- 1School of Electronic Information, Hunan First Normal University, Changsha, China
- 2College of Computer Science and Electronic Engineering, Hunan University, Changsha, China
- 3Wenzhou University of Technology, Wenzhou, China
- 4School of Computer Science, Hunan Institute of Technology, Hengyang, China
Metabolic diseases—including type 2 diabetes, obesity, non-alcoholic fatty liver disease, and certain cancers—pose major global public health challenges. These conditions share common mechanisms such as insulin resistance, chronic inflammation, and oxidative stress. Although medical advances have improved disease management, current treatments remain suboptimal. Natural medicines have gained increasing interest due to their safety, bioactivity, and diverse mechanisms. This study targets adenosine receptors (ARs), key regulators in glucose metabolism, lipid homeostasis, and cellular stress. As members of the G protein-coupled receptor (GPCR) family, ARs include four subtypes—A1, A2A, A2B, and A3—each with distinct pharmacological profiles. We developed a multimodal computational strategy to design natural drug candidates that simultaneously target A1 and A2A, using A2A-selective ligands as controls to explore subtype selectivity. To mitigate toxicity, we incorporated a filtering criterion for low hERG channel affinity. A random forest-based QSAR model was constructed using SMILES representations to predict compound activity. A stacked LSTM neural network was applied to generate plant-derived molecules, while reinforcement learning and Pareto optimization enabled multi-objective refinement. Evolutionary operations—crossover, mutation, and selection—were further introduced to enhance molecular diversity and performance. The proposed framework successfully generated compounds with high target selectivity, low toxicity, and it has good drug-likeness and synthetic accessibility. This work presents a robust and intelligent strategy for natural drug discovery in metabolic diseases and underscores the promising synergy between botanical medicine and artificial intelligence in therapeutic innovation.
1 Introduction
Metabolic diseases, such as type 2 diabetes (DeFronzo et al., 2015; Ahmad et al., 2022), obesity, non-alcoholic fatty liver disease and certain types of cancer, have become major global public health challenges due to their high incidence and complex pathological mechanisms. These diseases are often accompanied by metabolic homeostasis disorders such as insulin resistance, chronic inflammation and oxidative stress. Long-term reliance on traditional drug treatments has limitations in terms of efficacy and safety. Natural medicines, with their diverse structures, strong biological activities and wide range of action mechanisms, have shown unique advantages in the intervention of metabolic diseases. However, the traditional process of natural drug discovery is time-consuming and has weak target specificity, which limits the efficiency of clinical transformation (Atanasov et al., 2021). With the development of artificial intelligence and computational pharmacology, molecular generation methods combined with multi-objective optimization strategies are becoming important means for efficient screening and design of natural drug candidates (Vamathevan et al., 2019). Against this background, targeting key receptors involved in glucose metabolism, lipid regulation and cellular stress, such as adenosine receptors, provides new targets and research paths for the precise design of natural drugs.
In the field of multi-target pharmacology, drugs can be combined with multiple specific targets at the same time, aiming to enhance the therapeutic effect and reduce the development of drug resistance (Wei et al., 2024a). Studies have shown that multi-target suppression of a partial target is often more effective than complete suppression of a single target, and this strategy is especially suitable for complex and multifactorial disease conditions (Fu et al., 2025; Zhou et al., 2024). Recent global–local re-interpretation of drug–protein interactions further corroborates this view, indicating that balanced modulation across multiple targets can enhance therapeutic efficacy in metabolic disorders (Zhou et al., 2024).In addition, when the multiple targets mentioned are actually multiple mutant forms of a single target, the drug also has the ability to bind to these mutants simultaneously (Hopkins, 2008).
In addition, since different proteins may share common structures with similar functions, this increases the risk of non-specific binding to non-target proteins (Cai et al., 2025; Wang et al., 2025). Therefore, when working to develop highly targeted drug molecules, it is important to enhance the targeting selectivity of drugs to ensure that they avoid improper binding to non-target proteins. The key to achieving this goal is to accurately identify and target the uniqueness of the target protein, thereby minimizing the non-targeted effects and improving the overall safety and effectiveness of the drug (Tan et al., 2025). In this context, deep learning and reinforcement learning technologies, with their ability to predict drug binding affinity to target proteins and evaluate potential non-targeted effects, show great potential and promise in facilitating highly targeted and selective drug design (Zeng et al., 2024). Developing highly targeted drugs has become a central goal in the field of drug discovery, which can not only greatly optimize the treatment of complex diseases, but also effectively avoid unnecessary side effects, resulting in safer and more efficient treatment options for patients. Recent heterogeneous-graph approaches, such as the interpretable multi-instance model for circRNA–drug sensitivity prediction (Niu et al., 2025) and the deep multi-instance framework for drug–disease associations (Gu et al., 2025), have further demonstrated the value of integrating multi-omics and network information in computational drug discovery”.
As early as the 1970s, statistical mathematical modeling based on perceptrons has been used for computer-assisted medicine. Design, this kind of mathematical method belongs to supervised machine learning. However, until now, unsupervised deep learning drug design research has begun to emerge. Among them, generative deep learning can break through the technical bottleneck of traditional computer-aided drug design by extracting hidden features from molecules. In Zhavoronkov et al. (2019) used a deep generation model to successfully discover high-quality lead compounds from target screening to nanomolar activity in just 46 days, marking a milestone in the application of generative deep learning to new molecular design (Xu et al., 2025).
The core of the program is focused on developing drugs that can bind efficiently to specific targets. adenosine receptors (AR)(Ijzerman et al., 2022), as a class of receptors similar to G protein-coupled receptors (GPCRS)(Weis and Kobilka, 2018), are endogenous to adenosine (Wang et al., 2023). Adenosine and AR are widely distributed in various tissues of the human body, and their interaction triggers a wide range of physiological and pathological processes. The AR family consists of four subtypes, A1, A2A, A2B and A3, each of which exhibits unique pharmacological properties, tissue distribution patterns and effect coupling mechanisms (Fredholm, 2010; Chen et al., 2024). This project will focus on drug molecules that generate Adenosine receptor A1 and Adenosine receptor A2A, as well as drug molecules that only affine A2A while serving as control affinity A1. In addition, to reduce the risk of toxicity and adverse events, drugs should be designed to exhibit low affinity for member H 2 of the potassium voltage-gated channel subfamily (often referred to simply as the hERG channel, or human Ether-a-go-go-Related Gene Channel) (Cai et al., 2021). This can effectively prevent the drug from binding with non-target.
2 Methods
2.1 Data sets and molecular selection
The ChEMBL database (Gaulton et al., 2011) is a publicly available drug molecule database developed by the European Bioinformatics Institute (EBI) in collaboration with pharmaceutical companies and other partners. It contains a large number of small molecule compounds and their biological activity data, including the biological activity and pharmacological properties of the compounds, the structure and chemical information of the compounds, the structure and physiological function of biomolecules and other data (Zdrazil et al., 2023). These data are derived primarily from journals and papers, partner data, and are integrated with data on today’s approved drugs, current clinical development candidates in medicine, and other public databases. Together, it brings together chemical and biological information from multiple sources, covering multiple species and multiple disease domains. If the information in the ChEMBL database is fully utilized, it can help deep learning models for drug screening, design, and optimization.
At present, the latest version of the ChEMBL database is the ChEMBL35 database updated in December 2024 (Zdrazil, 2025). In order to ensure the reliability of the data, we need to reasonably control the number of data sets to meet the specific needs of the research, we choose ChEMBL34 as the data set for this research. ChEMBL34 contains data on approximately 2.4 million unique drug-like compounds and over 20 million bioactivity data points related to these compounds. After downloading the database file, the data set needs to be preprocessed: For charged molecules, the charge should be standardized, metals, small molecules and super-large molecules removed, the entire data set should be checked, and duplicate data should be removed. Finally, a total of about 2.1 million data were obtained, which was used as the ChEMBL data set for pre-training of the generation model, so that the generation model could generate legitimate drug molecules. Data preprocessing can be implemented using RDKit.
