- 1Department of Computer Science, University of Oxford, Oxford, United Kingdom
- 2R&D Preclinical Safety, Sanofi, Frankfurt, Germany
- 3R&D Preclinical Safety, Investigative Toxicology, Sanofi, Vitry-Sur-Seine, France
Introduction: Effective proarrhythmic and inotropic risk assessment is essential for pharmaceutical development, but current preclinical methods for assessment of cardiac inotropy are flawed and costly, particularly when combined with QTc prolongation studies. Ex vivo rabbit Langendorff isolated heart experiments provide valuable insights into cardiovascular effects and safety, but their high cost, experimental difficulty, and limited applicability to human physiology pose challenges. Human mechanistic in silico modelling and simulation has proven successful in risk assessments of both electrophysiological and cardiac inotropy assessment.
Methods: This study evaluates the feasibility of replacing ex vivo Langendorff experiments for contractility with human-based ventricular electromechanical modelling and simulations, based on 37 compounds.
Results: Results show 1) 86% of compounds show qualitative agreement using four channel data (IKr, ICaL, INa, Ito), with 73% showing quantitative agreement correlating with higher quality data, 2) sensitivity analysis identified hNCX1 and late hNaV1.5 currents as additional targets, which, when considered alongside the four channel data as input, improved agreement from 86% to 95% (at least qualitatively), 3) incomplete dose-response input data was the key reason for discrepancies between experiment and simulation, while noting only two compounds showed a complete disagreement. Incorporating patient variability through a population of N = 166 human ventricular cell models add further confidence, and highlights increasing inter-subject diversity with increasing concentrations.
Conclusion: This study supports the adoption of in silico new approach methodologies for accurate prediction of drug cardiotoxicity, and to refine, reduce and replace the use of ex vivo rabbit experiments.
1 Introduction
The recent emergence of New Approach Methodologies (NAMs), such as in silico models and advanced in vitro systems, are bridging preclinical and clinical biomedical research by offering higher throughput, better translatability to human patients, and alleviating ethical concerns (Robertson et al., 2025). In silico modelling refers to computational models grounded in mathematical representations of biological systems, informed by experimental data and patho-physiological knowledge. Simulations using these tools enable prediction and mechanistic exploration of physiological and pharmacological outcomes (Viceconti et al., 2021). Advances in human modelling and simulation have enabled in silico drug trials as powerful tools for predicting pro-arrhythmic risk (Mohr et al., 2022; Passini et al., 2017; Trovato et al., 2022; Varshneya et al., 2021) and more recently, inotropy assessments (Trovato et al., 2025). Human-based NAMs incorporating experimental-simulation approaches enable population variability studies and simultaneous evaluation of proarrhythmic and inotropic effects. Regulatory bodies like the United States Food and Drug Administration (FDA) and European Medicines Agency (EMA) endorse in silico methods for preclinical proarrhythmic risk assessments (EMA, 2022; Musuamba et al., 2021; Valentin and Leishman, 2023). However, NAM adoption in regulatory evaluations remains limited, highlighting the need for robust evaluation processes and better co-ordination (Valentin and Leishman, 2025).
Animal models (in vitro, in vivo and ex vivo) are widely used to evaluate proarrhythmic cardiotoxicity, using metrics like drug-induced repolarisation prolongation as surrogates for QT prolongation in the whole heart (Gintant et al., 2016). While current guidelines focus on QT prolongation and proarrhythmic risk, broader assessments of cardiotoxicity including inotropic risk are essential to improve safety assessments. Excessive inotropic cardiotoxicity has led to drug discontinuation (Mamoshina et al., 2021), yet inotropic evaluations lack equivalent rigour, potentially halting valuable compounds prematurely. Preclinical assessment of drug-induced cardiac inotropy remains challenging, as many animal-based or human induced pluripotent stem cell (iPSC)-derived cardiomyocyte models fail to capture human primary cardiomyocyte physiology (van Meer et al., 2016), or lack mature inotropic mechanisms (Pointon et al., 2015).
The Langendorff rabbit isolated heart model is widely used in cardiovascular research, enabling studies of the heart’s intrinsic properties in isolation of the body’s other dynamic systems. It facilitates investigation of contractile function, electrical activity and coronary blood flow in isolation which makes it valuable for pharmaceutical drug testing (Roche et al., 2010). However, in addition to ethical limitations, limited viability of isolated hearts mean experiments are time-sensitive, and species differences limit the translation of experimental outcomes to clinical risk assessments (Blanset et al., 2020; Van Norman, 2020).
The goal of this study is to quantitatively assess the usability, and optimise the translatability, of in silico simulations using human ventricular cardiomyocytes electromechanical models to replace ex vivo isolated rabbit experiments. Simulations for 37 compounds, containing both proprietary and reference compounds, are benchmarked with proprietary data from previously conducted rabbit Langendorff isolated heart experiments. Simulations incorporate variations in ionic conductance across the human population to support the development of “Phase 0” in silico cardiovascular studies, considering different patient populations. Sensitivity analysis identifies relevant currents beyond the four ion channel assay panel used in Comprehensive in vitro Proarrhythmic Assay (CiPA)-based studies (Colatsky et al., 2016) and identified by Zhou et al. (2020) as the minimum set required for pro-arrhythmia predictions. We quantify the importance of integrating high quality experimental data, which is crucial for in silico predictivity, and we pave the way towards the replacement of animal-based ex vivo experimentation.
2 Materials and methods
Figure 1 outlines the study’s methodology. A diverse selection of 37 unique compounds was analysed to measure drug-induced changes in both repolarisation and contractility properties in vitro in the isolated rabbit heart and in silico in human electromechanical cardiomyocyte simulations. These data were compared to evaluate and optimise the potential of human-based electromechanical simulations as alternatives to animal experimentation.
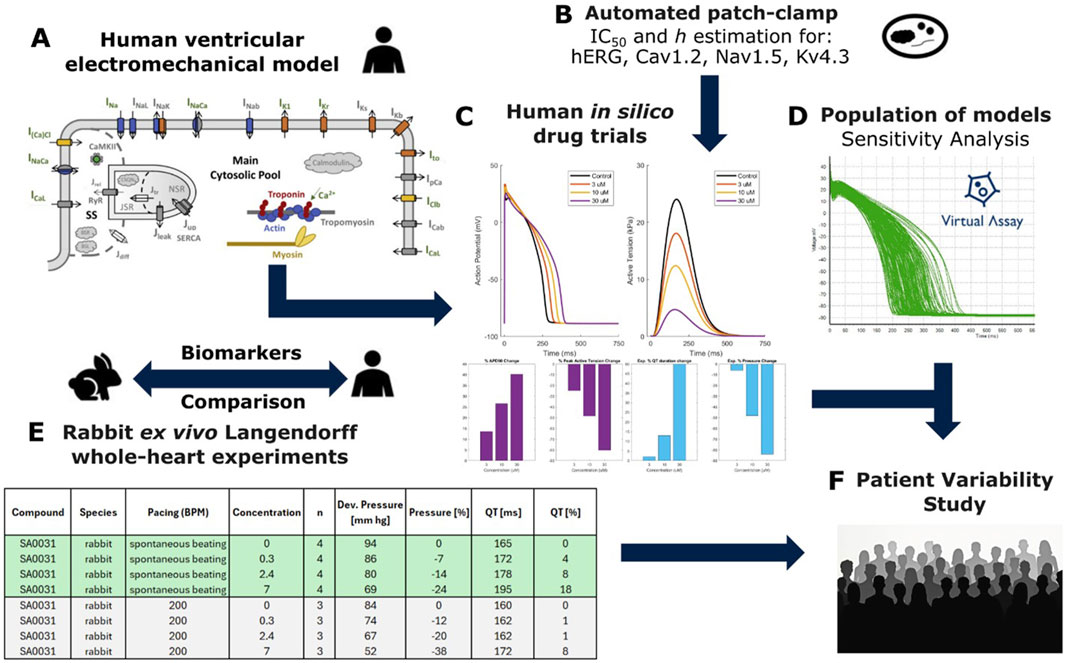
Figure 1. Combined experiment-simulation pipeline used to perform this study. (A) Structure of the biophysically detailed computational model used to simulate human ventricular cellular electromechanics (Margara et al., 2021). (B) In vitro estimation of IC50s and Hill coefficients through automated patch clamp. (C) Single-cell in silico drug trials performed using simple pore-block models, output traces showing action potential (AP) and active tension with comparison to rabbit experimental data. (D) Experimentally calibrated population of 166 models generated using the Virtual Assay Software (Oxford University Innovation © 2018); green traces show simulated AP. (E) In silico results were compared against pre-existing ex vivo recordings from whole-heart Langendorff rabbit experiments, (F) Drug models and calibrated population of models used to investigate patient variability in contractility for select compounds.
We employed the human-based electromechanical ventricular cardiomyocyte model (ToR-ORd-Land, Figure 1A), featuring biophysically detailed models of electrophysiology, excitation-contraction coupling and contractility mechanisms including ionic current, calcium dynamics and cross-bridge formation. This was performed through coupling of the ToR-ORd model (Tomek et al., 2019) with the Land et al. contractility model (Land et al., 2017) as in Margara et al. (2021), with additional studies also demonstrating validation through comparison with experimental data (Trovato et al., 2025). The choice of this model was based on extensive validation conducted through comparison with experimental data in control conditions and following pharmacological interventions and disease conditions (Tomek et al., 2019; Zhou et al., 2024b; Zhou et al., 2024a). Input data to the model included the automated patch-clamp quantifying the half-maximal inhibitory concentrations (IC50s) for four cardiac ion channels for each compound (Figure 1B).
Simulations yielded action potential, calcium, and active tension (AT) transients in single cells, in addition to all underlying variables such as ionic currents to mechanistically explore drug effects (Figure 1C). A sensitivity analysis, using Virtual Assay software (Passini et al., 2021), was performed using a population of human ventricular models (N = 166) to identify key determinants of repolarisation and contractility changes in healthy populations (Figure 1D). In silico predictions of action potential duration (APD) and peak active tension were compared with experimental rabbit whole-organ QT and pressure measurements (Figure 1E). Cardiac cell models further assessed patient variability in repolarization and contractility for selected compounds (Figure 1F). Single cell simulations were chosen in this study to minimize their computational cost (compared to whole ventricular simulations) and enable substantial exploration of compounds and patient variability.
2.1 Experimental data
2.1.1 In vitro ion channel data
Following the procedure in Trovato et al. (2022), we used automated patch-clamp platforms to study four ionic currents relevant to human repolarisation, as outlined by Zhou et al. (2020) as the minimum set of ion channels, marked by the CiPA Ion Channel Work Group, for reliable predictions of repolarisation abnormalities. These channels are the human ether-a-go-go related gene potassium channel also known as hERG channel, modulating the rapid inward rectifying potassium current; hCav1.2, modulating L-type calcium current; hNav1.5, modulating peak sodium current; and hKv4.3, modulating the transient outward potassium current. hKv4.3 notably has significant inter-species differences between human and rabbit (Oudit et al., 2001). The QPatch system (Sophion, Denmark) was used to investigate hERG, hNav1.5 and hKv4.3, and the SyncroPatch system (Nanion, Germany) was employed for hCav1.2. Recordings for all 37 compounds were performed at 21 °C.
