- 1Department of Oncology, 900th Hospital of PLA Joint Logistic Support Force, Fuzhou, Fujian, China
- 2Department of Oncology, Fuzong Clinical Medical College of Fujian Medical University, Fuzhou, Fujian, China
This case study details a 57-year-old woman with heavily pretreated, hormone receptor-positive (HR+), HER2-negative advanced breast cancer harboring a pathogenic germline BRCA2 mutation. Following progression on multiple prior therapies including endocrine therapy combined with a CDK4/6 inhibitor, chemotherapy, and an antibody-drug conjugate (resulting in liver metastases), blood-based next-generation sequencing (NGS) identified the gBRCA2 variant alongside persistent high ER expression. Guided by these molecular findings, treatment was initiated with the domestically developed, highly selective PARP inhibitor (PARPi) Fluzoparib (300 mg orally twice daily) combined with the aromatase inhibitor Exemestane (25 mg orally daily). The regimen was well-tolerated, with manageable grade 1–2 adverse events (anemia, nausea, rash). Follow-up imaging demonstrated complete resolution of the hepatic metastases. The patient achieved a remarkably prolonged progression-free survival (PFS) of 37 months on this combination therapy, representing the longest period of disease control in her metastatic course. Although eventual progression occurred (new axillary lymph node metastasis and suspected hepatic recurrence), this case demonstrates the exceptional efficacy and durable disease control achievable with Fluzoparib plus Exemestane in a pretreated patient with gBRCA2-mutated HR+/HER2-advanced breast cancer, highlighting a promising therapeutic approach for this molecularly defined population.
Introduction
Approximately 5%–10% of breast cancer patients harbor germline BRCA1/2 mutations (Saj et al., 2024). These patients face distinct therapeutic challenges. While gBRCA mutation has been historically associated with triple-negative breast cancer, however, a significant proportion (up to 60%) presents with HR+/HER2− disease (O'Shaughnessy et al., 2020). These tumors often exhibit aggressive behavior and may develop resistance to conventional therapies. PARP inhibitors (PARPis), which exploit synthetic lethality in homologous recombination repair (HRR)-deficient cells due to BRCA mutations, represent a major therapeutic advance (Albarrán et al., 2023). The FDA (Food and Drug Administration) has approved Olaparib and Talazoparib, and the NCCN (National Comprehensive Cancer Network) guidelines (Category 1) recommend them for treating HER2− advanced breast cancer with gBRCA1/2 mutations (Kang et al., 2025; Hennes et al., 2020).
Fluzoparib is a highly selective PARP1/2 inhibitor developed in China (Wang et al., 2019). Although preclinical data supports its potent activity (Wang et al., 2019), real-world clinical evidence in advanced breast cancer, particularly combined with endocrine therapy in the HR+/HER2− gBRCA-mutated subtype, remains scarce. This case details the molecularly guided use of Fluzoparib plus Exemestane in a patient with HR+/HER2− advanced breast cancer and a gBRCA2 mutation, achieving remarkable long-term disease control. This case exemplifies how molecular diagnostics directly inform treatment sequencing and align with NCCN principles. It also highlights a promising strategy that may be relevant for future guideline updates.
Case presentation
In 2015, a 57-year-old woman presented with a left breast mass and biopsy confirmed invasive ductal carcinoma. Immunohistochemistry (IHC) analysis revealed ER positivity at 95%, PR positivity at 30%, HER2 score of 1+ with FISH (fluorescence in situ hybridizatio) negative, and Ki-67 proliferation index of 35%. The tumor was staged as pT1cN2a.m.0 (IIIA), classified as Luminal B (HER2−). She underwent left modified radical mastectomy (2015), followed by adjuvant chemotherapy (Epirubicin + Cyclophosphamide - Taxane, EC-T), radiotherapy, and endocrine therapy (Leuprorelin plus Toremifene). The patient underwent bilateral salpingo-oophorectomy as castration in 2017; Toremifene continued until Jul. 2019 with a disease-free survival (DFS) of 51 months.
In Jul. 2019, multiple lung nodules were detected. VATS (Video-Assisted Thoracic Surgery Lobectomy resection) confirmed metastatic breast cancer (IHC: CK7+, Mammaglobin+, ER/PR retained). First-line therapy for advanced breast cancer was Fulvestrant plus Palbociclib (Sep. 2019-December 2020; PFS: 15 months). Upon bone progression (T11 vertebra, Dec. 2020), second-line therapy with Nab-paclitaxel plus Capecitabine (Dec. 2020-April 2021; 7 cycles) followed by Capecitabine maintenance (Apr. 2021-February 2022; PFS: 14 months total) was administered.
