Abstract
Hepatic fibrosis is a multifactorial process driven by hepatic stellate cell (HSCs) activation, participation of Kupffer cells and infiltrating immune cells, and profibrotic cytokine signaling (notably TGF-β), culminating in excessive extracellular matrix (ECM) and collagen deposition. Post-translational modifications (PTMs)—covalent changes added after protein synthesis—govern protein stability, localization, interactions, and activity. Common PTMs include phosphorylation, acetylation, ubiquitination, glycosylation, nitration, and methylation; collectively, they modulate fibrogenic pathways across disease stages. Despite available therapies, clinically effective and well-tolerated antifibrotic options remain limited. Natural products, with their structural diversity, relative safety, and broad accessibility, offer promising leads for antifibrotic drug discovery. This review delineates the central roles of PTMs in hepatic fibrosis, synthesizes how specific PTMs drive disease initiation and progression, and evaluates natural products that target PTM-regulated nodes of fibrogenesis. We also propose strategies to accelerate development of PTM-informed antifibrotic therapeutics.
1 Introduction
Hepatic fibrosis (HF) is a progressive pathological condition that substantially contributes to the global disease burden because it can advance to cirrhosis, liver failure, and hepatocellular carcinoma—each associated with high morbidity and mortality (Huang et al., 2023). Pathologically, HF features excessive deposition of collagen and other extracellular matrix (ECM) components that, while central to normal wound healing, become dysregulated in persistent injury and inflammation (Friedman, 2008; Huang et al., 2020; Seki and Schwabe, 2015). Such fibrotic remodeling impairs organ function and underlies major complications, including cirrhosis, renal failure, and myocardial fibrosis–related heart failure (McQuitty et al., 2020). In the liver, fibrosis represents a common, dynamic response across chronic liver diseases (CLDs) and is a key determinant of progression to cirrhosis, hepatocellular carcinoma, and ultimately liver failure (Zhou et al., 2020). Its pathogenesis reflects complex crosstalk among hepatocytes, hepatic stellate cells (HSCs), sinusoidal endothelial cells, and resident and infiltrating immune cells (Kisseleva and Brenner, 2021; Tsuchida and Friedman, 2017). Following acute injury, hepatocytes typically regenerate and replace necrotic or apoptotic cells to restore tissue integrity (Michalopoulos, 2017; Michalopoulos and Bhushan, 2021); with chronic injury, however, this regenerative capacity progressively fails, and parenchyma is increasingly replaced by ECM (Baiocchini et al., 2016; Ginès et al., 2021; Tsuchida and Friedman, 2017).
During fibrogenesis, virtually all hepatic cell types—parenchymal and non-parenchymal—undergo characteristic alterations (Seo and Jeong, 2016). Injured hepatocytes undergo apoptosis, whereas liver sinusoidal endothelial cells lose their fenestrations, resulting in sinusoidal capillarization (Canbay et al., 2004). Liver injury also activates Kupffer cells, the resident macrophages, which release cytokines and chemokines (Dixon et al., 2013; van der Heide et al., 2019). These mediators drive the transition of quiescent HSCs into an activated, myofibroblast-like state marked by de novo expression of platelet-derived growth factor (PDGF) receptors, transforming growth factor-β (TGF-β) receptors, and α-smooth muscle actin (α-SMA). Activated HSCs proliferate and secrete ECM components, ultimately depositing fibrotic scar tissue (Dewidar et al., 2019; Marcher et al., 2019; Ying et al., 2017).
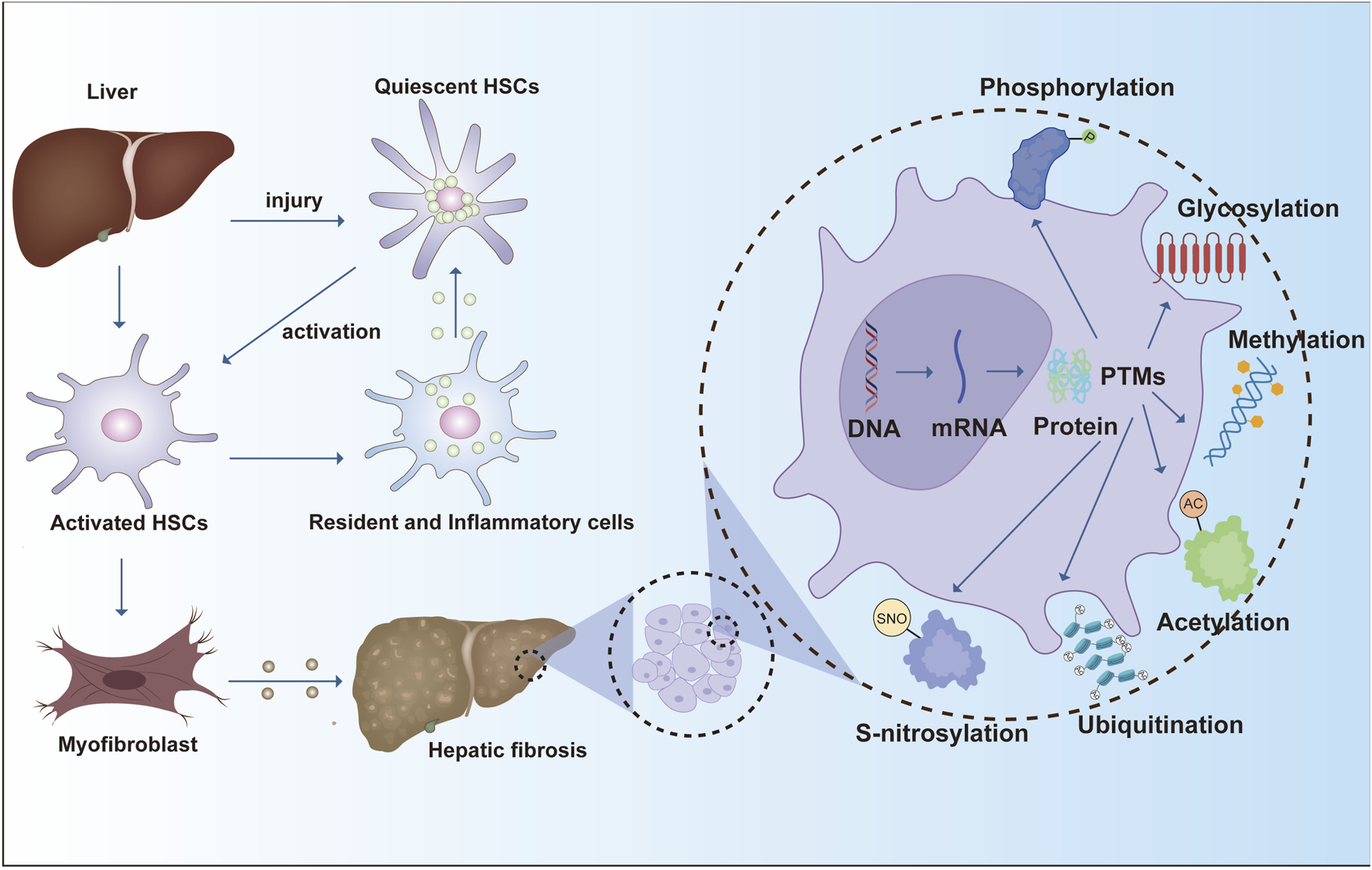
The progression of liver fibrosis is driven by diverse cellular programs and regulatory networks. Key molecular effectors in hepatic fibrogenesis include TGF-β receptors, SMAD transcription factors, and extracellular-matrix–modifying enzymes, which drive stellate-cell activation and matrix remodeling. Beyond synthesis, PTMs introduce specific chemical changes that reshape protein activity, stability, localization, and interactions, adding a crucial regulatory layer to the pathological remodeling of the liver. While gene transcription and translation establish the proteome, PTMs dynamically define protein function in context. Accordingly, the addition, removal, or rearrangement of functional groups can markedly alter protein behavior and thereby influence disease initiation and progression (Shu et al., 2023) (Figure 1).
FIGURE 1
Hepatic fibrosis progresses through hepatic stellate cell activation, leading to ECM deposition and fibrotic scarring. PTM such as phosphorylation, glycosylation, and ubiquitination regulate protein function and play critical roles in fibrosis pathogenesis.
More than 400 PTM types have been reported to date; among the most prevalent are phosphorylation, acetylation, ubiquitination, glycosylation, nitration, and methylation (Wu and Jankowski, 2022). Nevertheless, comprehensive mapping of PTM substrates and elucidation of their functional consequences—especially in liver disease—remain incomplete. This review synthesizes current advances on PTMs in hepatic fibrosis, outlines key knowledge gaps, and discusses how PTM-focused insights may inform novel therapeutic targets and drug development.
2 Literature screening and selection process
To comprehensively review the role of post-translational modifications (PTMs) in liver fibrosis and the regulatory effects of natural products on PTMs, a structured literature search was performed in accordance with the PRISMA 2020 guidelines.
2.1 Databases and time frame
Electronic databases including PubMed/MEDLINE, Web of Science Core Collection, Embase, and Scopus were searched for publications from January 2000 to June 2024. Additional references were retrieved by manually screening the bibliographies of relevant reviews and primary articles.
2.2 Search strategy
A combination of Medical Subject Headings (MeSH) and free-text terms was used to capture three major concepts:
Disease: “liver fibrosis” OR “hepatic fibrosis” OR “hepatic stellate cell*” OR “liver cirrhosis”.
Modification: “post-translational modification” OR “PTM” OR “phosphorylation” OR “acetylation” OR “ubiquitination” OR “SUMOylation” OR “methylation” OR “succinylation” OR “malonylation” OR “glycosylation”.
Natural products: natural product” OR “phytochemical” OR “herbal compound*” OR “traditional medicine” OR names of key classes (“flavonoid” OR “saponin” OR “alkaloid” OR “polyphenol”).
The three groups were combined using AND (e.g., liver fibrosis AND post-translational modification AND natural product). Searches were adapted for the syntax of each database.
2.3 Eligibility criteria
Inclusion: i. original experimental or clinical studies evaluating any PTM in the context of liver fibrosis and reporting modulation by a natural product or plant-derived compound; ii. in vitro (e.g., hepatic stellate cell activation), in vivo (animal models), or human clinical studies; iii. Articles in English with full text available.
Exclusion: reviews, editorials, conference abstracts without primary data; studies on liver cancer or metabolic liver disease lacking fibrosis/PTM endpoints; reports of genetic polymorphisms without PTM assessment.
2.4 Screening procedure
Two independent reviewers screened titles and abstracts for relevance. Full texts of potentially eligible studies were retrieved for detailed assessment. Disagreements were resolved through discussion or consultation with a third reviewer.
2.5 Data extraction and quality assessment
For each included study, we extracted: Type of natural product or compound (e.g., flavonoid, saponin, alkaloid); PTM type (phosphorylation, acetylation, ubiquitination, SUMOylation, methylation, succinylation, malonylation, etc.); Target proteins/enzymes (writers, erasers, readers) and signaling pathways.
3 Protein post-translational modifications in liver fibrosis
3.1 Phosphorylation modifications
Protein phosphorylation is a ubiquitous, deeply studied PTM that governs fundamental biological programs—including cell growth, differentiation, apoptosis, gene expression, and signal transduction (Bilbrough et al., 2022). In liver fibrosis, phosphorylation is tightly coupled to disease initiation and progression, largely through effects on ECM deposition and HSCs activation (Okuno et al., 2001). HSCs release and activate transforming growth factor-β1 (TGF-β1) and its intracellular Smad mediators, both of which are pivotal for driving collagen gene transcription (Inagaki et al., 2005; Okuno et al., 2001). TGF-β ligands also promote ECM accumulation and become sequestered within the matrix, thereby amplifying profibrotic signaling (Walton et al., 2017). Following injury, elevated TGF-β in the fibrotic niche activates SMAD2/3 via TGF-β receptors 1/2 (TGFBR1/2) (Budi et al., 2021). The phosphorylated SMAD2/3 complex then translocates to the nucleus to directly induce downstream targets (e.g., COL1A1, COL3A1, COL5A2), initiating ECM gene expression and advancing fibrogenesis (Walton et al., 2017).
Additional upstream cues converge on Smad signaling through canonical kinase cascades. For example, lipopolysaccharide (LPS) induces SMAD2 phosphorylation in HSC-T6 cells via PI3K/AKT and MAPK pathways, promoting ECM production and myofibroblastic transition (Kao et al., 2017). Renin/prorenin signaling increases prorenin receptor (PRR) expression and TGF-β1 production in LX-2 cells; PRR knockdown deactivates HSCs, reduces TGF-β1, and diminishes SMAD3 phosphorylation, thereby alleviating fibrotic responses (Hsieh et al., 2021).
Phosphorylation also regulates hepatocyte fate programs relevant to fibrosis. During hepatocyte apoptosis, caspase activity is modulated by phosphorylation—phospho-caspase-9, for instance, promotes apoptosis—typically accompanied by heightened inflammation and secondary HSC activation (Cardone et al., 1998; Cui et al., 2020; Riedl and Salvesen, 2007). Autophagy, another phosphorylation-tuned process, fuels HSC activation by mobilizing energy from retinoid-rich lipid droplets; its pharmacologic inhibition suppresses HSC activation and mitigates fibrosis in vitro and in vivo (Hernandez-Gea et al., 2012; Thoen et al., 2011). Mechanistically, Sestrin2 may restrain HSC activation by enhancing AMPK phosphorylation and dampening mTOR signaling (Hu Y. J. et al., 2024), whereas the BET inhibitor JQ-1 improves fibrosis by limiting HSC activation and proliferation through reduced JAK2/STAT3 phosphorylation (Song et al., 2023a).
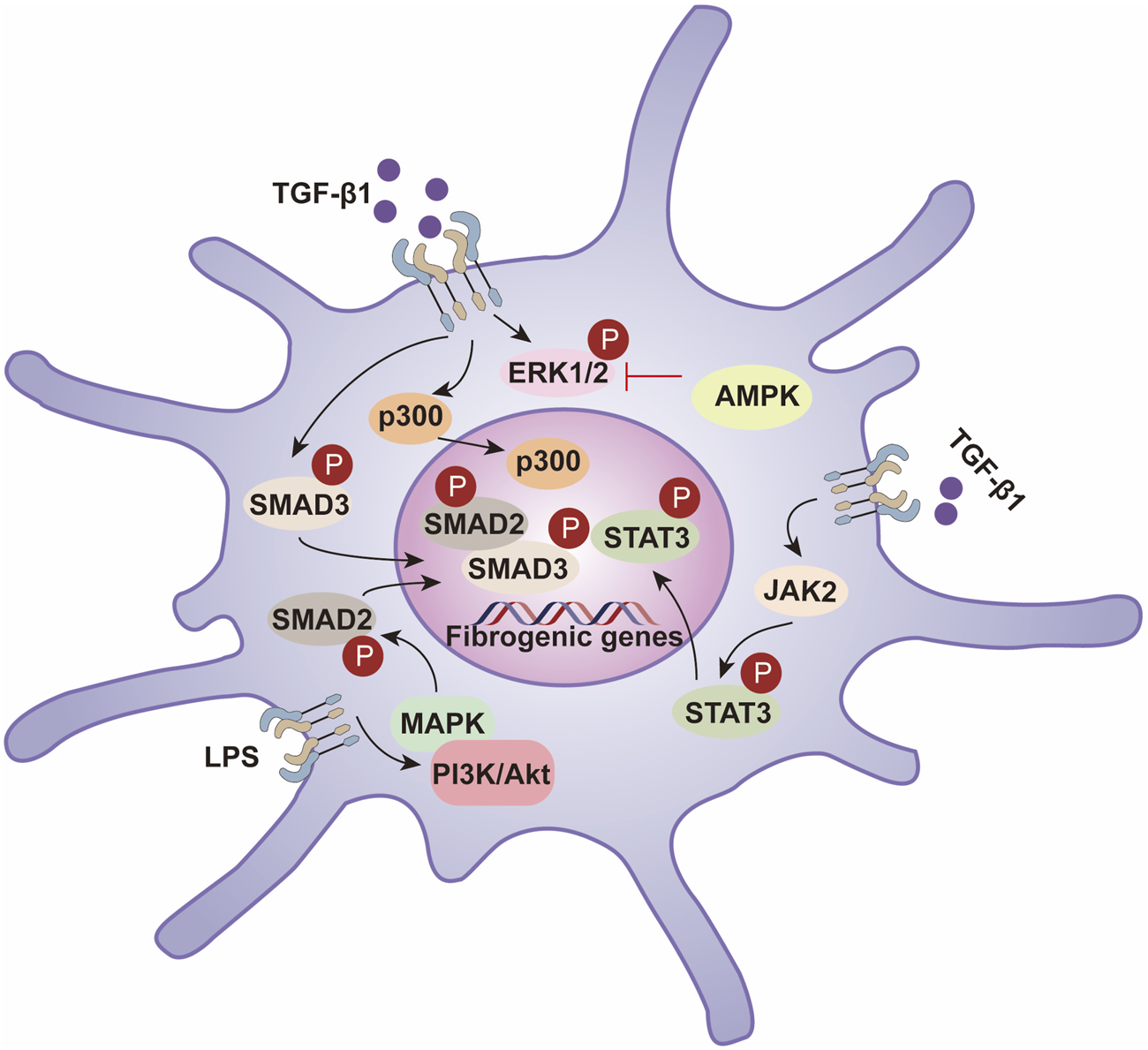
In sum, phosphorylation exerts broad, stage-spanning control over fibrogenesis by modulating key signaling axes (TGF-β/Smad, MAPK, NF-κB, PI3K/AKT) and by shaping HSC activation, proliferation, apoptosis, and autophagy (Dooley and ten Dijke, 2012; Hernandez-Gea and Friedman, 2011). Deeper delineation of these phosphorylation-dependent mechanisms—and their node-specific roles—will aid target identification and inform the rational development of antifibrotic therapies (Figure 2).
