- 1Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
- 2Athinoula A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States
Microglia are the resident immune cells of the central nervous system (CNS), playing a crucial role in maintaining brain homeostasis and mediating neuroimmune responses. The chemokine receptor CX3CR1, predominantly expressed on microglia, regulates microglial function via interactions with its neuronal ligand CX3CL1. The CX3CR1-CX3CL1 signaling exhibits complex, context-dependent roles in neurodegenerative diseases. In Alzheimer’s disease (AD) and Parkinson’s disease (PD) animal models, CX3CR1 deficiency shows paradoxical outcomes, attenuating or exacerbating amyloid-β (Aβ) and tau pathologies in AD, while consistently worsening α-synuclein-induced neurodegeneration in PD. Although CX3CR1 emerges as a promising therapeutic and diagnostic target, its complex role in microglial dynamics remains incompletely understood. Positron emission tomography (PET) imaging provides a powerful, noninvasive method for investigating biological processes in vivo. There is an urgent need to develop and validate new PET tracers targeting microglial CX3CR1 in the CNS, further offering new opportunities for the diagnosis and treatment of neuroinflammation-associated neurodegenerative diseases.
1 Introduction
Microglia are the resident immune cells in the CNS, responsible for regulating innate immunity and participating in adaptive immune responses (Garden and Möller, 2006). They originate from the early yolk sac and migrate into the CNS during the early embryonic development stage (around E9.5 and E10.5) (Ginhoux et al., 2010). Under physiological conditions, macroglia exhibit a highly ramified morphology in their homeostatic state. The branched structure enables them to sensitively monitor changes in the CNS microenvironment and maintain brain homeostasis by phagocytosing neuronal synapses, apoptotic cells, and cellular debris, while releasing trophic factors that support neuronal growth and survival (Bachiller et al., 2018). However, under pathological conditions, microglia rapidly adopt hypertrophic morphology characterized by an enlarged cell body and shortened cellular processes. As sentinels, microglia modulate the immune response to minimize potential CNS damage and promote tissue repair (Sadeghdoust et al., 2024). Since neuroinflammation plays a crucial role in the development and progression of neurodegenerative diseases, microglia have gained significant attention for mediating CNS immune responses, and their dysfunctional activation worsens disease progression (Figure 1).

Figure 1. Effects of CX3CR1-CX3CL1 signaling on microglial function. Both membrane-bound and soluble forms of CX3CL1 interact with CX3CR1, triggering G protein-coupled signaling cascades that regulate intracellular responses in microglia. Under physiological conditions, CX3CR1-CX3CL1 signaling suppresses pro-inflammatory cytokine production and maintains microglia in a homeostatic state. In pathological conditions, during the early stages of disease, CX3CR1-CX3CL1 activation exerts neuroprotective effects via the PI3K/Akt/NRF2 pathway, enhancing microglial phagocytosis and anti-inflammatory activity to support neuronal survival. As disease progresses, persistent neuroinflammation induces microglial hyperactivation, and CX3CR1-CX3CL1 signaling shifts toward the PI3K/Akt/NF-κB and MAPK/NF-κB pathways, promoting proinflammatory cytokine release, neuronal dysfunction, and death. These processes collectively exacerbate neurodegeneration in Alzheimer’s and Parkinson’s diseases. Figure created with BioRender.
The activation states of microglia are dynamic and context-dependent. They thus cannot be adequately studied through in vitro isolation or culturing cells, transcriptomic analysis, or biopsy sampling alone (Jain et al., 2020). Under these conditions, microglia may lose much of their distinctiveness and become prototype macrophages (Wolf et al., 2013). Given that microglia are highly sensitive cells that undergo rapid morphological and functional changes in vitro, in vivo microglial reporter mouse models have been developed to better study their behavior in the intact CNS environment (Gosselin et al., 2017). The CX3CR1-GFP knock-in mice (in which cDNA encoding green fluorescent protein (GFP) replaces one Cx3cr1 gene allele) are popular experimental models for studying microglial function of AD and PD (Guedes et al., 2018). However, there is an unmet need for noninvasive techniques that enable longitudinal in vivo visualization of neuroimmune responses, especially in humans. Positron emission tomography (PET) has been widely applied in both research and clinical settings to study biological processes in living subjects. It is an indispensable tool for early disease detection and diagnosis, real-time therapy monitoring, and patient stratification in clinical trials (Jain et al., 2020).
Microglial receptors play a crucial role in receiving external stimuli, regulating their function, and transmitting signals between cells. Some specific receptor-ligand interaction signaling axes play a key role in the pathogenesis of neurodegenerative diseases (Guedes et al., 2018; Dadwal and Heneka, 2024; Fu et al., 2025). Among them, CX3C chemokine receptor 1 (CX3CR1) stands out as a key molecule, specifically and predominantly expressed on microglia, and recognized by its sole endogenous ligand, CX3CL1 (Mizoue et al., 1999), which is primarily expressed on neurons. The CX3CR1-CX3CL1 axis plays a vital role in modulating microglial function within the CNS (Figure 1) (Dadwal and Heneka, 2024). Recently, overviews of the CX3CR1-CX3CL1 axis in neurodegenerative diseases (Subbarayan et al., 2022; Fu et al., 2025; Vida et al., 2025), drug and PET tracers targeting G protein-coupled receptors (GPCRs) (Pissarek, 2020; Sun et al., 2020; Wang et al., 2025) have been published. From the perspective of CX3CR1, this review provides a brief overview of its basic biology and the dynamics of microglial CX3CR1 in pathological conditions, as observed in mouse models of AD and PD. It discusses its potential as a microglia-specific therapeutic and imaging target in neurodegenerative diseases.
2 Biological and structural basis of CX3CR1
Chemokine receptors (CCRs), a subfamily of class A GPCRs, share a conserved seven-transmembrane (TM1-TM7) architecture and dynamic extracellular and intracellular loops (ECLs and ICLs) that enable ligand specificity and signal transduction (Wang et al., 2025). CX3CR1 (Combadiere et al., 1998), a conventional Gαi-coupled receptor and the sole member of the CX3C subfamily, features three amino acids separating two conserved cysteines (Wolf et al., 2013). The membrane-bound form of fractalkine functions as an adhesion molecule that maintains microglia in a quiescent state, whereas the soluble form acts as a chemoattractant, promoting microglial migration toward sites of injury or inflammation (Chidambaram et al., 2020). CX3CL1 binds to CX3CR1, activating intracellular signaling pathways such as PI3K/Akt, NRF2, and NF-κB via G-protein coupling. Under physiological conditions, CX3CR1-CX3CL1 signaling suppresses microglial activation, thereby maintaining a homeostatic phenotype. Under pathological conditions, chronic inflammation driven by the accumulation of Aβ, p-tau, and α-synuclein alters CX3CR1 expression, disrupting signaling balance and modulating microglial activation in a context-dependent manner, leading to both pro- and anti-inflammatory responses. Specifically, CX3CR1-CX3CL1 signaling exerts neuroprotective effects by activating the PI3K/Akt/NRF2 pathway to enhance microglial phagocytic and anti-inflammatory capacity, while exerting neurotoxicity through PI3K/Akt/NF-κB or MAPK/NF-κB to promote the release of the pro-inflammatory cytokines (Eugenín et al., 2023) (Figure 1).
