Abstract
Introduction:
Cervical cancer, the third most common cancer worldwide, is primarily caused by human papillomavirus (HPV) infection. This study aimed to develop natural extracts from the seeds of Carica papaya L. using the hydrodistillation method to evaluate their anticancer effects against HeLa cells.
Methods:
The phytochemical composition of Carica papaya seeds’ essential oil was identified using gas chromatography mass spectrometry, and their cytotoxicity on proliferation, apoptosis, cell cycle phases, migration, and colony formation of HeLa cancer cell lines was examined. Moreover, the effects of essential oil on the protein expression levels related to apoptosis, cell cycle regulation, and migration in the treated HeLa cells were examined.
Result and discussion:
The essential oil’s phytochemical composition was analyzed using gas chromatography-mass spectrometry (GC-MS), revealing benzyl isothiocyanate as the dominant compound (99.49%). Results demonstrated that the essential oil had a high cytotoxic effect, with an IC50 value of 16.78 µg/mL in the MTT assay. Apoptosis analysis indicated a significant increase in early and late apoptotic HeLa cells (23.45%). Flow cytometry revealed a G2/M phase arrest, which impeded cell division. The oil also exhibited a stronger inhibition of cancer cell migration (38.7%) than methotrexate (45.9%). Additionally, the clonogenic assay revealed a drastic reduction in colony formation (0.004% surviving fraction, 0.25% plating efficiency). ELISA results showed a profound effect on apoptosis-related proteins, reducing BCL-2, MMP-2, and CDK1/cyclin B1 expression, supported by molecular docking studies comparing its efficacy to methotrexate (−5.52, −6.29, and −5.75 vs −5.49, −5.44, and 5.18 kcal mol−, respectively). The findings suggest that Carica papaya seed essential oil may serve as a potential anticancer treatment for cervical cancer; however, further in vivo studies are required for validation in animal models.
1 Introduction
Cancer remains one of the most significant challenges to public health worldwide, with a profound impact on the healthcare system, individuals, and families (Nady et al., 2023; Chikkanna et al., 2024). Cancer is a group of diseases characterized by the uncontrolled growth and the spread of abnormal cells (Haber et al., 2023). Until now, there has been no absolute care for various types of cancers (Kong et al., 2021; Nady et al., 2024b). Cervical cancer is the third most common type of cancer all over the world, which is mainly caused by human papillomavirus infection (Praveena et al., 2017; Khan, 2021). Worldwide, it is the second most common type of cancer in women after breast cancer (Sari and Handayani, 2020), while in India, it is the leading type of cancer (Khan, 2021). Radiotherapy, surgery, hormone therapy, and chemotherapy are conventional cancer treatment methods (Haber et al., 2023; Jiao et al., 2023; Alzahrani et al., 2024), which are associated with several problems, including high costs (Alzahrani et al., 2024), increasing the multiple resistance (MDR) to drugs, dose-limiting systemic toxicity (Chandrasekaran et al., 2016), and the high cytotoxic effect on normal cells (Alotaibi et al., 2017). The several negative side effects associated with the conventional method have motivated researchers to search for new strategies for cancer treatment. In this context extensive research was focused on utilizing the plant and its extracts for cancer treatment (Varadarajan et al., 2022). Approximately one-third of Food and Drug Administration-approved drugs over the past two decades have been derived from natural products or their derivatives (Zhang et al., 2022).
At present, about 80% of individuals, mainly from developing countries, use traditional medicine derived from medicinal plants (Martin Paul et al., 2019; Abd El-Razek et al., 2023). In cancer treatment, natural compounds make up the majority of approved antitumor medications. Medicinal plants have been especially crucial in discovering and developing new agents for cancer prevention and therapy (Hegazy et al., 2021). Medicinal plants or extracted natural products have shown major advantages over synthetic ones, such as easy availability (Singh et al., 2020), being a cheaper alternative source (Lydia et al., 2016; Abdel-Halim et al., 2021), being more effective (Doughari and Manzara, 2007), having fewer adverse reactions, and having lower toxicity (Zhang et al., 2022), and finally, they offer a rich and renewable reservoir of bioactive compounds, including alkaloids, terpenoids, flavonoids, polyphenols, saponins, and glucosinolates that are essential for the treatment of many diseases (Saha et al., 2023; Hegazy et al., 2016; Varadarajan et al., 2022). One such medicinal plant is Carica papaya L.
C. papaya belongs to the Caricaceae family, commonly called pawpaw, papaya or papaw (Nirosha and Mangalanayaki, 2013; Doughari and Manzara, 2007; Balavijayalakshmi and Ramalakshmi, 2017). It is native to the tropical and semi-tropical regions of South America and Africa, respectively, but has now spread worldwide (Egbuonu et al., 2016; Abd-elkawy et al., 2019). C. papaya is an evergreen-herbaceous plant that looks like a tree but is semi-woody (Abd-elkawy et al., 2019), is marked with lead scars and rarely branches (Egbuonu et al., 2016). The pulp represents around 60% of the weight of C. papaya fruit, which is widely used in food products such as jams, juice, ice cream, jelly, and pepin production (Haber et al., 2023). The peel represents 12% of the weight, which is considered agricultural waste, and seeds represent about 20% of the fresh fruit weight, which is also considered agricultural waste (Gaikwad et al., 2023). But nowadays, C. papaya seeds are used for many purposes, as an alternative to black pepper, and are characterized by strong antibacterial, antioxidant, anticancer, anthelmintic, liver protection, nephroprotective, and typhoid treatment properties, so the seeds have more potent medicinal effects compared to other C. papaya parts (Memudu and Oluwole, 2021). Because of the high medicinal and nutritional values of C. papaya, a huge amount is consumed worldwide, which leads to the generation of a large amount of peels and seeds as waste, and these could be considered a significant cause of pollution if not utilized in another aspect (Amran et al., 2021; Phang et al., 2021).
The C. papaya is highly cultivated in India, and there are many studies using different parts of C. papaya against different cancer cell lines. India is one of the main origins of cultivated papaya around the world, and there are many studies of using Indian papaya extract against different cell lines, first using latex against human breast cancer (MCF-7), which shows a good anticancer response in a dose-dependent manner with IC50 equal to 19.88 µg/mL (Chandrasekaran et al., 2016). Using leaf extract against hepatocellular carcinoma (HepG-2) and breast carcinoma (MCF-7, A549, and MDA-MB-231) cell lines (Devanesan et al., 2021; Alzahrani et al., 2024). Also, using seed extracts against oral squamous carcinoma (SCC-25) and the HepG-2 cancer cell line (Anilkumar and Bhanu, 2022), which confirmed the anticancer effect of the seed extract, which exhibited G2/M phase arrest (Varadarajan et al., 2022), along with the potential to induce apoptotic change by downregulation of Bcl-2 protein and upregulation of P53 and Caspase-3 genes (Anilkumar and Bhanu, 2022). Also, pulp and peel extract against colon cancer (Praveena et al., 2017; Vundela et al., 2022), cervical cancer (Praveena et al., 2017), and breast cancer (John et al., 2021; Vundela et al., 2022). Furthermore, the seed extract from C. papaya cultivated in Taiwan significantly decreases the cell viability and migration of colorectal cancer cell lines (Chang et al., 2020; Lin et al., 2021). The seed extract from two types of C. papaya (California and Bangkok) was able to inhibit the growth of breast cancer cells without causing any toxic effects on normal cells (Alfarabi et al., 2022). Finally, C. papaya leaf extract cultivated in Egypt showed a moderate effect against hepatocellular carcinoma (HepG-2) and colon cancer (HCT-116) (Abdel-Halim et al., 2020). While the aqueous seed extract exhibited the anticancer effect against the human colorectal adenocarcinoma cell line (Caco-2) with an IC50 equal to 9.73 µg/mL, it regulated the expression of Caspase-3 and P53 genes, triggering cell cycle arrest and apoptosis (Mahrous and Noseer, 2023).
According to previous studies, the fixed oil of C. papaya seeds was found to be in a range of 13.9%–30.7%, with a color ranging from pale to dark yellow, and is flavorless and odorless. The most abundant fatty acids are oleic, palmitic, stearic, and linoleic acids (Roberta Malacrida et al., 2011; Yanty et al., 2014), while the essential oil “volatile aromatic” from C. papaya seeds has attracted the attention of the scientific community (Komal and Arya, 2013; Yusuf, 2018). Essential oil of C. papaya seeds was found to have an amount of benzyl isothiocyanate (BITC) (Nady et al., 2024a), which is mainly formed from benzyl glucosinolate in the presence of myrosinase enzyme, as shown in Figure 1 (Lee et al., 2011).
FIGURE 1
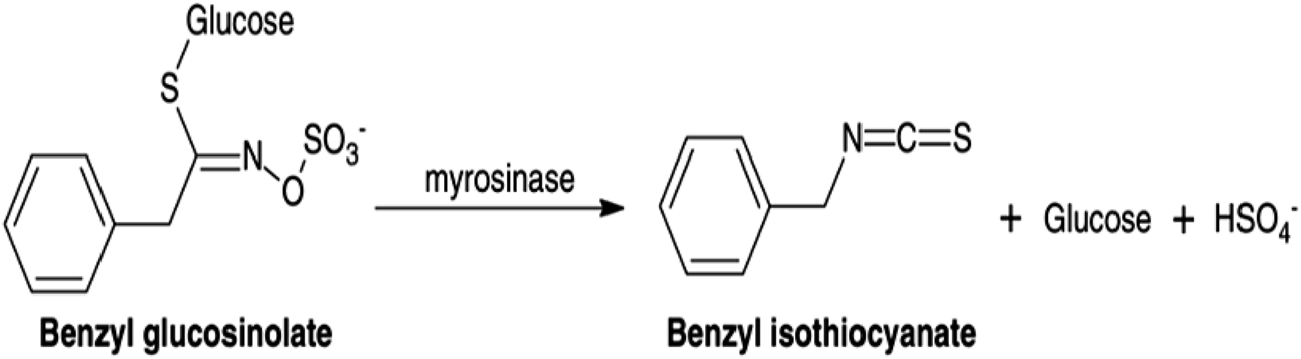
Schematic representation of the enzymatic conversion of benzyl glucosinolate into benzyl isothiocyanate (BITC) catalyzed by myrosinase.
Recent studies have highlighted the anticancer properties of benzyl isothiocyanate through multiple mechanisms. First, by inducing apoptosis, that is, by promoting programmed cell death by activating Caspase-3 and modulating pro-apoptotic proteins such as anti-apoptotic protein, as Bax (Rahaman et al., 2025; Sinha et al., 2025). By cell cycle arrest, that is, by disrupting the cell cycle progression, especially at the G2/M phase (Sinha et al., 2025). BITC tends to increase the intracellular reactive oxygen species (ROS), which leads to mitochondrial dysfunction and oxidative stress in cancer cells. Also, it influences oncogenic pathways, which are involved in stress response and cell survival (Rahaman et al., 2025). Finally, BITC has antimetastatic activity, which is by suppressing migration and invasion by downregulating epithelial-mesenchymal transition markers (EMT) and metalloproteinase markers (MMPs) (Sinha et al., 2025).
Although the anticancer potential of Carica papaya seed extract has been explored in various studies, the specific impact of the essential oil extracted from C. papaya cultivated in Egypt on human cervical cancer cells remains uninvestigated. Therefore, this study aims to characterize the chemical composition of Egyptian C. papaya seed essential oil using GC-MS analysis to evaluate its cytotoxic activity against HeLa cervical cancer cells via MTT assay, including the determination of the IC50 value. To further elucidate its anticancer effects, the study investigates the essential oil’s effect on apoptosis induction, cell cycle arrest, migration behavior, and clonogenic capacity using different methods such as Annexin V/PI analysis, flow cytometry, wound healing assay, and clonogenic assay, respectively. Additionally, the expression levels of key proteins involved in apoptosis, metastasis, and cell cycle regulation are quantified through ELISA, and their molecular interactions are explored via in silico docking analysis. The safety of the essential oil is also examined using the hemolysis assay.
2 Materials and methods
2.1 Chemicals and reagents
All medium components, chemicals, buffers, solvents, and reagents were purchased from Sigma Aldrich (United States).
2.2 Collection and preparation of plant samples
Fresh ripening fruits of C. papaya Linn. (family Caricaceae) were obtained from Gardens Resort at Kilometer 63 on the AL-Azizyah Alexandria desert road in July 2022. They were identified and certified by Dr. Kamal Zayed, professor of Ecology at Cairo University, with voucher specimens CAIRC [voucher No. 3237]. The fruits were washed with tap water, followed by distilled water. The fruits were then halved lengthwise, peeled using a kitchen knife, and the seeds were removed. The seeds underwent triple washing with distilled water to eliminate any impurities and dust (Anilkumar and Bhanu, 2022). The seeds were dried at room temperature in shade for 5 days to preserve the seeds’ physicochemical properties (Subbaiah Munagapati et al., 2022). Then dried for 48 h at a temperature of less than 60 °C in a convection oven until constant weight (Yanty et al., 2014; Aina et al., 2020). After that, they were pulverized into fine particles with a size of about 0.85 mm using the electric mill. Following that, the pulverized seeds were stored at (2 °C–3 °C) in a closed container until further use (Sultana et al., 2020). Finally, the percentage of the moisture content and yield were determined using the following Equations 1, 2, respectively (Adesola et al., 2019; Egbuonu et al., 2016):
2.3 Extraction of essential oil from Carica papaya seeds
The extraction of EO from C. papaya seeds was done utilizing a Clevenger-type apparatus for 3 h, using the previous protocol (Spyridopoulou et al., 2019; Saleh et al., 2020; Mohamed et al., 2022). A batch of 100 g of seeds was mixed with water (200 mL) at 70 °C, then the formed oily layer was extracted using diethyl ether. The collected essential oils were dried over anhydrous sodium sulfate (0.5 g) and then stored at −3 °C in a sealed, airtight glass vial until the next analysis. The weight and volume of the extracted essential oil were detected using the electric balance and pipette, respectively. The yield percentage of extracted oil was determined using the following Equation 3 (Toyin Oshin et al., 2021; Sultana et al., 2020):
2.4 GC-MS analysis
The extracted essential oils were analyzed using gas chromatography-mass spectrometry (GC-MS) gas chromatography-mass spectrometry. This apparatus consists of a TRACE GC Ultra Gas Chromatograph connected with a thermos mass spectrometer detector. The GC-MS system was outfitted with a TR-5 Ms column, and helium was used as a gas carrier. The program temperature was 60 °C for 1 min, rising by 4 °C/min to 240 °C and fixed for 1 min. The temperature of the detector and injector was kept at 210 °C. The sample was diluted by n-hexane (1:10 v/v), and 1 µL of the diluted mixture was injected (Reda et al., 2017; 2021).
2.4.1 Identification of the essential oil constituents
The chemical constituents of the extracted essential oils were characterized using Automated Mass Spectral Deconvolution and Identification (AMDIS) software and identified by following the Wiley spectral library collection, matching mass spectrum, and NIST library database with authentic standards, as well as their retention indices relative to n-alkanes (C8-C22) (Reda et al., 2017; Saleh et al., 2020). The mass spectra with those appraisals or published mass spectra with authentic standards are available in the authors’ laboratory (Adams, 2005). The schematic diagram for sample preparation and the extraction process is presented in Figure 2.
