- 1Department of Pharmacy, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
- 2Research Center for Clinical Pharmacy, Zhejiang University, Hangzhou, China
- 3Department of Medical Oncology, Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
Background: The management of off-label use (OLU) is an important aspect of standardizing and promoting the rational use of antitumor drugs. Tumor anti-angiogenic therapy (AAT) is essential in cancer treatment. However, OLU of anti-angiogenic drugs is common in clinical practice. Recognizing and standardizing the OLU of AAT is a significant challenge that needs to be addressed in the clinic.
Aim: We aim to collect and categorize the OLU of AAT in oncology based on clinical guidelines, providing practical guidance for drug management.
Methods: We established a multidisciplinary expert team to screen and include evidence-based OLU information.
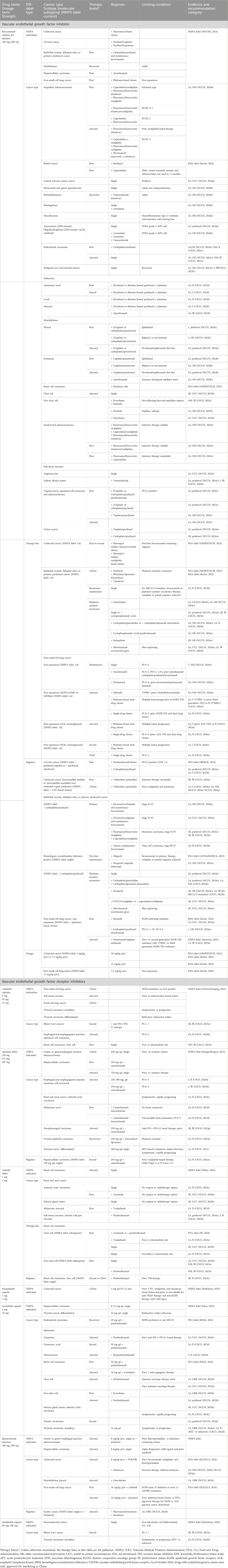
Results: By organizing the OLU information, it is evident that these 14 drugs of AAT have indications for 33 cancer types (36 items). Among them, 12 (85.7%) have OLU recommendations, totaling 215 items. These OLU recommendations were classified into four major categories. Cancer type is the most common OLU category, with 12 drugs covering 64 cancer types and 155 items. In addition, OLU in therapy lines, regimens, and dosage was identified in 3–4 drugs each. Bevacizumab, the drug most frequently involved in OLU, is associated with 12 cancer types, 3 therapy lines, 6 regimens, and 2 dosage-related OLU items. In addition, lenvatinib, pazopanib, sorafenib, apatinib, and sunitinib also have OLU recommendations for more than five cancer types.
Conclusion: Our work offers an updated reference for the OLU of tumor AAT and highlights the need for further exploration into specific management measures for OLU in clinical practice.
Introduction
Off-label use (OLU) of medications, also known as the unregistered use of a drug, occurs when a drug is prescribed for indications, dosages, treatment courses, routes of administration, or patient populations that fall outside the scope of its approved label as authorized by drug regulatory authorities (Maschke, 2018). In recent years, China has implemented a series of policies to regulate the OLU of anti-tumor drugs. The Law for Licensing Medical Practitioners of the People’s Republic of China (P.R.C.), originally enacted in 1999, was amended in 2021. This amendment marked the first legal protection for evidence-based off-label drug use in China. From 2018 to 2024, the National Health Commission (NHC) of the P.R.C. has issued annual guidelines for the clinical application of novel anti-tumor drugs, incorporating indications approved in other countries or regions. In 2021, the NHC released the Administrative Measures for the Clinical Application of Anti-Tumor Drugs (Trial) and the Guiding Principles for the Clinical Application of New Anti-Tumor Drugs to further standardize the management of off-label drug use. Scholars have also issued guidelines for off-label drug use recommendations, which primarily encompass general guidelines and those specifically tailored for pediatric or ophthalmic medications (Meng et al., 2022a; Li et al., 2022; Zuo et al., 2022; Singh et al., 2024; Meng et al., 2022b). Additionally, professional societies in China, such as the Guangdong Pharmaceutical Association, Zhejiang Pharmaceutical Association, Shandong Pharmaceutical Association, Sichuan Pharmaceutical Association, and Medical Association, have published several directories and expert consensuses on off-label drug use (Shi et al., 2024; International Medical Society and Breast Cancer Group Branch of Oncologist Chinese Medical Doctor Association, 2022), providing valuable references for the OLU of drugs. However, among these catalogs, one catalog with the most comprehensive inclusion of tumor anti-angiogenic therapy (AAT) lists a total of 19 off-label items for 7 drugs. Another catalog includes nine items for five drugs of tumor AAT. The remaining catalog does not include any drug for tumor AAT.
AAT represents a critical class of anticancer agents, which is widely used in clinical practice (Liu et al., 2023). Due to its unique mechanism of action, AAT can be effectively combined with cytotoxic drugs and tumor immunotherapy agents, offering broad application potential (Li and Fang, 2024). By October 2024, 14 drugs of AAT had been approved for use in China. However, the approved indications for drugs of AAT in China are fewer than those recommended by international guidelines, leading to the frequent OLU in real-world settings (Fontanals et al., 2023; Levêque, 2016). In clinical practice, identifying and managing the OLU of AAT remains challenging. One of the primary reasons is the slow progress in establishing hospital-level systems for documenting and approving OLU. Additionally, the lack of up-to-date reviews and summaries of OLU evidence further complicates this issue (Shi et al., 2024).