Recent cryo-EM structures of CX3CR1-Gi1 complexes in ligand-free and CX3CL1-bound states have elucidated (Lu et al., 2022). CX3CL1 engages CX3CR1 via a two-site binding mode; the globular domain binds the extracellular region (CRS1), while the N-terminal “hook” inserts into the transmembrane core (CRS2). The short β-hairpin loop of ECL2 limits contact with CX3CL1, contributing to the receptor’s unique recognition pattern. Upon binding to soluble or membrane-anchored CX3CL1 (Eugenín et al., 2023), CX3CR1 couples to Gi and transduces extracellular signals via conformational rearrangements. The Gi1-coupled structure exhibits a smaller outward movement of TM6, resulting in a narrower G-protein pocket, compensated by outward shifts of TM7 and helix VIII. The unique Gαi coupling conformation establishes extensive interactions among helices I-VIII and ICL2/ICL3, while cholesterol molecules further stabilize this compact active state by maintaining proximity between TM3 and TM6. These structural insights into ligand recognition and receptor activation provide a molecular framework to advance modern drug discovery by enabling the structure-based design of selective CX3CR1 allosteric modulators (Wang et al., 2025).
3 CX3CR1 in neurodegenerative diseases
CX3CR1 signaling (CX3CR1/CX3CL1 axis) plays a crucial role in regulating microglial morphology and function in response to neuroinflammation. This signaling pathway exhibits dual effects in neurological pathologies, exerting either neuroprotective or neurotoxic outcomes, depending on the variant microenvironment in the brain and stage of the disease (Luo et al., 2019; Suresh et al., 2021; Subbarayan et al., 2022; Zhao et al., 2023). CX3CR1 signaling confers neuroprotection by suppressing microglial hyperactivation and limiting the release of neurotoxic pro-inflammatory cytokines (e.g., TNF-α, IL-1β), thereby controlling CX3CR1 protein levels and maintaining microglial homeostasis. Conversely, disruptions in CX3CR1 signaling (e.g., CX3CR1 deficiency) lead to excessive microglial activation, heightened inflammatory responses, neuronal loss, and worsening of disease progression. As a result, the specific role of microglial CX3CR1 in neurodegenerative diseases remains controversial and warrants further investigation. This section provides a brief review of the complex roles of CX3CR1 in AD and PD.
3.1 CX3CR1 in Alzheimer’s disease
AD is characterized by the extracellular deposition of Aβ and the intracellular aggregation of hyperphosphorylated tau protein into neurofibrillary tangles (NFTs). In parallel, microglial activation-related neuroinflammation is a key contributor to the initiation and progression of AD pathology. Meanwhile, Single-nucleus RNA sequencing (snRNA-seq) studies have revealed distinct clusters of activated microglia associated with Aβ, NFTs, and inflammatory signaling pathways in AD (Nguyen et al., 2020; Gerrits et al., 2021). In human and 5xFAD mice frontal cortical samples, microglial CX3CR1 expression was increased in advanced AD, with CX3CR1 mRNA significantly upregulated at intermediate and advanced stages, indicating CX3CR1 as a potential therapeutic target in disease progression (González-Prieto et al., 2021). The expression of CX3CR1 also increased in the hippocampus of advanced AD patients (Bolós et al., 2017), and upregulation of CX3CR1 gene expression was observed in the hippocampus and frontal cortex of middle-aged 5xFAD mice (Liu et al., 2025). However, in vivo studies of CX3CR1 disruption remain challenging, with conflicting outcomes reported across transgenic AD mouse models (Table 1).
CX3CR1 deficiency has been associated with both neuroprotective and neurotoxic effects, depending on whether Aβ or tau pathology predominates (Chen, 2016). Systematic studies by Bruce’s group using Cx3cr1-deficient AD murine models have highlighted the divergent roles of neuron-microglial fractalkine signaling in modulating Aβ and tau pathologies (Bhaskar et al., 2010; Lee et al., 2010; Lee et al., 2014; Maphis et al., 2015; Puntambekar et al., 2022). Their findings suggest that CX3CR1 signaling modulates microglial activation triggered by Aβ deposition and further influences downstream neurotoxicity. In the early stages of disease in the APPPS1 and R1.40 mouse models, both complete and partial Cx3cr1 deficiency resulted in a gene-dose-dependent reduction in fibrillar Aβ burden, suggesting a protective role for CX3CR1 loss in Aβ pathology (Lee et al., 2010). Similarly, CRND8; Cx3cr1−/− mice exhibited lower levels of Aβ and reduced amyloid deposits through selective phagocytosis of protofibrillar Aβ (Liu et al., 2010). Recently, Bruce’s group investigated how CX3CR1-mediated microglial response to Aβ promotes sustained neurotoxicity in the 5xFAD amyloid model (Puntambekar et al., 2022). In 5xFAD; Cx3cr1−/− mice, the absence of CX3CR1 impaired microglial endolysosomal activity, reducing the uptake and degradation of fibrillar Aβ. This led to the accumulation of toxic Aβ species and further promoted a pro-inflammatory, neurodegenerative microglial morphology, characterized by dysregulated reactive oxygen species (ROS) metabolism and chronic microglial activation. Long-term, such dysfunction correlates with widespread neurodegeneration, including tau accumulation, synaptic loss, and disrupted neuronal homeostasis. These findings support the notion that the quality and abundance of extracellular Aβ can influence the extent of microglial dysfunction in the absence of CX3CR1 (Puntambekar et al., 2022).
Since most therapeutic interventions only achieve partial target inhibition, understanding the effects of partial CX3CR1 deficiency in AD models is particularly important (Hickman et al., 2019). Hickman et al. reassessed the impact of Cx3cr1 haploinsufficiency using the APPPS1 transgenic AD mouse model. Their results showed that partial CX3CR1 deficiency led to a significant reduction in Aβ deposition and levels across the early to advanced stages of disease, improved cognitive function, and increased levels of Aβ-degrading enzymes (insulysin and matrix metalloproteinase 9 (MMP9)) in the whole brain. These findings suggest that slight downregulation of CX3CR1 activity might be enough to influence neuron-microglia communication, restore neuronal Aβ-degrading enzymes, and ultimately slow the progression of AD-like pathology in APPPS1 mice (Hickman et al., 2019).
