- 1Department of Pharmacy, Affiliated Hospital of Southwest Jiaotong University, The Third People’s Hospital of Chengdu, Chengdu, Sichuan, China
- 2Department of Clinical Pharmacy and Pharmacy Administration, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan, China
- 3Department of Pharmacy, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan, China
Background: Gabapentin (GAB) is an adjunctive antiepileptic drug widely used in pediatric patients. However, little is known about its pharmacokinetics in pediatric patients under 3 years old or with renal impairment (RI). To address this, we developed a physiologically based pharmacokinetic (PBPK) model for precise dosing guidance.
Methods: A PBPK model for GAB was first developed in healthy adults using PK-Sim® and then extended to pediatric populations, accounting for age-related physiological changes. For RI simulations, reduced glomerular filtration and tubular secretion were incorporated based on adult RI models.
Results: The PBPK model accurately predicted GAB exposure in adults and children after single and multiple administration (geometric mean fold error <2). Plasma concentrations and PK parameters were similar in children under 3 years old and those aged 3-12. In pediatric RI patients under 12 years old, AUC0-
Conclusion: PBPK models provide better guidance for GAB dosing in pediatric patients with varying RI degrees, laying a foundation for precision therapy. This study is a significant step in optimizing GAB treatment for this high-risk pediatric population.
1 Introduction
Gabapentin (GAB) is a gamma-aminobutyric acid analogue that has been available since December 1993 (Swearingen et al., 2018). GAB is excreted in its original form from the systemic circulation through the kidneys, and its renal clearance (CL) is directly proportional to the creatinine clearance rate (Ccr). The changes in renal function will affect GAB pharmacokinetics (PK). When renal function is impaired, glomerular filtration rate (GFR) and tubular secretion are generally reduced due to kidney damage, leading to decreased GAB clearance and increased drug exposure (Blum et al., 1994). Based on guidance from the US Food and Drug Administration (FDA), the dosing of GBA in adult patients should be adjusted with a Ccr <60 mL/min (FDA, 2019). These dosing recommendations are a reduction in the total daily dose and a prolonged dosing interval for GAB.
The pharmacologic management of epilepsy in children is quite complex in clinical practice. GAB is indicated for use as an adjunctive antiepileptic drug in the treatment of complex partial seizures, with or without secondary generalization, in patients over 12 years of age. It is also approved for use as adjunctive therapy in treating partial seizures in pediatric patients aged 3–12 years (Tallian et al., 2004), but the use of GAB in children under 3 years of age is limited. The increasing use of GAB in pediatric centers necessitates further characterization of its PK in pediatric patients to determine dosing and optimize treatment, especially in pediatric patients with renal impairment (RI). However, in children with RI, appropriate dosing has not been established, which raises the risk of treatment.
Pediatric doses are typically derived from adult doses. Extrapolating adult dosages to children is challenging because it involves considering the developmental changes and maturation processes that affect drug absorption, distribution, metabolism, and excretion (Schijvens et al., 2020). There is no basis for extrapolating the recommended dose for adults with RI to children with RI. As a unique population, clinical PK trials tend to exclude children with or without RI and do not specifically analyze PK differences. Physiologically based pharmacokinetic (PBPK) models can be utilized to forecast PK profiles in simulated special populations and assist in making informed decisions about dosing strategies (Grimstein et al., 2019). PBPK models precisely assess age-related changes in anatomy and physiology, detailing the size, composition, and blood flow of various tissues and organs. This is particularly useful for understanding the change in CL in pediatric patients with limited data. The application of small-molecule drugs for pediatric patients has been extensively researched using PBPK models (Guo et al., 2023). A PBPK model for GAB has not been built for pediatric patients of different ages and renal function.
Therefore, this study aimed to create a PBPK model of GAB. This PBPK model was used to predict the PK of GAB in pediatric patients of differing ages and varying degrees of renal function and to establish a simulations-based dosage regimen. This approach aims to support extrapolated dose recommendations for GAB in children aged 1 month to 3 years or with RI.
2 Materials and methods
2.1 PBPK modeling development workflow and computer software
2.1.1 Workflow
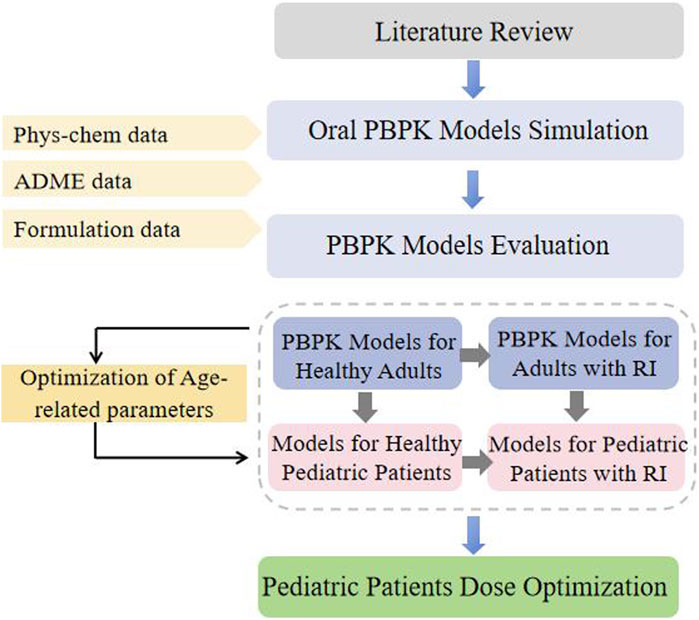
This study adopted a guidance-based workflow for PBPK modeling development in pediatric patients. A “top-down” strategy was used to facilitate modeling establishment (Figure 1). A PBPK modeling of GAB was developed and evaluated in healthy adults for p.o. Administration. The pediatric model is constructed by applying age-related changes in physiological parameters using the software’s built-in calculation methods. Afterward, the established healthy adults’ PBPK modeling of GAB was extrapolated to patients with RI by adjusting GFR, kidney size, and tubule secretion (TSspec). At this stage, GAB’s physicochemical and ADME properties remained the same. The simulated plasma exposure of virtual populations was compared with the observed concentration data to assess the accuracy of the PBPK modeling.
