- 1Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang, China
- 2Key Laboratory and Unit of Infertility in Chinese Medicine, Department of Obstetrics and Gynecology, First Affiliated Hospital, Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang, China
- 3Department of Obstetrics and Gynecology, The First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, China
- 4The First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China
Background: Polycystic ovary syndrome (PCOS) is one of the most common reproductive endocrine disorders affecting women of reproductive age. Insulin resistance (IR) is both a hallmark clinical feature and a key contributor to the pathophysiology of PCOS. Currently, metformin, along with other pharmaceuticals and lifestyle modifications, constitutes the primary approach to enhancing IR in PCOS. Despite demonstrating efficacy, some individuals exhibit suboptimal responses, and prolonged usage may lead to gastrointestinal side effects and other constraints. As an important complementary alternative medicine, recent research has highlighted traditional Chinese medicine (TCM) as a valuable adjunctive therapy for ameliorating IR in PCOS. The integration of TCM into the management of PCOS-related IR offers diverse therapeutic avenues, warranting comprehensive categorization and analysis.
Aim: This review systematically summarizes the pathogenesis and TCM interventions of IR in PCOS and its adverse clinical effects on patients at various stages. It primarily focuses on recent research findings, encompassing both animal studies and human studies, regarding the efficacy of TCM in ameliorating PCOS in conjunction with IR over the past 5 years.
Methods: This article collects relevant literature from databases such as PubMed, Web of Science, Embase, and Cochrane Library from the establishment to 2025. The search utilized the following keywords: Polycystic ovary syndrome, Insulin resistance, Polymorphism, Genetic, Epigenomics, Hyperandrogenism, Inflammation, Microbiota, Mitochondria. This review focuses on recent literature published within the last 5 years to maintain the research’s contemporary relevance. Additionally, classical studies are incorporated to uphold the theoretical framework’s integrity.
Results: The current evidence indicates that TCM contributes to the management of PCOS with IR primarily through modulation of gut microbiota equilibrium, suppression of inflammatory reactions (including reduction of inflammatory cytokines), amelioration of hyperandrogenism, and modulation of insulin signaling pathways.
Conclusion: This review examines current research on the treatment of PCOS complicated by IR using TCM. The findings confirm the efficacy of TCM in ameliorating IR. Discrepancies in dosages and treatment durations of TCM compounds and monomers, as well as batch-to-batch variability in TCM quality, may impact treatment efficacy. Additionally, the translation of animal study outcomes to clinical settings remains unvalidated, necessitating further investigation into the synergistic effects of combined TCM and modern medicine approaches. Future efforts should focus on establishing standardized research protocols and quality control measures, enhancing the evidence base for integrated TCM and Western medicine treatments, and facilitating the translation of basic research findings into clinical practice. These steps are crucial for optimizing the role of TCM in managing PCOS-IR.
1 Introduction
Polycystic ovary syndrome (PCOS) is one of the endocrine and metabolic disorders mainly characterized by polycystic ovarian changes, hyperandrogenemia, insulin resistance (IR), etc. (Joham et al., 2025). Its prevalence shows significant regional differences, with approximately 6%–10% in the Asian population, 5.3%–7.0% in Europe, and 5.0%–10.0% in the United States (Miazgowski et al., 2021; Wu et al., 2021; Chiaffarino et al., 2022; Kim et al., 2022; Yu et al., 2023; Meng et al., 2025). This difference may be related to racial genetics, lifestyle, and differences in diagnostic criteria (Wolf et al., 2018; Guo et al., 2021). IR and hyperinsulinemia (HI) are important clinical features of patients with PCOS. PCOS patients with varying degrees of IR exhibit more reproductive and endocrine abnormalities (Meirow et al., 1995; Belani et al., 2018; Hao et al., 2024). Currently, in clinical management of PCOS-related IR, treatment methods such as drugs like metformin and lifestyle interventions are commonly used (Teede et al., 2023). Although metformin has a definite effect in controlling symptoms, in clinical application, some patients may experience gastrointestinal adverse reactions (such as diarrhea and nausea), leading to a decrease in patient compliance (Melin et al., 2023; Hofmann et al., 2025; Saadati et al., 2025). Lifestyle interventions are greatly affected by individual behavioral habits and have poor long - term maintenance effects (Arentz et al., 2021; Al Wattar et al., 2022). Therefore, more complementary and alternative treatment methods need to be explored. Traditional Chinese medicine (TCM), as an important complementary and alternative medical approach, has a long history in the treatment of metabolic diseases and gynecological disorders. As early as the Spring and Autumn and Warring States periods (770 BC - 221 BC), the ancient Chinese medical classic “Huangdi Neijing” had recorded the basic understanding of the female menstrual cycle and gynecological diseases, providing a theoretical basis for the later treatment of gynecological diseases and metabolic disorders with TCM (Hammes, 2013; Ma et al., 2021). In recent years, the potential of TCM in improving PCOS and related metabolic and reproductive abnormalities has gradually attracted attention (Chen et al., 2023; Fu L.-W. et al., 2024). Each has its own advantages, providing multiple options for clinical treatment.
While previous research has begun to uncover the link between PCOS and IR, there is a lack of comprehensive descriptions of IR in PCOS within the current literature. This review aims to address this gap by reviewing the multifaceted pathogenesis of IR in PCOS, outlining the specific implications of IR for PCOS patients at various stages, and consolidating recent advancements in traditional Chinese medicines (both botanical drug formulas and their derived metabolites) for ameliorating PCOS-related IR. The objective is to bridge existing research deficiencies, amalgamate the aforementioned evidence, and establish a basis for the development of more targeted prevention and treatment approaches in clinical practice.
2 Pathological mechanism of IR in PCOS
2.1 Genetic factors involved in the development of IR in PCOS
The development of PCOS with IR is significantly influenced by genetic factors (Luo et al., 2024; Stamou et al., 2024). Gene polymorphism and epigenetics are the specific topics of discussion in this paragraph.
2.1.1 Genetic polymorphisms contributing to the development of IR in PCOS
Gene polymorphism refers to the presence of multiple genetic variations with a frequency exceeding 1% at a specific gene locus within a population (Townes, 1969; Fareed and Afzal, 2013). These variations can impact phenotypic traits such as disease susceptibility and drug response, serving as the foundation of genetic diversity (Ford, 1957; Townes, 1969). In a Finnish cohort, Zouali et al. (1993)initially documented a link between non-insulin-dependent diabetes and the A2 allele of the XbaI polymorphism in the glycogen synthase gene. Multiple receptor gene polymorphisms have been implicated in the pathophysiology of IR, according to subsequent research (Morris, 1997; Kadowaki et al., 2002). Researchers have discovered a growing number of genetic variants linked to the emergence of IR in PCOS in recent years.
People with the rs4784165GG + GT genotype were shown to have a greater risk of IR than those in the TOX3 rs4784165TT group in a study that included 2082 Chinese Han women with PCOS (Tian et al., 2020). Chen F. et al. (2021) used polymerase chain reaction (PCR) and other analytical methods to analyze 616 PCOS patients and 482 healthy women. In the obese subgroup of PCOS patients, they found that the GALNT2 gene rs4846914AA genotype was associated with significantly higher fasting insulin (FINS) and Homeostatic Model Assessment of Insulin Resistance (HOMA-IR) levels than the rs4846914GG or GA genotype in the non-obese subgroup. Nonetheless, the study did not establish a direct association between the GALNT2 gene rs4846914 and rs214430 Single Nucleotide Polymorphism (SNP) variants with PCOS risk. However, these variants were linked to metabolic traits such as IR. Furthermore, independent research has indicated that the rs1801278 polymorphism of the IRS1 gene, while not influencing susceptibility to PCOS, is correlated with IR (Rasool et al., 2022). Specifically, the GA and AA genotypes at rs1801278 of the IRS1 gene were notably linked to heightened HOMA-IR levels (Rasool et al., 2022). Nevertheless, not every genetic variation was linked to a higher incidence of IR in PCOS individuals. While some genetic variants were not linked to the development of IR, others were found to be linked to a lower risk of IR in PCOS individuals. For instance, the THADA rs13429458 polymorphism did not exhibit a difference in insulin resistance, while the DENND1A rs2479106GG and AG genotypes were linked to a lower risk of IR in PCOS individuals (Tian et al., 2020; Dadachanji et al., 2021).
2.1.2 Epigenetic mechanisms in the development of IR in PCOS
Independent of changes in DNA sequences, epigenetics includes the reversible genetic control of gene expression through processes like DNA methylation and non-coding RNA regulation (Guo et al., 2010; Bannister and Kouzarides, 2011; Greenberg and Bourc’his, 2019). These mechanisms play an essential part in the development and progression of IR in PCOS patients (Kang et al., 2015; Abbott and Dumesic, 2021). While methylation at loci 3 and 4 of the INSR gene was associated with HOMA-IR in PCOS patients, a recent case-control study found a significant correlation between HOMA-IR levels and methylation at locus 4 of the AMHRII gene (Zhong et al., 2021). In a familial case-control investigation, Gao et al. (2024) suggested that by increasing TGF-β1 gene expression, decreased methylation of the promoter region of the transforming growth factor-β1 (TGF-β1) gene may improve IR in PCOS. Zhou et al. (2022) used the KGN human granulosa cell line to either knock down FLOT2 using FLOT2-specific siRNAs or overexpress FTO by transfecting it with pcDNA-FTO plasmids. Their research showed that FTO increased the expression of FLOT2 by decreasing m6A methylation levels and enhancing the stability of FLOT2 mRNA, which in turn caused ovarian granulosa cells to induce IR (Zhou et al., 2022).
IR in PCOS is largely caused by non-coding RNAs, including circular RNAs (circRNAs), long-stranded non-coding RNAs (lncRNAs), and microRNAs (miRNAs) (Lin et al., 2018; Mu J. et al., 2021; Mu L. et al., 2021). By attaching to their target Messenger RNA (mRNA) at several regulatory stages, such as transcription, post-transcription, and translation, miRNAs alter the expression of genes (Guo et al., 2010; Kopp and Mendell, 2018). Zhang et al. (2020b) found that 58 miRNAs were differentially expressed in ovarian tissues in PCOS-IR animal models that were fed a diet high in fat and letrozole. These results provide fresh perspectives on possible treatment targets and approaches that involve ovarian miRNAs in the etiology of IR linked to PCOS. Cumulus cells from PCOS patients with IR and those without insulin resistance (NIR) were used in a recent case-control study (Hu et al., 2020). Molecular biology and bioinformatics analysis revealed that the two groups’ cumulus cells expressed 617 genes and 59 known miRNAs differently (Hu et al., 2020). The study specifically demonstrated how the control of the mitogen-activated protein kinase (MAPK) pathway by the miR-612/Rap1b axis contributes to the growth of IR in PCOS (Hu et al., 2020). Additionally, granulosa cells from PCOS patients showed higher expression of miR-133a-3p and suppression of the PI3K/AKT transmission axis in contrast to normal controls, according to Yang X. et al. (2022). Further research showed that miR-133a-3p affects the expression of proteins linked to glucose metabolism and reduces the activity of the PI3K/AKT pathway, which in turn causes ovarian IR (Yang X. et al., 2022). Furthermore, with the goal to shed light on the interaction between HA and IR in PCOS patients and to propose new research directions, Krentowska et al. (2024) identified miR-27a and miR-320 as prospective biomarkers for impaired glucose metabolism in PCOS with HA.
2.2 HA mediates the development of IR in PCOS
HA is a complex clinical condition characterized by elevated androgen levels, affecting a majority of individuals with PCOS (Stewart and Edwards, 1994; Azziz et al., 2004; Rosenfield and Ehrmann, 2016). This disorder is both a contributing factor and a clinical manifestation of PCOS. Early research by Burghen et al., in 1980 established a close relationship between IR and HA in PCOS (Burghen et al., 1980). Later research on human intervention provided more evidence in favor of the idea that anti-androgenic actions are essential for reducing IR (Elkind-Hirsch et al., 1993; Moghetti et al., 1996). Current understanding suggests that androgens contribute to the development of IR by directly or indirectly affecting the insulin signaling pathway (Cussen et al., 2025). Excessive androgens can directly influence insulin signaling through androgen receptors, while HA may also indirectly disrupt insulin signaling by promoting inflammation (Ignjatović et al., 2023; Su et al., 2025).
According to recent research, HA can also induce the occurrence and development of IR in PCOS by causing abnormalities in other indirect pathways (Zhai et al., 2020; Yuan et al., 2021; Aflatounian et al., 2022; Misra et al., 2024; Farhadi-Azar et al., 2025). HA reduces the level of Kisspeptin through the action of androgen receptor (AR), thereby disrupting the protective function of Kisspeptin in inhibiting the PERK/eIF2α pathway and calcium overload, leading to endoplasmic reticulum homeostasis and ultimately resulting in the occurrence of IR (Yuan et al., 2021). Zhai et al. (2020) noted that HA downregulates the levels of the circadian clock gene BMAL1 in the liver and adipose tissue, thereby inhibiting the function of the NAMPT/NAD/SIRT1 pathway, reducing the expression of glucose transporter4 (GLUT4), and finally results in the development of IR in an animal model of PCOS. The study by Aflatounian et al. (2022) found that the NAD+ level in skeletal muscle tissue mediates HA-induced IR. In addition, recent studies have proposed that prenatal androgen exposure is also one of the important pathophysiological factors for the formation and progression of IR. After constructing an environment of prenatal hyperandrogen exposure by injecting 5 mg of free testosterone into pregnant female rats on the 20th day of pregnancy, it was discovered that the corpulence of their female offspring increased significantly after birth, impaired glucose tolerance occurred at 3 months of age, and it gradually developed into insulin resistance with age (Farhadi-Azar et al., 2025). Interestingly, we found that not all studies seem to propose the theory that androgens are associated with impaired insulin sensitivity. A recent cross-sectional study showed that Dehydroepiandrosterone sulfate (DHEAS) and androstenedione in lean PCOS patients were positively related to insulin sensitivity. This suggests that androgens may protect insulin sensitivity in lean PCOS (Misra et al., 2024). However, the experimental sample was from a single region, and further studies are needed for the extrapolation of the conclusion.
In fact, the pathophysiology of HA in PCOS patients is significantly influenced by IR as well (Cussen et al., 2025; Su et al., 2025). Mechanistically, compensatory HI brought on by IR can increase the ovary’s luteinizing hormone (LH) receptors, making it more receptive to LH and encouraging the release of androgen (Cara and Rosenfield, 1988; Zhang et al., 2000; Rosenfield and Ehrmann, 2016). A key player in androgen biosynthesis, the CYP17-encoded enzyme P450c17α is increased in response to IR and HI, which increases androgen production (Rosenfield and Ehrmann, 2016; Wang et al., 2019; Sanchez-Garrido and Tena-Sempere, 2020; Zhang et al., 2020a). Additionally, HI linked to IR can inhibit the liver’s production of sex hormone-binding globulin (SHBG), increasing free testosterone and androgen biological activity (Deswal et al., 2018). Furthermore, “tissue-selective sensitivity” to insulin in the adrenal gland has been seen in PCOS-IR patients. As a result of IR, HI interacts with the adrenal gland’s insulin receptors, boosting CYP11B1 enzyme activity and subsequently raising the production of 11-oxygenated androgens generated from the adrenal gland (Walzer et al., 2022). The existence of this harmful loop is supported by recent studies. IR and HA in PCOS have a bidirectional pathological link, according to a recent network meta-analysis, and improving insulin sensitivity is essential to breaking this cycle (Xing et al., 2020).
2.3 Inflammation mediates the development of IR in PCOS
Actually, PCOS has long been thought to as a low-grade, chronic inflammation (Zhang Q. et al., 2024). The onset and progression of IR in PCOS individuals are significantly influenced by this persistent low-grade inflammation (Deng et al., 2024; Su et al., 2025). Through a case-control research, Kelly et al. hypothesized as early as 2001 that PCOS patients had a chronic low-grade inflammatory state that is inversely connected with insulin sensitivity (Kelly et al., 2001). A later prospective controlled study also revealed a strong correlation between IR and the inflammatory state in PCOS patients (González et al., 2006). Insulin signaling is primarily impacted by inflammation at the cellular and molecular levels via a variety of mechanisms (Hotamisligil et al., 1994; Yu et al., 2022; Olaniyi et al., 2024). First, by preventing the tyrosine phosphorylation of IRS, the inflammatory factor TNF-α reduces cellular sensitivity to insulin by blocking the downstream transmission of insulin signals (Hotamisligil et al., 1994). Through the JAK-STAT, HIF-1, and PI3K-Akt signaling pathways, inflammatory factors drive the development of PCOS-IR in overweight PCOS patients (Yu et al., 2022). Through the NrF2/HIF1-α pathway, hypothalamic inflammation and pyroptosis also contribute to PCOS-IR episodes (Olaniyi et al., 2024). According to a recent study, monocytes (MNC) from PCOS patients with saturated fat activate the NF-κB pathway, causing an overabundance of TNFα, IL-6, and IL-1β to be secreted. These inflammatory molecules then suppress insulin signaling, which results in PCOS-IR (González et al., 2020; Li X. et al., 2024). Therefore, moderately limiting saturated fat consumption can be a useful strategy for PCOS-IR patients to avoid the onset and progression of PCOS-IR.
2.4 Abnormal structure and function of gut microbiota contribute to the development of IR in PCOS
The diverse microbial population found in the human gut is referred to as the gut microbiota. In areas including immune system modulation, gastrointestinal mucosal barrier protection, and nutritional absorption and digestion, these microbial populations coexist harmoniously with the human body (Noverr and Huffnagle, 2004; Bäckhed et al., 2005). Thus, preserving the gut microbiota’s homeostasis is crucial to preserving the body’s regular physiological processes. Recent research has revealed that the structure and function of the gut microbiota are typically aberrant in PCOS patients (Morris, 2020; Li P. et al., 2023). Furthermore, some research has revealed that PCOS patients with IR and those without IR have quite different gut microbiotas (He and Li, 2021). As a result, there has been a lot of interest in the connection between the gut microbiota and IR, the main pathogenic characteristic of PCOS.
The pathogenic theory of “gut barrier-endotoxemia-inflammation” was put forth by Tremellen and Pearce (2012) in 2012. According to this view, intestinal mucosa tight junction protein function is initially compromised by gut microbial dysbiosis, which permits lipopolysaccharide (LPS) to pass from the gut into the bloodstream. After entering the bloodstream, LPS attaches itself to receptors on target cells, triggering intracellular signaling cascades that increase the release of cytokines that promote inflammation. In the end, this harms the insulin signaling system, which results in IR (Tremellen and Pearce, 2012). According to further research, Bacteroides vulgatus inhibits the production of interleukin-22 (IL-22) by lowering certain bile acids (such glycodeoxycholic acid) that cause the stomach to produce IL-22, which in turn causes PCOS with IR (Qi et al., 2019). According to Yang et al. (2021), there was a notable rise in Bacteroides abundance in PCOS patients and PCOS model mice, which was associated by a drop in cholic acid and an increase in farnesol. Insulin resistance results from this because the farnesoid X receptor (FXR) in the ileum is not sufficiently activated. By attaching to the aryl hydrocarbon receptor (AhR) on the surface of type 3 innate lymphoid cells (ILC3s), Aspergillus tubingensis and its secondary metabolite AT-C1 prevent endogenous ligand activation, suppress IL-22 release, and ultimately cause IR (Wu J. et al., 2025). Apart from the previously described microfunctional abnormalities, it has been established that PCOS-IR is intimately linked to macroabnormalities in the ecological structure of the microbiota and their metabolites. In PCOS patients, the degree of IR is positively connected with a decline in Prevotella_9 abundance (Chen J. et al., 2021). Impaired glucose tolerance is linked to an increase in the Firmicutes/Bacteroidetes ratio and a decrease in the gut microbiota’s α-diversity (Yue et al., 2022). The HOMA-IR and the amount of propionic acid in PCOS patients’ feces have a favorable correlation (Dong et al., 2024). But not every type of gut microbiota causes PCOS-IR to develop. By restoring gut microbiota diversity, increasing the abundance of beneficial bacteria (like Bifidobacterium, Lactobacillus, and Butyricoccus), decreasing the abundance of harmful bacteria (like Helicobacter and Bacteroides), and significantly lowering the levels of pro-inflammatory factors (like IL-6 and TNF-α), Bifidobacterium longum subsp. longum BL21 has recently been demonstrated to significantly improve IR in PCOS mice (Dong et al., 2025). Because the gut microbiota is diverse, strains within the same species may affect diseases differently, and future research into the precise pathogenic pathways is necessary.
Emerging research shows microbiota affects neuroendocrine signaling involved in PCOS. The gut microbiota plays a crucial role in regulating neuroendocrine signals via the “gut-brain axis”, contributing to the pathogenesis of PCOS and associated metabolic disorders, including insulin resistance (Babu et al., 2024; Gautam et al., 2024). Microbial metabolites, such as short-chain fatty acids and tryptophan derivatives, can modulate the central nervous system by circulating in the bloodstream (Eepho et al., 2023; Longo et al., 2023; Monroy et al., 2024). These metabolites influence the secretion pattern of gonadotropin-releasing hormone (GnRH) and disrupt the homeostasis of the hypothalamic-pituitary-ovarian axis (Eepho et al., 2023; Longo et al., 2023; Monroy et al., 2024). Consequently, they trigger metabolic dysregulations like energy metabolism perturbations and IR (Eepho et al., 2023; Longo et al., 2023; Monroy et al., 2024). This pathway of the “gut-brain axis” offers a novel perspective on the link between gut microbiota and PCOS with IR.
