- 1Chemistry Department, College of Science, Taibah University, Medina, Saudi Arabia
- 2Department of Forensic Medicine and Toxicology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt
- 3Department of Pharmaceutical Sciences, College of Pharmacy, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 4Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt
- 5Laboratory of Biotechnology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt
- 6Histology and Cytology Department, Faculty of Veterinary Medicine, Damanhour University, Damanhour, Egypt
- 7Polymers and Pigments Department, National Research Centre, Giza, Egypt
- 8Department of Pharmacy Practice, College of Pharmacy, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 9Department of Biological Sciences, College of Science, University of Jeddah, Jeddah, Saudi Arabia
Introduction: Microplastics (MPs), particularly polystyrene microplastics (PS-MPs), are emerging environmental contaminants that have been shown to accumulate in various organs, including the liver, leading to oxidative stress, inflammation, and metabolic dysregulation. However, the precise molecular mechanisms underlying PS-MP-induced hepatotoxicity and disruptions in lipid metabolism remain poorly understood. Taurine (Tau), a naturally occurring amino acid with known antioxidant and cytoprotective properties, may suggest a potential protective strategy against such toxicity. This study aimed to investigate the hepatotoxic effects of PS-MPs in a mouse model and to evaluate the potential ameliorative role of Tau.
Methods: Mice were exposed to PS-MPs with or without Tau supplementation over a 60-day experimental period. The groups were: control group, which received distilled water orally (0.5 mL/mouse). The Tau group was administered Tau at a dose of 200 mg/kg body weight. The PS-MPs group received PS-MPs at 10 mg/kg body weight, suspended in distilled water. The combination group (PS-MPs + Tau) received both Tau and PS-MPs at the same doses concurrently. Multiple endpoints were assessed, including oxidative stress biomarkers, liver function indicators, lipid and bilirubin profiles, histopathological alterations, and the expression of key genes involved in lipid metabolism and oxidative stress regulation.
Results: Exposure to PS-MPs resulted in notable hepatic injury, characterized by elevated oxidative stress, dysregulated lipid profiles, impaired antioxidant enzyme activity, and altered expression of genes related to lipogenesis and fatty acid oxidation compared to the control. Histological examination revealed congested central and portal veins, massive aggregations of lymphocytes, the hepatocytes appeared markedly swollen, disorganized arrangement, and exhibited large nuclei with strong basophilic staining consistent with these biochemical findings. Co-administration of Tau mitigated these adverse effects, improving antioxidant status, normalizing metabolic markers, and partially restoring gene expression patterns and tissue integrity.
Conclusion: Overall, the findings indicated that PS-MPs caused liver damage via oxidative stress and lipid metabolic disturbance, and that Tau supplementation had a protective effect, possibly via modulating oxidative and metabolic pathways. This experiment emphasized the necessity for additional research into Tau as a therapeutic agent in microplastic-related liver damage.
1 Introduction
In recent years, plastic pollution has become a pervasive environmental concern, with plastic products making up a substantial portion of marine debris due to their widespread use across industries and in everyday life (MacLeod et al., 2021). Over time, these plastics degrade through physical, chemical, and biological processes, resulting in the formation of microplastics (MPs), defined as plastic particles less than 5 mm in size (Ahmad et al., 2023). MPs have been widely detected across various ecosystems, including freshwater and marine environments, sediments, urban beaches, and wastewater systems (Bellasi et al., 2020). Due to their small size and resistance to degradation, MPs can be easily ingested and accumulate in the tissues of both aquatic and terrestrial organisms (Lin et al., 2023).
The increasing detection of MPs in human biological samples such as stool, placenta, and even lung tissues highlights growing concerns about their potential health impacts (Ragusa et al., 2021; Schwabl et al., 2019). PS-MPs, in particular, are among the most prevalent synthetic particles found in polluted environments (Yaseen et al., 2022). Several studies have reported that ingestion or inhalation of PS-MPs can lead to significant biological disturbances, including disruptions in gut microbiota (Qiao et al., 2021), liver metabolic dysfunction (Yin et al., 2022), renal damage (Wang et al., 2021), and neurotoxicity (Prüst et al., 2020).
Oxidative stress is one of the key mechanisms through which MPs exert toxic effects, resulting from an imbalance between reactive oxygen species (ROS) production and the body’s antioxidant defences (Jabeen et al., 2023). Excessive ROS can cause widespread cellular damage, including lipid peroxidation, protein denaturation, DNA mutations, and ultimately cell death through apoptosis or necrosis (Ehsan et al., 2023; Mostafa-Hedeab et al., 2023). The liver, as a central organ for detoxification and metabolism, is particularly vulnerable due to its high mitochondrial density, a major site of ROS generation (Mahfouz et al., 2023). Recent studies propose that oxidative stress is a key pathogenic mechanism in microplastic-induced hepatotoxicity (Blackburn and Green, 2022; Cheng et al., 2022). Studies using animal models such as mice and zebrafish have demonstrated that MPs can cross the intestinal barrier and subsequently accumulate in the liver, likely via translocation through the bloodstream (Ašmonaitė et al., 2018; Liu P. et al., 2020; Liu S. et al., 2020; Rubio et al., 2020). Exposure to PS-MPs, whether 5 μm or 70 nm in size, has been shown to trigger oxidative stress and inflammatory responses (Ijaz et al., 2024). Specifically, zebrafish (Pei et al., 2022) and mice (Zou et al., 2023) exposed to these particles displayed declined activities of antioxidant enzymes like catalase and superoxide dismutase (SOD), along with elevated malondialdehyde (MDA) levels and augmented expression of pro-inflammatory cytokines, such as TNF-α and IL-1β (Li et al., 2021; Lu et al., 2016). Moreover, microplastic exposure has been linked to hepatic steatosis and altered lipid metabolism in the liver of both species, indicating a typical profile of hepatotoxicity (Luo et al., 2019; Zheng et al., 2021).
Recent evidence indicates that microplastics, depending on their size and composition, may differentially affect liver function (Hu and Palić, 2020) Given the liver’s central role in systemic homeostasis and its susceptibility to microplastic accumulation, further investigation into the molecular mechanisms of PS-MP-induced hepatotoxicity is critical.
Taurine (Tau) is a sulfur-containing, conditionally essential amino acid that is among the most abundant in mammalian tissues, with high intracellular concentrations in various cell types, including hepatocytes (Lambert et al., 2015). It is both synthesized in the liver and acquired through the diet, and has a key role in a wide range of physiological processes such as bile acid conjugation, osmoregulation, membrane stabilization, calcium signalling, and detoxification of xenobiotics (Schaffer et al., 2000). Furthermore, dietary supplementation of Tau has been associated with a decreased hazard of several diseases, including diabetes (Piao et al., 2018), metabolic syndrome (Chen et al., 2016), skeletal muscle problems (De Luca et al., 2015), liver disease (Miyazaki and Matsuzaki, 2014), and central nervous system disorders (Menzie et al., 2014).
In the context of liver health, Tau has demonstrated potent antioxidant and anti-inflammatory properties. It scavenges reactive oxygen species (ROS) both directly and indirectly by increasing the activity of natural antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) (de Oliveira Ramos et al., 2018). Additionally, Tau contributes to maintaining mitochondrial integrity and reducing lipid peroxidation (Zhang et al., 2014). The anti-inflammatory effect is achieved via inhibiting pro-inflammatory cytokines such as TNF-α and IL-1β, as well as suppressing NF-κB activation, a central pathway in inflammation and immune activation in CCL4-induced hepatic damage (Abdel-Moneim et al., 2015). Tau chloramine, a derivative formed during immune responses, is known to modulate inflammation by downregulating excessive immune activity (Qaradakhi et al., 2020). Despite these established roles, the precise molecular targets of Tau in vivo remain under investigation, which has so far limited its full clinical application (Schaffer and Kim, 2018). Nonetheless, its multifaceted protective mechanisms make Tau a promising candidate for alleviating liver injury, particularly that induced by environmental pollutants such as microplastics.
Although the environmental and biological risks of MP exposure are increasingly recognized, our understanding of the underlying mechanisms of MP-induced organ toxicity, particularly hepatotoxicity, remains limited. PS-MPs, among the most common types of MPs, have been shown to accumulate in liver tissue and induce oxidative stress, inflammation, and metabolic disturbances in animal models. However, the specific impact of PS-MPs on hepatic lipid metabolism and liver function at the molecular level is still not well defined. Furthermore, there is a lack of effective mitigation strategies to counteract these toxic effects. Tau, a naturally occurring amino acid with known antioxidant properties, may represent a promising intervention, but its role in protecting against microplastic-induced liver injury has not yet been fully explored. Therefore, the present study was designed to investigate the hepatotoxic effects of PS-MPs in a mouse model by assessing oxidative stress biomarkers, lipid and bilirubin profiles, histopathological changes, and gene expression related to hepatic lipid metabolism. Additionally, we aim to evaluate the potential ameliorative effect of Tau in reducing PS-MP-induced liver damage. We hypothesize that PS-MP exposure leads to liver toxicity through oxidative stress and metabolic disruption, and that Tau supplementation can attenuate these effects and restore hepatic homeostasis.
2 Materials and methods
2.1 Preparation and characterization of PS-MP
PS-MPs were synthesized via the suspension polymerization method, as previously described in the literature (Cho et al., 2016). In brief, 50 mL of styrene monomer was mixed with 0.5 g of benzoyl peroxide (used as an initiator), and the mixture was added to 200 mL of ethanol containing 2 g of polyvinylpyrrolidone (PVP, MW: 40 kDa), which served as a stabilizer. The reaction mixture was continuously stirred and maintained at 70 °C in an oil bath equipped with a condenser for approximately 24 h. During the polymerization process, the formation of a white suspension indicated the generation of polystyrene microbeads. Upon completion of the reaction, the PS-MPs were collected by centrifugation at 5,000 rpm, washed successively with distilled water and ethanol to remove unreacted materials, and subsequently air-dried at room temperature to a constant weight.
The chemical composition and functional groups of the synthesized PS-MPs were analyzed using Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR) with an ALPHA-II FTIR spectrometer (Bruker Optik GmbH, Germany), operating over a spectral range of 4,000–400 cm-1 at a resolution of 4 cm-1 (Fang et al., 2010). Morphological characteristics and particle size were examined using a scanning electron microscope (QUANTA FEG 250, ESEM) under an accelerating voltage ranging from 200 V to 30 kV (Karanam et al., 2024). The hydrodynamic diameter of the PS-MPs was further measured using dynamic light scattering (DLS) analysis with a Zetasizer Nano-ZS (Malvern Instruments Ltd., UK, Zetasizer version 7.04).
2.2 Tested compound tau
Tau was purchased from Alfa Chemistry Co. (101–5 Colin Dr, Holbrook, NY 11741). Purity 98%, Catalog Number (ACM107357-5).
