- 1The People’s Hospital of Danyang, Affiliated Danyang Hospital of Nantong University, Danyang, China
- 2School of Pharmacy, Nantong University, Nantong, China
Photodynamic therapy (PDT) induces cancer cell death by utilizing photosensitizers to generate reactive oxygen species (ROS) upon light irradiation, which in turn trigger oxidative stress. However, the therapeutic efficacy of PDT is constrained by the short lifetimes and limited diffusion range of ROS, resulting in suboptimal outcomes and off-target effects. Specific organelle targeting, facilitated by rationally engineered photosensitizers and nanoplatforms with precise drug delivery capabilities that activate organelle-mediated cell death pathways, can maximize localized oxidative damage, enhance therapeutic efficacy, and minimize systemic toxicity. This review synthesizes advancements in organelle-targeted PDT, focusing on critical subcellular compartments (e.g., mitochondria, lysosomes, nuclei, cell membranes, ribosome, endoplasmic reticulum, golgi apparatus, autophagosome). It systematically summarizes the structural characteristics, design strategies, targeting mechanisms, and therapeutic effects of these organelle-targeted systems, with particular emphasis on organelle-mediated cell death signaling pathways. Ultimately, current challenges, prospective opportunities, and future research directions in organelle targeting are delineated, providing a strategic framework to advance organelle-targeted PDT toward precision therapy.
1 Introduction
The complexity and heterogeneity of malignant tumors are major contributors to their high mortality rate, making cancer treatment a critical challenge in nanomedicine and life sciences (Bian et al., 2024; Cantallops Vilà et al., 2024). Various approaches have been developed, including surgery, chemotherapy, radiotherapy, photodynamic therapy (PDT), immunotherapy, and gene therapy (Cortés-Guiral et al., 2021; Pan Y. et al., 2022; Xie R. et al., 2023; Qian et al., 2024). Among these, PDT has gained significant attention due to its spatiotemporal precision, low cost, ease of application, and minimal toxic side effects (Li et al., 2020; Di Bartolomeo et al., 2022). Notably, unlike conventional therapies, PDT rarely causes drug resistance or severe adverse effects (Sun B. et al., 2023; Hua et al., 2024). PDT relies on three essential components: a photosensitizer, light, and oxygen (O2) (Kolarikova et al., 2023; Wang et al., 2023). Upon absorption of light at specific wavelengths (typically matching its absorption spectrum), the photosensitizer undergoes a transition from the ground state to an excited singlet state (S1), followed by intersystem crossing to form a longer-lived triplet state (T1) (Fay and Limmer, 2024; Kim et al., 2024). This T1 species then mediates the two primary mechanisms of PDT (Figure 1A). Type II PDT is defined by energy transfer from the triplet-state photosensitizer to ground-state molecular oxygen (3O2), which is converted into highly cytotoxic singlet oxygen (1O2) (Shigemitsu et al., 2022; Yu L. et al., 2024). This process is strictly O2-dependent, as 1O2 generation directly relies on the availability of O2, and the short half-life of 1O2 limits its diffusion to a narrow region around the photosensitizer. In contrast, Type I PDT is characterized by relatively low O2 dependence, enabling it to retain efficacy even in hypoxic tumor regions. It operates through a distinct pathway where the excited photosensitizer, typically in its T1, undergoes electron transfer or hydrogen atom abstraction reactions with biological substrates, such as amino acids, lipids, or nucleic acids, in the tumor microenvironment. This substrate-mediated interaction leads to the generation of a suite of reactive oxygen species (ROS), including superoxide anion radicals (•O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH) (Wen et al., 2022; Zhen et al., 2025). Despite these differences in ROS generation mechanisms and oxygen requirements, both Type I and Type II PDT culminate in elevated oxidative stress in the tumor microenvironment, ultimately inducing cancer cell death (Yu Y. et al., 2023; Zhang et al., 2023d).
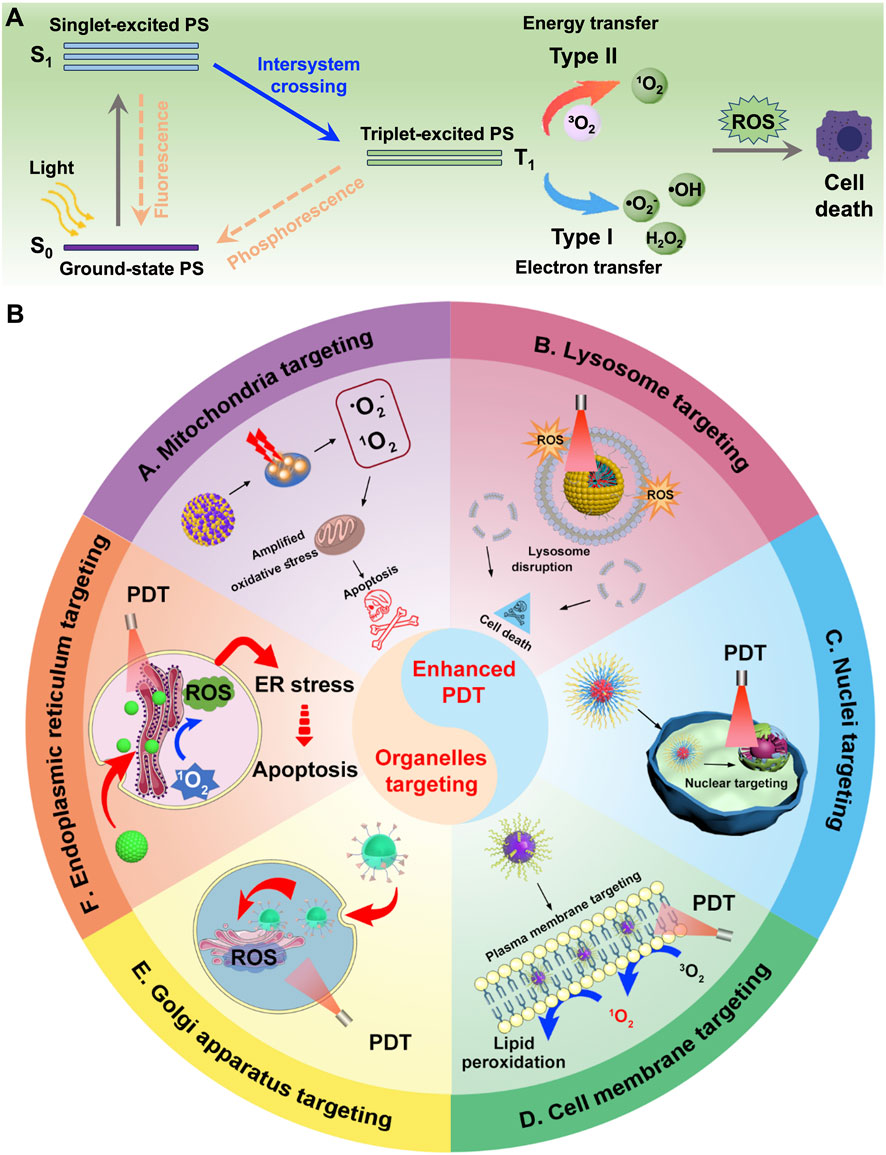
Figure 1. (A) Schematic illustration of type I and type II mechanism in the process of PDT. (B) Schematic illustration of different nanomedicines targeting various organelles, including mitochondria, lysosomes, nucleus, cell membrane, ER, and Golgi apparatus, to achieve enhanced PDT.
However, we must confront an undeniable fact: despite the increasing number of studies on PDT-based anti-tumor treatments in recent years, several critical issues still need to be addressed (Sun et al., 2022). One of the most prominent challenges is the extremely short half-life (0.03–0.18 µs) and limited action range (0.01–0.02 µm) of 1O2 generated by photosensitizers (Moan and Berg, 1991; Niedre et al., 2002). As a result, the oxidative effects of PDT-induced ROS are largely confined to areas in close proximity to the photosensitizer (Austin et al., 2025). To overcome this limitation, investigating the mechanism of PDT-induced immunogenic cell death (ICD) has emerged as a potentially effective strategy (Zhang et al., 2022b; Zhang T. et al., 2023). Nevertheless, an even more promising approach may lie in further enhancing the targeting capability of photosensitizer-based nanoplatforms, particularly their ability to selectively target various subcellular organelles (Sun et al., 2024). Achieving precise subcellular localization enables targeted disruption at critical sites, triggering a systemic effect that can ultimately lead to different modes of cancer cell death, including apoptosis, necrosis, or pyroptosis (Tong et al., 2024; Wang B. et al., 2024).
Cells are the fundamental units of life, and their normal metabolism is essential for maintaining health (Finley, 2023; Sun et al., 2025). Disruptions in cellular metabolism can lead to various diseases (Plikus et al., 2021; Lee et al., 2024). Such metabolic processes rely heavily on the coordinated function and communication among intracellular organelles (König and McBride, 2024; Zimmermann et al., 2024). Common organelles include mitochondria, lysosomes, endoplasmic reticulum (ER), Golgi apparatus, endosomes, and centrosomes, each playing a distinct role in cellular function (Bornens, 2021; Celik et al., 2023; Ebner et al., 2023; Meyer and Kravic, 2024; Sun S. et al., 2024). The cell membrane, as a phospholipid bilayer structure, plays a critical role in maintaining the separation and homeostasis between the intracellular and extracellular environments (Zhu X. et al., 2024). It is particularly susceptible to oxidative attack by ROS, leading to the generation of abundant lipid peroxidation (LPO) products (Pope and Dixon, 2023; Zhu et al., 2023). This not only exacerbates membrane damage but also provides a mechanistic basis for the synergistic enhancement of PDT with ferroptosis (Pan W. et al., 2022; Chen et al., 2024). Mitochondria generate adenosine triphosphate (ATP) through aerobic respiration, providing energy for cellular activities (Dong et al., 2023; Granath-Panelo and Kajimura, 2024). Upon exposure to ROS, mitochondria experience disruption of membrane potential and functional impairment, leading to reduced O2 consumption (Cho and Kim, 2024). This creates a favorable environment for O2-dependent PDT to generate increased levels of ROS, thereby enhancing therapeutic efficacy (Cen et al., 2023). Lysosomes degrade unwanted cellular materials via hydrolytic enzymes (Settembre and Perera, 2024; Sweet et al., 2025). Following ROS-induced damage, the permeability of the lysosomal membrane is altered, leading to the release of intracellular cathepsins that induce cell death. This mechanism opens up new avenues for cancer therapy (Yu Q. et al., 2023; Chen F. et al., 2025). The ER is crucial for the synthesis and transport of proteins and lipids, while the Golgi apparatus modifies, sorts, and transports proteins for secretion or delivery to other organelles (Weigel et al., 2021; Tang and Ginsburg, 2023). ROS-induced oxidative stress in the ER and the reduction of immunosuppressive factor secretion by the Golgi apparatus caused by ROS both provide new opportunities and mechanistic insights for enhanced PDT (Liu M. et al., 2022; Zhang X. et al., 2022). Given these critical roles, maintaining the structural and functional integrity of organelles is vital for cell survival (Yao et al., 2021; Harapas et al., 2022). Conversely, disrupting organelle function-such as by damaging membranes, altering membrane potential, or releasing destructive enzymes-can impair cellular metabolism and trigger programmed cell death, making organelles promising targets for therapeutic interventions (Šlachtová et al., 2023; Soukar et al., 2025).
Several reviews have summarized strategies for targeting specific organelles through material design to treat diseases, primarily focusing on the development of targeting agents (Yang et al., 2022; Shao et al., 2023; Ying et al., 2023; Hong et al., 2024). In contrast, this review highlights the structural features required for designing photosensitizers that target different organelles (Figure 1B). We also evaluate which organelles are most effective targets in PDT, particularly in overcoming challenges such as tumor hypoxia, drug resistance, metastasis, and recurrence. By analyzing current studies on organelle-targeted PDT, we aim to deepen the understanding of PDT’s mechanisms in inhibiting tumor growth and provide insights for its future clinical application.
2 Subcellular organelle-specific targeting mechanisms for enhanced PDT
Animal cells contain a variety of structurally and functionally distinct organelles that work in concert to maintain cellular homeostasis. These organelles not only carry out essential biological processes but also serve as potential targets for therapeutic interventions, including PDT (Liu X. et al., 2022; Xu Y. et al., 2025). Targeting specific organelles with photosensitizers can enhance PDT efficacy by inducing localized damage and activating cell death pathways (Chai et al., 2022). Below is an overview of key organelles and their functional roles relevant to PDT (Table 1).
The cell membrane acts as a selective barrier, regulating nutrient uptake and waste removal. It also plays a central role in signal transduction and intercellular communication (Bijonowski et al., 2025). Damage to the cell membrane via PDT can disrupt ion balance and trigger apoptosis (Fang R. H. et al., 2023). Moreover, it is well known that the therapeutic efficacy of PDT is not only limited by the short lifetime of ROS, but is also affected by intracellular reducing agents such as glutathione (GSH). The ROS generated during PDT can directly act on the cell membrane, inducing LPO and thereby achieving efficient tumor suppression (Deng et al., 2022). ROS can directly act on the cell membrane, effectively increasing membrane permeability, disrupting membrane integrity, and leading to the rapid release of intracellular contents, thereby inducing necroptosis cancer theranostics (Niu et al., 2022) and ICD (Tang et al., 2023).
Lysosomes are crucial for maintaining normal cellular turnover, and have been implicated in various diseases, including cancer (Zhu et al., 2020). The integrity of the lysosomal membrane is essential for its physiological functions (Cao et al., 2021). However, studies have shown that the permeability of the lysosomal membrane can be influenced by ROS, thereby inducing a cathepsin-mediated lysosomal cell death pathway, which operates distinctly from apoptosis mediated by caspases (Figure 2A) (Wu et al., 2022; Liu X. et al., 2023). Consequently, employing photosensitizers to generate ROS with the aim of altering the integrity and functionality of lysosomal membranes may emerge as a promising therapeutic strategy for cancer treatment (Chen Z. et al., 2025). In recent years, growing evidence has confirmed that lysosomes play a critical role in the activation of pyroptosis (Zheng Z. et al., 2021; Luo et al., 2024). Specifically, when lysosomes are damaged, they release cathepsin B, which subsequently activates the NLRP3 inflammasome and caspase-1, ultimately leading to pyroptotic cell death mediated by GSDMD-N (Liu et al., 2024c; Sun et al., 2024). Moreover, recent studies have indicated that alterations in lysosomal membrane permeability can impair the ability of lysosomes to maintain cellular homeostasis, ultimately leading to lysosome-mediated cell death mechanisms (Li et al., 2023; Xie Z. et al., 2023).
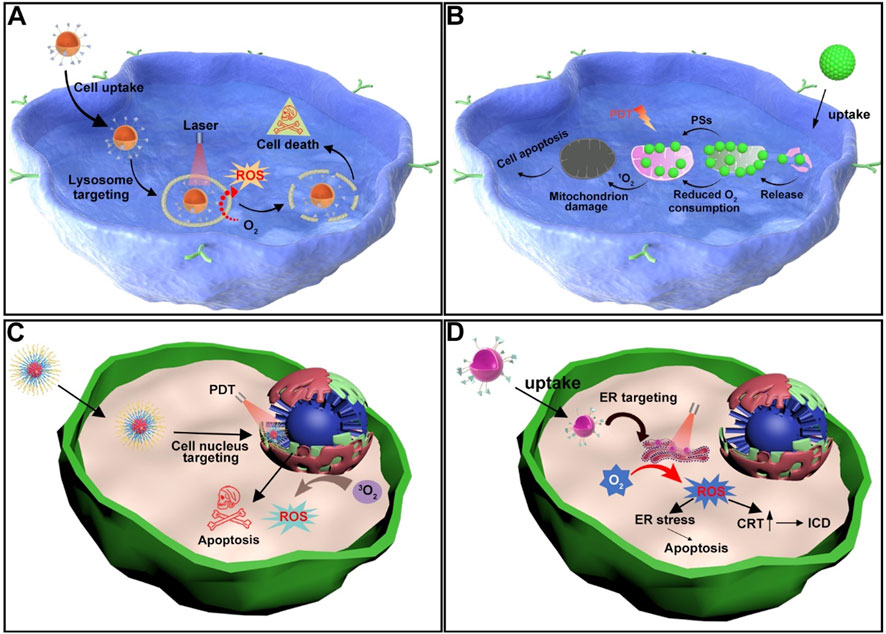
Figure 2. (A) Schematic illustration of the mechanism by which lysosome-targeting photosensitizers generate ROS upon light irradiation, leading to cancer cell death. (B) Schematic illustration of the mechanism by which mitochondrion-targeting photosensitizers produce ROS upon light irradiation, reducing the O2 consumption capacity of cancer cells and thereby enhancing PDT efficacy. (C) Schematic illustration of nucleus-targeting photosensitizers generating ROS upon light irradiation, resulting in apoptosis of cancer cells. (D) Schematic illustration of the mechanisms by which ER-targeting photosensitizers produce ROS upon light irradiation, leading to either apoptosis or ICD in cancer cells.
The Mitochondria could generate ATP through oxidative phosphorylation and play a central role in apoptosis regulation (Kilbride and Prehn, 2013; Yadav et al., 2015; Kuznetsov et al., 2022; Khaliulin et al., 2025). ROS generated during PDT can effectively disrupt the mitochondrial membrane potential, leading to mitochondrial dysfunction (Yang F. et al., 2023). This helps prevent further O2 consumption by mitochondria, thereby enhancing the efficiency of O2-dependent PDT in producing more ROS (Figure 2B). Moreover, studies have shown that mitochondria are highly enriched with GSH, an important antioxidant molecule (Liu Y. et al., 2023). Therefore, directly targeting mitochondria may offer a more direct way to impair cellular defense mechanisms. Mitochondrial damage is one of the most effective PDT targets due to its direct involvement in programmed cell death (Yan et al., 2024).
The Nucleus can store genetic material (DNA) and controls gene expression and cell proliferation (Sabari et al., 2020; Kalukula et al., 2022). Nuclear damage induced by PDT can lead to DNA fragmentation and irreversible cell cycle arrest (Zhang et al., 2023c). Moreover, studies have shown that photoactive materials targeting the nucleus can generate ROS upon light irradiation, which directly damage nuclear DNA (Figure 2C). The resulting cytosolic DNA fragments can then work in synergy with STING agonists to activate the innate anti-tumor immune response (Wu Y. et al., 2024; Feng et al., 2025).
Photosensitive agents targeting the ER can induce significant ER stress upon light exposure, leading to the surface expression of calreticulin (CRT) (Figure 2D) (Li et al., 2019). This process stimulates the antigen-presenting capability of dendritic cells and triggers ICD, thereby suppressing cancer cell proliferation. A number of studies have demonstrated that both ER stress and ROS production play critical roles in activating intracellular signaling pathways that regulate ICD (Deng H. et al., 2020). PDT with ER-targeting specificity can further enhance the antitumor therapeutic effect.
Research indicates that the Golgi apparatus serves as a key site for the production of various immunosuppressive cytokines. The generation of these cytokines relies on the structural integrity of the Golgi, making it an effective strategy to suppress their expression by disrupting this organelle. Therefore, PDT-induced ROS can impair Golgi function and thereby prevent the release of immunosuppressive factors, which would otherwise hinder the immune response triggered by PDT (Chen Y. J. et al., 2023). This approach enhances the overall efficacy of PDT in inhibiting cancer cell proliferation. Moreover, Golgi-targeted photosensitizers can upregulate the expression of NLRP3 upon light activation, promoting the release of pro-inflammatory cytokines such as IL-1β (Hu et al., 2023). This not only strengthens innate immunity but also boosts adaptive immune responses against tumor cells. Hence, a precise phototherapy strategy targeting the Golgi apparatus offers a promising new direction for effective tumor suppression (Shi et al., 2024).
By selectively delivering photosensitizers to specific subcellular organelles, PDT can be optimized to induce site-specific damage, thereby enhancing therapeutic efficacy and addressing key challenges such as hypoxia, drug resistance, immune suppression, and tumor recurrence (Wang et al., 2021; Tian et al., 2024). A deeper understanding of the unique biological functions and characteristics of each organelle lays the groundwork for developing more effective and precisely targeted PDT strategies.
3 Mitochondrial targeting for enhanced PDT
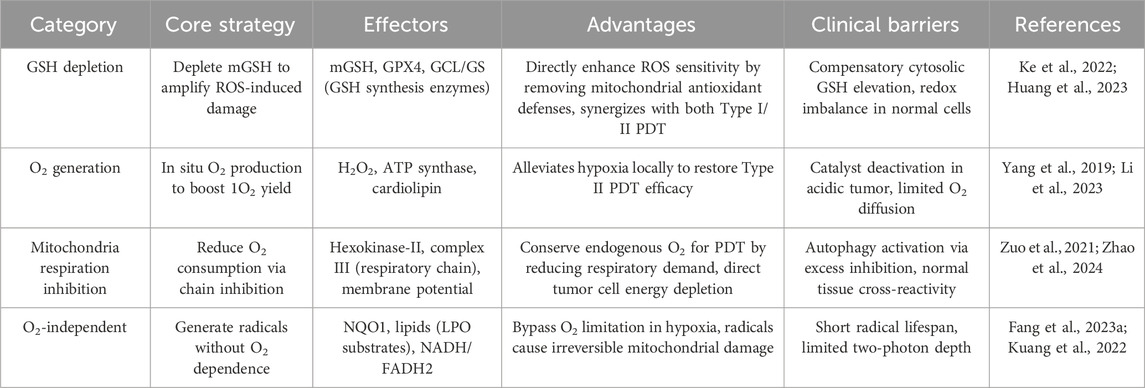
Cancer treatment remains one of the key research areas in the field of biomedicine (Shen et al., 2024; Wu D. et al., 2024; Ye et al., 2024; Yu et al., 2024b). The continuous development of effective and safe nanodrug delivery systems is not only crucial for advancing cancer therapy but also an urgent and ongoing research priority. Indeed, mitochondria play a pivotal role in cancer cellular energetics (Ikeda et al., 2021). Often referred to as the “powerhouses” of eukaryotic cells, these organelles are responsible for producing the majority of ATP, the cell’s universal energy currency (Wang H. et al., 2024). This process occurs through oxidative phosphorylation, a series of reactions that take place in the inner mitochondrial membrane (Vercellino and Sazanov, 2022; Decker and Funai, 2024). Developing mitochondria-targeted nanomedicine systems can enhance the therapeutic effects of PDT by leveraging the cascade of mechanisms triggered by mitochondrial dysfunction (Ding et al., 2025). A comprehensive overview of diverse mitochondria-targeted PDT strategies, spanning GSH depletion, O2 generation, mitochondria respiration inhibition, and O2-independent radical production, are systematically summarized in Table 2.