FIGURE 2
Hepatic stellate cell activation is driven by phosphorylation-dependent signaling pathways that regulate fibrogenesis. TGF-β1 activates the SMAD2/3 pathway, promoting fibrogenic gene expression through SMAD3 phosphorylation and interaction with p300. STAT3 activation via JAK2 and PI3K/Akt pathways enhances fibrosis, while LPS-induced MAPK signaling further contributes to this process. AMPK inhibits fibrosis by suppressing ERK1/2 phosphorylation, balancing profibrotic and antifibrotic mechanisms in liver fibrosis.
3.2 Protein glycosylation modification
Altered protein glycosylation is a key driver of hepatic fibrogenesis (Eichler, 2019). Glycosylation occurs co- and post-translationally as nascent proteins enter the endoplasmic reticulum, where enzymes install monosaccharides that are subsequently elaborated into oligosaccharide chains in a site-specific manner (Hebert et al., 2005). Two major classes predominate: N-glycosylation on the amide nitrogen of asparagine (Asn) and O-glycosylation on the hydroxyl groups of serine/threonine (Ser/Thr) (Kornfeld, 1998). In liver fibrosis, glycosylation shapes both matrix composition and profibrotic signaling. For example, glycan-dependent Galectin-1/NRP1 interactions potentiate TGF-β and PDGF-like pathways to activate HSCs, while the collagen O-galactosyltransferase GLT25D1 enhances collagen O-glycosylation and stabilizes fibrotic matrix architecture (Wang S. et al., 2023; Wu et al., 2017).
Glycosylation participates directly in ECM deposition by modifying collagens and thereby influencing their stability and susceptibility to degradation. It also regulates HSC activation (Caon et al., 2021; Ogawa and Okajima, 2019; Rockey et al., 2015). A particularly important cytosolic modification is O-GlcNAcylation, which decorates nuclear, cytoplasmic, and mitochondrial proteins. O-GlcNAc transferase (OGT) installs, and O-GlcNAcase (OGA) removes, this modification (Hahne et al., 2013; Ma and Hart, 2014; Ruan et al., 2012). In myofibroblast-like HSCs (MF-HSCs), global O-GlcNAcylation rises in parallel with α-SMA expression; similar increases occur in CCl4-induced liver injury in mice. Pharmacologic OGT inhibition (e.g., OSMI-1) lowers O-GlcNAcylation and downregulates collagen genes (Col1a1, Col1a2, Col3a1, Col5a2), indicating that O-GlcNAcylation is required for robust expression of fibrosis-related ECM genes and is a determinant of myofibroblast activation (Harvey and Chan, 2018; Housley et al., 2009; Wang et al., 2022).
Glycan-based biomarkers also mirror disease activity. Mac-2 binding protein glycoforms (M2BPGi)—a glycosylated variant of M2BP produced predominantly by HSCs—serve as serum indicators of liver fibrosis. M2BPGi engages Mac-2 on Kupffer cells, which in turn promotes HSC activation and elevates α-SMA expression (Bekki et al., 2017; Gantumur et al., 2021).
Glycosylation intersects with oxidative stress through advanced glycation end products (AGEs), non-enzymatic adducts formed between reducing sugars and proteins, lipids, or nucleic acids. AGE accumulation augments oxidative stress and activates pro-fibrotic, pro-inflammatory signaling via the receptor for AGEs (RAGE) (Hollenbach, 2017; Hyogo and Yamagishi, 2008). In vitro, glyceraldehyde-derived AGEs increase ROS, induce chronic injury signals, and drive HSC activation (Iwamoto et al., 2008). Consistently, RAGE expression is upregulated during HSC transdifferentiation to myofibroblasts, reinforcing profibrotic pathways (Fehrenbach et al., 2001).
Collectively, these findings position glycosylation—enzymatic glycans and non-enzymatic AGEs alike—as a central regulatory layer in hepatic fibrogenesis. Targeting specific glycosylation enzymes, lectin-mediated interactions, or AGE–RAGE signaling may yield promising antifibrotic strategies (Figure 3).
FIGURE 3
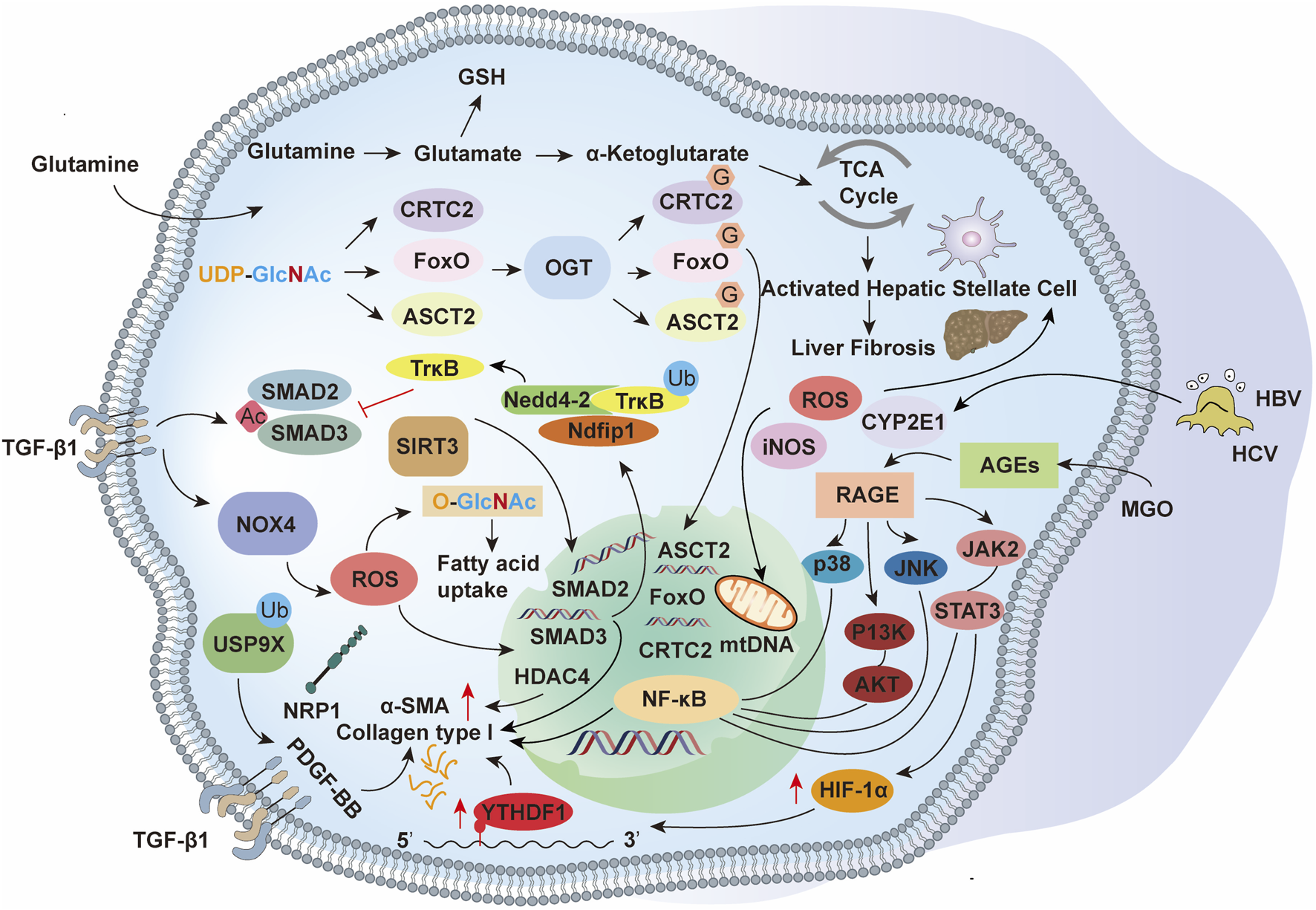
The activation of HSCs during liver fibrosis is orchestrated by PTMs, which regulate key signaling pathways and cellular processes. Acetylation modulates chromatin accessibility and transcriptional activity, ubiquitination governs protein turnover and degradation, and glycosylation influences protein folding, stability, and intercellular signaling. These modifications, in conjunction with TGF-β1-induced SMAD2/3 activation, oxidative stress, and metabolic reprogramming, drive HSC activation, extracellular matrix production, and fibrotic remodeling.
3.3 Protein acetylation modification
Protein acetylation—installed by acetyltransferases and removed by deacetylases—adds acetyl groups to specific residues, reshaping chromatin architecture, transcription, and signal transduction, and thereby influencing gene expression, protein function, cellular metabolism, and cell-cycle progression (Arnesen, 2011; Verdin and Ott, 2015; Xue et al., 2022). In hepatic fibrosis, epigenetic dysregulation driven by histone modifications is a major determinant of disease progression (Tessarz and Kouzarides, 2014; Tsukamoto et al., 2011). Activation and transdifferentiation of HSCs require broad epigenetic reprogramming that silences adipogenic programs while inducing genes supporting the myofibroblast-like phenotype (Barcena-Varela et al., 2019; Mann and Mann, 2013; Moran-Salvador and Mann, 2017).
A growing body of evidence implicates acetylation in HSC activation and fibrogenesis. Acetylation of C/EBP-α at K298, K302, and K326 enhances its interaction with Beclin-1 and promotes autophagy in activated HSCs (Hou et al., 2021). Pharmacologic inhibition of histone deacetylases with trichostatin A (TSA) alleviates CCl4-induced fibrosis, in part by increasing C/EBP-α acetylation and blocking its ubiquitin-dependent degradation (Ding et al., 2018). More broadly, hyperacetylation at promoters/enhancers of profibrotic genes associates with elevated TGF-β, collagen, and α-SMA expression. Acetylation also augments NF-κB activity, increasing TNF-α and IL-6 production that further activates HSCs; conversely, HDAC-mediated deacetylation dampens NF-κB signaling and mitigates inflammatory drive and fibrogenesis (Jimenez-Uribe et al., 2021; Joanna et al., 2009; Park et al., 2014). In LX-2 cells, nicotinamide riboside (NR) restrains TGF-β–induced activation by modulating acetylation within the Smad pathway and reduces CCl4-induced fibrosis in vivo (Jiang et al., 2019).
Acetylation also directly tunes mitochondrial function, influencing cellular energetics and oxidative stress (Anderson and Hirschey, 2012). The mitochondrial deacetylase SIRT3 deacetylates key antioxidant enzymes, attenuating oxidative stress and slowing fibrotic progression (Ning et al., 2024). Additionally, acetylation of apoptosis regulators within the Bcl-2 family can shift cell-death propensity. Some studies suggest that acetylation may increase the activity of pro-apoptotic proteins, promoting the apoptosis of HSCs and limiting the extent of fibrosis (Ma et al., 2022; Zhang et al., 2022). Together, these findings position acetylation—as both a chromatin-level and protein-level switch—as a tractable axis for antifibrotic intervention (Figure 3).
3.4 Protein ubiquitination modification
Ubiquitination is a covalent modification in which a small ubiquitin protein is conjugated to target proteins through a cascade of enzymatic reactions (Dikic and Schulman, 2023). This process plays a central role in protein degradation, signal transduction, cell cycle control, DNA repair, immune responses, and other cellular activities (Popovic et al., 2014). It requires the coordinated action of three enzymes: the E1 ubiquitin-activating enzyme, which activates ubiquitin; the E2 ubiquitin-conjugating enzyme, which transfers activated ubiquitin; and the E3 ubiquitin ligase, which attaches ubiquitin to lysine residues of substrate proteins, thereby conferring specificity (Pickart, 2001). Ubiquitination can occur as monoubiquitination (attachment of a single ubiquitin molecule) or polyubiquitination (formation of ubiquitin chains), with the type of linkage determining the fate of the substrate protein (Nakamura, 2018).
Accumulating evidence highlights the pivotal role of ubiquitination in liver fibrosis, regulating signaling pathways, protein turnover, cell activation, and programmed cell death (Filali-Mouncef et al., 2022; Rao et al., 2022; Shu B. et al., 2021; Wu et al., 2023). Ubiquitin has been identified as a biomarker of nonalcoholic liver fibrosis, frequently detected at cell boundaries and within the fibrotic matrix (Lachiondo-Ortega et al., 2019). The deubiquitinating enzyme UCHL1 is markedly upregulated in human HSCs and in livers of patients with alcoholic liver disease. Pharmacologic inhibition of UCHL1 with LDN-57444 mitigates fibrosis progression in CCl4-induced mouse models (Wilson et al., 2015). Similarly, the E3 ligase FBG1 degrades misfolded A1AT-Z mutants through the ubiquitin–proteasome system and autophagy, preventing their accumulation in the endoplasmic reticulum and attenuating fibrogenic stress (Wen et al., 2015).
Other ubiquitin-related mechanisms also contribute to fibrogenesis. Hepatocyte-specific deletion of the E3 ligase RNF5 exacerbates steatosis, inflammation, and fibrosis in dietary NASH models. Mechanistically, RNF5 binds HRD1 and promotes its K48/K33-linked ubiquitination, leading to proteasomal degradation; HRD1 knockdown reduces lipid accumulation and inflammatory signaling, underscoring the RNF5–HRD1 axis in hepatocyte injury and fibrosis (Yang et al., 2021). In HSCs, TGF-β signaling induces Ndfip1 expression, which recruits the E3 ligase Nedd4-2 to promote ubiquitination and degradation of TrkB. TrkB overexpression suppresses TGF-β/Smad signaling and limits HSC proliferation, suggesting that TrkB ubiquitination contributes to fibrosis progression (Song et al., 2023b). Moreover, Neuropilin-1 (NRP1) enhances HSC activation via TGF-β1, VEGFA, and PDGF-BB, while the deubiquitinase USP9X stabilizes NRP1. Thus, USP9X-mediated deubiquitination amplifies HSC activation, making the USP9X–NRP1 axis a promising therapeutic target (Zhao et al., 2023b).
Ubiquitination also intersects with hepatocyte injury and apoptosis. The transcription factor NRF2, a key antioxidant regulator, is degraded via ubiquitination by the CUL3–KEAP1 E3 ligase complex, a process dependent on neddylation (Zhang et al., 2004). Impaired neddylation disrupts NRF2 stability, induces mitochondrial dysfunction, and exacerbates oxidative stress, thereby promoting hepatocyte death and fibrosis (Xu et al., 2022). In addition, TRAF6 mediates K6-linked ubiquitination of apoptosis signal-regulating kinase 1 (ASK1), facilitating dissociation from thioredoxin and promoting ASK1 dimerization. This event activates the ASK1–JNK1/2–p38 cascade, stimulating pro-inflammatory and pro-fibrotic mediators that drive HSC activation and fibrogenesis (Wang et al., 2020b).
In summary, ubiquitination exerts multifaceted regulatory control over liver fibrosis by modulating HSC activation, ECM metabolism, inflammation, and apoptosis. Elucidating the precise ubiquitin-dependent mechanisms in fibrogenesis may reveal novel molecular targets and foster the development of innovative antifibrotic therapies (Figure 3).
3.5 Protein nitration modification
Protein S-nitrosylation is a covalent NO-dependent modification that regulates cell signaling by altering protein–protein interactions, subcellular localization, stability, and reactivity (Zhao et al., 2021). In parallel, protein nitration—most commonly tyrosine nitration mediated by peroxynitrite formed from NO and superoxide—affects transcriptional control, DNA-damage responses, cell growth, differentiation, and apoptosis; dysregulation of these NO-linked processes contributes to diverse diseases (Fernando et al., 2019).
