- 1Department of Bioscience and Biotechnology, Banasthali Vidyapith, Vanasthali, Rajasthan, India
- 2Department of Clinical Biochemistry, Kashmir University, Srinagar, India
- 3Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
Tecomella undulata (Sm.) Seem, an endangered plant, is native to India, Afghanistan, Iran, Oman, and Pakistan. Traditionally, in India, the stem bark is commonly used for the treatment of leucorrhea, pain, sexual disorders, digestive disorders, eczema, and skin infections. On the other hand, in Pakistan, both the flowers and stem bark are used as a remedy for different ailments (hepatitis, jaundice, sexual disorders, anorexia, constipation, and menstrual disorders). Phenolic metabolites and their derivatives, flavonoids, steroids, alkaloids, terpenoids, fatty acids and their derivatives, and quinones are the primary bioactive metabolites identified from this plant using different spectral and chromatographic techniques. T. undulata possesses hepatoprotective, antimicrobial, analgesic, antidiabetic, antioxidant, anti-obesity, acaricidal, and miticidal activities. However, these bioactivities have been partially validated scientifically. Thus, comprehensive reports exploring the mechanism of action of plant extracts/metabolites are needed to ascertain the therapeutic effect of T. undulata. The use of the plant in Ayurvedic formulations, as a source of timber, and in a few patents highlights their commercial importance. Preliminary toxicity studies suggest that the plant is reasonably safe; however, more in-depth data from animal models and clinical studies are needed to confirm its safety. There are a few reports on the micropropagation of this endangered plant, which can be used as a conservation strategy. With the plant being included in the Red Data Book, it becomes imperative to explore its tissue culture for the sustainable production of leading bioactive metabolites. Overall, this review compiles information on the ethnomedicinal uses, bioactive metabolites, pharmacology, commercial applications, toxicity, and micropropagation of T. undulata for further exploitation of the plant as a therapeutic agent.
1 Introduction
India is known to have a rich biodiversity of medicinal plants, which possess significant social, economic, and cultural value (Hamilton, 2004). Since ancient times, local communities have utilized botanical remedies for various health issues. Medicinal plants play a vital role in Ayurveda, Unani, Siddha, homeopathy, and naturopathy, offering numerous healing benefits. Moreover, they form a crucial component of the botanical drug industry and traditional medicine, providing income support to many in developing countries (Kumar et al., 2011). The therapeutic properties of plants are often attributed to the presence of bioactive metabolites, specifically secondary metabolites (Kumar and Janagam, 2011; Kandar, 2021). Even today, more than 60% of newly approved drugs are derived from natural sources, highlighting the relevance of secondary metabolites in both traditional and modern pharmacology (Li and Vederas, 2009).
The industrial relevance of medicinal plants has led to their depletion in the wild, which is a global issue. The current rate of extinction of plant species outnumbers the rate of natural extinction by 100–1,000 times (Wilson, 1988). The updated IUCN Red List includes 26,840 endangered species out of a total of 96,951 species (IUCN, 2021). Thus, it is essential to protect and rationally use the phyto-diversity for the sustainable development of human society (Orme et al., 2005). The enhanced extinction of endangered plants may seriously affect entire ecosystems and is a matter of concern for survival and human development. Thus, conservation of plant resources, including scarce and endangered species, is crucial for maintaining the diversity of Earth’s biological systems (Cyranoski, 2008). Plant tissue culture plays a pivotal role in agriculture, horticulture, metabolites, and conservation sectors (Pithiya et al., 2022). This technique involves the propagation of plants in vitro on a nutrient medium under aseptic conditions, allowing for the generation of multiple plants from a single explant (Vats et al., 2024).
Tecomella undulata (Sm.) Seem, commonly known as Rohida, honey tree, desert teak, Marwar teak, or white cedar, belongs to the Bignoniaceae family. This monotypic genus is native to India, Afghanistan, Iran, Oman, and Pakistan. T. undulata thrives in well-drained loamy to sandy loam soils with a pH range of 6.5–8.0, making it well-suited for arid environments. This species is adapted to low-rainfall areas, typically receiving between 150 and 500 mm of annual precipitation. It can endure significant temperature variations and shows remarkable tolerance to extreme cold, surviving temperatures as low as 0 °C to −2 °C in winter and reaching up to 48 °C–50 °C during summer (Singh et al., 2017). The plant has garnered interest in both classical and folk streams of the ancient medicinal system due to its therapeutic value (Ravishankar and Shukla, 2007), which is also mentioned in the ancient Samhitas of Ayurveda (Khare, 2004). Gelseminum undulatum (Sm.) Kuntze., Bignonia undulata (Sm.)., Tecoma undulata (Sm.) G.Don, Bignonia tropaeolum Jacquem. ex DC, and Tecoma glauca DC are synonyms of T. undulata (WFO, 2025). The monograph of the plant has been published in the Ayurvedic Pharmacopoeia of India (API, 2008), highlighting its therapeutic uses against helminthiasis, jaundice, skin diseases, obesity, constipation, leucorrhea, and other metabolic disorders. The tree yields good-quality timber. However, slow growth and overexploitation of this tree for medicinal and other commercial purposes have led to its classification as an endangered species (POWO, 2025).
Overall, T. undulata is an important medicinal plant, but there appears to be a dearth of manuscripts establishing the connection between its ethnopharmacological uses, phytochemistry, and modern pharmacological investigations. To date, no comprehensive review has been published to elucidate the limitations of studies on the plant, including its safety and toxicity, micropropagation strategies, and future perspectives. Therefore, this review aims to comprehensively summarize the ethnomedicinal importance, phytometabolites, bioactivities, toxicity, commercial importance, and in vitro propagation reports. The authors believe that this review is significant as it will help researchers identify research gaps and plan further strategies to establish T. undulata as a promising candidate for future drug discovery.
2 Geographical distribution and botanical description
The tree thrives in arid regions across parts of Afghanistan, Oman, southern Pakistan (Sindh and Baluchistan), and northwestern India (Rajasthan, Gujarat, Maharashtra, Punjab, and Haryana) (Tewari, 2007; Figure 1A). The majority of Rohida is found in western Rajasthan, particularly in districts such as Ajmer, Barmer, Bikaner, Churu, Jaisalmer, Jodhpur, Nagaur, Pali, and Sikar (Meena and Kant, 2022).
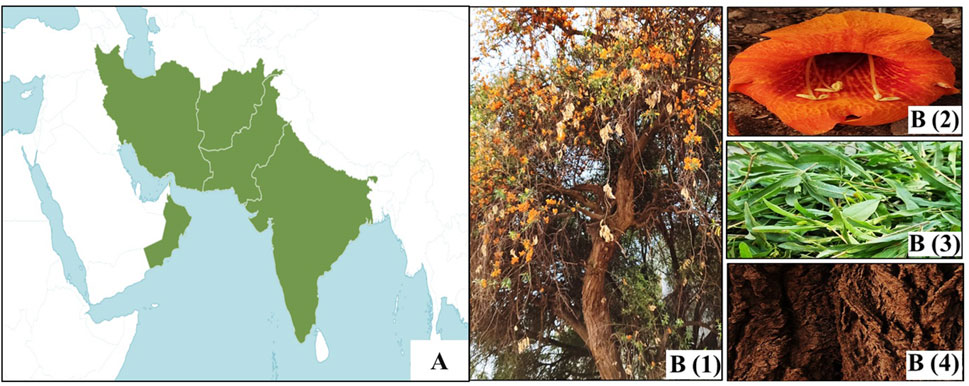
Figure 1. (A) Distribution of T. undulata (POWO, 2025). (B) Whole plant (1), flower (2), leaf (3), and bark (4) of T. undulata.
T. undulata has a curved trunk and drooping branches. The circumference measures 52 cm–80 cm, and the height varies from 4 to 10 m. In its natural habitat, it grows up to 8 m. The roots are deep-seated, and growth is slow. Leaves are greenish, thick, and coriaceous. Defoliation occurs from November until the end of March, but complete leaf shedding does not occur as new leaves begin to appear in mid-February (Kalia et al., 2014).
The tree produces large and showy flowers on shorter lateral branches (Figure 1B). The pedicle bases measure 1 cm–2 cm in length and are terete in shape. The calyx is yellow or green, 8 mm–9 mm long, ovate, campanulate, often recurved, and may have some black spots on the outer side. The corolla is yellow–orange (5 cm–7 cm long) and veined with five equal lobes. The anterior stamens are 10 mm–30 mm long, while the posterior stamens are 2.5 mm long, and they are exerted. The filaments are glabrous. There is a yellow annular disc ovary, a style (4.5 cm long), and a bilamellated stigma (3.6 mm long) and spathulate–oblong lobes (Arya et al., 1992). The botanical classification and morphological characteristics of T. undulata are summarized in Table 1.
3 Ethnomedicinal uses
T. undulata is an important plant used in traditional medicine. The stem bark of the plant is used in the preparation of various Ayurvedic formulations. The bark is also used in the preparation of various botanical formulations (Liv-52, Amlycure, Livo Plus, Herboliv, and Livosan) for the treatment of hepatic tissue (Jain et al., 2012). Ayurvedic massage oil and fairness masks are made from this plant, combined with other plants (Jain et al., 2012). Extracts or decoctions of powdered bark in clarified butter are beneficial in treating intestinal worms, jaundice, anemia, and urinary disorders, which may be attributed to an imbalance of pitta and kapha (Khare, 2004).
In India, the root pulp, along with rice water, is administered orally as treatment in the Churu district and the Shekhawati region of Rajasthan (Katewa and Galav, 2005). The Garasia tribe (Rajasthan) and tribal communities in Chhattisgarh use various parts of Rohida for treating syphilis and old wounds, respectively (Meena and Yadav, 2010). In the Aravalli Hills, the Meena tribes have reported its use in treating allergic reactions (Meena and Rao, 2010). Stem bark in combination with other plants is used to heal fractures (Paul and Prajapati, 2014). The bark oil is used to treat syphilis, eczema, and skin eruptions, and the heartwood is used for the treatment of diabetes. However, no mention of the ethnomedicinal use of oil from any part of the plant or the heartwood has been documented in Pakistan.
In Pakistan, women in the Khuzdar and Kalat regions use flowers to make tea, which sterile women consume during menstruation. A paste made from fresh leaves is applied to the forehead during headaches (Tareen et al., 2010). Bark powder (100 g) is administered daily as a tonic to procumbent animals until recovery. Bark powder is also taken with hot milk by women of the Samahni Valley for abortion (Muhammad et al., 2006). Syphilis, gonorrhea, hepatitis, conjunctivitis, infection, wounds, anorexia, jaundice, liver disorders, etc., are also treated using T. undulata (Table 2).
Overall, the plant is most commonly used in the treatment of liver disorders, followed by leucorrhea and syphilis. Oral administration of the traditional drugs was most common in India and Pakistan (Figure 2A). Considering the plant parts, it was observed that the stem bark was used extensively in India. On the other hand, in Pakistan, the use of stem bark and flowers was almost equal (Figure 2B). Most of the data did not specify the amount of plant parts used for the traditional preparation or the duration of administration. The ethnomedicinal uses of T. undulata have been partially validated scientifically through pharmacological studies, which further support its potential as a promising medicinal candidate (Alvala et al., 2013; Srinivas et al., 2023; Ravi et al., 2011).
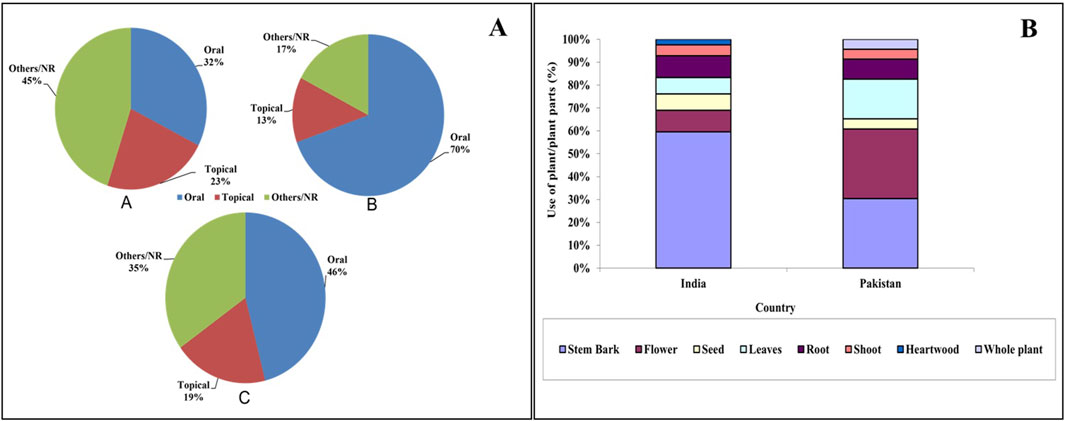
Figure 2. (A) Mode of administration of ethnomedicines from T. undulata. A: India; B: Pakistan; C: overall. (B) Use of plant parts (%) in the preparation of ethnomedicine in India and Pakistan.
