Abstract
Background:
Perioperative complications and emergence agitation (EA) are common after pediatric tonsillectomy and/or adenoidectomy (T&A), and may be influenced by the use of preoperative sedatives. The effectiveness of dexmedetomidine (Dex) in minimizing these risks is still debated.
Methods:
We searched EMBASE, PubMed, and the Cochrane Library for randomized controlled trials (RCTs) assessing the safety and effectiveness of Dex in pediatric T&A, with comparisons made against placebo and/or alternative comparators. The search included studies published before March 2025. Retrieved data included the incidence of EA, the percentage (%) of cases requiring rescue analgesics, and perioperative complications, such as hypotension and bradycardia, and perioperative respiratory adverse events (PRAEs). The meta-analysis was performed using RevMan 5.3.
Results:
Thirty-six RCTs including 3,773 children were included. Compared with placebo, benzodiazepines, and opioids, Dex significantly reduced the occurrence of EA [OR = 0.23, 95% CI (0.16, 0.32), I2 = 44%] [OR = 0.51, 95% CI (0.28, 0.93), I2 = 44%] [OR = 0.19, 95% CI (0.09, 0.39), I2 = 0%] (P < 0.05). Subgroup analysis of delivery methods, timing, and dosage (Dex ≥0.5 μg/kg) indicated that Dex significantly decreased the incidence of EA (P < 0.05). Furthermore, compared with placebo and benzodiazepines, Dex markedly decreased the incidence of patients necessitating rescue analgesia, while no statistically significant difference was noted versus opioids. Dex also significantly decreased the incidence of PRAEs (oxygen saturation (%) and laryngospasm) [OR = 0.41, 95% CI (0.25, 0.69), I2 = 0%] [OR = 0.38, 95% CI (0.19, 0.78), I2 = 0%] (P < 0.05) However, there was no significant difference in the incidence of hypotension or bradycardia [OR = 2.28, 95% CI (0.99, 5.23), I2 = 0%, P = 0.05] [OR = 2.00, 95% CI (1.00, 3.98), I2 = 2%, P = 0.05]. Finally, recovery time did not differ significantly between the Dex and control groups.
Conclusion:
Dex may mitigate EA and perioperative complications while enhancing recovery quality following T&A in pediatric patients.
1 Introduction
Tonsillectomy, with or without adenoidectomy (T&A), is a routinely performed operation in children under general anesthesia (Hall et al., 2017; Cho et al., 2018). Surgical procedures may result in throat irritation and considerable stress response, potentially linked to notable perioperative complications in 9.4% of cases, including emergence agitation (EA), perioperative complications (such as perioperative respiratory adverse events (PRAEs), nausea or vomiting, and severe pain) (Belyea et al., 2014). Despite their short duration, these occurrences may heighten the risk of self-harm, extend the stay in the PACU, demand more intensive nursing support, and increase healthcare expenditures (Zh et al., 2021). Effective perioperative management may reduce these complications, and numerous medications administered preoperatively or intraoperatively, such as dexmedetomidine (Dex), propofol, midazolam, opioids, ketofol, and ketamine, have been studied for their efficacy in preventing EA and perioperative complications in children (Urits et al., 2020). Nonetheless, considerable discrepancies in management practices persist (Steward et al., 2011).
Dex, characterized by its selectivity for α2-adrenoreceptors, exhibits multiple pharmacologic actions—sedation, analgesia, anesthesia, and sympatholysis—combined with vasoconstriction and minimal respiratory suppression, making it a valuable sedative-analgesic agent for children undergoing T&A under anesthesia (Mahmoud and Mason, 2015). The effectiveness of Dex in this setting has been documented in several clinical trials, that have employed various delivery methods and doses (Pestieau et al., 2011a; Li LQ. et al., 2018). Its role in mitigating EA has been the subject of numerous systematic reviews and meta-analyses (Cho et al., 2018; He et al., 2013). Nevertheless, current evaluations have not specifically addressed pediatric T&A. Previous meta-analyses predominantly contrasted Dex with opioids (e.g., morphine and fentanyl) in tonsillectomy operations (Cho et al., 2018; He et al., 2013; Rao et al., 2020); however, and their findings were limited by small sample sizes, significant heterogeneity, or the inclusion of nonrandomized trials. These comprehensive studies failed to account for the manner of delivery (continuous injection versus intranasal), the comparative target (placebo versus opioid), varying dosages, or PRAEs. Given the limited availability of recent randomized controlled trials (RCTs), the therapeutic profile of Dex in juvenile T&A has not yet been comprehensively reviewed. To address this gap, we incorporated trials utilizing delivery routes [intravenous (IV), intranasal, and oral] and varied timing of Dex administration (premedication, post-anesthesia induction, and prior to surgical closure) across low (<0.5 μg/kg), moderate (≥0.5 to <1 μg/kg), and high (≥1 μg/kg) dosing groups. The current meta-analysis is designed to evaluat the effects of Dex on various administration methods and dosages of Dex to enhance patient experience immediately following T&A, thereby providing evidence for healthcare professionals and pharmaceutical research and development.
2 Materials and methods
In conducting this meta-analysis, we complied with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria and applied procedures specified in the Cochrane Handbook (Higgins and Green, 2011).
2.1 Search methodology
Our search was conducted in the PubMed, Embase, and Cochrane Library databases for articles published before March 2025. Additional studies were identified through three clinical trial registry platforms: Clinical Trials.gov, the WHO Clinical Trials Registry Platform, and the Cochrane Central Registry of Controlled Trials. The search strategy was specific for each database and included a combination of medical subject headings and free-text terms (“Dex” or “Precedex”), pediatric populations, and tonsillectomy procedures.
2.2 Eligibility criteria
We included studies that (1) involved patients aged 0–18 years necessitating T&A procedures, classified as American Society of Anesthesiologists (ASA) I–III; (2) evaluated Dex against placebo and/or active comparators in pediatric T&A, with no restrictions on the route of administration; (3) placed no restrictions on the control group composition; (4) reported the frequency of EA and perioperative complications (e.g., nausea, vomiting, cough, laryngospasm, hypotension, bradycardia) as primary outcomes and the frequency of subjects requiring rescue analgesics and recovery time as secondary outcomes; and (5) were RCTs. We excluded studies that (1) involved intensive care unit patients; (2) included adults; (3) lacked extractable data; (4) were review articles, letters, or animal studies, or lacked a comparator; and (5) were duplicates of previously published work.
2.3 Data extraction
Two investigators separately retrieved data utilizing a preestablished extraction template. The information gathered included the study author, publication year, sample size, average age, intervention measure, dosage, surgical procedure, and relevant outcomes as per the inclusion criteria.
Two investigators also independently evaluated all titles and abstracts to select studies for full-text screening. Eligibility criteria were subsequently applied independently for final inclusion. Conflicts over article eligibility were addressed through deliberation, during which the reviewers articulated their reasoning and reached mutual agreement on inclusion or exclusion. If disagreements persisted, a third reviewer adjudicated the final inclusion decision.
2.4 Evaluation of bias risk
A bias assessment was conducted for the selected RCTs using the Cochrane risk-of-bias (RoB) tool (Higgins and Green, 2011).
2.5 Statistical analysis
The pooled analysis was implemented by use of Review Manager 5.3, and effect measures were calculated as either odds ratios (ORs) or standardized mean differences (SMDs), with 95% confidence intervals (95% CIs) provided.
We quantified heterogeneity by computing the I-squared (I2) value, and a fixed-effects model was employed. An I2 value greater than 50% was deemed indicative of significant heterogeneity; in such cases, contributing factors were explored, and a random-effects model was adopted as needed.
Furthermore, to examine the impact of Dex on EA occurrence, subgroup analyses were performed as per prior hypotheses from three aspects: varying administration routes (IV versus intranasal), differing administration times (post-induction of anesthesia, pre-surgery conclusion), and dosage variations (low (<0.5 μg/kg), moderate (≥0.5 to <1 μg/kg)), and high doses (≥1 μg/kg)]. We established six distinct subgroups according to several event types: vomiting, cough, hypotension, bradycardia, oxygen saturation (%), and laryngospasm.
3 Results
3.1 Literature search and study profile
From an initial pool of 384 screened articles, 36 relevant studies published from 2005 to 2024 were incorporated into this meta-analysis (Figure 1). a total of 3,773 children participated in this research. Dex was administered at 0.three to four μg/kg, which aligns with dosage guidelines for pediatric sedation during noninvasive operations and reflects contemporary clinical use (Mace et al., 2008; Aldamluji et al., 2021) (Table 1).
FIGURE 1
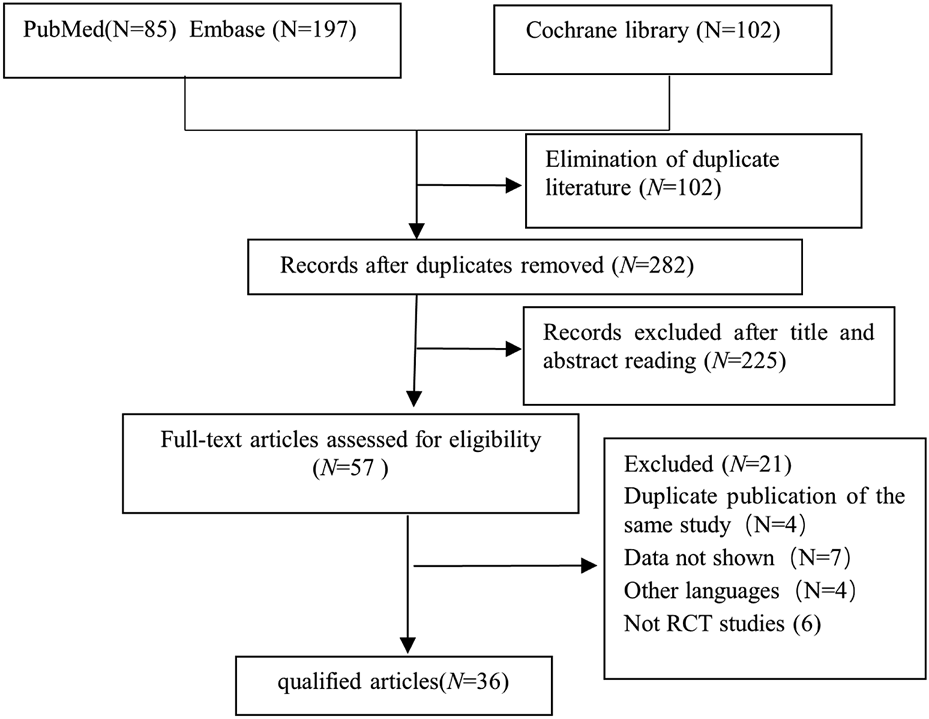
Study retrieval and selection workflow.
