- 1Department of Clinical Pharmacy, Xiangtan Central Hospital (The Affiliated Hospital of Hunan University), Xiangtan, China
- 2Department of Respiratory Medicine, Zhongshan Hospital of Traditional Chinese Medicine Afflilated to Guangzhou University of Chinese Medicine, Zhongshan, China
- 3Department of Pharmacy, Changsha Stomatological Hospital, Changsha, China
- 4State Key Laboratory of Chemo/Bio-Sensing and Chemometrics, School of Biomedical Sciences, Hunan University, Changsha, China
Background: Dasatinib-induced chylothorax is a rare but potentially serious complication, and its clinical features remain poorly defined. This study aimed to systematically evaluate its clinical characteristics, therapeutic approaches, and patient outcomes to provide practical guidance for clinical management.
Methods: We conducted a retrospective analysis by systematically retrieving case reports of dasatinib-induced chylothorax from relevant databases up to 31 May 2025, to achieve a comprehensive assessment.
Results: A total of 24 patients from 22 published case reports and case series were included in this analysis. The median age was 50 years (range 5, 79), and the median time to onset of chylothorax following dasatinib initiation was 41 months (range 1, 168). The most frequently reported clinical symptom was dyspnea (62.5%), followed by fever (25.0%), cough (16.7%), abdominal pain (8.3%), and chest pain (4.2%). Pleural fluid was typically described as white and milky in appearance (87.5%). Biochemical analysis revealed a median pleural fluid triglyceride concentration of 547.4 mg/dL and an albumin level of 4.4 g/dL. Dasatinib therapy was discontinued in 83.3% of patients. Alternative tyrosine kinase inhibitors were prescribed in selected cases, including nilotinib (33.3%), imatinib (20.8%), and bosutinib (16.7%). Supportive treatments consisted primarily of corticosteroids (45.8%), diuretics (45.8%), and thoracentesis (29.2%). Clinical improvement was observed in 91.7% of patients, with a median time to recovery of 12.2 weeks (range 1, 52). Among the four patients who underwent dasatinib rechallenge, three experienced recurrence of chylothorax.
Conclusion: Dasatinib-induced chylothorax is rare but often reversible. Early diagnosis, drug discontinuation, and appropriate supportive care are key to recovery. Rechallenge should be undertaken cautiously due to a high risk of recurrence.
Introduction
Dasatinib, an oral second-generation BCR-ABL tyrosine kinase inhibitor (TKI), received approval from the U.S. Food and Drug Administration in 2006 for patients with Philadelphia chromosome-positive (Ph+) chronic myeloid leukemia (CML) or acute lymphoblastic leukemia (ALL) who were resistant or intolerant to prior therapy, and was subsequently approved in 2010 for newly diagnosed chronic-phase CML (Foà et al., 2024; Advani et al., 2023). Compared with the first-generation TKI imatinib, dasatinib exhibits greater potency and broader kinase inhibition, targeting not only BCR-ABL but also a spectrum of kinases including Src-family kinases (SFKs), KIT proto-oncogene receptor tyrosine kinase (c-KIT), platelet-derived growth factor receptor beta (PDGFR-β), and ephrin type-A receptor 2 (EPHA2) (Keating, 2017). This expanded inhibitory profile underlies its superior therapeutic efficacy while also accounting for distinct adverse-event patterns. While adverse events are generally infrequent, pleural effusion has emerged as a notable complication, and in rare instances, this may evolve into chylothorax—an accumulation of chyle in the pleural space, typically presenting with milky effusion rich in triglycerides and lymphocytes (Huang et al., 2023). The underlying pathophysiology of dasatinib-induced chylothorax is not fully understood, but it is thought to involve off-target effects on lymphatic vessels and alterations in vascular permeability (Molina et al., 2020). To date, available data on dasatinib-induced chylothorax are primarily from case reports and small series, providing limited guidance for clinicians in terms of diagnosis and management. Understanding the clinical features, diagnostic strategies, and treatment options is crucial for improving patient outcomes. This study aims to retrospectively analyze the clinical characteristics, management approaches, and outcomes of patients with dasatinib-induced chylothorax, offering insights that may aid in the early recognition and effective management of this rare complication.
Methods
Study design and data collection
A systematic search was conducted in PubMed, EMBASE, Web of Science, and Chinese databases (WanFang, CNKI) for studies published up to 31 May 2025. The search used a combination of keywords: “Dasatinib” OR “Tyrosine kinase inhibitor” OR “Src-family kinases” AND “Chylothorax” OR “Pleural effusion” OR “pleural fluid” OR “chyle”. Only case reports, case series, and clinical studies reporting on dasatinib-induced chylothorax with detailed clinical data, were included. Duplicate studies were removed, and references from eligible articles were reviewed for additional relevant studies.
