Abstract
We present the case of a 66-year-old woman who developed weakness, nausea, and vomiting accompanied by markedly elevated creatine kinase levels after first treatment with an increased dose of tirzepatide. Laboratory findings were consistent with rhabdomyolysis and normalized within 4 days following discontinuation of tirzepatide and initiation of supportive intravenous fluid therapy. The temporal relationship strongly suggests tirzepatide as a likely trigger. Off-label use, particularly for weight loss, should be avoided and approached with caution. To the best of our knowledge, this is the first reported case of rhabdomyolysis following the initial administration of tirzepatide.
1 Introduction
Rhabdomyolysis is characterized by the breakdown of skeletal muscle fibers, resulting in the release of intracellular contents such as myoglobin and creatine kinase (CK) into the bloodstream (Cabral et al., 2020). The clinical presentation of this condition varies widely, ranging from asymptomatic elevations in muscle enzymes to severe complications, including electrolyte disturbances, acute kidney injury, and metabolic derangements (Cabral et al., 2020; Melli et al., 2005). Notably, up to 50% of cases may remain clinically silent (Cervellin et al., 2010). Drug-induced rhabdomyolysis is a well-recognized phenomenon, most associated with statin therapy (Montastruc, 2023). A literature search revealed two cases of rhabdomyolysis subsequent to glucagon-like peptide-1 (GLP-1) receptor agonist treatment. One case involved semaglutide and another case involved tirzepatide (Billings et al., 2023; Sonavane et al., 2025). Interestingly, in both cases GLP-1 receptor agonist treatment was administered for the purpose of weight reduction. In the case of tirzepatide muscle injury developed several months after treatment was started. Given tirzepatide’s increasing use in both diabetes management and weight reduction, the recognition and reporting of rare adverse events are essential. Rhabdomyolysis has not been reported in clinical trials leading to the approval of tirzepatide.
2 Case presentation
A 66-year-old woman presented to the emergency department with profound weakness, nausea, and repeated vomiting, 1 day after receiving her first subcutaneous dose of tirzepatide (Mounjaro®) at a supratherapeutic dose of 15 mg (recommended starting dose: 2.5 mg). The patient reported she did not adhere to the information in the package leaflet and used a higher dose to achieve faster weight loss.
The patient’s past medical history included neuropathic pain and hyperlipoproteinemia. Her current medications included atorvastatin, gabapentin, and amitriptyline had remained unchanged for over 3 years and were paused during the inpatient stay. Physical examination revealed no abnormalities. The patient denied myalgia, and neurological assessment showed preserved muscle strength. Initial laboratory investigations, performed approximately 24 h after tirzepatide administration, were within normal limits.
However, follow-up blood tests obtained approximately 48 h after tirzepatide injection revealed significant elevations in the following laboratory parameters: creatine kinase (CK) 4249 U/L (reference <170 U/L), myoglobin 472 ng/mL (reference 25–58 ng/mL), AST 87.5 U/L (reference <35 U/L), and ALT 37.4 U/L (reference <35 U/L) (Figure 1; Table 1). Renal function and inflammatory markers remained within normal limits. Chest X-ray and abdominal ultrasound showed no pathological findings. Further laboratory investigations revealed no evidence of active viral hepatitis. Tests for leptospira, Epstein–Barr virus (EBV), and cytomegalovirus (CMV) infection were negative. No bacterial or viral pathogens were detected in the throat swab, and there was no indication of a urinary tract infection. The patient was treated with isotonic intravenous crystalloids and antiemetics. Her symptoms resolved, CK levels declined (Figure 1), and all abnormal laboratory values normalized within 3 days. The clinical course strongly implicated tirzepatide as the causative agent. During an outpatient follow-up visit 8 weeks after discharge, the patient reported no complaints and routine blood tests were within normal limits.
