- 1National Drug Clinical Trial Institution of West China Second University Hospital, Sichuan University, Chengdu, China
- 2NMPA Key Laboratory for Technical Research on Drug Products In Vitro and In Vivo Correlation, Chengdu, China
- 3Key Laboratory of Birth Defects and Related Diseases of Women and Children, Sichuan University, Ministry of Education, Chengdu, China
- 4Chengdu Jingze Biopharmaceutical Co., Ltd., Chengdu Cross-Strait Science and Technology Industrial Development Park, Chengdu, China
Objective: This study aimed to evaluate the pharmacokinetic (PK) bioequivalence, safety, and immunogenicity of recombinant human follicle-stimulating hormone (rhFSH) for injection (code name: JZB30), developed by Chengdu Jingze Biopharmaceutical Co., Ltd., in comparison with Gonal-f® developed by Merck Serono in healthy adult Chinese female participants.
Methods: single-center, randomized, open-label, two-period crossover study for bioequivalence assessment enrolled 48 healthy adult female participants, who were equally and randomly assigned to two treatment sequences. Each participant received a single subcutaneous injection of either JZB30 or Gonal-f® at a dose of 225 IU on Day 1 and again on Day 11 as part of the crossover design. Blood concentrations of FSH were measured using a validated electrochemiluminescence immunoassay, followed by non-compartmental PK analysis. Safety and immunogenicity were systematically monitored throughout the study period, including adverse events and anti-FSH antibody testing.
Results: The geometric mean ratios (GMRs) of the primary PK parameters—Cmax, AUC0-t, and
Conclusion: JZB30 was demonstrated to be pharmacokinetically bioequivalent to Gonal-f® in healthy adult Chinese female participants, with comparable safety profiles and a low risk of immunogenicity. These findings provide evidence to support the clinical application of JZB30 as a biosimilar to Gonal-f®.
Clinical Trial Registration: The trial was registered on ClinicalTrials.gov under identifier NCT06778304.
1 Introduction
Assisted reproductive technology (ART) represents a critical strategy for managing female infertility, with controlled ovarian stimulation serving as a central component. Follicle-stimulating hormone (FSH) plays an essential role in this process by pharmacologically inducing the development and maturation of multiple ovarian follicles. FSH, a glycoprotein hormone secreted by the anterior pituitary gland, acts synergistically with luteinizing hormone (LH) to promote oocyte maturation in females. FSH is critical for follicular growth, which provides the structural and functional environment for oocyte development and maturation. It is also essential for triggering ovulation, steroid hormone synthesis in females, and spermatogenesis in males.Gonal-f®, a recombinant human follicle-stimulating hormone (rhFSH) injection developed by Merck Serono, has been widely used in ART since its approval in the United States in 1998, owing to its high purity and stability. JZB30, developed by Chengdu Jingze Biopharmaceutical Co., Ltd., shares the same active ingredient, formulation, specifications, indications, administration route, and dosage regimen as Gonal-f®. This study aims to evaluate the pharmacokinetics, safety, and immunogenicity of JZB30 compared with Gonal-f® in healthy adult Chinese female participants through a single-center, randomized, open-label, two-period, crossover bioequivalence trial, thereby providing evidence to support its rational clinical application.
2 Methods
2.1 Participants
This study was conducted at the Clinical Research Center of West China Second University Hospital of Sichuan University, Chengdu, Sichuan Province, China, from January 2021 to January 2022. All participants provided written informed consent. The main inclusion criteria were: healthy female participants aged 18–45 years (inclusive), body weight ≥45 kg, and body mass index (BMI) between 18 and 28 kg/m2;having a regular menstrual cycle length of 25–34 days (inclusive); sexually active but not seeking pregnancy; normal or clinically insignificant abnormalities in medical history, physical examination, laboratory tests, and gynecological examinations (including uterus and ovaries); normal sex hormone levels or deemed clinically insignificant by the investigator; voluntary participation in the clinical trial, understanding of the study procedures, and signed informed consent. The main exclusion criteria included: polycystic ovary syndrome; a history of ovarian hyperstimulation syndrome (OHSS); allergy to FSH or known allergy to gonadotropin-releasing hormone agonists (GnRH-a) or their analogs; clinically significant abnormalities in the history of ovarian, breast, uterine, hypothalamic, or pituitary diseases; history or current thromboembolic disease; use of hormonal contraceptives (oral short-acting contraceptives within 3 months or long-acting contraceptives within 6 months before screening); drug abuse; blood donation or loss of ≥400 mL within 3 months prior to screening.
2.2 Study design
This study was approved by the Ethics Committee of West China Second University Hospital of Sichuan University (approval number Y2020035). The study adhered to the Declaration of Helsinki (World Medical Association, 2013), the principles of Good Clinical Practice (National Medical Products Administration and National Health Commission of the People’s Republic of China, 2020), and all relevant Chinese laws and regulations.
Based on the bioequivalence studies of Gonal-f® biosimilars Ovaleap® (XM17) (Lammerich et al., 2015) and Bemfola® (Wolzt et al., 2016) in Europe, and the clinical use of Gonal-f® in China, the study design followed the European Medicines Agency (EMA) guidelines (European Medicines Agency EMA, 2013) for rhFSH biosimilar and the Chinese Center for Drug Evaluation (CDE) guideline (National Medical Products Administration NMPA, 2015). This Phase I study was a single-center, randomized, open-label, two-period, crossover, single-dose trial assessing the bioequivalence of two rhFSH formulations. A 225 IU (16.5 μg) subcutaneous injection was administered in the umbilical region (3–10 cm from the navel).