CX3CR1 deficiency has been associated with tau pathology. The hTau; Cx3cr1−/− mice exhibit enhanced microglial activation, and the loss of CX3CR1 accelerated neuronal microtubule-associated protein tau (MAPT) hyperphosphorylation and aggregation, as well as the mice’s behavioral impairments (Bhaskar et al., 2010; Kim et al., 2011). These activated microglia lacking CX3CR1 appear to promote the activation of p38 mitogen-activated protein kinase (MAPK) and MAPT hyperphosphorylation, especially upon exposure to microglia-derived interleukin-1β (IL-1β) (Bhaskar et al., 2010; Maphis et al., 2015; Guedes et al., 2018). In parallel, CX3CR1 signaling contributes to the microglial-mediated killing of neurons harboring intracellular tau, thereby facilitating the release and extracellular spread of tau. Furthermore, extracellular tau competes with CX3CL1 for binding to CX3CR1, promoting microglial uptake of tau. Thereby, CX3CR1 deficiency impairs the microglial phagocytosis of tau (Bolós et al., 2017; Chidambaram et al., 2020; Das et al., 2020; Chidambaram et al., 2024). Notably, in 5xTg or 3xTg models, which exhibit both Aβ and tau pathologies, Cx3cr1 deficiency appears to mitigate neuronal loss (Fuhrmann, 2010; Guedes et al., 2018). Increased Aβ load and plaque burden also affect the seeding and spread of pTau and further worsen memory deficits when pathological human-AD tau is injected into 5xFAD and APP-KI mice (He et al., 2018). Consistent with this, 5xFAD; Cx3cr1−/− mice study showed that Aβ-driven microglial dysfunction aggravates tau hyperphosphorylation (Puntambekar et al., 2022). These observations underscore the need for further investigation using AD models that incorporate both Aβ and tau pathologies better to understand the multifaceted role of CX3CR1 in disease progression (Yokoyama et al., 2022; Zhong M. Z, 2024).
Sex and age are major risk factors in AD, with a higher incidence of the disease in females (Laws et al., 2016; Mielke, 2019; Castro-Aldrete et al., 2023). Guillot-Sestier et al. investigated sex-related differences in microglia in APPPS1 mice and in postmortem tissue from AD patients (Guillot-Sestier et al., 2021). They found that upregulation of genotype-related microglial activation, increased rod-shaped microglial morphologies, and decreased microglial phagocytosis of Aβ were observed in female mice and AD patients, compared with age-matched males. Complementarily, Hemonnot-Girard et al. examined the impact of Cx3cr1 haplodeficiency in male and female APPswe/PSEN1dE9 mice across the early to more advanced stages of AD disease (Hemonnot-Girard et al., 2021). Their studies showed that sex and Cx3cr1 haplodeficiency affect Aβ plaque load in a disease-stage-dependent manner. However, sex and CX3CR1 haplodeficiency had little impact on microgliosis progression and cortical neuroinflammation in APPswe/PSEN1dE9 mice. Notably, they observed a higher Aβ plaque burden and slower learning abilities in females compared to age-matched males, highlighting a sex-dependent difference in disease pathology. Puntambekar et al. also reported that Cx3cr1 haplodeficiency accelerates plaque deposition with disease progression, exacerbates the accumulation/generation of neurotoxic soluble oligomeric Aβ species, and that female 5xFAD mice displayed significantly increased plaque burdens compared to males (Puntambekar et al., 2022). They also found that microglia from 5xFAD; Cx3cr1−/− mice brains express significantly increased mRNA levels of proinflammatory cytokines (e.g., IL-1β) and increased Apoe mRNA levels in females, indicating that CX3CR1 deficiency dysregulates microglial activation towards a neurotoxic phenotype.
3.2 CX3CR1 in Parkinson’s disease
PD is the most prevalent neurodegenerative movement disorder, defined pathologically by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and the abnormal accumulation of α-synuclein (α-SYN) in Lewy bodies and Lewy neurites (Schonhoff et al., 2023). Postmortem analyses of PD brains have consistently revealed robust microglial activation, suggesting a key role for neuroinflammation and microgliosis in disease pathogenesis (McGeer et al., 1988; Langston et al., 1999).
CX3CR1 has been reported to exert neuroprotective effects in α-synucleinopathy models of PD by limiting neurotoxicity and attenuating inflammatory responses (Table 2) (Tristão et al., 2016; Vida et al., 2025). In mouse models expressing human α-synuclein, dopaminergic neuronal loss occurred to a similar extent in both wild-type (Cx3cr1+/+) and Cx3cr1-deficient (Cx3cr1−/−) mice (Thome et al., 2015; Castro-Sánchez et al., 2018). However, in mice overexpressing the A53T mutant form of α-synuclein (α-SYNA53T), a mutation linked to familial PD neurodegeneration, the phenotype was significantly worsened in the absence of CX3CR1. This enhanced pathology was accompanied by elevated microgliosis, upregulation of pro-inflammatory mediators, and increased neuronal vulnerability, highlighting the critical role of CX3CR1 in modulating the microglial response to α-syn-induced stress. These findings underscore the importance of CX3CR1 signaling in regulating microglial phenotype and inflammatory cascades during PD progression, particularly in the context of α-synuclein mutations, such as A53T (Castro-Sánchez et al., 2018). Genetic deletion or functional disruption of Cx3cr1 has been shown to exacerbate dopaminergic neuronal loss, with corresponding increases in pro-inflammatory cytokine production and ROS activity in multiple PD mouse models (Nash et al., 2015; Castro-Sánchez et al., 2018). For example, in the MPTP neurotoxin model, Cx3cr1−/− mice exhibited significantly reduced numbers of tyrosine hydroxylase-immunoreactive (TH-IR) neurons in the SNpc, providing further evidence that CX3CR1 contributes to neuronal survival by suppressing microglia-driven neurotoxicity in vivo (Cardona et al., 2006). A study by Wang et al. further demonstrated that CX3CR1 expression is downregulated during the early stages of PD and gradually returns to baseline levels in the later stages in rAAV-hSYN-injected C57BL/6 mice, suggesting dynamic regulation of CX3CR1 during disease progression (Wang et al., 2020). These findings support the potential of CX3CR1 as a biomarker for disease staging and a candidate for therapeutic intervention. In parallel, work from Harms et al. (2018) using AAV-SYN; Cx3cr1+/GFP reporter mice confirmed that resident CNS microglia express high levels of CX3CR1 and actively respond to α-synuclein accumulation. However, their results also suggest that much of the observed microgliosis is driven by peripheral monocyte infiltration, complicating the distinction between intrinsic microglial activation and monocyte recruitment during neuroinflammatory responses (Harms et al., 2018).
4 Development of PET tracers targeting CX3CR1
The promiscuity of chemokines and their cognate chemokine receptors (CKRs) has long posed an obstacle to chemokine receptor-based drug development. Although CX3CR1 offers a unique advantage as a potential drug target due to its restricted ligand-receptor pairing with CX3CL1, the development of small-molecule modulators has remained slow and challenging (Lu et al., 2022). To date, only two small-molecule CX3CR1 antagonists have entered clinical evaluation (Figure 2). AZD8797, also known as KAND567 or Rugocrixan, is a potent and selective human CX3CR1 antagonist with high binding affinity (Ki = 3.9 nM, KB = 10 nM), 720-fold selectivity versus chemokine receptor CXCR2, and favorable physicochemical characteristics suitable for in vivo applications (Karlström et al., 2013; Ridderstad Wollberg et al., 2014). It functions as a non-competitive allosteric modulator that interferes with CX3CL1-induced receptor signaling and exhibits biased modulation of downstream G-protein and β-arrestin pathways (Cederblad et al., 2016).
