- 1School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2Key Laboratory of Blood-stasis-toxin Syndrome of Zhejiang Province, Hangzhou, China
- 3Traditional Chinese Medicine “Preventing Disease” Wisdom Health Project Research Center of Zhejiang, Hangzhou, China
- 4The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Province Hospital of Chinese Medicine), Hangzhou, Zhejiang, China
- 5Huzhou Central Hospital, Huzhou, Zhejiang, China
Background: Cancer remains a major global health burden. Combining traditional Chinese medicine (TCM) with immune checkpoint inhibitors (ICIs) may potentially mitigate treatment-related side effects and improve the quality of life for cancer patients. To critically evaluate the clinical efficacy of this combination therapy, a meta-analysis was performed.
Methods: A systematic search was conducted across six databases. Data were analyzed using RevMan 5.3 and Stata 12. Heterogeneity was explored through subgroup analysis and meta-regression. The robustness of results was assessed via sensitivity analysis and publication bias.
Results: 41 studies were included. The TCM + ICIs group demonstrated significantly superior outcomes compared to the ICIs group across multiple endpoints: Overall Response Rate (ORR) (RR: 1.34 [1.20, 1.49]), Disease Control Rate (DCR) (RR: 1.15 [1.10, 1.21]), CD4+/CD8+ T-cell ratio (WMD: 0.25 [0.15, 0.35]), Progression-Free Survival (PFS) (WMD: 0.96 [0.29, 1.63]), Overall Survival (OS) (WMD: 1.46 [0.62, 2.30]), Karnofsky Performance Status (KPS) (WMD: 6.35 [4.99, 7.70]), and TCM Therapeutic Evaluation (RR: 1.42 [1.30, 1.55]). Conversely, the TCM + ICIs group showed lower levels of tumor markers, including Alpha-Fetoprotein (AFP) (SMD: 0.75 [-1.49, −0.01]), Carcinoembryonic Antigen (CEA) (SMD: 0.72 [-1.08, −0.37]), Carbohydrate Antigen 125 (CA125) (SMD: 0.77 [-1.46, −0.08]), and a reduced incidence of adverse events (RR: 0.82 [0.69, 0.97]). There is high heterogeneity among CD4+T/CD8+T studies due to the type of tumor and whether it is combined with chemotherapy. The high heterogeneity among studies on KPS may be related to the type of ICIs. Sensitivity analysis and assessment of publication bias confirmed the robustness of the pooled results.
Conclusion: The combination of TCM with ICIs appears to enhance antitumor immunity, reduce adverse reactions, lower serum tumor marker levels, improve disease control, and ameliorate patient performance status. This combination strategy represents a promising therapeutic approach for various cancers and warrants further investigation.
Introduction
Cancer poses a significant global health challenge, exerting a profound impact on human life. According to Global Cancer Observatory (GCO) data, there were approximately 20 million new cancer cases and nearly 10 million cancer-related deaths worldwide in 2022. Projections indicate that by 2050, new cancer cases could exceed 35 million annually (Bray et al., 2024). Lung, liver, stomach, breast, and colon cancers are among the leading causes of cancer mortality (Cao et al., 2021). Current treatment modalities include local therapies such as surgical resection, radiotherapy, interventional therapy, and ablation, as well as systemic therapies like chemotherapy. However, these approaches often have limitations and can be associated with substantial side effects (Zeng, 2018).
Recent years have witnessed remarkable advances in cancer immunotherapy. Immune checkpoint inhibitors (ICIs), particularly monoclonal antibodies targeting programmed cell death protein 1 (PD-1), its ligand (PD-L1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), have become first-line treatments for various malignancies, significantly improving survival outcomes for many patients (Bagchi et al., 2021; Cunningham et al., 2024). ICIs function by blocking inhibitory immune signals, thereby potentiating T cell-mediated anti-tumor responses (Brahmer et al., 2021). Despite their efficacy, the widespread clinical application of ICIs has revealed a spectrum of immune-related adverse events (irAEs), such as myocarditis, pneumonitis, and hepatitis. The increasing incidence of these toxicities, along with the emergence of drug resistance, presents significant clinical challenges (Hu et al., 2022; Wang Z. et al., 2023).
Accumulating evidence suggests that various forms of Traditional Chinese Medicine (TCM) can inhibit the proliferation and metastasis of diverse tumor cells. TCM has shown notable benefits in treating cancers such as breast, lung, liver, and gastric cancer, potentially extending survival, improving quality of life, and enhancing the efficacy while reducing the toxicity of combined radiotherapy and chemotherapy (Wang K. et al., 2021; Xiang et al., 2019). Preclinical and clinical studies have begun to verify the synergistic effects of TCM and ICIs in cancers including lung cancer, breast cancer, and melanoma. Potential underlying mechanisms include modulation of the tumor microenvironment and regulation of gut microbiota (Yu YX. et al., 2023). Consequently, the combination of TCM and ICIs is gaining acceptance, although the precise mechanisms of action remain incompletely elucidated. This meta-analysis aims to clarify the efficacy and potential mechanisms of the TCM + ICIs combination in cancer treatment, with the goal of informing clinical practice and providing new perspectives for therapeutic development.
Methods
Literature search
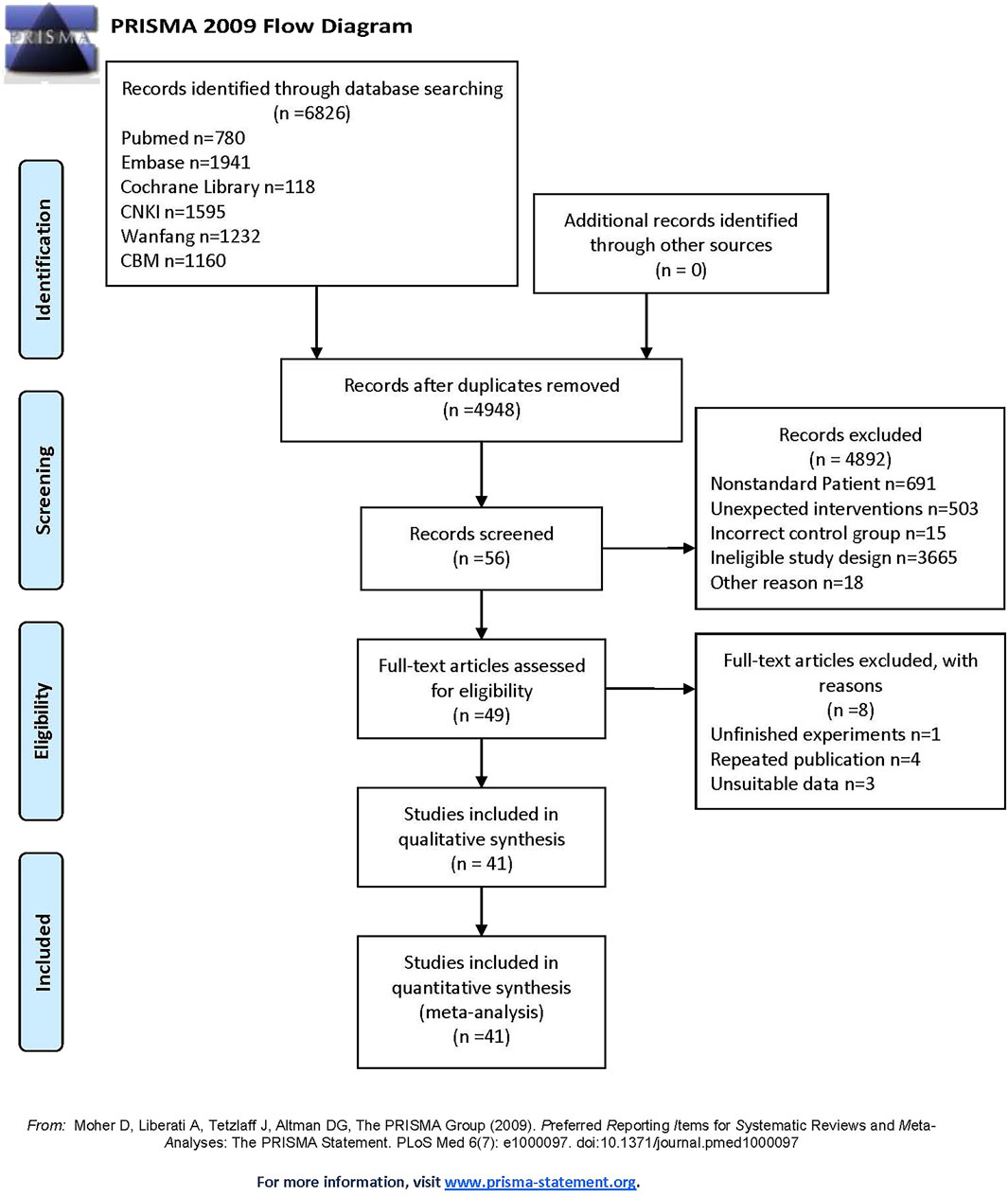
The protocol for this study has been registered on the PROSPERO website (https://www.crd.york.ac.uk/prospero/) with number CRD42024582055 in Additional File 1. Two independent investigators systematically searched six databases (PubMed, Embase, Cochrane Library, CNKI, Wanfang, and CBM) for literature published up to 7 October 2024. The search strategy combined Medical Subject Headings (MeSH) terms with free words, incorporating key concepts such as ‘Traditional Chinese Medicine’ (TCM), ‘immune checkpoint inhibitors’, ‘PD-1′, ‘PD-L1′, and ‘ICIs’. The detailed search strategy is provided in Additional File 2. Relevant references from the retrieved articles were also screened for inclusion. The entire process adhered strictly to the PRISMA guidelines (Moher et al., 2009) (see Additional File 3). Botanical drugs included in this study were verified using the Medicinal Plant Names Services (MPNS) portal (http://mpns.kew.org/mpns-portal/), while other traditional medicines were authenticated via the Zhong Hui Zhong Yao Wang database (https://www.zhzyw.com/).