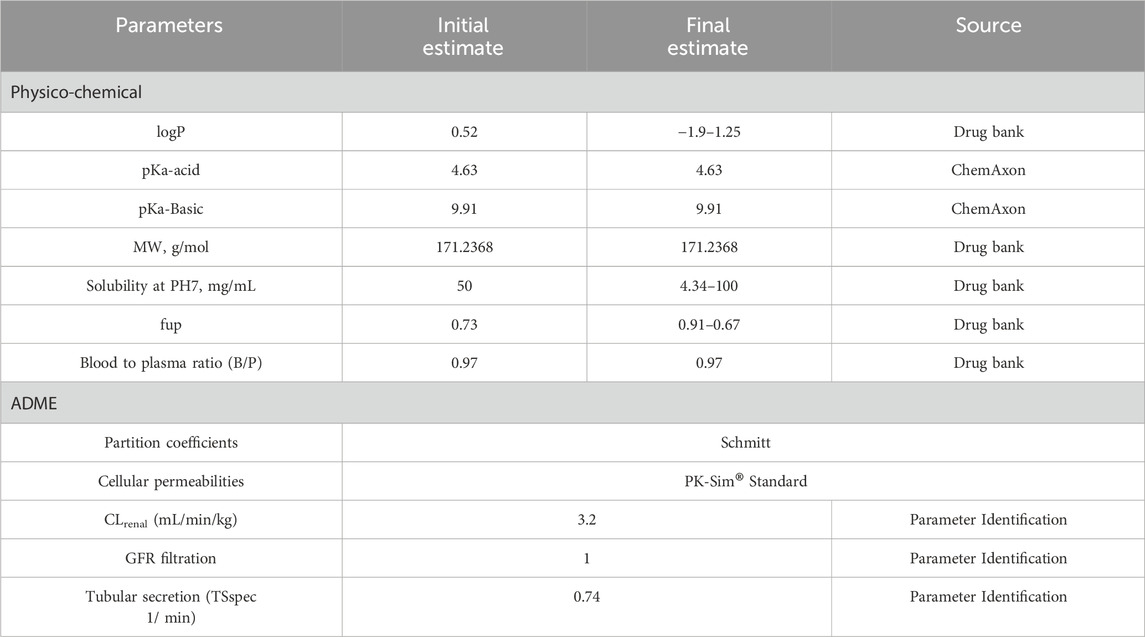
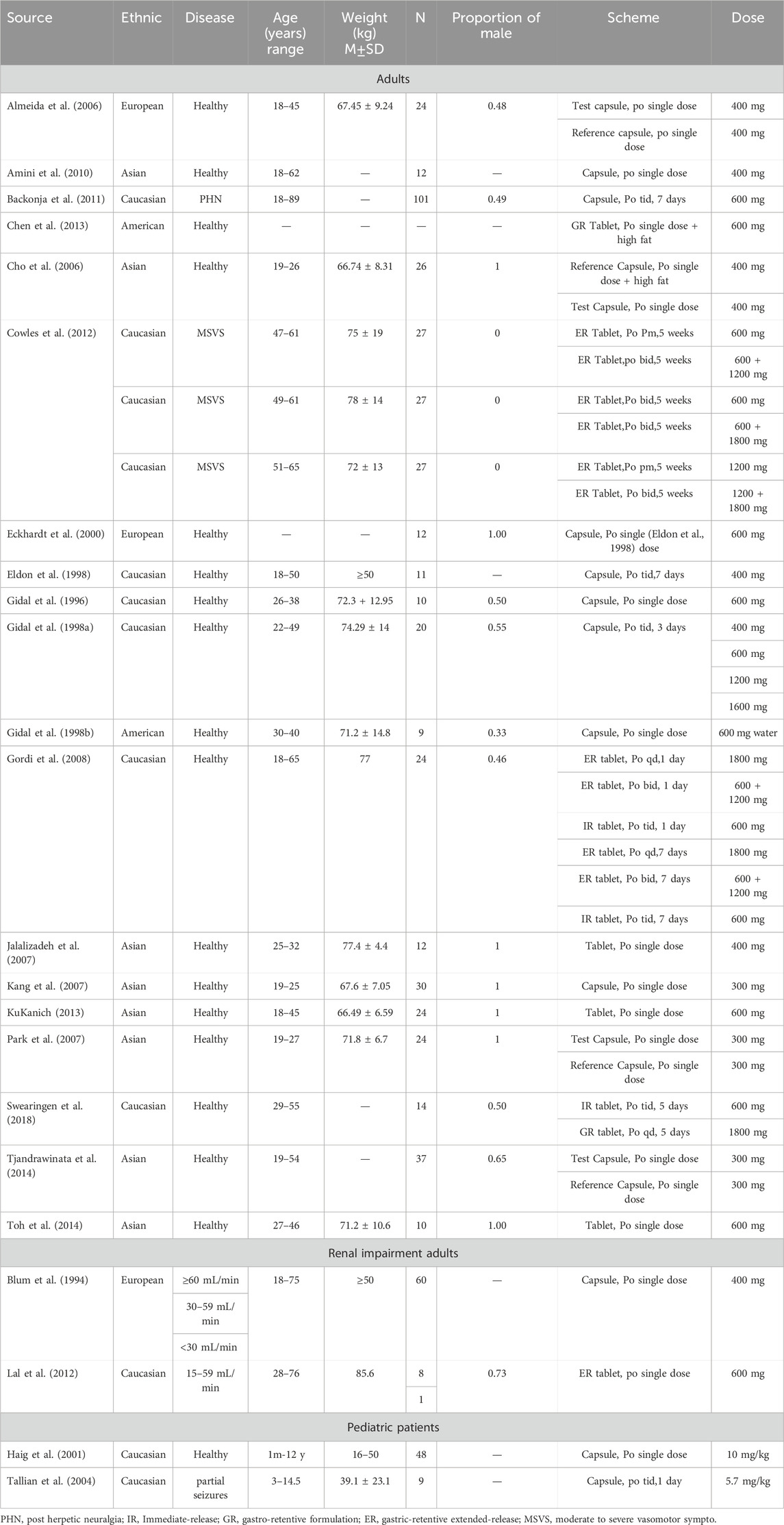
GAB’s physicochemical properties, ADME characteristics, and reported PK profiles following oral administration were obtained from literature searches in the DrugBank, Web of Science, PubMed, and Medline databases using the keywords “gabapentin” and “pharmacokinetics” (Table 1). The unique keywords for the pediatric patients were “pediatric,” “child,” “children,” “newborn,” “infant,” “adolescent,” or “teenager.” The collected literature was organized according to the dosing regimen and experimental data, and we excluded the study on determining GAB plasma concentration by the microbial method.
2.1.2 Software
PBPK Modeling was conducted using the whole-body PBPK framework in the PK-Sim software (version 9.0, 2020, www.open-systems-pharmacology.org). Plasma concentration-time profile data were obtained from published literature using GetData Graph Digitizer (S. Fedorov, version 2.25.0.32). Some optimization outputs were manually adjusted for specific parameters to enhance the visual fit of the data. The model’s performance was verified through visual inspection of the predicted and observed plasma concentration-time curves and by comparing predicted PK parameter values with those observed in clinical PK studies.
2.2 Gabapentin modeling development
2.2.1 PBPK model for healthy adults
We selected preexisting “healthy” populations and developed PBPK models for the oral administration of GAB in healthy adults. The represented GFR of healthy adults was 100 mL/min/1.73 m2. We used the Monte Carlo method to generate predicted values, and adopted a parameter identification method based on real-world observed data to estimate the renal clearance. We conducted single-dose simulations using GAB doses of 300 mg, 400 mg, and 600 mg. Additionally, we simulated multi-dose regimens for this population with the following doses: 600 mg and 1800 mg once daily (qd), 400 mg, 600 mg, 1200 mg, and 1600 mg three times daily (tid). We also included a regimen of 600 mg and a combination of 600 mg and 1200 mg taken twice daily (bid). The PBPK models for GAB were verified using clinical studies’ PK data.