4 Phytochemistry
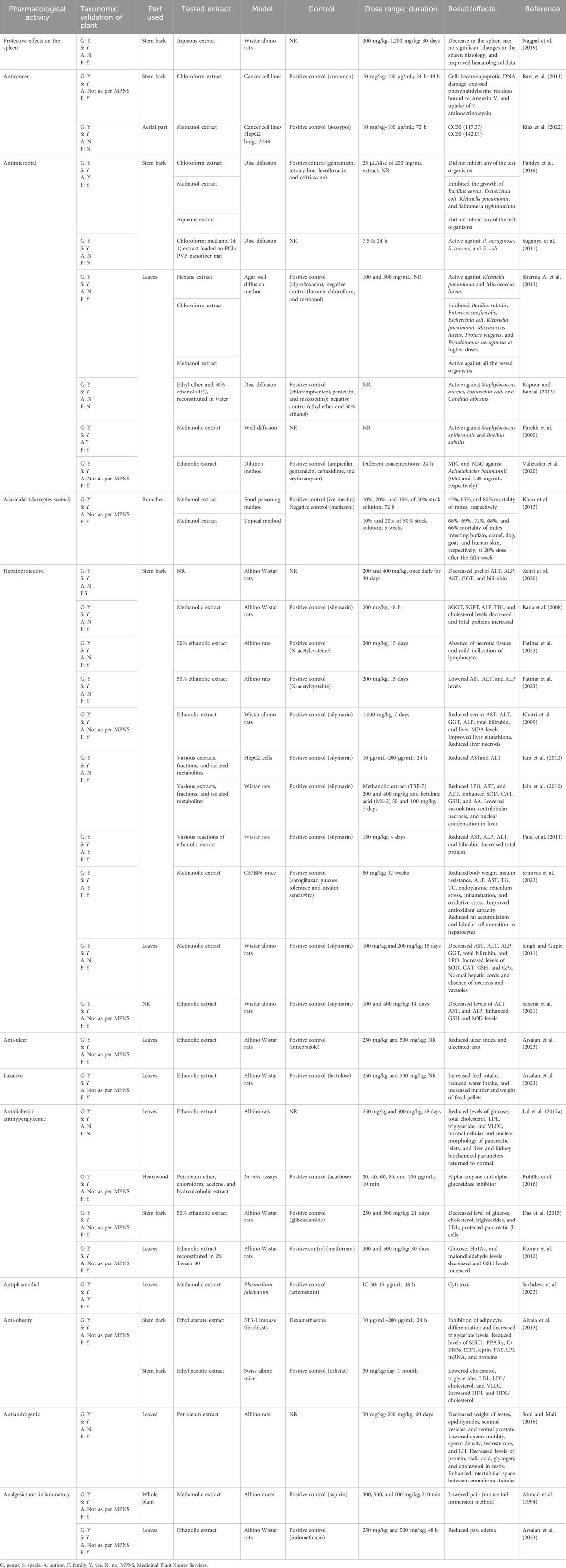
T. undulata has been reported to possess several bioactive metabolites. Fatty acids and their derivatives have been comprehensively explored, followed by quinones, phenolic acids and their derivatives, and alkaloids (Figure 3). The details of the individual metabolites with their bioactivities are presented in Table 3, and the structure of the metabolites is provided in Supplementary Figure S1.
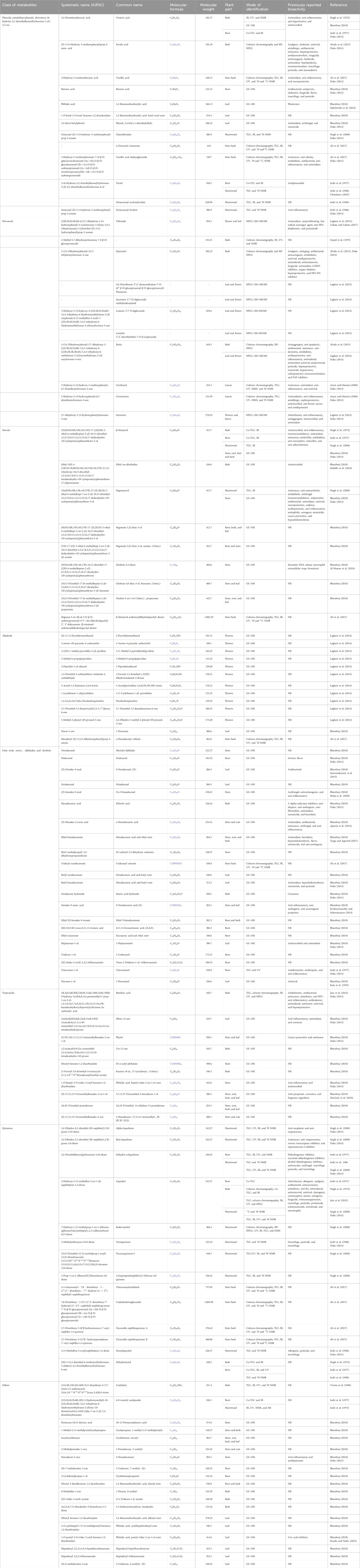
Table 3. Metabolites present in T. undulata (systematic names, molecular formula, and weight have been retrieved from pubchem.ncbi.nlm.nih.gov).
4.1 Phenolics and flavonoids
Phenolic metabolites and flavonoids show potent antioxidant activity by scavenging ROS and free radicals through enzyme inhibition, metal chelation, and hydrogen donation. These metabolites regulate the metabolism, inflammation, and immune responses and are also utilized in the treatment of diabetes, cardiovascular disorders, and viral infections (Yao et al., 2024).
Various metabolites belonging to different groups, including phenolics (vanillic acid and its derivatives, n-eicosanyl cinnamate), were isolated from the methanolic extract of the stem bark using column chromatography followed by thin-layer chromatography. The study used triple chemical fingerprinting methods to characterize the isolated metabolites (Ali et al., 2017). Alkaloid extract, when analyzed by GC–MS, yielded several phenolic metabolites, including veratric acid, benzoic acid, and phthalic acid, from different plant parts (Bhardwaj, 2018). In another study, the root was successively extracted with petroleum ether and acetone. The former extract yielded acidic and neutral metabolites. The latter showed the presence of tectol. Joshi et al. (1986) further identified tectol and octacosanyl ferulates in the heartwood using the above methodology. On extracting the ether-soluble fraction of the stem bark with sodium carbonate, further acidification showed the presence of veratric acid using TLC and other spectral techniques (Singh et al., 1972). Different extraction techniques were employed to isolate flavonoids from the hydroethanolic extracts of T. undulata flowers and leaves using HPLC–ESI–MS/MS. In all the techniques, leaves had more content of individual flavonoids except 5,6-dimethyoxy-3′,4′-dioxymethylene-7-O-(6″-β-D-glucopyranosyl-β-D-glucopyranosyl) flavanone, which had less content in leaves when extracted through Soxhlet, marination, sonication, and reflux. The highest content of rutin was found to be in flowers (28.1%) and leaves (28.2%). The lowest content was that of luteolin glucoside in leaves processed through marination (Laghari et al., 2013). Among the various extraction techniques, microwave-assisted extraction (MAE) yielded the highest flavonoid content in the shortest time. The MAE technique significantly reduces energy consumption, solvent usage, and processing time, making it a sustainable and environmentally friendly methodology. It should be preferably coupled with conventional extraction to enhance the extraction yield and purity (Alvi et al., 2022). Cirsimaritin and cirsilineol were reported in the petroleum ether extract of leaves separated through column chromatography (Azam and Ghanim, 2000). The ethyl acetate fraction of the stem bark was found to contain ferulic acid (4.95%), quercetin (0.72%), and rutin (0.18%), all of which have been shown to have anti-obesity activity (Alvala et al., 2013). Quercetin was also identified in the stem bark, having antiproliferative activity (Ravi et al., 2011).
4.2 Steroids
The perhydrocyclopentanophenanthrene nucleus forms the basic skeleton of steroid molecules. Numerous types of steroids exist due to variations in this fundamental skeleton, and the attachment of different functional groups results in structural diversity and biological activities among steroids (Atanasov et al., 2021).
The petroleum ether extract of heartwood (3 kg) was dissolved in ethyl acetate and extracted with sodium carbonate (Bhardwaj, 2018). The sodium carbonate-insoluble fraction yielded stigmasterol (1 g) and β-sitosterol (1.5 g). Sitosterol was commonly observed in the bark (Singh et al., 1972) and root (Joshi et al., 1977) following an almost similar extraction procedure. Steroidal arabinosyl diester was characterized from the methanolic extract of the stem bark using column chromatography with petroleum ether and chloroform in equal proportion, followed by TLC for further purification (Ali et al., 2017). Stigmasterol is utilized in the synthesis of semi-synthetic and synthetic pharmaceutical compounds. It demonstrates a broad spectrum of pharmacological effects. Similarly, β-sitosterol, a common dietary phytosterol, is found to inhibit tumor metastasis by enhancing gut immunity and also contributes to blood sugar regulation, immunomodulation, reproductive protection, and fever reduction (Rani et al., 2025).
4.3 Fatty acids, fatty esters, fatty aldehyde, and fatty alcohols
Ethyl hexadecanoate was commonly identified in the stem, root, and bark, with the highest content found in the stem bark. The GC–MS data revealed the maximum diversity in terms of the presence of fatty acids and their esters in various plant parts that were tested (Bhardwaj, 2018). Out of the five identified fatty aldehydes, the maximum content was found to be of cis-9-hexadecenal in the stem, and the minimum content was of octadecanal in the leaves. Two fatty alcohols, viz., 1-undecanol and trans-2-dodecen-1-ol, trifluoroacetate, were reported in the roots with peak areas of 2.38% and 3.71%, respectively. Triacontanol was reported by Joshi et al. (1977) in the roots of the plant. There are some limitations to the analysis of plant metabolites using GC–MS as it can only separate volatile metabolites, which are typically of low molecular weight. Non-volatile and polar metabolites should preferably be derivatized before analysis. Moreover, for proper chemical fingerprinting, it is always suggested to identify the metabolites using multiple spectral and chromatographic techniques (Heinrich et al., 2022).
4.4 Alkaloids
Alkaloids are nitrogen-containing bioactive substances that are promising candidates for drug development due to their significant biological and structural activity. These metabolites possess diverse therapeutic potential and are used in the treatment of cancer, inflammation, malaria, hypertension, diabetes, etc. (Rajput et al., 2022).
The flower (20 g) was processed to obtain an alkaloid fraction (0.5 g), which was then analyzed using GC–MS. During extraction, n-hexane was used instead of chloroform, which facilitated the removal of fatty metabolites and other interfering metabolites. Derivatization of the alkaloid fraction is a standard process used to achieve better results with GC–MS as it enhances the volatility, detection, and separation efficiency by chemically altering the functional groups on the original molecule (Wang et al., 2025). However, in this study, derivatization was not included. Almost 50% of the alkaloids were present in the fraction, and they were structurally diverse, comprising aromatic, cyclic, and bicyclic compounds, which is quite rare (Laghari et al., 2014). Out of 11 alkaloids identified, the largest peak area was observed for 4-formyl-1,3-dihydro-1,3-dimethyl-2H-imidazole-2-thione (16.63%), while the smallest area was for 1-piperidineethanol (1.44%). In leaves and stems, 1-docosene (Bhardwaj, 2018) and n-hexadecanyl caffeate, respectively, have also been reported (Ali et al., 2017). However, some alkaloids also have side effects/toxicity on human health (Rajput et al., 2022); thus, the use of plant-based medicines rich in alkaloids needs to undergo meticulous safety assessments.
4.5 Terpenoids
The defatted stem bark powder was extracted with methanol, leading to the isolation of betulinic acid, which was further purified (98%) using column chromatography and preparative TLC (Jain et al., 2012). Betulinic acid is a lupane-type pentacyclic triterpene having several bioactivities, including antidiabetic, anticancer, diuretic, antiviral, and immunomodulatory activities (Oliveira-Costa et al., 2022). Bhardwaj (2018) reported the presence of terpenoids in the alkaloid-rich fraction of plant parts. 3,7,11,15-Tetramethyl-2-hexadecen-1-ol and 2,6,10-trimethyl,14-ethylene-14-pentadecane were found to be present in all the plant parts. This study, compared to the work of Laghari et al. (2014), clearly demonstrates the importance of selecting and processing samples appropriately to obtain a fraction rich in the metabolite of interest. Moreover, the inclusion/modification of steps that remove most of the interfering molecules becomes crucial. Both studies aimed to identify alkaloids; however, Bhardwaj et al. (2014) found the presence of very few alkaloids in the alkaloid-rich fraction.
4.6 Quinones
The petroleum ether extract of the heartwood (3 kg) showed the presence of seven quinones. Radermachol (70 mg; rare pigment) and 2-isopropenylnaphtho [2,3-b]furan-4,9-quinone (30 mg) were reported for the first time in the genus Tecomella (Singh et al., 2008). Other naphthoquinone derivatives were also reported. Naphthoquinone derivatives (Tecomella naphthoquinone A and Tecomella naphthoquinone B) were reported for the first time in the stem bark of the plant by Ali et al. (2017). Dehydrotectol was reported in the bark (Singh et al., 1972) and root (Joshi et al., 1977; Joshi et al., 1986) of the plant. Lapachol is another quinone commonly found in the root, bark, and heartwood (Table 3). This metabolite has been reported to be toxic to monkeys (Willard and Murray, 2020).