Inclusion and exclusion criteria
We included clinical studies, case reports, and case series that (1) reported patients with dasatinib-induced chylothorax (Advani et al., 2023), provided comprehensive clinical data (e.g., demographic details, clinical presentation, pleural fluid analysis, treatment, and outcomes). Exclusion criteria were reviews, mechanism studies, animal studies, duplicate cases, and articles with insufficient data.
Data extraction
A standardized form was used to extract relevant information from each eligible article. The following data were collected: patient characteristics (age, sex), underlying disease (CML, Ph + ALL, etc.), dasatinib dosage and treatment duration, time to onset of chylothorax, presenting symptoms, imaging findings, pleural fluid characteristics (triglycerides, lymphocytes, albumin, lactate dehydrogenase), management strategies (discontinuation of dasatinib, use of alternative TKIs, supportive treatments), and patient outcomes (recovery, recurrence, or mortality).
Causality assessment
Causality was evaluated based on the WHO-UMC system, which classifies cases as “certain,” “probable,” “possible,” or “unlikely.” A “certain” classification was given when the onset was temporally related to dasatinib use, symptoms improved after discontinuation, and recurrence occurred upon rechallenge. “probable” cases had a reasonable temporal link, with improvement upon discontinuation but without rechallenge. “possible” cases had a reasonable temporal association, but other factors could not be ruled out. “unlikely” cases were those where the temporal relationship with dasatinib was unclear, and other explanations for the adverse event were more likely.
Statistical analysis
Descriptive statistics were used to summarize the data. Continuous variables were expressed as medians with ranges (minimum to maximum), while categorical variables were presented as percentages. All analyses were performed using SPSS version 25.0.
Results
Basic information
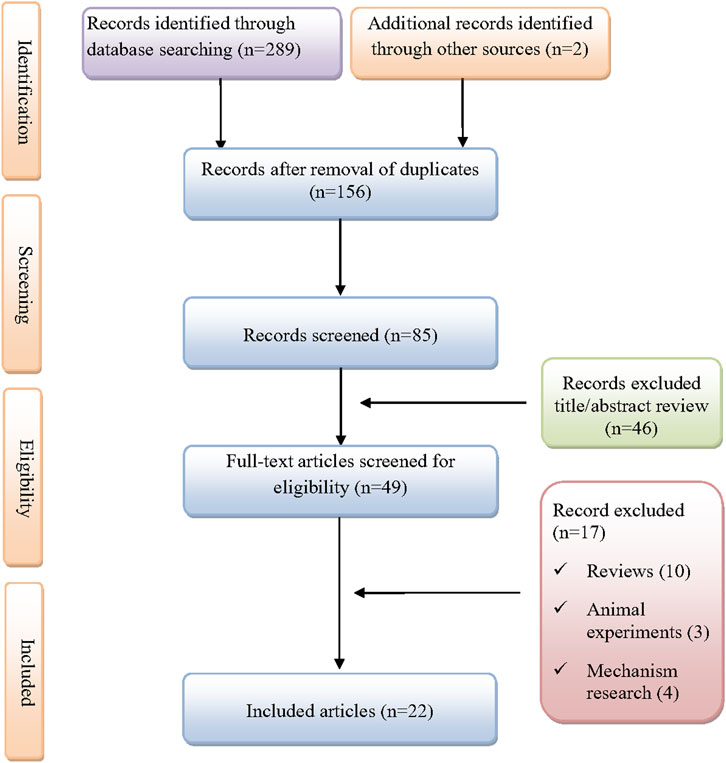
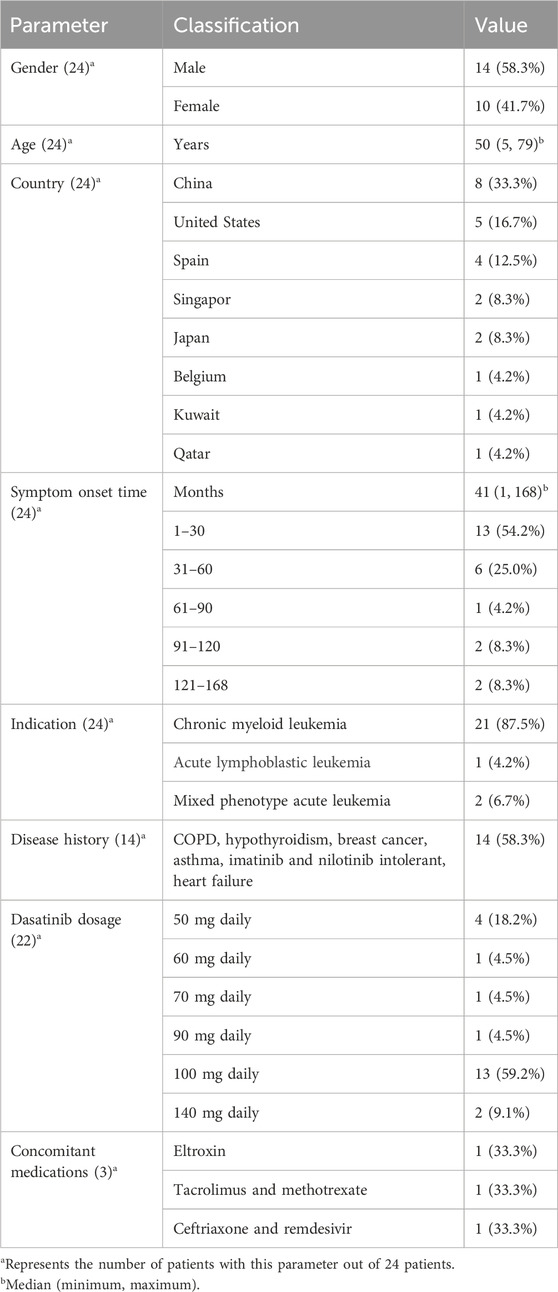