Study selection
Two reviewers independently screened the titles and abstracts of retrieved records, followed by full-text assessment for eligibility. A third reviewer resolved any discrepancies and made final judgments based on the pre-defined protocol. The specific inclusion criteria were as follows:
All included literature must meet the following: 1) Study participants were clinically diagnosed cancer patients aged 18 years or older, with both an experimental and a control group; 2) The intervention consisted of ICIs combined with TCM, which could include TCM monomers, single herbs, herb pairs, prescriptions, or moxibustion/fumigation therapies; 3) The control group received ICIs for cancer; 4) Reported outcomes included at least one of the following: Overall Response Rate (ORR), Disease Control Rate (DCR), CD4+/CD8+ T-cell ratio, Progression-Free Survival (PFS), Overall Survival (OS), Karnofsky Performance Status (KPS), levels of Alpha-Fetoprotein (AFP), Carcinoembryonic Antigen (CEA), Carbohydrate Antigen 125 (CA125), Carbohydrate Antigen 19-9 (CA199), incidence of adverse effects, or TCM therapeutic evaluation; 5) Study design was a randomized controlled trial (RCT) comparing ICI combination therapy with TCM versus ICI monotherapy. Concurrent conventional or basic supportive care was permitted in both groups. Common ICIs considered included Pembrolizumab, Nivolumab, Sintilimab, Tislelizumab, Camrelizumab, and so on.
Data extraction and quality assessment
Data extraction and quality assessment were performed independently by two reviewers. A third reviewer was consulted to reconcile disagreements and finalize the data synthesis. Extracted data encompassed: publication year, corresponding author’s country/region, cancer diagnostic criteria, baseline characteristics of participants (sample size, age, gender), details of interventions (types, dosage, administration of ICIs; types, formulation, administration of TCM; other concomitant treatments; treatment duration), and all pre-specified outcomes (ORR, DCR, CD4+/CD8+ ratio, PFS, OS, KPS, AFP, CEA, CA125, CA19-9, adverse effects, TCM evaluation). Corresponding authors were contacted for missing or unclear data. Furthermore, ORR was defined as the proportion of patients achieving a complete response (CR) or partial response (PR). DCR was defined as the proportion of patients achieving CR, PR, or stable disease (SD) (Upadhyay et al., 2025; Bae et al., 2022). Both ORR and DCR are key metrics for tumor response evaluation.
The methodological quality of the included RCTs was assessed using the Cochrane Risk of Bias tool (RoB 2.0) (Higgins et al., 2011). This tool evaluates six domains: i) randomization process, ii) allocation concealment, iii) blinding of participants and personnel, iv) blinding of outcome assessment, v) incomplete outcome data, and vi) selective reporting. Each domain was judged as ‘low risk of bias’, ‘some concerns’, or ‘high risk of bias’. To enhance the reporting transparency and reproducibility of the included TCM interventions, the ConPhyMP tool was referenced, following the approach of Heinrich et al.
Statistical analysis
Data analysis was conducted using RevMan (version 5.3) and Stata (version 12). For dichotomous outcomes, data were pooled and expressed as Risk Ratios (RR) with 95% confidence intervals (CI). Continuous outcomes were categorized based on their distribution. Data following a normal distribution were presented as mean ± standard deviation (SD). Non-normally distributed data were converted to mean ± SD using established methods (Shi et al., 2023; Luo et al., 2018; Wan et al., 2014) via https://www.math.hkbu.edu.hk/∼tongt/pages/median2mean.html. The Weighted Mean Difference (WMD) and 95% CI were applied when outcomes were measured on the same unit across studies; otherwise, the Standardized Mean Difference (SMD) and 95% CI were used. Heterogeneity among studies was quantitatively assessed using the I2 statistic and Cochran’s Q test, with results visualized using forest plots. An I2 value less than 50% indicated low heterogeneity, warranting the use of a fixed-effects model. An I2 value greater than 50% suggested substantial heterogeneity, leading to the adoption of a random-effects model (Schmidt et al., 2009; Higgins et al., 2003). If sufficient studies were available (n > 10), subgroup analysis or meta-regression was planned to explore potential sources of heterogeneity. Sensitivity analysis was performed by sequentially excluding each study to evaluate the robustness of the pooled results. Publication bias was assessed using Egger’s test (Egger et al., 1997) when more than five studies were included in a meta-analysis. A p-value <0.05 indicated potential publication bias, in which case the trim-and-filling method was employed to assess their stability.
Results
Study selection
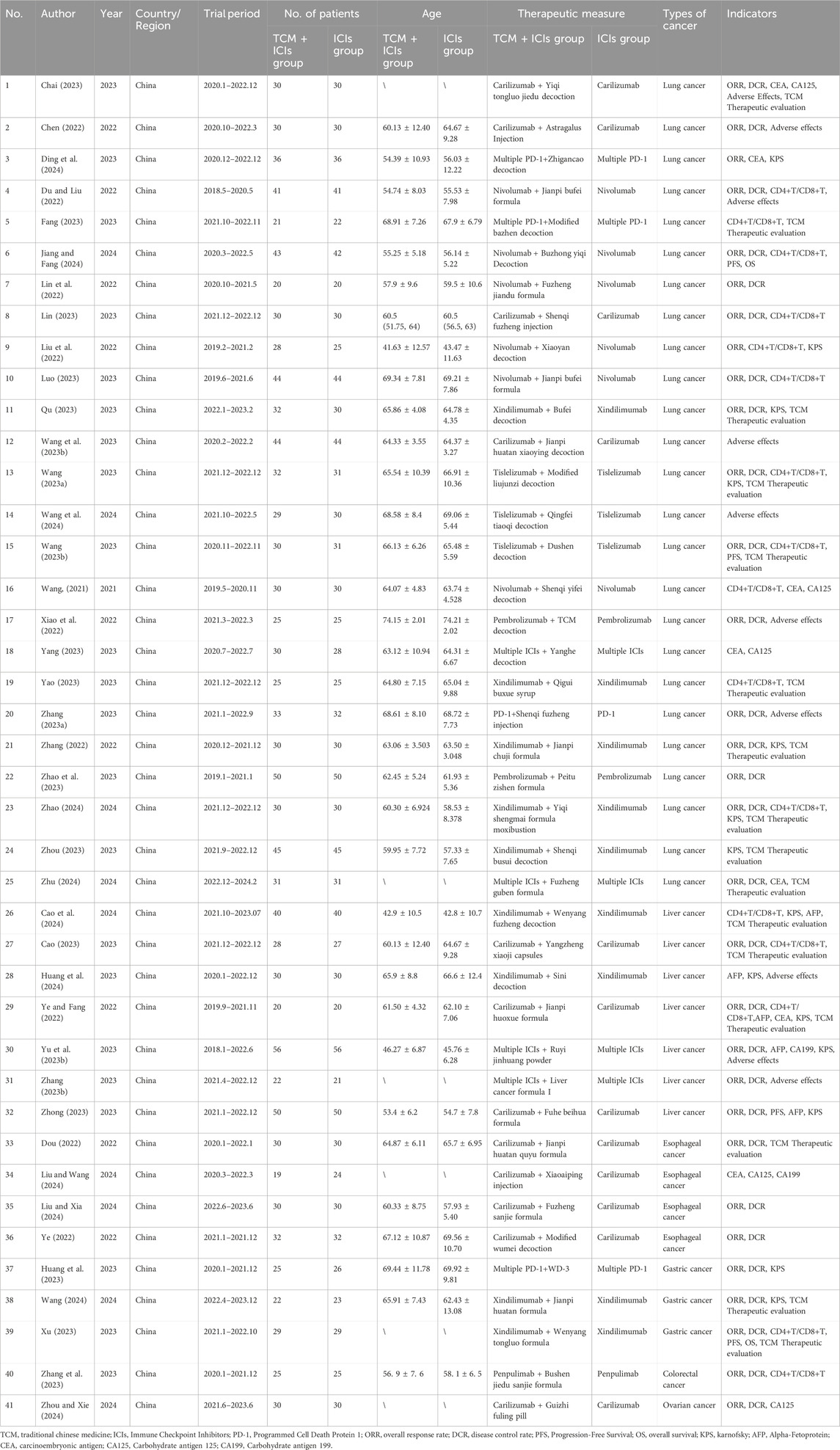
A total of 6,826 articles were retrieved from the six databases. After removal of duplicates, 4,948 articles remained for screening by two independent reviewers based on the inclusion and exclusion criteria. Ultimately, 41 studies (Chai, 2023; Chen, 2022; Ding et al., 2024; Du and Liu, 2022; Fang, 2023; Jiang and Fang, 2024; Lin et al., 2022; Lin, 2023; Liu et al., 2022; Luo, 2023; Qu, 2023; Wang L. et al., 2023; Wang Q., 2023; Wang et al., 2024; Wang XH., 2023; Wang, 2021; Xiao et al., 2022; Yang, 2023; Yao, 2023; Zhang J., 2023; Zhang, 2022; Zhao et al., 2023; Zhao, 2024; Zhou, 2023; Zhu, 2024; Cao et al., 2024; Cao, 2023; Huang et al., 2024; Ye and Fang, 2022; Yu D. et al., 2023; Zhang LL., 2023; Zhong, 2023; Dou, 2022; Liu and Wang, 2024; Liu and Xia, 2024; Ye, 2022; Huang et al., 2023; Wang, 2024; Xu, 2023; Zhang et al., 2023; Zhou and Xie, 2024) were included in the meta-analysis. All included studies were conducted in China, with only three published in English databases. This analysis encompassed 2,612 cancer patients, including cases of lung cancer (n = 1,631), liver cancer (n = 490), gastric cancer (n = 154), esophageal cancer (n = 227), colorectal cancer (n = 50), and ovarian cancer (n = 60). Various ICIs were used, such as Sintilimab, Nivolumab, Camrelizumab, and Tislelizumab. TCM interventions included prescriptions, moxibustion, injections, and other formulations. The publication years of the included studies ranged from 2020 to 2024, indicating a focus on recent research. The characteristics of the included studies are summarized in Table 1, and the study selection flow diagram is presented in Figure 1. The TCM included in each study are detailed in Additional File 4, with all drugs having been verified.