In Feb. 2022, abdominal CT suggested multiple liver metastases (Figure 1). Subsequently, the patient presented to our hospital. Ultrasound-guided biopsy confirmed metastatic breast cancer (IHC: ER 90%, PR 1%, HER2 IHC 2+) (Figure 2). FISH for HER2 was negative. Due to HER2 IHC 2+, Disitamab Vedotin was initiated as third-line therapy (March 2022-April 2022). Concurrently, Letrozole was added based on high ER expression. After two cycles, CT showed minimal response.
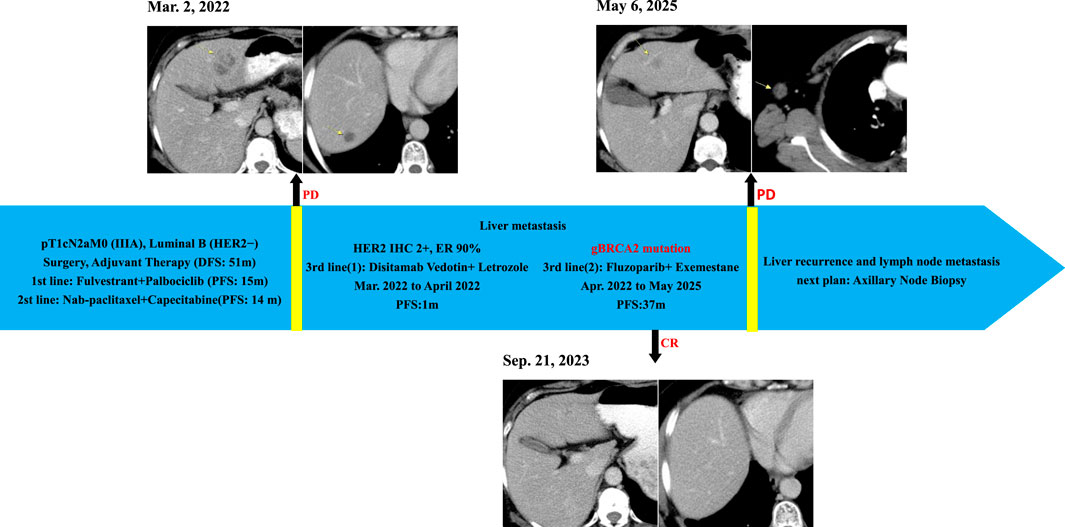
Figure 1. Clinical Course and Imaging Surveillance Timeline. 1) Contrast-enhanced CT (Mar. 2, 2022) showed multiple enhancing lesions consistent with hepatic metastases; 2) Contrast-enhanced CT (Sep. 21, 2023) demonstrated no evidence of hepatic metastasis; 3)Contrast-enhanced CT (6 May 2025) showed suspected recurrent metastatic lesion in the left hepatic lobe and metastatically enlarged right axillary lymph node.
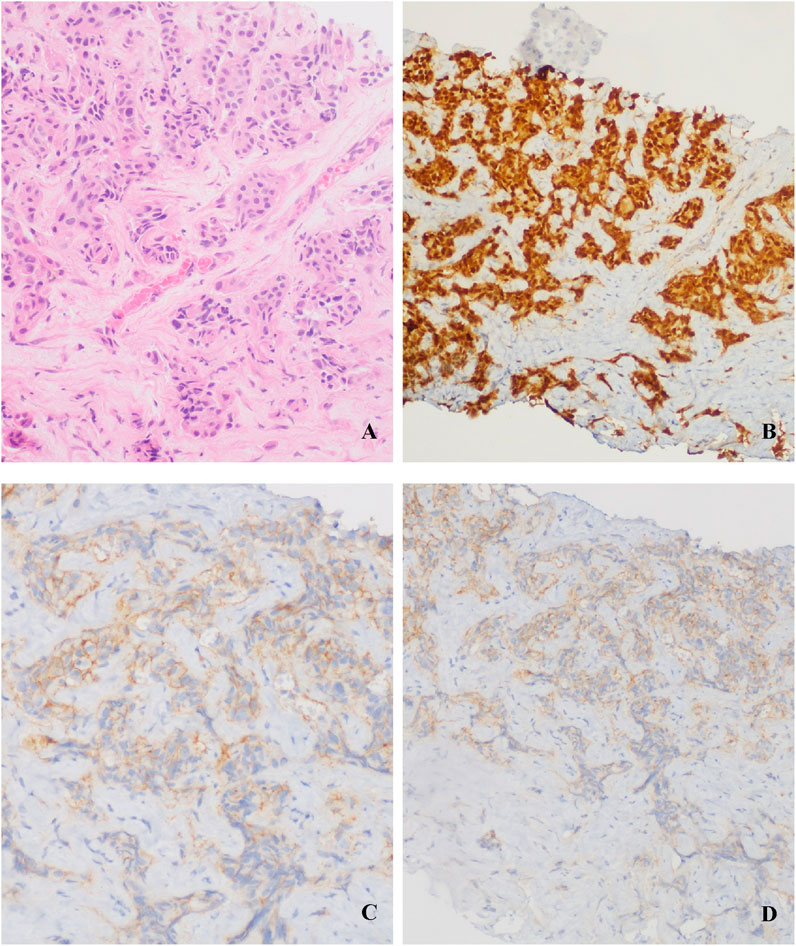
Figure 2. Liver metastasis biopsy: (A) Invasive carcinoma (HE staining); (B) ER 90%; (C) (D) HER2 IHC 2+.