TABLE 1
| Number | Study ID | Intervention | Sample size | Age (years) | Weight (kg) | Anesthesia | Time | Surgery | ASA | Recovery time (min) | EA (%) | Rescue analgesic frequency (%) | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cao et al. (2016) | DEX | 30 | 4.1 ± 1.5 | 20.9 ± 5.8 | intravenous (IV) dexmedetomidine 1ug/kg over 10 min, followed by 0.5ug/kg/h continuous infusion | until to 5 min before the end of surgery | tonsillectomy with or without adenoidectomy | ASA I or II | 15.2 ± 5.1∗ | NA | NA | NA |
| Group control | 30 | 3.9 ± 1.8 | 21.7 ± 7.9 | the same volume of 0.9% saline | 12.4 ± 3.5 | ||||||||
| 2 | Ali and Abdellatif (2013) | The control group (Group C) | 40 | 3.9 ± 1.6 | 18.7 ± 4.5 | received 10 mL NaCI 0.9% | About 5 min before the end of surgery | adenotonsillectomy | ASA I-II | NA | 12.5 | NA | ② |
| propofol group (Group P) | 40 | 4.2 ± 1.4 | 19.8 ± 4.6 | propofol 1 mg/kg | 10 | ||||||||
| Dexmedetomidine group (Group D) | 40 | 4.3 ± 1.3 | 19.5 ± 4.8 | IV dexmedetomidine 0.3 ug/kg diluted in 10 mL NaCI 0.9% | 12.5 | ||||||||
| 3 | Tsiotou et al. (2018) | Dexmedetomidine group (A) | 31 | 6.1 (2.6) | 22.8 (9.5) | IV dexmedetomidine 1 ug/kg | After the induction of anesthesia | tonsillectomy with and without adenoidectomy | ASA I or II | NA | 4 | NA | ① |
| Group control (B) | 29 | 6.3 (2.6) | 24.03 (10.9) | normal saline solution | 12 (41.4) | ||||||||
| 4 | Soliman and Alshehri (2015) | Group control (A) | 75 | 8.38 ± 3.00 | 21.12 ± 5.53 | the patients received sevoflurane 1%–3% during the surgery | after induction of anesthesia | adenotonsillectomy | ASA I–II | NA | 29 | NA | ②⑤⑥ |
| Dexmedetomidine group (B) | 75 | 8.56 ± 3.08 | 21.48 ± 3.99 | IV dexmedetomidine 0.5 ug/kg | 6 (8%) | ||||||||
| 5 | Bai et al. (2016) | Dexmedetomidine group | 62 | 9.8 ± 2.9 | 33.6 ± 11.0 | dexmedetomidine 0.5 μg/kg, intravenous | After stable anesthesia | Tonsillectomies | ASA I–II | NA | 5 | NA | ①②⑤⑥ |
| T group | 62 | 9.7 ± 3.3 | 33.5 ± 9.8 | the same amount of normal saline | 15 | ||||||||
| 6 | Mizrak et al. (2013) | Group D | 30 | 8.7 ± 3.6 | 28.0 ± 16.6 | dexmedetomidine 0.5 mg/kg | 10 min before the induction of anesthesia | undergoing adenotonsillectomy | ASAI or II. | 6.90 ± 2.92 | NA | NA | NA |
| Group C | 30 | 9.8 ± 4.0 | 23.9 ± 10.2 | placebo bolus | 6.0 ± 2.94 | ||||||||
| 7 | Li et al. (2018b) | group D | 30 | 5.1 6 ± 1.15 | 22.6 ± 7.09 | infused 0.2 ug/kg/hour dexmedetomidine | until the end of the surgery | undergoing tonsillectomy | ASAI or II | 36.70 ± 10.70 | NA | NA | ② |
| group C | 40 | 5.5 6 ± 1.17 | 22.4 ± 6.68 | placebo bolus | 40.68 ± 11.95 | ||||||||
| 8 | El-Hamid and Yassin (2017) | Group D | 43 | 4.4 ± 1.3 | 17.4 ± 3.4 | intranasal dexmedetomidine at 1 μg/kg | after the induction of general anesthesia |
tonsillectomy and/or adenoidectomy | ASA I and II | 35.93 ± 10.21 | 6.98% | NA | ② |
| Group C | 43 | 4.2 ± 0.93 | 18.6 ± 4.1 | received intranasal saline 0.9% | 39.17 ± 9.86 | 58% | |||||||
| 9 | Li et al. (2018c) | D1 groups | 30 | 4.47 ± 1.17 | 19.82 ± 5.51 | intranasally dexmedetomidine 1ug/kg |
25–40 min before surgery | adenoidectomy with or without tonsillectomy | ASA I and II | NA | 43.30% | NA | ② |
| D2 groups | 30 | 4.53 ± 1.55 | 20.05 ± 5.79 | intranasally dexmedetomidine 2 ug/kg |
30.00% | ||||||||
| S groups | 30 | 4.37 ± 1.30 | 18.67 ± 4.10 | saline of the same volume | 63.30% | ||||||||
| 10 | Wang et al. (2013) | group D1 | 20 | 4.2 ± 0.8 | 19.0 ± 3.7 | intranasal Dexmedetomidine 1 ug/kg | 30 min before anesthesia induction | adenotonsillectomy | ASA I or II | NA | NA | NA | NA |
| group D2 | 20 | 4.3 ± 1.1 | 18.9 ± 3.7 | intranasal Dexmedetomidine 2 ug/kg | |||||||||
| 11 | Yi et al. (2022) | dexmedetomidine 0.5 group | 58 | 6.06 ± 1.71 | 23.28 ± 7.42 | dexmedetomidine 0.5 μg/kg | After intubation | adenotonsillectomy | ASA I or II | 66.67 ± 16.12 |
NA | NA | ④ |
| dexmedetomidine 1 group | 62 | 6.17 ± 1.80 | 23.03 ± 6.74 | dexmedetomidine 1 μg/kg | 52.38 ± 15.33 |
||||||||
| 12 | Shafa et al. (2021) | dexmedetomidine 1ug/kg group | 35 | 6.5 ± 2.0 | 21.9 ± 6.8 | dexmedetomidine 1ug/kg | before the beginning of the operations |
denotonsillectomy | ASA I or II | 48.8 ± 6.6 | NA | NA | ⑥ |
| dexmedetomidine 2ug/kg group | 35 | 6.6 ± 2.01 | 21.6 ± 5.4 | dexmedetomidine 2ug/kg | 51.4 ± 7.5 | ||||||||
| Placebo group | 5 | 6.0 ± 2.1 | 21.7 ± 7.3 | saline of the same volume | 54.4 ± 7.3 | ||||||||
| 13 | Abo Elfadl et al. (2022) | Group L | 45 | 5.2 ± 1.3 | 20.32 ± 4.98 | levobupivacaine 0.25% | before the beginning of the operation | tonsillectomy with or without adenoidectomy | ASA I-II | 10.2 ± 1.67 | NA | NA | ②③⑤⑥ |
| Group LD | 45 | 5.1 ± 1.3 | 19.65 ± 4.41 | levobupivacaine plus dexmedetomidine 1 μg/kg | 10.8 ± 1.37 | ||||||||
| 14 | Guler et al. (2005) | Dexmedetomidine group | 30 | 4.7 ± 1.2 | 18.43 ± 3.47 | Dexmedetomidine (0.5ug/kg) | About 5 min before the end of surgery | adenotonsillectomy | ASA I | 9.30 ± 2.9* | 5* (17) | 7* (23) | ①② |
| Placebo group | 30 | 4.5 ± 1.2 | 17.46 ± 4.09 | the same volume of sodium chloride | 7.20 ± 2.7 | 17 (57) | 16 (53) | ||||||
| 15 | Abdel-Ghaffar et al. (2019) | Group C | 30 | 5 (2.5–6) | 15 (12–22) | saline placebo | preoperative premedication |
tonsillectomy | ASA I-II | NA | NA | NA | ① |
| Group dexmedetomidine I | 30 | 5 (3–6) | 15 (10–25) | buccal trans-mucosal dexmedetomidine 0.5 ug/kg | |||||||||
| Group dexmedetomidine II | 30 | 5 (3–6) | 18 (10–25) | buccal trans-mucosal dexmedetomidine 1 ug./kg | |||||||||
| 16 | Hao et al. (2020) | RL | 56 | 6.0 (2.1) | 19.6 (3.1) | 0.25% ropivacaine and 1 μg/kg dexmedetomidine | After intubation | The tonsillectomy and adenoidectomy | ASA I-II | NA | NA | NA | NA |
| R | 59 | 5.7 (2.0) | 21.0 (3.9) | 0.25% ropivacaine | |||||||||
| 17 | Yao et al. (2022) | Control | 30 | 4.3 ± 1.1 | 19.9 ± 4.5 | placebo | before induction | tonsillectomy and/or adenoidectomy | ASA I | 37.23 ± 7.71 | NA | NA | NA |
| PPIA group | 30 | 4.6 ± 1.2 | 20.9 ± 4.5 | a parent | 40.20 ± 7.28 | ||||||||
| Dexmedetomidine group | 30 | 4.4 ± 1.2 | 18.4 ± 4.9 | intranasal dexmedetomidine 1.0 μg/kg | 40.37 ± 7.61 | ||||||||
| PPIA + Dexmedetomidine group | 30 | 4.6 ± 1.4 | 19.7 ± 5.3 | intranasal dexmedetomidine 1.0 μg/kg+ a parent’s arms | 42.23 ± 6.78 | ||||||||
| 18 | Di et al. (2018) | Group D0 | 25 | 5.3 ± 1.3 | 19.5 ± 3.4 | saline infusion | over 10 min in pre-op area | tonsillectomy | ASA I-II | NA | NA | NA | ② |
| Group D1 | 26 | 5.0 ± 1.1 | 19.6 ± 3.7 | dexmedetomidine 1 μg/kg infusion | |||||||||
| Group D2 | 24 | 5.1 ± 1.0 | 19.8 ± 3.4 | dexmedetomidine 2 μg/kg infusion | |||||||||
| 19 | Golmohammadi et al. (2024) | Intervention group | 38 | 3.97 ± 1.04 | 13.88 ± 1.39 | an infusion of 0.5 μg/kg/h of dexmedetomidine | after induction of anesthesia | adenoidectomy | ASA I | 9.65 ± 5.14 | 34.21% | NA | ②④⑤⑥ |
| Control group | 38 | 3.62 ± 1.12 | 14.55 ± 1.5 | an equal volume of normal saline infusion | 7.31 ± 2.44 | 53.95% | |||||||
| 20 | Hadi et al. (2015) | KETODEX | 45 | 4.22 ± 1.32 | 18.52 ± 4.60 | dexmedetomidine 0.3 ug/kg i.v | About 10 min before the end of surgery | adenotonsillectomy | ASA I–II | NA | 11% | NA | ③④ |
| Control | 47 | 4.22 ± 1.12 | 18.37 ± 5.21 | volume-matched normal saline | 47% | ||||||||
| 21 | Shahhosseini et al. (2023) | A | 25 | 9 ± 2 | NA | infused in dose of 0.6 μg/kg | After induction | tonsillectomy | ASA I–II | 50 ± 9 | NA | NA | NA |
| B | 25 | 9 ± 2 | NA | infused in dose of 0.3 μg/kg | 67 ± 8 | ||||||||
| C | 25 | 9 ± 2 | NA | normal bolus saline | 75 ± 7 | ||||||||