2.5 Mitochondrial dysfunction mediates IR in PCOS
As an emerging area that has attracted much attention in the research on the mechanism of IR in PCOS in recent years, the exploration of mitochondrial dysfunction and its molecular regulatory mechanism provides a new perspective for understanding the pathology of the disease (Zhou et al., 2024). Specifically, mitochondrial gene polymorphisms, abnormal gene expression, and dysfunction form a cascading regulatory network that jointly promotes the occurrence and development of the disease (Deer et al., 2025; Kobayashi et al., 2025).
Firstly, in terms of mitochondrial gene polymorphism, specific polymorphisms in the mtDNA D-loop region may be involved in the occurrence and development of IR in patients with PCOS by influencing the association between body mass index and IR (He et al., 2023). Secondly, at the gene expression level, the reduced expression of nuclear-encoded genes in the mitochondrial oxidative phosphorylation (OXPHOS) system may mediate the occurrence and development of insulin resistance in the skeletal muscle tissue of PCOS patients, and the decreased expression of PGC-1α may be a key mediating factor (Skov et al., 2007). A recent study constructed an animal model of PCOS with IR and intervened with the mitochondrial-targeted antioxidant MitoQ (Ding et al., 2019). It was found that mitochondrial DNA (mtDNA) mutations, reduced ATP production, decreased membrane potential, and excessive reactive oxygen species (ROS) could promote the occurrence and development of IR in PCOS by triggering oxidative stress and abnormal apoptosis (Ding et al., 2019). This indicates that abnormal mitochondrial function also plays an important role in the occurrence and development of PCOS complicated with IR.
However, the relationship between mitochondrial dysfunction and IR is not unidirectional. IR, in turn, can exacerbate mitochondrial damage, forming an intertwined vicious cycle. A recent human study on women with PCOS found that compared with healthy women, the mtDNA content in PCOS patients was significantly reduced, accompanied by more obvious metabolic abnormalities (Rojo et al., 2024). After adjusting for the HOMA index, the differences in mtDNA content and oxidation levels between PCOS patients and healthy women lost statistical significance (Rojo et al., 2024). This suggests that IR may be the main driving factor for mitochondrial abnormalities in PCOS patients. Another study indicated that mitochondrial dysfunction (manifested as mtDNA deletion, decreased metabolic flexibility, etc.) is closely related to the occurrence and development of PCOS with IR and may form a vicious cycle through interaction with obesity and IR (Varhegyi et al., 2024). Human studies have shown that the high platelet reactivity in PCOS patients is closely related to mitochondrial dysfunction (impaired integrity, imbalance of fission - fusion) (Randriamboavonjy et al., 2015). After treating them with metformin, it was found that metformin can improve mitochondrial integrity through AMPKα1 - dependent Drp - 1 regulation, thereby reducing high platelet reactivity, and this effect is only observed in PCOS patients with IR (Randriamboavonjy et al., 2015). This provides a new perspective on the cardiovascular risk and mitochondrial dysfunction in PCOS with IR patients. Animal studies have also confirmed the negative effect of IR on mitochondrial function. IR leads to a decrease in the mtDNA copy number in mouse metaphase II (MII) oocytes, disordered mitochondrial distribution in germinal vesicle (GV) and MII oocytes, and abnormal mitochondrial inner membrane potential (increased in the GV stage and decreased in the MII stage), ultimately resulting in damage to mouse oocytes (Ou et al., 2012).
3 Dysregulation of insulin signaling pathways in PCOS involved in the development of IR
An essential biological mechanism underlying the onset and progression of PCOS with IR is the aberrant activation or inhibition of the insulin signaling system. The pathogenic process is significantly influenced by the following critical signaling pathways’ malfunction (Figure 1).
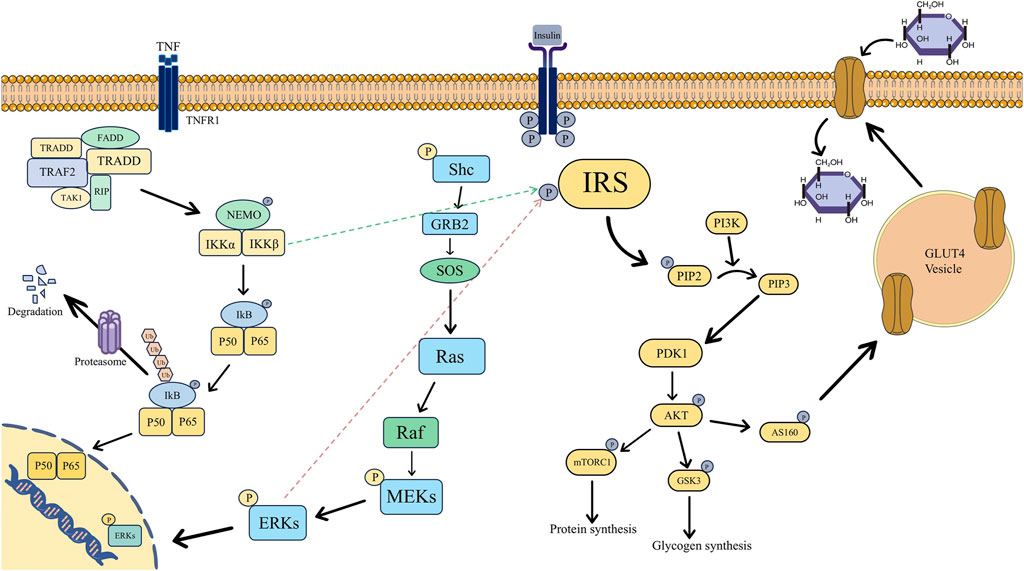
Figure 1. Mechanism diagram of insulin signaling pathways associated with insulin sensitivity in polycystic ovary syndrome, specifically involving the PI3K/AKT, NF-κB, and MAPK pathways. (Abbreviations: P: Phosphorylation, IRS: Insulin Receptor Substrate, PI3K: Phosphoinositide 3-Kinase, PIP2: Phosphatidylinositol 4,5-Bisphosphate, PIP3: Phosphatidylinositol 3,4,5-Trisphosphate, PDK1: 3-Phosphoinositide-Dependent Protein Kinase 1, AKT: Protein Kinase B, mTORC1: mammalian Target of Rapamycin Complex 1, GSK3: Glycogen Synthase Kinase 3, AS160: Akt Substrate of 160 kDa, GLUT4: Glucose Transporter 4, TNF:Tumor Necrosis Factor, TNFR1: Tumor Necrosis Factor Receptor 1, FADD: Fas-Associated Death Domain, TRADD: TNF Receptor-Associated Death Domain, TRAF2: TNF Receptor-Associated Factor 2, TAK1: Transforming Growth Factor-β-Activated Kinase 1, RIP: Receptor-Interacting Protein, NEMO: NF-κB Essential Modulator, IKK: IκB Kinase, IKB: Inhibitor of κB, Ub: Ubiquitin, Shc: Src Homology and Collagen, GRB2: Growth Factor Receptor-Bound Protein 2, SOS: Son of Sevenless, Ras: Rat Sarcoma Viral Oncogene Homolog, Raf: Rapidly Accelerated Fibrosarcoma, MEKs: Mitogen-Activated Protein Kinase Kinases, ERKs: Extracellular Signal-Regulated Kinases).
3.1 Abnormality of the PI3K/AKT signaling pathway mediates the development of IR in PCOS
Under normal circumstances, the tyrosine residues of the β-subunit of the insulin receptor undergo autophosphorylation upon the binding of insulin to its receptor, activating the receptor (Sun et al., 1991). IRS proteins are then recruited and phosphorylated by the active insulin receptor, resulting in their activation (Sun et al., 1991). PI3K is bound to and activated by the active IRS. The transformation of phosphatidylinositol 4,5 bisphosphate (PIP2) on the cell membrane into phosphatidylinositol 3,4,5 trisphosphate (PIP3) is then catalyzed by PI3K. AKT is drawn to the cell membrane by PIP3 (Folli et al., 1992; Hers et al., 2011). Phosphatidylinositol-dependent kinase-1 (PDK1) and mammalian target of rapamycin complex 2 (mTORC2) then work in concert to phosphorylate AKT’s Thr308 and Ser473 sites, which activates the protein (Hers et al., 2011; Huang et al., 2018). Phosphorylation of downstream target proteins is how the activated AKT carries out its actions. To lower blood glucose levels, AKT can also phosphorylate the AS160 protein, which encourages GLUT4 to move from the intracellular compartment to the cell membrane (Xu et al., 2016). This improves cellular glucose uptake and permits blood glucose to enter cells for storage or use. Conversely, GSK-3β is phosphorylated by AKT, which renders it inactive. This relieves the inhibition of GSK-3β, hence facilitating the synthesis of glycogen. Furthermore, AKT phosphorylates the target of rapamycin (mTOR) to stimulate protein synthesis (Cheatham and Kahn, 1995; Saltiel and Kahn, 2001; James et al., 2021).
According to recent studies, PCOS-IR develops and occurs as a result of anomalies at several locations along the insulin signaling pathway. As CPXM1 damages the insulin signaling system, it lowers the expression of IRS-1/2 and INSR while also downregulating the phosphorylation of Akt, a crucial component of the pathway (Pervaz et al., 2023). This results in IR in the ovary and adipose tissue. The primary mechanisms of IR in PCOS, as demonstrated by a recent animal study, are increased serine phosphorylation of IRS-1 and decreased phosphorylation of Akt in the insulin signaling pathway of perigonadal white adipose tissue (pgWAT). This abnormality is closely linked to the inflammatory response mediated by C-C chemokine receptor 5 (CCR5) (Seow et al., 2021). In liver tissue, targeting the reduction of neutrophil extracellular traps (NETs) is the key to improving their negative effect on the phosphorylation of AKT, a downstream signaling molecule of the insulin signaling pathway, and ultimately enhancing insulin sensitivity in the liver tissue (Lin et al., 2025). Src-associated in mitosis, 68 kDa (Sam68) in ovarian granulosa cells lowers the phosphorylation level of IRS-1, which results in inadequate activation of the downstream insulin signaling pathway and, eventually, IR (Vilariño-García et al., 2022).
3.2 Abnormality of the NF-κB signaling pathway mediates the development of IR in PCOS
Another important step in fostering the advancement of PCOS with IR is the activation of the classical NF-κB signaling pathway, which is mediated by tumor necrosis factor-α (TNF-α) (Moller, 2000; Shoelson et al., 2006). The NF-κB dimer is inactive in the cytoplasm and firmly binds to Inhibitor of NF-κB alpha (IκBα) under normal physiological conditions. The downstream IκB kinase (IKK) complex is activated in pathological conditions when TNF-α secretion rises sharply and binds to its receptor (TNFR). It then quickly attracts TNF receptor-associated death domain protein (TRADD) and TNF receptor-associated factor 2 (TRAF2) to form a membrane-bound signaling complex (Leong and Karsan, 2000; Oeckinghaus and Ghosh, 2009). When IKK phosphorylates the IκBα protein, it releases the active NF-κB dimer (like p50/p65) and causes the protein to be ubiquitinated and degraded. Following its translocation into the nucleus, NF-κB attaches itself to the κB site in the target gene’s promoter region and starts the transcription of pro-inflammatory proteins like IL-6 and IL-1β (Leong and Karsan, 2000; Oeckinghaus and Ghosh, 2009). These inflammatory factors, in turn, can further stimulate the secretion of TNF-α, forming an inflammatory cascade amplification effect.
From an IR perspective, IKKβ within the NF-κB signaling pathway phosphorylates Ser307 on IRS-1, reducing its capacity for insulin-triggered tyrosine phosphorylation. Consequently, insulin signal transduction is impeded, culminating in IR (Gao et al., 2002; de Alvaro et al., 2004; Liu et al., 2011). Inflammatory mediators like TNF-α, prompted by NF-κB activation, impede PI3K-AKT signaling, hindering GLUT4 translocation to the cell membrane and diminishing peripheral tissue sensitivity to insulin (Hotamisligil et al., 1996; Baker et al., 2011). Prolonged NF-κB activation can exacerbate IR, perturb glucose and lipid metabolism, fostering a detrimental cycle of “TNF-α-NF-κB-inflammation-IR” (Tan et al., 2024).
3.3 Abnormality of the MAPK signaling pathway mediates the development of IR in PCOS
Following its binding to target cell receptors, insulin triggers the receptors’ tyrosine kinase activity, which phosphorylates the tyrosine residues of the downstream adapter protein Shc in the pathological process of PCOS with IR (Diamanti-Kandarakis and Dunaif, 2012). Phosphorylated Shc binds to Grb2 and further recruits SOS, which promotes the activation of Ras. Subsequently, the Ras - MAPK cascade reaction composed of Raf - MEK - ERK is activated. Finally, the activated ERK enters the nucleus to phosphorylate transcription factors, thereby regulating the expression of genes related to cell growth and proliferation (de La Fernandes et al., 1999; Corbould et al., 2006; Rajkhowa et al., 2009; Makker et al., 2012; Zhao et al., 2015).
By further activating the pathway, HI in the presence of IR can worsen the development of the disease. After the MAPK pathway is activated, ERK phosphorylates serine sites on IRS-1/2, which prevents IRS from becoming tyrosine phosphorylated and lowers PI3K recruitment (Tanti and Jager, 2009). As a result, the PI3K-AKT pathway’s ability to regulate glucose uptake is impaired, which aids in the development and advancement of IR (Dunaif et al., 2001).
4 Negative effects of IR on health in patients with PCOS at different stages
4.1 Negative effects of IR on health in adolescent PCOS
IR causes an array of health hazards and worsens clinical characteristics in adolescent PCOS patients. Mechanistically, IR and compensatory HI increase the hypothalamic gonadotropin-releasing hormone (GnRH) pulsatile frequency, which causes the pituitary gland to secrete too much LH (Patel et al., 2003). It exacerbates pre-existing issues such high androgen levels, irregular ovulation, and menstrual cycle disorders by increasing ovarian androgen synthesis and ovulation disorders at the same time (Dunaif, 1997; Venkatesan et al., 2001; Diamanti-Kandarakis and Dunaif, 2012). Furthermore, biochemical hyperandrogenism may exacerbate the clinical manifestations of hyperandrogenism, including acne and hirsutism (Taketani and Mizuno, 1990; Stanczyk, 2006; Guo et al., 2023). As early as 2001, a clinical study demonstrated that most patients’ normal menstrual cycle and the hypothalamic-pituitary-ovarian axis function could be successfully restored, androgen levels could be decreased, after improving insulin sensitivity in white adolescent girls with PCOS with metformin and a high-protein, low-carb diet (Glueck et al., 2001). A retrospective observational study of adolescents with PCOS then examined metabolic markers of several menstruation types, such as oligomenorrhea (OM), secondary amenorrhea (SA), and primary amenorrhea (PA) (Javed et al., 2015). It was discovered that the PA group had a considerably higher HOMA-IR value and fasting insulin level than the SA and OM groups, and that the PA and SA groups had a significantly greater prevalence of metabolic syndrome than the OM group (Javed et al., 2015). This implies that IR is linked to a higher metabolic risk and may be particularly noticeable in teenage PCOS patients who have severe irregularities in their menstrual cycles, such amenorrhea. According to a recent meta-analysis and systematic review, the incidence of amenorrhea in adolescents with PCOS increases with body mass index (BMI) (Zuchelo et al., 2024). This indirectly reflects the correlation between abnormal glucose and lipid metabolism and abnormal menstrual cycles in adolescent PCOS patients.
IR is intimately linked to obesity and poor glucose metabolism in adolescents with PCOS (Bayramoğlu et al., 2021; Gupta et al., 2022; Fu et al., 2023). In adolescents with PCOS, elevated blood glucose and lipid levels are also strongly linked to the onset and progression of a number of metabolic disorders (Huang et al., 2010). Adolescent females with PCOS had higher triglyceride, cholesterol, and IR levels than do those without the condition (Patel-Sanchez et al., 2023). The incidence of non-alcoholic liver disease is greater in adolescents with PCOS who go on to develop diabetes (Patel-Sanchez et al., 2023). This indicates that IR is one of the major pathogenic causes for non-alcoholic liver disease in adolescents with PCOS, in addition to being intimately linked to aberrant glucose and lipid metabolism in these patients. Furthermore, a cross-sectional investigation of obese teenage PCOS patients revealed a strong correlation between fasting IR and the pancreatic fat fraction in teenage girls, which is unrelated to PCOS (Ware et al., 2022). Moreover, IR is significantly linked to poorer sleep quality (including sleep-disordered breathing and decreased sleep efficiency) in obese teenage PCOS patients, indicating that doctors should focus on the holistic care of both (Simon et al., 2020).
However, the psychological problems caused by metabolic disorders are also worthy of attention. Teenagers and young women with PCOS have higher levels of anxiety and sadness than those without the condition, according to a recent systematic review and meta-analysis (Nasiri-Amiri et al., 2023). The causes of negative psychology, including depression and anxiety, in adolescents with PCOS were then investigated in a number of clinical studies that used assessment techniques like questionnaire scale evaluation, group comparative analysis, and correlation analysis. These studies suggested that the presence of negative emotions in adolescents with PCOS may be connected to clinical symptoms like obesity, hirsutism, and irregular menstrual cycles linked to IR (Kara et al., 2022; Saei Ghare Naz et al., 2023). Therefore, the mental health of adolescents with PCOS-IR should be taken into consideration when treating their organic disorders in the future.
4.2 Negative effects of IR on the health of women of reproductive age with PCOS
In women with PCOS who are of reproductive age, IR can lead to both metabolic problems and malfunction of the reproductive system (Vatier et al., 2022). This disorder includes infertility, which is defined by anovulatory infertility and aberrant oocyte development, as well as pregnancy problems, such as decreased live birth rates and an increased chance of threatening miscarriage (Dumesic and Abbott, 2008; Sakumoto et al., 2010; Vatier et al., 2022).
On the one hand, IR can cause infertility in women of reproductive age with PCOS through multiple mechanisms. Heightened expression of miR-181d-5p in PCOS patients compared to healthy individuals has been observed (Sang et al., 2024). This upregulation of miR-181d-5p can impede granulosa cell proliferation by inhibiting SIRT1 activity, thereby compromising oocyte quality in PCOS-IR individuals (Sang et al., 2024). In terms of ovulation, IR stimulates the production of androgens (such as testosterone) by inducing compensatory hyperinsulinemia and acting synergistically with the elevated LH level on hypersensitive ovarian theca cells (Graham and Selgrade, 2017). The elevated testosterone induces the synthesis of LH in the pituitary gland and inhibits the function of FSH. Meanwhile, it increases the sensitivity of follicles to LH and causes premature luteinization, ultimately impairing ovulation ability (Graham and Selgrade, 2017). Furthermore, IR can reduce the sensitivity of the ovaries to gonadotropins in PCOS patients, obstructing the follicle development and ovulation processes and thus leading to ovulation disorders (Ezeh et al., 2021; Li Y. et al., 2023).
On the other hand, in women of reproductive age with PCOS, IR may lower the live birth rate and raise the frequency of pregnancy problems. By blocking the PI3K/AKT pathway and inhibiting glycolytic enzymes such as HK2 and PKM2, IR prevents embryo implantation by upsetting endometrial balance and energy supply (Zhang et al., 2024d). Patients with PCOS are therefore more likely to experience miscarriage and unsuccessful embryo implantation. Additionally, IR increases the endometrial inflammatory milieu by promoting the activation of natural killer (NK) cells and skewing macrophages toward the M2 anti-inflammatory phenotype (Liu S. et al., 2021). This chain reaction further hinders the implantation of embryos and jeopardizes the continuation of pregnancy. IR and the rate of prenatal abortions in PCOS are also tightly related. According to an examination of a retrospective cohort study, IR plays a significant role in early abortion in PCOS patients’ first embryo transfer cycle (Chen et al., 2022). According to another study, insulin resistance is linked to a higher risk of preterm delivery in PCOS patients, and PCOS is an independent risk factor for late abortion (Jie et al., 2022). Nevertheless, other research has also demonstrated that IR’s detrimental impact on the late pregnancy outcomes of induced pregnant women is not specific to PCOS patients (Yang T. et al., 2022). By interfering with endometrial homeostasis and energy availability, IR mechanistically reduces pregnancy stability (Zhang et al., 2024d). Additionally, IR can cause and exacerbate endometrial chronic inflammation, increasing the chance of miscarriage in PCOS patients (Liu S. et al., 2021). Additionally, the combined effects of IR and HA cause abnormal mitochondrial shape and function in the placenta and gravid uterus. By blocking the Nrf2/GPX4 pathway, this combination also upsets the balance between oxidation and antioxidation, which leads to unusual cell death pathways like ferroptosis and miscarriage (Hu et al., 2019; Zhang et al., 2019; Zhang Y. et al., 2020). The final result of the multi-link damage described above is a decrease in the live birth rate. Research has indicated that in patients with PCOS, IR is a risk factor in and of itself for the lower live birth rate following frozen-thawed embryo transfer (Jiang et al., 2021). According to another clinical investigation, pregnant women with metabolic syndrome are more likely to develop gestational diabetes and give birth to babies who are macrosomic, and metabolic syndrome has a detrimental effect on the clinical pregnancy rate and live birth rate in obese PCOS patients (Arya et al., 2021). These results highlight the negative effects of metabolic abnormalities, like IR, on the live birth rates and reproductive outcomes of women with PCOS who are of reproductive age.