2.2.2 PBPK model for adults with RI
We utilized three levels of RI as recommended by FDA guidelines: mild, moderate, and severe. These categories were classified based on the GFR ranges: 30-59, 15-29, and <15 mL/min/1.73 m2. We created a virtual population of four adult patients with RI and set the representative GFR to 30, 15, and 5 mL/min/1.73 m2, respectively. We estimated the renal filtration rate using the method of fup*GFR, which is the product of the concentration of unbound drugs in plasma (fup) and the glomerular filtration rate. However, this model could not satisfactorily simulate the clearance rate of GAB, which only considers GFR. Therefore, we added parameters for renal tubular secretion. We conducted both single-dose and multi-dose simulations at various levels of renal function. The accuracy of the PBPK models was validated by comparing them with existing clinical data.
2.2.3 PBPK model for pediatric patients with normal renal function
The PBPK models for healthy individuals with normal renal function were adjusted for age, height, and weight to create models suitable for pediatric patients with normal renal function. Age-related changes in physiological parameters were applied using the software’s built-in calculation methods. Based on a previous PK study, simulations were conducted for three age groups: children aged 1 month to less than 3 years, 3-12 years, and 12-17 years. Multi-dose simulations were performed for each age group, with simulated doses set at a single dose of 10 mg/kg and 5.7 mg/kg tid. The dosing regimens and demographics of pediatric patients with normal renal function are detailed in Table 2. Clinical observation data confirmed the accuracy of the models.
2.2.4 PBPK model for pediatric patients with RI
Based on the PBPK models for adults with RI and children with normal renal function, we developed PBPK models for pediatric patients with varying degrees of RI: mild, moderate, and severe. To simulate drug exposure in this population, we selected a dosing regimen of 5 mg/kg administered tid, which corresponds to the recommended dosage range for healthy children as specified on the GAB label. The alterations in other physiological parameters attributable to RI in pediatric patients were assessed using the default values provided in PK-sim.
2.3 GAB modeling verification
Population simulations were conducted using PK-Sim to evaluate the predictive performance of the final models. This evaluation involved comparing the model outcomes with clinically observed data from other literature following various dosing regimens. The predicted and observed concentration-time profiles were compared through visual inspection, and the plasma drug concentration data fit was assessed. PK parameters of observed values were obtained from geometric or arithmetic means reported in the literature. If no PK parameters were reported, the intercepted plasma drug concentration values were used to fit by non-compartment analysis. The Equations 1, 2 were used to compare the differences between predicted and observed values of PK parameters Cmax, tmax, area under the plasma concentration-time curve (AUC0-
According to conventional criteria, the model is considered acceptable if none of the predicted PK parameters exceed the corresponding observed value >2.0-fold. To further evaluate the model, we also conducted a (Equation 3) analysis for the GAB adult model using PK-Sim®. The sensitivity of parameters was calculated as follows.
An increase of 10% in the tested parameter (p) results in a corresponding 10% increase in the predicted PK parameter, according to a sensitivity value of +1.0.
2.4 Dose recommendations for pediatric patients
