- 1Department of Pharmacy, Affiliated Hospital of Southwest Jiaotong University, The Third People’s Hospital of Chengdu, Chengdu, Sichuan, China
- 2Department of Pharmacy, Affiliated Hospital of Sichuan University, Chengdu Second People’s Hospital, Chengdu, Sichuan, China
Objective: This study aimed to evaluate the impact of implementing a pre-audit system for inpatient medical orders based on the ORTCC (Objectives, Rules, Training, Check, Culture) management model in a tertiary hospital in Chengdu, China. The primary goals were to enhance the pass rate of medical orders, reduce medication errors (MEs), and improve patient safety regarding medication administration.
Methods: A pre-post intervention study was conducted using data from 2022 (pre-implementation) and 2024 (post-implementation). The Prescription Automatic Screening System (PASS) was employed to analyze medical orders, incorporating a “three review and three interception” model involving system alerts, pharmacist reviews, and dispensing checks. Key metrics included the qualification rate of medical orders, physician modification rates, and types of unreasonable orders. Statistical analysis was performed using SPSS (version 27), with chi-square tests for categorical data.
Results: Following implementation, unreasonable medical orders significantly decreased from 540,000 in 2022 to 79,514 in 2024. The physician modification rate increased from 8.59% to 31.86% (P < 0.001), while the final qualification rate improved by 21.31% (P < 0.001). Modules with frequent issues (e.g., dosage, administration routes, drug compatibility) showed reduced error proportions (P < 0.05). Targeted interventions in high-risk departments (e.g., cardiovascular, ICU) further reduced errors (P < 0.05).
Conclusion: The ORTCC-based pre-audit system significantly enhanced the rationality of medical orders, reduced MEs, and promoted safer medication practices. Continuous pharmacist training, dynamic rule updates, and advanced technologies are recommended to sustain improvements and address system limitations, such as false alerts.
1 Introduction
Medication errors (MEs) are any preventable event that can cause inappropriate medication use or harm to a patient, according to the National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP). This definition applies while the medication is in the possession of a healthcare professional, patient, or consumer (Edwards and Aronson, 2000; Classen et al., 2005; Ciapponi et al., 2021). In the United States, 53%–58% of adverse drug event (ADE) related hospitalizations were due to MEs. MEs are estimated to account for approximately 7,000 deaths annually; they represent 1 in 854 inpatient deaths and 1 in 131 outpatient deaths (Elshayib and Pawola, 2020). Meanwhile, the deaths of more than 2.5 million patients in approximately 50 million hospitalized patients each year in China are related to drug-induced adverse reactions (Ji et al., 2019). Research showed that approximately half of all MEs occurred at the stage of drug ordering (Kaushal et al., 2003). The main types of MEs include missed doses and incorrect dosages, frequencies, or routes of administration. The frequency and type of MEs identified often vary based on the detection methods. Research indicated that computerized physician order entry (CPOE) systems and clinical decision support systems (CDSS) can reduce MEs (Prgomet et al., 2017; Roumeliotis et al., 2019). CPOE systems vary; some provide medication lists, while others check for drug interactions, allergies, and lab-based prescription assessments (Ammenwerth et al., 2008).
Pharmacists play a vital role in preventing drug-related problems (DRPs) in both ambulatory care and hospital settings (Kaboli et al., 2006; Knudsen et al., 2007; Guignard et al., 2015). They are responsible for advising healthcare professionals, educating patients, and reviewing medications to ensure the quality of medicines provided to patients (Schnipper et al., 2006; Blouin and Adams, 2017; Bouchaud et al., 2022; Zaij et al., 2023). As national policies evolve, the daily responsibilities of pharmacists are changing, moving away from a product-focused approach to a patient-centered model of care (Kopp et al., 2007). In June 2018, China issued new prescription review specifications for medical institutions, outlining the principles of prescription review and emphasizing the pharmacist’s primary role in this process. Pharmacists must review all prescriptions and medical orders before processing payment and dispensing medications (Ji et al., 2019).