The molecules selected in this study have corresponding CHEMBL ID in ChEMBL: adenosine receptor A1 is CHEMBL226, adenosine receptor A2A is CHEMBL251, and hERG channel is CHEMBL240 (Mendez et al., 2018). By extracting CHEMBL ID, about 23,000 ligands that are biologically active to the above molecules are extracted from the processed ChEMBL data set and constructed into LIGAND data set for fine-tuning the generation model.
In order to concretely quantify the biological activity of drug molecules, the ChEMBL database also provides PCHEMBL VALUE (in the ChEMBL database, the value is given by the negative logarithm of IC50, EC50, XC50, AC50, Ki, Kd, Potency). (hereinafter referred to as pX value) for reference (Lenselink et al., 2017). In relevant studies, the threshold of biological activity was defined as
2.2 Deep learning
Today’s deep learning models show excellent performance in many areas, including widely used predictive models and generative model structures (Wei et al., 2024b). Predictive model and generative model are two important application directions of deep learning in drug molecule discovery. The predictive model is mainly trained for predictive analysis of a given molecule, including predicting the biological activity, drug efficacy, toxicity and other information of the molecule (Lai et al., 2025). Generative models automatically synthesize new molecular structures through deep learning models, providing important support for the design of new drugs (Wang et al., 2024).
In this project, the predictive model is trained first, aiming to make it have the ability to calculate

Figure 1. The computational flowchart of natural drugs for adenosine receptors. Arrows indicate process flow, and elements are labeled from (A-H).
2.2.1 Prediction model
Quantitative Structure-Activity Relationship (QSAR) model is one of the commonly used prediction models in drug molecular development (Kiralj and Ferreira, 2009). The regression QSAR model uses a series of molecular descriptors to describe the physical and chemical structural properties of drug molecules with the help of mathematical means. By constructing linear or nonlinear correlations between the structure and activity of molecular compounds, the model can predict the pharmacodynamic activity of new drug molecules. Regression QSAR model has been widely used in drug discovery field. It can screen drug molecules in the early drug development stage, improve screening efficiency and reduce costs. In addition, by analyzing the construction relationship of the regression QSAR model, researchers can better understand the relationship between drug molecular structure and activity, and provide guidance for further drug design.
In this project, we adopted a regression QSAR model as a predictive tool to predict the pX value of each molecule generated for a specific target. To enhance the fault tolerance of the QSAR model and enable it to make predictions about more chemical molecules, we added low-quality data to the dataset with no pChEMBL value, i.e., molecules labeled as not biologically active or with no defined pX value. For these data, define the pX value of these data to be 3.99, which is slightly less than 4, thus eliminating the imbalance of the data set and ensuring that the trained regression QSAR model has the ability to predict negative samples. In the model-training phase, we explicitly addressed the class imbalance between positive (
Labels such as CHEMBL ID, SMILES, pX value, comment, Standard Type, Standard Relation are extracted from the ligand data set for target molecules, and pX value is set to 3.99 for low-quality data. For each molecule its ECFP6 fingerprint is calculated by the RDKit Morgan fingerprint algorithm (Rogers and Hahn, 2010), as an input to the predictive model, with 2048 bits (i.e., a vector with 2048 dimensions). In addition, in order to describe the properties of molecules, it is necessary to add a 19-dimensional physicochemical descriptor: Molecular weight, logP, number of hydrogen bond acceptors and donors, number of rotatable bonds, number of amide bonds, number of bridgehead atoms, number of heteroatoms, number of helix atoms, number of heavy atoms, SP3 The fraction of hybrid carbon atoms, the number of alicycles, the number of saturated rings, the number of total rings, the number of aromatic rings, the number of heterocycles, the number of valence electrons, the polar surface area, and the Wildman-Crippen MR Value.
Therefore, each molecule in the data set will be converted into a 2067-dimensional feature vector. Before being submitted to the prediction model, the values of these eigenvectors are normalized to the interval
The mainstream directions of QSAR (Trinh et al., 2022) models mainly include: Statistics-based QSAR model: This type of model is based on statistical analysis of molecular structure and activity data, and analyzes and predicts by establishing mathematical models. The advantage is that it is easy to understand and implement, but the disadvantage is that it cannot deal with more complex molecular structures and characteristics. Typical examples of these models include partial least squares regression (PLS) (Xie et al., 2022) and multiple linear regression (MLR) (Shams et al., 2021). QSAR model based on machine learning: This type of model uses machine learning algorithms to map molecular structure and activity data to a high-dimensional space, and describes the relationship between them by building a nonlinear model. Its advantage is that it can handle relatively complex molecular structures and features, but it requires a large number of data sets and computational resources. Typical examples of such models are Support Vector Machine SVM) (El Morr et al., 2022), Random Forest (RF) (Belgiu and Draguţ, 2016) QSAR model based on deep learning: This type of model utilizes deep neural network algorithms to construct efficient nonlinear mapping relationships through multi-level feature extraction and abstraction. It has the advantage of being able to process large-scale molecular structure and activity data, but requires more computational resources and expertise. At present, common Deep Neural networks (DNN) (Pan et al., 2012) and Multi-task Deep Neural networks (MT-DNN) developed on this basis belong to this category. According to the relevant research on the target molecules in this subject (Liu et al., 2021), and considering the training time of the model and the available computing power, we choose to use the random forest algorithm to build the regression QSAR model, set the number of trees to 1000, take the Gini index as the segmentation standard, and realize it through Scikit-Learn.
The prediction model, which extracts the required labels from the ligand dataset for the target molecule. Each molecule is transformed into a 2067-dimensional vector. Subsequently, a MinMax operation is performed to normalize the vector values to the range of [0,1]. This normalized vector is then input into the QSAR model based on random forests, ultimately yielding the probability of the compound’s activity based on this vector.
Random Forest’s built-in out-of-bag error provides an unbiased internal validation, which is particularly advantageous for our highly imbalanced positive/negative sample ratio. These empirical and practical considerations collectively led us to adopt Random Forest as the QSAR engine. Random Forest was selected over XGBoost because, on our imbalanced dataset, it yielded 4% lower MAE and 6% higher AUROC in 10-fold cross-validation while requiring 30% less tuning time.
2.2.2 Generative model
Molecules characterized by the Simplified molecular Input Line input system (SMILES) (Weininger, 1988) are essentially sequences arranged according to specific rules, the hidden states that deep learning models want to learn are actually relationships between sequences, just like the objects that NLP problems deal with. Therefore, in order to learn the relationship of atoms within the entire drug molecule, which can be regarded as natural language text, using RNNs as a model for deep learning is a more appropriate choice. RNNs can accept SMILES strings as input, identify and understand the molecules represented by SMILES strings by examining them one by one, such as identifying the bonds, functional groups, etc., predict the next character, and continue the process to predict the entire molecule. Generative models build molecules in SMILES form, but generative models cannot generate molecules out of thin air and require us to provide SMILES dictionaries. Each drug molecule represented by SMILES in the CHEMBL and LIGAND data sets (Lagarde et al., 2015) is split into a series of markers, including bonds and roots. In this way, after processing all the data, we can extract all the markers that have appeared in the data set and collect all the markers that exist in the data set, thus forming the SMILES vocabulary of this topic. The final vocabulary contains 88 tokens, which are placed in order and the generative model is trained to form a valid SMILES sequence with the correct syntax.
