- 1The Affiliated Kangning Hospital of Wenzhou Medical University, Zhejiang Provincial Clinical Research Center for Mental Health, Wenzhou, China
- 2Wenzhou Kangning Hospital Group, Wenzhou, China
Background: Depression is a prevalent global disorder that imposes a significant burden on individuals worldwide. Berberine is a promising candidate for future antidepressant therapies; however, no comprehensive systematic evaluation has been conducted to date.
Methods: Five electronic databases—PubMed, Embase, Web of Science, OVID, and the Cochrane Library—were systematically searched to identify preclinical studies investigating the antidepressant effects of berberine. Outcomes were assessed using the standardized mean difference with 95% confidence intervals to evaluate overall effect sizes. Study quality was evaluated using the 10-item Systematic Review Centre for Laboratory Animal Experimentation risk of bias tool. Publication bias was assessed if more than 10 studies were included in an analysis.
Results: A total of 20 preclinical studies evaluating berberine‘s antidepressant effects were identified. Berberine administration was associated with reduced depression-like behaviors. Specifically, Berberine significantly: increased body weight (n = 7; SMD = 1.67; 95% CI: 0.57 to 2.76; P < 0.00001),Reduced immobility time in the tail suspension test (n = 9; SMD = −2.41; 95% CI: −3.15 to −1.67; P = 0.01),Increased sucrose consumption (n = 12; SMD = 1.82; 95% CI: 1.29 to 2.34; P = 0.02),Reduced immobility time in the forced swim test (n = 17; SMD = −2.35; 95% CI: −2.91 to −1.79; P < 0.00001),Increased total movement distance in the open field test (n = 7; SMD = 1.70; 95% CI: 0.58 to 2.81; P < 0.00001),Increased time spent in the open field test (n = 3; SMD = 1.02; 95% CI: 0.44 to 1.60; P = 0.92), Increased the number of crossings in the open field test (n = 4; SMD = 0.76; 95% CI: 0.20 to 1.33; P = 0.23). Furthermore, berberine was found to reduce levels of inflammatory markers, enhance neurotransmitter levels (excluding dopamine), and elevate brain-derived neurotrophic factor levels.
Conclusion: Berberine consistently demonstrated antidepressant-like effects in preclinical models and showed preliminary potential mechanisms of action. However, the limitations of current studies highlight the necessity for more comprehensive preclinical research and well-designed clinical trials.
1 Introduction
Depression is a widespread and formidable mental health affliction that impacts individuals worldwide. Between 1990 and 2019, the number of incident cases of depression increased by 49.86% (Liu et al., 2019). As of 2019, depression ranked among the top three causes of disability-adjusted life years among females and was the 13th leading cause of disability-adjusted life years across all age groups in 204 countries (GBD, 2019 Diseases and Injuries Collaborators, 2020). This condition imposes significant public health challenges and places a heavy burden on families. Depression is characterized by a high likelihood of recurrence throughout the lifespan (Assoc, 2013), can occur at any age (Alexopoulos, 2005; Donohue et al., 2019), and presents with a heterogeneous symptom profile (Fried and Nesse, 2015; Fried et al., 2014). To date, the underlying pathological and pharmacological mechanisms of depression remain complex and poorly understood. Various factors have been implicated in its onset and progression, including immune dysregulation (Bai et al., 2024; Bullmore, 2018; Drevets et al., 2022), monoamine imbalance (Malhi and Mann, 2018), age-specific neurofunctional changes (Bore et al., 2024), and gut microbiota metabolism (Aburto and Cryan, 2024; Zhao et al., 2024).
Currently, first-line treatments for depression include antidepressant medications and psychological therapies (Simon et al., 2024). In recent years, novel treatments targeting neurotransmitter systems have garnered increasing interest (De Risio et al., 2020; Njenga et al., 2024; Tang et al., 2025). However, depression remains a largely incurable condition, particularly in cases of treatment-resistant depression. The heterogeneity of depressive symptoms poses a major barrier to effective treatment (Fried, 2017), and a substantial proportion of patients fail to achieve meaningful improvement with existing therapies (Cuijpers et al., 2020). Moreover, the initiation of antidepressant medications is often associated with adverse effects, including weight changes (Gill et al., 2020), sexual dysfunction (Peleg et al., 2022), gastrointestinal disturbances (Oliva et al., 2021), and an increased risk of suicidality (Hetrick et al., 2021; Boaden et al., 2020).
Due to the limited efficacy of current treatments and the occurrence of serious adverse effects, there is an urgent need to identify innovative therapeutic approaches to combat depression. Recently, increasing attention has been directed toward berberine (BBR), an isoquinoline alkaloid with potential therapeutic benefits (Shayganfard, 2023). BBR is a bioactive compound isolated from medicinal herbs and has traditionally been used in the treatment of gastrointestinal disorders (Kong et al., 2004; Kulkarni and Dhir, 2010; Dong et al., 2022). Over the past 2 decades, BBR has demonstrated a wide range of pharmacological activities across various disease domains, including diabetes (Wang et al., 2024; Xie et al., 2022), cancer (Hsu et al., 2024; Sajeev et al., 2024; Yan et al., 2024), Parkinson’s disease (Wang et al., 2021), and cardiovascular disorders (Zhao et al., 2021). Given the complex multifactorial pathology of depression, BBR emerges as a promising therapeutic candidate due to its multiple pharmacological actions, including anti-inflammatory, antioxidant, and neuroprotective effects (Imanshahidi and Hosseinzadeh, 2008; Wang et al., 2017). Its multi-target mode of action is expected to overcome the limitations of conventional single-target drugs.
Recent in vivo and in vitro studies have provided positive therapeutic evidence suggesting that BBR holds significant potential for the treatment of depression (Chen and Zhang, 2025). Notably, previous research has demonstrated that BBR can enhance the effects of conventional antidepressants (Kulkarni and Dhir, 2008), primarily by modulating neurotransmitter levels and their associated receptor systems. BBR exerts its antidepressant effects through multiple pharmacological mechanisms. These include inhibition of the NLRP3 inflammasome (Qin et al., 2023), upregulation of brain-derived neurotrophic factor (BDNF) expression (Zhan et al., 2021), and improvement of hypothalamic-pituitary-adrenal axis function (Gao et al., 2024). After crossing the blood–brain barrier, BBR can enhance hippocampal neurogenesis (Yang et al., 2023) and exert neuroprotective effects (Wang et al., 2005; Yoo et al., 2006).
Taken together, these findings indicate that BBR may represent a novel, multimodal antidepressant that operates through mechanisms distinct from those of traditional antidepressant medications. Despite BBR’s diverse pharmacological and biochemical activities, its precise mechanisms of action remain unclear. Notably, no meta-analysis has yet been performed to synthesize and summarize the role of BBR in depression based on preclinical studies. To address this gap and enhance our understanding of BBR’s synergistic effects and underlying molecular mechanisms in depression, we systematically reviewed preclinical studies using animal models. This review endeavors to establish a robust and comprehensive body of evidence in support of future clinical investigations into the antidepressant properties of BBR.
2 Methods
The current systematic review and meta-analysis was designed and conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Vrabel, 2009; Shamseer et al., 2015). The study protocol, based on SYRCLE’s systematic review programme format for animal intervention studies (De Vries et al., 2015), was submitted to the INPLASY platform on 9 June 2025, and officially registered on 9 June 2025, under registration number INPLASY 202560037 (DOI:10.37766/inplasy2025.6.0037).
2.1 Literature search
Five online electronic databases—PubMed, OVID, Web of Science, Embase, and the Cochrane Library—were searched to obtain information on animal studies investigating the use of BBR for depression. Two separate searches were conducted on 31 March 2025, by two independent reviewers (Ling XJ and Chen GQ), once in the morning and once in the afternoon. To minimize the possible of omitting relevant studies, the reference lists of all retrieved studies were manually screened. The search strategy employed a predefined set of MeSH terms and keywords applied to the full text. These terms included both disease-related and compound-related keywords, such as “depress*,” “sadness,” “berberine,” and “huangliansu.” (The complete search strategies are shown in the Table 1).
2.2 Inclusion and exclusion criteria
After removing duplicates, two different reviewers (Ling XJ and Chen GQ) independently screened each article based on the PICOS criteria without mutual consultation. Any discrepancies were resolved by consulting a third independent reviewer (Long ZX).
2.2.1 Inclusion criteria
1) Results of studies published as an original article. 2) The subjects must be animals and there are no restrictions on the method of construction of the animal model, gender, size, species or sample size. 4) Studies with separate BBR treatment and control or model groups were available. 5) Outcome measures associated with depression -like behaviors 6) No restriction on the language.
2.2.2 Exclusion criteria
1) Reviews, patents, clinical studies, case reports, conference and book chapter. 2) No full-text articles 3) Repeatedly published literature. 4) Experimental findings in the articles were incomplete. 5) Outcome measures were unqualified. 6) Preclinical studies that were inconsistencies in the study purpose.
