- 1Department of Radiotherapy and Oncology, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, China
- 2Department of Development and Regeneration, Stem Cell Institute, Katholieke Universiteit (KU) Leuven, Leuven, Belgium
- 3Department of Gastrointestinal Surgery, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, China
Curcumin, a natural polyphenolic compound from Curcuma longa, has been extensively investigated for its potential role in colorectal cancer (CRC) prevention and therapy. Preclinical studies suggest that curcumin can modulate gut microbiota composition, influence immune cell subsets such as M1/M2 macrophages, Treg/Th17 cells, and CD8+ T cells, and interfere with oncogenic signaling cascades including NF-κB, PI3K/Akt, and Wnt/β-catenin. These findings collectively highlight curcumin as a biologically active compound with broad mechanistic relevance. However, most evidence derives from in vitro assays at supra-physiological concentrations or high-dose animal models, raising concerns about pharmacological validity and clinical translatability. Curcumin is also recognized as a pan-assay interfering compound (PAINS), which may account for part of its pleiotropic activity and complicates interpretation of preclinical findings. Clinical trials to date have largely confirmed safety and biomarker modulation but have not demonstrated clear improvements in progression-free or overall survival. In this review, we critically appraise the available preclinical and clinical evidence on curcumin in CRC, highlighting both its mechanistic promise and the substantial limitations that constrain its therapeutic relevance, while outlining priorities for future research.
1 Introduction
Colorectal cancer (CRC) is one of the most prevalent malignant tumors of the digestive system and continues to pose a major public health burden worldwide (Siegel et al., 2023). Despite advances in screening, surgery, radiotherapy, and chemotherapy that have improved overall survival, patients with advanced-stage disease still face poor outcomes, characterized by recurrence, metastasis, and limited response to conventional treatments (Nussbaum et al., 2022; Doxtater and Tripathi, 2023). Tumor heterogeneity, therapy resistance, and immune escape remain significant barriers, highlighting the urgent need for innovative and well-tolerated strategies (Lv et al., 2023). CRC pathogenesis extends far beyond isolated genetic mutations or uncontrolled proliferation. Instead, it reflects a complex interplay between gut microbiota, immune responses, and key oncogenic signaling pathways. The gut microbiota, the largest microbial ecosystem in humans, shapes host immunity and mucosal barrier function through metabolites such as short-chain fatty acids (SCFAs) and secondary bile acids (Dong et al., 2023; Hays et al., 2024). Dysbiosis not only promotes chronic inflammation but also reprograms immune surveillance, thereby fueling tumor progression (Yang et al., 2023a). Meanwhile, aberrant activation of signaling cascades such as NF-κB, Wnt/β-catenin, and PI3K/Akt orchestrates malignant transformation, stemness maintenance, and metastatic dissemination, often in close interaction with microbiota–immune crosstalk.
In this context, polyphenolic compounds from natural products have drawn increasing attention due to their multi-targeted actions and favorable safety profiles. Curcumin, the principal bioactive constituent of Curcuma longa, has long been used in traditional medicine and is now extensively investigated as an anticancer agent (Kunnumakkara et al., 2008; Wilken et al., 2011; Li et al., 2020). Preclinical studies suggest that curcumin induces apoptosis, inhibits invasion, remodels the tumor microenvironment, and modulates both microbiota and immune populations. It has been reported to promote beneficial bacteria (e.g., Lactobacillus and Bifidobacterium) while suppressing pathogenic taxa such as Clostridium difficile and Bacteroides fragilis, thereby indirectly influencing immune tone and tumor progression (Lamichhane et al., 2024; Zhu and He, 2024).
However, enthusiasm must be tempered by recognition of important limitations. Much of the mechanistic evidence derives from in vitro studies employing supra-physiological concentrations, often orders of magnitude above achievable plasma levels in humans. Moreover, curcumin has been classified as a pan-assay interfering compound (PAINS), raising the possibility that some reported anticancer activities reflect non-specific or artifactual effects rather than true pharmacological actions (Nelson et al., 2017). Clinical trials to date confirm safety and modest biomarker modulation but provide limited evidence for survival benefit, largely due to small sample sizes, heterogeneous cohorts, and underpowered study designs. Against this background, the present review aims to provide a critical appraisal of curcumin’s potential in CRC, integrating evidence across the microbiota–immune–signaling axis. We emphasize both mechanistic promise and pharmacological uncertainties, evaluate preclinical and clinical evidence with attention to dose ranges, models, and methodological rigor, and outline future directions for improving bioavailability, refining study designs, and strengthening translational relevance.
2 Mechanism of CRC development and challenges in precision treatment
CRC is a heterogeneous malignancy that progresses through a multistep adenoma–carcinoma sequence driven by genetic mutations, epigenetic modifications, chronic inflammation, and microbial dysbiosis (Okugawa et al., 2015; Seligmann et al., 2022; Fakih et al., 2023; Storandt et al., 2023). High-throughput omics technologies have revealed CRC to be far more complex than a linear genetic model, highlighting its molecular heterogeneity and the interplay of signaling, immune regulation, and gut microbiota within the tumor microenvironment (TME). While this growing knowledge has improved molecular classification, its direct impact on treatment strategies remains constrained by variability in study design and translational gaps.
2.1 Molecular mechanisms and precision therapy challenges
CRC development is classically explained by chromosomal instability (CIN), microsatellite instability (MSI), and CpG island methylator phenotype (CIMP) (Figure 1). CIN accounts for approximately 85% of sporadic CRC cases, involving APC and TP53 loss as well as KRAS or PIK3CA activation, which drive aberrant Wnt/β-catenin and PI3K/Akt signaling (Kudryavtseva et al., 2016; Hoevenaar et al., 2020; Al-Joufi et al., 2022). MSI, resulting from mismatch repair deficiency, occurs in 10%–15% of cases, and is associated with hypermutability and responsiveness to immune checkpoint blockade (The Cancer Genome Atlas Network, 2012; Dienstmann et al., 2017; Le et al., 2017). CIMP is characterized by widespread promoter methylation, frequently linked to BRAF mutations (Ichimura et al., 2015). The Cancer Genome Atlas (TCGA) initially provided a comprehensive molecular characterization of colorectal cancer (The Cancer Genome Atlas Network, 2012), and subsequent integrative analyses proposed four consensus molecular subtypes (CMS1–4) with distinct signaling, immune, and stromal profiles (The Cancer Genome Atlas Network, 2012; Guinney et al., 2015).
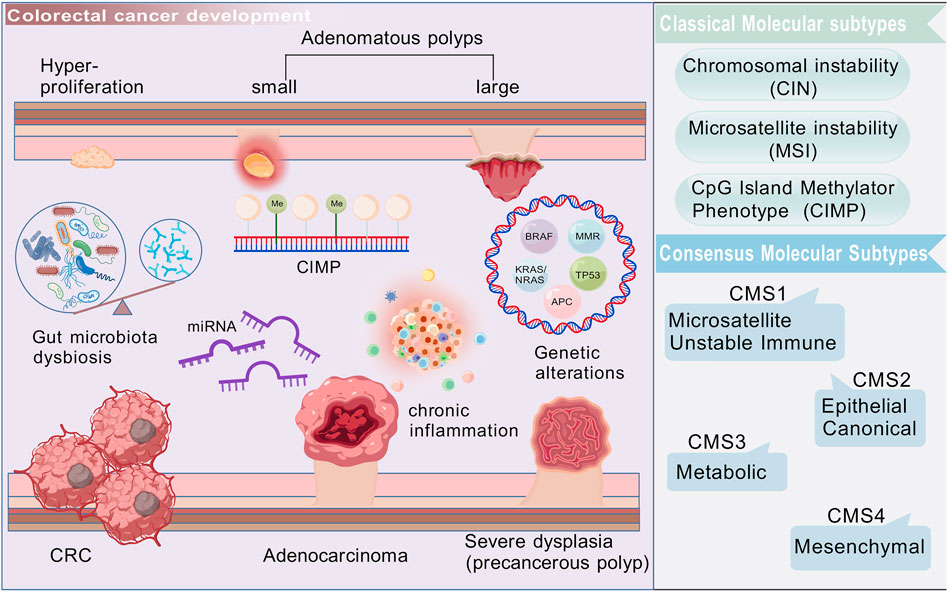
Figure 1. Colorectal cancer is a heterogeneous malignant tumor, originating from epithelial cells in the colon or rectum. Its development is a multistep process that involves the transformation of adenomatous polyps into invasive cancer, accompanied by complex pathological processes, such as gene mutations, epigenetic changes, inflammation, microenvironment shaping, and dysbiosis of gut microbiota. Created with BioGDP.com.