FIGURE 2
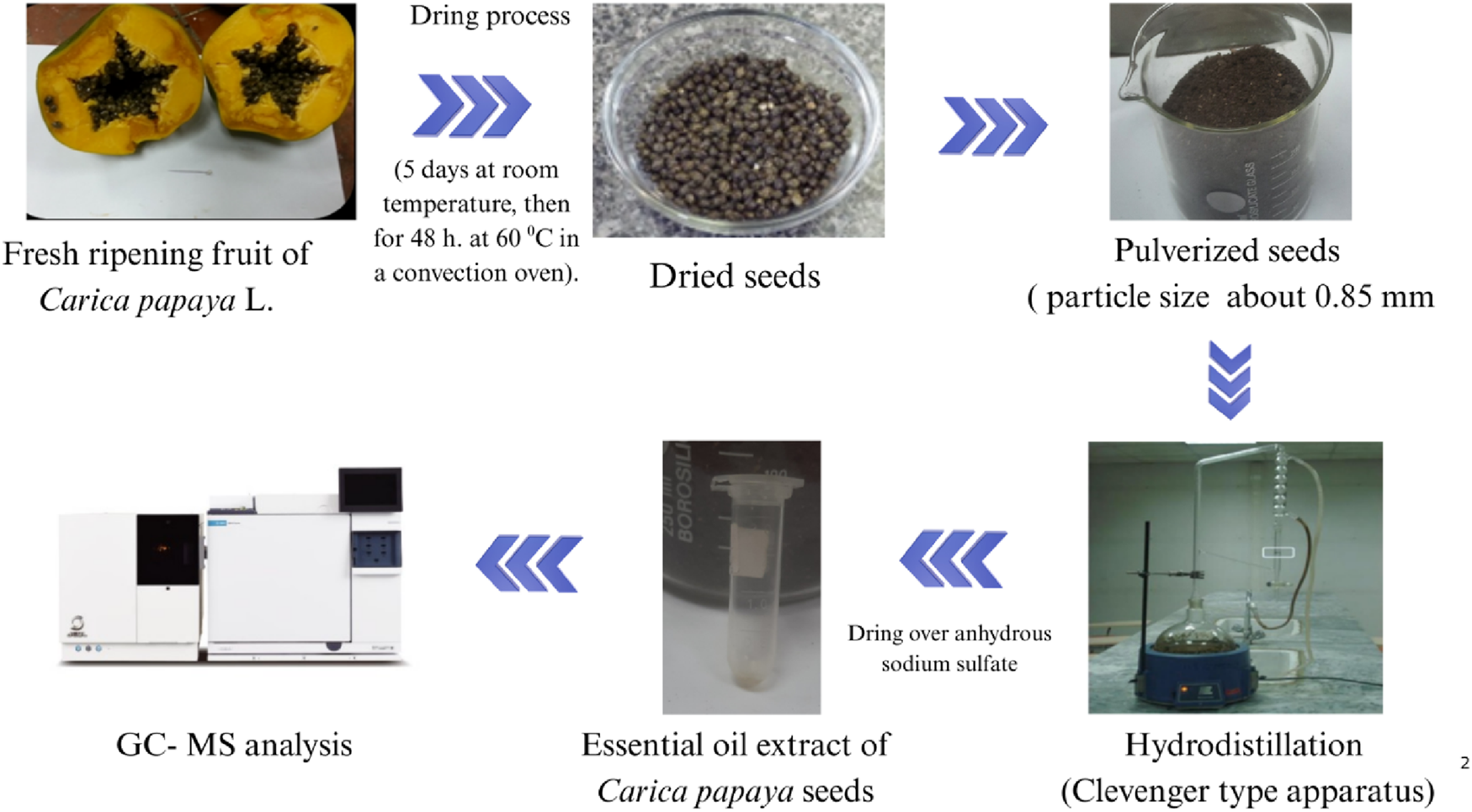
Workflow diagram of Carica papaya seed processing and essential oil extraction using the hydrodistillation method.
2.5 Anticancer properties
2.5.1 Preparation and maintenance of cell lines
The Hep-2 cell line (Human Laryngeal Carcinoma) (CCL-23) and the HeLa cell line (Human Cervical Carcinoma) (CCL-2) were purchased from the VACSERA tissue culture unit in Egypt. The cell lines were grown in (DMEM) Dulbecco’s Modified Eagle’s Medium, augmented with (FBS) fetal bovine serum (10%) and supplemented with two antibiotics (penicillin (100 mg/mL) and streptomycin (100 mg/mL)). Then preserved in a humidified atmosphere with 5% CO2 at 37 °C. Finally, the cell lines were trypsinized utilizing trypsin EDTA, then subcultured in the culture flasks to keep them and used in the following experiments.
2.5.2 MTT-based cytotoxicity assay
Cell cytotoxicity (cell viability) was detected by [3-(4,5-dimethylthiozol-2-yL)-2,5-diphenyl tetrazolium bromide] (MTT) assay (Moussa et al., 2025b; 2025a; Mustafa et al., 2025). The MTT assay is a colorimetric method used to determine the metabolically viable percentage of Hep-2 and HeLa cancer cells after treatment with the extracted essential oil from C. papaya seeds, as previously described (Hanna et al., 2023). In a nutshell, the 96-well tissue culture plate was incubated with 100 µL of cells (1 × 105) per well and incubated for 24 h at 37 °C that is, mainly to develop a complete monolayer sheet. Then, the cells were treated with different concentrations (7.81, 15.62, 31.25, 62.5, 125, 250, 500, and 1,000 µg/mL) of the extracted essential oil at 37 °C for an overnight incubation period. Subsequently, checking for any visual toxicity signs, like cell rounding, granulation, shrinkage, and complete or partial monolayer distribution in both the treated and control cells. After that, the 20 µL of MTT solution (5 mg/mL in PBS) was used to treat untreated cells (control) and the essential oil-treated cells for 4 h at an incubation temperature of 37 °C, that is to allow the metabolism of MTT into formazan which is an insoluble dark purple products that is directly proportional to the viable cells’ numbers. The intracellular reduction of MTT to form the formazan depends mainly on the mitochondrial dehydrogenase enzyme. Then, 200 µL of DMSO (dimethyl sulfoxide) was added to each well with shaking for 5 min at 150 rpm; that is to extract the MTT metabolic product (formazan crystal). Finally, the optical density of the formazan crystal was recorded at 560 nm using a microplate reader (Bio-Rad, United States). The effect of the concentration of the extracted essential oil on the proliferation of HeLa and Hep-2 cancer cells was represented as the percentage of cytotoxicity using the following Equations 4, 5 (Praveena et al., 2017; Anilkumar and Bhanu, 2022; El-Atawy et al., 2024):
The 50% inhibitory concentration (IC50) of the C. papaya seeds’ extracted essential oil was determined for 4 h as an incubation period at 37 °C. Where the IC50 concentration represents the concentration at which 50% of the cells are still alive.
2.5.3 Annexin V\PI analysis for apoptosis assay
The Annexin V/PI assay can be used to discriminate between necrotic and apoptotic cells using both FITC Annexin V-fluorescein isothiocyanate and (PI) propidium iodide stains, depending on the ability of FITC to bind to the phosphatidyl serine (PS), which allows it to transfer to the outer cell membrane from the inner plasma membrane after the early apoptotic process. Also, the permeabilized membrane of the necrotic cells was identified by using PI. The percentage of apoptotic/necrotic cells in the treated HeLa cells that were previously treated for 24 h with an IC50 concentration of the extracted essential oil from C. papaya seeds and untreated HeLa cells was detected by an Annexin V-FITC kit (BD Bioscience) as previously reported (Ahmed et al., 2024; Mustafa et al., 2025). In a nutshell, in a 6-well culture plate, HeLa cancer cells were harvested and incubated overnight; that is, it allowed the cells to adhere and proliferate. Then, the HeLa cells were treated with the extracted essential oil for 24 h. Then, the treated and untreated HeLa cells were trypsinized, centrifuged, and then washed with cold phosphate buffer saline twice. After that, the washed cells were reacted with the Annexin V binding buffer, and then into a culture tube, 100 µL of this cell solution was transferred for 15 min in the dark, allowing the Annexin V-FITC to incubate with stained cells and identify the presence of necrotic cells and also early and late apoptotic cells by using a BD Biosciences, BD FACS CaliburTm flow cytometer.
2.5.4 Examining the different phases of the cell cycle
Cell cycle analysis was examined to identify the cell division phases. The percentage of treated cells with the extracted essential oil and untreated cells through different stages of the cell cycle was evaluated using Abcan, a propidium iodide cytometry kit (catalog ab139418), and a flow cytometry kit, as mentioned earlier (Hanna et al., 2023). Briefly, in the 6-well culture plate, the HeLa cancer cells were harvested, and then the plate was left for 24 h, where the adhesion of cells occurred. Then the cells were incubated with the extracted essential oil for 48 h. After that, the cells were collected, trypsinized with trypsin EDTA, harvested using centrifugation at 1,500 rpm for 5 min, and finally fixed with 70% cold ethanol overnight. After the fixation process, the cells were centrifuged, washed with PBS twice, treated with RNase (100 mM/ml), added to a fluorescent propidium iodide solution, and left in the dark at 37 °C for 30 min, that is, for staining of the treated and untreated cells. In the last step, flow cytometry was used to measure the percentage of existing cells in each phase of the cell cycle (sub-G1, G0/G1, S, and G2/M phases) to quantify the DNA content.
2.5.5 Cell migration (the wound healing assay)
Malignant tumors proliferate uncontrollably and can spread throughout the whole body by migrating through the circulatory system. So, a wound healing assay was used to detect how the extracted essential oil can affect the ability of cervical cancer cells (HeLa) to migrate (Hanna et al., 2023). In brief, cells were incubated in a 6-well plate at 37 °C until they confluenced as a monolayer (90%). Then an artificial straight scratch was made by using a pipette tip, simulating a wound at 0 h. The formed scratch was imaged by using a light microscope and then washed with phosphate buffer saline three times. The scratched cells were seeded in fresh media containing the essential oil extracted from C. papaya seeds; untreated cells (negative control) were seeded in media containing sterile saline, while the positive control cells were cultured in fresh media containing methotrexate, then they were all incubated for 48 h at an incubation temperature of 37 °C at 5% CO2. After the incubation period (48 h), the migration of the negative control, treated cells, and positive control was imaged once more. Finally, the migration rate and the % of wound closure were calculated based on the width of cells scratched at 0 h and 48 h using the following Equations 6, 7, respectively.
Wi = average of the initial width of the wound (µm)
Wf = average of final width of the wound (µm)
T = span time of the assay in (hours)
At=0 = initial wound area.
At=∆t = wound area after n hours.
2.5.6 Colonogenic assay
The clonogenic assay is an in vitro technique used to assess cell survival, where the capacity of a single cell to proliferate and form a colony is evaluated (Ratnasari et al., 2023). To determine the effects of extracted oil on the clonogenic capacity of HeLa cancer cells, cells were planted in a 6-well plate and incubated for 24 h at an incubation temperature of 37 °C and 5% CO2. After the period of incubation, the cells were treated with the extracted essential oil using an IC50 concentration, and the culture was then incubated for one to 3 weeks or until a colony was formed. After that, the 6% glutaraldehyde was used to fix the cells, then stained with crystal violet (0.5%) for 30 min. After that, the cells were washed with tap water and then left at room temperature to dry. After the cells were dried, the colonies were observed using a stereomicroscope and a colony counter pen for automatic counting of the colonies. Finally, the plating efficiency (representing the ability to form colonies) and a surviving fraction (SF) were calculated using Equations 8, 9, respectively (El Habbash et al., 2017):
2.5.7 ELISA testing
The expression levels of the proteins involved in apoptosis (Bax, Bcl-2, and Cleaved Caspase-3), cell cycle regulation (Cyclin B1 and CDK1), and metastasis (MMP-9 and MMP-2) in the extracted essential oil-treated and untreated HeLa cells were evaluated and quantified using the ELISA technique. ELISA kits used included Bax (ab199080), Bcl-2 (ab272102), Cleaved Caspase-3 (ab220655), MMP-9 (ab246539), and MMP-2 (ab267813), all provided by Abcam (Cambridge, United Kingdom). These kits employed a sensitive 90-min sandwich ELISA technique with capturing antibodies attached to an affinity tag identified by an anti-tag antibody precoated on the ELISA plates. Treated and untreated HeLa cells were centrifuged, where the pellets were discarded, and the supernatants were used as samples. Equal volumes of the supernatant and an antibody cocktail mixture (containing detector antibodies and capture antibody diluted in Antibody Diluent 5BI) were dispensed into a 96-well ELISA plate and incubated for 1 h at room temperature. After multiple washes with (1X) washing buffer, the substrate solution tetramethylbenzidine (TMB) was added to each well (10 min), and the plate was kept in the dark. Finally, a stop solution was added after that, and the absorbance was measured using an automatic microplate reader at 450 nm. Additionally, the mitosis-regulating protein expression levels were detected using the ELISA Cyclin-Dependent Kinase 1 (CDK1) Kit (Catalog No: MBS458278; MyBioSource, United States) and ELISA Cyclin-B1 Kit (Catalog No: MBS765913; MyBioSource, United States). These kits were based on the sandwich ELISA double-antibody technique, with anti-human CDK1 and anti-human Cyclin-B1 precoated on 96-well plates. Test samples, standards, and biotin-conjugated detection antibodies were added to the wells and then washed using a washing buffer. After that, the wells were treated with HRP (horseradish peroxidase-streptavidin) and TMB substrate, which induced the blue color to turn yellow by adding an acidic stop solution. The yellow color absorbance was measured using an automated microplate reader at 450 nm, which directly correlated with the amount of human CDK1 and Cyclin-B1 in the treated samples, computed using a standard curve.
2.6 Analysis of the toxicity of the extracted essential oil using hemolysis assay
The antihemolytic activity of the extracted oil was analyzed using a spectrophotometric approach as previously described (Moussa et al., 2025b). The resulting pellet was washed with phosphate buffer saline (PBS) three times and then immersed in PBS (5% V/V). Then, the erythrocyte solution (400 µL) was added to the extracted essential oil (100 µL) and incubated for 30 min at 37 °C, then centrifuged at RT. At last, the level of hemolysis was measured by determining the absorbance of hemoglobin liberated from cells at 540 nm. The hemolysis percentage was determined using the following Equation 10:where A(test sample) represents the optical density of the sample containing the extracted essential oil, A(+sample) represents the absorbance of the positive control (containing erythrocytes treated with Triton X-100), and A(-sample) represents the absorbance of the negative control (containing erythrocyte solution in PBS only).
2.7 Molecular docking process
The structure of benzyl isothiocyanate, as well as the positive control, methotrexate (MTX), was downloaded as an SDF file from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). Then, converted SDF files to PDB format by using the free software Avogadro (https://avogadro.cc/). The PDB file for BCL-2 crystal structures (PDB ID: 1K3K) is a key player for pro- or anti-apoptotic activities, the PDB file for MMP-2 crystal structures (PDB ID: 1HOV) is a key player in the tumor ecosystem, and the PDB file for CDK1/cyclin B1 crystal structures (PDB ID: 4YC3) is a key player for arrest in G2/M. They were downloaded from the Protein Data Bank (http://www.rcsb.org/pdb/). These targets were selected based on their biological relevance: BCL-2 as a regulator of apoptosis, MMP-2 as a modulator of the tumor microenvironment, and CDK1/Cyclin B1 as a checkpoint controller of the G2/M cell cycle arrest, supported by prior literature (Petros et al., 2001; Quick et al., 2014; Brown et al., 2015; Hobani et al., 2017). As co-crystallized ligands were not available for 1K3K and 1HOV structures, blind docking was initially performed to identify potential binding regions. The convergence of benzyl isothiocyanate poses across multiple runs informed the prediction of the active site, which was Docking box dimensions were defined around this predicted site to ensure accurate redocking and pose evaluation. The box dimensions for 1K3K are 230.7 mm, 32.8 mm, and 116.9 mm for X, Y, and Z, respectively, while the box dimensions for 1HOV are 17.1 mm, 26.6 mm, and 19.7 mm for X, Y, and Z, respectively. The CDK1–Cyclin B1 complex (PDB ID: 4YC3) contains a co-crystallized ligand, enabling structural validation of the binding pocket. The active site was further predicted using Proteins Plus (https://proteins.plus), integrating geometric and chemical criteria to refine docking box dimensions and ensure accurate ligand placement. The box dimensions are 22.924, 5.297, and 168.970. Validation procedures included pose clustering analysis based on RMSD similarity and binding energy ranking. These metrics were used to assess conformational consistency and identify the most stable ligand orientations.
Then, converted SDF files to PDB format by using the free software Avogadro (https://avogadro.cc/). To calculate the standard deviations and mean value of the lowest binding energies, the docking process was performed three times independently. Validation procedures included pose clustering analysis based on RMSD similarity and binding energy ranking to assess conformational consistency and identify the most stable ligand orientations. The representation and graphical analyses were performed using the BIOVIA Discovery Studio Visualizer software. This study was used to prepare molecular docking simulations and protein structure by using AutodockTools (1.5.6rc316) software. Docking was performed using AutoDock4 using the Lamarckian algorithm as described before (Fukaya et al., 2018).
2.8 Statistical analysis
Each study was conducted three times, and the results are presented as mean ± standard deviation. Independent T-tests were used for all statistical analyses, performed with SPSS 17.0 software. Statistical significance was defined for p-values of p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001.
3 Results and discussion
3.1 Physicochemical properties of the extract
The quantity and quality of the extracted essential oil and other phyto-constituents of the natural extract (e.g., Carica papaya seed) differ according to the origin, seasonal variation, and ripening level, as well as the extraction technique (Shaheen et al., 2023). In this study, C. papaya seeds showed a high percentage of yield (67.36%), where the initial wet seeds weight is 297.1 g and the dry seeds weight is 200.12 g. Also, the moisture content of seeds was calculated to be equal to 32.53%. Besides the extraction process, the moisture content and drying process before the oil extraction from the seeds play a role in the yield of extracted oil (Tan, 2019). The C. papaya seeds’ essential oil extracted using the hydrodistillation extraction method was yellow, and the overall yield (based on the weight of dried seeds w/w (100 g)) was equal to 0.12%. In another study, the overall yield of extracted essential oil from Carica papaya seeds from China using the hydrodistillation method was 0.2% (w/w, based on the weight of dried seeds (300 g)) (He et al., 2017).