As shown in Figure 1, A total of 291 records were initially identified through database searches and manual screening. After removing duplicates and screening titles and abstracts, 22 studies were included for final analysis (Molina et al., 2020; Al-Abcha et al., 2019; Alqattan et al., 2022; Baloch et al., 2017; Bradt et al., 2022; Chen et al., 2020; Ferreiro et al., 2016; Garcia-Zamalloa et al., 2023; Hickman et al., 2020; Hsu et al., 2021; Huang et al., 2015; Justin et al., 2025; Kelly et al., 2022; Liu et al., 2023; Makimoto et al., 2022; Martínez et al., 2023; McGonegal and Baskar, 2024; Ng et al., 2024; Pai and Huang, 2025; Paul et al., 2021; Sasaki et al., 2019). A total of 24 patients with dasatinib-induced chylothorax were included in the analysis (Table 1). The median age was 50 years, ranging from 5 to 79 years, with a male predominance (58.3%). Most cases were reported from China (33.3%), followed by the United States (16.7%), Spain (12.5%), Singapore (8.3%), Japan (8.3%), Belgium (4.2%), Kuwait (4.2%), and Qatar (4.2%). The median time from dasatinib initiation to symptom onset was 41 months (range 1, 168), with over half of the patients (54.2%) developing symptoms within the first 30 months of therapy. The most common indication for dasatinib use was chronic myeloid leukemia (87.5%), followed by mixed phenotype acute leukemia (6.7%) and acute lymphoblastic leukemia (4.2%). Comorbidities were present in 58.3% of patients and included conditions such as chronic obstructive pulmonary disease, hypothyroidism, asthma, breast cancer, heart failure, and intolerance to imatinib or nilotinib. Among the 22 patients with reported dosing information, the most common dasatinib dose was 100 mg daily (59.2%), followed by 50 mg (18.2%), and other doses ranging from 60 mg to 140 mg. Concomitant medications were reported in three patients, including eltroxin, tacrolimus, methotrexate, ceftriaxone, and remdesivir.
Clinical manifestations
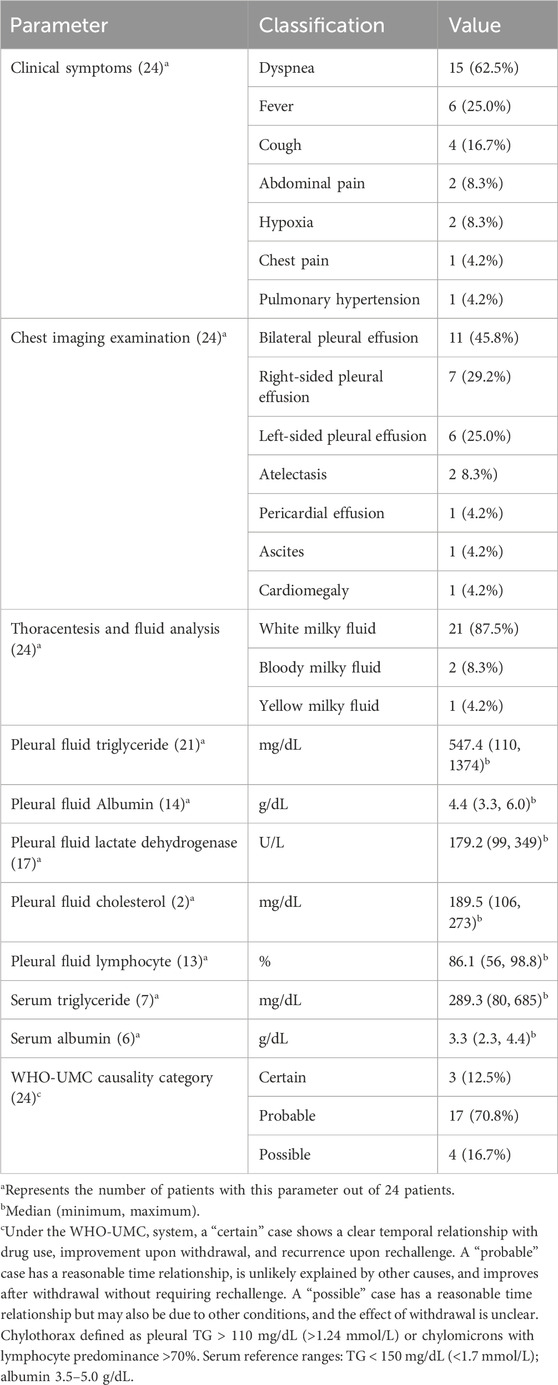
Respiratory symptoms were the most prominent clinical presentations of dasatinib-induced chylothorax (Table 2). Dyspnea was reported in 62.5% of patients, followed by fever (25.0%) and cough (16.7%). Less frequent symptoms included abdominal pain and hypoxia (8.3% each), as well as chest pain and pulmonary hypertension (4.2% each). Imaging findings revealed bilateral pleural effusions in 45.8% of patients, while right-sided and left-sided effusions were observed in 29.2% and 25.0%, respectively. Additional radiological abnormalities included atelectasis (8.3%), pericardial effusion (4.2%), ascites (4.2%), and cardiomegaly (4.2%). According to the WHO-UMC system, 12.5% of cases were classified as “certain,” 70.8% as “probable,” and 16.7% as “possible” (see Supplementary Data Table 1).