As mentioned above, IR affects oocyte development, ovulation function, and pregnancy outcomes in women of reproductive age with PCOS through the aforementioned multiple mechanisms, ultimately leading to infertility or increasing the risk of miscarriage. The various regulatory mechanisms discussed above are summarized in (Figure 2).
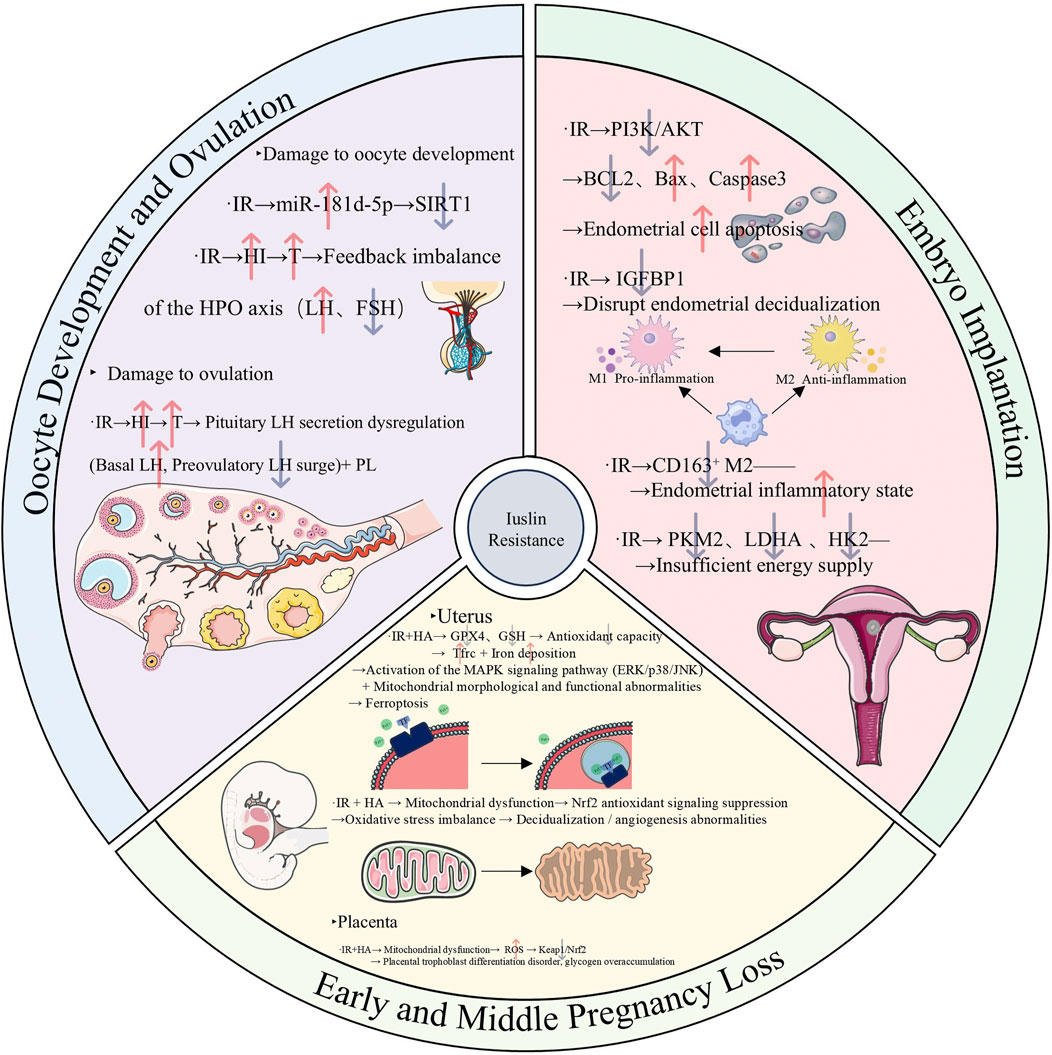
Figure 2. Mechanism diagram illustrating the mechanisms underlying the adverse effects of insulin resistance on the reproductive system in reproductive-aged women with polycystic ovary syndrome, specifically involving oocyte development, ovulation, embryo implantation, and early and middle pregnancy loss. (Abbreviations: IR: insulin resistance, miR-181d-5p: microRNA-181d-5p, SIRT1: sirtuin 1, HI: hyperinsulinemia, T: testosterone, LH: luteinizing hormone, FSH: Follicle-stimulating hormone, BCL2: B-cell lymphoma 2, Bax: Bcl-2-associated X protein, IGFBP1: Insulin-like Growth Factor Binding Protein 1, CD163: Cluster of Differentiation 163, PKM2: Pyruvate Kinase M2,LDHA: Lactate Dehydrogenase A, HK2: Hexokinase 2, GPX4: Glutathione Peroxidase 4, GSH: Glutathione, Tfrc: Transferrin Receptor, Nrf2: Nuclear Factor Erythroid 2-Related Factor 2, ROS: Reactive Oxygen Species, Keap: Kelch-Like ECH-Associated Protein 1).
4.3 Negative effects of IR on the health of postmenopausal women with PCOS
In patients with PCOS, IR is not only one of the core pathogenic factors in the pre-menopausal period but also continuously affects the health status after menopause, significantly increasing the risk of various long - term complications (Lambrinoudaki, 2011; Xu and Qiao, 2022). In fact, there are few studies on the multiple disease states of post - menopausal PCOS patients caused by single insulin resistance, and more studies focus on the synergistic effect of IR and other factors such as HA. The core mechanism of the synergistic effect between IR and HA lies in the vicious cycle of their interaction.
IR induced by HA worsens postmenopausal PCOS in rat models, resulting in significant abnormalities in glucose and lipid metabolism as well as cardiovascular and renal damage, including hypertension, decreased glomerular filtration rate, proteinuria, and tubular injury (Dalmasso et al., 2016). The negative effects of IR on postmenopausal cardiac health and glucose-lipid metabolism were highlighted by a recent study that used a postmenopausal PCOS rat model to show that treatment with a GLP-1 receptor agonist (liraglutide) effectively mitigated multiple cardiometabolic risk factors and improved IR (Torres Fernandez et al., 2019). More significantly, compared to people without PCOS, those with PCOS appear to be at a higher risk of developing cancer. With evidently aberrant glucose and lipid metabolism, postmenopausal PCOS patients have a larger chance of developing certain cancers than premenopausal PCOS patients (Meczekalski et al., 2020; Frandsen et al., 2023; Frandsen et al., 2024). The mechanism behind this could be linked to pathological diseases such adipokine imbalance and chronic inflammation brought on by IR alone or in conjunction with hyperandrogenism (Esposito et al., 2014; Slopien et al., 2018; Elibol et al., 2025).
5 TCM in the treatment of IR in PCOS
In the realm of complementary and alternative medicine, TCM is increasingly recognized for its significant role in treatment (Dubey and Kumar, 2025). Studies have highlighted the medicinal value of TCM, including compounds and monomers, in enhancing the management of PCOS and regulating endocrine and metabolic functions (Chen et al., 2023; Fu L.-W. et al., 2024). Research has shown promising outcomes in utilizing TCM to address PCOS, particularly in cases complicated by IR. The following paragraphs summarize the cutting-edge studies (covering animal and human studies) of TCM in improving PCOS with IR in recent years (especially in the past 5 years).
5.1 Application of TCM botanical drug products alone to improve IR in PCOS
5.1.1 Evidence from animal studies
5.1.1.1 TCM botanical drug formulas
The primary therapeutic modality in TCM clinical practice involves the utilization of compound formulas comprising diverse Chinese botanical drugs, known as “Fufang” in Chinese. Rooted in the holistic principles and the practice of syndrome differentiation and treatment within TCM, these compound formulas exhibit the capacity to ameliorate IR in PCOS through a multifaceted approach, leveraging the synergistic actions of numerous metabolites across various targets and pathways.
Cangfu Daotan Decoction (CFD) is a classic prescription in TCM, mainly composed of Atractylodes lancea, Cyperus rotundus, and Citrus reticulata. Animal experiments have shown that the basic formula of CFD or its modified formulas can effectively improve PCOS with IR (Figure 3). Wang et al. (2020a) observed the mechanism of action of CFD on PCOS-IR rats. The study found that CFD improves IR by activating the IGF-1-PI3K/Akt pathway (Wang et al., 2020b). Specifically, it upregulates the expression of IGF-1 and promotes the phosphorylation of PI3K and Akt, thereby enhancing insulin signal transduction and ultimately reducing the HOMA-IR value. Jiang et al. (2022) treated a PCOS-IR rat model with CFD or metformin and found that CFD can effectively improve insulin resistance in PCOS-IR model rats, and its efficacy is similar to that of metformin. In addition, the levels of pro-inflammatory factors (such as TNF-α and IL-1) in the serum of rats treated with CFD were significantly reduced. Mechanistically, it is speculated that CFD can effectively alleviate the negative effects of inflammation on the insulin signaling pathway. Liu et al. (2022)reported that treating a PCOS-IR rat model established by letrozole combined with a high-fat diet with Modified CFD can regulate the NF-κB/LCN-2 inflammatory signaling pathway, thereby inhibiting the release of inflammatory factors and upregulating insulin signal-related genes, and ultimately improving the insulin sensitivity of PCOS-IR rats.
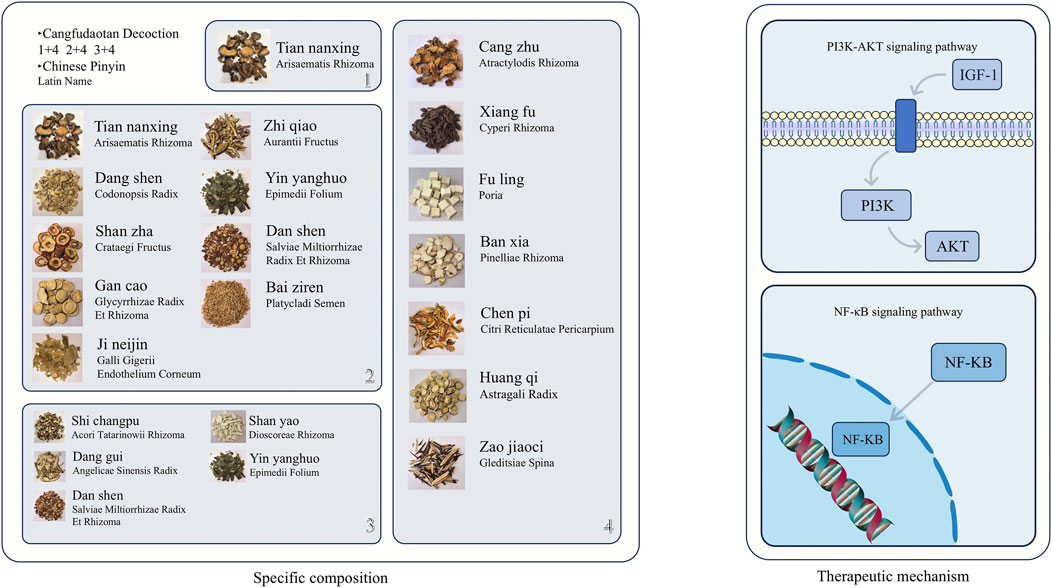
Figure 3. Schematic diagram of the specific composition of Cangfu Daotan Decoction (including Chinese herbal medicine images, Chinese pinyin, and Latin botanical names) and its mechanism of action in improving insulin resistance in polycystic ovary syndrome. The above Chinese Pinyin and Latin names are all sourced from the Chinese Pharmacopoeia (2020 Edition). The specific website of the Chinese Pharmacopoeia: https://ydz.chp.org.cn/#/.
HEQI San (HQS) is a widely utilized TCM compound comprising He ye (Lotus leaf), Huang qi (Astragalus), Ze lan (Zeran), Jue mingzi (Cassia seed), Dong guapi (Exocarpium benincasae), Bai zhu (Atractylodes macrocephala), Shan yao (Chinese yam), and Gan cao (Licorice), all botanical drugs. A recent animal study demonstrated that HQS effectively ameliorates IR in PCOS mice through diverse mechanisms (Figure 4). HQS suppresses NF-κB-mediated M1 polarization of macrophages, thereby reducing the secretion of inflammatory cytokines like IL-6 and TNF-α, consequently mitigating the disruptive impact of inflammation on the insulin signaling pathway (Li J. et al., 2024). Furthermore, HQS enhances insulin sensitivity in PCOS mice by modulating the composition of gut microbiota (Li J. et al., 2024).

Figure 4. The specific composition of HeQi San and a schematic diagram of its mechanism in improving insulin resistance in polycystic ovary syndrome. (Abbreviations: IL-6: Interleukin-6, TNF-α: Tumor Necrosis Factor-α). (Astragalus sp. (The specific species was not specified in the original study. According to the Chinese Pharmacopoeia, the medicinal Astragali radix includes two species: Astragalus membranaceus (Fisch.) Bge. var.mongholicus (Bge.) Hsiao and Astragalus membranaceus (Fisch.) Bge. Cassiae sp. (The specific species was not specified in the original study. Aaccording to the Chinese Pharmacopoeia, the medicinal Cassiae semen includes Cassia obtusifolia L. and Cassia tora L. Glycyrrhiza sp. (The specific species was not specified in the original study. According to the Chinese Pharmacopoeia, the medicinal Glycyrrhizae radix et rhizoma includes Glycyrrhiza uralensis Fisch., Glycyrrhiza glabra L. and Glycyrrhiza inflata Bat)). The Chinese pinyin, Latin names, generic names, and Latin scientific names of the above traditional Chinese medicines are from the Chinese Pharmacopoeia (2020 Edition), Kew Plants of the World Online, and Kew Medicinal Plant Names Services (MPNS). The website of the Chinese Pharmacopoeia: https://ydz.chp.org.cn/#/, the website of Kew Plants of the World Online: https://powo.science.kew.org, and the website of Kew Medicinal Plant Names Services (MPNS): https://www.kew.org/science/our-science/science-services/medicinal-plant-names-services.
In addition to the above TCM botanical drug compound prescriptions, recent studies have also indicated that other TCM botanical drug compound prescriptions can effectively improve IR in PCOS (Supplementary Table S1). These TCM botanical drug compound prescriptions are worthy of further development and utilization (An et al., 2025; Guan et al., 2024; Huang et al., 2024a; Huang et al., 2024b; Lian et al., 2020; Liu et al., 2025; Liu et al., 2021a; Qiu et al., 2020; Su et al., 2023; Wu et al., 2022b; Xu et al., 2021; Zhang et al., 2023b; Zhang et al.,2025; Zhang and Xu, 2021; Zhao et al., 2022b; Zheng et al., 2023).
5.1.1.2 TCM botanical drug metabolites
Advancements in TCM extraction and chemical structure analysis have enabled researchers to investigate the medicinal chemistry and pharmacology of TCM. Studies have revealed that certain TCM botanical drug active metabolites, including isoquinoline alkaloids and flavonoids, exhibit potential in ameliorating IR associated with PCOS.
TCM plants such as Phellodendron chinense Schneid. and Coptis chinensis Franch. yield berberine, an isoquinoline quaternary ammonium alkaloid with notable biological efficacy (Wu et al., 2019; Chen et al., 2020). Its clinical utility has garnered considerable interest owing to its robust antibacterial, anti-inflammatory, and metabolic regulatory attributes, coupled with minimal side effects and a high safety profile (Kong et al., 2020; Blais et al., 2023). Recent research indicates that berberine effectively ameliorates IR in PCOS patients through diverse mechanisms, including the activation of insulin-related pathways and the suppression of aberrant inflammatory factor release (Figure 5). On the one hand, increased insulin sensitivity results from berberine’s direct impact on the insulin signaling system. Zhang N. et al. (2020) demonstrated that berberine activates the PI3K/AKT pathway, boosting insulin signaling and increasing both mRNA and protein levels of GLUT4 in ovarian tissue in a dose-dependent manner. This augmentation enhances cellular glucose transport capacity, thereby ameliorating insulin resistance. Furthermore, berberine elevates the cellular levels of phosphorylated PI3K (p-PI3K) and phosphorylated AKT (p-AKT) in the ovarian tissue of rats with PCOS, thereby activating this pathway and improving insulin sensitivity. Notably, in vitro experiments revealed that inhibition of either PI3K or AKT negated the beneficial effects of berberine on IR, underscoring the pivotal role of the PI3K/AKT pathway in its mechanism of action (Yu et al., 2021). On the other hand, berberine enhances insulin sensitivity by inhibiting inflammatory factors. It achieves this by suppressing the activation of MAPK and NF-κB signaling pathways, leading to reduced expression of corresponding proteins in ovarian tissues (Zhao F.-Q. et al., 2022). This action alleviates local ovarian inflammation, thereby ameliorating insulin resistance. Furthermore, berberine downregulates the expression of TLR4 and NF-κB, consequently inhibiting the secretion of inflammatory factors and ultimately improving insulin sensitivity (Shen et al., 2021a). Berberine, in addition to activating insulin-related signaling pathways and inhibiting inflammatory factors, exerts its effects on improving PCOS-related IR by modulating the gut microbiota. Shen et al., 2021b demonstrated a significant decrease in the HOMA-IR index of PCOS-IR rats treated with berberine (150 mg/kg/d by gavage for 6 weeks), accompanied by a reduction in Firmicutes abundance and an increase in Bacteroidetes levels in the gut microbiota. Similarly, Xin et al. (2024) animal studies supported the efficacy of berberine in ameliorating PCOS-IR through restructuring the gut microbiota. However, not all findings align with these positive outcomes. Wang et al. (2021) observed that neither low nor high doses of berberine significantly improved insulin resistance in PCOS-IR model mice. The divergent results among these studies suggest that the dosage and duration of berberine intervention may play pivotal roles in determining treatment efficacy.
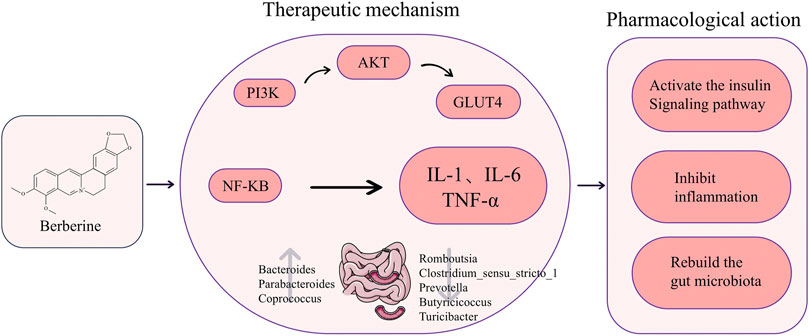
Figure 5. The chemical structure of berberine and a diagram of its specific mechanism in improving insulin resistance in polycystic ovary syndrome. (Abbreviations: PI3K: Phosphoinositide 3-Kinase, AKT: Protein Kinase B, GLUT4: Glucose Transporter 4, NF-KB:Nuclear Factor-kappa B, IL-1: Interleukin-1, IL-6: Interleukin-6, TNF-α: Tumor Necrosis Factor-α).
Quercetin, a ubiquitous flavonoid metabolite in TCM botanical drugs, exhibits diverse physiological functions including anti-inflammatory and antioxidant properties. Its efficacy in treating gynecological disorders such as endometriosis underscores its importance in human health (An et al., 2022; Hipólito-Reis et al., 2022; Shah et al., 2024). Additionally, quercetin demonstrates a beneficial impact on ameliorating IR in PCOS. Li M. et al. (2023) observed a notable decrease in PM20D1 levels in a PCOS-IR rat model, concomitant with evident insulin resistance. Subsequent administration of quercitrin to these rats demonstrated an amelioration of insulin resistance through the upregulation of PM20D1 expression and activation of the PI3K/Akt insulin pathway. In a separate study, Zheng L. et al. (2022) established a DHEA-induced PCOS rat model characterized by pronounced IR and elevated levels of inflammatory markers such as IL-6 and TNF-α. Treatment with quercetin significantly alleviated inflammation and IR in the PCOS rat model (Zheng S. et al., 2022). Mechanistically, quercetin is postulated to mitigate ovarian inflammation in PCOS model rats by reducing inflammatory markers, thereby enhancing insulin sensitivity. Furthermore, quercetin has been shown to lower androgen levels in PCOS model mice while improving IR (Shah et al., 2023). A recent systematic review and meta-analysis evaluating the efficacy of quercetin in PCOS animal models revealed its significant reduction of FINS, fasting blood glucose (FBG), and HOMA-IR levels, comparable to metformin (Su et al., 2024). These findings underscore the promising therapeutic potential of quercetin in addressing PCOS-related IR.