2.3 Data extraction
After an initial review of the titles and abstracts of all studies and the exclusion of duplicates, full-text articles eligible for qualitative data extraction were summarized, tabulated, and independently assessed by two reviewers (Chen GQ and Li XY). For studies reporting experimental data at multiple time points, only the data from the final time point were extracted in our analysis. A meta-analysis was performed after the collection at least 3 studies per group. Finally, the data include: 1) The first author of the articles and the year of publication. 2) The species, sex, weight range, and sample size of the subjective animals. 3) The modeling method of the animal model of depression. 4) The dose, duration of BBR treatment. 5) Method of vehicle or BBR administration 6) Medication for control variables in the control or model group, dose and duration of drugs used. 7) 15 of outcome indicators: Weight, Sucrose preference in sucrose preference test (SPT), The number of crossings in OFT (Open field test), Total distance of movement in OFT, Time duration of center square in OFT, Immobility time in FST (Forced swim test), Immobility time in TST (Tail suspension test), Interleukin 6 (IL-6) levels, Interleukin-1β (IL-1β) levels, Tumor necrosis factor α (TNF-α) levels, 5-hydroxytryptamine (5-HT) levels, Norepinephrine (NE) levels, dopamine (DA) levels; Brain-derived neurotrophic factor (BDNF) protein levels, BDNF mRNA levels. all of data in article were obtained from the tables or graphs by Engauge Digitizer software. All included data were presented as mean ± standard deviation (SD). If the original outcomes in the articles were reported as the standard error of the mean (SEM), they were converted to SD using the formula: SD = SEM *
2.4 Quality evaluation
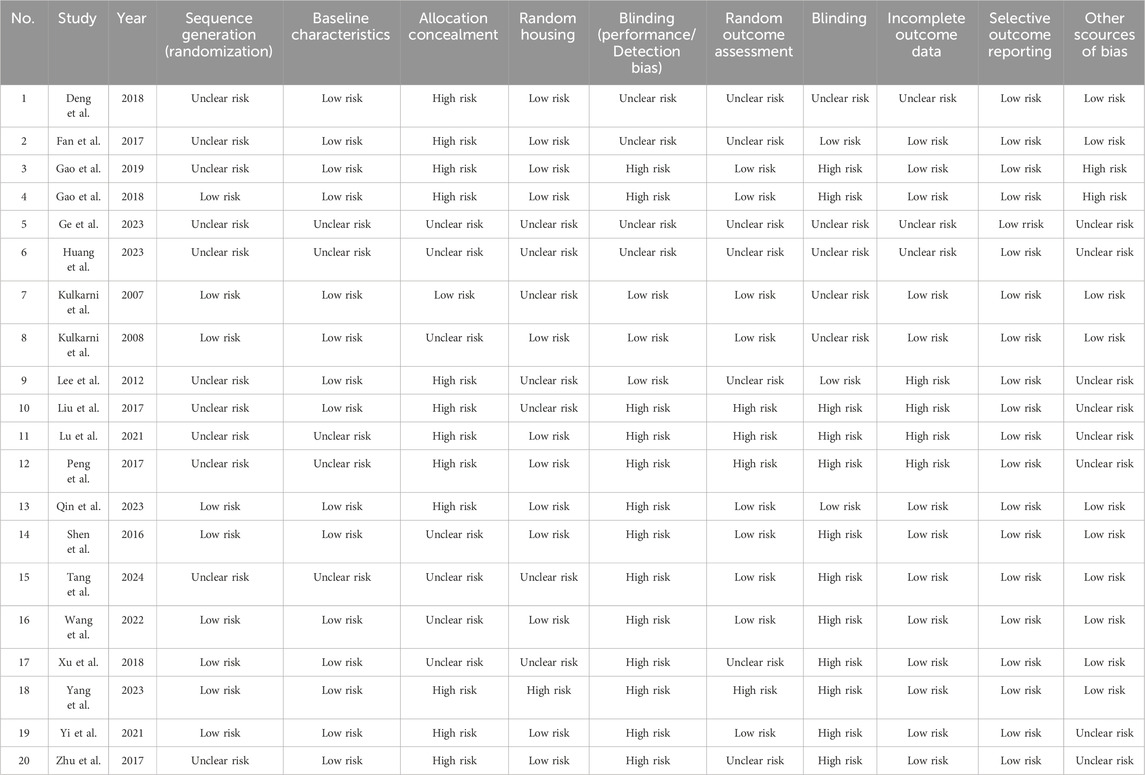
To assess the quality of the included studies, two reviewers (Chen GQ and Li XY) independently evaluated the risk of bias using the 10-item SYRCLE risk of bias tool developed by the Center for the Evaluation of Laboratory Animal Experiments (Hooijmans et al., 2014). The tool assesses the following domains: selection bias (sequence generation, allocation concealment, random housing), performance bias, detection bias (random outcome assessment, blinding), attrition bias, reporting bias, and other sources of bias. Each item was rated as “low risk,” “high risk,” or “unclear risk.” Any discrepancies during the quality assessment process were resolved through consultation with a third reviewer (Yao BF) to reach a consensus.
2.5 Statistical analysis
Statistical analyses were conducted using Review Manager (RevMan) version 5.4.1 and STATA version 15.1. As the outcome indicators were continuous variables, results were evaluated using standardized mean differences (SMDs) and their corresponding 95% confidence intervals (CIs) to estimate the overall effect size.
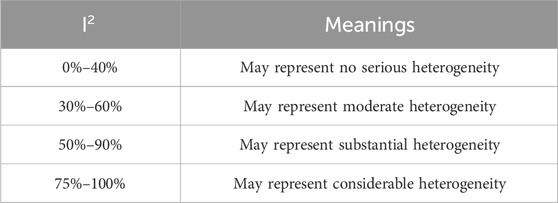
Due to variations among the included studies in terms of species, age, sample size, dosage or administration of BBR, and experimental duration, a random-effects model was employed. In line with recent proposals to address the replication crisis (Benjamin et al., 2017), we employed a stricter significance threshold of p < 0.005. This a priori decision was made to reduce the likelihood of false positives and to report only the most robust effects. Heterogeneity was assessed using the I2 statistic. However, following the updated Cochrane Handbook, I2 values were no longer used as the sole criterion for selecting the effects model. The general interpretation of I2 was shown in Table 2.
When ten or more studies reported the same outcome indicators, Begg’s test and Egger’s test were used to assess potential publication bias. Sensitivity analysis was conducted by sequentially excluding each individual study to evaluate the robustness of the overall findings and identify any potentially influential studies.
3 Results
3.1 Study selection
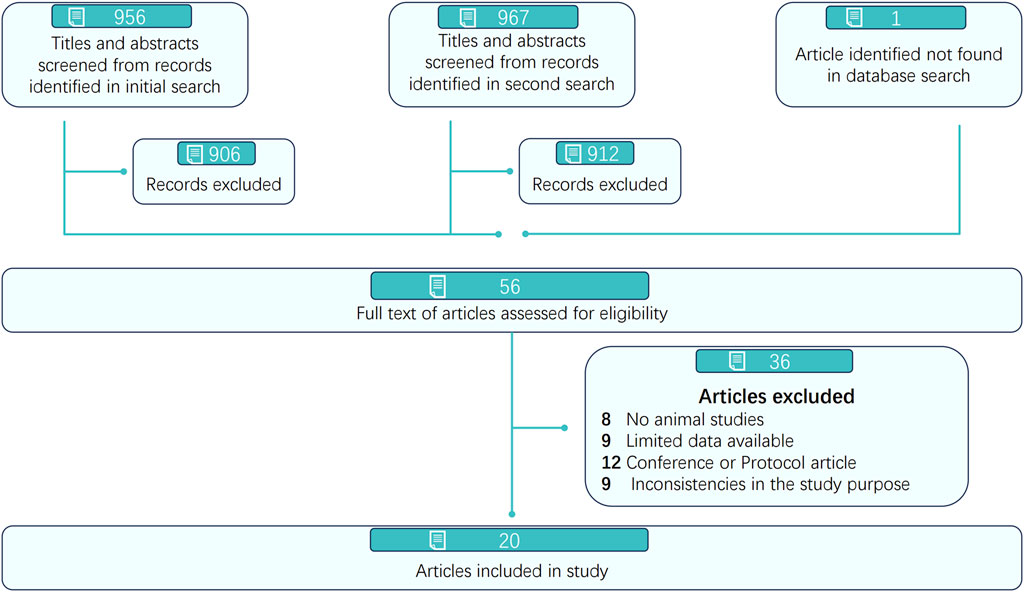
A total of 956 and 967 articles were identified from five electronic databases (PubMed, Embase, Web of Science, OVID, and the Cochrane Library) through two independent searches conducted on the same day at different times by two reviewers. After removing 359 and 362 duplicate articles, 597 and 605 articles remained and were screened by title and abstract by two reviewers (Ling XJ and Chen GQ), as detailed in Figure 1. Subsequently, 547 and 550 articles were excluded by each reviewer, respectively, resulting in 56 articles assessed for full-text eligibility. Ultimately, 20 articles published between 2007 and 2024 were included for methodological quality assessment and further analysis.
3.2 Article characteristics
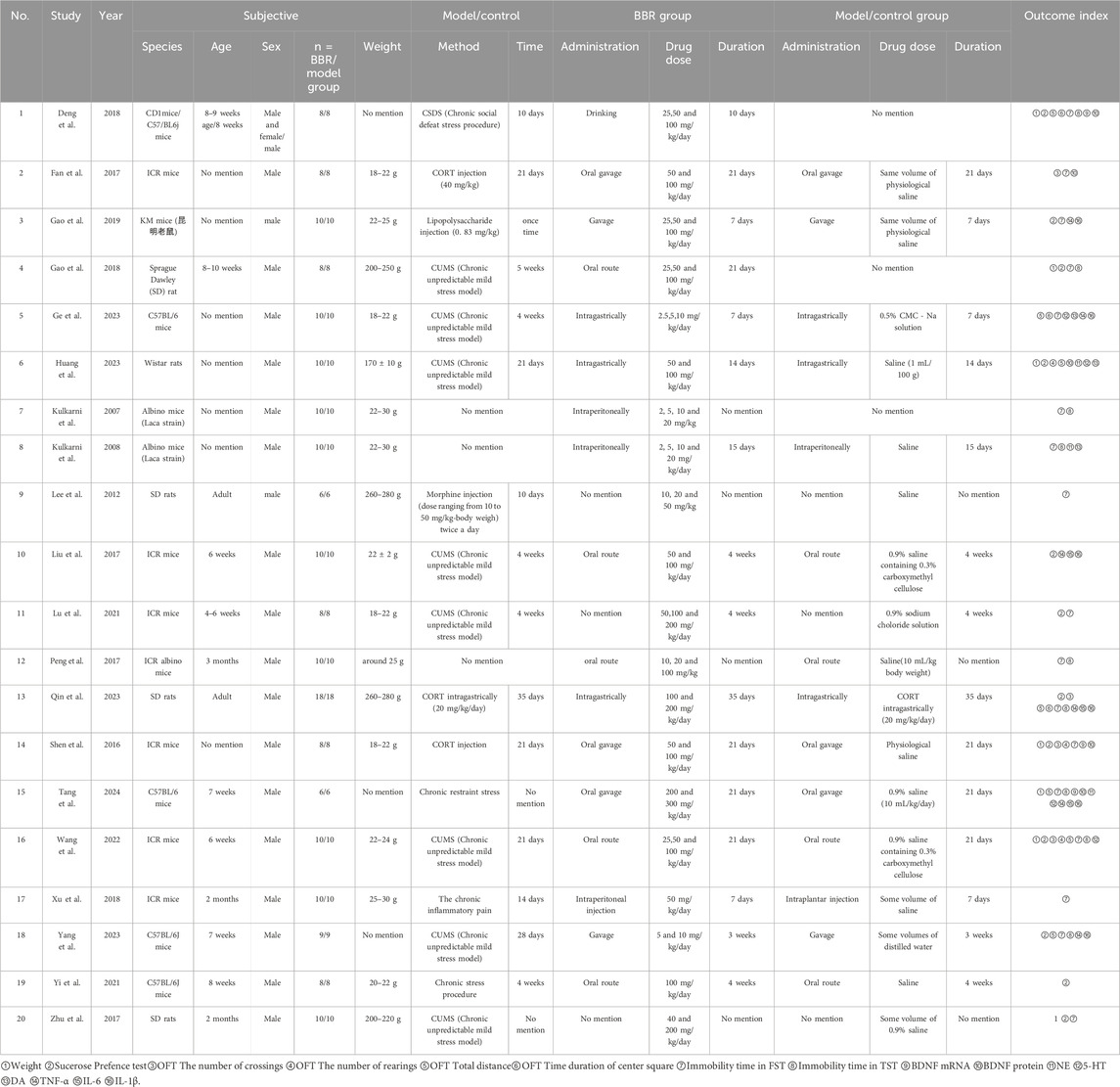
Across the 20 included studies, two species of laboratory animals were used: rats (n = 5) and mice (n = 15). Specifically, 7 mouse and rat strains were reported: CD1 mice (n = 1), C57BL/6 mice (n = 5), ICR mice (n = 7), SD rats (n = 4), KM mice (n = 1), Wistar rats (n = 1), and albino mice (n = 2). One study involved two different mouse strains. Experimental and control groups consisted of 6–18 animals per group. The reported ages of the animals ranged from 4 weeks (approximately 1 month) to 12 weeks (approximately 3 months). However, seven studies did not report the age of the animals, and two studies made only vague references to the animals being adults. Male animals were used exclusively in 19 studies, while only one study included both male and female subjects. Reported body weights varied considerably across studies, primarily due to the differences in species and strains used. The details are shown in the Table 3.