Although these classifications are valuable for research and biomarker discovery, their pharmacological relevance is still limited. Many studies report associations between CMS subtypes and treatment responses, yet most are retrospective and lack prospective stratification in clinical trials. Consequently, while molecular subtyping refines our understanding of CRC biology, its direct utility in precision therapy is still under active validation.
2.2 Gut microbiota in CRC development
Gut microbiota exerts profound effects on CRC through both protective and pathogenic species. Enrichment of Fusobacterium nucleatum, Bacteroides fragilis, and colibactin-producing Escherichia coli promotes tumorigenesis via TLR signaling, bacterial toxins, and barrier disruption (Lopez et al., 2021). Conversely, metabolites such as short-chain fatty acids (SCFAs) enhance mucosal immunity, modulate Treg activity, and inhibit HDACs, conferring anti-inflammatory and anti-tumor effects (Fellows et al., 2018; Mann et al., 2024). Secondary bile acids such as deoxycholic acid, however, can promote tumorigenesis by inducing oxidative stress and activating Wnt signaling (Ocvirk and O’Keefe, 2021).
Yet, most of these findings are derived from murine models or correlative human studies, where confounding factors such as diet and antibiotic use complicate interpretation. Moreover, metabolite concentrations reported to inhibit cancer cell growth in vitro often exceed physiological levels achievable in vivo. Therefore, while the microbiota is increasingly recognized as a driver and modifier of CRC, the causal mechanisms and pharmacological relevance in humans require more rigorous validation.
2.3 Tumor microenvironment and immune imbalance
The CRC tumor microenvironment (TME) integrates cancer cells with stromal fibroblasts, endothelial cells, immune infiltrates, and soluble mediators. Early immune surveillance can suppress tumor initiation, but tumors progressively achieve immune escape by recruiting Tregs and MDSCs, polarizing macrophages toward the M2 phenotype, and inducing T-cell exhaustion through PD-L1 and other checkpoints (Lei et al., 2020; Jancewicz et al., 2021). Effector populations such as CD8+ T cells and NK cells become functionally suppressed, while the balance between Th1/Th17 cells and Tregs strongly influences prognosis (Liu et al., 2021a; Calvo-Barreiro et al., 2023).
Despite extensive mechanistic insights, much of the evidence originates from murine models with limited comparability to human TME complexity. Importantly, the minimal effective concentrations of immunomodulatory factors or interventions are seldom reported, making pharmacological translation uncertain. Thus, while TME reshaping represents an attractive target, more rigorous studies with standardized immune readouts and clinically relevant dosing are needed.
2.4 Bottlenecks in precision medicine
Precision oncology has introduced immune checkpoint inhibitors (ICIs) and targeted therapies that yield remarkable benefits in defined CRC subgroups, such as MSI-high tumors (Le et al., 2017). However, heterogeneity remains a major barrier: CMS4 tumors, characterized by stromal activation and immune exclusion, show poor response to ICIs (Becht et al., 2016; Dienstmann et al., 2017), while accumulating evidence indicates that variations in gut microbiota strongly influence immunotherapy outcomes (Gopalakrishnan et al., 2018; Matson et al., 2018). Predictive biomarkers such as CD8+ T-cell infiltration, MSI status, or microbial composition are promising, but lack standardized validation across trials. Moreover, clinical trial evidence is often weakened by small sample sizes, heterogeneous cohorts, short follow-up, and inconsistent formulations of investigational agents such as curcumin. Microbiota profiling methodologies also vary widely, limiting reproducibility. Finally, immune escape mechanisms are highly redundant, suggesting that multi-target approaches will be required. These limitations highlight the gap between conceptual advances and clinical translation in CRC precision medicine.
3 Basic characteristics of curcumin and its anti-CRC mechanism: from signaling pathways to microecological remodeling
Curcumin has been extensively investigated for its anti-inflammatory, antioxidant, anticancer, immunomodulatory, and neuroprotective properties (Boroumand et al., 2018; Memarzia et al., 2021). In the CRC, curcumin’s therapeutic potential is not limited to direct inhibition of tumor proliferation or induction of apoptosis; preclinical studies show it also remodels the tumor microenvironment, modulates immune responses, and restores gut microbiota composition and metabolite profiles (Deng et al., 2024; Shakhpazyan et al., 2024; Zhou et al., 2025). This section reviews the structural basis and pharmacological activities of curcumin, focusing on its capacity to modulate oncogenic signaling pathways, immune responses, and intestinal microecology.
3.1 Structural characteristics and pharmacological activities of curcumin
Curcumin (C21H20O6) consists of two aromatic rings linked by an α,β-unsaturated β-diketone moiety (Priyadarsini, 2014). This conjugated structure underlies its strong resonance and free radical-scavenging ability, conferring antioxidant and anti-inflammatory effects. However, curcumin’s pharmacological utility is constrained by poor water solubility, low oral bioavailability, and rapid metabolic clearance. To address these challenges, advanced formulations—such as nano-emulsions, liposomes (Ternullo et al., 2019), solid lipid nanoparticles (Mulik et al., 2012; Yeo et al., 2022), cyclodextrin inclusion complexes, and phospholipid complexes (Maleki Dizaj et al., 2022)—have been developed. These systems significantly improve stability, absorption, and tissue targeting, though their comparative efficacy across clinical settings remains under evaluation. Importantly, pharmacological conclusions about curcumin’s activity must always be interpreted in light of formulation-dependent differences in bioavailability.
3.2 Regulatory roles in anti-tumor signaling pathways
Aberrant signaling is a hallmark of CRC, and curcumin is capable of intervening in several key pathways (Figure 2). The PI3K/Akt/mTOR axis, frequently activated in CRC, drives proliferation, survival, and therapy resistance (Stefani et al., 2021). Curcumin suppresses this pathway by inhibiting PI3K p110α and Akt phosphorylation, thereby downregulating mTOR activity and sensitizing resistant cells to chemotherapy (Chen et al., 2023; Deswal et al., 2024). While this suggests strong chemosensitizing potential, most evidence derives from in vitro studies at micromolar concentrations, which raises questions about conversion correlations. The Wnt/β-catenin pathway, central to stemness and metastasis in CRC, is another target. Curcumin accelerates β-catenin degradation and reduces downstream c-Myc and cyclin D1 expression, impairing clonogenicity and tumor-initiating capacity (Le and Kim, 2019; Liao et al., 2023). This mechanistic rationale is compelling, yet in vivo evidence remains limited, and dose-response relationships are not consistently defined.
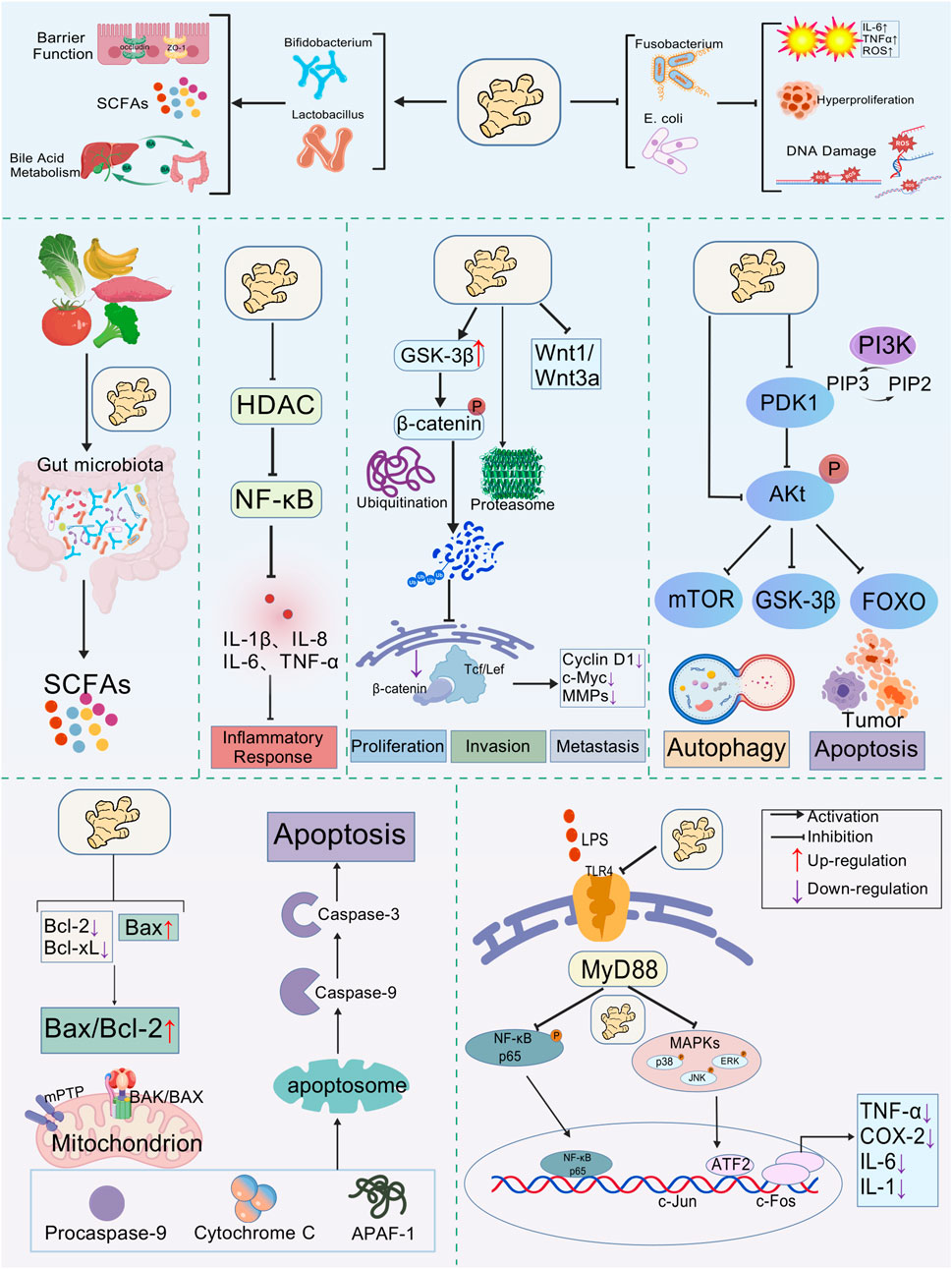
Figure 2. Multi-target mechanism framework of curcumin’s antitumor effects. Curcumin promotes the proliferation of beneficial gut bacteria (such as Lactobacilli and Bifidobacteria) while inhibiting pathogenic microorganisms (such as Escherichia coli and Fusobacterium). This effect is achieved by regulating the Bax/Bcl-2 balance and inhibiting carcinogenic signaling pathways (NF-κB, PI3K/AKT, Wnt/β-catenin), thus inducing tumor cell apoptosis and autophagy. Created with BioGDP.com.