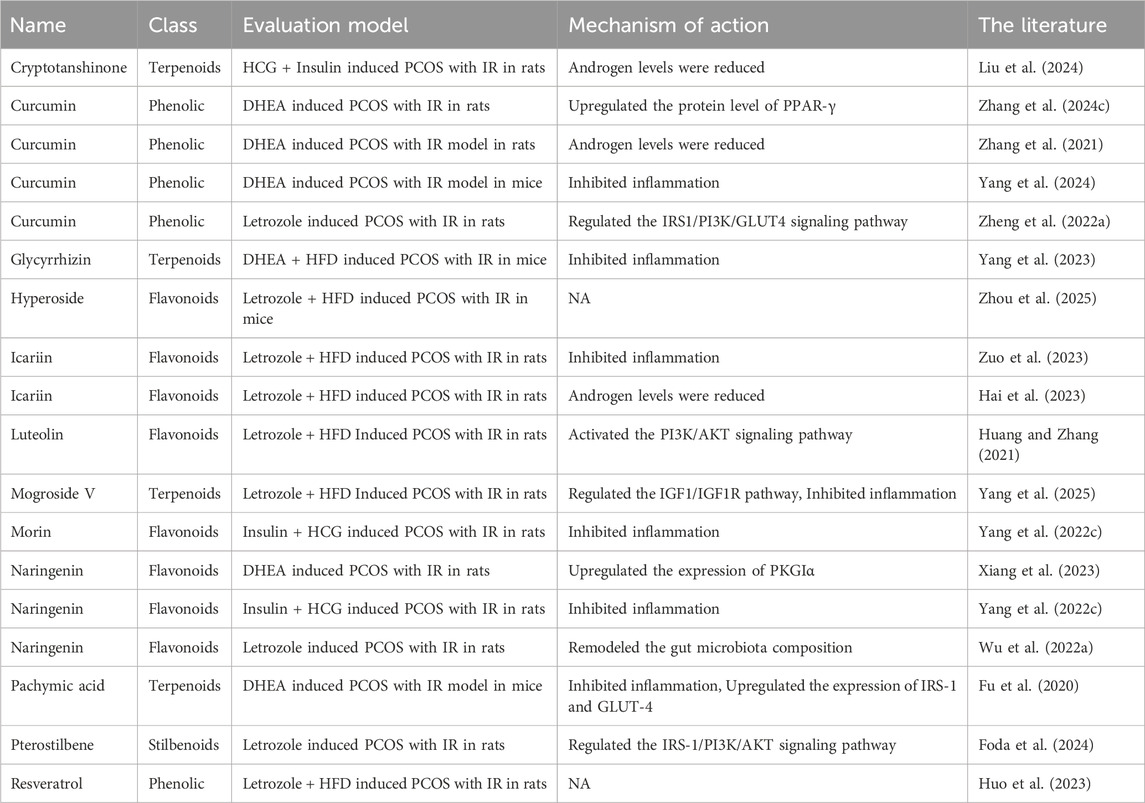
Apart from the TCM botanical drug metabolites previously discussed, additional metabolites from TCM botanical drugs have demonstrated efficacy in ameliorating PCOS-IR (Table 1). The underlying mechanism of its action may be associated with the activation of the insulin signaling pathway and the suppression of inflammation.
5.1.2 Evidence from human studies
While animal experiments have offered valuable insights into the mechanisms of TCM in treating PCOS with IR, the transition from laboratory research to clinical practice encounters several obstacles. Primarily, animal models typically represent a single etiological factor, limiting their ability to fully replicate the multifaceted pathological features of human PCOS with IR, including genetic and environmental influences (Ryu et al., 2019; Frangogiannis, 2022; Kamada et al., 2022). Furthermore, animal studies tend to concentrate on short-term molecular-level effects, whereas human PCOS with IR involves intricate metabolic-reproductive axis dysregulation (Finnie and Blumbergs, 2022; Frangogiannis, 2022; Kamada et al., 2022). The prolonged disease progression and individual variability in humans may result in disparities between animal study outcomes and clinical effectiveness (Finnie and Blumbergs, 2022; Frangogiannis, 2022; Kamada et al., 2022).
Although animal models have limitations, they offer valuable insights into the effects of TCM that are pertinent to clinical research. This discussion will now shift focus to human research findings to elucidate the practical impact of TCM on improving IR in PCOS patients. By bridging basic research with clinical application, this examination aims to underscore the relevance of TCM in treating PCOS-related IR in human subjects.
5.1.2.1 TCM botanical drug formulas
As previously mentioned, animal studies on PCOS with IR induced by different drugs have confirmed the significant effects of TCM botanical drug formulas on improving IR from multiple aspects. Recent human studies have further confirmed that TCM botanical drug formulas also have significant efficacy in improving IR in patients with PCOS-IR.
A recent clinical study indicated that the sole application of Dingkun Pill can effectively enhance insulin sensitivity in patients with PCOS (Deng et al., 2020). Moreover, the results of this study demonstrated that, in terms of improving insulin sensitivity and regulating lipid metabolism (reducing total cholesterol, low - density lipoprotein cholesterol, and free fatty acids), the sole use of Dingkun Pill outperforms the sole use of Diane - 35 (Deng et al., 2020). This suggests that in the treatment of PCOS, Dingkun Pill has certain application potential in improving metabolic abnormalities. Particularly for patients mainly presenting with IR and lipid metabolism disorders, it may be a treatment option worthy of attention. Another retrospective cohort study showed that the sole use of Zishen Yutai Pills can effectively improve IR in patients with PCOS, and its efficacy in improving IR in PCOS patients is similar to that of the sole use of metformin (Zhang Y. et al., 2024). Additionally, the levels of sex hormones such as LH and T in PCOS patients also improved after treatment with Zishen Yutai Pills (Zhang Y. et al., 2024). The specific drug compositions and other relevant information of the above-mentioned TCM botanical drug formulas are detailed in Table 2.
5.1.2.2 TCM botanical drug metabolites
In addition to animal experiments, the effectiveness of TCM botanical drug metabolites in improving PCOS with IR has also been confirmed by human studies.
Curcumin is a phenolic compound extracted from plants of Zingiberaceae, Araceae, etc. (such as Curcuma longa and Curcuma aromatica) (Garimella and Pradhan, 2024). Modern research has shown that curcumin has a wide range of pharmacological effects, including anti - inflammation, anti - oxidation, and anti - tumor effects. It can regulate multiple signaling pathways in the body and inhibit the release of inflammatory factors (Weng and Goel, 2022; Cox et al., 2022; Luo et al., 2023; Zhang J. et al., 2023). First, multiple RCTs have directly confirmed the independent efficacy of curcumin. A randomized double - blind placebo - controlled trial indicated that the sole application of curcumin in treatment can significantly improve insulin sensitivity in patients with PCOS (Jamilian et al., 2020). Another clinical study showed that the sole use of nano - curcumin can effectively improve insulin resistance in PCOS patients, but its efficacy is weaker than that of metformin (Feghhi et al., 2024). The combined application of metformin and nano - curcumin can more significantly improve IR in PCOS patients, which reflects the synergistic effect of the combined use of traditional Chinese and Western medicines (Feghhi et al., 2024). On this basis, systematic reviews and Meta - analyses based on RCTs have further strengthened the above conclusions. A systematic review and Meta - analysis based on RCTs stated that whether curcumin is used alone or on the basis of metformin, it can significantly improve IR in PCOS patients and effectively improve inflammatory markers, with no obvious adverse reactions (Shen et al., 2022). Another systematic review and Meta - analysis based on RCTs indicated that although curcumin can effectively improve IR in PCOS patients, its effectiveness in lipid regulation is still lacking (specifically, it has no significant impact on lipid parameters such as total cholesterol, LDL - C, HDL - C, and triglycerides) (Simental-Mendía et al., 2022). However, not all studies have reached consistent conclusions. Some studies have shown that although the application of curcumin can effectively reduce fasting blood glucose and dehydroepiandrosterone (DHEA) levels in PCOS patients, it has no obvious improvement on IR indicators (Heshmati et al., 2021). Combining the above research results, it can be seen that the improvement effect of curcumin on IR in PCOS patients may be related to factors such as the form of administration, the combined medication regimen, and individual differences among patients. Its specific mechanism of action and applicable conditions still need further research for clarification.
Recent studies have verified the efficacy of berberine in ameliorating IR in individuals with PCOS. A systematic review of clinical trials demonstrated that berberine administration alone effectively reduces IR in PCOS patients, albeit with a potential for mild gastrointestinal side effects (Mirzaee et al., 2021). Additionally, a network meta-analysis revealed that combining berberine with metformin yields superior improvements in IR compared to metformin monotherapy in PCOS patients (Zhao et al., 2021). These findings collectively support the significant efficacy of berberine, either as a standalone treatment or in conjunction with metformin, in enhancing IR in PCOS patients. This underscores the dual benefits of berberine in managing PCOS-related IR, positioning it as a versatile and dependable therapeutic option for addressing IR in PCOS patients.
Crocins, carotenoid compounds derived from the dried stigmas of Crocus sativus L. (saffron) in the Iridaceae family, exhibit diverse pharmacological properties, including anti-inflammatory effects and enhancement of microcirculation (Bahari et al., 2025; Hao et al., 2025). In a study by Rahimi et al. (2022), patients with PCOS were administered crocin or a placebo for 12 weeks. Results showed that compared to the placebo group, the crocin-treated group exhibited significant reductions in fasting insulin levels, HOMA-IR index, and IL-6 levels. Moreover, the crocin intervention led to a decrease in the atherosclerosis index and an increase in the cardioprotective index (Rahimi et al., 2022). These findings suggest that crocins effectively safeguard the cardiovascular system, ameliorate insulin resistance, and mitigate inflammation in women with PCOS.
The specific experimental details of the above research on the improvement of IR in PCOS patients by TCM botanical drug metabolites are summarized in Table 3.
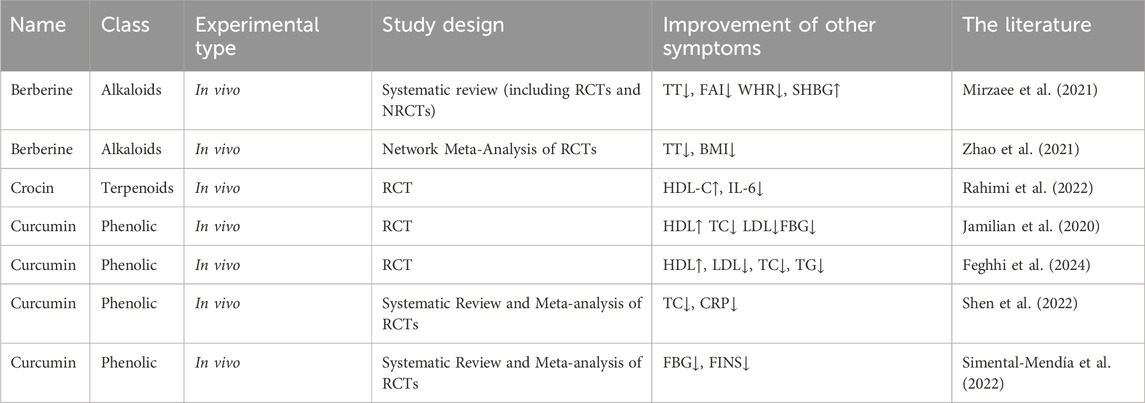
Table 3. Human studies on the improvement of IR in PCOS by single TCM botanical drug metabolites alone.
5.2 Combined application of TCM botanical drug products on the basis of conventional drugs to improve IR in PCOS
5.2.1 Evidence from animal studies
In animal studies investigating the enhancement of IR in PCOS through the integration of TCM with conventional pharmaceutical interventions, the limited yet emerging evidence underscores the synergistic benefits of this combined approach, delineated along two primary dimensions: TCM botanical drug formulas and TCM botanical drug metabolites. Specifically, experiments utilizing Chinese botanical drug formulas have demonstrated that the concurrent administration of the modern medication rosiglitazone with the botanical drug formulas Guizhi Fuling Pills (GFW) yields a more pronounced improvement in IR compared to rosiglitazone monotherapy (Ye et al., 2023). Furthermore, this combined regimen exhibits superior regulatory efficacy in various domains, including modulation of lipid metabolism (evidenced by reductions in total cholesterol, triglycerides, and low-density lipoprotein, alongside increases in high-density lipoprotein), amelioration of sex hormone imbalances (manifested by decreased testosterone and LH levels, and increased estradiol, progesterone, among others), and mitigation of inflammatory responses (attested by diminished levels of inflammatory markers such as CRP, IL-18, and TNF-α) (Ye et al., 2023). These findings underscore the synergistic and potentiated impact of integrating Chinese botanical drug formulas with modern pharmacotherapy in addressing the endocrine dysregulation characteristic of PCOS. Animal studies have shown promising outcomes when combining TCM botanical drug metabolites with conventional drugs. For instance, Zhang et al. (2021) investigated the impact of curcumin in conjunction with aerobic exercise on a DHEA-induced PCOS rat model. The findings revealed that this combined approach significantly ameliorated IR, leading to greater reductions in FBG, FINS, and the HOMA-IR compared to using curcumin or aerobic exercise alone (Zhang et al., 2021). Furthermore, the combined intervention exhibited superior effects in modulating sex hormone levels (such as decreasing T and LH while increasing FSH) and improving ovarian tissue pathology (including reducing atretic follicles and increasing corpora lutea count) (Zhang et al., 2021). These results suggest that integrating TCM botanical drug metabolites with standard therapy can holistically enhance various PCOS-related abnormalities through synergistic actions on multiple targets and pathways, underscoring the potential for optimizing PCOS treatment strategies.
In conclusion, despite the limited sample sizes in current animal studies, they consistently indicate that combining TCM botanical drug formulas or metabolites with conventional drugs can synergistically enhance the amelioration of IR in PCOS. This offers an experimental foundation for future clinical translation.
5.2.2 Evidence from human studies
5.2.2.1 TCM botanical drug formulas
In recent years, researchers have investigated the potential benefits of augmenting the effectiveness of conventional modern medicine treatments for IR in PCOS by incorporating TCM botanical drug formulas.
A prospective RCT demonstrated that combining Diane-35 with Dingkun Pill, in addition to Diane-35 alone, offers greater benefits in enhancing IR (Deng et al., 2020). Furthermore, the study revealed that combining Dingkun Pill with Diane-35, beyond Dingkun Pill alone, is more effective in decreasing total T levels and DHEAS, while increasing the rate of menstrual recovery (Deng et al., 2020). These findings underscore the synergistic and complementary therapeutic effects of the combined approach, providing a more comprehensive intervention targeting the multifaceted pathological aspects of PCOS. Shi et al. (2022) demonstrated that combining Jiawei Huanglian Wendan Decoction (JHWD) with metformin and Diane-35 in the treatment of PCOS patients with IR significantly enhances the patients’ IR status. This combined therapy also exhibits benefits in ameliorating additional parameters such as blood lipid levels (TC, triglycerides, LDL) and inflammatory markers (TNF-α, IL-6, IL-1) (Shi et al., 2022). These findings suggest that integrating TCM botanical drug formulas with standard treatment can comprehensively address metabolic irregularities and clinical manifestations in PCOS patients with IR.
Further clinical evidence reinforces the synergistic impact of integrating TCM botanical drug formulas with conventional modern medicine for managing IR in PCOS patients. Various studies, employing diverse experimental designs and outcome measures, consistently demonstrate the benefits of this combined approach in enhancing insulin sensitivity, managing metabolic irregularities, and addressing associated clinical manifestations. Specific details regarding drug compositions and trial characteristics are outlined in Table 4.

Table 4. Human studies on the combined application of TCM botanical drug formulas in improving IR in PCOS.
5.2.2.2 TCM botanical drug metabolites
Puerarin, an isoflavone derived from the roots of Pueraria lobata, a leguminous plant in traditional Chinese medicine, exhibits various pharmacological properties such as anti-inflammatory, IR improvement, and immunomodulatory effects (Wang S. et al., 2020; Wei et al., 2024; Zhang D. et al., 2024; Wu Q. et al., 2025). It is clinically utilized in treating cardiovascular and cerebrovascular diseases, diabetes, and its complications, as well as coronary heart disease (Wang et al., 2020b). A clinical trial involving Chinese women with PCOS demonstrated that supplementing the standard treatment of Diane-35 and metformin with puerarin effectively enhances insulin sensitivity in non-obese PCOS patients (Li et al., 2021). Additionally, the study revealed that puerarin supplementation elevates SHBG levels while reducing testosterone concentrations, indicating its potential in ameliorating HA in PCOS patients and supporting its adjunctive role in PCOS management (Li et al., 2021).
Multiple studies have confirmed the supplementary effectiveness of various TCM botanical drug metabolites, beyond puerarin, in ameliorating IR in individuals with PCOS. These TCM botanical drug metabolites engage in regulating metabolic disturbances associated with PCOS through diverse mechanisms, notably enhancing insulin sensitivity and exhibiting synergistic actions in modulating hormone profiles. Detailed experimental findings are outlined in Table 5.
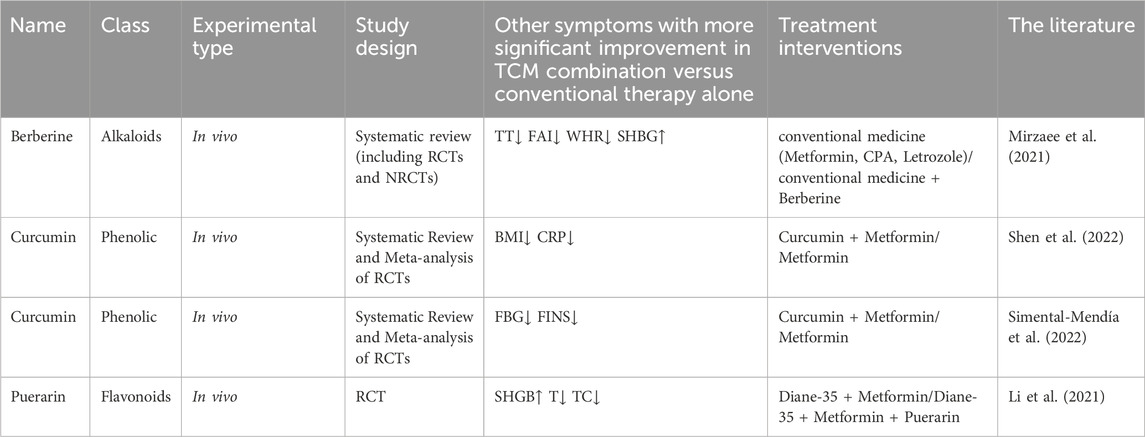
Table 5. Human studies on the combined application of TCM botanical drug metabolites to improve IR in PCOS.
6 Conclusion and prospects
This review validates the substantial therapeutic efficacy of TCM in ameliorating IR among with PCOS through both experimental and clinical investigations. Furthermore, it demonstrates that TCM can enhance and manage IR in PCOS patients through diverse mechanisms. Nevertheless, extant studies exhibit certain limitations. Primarily, the majority of current clinical investigations suffer from restricted geographical coverage in patient recruitment and relatively modest sample sizes, thereby necessitating further validation of the generalizability of the findings. Additionally, the follow-up durations in most clinical trials are brief or nonexistent, precluding the assessment of the enduring effects and safety profile of TCM in mitigating insulin resistance. However, animal models for PCOS often rely on hormone induction and high-fat diets, deviating from the natural disease progression in humans. This discrepancy hinders the accurate replication of the complex clinical pathology. In the realm of TCM research, challenges arise not only from the variability in the quality and composition of medicinal materials across different batches but also from the absence of standardized dosing regimens. Discrepancies in the dosage, frequency of administration, and duration of treatment with TCM monomers or compounds are commonly observed in animal studies, hindering the comparability of research findings and compromising the reliability and reproducibility of conclusions. This lack of standardization, spanning from the sourcing of medicinal materials to dosing protocols, not only complicates inter-study comparisons but also undermines the interpretability and clinical applicability of research outcomes. Additionally, the predominant use of rats and mice in research overlooks the potential benefits of employing animal models more closely resembling human physiology, such as non-human primates. Future investigations should prioritize large-scale, multi-center randomized clinical trials with extended follow-up periods. Standardizing the dose-response relationship of TCM is imperative. Emphasizing the selection of animal models that mirror human physiological characteristics can enhance the fidelity of PCOS research and facilitate a more nuanced exploration of dose-response dynamics. These efforts will enrich the therapeutic armamentarium for PCOS-relate IR.