The RNN model for SMILES sequence generation consists of six layers: an input layer, an embedding layer, three cyclic layers, and an output layer. After the drug molecule is represented as a sequence of characters, the RNN can receive it as a classification feature through the input layer. In the embedding layer, the vocabulary size is set to 88, consistent with the size of the SMILES vocabulary collected; The embedding dimension is set to 128, so that each drug molecule can be converted to a 128-dimensional vector. In the RNN model, Long Short-Term Memory (LSTM) (Van Houdt et al., 2020) is a commonly used cyclic unit. Compared with the traditional RNN model, LSTM can more effectively avoid the situation of gradient disappearance or local gradient explosion, thus improving the accuracy and generalization ability of the model. In addition, LSTM can better control the flow of information through Gated mechanisms and memory units, thereby improving the performance of the model, and compared to gated Recurrent units (GRUs) (Dey and Salem, 2017), it can effectively handle long-term dependencies and information transfer in string sequence data. Therefore, for the loop layer, we opt to use LSTM as the recurrent units, employing a 3-layer LSTM as the basic building block and stacking it up to 9 layers within the module of the generative model, while setting the number of hidden neurons to 512, instead of using GRU.In the output layer, the output for each position determines which character from the vocabulary is selected to increase the probability of SMILES strings.
Compared with ordinary language sentences, the length of SMILES molecules is obviously much longer than the character length of ordinary sentences. Therefore, in this topic, a single RNN model may not be able to learn the relationship between atoms in the whole molecule, and the training effect of using a single RNN model is relatively limited. If you want to improve training, the most straightforward way is to stack multiple RNNS, using the output and hidden state of the previous RNN model as the input of the next RNN model. In the forward propagator, the input is first renormalized to fit the dimensions of the embedding layer. Then, between each two layers of RNN model, the output of the RNN model is activated by Pytorch’s ReLU function, and the shape of the output is modified by a fully connected layer to the dimension size of the embedding layer, so that it can be used as the input of the next RNN model. In the training phase, we add a start flag (GO) to the front end of each data batch as an input signal, and set an end flag at the end of the same data batch. This ensures that the generation network can accurately select the appropriate label at each iteration based on the previously generated sequence information. For the loss function of RNN, we use the negative logarithmic likelihood function to build it to ensure that each marker in the output sequence is selected with maximum probability after training; At the same time, in order to optimize the model parameters, we used Adam algorithm instead of the traditional gradient descent process to optimize the loss function. In the training process of this subject, the learning rate is set to 10–3, the batch size is set to 512, and the training cycle is set to 1000.
2.2.3 Self-attention mechanism
Self-attention mechanism (Yang et al., 2016) is a widely used technique in deep learning, which has some flexibility and can be customized for different tasks and data to better meet the needs of different scenarios. In the field of NLP problems, self-attention mechanisms are also widely used. In language modeling, translation, summary generation, sentiment analysis and other tasks, the self-attention mechanism can help the model better understand the relationship between different parts of the input data, thereby improving the performance and accuracy of the model.
Similarly, as mentioned above, due to the similarity between drug molecule discovery research and NLP problem, self-attention mechanism can also be applied in deep learning of this subject, and has objective performance improvement.
Self-attention mechanisms can help generative models better understand the relationships between different atoms in a molecule and deal with long-distance dependencies in molecules. In drug molecular design, drug molecules are usually composed of many atoms, and the interactions between these atoms are very complex, and the interactions between different atoms may be affected by other atoms in the molecule, and may even involve distant parts of the molecule, which is difficult to capture with traditional neural network models. In the previous article, we represented each molecule as a vector, and the self-attention mechanism can weight them according to the relationships between different atoms, so as to better capture and predict the interactions between different atoms in the molecule, understand the relationships between different parts of the molecule, and thus better design drug molecules with specific functions and properties.
In this project, the self-attention mechanism can be implemented through Pytorch’s multi-head attention module (nn.MultiheadAtention). According to the article named of Attention is all you need published by Vaswani et al. (2017), the calculation formula is defined as the following Formulas 1, 2:
among that
And Attention is defined as the following Formula 3:
In the multi-head attention module, the input of the module includes three tensors: query (Q), key (K), and value (V). These tensors are usually of the shape (seq-len, batch size, embed-dim), where seq-len denotes the sequence length, batch size denotes the batch size, and embed dim denotes the embedding dimension. Where
2.3 Strategy optimization
After the pre-training and fine-tuning of the prediction model and the generative model respectively, in order to strengthen the generation strategy of the generative model, we use the multi-objective optimization strategy (MOO) for reinforcement learning, so that it can maximize each objective in each scene.
Building SMILES molecules within the framework of reinforcement learning can actually be seen as a series of decision steps. The generation model generates a batch of SMILES by progressively sampling tokens based on calculated probabilities; The generation model generates a batch of SMILES strings by sampling them step by step according to the calculated probabilities. These valid SMILES strings are then parsed into molecular structures by the predictive model and further encoded into descriptors. From these descriptors, the model is able to calculate the predicted
2.3.1 Object definition
In this topic, the objectives of reinforcement learning are defined as the following Formula 4:
Among them, the target molecules of reinforcement learning in this subject are A1, A2A, hERG, and 3 molecules, that is, n is 3 in this subject.
Where
In the case of no affinity to A1 and only affinity to A2A,
2.3.2 Multi-objective optimization
For these three indicators, I use two MOO schemes to make decisions, namely, weighted scheme and Pareto optimization scheme. In the weight-based scheme, for the ith target molecule (total n = 3 in this subject), the weight wi of the ith target is determined according to the ratio of the number of generated molecules whose reward score is less than and greater than the threshold value, and its weight is defined the following Formula 8:
Where N represents the total number of molecules generated, and
Where
In this way, in a training cycle, the generative model can set weights for target molecules according to this batch of generated molecules, so as to balance their contributions in the reward score, so as to achieve the purpose of multi-objective optimization.
In an optimization scheme based on Pareto frontier, given two solutions
When
Among them,
After determining the dominant relationship among all solutions, a non-dominant sorting algorithm is used to obtain Pareto frontiers of different levels consisting of a set of solutions (Deb et al., 2000). The solution at the top is constrained by the other solutions at the bottom. After the frontier order from the dominant solution to another dominant solution is determined, we no longer limit ourselves to comparing the crowding distance between molecules within the same boundary, but order the molecules according to the average value of the local distance. Specifically, molecules with larger valley local distances will be assigned higher rankings. The final reward
Where
For each step in the generation process, the generation model calculates the probability that each tag in the vocabulary is selected based on the sequence generated in the previous step. By applying the expected final reward obtained from the prediction model, its parameters are updated via the policy gradient. The objective function is as the following Formula 14:
Maximizing this function optimizes the parameters in the generative model to ensure that, after the generative model is trained, it is able to construct the desired SMILES sequences that result in the highest reward score.
2.3.3 Crossover, mutation and selection
In the above method, this paper constructed an executable neural network model for drug molecule design research. However, in the initial attempt, we found a problem: although the training effect was quite good, the molecules generated by the generative model began to show a trend of convergence after a relatively short training cycle: Within a training cycle, the molecules produced by the generative model are almost identical, with differences in only a few atoms, which is obviously unsatisfactory. To increase the diversity of generated molecules, we adopted the following strategies.
Evolutionary algorithms (EAs) (Vikhar, 2016) are a class of optimization techniques inspired by the mechanisms of biological evolution. By simulating natural selection processes, EAs iteratively select individuals with the highest fitness within a population and employ genetic operations such as crossover and mutation to generate new individuals, thereby continuously optimizing the objective function. In the field of deep learning, evolutionary algorithms have found widespread application, with genetic algorithms (GAs) and evolutionary strategy (ES) algorithms being particularly prominent. These methods are commonly used to optimize neural network architectures, structural weights, and hyperparameters, thereby enhancing model performance. Drawing inspiration from the work of Professor Xuhan Liu (Liu et al., 2021), this study leverages evolutionary algorithms to improve the diversity of generated molecules. Specifically, we adapt the core principles of selection, crossover, and mutation from EAs, with a modification that applies the selection step after crossover and mutation, prior to integrating these operations into the training of generative models. During the training process, three models—agent, prior, and crover—are employed. These models share the same Recurrent Neural Network (RNN) architecture and are initialized using pre-trained weights from the generative model, as well as fine-tuned weights saved during previous training iterations. The agent and crover models are loaded with the fine-tuned weights specific to the target molecule, where the agent’s parameters are derived from the most recent reinforcement learning checkpoint. In contrast, the prior model is initialized with pre-trained weights without fine-tuning. Throughout the reinforcement learning phase, the prior model remains static, with its parameters fixed, and serves solely as a variation factor to introduce diversity into the training process. During each training cycle, the agent model is updated based on reinforcement learning objectives, and its parameters are saved at checkpoints corresponding to the highest reward scores. At predefined intervals, both the agent and crover models synchronize their parameters with those of the saved optimal model to further refine training outcomes. This iterative process ensures continuous improvement in the quality and diversity of generated molecules, aligning with the overarching goal of optimizing molecular design through evolutionary-inspired deep learning techniques.