FIGURE 1
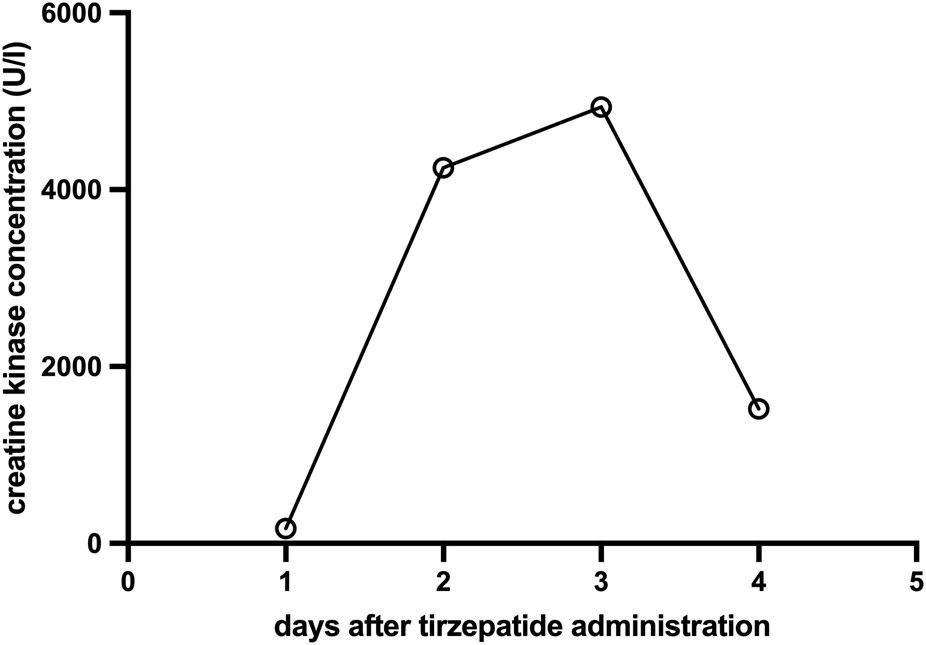
Evolution of creatine kinase concentration (U/L) over time (days) after a single subcutaneous dose of 15 mg tirzepatide on day 0 (creatine kinase reference <170 U/L).
TABLE 1
| Parameter | Reference | day1 | day2 | day3 | day4 |
|---|---|---|---|---|---|
| Hemoglobin | 7.4–9.9 mmol/L | 8.8 mmol/L | 8.6 mmol/L | 7.8 mmol/L | 8.1 mmol/L |
| Hematocrit | 0.35–0.47 | 0.43 | 0.42 | 0.36 | 0.39 |
| Platelets | 150–400 × 109/l | 194 × 109/l | 205 × 109/l | 152 × 109/l | 145 x10 9 /l (−) |
| White blood cell count | 4–9 x109/l | 12.5 x10 9 /l (+) | 15.1 x10 9 /l (+) | 9.29 x10 9 /l (+) | 10.0 x10 9 /l (+) |
| Sodium | 136–145 mmol/L | 143 mmol/L | 143 mmol/L | 141 mmol/L | 136 mmol/L |
| Potassium | 3.4–4.5 mmol/L | 3.8 mmol/L | 4.4 mmol/L | 4.0 mmol/L | 3.0 mmol/L (−) |
| Calcium | 2.20–2.35 mmol/L | 2.35 mmol/L | 2.40 mmol/L | 2.26 mmol/L | 2.15 mmol/L (−) |
| Phosphate | 0.81–1.45 mmol/L | Not measured | Not measured | Not measured | 0.57 mmol/L (−) |
| Alanine aminotransferase (ALT) | <35 U/L | 23.5 U/L | 37.4 U/L (+) | 67.6 U/L (+) | 86.7 U/L (+) |
| Aspartate aminotransferase (AST) | <35 U/L | 26.5 U/L | 87.5 U/L (+) | 150 U/L (+) | 108 U/L (+) |
| Alkaline Phosphatase (AP) | 35–104 U/L | 116 U/L (+) | 106 U/L (+) | 94.7 U/L | Not measured |
| Gamma-glutamyl Transferase | <40 U/L | 14.5 U/L | 16.9 U/L | 28.0 U/L | Not measured |
| Bilirubin total | <15 μmol/L | 6.87 μmol/L | 11.2 μmol/L | 8.69 μmol/L | Not measured |
| Lactate dehydrogenase (LDH) | 135–214 U/L | 214 U/L | 312 U/L (+) | 312 U/L (+) | Not measured |
| Creatine Kinase (CK) | <170 U/L | 167 U/L | 4249 U/L (+) | 4934 U/L (+) | 1520 U/L (+) |
| Myoglobin | 25–58 ng/mL | Not measured | Not measured | 472 ng/mL (+) | 99.2 ng/mL (+) |
| Creatinine | 45–84 μmol/L | 57.4 μmol/L | 61.6 μmol/L | 60.7 μmol/L | 50.1 μmol/L |
| C-reactive protein (CRP) | <5 mg/L | 2.56 mg/L | 2.77 mg/L | 3.07 mg/L | 2.67 mg/L |
| Procalcitonin (PCT) | <0.046 ng/mL | Not measured | Not measured | 0.038 ng/mL | Not measured |
| Ceruloplasmin | 0.2–0.6 g/L | Not measured | Not measured | Not measured | 0.243 g/L |
| Ferritin | 13–300 μg/L | Not measured | Not measured | Not measured | 207 μg/L |
| HbA1c | <6.0% | Not measured | Not measured | 5.7% | Not measured |
| Thyroid-stimulating hormone (TSH) | 0.27–4.2 µIU/mL | Not measured | Not measured | 1.63 µIU/mL | Not measured |
Overview of all laboratory parameters following tirzepatide administration on day 0 (abnormal values are indicated in bold).
3 Discussion