Furthermore, in vitro potency for 11 compounds was assessed on the NCX isoforms using a cell-based calcium mobilization assay on CHO cell lines expressing NCX1, NCX2 or NCX3. Measurements of intracellular calcium concentration employed a fluorescent imaging plate reader (Molecular Devices, United States) with the calcium-sensitive dye Fluo4-AM. Detailed methods are described in Pelat et al. (2021).
IC50 data for all compounds are summarised in Supplementary Table S1. When a Hill coefficient was not available, it was approximated as 1. Compounds that did not inhibit a channel within the tested concentrations were interpreted as having no effect and are given no value. This is relevant for Kv4.3, in which more than half of the compounds showed no effect. Some compounds showed a visible inhibition, but an IC50 was not achieved. These compounds were taken at their maximum possible concentration, or with sufficient data, an IC50 was estimated with a Hill coefficient of 1.
2.1.2 Ex vivo rabbit whole-heart Langendorff experiments
Routine experimental measurements used in this study were retrieved from already existing datasets collected and curated by Sanofi, contributing to the 3R principles in animal experimentation: replacement, reduction and refinement. All the procedures described in the present study were performed in agreement with the European regulation (2010/63/EU) and under the approval and control of Sanofi’s ethics committee. All procedures were performed in Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited facilities, in full compliance with the standards for the care and use of laboratory animals and in accordance with the French Ministry for Research. Hearts were excised from anesthetized rabbits (Medotomidine (Domitor®): 150 μg/kg, Ketamine (Imalgene 1,000®): 10 mg/kg), washed in heparinized physiological solution at room temperature, and perfused through the aorta using the Langendorff method (EMKA-Technologies, France). The physiological solution (in mmol/L: NaCl 120; KCl 4; MgCl2 1; NaH2PO4 1.8; NaHCO3 25; glucose 11; CaCl2 1.8; pH = 7.4) was maintained at 37 °C ± 0.5 °C, gassed with 95% O2/5% CO2 and delivered into the heart through a cannula inserted into the ascending aorta. Retrograde flow in the aorta closed the leaflets of the aortic valve, and consequently, the entire perfusate entered the coronary arteries via the ostia at the aortic root. After passing through the coronary circulation, the perfusate drained into the right atrium via the coronary sinus. The ventricles were empty of perfusate throughout the experiment. The experimental chamber was closed to maintain a wet atmosphere.
Hearts were spontaneously beating. Left ventricular contractility was derived from the developed left ventricular pressure using both developed pressure and maximal rate of rise. The left ventricular pressure was evaluated using a pressure transducer within a saline-filled latex balloon inserted into the ventricular chamber. The saline volume allowed a resting pressure of 10–20 mmHg (preload). The electrocardiogram was recorded with two flexible electrodes gently pressed on the epicardium. The ECG parameters PQ, QRS, and QT durations were calculated using specific software (HEM-Notocord systems).
After stabilization, pressure was recorded with the vehicle for 15 min and used as a control period. Then, compounds were consecutively perfused at four concentrations (C1, C2, C3, and C4) during four successive periods of 10 min, followed by a 30-min washout period after the last concentration.
2.2 Simulating human cellular electrophysiology and contractility
Human ventricular electrophysiology, calcium dynamics and active contraction were simulated with the ToR-ORd model (Tomek et al., 2019) coupled with the Land contractility model (Land et al., 2017). The ToR-ORd model comprises of 17 ionic currents and fluxes, and has been validated under healthy, disease and drug conditions with extensive experimental data. It can produce crucial arrhythmia mechanisms at the cell level, including early after-depolarisations, an established, mechanistically-sound metric to quantify pro-arrhythmic cardiotoxicity in silico (Passini et al., 2021). As described in Margara et al. (2021), the ToR-ORd model is bidirectionally coupled to the Land model through the free intracellular calcium concentration. This is computed in the ToR-ORd model and used as input in the Land model to generate active tension, while the amount of calcium bound to troponin C is fed back to the ToR-ORd model and used to update the free intracellular calcium concentration at each time step.
A virtual population of 500 human ventricular endocardial cellular electromechanical models based on the ToR-ORd-Land model was generated by scaling ionic current conductivities (50%–150%) using Latin Hypercube Sampling. After 500 stimuli (1 Hz), 166 models meeting experimentally obtained criteria for human ventricular action potential morphology, calcium transient and tension biomarkers (Table 1) were retained for simulations (Figure 1D).
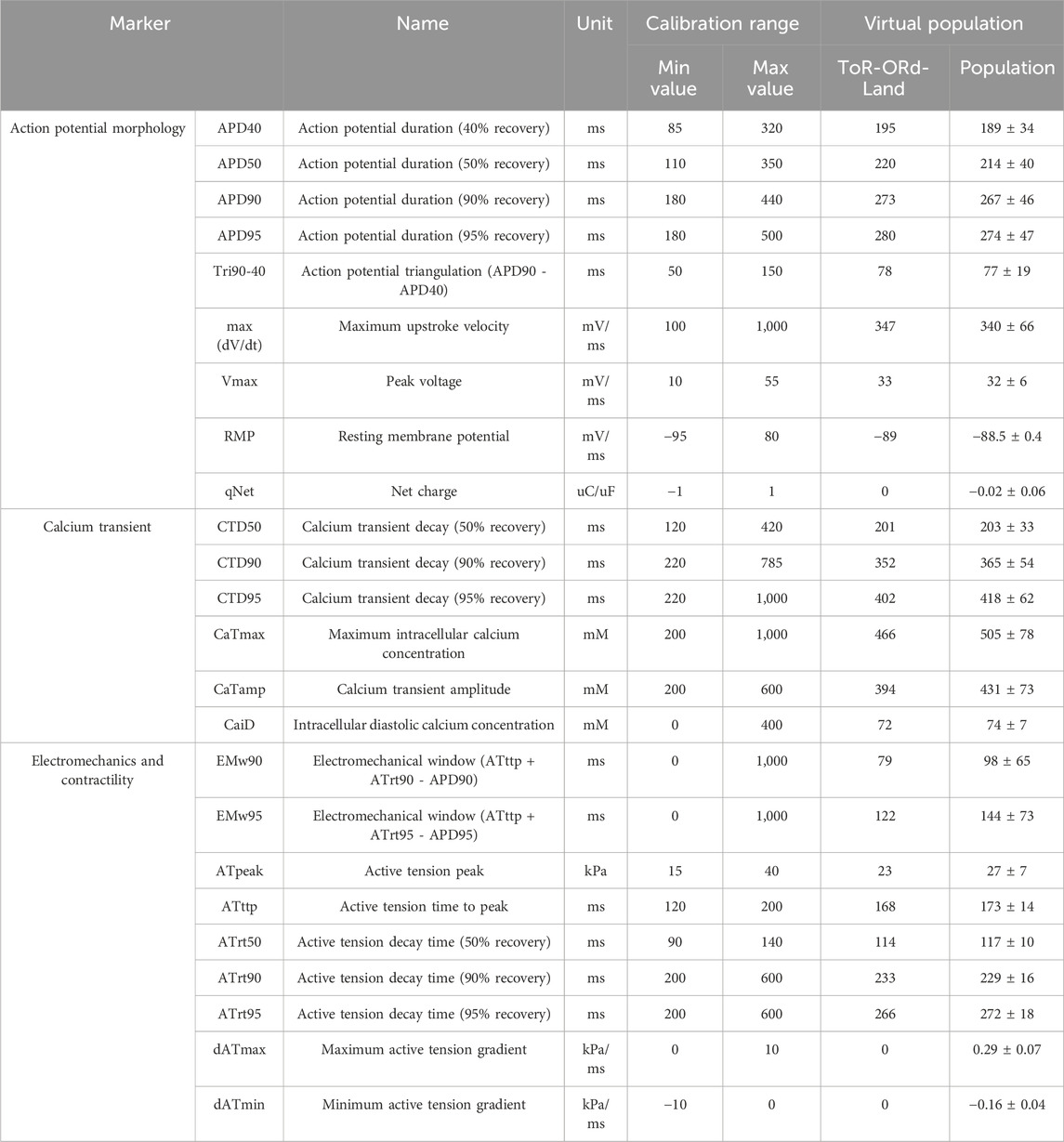
Table 1. Calibration criteria and calibrated biomarkers for the population of healthy adult cardiomyocyte models. Calibration criteria for healthy adult ventricular myocytes and simulated biomarker outputs from the calibrated virtual population. Action potential morphology, calcium transient, electromechanical, and contractile biomarkers in control for the ToR-ORd-Land model (Margara et al., 2021). Mean and standard deviations calculated from the virtual population of 166 cell models are provided. The baseline values are similar or identical to the population mean for all biomarkers.
2.3 Human in silico drug trials
Drug-induced inhibition of ion channel function was simulated through conductance scaling using a simple pore-block model (Brennan et al., 2009), with the experimental IC50, hill coefficients and drug concentrations reported in Supplementary Table S1 for the minimum set of ion channels required for reliable predictions of repolarisation abnormalities set out by Zhou et al. (2020): IKr, ICaL, INa, Ito and additionally INCX and INaL which were determined as important through a sensitivity analysis. Figure 2 shows a visual representation of the conductivities of these currents following the application of the drug model for each compound at each concentration.
