Abstract
Background:
The increasing macrolide resistance in Mycoplasma pneumoniae is mainly driven by mutations in the V domain of 23S rRNA (A2063G/A2064G), which impairs the efficacy of first-line treatment. Previous meta-analyses failed to distinguish between mutation subtypes or quantify age-specific susceptibility, blurring the clinical significance of different mutation burdens.
Objective:
To quantify the differential impact of single mutation (A2063G) and double mutation (A2063G + A2064G) on core clinical outcomes and to dissect the age-adjusted effects between children and adults.
Methods:
We searched PubMed, Web of Science, Embase, Scopus, and CNKI databases (up to June 2025). The Newcastle-Ottawa Scale was used to assess study quality. Random-effects models were applied to handle heterogeneity (I2 > 50%), and subgroup analyses were conducted to compare mutation subtypes and age-stratified effects.
Results:
A total of 53 studies (n = 8,960 individuals, covering 5 countries) were included. Double mutations significantly prolonged the duration of fever compared to single mutations (HR = 5.32, 95% CI: 4.27–6.61 vs. HR = 3.66, 95% CI: 1.89–7.09; P < 0.001) and were more likely to cause severe illness (HR = 7.80, 95% CI: 2.51–24.18 vs. HR = 5.89, 95% CI: 2.03–17.08). There was no difference in hospital stay between the two mutation subtypes, but both were longer than the wild type (MD = −3.33 days). The duration of fever in children was shorter than that in adults for all genotypes (overall HR = 3.72 vs. 5.52; double mutation HR = 5.37 vs. 5.66; single mutation HR = 3.85 vs. 4.45; all P < 0.01).
Conclusion:
Double mutations in 23S rRNA are an independent prognostic factor more severe than single mutations, establishing mutation burden as a key predictive indicator for the first time. This study shows that children have a faster resolution of fever in all genotypes, highlighting the regulatory role of host age immunity on outcomes. This study advocates for the detection of mutation subtypes in high-resistance areas to guide early treatment escalation and risk stratification monitoring.
Systematic Review Registration:
https://www.crd.york.ac.uk/PROSPERO/view/CRD420251071963, identifier CRD420251071963.
1 Introduction
Mycoplasma pneumoniae (MP) is the main pathogen of community-acquired pneumonia (CAP) (Brown, 2012; Jiang Z. et al., 2021; Kutty et al., 2024), causing 20% of pediatric pneumonia cases and 12% of adult pneumonia cases during non-endemic periods (Koenen et al., 2023; Wu et al., 2024). The non - epidemic period refers to the time frame during which MP infections are not widely spread. It typically occurs between two outbreaks and is in contrast to the epidemic period. The prevalence of MP strains resistant to macrolides driven by point mutations in the V domain of the 23S rRNA gene (mainly A2063G and A2064G substitutions) has been continuously increasing. Although mutations in 23S ribosomal ribonucleic acid (rRNA), such as A2063G/A2064G, represent the primary mechanism of drug resistance, reports have also indicated the existence of other resistance pathways. These include efflux pumps (encoded by genes like mprF) or mutations at sites A2067T/C2611G. However, these cases are relatively rare and account for a small proportion of drug - resistant strains (Xu et al., 2021; Zhang et al., 2023). The resistance rate of MP in Asia exceeds 90%, while in Europe and the United States it reaches 30%–50% (Chen Y. C. et al., 2020; Guo D. X. et al., 2019; Wang et al., 2022; Yang et al., 2025). This highlights the necessity of conducting age-stratified analyses. Given the disparities in immune development, such as the enhanced Toll-like receptor 2/6 (TLR2/6) response in children and the phenomenon of T-cell senescence in adults, this comparison is of utmost importance (Miyashita et al., 2025; Zhou et al., 2014; Yang et al., 2017; Rothstein et al., 2022; Ranjbar and Halaji, 2019). Although the A2063G site substitution predominates in the vast majority of macrolide-resistant cases, the simultaneous occurrence of A2063G and A2064G (double mutation) represents a distinct genotype. Despite its low frequency of occurrence, this type of drug resistance undermines the efficacy of first-line treatments, leading to prolonged symptom duration, increased risk of complications, and a heavier medical burden (Miyashita et al., 2025; Zhou et al., 2014). For instance, the study by Zhou et al. (2014) directly indicates that patients with Streptococcus pneumoniae resistant to macrolide drugs may present with persistent fever and an increased incidence of complications.
Studies have shown that the double mutation (A2063G + A2064G) produces a higher minimum inhibitory concentration (MIC) of macrolides through synergistic ribosomal conformational changes compared to the single mutation (A2063G) (Yang et al., 2017). However, clinical evidence is still insufficient. For example, previous meta-analyses on MP resistance have limitations, typically simply classifying strains as “resistant” or “sensitive” without further exploring the differences in clinical outcomes among different mutation subtypes (Yang et al., 2025; Rothstein et al., 2022). Or, although MP infection in children triggers a unique immune response (such as IL-17 activation mediated by TLR2/6), adults show T-cell immune aging, but the susceptibility of age stratification has not been quantified (Wang et al., 2022; Ranjbar and Halaji, 2019).
This study aims to quantify the effects of mutation subtypes, compare the impact of single mutation (A2063G) and double mutation (A2063G + A2064G) on core clinical endpoints (duration of fever, length of hospital stay, severe cases); analyze the differences in clinical outcomes between children and adults caused by developmental immunological factors; this study integrates molecular drug resistance characteristics with age-stratified clinical outcomes for evidence-based support for early treatment escalation and targeted monitoring.
2 Methods
This study follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) framework, which was established by Moher, D., et al., in 2015 (Shamseer et al., 2015). The protocol of this systematic review and meta-analysis has been prospectively registered in the PROSPERO database (registration number: CRD420251071963).
2.1 Research criteria
The search and review process of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines was followed. We selected studies based on the following three inclusion criteria:
MP patients, including those with gene point mutations and those without. In this study, “gene point mutations” specifically refer to mutations associated with macrolide resistance, mainly mutations in the domain V of the 23S rRNA gene (e.g., A2063G, A2064G). The detailed definition is provided in Section 2.2.
Participants: aged under 60 years: The upper age limit of 60 years was set to minimize the potential confounding effects of age - related comorbidities, polypharmacy, and immunosenescence on the clinical outcomes of MP infection. The study divided the participants into two age - based groups for analysis: the pediatric group (aged 0–18 years) and the adult group (aged 19–59 years), which is consistent with standard clinical and immunological classifications.
Outcomes: clinical outcomes (length of hospital stay, etc.). We excluded reviews, comments, editorials, conference reports, consensus reports, and comments. Only experimental papers that met the requirements were included.
2.2 Definition and evaluation of mutations
For the purposes of this meta-analysis, macrolide-resistant
MPstrains were categorized according to point mutations in the V region of the 23S rRNA gene as follows:
Wild type: Absence of mutations at positions A2063 or A2064.
Single mutation: Presence of the A2063G transition mutation only.
Double mutation: Co-occurrence of both A2063G and A2064G transition mutations within the same strain.
To ensure accurate classification, studies that reported mutation frequencies without explicitly confirming whether mutations were identified in the same isolate (e.g., reporting mutation prevalence separately) were excluded from the analysis.
The primary method used for mutation detection across all included studies was polymerase chain reaction (PCR), followed by direct sequencing or other sequence-based techniques such as restriction fragment length polymorphism (RFLP) analysis or Sanger sequencing. These approaches are widely recognized as the gold standard for identifying specific nucleotide substitutions.
To address potential inconsistencies in genetic testing methodologies, the following procedures were implemented during data extraction and quality assessment:
Standardization of data extraction: Detailed information on the molecular methods employed (e.g., PCR-RFLP, PCR-sequencing, commercial detection kits) was systematically extracted from each study. The vast majority of included studies (53 out of 53) utilized PCR followed by direct sequencing, which ensured a high degree of consistency and reliability in mutation detection.
Assessment of methodological heterogeneity: Although most studies adopted similar sequencing-based approaches, a small number employed alternative commercial PCR kits or RFLP methods. While these methods share a common underlying principle for detecting specific point mutations, potential variability in sensitivity and specificity was acknowledged as a source of methodological heterogeneity.