These three models come into play when reinforcement learning generates SMILES sequences: Set the cross-change rate to a random number
First, the selection operation is carried out. From the current population (i.e., the set of generated molecules), molecules on the Pareto front are preferentially screened based on optimization objectives. These molecules represent the top-ranked, high-quality solutions in the current multi-objective optimization problem.
Next, the crossover operation is implemented. The crover model (another model with molecule generation capabilities) is employed to mix the SMILES sequences of two parent molecules. Through this crossover and mixing process, it is expected to integrate the advantageous characteristics of the parent molecules and generate new molecules with novel properties.
Subsequently, the mutation operation is conducted. For the molecules selected after the crossover operation, local modifications are carried out. Specifically, operations such as atom substitution and fragment insertion can be employed to fine-tune the molecular structures, thereby further expanding the search space of molecules and increasing the likelihood of obtaining superior molecules. Finally, the new population generation operation is executed. The molecules obtained after a series of evolutionary operations, including selection, crossover, and mutation, are added to the training pool for the next round, providing a basis for subsequent iterative optimization. Throughout the evolutionary process, the crossover threshold is set to 0.5 to control the probability of crossover operations. Meanwhile, a random number within the range of (0, 1) is designated as the mutation rate
3 Experiments
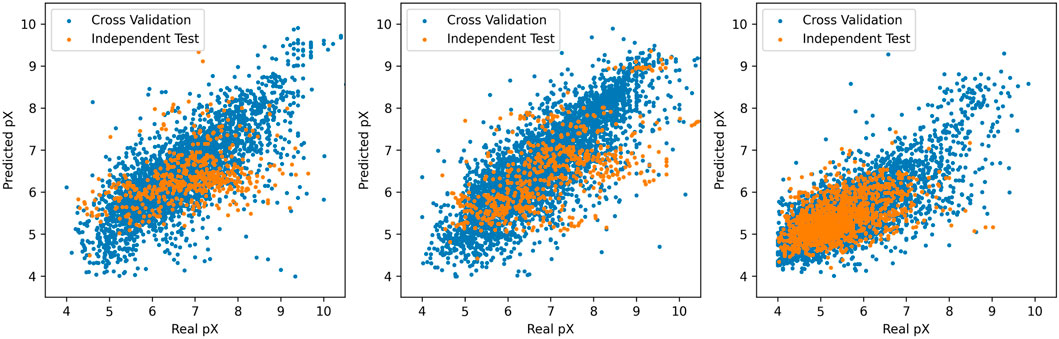
The training of the prediction model based on the random forest algorithm took about 15 h. After 1000 training cycles, the error rate of the prediction model has been reduced to less than 5
The three graphs from left to right show the relationship between the predicted
3.1 The feasibility and effectiveness
In the training process of generating the model, we mainly judge the quality of the model training effect through the feasibility and effectiveness, and independence is mainly used in the internal training of the model and the gradient strategy. Whether the molecules generated by the generative model are effective, whether they are biologically active and have the affinity we need, that’s what matters.
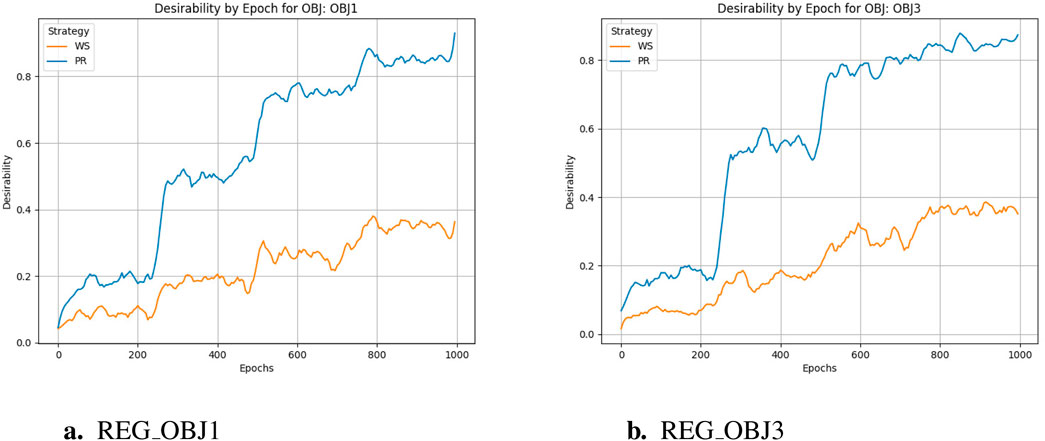
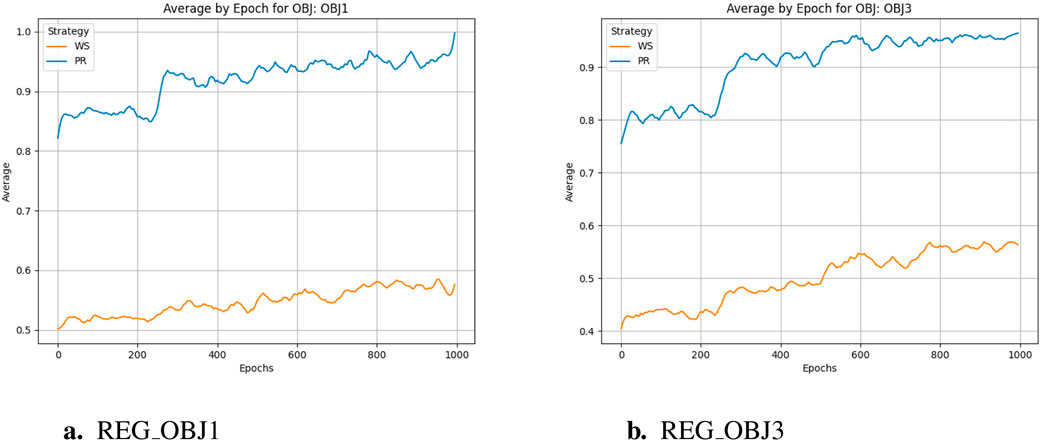
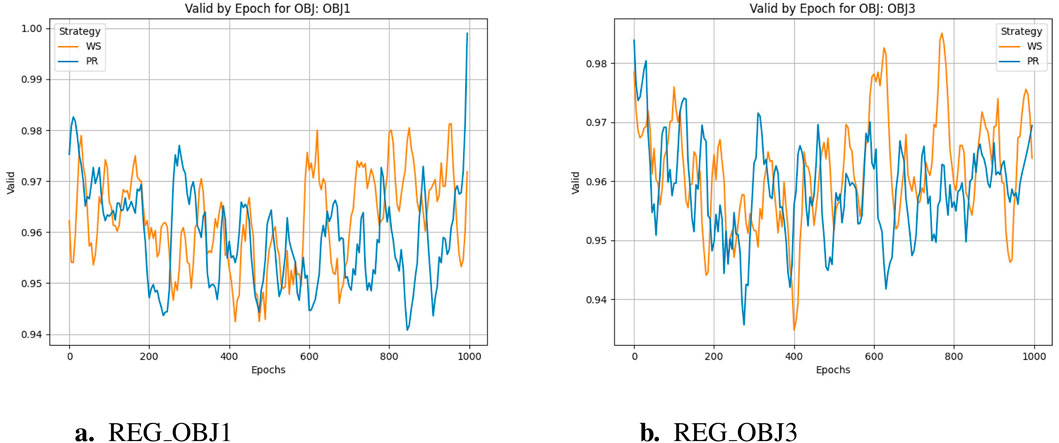
The training cycle is 1000 for multiple targets and 1500 for specific targets. When applying crossover and variation, crover’s parameters are updated every 250 cycles. REG OBJ1 and REG OBJ3 denote reinforcement-learning objectives targeting A1/A2A and A2A-only selectivity, respectively. As can be seen from Figure 3a, the effectiveness of the Pareto optimization strategy during the training process is notably higher than that of the weighted strategy. Its average performance begins at a higher level and rises more swiftly, ultimately nearing 1.0. In comparison, the weighted strategy’s average performance improves at a slower pace and plateaus around 0.4, which is considerably less than the Pareto optimization strategy’s performance. Consequently, it is evident that the Pareto optimization strategy is superior to the weighted strategy. There were two large jumps in desirability during the initial training, presumably because crover’s parameters were updated to improve the crossover effect in the evolutionary algorithm. From Figure 3b, we can observe the following:the Pareto optimization strategy significantly outperforms the weighted strategy during the training process. Its desirability not only starts at a higher level but also increases more rapidly, eventually exceeding 0.8. In contrast, the desirability of the weighted strategy increases more slowly and stabilizes around 0.4, which is significantly lower than that of the Pareto optimization strategy. This figure clearly demonstrates the performance difference between the two strategies during the training process, with the Pareto optimization strategy showing markedly better performance for the targe. According to speculation, when the Epoch is equal to 750, there should be a significant jump in desirability, but it does not appear in the actual situation, indicating that the improvement of model training has basically achieved enough excellent results, and the update of crover parameters involved in the cross has not significantly improved. However, if we only look at the weight-based scheme, in the optimization of multiple objectives and specific objectives, there is also a small jump in the corresponding nodes, but the improvement rate is much lower than that of the Pareto scheme, indicating that the improvement effect of the evolutionary algorithm is not so obvious in the weighted scheme.
