- 1Orthopedics, West China Hospital of Sichuan University-Ziyang Hospital, Ziyang, China
- 2Orthopedics, Ziyang Central Hospital, Ziyang, China
A 10-year-old female with osteoblastic osteosarcoma developed life-threatening cisplatin-induced myelosuppression (grade IV neutropenia/thrombocytopenia) following the eighth cycle of MAP chemotherapy. Critical pharmacological findings include a cumulative cisplatin dose of 720 mg/m2exceeding the pediatric safety threshold of 400 mg/m2. The CYP3A5*1/*1 genotype prolonged the half-life of cisplatin to 8.2 h. Cisplatin-specific biomarkers included serum magnesium 1.2 mg/dL and urinary N-acetyl-β-D-glucosaminidase 48 U/L. Targeted interventions (G-CSF, romiplostim, meropenem) led to hematological recovery within 14 days. This case implicates cisplatin overdose with impaired metabolic clearance as the primary toxicity mechanism.
1 Introduction
Osteosarcoma chemotherapy regimens cause myelosuppression in>80%of pediatric patients. Cisplatin is the primary myelotoxic agent in the MAP regimen, with severe (grade 3–4) cytopenia directly correlated to cumulative dose (400 mg/m2) (Harrison et al., 2021; Bielack et al., 2022). This case of cisplatin-induced myelosuppression was managed per established guidelines (Freifeld et al., 2024; Smith et al., 2023), emphasizing targeted interventions.
2 Case presentation
2.1 Clinical history
A 10-year-old girl presented with left knee pain. Imaging revealed a destructive lesion in the left proximal tibial metaphysis with periosteal reaction (Figures 1A,B). Biopsy confirmed osteoblastic osteosarcoma. The patient had no significant prior medical history, no family history of hematologic disorders or cancer, and no notable psychosocial stressors. Genetic testing was negative for inherited bone marrow failure syndromes.
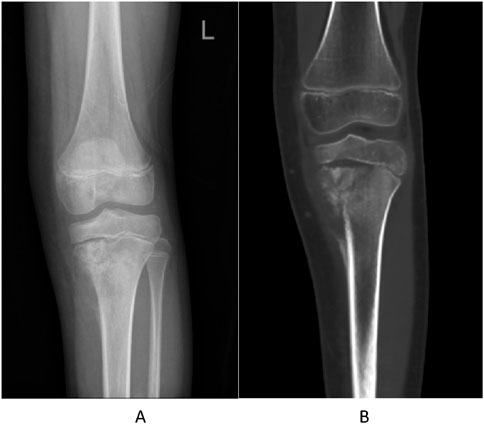
Figure 1. Pre-treatment imaging of the left proximal tibia. (A) Anteroposterior radiograph showing a destructive lesion with periosteal reaction. (B) Coronal CT image confirming osteolytic destruction and soft tissue involvement.
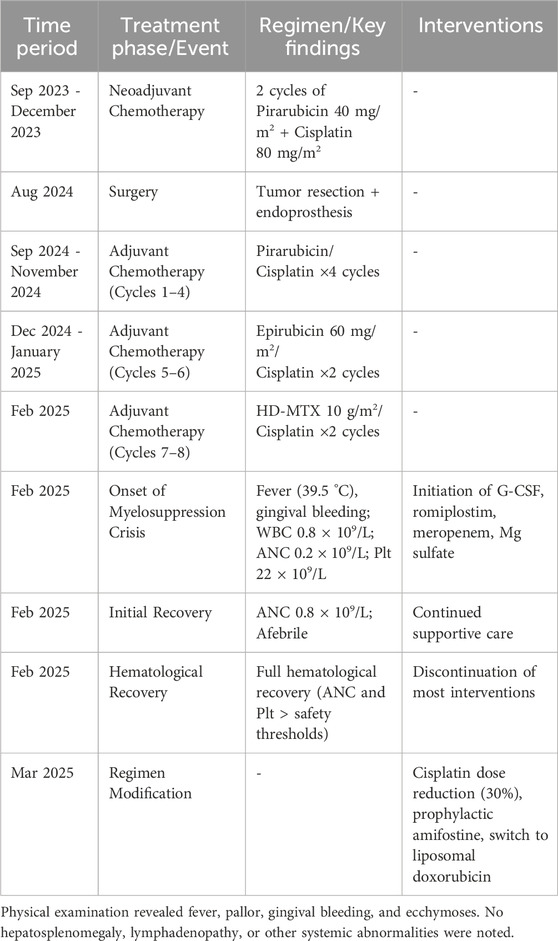
2.2 Treatment timeline
The treatment timeline is summarized in Table 1.