3.1 Targeted GSH depletion in mitochondria for enhanced PDT efficacy
Mitochondria-often referred to as the cell’s energy powerhouse-are particularly vulnerable to oxidative stress (Zhu et al., 2022). GSH is a tripeptide antioxidant present in all cells that maintains cellular redox homeostasis, with mitochondrial GSH (mGSH) playing a unique role in protecting mitochondrial DNA and respiratory chain proteins from oxidative damage (Li B. et al., 2025). Notably, mGSH is spatially and functionally distinct from cytosolic GSH: it is synthesized in the cytosol and transported into mitochondria via the glutamate-cystine antiporter (GC1), forming a relatively independent pool that accounts for ∼10–15% of total cellular GSH but is critical for mitochondrial redox defense (Lu et al., 2022; Xu T. et al., 2025). Tumor cells exhibit higher mGSH levels than normal cells, enabling them to scavenge ROS generated during PDT and resist oxidative stress (Song et al., 2024). Thus, selective depletion of mGSH rather than global GSH reduction avoids disrupting cytosolic redox balance and enhances PDT specificity (Zhang et al., 2025).
Ke et al. developed an iridium (III) complex containing thiols at both ends (Ir-SH) to construct self-assembled nanoparticles (IrS NPs) for targeted GSH depletion (Figure 3A) (Ke et al., 2022). Surface modification with Biotin, a small molecule ligand that specifically binds to overexpressed biotin receptors on tumor cells, enabling active targeting cellular uptake. Intrinsic lipophilic cationic properties of Ir-SH, which facilitates accumulation in mitochondria via electrostatic attraction to the negatively charged inner mitochondrial membrane. These nanoparticles self-assemble via disulfide bonds, which are selectively cleaved by mGSH. Upon cleavage, Ir3+ complexes are released, and simultaneously lowering endogenous GSH levels. Under two-photon laser irradiation, both the nanoparticles and released Ir3+ generate a mixture of 1O2 and •O2−, thereby amplifying intracellular oxidative stress. The depletion of GSH combined with increased ROS production inhibits the biosynthesis of glutathione peroxidase 4 (GPX4), a key lipid repair enzyme (Zhang Y. et al., 2021). Downregulation of GPX4 is widely recognized as a hallmark of ferroptosis (Zou et al., 2024a). This study demonstrates that IrS NPs precisely trigger ferroptosis by reducing mitochondrial GSH levels and promoting ROS generation. This work offers a novel strategy for enhancing PDT through organelle-targeted approaches, potentially overcoming limitations associated with conventional apoptosis-inducing therapies and holding significant clinical potential.
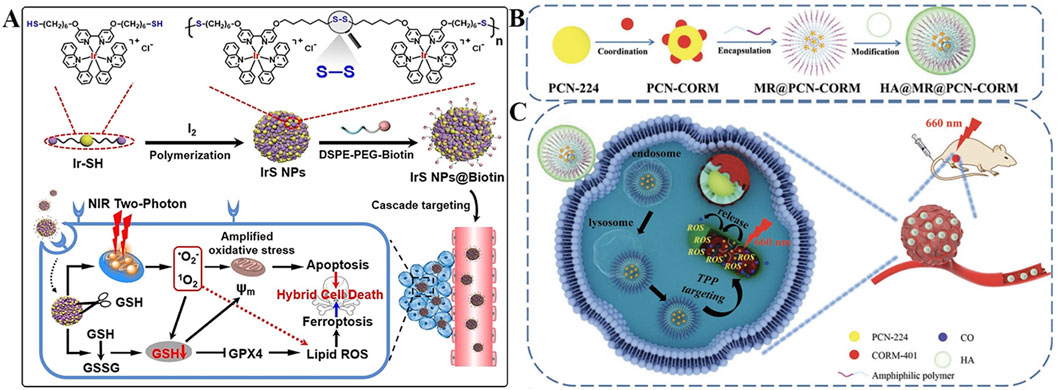
Figure 3. (A) Preparation of the IrS NPs@Biotin nanoplatform with cascade targeting and mGSH-responsive capabilities, along with its antitumor mechanism for enhanced PDT/ferroptosis. Reproduced with permission from Ref. Ke et al. (2022). Copyright © 2022 WILEY-VCH Verlag GmbH and Co. KGaA. (B) Preparation process of the HA@MR@PCN-CORM nanoplatform featuring hierarchical targeting and ROS-triggered CO release ability. (C) Schematic illustration of the multi-layered targeting HA@MR@PCN-CORM system for augmented ROS production in cancer cells. Reproduced with permission from Ref. Yang F. et al. (2023). Copyright © 2023 WILEY-VCH Verlag GmbH and Co. KGaA.
Besides the direct depletion of intracellular GSH by disulfide bonds, delivering carbon monoxide (CO) has also been shown to enhance the therapeutic effect of PDT by suppressing mitochondrial biosynthesis. High doses of CO can induce apoptosis in cancer cells by inhibiting mitochondrial respiration and interfering with protein synthesis (Dong et al., 2024; Yu et al., 2024c). Porphyrin-based nMOF materials have become a major focus in phototherapy research in recent years (Zhang et al., 2024; Zou et al., 2024b; Zou et al., 2025a). However, achieving more precise treatment by targeting specific intracellular organelles may serve as a powerful driving force for further advancing the biomedical applications of nMOFs. Yang et al. designed a multi-layered targeting nanosystem (HA@MR@PCN-CORM) with hierarchical targeting strategies (Figure 3B), in which CORM-40-a transition metal carbonyl compound capable of controlled CO release-was loaded into the hollow structure of porphyrin-based PCN-224 (Yang F. et al., 2023). By surface coating with hyaluronic acid (HA), a natural polysaccharide that binds to CD44 receptors overexpressed on tumor cells, the nanosystem could realize active tumor targeting. Moreover, incorporation of a mitochondria-targeted ROS-responsive amphiphilic copolymer (MR), which contains triphenylphosphonium (TPP) groups, it could accumulate in mitochondria. The TPP groups are initially shielded by HA, preventing premature mitochondrial targeting in normal tissues. Upon HA degradation by tumor-associated hyaluronidase, TPP is exposed to mediate secondary mitochondrial targeting (Figure 3C). The core of the system is PCN-224, a porphyrin-based MOF loaded with CORM-40, a CO-releasing molecule. Under 660 nm laser irradiation, PCN-224 generates ROS, which cleaves the thioketal linker in MR, triggering controlled CO release. CO then downregulates glutamate-cysteine ligase and glutathione synthetase in 4T1 cells, inhibiting mGSH synthesis. In xenograft tumor models, HA@MR@PCN-CORM treatment led to significantly reduced levels of GPX4 and glutamate-cysteine ligase proteins, further confirming that mitochondrial CO release can sensitize cancer cells to ferroptosis through GSH suppression, thereby enhancing apoptosis and achieving effective antitumor activity.
3.2 Direct O2 generation in mitochondria alleviates hypoxia and enhances PDT efficacy
Hypoxic tumor microenvironment remains a major obstacle limiting the efficacy of O2-dependent PDT in suppressing cancer cell proliferation (Shen et al., 2022; Xie et al., 2024). Therefore, direct strategies to generate O2 within tumor cells can significantly enhance PDT outcomes (Zhang et al., 2022a). Mitochondria are both major O2 consumers via oxidative phosphorylation and key PDT targets, therefore, in situ O2 generation within mitochondria can simultaneously replenish O2 for 1O2 production and protect this organelle from hypoxic damage. The mitochondrial matrix’s unique microenvironment, such as high H2O2 levels and acidic pH, makes it an ideal site for catalytic O2 generation. Numerous approaches have been developed, such as using inorganic catalysts like Mn3O4 and Pt nanoparticles, which react with endogenous H2O2 to produce O2 (Xu et al., 2018; Zhao et al., 2023). Alternatively, catalase enzymes can be employed to directly convert H2O2 into O2 (Cheng et al., 2020; Sun H. et al., 2023).
For example, Guan et al. developed mesoporous silica nanoparticles co-loaded with lipophilic cation IR780 and Mn3O4 nanoparticles (Figure 4A) (Yang et al., 2019). IR780, a lipophilic cationic cyanine dye, serves as the mitochondrial targeting ligand. IR780’s positive charge drives accumulation in mitochondria, while its hydrophobic structure enables insertion into the inner mitochondrial membrane. The mesoporous silica core acts as a carrier for Mn3O4 nanoparticles, protecting them from premature degradation and ensuring co-delivery with IR780 to mitochondria. With pH-dependent activity, Mn3O4 reacts with H2O2 to generate O2 in mitochondrial matrix-mimicking conditions. This O2 supply strategy enhances IR780-mediated 1O2 generation, while PDT-induced mitochondrial damage reduces O2 consumption, creating a “self-amplifying” effect. Zhu et al. fabricated a hybrid nanoplatform by combining upconversion nanoparticles (UCNPs) with MOFs, and decorated the surface with platinum nanoparticles and mitochondria-targeting TPP groups (Figure 4B) (Chen Y. et al., 2023). In this system, Pt efficiently catalyzes O2 generation from H2O2, alleviating tumor hypoxia and boosting PDT. Additionally, mitochondrial targeting leads to membrane depolarization and activation of apoptotic pathways, further enhancing therapeutic performance.
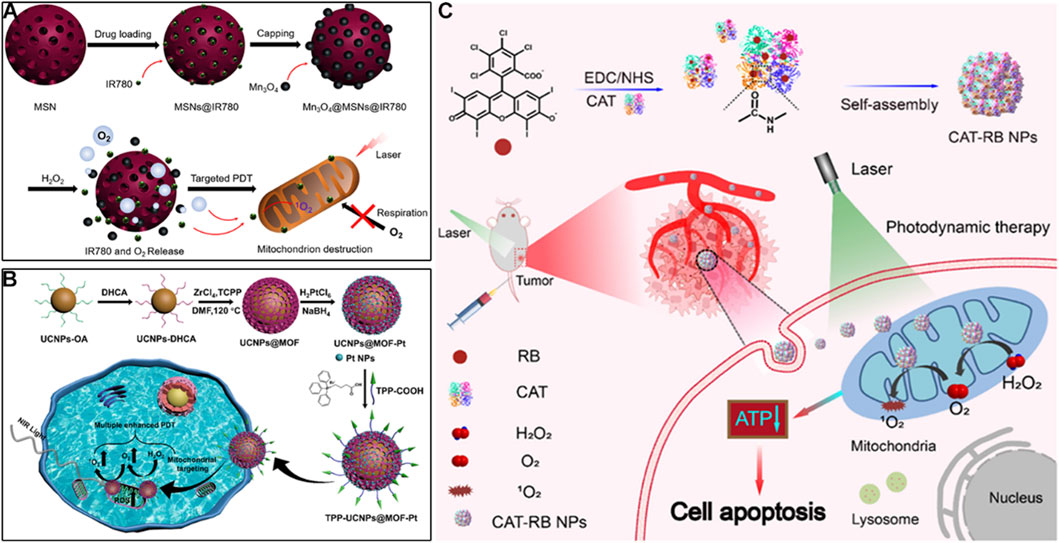
Figure 4. (A) Preparation of the Mn3O4@MSN@IR780 nanoplatform with mitochondrial targeting via IR780 and pH-responsive O2-generating capability, along with its mechanism for enhanced PDT. Reproduced with permission from Ref. Yang et al. (2019). Copyright © 2019 Ivyspring International Publisher. (B) Preparation process of the TPP-UCNP@MOF-Pt nanoplatform featuring TPP-mediated mitochondrial targeting and Pt-catalyzed O2 production, and its mechanism in boosting PDT. Reproduced with permission from Ref. Chen et al. (2023c). Copyright © 2023 American Chemical Society. (C) Schematic illustration of the CAT-RB NP system with cardiolipin-mediated mitochondrial targeting for catalase-driven O2 generation and augmented 1O2 production in PDT. Reproduced with permission from Ref. Xu et al. (2023b). Copyright © 2023 Chinese Chemical Society.
Beyond inorganic catalysts, enzymatic O2 generation offers higher specificity, as catalase directly converts H2O2 to O2 with high efficiency. Xu et al. catalase with rose bengal (RB) to form CAT-RB nanoparticles, where RB acts as both a photosensitizer and a mitochondrial targeting moiety via its high affinity for cardiolipin, a unique phospholipid enriched in the inner mitochondrial membrane (Figure 4C) (Xu X. et al., 2023). This cardiolipin-RB interaction ensures higher mitochondrial colocalization far exceeding cytosolic distribution. The covalent conjugation of catalase to RB through a PEG linker ensures that O2 generated by catalase is locally retained in mitochondria, maximizing its efficacy for 1O2 production. After cellular uptake, CAT-RB specifically accumulate in mitochondria, maintaining high catalase activity to converts H2O2 into O2, and increasing 1O2 production during PDT under 546 nm irradiation. The generated ROS further damage mitochondria and reduce ATP levels, promoting cancer cell apoptosis. These O2-generating strategies are straightforward and effective, offering promising solutions to overcome hypoxia-associated limitations in PDT and significantly improving its anticancer potential.
3.3 Reduce mitochondrial respiration in mitochondria for enhanced PDT
Mitochondria is a crucial organelle targeted by PDT, and excessive ROS accumulation can initiate the mitochondrial apoptosis pathway, resulting in cell death (Zuo et al., 2021). Positioning fundamental elements of PDT into mitochondria has received considerable attention for improving ROS transportation, thus realizing PDT with high specificity (Li et al., 2021). Due to the high consumption of O2 caused by mitochondrial respiration, sabotaging mitochondria and eliminating mitochondrial respiration are expected effective strategies to amplify the antitumor efficacy of PDT (Guo et al., 2021; Hu et al., 2024). Inhibiting respiration reduces O2 consumption, increasing local O2 availability for Type II PDT, while simultaneously sensitizing cells to ROS by disrupting mitochondrial membrane potential and reducing ATP-dependent repair mechanisms.
To enhance the accumulation of O2 and photosensitizers within mitochondria precisely, hexyl 5-aminolevulinate hydrochloride (HAL) and 3-bromopyruvic acid (3BP) were simultaneously encapsulated into microparticles of X-ray irradiated tumor cells (X-MP) to prepareHAL/3BP@X-MP for potent PDT (Figure 5A) (Zuo et al., 2021). Leveraging homotypic targeting, X-MP retains tumor cell surface antigens (e.g., integrins, E-cadherin), enabling specific recognition and uptake by homologous tumor cells. The payload 3BP could serve as a small-molecule inhibitor target to hexokinase-II (HK-II), an enzyme that binds to the outer mitochondrial membrane via voltage-dependent anion channel interactions, linking glycolysis to mitochondrial respiration. 3BP covalently modifies HK-II’s active site, significantly increasing local O2 supply by inhibiting mitochondrial respiration and glycolysis. Concurrently, HAL promotes the localized biosynthesis and effective accumulation of protoporphyrin IX (PpIX) in mitochondria via the heme biosynthesis pathway, resulting in high PpIX accumulation in mitochondria. Additionally, PpIX can produce sufficient ROS to directly destroy the mitochondria of cancer cells, leading to outstanding antitumor effects of PDT. In both in vitro/vivo experiments, the remarkable antitumor capability of HAL/3BP@X-MP could be observed without obvious side effects, providing a prospect for conquering the limitations of the PDT modality to fight hypoxic tumors.
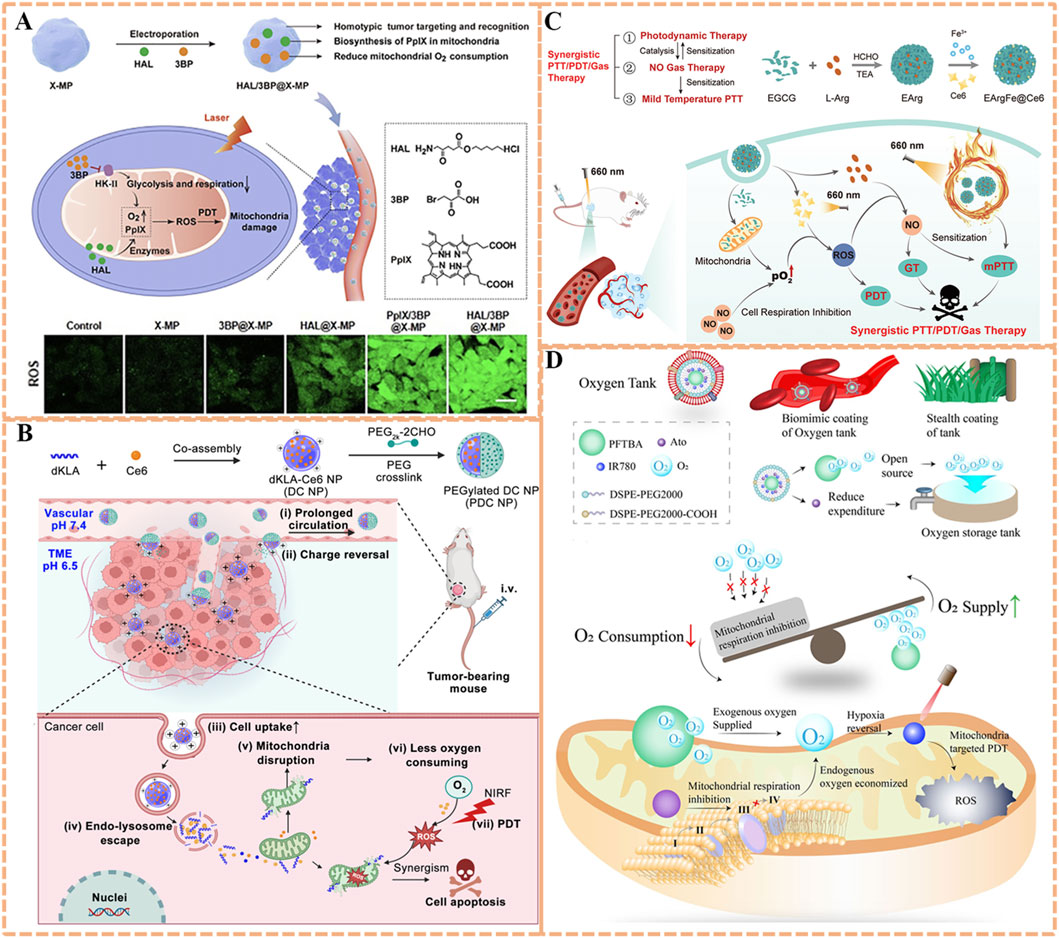
Figure 5. (A) Preparation of the HAL/3BP@X-MP nanoplatform with homotypic tumor targeting and HK-II-inhibiting capability, along with its mechanism for potent PDT via O2 conservation. Reproduced with permission from Ref. Zuo et al. (2021). Copyright © 2021 WILEY-VCH Verlag GmbH and Co. KGaA. (B) Preparation process of the PDC NP system featuring pH-responsive tumor accumulation and dKLA-mediated mitochondrial targeting, and its mechanism in synergistic PDT/peptide therapy. Reproduced with permission from Ref. Qu et al. (2023). Copyright © 2023 Springer-Verlag. (C) Schematic illustration of the EArgFe@Ce6 nanoplatform with EGCG-Fe3+-mediated mitochondrial targeting for combined PDT/PTT/gas therapy via respiration inhibition. Reproduced with permission from Ref. Shi et al. (2023). Copyright © 2023 WILEY-VCH Verlag GmbH and Co. KGaA. (D) Preparation of the O2 Tank nanoplatform with RBCm-enabled prolonged circulation and dual-mode hypoxia alleviation, along with its mechanism for enhanced mitochondrial PDT. Reproduced with permission from Ref. Li et al. (2022b). Copyright © 2022 BioMed Central.
Peptide-based respiration inhibitors could also offer high specificity. d-(KLAKLAK)2 (dKLA), a 14-amino-acid α-helical proapoptotic peptide, has been demonstrated to damage mitochondria of eukaryotic cells, abrogate mitochondria respiration, reduce O2 consumption (Qu et al., 2023). Accordingly, dKLA is conductive to improve the photodynamic therapeutic effect when combined with photosensitizers. Inspired by this, a charge-reversible crosslinked nanoparticle (PDC NP) based on photosensitizer chlorin e6 (Ce6) and dKLA was developed for strengthened peptide delivery efficiency and synergistic antitumor effect (Qu et al., 2023). As illustrated in Figure 5B, the proapoptotic dKLA was co-assembled with Ce6 to construct pure drug nanoparticles (DC NP) with drug loading capacity of 100%. Subsequently, a dual-aldehyde terminated PEG2k-2CHO was utilized to crosslink the DC NP to obtain the surface charge reversal PEGylated DC NPs (PDC NP, 68.3% drug loading). Crosslinking with a dual-aldehyde PEG, theses pH-responsive NPs confer a slightly negative surface charge in blood for prolonged circulation, and undergoes charge reversal to positive in acidic tumor microenvironments, promoting cellular internalization. dKLA’s targeting mechanism relies on its cationic α-helical structure, which interacts with anionic phospholipids in the outer mitochondrial membrane and inserts into the membrane, causing depolarization. The dKLA could target and disrupt the mitochondrial membrane, and next inhibit the mitochondrial respiratory chain to reduce the consumption of local O2. In addition, the concentration of Ce6 around mitochondria would increase in company with the targeting delivery of dKLA, thus extensively augmenting 1O2 production mediated by Ce6 due to increased O2 availability. This strategy may offer a valuable approach for magnifying the drug delivery capability of peptides and optimizing mitochondrial-targeted PDT efficiency.
Apart from the well-known effect on tumor cell-killing directly, the antitumor effect induced by nitric oxide (NO) involves multiple pathways, such as nitrosation of mitochondria and DNA, as well as inhibition of cellular respiration, which is effective for overcoming drug resistance in PDT (Xu et al., 2021; Jiang et al., 2022). A mitochondria-targeted NO nanogenerator termed EArgFe@Ce6 was developed to achieve synergistic photodynamic/gas/photothermal tumor therapy (Figure 5C) (Shi et al., 2023). EGCG-Arg nanoparticles (EArg) were formed via chemical assembly of epigallocatechin gallate (EGCG) and NO donor l-Arginine (l-Arg), then EArgFe was prepared by adding ferric ions (Fe3+) into EArg to enable the coordination between Fe3+ and EGCG, and finally Ce6 was physically decorated on its surface to construct EArgFe@Ce6 (Figure 8B). Upon 660 nm light irradiation, the coordination of EGCG and Fe3+ endows nanogenerator with prominent photothermal capability for low-temperature PTT (Figure 8C). EGCG has verified hypoxia alleviation by inhibiting mitochondrial respiration, therefore EArgFe@Ce6 could target to and accumulate in mitochondria to suppress cell respiration, favoring the improvement of PDT. The ample ROS produced by PDT would in-turn catalyze l-Arg to synthesize considerable NO for gas therapy. The mitochondrial localized NO can suppress cell respiration and further sensitize PDT and low-temperature PTT. Together, the nanogenerator has been manifested with superior photodynamic/gas/photothermal therapeutic outcomes in in vitro and in vivo experiments, which almost achieve complete tumor ablation.