The TLR4/MyD88/NF-κB pathway, which links microbial dysbiosis to chronic inflammation and carcinogenesis, is also suppressed by curcumin. It blocks TLR4 recognition of pathogenic ligands, prevents NF-κB activation, and reduces pro-inflammatory mediators such as IL-6, TNF-α, and COX-2 (Boozari et al., 2019; Guo et al., 2021). Although these findings support the concept of an “anti-inflammatory shield,” they often rely on models with artificial stimulation or supraphysiological curcumin exposure. Therefore, while curcumin’s multi-targeted signaling modulation is well established, the pharmacological strength of evidence varies depending on the model system and achievable concentrations.
3.3 Tumor immune regulatory effects
Curcumin exerts multi-faceted immunomodulatory effects. It enhances CD8+ T-cell proliferation and cytotoxic activity, increasing perforin and granzyme B secretion (Liu et al., 2021b). Furthermore, it rebalances CD4+ T-cell subsets by reducing Tregs and promoting Th1/Th17 responses (Zou et al., 2018; Shafabakhsh et al., 2019; Fu et al., 2021). Particularly noteworthy is the ability of curcumin to convert Foxp3+ Tregs into Th1-like cells, thereby reinforcing anti-tumor immunity (Zou et al., 2018). Macrophage polarization is another critical target, CRC TMEs are enriched with pro-tumorigenic M2 macrophages, while curcumin suppresses M2 markers (IL-10, ARG1) and activates STAT6/MAO-A-related switches, shifting toward an M1 phenotype (Jiang et al., 2022).
Nevertheless, immune regulation by curcumin is context- and dose-dependent. In vitro, curcumin often suppresses dendritic cell (DC) maturation, reducing co-stimulatory molecule expression and T-cell priming capacity (Shirley et al., 2008). In contrast, in vivo studies using high-bioavailability formulations demonstrate enhanced DC antigen presentation via STAT3 inhibition (Hayakawa et al., 2020). These conflicting results underscore the importance of formulation, concentration, and biological context, and highlight the need for dose-optimized strategies in clinical applications.
3.4 Gut microbiota remodeling and immune-metabolic regulation
Curcumin also exerts its effects indirectly by remodeling gut microbiota. It increases beneficial genera such as Bifidobacterium and Lactobacillus while reducing pro-inflammatory taxa such as Fusobacterium, Prevotella, and Enterobacteriaceae (Pluta et al., 2020; Zhu and He, 2024). These changes shift microbial metabolism toward greater short-chain fatty acid (SCFA) production, particularly butyrate, which supports epithelial energy metabolism, reinforces barrier function, and epigenetically regulates T-cell differentiation (Van Deuren et al., 2022). Curcumin also strengthens barrier integrity by upregulating tight junction proteins (ZO-1, occludin, claudin-1), thereby limiting lipopolysaccharide (LPS) translocation and systemic inflammation (Burge et al., 2019; Cao et al., 2020). This creates a feedback loop in which microbiota restoration, metabolic regulation, and immune modulation act synergistically to restrain tumorigenesis.
However, the majority of microbiota studies rely on rodent models, with results influenced by diet, housing, and antibiotic exposure. Human evidence is relatively sparse, and mechanistic links remain associative rather than causal. Moreover, the concentrations of curcumin required to shift microbial communities are not always aligned with pharmacologically achievable levels. Therefore, while gut microbiota remodeling is a promising dimension of curcumin’s activity, its clinical translation requires rigorous validation through standardized multi-omics studies and controlled interventions.
4 Progress in preclinical and translational research on curcumin
Despite extensive research into the molecular mechanisms underlying curcumin’s anti-CRC effects, its clinical translation ultimately depends on the robustness of preclinical evidence across multiple experimental levels. Chemically induced CRC models, such as the azoxymethane/dextran sulfate sodium (AOM/DSS) inflammation-associated system, have consistently demonstrated that oral or gavage administration of curcumin reduces tumor number and volume, alleviates mucosal ulceration, and decreases dysplastic gland formation (Deng et al., 2024). Mechanistic analyses highlight suppression of the TLR4/MyD88–NF-κB pathway and downregulation of inflammatory mediators, including IL-6, TNF-α, and COX-2 (Buhrmann et al., 2019; Liu et al., 2020; Iglesias et al., 2022; Yang et al., 2023b). These results provide strong biological plausibility for curcumin as an inflammation-modulating agent. However, it must be emphasized that the doses applied in rodents (often >100 mg/kg/day) are much higher than typical human exposures, and the curcumin formulations used are heterogeneous, limiting extrapolation to human pharmacology (Anand et al., 2007; Sharma et al., 2007).
In xenograft models using human CRC cells, curcumin has been shown to suppress tumor growth and enhance sensitivity to 5-fluorouracil (5-FU), oxaliplatin, and irinotecan (Howells et al., 2019; Zhao et al., 2020; Zheng et al., 2021). Mechanistically, this effect is associated with inhibition of PI3K/Akt signaling (Astinfeshan et al., 2019; Jin et al., 2021; Liu et al., 2021b), suppression of cancer stemness markers (SOX2, OCT4, CD44, CD133, LGR5), and attenuation of IL-6/STAT3-and NF-κB-driven survival signaling (Prud’homme, 2012; Hu et al., 2019; Brockmueller et al., 2023; Kubatka et al., 2024). Furthermore, curcumin downregulates anti-apoptotic proteins and modulates ATP-binding cassette (ABC) transporters, such as P-glycoprotein (P-gp), thereby reducing drug efflux and increasing intracellular concentrations of chemotherapeutics (Fathy Abd-Ellatef et al., 2020). These findings suggest a strong chemosensitizing role for curcumin. Yet, most xenograft studies involve immunodeficient mice that lack a functional adaptive immune system (Sharma et al., 2005; Olive and Tuveson, 2006), preventing evaluation of immune-dependent mechanisms that are central to human CRC biology. Moreover, the curcumin concentrations applied in vitro (often 10–50 μM) far exceed clinically achievable plasma levels (<1 μM), raising questions about translational pharmacological relevance (Anand et al., 2007; Sharma et al., 2007; Wilken et al., 2011).
Patient-derived tumor organoids (PDOs) represent an important step toward bridging preclinical and clinical research. Studies have shown that even relatively low micromolar concentrations of curcumin can inhibit organoid proliferation, suppress ERK phosphorylation, and reduce expression of stemness markers such as CD44, CD133, and LGR5 (Elbadawy et al., 2021). Compared with cell-line xenografts, organoids preserve inter-patient heterogeneity and more closely mimic clinical tumor responses, making them attractive platforms for personalized drug testing and screening of combination regimens. However, PDO studies remain scarce, with small sample sizes and variable culture conditions, limiting their generalizability. In addition, most work has focused on acute responses (≤72 h) rather than long-term resistance dynamics, which may underestimate the challenges of clinical translation (Elbadawy et al., 2021; Furbo et al., 2022).
