81
Yu L. Zhang Y. Chen Q. He Y. Zhou H. Wan H. et al (2022). Formononetin protects against inflammation associated with cerebral ischemia-reperfusion injury in rats by targeting the JAK2/STAT3 signaling pathway. Biomed. Pharmacother.149, 112836. 10.1016/j.biopha.2022.112836
82
Yang P. Tian D. Han X. Y. Zou Q. J. Ma L. J. Wei M. et al (2024). Optimal harvest period and quality control markers of cultivated Flos chrysanthemi indici using untargeted/targeted metabolomics, chemometric analysis and in vivo study. J. Ethnopharmacol.334, 118533. 10.1016/j.jep.2024.118533
83
Yang S. Duan H. Zeng J. Yan Z. Niu T. Ma X. et al (2025). Luteolin modulates macrophage phenotypic switching via the AMPK-PPARγ pathway to alleviate ulcerative colitis in mice. J. Ethnopharmacol.339, 119157. 10.1016/j.jep.2024.119157
84
Yi L. Cui J. Wang W. Tang W. Teng F. Zhu X. et al (2020). Formononetin attenuates airway inflammation and oxidative stress in murine allergic asthma. Front. Pharmacol.11, 533841. 10.3389/fphar.2020.533841
85
Yuan W. Y. Li L. Q. Chen Y. Y. Zhou Y. J. Bao K. F. Zheng J. et al (2020). Frontline science: two flavonoid compounds attenuate allergic asthma by regulating epithelial barrier via G protein-coupled estrogen receptor: probing a possible target for allergic inflammation. J. Leukoc. Biol.108 (1), 59–71. 10.1002/JLB.3HI0220-342RR
86
Yuan J. Liao Z. Zhu X. Zhu Y. Wu S. Guo L. et al (2025). PM2.5 exacerbates nasal epithelial barrier dysfunction in allergic rhinitis by inducing NLRP3-mediated pyroptosis via the AhR/CYP1A1/ROS axis. J. Hazard Mater.492, 138145. 10.1016/j.jhazmat.2025.138145
87
Zhang B. Nan T. Zhan Z. Kang L. Yang J. Yuan Y. et al (2016). Development of a monoclonal antibody-based enzyme-linked immunosorbent assay for luteoloside detection in flos Lonicerae japonicae. Anal. Bioanal. Chem.408 (22), 6053–6061. 10.1007/s00216-016-9396-0
88
Zhang Y. Lan F. Zhang L. (2021a). Advances and highlights in allergic rhinitis. Allergy76 (11), 3383–3389. 10.1111/all.15044
89
Zhang Z. T. Zhang D. Y. Xie K. Wang C. J. Xu F. (2021b). Luteolin activates tregs to promote IL-10 expression and alleviating caspase-11-dependent pyroptosis in sepsis-induced lung injury. Int. Immunopharmacol.99, 107914. 10.1016/j.intimp.2021.107914
90
Zhang C. Guo W. Yao X. Xia J. Zhang Z. Li J. et al (2022a). Database mining and animal experiment-based validation of the efficacy and mechanism of Radix astragali (huangqi) and rhizoma atractylodis macrocephalae (baizhu) as core drugs of Traditional Chinese medicine in cancer-related fatigue. J. Ethnopharmacol.285, 114892. 10.1016/j.jep.2021.114892
91
Zhang L. Fan C. Jiao H. C. Zhang Q. Jiang Y. H. Cui J. et al (2022b). Calycosin alleviates doxorubicin-induced cardiotoxicity and pyroptosis by inhibiting NLRP3 inflammasome activation. Oxid. Med. Cell Longev.2022, 1733834. 10.1155/2022/1733834
92
Zhang Y. Deng J. Chen T. Liu S. Tang Y. Zhao J. R. et al (2024a). Formononetin alleviates no reflow after myocardial ischemia-reperfusion via modulation of gut microbiota to inhibit inflammation. Life Sci.358, 123110. 10.1016/j.lfs.2024.123110
93
Zhang X. Yu X. Sun X. Meng X. Fan J. Zhang F. et al (2024b). Comparative study on chemical constituents of different medicinal parts of Lonicera japonica thunb. Based on LC-MS combined with multivariate statistical analysis. Heliyon10 (12), e31722. 10.1016/j.heliyon.2024.e31722
94
Zheng J. Yao L. Zhou Y. Gu X. Wang C. Bao K. et al (2021). A novel function of NLRP3 independent of inflammasome as a key transcription factor of IL-33 in epithelial cells of atopic dermatitis. Cell Death Dis.12 (10), 871. 10.1038/s41419-021-04159-9
95
Zhou H. Wang L. Lv W. Yu H. (2024). The NLRP3 inflammasome in allergic diseases: mechanisms and therapeutic implications. Clin. Exp. Med.24 (1), 231. 10.1007/s10238-024-01492-z
96
Zou B. Long Y. Gao R. Liu Q. Tian X. Liu B. et al (2024). Nanodelivery system of traditional Chinese medicine bioactive compounds: application in the treatment of prostate cancer. Phytomedicine135, 155554. 10.1016/j.phymed.2024.155554
Summary
Keywords
allergic rhinitis, luteolin, calycosin, formononetin, NLRP3/IL-33/ILC2s signaling axis
Citation
Jia M, Lei X, Jiang F and Li D (2025) The regulatory effects of luteolin, calycosin, and formononetin on the NLRP3/IL-33/ILC2s axis in the treatment of allergic rhinitis: mechanistic analysis and therapeutic potential. Front. Pharmacol. 16:1658772. doi: 10.3389/fphar.2025.1658772
Received
03 July 2025
Accepted
17 September 2025
Published
26 September 2025
Volume
16 - 2025
Edited by
Jean Sylvia Marshall, Dalhousie University, Canada
Reviewed by
Sidharth Mehan, Indo-Soviet Friendship College of Pharmacy, India
Peixin Guo, Yunnan University of Chinese Medicine, China
Updates
Copyright
© 2025 Jia, Lei, Jiang and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Detang Li, lidetang2002@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.