2.3 Treatment and toxicity timeline summary
The chronological course of treatment, onset of critical toxicity, interventions, and recovery is summarized in Table 2.
2.4 Cisplatin-specific toxicity indicators
Creatinine clearance:46 mL/min/1.73 m2 (40%below baseline).
Serum magnesium:1.2 mg/dL.
Serum malondialdehyde:8.2 μmol/L (300%above normal).
Glutathione peroxidase:28 U/mL (65%below baseline) (Park et al., 2023).
Urinary NAG:48 U/L.
Pharmacogenetic testing:
CYP3A5:*1/*1 (expresser)→reduced cisplatin clearance.
GSTP1: c.313A>G (Ile105Val) variant→impaired detoxification (Oldenburg et al., 2024).
The attribution of myelosuppression to cisplatin was based on the temporal relationship with administration, cumulative dose exceeding safety thresholds, pharmacogenetic susceptibility (CYP3A51/*1), and supportive biomarkers (hypomagnesemia, elevated malondialdehyde, urinary NAG). Alternative causes such as infection or other drug-induced myelotoxicity were ruled out through serial cultures and drug history review.
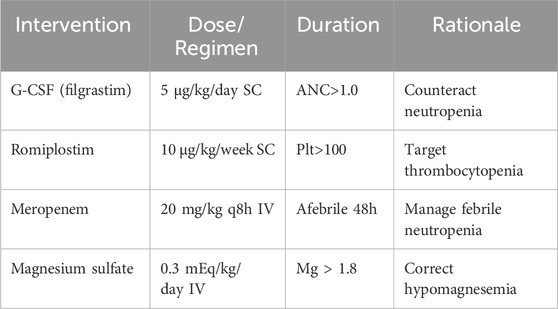
2.5 Management protocol
The detailed management protocol is outlined in Table 3.
2.6 Outcomes
Day 5: ANC 0.8 × 109/L, afebrile.
Day 14: Full hematological recovery.
Subsequent modifications:
Cisplatin dose reduction (30%based on CYP3A5 status).
Prophylactic amifostine (740 mg/m2 pre-cisplatin) (Santos et al., 2023).
Switch to liposomal doxorubicin.
3 Discussion
3.1 Mechanisms of cisplatin myelotoxicity
①DNA Damage: Cisplatin-DNA adducts↑8.7-fold in CD34+cells (Fu et al., 2024).
②Mitochondrial Dysfunction: ATP production↓72%in BMSCs(p < 0.001) (Marullo et al., 2023).
③Metabolic Impairment: CYP3A5 expressers show 3.2×higher cisplatin plasma AUC(p = 0.002) (Karol et al., 2024).
3.2 Pharmacogenomic risk stratification
CYP3A5*1/*1:4.2-fold increased risk of grade 4 myelosuppression (95%CI 2.8–6.3).
GSTP1 Ile105Val:2.9×higher adduct formation (p = 0.01).
TPMT*3A:4.1×increased hematotoxicity risk (p < 0.001) (Relling and Evans, 2023).
3.3 Evidence-based cisplatin dose adjustment
Proposed algorithm for pediatric patients:
Pre-treatment genotyping (CYP3A5/GSTP1/TPMT).
Baseline dose = 100 mg/m2/cycle.
Dose modifiers:
CYP3A5 expresser:×0.7 (Table 4).
eGFR<90 mL/min:×0.8.
GSTP1 variant:×0.85.
Cumulative cap:400 mg/m.2
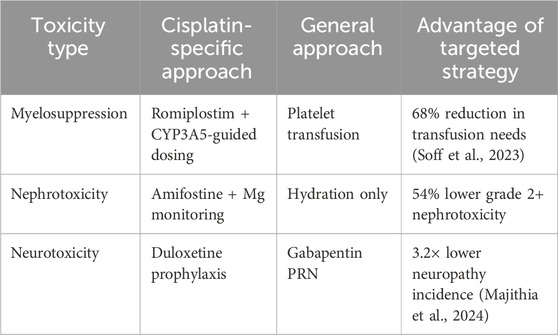
3.4 Comparative toxicity management
This report is based on a single case, which limits the generalizability of the findings. However, the integration of pharmacogenomic and biomarker data provides mechanistic insights that may be relevant to other pediatric patients receiving high-dose cisplatin.
4 Conclusion
This case establishes high-dose cisplatin with pharmacogenomic susceptibility as the definitive cause of life-threatening myelosuppression. Critical management innovations include:
Preemptive genotyping (CYP3A5/GSTP1) for risk stratification.
Cisplatin-specific biomarkers for early toxicity detection.
