- 1Xinjiang Medical University, Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, China
- 2Huashan Hospital Fudan University, Shanghai, China
- 3Sixth People’s Hospital of Xinjiang Uygur Autonomous Region, Urumqi, Xinjiang, China
- 4Shandong Public Health Clinical Center, Shandong University, Jinan, Shandong, China
Background: Low molecular weight heparin (LMWH) and fondaparinux (FPX) are commonly used to prevent deep vein thrombosis (DVT) following total knee arthroplasty (TKA). This study aimed to compare the efficacy and safety of tranexamic acid (TXA) combined with LMWH versus TXA combined with FPX in preventing DVT among TKA patients.
Methods: A retrospective cohort study was conducted involving patients who underwent unilateral TKA at our institution between January 2020 and December 2023. Patients were divided into two groups based on their anticoagulation regimen: the TXA + LMWH group (n = 150) and the TXA + FPX group (n = 130). Perioperative indicators (blood loss, hospital stay, operative time, transfusion rate, transfusion volume, and total hospitalization costs), complications (DVT, muscular calf vein thrombosis [MCVT], surgical site infection, pulmonary thromboembolism, and postoperative hematoma), adverse reactions, coagulation parameters (D-dimer, prothrombin activity, INR, fibrinogen), and routine blood parameters (platelet count, hemoglobin, hematocrit) were compared between groups.
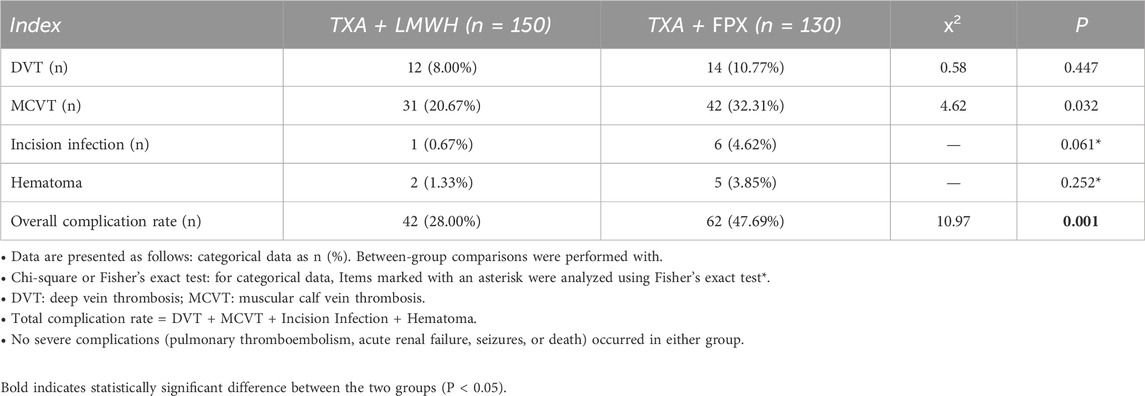
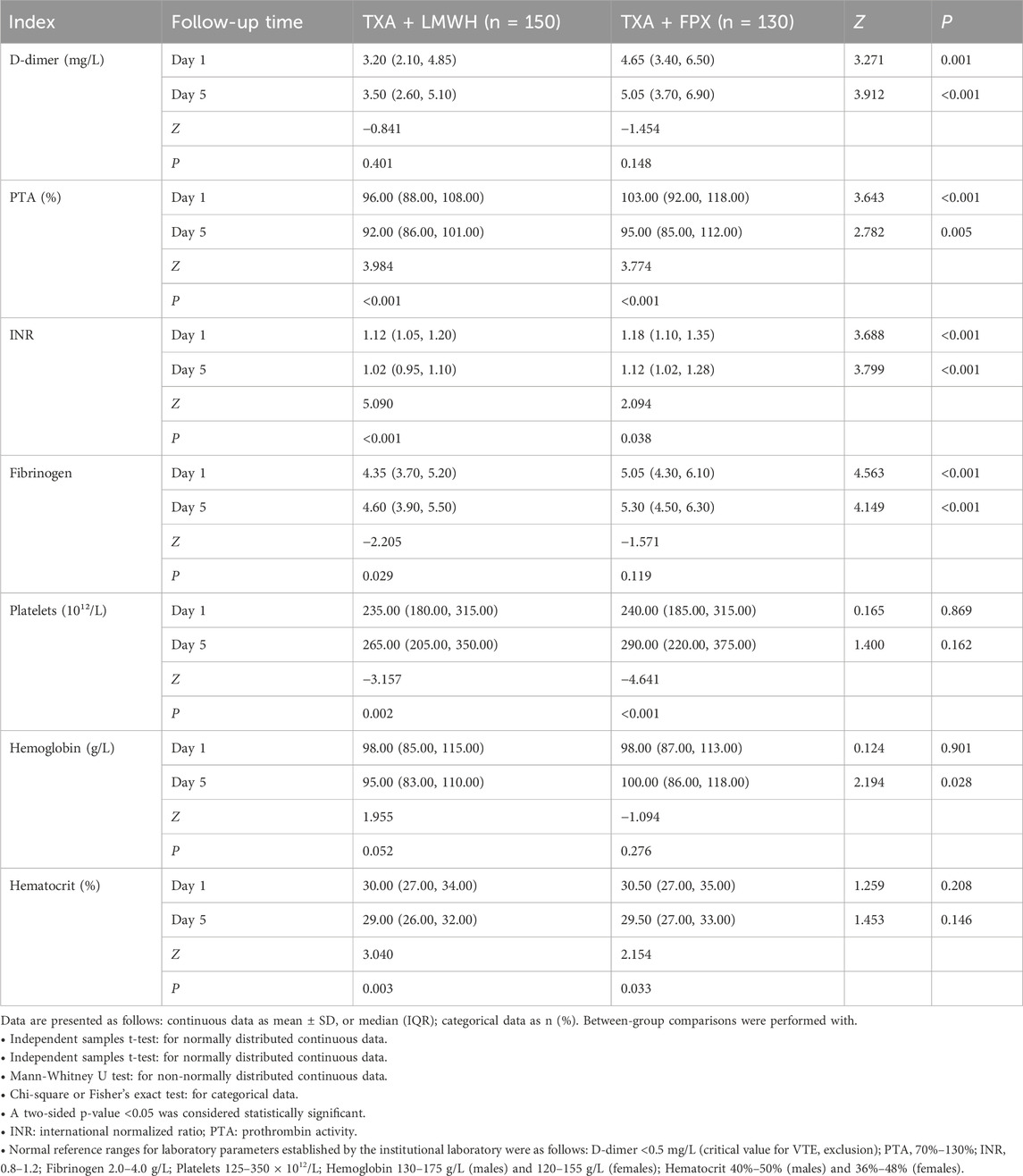
Results: No significant differences were observed between groups in perioperative blood loss, operative time, hospital stay, transfusion rate, or volume (P > 0.05). While most preoperative baseline characteristics were comparable, the TXA + FPX group had significantly better baseline renal function (P < 0.05)0. On postoperative days 1 and 5, levels of D-dimer, prothrombin activity, INR, and fibrinogen were significantly lower in the TXA + LMWH group compared to the TXA + FPX group (P < 0.05). Total hospitalization costs were significantly lower in the TXA + LMWH group (P < 0.05). Additionally, the TXA + LMWH group exhibited a significantly lower overall complication rate (28.00% vs. 47.69%, P < 0.05) and lower incidence of MCVT (20.67% vs. 32.31%, P < 0.05). No significant differences were found in rates of DVT, surgical site infection, or postoperative hematoma (P > 0.05). No severe complications, such as pulmonary thromboembolism, acute renal failure, seizures, or death, occurred in either group.