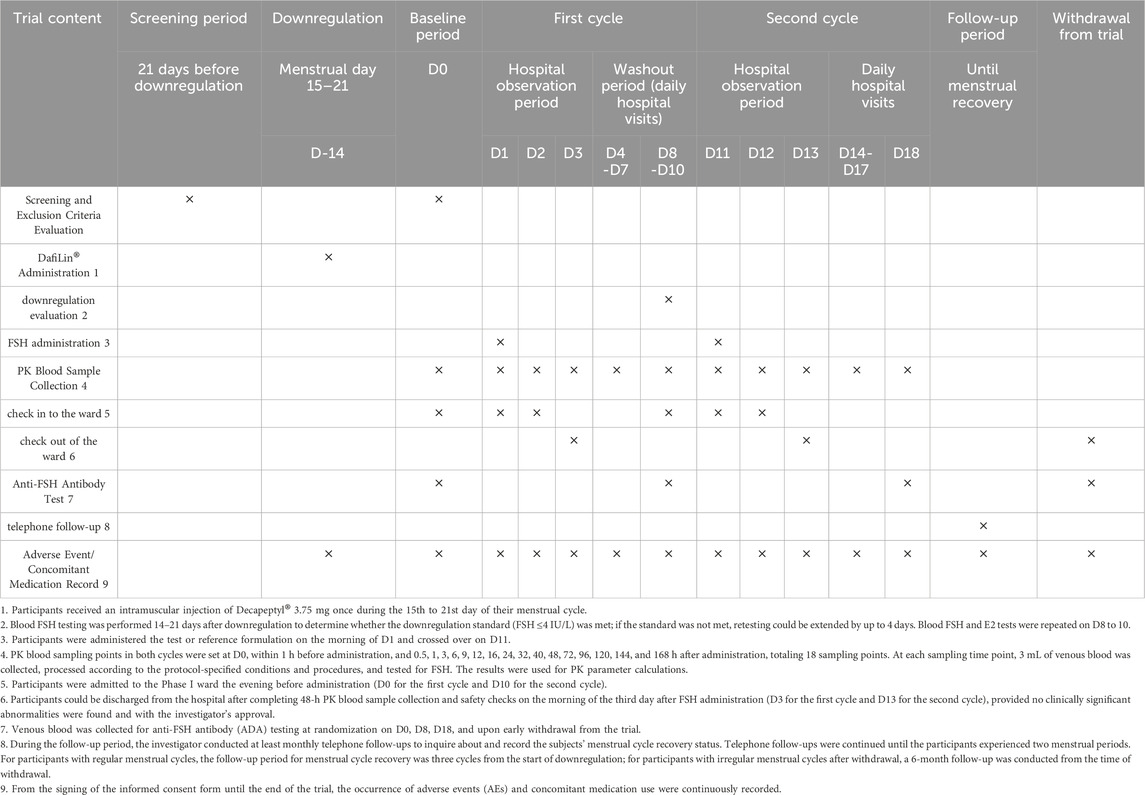
We planned to enroll 48 healthy adult female participants. Screening was conducted over 21 days. Eligible participants entered the downregulation phase. On Day −14 (days 15–21 of the menstrual cycle), participants received a single intramuscular injection of triptorelin acetate for injection (Decapeptyl®) 3.75 mg. This was performed to achieve pituitary downregulation, suppressing the secretion of endogenous FSH and thus minimizing its interference on the PK assessment of the exogenous rhFSH formulations. On Day 0, PK blood samples and serum FSH levels were collected (if the downregulation requirement was not met, retesting could be extended up to 4 days). If the participant met the downregulation requirement (FSH ≤4 IU/L) (Lammerich et al., 2015), the inclusion/exclusion criteria were re-evaluated. Those who still met the criteria were randomly assigned to Group A (T-R group) or Group B (R-T group) and underwent baseline assessments. If the participant did not meet the downregulation requirement, she was excluded from the study.
On Day 1, participants received a single subcutaneous injection of the test drug or reference drug (injection site: 3–10 cm around the umbilicus) at a dose of 225 IU. Participants were required to fast for 10 h before and 2 h after each dose in both periods. After an 8–10-day washout period, the second period of crossover dosing began on Day 11. Blood samples for PK analysis were collected at specified time points (see Section 2.3 Sample collection and analysis).
Clinical safety assessments were conducted throughout the trial. Vital signs were monitored daily from the screening period to Day 18 and at the end of the trial. Physical examinations and 12-lead electrocardiograms were performed at screening, Day 0, Day 18, and at the end of the trial (cover both the scheduled end-of-study visit and unscheduled follow-up visits triggered by special events, such as a positive antibody result). Routine blood tests, urinalysis, biochemical tests, and coagulation function tests were conducted at screening, Day 18, and at the end of the trial. Venous blood samples (3 mL) for anti-FSH antibody testing were collected at screening, Day 0, Day 8, Day 18, and at the end of the trial. Adverse events (AEs) were monitored and recorded throughout the trial, with follow-up conducted until resolution of any AE. The detailed procedure is shown in Table 1.