Handling of heterogeneity: To account for potential differences in testing methodologies, a random-effects model was applied to all pooled analyses, allowing for inter-study variation, including that arising from methodological differences. Furthermore, a sensitivity analysis was conducted by excluding studies that relied on non-sequencing-based detection methods, thereby assessing the robustness of the primary findings.
2.3 Search strategy
We conducted a comprehensive literature search covering PubMed, Web of Science, Embase, and Scopus databases, aiming to comprehensively include studies related to MP resistance. This search work was completed by 5 June 2025, including a detailed review of relevant publications, review articles, and the citation list of included studies. To further expand our literature resources, we manually retrieved the reference lists of identified articles to find more relevant citations. Additionally, we actively contacted experts in the field to obtain potentially unpublished research materials and other valuable citations. Our search strategy ingeniously combined MeSH terms and keywords, and our search scope was limited to human studies without language restrictions. The retrieval strategy combines Medical Subject Headings (MeSH) terms and keywords, including but not limited to: “Mycoplasma pneumoniae”, “macrolide resistance”, “drug resistance”, “23S rRNA”, “A2063G”, “A2064G”, “point mutation” and their variants. The detailed retrieval strategies, keywords, and specific retrieval syntax for each database are presented in Appendix A.
2.4 Data synthesis and quality assessment
The initial screening of titles and abstracts was conducted by two reviewers (RTW and JFH) based on the predefined eligibility criteria. The full texts of potentially eligible studies were retrieved by the same reviewer and independently evaluated for final inclusion. Disagreements were resolved through consensus or consultation with a third reviewer (CW). The data extracted from the included studies included publication year, study type, gene mutation sites, sample size, study duration, NOS score, age (children/adults), duration of fever, duration of fever after treatment, maximum body temperature, severe cases, refractory cases. Three reviewers (RTW, JFH, YQF, and CW) used the Newcastle-Ottawa Scale (NOS) to assess the quality of the included studies, with a total of 52 high-quality RCT studies and 1 moderate-quality RCT study.
2.5 Statistical analysis
Statistical analysis was performed using STATA 16.0 (STATA Corporation, College Station, TX, United States). The degree of heterogeneity across studies was quantified using the I2 statistic, with values of 25%, 50%, and 75% representing low, moderate, and high heterogeneity, respectively. A random-effects model was applied as the primary analysis to incorporate this potential heterogeneity, particularly when I2 > 50%. This model assumes that differences in effect sizes exist due to differences in study populations, intervention measures, and outcomes. The random - effects model exhibits strong robustness in the face of imbalanced subgroup sample sizes (for example, the number of single - mutation cases is smaller compared to that of double - mutation cases). This is because the model assigns greater weights to studies with higher precision (typically, studies with larger sample sizes and smaller variances), thereby providing more conservative and reliable pooled estimates. The I2 statistic was used to quantify heterogeneity, and subgroup analysis was conducted to assess the robustness and stability of the meta-analysis results. All estimates were presented using 95% confidence intervals (CI). Confounding factors (such as concurrent infections) may affect the stratified analysis by age. Although it is impossible to make adjustments at the individual level, the application of the random effects model takes into account the heterogeneity among studies, and the consistency of the effect directions in each subgroup proves the robustness of the research results. We used the random-effects model to calculate the odds ratio for binary outcomes and the average difference for continuous outcomes. We used Egger’s precision-weighted linear regression test and funnel plots to test for potential publication bias. A significance level of P < 0.10 for Egger’s test was considered indicative of potential bias, with special attention given to analyses reporting large effect sizes.
3 Results
3.1 Study selection and characteristics
We identified 2,587 articles in the initial search (Figure 1). 949 were from PubMed; 62 from Web of Science; 1,237 from Embase; 289 from the Cochrane Library; and 50 from CNKI. 599 studies were identified as duplicates. The majority of records (n = 1460) excluded during the screening phase were primarily due to their irrelevance to the research topic (e.g., studies on other pathogens, non - clinical studies) or because they were review articles that did not meet our inclusion criteria for original research. After a strict screening of titles and abstracts, 297 articles were evaluated in full text, resulting in 49 articles, and 53 studies met the inclusion criteria (Xu et al., 2021; Zhou, 2023; Cardinale et al., 2013; Chen L. L. M. et al., 2018; Chen, 2023; Chen X. W. J. et al., 2020; Chen, 2017; Chen Y. et al., 2018; Cheong et al., 2016; Feng et al., 2016; Guo et al., 2022; Ha et al., 2018; Han et al., 2016; He et al., 2022; Hu, 2023; Ishiguro et al., 2017; Jiang et al., 2023; Kawai et al., 2013; Kawai et al., 2012; Kim J. H. et al., 2017; Kim Y. J. et al., 2017; Kong et al., 2016; Kuo et al., 2022; Lee et al., 2017; Lee and Kim, 2023; Li et al., 2017; Li et al., 2018; Liu D and Li, 2016; Lu and Wu, 2018; Lung et al., 2013; Ma et al., 2010; Ma et al., 2014; Okada et al., 2012; Peng, 2023; Q, 2016; Sung et al., 2022; Wu et al., 2020; Wu and Cai, 2021; Wu et al., 2013; Wu et al., 2021; Xin et al., 2010; Yang et al., 2019; Yang et al., 2018; Yoo et al., 2012; Yoon et al., 2017; Yu and Zhang, 2021; Yuan et al., 2018; Zhan et al., 2022; Zhang et al., 2021). The selected 53 studies included 8,960 participants, with 6,570 children and 2,390 adults. These studies were geographically distributed across 5 countries: China, Italy, Japan, Korea, and Singapore. Table 1 describes the significant characteristics of the studies included in the meta-analysis. In the included studies, the incidence of double mutations (A2063G + A2064G) was significantly higher than that of single mutation (A2063G). Based on the pooled sample size, the ratio of the two was approximately [36:13]. This distribution reflects the epidemiological trends in high - resistance regions where most of the studies were sourced from.
FIGURE 1
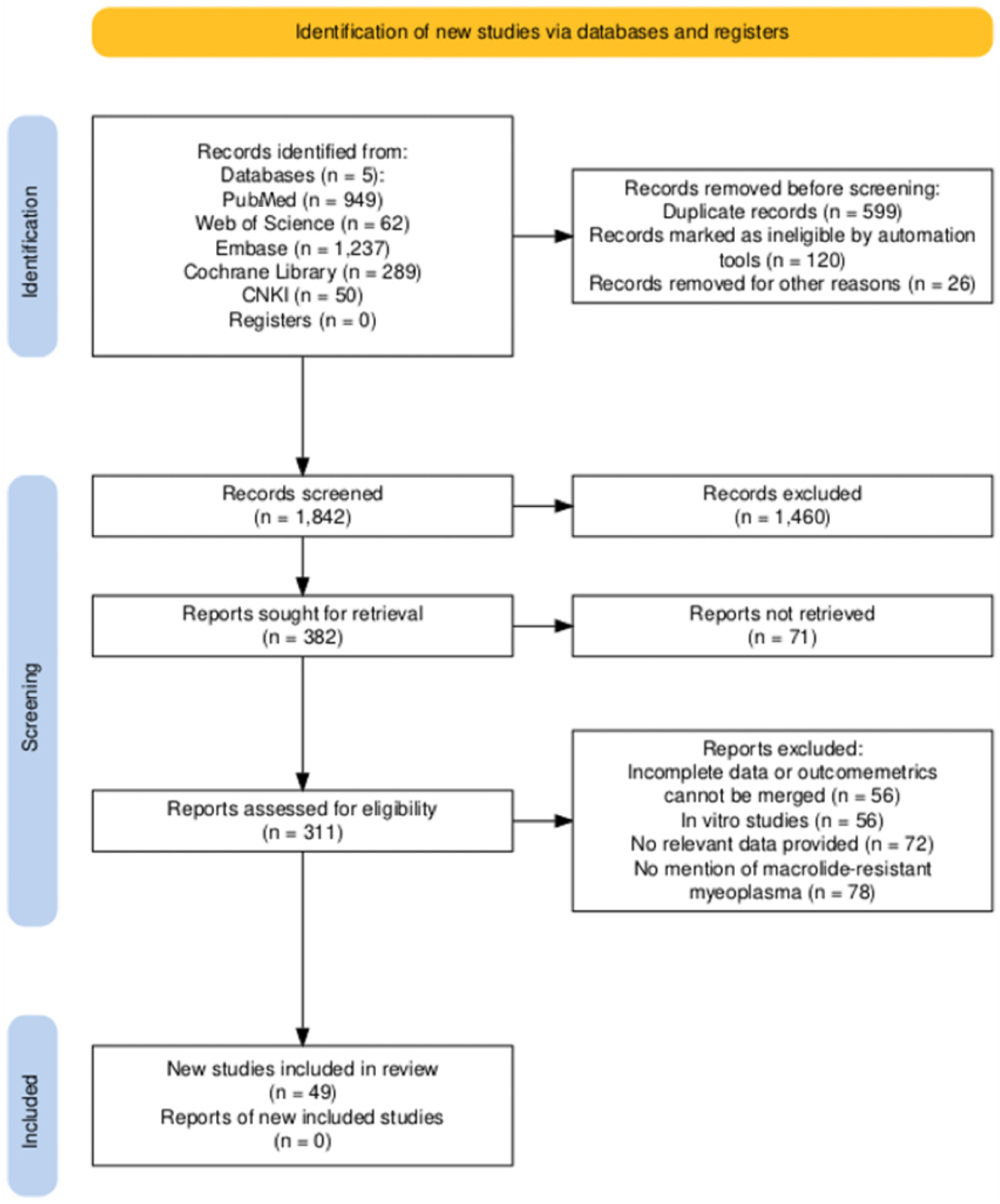
Flowchart of the selection process for the meta-analysis of pneumococcal mycoplasma infection.