4.7 Other metabolites
Undulatin, an iridoid glucoside, was identified in the stem bark of the plant. The defatted powdered sample was extracted with ethanol, followed by ethyl acetate, to yield undulatin (50 mg). The metabolite was characterized using IR, UV, and 1H NMR spectroscopy (Verma et al., 1986). Another iridoid glucoside, 6-0-veratryl catalposide, was isolated from the ether-insoluble acetone extract of the heartwood (Joshi et al., 1975) and the root of the plant (Joshi et al., 1977). Undecanyl stearate was identified from the methanolic extract of the stem bark with a 0.034% yield. Using GC–MS, Bhardwaj (2018) identified diacetylene (stem), cyclopropane derivatives (stem and bark), alkene (stem and root), ketone (stem), primary alcohol (root), phthalate esters (root, bark, and leaves), alkene (bark), anhydride (bark), fluoroalkyl (leaves), and esters (leaves).
5 Bioactivities
T. undulata has traditionally been used by indigenous healers and herbalists to treat diseases in humans and animals. The scientific validation of traditional wisdom and experiences has often highlighted the mechanisms and modes of action of plants or their extracts and confirmed the effectiveness of bioactive products. The various pharmacological activities exhibited by different parts of the plant, along with their reported effects, are listed in Table 4.
5.1 Hepatoprotective activity
T. undulata is reported to have hepatoprotective activity against isoniazid-induced liver damage. The stem bark extract significantly lowered the elevated levels of AST (aspartate aminotransferase), ALT (alanine transaminase), ALP (alkaline phosphatase), GGT (gamma-glutamyl transferase), and bilirubin (Zehri et al., 2020). These enzymes are released due to the membrane damage of liver cells. Thus, the plant has a membrane-stabilizing effect. In a damaged state, the liver cannot properly release bilirubin through the bile. This may lead to its leakage into the blood. High levels of bilirubin may lead to jaundice (Bajaj et al., 2022). However, there is no clarity regarding the determination of the dose and the authentication of the plant samples. Additionally, the inclusion of histopathological studies and other markers of liver damage could have made the investigation more comprehensive. The methanolic extract of the plant reduced the levels of liver enzymes, including cholesterol, in the experimental model compared to those in the CCl4 group. However, the amount of protein and albumin increased. It is reported that damage to the ER leads to a loss of P540, resulting in lower protein synthesis. Moreover, CCl4 inhibits the synthesis of bile from cholesterol, resulting in its accumulation. Histological sections revealed that the cellular architecture improved following the administration of the plant extract (Rana et al., 2008).
In related studies, the ameliorative effect of T. undulata stem bark on acetaminophen-induced toxicity in rats was observed. Acetaminophen forms N-acetyl P benzoquinone (NAPQI), which is toxic. Overproduction of NAPQI generates free radicals, which damage mitochondrial DNA and increase membrane permeability, thereby adversely affecting hepatocytes. The bark extract improved liver histology, which was characterized by a reduced presence of inflammatory cells (Fatima et al., 2022), and lowered hepatic enzyme levels (Fatima et al., 2023). The leaves of the plant showed protective effects against alcohol-induced hepatotoxicity. The occurrence of liver marker enzymes decreased in the serum, and the liver GSH (glutathione), GPx (glutathione peroxidase), and SOD (superoxide dismutase) levels increased. Moreover, lipid peroxidation was also reduced. Fatima et al. did not justify the need to present the related data (histopathological and liver marker enzymes) in separate articles, which is questionable. Similar effects of the leaf extract were observed in rats with paracetamol-induced liver damage (Singh and Gupta, 2011). The extract may possess antioxidants that minimize lipid peroxidation of the membrane, and thus, the presence of marker enzymes is reduced in the serum. Alcohol decreases the production of antioxidant enzymes due to the adverse effects of free radicals or due to the production of acetaldehyde as a result of alcohol oxidation (Das et al., 2005). Jain et al. (2012) evaluated the hepatoprotective activity of the plant’s stem bark on HepG2 cells and rats and concluded that this may be due to the presence of betulinic acid (triterpenoid). TSB-2 fraction showed the highest degree of cytotoxicity, which may be due to the presence of lapachol (Almeid, 2009). TSB-7 fraction (containing betulinic acid) showed lower cytotoxicity and was assessed in an animal model. Betulinic acid is reported to be cytotoxic against several animal cell lines (Eichenmüller et al., 2009); however, HepG2 cells were less adversely affected, which may be due to the expression of survivin and Bcl2 (survival factors). The positive effect of the plant extract on liver marker enzymes is indicative of reduced liver damage and a membrane-stabilizing effect.
Non-alcoholic fatty liver disease (NAFLD) is a primary concern globally, which is caused by poor eating habits, a sedentary lifestyle, and obesity. It mainly leads to non-alcoholic steatohepatitis (NASH). Mice were fed with a Western diet sugar water (WDSW), leading to nonalcoholic steatohepatitis. In a preclinical study, the stem bark showed a positive effect on liver marker enzymes, total cholesterol levels, triglyceride levels, and insulin resistance (Srinivas et al., 2023). The mouse model mimics the effects of diet and the incidence and progression of disease in humans. The dose of the experiment was determined according to guidelines established by the US Food and Drug Administration. A pharmacopoeia evaluation of the plant material was conducted to assess its pharmaceutical quality. Overall, important factors such as the dose, mode of administration, timing of the intervention, extent of exposure, and endpoint assessments were meticulously selected to evaluate the effect of Tecomella in treating NASH. Oxidative stress, inflammation, and ER stress were found to be reduced, which was nearly equivalent to that of saroglitazar. Downregulation of ER stress markers [C/EBP homologous protein (CHOP), 78-kDa glucose-regulated protein (Grp78), and unfolded protein response (UPR)] was observed. The reduction in inflammation was primarily due to a decrease in pro-inflammatory markers, specifically tumor necrosis factor α (TNF-α) and interleukin-1β (IL-1β). The expression of c-Jun N-terminal kinase (JNK) and extracellular signal-regulated protein kinase (ERK1/2), which are markers of inflammation and steatosis, was observed (Urano et al., 2000). Thus, a reduction in oxidative and ER stress could have lowered the levels of cholesterol and lipids in the liver.
5.2 Analgesic activity
T. undulata exhibits notable pain-relieving properties. In a study, the whole plant was processed to obtain an extract using absolute methanol. Significant analgesic activity was observed, as assessed by the hot-water tail immersion test in mice. However, the results were not dose-dependent. The extract was not able to exert significant anti-inflammatory activity on paw edema induced by carrageenan (Ahamad et al., 1994). Carrageenan, as an irritant substance, is used to cause edema. It induces the secretion of cytokinins under the influence of bradykinin (Vats et al., 2024). The variation in results may be due to the presence and concentration of bioactive metabolite/s and pharmacokinetic variations. Since the methanolic extract was administered to animal models, it becomes imperative to include a negative control, which was not mentioned in the study.
Arsalan et al. (2023) highlighted the anti-inflammatory potential of T. undulata leaves. The study revealed that the plant extract was effective in both the initial and later phases of edema (Akinloye et al., 2020; Zahra et al., 2020). This may be due to the presence of phenolic metabolites in the ethanolic extract, which might have worked synergistically and antagonistically with anti- and pro-inflammatory markers. The study was carried out on formalin- and carrageenan-induced paw edema in rats. However, the study is too preliminary, and further molecular and biochemical studies are needed to establish the efficacy of the plant. However, the paw edema test is a very preliminary study and does not conclusively establish the bioactivity of the plant extract.
5.3 Anticancer/antimutagenic activity
Ravi et al. (2011) studied the anti-proliferative activity of the plant bark against cancer cell lines with a promising IC50 value (30 µg/mL) in K562 cells. The cell line exhibited characteristic features of apoptotic cells, including membrane blebs, cell shrinkage, and DNA damage. Moreover, phosphatidylserine (PS) residues were bound to Annexin V, which enhanced the uptake of 7-aminoactinomycin (7-AA). In normal cells, PS is present on the inner surface of the membrane; however, during early apoptosis, it becomes exposed on the cell surface. Annexin V is a phospholipid-binding protein with high affinity to PS (Robinson et al., 2020). On the other hand, uptake of 7-AA signifies a late apoptotic event (Wang T. et al., 2022). The chloroform extract showed the presence of a metabolite with an identical retention time to quercetin. Isolation, characterization, quantification, and evaluation of the antitumor potential of these metabolites may result in the identification of the lead target molecule.
Riaz et al. (2022) reported a bioassay-guided study of T. undulata, which showed significant cytotoxic, antimutagenic, and anticancer potential. The hexane extract had the greatest effect on the locomotion of Caenorhabditis elegans, while the methanolic extract had the least. Salmonella typhimurium strains TA98 and TA100 were modified for frame-shift and base-pair substitutions, respectively. The growth of these strains is inhibited in the absence of histidine in the culture medium. Plant extracts are tested in the presence of a mutagen to evaluate their antimutagenic potential, which is calculated according to the number of revertant colonies. The methanolic extract of Tecomella showed the highest antimutagenic potential, which may be due to the presence of flavonoids and other phenolic metabolites in the extract. Thus, the plant showed potential to prevent or inhibit the carcinogenic effect of mutagens (De Silva and Alcorn, 2019).
An MTT assay was performed to assess the cellular toxicity. The methanolic extract showed good activity against HepG2 tumor cell lines (68.17%). A significant difference in the inhibition of HepG2 cell lines was observed when comparing the data of the chloroform (Ravi et al., 2011) and dichloromethane extracts (Riaz et al., 2022) of the plant. These two solvents differ slightly in their polarity index, and the difference in their activity may be due to the time of collection and geographical location, which affect the concentrations of plant metabolites (Heinrich et al., 2022). The resazurin assay (simple, rapid, and sensitive) was performed on various cancer cell lines. The methanolic extract was found to be significantly effective on liver HepG2 and lung A549 cell lines, with CC50 values of 117.37 and 142.01 µg/mL, respectively. Resazurin, also known as Alamar blue, is an indicator dye used to measure cell viability. The extract was found to possess a decent selectivity index when its cytotoxicity on cancer cells was compared with that on normal cells. Such an extract or metabolite is considered suitable for further anticancer studies. The metabolites present in the extract also showed potential against cancer-related proteins, as revealed through docking studies.
5.4 Antidiabetic activity
The plant was reported to have a mild blood glucose-lowering effect in an acute study. The effect was quite significant in a chronic study wherein the ethanolic leaf extract was administered for 30 days. Moreover, glucose tolerance improved in streptozotocin-treated rats (Kumar et al., 2012). This may be due to the increased secretion of insulin from pancreatic cells or the peripheral utilization of glucose. In the long term, diabetes glycosylation of proteins, including hemoglobin, is observed. A high level of glycosylated hemoglobin (HbA1c) is a marker for poor glycemic control and is associated with diabetes-related disorders (Lau and Aw, 2020). The plant extract helped bring the enhanced levels of HbA1c to nearly normal levels. Insulin activates glycogen synthase to form more glycogen (Norton et al., 2022). Hepatic glycogen was found to increase after the treatment with the plant extract. This may be attributed to the reactivation of glycogen synthase in test animals. Lipid peroxidation was observed to be reduced, as evidenced by the lower levels of malondialdehyde in diabetic rats treated with T. undulata. Additionally, GSH levels increased in streptozotocin-induced diabetic rats. The data projects the antioxidant potential of the plant. It is known that oxidative stress occurs in diabetes. Glycosylation of proteins can lead to the generation of reactive oxygen species (ROS) (Gupta et al., 1997). In individuals with diabetes, glucose is channeled toward a pathway that requires NADPH. GSH reductase forms reduced glutathione, involving NADPH. Thus, diabetes leads to GSH depletion and enhances oxidative stress (Sha et al., 2021). However, the study authors did not highlight dose determination and toxicity analyses, which are essential in animal studies using plant extracts.
In a study conducted by Das et al. (2015), the hydroethanolic extract of the heartwood of T. undulata lowered blood glucose levels and serum triglycerides, total cholesterol, and low-density lipoproteins and increased high-density lipoproteins. Increased free fatty acids in diabetes are associated with decreased glucose tolerance and impaired β-cell function (Wismayer et al., 2023). Bioactives present in the plant extract may have partially reversed the damage caused to the pancreas by streptozotocin (Papuc et al., 2021), as evidenced by the histopathological study of the pancreas. A similar effect of the hydroethanolic extract was reported in streptozotocin-induced diabetic rats using the leaves (Lal et al., 2017a) and roots (Lal et al., 2017b) of the plant. Tecomella extract induced the rearrangement of peripheral tissue and the normalcy of islets. The biochemical studies also supported the plant’s antidiabetic efficacy. However, the mechanism of action and the identification of lead antidiabetic metabolites still need to be explored.
5.5 Antioxidant activity
Free radicals are byproducts of cellular metabolism. However, if their production exceeds the cell’s neutralization capacity, it leads to oxidative stress (Masenga et al., 2023). Thus, external supplementation with antioxidants is recommended, and the use of plant-based products is a matter of personal choice (Vats, 2016; Baroni et al., 2021). Bhardwaj et al. (2014) analyzed the methanolic extract of different plant parts of T. undulata for its antioxidant activity. The total phenolic content was found to be the highest in the stem (12.70 GAE/g DW). Meanwhile, the maximum flavonoid content was observed in leaves (71.87 mg QE/g DW). The best ferric reducing antioxidant power was observed in leaves (96.66 mM/L/g). The best antioxidant potential with respect to the ABTS assay was shown by the leaves. The results of the antioxidant assays are in accordance with the occurrence of the highest total flavonoid content in leaves. These in vitro antioxidant assays only define the chemical profile of a preparation and require further evaluation through pharmacological experiments to validate its antioxidant efficacy.