2.3 Sample collection and analysis
Blood sampling for PK analysis was conducted at the following time points: pre-dose (1 day prior and within 1 h before dosing), and 0.5, 1, 3, 6, 9, 12, 16, 24, 32, 40, 48, 72, 96, 120, 144, and 168 h post-dose, totaling 18 time points. Anti-drug antibody (ADA) samples were obtained pre-dose on Day 0, at 168 h post-dose in the first period (Day 8), at 168 h post-dose in the second period (Day 18), and at the end of the trial. At each time point, 3 mL of venous blood was collected in a clot-activator tube. If a venous indwelling needle was used, approximately 0.5 mL of blood was discarded prior to sampling to avoid contamination, and the catheter was flushed with saline after each collection. Blood samples were left to stand upright for 30 min, then centrifuged at 2,500 g for 10 min at 4 °C. The separated serum was stored at −90 °C ∼ -60 °C in ultra-low temperature freezers until PK analysis.
The serum concentration of FSH was determined using a validated electrochemiluminescence method based on the Meso Scale Discovery (MSD) platform, with two analytical linear ranges: 160 pg/mL to 16,200 pg/mL and 134 pg/mL to 4,710 pg/mL.
2.4 PK analysis
PK parameters were analyzed using Phoenix™ WinNonlin® software (version 8.3) with a non-compartmental analysis model. The primary PK parameters included Cmax, AUC0-t, and
2.5 Safety assessment
Safety was evaluated throughout the trial, including spontaneously reported or directly observed (AEs, abnormalities in vital signs, physical examinations, and 12-lead electrocardiograms, and clinically significant laboratory test abnormalities. ADA testing was also included in the safety assessment.
2.6 Sample size and statistical analysis
According to the EMA guidelines on nonclinical and clinical development of rhFSH-containing biosimilars, healthy female participants should be chosen for clinical PK comparison studies, and the sample size should ensure sufficient statistical power for bioequivalence evaluation. Considering the geometric mean ratio (GMR) of the test and reference formulations was 1.03, the estimated within-subject coefficient of variation (CV) was 30%, and the power (1-β) was 90%, the required sample size was calculated as 44 participants using a 2 × 2 crossover design. Assuming a 10% dropout rate during the trial, 48 participants were enrolled.