Author contributions
ZS: Conceptualization, Formal Analysis, Visualization, Writing – original draft, Writing – review and editing. BJ: Conceptualization, Formal Analysis, Writing – review and editing. HH: Visualization, Writing – review and editing. ZQ: Visualization, Writing – review and editing. YS: Writing – review and editing. YZ: Funding acquisition, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was financially supported by the National Natural Science Foundation of China (82374286), Project of the Traditional Chinese Medicine Innovation Team and Talent Support Program in Heilongjiang Province (Collaborative Innovation Team for Prevention and Treatment of Polycystic Ovary Syndrome with Traditional Chinese Medicine), Program for Supporting Exceptional Young Academic Leaders at Heilongjiang University of Chinese Medicine (Novel Investigations into the Pathogenesis of Polycystic Ovary Syndrome via Tissue-Specific Androgen Receptor and the Mechanism of Action of Cryptotanshinone, a Chinese Herbal Medicine).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1661806/full#supplementary-material
References
Abbott, D. H., and Dumesic, D. A. (2021). Passing on PCOS: new insights into its epigenetic transmission. Cell Metab. 33, 463–466. doi:10.1016/j.cmet.2021.02.008
Aflatounian, A., Paris, V. R., Richani, D., Edwards, M. C., Cochran, B. J., Ledger, W. L., et al. (2022). Declining muscle NAD+ in a hyperandrogenism PCOS mouse model: possible role in metabolic dysregulation. Mol. Metab. 65, 101583. doi:10.1016/j.molmet.2022.101583
Al Wattar, B. H., M Hussain, N., and S Khan, K. (2022). Lifestyle interventions in women with polycystic ovary syndrome: a scoping systematic review of randomised evidence. Semergen 48, 186–194. doi:10.1016/j.semerg.2021.10.010
An, P., Wan, S., Luo, Y., Luo, J., Zhang, X., Zhou, S., et al. (2022). Micronutrient supplementation to reduce cardiovascular risk. J. Am. Coll. Cardiol. 80, 2269–2285. doi:10.1016/j.jacc.2022.09.048
An, J., Lin, T., Guo, X., Cao, Z., and Lu, Q. (2025). Regulation of the EGFR/PI3K/AKT signaling cascade using the Shengui Yangrong Decoction improves ovulation dysfunction and insulin resistance in polycystic ovary syndrome. Fitoterapia 182, 106407. doi:10.1016/j.fitote.2025.106407
Arentz, S., Smith, C. A., Abbott, J., and Bensoussan, A. (2021). Perceptions and experiences of lifestyle interventions in women with polycystic ovary syndrome (PCOS), as a management strategy for symptoms of PCOS. BMC Womens Health 21, 107. doi:10.1186/s12905-021-01252-1
Arya, S., Hansen, K. R., Peck, J. D., and Wild, R. A.National Institute of Child Health and Human Development Reproductive Medicine Network (2021). Metabolic syndrome in obesity: treatment success and adverse pregnancy outcomes with ovulation induction in polycystic ovary syndrome. Am. J. Obstet. Gynecol. 225, 280.e1–280.e11. doi:10.1016/j.ajog.2021.03.048
Azziz, R., Sanchez, L. A., Knochenhauer, E. S., Moran, C., Lazenby, J., Stephens, K. C., et al. (2004). Androgen excess in women: experience with over 1000 consecutive patients. J. Clin. Endocrinol. Metab. 89, 453–462. doi:10.1210/jc.2003-031122
Babu, A., Devi Rajeswari, V., Ganesh, V., Das, S., Dhanasekaran, S., Usha Rani, G., et al. (2024). Gut microbiome and polycystic ovary syndrome: interplay of associated microbial-metabolite pathways and therapeutic strategies. Reprod. Sci. 31, 1508–1520. doi:10.1007/s43032-023-01450-2
Bäckhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A., and Gordon, J. I. (2005). Host-bacterial mutualism in the human intestine. Science 307, 1915–1920. doi:10.1126/science.1104816
Bahari, H., Shahraki Jazinaki, M., Aghakhani, L., Amini, M. R., Noushzadeh, Z., Khodashahi, R., et al. (2025). Crocin supplementation on inflammation and oxidative stress: a systematic review and meta-analysis. Phytother. Res. 39, 465–479. doi:10.1002/ptr.8380
Baker, R. G., Hayden, M. S., and Ghosh, S. (2011). NF-κB, inflammation, and metabolic disease. Cell Metab. 13, 11–22. doi:10.1016/j.cmet.2010.12.008
Bannister, A. J., and Kouzarides, T. (2011). Regulation of chromatin by histone modifications. Cell Res. 21, 381–395. doi:10.1038/cr.2011.22
Bayramoğlu, E., Çetinkaya, S., Özalkak, S., Kurnaz, E., Demirci, G., Öztürk, H. S., et al. (2021). Evaluation of the pathophysiological role of Fetuin A levels in adolescents with polycystic ovary syndrome. J. Pediatr. Endocrinol. Metab. 34, 911–916. doi:10.1515/jpem-2020-0524
Belani, M., Deo, A., Shah, P., Banker, M., Singal, P., and Gupta, S. (2018). Differential insulin and steroidogenic signaling in insulin resistant and non-insulin resistant human luteinized granulosa cells-A study in PCOS patients. J. Steroid Biochem. Mol. Biol. 178, 283–292. doi:10.1016/j.jsbmb.2018.01.008
Blais, J. E., Huang, X., and Zhao, J. V. (2023). Overall and sex-specific effect of berberine for the treatment of dyslipidemia in adults: a systematic review and meta-analysis of randomized placebo-controlled trials. Drugs 83, 403–427. doi:10.1007/s40265-023-01841-4
Burghen, G. A., Givens, J. R., and Kitabchi, A. E. (1980). Correlation of hyperandrogenism with hyperinsulinism in polycystic ovarian disease. J. Clin. Endocrinol. Metab. 50, 113–116. doi:10.1210/jcem-50-1-113
Cara, J. F., and Rosenfield, R. L. (1988). Insulin-like growth factor I and insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology 123, 733–739. doi:10.1210/endo-123-2-733
Cheatham, B., and Kahn, C. R. (1995). Insulin action and the insulin signaling network. Endocr. Rev. 16, 117–142. doi:10.1210/edrv-16-2-117
Chen, Y.-X., Gao, Q.-Y., Zou, T.-H., Wang, B.-M., Liu, S.-D., Sheng, J.-Q., et al. (2020). Berberine versus placebo for the prevention of recurrence of colorectal adenoma: a multicentre, double-blinded, randomised controlled study. Lancet Gastroenterol. Hepatol. 5, 267–275. doi:10.1016/S2468-1253(19)30409-1
Chen, F., Chen, Z., Chen, M., Chen, G., Huang, Q., Yang, X., et al. (2021a). Reduced stress-associated FKBP5 DNA methylation together with gut microbiota dysbiosis is linked with the progression of obese PCOS patients. NPJ Biofilms Microbiomes 7, 60. doi:10.1038/s41522-021-00231-6
Chen, J., Guan, L., Liu, H., Liu, Q., Fan, P., and Bai, H. (2021b). GALNT2 gene variant rs4846914 is associated with insulin and insulin resistance depending on BMI in PCOS patients: a case-control study. Reprod. Sci. 28, 1122–1132. doi:10.1007/s43032-020-00380-7
Chen, Y., Guo, J., Zhang, Q., and Zhang, C. (2022). Insulin resistance is a risk factor for early miscarriage and macrosomia in patients with polycystic ovary syndrome from the first embryo transfer cycle: a retrospective cohort study. Front. Endocrinol. (Lausanne) 13, 853473. doi:10.3389/fendo.2022.853473
Chen, H., Deng, C., Meng, Z., and Meng, S. (2023). Effects of TCM on polycystic ovary syndrome and its cellular endocrine mechanism. Front. Endocrinol. (Lausanne) 14, 956772. doi:10.3389/fendo.2023.956772
Chiaffarino, F., Cipriani, S., Dalmartello, M., Ricci, E., Esposito, G., Fedele, F., et al. (2022). Prevalence of polycystic ovary syndrome in European countries and USA: a systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 279, 159–170. doi:10.1016/j.ejogrb.2022.10.020
Corbould, A., Zhao, H., Mirzoeva, S., Aird, F., and Dunaif, A. (2006). Enhanced mitogenic signaling in skeletal muscle of women with polycystic ovary syndrome. Diabetes 55, 751–759. doi:10.2337/diabetes.55.03.06.db05-0453
Cox, F. F., Misiou, A., Vierkant, A., Ale-Agha, N., Grandoch, M., Haendeler, J., et al. (2022). Protective effects of curcumin in cardiovascular diseases-impact on oxidative stress and mitochondria. Cells 11, 342. doi:10.3390/cells11030342
Cussen, L., McDonnell, T., Miller, C., McIlroy, M., and O’Reilly, M. W. (2025). Polycystic ovary syndrome: origins and implications: polycystic ovary syndrome: the impact of androgen excess on metabolic health. Reproduction 170, e250102. doi:10.1530/REP-25-0102
Dadachanji, R., Sawant, D., Patil, A., and Mukherjee, S. (2021). Replication study of THADA rs13429458 variant with PCOS susceptibility and its related traits in Indian women. Gynecol. Endocrinol. 37, 716–720. doi:10.1080/09513590.2021.1906854
Dalmasso, C., Maranon, R., Patil, C., Bui, E., Moulana, M., Zhang, H., et al. (2016). Cardiometabolic effects of chronic hyperandrogenemia in a new model of postmenopausal polycystic ovary syndrome. Endocrinology 157, 2920–2927. doi:10.1210/en.2015-1617
de Alvaro, C., Teruel, T., Hernandez, R., and Lorenzo, M. (2004). Tumor necrosis factor alpha produces insulin resistance in skeletal muscle by activation of inhibitor kappaB kinase in a p38 MAPK-dependent manner. J. Biol. Chem. 279, 17070–17078. doi:10.1074/jbc.M312021200
de La Fernandes, M. L., Saad, M. J., and Velloso, L. A. (1999). Insulin induces tyrosine phosphorylation of the insulin receptor and SHC, and SHC/GRB2 association in cerebellum but not in forebrain cortex of rats. Brain Res. 826, 74–82. doi:10.1016/s0006-8993(99)01118-x
Deer, E., LaMarca, B., Reckelhoff, J. F., Shawky, N. M., and Edwards, K. (2025). The role of mitochondrial dysfunction and oxidative stress in women’s reproductive disorders: implications for polycystic ovary syndrome and preeclampsia. Int. J. Mol. Sci. 26, 6439. doi:10.3390/ijms26136439
Deng, Y., Wang, Y.-F., Zhu, S.-Y., Ma, X., Xue, W., Ma, R.-L., et al. (2020). Is there an advantage of using dingkun pill alone or in combination with Diane-35 for management of polycystic ovary syndrome? A randomized controlled trial. Chin. J. Integr. Med. 26, 883–889. doi:10.1007/s11655-020-3097-4
Deng, H., Chen, Y., Xing, J., Zhang, N., and Xu, L. (2024). Systematic low-grade chronic inflammation and intrinsic mechanisms in polycystic ovary syndrome. Front. Immunol. 15, 1470283. doi:10.3389/fimmu.2024.1470283
Deswal, R., Yadav, A., and Dang, A. S. (2018). Sex hormone binding globulin - an important biomarker for predicting PCOS risk: a systematic review and meta-analysis. Syst. Biol. Reprod. Med. 64, 12–24. doi:10.1080/19396368.2017.1410591
Diamanti-Kandarakis, E., and Dunaif, A. (2012). Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr. Rev. 33, 981–1030. doi:10.1210/er.2011-1034
Ding, Y., Jiang, Z., Xia, B., Zhang, L., Zhang, C., and Leng, J. (2019). Mitochondria-targeted antioxidant therapy for an animal model of PCOS-IR. Int. J. Mol. Med. 43, 316–324. doi:10.3892/ijmm.2018.3977
Dong, S., Yao, X., Jiao, J., Lin, B., Yan, F., and Wang, X. (2024). Fecal propionate is a signature of insulin resistance in polycystic ovary syndrome. Front. Cell Infect. Microbiol. 14, 1394873. doi:10.3389/fcimb.2024.1394873
Dong, Y., Yang, S., Zhang, S., Zhao, Y., Li, X., Han, M., et al. (2025). Modulatory impact of Bifidobacterium longum subsp. longum BL21 on the gut-brain-ovary axis in polycystic ovary syndrome: insights into metabolic regulation, inflammation mitigation, and neuroprotection. mSphere 10, e0088724. doi:10.1128/msphere.00887-24
Dubey, R. K., and Kumar, B. (2025). A comprehensive overview of traditional chinese medicine (TCM). Zhongguo Ying Yong Sheng Li Xue Za Zhi 41, e20250015. doi:10.62958/j.cjap.2025.015
Dumesic, D. A., and Abbott, D. H. (2008). Implications of polycystic ovary syndrome on oocyte development. Semin Reprod Med. 26 (1):53–61. doi:10.1055/s-2007-992925
Dunaif, A. (1997). Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr. Rev. 18, 774–800. doi:10.1210/edrv.18.6.0318
Dunaif, A., Wu, X., Lee, A., and Diamanti-Kandarakis, E. (2001). Defects in insulin receptor signaling in vivo in the polycystic ovary syndrome (PCOS). Am. J. Physiol. Endocrinol. Metab. 281, E392–E399. doi:10.1152/ajpendo.2001.281.2.E392
Eepho, O.-I., Bashir, A.-A. M., Oniyide, A. A., Aturamu, A., Owolabi, O. V., Ajadi, I. O., et al. (2023). Modulation of GABA by sodium butyrate ameliorates hypothalamic inflammation in experimental model of PCOS. BMC Neurosci. 24, 62. doi:10.1186/s12868-023-00834-z
Elibol, E., Eski, S., Gez, E., and Çamdeviren, G. (2025). The impact of carbohydrate quality index on menopausal symptoms and quality of life in postmenopausal women. BMC Womens Health 25, 262. doi:10.1186/s12905-025-03822-z
Elkind-Hirsch, K. E., Valdes, C. T., and Malinak, L. R. (1993). Insulin resistance improves in hyperandrogenic women treated with Lupron. Fertil. Steril. 60, 634–641. doi:10.1016/s0015-0282(16)56213-x
Esposito, K., Chiodini, P., Capuano, A., Bellastella, G., Maiorino, M. I., and Giugliano, D. (2014). Metabolic syndrome and endometrial cancer: a meta-analysis. Endocrine 45, 28–36. doi:10.1007/s12020-013-9973-3
Ezeh, U., Ezeh, C., Pisarska, M. D., and Azziz, R. (2021). Menstrual dysfunction in polycystic ovary syndrome: association with dynamic state insulin resistance rather than hyperandrogenism. Fertil. Steril. 115, 1557–1568. doi:10.1016/j.fertnstert.2020.12.015
Fareed, M., and Afzal, M. (2013). Single nucleotide polymorphism in genome-wide association of human population: a tool for broad spectrum service. Egypt. J. Med. Hum. Genet. 14, 123–134. doi:10.1016/j.ejmhg.2012.08.001
Farhadi-Azar, M., Noroozzadeh, M., Mousavi, M., Saei Ghare Naz, M., and Ramezani Tehrani, F. (2025). Impaired glucose tolerance and insulin resistance in a prenatally-androgenized rat model of polycystic ovary syndrome in later life. Exp. Physiol. 110, 410–423. doi:10.1113/EP091912
Feghhi, F., Ghaznavi, H., Sheervalilou, R., Razavi, M., and Sepidarkish, M. (2024). Effects of metformin and curcumin in women with polycystic ovary syndrome: a factorial clinical trial. Phytomedicine 135, 156160. doi:10.1016/j.phymed.2024.156160
Finnie, J. W., and Blumbergs, P. C. (2022). Animal models of pediatric abusive head trauma. Childs Nerv. Syst. 38, 2317–2324. doi:10.1007/s00381-022-05577-6
Foda, A. M., Ibrahim, S. S., Ibrahim, S. M., and Elbaz, E. M. (2024). Pterostilbene ameliorates cognitive impairment in polycystic ovary syndrome rat model through improving insulin resistance via the IRS-1/PI3K/Akt/GSK-3β pathway: a comparative study with metformin. ACS Chem. Neurosci. 15, 3064–3077. doi:10.1021/acschemneuro.4c00352
Folli, F., Saad, M. J., Backer, J. M., and Kahn, C. R. (1992). Insulin stimulation of phosphatidylinositol 3-kinase activity and association with insulin receptor substrate 1 in liver and muscle of the intact rat. J. Biol. Chem. 267, 22171–22177. doi:10.1016/s0021-9258(18)41650-x
Ford, E. B. (1957). Polymorphism in plants, animals and man. Nature 180, 1315–1319. doi:10.1038/1801315a0
Frandsen, C. L. B., Svendsen, P. F., Nøhr, B., Viuff, J. H., Maltesen, T., Kjaer, S. K., et al. (2023). Risk of epithelial ovarian tumors among women with polycystic ovary syndrome: a nationwide population-based cohort study. Int. J. Cancer 153, 958–968. doi:10.1002/ijc.34574
Frandsen, C. L. B., Gottschau, M., Nøhr, B., Viuff, J. H., Maltesen, T., Kjær, S. K., et al. (2024). Polycystic ovary syndrome and endometrial cancer risk: results from a nationwide cohort study. Am. J. Epidemiol. 193, 1399–1406. doi:10.1093/aje/kwae061
Frangogiannis, N. G. (2022). Why animal model studies are lost in translation. J. Cardiovasc Aging 2, 22. doi:10.20517/jca.2022.10
Fu, X.-P., Xu, L., Fu, B.-B., Wei, K.-N., Liu, Y., Liao, B.-Q., et al. (2020). Pachymic acid protects oocyte by improving the ovarian microenvironment in polycystic ovary syndrome mice. Biol. Reprod. 103, 1085–1098. doi:10.1093/biolre/ioaa141
Fu, L., Xie, N., Qu, F., Zhou, J., and Wang, F. (2023). The association between polycystic ovary syndrome and metabolic syndrome in adolescents: a systematic review and meta-analysis. Reprod. Sci. 30, 28–40. doi:10.1007/s43032-022-00864-8
Fu, L.-W., Gao, Z., Zhang, N., Yang, N., Long, H.-Y., Kong, L.-Y., et al. (2024a). Traditional Chinese medicine formulae: a complementary method for the treatment of polycystic ovary syndrome. J. Ethnopharmacol. 323, 117698. doi:10.1016/j.jep.2023.117698
Fu, Y., Xie, P., Yang, Q., Chen, P., and Yu, J. (2024b). Analysis on the therapeutic effect of Cangfu Daotan Decoction combined with drospirenone and ethinylestradiol tablets (II) on patients with polycystic ovary syndrome. Prostagl. Other Lipid Mediat 170, 106801. doi:10.1016/j.prostaglandins.2023.106801
Gao, Z., Hwang, D., Bataille, F., Lefevre, M., York, D., Quon, M. J., et al. (2002). Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J. Biol. Chem. 277, 48115–48121. doi:10.1074/jbc.M209459200
Gao, M., Liu, X., Gu, H., Xu, H., Zhong, W., Wei, X., et al. (2024). Association between single nucleotide polymorphisms, TGF-β1 promoter methylation, and polycystic ovary syndrome. BMC Pregnancy Childbirth 24, 5. doi:10.1186/s12884-023-06210-3
Garimella, J. N., and Pradhan, R. C. (2024). Effect of (multi pin) atmospheric cold plasma treatment on curcumin extraction and investigating phytochemicals, antioxidants, physical and morphological properties of turmeric (Curcuma longa L.) powder. Food Chem. 449, 139233. doi:10.1016/j.foodchem.2024.139233
Gautam, R., Maan, P., Patel, A. K., Vasudevan, S., and Arora, T. (2024). Unveiling the complex interplay between gut microbiota and polycystic ovary syndrome: a narrative review. Clin. Nutr. 43, 199–208. doi:10.1016/j.clnu.2024.10.028
Glueck, C. J., Wang, P., Fontaine, R., Tracy, T., and Sieve-Smith, L. (2001). Metformin to restore normal menses in oligo-amenorrheic teenage girls with polycystic ovary syndrome (PCOS). J. Adolesc. Health 29, 160–169. doi:10.1016/s1054-139x(01)00202-6
González, F., Rote, N. S., Minium, J., and Kirwan, J. P. (2006). Increased activation of nuclear factor kappaB triggers inflammation and insulin resistance in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 91, 1508–1512. doi:10.1210/jc.2005-2327