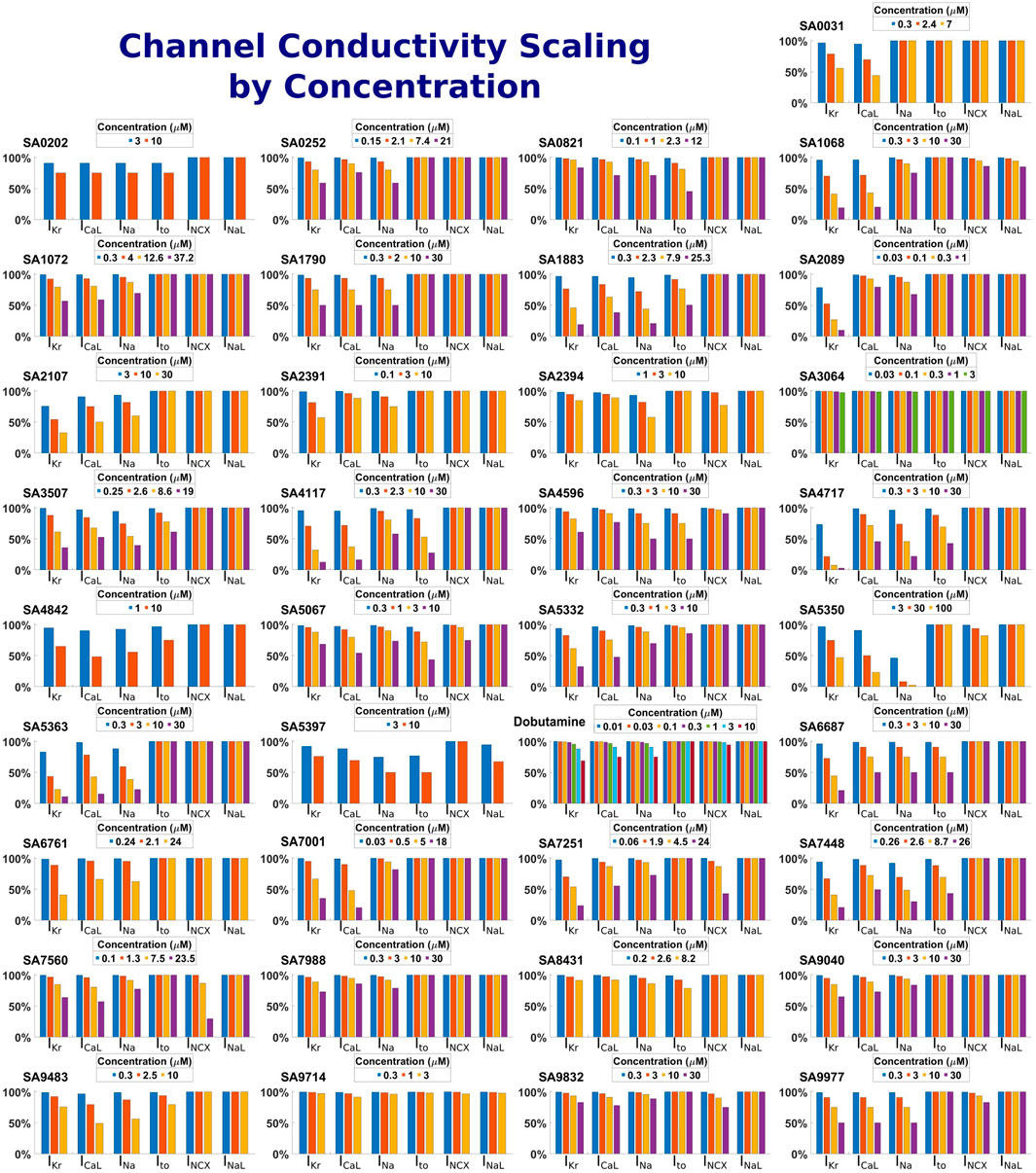
Figure 2. Summary of drug-induced effects on six cardiac ion channels. Channel modulation was calculated using a simple pore-block model. Each panel shows one of the 37 compounds, both reference and proprietary, with different bars representing the residual current as a percentage following drug-application for each ion channel and drug concentration.
Starting from the steady state described above, the models were paced for 200 beats at 1 Hz using a stimulus current, with AP, intracellular calcium transient (CaT) and active tension biomarkers computed using the last beat. The final 10 beats were checked for repolarisation abnormalities, defined as the existence of a positive change in membrane potential with respect to time greater than 0.01 mV/ms occurring after the first 100 ms of the beat. If a repolarisation abnormality was observed, the biomarkers were calculated for the last beat in normal sinus rhythm. Drugs were initially simulated using the minimum four ion channels needed as detailed in Section 2.1.1, and then the 12 compounds for which hNCX1 and late hNav1.5 inhibitions were experimentally measured were simulated again including these additional drug/ionic current interactions.
All simulations were performed on a regular laptop (Intel Core i7-1185G7, 16 GB RAM). The time required to simulate one drug at one concentration (500 beats at 1 Hz) on all 166 cell models was 14 min.
2.4 Metrics for comparison of experiments and simulations
Baseline biomarker measurements were established in control conditions for both ex vivo experiments and in silico simulations, benchmarking compound results at each concentration against these respective controls. Percent variations of means for experiments and simulations were calculated. In the population, these variations were measured against each cell’s control, and the group mean is calculated.
For repolarisation assessment, in silico APD90 prolongation was compared to experimental QT prolongation ex vivo, as these biomarkers are both related to ventricular repolarisation (Ducroq et al., 2007; Omata et al., 2005; Redfern et al., 2003; Romero et al., 2018). Contractile assessment compared in silico peak active tension with ex vivo pressure. Increased ventricular pressure results from myocardial contraction-generated tension, measured at the left ventricular wall. The measured pressure is the result of the collective forces of active (contractility) and passive tension (preload). These factors–active contractility, passive tension, blood volume, heart rate and arterial pressure–all influence pressure, though arterial pressure is less relevant in Langendorff heart experiments with retrograde fixed perfusion at 60 mmHg.
To evaluate consistency between experimental outcomes and human in silico trial predictions, we used a quantitative metric based on the percentage difference between experimental QT prolongation and simulated APD prolongation, as well as experimental contractility and simulated active tension under drug influence at each concentration. Three outcomes were defined: 1) quantitative agreement, for matching trends with ≤25% difference (increase, decrease or negligible response, where negligible was defined as a change of no more than ±10% from control at every concentration tested); 2) qualitative agreement, for matching trends but >25% difference; and 3) disagreement, for differing trends, excluding negligible differences (e.g., negligible increases vs. decreases). A compound’s match was determined by the majority of concentrations tested. For some compounds, the highest concentration produced a severe change in active tension or APD, inconsistent with the general trend, and caused by general depolarisation/contraction failure. In these cases, we excluded the highest concentration from the analysis, as it was not representative of the general trend.
Repolarisation and contractility assessments were analysed separately. Compounds were classified as full matches (both assessments in qualitative or quantitative agreement), partial matches (one match in disagreement) or mismatches (both in disagreement).
2.5 Verification, validation and uncertainty quantification strategy for credibility assessment
This study aims to assess and optimise a translational model linking ex vivo Langendorff rabbit heart experiments and human-based in silico trials. Comparing simulation outcomes with experimental data and established methods is critical for validating the computational framework’s credibility in assessing drug effects and potential cardiotoxicity.
The ToR-ORd model, coupled with the Land model, was evaluated against a wide range of experimental data in previous studies with drug action as a specific focus (Tomek et al., 2019; Margara et al., 2021). We also confirmed its consistency in the Virtual Assay software through comparison with MATLAB implementations. The computational pipeline accurately reproduced drug-induced alterations in cell markers for reference compounds in the dataset, including hydroxychloroquine, loperamide, clozapide, lidocaine and cannabidiol.
Moreover, a sensitivity analysis (SA) was conducted on ion channel conductivities of the four primary currents (IKr, ICaL, INa, Ito) and extended to a total of 10 key currents (IKs, IK1, INCX, INaL, Jrel, Jup) in the human ventricular cardiomyocyte. By modulating conductance from 10%–100% of the baseline value, the SA identified key mechanisms influencing contractile and repolarisation biomarkers in healthy human ventricular cell models (described in Section 2.2).
3 Results
3.1 In silico predictions using drug block for four ionic currents as input compared with ex vivo observations
Figure 3 summarizes the comparison of experiment and simulation results for the 37 compounds, characterised by a minimum dataset of four ion currents (IKr, ICaL, INa and Ito). Individual results are presented in Supplementary Table S1. All compounds with ex vivo data show agreement in at least either electrophysiology or contractility, and 68% of compounds show matches for both. Matches with only contractility or repolarisation agreement account for 13% and 8%, respectively. Quantitative matches were found for 73% of compounds. Of the 11% missing experimental data for either repolarisation or contractility, all but one compound exhibited at least a qualitative match.
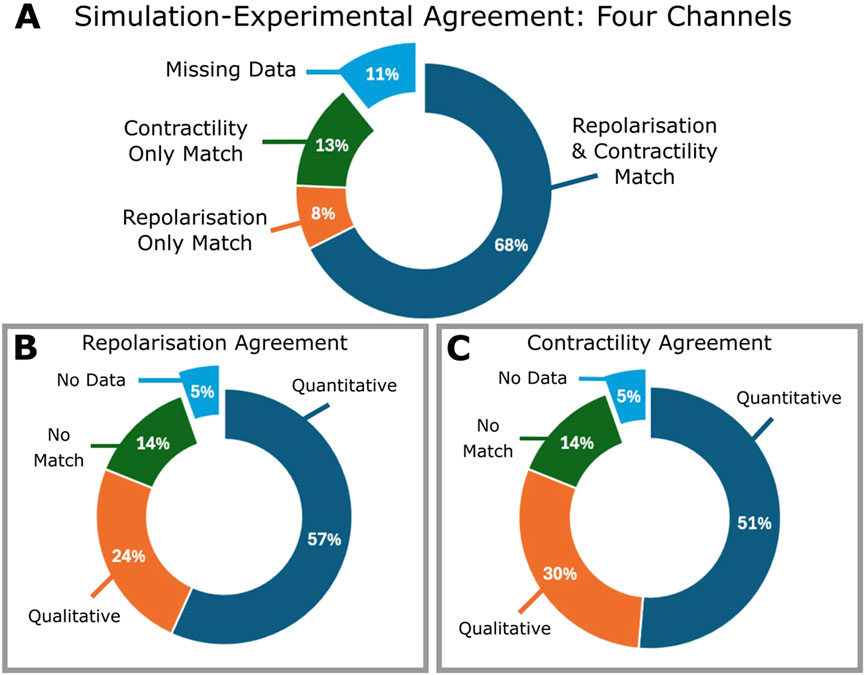
Figure 3. Summary of the comparison between ex vivo experiments and in silico testing based on blocking four ion currents (IKr, ICaL, INa and Ito) of all 37 compounds. Percentages are calculated including compounds for which experimental inotropic or ECG data is missing. (A) Pie chart showing percentages of compounds which demonstrated a match one or both of APD prolongation or contractility; for all compounds evaluated, one compound was found to exhibit no match between either APD or contractile comparisons. (B) Contractility match breakdown into quantitative (within 25%) or qualitative agreement, no match, or missing data. (C) APD match breakdown into quantitative, qualitative, no agreement or missing data.
Excluding compounds without contractility data, 86% showed agreement, of these, 54% were quantitative, 31% were qualitative and 14% (5 compounds) had no match. Similarly, excluding those without repolarisation data (Figure 3C), 86% demonstrated agreement: 60% quantitative, 26% qualitative, and 5 compounds (14%) presented no match.
Figure 4 illustrates four examples comparing experimental and simulated drug effects. For hydroxychloroquine (Figure 4A), simulations reveal a −58% decrease in contractile force, aligning with observed contractility loss in porcine heart slices (Wu et al., 2023) and human ventricular cardiomyocytes (Jordaan et al., 2021). A 26% APD90 prolongation at 10 µM quantitatively agreed with experiments in guinea-pigs (Wang et al., 2021) and engineered cardiac tissue from human stem cell cardiomyocytes (Wong et al., 2021). Additionally, Figure 4 also presents three proprietary compounds further exemplifying simulation-experimental agreement. SA7448 (Figure 4A) shows quantitative agreement for both repolarisation and contractile modulation across all doses. SA5397 (Figure 4B) reproduced contractile effects but predicted dose-dependent APD prolongation absent in ex vivo QT data due to negligible effects on the QT interval. SA7251 (Figure 4C) matched QTc and simulated APD, while simulated contractility contradicted experimental pressure measurements. This was linked to missing L-type calcium channel inhibition data for SA7251 (Supplementary Table S1).