The Oncology Pharmacy Group of the Hospital Pharmacy Committee of the Zhejiang Pharmaceutical Society has issued a series of consensus statements on the off-label use of specific drugs of AAT, such as recombinant human endostatin, anlotinib, pazopanib, and regorafenib in China. However, with the rapid accumulation of new clinical evidence and the increasing number of AAT drugs entering the market, the existing off-label consensus requires updating. To address this need, we established a multidisciplinary expert writing group to systematically classify and summarize the evidence for 14 drugs of tumor AAT. This initiative aims to provide an updated and comprehensive reference for the clinical OLU of AAT in oncology.
Aim
In this narrative review, we screened and collected the most recent drug label information and guideline recommendations for the use of 14 drugs of AAT in oncology. These findings were systematically organized and summarized by drug, with a particular focus on OLU details, recommended sources, and the corresponding levels of evidence.
Methods
Data sources
Two investigators independently collected drug names, drug labels, and guideline documents according to the following data collection steps. After completion, a third investigator compared and verified the completeness of the collected data.
We identified 14 AAT drugs approved and marketed in China before October 2024 through the National Medical Products Administration (NMPA) database (https://english.nmpa.gov.cn/database.html). For each drug, we obtained the latest prescribing information (current as of October 2024) from China (https://db.yaozh.com/instruct), the United States (https://www.fda.gov/drugs), and the European Union (https://www.ema.europa.eu). Details of drug label versions/dates are provided in Supplementary Table S1.
We collected the latest tumor treatment guidelines (current as of October 2024). The primary guidelines included the following: Chinese Society of Clinical Oncology (CSCO) guidelines (https://www.csco.org.cn), National Comprehensive Cancer Network (NCCN) guidelines (https://www.nccn.org), and oncology diagnostic and therapeutic guidelines issued by the China National Health Commission (China, 2023).
Guideline and drug label inclusion/exclusion criteria
Inclusion criteria were as follows: NMPA labels for the 14 drugs of AAT available at that time; FDA and EMA labels in cases where discrepancies existed in indications, treatment lines, regimens, or dosage, compared with the NMPA labels; and guidelines that formally recommended any of the 14 AAT drugs as oncology treatment regimens. Exclusion criteria were as follows: drug labels from non-originator manufacturers; historical versions of labels; FDA and EMA product information consistent with the NMPA labeling; and guidelines with recommendations not involving OLU of the 14 AAT drugs. The full process of the guideline and label search is shown in Figure 1.
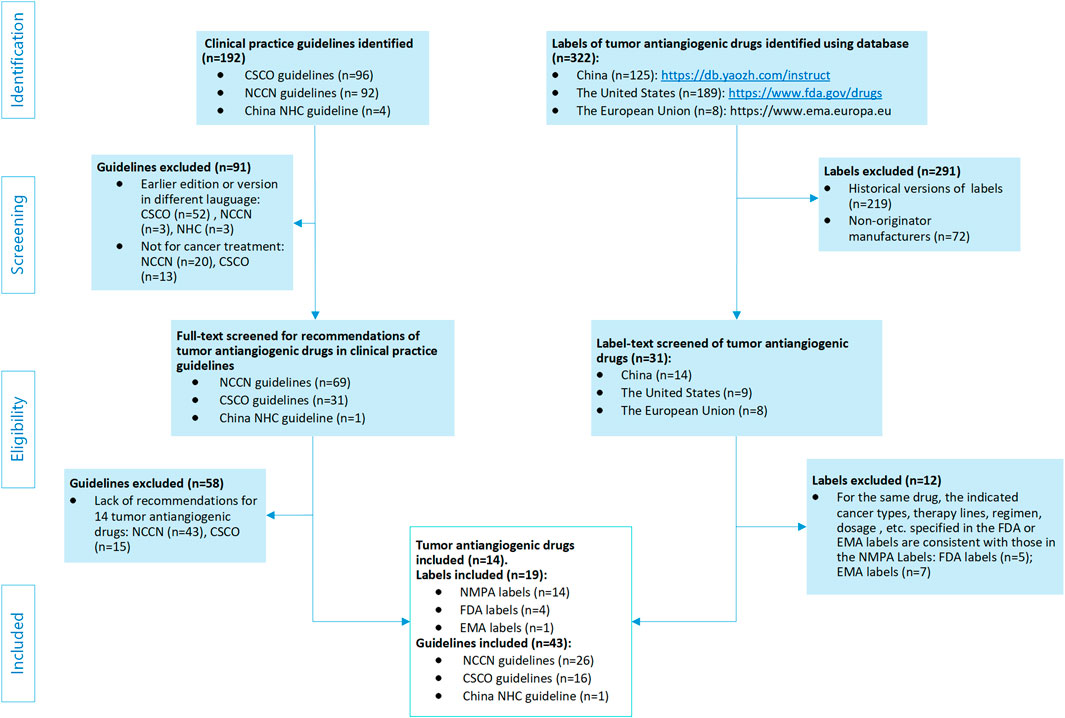
Figure 1. Flowchart of the guideline and drug-label screening process. NMPA, P.R.C National Medical Products Administration; FDA, U.S. Food and Drug Administration; EMA, European Medicines Agency; CSCO, Chinese Society of Clinical Oncology; NCCN, National Comprehensive Cancer Network; NHC, China National Health Commission.