Collectively, preclinical evidence consistently indicates that curcumin exerts anti-CRC effects through multi-targeted signaling suppression, immune modulation, and chemosensitization. Nevertheless, the majority of findings are constrained by supraphysiological dosing, variability in curcumin formulations, limited immune-competent models, and small-scale PDO studies. Thus, while curcumin emerges as a promising adjunct or synergistic therapy, rigorous pharmacological assessment—encompassing standardized dosing protocols, bioavailability optimization, immune-competent animal studies, and biomarker-guided patient stratification—is required before reliable clinical translation can be achieved.
5 Limitations and pharmacological appraisal of curcumin in CRC
Although curcumin has been widely studied for its potential in CRC, a rigorous pharmacological appraisal reveals important limitations that temper enthusiasm for its therapeutic translation (Table 1). Without such critical analysis, there is a risk of overestimating curcumin’s value based on preclinical findings alone. A first concern relates to curcumin’s chemical nature as a PAINS (Baker, 2017; Nelson et al., 2017; Padmanaban and Nagaraj, 2017). Such compounds are notorious for producing false-positive results in diverse bioassays due to intrinsic properties such as redox activity, fluorescence interference, nonspecific protein binding, or covalent modification of nucleophilic residues. These features complicate the interpretation of experiments that report modulation of key signaling pathways, including NF-κB, PI3K/Akt, Wnt/β-catenin, and STAT3. Many of the supposed “multi-targeted” effects may reflect assay artifacts rather than genuine pharmacological specificity. Unless confirmed through orthogonal assays or validated in vivo, such mechanistic claims should be regarded with caution.
The limitations become even more evident when examining in vitro studies. Anti-proliferative or chemosensitizing effects of curcumin in CRC cell lines such as HCT116, HT-29, or SW480 are typically observed at concentrations between 5 and 50 μM, with minimal active concentrations rarely below 5 μM (Link et al., 2013; Li et al., 2021; Yang et al., 2022). Yet, pharmacokinetic studies consistently show that even with oral administration of 8–12 g/day in humans, plasma concentrations seldom exceed 1–2 μM, and circulating curcumin largely exists in conjugated forms rather than as free active compound (Vareed et al., 2008; Urošević et al., 2022). This discrepancy underscores a key translational gap, many effects reported in vitro are unlikely to occur in vivo at physiologically achievable exposures. Furthermore, in vitro experiments often lack rigorous pharmacological controls, with positive and negative comparators inconsistently included and standardized pharmacodynamic parameters such as IC50 or therapeutic index rarely reported. The heterogeneity of exposure times—ranging from short-term assays to long-term clonogenic models—further complicates interpretation and limits extrapolation to clinical contexts.
Animal models provide supportive but similarly constrained evidence. Xenograft models using immunodeficient mice have demonstrated that curcumin can inhibit tumor growth or enhance the effects of chemotherapy, yet these studies often rely on doses such as 100 mg/kg (Shaikh et al., 2021), which correspond to several grams per day in human equivalent dosing, levels not feasible in clinical practice. In addition, many animal studies employ formulations with artificially enhanced bioavailability, including nanoparticles or curcumin–piperine combinations, which are not consistently available or standardized for human use. A further limitation is the lack of pharmacokinetic monitoring in tumor tissues, making it unclear whether observed effects are attributable to direct tumor exposure or to systemic anti-inflammatory activity (Dhillon et al., 2008; Ozawa-Umeta et al., 2020). The frequent reliance on immunodeficient hosts also omits critical contributions of immune and microbiota pathways, both of which are proposed to be central to curcumin’s mechanisms in CRC.
Clinical trials, though offering the most relevant data, remain small, heterogeneous, and often underpowered. Early pharmacokinetic and pharmacodynamic studies confirmed that curcumin is safe at doses up to 3.6 g/day and demonstrated reductions in biomarkers such as DNA adducts, but these were limited to fewer than 20 patients and focused on surrogate endpoints of uncertain predictive value (Sharma et al., 2004; Garcea et al., 2005). Phase IIa studies, including those assessing aberrant crypt foci (ACF) or familial adenomatous polyposis (FAP), suggested modest benefits but were limited by small sample sizes and endpoints not directly linked to long-term clinical outcomes (Carroll et al., 2011). More recent studies combining curcumin with chemotherapy in metastatic CRC have shown acceptable safety but no clear improvements in progression-free or overall survival, while randomized placebo-controlled trials in locally advanced rectal cancer failed to demonstrate clinical benefit and in some cases even suggested numerically worse complete response rates in curcumin-treated groups (Gunther et al., 2022). These results highlight a striking disconnect between preclinical promise and clinical reality.
Taken together, the current body of evidence illustrates that while curcumin is biologically active in CRC-related systems, its translation into clinically meaningful efficacy remains unproven. The PAINS nature of curcumin raises the possibility of false-positive mechanistic findings, in vitro studies rely on supraphysiological concentrations, animal models use doses or formulations not applicable to patients, and clinical trials remain exploratory and inconclusive. This evidence landscape underscores the need for a more rigorous and standardized research framework. Future work should emphasize dose–response characterization, tissue-level pharmacokinetic–pharmacodynamic correlation, the use of clinically relevant and immunocompetent models, and adequately powered randomized trials with standardized formulations and hard endpoints such as progression-free survival and overall survival. Equally important is the incorporation of biomarker-driven patient stratification, allowing identification of subgroups most likely to benefit from curcumin-based interventions. In summary, curcumin research in CRC offers both opportunities and cautionary lessons. It demonstrates the potential of natural products to modulate complex biological systems, but also illustrates the risks of overinterpreting descriptive or artifact-prone data. Only by adopting pharmacologically rigorous and clinically robust approaches can the field determine with confidence whether curcumin holds genuine therapeutic relevance for colorectal cancer.
6 Future directions
Although curcumin has long been celebrated as a natural product with broad pharmacological potential, its clinical translation in CRC continues to face major obstacles. The accumulated literature demonstrates that curcumin can influence the microbiota–immune–signaling axis and reshape the tumor microenvironment, yet the strength of these findings remains uncertain. A critical challenge arises from the recognition that curcumin belongs to the class of PAINS, which are notorious for producing assay artifacts and nonspecific signals in biochemical and cellular experiments. This raises the risk that many of the reported “anticancer” effects may reflect in vitro artifacts rather than pharmacologically meaningful mechanisms. Future research must therefore adopt a more rigorous and hypothesis-driven approach to establish the true scope and relevance of curcumin’s biological activity.
One of the most pressing needs is to move beyond descriptive evidence toward rigorous pharmacological standards. Current reports often claim that curcumin inhibits NF-κB activation, regulates CD8+ T cells, or alters gut microbiota composition, but such conclusions are frequently unsupported by systematic dose–response analyses or pharmacokinetic validation. The use of concentrations far above clinically achievable levels undermines translational credibility. It is essential that future studies define minimal effective concentrations, establish concentration–effect curves, and incorporate appropriate controls to exclude nonspecific PAINS-related effects (Nelson et al., 2017). Moreover, detailed reporting of formulation, purity, and delivery systems must become standard to enable reproducibility and cross-study comparison. Without this level of rigor, mechanistic claims risk remaining anecdotal rather than clinically actionable. Equally important is the need to bridge the persistent gap between in vitro assays and in vivo relevance. Curcumin’s proposed mechanisms of action, ranging from immune modulation to microbiota reshaping, cannot be meaningfully assessed in conventional tumor cell lines alone. More advanced models are required, including patient-derived organoids co-cultured with immune components, immune-competent mouse models, and germ-free or microbiota-humanized systems that capture the host–microbiota–immune interplay. These platforms will help clarify whether curcumin’s observed effects reflect direct cytotoxicity, immunomodulation, microbial reshaping, or artifacts of simplified assay systems. Without this integration, the field risks overinterpreting results from reductionist models. Another critical issue lies in curcumin’s unfavorable pharmacokinetic profile. Poor solubility, extensive metabolism, and rapid clearance limit systemic exposure, raising the paradox of how curcumin exerts strong in vitro activity at concentrations never attained in human plasma. Although formulation strategies such as nanoparticles, liposomes, and phytosomal complexes have improved bioavailability, systematic pharmacokinetic/pharmacodynamic (PK/PD) correlations remain rare. Future research should consistently report plasma and tissue concentrations alongside pharmacodynamic readouts, calculate human equivalent doses when extrapolating from animal models, and explore localized delivery systems that exploit curcumin’s potential activity in the gut mucosa without requiring high systemic levels. Only through this type of pharmacological discipline can the field resolve the disconnect between laboratory efficacy and clinical plausibility.