5.6 Antimicrobial activity
The methanolic extract of leaves showed better activity than the aqueous extract against Staphylococcus epidermidis and Bacillus subtilis (Parekh et al., 2005). This clearly reveals the importance of solvent selection in extracting active metabolites against a specific microbe (Nortjie et al., 2022). In another study, Sharma A. et al. (2013) reported that, among the different solvents tested, the methanolic extract exhibited the best antimicrobial potential. The least MIC (0.01 mg/mL) was observed against Klebsiella pneumoniae, and the highest MIC (4 mg/mL) was observed against B. subtilis. The antimicrobial potential of the methanolic extract of the stem bark was found to be better than that of the chloroform and aqueous extracts, as reported by Pandya et al. (2019). It is essential to note that the former study did not demonstrate any positive effect against Salmonella typhi; however, the latter study was found to be effective against the test organism of typhoid. The difference in the results may be due to the solubility of different bioactive metabolites in both solvents. Additionally, different plant parts may vary in terms of the type and concentration of metabolites. Moreover, the better diffusion of the methanolic extract in the microbial medium may be another reason for the differential activity (Parekh et al., 2005). Suganya et al. (2011) reported the antibacterial potential of PCL/PVP (polycaprolactone/polyvinylpyrrolidone) mats loaded with T. undulata extract. The medicated fibers remained stable even after high electrical voltage. The study highlighted the use of mats as both a drug carrier and a wound dressing material loaded with antimicrobials.
5.7 Miticidal/acaricidal/antiplasmodial activity
Khan et al. (2013) observed the maximum acaricidal activity of the methanolic extract in goats and camels, followed by humans. However, the results were better in the in vitro assay, wherein the plant extract (30%) showed 80% mortality. The miticidal activity may be due to the presence of lapachol, which interferes with cellular respiration and the generation of free radicals (Rahman et al., 2022). However, the actual molecular mechanism involved in the acaricidal activity needs to be further explored. Antiplasmodial activity of 17 plants was studied by Sachdeva et al. (2023). It was observed that T. undulata was the most effective with IC50 values of 15 and 15.3 µg/mL against Plasmodium falciparum 3D7 and P. falciparum INDO, respectively. Some metabolites, such as quercetin, rutin, ursolic acid, lapachol, and betulinic acid (Table 4), may be responsible for the antimalarial activity of the plant extract.
5.8 Anti-obesity activity
Obesity is prevalent in most parts of the world. Obesity is associated with the incidence of other disorders, viz., diabetes, cardiac disorders, and cancer (Chen et al., 2020). It has been reported that during weight loss, there is mainly a reduction in the volume of adipocytes rather than a reduction in their number (Spalding et al., 2008). The expansion of adipose tissue through hyperplasia is quite challenging to reverse as adipocytes are resistant to apoptosis. Thus, anti-obesity drugs essentially should target hyperplasia. Alvala et al. (2013) sequentially extracted the bark of T. undulata and further fractionated the ethyl acetate fraction. Fraction 1 (F1) significantly inhibited the division and accumulation of triglycerides in adipocytes (3T3-L1 mouse fibroblast cells). This was achieved through the activation of Sirtuin 1 (SIRT1) mRNA and proteins. In addition, downregulation of peroxisome proliferator-activated receptor gamma (PPARγ) and CCAAT/enhancer-binding protein alpha (C/EBPα) was observed. A reduction in the mRNA expression of E2F1, leptin, FAS, and LPL was also observed. On the other hand, levels of adiponectin increased. SIRT1 is known to regulate transcription factors, which affect the expression of PPARγ, C/EBPα, and leptin, among others, which in turn regulate fat metabolism. PPARγ and C/EBPα stimulate adipocyte-specific genes and regulate adipocyte differentiation. LPL expression is a marker of lipid accumulation in adipocytes. FAS enzyme catalyzes the synthesis of long-chain fatty acids, and it is upregulated during adipogenesis (Ortega et al., 2010). E2Fs regulate adipogenesis through changes in the expression of the nuclear receptor PPARγ (Chen et al., 2020). Leptin is a hormone that plays a significant role in energy balance (Tucker et al., 2024). Adiponectin is a hormone derived from fat that has been shown to affect obesity negatively (Maeda et al., 2020). Thus, agents that regulate SIRT1 activity can be essential candidates for treating obesity and its related disorders. An in vivo study supported the data obtained in cell lines. A significant decrease in cholesterol, triglycerides, LDL, LDL/cholesterol, and VLDL was noticed. Additionally, an increase in HDL and HDL/cholesterol was observed in mice treated with the plant extract compared to obese mice fed a high-fat diet. The F1 was found to be rich in ferulic acid (4.95%), and the metabolite has been reported to possess anti-obesity properties (Wang O. et al., 2022). However, the study did not specify how the dose administered to mice was determined.
5.9 Other activities
A stomach ulcer was induced in rats using ethanol, and the ulcer index was found to be 7, with an ulcerated area of 1.10 cm2–0.3 cm2 (Arsalan et al., 2023). T. undulata extract significantly reduced ulcer index (2) and ulcerated area (0.3 cm2–0.1 cm2). Ethanol-induced gastric ulcers cause a decline in bicarbonate secretion and a reduction in the mucus present in the gastric wall (Ibrahim et al., 2022). Ethanol induces the production of free radicals, such as the superoxide anion and hydroxyl radical, and enhances lipid peroxidation, leading to the impairment of the stomach mucosa (Ibrahim et al., 2022). Thus, the plant extract may have reduced oxidative stress, leading to decreased capillary injury, vascular permeability, and the production of inflammatory markers (Akmal et al., 2023). Furthermore, constipation was induced in rats using loperamide (Arsalan et al., 2023), which reduces peristaltic movement in the intestine, including water secretion (Parkar et al., 2024). The plant extract increased the weight and number of fecal matter, suggesting improved colon movement (Katsirma et al., 2021). This may be due to the presence of glycosides, which possess laxative activity, and their presence was also reported in the study.
The plant was projected as an antifertility agent by Soni and Mali (2016). Administration of T. undulata extract resulted in a 70% reduction in the weight of male reproductive organs, accompanied by a decrease in fertility rate. The sialic acid content, which facilitates the seamless movement of sperm, was found to be reduced. This may have affected the acrosomal membrane and the fertilization ability of sperm (Aslan Çetin et al., 2022). Reduced levels of luteinizing hormone and testosterone were observed. LH induces the production of testosterone. Testosterone plays a crucial role in spermatogenesis (Oduwole et al., 2021). The analysis of the phytochemicals present in the extract and the determination of the dose were not mentioned by the authors.
The ameliorative effect of T. undulata on CdCl2 (cadmium chloride)-induced splenomegaly was investigated by Nagpal et al. (2019). In rats treated with the plant leaf extract, the size of the spleen was comparable to that of the control group. The complete bold count suggested a positive effect of the plant’s extract. The hemoglobin, red blood cell, platelet, and packed cell volume data were found to be almost equal to those of the control group and better than those of the CdCl2-treated group. The histological studies revealed that the spleen of rats administered with CdCl2 showed necrosis, hyperplasia, swollen and dead cells in the pulp, and sinus congestion. These features were improved in the group treated with T. undulata. In the above-studied parameters, a lower dose of the plant’s extract did not prove to be effective; however, a higher dose (>600 mg/kg/day) showed promising results.
Botanical extracts, in general, are complex, and seasonal/geographical differences significantly affect the concentrations of individual metabolites, thereby affecting their efficacy. These changes are also due to the use of agrochemicals during cultivation and other variables affecting plant growth (Heinrich et al., 2022). However, none of the above-mentioned studies have provided these details.
6 Commercial uses
A constant surge in the demand for medicinal and aromatic plants (MAPs) has been observed globally over the past decade. Recent data suggest that the market value of MAPs was estimated to be $201 billion in 2023 and is expected to increase to $375.6 billion by 2032, with a significant compound annual growth rate (CAGR) of 7.22%. China contributed the most (22.98%) to the total worldwide exports, followed by India (10.54%). This clearly demonstrates the commercial relevance and growing interest in natural products among the general public (Zamani et al., 2025).
The bark of Rohida is extensively used in the preparation of various Ayurvedic formulations, including Rohitaka Ghrita, Rohitaka Loha, Rohitaka Rishta, and Rohitaka Dyachoorna (Jain et al., 2012). Rohitaka Rishta is effective in treating the liver, spleen, stomach, and skin disorders (Ullah et al., 2010). In addition, the wood of desert teak is commercially very important. It is a useful building material mainly due to its strength and durability. Thus, it is commonly used in the manufacture of furniture, such as cabinets, doors, and window frames (Kalia et al., 2014). One of the metabolites, vanillic acid, which was identified in the plant, is an oxidized form of vanillin and is used as a flavoring agent in the food industry. It is also used in the production of vanillin and the synthesis of different pharmaceutical agents. The production rate of vanillic acid has increased significantly due to the gradual increase in its demand (Kaur et al., 2022), with an estimated average growth rate of more than 1.5% (2012–2016). Moreover, the plant is utilized as an essential component in botanical formulations, which are patented and exhibit various therapeutic properties, including immune-boosting, antioxidant, anti-aging, anticancer, and antiviral effects (Table 5).
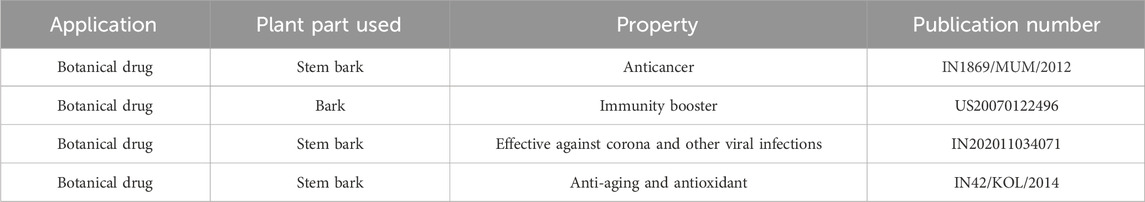
Table 5. Patents related to T. Undulata and their claimed properties (Google patents and https://www.wipo.int/patentscope/en/).
7 Toxicity
Cellular toxicity of the ethyl acetate fraction of T. undulata stem bark was assessed on 3T3-L1 mouse fibroblasts. The extract was found to be non-toxic even at a concentration of 200 µg/mL (Alvala et al., 2013). The petroleum ether extract (250 µg/mL) of the stem bark showed less than 50% viability of HepG2 cells (Jain et al., 2012); however, the methanolic extract and its fractions were found to be more viable (65%). The methanolic extract of the leaves was non-toxic to HEK293 mammalian cell lines at a concentration of 200 µg/mL (Sachdeva et al., 2023). The ethanolic extract (50%) of the plant did not cause toxicity, even at a dose of 2,000 mg/kg body weight in Wistar albino rats (Das et al., 2015). The methanolic extract and one of its metabolites, MS-2, did not exhibit toxicity at doses of 5,000 mg/kg and 1,000 mg/kg body weight, respectively (Jain et al., 2012). The aqueous extract was found to be safe up to a dose of 2,000 mg/kg body weight; however, it was toxic at doses of 4,000 and 8,000 mg/kg body weight (Nagpal et al., 2019). The methanolic extract of the bark was found to be safe up to 2,000 mg/kg body weight in terms of acute toxicity (Rana et al., 2008). Saxena et al. (2021) reported that the ethanolic extract of the bark did not exhibit toxicity up to a dose of 4,000 mg/kg body weight. One of the identified metabolites in the plant, lapachol, possesses anti-vitamin K activity. Long-term administration of lapachol (0.0625 g/kg/day–0.25 g/kg/day) in monkeys caused anemia (Willard and Murray, 2020). It is reported to induce reproductive toxicity affecting the seminal vesicle in male Wistar rats (De Cássia da Silveira e Sá and de Oliveira Guerra, 2007). Although generally safe at low concentrations, benzoic acid exhibits dose-dependent toxicities, including dermatological irritation, hypersensitivity reactions, gastrointestinal disturbances, and metabolic acidosis. At higher exposures, it may cause glycine depletion, neurological impairment, and bilirubin displacement, leading to neonatal encephalopathy (Issa and Mohammed, 2025). β-Sitosterol is generally considered safe in healthy individuals, but it may cause mild gastrointestinal effects or reduced absorption of fat-soluble vitamins at high doses (Paniagua-Pérez et al., 2005).
Even if preliminary studies on toxicity reveal that the plant is less toxic, further comprehensive studies involving animals and clinical trials are needed to ascertain the safety of T. undulata as a medicinal agent in healthcare. Additionally, it is worth noting that a metabolite may exhibit different activity/toxicity when administered individually or in combination with other metabolites (extract).
8 Micropropagation
Endangered medicinal plants typically exhibit slow growth, narrow distribution, low fruiting, poor seed development, and challenging germination. Their survival is further threatened by overharvesting and ecological changes, which hinder both slow natural regeneration and artificial reproduction. Therefore, ex vivo propagation is vital. Micropropagation is an important technique as it enables the rapid and large-scale production of genetically uniform, disease-free plantlets, supporting both the conservation and sustainable use of endangered medicinal species (Zheng et al., 2023).