In Apr. 2022, blood-based NGS detected a pathogenic gBRCA2 exon 11 variant (c.6462_6463del; p.Q2157Ifs*18; VAF 49.7%), confirming HRR deficiency. Based on this molecular evidence combined with persistent high ER expression—and considering the patient’s preference for oral therapy and economic factors favoring domestically developed agents—treatment was transitioned to oral Fluzoparib (300 mg twice daily) plus Exemestane (25 mg daily) in Apr. 2022.
Results of molecularly guided therapy
Follow-up imaging (CT) demonstrated complete resolution of the previously identified liver metastases (Figure 1). The patient achieved a PFS of 37 months on Fluzoparib plus Exemestane. This duration represents the longest period of disease control in her metastatic course. Treatment was well-tolerated. Adverse events included: anemia (CTCAE Grade 2, managed with intermittent Erythropoietin), nausea (CTCAE Grade 1, managed with Ondansetron prn), and rash (CTCAE Grade 1, managed with topical steroids). No dose reductions or treatment interruptions were required.
Disease progression occurred in May 2025, evidenced by a new right axillary lymph node metastas and possible recurrence in the left liver lobe on CT (Figure 1), meeting RECIST 1.1 criteria. Planned evaluations include liver MRI, biopsy of the axillary node for IHC (ER, PR, CerbB2, PD-L1, MMR, TROP2) and genetic analysis (BRCA1/2 reversion, HER2, PIK3CA, ESR1) to guide next-line precision therapy.
Discussion
This case powerfully illustrates the impact of molecular diagnostics on clinical outcomes in advanced breast cancer. The identification of a gBRCA2 mutation through timely blood-based NGS upon liver metastasis progression was the pivotal factor enabling a highly effective, targeted therapeutic strategy. The selection of Fluzoparib plus Exemestane, driven by the molecular finding of HRR deficiency (gBRCA2 mutation) and sustained HR positivity, yielded an exceptional PFS of 37 months in the fourth-line setting. This result significantly exceeded the median PFS reported for single-agent Olaparib (7.0 months) and Talazoparib (8.6 months) in registration trials for gBRCAm advanced breast cancer (Robson et al., 2017; Litton et al., 2018), and greatly surpassed the benefit seen with prior treatment lines in this patient.
The profound and durable response observed underscores the central role of synthetic lethality achieved by PARPis in BRCA-deficient cells (Faraoni and Graziani, 2018). Fluzoparib’s high selectivity for PARP1/2 potently inhibits base excision repair, leading to accumulation of DNA double-strand breaks that cannot be repaired in HRR-deficient tumor cells (Kutuzov et al., 2021). The synergy with endocrine therapy (Exemestane) is mechanistically plausible because PARPi-induced DNA damage may impair the ER pathway, and ER blockade can downregulate critical DNA repair genes (e.g., BRCA1, RAD51), together creating a “dual hit” that exacerbates genomic instability and enhances tumor cell death in HR + disease (Sottnik et al., 2025; Wu et al., 2020). This combination strategy warrants dedicated clinical investigation, particularly in the HR+/HER2−/gBRCAm subtype which constitutes a substantial fraction of gBRCAm advanced breast cancer.