Clinical evidence to date, while encouraging in isolated trials, remains fragmented and underpowered. Small sample sizes, heterogeneous formulations, and inconsistent endpoints have limited the interpretability of curcumin trials in CRC. Future studies must be designed as adequately powered, biomarker-driven randomized controlled trials, ideally stratifying patients by molecular subtype, immune contexture, or microbiota composition. Integration of multi-omics biomarkers into trial design could transform exploratory observations into precision-guided interventions, identifying patient subgroups most likely to benefit. Equally important is the use of standardized clinical endpoints such as progression-free survival, overall survival, and validated biomarker changes, rather than reliance on surrogate markers alone.
Avoiding the PAINS trap also requires cultural change within the field. Researchers must implement PAINS-aware assay design, including aggregation counterscreens and orthogonal validation methods. Furthermore, rather than continuing to portray curcumin as a universal anticancer agent, future research should focus on contexts where its effects are most reproducible and mechanistically plausible, for example, in inflammation-associated CRC where microbiota modulation and mucosal barrier protection may be more relevant than direct tumor cytotoxicity.
Taken together, these considerations point to a research roadmap that prioritizes standardization and reproducibility in the near term, biomarker discovery and early-phase stratified trials in the medium term, and large-scale precision-guided randomized trials in the long term (Figure 3). Such a staged approach will allow the field to move from descriptive enthusiasm toward rigorous evidence-based evaluation. The ultimate goal is not merely to confirm whether curcumin has anticancer activity, but to define under what conditions, in which patient subgroups, and through which validated mechanisms such activity can be reliably observed. In conclusion, curcumin represents both a cautionary tale and a continuing opportunity in natural product research. Its wide-ranging mechanistic effects have attracted enormous scientific attention, yet the translational significance of these findings remains uncertain without stronger pharmacological foundations. By adopting rigorous assay standards, bridging the in vitro–in vivo gap, addressing pharmacokinetic limitations, and embedding biomarker-guided design into clinical research, the field can move beyond descriptive speculation and toward true precision medicine. Only under such conditions can curcumin be repositioned from a widely cited but weakly validated compound into a scientifically credible candidate for colorectal cancer prevention or therapy.
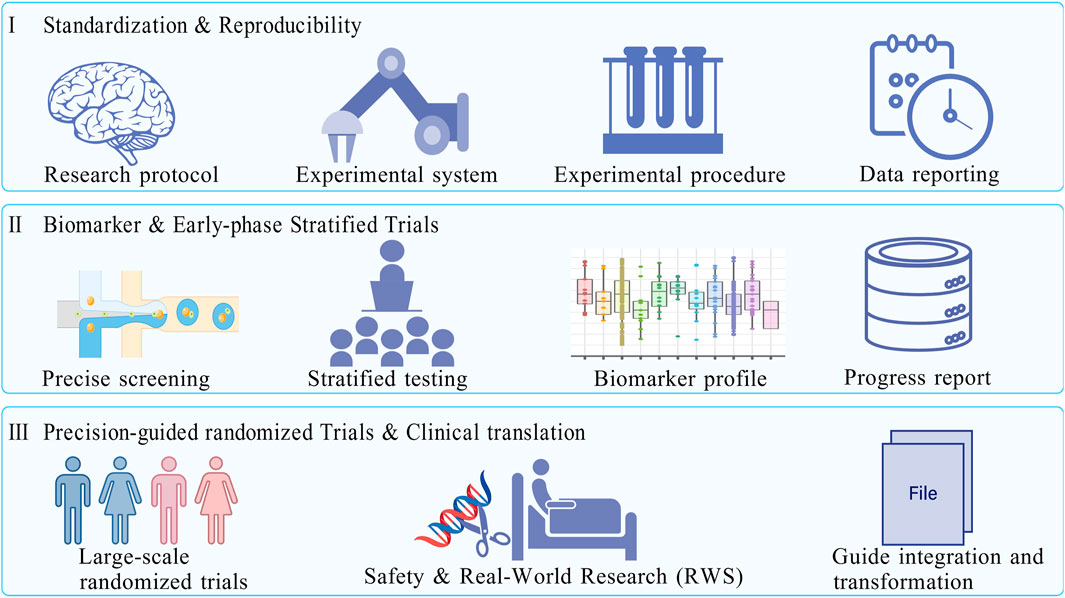
Figure 3. A phased roadmap for curcumin in colorectal cancer research. Based on the current status of curcumin research (safety verified, bioavailability and mechanism need to be deepened, and clinical evidence needs to be strengthened), the roadmap is divided into three research stages to promote the transformation of curcumin from basic research to clinical practice. Phase I: Focus on the standardization of experimental systems and cross-laboratory reproducibility verification; Phase II.: Explore predictive biomarkers related to curcumin efficacy, develop a “patient stratification strategy”, and carry out early clinical stratification trials with small samples and precise enrollment; Phase III: Conduct a large-scale precision guidance randomized trial (Phase III) to verify the survival benefits of curcumin combined with chemotherapy, and promote its inclusion in clinical guidelines in combination with real-world research to ensure research translation. Created with BioGDP.com.
Author contributions
S-QL: Conceptualization, Writing – original draft. X-RZ: Formal Analysis, Methodology, Supervision, Writing – review and editing. B-CQ: Software, Writing – review and editing. M-BC: Funding acquisition, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation (grant no. 82072712); China Scholarship Council program (Project ID: 202408320405); Health Commission Medical Research Program of Jiangsu Province (Z2023096); Suzhou Clinical Key Disease Diagnosis and Treatment Technology Program (LCZX202339); Suzhou Science and Technology Development Program (SLT2023020; SKY2023093); Key Healthcare Talent in Gusu District (054).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Joufi, F., Setia, A., Salem-Bekhit, M., Sahu, R., Alqahtani, F., Widyowati, R., et al. (2022). Molecular pathogenesis of colorectal cancer with an emphasis on recent advances in biomarkers, as well as nanotechnology-based diagnostic and therapeutic approaches. Nanomaterials 12, 169. doi:10.3390/nano12010169
Anand, P., Kunnumakkara, A. B., Newman, R. A., and Aggarwal, B. B. (2007). Bioavailability of curcumin: problems and promises. Mol. Pharm. 4, 807–818. doi:10.1021/mp700113r
Astinfeshan, M., Rasmi, Y., Kheradmand, F., Karimipour, M., Rahbarghazi, R., Aramwit, P., et al. (2019). Curcumin inhibits angiogenesis in endothelial cells using downregulation of the PI3K/akt signaling pathway. Food Biosci. 29, 86–93. doi:10.1016/j.fbio.2019.04.005
Baker, M. (2017). Deceptive curcumin offers cautionary tale for chemists. Nature 541, 144–145. doi:10.1038/541144a
Becht, E., De Reyniès, A., Giraldo, N. A., Pilati, C., Buttard, B., Lacroix, L., et al. (2016). Immune and stromal classification of colorectal cancer is associated with molecular subtypes and relevant for precision immunotherapy. Clin. Cancer Res. 22, 4057–4066. doi:10.1158/1078-0432.CCR-15-2879
Boozari, M., Butler, A. E., and Sahebkar, A. (2019). Impact of curcumin on toll-like receptors. J. Cell. Physiol. 234, 12471–12482. doi:10.1002/jcp.28103
Boroumand, N., Samarghandian, S., and Hashemy, S. I. (2018). Immunomodulatory, anti-inflammatory, and antioxidant effects of curcumin. J. Herbmed Pharmacol. 7, 211–219. doi:10.15171/jhp.2018.33
Brockmueller, A., Samuel, S. M., Mazurakova, A., Büsselberg, D., Kubatka, P., and Shakibaei, M. (2023). Curcumin, calebin a and chemosensitization: how are they linked to colorectal cancer? Life Sci. 318, 121504. doi:10.1016/j.lfs.2023.121504
Buhrmann, C., Popper, B., Kunnumakkara, A. B., Aggarwal, B. B., and Shakibaei, M. (2019). Evidence that calebin a, a component of curcuma longa suppresses NF-κB mediated proliferation, invasion and metastasis of human colorectal cancer induced by TNF-β (lymphotoxin). Nutrients 11, 2904. doi:10.3390/nu11122904
Burge, K., Gunasekaran, A., Eckert, J., and Chaaban, H. (2019). Curcumin and intestinal inflammatory diseases: molecular mechanisms of protection. IJMS 20, 1912. doi:10.3390/ijms20081912
Calvo-Barreiro, L., Zhang, L., Abdel-Rahman, S. A., Naik, S. P., and Gabr, M. (2023). Gut microbial-derived metabolites as immune modulators of T helper 17 and regulatory T cells. Int. J. Mol. Sci. 24, 1806. doi:10.3390/ijms24021806