| 22 | Zhang et al. (2022) | Control | 20 | 4.53 ± 1.32 | 21.35 ± 9.69 | normal saline | from the induction | adenoidectomy and tonsillectomy | ASA I or II | 14.95 ± 3.57 | 10 (50%) | 15 | ①②③⑥ |
| Dexmedetomidine | 20 | 4.81 ± 1.09 | 21.60 ± 5.12 | intravenously 0.4 μg/kg dexmedetomidine | 14.86 ± 3.89 | 5 (25%) | 7 | ||||||
| Dexmedetomidine + Alf1 | 20 | 5.13 ± 1.29 | 23.15 ± 9.31 | intravenously with 0.4 μg/kg dexmedetomidine and alfentanil (10 μg/kg) |
15.61 ± 4.59 | 1 (5%) * | 3 | ||||||
| Dexmedetomidine + Alf2 | 20 | 5.11 ± 1.23 | 22.69 ± 9.83 | intravenously with 0.4 μg/kg dexmedetomidine and alfentanil (20 μg/kg) | 19.25 ± 4.38 | 0 (0) * | 2 | ||||||
| 23 | Abdel-ghaffar and Abdel-Haleem (2011) | Placebo group | 28 | 8.92 ± 2.53 | 30.60 ± 6.61 | 50 mL saline 0.9% iv | after intubation 3– 5 min before start of surgery |
Adenoidectomy/tonsillectomy | ASA I---II | NA | NA | NA | NA |
| Dexmedetomidine IV | 28 | 8.26 ± 2.35 | 28.85 ± 8.35 | 1ug/kg dexmedetomidine given by iv. infusion | |||||||||
| dexmedetomidine.PT | 28 | 8.60 ± 2.31 | 30.28 ± 8.70 | l ug/kg dexmedetomidine | |||||||||
| 24 | Shen et al. (2022) | Normal saline | 125 | 12 (9.6) | 17.2 (15.4–19.1) | 1 mL of 0.9%saline | anesthesia induction 前 | Tonsillectomy/Adenoidectomy | ASA I or II | 15.0 (12.0–17.0) | 27 (21.6) | 23 (18.4) | ①②③ |
| Midazolam | 124 | 17 (13.7) | 15.9 (14.6–18.3) | intranasal midazolam (0.1 mg/kg) | 14.0 (12.0–16.0) | 36 (29.0) | 30 (24.2) | ||||||
| Dexmedetomidine | 124 | 16 (12.9) | 16.3 (14.6–18.4) | intranasal Dexmedetomidine 2.0 μg/kg | 15.0 (12.0–17.0) | 12 (9.7) | 14 (11.3) | ||||||
| 25 | Cho et al. (2019) | Midazolam | 32 | 7.2 ± 2.2 | 28.9 ± 11.3 | 0.03 mg/kg midazolam, IV | Five minutes before the end of surgery | elective tonsillectomy | ASA I or II | 19.0 [13.0–23.0] | 10 (31.3%) | 5 (15.6%) | ② |
| Dexmedetomidine | 34 | 6.7 ± 2.4 | 26.3 ± 10.0 | Dexmedetomidine 0.3 μg/kg, IV | 18.5 [15.0–25.0] | 9 (26.5%) | 3 (8.8%) | ||||||
| 26 | Mahfouz et al. (2011) | Group D | 60 | 8.2 ± 1.4 | 18.40 ± 4.74 | intranasal 1 ug/kg dexmedetomidine | before induction of anesthesia | adenotonsillectomy | ASA I | 38.27 ± 4.31 | NA | 8 (13) | NA |
| Group M | 60 | 8.1 ± 2.3 | 17.9 ± 5.89 | 10 mL apple juice orally as a placebo | 36.77 ± 4.62 | 15 (25)* | |||||||
| 27 | Akin et al. (2012) | Midazolam | 45 | 6 (2–9) | 19.5 (11–35) | 0.2 mg/kg of intranasal midazolam | before the induction of anesthesia | adenotonsillectomy | ASA I | NA | NA | 15 (33.3) | ②③ |
| Dexmedetomidine | 45 | 5 (3–9) | 18.5 (11–35) | intranasal 1 ug/kg dexmedetomidine | 6 (13.3) | ||||||||
| 28 | Elagamy et al. (2020) | Group (Dexmedetomidine) | 80 | 4.5 ± 0.81 | 17.18 ± 2.5 | 0.5 μg/kg Dexmedetomidine by IV infusion | over 10 min after induction of anesthesia | adenotonsillectomy | ASA I or II | 40.38 ± 7.43 | NA | NA | ②⑤⑥ |
| Group (Nal) | 80 | 4.7 ± 1.2 | 16 ± 2.53 | 0.9% normal saline IV | 37.16 ± 9.38 | ||||||||
| 29 | Zhuang et al. (2011) | Morphine | 30 | 5.0 (2.5) | 21.9 (9.4) | Intravenous dexmedetomidine 1 ug/kg | anaesthetic induction | adenotonsillectomy | ASA I or II | NA | NA | 30% | ② |
| Dexmedetomidine | 30 | 4.5 (1.7) | 22.6 (7.9) | Intravenous dmorphine 100 ug/kg | 57% | ||||||||
| 30 | Bedirli et al. (2017a) | Group T | 39 | 8.4 ± 2.1 | 28.3 ± 3.7 | 2 mg/kg tramadol | After intubation | Adenotonsillectomy | ASA I–II | 15.2 ± 4.7 | NA | 19 | ② |
| Group D | 38 | 6.7 ± 3.1 | 27.1 ± 2.7 | 1 μg/kg dexmedetomidine | 37.6 ± 5.4 | 17 | |||||||
| 31 | Koceroglu et al. (2019) | Dexmedetomidine group | 30 | 6.17 ± 2.07 | 22.77 ± 5.94 | 1 μg/kg dexmedetomidine | tthe end of surgery | adenotonsillectomies | ASA I -II | NA | NA | NA | ②④ |
| Tramadol group | 30 | 5.4 ± 2.19 | 19.6 ± 7.24 | 1.5 mg/kg tramadol | |||||||||
| 32 | Modir et al. (2024) | Dexmedetomidine -ropivacaine | 54 | 6.97 ± 1.45 | NA | 0.25% ropivacaine +1 μg/kg dexmedetomidine | before surgical incision | tonsillectomy | ASA I -II | NA | NA | 7 (21.21) | ② |
| Tramadol-ropivacaine | 54 | 6.97 ± 1.45 | NA | the same ropivacaine solution +2 mg/kg tramadol | 29 (87.87) | ||||||||
| Placebo-ropivacaine | 54 | 6.97 ± 1.45 | NA | the same solution + normal saline | 33 (100) | ||||||||
| 33 | Patel et al. (2010) | Group D (Dexmedetomidine) | 61 | 4.2 ± 2.1 | 18.3 ± 5.7 | IV dexmedetomidine 2 μg/kg | 5 min before the end of the surgery) | Tonsillectomy and Adenoidectomy | ASA II–III | 7.18 ± 4.05 | NA | 22 (36.1) | ④ |
| Group F (fentanyl) | 61 | 3.8 ± 1.5 | 20.4 ± 8.6 | IV fentanyl bolus 1 μg/kg | 8.75 ± 4.06 | NA | 6 (9.8) | ||||||
| Dexmedetomidine | 20 | 4.81 ± 1.09 | 21.60 ± 5.12 | intravenously 0.4 μg/kg dexmedetomidine | 14.86 ± 3.89 | 25.00% | |||||||
| Dexmedetomidine + Alf1 group | 20 | 5.13 ± 1.29 | 23.15 ± 9.31 | intravenously with 0.4 μg/kg dexmedetomidine and alfentanil (10 μg/kg) | 15.61 ± 4.59 | 5.00% | |||||||
| Dexmedetomidine + Alf2 group | 20 | 5.11 ± 1.23 | 22.69 ± 9.83 | intravenously with 0.4 μg/kg dex and alfentanil (20 μg/kg) | 19.25 ± 4.38 | 0.00% | |||||||
| 34 | Pestieau et al. (2011b) | fentanyl 1 μg/kg (Group 1) | 26 | 4 (2–9.9) | 17.4 (4.0) | fentanyl 1 μg/kg | immediately after endotracheal intubation | Tonsillectomy with or without adenoidectomy | ASA I or II | NA | 15 (58) | 25 (96) | NA |
| fentanyl 2 μg/kg (Group 2) | 25 | 4.7 (2.1–11.8) | 16.6 (2.8) | fentanyl 2 μg/kg | 14 (56) | 18 (72) | |||||||
| Dex 2 μg/kg (Group 3) | 25 | 5.3 (2.3–12.9) | 17.1 (3.4) | dexmedetomidine 2 μg/kg | 5 (20) | 7 (28) | |||||||
| Dex 4 μg/kg (Group 4) | 25 | 4.3 (2.2–11.9) | 18.1 (3.5) | dexmedetomidine 4 μg/kg | 4 (16) | 7 (28) | |||||||
| 35 | Anjana et al. (2021) | Group F (fentanyl) | 60 | 7 | 24 (11) | fentanyl 2 μg/kg intravenously | premedication | tonsillectomy | ASA I or II | 14 (6.5) | NA | NA | ① |
| Group D (Dex) | 60 | 7.5 | 24 (12) | dexmedetomidine 0.5 μg/kg as intravenous infusion | 13 (4) | ||||||||
| 36 | Erdil et al. (2009) | Group C | 30 | 4.2 ± 1.3 | 17.3 ± 4.0 | saline solution | After induction | adenoidectomy with or without bilateral myringotomy | ASA I | 12.0 ± 4.2 | 47% (14/30) | 13 | ② |
| Group F (fentanyl) | 30 | 4.6 ± 1.4 | 17.0 ± 3.6 | fentanyl 2.5 μg/kg | 16.1 ± 5.3 | 13% (4/30) | 4 | ||||||
| Group D (Dexmedetomidine) | 30 | 4.7 ± 1.4 | 17.9 ± 3.2 | dexmedetomidine 0.5 μg/kg | 12.7 ± 3.2 | 17% (5/30) | 5 |
Characteristics of included randomized-controlled trial.
① Cough; ② Nausea and vomiting; ③ Laryngospasm; ④ Low oxygen saturation; ⑤ Hypotension; ⑥ Bradycardia.
3.2 Quality assessment (RoB tool)
The Cochrane RoB tool assessed allocation concealment, random sequence generation, participant and personnel outcome assessment blinding, selective reporting, insufficient outcome data, and additional biases. Two reviewers, Xianghong Lian and Ting Luo, engaged in the process, and when conflicts arose between them, they deliberated, discovered the underlying causes, and then reached a final judgment. If an agreement could not be reached, the ultimate decision was rendered by a third evaluator.
All investigations (36/36) employed an appropriate approach, using either manual or computerized random number tables. Of these, 29 explicitly addressed allocation concealment. Blinding of participants and research staff was implemented in 83.33% of the trials (30 out of 36). All trials (36/36) provided complete outcome data, and 97.22% of studies (35 out of 36) indicated no selective reporting upon review procedures. Blinding of outcome evaluation was conducted in 94.44% of trials (34 out of 36). Assessment of other biases was inconclusive in most trials (Figure 2).