This case carries significant implications for the guideline-aligned practice:
1. Timeliness of Molecular Testing: The gBRCA2 mutation was detected only after three lines of metastatic therapy. The NCCN guidelines strongly recommend gBRCA1/2 testing for all patients with HER2− advanced breast cancer at diagnosis (Brugioni et al., 2023). Earlier detection could have allowed PARPi initiation at an earlier line, potentially maximizing the benefit, as suggested by exploratory analyses of the OlympiAD trial (Im et al., 2020). This case reinforces the critical recommendation for prompt gBRCA testing at advanced breast cancer diagnosis.
2. Therapeutic Options for gBRCAm HR+/HER2− advanced breast cancer: While PARPi monotherapy is standard, this case provides compelling real-world evidence supporting the efficacy and tolerability of Fluzoparib within this molecularly defined population. Furthermore, it highlights the potential of combining PARPis with endocrine therapy in HR + disease. Additionally, Fluzoparib offers a valuable option, particularly in regions where access to or cost of other PARPis is a barrier. This data supports the consideration of Fluzoparib within the treatment framework and encourages exploration of PARPi/endocrine therapy combinations in clinical trials and guidelines.
3. Overcoming Resistance & Future Directions: The eventual progression after 37 months suggests acquired resistance mechanisms, possibly including BRCA2 reversion mutations, restoration of HRR, or activation of bypass pathways (e.g., PI3K/AKT/mTOR) (Seed et al., 2024). Planned re-biopsy and genomic profiling align with the principles recommending repeat molecular testing at progression to guide therapy. This case underscores the need for research into optimal sequencing and combination strategies post-PARPi progression.
Conclusion
This molecularly guided case demonstrates exceptional efficacy (PFS: 37 months) and tolerability of the combination of Fluzoparib and Exemestane in a patient with heavily pretreated HR+/HER2− advanced breast cancer harboring a gBRCA2 mutation. The pivotal role of blood-based NGS in identifying the targetable gBRCA2 alteration directly enabled this successful therapeutic strategy. The profound clinical benefit achieved significantly surpasses outcomes typically seen with approved PARPis in similar settings and highlights the synergistic potential of combining PARPis with endocrine blockade in HR + gBRCAm disease.
This experience strongly reinforces the guideline recommendation for prompt gBRCA1/2 testing in all patients with HER2− advanced breast cancer at diagnosis, because earlier identification could optimize treatment sequencing. It provides robust real-world evidence supporting the clinical utility of Fluzoparib as an effective PARPi option for gBRCA-mutated advanced breast cancer. The remarkable PFS observed warrants further clinical investigation of Fluzoparib, particularly in combination with endocrine therapy, for HR+/HER2− gBRCAm advanced breast cancer, potentially informing future refinements to treatment algorithms. This case exemplifies the transformative impact of molecular insights on precision oncology.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by Ethics Committee of the 900th Hospital of PLA Joint Logistic Support Force. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DY: Writing – original draft, Writing – review and editing. XZ: Writing – original draft. XH: Data curation, Writing – original draft. SL: Visualization, Writing – original draft. NY: Data curation, Writing – original draft. XL: Data curation, Writing – original draft. QC: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – review and editing. XC: Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This case report was supported by the project of Joint Logistic Medical Quality Specialty (No. LQYZ-ZL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albarrán, V., Chamorro, J., Pozas, J., San Román, M., Rosero, D. I., Saavedra, C., et al. (2023). Maintained complete response to talazoparib in a BRCA-2 mutated metastatic luminal breast cancer: case report and review of literature. Front. Oncol. 13, 1158981. doi:10.3389/fonc.2023.1158981