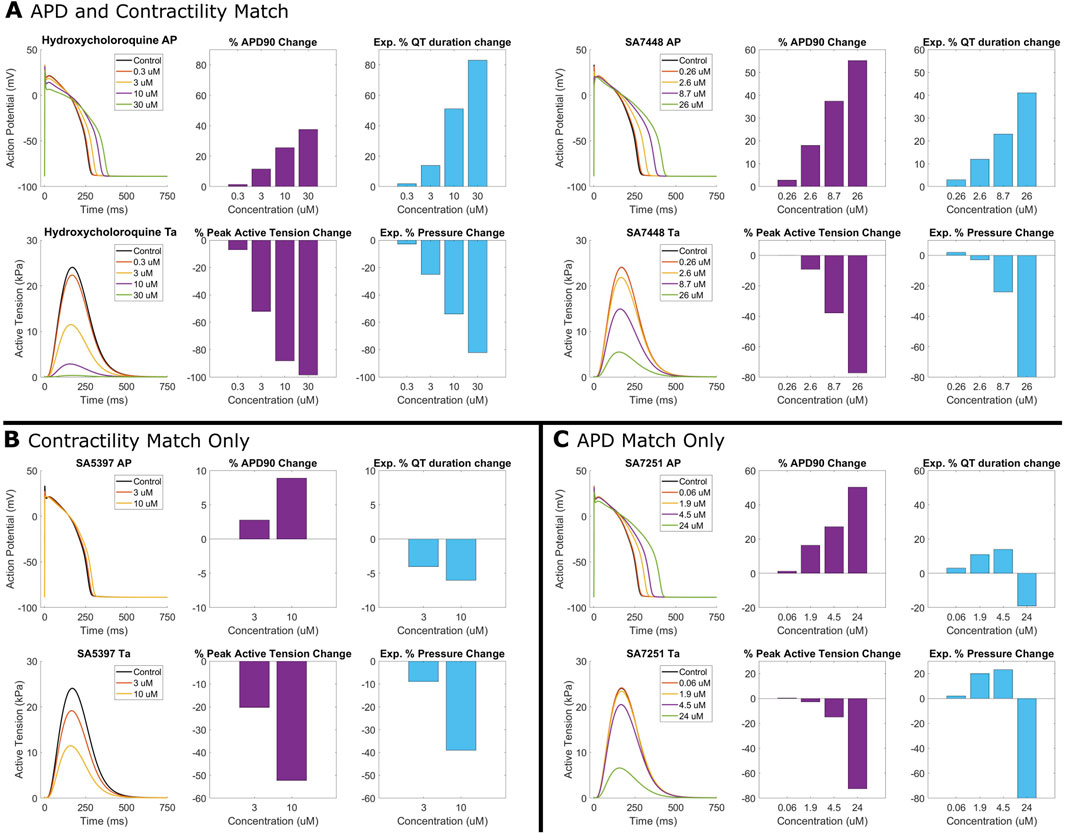
Figure 4. Comparison between human in silico and rabbit ex vivo biomarkers. Percentage modification of QT prolongation and pressure measurements taken from ex vivo Langendorff isolated rabbit heart experiments (purple) are compared with APD prolongation and active tension from simulated human ventricular myocytes (blue). (A) Quantitative match for both APD prolongation and contractility for hydroxychloroquine (right), and SA7448, a proprietary compound. (B) Quantitative agreement for contractility for SA5397 but only a qualitative agreement for APD/QT prolongation. (C) Quantitative agreement for APD/QT prolongation for SA7251, but opposite trend in contractile modulation.
Figure 5 demonstrates effect of two reference compounds, hydroxychloroquine and clozapine on populations of human ventricular myocytes, both showing quantitative agreement with internal experimental data from 0.3 to 10 µM, and quantitative agreement at 30 µM. APD variability increases at higher concentrations. Clozapine shows a +1.70% [IQR: (+1.14%, +2.01%)] APD prolongation at 0.3 µM, increasing to +39.17% [IQR: (+32.11%, +45.30%)] at 10 µM. Clinical data shows Clozapine produced a dose-dependent QTc prolongation, but clinically significant prolongation is rare; published studies show either minor (∼10 ms) or no QTc prolongation at indicted dosages (Grande et al., 2011; Wenzel-Seifert et al., 2011). Hydroxychloroquine simulations predict a +0.53% [IQR: (+0.08%, +0.89%)] APD increase at 0.3 µM, rising to +33.53% [IQR: (+24.48%, +41.98%)] at 30µM, with greater response variability. Simulated APD prolongation via hydroxychloroquine on QT matches with clinical data (N = 1890, 86% male; El Kadri et al., 2022) suggesting hydroxychloroquine has a low incidence of severe QTc prolongation at indicted dosages (up to approximately 1 µM), and an average 11 ms increase in QTc when administered in isolation (El Kadri et al., 2022; Morrisette et al., 2020). Cell population simulation results align with single-cell simulations (red diamonds in Figure 5), yet greater variations at higher concentrations emphasizes the need to account for population variability.
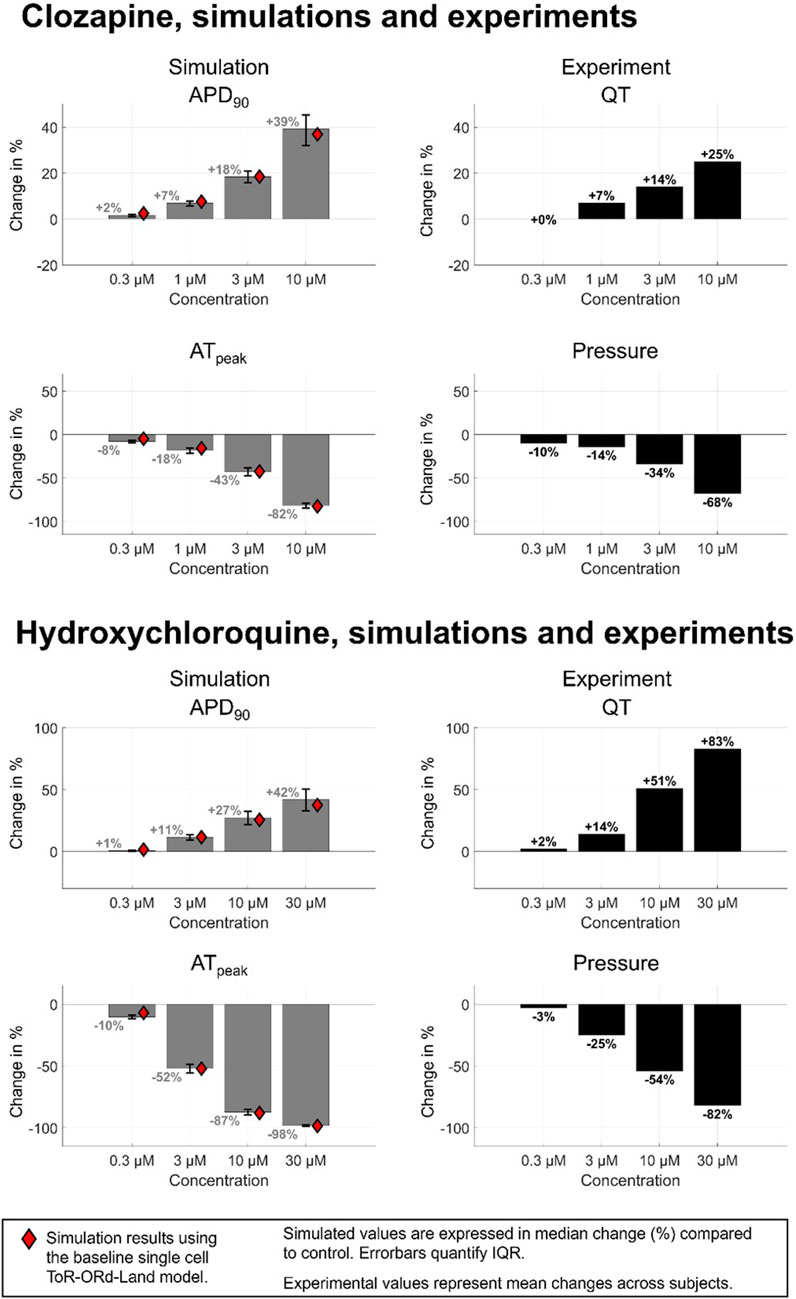
Figure 5. Comparison between human in silico and rabbit ex vivo biomarkers for 2 compounds presenting a quantitative match for both APD and contractility changes in single cell: clozapine and hydroxychloroquine. In silico markers (grey bars) quantified from a healthy-calibrated population of human ventricular cardiomyocytes (N = 155), values shown is the median, error bars show the interquartile range, and red rhombus represent the measurements obtained from single cell using the baseline ToR-ORd-Land model. The inclusion of biological variability provides further confidence in the robustness of the simulation results. IQR = Inter-quartile range.
3.2 Optimisation of translational model through sensitivity analysis
The sensitivities of APD90, peak active tension (ATpeak), time to peak active tension (ATttp) and decay to 90% active tension (ATrt90) to the reduction of 10 ionic currents are shown in Table 2. In our analyses, APD90 has the largest dependence on IKr (+34.5% at 50% block), followed by ICaL, INaL and INCX where inhibition corresponded with reductions in APD90. ATpeak was strongly dependent on INCX where inhibition led to largely increased peak tension (+97.6% at 50% block) and ICaL where inhibition reduced peak tension (−85.4% at 50%). ATpeak had lesser dependencies on Jrel, Jup, and potassium currents. ATttp and ATrt90 is mostly influenced by calcium currents (ICaL, Jup).