3.3 Risk of bias
The SYRCLE risk of bias assessments for all included studies are summarized in Supplementary Table S4. Most studies demonstrated either a low risk or an unclear risk in the domains of sequence generation and baseline characteristics. With the exception of the study by Kulkarni and Dhir (2007), all others exhibited either unclear or high risk in these domains. Regarding random housing, all studies showed either unclear or low risk, except for the study by Yang L et al., which presented a higher risk. All studies showed good control in the domain of selective outcome reporting. For other domains, the risk of bias varied among individual studies. The details are shown in the Table 4.
3.4 Meta-analysis results
3.4.1 Depression-like behaviors
This analysis included seven behavioral indicators related to depression-like symptoms reported across the 20 included studies: body weight, sucrose preference in the SPT, number of crossings in the OFT, total distance moved in the OFT, time spent in the center square in the OFT, immobility time in the FST, and immobility time in the TST.
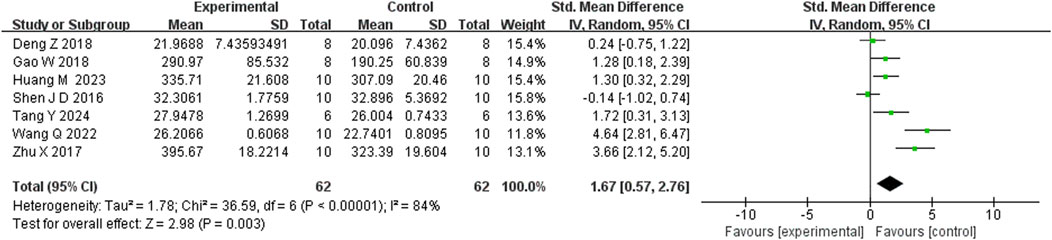
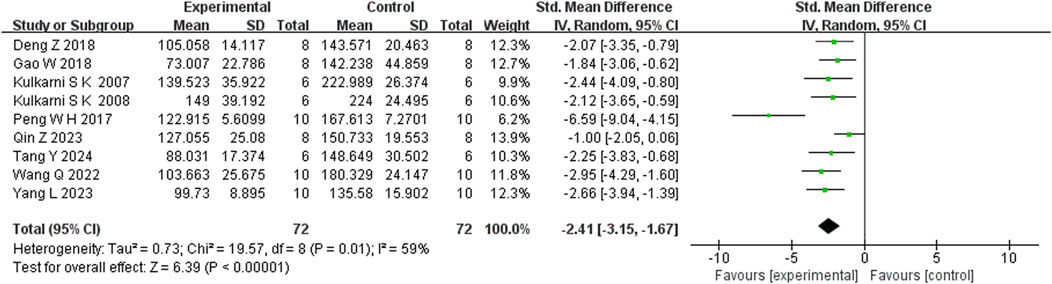
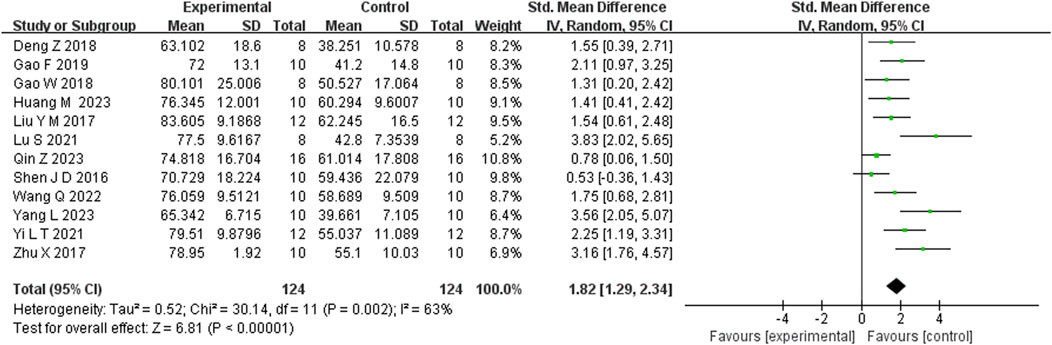
Seven studies reported that BBR significantly increased body weight compared to controls (n = 7; SMD = 1.67; 95% CI: 0.57 to 2.76; heterogeneity: I2 = 84%, P < 0.00001; Figure 2). Nine studies showed that BBR reduced immobility time in the TST (n = 9; SMD = −2.41; 95% CI: 3.15 to −1.67; I2 = 59%, P = 0.01; Figure 3). Twelve studies demonstrated that BBR significantly increased sucrose preference (n = 12; SMD = −1.82; 95% CI: 2.34 to −1.29; I2 = 63%, P = 0.02; Figure 4).
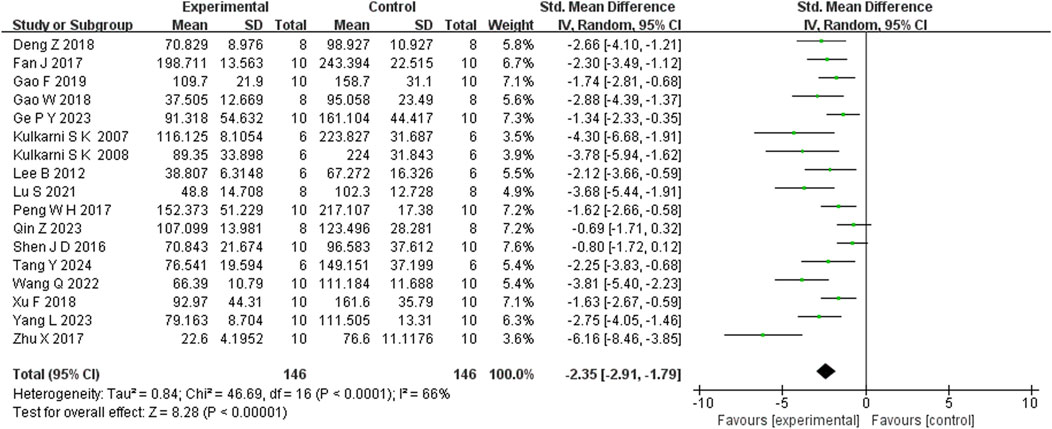
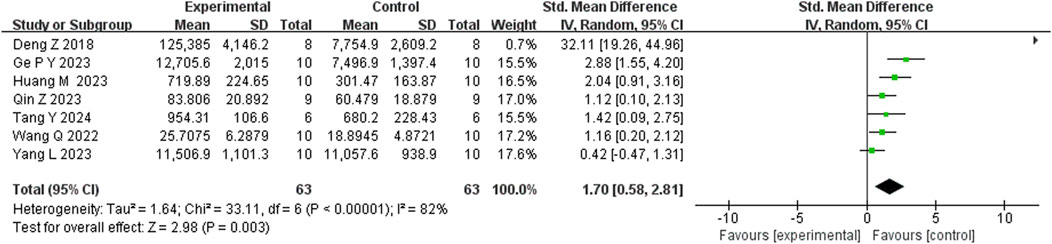
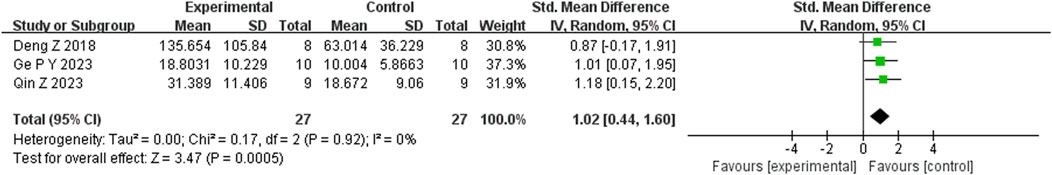
Seventeen studies reported that BBR reduced immobility time in the FST (n = 17; SMD = −2.35; 95% CI: 2.91 to −1.79; I2 = 82%, P < 0.00001; Figure 5). Seven studies showed that BBR increased total distance moved in the OFT (n = 7; SMD = 1.70; 95% CI: 0.58 to 2.81; I2 = 83%, P < 0.00001; Figure 6). Three studies indicated that BBR increased time spent in the center square of the OFT (n = 3; SMD = 1.02; 95% CI: 0.44 to 1.60; I2 = 0%, P = 0.92; Figure 7). Finally, four studies demonstrated an increase in the number of crossings in the OFT with BBR treatment (n = 4; SMD = 0.76; 95% CI: 0.20 to 1.33; I2 = 30%, P = 0.23; Figure 8).