González, F., Considine, R. V., Abdelhadi, O. A., and Acton, A. J. (2020). Lipid-induced mononuclear cell cytokine secretion in the development of metabolic aberration and androgen excess in polycystic ovary syndrome. Hum. Reprod. 35, 1168–1177. doi:10.1093/humrep/deaa056
Graham, E. J., and Selgrade, J. F. (2017). A model of ovulatory regulation examining the effects of insulin-mediated testosterone production on ovulatory function. J. Theor. Biol. 416, 149–160. doi:10.1016/j.jtbi.2017.01.007
Greenberg, M. V. C., and Bourc’his, D. (2019). The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 20, 590–607. doi:10.1038/s41580-019-0159-6
Guan, H.-R., Li, B., Zhang, Z.-H., Wu, H.-S., Wang, N., Chen, X.-F., et al. (2024). Exploring the efficacy and mechanism of bailing capsule to improve polycystic ovary syndrome in mice based on intestinal-derived LPS-TLR4 pathway. J. Ethnopharmacol. 331, 118274. doi:10.1016/j.jep.2024.118274
Guo, H., Ingolia, N. T., Weissman, J. S., and Bartel, D. P. (2010). Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840. doi:10.1038/nature09267
Guo, L., Gordon, N. P., Chandra, M., Dayo, O., and Lo, J. C. (2021). The risks of polycystic ovary syndrome and diabetes vary by ethnic subgroup among young Asian women. Diabetes Care 44, e129–e130. doi:10.2337/dc21-0373
Guo, Z., Jin, F., Chen, S., Hu, P., Hao, Y., and Yu, Q. (2023). Correlation between biochemical and clinical hyperandrogenism parameter in polycystic ovary syndrome in relation to age. BMC Endocr. Disord. 23, 89. doi:10.1186/s12902-023-01346-x
Gupta, J., Antal, Z., Mauer, E., Gerber, L. M., An, A., and Censani, M. (2022). Dysglycemia screening with oral glucose tolerance test in adolescents with polycystic ovary syndrome and relationship with obesity. BMC Endocr. Disord. 22, 180. doi:10.1186/s12902-022-01098-0
Hai, Y., Zuo, L., Wang, M., Zhang, R., Wang, M., Ren, L., et al. (2023). Icariin alleviates nonalcoholic fatty liver disease in polycystic ovary syndrome by improving liver fatty acid oxidation and inhibiting lipid accumulation. Molecules 28, 517. doi:10.3390/molecules28020517
Hammes, M. (2013). Huang Di nei jing su wen — an Annotated Translation of Huang Di’s Inner Classic — basic Questions. Dtsch. Z Akupunkt. 56, 55. doi:10.1016/j.dza.2013.06.025
Hao, X., Ma, J., Zhang, L., Meng, T., and Ma, Q. (2024). The relationship between thyroid hormones and insulin resistance in polycystic ovary syndrome women. Gynecol. Obstet. Invest. 89, 512–519. doi:10.1159/000539361
Hao, C., Hu, K., Xie, J., Tong, X., Zhang, X., Qi, Z., et al. (2025). Recent advancements in the biomanufacturing of crocetin and crocins: key enzymes and metabolic engineering. J. Agric. Food Chem. 73, 6400–6415. doi:10.1021/acs.jafc.4c12576
He, F., and Li, Y. (2021). The gut microbial composition in polycystic ovary syndrome with insulin resistance: findings from a normal-weight population. J. Ovarian Res. 14, 50. doi:10.1186/s13048-021-00799-9
He, S., Ji, D., Liu, Y., Deng, X., Zou, W., Liang, D., et al. (2023). Polymorphisms of mtDNA in the D-loop region moderate the associations of BMI with HOMA-IR and HOMA-β among women with polycystic ovary syndrome: a cross-sectional study. J. Assist. Reprod. Genet. 40, 1983–1993. doi:10.1007/s10815-023-02843-7
Hers, I., Vincent, E. E., and Tavaré, J. M. (2011). Akt signalling in health and disease. Cell. Signal. 23, 1515–1527. doi:10.1016/j.cellsig.2011.05.004
Heshmati, J., Moini, A., Sepidarkish, M., Morvaridzadeh, M., Salehi, M., Palmowski, A., et al. (2021). Effects of curcumin supplementation on blood glucose, insulin resistance and androgens in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Phytomedicine 80, 153395. doi:10.1016/j.phymed.2020.153395
Hipólito-Reis, M., Neto, A. C., and Neves, D. (2022). Impact of curcumin, quercetin, or resveratrol on the pathophysiology of endometriosis: a systematic review. Phytother. Res. 36, 2416–2433. doi:10.1002/ptr.7464
Hofmann, K., Singer, S., Theis, S., Dionysopoulou, A., Schiestl, L., Degirmenci, Y., et al. (2025). Mental state and health-related quality of life in patients with polycystic ovary syndrome under metformin therapy - a prospective study. J. Psychosom. Obstet. Gynaecol. 46, 2516669. doi:10.1080/0167482X.2025.2516669
Hotamisligil, G. S., Budavari, A., Murray, D., and Spiegelman, B. M. (1994). Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J. Clin. Invest. 94, 1543–1549. doi:10.1172/JCI117495
Hotamisligil, G. S., Peraldi, P., Budavari, A., Ellis, R., White, M. F., and Spiegelman, B. M. (1996). IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271, 665–668. doi:10.1126/science.271.5249.665
Hu, M., Zhang, Y., Guo, X., Jia, W., Liu, G., Zhang, J., et al. (2019). Hyperandrogenism and insulin resistance induce gravid uterine defects in association with mitochondrial dysfunction and aberrant reactive oxygen species production. Am. J. Physiol. Endocrinol. Metab. 316, E794–E809. doi:10.1152/ajpendo.00359.2018
Hu, M.-H., Zheng, S.-X., Yin, H., Zhu, X.-Y., Lu, F.-T., Tong, X.-H., et al. (2020). Identification of microRNAs that regulate the MAPK pathway in human cumulus cells from PCOS women with insulin resistance. Reprod. Sci. 27, 833–844. doi:10.1007/s43032-019-00086-5
Huang, Y., and Zhang, X. (2021). Luteolin alleviates polycystic ovary syndrome in rats by resolving insulin resistance and oxidative stress. Am. J. Physiol. Endocrinol. Metab. 320, E1085–E1092. doi:10.1152/ajpendo.00034.2021
Huang, J., Ni, R., Chen, X., Huang, L., Mo, Y., and Yang, D. (2010). Metabolic abnormalities in adolescents with polycystic ovary syndrome in south China. Reprod. Biol. Endocrinol. 8, 142. doi:10.1186/1477-7827-8-142
Huang, X., Liu, G., Guo, J., and Su, Z. (2018). The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 14, 1483–1496. doi:10.7150/ijbs.27173
Huang, J.-G., Zhang, J., Xiang, R.-J., Wu, Y.-Y., He, T.-Y., Zhao, M.-N., et al. (2024a). Mechanism of kuntai capsules in treatment of polycystic ovary syndrome rat models based on AGE-RAGE signal pathway. Zhongguo Zhong Yao Za Zhi 49, 1082–1090. doi:10.19540/j.cnki.cjcmm.20231115.501
Huang, Q., Li, Y., Chen, Z., Ou, H., Tan, Y., and Lin, H. (2024b). Bushenhuoluo decoction improves polycystic ovary syndrome by regulating exosomal miR-30a-5p/SOCS3/mTOR/NLRP3 signaling-mediated autophagy and pyroptosis. J. Ovarian Res. 17, 29. doi:10.1186/s13048-024-01355-x
Huo, P., Li, M., Le, J., Zhu, C., Yao, J., and Zhang, S. (2023). Resveratrol improves follicular development of PCOS rats via regulating glycolysis pathway and targeting SIRT1. Syst. Biol. Reprod. Med. 69, 153–165. doi:10.1080/19396368.2022.2125855
Ignjatović, Đ., Tovilović-Kovačević, G., Mićić, B., Tomić, M., Djordjevic, A., Macut, D., et al. (2023). Effects of early life overnutrition and hyperandrogenism on spatial learning and memory in a rat model of polycystic ovary syndrome. Horm. Behav. 153, 105392. doi:10.1016/j.yhbeh.2023.105392
James, D. E., Stöckli, J., and Birnbaum, M. J. (2021). The aetiology and molecular landscape of insulin resistance. Nat. Rev. Mol. Cell Biol. 22, 751–771. doi:10.1038/s41580-021-00390-6
Jamilian, M., Foroozanfard, F., Kavossian, E., Aghadavod, E., Shafabakhsh, R., Hoseini, A., et al. (2020). Effects of curcumin on body weight, glycemic control and serum lipids in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Clin. Nutr. ESPEN 36, 128–133. doi:10.1016/j.clnesp.2020.01.005
Javed, A., Kumar, S., Simmons, P. S., and Lteif, A. N. (2015). Phenotypic characterization of polycystic ovary syndrome in adolescents based on menstrual irregularity. Horm. Res. Paediatr. 84, 223–230. doi:10.1159/000435883
Jiang, X., Liu, R., Liao, T., He, Y., Li, C., Guo, P., et al. (2021). A predictive model of live birth based on obesity and metabolic parameters in patients with PCOS undergoing frozen-thawed embryo transfer. Front. Endocrinol. (Lausanne) 12, 799871. doi:10.3389/fendo.2021.799871
Jiang, X.-L., Tai, H., Xiao, X.-S., Zhang, S.-Y., Cui, S.-C., Qi, S.-B., et al. (2022). Cangfudaotan decoction inhibits mitochondria-dependent apoptosis of granulosa cells in rats with polycystic ovarian syndrome. Front. Endocrinol. (Lausanne) 13, 962154. doi:10.3389/fendo.2022.962154
Jie, H.-Y., Zhou, X., Zhao, M.-P., Hu, M., Mai, Q.-Y., and Zhou, C.-Q. (2022). Pregnancy outcomes in patients with polycystic ovary syndrome who conceived after single thawed blastocyst transfer: a propensity score-matched study. BMC Pregnancy Childbirth 22, 718. doi:10.1186/s12884-022-05011-4
Joham, A. E., Rees, D. A., Shinkai, K., Forslund, M., Tay, C. T., and Teede, H. J. (2025). Polycystic ovary syndrome: origins and implications: epidemiological aspects of polycystic ovary syndrome. Reproduction 170, e250121. doi:10.1530/REP-25-0121
Kadowaki, T., Hara, K., Kubota, N., Tobe, K., Terauchi, Y., Yamauchi, T., et al. (2002). The role of PPARgamma in high-fat diet-induced obesity and insulin resistance. J. Diabetes Complicat. 16, 41–45. doi:10.1016/s1056-8727(01)00206-9
Kamada, S., Yamamoto, Y., Aoki, H., Tamura, K., Takeda, A., Minato, S., et al. (2022). A novel PCOS rat model and an evaluation of its reproductive, metabolic, and behavioral phenotypes. Reprod. Med. Biol. 21, e12416. doi:10.1002/rmb2.12416
Kang, S., Tsai, L. T., Zhou, Y., Evertts, A., Xu, S., Griffin, M. J., et al. (2015). Identification of nuclear hormone receptor pathways causing insulin resistance by transcriptional and epigenomic analysis. Nat. Cell Biol. 17, 44–56. doi:10.1038/ncb3080
Kara, O., Kaymaz, N., and Uzun, M. E. (2022). The effect of hyperandrogenism and obesity on mindfulness and metacognition in adolescents with polycystic ovary syndrome. Arch. Womens Ment. Health 25, 911–921. doi:10.1007/s00737-022-01264-2
Kelly, C. C., Lyall, H., Petrie, J. R., Gould, G. W., Connell, J. M., and Sattar, N. (2001). Low grade chronic inflammation in women with polycystic ovarian syndrome. J. Clin. Endocrinol. Metab. 86, 2453–2455. doi:10.1210/jcem.86.6.7580
Kim, J. H., Jung, M. H., Hong, S. H., Moon, N., and Kang, D. R. (2022). Age-adjusted prevalence and characteristics of women with polycystic ovarian syndrome in Korea: a nationwide population-based study (2010-2019). Yonsei Med. J. 63, 794–798. doi:10.3349/ymj.2022.63.8.794
Kobayashi, H., Matsubara, S., Yoshimoto, C., Shigetomi, H., and Imanaka, S. (2025). A comprehensive review of the contribution of mitochondrial DNA mutations and dysfunction in polycystic ovary syndrome, supported by secondary database analysis. Int. J. Mol. Sci. 26, 1172. doi:10.3390/ijms26031172
Kong, W.-J., Vernieri, C., Foiani, M., and Jiang, J.-D. (2020). Berberine in the treatment of metabolism-related chronic diseases: a drug cloud (dCloud) effect to target multifactorial disorders. Pharmacol. Ther. 209, 107496. doi:10.1016/j.pharmthera.2020.107496
Kopp, F., and Mendell, J. T. (2018). Functional classification and experimental dissection of long noncoding RNAs. Cell 172, 393–407. doi:10.1016/j.cell.2018.01.011
Krentowska, A., Ponikwicka-Tyszko, D., Łebkowska, A., Adamska, A., Sztachelska, M., Milewska, G., et al. (2024). Serum expression levels of selected microRNAs and their association with glucose metabolism in young women with polycystic ovary syndrome. Pol. Arch. Intern Med. 134, 16637. doi:10.20452/pamw.16637
Lambrinoudaki, I. (2011). Cardiovascular risk in postmenopausal women with the polycystic ovary syndrome. Maturitas 68, 13–16. doi:10.1016/j.maturitas.2010.09.005
Leong, K. G., and Karsan, A. (2000). Signaling pathways mediated by tumor necrosis factor alpha. Histol. Histopathol. 15, 1303–1325. doi:10.14670/HH-15.1303
Li, W., Hu, H., Zou, G., Ma, Z., Liu, J., and Li, F. (2021). Therapeutic effects of puerarin on polycystic ovary syndrome: a randomized trial in Chinese women. Med. (Baltimore) 100, e26049. doi:10.1097/MD.0000000000026049
Li, M., Gao, S., Kang, M., Zhang, X., Lan, P., Wu, X., et al. (2023a). Quercitrin alleviates lipid metabolism disorder in polycystic ovary syndrome-insulin resistance by upregulating PM20D1 in the PI3K/Akt pathway. Phytomedicine 117, 154908. doi:10.1016/j.phymed.2023.154908
Li, P., Shuai, P., Shen, S., Zheng, H., Sun, P., Zhang, R., et al. (2023b). Perturbations in gut microbiota composition in patients with polycystic ovary syndrome: a systematic review and meta-analysis. BMC Med. 21, 302. doi:10.1186/s12916-023-02975-8
Li, Y., Wang, Y., Liu, H., Zhang, S., and Zhang, C. (2023c). Association between HOMA-IR and ovarian sensitivity index in women with PCOS undergoing ART: a retrospective cohort study. Front. Endocrinol. (Lausanne) 14, 1117996. doi:10.3389/fendo.2023.1117996
Li, J., Liu, D., Zhao, H., Zhang, P., Cai, F., Li, H., et al. (2024a). Chinese medicine compound prescription HeQi San ameliorates chronic inflammatory states and modulates gut flora in dehydroepiandrosterone-induced polycystic ovary syndrome mouse model. Int. Immunopharmacol. 137, 112491. doi:10.1016/j.intimp.2024.112491
Li, X., Yi, Y., Ren, Y., Zhang, Y., Wang, C. C., Liu, C., et al. (2024b). Zishen Qingre Lishi Huayu recipe may ameliorate the symptoms of PCOS model rats via alleviating systemic and ovarian inflammation. Am. J. Reprod. Immunol. 92, e13918. doi:10.1111/aji.13918
Lian, Y., Zhao, F., and Wang, W. (2020). Use of Bao Gui capsule in treatment of a polycystic ovary syndrome rat model. Mol. Med. Rep. 21, 1461–1470. doi:10.3892/mmr.2020.10953
Lin, H., Xing, W., Li, Y., Xie, Y., Tang, X., and Zhang, Q. (2018). Downregulation of serum long noncoding RNA GAS5 may contribute to insulin resistance in PCOS patients. Gynecol. Endocrinol. 34, 784–788. doi:10.1080/09513590.2018.1459548
Lin, S., Li, Y., Liu, W., Du, Y., and Tao, T. (2025). Metabolic implications of elevated neutrophil extracellular traps in polycystic ovary syndrome: a focus on hepatic glycolysis. Biomolecules 15, 572. doi:10.3390/biom15040572
Liu, K., Luo, T., Zhang, Z., Wang, T., Kou, J., Liu, B., et al. (2011). Modified Si-Miao-San extract inhibits inflammatory response and modulates insulin sensitivity in hepatocytes through an IKKβ/IRS-1/Akt-dependent pathway. J. Ethnopharmacol. 136, 473–479. doi:10.1016/j.jep.2011.01.051
Liu, M., Zhu, H., Zhu, Y., and Hu, X. (2021a). Guizhi Fuling Wan reduces autophagy of granulosa cell in rats with polycystic ovary syndrome via restoring the PI3K/AKT/mTOR signaling pathway. J. Ethnopharmacol. 270, 113821. doi:10.1016/j.jep.2021.113821
Liu, S., Hong, L., Mo, M., Xiao, S., Chen, C., Li, Y., et al. (2021b). Evaluation of endometrial immune status of polycystic ovary syndrome. J. Reprod. Immunol. 144, 103282. doi:10.1016/j.jri.2021.103282
Liu, S., Zhang, Y., Yang, F., Gu, J., Zhang, R., Kuang, Y., et al. (2022). Modified Cangfu Daotan decoction ameliorates polycystic ovary syndrome with insulin resistance via NF-κB/LCN-2 signaling pathway in inflammatory microenvironment. Front. Endocrinol. (Lausanne) 13, 975724. doi:10.3389/fendo.2022.975724
Liu, Y.-C., Wang, J.-W., Li, J., Guo, Y., Han, F.-J., Lu, W.-H., et al. (2024). Mechanism of cryptotanshinone to improve endocrine and metabolic functions in the endometrium of PCOS rats. J. Ethnopharmacol. 319, 117346. doi:10.1016/j.jep.2023.117346
Liu, H., Wang, G., Sui, C., Guo, Y., and He, X. (2025). Woxuanzhongzhou formula improves DHEAS and high-fat diet-induced IR and anovulatory mice via AMPK/PGC1- α/Irisin pathway. J. Ovarian Res. 18, 7. doi:10.1186/s13048-025-01587-5
Longo, S., Rizza, S., and Federici, M. (2023). Microbiota-gut-brain axis: relationships among the vagus nerve, gut microbiota, obesity, and diabetes. Acta Diabetol. 60, 1007–1017. doi:10.1007/s00592-023-02088-x
Luo, W., Bai, L., Zhang, J., Li, Z., Liu, Y., Tang, X., et al. (2023). Polysaccharides-based nanocarriers enhance the anti-inflammatory effect of curcumin. Carbohydr. Polym. 311, 120718. doi:10.1016/j.carbpol.2023.120718
Luo, X., Dong, Y., Zheng, H., Zhou, X., Rong, L., Liu, X., et al. (2024). CAPN2 correlates with insulin resistance states in PCOS as evidenced by multi-dataset analysis. J. Ovarian Res. 17, 79. doi:10.1186/s13048-024-01407-2
Ma, D., Wang, S., Shi, Y., Ni, S., Tang, M., and Xu, A. (2021). The development of traditional Chinese medicine. J. Traditional Chin. Med. Sci. 8, S1–S9. doi:10.1016/j.jtcms.2021.11.002
Makker, A., Goel, M. M., Das, V., and Agarwal, A. (2012). PI3K-Akt-mTOR and MAPK signaling pathways in polycystic ovarian syndrome, uterine leiomyomas and endometriosis: an update. Gynecol. Endocrinol. 28, 175–181. doi:10.3109/09513590.2011.583955
Meczekalski, B., Pérez-Roncero, G. R., López-Baena, M. T., Chedraui, P., and Pérez-López, F. R. (2020). The polycystic ovary syndrome and gynecological cancer risk. Gynecol. Endocrinol. 36, 289–293. doi:10.1080/09513590.2020.1730794
Meirow, D., Yossepowitch, O., Rösler, A., Brzezinski, A., Schenker, J. G., Laufer, N., et al. (1995). Insulin resistant and non-resistant polycystic ovary syndrome represent two clinical and endocrinological subgroups. Hum. Reprod. 10, 1951–1956. doi:10.1093/oxfordjournals.humrep.a136215
Melin, J., Forslund, M., Alesi, S., Piltonen, T., Romualdi, D., Spritzer, P. M., et al. (2023). The impact of metformin with or without lifestyle modification versus placebo on polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Eur. J. Endocrinol. 189, S37–S63. doi:10.1093/ejendo/lvad098
Meng, Y., Zhao, T., Zhang, R., Zhu, X., Ma, C., and Shi, Q. (2025). Global burden of polycystic ovary syndrome among women of childbearing age, 1990-2021: a systematic analysis using the global burden of disease study 2021. Front. Public Health 13, 1514250. doi:10.3389/fpubh.2025.1514250