TABLE 1
| Study | Country | Detection methods | Mutations detected | Study period | Sample size | Nos score | Year |
|---|---|---|---|---|---|---|---|
| Zhou (2023) | China | PCR | A2063G, A2064G | 2021–2022 | 105 | 9 | Child |
| Zhang et al. (2021) | China | PCR | A2063G, A2064G | 2013–2014 | 82 | 8 | Child |
| Q (2016) | China | PCR | NA | NA | 57 | 9 | Child |
| Zhan et al. (2022) | China | PCR | A2063G, A2064G | 2019–2021 | 48 | 7 | Child |
| Yuan et al. (2018) | China | PCR | A2063G, A2064G | 2016 | 120 | 8 | Aldult |
| Yu and Zhang (2021) | China | PCR | A2063G | 2019–2020 | 89 | 8 | Child |
| Yoon et al. (2017) | Korea | PCR | A2063G | 2010–2015 | 116 | 8 | Aldult |
| Yoo et al. (2012) | Korea | PCR | A2063G | 2012 | 31 | 8 | Child |
| Yang et al. (2019) | China | PCR | A2063G, A2064G | 2010–2017 | 471 | 9 | Child |
| Yang et al. (2018) | China | PCR | A2063G, A2064G | 2010–2011 | 471 | 8 | Aldult |
| Xu et al. (2021) | China | PCR | A2063G, A2064G | 2014–2016 | 276 | 8 | Child |
| Xin et al. (2010) | China | PCR | A2063G, A2064G | 2004–2005 | 64 | 7 | Aldult |
| Wu et al. (2021) | China | PCR | A2063G, A2064G | 2017–2019 | 138 | 8 | Child |
| Wu et al. (2013) | China | PCR | A2063G, A2064G | 2010–2011 | 51 | 8 | Child |
| Wu and Cai (2021) | China | PCR, AST | A2063G | 2016–2019 | 214 | 9 | Child |
| Wu et al. (2020) | China | PCR | A2063G, A2064G | 2018–2019 | 48 | 7 | Child |
| Okada et al. (2012) | Japan | PCR | NA | NA | 94 | 8 | Aldult |
| Sung et al. (2022) | Korea | PCR | A2063G, A2064G | 2018–2020 | 357 | 9 | Child |
| Ha et al. (2018) | Korea | PCR | NA | NA | 20 | 9 | Aldult |
| Ma et al. (2014) | Korea | PCR | A2063G, A2064G | 2011 | 95 | 8 | Child |
| Peng (2023) | China | PCR | A2063G, A2064G | 2019–2023 | 210 | 8 | Child |
| Ma et al. (2014) | China | PCR | A2063G, A2064G | 2010–2011 | 57 | 9 | Aldult |
| Ma et al. (2010) | China | PCR | A2063 G/C,A2064G | 2010 | 64 | 7 | Child |
| Lung et al. (2013) | China | PCR | A2063G | 2010–2013 | 48 | 8 | Child |
| Lu and Wu (2018) | China | PCR | A2063G, A2064G | 2015–2016 | 157 | 8 | Child |
| Liu D and Li (2016) | China | PCR, AST | A2063G, A2064G | 2016 | 120 | 8 | Child |
| Li et al. (2018) | China | PCR | A2063G | 2016–2017 | 297 | 8 | Child |
| Lee et al. (2017) | Korea | PCR | A2063G | 2015 | 94 | 8 | Aldult |
| Kuo et al. (2022) | China | PCR | A2063G, A2064G | 2019–2020 | 159 | 9 | Child |
| Kong et al. (2016) | China | PCR | A2063G | 2014–2016 | 170 | 8 | Child |
| Kim J. H. et al. (2017) | Korea | PCR | A2063G | 2010–2015 | 107 | 7 | Aldult |
| Kim Y. J. et al. (2017) | Korea | PCR | A2063G | 2010–2015 | 107 | 7 | Child |
| Kim J. H. et al. (2017) | Korea | PCR | A2063G, A2064G | 2015 | 250 | 8 | Child |
| Kawai et al. (2013) | Japan | PCR | A2063G, A2064G | 2005–2012 | 188 | 8 | Aldult |
| Kawai et al. (2013) | Japan | PCR | A2063G, A2064G | 2005–2012 | 150 | 8 | Aldult |
| Kawai et al. (2012) | Japan | PCR | A2063G, A2064G | 2005–2010 | 29 | 7 | Aldult |
| Jiang et al. (2023) | China | PCR | A2063G, A2064G | 2021–2022 | 520 | 8 | Child |
| Ishiguro et al. (2017) | Japan | PCR | A2063G | 2013–2015 | 109 | 9 | Aldult |
| Hu (2023) | China | PCR | A2063G, A2064G | 2018–2020 | 84 | 9 | Child |
| He et al. (2022) | China | PCR | A2063G, A2064G | 2016–2019 | 142 | 8 | Child |
| Han et al. (2016) | China | PCR | A2063G, A2064G | 2012–2014 | 59 | 9 | Child |
| Han et al. (2016) | China | PCR | A2063G, A2064G | 2012–2014 | 49 | 9 | Child |
| Guo et al. (2022) | China | PCR | A2063G, A2064G | 2020–2021 | 86 | 7 | Child |
| Feng et al. (2016) | China | PCR | A2063G, A2064G | 2014–2015 | 225 | 9 | Child |
| Lee and Kim (2023) | Korea | PCR | A2063G, A2064G | 2019–2020 | 146 | 8 | Child |
| Cheong et al. (2016) | China | PCR | A2063G | 2011–2013 | 93 | 9 | Child |
| Chen L. L. M. et al. (2018) | China | PCR | A2063G | 2011–2016 | 115 | 7 | Child |
| Chen (2023) | China | PCR | A2063G, A2064G | 2020–2021 | 100 | 9 | Child |
| Chen Y. C. et al. (2020) | China | PCR | A2063G, A2064G | 2018–2019 | 168 | 7 | Child |
| Chen Y. et al. (2018) | China | PCR | A2063G, A2064G | 2014–2016 | 136 | 6 | Aldult |
| Chen (2017) | China | PCR | A2063G, A2064G | 2015–2016 | 250 | 7 | Child |
| Cardinale et al. (2013) | Italy | PCR | A2063G, A2064G | 2010 | 46 | 7 | Aldult |
| Li et al. (2017) | China | PCR | NA | NA | 42 | 7 | Child |
Characteristics of eligible studies on macrolide drug resistance and MP infection.