We used a PBPK model to establish the appropriate administration regimen for GAB in children under 3 years old. Next, we compared the drug exposure resulting from a 300 mg tid dose in adolescents over 12 years old. Finally, we evaluated potential dosing regimens for pediatric patients with different levels of renal function. Using the PBPK model, we simulated the recommended GAB dose for those with RI and compared their drug exposure to that of healthy children receiving a dose of 5 mg/kg tid. We predicted Cmax and AUC values for various regimens in a simulation involving 1,000 subjects.
3 Results
3.1 PBPK modeling for healthy adult
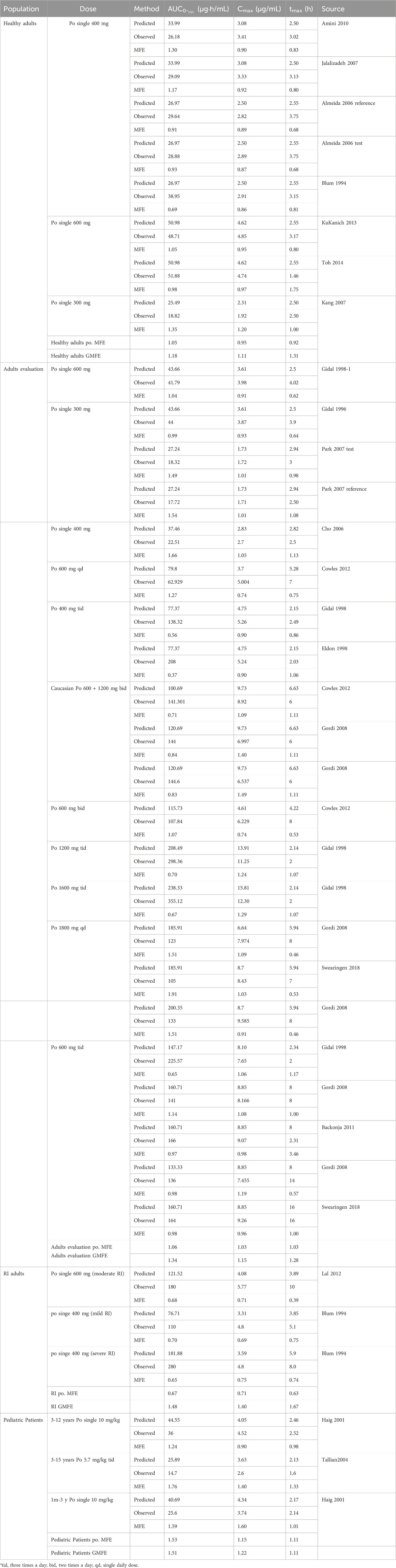
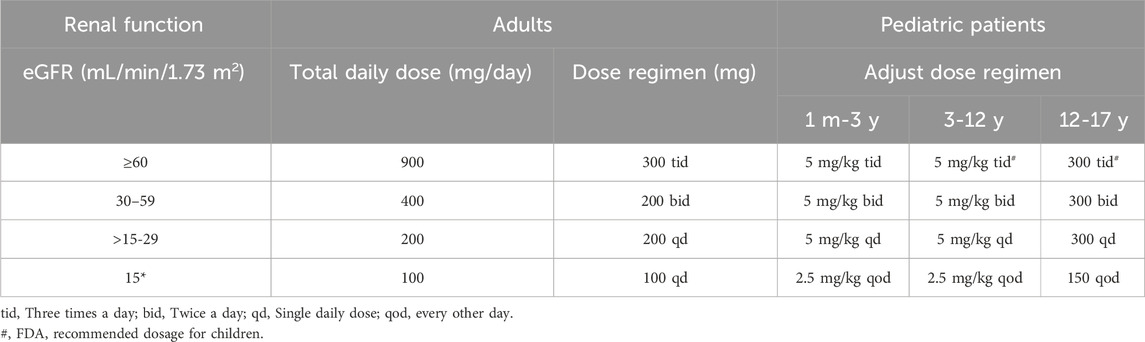
We successfully constructed a PBPK model for healthy adults and validated it using clinical PK data. The established adult GAB PBPK model is shown in Supplementary Figure S1, which fits a single p.o well—administration at different doses of GAB on visual inspection. The GMFE of AUC0-
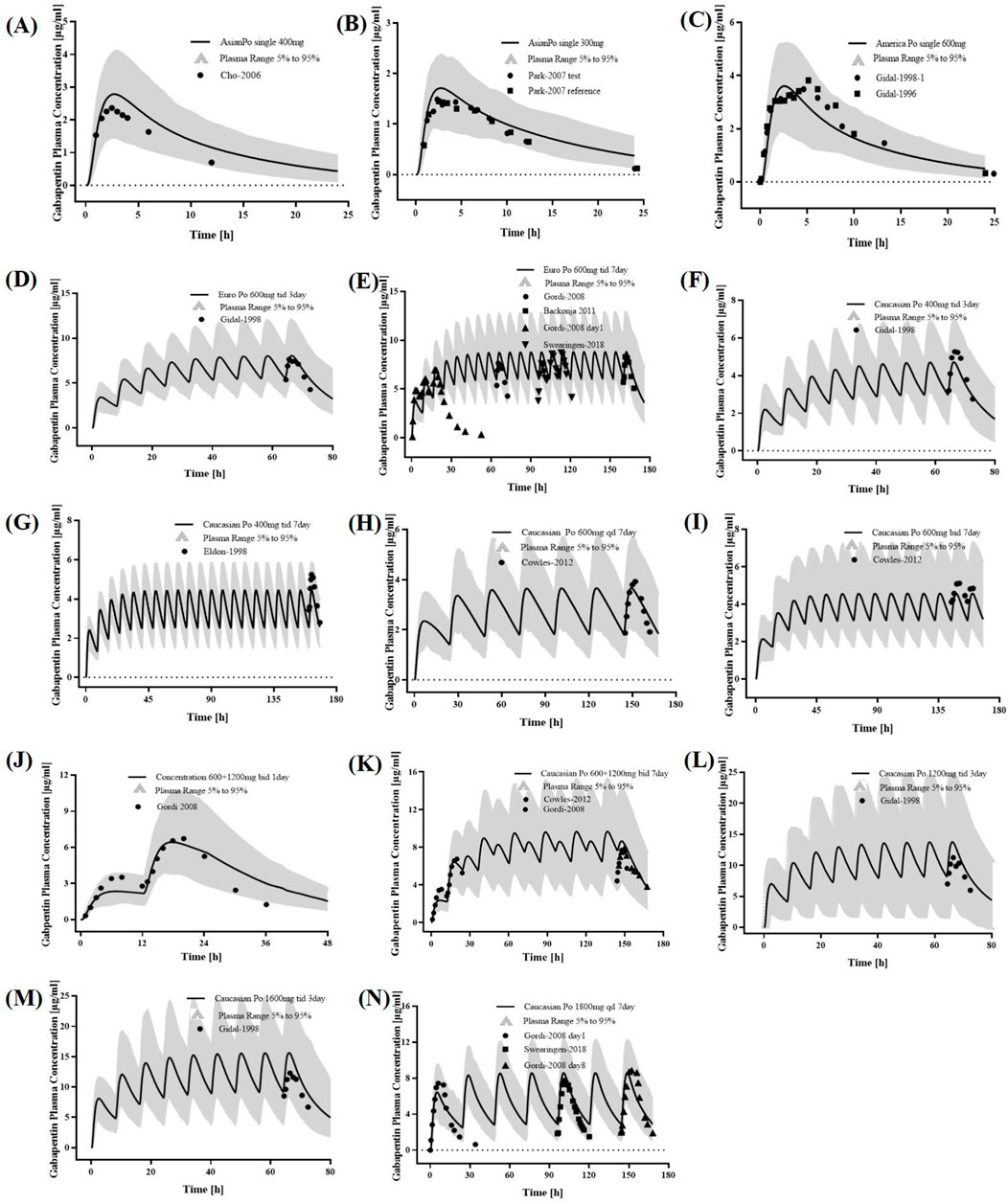
Figure 2. PK profiles in the healthy adult population. The po. administration at a single dose of (A) 400 mg, (B) 300 mg gabapentin in Asians; The po. administration at a single dose of (C) 600 mg gabapentin in White Americans; The po. administration at a dose of (D) 600 mg tid 3 days and (E) 600 mg tid 7 days gabapentin in Europeans; The po. administration at a dose of (F) 400 mg tid 3 days, (G) 400 mg tid 7 days, (H) 600 mg qd 7 days, (I) 600 mg bid 7 days, (J) 600 + 1,200 mg bid 1 day, (K) 600 + 1,200 mg bid 7 days, (L) 1,200 mg tid 3 days, (M) 1600 mg tid 3 days, and (N) 1800 mg qd 7 days gabapentin in caucasian. The observed concentration data were provided as the arithmetic mean values extracted from references. The solid line represented the predicted mean concentration, and the shaded area represented the predicted 5th to 95th percentile range.
3.2 PBPK modeling for adults with RI
The PBPK model incorporated key physiological parameter changes associated with RI, including GFR, kidney size, and TSspec. As renal dysfunction intensifies, its related parameters decrease more (Supplementary Table S2). The plasma concentration predicted by the PBPK model for adults with RI aligned well with the observed values (Figure 3). The GMFE of PK parameters AUC0-
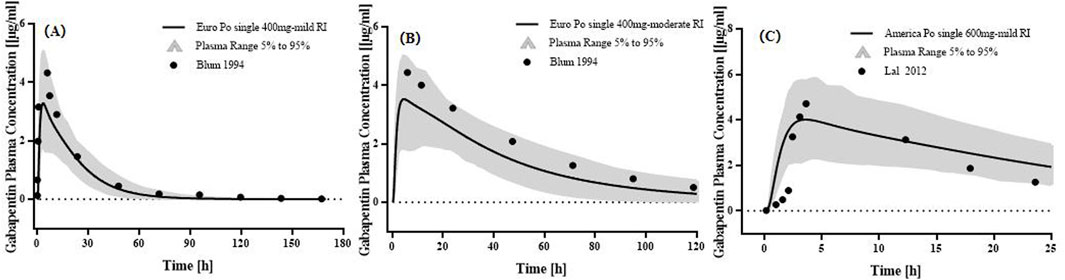
Figure 3. PK profiles in the adults with different levels of RI. The po. administration at a singe dose of (A) 400 mg gabapentin in Europeans with mild RI; The po. administration at a single dose of (B) 400 mg gabapentin in Europeans with moderate RI; The po. administration at a singe dose of (C) 600 mg gabapentin in White Americans with mild RI. The observed concentration data were provided as the arithmetic mean values extracted from references. The solid line represented the predicted mean concentration, and the shaded area represented the predicted 5th to 95th percentile range.
3.3 PBPK modeling for pediatric patients with or without RI
Two studies of p.o. The administration of GAB in Caucasians was used to optimize physiological parameters (Figure 4). The simulation results showed that the placement of age-related physiological parameters is suitable for GAB in pediatric patients. The GMFE of PK parameters AUC0-
Figure 4. PK profiles in the pediatric patients with normal renal function. The po. administration at (A) a single dose of 10 mg/kg gabapentin in 1 month-3 years Americans; The po. administration at (B) a single dose of 10 mg/kg gabapentin in 3–12 years Americans; The po. administration at (C) a dose of 5.7 mg/kg tid gabapentin in 3–15 years Americans. The observed concentration data were provided as the arithmetic mean values extracted from references. The solid line represented the predicted mean concentration, and the shaded area represented the predicted 5th to 95th percentile range.