FIGURE 2
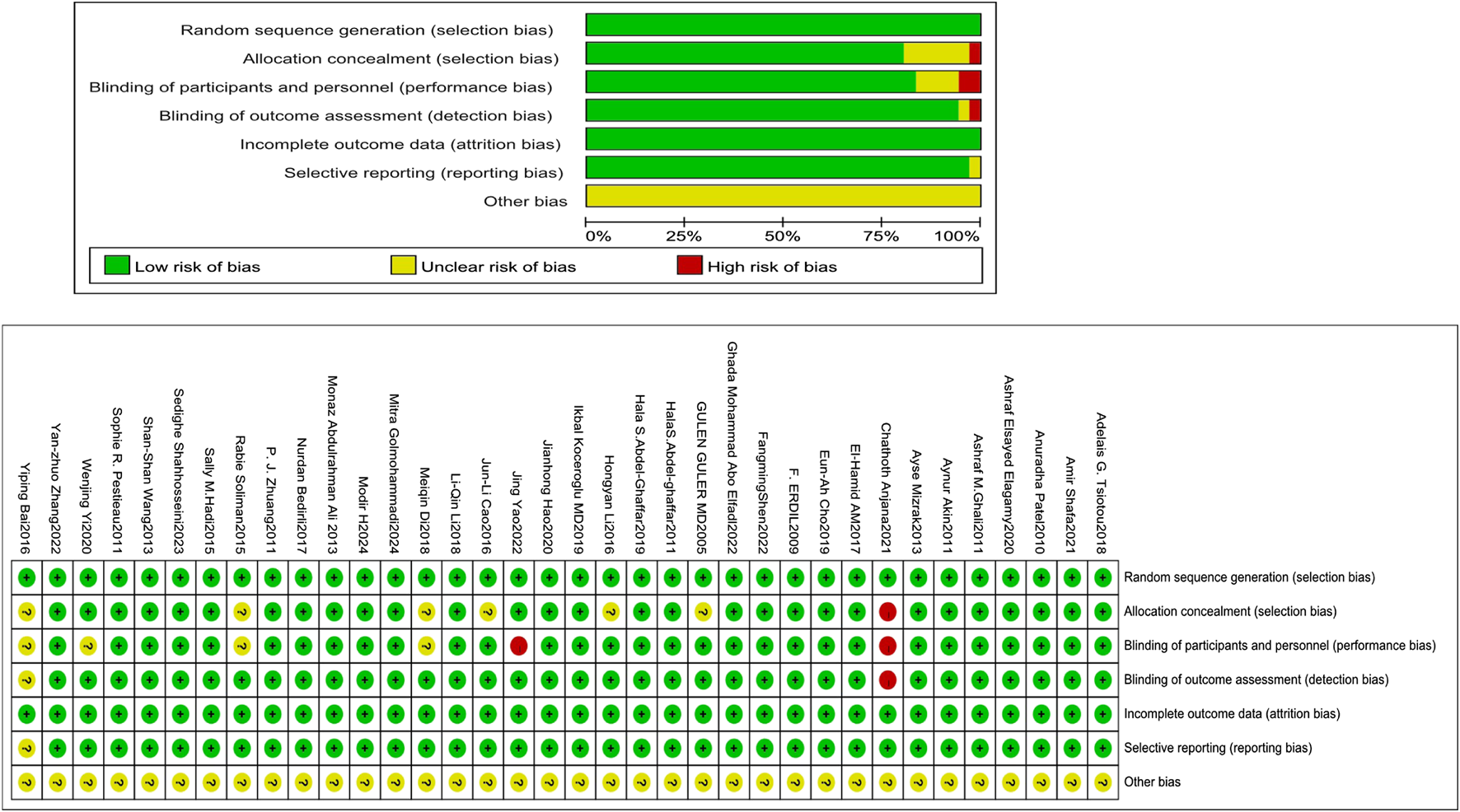
Evaluation of the quality of the included studies.
3.3 Data examination
3.3.1 Incidence of EA
Fourteen studies (Golmohammadi et al., 2024; Shen et al., 2022; Ali and Abdellatif, 2013; Tsiotou et al., 2018; Soliman and Alshehri, 2015; Bai et al., 2016; El-Hamid and Yassin, 2017; Li L-Q. et al., 2018; Guler et al., 2005; Hadi et al., 2015; Zhang et al., 2022; Cho et al., 2019; Pestieau et al., 2011b; Erdil et al., 2009) with 1334 patients evaluated the efficacy of Dex relative to that of three comparators in mitigating the risk of EA in children. Dex significantly reduced the incidence of EA compared with placebo, benzodiazepines, and opioids [OR = 0.23, 95% CI (0.16, 0.32), I2 = 44% [OR = 0.51, 95% CI (0.28, 0.93), I2 = 44%] [OR = 0.19, 95% CI (0.09, 0.39), I2 = 0%] (P < 0.0001) (Figure 3). No differences in significance levels emerged from the sensitivity analyses performed for each comparison.
FIGURE 3
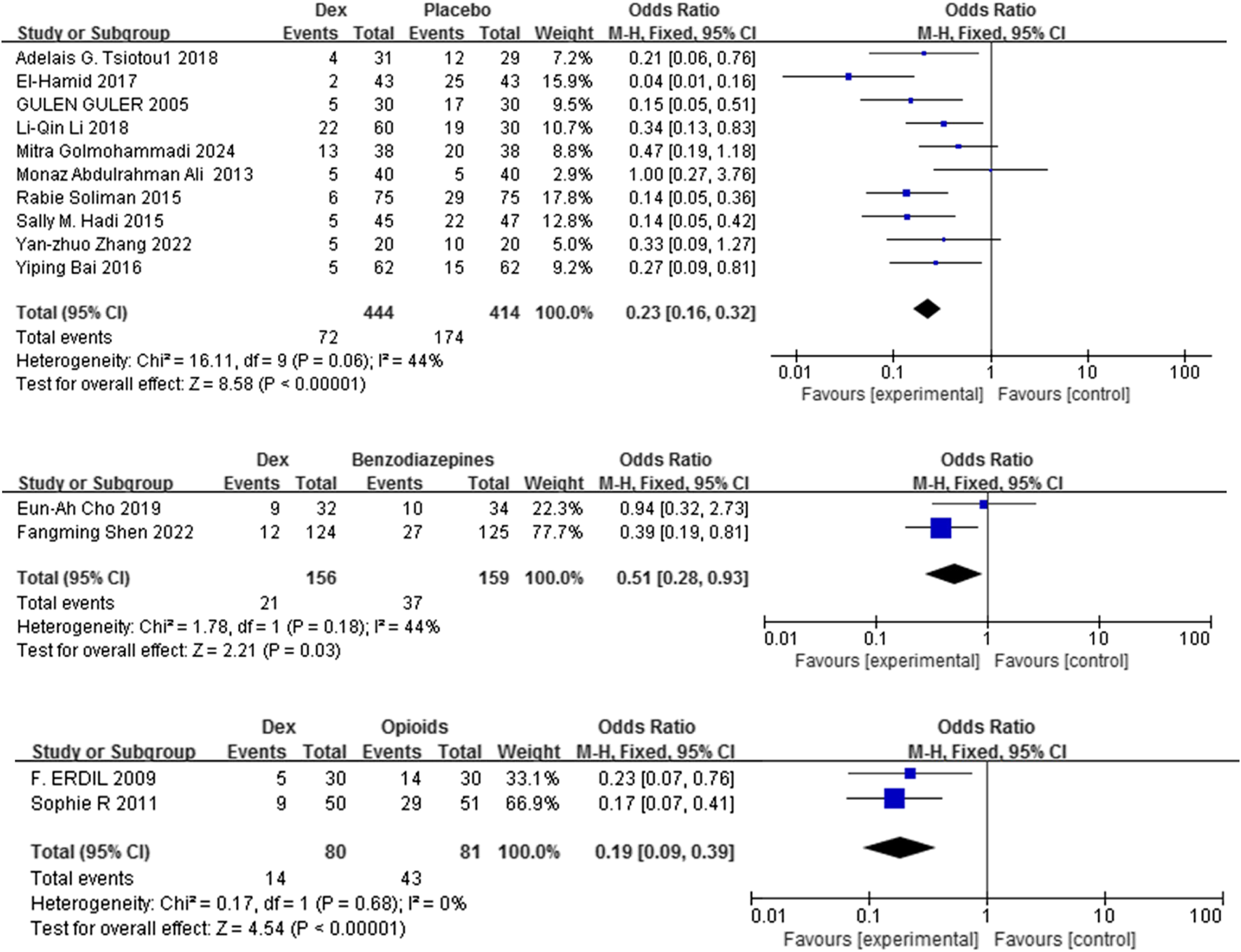
Forest plot illustrating EA incidence: Dex group versus control group.
3.3.2 Frequency of rescue analgesic use
Eleven trials (Shen et al., 2022; Guler et al., 2005; Zhang et al., 2022; Mahfouz et al., 2011; Akin et al., 2012; Zhuang et al., 2011; Bedirli et al., 2017a; Modir et al., 2024; Patel et al., 2010; Pestieau et al., 2011b; Erdil et al., 2009) including 1320 patients compared Dex with control (placebo, benzodiazepines, and opioids) on the frequency of rescue analgesic use. Dex substantially reduced the incidence of rescue analgesics compared with placebo, and benzodiazepines [OR = 0.17, 95% CI (0.10, 0.30), I2 = 0%,] [OR = 0.47, 95% CI (0.29, 0.76), I2 = 0%] (P < 0.0001) (Figure 4).