Most of the previous studies of the fixed oil content of C. papaya seeds showed that the fixed oil (fats) content of C. papaya seeds ranges between 13.9% and 30.7%, and the color ranges from pale to dark yellow and is almost flavorless and odorless (Yanty et al., 2014). Also, they found that the functional and nutritional properties of fixed oil extracted from C. papaya seeds are highly similar to olive oil (Tan, 2019). It has a high level of monounsaturated fatty acids (MUFA) and a low level of total tocopherol (Roberta Malacrida et al., 2011), so C. papaya seed oil represents a good prospective source of oil (Tan, 2019). Nowadays, the extracted essential oil has attracted a lot of attention due to having many applications in different fields like food, cosmetics, industry, and medicine. That EOs have several biological activities, including antitumor, anti-inflammatory, antiviral, antioxidant, and antibacterial activity (Shakeri et al., 2016; He et al., 2017; Yusuf, 2018).
3.1.1 Chemical composition of essential oil
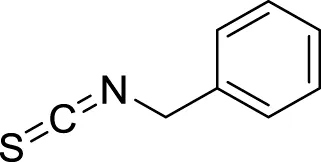
The yield of the extracted essential oil was 0.12% v/w (yellow), which was obtained after the extraction of oil from C. papaya seeds using hydrodistillation. Compound identification was achieved based on their mass spectral data (MS) to compare with the NIST library databases, the Wiley spectral library collection, and GNPS. There were an estimated 99.87% of total pointed-out molecules, Figure 3. The chemical components of the extracted essential oils, along with their retention times (RT), experimentally determined Kovats retention indices (KI Exp.), and Kovats retention indices from literature, are summarized in Table 1. The essential oil’s chemical composition closely matches previous studies, predominantly consisting of isothiocyanates (ITCs). Benzyl isothiocyanate (BITC) is the primary isothiocyanate, representing 99.49% of the composition (He et al., 2017), as shown in Figure 4. Isothiocyanates (ITCs), present in cruciferous vegetables like broccoli and cabbage, possess a range of properties, including antibacterial, antifungal, antioxidant, and cytoprotective effects (Ramakrishnan et al., 2024). The rest of the classes of essential oil constituents are present as traces (0.38%). They were identified as hydrocarbons (0.15%), alkaloids (0.02%), amino acids (0.12%), oxygenated sesquiterpenes (0.03%), and steroids (0.01%). Their biological activities are mentioned in Table 1.
FIGURE 3
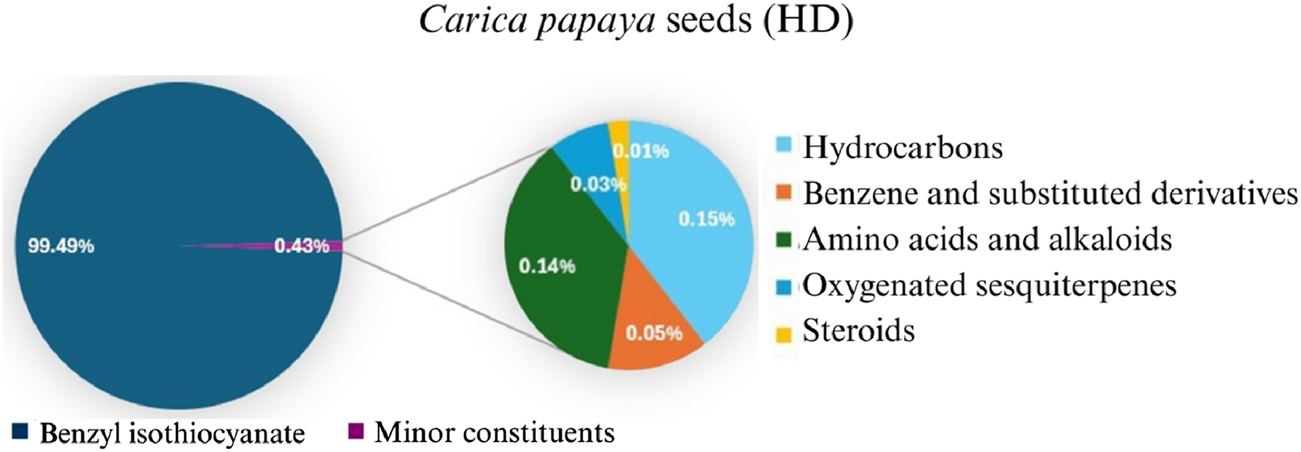
Pie chart illustrates the chemical composition of Carica papaya seeds extract obtained via hydrodistillation. Benzyl isothiocyanate constitutes the major component (99.49%), while minor constituents, including hydrocarbons, benzene derivatives, amino acids, oxygenated sesquiterpenes, and steroids, collectively account for 0.43% of the total composition.
TABLE 1
| No. | RT (min) | Compound name | MF | MW (g\mol) | Structure | KLit | KExp | Peak area (%) | Base peak (m/z) | Key fragments (m/z) | Biological activity | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrocarbons | ||||||||||||
| 1 | 3.02 | Heptane | C7H16 | 100 |

|
700 | 695 | 0.02 | 43 | 71, 57, 41 | ||
| 2 | 3.26 | Methylcyclohexane | C7H14 | 98 |
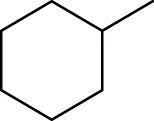
|
720 | 212 | 0.13 | 83 | 98, 69, 55, 41 | - | (Macleod and Pieris, 1983) |
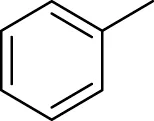
| 3 | 3.65 | Toluene | C7H8 | 92 |
|
770 | 763 | 0.05 | 91 | 92, 65, 51, 39 | - | Macleod and Pieris (1983) |
| Total hydrocarbons | 0.20 | |||||||||||
| Alkaloids | ||||||||||||
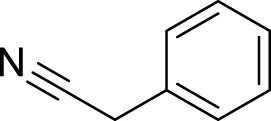
| 4 | 13.32 | 2-phenylacetonitrile | C8H7N | 117 |
|
1,148 | 1,145 | 0.12 | 117 | 116 90, 51 | - | (Macleod and Pieris, 1983; MacLeod and Pieris, 1983)[2][4] |
| 5 | 23.23 | Benzyl isothiocyanate | C8H7NS | 149 |
|
1,353–1,371 | 1,360 | 99.49 | 91 | 92, 65, 61 | Anticarcinogenic, anthelmintic, and antibacterial activity | (Chandra Kala and Ammani, 2017; Dahham et al., 2015) |
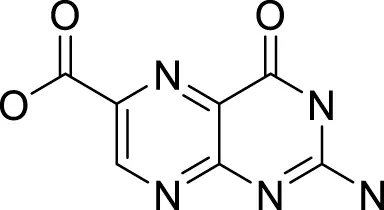
| 6 | 43.47 | Pterin-6-carboxylic acid | C7H5N5O3 | 207 |
|
2,453 | 2,455 | 0.02 | 44 | 207, 163, 149 | Anti-Tumor Activity | (Ananthanayagi et al., 2022; Dinh et al., 2021; Li et al., 2021) |
| Total alkaloids | 99.63 | |||||||||||
| Oxygenated sesquiterpenes | ||||||||||||
| 7 | 35.31 | Caryophyllene oxide | C15H24O | 220 |
|
1,583 | 1,580 | 0.01 | 43 | 220, 121, 79 | Antioxidant, anticancer antibacterial, anti-inflammatory and anesthetic activity | (Ulubelen et al., 1994; Kadhim et al., 2016; Almanaa et al., 2022; Youssef et al., 2023) |
| 8 | 35.53 | tau-Cadinol | C15H26O | 222 |
|
1,640 | 1,635 | 0.02 | 161 | 204, 189, 134 | Antibacterial activity | Abd-ElGawad et al. (2022) |
| Total Oxygenated Sesquiterpenes | 0.03 | |||||||||||
| Steroids | ||||||||||||
| 9 | 47.76 | Ethyl iso-allocholate | C26H44O5 | 436 |
|
2,478 | 2,477 | 0.01 | 354 | 400, 280, 200 | Antioxidant, antimicrobial, anti-inflammatory, anti-arthritic, and antiasthmatic activity | (Elsharkawy et al., 2021; Taha Mohamed et al., 2021) |
| Total Steroids | 0.01 | |||||||||||
| Total identified compounds | 99.87 | |||||||||||
| Total unidentified compounds | 0.13 | |||||||||||
Identification of bioactive constituents in the essential oil of Carica papaya seeds obtained via hydrodistillation, as determined by GC-MS analysis. The table highlights the major compounds with known biological activity, including their retention times, molecular formulas, and pharmacological activity.
RT, is the retention time, MF, is the molecular formula, Mw is the molecular weight, Kilit “is the published Kovats Retention indices”, Kiexp “is the Kovats index determined experimentally relative to n-alkanes (C8-C28)” and indicates no data available.
FIGURE 4
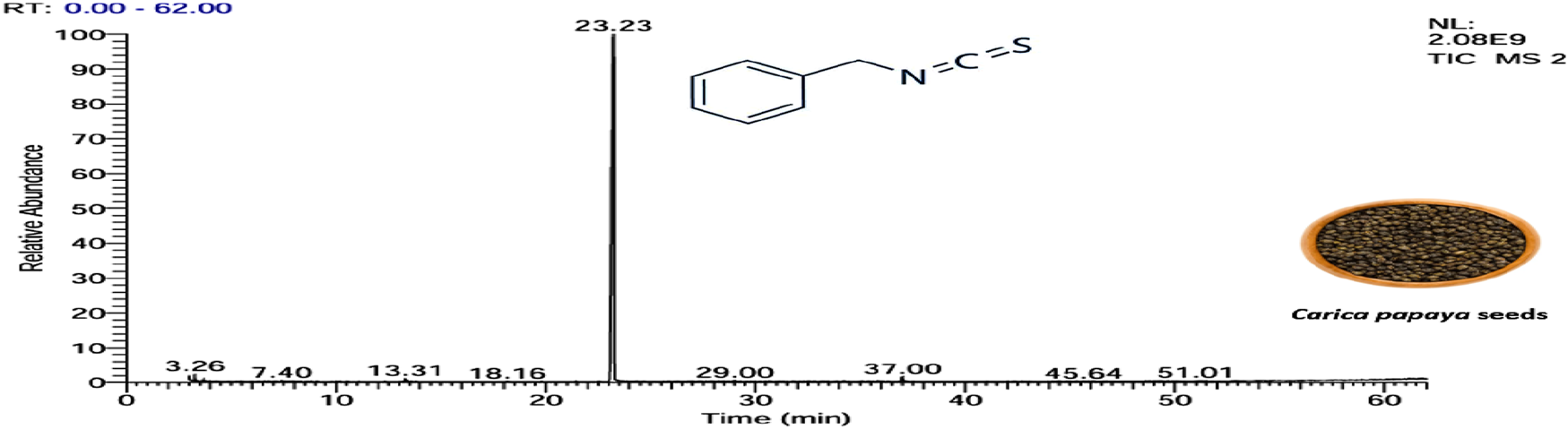
Essential oil extracted from Carica papaya seeds, as shown by the GC–MS.
The identification of benzyl isothiocyanate as a major compound in the extracted essential oil from papaya seeds. Consistent with the research of He et al. (2017), who demonstrated that three components were identified in the extracted essential oils from papaya seeds. The major compound was BITC, representing 99.36% of the total extract, and the other two compounds are benzyl nitrile and benzaldehyde, accounting for 0.31% and 0.33%, respectively (He et al., 2017). Also, this is in agreement with Yi et al. (2022), who reported BITC as a mainly chemical component of the extracted essential oils of C. papaya seed from China, with a content accounting for 97.43% (Yi et al., 2022).
BITC is a bioactive compound that has been used in different areas because of its wide applications, which range from vascular relaxation to cancer proliferation inhibition (Toyin Oshin et al., 2021; Barroso et al., 2016). Studies on the effect of BITC as a pure compound or extracted from natural products against different types of cancer such as lung cancer, prostate cancer, leukemia, colon cancer, breast cancer, hepatocellular carcinoma, and pancreatic cancer (Ranjan et al., 2019), reported that BITC has anticancer activities that are mediated by modulation of various signaling pathways, including cell proliferation, cell migration, cell cycle arrest, apoptosis, and invasion (Lee et al., 2011; Yi et al., 2022). However, the application of BITC in food, medicine, industry, and other fields is restricted by its easy degradation, instability, volatility, and poor water solubility (Zheng et al., 2025).
3.2 Efficiency of the prepared oil extract on the proliferation of the HeLa cancer cell line
Nowadays, it is vital to replace synthetic anticancer agents with natural ones. Therefore, the current work aims to investigate the essential oil extracted from C. papaya seeds, composed mainly of benzyl isothiocyanate (aromatic isothiocyanate), as mentioned above. Benzyl isothiocyanate is considered to be the hallmark of cancer, which is due to its ability to modulate various signaling pathways, including cell cycle arrest, apoptosis, and cell proliferation (Ranjan et al., 2019; Yi et al., 2022).
3.2.1 MTT cytotoxicity assay
The MTT assay is used to investigate the in vitro cell viability assay, which is important to measure the cellular response to the drug or toxicant and gives information about cell death, survival, and metabolic activity (Chandrasekaran et al., 2016). The present study aimed to examine the antiproliferative activity of the extracted essential oil against two types of human cancer cells and determine the effective concentration (IC50) that causes a 50% loss of cell viability. The efficacy of the extracted essential oil on the growth and morphology of Hep-2 and HeLa cancer cells using the MTT assay is represented in Figures 5, 6, respectively.
FIGURE 5
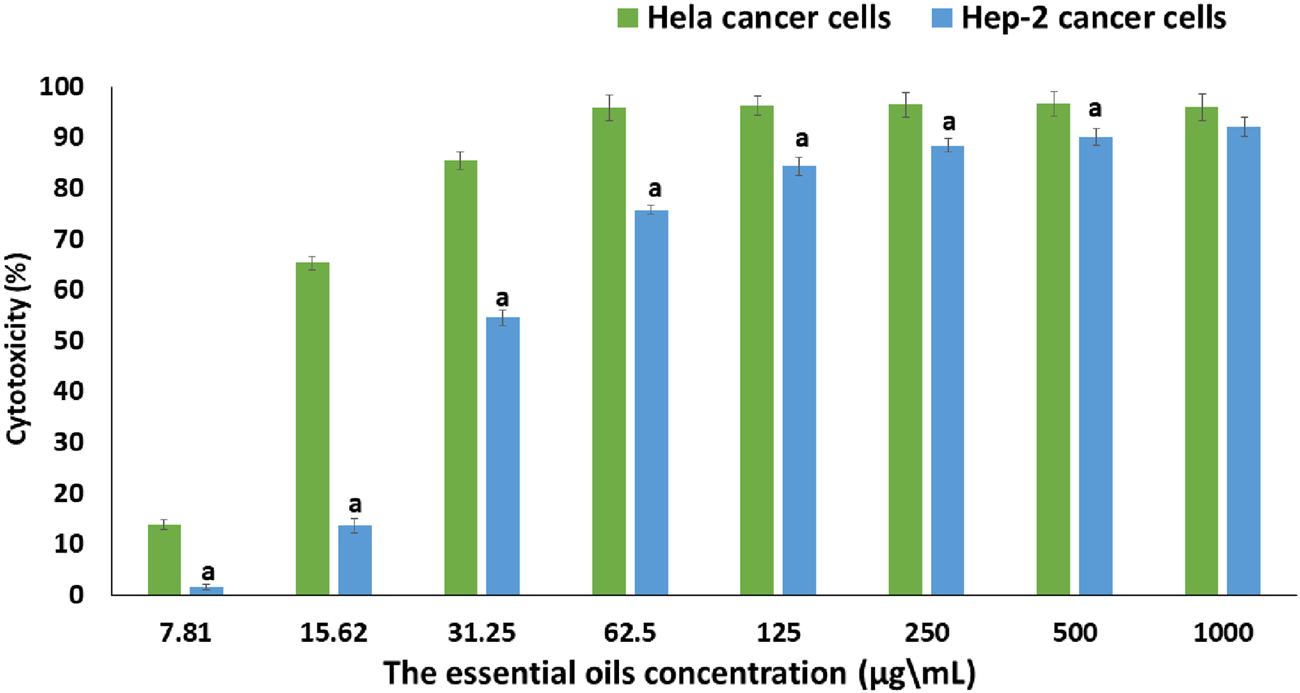
The cytotoxic effect of extracted essential oils at various concentrations (7.81–1,000 µg/mL) on HeLa and Hep-2 cancer cell lines using the MTT test. Data are presented as mean ± SD of three separate analyses. The statistical significance was assessed using an independent T-test to compare the studied groups. Statistically significant (p < 0.05) differences are shown by lowercase letters. a: (p < 0.05) with respect to HeLa cancer cell lines.