Quality assessment and data extraction
The methodological quality of the 41 included studies, assessed using the Cochrane RoB 2.0 tool, is summarized in Figure 2 and detailed in Additional File 5. Most studies reported random allocation of participants to the case or control group; however, only two studies specified the involvement of a third researcher in the randomization process. Blinding procedures were seldom mentioned, leading to potential performance and detection bias. Several studies reported participant dropouts, with reasons for attrition not always being consistent between groups. All studies adhered to their pre-specified outcome measures without selective reporting. Overall, the quality of the included studies was variable, with some demonstrating robust methodology and others having unclear reporting of design elements. The assessment of TCM preparation reporting quality using the ConPhyMP tool is provided in Additional Files 6 and 7.
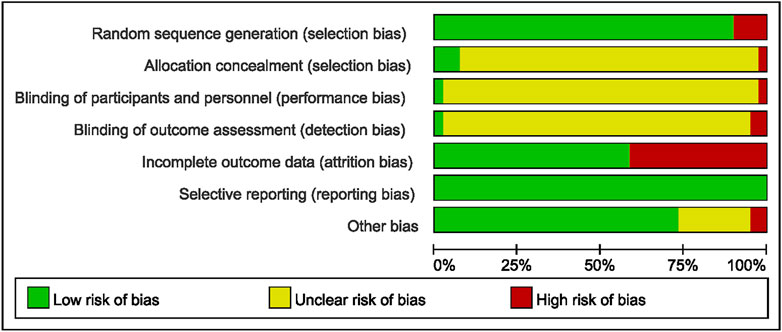
Figure 2. Risk of bias analysis across studies: Risk of bias item presented as a percentage across all included trials (n = 41).
TCM + ICIs vs. ICIs in cancer—Overall Response Rate (ORR)
The comparison of ORR between two groups of patients is shown in Figure 3. The heterogeneity among 31 studies was low (p = 0.79, I2 = 0%), therefore a fixed-effect model was chosen. The results showed that the patients in TCM + ICIs group had higher ORR values (RR = 1.34 [1.20–1.49]) compared to those in ICIs group, with RR = 1 as the reference. In addition, exploration on different cancers were also conducted (see Table 2), and the results showed that the TCM + ICIs group had higher ORR values in lung cancer, gastric cancer, esophageal cancer, and other tumors (RR = 1.37 [1.19,1.58], RR = 2.07 [1.05,4.08], RR = 1.63 [1.10,2.41], RR = 2.43 [1.09,5.40], respectively).
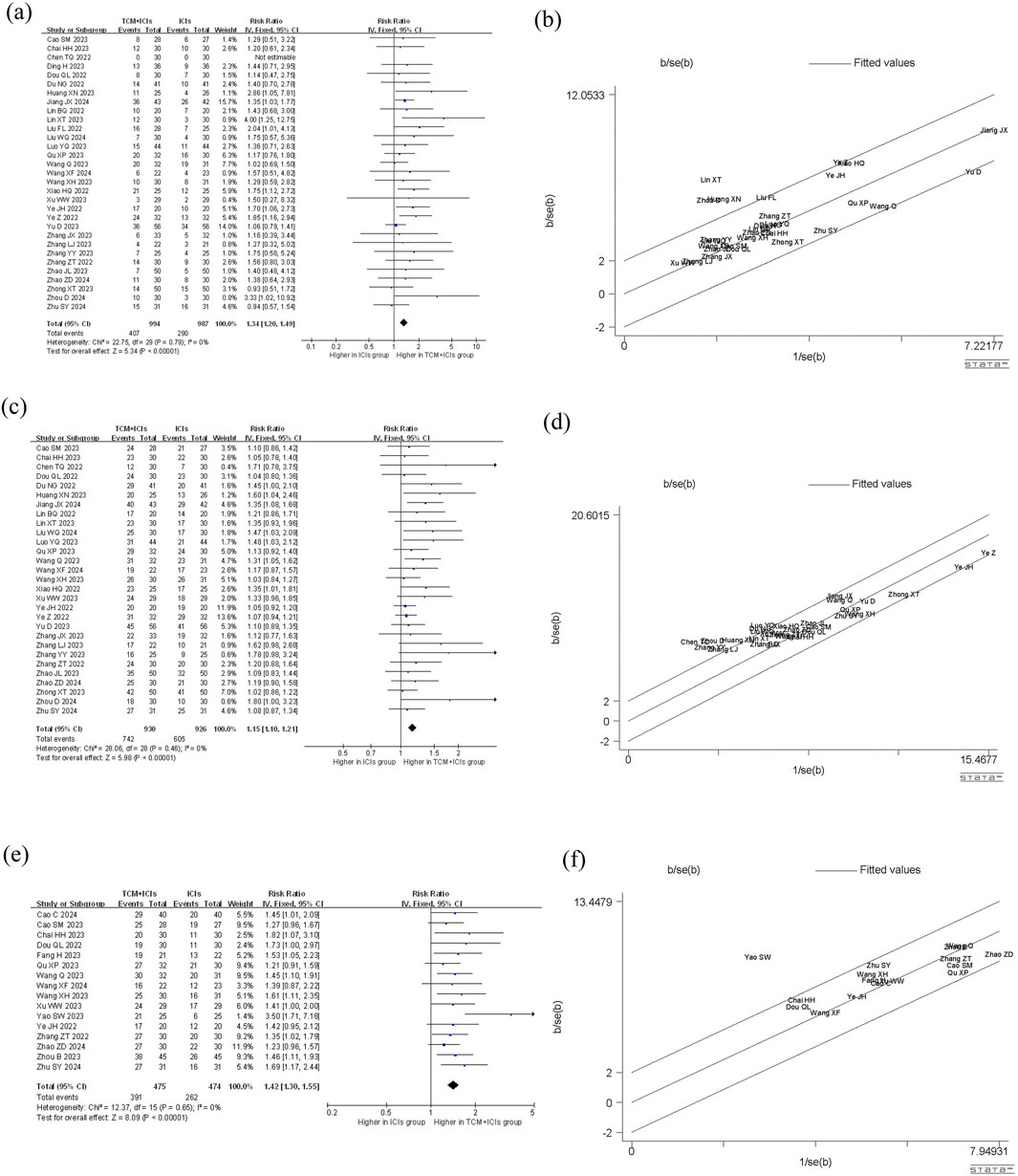
Figure 3. Meta-analysis and Galbr analysis of TCM + ICIs vs. ICIs in cancer—ORR, DCR, TCM Therapeutic Evaluation: (a) Meta-analysis of TCM + ICIs vs. ICIs in cancer—ORR; (b) Galbr analysis of TCM + ICIs vs. ICIs in cancer—ORR; (c) Meta-analysis of TCM + ICIs vs. ICIs in cancer—DCR; (d) Galbr analysis of TCM + ICIs vs. ICIs in cancer—DCR; (e) Meta-analysis of TCM + ICIs vs. ICIs in cancer—TCM Therapeutic Evaluation; (f) Galbr analysis of TCM + ICIs vs. ICIs in cancer—TCM Therapeutic Evaluation.
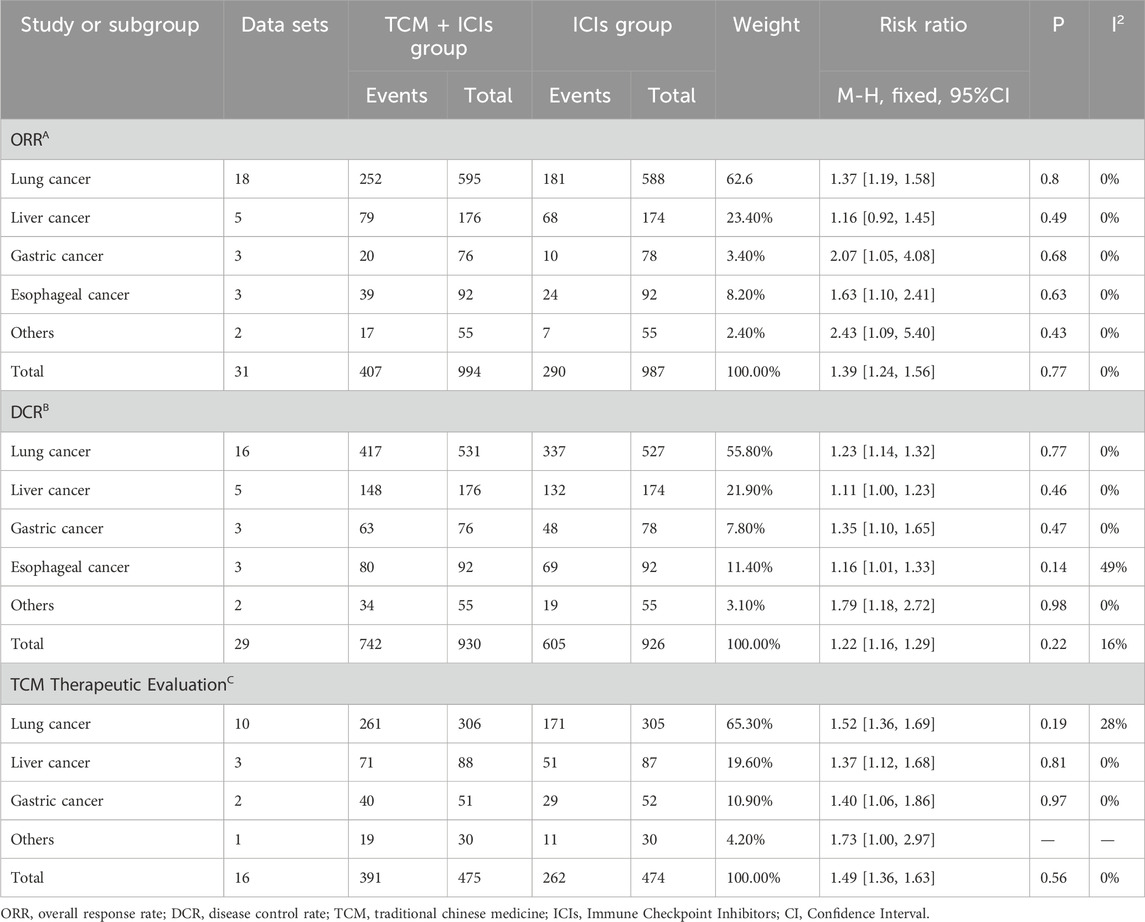
Table 2. Subgroup analysis of TCM + ICIs vs. ICIs in cancer. (A)ORR; (B)DCR; (C)TCM Therapeutic evaluation.