3.4.2 Inflammation indicators
This analysis included three inflammatory markers—TNF-α, IL-1β, and IL-6—reported in the 20 included studies.
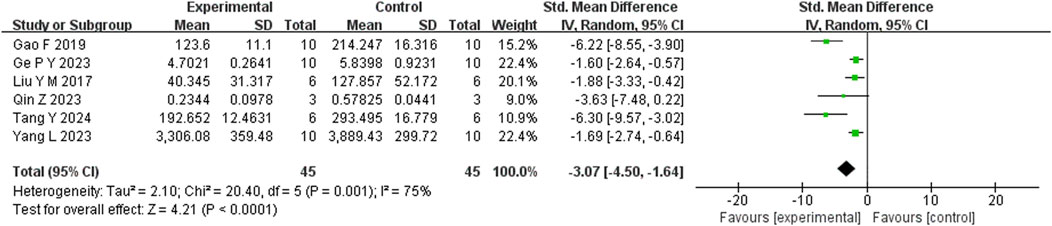
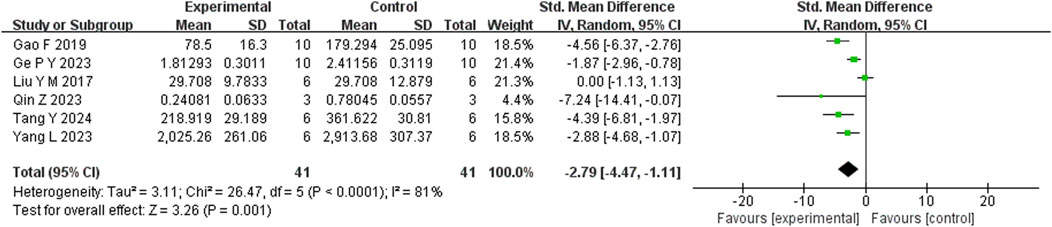
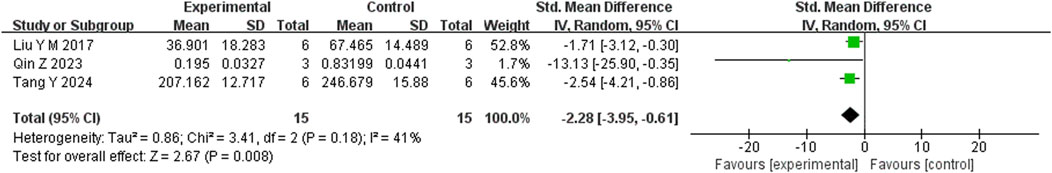
Six studies reported that BBR significantly reduced TNF-α levels compared to the control group (n = 6; SMD = −3.07; 95% CI: 4.50 to −1.64; heterogeneity: I2 = 75%, P = 0.001; Figure 9). Another six studies demonstrated that BBR significantly decreased IL-1β levels (n = 6; SMD = −2.79; 95% CI: 2.79 to −1.11; I2 = 81%, P < 0.00001; Figure 10). Additionally, three studies showed that BBR reduced IL-6 levels compared to controls (n = 3; SMD = −2.28; 95% CI: 3.95 to −0.61; I2 = 41%, P = 0.18; Figure 11).
3.4.3 Neurotransmitters
This analysis included three neurotransmitter indicators—5-hydroxytryptamine (5-HT), norepinephrine (NE), and dopamine (DA)—reported across the 20 included studies.
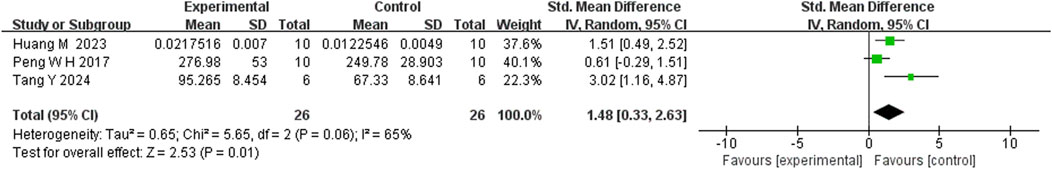
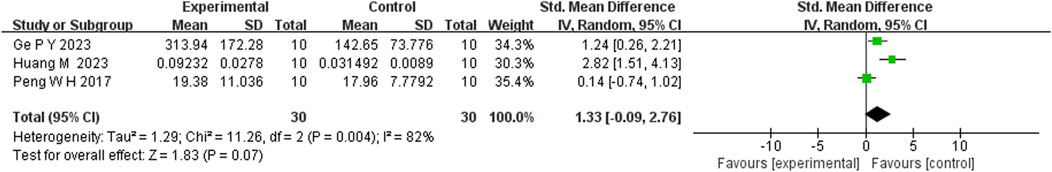
Five studies reported that berberine (BBR) increased 5-HT levels compared to the control group (n = 5; SMD = 1.82; 95% CI: 1.27 to 2.37; heterogeneity: I2 = 0%, P = 0.45; Figure 12). Three studies showed that BBR significantly elevated NE levels (n = 3; SMD = 1.48; 95% CI: 0.33 to 2.63; I2 = 65%, P = 0.06; Figure 13). Regarding DA levels, three studies reported minimal differences between the BBR-treated and control groups (n = 3; SMD = 1.33; 95% CI: 0.09 to 2.76; I2 = 82%, P = 0.004; Figure 14).
3.4.4 Brain-derived neurotrophic factor
This analysis included two indicators—BDNF protein levels and BDNF mRNA levels—to represent changes in neuroplasticity-related outcomes reported across the 20 included studies.
Four studies reported that berberine (BBR) significantly increased BDNF protein levels compared to controls (n = 4; SMD = 2.13; 95% CI: 1.01 to 3.26; heterogeneity: I2 = 68%, P = 0.01; Figure 15). An increase in BDNF mRNA levels following BBR treatment was observed in three studies (n = 3; SMD = 1.86; 95% CI: 0.99 to 2.72; I2 = 0%, P = 0.39; Figure 16).
3.5 Subgroup analysis
The subgroup analyses revealed that animal body weight, BBR dosage, and administration route significantly influenced specific outcomes. In the body weight subgroup, animals weighing <100 g showed larger effect sizes in the tail suspension test (SMD = −3.03 vs. −1.36, P-between = 0.025) and BDNF protein levels, while the ≥100 g subgroup demonstrated greater effects in dopamine levels (P-between = 0.013). In the dosage subgroup, lower BBR dosage (<100 mg/kg) produced greater effects on body weight (SMD = 4.64, P-between <0.001), while higher dosage (>100 mg/kg) showed stronger effects on TNF-α reduction (SMD = −5.16, P-between = 0.025). Significant dopamine improvement was only observed at 100 mg/kg (SMD = 2.82). Regarding administration routes, intragastric administration was most effective for IL-1β reduction (P-between <0.001) while gavage administration showed the greatest effects on dopamine levels (P-between = 0.044). Most behavioral tests showed no significant subgroup differences. Considerable heterogeneity (I2 > 50%) was observed in several subgroups (detailed results provided in Supplementary Figures S17–S55).
3.6 Publication bias
Publication bias was assessed using two approaches. For outcomes reported in more than 10 studies, both Begg’s test and Egger’s test were performed. Additionally, sensitivity analyses were conducted for these outcomes. For indicators reported in fewer than 10 studies, no further bias analysis was conducted.
3.6.1 Begg’s test and Egger’s test
Two indicators—sucrose preference test (SPT) and immobility time in the forced swim test (FST)—were reported in more than 10 studies. Therefore, Begg’s test and Egger’s test were conducted to assess potential publication bias. For the SPT, Begg’s test indicated significant publication bias (P < 0.0005; P = 0.004). Similarly, both Begg’s test and Egger’s test for immobility time in the FST also indicated significant publication bias (Begg’s test: P < 0.0005; P < 0.001; Egger’s test: P < 0.0005; P = 0.000). These results suggest the presence of significant publication bias in these indicators.
3.6.2 Sensitivity analysis
Meanwhile, sensitivity analysis was conducted for 2 indicators (SPT, Immobility time in FST) (the details in Supplementary Figures S56, S57). The analysis indicates that the 2 experimental results exhibit a certain degree of robustness.
4 Discussion
The aim of this study was to synthesize preclinical studies to evaluate the efficacy and potential mechanisms of BBR in the treatment of depression. Our findings provide further evidence of BBR’s effectiveness in promoting weight gain and producing antidepressant-like effects in animal models. We also found that BBR reduces levels of inflammatory markers (IL-6, IL-1β, TNF-α), enhances neurotransmitter levels (5-HT, NE—but not DA), and increases neuroprotective factors, including BDNF protein and BDNF mRNA expression.
The results suggest that BBR may increase body weight and modulate various depression-like behaviors, indicating its potential to alleviate different depressive symptoms to varying degrees.
Though, patients with depression exhibit heterogeneity in body weight changes. In this study, depressive model animals exhibited the expected reduction in body weight, while BBR treatment restored their body weight to within the normal control range. This “restorative increase” in body weight, which’s it to normal levels, suggests that BBR may exert its therapeutic effects by correcting depression-related physiological disturbances, such as appetite loss. Furthermore, not all studies in our review reported weight gain following BBR administration (Shen et al., 2016), possibly due to the non-dose-dependent nature of BBR’s antidepressant effects (Kulkarni and Dhir, 2007; Kulkarni and Dhir, 2008) and our subgroup analysis based on animal body weight further corroborates this observation. Reduced sucrose preference is widely used as a proxy for anhedonia, a core symptom of depression (Riaz et al., 2015; Willner, 2005). The observed enhancement of sucrose preference following BBR treatment suggests a potential benefit for anhedonia. In previous research, the FST and tail TST have been validated as predictors of antidepressant activity (Kulkarni and Dhir, 2007) and are associated with behavioral despair (Xing et al., 2019). In our analysis, BBR significantly reduced immobility time in both tests, indicating its ability to mitigate despair-like behaviors. In contrast to previous reports (Kulkarni and Dhir, 2007; Kulkarni and Dhir, 2008), this effect demonstrated no association with a linear dose-response relationship but was significantly influenced by animal body weight.