Thoracentesis and fluid analysis
All patients underwent thoracentesis. Pleural findings were interpreted against standard thresholds for chylothorax—pleural triglycerides (TG) > 110 mg/dL (>1.24 mmol/L) or chylomicrons present, with lymphocyte predominance >70% supporting the diagnosis. As summarized in Table 2, the pleural fluid was predominantly white and milky in appearance (87.5%), consistent with chylous effusion, while a smaller proportion appeared bloody milky (8.3%) or yellow milky (4.2%). Biochemically, pleural TG was markedly elevated, with a median of 547.4 mg/dL and a range of 110–1374 mg/dL, and the differential showed lymphocyte predominance (median 86.1%, range 56.0%–98.8%). The median pleural fluid albumin level was 4.4 g/dL, and lactate dehydrogenase (LDH) had a median value of 179.2 U/L. For serum measurements, we applied widely recognized reference ranges (triglycerides <150 mg/dL [<1.7 mmol/L]; albumin 3.5–5.0 g/dL) to aid interpretation. Among cases with available data, the median serum triglyceride level was 289.3 mg/dL (3.27 mmol/L) and serum albumin was 3.3 g/dL, indicating hypertriglyceridemia with mild hypoalbuminemia.
Treatment and prognosis
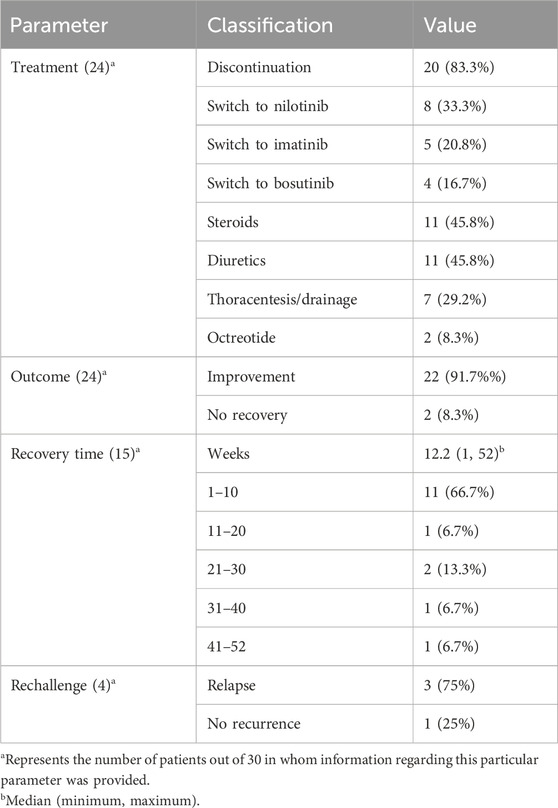
As shown in Table 3, following the diagnosis of chylothorax, dasatinib was discontinued in most cases (83.3%, n = 20). Among these, 33.3% of patients (n = 8) were switched to nilotinib, 20.8% (n = 5) to imatinib, and 16.7% (n = 4) to bosutinib as alternative tyrosine kinase inhibitors. Supportive treatment was commonly administered and included corticosteroids and diuretics in 45.8% of patients (n = 11 each), while thoracentesis or pleural drainage was performed in 29.2% (n = 7). Octreotide was used in two cases (8.3%). Clinical improvement was achieved in 91.7% of patients (n = 22). Among the 15 patients with available recovery time data, the median time to resolution was 12.2 weeks (range: 1–52). Most patients (66.7%, n = 11) recovered within the first 10 weeks, while a smaller proportion required prolonged treatment duration, with recovery extending up to 52 weeks in isolated cases. Rechallenge with dasatinib was attempted in four patients, of whom three (75.0%) experienced recurrence of chylothorax, highlighting a high risk of relapse upon re-exposure.