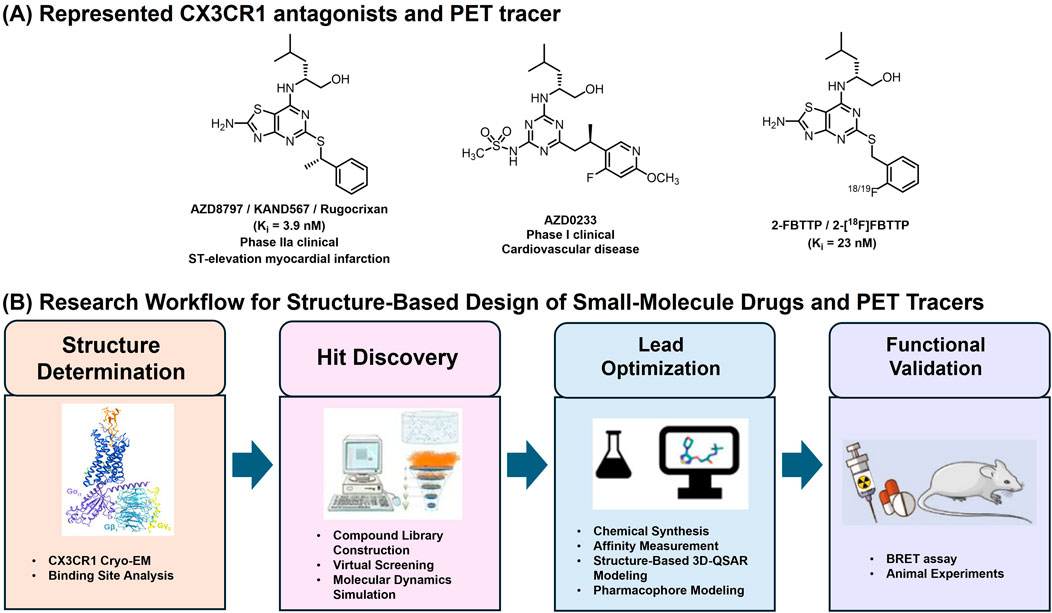
Figure 2. (A) Represented CX3CR1 antagonists and PET tracer; (B) General Research Workflow for Structure-Based Design of Small-Molecule Drugs and PET Tracers Targeting CX3CR1. The experimental workflow comprises four main parts: cryo-EM CX3CR1 structure determination; structure-based small-molecule Hit discovery; lead optimization and functional validation.
Preclinical studies have demonstrated that AZD8797 (I.P., 80 μg/kg/day) exerts therapeutic effects by inhibiting the CX3CR1-CX3CL1 signaling pathway, thereby suppressing inflammatory responses and promoting tissue repair in CNS inflammatory animal models of spinal cord injury (SCI) (Chen et al., 2020) and eosinophilic meningitis (Lai et al., 2025). For instance, in mice infected with A. cantonensis, microglial activation and neuronal degeneration were accompanied by increased p-tau accumulation and elevated CX3CR1 expression in brain tissue; these pathological changes were markedly attenuated following intraperitoneal administration of AZD8797 (Lai et al., 2025). Beyond its effects on neuroinflammation, AZD8797 suppresses CX3CR1-positive monocyte-mediated tumor growth in chronic lymphocytic leukemia (CLL) cells (Zhong W, 2024). It is currently under Phase IIa evaluation in patients with ST-elevation myocardial infarction following percutaneous coronary intervention (Ekinci et al., 2024). Recently, AstraZeneca disclosed a new CX3CR1 antagonist, AZD0233 (Guo et al., 2025). Oral administration of the compound (100 mg/kg/day) can improve cardiac function and reduce fibrotic scar tissue in a mouse model of dilated cardiomyopathy. This compound has advanced into single-ascending dose (SAD) and multiple-ascending dose (MAD) Phase I clinical studies for dilated cardiomyopathy and heart failure. As the development of PET tracers is closely intertwined with advances in drug discovery, 2-[18F]FBTTP remains the only reported radiotracer specifically targeting microglial CX3CR1, derived directly from the small-molecule antagonist 2-FBTTP (Ki = 23 nM) (Figure 2) (Karlström et al., 2013; Pissarek, 2020). Designed to enable noninvasive visualization of neuroinflammation, 2-[18F]FBTTP has demonstrated sufficient blood-brain barrier permeability in healthy mice, supporting its potential for CNS imaging. However, comprehensive biodistribution studies and validation in disease-relevant neuroinflammatory models are still required to establish its translational applicability.
Different target-based small-molecule PET tracers have been developed to image microglial activation. Among these, PET radiotracers targeting the 18 kDa translocator protein (TSPO) have been traditionally and widely used to assess neuroinflammation by visualizing microglial activation in AD, PD, and other neurodegenerative disorders. However, TSPO tracers exhibit several notable limitations, including high nonspecific binding, low brain penetration, and interspecies polymorphic differences that affect binding affinity between rodents and humans (Janssen et al., 2018; Jain et al., 2020; Beaino et al., 2021). Specifically, although TSPO expression increases in activated microglia in mouse models of brain disease, it remains essentially unchanged in human patients, indicating that TSPO-PET signals in humans reflect only the density of inflammatory cells (e.g., microglia/macrophages) rather than the activation state of microglia (Nutma et al., 2023).
To overcome these limitations, alternative microglia-specific imaging targets have been explored (Janssen et al., 2018; Jain et al., 2020; Beaino et al., 2021). Among them, the purinergic P2Y12 receptor (P2Y12R), a G-protein-coupled receptor selectively expressed on homeostatic microglia, has emerged as a promising biomarker for distinguishing microglial phenotypic states during neuroinflammation (Mildner et al., 2017; Pissarek, 2020). Recently, two nicotinate-based PET radiotracers with favorable brain permeability and high binding affinity for P2Y12R have been developed. Yao et al. (2025) reported [18F]QTFT (Yao et al., 2025), which enables quantitative imaging of P2Y12 expression on anti-inflammatory microglia across diverse preclinical models and in patients with neuroinflammatory diseases; however, further structural optimization is required to minimize hepatic uptake and off-target binding. Similarly, Joseph et al. described another P2Y12 PET tracer [18F]12 (Joseph et al., 2025), enabling images of P2Y12-positive microglia, although additional modifications are still needed to improve in vivo stability and reduce nonspecific binding.