Furthermore, several OFT indicators—including total distance traveled, time spent in the center, and number of crossings—are commonly interpreted as behavioral responses to psychotropic treatments (Schulz et al., 2023). Our findings suggest that BBR administration improves anxiety- and fear-related behaviors (Kraeuter et al., 2019; Walz et al., 2016). Collectively, these results support the potential of BBR for future clinical use in the prevention and treatment of depressive disorders.
In terms of mechanisms, BBR has been shown to influence three major biological systems relevant to depression. However, the underlying mechanisms remain complex and not fully understood. One of the leading hypotheses for the pathophysiology of depression involves inflammatory pathways, first proposed in 1987 (Renault et al., 1987). Increasing evidence suggests that both peripheral and central inflammation contribute significantly to the risk and susceptibility to depression (Beurel et al., 2020; Colasanto et al., 2020; Enache et al., 2019). Pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α are widely recognized as classic biomarkers of inflammation (Felger and Lotrich, 2013). Moreover, the relationship between inflammation and depression appears to be biphasic (Beurel et al., 2020; Kiecolt-Glaser et al., 2015). Notably, some antidepressants have also demonstrated anti-inflammatory effects (Johnston et al., 2023; Köhler et al., 2018). Although a few studies have suggested that BBR may exacerbate the inflammatory response (Zhu and Qian, 2006), our meta-analysis aligns with major previous reports (Gong et al., 2024; Jeong et al., 2009) by supporting the anti-inflammatory role of BBR in regulating typical pro-inflammatory cytokines such as IL-6, IL-1β, and TNF-α.
Despite these findings, the precise mechanisms by which BBR exerts its anti-inflammatory effects remain unclear. Recent studies have implicated several molecular pathways and regulators, including acetylation of p65 at Lys310 by p300 in macrophages (Zhang et al., 2023), EIF2AK2 (Wei et al., 2023), the NF-κB signaling pathway (Yu et al., 2019; Tang et al., 2021), the NLRP3 inflammasome pathway (Yang et al., 2023), and the ADK/AMPK/Nrf2 signaling axis (Cheng et al., 2024). Additionally, BBR may help maintain immunodynamic homeostasis through multiple immune-related mechanisms (Vita and Pullen, 2022; Wang et al., 2020; Xia et al., 2024).
Secondly, it is widely believed that depression results from an imbalance of 5-HT, NE, DA, or other neurochemical substances in the brain (Pilkington et al., 2018). However, our findings partially contradict previous studies (Mohi-Ud-Din et al., 2022), as DA levels did not increase following BBR treatment. Our subgroup analysis confirms the non-dose-dependent pharmacology of BBR reported in previous studies (Huang et al., 2023; Peng et al., 2007): DA levels increased significantly only at the 100 mg/kg dosage. This finding underscores the importance of methodological considerations in interpreting BBR’s complex interactions with neurotransmitter systems. Different doses of BBR may exert varying effects on specific neurotransmitters (Arora and Chopra, 2013; Kulkarni and Dhir, 2008). For instance, BBR has been shown to inhibit monoamine oxidase (Peng et al., 2007) and influence organic cation transporter 2 and 3 activity (Sun et al., 2014), which may contribute to increased levels of certain neurotransmitters. The BBR-mediated enhancement of neurotransmitters may represent one step in a broader cascade of events leading to its antidepressant effects (Arora and Chopra, 2013). Moreover, recent studies suggest that BBR may exert synergistic effects when combined with classical antidepressants (Sun et al., 2014).
Notably, BBR is capable of rapidly crossing the blood–brain barrier to exert neuroprotective effects (Kulkarni and Dhir, 2010; Tian et al., 2023; L. Wang et al., 2021; Yang et al., 2018). The relationship between BBR and BDNF has been increasingly studied over the past decade. BDNF is a key neurotrophin widely distributed in the brain and is essential for neuronal survival and plasticity (Zhang et al., 2016). It has been demonstrated that one of the mechanisms through which BBR exerts its effects is by increasing BDNF levels, thereby promoting neuronal nourishment, conferring anti-seizure activity (Jivad et al., 2024), preventing neurodegeneration (Begh et al., 2025), and offering cognitive protection (Begh et al., 2025; Shaker et al., 2021). Furthermore, BBR’s effect on BDNF expression resembles that of certain antidepressants (Hess et al., 2022). This regulatory effect may occur through modulation of the PI3K/AKT signaling pathway (Tang et al., 2024), inhibition of the NF-κB signaling pathway (Yu et al., 2019), and activation of the cAMP response element-binding protein (Tang et al., 2024; Yu et al., 2019). These pathways ultimately influence BDNF expression. However, the precise molecular mechanisms by which BBR upregulates BDNF remain unclear. Importantly, BDNF has also been implicated in the regulation of inflammatory responses (Kim et al., 2023), further supporting BBR’s potential anti-inflammatory effects. Additionally, some studies have shown that BDNF may enhance the levels of neurotransmitters (Bastioli et al., 2022), highlighting its broader role in neuropsychiatric regulation.
4.1 Advantages
There is an urgent need for new antidepressant therapies. This study provides a comprehensive summary of preclinical findings on the effects of BBR in the treatment of depression, offering foundational evidence to support its potential therapeutic use. Although the exact mechanisms underlying BBR’s antidepressant-like effects remain unclear and were not definitively established in this study, our findings represent an important step forward. This work offers both theoretical insights and practical guidance for future research on BBR, potentially accelerating the development of novel antidepressant agents targeting diverse etiologies of depression (Xu et al., 2018).
4.2 Limitation
Several important considerations should be noted before interpreting the findings of this study:
1. The near-exclusive use of male animals in the included studies limits the generalizability of our findings to both sexes, as it fails to account for potential sex-based differences. This constraint necessitates caution when extrapolating the results to clinical settings, particularly given the well-documented sex-specific characteristics of depression (Salk et al., 2017).
2. Prior studies have shown that inflammatory markers, neurotransmitters, and BDNF interact with one another (Bastioli et al., 2022; Hodo et al., 2020; Kim et al., 2023; Oshaghi et al., 2023; Zhu et al., 2022). In our study, it remains unclear whether the observed effects are due to these interactions or if causal relationships exist among these factors. Further research is needed to clarify these complex linkages.
3. While this study contributes a novel perspective on the treatment of depression, the mechanism by which BBR exerts its antidepressant effects is still not fully understood, and current findings are limited to animal models. Before BBR can be translated into clinical practice, it is crucial to recognize that animal experimental results cannot be directly extrapolated to humans. Three key issues require resolution: species differences preventing direct translation of effective dosage, unknown drug interaction mechanisms, and unverified long-term safety profiles. These inherent limitations determine that the current findings can only serve as reference for subsequent clinical work.
4. This meta-analysis is limited by substantial heterogeneity, reflecting methodological variations in BBR sources, animal models, administration routes, and detection methods among included studies. The findings should therefore be interpreted as representing a range of potential effects under different experimental conditions. Future studies would benefit from standardized protocols and complete methodological reporting to improve evidence synthesis.
5. This study has important limitations, including concerns regarding the high risk of bias in most included studies and the presence of publication bias, as evidenced by significant Begg’s and Egger’s tests for SPT and FST (P < 0.05). These issues call for cautious interpretation of the findings.
5 Conclusion
In summary, our analysis indicates that BBR may be effective in reducing depression-like behaviors across various animal models. Moreover, the findings suggest that BBR has the potential to modulate inflammatory factors, neurotransmitters, and BDNF. However, further investigation is needed to elucidate the complex mechanisms through which BBR regulates these systems and to determine whether additional interrelationships exist among them. Importantly, it remains unclear whether the effects observed in preclinical studies can be replicated in clinical settings, and the safety profile of BBR in humans warrants further exploration. Therefore, more rigorous and comprehensive evidence is required to support the translation of these findings into clinical practice and to realize potential therapeutic benefits for patients with depression.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
BY: Formal Analysis, Software, Writing – original draft. ZL: Formal Analysis, Software, Supervision, Visualization, Writing – original draft. XjL: Investigation, Writing – original draft. GC: Investigation, Writing – original draft. XyL: Writing – original draft. ZY: Resources, Writing – original draft. JL: Supervision, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1664784/full#supplementary-material
Abbreviations
5-HT, 5-hydroxytryptamine; BBR, berberine; BDNF, brain-derived neurotrophic factor; CI, confidence interval; CIs, confidence intervals; DA, dopamine; FST, forced swim test; IL-1β, Interleukin-1β; IL-6, Interleukin 6; NE, Norepinephrine; OFT, open field test; SD, standard deviation; SMD, standardized mean difference; SMDs, standardized mean differences; SPT, Sucrose preference in sucrose preference test; TNF-α, Tumor necrosis factor α; TST, Tail suspension test.