The pre-prescription review has emerged as a new model for hospitals in recent years, promoting patient safety and the rational use of medications compared to traditional prescription reviews. During the initial phase of the work, an automatic prescription Screening System (PASS) was officially launched in 2019, and the pre-approval process for prescriptions was officially launched in January 2020. The results indicated a marked improvement: the pass rate for outpatient prescriptions increased from 84% in 2019 to 97% in 2020, demonstrating significant success. Building on this foundation, in 2023, our hospital implemented a pre-audit of inpatient medical orders based on the ORTCC (Objectives, Rules, Training, Check, and Culture) management model. This study evaluated key indicators, such as the pass rate of medical orders and the incidence of unreasonable issues, both before and after implementing the pre-audit system. This aims to enhance the efficiency of prescription reviews further, standardize the rational use of drugs, reduce MEs caused while prescribing medical orders, and identify areas for improvement and actionable measures.
2 Materials and methods
2.1 Medical records
This study was conducted at a grade III tertiary hospital in Chengdu, China. We initiated a pre-audit of inpatient medical orders in 2023, implementing this process departmentally and in batches. By the end of 2023, we aim to achieve full coverage of hospitalization audits. In this study, we utilized the Hospital Information Management System (HIS) and PASS to query and analyze data on the qualification rate of inpatient orders before and after the pre-audit process. The data analyzed was from 2022 (before the system launch) and 2024 (after the system launch). We also examined the modification rate of doctors’ orders following the system audit and the proportion of unreasonable order types.
2.2 Methods
The ORTCC model serves as the theoretical foundation for refined management, comprising five key elements: Objectives, Rules, Training, Check, and Culture.
2.2.1 Set objectives
To enhance the rationality of medical orders, improve the pass rate of these orders, reduce MEs caused while prescribing medical orders, and ensure patient medication safety.
2.2.2 The establishment and deployment of the medical order review system rules
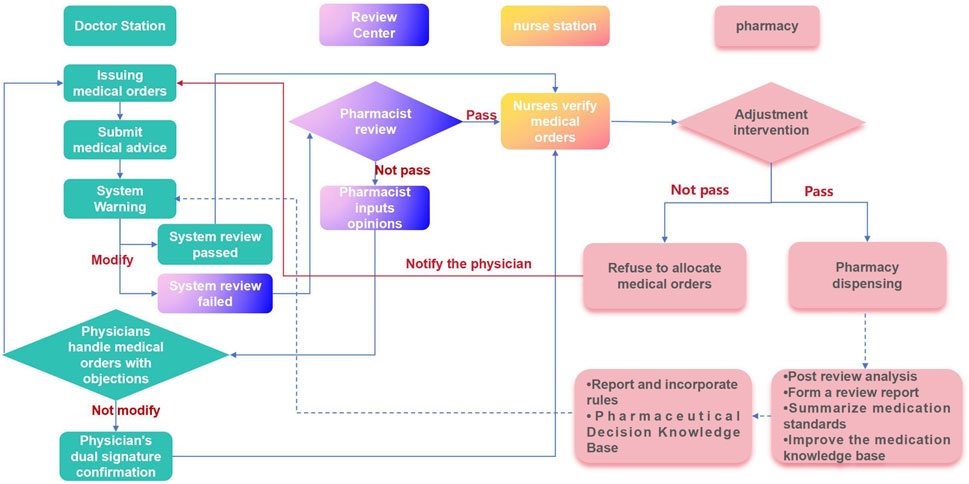
A medical order review working group comprising clinical pharmacists, full-time prescription review pharmacists, dispensing pharmacists, and information pharmacists should be established. This group will establish and maintain a process for reviewing inpatient medical orders and associated rules. We employ a “three review and three interception” model for medical order review (Figure 1). Initially, the PASS system intercepts physician orders for an initial review. If the system identifies an order as potentially unreasonable, it is returned to the physician for modification or review. Subsequently, a reviewing pharmacist conducts a second review to differentiate between true and false positive alerts. Only pharmacist-approved medical orders are then transferred to the nurse station. Finally, when all medical orders, including those initially misdiagnosed or falsely negative, are processed for inpatient dispensing, the dispensing pharmacist conducts a third and final review to intercept potential errors.