The development of PET tracers targeting CX3CR1 remains at an early stage. The structure-based drug design (SBDD) (Yang et al., 2021; Cao et al., 2024; Cebi et al., 2024) has become an increasingly precise and efficient approach in modern drug discovery, enabling rational optimization based on atomic-level structural insights (Figure 2). Both AZD8797 and AZD0233, representative CX3CR1 antagonists sharing a thiazolopyrimidine scaffold, likely bind to a similar receptor pocket, underscoring the need for new chemotypes to expand the chemical diversity of CX3CR1 ligands. The elucidation of the CX3CR1-Gi1 complex provides a structural framework for SBDD, enabling the rational development of allosteric modulators and antagonists guided by the receptor’s distinct extracellular topology, compact intracellular conformation, and cholesterol-binding interfaces. Complementary molecular dynamics (MD) simulations (Goode-Romero and Dominguez, 2022; Piloto et al., 2024) further characterize receptor flexibility, transient pockets, and ligand-binding stability. Together, the integration of cryo-EM structures and MD-guided modeling accelerates rational discovery of CX3CR1-targeted allosteric modulators, facilitating the development of highly specific PET tracers for imaging microglial activation and neuroinflammation in the CNS.
5 Discussion
CX3CR1 is a key microglial homeostatic gene that regulates motility, phagocytosis, and inflammatory responses in the CNS. It is selectively expressed on microglia, and the CX3CR1-CX3CL1 axis mediates neuron and microglia communication, maintaining microglial quiescence and immune balance. Notably, CX3CR1 expression is upregulated in the AD patient’s brain, particularly in the frontal cortex and hippocampus, reflecting microglial proliferation and sustained activation in these regions. In contrast, the inconsistent findings observed in experimental models may be partly attributed to CX3CR1 polymorphisms, as human variants (e.g., V249I and T280M) have been associated with altered receptor function and differential susceptibility to AD and PD (Kalinderi et al., 2012; López-López et al., 2018; Meyer, 2025). Although TSPO tracers are widely used, they lack cellular specificity and are strongly influenced by gene polymorphisms (Janssen et al., 2018; Jain et al., 2020; Kreisl et al., 2020; Beaino et al., 2021). Similarly, P2RY12, while highly microglia-specific (Honarpisheh et al., 2020), is markedly downregulated upon activation, and its reduced expression and regional heterogeneity in AD brain tissue further limit its translational potential (Mildner et al., 2017; Walker et al., 2020). Compared with other microglial imaging targets, CX3CR1 offers distinct advantages. It remains microglia-specific and functionally involved in neuron–microglia signaling, and its expression increases throughout disease progression. These characteristics make CX3CR1 a particularly promising target for microglial imaging. However, challenges remain in achieving the optimal balance between binding affinity, selectivity, and blood–brain barrier permeability required for the development of effective PET tracers. Ongoing research aims to develop CX3CR1-targeted PET tracers to noninvasively assess microglial activity and neuroinflammatory dynamics in vivo, particularly in AD and PD patients.
Author contributions
HY: Writing – review and editing, Writing – original draft. YW: Writing – review and editing. YX: Writing – review and editing. CW: Writing – review and editing, Writing – original draft.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bachiller, S., Jiménez-Ferrer, I., Paulus, A., Yang, Y., Swanberg, M., Deierborg, T., et al. (2018). Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front. Cell. Neurosci. 12, 488. doi:10.3389/fncel.2018.00488
Beaino, W., Janssen, B., Vugts, D. J., de Vries, H. E., and Windhorst, A. D. (2021). Towards PET imaging of the dynamic phenotypes of microglia. Clin. Exp. Immunol. 206 (3), 282–300. doi:10.1111/cei.13649
Bhaskar, K., Konerth, M., Kokiko-Cochran, O. N., Cardona, A., Ransohoff, R. M., and Lamb, B. T. (2010). Regulation of tau pathology by the microglial fractalkine receptor. Neuron 68 (1), 19–31. doi:10.1016/j.neuron.2010.08.023
Bolós, M., Llorens-Martín, M., Perea, J. R., Jurado-Arjona, J., Rábano, A., Hernández, F., et al. (2017). Absence of CX3CR1 impairs the internalization of tau by microglia. Mol. Neurodegener. 12 (1), 59. doi:10.1186/s13024-017-0200-1
Cao, D., Zhang, P., and Wang, S. (2024). Advances in structure-based drug design: the potential for precision therapeutics in psychiatric disorders. Neuron 112 (4), 526–538. doi:10.1016/j.neuron.2024.01.004
Cardona, A. E., Pioro, E. P., Sasse, M. E., Kostenko, V., Cardona, S. M., Dijkstra, I. M., et al. (2006). Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9 (7), 917–924. doi:10.1038/nn1715
Castro-Aldrete, L., Moser, M. V., Putignano, G., Ferretti, M. T., Schumacher Dimech, A., and Santuccione Chadha, A. (2023). Sex and gender considerations in Alzheimer’s disease: the Women’s brain project contribution. Front. Aging Neurosci. 15, 1105620. doi:10.3389/fnagi.2023.1105620
Castro-Sánchez, S., García-Yagüe, Á. J., López-Royo, T., Casarejos, M., Lanciego, J. L., and Lastres-Becker, I. (2018). Cx3cr1-deficiency exacerbates alpha-synuclein-A53T induced neuroinflammation and neurodegeneration in a mouse model of parkinson's disease. Glia 66 (8), 1752–1762. doi:10.1002/glia.23338