Discussion
Chylothorax primarily occurs following disruption of the thoracic duct, which may result from traumatic injury such as cardiac surgery, or from non-traumatic causes including lymphatic obstruction associated with sarcoidosis, lymphoma, or superior vena cava syndrome (Armas-Villalba and Jimenez, 2025). In contrast, pleural effusion is a far more common non-hematologic toxicity of dasatinib, occurring in 20%–30% of CML patients, often within the first year of therapy, and is associated with immune-mediated mechanisms, endothelial permeability changes, and fluid retention (Breccia et al., 2011; Jain et al., 2024). Chylothorax, however, is exceedingly rare and generally develops after prolonged exposure. Eskazan et al. reported that dasatinib-related pleural effusion may be accompanied by pericardial effusion but rarely by ascites, suggesting selective serosal involvement (Küçükyurt et al., 2024). These findings highlight chylothorax as a rare and distinct complication of dasatinib, with timing, mechanisms, and clinical implications differing from pleural effusion. In clinical practice, diagnostic confirmation of chylothorax is based on pleural fluid characteristics, which often present as a milky effusion with elevated triglycerides and predominance of lymphocytes. Widely accepted criteria include triglyceride levels >110 mg/dL (>1.24 mmol/L) or the presence of chylomicrons, with nucleated cell counts showing >70% lymphocytes further supporting a chylous etiology (Armas-Villalba and Jimenez, 2025; Riley and Ataya, 2019). While medication-induced chylothorax is rare, dasatinib is recognized as an uncommon pharmacological cause, representing one of the few drug-related etiologies identified to date (Agrawal et al., 2022). As noted in our analysis, the majority of patients developed chylothorax after prolonged exposure to dasatinib, with the median time to symptom onset being 41 months. This finding indicating that chylothorax typically arises in the later stages of anti-myeloid leukemia treatment following extended drug exposure. Noteworthy, reported cases span a broad age range, from pediatric to elderly patients, underscoring the need for vigilance across age groups. Given the limited pediatric evidence, decisions regarding dose adjustment, switching to alternative TKIs, and the appropriateness of rechallenge should be individualized and carefully balanced against the risk of recurrence. Although the exact pathophysiological mechanisms remain unclear, they are thought to involve dasatinib-induced disruption of lymphatic function and vascular integrity, potentially through the inhibition of PDGFR-β and SFKs, both of which are crucial for maintaining lymphatic drainage and vascular stability (Huang et al., 2015; Bergers et al., 2003). Mechanistically, PDGFR-β regulates lymphangiogenesis, and its inhibition leads to the formation of abnormal lymphatic vessels, resulting in leakage into the pleural space (Zhang et al., 2024). Similarly, SFKs inhibition can modulate endothelial barrier function and increase vascular permeability, potentially destabilizing the pleural microvasculature and favoring effusion formation (Cai et al., 2020). However, the specific reasons behind dasatinib’s heightened affinity for the vasculature and lymphatics remain unclear.
Dasatinib has a half-life of approximately 3–5 h and is primarily metabolized by the liver via the CYP3A4 enzyme, with excretion occurring through bile and urine (Advani et al., 2023). The typical dosage of dasatinib is administered once daily, usually at doses of 50 mg or 100 mg, adjusted based on the patient’s specific condition and tolerance (Pontarollo and Reinhardt, 2023). Notably, dasatinib is associated with a broad adverse-event profile that includes hematologic toxicities (e.g., neutropenia, anemia, thrombocytopenia), cardiopulmonary events (e.g., pleural effusion, pulmonary arterial hypertension, arrhythmias), hepatic dysfunction, cutaneous reactions, and fluid-retention syndromes, as well as systemic symptoms such as headache and fatigue (Pontarollo and Reinhardt, 2023). In our analysis of 24 patients, the most commonly reported clinical manifestations were dyspnea (62.5%), followed by fever (25.0%), cough (16.7%), and abdominal pain and hypoxia. The diagnosis of dasatinib-induced chylothorax is confirmed through pleural fluid analysis, which typically shows a characteristic milky appearance and elevated triglyceride levels, findings that were observed in the majority of our cases. The pleural fluid analysis also showed a predominance of lymphocytes, which is consistent with the typical presentation of chylothorax. This highlights the critical role of pleural fluid analysis in differentiating dasatinib-induced chylothorax from other potential causes of pleural effusion, such as malignancy or infection. Given that this complication is relatively rare, clinicians must maintain a high degree of suspicion when faced with patients on dasatinib therapy presenting with pleural effusion.