To our knowledge, this is the second reported case implicating tirzepatide as a causative agent for rhabdomyolysis. The temporal association between drug administration and symptom onset, along with the exclusion of other potential causes—such as trauma, current infections, electrolyte disturbances, and drug abuse—strongly supports this link. The rapid onset of the typical symptoms such as nausea and vomiting within 24 h of drug initiation, the subsequent development of rhabdomyolysis and clinical improvement following drug discontinuation support a causal relationship.
Although drug-induced rhabdomyolysis is well established—particularly in association with statins—tirzepatide itself was not described as an inducer of rhabdomyolysis (Thomsen et al., 2025). As a dual GLP-1 and GIP receptor agonist, tirzepatide modulates insulin sensitivity, lipid metabolism, and inflammatory pathways (Nauck and Müller, 2023). Its pharmacodynamic effects include delayed gastric emptying and modulation of incretin activity, which may indirectly impact systemic homeostasis and muscle metabolism (Müller et al., 2019). It is conceivable that supratherapeutic activation of GLP-1 and GIP receptors may provoke exaggerated metabolic responses and cellular stress in skeletal muscle tissue, particularly in the presence of additional risk factors such as concomitant statin use. Although this mechanism alone may not fully account for the clinical course, initiation of tirzepatide at a supratherapeutic starting dose of 15 mg subcutaneously may have been a contributing factor. While atorvastatin is known to cause muscle toxicity, the patient had tolerated it for over 3 years without prior complications. Dehydration secondary to tirzepatide induced nausea and vomiting may also have played a role in the development of rhabdomyolysis, as volume depletion can lead to reduced muscle perfusion, electrolyte disturbances, and metabolic stress—all recognized risk factors for myocyte injury (Singhal et al., 1992).
The increasing off-label use of tirzepatide for cosmetic weight loss, particularly in patients without metabolic disease, underscores the need for vigilant post-marketing surveillance. In this case, tirzepatide was prescribed off-label for weight reduction, despite the patient’s BMI being 25.3 kg/m2. According to the manufacturer’s guidelines, a BMI of ≥27 kg/m2 is required for weight management indications. While rhabdomyolysis has previously been reported after several months of tirzepatide therapy, the present case is notable for the onset of this adverse effect following a single supratherapeutic dose (Sonavane et al., 2025).