Extensive evidence implicates NO-mediated modifications in hepatic fibrogenesis. Svegliati-Baroni et al. showed that the exogenous NO donor S-nitroso-N-acetylpenicillamine (SNAP) suppresses HSC activation and proliferation by scavenging ROS, thereby limiting the onset of hepatic fibrosis and cirrhosis (Svegliati-Baroni et al., 2001). Within the space of Disse, liver sinusoidal endothelial cells (LSECs) release VEGF, which supports HSC proliferation and angiogenesis; under physiological conditions, VEGF-stimulated eNOS activity in LSECs generates NO that helps revert activated HSCs toward quiescence (Deleve et al., 2008). Consistently, eNOS-derived NO is generally protective, whereas iNOS-derived NO is linked to pathological nitrosative stress and disease progression (Iwakiri and Kim, 2015).
Nitrosative stress is elevated in obesity-related NASH and in models of chronic fructose exposure, as evidenced by increased CYP2E1, iNOS, and protein nitration, changes that associate with fibrotic remodeling (Cho et al., 2021). In HepG2 cells replicating HBV or expressing HBx, mitochondrial superoxide and peroxynitrite rise, leading to mtDNA damage, nitration of respiratory-chain complexes—especially complex I—and bioenergetic impairment; superoxide scavenging (Mito-Tempo) or iNOS inhibition prevents these lesions, implicating mitochondrial nitrosative damage in inflammation and profibrotic signaling (Loureiro et al., 2023). Heat-shock protein 90 (HSP90), highly expressed in hepatocytes, is a nitration target: in aged wild-type mice, HSP90 nitration accompanies oxidative DNA damage, increased mitochondrial nitrosative stress, and alterations in complexes III/IV, culminating in age-dependent steatosis, apoptosis, and fibrosis (Abdelmegeed et al., 2016).
Given the central role of NO-driven S-nitrosylation and nitration in liver fibrosis, therapeutic strategies that rebalance these pathways are promising: enhancing eNOS-derived cytoprotective NO, limiting iNOS-driven nitrosative stress, curbing peroxynitrite formation, and/or strengthening antioxidant defenses may attenuate fibrogenic progression (Figure 3).
3.6 Methylation modification
Methylation is a major post-translational modification occurring predominantly on lysine and arginine residues of histone and non-histone proteins. By shaping chromatin architecture, gene expression, and intracellular signaling, it exerts wide-ranging control over cellular phenotypes; its dysregulation is closely linked hepatic HSCs activation and liver fibrosis (Mattei et al., 2022; Menezo et al., 2020).
Emerging evidence indicates that the methylation status of fibrotic regulators serves not only as a biomarker but also as a functional driver of disease progression (Yang et al., 2020). For example, PTEN antagonizes PI3K lipid signaling and thereby restrains PI3K/AKT and ERK pathways implicated in HSC activation (Kumar et al., 2018). In TGF-β–stimulated HSCs, the DNA methylation inhibitor 5-Aza sustains PTEN expression, attenuating HSC activation and alleviating fibrosis (Bian et al., 2013). In a CCl4 model, osteopontin (Spp1) is markedly upregulated; hypomethylation of the Spp1 promoter enhances its transcription, activates PI3K/AKT, promotes profibrogenic mediators (including TGF-β), and increases type I collagen and α-SMA, thereby accelerating HSC activation and matrix remodeling. Persistent activation of this axis has also been implicated in the transition from fibrosis to hepatocarcinogenesis (Song et al., 2019).
DNA methyltransferases (DNMTs) further integrate methylation cues into fibrogenic programs. DNMT1—the maintenance methyltransferase—is elevated in human cirrhotic livers, murine fibrosis, and primary mouse HSCs; in human HSCs, TGF-β1 recruits DNMT1 to chromatin. Genetic DNMT1 knockdown or pharmacologic disruption of the G9a/DNMT1 complex with CM272 suppresses TGF-β1–driven fibrotic responses and mitigates fibrosis (Barcena-Varela et al., 2021). DNMT3b also contributes by repressing SUN2: during CCl4-induced fibrosis, CpG hypermethylation coincides with low SUN2 expression. AAV9-mediated SUN2 overexpression reduces fibrotic markers in vivo, and SUN2 overexpression in TGF-β1–activated HSC-T6 cells dampens HSC activation (Chen et al., 2018).
Beyond DNA, RNA methylation intersects with fibrogenesis. Coordinated waves of 5-methylcytosine (5 mC, DNA) and N6-methyladenosine (m6A, RNA) modifications align with distinct phases of HSC activation (Luo H. et al., 2023). During initiation, promoter 5 mC hypermethylation at SOCS3 and PPARγ facilitates STAT3-dependent metabolic reprogramming and lipid loss. During maintenance, m6A hypermethylation of collagen transcripts enhances mRNA stability via YTHDF1, driving excessive ECM production (Feng et al., 2023).
The dynamic—and reversible—nature of methylation makes it an attractive therapeutic axis. Modulating methyltransferases/demethylases can reset profibrotic programs: DNA demethylating agents or histone demethylase inhibitors have been shown to restore more physiological methylation states at key loci (e.g., TGF-β1), reduce overexpression of profibrotic genes, and ameliorate fibrosis in animal models. Collectively, these findings position protein/DNA/RNA methylation as a convergent regulatory layer in hepatic fibrogenesis and a promising target space for antifibrotic drug development (Figure 3).
4 Natural products targeting PTMs in liver fibrosis
Natural products have long served as a valuable reservoir for drug discovery and development. Flavonoids, phenolics, terpenoids, polysaccharides, and alkaloids derived from plants exhibit diverse pharmacological activities, including anti-inflammatory, antioxidant, apoptosis-regulating, and antifibrotic effects. Importantly, many of these compounds exert their therapeutic benefits by modulating post-translational modifications (PTMs) (Table 1).
TABLE 1
| Natural products | Metabolite | Source | Mechanisms | Mode of action | Model | Dosages | References |
|---|---|---|---|---|---|---|---|
| Flavonoids | Total flavonoids from Scabiosa comosa | Scabiosa comosa Fisch. ex Roem. and Schult. [Caprifoliaceae] | Phosphorylation | ↓ p-Smad3; ↓ Smad3-TβRI interaction | In a rat model of hepatic fibrosis induced by CCl4; Primary mouse HSCs | 50,100, and 200 mg/kg for 7 days; 25, 50 and 100 µg/mL for 1 h | Ma et al. (2018) |
| Luteolin | Limnophila aromatica (Lam.) Merr. [Plantaginaceae] | Phosphorylation | ↓ AKT/mTOR/p70S6K signalling pathways; ↓ TGFβ/Smad signaling pathways | In mice model of hepatic fibrosis induced by CCl4, DMN or BDL; HSC-T6 cells treated with TGF-β1 | 150 mg/kg for 12 weeks in CCl4 model, 150 mg/kg for 2 weeks in DMN and BDL model; 10, 20 and 40 µg/mL for 24 h | Li et al. (2015) | |
| Naringin | Citrus × aurantium f. aurantium [Rutaceae] | Phosphorylation | ↓ PI3K/Akt signaling pathway | In a rat model of hepatic fibrosis induced by TAA | 40 mg/kg for 6 weeks | El-Mihi et al. (2017) | |
| Myricetin | Myrica rubra (Lour.) Siebold and Zucc. [Myricaceae] | Phosphorylation | ↓ p-Smad2, ↓ p-AKT, ↓ p-ERK and ↓ p-P38 MAPK | In a mice model of hepatic fibrosis induced by CCl4; CFSC-8B Cells treated with TGF-β1 or PDGF-BB | 50 mg/kg for 2 weeks; 12, 25 and 50 µg/mL for 2 h | Geng et al. (2017) | |
| Quercetin | Houttuynia cordata Thunb. [Saururaceae] | Phosphorylation | ↓ NF-κB and p38 MAPK signaling pathways; ↓ Bcl-2/Bax anti-apoptosis signaling pathway | In a rat model of hepatic fibrosis induced by CCl4 | 5 and 15 mg/kg for 8 weeks | Wang et al. (2017) | |
| Limonin | Citrus × aurantium f. aurantium [Rutaceae] | Phosphorylation | ↑ p-Smad7; ↓ p-Smad2/3 | In a rat model of hepatic fibrosis induced by CCl4; AML-12 cell and LX-2 HSCs cell treated with TGF-β | 5 and 15 μM for 24 h; 10 and 20 mg/kg for 4 weeks | Shu et al. (2022) | |
| Isoliquiritigenin | Glycyrrhiza uralensis Fisch. ex DC. [Fabaceae] | Phosphorylation | ↓ p-STAT3; ↓ ANXA2 and SPHKs/S1P/IL-17 signals pathway | In a mice model of alcoholic liver fibrosis induced by alcohol feeding combined with 5% CCl4; HSC-T6 cells treated with alcohol | 10 and 20 mg/kg for 10 days; 4, 8 and 16 μmol/L for 24 h | Liu et al. (2023) | |
| (−)-Catechin-7-O-β-d-apiofuranoside | Ulmus davidiana var. japonica (Rehder) Nakai [Ulmaceae] | Phosphorylation | ↓ p-STAT3 | In a rat model of hepatic fibrosis induced by TAA LX-2 cells treated with TGF-β1 | 40 mg/kg for 3 weeks; 2.5, 5, 10 and 20 μg/mL for 48 h | Park et al. (2019) | |
| Luteolin-7-diglucuronide | Perilla frutescens (L.) Britton [Lamiaceae] | phosphorylation | ↑ p-AMPK | In a mice model of hepatic fibrosis induced by CCl4 alone or in combination with HFHC diet; Primary HSCs cells and LX-2 cells treated with TGF-β1 | 40 and 150 mg/kg for 4 or 8 weeks; 5, 20, 50 µM for 24 h | Tang et al. (2024) | |
| Ampelopsin | Nekemias grossedentata (Hand.-Mazz.) J.Wen and Z.L.Nie [Vitaceae] | Phosphorylation | ↓ SIRT1/TGF-β1/Smad3 signaling pathway; ↓ AKT/mTOR signaling pathway | In a mice model of hepatic fibrosis induced by CCl4; Primary HSCs cells treated with TGF-β1 | 125 and 250 mg/kg for 10 weeks; 25, 50 and 100 μM for 24 h | Ma et al. (2019) | |
| Physalin B | Physalis L.[Solanaceae] | Acetylation | ↓ GLI1 deacetylation | In a mice model of hepatic fibrosis induced by CCl4 and BDL; Primary HSCs cells and LX-2 cells treated with TGF-β1 | 1, 2.5 and 5 mg/kg for 4 weeks; 0.25, 0.5 and 1 μM for 24 h | Zhu X. et al. (2021) | |
| Phenolic Compounds | Capsaicin | Capsicum cardenasii Heiser and P.G.Sm. and Capsicum L. [Solanaceae] | Phosphorylation | ↑ PPAR-γ; ↓ TGF-β1/Smad Pathway | In a rat model of hepatic fibrosis induced by DMN; HSC-T6 cells treated with TGF-β1 | 0.5 and 1.0 mg/kg for 4 weeks; 0.1, 1 and 10 μM for 24 h | Choi et al. (2017) |
| Ferulic acid | Angelica sinensis (Oliv.) Diels [Apiaceae] | Phosphorylation | ↓ p-Smad2; ↓ p-Smad3 | In a rat model of hepatic fibrosis induced by CCl4; LX-2 cells treated with TGF-β1 | 10 mg/kg/day for 8 weeks; 50 μM for 24 h | Mu et al. (2018) | |
| Honokiol | Magnolia officinalis Rehder and E.H.Wilson [Magnoliaceae] | Phosphorylation | ↑ E-cadherin/GSK3β/JNK signaling pathway; ↓ AKT/ERK/p38/β-catenin/TMPRSS4 signaling pathway | In a mice model of hepatic fibrosis induced by CCl4; AML-2 cells treated with TGF-β1 | 1 mg/kg for 6 weeks; 12, 24, 36, and 48 μM for 24 h | Seo et al. (2023) | |
| Epigallocatechin (ECG), Epicatechin-3-O-gallate (EGC) and Epigallocatechin-3-O-gallate (EGCG) | Camellia sinensis (L.) Kuntze [Theaceae] | Phosphorylation | ↓ p-ERK; ↓ p-Smad1/2 | In a rat model of hepatic fibrosis induced by CCl4 | ECG (100 and 300 mg/kg), EGC (100 and 300 mg/kg),EGCG (300 mg/kg) | Wang et al. (2019) | |
| Astragalus and Salvia miltiorrhiza extract | Astragalus L. [Fabaceae] and Salvia miltiorrhiza Bunge [Lamiaceae] | Phosphorylation | ↓ p-ERK, p-JNK p-P38; ↓ p-Smad2C/L, p-Smad3L, Smad4, Imp7/8 | In a rat model of hepatic fibrosis induced by DEN; Primary HSCs cells treated with TGF-β1 and HepG2 cells | 60,120 and 240 mg/kg for 12 or 16 weeks; 20, 40 and 80 mg/mL | Boye et al. (2015) | |
| Danshensu | Salvia miltiorrhiza Bunge [Lamiaceae] | Phosphorylation | ↓ p-STAT3; ↓ JAK2-STAT3 signaling pathway | In a rat model of hepatic fibrosis induced by CCl4; HSC-T6 cells treated with TGF-β1 | 10, 30 and 60 mg/kg for 6 weeks; 1, 2, and 3 μM for 12, 24 and 48 h | Cao et al. (2019) | |
| Salvianolic acid B | Salvia miltiorrhiza Bunge [Lamiaceae] | Phosphorylation | ↓ p-Smad2/3L, ↓ p-Smad2C, ↑ p-Smad3C; ↓ MAPK | In a mice model of hepatic fibrosis induced by DEN; HSC-T6 cells and LX-2 cells treated with TGF-β1 | 15 and 30 mg/kg for 12 weeks; 20, 50 and 100 μM for 24 h | Wu et al. (2019) | |
| Methylation | ↓ DNMT1, ↑ PTCH1 | In a mice model of hepatic fibrosis induced by CCl4; Primary HSCs cells treated with TGF-β1 | 100 mg/kg for 8 weeks; 100 μM for 48 h | Yu et al. (2015) | |||
| Oroxylin A | Scutellaria baicalensis Georgi [Lamiaceae] | Methylation | ↑ cGAS and STING, ↓ cGAS gene methylation; ↓ DNMT3A | In a mice model of hepatic fibrosis induced by CCl4; HSC-T6 cells and LX-2 cells treated with TGF-β1 | 40 mg/kg for 8 weeks; 20, 30 and 40 μM for 24 h | Zhao et al. (2023) | |
| Terpenoids | Total astragalus saponins (AST); Glycyrrhizic acid (GA) | Astragalus mongholicus Bunge [Fabaceae]; Glycyrrhiza uralensis Fisch. ex DC. [Fabaceae] | Phosphorylation | ↓ p-Smad2/3 and TGF-β1 pathway | In a rat model of hepatic fibrosis induced by DEN and Bile duct ligation; JS-1 cells and AML-12 cells | AST (164 mg/kg)+GA (48 mg/kg), AST (164 mg/kg), GA (48 mg/kg) for 2 or 3 weeks; AST (10、20 and 40 μg/mL), GA (25、50 and 100 μM), AST: 20 μg/mL + GA: 50 μM | Zhou et al. (2016) |
| Corosolic acid | Crataegus pinnatifida var. pinnatifida [Rosaceae] | Phosphorylation | ↓ TGF-β1/Smad2, NF-κB, and AMPK signaling pathways | In a mice model of hepatic fibrosis induced by HFD diet and CCl4; LX-2 cells treated TGF-β1 and HepG2 cells | 10, 20 and 30 mg/kg for 9 weeks; 5, 10 and 20 μM for 24 h | Liu et al. (2021) | |
| Cryptotanshinone | Salvia miltiorrhiza Bunge [Lamiaceae] | Phosphorylation | ↓ p-STAT3; ↓ CTP1A fatty acid metabolism | In a mice model of hepatic fibrosis induced by CCl4; Primary HSCs cells and LX-2 cells treated TGF-β | 40 mg/kg for 5 weeks; 1, 5 and 10 μM for 48 h | Li et al. (2024) | |
| Asiatic acid | Centella asiatica (L.) Urb. [Apiaceae] | Phosphorylation | ↓ NF-κB/IκBα and JAK1/STAT3 signaling pathway | In a rat model of hepatic fibrosis induced by CCl4 | 5 and 15 mg/kg for 6 weeks | Fan et al. (2018) | |
| Demethylzeylasteral | Tripterygium wilfordii Hook.f. [Celastraceae] | Phosphorylation | ↓ AGAP2, ↓ p-FAK, ↓ p-AKT | In a rat model of hepatic fibrosis induced by CCl4; Primary HSCs, LX-2 and HSC-T6 cells treated with TGF-β1 | 20 mg/kg for 4 weeks; 0.5, 1 and 2 μM for 24 h | Chen et al. (2022) | |