Bioequivalence evaluation was performed using SAS® 9.4 software. Statistical analyses were conducted on the PK parameters AUC0-t,
If the 90% confidence intervals of the GMRs of the PK parameters (AUC0-t,
FSH is an endogenous substance that plays important physiological roles in the body. Its secretion varies cyclically and is influenced by circadian rhythms and individual differences. The primary objective of this study was to compare the PK parameters and evaluate the bioequivalence between the test and reference formulations. Therefore, adjustments or removal of non-drug factors that could affect the bioequivalence evaluation were made. This trial employed downregulation treatment using a GnRH-a, as described in Section 2.2, to suppress endogenous FSH levels and establish a stable baseline for bioequivalence evaluation.
Sensitivity analysis was used to assess the sensitivity of the results to certain parameter changes. PK parameters and bioequivalence statistical analyses were performed on uncorrected FSH concentrations as a sensitivity analysis to explore the impact of baseline FSH on the results.
3 Results
3.1 Participants’ demographics and baseline characteristics
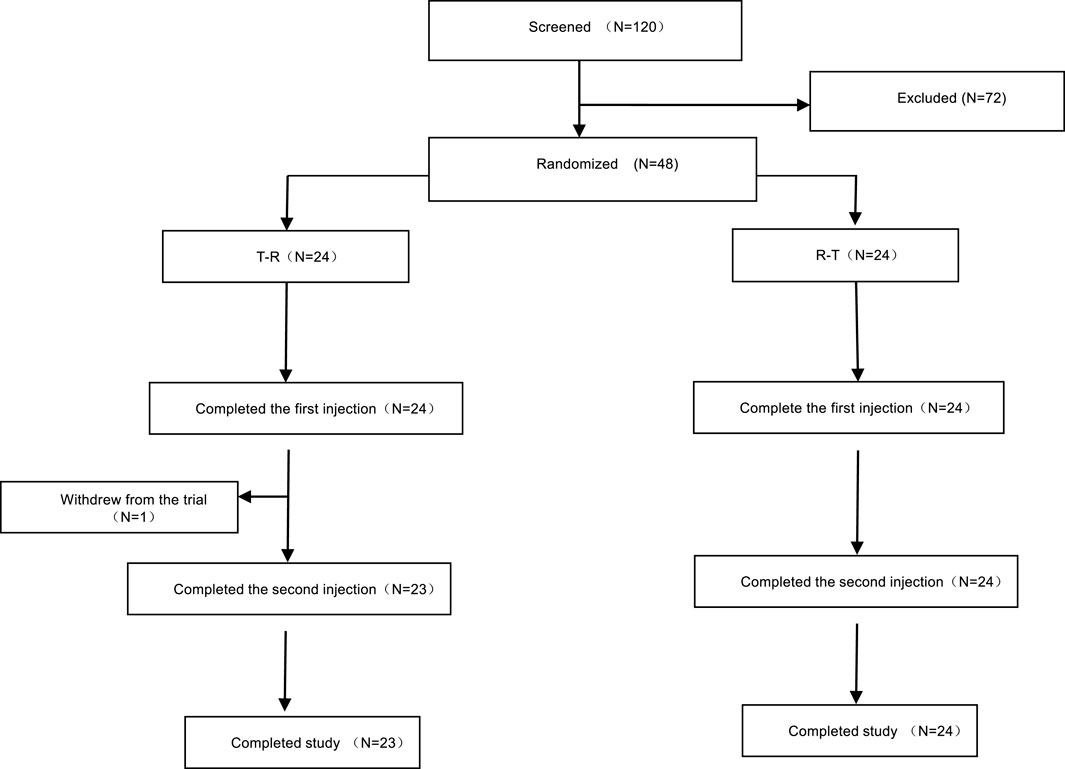
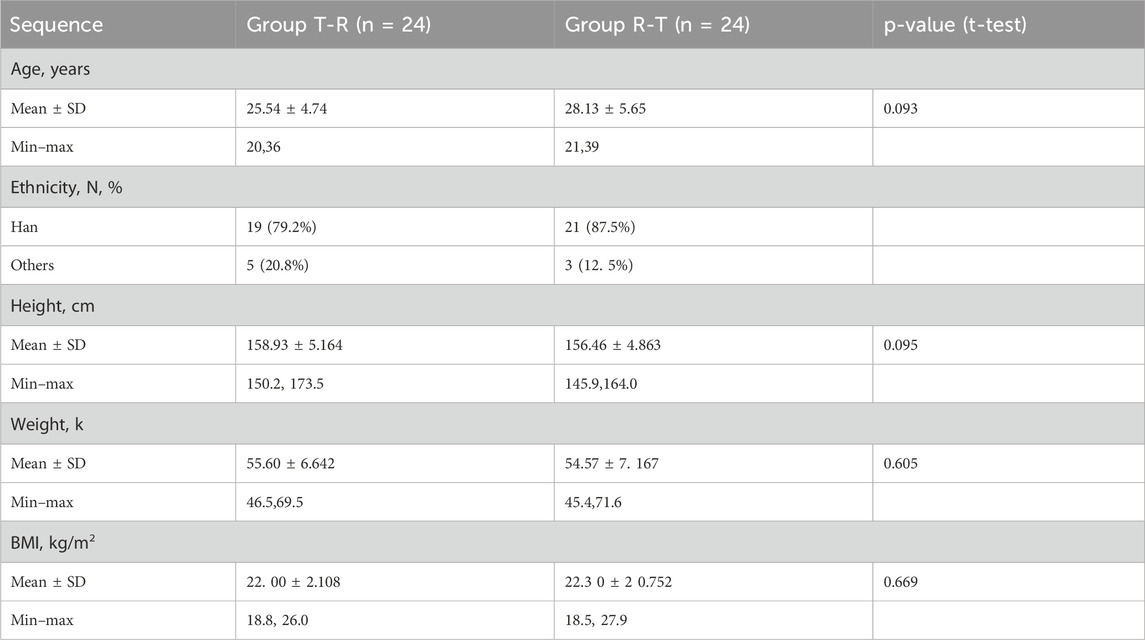
A total of 120 participants were screened, of whom 72 were excluded, and 48 were enrolled and randomly assigned to treatment groups. Group A (T-R sequence) and Group B (R-T sequence) each enrolled 24 participants. All participants in Group B completed the trial, whereas one participant (R038) in Group A withdrew early, and the remaining 23 participants in Group A completed the trial. Details are shown in Figure 1. Demographic and baseline characteristics are summarized in Table 2 and were well balanced between the two groups.
3.2 PK properties
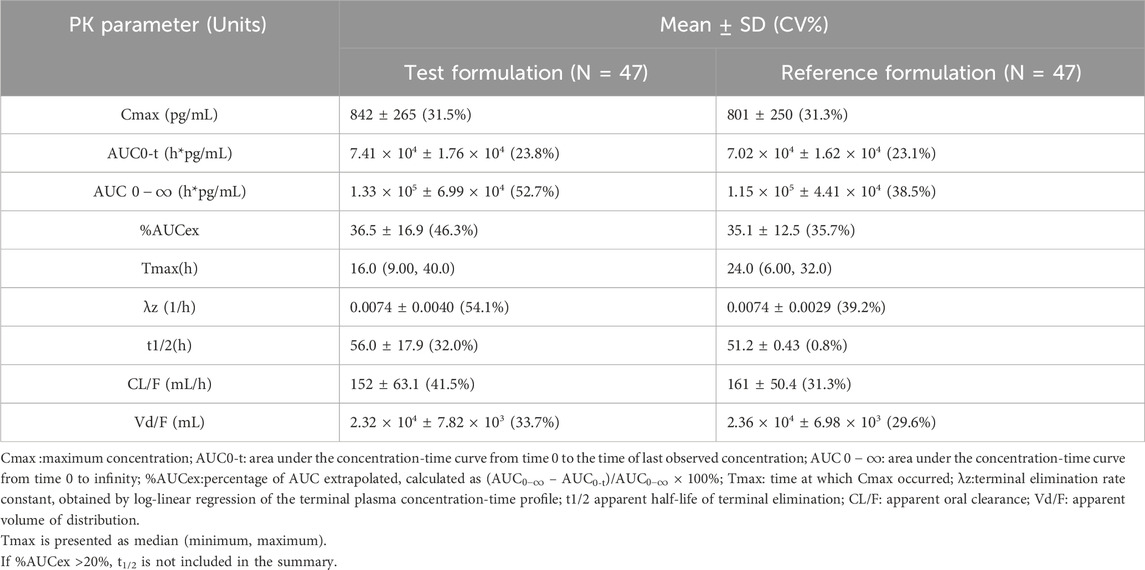
Following administration, the mean serum concentrations of the test and reference formulations gradually increased, reached a peak, and then slowly declined, demonstrating comparable PK profiles (Figure 2). PK parameters included Cmax, AUC0-t,
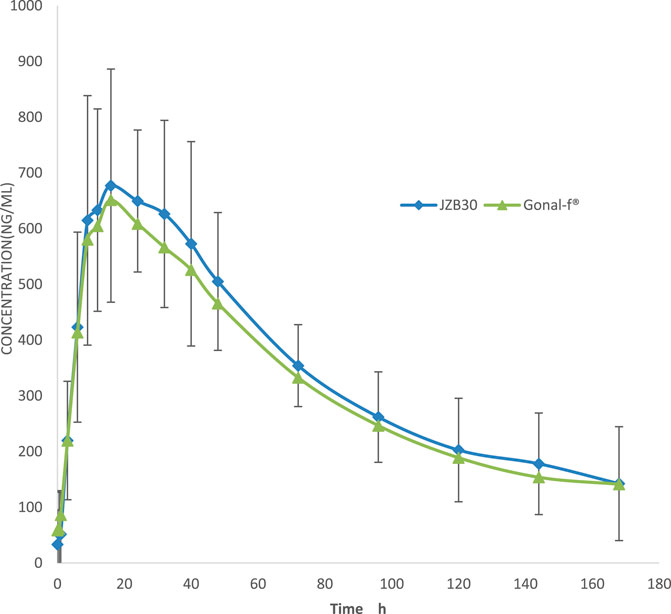
Figure 2. Serum concentration–time curves. The mean (±SD) serum concentration–time curves of JZB30 and Gonal-f®.