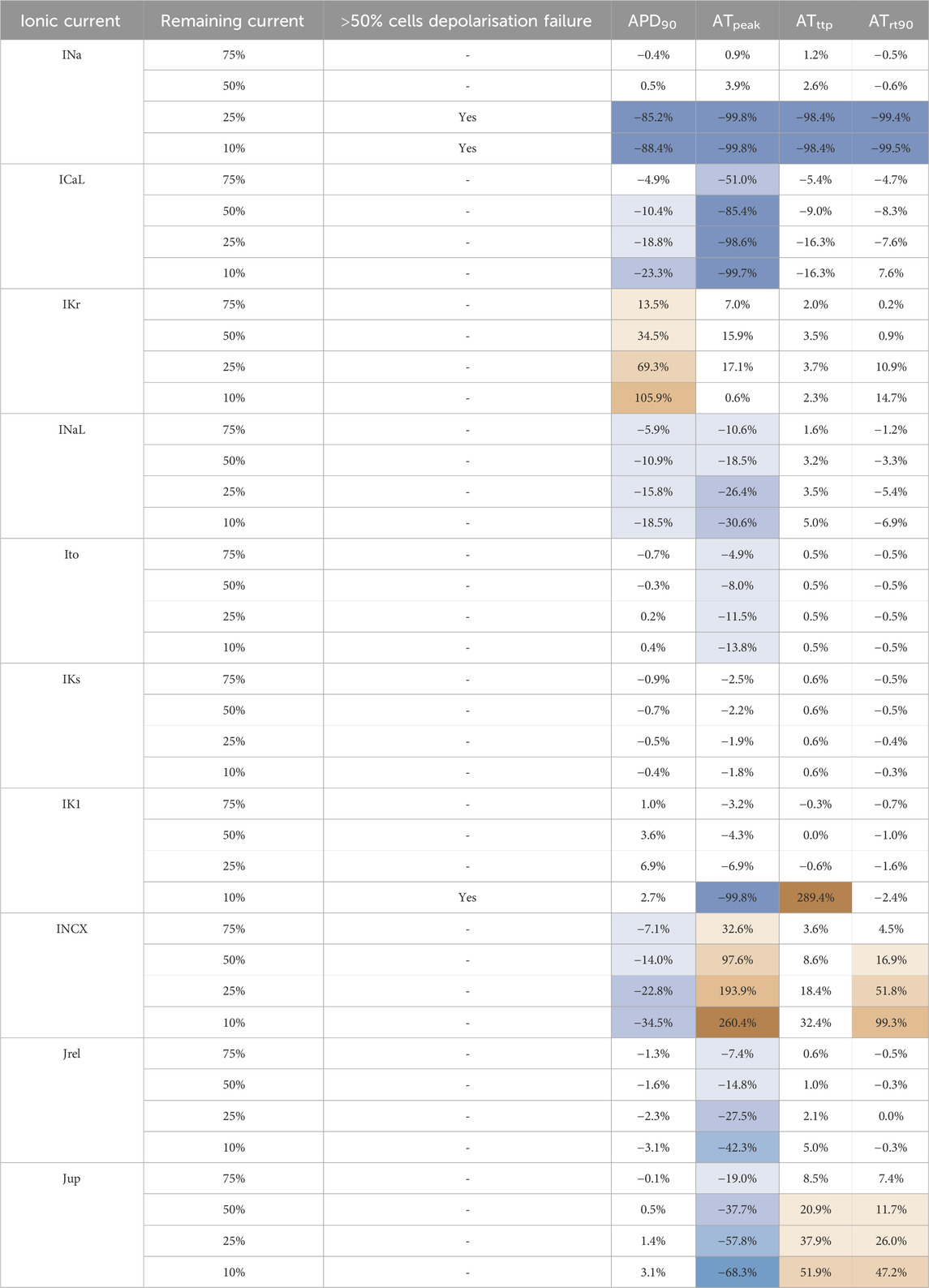
Table 2. Sensitivity analysis of APD and AT biomarkers with respect to changes in the conductivities of the main ionic currents in the human ventricular cardiomyocytes, conducted on a N = 166 variants of the ToR-ORd-Land model. For each current, conductance was modulated to 75%, 50%, 25% and 10% of the baseline value; changes in APD and contractility markers (ATpeak, ATttp, ATrt90) are quantified as change (%) in comparison to baseline. The divergent color map represents the magnitude and direction of the marker change (red: increase, blue: decrease).
The currents in the standard CiPA assay panel (INa, ICaL, IKr and Ito) are routinely quantified. In our analyses, INa, ICaL and IKr show significant effects on electrophysiology and contractile markers. Severe INa block caused depolarization failure (<25% remaining current). ICaL block leads to mild APD90 reduction, but critical contractility failure. IKr block led to large APD prolongation, and mild effects on contractility. Ito block was only shown to mildly reduce ATpeak.
These results highlight INaL and INCX as additional currents with significant impact on our selected biomarkers. INaL block moderately reduced APD90 (−10.9% at 50% block) and ATpeak (−18.5% at 50% block). INCX block also reduced APD90 moderately (−14% at 50% block) but large ATpeak increase (+97.6% at 50% block) and a relevant impact on ATrt90 (+16.9% at 50% block). We performed further experiments exploring the impact of including INCX and INaL in 12 compounds, focused on those with lesser agreements.
3.3 Inclusion of hNCX1 and late hNav1.5 inhibition in vitro data improves agreement between simulations and experiments
We tested the hypothesis that consideration of INCX and INaL inhibition would improve the match between experiments and simulations. Of the 12 compounds which displayed qualitative, or no agreement based on 4 ion channel data, we found that 6 compounds notably inhibit either INCX and INaL (Supplementary Table S1). Furthermore, Figure 6A summarises simulation-experiment agreement after including INCX and INaL inhibition. Quantitative agreement between simulated APD and experimental QT improved for one compound, and contractility agreement increased for three compounds–shifting from no match to qualitative agreement. This increased the number of compounds with agreement to 95% (35 of 37).
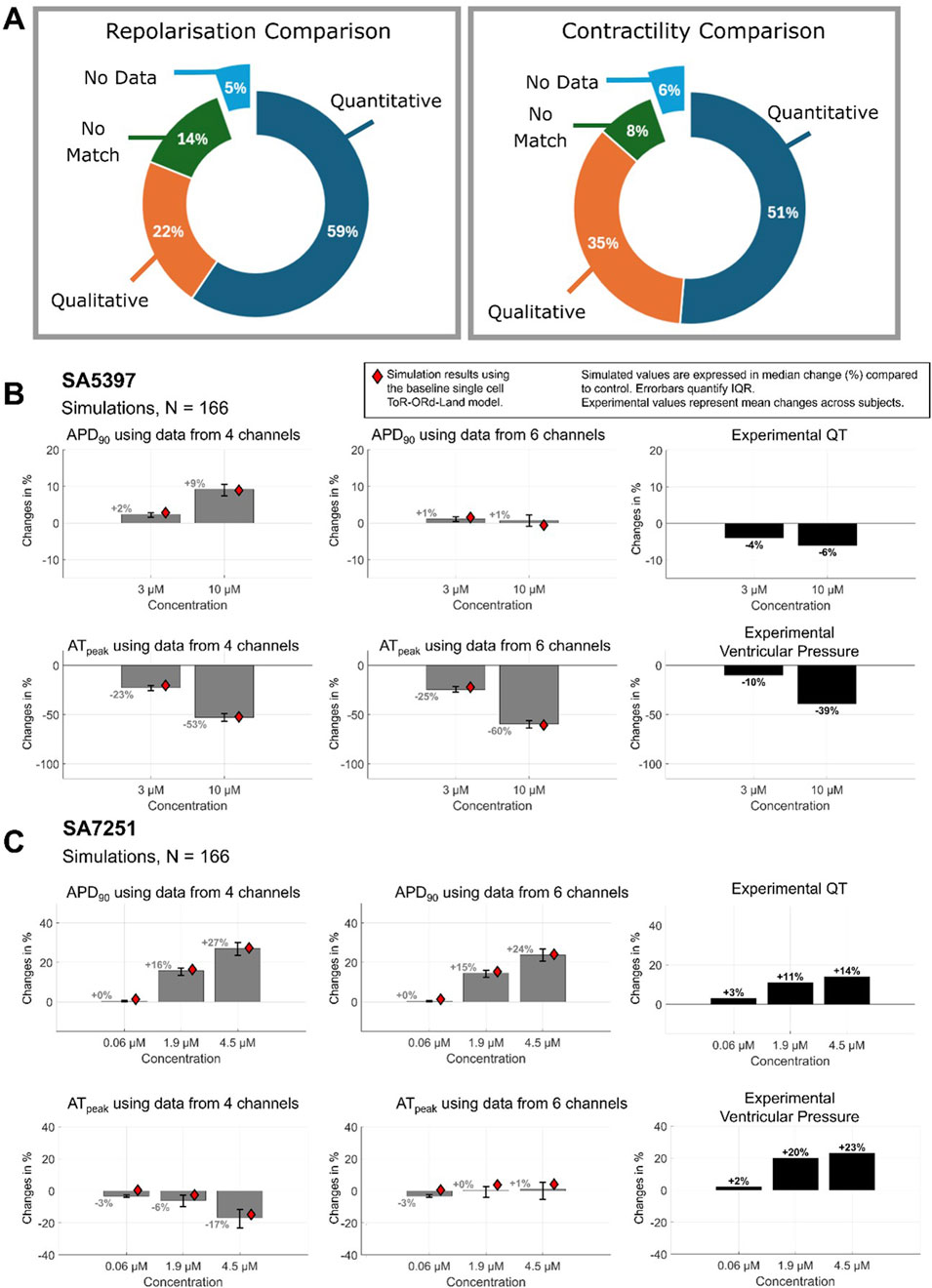
Figure 6. Simulation-Experimental Agreement with 6 channels. (A) Inclusion of NaV1.5 late sodium inhibition (late hNav1.5) and NCX1 data improves agreement for compounds (B) SA5397 and (C) SA7251, respectively. Compared to Figure 2 without this data, we observe a minor increase in contractile agreement and a major increase in APD-QT prolongation agreement.
Significant improvement was observed for four compounds (SA9714, SA7251, SA5397, SA5067) and minor improvement for SA2394. In 4 out of 6 cases where INCX or INaL block was observed to be significant (IC50 < 30 µM), agreement classification improved. Results for SA5397 and SA7251 are illustrated in Figures 6B,C, and for other compounds, data can be found in Supplementary Table S1. Inclusion of INaL inhibition data significantly improved the match for SA5397 (Figure 6B), resolving prior discrepancies between simulated APD prolongation and experimental QT recordings. Simulations now align with experimental repolarization results (−6% at 10 µM) as no change in APD was observed, achieving quantitative agreement. Similarly, the inclusion of INCX inhibition improved agreement for SA7251 (Figure 6C). INCX inhibition reduced APD prolongation from +27.3% to +24.1% at 4.5 µM; experimental: +14%) and peak active tension (from −14.8% to +4.1%), aligning contractility predictions with experimental results (+23%).
The inclusion of NCX inhibition data demonstrated complex effects across the other 6 compounds, with examples improving one match (e.g., repolarisation) at the expense of the other. The specificity of IC50 data is critical when modelling NCX inhibition due to the model’s sensitivity to NCX conductance (as shown in the sensitivity analysis) and the bimodal nature of NCX current, which influences both contractility and repolarisation (Tomek et al., 2019).
Two unmatched compounds when using 6 channel data, dobutamine and SA7560 did not have specific ion channel data for key ionic channels which drive repolarisation and contractile changes. SA7560 only had specific data for INCX inhibition and all other inhibitions were estimated. Dobutamine only has contractility data for comparison, and no specific IC50 for ICaL where dobutamine was shown to increase contractility in our ex vivo investigation; thus, it is expected that we would not observe a match.
4 Discussion
This study highlights the potential of NAMs, specifically human in silico drug trials, to replace conventional animal experiments for assessment of drug-induced effects on electrophysiology and contractility. Simulated action potential and active tension in human ventricular cardiomyocytes with four ionic current input data show predominantly quantitative agreement for 37 compounds across multiple concentrations with ex vivo rabbit whole-heart Langendorff drug screening. Consideration of additional input data on drug effects on sodium-calcium exchanger and late sodium current further improve the simulation-experimental match, particularly for contractility, as identified by a sensitivity analysis using populations of models to account for variability.
The main findings of this study are:
1. In silico predictions using the human electromechanical ventricular model (Margara et al., 2021) achieved qualitative matches for 92% of compounds, and quantitative contractility matches for 76%. For drug-induced repolarisation modulation, 85% and 64% of compounds showed qualitative and quantitative agreements respectively. Lower quantitative repolarisation matches often stemmed from strong hERG block, potentially reflecting species differences (Vandenberg et al., 2012), or lower-quality data.
2. Data quality was pivotal for accurate predictions. Compounds with complete dose-response curves and IC50 measurements yielded more quantitative agreements than those with incomplete or estimated data. For unmatched compounds, missing data (e.g., dobutamine lacking hCav1.2 IC50, or experimental APD data) was a key factor.