| Triptolide | Tripterygium wilfordii Hook.f. [Celastraceae] | Phosphorylation | ↑ p-AMPK, ↑ p-ACC1 lipid metabolism | In a mice were fed a methionine/choline-supplied (MCS) or MCD diet | 50 μg/kg and 100 μg/kg for 10 weeks | Huang et al. (2021) | |
| Celastrol | Tripterygium wilfordii Hook.f. [Celastraceae] | Phosphorylation | ↑ p-AMPK, ↑ p-SIRT3 | In a rat model of hepatic fibrosis induced by CCl4; Primary HSCs cells treated with PDGR-BB | 0.25, 0.5 and 1 mg/kg for 8 weeks; 10, 20 and 40 μM for 24 h | Wang et al. (2020a) | |
| Saponin extract of P. japonicus rhizomes | Panax japonicus (T.Nees) C.A.Mey. [Araliaceae] | Phosphorylation | ↑ p-Akt, ↑ p-GSK3β; ↑ Akt/GSK3β/Nrf2 cascade | In a mice model of hepatic fibrosis induced by CCl4; AML-12 cells treated with TGF-β | 50 and 100 mg/kg for 4 weeks; 30 and 100 μg/mL for 24 h | Dai et al. (2021) | |
| Carnosol | Salvia rosmarinus Spenn. [Lamiaceae] | Acetylation | ↑ SIRT1; ↓ EZH2 acetylation | In a rat model of hepatic fibrosis induced by CCl4; Primary HSCs cells and LX-2 cells treated with TGF-β1 | 25 and 30 mg/kg for 4 weeks; 10 μM for 24 h | Zhao et al. (2018) | |
| Sclareol | Salvia sclarea L. [Lamiaceae] | Ubiquitylation | ↓ SENP1, ↑ VEGFR2 SUMOylation, ↓ VEGFR2–STAT3 interaction, ↓ p-STAT3 | In a rat model of hepatic fibrosis induced by CCl4 and Bile duct ligation; LX-2 cells treated TGF-β1 | 300 mg/kg for 4 weeks; 10 and 20 μM for 24 h | Ge et al. (2023) | |
| Ginsenoside Rg1 | Panax ginseng C.A.Mey. [Araliaceae] | Methylation | ↓ DNMT1-mediated Smad7 methylation | In a mice model of hepatic fibrosis induced by CCl4; Primary HSCs treated TGF-β | 40 mg/kg for 8 weeks, 50 μM for 24 h | Zhang et al. (2023) | |
| Ginsenoside Rg3 | Panax ginseng C.A.Mey. [Araliaceae] | Methylation | ↑ ACSL4, ↓ DNMT3B-mediated ACSL4 methylation | In a mice model of hepatic fibrosis induced by CCl4; Primary HSCs cells treated with TGF-β1 | 10 and 20 mg/kg for 8 weeks; 20 and 40 μM for 24 h | Hu Y. et al. (2024) | |
| Alkaloids | Matrine (MT) Thio derivative of MT (MD-1) | Sophora flavescens Aiton [Fabaceae] | Phosphorylation | ↓ p-EGFR and p-AKT | In a rat model of hepatic fibrosis induced by DMN; HSC-T6 cells treated with TGF-β1 | MT and MD-1 (62 μmol/L/kg) for 4 weeks; MD-1 (62 µmol/L), MT (128 µmol/L) for 24 h | Feng et al. (2016) |
| Berberine | Coptis chinensis Franch. [Ranunculaceae]is | Phosphorylation | ↑ p-AMPK, ↓ p-Akt, ↓ Nox4, ↓ TGF-β1 | In a mice model of hepatic fibrosis induced by CCl4; CFSC-2G cells | 25 and 50 mg/kg for 4 weeks; 12.5 μM for 24 h | Li et al. (2014) | |
| Neferine | Nelumbo nucifera Gaertn. [Nelumbonaceae] | Phosphorylation | ↑ p-AMPK and p-ACC; ↑ p-Smad2/3, TGF-β | In a mice model of hepatic fibrosis induced by HFD diet and CCl4; LX-2 cells treated with TGF-β and HepG2 cells | 5 and 10 mg/kg for 4 weeks; 12.5 and 25 μM for 24 h | Wang M. Y. et al. (2023) | |
| Piperine | Piper nigrum L. [Piperaceae] | Phosphorylation | ↑ Nrf2, ↓ p-Smad2/3, ↑ Smad7 | In a mice model of hepatic fibrosis induced by CCl4; AML-12 cells and LX-2 cells treated with TGF-β | 20 and 40 mg/kg for 4 weeks; 20 and 40 μM for 48 h | Shu G. et al. (2021) | |
| Other Natural Drugs | Ganoderma lucidum polysaccharide | Rhinacanthus nasutus (L.) Kurz [Acanthaceae] | Phosphorylation | ↓ TGF-β, p-Smad2 and p-Smad3 | In a mice model of hepatic fibrosis induced by CCl4; HSC-T6 cells treated with TGF-β1 | 150 and 300 mg/kg for 6 weeks; 1.25, 2.5 and 5 mg/mL for 24 h | Chen C. et al. (2023) |
| S. glauca extract (SGE) | Suaeda glauca (Bunge) Bunge [Amaranthaceae] | Phosphorylation | ↓ p-Smad2/3 and Smad2/3 nuclear translocation | In a mice model of hepatic fibrosis induced by CCl4; Primary HSCs cells and LX-2 cells treated TGF-β | 30 and 100 mg/kg for 6 weeks; 100 and 300 μg/mL for 30 min, 72 h or 5 days | Hong et al. (2023) | |
| Amygdalin | Prunus armeniaca L. [Rosaceae]] | Phosphorylation | ↓ p-Smad2 and p-Smad3; ↓ p-p65 (NF-κB) | In a rat model of hepatic fibrosis induced by CCl4; LX-2 cells treated with TGF-β1 | 3 mg/kg for 8 weeks; 1.25, 2.5 and 5 mg/mL for 48 h | Wang et al. (2021) | |
| Cordycepin | Cordyceps militaris (L.ex Fr.) Link.[clavicipitaceae] | Phosphorylation | ↑ p-AMPKα and p-ACC | In a mice model of hepatic fibrosis induced by HFHC-fed; L02 cells | 100 and 200 mg/kg for 16 weeks; 50 μM for 12 h | Lan et al. (2021) | |
| Plumbagin | Plumbago zeylanica L. [Plumbaginaceae] | Phosphorylation | ↓ p-IκB and NF-κB p65 nuclear translocation | In a rat model of hepatic fibrosis induced by CCl4; Primary HSCs cells | 2, 4 and 8 mg/kg for 4 weeks; 2, 4 and 8 μM for 24 h | Chen et al. (2019) | |
| Inchin-ko-to | Artemisia capillaris Thunb. [Asteraceae]; Gardenia jasminoides J.Ellis [Rubiaceae] and Rheum rhabarbarum L. [Polygonaceae] | Phosphorylation | ↓ phosphorylation of PDGFRβ, c-Raf, MEK1/2, ERK1/2 and Akt | In a rat model of hepatic fibrosis induced by TAA; Primary HSCs cells treated with PDGF-BB | 1 mg/g for 6 weeks; 10, 50 and 100 μg/mL for 24 h or 48 h | Imanishi et al. (2004) | |
| Emodin | Rheum officinale Baill. [Polygonaceae] | Methylation | ↓ p-ERK, ↓ p-Nur77, ↓ Nur77/DNMT3b interaction, ↓ GLS1 promoter methylation | In a mice model of hepatic fibrosis induced by CCl4; HSC-T6 cells and LX-2 cells treated with TGF-β1 | 30 mg/kg for 4 weeks; 10, 20 and 40 μM, or a single concentration of 20 μM for 24 h | Chen L. et al. (2023) | |
| Sennoside A | Rheum officinale Baill. [Polygonaceae] | Methylation | ↑ SOCS1, ↓ DNMT1 | In a rat model of hepatic fibrosis induced by CCl4; HSC-T6 cells treated with TGF-β1 | 30 mg/kg for 8 weeks; 20 nM for 24 h | Zhu H. et al. (2021) |
Post-translational modifications of key compounds in liver fibrosis treatment.
4.1 Flavonoids
Flavonoids are widely distributed plant-derived compounds with multiple pharmacological properties, such as anti-inflammatory, antioxidant, anti-apoptotic, and lipid-regulating effects (Li et al., 2016; Pourcel et al., 2007; Serafini et al., 2010). They have been shown to ameliorate liver diseases, including acute liver injury and fatty liver (Blankson et al., 2000; Luo S. et al., 2023).
Recent studies demonstrate that flavonoids exert antifibrotic effects primarily through the regulation of PTMs, especially phosphorylation. For example, total flavonoids from Scabiosa comosa (TF-SC) selectively inhibit TGF-β1–induced Smad3 phosphorylation by blocking the TβRI–Smad3 interaction, thereby attenuating CCl4-induced liver fibrosis and identifying TF-SC as a potential TGF-β1/Smad3 pathway inhibitor (Ma et al., 2018). Similarly, luteolin promotes HSC apoptosis by activating caspase-3 and upregulating p53, while downregulating bcl-2, cyclin E, and p-Cdk2. In vivo, luteolin alleviates fibrotic injury by suppressing PDGF- and TGF-β1-mediated AKT and Smad phosphorylation (Li et al., 2015). Naringin inhibits PI3K/AKT signaling, decreases fibronectin and TGF-β1 expression, and induces caspase-3–dependent apoptosis to mitigate fibrosis (El-Mihi et al., 2017). Myricetin suppresses TGF-β1–induced phosphorylation of Smad2, p38 MAPK, ERK, and AKT, and dose-dependently inhibits PDGF-BB–induced ERK/AKT activation. In CCl4 models, myricetin reduces α-SMA expression and collagen deposition, indicating multitarget regulation of PTMs (Geng et al., 2017). Quercetin attenuates HSC activation by downregulating p38 MAPK phosphorylation and modulating the NF-κB/IκBα axis (Wang et al., 2017).
Other flavonoids demonstrate similar PTM-mediated mechanisms. Limonin blocks TGF-β–induced Smad2/3 phosphorylation and nuclear translocation, while enhancing Smad7 expression to suppress epithelial–mesenchymal transition (EMT) and HSC activation (Shu et al., 2022). Isoliquiritigenin inhibits STAT3 phosphorylation by targeting ANXA2 and the SPHK/S1P/IL-17 pathway, reversing HSC activation (Liu et al., 2023). Catechin-7-O-β-d-apiofuranoside (C7A) represses TGF-β1–induced STAT3 phosphorylation and downstream ECM gene expression, reducing fibrosis (Park et al., 2019). In addition, luteolin-7-diglucuronide (L7DG) has been identified as an inhibitor of protein tyrosine phosphatase 1B (PTP1B), which promotes AMPK phosphorylation and suppresses cell activation in TGF-β1–stimulated HSCs (Tang et al., 2024).
Beyond phosphorylation, other PTMs also play important roles in the anti-fibrotic mechanisms of flavonoids. Ampelopsin (Amp) has been shown to exert regulatory effects largely through deacetylation. Specifically, Amp downregulates collagen I, α-SMA, TIMP1, TGF-β1, and p-Smad3 expression, while promoting the upregulation of MMP9 and SIRT1, thereby inhibiting the sustained activation of HSCs. Importantly, SIRT1 activation is central to this process, as its inhibition reverses the protective effects of Amp. Moreover, Amp induces autophagy by upregulating LC3-II and Beclin-1, whereas the autophagy inhibitor 3-MA partially abrogates its anti-fibrotic effects (Ma et al., 2019). Physalin B enhances the acetylation of the transcription factor GLI1, preventing its interaction with the LAP2α/HDAC1 complex, leading to its inactivation, downregulation of α-SMA and COL1A1 expression, and ultimately exerting anti-fibrotic activity (Zhu X. et al., 2021).
In summary, flavonoids and related bioactive compounds exert anti-fibrotic effects not only through classical signaling pathways such as TGF-β/Smad, PI3K/AKT, MAPK, STAT3, and NF-κB, but more importantly through the regulation of diverse PTMs. Phosphorylation is central to blocking the Smad, AKT, and MAPK axes; deacetylation and acetylation regulate HSC fate via SIRT1 activation and GLI1 inactivation, respectively; and autophagy induction contributes to the suppression of fibrosis progression. These findings highlight PTMs as critical therapeutic targets of natural compounds, underscoring their multi-target and multi-pathway advantages and providing a solid theoretical foundation for the development of novel PTM-oriented anti-fibrotic drugs.
4.2 Phenolic compounds
Phenolic compounds are naturally occurring substances abundant in fruits, vegetables, grains, legumes, chocolate, and beverages such as tea and wine (Spencer et al., 2008). They display diverse pharmacological properties, including anti-inflammatory, antioxidant, anti-proliferative, lipid-regulating, and anti-aging activities (Toma et al., 2020; Urso and Clarkson, 2003; Van Hung, 2016). Clinically, phenolics have been applied in the management of hypertension, metabolic disorders, infections, and neurodegenerative diseases (Lin et al., 2016; Rahman et al., 2021). Increasing evidence supports their therapeutic role in HF through the regulation of PTMs and epigenetic processes.
Capsaicin (CPS) has been shown to upregulate Smad7 expression through the activation of PPAR-γ, thereby suppressing DMN-induced TGF-β1 production. In HSCs, CPS effectively reduced TGF-β1–mediated α-SMA and collagen I expression via this pathway, suggesting that it ameliorates fibrosis by negatively regulating the TGF-β1/Smad signaling axis through the PPAR-γ/Smad7 pathway (Choi et al., 2017). Similarly, ferulic acid (FA) markedly inhibits the phosphorylation of Smad2/3 and the downstream signal transduction of Smad4, thereby attenuating TGF-β1–induced HSC activation and contributing to the reversal of fibrosis progression (Mu et al., 2018). Honokiol exhibits broader multi-pathway regulatory effects. In HSCs, honokiol reduces the expression of α-SMA, TGF-β1, p-Smad3, p-AKT, Cyclin D1, c-Myc, and Wnt3a/β-catenin, while inhibiting the phosphorylation of GSK3β, which leads to GSK3β activation, blockade of Wnt3a/β-catenin signaling, and apoptosis induction (Lee et al., 2021). Further studies revealed that honokiol also suppresses non-canonical TGF-β1 pathways (AKT, ERK, and p38) while promoting GSK3β/JNK phosphorylation, which enhances E-cadherin expression and inhibits EMT progression. In vivo, honokiol has been shown to attenuate CCl4-induced hepatic fibrosis and necrosis (Seo et al., 2023).
Tea polyphenols also confer hepatoprotection. Green tea catechins [epigallocatechin (EGC), epicatechin-3-O-gallate (ECG), and epigallocatechin-3-O-gallate (EGCG)] significantly reduce desmin, α-SMA, and TGF-β expression, while inhibiting the phosphorylation of ERK1/2 and Smad1/2, thereby ameliorating fibrosis (Wang et al., 2019). Sugarcane polyphenol extract (SPE) has also been shown to inhibit the phosphorylation of p38 and JNK1/2 and downregulate α-SMA expression in TGF-β1–induced HSCs (Wang L. et al., 2018). In addition, the compound extract of Astragalus and Salvia miltiorrhiza (CASE) suppresses linker-region phosphorylation of Smad2/3 and the nuclear import of Smad4 and Imp7/8, thereby reducing the transcriptional activity of PAI-1, a key target gene of TGF-β signaling. This effect is accompanied by inhibition of the MAPK pathway (pERK, pJNK), ultimately suppressing the fibrotic response of HSCs (Boye et al., 2015).
Among active compounds from S. miltiorrhiza, Danshensu (DSS) is identified as an inhibitor of indoleamine 2,3-dioxygenase 1 (IDO1). DSS downregulates JAK2/STAT3 signaling by reducing JAK2/STAT3 phosphorylation and STAT3 nuclear localization, thereby inhibiting ECM deposition and liver injury. Overexpression of IDO1 reverses these effects, confirming its role in fibrosis (Cao et al., 2019). Salvianolic acid B (Sal B) suppresses fibrosis via the MAPK/Smad axis and Hedgehog pathway, significantly reducing p-ERK1/2, p-JNK1/2, p-p38, p-Smad2/3, and PAI-1 levels (Wu et al., 2019). In addition, Sal B attenuates HSC activation by regulating miR-152/DNMT1-mediated DNA methylation, thereby suppressing the hypermethylation of PTCH1 and restoring its expression (Yu et al., 2015). Oroxylin A further demonstrates epigenetic action by inhibiting DNMT3A-mediated methylation of the cGAS promoter. This activates the cGAS–STING pathway and induces cellular senescence, while DNMT3A overexpression reverses these effects (Zhao et al., 2023).