Management of dasatinib-induced chylothorax primarily involves discontinuing the drug, which was associated with clinical improvement in the majority of patients in our analysis. Alternative TKIs such as nilotinib, imatinib, and bosutinib were frequently used in place of dasatinib, with no recurrence of chylothorax in most cases. Nilotinib is generally considered a suitable alternative owing to its efficacy and lower incidence of pleural effusion compared with dasatinib (Jain et al., 2024). Imatinib, although less potent, remains a well-tolerated option, particularly in patients with stable disease or those intolerant to other second-generation TKIs (Jain et al., 2024; Cortes et al., 2016; Paydas, 2014). Bosutinib, another second-generation TKI, has been associated with gastrointestinal toxicities but rarely with pleural or chylous effusions, making it a reasonable alternative in selected cases (Cortes et al., 2018). Clinicians should individualize the choice of an alternative TKI based on prior therapy, comorbidities, and overall risk-benefit assessment. In addition to drug discontinuation, supportive treatments, including corticosteroids, diuretics, and thoracentesis, were employed and were effective in most patients. These interventions contributed significantly to symptom resolution, with the median recovery time being 12.2 weeks. Although most patients recovered within 10 weeks, some required a prolonged treatment course, with recovery extending up to 52 weeks in a few isolated cases. Of particular concern is the high rate of recurrence of chylothorax upon rechallenge with dasatinib. Notably, three out of four patients who were rechallenged with dasatinib experienced a recurrence of symptoms, highlighting the high risk of relapse with re-exposure to the drug. This finding underscores the high risk of relapse with rechallenge and suggests that rechallenging with dasatinib should be avoided once chylothorax has developed. In refractory cases, additional therapeutic approaches have been described. For instance, a single case report documented the use of Goreisan combined with diuretics, which achieved control of dasatinib-induced chylothorax (Sasaki et al., 2019). Nevertheless, because of the concomitant treatment, the independent efficacy of Goreisan remains uncertain and warrants further evaluation.
Limitations of the study
This study has several limitations that should be acknowledged. First, the analysis is based on a limited number of published case reports, which may lead to selection and publication bias. Secondly, incomplete clinical data in some reports, particularly the absence of prognostic information in certain patients, may limit the accuracy of the analysis. Third, the retrospective nature of the study precludes standardized data collection and limits the ability to evaluate causality or treatment efficacy in a controlled manner. Finally, due to variability in diagnostic approaches and management strategies across reports, it remains difficult to establish uniform recommendations. Nevertheless, this study summarizes current evidence and may aid clinicians in the early recognition and appropriate management of dasatinib-induced chylothorax.
Conclusion
In conclusion, dasatinib-induced chylothorax is a rare but serious complication that requires early recognition and prompt management. Discontinuation of dasatinib, combined with appropriate supportive therapies, remains the cornerstone of treatment. The high risk of recurrence upon rechallenge underscores the need for caution when considering re-exposure to the drug. Further research is needed to explore alternative treatment options and to understand the underlying mechanisms of this complication.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the studies involving humans because this study is a data analysis based on previously published literature and therefore does not require ethical review. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because this study is a data analysis based on previously published literature and therefore does not require ethical review. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because this study is a data analysis based on previously published literature and therefore does not require ethical review.
Author contributions
YL: Conceptualization, Validation, Writing – original draft, Writing – review and editing. YH: Conceptualization, Validation, Writing – review and editing. JG: Validation, Visualization, Writing – review and editing. XL: Funding acquisition, Supervision, Validation, Writing – review and editing. YH: Funding acquisition, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Hunan Provincial Natural Science Foundation of China (No.2021JJ40549 and No.2024JJ9532) and the research project of Health Commission of Hunan Province (No. D202313018969).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1665484/full#supplementary-material
References
Advani, A. S., Moseley, A., O'Dwyer, K. M., Wood, B. L., Park, J., Wieduwilt, M., et al. (2023). Dasatinib/prednisone induction followed by blinatumomab/dasatinib in Ph+ acute lymphoblastic leukemia. Blood Adv. 7 (7), 1279–1285. doi:10.1182/bloodadvances.2022008216
Agrawal, A., Chaddha, U., Kaul, V., Desai, A., Gillaspie, E., and Maldonado, F. (2022). Multidisciplinary management of chylothorax. Chest 162 (6), 1402–1412. doi:10.1016/j.chest.2022.06.012
Al-Abcha, A., Iftikhar, M. H., Abu Rous, F., and Laird-Fick, H. (2019). Chylothorax: complication attributed to dasatinib use. BMJ Case Rep. 12 (12), e231653. doi:10.1136/bcr-2019-231653
Alqattan, Y., Ali, S., Almohammad, R., Kayali, N., and Alhuraiji, A. (2022). Dasatinib-induced chylothorax in chronic myeloid leukemia. Gulf J. Oncol. 1 (40), 74–77.