FIGURE 4
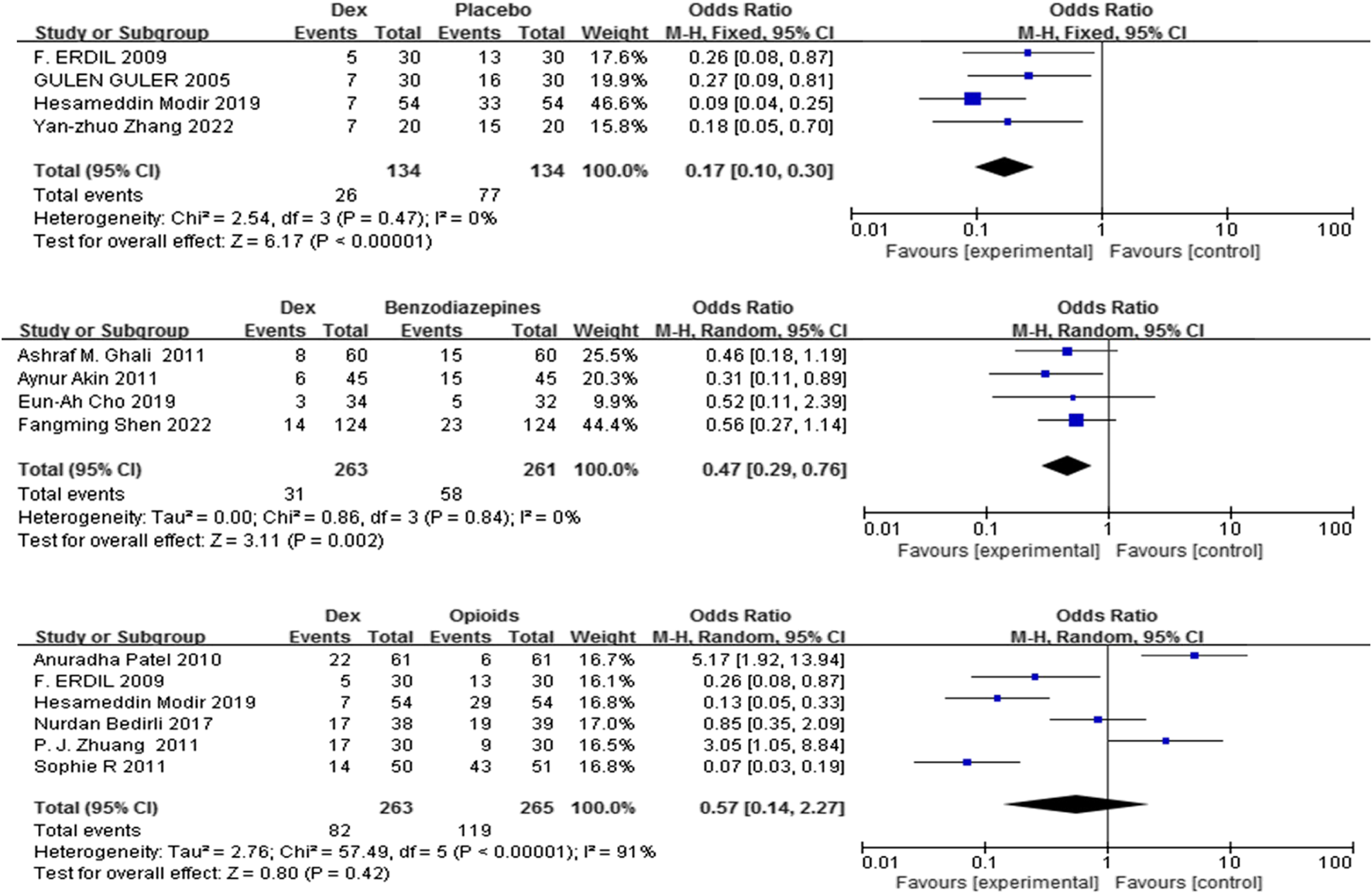
Forest plot comparing Dex and control groups on the frequency of patients who needed rescue analgesia.
In contrast, no significant difference was found in the frequency of rescue analgesic use (%) between the Dex and opioid groups [OR = 0.57, 95% CI (0.14, 2.27), I2 = 91%, P = 0.42] (Figure 4).
3.3.3 Recovery duration
Recovery duration was defined as the period between the cessation of anesthesia and the patient’s eyes openings upon a verbal command. Fifteen studies (Golmohammadi et al., 2024; Shen et al., 2022; Shafa et al., 2021; Cao et al., 2016; Mizrak et al., 2013; Li H. et al., 2018; El-Hamid and Yassin, 2017; Guler et al., 2005; Zhang et al., 2022; Cho et al., 2019; Mahfouz et al., 2011; Elagamy et al., 2020; Patel et al., 2010; Anjana et al., 2021; Erdil et al., 2009) with 1320 patients were included, and the impact of Dex relative to a control group on recovery duration was evaluated. Recovery time was comparable between Dex and placebo, benzodiazepines, and opioids [SMD = 0.15, 95% CI (−0.08, 0.37), I2 = 68%, P = 0.20] [SMD = 0.10, 95% CI (−0.08, 0.28), I2 = 61%, P = 0.28] [SMD = −0.19, 95% CI (−0.42, 0.04), I2 = 38%, P = 0.10] (Figure 5).
FIGURE 5
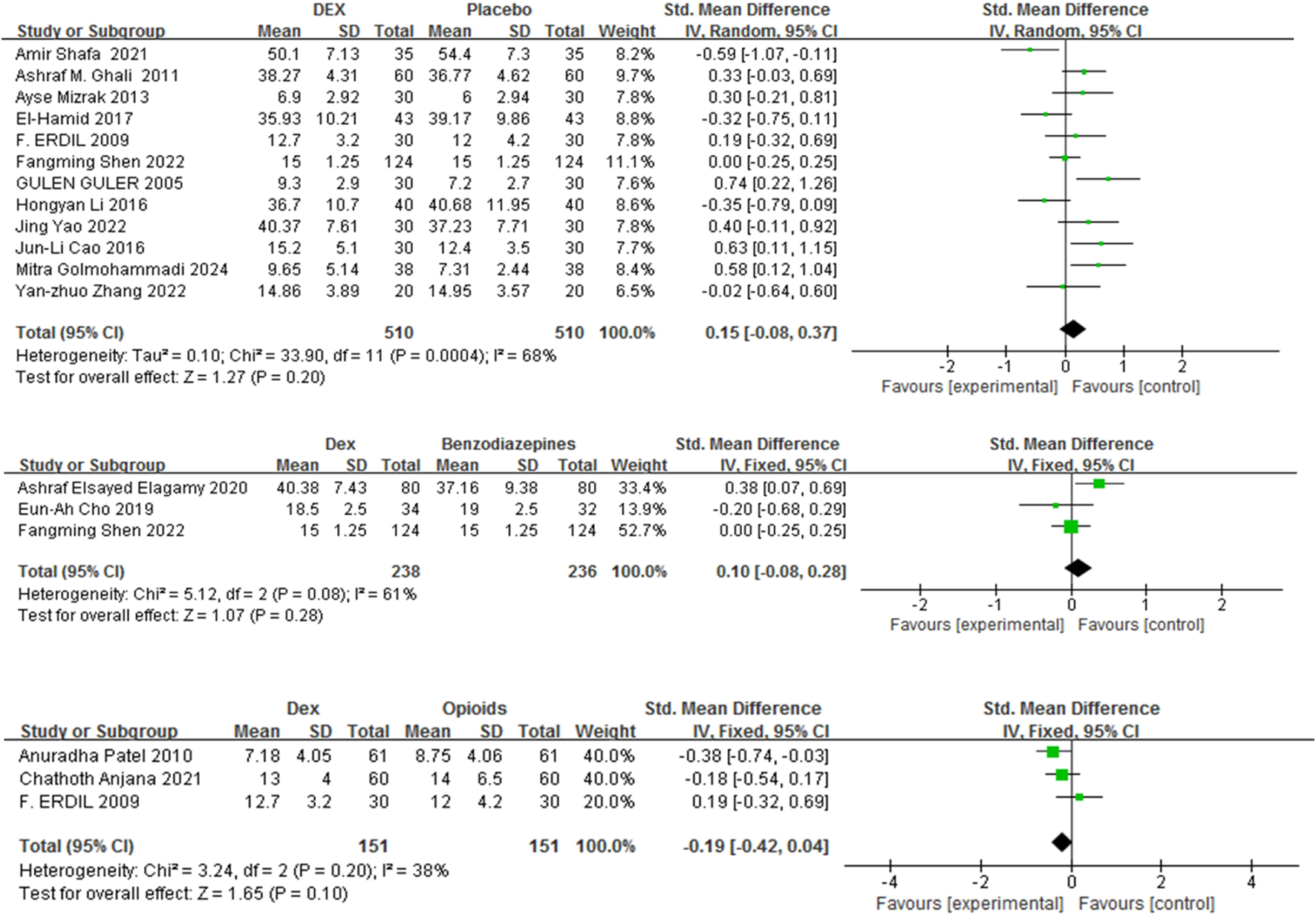
Forest plot comparing recovery time between Dex and control groups.
3.3.4 Perioperative complications
Among the 36 RCTs, 24 studies (Golmohammadi et al., 2024; Shen et al., 2022; Ali and Abdellatif, 2013; Tsiotou et al., 2018; Soliman and Alshehri, 2015; Bai et al., 2016; Li H. et al., 2018; El-Hamid and Yassin, 2017; Li L-Q. et al., 2018; Abo Elfadl et al., 2022; Guler et al., 2005; Abdel-Ghaffar et al., 2019; Di et al., 2018; Hadi et al., 2015; Zhang et al., 2022; Cho et al., 2019; Mahfouz et al., 2011; Akin et al., 2012; Zhuang et al., 2011; Bedirli et al., 2017a; Modir et al., 2024; Patel et al., 2010; Pestieau et al., 2011b; Erdil et al., 2009) including 2,294 children were analyzed. Compared with placebo, benzodiazepines, and opioids, Dex markedly reduced the incidence of perioperative complications [OR = 0.58, 95% CI (0.45, 0.75), I2 = 45%] [OR = 0.24, 95% CI (0.16, 0.36), I2 = 0%] [OR = 0.21, 95% CI (0.13, 0.33), I2 = 45%] (P < 0.0001) (Figure 6).
FIGURE 6
![Forest plot showcasing three meta-analyses comparing Dex against placebo, benzodiazepines, and opioids. Each section lists studies, event numbers, totals, and weights. The horizontal lines represent confidence intervals, with diamonds showing overall effect sizes. Dex is favored across all comparisons with odds ratios: 0.58 [0.45, 0.75] for placebo, 0.24 [0.16, 0.36] for benzodiazepines, and 0.21 [0.13, 0.33] for opioids. Heterogeneity and statistical significance are noted for each analysis.](https://www.frontiersin.org/files/Articles/1681936/xml-images/fphar-16-1681936-g006.webp)
Occurrence of perioperative complications in Dex versus the control groups.
3.3.4.1 Occurrence of perioperative complications
Dex reduced the risk of vomiting, cough, oxygen saturation (%), and laryngospasm compared with controls [OR = 0.54, 95% CI (0.40, 0.73), I2 = 21%,] [OR = 0.54, 95% CI (0.37, 0.77), I2 = 45%] [OR = 0.41, 95% CI (0.25, 0.69), I2 = 0%] [OR = 0.38, 95% CI (0.19, 0.78), I2 = 0%] (P < 0.05) (Figure 7). No significant difference was observed between the Dex and control groups regarding the risk of hypotension and bradycardia [OR = 2.28, 95% CI (0.99, 5.23), I2 = 0%, P = 0.05] [OR = 2.00, 95% CI (1.00, 3.98), I2 = 2%, P = 0.05] (Figure 7).
FIGURE 7
![Forest plot from a meta-analysis showing the effects of an intervention (DEX) versus placebo on various outcomes like nausea, vomiting, coughing, etc. Each study's odds ratio and confidence interval are depicted, with diamonds summarizing overall effects. The plot indicates significant favoring of DEX in most outcomes, with total odds ratio of 0.61 [0.51, 0.73]. Heterogeneity statistics are provided for each subgroup.](https://www.frontiersin.org/files/Articles/1681936/xml-images/fphar-16-1681936-g007.webp)
Perioperative complications associated with different administration types.
3.3.5 Subgroup analyses
Guided by predefined hypotheses, subgroup analyses were performed to examine how Dex affects EA: stratifying studies by routes of administration (IV versus intranasal), timing of administration (post-anesthesia induction versus pre-surgery conclusion), and dosage variations [low (<0.5 μg/kg), moderate (≥0.5 to <1 μg/kg), and high doses (≥1 μg/kg)]. Table 2 presents the findings derived from subgroup analyses.