In summary, phenolic compounds—including alkaloids, phenolic acids, polyphenols, and herbal extracts—exert potent antifibrotic effects by targeting both canonical and noncanonical pathways (TGF-β/Smad, MAPK, PI3K/AKT, Wnt/β-catenin, and JAK/STAT), as well as by modulating DNA methylation and other epigenetic processes. Their ability to act at multiple levels of regulation underscores PTMs and epigenetic remodeling as promising therapeutic intervention points, providing a strong foundation for the development of novel antifibrotic strategies.
4.3 Terpenoids
Terpenoids are a large and structurally diverse class of bioactive natural compounds found in plants, animals, marine organisms, and microorganisms. They exhibit a wide spectrum of pharmacological properties, including antitumor cytotoxicity, neuroprotection, anti-inflammatory activity, regulation of lipid metabolism, as well as hepatoprotective and hypoglycemic effects (Cassiano et al., 2014; El Omari et al., 2021; Hua et al., 2022; Yao and Liu, 2022).
Co-administration of total astragalosides (AST) and glycyrrhizic acid (GA) markedly inhibits HSC activation, lowers α-SMA and COL1A1 expression, and suppresses transcription and phosphorylation of TGF-β1 and Smad2/3, thereby reversing dimethylnitrosamine (DMN)– or bile duct ligation (BDL)–induced hepatic fibrosis (Zhou et al., 2016). Similarly, corosolic acid (CA) mitigates fibrosis by blocking TGF-β1/Smad2 phosphorylation and concomitantly regulating the AMPK and NF-κB pathways, which decreases ECM deposition and inflammation (Liu et al., 2021). Ginsenoside Rg1 restores Smad7 expression through promoter demethylation, blocking TGF-β/Smad signaling and suppressing epithelial–mesenchymal transition (EMT); consistent with this mechanism, the DNMT inhibitor 5-Aza also enhances Smad7 demethylation and expression (Zhang et al., 2023).
Several terpenoids act through STAT3 modulation. Cryptotanshinone (CTS), a derivative of tanshinone IIA, inhibits STAT3 nuclear translocation, reduces CPT1A-dependent fatty acid oxidation, and promotes an adipocyte-like phenotype in HSCs, collectively producing antifibrotic effects. In CCl4-induced fibrosis, p-STAT3 co-localizes with the HSC activation marker α-SMA; CTS reduces p-STAT3 and favors HSC adipogenic reprogramming, thereby attenuating fibrosis (Li et al., 2024). Similarly, Asiatic acid (AA) further suppresses JAK1/STAT3 phosphorylation, preventing persistent pathway activation and ameliorating fibrosis (Fan et al., 2018). Sclareol (SCL) suppresses SENP1 expression, augments VEGFR2 SUMOylation, and disrupts the VEGFR2–STAT3 interaction, thereby inhibiting downstream STAT3 phosphorylation and providing new evidence that SUMOylation contributes to antifibrotic effects (Ge et al., 2023).
Other terpenoids target cytoskeletal and metabolic signaling. Demethylzeylasteral (T-96), from Tripterygium wilfordii, selectively inhibits FAK and AKT phosphorylation and disrupts the AGAP2–FAK interaction, suppressing HSC proliferation and migration (Chen et al., 2022). Triptolide acts as an AMPK agonist, increases AMPK Thr172 phosphorylation and phosphorylation of its downstream substrate ACC1, thereby attenuating fibrosis (Huang et al., 2021). Similarly, celastrol mitigates hepatic fibrosis by activating the AMPK–SIRT3 axis, improving mitochondrial homeostasis and anti-inflammatory defenses (Wang et al., 2020a). Likewise, the ginsenoside metabolite 20(S)-protopanaxadiol (20S-PPD) activates LKB1-dependent AMPK Thr172 phosphorylation, downregulates the mTOR/S6K pathway, and promotes HSC apptosis (Park et al., 2017).
Other mechanisms have also been reported. The saponin extract of Panax japonicus rhizomes (SEPJ) augments phosphorylation of AKT and GSK-3β to activate NRF2 signaling, upregulates NRF2 and its downstream antioxidant genes, thereby inhibiting EMT and HSC activation, and demonstrates antifibrotic efficacy in vitro and in vivo (Dai et al., 2021). Carnosol (CS) activates SIRT1 to reduce EZH2 acetylation and stability, limiting myofibroblast differentiation and ECM accumulation (Zhao et al., 2018). Ginsenoside Rg3 augments ferroptosis through ACSL4 demethylation to restrain HSC activation; this effect is mediated via the miR-6945-3p/DNMT3B axis, unveiling a ferroptosis-based mechanism underlying Rg3’s antifibrotic activity (Hu Y. et al., 2024).
4.4 Alkaloids
Alkaloids are nitrogen-containing basic compounds with broad pharmacological activity. More than 18,000 alkaloids have been identified across >300 plant families as well as from microorganisms, marine invertebrates, insects, and other sources (Elissawy et al., 2021; Nair and van Staden, 2019). Research has demonstrated that various alkaloids possess pharmacological effects such as antihypertensive, anti-inflammatory, anticancer, and anti-fibrotic properties (Bhambhani et al., 2021; Feng et al., 2016; Halim et al., 2019).
Mechanistically, representative alkaloids modulate key PTM-linked signaling nodes to restrain HSC activation and ECM accumulation. The matrine derivative MD-1 engages EGFR on HSC-T6 cells to suppress EGFR/AKT phosphorylation, downregulate cyclin D1, and block persistent HSC activation; in DMN–induced rat fibrosis, MD-1 attenuates disease progression, improves liver function, and preserves hepatocyte integrity (Feng et al., 2016). Berberine (BBR) exerts antifibrotic activity predominantly via AMPK activation with concomitant repression of NOX4/AKT expression, thereby reducing oxidative stress and ECM deposition (Li et al., 2014). Neferine (NEF) protects against NASH-associated fibrosis by inhibiting TGF-β/Smad2/3 signaling, preventing HSC activation and downregulating profibrotic genes (Wang M. Y. et al., 2023). In AML-12 hepatocytes and LX-2 HSCs, piperine (PIP) drives NRF2 nuclear translocation and antioxidant gene transcription to limit TGF-β1–elicited ROS; concurrently it increases SMAD7 while restraining SMAD2/3 phosphorylation/nuclear entry, an effect blunted by NRF2 knockdown, implicating NRF2 as a key mediator (Shu G. et al., 2021).
Collectively, these alkaloids converge on receptor signaling (EGFR), metabolic/oxidative pathways (AMPK/NOX4), and canonical profibrotic axes (TGF-β/Smad) with NRF2 crosstalk, highlighting PTM-centered mechanisms as tractable targets for antifibrotic intervention.
4.5 Other natural drugs
Accumulating evidence indicates that natural products of varied origin mitigate hepatic fibrosis by tuning PTMs across key signaling nodes. Ganoderma lucidum polysaccharide (GLP) lowers hepatic TGF-β and SMAD2/3 phosphorylation in CCl4-injured mice while restoring SMAD7, thereby preventing activation of HSC-T6 cells (Chen C. et al., 2023). Likewise, Suaeda glauca extract (SGE) suppresses TGF-β1–evoked SMAD2/3 phosphorylation and nuclear translocation and reduces SBE-dependent transcriptional activity—without affecting JNK or ERK—implicating selective blockade of the TGF-β/SMAD axis (Hong et al., 2023). Moreover, amygdalin diminishes TGF-β/SMAD2/3 phosphorylation and downregulates profibrotic gene expression, thereby curbing HSC activation and ECM deposition and mitigating CCl4-induced hepatic injury (Wang et al., 2021).
Several agents act through coordinated pathway inhibition. In HSCs, emodin reduces TGF-β1, TβRI/TβRII, and SMAD4 expression and markedly suppresses SMAD-responsive luciferase activity and p38 MAPK activation. Notably, single inhibition of SMAD4 or p38 only partially attenuates emodin’s effect, whereas combined inhibition abolishes it, indicating that emodin represses collagen gene transcription via cooperative blockade of SMAD and p38 MAPK signaling (Wang X. et al., 2018). Chrysophanol 8-O-glucoside (C8G) selectively inhibits p38 phosphorylation and blocks STAT3 activation/nuclear import, reducing MMP2 expression and ECM accumulation (Park et al., 2020). In NASH, cordycepin augments AMPK phosphorylation to restrain NF-κB activation and, via ACC phosphorylation, corrects lipid dysregulation, yielding anti-inflammatory and antifibrotic benefits; AMPK inhibition abrogates these effects, underscoring AMPK dependence (Lan et al., 2021). Plumbagin (PL) lowers IκB phosphorylation in CCl4-injured rat liver and IL-1β–stimulated HSCs, preventing NF-κB nuclear entry/transactivation and thus mitigating inflammation and ECM build-up (Chen et al., 2019). The Kampo formula Inchin-ko-to (TJ-135) decreases collagen deposition and α-SMA in fibrotic rats and represses COL1A1, COL3A1, and fibronectin transcription in HSCs by inhibiting PDGFRβ phosphorylation and downstream signaling (Imanishi et al., 2004).
Epigenetic targeting is also prominent. Emodin suppresses ERK/Nur77 signaling, drives Nur77 nuclear translocation and DNMT3b binding, increases GLS1 promoter methylation, inhibits glutaminolysis, triggers energetic stress and HSC senescence, and thereby exerts antifibrotic activity (Chen L. et al., 2023). Sennoside A (SA) upregulates SOCS1 in a DNMT1-dependent manner and suppresses macrophage pro-inflammatory cytokines, indirectly limiting HSC proliferation and ECM deposition; SOCS1 blockade diminishes efficacy, highlighting a pivotal epigenetic component in SA’s antifibrotic action (Zhu H. et al., 2021).
These natural products—polysaccharides, halophyte extracts, anthraquinones, nucleosides, naphthoquinones, and multi-component formulas—converge on PTM-regulated nodes (e.g., SMAD, p38, STAT3, AMPK/ACC, NF-κB, PDGFRβ) and epigenetic machinery (DNMT1/DNMT3b, promoter methylation) to suppress HSC activation, inflammation, and ECM deposition. Their multi-target profiles reinforce PTMs and epigenetic remodeling as tractable intervention points for antifibrotic drug development.
5 The prospects and challenges of traditional medicine in the treatment of liver fibrosis
Natural products have long served as a vital source for drug discovery because of their broad biological activities. Effective antifibrotic constituents are generally categorized into flavonoids, saponins, alkaloids, and other classes (Liu et al., 2022; Liu et al., 2020; Sharma et al., 2024; Zhao et al., 2023a). Their antifibrotic mechanisms include inhibition of hepatic inflammation, suppression of lipid-peroxidation injury, regulation of the synthesis and secretion of profibrotic factors, modulation of ECM synthesis and degradation, and inhibition of HSCs activation and proliferation. These effects are tightly linked to the regulation of PTMs, and this multi-target strategy offers distinct advantages for addressing the complexity of liver fibrosis (Li et al., 2023; Ma et al., 2020).
A growing body of in vivo and in vitro evidence indicates that natural products can attenuate fibrosis by tuning PTMs (phosphorylation, acetylation, methylation, SUMOylation). However, current PTM research is heavily skewed toward phosphorylation, whereas other PTMs remain sparsely studied. This imbalance reflects not only biological interest but also methodological convenience: phosphoproteomic enrichment and mass-spectrometry workflows are mature and widely accessible, whereas systematic profiling of lysine acetylation, ubiquitination, or emerging PTMs such as succinylation or malonylation requires higher cost, more complex sample preparation, and specialized antibodies or chemical probes. As a result, kinase/phosphatase axes dominate the mechanistic landscape, potentially obscuring druggable “writers,” “erasers,” and “readers” of other PTMs and underestimating crosstalk among PTM layers. Such bias limits discovery of alternative regulatory nodes in HSC activation and ECM dynamics and may hinder translation if therapeutic development focuses narrowly on kinase pathways.
Clinical translation remains nascent. Small randomized trials in non-alcoholic fatty liver disease (NAFLD) suggest that certain flavonoids (e.g., hesperidin) improve liver and metabolic indices (El-Mihi et al., 2017). Human studies of the green-tea catechin EGCG report heterogeneous effects on steatosis and inflammation, and dose-related hepatotoxicity has been documented (Wang et al., 2019; Yu et al., 2015). Components of Salvia miltiorrhiza (e.g., salvianolic acid B, danshensu) and the alkaloid berberine show partial clinical benefit in metabolic or cardiovascular settings, yet evidence specific to liver fibrosis remains largely preclinical or exploratory (Wu et al., 2019; Li et al., 2014). From an evidence-quality perspective, major limitations persist: small sample sizes, inadequate randomization or controls, and pronounced heterogeneity in dosing, administration routes, and experimental models, all of which compromise reproducibility and cross-study comparison. Variability in extraction, purity, and chemical characterization further contributes to divergent findings. Conflicting effects—such as inconsistent impacts of green-tea catechins on HSC activation and ECM deposition—raise concerns about model dependence, selective reporting, and publication bias (Spencer et al., 2008; Van Hung, 2016). Safety is equally critical: EGCG-associated hepatotoxicity underscores the need for integrated toxicology and drug–drug interaction assessments alongside clinical evaluation (Wang et al., 2019).
Pharmacokinetic and formulation barriers compound these challenges. Many flavonoids, phenolics, and saponins display poor oral bioavailability, rapid metabolism, and variable systemic exposure, making it difficult to achieve therapeutic concentrations in vivo (Zhang et al., 2023; Zhao et al., 2018). Nanodelivery systems, liposomal encapsulation, and prodrug strategies have improved exposure and efficacy in animal models, but scale-up manufacturing and long-term safety remain to be validated (Hu Y. et al., 2024).
5.1 Future directions
To overcome these limitations and correct the PTM imbalance, priority areas include: i. expanding research from single-PTM (phosphorylation) analysis to multi-PTM network interrogation using integrated phosphoproteomics, acetylomics, ubiquitinomics, and emerging succinyl/malonyl modifications; ii. systematically evaluating PTM “writers,” “erasers,” and “readers” (e.g., deacetylases, ubiquitin ligases, SUMO E3 ligases, methyltransferases) as potential therapeutic targets; iii. validating candidate PTM changes not only in animal and cell models but also in human liver tissues and circulating biomarkers; iv. advancing compounds with defined pharmacokinetics, acceptable safety, and concordant in vitro/in vivo mechanisms into standardized Phase I/II trials (dose finding, PK/PD, interaction studies), followed by multicenter randomized trials with long-term antifibrotic endpoints; and v. implementing rigorous standards for extraction, chemical characterization, and reporting of PTM data to reduce selective reporting and publication bias. Only by broadening the PTM perspective beyond phosphorylation, integrating high-throughput epigenomics and systems biology, and coupling mechanistic insight with early pharmacokinetic/toxicological evaluation can natural-product discoveries be efficiently translated into clinically useful antifibrotic therapeutics.