Brugioni, E., Cathcart-Rake, E., Metsker, J., Gustafson, E., Douglass, L., and Pluard, T. J. (2023). Germline BRCA-mutated HER2-Negative advanced breast cancer: overcoming challenges in genetic testing and clinical considerations when using talazoparib. Clin. Breast Cancer 23 (5), 469–477. doi:10.1016/j.clbc.2023.04.006
Faraoni, I., and Graziani, G. (2018). Role of BRCA mutations in cancer treatment with Poly(ADP-ribose) polymerase (PARP) inhibitors. Cancers (Basel) 10 (12), 487. doi:10.3390/cancers10120487
Hennes, E. R., Dow-Hillgartner, E. N., Bergsbaken, J. J., and Piccolo, J. K. (2020). PARP-Inhibitor potpourri: a comparative review of class safety, efficacy, and cost. J. Oncol. Pharm. Pract. 26 (3), 718–729. doi:10.1177/1078155219895066
Im, S. A., Xu, B., Li, W., Robson, M., Ouyang, Q., Yeh, D. C., et al. (2020). Olaparib monotherapy for Asian patients with a germline BRCA mutation and HER2-negative metastatic breast cancer: olympiad randomized trial subgroup analysis. Sci. Rep. 10 (1), 8753. doi:10.1038/s41598-020-63033-4
Kang, I., Naghi, L., Yost, S. E., and Mortimer, J. (2025). Clinical actionability of molecular targets in multi-ethnic breast cancer patients: a retrospective single-institutional study. Mol. Diagn Ther. 29 (3), 393–405. doi:10.1007/s40291-025-00777-7
Kutuzov, M. M., Belousova, E. A., Kurgina, T. A., Ukraintsev, A. A., Vasil'eva, I. A., Khodyreva, S. N., et al. (2021). The contribution of PARP1, PARP2 and poly(ADP-ribosyl)ation to base excision repair in the nucleosomal context. Sci. Rep. 11 (1), 4849. doi:10.1038/s41598-021-84351-1
Litton, J. K., Rugo, H. S., Ettl, J., Hurvitz, S. A., Gonçalves, A., Lee, K. H., et al. (2018). Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N. Engl. J. Med. 379 (8), 753–763. doi:10.1056/NEJMoa1802905
O'shaughnessy, J., Brezden-Masley, C., Cazzaniga, M., Dalvi, T., Walker, G., Bennett, J., et al. (2020). Prevalence of germline BRCA mutations in HER2-negative metastatic breast cancer: global results from the real-world, observational BREAKOUT study. Breast Cancer Res. 22 (1), 114. doi:10.1186/s13058-020-01349-9
Robson, M., Im, S. A., Senkus, E., Xu, B., Domchek, S. M., Masuda, N., et al. (2017). Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 377 (6), 523–533. doi:10.1056/NEJMoa1706450
Saj, F., Nag, S., Nair, N., and Sirohi, B. (2024). Management of BRCA-Associated breast cancer patients in low and middle-income countries: a review. Ecancermedicalscience 18, 1744. doi:10.3332/ecancer.2024.1744
Seed, G., Beije, N., Yuan, W., Bertan, C., Goodall, J., Lundberg, A., et al. (2024). Elucidating acquired PARP inhibitor resistance in advanced prostate cancer. Cancer Cell 42 (12), 2113–2123.e4. doi:10.1016/j.ccell.2024.10.015
Sottnik, J. L., Shackleford, M. T., Nesiba, C. S., Richer, A. L., Fleischmann, Z., Swartz, J. M., et al. (2025). Co-regulator activity of mediator of DNA damage checkpoint 1 (MDC1) is associated with DNA repair dysfunction and PARP inhibitor sensitivity in lobular carcinoma of the breast. BioRxiv, 2023.10.29.564555. doi:10.1101/2023.10.29.564555
Wang, L., Yang, C., Xie, C., Jiang, J., Gao, M., Fu, L., et al. (2019). Pharmacologic characterization of fluzoparib, a novel poly(ADP-ribose) polymerase inhibitor undergoing clinical trials. Cancer Sci. 110 (3), 1064–1075. doi:10.1111/cas.13947
Keywords: fluzoparib, BRCA, mutation, breast cancer, PARP inhibitor, case report
Citation: Yang D, Zhang X, Hu X, Lin S, Yu N, Lin X, Chen Q and Chen X (2025) Case Report: Fluzoparib combined Exemestane in gBRCA2-mutated HR+/HER2− advanced breast cancer. Front. Pharmacol. 16:1673418. doi: 10.3389/fphar.2025.1673418
Received: 25 July 2025; Accepted: 29 September 2025;
Published: 09 October 2025.
Edited by:
Ajit Prakash, University of North Carolina at Chapel Hill, United StatesReviewed by:
Maoben Sun, Dongguan Binhaiwan Central Hospital, ChinaSai Kumar Badam, Gandhi Institute of Technology and Management (GITAM), India
Copyright © 2025 Yang, Zhang, Hu, Lin, Yu, Lin, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qunxiang Chen, NTU0MTYwODYzQHFxLmNvbQ==
 Dunya Yang1,2
Dunya Yang1,2 Qunxiang Chen
Qunxiang Chen Xi Chen
Xi Chen