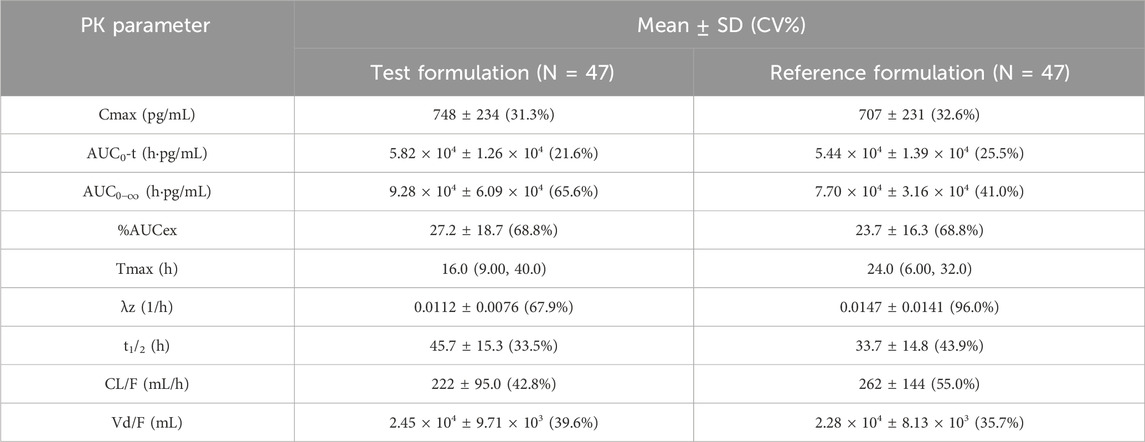
Table 4. PK parameters of recombinant human follicle-stimulating hormone injection after baseline correction.
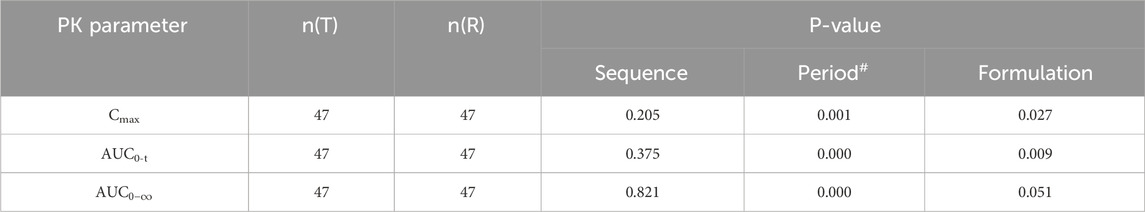
Table 5. ANOVA results for major PK parameters of recombinant human follicle stimulating hormone injection.
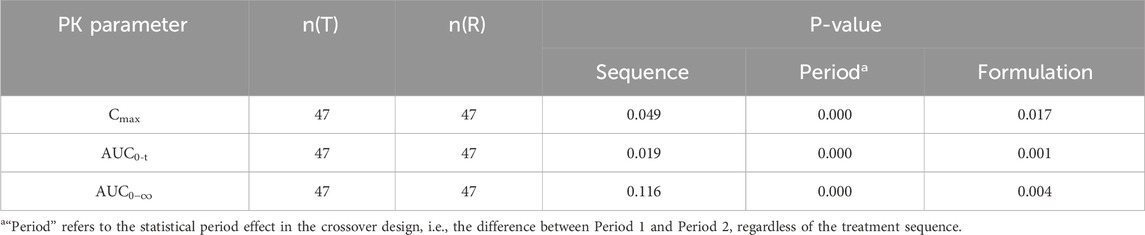
Table 6. ANOVA results for baseline-corrected major PK parameters of recombinant human follicle stimulating hormone injection.