Statements
Author contributions
QN: Writing – review and editing, Investigation, Writing – original draft. YM: Writing – review and editing, Writing – original draft. KW: Data curation, Investigation, Writing – review and editing. HD: Writing – review and editing, Data curation, Investigation. ZZ: Conceptualization, Writing – review and editing, Investigation. HL: Writing – review and editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (No. 82274323) and Key research and development project of Science and Technology Department of Sichuan Province (No. 2024YFFK0150). Key project of Sichuan Provincial Administration of Traditional Chinese Medicine (25ZDIZX026).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abdelmegeed M. A. Choi Y. Ha S. K. Song B. J. (2016). Cytochrome P450-2E1 promotes aging-related hepatic steatosis, apoptosis and fibrosis through increased nitroxidative stress. Free Radic. Biol. Med.91, 188–202. 10.1016/j.freeradbiomed.2015.12.016
2
Anderson K. A. Hirschey M. D. (2012). Mitochondrial protein acetylation regulates metabolism. Essays Biochem.52, 23–35. 10.1042/bse0520023
3
Arnesen T. (2011). Towards a functional understanding of protein N-terminal acetylation. PLoS Biol.9 (5), e1001074. 10.1371/journal.pbio.1001074
4
Baiocchini A. Montaldo C. Conigliaro A. Grimaldi A. Correani V. Mura F. et al (2016). Extracellular matrix molecular remodeling in human liver fibrosis evolution. PLoS One11 (3), e0151736. 10.1371/journal.pone.0151736
5
Barcena-Varela M. Colyn L. Fernandez-Barrena M. G. (2019). Epigenetic mechanisms in hepatic stellate cell activation during liver fibrosis and carcinogenesis. Int. J. Mol. Sci.20 (10), 2507. 10.3390/ijms20102507
6
Barcena-Varela M. Paish H. Alvarez L. Uriarte I. Latasa M. U. Santamaria E. et al (2021). Epigenetic mechanisms and metabolic reprogramming in fibrogenesis: dual targeting of G9a and DNMT1 for the inhibition of liver fibrosis. Gut70 (2), 388–400. 10.1136/gutjnl-2019-320205
7
Bekki Y. Yoshizumi T. Shimoda S. Itoh S. Harimoto N. Ikegami T. et al (2017). Hepatic stellate cells secreting WFA(+) -M2BP: its role in biological interactions with kupffer cells. J. Gastroenterol. Hepatol.32 (7), 1387–1393. 10.1111/jgh.13708
8
Bhambhani S. Kondhare K. R. Giri A. P. (2021). Diversity in chemical structures and biological properties of plant alkaloids. Molecules26 (11), 3374. 10.3390/molecules26113374
9
Bian E. B. Huang C. Wang H. Wu B. M. Zhang L. Lv X. W. et al (2013). DNA methylation: new therapeutic implications for hepatic fibrosis. Cell Signal25 (1), 355–358. 10.1016/j.cellsig.2012.10.007
10
Bilbrough T. Piemontese E. Seitz O. (2022). Dissecting the role of protein phosphorylation: a chemical biology toolbox. Chem. Soc. Rev.51 (13), 5691–5730. 10.1039/d1cs00991e
11
Blankson H. Grotterød E. M. Seglen P. O. (2000). Prevention of toxin-induced cytoskeletal disruption and apoptotic liver cell death by the grapefruit flavonoid, naringin. Cell Death Differ.7 (8), 739–746. 10.1038/sj.cdd.4400705
12
Boye A. Wu C. Jiang Y. Wang J. Wu J. Yang X. et al (2015). Compound astragalus and Salvia miltiorrhiza extracts modulate MAPK-regulated TGF-β/Smad signaling in hepatocellular carcinoma by multi-target mechanism. J. Ethnopharmacol.169, 219–228. 10.1016/j.jep.2015.04.013
13
Budi E. H. Schaub J. R. Decaris M. Turner S. Derynck R. (2021). TGF-β as a driver of fibrosis: physiological roles and therapeutic opportunities. J. Pathol.254 (4), 358–373. 10.1002/path.5680
14
Canbay A. Friedman S. Gores G. J. (2004). Apoptosis: the nexus of liver injury and fibrosis. Hepatology39 (2), 273–278. 10.1002/hep.20051
15
Cao G. Zhu R. Jiang T. Tang D. Kwan H. Y. Su T. (2019). Danshensu, a novel indoleamine 2,3-dioxygenase1 inhibitor, exerts anti-hepatic fibrosis effects via inhibition of JAK2-STAT3 signaling. Phytomedicine63, 153055. 10.1016/j.phymed.2019.153055
16
Caon I. Parnigoni A. Viola M. Karousou E. Passi A. Vigetti D. (2021). Cell energy metabolism and hyaluronan synthesis. J. Histochem Cytochem69 (1), 35–47. 10.1369/0022155420929772
17
Cardone M. H. Roy N. Stennicke H. R. Salvesen G. S. Franke T. F. Stanbridge E. et al (1998). Regulation of cell death protease caspase-9 by phosphorylation. Science282 (5392), 1318–1321. 10.1126/science.282.5392.1318
18
Cassiano C. Esposito R. Tosco A. Zampella A. D'Auria M. V. Riccio R. et al (2014). Heteronemin, a marine sponge terpenoid, targets TDP-43, a key factor in several neurodegenerative disorders. Chem. Commun. (Camb)50 (4), 406–408. 10.1039/c3cc45454a
19
Chen X. Li W. X. Chen Y. Li X. F. Li H. D. Huang H. M. et al (2018). Suppression of SUN2 by DNA methylation is associated with HSCs activation and hepatic fibrosis. Cell Death Dis.9 (10), 1021. 10.1038/s41419-018-1032-9
20
Chen Y. Zhao C. Liu X. Wu G. Zhong J. Zhao T. et al (2019). Plumbagin ameliorates liver fibrosis via a ROS-mediated NF-кB signaling pathway in vitro and in vivo. Biomed. Pharmacother.116, 108923. 10.1016/j.biopha.2019.108923
21
Chen K. Guo W. Li R. Han Y. Gao Q. Wang S. (2022). Demethylzeylasteral attenuates hepatic stellate cell activation and liver fibrosis by inhibiting AGAP2 mediated signaling. Phytomedicine105, 154349. 10.1016/j.phymed.2022.154349
22
Chen C. Chen J. Wang Y. Fang L. Guo C. Sang T. et al (2023). Ganoderma lucidum polysaccharide inhibits HSC activation and liver fibrosis via targeting inflammation, apoptosis, cell cycle, and ECM-receptor interaction mediated by TGF-β/Smad signaling. Phytomedicine110, 154626. 10.1016/j.phymed.2022.154626
23
Chen L. Liang B. Xia S. Wang F. Li Z. Shao J. et al (2023). Emodin promotes hepatic stellate cell senescence and alleviates liver fibrosis via a nuclear receptor (Nur77)-mediated epigenetic regulation of glutaminase 1. Br. J. Pharmacol.180 (19), 2577–2598. 10.1111/bph.16156
24
Cho Y. E. Kim D. K. Seo W. Gao B. Yoo S. H. Song B. J. (2021). Fructose promotes leaky gut, endotoxemia, and liver fibrosis through ethanol-inducible cytochrome P450-2E1-Mediated oxidative and nitrative stress. Hepatology73 (6), 2180–2195. 10.1002/hep.30652
25
Choi J. H. Jin S. W. Choi C. Y. Kim H. G. Lee G. H. Kim Y. A. et al (2017). Capsaicin inhibits dimethylnitrosamine-induced hepatic fibrosis by inhibiting the TGF-β1/Smad pathway via peroxisome proliferator-activated receptor gamma activation. J. Agric. Food Chem.65 (2), 317–326. 10.1021/acs.jafc.6b04805
26
Cui J. Shen H. M. Lim L. H. K. (2020). The role of autophagy in liver cancer: crosstalk in signaling pathways and potential therapeutic targets. Pharm. (Basel)13 (12), 432. 10.3390/ph13120432
27
Dai C. Yusuf A. Sun H. Shu G. Deng X. (2021). A characterized saponin extract of Panax japonicus suppresses hepatocyte EMT and HSC activation in vitro and CCl(4)-provoked liver fibrosis in mice: roles of its modulatory effects on the Akt/GSK3β/Nrf2 cascade. Phytomedicine93, 153746. 10.1016/j.phymed.2021.153746
28
Deleve L. D. Wang X. Guo Y. (2008). Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology48 (3), 920–930. 10.1002/hep.22351
29
Dewidar B. Meyer C. Dooley S. Meindl-Beinker A. N. (2019). TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated 2019. Cells8 (11), 1419. 10.3390/cells8111419
30
Dikic I. Schulman B. A. (2023). An expanded lexicon for the ubiquitin code. Nat. Rev. Mol. Cell Biol.24 (4), 273–287. 10.1038/s41580-022-00543-1
31
Ding D. Chen L. L. Zhai Y. Z. Hou C. J. Tao L. L. Lu S. H. et al (2018). Trichostatin A inhibits the activation of hepatic stellate cells by increasing C/EBP-α acetylation in vivo and in vitro. Sci. Rep.8 (1), 4395. 10.1038/s41598-018-22662-6
32
Dixon L. J. Barnes M. Tang H. Pritchard M. T. Nagy L. E. (2013). Kupffer cells in the liver. Compr. Physiol.3 (2), 785–797. 10.1002/cphy.c120026
33
Dooley S. ten Dijke P. (2012). TGF-beta in progression of liver disease. Cell Tissue Res.347 (1), 245–256. 10.1007/s00441-011-1246-y
34
Eichler J. (2019). Protein glycosylation. Curr. Biol.29 (7), r229–r231. 10.1016/j.cub.2019.01.003
35
El Omari N. Bakrim S. Bakha M. Lorenzo J. M. Rebezov M. Shariati M. A. et al (2021). Natural bioactive compounds targeting epigenetic pathways in cancer: a review on alkaloids, terpenoids, quinones, and isothiocyanates. Nutrients13 (11), 3714. 10.3390/nu13113714
36
El-Mihi K. A. Kenawy H. I. El-Karef A. Elsherbiny N. M. Eissa L. A. (2017). Naringin attenuates thioacetamide-induced liver fibrosis in rats through modulation of the PI3K/Akt pathway. Life Sci.187, 50–57. 10.1016/j.lfs.2017.08.019
37
Elissawy A. M. Soleiman Dehkordi E. Mehdinezhad N. Ashour M. L. Mohammadi Pour P. (2021). Cytotoxic alkaloids derived from marine sponges: a comprehensive review. Biomolecules11 (2), 258. 10.3390/biom11020258
38
Fan J. Chen Q. Wei L. Zhou X. Wang R. Zhang H. (2018). Asiatic acid ameliorates CCl(4)-induced liver fibrosis in rats: involvement of Nrf2/ARE, NF-κB/IκBα, and JAK1/STAT3 signaling pathways. Drug Des. Devel Ther.12, 3595–3605. 10.2147/dddt.S179876
39
Fehrenbach H. Weiskirchen R. Kasper M. Gressner A. M. (2001). Up-regulated expression of the receptor for advanced glycation end products in cultured rat hepatic stellate cells during transdifferentiation to myofibroblasts. Hepatology34 (5), 943–952. 10.1053/jhep.2001.28788
40
Feng Y. Ying H. Y. Qu Y. Cai X. B. Xu M. Y. Lu L. G. (2016). Novel matrine derivative MD-1 attenuates hepatic fibrosis by inhibiting EGFR activation of hepatic stellate cells. Protein Cell7 (9), 662–672. 10.1007/s13238-016-0285-2
41
Feng Y. Guo S. Zhao Y. Dong H. Qian J. Hu Y. et al (2023). DNA 5mC and RNA m(6)A modification successively facilitates the initiation and perpetuation stages of HSC activation in liver fibrosis progression. Cell Death Differ.30 (5), 1211–1220. 10.1038/s41418-023-01130-3
42
Fernando V. Zheng X. Walia Y. Sharma V. Letson J. Furuta S. (2019). S-Nitrosylation: an emerging paradigm of redox signaling. Antioxidants (Basel)8 (9), 404. 10.3390/antiox8090404
43
Filali-Mouncef Y. Hunter C. Roccio F. Zagkou S. Dupont N. Primard C. et al (2022). The ménage à trois of autophagy, lipid droplets and liver disease. Autophagy18 (1), 50–72. 10.1080/15548627.2021.1895658
44
Friedman S. L. (2008). Mechanisms of hepatic fibrogenesis. Gastroenterology134 (6), 1655–1669. 10.1053/j.gastro.2008.03.003
45
Gantumur D. Harimoto N. Muranushi R. Hoshino K. Batbayar C. Hagiwara K. et al (2021). Hepatic stellate cell as a Mac-2-binding protein-producing cell in patients with liver fibrosis. Hepatol. Res.51 (10), 1058–1063. 10.1111/hepr.13648
46
Ge M. X. Niu W. X. Bao Y. Y. Lu Z. N. He H. W. (2023). Sclareol attenuates liver fibrosis through SENP1-mediated VEGFR2 SUMOylation and inhibition of downstream STAT3 signaling. Phytother. Res.37 (9), 3898–3912. 10.1002/ptr.7845
47
Geng Y. Sun Q. Li W. Lu Z. M. Xu H. Y. Shi J. S. et al (2017). The common dietary flavonoid myricetin attenuates liver fibrosis in carbon tetrachloride treated mice. Mol. Nutr. Food Res.61 (4), 1600392. 10.1002/mnfr.201600392
48
Ginès P. Krag A. Abraldes J. G. Solà E. Fabrellas N. Kamath P. S. (2021). Liver cirrhosis. Lancet398 (10308), 1359–1376. 10.1016/s0140-6736(21)01374-x
49
Hahne H. Sobotzki N. Nyberg T. Helm D. Borodkin V. S. van Aalten D. M. et al (2013). Proteome wide purification and identification of O-GlcNAc-modified proteins using click chemistry and mass spectrometry. J. Proteome Res.12 (2), 927–936. 10.1021/pr300967y
50
Halim C. E. Xinjing S. L. Fan L. Bailey Vitarbo J. Arfuso F. Tan C. H. et al (2019). Anti-cancer effects of oxymatrine are mediated through multiple molecular mechanism(s) in tumor models. Pharmacol. Res.147, 104327. 10.1016/j.phrs.2019.104327
51
Harvey L. D. Chan S. Y. (2018). YAPping about glutaminolysis in hepatic fibrosis. Gastroenterology154 (5), 1231–1233. 10.1053/j.gastro.2018.03.007
52
Hebert D. N. Garman S. C. Molinari M. (2005). The glycan code of the endoplasmic reticulum: asparagine-linked carbohydrates as protein maturation and quality-control tags. Trends Cell Biol.15 (7), 364–370. 10.1016/j.tcb.2005.05.007
53
Hernandez-Gea V. Friedman S. L. (2011). Pathogenesis of liver fibrosis. Annu. Rev. Pathol.6, 425–456. 10.1146/annurev-pathol-011110-130246
54
Hernandez-Gea V. Ghiassi-Nejad Z. Rozenfeld R. Gordon R. Fiel M. I. Yue Z. et al (2012). Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology142 (4), 938–946. 10.1053/j.gastro.2011.12.044
55
Hollenbach M. (2017). The role of Glyoxalase-I (Glo-I), advanced glycation endproducts (AGEs), and their receptor (RAGE) in chronic liver disease and hepatocellular carcinoma (HCC). Int. J. Mol. Sci.18 (11), 2466. 10.3390/ijms18112466
56
Hong Y. J. Kim G. H. Park Y. Jo H. J. Nam M. W. Kim D. G. et al (2023). Suaeda glauca attenuates liver fibrosis in mice by inhibiting TGFβ1-Smad2/3 signaling in hepatic stellate cells. Nutrients15 (17), 3740. 10.3390/nu15173740
57
Hou C. Lu S. Su Y. Ding D. Tao L. Wang M. et al (2021). C/EBP-α induces autophagy by binding to Beclin1 through its own acetylation modification in activated hepatic stellate cells. Exp. Cell Res.405 (2), 112721. 10.1016/j.yexcr.2021.112721
58
Housley M. P. Udeshi N. D. Rodgers J. T. Shabanowitz J. Puigserver P. Hunt D. F. et al (2009). A PGC-1alpha-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose. J. Biol. Chem.284 (8), 5148–5157. 10.1074/jbc.M808890200
59
Hsieh Y. C. Lee K. C. Lei H. J. Lan K. H. Huo T. I. Lin Y. T. et al (2021). (Pro)renin receptor knockdown attenuates liver fibrosis through inactivation of ERK/TGF-β1/SMAD3 pathway. Cell Mol. Gastroenterol. Hepatol.12 (3), 813–838. 10.1016/j.jcmgh.2021.05.017
60
Hu Y. Lang Z. Li X. Lin L. Li Y. Zhang R. et al (2024). Ginsenoside Rg3 promotes hepatic stellate cell ferroptosis by epigenetically regulating ACSL4 to suppress liver fibrosis progression. Phytomedicine124, 155289. 10.1016/j.phymed.2023.155289
61
Hu Y. J. Zhang X. Lv H. M. Liu Y. Li S. Z. (2024). Protein O-GlcNAcylation: the sweet hub in liver metabolic flexibility from a (patho)physiological perspective. Liver Int.44 (2), 293–315. 10.1111/liv.15812