Off-label use categorization framework
Two independent investigators evaluated all collected data using an abstraction form (Supplementary Table S2). OLU was defined as meeting any of the following criteria compared with NMPA-approved indications: cancer type(s) not included in the approved indications; treatment lines differing from the approved sequence; combination therapy regimens not specified in the label; dosage regimens deviating from the approved prescribing information.
As shown in Figure 2, identified OLU cases were hierarchically categorized based on the scope of divergence (broad to narrow): cancer type (unapproved indication), therapy line (unapproved treatment sequence), regimen (unapproved combination therapy), and dosage (unapproved dosing). Each case was assigned exclusively to the most comprehensive applicable category. Dosage OLU implied on-label cancer type, therapy line, and regimen. Regimen OLU implied on-label cancer type and therapy line. Therapy line OLU implied on-label cancer type. This hierarchical classification ensured mutually exclusive categorization across all OLU types. Python 3.12 software was used to perform a kappa agreement test on the OLU classifications of the two investigators. For OLU classification items with disputes, the final category was determined through joint discussion by the article writing team in accordance with the initial workflow criteria.
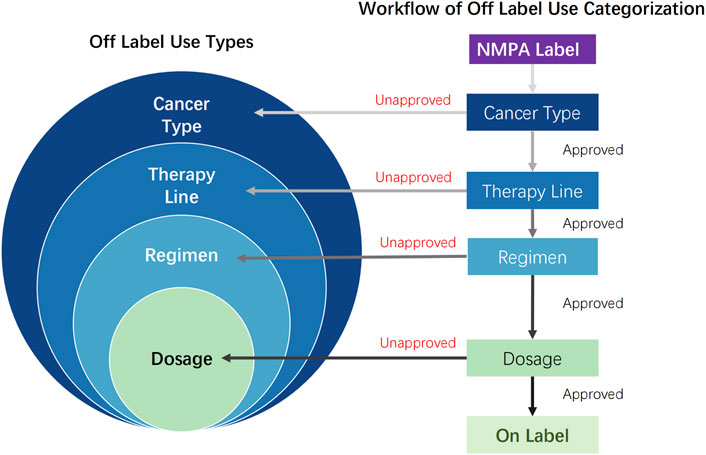
Figure 2. Off-label use types and workflow of off-label use categorization: off-label use cases were hierarchically categorized based on the scope of divergence (broad to narrow), cancer type (unapproved indication), therapy line (unapproved treatment sequence and on-label cancer type), regimen (unapproved combination therapy, on-label cancer type, and therapy line), and dosage (unapproved dosing, on-label cancer type, therapy line, and regimen). NMPA, P.R.C National Medical Products Administration; approved, refers to content described in the NMPA drug label; unapproved, content not included in the NMPA drug label.
Evidence classification and recommendation grading adhered to the original guideline standards. When an OLU entry has recommendations from different guidelines, we list all the recommendations. Where recommendations lacked explicit evidence levels, we applied the CSCO evidence classification system. For a quick understanding of the classification of recommendations and evidence, we compiled a table (Supplementary Table S3) that outlines the corresponding recommendation categories and evidence levels. For detailed information, please check the official websites.
Results
Initially, we identified 192 oncology clinical guidelines and 322 drug product information documents. After multi-round screening (Figure 1), we finally included 19 AAT drug labels [14 NMPA labels (ROCHE, 2024; ChiaTaiTianqing, 2024; JiangsuHengrui, 2023; Pfizer, 2024; Hutchison, 2023; Eisai, 2022; EliLilly, 2022; Hutchison, 2024; SuzhouZelgen, 2022; Novartis, 2021; Bayer, 2021; Bayer, 2018; PFIZER, 2022; Simcere, 2020) and 5 AAT labels from other regions (Roche, 2022; GENENTECH, 2022; PF, 2024; EISAI, 2024; ELILILLY, 2024; NOVARTIS, 2024; CPPI, 2024)], 3 non-AAT labels (MERCK, 2024; Innovent, 2023; ASTRAZENECA, 2023), and 43 guidelines (NCCN, 2024b; NCCN, 2024d, NCCN, 2024p, NCCN, 2024v; CSCO, 2023c, NCCN, 2024o; CSCO, 2023h; CSCO, 2023f, NCCN, 2024l, NCCN, 2024k; NCCN, 2025b; CSCO, 2023e, NCCN, 2024r, NCCN, 2024s; CSCO, 2023b; NCCN, 2025c, NCCN, 2024w, NCCN, 2024n; CSCO, 2024e; CSCO, 2024a; NCCN, 2024e, NCCN, 2024q; CSCO, 2023a; CSCO, 2024b; CSCO, 2024c; CSCO, 2023g; CSCO, 2021; CSCO, 2022a; NCCN, 2024h; NCCN, 2024i, NCCN, 2024t, NCCN, 2024u; CSCO, 2022b; NCCN, 2024f; NCCN, 2025a; NCCN, 2024g; CSCO, 2023d, NCCN, 2024j; NCCN, 2024c; CSCO, 2024d; NCCN, 2024a, NCCN, 2024m) for OLU evaluation. After aggregation, the tumor types across the indications of the 14 drugs of tumor AAT were summed, resulting in a total of 33 tumor types involving 36 entries. Among the 14 drugs, 12 had OLU recommendations, with a total of 215 OLU entries determined (Cohen’s kappa = 0.917) (Supplementary Table S4, where each row, except NMPA labels in the table, is considered one entry or item).