TCM + ICIs vs. ICIs in cancer—Disease Control Rate (DCR)
There are a total of 29 studies involving DCR, and the results of meta-analysis and heterogeneity analysis are shown in Figure 3. The fixed-effect model was applied (p = 0.46, I2 = 0%). The results showed that the DCR values of TCM + ICIs group were significantly higher than those of ICIs group, with RR 1.15 (1.10, 1.21). The subgroup analysis results of different cancers also demonstrated that regardless of the type of cancer, patients in TCM + ICIs group have higher DCR in Table 2.
TCM + ICIs vs. ICIs in cancer—Cd4+T/CD8+T
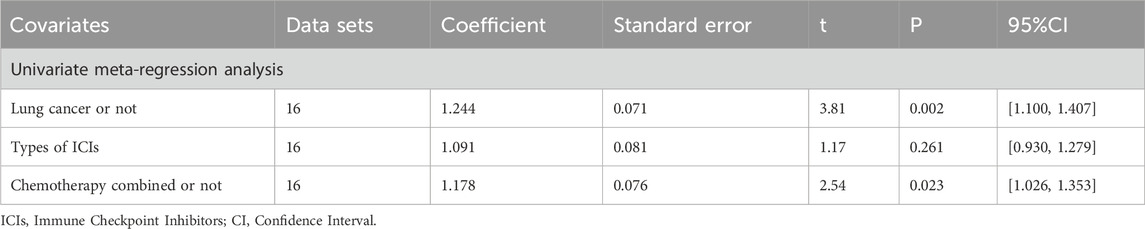
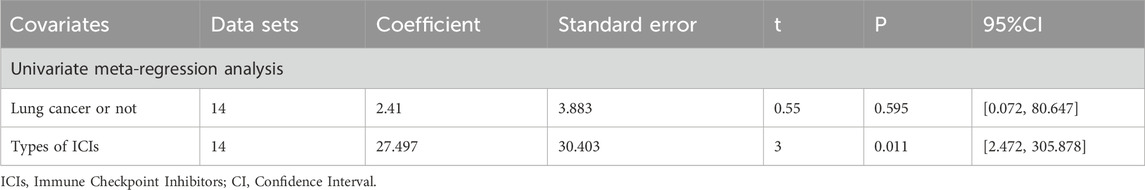
The meta-analysis and heterogeneity analysis of 16 studies are shown in Figure 4. There is obvious heterogeneity (p < 0.05, I2 = 96%), so the random-effect model is the best choice. The CD4+T/CD8+T ratio in TCM + ICIs group was significantly higher than that in ICIs group, with WMD 0.25 (0.15, 0.35). Due to the presence of high heterogeneity, subgroup analysis and meta-regression were performed to further explore its sources. The results in Table 3 showed that heterogeneity among subgroups decreased when grouped based on different cancers or ICIs, but it cannot be determined whether it is the source of heterogeneity. Therefore, the results of meta-regression are particularly important. The results in Table 4 suggest that whether it is lung cancer and whether both groups receive chemotherapy regimens may be sources of high heterogeneity (p < 0.05). Unfortunately, further multi-meta-regression was not performed.
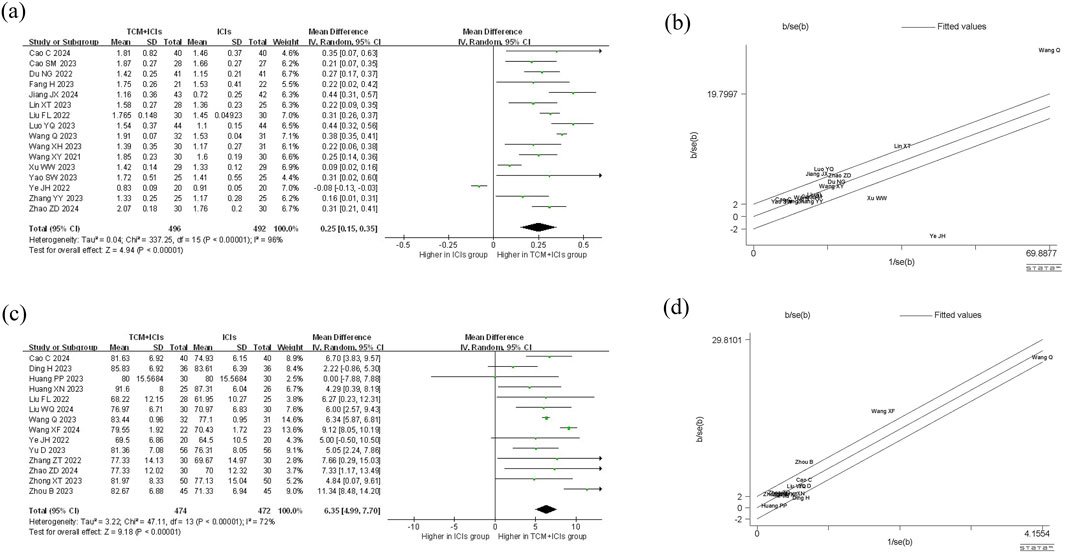
Figure 4. Meta-analysis and Galbr analysis of TCM + ICIs vs. ICIs in cancer—CD4+T/CD8+T or KPS: (a) Meta-analysis of TCM + ICIs vs. ICIs in cancer—CD4+T/CD8+T; (b) Galbr analysis of TCM + ICIs vs. ICIs in cancer—CD4+T/CD8+T; (c) Meta-analysis of TCM + ICIs vs. ICIs in cancer—KPS; (d) Galbr analysis of TCM + ICIs vs. ICIs in cancer—KPS.
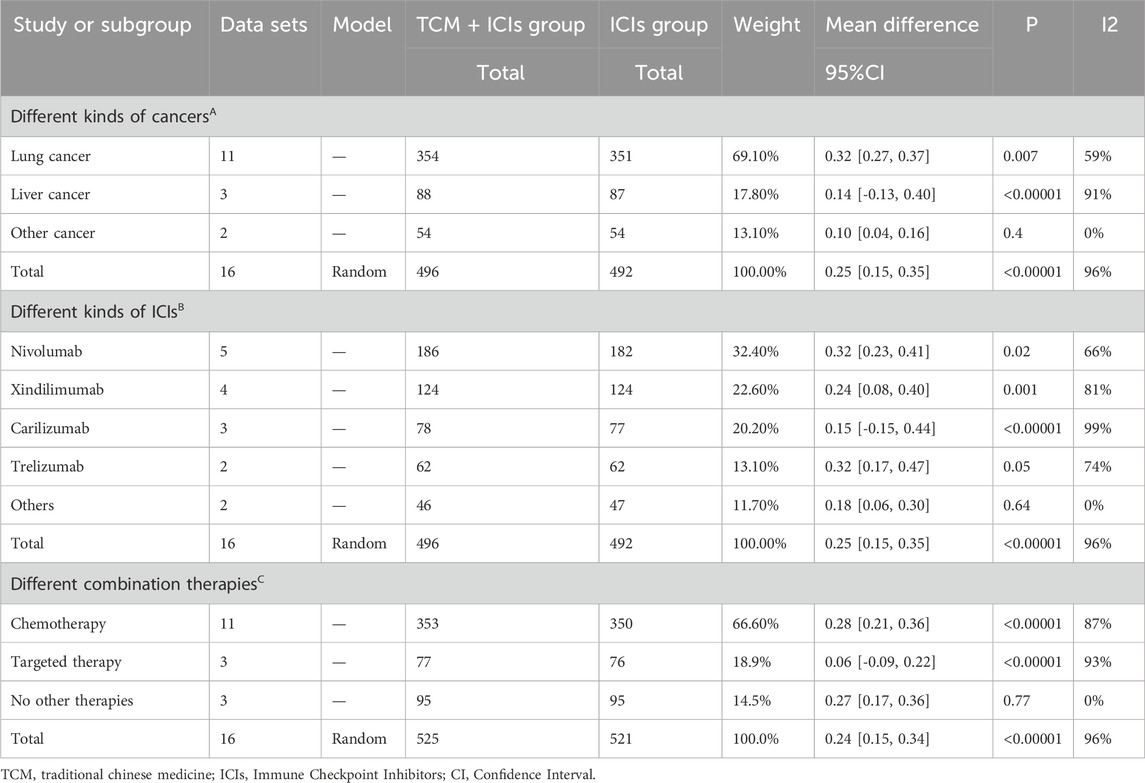
Table 3. Subgroup analysis of TCM + ICIs vs. ICIs in cancer—CD4+T/CD8+T by different factors. (A) Different kinds of cancers; (B) Different kinds of ICIs; (C) Different combination therapies.
TCM + ICIs vs. ICIs in cancer—Progression-Free Survival (PFS)/Overall Survival (OS)
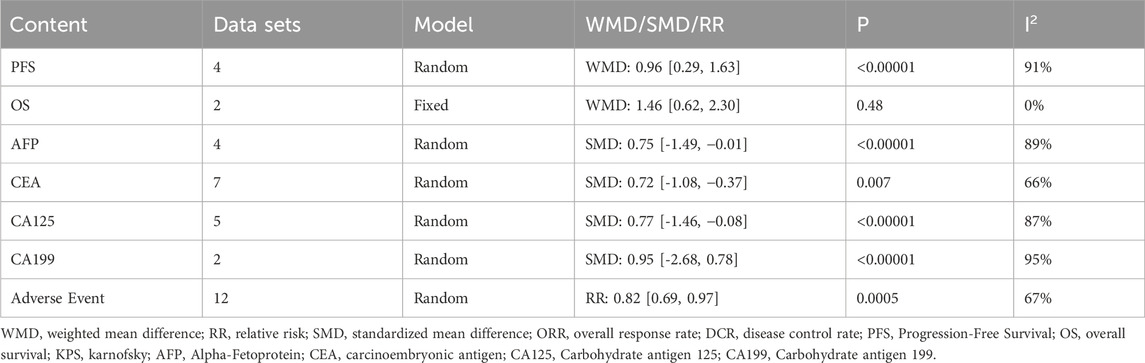
Table 5 contains a comparison of PFS and OS between two groups of patients. The meta-analysis of PFS used a random-effect model (p < 0.05, I2 = 91%), and the results showed that the PFS of TCM + ICIs group was significantly higher than that of ICIs group (WMD = 0.96 [0.29, 1.63]). Due to the small number of studies included (n = 4), heterogeneity exploration was not conducted. Meanwhile, only two studies involved OS, and the heterogeneity among them was not high (p = 0.48, I2 = 0%). After applying the fixed-effect model, the results showed that TCM + ICIs group had a longer OS period, with WMD 1.46 (0.62, 2.30).