TABLE 2
| Subgroup outcomes |
Number of studies | Results of heterogeneity test | Meta analysis results | ||
|---|---|---|---|---|---|
| P value | I2 | OR (95% CI) | P value | ||
| (A) Different administration routes | |||||
| Intravenous | 12 | 0.14 | 32% | 0.27 (0.18,0.41) | <0.0001 |
| Intranasal | 3 | 0.03 | 71% | 0.22 (0.18,0.38) | <0.0001 |
| (B) Different administration time | |||||
| Post-anesthesia induction | 7 | 0.12 | 41% | 0.24 (0.17,0.35) | <0.0001 |
| Pre-surgery conclusion | 5 | 0.06 | 56% | 0.34 (0.21,0.54) | <0.0001 |
| (C) Different doses | |||||
| Low (<0.5 μg/kg) | 2 | 0.22 | 34% | 0.50 (0.24,1.04) | 0.06 |
| Moderate (≥0.5 to <1 μg/kg) | 5 | 0.42 | 0% | 0.23 (0.15,0.37) | <0.0001 |
| High (≥1 μg/kg) | 4 | 0.16 | 41% | 0.17 (0.10,0.29) | <0.0001 |
Subgroup analysis results of the effect of Dex on the incidence of EA.
In the subgroup analyses, Dex significantly decreased the frequency of EA, irrespective of whether it was administered via IV or intranasal routes [OR = 0.27, 95% CI (0.18,0.41), I2 = 32%] [OR = 0.22, 95% CI (0.18,0.38), I2 = 71%] (P < 0.0001) (Table 2). Timing of administration had consistent effects on both post-anesthesia induction and pre-surgery conclusion [OR = 0.24, 95% CI (0.17,0.35), I2 = 41%] [OR = 0.34, 95% CI (0.21,0.54), I2 = 56%] (P < 0.0001).
Furthermore, Dex markedly reduced EA at both moderate (≥0.5 to <1 μg/kg) and high doses (≥1 μg/kg) [OR = 0.23, 95% CI (0.15,0.37), I2 = 0%] [OR = 0.17, 95% CI (0.10,0.29), I2 = 41%] (P < 0.0001). In contrast, low-dose Dex (<0.5 μg/kg) did not significantly differ from the control [OR = 0.50, 95% CI (0.24, 1.04), I2 = 34%, P = 0.06].
4 Discussion
T&A in pediatric patients is a prevalent surgical procedure (Papp et al., 1998). Given its brevity, the anesthetics employed should demonstrate quick anesthesia induction, consistent anesthetic effects, minimal respiratory tract irritation, fast recovery, and a low incidence of complications (Thomsen and Gower, 2002; Kulka et al., 2001; D et al., 2010; Park et al., 2014). Consequently, the choice of a suitable anesthetic is crucial to mitigate complication risks and enhance the quality of anesthesia (Lodes, 1999; Maze and Tranquilli, 1991). Dex is recognized for its high selectivity toward α2-adrenoreceptors, enabling it to induce sedation, analgesia, and anxiolysis. It has a short half-life (1.8 h) and does not induce respiratory depression, which has supported its widespread use in several therapeutic contexts (Zhu et al., 2015). Owing to its dual analgesic and sedative properties, dexmedetomidine can serve as a viable adjunct or alternative agent for perioperative management in children undergoing T&A. Certain studies indicate that the prudent application of Dex and multimodal analgesia may lead to decreased opioid consumption or possibly its avoidance (Mann et al., 2021; Franz et al., 2019; Adler et al., 2021; Shi et al., 2019; Zhang et al., 2019; Sun et al., 2014). Consequently, an essential aspect in analyzing these results is the extent to which pain and agitation may be clinically intertwined.
This study demonstrates that, compared with placebo, benzodiazepines, and opioids, Dex was more effective in lowering the incidence of EA (Figure 3). This meta-analysis is the first to perform a specific subgroup analysis on the efficacy of Dex in preventing EA, providing novel, granular evidence on its optimal use that was not available in previous pooled analyses. Moreover, other measures have been employed to evaluate EA, including the Pediatric Anesthesia Emergence Delirium (PAED) scale developed by Sikich and Lerman, as well as five scales validated by Cole et al. (He et al., 2013; Hauber et al., 2015), which are extensively utilized. We incorporated the PAED scale into our study, and the results indicate that Dex significantly decreased PAED scores at 15, 30, and 45 min post-administration (Supplementary Figure S1), corroborating prior findings that Dex decreases the frequency of EA.
Pain, while not the only cause of EA, is a significant etiological element, and alleviating pain is often regarded as a means to reduce the frequency of EA linked to general anesthesia (Sun et al., 2014; Bedirli et al., 2017b). This review highlights the use of acetaminophen, NSAIDs, and a single steroid dose in pediatric T&A anesthesia (Mann et al., 2021). Compared with placebo or benzodiazepines, Dex decreased the need for rescue analgesics, reinforcing the analgesic properties of Dex in mitigating EA (Figure 2). In comparison to opioids, Dex appeared to lower EA. However, this assessment was derived from the analysis of only two studies. The observed lack of significant difference in rescue analgesic use [OR = 0.57, 95% CI (0.14, 2.27), I2 = 91%, P = 0.42] suggests notable uncertainty surrounding the comparative pain control benefits of Dex versus opioids. Consequently, these findings warrant cautious interpretation, and further empirical evidence is needed for confirmation.
Furthermore, recovery time was comparable between the Dex group and the control group, indicating that Dex does not delay or increase recovery to discharge time in the PACU. Several factors might account for these results. First, patients who did not receive Dex utilized supplementary medications, including opioids, for EA management (Zhuang et al., 2011; Modir et al., 2024; Albornoz et al., 2024). Second, the short half-life (under 2 h) of administered Dex may also inhibit an extended recovery duration.
PRAEs are the most prevalent complications associated with pediatric anesthesia. In pediatric cases, airway trauma from surgery induces edema in the upper respiratory tract and adjacent tissues in children, thus leading to the retention of secretions in the airway, and significantly increasing the risk of PRAEs (Shen et al., 2022). A significant percentage of children who had tonsillectomies encounter PRAEs, with the incidence reaching 50%. Dex has demonstrated efficacy in decreasing the incidence of PRAEs in pediatric patients with congenital heart disease (Zhang et al., 2020; von Ungern-Sternberg et al., 2013; von Ungern-Sternberg et al., 2019); however, conclusive data from rigorous assessments on its preoperative use for T&A-related PRAEs are currently insufficient. Our findings indicate that the occurrence of oxygen desaturation and laryngospasm dramatically decreased with Dex administration (Figure 7). Multiple pathways may contribute to this advantageous effect. First, Dex may increase the anesthetic level, thereby dampening airway reflex activity (Najafi et al., 2016; Wang et al., 2014). Second, its immunomodulatory effects, demonstrated through decreased interleukin-6 and tumor necrosis factor–α levels, may reduce airway inflammation and sensitivity (Tang et al., 2015). Third, Dex may correlate with reduced coughing and desaturation by decreasing the need for analgesics, attributable to its opioid-sparing properties. These findings indicate that the opioid-sparing properties of Dex may be advantageous for high-risk T&A patients. Moreover, hypotension or bradycardia occurred at similar rates in the Dex and control groups. Dex is known to induce hypotension, which may occasionally be preceded strangely by hypertension. This effect can be alleviated by avoiding fast infusion and bolus dosing. In studies with strict protocol adherence, Dex—used at conservative doses and not delivered intravenously—demonstrated a safety profile similar to the control group concerning hypotension and bradycardia occurrence (Ebert et al., 2000). In addition, due to its risks of hypotension and bradycardia pharmacological effects, it should only be used by healthcare professionals in settings equipped with medical monitoring facilities. Additionally, patients receiving this infusion should be under continuous monitoring, and should be discharged after demonstrating recovery from anesthesia and meeting established discharge criteria.
Subgroup studies of EA incidence were conducted to discern variations in the effects of administration route, timing, and dose. Both administration strategies and time points improved the incidence of EA. Furthermore, our findings indicated that compared with high doses (Dex ≥1 μg/kg), moderate doses (Dex ≥0.5, <1 μg/kg) markedly decreased the incidence of EA. Despite the results of the subgroup analyses, compared with the control treatment, low-dose Dex (<0.5 μg/kg) failed to significantly reduce the incidence of EA. Dex has dose-dependent effects on analgesia and sedation; lower dosages are associated with lower sedative efficacy, leading to an increased incidence of EA, similar to prior findings (Zh et al., 2021).
This study has several limitations. The exclusive focus on RCTs, while methodologically rigorous, may omit insights from other study designs. Heterogeneity in Dex regimens, adjuvant therapies, and small subgroup samples may affect generalizability. Although funnel plots revealed no publication bias (Supplementary Figure S2), language bias is possible given the exclusion of non-English studies. Moreover, the majority of RCTs have documented only these monitoring indicators within the post-anesthesia care unit, leaving the analgesic impact and its implications on neurological features post-discharge unexamined. Well-designed RCTs are essential for determining both the analgesic benefits and the post-discharge neurocognitive risks of Dex, especially regarding mood and focus capacity.
Finally, systematic studies comparing different administration routes, dosing timings, and dose regimens of Dex are currently lacking. Therefore, optimal routes of administration, specific doses, or timing strategies for Dex cannot yet be determined, and further research is needed.