Off-target respiratory inhibition remains a challenge, as systemic delivery can affect normal tissues (e.g., heart, brain) with high O2 demand (Xu et al., 2022; Zeng et al., 2024a). Li et al. addressed this with an “O2 Tank” system: red blood cell membrane (RBCm)-coated perfluorocarbon (PFC) liposomes co-loaded with atovaquone (ATO, a Complex III inhibitor) and IR780 (Figure 5D) (Li X. et al., 2022). By a biomimetic stealth strategy, RBCm coating provides immune evasion and prolonged circulation, while PFC’s high O2 solubility acts as an exogenous O2 reservoir. Essentially, ATO, a lipophilic drug, passively accumulates in mitochondria via partitioning into lipid membranes, where it selectively inhibits Complex III of the respiratory chain, reducing tumor O2 depletion without affecting normal tissues. IR780, as a mitochondrial-targeted photosensitizer, utilizes both endogenous (respiration-inhibited) and exogenous (PFC-delivered) O2 to generate 1O2, thus boosting PDT for amplified tumor growth inhibition. This current strategy concentrates on synergistic tumor hypoxia modulation via exogenous O2 supply and inherent O2 metabolism, exerting the potential to augment outcomes of various therapies with hypoxia-associated resistance.
3.4 PDT with O2-independent pathways
The therapeutic effect of photosensitizers in PDT largely depends on the ROS production efficiency and tissue penetration depth, therefore, type II PDT which relies on O2 to produce 1O2 always displays insufficient effect in deep hypoxic region (Zhuang et al., 2025). In contrast, Type I PDT circumvents O2 dependence by generating cytotoxic radicals (e.g., •O2−, •OH) via electron/proton transfer reactions with endogenous donors (e.g., NADH, GSH), maintaining ROS production even when O2 is depleted (Zheng X. et al., 2021). This O2 independence makes Type I photosensitizers particularly valuable for treating hypoxic tumors, where they can exploit the highly reducing mitochondrial matrix (rich in redox-active molecules) to amplify radical generation (Zhuang et al., 2023).
To achieve a satisfactory PDT effect in aerobic and hypoxic scenarios simultaneously, three types of red light-emissive CDs (RCDs) derived from hypericum perforatum extract with adjustable type I/II ROS production have been developed (Li M. et al., 2025). These RCDs exhibit intrinsic mitochondrial targeting via surface amine groups, which interact electrostatically with the negatively charged inner mitochondrial membrane, achieving high mitochondrial colocalization. Their unique structural design enables dual-mode ROS generation, the RCDs exhibit tunable ROS generation with equal •O2− via type I PDT and incremental 1O2 via type II PDT, which could be adopted for deep red fluorescence imaging-guided type I/II PDT. On one hand, the unaltered core sizes of RCDs donated their same redox potentials, resulting in the production of equal •O2− (Figure 6A) (Zhang et al., 2023d). On the other hand, the enhancement of 1O2 production was demonstrated to be ascribed to their surface states, thus reducing the energy gaps between S1 to T1. With inherent mitochondria targeting ability, RCDs could precision attack towards tumors. In vitro and in vivo studies demonstrated RCDs with tunable type I/II ROS production capability, realizing a remarkable anticancer eradication even under harsh hypoxic tumor microenvironment. This work offers considerable prospects for developing versatile CDs as photosensitizers with adjustable ROS production to overcome the limitation of current single-type PDT, exhibiting substantial potential in antitumor applications.
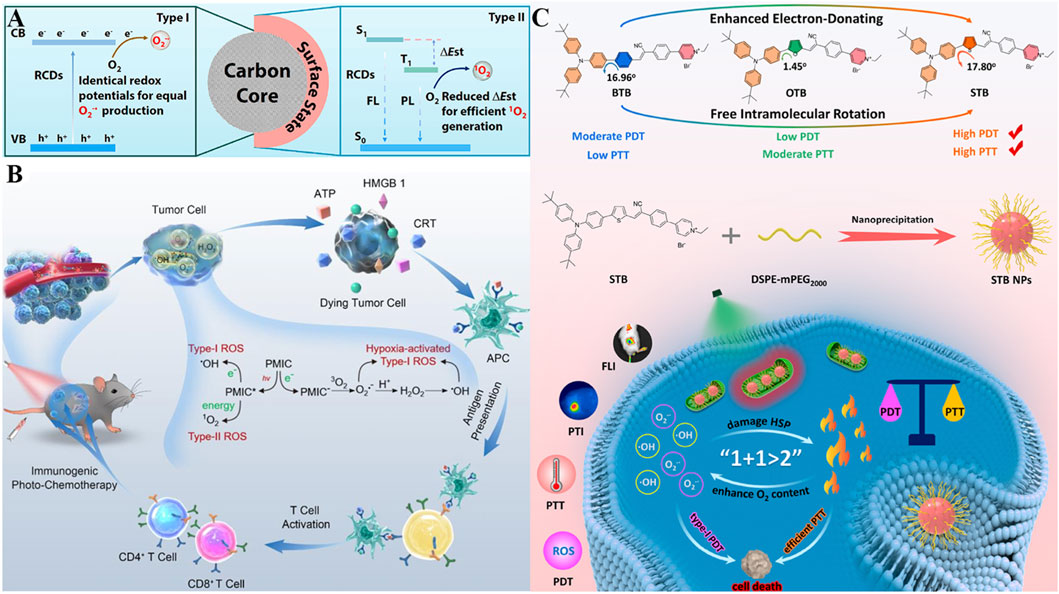
Figure 6. (A) Mechanism of the RCD nanoplatform with tunable Type I/II ROS production capability via surface state regulation, along with its dual-mode PDT mechanism. Reproduced with permission from Ref. (Zhang et al., 2023d). Copyright © 2023 Elsevier Ltd. (B) Schematic of the PMIC-NC system featuring NIR light-activated Type I/II ROS generation, hypoxia-triggered Type I ROS burst, and ICD induction for synergistic antitumor therapy. Reproduced with permission from Ref. Lou et al. (2023). Copyright © 2023 WILEY-VCH Verlag GmbH and Co. KGaA. (C) Preparation of π bridge-engineered STB phototheranostic agents with enhanced Type I PDT and PTT performance, along with their mechanism for synergistic therapy. Reproduced with permission from Ref. Fang et al. (2023a). Copyright © 2023 American Chemical Society.
Immunotherapy has been widely studied for cancer treatment in recent years (Chen and Wang, 2020; Hu et al., 2020; Song et al., 2022; Mao et al., 2023; Zhao et al., 2024). The combination of PDT with immunotherapy offers a promising new direction for the treatment of malignant cancer cells (Ji et al., 2022; Liu et al., 2025). Aside from directly inducing tumor cell death by PDT, ROS can further induce ICD effect for efficient tumor ablation (Huang et al., 2022; Wei et al., 2022). To improve the ROS-induced ICD effect, one soluble perylene monoimide derivative bearing tertiary ammonium functions at the imide and peri positions (PMIC-NC) was synthesized for augmented PDT therapy. Serving as a ROS supergenerator, the prepared PMIC-NC could induce Type-I/II ROS generation through electron/energy transfer upon 660 nm laser irradiation as well as trigger endogenous burst of Type I ROS assisted by proton transients in tumor cells. The excessive ROS could directly kill cells by PDT on the primary tumor, furthermore elicit vigorous ICD effect to awake antitumor immunity (cytokine secretion, DCs maturation, cytotoxic T lymphocytes activation) and thereby curb the proliferation of distant or metastasis tumors (Figure 6B) (Lou et al., 2023). After therapies, PMIC-NC supplied preferable antitumor effects on both primary tumors (suppression rate of 85.4%) and pulmonary metastasis (suppression rate of 58.1%) upon NIR irradiation. This work thus provides one proof-of-concept for the design of ROS generators which incorporates NIR light-activated Type-I/II PDT, hypoxia-triggered Type-I ROS burst and ICD effect, to realize immunogenic photochemotherapy towards hypoxic tumors.
Synergizing PDT with PTT leverages photon energy for both ROS generation and hyperthermia, with mitochondria as a critical co-target due to their sensitivity to thermal damage (Cui et al., 2023). Given that the combinational therapy of PDT and PTT may maximize treatment outcomes, Fang et al. have synthesized three mitochondria-targeting phototheranostic agents for multimodal imaging-guided PDT/PTT synergistic therapy (Figure 6C) (Fang L. et al., 2023). Attributed to the cationic pyridinium moiety, (Z)-4-(4-(2-(5-(4-(bis(4-(tert-butyl)phenyl)amino)phenyl)thiophen-2-yl)-1-cyanovinyl)phenyl)-1-ethylpyridin-1-ium bromide (STB) shows mitochondria-targeting ability via electrostatic interactions with the inner mitochondrial membrane. Employing thiophene as π bridge, STB strengthens robust donor-accepter (D-A) interactions, separates the distribution of HOMO and LUMO, enabling it with prominent NIR fluorescence emission. Moreover, the decrease of the S1-T1 energy gap facilitates the intersystem crossing (ISC), allowing free intramolecular rotation and resulting in massive amounts of •O2− and •OH production by optimal type I PDT. In the meantime, an ideal molar extinction coefficient and adequate intramolecular motions are beneficial for the enhancement of photothermal conversion efficiency (up to 51.9%). Based on these, STB NPs could actively target to and accumulate in the tumor site, realize real-time in vivo NIR fluorescence imaging, and obviously inhibit tumor growth with negligible side effects. This work subtly established a practical strategy by π bridge engineering, for designing phototheranostic agents with the enhancement of PDT/PTT performance, accelerating the progress of multimodal imaging-guided phototherapy against tumors.
Despite their hypoxia resistance, Type I photosensitizers face challenges: short radical lifetimes and limited intratumoral accumulation (Xiong et al., 2025). To address this, an injectable hydrogel (TSH) was engineered to co-deliver TDCAc (a Type I photosensitizer) and (NH4)2S (a donor of H2S), aiming at sustained ROS generation (Figure 7A) (Zhang T. et al., 2022). Upon 660 nm laser irradiation, TDCAc would rapidly gain heat due to PTT effect, promoting the dissolution of agarose hydrogel, accelerating the release of (NH4)2S and TDCAc in the tumor. Afterward, H2S gas was generated by (NH4)2S in an acidic tumor microenvironment, diffused into tumor cells, and then repressed intracellular catalase activity. Due to reduced H2O2 consumption, the in-turn accumulation of H2O2 could induce continuous •OH generation via the Fenton reaction by utilizing the endogenous labile iron pool in cancer cells. Notably, H2S also upregulates mitochondrial Fe2+ transporters (e.g., DMT1), amplifying Fenton reactions in a feedforward loop and increasing •OH levels. The uninterrupted ROS production in cancer cells and distinguished tumor ablation have been demonstrated in vitro and in vivo. In recent years, drug delivery research based on hydrogel carriers has emerged as a promising field (Xia et al., 2023; Xu L. et al., 2023; Zhang H. et al., 2023). Combining hydrogels with phototherapy may offer new insights and strategies for the treatment of malignant cancers (Chen et al., 2021).
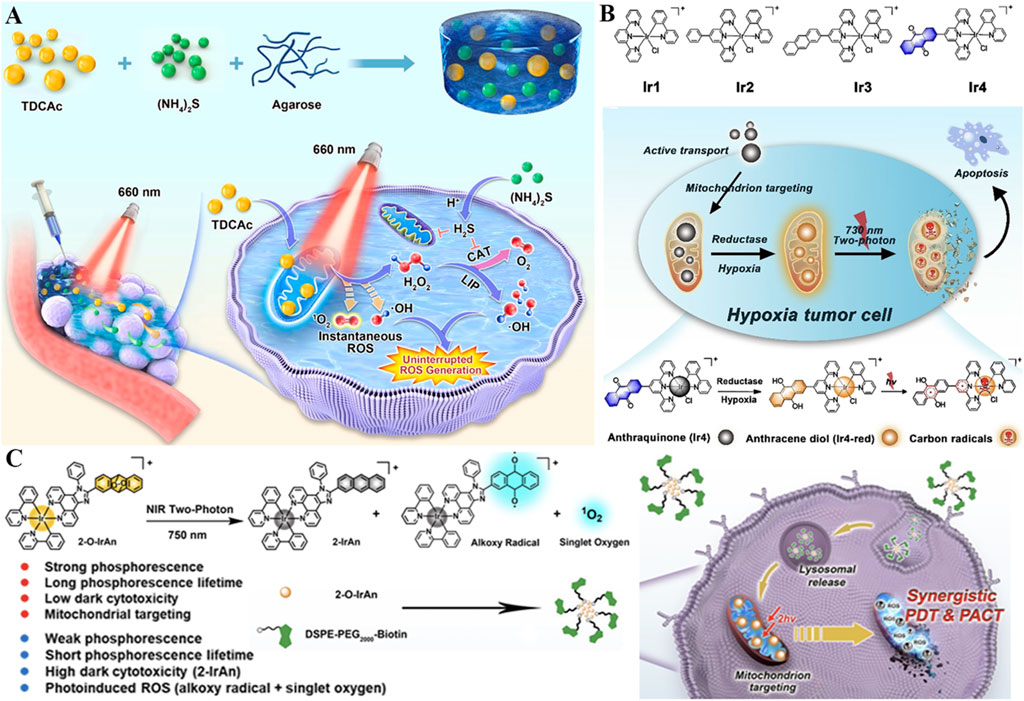
Figure 7. (A) Schematic of the TSH hydrogel-based uninterrupted ROS generator with H2S-mediated catalase inhibition, along with its mechanism for combined H2S gas therapy and Type I PDT. Reproduced with permission from Ref. Zhang et al. (2022c). Copyright © 2022 Elsevier Ltd. (B) Mechanism of the Ir4 system with hypoxia-activated two-photon excitation and mitochondrial carbon radical generation for PDT in hypoxic tumor cells. Reproduced with permission from Ref. Kuang et al. (2020). Copyright © 2020 WILEY-VCH Verlag GmbH and Co. KGaA. (C) Illustration of the Ir(III) complex-based system featuring synergistic PDT and photoactivated chemotherapy via NIR-triggered ROS release and cytotoxic precursor activation. Reproduced with permission from Ref. Kuang et al. (2022). Copyright © 2022 American Chemical Society.
Two-photon excited PDT (2PE-PDT) utilizes a photosensitizer with short wavelength absorption to absorb two NIR photons and subsequently trigger ROS production, which offers an enormous possibility for precise treatment against deep tumor tissues (Wu et al., 2021; Tan et al., 2025). In this context, a series of iridium (III) complexes (Ir1-4) were synthesized, and anthraquinone was inserted in Ir4 as a mitochondrion-localized carbon radical initiator and an emission quenching group (Figure 7B) (Kuang et al., 2020). Under hypoxic conditions, this anthraquinone group within Ir4 could be specifically reduced by reductase and formed anthracene diol (Ir4-red), thus turning on the two-photon excited capability of complexes. Subsequent irradiation results in mitochondrion-localized carbon radical production, excessive intracellular ROS accumulation, and damage to mitochondrial membrane potential, resulting in cell death (IC50light = 2.1 μM). Due to the hypoxia-activated and mitochondrion-targeted merits of two-photon excitation, Ir4 has demonstrated dual functions of two-photon excited imaging (lex = 730 nm) and remarkably PDT therapy in vivo against hypoxic conditions. This metal complex-based two-photon photosensitizer could generate carbon radicals in an O2-independent way, which is promising as a candidate for clinical treatment of hypoxic tumors.
On this basis, Kuang et al. reported a novel photoactivated prodrug (2-O-IrAn) based on the iridium (III) endoperoxide complex, which effectively united the characteristics of two-photon photosensitizers and photoactivated chemotherapy prodrugs with low dark toxicity. 2-O-IrAn targets mitochondria via its lipophilic cationic structure, achieving high colocalization with mitochondria, and is activated by NIR two-photon irradiation to release 1O2, alkoxy radicals, and cytotoxic precursor 2-IrAn (a mitochondrial Complex I inhibitor) (Figure 7C) (Kuang et al., 2022). This photoactivation reaction can disrupt the mitochondrial membrane and ultimately cause tumor cell death efficiently under both normal O2 (PI = 959.1) and anaerobic conditions (PI = 690.3), appreciably enhancing the therapeutic effect of hypoxic PDT and photoactivated chemotherapy. In addition, DSPE-PEG-Biotin was introduced to endow DSPE-PEG-Biotin@2-O-IrAn nanoparticles with excellent tumor targeting and prolonged blood half-life, which could realize complete tumor eradication in vivo. This synergistic strategy of NIR two-photon photoactivated chemotherapy and PDT provides a novel guideline for the treatment of hypoxic tumors.
However, mitochondria-targeted PDT is also constrained by multi-dimensional limitations: In terms of targeting specificity, membrane potential-dependent carriers exhibit reduced localization efficiency in hypoxic tumor cells due to decreased mitochondrial membrane potential, and may be affected by variations in tumor metabolic phenotypes. At the level of carriers, metal-based nanoparticles (e.g., Pt, Ir) may interfere with mitochondrial iron-sulfur cluster synthesis to trigger chronic toxicity, peptide drugs are susceptible to protease degradation, and enzymatic catalysts show poor stability in acidic tumor microenvironments. In terms of therapeutic mechanisms, mGSH depletion may trigger compensatory elevation of cytosolic GSH, short-lived radicals tend to induce Nrf2-mediated antioxidant responses, and respiratory inhibitors may carry the risk of off-target toxicity. Additionally, excess inhibition of mitochondrial respiration may activate protective autophagy, thereby weakening therapeutic effects. These challenges collectively restrict the clinical translation and efficacy optimization of mitochondria-targeted PDT.
4 Targeting lysosome for enhanced PDT
Lysosomes are organelles that contain large amounts of cathepsin to perform key cellular catabolic processes, which are essential for maintaining cell homeostasis and regulating various cellular processes (Zhang Z. et al., 2021; Gros and Muller, 2023). Different from the normal cells, lysosomes in tumor cells are more plentiful with altered morphology, which upregulated lysosomal activity in tumor tissue is related to the metabolic need of fast proliferating tumors (He et al., 2023; Jia et al., 2023). In tumor tissue, photosensitizers localized in the lysosome may cause selective damage to lysosomes and induce subsequent cathepsin release into the cytoplasm, trigger cell death, and finally postpone the progression and metastasis of tumors.
The alteration of lysosomal membrane permeabilization (LMP) can disrupt the function of lysosomes and even cause lysosome-dependent cell death (Tanaka et al., 2022). By inhibiting the leakage of photosensitizers from lysosomes, constant LMP and unrecoverable damage to cancer cells could be motived under light irradiation, thus the efficiency of PDT substantially improved. Encourage by this, Li et al. reported lysosome-targeting nanophotosensitizer (BDQ-NP) based on boron-dipyrromethene (BOD) derivatives with an amphiphilic structure (synthesized via a reaction involving BOD core modification and hydrophobic chain conjugation) for highly effective PDT (Figure 8A) (Li et al., 2023). Amphiphilic BDQ could be synthesized in four steps by BOD and subsequently self-assembled into nano-sized particles (BDQ-NP) in aqueous solution at a concentration of 0.5 mg/mL under mild stirring (200 rpm) for 2 h. Owing to the pH-dependent lysosome-targeting property (facilitated by protonation of amino groups in the acidic lysosomal environment), BDQ powerfully incorporates into the lipid bilayers of lysosomes and induces continuous LMP through ROS-mediated LPO. Under light irradiation (660 nm, 10 mW/cm2), BDQ-NP produced a substantial amount of ROS, and disrupted functions of lysosome and mitochondria via activating the cathepsin B-mediated mitochondrial damage pathway. Specifically, cathepsin B cleaves pro-apoptotic proteins to trigger outer membrane permeabilization, releasing cytochrome c and initiating caspase-dependent apoptosis. After intravenous injection, BDQ-NP achieved high accumulation in tumors as well as a fantastic PDT effect on subcutaneous colorectal CT26 tumors and orthotopic 4T1 breast carcinomas with low systemic toxicity. In addition, PDT mediated by BDQ-NP also efficiently hindered lung metastasis of 4T1 breast carcinomas by reducing MMP-9 expression (downregulated by 42%) via lysosomal damage. This work highlights the potential value of self-assembled lysosome-targeted photosensitizers for augmented PDT effect against tumor metastasis.
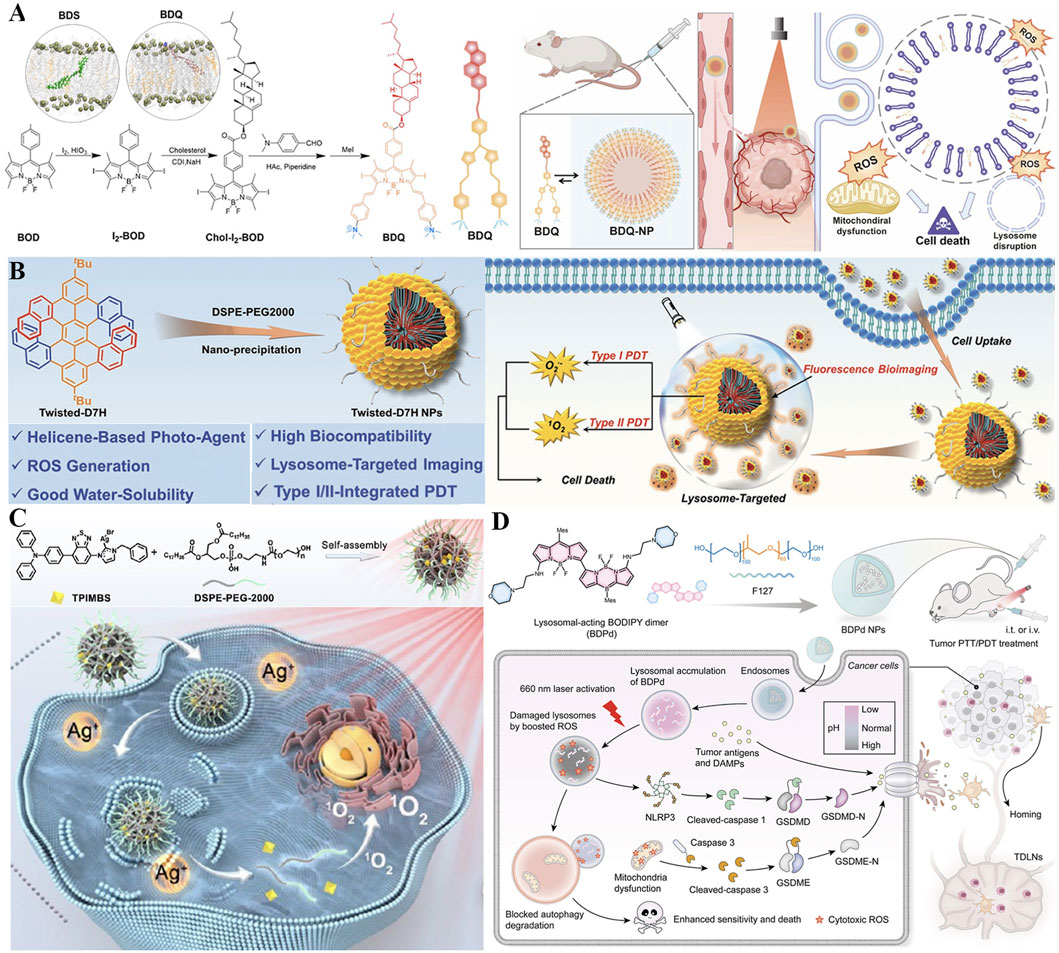
Figure 8. (A) Preparation of the BDQ-NP nanoplatform with pH-dependent lysosomal targeting via protonated amino groups and ROS-mediated lipid peroxidation capability, along with its mechanism for enhanced PDT and metastasis inhibition. Reproduced with permission from Ref. Li et al. (2023). Copyright © 2023 Royal Society of Chemistry. (B) Preparation process of the Twisted-D7H NP system featuring DSPE-PEG2000-enabled water solubility and lysosome-specific accumulation, and its mechanism in Type I/II PDT. Reproduced with permission from Ref. Zhao et al. (2022). Copyright © 2022 WILEY-VCH Verlag GmbH and Co. KGaA. (C) Preparation of the TPIMBS NP nanoplatform with lysosomal targeting and photoactivable Ag+ release capability, along with its mechanism for synergistic PDT/chemotherapy and prolonged bioimaging. Reproduced with permission from Ref. Liu et al. (2023a). Copyright © 2022 American Chemical Society. (D) Preparation process of BDPd NP system featuring morpholine-mediated lysosomal targeting and NIR-responsive dual PDT/PTT functionality, and its mechanism in synergistic therapy. Reproduced with permission from Ref. Sun et al. (2024). Copyright © 2023 American Chemical Society.