TCM + ICIs vs. ICIs in cancer—Karnofsky Performance Status (KPS)
14 studies are related to KPS, and there is significant heterogeneity among them (p < 0.05, I2 = 72%). As shown in Figure 4, the results of the random-effect model demonstrate that the KPS score of TCM + ICIs group is significantly higher than that of the ICIs group, with WMD 6.35 (4.99, 7.70). The Galbr plot also showed high heterogeneity among studies. Thus, subgroup analysis and meta-regression were also performed. Through subgroup analysis of different cancers and ICIs, heterogeneity of each group has decreased, shown in Table 6, suggesting that they may be the source of heterogeneity (Table 7). Univariate meta-regression suggests that different types of ICIs are indeed sources of high heterogeneity (p < 0.05), which deserves further research.
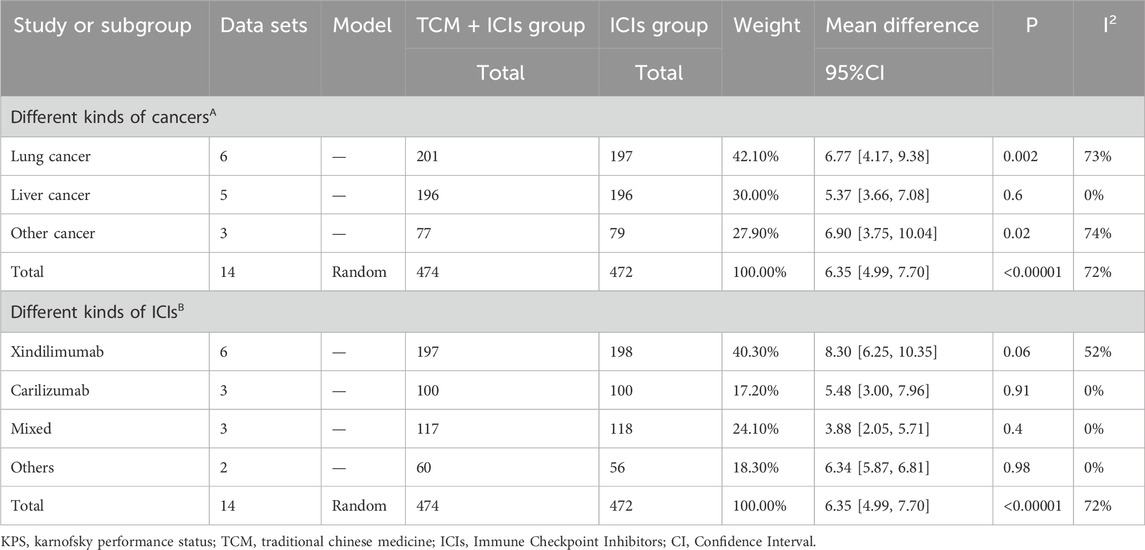
Table 6. Subgroup analysis of TCM + ICIs vs. ICIs in cancer—KPS by different factors. (A) Different kinds of cancers; (B) Different kinds of ICIs.
TCM + ICIs vs. ICIs in cancer—Alpha-Fetoprotein (AFP)
Only four studies involved AFP (Table 5), and there was significant heterogeneity (p < 0.05, I2 = 89%). After the application of the random-effect model, the AFP level in TCM + ICIs group was significantly lower than that in ICIs group, with SMD -0.75 (- 1.49, − 0.01). The source of heterogeneity has not been explored.
TCM + ICIs vs. ICIs in cancer—Carcinoembryonic Antigen (CEA)/carbohydrate antigen 125 (CA125)/carbohydrate antigen 199 (CA199)
Nine studies are included in this section (Table 5), including seven on CEA, five on CA125, and two on CA199. Due to the presence of high heterogeneity, they all adopted a random-effect model (p < 0.05, I2 = 66%; p < 0.05, I2 = 87%; p < 0.05, I2 = 95%, respectively). All results demonstrated that the levels of CEA and CA125 in TCM + ICIs group were significantly lower than those in ICIs group (SMD = −0.72 [-1.08, −0.37]; SMD = −0.77 [-1.46, −0.08], respectively), and there was no significant difference in CA199 levels between the two groups (SMD = −0.95 [-2.68, 0.78]). However, the number of studies included in this section is limited, which also affects the reliability of the conclusion.
TCM + ICIs vs. ICIs in cancer—Adverse event
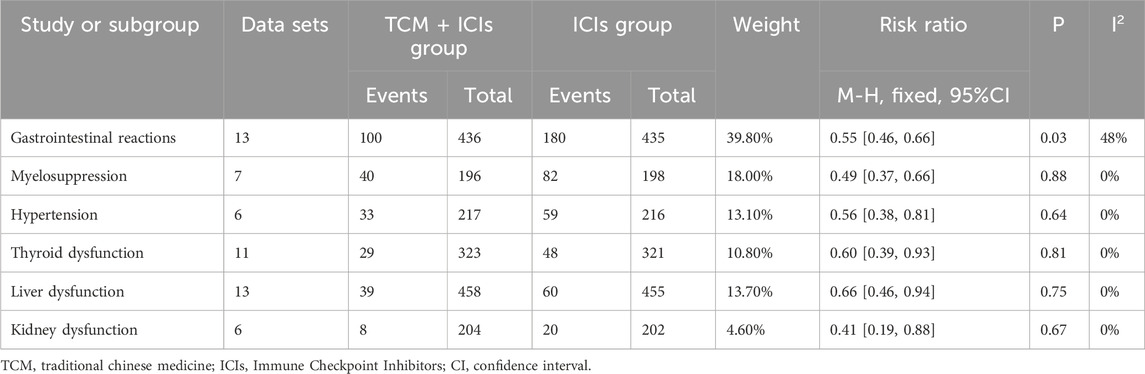
The results of the adverse events of the two groups are presented in Table 5. There was high heterogeneity among the 12 included studies (p = 0.0005, I2 = 67%), therefore a random-effect model was selected. The incidence of adverse events in TCM + ICIs group was significantly lower than that in ICIs group (RR = 0.82 [0.69, 0.97]). Further analysis was conducted based on different adverse events, including gastrointestinal reactions, myelosuppression, hypertension, thyroid dysfunction, liver dysfunction and kidney dysfunction. The final results showed that the various adverse reactions in TCM + ICIs group were significantly lower than those in ICIs group shown in Table 8.
TCM + ICIs vs. ICIs in cancer—TCM therapeutic evaluation
Figure 3 shows the TCM Therapeutic Evaluation of two groups, which includes 16 related studies. Due to insignificant heterogeneity (p = 0.65, I2 = 0%), a fixed-effect model was chosen. The overall TCM therapeutic evaluation of TCM + ICIs group was higher than that of ICIs group, with RR 1.42 (1.30, 1.55). Regardless of the type of cancer, the TCM therapeutic evaluation of TCM + ICIs group was significantly increased shown in Table 2.
Sensitivity analysis
The results of all sensitivity analyses can be found in Additional File 8. The stability of each meta-analysis can be determined by whether the changes of the results are significant or whether there is a reversal after removing each study one by one. After comprehensive evaluation, it was found that all 11 meta-analyses in this study have good stability (with no significant changes in the results).
Publication bias
Only meta-analyses with more than 5 included studies were evaluated for publication bias. The publication bias results of these seven meta-analyses were based on Eggar’s Test (Additional File 9). When it comes to ORR, DCR, CD4+T/CD8+T, Adverse Event, and TCM therapeutic evaluation, significant publication bias cannot be ignored (p < 0.05), while the other two meta-analyses are not. Therefore, the trim-and-filling method was executed to further evaluate whether the presence of publication bias affects the reliability and robustness of the results. This adjustment did not substantially alter or reverse the original pooled results, supporting the robustness and representativeness of the findings despite the presence of publication bias.
Discussion
The immune system plays a critical role in anti-tumor defense by identifying and eliminating mutated cells through immune surveillance, thereby preventing tumor development (Gao et al., 2022). Cancer immunotherapy represents a milestone in oncology, shifting the therapeutic focus from solely targeting malignant cells to modulating the tumor microenvironment (Rui et al., 2023). ICIs, a class of immunotherapy, function by reactivating T cells in the tumor microenvironment and enhancing natural killer (NK) cell activity. This process prevents tumor immune escape and facilitates the conversion of immunologically “cold” tumors to “hot” tumors (Zhang et al., 2022). ICIs are monoclonal antibodies targeting inhibitory checkpoint molecules expressed on antigen-presenting cells (APCs) and CD4+ T cells (Marei et al., 2023). Currently, FDA-approved ICIs include CTLA-4 inhibitors (e.g., ipilimumab), PD-1 inhibitors (e.g., nivolumab, pembrolizumab, cemiplimab), and PD-L1 inhibitors (e.g., atezolizumab, durvalumab, avelumab) (Vafaei et al., 2022). By blocking ligand-receptor interactions on T cells, ICIs reverse immunosuppression and inhibit tumor growth (Andrews et al., 2019). Evidence indicates that ICIs enhance anti-tumor immunity by modulating the PD-1/PD-L1 and CTLA-4/CD80/86 pathways, strengthening tumor antigen recognition and ultimately inducing tumor cell death (Seto et al., 2019). However, ICIs can disrupt peripheral self-tolerance, triggering autoimmune-like inflammatory responses known as immune-related adverse events (irAEs) (Zhou et al., 2024). These irAEs may involve multiple organ systems, such as cardiac, endocrine, gastrointestinal, dermatologic, and renal, and can lead to significant inflammation and visceral toxicity (Ramos-Casals et al., 2022). Identifying strategies to mitigate the incidence and severity of irAEs remains a key research priority.