3. The sodium-calcium exchanger (NCX) emerged as an important factor for reproducing both repolarisation and contractility in silico. Including IC50 measurements for INCX improved agreement for several compounds with prior mismatches. Similarly, the inclusion of INaL inhibition data for the compound it was found to be significant led to a categorical improvement in agreement.
4.1 Human single cell in silico vs. whole-organ rabbit ex vivo
This study showcases the strong translatability of human-based simulations and ex vivo rabbit experiments in assessing electrophysiological and inotropic drug responses. Human-based simulations effectively predict clinical arrhythmogenesis and inotropic risk (Margara et al., 2022) offering a robust alternative to animal experiments while enhancing pharmaceutical discovery throughput (Musuamba et al., 2021). By capturing repolarisation and inotropic modulation concurrently, these simulations enable comprehensive evaluation of drugs with complex electrogenic and inotropic effects, such as Verapamil, Quinidine and Digoxin.
Sensitivity analysis identified the sodium-calcium exchanger current (INCX) and the late sodium current (INaL) as key drivers. Including hNCX1 and late NaV1.5 inhibition improved quantitative agreements for repolarisation and inotropic changes. High-quality IC50 data for pore block models yielded consistent quantitative matches, while mismatches correlated strongly with missing data, or estimated IC50 values. Dobutamine was the sole compound with no agreement, lacking experimental QT data, and hCav1.2 inhibition data. Simulations incorporating hNCX1 inhibition emphasized the need for high-quality inhibition data due to its dual-mode modulation of repolarisation and contractility, which affects both forward and reverse NCX currents.
4.2 Quantitative assessment between in silico and ex vivo
Rabbits are a widely used ventricular drug development model due to their similarly shaped action potential (Zicha et al., 2003), repolarisation reserve (Varró et al., 1993), and comparable cardiac tissue size relative to excitation wavelength (Panfilov, 2006). These characteristics make rabbits suitable for arrhythmic risk assessments (Blackwell et al., 2022) and inotropic evaluations as both species exhibit a positive force-frequency relationship (Milani-Nejad and Janssen, 2014).
Key disparities include higher and more variable Kv4.3 channel activity (Workman et al., 2012), increased SERCA2a flux (Su et al., 2003), faster heart rates, faster hERG channel deactivation and differences in IKr contributions to repolarisation (Ágoston et al., 2024). Furthermore, the input data used for ion channel drug block assume functional invariance of ion channel IC50 values between humans and rabbits (Tveito et al., 2020). Considering inter-species differences, our criteria of a similar trend within ±25% of the experimental value is a valid benchmark for quantitative agreement.
4.2.1 Explaining disagreement between simulation and experiment for only one compound
Dobutamine was the sole compound showing no agreement, with specific IC50 data only for hERG and a non-specific IC50 for hCav1.2, hNav1.2 and hNCX1 channels. In healthy volunteers, studies on dobutamine have previously shown complex relations between plasma concentration, heart rate, blood pressure and contractility (Ahonen et al., 2008; Dubin and Mugno, 2024), as well as biphasic responses in stroke volume across multiple studies. These variable responses may contribute towards the inability to predict the dobutamine response using our input data. Ex vivo rabbit experiments showed a 17% increase in ventricular pressure at 0.1µM, consistent with findings from (Trovato et al., 2025), where dobutamine increased L-type calcium current by a factor of 1.22 at EC50. Our sensitivity analysis predicts that a similar gain-of-function increase of L-type calcium current conductivity would increase simulated peak active tension by ∼40%. Using the gain-of-function data in Trovato et al. (2025) to simulate dobutamine would have resulted in a quantitative agreement with our ex vivo rabbit data.
Compounds utilising NCX data, such as SA7251, showed increasing inotropic effects with concentration, while dobutamine, which does not inhibit NCX, highlights the need for gain-of-function data to improve agreement. Compounds matching only APD or inotropic modulation between simulation and experiment often lack specific data for hERG or hCav1.2 channels–the primary drivers of repolarisation and contractility. Accurate IC50 assessment from in vitro assays is critical for evaluating ion channel blockers in silico (Trovato et al., 2022).
4.3 Advantages of in silico approaches
High-throughput computational simulations offer a powerful alternative to animal experimentation by enabling the study of patient variability through virtual cohorts. Incorporating biological variability via a population of in silico ventricular cardiomyocyte models improves prediction reliability and robustness. For selected compounds, in silico trials were conducted on 166 human ventricular cardiomyocyte models with healthy phenotypes, incorporating variability through modified ionic current conductance. This study demonstrates how drug effects vary within a calibrated healthy adult human population, with increasing concentrations amplifying response diversity. Simulations with the population of models are consistent with the single cell simulations, reinforcing our confidence in the findings. The growing variability at higher drug concentrations highlights the necessity of considering patient differences when assessing therapeutic options. This approach can extend to specific cohorts, such as unhealthy individuals with myocardial infarction and heart failure (Zhou et al., 2024b; Zhou et al., 2024a; Qu et al., 2021) or hypertrophic cardiomyopathy (Doste et al., 2022; Passini et al., 2016) and include demographic factors including advanced age, sex or ethnicity (Holmes et al., 2025; Vicente et al., 2015). Expanding simulations to cover diverse drug conditions and cohort demographics enhances the detection of arrhythmogenic or inotropic risks.
Human-based simulations provide advantages over conventional ex vivo Langendorff experimentation. These non-animal methods align with the 3R’s principles—reduction, refinement, and replacement—advancing the goals of organizations like the UK government, European Commission, and World Health Organisation. NAMs streamline drug safety assessments, increase compound throughput, and accelerate development, overcoming experimental challenges such as drug solubility and enabling testing across diverse conditions (e.g., pacing frequencies, biological sex, pregnancy) and broader populations (Cui et al., 2018; Dönertaş et al., 2019; Kelleci Çelik and Karaduman, 2023). These in silico methodologies are cost-effective, utilizing standard computational equipment without the need for additional technologies, making them superior alternatives to expensive animal experiments.
4.4 Limitations and design choices
Comparative study between animal species and human has led to useful insights into key physiological mechanisms but using animal models quantitatively has been challenging due to inherent species differences (Tveito et al., 2020). This study provides a quantitative comparison between human in silico simulations and existing rabbit Langendorff drug screening data using high-throughput assessment often used in preclinical safety assessment.
The data generated by automated patch-clamp technique was taken from experiments performed at room temperature (21 °C), rather than physiological temperatures (33 °C or higher) due to increased success rate and stability in measured current block, and better agreement with published datasets (Trovato et al., 2022). It is known that electrophysiological parameters, including APD, are temperature dependent, which may have some impact on results; however, Syren et al. (2025) show a non-significant difference between APD measured at 28 °C and 37 °C, suggesting that APD may be relatively robust within this range.
We compared simulated APD90 outputs from fast single cell simulations to rabbit Langendorff QT intervals, isolating ionic-level drug effects. QT measurements inherently integrate transmural dispersion, lead placement and autonomic tone (Boukens et al., 2015) – factors excluded in our simulations which allow for a controlled comparison. Previous works demonstrate that changes in QTc track changes in APD90 closely (∆QTc/∆APD90 ∼ 0.8–1.1) (Boukens et al., 2015; Franz et al., 1987; Sedgwick et al., 1992). Similarly, we compared peak active tension with rabbit Langendorff ventricular pressure–focusing on myofilament response without confounding factors (preload, afterload and chamber geometry). Under isometric or tightly load-controlled conditions, changes in ventricular pressure are proportional to changes in active tension, scaled by geometry (Land et al., 2017; Walley, 2016), however spatial heterogeneity of ventricular wall stress is an important factor to consider and may play a role in only qualitative agreement for some compounds. The ±25% quantitative agreement margin sufficiently contains anticipated inter-beat and inter-observer variability under standard Langendorff perfusion (King et al., 2022; Louradour et al., 2023) and is comparable to other studies of this type (Passini et al., 2017; Trovato et al., 2025).
Our results demonstrate consistency between human-based in silico simulations and rabbit Langendorff experimental recordings across both repolarisation and contractility biomarkers. The simple Hill equation used in this study is sufficient for reproducing the drug effects in silico for most compounds; however, our simulations with dobutamine reveal that more complex models including gain-of-function would improve simulation accuracy. Furthermore, with additional experimental data, more complex compound-protein binding, for example, with Markov models, could reduce uncertainty in binding mechanisms (Lei et al., 2024) and may further improve the accurate simulation of drug effects in silico (Li et al., 2017). This however comes at an increase cost, with potential benefits that need to be evaluated further. The assumption of functional invariance on the ionic current response between species was used, as done in prior human-animal comparative studies (Passini et al., 2017; Tveito et al., 2020). Refining these assumptions with additional data could improve the in silico predictions of drug response (Joukar, 2021).
The sensitivity analysis presented INCX and INaL as two drivers of repolarization and contractile modulation which improved the matching of several compounds. Additionally, Jup (SR calcium uptake) was also significant in our sensitivity analyses, but their measurements were excluded as measurements of these fluxes would require more complex additional experimental procedures. Inclusion of additional data on these channels may further improve future in silico drug studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Sanofi’s Internal Animal Ethics Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MH: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review and editing. HM-N: Conceptualization, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review and editing. MM: Conceptualization, Data curation, Investigation, Methodology, Writing – review and editing. J-MC: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing – review and editing. VB: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing – review and editing. EV: Conceptualization, Data curation, Investigation, Methodology, Resources, Writing – review and editing. AG: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – review and editing. FS: Conceptualization, Data curation, Investigation, Methodology, Writing – review and editing. BR: Conceptualization, Formal Analysis, Funding acquisition, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. MH, HM-N and BR are supported by a Wellcome Trust Fellowship in Basic Biomedical Sciences to Blanca Rodriguez (214290/Z/18/Z), and an industry funded project by Sanofi-Aventis Deutschland GmbH. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. This work is supported by the EPSRC Impact Acceleration Account Technology Fund 0016113 on Virtual Assay development and refinement.
Acknowledgments
We would like to thank Sanofi colleagues C. Chantoiseau, F. Chesney, AJ. Garnier and E. Tagat for performing the experimental ion channel electrophysiological assays. We would also like to thank Oxford colleague, Jenny Zhinuo Wang, for stimulating dialogues and insight on contractile simulations at the project’s conception.