2.3 Animals and experimental design
Eighty healthy and pathogen-free Swiss male mice (20–25 g body weight, 6 weeks old) were procured from the Laboratory Animal Housing Unit at the Faculty of Veterinary Medicine at Zagazig University in Egypt. Mice were housed in stainless steel cages with free access to food and water in a well-ventilated room with a light/dark cycle of 12 h each day. Before using in any of the experiments described here, the experimental animals were adapted to the laboratory setting for 14 days pre-dosing.
2.4 Experimental scheme
Eighty Swiss mice were randomly divided into four groups (n = 20/group). The control group received distilled water orally (0.5 mL/mouse). The Tau group was administered taurine (Tau) at a dose of 200 mg/kg body weight, following previously well-known protocols (Ma et al., 2021; Song et al., 2024). The Ps-MPs group received Ps-MPs at 10 mg/kg body weight, prepared in distilled water; the volume administered was calculated using the formula: Volume (L) = Required dose (mg/kg)/Concentration of solution (mg/L). The combination group (PS-MPs + Tau) received both treatments at the same respective doses for 60 consecutive days. The tested compounds, including PS-MPs and Tau, were administered orally by gavage using a feeding needle to ensure accurate and consistent dosing. In the combination group, PS-MPs and taurine were administered with a 1-h interval between the two substances to guarantee precise dosage delivery and to minimize the possibility of direct interaction. Mice were weighed weekly to adjust doses based on weight changes, and they were observed daily for signs of distress, abnormal behaviour, respiratory abnormalities, mucous membrane colour changes, sickness, or death.
2.5 Tau dose selection
A dose of 200 mg/kg body weight (bwt) of Tau was selected based on prior experimental evidence demonstrating its efficacy in modulating oxidative stress, inflammation, and apoptosis in murine models (Niu et al., 2018). Specifically, the dose, corresponding to approximately 200 μmol/kg, has been previously reported to exhibit significant antioxidant, anti-inflammatory, and anti-apoptotic properties, particularly in the context of cardiovascular protection (Song et al., 2024). Based on this, we hypothesized that Tau at this effective dose may also confer hepatoprotective effects, particularly against oxidative stress, hepatocellular damage, and inflammation induced by PS-MPs exposure.
2.6 PS-MPs dose selection
Oral gavage was selected as the mode of administration, reflecting ingestion as the primary route of microplastic exposure in humans (Allouzi et al., 2021; Campanale et al., 2020). The administered dose of 10 mg/kg body weight (equivalent to roughly 0.6 g for a 60 kg adult) falls within the estimated human intake range of microplastics, reported to be between 0.1 and 5 g per week (Senathirajah et al., 2021).
2.7 Detection of hepatosomatic index
Hepatosomatic index (HSI) was calculated to assess relative liver size and potential hepatomegaly. The livers were carefully excised, cleaned of adherent fat and connective tissue, and weighed using a digital analytical balance with 0.01 g precision. The HSI was determined using the following formula: [Liver weight (g)/body weight (g) ]x 100.
Where:
• Liver weight (g) is the absolute wet weight of the liver immediately after dissection.
• Body weight (g) is the final body weight of the mouse recorded prior to sacrifice.
2.8 Sample collection
At the end of the exposure period, mice were weighed, and isoflurane was administered in an induction chamber until loss of consciousness, then cervical dislocation was applied. Blood samples were collected from the orbital sinus without an anticoagulant, and serum was separated by centrifugation at 3,000 rpm for 10 min. The serum was stored at −20 °C for subsequent biochemical analyses, including liver enzymes, lipid profile, and bilirubin levels. Liver tissues were carefully excised, rinsed with ice-cold saline to remove blood residues, and divided into three portions for comprehensive analysis. The first portion was homogenized at 4 °C (15 min at 664 × g) for the assessment of antioxidant enzyme activities and lipid peroxidation levels (MDA). The second portion was fixed in 10% neutral-buffered formalin for histopathological evaluation. The third portion was preserved at −80 °C for total RNA extraction and subsequent quantitative real-time PCR (qRT-PCR) to assess gene expression.
2.9 Evaluation of hepatocellular enzymes
Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were determined using colorimetric commercial kits (Sigma-Aldrich, Cat. No. Cat. No. MAK467 and MAK052) based on the spectrophotometric method described by Reitman and Frankel (1957). Absorbance was read using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA). Also, Gamma glutamyl transferase (GGT) was diagnosed via colorimetric kits of My BioSource (San Diego, USA), catalogue no. MBS165115.
2.10 Estimation of bilirubin content, lipid profile, and total protein content
Bilirubin content, serum triglycerides, and serum cholesterol were detected in the sera of all experimental groups via the methods of Walters and Gerarde (1970), Foster and Dunn (1973), and Zlatkis et al. (1953), respectively, using a biochemistry analyzer system (Synchron Clinical System Lx 20; Beckman Coulter Inc., Fullerton, CA, USA). Additionally, the low-density lipoprotein cholesterol (LDL-C) fraction was estimated using the formula of Friedewald et al. (1972).
2.11 Evaluation of the liver homogenate malondialdehyde and antioxidants (MDA)
Each frozen liver tissue sample was homogenized in an ice bath for 10 min using a Polytron PT one200 E homogenizer with 10 volumes of a 50 mM Tris buffer containing 10 mM EDTA (pH 7.5). The homogenate was then centrifuged at 1,000 × g for 10 min at 4 °C. The homogenate was used for the assessment of oxidative stress markers. Malondialdehyde (MDA) levels were determined using the Thiobarbituric Acid Reactive Substances (TBARS) assay, Ohkawa et al. (1979).
The superoxide dismutase (SOD) activities were detected using Bio-diagnostic colorimetric kits (Giza, Egypt) with Catalogue numbers SD 25 21. EnzyChromTM GSH/GSSG Assay Kit (EGTT-100) obtained from BioAssay Systems (Hayward, CA, USA) was selected to determine the GSH/GSSG ratio. Catalase (CAT) levels were estimated in our experiment using Bio-diagnostic Co., Egypt kits, based on the protocol described by Beutler et al. (1963).
2.12 Gene expression
The expression of several key hepatic genes AMPK-1 (AMP-activated protein kinase alpha-1), ACC (acetyl-CoA carboxylase), CPT-1 (carnitine palmitoyltransferase-1), SREBP-1 (sterol regulatory element-binding protein-1c), PPAR-α (peroxisome proliferator-activated receptor alpha), CYP2E1 (cytochrome P450 2E1), and NR4A1 (nuclear receptor subfamily four group A member 1) was evaluated in liver tissues from the experimental groups. These genes collectively regulate crucial metabolic pathways, including lipid metabolism, fatty acid oxidation, energy homeostasis, oxidative stress response, and inflammation. Total RNA was extracted from homogenized liver samples using TRIzol™ Reagent (Invitrogen, Thermo Fisher Scientific), followed by DNase I treatment and purification with QIAGEN columns. RNA purity was assessed using a NanoDrop™ One UV–Vis spectrophotometer (Thermo Scientific, USA). Complementary DNA (cDNA) was synthesized from the purified RNA for quantitative PCR (qPCR) using the QuantiTect® SYBR® Green PCR kit on a Rotor-Gene Q instrument (Qiagen, Germany). Primer efficiency was validated through standard curves generated from serial cDNA dilutions, showing efficiencies between 95% and 104% with R2 values ≥0.98. Specific amplification and absence of primer-dimers were confirmed via melt curve analysis and agarose gel electrophoresis, and no-template controls were included to check for contamination; primer sequences are listed in Supplementary Table 1. Real-time PCR was performed using the QuantStudio™ 6 Flex Real-Time PCR System (Applied Biosystems). Gene expression levels were normalized to the Glyceraldehyde-3-Phosphate Dehydrogenase (GABDH), selected from four reference genes based on stability analysis using the geNorm algorithm (Vandesompele et al., 2002). Relative expression was calculated according to the method described by Schmittgen and Livak (2008), incorporating primer efficiencies in the analysis.
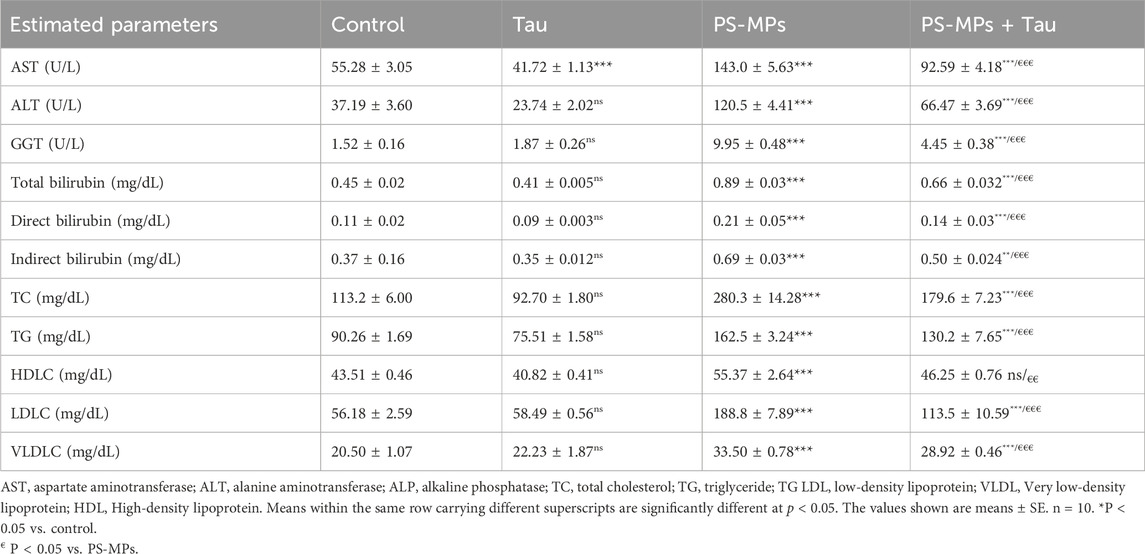
Table 1. Effect of Polystyrene microplastics (PS-MPs) (10 mg/kg b.wt) and Tau (200 mg/kg b.wt) oral dosing on hepatic enzymes, bilirubin, and lipid profile of adult male Swiss mice for 60 days.
2.13 Histopathological evaluations
The specimens were then processed using the routine paraffin embedding method. Tissue sections, each 4 µm thick, were stained with Hematoxylin and Eosin (H&E) according to the protocol described by Bancroft and Layton (2013). Hepatic lesions were evaluated using a semiquantitative scoring system based on the criteria of Gibson-Corley et al. (2013). Ten randomly selected fields at ×40 magnification (Olympus BX53, Leica Application Suite) were assessed, and the scores were averaged. The evaluation was performed in a blinded manner using the following scale: 0 = normal; 1 = ≤25% affected area; 2 = 26–50%; 3 = 51–75%; and 4 = 76–100%.