In China, Traditional Chinese Medicine (TCM) represents a major therapeutic modality for cancer treatment. TCM derives from three primary sources: botanical, animal, and microbial materials. Its therapeutic effects are not attributable to isolated drugs but arise from the synergistic interactions among drug combinations and complex interactions, producing multi-target regulatory activities that collectively exert anti-tumor effects. Numerous studies have confirmed that various traditional Chinese medicines and their main metabolites can inhibit tumor progression, alleviate radiotherapy- and chemotherapy-induced side effects, and improve survival in cancer patients (Li et al., 2012). Proposed anti-tumor mechanisms of TCM include the suppression of cancer cell proliferation, migration, and invasion, promotion of tumor vascular normalization, and interference with metastatic processes (Cheng et al., 2021; Chen et al., 2022). A growing body of clinical and preclinical evidence supports the combined use of TCM with immunotherapy, demonstrating that such combinations can enhance efficacy, reverse drug resistance, and reduce adverse reactions (Zhang et al., 2020). It was confirmed through in vivo and in vitro experiments that bufonidin can reverse the activation of phosphatidylinositol 3-kinase (PI3K)/(Serine/Threonine Kinase B) AKT/(mammalian target of rapamycin) mTOR signaling pathway induced by bifunctional apoptosis regulator (BFAR), and enhance the efficacy of combination with ICIs (Chen G. et al., 2023). Cordycepin, combined with anti–CTLA-4 therapy, alleviated CD8+ T cell exhaustion in the tumor microenvironment by upregulating chemokine expression (Chen L. et al., 2023). Similarly, the combination of evodiamine and anti–PD-1 therapy more effectively suppressed Lewis lung cancer growth by increasing CD8+ T cell infiltration in blood, tumor, and spleen, reducing Treg proportions, and promoting the secretion of cytokines such as Tumor Necrosis Factor-alpha (TNF-α), Recombinant Granzyme B (GZMB), and Interferon-gamma (IFN-γ) (Jiang et al., 2020). These findings collectively indicate that TCM and ICIs can act synergistically, suggesting a promising approach for enhancing cancer treatment outcomes.
This meta-analysis evaluated 41 randomized controlled trials investigating the efficacy of TCM combined with ICIs in cancer treatment. All included studies were conducted in China and published within the past 5 years. Although most reported random allocation, blinding procedures were rarely described. The results showed that the ORR, DCR, CD4+T/CD8+T, PFS, OS, KPS, adverse events, and TCM therapeutic evaluation of the TCM + ICIs group were significantly higher than those of the ICIs group. On the contrary, the AFP, CEA, and CA125 levels in TCM + ICIs group were significantly lower than those in ICIs group, while CA199 showed no significant difference. These findings suggest that TCM combination therapy may enhance immune function, improve performance status, reduce tumor marker levels, and ameliorate treatment-related toxicity. However, the efficacy of TCM + ICIs appears to vary by cancer type. The present analysis primarily included studies on respiratory and digestive tract tumors. For respiratory cancers, TCM + ICIs consistently outperformed ICIs monotherapy. Among gastrointestinal cancers, effects were more heterogeneous; notably, in liver cancer, no significant differences were observed in ORR, DCR, or CD4+/CD8+ ratio between groups, whereas benefits were evident in other digestive malignancies. These discrepancies may reflect the limited number of available studies or differential tumor biology and TCM sensitivity, highlighting the need for further clinical validation. Furthermore, the overall incidence of adverse reactions and the incidence of adverse reactions in each system in TCM + ICIs group were lower than those in ICIs group. From the perspective of modern pharmacological research, some active metabolites in TCM can exert a synergistic effect with immunosuppressants to reduce the occurrence of adverse reactions. TCM can affect enzyme activity, regulating the metabolic rate of immunosuppressants in the body, avoiding drug accumulation, and reducing adverse reactions such as liver and kidney damage (Wang S. et al., 2021). Meanwhile, it can alleviate excessive inflammation caused by immunosuppressants and reduce inflammation related tissue damage by relying on its own anti-inflammatory and oxygen free radical scavenging effects (Zhu et al., 2022). In addition, the protective effect of TCM on organs can weaken the direct stimulation and damage of immunosuppressants, thereby reducing adverse reactions (Ren et al., 2021).
The heterogeneity among studies on ORR, DCR, OS, and TCM therapeutic evaluation is not high, so fixed-effect models have been well applied. However, significant heterogeneity was observed in other outcome analyses, necessitating investigation into its sources. Given the varying number of studies across outcomes, subgroup analysis and meta-regression were only feasible for selected endpoints. When CD4+T/CD8+T is grouped based on tumor type and ICIs type, the heterogeneity among different subgroups decreases. The conclusion drawn from meta-regression is that whether it is lung cancer and whether chemotherapy treatment is applied may both be sources of heterogeneity, suggesting that TCM treatment may be more effective for lung cancer and the possible clinical efficacy of TCM with immunotherapy and chemotherapy. During the subgroup analysis of KPS, the heterogeneity among subgroups of different tumors or ICIs was significantly reduced. Further meta-regression analysis shows that the source of heterogeneity is the type of ICIs. These findings suggest that TCM’s therapeutic effects may vary by tumor type and ICI agent. Tumor-specific pathophysiology and ICI mechanism may influence TCM compatibility and efficacy. Currently, TCM + ICIs show consistent benefit in lung cancer across ICI types. As ICI diversity and indication breadth continue to expand, future research should clarify which ICIs are most suitable for specific patient subgroups and baseline characteristics, enabling treatment personalization and optimized resource use. If more systematic and comprehensive research can be conducted in the later stage, it is an essential part of improving clinical efficacy and avoiding resource waste. The reliability and robustness of the results are crucial for drawing correct conclusions. Sensitivity analysis confirmed the stability of all meta-analyses. However, Egger’s test indicated potential publication bias for ORR, DCR, CD4+/CD8+T, adverse events, and TCM therapeutic evaluation. Subsequent trim-and-filling analysis confirmed that the pooled results remained robust despite such bias, underscoring the clinical relevance of this study.
As the first meta-analysis to assess the clinical efficacy of TCM combined with ICIs for cancer, this review has several limitations. First, the number of included studies remains limited, with strong representation of lung cancer but relatively few trials on other common malignancies such as liver or gastric cancer. Second, all studies were conducted in China; although they cover multiple regions, potential ethnic variations in treatment response cannot be ruled out. As numerous preclinical studies support TCM’s anti-tumor properties, international clinical trials are warranted to assess the generalizability of TCM + ICI therapy. Third, in the quality assessment, most studies were rated as having “unclear” risk of bias across several domains, which may compromise the overall credibility of the evidence and limit its direct clinical applicability. Finally, although this meta-analysis evaluated multiple efficacy endpoints, incomplete reporting in many publications constrained deeper subgroup analysis. It is suggested that future clinical studies should adopt more comprehensive and standardized reporting to facilitate more precise evidence synthesis.
Conclusion
Compared with ICIs monotherapy, the combination of TCM and ICIs significantly improves ORR, DCR, CD4+/CD8+ T-cell ratio, PFS, OS, KPS, and TCM therapeutic evaluation, while reducing levels of AFP, CEA, and CA125 and the incidence of adverse events. Subgroup analysis and meta-regression revealed that heterogeneity in CD4+/CD8+ T-cell ratio was influenced by tumor type and concomitant chemotherapy, while heterogeneity in KPS was associated with ICI type. Combining sensitivity analysis, publication bias analysis, and the trim-and-filling method, the results of our studies are robust and reliable. However, our research is still limited in terms of quantity and region, and we hope more clinicians and researchers will engage in this field to contribute higher-quality data and more comprehensive insights.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
YK: Software, Formal Analysis, Writing – original draft. YP: Formal Analysis, Software, Writing – original draft. XH: Writing – original draft, Data curation, Validation. XB: Writing – original draft, Data curation, Validation. XL: Writing – original draft, Investigation, Methodology. MZ: Methodology, Investigation, Writing – original draft. YW: Writing – original draft, Methodology, Investigation. TJ: Supervision, Project administration, Writing – review and editing, Conceptualization, Writing – original draft. GZ: Writing – review and editing, Supervision, Writing – original draft, Conceptualization, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Youth Fund of the National Natural Science Foundation of China, No. 82204950. Zhejiang Provincial Natural Science Foundation Youth Project, No. LQ23H270013. Zhejiang Province Traditional Chinese Medicine Science and Technology Plan Youth Talent Support Program Project, No. 2025ZR102. State Administration of Traditional Chinese Medicine Science and Technology Department - Zhejiang Provincial Administration of Traditional Chinese Medicine joint science and technology plan key research project, No. GZY-ZJ-KJ-23094. Chunyan Traditional Chinese Medicine Development Special Fund Achievement Transformation Research Project, No. CY202302.
Acknowledgments
We would like to deliver our thanks to Shan XL and Jie Hu from the first affiliated hospital of Zhejiang Chinese Medical University for their encouragement and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1661503/full#supplementary-material
References
Andrews, L. P., Yano, H., and Vignali, D. A. A. (2019). Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat. Immunol. 20, 1425–1434. doi:10.1038/s41590-019-0512-0
Bae, W. K., Lee, B. C., Kim, H. J., Lee, J. J., Chung, I. J., Cho, S. B., et al. (2022). A phase I study of locoregional high-dose autologous natural killer cell therapy with hepatic arterial infusion chemotherapy in patients with locally advanced hepatocellular carcinoma. Front. Immunol. 13, 879452. doi:10.3389/fimmu.2022.879452
Bagchi, S., Yuan, R., and Engleman, E. G. (2021). Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 16, 223–249. doi:10.1146/annurev-pathol-042020-042741
Brahmer, J. R., Abu-Sbeih, H., Ascierto, P. A., Brufsky, J., Cappelli, L. C., Cortazar, F. B., et al. (2021). Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer, 9.
Bray, F., Laversanne, M., Sung, H., Ferlay, J., Siegel, R. L., Soerjomataram, I., et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263. doi:10.3322/caac.21834
Cao, S. (2023). Clinical efficacy observation of Yangzheng Xiaoji Capsules Combined with camrelizumab and apatinib in the treatment if middle and advanced hepatocellular carcinoma. Master's thesis from Anhui University of Traditional Chinese Medicine.