Cebi, E., Lee, J., Subramani, V. K., Bak, N., Oh, C., and Kim, K. K. (2024). Cryo-electron microscopy-based drug design. Front. Mol. Biosci. 11, 1342179. doi:10.3389/fmolb.2024.1342179
Cederblad, L., Rosengren, B., Ryberg, E., and Hermansson, N. O. (2016). AZD8797 is an allosteric non-competitive modulator of the human CX3CR1 receptor. Biochem. Journal 473 (5), 641–649. doi:10.1042/BJ20150520
Chen, P., Zhao, W., Guo, Y., Xu, J., and Yin, M. (2016). CX3CL1/CX3CR1 in alzheimer’s disease: a target for neuroprotection. Biomed. Res. Int. 2016, 8090918. doi:10.1155/2016/8090918
Chen, G., Zhou, Z., Sha, W., Wang, L., Yan, F., Yang, X., et al. (2020). A novel CX3CR1 inhibitor AZD8797 facilitates early recovery of rat acute spinal cord injury by inhibiting inflammation and apoptosis. Int. J. Mol. Med. Prepr. 45, 1373–1384. doi:10.3892/ijmm.2020.4509
Chidambaram, H., Das, R., and Chinnathambi, S. (2020). Interaction of tau with the chemokine receptor, CX3CR1 and its effect on microglial activation, migration and proliferation. Cell and Biosci. 10 (1), 109–9. doi:10.1186/s13578-020-00474-4
Chidambaram, H., Desale, S. E., Suri, K., Murugan N, A., and Chinnathambi, S. (2024). Microglia undergoes chemokine receptor, CX3CR1-mediated internalization of extracellular tau. Cell Biol. doi:10.1101/2024.12.03.626734
Combadiere, C., Salzwedel, K., Smith, E. D., Tiffany, H. L., Berger, E. A., and Murphy, P. M. (1998). Identification of CX3CR1. A chemotactic receptor for the human CX3C chemokine fractalkine and a fusion coreceptor for HIV-1. J. Biol. Chem. 273 (37), 23799–23804. doi:10.1074/jbc.273.37.23799
Dadwal, S., and Heneka, M. T. (2024). Microglia heterogeneity in health and disease. FEBS Open Bio 14 (2), 217–229. doi:10.1002/2211-5463.13735
Das, R., Balmik, A. A., and Chinnathambi, S. (2020). Phagocytosis of full-length tau oligomers by Actin-remodeling of activated microglia. J. Neuroinflammation 17 (1), 10–15. doi:10.1186/s12974-019-1694-y
Ekinci, Y., Richardson, G., and Spyridopoulos, I. (2024). A phase IIa clinical trial of KAND567, fractalkine receptor inhibitor, in patients with ST-Elevation acute myocardial infarction after percutaneous coronary intervention. J. Pharmacol. Exp. Ther. 389, 472–473. doi:10.1124/jpet.472.935010
Eugenín, J., Eugenín-von Bernhardi, L., and Von Bernhardi, R. (2023). Age-dependent changes on fractalkine forms and their contribution to neurodegenerative diseases. Front. Mol. Neurosci. 16, 1249320. doi:10.3389/fnmol.2023.1249320
Fu, J., Wang, R., He, J., Liu, X., Wang, X., Yao, J., et al. (2025). Pathogenesis and therapeutic applications of microglia receptors in Alzheimer’s disease. Front. Immunol. 16, 1508023. doi:10.3389/fimmu.2025.1508023
Fuhrmann, M., Bittner, T., Jung, C. K. E., Burgold, S., Page, R. M., Mitteregger, G., et al. (2010). Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat. Neuroscience 13 (4), 411–413. doi:10.1038/nn.2511
Garden, G. A., and Möller, T. (2006). Microglia biology in health and disease. J. Neuroimmune Pharmacol. 1 (2), 127–137. doi:10.1007/s11481-006-9015-5
Gerrits, E., Brouwer, N., Kooistra, S. M., Woodbury, M. E., Vermeiren, Y., Lambourne, M., et al. (2021). Distinct amyloid-β and tau-associated microglia profiles in Alzheimer’s disease. Acta Neuropathol. 141 (5), 681–696. doi:10.1007/s00401-021-02263-w
Ginhoux, F., Greter, M., Leboeuf, M., Nandi, S., See, P., Gokhan, S., et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330 (6005), 841–845. doi:10.1126/science.1194637
González-Prieto, M., Gutiérrez, I. L., García-Bueno, B., Caso, J. R., Leza, J. C., Ortega-Hernández, A., et al. (2021). Microglial CX3CR1 production increases in Alzheimer’s disease and is regulated by noradrenaline. Glia 69 (1), 73–90. doi:10.1002/glia.23885
Goode-Romero, G., and Dominguez, L. (2022). Computational study of the conformational ensemble of CX3C chemokine receptor 1 (CX3CR1) and its interactions with antagonist and agonist ligands. J. Mol. Graph. Model. 117, 108278. doi:10.1016/j.jmgm.2022.108278
Gosselin, D., Skola, D., Coufal, N. G., Holtman, I. R., Schlachetzki, J. C. M., Sajti, E., et al. (2017). An environment-dependent transcriptional network specifies human microglia identity. Sci. (New York, N.Y.) 356 (6344), eaal3222. doi:10.1126/science.aal3222
Guedes, J. R., Lao, T., Cardoso, A. L., and El Khoury, J. (2018). Roles of microglial and monocyte chemokines and their receptors in regulating alzheimer’s disease-associated Amyloid-β and tau pathologies. Front. Neurology 9, 549. doi:10.3389/fneur.2018.00549
Guillot-Sestier, M.-V., Araiz, A. R., Mela, V., Gaban, A. S., O'Neill, E., Joshi, L., et al. (2021). Microglial metabolism is a pivotal factor in sexual dimorphism in Alzheimer’s disease. Commun. Biol. 4 (1), 711. doi:10.1038/s42003-021-02259-y
Guo, L., Behrendt, M., Bao, W., Lasky, G., Ryden-Markinhutha, K., Holmgren, G., et al. (2025). Abstract 4357315: AZD0233: a CX3CR1 modulator that regulates immune cells, improves cardiac function, and alleviates myocardial fibrosis in a mouse model of dilated cardiomyopathy. Circulation 152 (Suppl. l_3), A4357315. doi:10.1161/circ.152.suppl_3.4357315
Harms, A. S., Thome, A. D., Yan, Z., Schonhoff, A. M., Williams, G. P., Li, X., et al. (2018). Peripheral monocyte entry is required for alpha-Synuclein induced inflammation and neurodegeneration in a model of parkinson disease. Exp. Neurol. 300, 179–187. doi:10.1016/j.expneurol.2017.11.010
He, Z., Guo, J. L., McBride, J. D., Narasimhan, S., Kim, H., Changolkar, L., et al. (2018). Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med. 24 (1), 29–38. doi:10.1038/nm.4443