The characteristics of inherent chirality, nonplanarity, and structural controllability make helicenes promising candidates in the field of optoelectronics and biomedical applications (Bouz et al., 2025). Nevertheless, the poor water solubility of helicenes strictly restricts their biological applications for organelle-targeted type I and II PDT (Yang et al., 2024). To overcome these limitations, water-soluble nanoparticles named D7H-NPs comprising twisted double [7]carbohelicene (D7H) were fabricated for lysosome-targeted bioimaging and advanced PDT (Zhao et al., 2022). As shown in Figure 8B, D7H was reprecipitated with amphiphilic 1,2-distearoyl-sn-glycero-3-phosphoethanolamine N-[methoxy (polyethylene glycol)-2000] (DSPE-PEG2000) to prepare self-assembled D7H-NPs via the nanoprecipitation method (Zhao et al., 2022). The resulting D7H-NPs displayed a uniform size of 46 ± 2 nm and exhibited superior water solubility. Especially, D7H-NPs could specifically gather in the lysosomes of 4T1 tumor cells, suggesting their ability of lysosome-targeted bioimaging. Moreover, the extensive production of •O2− and 1O2 derived from D7H-NPs was noticed upon light excitation, thereby triggering tumor cell apoptosis. This work contributes a promising phototherapeutic agent based on helicenes concerning type I/II PDT, manifesting a facile strategy for organelle-targeted enhanced PDT therapy.
On account of light-triggered lysosomal disruption is beneficial for tumor therapy, a photo-activable theranostic nanoplatform (TPIMBS NPs) based on a synthetic multifunctional molecule (TPIMBS, chemical structure containing a cyanine dye core, organosilver moiety, and PEGylated segment) was constructed for spatiotemporally-controllable synergistic tumor therapy (Figure 8C) (Liu X. et al., 2023). With rational design, the synthetic TPIMBS photosensitizer exhibited function of aggregation-induced NIR emission and utilized organosilver as a chemotherapeutic agent. The amphiphilic block copolymers (DSPE-PEG-2000) at a mass ratio of 1:3 (TPIMBS:polymer) was introduced to assisted TPIMBS self-assemble into nanoparticles, rendering TPIMBS NPs a prolonged blood circulation half-life time of 12.5 h as well as expanded tumor accumulation via passive targeting. Through photochemical internalization, TPIMBS NPs favorably focused on the lysosomes of tumor cells and created ROS under light irradiation, leading to lysosomal disruption and release of Ag+ and cathepsin B from TPIMBS NPs. Cathepsin B contributes to both mitochondrial damage and inflammasome activation via the NLRP3 inflammasome/caspase-1/GSDMD-N pathway. Moreover, the release Ag+ could serve as a chemotherapeutic agent, which inhibits thioredoxin reductase and DNA polymerase to block multiple enzymatic pathways and result in cell apoptosis, significantly boosting the systemic antitumor efficacy by synergism with PDT (tumor inhibition rate = 91% vs. 65% for monotherapy). Notably, TPIMBS NPs with excellent stability can persevere accumulation at the tumor site for up to 36 h for prolonged bioimaging, and possess the desirable capability for tumor elimination in in vivo experiments. This nanoplatform exploits the combination of lysosomal-targeted ROS production with the photoactivable release of chemotherapeutic Ag+, to realize a spatiotemporally-controllable antitumor effect.
Accumulating evidence demonstrates that regular lysosomal function plays a critical role in autophagic degradation and the activation of pyroptosis (Liu Y. et al., 2024; Zhu et al., 2025). Inspired by the function of lysosome closely related to pyroptosis and autophagy, Sun et al. rationally synthesized a boron-dipyrromethene dimer (BDPd) comprising two lysosomal-targeted morpholine groups as NIR photosensitizer to augment PDT/PTT (Figure 8D) (Sun et al., 2024). To facilitate internalization into cancer cells, the synthesized BDPd was further self-assembled together with amphiphilic triblock copolymer (Pluronic F127) to prepare nanosized micelles (named BDPd NPs). BDPd NPs exhibited good biocompatibility, high ROS production yield, and excellent photothermal abilities. The perfect lysosomal-targeting capability endows BDPd NPs accumulating in lysosomes and stimulates vigorous lysosomal damage by boosted ROS under NIR laser irradiation, thus increasing the killing efficiency of 4T1 cancer cells. Mechanistically, BDPd NPs could efficiently damage the lysosomal and mitochondrial of cancer cells, activate ICD marker exposure, and trigger pyroptosis through simultaneously stimulating caspase-3/GSDME and NLRP3/GSDMD pathway, subsequently promoting DCs maturation. More importantly, the destruction of lysosomal functions in-turn blocks self-protective autophagic degradation, thus losing cytoprotection towards cancer cells. Either intratumoral or intravenous injection, BDPd NPs substantially suppressed tumor growth upon NIR light activation, provoked robust antitumor immune responses, and prevented tumor recurrence in breast tumor-bearing mouse models. Collectively, this work highlights the close relationship between subcellular organelles and PTT/PDT with advanced nanotechnology, to amplify the therapeutic outcomes in the future.
5 Targeting nucleus for enhanced PDT
Metabolism imbalance advances the tumor aggressiveness and resistance towards therapies, therefore cell nucleus regulating cellular metabolism and heredity becomes a brightening subcellular therapeutic location (Pham et al., 2023). Nucleus-targeted photosensitizers can apply pressure to DNA replication, facilitate apoptosis of cancer cells, as well as provoke inflammatory cells via secreting extensively proinflammatory factors and stimulate antitumor immune response (Liu et al., 2021). Targeting the DNA damage response and DNA repair capacity in cancer cells has been recognized as an important anti-cancer strategy in recent years (Chen M. et al., 2023). Accordingly, manners to target the nucleus of tumor cells are considered a perfect target for opening the complete potential of tumor phototherapy (Wang P. et al., 2024). However, nucleus-targeting drugs with high efficiency and biosafety characteristic are recently rare.
Encouraged by nucleus-targeting photosensitizers-mediated PDT can elicit innate immune response for long-term immunotherapy, the combination of aPD-L1 with photoimmunotherapy is promising for reversing the immunosuppressive microenvironment and minimizing tumor recurrence (Zhang et al., 2023c). Zhang et al. constructed a novel nanomaterial named as PZGE by employing graphene quantum dots (GQDs) to loaded PD-L1 molecular antibody (aPD-L1) and zinc phthalocyanine (ZnPc) to promote innate immune response and transpose immunosuppressive microenvironment (Figure 9A) (Zhang et al., 2023c). To investigate the relationship between nucleus entry capacity and nanoparticle size, PZGE with three different particle sizes (5 nm, 32 nm, 200 nm) were synthesized. Then, the CCK-8 assay was employed to quantify their phototoxicity (Xiong et al., 2020; Cheng et al., 2021; Hu et al., 2021). Notably, PZGE with particle size of 5 nm exhibited the best nucleus enrichment ability due to its ultrasmall size, and amplified DNA destruction by nucleus-located PDT. Antitumor immunity was activated via cGAS/STING/IFN I pathway, followed by cytotoxic T lymphocyte infiltration and PD-L1 expression reversion. Besides, PZGE upon light irradiation effective inhibited the oral squamous cell carcinoma in vivo. Immunotherapy has introduced innovative design concepts and broad application prospects for the treatment of malignant tumors (Ge et al., 2023; Huang et al., 2023). The combination of phototherapy and immunotherapy offers a promising new approach in the fight against cancer (Liang et al., 2023). This work displays a size-dependent strategy of nucleus-targeted PDT and provides new insight into nucleus-targeting photo-mediated immunotherapy for “immune cold” tumors.
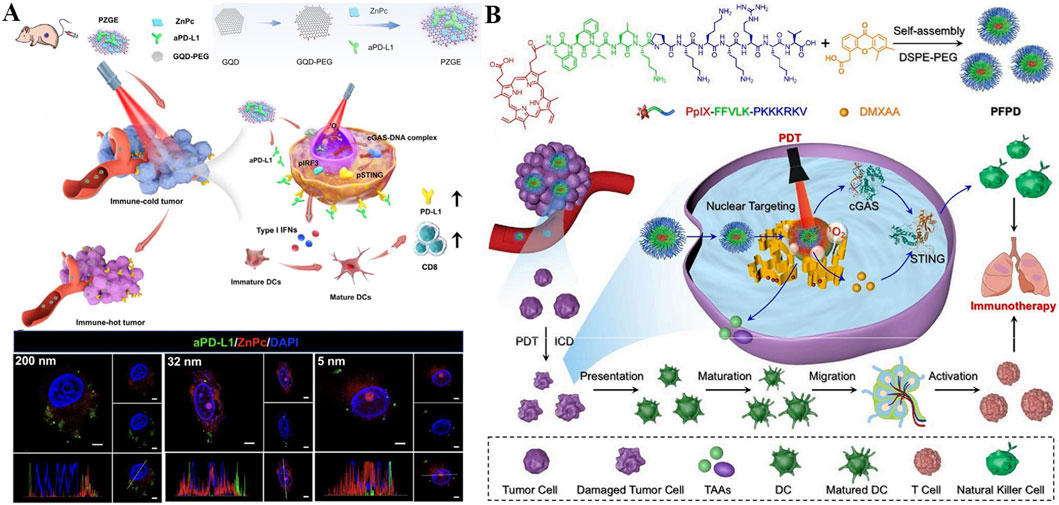
Figure 9. (A) Schematic illustration of PZGE for nucleus-targeted PDT-mediated photoimmunotherapy. Reproduced with permission from Ref. Zhang et al. (2023c). Copyright © 2023 Elsevier Ltd. (B) Schematic diagram of PFPD with nucleus-targeting ability for improved innate antitumor immunity via nucleus-targeted PDT and STING stimulation. Reproduced with permission from Ref. Wu et al. (2024b). Copyright © 2024 Elsevier Ltd.
The combination of PDT and STING agonists has been shown to strongly augment the efficacy of antitumor therapy, so local delivery of photosensitizers and STING agonists based on nanoplatforms is expected to maximize tumor elimination (Ding et al., 2024). A nucleus-targeted chimeric peptide nanorod referred PFPD was constructed for antitumor precision therapy, which could enhance innate immunity through PDT-mediated local DNA damage and STING activation. Utilizing intermolecular forces, a photosensitizer (protoporphyrin IX, PpIX) was coupled onto multifunctional FFVLKPKKKRKV peptide and a novel chimeric peptide (PpIX-FFVLK-PKKKPRV) produced, which was self-assembled to form nanorods and loaded with DMXAA (a STING agonist) (Figure 9B) (Wu Y. et al., 2024). PFPD has a uniform and small size distribution with good stability, rendering its intratumor accumulation, cell internalization, and nucleus-targeted delivery of payloads. When exposure to laser, large amounts of 1O2 produced by PFPD induces in situ DNA fragmentation in nucleus, while the released cytoplasmic DNA fragments subsequently combined with the STING agonist to stimulate innate antitumor immunity. In addition, PFPD triggered ICD effect through nucleus-localized PDT, activated natural killer cells (NKs) and T cells, thus effectively eradicating primary and metastatic tumors with ignorable side effects. This research offers valuables for precise drug delivery and combination therapy against metastatic tumors with poor prognosis.
To deliver drugs to the nucleus of tumor cells more accurately, a hierarchical targeting cascade system towards tumor cells and the nucleus has been developed for tumor eradication through self-enhancement of PDT and chemotherapy. Specifically, a nucleus-targeting nanoprodrugs named HPC-CAT/CL were prepared based on human serum albumin (HSA), Ce6, a cisplatin prodrug (Pt (IV)), catalase (CAT), and b-cyclodextrin-lysine (CL) (Yang et al., 2023c). Then, the surface functionalization of HPC-CAT/CL was performed by a targeting agent (AS1411 aptamer), and the resulting HPC-CAT/CL-AP could actively target tumors, thus achieving self-reinforcing cascade photochemotherapy. HPC-CAT/CL-AP were efficiently taken up by tumor cells and accumulated in the nucleus, and non-toxic Pt (IV) prodrug was effectively reduced to its active state Pt (II) and supplied H2O2 simultaneously when exposed to overexpressed GSH in tumor cells and nucleus. Noteworthy, H2O2 could be decomposed by CAT to produce O2, which effectively alleviated the influence of anaerobic environment on the curative effect of PDT, and accordingly realized the synergistic photochemotherapy effect. The in vitro and in vivo experiments showed that HPC-CAT/CL-AP was highly successful to kill tumor cells, significantly repressing tumor growth and lung metastasis. This nucleus-targeted self-augmenting cascade photochemotherapy strategy has enormous potential in overcoming recurrence and metastasis of tumors.
Beyond traditional nucleus-targeting strategies (e.g., size control, peptide-mediated delivery, and oligonucleotide aptamers targeting nuclear proteins), recent advances in s in nucleus-targeted PDT have focused on specific recognition of nuclear nucleic acids, including G-quadruplexes (G4s) and general RNA/DNA, to enhance targeting precision and therapeutic efficacy (Long et al., 2024; Chen W. et al., 2025). G4s are stable secondary structures formed by guanine-rich nucleic acids in oncogene promoters and telomeres, making them attractive targets for selective tumor intervention (Liu et al., 2024a). Building on this foundation, an advanced donor-π-acceptor (D-π-A) acridinium-based Type I photosensitizer was developed for RNA G4s targeting (Chen W. et al., 2023). Its structure integrates triphenylamine (electron donor) and pyridinium (electron acceptor) to achieve NIR absorption and efficient electron transfer. This acridinium derivative specifically recognizes RNA G4s through hydrogen bonding, with negligible affinity for double-stranded DNA or single-stranded RNA. Upon light irradiation, it predominantly generates •O2−, instead of 1O2, overcoming hypoxia-induced PDT resistance and enhancing ICD in tumors.
Additionally, direct targeting of nuclear RNA/DNA via sequence complementarity or intercalation has also advanced PDT precision. A nucleus-targeted activatable photosensitive probe (CMT-I) was designed and synthesized for DNA targeting (Liao et al., 2025). Molecular docking analyses indicate that CMT-I binds to DNA selectively, relying on hydrogen bonds and π-π conjugation for interaction. In vitro spectral tests show that ct-DNA specifically activates CMT-I, leading to a notable fluorescence enhancement upon binding. When irradiated with light, CMT-I proves effective at generating 1O2 RNA sequencing studies reveal that the PDT induced by CMT-I activates tumor cell immunity, initiating an adaptive immune response. In vivo therapeutic trials further confirm that CMT-I boosts antitumor immunity, a key factor in successfully eliminating immunologically cold tumors, and underscores the value of nucleus-targeted PDT for precise cancer treatment. Together, these G4-and nucleic acid-targeted approaches represent a paradigm shift from broad nuclear localization to molecularly precise damage, with successive advancements significantly improving PDT efficacy and reducing systemic toxicity.
6 Targeting cell membrane for enhanced PDT
Due to ROS possess a short half-life and a restricted effective diffusion radius, the introduction of photosensitizers to produce ROS in situ on target organelles is essential to avoid their rapid decay. Among these, the plentiful unsaturated lipids of the plasma membrane can stimulate LPO under the action of ROS produced by PDT, thus damaging the cell integrity (Fan et al., 2021). Therefore, photosensitizer-based cell membrane-targeted delivery systems can improve membrane permeability, cause LPO, disrupt membrane integrity, and ultimately result in cell membrane disruption and release of cell payloads (Wang M. et al., 2022). In recent years, membrane-targeted photosensitizer systems have been established, which could deliver photosensitizers to therapeutic target organelles to enhance the efficiency of PDT (Yang et al., 2023b).
To amerliorate phototherapy-mediated tumor metastasis suppression, self-delivery chimeric peptide (C16-CypateRRKK-PEG8-COOH, CCP) was designed for cell membrane-targeted low-temperature PTT and PDT combination therapy. As depicted in Figure 10A, CCP was constructed from palmtic acid-modified NIR dye (Cypate, hydrophobic) and chimeric peptide Arg-Arg-Lys-Lys-PEG8 (RRKK-PEG8-COOH, hydrophilic), whose amphiphilicity gives it the ability to self-assemble into nanoparticles (CCP NPs) (Chen et al., 2022). Among them, Cypate is a photosensitive agent for clinical use with dual effects of PDT and PTT (Zou et al., 2025b), positively charged RRKK fragment and alkyl chain of palmitic acid enable CPPs to target and insert into the cell membrane, and PEG chain endows prolong the blood circulation time of CCP NPs. CCP NPs exhibited strong tumor targeting and accumulation capability, which could effectively kill tumor cells. Under NIR laser irradiation, CCP NPs can produce numerous ROS and mild heat of less than 45 °C locally in the cell membrane, destroy the cancer cell membrane, effectively induce ICD, and activate antitumor immune response, thereby eliminating residual tumor cells. Under single administration and NIR light irradiation, CCP NPs significantly inhibited tumor growth and lung metastasis, thus extending the survival time of mice. This unique cell membrane-targeted phototherapy strategy helps to achieve tumor clearance and inhibit metastasis in a safe and effective manner.
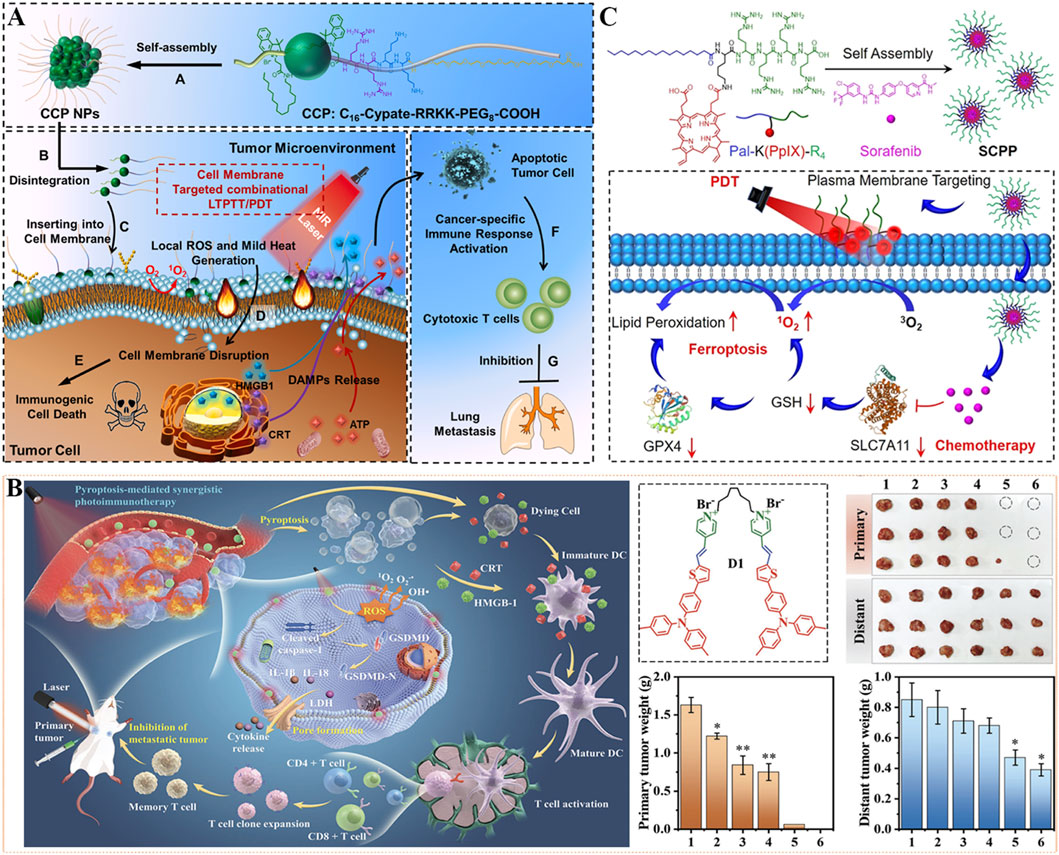
Figure 10. (A) The illustration of cell membrane-targeting CCP NPs for antimetastatic combination low-temperature PTT/PDT therapy. Reproduced with permission from Ref. (Chen et al., 2022). Copyright © 2022 Elsevier Ltd. (B) Schematic illustration of photosensitive dimer D1-based pyroptosis-mediated synergistic photodynamic and photothermal immunotherapy for inhibition of primary and distant tumor. 1: Control, 2: Laser, 3: M1, 4: D1, 5: M1+Laser, 6: D1+Laser. M1: monomer. Reproduced with permission from Ref. Tang et al. (2023). Copyright © 2023 WILEY-VCH Verlag GmbH & Co. KGaA. (C) The preparation and antitumor mechanism of SCPP utilized for plasma membrane-targeted combination therapy. Reproduced with permission from Ref. Deng et al. (2022). Copyright © 2022 American Chemical Society.
As a form of programmed cell death caused by inflammasome, pyroptosis is an important means to overcome drug resistance towards apoptosis and fight against immunosuppressive tumor microenvironment, and ultimately inhibit tumor growth (Loveless et al., 2021; Zhu Y. et al., 2024). However, the current pyroptosis-induced drugs have some limitations, such as high toxicity, poor stability, and low intracellular accumulation. In view of this, a cell membrane-targeting photosensitizer dimer (D1) with aggregation-induced emission properties was constructed for synergistic pyroptosis-mediated photodynamic and photothermal immunotherapy. In detail, a π-conjugated chromophore with strong intramolecular charge transfer ability was formed by thiophene, triphenylamine, and pyridinium groups, and then linked two chromophores with one flexible octyl group to construct the dimeric photosensitizer D1 (Figure 10B) (Tang et al., 2023). This photosensitive dimer D1 could specially accumulate on the cell membrane, and generate ROS by type I PDT as well as heat energy by PTT under light irradiation, enabling a reinforced pyroptosis effect. Additionally, the pyroptosis triggered by synergistic phototherapy effectively triggered the discharge of inflammatory cytokines and intracellular contents, promoted tumor-specific antigen induction and DCs maturation, and stimulated T cell proliferation, thus activating systemic antitumor immunity. In mechanism, D1 exhibits improved permeability and retention effect in the tumor cell membrane, high ROS-generated efficiency, and strong photothermal effect, which accelerates pyroptosis-mediated synergistic PDT/PTT/immunotherapy in mouse models. After 7 days of treatment with laser irradiation, D1 was found to completely eliminate the primary tumors and effectively inhibit the distant tumors. This work provides a promising strategy for a satisfactory antitumor effect and rouses systemic immune response against tumor metastasis.