Cao, W., Chen, H. D., Yu, Y. W., Li, N., and Chen, W. Q. (2021). Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin. Med. J. Engl. 134, 783–791. doi:10.1097/CM9.0000000000001474
Cao, C., Liu, Z., Zhang, X., and Chen, K. (2024). A clinical study on the effect of Wenyang Fuzheng Formula combined with PD-1 immunotherapy on the microenvironment of Yang deficiency type of liver cancer. Labeled Immunoassays and Clin Med 31, 1042–1048.
Chai, H. (2023). Efficacy and safety of Yiqi Tongluo Jiedu decoction combined with chemotherapy and immunotherapy for advanced lung adenocarcinoma. Master's thesis Anhui Univ. Traditional Chin. Med.
Chen, T. (2022). Clinical Study of astragalus polysaccharide injection combined with camrelizumab and apatinib in the treatment of Qi-deficiency lung cancer. Master's thesis from Tianjin University of Traditional Chinese Medicine.
Chen, F., Kolben, T., Meister, S., Czogalla, B., Kolben, T. M., Hester, A., et al. (2022). The role of resveratrol, Sirtuin1 and RXRα as prognostic markers in ovarian cancer. Arch. Gynecol. Obstet. 305, 1559–1572. doi:10.1007/s00404-021-06262-w
Chen, G., Zhang, H., Sun, H., Ding, X., Liu, G., Yang, F., et al. (2023a). Bufalin targeting BFAR inhibits the occurrence and metastasis of gastric cancer through PI3K/AKT/mTOR signal pathway. Apoptosis 28, 1390–1405. doi:10.1007/s10495-023-01855-z
Chen, L., Zheng, X., Huang, H., Feng, C., Wu, S., Chen, R., et al. (2023b). Cordycepin synergizes with CTLA-4 blockade to remodel the tumor microenvironment for enhanced cancer immunotherapy. Int. Immunopharmacol. 124, 110786. doi:10.1016/j.intimp.2023.110786
Cheng, L., Liu, W., Zhong, C., Ni, P., Ni, S., Wang, Q., et al. (2021). Remodeling the homeostasis of pro- and anti-angiogenic factors by Shenmai injection to normalize tumor vasculature for enhanced cancer chemotherapy. J. Ethnopharmacol. 270, 113770. doi:10.1016/j.jep.2020.113770
Cunningham, M., Gupta, R., and Butler, M. (2024). Checkpoint inhibitor hepatotoxicity: pathogenesis and management. Hepatology 79, 198–212. doi:10.1097/HEP.0000000000000045
Ding, H., Chen, Z., Yu, H., Feng, Z., and Shi, L. (2024). Efficacy of Fried Licorice Decoction Plus-minus combined with PD-1 for advanced non-small cell lung cancer with Qi-Yin deficiency. Chin. J. Clin. Oncol. 51, 461–466.
Dou, Q. (2022). “Clinical Study of Jianpi Huatan Quyu recipe combined with nab-paclitaxel,” in Platinum and camrelizumab in the treatment of advanced esophageal squamous cell carcinoma. Master's thesis from. Nanjing University of Chinese Medicine.
Du, N., and Liu, Q. (2022). The efficacy and immune function of the combination of spleen tonifying and lung tonifying therapy and PD-1 monoclonal antibody in neoadjuvant chemotherapy for advanced non-small cell lung cancer. J. Clin. Res. 39, 294–297.
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. Bmj 315, 629–634. doi:10.1136/bmj.315.7109.629
Fang, H. (2023). From the perspective of immune regulation to explore the eight flavor soup effect on immunocombined with chemotherapy. Master's thesis from Anhui University of Traditional Chinese Medicine.
Gao, W., Wang, X., Zhou, Y., Wang, X., and Yu, Y. (2022). Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target Ther. 7, 196. doi:10.1038/s41392-022-01046-3
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. Bmj 327, 557–560. doi:10.1136/bmj.327.7414.557
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Bmj 343, d5928. doi:10.1136/bmj.d5928
Hu, M., Yao, W., and Shen, Q. (2022). Advances and challenges of immunocheckpoint inhibitors in the treatment of primary liver cancer. Front. Genet. 13, 1005658. doi:10.3389/fgene.2022.1005658
Huang, X. N., Zhu, C., Li, Y., and Jin, C. (2023). Weidiao-3 mixture improves the clinical efficacy of immunotherapy for advanced gastric cancer by regulating intestinal flora. Acta Acad. Med. Sin. 45, 581–590. doi:10.3881/j.issn.1000-503X.15496
Huang, P. P., Shu, Y., Sun, H., Chen, T., Jiang, Y., Ma, M., et al. (2024). Clinical Study on the use of sini decoction in the treatment of 30 cases of advanced primary liver cancer with Yang deficiency. Jiangsu J. Traditional Chin. Med. 56, 39–42.
Jiang, J., and Fang, Y. (2024). Application value of Buzhong Yiqi decoction in immunotherapy of NSCLC with negative driver gene. Int. Med. Health Guid. News 2, 305–309.
Jiang, Z. B., Huang, J. M., Xie, Y. J., Zhang, Y. Z., Hang, Y. Z., Chang, C., et al. (2020). Evodiamine suppresses non-small cell lung cancer by elevating CD8(+) T cells and downregulating the MUC1-C/PD-L1 axis. J. Exp. Clin. Cancer Res. 39, 249. doi:10.1186/s13046-020-01741-5
Li, J., Li, L., Liu, R., and Lin, H. S. (2012). Establishing Chinese medicine characteristic tumor response evaluation system is the key to promote internationalization of Chinese medicine oncology. Chin. J. Integr. Med. 18, 730–736. doi:10.1007/s11655-012-1254-0
Lin, X. T. (2023). Study of shenqi Fuzheng Injection combined camrelizumab with chemotherapy in the treatment of advanced non-small cell lung cancer. Master's thesis from Guangzhou University of Chinese Medicine.
Lin, B. Q., Zeng, L., and Li, S. (2022). Effect of Fuzheng Jiandu formula regulating Thl/Th2 immune balance on immunotherapy of advanced lung cancer and Study on its mechanism. Shanxi J TCM. 38, 18–20.
Liu, T., and Wang, J. (2024). The effect of xiaoaiping combined with camrelizumab on Serum VEGF and tumor markers in advanced esophageal cancer. China Mod. Dr. 62, 71–73.
Liu, W., and Xia, L. (2024). Efficacy observation of Fuzheng Sanjie Formula, camrelizumab and apatinib on advanced metastatic esophageal squamous carcinoma with syndrome of zheng-deficiency-phlegm-stasis. Clin. J. Traditional Chin. Med. 36, 939–944.
Liu, F. L., Ma, L., Song, R., and Huang, Y. (2022). The effect of modified Xiaoyan decoction combined with PD-1 monoclonal antibody in neoadjuvant therapy for advanced NSCLC and its significance on immune function. Chin. J. Lung Dis. 15, 79–81.
Luo, Y. (2023). Observation of the therapeutic effect of Jianpi Bufei formula combined with PD-1 monoclonal antibody and DP regimen in the treatment of non-small cell lung cancer. J. Sichuan Traditional Chin. Med. 41, 98–101.
Luo, D., Wan, X., Liu, J., and Tong, T. (2018). Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 27, 1785–1805. doi:10.1177/0962280216669183
Marei, H. E., Hasan, A., Pozzoli, G., and Cenciarelli, C. (2023). Cancer immunotherapy with immune checkpoint inhibitors (ICIs): potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int. 23, 64. doi:10.1186/s12935-023-02902-0
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097. doi:10.1371/journal.pmed.1000097
Qu, X. P. (2023). Observation on the clinical efficacy of Bufei decoction combined with chemotherapy and immunotherapy for advanced non-small cell lung cancer with lung Qi deficiency syndrome. Master's thesis Hubei Univ. Chin. Med.
Ramos-Casals, M., Brahmer, J. R., Callahan, M. K., Flores-Chávez, A., Keegan, N., Khamashta, M. A., et al. (2022). Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 6, 38. doi:10.1038/s41572-020-0160-6
Ren, W., Liang, P., Ma, Y., Sun, Q., Pu, Q., Dong, L., et al. (2021). Research progress of traditional Chinese medicine against COVID-19. Biomed. Pharmacother. 137, 111310. doi:10.1016/j.biopha.2021.111310
Rui, R., Zhou, L., and He, S. (2023). Cancer immunotherapies: advances and bottlenecks. Front. Immunol. 14, 1212476. doi:10.3389/fimmu.2023.1212476
Schmidt, F. L., Oh, I. S., and Hayes, T. L. (2009). Fixed-versus random-effects models in meta-analysis: model properties and an empirical comparison of differences in results. Br. J. Math. Stat. Psychol. 62, 97–128. doi:10.1348/000711007X255327
Seto, T., Sam, D., and Pan, M. (2019). Mechanisms of primary and secondary resistance to immune checkpoint inhibitors in cancer. Med. Sci. (Basel). 7.
Shi, J., Luo, D., Wan, X., Liu, Y., Liu, J., Bian, Z., et al. (2023). Detecting the skewness of data from the five-number summary and its application in meta-analysis. Stat. Methods Med. Res. 32, 1338–1360. doi:10.1177/09622802231172043
Upadhyay, R., Elguindy, A. N. M., Salts, L., Donovan, K., Sengupta, S., Wang, K., et al. (2025). Boswellia Serrata for cerebral radiation necrosis after radiosurgery for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 122, 1282–1291. doi:10.1016/j.ijrobp.2025.02.016
Vafaei, S., Zekiy, A. O., Khanamir, R. A., Zaman, B. A., Ghayourvahdat, A., Azimizonuzi, H., et al. (2022). Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 22, 2. doi:10.1186/s12935-021-02407-8
Wan, X., Wang, W., Liu, J., and Tong, T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 14, 135. doi:10.1186/1471-2288-14-135
Wang, X. Y. (2021). Clinical observation of shenqi Yifei decoction in treating advanced non-small cell lung cancer with deficiency of Qi and yin. Master's thesis from Shandong University of Traditional Chinese Medicine.
Wang, Q. (2023a). Clinical observation of modified Liujunzi decoction combined with tirelizumab an chemotherapy in the first-line treatment of advanced lung squamous cell carcinoma. Master's thesis Anhui Univ. Traditional Chin. Med.
Wang, X. H. (2023b). Clinical and mechanism Study of dushen decoction combined with chemotherapy and immunotherapy for advanced lung squamous cell carcinoma. Master's thesis Shandong Univ. Traditional Chin. Med.