Armas-Villalba, A. J., and Jimenez, C. A. (2025). Chylothorax. Clin. Chest Med. 46 (2), 251–260. doi:10.1016/j.ccm.2025.02.004
Baloch, Z. Q., Abbas, S. A., Bhatti, H., Braver, Y., and Ali, S. K. (2017). Dasatinib-induced chylothorax in chronic myeloid leukemia. Proc. Bayl Univ. Med. Cent. 30 (1), 71–73. doi:10.1080/08998280.2017.11929535
Bergers, G., Song, S., Meyer-Morse, N., Bergsland, E., and Hanahan, D. (2003). Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 111 (9), 1287–1295. doi:10.1172/jci17929
Bradt, N., Olbrechts, F., Alexander, P., and Salembier, A. (2022). A dasatinib-induced chylothorax persisting after the discontinuation of dasatinib. Eur. J. Case Rep. Intern. Med. 9 (10), 003601. doi:10.12890/2022_003601
Breccia, M., Latagliata, R., Stagno, F., Luciano, L., Gozzini, A., Castagnetti, F., et al. (2011). Charlson comorbidity index and adult comorbidity evaluation-27 scores might predict treatment compliance and development of pleural effusions in elderly patients with chronic myeloid leukemia treated with second-line dasatinib. Haematologica 96 (10), 1457–1461. doi:10.3324/haematol.2011.041251
Cai, M. L., Wang, M. Y., Zhang, C. H., Wang, J. X., Liu, H., He, H. W., et al. (2020). Role of Co- and post-translational modifications of sfks in their kinase activation. J. Drug Target 28 (1), 23–32. doi:10.1080/1061186x.2019.1616297
Chen, B., Wu, Z., Wang, Q., Li, W., and Cheng, D. (2020). Dasatinib-induced chylothorax: report of a case and review of the literature. Invest. New Drugs 38 (5), 1627–1632. doi:10.1007/s10637-020-00932-3
Cortes, J. E., Saglio, G., Kantarjian, H. M., Baccarani, M., Mayer, J., Boqué, C., et al. (2016). Final 5-year study results of dasision: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J. Clin. Oncol. 34 (20), 2333–2340. doi:10.1200/jco.2015.64.8899
Cortes, J. E., Gambacorti-Passerini, C., Deininger, M. W., Mauro, M. J., Chuah, C., Kim, D. W., et al. (2018). Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized bfore trial. J. Clin. Oncol. 36 (3), 231–237. doi:10.1200/jco.2017.74.7162
Ferreiro, L., San-José, E., Suárez-Antelo, J., and Valdés, L. (2016). Dasatinib-induced pleural effusion: chylothorax, an option to consider. Ann. Thorac. Med. 11 (4), 289–293. doi:10.4103/1817-1737.191871
Foà, R., Bassan, R., Elia, L., Piciocchi, A., Soddu, S., Messina, M., et al. (2024). Long-term results of the dasatinib-blinatumomab protocol for adult philadelphia-positive all. J. Clin. Oncol. 42 (8), 881–885. doi:10.1200/jco.23.01075
Garcia-Zamalloa, A., Basauri, B., Urcelay, G., and Sainz, A. (2023). Dasatinib-induced chylothorax beyond 5 Years of treatment: is there actually any limit? Eur. J. Case Rep. Intern Med. 10 (7), 003921. doi:10.12890/2023_003921
Hickman, K., Diaz, E., Graham, R., Adams, R., and Ngwube, A. (2020). Dasatinib-induced chylothorax in chronic myelogenous leukemia in pediatric patient: report of a case and review of literature. J. Pediatr. Hematol. Oncol. 42 (7), e665–e667. doi:10.1097/mph.0000000000001619
Hsu, C. C., Hsu, J. F., and Wu, K. L. (2021). Dasatinib-induced chylothorax in a patient with chronic myeloid leukaemia: a case report and literature review. Respirol. Case Rep. 9 (6), e00753. doi:10.1002/rcr2.753
Huang, Y. M., Wang, C. H., Huang, J. S., Yeh, K. Y., Lai, C. H., Wu, T. H., et al. (2015). Dasatinib-related chylothorax. Turk J. Haematol. 32 (1), 68–72. doi:10.4274/tjh.2012.0196
Huang, J., Cai, J., Ye, Q., Jiang, Q., Lin, H., and Wu, L. (2023). Fluid retention-associated adverse events in patients treated with bcr::abl1 inhibitors based on fda adverse event reporting system (faers): a retrospective pharmacovigilance study. BMJ Open 13 (8), e071456. doi:10.1136/bmjopen-2022-071456
Jain, A. G., Gesiotto, Q., Ball, S., Nodzon, L., Rodriguez, A., Chan, O., et al. (2024). Incidence of pleural effusion with dasatinib and the effect of switching therapy to a different tki in patients with chronic phase cml. Ann. Hematol. 103 (6), 1941–1945. doi:10.1007/s00277-024-05760-6