By self-assembly of chemotherapeutic agent sorafenib and plasma membrane-targeted amphiphilic chimeric peptide (Pal-K(PpIX)-R4), Deng et al. have successfully prepared a nanoscale photooxidant named as SCPP (Figure 10C) (Deng et al., 2022). Among them, the palmitic part and positively charged peptide of Pal-K(PpIX)-R4 (hydrophobic) given the SCPP plasma membrane-targeting capability. SCPP displayed excellent stability and ideal size distribution, which could enhance localized LPO production by in situ PDT with laser. In addition, intracellular accumulation of the chemotherapy drug (sorafenib) can downregulate the levels of GSH and glutathione peroxidase 4 (GPX4) by blocking the expression of cystine/glutamate antiporter (SLC7A11), thereby amplifying the PDT effect and disrupting the antioxidant defense system. Therefore, SCPP could trigger LPO through plasma membrane-targeting PDT, and activate ferroptosis of tumor cells through sorafenib-mediated chemotherapy, finally exhibiting superior tumor inhibition ability in vivo. This work may afford scientific direction for plasma membrane-targeted synergistic treatment of tumors when exposure to disadvantage conditions.
7 Targeting ribosome for enhanced PDT
Ribosomes are highly complex cellular machines, primarily composed of ribosomal RNA (rRNA) and dozens of different ribosomal proteins (R-proteins); the exact number varies slightly between species (Cheng et al., 2024). These ribosomal proteins and rRNA are arranged into two subunits of different sizes, commonly referred to as the small and large subunits of the ribosome (Elhamamsy et al., 2022). These subunits work together to convert mRNA into polypeptide chains during protein synthesis (Jiao et al., 2023). It has been reported that cancer stem cells (CSCs) have a higher abundance of ribosomes than those found in normal cells, which is one of the important markers of CSCs (Xue et al., 2024). The proportion of ribosomes in the body determines the total proteome and the rate of cell division, thus influencing the cell cycle and its fate. Due to the negative charges of ribosomes, positively charged materials interact strongly with ribosomes to achieve targeting. Qin’s group (Figure 11) first synthesized a positively charged photosensitizer (meso-Tetra (4-aminophenyl)Porphyrin, m-TAPP) and small peptides (N-fluorenylmethoxycarbonyl-leucine-leucine-leucine-OMe, Fmoc-L3-OMe) that co-assemble into nanoparticles based on π-π interactions (Wang J. et al., 2022). The pH of tumor cells is 6.5 lower than that of normal cells, which allows the positively charged co-assembly to interact strongly with ribosomes for effective targeting. The co-assembly exhibits strong ROS production under light irradiation, significantly reducing tumor stem cells and deactivating ribosomes, and inhibiting tumor cell growth both in vitro and in vivo.
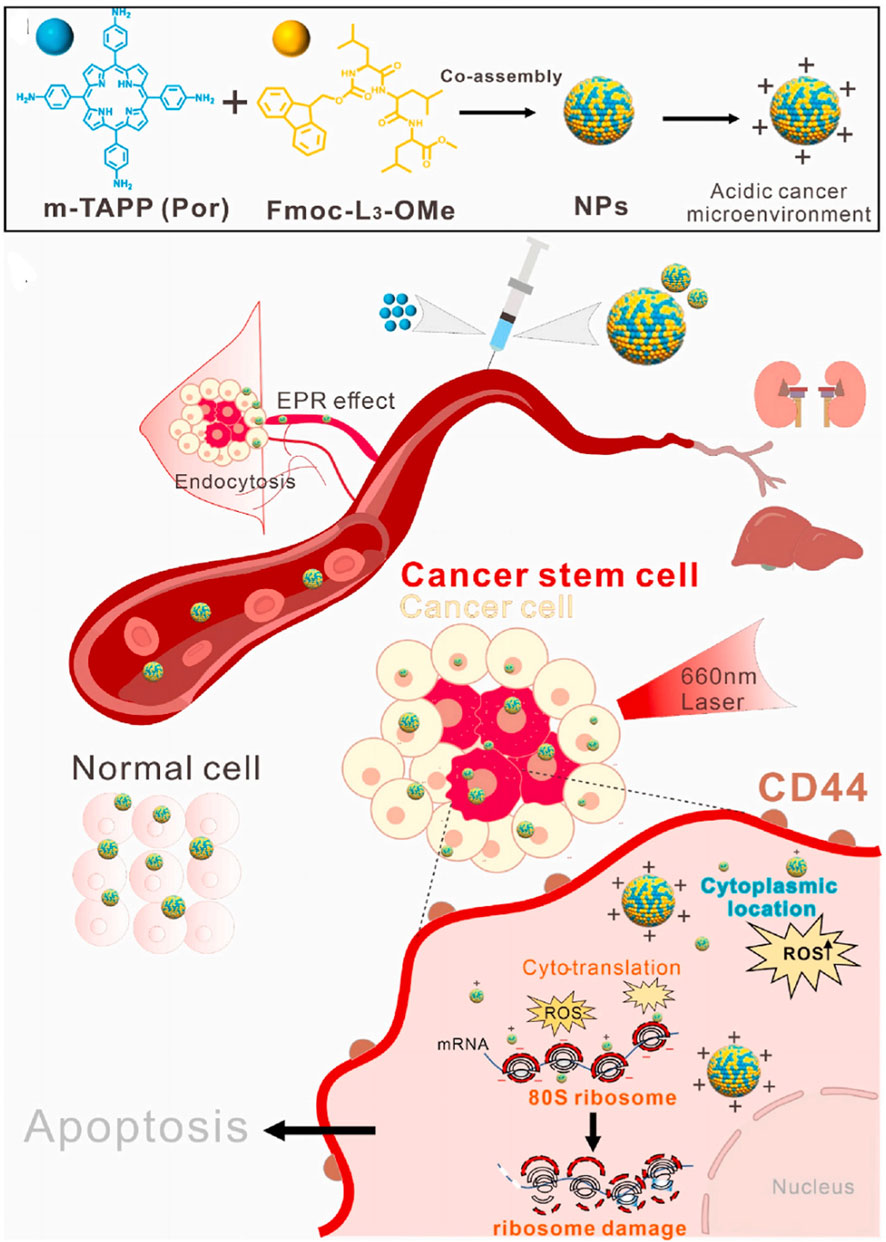
Figure 11. Schematic illustration that showing NPs targeted to cytosolic ribosomes and inhibited the CSCs upon light irradiation. Reproduced with permission from Ref. Wang et al. (2022a). Copyright © 2022 Elsevier Ltd.
8 Targeting endoplasmic reticulum for enhanced PDT
The ER is a multifunctional organelle responsible for maintaining cell homeostasis, protein synthesis, folding, and secretion. The production of ROS by PDT could disrupt the protein-folding capacity of this organelle and trigger ER stress, which leads to tumor cell death (Diao et al., 2025). Therefore, targeting the ER is critical to evoke ER stress.
8.1 Paraxin
Pardaxin, served as ER-targeting peptides, combined with a photosensitizer, were delivered to the ER to induce ICD via ER stress. You et al. designed pardaxin-modified and indocyanine green (ICG) conjugated multifunctional nanoparticles (Figure 12A), as well as O2-carrying hemoglobin (Hb) liposomes (FAL-Hb lipo) to reverse hypoxia (Li et al., 2019). In the hypoxic tumor environment, this system showed a special affinity for the ER and produced 1O2 species under light irradiation. The ER-localized 1O2 species triggered strong ER stress and calreticulin (CRT) exposure on the cell surface as an “eat me” signal. CRT, a biomarker of ICD, promotes the maturation of dendritic cells (DCs) and further induces an immunological response, including CD8+ T cell proliferation and cytotoxic cytokine secretion. Finally, the ER-targeting nanosystem achieves enhanced antitumor effects. In 2022, this group reported another ER-targeting nanoparticle (Pardaxin-ICG-Liposome) (Figure 12B) fabricated through microfluidic techniques (Liu X. et al., 2022). Under near-infrared (NIR) irradiation, Pardaxin-ICG-Liposome could produce an ICD effect and activate the immune system by ER-targeting PDT.
Figure 12. (A) Schematic illustration of enhanced immunogenic cancer cell death and anticancer effect induced by ER-targeting photothermal/photodynamic therapy. Reproduced with permission from Ref. Li et al. (2019). Copyright © 2019 Springer Nature. (B) Graphic illustration of Pardaxin-ICG-Liposome mediated ER-targeting PDT under NIR irradiation, which could induce immunogenic tumor cell death, acting as an in situ tumor vaccination. Reproduced with permission from Ref. Liu et al. (2022b). Copyright © 2022 American Chemical Society. (C) Schematic diagram of efficient ER-targeting PS TCPP-TER to induce ER stress and amplify ICD. Reproduced with permission from Ref. Deng et al. (2020a). Copyright © 2020 American Chemical Society. (D) Schematic illustration depicting the molecular mechanisms of ER-ZS for diagnosing NAFLD, and for photodynamic cancer therapy by activating tumor cell pyroptosis. Reproduced with permission from Ref. Zeng et al. (2024b). Copyright © 2024 Wiley-VCH GmbH.
8.2 p-Toluenesulfonamide
p-Toluenesulfonamide has been reported to target ER by binding to the sulfonamide receptor (Zhu et al., 2021). Song et al. synthesized reduction-responsive Ds-sP nanoparticles encapsulating an ER-targeting photosensitizer (Figure 12C), TCPP-TER (Deng H. et al., 2020). The Ds-sP/TCPP-TER nanoparticles could accumulate in the ER and generate ROS to trigger ER stress under 670 nm laser irradiation, resulting in an enhanced ICD effect. The amplified ICD then induced dendritic cell (DC) activation, leading to an augmented immune response, including the infiltration of CD8+ T cells and enhanced secretion of cytokines. Therefore, the ER-targeting Ds-sP/TCPP-TER exhibited a better antitumor effect compared to Ds-sP/TCPP. Additionally, Yoon et al. designed p-Toluenesulfonamide-modified hemicyanine dyes (ER-ZS) (Figure 12D), which are type I photosensitizers, capable of producing •O2− and •OH radicals under light irradiation (Zeng et al., 2024b). The numerous ROS generated in the ER could effectively damage it, activating the pyroptosis cascade pathway, and subsequently causing DNA breakage and effectively inhibiting tumor growth.
8.3 Others
Wong and colleagues developed a rhodamine-decorated iridium (III) complex by modifying the cyclometallating ligand to enhance the production of ROS and to achieve specific localization in the ER (Figure 13A) (Zhou et al., 2020). The replacement of the bipyridine (ppy) chelating ligand with 2,3-diphenylquinoxaline (dpqx) resulted in the complex Ir-Rho-G2, which demonstrated a higher 1O2 generation quantum yield and a lower luminescence quantum yield compared to Ir-Rho, thereby enhancing its ROS generation capability. Additionally, the dpqx ligand in Ir-Rho-G2 conferred a predominant and specific intracellular localization to the ER. Consequently, the cytotoxicity of Ir-Rho-G2 was approximately three times greater than that of Ir-Rho under lamp irradiation, attributable to its ability to disrupt protein folding in the ER and to activate ER stress-induced apoptosis pathways. In vivo PDT experiments, Ir-Rho-G2 effectively targeted the tumor site and suppressed tumor growth.
Figure 13. (A) Schematic illustration of rhodamine-decorated iridium (III) complex, Ir-Rho-G2, with ER targeting and enhanced 1O2 generation showing high efficiency-induced apoptosis of cancer cells for efficient PDT. Reproduced with permission from Ref. Zhou et al. (2020). Copyright © 2020 The Royal Society of Chemistry. (B) Schematic illustration of the cytological process of PDT treatment mediated by PIO-based fluorogens, and the structures of α-TPA-PIO and β-TPA-PIO. Reproduced with permission from Ref. Zhuang et al. (2020). Copyright © 2020 The Royal Society of Chemistry. (C) Schematic of the ER localizer mediating precise construction of the photosensitizer for the ER and the synthesis of TCy5 derivatives. Reproduced with permission from Ref. Ma et al. (2022). Copyright © 2022 American Chemical Society.
Tang’s group (Figure 13B) described two Type I photosensitizers, α-TPA-PIO and β-TPA-PIO, both derived from phosphindole oxide (Zhuang et al., 2020). These photosensitizers demonstrated selective accumulation in the ER, with β-TPA-PIO exhibiting a higher tumor inhibition efficiency under light irradiation than α-TPA-PIO, owing to its enhanced ROS generation capacity. In vivo, β-TPA-PIO was shown to effectively destroy tumor cells while maintaining good biocompatibility. The ER stress elicited by the excessive production of local ROS could activate apoptosis and autophagy pathways, thereby contributing to a significant PDT effect.
Peng et al. designed three types of fluorinated thio-pentamethine dyes, with TCy5-Ph-3F exhibiting the highest 1O2 generation efficiency and cell killing ability compared to TCy5-Ph-2F and TCy5-Ph-CF3 (Figure 13C) (Ma et al., 2022). The half-maximal inhibitory concentration (IC50) of TCy5-Ph-3F was 125 nM following LED light irradiation for 10 min, significantly lower than that of TCy5-Ph-2F (IC50 ∼0.5 μM) and TCy5-Ph-CF3 (IC50 > 1 μM). TCy5-Ph-3F localized spontaneously to the ER with a Pearson correlation coefficient of 0.96. In vitro studies demonstrated that ROS production by TCy5-Ph-3F within the ER can induce enhanced ER stress and trigger the ICD cascade in cancer cells. In vivo, the efficacy of TCy5-Ph-3F in inhibiting primary and distant tumor growth under light irradiation exceeded that of the TCy5-Ph-2F group, attributable to an ER-targeting activated immune response.
9 Targeting golgi apparatus for enhanced PDT
The Golgi apparatus (GA) functions as a vital organelle in the intracellular membrane transport network, acting as a crossroads for extracellular and intracellular pathways (Xu et al., 2024). It is fundamental in the processing, sorting, and trafficking of proteins synthesized within the cell, ensuring their proper delivery to the intended destinations. The generation of ROS can adversely affect the structure and function of the GA, potentially leading to modifications in stress signaling pathways downstream (Hu et al., 2023). Such alterations may play a role in inhibiting tumor cell growth, contributing to the activation of cellular stress responses such as apoptosis or cell cycle arrest.
9.1 Chondroitin sulfate
Chondroitin sulfate, a polysaccharide abundant in the extracellular matrix and on cell surfaces, possesses a sugar chain structure consisting of glucuronic acid and N-acetylgalactosamine (GalNAc) (Li H. et al., 2022). This structure enables it to bind to the surface receptor GalNAc-T1, specifically targeting the Golgi apparatus of tumor cells. For example, Gong and colleagues designed a nanodrug based on chondroitin sulfate that encapsulates the photosensitizer chlorin e6 (Ce6), retinoic acid (RA), and a CpG oligodeoxynucleotide (Figure 14A) (Li H. et al., 2022). The production of ROS by this nanodrug can damage tumor cells, and the immunosuppressive effects of PDT are mitigated by targeting and destroying the Golgi apparatus. In a subsequent study, Dong’s group developed chondroitin sulfate-based nanoparticles (ChS-Ce6) where the photosensitizer Ce6 was covalently attached to chondroitin sulfate (Figure 14B) (Hu et al., 2023). The ChS-Ce6 nanoparticles were directed to the Golgi apparatus due to the strong interaction between chondroitin sulfate and GalNAc-T1 on the Golgi apparatus. Consequently, tumor cell growth was significantly inhibited in the ChS-Ce6 group upon laser irradiation, as chondroitin sulfate enhanced ROS production by Ce6. This increase in ROS upregulated the nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3), promoting the release of inflammatory contents, generating an inflammatory environment, and triggering downstream classical caspase-1-dependent pyroptosis.
Figure 14. (A) Schematic illustration of the in vivo application of the Golgi apparatus-targeted prodrug Ce6-CS-RA nanoparticles for enhanced photodynamic immunotherapy. Reproduced with permission from Ref. Li et al. (2022a). Copyright © 2022 Elsevier Ltd. (B) A schematic representation of the preparation and use of Golgi apparatus-targeted ChS-Ce6 nanovesicles for the treatment of NSCLC-SM. Reproduced with permission from Ref. Hu et al. (2023). Copyright © 2023 American Chemical Society. (C) GA targeting and ROS generation capacity of the AIEgens, as well as the apoptosis pathway caused by Golgi oxidative stress. Reproduced with permission from Ref. Liu et al. (2022a). Copyright © 2022 Springer Nature. (D) COX-2 inhibition mediated TTPI exerted enhanced PDT to kill cancer cells. Reproduced with permission from Ref. Wang et al. (2024d). Copyright © 2023 Elsevier Ltd.
9.2 Others
While other materials for targeting the Golgi apparatus do exist, Guo’s group designed aggregation-induced emission (AIE) photosensitizers, termed TPE-PyT-CPS, specifically for targeting the Golgi apparatus (Figure 14C) (Liu M. et al., 2022). These photosensitizers, modified with a CN group, are capable of forming large rod-like aggregates ranging from 200 to 400 nm in size and can target the Golgi apparatus via caveolin/raft-mediated endocytosis. The TPE-PyT-CPS, with its Golgi apparatus-targeting capability, induces oxidative stress within the Golgi apparatus and triggers mitochondrial-dependent apoptosis. This study demonstrates that photosensitizers with the ability to target the Golgi apparatus can more effectively inhibit tumor growth, providing a novel strategy for precise and efficient tumor therapy.
Qi’s group developed an aggregation-induced emission (AIE) photosensitizer, TPPI, which combines a cationic triphenylamine-based photosensitizer with the cyclooxygenase (COX-2) inhibitor Indomethacin (IMC) (Figure 15D) (Wang X. et al., 2024). COX-2 is overproduced and accumulated in the Golgi apparatus, allowing TPPI to specifically target the Golgi apparatus by recognizing COX-2 through IMC. Upon light irradiation, TPPI produces an abundance of ROS, leading to the destruction of the Golgi apparatus and cell death, thereby enhancing PDT through targeted action on the Golgi apparatus. Furthermore, Wu et al. created a Golgi apparatus-targeting Ru-SL complex consisting of Ru(II) pyridine and sphingosine lipid (Zhao et al., 2021). This complex effectively localizes within the Golgi apparatus and, upon light exposure, induces damage to the apparatus, resulting in the killing of tumor cells.
Figure 15. (A) Schematic Illustration of SQU@PCN Preparation and the Tumor Cell Death Process by Homeostatic Perturbation Therapy and Sensitized Photodynamic Therapy. Reproduced with permission from Ref. Wan et al. (2019). Copyright © 2019 American Chemical Society. (B) Schematics of the synthesis and fabrication of CD-Ce6-3BP NPs induced pro-death autophagy to enhance photodynamic therapy against hypoxic tumor. Reproduced with permission from Ref. Deng et al. (2020b). Copyright © 2020 American Chemical Society.
10 Targeting autophagosome for enhanced PDT
Autophagy is a highly conserved lysosomal-mediated degradation process in eukaryotes. Autophagosomes, which are double-membrane vesicles formed during autophagy, encapsulate autophagy substrates and transport them to lysosomes for degradation (Liu et al., 2024b). This process is crucial for cell survival, growth, and homeostasis. Reports indicate that PDT efficacy is enhanced in tumor cells when autophagy is suppressed by inhibitors. Zhang et al. developed an adenosine-triphosphate (ATP)-regulated ion transport nanosystem, comprising a porphyrinic porous coordination network (PCN) and squaramide (SQU) (Figure 15A) (Wan et al., 2019). The release of SQU in response to high ATP levels inhibits autophagy by disrupting cellular ion homeostasis, sensitizing PDT, and improving its therapeutic efficacy. Conversely, Jin’s group studied acid-sensitive CD-Ce6-3BP nanoparticles loaded with the respiration inhibitor 3-bromopyruvate (3BP) and the photosensitizer Ce6, facilitated by host-guest interaction (Figure 15B) (Deng Y. et al., 2020). 3BP reduces intracellular O2 consumption, alleviating tumor hypoxia, and converting autophagy from a pro-survival to a pro-death process. This excessive activation of autophagy enhances the PDT effect.
11 Conclusion
In recent years, PDT has emerged as a minimally invasive, highly regulable, and immunogenic therapeutic strategy that holds great promise in the field of cancer treatment. Despite its growing popularity and notable achievements in preclinical studies, several critical challenges remain before PDT can be widely adopted in clinical practice-particularly in improving therapeutic efficiency, enhancing targeting specificity, and overcoming tumor microenvironmental limitations. This review systematically summarizes recent advances in subcellular organelle-targeted PDT strategies aimed at enhancing therapeutic outcomes. Particular emphasis is placed on nanoplatforms designed to target mitochondria, lysosomes, the nucleus, cell membrane, Golgi apparatus, and ribosomes, with detailed discussion of their targeting mechanisms and underlying pathways that contribute to enhanced PDT efficacy. These approaches not only improve the accumulation of photosensitizers at specific organelles but also modulate key intracellular signaling and oxidative stress responses, thereby potentiating antitumor effects.
Although numerous studies have demonstrated the therapeutic potential of PDT, several key issues still need to be addressed. First, in terms of drug delivery, many currently used photosensitizers suffer from poor water solubility and limited stability in vivo, often requiring complex delivery systems for effective administration. This increases formulation complexity and may compromise bioavailability. Secondly, most photosensitizers inherently lack targeting ability and must be conjugated-either covalently or non-covalently-to targeting ligands such as peptides, antibodies, or small molecules to achieve precise subcellular localization. Such multi-step functionalization processes further complicate preparation and may lead to batch-to-batch variability. Moreover, the hypoxic tumor microenvironment remains a major obstacle to efficient ROS generation during PDT. While various strategies-such as O2-carrying materials, catalytic H2O2 decomposition, or mitochondrial respiration inhibition and regulation-have shown promising results in cellular and animal models, their safety and efficacy in humans remain to be fully validated. Additionally, most conventional photosensitizers rely on visible light excitation, which limits tissue penetration and restricts their application to superficial tumors. Therefore, developing photosensitizing systems responsive to NIR or X-ray irradiation could significantly expand the clinical applicability of PDT. Finally, the high interstitial pressure and dense extracellular matrix within solid tumors pose significant barriers to effective drug delivery. As a result, the concentration of systemically administered nanomedicines reaching the tumor site is often insufficient. To address this challenge, biomimetic delivery systems-such as cell membrane-coated nanoparticles or exosome-based carriers-along with active targeting strategies may offer promising solutions to enhance tumor accumulation and therapeutic performance.