References
Aburto, M. R., and Cryan, J. F. (2024). Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota-gut-brain axis. Nat. Rev. Gastroenterol. Hepatol. 21 (4), 222–247. doi:10.1038/s41575-023-00890-0
Alexopoulos, G. S. (2005). Depression in the elderly. Lancet 365 (9475), 1961–1970. doi:10.1016/s0140-6736(05)66665-2
Arora, V., and Chopra, K. (2013). Possible involvement of oxido-nitrosative stress induced neuro-inflammatory Cascade and monoaminergic pathway: underpinning the correlation between nociceptive and depressive behaviour in a rodent model. J. Affect Disord. 151 (3), 1041–1052. doi:10.1016/j.jad.2013.08.032
Bai, Y., Cai, Y., Chang, D., Li, D., Huo, X., and Zhu, T. (2024). Immunotherapy for depression: recent insights and future targets. Pharmacol. Ther. 257, 108624. doi:10.1016/j.pharmthera.2024.108624
Bastioli, G., Arnold, J. C., Mancini, M., Mar, A. C., Gamallo-Lana, B., Saadipour, K., et al. (2022). Voluntary exercise boosts striatal dopamine release: evidence for the necessary and sufficient role of BDNF. J. Neurosci. 42 (23), 4725–4736. doi:10.1523/jneurosci.2273-21.2022
Begh, M. Z. A., Amin, M. A., Shatu, M. M., Sweilam, S. H., Puri, S., Ramesh, R. B., et al. (2025). Unraveling berberine's molecular mechanisms in neuroprotection against neurodegeneration. Chem. Biodivers. 22, e202500170. doi:10.1002/cbdv.202500170
Benjamin, D. J., Berger, J. O., Johannesson, M., Nosek, B. A., and Johnson, V. E. (2017). Redefine statistical significance. Nat. Hum. Behav. 2 (1). doi:10.1038/s41562-017-0189-z
Beurel, E., Toups, M., and Nemeroff, C. B. (2020). The bidirectional relationship of depression and inflammation: double trouble. Neuron 107 (2), 234–256. doi:10.1016/j.neuron.2020.06.002
Boaden, K., Tomlinson, A., Cortese, S., and Cipriani, A. (2020). Antidepressants in children and adolescents: meta-review of efficacy, tolerability and suicidality in acute treatment. Front. Psychiatry 11, 717. doi:10.3389/fpsyt.2020.00717
Bore, M. C., Liu, X., Huang, X., Kendrick, K. M., Zhou, B., Zhang, J., et al. (2024). Common and separable neural alterations in adult and adolescent depression - evidence from neuroimaging meta-analyses. Neurosci. Biobehav. Rev. 164, 105835. doi:10.1016/j.neubiorev.2024.105835
Bullmore, E. (2018). The art of medicine: inflamed depression. Lancet 392 (10154), 1189–1190. doi:10.1016/s0140-6736(18)32356-0
Chen, C.-Y., and Zhang, Y. (2025). Berberine: an isoquinoline alkaloid targeting the oxidative stress and gut-brain axis in the models of depression. Eur. J. Med. Chem., 290, 117475. doi:10.1016/j.ejmech.2025.117475
Cheng, J., Yan, G., Tan, W., Qin, Z., Xie, Q., Liu, Y., et al. (2024). Berberine alleviates fructose-induced hepatic injury via ADK/AMPK/Nrf2 pathway: a novel insight. Biomed. Pharmacother. 179, 117361. doi:10.1016/j.biopha.2024.117361
Colasanto, M., Madigan, S., and Korczak, D. J. (2020). Depression and inflammation among children and adolescents: a meta-analysis. J. Affect Disord. 277, 940–948. doi:10.1016/j.jad.2020.09.025
Cuijpers, P., Stringaris, A., and Wolpert, M. (2020). Treatment outcomes for depression: challenges and opportunities. Lancet Psychiatry 7 (11), 925–927. doi:10.1016/s2215-0366(20)30036-5
De Risio, L., Borgi, M., Pettorruso, M., Miuli, A., Ottomana, A. M., Sociali, A., et al. (2020). Recovering from depression with repetitive transcranial magnetic stimulation (rTMS): a systematic review and meta-analysis of preclinical studies. Transl. Psychiatry 10 (1), 393. doi:10.1038/s41398-020-01055-2
De Vries, R. B. M., Hooijmans, C. R., Langendam, M. W., Van Luijk, J., Leenaars, M., Ritskes-Hoitinga, M., et al. (2015). A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evidence-based Preclin. Med. 2 (1), 1–9. doi:10.1002/ebm2.7
Dong, Y., Fan, H., Zhang, Z., Jiang, F., Li, M., Zhou, H., et al. (2022). Berberine ameliorates DSS-Induced intestinal mucosal barrier dysfunction through microbiota-dependence and Wnt/β-catenin pathway. Int. J. Biol. Sci. 18 (4), 1381–1397. doi:10.7150/ijbs.65476
Donohue, M. R., Whalen, D. J., Gilbert, K. E., Hennefield, L., Barch, D. M., and Luby, J. (2019). Preschool depression: a diagnostic reality. Curr. Psychiatry Rep. 21 (12), 128. doi:10.1007/s11920-019-1102-4
Drevets, W. C., Wittenberg, G. M., Bullmore, E. T., and Manji, H. K. (2022). Immune targets for therapeutic development in depression: towards precision medicine. Nat. Rev. Drug Discov. 21 (3), 224–244. doi:10.1038/s41573-021-00368-1
Enache, D., Pariante, C. M., and Mondelli, V. (2019). Markers of central inflammation in major depressive disorder: a systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav. Immun. 81, 24–40. doi:10.1016/j.bbi.2019.06.015
Felger, J. C., and Lotrich, F. E. (2013). Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246, 199–229. doi:10.1016/j.neuroscience.2013.04.060
Fried, E. I. (2017). Moving forward: how depression heterogeneity hinders progress in treatment and research. Expert Rev. Neurother. 17 (5), 423–425. doi:10.1080/14737175.2017.1307737
Fried, E. I., and Nesse, R. M. (2015). Depression is not a consistent syndrome: an investigation of unique symptom patterns in the STAR*D study. J. Affect Disord. 172, 96–102. doi:10.1016/j.jad.2014.10.010
Fried, E. I., Nesse, R. M., Zivin, K., Guille, C., and Sen, S. (2014). Depression is more than the sum score of its parts: individual DSM symptoms have different risk factors. Psychol. Med. 44 (10), 2067–2076. doi:10.1017/s0033291713002900
Gao, Y., Nie, K., Wang, H., Dong, H., and Tang, Y. (2024). Research progress on antidepressant effects and mechanisms of berberine. Front. Pharmacol. 15, 1331440. doi:10.3389/fphar.2024.1331440
GBD 2019 Diseases and Injuries Collaborators (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 396 (10258), 1204–1222. doi:10.1016/s0140-6736(20)30925-9
Gill, H., Gill, B., El-Halabi, S., Chen-Li, D., Lipsitz, O., Rosenblat, J. D., et al. (2020). Antidepressant medications and weight change: a narrative review. Obes. (Silver Spring) 28 (11), 2064–2072. doi:10.1002/oby.22969
Gong, S., Chen, J., Zheng, X., Lu, X., Chen, M., Li, J., et al. (2024). Kidney targeting and modulating macrophage polarization through AMPK signaling: therapeutic mechanism of berberine in uric acid nephropathy. Int. Immunopharmacol. 138, 112632. doi:10.1016/j.intimp.2024.112632
Hess, E. M., Riggs, L. M., Michaelides, M., and Gould, T. D. (2022). Mechanisms of ketamine and its metabolites as antidepressants. Biochem. Pharmacol. 197, 114892. doi:10.1016/j.bcp.2021.114892
Hetrick, S. E., McKenzie, J. E., Bailey, A. P., Sharma, V., Moller, C. I., Badcock, P. B., et al. (2021). New generation antidepressants for depression in children and adolescents: a network meta-analysis. Cochrane Database Syst. Rev. 5 (5), Cd013674. doi:10.1002/14651858.CD013674.pub2
Hodo, T. W., de Aquino, M. T. P., Shimamoto, A., and Shanker, A. (2020). Critical neurotransmitters in the neuroimmune network. Front. Immunol. 11, 1869. doi:10.3389/fimmu.2020.01869
Hooijmans, C. R., Rovers, M. M., de Vries, R. B. M., Leenaars, M., Ritskes-Hoitinga, M., and Langendam, M. W. (2014). SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14 (1), 43. doi:10.1186/1471-2288-14-43
Hsu, C. Y., Pallathadka, H., Gupta, J., Ma, H., Al-Shukri, H. H. K., Kareem, A. K., et al. (2024). Berberine and berberine nanoformulations in cancer therapy: focusing on lung cancer. Phytother. Res. 38 (8), 4336–4350. doi:10.1002/ptr.8255
Huang, M., He, Y., Tian, L., Yu, L., Cheng, Q., Li, Z., et al. (2023). Gut microbiota-SCFAs-brain axis associated with the antidepressant activity of berberine in CUMS rats. J. Affect Disord. 325, 141–150. doi:10.1016/j.jad.2022.12.166
Imanshahidi, M., and Hosseinzadeh, H. (2008). Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother. Res. 22 (8), 999–1012. doi:10.1002/ptr.2399
Jeong, H. W., Hsu, K. C., Lee, J. W., Ham, M., Huh, J. Y., Shin, H. J., et al. (2009). Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am. J. Physiol. Endocrinol. Metab. 296 (4), E955–E964. doi:10.1152/ajpendo.90599.2008
Jivad, N., Heidari-Soureshjani, S., Bagheri, H., Sherwin, C. M. T., and Rostamian, S. (2024). Anti-seizure effects and mechanisms of berberine: a systematic review. Curr. Pharm. Biotechnol. 25 (17), 2253–2265. doi:10.2174/0113892010283237240107121749