Miazgowski, T., Martopullo, I., Widecka, J., Miazgowski, B., and Brodowska, A. (2021). National and regional trends in the prevalence of polycystic ovary syndrome since 1990 within Europe: the modeled estimates from the global burden of disease study 2016. Arch. Med. Sci. 17, 343–351. doi:10.5114/aoms.2019.87112
Mirzaee, F., Razmjouei, P., Shahrahmani, H., Vafisani, F., Najaf Najafi, M., and Ghazanfarpour, M. (2021). The effect and safety of berberine on polycystic ovary syndrome: a systematic review. J. Obstet. Gynaecol. 41, 684–689. doi:10.1080/01443615.2020.1787964
Misra, S., Gada, J., Dhole, C., Varthakavi, P., and Bhagwat, N. (2024). Comparative study of insulin sensitivity and resistance and their correlation with androgens in lean and Obese women with polycystic ovary syndrome. Reprod. Sci. 31, 754–763. doi:10.1007/s43032-023-01374-x
Moghetti, P., Tosi, F., Castello, R., Magnani, C. M., Negri, C., Brun, E., et al. (1996). The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J. Clin. Endocrinol. Metab. 81, 952–960. doi:10.1210/jcem.81.3.8772557
Moller, D. E. (2000). Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol. Metab. 11, 212–217. doi:10.1016/s1043-2760(00)00272-1
Monroy, J., Cortés, O. D., Domínguez, R., Mendoza-Garrido, M. E., Gallegos, E., Cárdenas, M., et al. (2024). The differential sensitivity of the hypothalamic-hypophysial-ovarian axis to 5-hydroxytryptophan alters the secretion of estradiol. Exp. Physiol. 109, 365–379. doi:10.1113/EP091158
Morris, B. J. (1997). Insulin receptor gene in hypertension. Clin. Exp. Hypertens. 19, 551–565. doi:10.3109/10641969709083169
Morris, A. (2020). Changes to microbiota in girls with PCOS. Nat. Rev. Endocrinol. 16, 196–197. doi:10.1038/s41574-020-0332-1
Mu, J., Yu, P., and Li, Q. (2021a). microRNA-103 contributes to progression of polycystic ovary syndrome through modulating the IRS1/PI3K/AKT signal axis. Arch. Med. Res. 52, 494–504. doi:10.1016/j.arcmed.2021.01.008
Mu, L., Sun, X., Tu, M., and Zhang, D. (2021b). Non-coding RNAs in polycystic ovary syndrome: a systematic review and meta-analysis. Reprod. Biol. Endocrinol. 19, 10. doi:10.1186/s12958-020-00687-9
Nasiri-Amiri, F., Faramarzi, M., Omidvar, S., and Alizadeh-Navaei, R. (2023). Depression and anxiety in adolescents and young women with polycystic ovary syndrome: a systematic review and meta-analysis. Int. J. Adolesc. Med. Health 35, 233–242. doi:10.1515/ijamh-2022-0065
Noverr, M. C., and Huffnagle, G. B. (2004). Does the microbiota regulate immune responses outside the gut? Trends Microbiol. 12, 562–568. doi:10.1016/j.tim.2004.10.008
Oeckinghaus, A., and Ghosh, S. (2009). The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 1, a000034. doi:10.1101/cshperspect.a000034
Olaniyi, K. S., Agan, S. U., Areloegbe, S. E., Sabinari, I. W., Oniyide, A. A., Enye, L. A., et al. (2024). Acetate attenuates hypothalamic pyroptosis in experimentally induced polycystic ovarian syndrome. BMC Res. Notes 17, 260. doi:10.1186/s13104-024-06921-6
Ou, X.-H., Li, S., Wang, Z.-B., Li, M., Quan, S., Xing, F., et al. (2012). Maternal insulin resistance causes oxidative stress and mitochondrial dysfunction in mouse oocytes. Hum. Reprod. 27, 2130–2145. doi:10.1093/humrep/des137
Patel, K., Coffler, M. S., Dahan, M. H., Yoo, R. Y., Lawson, M. A., Malcom, P. J., et al. (2003). Increased luteinizing hormone secretion in women with polycystic ovary syndrome is unaltered by prolonged insulin infusion. J. Clin. Endocrinol. Metab. 88, 5456–5461. doi:10.1210/jc.2003-030816
Patel-Sanchez, N., Perito, E., Tsai, P., Raymond-Flesch, M., Lodish, M., and Sarkar, M. (2023). Prevalence of nonalcoholic fatty liver disease increased with type 2 diabetes mellitus in overweight/obese youth with polycystic ovary syndrome. J. Pediatr. Endocrinol. Metab. 36, 441–446. doi:10.1515/jpem-2022-0527
Pervaz, S., Ullah, A., Adu-Gyamfi, E. A., Lamptey, J., Sah, S. K., Wang, M.-J., et al. (2023). Role of CPXM1 in impaired glucose metabolism and ovarian dysfunction in polycystic ovary syndrome. Reprod. Sci. 30, 526–543. doi:10.1007/s43032-022-00987-y
Qi, X., Yun, C., Sun, L., Xia, J., Wu, Q., Wang, Y., et al. (2019). Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 25, 1225–1233. doi:10.1038/s41591-019-0509-0
Qiu, Z., Dong, J., Xue, C., Li, X., Liu, K., Liu, B., et al. (2020). Liuwei dihuang pills alleviate the polycystic ovary syndrome with improved insulin sensitivity through PI3K/Akt signaling pathway. J. Ethnopharmacol. 250, 111965. doi:10.1016/j.jep.2019.111965
Rahimi, G., Shams, S., and Aslani, M. R. (2022). Effects of crocin supplementation on inflammatory markers, lipid profiles, insulin and cardioprotective indices in women with PCOS: a randomized, double-blind, placebo-controlled trial. Phytother. Res. 36, 2605–2615. doi:10.1002/ptr.7474
Rajkhowa, M., Brett, S., Cuthbertson, D. J., Lipina, C., Ruiz-Alcaraz, A. J., Thomas, G. E., et al. (2009). Insulin resistance in polycystic ovary syndrome is associated with defective regulation of ERK1/2 by insulin in skeletal muscle in vivo. Biochem. J. 418, 665–671. doi:10.1042/BJ20082176
Randriamboavonjy, V., Mann, W. A., Elgheznawy, A., Popp, R., Rogowski, P., Dornauf, I., et al. (2015). Metformin reduces hyper-reactivity of platelets from patients with polycystic ovary syndrome by improving mitochondrial integrity. Thromb. Haemost. 114, 569–578. doi:10.1160/TH14-09-0797
Rasool, S. U. A., Nabi, M., Ashraf, S., and Amin, S. (2022). Insulin receptor substrate 1 Gly972Arg (rs1801278) polymorphism is associated with obesity and insulin resistance in Kashmiri women with polycystic ovary syndrome. Genes (Basel) 13, 1463. doi:10.3390/genes13081463
Rojo, M., Pérez, H., Millán, A. L., Pautasso, M. C., Duarte, A., Abruzzese, G. A., et al. (2024). Mitochondrial DNA oxidation and content in different metabolic phenotypes of women with polycystic ovary syndrome. Front. Endocrinol. (Lausanne) 15, 1501306. doi:10.3389/fendo.2024.1501306
Rong, A., Ta, N., E, L., and Meng, W. (2022). Add-on effect of the Guizhi Fuling formula for management of reduced fertility potential in women with polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Front. Endocrinol. (Lausanne) 13, 995106. doi:10.3389/fendo.2022.995106
Rosenfield, R. L., and Ehrmann, D. A. (2016). The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr. Rev. 37, 467–520. doi:10.1210/er.2015-1104
Ryu, Y., Kim, S. W., Kim, Y. Y., and Ku, S.-Y. (2019). Animal models for human polycystic ovary syndrome (PCOS) focused on the use of indirect hormonal perturbations: a review of the literature. Int. J. Mol. Sci. 20, 2720. doi:10.3390/ijms20112720
Saadati, S., Mason, T., Godini, R., Vanky, E., Teede, H., and Mousa, A. (2025). Metformin use in women with polycystic ovary syndrome (PCOS): opportunities, benefits, and clinical challenges. Diabetes Obes. Metab. 27 (Suppl. 3), 31–47. doi:10.1111/dom.16422
Saei Ghare Naz, M., Ozgoli, G., Mousavi, M., and Ramezani Tehrani, F. (2023). Polycystic ovary syndrome and body image concerns during adolescence. J. Pediatr. Nurs. 72, e1–e9. doi:10.1016/j.pedn.2023.05.009
Sakumoto, T., Tokunaga, Y., Tanaka, H., Nohara, M., Motegi, E., Shinkawa, T., et al. (2010). Insulin resistance/hyperinsulinemia and reproductive disorders in infertile women. Reprod. Med. Biol. 9, 185–190. doi:10.1007/s12522-010-0062-5
Saltiel, A. R., and Kahn, C. R. (2001). Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806. doi:10.1038/414799a
Sanchez-Garrido, M. A., and Tena-Sempere, M. (2020). Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol. Metab. 35, 100937. doi:10.1016/j.molmet.2020.01.001
Sang, M., Yu, Y., Zhang, Y., and Chang, H. (2024). The role and diagnostics of miR-181d-5p in polycystic ovary syndrome in insulin-resistant women. Endokrynol. Pol. 75, 655–664. doi:10.5603/ep.99021
Seow, K.-M., Liu, P.-S., Chen, K.-H., Chen, C.-W., Chen, L.-K., Ho, C.-H., et al. (2021). Cysteine-cysteine motif chemokine receptor 5 expression in letrozole-induced polycystic ovary syndrome mice. Int. J. Mol. Sci. 23, 134. doi:10.3390/ijms23010134
Shah, M. Z. U. H., Shrivastva, V. K., Mir, M. A., Sheikh, W. M., Ganie, M. A., Rather, G. A., et al. (2023). Effect of quercetin on steroidogenesis and folliculogenesis in ovary of mice with experimentally-induced polycystic ovarian syndrome. Front. Endocrinol. (Lausanne) 14, 1153289. doi:10.3389/fendo.2023.1153289
Shah, M. A., Faheem, H. I., Hamid, A., Yousaf, R., Haris, M., Saleem, U., et al. (2024). The entrancing role of dietary polyphenols against the most frequent aging-associated diseases. Med. Res. Rev. 44, 235–274. doi:10.1002/med.21985
Shen, H.-R., Xu, X., and Li, X.-L. (2021a). Berberine exerts a protective effect on rats with polycystic ovary syndrome by inhibiting the inflammatory response and cell apoptosis. Reprod. Biol. Endocrinol. 19, 3. doi:10.1186/s12958-020-00684-y
Shen, H.-R., Xu, X., Ye, D., and Li, X.-L. (2021b). Berberine improves the symptoms of DHEA-induced PCOS rats by regulating gut microbiotas and metabolites. Gynecol. Obstet. Invest. 86, 388–397. doi:10.1159/000518040
Shen, W., Qu, Y., Jiang, H., Wang, H., Pan, Y., Zhang, Y., et al. (2022). Therapeutic effect and safety of curcumin in women with PCOS: a systematic review and meta-analysis. Front. Endocrinol. (Lausanne) 13, 1051111. doi:10.3389/fendo.2022.1051111
Shi, N., Zhou, Y., and Ma, H. (2022). A network pharmacology study of mechanism and efficacy of Jiawei Huanglian-Wendan decoction in polycystic ovary syndrome with insulin resistance. Med. (Baltimore) 101, e32057. doi:10.1097/MD.0000000000032057
Shoelson, S. E., Lee, J., and Goldfine, A. B. (2006). Inflammation and insulin resistance. J. Clin. Invest. 116, 1793–1801. doi:10.1172/JCI29069
Simental-Mendía, L. E., Shah, N., Sathyapalan, T., Majeed, M., Orekhov, A. N., Jamialahmadi, T., et al. (2022). Effect of curcumin on glycaemic and lipid parameters in polycystic ovary syndrome: a systematic review and meta-analysis of randomized controlled trials. Reprod. Sci. 29, 3124–3133. doi:10.1007/s43032-021-00761-6
Simon, S., Rahat, H., Carreau, A.-M., Garcia-Reyes, Y., Halbower, A., Pyle, L., et al. (2020). Poor sleep is related to metabolic syndrome severity in adolescents with PCOS and obesity. J. Clin. Endocrinol. Metab. 105, e1827–e1834. doi:10.1210/clinem/dgz285
Skov, V., Glintborg, D., Knudsen, S., Jensen, T., Kruse, T. A., Tan, Q., et al. (2007). Reduced expression of nuclear-encoded genes involved in mitochondrial oxidative metabolism in skeletal muscle of insulin-resistant women with polycystic ovary syndrome. Diabetes 56, 2349–2355. doi:10.2337/db07-0275
Slopien, R., Wender-Ozegowska, E., Rogowicz-Frontczak, A., Meczekalski, B., Zozulinska-Ziolkiewicz, D., Jaremek, J. D., et al. (2018). Menopause and diabetes: EMAS clinical guide. Maturitas 117, 6–10. doi:10.1016/j.maturitas.2018.08.009
Stamou, M. I., Smith, K. T., Kim, H., Balasubramanian, R., Gray, K. J., and Udler, M. S. (2024). Polycystic ovary syndrome physiologic pathways implicated through clustering of genetic loci. J. Clin. Endocrinol. Metab. 109, 968–977. doi:10.1210/clinem/dgad664
Stanczyk, F. Z. (2006). Diagnosis of hyperandrogenism: biochemical criteria. Best. Pract. Res. Clin. Endocrinol. Metab. 20, 177–191. doi:10.1016/j.beem.2006.03.007
Stewart, P. M., and Edwards, C. R. (1994). Hyperandrogenism in polycystic ovary syndrome. N. Engl. J. Med. 331, 131–132. doi:10.1056/NEJM199407143310216
Su, Y.-N., Wang, M.-J., Yang, J.-P., Wu, X.-L., Xia, M., Bao, M.-H., et al. (2023). Effects of Yulin Tong Bu formula on modulating gut microbiota and fecal metabolite interactions in mice with polycystic ovary syndrome. Front. Endocrinol. (Lausanne) 14, 1122709. doi:10.3389/fendo.2023.1122709
Su, P., Chen, C., Pang, L., Wu, K., and Sun, Y. (2024). Effects of quercetin on polycystic ovary syndrome in animal models: a systematic review and meta-analysis. Reprod. Biol. Endocrinol. 22, 46. doi:10.1186/s12958-024-01220-y
Su, P., Chen, C., and Sun, Y. (2025). Physiopathology of polycystic ovary syndrome in endocrinology, metabolism and inflammation. J. Ovarian Res. 18, 34. doi:10.1186/s13048-025-01621-6
Sun, X. J., Rothenberg, P., Kahn, C. R., Backer, J. M., Araki, E., Wilden, P. A., et al. (1991). Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 352, 73–77. doi:10.1038/352073a0
Taketani, Y., and Mizuno, M. (1990). A syndrome of hyperandrogenism, insulin resistance, and acanthosis nigricans associated with polycystic ovary syndrome: clinical and laboratory features. Horm. Res. 33 (Suppl. 2), 27–30. doi:10.1159/000181562
Tan, W., Zhang, J., Dai, F., Yang, D., Gu, R., Tang, L., et al. (2024). Insights on the NF-κB system in polycystic ovary syndrome, attractive therapeutic targets. Mol. Cell Biochem. 479, 467–486. doi:10.1007/s11010-023-04736-w
Tanti, J.-F., and Jager, J. (2009). Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr. Opin. Pharmacol. 9, 753–762. doi:10.1016/j.coph.2009.07.004
Teede, H. J., Tay, C. T., Laven, J. J. E., Dokras, A., Moran, L. J., Piltonen, T. T., et al. (2023). Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 108, 2447–2469. doi:10.1210/clinem/dgad463
Tian, Y., Li, J., Su, S., Cao, Y., Wang, Z., Zhao, S., et al. (2020). PCOS-GWAS susceptibility variants in THADA, INSR, TOX3, and DENND1A are associated with metabolic syndrome or insulin resistance in women with PCOS. Front. Endocrinol. (Lausanne) 11, 274. doi:10.3389/fendo.2020.00274
Torres Fernandez, E. D., Huffman, A. M., Syed, M., Romero, D. G., and Yanes Cardozo, L. L. (2019). Effect of GLP-1 receptor agonists in the cardiometabolic complications in a rat model of postmenopausal PCOS. Endocrinology 160, 2787–2799. doi:10.1210/en.2019-00450
Townes, P. L. (1969). Human polymorphism. Med. Clin. North Am. 53, 887–901. doi:10.1016/s0025-7125(16)32739-0
Tremellen, K., and Pearce, K. (2012). Dysbiosis of gut microbiota (DOGMA)--a novel theory for the development of polycystic ovarian syndrome. Med. Hypotheses 79, 104–112. doi:10.1016/j.mehy.2012.04.016
Varhegyi, V., Modos, A., Trager, D., Gerszi, D., Horvath, E. M., Sipos, M., et al. (2024). GDF-15 and mtDNA deletions are useful biomarkers of mitochondrial dysfunction in insulin resistance and PCOS. Int. J. Mol. Sci. 25, 10916. doi:10.3390/ijms252010916
Vatier, C., Christin-Maitre, S., and Vigouroux, C. (2022). Role of insulin resistance on fertility - focus on polycystic ovary syndrome. Ann. Endocrinol. (Paris) 83, 199–202. doi:10.1016/j.ando.2022.04.004
Venkatesan, A. M., Dunaif, A., and Corbould, A. (2001). Insulin resistance in polycystic ovary syndrome: progress and paradoxes. Recent Prog. Horm. Res. 56, 295–308. doi:10.1210/rp.56.1.295
Vilariño-García, T., Guadix, P., Dorado-Silva, M., Sánchez-Martín, P., Pérez-Pérez, A., and Sánchez-Margalet, V. (2022). Decreased expression of Sam68 is associated with insulin resistance in granulosa cells from PCOS patients. Cells 11, 2821. doi:10.3390/cells11182821
Walzer, D., Turcu, A. F., Jha, S., Abel, B. S., Auchus, R. J., Merke, D. P., et al. (2022). Excess 11-Oxygenated androgens in women with severe insulin resistance are mediated by adrenal insulin receptor signaling. J. Clin. Endocrinol. Metab. 107, 2626–2635. doi:10.1210/clinem/dgac365
Wang, J., Wu, D., Guo, H., and Li, M. (2019). Hyperandrogenemia and insulin resistance: the chief culprit of polycystic ovary syndrome. Life Sci. 236, 116940. doi:10.1016/j.lfs.2019.116940
Wang, C., Ding, C., Hua, Z., Chen, C., and Yu, J. (2020a). Cangfudaotan decoction alleviates insulin resistance and improves follicular development in rats with polycystic ovary syndrome via IGF-1-PI3K/Akt-Bax/Bcl-2 pathway. Mediat. Inflamm. 2020, 8865647. doi:10.1155/2020/8865647
Wang, C., Yao, J., Ju, L., Wen, X., and Shu, L. (2020b). Puerarin ameliorates hyperglycemia in HFD diabetic mice by promoting β-cell neogenesis via GLP-1R signaling activation. Phytomedicine 70, 153222. doi:10.1016/j.phymed.2020.153222
Wang, S., Zhang, S., Wang, S., Gao, P., and Dai, L. (2020c). A comprehensive review on pueraria: insights on its chemistry and medicinal value. Biomed. Pharmacother. 131, 110734. doi:10.1016/j.biopha.2020.110734
Wang, Z., Nie, K., Su, H., Tang, Y., Wang, H., Xu, X., et al. (2021). Berberine improves ovulation and endometrial receptivity in polycystic ovary syndrome. Phytomedicine 91, 153654. doi:10.1016/j.phymed.2021.153654
Ware, M. A., Kaar, J. L., Diniz Behn, C., Bartlette, K., Carreau, A.-M., Lopez-Paniagua, D., et al. (2022). Pancreatic fat relates to fasting insulin and postprandial lipids but not polycystic ovary syndrome in adolescents with obesity. Obes. (Silver Spring) 30, 191–200. doi:10.1002/oby.23317
Wei, H., Sun, M., Wang, R., Zeng, H., Zhao, B., and Jin, S. (2024). Puerarin mitigated LPS-ATP or HG-primed endothelial cells damage and diabetes-associated cardiovascular disease via ROS-NLRP3 signalling. J. Cell Mol. Med. 28, e18239. doi:10.1111/jcmm.18239
Weng, W., and Goel, A. (2022). Curcumin and colorectal cancer: an update and current perspective on this natural medicine. Semin. Cancer Biol. 80, 73–86. doi:10.1016/j.semcancer.2020.02.011
Wolf, W. M., Wattick, R. A., Kinkade, O. N., and Olfert, M. D. (2018). Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int. J. Environ. Res. Public Health 15, 2589. doi:10.3390/ijerph15112589
Wu, L., Xia, M., Duan, Y., Zhang, L., Jiang, H., Hu, X., et al. (2019). Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 10, 468. doi:10.1038/s41419-019-1706-y
Wu, Q., Gao, J., Bai, D., Yang, Z., and Liao, Q. (2021). The prevalence of polycystic ovarian syndrome in Chinese women: a meta-analysis. Ann. Palliat. Med. 10, 74–87. doi:10.21037/apm-20-1893