Wang, X. F. (2024). Clinical observation of the combination of Jianpi Huatan prescription with PD-1 inhibitor and the SOX regimen in the treatment of advanced gastric cancer with spleen deficiency combined with phlegm and stasis syndrome. Master's thesis from Nanjing University of Chinese Medicine.
Wang, K., Chen, Q., Shao, Y., Yin, S., Liu, C., Liu, Y., et al. (2021a). Anticancer activities of TCM and their active components against tumor metastasis. Biomed. Pharmacother. 133, 111044. doi:10.1016/j.biopha.2020.111044
Wang, S., Fu, J. L., Hao, H. F., Jiao, Y. N., Li, P. P., and Han, S. Y. (2021b). Metabolic reprogramming by traditional Chinese medicine and its role in effective cancer therapy. Pharmacol. Res. 170, 105728. doi:10.1016/j.phrs.2021.105728
Wang, Z., Wang, Y., Gao, P., and Ding, J. (2023a). Immune checkpoint inhibitor resistance in hepatocellular carcinoma. Cancer Lett. 555, 216038. doi:10.1016/j.canlet.2022.216038
Wang, L., Wang, M., and Kan, C. (2023b). Effects of Jianpi Huatan xiaoying decoction on autoimmunity, swallowing function and Serum MIP-1, sIL-2R and S-TKl in patients with thyroid dysfunction induced by immunotherapy of lung cancer. Eval. analysis drug-use Hosp. China 23, 935–939.
Wang, X., Chen, H., Hong, W., Yang, Y., Wang, S., Wang, C., et al. (2024). Qingfei tiaoqi decoction can reduce the immune checkpoint inhibitor-related adverse events in lung cancer patients by regulating the intestinal flora structure. Mod. Oncol. 32, 890–895.
Xiang, Y., Guo, Z., Zhu, P., Chen, J., and Huang, Y. (2019). Traditional Chinese medicine as a cancer treatment: modern perspectives of ancient but advanced science. Cancer Med. 8, 1958–1975. doi:10.1002/cam4.2108
Xiao, H. Q., Li, X., Xie, J., and Xie, S. (2022). Combination of traditional Chinese medicine decoction and immunotherapy for advanced lung cancer in elderly patients research on the application effect in patients. Electron. J. Clin. Med. Literature 48, 52–54.
Xu, W. W. (2023). Clinical observation on the third-line treatment of advanced gastric cancer with Wenyang Tongluo Formula combined with PD-1 inhibitor, apatinib and tegafur. Master's thesis from Anhui University of Traditional Chinese Medicine.
Yang, H. (2023). The clinical research of the treatment on NSCLC patients with Yanghe decoction combined with immune checkpoint inhibitors. Master's thesis from Jiangxi University of Chinese Medicine.
Yao, S. W. (2023). Clinical observation of qigui Buxue syrup on cancer-related fatigue after immunotherapy for non-small cell lung cancer of lung-spleen deficiency type. Master's thesis from Chengde Medical University.
Ye, Z. (2022). Clinical observation of modified Wumei decoction combined with chemotherapy and immunotherapy for mixed cold and heat esophageal cancer. Master's thesis from Henan University of Chinese Medicine.
Ye, J. H., and Fang, Z. (2022). Efficacy observation of Jianpi Huoxue formula and camrelizumab and Lenvatinib on liver cancer. Shanxi J TCM. 38, 35–37.
Yu, Y. X., Wang, S., Liu, Z. N., Zhang, X., Hu, Z. X., Dong, H. J., et al. (2023a). Traditional Chinese medicine in the era of immune checkpoint inhibitor: theory, development, and future directions. Chin. Med. 18, 59. doi:10.1186/s13020-023-00751-7
Yu, D., He, S., Shen, J., Hu, N., Cai, Y., and Cao, T. (2023b). Clinical observation of ruyi Jinhuang Powder combined with ICIs in the treatment of advanced liver cancer complicated with dampness and heat syndrome of liver and gallbladder. China Pharm. 34, 1488–1492.
Zeng, Y. (2018). Advances in mechanism and treatment strategy of cancer. Cell Mol. Biol. (Noisy-le-grand) 64, 1–3.
Zhang, Z. (2022). Clinical observation of jianpi chuji formula combined with immune-checkpoint inhibitors in the treatment of advanced non-small cell lung cancer. Master's thesis from Chengde Medical University.
Zhang, J. (2023a). Clinical Study of shenqi Fuzheng injection combined with endostar and PD-1 inhibitor in the treatment of driver gene negative NSCLC. Master's thesis from Tianjin University of traditional Chinese medicine.
Zhang, L. L. (2023b). Efficacy and safety of hepatoma I formula plus minus plus and minus plus PD-1 inhibitor in the treatment of advanced primary hepatoma. Master's thesis from Gansu University of Chinese Medicine.
Zhang, Y., Lou, Y., Wang, J., Yu, C., and Shen, W. (2020). Research status and molecular mechanism of the traditional Chinese medicine and antitumor therapy combined strategy based on tumor microenvironment. Front. Immunol. 11, 609705. doi:10.3389/fimmu.2020.609705
Zhang, J., Huang, D., Saw, P. E., and Song, E. (2022). Turning cold tumors hot: from molecular mechanisms to clinical applications. Trends Immunol. 43, 523–545. doi:10.1016/j.it.2022.04.010
Zhang, Y. Y., Han, F., Cao, Y., Zhang, Y., Zhang, X., Hu, J., et al. (2023). Effect of Bushen Jiedu Sanjie recipe on Immune Micro-environment of patients with advanced colorectal cancer based on PD-1/PD-L1 pathway. Pract. Pharm. And Clin. Remedies 26, 306–310.
Zhao, Z. D. (2024). Clinical observation on the therapeutic effect of Yiqi shengmai formula combined with herbal moxibustion and immunotherapy on advanced non-small cell lung cancer with Qi and yin deficiency type. Master's thesis from Chengde Medical University.
Zhao, J. L., Xu, H., Yu, X., Lu, H., Wang, X., and Chen, L. (2023). Clinical effect of peitu zishen decoction combined with immunotherapy in the treatment of advanced non-small. Cell Lung Cancer 61, 80–83.
Zhong, X. T. (2023). Clinical observation on the therapeutic effect of Fuhe Beihua Formula combined with Karelizumab and apatinib in the treatment of advanced liver cancer with liver depression and spleen deficiency syndrome after TACE surgery. Master's thesis from Guangxi University of Chinese Medicine.
Zhou, B. (2023). Clinical effect of shenqi busui decoction in treatment of Qi-blood deficiency-type advanced non-small cell lung cancer without driver mutations:an analysis of 45 cases. Master's thesis Hunan J. Traditional Chin. Med.
Zhou, D., and Xie, L. (2024). Clinical Study of guizhifuling pill combined with PD-1 in the treatment of advanced ovarian cancer. Electron. JournaI Pract. GynecoIogical EndocrinoIogy 11, 68–70.
Zhou, P., Gao, Y., Kong, Z., Wang, J., Si, S., Han, W., et al. (2024). Immune checkpoint inhibitors and acute kidney injury. Front. Immunol. 15, 1353339. doi:10.3389/fimmu.2024.1353339
Zhu, S. Y. (2024). Study on the clinical and experimental of Fuzheng Guben Decoction on immune maintenance in advanced lung cancer. Doctor’s thesis from Changchun University of Chinese Medicine.
Zhu, C., Li, K., Peng, X. X., Yao, T. J., Wang, Z. Y., Hu, P., et al. (2022). Berberine a traditional Chinese drug repurposing: its actions in inflammation-associated ulcerative colitis and cancer therapy. Front. Immunol. 13, 1083788. doi:10.3389/fimmu.2022.1083788
Glossary
GCO Global Clinical Operations
ICIs Immune Checkpoint Inhibitors
PD-1 Programmed Cell Death Protein 1
PD-L1 Programmed Death Ligand 1
CTLA-4 Cytotoxic T Lymphocyte Antigen-4
TCM Traditional Chinese Medicine
ORR Overall Response Rate
DCR Disease Control Rate
PFS Progression-Free Survival
OS Overall Survival
KPS Karnofsky Performance Status
AFP Alpha-Fetoprotein
CEA Carcinoembryonic Antigen
CA125 Carbohydrate antigen 125
CA199 Carbohydrate antigen 199
CR Complete remission
PR Partial remission
SD Stable disease
RR Relative Risk
WMD Weighted Mean Difference
CI Confidence Intervals
SMD Standardized Mean Difference
mAb Monoclonal Antibody
APC Antigen Presenting Cells
FDA Food and Drug Administration
irAEs immune related Adverse Events
PI3K Phosphatidylinositol 3-kinase
AKT Serine/Threonine Kinase B
mTOR mammalian target of rapamycin
BFAR Bifunctional Apoptosis Regulator
TME Tumor Microenvironment
TNF-α Tumor Necrosis Factor-alpha
GZMB Recombinant Granzyme B
IFN-γ Interferon-gamma
Keywords: TCM, traditional Chinese medicine, ICIS, immune checkpoint inhibitors, cancer, meta-analysis
Citation: Ke Y, Pan Y, Huang X, Bai X, Liu X, Zhang M, Wei Y, Jiang T and Zhang G (2025) Efficacy and safety of traditional Chinese medicine (TCM) combined with immune checkpoint inhibitors (ICIs) for the treatment of cancer: a systematic review and meta-analysis. Front. Pharmacol. 16:1661503. doi: 10.3389/fphar.2025.1661503
Received: 23 July 2025; Accepted: 17 October 2025;
Published: 31 October 2025.
Edited by:
Rajiv Pathak, Albert Einstein College of Medicine, United StatesReviewed by:
Anusha Aditya, Columbia University, United StatesSoundhar Ramasamy, Kyoto University, Japan
Copyright © 2025 Ke, Pan, Huang, Bai, Liu, Zhang, Wei, Jiang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangji Zhang, emdqdGNtQHpjbXUuZWR1LmNu; Tao Jiang, anR0Y21AemNtdS5lZHUuY24=
†These authors share first authorship
 Yani Ke
Yani Ke Yuyan Pan2,3,4†
Yuyan Pan2,3,4† Guangji Zhang
Guangji Zhang