Cao, S., Wang, C., Yan, J., Li, X., Wen, J., and Hu, C. (2020). Curcumin ameliorates oxidative stress-induced intestinal barrier injury and mitochondrial damage by promoting parkin dependent mitophagy through AMPK-TFEB signal pathway. Free Radic. Biol. Med. 147, 8–22. doi:10.1016/j.freeradbiomed.2019.12.004
Carroll, R. E., Benya, R. V., Turgeon, D. K., Vareed, S., Neuman, M., Rodriguez, L., et al. (2011). Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 4, 354–364. doi:10.1158/1940-6207.CAPR-10-0098
Chen, M., Tan, A., and Li, J. (2023). Curcumin represses colorectal cancer cell proliferation by triggering ferroptosis via PI3K/akt/mTOR signaling. Nutr. Cancer 75, 726–733. doi:10.1080/01635581.2022.2139398
Cruz–Correa, M., Shoskes, D. A., Sanchez, P., Zhao, R., Hylind, L. M., Wexner, S. D., et al. (2006). Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 4, 1035–1038. doi:10.1016/j.cgh.2006.03.020
Deng, W., Xiong, X., Lu, M., Huang, S., Luo, Y., Wang, Y., et al. (2024). Curcumin suppresses colorectal tumorigenesis through restoring the gut microbiota and metabolites. BMC Cancer 24, 1141. doi:10.1186/s12885-024-12898-z
Deswal, B., Bagchi, U., and Kapoor, S. (2024). Curcumin suppresses M2 macrophage-derived paclitaxel chemoresistance throughInhibition of PI3K-AKT/STAT3 signaling. Anti-Cancer Agents Med. Chem. 24, 146–156. doi:10.2174/0118715206275259231105184959
Dhillon, N., Aggarwal, B. B., Newman, R. A., Wolff, R. A., Kunnumakkara, A. B., Abbruzzese, J. L., et al. (2008). Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 14, 4491–4499. doi:10.1158/1078-0432.CCR-08-0024
Dienstmann, R., Vermeulen, L., Guinney, J., Kopetz, S., Tejpar, S., and Tabernero, J. (2017). Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer 17, 79–92. doi:10.1038/nrc.2016.126
Dong, Y., Zhang, K., Wei, J., Ding, Y., Wang, X., Hou, H., et al. (2023). Gut microbiota-derived short-chain fatty acids regulate gastrointestinal tumor immunity: a novel therapeutic strategy? Front. Immunol. 14, 1158200. doi:10.3389/fimmu.2023.1158200
Doxtater, K. D., and Tripathi, M. K. (2023). Abstract 2854: LncRNA UCA1 as a potential therapeutic target for drug resistance in colorectal cancer. Cancer Res. 83, 2854. doi:10.1158/1538-7445.AM2023-2854
Elbadawy, M., Hayashi, K., Ayame, H., Ishihara, Y., Abugomaa, A., Shibutani, M., et al. (2021). Anti-cancer activity of amorphous curcumin preparation in patient-derived colorectal cancer organoids. Biomed. Pharmacother. 142, 112043. doi:10.1016/j.biopha.2021.112043
Fakih, M., Ye, J., Wang, C., Ashok, A., Mauer, E., and Chao, C. Y. (2023). Comparative analysis of tumor immune microenvironment in primary tumors vs metastatic tissue in microsatellite stable metastatic colorectal cancer. J. Clin. Oncol. 41, 187. doi:10.1200/JCO.2023.41.4_suppl.187
Fathy Abd-Ellatef, G.-E., Gazzano, E., Chirio, D., Ragab Hamed, A., Belisario, D. C., Zuddas, C., et al. (2020). Curcumin-loaded solid lipid nanoparticles bypass P-glycoprotein mediated doxorubicin resistance in triple negative breast cancer cells. Pharmaceutics 12, 96. doi:10.3390/pharmaceutics12020096
Fellows, R., Denizot, J., Stellato, C., Cuomo, A., Jain, P., Stoyanova, E., et al. (2018). Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat. Commun. 9, 105. doi:10.1038/s41467-017-02651-5
Fu, X., He, Y., Li, M., Huang, Z., and Najafi, M. (2021). Targeting of the tumor microenvironment by curcumin. BioFactors 47, 914–932. doi:10.1002/biof.1776
Furbo, S., Urbano, P. C. M., Raskov, H. H., Troelsen, J. T., Kanstrup Fiehn, A.-M., and Gögenur, I. (2022). Use of patient-derived organoids as a treatment selection model for colorectal cancer: a narrative review. Cancers 14, 1069. doi:10.3390/cancers14041069
Garcea, G., Berry, D. P., Jones, D. J. L., Singh, R., Dennison, A. R., Farmer, P. B., et al. (2005). Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomarkers Prev. 14, 120–125. doi:10.1158/1055-9965.120.14.1
Gopalakrishnan, V., Spencer, C. N., Nezi, L., Reuben, A., Andrews, M. C., Karpinets, T. V., et al. (2018). Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 359, 97–103. doi:10.1126/science.aan4236
Guinney, J., Dienstmann, R., Wang, X., De Reyniès, A., Schlicker, A., Soneson, C., et al. (2015). The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356. doi:10.1038/nm.3967
Gunther, J. R., Chadha, A. S., Guha, S., Raju, G. S., Maru, D. M., Munsell, M. F., et al. (2022). A phase II randomized double blinded trial evaluating the efficacy of curcumin with pre-operative chemoradiation for rectal cancer. J. Gastrointest. Oncol. 13, 2938–2950. doi:10.21037/jgo-22-259
Guo, C., Guo, D., Fang, L., Sang, T., Wu, J., Guo, C., et al. (2021). Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 267, 118231. doi:10.1016/j.carbpol.2021.118231
Hayakawa, T., Yaguchi, T., and Kawakami, Y. (2020). Enhanced anti-tumor effects of the PD-1 blockade combined with a highly absorptive form of curcumin targeting STAT3. Cancer Sci. 111, 4326–4335. doi:10.1111/cas.14675
Hays, K. E., Pfaffinger, J. M., and Ryznar, R. (2024). The interplay between gut microbiota, short-chain fatty acids, and implications for host health and disease. Gut Microbes 16, 2393270. doi:10.1080/19490976.2024.2393270
Hoevenaar, W. H. M., Janssen, A., Quirindongo, A. I., Ma, H., Klaasen, S. J., Teixeira, A., et al. (2020). Degree and site of chromosomal instability define its oncogenic potential. Nat. Commun. 11, 1501. doi:10.1038/s41467-020-15279-9
Howells, L. M., Iwuji, C. O. O., Irving, G. R. B., Barber, S., Walter, H., Sidat, Z., et al. (2019). Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. J. Nutr. 149, 1133–1139. doi:10.1093/jn/nxz029
Hu, C., Li, M., Guo, T., Wang, S., Huang, W., Yang, K., et al. (2019). Anti-metastasis activity of curcumin against breast cancer via the inhibition of stem cell-like properties and EMT. Phytomedicine 58, 152740. doi:10.1016/j.phymed.2018.11.001
Ichimura, N., Shinjo, K., Ohka, F., Katsushima, K., Hatanaka, A., Tojo, M., et al. (2015). Abstract 1054: aberrant TET1 methylation closely associated with CpG island methylator phenotype in colorectal cancer. Cancer Res. 75, 1054. doi:10.1158/1538-7445.AM2015-1054
Iglesias, D. E., Cremonini, E., Oteiza, P. I., and Fraga, C. G. (2022). Curcumin mitigates TNFα-induced caco-2 cell monolayer permeabilization through modulation of NF-κB, ERK1/2, and JNK pathways. Mol. Nutr. Food Res. 66, 2101033. doi:10.1002/mnfr.202101033
James, M. I., Iwuji, C., Irving, G., Karmokar, A., Higgins, J. A., Griffin-Teal, N., et al. (2015). Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett. 364, 135–141. doi:10.1016/j.canlet.2015.05.005
Jancewicz, I., Szarkowska, J., Konopinski, R., Stachowiak, M., Swiatek, M., Blachnio, K., et al. (2021). PD-L1 overexpression, SWI/SNF complex deregulation, and profound transcriptomic changes characterize cancer-dependent exhaustion of persistently activated CD4+ T cells. Cancers 13, 4148. doi:10.3390/cancers13164148