2.14 Data analysis
Prior to statistical evaluation, the dataset was subjected to preliminary assessments to verify the assumptions of normality and homogeneity. The Shapiro-Wilk test was employed to assess the normal distribution of the data, while Levene’s test was used to examine the equality of variances. Data were presented as mean ± standard error of the mean (SEM). Statistical analyses were performed using GraphPad Prism® version 7 (GraphPad Software Inc., San Diego, CA, USA). One-way analysis of variance (ANOVA) was conducted to detect group differences, and post hoc comparisons were made using Tukey’s multiple comparison test. A p-value of less than 0.05 was considered statistically significant. Standardized mean differences (Hedges’ g) with 95% CIs were also calculated for final body weight, liver weight, and HSI and visualized using a Forest plot.
3 Results
3.1 Characterization of PS-MP
ATR-FTIR analysis (Figure 1) confirmed the chemical structure of the synthesized PS-MPs. The spectrum showed characteristic peaks at 3,059 and 3,024 cm-1, corresponding to aromatic C–H stretching vibrations of the benzene ring. Additional peaks at 2,988 and 2,921 cm1 were assigned to aliphatic C–H and CH2 stretching. Peaks at 1,601, 1,492, and 1,451 cm-1 indicated C=C aromatic ring vibrations. Absorption bands at 755 and 696 cm-1 were attributed to out-of-plane C–H bending, consistent with monosubstituted aromatic rings. The absence of a peak between 1,640 and 1,680 cm-1 confirmed the full consumption of vinyl double bonds, indicating complete polymerization.
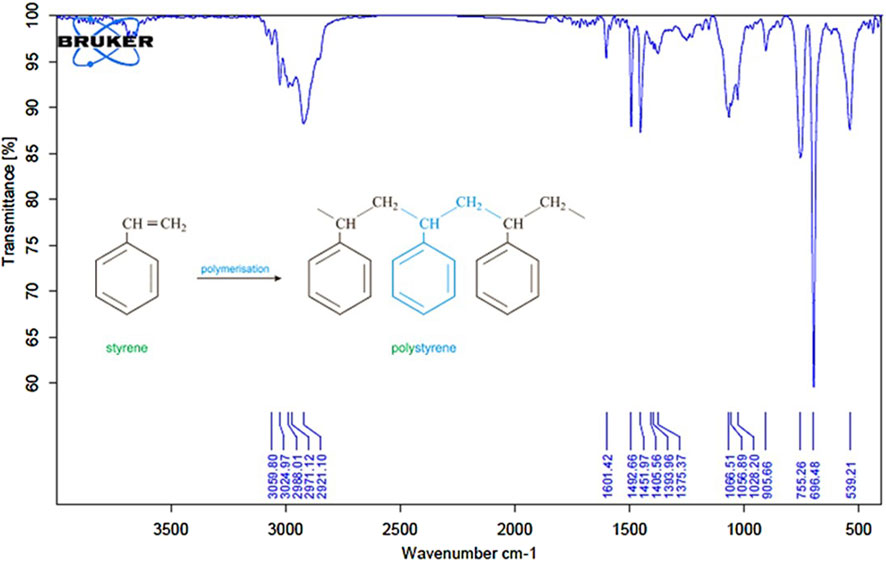
Figure 1. ATR-FTIR spectrum of synthesized polystyrene microplastics (PS-MPs). The spectrum confirms the characteristic functional groups of polystyrene.
Scanning Electron Microscopy (Figure 2) revealed that the synthesized particles were predominantly spherical with smooth surfaces and a narrow size distribution. The average particle diameter was approximately 1.8 µm.
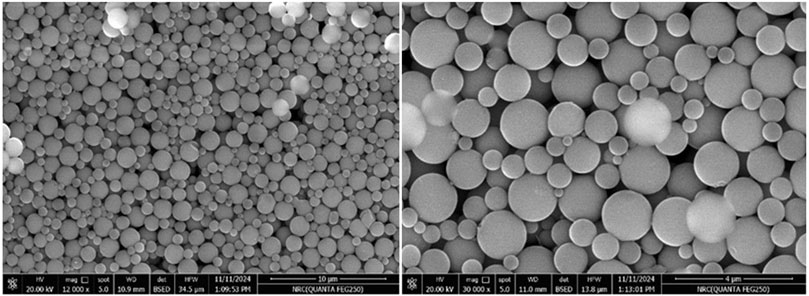
Figure 2. Scanning Electron Microscope (SEM) image of synthesized polystyrene micro-plastic (PS-MPs) at two different magnifications.
Dynamic Light Scattering (DLS) (Figure 3) analysis supported the Scanning Electron Microscopy findings, confirming an average particle size of 1.8 µm. The particles displayed a low polydispersity index (PDI = 0.133), indicating a uniform size distribution.
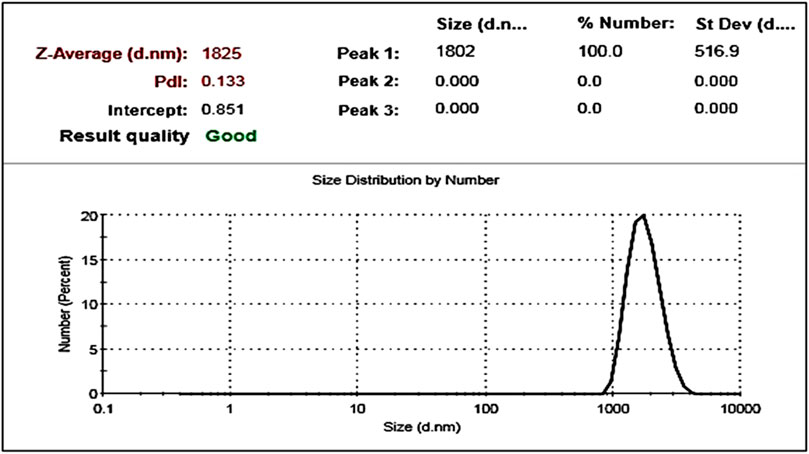
Figure 3. Particle size distribution of synthesized polystyrene microplastics (PS-MPs) as determined by Dynamic Light Scattering (DLS).
3.2 Body weight and hepatosomatic index
Figure 4 illustrates the impact of oral exposure to PS-MPs; 10 mg/kg b. wt) and taurine (Tau; 200 mg/kg b. wt) on final body weight, liver weight, and hepatosomatic index (HSI) in adult male Swiss mice over a period of 60 days. Compared to the control group, PS-MPs exposure resulted in a 9.7% reduction in final body weight, while co-administration with Tau improved it by 6.4% relative to the PS-MPs group, indicating partial recovery.
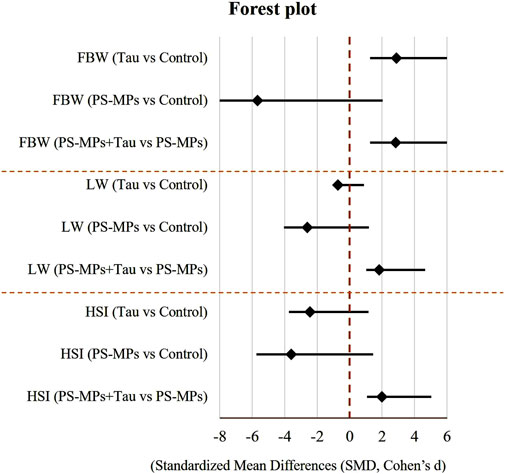
Figure 4. Forest plot of standardized mean differences (Hedges’ g) with 95% confidence intervals for the effects of taurine (Tau) and polystyrene microplastics (PS-MPs) on final body weight (FBW), liver weight (LW), and hepatosomatic index (HSI) on adult male Swiss mice after 60 days of oral dosing. A vertical dashed line at 0 indicates no effect relative to Control. Negative values indicate a reduction compared to Control, while positive values indicate an increase.
Liver weight was reduced by 19.3% in the PS-MPs group compared to the control. However, in the co-treated group, liver weight increased by 10.5% compared to the PS-MPs group, suggesting a protective role of Tau.
Regarding the hepatosomatic index, PS-MPs caused a 27.1% decrease compared to the control group. Tau co-treatment improved the index by 15.0% relative to the PS-MPs group, reflecting a mitigating effect of Tau against PS-MP-induced hepatic impairment.
3.3 Hepatic enzymes, lipid, and bilirubin profile
Exposure to PS-MPs significantly disrupted liver function and lipid metabolism in adult male Swiss mice after 60 days of oral administration. Compared to the control group (Table 1), PS-MPs significantly (P < 0.001) elevated serum levels of AST, ALT, and GGT by approximately 159%, 224%, and more than 6.5-fold, respectively, indicating severe hepatocellular injury. Co-administration of Tau notably mitigated these elevations, reducing AST by 35%, ALT by 45%, and GGT by 55% compared to the PS-MPs group (P < 0.001), suggesting a potent hepatoprotective effect. Interestingly, Tau alone resulted in a significant (P < 0.001) 24% decrease in AST activity relative to control, reflecting its baseline liver-protective potential.
The presented data in Table 1, PS-MPs induced a significant (P < 0.001) increase in total, direct, and indirect bilirubin levels by 98%, 91%, and 86%, respectively, indicating impaired hepatic clearance or conjugation. Tau co-treatment improved these alterations, lowering total bilirubin by 26%, direct bilirubin by 33%, and indirect bilirubin by 28% compared to the PS-MPs group, pointing to Tau’s ability to restore hepatic excretory function. Lipid profile parameters were also adversely affected by PS-MPs exposure. Total cholesterol (TC) and triglyceride (TG) levels rose by 147% and ∼80%, respectively, while low-density lipoprotein cholesterol (LDL-C) increased dramatically by 236%. Although high-density lipoprotein cholesterol (HDL-C) increased by 27%, this likely reflects a compensatory response rather than a beneficial effect. Tau co-administration significantly reduced TC, TG, and LDL-C levels by 36%, 20%, and 40%, respectively, when compared to the PS-MPs group, indicating notable correction of dyslipidemia. Moreover, very low-density lipoprotein cholesterol (VLDL-C) levels, which were elevated by 63% in the PS-MPs group, were decreased by 14% following Tau treatment. The Tau-only group exhibited a moderate 18% reduction in TC compared to the control, with no significant effects on the other lipid fractions. PS-MPs induce pronounced liver damage and lipid abnormalities, while Tau offers considerable protection against such disturbances.