Conclusion: TXA combined with LMWH demonstrates significant advantages over TXA combined with FPX in reducing overall complications, particularly MCVT, and lowering hospitalization costs, with favorable improvements in coagulation parameters. Both regimens showed comparable efficacy in managing perioperative blood loss, operative time, hospital stay, and transfusion requirements in TKA patients. Given the retrospective design and limited sample size, further validation through high-quality, large-scale prospective studies is warranted.
Introduction
Total knee arthroplasty (TKA) is a highly effective treatment for end-stage knee osteoarthritis, substantially improving patients’ quality of life. However, the procedure is associated with significant perioperative blood loss and an increased risk of venous thromboembolism (VTE), particularly DVT (Zheng and Qiu, 2017; Ning, 2021). These complications remain major concerns affecting postoperative recovery.
TXA, an antifibrinolytic agent, is now widely utilized for managing blood loss associated with TKA, effectively reducing transfusion requirements without substantially increasing thrombotic risk (Yao et al., 2018; Agarwal et al., 2014). Meanwhile, routine thromboprophylaxis with anticoagulants is essential for VTE prevention. Low-molecular-weight heparin (LMWH) and FPX are standard anticoagulants recommended by clinical guidelines (Bannuru et al., 2019; Katz et al., 2013). Fondaparinux (FPX), a selective factor Xa inhibitor, provides potent anticoagulation, while LMWH, with its broader inhibitory profile, remains widely used due to proven efficacy and cost-effectiveness (NIH, 2022).
The concomitant use of a hemostatic agent (TXA) and an anticoagulant presents a clinical challenge due to their seemingly opposing mechanisms. Although the safety and efficacy of TXA combined with various anticoagulants have been demonstrated, direct comparisons of TXA + LMWH versus TXA + FPX within the same patient population remain scarce (Kakar et al., 2009). Critical questions regarding their comparative impact on coagulation parameters, specific thrombotic complications (such as MCVT), and overall cost-effectiveness require further exploration.
Therefore, this retrospective cohort study aims to compare the clinical efficacy and safety of TXA combined with LMWH versus TXA combined with FPX in patients undergoing TKA, focusing specifically on perioperative blood loss, thrombotic and bleeding complications, coagulation profiles, and hospitalization costs.
Materials and methods
Study population
This study was conducted at the Department of Orthopedics, Our hospital between January 2020 and December 2023. Eligibility criteria were as follows:
Inclusion Criteria: ① Diagnosed osteoarthritis according to established criteria (Guidelines for the diagnosis and treatment of osteoarthritis, 2007) and age between 60 and 85 years; ② No previous history of TKA; ③ Complete clinical data and follow-up results available.
Exclusion Criteria: ① Revision surgery or bilateral TKA; ② American Society of Anesthesiologists (ASA) classification > Grade 3 (Hao et al., 2024); ③ Contraindications to LMWH or FPX, including allergy within the past 6 months or previous thrombotic events; ④ Preoperative anemia (hemoglobin <12 g/dL for females, <13 g/dL for males) (Xiong et al., 2023); ⑤ Body mass index (BMI) > 35 kg/m2; ⑥ Immunodeficiency or immune dysfunction; ⑦ Pregnancy or lactation; ⑧ Severe comorbidities.
LMWH group
Patients received a subcutaneous injection of 4,000 AXaIU, administered once daily at 4–6 h postoperatively.
FPX group
Patients received a subcutaneous injection of 2.5 mg, administered once daily beginning 6 h postoperatively.
A sequential thromboprophylactic regimen lasting 14 days was employed for all patients. This included initial in-hospital administration of LMWH or FPX, followed by oral rivaroxaban upon discharge. The choice of injectable anticoagulant during hospitalization was determined through shared decision-making between physicians and patients. The dosing regimen adhered to national expert consensus and recommendations from our hospital’s multidisciplinary team (Weber et al., 2017).
This study was approved by the Ethics Committee of the Sixth People’s Hospital of Xinjiang Uygur Autonomous Region, and written informed consent was obtained from all participants.
Surgical procedures
All patients undergoing TKA underwent standardized preoperative assessments, including X-ray, CT, MRI, routine blood tests, liver and kidney function tests, and comprehensive physical examinations. All surgeries were conventional, cemented TKAs performed by the same surgical team, consisting exclusively of attending surgeons and anesthesiologists ranked associate senior attending or higher. A medial parapatellar surgical approach was uniformly employed, with anesthesia administered either generally or epidurally. A pneumatic tourniquet was consistently used at a pressure of 53.0 kPa, inflated prior to skin incision and released immediately after wound closure.All knee prostheses were cemented ceramic femoral component models from the ATTUNE® Knee System (Johnson & Johnson, Shanghai, China), coupled with cemented tibial trays. The tibial construct included offset tibial baseplates, femoral condyles, and locking lugs, which were fabricated using additive manufacturing technologies to optimize the osseointegative interface. Prior to joint capsule closure, a standardized periarticular injection (50 mL “cocktail mixture”) was administered uniformly, consisting of: 1 mg Compound Betamethasone Injection (Tianjin Kingyork Group Hubei Tianyao Pharmaceuticals Co., Ltd., Approval No. H42020019), 10 mL (20 mg) Ropivacaine (Yichang Humanwell Pharmaceutical Co., Ltd., Approval No. H20103552), and 0.3 mL (0.3 mg) Epinephrine Hydrochloride Injection (Grand Pharma [China] Co., Ltd., Approval No. H42021700), diluted with normal saline to a total volume of 50 mL. The TXA protocol (5 mL: 0.5 g, Yangtze River Pharmaceutical Group Nanjing Hailing Pharmaceutical Co., Ltd., Approval No. H20123005) included an intravenous infusion of 1 g TXA in 250 mL normal saline administered 30 min before tourniquet inflation, followed by topical application of another 1 g TXA in 250 mL normal saline via soaking after successful prosthesis implantation. The dosing regimen was established based on national expert consensus and recommendations from our hospital’s anesthesia team.
Postoperative management
1. Within 2 h postoperatively, all patients routinely received an infusion of 5% Glucose Injection (500 mL: 25 g, Sichuan Kelun Pharmaceutical Co., Ltd.) combined with Sodium Lactate Ringer’s Injection (500 mL, Xinjiang Huashidan Pharmaceutical Co., Ltd.) for volume expansion and electrolyte balance maintenance. Patients were subsequently transferred to the post-anesthesia care unit (PACU) for a standard 2-h observation period before returning to the inpatient ward.