Jiang, M., Qi, Y., Huang, W., Lin, Y., and Li, B. (2022). Curcumin reprograms TAMs from a protumor phenotype towards an antitumor phenotype via inhibiting MAO-a/STAT6 pathway. Cells 11, 3473. doi:10.3390/cells11213473
Jin, M., Kong, L., Han, Y., and Zhang, S. (2021). Gut microbiota enhances the chemosensitivity of hepatocellular carcinoma to 5-fluorouracil in vivo by increasing curcumin bioavailability. Phytotherapy Res. 35, 5823–5837. doi:10.1002/ptr.7240
Kubatka, P., Koklesova, L., Mazurakova, A., Brockmueller, A., Büsselberg, D., Kello, M., et al. (2024). Cell plasticity modulation by flavonoids in resistant breast carcinoma targeting the nuclear factor kappa B signaling. Cancer Metastasis Rev. 43, 87–113. doi:10.1007/s10555-023-10134-x
Kudryavtseva, A. V., Lipatova, A. V., Zaretsky, A. R., Moskalev, A. A., Fedorova, M. S., Rasskazova, A. S., et al. (2016). Important molecular genetic markers of colorectal cancer. Oncotarget 7, 53959–53983. doi:10.18632/oncotarget.9796
Kunnumakkara, A. B., Diagaradjane, P., Guha, S., Deorukhkar, A., Shentu, S., Aggarwal, B. B., et al. (2008). Curcumin sensitizes human colorectal cancer xenografts in nude mice to gamma-radiation by targeting nuclear factor-kappaB-regulated gene products. Clin. Cancer Res. 14, 2128–2136. doi:10.1158/1078-0432.CCR-07-4722
Lamichhane, G., Olawale, F., Liu, J., Lee, D.-Y., Lee, S.-J., Chaffin, N., et al. (2024). Curcumin mitigates gut dysbiosis and enhances gut barrier function to alleviate metabolic dysfunction in obese, aged mice. Biology 13, 955. doi:10.3390/biology13120955
Le, T. T., and Kim, D. (2019). Folate-PEG/hyd-curcumin/C18-g-PSI micelles for site specific delivery of curcumin to colon cancer cells via wnt/β-catenin signaling pathway. Mater. Sci. Eng. C 101, 464–471. doi:10.1016/j.msec.2019.03.100
Le, D. T., Durham, J. N., Smith, K. N., Wang, H., Bartlett, B. R., Aulakh, L. K., et al. (2017). Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357, 409–413. doi:10.1126/science.aan6733
Lei, X., Lei, Y., Li, J.-K., Du, W.-X., Li, R.-G., Yang, J., et al. (2020). Immune cells within the tumor microenvironment: biological functions and roles in cancer immunotherapy. Cancer Lett. 470, 126–133. doi:10.1016/j.canlet.2019.11.009
Li, H., Sureda, A., Devkota, H. P., Pittalà, V., Barreca, D., Silva, A. S., et al. (2020). Curcumin, the golden spice in treating cardiovascular diseases. Biotechnol. Adv. 38, 107343. doi:10.1016/j.biotechadv.2019.01.010
Li, G., Fang, S., Shao, X., Li, Y., Tong, Q., Kong, B., et al. (2021). Curcumin reverses NNMT-induced 5-fluorouracil resistance via increasing ROS and cell cycle arrest in colorectal cancer cells. Biomolecules 11, 1295. doi:10.3390/biom11091295
Liao, W., Zhang, L., Chen, X., Xiang, J., Zheng, Q., Chen, N., et al. (2023). Targeting cancer stem cells and signalling pathways through phytochemicals: a promising approach against colorectal cancer. Phytomedicine 108, 154524. doi:10.1016/j.phymed.2022.154524
Link, A., Balaguer, F., Shen, Y., Lozano, J. J., Leung, H.-C. E., Boland, C. R., et al. (2013). Curcumin modulates DNA methylation in colorectal cancer cells. PLOS One 8, e57709. doi:10.1371/journal.pone.0057709
Liu, G., Lu, Y., Shi, L., Ren, Y., Kong, J., Zhang, M., et al. (2020). TLR4-MyD88 signaling pathway is responsible for acute lung inflammation induced by reclaimed water. J. Hazard. Mater. 396, 122586. doi:10.1016/j.jhazmat.2020.122586
Liu, J., Geng, X., Hou, J., and Wu, G. (2021a). New insights into M1/M2 macrophages: key modulators in cancer progression. Cancer Cell Int. 21, 389. doi:10.1186/s12935-021-02089-2
Liu, L., Lim, M. A., Jung, S.-N., Oh, C., Won, H.-R., Jin, Y. L., et al. (2021b). The effect of curcumin on multi-level immune checkpoint blockade and T cell dysfunction in head and neck cancer. Phytomedicine 92, 153758. doi:10.1016/j.phymed.2021.153758
Lopez, L. R., Bleich, R. M., and Arthur, J. C. (2021). Microbiota effects on carcinogenesis: initiation, promotion, and progression. Annu. Rev. Med. 72, 243–261. doi:10.1146/annurev-med-080719-091604
Lv, M., Cai, D., Li, C., Chen, J., Li, G., Hu, C., et al. (2023). Senescence-based colorectal cancer subtyping reveals distinct molecular characteristics and therapeutic strategies. Medcomm 4, e333. doi:10.1002/mco2.333
Maleki Dizaj, S., Alipour, M., Dalir Abdolahinia, E., Ahmadian, E., Eftekhari, A., Forouhandeh, H., et al. (2022). Curcumin nanoformulations: beneficial nanomedicine against cancer. Phytother. Res. 36, 1156–1181. doi:10.1002/ptr.7389
Mann, E. R., Lam, Y. K., and Uhlig, H. H. (2024). Short-chain fatty acids: linking diet, the microbiome and immunity. Nat. Rev. Immunol. 24, 577–595. doi:10.1038/s41577-024-01014-8
Matson, V., Fessler, J., Bao, R., Chongsuwat, T., Zha, Y., Alegre, M.-L., et al. (2018). The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108. doi:10.1126/science.aao3290
Memarzia, A., Khazdair, M. R., Behrouz, S., Gholamnezhad, Z., Jafarnezhad, M., Saadat, S., et al. (2021). Experimental and clinical reports on anti-inflammatory, antioxidant, and immunomodulatory effects of Curcuma longa and curcumin, an updated and comprehensive review. BioFactors 47, 311–350. doi:10.1002/biof.1716
Mulik, R. S., Mönkkönen, J., Juvonen, R. O., Mahadik, K. R., and Paradkar, A. R. (2012). Apoptosis-induced anticancer effect of transferrin-conjugated solid lipid nanoparticles of curcumin. Cancer Nanotechnol. 3, 65–81. doi:10.1007/s12645-012-0031-2
Nelson, K. M., Dahlin, J. L., Bisson, J., Graham, J., Pauli, G. F., and Walters, M. A. (2017). The essential medicinal chemistry of curcumin: miniperspective. J. Med. Chem. 60, 1620–1637. doi:10.1021/acs.jmedchem.6b00975
Nussbaum, Y. I., Manjunath, Y., Kaifi, J. T., Warren, W., and Mitchem, J. B. (2022). Analysis of tumor-associated macrophages’ heterogeneity in colorectal cancer patients using single-cell RNA-seq data. J. Clin. Oncol. 40, 146. doi:10.1200/JCO.2022.40.4_suppl.146
Ocvirk, S., and O’Keefe, S. J. D. (2021). Dietary fat, bile acid metabolism and colorectal cancer. Semin. Cancer Biol. 73, 347–355. doi:10.1016/j.semcancer.2020.10.003
Okugawa, Y., Grady, W. M., and Goel, A. (2015). Epigenetic alterations in colorectal cancer: emerging biomarkers. Gastroenterology 149, 1204–1225.e12. doi:10.1053/j.gastro.2015.07.011
Olive, K. P., and Tuveson, D. A. (2006). The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin. Cancer Res. 12, 5277–5287. doi:10.1158/1078-0432.CCR-06-0436
Ozawa-Umeta, H., Kishimoto, A., Imaizumi, A., Hashimoto, T., Asakura, T., Kakeya, H., et al. (2020). Curcumin β-D-glucuronide exhibits anti–tumor effects on oxaliplatin-resistant colon cancer with less toxicity in vivo. Cancer Sci. 111, 1785–1793. doi:10.1111/cas.14383
Padmanaban, G., and Nagaraj, V. A. (2017). Curcumin may defy medicinal chemists. ACS Med. Chem. Lett. 8, 274. doi:10.1021/acsmedchemlett.7b00051
Pluta, R., Januszewski, S., and Ułamek-Kozioł, M. (2020). Mutual two-way interactions of curcumin and gut microbiota. Int. J. Mol. Sci. 21, 1055. doi:10.3390/ijms21031055
Priyadarsini, K. (2014). The chemistry of curcumin: from extraction to therapeutic agent. Molecules 19, 20091–20112. doi:10.3390/molecules191220091