Hemonnot-Girard, A.-L., Valverde, A. J., Hua, J., Delaygue, C., Linck, N., Maurice, T., et al. (2021). Analysis of CX3CR1 haplodeficiency in male and female APPswe/PSEN1dE9 mice along alzheimer disease progression. Brain, Behav. Immun. 91, 404–417. doi:10.1016/j.bbi.2020.10.021
Hickman, S. E., Allison, E. K., Coleman, U., Kingery-Gallagher, D., and Khoury, J. E. (2019). Heterozygous CX3CR1 deficiency in microglia restores neuronal β-Amyloid clearance pathways and slows progression of Alzheimer’s like-disease in PS1-APP mice. Front Immunol. 10, 498281. doi:10.3389/fimmu.2019.02780
Honarpisheh, P., Lee, J., Banerjee, A., Blasco-Conesa, M. P., Honarpisheh, P., d'Aigle, J., et al. (2020). Potential caveats of putative microglia-specific markers for assessment of age-related cerebrovascular neuroinflammation. J. Neuroinflammation 17 (1), 366. doi:10.1186/s12974-020-02019-5
Jain, P., Chaney, A. M., Carlson, M. L., Jackson, I. M., Rao, A., and James, M. L. (2020). Neuroinflammation PET imaging: current opinion and future directions. J. Nucl. Med. 61 (8), 1107–1112. doi:10.2967/jnumed.119.229443
Janssen, B., Vugts, D. J., Windhorst, A. D., and Mach, R. H. (2018). PET imaging of microglial activation—beyond targeting TSPO. Molecules 23 (3), 607. doi:10.3390/molecules23030607
Joseph, E., Kunze, L. H., Schaefer, R., Palumbo, G., Kugelmann, B., Wagner, S., et al. (2025). Design, synthesis and preclinical evaluation of a brain-permeable PET tracer for P2Y12 receptor imaging in the brain. J. Med. Chem. 68 (15), 15543–15562. doi:10.1021/acs.jmedchem.5c00457
Kalinderi, K., Bostantjopoulou, S., Katsarou, Z., Clarimón, J., and Fidani, L. (2012). Lack of association between CX3CR1 V249I and T280M polymorphisms and risk of parkinson’s disease in a Greek population. Genet. Test. Mol. Biomarkers 16 (8), 974–977. doi:10.1089/gtmb.2011.0330
Karlström, S., Nordvall, G., Sohn, D., Hettman, A., Turek, D., Åhlin, K., et al. (2013). Substituted 7-Amino-5-thio-thiazolo[4,5- d ]pyrimidines as potent and selective antagonists of the fractalkine receptor (CX3 CR1). J. Med. Chem. 56 (8), 3177–3190. doi:10.1021/jm3012273
Kim, K.-W., Vallon-Eberhard, A., Zigmond, E., Farache, J., Shezen, E., Shakhar, G., et al. (2011). In vivo structure/function and expression analysis of the CX3C chemokine fractalkine. Blood 118 (22), e156–e167. doi:10.1182/blood-2011-04-348946
Kreisl, W. C., Kim, M. J., Coughlin, J. M., Henter, I. D., Owen, D. R., and Innis, R. B. (2020). PET imaging of neuroinflammation in neurological disorders. Lancet Neurology 19 (11), 940–950. doi:10.1016/S1474-4422(20)30346-X
Lai, S.-C., Wang, Y. H., Lu, C. Y., Huang, S. S., Chen, A. C., and Chen, K. M. (2025). CX3CL1/CX3CR1 involved modulation of microglial activation and eosinophilic meningoencephalitis in angiostrongyliasis. Food Waterborne Parasitol. 41, e00284. doi:10.1016/j.fawpar.2025.e00284
Langston, J. W., Forno, L. S., Tetrud, J., Reeves, A. G., Kaplan, J. A., and Karluk, D. (1999). Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann. Neurology 46 (4), 598–605. doi:10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f
Laws, K. R., Irvine, K., and Gale, T. M. (2016). Sex differences in cognitive impairment in Alzheimer’s disease. World J. Psychiatry 6 (1), 54–65. doi:10.5498/wjp.v6.i1.54
Lee, S., Varvel, N. H., Konerth, M. E., Xu, G., Cardona, A. E., Ransohoff, R. M., et al. (2010). CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am. Journal Pathology 177 (5), 2549–2562. doi:10.2353/ajpath.2010.100265
Lee, S., Xu, G., Jay, T. R., Bhatta, S., Kim, K. W., Jung, S., et al. (2014). Opposing effects of membrane-anchored CX3CL1 on amyloid and tau pathologies via the p38 MAPK pathway. J. Neurosci. 34 (37), 12538–12546. doi:10.1523/JNEUROSCI.0853-14.2014
Liu, Z., Condello, C., Schain, A., Harb, R., and Grutzendler, J. (2010). CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar Amyloid-β phagocytosis. J. Neurosci. 30 (50), 17091–17101. doi:10.1523/JNEUROSCI.4403-10.2010
Liu, J., Sun, Z., Liu, X., Chiu, K., Ma, L., and Wang, J. (2025). CX3CR1 upregulation modulates microglial activation and preserves synapses in the hippocampus and frontal cortex of middle-aged mice. Front. Aging 6, 1549848. doi:10.3389/fragi.2025.1549848
López-López, A., Gelpi, E., Lopategui, D. M., and Vidal-Taboada, J. M. (2018). Association of the CX3CR1-V249I variant with neurofibrillary pathology progression in late-onset alzheimer’s disease. Mol. Neurobiol. 55 (3), 2340–2349. doi:10.1007/s12035-017-0489-3
Lu, M., Zhao, W., Han, S., Lin, X., Xu, T., Tan, Q., et al. (2022). Activation of the human chemokine receptor CX3CR1 regulated by cholesterol. Sci. Adv. 8 (26), eabn8048. doi:10.1126/sciadv.abn8048
Luo, P., Chu, S. F., Zhang, Z., Xia, C. Y., and Chen, N. H. (2019). Fractalkine/CX3CR1 is involved in the cross-talk between neuron and glia in neurological diseases. Brain Res. Bull. 146, 12–21. doi:10.1016/j.brainresbull.2018.11.017
Maphis, N., Xu, G., Kokiko-Cochran, O. N., Jiang, S., Cardona, A., Ransohoff, R. M., et al. (2015). Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain 138 (6), 1738–1755. doi:10.1093/brain/awv081
McGeer, P. L., Itagaki, S., Boyes, B. E., and McGeer, E. G. (1988). Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38 (8), 1285–1291. doi:10.1212/WNL.38.8.1285
Meyer, J. (2025). “Human induced pluripotent stem cell-derived microglia with a CX3CR1-V249I genetic variant exhibit dysfunctional phenotypes and modulate neuronal growth and function.”