In conclusion, the underlying pathophysiological mechanism remains uncertain. While common adverse effects such as gastrointestinal symptoms are well documented (Jastreboff et al., 2022), rare and serious events like rhabdomyolysis may only emerge with broader clinical use. This case underscores the importance of informed consent, individualized risk-benefit assessment, and close monitoring, particularly when tirzepatide is used outside of approved indications.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans. Written informed consent was obtained from the patient for publication of this case report. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JB: Writing – review and editing, Investigation, Methodology, Writing – original draft, Data curation, Conceptualization. IM: Methodology, Writing – review and editing, Investigation, Data curation. ML: Supervision, Conceptualization, Writing – review and editing. CF: Conceptualization, Supervision, Project administration, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Billings S. A. Felix H. M. Prier C. C. Hedges M. S. (2023). Rhabdomyolysis associated with semaglutide therapy: a case report. Cureus15 (12), e50227. 10.7759/cureus.50227
2
Cabral B. M. I. Edding S. N. Portocarrero J. P. Lerma E. V. (2020). Rhabdomyolysis. Dis. Mon.66 (8), 101015. 10.1016/j.disamonth.2020.101015
3
Cervellin G. Comelli I. Lippi G. (2010). Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin. Chem. Lab. Med.48 (6), 749–756. 10.1515/CCLM.2010.151
4
Jastreboff A. M. Aronne L. J. Ahmad N. N. Wharton S. Connery L. Alves B. et al (2022). Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med.387 (3), 205–216. 10.1056/NEJMoa2206038
5
Melli G. Chaudhry V. Cornblath D. R. (2005). Rhabdomyolysis: an evaluation of 475 hospitalized patients. Med. Baltim.84 (6), 377–385. 10.1097/01.md.0000188565.48918.41
6
Montastruc J.-L. (2023). Rhabdomyolysis and statins: a pharmacovigilance comparative study between statins. Br. J. Clin. Pharmacol.89 (8), 2636–2638. 10.1111/bcp.15757
7
Müller T. D. Finan B. Bloom S. R. D'Alessio D. Drucker D. J. Flatt P. R. et al (2019). Glucagon-like peptide 1 (GLP-1). Mol. Metab.30, 72–130. 10.1016/j.molmet.2019.09.010
8
Nauck M. A. Müller T. D. (2023). Incretin hormones and type 2 diabetes. Diabetologia66 (10), 1780–1795. 10.1007/s00125-023-05956-x
9
Singhal P. C. Kumar A. Desroches L. Gibbons N. Mattana J. (1992). Prevalence and predictors of rhabdomyolysis in patients with hypophosphatemia. Am. J. Med.92 (5), 458–464. 10.1016/0002-9343(92)90740-3
10
Sonavane K. Shirsat P. Agrawal G. Agarwal B. (2025). Rhabdomyolysis associated with the use of tirzepatide. Eur. J. Case Rep. Intern Med.12 (5), 005392. 10.12890/2025_005392
11
Thomsen R. W. Mailhac A. Løhde J. B. Pottegård A. (2025). Real-world evidence on the utilization, clinical and comparative effectiveness, and adverse effects of newer GLP-1RA-based weight-loss therapies. Diabetes Obes. Metab.27 (Suppl. 2), 66–88. 10.1111/dom.16364
Summary
Keywords
tirzepatide, rhabdomyolysis, GLP-1 receptor agonist, adverse drug reaction, muscle injury
Citation
Bodanowitz JM, Mattes I, Loebermann M and Fritzsche C (2025) Case Report: Rhabdomyolysis following initiation of tirzepatide. Front. Pharmacol. 16:1660785. doi: 10.3389/fphar.2025.1660785
Received
06 July 2025
Accepted
05 August 2025
Published
20 August 2025
Volume
16 - 2025
Edited by
Andres F. Zuluaga, Universidad de Antioquia, Colombia
Reviewed by
César Augusto Trinta Weber, Lutheran University of Brazil, Brazil
Zae Kim, Nassau University Medical Center, United States
Updates
Copyright
© 2025 Bodanowitz, Mattes, Loebermann and Fritzsche.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonas Michael Bodanowitz, jonasmichael.bodanowitz@med.uni-rostock.de
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.