Conflict of interest
MM, FS, J-MC, VB, EV and AG were employees of Sanofi and may hold stock/stock options in the company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1671199/full#supplementary-material
References
Ágoston, M., Kohajda, Z., Virág, L., Baláti, B., Nagy, N., Lengyel, C., et al. (2024). A comparative study of the rapid (IKr) and slow (IKs) delayed rectifier potassium currents in undiseased human, dog, rabbit, and Guinea pig cardiac ventricular preparations. Pharmaceuticals 17, 1091. doi:10.3390/ph17081091
Ahonen, J., Aranko, K., Iivanainen, A., Maunuksela, E.-L., Paloheimo, M., and Olkkola, K. T. (2008). Pharmacokinetic-pharmacodynamic relationship of dobutamine and heart rate, stroke volume and cardiac output in healthy volunteers. Clin. Drug Investig. 28, 121–127. doi:10.2165/00044011-200828020-00006
Blackwell, D. J., Schmeckpeper, J., and Knollmann, B. C. (2022). Animal models to study cardiac arrhythmias. Circ. Res. 130, 1926–1964. doi:10.1161/CIRCRESAHA.122.320258
Blanset, D., Hutt, J., and Morgan, S. (2020). Current use of animal models of disease for nonclinical safety testing. Curr. Opin. Toxicol. Transl. Toxicol. 23 (24), 11–16. doi:10.1016/j.cotox.2020.02.005
Boukens, B. J., Sulkin, M. S., Gloschat, C. R., Ng, F. S., Vigmond, E. J., and Efimov, I. R. (2015). Transmural APD gradient synchronizes repolarization in the human left ventricular wall. Cardiovasc. Res. 108, 188–196. doi:10.1093/cvr/cvv202
Brennan, T., Fink, M., and Rodriguez, B. (2009). Multiscale modelling of drug-induced effects on cardiac electrophysiological activity. Eur. J. Pharm. Sci., Biosimulations for Pharmaceutical Sciences 36, 62–77. doi:10.1016/j.ejps.2008.09.013
Colatsky, T., Fermini, B., Gintant, G., Pierson, J. B., Sager, P., Sekino, Y., et al. (2016). The comprehensive in vitro proarrhythmia assay (CiPA) initiative — update on progress. J. Pharmacol. Toxicol. Methods, Focus. Issue Saf. Pharmacol. 81, 15–20. doi:10.1016/j.vascn.2016.06.002
Cui, C., Huang, C., Liu, K., Xu, G., Yang, J., Zhou, Y., et al. (2018). Large-scale in silico identification of drugs exerting sex-specific effects in the heart. J. Transl. Med. 16, 236. doi:10.1186/s12967-018-1612-6
Dönertaş, H. M., Fuentealba, M., Partridge, L., and Thornton, J. M. (2019). Identifying potential ageing-modulating drugs in silico. Trends Endocrinol. Metab. 30, 118–131. doi:10.1016/j.tem.2018.11.005
Doste, R., Coppini, R., and Bueno-Orovio, A. (2022). Remodelling of potassium currents underlies arrhythmic action potential prolongation under beta-adrenergic stimulation in hypertrophic cardiomyopathy. J. Mol. Cell. Cardiol. 172, 120–131. doi:10.1016/j.yjmcc.2022.08.361
Dubin, A., and Mugno, M. (2024). The effects of dobutamine in septic shock: an updated narrative review of clinical and experimental studies. Med. (Mex.) 60, 751. doi:10.3390/medicina60050751
Ducroq, J., Printemps, R., Guilbot, S., Gardette, J., Salvetat, C., and Le Grand, M. (2007). Action potential experiments complete hERG assay and QT-interval measurements in cardiac preclinical studies. J. Pharmacol. Toxicol. Methods 56, 159–170. doi:10.1016/j.vascn.2007.03.009
El Kadri, M., Al Falasi, O., Ahmed, R., Al Awadhi, A., Altaha, Z., Hillis, A., et al. (2022). Changes in QTc interval after hydroxychloroquine therapy in patients with COVID-19 infection: a large, retrospective, multicentre cohort study. BMJ Open 12, e051579. doi:10.1136/bmjopen-2021-051579
EMA (2022). ICH guideline E14/S7B, 2022. European Medicines Agency. Available online at: https://www.ema.europa.eu/en/ich-guideline-e14-s7b-clinical-nonclinical-evaluation-qt-qtc-interval-prolongation-proarrhythmic-potential-questions-answers-scientific-guideline (Accessed May 02, 2025).
Franz, M. R., Bargheer, K., Rafflenbeul, W., Haverich, A., and Lichtlen, P. R. (1987). Monophasic action potential mapping in human subjects with normal electrocardiograms: direct evidence for the genesis of the T wave. Circulation 75, 379–386. doi:10.1161/01.CIR.75.2.379
Gintant, G., Sager, P. T., and Stockbridge, N. (2016). Evolution of strategies to improve preclinical cardiac safety testing. Nat. Rev. Drug Discov. 15, 457–471. doi:10.1038/nrd.2015.34
Grande, I., Pons, A., Baeza, I., Torras, Á., and Bernardo, M. (2011). QTc prolongation: is clozapine safe? Study of 82 cases before and after clozapine treatment. Hum. Psychopharmacol. Clin. Exp. 26, 397–403. doi:10.1002/hup.1221
Holmes, M., Wang, Z. J., Doste, R., Camps, J., Martinez-Navarro, H., Smith, H., et al. (2025). Sex-specific human electromechanical multiscale in-silico models for virtual therapy evaluation. bioRxiv 2025.03.14.643310. doi:10.1101/2025.03.14.643310
Jordaan, P., Dumotier, B., Traebert, M., Miller, P. E., Ghetti, A., Urban, L., et al. (2021). Cardiotoxic potential of hydroxychloroquine, chloroquine and azithromycin in adult human primary cardiomyocytes. Toxicol. Sci. 180, 356–368. doi:10.1093/toxsci/kfaa194
Joukar, S. (2021). A comparative review on heart ion channels, action potentials and electrocardiogram in rodents and human: extrapolation of experimental insights to clinic. Lab. Anim. Res. 37, 25. doi:10.1186/s42826-021-00102-3
Kelleci Çelik, F., and Karaduman, G. (2023). In silico QSAR modeling to predict the safe use of antibiotics during pregnancy. Drug Chem. Toxicol. 46, 962–971. doi:10.1080/01480545.2022.2113888
King, D. R., Hardin, K. M., Hoeker, G. S., and Poelzing, S. (2022). Reevaluating methods reporting practices to improve reproducibility: an analysis of methodological rigor for the langendorff whole heart technique. Am. J. Physiol.-Heart Circ. Physiol. 323, H363–H377. doi:10.1152/ajpheart.00164.2022
Land, S., Park-Holohan, S.-J., Smith, N. P., Dos Remedios, C. G., Kentish, J. C., and Niederer, S. A. (2017). A model of cardiac contraction based on novel measurements of tension development in human cardiomyocytes. J. Mol. Cell. Cardiol. 106, 68–83. doi:10.1016/j.yjmcc.2017.03.008
Lei, C. L., Whittaker, D. G., and Mirams, G. R. (2024). The impact of uncertainty in hERG binding mechanism on in silico predictions of drug-induced proarrhythmic risk. Br. J. Pharmacol. 181, 987–1004. doi:10.1111/bph.16250
Li, Z., Dutta, S., Sheng, J., Tran, P. N., Wu, W., Chang, K., et al. (2017). Improving the in silico assessment of proarrhythmia risk by combining hERG (human Ether-à-go-go-Related gene) channel–drug binding kinetics and multichannel pharmacology. Circ. Arrhythm. Electrophysiol. 10, e004628. doi:10.1161/CIRCEP.116.004628
Louradour, J., Ottersberg, R., Segiser, A., Olejnik, A., Martínez-Salazar, B., Siegrist, M., et al. (2023). Simultaneous assessment of mechanical and electrical function in Langendorff-perfused ex-vivo mouse hearts. Front. Cardiovasc. Med. 10, 1293032. doi:10.3389/fcvm.2023.1293032
Mamoshina, P., Rodriguez, B., and Bueno-Orovio, A. (2021). Toward a broader view of mechanisms of drug cardiotoxicity. Cell Rep. Med. 2, 100216. doi:10.1016/j.xcrm.2021.100216
Margara, F., Wang, Z. J., Levrero-Florencio, F., Santiago, A., Vázquez, M., Bueno-Orovio, A., et al. (2021). In-silico human electro-mechanical ventricular modelling and simulation for drug-induced pro-arrhythmia and inotropic risk assessment. Prog. Biophys. Mol. Biol. Mechanobiol. Cardiovasc. Syst. 159, 58–74. doi:10.1016/j.pbiomolbio.2020.06.007
Margara, F., Psaras, Y., Wang, Z. J., Schmid, M., Doste, R., Garfinkel, A. C., et al. (2022). Mechanism based therapies enable personalised treatment of hypertrophic cardiomyopathy. Sci. Rep. 12, 22501. doi:10.1038/s41598-022-26889-2
Milani-Nejad, N., and Janssen, P. M. L. (2014). Small and large animal models in cardiac contraction research: advantages and disadvantages. Pharmacol. Ther. 141, 235–249. doi:10.1016/j.pharmthera.2013.10.007
Mohr, M., Chambard, J.-M., Ballet, V., and Schmidt, F. (2022). Accurate in silico simulation of the rabbit Purkinje fiber electrophysiological assay to facilitate early pharmaceutical cardiosafety assessment: dream or reality? J. Pharmacol. Toxicol. Methods 115, 107172. doi:10.1016/j.vascn.2022.107172
Morrisette, T., Lodise, T. P., Scheetz, M. H., Goswami, S., Pogue, J. M., and Rybak, M. J. (2020). The pharmacokinetic and pharmacodynamic properties of hydroxychloroquine and dose selection for COVID-19: putting the cart before the horse. Infect. Dis. Ther. 9, 561–572. doi:10.1007/s40121-020-00325-2
Musuamba, F. T., Skottheim Rusten, I., Lesage, R., Russo, G., Bursi, R., Emili, L., et al. (2021). Scientific and regulatory evaluation of mechanistic in silico drug and disease models in drug development: building model credibility. Syst. Pharmacol. 10, 804–825. doi:10.1002/psp4.12669
Omata, T., Kasai, C., Hashimoto, M., Hombo, T., and Yamamoto, K. (2005). QT PRODACT: comparison of non-clinical studies for drug-induced delay in ventricular repolarization and their role in safety evaluation in humans. J. Pharmacol. Sci. 99, 531–541. doi:10.1254/jphs.QT-C12
Oudit, G. Y., Kassiri, Z., Sah, R., Ramirez, R. J., Zobel, C., and Backx, P. H. (2001). The molecular physiology of the cardiac transient outward potassium current (Ito) in normal and diseased myocardium. J. Mol. Cell. Cardiol. 33, 851–872. doi:10.1006/jmcc.2001.1376
Panfilov, A. V. (2006). Is heart size a factor in ventricular fibrillation? Or how close are rabbit and human hearts? Heart Rhythm 3, 862–864. doi:10.1016/j.hrthm.2005.12.022
Passini, E., Mincholé, A., Coppini, R., Cerbai, E., Rodriguez, B., Severi, S., et al. (2016). Mechanisms of pro-arrhythmic abnormalities in ventricular repolarisation and anti-arrhythmic therapies in human hypertrophic cardiomyopathy. J. Mol. Cell. Cardiol. 96, 72–81. doi:10.1016/j.yjmcc.2015.09.003