3.4 Oxidative stress and antioxidants
Exposure to PS-MPs led to a significant impairment in antioxidant defense and a pronounced increase in oxidative stress markers in liver tissues. SOD activity showed a significant decrease (P < 0.001) of approximately 87% in the PS-MPs group compared to the control, reflecting severe oxidative burden. Co-administration of Tau significantly (P < 0.001) restored SOD activity, increasing it by around 4.5-fold compared to the PS-MPs group, although it remained lower than the control. Tau alone also significantly enhanced SOD activity by ∼17% compared to the control. CAT activity was also markedly (P < 0.001) reduced in the PS-MPs group, showing an approximate 89% decrease relative to the control. Tau co-treatment substantially improved CAT activity, with a more than 4.5-fold increase compared to the PS-MPs group (Figure 5). While still below the control level, this increase signifies partial recovery of the enzymatic antioxidant capacity. The Tau group demonstrated a modest but statistically significant increase (13%) in CAT activity relative to controls.
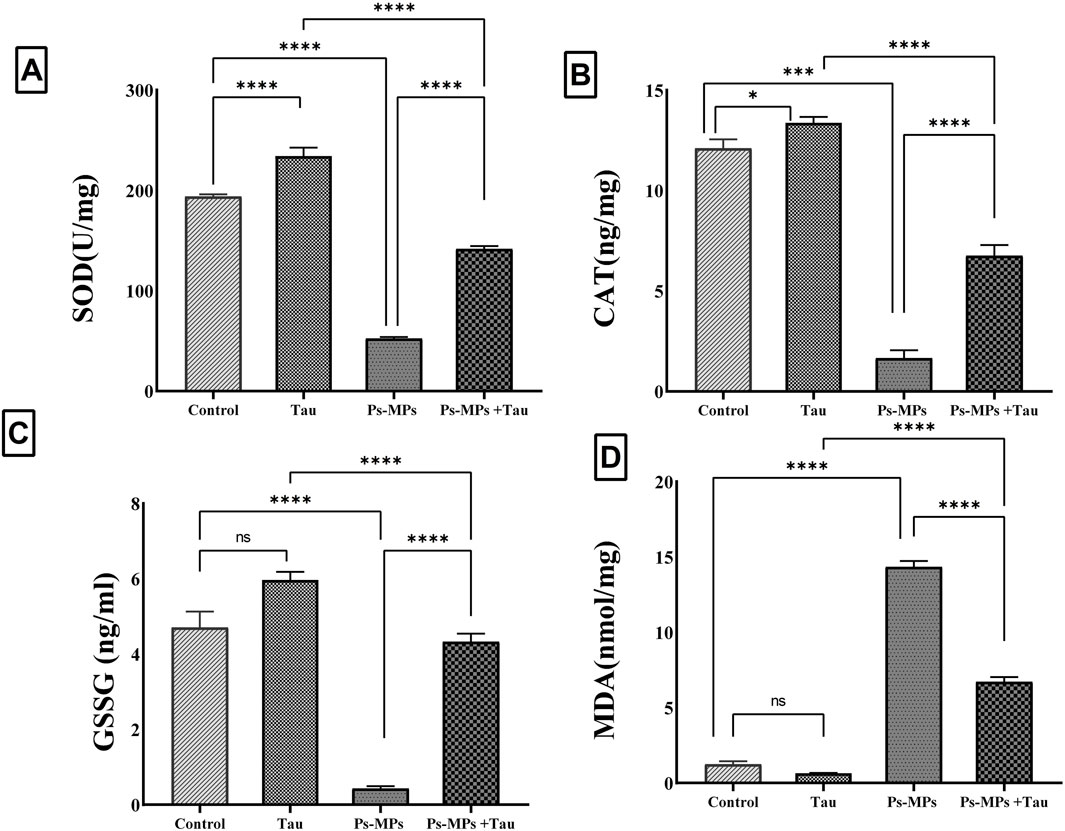
Figure 5. Effect of polystyrene microplastics (PS-MPs) and/or Taurine (Tau) exposure on hepatic tissue levels of Superoxide dismutase (SOD) (A), Catalase (CAT) (B), Glutathione disulfide (GSSG) (C), and Malondialdehyde (MDA) (D) in a mouse model. Data expressed as mean ± SE, n = 10 for each group. *P<0.05, ***P < 0.0001, and “ns” means “non-significant”.
For glutathione disulfide (GSSG), a marker of oxidative glutathione consumption, PS-MPs exposure resulted in a sharp 90% reduction compared to controls, indicating severe oxidative depletion. Tau administration alongside PS-MPs significantly (P < 0.001) restored GSSG levels, increasing them by approximately 7.3-fold versus the PS-MPs group (Figure 5). Tau alone induced a slight, non-significant (P > 0.05) increase in GSSG relative to control. Malondialdehyde (MDA), was profoundly elevated (P < 0.001) in the PS-MPs group, showing a more than 10-fold increase compared to control animals, reflecting intense membrane oxidative damage. Tau co-treatment significantly attenuated MDA levels by over 60% compared to the PS-MPs group.
3.5 Hepatic gene expression alteration and inter-group comparisons
The hepatic expression of AMPK-1 (an energy sensor that regulates cellular energy homeostasis) mRNA was significantly (P < 0.0001) altered following the various treatments. Exposure to PS-MPs led to a marked downregulation in AMPK-1 expression by approximately 82.2% compared to the control group. In contrast, Tau administration alone resulted in an upregulation of AMPK-1 by 33.3%. Notably, co-administration of Tau with PS-MPs significantly (P < 0.0001) restored AMPK-1 expression, with an increase of approximately 362.5% relative to the PS-MPs group (Figure 6).

Figure 6. Effect of polystyrene micro-plastic (PS-MPs) and/or Taurine (Tau) exposure on hepatic mRNA expression of AMPK-1 (AMP-activated protein kinase alpha-1) (A), ACC (acetyl-CoA carboxylase) (B), CPT-1 (carnitine palmitoyltransferase-1) (C), SREBP-1 (sterol regulatory element-binding protein-1c) (D), PPAR-α (peroxisome proliferator-activated receptor alpha) (E), CYP2E1 (cytochrome P450 2E1) (F), and NR4A1 (nuclear receptor subfamily 4 group A member 1)(G) against the normalizing gene GAPDH: glyceraldehyde-3-phosphate dehydrogenase. Data expressed as mean ± SE, n = 10 for each group. ****P < 0.0001, and *ns“ means ”non“significant”.
Expression levels of ACC mRNA, a key enzyme in fatty acid biosynthesis, remained statistically unchanged in the Tau group compared to the control. However, a significant upregulation of 5.02-fold was observed in the PS-MPs group. Co-treatment with Tau significantly attenuated this increase, reducing ACC expression by 58.5% compared to the PS-MPs group (Figure 6).
The expression of CPT-1 mRNA (Figure 6), which is involved in mitochondrial fatty acid oxidation, was elevated by 50% in the Tau group versus the control. In contrast, PS-MPs exposure suppressed CPT-1 expression by 75% versus control (P < 0.0001), while Tau co-administration restored its expression by 1.7-fold compared to the PS-MPs group.
A substantial increase (P < 0.0001) in SREBP-1 mRNA expression, approximately 6-fold, was detected in the PS-MPs group compared to the control, suggesting enhanced lipogenesis. Tau alone caused only a minor, non-significant (P > 0.05) increase of 22.2%. Co-treatment with Tau markedly decreased the PS-MP-induced upregulation by 46.6%, indicating a mitigating effect.
Expression of PPAR-α mRNA, a key regulator of lipid catabolism, was upregulated by 27.3% in the Tau group, whereas PS-MPs caused a significant suppression by 76.4% compared to the control. Tau co-administration significantly counteracted this suppression, resulting in a 154.5% increase relative to the PS-MPs group.
The oxidative stress marker CYP2E1 mRNA was dramatically upregulated by 4.5-fold in the PS-MPs group. Tau supplementation in combination with PS-MPs significantly reduced this expression by 53.6%, suggesting a protective effect. Tau alone slightly downregulated CYP2E1 by 11.7% compared to control.
Finally, Nrf4-A1 mRNA expression was elevated by approximately 7.5-fold following PS-MPs exposure. Tau co-administration significantly reduced this level by 54.2% compared to PS-MPs alone, while taurine alone caused a minor downregulation of 17.4% versus control.
3.6 Histopathological alterations of the mouse liver in different experimental groups
No histopathological hepatic lesions were observed in the negative control and Tau groups, and the hepatocytes displayed a healthy cord-like arrangement (Figures 7A,B). In contrast, liver samples from the PS-MPs group showed congested central and portal veins, massive lymphocytic infiltrations, and hepatocytes that appeared markedly swollen, disorganized, and contained large nuclei with intense basophilic staining (Figures 7C–F). Mice treated with PS-MPs + Tau exhibited an improved hepatic architecture compared to the PS-MPs group (Figure 7E).