FIGURE 6
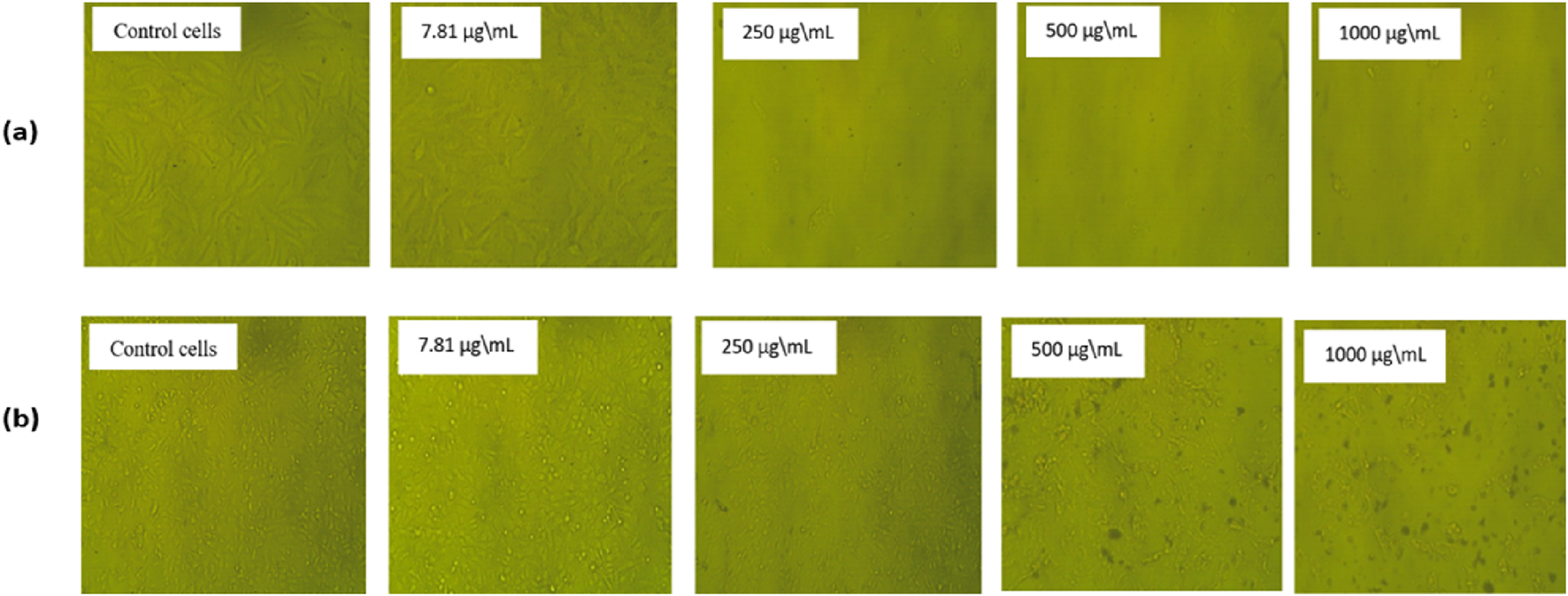
The morphological alterations in HeLa cells (a) and Hep-2 cells (b) after 24 h treatment with various concentrations of the extracted essential oil compound compared to normal control cells. The control group of both cell lines has not been treated and has a regular form. (b) show minimal morphological alterations in Hep-2 cells treated with extracted essential oil, in contrast to (a), which showed substantial shrinkage of cells and detachment, indicating cytotoxic impacts, especially for 1,000 µg/mL, showing a significant number of cell deaths, which means that the extracted essential oil effectively eliminates cervical carcinoma cells in greater quantities.
By comparing with the control untreated cells (Figure 5), the percentage of cytotoxicity was significantly increased with increasing concentration of the extracted essential oil in both cell lines (7.81–1,000 µg/mL), where the extracted essential oil showed a stronger cytotoxicity effect against HeLa cells than Hep-2 cells at each tested concentration. So, at the highest concentration (1,000 µg/mL), the percentage of cytotoxicity on the HeLa and Hep-2 cells was found to be 96.07% ± 0.001% and 92.16% ± 0.002%, respectively. Also, at the lowest concentration (7.81 µg/mL), the percentage of cytotoxicity of the extracted oil on the HeLa and Hep-2 cells was found to be 13.87% ± 0.01% and 0.23% ± 0.01%, respectively. Moreover, the extracted oil had a stronger IC50 value (16.78 ± 0.38 µg/mL) against the HeLa cell line than the Hep-2 cell line (37.84 ± 0.64 µg/mL). According to the National Cancer Institute’s guidelines and criteria, if the IC50 of the tested sample had an amount less than 20 µg/mL, it was classified as a potent sample for cancer cell treatment.
The morphological change of the cells, such as rounding or shrinking of the cells, granulation, and vacuolization in the cytoplasm of the cell, can be detected on direct microscopic observation and is considered an indicator of cytotoxicity (Praveena et al., 2017). As displayed in Figure 6, in comparison to the untreated cells, which showed a strong adherent cell to the surface and developed on an epithelial monolayer, the extracted essential oil showed higher morphological apoptotic variations in HeLa cells (Figure 6a) in Hep-2 cells (Figure 6b), such as cell shrinkage, phase-dense nucleus, rounded cell, and a decrease in the number of cells by increasing the extract concentration. By comparing with the control untreated cells, the percentage of cell viability was significantly decreased with an increase in concentration of the extracted essential oil (7.81–1,000 µg/mL) in both cell lines. At the highest concentration (1,000 µg/mL), the percentage of cell viability on HeLa and Hep-2 cells was found to be 3.93% ± 0.001% and 7.85% ± 0.002%, respectively. Also, at the lowest concentration (7.81 µg/mL), the percentage of cell viability of the extracted oil on HeLa and Hep-2 cells was found to be 86.13% ± 0.01% and 99.77% ± 0.01%, respectively.
In the past, various studies evaluated the anticancer potential of the different C. papaya extracts against different cell lines. First, different studies investigate the cytotoxic effect of different C. papaya extracts against different hepatocellular cancer cell lines. Based on a study conducted by Tessy John et al., the C. papaya peel extract loaded on silver nanoparticles has a cytotoxic effect against the Hep-2 cell line with an IC50 equal to 83.06 µg/mL (John et al., 2021). Also, different studies evaluated the effect of different C. papaya extracts, like fruit juice, lycopene, leaves, and seeds, on the Hep-2 cell line, which showed a significant inhibition in a dose-dependent manner (Salla et al., 2016; Abdel-Halim et al., 2020; Devanesan et al., 2021; Anilkumar and Bhanu P A, 2022).
Second, various investigations evaluated the anticancer potential of various papaya extracts against the cervical cancer cell line. Thanigaimalai et al. (2025) investigated the cytotoxic effect of ethanolic extract of Carica papaya leaves, roots, and seeds, which inhibited the proliferation of HeLa cell lines up to 19.57%, 25.76%, and 29.21%, respectively, at 100 µg/mL concentration. The IC50 value of the ethanolic leaf extract is found to be about 19.57 ± 0.048 µg/mL, indicating a greater cytotoxic effect in cervical cancer cells (Thanigaimalai et al., 2025). In another study, the in vitro cytotoxic study of C. papaya bark extract on the HeLa cancer cell line showed a dose-dependent cell death with an IC50 value of 18 ± 0.4 µg/mL, compared to Doxorubicin with an IC50 value of 11 ± 0.5 µg/mL (Altamimi et al., 2025).
3.2.2 Estimation of the effect of oil extract on cell apoptosis
Cancer cells proliferate excessively, can survive in an overstretched environment, and have a high ability to resist therapies. Whereas conceiving the tumor cells’ apoptosis has appeared as a useful treatment method for cancer (Alzahrani et al., 2024). By using Annexin V-FITC/PI staining, the proportion of apoptosis in the untreated cells (control) and treated cells with extracted oil was investigated. As seen in Figures 7, 8, there was a significant increase in the percentage of necrotic, early apoptotic, and late apoptotic cells in the HeLa cancer cells treated with the extracted essential oil (6.16%, 8.06%, and 15.39%, respectively) when compared to control cells (1.24, 0.31%, and 0.18%, respectively). The results indicated that the extracted essential oil from C. papaya seeds could induce the apoptosis pathway in HeLa-treated cells.
FIGURE 7
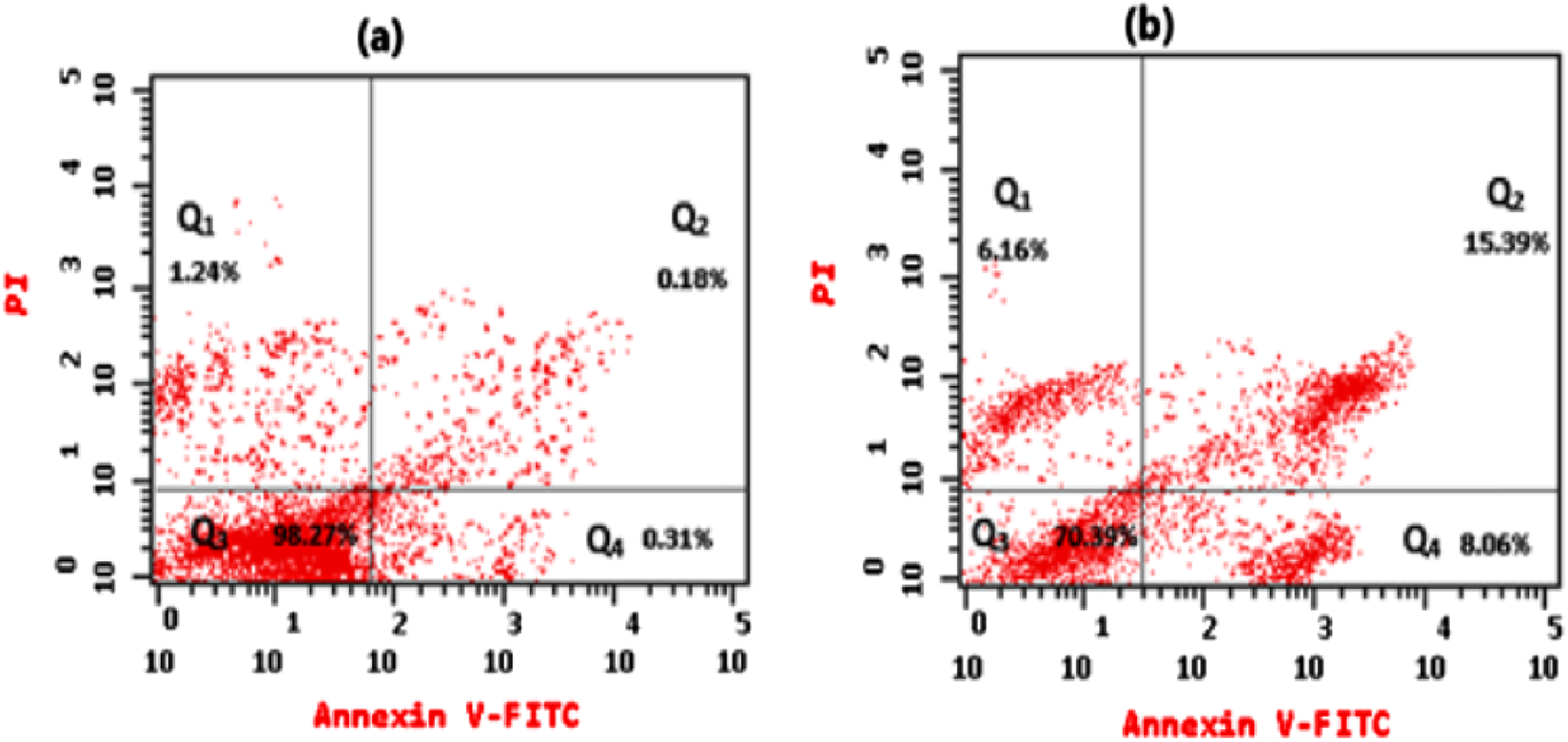
Flow cytometry assessed the apoptotic pattern of HeLa cells, showing Q1 (An−, PI+) percentage of necrotic cells, Q2 (An+, PI+) percentage of late apoptotic cells, Q3 (An−, PI−) percentage of viable cells, and Q4 (An+, PI−) percentage of early apoptotic cells for treated cells with an IC50 dose of essential oil for 24 h (b) in comparison to untreated HeLa cells (a).
FIGURE 8
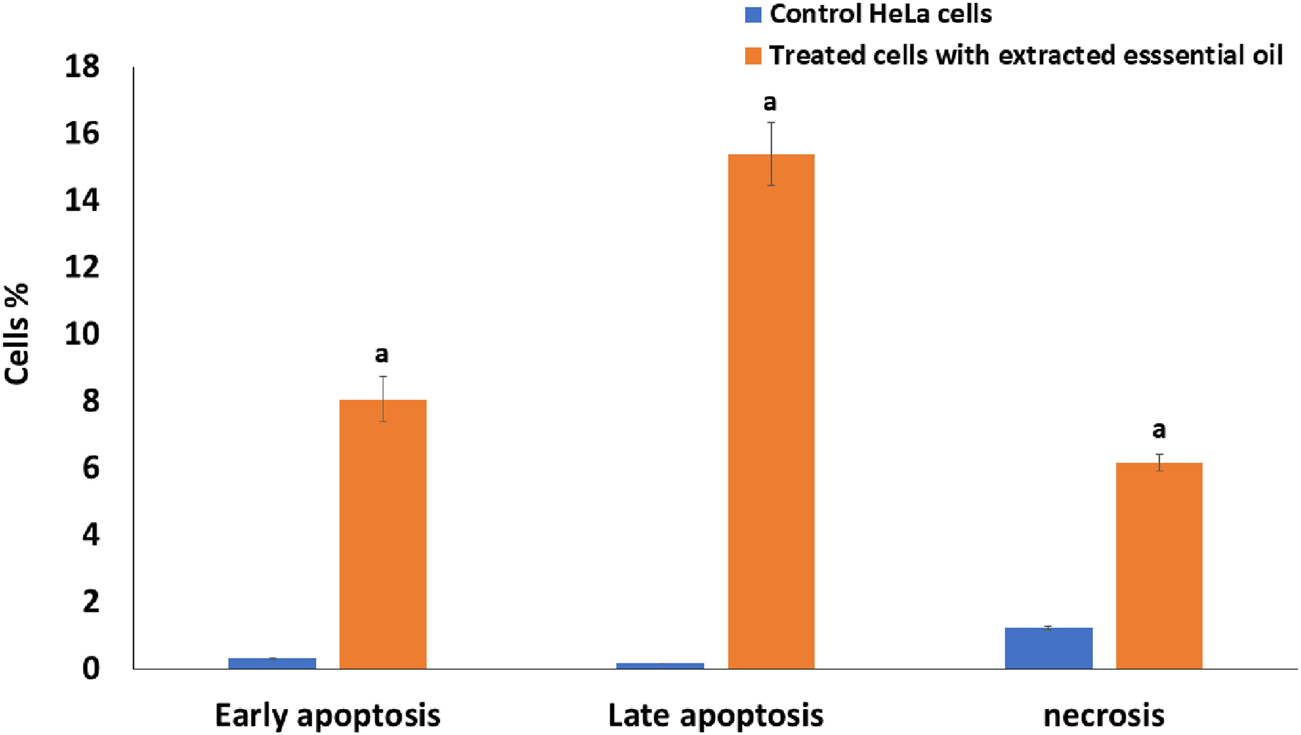
Comparative analysis of early apoptosis, late apoptosis, and necrosis percentage in HeLa cells treated with IC50 of the extracted essential oil for 24 h using the Annexin V-FITC/PI staining method versus untreated control cells. The statistical significance was assessed using an independent T-test to compare the studied groups. Statistically significant (p < 0.05) differences are shown by lowercase letters. a: (p < 0.05) with respect to the control untreated cells.