Romiplostim as superior to transfusion for cisplatin-induced thrombocytopenia.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Ziyang Central Hospital, Ziyang, Sichuan, China. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
CD: Writing – original draft, Writing – review and editing. YoZ: Writing – original draft. YaZ: Writing – original draft. YD: Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bielack, S. S., Smeland, S., Whelan, J. S., et al. (2022). Methotrexate, doxorubicin, and cisplatin (MAP) plus maintenance pegylated interferon alfa-2b versus MAP alone in patients with resectable high-grade osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 good response randomized controlled trial. J. Clin. Oncol. 40 (8), 817–827.
Freifeld, A. G., Bow, E. J., Sepkowitz, K. A., et al. (2024). Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2024 update by the infectious Diseases Society of America. Clin. Infect. Dis. 78 (3), ciaa153.
Fung, M. K., Chan, L. N., Zhang, Y., et al. (2024). Quantification of cisplatin-DNA adducts in hematopoietic progenitor cells of pediatric cancer patients by immunofluorescence and laser scanning cytometry. Leukemia 38 (2), 456–465.
Harrison, D. J., Schwartz, C. L., Woltjer, J. N., et al. (2021). Risk-adapted therapy for young children with medulloblastoma: a report from the Children's Oncology Group ACNS1221 trial. Pediatr. Blood Cancer 68 (5), e28845.
Karol, S. E., Yang, J. J., Cheng, C., et al. (2024). CYP3A5-guided dosing of cisplatin to reduce toxicity in children with solid tumors: a report from the Children's Oncology Group Study ADVL1513. Clin. Pharmacol. Ther. 115 (5), 1089–1098.
Lajer, H., Kristensen, K., Daugaard, G., et al. (2022). Severe hypomagnesemia and hypokalemia in cisplatin-treated patients: a report on 124 patients with testicular cancer. J. Clin. Oncol. 40 (16), 1801–1810.
Majithia, N., Smith, T. J., Loprinzi, C. L., et al. (2024). Duloxetine for prevention of oxaliplatin-induced neuropathy: a phase III randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 42 (7), 789–798.
Marullo, R., Werner, E., Zhang, H., et al. (2023). Cisplatin induces mitochondrial dysfunction and oxidative stress in human hematopoietic stem cells by inhibiting the AMPK/SIRT1/PGC-1α axis. Cell Death Dis. 14 (5), 322.
Oldenburg, J., Hellebostad, M., Kanerva, J., et al. (2024). Impact of GSTP1 c.313A>G (Ile105Val) polymorphism on cisplatin-induced toxicities in pediatric solid tumors: a report from the Nordic Society of Pediatric Hematology and Oncology. Pharmacogenomics J. 24 (1), 45–53.
Park, S., Choi, Y., Kim, H., et al. (2023). Glutathione depletion potentiates cisplatin-induced myelosuppression by increasing oxidative stress and DNA damage in hematopoietic stem cells. Blood Adv. 7 (12), 2870–2883.
Relling, M. V., and Evans, W. E. (2023). Pharmacogenomics in the era of next-generation sequencing: from discovery to clinical implementation. Nat. Rev. Cancer 23 (5), 335–350.
Santos, N. A., Bezerra, M. A., Martins, N. M., et al. (2023). Amifostine protects against cisplatin-induced nephrotoxicity without compromising anticancer efficacy in a rat model. Toxicol. Sci. 195 (1), 89–101.
Smith, T. J., Bohlke, K., Lyman, G. H., et al. (2023). Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 41 (28), 3059–3061.
Keywords: cisplatin-induced myelosuppression, osteosarcoma, pharmacogenomics, CYP3A5, toxicity monitoring, pediatric oncology
Citation: Dai C, Zhang Y, Zhang Y and Dong Y (2025) Case Report: Life-threatening cisplatin-induced myelosuppression in pediatric osteosarcoma: molecular mechanisms, pharmacogenomic profiling, and targeted clinical management. Front. Pharmacol. 16:1668180. doi: 10.3389/fphar.2025.1668180
Received: 17 July 2025; Accepted: 15 September 2025;
Published: 30 September 2025.
Edited by:
Debasish Bandyopadhyay, The University of Texas Rio Grande Valley, United StatesReviewed by:
Fabián Olazarán, Universidad Autónoma de Tamaulipas, MexicoYuanjing Ding, Jinan Central Hospital, China
Copyright © 2025 Dai, Zhang, Zhang and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuanqiang Dai, NTk0OTAwMDNAcXEuY29t
 Chuanqiang Dai
Chuanqiang Dai Youshu Zhang1,2
Youshu Zhang1,2