3.3 Bioequivalence assessment
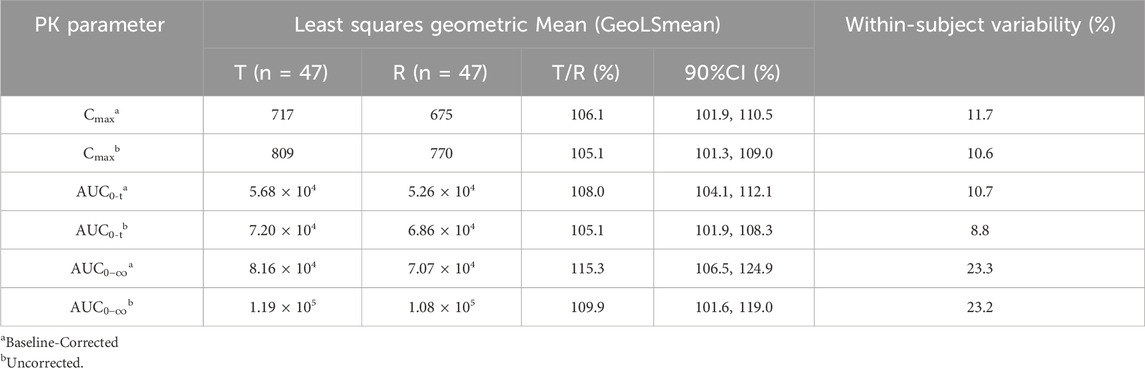
Based on the bioequivalence analysis set (BES, N = 47), the primary PK parameters (Cmax, AUC0-t,
The GMR of Cmax was 106.12%; the GMR of AUC0-t was 108.04%; and the GMR of AUC
Sensitivity analysis was performed on uncorrected FSH concentrations, and the results are shown in Table 7. The sensitivity analysis results were consistent with the main analysis results, indicating the robustness of the bioequivalence conclusion.
3.4 Safety assessment
Safety analysis was conducted only on adverse events occurring after the administration of the test or reference formulations, in accordance with the protocol. Therefore, the safety analysis population included 48 participants who received the test or reference formulations, and safety events in four participants who only received Decapeptyl were reported separately. All adverse events observed during the trial were systematically assessed for causality in relation to the investigational products (JZB30 or Gonal-f®). This causality assessment was conducted by the investigators based on temporal relationship, known pharmacological effects of rhFSH, and clinical judgment. Some adverse events were assessed as “unrelated” to the study drugs. These included common non-specific events such as High Blood Triglyceride levels and elevated blood creatine phosphokinase. In contrast, events related to the reproductive system (e.g., vaginal bleeding) were assessed as “possibly related” to the study drugs. This classification was based on the known pharmacological action of FSH in stimulating ovarian activity. It is important to emphasize that even these “possibly related” events were mild (Grade 1), transient, and resolved spontaneously without any medical intervention.
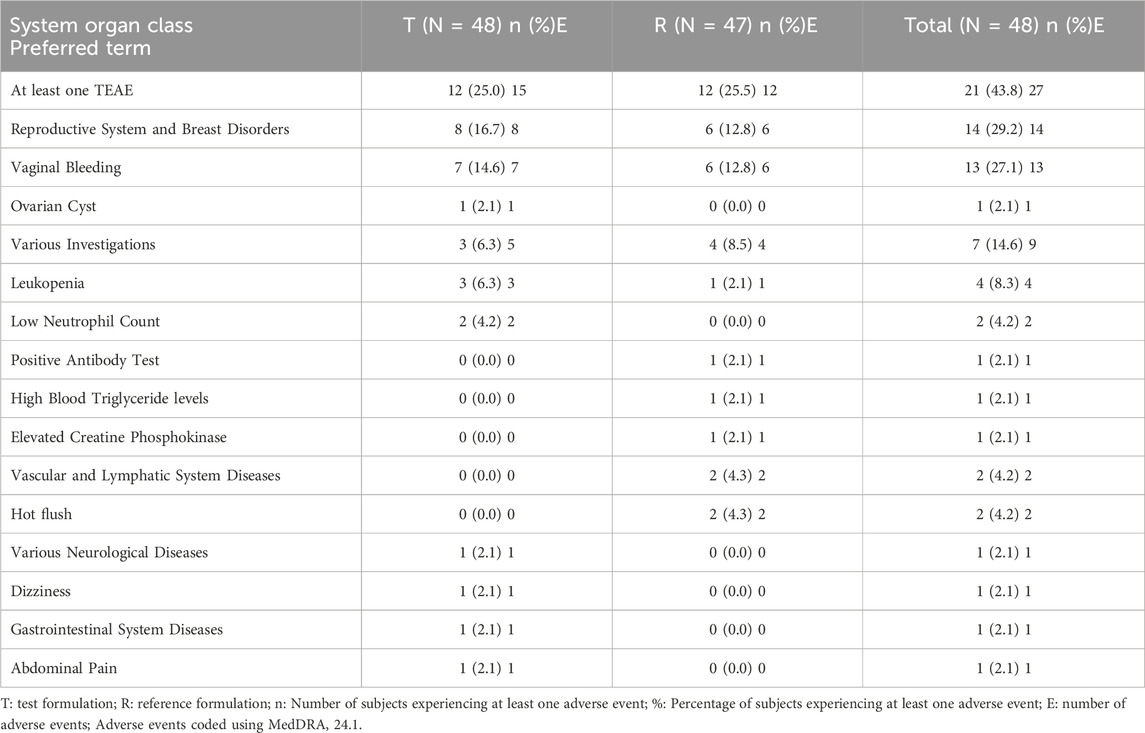
Safety analysis results showed that 21 participants experienced 27 adverse events, with an overall adverse event incidence of 43.8%. Among those receiving the test formulation, 12 participants experienced 15 adverse events, with an adverse event incidence of 25.0% and an adverse reaction incidence of 20.8%. Among those receiving the reference formulation, 12 participants experienced 12 adverse events, with an adverse event incidence of 25.5% and an adverse reaction incidence of 17.0%. The incidence and severity of adverse events were comparable between the test and reference formulations.