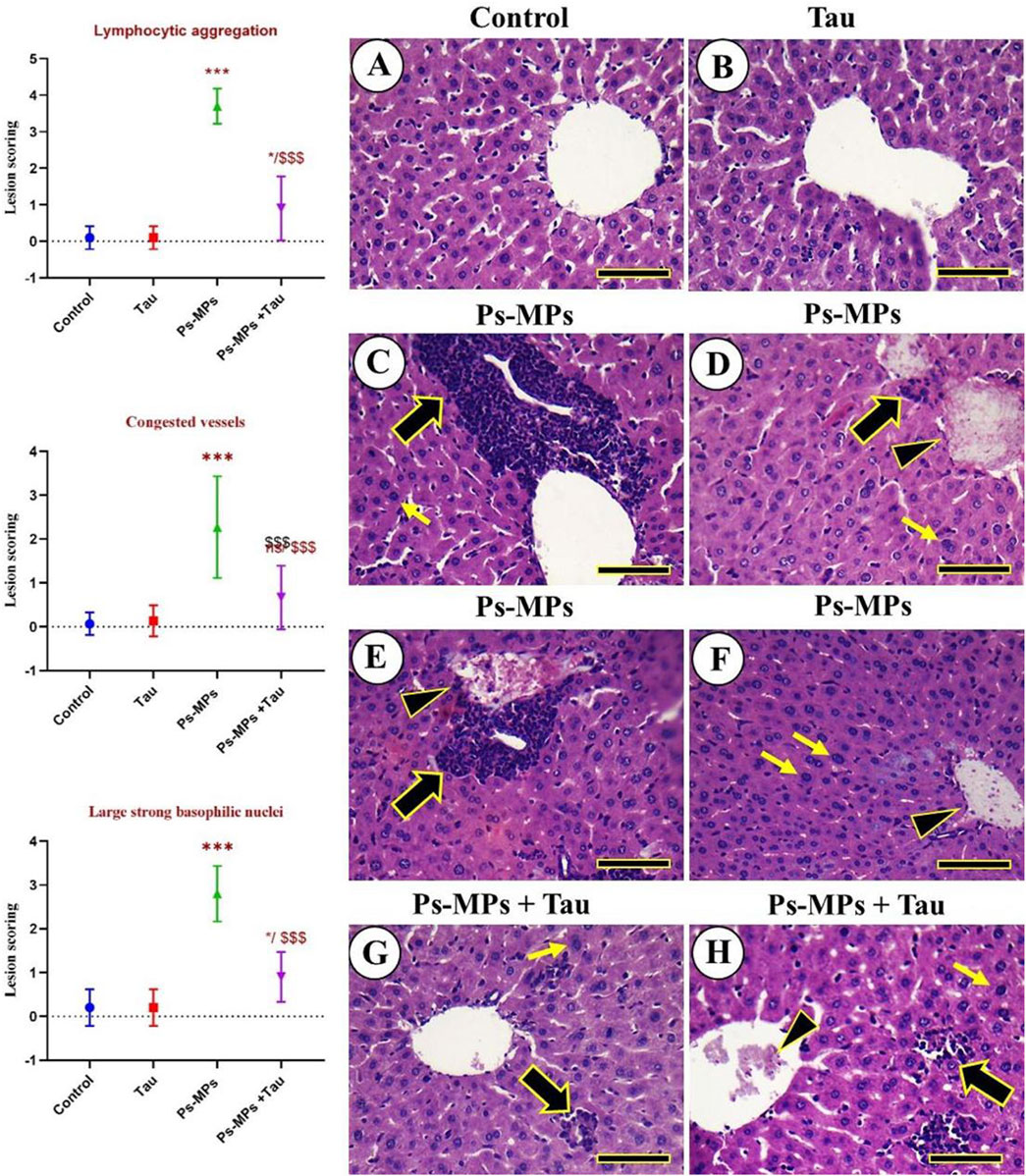
Figure 7. Histopathological examination of the liver of mice. (A) Negative control. (B) Tau. (C–F) Ps-MPs group revealing congested central and portal veins, massive aggregations of lymphocytic cells, and the hepatocytes appeared markedly swollen, with a disorganized arrangement, and exhibited strong, large nuclei with strong basophilic staining. (G,H) Ps-MPs + Tau group. Congested veins (arrowheads), aggregated lymphocytic aggregations (thick black arrows), and large nuclei with strong basophilic staining (thin yellow arrows). Scale bar =50 mm. Moreover, the figure explains the lesion scoring in the hepatic tissue of mice in all experimental groups in the form of a histogram, which indicates the different lesion scoring, including Lymphocytic aggregation, Congested vessels, and large, strong basophilic nuclei.
The scoring results demonstrated significant hepatic alterations in the PS-MPs group compared to the control and Tau groups. Lymphocytic aggregation in the PS-MPs group exhibited a marked increase in lymphocytic infiltration compared to the control and Tau groups (***p < 0.001). In the PS-MPs + Tau group, lymphocytic aggregation was significantly reduced compared to the PS-MPs group (###p < 0.001), although it remained elevated relative to controls. Severe vascular congestion was observed in the PS-MPs group, with lesion scores significantly higher than in the control and Tau groups (***p < 0.001). Co-administration with Tau significantly alleviated vascular congestion (###p < 0.001 vs PS-MPs), though not to control levels. Lastly, regarding the Large basophilic nuclei Hepatocytes with enlarged, strongly basophilic nuclei were significantly more frequent in the PS-MPs group compared to the control and Tau groups (**p < 0.01). In contrast, Tau supplementation markedly attenuated this alteration vs. PS-MPs, suggesting a protective effect.
4 Discussion
The global production of plastic has risen dramatically over recent decades, resulting in plastic pollution becoming a critical environmental issue worldwide (Chen et al., 2021; Horton et al., 2017). PS-MPs are among the most commonly used polymers in personal care products and are also prevalent in marine environments (Akanyange et al., 2022). Furthermore, PS-MPs rank among the most frequently identified microplastic types in food, drinking water, and other beverages (Vitali et al., 2023). Following their entry into the circulatory system, microplastics are largely transported to and processed by the liver and kidneys (Goodman et al., 2022). Consequently, the liver is recognized as a key target organ for microplastic-related toxicity. These particles can reach the liver via several routes, including gastrointestinal absorption, penetration through the skin, or direct transport through the bloodstream (Ma et al., 2021). Studies have indicated that the accumulation of microplastics within hepatic tissue can initiate autophagy and programmed cell death (apoptosis) in liver cells (Lu et al., 2016). Moreover, such accumulation can interfere with lipid metabolic processes, exacerbate hepatic fibrosis, and play a role in the development of non-alcoholic fatty liver disease (NAFLD) (Auguet et al., 2022; Lai et al., 2021).
4.1 Body weight and hepatosomatic index
In the present study, exposure to PS-MPs (10 mg/kg b. wt) led to reductions in body weight, liver weight, and HSI by the end of the experimental period compared to the control group. These findings are in agreement with those reported by Lu et al. (2018). Conversely, a study by Huang et al. (2023) demonstrated that mice exposed to relatively low doses of microplastics over a 10-week period became overweight. This was attributed to enhanced appetite and increased feed intake, likely driven by altered lipid metabolism and reduced physical activity. However, the same study reported the opposite effect at higher microplastic doses (100 μg/mL), where exposed mice exhibited significant declines in body weight relative to controls. Furthermore, it has been previously found that PS-MP exposure leads to gut microbiota dysbiosis, intestinal inflammation, and impaired barrier integrity, which reduce nutrient absorption and caloric uptake (Xu et al., 2024). These processes damage hepatocytes, contributing to cell death and reduced liver mass, thereby lowering HSI. Furthermore, our results indicated that PS-MPs played a role in the alteration of lipid metabolism and lipid profile detected in the exposed group.
Yet, our findings revealed that co-treatment with Tau could elevate the body weight, liver weight, and HSI when it is administered with PS-MPS for 60 days. This is a lightened point which proves that Tau could be a candidate for supporting health issues correlated with PS-MPs administration and toxicity. Tau has been shown to enhance energy metabolism and glucose tolerance by improving insulin sensitivity and glucose uptake in the liver and peripheral tissues. One mechanism involves increased phosphorylation of Akt (protein kinase B), a central regulator in the insulin signalling pathway. Enhanced Akt activity promotes glucose uptake, glycogen synthesis, and lipid metabolism, which collectively contribute to better energy utilization and reduced fat accumulation.
Importantly, improved glucose handling and metabolic efficiency reduce the metabolic burden on the liver, preventing hepatic steatosis and maintaining overall body weight (Musso et al., 2012). Thus, Tau’s ability to improve liver glucose control even more so in the Tau group, combined with PS-MPs, directly supports the restoration of body weight, as it helps reverse or prevent the metabolic dysregulation (e.g., insulin resistance, altered lipid storage) often caused by microplastic exposure.
4.2 Liver enzymes and bilirubin profile
In the present study, oral exposure to PS-MPs led to significant elevations in serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), which are well-established biomarkers of hepatocellular injury. The marked increase in these enzymes suggests compromised hepatocyte membrane integrity and leakage of intracellular contents into circulation, reflecting liver dysfunction (Hatipoglu et al., 2024). These findings align with those reported by Mu et al. (2022), who demonstrated PS-MPs-induced hepatotoxicity in mice, likely mediated by oxidative stress and inflammatory responses, which is indicated in the current study by suppression of antioxidants, enhanced lipid peroxidation, and upregulated expression of inflammatory genes. Furthermore, the current data showed a concurrent rise in total, direct (conjugated), and indirect (unconjugated) bilirubin levels in the PS-MPs-exposed group. Elevated bilirubin, particularly the direct fraction, often indicates hepatocellular damage or impaired bilirubin excretion, while an increase in indirect bilirubin may suggest disruptions in hepatic uptake or conjugation processes (Alqahtani et al., 2023).
4.3 Oxidative stress
In the current study, exposure to PS-MPs induced marked oxidative stress in the liver, as demonstrated by a significant decrease in the activities of key antioxidant enzymes, including SOD and CAT, and a notable increase in MDA levels, a marker of lipid peroxidation. In addition, GSSG levels were significantly reduced in the PS-MPs group, indicating impaired redox homeostasis. These findings are consistent with previous studies, such as Wan et al. (2019) and Wang et al. (2022), which reported decreased antioxidant capacity and elevated oxidative damage following PS-MPs exposure in zebrafish and mudskipper larvae, respectively. Although Deng et al. (2017) observed increased SOD and GSH-Px activity with decreased CAT in mice, such discrepancies across studies have been attributed to differences in particle size, concentration, exposure duration, and species or tissue-specific responses, as suggested by Prokić et al. (2019).
In our study, co-treatment with Tau significantly restored antioxidant enzyme activities and reduced MDA levels compared to the PS-MPs-only group. This antioxidant effect is likely linked to Tau’s well-documented capacity to scavenge ROS, stabilize cellular membranes, and upregulate endogenous antioxidant defenses (Surai et al., 2021). Tau has been shown to enhance mitochondrial function and reduce oxidative injury in various models (Baliou et al., 2021), including hepatic tissue, thereby mitigating PS-MPs-induced oxidative damage. Tau is found to counteract oxidative stress damage in liver tissue in several previous studies.
4.4 Gene expression
Our findings demonstrate that exposure to PS-MPs leads to significant dysregulation of genes involved in hepatic energy metabolism, lipid homeostasis, and oxidative stress, with clear alterations in the AMPK/NR4A1 signalling axis. As shown in the current study (Figure 5), PS-MPs exposure markedly downregulated hepatic AMPK-1, CPT-1, and PPAR-α expression, while significantly upregulating ACC, SREBP-1, CYP2E1, and NR4A1. These changes suggest suppressed fatty acid oxidation, enhanced lipogenesis, and increased oxidative burden. The published study supports this mechanistic insight, showing that PS-MPs activate NR4A1 (Chiu et al., 2025), a nuclear receptor that acts upstream of AMPK signalling, contributing to an energy metabolism imbalance and hepatic steatosis. In contrast to expected AMPK activation in energy-stressed cells (Feng et al., 2024). Our results show reduced AMPK-1 expression in PS-MPs-exposed mice livers, possibly reflecting a disrupted or maladaptive NR4A1-AMPK signalling loop. The increased ACC and SREBP-1 promote lipogenesis (Li et al., 2014), while reduced CPT-1 and PPAR-α expression impairs mitochondrial fatty acid β-oxidation (Hardwick et al., 2009), further exacerbating lipid accumulation. Elevated CYP2E1, a ROS-generating enzyme, and upregulated NR4A1 expression suggest oxidative stress and pro-inflammatory responses (Ma et al., 2024). Importantly, co-administration of taurine significantly reversed most of these gene expression changes, restoring AMPK-1, PPAR-α, and CPT-1 levels, and suppressing NR4A1, ACC, and SREBP-1, which implies that Tau may counteract PS-MPs-induced metabolic disruption through modulation of the NR4A1/AMPK/PPAR-α pathway.