The Annexin V/PI flow cytometry assay revealed that the extracted essential oil primarily induced apoptosis, as evidenced by the substantial increase in early apoptotic cells (15.39%) compared with control cells (0.18%). While a necrotic population of ∼8.06% was detected, this is most likely attributable to secondary necrosis, a common outcome of apoptosis in vitro when apoptotic bodies are not removed by phagocytosis (Galluzzi et al., 2015; Costigan et al., 2023; Berghe et al., 2014). These findings indicate that programmed cell death via apoptosis represents the predominant mechanism of cytotoxicity induced by the essential oil, while necrosis constitutes only a minor secondary event. Several essential oils have been shown to trigger cell death primarily through apoptotic pathways, with necrotic cell death playing only a minimal role. For instance, Pallines spinosa flower and leaf essential oils (F-PSEO, L-PSEO) induced apoptosis in MCF-7 and MDA-MB-231 breast cancer cells—80%–90% and 30%–50%, respectively —with necrosis remaining below 10% (Berghe et al., 2014; Galluzzi et al., 2015; Costigan et al., 2023).
3.2.3 Cell cycle analysis
Cell cycle regulation is the elementary process for organizing cell proliferation, where cells have three checkpoints: G1 (gap 1), S (synthesis of DNA), and G2/M (mitosis, gap 2) as control mechanisms to ensure that the switch from one phase to another occurs in an ordered way (Alzahrani et al., 2024). The major characteristic of cancer cells is the disruption of the cell cycle flow (Alzahrani et al., 2024), so using flow cytometry is considered a possible analysis to find the impact of the extracted essential oil on regulating the HeLa cell cycle phases.
As shown in Figure 9, there was a significant increase in the cell percentages in the extracted essential oil-treated HeLa cells (Figure 9b) in the G2/M phase (26.54%) when compared with the percentages of cells in the control cells (Figure 9a) (5.02%), P < 0.001. But there was a significant decrease in the treated HeLa cells with extracted essential oil in G0/G1 and S phases (44.19% and 29.27%, respectively) when compared to control cells (58.43% and 36.55%, respectively), P < 0.001. Also, the percentages of the cells in each phase of the cell cycle in the extracted treated HeLa cells and control cells are represented in a representative histogram as shown in Figure 9c. The results revealed that the extracted essential oil induced the cell cycle arrest (DNA accumulation) at the G2/M phase in HeLa cancer cells. In a previous study, Praveeno P. et al demonstrated the effect of hydroethanolic extract of unripe fruit of C. papaya, where the extract showed high activity against cervical cancer cell lines, suggesting the inhibition and the interaction of various components participating in cell cycle regulation, and the IC50 value was found to be equal to 75.88 µg/mL (Praveena et al., 2017). Also, different extracts of C. papaya leaves showed a cytotoxic effect against HeLa cancer cells in a concentration-dependent manner (Bhuiyan et al., 2020; Haber et al., 2023).
FIGURE 9
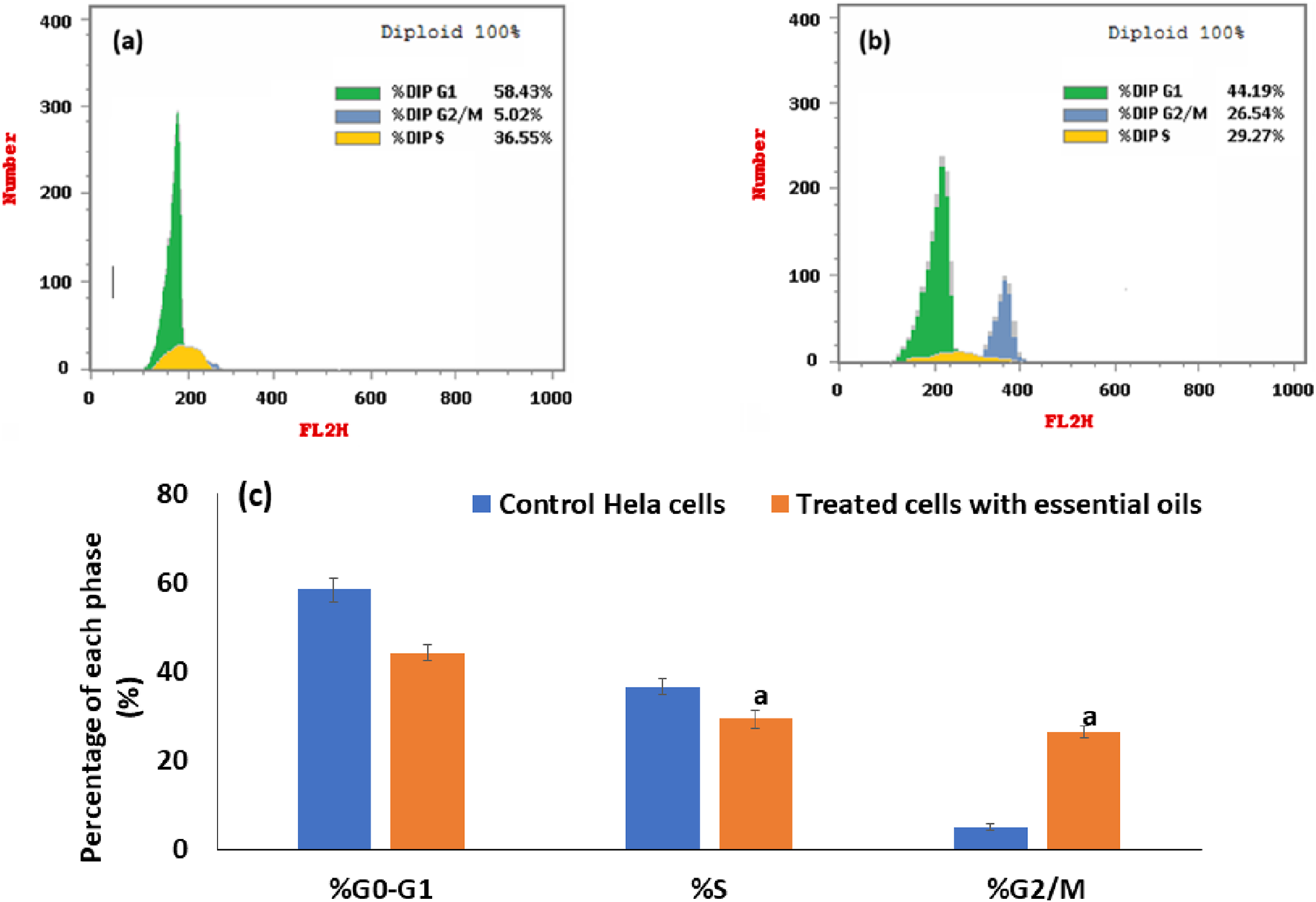
Flow cytometric analysis of the distribution of the cell cycle at different stages in control cells (a) and in HeLa cells that had been treated with an IC50 dose of essential oil for 24 h (b). An illustration of a histogram that displays the percentage of cells in each stage of the cell cycle in untreated and essential oil-treated cells (c). The statistical significance was assessed using an independent T-test to compare the studied groups. Statistically significant (p < 0.05) differences are shown by lowercase letters. a: (p < 0.05) with respect to the control untreated cells.
3.2.4 Effectiveness of the extracted essential oil in preventing HeLa cells metastasis
Metastasis is a complex process that occurs through several interconnected processes, such as migration, invasion, and adhesion of cancer cells. So, it has been hypothesized that interfering with the motility, adherence, and invasion of the tumor cells to the target organ is one way to inhibit the cancer spread. The ability of cancer cells to migrate is considered the essential process for cancer invasion and metastasis (Spyridopoulou et al., 2019). Therefore, using a wound healing assay, this study investigated whether the extracted essential oil from the C. papaya seeds could attenuate the HeLa cervical cancer cell migration rate in vitro.
The attained results indicated that the open area (wound) closed earlier in untreated cells (negative control) with a migration rate of 14.1 µm compared to the migration rate in the cells treated with methotrexate, positive control (7.89 µm), and the cells treated with the extracted essential oil (6.66 µm). As shown in Figure 10, the percentage of wound closure was 38.78% and 45.9% in scratched HeLa cells treated with essential oil and methotrexate, respectively compared to the wound closure % in the untreated cells (81.98%).
FIGURE 10
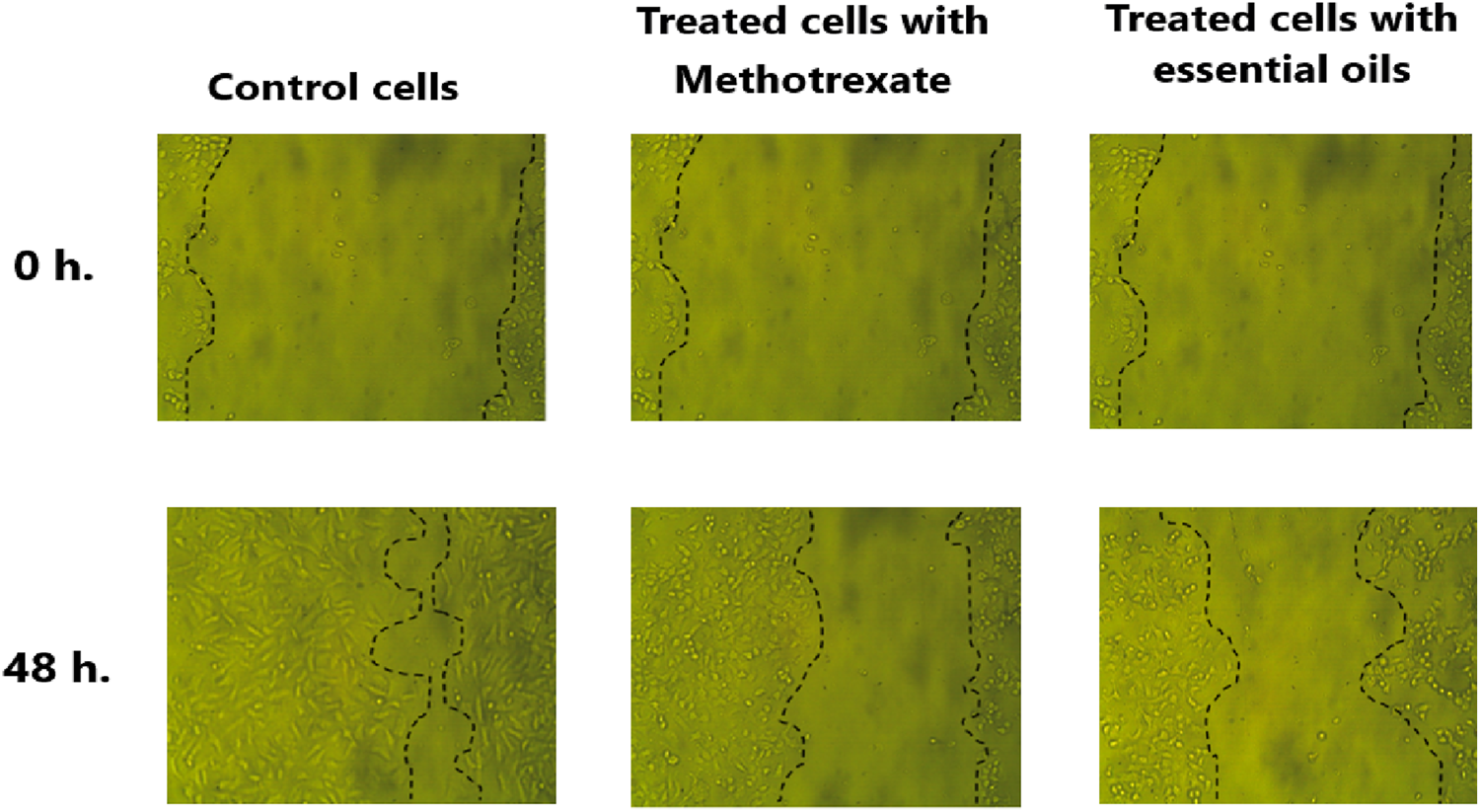
Microscopic examination of HeLa cells’ migration was assessed via a wound healing assay over a 48 h period. After scratching the HeLa cells, the migration of untreated cells (control), treated cells with methotrexate (positive control), and treated cells with extracted essential oil to the wound area was imaged at 0 h and following 48 h. The dashed lines mark the wound boundaries, and the degree of closure reflects the migration response under each condition.
3.2.5 Clonogenic assay
The clonogenic assay is an in vitro cell survival assay that depends mainly on the ability of a single cell to form a colony. In contrast, it is the only assay that monitors the ability of cancer cells to form a viable colony (Ratnasari et al., 2023). Commonly, the cancer cells have a proclivity to grow in colonies, where the loss of the connection between cancer cells and the adjacent cells leads to the death of the cancer cells. The obtained results reported that the extracted essential oil significantly inhibits the ability of HeLa cells to form colonies with a plating efficiency (PE) of 0.25% ± 0.29% and a surviving fraction (SF) of 0.004% ± 0.29% compared to the plating efficiency (61 ± 1.49) and the surviving fraction (1%) of HeLa cells before treatment. Moreover, using a stereomicroscope, the examination of the plating efficiency and survival of essential oil on the HeLa cells was displayed in Figure 11, showing a significant decrease in density and number of the HeLa cells after treatment with the extracted essential oil compared to the HeLa cells before treatment.
FIGURE 11
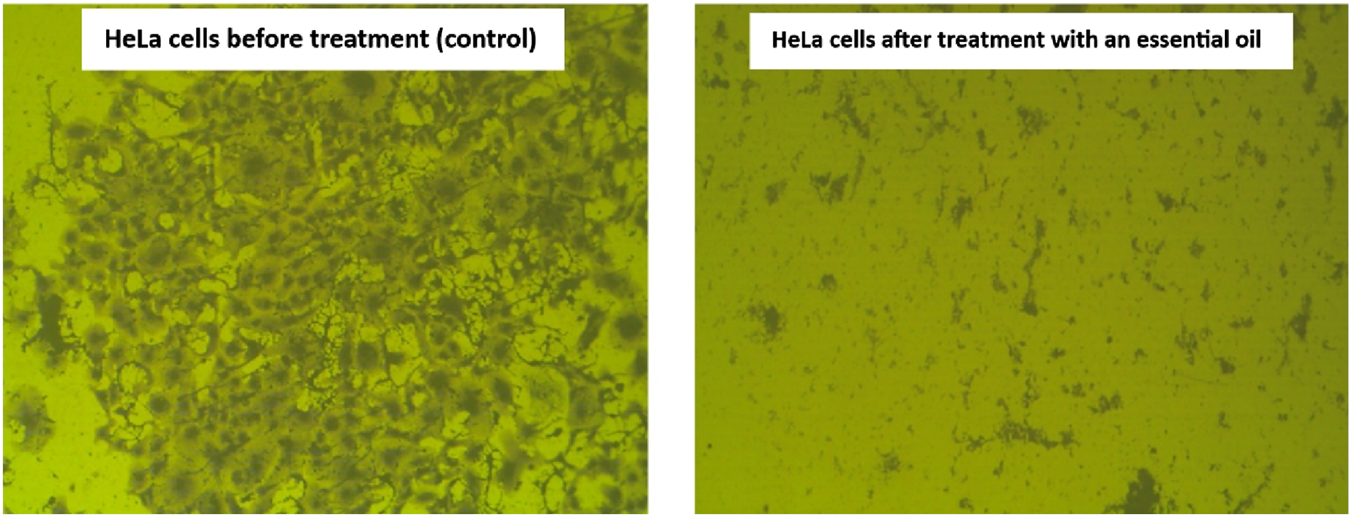
Microscopic examination demonstrating the effect of the extracted essential oil on HeLa cells’ survival. The control cells show a dense colony formation, while the treated cells with extracted oil display a marked reduction in colony density. This reduction reflects the inhibitory effect of the extracted oil on cell proliferation and viability.
In the clonogenic assay, treatment with the essential oil at the IC50 concentration (determined by the MTT assay) resulted in almost complete ablation of colony formation (SF = 0.004%). This outcome reflects loss of reproductive viability, a recognized hallmark of cytotoxicity (Franken et al., 2006), rather than a nonspecific effect of excessive dosing. Supporting this interpretation, Annexin V/PI analysis demonstrated apoptosis as the predominant mode of death (21.55% apoptotic vs 8.06% necrotic), while cell cycle analysis revealed G2/M arrest. Furthermore, migration assays indicated suppression of motility, suggesting a cytostatic contribution. These findings align with previous reports that essential oils exert anticancer effects mainly through apoptosis induction with ancillary cytostatic activity (Bayala et al., 2014; Edris and Fairag, 2003). Collectively, the results demonstrate that the essential oil induces an apoptosis-predominant cytotoxic response with additional cytostatic effects, thereby explaining the dramatic reduction in clonogenic survival (Bayala et al., 2014; Franken et al., 2006).