Johnston, J. N., Greenwald, M. S., Henter, I. D., Kraus, C., Mkrtchian, A., Clark, N. G., et al. (2023). Inflammation, stress and depression: an exploration of ketamine's therapeutic profile. Drug Discov. Today 28 (4), 103518. doi:10.1016/j.drudis.2023.103518
Kiecolt-Glaser, J. K., Derry, H. M., and Fagundes, C. P. (2015). Inflammation: depression fans the flames and feasts on the heat. Am. J. Psychiatry 172 (11), 1075–1091. doi:10.1176/appi.ajp.2015.15020152
Kim, J. H., Irfan, M., Hossain, M. A., George, A., and Chung, S. (2023). BDNF/trkB is a crucial regulator in the inflammation-mediated odontoblastic differentiation of dental pulp stem cells. Cells 12 (14), 1851. doi:10.3390/cells12141851
Köhler, C. A., Freitas, T. H., Stubbs, B., Maes, M., Solmi, M., Veronese, N., et al. (2018). Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol. Neurobiol. 55 (5), 4195–4206. doi:10.1007/s12035-017-0632-1
Kong, W., Wei, J., Abidi, P., Lin, M., Inaba, S., Li, C., et al. (2004). Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 10 (12), 1344–1351. doi:10.1038/nm1135
Kraeuter, A. K., Guest, P. C., and Sarnyai, Z. (2019). The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol. Biol. 1916, 99–103. doi:10.1007/978-1-4939-8994-2_9
Kulkarni, S. K., and Dhir, A. (2007). Effect of various classes of antidepressants in behavioral paradigms of despair. Prog. Neuropsychopharmacol. Biol. Psychiatry 31 (6), 1248–1254. doi:10.1016/j.pnpbp.2007.05.002
Kulkarni, S. K., and Dhir, A. (2007). Possible involvement of L-arginine-nitric oxide (NO)-Cyclic guanosine monophosphate (cGMP) signaling pathway in the antidepressant activity of berberine chloride. Eur. J. Pharmacol. 569 (1-2), 77–83. [Article]. doi:10.1016/j.ejphar.2007.05.002
Kulkarni, S. K., and Dhir, A. (2008). On the mechanism of antidepressant-like action of berberine chloride. Eur. J. Pharmacol. 589 (1-3), 163–172. doi:10.1016/j.ejphar.2008.05.043
Kulkarni, S. K., and Dhir, A. (2010). Berberine: a plant alkaloid with therapeutic potential for central nervous system disorders. Phytother. Res. 24 (3), 317–324. doi:10.1002/ptr.2968
Lee, D. K., In, J., and Lee, S. (2015). Standard deviation and standard error of the mean. Korean J. Anesthesiol. 68 (3), 220–223. doi:10.4097/kjae.2015.68.3.220
Liu, Q., He, H., Yang, J., Feng, X., and Lyu, J. (2019). Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J. Psychiatric Res. 126, 134–140. doi:10.1016/j.jpsychires.2019.08.002
Malhi, G. S., and Mann, J. J. (2018). Depression. Lancet 392 (10161), 2299–2312. doi:10.1016/s0140-6736(18)31948-2
Mohi-Ud-Din, R., Mir, R. H., Wani, T. U., Shah, A. J., Banday, N., and Pottoo, F. H. (2022). Berberine in the treatment of neurodegenerative diseases and nanotechnology enabled targeted delivery. Comb. Chem. High. Throughput Screen 25 (4), 616–633. doi:10.2174/1386207324666210804122539
Njenga, C., Ramanuj, P. P., de Magalhães, F. J. C., and Pincus, H. A. (2024). New and emerging treatments for major depressive disorder. Bmj 386, e073823. doi:10.1136/bmj-2022-073823
Oliva, V., Lippi, M., Paci, R., Del Fabro, L., Delvecchio, G., Brambilla, P., et al. (2021). Gastrointestinal side effects associated with antidepressant treatments in patients with major depressive disorder: a systematic review and meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 109, 110266. doi:10.1016/j.pnpbp.2021.110266
Oshaghi, M., Kourosh-Arami, M., and Roozbehkia, M. (2023). Role of neurotransmitters in immune-mediated inflammatory disorders: a crosstalk between the nervous and immune systems. Neurol. Sci. 44 (1), 99–113. doi:10.1007/s10072-022-06413-0
Peleg, L. C., Rabinovitch, D., Lavie, Y., Rabbie, D. M., Horowitz, I., Fruchter, E., et al. (2022). Post-SSRI sexual dysfunction (PSSD): biological plausibility, symptoms, diagnosis, and presumed risk factors. Sex. Med. Rev. 10 (1), 91–98. doi:10.1016/j.sxmr.2021.07.001
Peng, W.-H., Lo, K.-L., Lee, Y.-H., Hung, T.-H., and Lin, Y.-C. (2007). Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci. 81 (11), 933–938. doi:10.1016/j.lfs.2007.08.003
Pilkington, P. D., Reavley, N. J., and Jorm, A. F. (2018). The Australian public's beliefs about the causes of depression: associated factors and changes over 16 years. J. Affect. Disord. 2013年150卷2期, 356–362. doi:10.1016/j.jad.2013.04.019
Qin, Z., Shi, D.-D., Li, W., Cheng, D., Zhang, Y.-D., Zhang, S., et al. (2023). Berberine ameliorates depression-like behaviors in mice via inhibiting NLRP3 inflammasome-mediated neuroinflammation and preventing neuroplasticity disruption. J. Neuroinflammation, 20, 54(1). doi:10.1186/s12974-023-02744-7
Renault, P. F., Hoofnagle, J. H., Park, Y., Mullen, K. D., Peters, M., Jones, D. B., et al. (1987). Psychiatric complications of long-term interferon alfa therapy. Arch. Intern. Med. 147 (9), 1577–1580. doi:10.1001/archinte.1987.00370090055011
Riaz, M. S., Bohlen, M. O., Gunter, B. W., Quentin, H., Stockmeier, C. A., and Paul, I. A. (2015). Attenuation of social interaction-associated ultrasonic vocalizations and spatial working memory performance in rats exposed to chronic unpredictable stress. Physiol. Behav. 152 (Pt A), 128–134. doi:10.1016/j.physbeh.2015.09.005
Sajeev, A., Sailo, B., Unnikrishnan, J., Talukdar, A., Alqahtani, M. S., Abbas, M., et al. (2024). Unlocking the potential of berberine: advancing cancer therapy through chemosensitization and combination treatments. Cancer Lett. 597, 217019. doi:10.1016/j.canlet.2024.217019
Salk, R. H., Hyde, J. S., and Abramson, L. Y. (2017). Gender differences in depression in representative national samples: meta-analyses of diagnoses and symptoms. Psychol. Bull. 143 (8), 783–822. doi:10.1037/bul0000102
Schulz, M., Zieglowski, L., Kopaczka, M., and Tolba, R. H. (2023). The open field test as a tool for behaviour analysis in pigs: recommendations for Set-Up standardization - a systematic review. Eur. Surg. Res. 64 (1), 7–26. doi:10.1159/000525680
Shaker, F. H., El-Derany, M. O., Wahdan, S. A., El-Demerdash, E., and El-Mesallamy, H. O. (2021). Berberine ameliorates doxorubicin-induced cognitive impairment (chemobrain) in rats. Life Sci. 269, 119078. doi:10.1016/j.lfs.2021.119078
Shamseer, L., Moher, D., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj 350, g7647. doi:10.1136/bmj.g7647
Shayganfard, M. (2023). Berberine: is it a promising agent for mental disorders treatment? Curr. Mol. Pharmacol. 16 (3), 307–320. doi:10.2174/1874467215666220509213122
Shen, J.-d., Ma, L.-g., Hu, C.-y., Pei, Y.-y., Jin, S.-l., Fang, X.-y., et al. (2016). Berberine up-regulates the BDNF expression in hippocampus and attenuates corticosterone-induced depressive-like behavior in mice. Neurosci. Lett. 614, 77–82. doi:10.1016/j.neulet.2016.01.002
Simon, G. E., Moise, N., and Mohr, D. C. (2024). Management of depression in adults: a review. Jama 332 (2), 141–152. doi:10.1001/jama.2024.5756
Sun, S., Wang, K., Lei, H., Li, L., Tu, M., Zeng, S., et al. (2014). Inhibition of organic cation transporter 2 and 3 may be involved in the mechanism of the antidepressant-like action of berberine. Prog. Neuro-Psychopharmacology and Biol. Psychiatry 49, 1–6. [Article]. doi:10.1016/j.pnpbp.2013.11.005
Tang, M., Yuan, D., and Liao, P. (2021). Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-κB/MAPK signaling pathway in deoxynivalenol-challenged piglets. Environ. Pollut. 289, 117865. doi:10.1016/j.envpol.2021.117865
Tang, Y., Su, H., Nie, K., Wang, H., Gao, Y., Chen, S., et al. (2024). Berberine exerts antidepressant effects in vivo and in vitro through the PI3K/AKT/CREB/BDNF signaling pathway. Biomed. Pharmacother. 170, 116012. doi:10.1016/j.biopha.2023.116012
Tang, M., Zheng, Y., Zhang, X., and Fan, X. (2025). Non-invasive neuromodulation treatment for depression in adolescents: a systematic review and meta-analysis. Psychiatry Res. 344, 116329. doi:10.1016/j.psychres.2024.116329
Tian, E., Sharma, G., and Dai, C. (2023). Neuroprotective properties of berberine: molecular mechanisms and clinical implications. Antioxidants (Basel) 12 (10), 1883. doi:10.3390/antiox12101883
Vita, A. A., and Pullen, N. A. (2022). Exploring the mechanism of berberine-mediated t(fh) cell immunosuppression. Phytomedicine 105, 154343. doi:10.1016/j.phymed.2022.154343
Vrabel, M. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Rev. Espaola De. Nutr. Humana Y Dietética 18 (3), e123.