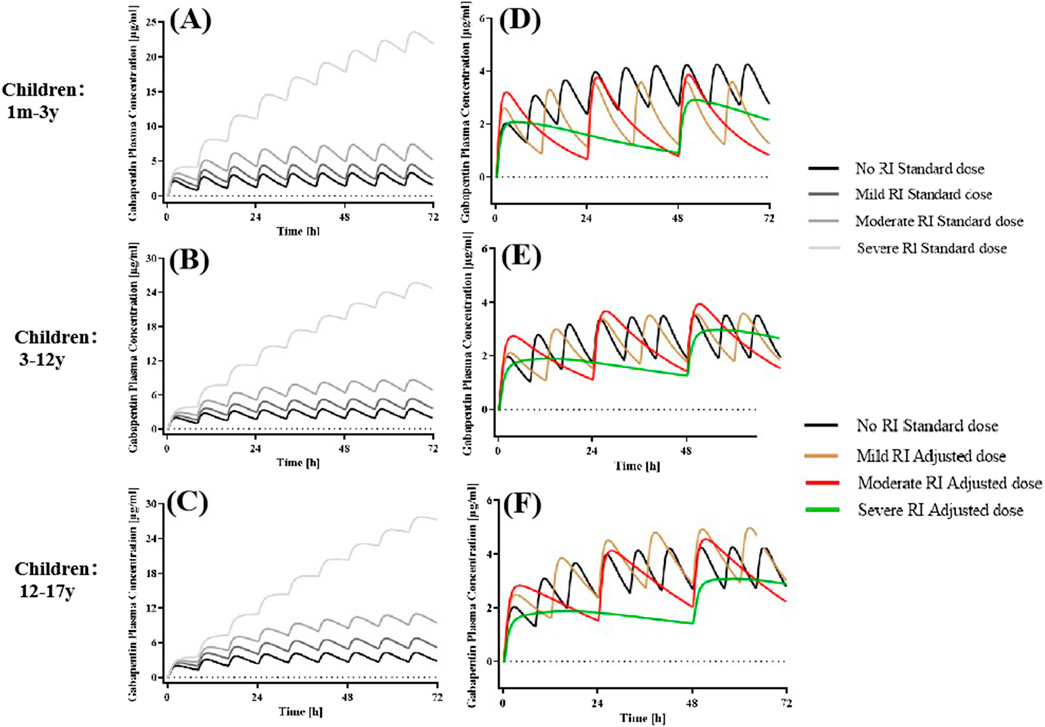
Figure 5. Simulated plasma concentration-time profiles in pediatric patients without RI and various degrees of RI receiving standard and adjusted dosages. The po. administration at a dose of 5 mg/kg tid GAB in (A) 1 m-3 y, (B) 3-12 years, and 300 mg tid in (C) 12-17 years with different degrees of RI. (D–F) Drug concentration-time profile of GAB following oral administration of 5 mg/kg (1m-12 y), 300 mg (12-17 years) bid in mild RI, 5 mg/kg (1 m-12 y), 300 mg (12-17 years) qd in moderate RI, and 2.5 mg/kg (1 m-12 y), 150 mg (12-17 years) qod in severe RI.
3.4 Dose recommendations for pediatric patients
The simulated plasma concentrations and PK parameters of GAB showed no significant differences between children below 3 years of age and those aged 3–12 years under identical dosing regimens (Figure 6). This suggests that a dosing regimen (5 mg/kg) based on body weight can be uniformly applied in pediatric patients up to 12 years of age. In addition, we categorized children into three groups according to international age classifications: young children (1 month–2 years), preschool children (2–6 years), and school-age children (6–12 years) (San and trock, 2017). The simulation results showed that the exact dosage was recommended for newborns and preschool children, consistent with previous research findings (Supplementary Figure S5).
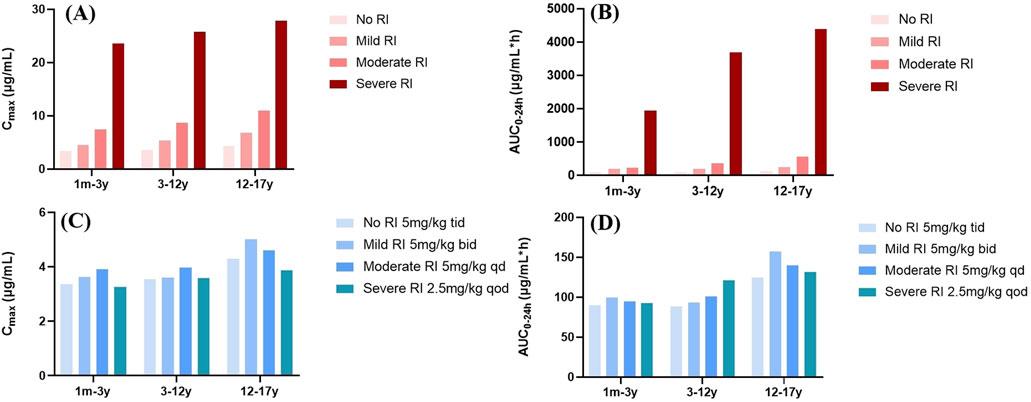
Figure 6. PK parameter profile of GAB in pediatric patients with different degrees of RI. (A,B) Mean Cmax and AUC0–24h profile of GAB following oral administration of 5 mg/kg (1m-12 y), 300 mg (12-17 years) tid in different RI. (C,D) Mean Cmax and AUC0–24h profile of GAB following oral administration of 5 mg/kg (1m-12 y), 300 mg (12-17 years) bid in mild RI, 5 mg/kg (1m-12 y), 300 mg (12-17 years) qd in moderate RI, and 2.5 mg/kg (1m-12 y), 150 mg (12-17 years) qod in severe RI.
Administration of the standard dose of GAB to pediatric virtual RI populations resulted in varying proportional increases in AUC0–24h and Cmax (Figure 6). Our findings demonstrated that GAB exposure was significantly higher in pediatric patients with moderate and severe RI compared to those with normal renal function. Consequently, based on the results of population simulations, we recommend a prolonged dosing interval for GAB in pediatric patients with RI to reduce the total daily dose. Specifically, for pediatric patients with mild, moderate, and severe RI, the frequency of administration was reduced to bid, qd, and qod, respectively. Moreover, the dose of pediatric patients with severe RI should also be halved (2.5 mg/kg). The results showed that PK parameters (AUC0–24h and Cmax) of pediatric patients with RI were comparable after dose adjustment (Figure 6; Table 4).
4 Discussion
The PBPK model has become an indispensable tool in drug development, especially in the study of children. Not only that, the application of the PBPK model in clinical practice is increasingly widespread, and its prediction of PK in special populations has also been widely accepted. Our PBPK models accurately predicted GAB exposures in adult and pediatric subjects with or without RI. The results of our study fill the blank of the use of GAB in pediatric patients with RI and have important significance for guiding clinical drug use.