Off-label use of vascular endothelial growth factor inhibitors
Currently, there are two anti-vascular endothelial growth factor (VEGF) antibody drugs available: bevacizumab, approved by the U.S. Food and Drug Administration (FDA) in 2004, and aflibercept, approved in 2011 (Hida et al., 2024). Bevacizumab and aflibercept were approved for marketing in China in 2010 and 2013, respectively. Bevacizumab is widely used in oncological treatments, while aflibercept is primarily indicated for ophthalmological conditions in China (Hang et al., 2023).
In the field of antitumor therapy, the main OLU of bevacizumab involves indications (Table 1). There are 12 cancer types and 57 items involving bevacizumab recommended by guidelines that are not included in the NMPA-approved indications. Additionally, OLU related to treatment regimens includes 22 entries across 6 therapy lines in 4 cancer types. Exceeding the recommended therapy lines was observed in the palliative treatment of three cancer types. Furthermore, differences in dosing practices for specific indications across geographical regions have resulted in variations in the recommended dosages specified in product inserts.
Off-label use of vascular endothelial growth factor receptor inhibitors
The scope of vascular endothelial growth factor receptor (VEGFR) inhibitors in China includes a total of seven drugs: anlotinib, apatinib, axitinib, fruquintinib, lenvatinib, ramucirumab, and surufatinib. The predominant form of OLU among these drugs was related to indications (Table 1). OLU involving exceeding standard treatment regimens or therapy lines was relatively infrequent. Notably, there were no OLU recommendations for fruquintinib.
Among the VEGFR inhibitors, lenvatinib had the highest number of OLU recommendations for cancer types, with a total of seven uses beyond its labeled indications. This was followed by apatinib, which had six off-label recommendations for cancer types. Anlotinib, axitinib, and ramucirumab each had three off-label recommended cancer types, while surufatinib had two.
In terms of treatment regimens, anlotinib, axitinib, and ramucirumab each had one recommended regimen item that constituted OLU. Recommendations for OLU related to therapy lines were rare among VEGFR inhibitors, with axitinib being the only drug for which a recommendation transitioned subsequent lines of therapy to first-line use.
Off-label use of multi-kinase targeted tumor anti-angiogenic drugs
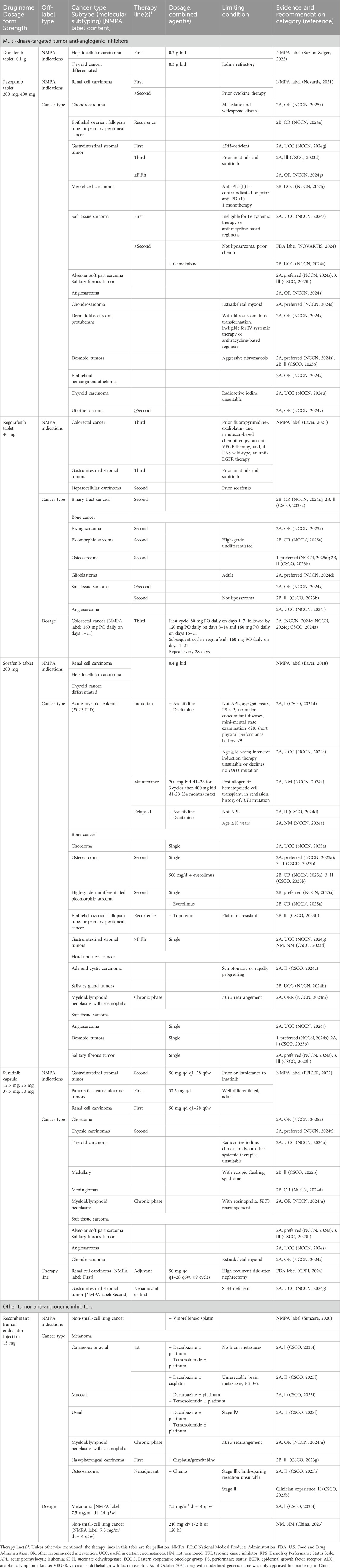
The multi-kinase targeted included a total of five drugs: donafenib, pazopanib, regorafenib, sorafenib, and sunitinib.
Except for donafenib, which had no OLU recommendations, the remaining four drugs had recommendations for use beyond their approved tumor types (Table 2). Notably, pazopanib and sorafenib had off-label recommendations for seven distinct cancer types. Sunitinib followed closely, with OLU recommendations for six cancer types and nine items. Regorafenib was recommended for OLU in four cancer types, comprising eight items, and also had one OLU recommendation related to dosage.
Among these five drugs, sunitinib was the only one with OLU recommendations for therapy lines, specifically for adjuvant or neoadjuvant therapies in two cancer types.
Off-label use of other tumor anti-angiogenic inhibitors
Endostatin is a potent endogenous inhibitor of angiogenesis. It is a 20 kDa C-terminal fragment of collagen XVIII, a component of the basement membrane. Endostar, a recombinant human endostatin, was launched in China on 23 July 2006 (Han et al., 2011).