No adverse events led to withdrawal, no serious adverse events occurred, and no adverse events were graded as three or higher. Adverse events by system organ class and preferred term for T and R are presented in Table 8
3.5 Immunogenicity assessment
48 participants were enrolled in the trial. One participant (R038, T-R) withdrew from the study 6 h after the first dose, having only provided a baseline ADA sample. The remaining 47 participants completed the two dosing periods and provided ADA samples at baseline, Day 8, and Day 18.
One participant (R044, T-R) tested positive for anti-FSH antibodies at Day 18. No anti-FSH antibodies were detected after administration of the test formulation, and only one participant [1/47] tested positive after receiving the reference formulation. The antibody response turned negative after approximately 6 months without any intervention. These results indicate that the drug exhibited low immunogenicity in the majority of participants.
4 Discussion
4.1 FSH endogenous characteristics
FSH is an endogenously produced substance. In the bioequivalence studies of Gonal-f® biosimilars Ovaleap® and Bemfola®, gonadotropin-releasing hormone agonists (GnRH-a) were used to downregulate endogenous FSH to prevent individual variations in endogenous FSH secretion from affecting the bioequivalence evaluation. GnRH-a acts by continuously stimulating GnRH receptors, leading to receptor downregulation and subsequent suppression of pituitary gonadotropin secretion, including FSH and LH. This mechanism effectively reduces endogenous hormone levels, providing a stable baseline for PK assessment.
According to the “Expert Consensus on Ovulation Induction Drugs in Assisted Reproduction” (Le Contonnec et al., 1994) published by the Chinese Society of Reproductive Medicine in 2015, GnRH-a can be used to downregulate FSH and LH in controlled ovarian stimulation (COS) protocols. After 7–14 days of GnRH-a administration, drug-induced pituitary-ovarian suppression is achieved, reducing endogenous FSH and LH secretion to low levels. The 14-day time point was chosen for downregulation evaluation because it corresponds to the period when GnRH-a typically reaches its maximal suppressive effect. In this study, 3.75 mg of triptorelin acetate for injection (Decapeptyl®) was administered 14 days before the first dose to achieve downregulation. On Day 0, participants were evaluated for downregulation, and those meeting the requirement (FSH ≤4 IU/L) proceeded to the subsequent dosing of the test and reference formulations. The threshold of FSH≤ 4 IU/L, which has been applied in previous pivotal bioequivalence studies of rhFSH biosimilars (Lammerich et al., 2015) (Lugan et al., 2005) indicates effective suppression of endogenous FSH, minimizing individual hormonal fluctuations that could confound PK and bioequivalence evaluations.
4.2 Dose selection rationality
According to the PK study of Ovaleap®, the linear PK range for a single-dose subcutaneous injection of rhFSH spans at least from 37.5 to 300 IU. The “Expert Consensus on Ovulation Induction Drugs in Assisted Reproduction” published by the Chinese Society of Reproductive Medicine in 2015 recommends that the maximum daily dose of FSH in ovulation induction (OI) protocols should not exceed 225 IU, while the initial dose range in COS protocols is 112.5–300 IU/day. According to the EMA guidelines for the nonclinical and clinical development of rhFSH-containing biosimilars, a dose within the linear portion of the dose-response curve should be selected to sensitively detect potential differences. In our study, PK parameters were used as endpoints to evaluate the bioequivalence between the test and reference formulations, with the linear PK range serving as a partial surrogate for the linear dose-response range. Considering the product specification of 5.5 μg (75 IU) and the clinical dosing practices, including the maximum dose used in China, a dose of 225 IU was chosen for this study. Therefore, a single subcutaneous injection of 225 IU was administered as the dosing regimen.
4.3 Data handling method and its impact
In bioequivalence studies, the choice of data handling methods is crucial for the accuracy and reliability of the results (Dis and sanayake, 2010). FSH is an endogenous hormone whose circulating levels exhibit natural fluctuations influenced by the menstrual cycle, circadian rhythms, and individual physiological differences. Although downregulation was successful (FSH ≤4 IU/L), low levels of endogenous FSH persist and demonstrate minor intra- and inter-individual variations. Baseline correction is performed precisely to isolate the PK signal attributable solely to the injected drug from this residual background “noise.” Baseline correction was performed for the bioequivalence (BE) studies of the Gonal-f® biosimilars Ovaleap® and Bemfola®. Although baseline correction can improve statistical power, in some cases, uncorrected data analysis may already provide reliable conclusions. When correction methods are complex or not applicable, using uncorrected data may be more reasonable. In this study, baseline-corrected data were used for evaluation, and sensitivity analysis was performed using uncorrected data to assess the impact of baseline correction on the study results. This approach allowed a comprehensive assessment of the similarity between the biosimilar and the reference drug, leading to more robust conclusions.
Variance analysis was conducted on sequence, period, and formulation factors to evaluate their impact on the primary PK parameters (Cmax, AUC0-t). Before baseline correction, period and formulation had statistically significant effects on Cmax and AUC0-t (P < 0.05), indicating these factors significantly affected the PK parameters without correction. The formal statistical test for carryover effects, manifested as the sequence effect in the ANOVA, demonstrated no significant sequence effect for any primary PK parameter in the uncorrected data (all P > 0.05, Table 5). This provides key statistical evidence against the presence of a clinically meaningful carryover effect. Although the sequence factor reached nominal significance for some parameters after baseline correction, this is more likely attributable to a chance imbalance in baseline FSH levels between the sequence groups, an difference that was accentuated by the correction procedure itself. This phenomenon may be related to natural fluctuations in follicle-stimulating hormone (FSH) levels, which vary over time and physiological status, especially during the menstrual cycle in women. Therefore, when designing the dosing sequence, it is essential to consider these natural fluctuations to minimize their impact on trial results. The inclusion criteria for this study specified a menstrual cycle length of 25–34 days (inclusive) and scheduled downregulation between days 15 and 21 of the menstrual cycle. This design aimed to standardize the downregulation process and reduce interference from natural fluctuations in endogenous FSH levels on the trial outcomes.