5 Conclusion
Our study revealed that compared with different targets, Dex significantly reduced the overall occurrence of EA and perioperative complications. Furthermore, recovery time was comparable between subjects in the Dex group and those in the control group, indicating that Dex does not delay awakening to discharge readiness in the PACU. The present meta-analysis demonstrated the protective effect of Dex on EA and perioperative complications. Dex could be a useful analgesic option for children undergoing tonsillectomy with or without adenoidectomy. However, additional studies are needed to confirm these findings. Furthermore, high-quality research with a standard definition for EA is needed to explore the optimal administration route, dosage, and timing of Dex in pediatric anesthesia. Well-designed RCTs are essential for determining both the analgesic benefits and the post-discharge neurocognitive risks of Dex, especially regarding mood and focus capacity. Finally, further research is needed to compare the effects of different Dex doses in T&A.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JH: Methodology, Writing – original draft, Data curation. XL: Investigation, Data curation, Writing – review and editing, Methodology. TL: Investigation, Writing – review and editing, Conceptualization, Formal Analysis.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1681936/full#supplementary-material
References
1
Abdel-ghaffar H. S. Abdel-Haleem A. K. (2011). Efficacy and safety of intraoperative dexmedetomidine in pediatric posttonsillectomy pain: peritonsillar versus intravenous administration. Egypt. J. Anaesth.27, 219–225. 10.1016/j.egja.2011.08.001
2
Abdel-Ghaffar H. S. Abdel-Wahab A. H. Roushdy M. M. (2019). Oral trans-mucosal dexmedetomidine for controlling of emergence agitation in children undergoing tonsillectomy: a randomized controlled trial. Braz. J. Anesthesiol. Engl. Ed.69 (69), 469–476. 10.1016/j.bjan.2019.06.012
3
Abo Elfadl G. M. AbdelRady M. M. Osman H. M. Gad M. O. Abd El-Rady N. M. Ali W. N. (2022). Efficacy of levobupivacaine versus levobupivacaine plus dexmedetomidine infiltration for post-tonsillectomy analgesia: a randomized controlled trial. Pain Res. Manag.2022, 9958668–7. 10.1155/2022/9958668
4
Adler A. C. Daszkowski A. Tan J. C. Poliner A. D. Wei E. Z. Nathanson B. H. et al (2021). The association of dexmedetomidine on perioperative opioid consumption in children undergoing adenotonsillectomy with and without obstructive sleep apnea. Anesth. Analg.133, 1260–1268. 10.1213/ANE.0000000000005410
5
Akin A. Bayram A. Esmaoglu A. Tosun Z. Aksu R. Altuntas R. et al (2012). Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Pediatr. Anesth.22, 871–876. 10.1111/j.1460-9592.2012.03802.x
6
Albornoz A. E. Rana M. Hayes J. Englesakis M. Tsang M. Amin R. et al (2024). Perioperative clinical practice recommendations for pediatric tonsillectomy: a systematic review. Can. J. Anaesth.71 (2), 187–200. 10.1007/s12630-023-02668-z
7
Aldamluji N. Burgess A. Pogatzki-Zahn E. Raeder J. Beloeil H. (2021). PROSPECT guideline for tonsillectomy: systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia76, 947–961. 10.1111/anae.15299
8
Ali M. A. Abdellatif A. A. (2013). Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: a comparison of dexmedetomidine and propofol. Saudi J. Anaesth.7, 296–300. 10.4103/1658-354X.115363
9
Anjana C. Indu S. Vineetha P. (2021). Effects of dexmedetomidine and fentanyl premedication on quality of extubation in children undergoing tonsillectomy: a prospective cohort study. J. Clin. Diagnostic Res.10.7860/JCDR/2021/47441.14469
10
Bai Y. Yu H. Wang M. (2016). Effect of intraoperative application of dexmedetomidine on early postoperative cognitive function and serum brain-derived neurotrophic factor (BDNF) in children undergoing tonsillectomy. Int. J. Clin. Exp. Med.9, 8482–8489.
11
Bedirli N. Akçabay M. Emik U. (2017a). Tramadol vs dexmedetomidine for emergence agitation control in pediatric patients undergoing adenotonsillectomy with sevoflurane anesthesia: prospective randomized controlled clinical study. BMC Anesthesiol.17, 41. 10.1186/s12871-017-032-4
12
Bedirli N. Akçabay M. Emik U. (2017b). Tramadol vs dexmedetomidine for emergence agitation control in pediatric patients undergoing adenotonsillectomy with sevoflurane anesthesia: prospective randomized controlled clinical study. BMC Anesthesiol.17, 41. 10.1186/s12871-017-0332-4
13
Belyea J. Chang Y. Rigby M. H. Corsten G. Hong P. (2014). Post-tonsillectomy complications in children less than three years of age: a case-control study. Int. J. Pediatr. Otorhinolaryngol.78, 871–874. 10.1016/j.ijporl.2014.02.029
14
Cao J. L. Pei Y. P. Wei J. Q. Zhang Y. Y. (2016). Effects of intraoperative dexmedetomidine with intravenous anesthesia on postoperative emergence agitation/delirium in pediatric patients undergoing tonsillectomy with or without adenoidectomy: a CONSORT-prospective, randomized, controlled clinical trial. Med. Baltim.95, 95:e5566. 10.1097/MD.0000000000005566
15
Cho H. K. Yoon H. Y. Jin H. J. Hwang S. H. (2018). Efficacy of dexmedetomidine for perioperative morbidities in pediatric tonsillectomy: a metaanalysis. Laryngoscope128, E184–E193. 10.1002/lary.26888
16
Cho E.-A. Cha Y.-B. Shim J.-G. Ahn J.-H. Lee S. H. Ryu K.-H. (2019). Comparison of single minimum dose administration of dexmedetomidine and midazolam for prevention of emergence delirium in children: a randomized controlled trial. J. Anesth.34, 59–65. 10.1007/s00540-019-02705-6
17
Dahmani S. Stany I. Brasher C. Lejeune C. Bruneau B. Wood C. et al (2010). Pharmacological prevention of sevoflurane- and desflurane-related emergence agitation in children: a meta-analysis of published studies. Br. J. Anaesth.104, 216–223. 10.1093/bja/aep376
18
Di M. Yang Z. Qi D. Lai H. Wu J. Liu H. et al (2018). Intravenous dexmedetomidine pre-medication reduces the required minimum alveolar concentration of sevoflurane for smooth tracheal extubation in anesthetized children: a randomized clinical trial. BMC Anesthesiol.18, 9. 10.1186/s12871-018-0469-9
19
Ebert T. J. Hall J. E. Barney J. A. Uhrich T. D. Colinco M. D. (2000). The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology93, 382–394. 10.1097/00000542-200008000-00016
20
El-Hamid A. M. A. Yassin H. M. (2017). Effect of intranasal dexmedetomidine on emergence agitation after sevoflurane anesthesia in children undergoing tonsillectomy And/or adenoidectomy. Saudi J. Anaesth.11, 137–143. 10.4103/1658-354X.203020
21
Elagamy A. E. Mahran M. G. Mahmoud A. Z. (2020). Dexmedetomidine versus nalbuphine in prevention of emergence agitation following adenotonsillectomy in pediatrics. Egypt. J. Anaesth.36, 24–29. 10.1080/11101849.2020.1728865
22
Erdil F. Demirbilek S. Begec Z. Ozturk E. Ulger M. H. Ersoy M. O. (2009). The effects of dexmedetomidine and fentanyl on emergence characteristics after adenoidectomy in children. Anaesth. Intensive Care37, 571–576. 10.1177/0310057X0903700405
23
Franz A. M. Dahl J. P. Huang H. Verma S. T. Martin L. D. Martin L. D. et al (2019). The development of an opioid sparing anesthesia protocol for pediatric ambulatory tonsillectomy and adenotonsillectomy surgery-A quality improvement project. Paediatr. Anaesth.29 (7), 682–689. 10.1111/pan.13662
24
Golmohammadi M. Sane S. Ghavipanjeh R. S. Hosseini R. Alwaily E. R. Hussien B. M. et al (2024). Investigating the effect of dexmedetomidine in controlling postoperative emergence agitation in children under Sevoflurane Anesthesia. Anesthesiol. Res. Pract.2024, 6418429. 10.1155/2024/6418429
25
Guler G. Akin A. Tosun Z. Ors S. Esmaoglu A. Boyaci A. (2005). Single‐dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Pediatr. Anesth.15, 762–766. 10.1111/j.1460-9592.2004.01541.x
26
Hadi S. M. Saleh A. J. Tang Y. Z. Daoud A. Mei X. Ouyang W. (2015). The effect of KETODEX on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane based-anesthesia. Int. J. Pediatr. Otorhinolaryngology79, 671–676. 10.1016/j.ijporl.2015.02.012
27
Hall M. J. Schwartzman A. Zhang J. Liu X. (2017). Ambulatory surgery data from hospitals and ambulatory surgery centers: united States, 2010. Natl. Health Stat. Rep.102, 1–15.
28
Hao J. Wu Z. Luo Z. Dong B. (2020). Addition of dexmedetomidine to ropivacaine for local infiltration anaesthesia improves analgesic efficacy after tonsillectomy and adenoidectomy: a randomized controlled trial. Int. J. Pediatr. Otorhinolaryngology137, 110168. 10.1016/j.ijporl.2020.110168
29
Hauber J. A. Davis P. J. Bendel L. P. Martyn S. V. McCarthy D. L. Evans M. C. et al (2015). Dexmedetomidine as a rapid bolus for treatment and prophylactic prevention of emergence agitation in anesthetized children. Anesth. Analg.121, 1308–1315. 10.1213/ANE.0000000000000931
30
He X. Y. Cao J. P. Shi X. Y. Zhang H. (2013). Dexmedetomidine versus morphine or fentanyl in the management of children after tonsillectomy and adenoidectomy: a meta-analysis of randomized controlled trials. Ann. Otol. Rhinol. Laryngol.122, 114–120. 10.1177/000348941312200207
31
Higgins J. P. T. Green S. (2011). Cochrane handbook for systematic reviews of interventions. TheCochrane Collaboration. Available online at: www.cochrane-handbook.org (Accessed March, 2 2011).
32
Koceroglu I. Devrim S. Bingol Tanriverdi T. Gura Celik M. (2019). The effects of dexmedetomidine and tramadol on post‐operative pain and agitation, and extubation quality in paediatric patients undergoing adenotonsillectomy surgery: a randomized trial. J. Clin. Pharm. Ther.45, 340–346. 10.1111/jcpt.13080
33
Kulka P. J. Bressem M. Tryba M. (2001). Clonidine prevents sevoflurane-induced agitation in children. Anesth. Analg.93 (2), 335–338. 10.1213/00000539-200108000-00019
34
Li L. Q. Wang C. Xu H. Y. Lu H. L. Zhang H. Z. (2018a). Effects of different doses of intranasal dexmedetomidine on preoperative sedation and postoperative agitation in pediatric with total intravenous anesthesia undergoing adenoidectomy with or without tonsillectomy. Med. Baltim.97, e12140. 10.1097/MD.000000000012140
35
Li H. Zhang L. Shi M. Yang S. Li S. Gao S. (2018b). Impact of dexmedetomidine on pediatric agitation in the Postanesthesia care unit. J. PeriAnesthesia Nurs.33 (1), 53–57. 10.1016/j.jopan.2016.03.005
36
Li L.-Q. Wang C. Xu H.-Y. Lu H.-L. Zhang H.-Z. (2018c). Effects of different doses of intranasal dexmedetomidine on preoperative sedation and postoperative agitation in pediatric with total intravenous anesthesia undergoing adenoidectomy with or without tonsillectomy. Medicine97, e12140. 10.1097/MD.0000000000012140
37
Lodes U. (1999). Total intravenous anesthesia (TIVA) and balanced anesthesia with short-acting anesthetics for ENT surgery in children. Anaesthesiol. Reanim.24, 13–18.