The OLU recommendations for recombinant human endostatin primarily involved indications beyond the approved cancer types and dosages. There were 4 cancer types and 11 items recommended for OLU. Additionally, there were two tumor types with OLU recommendations related to dosage.
Discussion
AAT is an essential component of malignant tumor treatment (Liu et al., 2023). However, some AAT drugs, particularly those with limited market availability, insufficient evidence, or limited clinical experience, are frequently used off-label (Fontanals et al., 2023; Van Norman, 2023; Zarkavelis et al., 2023; Liu et al., 2024). This comprehensive narrative review shows that OLU is highly prevalent across a diverse range of tumor AAT, primarily manifesting through four distinct aspects. The use of AAT drugs beyond approved labels reflects the rapid evolution of clinical evidence and the necessity of addressing complex and diverse patient needs in oncology (Gray et al., 2009).
Based on the OLU table, the most common type of OLU is cancer types. This is also the type of OLU that clinical practice pays the most attention to. There are a total of 64 recommendations for the OLU of cancer type for 14 drugs. Although we had only included recommendations for OLU of AAT in oncology, bevacizumab has emerged as the drug most frequently recommended for OLU, with 12 cancer types and 57 documented items. Lenvatinib, pazopanib, and sorafenib also show significant OLU, mainly within expanded indications. This trend highlights the clinicians’ efforts to explore new therapeutic applications for these agents beyond their currently approved cancer types, potentially offering new hope to patients with challenging diseases (Fontanals et al., 2023; Wang et al., 2025).
In contrast, the OLUs for therapy line, regimen, and dosage were relatively sparse, with each OLU category typically involving 3–4 drugs. Furthermore, in most instances, the number of associated items was also limited. This may be attributed to our setting, wherein each OLU situation could only be assigned to one type of OLU, following a classification method that prioritizes cancer type first, then therapy line, followed by regimen, and ultimately dosage. Although the span of OLU of the therapy line is considered smaller than the cancer type, it is still important to clarify whether the earlier use of later-line treatment drugs can bring clinical benefits. In addition, there should be sufficient evidence to determine whether AAT should be continued in the subsequent line of treatment following the failure of the previous drug regimen. Modification of combination therapies involves the strategic use of AAT in conjunction with chemotherapy, immunotherapy, or other agents in treatment regimens not explicitly specified in the prescribing information. Such modifications reflect the attempts to optimize treatment efficacy by leveraging the synergistic effects of different therapeutic modalities. These combinations may provide enhanced anti-tumor activity and potentially improve patient outcomes in complex clinical scenarios. Adjustments in dosage and administration are commonly implemented based on careful assessments of treatment efficacy and patient tolerance, particularly when the drugs are used in combination with other therapies (Overbeek et al., 2023). Scholars have previously proposed a nine-category OLU classification method, which more comprehensively encompasses the content of statutory product information (Chen et al., 2024). However, since some categories have minimal relevance to OLU recommendations for AAT, we adopted a more concise four-category method in this study. Future investigations may warrant consideration of a more comprehensive OLU evaluation framework.
The lack of high-quality evidence is a significant concern (Liang and Cai, 2025) as many off-label uses are based on small studies or case reports, lacking the support of large-scale randomized controlled trials. Safety concerns arise as off-label uses may increase the risk of adverse reactions, such as hypertension, proteinuria, and bleeding. Ethical and legal issues also loom large as off-label uses can lead to medical disputes, particularly in the absence of clear informed consent (Liang and Cai, 2025). Additionally, economic burdens can be substantial as anti-angiogenic drugs are expensive, potentially increasing the financial burden on patients (Shi et al., 2024; Gordon et al., 2021). Thus, OLU should adhere to the following principles: in the absence of other effective treatment options, OLU should be applied for therapeutic purposes, prioritizing the patient’s best interests; physicians must inform patients of the specific drug, reasons, and risks associated with OLU and obtain explicit informed consent; off-label practices must be supported by safety and efficacy evidence from evidence-based medicine; medical institutions should establish management mechanisms and strictly follow off-label drug use procedures; and OLU should not be conducted for the purpose of clinical trials, scientific research, or the personal interests of medical staff (Liang and Cai, 2025).
Limitations
Although we attempted to include the most current and up-to-date recommended evidence, readers are reminded that this is not a systematic review. The perspective on off-label recommendations presented in our review is based on authoritative guidelines and, as such, may lack the most cutting-edge research evidence. Furthermore, the tumor anti-angiogenic drugs included in this review were all products marketed in China, which may differ from those in other regions.
Conclusion
Our work offers an updated reference for the OLU of tumor AAT and highlights the need for further exploration into specific management measures for OLU in clinical practice.
Author contributions
DY: Writing – original draft, Writing – review and editing, Funding acquisition, Formal analysis, Data curation, Conceptualization. YH: Methodology, Data curation, Software, Writing – review and editing. SW: Project administration, Writing – review and editing, Supervision, Validation, Conceptualization, Resources. TW: Validation, Writing – review and editing, Formal analysis, Software, Data curation. TZ: Validation, Writing – review and editing, Investigation, Data curation. LL: Formal analysis, Data curation, Validation, Writing – review and editing. HL: Data curation, Formal analysis, Writing – review and editing, Investigation. WH: Writing – review and editing, Project administration, Conceptualization, Methodology. HD: Conceptualization, Writing – review and editing, Methodology, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Zhejiang Provincial Natural Science Foundation of China under the grant no. ZCLQ24H0901 and the Research Project on High-Quality Development of Hospital Pharmacy, National Institute of Hospital Administration, NHC, China (no. NIHAYSZX2533).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1668620/full#supplementary-material
References
ASTRAZENECA (2023). Olaparib Tablets package insert. U.S. Food Drug Adm. website. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/208558s028lbl.pdf (Accessed June 30, 2024).