62
Hua F. Shi L. Zhou P. (2022). Phenols and terpenoids: natural products as inhibitors of NLRP3 inflammasome in cardiovascular diseases. Inflammopharmacology30 (1), 137–147. 10.1007/s10787-021-00918-4
63
Huang E. Peng N. Xiao F. Hu D. Wang X. Lu L. (2020). The roles of immune cells in the pathogenesis of fibrosis. Int. J. Mol. Sci.21 (15), 5203. 10.3390/ijms21155203
64
Huang R. Guo F. Li Y. Liang Y. Li G. Fu P. et al (2021). Activation of AMPK by triptolide alleviates nonalcoholic fatty liver disease by improving hepatic lipid metabolism, inflammation and fibrosis. Phytomedicine92, 153739. 10.1016/j.phymed.2021.153739
65
Huang D. Q. Terrault N. A. Tacke F. Gluud L. L. Arrese M. Bugianesi E. et al (2023). Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat. Rev. Gastroenterol. Hepatol.20 (6), 388–398. 10.1038/s41575-023-00759-2
66
Hyogo H. Yamagishi S. (2008). Advanced glycation end products (AGEs) and their involvement in liver disease. Curr. Pharm. Des.14 (10), 969–972. 10.2174/138161208784139701
67
Imanishi Y. Maeda N. Otogawa K. Seki S. Matsui H. Kawada N. et al (2004). Herb medicine inchin-ko-to (TJ-135) regulates PDGF-BB-dependent signaling pathways of hepatic stellate cells in primary culture and attenuates development of liver fibrosis induced by thioacetamide administration in rats. J. Hepatol.41 (2), 242–250. 10.1016/j.jhep.2004.04.005
68
Inagaki Y. Kushida M. Higashi K. Itoh J. Higashiyama R. Hong Y. Y. et al (2005). Cell type-specific intervention of transforming growth factor Beta/smad signaling suppresses collagen gene expression and hepatic fibrosis in mice. Gastroenterology129 (1), 259–268. 10.1053/j.gastro.2005.03.088
69
Iwakiri Y. Kim M. Y. (2015). Nitric oxide in liver diseases. Trends Pharmacol. Sci.36 (8), 524–536. 10.1016/j.tips.2015.05.001
70
Iwamoto K. Kanno K. Hyogo H. Yamagishi S. Takeuchi M. Tazuma S. et al (2008). Advanced glycation end products enhance the proliferation and activation of hepatic stellate cells. J. Gastroenterol.43 (4), 298–304. 10.1007/s00535-007-2152-7
71
Jiang R. Zhou Y. Wang S. Pang N. Huang Y. Ye M. et al (2019). Nicotinamide riboside protects against liver fibrosis induced by CCl(4) via regulating the acetylation of smads signaling pathway. Life Sci.225, 20–28. 10.1016/j.lfs.2019.03.064
72
Jimenez-Uribe A. P. Gomez-Sierra T. Aparicio-Trejo O. E. Orozco-Ibarra M. Pedraza-Chaverri J. (2021). Backstage players of fibrosis: NOX4, mTOR, HDAC, and S1P; companions of TGF-β. Cell Signal87, 110123. 10.1016/j.cellsig.2021.110123
73
Joanna F. van Grunsven L. A. Mathieu V. Sarah S. Sarah D. Karin V. et al (2009). Histone deacetylase inhibition and the regulation of cell growth with particular reference to liver pathobiology. J. Cell Mol. Med.13 (9B), 2990–3005. 10.1111/j.1582-4934.2009.00831.x
74
Kao Y. H. Chen P. H. Wu T. Y. Lin Y. C. Tsai M. S. Lee P. H. et al (2017). Lipopolysaccharides induce Smad2 phosphorylation through PI3K/Akt and MAPK cascades in HSC-T6 hepatic stellate cells. Life Sci.184, 37–46. 10.1016/j.lfs.2017.07.004
75
Kisseleva T. Brenner D. (2021). Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol.18 (3), 151–166. 10.1038/s41575-020-00372-7
76
Kornfeld S. (1998). Diseases of abnormal protein glycosylation: an emerging area. J. Clin. Invest101 (7), 1293–1295. 10.1172/jci3140
77
Kumar P. Raeman R. Chopyk D. M. Smith T. Verma K. Liu Y. et al (2018). Adiponectin inhibits hepatic stellate cell activation by targeting the PTEN/AKT pathway. Biochim. Biophys. Acta Mol. Basis Dis.1864 (10), 3537–3545. 10.1016/j.bbadis.2018.08.012
78
Lachiondo-Ortega S. Mercado-Gómez M. Serrano-Maciá M. Lopitz-Otsoa F. Salas-Villalobos T. B. Varela-Rey M. et al (2019). Ubiquitin-like post-translational modifications (Ubl-PTMs): small peptides with huge impact in liver fibrosis. Cells8 (12), 1575. 10.3390/cells8121575
79
Lan T. Yu Y. Zhang J. Li H. Weng Q. Jiang S. et al (2021). Cordycepin ameliorates nonalcoholic steatohepatitis by activation of the AMP-activated protein kinase signaling pathway. Hepatology74 (2), 686–703. 10.1002/hep.31749
80
Lee I. H. Im E. Lee H. J. Sim D. Y. Lee J. H. Jung J. H. et al (2021). Apoptotic and antihepatofibrotic effect of honokiol via activation of GSK3β and suppression of Wnt/β-catenin pathway in hepatic stellate cells. Phytother. Res.35 (1), 452–462. 10.1002/ptr.6824
81
Li J. Pan Y. Kan M. Xiao X. Wang Y. Guan F. et al (2014). Hepatoprotective effects of berberine on liver fibrosis via activation of AMP-Activated protein kinase. Life Sci.98 (1), 24–30. 10.1016/j.lfs.2013.12.211
82
Li J. Li X. Xu W. Wang S. Hu Z. Zhang Q. et al (2015). Antifibrotic effects of luteolin on hepatic stellate cells and liver fibrosis by targeting AKT/mTOR/p70S6K and TGFβ/Smad signalling pathways. Liver Int.35 (4), 1222–1233. 10.1111/liv.12638
83
Li H. Zhou X. Wu M. Deng M. Wang C. Hou J. et al (2016). The cytotoxicity and protective effects of Astragalus membranaceus extracts and butylated hydroxyanisole on hydroxyl radical-induced apoptosis in fish erythrocytes. Anim. Nutr.2 (4), 376–382. 10.1016/j.aninu.2016.08.004
84
Li J. Z. Chen N. Ma N. Li M. R. (2023). Mechanism and progress of natural products in the treatment of NAFLD-related fibrosis. Molecules28 (23), 7936. 10.3390/molecules28237936
85
Li Z. Zheng Y. Zhang L. Xu E. (2024). Cryptotanshinone alleviates liver fibrosis via inhibiting STAT3/CPT1A-dependent fatty acid oxidation in hepatic stellate cells. Chem. Biol. Interact.399, 111119. 10.1016/j.cbi.2024.111119
86
Lin D. Xiao M. Zhao J. Li Z. Xing B. Li X. et al (2016). An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules21 (10), 1374. 10.3390/molecules21101374
87
Liu X. Mi X. Wang Z. Zhang M. Hou J. Jiang S. et al (2020). Ginsenoside Rg3 promotes regression from hepatic fibrosis through reducing inflammation-mediated autophagy signaling pathway. Cell Death Dis.11 (6), 454. 10.1038/s41419-020-2597-7
88
Liu G. Cui Z. Gao X. Liu H. Wang L. Gong J. et al (2021). Corosolic acid ameliorates non-alcoholic steatohepatitis induced by high-fat diet and carbon tetrachloride by regulating TGF-β1/Smad2, NF-κB, and AMPK signaling pathways. Phytother. Res.35 (9), 5214–5226. 10.1002/ptr.7195
89
Liu G. Wei C. Yuan S. Zhang Z. Li J. Zhang L. et al (2022). Wogonoside attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis through SOCS1/P53/SLC7A11 pathway. Phytother. Res.36 (11), 4230–4243. 10.1002/ptr.7558
90
Liu N. Liu M. Jiang M. Li Z. Chen W. Wang W. et al (2023). Isoliquiritigenin alleviates the development of alcoholic liver fibrosis by inhibiting ANXA2. Biomed. Pharmacother.159, 114173. 10.1016/j.biopha.2022.114173
91
Loureiro D. Tout I. Narguet S. Bed C. M. Roinard M. Sleiman A. et al (2023). Mitochondrial stress in advanced fibrosis and cirrhosis associated with chronic hepatitis B, chronic hepatitis C, or nonalcoholic steatohepatitis. Hepatology77 (4), 1348–1365. 10.1002/hep.32731
92
Luo S. Yang Y. Zhao T. Zhang R. Fang C. Li Y. et al (2023). Albumin-based silibinin nanocrystals targeting activated hepatic stellate cells for liver fibrosis therapy. ACS Appl. Mater Interfaces15 (6), 7747–7758. 10.1021/acsami.2c19269
93
Luo H. Cortés-López M. Tam C. L. Xiao M. Wakiro I. Chu K. L. et al (2023). SON is an essential m(6)A target for hematopoietic stem cell fate. Cell Stem Cell30 (12), 1658–1673.e1610. 10.1016/j.stem.2023.11.006
94
Ma J. Hart G. W. (2014). O-GlcNAc profiling: from proteins to proteomes. Clin. Proteomics11 (1), 8. 10.1186/1559-0275-11-8
95
Ma Y. Yuan H. Jin R. Bao X. Wang H. Su X. et al (2018). Flavonoid-rich Scabiosa comosa inflorescence extract attenuates CCl(4)-induced hepatic fibrosis by modulating TGF-β-induced Smad(3) phosphorylation. Biomed. Pharmacother.106, 426–433. 10.1016/j.biopha.2018.06.118
96
Ma J. Q. Sun Y. Z. Ming Q. L. Tian Z. K. Yang H. X. Liu C. M. (2019). Ampelopsin attenuates carbon tetrachloride-induced mouse liver fibrosis and hepatic stellate cell activation associated with the SIRT1/TGF-β1/Smad3 and autophagy pathway. Int. Immunopharmacol.77, 105984. 10.1016/j.intimp.2019.105984
97
Ma X. Jiang Y. Wen J. Zhao Y. Zeng J. Guo Y. (2020). A comprehensive review of natural products to fight liver fibrosis: Alkaloids, terpenoids, glycosides, coumarins and other compounds. Eur. J. Pharmacol.888, 173578. 10.1016/j.ejphar.2020.173578
98
Ma T. Cheng H. Li T. Chen Y. Cai T. Bai J. et al (2022). N-Acetyl-l-tryptophan inhibits CCl4-induced hepatic fibrogenesis via regulating TGF-β1/SMAD and Hippo/YAP1 signal. Bioorg Chem.126, 105899. 10.1016/j.bioorg.2022.105899
99
Mann J. Mann D. A. (2013). Epigenetic regulation of wound healing and fibrosis. Curr. Opin. Rheumatol.25 (1), 101–107. 10.1097/BOR.0b013e32835b13e1
100
Marcher A. B. Bendixen S. M. Terkelsen M. K. Hohmann S. S. Hansen M. H. Larsen B. D. et al (2019). Transcriptional regulation of hepatic stellate cell activation in NASH. Sci. Rep.9 (1), 2324. 10.1038/s41598-019-39112-6
101
Mattei A. L. Bailly N. Meissner A. (2022). DNA methylation: a historical perspective. Trends Genet.38 (7), 676–707. 10.1016/j.tig.2022.03.010
102
McQuitty C. E. Williams R. Chokshi S. Urbani L. (2020). Immunomodulatory role of the extracellular matrix within the liver disease microenvironment. Front. Immunol.11, 574276. 10.3389/fimmu.2020.574276
103
Menezo Y. Clement P. Clement A. Elder K. (2020). Methylation: an ineluctable biochemical and physiological process essential to the transmission of life. Int. J. Mol. Sci.21 (23), 9311. 10.3390/ijms21239311
104
Michalopoulos G. K. (2017). Hepatostat: liver regeneration and normal liver tissue maintenance. Hepatology65 (4), 1384–1392. 10.1002/hep.28988
105
Michalopoulos G. K. Bhushan B. (2021). Liver regeneration: biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol.18 (1), 40–55. 10.1038/s41575-020-0342-4
106
Moran-Salvador E. Mann J. (2017). Epigenetics and liver fibrosis. Cell Mol. Gastroenterol. Hepatol.4 (1), 125–134. 10.1016/j.jcmgh.2017.04.007
107
Mu M. Zuo S. Wu R. M. Deng K. S. Lu S. Zhu J. J. et al (2018). Ferulic acid attenuates liver fibrosis and hepatic stellate cell activation via inhibition of TGF-β/Smad signaling pathway. Drug Des. Devel Ther.12, 4107–4115. 10.2147/dddt.S186726
108
Nair J. J. van Staden J. (2019). The amaryllidaceae as a source of antiplasmodial crinane alkaloid constituents. Fitoterapia134, 305–313. 10.1016/j.fitote.2019.02.009
109
Nakamura N. (2018). Ubiquitin system. Int. J. Mol. Sci.19 (4), 1080. 10.3390/ijms19041080
110
Ning Y. Dou X. Wang Z. Shi K. Wang Z. Ding C. et al (2024). SIRT3: a potential therapeutic target for liver fibrosis. Pharmacol. Ther.257, 108639. 10.1016/j.pharmthera.2024.108639
111
Ogawa M. Okajima T. (2019). Structure and function of extracellular O-GlcNAc. Curr. Opin. Struct. Biol.56, 72–77. 10.1016/j.sbi.2018.12.002
112
Okuno M. Akita K. Moriwaki H. Kawada N. Ikeda K. Kaneda K. et al (2001). Prevention of rat hepatic fibrosis by the protease inhibitor, camostat mesilate, via reduced generation of active TGF-beta. Gastroenterology120 (7), 1784–1800. 10.1053/gast.2001.24832
113
Park K. C. Park J. H. Jeon J. Y. Kim S. Y. Kim J. M. Lim C. Y. et al (2014). A new histone deacetylase inhibitor improves liver fibrosis in BDL rats through suppression of hepatic stellate cells. Br. J. Pharmacol.171 (21), 4820–4830. 10.1111/bph.12590
114
Park S. M. Jung E. H. Kim J. K. Jegal K. H. Park C. A. Cho I. J. et al (2017). 20S-Protopanaxadiol, an aglycosylated ginsenoside metabolite, induces hepatic stellate cell apoptosis through liver kinase B1-AMP-activated protein kinase activation. J. Ginseng Res.41 (3), 392–402. 10.1016/j.jgr.2017.01.012
115
Park Y. J. Kim D. M. Jeong M. H. Yu J. S. So H. M. Bang I. J. et al (2019). (-)-Catechin-7-O-β-d-Apiofuranoside inhibits hepatic stellate cell activation by suppressing the STAT3 signaling pathway. Cells9 (1), 30. 10.3390/cells9010030
116
Park Y. J. Lee K. H. Jeon M. S. Lee Y. H. Ko Y. J. Pang C. et al (2020). Hepatoprotective potency of chrysophanol 8-O-Glucoside from Rheum palmatum L. against hepatic fibrosis via regulation of the STAT3 signaling pathway. Int. J. Mol. Sci.21 (23), 9044. 10.3390/ijms21239044
117
Pickart C. M. (2001). Mechanisms underlying ubiquitination. Annu. Rev. Biochem.70, 503–533. 10.1146/annurev.biochem.70.1.503
118
Popovic D. Vucic D. Dikic I. (2014). Ubiquitination in disease pathogenesis and treatment. Nat. Med.20 (11), 1242–1253. 10.1038/nm.3739
119
Pourcel L. Routaboul J. M. Cheynier V. Lepiniec L. Debeaujon I. (2007). Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci.12 (1), 29–36. 10.1016/j.tplants.2006.11.006
120
Rahman M. M. Rahaman M. S. Islam M. R. Rahman F. Mithi F. M. Alqahtani T. et al (2021). Role of phenolic compounds in human disease: current knowledge and future prospects. Molecules27 (1), 233. 10.3390/molecules27010233
121
Rao J. Wang H. Ni M. Wang Z. Wang Z. Wei S. et al (2022). FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut71 (12), 2539–2550. 10.1136/gutjnl-2021-325150
122
Riedl S. J. Salvesen G. S. (2007). The apoptosome: signalling platform of cell death. Nat. Rev. Mol. Cell Biol.8 (5), 405–413. 10.1038/nrm2153
123
Rockey D. C. Bell P. D. Hill J. A. (2015). Fibrosis--A common pathway to organ injury and failure. N. Engl. J. Med.373 (1), 96. 10.1056/NEJMc1504848
124
Ruan H. B. Han X. Li M. D. Singh J. P. Qian K. Azarhoush S. et al (2012). O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1α stability. Cell Metab.16 (2), 226–237. 10.1016/j.cmet.2012.07.006
125
Seki E. Schwabe R. F. (2015). Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology61 (3), 1066–1079. 10.1002/hep.27332
126
Seo W. Jeong W. I. (2016). Hepatic non-parenchymal cells: master regulators of alcoholic liver disease?World J. Gastroenterol.22 (4), 1348–1356. 10.3748/wjg.v22.i4.1348
127