As can be seen from Figure 4, in the training process of reinforcement learning, reward scores gradually increase with the increase of training cycle, which is basically consistent with the change trend of acceptability. This shows that in reinforcement learning, no matter based on Pareto frontier or weighted scheme, multi-objective optimization strategy is effective. However, it is worth noting that through the comparison within the figures and observing between Figures 4a,b, it can be found that compared to the latter, the former not only achieved a higher reward score at the end of training but also showed better improvement compared to the beginning of training, and reaches the critical value in fewer training cycles. However, from the trends in the following two graphs, it is reasonable to infer that if the weight-based scheme continues to train, it can also get a relatively reasonable result, but the training period is much longer than the normal requirements.
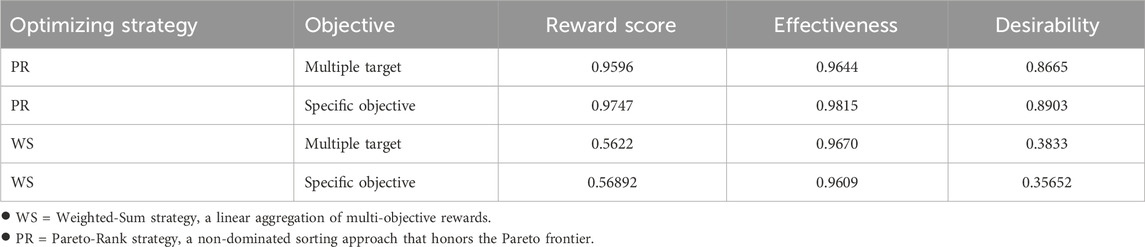
The final training results are shown in Table 1. In order to reduce errors, we selected the training results of the last 10 training cycles, calculated their average values for comparison, and finally obtained the following results.
It can be clearly seen that the reward scores obtained by the optimization strategy using Pareto frontier and the feasibility of generating molecules are far superior to the optimization strategy based on weight, which proves that our choice is correct. However, it is worth noting that although the difference is not large, the effectiveness of the former is slightly less than that of the latter. But taking into account other metrics and performance improvements, a small lag in this single area is acceptable.
According to the analysis in Figure 5, it can be found that there is little improvement in the effectiveness of the generative model to generate molecules in the reinforcement learning stage. Although in the early stages of training, reinforcement learning can help the model find polymer generation strategies more quickly, as the training progresses, the generative model has been able to generate higher quality molecules, so the effect of reinforcement learning on it is no longer obvious.
3.2 Comparative experiment
Above shows that the early pre-training stage is very important in the training process of molecular generative model, and through pre-training, the generative model can generate high-quality molecules faster and more accurately. The role of reinforcement learning in the training of molecular generative models requires more specific analysis and evaluation to determine its impact on the generation of high-quality molecules. After the pre-training is completed, the molecular effectiveness generated by the generative model itself is already close to quite high, and there is not much room for improvement. Although the effectiveness of the model fluctuates somewhat during the training process, most of them are above 0.95, indicating that the pre-training has made the model achieve a very good effect in the generation of molecules, and it is acceptable that the effectiveness is not significantly improved.
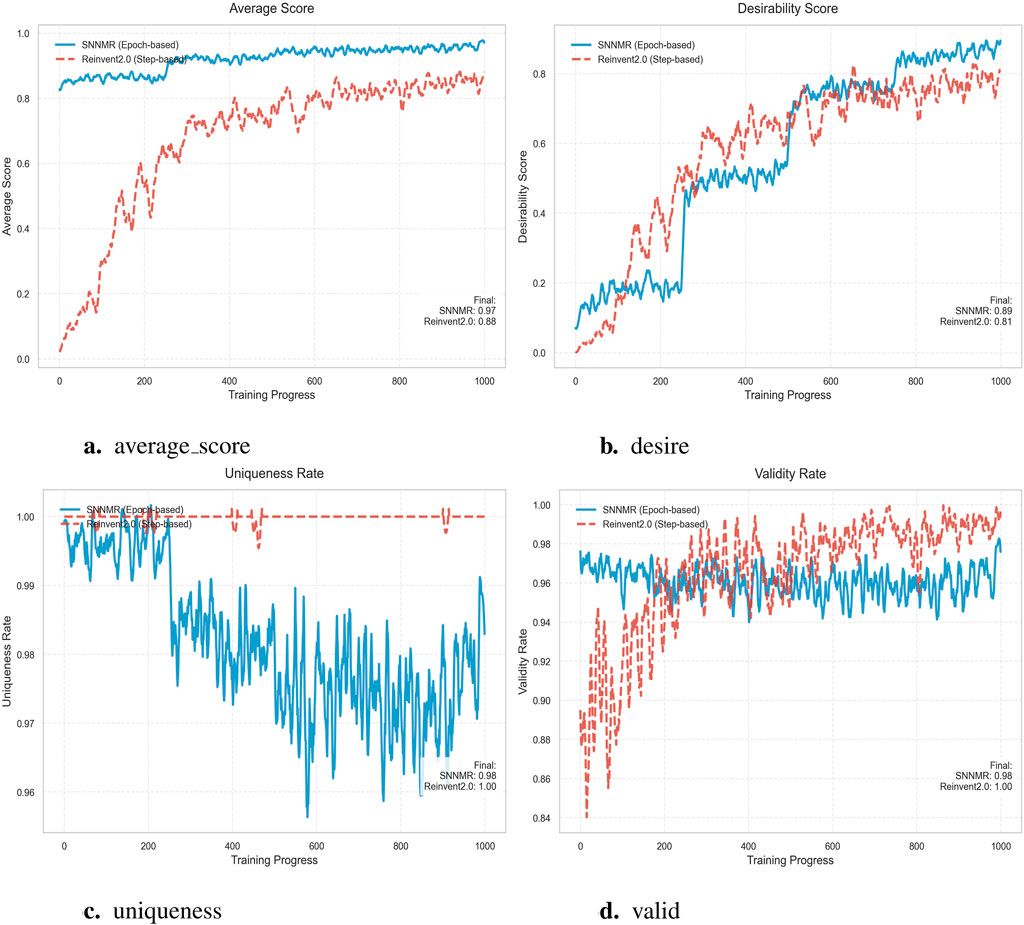
After in-depth analysis of the data presented in the four subgraphs, we can clearly see the superior advantages of the SNNMR model across a number of key performance indicators. First of all, from the core indicator of average score, SNNMR model performs well in multiple training stages. In Figure 6a, SNNMR has a slightly higher average score (0.97) than Reinvent2.0 (0.88), which initially shows the potential advantages of SNNMR for specific tasks. Looking further at Figure 6b, although the mean score of SNNMR (0.98) is very close to that of Reinvent2.0 (1.00), SNNMR scores change more smoothly over the course of training, showing greater stability and reliability. In Figure 6c, SNNMR’s average score (0.89) is once again higher than that of Reinvent2.0 (0.81), further cementing SNNMR’s lead in performance. Finally, in Figure 6d, the smoothness of the SNNMR score curve once again highlights its stable training process and good generalization, although the average scores of the two are close again. In addition, the stability of SNNMR models during training also deserves special mention. In the four subgraphs, SNNMR score curves show a smooth and stable trend, which indicates that the model can adapt to data changes well in the training process and avoid overfitting or underfitting problems. This stability not only helps to improve the generalization ability of the model, but also reduces the cost of model adjustment and optimization in practical applications. Subsequently, a comparison of the various indicators between SNNMR and GENTRL (Zhavoronkov et al., 2019), Reinvent2.0 (Blaschke et al., 2020), as well as Diff - AMP (Wang et al., 2024) will be conducted respectively.