BAYER (2018). Sorafenib tosylate tablets [package insert]. P.R.C national medical products administration. Bayer. Ag., 11–16.
BAYER (2021). Regorafenib tablets [package insert]. P.R.C national medical products administration: bayer AG, 7–29.
Chen, X., Ou, S., Luo, J., He, Z., and Jiang, Q. (2024). Advancing perspectives on the off-label use of anticancer drugs: an updated classification and exploration of categories. Front. Pharmacol., 15–2024. doi:10.3389/fphar.2024.1374549
CHIATAITIANQING (2024). Anlotinib hydrochloride capsules [package insert]. Beijing: P.R.C national medical products administration: chia tai tianqing pharmaceutical group Co. Ltd.
CPPI (2024). Sunitinib malate capsules [package insert]. U.S. Food Drug Adm. website. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021938s039lbledt.pdf (Accessed June 30, 2024).
CSCO (2021). CSCO guidelines for diagnosis and treatment of differentiated thyroid cancer 2021. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2022a). CSCO guidelines for diagnosis and treatment of hepatocellular carcinoma 2022. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2022b). CSCO guidelines for diagnosis and treatment of medullary thyroid carcinoma 2022. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2023a). CSCO guidelines for diagnosis and treatment of biliary tract cancer 2023. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2023b). CSCO guidelines for diagnosis and treatment of bone and soft tissue tumors 2023. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2023c). CSCO guidelines for diagnosis and treatment of endometrial carcinoma 2023. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2023d). CSCO guidelines for diagnosis and treatment of gastrointestinal stromal tumors 2023. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2023e). CSCO guidelines for diagnosis and treatment of kidney cancer 2023. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2023f). CSCO guidelines for diagnosis and treatment of melanoma 2023. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2023g). CSCO guidelines for diagnosis and treatment of nasopharyngeal carcinoma 2023. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2023h). CSCO guidelines for Diagnosis and treatment of Ovarian cancer 2023. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2024a). CSCO guidelines for Diagnosis and treatment of Colorectal cancer 2024. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2024b). CSCO guidelines for diagnosis and treatment of esophageal cancer 2024. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2024c). CSCO guidelines for diagnosis and treatment of Head and Neck cancer 2024. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2024d). CSCO guidelines for Diagnosis and treatment of Hematological malignancies 2024. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
CSCO (2024e). CSCO guidelines for Diagnosis and treatment of non-small cell lung cancer 2024. Available online at: https://www.csco.org.cn (Accessed October 31, 2024).
EISAI (2022). Lenvatinib Mesilate Capsules [package insert]. P.R.C National medical products administration: Eisai GmbH, 2022–29.
EISAI (2024). “Lenvatinib Capsules [package insert],”. U.S. Food Drug Adm. website. EISAI INC. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/206947s031lbl.pdf (Accessed October 30, 2024).
ELILILLY (2022). Ramucirumab Injection [package insert]. Beijing: P.R.C National medical products administration. Eli Lilly and Company, 2022–2030.
ELILILLY (2024). “Ramucirumab Injection [package insert],”. U.S. Food Drug Adm. website. ELI LILLY AND CO. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125477s042lbl.pdf (Accessed June 30, 2024).
Fontanals, S., Esteve, A., GonzáLEZ, A., IbáñEZ, C., MartíNEZ, J., MesíA, R., et al. (2023). Real-world treatment outcomes of medicines used in special situations (off-label and compassionate use) in oncology and hematology: a retrospective study from a comprehensive cancer institution. Cancer Med. 12, 17112–17125. doi:10.1002/cam4.6360
GENENTECH (2022). Bevacizumab Injection [package insert]. U.S. Food Drug Adm. website. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125085s340lbl.pdf (Accessed June 30, 2024).
Gordon, N., Goldstein, D. A., Tadmor, B., Stemmer, S. M., and Greenberg, D. (2021). Factors associated with off-label oncology prescriptions: the role of cost and financing in a universal healthcare system. Front. Pharmacol., 12–2021. doi:10.3389/fphar.2021.754390
Gray, S. W., Armstrong, K., Demichele, A., Schwartz, J. S., and Hornik, R. C. (2009). Colon cancer patient information seeking and the adoption of targeted therapy for on-label and off-label indications. Cancer 115, 1424–1434. doi:10.1002/cncr.24186
Han, B., Xiu, Q., Wang, H., Shen, J., Gu, A., Luo, Y., et al. (2011). A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy of paclitaxel-carboplatin alone or with endostar for advanced non-small cell lung cancer. J. Thorac. Oncol. 6, 1104–1109. doi:10.1097/JTO.0b013e3182166b6b
Hang, A., Feldman, S., Amin, A. P., Ochoa, J. A. R., and Park, S. S. (2023). Intravitreal anti-vascular endothelial growth factor therapies for Retinal Disorders. Pharm. (Basel) 16, 1140. doi:10.3390/ph16081140
Hida, K., Maishi, N., Matsuda, A., and Yu, L. (2024). Beyond starving cancer: anti-angiogenic therapy. J. Med. Ultrasonics 51, 605–610. doi:10.1007/s10396-023-01310-1
HUTCHISON (2023). Fruquintinib Capsules [package insert]. Beijing: P.R.C National medical products administration: Hutchison Medi Pharma(Shanghai) Co. Ltd., 2023–19.