In contrast to some previous studies that reported downregulation of SREBP-1 and upregulation of lipolytic regulators such as PPAR-α and PGC-1α, our current findings demonstrated a marked increase in hepatic SREBP-1 expression along with a significant suppression of PPAR-α and AMPK-1 following PS-MPs exposure. This expression pattern indicates a metabolic shift toward enhanced lipogenesis and impaired fatty acid oxidation, contributing to lipid accumulation in hepatic tissue (Garcia and Shaw, 2017). Given that AMPK is a known inhibitor of SREBP-1 and an activator of both PPAR-α and PGC-1α (de Souza et al., 2017), the observed reduction in AMPK-1 expression likely contributes to the upregulation of SREBP-1 and downregulation of PPAR-α. These results highlight a disrupted AMPK-mediated regulatory axis in the liver following PS-MPs exposure, favouring lipid synthesis and energy imbalance.
Herein, the obtained results show that Tau exerts a significant protective effect against PS-MPs-induced hepatic gene dysregulation by modulating key metabolic and oxidative stress pathways. In the present study, Tau co-administration effectively restored the expression of AMPK-1, PPAR-α, and CPT-1, while suppressing NR4A1, ACC, and SREBP-1, indicating its role in rebalancing energy metabolism and lipid homeostasis.
One of the central mechanisms by which Tau exerts this effect is through activation of AMP-activated protein kinase (AMPK), a master regulator of cellular energy status. As reported by Morsy et al. (2021), Tau enhances AMPK activity, which in turn inhibits lipogenesis by downregulating SREBP-1 and ACC, and promotes fatty acid β-oxidation via upregulation of PPAR-α and CPT-1. Moreover, Tau’s antioxidant properties contribute to its regulatory role. Elevated NR4A1 and CYP2E1 expressions, both linked to oxidative stress and inflammation, were significantly reduced following taurine treatment. Yao et al. (2009) reported that Tau decreases CYP2E1 expression and suppresses ROS production, thereby mitigating oxidative damage. Additionally, Zhang et al. (2023) demonstrated that Tau negatively regulates NR4A1, potentially disrupting its pathological interaction with AMPK and restoring energy balance.
Through the activation of AMPK, enhancing fatty acid oxidation, inhibiting lipogenesis, and reducing oxidative stress, Tau effectively re-establishes a normal hepatic gene expression profile. This highlights its therapeutic potential in alleviating microplastic-induced metabolic disturbances in the liver.
4.5 Histopathological alterations
The histopathological findings in PS-MPs-exposed liver tissue revealed significant structural damage, including congestion of central and portal veins, lymphocytic infiltration, and marked hepatocellular swelling and disorganization, accompanied by enlarged, basophilic nuclei, hallmarks of hepatic inflammation, oxidative injury, and cellular stress. These alterations reflect the hepatotoxic potential of PS-MPs, which may disrupt vascular integrity, initiate immune responses, and impair hepatocyte morphology and function (Elsheikh et al., 2023). However, co-administration of Tau markedly alleviated these pathological features, with notable preservation of hepatic architecture and reduction in inflammatory cell infiltration and hepatocellular degeneration. These protective effects are consistent with Tau’s well-established cytoprotective, anti-inflammatory, and antioxidant properties (Liu et al., 2017).
Furthermore, Tau’s capacity to regulate intracellular calcium homeostasis and osmoregulation (El Idrissi and Trenkner, 2003; Schaffer et al., 1980) helps maintain hepatocyte structural integrity, preventing the swelling and nuclear abnormalities observed in PS-MPs-exposed tissues (de Oliveira Ramos et al., 2018).
5 Conclusion
This study reveals that chronic exposure to PS-MPs at an average particle diameter of approximately 1.8 µm induces significant hepatic toxicity in mice, as evidenced by disrupted liver function, oxidative stress, lipid metabolic imbalance, and altered expression of key regulatory genes. The observed hepatotoxicity was marked by elevated liver enzymes, lipid abnormalities, oxidative damage, and histopathological alterations. Notably, co-administration of Tau effectively mitigated these deleterious effects. Tau’s protective role appears to be mediated through enhancement of antioxidant defenses and restoration of energy and lipid metabolic pathways, including modulation of AMPK-1, CPT-1, SREBP-1, PPAR-α, and related genes. These findings support the potential of Tau as a promising therapeutic agent against microplastic-induced hepatic injury. Also, these findings underscore the possibility that chronic microplastic exposure may contribute to the growing burden of metabolic and cardiovascular diseases in humans, particularly in populations with high environmental exposure. Further research is warranted to evaluate these risks and explore taurine or related interventions as potential protective strategies in the context of human health and environmental toxicology.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Ethics Committee of the Faculty of Veterinary Medicine at Zagazig University, Egypt (Approval No. ZU-IACUC/2/F/33/2025). The study adhered to the principles outlined in the U.K. Animals (Scientific Procedures) Act of 1986, the EU Directive 2010/63/EU on the protection of animals used for scientific purposes, and the Guide for the Care and Use of Laboratory Animals by the National Research Council. Additionally, all experimental protocols were documented in line with the ARRIVE guidelines. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AAE: Formal Analysis, Investigation, Resources, Visualization, Methodology, Writing – original draft. AA-RM: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review and editing. BA: Conceptualization, Data curation, Resources, Validation, Writing – review and editing. YAE-H: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – review and editing. TK: Formal Analysis, Investigation, Methodology, Software, Writing – original draft. AEN: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. AEA: Data curation, Investigation, Methodology, Validation, Writing – original draft. NA: Conceptualization, Resources, Supervision, Writing – original draft. LA: Data curation, Investigation, Methodology, Resources, Validation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R73), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia provided the authors with administrative assistance, access to research resources, and intellectual contributions from colleagues.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. The author(s) verify and take full responsibility for the use of generative AI in the preparation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1665161/full#supplementary-material
References
Abdel-Moneim, A. M., Al-Kahtani, M. A., El-Kersh, M. A., and Al-Omair, M. A. J. P. o. (2015). Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS One 10, e0144509. doi:10.1371/journal.pone.0144509
Ahmad, M., Ehsan, N., Khan, H. A., Hamza, A., Azmat, R., and Ijaz, M. U. (2023). Ameliorative effects of rhamnetin against polystyrene microplastics-induced nephrotoxicity in rats.
Akanyange, S. N., Zhang, Y., Zhao, X., Adom-Asamoah, G., Ature, A.-R. A., Anning, C., et al. (2022). A holistic assessment of microplastic ubiquitousness: pathway for source identification in the environment. Sustain. Prod. Consum. 33, 113–145. doi:10.1016/j.spc.2022.06.020
Allouzi, M. M. A., Tang, D. Y. Y., Chew, K. W., Rinklebe, J., Bolan, N., Allouzi, S. M. A., et al. (2021). Micro (Nano) plastic pollution: the ecological influence on soil-plant system and human health. Sci. Total Environ. 788, 147815. doi:10.1016/j.scitotenv.2021.147815
Alqahtani, L. S., Abd-Elhakim, Y. M., Mohamed, A.A.-R., Khalifa, N. E., Khamis, T., Alotaibi, B. S., et al. (2023). Curcumin-loaded chitosan nanoparticles alleviate fenpropathrin-induced hepatotoxicity by regulating lipogenesis and pyroptosis in rats. Food Chem. Toxicol. 180, 114036. doi:10.1016/j.fct.2023.114036
Ašmonaitė, G., Sundh, H., Asker, N., and Carney Almroth, B. J. E. s. (2018). Rainbow trout maintain intestinal transport and barrier functions following exposure to polystyrene microplastics. Environ. Sci. Technol. 52, 14392–14401. doi:10.1021/acs.est.8b04848
Auguet, T., Bertran, L., Barrientos-Riosalido, A., Fabregat, B., Villar, B., Aguilar, C., et al. (2022). Are ingested or inhaled microplastics involved in nonalcoholic fatty liver disease? Int. J. Environ. Res. public health 19, 13495. doi:10.3390/ijerph192013495
Baliou, S., Adamaki, M., Ioannou, P., Pappa, A., Panayiotidis, M. I., Spandidos, D. A., et al. (2021). Protective role of taurine against oxidative stress. 24, 1–19.
Bancroft, J. D., and Layton, C. (2013). “The hematoxylin and eosin, connective and mesenchymal tissues with their stains,” in Bancroft s theory and practice of histological techniques. 7th ed. (Philadelphia: Churchill Livingstone), 173–186.
Bellasi, A., Binda, G., Pozzi, A., Galafassi, S., Volta, P., and Bettinetti, R. J. E. (2020). Microplastic contamination in freshwater environments: a review, focusing on interactions with sediments and benthic organisms. Environ. (Basel). 7, 30. doi:10.3390/environments7040030
Beutler, E., Duron, O., and Kelly, B. M. (1963). Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 61, 882–888.
Blackburn, K., and Green, D. J. A. (2022). The potential effects of microplastics on human health: what is known and what is unknown. Ambio 51, 518–530. doi:10.1007/s13280-021-01589-9
Campanale, C., Massarelli, C., Savino, I., Locaputo, V., and Uricchio, V. F. (2020). A detailed review study on potential effects of microplastics and additives of concern on human health. Int. J. Environ. Res. Public Health 17, 1212. doi:10.3390/ijerph17041212
Chen, W., Guo, J., Zhang, Y., and Zhang, J. J. F. (2016). The beneficial effects of taurine in preventing metabolic syndrome. Food Funct. 7, 1849–1863. doi:10.1039/c5fo01295c
Chen, H., Hua, X., Li, H., Wang, C., Dang, Y., Ding, P., et al. (2021). Transgenerational neurotoxicity of polystyrene microplastics induced by oxidative stress in Caenorhabditis elegans. Chemosphere 272, 129642. doi:10.1016/j.chemosphere.2021.129642
Cheng, W., Li, X., Zhou, Y., Yu, H., Xie, Y., Guo, H., et al. (2022). Polystyrene microplastics induce hepatotoxicity and disrupt lipid metabolism in the liver organoids. Sci. Total Environ. 806, 150328. doi:10.1016/j.jep.2021.114529
Chiu, H.-W., Chu, C.-W., Huang, C.-C., Chia, Z.-C., Wang, Y.-L., and Lee, Y.-H. J. E. P. (2025). Polystyrene microplastics induce hepatic lipid metabolism and energy disorder by upregulating the NR4A1-AMPK signaling pathway. Environ. Pollut. 369, 125850. doi:10.1016/j.envpol.2025.125850
Cho, Y.-S., Shin, C. H., and Han, S. J. N. r.l. (2016). Dispersion polymerization of polystyrene particles using alcohol as reaction medium. Nanoscale Res. Lett. 11, 46–49. doi:10.1186/s11671-016-1261-8
De Luca, A., Pierno, S., and Camerino, D. C. J. J. o.t.m. (2015). Taurine: the appeal of a safe amino acid for skeletal muscle disorders. J. Transl. Med. 13, 243–18. doi:10.1186/s12967-015-0610-1
de Oliveira Ramos, C., Campos, K. K. D., de Paula Costa, G., Cangussu, S. D., Talvani, A., and Bezerra, F. S. J. R. T. (2018). Taurine treatment decreases inflammation and oxidative stress in lungs of adult mice exposed to cigarette smoke. Regul. Toxicol. Pharmacol. 98, 50–57. doi:10.1016/j.yrtph.2018.07.008
de Souza, C. O., Teixeira, A. A., Biondo, L. A., Lima Junior, E. A., Batatinha, H. A., and Rosa Neto, J. C. J. J. o.c.p. (2017). Palmitoleic acid improves metabolic functions in fatty liver by PPARα-dependent AMPK activation. J. Cell. Physiol. 232, 2168–2177. doi:10.1002/jcp.25715
Deng, Y., Zhang, Y., Lemos, B., and Ren, H. (2017). Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 7, 46687. doi:10.1038/srep46687
Ehsan, N., Ali, S., Hamza, A., Batool, M., Al-Ghanim, K. A., Atique, U., et al. (2023). Attenuative effects of ginkgetin against polystyrene microplastics-induced renal toxicity in rats. 43.