2. All patients received standardized postoperative care and thromboprophylaxis prior to ambulation. Mobilization and strength training commenced on postoperative day 1. After discharge, oral rivaroxaban (Xarelto, 10 mg, Bayer AG) was prescribed for 15 days to continue thromboprophylaxis. Doppler ultrasound was routinely performed to screen for DVT at discharge, at the 1-month follow-up, or upon clinical suspicion of DVT. Chest CT pulmonary angiography (CT-PA) was conducted to detect pulmonary embolism when clinically indicated.
Transfusion Protocol: Blood transfusion was indicated for patients with hemoglobin levels below 70 g/L or for those with hemoglobin levels between 70 and 100 g/L accompanied by symptomatic anemia (e.g., dizziness, palpitations, dyspnea, or reduced exercise tolerance).
Outcomes
Baseline data
Baseline data, including age, gender, BMI, surgical site, type of surgery, ASA Physical Status Classification, Age-Adjusted Charlson Comorbidity Index (ACCI), comorbidities (hypertension, diabetes, coronary heart disease, neurological diseases, respiratory diseases, and metabolic disorders), and preoperative laboratory indicators, were collected from electronic medical records (EMRs).
The ASA Physical Status Classification System (Shivali and Thiagarajan, 2022), established by the American Society of Anesthesiologists (ASA), is a standardized preoperative health assessment tool designed to evaluate anesthetic risk by categorizing patients into six classes based on physiological status and surgical risk. Class I includes healthy patients, whereas Class II comprises patients with mild systemic disease without functional impairment; both classes generally tolerate anesthesia and surgery well. Class III encompasses patients with severe systemic disease causing functional limitations. Class IV includes patients with severe, life-threatening systemic disease resulting in significant impairment of daily living activities and elevated perioperative mortality risk. Class V represents moribund patients unlikely to survive without surgery, and Class VI denotes brain-dead organ donors.
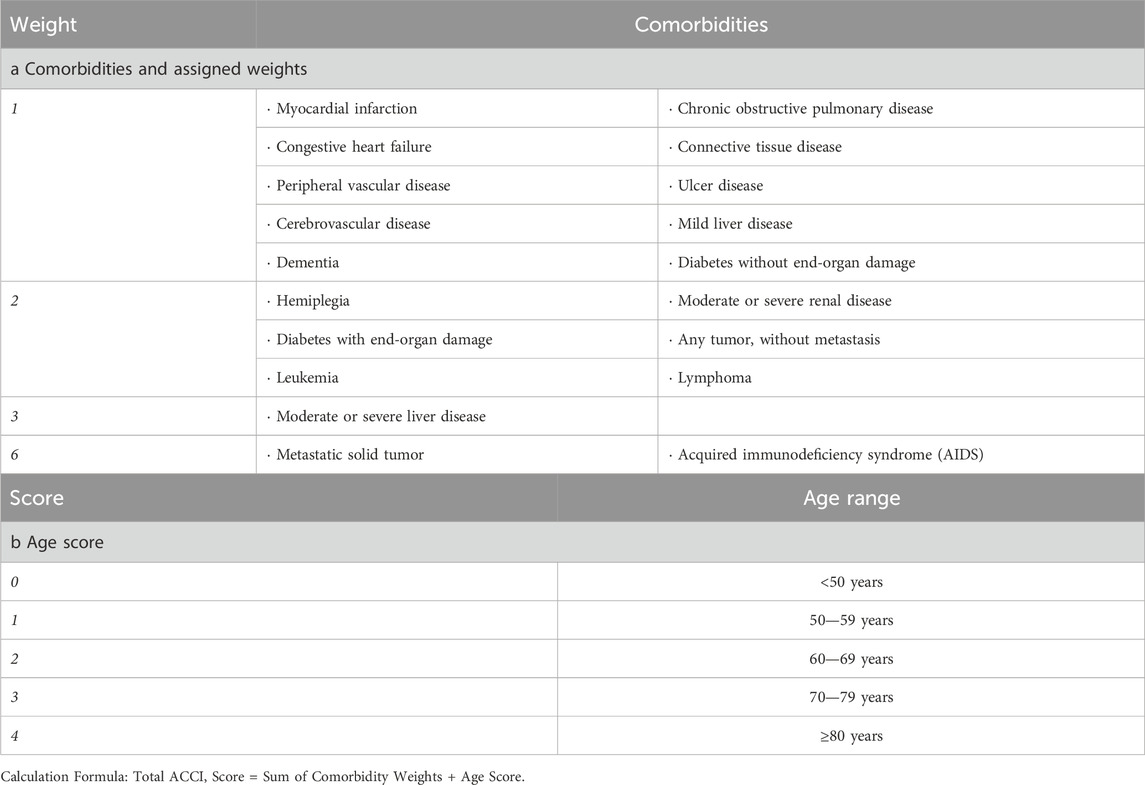
The ACCI (Charlson et al., 1994) incorporates patient age into the original Charlson Index score. This practical assessment tool can be quickly administered during admission history-taking or derived from medical records, enabling comprehensive evaluation. It remains the most widely adopted comorbidity scoring system. The ACCI score is calculated by summing individual comorbidity weights and adjusting for age by adding one point per decade after age 40 (1 point for ages 50–59, 2 points for 60–69, 3 points for 70–79, and 4 points for ≥80 years). Details are provided in Table 1.
Primary outcomes
The primary outcomes included perioperative indicators (blood loss, length of hospital stay, operative time, blood transfusion rate, transfusion volume, and total hospitalization costs) and complication rates (DVT of the lower extremities, MCVT, surgical site infection, PTE, and postoperative hematoma).
Total Blood Loss was Calculated using the Gross equation (Forster and Stewart, 2016) and Nadler equation (Sun et al., 2019). Total blood volume (TBV) was calculated as: TBV = k1 × height (m)3 + k2 × weight (kg) + k3, with coefficients k1 = 0.3669, k2 = 0.03219, k3 = 0.6041 for males; and k1 = 0.3561, k2 = 0.03308, k3 = 0.1833 for females. Total blood loss was then calculated using: TBV × (preoperative hematocrit–lowest postoperative hematocrit)/average hematocrit. Visible blood loss was defined as the sum of intraoperative blood loss and postoperative drainage volume. Hidden blood loss was calculated as: total blood loss–visible blood loss + volume of transfused red blood cells.
Diagnosis of Lower Extremity DVT: Lower extremity DVT diagnosis followed the “Guidelines for the Diagnosis and Treatment of DVT (Third Edition).” If marked asymmetric edema of the lower limbs occurred or if the patient complained of unilateral lower limb swelling and pain, thigh circumference (10 cm above the midpoint of the patella) and calf circumference (10 cm below the midpoint of the patella) were measured bilaterally. A side-to-side circumference difference exceeding 2 cm indicated possible DVT, prompting measurement of serum D-dimer levels. A D-dimer level exceeding 0.5 mg/L raised suspicion of lower extremity DVT, necessitating further evaluation with deep venous ultrasonography. DVT was definitively diagnosed if ultrasonography revealed a solid echogenic filling defect within the deep venous lumen, accompanied by interruption of color Doppler flow signals.