HUTCHISON (2024). Surufatinib Capsules [package insert]. Beijing: P.R.C National medical products administration: Hutchison Medi Pharma(Shanghai) Co. Ltd., 2024–15.
INNOVENT (2023). Sintilimab Injection [package insert]. Beijing: P.R.C National medical products administration: Innovent Biologics (Suzhou) Co. Ltd., 2023–16.
International Medical SocietyBreast Cancer Group, Branch of Oncologist, Chinese Medical Doctor Association (2022). Expert consensus on off-label use of small molecule anti-angiogenic drugs in the treatment of metastatic breast cancer. Zhonghua Zhong Liu Za Zhi 44, 523–530. doi:10.3760/cma.j.cn112152-20220310-00168
JIANGSUHENGRUI (2023). “Apatinib Mesylate Tablets [package insert]. Beijing: P.R.C National medical products administration,”, 2023-3-3. Jiangsu Hengrui Pharmaceuticals Co., Ltd.
Levêque, D. (2016). Off-label use of targeted therapies in oncology. World J. Clin. Oncol. 7, 253–257. doi:10.5306/wjco.v7.i2.253
Li, A.-Q., and Fang, J.-H. (2024). Anti-angiogenic therapy enhances cancer immunotherapy: mechanism and clinical application. Interdiscip. Med. 2, e20230025. doi:10.1002/inmd.20230025
Li, G., Wang, N., Zhang, Y., Wei, W., Lu, H., Zhai, S., et al. (2022). Recommendations for off-label drug Use in Ophthalmology in China: a clinical practice guideline. Front. Pharmacol. 13, 919688. doi:10.3389/fphar.2022.919688
Liang, S., and Cai, F. (2025). Off-label drug use in China after the Physician Law (2021): legal challenges and solutions. Front. Pharmacol., 16–2025. doi:10.3389/fphar.2025.1547418
Liu, Z.-L., Chen, H.-H., Zheng, L.-L., Sun, L.-P., and Shi, L. (2023). Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 8, 198. doi:10.1038/s41392-023-01460-1
Liu, R., Wang, L., Rizzo, S., Garmhausen, M. R., Pal, N., Waliany, S., et al. (2024). Systematic analysis of off-label and off-guideline cancer therapy usage in a real-world cohort of 165,912 US patients. Cell Rep. Med. 5, 101444. doi:10.1016/j.xcrm.2024.101444
Meng, M., Liu, E., Zhang, B., Lu, Q., Zhang, X., Ge, B., et al. (2022a). Guideline for the management of pediatric off-label use of drugs in China (2021). BMC Pediatr. 22, 442. doi:10.1186/s12887-022-03457-1
Meng, M., Zhou, Q., Lei, W., Tian, M., Wang, P., Liu, Y., et al. (2022b). Recommendations on off-label drug use in pediatric guidelines. Front. Pharmacol., 13–2022. doi:10.3389/fphar.2022.892574
MERCK (2024). “Pembrolizumab Injection package insert,”. U.S. Food Drug Adm. website. MERCK SHARP DOHME. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/125514s155lbl.pdf (Accessed June 30, 2024).
NCCN (2024a). NCCN clinical practice guidelines in oncology: acute Myeloid leukemia (version 3. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024b). NCCN clinical practice guidelines in oncology: Ampullary Adenocarcinoma (V.2.2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024c). NCCN clinical practice guidelines in oncology: Biliary Tract cancers (version 4. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024d). NCCN clinical practice guidelines in oncology: Central Nervous system cancers (version 3. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024e). NCCN clinical practice guidelines in oncology: Colon cancer (version 5. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024f). NCCN clinical practice guidelines in oncology: Gastric cancer (version 4. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024g). NCCN clinical practice guidelines in oncology: Gastrointestinal Stromal tumors (version 2. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024h). NCCN clinical practice guidelines in oncology: Head and Neck cancers (version 4. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024i). NCCN clinical practice guidelines in oncology: Melanoma: Cutaneous (version 3. 2024) Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024j). NCCN clinical practice guidelines in oncology: Merkel cell Carcinoma (version 1. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024k). NCCN clinical practice guidelines in oncology: Mesothelioma: Peritoneal (version 1. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024l). NCCN clinical practice guidelines in oncology: Mesothelioma: Pleural (version 2. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024m). NCCN clinical practice guidelines in oncology: Myeloid/Lymphoid neoplasms with eosinophilia and tyrosine kinase gene fusions (version 2. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024n). NCCN clinical practice guidelines in oncology: non-small cell lung cancer (version 11. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024o). NCCN clinical practice guidelines in oncology: ovarian cancer/fallopian tube cancer/primary peritoneal cancer (version 3. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024p). NCCN clinical practice guidelines in oncology: pediatric central nervous system cancers (version 1. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024q). NCCN clinical practice guidelines in oncology: rectal cancer (version 4. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024r). NCCN clinical practice guidelines in oncology: small bowel Adenocarcinoma (version 5. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024s). NCCN clinical practice guidelines in oncology: soft tissue Sarcoma (version 3. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024t). NCCN clinical practice guidelines in oncology: Thymomas and Thymic Carcinomas (version 1. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024u). NCCN clinical practice guidelines in oncology: Thyroid carcinoma (version 4. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024v). NCCN clinical practice guidelines in oncology: Uterine Neoplasms (version 3. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2024w). NCCN clinical practice guidelines in oncology: vulvar cancer (version 4. 2024). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2025a). NCCN clinical practice guidelines in oncology: bone cancer (version 1. 2025). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2025b). NCCN clinical practice guidelines in oncology: kidney cancer (version 2. 2025). Available online at: www.nccn.org (Accessed October 31, 2024).