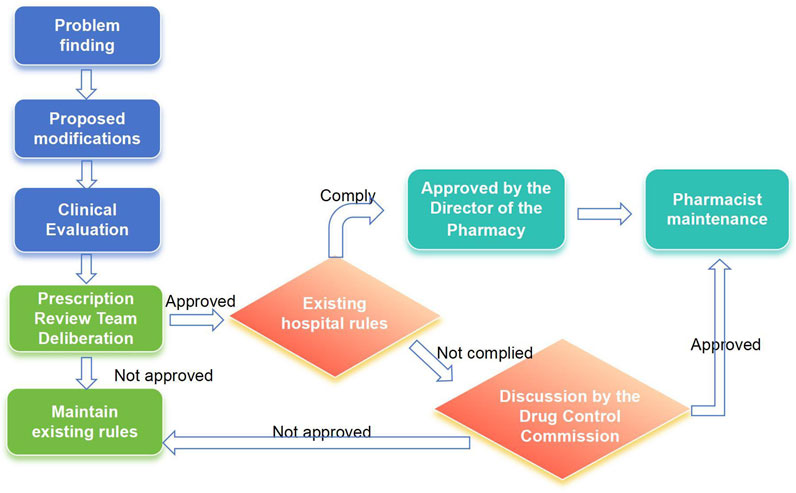
Pharmacists can apply to modify audit rules found to be missing in the system during the review, evaluation, and pharmaceutical monitoring of medical orders, as well as audit rules that clinical departments consider unsuitable for daily medical work. The applicant for a rule modification should consult relevant laws, regulations, and evidence-based medicine resources. They must then conduct an evidence-based evaluation of the proposed rule modification. The medical order review working group will then review these evaluation opinions. If approved, the rule maintenance pharmacist will modify the rules. If the review fails, the existing rules will be maintained (Figure 2). This medical practice evidence evaluation of the modification basis should include an assessment of effectiveness, recommendations, and evidence levels, all grounded in evidence-based medicine principles. The PASS utilizes a dynamic, evidence-based knowledge base, incorporating product information, clinical guidelines and conventions, clinical pathways, and national formularies. The evaluation results of this medical practice evidence determine our hospital’s refined maintenance principle.
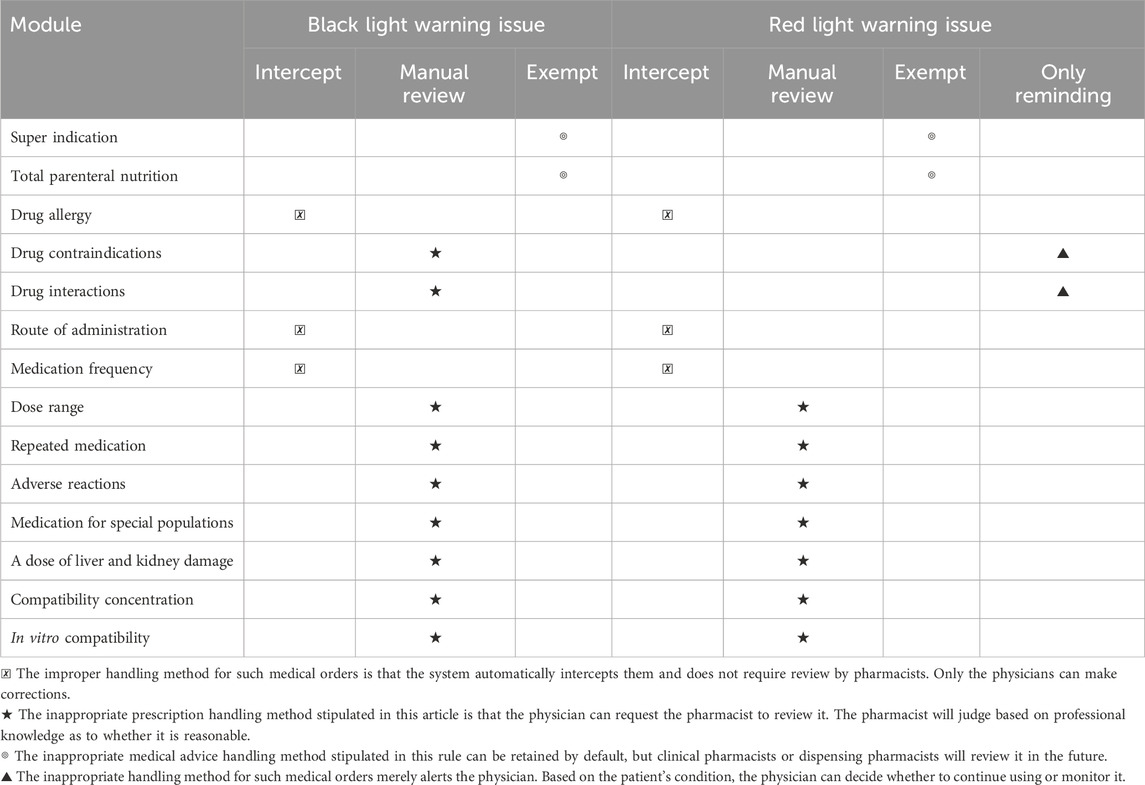
The PASS system categorizes warning issues into black, red, orange, and yellow alerts based on risk level, with black alerts indicating the highest risk. We have directly blocked the red and black lights in the drug administration routes and frequencies, bypassing the pharmacist’s review. Medical orders that fail system review must be returned to the physician for modification. Meanwhile, Warnings below the red alert level in the drug contraindication module are intended for physician review, while black alerts require consultation with a pharmacist for professional judgment. Table 1 details the processing path for inpatient medical orders.