38
Mace S. E. Brown L. A. Francis L. Godwin S. A. Hahn S. A. Howard P. K. et al (2008). Clinical policy: critical issues in the sedation of pediatric patients in the emergency department. J. Emerg. Nurs.34, e33–e107. 10.1016/j.jen.2008.04.018
39
Mahfouz A. Ghali A. Al-Bahrani M. (2011). Preanesthetic medication in children: a comparison of intranasal dexmedetomidine versus oral midazolam. Saudi J. Anaesth.5, 387–391. 10.4103/1658-354X.87268
40
Mahmoud M. Mason K. P. (2015). Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br. J. Anaesth.115, 171–182. 10.1093/bja/aev226
41
Mann G. E. Flamer S. Z. Nair S. Maher J. N. Cowan B. Streiff A. et al (2021). Opioid-free anesthesia for adenotonsillectomy in children. Int. J. Pediatr. Otorhinolaryngol.140, 110501. 10.1016/j.ijporl.2020.110501
42
Maze M. Tranquilli W. (1991). Alpha-2 adrenoceptor agonists: defining the role in clinical anesthesia. Anesthesiology74, 581–605. 10.1097/00000542-199103000-00029
43
Mizrak A. Karatas E. Saruhan R. Kara F. Oner U. Saricicek V. et al (2013). Does dexmedetomidine affect intraoperative blood loss and clotting tests in pediatric adenotonsillectomy patients?J. Surg. Res.179, 94–98. 10.1016/j.jss.2012.09.014
44
Modir H. Moshiri E. Naghavi F. (2024). Efficacy of peritonsillar infiltration with dexmedetomidine versus tramadol in comparison to placebo for pain control and sedation after tonsillectomy in pediatric patients: a randomized clinical trial. Natl. J. Maxillofac. Surg.15, 40–46. 10.4103/njms.njms_507_21
45
Najafi N. Veyckemans F. Van de Velde A. Poelaert J. (2016). Usability of dexmedetomidine for deep sedation in infants and small children with respiratory morbidities. Acta Anaesthesiol. Scand.60, 865–873. 10.1111/aas.12715
46
Pappas A. L. Sukhani R. Hotaling A. J. Mikat-Stevens M. Javorski J. J. Donzelli J. et al (1998). The effect of preoperative dexamethasone on the immediate and delayed postoperative morbidity in children undergoing adenotonsillectomy. Anesth. Analg.87, 57–61. 10.1097/00000539-199807000-00013
47
Park J. H. Lim B. G. Kim H. Z. Kong M. H. Lim S. H. Kim N. S. et al (2014). Comparison of emergence agitation between sevoflurane/nitrous oxide administration and sevoflurane administration alone in children undergoing adenotonsillectomy with preemptive ketorolac. Korean J. Anesthesiol.66, 34–38. 10.4097/kjae.2014.66.1.34
48
Patel A. Davidson M. Tran M. C. J. Quraishi H. Schoenberg C. Sant M. et al (2010). Dexmedetomidine infusion for Analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth. and Analgesia111, 1004–1010. 10.1213/ANE.0b013e3181ee82fa
49
Pestieau S. R. Quezado Z. M. Johnson Y. J. Anderson J. L. Cheng Y. I. McCarter R. J. et al (2011a). High-dose dexmedetomidine increases the opioid-free interval and decreases opioid requirement after tonsillectomy in children. Can. J. Anaesth.58, 540–550. 10.1007/s12630-011-993-7
50
Pestieau S. R. Quezado Z. M. N. Johnson Y. J. Anderson J. L. Cheng Y. I. McCarter R. J. et al (2011b). High-dose dexmedetomidine increases the opioid-free interval and decreases opioid requirement after tonsillectomy in children. Can. J. Anesthesia/Journal Can. d'anesthésie.58, 540–550. 10.1007/s12630-011-9493-7
51
Rao Y. Zeng R. Jiang X. Li J. Wang X. (2020). The effect of dexmedetomidine on emergence agitation or delirium in children after Anesthesia-A systematic review and meta-analysis of clinical studies. Front. Pediatr.8, 329. 10.3389/fped.2020.00329
52
Shafa A. Aledavud H. Shetabi H. Shahhosseini S. (2021). Effects of the two doses of dexmedetomidine on sedation, agitation, and bleeding during pediatric adenotonsillectomy. Anesth. Pain Med.11, e118424. 10.5812/aapm.118424
53
Shahhosseini S. Naderi Boldaji H. Shetabi H. Shafa A. (2023). Comparative Study of the effect of two different doses of dexmedetomidine to prevent emergence agitation in tonsillectomy in children aged 2 to 12 years old. Adv. Biomed. Res.12, 57. 10.4103/abr.abr_30_21
54
Shen F. Zhang Q. Xu Y. Wang X. Xia J. Chen C. et al (2022). Effect of intranasal dexmedetomidine or midazolam for premedication on the occurrence of respiratory adverse events in children undergoing tonsillectomy and adenoidectomy: a randomized clinical trial. JAMA Netw. Open5, e2225473. 10.1001/jamanetworkopen.2022.25473
55
Shi M. Miao S. Gu T. Wang D. Zhang H. Liu J. (2019). Dexmedetomidine for the prevention of emergence delirium and postoperative behavioral changes in pediatric patients with sevoflurane anesthesia: a double-blind, randomized trial. Drug Des. Devel Ther.13, 897–905. 10.2147/DDDT.S196075
56
Soliman R. Alshehri A. (2015). Effect of dexmedetomidine on emergence agitation in children undergoing adenotonsillectomy under sevoflurane anesthesia: a randomized controlled study. Egypt. J. Anaesth.31, 283–289. 10.1016/j.egja.2015.04.006
57
Steward D. L. Grisel J. Meinzen-Derr J. (2011). Steroids for improving recovery following tonsillectomy in children. Cochrane Database Syst. Rev.2011, Cd003997. 10.1002/14651858.CD003997.pub2
58
Sun L. Guo R. Sun L. (2014). Dexmedetomidine for preventing sevoflurane-related emergence agitation in children: a meta-analysis of randomized controlled trials. Acta Anaesthesiol. Scand.58, 642–650. 10.1111/aas.12292
59
Tang C. Huang X. Kang F. Chai X. Wang S. Yin G. et al (2015). Intranasal dexmedetomidine on stress hormones, inflammatory markers, and Postoperative Analgesia after functional endoscopic sinus surgery. Mediat. Inflamm.2015, 939431. 10.1155/2015/939431
60
Thomsen J. Gower V. (2002). Adjuvant therapies in children undergoing adenotonsillectomy. Laryngoscope112, 32–34. 10.1002/lary.5541121412
61
Tsiotou A. G. Malisiova A. Kouptsova E. Mavri M. Anagnostopoulou M. Kalliardou E. (2018). Dexmedetomidine for the reduction of emergence delirium in children undergoing tonsillectomy with propofol anesthesia: a double-blind, randomized study. Paediatr. Anaesth.28, 632–638. 10.1111/pan.13397
62
Urits I. Peck J. Giacomazzi S. Patel R. Wolf J. Mathew D. et al (2020). Emergence delirium in perioperative pediatric care: a review of Current evidence and new directions. Adv. Ther.37, 1897–1909. 10.1007/s12325-020-01317-x
63
von Ungern-Sternberg B. S. Davies K. Hegarty M. Erb T. O. Habre W. (2013). The effect of deep vs. awake extubation on respiratory complications in high-risk children undergoing adenotonsillectomy: a randomised controlled trial. Eur. J. Anaesthesiol.30, 529–536. 10.1097/EJA.0b013e32835df608
64
von Ungern-Sternberg B. S. Sommerfield D. Slevin L. Drake-Brockman T. F. E. Zhang G. Hall G. L. (2019). Effect of albuterol premedication vs placebo on the occurrence of respiratory adverse events in children undergoing tonsillectomies: the REACT randomized clinical trial. JAMA Pediatr.173, 527–533. 10.1001/jamapediatrics.2019.0788
65
Wang S. S. Zhang M. Z. Sun Y. Wu C. Xu W. Y. Bai J. et al (2013). The sedative effects and the attenuation of cardiovascular and arousal responses during anesthesia induction and intubation in pediatric patients: a randomized comparison between two different doses of preoperative intranasal dexmedetomidine. Pediatr. Anesth.24, 275–281. 10.1111/pan.1284
66
Wang S. S. Zhang M. Z. Sun Y. Wu C. Xu W. Y. Bai J. et al (2014). The sedative effects and the attenuation of cardiovascular and arousal responses during anesthesia induction and intubation in pediatric patients: a randomized comparison between two different doses of preoperative intranasal dexmedetomidine. Paediatr. Anaesth.24, 275–281. 10.1111/pan.12284
67
Yao J. Gong H. Zhao X. Peng Q. Zhao H. Yu S. (2022). Parental presence and intranasal dexmedetomidine for the prevention of anxiety during anesthesia induction in children undergoing tonsillectomy and/or adenoidectomy surgery: a randomized controlled trial. Front. Pharmacol.13, 1015357. 10.3389/fphar.2022.1015357
68
Yi W. Li J. Zhuang Y. Wan L. Li W. Jia J. (2022). The effect of two different doses of dexmedetomidine to prevent emergence agitation in children undergoing adenotonsillectomy: a randomized controlled trial. Braz. J. Anesthesiol. Engl. Ed.72 (72), 63–68. 10.1016/j.bjane.2021.08.019
69
Zhang X. Bai Y. Shi M. Ming S. Jin X. Xie Y. (2021). Effect of different administration and dosage of dexmedetomidine in the reduction of emergence agitation in children: a meta-analysis of randomized controlled trials with sequential trial analysis. Transl. Pediatr.10, 929–957. 10.21037/tp-21-105
70
Zhang Y. Z. Wang X. Wu J. M. Song C. Y. Cui X. G. (2019). Optimal dexmedetomidine dose to prevent emergence agitation under Sevoflurane and Remifentanil Anesthesia during pediatric tonsillectomy and adenoidectomy. Front. Pharmacol.10, 1091. 10.3389/fphar.2019.01091
71
Zhang S. Zhang R. Cai M. Zhang K. Zhang M. Zheng J. (2020). Intranasal dexmedetomidine premedication in children with recent upper respiratory tract infection undergoing interventional cardiac catheterisation: a randomised controlled trial. Eur. J. Anaesthesiol.37, 85–90. 10.1097/EJA.0000000000001097
72
Zhang Y.-z. Wei X.-l. Tang B. Qin Y. Y. Ou M. Jiang X. H. et al (2022). The effects of different doses of alfentanil and dexmedetomidine on prevention of emergence agitation in pediatric tonsillectomy and adenoidectomy surgery. Front. Pharmacol.13, 648802. 10.3389/fphar.2022.648802
73
Zhu M. Wang H. Zhu A. Niu K. Wang G. (2015). Meta-analysis of dexmedetomidine on emergence agitation and recovery profiles in children after sevoflurane anesthesia: different administration and different dosage. PLoS One10, e0123728. 10.1371/journal.pone.0123728
74
Zhuang P. J. Wang X. Zhang X. F. Zhou Z. J. Wang Q. (2011). Postoperative respiratory and analgesic effects of dexmedetomidine or morphine for adenotonsillectomy in children with obstructive sleep apnoea. Anaesthesia66, 989–993. 10.1111/j.1365-2044.2011.06817.x
Summary
Keywords
dexmedetomidine, pediatric, tonsillectomy, adenoidectomy, perioperative complications, emergence agitation, meta-analysis
Citation
He J, Lian X and Luo T (2025) Evaluating dexmedetomidine in mitigating emergence agitation and perioperative complications in pediatric tonsillectomy and/or adenoidectomy: a systematic review and meta-analysis. Front. Pharmacol. 16:1681936. doi: 10.3389/fphar.2025.1681936
Received
08 August 2025
Accepted
13 October 2025
Published
29 October 2025
Volume
16 - 2025
Edited by
Cristian Sandoval, University of La Frontera, Chile
Reviewed by
Behzad Kazemi Haki, University Hospital Southampton NHS Foundation Trust, United Kingdom
Samuel Nuhu, University of Jos, Plateau State Nigeria, Nigeria
Updates
Copyright
© 2025 He, Lian and Luo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Luo, 578490169@qq.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.