4.4 Immunogenicity assessment
Immunogenicity assessment in this study revealed a single, transient anti-FSH antibody positive case following administration of the reference product, which reverted to negative spontaneously during the approximately 6-month follow-up period without associated PK alterations or clinical adverse events. This finding aligns with the well-documented low immunogenicity profile of marketed rhFSH products, largely attributable to their high structural homology with endogenous FSH.
Although the theoretical risk exists that persistent neutralizing antibodies could impact the bioactivity and efficacy of exogenous FSH, accumulated clinical evidence suggests that antibody events associated with rhFSH are predominantly transient and non-neutralizing. The isolated event in our study did not impact the bioequivalence conclusion, underscoring its likely limited clinical relevance. Considering the demonstrated PK bioequivalence, comparable safety profile, and this self-limiting immunogenicity event between JZB30 and the reference product in healthy women, JZB30 exhibits a similarly low immunogenicity risk profile.
5 Conclusion
This study demonstrates that JZB30 is bioequivalent to Gonal-f® in healthy adult Chinese female participants, with a comparable safety profile and a low incidence of immunogenicity in the short term. It is important to note that the immunogenicity data were derived from a Phase I study with limited dosing and observation period. The final confirmation of the immunogenicity profile of JZB30 will require evaluation in the target patient population undergoing multiple treatment cycles in longer-term Phase III clinical studies.These findings provide strong scientific support for the clinical application of JZB30 as a biosimilar to Gonal-f®.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethics Committee of the West China Second Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZC: Writing – original draft, Writing – review and editing, Formal Analysis, Investigation, Methodology. QY: Conceptualization, Project administration, Writing – review and editing. SF: Formal Analysis, Project administration, Writing – review and editing, Supervision. LM: Funding acquisition, Resources, Writing – review and editing. LC: Data curation, Investigation, Writing – review and editing. CD: Data curation, Resources, Validation, Writing – review and editing. DD: Data curation, Validation, Writing – review and editing. QZ: Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors declare that this study received funding from Chengdu Jingze Biopharmaceutical Co., Ltd. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
Author LM was employed by Chengdu Jingze Biopharmaceutical Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Dissanayake, S. (2010). Assessing the bioequivalence of analogues of endogenous substances ('endogenous drugs'): considerations to optimize study design. Br. J. Clin. Pharmacol. 69 (3), 238–244. doi:10.1111/j.1365-2125.2009.03585.x
European Medicines Agency (EMA) (2013). Guideline on non-clinical and clinical development of similar biological medicinal products containing recombinant human follicle-stimulating hormone (rhFSH).
Lammerich, A., Mueller, A., and Bias, P. (2015). Phase I, two-way, crossover study to demonstrate bioequivalence and to compare safety and tolerability of single-dose XM17 vs Gonal-f® in healthy women after follicle-stimulating hormone downregulation. Reproductive Biol. Endocrinol. 13 (1), 130–138. doi:10.1186/s12958-015-0124-y
Le Contonnec, J. Y., Porchet, H. C., Beltrami, V., Khan, A., Toon, S., and Rowland, M. (1994). Clinical pharmacology of recombinant human follicle-stimulating hormone. II. Single doses and steady state pharmacokinetics. Fertil. Steril. 61 (4), 679–686. doi:10.1016/s0015-0282(16)56645-x
Lugan, I., Febbraro, S., Lecuelle, H., Papasouliotis, O., Ho-Nguyena, Q., and Buraglio, M. (2005). Bioequivalence of liquid and freeze-dried recombinant human follicle-stimulating hormone. Curr. Med. Res. Opin. 21, 121–125. doi:10.1185/030079904x18027
National Medical Products Administration (NMPA) (2015). Technical guidelines for the development and evaluation of biosimilar medicinal products (trial). Available online at: https://www.cde.org.cn/zdyz/downloadAtt?idCODE=5097ae71b10504a18ef6c90c7a6969ab.
Keywords: recombinant human follicle-stimulating hormone, pharmacokinetics, bioequivalence, safety, biosimilar
Citation: Chen Z, Yu Q, Feng S, Mo L, Cai L, Du C, Du D and Zou Q (2025) A single-center, randomized, open-label, two-period, crossover, fasted bioequivalence study: comparing recombinant human follicle-stimulating hormone for injection (JZB30) with Gonal-f® in healthy Chinese female participants. Front. Pharmacol. 16:1678830. doi: 10.3389/fphar.2025.1678830
Received: 03 August 2025; Accepted: 27 October 2025;
Published: 10 November 2025.
Edited by:
Zhe-Sheng Chen, St. John’s University, United StatesReviewed by:
Ping Du, Capital Medical University, ChinaJin Niu, Janssen Pharmaceuticals, Inc., United States
Copyright © 2025 Chen, Yu, Feng, Mo, Cai, Du, Du and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Yu, eXVxaW5Ac2N1LmVkdS5jbg==
 Zhuo Chen
Zhuo Chen Qin Yu1,2,3*
Qin Yu1,2,3*