2.2.3 Building a training system
Clinical pharmacists and department liaison pharmacists offer personalized training to department staff, ensuring they become proficient in system operation. This training also facilitates effective communication and the exchange of feedback or medical advice on relevant issues.
2.2.4 Check the assessment system
Every month, we review approximately 3,000 hospitalization records, covering types 1 and 2 incisions, key monitoring of medications, etc. Based on established drug review standards, clinical pharmacists comprehensively evaluate the rationality of medication indications and treatment duration. This evaluation is particularly important for reviewing medical orders flagged by the system when pharmacists are offline. The pharmacists also organize and continuously update the system’s rules to correct imperfections. The medical department reviews these evaluation results quarterly, publishes them regularly, and discloses any identified unreasonable medical orders. These findings serve as a basis for penalties, which are incorporated into the performance evaluations and annual assessments of relevant departments and staff.
2.2.5 Creating a cultural system
Integrating the concept of pre-control into rational drug use involves more than just evaluating prescription rationality. Effective management requires clarifying the responsibilities at each level of pharmaceutical oversight. Furthermore, it is crucial to provide in-depth clinical training for doctors on rational drug use. Before going online, we provided systematic training for all physicians and pharmacists in each department. Moreover, simultaneously, a robust, cyclical process of supervision, evaluation, feedback, intervention, and repeated cycles will enable doctors to master the PASS system’s prescription norms and promote rational drug use.
2.3 Statistical analysis
The SPSS software (IBM, version 27) was used for all statistical analyses. Descriptive statistics (number and frequency) were performed to assess system audit tasks, the rate of unreasonable system audits, the number of intercepted system tasks, and the doctor modification rate after system audits. Group comparisons were conducted using the chi-square test. Statistical significance was defined as a P < 0.05.
3 Results
3.1 Comparison of key indicators before and after the implementation of the plan
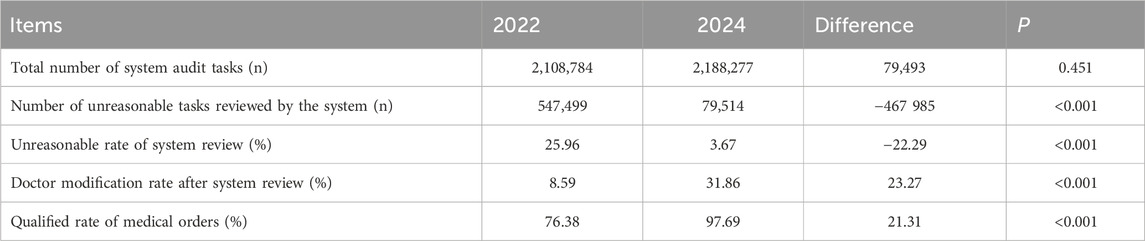
In 2022 and 2024, the total system audit tasks reached approximately 2.1 million. The system experienced a significant reduction in unreasonable tasks following the implementation of the plan. In 2022, the system identified 540,000 unreasonable tasks. Following the plan’s implementation, this number decreased to only 79,514 for the entire year, representing a reduction of approximately 460,000. Post-implementation, the modification rate of medical orders significantly increased, from 8.59% in 2022 to 31.86% in 2024, P < 0.001. The final qualification rate increased 21.31%, P < 0.001 (Table 2).