From the clinical translation perspective, subcellular organelle-targeted PDT faces multiple bottlenecks hindering its transition from lab to clinic. Insufficient pharmacokinetic data on nano-photosensitizers impedes precise control of dosage and frequency, raising clinical risks. The lack of long-term biosafety assessments is a major concern, as some nanomaterials may accumulate in vivo, causing chronic toxicity or immune reactions that require long-term monitoring. Additionally, standardizing light irradiation doses in multi-center trials is challenging, in which variations in equipment, duration, and intensity reduce result comparability, hampering unified clinical standards. Notably, clinical experience with second-generation photosensitizers such as ALA in treating condyloma acuminatum, encompassing administration protocols, irradiation parameters, and efficacy assessment, provides a valuable reference for the design of subcellular PDT. The steps for promoting clinical translation involve strict screening of biocompatible, stable nanocarriers (material level), comprehensive evaluation of tumor targeting/killing (cellular level), in-depth in vivo pharmacokinetic and toxicity studies (animal level), and well-designed multi-center trials (clinical level). Collaborating with regulators to develop tailored approval standards will accelerate translation of photosensitizers to clinical use.
In terms of emerging trends, interdisciplinary integration drives PDT development. Deep integration with artificial intelligence enables accurate prediction of patient responses to PDT by building tumor models and analyzing clinical data, optimizing light parameters for personalized treatment based on tumor heterogeneity. Combining with immune checkpoint inhibitors creates synergy: PDT releases tumor-associated antigens to activate immunity, while inhibitors enhance the immune system’s ability to recognize and eliminate tumor cells, strengthening systemic anti-tumor immunity. Technically, activatable photosensitizers remain inert in normal physiology but activate to generate ROS upon sensing tumor microenvironment signals (e.g., low pH, high ATP, specific enzymes), achieving precise tumor killing. Multimodal platforms integrate imaging, PDT, and PTT, with real-time imaging monitoring tumor location, size, and treatment changes to guide therapies and improve efficacy. Additionally, PDT’s applications extend beyond tumors, encompassing effective killing of bacteria, fungi, and viruses (especially drug-resistant strains) without inducing resistance, as well as clearing abnormally aggregated proteins and reducing neuroinflammation in neurodegenerative diseases.
In conclusion, by deeply exploring subcellular targeting mechanisms and combining advanced materials science with biological insights, the performance of PDT is expected to be further optimized. Especially under the premise of overcoming the above challenges, accelerating clinical translation, and keeping up with emerging trends, subcellular organelle-targeted PDT is expected to break through the limitations of existing clinical treatments in the future, achieve breakthrough expansion of clinical indications, gradually expand from the current treatment of some cancers to more disease fields, transition to a wider range of clinical applications, and make greater contributions to the cause of human health.
Author contributions
JT: Writing – original draft, Writing – review and editing. ZY: Writing – original draft, Writing – review and editing. MZ: Funding acquisition, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Undergraduate Innovation and Entrepreneurship Training Program of Jiangsu Province (202310304155Y).
Acknowledgments
The authors are grateful to all the reviewers of this article for their comments and suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Austin, E., Wang, J. Y., Ozog, D. M., Zeitouni, N., Lim, H. W., and Jagdeo, J. (2025). Photodynamic therapy: overview and mechanism of action. J. Am. Acad. Dermatol. doi:10.1016/j.jaad.2025.02.037
Bian, X., Wang, W., Abudurexiti, M., Zhang, X., Ma, W., Shi, G., et al. (2024). Integration analysis of single-cell multi-omics reveals prostate cancer heterogeneity. Adv. Sci. 11 (18), e2305724. doi:10.1002/advs.202305724
Bijonowski, B. M., Park, J., Bergert, M., Teubert, C., Diz-Muñoz, A., Galic, M., et al. (2025). Intercellular adhesion boots collective cell migration through elevated membrane tension. Nat. Commun. 16 (1), 1588. doi:10.1038/s41467-025-56941-4
Bornens, M. (2021). Centrosome organization and functions. Curr. Opin. Struct. Biol. 66, 199–206. doi:10.1016/j.sbi.2020.11.002
Bouz, G., Žádný, J., Storch, J., and Vacek, J. (2025). Chiral helical scaffolds: unlocking their potential in biomolecular interactions and biomedical applications. Biotechnol. Adv. 79, 108513. doi:10.1016/j.biotechadv.2024.108513
Cantallops Vilà, P., Ravichandra, A., Agirre Lizaso, A., Perugorria, M. J., and Affò, S. (2024). Heterogeneity, crosstalk, and targeting of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 79 (4), 941–958. doi:10.1097/hep.0000000000000206
Cao, M., Luo, X., Wu, K., and He, X. (2021). Targeting lysosomes in human disease: from basic research to clinical applications. Signal Transduct. Target. Ther. 6 (1), 379. doi:10.1038/s41392-021-00778-y
Celik, C., Lee, S. Y. T., Yap, W. S., and Thibault, G. (2023). Endoplasmic reticulum stress and lipids in health and diseases. Prog. Lipid Res. 89, 101198. doi:10.1016/j.plipres.2022.101198
Cen, Y., Chen, X., Liu, Y., Yu, B., Yan, M., Yang, N., et al. (2023). Drug induced mitochondria dysfunction to enhance photodynamic therapy of hypoxic tumors. J. Control. Release 358, 654–666. doi:10.1016/j.jconrel.2023.05.023
Chai, C., Zhou, T., Zhu, J., Tang, Y., Xiong, J., Min, X., et al. (2022). Multiple light-activated photodynamic therapy of tetraphenylethylene derivative with AIE characteristics for hepatocellular carcinoma via dual-organelles targeting. Pharmaceutics 14 (2), 459. doi:10.3390/pharmaceutics14020459
Chen, D., and Wang, H. (2020). The clinical and immune features of CD14 in colorectal cancer identified via large-scale analysis. Int. Immunopharmacol. 88, 106966. doi:10.1016/j.intimp.2020.106966
Chen, G., Ullah, A., Xu, G., Xu, Z., Wang, F., Liu, T., et al. (2021). Topically applied liposome-in-hydrogels for systematically targeted tumor photothermal therapy. Drug Deliv. 28 (1), 1923–1931. doi:10.1080/10717544.2021.1974607
Chen, P.-L., Huang, P.-Y., Chen, J.-Y., Shi, Q.-Y., Zhu, Y.-Y., Chen, Y., et al. (2022). A self-delivery chimeric peptide for high efficient cell membrane-targeting low-temperature photothermal/photodynamic combinational therapy and metastasis suppression of tumor. Biomaterials 286, 121593. doi:10.1016/j.biomaterials.2022.121593
Chen, M., Zhao, E., Li, M., Xu, M., Hao, S., Gao, Y., et al. (2023a). Kaempferol inhibits non-homologous end joining repair via regulating Ku80 stability in glioma cancer. Phytomedicine 116, 154876. doi:10.1016/j.phymed.2023.154876
Chen, W., Zhang, Y., Yi, H. B., Wang, F., Chu, X., and Jiang, J. H. (2023b). Type I photosensitizer targeting G-quadruplex RNA elicits augmented immunity for cancer ablation. Angew. Chem. Int. Ed. 62 (17), e202300162. doi:10.1002/anie.202300162
Chen, Y., Yang, Y., Du, S., Ren, J., Jiang, H., Zhang, L., et al. (2023c). Mitochondria-targeting upconversion nanoparticles@MOF for multiple-enhanced photodynamic therapy in hypoxic tumor. ACS Appl. Mater. Interfaces 15 (30), 35884–35894. doi:10.1021/acsami.3c05447
Chen, Y. J., Li, G. N., Li, X. J., Wei, L. X., Fu, M. J., Cheng, Z. L., et al. (2023d). Targeting IRG1 reverses the immunosuppressive function of tumor-associated macrophages and enhances cancer immunotherapy. Sci. Adv. 9 (17), eadg0654. doi:10.1126/sciadv.adg0654
Chen, J., Duan, Z., Deng, L., Li, L., Li, Q., Qu, J., et al. (2024). Cell membrane-targeting type I/II photodynamic therapy combination with FSP1 inhibition for ferroptosis-enhanced photodynamic immunotherapy. Adv. Healthc. Mater. 13 (16), e2304436. doi:10.1002/adhm.202304436
Chen, F., Tian, X. F., Yang, T., Dai, Y. J., Chen, D. Y., Chen, H. B., et al. (2025a). Lysosomal cathepsin S escape facilitates near infrared light-triggered pyroptosis via an antibody-indocyanine green conjugate. Adv. Sci. e04851. doi:10.1002/advs.202504851
Chen, W., Mao, X. Q., Wang, X. Z., Liao, Y. C., Yin, X. Y., Wu, H. L., et al. (2025b). Data-driven discovery of near-infrared type I photosensitizers for RNA-Targeted tumor photodynamic therapy. Chem. Sci. 16, 14455–14467. doi:10.1039/d5sc03648h
Chen, Z., Wang, Y., Li, Z., Chen, M., Li, Y., Lu, C., et al. (2025c). Improving ferroptosis-mediated immunotherapy for colorectal cancer through lysosome-targeted photodynamic therapy. Mater Today Bio 31, 101552. doi:10.1016/j.mtbio.2025.101552
Cheng, X., He, L., Xu, J., Fang, Q., Yang, L., Xue, Y., et al. (2020). Oxygen-producing catalase-based prodrug nanoparticles overcoming resistance in hypoxia-mediated chemo-photodynamic therapy. Acta Biomater. 112, 234–249. doi:10.1016/j.actbio.2020.05.035
Cheng, Q., Chen, J., Guo, H., Lu, J.-l., Zhou, J., Guo, X.-y., et al. (2021). Pyrroloquinoline quinone promotes mitochondrial biogenesis in rotenone-induced Parkinson’s disease model via AMPK activation. Acta Pharmacol. Sin. 42 (5), 665–678. doi:10.1038/s41401-020-0487-2
Cheng, Y., Wang, S., Zhang, H., Lee, J. S., Ni, C., Guo, J., et al. (2024). A non-canonical role for a small nucleolar RNA in ribosome biogenesis and senescence. Cell 187 (17), 4770–4789.e23. doi:10.1016/j.cell.2024.06.019
Cho, Y., and Kim, Y. K. (2024). ROS-Mediated cytoplasmic localization of CARM1 induces mitochondrial fission through DRP1 methylation. Redox Biol. 73, 103212. doi:10.1016/j.redox.2024.103212
Cortés-Guiral, D., Hübner, M., Alyami, M., Bhatt, A., Ceelen, W., Glehen, O., et al. (2021). Primary and metastatic peritoneal surface malignancies. Nat. Rev. Dis. Prim. 7 (1), 91. doi:10.1038/s41572-021-00326-6
Cui, M., Tang, D., Wang, B., Zhang, H., Liang, G., and Xiao, H. (2023). Bioorthogonal guided activation of cGAS-STING by AIE photosensitizer nanoparticles for targeted tumor therapy and imaging. Adv. Mater. 35 (52), e2305668. doi:10.1002/adma.202305668
Decker, S. T., and Funai, K. (2024). Mitochondrial membrane lipids in the regulation of bioenergetic flux. Cell Metab. 36 (9), 1963–1978. doi:10.1016/j.cmet.2024.07.024
Deng, H., Zhou, Z., Yang, W., Lin, L.-s., Wang, S., Niu, G., et al. (2020a). Endoplasmic reticulum targeting to amplify immunogenic cell death for cancer immunotherapy. Nano Lett. 20 (3), 1928–1933. doi:10.1021/acs.nanolett.9b05210
Deng, Y., Song, P., Chen, X., Huang, Y., Hong, L., Jin, Q., et al. (2020b). 3-Bromopyruvate-conjugated nanoplatform-induced pro-death autophagy for enhanced photodynamic therapy against hypoxic tumor. ACS Nano 14 (8), 9711–9727. doi:10.1021/acsnano.0c01350
Deng, F.-A., Yan, M.-Y., Liu, Y.-B., Yu, B.-X., Huang, J.-Q., Wang, C., et al. (2022). Plasma membrane-targeted photooxidant for chemotherapy-enhanced lipid peroxidation. ACS Appl. Bio Mater. 5 (9), 4523–4530. doi:10.1021/acsabm.2c00597
Di Bartolomeo, L., Altavilla, D., Vaccaro, M., Vaccaro, F., Squadrito, V., Squadrito, F., et al. (2022). Photodynamic therapy in pediatric age: current applications and future trends. Front. Pharmacol. 13, 879380. doi:10.3389/fphar.2022.879380
Diao, S., He, X., Wu, Y., Yin, L., Huang, Y., Zhou, W., et al. (2025). Endoplasmic reticulum-targeting activatable nanophotosensitizers for hypoxia relief and enhanced photodynamic therapy. Chem. Sci. 16 (24), 10909–10917. doi:10.1039/d5sc00534e
Ding, F., Liu, J., Ai, K., Xu, C., Mao, X., Liu, Z., et al. (2024). Simultaneous activation of pyroptosis and cGAS-STING pathway with epigenetic/photodynamic nanotheranostic for enhanced tumor photoimmunotherapy. Adv. Mater. 36 (7), e2306419. doi:10.1002/adma.202306419
Ding, Q., Rha, H., Yoon, C., Kim, Y., Hong, S. J., Kim, H. J., et al. (2025). Regulated cell death mechanisms in mitochondria-targeted phototherapy. J. Control. Release 382, 113720. doi:10.1016/j.jconrel.2025.113720
Dong, W. T., Long, L. H., Deng, Q., Liu, D., Wang, J. L., Wang, F., et al. (2023). Mitochondrial fission drives neuronal metabolic burden to promote stress susceptibility in male mice. Nat. Metab. 5 (12), 2220–2236. doi:10.1038/s42255-023-00924-6
Dong, X., Zhang, Z., Wang, R., Sun, J., Dong, C., Sun, L., et al. (2024). RSS and ROS sequentially activated carbon monoxide release for boosting NIR imaging-guided on-demand photodynamic therapy. Small 20 (22), e2309529. doi:10.1002/smll.202309529
Ebner, M., Puchkov, D., López-Ortega, O., Muthukottiappan, P., Su, Y., Schmied, C., et al. (2023). Nutrient-regulated control of lysosome function by signaling lipid conversion. Cell 186 (24), 5328–5346.e5326. doi:10.1016/j.cell.2023.09.027
Elhamamsy, A. R., Metge, B. J., Alsheikh, H. A., Shevde, L. A., and Samant, R. S. (2022). Ribosome biogenesis: a central player in cancer metastasis and therapeutic resistance. Cancer Res. 82 (13), 2344–2353. doi:10.1158/0008-5472.Can-21-4087
Fan, G. L., Deng, F. A., Zhou, X., Liu, L. S., Huang, J. Q., Zhang, D. W., et al. (2021). Plasma membrane targeted photodynamic O(2) economizer for hypoxic tumor therapy. Biomaterials 273, 120854. doi:10.1016/j.biomaterials.2021.120854
Fang, L., Meng, Q., Zhang, Y., Su, R., Xing, F., Yang, H., et al. (2023a). π Bridge engineering-boosted dual enhancement of type-I photodynamic and photothermal performance for mitochondria-targeting multimodal phototheranostics of tumor. ACS Nano 17 (21), 21553–21566. doi:10.1021/acsnano.3c06542
Fang, R. H., Gao, W., and Zhang, L. (2023b). Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 20 (1), 33–48. doi:10.1038/s41571-022-00699-x
Fay, T. P., and Limmer, D. T. (2024). Unraveling the mechanisms of triplet state formation in a heavy-atom free photosensitizer. Chem. Sci. 15 (18), 6726–6737. doi:10.1039/d4sc01369g
Feng, D., Kang, X., Wang, H., He, Z., Xu, H., Li, Y., et al. (2025). Photochemical bomb: precision nuclear targeting to activate cGAS-STING pathway for enhanced bladder cancer immunotherapy. Biomaterials 318, 123126. doi:10.1016/j.biomaterials.2025.123126
Finley, L. W. S. (2023). What is cancer metabolism? Cell 186 (8), 1670–1688. doi:10.1016/j.cell.2023.01.038
Ge, C., Yang, X., Xin, J., Gong, X., Wang, X., and Kong, L. (2023). Recent advances in antitumor dendritic cell vaccines. Cancer Biotherapy Radiopharm. 38 (7), 450–457. doi:10.1089/cbr.2023.0041
Granath-Panelo, M., and Kajimura, S. (2024). Mitochondrial heterogeneity and adaptations to cellular needs. Nat. Cell Biol. 26 (5), 674–686. doi:10.1038/s41556-024-01410-1
Gros, F., and Muller, S. (2023). The role of lysosomes in metabolic and autoimmune diseases. Nat. Rev. Nephrol. 19 (6), 366–383. doi:10.1038/s41581-023-00692-2
Guo, X., Yang, N., Ji, W., Zhang, H., Dong, X., Zhou, Z., et al. (2021). Mito-bomb: targeting mitochondria for cancer therapy. Adv. Mater. 33 (43), e2007778. doi:10.1002/adma.202007778
Harapas, C. R., Idiiatullina, E., Al-Azab, M., Hrovat-Schaale, K., Reygaerts, T., Steiner, A., et al. (2022). Organellar homeostasis and innate immune sensing. Nat. Rev. Immunol. 22 (9), 535–549. doi:10.1038/s41577-022-00682-8
He, L., Chen, J., Deng, P., Huang, S., Liu, P., Wang, C., et al. (2023). Lysosomal cyst(e)ine storage potentiates tolerance to oxidative stress in cancer cells. Mol. Cell 83 (19), 3502–3519.e11. doi:10.1016/j.molcel.2023.08.032
Hong, W. L., Huang, H., Zeng, X., and Duan, C. Y. (2024). Targeting mitochondrial quality control: new therapeutic strategies for major diseases. Mil. Med. Res. 11 (1), 59. doi:10.1186/s40779-024-00556-1
Hu, M., Zhou, W., Wang, Y., Yao, D., Ye, T., Yao, Y., et al. (2020). Discovery of the first potent proteolysis targeting chimera (PROTAC) degrader of indoleamine 2,3-dioxygenase 1. Acta Pharm. Sin. B 10 (10), 1943–1953. doi:10.1016/j.apsb.2020.02.010
Hu, Y., Li, D., Wei, H., Zhou, S., Chen, W., Yan, X., et al. (2021). Neurite extension and orientation of spiral ganglion neurons can be directed by superparamagnetic iron oxide nanoparticles in a magnetic field. Int. J. Nanomedicine 16 (null), 4515–4526. doi:10.2147/IJN.S313673
Hu, Z. C., Wang, B., Zhou, X. G., Liang, H. F., Liang, B., Lu, H. W., et al. (2023). Golgi apparatus-targeted photodynamic therapy for enhancing tumor immunogenicity by eliciting NLRP3 protein-dependent pyroptosis. ACS Nano 17 (21), 21153–21169. doi:10.1021/acsnano.3c05005
Hu, X., Zhang, M., Quan, C., Ren, S., Chen, W., and Wang, J. (2024). ROS-responsive and triple-synergistic mitochondria-targeted polymer micelles for efficient induction of ICD in tumor therapeutics. Bioact. Mater. 36, 490–507. doi:10.1016/j.bioactmat.2024.06.038
Hua, Y., Tian, X., Zhang, X., Song, G., Liu, Y., Zhao, Y., et al. (2024). Applications and challenges of photodynamic therapy in the treatment of skin malignancies. Front. Pharmacol. 15, 1476228. doi:10.3389/fphar.2024.1476228
Huang, C., Lin, B., Chen, C., Wang, H., Lin, X., Liu, J., et al. (2022). Synergistic reinforcing of immunogenic cell death and transforming tumor-associated macrophages via a multifunctional cascade bioreactor for optimizing cancer immunotherapy. Adv. Mater. 34 (51), e2207593. doi:10.1002/adma.202207593
Huang, P., Wang, M., Lu, Z., Shi, S., Wei, X., Bi, C., et al. (2023). Putrescine accelerates the differentiation of bone marrow derived dendritic cells via inhibiting phosphorylation of STAT3 at Tyr705. Int. Immunopharmacol. 116, 109739. doi:10.1016/j.intimp.2023.109739
Ikeda, G., Santoso, M. R., Tada, Y., Li, A. M., Vaskova, E., Jung, J. H., et al. (2021). Mitochondria-rich extracellular vesicles from autologous stem cell-derived cardiomyocytes restore energetics of ischemic myocardium. J. Am. Coll. Cardiol. 77 (8), 1073–1088. doi:10.1016/j.jacc.2020.12.060
Ji, B., Wei, M., and Yang, B. (2022). Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 12 (1), 434–458. doi:10.7150/thno.67300
Jia, P., Tian, T., Li, Z., Wang, Y., Lin, Y., Zeng, W., et al. (2023). CCDC50 promotes tumor growth through regulation of lysosome homeostasis. Eur. Mol. Biol. Organ. Rep. 24 (10), e56948. doi:10.15252/embr.202356948
Jiang, L., Chen, D., Jin, Z., Xia, C., Xu, Q., Fan, M., et al. (2022). Light-triggered nitric oxide release and structure transformation of peptide for enhanced intratumoral retention and sensitized photodynamic therapy. Bioact. Mater. 12, 303–313. doi:10.1016/j.bioactmat.2021.09.035
Jiao, L., Liu, Y., Yu, X. Y., Pan, X., Zhang, Y., Tu, J., et al. (2023). Ribosome biogenesis in disease: new players and therapeutic targets. Signal Transduct. Target. Ther. 8 (1), 15. doi:10.1038/s41392-022-01285-4
Kalukula, Y., Stephens, A. D., Lammerding, J., and Gabriele, S. (2022). Mechanics and functional consequences of nuclear deformations. Nat. Rev. Mol. Cell Biol. 23 (9), 583–602. doi:10.1038/s41580-022-00480-z
Ke, L., Wei, F., Xie, L., Karges, J., Chen, Y., Ji, L., et al. (2022). A biodegradable iridium(III) coordination polymer for enhanced two-photon photodynamic therapy using an apoptosis–ferroptosis hybrid pathway. Angew. Chem. Int. Ed. 61 (28), e202205429. doi:10.1002/anie.202205429