T. undulata is commercially very important due to its medicinal and timber value. However, the sluggish growth and excessive cutting of this tree for commercial purposes have made it endangered. Cross-pollination has resulted in significant variability (Rathore et al., 1991) in the plant; therefore, clonal propagation of selected germplasm is desirable for conservation and for its valuable timber and medicinal properties. An overview of tissue culture studies on T. undulata is presented in Table 6.
8.1 Shooting
Explants collected in August and September showed the best response and the highest number of shoot inductions. The maximum number of shoots was observed in MS + BAP (5 mg/mL) + IAA (0.05 mg/mL); however, the shoots were very short in length. This shows that higher concentrations of BAP inhibited the adequate differentiation of shoots (Rathore et al., 1991). It was observed that the response was better at 31 °C, possibly due to the physiological nature of the plant, which propagates optimally in a semi-arid environment. Kinetin-induced leafy shoots were not found to be better for further micropropagation steps. Furthermore, the sub-culturing of the shoots was performed on a medium with lower concentrations of auxins to inhibit callusing (Vats and Kamal, 2013; 2014; Vats, 2018). Aghdaei et al. (2012) investigated the impact of silver nanoparticles on plant culture. Nanoparticles, when used alone, showed a mild response in terms of the induction of the number of shoots (<2). In combination with hormones (BAP + IAA), the response was even poorer. When nanoparticles were used in combination with thidiazuron, the response was found to be slightly better with two shoots per explant. Thus, silver nanoparticles had no significant effect on shoot induction. This may be due to seasonal variations in terms of explant collection, region of collection, and the hormones used (Cheng et al., 2024). Aslam et al. (2006) observed a positive effect of IAA and BAP on shoot induction from cotyledonary nodes. Better shoot induction was observed when IAA and BAP were used in combination, with a superior percentage of explant response. A higher concentration of cytokinin than auxin generally promotes shoot growth (Wu et al., 2022). Chhajer and Kalia (2017) used Schenk and Hilderbrandt medium supplemented with BAP, KN, and ascorbic acid for multiple shootings. Antioxidants have been used in the medium to avoid the production of phenolic metabolites in the culture and leaching in the medium (Vats, 2018).
8.2 Rooting
Rooting from shoots was induced using a liquid medium supplemented with IBA for 2 days, and then the plants were transferred to half-strength MS medium (Rathore et al., 1991). The survival rate after acclimatization was evaluated to be 46%, which is comparatively lower. Aslam et al. (2006) and Aslam et al. (2009) reported a stepwise process for efficient rooting, which involved transferring in vitro shoots to a liquid medium containing different concentrations of NAA, IAA, and IBA, either alone or in combination, for 36 h, followed by half-strength MS medium without hormones. Many workers suggest that the two-step method reduces the number of days needed for root initiation and yields a better average number of shoots (Rathore et al., 1991; Bhansali, 1993). In vitro-generated shoots were cultured on Schenk and Hildebrandt (SH) medium supplemented with IBA and ascorbic acid for rooting. According to Tyagi and Tomar (2013), the most suitable medium for in vitro rooting was ½ B5 medium, possibly due to its lower content of ammonium nitrate and potassium nitrate than that of the MS medium. The addition of ascorbic acid improved the rooting because it acts as an antioxidant, which minimizes phenolic production involved in retarding the growth of cultures.
8.3 Callus
Indirect micropropagation was carried out using seedlings and nodal explants from the tree. Callus obtained from explants of the tree was hard, compact, and green; however, it was fragile and light brown when seedlings were used as explants (Nandwani et al., 1996). NAA, in combination with kinetin, had no effect on shoot induction in callus generated from both explants; however, IAA and BAP proved to be effective in this regard. Better shooting was observed in the seedling-derived callus. The two-step method was followed for root induction. Instead of supplementing hormones in agar containing MS medium, pretreatment was carried out in a hormone-containing liquid medium, and thereafter, the cells were transferred to ½ MS medium. Auxins (NAA, IBA, and IAA) were used either alone or in combination, and the best response was observed when all three hormones were used in combination at a concentration of 5 ppm. As mentioned earlier, Rathore et al. (1991) also used a two-step method; however, the response was poor. Variation in the result may be due to the difference in genotype (Holmes et al., 2021).
9 Conclusion and future perspectives
The review summarizes the data on the botany, ethnopharmacology, phytochemicals, pharmacology, toxicity, and micropropagation of T. undulata. Traditionally, the plant is used to treat leucorrhea, sexual disorders, digestive disorders, liver disorders, and skin infections by the people of India and Pakistan. These activities are mainly attributed to the presence of various metabolites such as phenolic metabolites and their derivatives, flavonoids, steroids, alkaloids, terpenoids, fatty acids and their derivatives, and quinones. Certain bioactivities (hepatoprotective, antimicrobial, analgesic, antidiabetic, antioxidant, anti-obesity, acaricidal, and miticidal) have been partially validated scientifically, and a probable mode of action is provided in Figure 4. Since the plant is endangered, the review also focuses on the in vitro propagation techniques.
However, the following points require attention. The pharmacological activities explored in the plant are primarily focused on the stem bark, followed by the leaves. There is a dearth of reports on the bioactivities of the flowers, which have comprehensive traditional value in Pakistan. Some metabolites have been identified in T. undulata, but these studies may represent only a limited spectrum of the total metabolic profile. Phytochemical research primarily focuses on fatty acids, quinones, and phenolic acids, while studies on flavonoids, steroids, and terpenoids are relatively scarce. To substantiate the ethnomedicinal properties effectively, further animal and human clinical trials are required to determine the appropriate dose for treating various ailments. Bioassay-guided isolation of bioactive metabolites from different plant parts, pharmacokinetic investigations, computer-aided drug design, and elucidation of a potential mode of action are essential for exploring the therapeutic potential of key drug leads. The toxicity studies suggest that the plant is relatively safe; however, comprehensive chronic, sub-chronic, reproductive, and genotoxicity studies remain scarce. Investigating the pharmacodynamics and metabolic mechanisms will support the safe clinical use and development of more effective plant-based therapeutics.
The bioactive metabolites from the callus culture of T. undulata have not been identified yet, and elicitation strategies to enhance the production of secondary metabolites also remain unexplored. This will provide a comparatively sustainable approach to producing valuable metabolites.
In addition, meticulous analyses of the research articles included in the present review suggest that many studies conducted on the plant extract did not follow the guidelines of ConPhyMP (consensus-based reporting guidelines for the phytochemical characterization of medicinal plant extracts). Furthermore, studies should focus on validating the traditional uses of the plant through appropriate models (animal and cell cultures), determining the dose and selecting the most effective one, exploring the mechanism of action, and investigating various delivery systems, preferably based on nanotechnology, to ensure bioavailability and target specificity. For an unambiguous representation of data about the plant name, synonyms, identification, and distribution of plants, MPNS (The Medicinal Plant Names Services) must be referred to. These guidelines will help create an appropriate work plan, and the preclinical experimental data thus obtained can be effectively used/translated for safe and realistic clinical trials.
In summary, T. undulata has important ethnomedicinal and pharmacological activities. There are significant challenges related to the therapeutic application of this plant. Future studies should integrate proper taxonomic validation of the plant, mechanistic pharmacological studies, multi-omics platforms, and advanced chromatographic and spectral techniques for metabolite identification and characterization. This approach will add scientific value to the obtained data, which can further be used for clinical trials and global applications. The authors believe that this review will generate considerable interest within the scientific community and serve as a valuable reference for the future development and application of T. undulata.
Author contributions
SV: Conceptualization, Writing – original draft, Writing – review and editing. NiB: Data curation, Writing – original draft. SG: Writing – review and editing. MAM: Funding acquisition, Writing - review and editing, NaB: Funding acquisition, Writing - review and editing
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Large Research Project under grant number RGP2/264/46, King Khalid University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor J.E. declared a past co-authorship with the authors S.V.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1665446/full#supplementary-material
References
Agarwal, R., and Rijhwani, S. (2021). Diversity of economically useful wild plants of jhalana forest, jaipur. Int. J. Life Sci. Pharma. Res. 11 (1), 38–43. doi:10.22376/ijpbs/lpr.2021.11.1.L38-43
Aghdaei, M., Salehi, H., and Sarmast, M. K. (2012). Effects of silver nanoparticles on tecomella undulate (roxh.) seem. Micropropagation. Adv. Hortic. Sci. 26 (1), 21–24. doi:10.13128/ahs-12748
Ahmad, F., Khan, R. A., and Rasheed, S. (1994). Preliminary screening of methanolic extracts of Celastrus paniculatus and Tecomella undulata for analgesic and anti-inflammatory activities. J. Ethnopharmacol. 42 (3), 193–198. doi:10.1016/0378-8741(94)90085-X
Akinloye, O. A., Alagbe, O. A., Ugbaja, R. N., and Omotainse, S. O. (2020). Evaluation of the modulatory effects of Piper guineense leaves and seeds on egg albumin-induced inflammation in experimental rat models. J. Ethnopharmacol. 255, 112762. doi:10.1016/j.jep.2020.112762
Akmal, M. N., Abdel Aziz, I., and Nur Azlina, M. F. (2023). Piper sarmentosumRoxb. Methanolic extract prevents stress-induced gastric ulcer by modulating oxidative stress and inflammation. Front. Pharmacol. 13, 971443. doi:10.3389/fphar.2022.971443
Al-Hassan, J. M., Afzal, M., Oommen, S., Liu, Y. F., Khan, M., and Pace-Asciak, C. (2024). Oxidized cholesterol derivatives in fraction B prepared from gulf catfish (Arius bilineatus, Val.) skin regulate calcium response and neutrophil extracellular traps (NETs) formation. Biomedicines 12 (7), 1380. doi:10.3390/biomedicines12071380
Ali, M., Abra, H. H., Sultana, and S., and Mir, S. R. (2017). Phytochemical investigation of the stem bark of Tecomella undulata (Sm.) seem. Mod. Org. Chem. Res. 2 (4), 159–171. doi:10.22606/mocr.2017.24002
Almeid, E. R. (2009). Preclinical and clinical studies of lapachol and beta-lapachone. Open Nat. Prod. J. 2, 42–47. doi:10.2174/1874848100902010042
Alvala, R., Alvala, M., Sama, V., Dharmarajan, S., Ullas, J. V., and Reddy, M. (2013). Scientific evidence for traditional claim of anti-obesity activity of Tecomella undulata bark. J. Ethnopharmacol. 148 (2), 44 1–448. doi:10.1016/j.jep.2013.04.033
Alvi, T., Asif, Z., and Khan, M. K. I. (2022). Clean label extraction of bioactive compounds from food waste through microwave-assisted extraction technique-A review. Food Biosci. 46, 101580. doi:10.1016/j.fbio.2022.101580
Aparna, V., Dileep, K. V., Mandal, P. K., Karthe, P., Sadasivan, C., and Haridas, M. (2012). Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem. Biol. Drug Des. 80 (3), 434–439. doi:10.1111/j.1747-0285.2012.01418.x
API (Ayurvedic Pharmacopoeia of India) (2008). Government of India, ministry of health and family welfare, department of ayush. Part-I, Volume-I, First Edition. New Delhi. doi:10.1016/j.jep.2016.07.030
Arsalan, M., Azhar, I., Muhammad, S., Imam, S., Razzaque, G., Jabbar, A., et al. (2023). Evaluation of anti-ulcer, laxative and anti-inflammatory activities of Tecomella undulata (ROXB.). J. Popul. Ther. Clin. Pharmacol. 30 (19), 1136–1146. doi:10.53555/jptcp.v30i19.3866
Arya, S., Toky, O. P., Harris, S. M., and Harris, P. J. C. (1992). Tecomella undulata (Rohira): a valuable tree of thethar desert. Int. Tree Crops J. 7 (3), 141–147. doi:10.1080/01435698.1992.9752912
Aslam, M., Singh, R., Negi, P. S., Bhakuni, D. S., and Das, S. C. (2006). Enhanced in-vitro regeneration from cotyledonary node explants of Tecomella undulata (Smith) seem. Proc. Nat. Acad. Sci. India Sect. B 76 (3), 281–285.
Aslam, M., Singh, R., Anandhan, S., Pande, V., and Ahmed, Z. (2009). Development of a transformation protocol for Tecomella undulata (Smith) seem from cotyledonary node explants. Sci. Hortic. 121 (1), 119–121. doi:10.1016/j.scienta.2009.01.007
Aslan Çetin, B., Ocal, P., Irez, T., Uslu, E., Irmak, K., and Karataş, S. (2022). The association between follicular fluid sialic acid levels, oocyte quality, and pregnancy rates. Reprod. Sci. 29 (2), 633–638. doi:10.1007/s43032-021-00688-y
Atanasov, A. G., Zotchev, S. B., Dirsch, V. M., and Supuran, C. T. (2021). Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug. Discov. 20 (3), 200–216. doi:10.1038/s41573-020-00114-z
Azam, M. M., and Ghanim, A. (2000). Flavones from leaves of Tecomella undulata (Bignoniaceae). Biochem. Syst. Ecol. 28 (8), 803–804. doi:10.1016/s0305-1978(99)00116-7
Bajaj, J. S., O'Leary, J. G., Lai, J. C., Wong, F., Long, M. D., Wong, R. J., et al. (2022). Acute-on-chronic liver failure clinical guidelines. Am. J. Gastroenterol. 117 (2), 225–252. doi:10.14309/ajg.0000000000001595
Baroni, L., Sarni, A. R., and Zuliani, C. (2021). Plant foods rich in antioxidants and human cognition: a systematic review. Antioxidants 10 (5), 714. doi:10.3390/antiox10050714
Bhansali, R. R. (1993). Bud culture for shoot multiplication and plantlet formation of Tecomella undulata (rohida), a woody tree of the arid zone. Trop. Sci. 33 (1), 1–8.