MCVT (Schwarz et al., 2010): Thrombosis confined to the gastrocnemius or soleus venous plexus, without involvement of the central deep veins, confirmed by ultrasound.
Incision infection (Berríos-Torres et al., 2017): Infection was defined according to CDC guidelines as either a superficial infection (incision redness or purulence within 30 days postoperatively) or a deep infection (purulence in deep tissues, a positive pathogen culture, or occurrence within 90 days postoperatively).
Postoperative hematoma (Streubel et al., 2011): Required meeting two criteria: (1) ultrasound or MRI confirmation of a periarticular fluid collection or hematoma with a diameter ≥5 cm, and (2) a hemoglobin drop ≥2 g/dL or requirement for surgical intervention.
All events were adjudicated by an independent committee based on imaging and clinical data. Monitoring for DVT and PTE continued until postoperative day 90, whereas infections and hematomas were assessed until hospital discharge.
Secondary outcomes
Secondary outcomes included the following postoperative laboratory parameters: D-dimer, prothrombin activity, international normalized ratio (INR), fibrinogen, platelet count, hemoglobin, and hematocrit.
Statistical analysis
Statistical analyses were performed using SPSS version 26.0. Continuous variables are presented as mean ± standard deviation (x̄ ± s). Data normality was assessed using the Kolmogorov–Smirnov test and normal Q–Q plots. Intergroup comparisons for normally distributed data were conducted using the independent samples t-test. For non-normally distributed data, the Mann–Whitney U test was employed. Categorical variables were analyzed using the chi-square test or Fisher’s exact test, as appropriate. A two-sided P value <0.05 was considered statistically significant.
Results
Comparison of general data
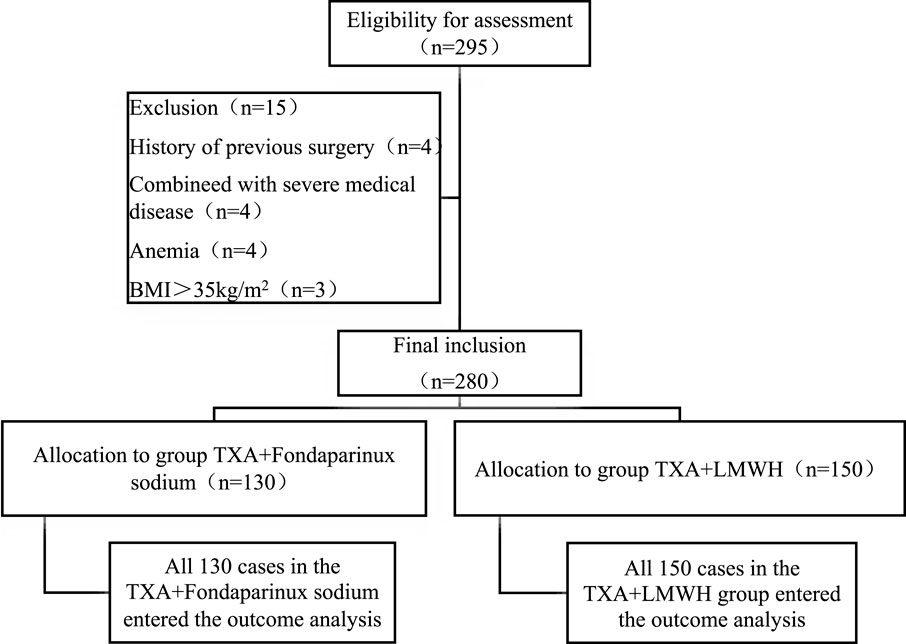
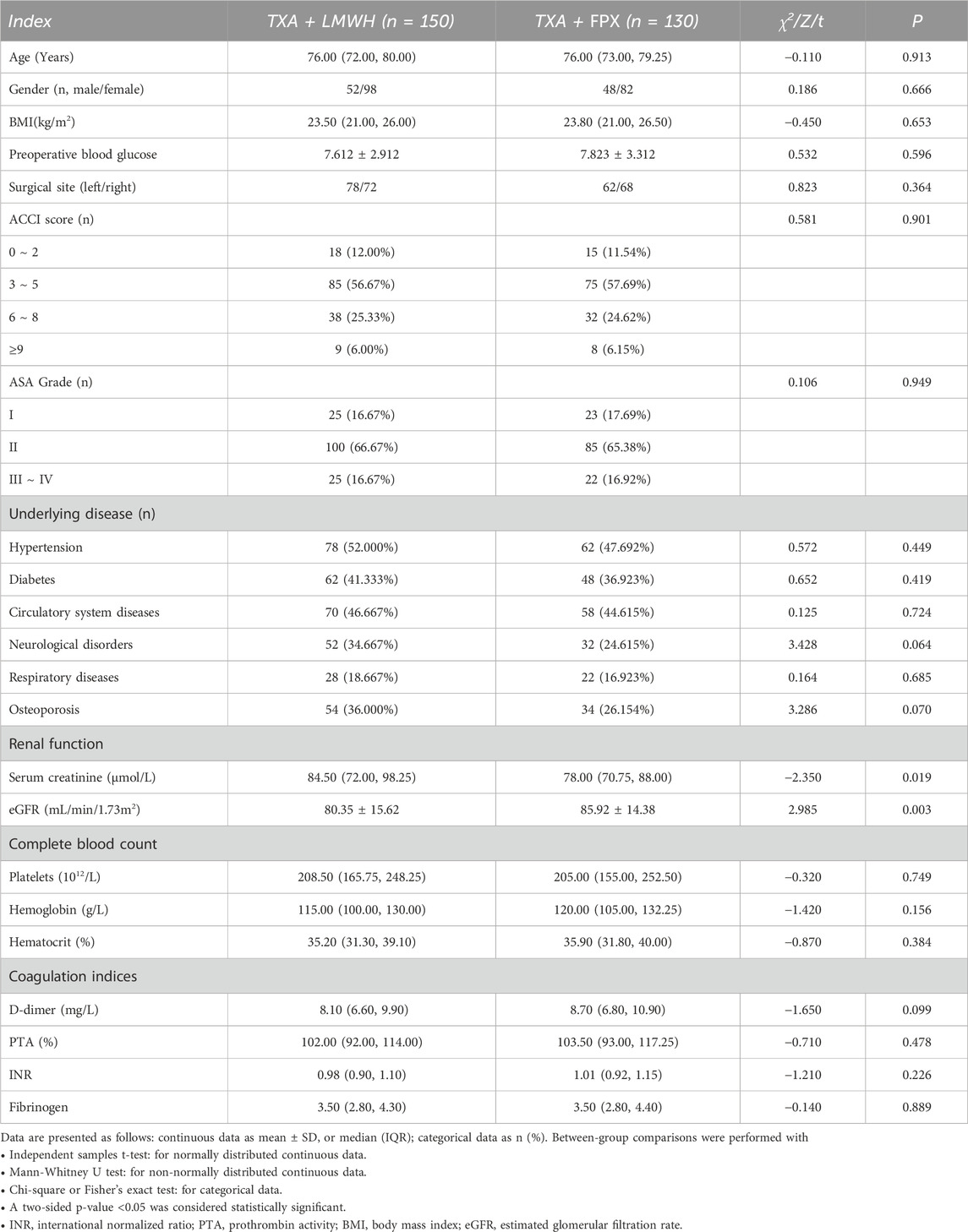
A total of 280 patients undergoing TKA were allocated to the TXA + LMWH (n = 150) or TXA + FPX (n = 130) groups, with no dropouts (Figure 1). Baseline characteristics were comparable between groups except for renal function parameters, which were significantly better in the TXA + FPX group (P < 0.05; Table 2).