Khaliulin, I., Hamoudi, W., and Amal, H. (2025). The multifaceted role of mitochondria in autism spectrum disorder. Mol. Psychiatry 30 (2), 629–650. doi:10.1038/s41380-024-02725-z
Kilbride, S. M., and Prehn, J. H. M. (2013). Central roles of apoptotic proteins in mitochondrial function. Oncogene 32 (22), 2703–2711. doi:10.1038/onc.2012.348
Kim, D., Rosko, M. C., Castellano, F. N., Gray, T. G., and Teets, T. S. (2024). Long excited-state lifetimes in three-coordinate copper(I) complexes via triplet-triplet energy transfer to pyrene-decorated isocyanides. J. Am. Chem. Soc. 146 (28), 19193–19204. doi:10.1021/jacs.4c04288
Kolarikova, M., Hosikova, B., Dilenko, H., Barton-Tomankova, K., Valkova, L., Bajgar, R., et al. (2023). Photodynamic therapy: innovative approaches for antibacterial and anticancer treatments. Med. Res. Rev. 43 (4), 717–774. doi:10.1002/med.21935
König, T., and McBride, H. M. (2024). Mitochondrial-derived vesicles in metabolism, disease, and aging. Cell Metab. 36 (1), 21–35. doi:10.1016/j.cmet.2023.11.014
Kuang, S., Sun, L., Zhang, X., Liao, X., Rees, T. W., Zeng, L., et al. (2020). A mitochondrion-localized two-photon photosensitizer generating carbon radicals against hypoxic tumors. Angew. Chem. Int. Ed. 59 (46), 20697–20703. doi:10.1002/anie.202009888
Kuang, S., Wei, F., Karges, J., Ke, L., Xiong, K., Liao, X., et al. (2022). Photodecaging of a mitochondria-localized iridium(III) endoperoxide complex for two-photon photoactivated therapy under hypoxia. J. Am. Chem. Soc. 144 (9), 4091–4101. doi:10.1021/jacs.1c13137
Kuznetsov, A. V., Margreiter, R., Ausserlechner, M. J., and Hagenbuchner, J. (2022). The complex interplay between mitochondria, ROS and entire cellular metabolism. Antioxidants 11 (10), 1995. doi:10.3390/antiox11101995
Lee, L. E., Doke, T., Mukhi, D., and Susztak, K. (2024). The key role of altered tubule cell lipid metabolism in kidney disease development. Kidney International 106 (1), 24–34. doi:10.1016/j.kint.2024.02.025
Li, W., Yang, J., Luo, L., Jiang, M., Qin, B., Yin, H., et al. (2019). Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat. Commun. 10 (1), 3349. doi:10.1038/s41467-019-11269-8
Li, X., Lovell, J. F., Yoon, J., and Chen, X. (2020). Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 17 (11), 657–674. doi:10.1038/s41571-020-0410-2
Li, X., Zhao, Y., Zhang, T., and Xing, D. (2021). Mitochondria-specific agents for photodynamic cancer therapy: a key determinant to boost the efficacy. Adv. Healthc. Mater. 10 (3), e2001240. doi:10.1002/adhm.202001240
Li, H., Deng, C., Tan, Y., Dong, J., Zhao, Y., Wang, X., et al. (2022a). Chondroitin sulfate-based prodrug nanoparticles enhance photodynamic immunotherapy via golgi apparatus targeting. Acta Biomater. 146, 357–369. doi:10.1016/j.actbio.2022.05.014
Li, X., Wang, H., Li, Z., Li, D., Lu, X., Ai, S., et al. (2022b). Oxygen tank for synergistic hypoxia relief to enhance mitochondria-targeted photodynamic therapy. Biomaterials Res. 26 (1), 47. doi:10.1186/s40824-022-00296-0
Li, Y., Han, W., Gong, D., Luo, T., Fan, Y., Mao, J., et al. (2023). A self-assembled nanophotosensitizer targets lysosomes and induces lysosomal membrane permeabilization to enhance photodynamic therapy. Chem. Sci. 14 (19), 5106–5115. doi:10.1039/D3SC00455D
Li, B., Ming, H., Qin, S., Nice, E. C., Dong, J., Du, Z., et al. (2025a). Redox regulation: mechanisms, biology and therapeutic targets in diseases. Signal Transduct. Target. Ther. 10 (1), 72. doi:10.1038/s41392-024-02095-6
Li, M., Xiong, J., Zhang, Y., Yu, L., Yue, L., Yoon, C., et al. (2025b). New guidelines and definitions for type I photodynamic therapy. Chem. Soc. Rev. 54, 7025–7057. doi:10.1039/d1cs01079d
Liang, Y., Wang, P., Liu, Z., Sun, H., Wang, Q., Sun, G., et al. (2023). Dual stimuli-responsive micelles for imaging-guided mitochondrion-targeted photothermal/photodynamic/chemo combination therapy-induced immunogenic cell death. Int. J. Nanomed. 18, 4381–4402. doi:10.2147/IJN.S410047
Liao, Y., Lin, X., He, Z., Chen, J., Tang, S., Wang, W., et al. (2025). Construction of nucleus-targeted photosensitizer and highly effective photodynamic immunotherapy for cancer. Bioorg. Chem. 154, 108022. doi:10.1016/j.bioorg.2024.108022
Liu, J., Yin, Y., Yang, L., Lu, B., Yang, Z., Wang, W., et al. (2021). Nucleus-targeted photosensitizer nanoparticles for photothermal and photodynamic therapy of breast carcinoma. Int. J. Nanomedicine 16, 1473–1485. doi:10.2147/ijn.S284518
Liu, M., Chen, Y., Guo, Y., Yuan, H., Cui, T., Yao, S., et al. (2022a). Golgi apparatus-targeted aggregation-induced emission luminogens for effective cancer photodynamic therapy. Nat. Commun. 13 (1), 2179. doi:10.1038/s41467-022-29872-7
Liu, X., Liu, Y., Li, X., Huang, J., Guo, X., Zhang, J., et al. (2022b). ER-Targeting PDT converts tumors into in situ therapeutic tumor vaccines. ACS Nano 16 (6), 9240–9253. doi:10.1021/acsnano.2c01669
Liu, X., Fan, D., Ren, Y., Huang, S., Ding, J., Liu, M., et al. (2023a). Photo-activable organosilver nanosystem facilitates synergistic cancer theranostics. ACS Appl. Mater. Interfaces 15 (1), 711–722. doi:10.1021/acsami.2c21004
Liu, Y., Liu, S., Tomar, A., Yen, F. S., Unlu, G., Ropek, N., et al. (2023b). Autoregulatory control of mitochondrial glutathione homeostasis. Science 382 (6672), 820–828. doi:10.1126/science.adf4154
Liu, J., Sun, L., Hong, Y., Deng, J., Luo, Q., Zeng, R., et al. (2024a). Near-infrared fluorescent probe for sensitive detection and imaging of DNA G4s in living cells. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 318, 124489. doi:10.1016/j.saa.2024.124489
Liu, J., Wu, Y., Meng, S., Xu, P., Li, S., Li, Y., et al. (2024b). Selective autophagy in cancer: mechanisms, therapeutic implications, and future perspectives. Mol. Cancer 23 (1), 22. doi:10.1186/s12943-024-01934-y
Liu, J., Yan, Y., Zhang, Y., Pan, X., Xia, H., Zhou, J., et al. (2024c). Lysosome-mitochondria cascade targeting nanoparticle drives robust pyroptosis for cancer immunotherapy. J. Am. Chem. Soc. 146 (50), 34568–34582. doi:10.1021/jacs.4c12264
Liu, Y., Guo, Y., Zeng, Q., Hu, Y., He, R., Ma, W., et al. (2024d). Prosapogenin a induces GSDME-dependent pyroptosis of anaplastic thyroid cancer through vacuolar ATPase activation-mediated lysosomal over-acidification. Cell death Dis. 15 (8), 586. doi:10.1038/s41419-024-06985-z
Liu, X., Huang, J., Zhou, H., Wang, S., Guo, X., Mao, J., et al. (2025). Inhibition of PDT-induced PGE2 surge for enhanced photo-immunotherapy. Biomaterials 317, 123116. doi:10.1016/j.biomaterials.2025.123116
Long, S., Zhou, J., Zheng, Y., Xiao, Q., Li, Y., Yin, Z., et al. (2024). A NIR fluorescent probe with large stokes shift for sensitive detection and imaging of G4s in live cells. J. Photochem. Photobiol. A Chem. 455, 115783. doi:10.1016/j.jphotochem.2024.115783
Lou, X., Wang, H., Liu, Y., Huang, Y., Liu, Z., Zhang, W., et al. (2023). Perylene-based reactive oxygen species supergenerator for immunogenic photochemotherapy against hypoxic tumors. Angew. Chem. Int. Ed. 62 (11), e202214586. doi:10.1002/anie.202214586
Loveless, R., Bloomquist, R., and Teng, Y. (2021). Pyroptosis at the forefront of anticancer immunity. Journal Exp. Clin. Cancer Res. 40 (1), 264. doi:10.1186/s13046-021-02065-8
Lu, J., Mao, Y., Feng, S., Li, X., Gao, Y., Zhao, Q., et al. (2022). Biomimetic smart mesoporous carbon nanozyme as a dual-GSH depletion agent and O(2) generator for enhanced photodynamic therapy. Acta Biomater. 148, 310–322. doi:10.1016/j.actbio.2022.06.001
Luo, Y., Guan, B., Deng, X., Bai, P., Huang, H., Miao, C., et al. (2024). Methuosis inducer SGI-1027 cooperates with everolimus to promote apoptosis and pyroptosis by triggering lysosomal membrane permeability in renal cancer. Adv. Sci. 11 (38), e2404693. doi:10.1002/advs.202404693
Ma, H., Lu, Y., Huang, Z., Long, S., Cao, J., Zhang, Z., et al. (2022). ER-targeting cyanine dye as an NIR photoinducer to efficiently trigger photoimmunogenic cancer cell death. J. Am. Chem. Soc. 144 (8), 3477–3486. doi:10.1021/jacs.1c11886
Mao, L., Yin, M., Ji, C., Ma, Q., Xia, D., and Yang, L. (2023). Targeted delivery of T-cell agonists for enhancing immunotherapy. J. Drug Deliv. Sci. Technol. 89, 105074. doi:10.1016/j.jddst.2023.105074
Meyer, H., and Kravic, B. (2024). The endo-lysosomal damage response. Annu. Rev. Biochem. 93 (1), 367–387. doi:10.1146/annurev-biochem-030222-102505
Moan, J., and Berg, K. (1991). The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 53 (4), 549–553. doi:10.1111/j.1751-1097.1991.tb03669.x
Niedre, M., Patterson, M. S., and Wilson, B. C. (2002). Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem. Photobiol. 75 (4), 382–391. doi:10.1562/0031-8655(2002)0750382DNILDO2.0.CO2
Niu, N., Yu, Y., Zhang, Z., Kang, M., Wang, L., Zhao, Z., et al. (2022). A cell membrane-targeting AIE photosensitizer as a necroptosis inducer for boosting cancer theranostics. Chem. Sci. 13 (20), 5929–5937. doi:10.1039/D2SC01260J
Pan, W. L., Tan, Y., Meng, W., Huang, N. H., Zhao, Y. B., Yu, Z. Q., et al. (2022a). Microenvironment-driven sequential ferroptosis, photodynamic therapy, and chemotherapy for targeted breast cancer therapy by a cancer-cell-membrane-coated nanoscale metal-organic framework. Biomaterials 283, 121449. doi:10.1016/j.biomaterials.2022.121449
Pan, Y., Tang, W., Fan, W., Zhang, J., and Chen, X. (2022b). Development of nanotechnology-mediated precision radiotherapy for anti-metastasis and radioprotection. Chem. Soc. Reviews 51 (23), 9759–9830. doi:10.1039/d1cs01145f
Pham, T. C., Hoang, T. T. H., Tran, D. N., Kim, G., Nguyen, T. V., Pham, T. V., et al. (2023). Imidazolium-based heavy-atom-free photosensitizer for nucleus-targeted fluorescence bioimaging and photodynamic therapy. ACS Appl. Mater. Interfaces 15 (41), 47969–47977. doi:10.1021/acsami.3c10200
Plikus, M. V., Wang, X., Sinha, S., Forte, E., Thompson, S. M., Herzog, E. L., et al. (2021). Fibroblasts: origins, definitions, and functions in health and disease. Cell 184 (15), 3852–3872. doi:10.1016/j.cell.2021.06.024
Pope, L. E., and Dixon, S. J. (2023). Regulation of ferroptosis by lipid metabolism. Trends Cell Biol. 33 (12), 1077–1087. doi:10.1016/j.tcb.2023.05.003
Qian, J., Liao, G., Chen, M., Peng, R. W., Yan, X., Du, J., et al. (2024). Advancing cancer therapy: new frontiers in targeting DNA damage response. Front. Pharmacol. 15, 1474337. doi:10.3389/fphar.2024.1474337
Qu, H., Chen, H., Cheng, W., Pan, Y., Duan, Z., Wang, Y., et al. (2023). Charge-reversible crosslinked nanoparticle for pro-apoptotic peptide delivery and synergistic photodynamic cancer therapy. Nano Res. 16 (12), 13267–13282. doi:10.1007/s12274-023-5912-7
Sabari, B. R., Dall'Agnese, A., and Young, R. A. (2020). Biomolecular condensates in the nucleus. Trends Biochem. Sci. 45 (11), 961–977. doi:10.1016/j.tibs.2020.06.007
Settembre, C., and Perera, R. M. (2024). Lysosomes as coordinators of cellular catabolism, metabolic signalling and organ physiology. Nat. Rev. Mol. Cell Biol. 25 (3), 223–245. doi:10.1038/s41580-023-00676-x
Shao, X., Meng, C., Song, W., Zhang, T., and Chen, Q. (2023). Subcellular visualization: organelle-specific targeted drug delivery and discovery. Adv. Drug Deliv. Rev. 199, 114977. doi:10.1016/j.addr.2023.114977
Shen, W., Han, G., Yu, L., Yang, S., Li, X., Zhang, W., et al. (2022). Combined prussian blue nanozyme carriers improve photodynamic therapy and effective interruption of tumor metastasis. Int. J. Nanomedicine 17 (null), 1397–1408. doi:10.2147/IJN.S359156
Shen, J., Qian, N., Xu, G., Dou, X., An, Y., Yang, C., et al. (2024). IMT030122, A novel engineered EpCAM/CD3/4-1BB tri-specific antibody, enhances T-cell recruitment and demonstrates anti-tumor activity in mouse models of colorectal cancer. Int. Immunopharmacol. 137, 112424. doi:10.1016/j.intimp.2024.112424
Shi, H., Xiong, C., Zhang, L., Cao, H., Wang, R., Pan, P., et al. (2023). Light-triggered nitric oxide nanogenerator with high l-arginine loading for synergistic photodynamic/gas/photothermal therapy. Adv. Healthc. Mater. 12 (20), 2300012. doi:10.1002/adhm.202300012
Shi, M., Fu, Z., Pan, W., Wang, K., Liu, X., Li, N., et al. (2024). A golgi apparatus-targeted photothermal agent with protein anchoring for enhanced cancer photothermal therapy. Adv. Healthc. Mater. 13 (17), e2303749. doi:10.1002/adhm.202303749
Shigemitsu, H., Ohkubo, K., Sato, K., Bunno, A., Mori, T., Osakada, Y., et al. (2022). Fluorescein-based type I supramolecular photosensitizer via induction of charge separation by self-assembly. JACS Au 2 (6), 1472–1478. doi:10.1021/jacsau.2c00243
Šlachtová, V., Chovanec, M., Rahm, M., and Vrabel, M. (2023). Bioorthogonal chemistry in cellular organelles. Top. Curr. Chem. 382 (1), 2. doi:10.1007/s41061-023-00446-5
Song, L., Hao, Y., Wang, C., Han, Y., Zhu, Y., Feng, L., et al. (2022). Liposomal oxaliplatin prodrugs loaded with metformin potentiate immunotherapy for colorectal cancer. J. Control. Release 350, 922–932. doi:10.1016/j.jconrel.2022.09.013
Song, W., Yang, H., Wang, Y., Chen, S., Zhong, W., Wang, Q., et al. (2024). Glutathione-sensitive photosensitizer-drug conjugates target the mitochondria to overcome multi-drug resistance in cancer. Adv. Sci. 11 (30), e2307765. doi:10.1002/advs.202307765
Soukar, J., Peppas, N. A., and Gaharwar, A. K. (2025). Organelle-targeting nanoparticles. Adv. Sci. 12 (7), e2411720. doi:10.1002/advs.202411720
Sun, B., Bte Rahmat, J. N., and Zhang, Y. (2022). Advanced techniques for performing photodynamic therapy in deep-seated tissues. Biomaterials 291, 121875. doi:10.1016/j.biomaterials.2022.121875
Sun, B., Liu, J., Kim, H. J., Rahmat, J. N. B., Neoh, K. G., and Zhang, Y. (2023a). Light-responsive smart nanocarriers for wirelessly controlled photodynamic therapy for prostate cancers. Acta Biomater. 171, 553–564. doi:10.1016/j.actbio.2023.09.031
Sun, H., Xu, J., Wang, Y., Shen, S., Xu, X., Zhang, L., et al. (2023b). Bone microenvironment regulative hydrogels with ROS scavenging and prolonged oxygen-generating for enhancing bone repair. Bioact. Mater. 24, 477–496. doi:10.1016/j.bioactmat.2022.12.021
Sun, Q., Yang, J., Wu, Q., Shen, W., Yang, Y., and Yin, D. (2024). Targeting lysosome for enhanced cancer photodynamic/photothermal therapy in a “one stone two birds” pattern. ACS Appl. Mater. Interfaces 16 (1), 127–141. doi:10.1021/acsami.3c13162
Sun, S., Zhao, G., Jia, M., Jiang, Q., Li, S., Wang, H., et al. (2024c). Stay in touch with the endoplasmic reticulum. Sci. China Life Sci. 67 (2), 230–257. doi:10.1007/s11427-023-2443-9
Sun, F., Li, H., Sun, D., Fu, S., Gu, L., Shao, X., et al. (2025). Single-cell omics: experimental workflow, data analyses and applications. Sci. China Life Sci. 68 (1), 5–102. doi:10.1007/s11427-023-2561-0
Sweet, M. J., Ramnath, D., Singhal, A., and Kapetanovic, R. (2025). Inducible antibacterial responses in macrophages. Nat. Rev. Immunol. 25 (2), 92–107. doi:10.1038/s41577-024-01080-y
Tan, Y., Hu, G., Li, M., An, Y., Wang, Z., Liu, R., et al. (2025). Two-photon photosensitizer for specific targeting and induction of tumor pyroptosis to elicit systemic immunity-boosting anti-tumor therapy. Biomaterials 317, 123108. doi:10.1016/j.biomaterials.2025.123108
Tanaka, T., Warner, B. M., Michael, D. G., Nakamura, H., Odani, T., Yin, H., et al. (2022). LAMP3 inhibits autophagy and contributes to cell death by lysosomal membrane permeabilization. Autophagy 18 (7), 1629–1647. doi:10.1080/15548627.2021.1995150
Tang, V. T., and Ginsburg, D. (2023). Cargo selection in endoplasmic reticulum-to-golgi transport and relevant diseases. J. Clin. Investigation 133 (1), e163838. doi:10.1172/jci163838
Tang, Y., Bisoyi, H. K., Chen, X.-M., Liu, Z., Chen, X., Zhang, S., et al. (2023). Pyroptosis-mediated synergistic photodynamic and photothermal immunotherapy enabled by a tumor-membrane-targeted photosensitive dimer. Adv. Mater. 35 (25), 2300232. doi:10.1002/adma.202300232
Tian, H., Li, W., Wang, G., Tian, Y., Yan, J., Yu, X., et al. (2024). Metal-phenolic nanomaterial with organelle-level precision primes antitumor immunity via mtDNA-dependent cGAS-STING activation. Angew. Chem. Int. Ed. 63 (50), e202411498. doi:10.1002/anie.202411498
Tong, F., Wang, Y., Xu, Y., Zhou, Y., He, S., Du, Y., et al. (2024). MMP-2-triggered, mitochondria-targeted PROTAC-PDT therapy of breast cancer and brain metastases inhibition. Nat. Commun. 15 (1), 10382. doi:10.1038/s41467-024-54854-2
Vercellino, I., and Sazanov, L. A. (2022). The assembly, regulation and function of the mitochondrial respiratory chain. Nat. Rev. Mol. Cell Biol. 23 (2), 141–161. doi:10.1038/s41580-021-00415-0
Wan, S.-S., Zhang, L., and Zhang, X.-Z. (2019). An ATP-regulated ion transport nanosystem for homeostatic perturbation therapy and sensitizing photodynamic therapy by autophagy inhibition of tumors. ACS Central Sci. 5 (2), 327–340. doi:10.1021/acscentsci.8b00822
Wang, R., Li, X., and Yoon, J. (2021). Organelle-targeted photosensitizers for precision photodynamic therapy. ACS Appl. Mater. Interfaces 13 (17), 19543–19571. doi:10.1021/acsami.1c02019
Wang, J., Yang, B., Lv, C., Chen, T., Sun, L., Sun, L., et al. (2022a). Amino porphyrin-peptide assemblies induce ribosome damage and cancer stem cell inhibition for an enhanced photodynamic therapy. Biomaterials 289, 121812. doi:10.1016/j.biomaterials.2022.121812
Wang, M., Wu, M., Liu, X., Shao, S., Huang, J., Liu, B., et al. (2022b). Pyroptosis remodeling tumor microenvironment to enhance pancreatic cancer immunotherapy driven by membrane anchoring photosensitizer. Adv. Sci. 9 (29), e2202914. doi:10.1002/advs.202202914
Wang, W., Zhu, C., Zhang, B., Feng, Y., Zhang, Y., and Li, J. (2023). Self-assembled nano-PROTAC enables near-infrared photodynamic proteolysis for cancer therapy. J. Am. Chem. Soc. 145 (30), 16642–16649. doi:10.1021/jacs.3c04109
Wang, B., Zhou, H., Chen, L., Ding, Y., Zhang, X., Chen, H., et al. (2024a). A mitochondria-targeted photosensitizer for combined pyroptosis and apoptosis with NIR-II imaging/photoacoustic imaging-guided phototherapy. Angew. Chem. Int. Ed. 63 (39), e202408874. doi:10.1002/anie.202408874
Wang, H., Vant, J. W., Zhang, A., Sanchez, R. G., Wu, Y., Micou, M. L., et al. (2024b). Organization of a functional glycolytic metabolon on mitochondria for metabolic efficiency. Nat. Metab. 6 (9), 1712–1735. doi:10.1038/s42255-024-01121-9
Wang, P., Bai, Q., Liu, X., Zhao, M., Chen, L., Hu, F., et al. (2024c). Nucleus-targeting photosensitizers enhance neutrophil extracellular traps for efficient eradication of multidrug-resistant bacterial infections. Adv. Mater. 36 (50), e2400304. doi:10.1002/adma.202400304
Wang, X., Wang, X., Li, Y., and Qi, Z. (2024d). COX-2 inhibition mediated aggregation-induced emission photosensitizer target the golgi apparatus for selective imaging of cancer cells and enhanced photodynamic therapy. Dyes Pigments 222, 111897. doi:10.1016/j.dyepig.2023.111897
Wei, X., Song, M., Jiang, G., Liang, M., Chen, C., Yang, Z., et al. (2022). Progress in advanced nanotherapeutics for enhanced photodynamic immunotherapy of tumor. Theranostics 12 (12), 5272–5298. doi:10.7150/thno.73566