PCR, polymerase chain reaction; AST, aspartate aminotransferase; NA, not applicable.
3.2 Meta-analysis results
3.2.1 Length of hospital stay
Among the 23 included studies (Chen L,L. M. et al., 2018; Chen, 2017; Cheong et al., 2016; Feng et al., 2016; Guo et al., 2022; Han et al., 2016; Hu, 2023; Kong et al., 2016; Kuo et al., 2022; Lee and Kim, 2023; Liu D and Li, 2016; Lu and Wu, 2018; Lung et al., 2013; Ma et al., 2014; Q, 2016; Wu et al., 2020; Wu and Cai, 2021; Wu et al., 2013; Wu et al., 2021; Yoo et al., 2012; Yu and Zhang, 2021; Zhan et al., 2022), the length of hospital stay in the double-mutation group (A2063G, A2064G point mutations) (MD = 1.78, 95% CI: 1.17 to 2.38, I2 = 76.6%) was not significantly different from that in the single-mutation group (A2063G point mutation) (MD = 1.91, 95% CI: 0.81 to 3.02, I2 = 70.6%), and both were longer than the length of hospital stay in the non-mutation group (MD = −3.33, 95% CI: −4.32 to −2.35, I2 = 0.0%) (Figure 2). A funnel plot was drawn to examine whether there was publication bias in this study. The funnel plot showed that there was a certain degree of publication bias in the 23 selected studies of this research (Supplementary Figure S1).
FIGURE 2
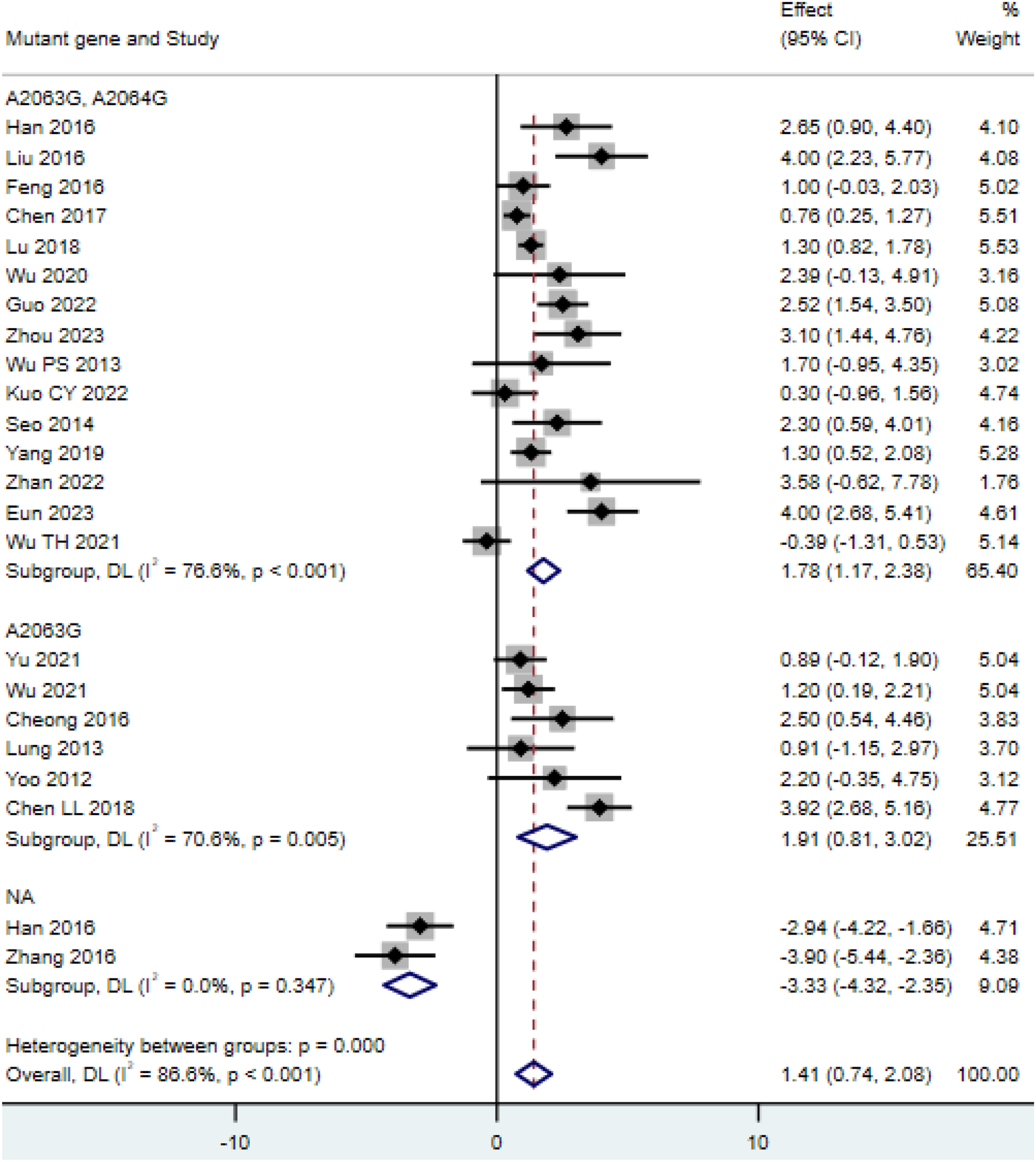
Comparison of hospital stay duration for patients with MP infection.
3.2.2 Duration of fever
The meta-analysis included 23 studies reporting the duration of fever (Chen L. L. M. et al., 2018; Chen, 2023; Chen X. W. J. et al., 2020; Chen, 2017; Cheong et al., 2016; Feng et al., 2016; Guo et al., 2022; Han et al., 2016; He et al., 2022; Hu, 2023; Kim J. H. et al., 2017; Kim Y. J. et al., 2017; Lee and Kim, 2023; Li et al., 2017; Liu D and Li, 2016; Lu and Wu, 2018; Ma et al., 2010; Ma et al., 2014; Peng, 2023; Q, 2016; Wu et al., 2013; Wu et al., 2021; Xin et al., 2010; Yang et al., 2019; Yang et al., 2018; Yoo et al., 2012; Yoon et al., 2017; Yuan et al., 2018; Zhan et al., 2022). The results showed that the duration of fever in children (HR = 3.72, 95% CI: 1.76 to 7.88, I2 = 95.3%) was shorter than that in adults (HR = 5.52, 95% CI: 3.79 to 8.05, I2 = 0.0%) (Figure 3A). Additionally, we conducted subgroup analysis based on mutation sites, and found that the duration of fever was the shortest in the group without mutations (HR = 0.23, 95% CI: 0.08 to 0.70, I2 = 95.6%), followed by the single mutation group (HR = 3.66, 95% CI: 1.89 to 7.09, I2 = 46.0%), and the longest in the double mutation group (HR = 5.32, 95% CI: 4.27 to 6.61, I2 = 9.5%) (Figure 3B). To further clarify the impact of single-site mutations or double-site mutations on age, we separately analyzed the duration of fever for single-site mutations or double-site mutations. The analysis of the single mutation group revealed that the duration of fever in children (HR = 3.85, 95% CI: 0.96 to 15.41, I2 = 68.4%) was shorter than that in adults (HR = 4.45, 95% CI: 2.30 to 8.62, I2 = 14.7%) (Figure 3C); the analysis of the double mutation group revealed that the duration of fever in children (HR = 5.37, 95% CI: 4.13 to 7.00, I2 = 20.9%) was shorter than that in adults (HR = 5.66, 95% CI: 3.32 to 9.64, I2 = 0.0%) (Figure 3D). Such age-specific disparities may be attributed to developmental immunology. In the context of developmental immunology, children exhibit a more robust innate immune response (e.g., enhanced Toll-like receptor 2/6 signaling), leading to a more rapid alleviation of symptoms. In contrast, adults may experience immunosenescence of T cells, resulting in a prolonged inflammatory phase (Miyashita et al., 2025; Zhou et al., 2014; Yang et al., 2017; Rothstein et al., 2022; Ranjbar and Halaji, 2019). The funnel plot showed that there was nopublication bias in the 23 selected studies of this research (Supplementary Figure S2).