3.2 Comparison of unreasonable problem types before and after the plan’s implementation
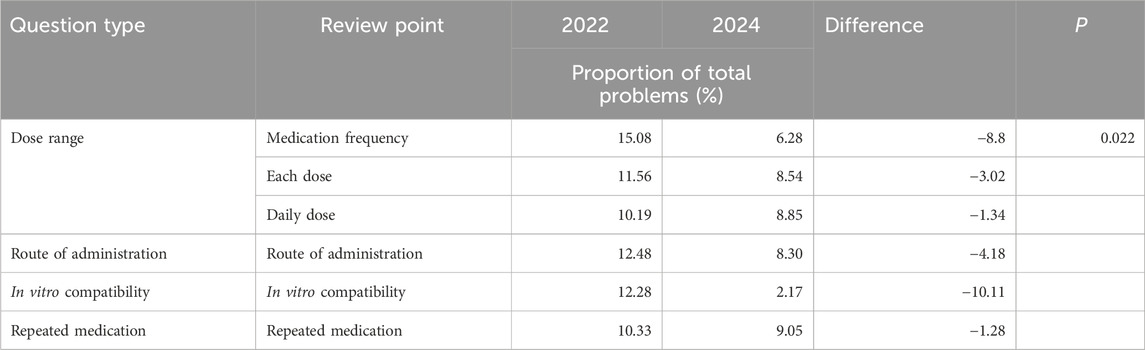
After reviewing medical orders, the modules with the most frequent issues are indications, administration routes, in vitro compatibility, and repeated use. As demonstrated in Table 3, the proportion of unreasonable issues in these modules decreased after implementing the comparative plan, P < 0.05.
3.3 Classification of system audit intervention before and after plan implementation - top 10 departments
A review of medical orders identified the cardiovascular and cerebrovascular ward, ICU, and respiratory ICU as the top 10 departments with the highest number of unreasonable orders. Following this finding, the project will prioritize interventions in these specific wards. Table 4 demonstrates a decrease in unreasonable medical orders compared to 2022. Among these 10 departments, except for the slight difference in the total number of tasks (P > 0.05), the improvements in the number of unreasonable tasks, the number of tasks modified by doctors, the modification rate, and the qualified rate of medical orders were all significant (P < 0.05).
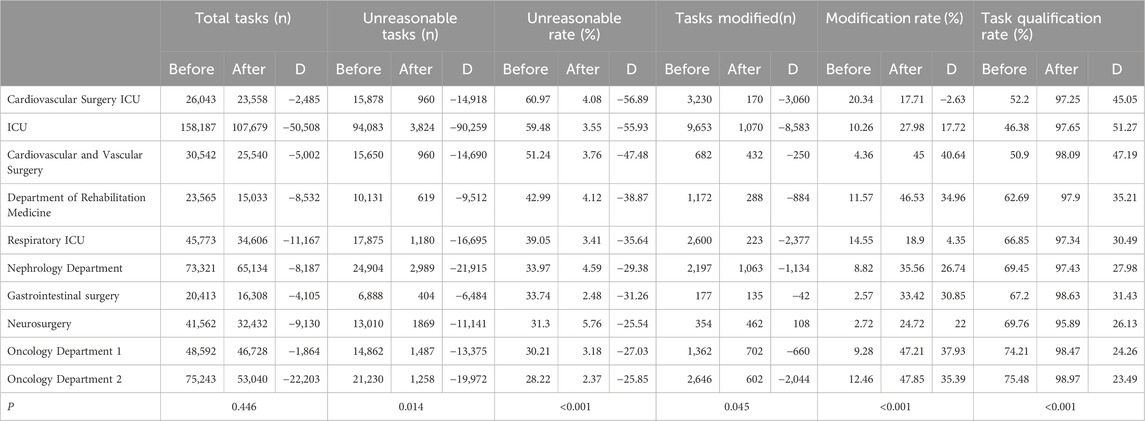
Table 4. Classification of system audit intervention before and after plan implementation - top 10 departments.