Passini, E., Britton, O. J., Lu, H. R., Rohrbacher, J., Hermans, A. N., Gallacher, D. J., et al. (2017). Human in silico drug trials demonstrate higher accuracy than animal models in predicting clinical pro-arrhythmic cardiotoxicity. Front. Physiol. 8, 668. doi:10.3389/fphys.2017.00668
Passini, E., Zhou, X., Trovato, C., Britton, O. J., Bueno-Orovio, A., and Rodriguez, B. (2021). The virtual assay software for human in silico drug trials to augment drug cardiac testing. J. Comput. Sci. 52, 101202. doi:10.1016/j.jocs.2020.101202
Pelat, M., Barbe, F., Daveu, C., Ly-Nguyen, L., Lartigue, T., Marque, S., et al. (2021). SAR340835, a novel selective Na+/Ca2+ exchanger inhibitor, improves cardiac function and restores sympathovagal balance in heart failure. J. Pharmacol. Exp. Ther. 377, 293–304. doi:10.1124/jpet.120.000238
Pointon, A., Harmer, A. R., Dale, I. L., Abi-Gerges, N., Bowes, J., Pollard, C., et al. (2015). Assessment of cardiomyocyte contraction in human-induced pluripotent stem cell-derived cardiomyocytes. Toxicol. Sci. Off. J. Soc. Toxicol. 144, 227–237. doi:10.1093/toxsci/kfu312
Qu, Y., Gao, B., Arimura, Z., Fang, M., and Vargas, H. M. (2021). Comprehensive in vitro pro-arrhythmic assays demonstrate that omecamtiv mecarbil has low pro-arrhythmic risk. Clin. Transl. Sci. 14, 1600–1610. doi:10.1111/cts.13039
Redfern, W. S., Carlsson, L., Davis, A. S., Lynch, W. G., MacKenzie, I., Palethorpe, S., et al. (2003). Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc. Res. 58, 32–45. doi:10.1016/S0008-6363(02)00846-5
Robertson, A. S., Latif, N., Bousnina, I., Boyce, D., Carl, K., Curtis, S. P., et al. (2025). Accelerating adoption of new approach methodologies in regulatory decision making: an industry perspective. Nat. Rev. Drug Discov. 24, 401–402. doi:10.1038/d41573-025-00038-6
Roche, M., Renauleaud, C., Ballet, V., Doubovetzky, M., and Guillon, J.-M. (2010). The isolated rabbit heart and Purkinje fibers as models for identifying proarrhythmic liability. J. Pharmacol. Toxicol. Methods 61, 238–250. doi:10.1016/j.vascn.2010.01.011
Romero, L., Cano, J., Gomis-Tena, J., Trenor, B., Sanz, F., Pastor, M., et al. (2018). In silico QT and APD prolongation assay for early screening of drug-induced proarrhythmic risk. J. Chem. Inf. Model. 58, 867–878. doi:10.1021/acs.jcim.7b00440
Sedgwick, M. L., Rasmussen, H. S., and Cobbe, S. M. (1992). Effects of the class III antiarrhythmic drug dofetilide on ventricular monophasic action potential duration and QT interval dispersion in stable angina pectoris. Am. J. Cardiol. 70, 1432–1437. doi:10.1016/0002-9149(92)90295-A
Su, Z., Li, F., Spitzer, K. W., Yao, A., Ritter, M., and Barry, W. H. (2003). Comparison of sarcoplasmic reticulum Ca2+-ATPase function in human, dog, rabbit, and mouse ventricular myocytes. J. Mol. Cell. Cardiol. 35, 761–767. doi:10.1016/s0022-2828(03)00119-6
Syren, P., Zlatopolskaia, A., Bruehl, C., Schöffel, A., Caspari, T., Heß, C., et al. (2025). Heterogeneity of ventricular action potentials in neonatal rat cardiomyocytes and methodological aspects of patch clamp measurements. Front. Physiol. 16, 1537345. doi:10.3389/fphys.2025.1537345
Tomek, J., Bueno-Orovio, A., Passini, E., Zhou, X., Minchole, A., Britton, O., et al. (2019). Development, calibration, and validation of a novel human ventricular myocyte model in health, disease, and drug block. eLife 8, e48890. doi:10.7554/eLife.48890
Trovato, C., Mohr, M., Schmidt, F., Passini, E., and Rodriguez, B. (2022). Cross clinical-experimental-computational qualification of in silico drug trials on human cardiac purkinje cells for proarrhythmia risk prediction. Front. Toxicol. 4, 992650. doi:10.3389/ftox.2022.992650
Trovato, C., Longobardi, S., Passini, E., Beattie, K. A., Holmes, M., Chaudhary, K., et al. (2025). In silico predictions of drug-induced changes in human cardiac contractility align with experimental recordings. Front. Pharmacol. 16, 1500668. doi:10.3389/fphar.2025.1500668
Tveito, A., Jæger, K. H., Maleckar, M. M., Giles, W. R., and Wall, S. (2020). Computational translation of drug effects from animal experiments to human ventricular myocytes. Sci. Rep. 10, 10537. doi:10.1038/s41598-020-66910-0
Valentin, J.-P., and Leishman, D. (2023). 2000–2023 over two decades of ICH S7A: has the time come for a revamp? Regul. Toxicol. Pharmacol. 139, 105368. doi:10.1016/j.yrtph.2023.105368
Valentin, J.-P., and Leishman, D. (2025). Points to consider for revising the ICH S7A guideline on safety and secondary pharmacology. Regul. Toxicol. Pharmacol. 159, 105795. doi:10.1016/j.yrtph.2025.105795
van Meer, B. J., Tertoolen, L. G. J., and Mummery, C. L. (2016). Concise review: measuring physiological responses of human pluripotent stem cell derived cardiomyocytes to drugs and disease. Stem Cells 34, 2008–2015. doi:10.1002/stem.2403
Van Norman, G. A. (2020). Limitations of animal studies for predicting toxicity in clinical trials: part 2: potential alternatives to the use of animals in preclinical trials. JACC Basic Transl. Sci. 5, 387–397. doi:10.1016/j.jacbts.2020.03.010
Vandenberg, J. I., Perry, M. D., Perrin, M. J., Mann, S. A., Ke, Y., and Hill, A. P. (2012). hERG K+ channels: structure, function, and clinical significance. Physiol. Rev. 92, 1393–1478. doi:10.1152/physrev.00036.2011
Varró, A., Lathrop, D. A., Hester, S. B., Nánási, P. P., and Papp, J. G. (1993). Ionic currents and action potentials in rabbit, rat, and Guinea pig ventricular myocytes. Basic Res. Cardiol. 88, 93–102. doi:10.1007/BF00798257
Varshneya, M., Mei, X., and Sobie, E. A. (2021). Prediction of arrhythmia susceptibility through mathematical modeling and machine learning. Proc. Natl. Acad. Sci. 118, e2104019118. doi:10.1073/pnas.2104019118
Viceconti, M., Pappalardo, F., Rodriguez, B., Horner, M., Bischoff, J., and Musuamba Tshinanu, F. (2021). In silico trials: verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods, Methods Simul. Biomed. 185, 120–127. doi:10.1016/j.ymeth.2020.01.011
Vicente, J., Johannesen, L., Mason, J. W., Pueyo, E., Stockbridge, N., and Strauss, D. G. (2015). Sex differences in drug-induced changes in ventricular repolarization. J. Electrocardiol. 48, 1081–1087. doi:10.1016/j.jelectrocard.2015.08.004
Walley, K. R. (2016). Left ventricular function: time-varying elastance and left ventricular aortic coupling. Crit. Care 20, 270. doi:10.1186/s13054-016-1439-6
Wang, G., Lu, C.-J., Trafford, A. W., Tian, X., Flores, H. M., Maj, P., et al. (2021). Electrophysiological and proarrhythmic effects of hydroxychloroquine challenge in guinea-pig hearts. ACS Pharmacol. Transl. Sci. 4, 1639–1653. doi:10.1021/acsptsci.1c00166
Wenzel-Seifert, K., Wittmann, M., and Haen, E. (2011). QTc Prolongation by Psychotropic Drugs and the Risk of Torsade de Pointes. Dtsch. Ärztebl. Int. 108, 687–693. doi:10.3238/arztebl.2011.0687
Wong, A.O.-T., Gurung, B., Wong, W. S., Mak, S. Y., Tse, W. W., Li, C. M., et al. (2021). Adverse effects of hydroxychloroquine and azithromycin on contractility and arrhythmogenicity revealed by human engineered cardiac tissues. J. Mol. Cell. Cardiol. 153, 106–110. doi:10.1016/j.yjmcc.2020.12.014
Workman, A. J., Marshall, G. E., Rankin, A. C., Smith, G. L., and Dempster, J. (2012). Transient outward K+ current reduction prolongs action potentials and promotes afterdepolarisations: a dynamic-clamp study in human and rabbit cardiac atrial myocytes. J. Physiol. 590, 4289–4305. doi:10.1113/jphysiol.2012.235986
Wu, Q., Ross, A. J., Ipek, T., Thompson, G. H., Johnson, R. D., Wu, C., et al. (2023). Hydroxychloroquine and azithromycin alter the contractility of living porcine heart slices. Front. Pharmacol. 14, 1127388. doi:10.3389/fphar.2023.1127388
Zhou, X., Qu, Y., Passini, E., Bueno-Orovio, A., Liu, Y., Vargas, H. M., et al. (2020). Blinded in silico Drug Trial Reveals the Minimum Set of Ion Channels for Torsades de Pointes Risk Assessment. Front. Pharmacol. 10, 1643. doi:10.3389/fphar.2019.01643
Zhou, X., Levesque, P., Chaudhary, K., Davis, M., and Rodriguez, B. (2024a). Lower diastolic tension may be indicative of higher proarrhythmic propensity in failing human cardiomyocytes. Sci. Rep. 14, 17351. doi:10.1038/s41598-024-65249-0
Zhou, X., Wang, Z. J., Camps, J., Tomek, J., Santiago, A., Quintanas, A., et al. (2024b). Clinical phenotypes in acute and chronic infarction explained through human ventricular electromechanical modelling and simulations. eLife 13. doi:10.7554/eLife.93002
Keywords: modelling and simulation, new approach methodologies, cardiac electromechanics contractility, drug modelling, cardiotoxicity, cardiac risk assessment, digital twin
Citation: Holmes M, Martinez-Navarro H, Mohr M, Chambard J-M, Ballet V, Vermersch E, Garry A, Schmidt F and Rodriguez B (2025) Quantitative assessment of the usability of electromechanical human-based modelling and simulation to replace Langendorff isolated rabbit heart experiments in the preclinical setting. Front. Pharmacol. 16:1671199. doi: 10.3389/fphar.2025.1671199
Received: 22 July 2025; Accepted: 30 September 2025;
Published: 24 October 2025.
Edited by:
Simone Brogi, University of Pisa, ItalyReviewed by:
Vladimír Sobota, Masaryk University, CzechiaTareg Bey, University of Texas MD Anderson Cancer Center, United States
Copyright © 2025 Holmes, Martinez-Navarro, Mohr, Chambard, Ballet, Vermersch, Garry, Schmidt and Rodriguez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maxx Holmes, bWF4eC5ob2xtZXNAY3Mub3guYWMudWs=
†These authors have contributed equally to this work and share first authorship
 Maxx Holmes
Maxx Holmes Hector Martinez-Navarro
Hector Martinez-Navarro Marcel Mohr
Marcel Mohr Jean-Marie Chambard
Jean-Marie Chambard Veronique Ballet3
Veronique Ballet3 Eva Vermersch
Eva Vermersch Ambroise Garry
Ambroise Garry Friedemann Schmidt
Friedemann Schmidt Blanca Rodriguez
Blanca Rodriguez