3.2.6 Quantification analysis using the ELISA assay
The expression of metastasis-related proteins, apoptosis-associated proteins, and proteins that control the cell cycle in essential oil-treated cells was quantified and evaluated using the ELISA technique. As seen in Figure 12, the essential oil had a significantly higher influence on the upregulation of protein levels of expression of active cleaved Bax and Caspase 3, coupled with a substantial downregulation of protein levels of Cyclin B1, Bcl-2 CDK1, MMP-9, and MMP-2 in essential oil-treated cells versus untreated ones.
FIGURE 12
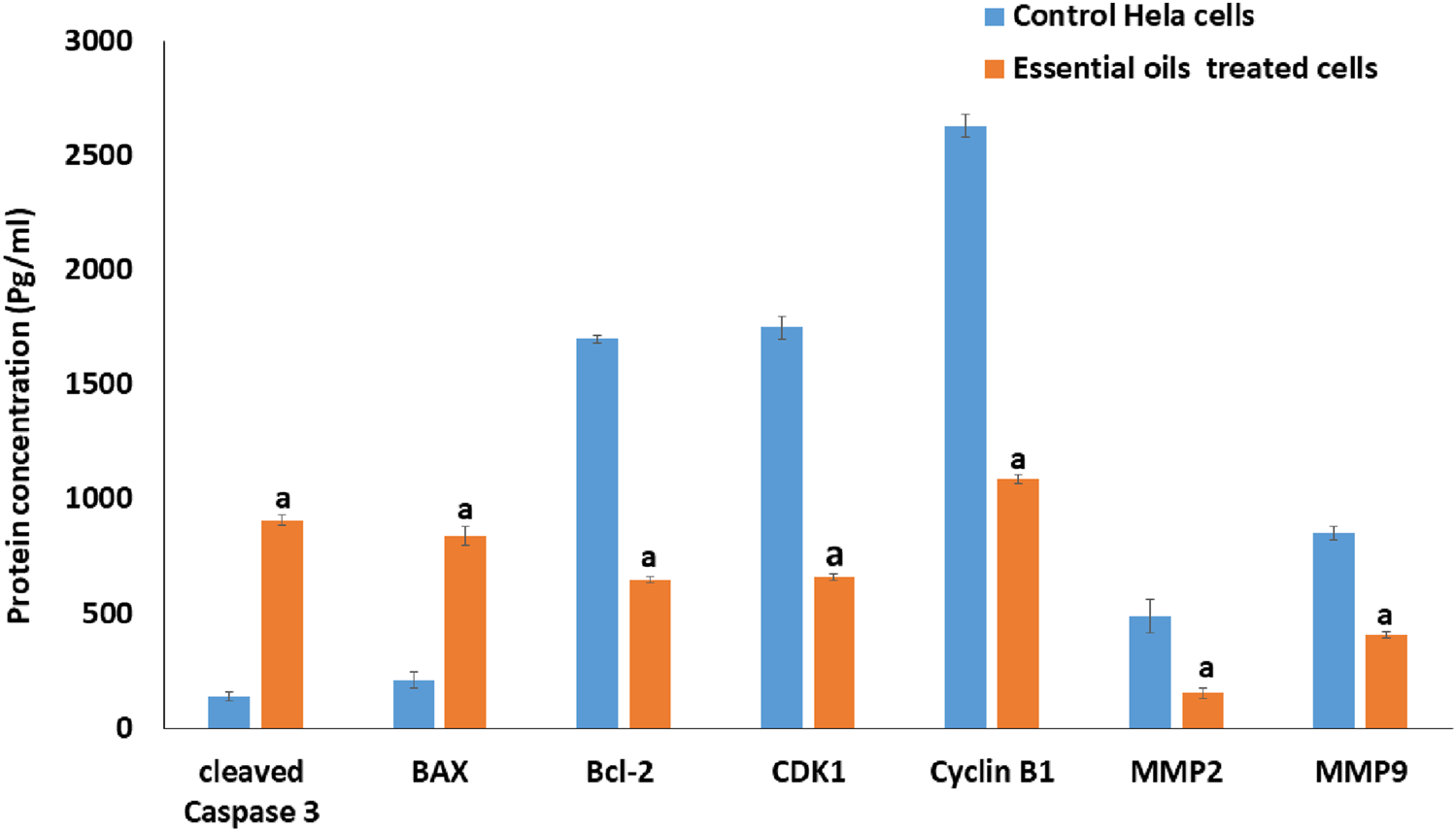
The effect of the IC50 dose of essential oil on the protein content of cleaved caspase-3, BAX, BCL-2, CDK1, cyclin B1, MMP-2, and MMP-9 in HeLa-treated cells compared to the protein content of the control cells, utilizing the ELISA method. The statistical significance was assessed using an independent T-test to compare the studied groups. Statistically significant (p < 0.05) differences are shown by lowercase letters. a: (p < 0.05) with respect to the control untreated cells.
According to the reports (Hanna et al., 2023), the BCL-2 protein family plays a crucial role in regulating apoptosis, with members acting as either antiapoptotic controllers, such as BCL-2, or proapoptotic controllers such as BAX. Where the overexpression of BAX leads to permeabilization of the mitochondria, which leads to enhancing the activation of caspase-3 and stimulates the liberation of cytochrome c to the cytoplasm (Hanna et al., 2023). Consequently, the activation of Caspase-3 leads to DNA damage, apoptosis execution, and a decrease in BCL-2 gene expression levels. In this study, the effect of the prepared essential oil on the expression of apoptosis-related proteins (BCL-2, BAX, and activated Caspase-3) was examined. The findings showed a significant increase in the expression levels of BAX and activated Caspase-3, along with a marked decrease in BCL-2 levels compared to untreated cells (Figure 12). These results suggest that apoptosis induction in the essential oil-treated HeLa cells likely occurred via the mitochondrial pathway.
Also, it is considered that the first important step to prevent the metastasis of cancer cells is the lowering of MMP expression levels (Johansson et al., 2000). To determine whether the observed antimetastatic effect was associated with a reduction in MMP expression, the impact of the essential oil on expression levels of MMP-9 and MMP-2 proteins in HeLa-treated cells was examined. As shown in Figure 12, the essential oil significantly decreased the expression levels of MMP-9 and MMP-2 compared to control cells, suggesting its potential to inhibit HeLa cell migration by reducing these protein levels.
Quantitatively, the migration rate in essential oil–treated cells was reduced to 6.66 µm, compared with 7.89 µm in methotrexate-treated cells. Similarly, wound closure was limited to 38.78% with the essential oil versus 45.9% in methotrexate-treated cells, indicating a stronger inhibition of cell motility. At the molecular level, MMP-2 levels decreased from 490 pg/mL (untreated cells) to 156 pg/mL (≈68% reduction) in treated cells, and MMP-9 levels decreased from 854 pg/mL (untreated cells) to 410 pg/mL (≈52% reduction) in treated cells. These magnitudes of suppression are therapeutically relevant, as even partial inhibition of MMP-2 and MMP-9 has been shown to significantly impair tumor invasion and metastasis (Cathcart et al., 2015; Pavlova et al., 2022).
For context, classical synthetic MMP inhibitors such as marimastat and prinomastat potently inhibit MMP catalytic activity, with reported sub- to low-nanomolar IC50 values in biochemical assays (Whittaker et al., 1999). While direct comparison is limited by methodological differences—the data reflect secreted protein concentrations and cellular migration rather than purified enzyme activity—the level of suppression observed with the essential oil falls within ranges reported for other MMP-targeting strategies that attenuate metastatic behavior in preclinical models. Importantly, whereas broad-spectrum MMP inhibitors in clinical trials faced toxicity challenges, the natural product demonstrated a substantial inhibitory effect on MMP expression and migration, suggesting a potentially safer anti-metastatic profile.
Moreover, it has been proposed that inhibition of different Cdk complexes, such as cyclin B/CDK1 and cyclin A/CDK1, at the G2-phase checkpoint creates different regulatory cell cycle checkpoints. Previous studies have shown that DNA damage can inhibit Cdks by preventing Cdk-cyclin complex formation, cyclin downregulation, and limiting tyrosine phosphorylation of Cdks, thus inhibiting their activating phosphorylation. Thus, the inhibition of the CDK1/cyclin B1 complex activity may explain the G2 phase arrest observed in this study. Therefore, the expression levels of cyclin B1 and CDK1 were examined in essential oil-treated cells. The results revealed that the essential oil significantly reduced the expression levels of cyclin B1 and CDK1 (Figure 12), suggesting that the essential oil induced a G2 phase arrest in the treated HeLa cells through inhibition of the activity of the CDK1/cyclin B1 complex.
3.3 Antihemolytic effectiveness
The effect of the extracted essential oil on healthy tissues must be confirmed to assess the safety of this extract for therapeutic purposes. The hemolysis test, which accurately depicts how the tested substance interacts with human erythrocytes, is a well-known and frequently used approach for first assessments of the biocompatibility and toxicity of natural substances on normal mammalian cells (1). Subsequently, the biocompatibility of the extracted essential oil against fresh human red blood cells was examined using a hemolysis assay, and the obtained results displayed that the positive control, extracted essential oil, and negative control absorbance values were 0.97 ± 0.05, 0.49 ± 0.03, and 0.43 ± 0.01, respectively. Thus, the hemolysis percentage of the essential oil extracted from C. papaya seeds was 11.1% ± 1.6%, which falls below the generally accepted threshold of 25% for hemocompatible materials (Zhou and Xu, 2015). This indicates that the obtained essential oil demonstrates acceptable safety margins, which confirms the biocompatibility of the extracted essential oil against normal cells.
3.4 Molecular docking
All selected structures met quality standards suitable for reliable docking. For NMR-derived structures, representative conformers were chosen based on structural completeness, low ensemble RMSD, and relevance to the biologically active state, allowing confident prediction of ligand interactions. While for X-ray crystallographic models, resolution values were below 2.5 Å, ensuring accurate atomic positioning. This strategic selection ensures that benzyl isothiocyanate was evaluated against conformations most likely to reflect its physiological binding behavior, thereby reinforcing the validity of the docking results. The docking study of essential oil was carried out with BCL-2, MMP-2, and CDK1/cyclin B1 to determine whether benzyl isothiocyanate would bind to these receptors. It has been known that the BCL-2 protein family has an important role in the apoptosis process (Anilkumar and Bhanu P A, 2022; Alzahrani et al., 2024). Also, it is expected that one of the most important processes to prevent the metastasis of cancer cells is the lowering of MMP protein levels . The docking score, area of contact, and atomic contact energy values of benzyl isothiocyanate compared with standard compounds (methotrexate) were determined. The results showed that benzyl isothiocyanate exhibited higher docking scores with the receptor BCL-2 (−5.52 kcal mol−) compared to methotrexate (−5.49 kcal mol−); it formed hydrogen bonds with SER77 and ALA122 along with engaging in a pi-pi stacking interaction, as depicted in Figure 13a. While it exhibited the strongest binding score with MMP-2, measuring −6.29 kcal mol− compared to methotrexate (−5.44 kcal mol−). Furthermore, it formed a hydrogen bond with GLU78 and TRP68, as shown in Figure 13b. Benzyl isothiocyanate displayed significant inhibition against CDK1/cyclin B1 (−5.75 kcal mol−) compared to methotrexate (−5.18 kcal mol−). It formed three hydrogen bonds with ARG123, ARG151, and GLY154 in its interaction with CDK1/cyclin B1 (Figure 13c). These interaction studies indicate that the benzyl isothiocyanate present in the essential oil from C. papaya seeds could be used as a therapeutic drug against cervical cancer cells. Binding energies obtained from molecular docking were interpreted using established empirical thresholds to assess ligand affinity. Values below −6.0 kcal/mol are generally considered indicative of strong binding potential, while those between −4.0 and −6.0 kcal/mol suggest moderate binding affinity (Hobani et al., 2017). Benzyl isothiocyanate exhibited binding energies in the moderate range, slightly more favorable than methotrexate (which served as a reference inhibitor). Although neither compound reached the strong binding threshold, the comparative docking scores suggest that benzyl isothiocyanate may exert competitive inhibitory effects, reinforce its biological plausibility, and support its potential role as a natural therapeutic candidate.
FIGURE 13
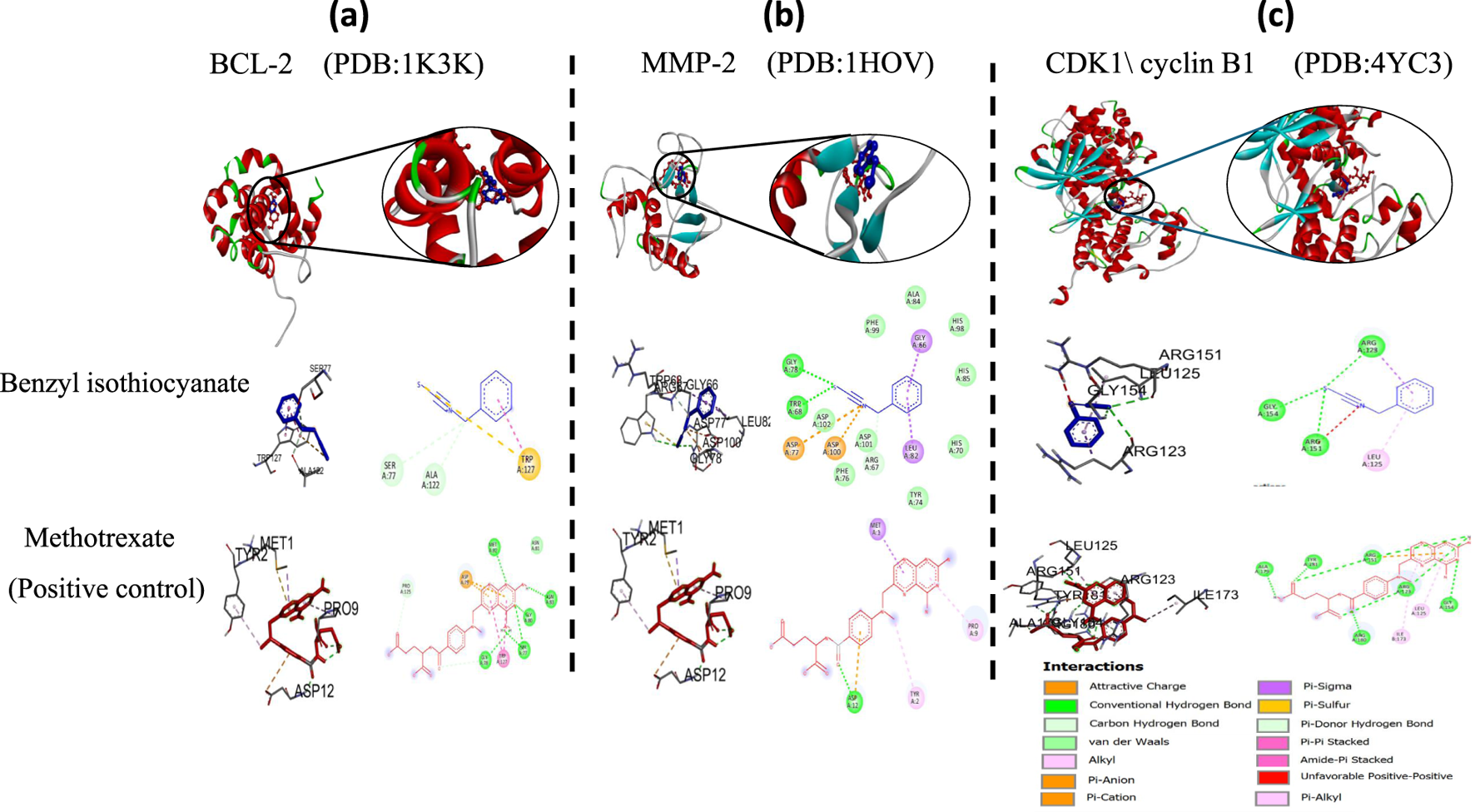
2D and 3D representations of the predicted binding modes of (a) BCL2 crystal structure (PDB ID: 1K3K), (b) MMP-2 crystal structure (PDB ID: 1HOV), (c) CDK1/cyclin B1 crystal structure (PDB ID: 4YC3) for benzyl isothiocyanate (blue), compared to the docked methotrexate (red) as a positive control.
4 Conclusion
Carica papaya is widely consumed for its nutritional and medicinal properties, leading to the generation of considerable seed and peel waste that poses environmental challenges if not effectively utilized. This study demonstrates that essential oil extracted from papaya seeds, rich in benzyl isothiocyanate, exhibits potent anticancer activity against HeLa cervical cancer cells. The oil markedly reduced cell viability (IC50 = 16.78 ± 0.38 µg/mL), induced apoptosis as confirmed by Annexin V-FITC/PI analysis, and triggered G2/M cell cycle arrest. Moreover, it significantly inhibited cell migration (38.7%), with an efficacy comparable to the standard drug methotrexate (45.9%), and drastically suppressed clonogenic survival, indicating near-complete loss of long-term proliferative capacity. At the molecular level, the oil downregulated proteins involved in apoptosis regulation, cell cycle progression, and metastatic potential. Importantly, it displayed acceptable hemocompatibility, with low hemolytic activity against normal erythrocytes (11.1% ± 1.6%), though comprehensive toxicological evaluations remain necessary.