4 Discussion
In 2020, driven by China’s ongoing medical reforms and the demand for high-quality pharmaceutical services, we implemented the PASS system. It significantly improves over traditional prescription review methods due to its rapid response time, averaging approximately 5 s per task, substantially faster than manual review. Furthermore, the PASS system provides a comprehensive and precise audit scope, encompassing patient-specific factors such as age, gender, and diagnosis, as well as therapy regimens including medication selection, dosage, frequency, route of administration, and potential drug-drug interactions. Following the implementation of this pre-validation system, we observed a marked improvement in the pass rate of outpatient prescriptions, demonstrating its positive impact.
To enhance drug quality control and minimize MEs, this study implemented the pre-audit of inpatient medical orders based on the ORTCC model, across five key aspects: Objectives, Rules, Training, Check, and Culture. The effectiveness of this pre-audit system for medical orders was evaluated by comparing data before and after its implementation. The results demonstrate a significant decrease in unqualified medical order tasks in hospitalization, from 547,499 in 2022 to 79,514 in 2024. Concurrently, the qualified rate of inpatient medical orders significantly improved, rising from 76.38% in 2022 to 97.69% in 2024 (P < 0.001), indicating statistical significance. Moreover, the system effectively addressed frequently occurring issues in medical orders. Modules with a high prevalence of problems, such as dosage range, administration route, in vitro compatibility, and repeated use, showed a reduced proportion of unreasonable issues after the system’s implementation. Notably, the administration route and frequency presented a unique case. Communication with physicians revealed that these errors were primarily due to oversight. Consequently, the system was configured to directly intercept these specific errors without requiring pharmacist review, streamlining the workflow for pharmacists and preventing potentially unreasonable orders from being missed during offline periods.
Furthermore, analysis of the top 10 departments with the highest number of unreasonable medical orders before system implementation showed a significant reduction in unreasonable audits and task irrationality rates (P < 0.05), demonstrating statistical significance. While the number of audit tasks remained relatively stable, the doctors’ modification rate doubled, suggesting a heightened awareness and correction of errors. Overall, the plan led to a noticeable change in doctor behaviour; previously, system prompts were often ignored. However, post-implementation, the modification rate of medical orders significantly increased, from 8.59% in 2022 to 31.86% in 2024, P < 0.001. These findings suggest that the PASS system has significantly enhanced the rationality of medical orders, positively impacting rational drug use in the hospital’s clinical practice. This ultimately ensures safer medication practices for patients and reduces the incidence of MEs.
The PASS system requires comprehensive drug information, including usage and dosage, adverse reactions, incompatibilities, and interactions, supported by big data. This software is updated annually with data from guidelines and expert consensus. However, a qualified prescription review software should allow users to personalize the configuration, incorporating information from sources like instructions, clinical trials, and post-clinical demonstration data on rational medication use. Over time, rational drug software will continuously improve and integrate further into hospitals (Liu et al., 2021). By the end of 2024, we have manually maintained approximately 90,000 rules to personalize the system and better align it with clinical needs. A key aspect of inpatient diagnosis logic is that it reflects the patient’s condition upon admission, not necessarily their current state. For instance, a patient admitted with gastrointestinal bleeding may require discontinuing nonsteroidal anti-inflammatory drugs. However, the system retains the gastrointestinal bleeding diagnosis even after the bleeding resolves or the patient is discharged. To address this, we have configured the drug contraindication module with different warning levels. A red light warning serves as a prompt for the doctor, while a black light warning triggers a review by the pharmacist. The pharmacist then conducts a detailed review of the medical record to determine the appropriateness of the drug. Similarly, for medications prescribed without clear indications, we bypass pre-hospitalization review and instead rely on continuous drug rationality review by clinical pharmacists. Moreover, a dedicated pharmacist in the intravenous configuration center reviews parenteral nutrition orders, which the PASS system does not intercept separately. These personalized rule settings enhance patient medication safety, reduce pharmacist workload, minimize unnecessary alerts for doctors, and improve the system’s overall usability.
After applying the pre-audit of inpatient medical orders, the relative number of irrational medical orders was reduced. However, the errors brought about by the system itself cannot be ignored. It is crucial to consider the possibility of false negative alerts and false positive medical orders and the risk of alert fatigue when frequent, clinically irrelevant alerts are ignored. Therefore, frontline pharmacists should perform a final check (Hincapie et al., 2014).