FIGURE 3
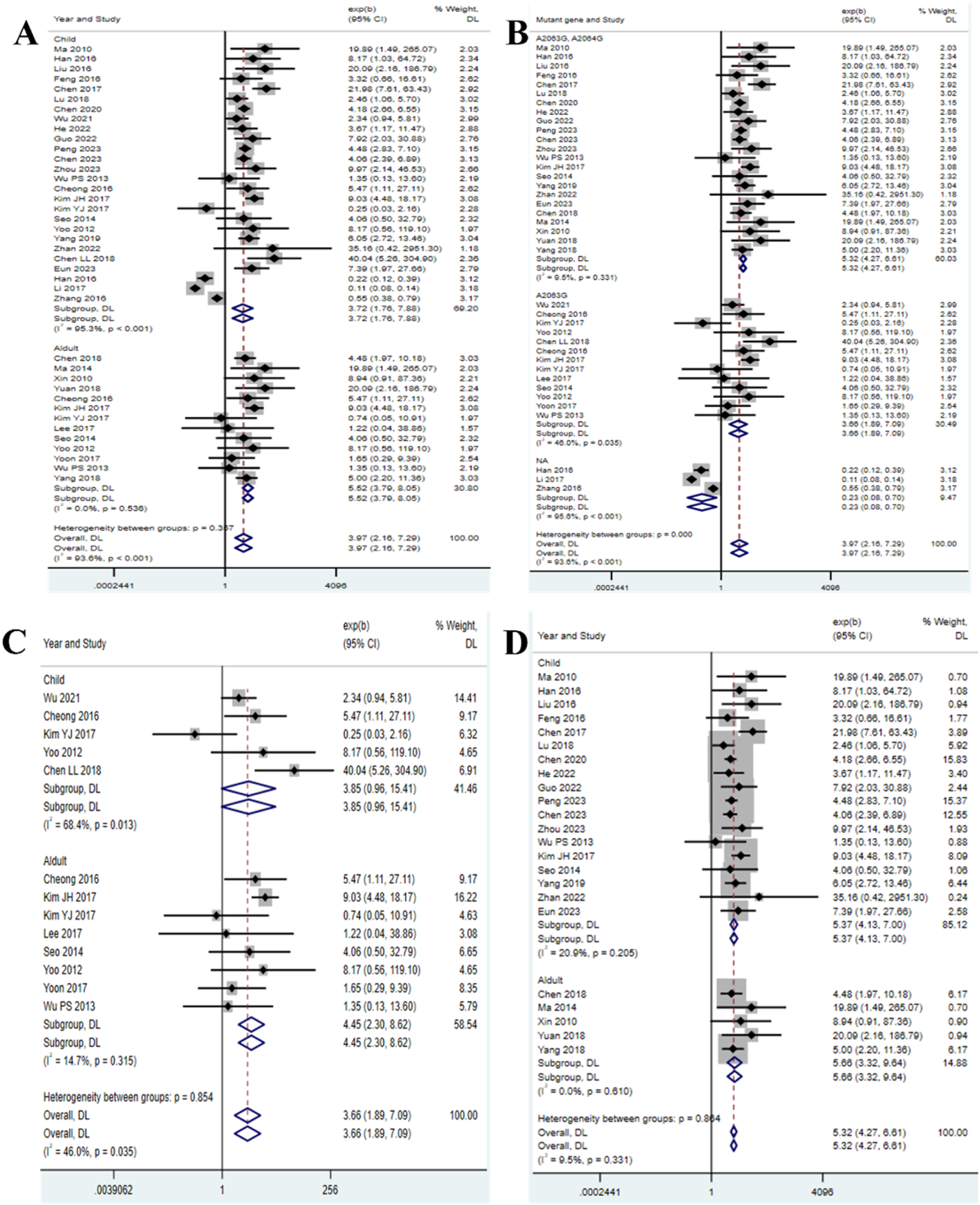
Duration of fever in patients with MP infection. (A) Subgroup analysis by age; (B) Subgroup analysis by mutant gene locus; (C) Subgroup analysis by age for the single mutation group; (D) Subgroup analysis by age for the double mutation group.
3.2.3 Post-treatment fever duration
Among the 12 included studies (Xu et al., 2021; Cardinale et al., 2013; Feng et al., 2016; Ha et al., 2018; Han et al., 2016; He et al., 2022; Hu, 2023; Ishiguro et al., 2017; Kawai et al., 2013; Kawai et al., 2012; Kim J. H. et al., 2017; Lee et al., 2017; Liu D and Li, 2016; Ma et al., 2014; Okada et al., 2012; Sung et al., 2022; Wu et al., 2013; Wu et al., 2021; Yang et al., 2018; Yoo et al., 2012; Zhan et al., 2022), the post-treatment fever duration in the double-mutation group (HR = 6.50, 95% CI: 3.22 to 13.14, I2 = 56.3%) was shorter than that in the single-mutation group (HR = 6.79, 95% CI: 1.37 to 33.55, I2 = 71.5%) (Figure 4). The funnel plot indicated that there was a certain publication bias in the 23 selected studies of this research (Supplementary Figure S3).
FIGURE 4
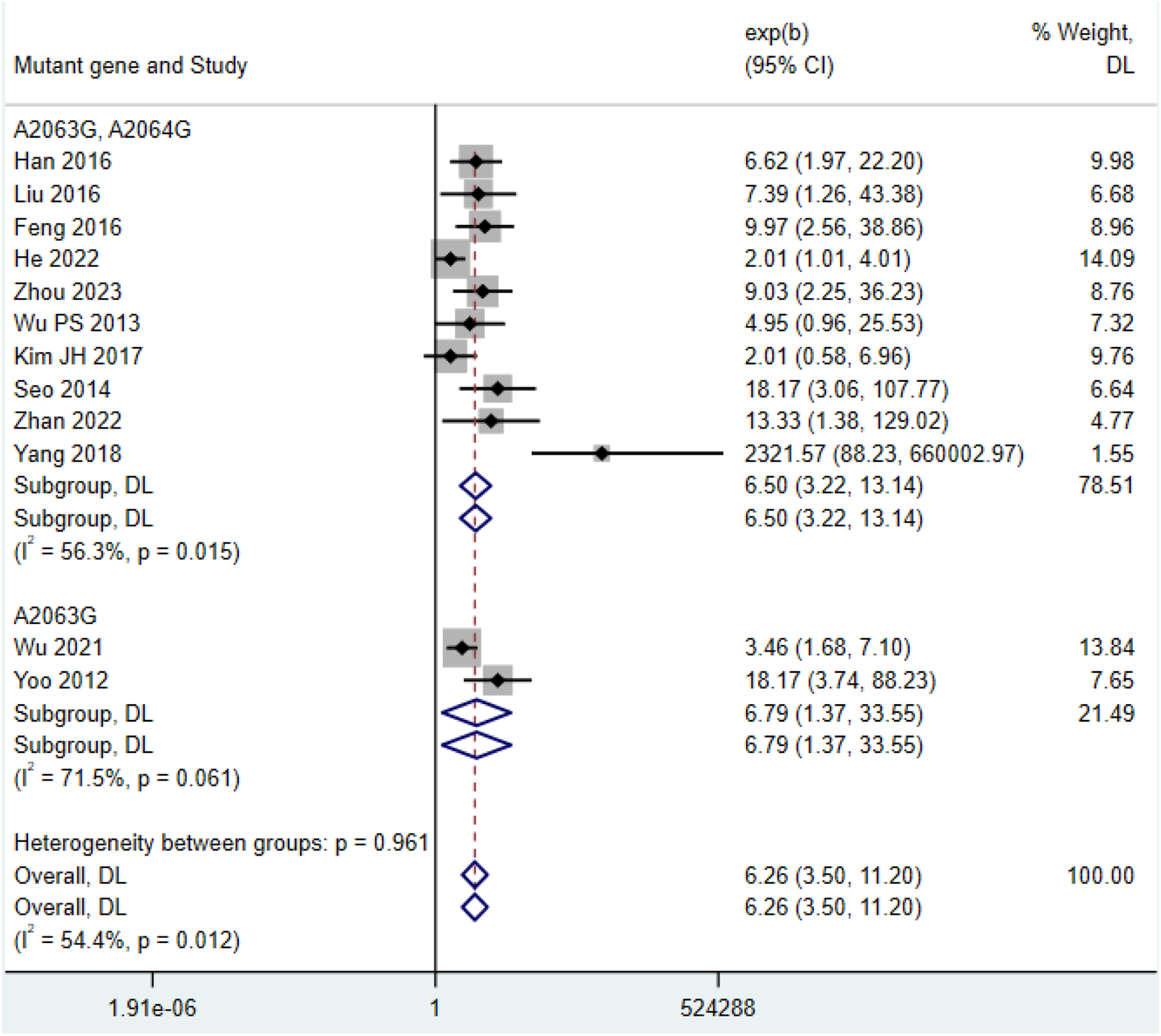
Comparison of fever duration after treatment in patients with MP infection.