El Idrissi, A., and Trenkner, E. (2003). “Taurine regulates mitochondrial calcium homeostasis,” in Taurine 5: beginning the 21st century. Springer, 527–536.
Elsheikh, A. A., Alnasser, S. M., Shalaby, A. M., Alabiad, M. A., Abd-Almotaleb, N. A., Alorini, M., et al. (2023). Polystyrene microplastic particles induced hepatotoxic injury via pyroptosis, oxidative stress, and fibrotic changes in adult Male albino rats; the therapeutic role of silymarin. Toxicol. Mech. Methods 33, 512–528. doi:10.1080/15376516.2023.2191270
Fang, J., Xuan, Y., and Li, Q. J. S. C. T. S. (2010). Preparation of polystyrene spheres in different particle sizes and assembly of the PS colloidal crystals. Sci. China Technol. Sci. 53, 3088–3093. doi:10.1007/s11431-010-4110-5
Feng, Y., Chen, Y., Wu, X., Chen, J., Zhou, Q., Liu, B., et al. (2024). Interplay of energy metabolism and autophagy. Autophagy 20, 4–14. doi:10.1080/15548627.2023.2247300
Foster, L. B., and Dunn, R. T. (1973). Stable reagents for determination of serum triglycerides by a colorimetric hantzsch condensation method. Clin. Chem. 19, 338–340. doi:10.1093/clinchem/19.3.338
Friedewald, W. T., Levy, R. I., and Fredrickson, D. S. (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502. doi:10.1093/clinchem/18.6.499
Garcia, D., and Shaw, R. J. (2017). AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 66, 789–800. doi:10.1016/j.molcel.2017.05.032
Gibson-Corley, K. N., Olivier, A. K., and Meyerholz, D. K. (2013). Principles for valid histopathologic scoring in research. Veterinary Pathol. 50, 1007–1015. doi:10.1177/0300985813485099
Goodman, K. E., Hua, T., and Sang, Q. A. (2022). Effects of polystyrene microplastics on human kidney and liver cell morphology, cellular proliferation, and metabolism. ACS omega 7, 34136–34153. doi:10.1021/acsomega.2c03453
Hardwick, J. P., Osei-Hyiaman, D., Wiland, H., Abdelmegeed, M. A., and Song, B.-J. J. P. r. (2009). PPAR/RXR regulation of fatty acid metabolism and fatty acid omega-Hydroxylase (CYP4) isozymes: implications for prevention of lipotoxicity in fatty liver disease. PPAR Res. 2009, 952734. doi:10.1155/2009/952734
Hatipoglu, D., Ates, M. B., Bulut, A., Gezgic, B., and Dik, B. J. P. V. J. (2024). Efficacy of resatorvid and alpha-lipoic acid in ameliorating gentamicin-induced liver injury: insights from rat model study. 44.
Horton, A. A., Walton, A., Spurgeon, D. J., Lahive, E., and Svendsen, C. (2017). Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 586, 127–141. doi:10.1016/j.scitotenv.2017.01.190
Hu, M., and Palić, D. (2020). Micro-and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 37, 101620. doi:10.1016/j.redox.2020.101620
Huang, H., Wei, F., Qiu, S., Xing, B., and Hou, J. J. S. o.T. T. E. (2023). Polystyrene microplastics trigger adiposity in mice by remodeling gut microbiota and boosting fatty acid synthesis. Sci. Total Environ. 890, 164297. doi:10.1016/j.scitotenv.2023.164297
Ijaz, M. U., Nadeem, A., Hayat, M. F., Ehsan, N., Al-Ghanim, K. A., and Atique, U. J. P. V. J. (2024). Evaluation of possible ameliorative role of robinetin to counteract polystyrene microplastics instigated renal toxicity in rats. 44.
Jabeen, Z., Bukhari, S. A., Malik, S. A., Hussain, G., and Kamal, S. J. P. V. J. (2023). Improved gut microbiota escalates muscle function rehabilitation and ameliorates oxidative stress following mechanically induced peripheral nerve injury in mice. 43.
Karanam, K., Okoffo, E. D., Shukla, P., Thomas, K. V., and Rudolph, V. J. S. C. f.t.E. (2024). Preparation of polystyrene microplastic particles by solvent-dissolution-precipitation. Sustain. Chem. Environ. 7, 100144. doi:10.1016/j.scenv.2024.100144
Lai, W., Xu, D., Li, J., Wang, Z., Ding, Y., Wang, X., et al. (2021). Dietary polystyrene nanoplastics exposure alters liver lipid metabolism and muscle nutritional quality in carnivorous marine fish large yellow croaker (Larimichthys crocea). J. Hazard. Mater. 419, 126454. doi:10.1016/j.jhazmat.2021.126454
Lambert, I., Kristensen, D., Holm, J., and Mortensen, O. J. A. P. (2015). Physiological role of taurine–from organism to organelle. Acta Physiol. 213, 191–212. doi:10.1111/apha.12365
Li, X., Li, Y., Yang, W., Xiao, C., Fu, S., Deng, Q., et al. (2014). SREBP-1c overexpression induces triglycerides accumulation through increasing lipid synthesis and decreasing lipid oxidation and VLDL assembly in bovine hepatocytes. J. Steroid Biochem. Mol. Biol. 143, 174–182. doi:10.1016/j.jsbmb.2014.02.009
Li, S., Shi, M., Wang, Y., Xiao, Y., Cai, D., and Xiao, F. J. J. o.h.m. (2021). Keap1-Nrf2 pathway up-regulation via hydrogen sulfide mitigates polystyrene microplastics induced-hepatotoxic effects. J. Hazard. Mater. 402, 123933. doi:10.1016/j.jhazmat.2020.123933
Lin, Y.-D., Huang, P.-H., Chen, Y.-W., Hsieh, C.-W., Tain, Y.-L., Lee, B.-H., et al. (2023). Sources, degradation, ingestion and effects of microplastics on humans: a review. Toxics 11, 747. doi:10.3390/toxics11090747
Liu, Y., Li, F., Zhang, L., Wu, J., Wang, Y., and Yu, H. J. M. m.r. (2017). Taurine alleviates lipopolysaccharide-induced liver injury by anti-inflammation and antioxidants in rats. Mol. Med. Rep. 16, 6512–6517. doi:10.3892/mmr.2017.7414
Liu, P., Wu, X., Liu, H., Wang, H., Lu, K., and Gao, S. J. J. o.h.m. (2020a). Desorption of pharmaceuticals from pristine and aged polystyrene microplastics under simulated gastrointestinal conditions. J. Hazard. Mater. 392, 122346. doi:10.1016/j.jhazmat.2020.122346
Liu, S., Wu, X., Gu, W., Yu, J., and Wu, B. J. C. (2020b). Influence of the digestive process on intestinal toxicity of polystyrene microplastics as determined by in vitro Caco-2 models. Chemosphere 256, 127204. doi:10.1016/j.chemosphere.2020.127204
Lu, Y., Zhang, Y., Deng, Y., Jiang, W., Zhao, Y., Geng, J., et al. (2016). Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. and Technol. 50, 4054–4060. doi:10.1021/acs.est.6b00183
Lu, L., Wan, Z., Luo, T., Fu, Z., and Jin, Y. (2018). Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Sci. Total Environ. 631-632, 449–458. doi:10.1016/j.scitotenv.2018.03.051
Luo, T., Zhang, Y., Wang, C., Wang, X., Zhou, J., Shen, M., et al. (2019). Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ. Pollut. 255, 113122. doi:10.1016/j.envpol.2019.113122
Ma, C., Chen, Q., Li, J., Li, B., Liang, W., Su, L., et al. (2021). Distribution and translocation of micro- and nanoplastics in fish. Crit. Rev. Toxicol. 51, 740–753. doi:10.1080/10408444.2021.2024495
Ma, T., Huang, W., Ding, Y., Ji, R., Ge, S., Liu, Q., et al. (2024). AIBP protects drug-induced liver injury by inhibiting MAPK-Mediated NR4A1 expression. iScience 27, 110873. doi:10.1016/j.isci.2024.110873
MacLeod, M., Arp, H. P. H., Tekman, M. B., and Jahnke, A. J. S. (2021). The global threat from plastic pollution. Sci. (1979). 373, 61–65. doi:10.1126/science.abg5433
Mahfouz, H., Dahran, N., Abdel-Rahman Mohamed, A., Abd El-Hakim, Y. M., Metwally, M. M. M., Alqahtani, L. S., et al. (2023). Stabilization of glutathione redox dynamics and CYP2E1 by green synthesized moringa oleifera-mediated zinc oxide nanoparticles against acrylamide induced hepatotoxicity in rat model: Morphometric and molecular perspectives. Food Chem. Toxicol. 176, 113744. doi:10.1016/j.fct.2023.113744
Menzie, J., Pan, C., Prentice, H., and Wu, J.-Y. J. A. a. (2014). Taurine and central nervous system disorders. Amino Acids 46, 31–46. doi:10.1007/s00726-012-1382-z
Miyazaki, T., and Matsuzaki, Y. J. A. a. (2014). Taurine and liver diseases: a focus on the heterogeneous protective properties of taurine. Amino Acids 46, 101–110. doi:10.1007/s00726-012-1381-0
Morsy, M. D., Aboonq, M. S., Alsleem, M. A., Abusham, A. A. J. C., and Pharmacology, E. (2021). Taurine prevents high-fat diet-induced-hepatic steatosis in rats by direct inhibition of hepatic sterol regulatory element-binding proteins and activation of AMPK. Clin. Exp. Pharmacol. Physiol. 48, 72–85. doi:10.1111/1440-1681.13387
Mostafa-Hedeab, G., Behairy, A., Abd-Elhakim, Y. M., Mohamed, A. A. R., Noreldin, A. E., Dahran, N., et al. (2023). Green synthesized zinc oxide nanoparticles using moringa olifera ethanolic extract lessens acrylamide-induced testicular damage, apoptosis, and steroidogenesis-related gene dysregulation in adult rats. Antioxidants 12, 361. doi:10.3390/antiox12020361
Mu, Y., Sun, J., Li, Z., Zhang, W., Liu, Z., Li, C., et al. (2022). Activation of pyroptosis and ferroptosis is involved in the hepatotoxicity induced by polystyrene microplastics in mice. Chemosphere 291, 132944. doi:10.1016/j.chemosphere.2021.132944
Musso, G., Cassader, M., Rosina, F., and Gambino, R. J. D. (2012). Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia 55, 885–904. doi:10.1007/s00125-011-2446-4
Niu, X., Zheng, S., Liu, H., and Li, S. J. M. m.r. (2018). Protective effects of taurine against inflammation, apoptosis, and oxidative stress in brain injury. Mol. Med. Rep. 18, 4516–4522. doi:10.3892/mmr.2018.9465
Ohkawa, H., Ohishi, N., and Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95, 351–358. doi:10.1016/0003-2697(79)90738-3
Pei, X., Heng, X., and Chu, W. J. M. p. (2022). Polystyrene nano/microplastics induce microbiota dysbiosis, oxidative damage, and innate immune disruption in zebrafish. Microb. Pathog. 163, 105387. doi:10.1016/j.micpath.2021.105387
Piao, F., Aadil, R. M., Suleman, R., Li, K., Zhang, M., Wu, P., et al. (2018). Ameliorative effects of taurine against diabetes. a Rev. 50, 487–502.