Comparative analysis demonstrates SNNMR’s superior performance with 98.15
Comprehensive evaluation of Table 2 shows that SNNMR outperforms Reinvent2.0 across all key metrics. First, its Desire (target-achievement rate) reaches 89.00
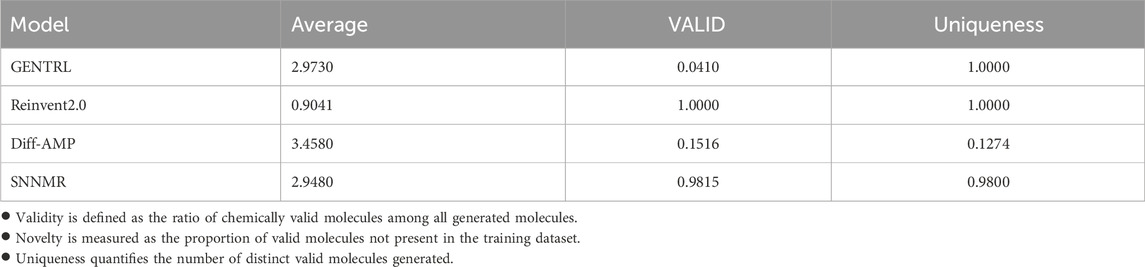
In the comparative experiment between the SNNMR and Diff - AMP models, distinct performance characteristics are observed. The SNNMR model demonstrates a VALID value of 0.9815, indicating a high degree of validity in its outputs, which signifies that the majority of its generated results are effective and accurate. In contrast, the Diff - AMP model has a VALID value of only 0.1516, reflecting a relatively low level of effectiveness and a higher proportion of invalid or inaccurate outputs. Regarding uniqueness, the SNNMR model achieves a Uniqueness value of 0.9800, showcasing a strong ability to produce diverse and non - repetitive results. On the other hand, the Diff - AMP model’s Uniqueness value is a mere 0.1274, indicating a significant lack of uniqueness in its outputs. Although the Diff - AMP model has a higher average value (3.4580) compared to SNNMR’s 2.9480, this higher average may imply greater performance volatility. Overall, the SNNMR model outperforms the Diff - AMP model in terms of both validity and uniqueness, making it a more reliable and versatile choice for the task at hand.
In the comparative study of the GENTRL, Reinvent2.0, Diff-AMP, and SNNMR models, distinct performance characteristics are evident. The GENTRL model, despite achieving a perfect Uniqueness score of 1.0000, suffers from a very low VALID value of 0.0410, which significantly undermines its overall utility. The Reinvent2.0 model, with both VALID and Uniqueness values at 1.0000, demonstrates high levels of effectiveness and diversity, but its relatively low average value of 0.9041 may imply a certain degree of conservatism in performance. The Diff-AMP model has a high average value of 3.4580, yet its low VALID value of 0.1516 and extremely low Uniqueness value of 0.1274 reveal substantial shortcomings in terms of output validity and diversity. In stark contrast, the SNNMR model excels in multiple aspects. It achieves a high VALID value of 0.9815, ensuring a large proportion of valid outputs, and a commendable Uniqueness value of 0.9800, indicating a strong ability to generate diverse results. Moreover, its average value of 2.9480 reflects a stable and well-balanced performance. Overall, the SNNMR model stands out as the most promising approach among the four, as it effectively combines high validity, good uniqueness, and stable performance, making it a superior choice for the task at hand.
3.3 Case analysis
In this experiment, our model will conduct screening and validation of natural compounds targeting the Adenosine A2A receptor (A2A receptor), aiming to generate novel molecules from the Data Set and verify their potential as A2A receptor inhibitors.
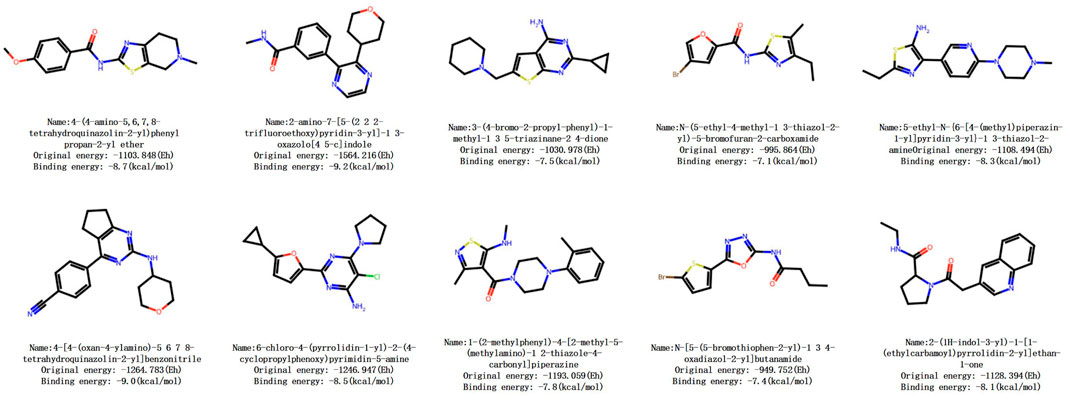
Firstly, brand-new molecular structures are generated to ensure novelty. Subsequently, based on the reward score, effectiveness, and uniqueness, Pareto optimization ranking is performed using the composite score formula (composite score = (desirability
Taking a certain molecule as an example, its binding free energy is −6.18 kcal/mol, indicating spontaneous binding and falling within the acceptable range of drug binding energy (−6 to −12 kcal/mol). It shows a significant improvement of 73
In conclusion, the preliminary activity of this compound is favorable, indicating that our model is effective and reliable in generating drug molecules and has good prospects for pharmaceutical applications.
4 Conclusion
In this study, we present an advanced methodology for drug molecular design based on multi-objective optimization. A robust predictive model was developed using the Random Forest algorithm, which is widely recognized for its efficacy in handling complex datasets. To enhance the generative capabilities, we refined the traditional Recurrent Neural Network (RNN) architecture by integrating Long Short-Term Memory (LSTM) layers and incorporating a self-attention mechanism, thereby significantly improving the model’s ability to capture long-range dependencies and intricate molecular patterns. Additionally, we employed evolutionary algorithms, utilizing crossover, mutation, and selection operations, to iteratively optimize the quality of generated molecular structures. Comprehensive ablation studies were conducted to validate the proposed methodology, with results unequivocally demonstrating the superior performance and effectiveness of our approach in generating high-quality drug-like molecules. Analogous hypervolume-driven multi-objective frameworks have recently been leveraged for antimicrobial peptide discovery, underscoring the broad applicability of our attention-based evolutionary strategy (Wang et al., 2025).