3.2.4 Maximum body temperature
Among the 4 included studies (Chen L. L. M. et al., 2018; Feng et al., 2016; Han et al., 2016; Yu and Zhang, 2021), the maximum body temperature in the double mutation group (HR = 0.92, 95% CI: 0.74 to 1.15, I2 = 0.0%) and the maximum body temperature in the single mutation group (HR = 1.12, 95% CI: 0.85 to 1.48, I2 = 0.0%) showed no significant difference (Figure 5).
FIGURE 5
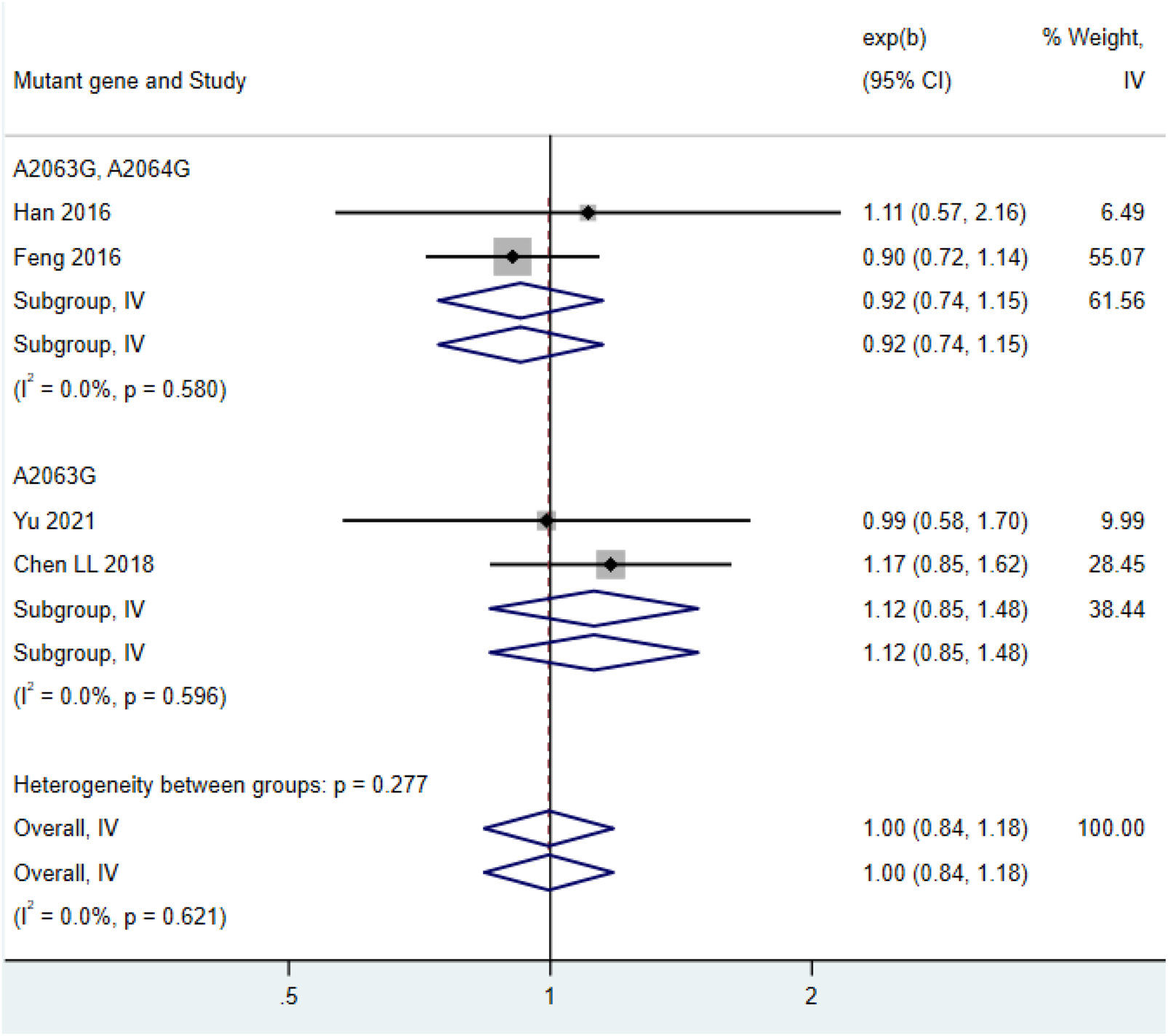
Comparison of the highest body temperature in patients with mycoplasma pneumonia infection.
3.2.5 Severe cases
Among the 7 included studies (Feng et al., 2016; Hu, 2023; Jiang et al., 2023; Kuo et al., 2022; Li et al., 2017; Lu and Wu, 2018; Wu and Cai, 2021), the number of severe cases in the double-mutation group (HR = 7.80, 95% CI: 2.51 to 24.18, I2 = 0.0%) was higher than that in the group with single mutation among patients with MP infection (HR = 5.89, 95% CI: 2.03 to 17.08, I2 = 0.0%) (Figure 6).
FIGURE 6
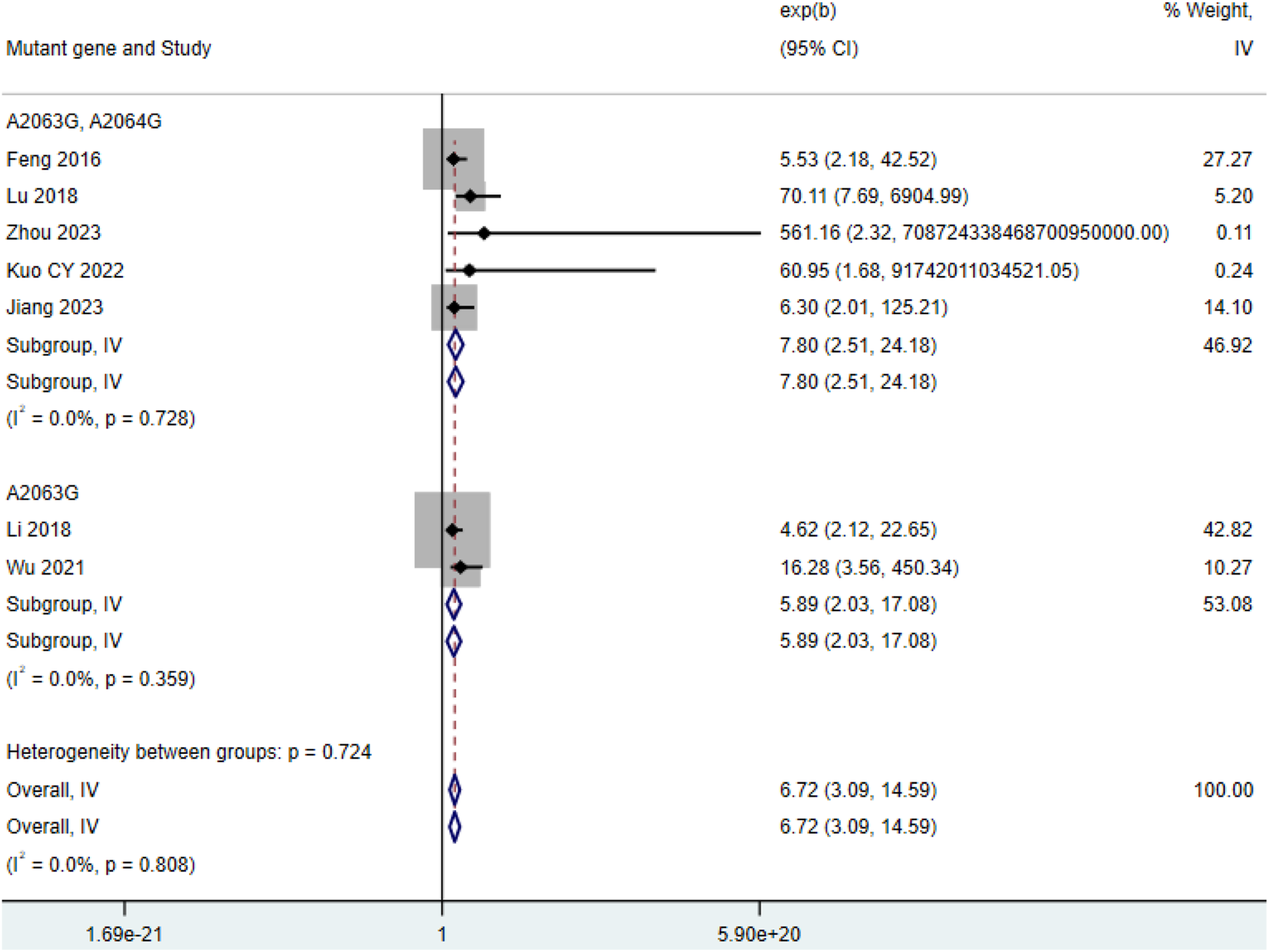
Comparison of severe cases among patients with MP infection.