Prokić, M. D., Radovanović, T. B., Gavrić, J. P., and Faggio, C. (2019). Ecotoxicological effects of microplastics: examination of biomarkers, current state and future perspectives. TrAC Trends Anal. Chem. 111, 37–46. doi:10.1016/j.trac.2018.12.001
Prüst, M., Meijer, J., and Westerink, R. H. J. P. (2020). The plastic brain: neurotoxicity of micro-and nanoplastics. 17, 1–16.
Qaradakhi, T., Gadanec, L. K., McSweeney, K. R., Abraham, J. R., Apostolopoulos, V., and Zulli, A. J. N. (2020). The anti-inflammatory effect of taurine on cardiovascular disease. Nutrients 12, 2847. doi:10.3390/nu12092847
Qiao, J., Chen, R., Wang, M., Bai, R., Cui, X., Liu, Y., et al. (2021). Perturbation of gut microbiota plays an important role in micro/nanoplastics-induced gut barrier dysfunction. Nanoscale 13, 8806–8816. doi:10.1039/d1nr00038a
Ragusa, A., Svelato, A., Santacroce, C., Catalano, P., Notarstefano, V., Carnevali, O., et al. (2021). Plasticenta: first evidence of microplastics in human placenta. Environ. Int. 146, 106274. doi:10.1016/j.envint.2020.106274
Reitman, S., and Frankel, S. (1957). A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathology 28, 56–63. doi:10.1093/ajcp/28.1.56
Rubio, L., Marcos, R., Hernández, A. J. J. o.T., and Environmental Health, P. B. (2020). Potential adverse health effects of ingested micro-and nanoplastics on humans. Lessons learned from in vivo and in vitro Mammalian models. J. Toxicol. Environ. Health. B Crit. Rev. 23, 51–68. doi:10.1080/10937404.2019.1700598
Schaffer, S., and Kim, H. W. J. B. (2018). Effects and mechanisms of taurine as a therapeutic agent. Biomol. Ther. 26, 225–241. doi:10.4062/biomolther.2017.251
Schaffer, S. W., Kramer, J., and Chovan, J. P. (1980). Regulation of calcium homeostasis in the heart by taurine. Fed. Proc. 39, 2691–2694.
Schaffer, S., Takahashi, K., and Azuma, J. J. A. a. (2000). Role of osmoregulation in the actions of taurine. Amino Acids 19, 527–546. doi:10.1007/s007260070004
Schmittgen, T. D., and Livak, K. J. J. N. p. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. doi:10.1038/nprot.2008.73
Schwabl, P., Köppel, S., Königshofer, P., Bucsics, T., Trauner, M., Reiberger, T., et al. (2019). Detection of various microplastics in human stool: a prospective case series. Ann. Intern. Med. 171, 453–457. doi:10.7326/M19-0618
Senathirajah, K., Attwood, S., Bhagwat, G., Carbery, M., Wilson, S., and Palanisami, T. (2021). Estimation of the mass of microplastics ingested – a pivotal first step towards human health risk assessment. J. Hazard. Mater. 404, 124004. doi:10.1016/j.jhazmat.2020.124004
Song, S., He, S., Lin, Q., and Jin, H. J. F. B. (2024). Synergistic effect of collagen peptide LR-7 and taurine on the prevention of cardiovascular injury in high salt-induced hypertensive mice. Food Biosci. 61, 104907. doi:10.1016/j.fbio.2024.104907
Surai, P. F., Earle-Payne, K., and Kidd, M. T. J. A. (2021). Taurine as a natural antioxidant: from direct antioxidant effects to protective action in various toxicological models. Antioxidants (Basel). 10, 1876. doi:10.3390/antiox10121876
Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034–12. doi:10.1186/gb-2002-3-7-research0034
Vitali, C., Peters, R. J., Janssen, H.-G., and Nielen, M. W. J. T. T. i.A. C. (2023). Microplastics and nanoplastics in food, water, and beverages; part I. Occurrence. Occurrence 159, 116670. doi:10.1016/j.trac.2022.116670
Walters, M. I., and Gerarde, H. (1970). An ultramicromethod for the determination of conjugated and total bilirubin in serum or plasma. Microchem. J. 15, 231–243. doi:10.1016/0026-265x(70)90045-7
Wan, Z., Wang, C., Zhou, J., Shen, M., Wang, X., Fu, Z., et al. (2019). Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 217, 646–658. doi:10.1016/j.chemosphere.2018.11.070
Wang, Y.-L., Lee, Y.-H., Hsu, Y.-H., Chiu, I.-J., Huang, C.C.-Y., Huang, C.-C., et al. (2021). The kidney-related effects of polystyrene microplastics on human kidney proximal tubular epithelial cells HK-2 and male C57BL/6 mice. Environ. Health Perspect. 129, 057003. doi:10.1289/EHP7612
Wang, X., Jian, S., Zhang, S., Wu, D., Wang, J., Gao, M., et al. (2022). Enrichment of polystyrene microplastics induces histological damage, oxidative stress, Keap1-Nrf2 signaling pathway-related gene expression in loach juveniles (Paramisgurnus dabryanus). Ecotoxicol. Environ. Saf. 237, 113540. doi:10.1016/j.ecoenv.2022.113540
Xu, R., Cao, J. W., Lv, H. L., Geng, Y., and Guo, M. Y. (2024). Polyethylene microplastics induced gut microbiota dysbiosis leading to liver injury via the TLR2/NF-κB/NLRP3 pathway in mice. Sci. total Environ. 917, 170518. doi:10.1016/j.scitotenv.2024.170518
Yao, H.-T., Lin, P., Chang, Y.-W., Chen, C.-T., Chiang, M.-T., Chang, L., et al. (2009). Effect of taurine supplementation on cytochrome P450 2E1 and oxidative stress in the liver and kidneys of rats with streptozotocin-induced diabetes. Food Chem. Toxicol. 47, 1703–1709. doi:10.1016/j.fct.2009.04.030
Yaseen, A., Assad, I., Sofi, M. S., Hashmi, M. Z., and Bhat, S. U. (2022). A global review of microplastics in wastewater treatment plants: Understanding their occurrence, fate and impact. Environ. Res. 212, 113258.
Yin, J., Ju, Y., Qian, H., Wang, J., Miao, X., Zhu, Y., et al. (2022). Nanoplastics and microplastics may be damaging our livers. Toxics 10, 586. doi:10.3390/toxics10100586
Zhang, Z., Liu, D., Yi, B., Liao, Z., Tang, L., Yin, D., et al. (2014). Taurine supplementation reduces oxidative stress and protects the liver in an iron-overload murine model. Mol. Med. Rep. 10, 2255–2262. doi:10.3892/mmr.2014.2544
Zhang, Y., Weng, J., Huan, L., Sheng, S., and Xu, F. J. F. i.i. (2023). Mitophagy in atherosclerosis: from mechanism to therapy. Front. Immunol. 14, 1165507. doi:10.3389/fimmu.2023.1165507
Zheng, H., Wang, J., Wei, X., Chang, L., and Liu, S. J. S. o.t.T. E. (2021). Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Sci. Total Environ. 750, 143085. doi:10.1016/j.scitotenv.2020.143085
Zlatkis, A., Zak, B., and Boyle, A. J. (1953). A new method for the direct determination of serum cholesterol. J. Lab. Clin. Med. 41 (3), 486℃492.
Keywords: liver, gene expression, microplastics, oxidative stress, taurine, lipid metabolic disturbances, histological assessments
Citation: A. Eskandrani A, Abdel-Rahman Mohamed A, Alotaibi BS, Abd El-Hakim YM, Khamis T, E. Noreldin A, E Abdelhamid A, Alsubaie N and Alqahtani LS (2025) Palliative effect of taurine against hepatic injury induced by polystyrene microplastics through antioxidant and metabolic pathway modulation in mice. Front. Pharmacol. 16:1665161. doi: 10.3389/fphar.2025.1665161
Received: 13 July 2025; Accepted: 15 September 2025;
Published: 02 October 2025; Corrected: 09 October 2025.
Edited by:
Andrés Trostchansky, Universidad de la República, UruguayReviewed by:
Rocio Gomez, Center for Research and Advanced Studies (CINVESTAV), MexicoAisha Khatoon, University of Agriculture, Pakistan
Copyright © 2025 A. Eskandrani, Abdel-Rahman Mohamed, Alotaibi, Abd El-Hakim, Khamis, E. Noreldin, E Abdelhamid, Alsubaie and Alqahtani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Badriyah S. Alotaibi, YnNhbG90YWliaUBwbnUuZWR1LnNh
 Areej A. Eskandrani1
Areej A. Eskandrani1 Amany Abdel-Rahman Mohamed
Amany Abdel-Rahman Mohamed Badriyah S. Alotaibi
Badriyah S. Alotaibi Yasmina M. Abd El-Hakim
Yasmina M. Abd El-Hakim Tarek Khamis
Tarek Khamis Ahmed E. Noreldin
Ahmed E. Noreldin Nawal Alsubaie
Nawal Alsubaie Leena S. Alqahtani
Leena S. Alqahtani