However, we acknowledge that equipment constraints have limited the full potential of our methodologies. Specifically, the stacking of neural networks has prolonged training durations, and the inclusion of multi-head attention modules has necessitated greater memory resources than initially anticipated. Consequently, optimizing the trade-off between training efficiency and performance within constrained computational resources remains a critical research challenge. Additionally, while the evolutionary algorithm inherently exhibits adaptive tendencies towards optimality, its full potential has yet to be fully realized in this context. Our current implementation primarily employs crossover and mutation strategies to enhance molecular diversity. A more comprehensive integration of the evolutionary algorithm with reinforcement learning’s policy gradients holds promise for theoretically superior outcomes. A notable limitation is that the model was trained solely on ChEMBL34, leaving its performance on proprietary or newly released databases unverified; furthermore, all bioactivity and toxicity predictions were generated in silico, with no accompanying in-vitro or in-vivo validation.
Despite these challenges, this project has successfully established a deep learning framework for drug molecular design. The results achieved not only meet the project requirements but also surpass our initial expectations, highlighting the feasibility and potential of our methodology. This underscores the significant promise of our approach in advancing the field of drug discovery and molecular design.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
PW: Methodology, Writing – review and editing, Supervision, Funding acquisition, Writing – original draft, Data curation, Resources, Conceptualization. ZX: Software, Writing – review and editing, Formal analysis, Data curation. YD: Writing – original draft, Software, Visualization. HY: Writing – review and editing, Validation, Supervision, Investigation. YW: Writing – original draft, Validation, Investigation. JJ: Writing – review and editing, Validation, Investigation. ZhL: Writing – review and editing, Resources. ZeL: Writing – review and editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is partially supported by Hunan Province Three Point Science and Technology Innovation Plan Youth Science and Technology Talent Project (Grant No. 2022RC1101) and the National Natural Science Foundation of China (No. 62202153,62172158).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmad, E., Lim, S., Lamptey, R., Webb, D. R., and Davies, M. J. (2022). Type 2 diabetes. Lancet 400, 1803–1820. doi:10.1016/S0140-6736(22)01655-5
Atanasov, A. G., Zotchev, S. B., Dirsch, V. M., and Supuran, C. T. (2021). Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 20, 200–216. doi:10.1038/s41573-020-00114-z
Belgiu, M., and Draguţ, L. (2016). Random forest in remote sensing: a review of applications and future directions. ISPRS J. Photogrammetry Remote Sens. 114, 24–31. doi:10.1016/j.isprsjprs.2016.01.011
Blaschke, T., Arús-Pous, J., Chen, H., Margreitter, C., Tyrchan, C., Engkvist, O., et al. (2020). REINVENT 2.0 – an AI Tool for de novo Drug Design. ChemRxiv 60, 5918–5922. doi:10.1021/acs.jcim.0c00915
Cai, L., Wang, L., Fu, X., and Zeng, X. (2021). Active semisupervised model for improving the identification of anticancer peptides. ACS omega 6, 23998–24008. doi:10.1021/acsomega.1c03132
Cai, L., Yue, G., Chen, Y., Wang, L., Yao, X., Zou, Q., et al. (2025). Et-protacs: modeling ternary complex interactions using cross-modal learning and ternary attention for accurate protac-induced degradation prediction. Briefings Bioinforma. 26, bbae654. doi:10.1093/bib/bbae654
Chen, Y., Wang, J., Zou, Q., Niu, M., Ding, Y., Song, J., et al. (2024). Drugdagt: a dual-attention graph transformer with contrastive learning improves drug-drug interaction prediction. BMC Biol. 22, 233. doi:10.1186/s12915-024-02030-9
Deb, K., Agrawal, S., Pratap, A., and Meyarivan, T. (2000). “A fast elitist non-dominated sorting genetic algorithm for multi-objective optimization: nsga-Ii,” in Parallel Problem Solving from Nature PPSN VI: 6th International Conference Paris, France, September 18–20, 2000 Proceedings 6 (Springer), 849–858. doi:10.1007/3-540-45356-3_83
DeFronzo, R. A., Ferrannini, E., Groop, L., Henry, R. R., Herman, W. H., Holst, J. J., et al. (2015). Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 1, 15019–15022. doi:10.1038/nrdp.2015.19
Dey, R., and Salem, F. M. (2017). “Gate-variants of gated recurrent unit (gru) neural networks,” in 2017 IEEE 60th international Midwest Symposium on Circuits and Systems (MWSCAS), 1597–1600. doi:10.1109/MWSCAS.2017.8053243
El Morr, C., Jammal, M., Ali-Hassan, H., and El-Hallak, W. (2022). Support Vector machine. Cham: Springer International Publishing, 385–411. doi:10.1007/978-3-031-16990-8_13
Fredholm, B. B. (2010). Adenosine receptors as drug targets. Exp. Cell. Res. 316, 1284–1288. doi:10.1016/j.yexcr.2010.02.004
Fu, X., Du, Z., Chen, Y., Chen, H., Zhuo, L., Lu, A., et al. (2025). Drugkans: a paradigm to enhance drug-target interaction prediction with kans. IEEE J. Biomed. Health Inf., 1–12doi. doi:10.1109/JBHI.2025.3566931
Gaulton, A., Bellis, L. J., Bento, A. P., Chambers, J., Davies, M., Hersey, A., et al. (2011). Chembl: a large-scale bioactivity database for drug discovery. Nucleic Acids Res. 40, D1100–D1107. doi:10.1093/nar/gkr777
Gu, Y., Zheng, S., Zhang, B., Kang, H., Jiang, R., and Li, J. (2025). Deep multiple instance learning on heterogeneous graph for drug–disease association prediction. Comput. Biol. Med. 184, 109403. doi:10.1016/j.compbiomed.2024.109403
Hopkins, A. L. (2008). Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 4, 682–690. doi:10.1038/nchembio.118
Ijzerman, A. P., Jacobson, K. A., Müller, C. E., Cronstein, B. N., and Cunha, R. A. (2022). International union of basic and clinical pharmacology. cxii: adenosine receptors: a further update. Pharmacol. Rev. 74, 340–372. doi:10.1124/pharmrev.121.000445
Kiralj, R., and Ferreira, M. M. C. (2009). Basic validation procedures for regression models in qsar and qspr studies: theory and application. J. Braz. Chem. Soc. 20, 770–787. doi:10.1590/s0103-50532009000400021
Lagarde, N., Zagury, J.-F., and Montes, M. (2015). Benchmarking data sets for the evaluation of virtual ligand screening methods: review and perspectives. J. Chem. Inf. Model. 55, 1297–1307. doi:10.1021/acs.jcim.5b00090
Lai, L., Liu, Y., Song, B., Li, K., and Zeng, X. (2025). Deep generative models for therapeutic peptide discovery: a comprehensive review. ACM Comput. Surv. 57, 1–29. doi:10.1145/3714455
Lenselink, E. B., Ten Dijke, N., Bongers, B., Papadatos, G., Van Vlijmen, H. W., Kowalczyk, W., et al. (2017). Beyond the hype: deep neural networks outperform established methods using a chembl bioactivity benchmark set. J. cheminformatics 9, 45–14. doi:10.1186/s13321-017-0232-0