Pre-audit review is a strategy to improve medical service quality by promoting rational drug use. For example, pharmacists must assess the reasonableness of the system’s daily dose limit reminders, especially when patients have long-term and temporary medical orders. Furthermore, if a child’s single dose is calculated based on their weight in kilograms, it may exceed the specified dosage of the drug. Consequently, pharmacists must carefully review and examine these calculations during the review process. By integrating the ORTCC management model with the PASS system, we conducted a pre-audit of inpatient orders, resulting in more closed-loop management of rational drug use. There are ORTCC models available for other medical quality research in the market, as well as studies related to pre-hospitalization review. However, integrating the ORTCC model with PASS is not available. This is also a novelty of this research.
Our study enhances pharmacists’ efficiency and accuracy, and proposes new ideas for improving the rational use of drugs among patients. Nevertheless, several limitations should be acknowledged. First, relying solely on single-center studies is insufficient; more data are needed for verification. In addition, PASS technology may introduce errors. The system still has issues with false negatives and false positives, such as incorrect dosing or route recommendations. To mitigate these risks, we plan to utilize more data, implement frequent updates, and adopt advanced technologies to enhance the accuracy of the review system.
5 Conclusion
Effective management of MEs and rational drug use are important global issues. The integration of the ORTCC model and the PASS system has improved inpatient prescription practices and ensured patient medication safety. To enhance drug use and patient safety, it is recommended that these systems be incorporated into the healthcare framework.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XQ: Conceptualization, Data curation, Writing – original draft, Writing – review and editing. SL: Conceptualization, Data curation, Software, Writing – review and editing. HX: Conceptualization, Data curation, Methodology, Software, Writing – review and editing. MX: Conceptualization, Data curation, Investigation, Methodology, Writing – review and editing. YY: Conceptualization, Investigation, Writing – review and editing, Methodology. QH: Conceptualization, Data curation, Methodology, Validation, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
All authors are thankful to the engineers who provided consulting assistance for PASS.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ME, medication error; ADE, adverse drug event; NCC MERP, National Coordinating Council for Medication Error Reporting and Prevention; CPOE, computerized physician order entry system; CDSS, clinical decision support system; DRP, drug-related problem; PASS, prescription automatic screening system; HIS, hospital information management system.
References
Ammenwerth, E., Schnell-Inderst, P., Machan, C., and Siebert, U. (2008). The effect of electronic prescribing on medication errors and adverse drug events: a systematic review. J. Am. Med. Inf. Assoc. 15 (5), 585–600. doi:10.1197/jamia.M2667
Blouin, R. A., and Adams, M. L. (2017). The role of the pharmacist in health care: expanding and evolving. N. C. Med. J. 78 (3), 165–167. doi:10.18043/ncm.78.3.165
Bouchaud, L., Bluze, E., Dussart, C., Massoubre, B., and Boulliat, C. (2022). The role of the community and hospital pharmacist in public health prevention in France. Ann. Pharm. Fr. 80 (6), 769–777. doi:10.1016/j.pharma.2022.02.004
Ciapponi, A., Fernandez Nievas, S. E., Seijo, M., Rodriguez, M. B., Vietto, V., Garcia-Perdomo, H. A., et al. (2021). Reducing medication errors for adults in hospital settings. Cochrane Database Syst. Rev. 11 (11), CD9985. doi:10.1002/14651858.CD009985.pub2
Classen, D. C., Pestotnik, S. L., Evans, R. S., and Burke, J. P. (2005). Computerized surveillance of adverse drug events in hospital patients. Qual. Saf. Health Care 14 (3), 221–225. doi:10.1136/qshc.2002.002972/10.1136/qshc.2005.014522