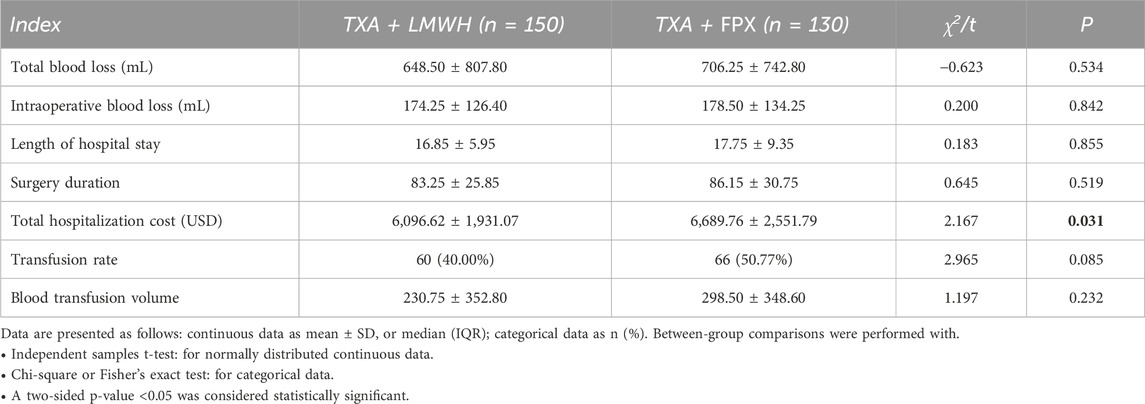
Comparison of Perioperative Outcomes between groups: No statistically significant differences were observed between the two groups in terms of perioperative blood loss, operative duration, hospital stay length, or transfusion requirements. However, total hospitalization costs were significantly lower in the TXA + LMWH group than in the TXA + FPX group (P < 0.05; Table 3).
Comparison of complications between groups: The TXA + FPX group exhibited a significantly higher total complication rate (47.69%, 62/130) compared to the TXA + LMWH group (28.00%, 42/150; χ2 = 10.970, P < 0.05). Specifically, the incidence of MCVT was significantly higher in the TXA + FPX group (32.31% vs. 20.67%; χ2 = 4.620, P < 0.05). No statistically significant differences were observed between groups regarding DVT (10.77% vs. 8.00%; χ2 = 0.580), incision infection (4.62% vs. 0.67%), or hematoma (3.85% vs. 1.33%; all P > 0.05; Table 4; Figure 2). No severe complications, including pulmonary thromboembolism, acute renal failure, seizures, or death, occurred in either group, indicating comparable safety profiles for both anticoagulation regimens.
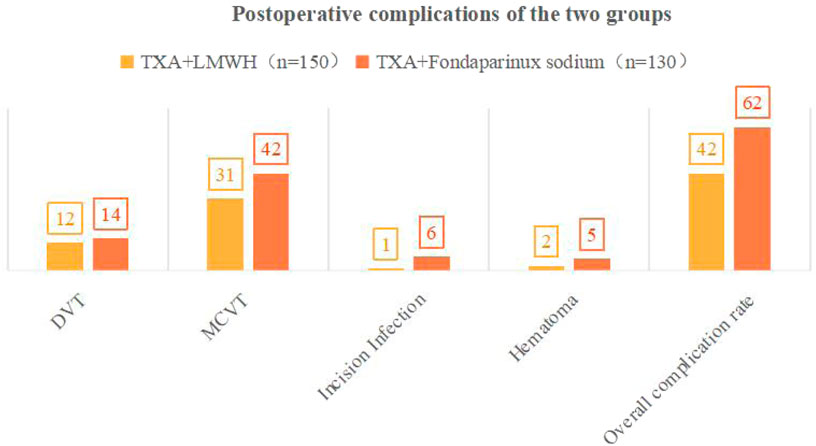
Figure 2. Postoperative complications between groups. Notes:·DVT, Deep Vein Thrombosis; MCVT, Muscular Calf Vein Thrombosis.
Comparison of coagulation and routine blood parameters between groups on postoperative days 1 and 5: On postoperative day 1, significantly higher levels of D-dimer, prothrombin activity (PTA), INR, and fibrinogen were observed in the TXA + FPX group compared to the TXA + LMWH group (P < 0.05). These differences persisted on day 5, with an additional significant difference noted in hemoglobin (all P < 0.05). No significant differences were detected in platelet count and hematocrit between the two groups at either time point (P > 0.05). Longitudinally, significant changes occurred from day 1 to day 5 in PTA, INR, platelet count, and hematocrit in both groups (all P < 0.05). However, hemoglobin levels did not change significantly in the TXA + FPX group (P > 0.05; Table 5).
Discussion
TXA effectively reduces blood loss and transfusion requirements in major orthopedic surgery. Evidence indicates that appropriate anticoagulation following TXA administration does not increase the risk of VTE (Weng et al., 2023; Zhao et al., 2014). Therefore, postoperative anticoagulation must balance efficacy and safety, with LMWH remaining the guideline-recommended option (Hill et al., 2010; Lin et al., 2011; Migliorini et al., 2024; Simon et al., 2023). Consistent with previous findings, LMWH is the most frequently used anticoagulant alongside TXA following TKA, although warfarin and dabigatran etexilate are also common (Tantavisut et al., 2025; Gillette et al., 2013). This retrospective cohort study provided a direct comparative analysis of TXA + LMWH versus TXA + FPX, addressing a notable gap in the literature. The novelty of our study lies in its integrated evaluation of clinical complications, coagulation profiles, and economic outcomes. While the two regimens showed similar results in perioperative blood loss, operative time, hospital stay, and transfusion rate (P > 0.05), the TXA + LMWH regimen was associated with significantly fewer overall complications (particularly MCVT), lower hospitalization costs (P < 0.05), and more favorable postoperative coagulation parameters (D-dimer, PTA, INR, and fibrinogen; P < 0.05). This multi-dimensional assessment suggests that TXA combined with LMWH may represent an optimized hemostatic--anticoagulant strategy following TKA.