Mildner, A., Huang, H., Radke, J., Stenzel, W., and Priller, J. (2017). P2Y12 receptor is expressed on human microglia under physiological conditions throughout development and is sensitive to neuroinflammatory diseases: P2Y12 expression on human microglia. Glia 65 (2), 375–387. doi:10.1002/glia.23097
Mizoue, L. S., Bazan, J. F., Johnson, E. C., and Handel, T. M. (1999). Solution structure and dynamics of the CX3 C chemokine domain of fractalkine and its interaction with an N-Terminal fragment of CX3 CR1. Biochemistry 38 (5), 1402–1414. doi:10.1021/bi9820614
Nash, K. R., Moran, P., Finneran, D. J., Hudson, C., Robinson, J., Morgan, D., et al. (2015). Fractalkine over expression suppresses α-Synuclein-mediated neurodegeneration. Mol. Ther. 23 (1), 17–23. doi:10.1038/mt.2014.175
Nguyen, A. T., Wang, K., Hu, G., Wang, X., Miao, Z., Azevedo, J. A., et al. (2020). APOE and TREM2 regulate amyloid-responsive microglia in Alzheimer’s disease. Acta Neuropathol. 140 (4), 477–493. doi:10.1007/s00401-020-02200-3
Nutma, E., Fancy, N., Weinert, M., Tsartsalis, S., Marzin, M. C., Muirhead, R. C. J., et al. (2023). Translocator protein is a marker of activated microglia in rodent models but not human neurodegenerative diseases. Nat. Commun. 14 (1), 5247. doi:10.1038/s41467-023-40937-z
Piloto, J. V., Dias, R. V. R., Mazucato, W. S. A., Fossey, M. A., de Melo, F. A., Almeida, F. C. L., et al. (2024). Computational insights into the interaction of the conserved cysteine-noose domain of the human respiratory syncytial virus G protein with the canonical fractalkine binding site of transmembrane receptor CX3CR1 isoforms. Membranes 14 (4), 84. doi:10.3390/membranes14040084
Pissarek, M. (2020). Positron emission tomography in the inflamed cerebellum: addressing novel targets among G protein-coupled receptors and immune receptors. Pharmaceutics 12 (10), 925. doi:10.3390/pharmaceutics12100925
Puntambekar, S. S., Moutinho, M., Lin, P. B. C., Jadhav, V., Tumbleson-Brink, D., Balaji, A., et al. (2022). CX3CR1 deficiency aggravates amyloid driven neuronal pathology and cognitive decline in Alzheimer’s disease. Mol. Neurodegener. 17, 47. doi:10.1186/s13024-022-00545-9
Ridderstad Wollberg, A., Ericsson-Dahlstrand, A., Juréus, A., Ekerot, P., Simon, S., Nilsson, M., et al. (2014). Pharmacological inhibition of the chemokine receptor CX3CR1 attenuates disease in a chronic-relapsing rat model for multiple sclerosis. Proc. Natl. Acad. Sci. 111 (14), 5409–5414. doi:10.1073/pnas.1316510111
Sadeghdoust, M., Das, A., and Kaushik, D. K. (2024). Fueling neurodegeneration: metabolic insights into microglia functions. J. Neuroinflammation 21 (1), 300–318. doi:10.1186/s12974-024-03296-0
Schonhoff, A. M., Figge, D. A., Williams, G. P., Jurkuvenaite, A., Gallups, N. J., Childers, G. M., et al. (2023). Border-associated macrophages mediate the neuroinflammatory response in an alpha-synuclein model of parkinson disease. Nat. Commun. 14 (1), 3754–16. doi:10.1038/s41467-023-39060-w
Subbarayan, M. S., Joly-Amado, A., Bickford, P. C., and Nash, K. R. (2022). CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases. Pharmacol. and Ther. 231, 107989. doi:10.1016/j.pharmthera.2021.107989
Sun, M.-J., Liu, F., Zhao, Y. F., and Wu, X. A. (2020). In vivo positron emission tomography imaging of adenosine A2A receptors. Front. Pharmacol. 11, 599857. doi:10.3389/fphar.2020.599857
Suresh, P., Phasuk, S., and Liu, I. Y. (2021). Modulation of microglia activation and Alzheimer’s disease: CX3 chemokine ligand 1/CX3CR and P2X7R signaling. Tzu Chi Med. J. 33 (1), 1–6. doi:10.4103/tcmj.tcmj_144_20
Thome, A. D., Standaert, D. G., and Harms, A. S. (2015). Fractalkine signaling regulates the inflammatory response in an α-Synuclein model of parkinson disease. PLOS ONE 10 (10), e0140566. doi:10.1371/journal.pone.0140566
Tristão, F. S. M., Lazzarini, M., Martin, S., Amar, M., Stühmer, W., Kirchhoff, F., et al. (2016). CX3CR1 disruption differentially influences dopaminergic neuron degeneration in parkinsonian mice depending on the neurotoxin and route of administration. Neurotox. Res. 29 (3), 364–380. doi:10.1007/s12640-015-9557-5
Vida, H., Sahar, M., Nikdouz, A., and Arezoo, H. (2025). Chemokines in neurodegenerative diseases. Immunol. and Cell Biol. 103 (3), 275–292. doi:10.1111/imcb.12843
Walker, D. G., Tang, T. M., Mendsaikhan, A., Tooyama, I., Serrano, G. E., Sue, L. I., et al. (2020). Patterns of expression of purinergic receptor P2RY12, a putative marker for non-activated microglia, in aged and alzheimer’s disease brains. Int. J. Mol. Sci. 21 (2), 678. doi:10.3390/ijms21020678
Wang, L., Liu, Y., Yan, S., Du, T., Fu, X., Gong, X., et al. (2020). Disease progression-dependent expression of CD200R1 and CX3CR1 in mouse models of parkinson’s disease. Aging Disease 11 (2), 254–268. doi:10.14336/AD.2019.0615
Wang, J., Qu, C., Xiao, P., Liu, S., Sun, J. P., and Ping, Y. Q. (2025). Progress in structure-based drug development targeting chemokine receptors. Front. Pharmacol. 16, 1603950. doi:10.3389/fphar.2025.1603950
Wolf, Y., Yona, S., Kim, K. W., and Jung, S. (2013). Microglia, seen from the CX3CR1 angle. Front. Cell. Neurosci. 7, 26. doi:10.3389/fncel.2013.00026
Yang, D., Zhou, Q., Labroska, V., Qin, S., Darbalaei, S., Wu, Y., et al. (2021). G protein-coupled receptors: Structure- and function-based drug discovery. Signal Transduct. Target. Ther. 6 (1), 7. doi:10.1038/s41392-020-00435-w
Yao, B., Kong, Y., Li, J., Xu, F., Deng, Y., Chen, Y., et al. (2025). Synthesis, preclinical evaluation and pilot clinical study of a P2Y12 receptor targeting radiotracer [18F]QTFT for imaging brain disorders by visualizing anti-inflammatory microglia. Acta Pharm. Sin. B 15 (2), 1056–1069. doi:10.1016/j.apsb.2025.01.009
Yokoyama, M., Kobayashi, H., Tatsumi, L., and Tomita, T. (2022). Mouse models of alzheimer’s disease. Front. Mol. Neurosci. 15, 912995. doi:10.3389/fnmol.2022.912995
Zhao, J., Li, Q., Ouyang, X., Wang, F., Li, Q., Xu, Z., et al. (2023). The effect of CX3CL1/CX3CR1 signal axis on microglia in central nervous system diseases. J. Neurorestoratology 11 (1), 100042. doi:10.1016/j.jnrt.2023.100042
Zhong M. Z, M. Z., Peng, T., Duarte, M. L., Wang, M., and Cai, D. (2024). Updates on mouse models of Alzheimer’s disease. Mol. Neurodegener. 19 (1), 23–33. doi:10.1186/s13024-024-00712-0
Keywords: microglia, CX3CR1, Alzheimer’s disease, Parkinson’s disease, positron emission tomography tracers
Citation: Yang H, Wang Y, Xu Y and Wang C (2025) CX3CR1: a potential microglia-specific PET imaging target in Alzheimer’s and Parkinson’s diseases. Front. Pharmacol. 16:1678159. doi: 10.3389/fphar.2025.1678159
Received: 01 August 2025; Accepted: 19 November 2025;
Published: 04 December 2025.
Edited by:
Harish Darasaguppe Ramachandra, Indian Council of Medical Research, IndiaReviewed by:
Vishal Patil, KLE Academy of Higher Education and Research, IndiaJagdish Chand, JSS Academy of Higher Education and Research, India
Chang Liu, Emory University School of Medicine, United States
Copyright © 2025 Yang, Wang, Xu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changning Wang, Y3dhbmcxNUBtZ2guaGFydmFyZC5lZHU=
 Hongzhi Yang
Hongzhi Yang Yanli Wang
Yanli Wang Yulong Xu
Yulong Xu Changning Wang
Changning Wang