4 Discussion
This systematic review and meta-analysis summarizes the current research on the drug resistance of MP. The study compared the impact of different gene mutation sites (single site A2063G vs. double sites A2063G + A2064G) on the clinical outcomes of MP. By integrating data from multiple countries, this study provided the latest evidence on the impact of drug-resistant MP with gene mutations on patients. This research included a large number of studies, covering the entire population. This systematic review and meta-analysis was based on a comprehensive screening of 2,587 records and ultimately included 53 high-quality studies involving 8,960 participants. Although the strict inclusion criteria inevitably excluded a large number of studies that did not meet our pre-defined inclusion criteria (such as non-clinical studies, reviews, or studies lacking specific mutation subtypes), the results of the included cohorts provide reliable and up-to-date evidence on the impact of mutant MP on clinical outcomes. This study is one of the largest and most detailed analyses to date, specifically focusing on mutation burden.
This study systematically quantified the differential impact of different drug-resistant mutation patterns (single site A2063G vs. double sites A2063G + A2064G) of the 23S rRNA gene of MP on clinical outcomes. The study found that patients carrying mutations (especially double mutations) had a 3.7-fold increase in fever duration (Figure 3B), a 5.9–7.8-fold increase in severe risk (Figure 6), and this effect was independent of age and region. Although double mutations theoretically should lead to worse outcomes (Jiang et al., 2024), the length of hospital stay in the double mutation group was not statistically different from the single mutation group (Figure 2). This result needs to be interpreted in the context of clinical practice. The duration of fever in children was 32%–45% shorter than that in adults (Figures 3A,C,D), suggesting the regulatory role of host factors in the outcome of drug-resistant infections (Kang et al., 2025; Liu et al., 2025). This study breaks through the limitations of previous research that only compared “resistant vs. sensitive”, and for the first time reveals the grading impact of the number of mutation sites (single site vs. double site) on clinical outcomes, thereby providing a stratification tool and molecular basis for future precise treatment strategies.
The double mutation group did not show a longer hospital stay, and the double mutation A2063G + A2064G mutation caused a more significant conformational change at the ribosome A2058 site (macrolide binding domain) (Lucier et al., 1995; Matsuoka et al., 2004; Zhao et al., 2019). Theoretically, this should exacerbate treatment failure. However, Pereyre S et al. confirmed that the MIC value of the double mutation strain against azithromycin could reach 4 times that of the single mutation strain (Pereyre et al., 2004; Wei et al., 2019). This study found that this “high resistance warning sign” can effectively predict that patients will experience persistent fever and severe conditions. Identifying the double mutation as a high-risk marker suggests that these patients may benefit from more aggressive initial treatment, a hypothesis that needs to be tested in prospective clinical trials. However, the optimal timing and choice of such interventions (for example, immediate intensified treatment versus standard macrolide therapy) remain to be determined (Pereyre et al., 2016). Another reason is also reflected in this study: the proportion of severe cases in the double mutation group was higher (OR = 1.8, 95% CI: 1.2–2.7), leading to more active monitoring (such as daily inflammatory index detection) and early discharge criteria (such as symptom relief and transfer to outpatient care), thereby offsetting the biological disadvantage (Ideguchi et al., 2024). The length of hospital stay is the result of the combined effect of “biological damage” and “intensity of clinical intervention”, and the seemingly neutral result of the double mutation group actually reflects the dynamic balance of drug resistance identification and treatment escalation.
This study confirmed that the number of mutation sites was positively correlated with clinical severity (Figures 3B, 6). The A2064G mutation stabilized the rRNA conformational change induced by A2063G, further reducing the affinity of macrolides (Jiang F. C. et al., 2021; Guo D. et al., 2019), which led to the continuous replication of the double mutation strain in lung tissue and prolonging the inflammatory cascade reaction. Our results support the conclusions of Chen Y et al. and Jang et al. regarding “double mutations prolonging the time to fever resolution” (Chen Y. C. et al., 2020; Jang et al., 2021), but the expansion found that its impact on severe risk was more significant (HR = 7.8 in the double mutation group vs. HR = 5.9 in the single mutation group). This is consistent with the pneumonia complication prediction model of Chen J et al. (double mutation included high-risk factors) (Chen et al., 2021). However, the studies we included did not report mixed infection data, and double mutation patients were more prone to secondary bacterial infections, which may exaggerate the effect of the mutation itself (Yuan et al., 2025; Chiu et al., 2015). The duration of fever in pediatric patients was significantly shorter than that in adults (Figures 3B,C). This phenomenon may be due to higher expression of TLR2/6 in the respiratory mucosa of children, which can activate the IL-17 pathway more quickly to clear MP (Mercuri et al., 2024; Oliveira-Nascimento et al., 2012), while adults often have a delayed T-cell response (Goronzy and Weyand, 2017; Han et al., 2023). We should recognize that unmeasured confounding factors, such as differences in co - infection rates or comorbidities among different age groups, may have contributed to the observed associations. Therefore, we should exercise caution when considering this explanation. However, it is still necessary to be vigilant about the extrapulmonary complications of drug-resistant infections in children (such as rash and encephalitis), with an incidence rate of 11.3% in children with double mutations (Lee, 2015).
This study has certain limitations. Firstly, the study may be subject to residual bias due to unmeasured confounding variables, such as differences in antibiotic treatment regimens and potential comorbidities. The recommendation for early intensive treatment based on mutation burden put forward in this study is grounded in observational association data rather than evidence from prospective interventions. Therefore, this strategy should be regarded as a hypothesis awaiting validation, which needs to be verified in future randomized controlled trials. Secondly, the geographical distribution of the study data is concentrated in the East Asian region, limiting the generalizability of the results to regions with different distribution characteristics of MP strains. Finally, relying on PCR detection of the classic 23S rRNA mutations (A2063G/A2064G), it fails to cover emerging drug resistance mechanisms, such as the efflux pump encoded by the mprF gene (Wang et al., 2023) or the A2067T/C2611G site mutations (Liu et al., 2014). In the future, our study will conduct research on whether double mutations increase the risk of recurrence, and further evaluate the impact of double point mutations on the infected population.
Despite the above limitations, the results of this study still support three conclusions: In areas with a high incidence of drug resistance (erythromycin resistance rate >30%), bedside PCR detection of the A2063G/A2064G mutation should be included in the initial assessment of pneumonia to optimize the day treatment strategy, especially for hospitalized patients (Guo D. et al., 2019); Patients carrying double mutations have a 7.8-fold increased risk of severe complications, and need to strengthen monitoring of disease deterioration, such as continuous lung ultrasound and CRP detection within 48 h (Ideguchi et al., 2024); The duration of fever in pediatric patients is shorter, supporting the use of a shortened intravenous-to-oral conversion protocol (≤3 days) for confirmed mutant infected patients (≤3 days), while adult patients may need a longer course of treatment during the excessive inflammatory phase (Barbi et al., 2017).