Justin, W. L. Y., Mei Lam, J. C., and Teoh, O. H. (2025). Dasatinib-induced chylothorax treated with diuretics and steroids. Pediatr. Pulmonol. 60 (1), e27503. doi:10.1002/ppul.27503
Keating, G. M. (2017). Dasatinib: a review in chronic myeloid leukaemia and Ph+ acute lymphoblastic leukaemia. Drugs 77 (1), 85–96. doi:10.1007/s40265-016-0677-x
Kelly, R. L., Bae, J. Y., D'Annunzio, S., and Montanari, F. (2022). Diagnostic pitfalls of chylothorax after dasatinib treatment of chronic myeloid leukemia. Am. J. Case Rep. 23, e938319. doi:10.12659/ajcr.938319
Küçükyurt, S., Eşkazan, T., Ayer, M., Kılıçkıran Avcı, B., Hatemi, İ., and Eşkazan, A. E. (2024). Ascites does not accompany pleural effusion developing under dasatinib therapy in patients with cml-cp. Pleura Perit. 9 (1), 39–43. doi:10.1515/pp-2023-0016
Liu, Q., Fu, J., and Zhang, A. (2023). Dasatinib-associated chylothorax in a pediatric patient with chronic myeloid leukemia: a case report and literature review. Transl. Cancer Res. 12 (1), 194–200. doi:10.21037/tcr-22-1983
Makimoto, G., Misawa, M., Maeda, Y., and Kiura, K. (2022). Dasatinib-induced massive left chylothorax in a patient with chronic myeloid leukemia. Respir. Med. Case Rep. 37, 101662. doi:10.1016/j.rmcr.2022.101662
Martínez, R. J. G., Gea, C. S., Martin-Riera, V., Ruiz, A. R. G., Barzanti, R. P., Ferrer-Costa, R., et al. (2023). Dasatinib-induced chylothorax: a clinical laboratory's perspective. Ejifcc 34 (1), 76–80.
McGonegal, C. E., and Baskar, S. (2024). Dasatinib-induced bilateral chylothorax: a case report. Cureus 16 (9), e68479. doi:10.7759/cureus.68479
Molina, V., Vañes, S., Castelló, C., and Chiner, E. (2020). Chylothorax secondary to dasatinib. Arch. Bronconeumol. Engl. Ed. 56 (9), 599–601. doi:10.1016/j.arbres.2020.05.001
Ng, I. K., Ruparel, M., Chan, E. H., and Khoo, K. L. (2024). Drug-induced chylothorax as a rare pleural complication in dasatinib therapy for chronic myeloid leukaemia. J. R. Coll. Physicians Edinb. 54 (1), 44–47. doi:10.1177/14782715241237577
Pai, T. W., and Huang, C. W. (2025). Low-dose dasatinib-induced chylothorax, pulmonary hypertension, and pericardial effusion in a patient with chronic myeloid leukemia: a case report and literature review. Med. Baltim. 104 (3), e41328. doi:10.1097/md.0000000000041328
Paul, T., Ellahie, A. Y., Almohtasib, Y. S., Sinha, U., and El Omri, H. (2021). Dasatinib-induced chylothorax: an unusual presentation of a common adverse event-a case report with literature review. EJHaem 2 (3), 545–550. doi:10.1002/jha2.226
Paydas, S. D. (2014). Large granular lymphocytosis, and pleural effusion: useful or adverse effect? Crit. Rev. Oncol. Hematol. 89 (2), 242–247. doi:10.1016/j.critrevonc.2013.10.005
Pontarollo, G., and Reinhardt, C. (2023). The hemorrhage risk of dasatinib therapy. Blood 141 (24), 2917–2918. doi:10.1182/blood.2023020399
Riley, L. E., and Ataya, A. (2019). Clinical approach and review of causes of a chylothorax. Respir. Med. 157, 7–13. doi:10.1016/j.rmed.2019.08.014
Sasaki, H., Kimizuka, Y., Ogata, H., Okada, Y., Ota, S., Sano, T., et al. (2019). Successful control of dasatinib-related chylothorax by the Japanese herbal medicine goreisan. Intern Med. 58 (21), 3139–3141. doi:10.2169/internalmedicine.3002-19
Keywords: dasatinib, chylothorax, chronic myeloid leukemia, pleural effusion, diagnosis
Citation: Liu Y, Huang Y, Guo J, Liu X and Hu Y (2025) Clinical characteristics and management of dasatinib-induced chylothorax: retrospective analysis based on case reports. Front. Pharmacol. 16:1665484. doi: 10.3389/fphar.2025.1665484
Received: 14 July 2025; Accepted: 15 September 2025;
Published: 29 September 2025.
Edited by:
Zhiyu Zhang, Fourth Affiliated Hospital of China Medical University, ChinaReviewed by:
Aklank Jain, Central University of Punjab, IndiaGarcía-Martínez Rafael J., Hospital Clínico Universitario de Valladolid, Spain
Copyright © 2025 Liu, Huang, Guo, Liu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yixiang Hu, YWFoeXhhYUAxNjMuY29t, eWl4aWFuZ2h1QGhudS5lZHUuY24=; Xiang Liu, bGl1eGlhbmc4NzZAMTYzLmNvbQ==
 Ya Liu
Ya Liu Ying Huang2
Ying Huang2 Xiang Liu
Xiang Liu Yixiang Hu
Yixiang Hu