Walz, N., Mühlberger, A., and Pauli, P. (2016). A human open field test reveals thigmotaxis related to agoraphobic fear. Biol. Psychiatry 80 (5), 390–397. doi:10.1016/j.biopsych.2015.12.016
Wang, X., Wang, R., Xing, D., Su, H., Ma, C., Ding, Y., et al. (2005). Kinetic difference of berberine between hippocampus and plasma in rat after intravenous administration of coptidis rhizoma extract. Life Sci. 77 (24), 3058–3067. doi:10.1016/j.lfs.2005.02.033
Wang, K., Feng, X., Chai, L., Cao, S., and Qiu, F. (2017). The metabolism of berberine and its contribution to the pharmacological effects. Drug Metab. Rev. 1. doi:10.1080/03602532.2017.1306544
Wang, Y., Zhou, X., Zhao, D., Wang, X., Gurley, E. C., Liu, R., et al. (2020). Berberine inhibits free fatty acid and LPS-Induced inflammation via modulating ER stress response in macrophages and hepatocytes. PLoS One 15 (5), e0232630. doi:10.1371/journal.pone.0232630
Wang, L., Sheng, W., Tan, Z., Ren, Q., Wang, R., Stoika, R., et al. (2021). Treatment of parkinson's disease in Zebrafish model with a berberine derivative capable of crossing blood brain barrier, targeting mitochondria, and convenient for bioimaging experiments. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 249, 109151. doi:10.1016/j.cbpc.2021.109151
Wang, Y., Tong, Q., Ma, S. R., Zhao, Z. X., Pan, L. B., Cong, L., et al. (2021). Oral berberine improves brain dopa/dopamine levels to ameliorate parkinson's disease by regulating gut microbiota. Signal Transduct. Target Ther. 6 (1), 77. doi:10.1038/s41392-020-00456-5
Wang, J., Bi, C., Xi, H., and Wei, F. (2024). Effects of administering berberine alone or in combination on type 2 diabetes mellitus: a systematic review and meta-analysis. Front. Pharmacol. 15, 1455534. doi:10.3389/fphar.2024.1455534
Wei, W., Zeng, Q., Wang, Y., Guo, X., Fan, T., Li, Y., et al. (2023). Discovery and identification of EIF2AK2 as a direct key target of berberine for anti-inflammatory effects. Acta Pharm. Sin. B 13 (5), 2138–2151. doi:10.1016/j.apsb.2022.12.009
Willner, P. (2005). Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52 (2), 90–110. doi:10.1159/000087097
Xia, S., Jing, R., Shi, M., Yang, Y., Feng, M., Deng, L., et al. (2024). BBR affects macrophage polarization via inhibition of NF-κB pathway to protect against T2DM-associated periodontitis. J. Periodontal Res. 59 (4), 728–737. doi:10.1111/jre.13246
Xie, W., Su, F., Wang, G., Peng, Z., Xu, Y., Zhang, Y., et al. (2022). Glucose-lowering effect of berberine on type 2 diabetes: a systematic review and meta-analysis. Front. Pharmacol. 13, 1015045. doi:10.3389/fphar.2022.1015045
Xing, H., Zhang, X., Xing, N., Qu, H., and Zhang, K. (2019). Uncovering pharmacological mechanisms of zhi-zi-hou-po decoction in chronic unpredictable mild stress induced rats through pharmacokinetics, monoamine neurotransmitter and neurogenesis. J. Ethnopharmacol. 243, 112079. doi:10.1016/j.jep.2019.112079
Xu, F., Yang, J., Meng, B., Zheng, J. W., Liao, Q., Chen, J. P., et al. (2018). The effect of berberine on ameliorating chronic inflammatory pain and depression. Zhonghua Yi Xue Za Zhi 98 (14), 1103–1108. doi:10.3760/cma.j.issn.0376-2491.2018.14.011
Yan, X., Yuan, C., Wang, Z., Xu, Z., Wu, Z., Wang, M., et al. (2024). Berberine modulates ovarian cancer autophagy and glycolysis through the LINC01123/P65/MAPK10 signaling axis. Phytomedicine 135, 156121. doi:10.1016/j.phymed.2024.156121
Yang, J., Yan, H., Li, S., and Zhang, M. (2018). Berberine ameliorates MCAO induced cerebral ischemia/reperfusion injury via activation of the BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem. Res. 43 (3), 702–710. doi:10.1007/s11064-018-2472-4
Yang, L., Huang, Y., Chen, F., Wang, Y., Su, K., Zhao, M., et al. (2023). Berberine attenuates depression-like behavior by modulating the hippocampal NLRP3 ubiquitination signaling pathway through Trim65. Int. Immunopharmacol. 123, 110808. doi:10.1016/j.intimp.2023.110808
Yoo, K. Y., Hwang, I. K., Lim, B. O., Kang, T. C., Kim, D. W., Kim, S. M., et al. (2006). Berberry extract reduces neuronal damage and N-Methyl-D-aspartate receptor 1 immunoreactivity in the gerbil hippocampus after transient forebrain ischemia. Biol. Pharm. Bull. 29 (4), 623–628. doi:10.1248/bpb.29.623
Yu, Z. C., Cen, Y. X., Wu, B. H., Wei, C., Xiong, F., Li, D. F., et al. (2019). Berberine prevents stress-induced gut inflammation and visceral hypersensitivity and reduces intestinal motility in rats. World J. Gastroenterol. 25 (29), 3956–3971. doi:10.3748/wjg.v25.i29.3956
Zhan, Y., Han, J., Xia, J., and Wang, X. (2021). Berberine suppresses mice depression behaviors and promotes hippocampal neurons growth through regulating the miR-34b-5p/miR-470-5p/BDNF axis. Neuropsychiatr. Dis. Treat. 17, 613–626. doi:10.2147/ndt.S289444
Zhang, J. C., Yao, W., and Hashimoto, K. (2016). Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr. Neuropharmacol. 14 (7), 721–731. doi:10.2174/1570159x14666160119094646
Zhang, S., Xu, P., Zhu, Z., Zhou, L., Li, J., Zhou, R., et al. (2023). Acetylation of p65(Lys310) by p300 in macrophages mediates anti-inflammatory property of berberine. Redox Biol. 62, 102704. doi:10.1016/j.redox.2023.102704
Zhao, J. V., Yeung, W. F., Chan, Y. H., Vackova, D., Leung, J. Y. Y., Ip, D. K. M., et al. (2021). Effect of berberine on cardiovascular disease risk factors: a mechanistic randomized controlled trial. Nutrients 13 (8), 2550. doi:10.3390/nu13082550
Zhao, M., Ren, Z., Zhao, A., Tang, Y., Kuang, J., Li, M., et al. (2024). Gut bacteria-driven homovanillic acid alleviates depression by modulating synaptic integrity. Cell Metab. 36 (5), 1000–1012.e6. doi:10.1016/j.cmet.2024.03.010
Zhu, F., and Qian, C. (2006). Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer's disease. BMC Neurosci. 7, 78. doi:10.1186/1471-2202-7-78
Keywords: depression, berberine, preclinical, mechanisms, meta-analysis
Citation: Yao B, Long Z, Lin X, Chen G, Li X, Ye Z and Liu J (2025) The potential value of the use of berberine in depression: a systematic review and meta-analysis of preclinical studies. Front. Pharmacol. 16:1664784. doi: 10.3389/fphar.2025.1664784
Received: 12 July 2025; Accepted: 13 October 2025;
Published: 03 November 2025.
Edited by:
Javier Echeverria, University of Santiago, ChileReviewed by:
Giovanni Lentini, University of Bari Aldo Moro, ItalyRamdas Bhat, Department of Pharmacology at Srinivas college of Pharmacy, India
Copyright © 2025 Yao, Long, Lin, Chen, Li, Ye and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiahong Liu, YWxsYW5hbnk5OThAMTYzLmNvbQ==
†ORCID: Guangqiang Chen, orcid.org/0009-0000-4800-2679
 Bifang Yao
Bifang Yao Zhengxiang Long2
Zhengxiang Long2 Jiahong Liu
Jiahong Liu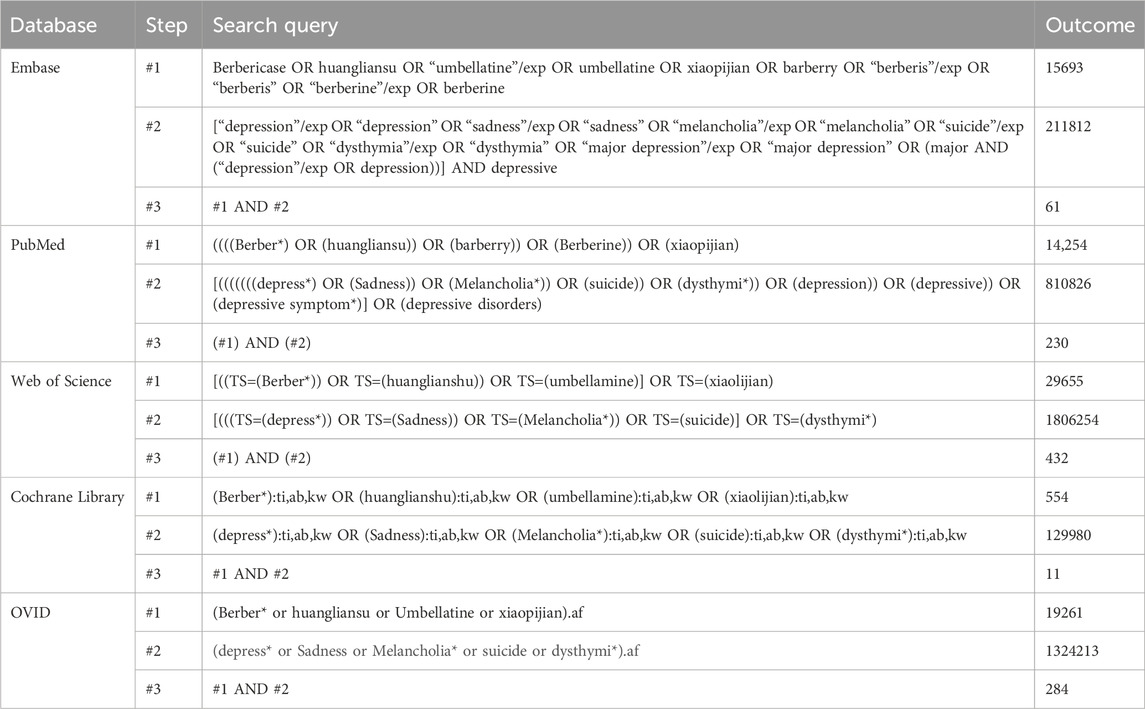
![Forest plot illustrating a meta-analysis comparing experimental and control groups from four studies. Each study is represented by squares with lines indicating confidence intervals: Fan J 2017, Qin Z 2023, Shen J D 2016, and Wang Q 2022. The combined effect is shown as a diamond at 0.76 with a 95% confidence interval of [0.20, 1.33]. The heterogeneity test shows Tau-squared equals 0.10, Chi-squared equals 4.28, degrees of freedom equals 3, with an I-squared of 30%. Overall effect test: Z equals 2.65 (P equals 0.008).](https://www.frontiersin.org/files/Articles/1664784/fphar-16-1664784-HTML-r1/image_m/fphar-16-1664784-g008.jpg)
![Forest plot comparing experimental and control groups across five studies. It shows standardized mean differences and 95% confidence intervals. Studies include Ge P Y 2023, Huang M 2023, Peng W H 2017, Tang Y 2024, and Wang Q 2022. The total effect shows a standardized mean difference of 1.82 [1.27, 2.37]. Heterogeneity is low with I² = 0%. The overall effect is significant with Z = 6.50 and p < 0.00001.](https://www.frontiersin.org/files/Articles/1664784/fphar-16-1664784-HTML-r1/image_m/fphar-16-1664784-g012.jpg)
![Forest plot showing the standard mean difference between experimental and control groups in five studies. Horizontal lines represent confidence intervals, with green squares for each study's weight and a diamond for the overall effect. The overall effect size is 2.13, with confidence interval [1.01, 3.26]. There is significant heterogeneity (I² = 68%). The plot favors the experimental group.](https://www.frontiersin.org/files/Articles/1664784/fphar-16-1664784-HTML-r1/image_m/fphar-16-1664784-g015.jpg)
![Forest plot displaying results from three studies (Deng Z 2018, Shen J D 2016, Tang Y 2024) comparing experimental and control groups. The standardized mean differences (SMD) are shown with 95% confidence intervals: Deng Z (1.64 [0.46, 2.81]), Shen J D (1.73 [0.32, 3.14]), Tang Y (3.85 [0.86, 6.83]). The total effect SMD is 1.86 [0.99, 2.72]. Heterogeneity is minimal (I²=0%). A diamond represents the overall effect estimate.](https://www.frontiersin.org/files/Articles/1664784/fphar-16-1664784-HTML-r1/image_m/fphar-16-1664784-g016.jpg)