Bhardwaj, R. (2018). GC-MS analysis and antimicrobial activity of alkaloids of Tecomella undulata.J. Med. Plant Stud 6 (6), 68–72.
Bhardwaj, R., Yadav, A., and Sharma, R. (2014). Tecomella undulata-phenolic compounds and antioxidant activities. Res. J. Med. Plant 8 (5), 223–230. doi:10.3923/rjmp.2014.223.230
Chemfaces (2025). Chemfaces. Available online at: https://www.chemfaces.com›natural›Tectol-CFN91054 (Accessed on March 6, 2025).
Chen, J., Yang, Y., Li, S., Yang, Y., Dai, Z., Wang, F., et al. (2020). E2F1 regulates adipocyte differentiation and adipogenesis by activating ICAT. Cells 9 (4), 1024. doi:10.3390/cells9041024
Cheng, Y., Cui, Y., Shang, X., and Fu, X. (2024). Fitting levels of 6-benzylademine matching seasonal explants effectively stimulate adventitious shoot induction in cyclocaryapaliurus (Batal.) iljinskaja. Plant Cell Tissue Organ Cult. (PCTOC) 156 (2), 38. doi:10.1007/s11240-023-02620-5
Chhajer, S., and Kalia, R. K. (2017). Seasonal and micro-environmental factors controlling clonal propagation of mature trees of marwar teak [Tecomella undulata (Sm.) Seem]. Acta Physiol. Plant 39, 60–15. doi:10.1007/s11738-017-2364-2
Das, D., Mukherjee, S., Mukherjee, M., Das, A. S., and Mitra, C. (2005). Aqueous extract of black tea (Camellia sinensis) prevents chronic ethanol toxicity. Curr. Sci. 88 (6), 952–961.
Das, T., Das, B., Saha, D., and Mishra, S. B. (2015). Anti-hyperglycemic effect of Tecomella undulata extract by ameliorating pancreatic dysfunction in streptozotocin induced diabetic albino rats. J. Appl. Pharm. Sci. 5 (11), 090–094. doi:10.7324/JAPS.2015.501115
De Cássia da Silveira e Sá, R., and de Oliveira Guerra, M. (2007). Reproductive toxicity of lapachol in adult male wistar rats submitted to short-term treatment. Phytother. Res. 21 (7), 658–662. doi:10.1002/ptr.2141
De Silva, S. F., and Alcorn, J. (2019). Flaxseed lignans as important dietary polyphenols for cancer prevention and treatment: chemistry, pharmacokinetics, and molecular targets. Pharm 12 (2), 68. doi:10.3390/ph12020068
Dhir, R., and Shekhawat, G. S. (2012). Critical review on Tecomella undulata: a medicinally potent endangered plant species of Indian thar desert. Int. J. Curr. Res. 4 (6), 36–44. doi:10.1002/med.20095
Duke, J. (2014). Dr Duke’s phytochemical and ethnobotanical databases. Available online at: http://www.ars-grin.gov/duke/.
Eichenmüller, M., Von Schweinitz, D., and Kappler, R. (2009). Betulinic acid treatment promotes apoptosis in hepatoblastoma cells. Int. J. Oncol. 35 (4), 873–879. doi:10.3892/ijo_00000402
Fatima, M., Kamran, M., Zehra, S., Jamil, N., and Zaidi, I. H. (2022). Histopathological effects with marwar teak (Tecomella undulata) bark extract and N-acetylcysteine on acetaminophen induced hepatotoxicity in albino rats. Prof. Med. J. 29 (03), 345–350. doi:10.29309/TPMJ/2022.29.03.6669
Fatima, M., Arslaan, M., Zehra, S., Sayyar, H. T., Kamran, M., and Zaidi, I. H. (2023). Hepatoprotective role of Tecomella undulata bark extract in comparison with n-acetylcysteine on acetaminophen induced hepatotoxicity in albino rats. Prof. Med. J. 30 (06), 758–763. doi:10.29309/TPMJ/2023.30.06.7435
Gujral, V. K., Gupta, S. R., and Verma, K. S. (1979). A new chromone glucoside from Tecomella undulata. Phytochem 18 (1), 181–182. doi:10.1016/S0031-9422(00)90945-2
Gupta, B. C., Nehal, M., and Banquet, N. Z. (1997). Effect of experimental diabetes on the activity of hexokinase, glucose-6-phosphate dehydrogenase and catecholamines in rat erythrocytes of different ages. Indian. J. Exp. Biol. 35 (7), 792–795.
Hamilton, A. C. (2004). Medicinal plants, conservation and livelihoods. Biodivers. Conserv. 13, 1477–1517. doi:10.1023/B:BIOC.0000021333.23413.42
Heinrich, M., Jalil, B., Abdel-Tawab, M., Echeverria, J., Kulić, Ž., McGaw, L. J., et al. (2022). Best practice in the chemical characterisation of extracts used in pharmacological and toxicological research—the ConPhyMP—guidelines. Front. Pharmacol. 13, 953205. doi:10.3389/fphar.2022.953205
Hoda, S., Gupta, L., Shankar, J., Gupta, A. K., and Vijayaraghavan, P. (2020). cis-9-hexadecenal, a natural compound targeting cell wall organization, critical growth factor, and virulence of Aspergillus fumigatus. ACS Omega 5 (17), 10077–10088. doi:10.1021/acsomega.0c00615
Holmes, J. E., Lung, S., Collyer, D., and Punja, Z. K. (2021). Variables affecting shoot growth and plantlet recovery in tissue cultures of drug-type Cannabis sativa L. Front. Plant Sci. 12, 732344. doi:10.3389/fpls.2021.732344
Ibrahim, I. A. A., Hussein, A. I., Muter, M. S., Mohammed, A. T., Al-Medhtiy, M. H., Shareef, S. H., et al. (2022). Effect of nano silver on gastroprotective activity against ethanol-induced stomach ulcer in rats. Biomed. Pharmacother. 154, 113550. doi:10.1016/j.biopha.2022.113550
Issa, H. M., and Mohammed, D. H. (2025). A critical review on the journey of benzoic acid in the pharmaceutical industry from manufacturing processes through various uses to disposal: an environmental perspective. Environ. Anal. Health Toxicol. 40, 2025007. doi:10.5620/eaht.2025007
Jain, M., Kapadia, R., Jadeja, R. N., Thounaojam, M. C., Devkar, R. V., and Mishra, S. H. (2012). Hepatoprotective potential of Tecomella undulata stem bark is partially due to the presence of betulinic acid. J. Ethnopharmacol. 143 (1), 194–200. doi:10.1016/j.jep.2012.06.023
Jeph, A., and Khan, J. B. (2020). Ethnomedicinal study in reserve forest area of jhunjhunu district, Rajasthan, India. Trop. Plant Res. 7 (2), 379–387. doi:10.22271/tpr.2020.v7.i2.044
Joshi, K. C., Prakash, L., and Singh, L. B. (1975). 6-O-veratryl catalposide: a new iridoid glucoside from Tecomella undulata. Phytochemistry 14 (5-6), 1441–1442. doi:10.1016/s0031-9422(00)98654-0
Joshi, K. C., Singh, P., and Pardasani, R. T. (1977). Quinones and other constituents from the roots of Tecomella undulata. Planta Med. 31 (1), 14–16. doi:10.1055/s-0028-1097481
Joshi, K. C., Sharma, A. K., and Singh, P. (1986). A new ferulic ester from Tecomella undulata. Planta Med. 52 (1), 71–72. doi:10.1055/s-2007-969078
Kalia, R. K., Rai, M. K., Sharma, R., and Bhatt, R. K. (2014). Understanding Tecomella undulata: an endangered pharmaceutically important timber species of hot arid regions. Genet. Resour. Crop Evol. 61, 1397–1421. doi:10.1007/s10722-014-0140-3
Kandar, C. C. (2021). “Secondary metabolites from plant sources,” in Bioactive natural products for pharmaceutical applications. Editors D. Pal, and A. K. Nayak (Cham: Springer), 140, 329–377. doi:10.1007/978-3-030-54027-2_10
Kapoor, B. B. S., and Bansal, R. (2013). Antimicrobial screening of some medicinal tree species of nagaur district of Rajasthan. Int. J. Herb. Med. 1 (4), 10–11. doi:10.32859/era.18.32.1-18
Katewa, S. (2009). “Indigenous people and forests: perspectives of an ethnobotanical study from Rajasthan (india),” in Herbal drugs: ethnomedicine to modern medicine. Editor K. Ramawat (Berlin, Heidelberg: Springer), 33–56. doi:10.1007/978-3-540-79116-4_3
Katewa, S. S., and Galav, P. K. (2005). Traditional herbal medicines from shekhawati region of Rajasthan. Indian J. Tradit. Knowl. 4 (3), 237–245.
Katsirma, Z., Dimidi, E., Rodriguez-Mateos, A., and Whelan, K. (2021). Fruits and their impact on the gut microbiota, gut motility and constipation. Food Funct. 12 (19), 8850–8866. doi:10.1039/D1FO01125A
Katz, D. H., Marcelletti, J. F., Khalil, M. H., Pope, L. E., and Katz, L. R. (1991). Antiviral activity of 1-docosanol, an inhibitor of lipid-enveloped viruses including herpes simplex. Proc. Natl. Acad. Sci. USA. 88 (23), 10825–10829. doi:10.1073/pnas.88.23.10825
Kaur, J., Gulati, M., Singh, S. K., Kuppusamy, G., Kapoor, B., Mishra, V., et al. (2022). Discovering multifaceted role of vanillic acid beyond flavours: nutraceutical and therapeutic potential. Trends Food. Sci. Technol. 122, 187–200. doi:10.1016/j.tifs.2022.02.023
Khan, M. A., Shah, A. H., Maqbol, A., Khan, S. B., Sadique, U., and Idress, M. (2013). Study of Tecomella undulata G. Don. methanolic extract against Sarcoptes scabiei L. in vivo and in vitro. J. Anim. Plant Sci. 23 (1), 47–53.
C. P. Khare (2004). Indian herbal remedies: rational Western therapy, ayurvedic, and other traditional usage, botany (Berlin Hiedelberg New York: Springer Verlag). doi:10.1007/978-3-642-18659-2
Khatri, A., Garg, A., and Agrawal, S. S. (2009). Evaluation of hepatoprotective activity of aerial parts of Tephrosia purpurea L. and stem bark of Tecomella undulata. J. Ethnopharmacol. 122 (1), 1–5. doi:10.1016/j.jep.2008.10.043
Krishnamoorthy, K., and Subramaniam, P. (2014). Phytochemical profiling of leaf, stem, and tuber parts of Solena amplexicaulis (Lam.) gandhi using GC-MS. Int. Sch. Res. Not. 2014 (1), 567409. doi:10.1155/2014/567409
Kumar, M. R., and Janagam, D. (2011). Export and import pattern of medicinal plants in India. Indian J. Sci. Technol. 4 (3), 245–248.
Kumar, N., and Khan, J. B. (2023). Ethnobotanical survey of traditional medicinal plants in shekhawati region, Rajasthan, India. Int. J. Multidiscip. Res. 5 (6), 1–17. doi:10.36948/ijfmr.2023.v05i06.9940
Kumar, S., Goyal, S., and Parveen, F. (2004). Ethno-veterinary plants in the Indian arid zone. Ethnobot 16, 91–95.
Kumar, S., Kumar, R., and Khan, A. (2011). Medicinal plant resources: manifestation and prospects of life-sustaining healthcare system. Cont. J. Biol. Sci. 4 (1), 19–29. doi:10.5281/zenodo.1310750
Kumar, S., Sharma, S., Vasudeva, N., and Ranga, V. (2012). In vivo anti-hyperglycemic and antioxidant potentials of ethanolic extract from Tecomella undulata. Diabetol. Metab. Syndr. 4 (33), 33–37. doi:10.1186/1758-5996-4-33
Kundu, C., and Sinha, S. K. (2023). Evaluation of phytochemicals from Morindacitrifolia (noni) fruit extracts against nicotine-induced different physiological functions: an experimental study on albino rat model. Indian J. Physiol. Allied Sci. 75 (02), 21–28. doi:10.55184/ijpas.v75i02.153
Laghari, A. Q., Memon, S., Nelofar, A., and Laghari, A. H. (2013). Tecomella undulata G. Don: a rich source of flavonoids. Ind. Crops Prod. 43, 213–217. doi:10.1016/j.indcrop.2012.07.025
Laghari, A. Q., Memon, S., Nelofar, A., and Laghari, A. H. (2014). Structurally diverse alkaloids from Tecomella undulata G. Don flowers. J. King Saud. Univ. Sci. 26 (4), 300–304. doi:10.1016/j.jksus.2014.02.005
Lal, K., Purohit, A., and Ram, H. (2017a). Insulin mimetic and pancreas-protective effect of Tecomella undulata leaves extract in diabetic rats. World J. Pharm. Pharm. Sci. 6 (2), 924–938. doi:10.20959/wjpps20172-8559
Lal, K., Purohit, A., and Ram, H. (2017b). Glucose homeostatic and pancreas protective potential of Tecomella undulata root extract in streptozotocin-induced diabetic rats. Asian J. Pharm. Clin. Res. 10 (6), 292–297. doi:10.22159/ajpcr.2017.v10i6.17997
Lau, C. S., and Aw, T. C. (2020). HbA1c in the diagnosis and management of diabetes mellitus: an update. Diabetes Updat. 6, 1–4. doi:10.15761/du.1000137
Li, J. W. H., and Vederas, J. C. (2009). Drug discovery and natural products: end of an era or an endless frontier? Science 325 (5937), 161–165. doi:10.1126/science.1168243
Luhata, L. P., and Luhata, W. G. (2017). Tiliroside: biosynthesis, bioactivity and structure activity relationship (SAR)-A review. J. Phytopharm. 6 (6), 343–348. doi:10.31254/phyto.2017.6607
Maeda, N., Funahashi, T., Matsuzawa, Y., and Shimomura, I. (2020). Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis 292, 1–9. doi:10.1016/j.atherosclerosis.2019.10.021
Malathi, K., Anbarasu, A., and Ramaiah, S. (2016). Ethyl iso-allocholate from a medicinal rice karungkavuni inhibits dihydropteroate synthase in Escherichia coli: a molecular docking and dynamics study. Indian J. Pharm. Sci. 78 (6), 780–788. doi:10.4172/pharmaceutical-sciences.1000184
Maru, R. N., and Patel, R. S. (2014). Certain ethno-medicinal plants used to treat gynaecological disorders by tribal people of Jhalodtaluka of dahod district, Gujarat, India. Life Sci. Leafl. 58, 26–34.