FPX, a selective factor Xa inhibitor, produces a linear antithrombin-mediated effect and does not bind Platelet factor IV, thereby reducing the risk of over-anticoagulation and thrombocytopenia (Tantavisut et al., 2025; Gillette et al., 2013). Despite its stronger anticoagulant activity, our results show that FPX combined with TXA did not significantly increase total perioperative blood loss compared with LMWH (648.50 ± 807.80 mL vs. 706.25 ± 742.80 mL, P = 0.534). However, the FPX group exhibited higher rates of postoperative hematoma (3.85% vs. 1.33%) and surgical site infection (4.62% vs. 0.67%), along with a significantly higher overall complication rate (47.69% vs. 28.00%) and MCVT incidence (32.31% vs. 20.67%, P < 0.05). These observations are consistent with its pharmacological profile as a potent anticoagulant and align with meta-analyses reporting an increased risk of surgical site bleeding (OR = 1.43) (Kumar et al., 2019; Haibier et al., 2023; Smith et al., 2010; Fu et al., 2022; Mutlu et al., 2025).
Although FPX was associated with higher MCVT risk, no significant differences were observed in major safety outcomes between the two groups. Neither regimen caused severe complications, and bleeding risks remained well-controlled. This suggests that the standardized use of TXA effectively counteracted the inherent bleeding risks of both anticoagulants. Elevated postoperative coagulation activation markers (D-dimer and fibrinogen) in the FPX group indicate a possible disturbance in the coagulation–fibrinolysis balance, potentially increasing the risk of microcirculatory thrombosis (Kumar et al., 2019; Haibier et al., 2023; Smith et al., 2010; Fu et al., 2022; Mutlu et al., 2025; Karayiannis et al., 2022; Yang et al., 2021). Recent network meta-analyses further confirm that LMWHs maintain a more favorable bleeding profile than other anticoagulants, including FPX, in joint arthroplasty (Kumar et al., 2019).
The TXA + LMWH group also demonstrated significantly lower total hospitalization costs. Coupled with the reduced complication rate, LMWH showed superior cost-effectiveness, which is particularly relevant in resource-limited settings (Chen et al., 2022). These findings indicate that FPX may not be a cost-effective “gold standard” for post-TKA thromboprophylaxis when used with TXA. While meta-analyses support its efficacy in preventing proximal DVT, our data suggest that LMWH performs better in reducing distal MCVT. At our institution, the cost per dose of FPX (2.5 mg) is approximately $6.60 USD, compared with $2.70 USD for LMWH (enoxaparin 4000 IU) [exchange rate: 1 USD ≈7.25 CNY]. Although absolute costs vary across systems, FPX remains roughly 2.4 times more expensive, representing a key finding in cost-effectiveness analysis. Over a 2-week prophylactic course, this translates to substantial savings for the LMWH regimen. The higher incidence of MCVT in the FPX group may further increase costs due to additional imaging (e.g., Doppler ultrasound, ∼$16.50 USD per scan) and prolonged hospitalization. Thus, routine FPX use over LMWH is not economically justified in this context (Defrancesco et al., 2023; Huvers et al., 2005; Cheok et al., 2024). This multi-dimensional assessment suggests that TXA combined with LMWH may represent an optimized hemostatic–anticoagulant strategy following TKA. Our findings align with a previous study, which also reported that in patients undergoing joint replacement, compared to TXA combined with another anticoagulant, TXA + LMWH provided comparable clinical efficacy while demonstrating superior thromboprophylactic efficacy and lower hospitalization costs (Haibier et al., 2025).
However, an important consideration in interpreting these results is the potential for selection bias, inherent in our retrospective design. Fondaparinux is contraindicated in patients with severe renal impairment (creatinine clearance <30 mL/min) and requires dose adjustment or caution in moderate impairment, whereas LMWH has a broader therapeutic window (Ortel et al., 2020; Nutescu et al., 2009). In our cohort, the TXA + FPX group had significantly better baseline renal function, as evidenced by lower serum creatinine and higher estimated glomerular filtration rate (Table 2). This likely reflects clinical practice where physicians preferentially prescribe FPX to patients with preserved renal function. This systematic difference confounds the comparison: the group receiving the more potent anticoagulant (FPX) was also comprised of patients with inherently lower thrombotic risk due to better renal function. Consequently, the observed higher MCVT incidence in the TXA + FPX group might actually be an underestimation of its true risk relative to LMWH in a comparable patient population. This potential bias necessitates a more cautious interpretation of the complication rates and reinforces the conclusion that LMWH remains a robust and versatile option, particularly in unselected or renally impaired TKA populations.
This study was conducted in a unique geographic and demographic setting. The high altitude of Urumqi may predispose patients to elevated hematocrit and a hypercoagulable state, potentially increasing thrombotic risk such as MCVT. Moreover, the multi-ethnic composition of Xinjiang, including Uyghur, Han, and other groups, may contribute to genetic variability in thrombosis susceptibility and drug metabolism. These population-specific factors might have influenced the observed protective association of LMWH against MCVT and may limit generalizability to other regions or populations.
Several limitations should be acknowledged. First, the retrospective, single-center design and non-randomized anticoagulant allocation may introduce selection bias and limit generalizability. Second, the sequential thromboprophylactic regimen (in-hospital LMWH/FPX followed by post-discharge rivaroxaban) means that the outcomes reflect the entire prophylactic strategy rather than the in-hospital anticoagulant alone. This limits the direct attribution of effects, particularly beyond the hospitalization period, specifically to LMWH or FPX. Third, although the sample size was sufficient for primary outcomes, it was underpowered for detecting rare adverse events such as symptomatic pulmonary embolism. Fourth, laboratory evaluations were confined to postoperative days 1 and 5, providing only an early snapshot of coagulation status and missing potential later fluctuations or long-term normalization trends.In addition, the absence of anti-Xa monitoring prevented dose–response analysis, and the 90-day follow-up period was insufficient to assess long-term thrombotic risks.