GAB is used as adjuvant therapy for partial seizures (with or without secondary generalization). We have observed that the European Medicines Agency (EMA) currently approves GAB for children 6 years and older. The recommended starting dose is 10–15 mg/kg daily. The effective dose is typically between 25 and 35 mg/kg per day for children in this age group (EMA, 2021). While, the FDA approves GAB for use in children aged 3 years and older, with a starting dose that also ranges from 10 to 15 mg/kg per day and the effective dose ranging from 25 to 40 mg/kg per day, which (EMA, 2021). Several randomized controlled trials (RCTs) have established that GAB can be safely used in children under 3 years old (de Leeuw et al., 2019; Kaguelidou et al., 2019). However, there is currently no specific dosage recommendation available. Previous studies have suggested that drug exposure in older children when GAB is administered on a milligram per kilogram basis (Haig et al., 2001). Traditionally, pediatric dosages were often extrapolated from adult dosages based on age, weight, or body surface area. Applying PBPK modeling to determine initial pediatric clinical trial doses has gained increasing traction and regulatory acceptance in recent years (Heimbach et al., 2019; Johnson et al., 2021). Our PBPK modeling demonstrated that GAB plasma concentrations and PK parameters at standard doses are similar between children under 3 and those aged 3 to 12. This study established a GAB model for children under 3 years old, providing valuable evidence for pediatric practice.
GAB is a drug eliminated through glomerular filtration and tubular secretion (Beydoun et al., 1995). Human PK studies indicated that GAB is neither metabolized nor bound to serum proteins, and it is cleared solely through renal excretion (Blum et al., 1994). Consequently, GAB PK was susceptible to alterations caused by diminished renal function. Specifically, reduced renal function impairs GAB excretion, leading to its accumulation in the body (Zand et al., 2010). This suggests that dosage adjustments are necessary for individuals with RI (Randinitis et al., 2003; Ke et al., 2022). The FDA instructions of GAB explicitly state that GAB dosage should be adjusted based on CrCl in patients 12 years and older with RI. It is worth noting that in the period of RI, CrCl is affected by renal tubule secretion and overestimates GFR. Several studies have successfully applied PBPK modeling to simulate the PK of really excreted drugs in pediatric chronic kidney disease (CKD) populations (Yoon et al., 2019; Xu et al., 2022; Zhou et al., 2021). However, these investigations primarily focused on compounds without active tubular secretion, such as lamivudine and emtricitabine. Ye et al. utilized PBPK modeling to predict the PK of ertapenem in pediatric populations with CKD, considering glomerular filtration and tubular secretion (Ye et al., 2020). Our model incorporates GFR, kidney size, and TSspec into the design of a representative individual (RI) population, which aligns with Ye’s considerations. This method assumes that a decline in glomerular filtration correlates with a reduction in tubular secretion, reflecting an overall loss of nephron function. Our study considered the advantages of renal tubular secretion, which is more comprehensive.
Interestingly, we found that the dose-adjustment protocol for RI patients in children was less consistent with that for adults, mainly the frequency in patients with severe RI. This difference arises because GAB drug exposure increases more substantially in children with severe RI than in adults. Children with severe RI showed a 31.67-fold increase in drug exposure compared to individuals with normal kidney function. The frequency and dose of severe RI in children should be further reduced compared to the dose-adjustment regimen of moderate RI. The core issue stems from the dynamic changes in children’s physiological parameters, resulting in a lack of pediatric-specific data. For example, the GFR of newborns is only 30% of that of adults, and it only approaches adult levels at the age of 2. When children have renal insufficiency, this immature glomerular function will be further damaged, leading to a significant decrease in drug clearance rate. In addition, there are differences in the distribution of body fluids, with children having a higher proportion of body fluids (75% in newborns compared to 60% in adults). The distribution volume of water-soluble drugs increases, but they are prone to accumulation when renal dysfunction occurs. Furthermore, children with low protein binding rates have lower plasma protein levels, especially in nephrotic syndrome, where the concentration of free drugs increases and the dosage needs to be adjusted more strictly (Edginton et al., 2006). Consequently, directly extrapolating dosage recommendations for adults with RI to children with RI is not advisable. PBPK modeling offers a robust scientific foundation for GAB dose adjustment strategies tailored to children with RI (Khalid et al., 2023), demonstrating its significant value in supporting medication decisions for pediatric populations (Freriksen et al., 2023).
While our study provides valuable insights into GAB dosing in pediatric RI patients, several limitations should be acknowledged. The model evaluation was constrained by the limited availability of pediatric data, with only three relevant studies identified through a comprehensive literature review. To support these findings, future validation through targeted clinical pharmacokinetic studies or therapeutic drug monitoring will be crucial to confirm the safety and efficacy of GFR-adjusted GAB regimens in children, particularly those under 3 years of age and RI patients. While developing this PBPK model represents a pivotal first step in pediatric dose determination for GBA therapy, the scientific approach must be complemented by concurrent evaluation of PK variations and age-related maturation of pharmacodynamic (PD) pathways that govern drug effects. Successful therapeutic optimization in pediatric populations necessitates a holistic assessment of PK/PD parameters, as the interplay between drug exposure dynamics and evolving biological responses fundamentally determines the risk-benefit profile. In addition, most GAB preparations on the market are tablets and capsules, often administered to children based on their body weight. However, these forms can be inconvenient for young patients. It is advisable to introduce more granules and solutions that would suit children better.
5 Conclusion
The PBPK models of GAB in adults were developed and adapted for young children, children, and adolescents with mild, moderate, and severe RI, incorporating age-related physiology, GFR, kidney size, and TSspec adjustments and for pediatric patients with severe RI required careful consideration of both dosing frequency and amount. Our approach was particularly valuable when establishing clinical recommendations for drug administration of GAB in children.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
XQ: Conceptualization, Writing – original draft, Writing – review and editing. CS: Data curation, Formal Analysis, Methodology, Writing – review and editing. ZL: Data curation, Formal Analysis, Methodology, Writing – review and editing. YY: Conceptualization, Funding acquisition, Methodology, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. YY is partially supported by National Natural Science Foundation of China (No. 82404760).
Acknowledgments
All authors are thankful to the subjects involved in this study and all Open Systems Pharmacology community members on GitHub. The authors also thank Liang Zheng (The Second Hospital of Anhui Medical University) for providing proper instructions and advice while learning PBPK modeling.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1669990/full#supplementary-material
Abbreviations
GAB, gabapentin; RI, renal impairment; PBPK, physiologically-based pharmacokinetic; CL, renal clearance; Ccr, creatinine clearance rate; PK, pharmacokinetics; PD, pharmacodynamic; GFR, glomerular filtration rate; FDA, US Food and Drug Administration; EMA, European Medicines Agency; qd, once daily; tid, three times daily; bid, twice daily; MFE, mean fold error; GMFE, geometric mean fold error; RCTs, randomized controlled trials; CKD, chronic kidney disease.