Liu, X., Ye, K., van Vlijmen, H. W., Emmerich, M. T., Ijzerman, A. P., and van Westen, G. J. (2021). Drugex v2: de novo design of drug molecules by pareto-based multi-objective reinforcement learning in polypharmacology. J. cheminformatics 13, 85. doi:10.1186/s13321-021-00561-9
Mendez, D., Gaulton, A., Bento, A. P., Chambers, J., De Veij, M., Félix, E., et al. (2018). Chembl: towards direct deposition of bioassay data. Nucleic Acids Res. 47, D930–D940. doi:10.1093/nar/gky1075
Niu, M., Wang, C., Chen, Y., Zou, Q., and Luo, X. (2025). Interpretable multi-instance heterogeneous graph network learning modelling CircRNA–drug sensitivity association prediction. BMC Biol. 23, 131. doi:10.1186/s12915-025-02223-w
Pan, J., Liu, C., Wang, Z., Hu, Y., and Jiang, H. (2012). “Investigation of deep neural networks (dnn) for large vocabulary continuous speech recognition: why dnn surpasses gmms in acoustic modeling,” in 2012 8th international symposium on Chinese spoken Language processing, 301–305. doi:10.1109/ISCSLP.2012.6423452
Rogers, D., and Hahn, M. (2010). Extended-connectivity fingerprints. J. Chem. Inf. Model. 50, 742–754. doi:10.1021/ci100050t
Shams, S. R., Jahani, A., Kalantary, S., Moeinaddini, M., and Khorasani, N. (2021). The evaluation on artificial neural networks (ann) and multiple linear regressions (mlr) models for predicting so2 concentration. Urban Clim. 37, 100837. doi:10.1016/j.uclim.2021.100837
Tan, L., Wang, L., Ren, X., Zou, Q., Yao, X., Zeng, X., et al. (2025). Sq-diffupep: a multimodal information-guided quantitative latent diffusion model for antimicrobial peptide discovery. Inf. Fusion 121, 103119. doi:10.1016/j.inffus.2025.103119
Trinh, T. X., Seo, M., Yoon, T. H., and Kim, J. (2022). Developing random forest based qsar models for predicting the mixture toxicity of tio2 based nano-mixtures to daphnia magna. NanoImpact 25, 100383. doi:10.1016/j.impact.2022.100383
Vamathevan, J., Clark, D., Czodrowski, P., Dunham, I., Ferran, E., Lee, G., et al. (2019). Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 18, 463–477. doi:10.1038/s41573-019-0024-5
Van Houdt, G., Mosquera, C., and Nápoles, G. (2020). A review on the long short-term memory model. Artif. Intell. Rev. 53, 5929–5955. doi:10.1007/s10462-020-09838-1
Vaswani, A., Shazeer, N., Parmar, N., Uszkoreit, J., Jones, L., Gomez, A. N., et al. (2017). Attention is all you need. Adv. neural Inf. Process. Syst. 30. doi:10.48550/arXiv.1706.03762
Vikhar, P. A. (2016). “Evolutionary algorithms: a critical review and its future prospects,” in 2016 International conference on global trends in signal processing, information computing and communication (ICGTSPICC), 261–265. doi:10.1109/ICGTSPICC.2016.7955308
Wang, R., Zhou, Z., Wu, X., Jiang, X., Zhuo, L., Liu, M., et al. (2023). An effective plant small secretory peptide recognition model based on feature correction strategy. J. Chem. Inf. Model. 64, 2798–2806. doi:10.1021/acs.jcim.3c00868
Wang, R., Wang, T., Zhuo, L., Wei, J., Fu, X., Zou, Q., et al. (2024). Diff-amp: tailored designed antimicrobial peptide framework with all-in-one generation, identification, prediction and optimization. Briefings Bioinforma. 25, bbae078. doi:10.1093/bib/bbae078
Wang, L., Liu, Y., Fu, X., Ye, X., Shi, J., Yen, G. G., et al. (2025). Hmamp: designing highly potent antimicrobial peptides using a hypervolume-driven multiobjective deep generative model. J. Med. Chem. 68, 8346–8360. doi:10.1021/acs.jmedchem.4c03073
Wei, J., Zhu, Y., Zhuo, L., Liu, Y., Fu, X., and Li, F. (2024a). Efficient deep model ensemble framework for drug-target interaction prediction. J. Phys. Chem. Lett. 15, 7681–7693. doi:10.1021/acs.jpclett.4c01509
Wei, J., Zhuo, L., Fu, X., Zeng, X., Wang, L., Zou, Q., et al. (2024b). Drugrealign: a multisource prompt framework for drug repurposing based on large language models. BMC Biol. 22, 226. doi:10.1186/s12915-024-02028-3
Weininger, D. (1988). Smiles, a chemical language and information system. 1. introduction to methodology and encoding rules. J. Chem. Inf. Comput. Sci. 28, 31–36. doi:10.1021/ci00057a005
Weis, W. I., and Kobilka, B. K. (2018). The molecular basis of g protein–coupled receptor activation. Annu. Rev. Biochem. 87, 897–919. doi:10.1146/annurev-biochem-060614-033910
Xie, Z., Feng, X., and Chen, X. (2022). Partial least trimmed squares regression. Chemom. Intelligent Laboratory Syst. 221, 104486. doi:10.1016/j.chemolab.2021.104486
Xu, J., Lu, C., Jin, S., Meng, Y., Fu, X., Zeng, X., et al. (2025). Deep learning-based cell-specific gene regulatory networks inferred from single-cell multiome data. Nucleic Acids Res. 53, gkaf138. doi:10.1093/nar/gkaf138
Yang, Z., Yang, D., Dyer, C., He, X., Smola, A., and Hovy, E. (2016). “Hierarchical attention networks for document classification,” in Proceedings of the 2016 conference of the North American chapter of the association for computational linguistics: human language technologies, 1480–1489. doi:10.18653/v1/n16-1174
Zdrazil, B. (2025). Fifteen years of chembl and its role in cheminformatics and drug discovery. J. Cheminform 17, 32. doi:10.1186/s13321-025-00963-z
Zdrazil, B., Felix, E., Hunter, F., Manners, E. J., Blackshaw, J., Corbett, S., et al. (2023). The chembl database in 2023: a drug discovery platform spanning multiple bioactivity data types and time periods. Nucleic Acids Res. 52, D1180–D1192. doi:10.1093/nar/gkad1004
Zeng, P., Zhang, B., Liu, A., Meng, Y., Tang, X., Yang, J., et al. (2024). Drug repositioning based on tripartite cross-network embedding and graph convolutional network. Expert Syst. Appl. 252, 124152. doi:10.1016/j.eswa.2024.124152
Zhavoronkov, A., Ivanenkov, Y. A., Aliper, A., Veselov, M. S., Aladinskiy, V. A., Aladinskaya, A. V., et al. (2019). Deep learning enables rapid identification of potent ddr1 kinase inhibitors. Nat. Biotechnol. 37, 1038–1040. doi:10.1038/s41587-019-0224-x
Keywords: metabolic diseases, adenosine receptors, natural medicines, computational strategy, artificial intelligence
Citation: Wang P, Xu Z, Deng Y, Yuan H, Wu Y, Jiang J, Lyu Z and Li Z (2025) Computational discovery of natural medicines targeting adenosine receptors for metabolic diseases. Front. Pharmacol. 16:1671415. doi: 10.3389/fphar.2025.1671415
Received: 23 July 2025; Accepted: 18 August 2025;
Published: 02 September 2025.
Edited by:
Yansu Wang, University of Electronic Science and Technology of China, ChinaReviewed by:
Chen-Huan Yu, Chinese Academy of Sciences (CAS), ChinaChu Pan, University Health Network (UHN), Canada
Mengting Niu, University of Electronic Science and Technology of China, China
Copyright © 2025 Wang, Xu, Deng, Yuan, Wu, Jiang, Lyu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaohui Lyu, MTM5MDY2NDUyODdAMTM5LmNvbQ==; Zejun Li, bHpqZm94QGhuaXQuZWR1LmNu
 Peng Wang
Peng Wang Zhiyu Xu1
Zhiyu Xu1 Yuqing Deng
Yuqing Deng Huiping Yuan
Huiping Yuan Zejun Li
Zejun Li