In conclusion, this retrospective analysis suggests that for TKA patients, TXA combined with LMWH provides comparable perioperative hemostatic efficacy to TXA combined with FPX, while being associated with significantly lower complication rates, better coagulation recovery, and reduced hospitalization costs. The observed advantage of the LMWH regimen, particularly in reducing MCVT, may be even more pronounced given the better baseline renal profile of the FPX group. Although FPX remains an effective anticoagulant, our data indicate that LMWH with TXA may represent a more cost-effective and safer option in a broad clinical setting. Given the potential for selection bias and the unique demographic features of our cohort, these findings should be interpreted with caution, and their generalizability requires validation in prospective, randomized studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The study protocol was reviewed and approved by Sixth People’s Hospital of Xinjiang Uygur Autonomous Region, Institutional Review Board (IRB Approval No. M2021-0510). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AH: Investigation, Visualization, Software, Funding acquisition, Data curation, Conceptualization, Resources, Writing – review and editing, Supervision, Project administration, Methodology, Writing – original draft, Validation, Formal Analysis. MP: Funding acquisition, Resources, Writing – original draft, Writing – review and editing, Formal Analysis, Software, Visualization, Conceptualization, Project administration, Validation, Data curation, Investigation, Supervision. DA: Investigation, Visualization, Software, Funding acquisition, Data curation, Conceptualization, Resources, Writing – review and editing, Supervision, Project administration, Methodology, Writing – original draft, Validation, Formal Analysis. PM: Funding acquisition, Conceptualization, Project administration, Validation, Investigation, Supervision, Writing – review and editing, Methodology, Visualization, Software, Writing – original draft, Formal Analysis, Data curation, Resources.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TKA, Total knee arthroplasty; DVT, Deep vein thrombosis; TXA, Tranexamic acid; LMWH, Low-molecular-weight heparin; INR, International normalized ratio; MCVT, Muscular calf vein thrombosis; PTE, Pulmonary thromboembolism; ASA, American Society of Anesthesiologists; BMI, Body mass index; EMR, Electronic medical record; EBV, Estimated blood volume; PTA, Prothrombin activity; CDC, Centers for Disease Control and Prevention; CT, Computed tomography; MRI, Magnetic resonance imaging; PACU, Post-anesthesia care unit; CT-PA, CT pulmonary angiography.
References
Agarwal, S., Sharma, R. K., and Jain, J. K. (2014). Periprosthetic fractures after total knee arthroplasty. J. Orthop. Surg. Hong. Kong 22 (1), 24–29. doi:10.1177/230949901402200108
Bannuru, R. R., Osani, M. C., Vaysbrot, E. E., Arden, N. K., Bennell, K., Bierma-Zeinstra, S. M. A., et al. (2019). OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 27 (11), 1578–1589. doi:10.1016/j.joca.2019.06.011
BerríOS-Torres, S. I., Umscheid, C. A., Bratzler, D. W., Leas, B., Stone, E. C., Kelz, R. R., et al. (2017). Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 152 (8), 784–791. doi:10.1001/jamasurg.2017.0904
Charlson, M., Szatrowski, T. P., Peterson, J., and Gold, J. (1994). Validation of a combined comorbidity index. J. Clin. Epidemiol. 47 (11), 1245–1251. doi:10.1016/0895-4356(94)90129-5
Chen, L. Y., Khan, N., Lindenbauer, A., and Nguyen, T. H. (2022). When will fondaparinux induce thrombocytopenia? Bioconjug Chem. 33 (8), 1574–1583. doi:10.1021/acs.bioconjchem.2c00316
Cheok, T., Beveridge, A., Berman, M., Coia, M., Campbell, A., Tse, T. T. S., et al. (2024). Efficacy and safety of commonly used thromboprophylaxis agents following hip and knee arthroplasty. Bone Jt. J. 106-B (9), 924–934. doi:10.1302/0301-620X.106B9.BJJ-2023-1252.R2
Defrancesco, C. J., Reichel, J. F., Gbaje, E., Popovic, M., Freeman, C., Wong, M., et al. (2023). Effectiveness of oral versus intravenous tranexamic acid in primary total hip and knee arthroplasty: a randomised, non-inferiority trial. Br. J. Anaesth. 130 (2), 234–241. doi:10.1016/j.bja.2022.11.003
NIH (2022). Expert consensus on perioperative management strategies for accelerated recovery after hip and knee replacement in China. J. Chin. Orthop. Jt. Surg. 15(1):1–9.
Forster, R., and Stewart, M. (2016). Anticoagulants (extended duration) for prevention of venous thromboembolism following total hip or knee replacement or hip fracture repair. Cochrane Database Syst. Rev. 3 (3), Cd004179. doi:10.1002/14651858.CD004179.pub2
Fu, D., Li, L., Li, Y., Liu, X., Chen, H., Wu, N., et al. (2022). Fondaparinux sodium and low molecular weight heparin for venous thromboembolism prophylaxis in Chinese patients with major orthopedic surgery or trauma: a real-world study. BMC Surg. 22 (1), 243. doi:10.1186/s12893-022-01652-6
Gillette, B. P., Desimone, L. J., Trousdale, R. T., Pagnano, M. W., and Sierra, R. J. (2013). Low risk of thromboembolic complications with tranexamic acid after primary total hip and knee arthroplasty. Clin. Orthop. Relat. Res. 471 (1), 150–154. doi:10.1007/s11999-012-2488-z
Guidelines for the diagnosis and treatment of osteoarthritis (2007). Chin. J. Orthop. Surg. 22 (03), 287–288.
Haibier, A., Yusufu, A., Lin, H., Kayierhan, A., Abudukelimu, Y., and Abudurexiti, T. (2023). Efficacy and safety study of low-molecular-weight heparin and fondaparinux sodium after hip arthroplasty: a retrospective cohort study. Orthop. Res. Rev. 15, 253–261. doi:10.2147/ORR.S431372
Haibier, A., Aierxiding, S., Yusufu, A., and Lin, H. (2025). Efficacy and safety study of tranexamic acid combined with low-molecular-weight heparin and nadroparin calcium in postoperative application after joint replacement: a retrospective cohort study. BMC Musculoskelet. Disord. 26 (1), 826. doi:10.1186/s12891-025-08605-z
Hao, J., Pang, P., Liu, X., Chi, W., Luo, Z., Cai, W., et al. (2024). Can the lung ultrasound score predict pulmonary complications after non-thoracic surgery in patients with blunt thoracic trauma: a single-center observational study. J. Clin. Anesth. 99, 111675. doi:10.1016/j.jclinane.2024.111675
Harris, R. N., Moskal, J. T., and Capps, S. G. (2015). Does tranexamic acid reduce blood transfusion cost for primary total hip arthroplasty? A case-control study. J. Arthroplasty 30 (2), 192–195. doi:10.1016/j.arth.2014.08.020
Hill, J., and Treasure, T., and National Clinical Guideline Centre for Acute and Chronic Conditions (2010). Reducing the risk of venous thromboembolism in patients admitted to hospital: summary of NICE guidance. BMJ 340, c95. doi:10.1136/bmj.c95
Huvers, F., Slappendel, R., Benraad, B., van Hellemondt, G., and van Kraaij, M. (2005). Treatment of postoperative bleeding after fondaparinux with rFVIIa and tranexamic acid. Neth J. Med. 63 (5), 184–186.
Kakar, P. N., Gupta, N., Govil, P., and Shah, V. (2009). Efficacy and safety of tranexamic acid in control of bleeding following TKR: a randomized clinical trial. Indian J. Anaesth. 53 (6), 667–671.