References
Almeida, S., Filipe, A., Almeida, A., Antonijoan, R., García-Gea, C., Gich, I., et al. (2006). Comparative study on the bioequivalence of two different gabapentin formulations. A randomised, two-period, two-sequence, crossover clinical trial in healthy volunteers. Arzneimittel schung 56 (2), 59–63. doi:10.1055/s-0031-1296702
Amini, M., Rouini, M. R., Asad-Paskeh, A., and Shafiee, A. (2010). A new pre-column derivatization method for determination of gabapentin in human serum by HPLC using UV detection. J. Chromatogr. Sci. 48 (5), 358–361. doi:10.1093/chromsci/48.5.358
Backonja, M. M., Canafax, D. M., and Cundy, K. C. (2011). Efficacy of gabapentin enacarbil vs placebo in patients with postherpetic neuralgia and a pharmacokinetic comparison with oral gabapentin. Pain Med. 12 (7), 1098–1108. doi:10.1111/j.1526-4637.2011.01139.x
Beydoun, A., Uthman, B. M., and Sackellares, J. C. (1995). Gabapentin: pharmacokinetics, efficacy, and safety. Clin. Neuropharmacol. 18 (6), 469–481. doi:10.1097/00002826-199512000-00001
Blum, R. A., Comstock, T. J., Sica, D. A., Schultz, R. W., Keller, E., Reetze, P., et al. (1994). Pharmacokinetics of gabapentin in subjects with various degrees of renal function. Clin. Pharmacol. Ther. 56 (2), 154–159. doi:10.1038/clpt.1994.118
Chen, C., Han, C. H., Sweeney, M., and Cowles, V. E. (2013). Pharmacokinetics, efficacy, and tolerability of a once-daily gastroretentive dosage form of gabapentin for the treatment of postherpetic neuralgia. J. Pharm. Sci. 102 (4), 1155–1164. doi:10.1002/jps.23467
Cho, H. Y., Kang, H. A., and Lee, Y. B. (2006). Pharmacokinetics and bioequivalence evaluation of two gabapentin preparations after a single oral dose in healthy Korean volunteers. Int. J. Clin. Pharmacol. Ther. 44 (8), 386–392. doi:10.5414/cpp44386
Cowles, V. E., Gordi, T., and Hou, S. Y. (2012). Steady-state pharmacokinetics of gabapentin after administration of a novel gastroretentive extended-release formulation in postmenopausal women with vasomotor symptoms. Clin. Drug Investig. 32 (9), 593–601. doi:10.1007/BF03261914
de Leeuw, T. G., Mangiarini, L., Lundin, R., Kaguelidou, F., van der Zanden, T., Pasqua, O. D., et al. (2019). Gabapentin as add-on to morphine for severe neuropathic or mixed pain in children from age 3 months to 18 years - evaluation of the safety, pharmacokinetics, and efficacy of a new gabapentin liquid formulation: study protocol for a randomized controlled trial. Trials 20 (1), 49. doi:10.1186/s13063-018-3169-3
Eckhardt, K., Ammon, S., Hofmann, U., Riebe, A., Gugeler, N., and Mikus, G. (2000). Gabapentin enhances the analgesic effect of morphine in healthy volunteers. Anesth. Analg. 91 (1), 185–191. doi:10.1097/00000539-200007000-00035
Edginton, A. N., Schmitt, W., and Willmann, S. (2006). Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin. Pharmacokinet. 45 (10), 1013–1034. doi:10.2165/00003088-200645100-00005
Eldon, M. A., Underwood, B. A., Randinitis, E. J., and Sedman, A. J. (1998). Gabapentin does not interact with a contraceptive regimen of norethindrone acetate and ethinyl estradiol. Neurology 50 (4), 1146–1148. doi:10.1212/wnl.50.4.1146
FDA (2019). FDA-Label-gabapentin. Available online at: https://nctr-crs.fda.gov/fdalabel/services/spl/set-ids/3255245c-6ae6-46ab-a1c1-0b8d4d4cdaa2/spl-doc (Accessed August 16, 2019).
Freriksen, J., van der Heijden, J., de Hoop-Sommen, M. A., Greupink, R., and de Wildt, S. N. (2023). Physiologically based pharmacokinetic (PBPK) model-informed dosing guidelines for pediatric clinical care: a pragmatic approach for a special population. Paediatr. Drugs 25 (1), 5–11. doi:10.1007/s40272-022-00535-w
Gidal, B. E., Maly, M. M., Budde, J., Lensmeyer, G. L., Pitterle, M. E., and Jones, J. C. (1996). Effect of a high-protein meal on Gabapentin pharmacokinetics. Epilepsy Res. 23 (1), 71–76. doi:10.1016/0920-1211(95)00051-8
Gidal, B. E., DeCerce, J., Bockbrader, H. N., Gonzalez, J., Kruger, S., Pitterle, M. E., et al. (1998a). Gabapentin bioavailability: effect of dose and frequency of administration in adult patients with epilepsy. Epilepsy Res. 31 (2), 91–99. doi:10.1016/s0920-1211(98)00020-5
Gidal, B. E., Maly, M. M., Kowalski, J. W., Rutecki, P. A., Pitterle, M. E., and Cook, D. E. (1998b). Gabapentin absorption: effect of mixing with foods of varying macronutrient composition. Ann. Pharmacother. 32 (4), 405–409. doi:10.1345/aph.17281
Gordi, T., Hou, E., Kasichayanula, S., and Berner, B. (2008). Pharmacokinetics of gabapentin after a single day and at steady state following the administration of gastric-retentive- extended-release and immediate-release tablets: a randomized, open-label, multiple-dose, three-way crossover, exploratory study in healthy subjects. Clin. Ther. 30 (5), 909–916. doi:10.1016/j.clinthera.2008.05.008
Grimstein, M., Yang, Y., Zhang, X., Grillo, J., Huang, S. M., Zineh, I., et al. (2019). Physiologically based pharmacokinetic modeling in regulatory science: an update from the U.S. food and drug administration's office of clinical pharmacology. J. Pharm. Sci. 108 (1), 21–25. doi:10.1016/j.xphs.2018.10.033
Guo, G., You, X., Wu, W., Chen, J., Ke, M., Lin, R., et al. (2023). Physiologically-based pharmacokinetic modeling of omalizumab to predict the pharmacokinetics and pharmacodynamics in pediatric patients. Clin. Pharmacol. Ther. 113 (3), 724–734. doi:10.1002/cpt.2815
Haig, G. M., Bockbrader, H. N., Wesche, D. L., Boellner, S. W., Ouellet, D., Brown, R. R., et al. (2001). Single-dose Gabapentin pharmacokinetics and safety in healthy infants and children. J. Clin. Pharmacol. 41 (5), 507–514. doi:10.1177/00912700122010384
Heimbach, T., Lin, W., Hourcade-Potelleret, F., Tian, X., Combes, F. P., Horvath, N., et al. (2019). Physiologically based pharmacokinetic modeling to supplement nilotinib pharmacokinetics and confirm dose selection in pediatric patients. J. Pharm. Sci. 108 (6), 2191–2198. doi:10.1016/j.xphs.2019.01.028