Collectively, these findings suggest that papaya seed essential oil exerts an apoptosis-dominant cytotoxic effect, supplemented by cytostatic mechanisms including cell cycle arrest and anti-migratory action. This multifaceted activity underscores its strong potential as a natural anti-cervical cancer agent. Nonetheless, further research should focus on elucidating its intracellular signaling targets, pharmacokinetic behavior, and in vivo safety profile to support its future therapeutic application.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. Ethical approval was not required for the studies on animals in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
DS: Data curation, Formal Analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review and editing. SA-H: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review and editing. MH: Conceptualization, Formal Analysis, Investigation, Validation, Writing – original draft. SA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. AA: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft. ME-D: Investigation, Methodology, Supervision, Writing – original draft, Writing – review and editing. HA: Data curation, Resources, Writing – original draft. MJ: Conceptualization, Data curation, Formal Analysis, Investigation, Resources, Visualization, Writing – original draft. AE: Conceptualization, Data curation, Formal Analysis, Resources, Software, Visualization, Writing – original draft. DH: Conceptualization, Data curation, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abd El-Razek M. H. Mohamed T. A. Abdel-Halim S. Bata S. M. Kubacy T. M. (2023). Comprehensive NMR reassignments of lignans derived from commiphora myrrha. Egypt J. Chem.66, 45–57. 10.21608/ejchem.2023.187893.7469
2
Abd-ElGawad A. M. Elshamy A. I. Elgorban A. M. Hassan E. M. Zaghloul N. S. Alamery S. F. et al (2022). Essential oil of ipomoea carnea: Chemical profile, chemometric analysis, free radical scavenging, and antibacterial activities. Sustain. Switz.14, 9504. 10.3390/su14159504
3
Abd-elkawy M. M. A Khairy H. (2019). Numerical and economic evaluation of some papaya genotype trees grown in Qalyubia region. Middle East J. Agric. Res.8, 1392–1402. 10.36632/mejar/2019.8.4.40
4
Abdel-Halim S. A. Ibrahim M. T. Mohsen M. M. A. Abou-Setta L. M. Sleem A. A. Morsy F. A. et al (2020). Phytochemical and biological investigation of Carica papaya Linn. Leaves cultivated in Egypt (family caricaceae). J. Pharmacogn. Phytochem.9, 47–54. 10.22271/phyto.2020.v9.i5a.12421
5
Abdel-Halim S. Ibrahim M. Abdel Mohsen M. Abou-Setta L. Sleem A. El-Missiry M. (2021). The influence of the extraction method on polyphenols, flavonoids composition and anti-hyperlipidemic properties of papaya leaves (Carica papaya Linn). Bull. Natl. Res. Cent.45, 85. 10.1186/s42269-021-00548-4
6
Adams R. P. (2005). Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy. J. American Soc. Mass Spectro.16, (11).
7
Adesola M. O. Akande E. A. Adejuyitan A. (2019). Effect of fermentation on the chemical composition of pawpaw (Carica papaya) seeds. Int. J. Gener. Eng. Tech.9, 1–8.
8
Ahmed T. A. Ahmed S. M. Elkhenany H. El-Desouky M. A. Magdeldin S. Osama A. et al (2024). The cross talk between type II diabetic microenvironment and the regenerative capacities of human adipose tissue-derived pericytes: a promising cell therapy. Stem Cell Res. Ther.15, 36. 10.1186/s13287-024-03643-1
9
Aina A. D. Owolo O. Adeoye-Isijola M. Olukanni O. D. Lateef A. Egbe T. et al (2020). “Ecofriendly production of silver nanoparticles from the seeds of Carica papaya and its larvicidal and antibacterial efficacy against some selected bacterial pathogens”, in IOP conference series: materials science and engineering (Institute of Physics Publishing). 805, 012038. 10.1088/1757-899X/805/1/012038
10
Alfarabi M. Siagian F. E. Cing J. M. Suryowati T. Turhadi Suyono M. S. et al (2022). Bioactivity and metabolite profile of papaya (Carica papaya) seed extract. Biodiversitas23, 45894600. 10.13057/biodiv/d230926
11
Almanaa T. N. Rabie G. El-Mekkawy R. M. Yassin M. A. Saleh N. El-Gazzar N. (2022). Antioxidant, antimicrobial and antiproliferative activities of fungal metabolite produced by Aspergillus flavus on in vitro study. Food Sci. Technol. Braz.42, e01421. 10.1590/fst.01421
12
Alotaibi K. S. Li H. Rafi R. Siddiqui R. A. (2017). Papaya Black seeds have beneficial anticancer effects on PC-3 prostate cancer cells. J. Cancer Metastasis Treat.3, 161. 10.20517/2394-4722.2017.33
13
Altamimi H. N. Bepari A. Niazi S. K. Al-Zharani M. Shaikh M. A. Assiri G. A. et al (2025). Chitosan-coated superparamagnetic iron oxide nanoparticles synthesized using Carica papaya bark extract: evaluation of antioxidant, antibacterial, and anticancer activity of HeLa cervical cancer cells. Green Process. Synthesis14, 20250036. 10.1515/gps-2025-0036
14
Alzahrani B. Elderdery A. Y. Alsrhani A. Alzerwi N. A. N. Althobiti M. M. Rayzah M. et al (2024). Manganese and copper-coated nickel oxide nanoparticles synthesized from Carica papaya leaf extract induce antimicrobial activity and breast cancer cell death by triggering mitochondrial caspases and p53. Green Process. Synthesis13, 20230087. 10.1515/gps-2023-0087
15
Amran A. H. Zaidi N. S. Syafiuddin A. Zhan L. Z. Bahrodin M. B. Mehmood M. A. et al (2021). Potential of carica papaya seed-derived bio-coagulant to remove turbidity from polluted water assessed through experimental and modeling-based study. Appl. Sci. Switz.11, 5715. 10.3390/app11125715
16
Ananthanayagi N. Navamathavan R. Nirmala R. (2022). Pharmacological activities and bioactive compounds of papaya (Carica papaya L.): a mini topical review. Int. J. Green Pharm.16 (1). 10.22377/ijgp.v16i1.3213
17
Anilkumar A. Bhanu P. A. A. (2022). In vitro anticancer activity of “Methanolic extract of papaya blackseeds” (MPB) in Hep G2 cell lines and its effect in the regulation of bcl-2, caspase-3 and p53 gene expression. Adv. Cancer Biol. - Metastasis4, 100025. 10.1016/j.adcanc.2021.100025
18
Balavijayalakshmi J. Ramalakshmi V. (2017). Carica papaya peel mediated synthesis of silver nanoparticles and its antibacterial activity against human pathogens. J. Appl. Res. Technol.15, 413–422. 10.1016/j.jart.2017.03.010
19
Barroso P. T. W. de Carvalho P. P. Rocha T. B. Pessoa F. L. P. Azevedo D. A. Mendes M. F. (2016). Evaluation of the composition of Carica papaya L. seed oil extracted with supercritical CO2. Biotechnol. Rep.11, 110–116. 10.1016/j.btre.2016.08.004
20
Bayala B. Bassolé I. H. Scifo R. Gnoula C. Morel L. Lobaccaro J. M. (2014). Anticancer activity of essential oils and their chemical components - a review. American J. Cancer Res.4 (6), 591–607.
21
Berghe T. V. Linkermann A. Jouan-Lanhouet S. Walczak H. Vandenabeele P. (2014). Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol.15, 135–147. 10.1038/nrm3737
22
Bhuiyan M. S. H. Miah M. Y. Paul S. C. Aka T. D. Saha O. Rahaman M. M. et al (2020). Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon6, e04603. 10.1016/j.heliyon.2020.e04603
23
Brown N. R. Korolchuk S. Martin M. P. Stanley W. A. Moukhametzianov R. Noble M. E. M. et al (2015). CDK1 structures reveal conserved and unique features of the essential cell cycle CDK. Nat. Commun.6, 6769. 10.1038/ncomms7769
24
Cathcart J. Pulkoski-Gross A. Cao J. (2015). Targeting matrix metalloproteinases in cancer: bringing new life to old ideas. Genes Dis.2, 26–34. 10.1016/j.gendis.2014.12.002
25
Chandra Kala S. Ammani K. (2017). GC-MS analysis of biologically active compounds in Canthium parviflorum Lam. leaf and callus extracts. Int. J. Chem Tech Res.10, 1039–1058.
26
Chandrasekaran R. Gnanasekar S. Seetharaman P. Keppanan R. Arockiaswamy W. Sivaperumal S. (2016). Formulation of Carica papaya latex-functionalized silver nanoparticles for its improved antibacterial and anticancer applications. J. Mol. Liq.219, 232–238. 10.1016/j.molliq.2016.03.038
27
Chang Y.-X. Liu Y.-T. Chen C.-C. Lin Y.-S. Hung Y.-C. Lin C.-Y. et al (2020). Inhibition activities of papaya black seed extracts on colorectal cancer cell viability and migration. Adapt. Med.12, 59–63. 10.4247/AM.2020.ACA248
28
Chikkanna U. Ramaiah M. B. Seetharaman S. Venugopal V. Elumalai A. Venkatesh C. et al (2024). A review on herbal power bamboo, papaya, ginger and plectranthus in cancer, bugs and oxidant combat. Int. J. Pharm. Investig.14, 681–695. 10.5530/ijpi.14.3.79
29
Costigan A. Hollville E. Martin S. J. (2023). Discriminating between apoptosis, necrosis, necroptosis, and ferroptosis by microscopy and flow cytometry. Curr. Protoc.3, e951. 10.1002/cpz1.951
30
Dahham S. S. Tabana Y. M. Iqbal M. A. Ahamed M. B. K. Ezzat M. O. Majid A. S. A. et al (2015). The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules20, 11808–11829. 10.3390/molecules200711808
31
Devanesan S. Jayamala M. AlSalhi M. S. Umamaheshwari S. Ranjitsingh A. J. A. (2021). Antimicrobial and anticancer properties of Carica papaya leaves derived di-methyl flubendazole mediated silver nanoparticles. J. Infect. Public Health14, 577–587. 10.1016/j.jiph.2021.02.004
32
Dinh T. N. Parat M. O. Ong Y. S. Khaw K. Y. (2021). Anticancer activities of dietary benzyl isothiocyanate: a comprehensive review. Pharmacol. Res.169, 105666. 10.1016/j.phrs.2021.105666
33
Doughari J. H. Manzara A. M. (2007). Studies on the antibacterial activity of root extracts of Carica papaya L. African J. Microbiol. Res.12, 37–41.
34
Edris A. E. Farrag E. S. (2003). Antifungal activity of peppermint and sweet basil essential oils and their major aroma constituents on some plant pathogenic fungi from the vapor phase. Die Nahrung.47 (2), 117–121. 10.1002/food.200390021
35
Egbuonu A. Harry E. Orji I. (2016). Comparative proximate and antibacterial properties of milled Carica papaya (pawpaw) peels and seeds. Br. J. Pharm. Res.12, 1–8. 10.9734/bjpr/2016/26808
36
El Habbash A. I. Hashim N. M. Ibrahim M. Y. Yahayu M. Elmutaal Omer F. A. Rahman M. A. et al (2017). In vitro assessment of anti-proliferative effect induced by α-mangostin from Cratoxylum arborescens on HeLa cells. PeerJ2017. 10.7717/peerj.3460
37
El-Atawy M. A. Hanna D. H. Bashal A. H. Ahmed H. A. Alshammari E. M. Hamed E. A. et al (2024). Synthesis, characterization, antioxidant, and anticancer activity against Colon cancer cells of some cinnamaldehyde-based chalcone derivatives. Biomolecules14, 216. 10.3390/biom14020216
38
Elsharkawy E. R. Alghanem S. M. Elmorsy E. (2021). Effect of habitat variations on the chemical composition, antioxidant, and antimicrobial activities of Achillea fragrantissima (forssk) Sch. Bip. Biotechnol. Rep.29, e00581. 10.1016/j.btre.2020.e00581
39
Franken N. A. P. Rodermond H. M. Stap J. Haveman J. van Bree C. (2006). Clonogenic assay of cells in vitro. Nat. Protoc.1, 2315–2319. 10.1038/nprot.2006.339
40
Fukaya M. Nakamura S. Hegazy M. E. F. Sugimoto Y. Hayashi N. Nakashima S. et al (2018). Cytotoxicity of sesquiterpene alkaloids from: nuphar plants toward sensitive and drug-resistant cell lines. Food Funct.9, 6279–6286. 10.1039/c8fo01804a
41
Gaikwad A. B. Tiwari R. K. Choudhary P. Kaur N. (2023). A comprehensive review on papaya seed oil extraction and recent applications in food industry. Pharma Innov.12, 1520–1527. 10.22271/tpi.2023.v12.i5s.20106
42
Galluzzi L. Bravo-San Pedro J. M. Vitale I. Aaronson S. A. Abrams J. M. Adam D. et al (2015). Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ.22, 58–73. 10.1038/cdd.2014.137
43
Haber R. A. Garcia R. D. Hernandez J. N. Jamieson S. Mondal A. Bishayee A. (2023). Papaya (Carica papaya L.) for cancer prevention: progress and promise. Crit. Rev. Food Sci. Nutr.63, 10499–10519. 10.1080/10408398.2022.2079607
44
Hanna D. H. Osailan R. Ahmed H. A. (2023). Stevia rebaudiana methanolic leaf extract in Egypt: phytochemical analysis, antioxidant, antilipid peroxidation, antihemolytic, antimetastatic, and anticancer properties. J. Food Biochem.2023, 1–22. 10.1155/2023/7161091
45
He X. Ma Y. Yi G. Wu J. Zhou L. Guo H. (2017). Chemical composition and antifungal activity of Carica papaya Linn. seed essential oil against candida spp. Lett. Appl. Microbiol.64, 350–354. 10.1111/lam.12711
46
Hegazy M. E. F. Mohamed T. A. Elshamy A. I. Hassanien A. A. Abdel-Azim N. S. Shreadah M. A. et al (2016). A new steroid from the Red Sea soft coral lobophytum lobophytum. Nat. Prod. Res.30, 340–344. 10.1080/14786419.2015.1046871
47
Hegazy M. E. F. Dawood M. Mahmoud N. Elbadawi M. Sugimoto Y. Klauck S. M. et al (2021). 2α-Hydroxyalantolactone from pulicaria undulata: activity against multidrug-resistant tumor cells and modes of action. Phytomedicine81, 153409. 10.1016/j.phymed.2020.153409
48
Hobani Y. Jerah A. Bidwai A. (2017). A comparative molecular docking study of curcumin and methotrexate to dihydrofolate reductase. Bioinformation13, 6366. 10.6026/97320630013063
49
Jiao M. Liu C. Prieto M. A. Lu X. Wu W. Sun J. et al (2023). Biological functions and utilization of different part of the papaya: a review. Food Rev. Int.39, 6781–6804. 10.1080/87559129.2022.2124415
50
Johansson N. Ahonen M. Kähäri V.-M. (2000). Matrix metalloproteinases in tumor invasion. Cell. Mol. Life Sci. CMLS57, 5–15. 10.1007/s000180050495
51
John T. Parmar K. A. Kotval S. C. Jadhav J. (2021). Synthesis, characterization, antibacterial and anticancer properties of silver nanoparticles synthesized from Carica papaya peel extract. Int. J. Nanosci. Nanotech.17, 23–32.