Karayiannis, P. N., Agus, A., Bryce, L., Hill, J. C., and Beverland, D. (2022). Using tranexamic acid for an additional 24 hours postoperatively in hip and knee arthroplasty saves money: a cost analysis from the TRAC-24 randomized control trial. Bone Jt. Open 3 (7), 536–542. doi:10.1302/2633-1462.37.BJO-2021-0213.R1
Katz, J. N., Brophy, R. H., Chaisson, C. E., de Chaves, L., Cole, B. J., Dahm, D. L., et al. (2013). Surgery versus physical therapy for a meniscal tear and osteoarthritis. N. Engl. J. Med. 368 (18), 1675–1684. doi:10.1056/NEJMoa1301408
Kumar, A., Talwar, A., Farley, J. F., Muzumdar, J., Schommer, J. C., Balkrishnan, R., et al. (2019). Fondaparinux sodium compared with low-molecular-weight heparins for perioperative surgical thromboprophylaxis: a systematic review and meta-analysis. J. Am. Heart Assoc. 8 (17), e012184. doi:10.1161/JAHA.119.012184
Lin, P. C., Hsu, C. H., Chen, W. S., and Wang, J. W. (2011). Does tranexamic acid save blood in minimally invasive total knee arthroplasty? Clin. Orthop. Relat. Res. 469 (7), 1995–2002. doi:10.1007/s11999-011-1789-y
Migliorini, F., Maffulli, N., Velaj, E., Bell, A., Kämmer, D., Eschweiler, J., et al. (2024). Antithrombotic prophylaxis following total knee arthroplasty: a level I Bayesian network meta-analysis. Eur. J. Orthop. Surg. Traumatol. 34 (6), 2881–2890. doi:10.1007/s00590-024-04071-w
Mutlu, T., Arican, M., Karaduman, Z. O., Turhan, Y., Kaban, İ., Dalaslan, R. E., et al. (2025). Effect of oral + topical and only topical tranaxamic acid application on blood loss and postoperative transfusion in primary total hip arthroplasty. J. Clin. Med. 14 (4), 1275. doi:10.3390/jcm14041275
Nutescu, E. A., Spinler, S. A., Wittkowsky, A., and Dager, W. E. (2009). Low-molecular-weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical settings. Ann. Pharmacother. 43 (6), 1064–1083. doi:10.1345/aph.1L194
Ortel, T. L., Neumann, I., Ageno, W., Beyth, R., Clark, N. P., Cuker, A., et al. (2020). American society of hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 4 (19), 4693–4738. doi:10.1182/bloodadvances.2020001830
Schwarz, T., Buschmann, L., Beyer, J., Halbritter, K., Rastan, A., and Schellong, S. (2010). Therapy of isolated calf muscle vein thrombosis: a randomized, controlled study. J. Vasc. Surg. 52 (5), 1246–1250. doi:10.1016/j.jvs.2010.05.094
Shivali, S., and Thiagarajan, P. (2022). A practical guide to the American society of Anesthesiologists-physical status classification (ASA-PS). Indian J. Anaesth. 66 (4), 299–300. doi:10.4103/ija.ija_526_21
Simon, S. J., Patell, R., Zwicker, J. I., Kazi, D. S., and Hollenbeck, B. L. (2023). Venous thromboembolism in total hip and total knee arthroplasty. JAMA Netw. Open 6 (12), e2345883. doi:10.1001/jamanetworkopen.2023.45883
Smith, S. B., Geske, J. B., Maguire, J. M., Zane, N. A., Carter, R. E., and Morgenthaler, T. I. (2010). Early anticoagulation is associated with reduced mortality for acute pulmonary embolism. Chest 137 (6), 1382–1390. doi:10.1378/chest.09-0959
Streubel, P. N., Ricci, W. M., Wong, A., and Gardner, M. J. (2011). Mortality after distal femur fractures in elderly patients. Clin. Orthop. Relat. Res. 469 (4), 1188–1196. doi:10.1007/s11999-010-1530-2
Sun, G., Wu, J., Wang, Q., Liang, Q., Jia, J., Cheng, K., et al. (2019). Factor Xa inhibitors and direct thrombin inhibitors versus low-molecular-weight heparin for thromboprophylaxis after total hip or total knee arthroplasty: a systematic review and meta-analysis. J. Arthroplasty 34 (4), 789–800. doi:10.1016/j.arth.2018.11.029
Tantavisut, S., Artykbay, S., Tangwiwat, P., and Susantitaphong, P. (2025). Topical tranexamic acid in hip and knee surgery: a meta-analysis of randomized-controlled trials. EFORT Open Rev. 10 (7), 454–465. doi:10.1530/EOR-2024-0152
Weber, P., SteinbrüCK, A., Paulus, A. C., Woiczinski, M., Schmidutz, F., Fottner, A., et al. (2017). Partial exchange in total hip arthroplasty: what can we combine. Orthopade 46 (2), 142–147. doi:10.1007/s00132-016-3380-4
Weng, N., Gou, Y., and Kuang, F. (2023). Efficacy and safety of tranexamic acid in unicompartmental knee arthroplasty: a systematic review and meta-analysis. Asian J. Surg. 46 (8), 3033–3045. doi:10.1016/j.asjsur.2022.10.078
Xiong, X., Li, T., and Cheng, B. (2023). Anemia and formation of deep vein thrombosis before operation in patients with knee osteoarthritis: a cross-sectional study. J. Orthop. Surg. Res. 18 (1), 33. doi:10.1186/s13018-023-03518-w
Yang, Y., Wang, Z., Wang, F., Zhao, X., Yang, K., He, J., et al. (2021). Prospective, randomised, controlled study on the efficacy and safety of different strategies of tranexamic acid with total blood loss, blood transfusion rate and thrombogenic biomarkers in total knee arthroplasty: study protocol. BMJ Open 11 (2), e038399. doi:10.1136/bmjopen-2020-038399
Yao, M. H., Xu, W. J., and Shen, W. Y. (2018). Application of rapid rehabilitation concept in single meniscus replacement patients. J. Qilu Nurs. 24 (20), 68–71.
Zhao, Y. C., Yan, G., Wei, C., Yuejv, L., and Ying-Ze, Z. (2014). Reduced blood loss after intra-articular tranexamic acid injection during total knee arthroplasty: a meta-analysis of the literature. Knee Surg. Sports Traumatol. Arthrosc. 22 (12), 3181–3190. doi:10.1007/s00167-013-2814-3
Keywords: osteoarthritis, total knee arthroplasty, low molecular weight heparin, fondaparinux, coagulation parameters, thrombosis
Citation: Haibier A, Pan M, Anwar D and Ma P (2025) Efficacy and safety of tranexamic acid combined with low molecular weight heparin versus fondaparinux sodium following total knee arthroplasty: a retrospective cohort study. Front. Pharmacol. 16:1667528. doi: 10.3389/fphar.2025.1667528
Received: 19 August 2025; Accepted: 06 November 2025;
Published: 25 November 2025.
Edited by:
Corrado Ciatti, Guglielmo da Saliceto Hospital, ItalyReviewed by:
Yan Liu, Lanzhou University, ChinaMücahid Osman Yücel, Duzce University, Türkiye
Tim Cheok, Flinders University, Australia
Copyright © 2025 Haibier, Pan, Anwar and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pengcheng Ma, bWFwZW5nY2hlbmcwNTE4QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Abuduwupuer Haibier1†
Abuduwupuer Haibier1† Dilxat Anwar
Dilxat Anwar