Wu, Y.-X., Yang, X.-Y., Han, B.-S., Hu, Y.-Y., An, T., Lv, B.-H., et al. (2022a). Naringenin regulates gut microbiota and SIRT1/PGC-1ɑ signaling pathway in rats with letrozole-induced polycystic ovary syndrome. Biomed. Pharmacother. 153, 113286. doi:10.1016/j.biopha.2022.113286
Wu, Y.-Y., Li, S.-Y., Zhu, H.-Q., Zhuang, Z.-M., Shao, M., Chen, F.-L., et al. (2022b). Network pharmacology integrated with experimental validation reveals the regulatory mechanism of action of Hehuan Yin decoction in polycystic ovary syndrome with insulin resistance. J. Ethnopharmacol. 289, 115057. doi:10.1016/j.jep.2022.115057
Wu, J., Wang, K., Qi, X., Zhou, S., Zhao, S., Lu, M., et al. (2025a). The intestinal fungus Aspergillus tubingensis promotes polycystic ovary syndrome through a secondary metabolite. Cell Host Microbe 33, 119–136.e11. doi:10.1016/j.chom.2024.12.006
Wu, Q., Chen, K., Ma, L., and Man, S. (2025b). Pueraria: a comprehensive review of chemical composition, health benefits, and current applications. Compr. Rev. Food Sci. Food Saf. 24, e70177. doi:10.1111/1541-4337.70177
Xiang, Y., Wang, M., Yu, G., Wan, L., Song, Y., Li, Y., et al. (2023). Naringenin alleviates the excessive lipid deposition of polycystic ovary syndrome rats and insulin-resistant adipocytes by promoting PKGIα. Am. J. Reprod. Immunol. 90, e13795. doi:10.1111/aji.13795
Xin, G., Zhang, L. Y., Qiu, H. R., Wang, Y. C., Sui, Y. H., Xue, B. G., et al. (2024). Investigation of the effects and mechanisms of berberine on a mouse model of polycystic ovary syndrome: based on intestinal flora analysis. Zhonghua Fu Chan Ke Za Zhi 59, 215–226. doi:10.3760/cma.j.cn112141-20231222-00272
Xing, C., Li, C., and He, B. (2020). Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J. Clin. Endocrinol. Metab. 105, 2950–2963. doi:10.1210/clinem/dgaa337
Xu, Y., and Qiao, J. (2022). Association of insulin resistance and elevated androgen levels with polycystic ovarian syndrome (PCOS): a review of literature. J. Healthc. Eng. 2022, 9240569. doi:10.1155/2022/9240569
Xu, Y., Nan, D., Fan, J., Bogan, J. S., and Toomre, D. (2016). Optogenetic activation reveals distinct roles of PIP3 and Akt in adipocyte insulin action. J. Cell Sci. 129, 2085–2095. doi:10.1242/jcs.174805
Xu, Y., Tang, J., Guo, Q., Xu, Y., Yan, K., Wu, L., et al. (2021). Traditional Chinese medicine formula FTZ protects against polycystic ovary syndrome through modulating adiponectin-mediated fat-ovary crosstalk in mice. J. Ethnopharmacol. 268, 113587. doi:10.1016/j.jep.2020.113587
Yang, Y.-L., Zhou, W.-W., Wu, S., Tang, W.-L., Wang, Z.-W., Zhou, Z.-Y., et al. (2021). Intestinal flora is a key factor in insulin resistance and contributes to the development of polycystic ovary syndrome. Endocrinology 162, bqab118. doi:10.1210/endocr/bqab118
Yang, T., Yang, Y., Zhang, Q., Liu, D., Liu, N., Li, Y., et al. (2022a). Homeostatic model assessment for insulin resistance is associated with late miscarriage in non-dyslipidemic women undergoing fresh IVF/ICSI embryo transfer. Front. Endocrinol. (Lausanne) 13, 880518. doi:10.3389/fendo.2022.880518
Yang, X., Wang, K., Lang, J., Guo, D., Gao, H., Qiu, Y., et al. (2022b). Up-regulation of miR-133a-3p promotes ovary insulin resistance on granulosa cells of obese PCOS patients via inhibiting PI3K/AKT signaling. BMC Womens Health 22, 412. doi:10.1186/s12905-022-01994-6
Yang, Y., Liu, J., and Xu, W. (2022c). Naringenin and morin reduces insulin resistance and endometrial hyperplasia in the rat model of polycystic ovarian syndrome through enhancement of inflammation and autophagic apoptosis. Acta Biochim. Pol. 69, 91–100. doi:10.18388/abp.2020_5722
Yang, J.-P., Ullah, A., Su, Y.-N., Otoo, A., Adu-Gyamfi, E. A., Feng, Q., et al. (2023). Glycyrrhizin ameliorates impaired glucose metabolism and ovarian dysfunction in a polycystic ovary syndrome mouse model. Biol. Reprod. 109, 83–96. doi:10.1093/biolre/ioad048
Yang, Q., Wan, Q., and Wang, Z. (2024). Curcumin mitigates polycystic ovary syndrome in mice by suppressing TLR4/MyD88/NF-κB signaling pathway activation and reducing intestinal mucosal permeability. Sci. Rep. 14, 29848. doi:10.1038/s41598-024-81034-5
Yang, W., Ma, Y., Wu, Y., Lei, X., Zhang, J., and Li, M. (2025). Study on the effects of mogroside V in inhibiting NLRP3-mediated granulosa cell pyroptosis and insulin resistance to improve PCOS. J. Ovarian Res. 18, 10. doi:10.1186/s13048-024-01563-5
Ye, Y., Zhou, W., Ren, Y., Lu, J., Chen, A., Jin, R., et al. (2023). The ameliorating effects of Guizhi Fuling Wan combined with rosiglitazone in a rat ovarian model of polycystic ovary syndrome by the PI3K/AKT/NF-κB and Nrf2/HO-1 pathways. Gynecol. Endocrinol. 39, 2254848. doi:10.1080/09513590.2023.2254848
Yu, J., Ding, C., Hua, Z., Jiang, X., and Wang, C. (2021). Protective effects of berberine in a rat model of polycystic ovary syndrome mediated via the PI3K/AKT pathway. J. Obstet. Gynaecol. Res. 47, 1789–1803. doi:10.1111/jog.14730
Yu, J., Zhou, Y., Ding, J., Zhang, D., Yu, C., and Huang, H. (2022). Characteristics and possible mechanisms of metabolic disorder in overweight women with polycystic ovary syndrome. Front. Endocrinol. (Lausanne) 13, 970733. doi:10.3389/fendo.2022.970733
Yu, O., Christ, J. P., Schulze-Rath, R., Covey, J., Kelley, A., Grafton, J., et al. (2023). Incidence, prevalence, and trends in polycystic ovary syndrome diagnosis: a United States population-based study from 2006 to 2019. Am. J. Obstet. Gynecol. 229 (39), 39.e1–39.e12. doi:10.1016/j.ajog.2023.04.010
Yuan, C., Huang, W. Q., Guo, J. H., Liu, X. Y., Yang, J. Z., Chen, J. J., et al. (2021). Involvement of kisspeptin in androgen-induced hypothalamic endoplasmic reticulum stress and its rescuing effect in PCOS rats. Biochim. Biophys. Acta Mol. Basis Dis. 1867, 166242. doi:10.1016/j.bbadis.2021.166242
Yue, F., Xing, L., Wu, S., Wei, L., Zhou, Z., Shi, Y., et al. (2022). Constant light exposure alters gut microbiota and short-/medium-chain fatty acids and aggravates PCOS-like traits in HFD-fed rats. Obes. (Silver Spring) 30, 694–706. doi:10.1002/oby.23380
Zhai, J., Li, S., Hu, M., Di, F., Liu, J., and Du, Y. (2020). Decreased brain and muscle ARNT-like protein 1 expression mediated the contribution of hyperandrogenism to insulin resistance in polycystic ovary syndrome. Reprod. Biol. Endocrinol. 18, 32. doi:10.1186/s12958-020-00592-1
Zhang, Y., and Xu, L. (2021). Preliminary study of Yulin mixture affecting the miR-320/SF-1/Cyp19a1 on mouse polycystic ovary syndrome model. Gynecol. Endocrinol. 37, 546–553. doi:10.1080/09513590.2020.1843623
Zhang, G., Garmey, J. C., and Veldhuis, J. D. (2000). Interactive stimulation by luteinizing hormone and insulin of the steroidogenic acute regulatory (StAR) protein and 17alpha-hydroxylase/17,20-lyase (CYP17) genes in porcine theca cells. Endocrinology 141, 2735–2742. doi:10.1210/endo.141.8.7595
Zhang, Y., Zhao, W., Xu, H., Hu, M., Guo, X., Jia, W., et al. (2019). Hyperandrogenism and insulin resistance-induced fetal loss: evidence for placental mitochondrial abnormalities and elevated reactive oxygen species production in pregnant rats that mimic the clinical features of polycystic ovary syndrome. J. Physiol. 597, 3927–3950. doi:10.1113/JP277879
Zhang, C., Hu, J., Wang, W., Sun, Y., and Sun, K. (2020a). HMGB1-induced aberrant autophagy contributes to insulin resistance in granulosa cells in PCOS. FASEB J. 34, 9563–9574. doi:10.1096/fj.202000605RR
Zhang, C., Yu, C., Lin, Z., Pan, H., Li, K., and Ma, H. (2020b). MiRNAs expression profiling of rat ovaries displaying PCOS with insulin resistance. Arch. Gynecol. Obstet. 302, 1205–1213. doi:10.1007/s00404-020-05730-z
Zhang, N., Liu, X., Zhuang, L., Liu, X., Zhao, H., Shan, Y., et al. (2020c). Berberine decreases insulin resistance in a PCOS rats by improving GLUT4: dual regulation of the PI3K/AKT and MAPK pathways. Regul. Toxicol. Pharmacol. 110, 104544. doi:10.1016/j.yrtph.2019.104544
Zhang, Y., Hu, M., Jia, W., Liu, G., Zhang, J., Wang, B., et al. (2020d). Hyperandrogenism and insulin resistance modulate gravid uterine and placental ferroptosis in PCOS-like rats. J. Endocrinol. 246, 247–263. doi:10.1530/JOE-20-0155
Zhang, Y., Weng, Y., Wang, D., Wang, R., Wang, L., Zhou, J., et al. (2021). Curcumin in combination with aerobic exercise improves follicular dysfunction via inhibition of the hyperandrogen-induced IRE1α/XBP1 endoplasmic reticulum stress pathway in PCOS-like rats. Oxid. Med. Cell Longev. 2021, 7382900. doi:10.1155/2021/7382900
Zhang, J., Sun, J., Li, C., Qiao, H., and Hussain, Z. (2023a). Functionalization of curcumin nanomedicines: a recent promising adaptation to maximize pharmacokinetic profile, specific cell internalization and anticancer efficacy against breast cancer. J. Nanobiotechnology 21, 106. doi:10.1186/s12951-023-01854-x
Zhang, Q., Ren, J., Wang, F., Li, M., Pan, M., Zhang, H., et al. (2023b). Chinese herbal medicine alleviates the pathogenesis of polycystic ovary syndrome by improving oxidative stress and glucose metabolism via mitochondrial Sirtuin 3 signaling. Phytomedicine 109, 154556. doi:10.1016/j.phymed.2022.154556
Zhang, D., Xu, D., Huang, X., Wei, Y., Tang, F., Qin, X., et al. (2024a). Puerarin-loaded electrospun patches with anti-inflammatory and pro-collagen synthesis properties for pelvic floor reconstruction. Adv. Sci. (Weinh) 11, e2308590. doi:10.1002/advs.202308590
Zhang, Q., Yang, Z., Ou, X., Zhang, M., Qin, X., and Wu, G. (2024b). The role of immunity in insulin resistance in patients with polycystic ovary syndrome. Front. Endocrinol. (Lausanne) 15, 1464561. doi:10.3389/fendo.2024.1464561
Zhang, W., Peng, C., Xu, L., Zhao, Y., Huang, C., and Lu, L. (2024c). The therapeutic effects of curcumin on polycystic ovary syndrome by upregulating PPAR-γ expression and reducing oxidative stress in a rat model. Front. Endocrinol. (Lausanne) 15, 1494852. doi:10.3389/fendo.2024.1494852
Zhang, W., Zhang, J., Xue, H., Chen, X., Li, M., Chen, S., et al. (2024d). Nicotinamide mononucleotide improves endometrial homeostasis in a rat model of polycystic ovary syndrome by decreasing insulin resistance and regulating the glylytic pathway. Mol. Nutr. Food Res. 68, e2400340. doi:10.1002/mnfr.202400340
Zhang, Y., Cao, S., Liang, J.-X., Hu, S.-H., Guo, X.-F., Chun-Jing, S., et al. (2024e). Effects of Zishen Yutai pills combined with metformin on women with polycystic ovary syndrome undergoing in vitro fertilization. Med. (Baltimore) 103, e39030. doi:10.1097/MD.0000000000039030
Zhang, X., Ji, D., Zhang, Y., Du, C., Liang, L., Ahmad, A., et al. (2025). Study on the mechanism of action of berberine combined with Jianpi Yishen Huazhuo formulation in treating obese polycystic ovary syndrome by activating PI3K/AKT signaling pathway. Gynecol. Endocrinol. 41, 2462068. doi:10.1080/09513590.2025.2462068
Zhao, X., Ponomaryov, T., Ornell, K. J., Zhou, P., Dabral, S. K., Pak, E., et al. (2015). RAS/MAPK activation drives resistance to smo inhibition, metastasis, and tumor evolution in Shh pathway-dependent tumors. Cancer Res. 75, 3623–3635. doi:10.1158/0008-5472.CAN-14-2999-T
Zhao, H., Xing, C., Zhang, J., and He, B. (2021). Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: a network meta-analysis. Reprod. Health 18, 171. doi:10.1186/s12978-021-01207-7
Zhao, F.-Q., Zhao, Y., Liu, J.-Y., Gou, Y.-J., and Yang, Y.-Q. (2022a). Effects of berberine on LPS/NF-κB and MAPK signaling pathways in PCOS model rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 38, 181–186. doi:10.12047/j.cjap.6229.2022.029
Zhao, H., Chen, R., Zheng, D., Xiong, F., Jia, F., Liu, J., et al. (2022b). Modified Banxia Xiexin decoction ameliorates polycystic ovarian syndrome with insulin resistance by regulating intestinal microbiota. Front. Cell Infect. Microbiol. 12, 854796. doi:10.3389/fcimb.2022.854796
Zheng, L., Chen, P.-F., Dai, W.-C., Zheng, Z.-Q., and Wang, H.-L. (2022a). Curcumin alleviates hyperandrogenism and promotes follicular proliferation in polycystic ovary syndrome rats: insights on IRS1/PI3K/GLUT4 and PTEN modulations. Chin. J. Integr. Med. 28, 1088–1095. doi:10.1007/s11655-022-3582-z
Zheng, S., Chen, Y., Ma, M., and Li, M. (2022b). Mechanism of quercetin on the improvement of ovulation disorder and regulation of ovarian CNP/NPR2 in PCOS model rats. J. Formos. Med. Assoc. 121, 1081–1092. doi:10.1016/j.jfma.2021.08.015
Zheng, R., Shen, H., Li, J., Zhao, J., Lu, L., Hu, M., et al. (2023). Qi Gong Wan ameliorates adipocyte hypertrophy and inflammation in adipose tissue in a PCOS mouse model through the Nrf2/HO-1/Cyp1b1 pathway: integrating network pharmacology and experimental validation in vivo. J. Ethnopharmacol. 301, 115824. doi:10.1016/j.jep.2022.115824
Zhong, X., Jin, F., Huang, C., Du, M., Gao, M., and Wei, X. (2021). DNA methylation of AMHRII and INSR gene is associated with the pathogenesis of polycystic ovary syndrome (PCOS). Technol. Health Care 29, 11–25. doi:10.3233/THC-218002
Zhou, L., Han, X., Li, W., Wang, N., Yao, L., Zhao, Y., et al. (2022). N6-methyladenosine demethylase FTO induces the dysfunctions of ovarian granulosa cells by upregulating flotillin 2. Reprod. Sci. 29, 1305–1315. doi:10.1007/s43032-021-00664-6
Zhou, X., Ma, Q., Yan, Z., Wang, Y., Qin, J., Tong, T., et al. (2023). Efficacy and safety of Chinese patent medicine Xiao Yao San in polycystic ovary syndrome: a systematic review and meta-analysis. J. Ethnopharmacol. 313, 116517. doi:10.1016/j.jep.2023.116517
Zhou, Y., Jin, Y., Wu, T., Wang, Y., Dong, Y., Chen, P., et al. (2024). New insights on mitochondrial heteroplasmy observed in ovarian diseases. J. Adv. Res. 65, 211–226. doi:10.1016/j.jare.2023.11.033
Zhou, Q., Tang, H., Wang, Y., Hua, Y., Ouyang, X., and Li, L. (2025). Hyperoside mitigates PCOS-associated adipogenesis and insulin resistance by regulating NCOA2-mediated PPAR-γ ubiquitination and degradation. Life Sci. 364, 123417. doi:10.1016/j.lfs.2025.123417
Zouali, H., Velho, G., and Froguel, P. (1993). Polymorphism of the glycogen synthase gene and non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 328, 1569. doi:10.1056/NEJM199305273282113
Zuchelo, L. T. S., Alves, M. S., Baracat, E. C., Sorpreso, I. C. E., and Soares, J. M. (2024). Menstrual pattern in polycystic ovary syndrome and hypothalamic-pituitary-ovarian axis immaturity in adolescents: a systematic review and meta-analysis. Gynecol. Endocrinol. 40, 2360077. doi:10.1080/09513590.2024.2360077
Zuo, L., Hai, Y., Zhang, R., Zuo, B., Tian, J., Li, P., et al. (2023). Therapeutic potential of icariin in rats with letrozole and high-fat diet-induced polycystic ovary syndrome. Eur. J. Pharmacol. 953, 175825. doi:10.1016/j.ejphar.2023.175825
Glossary
AhR aryl hydrocarbon receptor
AKT Protein Kinase B
AR androgen receptor
BM I, body mass index
CCR5 C-C chemokine receptor 5
CFD Cangfu Daotan Decoction
circRNAs circular RNAs
DHEA Dehydroepiandrosterone
DHEAS Dehydroepiandrosterone sulfate
ERK Extracellular Signal-Regulated Kinase
FBG fasting blood glucose
FINS fasting insulin
FSH Follicle-Stimulating Hormone
FXR farnesoid X receptor
GFW Guizhi Fuling Pills
GLP-1 Glucagon-Like Peptide-1
GLUT4 glucose transporter4
GnRH gonadotropin-releasing hormone
GPX4 Glutathione Peroxidase 4
GV germinal vesicle
HA Hyperandrogenism
HDL-C High-Density Lipoprotein Cholesterol
HI hyperinsulinemia
HOMA-IR Homeostatic Model Assessment of Insulin Resistance
HQS HEQI San
IκBα Inhibitor of Nuclear Factor-kappa B alpha
IKK IκB Kinase
IKKβ IκB Kinase beta
IL-1 Interleukin-1
IL-1β Interleukin-1 beta
IL-6 interleukin-6
IL-22 interleukin-22
lncRNAs long-stranded non-coding RNAs
IR insulin resistance
IRS Insulin Receptor Substrate
LCN-2 Lipocalin-2
LDL-C Low-Density Lipoprotein Cholesterol
LH luteinizing hormone
LPS lipopolysaccharide
MAPK mitogen-activated protein kinase
miRNA microRNA
mRNA Messenger RNA
mtDNA mitochondrial DNA
mTORC2 mammalian target of rapamycin complex 2
NETs neutrophil extracellular traps
NF-κ B, Nuclear Factor-kappa B
NK natural killer
Nrf2 Nuclear Factor Erythroid 2-Related Factor 2
OM oligomenorrhea (OM)
p-AKT phosphorylated AKT
PCOS Polycystic ovary syndrome
PCR polymerase chain reaction
PDK1 Phosphatidylinositol-dependent kinase-1
pgWAT perigonadal white adipose tissue
PIP2 phosphatidylinositol 4,5 bisphosphate
PIP3 phosphatidylinositol 3,4,5 trisphosphate
PI3K Phosphatidylinositol 3-Kinase
p-PI3K phosphorylated PI3K
Ras Rat Sarcoma Viral Oncogene Homolog
RCT Randomized Controlled Trial
ROS reactive oxygen species
SA secondary amenorrhea
SHGB sex hormone-binding globulin
SNP Single Nucleotide Polymorphism
T Testosterone
TC Total Cholesterol
TCM Traditional Chinese medicine
TGF-β1 transforming growth factor-β1
TNF-α tumor necrosis factor-α
TNFR Tumor Necrosis Factor Receptor
TRADD Tumor Necrosis Factor Receptor-Associated Death Domain Protein
TRAF2 Tumor Necrosis Factor Receptor-Associated Factor 2
Keywords: polycystic ovary syndrome, insulin resistance, traditional chinesemedicine, pathogenesis, signal pathway
Citation: Sun Z, Jin B, Han H, Qin Z, Shi Y and Zhang Y (2025) Research progress on the pathogenesis, clinical impact, and traditional Chinese medicine treatment of polycystic ovary syndrome complicated by insulin resistance. Front. Pharmacol. 16:1661806. doi: 10.3389/fphar.2025.1661806
Received: 08 July 2025; Accepted: 25 August 2025;
Published: 11 September 2025.
Edited by:
Ramith Ramu, JSS Academy of Higher Education and Research, IndiaReviewed by:
Phiwayinkosi V. Dludla, University of Zululand, South AfricaRamoji Kosuru, Versiti Blood Research Institute, United States
Copyright © 2025 Sun, Jin, Han, Qin, Shi and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuehui Zhang, Y2hpemlzaHVpLTA0QDE2My5jb20=
 Zepu Sun
Zepu Sun Bao Jin
Bao Jin Han Han4
Han Han4 Zhen Qin
Zhen Qin Yuqian Shi
Yuqian Shi Yuehui Zhang
Yuehui Zhang