Seo J. H. Lee H. J. Sim D. Y. Park J. E. Ahn C. H. Park S. Y. et al (2023). Honokiol inhibits epithelial-mesenchymal transition and hepatic fibrosis via activation of Ecadherin/GSK3β/JNK and inhibition of AKT/ERK/p38/β-catenin/TMPRSS4 signaling axis. Phytother. Res.37 (9), 4092–4101. 10.1002/ptr.7871
128
Serafini M. Peluso I. Raguzzini A. (2010). Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc.69 (3), 273–278. 10.1017/s002966511000162x
129
Sharma N. Sistla R. Andugulapati S. B. (2024). Yohimbine ameliorates liver inflammation and fibrosis by regulating oxidative stress and Wnt/β-catenin pathway. Phytomedicine123, 155182. 10.1016/j.phymed.2023.155182
130
Shu B. Zhou Y. X. Li H. Zhang R. Z. He C. Yang X. (2021). The METTL3/MALAT1/PTBP1/USP8/TAK1 axis promotes pyroptosis and M1 polarization of macrophages and contributes to liver fibrosis. Cell Death Discov.7 (1), 368. 10.1038/s41420-021-00756-x
131
Shu G. Dai C. Yusuf A. Sun H. Deng X. (2022). Limonin relieves TGF-β-induced hepatocyte EMT and hepatic stellate cell activation in vitro and CCl(4)-induced liver fibrosis in mice via upregulating Smad7 and subsequent suppression of TGF-β/Smad cascade. J. Nutr. Biochem.107, 109039. 10.1016/j.jnutbio.2022.109039
132
Shu F. Xiao H. Li Q. N. Ren X. S. Liu Z. G. Hu B. W. et al (2023). Epigenetic and post-translational modifications in autophagy: biological functions and therapeutic targets. Signal Transduct. Target Ther.8 (1), 32. 10.1038/s41392-022-01300-8
133
Shu G. Yusuf A. Dai C. Sun H. Deng X. (2021). Piperine inhibits AML-12 hepatocyte EMT and LX-2 HSC activation and alleviates mouse liver fibrosis provoked by CCl(4): roles in the activation of the Nrf2 cascade and subsequent suppression of the TGF-β1/Smad axis. Food Funct.12 (22), 11686–11703. 10.1039/d1fo02657g
134
Song S. Z. Lin S. Liu J. N. Zhang M. B. Du Y. T. Zhang D. D. et al (2019). Targeting of SPP1 by microRNA-340 inhibits gastric cancer cell epithelial-mesenchymal transition through inhibition of the PI3K/AKT signaling pathway. J. Cell Physiol.234 (10), 18587–18601. 10.1002/jcp.28497
135
Song Y. Wei J. Li R. Fu R. Han P. Wang H. et al (2023a). Tyrosine kinase receptor B attenuates liver fibrosis by inhibiting TGF-β/SMAD signaling. Hepatology78 (5), 1433–1447. 10.1097/HEP.0000000000000319
136
Song Y. Wei J. Li R. Fu R. Han P. Wang H. et al (2023b). Tyrosine kinase receptor B attenuates liver fibrosis by inhibiting TGF-β/SMAD signaling. Hepatology78 (5), 1433–1447. 10.1097/hep.0000000000000319
137
Spencer J. P. Abd El Mohsen M. M. Minihane A. M. Mathers J. C. (2008). Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br. J. Nutr.99 (1), 12–22. 10.1017/s0007114507798938
138
Svegliati-Baroni G. Saccomanno S. van Goor H. Jansen P. Benedetti A. Moshage H. (2001). Involvement of reactive oxygen species and nitric oxide radicals in activation and proliferation of rat hepatic stellate cells. Liver21 (1), 1–12. 10.1034/j.1600-0676.2001.210101.x
139
Tang B. X. Zhang Y. Sun D. D. Liu Q. Y. Li C. Wang P. P. et al (2024). Luteolin-7-diglucuronide, a novel PTP1B inhibitor, ameliorates hepatic stellate cell activation and liver fibrosis in mice. Acta Pharmacol. Sin.46, 122–133. 10.1038/s41401-024-01351-3
140
Tessarz P. Kouzarides T. (2014). Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol.15 (11), 703–708. 10.1038/nrm3890
141
Thoen L. F. Guimaraes E. L. Dolle L. Mannaerts I. Najimi M. Sokal E. et al (2011). A role for autophagy during hepatic stellate cell activation. J. Hepatol.55 (6), 1353–1360. 10.1016/j.jhep.2011.07.010
142
Toma L. Sanda G. M. Niculescu L. S. Deleanu M. Sima A. V. Stancu C. S. (2020). Phenolic compounds exerting lipid-regulatory, anti-inflammatory and epigenetic effects as complementary treatments in cardiovascular diseases. Biomolecules10 (4), 641. 10.3390/biom10040641
143
Tsuchida T. Friedman S. L. (2017). Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol.14 (7), 397–411. 10.1038/nrgastro.2017.38
144
Tsukamoto H. Zhu N. L. Asahina K. Mann D. A. Mann J. (2011). Epigenetic cell fate regulation of hepatic stellate cells. Hepatol. Res.41 (7), 675–682. 10.1111/j.1872-034X.2011.00804.x
145
Urso M. L. Clarkson P. M. (2003). Oxidative stress, exercise, and antioxidant supplementation. Toxicology189 (1-2), 41–54. 10.1016/s0300-483x(03)00151-3
146
van der Heide D. Weiskirchen R. Bansal R. (2019). Therapeutic targeting of hepatic macrophages for the treatment of liver diseases. Front. Immunol.10, 2852. 10.3389/fimmu.2019.02852
147
Van Hung P. (2016). Phenolic compounds of cereals and their antioxidant capacity. Crit. Rev. Food Sci. Nutr.56 (1), 25–35. 10.1080/10408398.2012.708909
148
Verdin E. Ott M. (2015). 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat. Rev. Mol. Cell Biol.16 (4), 258–264. 10.1038/nrm3931
149
Walton K. L. Johnson K. E. Harrison C. A. (2017). Targeting TGF-beta mediated SMAD signaling for the prevention of fibrosis. Front. Pharmacol.8, 461. 10.3389/fphar.2017.00461
150
Wang R. Zhang H. Wang Y. Song F. Yuan Y. (2017). Inhibitory effects of quercetin on the progression of liver fibrosis through the regulation of NF-кB/IкBα, p38 MAPK, and Bcl-2/Bax signaling. Int. Immunopharmacol.47, 126–133. 10.1016/j.intimp.2017.03.029
151
Wang L. Yang G. Yuan L. Yang Y. Zhao H. Ho C. T. et al (2019). Green tea catechins effectively altered hepatic fibrogenesis in rats by inhibiting ERK and Smad1/2 phosphorylation. J. Agric. Food Chem.67 (19), 5437–5445. 10.1021/acs.jafc.8b05179
152
Wang Y. Li C. Gu J. Chen C. Duanmu J. Miao J. et al (2020a). Celastrol exerts anti-inflammatory effect in liver fibrosis via activation of AMPK-SIRT3 signalling. J. Cell Mol. Med.24 (1), 941–953. 10.1111/jcmm.14805
153
Wang Y. Wen H. Fu J. Cai L. Li P. L. Zhao C. L. et al (2020b). Hepatocyte TNF receptor-associated factor 6 aggravates hepatic inflammation and fibrosis by promoting lysine 6-Linked polyubiquitination of apoptosis signal-regulating kinase 1. Hepatology71 (1), 93–111. 10.1002/hep.30822
154
Wang R. Zhang D. Tang D. Sun K. Peng J. Zhu W. et al (2021). Amygdalin inhibits TGFβ1-induced activation of hepatic stellate cells (HSCs) in vitro and CCl(4)-induced hepatic fibrosis in rats in vivo. Int. Immunopharmacol.90, 107151. 10.1016/j.intimp.2020.107151
155
Wang F. Chen L. Zhang B. Li Z. Shen M. Tang L. et al (2022). O-GlcNAcylation coordinates glutaminolysis by regulating the stability and membrane trafficking of ASCT2 in hepatic stellate cells. J. Clin. Transl. Hepatol.10 (6), 1107–1116. 10.14218/JCTH.2021.00413
156
Wang M. Y. Zhang S. S. An M. F. Xia Y. F. Fan M. S. Sun Z. R. et al (2023). Neferine ameliorates nonalcoholic steatohepatitis through regulating AMPK pathway. Phytomedicine114, 154798. 10.1016/j.phymed.2023.154798
157
Wang S. He L. Xiao F. Gao M. Wei H. Yang J. et al (2023). Upregulation of GLT25D1 in hepatic stellate cells promotes liver fibrosis via the TGF-β1/SMAD3 pathway in vivo and in vitro. J. Clin. Transl. Hepatol.11 (1), 1–14. 10.14218/jcth.2022.00005
158
Wang L. Pan M. H. Lo C. Y. Zhao H. Li S. Ho C. T. et al (2018). Anti-fibrotic activity of polyphenol-enriched sugarcane extract in rats via inhibition of p38 and JNK phosphorylation. Food Funct.9 (2), 951–958. 10.1039/c7fo01617d
159
Wang X. Niu C. Zhang X. Dong M. (2018). Emodin suppresses activation of hepatic stellate cells through p38 mitogen-activated protein kinase and smad signaling pathways in vitro. Phytother. Res.32 (12), 2436–2446. 10.1002/ptr.6182
160
Wen J. H. Wen H. Gibson-Corley K. N. Glenn K. A. (2015). FBG1 is the final arbitrator of A1AT-Z degradation. PLoS One10 (8), e0135591. 10.1371/journal.pone.0135591
161
Wilson C. L. Murphy L. B. Leslie J. Kendrick S. French J. Fox C. R. et al (2015). Ubiquitin C-terminal hydrolase 1: a novel functional marker for liver myofibroblasts and a therapeutic target in chronic liver disease. J. Hepatol.63 (6), 1421–1428. 10.1016/j.jhep.2015.07.034
162
Wu M. H. Chen Y. L. Lee K. H. Chang C. C. Cheng T. M. Wu S. Y. et al (2017). Glycosylation-dependent galectin-1/neuropilin-1 interactions promote liver fibrosis through activation of TGF-β- and PDGF-like signals in hepatic stellate cells. Sci. Rep.7 (1), 11006. 10.1038/s41598-017-11212-1
163
Wu C. Chen W. Ding H. Li D. Wen G. Zhang C. et al (2019). Salvianolic acid B exerts anti-liver fibrosis effects via inhibition of MAPK-mediated phospho-Smad2/3 at linker regions in vivo and in vitro. Life Sci.239, 116881. 10.1016/j.lfs.2019.116881
164
Wu Z. Jankowski J. (1953). Impact of post-translational modification on the genesis and progression of diseases. Mol. Aspects Med.86, 101105. 10.1016/j.mam.2022.101105
165
Wu N. N. Wang L. Wang L. Xu X. Lopaschuk G. D. Zhang Y. et al (2023). Site-specific ubiquitination of VDAC1 restricts its oligomerization and mitochondrial DNA release in liver fibrosis. Exp. Mol. Med.55 (1), 269–280. 10.1038/s12276-022-00923-9
166
Xu C. Zhou H. Jin Y. Sahay K. Robicsek A. Liu Y. et al (2022). Hepatic neddylation deficiency triggers fatal liver injury via inducing NF-κB-inducing kinase in mice. Nat. Commun.13 (1), 7782. 10.1038/s41467-022-35525-6
167
Xue M. Feng T. Chen Z. Yan Y. Chen Z. Dai J. (2022). Protein acetylation going viral: implications in antiviral immunity and viral infection. Int. J. Mol. Sci.23 (19), 11308. 10.3390/ijms231911308
168
Yang J. J. Yang Y. Zhang C. Li J. Yang Y. (2020). Epigenetic silencing of LncRNA ANRIL enhances liver fibrosis and HSC activation through activating AMPK pathway. J. Cell Mol. Med.24 (4), 2677–2687. 10.1111/jcmm.14987
169
Yang Q. Chen X. Zhang Y. Hu S. Hu F. Huang Y. et al (2021). The E3 ubiquitin ligase ring finger protein 5 ameliorates NASH through ubiquitin-mediated degradation of 3-Hydroxy-3-Methylglutaryl CoA reductase degradation protein 1. Hepatology74 (6), 3018–3036. 10.1002/hep.32061
170
Yao P. Liu Y. (2022). Terpenoids: natural compounds for non-alcoholic fatty liver disease (NAFLD) therapy. Molecules28 (1), 272. 10.3390/molecules28010272
171
Ying H. Z. Chen Q. Zhang W. Y. Zhang H. H. Ma Y. Zhang S. Z. et al (2017). PDGF signaling pathway in hepatic fibrosis pathogenesis and therapeutics (review). Mol. Med. Rep.16 (6), 7879–7889. 10.3892/mmr.2017.7641
172
Yu F. Lu Z. Chen B. Wu X. Dong P. Zheng J. (2015). Salvianolic acid B-induced microRNA-152 inhibits liver fibrosis by attenuating DNMT1-mediated Patched1 methylation. J. Cell Mol. Med.19 (11), 2617–2632. 10.1111/jcmm.12655
173
Zhang D. D. Lo S. C. Cross J. V. Templeton D. J. Hannink M. (2004). Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell Biol.24 (24), 10941–10953. 10.1128/mcb.24.24.10941-10953.2004
174
Zhang T. Liu Q. Gao W. Sehgal S. A. Wu H. (2022). The multifaceted regulation of mitophagy by endogenous metabolites. Autophagy18 (6), 1216–1239. 10.1080/15548627.2021.1975914
175
Zhang R. Li X. Gao Y. Tao Q. Lang Z. Zhan Y. et al (2023). Ginsenoside Rg1 epigenetically modulates Smad7 expression in liver fibrosis via MicroRNA-152. J. Ginseng Res.47 (4), 534–542. 10.1016/j.jgr.2022.12.005
176
Zhao H. Wang Z. Tang F. Zhao Y. Feng D. Li Y. et al (2018). Carnosol-mediated sirtuin 1 activation inhibits enhancer of zeste homolog 2 to attenuate liver fibrosis. Pharmacol. Res.128, 327–337. 10.1016/j.phrs.2017.10.013
177
Zhao Q. Ma J. Xie F. Wang Y. Zhang Y. Li H. et al (2021). Recent advances in predicting protein S-Nitrosylation sites. Biomed. Res. Int.2021, 5542224. 10.1155/2021/5542224
178
Zhao D. Gao Y. Su Y. Zhou Y. Yang T. Li Y. et al (2023). Oroxylin A regulates cGAS DNA hypermethylation induced by methionine metabolism to promote HSC senescence. Pharmacol. Res.187, 106590. 10.1016/j.phrs.2022.106590
179
Zhao J. Bai D. Qi L. Cao W. Du J. Gu C. et al (2023a). The flavonoid GL-V9 alleviates liver fibrosis by triggering senescence by regulating the transcription factor GATA4 in activated hepatic stellate cells. Br. J. Pharmacol.180 (8), 1072–1089. 10.1111/bph.15997
180
Zhao J. Bai J. Peng F. Qiu C. Li Y. Zhong L. (2023b). USP9X-mediated NRP1 deubiquitination promotes liver fibrosis by activating hepatic stellate cells. Cell Death Dis.14 (1), 40. 10.1038/s41419-022-05527-9
181
Zhou Y. Tong X. Ren S. Wang X. Chen J. Mu Y. et al (2016). Synergistic anti-liver fibrosis actions of total astragalus saponins and glycyrrhizic acid via TGF-β1/Smads signaling pathway modulation. J. Ethnopharmacol.190, 83–90. 10.1016/j.jep.2016.06.011
182
Zhou J. Zhou F. Wang W. Zhang X. J. Ji Y. X. Zhang P. et al (2020). Epidemiological features of NAFLD from 1999 to 2018 in China. Hepatology71 (5), 1851–1864. 10.1002/hep.31150
183
Zhu H. Zhao H. Xu S. Zhang Y. Ding Y. Li J. et al (2021). Sennoside A alleviates inflammatory responses by inhibiting the hypermethylation of SOCS1 in CCl(4)-induced liver fibrosis. Pharmacol. Res.174, 105926. 10.1016/j.phrs.2021.105926
184
Zhu X. Ye S. Yu D. Zhang Y. Li J. Zhang M. et al (2021). Physalin B attenuates liver fibrosis via suppressing LAP2α-HDAC1-mediated deacetylation of the transcription factor GLI1 and hepatic stellate cell activation. Br. J. Pharmacol.178 (17), 3428–3447. 10.1111/bph.15490
Summary
Keywords
hepatic fibrosis, post-translational modifications, natural products, hepatic stellate cells, extracellular matrix, TGF-β, antifibrotic therapy
Citation
Niu Q, Mou Y, Wang K, Dong H, Zeng Z and Li H (2025) Natural medicines for treating liver fibrosis by modulating post-translational modifications. Front. Pharmacol. 16:1660908. doi: 10.3389/fphar.2025.1660908
Received
07 July 2025
Revised
22 October 2025
Accepted
23 October 2025
Published
10 November 2025
Volume
16 - 2025
Edited by
Javier Echeverria, University of Santiago, Chile
Reviewed by
Wei Chen, Capital Medical University, China
Zheyun Peng, Indiana University, Purdue University Indianapolis, United States
Updates
Copyright
© 2025 Niu, Mou, Wang, Dong, Zeng and Li.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Li, lihui@cdutcm.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.