Masenga, S. K., Kabwe, L. S., Chakulya, M., and Kirabo, A. (2023). Mechanisms of oxidative stress in metabolic syndrome. Int. J. Mol. Sci. 24 (9), 7898. doi:10.3390/ijms24097898
McGinty, D., Letizia, C. S., and Api, A. M. (2010). Fragrance material review on 2-hexadecen-1-ol, 3, 7, 11, 15-tetramethyl. Food Chem. Toxicol. 48, S101–S102. doi:10.1016/j.fct.2009.11.023
Meena, D., and Kant, T. (2022). Assignment of genotypes to populations and assessment of genetic diversity of Tecomella undulata trees of Rajasthan (india) using ISSR markers. Vegetos 35 (2), 317–329. doi:10.1007/s42535-021-00294-y
Meena, A. K., and Rao, M. M. (2010). Folk herbal medicines used by the meena community in Rajasthan. Asian J. Tradit. Med. 5 (1), 19–31.
Meena, K. L., and Yadav, B. L. (2010). Studies on ethnomedicinal plants conserved by garasia tribes of sirohi district, Rajasthan, India. Indian J. Nat. Prod. Resour. 1 (4), 500–506.
Mewada, K. B., Ant, H. M., and Yadav, R. S. (2021). Ethnobotanical uses and population status of selected medicinal plants found in the polo forests of sabarkantha district, Gujarat, India. Plant Arch. 21 (2), 608–613. doi:10.1016/j.atherosclerosis.2019.10.021
Muhammad, I. C., Khan, M., and Hanif, W. (2006). An ethnomedicinal inventory of plants used for family planning and sex diseases treatment in samahni valley, (A.K.) Pakistan. Pak. J. Biol. Sci. 9, 2546–2555. doi:10.3923/pjbs.2006.2546.2555
Muneeb, A., Ahmad, I., Ahmad, M. S. A., Fatima, S., Hameed, M., Ahmad, F., et al. (2023). Ethnobotanical and economic uses of some medicinal plants from native saline areas. Intl. J. Appl. Exp. Biol. 2 (2), 147–154. doi:10.56612/ijaeb.v1i1.45
Nagpal, N., Gill, G. K., Singh, A., and Kapoor, R. (2019). Ameliorative effect of Tecomella undulata stem bark on the cadmium chloride induced splenomegaly and its associated changes in wistar rats. J. Med. Plants Stud. 7 (4), 203–206.
Nandwani, D., Sharma, R., and Ramawat, K. G. (1996). High frequency regeneration in callus cultures of a tree – Tecomella undulate. Gartenbauwissenschaft 61 (3), 147–150.
Nortjie, E., Basitere, M., Moyo, D., and Nyamukamba, P. (2022). Extraction methods, quantitative and qualitative phytochemical screening of medicinal plants for antimicrobial textiles: a review. Plants 11 (15), 2011. doi:10.3390/plants11152011
Norton, L., Shannon, C., Gastaldelli, A., and DeFronzo, R. A. (2022). Insulin: the master regulator of glucose metabolism. Metab. Clin. Exp. 129, 155142. doi:10.1016/j.metabol.2022.155142
Oduwole, O. O., Huhtaniemi, I. T., and Misrahi, M. (2021). The roles of luteinizing hormone, follicle-stimulating hormone and testosterone in spermatogenesis and folliculogenesis revisited. Int. J. Mol. Sci. 22 (23), 12735. doi:10.3390/ijms222312735
Oliveira-Costa, J. F., Meira, C. S., Neves, M. V. G. D., Dos Reis, B. P. Z. C., and Soares, M. B. P. (2022). Anti-inflammatory activities of betulinic acid: a review. Front. Pharmacol. 13, 883857. doi:10.3389/fphar.2022.883857
Orme, C. D. L., Davies, R. G., Burgess, M., Eigenbrod, F., Pickup, N., Olson, V. A., et al. (2005). Global hotspots of species richness are not congruent with endemism or threat. Nature 436 (7053), 1016–1019. doi:10.1038/nature03850
Ortega, F. J., Moreno-Navarrete, J. M., Pardo, G., Sabater, M., Hummel, M., Ferrer, A., et al. (2010). MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PloS One 5 (2), e9022. doi:10.1371/journal.pone.0009022
Pandya, K. B., Patel, H. B., Bhatt, P. R., Patel, U. D., and Modi, C. M. (2019). In vitro antibacterial activity of sixteen medicinal plants collected from nearby region of junagadh, Gujarat (india). J. Pharm. Innov. 8 (3), 662–667.
Panhwar, A. Q., and Abro, H. (2007). Ethnobotanical studies of mahal kohistan (khirthar national park). Pak. J. Bot. 39 (7), 2301–2315.
Paniagua-Pérez, R., Madrigal-Bujaidar, E., Reyes-Cadena, S., Molina-Jasso, D., Gallaga, J. P., Silva-Miranda, A., et al. (2005). Genotoxic and cytotoxic studies of beta-sitosterol and pteropodine in mouse. Biomed. Res. Int. 2005 (3), 242–247. doi:10.1155/JBB.2005.242
Papuc, C., Goran, G. V., Predescu, C. N., Tudoreanu, L., and Ștefan, G. (2021). Plant polyphenols mechanisms of action on insulin resistance and against the loss of pancreatic beta cells. Crit. Rev. Food Sci. Nutr. 62 (2), 325–352. doi:10.1080/10408398.2020.1815644
Pareek, A., and Sharma, N. (2017). Indigenous people and plants-perspectives of an ethnobotanical study from jaipur district (rajasthan). J. Phytol. Res. 30 (2), 7–17.
Parekh, J., and Chanda, S. (2007). In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk. J. Biol. 31 (1), 53–58.
Parekh, J., Jadeja, D., and Chanda, S. (2005). Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk. J. Biol. 29 (4), 203–210.
Parkar, N., Spencer, N. J., Wiklendt, L., Olson, T., Young, W., Janssen, P., et al. (2024). Novel insights into mechanisms of inhibition of colonic motility by loperamide. Front. Neurosci. 18, 1424936. doi:10.3389/fnins.2024.1424936
Patel, S., and Khare, N. (2023). Ethnobotanical studies of jessore wildlife sanctuary, banaskantha, Gujarat, India. Int. J. Environ. Clim. Change 13 (9), 2216–2226. doi:10.9734/ijecc/2023/v13i92455
Patel, K. N., Gupta, G. A., Goyal, M., and Nagori, B. P. (2011). Assessment of hepatoprotective effect of Tecomella undulata (Sm.) Seem., bignoniaceae, on paracetamol-induced hepatotoxicity in rats. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 21 (1), 133–138. doi:10.1590/S0102-695X2011005000020
Paul, T., and Prajapati, M. M. (2014). Ethno-therapeutic remedies for bone fracture, dang Dt. Gujarat. India. IOSR J. Pharm. Biol. Sci. 9 (2), 45–50. doi:10.9790/3008-09224550
Perveen, A., Wei, C. R., Bokhari, S. W. A., Ijaz, S., Iqbal, J., Ashraf, S., et al. (2024). Ethnobotany and urban life: medicinal and food use of plants from karachi (Pakistan’s largest metropolis). Ethnobot. Res. Appl. 28 (40), 1–26. doi:10.32859/era.28.40.1-26
Pithiya, M. B., Sharma, S. K., Sharma, M., Sharma, M., and Kotwal, N. (2022). Advancements and challenges in plant tissue culture: a comprehensive overview. J. Plant Biota 1 (1), 12–16. doi:10.51470/JPB.2022.1.1.12
POWO (2025). Plants of the world online. Facilitated by the royal botanic gardens, Kew. Available online at: http://www.plantsoftheworldonline.org/June 06, 2025).
Rahim, S., Shah, A., and Iqbal, S. (2023). Ethnobotany of medicinal plants in surghar range of Pakistan. Ethnobot. Res. Appl. 26 (6), 1–72. doi:10.32859/era.26.6.1-72
Rahman, M. M., Islam, M. R., Akash, S., Shohag, S., Ahmed, L., Supti, F. A., et al. (2022). Naphthoquinones and derivatives as potential anticancer agents: an updated review. Chem. Biol. Interact. 368, 110198. doi:10.1016/j.cbi.2022.110198
Rajput, A., Sharma, R., and Bharti, R. (2022). Pharmacological activities and toxicities of alkaloids on human health. Mater. Today. Proc. 48, 1407–1415. doi:10.1016/j.matpr.2021.09.189
Rana, M. G., Katbamna, R. V., Dudhrejiya, A. V., and Sheth, N. R. (2008). Hepatoprotection of Tecomella undulata against experimentally induced liver injury in rats. Pharmacologyonline 3, 674–682.
Rani, A., Singh, J., Jangra, A., Bhatia, M., Raghavendra, P. S., and Kumar, D. (2025). Gomphrena celosioides Mart.: a review of traditional uses, phytochemistry and pharmacology. Pharmacol. Res. Nat. Prod., 100366. doi:10.1016/j.prenap.2025.100366
Rathore, T. S., Singh, R. P., and Shekhawat, N. S. (1991). Clonal propagation of desert teak (Tecomella undulata) through tissue culture. Plant Sci. 79 (2), 217–222. doi:10.1016/0168-9452(91)90108-K
Ravi, A., Mallika, A., Sama, V., Begum, A. S., Khan, R. S., and Reddy, B. M. (2011). Antiproliferative activity and standardization of Tecomella undulata bark extract on K562 cells. J. Ethnopharmacol. 137 (3), 1353–1359. doi:10.1016/j.jep.2011.07.067
Ravishankar, B., and Shukla, V. J. (2007). Indian systems of medicine: a brief profile. Afr. J. Tradit. Complement. Altern. Med. 4 (3), 319–337. doi:10.4314/ajtcam.v4i3.31226
Rehman, S., Iqbal, Z., Qureshi, R., Ur Rahman, I., Khan, M. A., Elshaer, M. M., et al. (2022). Ethnogynaecological knowledge of traditional medicinal plants used by the indigenous communities of north waziristan, Pakistan. Evid. Based Complement. Altern. Med. 2022 (1), 6528264. doi:10.1155/2022/6528264
Riaz, S., Javed, M. A., Nawaz, I., and Javed, T. (2022). Biochemical characterization, cytotoxic, antimutagenic, anticancer and molecular docking studies on Tecomella undulata. Saudi J. Biol. Sci. 29 (4), 2421–2431. doi:10.1016/j.sjbs.2021.12.015
Robinson, J., Berselli, G. B., Ryadnov, M. G., and Keyes, T. E. (2020). Annexin V drives stabilization of damaged asymmetric phospholipid bilayers. Langmuir 36 (19), 5454–5465. doi:10.1021/acs.langmuir.0c00035
Rohilla, R., Garg, T., Goyal, A. K., and Rath, G. (2016). Herbal and polymeric approaches for liver-targeting drug delivery: novel strategies and their significance. Drug Deliv. 23 (5), 1645–1661. doi:10.3109/10717544.2014.945018
Sachdeva, C., Dhir, S., Kaushik, A., Kaswan, V., Walia, Y., and Kumar, N. (2023). Evaluation of in-vitro antiplasmodial activity of selected ethnobotanically important medicinal plant extracts. Int. J. Pharm. Sci. Res. 14 (2), 988–996. doi:10.13040/IJPSR.0975-8232.14(2).988-96
Saggoo, M., Kaur, N., and Gill, A. (2014). Variable response of three morphotypes of Tecomella undulata (sm.) seem towards human pathogenic bacteria. Int. J. Pharm. Pharm. Sci. 6 (4), 428–431.