Jalalizadeh, H., Souri, E., Tehrani, M. B., and Jahangiri, A. (2007). Validated HPLC method for the determination of gabapentin in human plasma using pre-column derivatization with 1-fluoro-2,4-dinitrobenzene and its application to a pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 854 (1-2), 43–47. doi:10.1016/j.jchromb.2007.03.039
Johnson, T. N., Abduljalil, K., Nicolas, J. M., Muglia, P., Chanteux, H., Nicolai, J., et al. (2021). Use of a physiologically based pharmacokinetic-pharmacodynamic model for initial dose prediction and escalation during a paediatric clinical trial. Br. J. Clin. Pharmacol. 87 (3), 1378–1389. doi:10.1111/bcp.14528
Kaguelidou, F., Le Roux, E., Mangiarini, L., Lundin, R., de Leeuw, T. G., Della Pasqua, O., et al. (2019). Non-inferiority double-blind randomised controlled trial comparing gabapentin versus tramadol for the treatment of chronic neuropathic or mixed pain in children and adolescents: the GABA-1 trial-a study protocol. BMJ Open 9 (2), e023296. doi:10.1136/bmjopen-2018-023296
Kang, H. A., Cho, H. Y., and Lee, Y. B. (2007). The effect of MDR1 G2677T/A polymorphism on pharmacokinetics of gabapentin in healthy Korean subjects. Arch. Pharm. Res. 30 (1), 96–101. doi:10.1007/BF02977784
Ke, C., You, X., Lin, C., Chen, J., Guo, G., Wu, W., et al. (2022). Development of physiologically based pharmacokinetic model for Pregabalin to predict the pharmacokinetics in pediatric patients with renal impairment and adjust dosage regimens: PBPK model of Pregabalin in pediatric patients with renal impairment. J. Pharm. Sci. 111 (2), 542–551. doi:10.1016/j.xphs.2021.10.026
Khalid, S., Rasool, M. F., Masood, I., Imran, I., Saeed, H., Ahmad, T., et al. (2023). Application of a physiologically based pharmacokinetic model in predicting captopril disposition in children with chronic kidney disease. Sci. Rep. 13 (1), 2697. doi:10.1038/s41598-023-29798-0
KuKanich, B. (2013). Outpatient oral analgesics in dogs and cats beyond nonsteroidal antiinflammatory drugs: an evidence-based approach. Vet. Clin. North Am. Small Anim. Pract. 43 (5), 1109–1125. doi:10.1016/j.cvsm.2013.04.007
Lal, R., Ellenbogen, A., Chen, D., Zomorodi, K., Atluri, H., Luo, W., et al. (2012). A randomized, double-blind, placebo-controlled, dose-response study to assess the pharmacokinetics, efficacy, and safety of gabapentin enacarbil in subjects with restless legs syndrome. Clin. Neuropharmacol. 35 (4), 165–173. doi:10.1097/WNF.0b013e318259eac8
Park, J. H., Jhee, O. H., Park, S. H., Lee, J. S., Lee, M. H., Shaw, L. M., et al. (2007). Validated LC-MS/MS method for quantification of gabapentin in human plasma: application to pharmacokinetic and bioequivalence studies in Korean volunteers. Biomed. Chromatogr. 21 (8), 829–835. doi:10.1002/bmc.826
Randinitis, E. J., Posvar, E. L., Alvey, C. W., Sedman, A. J., Cook, J. A., and Bockbrader, H. N. (2003). Pharmacokinetics of pregabalin in subjects with various degrees of renal function. J. Clin. Pharmacol. 43 (3), 277–283. doi:10.1177/0091270003251119
Schijvens, A. M., de Wildt, S. N., and Schreuder, M. F. (2020). Pharmacokinetics in children with chronic kidney disease. Pediatr. Nephrol. 35 (7), 1153–1172. doi:10.1007/s00467-019-04304-9
Swearingen, D., Aronoff, G. M., Ciric, S., and Lal, R. (2018). Pharmacokinetics of immediate release, extended release, and gastric retentive gabapentin formulations in healthy adults. Int. J. Clin. Pharmacol. Ther. 56 (5), 231–238. doi:10.5414/CP203166
Tallian, K. B., Nahata, M. C., Lo, W., and Tsao, C. Y. (2004). Pharmacokinetics of gabapentin in paediatric patients with uncontrolled seizures. J. Clin. Pharm. Ther. 29 (6), 511–515. doi:10.1111/j.1365-2710.2004.00596.x
Tjandrawinata, R. R., Setiawati, E., Putri, R. S., Yunaidi, D. A., Amalia, F., and Susanto, L. W. (2014). Single dose pharmacokinetic equivalence study of two gabapentin preparations in healthy subjects. Drug Des. Devel Ther. 8, 1249–1255. doi:10.2147/DDDT.S69326
Toh, D. S., Limenta, L. M., Yee, J. Y., Wang, L. Z., Goh, B. C., Murray, M., et al. (2014). Effect of mushroom diet on pharmacokinetics of gabapentin in healthy Chinese subjects. Br. J. Clin. Pharmacol. 78 (1), 129–134. doi:10.1111/bcp.12273
Xu, J., Lin, R., Chen, Y., You, X., and Huang, P. (2022). Physiologically based pharmacokinetic modeling and dose adjustment of teicoplanin in pediatric patients with renal impairment. J. Clin. Pharmacol. 62 (5), 620–630. doi:10.1002/jcph.2000
Ye, L., Ke, M., You, X., Huang, P., and Lin, C. (2020). A physiologically based pharmacokinetic model of ertapenem in pediatric patients with renal impairment. J. Pharm. Sci. 109 (9), 2909–2918. doi:10.1016/j.xphs.2020.06.010
Yoon, S., Yi, S., Rhee, S. J., Lee, H. A., Kim, Y., Yu, K. S., et al. (2019). Development of a physiologically-based pharmacokinetic model for cyclosporine in Asian children with renal impairment. Transl. Clin. Pharmacol. 27 (3), 107–114. doi:10.12793/tcp.2019.27.3.107
Zand, L., McKian, K. P., and Qian, Q. (2010). Gabapentin toxicity in patients with chronic kidney disease: a preventable cause of morbidity. Am. J. Med. 123 (4), 367–373. doi:10.1016/j.amjmed.2009.09.030
Keywords: gabapentin, pediatric, renal impairment, physiologically based pharmacokinetic, dose adjustment
Citation: Qin X, Shen C, Li Z and Yang Y (2025) Optimization of gabapentin dosage in pediatric patients with renal impairment: a physiologically based pharmacokinetic modeling approach. Front. Pharmacol. 16:1669990. doi: 10.3389/fphar.2025.1669990
Received: 21 July 2025; Accepted: 09 September 2025;
Published: 26 September 2025.
Edited by:
Anna Maria Siebel, Federal University of Paraná, BrazilReviewed by:
GuoLin Li, China Pharmaceutical University, ChinaFiras Al-Zubaydi, University of Baghdad, Iraq
Copyright © 2025 Qin, Shen, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yujie Yang, c2N1eWFuZ3l1amllQDE2My5jb20=
†ORCID: Xiaoli Qin, orcid.org/0009-0008-5965-2464; Chaozhuang Shen, orcid.org/0000-0002-2263-2709; Zhimin Li, orcid.org/0009-0006-2712-2931; Yujie Yang, orcid.org/0000-0001-6941-0380
 Xiaoli Qin
Xiaoli Qin Chaozhuang Shen
Chaozhuang Shen Zhimin Li
Zhimin Li Yujie Yang
Yujie Yang