NCCN (2025c). NCCN clinical practice guidelines in oncology: vaginal cancer (version 2. 2025). Available online at: www.nccn.org (Accessed October 31, 2024).
NOVARTIS (2021). Pazopanib Tablets [package insert]. Beijing: P.R.C National medical products administration. Novartis Pharma Schweiz AG, 12–31.
NOVARTIS (2024). Pazopanib Tablets [package insert]. U.S. Food Drug Adm. website. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/022465s036lbl.pdf (Accessed October 30, 2024).
Overbeek, J. K., Ter Heine, R., Verheul, H. M. W., Chatelut, E., Rudek, M. A., Gurney, H., et al. (2023). Off-label, but on target: the evidence needed to implement alternative dosing regimens of anticancer drugs. ESMO Open 8, 100749. doi:10.1016/j.esmoop.2022.100749
PF (2024). Axitinib Tablets package insert. U.S. Food Drug Adm. website. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/202324s016lbl.pdf (Accessed October 30, 2024).
PFIZER (2022). “Sunitinib malate Capsules [package insert]. Beijing: P.R.C National medical products administration,”, 2022-7-29. PFIZER EUROPE MA EEIG.
PFIZER (2024). Axitinib Tablets [package insert]. P.R.C National medical products administration. Pfizer Eur. MA EEIG.
ROCHE (2022). “Bevacizumab 25 mg/ml concentrate for solution for infusion [package insert],”. Amsterdam: European Medicines Agency, 11–29.
ROCHE (2024). Bevacizumab Injection [package insert]. P.R.C National medical products administration: roche Pharma(Schweiz)AG; 2024-10-11.
Shi, C., Yang, Y., Wu, C., Wang, L., Dong, Y., Qi, Y., et al. (2024). Guidance documents for off-label drug use management for Chinese health care institutions: a scoping review. J. Evid. Based Med. 17, 808–821. doi:10.1111/jebm.12669
SIMCERE (2020). Recombinant human endostatin injection [package insert]. Beijing: P.R.C National medical products administration: Shandong Simcere Bio-pharmaceutical Co. Ltd., 12–30.
Singh, S., Saxena, S., Akduman, L., and Meyer, C. H. (2024). Off-label use of intravitreal bevacizumab: a global conundrum. Indian J. Ophthalmol. 72, 617–619. doi:10.4103/IJO.IJO_2166_23
SUZHOUZELGEN (2022). Donafenib Tosilate Tablets [package insert]. Beijing: P.R.C national medical products administration: suzhou zelgen biopharmaceuticals Co. Ltd., 2022–16.
Van Norman, G. A. (2023). Off-label use vs off-label marketing of drugs: Part 1: off-label use—patient harms and prescriber Responsibilities. Basic Transl. Sci. 8, 224–233. doi:10.1016/j.jacbts.2022.12.011
Wang, G., Wang, Y., Jin, C., and Sun, X. (2025). Off-label use of anlotinib in malignancies’ treatment: efficacy and management of adverse reactions. Pharmacol. Rep. 77, 392–408. doi:10.1007/s43440-025-00700-1
Zarkavelis, G., Amylidi, A. L., Verbaanderd, C., Cherny, N. I., Metaxas, Y., DE Vries, E. G. E., et al. (2023). Off-label despite high-level evidence: a clinical practice review of commonly used off-patent cancer medicines. ESMO Open 8, 100604. doi:10.1016/j.esmoop.2022.100604
Keywords: anti-angiogenic therapy, off-label use, drug label, guidelines, drug management
Citation: Yao D, Hu Y, Weng S, Wang T, Zhu T, Liu L, Luo H, He W and Dai H (2025) Off-label use catalogue of tumor anti-angiogenic drugs in China: a narrative review. Front. Pharmacol. 16:1668620. doi: 10.3389/fphar.2025.1668620
Received: 18 July 2025; Accepted: 02 September 2025;
Published: 14 October 2025.
Edited by:
Nor Eddine Sounni, University of Liège, BelgiumReviewed by:
Xiaoqiang Xiang, Fudan University, ChinaCopyright © 2025 Yao, Hu, Weng, Wang, Zhu, Liu, Luo, He and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haibin Dai, aGFpYmluZGFpQHpqdS5lZHUuY24=; Wei He, MjE5ODAzOUB6anUuZWR1LmNu; Difei Yao, eWFvZGlmZWkxMDIxQHpqdS5lZHUuY24=
 Difei Yao
Difei Yao Yangmin Hu
Yangmin Hu Shanshan Weng
Shanshan Weng Tiantian Wang1
Tiantian Wang1 Huan Luo
Huan Luo Haibin Dai
Haibin Dai