Saravanakumar, M., Raja, B., Manivannan, J., Silambarasan, T., Prahalathan, P., Kumar, S., et al. (2015). Oral administration of veratric acid, a constituent of vegetables and fruits, prevents cardiovascular remodelling in hypertensive rats: a functional evaluation. Br. J. Nutr. 114 (9), 1385–1394. doi:10.1017/S0007114515003086
Saxena, P. K., Nanda, D., and Gupta, R. (2021). Hepatoprotective potential of Tecomella undulata bark on paracetamol and CCL4 induced hepatotoxicity in rats: invitro analysis. J. Pharm. Res. Int. 33 (42A), 307–322. doi:10.9734/JPRI/2021/v33i42A32409
Sen, U. K., and Bhakat, R. K. (2019). Ethnobotanical study on sand-dune based medicinal plants and traditional therapies in coastal purba medinipur district, West Bengal, India. Eur. J. Med. Plants 26 (2), 1–19. doi:10.9734/EJMP/2018/46231
Sha, W., Hu, F., Xi, Y., Chu, Y., and Bu, S. (2021). Mechanism of ferroptosis and its role in type 2 diabetes mellitus. J. Diabetes Res. 2021 (1), 9999612. doi:10.1155/2021/9999612
Sharma, A., Ujwala, P., Shivani, K., and Meeta, B. (2013). Evaluation of antibacterial activity of Tecomella undulata leaves crude extracts. Int. Res. J. Biol. Sci. 2 (6), 60–62.
Singh, A. K. (2004). Endangered economic species of Indian desert. Genet. Resour. Crop Evol. 51, 371–380. doi:10.1023/B:GRES.0000023452.91250.52
Singh, D., and Gupta, R. S. (2011). Hepatoprotective activity of methanol extract of Tecomella undulata against alcohol and paracetamol induced hepatotoxicity in rats. Life Sci. Med. Res. 26, 1–8.
Singh, P., Prakash, L., and Joshi, K. C. (1972). Lapachol and other constituents from the bignoniaceae. Phytochem 11 (4), 1498. doi:10.1016/S0031-9422(00)90114-6
Singh, P., Khandelwal, P., Hara, N., Asai, T., and Fujimoto, Y. (2008). Radermachol and naphthoquinone derivatives from Tecomella undulata: complete 1H and 13C NMR assignments of radermachol with the aid of computational 13C shift prediction. Indian J. Chem. 47 (B), 1865–1870.
Singh, G., Singh, B., Tomar, U. K., and Sharma, S. (2017). A manual for dryland afforestation and management. Jodhpur: Scientific Publishers-AFARI.
Sokołowski, A., Kończak, M., Oleszczuk, P., Gao, Y., and Czech, B. (2024). Environmental and food contamination by phthalic acid esters (PAEs): overview. Water Air Soil Pollut. 235 (5), 313. doi:10.1007/s11270-024-07121-5
Soni, P. K., and Mali, P. C. (2016). Oral administration of petroleum ether extract of Tecomellaundulata leaves affects spermatogenesis and fertility of male rats. Eur. J. Biomed. Pharm. Sci. 3 (9), 339–344.
Soni, H., Kanjariya, K. V., and Patel, R. S. (2018). Angiospermic plants used medicinally, by local people of vijapur taluka of mehsana district, Gujarat, India. Int. J. S. Res. Sci. Tech. 4 (5), 661–668.
Spalding, K. L., Arner, E., Westermark, P. O., Bernard, S., Buchholz, B. A., Bergmann, O., et al. (2008). Dynamics of fat cell turnover in humans. Nature 453 (5), 783–787. doi:10.1038/nature06902
Srinivas, A. N., Suresh, D., Suvarna, D., Pathak, P., Giri, S., Satish, S., et al. (2023). Unraveling the potential role of Tecomella undulata in experimental NASH. Int. J. Mol. Sci. 24 (4), 3244. doi:10.3390/ijms24043244
Suganya, S., Senthil Ram, T., Lakshmi, B. S., and Giridev, V. R. (2011). Herbal drug incorporated antibacterial nanofibrous mat fabricated by electrospinning: an excellent matrix for wound dressings. J. Appl. Polym. Sci. 121 (5), 2893–2899. doi:10.1002/app.33915
Tareen, R. B., Bibi, T., Khan, M. A., Ahmad, M., Zafar, M., and Hina, S. (2010). Indigenous knowledge of folk medicine by the women of kalat and khuzdar regions of Balochistan, Pakistan. Pak. J. Bot. 42 (3), 1465–1485.
Tewari, V. P. (2007). Comparing the model forms estimating generalised diameter-height relationships in Tecomella undulata plantations in hot arid region of India. J. For. Res. 18 (4), 255–260. doi:10.1007/s11676-007-0052-6
Tripathi, Y. C., Sharma, H. K., and Arya, R. (2000). Ethnomedicinal appraisal of traditional phytomedicines of arid Rajasthan. J. Med. Aromat. Plant Sci. 22 (23), 487–498.
Tucker, J. A., Bornath, D. P., McCarthy, S. F., and Hazell, T. J. (2024). Leptin and energy balance: exploring Leptin’s role in the regulation of energy intake and energy expenditure. Nutr. Neurosci. 27 (1), 87–95. doi:10.1080/1028415X.2022.2161135
Tyagi, T., and Agarwal, M. (2017). Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) solms. J. Pharmacogn. Phytochem. 6 (1), 195–206.
Tyagi, H., and Tomar, U. K. (2013). Factors affecting in vitro shoot proliferation and rooting of mature Tecomella undulata (Sm.) seem tree. Res. Plant Sci. 1 (2), 38–44. doi:10.12691/plant-1-2-6
Ullah, M. O., Hamid, K., Rahman, K. A., and Choudhuri, M. (2010). Effect of Rohitakarista (RHT), an ayurvedic formulation, on the lipid profile of rat plasma after chronic administration. Biol. Med. 2 (2), 26–31. doi:10.0001/164
Ullah, I., Ullah, I., Ali, M., Durrani, F., Khan, S. U., Hussain, D., et al. (2023). Quantitative study of medicinal plants and biological activities of two common species used by inhabitants of district Bannu, Pakistan. Acta Ecol. Sin. 43 (2), 271–287. doi:10.1016/j.chnaes.2021.08.006
Urano, F., Wang, X., Bertolotti, A., Zhang, Y., Chung, P., Harding, H. P., et al. (2000). Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287 (5453), 664–666. doi:10.1126/science.287.5453.664
Valizadeh, M., Beigomi, M., and Fazeli-Nasab, B. (2020). Antibacterial and anti biofilm effects of ethanol and aceton leaf extract of Momordica charantia and Tecomella undulata against Acinetobacter baumannii. Int. J. Adv. Biol. Biomed. Res. 8 (4), 403–418. doi:10.33945/SAMI/IJABBR.2020.4.6
Varshney, A., and Anis, M. (2012). Improvement of shoot morphogenesis in vitro and assessment of changes of the activity of antioxidant enzymes during acclimation of micropropagated plants of desert teak. Acta Physiol. Plant. 34, 859–867. doi:10.1007/s11738-011-0883-9
Vats, S. (2016). Effect of initial temperature treatment on phytochemicals and antioxidant activity of Azadirachta indica A. Juss. Appl. Biochem. Biotechnol. 178, 504–512. doi:10.1007/s12010-015-1890-x
Vats, S. (2018). Larvicidal activity and in vitro regulation of rotenoids from Cassia tora L. 3 Biotech. 8 (1), 13. doi:10.1007/s13205-017-1038-5
Vats, S., and Kamal, R. (2013). In vivo and in vitro evaluation of sterols from Gymnemabsylvestrte R. Br. Pak. J. Biol. Sci. 16 (23), 1771–1775. doi:10.3923/pjbs.2013.1771.1775
Vats, S., and Kamal, R. (2014). Cassia occidentalis L. (a new source of rotenoids): its in vitro regulation by feeding precursors and larvicidal efficacy. Plant Cell. Tiss. Organ Cult. 116, 403–409. doi:10.1007/s11240-013-0409-9
Vats, S., Kaushal, C., Timko, M. P., and Ganie, S. A. (2024). Uraria picta: a review on its ethnobotany, bioactive compounds, pharmacology and commercial relevance. S. Afr. J. Bot. 167, 333–354. doi:10.1016/j.sajb.2024.02.008
Verma, K. S., Jain, A. K., and Gupta, S. R. (1986). Structure of undulatin: a new iridoid glucoside from Tecomella undulata. Planta Med. 52 (5), 359–362. doi:10.1055/s-2007-969184
Wagh, V. V., and Jain, A. K. (2019). Arboreal ethnoflora of Western Madhya Pradesh India. Indian J. Nat. Prod. Resour. 10 (4), 68–80.
Wang, T., Remberger, M., Björklund, A., and Watz, E. (2022a). The impact of transportation time on apoptosis in allogeneic stem cell grafts and the clinical outcome in malignant patients with unrelated donors. Cytotherapy 24 (5), 508–515. doi:10.1016/j.jcyt.2021.11.008
Wang, O., Zhang, N., Han, C., and Huang, J. (2022b). Regular exercise combined with ferulic acid exhibits antiobesity effect and regulates metabolic profiles in high-fat diet-induced mice. Front. Nutr. 9, 957321. doi:10.3389/fnut.2022.957321
Wang, D., Xiao, H., Lv, X., Chen, H., and Wei, F. (2025). Mass spectrometry based on chemical derivatization has brought novel discoveries to lipidomics: a comprehensive review. Crit. Rev. Anal. Chem. 55 (1), 21–52. doi:10.1080/10408347.2023.2261130
WFO (2025). Tecomella undulata (Sm.) seem. Available online at: https://www.worldfloraonline.org/taxon/wfo-0000780043 (Accessed on June 06, 2025).
Willard, T., and Murray, M. T. (2020). “Tabebuia avellanedae (syn. T. impetiginosa, lapacho, pau d’Arco, ipe roxo),” in Textbook of natural medicine (Churchill Livingstone), 868–872. doi:10.1016/B978-0-323-43044-9.00115-1
Wismayer, D. S., Laurenti, M. C., Song, Y., Egan, A. M., Welch, A. A., Bailey, K. R., et al. (2023). Effects of acute changes in fasting glucose and free fatty acid concentrations on indices of β-cell function and glucose metabolism in subjects without diabetes. Am. JPhysiol. Endocrinol. Metab. 325, 2 E119–E131. doi:10.1152/ajpendo.00043.2023
Wu, L. Y., Shang, G. D., Wang, F. X., Gao, J., Wan, M. C., Xu, Z. G., et al. (2022). Dynamic chromatin state profiling reveals regulatory roles of auxin and cytokinin in shoot regeneration. Dev. Cell 57 (4), 526–542.e7. doi:10.1016/j.devcel.2021.12.019
Yao, Y. X., Yu, Y. J., Dai, S., Zhang, C. Y., Xue, X. Y., Zhou, M. L., et al. (2024). Kaempferol efficacy in metabolic diseases: molecular mechanisms of action in diabetes mellitus, obesity, non-alcoholic fatty liver disease, steatohepatitis, and atherosclerosis. Biomed. Pharmacother. 175, 116694. doi:10.1016/j.biopha.2024.116694
Yaseen, G., Ahmad, M., Potter, D., Zafar, M., Sultana, S., and Mir, S. (2018). “Ethnobotany of medicinal plants for livelihood and community health in deserts of Sindh-Pakistan,” in Plant and human health. Editors M. Ozturk, and K. Hakeem (Cham: Springer), 1, 767–792. doi:10.1007/978-3-319-93997-1_24
Zahra, Z., Khan, M. R., Shah, S. A., Maryam, S., Majid, M., Younis, T., et al. (2020). Vincetoxicumarnottianum ameliorate inflammation by suppressing oxidative stress and pro-inflammatory mediators in rat. J. Ethnopharmacol. 252, 112565. doi:10.1016/j.jep.2020.112565
Zamani, S., Fathi, M., Ebadi, M. T., and Máthé, Á. (2025). Global trade of medicinal and aromatic plants. A review. J. Agr. Food Res. 21, 101910. doi:10.1016/j.jafr.2025.101910
Zehri, Z. U., Zehra, S., Mahesar, A. L., Anwar, M., Zaidi, S. I. H., and Ashraf, M. (2020). Protective effects of Tecomella undulata stem bark extract on isoniazid induced hepatotoxicity: based on liver enzymes and histopathology in rat model. J. Bahria Univ. Med. Dent. Coll. 10 (1), 49–52. doi:10.51985/JBUMDC2019124
Keywords: T. undulata, endangered plant, ethnomedicine, pharmacology, phytochemistry, conservation
Citation: Vats S, Bhandari N, Ganie SA, Mir MA and Bashir N (2025) Ethnomedicinal, phytochemical, pharmacological, and conservation studies of an endangered plant: the desert teak (Tecomella undulata (Sm.) Seem.). Front. Pharmacol. 16:1665446. doi: 10.3389/fphar.2025.1665446
Received: 14 July 2025; Accepted: 07 October 2025;
Published: 17 November 2025.
Edited by:
Javier Echeverria, University of Santiago, ChileReviewed by:
Supriya Sharma, Abhilashi College of Pharmacy, IndiaMustofa Ahda, Universitas Ahmad Dahlan Fakultas Farmasi, Indonesia
Copyright © 2025 Vats, Bhandari, Ganie, Mir and Bashir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sharad Vats, dmF0c19zaGFyYWRAeWFob28uY28uaW4=
 Sharad Vats
Sharad Vats Nikkee Bhandari1
Nikkee Bhandari1 Showkat Ahmad Ganie
Showkat Ahmad Ganie