Edwards, I. R., and Aronson, J. K. (2000). Adverse drug reactions: definitions, diagnosis, and management. Lancet 356 (9237), 1255–1259. doi:10.1016/S0140-6736(00)02799-9
Elshayib, M., and Pawola, L. (2020). Computerized provider order entry-related medication errors among hospitalized patients: an integrative review. Health Inf. J. 26 (4), 2834–2859. doi:10.1177/1460458220941750
Guignard, B., Bonnabry, P., Perrier, A., Dayer, P., Desmeules, J., and Samer, C. F. (2015). Drug-related problems identification in general internal medicine: the impact and role of the clinical pharmacist and pharmacologist. Eur. J. Intern Med. 26 (6), 399–406. doi:10.1016/j.ejim.2015.05.012
Hincapie, A. L., Warholak, T., Altyar, A., Snead, R., and Modisett, T. (2014). Electronic prescribing problems reported to the Pharmacy and Provider ePrescribing Experience Reporting (PEER) portal. Res. Soc. Adm. Pharm. 10 (4), 647–655. doi:10.1016/j.sapharm.2013.08.007
Ji, Z., Song, W., Yan, X., and Ai, C. (2019). Practice and evaluation of pre-prescription review mode. Pharm. Sci. Technol. 3 (1), 1–6. doi:10.11648/j.pst.20190301.11
Kaboli, P. J., Hoth, A. B., McClimon, B. J., and Schnipper, J. L. (2006). Clinical pharmacists and inpatient medical care: a systematic review. Arch. Intern Med. 166 (9), 955–964. doi:10.1001/archinte.166.9.955
Kaushal, R., Shojania, K. G., and Bates, D. W. (2003). Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch. Intern Med. 163 (12), 1409–1416. doi:10.1001/archinte.163.12.1409
Knudsen, P., Herborg, H., Mortensen, A. R., Knudsen, M., and Hellebek, A. (2007). Preventing medication errors in community pharmacy: frequency and seriousness of medication errors. Qual. Saf. Health Care 16 (4), 291–296. doi:10.1136/qshc.2006.018770
Kopp, B. J., Mrsan, M., Erstad, B. L., and Duby, J. J. (2007). Cost implications of and potential adverse events prevented by interventions of a critical care pharmacist. Am. J. Health Syst. Pharm. 64 (23), 2483–2487. doi:10.2146/ajhp060674
Liu, J., Zhang, Y., Chen, N., Li, L., Wu, Y., Guan, C., et al. (2021). Remote pharmacy service in primary care: the implementation of a cloud-based pre-prescription review system. J. Am. Pharm. Assoc. 61 (2), e176–e182. doi:10.1016/j.japh.2020.12.008
Prgomet, M., Li, L., Niazkhani, Z., Georgiou, A., and Westbrook, J. I. (2017). Impact of commercial computerized provider order entry (CPOE) and clinical decision support systems (CDSSs) on medication errors, length of stay, and mortality in intensive care units: a systematic review and meta-analysis. J. Am. Med. Inf. Assoc. 24 (2), 413–422. doi:10.1093/jamia/ocw145
Roumeliotis, N., Sniderman, J., Adams-Webber, T., Addo, N., Anand, V., Rochon, P., et al. (2019). Effect of electronic prescribing strategies on medication error and harm in hospital: a systematic review and meta-analysis. J. Gen. Intern Med. 34 (10), 2210–2223. doi:10.1007/s11606-019-05236-8
Schnipper, J. L., Kirwin, J. L., Cotugno, M. C., Wahlstrom, S. A., Brown, B. A., Tarvin, E., et al. (2006). Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch. Intern Med. 166 (5), 565–571. doi:10.1001/archinte.166.5.565
Keywords: adverse drug events, medication errors, prescription automatic screening system, ORTCC model, clinical decision support system
Citation: Qin X, Luo S, Xi H, Xu M, Yang Y and He Q (2025) Evaluation of the implementation effect of pre-audit of inpatient medical orders: based on the ORTCC model. Front. Pharmacol. 16:1681245. doi: 10.3389/fphar.2025.1681245
Received: 07 August 2025; Accepted: 22 September 2025;
Published: 01 October 2025.
Edited by:
Zhiyao He, Sichuan University, ChinaReviewed by:
Sondang Khairani, Universitas Pancasila, IndonesiaSajal Pandya, Government Medical College, Surat, India
Copyright © 2025 Qin, Luo, Xi, Xu, Yang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin He, eWpraHFAc2luYS5jb20=
 Xiaoli Qin
Xiaoli Qin Shanhong Luo2
Shanhong Luo2 Heng Xi
Heng Xi Yujie Yang
Yujie Yang