Prud’homme, J. (2012). Cancer stem cells and novel targets for antitumor strategies. Curr. Pharm. Des. 18, 2838–2849. doi:10.2174/138161212800626120
Seligmann, J. F., Domingo, E., Fisher, D., Elliott, F., Brown, L. C., Seymour, M. T., et al. (2022). The clinical relevance of tumor RAS/TP53 dual mutation in early and metastatic colorectal cancer (CRC). J. Clin. Oncol. 40, 3540. doi:10.1200/JCO.2022.40.16_suppl.3540
Shafabakhsh, R., Pourhanifeh, M. H., Mirzaei, H. R., Sahebkar, A., Asemi, Z., and Mirzaei, H. (2019). Targeting regulatory T cells by curcumin: a potential for cancer immunotherapy. Pharmacol. Res. 147, 104353. doi:10.1016/j.phrs.2019.104353
Shaikh, S., Shaikh, J., Naba, Y. S., Doke, K., Ahmed, K., and Yusufi, M. (2021). Curcumin: reclaiming the lost ground against cancer resistance. CDR 4, 298–320. doi:10.20517/cdr.2020.92
Shakhpazyan, N. K., Mikhaleva, L. M., Bedzhanyan, A. L., Gioeva, Z. V., Mikhalev, A. I., Midiber, K. Y., et al. (2024). Exploring the role of the gut microbiota in modulating colorectal cancer immunity. Cells 13, 1437. doi:10.3390/cells13171437
Shakibaei, M., Buhrmann, C., Kraehe, P., Shayan, P., Lueders, C., and Goel, A. (2014). Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLOS One 9, e85397. doi:10.1371/journal.pone.0085397
Sharma, R. A., Euden, S. A., Platton, S. L., Cooke, D. N., Shafayat, A., Hewitt, H. R., et al. (2004). Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res. 10, 6847–6854. doi:10.1158/1078-0432.CCR-04-0744
Sharma, R. A., Gescher, A. J., and Steward, W. P. (2005). Curcumin: the story so far. Eur. J. Cancer 41, 1955–1968. doi:10.1016/j.ejca.2005.05.009
Sharma, R. A., Steward, W. P., and Gescher, A. J. (2007). Pharmacokinetics and pharmacodynamics of curcumin. In: B. B. Aggarwal, Y.-J. Surh, and S. Shishodia, editors. The molecular targets and therapeutic uses of curcumin in health and disease. Boston, MA: Springer US. p. 453–470. doi:10.1007/978-0-387-46401-5_20
Shirley, S. A., Montpetit, A. J., Lockey, R. F., and Mohapatra, S. S. (2008). Curcumin prevents human dendritic cell response to immune stimulants. Biochem. Biophys. Res. Commun. 374, 431–436. doi:10.1016/j.bbrc.2008.07.051
Siegel, R. L., Wagle, N. S., Cercek, A., Smith, R. A., and Jemal, A. (2023). Colorectal cancer statistics, 2023. Ca. Cancer J. Clin. 73, 233–254. doi:10.3322/caac.21772
Stefani, C., Miricescu, D., Stanescu-Spinu, I.-I., Nica, R. I., Greabu, M., Totan, A. R., et al. (2021). Growth factors, PI3K/AKT/mTOR and MAPK signaling pathways in colorectal cancer pathogenesis: where are we now? Int. J. Mol. Sci. 22, 10260. doi:10.3390/ijms221910260
Storandt, M., Rogen, K., Iyyangar, A., Mitchell, J., Hubbard, J., Sinicrope, F., et al. (2023). P-142 completion of genetic testing and incidence of pathogenic germline mutation among patients with early-onset colorectal cancer, a single institution retrospective analysis. Ann. Oncol. 34, S65. doi:10.1016/j.annonc.2023.04.198
Ternullo, S., Gagnat, E., Julin, K., Johannessen, M., Basnet, P., Vanić, Ž., et al. (2019). Liposomes augment biological benefits of curcumin for multitargeted skin therapy. Eur. J. Pharm. Biopharm. 144, 154–164. doi:10.1016/j.ejpb.2019.09.016
The Cancer Genome Atlas Network (2012). Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337. doi:10.1038/nature11252
Toden, S., Okugawa, Y., Jascur, T., Wodarz, D., Komarova, N. L., Buhrmann, C., et al. (2015). Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 36, 355–367. doi:10.1093/carcin/bgv006
Urošević, M., Nikolić, L., Gajić, I., Nikolić, V., Dinić, A., and Miljković, V. (2022). Curcumin: biological activities and modern pharmaceutical forms. Antibiotics 11, 135. doi:10.3390/antibiotics11020135
Van Deuren, T., Blaak, E. E., and Canfora, E. E. (2022). Butyrate to combat obesity and obesity-associated metabolic disorders: current status and future implications for therapeutic use. Obes. Rev. 23, e13498. doi:10.1111/obr.13498
Vareed, S. K., Kakarala, M., Ruffin, M. T., Crowell, J. A., Normolle, D. P., Djuric, Z., et al. (2008). Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev. 17, 1411–1417. doi:10.1158/1055-9965.EPI-07-2693
Wilken, R., Veena, M. S., Wang, M. B., and Srivatsan, E. S. (2011). Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 10, 12. doi:10.1186/1476-4598-10-12
Yang, J., He, C., and Liu, N. (2022). Proteomic analysis of the chemosensitizing effect of curcumin on CRC cells treated with 5-FU. Front. Med. 9, 1032256. doi:10.3389/fmed.2022.1032256
Yang, J., Yang, H., and Li, Y. (2023a). The triple interactions between gut microbiota, mycobiota and host immunity. Crit. Rev. Food Sci. Nutr. 63, 11604–11624. doi:10.1080/10408398.2022.2094888
Yang, Y., Liu, Z., Lyu, H., Guo, X., Jiang, H., Liu, L., et al. (2023b). Traditional Chinese medicine-induced treatment in colitis-associated colorectal cancer. Chin. Med. J. 136, 1249–1250. doi:10.1097/CM9.0000000000002667
Yeo, S., Kim, M. J., Shim, Y. K., Yoon, I., and Lee, W. K. (2022). Solid lipid nanoparticles of curcumin designed for enhanced bioavailability and anticancer efficiency. ACS Omega 7, 35875–35884. doi:10.1021/acsomega.2c04407
Zhao, Y., Feng, X., Chen, Y., Selfridge, J. E., Gorityala, S., Du, Z., et al. (2020). 5-Fluorouracil enhances the antitumor activity of the glutaminase inhibitor CB-839 against PIK3CA-mutant colorectal cancers. Cancer Res. 80, 4815–4827. doi:10.1158/0008-5472.CAN-20-0600
Zheng, X., Yang, X., Lin, J., Song, F., and Shao, Y. (2021). Low curcumin concentration enhances the anticancer effect of 5-fluorouracil against colorectal cancer. Phytomedicine 85, 153547. doi:10.1016/j.phymed.2021.153547
Zhou, H., Zhuang, Y., Liang, Y., Chen, H., Qiu, W., Xu, H., et al. (2025). Curcumin exerts anti-tumor activity in colorectal cancer via gut microbiota-mediated CD8+ T cell tumor infiltration and ferroptosis. Food Funct. 16, 3671–3693. doi:10.1039/D4FO04045G
Zhu, J., and He, L. (2024). The modulatory effects of curcumin on the gut microbiota: a potential strategy for disease treatment and health promotion. Microorganisms 12, 642. doi:10.3390/microorganisms12040642
Keywords: curcumin, colorectal cancer, gut microbiota, immune response, signaling pathways, chemoresistance, pharmacological limitations, translational research
Citation: Li S-Q, Zhu X-R, Qin B-C and Chen M-B (2025) Curcumin in colorectal cancer: mechanistic insights, pharmacological limitations, and translational perspectives. Front. Pharmacol. 16:1667731. doi: 10.3389/fphar.2025.1667731
Received: 17 July 2025; Accepted: 19 September 2025;
Published: 29 September 2025.
Edited by:
Chenghao Fei, Nanjing Agricultural University, ChinaReviewed by:
Fangnan Lv, China Pharmaceutical University, ChinaYipeng Pang, Jiangsu Normal University, China
Copyright © 2025 Li, Zhu, Qin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min-Bin Chen, Y2hlbm1iQGtzcm15eS5vcmc=
†These authors have contributed equally to this work
 Si-Qi Li
Si-Qi Li Xiao-Ren Zhu
Xiao-Ren Zhu Bai-Chun Qin3
Bai-Chun Qin3