52
Kadhim M. J. Mohammed G. J. Hameed I. H. (2016). In vitro antibacterial, antifungal and phytochemical analysis of methanolic extract of fruit Cassia fistula. Orient. J. Chem.32, 1329–1346. 10.13005/ojc/320307
53
Khan N. H. (2021). Phytochemical analysis, antioxidant and antibacterial activity determination of ethanolic extract of Carica papaya seeds. Biomed. J. Sci. Tech. Res.33. 10.26717/bjstr.2021.33.005459
54
Komal R. Arya V. (2013). Biosynthesis and characterization of silver nanoparticles from aqueous leaf extracts of carica papaya and its antibacterial activity. Int. J. Nanomater. Biostructures3, 17–20. 10.2147/IJN.S79984
55
Kong Y. R. Jong Y. X. Balakrishnan M. Bok Z. K. Weng J. K. K. Tay K. C. et al (2021). Beneficial role of carica papaya extracts and phytochemicals on oxidative stress and related diseases: a mini review. Biol. (Basel)10, 287. 10.3390/biology10040287
56
Lee W. J. Lee M. H. Su N. W. (2011). Characteristics of papaya seed oils obtained by extrusion-expelling processes. J. Sci. Food Agric.91, 2348–2354. 10.1002/jsfa.4466
57
Li P. Zhao Y. Wang C. Zhu H. (2021). Antibacterial activity and main action pathway of benzyl isothiocyanate extracted from papaya seeds. J. Food Sci.86, 169–176. 10.1111/1750-3841.15539
58
Lin Y.-H. Chang Y.-X. Chi T.-Y. Chen C.-C. Hung Y.-C. Chen H.-Y. et al (2021). Comparison of tumoral inhibition abilities between two colorectal cancer cell lines after supercritical fluid extracted-papaya seed extract treatments. United J. Agri. Sci. Res.1 (3), 1–5. 10.47829/UJASR.2021.1301
59
Lydia E. Riyazudin M. John S. Thiyagarajan S. (2016). Investigation on the phytochemicals present in the fruit peel of Carica papaya and evaluation of its antioxidant properties. Int. J. Health and Allied Sci.5, 247. 10.4103/2278-344x.194127
60
Macleod A. J. Pieris N. M. (1983). Volatile components of papaya (Carica papaya L.) with particular reference to glucosinolate products. J. Agric. Food Chem.31, 1005–1008. 10.1021/jf00119a021
61
Mahrous N. S. Noseer E. A. (2023). Anticancer potential of Carica papaya Linn black seed extract against human colon cancer cell line: in vitro study. BMC Complement. Med. Ther.23, 271. 10.1186/s12906-023-04085-7
62
Martin Paul A. Jayanthi D. Thamizhseran N. (2019). Bio-physicochemical studies on water calyx fluid in the African tulip tree, Spathodea campanulata P. Beauv. Pharmacogn. J.11, 594–599. 10.5530/pj.2019.11.94
63
Memudu A. E. Oluwole T. J. (2021). The contraceptive potential of carica papaya seed on oestrus cycle, progesterone, and histomorphology of the utero-ovarian tissue of adult wistar rats. J. Bras. Reprod. Assist.25, 34–43. 10.5935/1518-0557.20200023
64
Mohamed T. A. Saleh I. Ali S. Hussien T. Hegazi N. M. Abdel-Halim S. et al (2022). A comparative evaluation of the antimicrobial activities of the essential oils of three salvia species growing in Egypt, obtained by hydrodistillation and microwave-assisted hydro-distillation. J. Essent. Oil-Bearing Plants25, 1109–1121. 10.1080/0972060X.2022.2135388
65
Moussa A. K. Abd El-Rahman H. A. Mohamed R. R. Hanna D. H. (2025a). Hyaluronic acid-based pH-Sensitive nanogel: synthesis, characterization, and assessment for acyclovir delivery in vitro and in vivo. Biomacromolecules26, 341–362. 10.1021/acs.biomac.4c01189
66
Moussa A. K. Abd El-Rahman H. A. Mohamed R. R. Hanna D. H. (2025b). Multifunctional plasticized hyaluronic-acid-based nanogel dressing for accelerating diabetic and nondiabetic wounds. Biomacromolecules26, 3495–3513. 10.1021/acs.biomac.5c00126
67
Mustafa N. N. El-Desouky M. A. Shawush N. A. Hanna D. H. (2025). Apoptosis induction in ascorbic acid treated human colorectal cancer cell lines (Caco-2). J. Biol. Act. Prod. Nat.15 (1), 56–71. 10.1080/22311866.2025.2465679
68
Nady D. S. Bakowsky U. Fahmy S. A. (2023). Recent advances in brain delivery of synthetic and natural nano therapeutics: reviving hope for Alzheimer’s disease patients. J. Drug Deliv. Sci. Technol.89, 105047. 10.1016/j.jddst.2023.105047
69
Nady D. S. Abdel-Halim S. Hegazy M. E. F. El-Desouky M. A. Hanna D. H. (2024a). Use of Carica papaya waste as bio-adsorbent for sewage wastewater treatment. Biomass Convers. Biorefin15, 15921–15938. 10.1007/s13399-024-06300-y
70
Nady D. S. Hassan A. Amin M. U. Bakowsky U. Fahmy S. A. (2024b). Recent innovations of mesoporous silica nanoparticles combined with photodynamic therapy for improving cancer treatment. Pharmaceutics16, 14. 10.3390/pharmaceutics16010014
71
Nirosha N. Mangalanayaki R. (2013). Antibacterial activity of leaves and stem extract of Carica papaya L. Int. J. Adv. Pharm. Biol. Chem.2, 2277–4688.
72
Pavlova N. Demin S. Churnosov M. Reshetnikov E. Aristova I. Churnosova M. et al (2022). Matrix metalloproteinase gene polymorphisms are associated with breast cancer in the caucasian women of Russia. Int. J. Mol. Sci.23, 12638. 10.3390/ijms232012638
73
Petros A. M. Medek A. Nettesheim D. G. Kim D. H. Yoon H. S. Swift K. et al (2001). Solution structure of the antiapoptotic protein bcl-2. Proc. Natl. Acad. Sci. U. S. A.98, 3012–3017. 10.1073/pnas.041619798
74
Phang Y. K. Aminuzzaman M. Akhtaruzzaman M. Muhammad G. Ogawa S. Watanabe A. et al (2021). Green synthesis and characterization of CuO nanoparticles derived from papaya peel extract for the photocatalytic degradation of palm oil mill effluent (POME). Sustain. Switz.13, 796–15. 10.3390/su13020796
75
Praveena P. Jethinlalkhosh J. P. Arokia Doss V. (2017). Evaluation of antiproliferative activity of Hydro-ethanolic extract of unripe fruit of carica papaya l. using various cell lines. Available online at: www.jasem.in.
76
Quick M. Dobryakov A. L. Gerecke M. Richter C. Berndt F. Ioffe I. N. et al (2014). Photoisomerization dynamics and pathways of trans - and cis -azobenzene in solution from broadband femtosecond spectroscopies and calculations. J. Phys. Chem. B118, 8756–8771. 10.1021/jp504999f
77
Rahaman M. H. A. Azlan N. A. A. Alam M. Kadir N. H. A. (2025). Synergistic targeting of cancer-related proteins by benzyl isothiocyanate and caffeic acid: MLSD and cytotoxic mechanisms in MCF-7 cells. 3 Biotech.15, 291. 10.1007/s13205-025-04469-1
78
Ramakrishnan M. Fahey J. W. Zimmerman A. W. Zhou X. Panjwani A. A. (2024). The role of isothiocyanate-rich plants and supplements in neuropsychiatric disorders: a review and update. Front. Nutr.11, 1448130. 10.3389/fnut.2024.1448130
79
Ranjan A. Ramachandran S. Gupta N. Kaushik I. Wright S. Srivastava S. et al (2019). Role of phytochemicals in cancer prevention. Int. J. Mol. Sci.20, 4981. 10.3390/ijms20204981
80
Ratnasari J. Tan M. I. Esyanti R. R. Juliawaty L. D. (2023). Cryptobrachytone C from Cryptocarya pulchrinervia (kosterm) leaves on proliferation, apoptosis, migration and clonogenicity of MCF-7 and T47D cell lines. Trop. Life Sci. Res.34, 223–241. 10.21315/tlsr2023.34.2.11
81
Reda E. H. Saleh I. A. Nasser A. Gendy G.E. Talaat Z. A. Hegazy M.-E. F. et al (2017). Chemical constituents of Euphorbia sanctae-catharinae fayed essential oil: a comparative study of hydro-distillation and microwave-assisted extraction. J. Adv. Pharm. Res. 10.21608/aprh.2017.3383
82
Reda E. H. Abdel Shakour Z. T. El-Halawany A. M. El-Kashoury E. S. A. Shams K. A. Mohamed T. A. et al (2021). Comparative study on the essential oils from five wild egyptian centaurea species: effective extraction techniques, antimicrobial activity and in-silico analyses. Antibiotics10, 252–18. 10.3390/antibiotics10030252
83
Roberta Malacrida C. Kimura M. Jorge N. (2011). Characterization of a high oleic oil extracted from papaya (Carica papaya L.) seeds. Ciênc. Tecnol. Aliment.31, 929–934. 10.1590/s0101-20612011000400016
84
Saha M. Sengupta S. Chatterjee S. (2023). Papaya seed waste: a potential source of therapeutic diet. J. Pharm. Sci. Res.15, 978–984.
85
Saleh I. Abd-Elgawad A. Gendy A. E. El Aty A. El Mohamed T. Kassem H. et al (2020). Phytotoxic and antimicrobial activities of teucrium polium and thymus decussatus essential oils extracted using hydrodistillation and microwave-assisted techniques. Plants9, 716. 10.3390/plants9060716
86
Salla S. Sunkara R. Ogutu S. Walker L. T. Verghese M. (2016). Antioxidant activity of papaya seed extracts against H2O2 induced oxidative stress in HepG2 cells. LWT66, 293–297. 10.1016/j.lwt.2015.09.008
87
Sari G. N. F. Handayani S. R. (2020). The effect cytotoxic and apoptotic propagation chloroform fraction of Ocimum sanctum L. towards Hela cells line culture. Biomedika13, 31–39. 10.31001/biomedika.v13i1.774
88
Shaheen S. Galanakis C. M. Farag M. A. (2023). Carica papaya biowaste valorization: biorefinery advances and extraction optimization. Food Rev. Int.39, 4745–4760. 10.1080/87559129.2022.2057527
89
Shakeri A. Khakdan F. Soheili V. Sahebkar A. Shaddel R. Asili J. (2016). Volatile composition, antimicrobial, cytotoxic and antioxidant evaluation of the essential oil from Nepeta sintenisii bornm. Ind. Crops Prod.84, 224–229. 10.1016/j.indcrop.2015.12.030
90
Singh S. P. Kumar S. Mathan S. V. Singh Tomar M. Verma P. K. Singh K. et al (2020). Therapeutic application of Carica papaya leaf extract in the management of human diseases. Daru28, 735–744. 10.1007/s40199-020-00348-7
91
Sinha A. Karan G. Das S. Maity T. Choudhury S. M. (2025). Harnessing the antineoplastic potential of benzyl isothiocyanate: phytochemical-based therapeutic strategy towards cancer. Int. J. Innov. Sci. Res. Technol., 771–784. 10.38124/ijisrt/25jul270
92
Spyridopoulou K. Fitsiou E. Bouloukosta E. Tiptiri-Kourpeti A. Vamvakias M. Oreopoulou A. et al (2019). Extraction, chemical composition, and anticancer potential of Origanum onites L. essential oil. Molecules24, 2612. 10.3390/molecules24142612
93
Subbaiah Munagapati V. Wen H. Y. Gollakota A. R. K. Wen J. C. Shu C. M. Andrew Lin K. Y. et al (2022). Magnetic Fe3O4 nanoparticles loaded papaya (Carica papaya L.) seed powder as an effective and recyclable adsorbent material for the separation of anionic azo dye (congo red) from liquid phase: evaluation of adsorption properties. J. Mol. Liq.345, 118255. 10.1016/j.molliq.2021.118255
94
Sultana N. Hossain S. M. Z. Taher S. Khan A. Razzak S. A. Haq B. et al (2020). Modeling and optimization of non-edible papaya seed waste oil synthesis using data mining approaches. S Afr. J. Chem. Eng.33, 151–159. 10.1016/j.sajce.2020.07.009
95
Taha Mohamed N. Abdelsalam D. H. Salem El-Ebiarie A. Elaasser M. (2021). Separation of bioactive compounds from haemolymph of scarab beetle Scarabaeus sacer (coleoptera: scarabaeidae) by GC-MS and determination of its antimicrobial activity. Int. J. Appl. Biol. Pharm.12. 10.26502/ijabpt.202113
96
Tan S. S. (2019). “Papaya (Carica papaya L.) seed oil”, in Fruit oils: chemistry and functionality (Springer International Publishing), 615–626. 10.1007/978-3-030-12473-1_31
97
Thanigaimalai M. Nainangu P. Panda S. P. Shaik M. R. Hussain S. A. Antonyrai A. P. et al (2025). The extracts of Carica papaya (Linn.): phytochemistry studies, anti-infective, antioxidant, and cytotoxic properties against cervical carcinoma. South African J. Bot.177, 604–616.
98
Toyin Oshin T. Kareem R. A. Fadayini O. Momoh J. O. (2021). Histological and cytotoxic evaluation of Carica papaya seed oil and its potential biodiesel feedstock. arXiv13, 43–52. 10.5897/JECE2020.0468
99
Ulubelen A. Topcu G. Eri$ C. %nmez U. ! Kartal M. Kurucu∼ S. et al (1994). Terpenoids from Salvia sclarea. Phytochemistry36, 971–974. 10.1016/s0031-9422(00)90474-6
100
Varadarajan S. Madapusi B. T. Narasimhan M. Pandian C. D. Dhanapal S. (2022). Anticancer effects of Carica papaya L. and benzyl isothiocyanate on an oral squamous cell carcinoma cell line: an in vitro study. J. Contemp. Dent. Pract.23, 839–844. 10.5005/jp-journals-10024-3384
101
Vundela S. R. Kalagatur N. K. Nagaraj A. Kadirvelu K. Chandranayaka S. Kondapalli K. et al (2022). Multi-biofunctional properties of phytofabricated selenium nanoparticles from Carica papaya fruit extract: Antioxidant, antimicrobial, antimycotoxin, anticancer, and biocompatibility. Front. Microbiol.12, 769891. 10.3389/fmicb.2021.769891
102
Whittaker M. Floyd C. D. Brown P. Gearing A. J. H. (1999). Design and therapeutic application of matrix metalloproteinase inhibitors. Chem. Rev.99, 2735–2776. 10.1021/cr9804543
103
Yanty N. A. M. Marikkar J. M. N. Nusantoro B. P. Long K. Ghazali H. M. (2014). Physico-chemical characteristics of papaya (Carica papaya L.) seed oil of the hong kong/sekaki variety. J. Oleo Sci.63, 885–892. 10.5650/jos.ess13221
104
Yi G. Yin C. Lao Y. Shi Z. He X. Wu J. et al (2022). Antibacterial and antitumor activities of chitosan/polyvinyl alcohol films containing microemulsion of papaya seed essential oil. Mater Today Commun.31, 103475. 10.1016/j.mtcomm.2022.103475
105
Youssef A. M. M. Maaty D. A. M. Al-Saraireh Y. M. (2023). Phytochemical analysis and profiling of antioxidants and anticancer compounds from Tephrosia purpurea (L.) subsp. apollinea family fabaceae. Molecules28, 3939. 10.3390/molecules28093939
106
Yusuf A. (2018). A review of methods used for seed oil extraction. Int. J. Sci. Res. (IJSR)7, 233–238. 10.21275/1121804
107
Zhang Y. Huang H. Jin L. Lin S. (2022). Anticarcinogenic effects of isothiocyanates on hepatocellular carcinoma. Int. J. Mol. Sci.23, 13834. 10.3390/ijms232213834
108
Zheng Z. Li Z. Yu M. Ma X. Gao J. Wang Y. et al (2025). Enhanced in vitro and in vivo antifungal efficacy against Candida albicans of nanostructured lipid carrier loaded with benzyl isothiocyanate extracted from Carica papaya L. seeds. Drug Deliv.32, 2544687. 10.1080/10717544.2025.2544687
109
Zhou Y. Xu H. (2015). Hemolysis of red blood cells induced by nanomaterials. J. Nanosci. Nanotech.15 (1), 2–12.
Summary
Keywords
Carica papaya , essential oil, HeLa cancer cell line, apoptosis, migration, molecular docking
Citation
Sayed Nady D, Abdel-Halim SA, Hegazy M-EF, Aljohani S, AlRashidi AA, El-Desouky MA, Ahmed HA, Jaremko M, Emwas A-H and Hanna DH (2025) In Vitro antiproliferative potential of essential oil extract from Carica papaya L. seeds against cervical cancer. Front. Pharmacol. 16:1676006. doi: 10.3389/fphar.2025.1676006
Received
29 July 2025
Accepted
04 September 2025
Published
02 October 2025
Volume
16 - 2025
Edited by
Mónica Vieira, Polytechnic Institute of porto, Portugal
Reviewed by
Himanshu Kathuria, Nusmetics Pte Ltd., Singapore
Arif Setiawansyah, Cendikia Farma Husada College of Pharmacy, Indonesia
Updates
Copyright
© 2025 Sayed Nady, Abdel-Halim, Hegazy, Aljohani, AlRashidi, El-Desouky, Ahmed, Jaremko, Emwas and Hanna.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Demiana H. Hanna, dhelmy@sci.cu.edu.eg
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.