Weigel, A. V., Chang, C. L., Shtengel, G., Xu, C. S., Hoffman, D. P., Freeman, M., et al. (2021). ER-to-Golgi protein delivery through an interwoven, tubular network extending from ER. Cell 184 (9), 2412–2429.e16. doi:10.1016/j.cell.2021.03.035
Wen, K., Tan, H., Peng, Q., Chen, H., Ma, H., Wang, L., et al. (2022). Achieving efficient NIR-II type-I photosensitizers for photodynamic/photothermal therapy upon regulating chalcogen elements. Adv. Mater. 34 (7), e2108146. doi:10.1002/adma.202108146
Wu, L., Liu, J., Li, P., Tang, B., and James, T. D. (2021). Two-photon small-molecule fluorescence-based agents for sensing, imaging, and therapy within biological systems. Chem. Soc. Reviews 50 (2), 702–734. doi:10.1039/d0cs00861c
Wu, C., Wang, C., Zhang, T., Gao, G., Wei, M., Chen, Y., et al. (2022). Lysosome-targeted and fluorescence-turned “on” cytotoxicity induced by alkaline phosphatase-triggered self-assembly. Adv. Healthc. Mater. 11 (1), 2101346. doi:10.1002/adhm.202101346
Wu, D., Huang, C., and Guan, K. (2024a). Mechanistic and therapeutic perspectives of miRNA-PTEN signaling axis in cancer therapy resistance. Biochem. Pharmacol. 226, 116406. doi:10.1016/j.bcp.2024.116406
Wu, Y., Li, Y., Yan, N., Huang, J., Li, X., Zhang, K., et al. (2024b). Nuclear-targeted chimeric peptide nanorods to amplify innate anti-tumor immunity through localized DNA damage and STING activation. J. Control. Release 369, 531–544. doi:10.1016/j.jconrel.2024.04.008
Xia, D., Zhang, X., Hao, H., Jiang, W., Chen, C., Li, H., et al. (2023). Strategies to prolong drug retention in solid tumors by aggregating Endo-CMC nanoparticles. J. Control. Release 360, 705–717. doi:10.1016/j.jconrel.2023.07.006
Xie, R., Huang, H., Chen, T., Huang, X., and Chen, C. (2023a). Effectiveness and safety of pelareorep plus chemotherapy versus chemotherapy alone for advanced solid tumors: a meta-analysis. Front. Pharmacol. 14, 1228225. doi:10.3389/fphar.2023.1228225
Xie, Z., Zhao, M., Yan, C., Kong, W., Lan, F., Narengaowa, , et al. (2023b). Cathepsin B in programmed cell death machinery: mechanisms of execution and regulatory pathways. Cell death Dis. 14 (4), 255. doi:10.1038/s41419-023-05786-0
Xie, X., Sun, T., Pan, H., Ji, D., Xu, Z., Gao, G., et al. (2024). Development of novel β-carboline/furylmalononitrile hybrids as type I/II photosensitizers with chemo-photodynamic therapy and minimal toxicity. Mol. Pharm. 21 (7), 3553–3565. doi:10.1021/acs.molpharmaceut.4c00238
Xiong, S., Song, D., Xiang, Y., Li, Y., Zhong, Y., Li, H., et al. (2020). Reactive oxygen species, not Ca2+, mediates methotrexate-induced autophagy and apoptosis in spermatocyte cell line. Basic Clin. Pharmacol. Toxicol. 126 (2), 144–152. doi:10.1111/bcpt.13306
Xiong, T., Chen, Y., Li, M., Chen, X., and Peng, X. (2025). Recent progress of molecular design in organic type I photosensitizers. Small 21 (22), e2501911. doi:10.1002/smll.202501911
Xu, S., Zhu, X., Zhang, C., Huang, W., Zhou, Y., and Yan, D. (2018). Oxygen and Pt(II) self-generating conjugate for synergistic photo-chemo therapy of hypoxic tumor. Nat. Communications 9 (1), 2053. doi:10.1038/s41467-018-04318-1
Xu, Y., Wang, S., Chen, Z., Hu, R., Zhao, Y., Wang, K., et al. (2021). Nitric oxide release activated near-infrared photothermal agent for synergistic tumor treatment. Biomaterials 276, 121017. doi:10.1016/j.biomaterials.2021.121017
Xu, Y., Liu, R., Yang, H., Qu, S., Qian, L., and Dai, Z. (2022). Enhancing photodynamic therapy efficacy against cancer metastasis by ultrasound-mediated oxygen microbubble destruction to boost tumor-targeted delivery of oxygen and renal-clearable photosensitizer micelles. ACS Appl. Mater. Interfaces 14 (22), 25197–25208. doi:10.1021/acsami.2c06655
Xu, L., Bai, E., Zhu, Y., Qin, J., Du, X., and Huang, H. (2023a). pH-responsive hydrogel as a potential oral delivery system of baicalin for prolonging gastroprotective activity. Pharmaceutics 15 (1), 257. doi:10.3390/pharmaceutics15010257
Xu, X., Li, Z., Zhao, J., Liu, Y., Xu, Y., Jia, Y., et al. (2023b). Catalase-conjugated rose bengal biological nanoparticles with mitochondrial selectivity toward photodynamic therapy. CCS Chem. 5 (12), 2877–2887. doi:10.31635/ccschem.023.202202525
Xu, S., Yan, K. C., Xu, Z. H., Wang, Y., and James, T. D. (2024). Fluorescent probes for targeting the golgi apparatus: design strategies and applications. Chem. Soc. Rev. 53 (14), 7590–7631. doi:10.1039/d3cs00171g
Xu, T., Ma, Q., Zhang, C., He, X., Wang, Q., Wu, Y., et al. (2025a). A novel nanomedicine for osteosarcoma treatment: triggering ferroptosis through GSH depletion and inhibition for enhanced synergistic PDT/PTT therapy. J. Nanobiotechnology 23 (1), 323. doi:10.1186/s12951-025-03380-4
Xu, Y., An, D., Zhang, T., Wu, X., Wang, S., Shao, J., et al. (2025b). Mitochondrion-targeted type I photodynamic therapy for agonist independent cGAS-STING activation. Adv. Mater. 37 (14), e2418894. doi:10.1002/adma.202418894
Xue, M., Dong, L., Zhang, H., Li, Y., Qiu, K., Zhao, Z., et al. (2024). METTL16 promotes liver cancer stem cell self-renewal via controlling ribosome biogenesis and mRNA translation. J. Hematol. Oncol. 17 (1), 7. doi:10.1186/s13045-024-01526-9
Yadav, N., Kumar, S., Marlowe, T., Chaudhary, A. K., Kumar, R., Wang, J., et al. (2015). Oxidative phosphorylation-dependent regulation of cancer cell apoptosis in response to anticancer agents. Cell Death Dis. 6 (11), e1969. doi:10.1038/cddis.2015.305
Yan, Y., Li, S., Su, L., Tang, X., Chen, X., Gu, X., et al. (2024). Mitochondrial inhibitors: a new horizon in breast cancer therapy. Front. Pharmacol. 15, 1421905. doi:10.3389/fphar.2024.1421905
Yang, Z., Wang, J., Ai, S., Sun, J., Mai, X., and Guan, W. (2019). Self-generating oxygen enhanced mitochondrion-targeted photodynamic therapy for tumor treatment with hypoxia scavenging. Theranostics 9 (23), 6809–6823. doi:10.7150/thno.36988
Yang, J., Griffin, A., Qiang, Z., and Ren, J. (2022). Organelle-targeted therapies: a comprehensive review on system design for enabling precision oncology. Signal Transduct. Target. Ther. 7 (1), 379. doi:10.1038/s41392-022-01243-0
Yang, F., Yu, W., Yu, Q., Liu, X., Liu, C., Lu, C., et al. (2023a). Mitochondria-targeted nanosystem with reactive oxygen species-controlled release of CO to enhance photodynamic therapy of PCN-224 by sensitizing ferroptosis. Small 19 (16), 2206124. doi:10.1002/smll.202206124
Yang, L., Chen, Q., Gan, S., Huang, C., Zhang, H., and Sun, H. (2023b). Rational design of self-reporting photosensitizers for cell membrane-targeted photodynamic therapy. Anal. Chem. 95 (32), 11988–11996. doi:10.1021/acs.analchem.3c01659
Yang, L., Ma, H., Liu, Y., Cao, R., Chen, S., Wang, J., et al. (2023c). Nucleus-selective self-augmenting cascade nanoassemblies for targeted synergistic photo-chemo therapy of tumors. Chem. Commun. 59 (73), 10940–10943. doi:10.1039/D3CC03331G
Yang, W. W., Ren, Z. H., Feng, J., Lv, Z. B., Cheng, X., Zhang, J., et al. (2024). A deep-red emissive sulfur-doped double [7]helicene photosensitizer: synthesis, structure and chiral optical properties. Angew. Chem. Int. Ed. 63 (45), e202412681. doi:10.1002/anie.202412681
Yao, R. Q., Ren, C., Xia, Z. F., and Yao, Y. M. (2021). Organelle-specific autophagy in inflammatory diseases: a potential therapeutic target underlying the quality control of multiple organelles. Autophagy 17 (2), 385–401. doi:10.1080/15548627.2020.1725377
Ye, Z., Liu, J., Liu, Y., Zhao, Y., Li, Z., Xu, B., et al. (2024). Hybrid nanopotentiators with dual cascade amplification for glioma combined interventional therapy. J. Control. Release 372, 95–112. doi:10.1016/j.jconrel.2024.06.016
Ying, Z., Ye, N., Ma, Q., Chen, F., Li, N., and Zhen, X. (2023). Targeted to neuronal organelles for CNS drug development. Adv. Drug Deliv. Rev. 200, 115025. doi:10.1016/j.addr.2023.115025
Yu, Q., Shi, H., Ding, Z., Wang, Z., Yao, H., and Lin, R. (2023a). The E3 ubiquitin ligase TRIM31 attenuates NLRP3 inflammasome activation in Helicobacter pylori-associated gastritis by regulating ROS and autophagy. Cell Commun. Signal. 21 (1), 1. doi:10.1186/s12964-022-00954-9
Yu, Y., Xiang, L., Zhang, X., Zhang, L., Ni, Z., Zhu, Z. H., et al. (2023b). Pure organic AIE nanoscintillator for X-ray mediated type I and type II photodynamic therapy. Adv. Sci. 10 (26), e2302395. doi:10.1002/advs.202302395
Yu, L., Liu, Z., Xu, W., Jin, K., Liu, J., Zhu, X., et al. (2024a). Towards overcoming obstacles of type II photodynamic therapy: endogenous production of light, photosensitizer, and oxygen. Acta Pharm. Sin. B 14 (3), 1111–1131. doi:10.1016/j.apsb.2023.11.007
Yu, Y., Liu, M., Wang, Z., Liu, Y., Yao, M., Wang, L., et al. (2024b). Identification of oxidative stress signatures of lung adenocarcinoma and prediction of patient prognosis or treatment response with single-cell RNA sequencing and bulk RNA sequencing data. Int. Immunopharmacol. 137, 112495. doi:10.1016/j.intimp.2024.112495
Yu, Y., Zhang, L., Jia, H., Ji, C., Liu, Y., Zhao, Z., et al. (2024c). Dual-mode reactive oxygen species-stimulated carbon monoxide release for synergistic photodynamic and gas tumor therapy. ACS Nano 18 (45), 31286–31299. doi:10.1021/acsnano.4c10277
Zeng, S., Chen, J., Gao, R., Chen, R., Xue, Q., Ren, Y., et al. (2024a). NIR-II photoacoustic imaging-guided oxygen delivery and controlled release improves photodynamic therapy for hepatocellular carcinoma. Adv. Mater. 36 (4), e2308780. doi:10.1002/adma.202308780
Zeng, S., Wang, Y., Chen, C., Kim, H., Liu, X., Jiang, M., et al. (2024b). An ER-targeted, viscosity-sensitive hemicyanine dye for the diagnosis of nonalcoholic fatty liver and photodynamic cancer therapy by activating pyroptosis pathway. Angew. Chem. Int. Ed. 63 (9), e202316487. doi:10.1002/anie.202316487
Zhang, Y., Swanda, R. V., Nie, L., Liu, X., Wang, C., Lee, H., et al. (2021a). mTORC1 couples cyst(e)ine availability with GPX4 protein synthesis and ferroptosis regulation. Nat. Commun. 12 (1), 1589. doi:10.1038/s41467-021-21841-w
Zhang, Z., Yue, P., Lu, T., Wang, Y., Wei, Y., and Wei, X. (2021b). Role of lysosomes in physiological activities, diseases, and therapy. J. Hematol. Oncol. 14 (1), 79. doi:10.1186/s13045-021-01087-1
Zhang, M., Liu, X., Mao, Y., He, Y., Xu, J., Zheng, F., et al. (2022a). Oxygen-generating hydrogels overcome tumor hypoxia to enhance photodynamic/gas synergistic therapy. ACS Appl. Mater. Interfaces 14 (24), 27551–27563. doi:10.1021/acsami.2c02949
Zhang, M., Zhao, Y., Ma, H., Sun, Y., and Cao, J. (2022b). How to improve photodynamic therapy-induced antitumor immunity for cancer treatment? Theranostics 12 (10), 4629–4655. doi:10.7150/thno.72465
Zhang, T., Liu, Z., Tang, W., Zhu, D., Lyu, M., Lam, J. W. Y., et al. (2022c). Mitochondria-targeting type I AIE photosensitizer combined with H2S therapy: uninterrupted hydroxyl radical generation for enhancing tumor therapy. Nano Today 46, 101620. doi:10.1016/j.nantod.2022.101620
Zhang, X., Wan, J., Mo, F., Tang, D., Xiao, H., Li, Z., et al. (2022d). Targeting bone tumor and subcellular endoplasmic reticulum via near infrared II fluorescent polymer for photodynamic-immunotherapy to break the step-reduction delivery dilemma. Adv. Sci. 9 (24), e2201819. doi:10.1002/advs.202201819
Zhang, H., Zhou, P., Jiang, Y., Li, L., Ju, F., Cheng, Q., et al. (2023a). Sustained-release esketamine based nanoparticle-hydrogel delivery system for neuropathic pain management. Int. J. Nanomedicine 18 (null), 1131–1143. doi:10.2147/IJN.S400798
Zhang, T., Yang, X., Ou, X., Lee, M. M. S., Zhang, J., Xu, C., et al. (2023b). Tailoring the amphiphilic structure of zwitterionic AIE photosensitizers to boost antitumor immunity. Adv. Mater. 35 (38), e2303186. doi:10.1002/adma.202303186
Zhang, X., Yi, C., Zhang, L., Zhu, X., He, Y., Lu, H., et al. (2023c). Size-optimized nuclear-targeting phototherapy enhances the type I interferon response for “cold” tumor immunotherapy. Acta Biomater. 159, 338–352. doi:10.1016/j.actbio.2023.01.023
Zhang, Y., Jia, Q., Nan, F., Wang, J., Liang, K., Li, J., et al. (2023d). Carbon dots nanophotosensitizers with tunable reactive oxygen species generation for mitochondrion-targeted type I/II photodynamic therapy. Biomaterials 293, 121953. doi:10.1016/j.biomaterials.2022.121953
Zhang, Q., Wang, X., Chen, J., Wu, J., Zhou, M., Xia, R., et al. (2024). Recent progress of porphyrin metal–organic frameworks for combined photodynamic therapy and hypoxia-activated chemotherapy. Chem. Commun. 60 (93), 13641–13652. doi:10.1039/D4CC04512B
Zhang, P., Mukwaya, V., Guan, Q., Xiong, S., Tian, Z., Levi-Kalisman, Y., et al. (2025). Dextran-based nanodrugs with mitochondrial targeting/glutathione depleting synergy for enhanced photodynamic therapy. Carbohydr. Polymers 348 (Pt A), 122854. doi:10.1016/j.carbpol.2024.122854
Zhao, F., Wang, W., and Wu, W. (2021). A novel ruthenium polypyridyl complex for the selective imaging and photodynamic targeting of the golgi apparatus. Dalton Trans. 50 (10), 3536–3541. doi:10.1039/D1DT00216C
Zhao, H., Xu, X., Zhou, L., Hu, Y., Huang, Y., and Narita, A. (2022). Water-soluble nanoparticles with twisted double [7]carbohelicene for lysosome-targeted cancer photodynamic therapy. Small 18 (1), e2105365. doi:10.1002/smll.202105365
Zhao, Y., Huang, T., Wang, S., Yao, S., Hu, Q., Wan, X., et al. (2023). Manganese oxide-modified bismuth oxychloride piezoelectric nanoplatform with multiple enzyme-like activities for cancer sonodynamic therapy. J. Colloid Interface Sci. 640, 839–850. doi:10.1016/j.jcis.2023.03.008
Zhao, Y., Liu, Z., Deng, K., Qu, H., Zhang, Q., Zhou, P., et al. (2024). Identification of TAP1 as a T-cell related therapeutic target in gastric cancer by mediating oxalipliatin-related synergistic enhancement of immunotherapy. Int. Immunopharmacol. 132, 111998. doi:10.1016/j.intimp.2024.111998
Zhen, S., Xu, Z., Suo, M., Zhang, T., Lyu, M., Li, T., et al. (2025). NIR-II AIE liposomes for boosting type-I photodynamic and mild-temperature photothermal therapy in breast cancer treatment. Adv. Mater. 37 (3), e2411133. doi:10.1002/adma.202411133
Zheng, X., Jin, Y., Liu, X., Liu, T., Wang, W., and Yu, H. (2021a). Photoactivatable nanogenerators of reactive species for cancer therapy. Bioact. Mater. 6 (12), 4301–4318. doi:10.1016/j.bioactmat.2021.04.030
Zheng, Z., Deng, W., Bai, Y., Miao, R., Mei, S., Zhang, Z., et al. (2021b). The lysosomal rag-ragulator complex licenses RIPK1 and caspase-8-mediated pyroptosis by yersinia. Science 372 (6549), eabg0269. doi:10.1126/science.abg0269
Zhou, L., Wei, F., Xiang, J., Li, H., Li, C., Zhang, P., et al. (2020). Enhancing the ROS generation ability of a rhodamine-decorated iridium(iii) complex by ligand regulation for endoplasmic reticulum-targeted photodynamic therapy. Chem. Sci. 11 (44), 12212–12220. doi:10.1039/D0SC04751A
Zhu, S.-y., Yao, R.-q., Li, Y.-x., Zhao, P.-y., Ren, C., Du, X.-h., et al. (2020). Lysosomal quality control of cell fate: a novel therapeutic target for human diseases. Cell Death Dis. 11 (9), 817. doi:10.1038/s41419-020-03032-5
Zhu, Z., Wang, Q., Liao, H., Liu, M., Liu, Z., Zhang, Y., et al. (2021). Trapping endoplasmic reticulum with amphiphilic AIE-active sensor via specific interaction of ATP-sensitive potassium (K(ATP)). Natl. Sci. Rev. 8 (6), nwaa198. doi:10.1093/nsr/nwaa198
Zhu, J., Jiao, A., Li, Q., Lv, X., Wang, X., Song, X., et al. (2022). Mitochondrial Ca(2+)-overloading by oxygen/glutathione depletion-boosted photodynamic therapy based on a CaCO(3) nanoplatform for tumor synergistic therapy. Acta Biomater. 137, 252–261. doi:10.1016/j.actbio.2021.10.016
Zhu, Y., Gong, P., Wang, J., Cheng, J., Wang, W., Cai, H., et al. (2023). Amplification of lipid peroxidation by regulating cell membrane unsaturation to enhance chemodynamic therapy. Angew. Chem. Int. Ed. 62 (12), e202218407. doi:10.1002/anie.202218407
Zhu, X., Shi, Z., Mao, Y., Lächelt, U., and Huang, R. (2024a). Cell membrane perforation: patterns, mechanisms and functions. Small 20 (24), e2310605. doi:10.1002/smll.202310605
Zhu, Y., Wang, X., Feng, L., Zhao, R., Yu, C., Liu, Y., et al. (2024b). Intermetallics triggering pyroptosis and disulfidptosis in cancer cells promote anti-tumor immunity. Nat. Commu. 15 (1), 8696. doi:10.1038/s41467-024-53135-2
Zhu, L., Hu, J., Wu, X., Zhang, J., Xu, X., Huang, X., et al. (2025). Programmed enhancement of endogenous iron-mediated lysosomal membrane permeabilization for tumor ferroptosis/pyroptosis dual-induction. Nat. Commu. 16 (1), 3017. doi:10.1038/s41467-025-58124-7
Zhuang, Z., Dai, J., Yu, M., Li, J., Shen, P., Hu, R., et al. (2020). Type I photosensitizers based on phosphindole oxide for photodynamic therapy: apoptosis and autophagy induced by endoplasmic reticulum stress. Chem. Sci. 11 (13), 3405–3417. doi:10.1039/D0SC00785D
Zhuang, J., Wang, B., Chen, H., Zhang, K., Li, N., Zhao, N., et al. (2023). Efficient NIR-II type-I AIE photosensitizer for mitochondria-targeted photodynamic therapy through synergistic apoptosis-ferroptosis. ACS Nano 17 (10), 9110–9125. doi:10.1021/acsnano.2c12319
Zhuang, J., Liu, S., Li, B., Li, Z., Wu, C., Xu, D., et al. (2025). Electron transfer mediator modulates type II porphyrin-based metal-organic framework photosensitizers for type I photodynamic therapy. Angew. Chem. Int. Ed. 64 (9), e202420643. doi:10.1002/anie.202420643
Zimmermann, J. A., Lucht, K., Stecher, M., Badhan, C., Glaser, K. M., Epple, M. W., et al. (2024). Functional multi-organelle units control inflammatory lipid metabolism of macrophages. Nat. Cell Biol. 26 (8), 1261–1273. doi:10.1038/s41556-024-01457-0
Zou, Y., Chen, J., Luo, X., Qu, Y., Zhou, M., Xia, R., et al. (2024a). Porphyrin-engineered nanoscale metal-organic frameworks: enhancing photodynamic therapy and ferroptosis in oncology. Front. Pharmacol. 15, 1481168. doi:10.3389/fphar.2024.1481168
Zou, Y., Wu, J., Zhang, Q., Chen, J., Luo, X., Qu, Y., et al. (2024b). Recent advances in cell membrane-coated porphyrin-based nanoscale MOFs for enhanced photodynamic therapy. Front. Pharmacol. 15, 1505212–2024. doi:10.3389/fphar.2024.1505212
Zou, Y., Chen, J., Qu, Y., Luo, X., Wang, W., and Zheng, X. (2025a). Evolution of nMOFs in photodynamic therapy: from porphyrins to chlorins and bacteriochlorins for better efficacy. Front. Pharmacol. 16, 1533040. doi:10.3389/fphar.2025.1533040
Zou, Y., Zhang, Q., Chen, J., Wang, W., and Zheng, X. (2025b). Metal-organic frameworks-loaded indocyanine green for enhanced phototherapy: a comprehensive review. Front. Bioeng. Biotechnol. 13, 1601476. doi:10.3389/fbioe.2025.1601476
Keywords: photodynamic therapy, photosensitizers, reactive oxygen species, cellular organelles, subcellular organelles targeting
Citation: Tao J, Yuan Z and Zhou M (2025) Targeted photodynamic therapy: enhancing efficacy through specific organelle engagement. Front. Pharmacol. 16:1667812. doi: 10.3389/fphar.2025.1667812
Received: 17 July 2025; Accepted: 11 August 2025;
Published: 25 August 2025.
Edited by:
Jean-Michel Escoffre, Université de Tours, FranceReviewed by:
Wen Chen, Hengyang Normal University, ChinaSabarinathan Rangasamy, PSG College of Technology, India
Copyright © 2025 Tao, Yuan and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mengjiao Zhou, bWp6aG91MDIwN0BudHUuZWR1LmNu
 Jiawen Tao1
Jiawen Tao1 Mengjiao Zhou
Mengjiao Zhou