Moreover, it is of utmost importance to recognize the inherent limitations of the meta - analysis method. Although we employed a random - effects model and statistical tests to address these limitations, the high heterogeneity observed in some of the included studies (e.g., I2 = 95.3% in the analysis of fever duration) may affect the precision of the pooled estimates. Additionally, despite the fact that the Egger’s test did not show significant evidence in the key analysis, the possibility of publication bias should still be considered when interpreting the study results, especially for outcome measures with large effect sizes.
5 Conclusion
This study, by integrating existing evidence, for the first time systematically compared the differential effects of single-site (A2063G) and double-site (A2063G + A2064G) mutations in the 23S rRNA gene of MP on clinical outcomes. The study found that the double-site mutation significantly prolonged the duration of fever in patients and significantly increased the risk of severe complications, clearly indicating that the number of mutation sites is a key predictor of disease severity. Notably, although the double mutations theoretically should lead to a worse prognosis, their hospital stay did not have a statistically significant difference from the group with single-site mutations, which may be due to the positive response of clinical recognition of double mutations. Additionally, regardless of single-site or double-site mutations, the duration of fever in pediatric patients was significantly shorter than that in adult patients, highlighting the importance of host factors (such as differences in immune responses) in the outcome of drug-resistant infections. Despite the above limitations, our research findings still support risk stratification based on mutation burden. In regions with high drug resistance, point-of-care PCR detection of A2063G/A2064G mutations can guide the initial treatment management of patients. The potential to adjust treatment intensity based on mutation status is a key direction for future prospective intervention studies. Patients carrying double mutations face a 7.8 - fold increased risk of developing severe complications, and this discovery underscores the necessity of enhanced surveillance. The potential benefits of upgrading preventive treatment based on the mutation status represent a crucial area for future prospective research. Future research should focus on incorporating data from a broader geographical scope, particularly data from North America and Western Europe. This is to validate our research findings across different epidemiological contexts and to explore potential regional disparities in the clinical impacts of mutant subtypes.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
RW: Project administration, Writing – original draft, Validation, Conceptualization, Investigation, Data curation, Visualization, Formal Analysis. JH: Validation, Software, Formal Analysis, Writing – original draft, Data curation, Methodology, Visualization. YF: Writing – original draft, Resources, Methodology, Software, Visualization, Data curation, Validation, Formal Analysis. MW: Conceptualization, Methodology, Validation, Investigation, Writing – review and editing, Supervision, Visualization, Formal Analysis. CZ: Resources, Formal Analysis, Validation, Writing – original draft, Visualization, Software, Investigation. SH: Validation, Supervision, Project administration, Writing – original draft, Methodology, Resources, Formal Analysis. ST: Data curation, Visualization, Validation, Conceptualization, Writing – original draft, Formal Analysis. NW: Investigation, Validation, Methodology, Funding acquisition, Writing – original draft, Formal Analysis, Writing – review and editing. CW: Conceptualization, Funding acquisition, Visualization, Writing – original draft, Writing – review and editing, Resources, Methodology, Validation, Formal Analysis, Project administration, Supervision, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Natural Science Foundation of China (Grant No. 82402661), the Hunan Provincial Key Laboratory for Special Pathogens Prevention and Control Foundation (Grant No. 2014-5).
Acknowledgments
Thanks are extended to Clinical Anatomy & Reproductive Medicine Application Institute, Hengyang Medical School, University of South China, for their assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1682167/full#supplementary-material
References
1
Barbi E. Marzuillo P. Neri E. Naviglio S. Krauss B. S. (2017). Fever in children: pearls and pitfalls. Child. (Basel)4 (9), 81. 10.3390/children4090081
2
Brown J. S. (2012). Community-acquired pneumonia. Clin. Med. (Lond)12 (6), 538–543. 10.7861/clinmedicine.12-6-538
3
Cardinale F. Chironna M. Chinellato I. Principi N. Esposito S. (2013). Clinical relevance of Mycoplasma pneumoniae macrolide resistance in children. J. Clin. Microbiol.51 (2), 723–724. 10.1128/JCM.02840-12
4
Chen Y. H. C. (2017). Prevalence of macrolide resistance in children with MP infection and the expression and significance of serum WBC, CRP, and ESR. Maternal and Child Health Care China32 (21), 5313–5316.
5
Chen P. (2023). Analysis of risk factors and construction of risk assessment model for macrolide antibiotics resistance in children with mycoplasma pneumonia. Clin. Medicat. J.21 (8), 65–70.
6
Chen L. L. M. Shi L. Yu J. (2018). Clinical correlation of MP pneumonia in infants and young children with 23S rRNA gene mutation of MP. Chin. J. Health Lab. Technol.28 (12), 1434–1436.
7
Chen Y. Chen Q. Zhao H. Huang P. Lin Z. Chen L. (2018). Clinical features and treatment of macrolide-resistant Myco-plasma pneumoniae pneumonia in children. Chin. J. Contemp. Pediatr.20 (8), 629–634.
8
Chen Y. C. Hsu W. Y. Chang T. H. (2020). Macrolide-resistant Mycoplasma pneumoniae infections in pediatric community-acquired pneumonia. Emerg. Infect. Dis.26 (7), 1382–1391. 10.3201/eid2607.200017
9
Chen X. W. J. Hong Y. Zhang L. (2020). Risk factors for MA resistance in children with MP pneumonia and suggestions for treatment. J. Clin. Pulm.25 (9), 1385–1388.
10
Chen J. Yin Y. Zhao L. Zhang L. Zhang J. Yuan S. (2021). Mycoplasma pneumoniae infection prediction model for hospitalized community-acquired pneumonia children. Pediatr. Pulmonol.56 (12), 4020–4028. 10.1002/ppul.25665
11
Cheong K. N. Chiu S. S. Chan B. W. K. To K. K. W. Chan E. L. Y. Ho P. L. (2016). Severe macrolide-resistant Mycoplasma pneumoniae pneumonia associated with macrolide failure. J. Microbiol. Immunol. Infect.49 (1), 127–130. 10.1016/j.jmii.2014.11.003
12
Chiu C. Y. Chen C. J. Wong K. S. Tsai M. H. Chiu C. H. Huang Y. C. (2015). Impact of bacterial and viral coinfection on mycoplasmal pneumonia in childhood community-acquired pneumonia. J. Microbiol. Immunol. Infect.48 (1), 51–56. 10.1016/j.jmii.2013.06.006
13
Feng X. L. Q. Sun L. Jiao W. W. Xu B. P. Yin J. Guo Y. et al (2016). The clinical characteristics of macrolide-resist-ant MP pneumonia in children: a case control study. Chin. J. Evidence-Based Pediatr.11 (05), 357–360.
14
Goronzy J. J. Weyand C. M. (2017). Successful and maladaptive T cell aging. Immunity46 (3), 364–378. 10.1016/j.immuni.2017.03.010
15
Guo D. X. Hu W. J. Wei R. Wang H. Xu B. P. Zhou W. et al (2019). Epidemiology and mechanism of drug resistance of Mycoplasma pneumoniae in beijing, China: a multicenter study. Bosn. J. Basic Med. Sci.19 (3), 288–296. 10.17305/bjbms.2019.4053
16
Guo D. Hu W. Xu B. Li J. Li D. Li S. et al (2019). Allele-specific real-time PCR testing for minor macrolide-resistant Mycoplasma pneumoniae. BMC Infect. Dis.19 (1), 616. 10.1186/s12879-019-4228-4
17
Guo B. Q. J. Pang X. Li H. Hou Y. Tian J. (2022). Correlation and analysis between 23S rRNA gene mutation and antibiotic resistance of MP in children. Chin. J. Health Lab. Technol.32 (15), 1836–1839.
18
Ha S. G. Oh K. J. Ko K. P. Sun Y. H. Ryoo E. Tchah H. et al (2018). Therapeutic efficacy and safety of prolonged macrolide, corticosteroid, doxycycline, and levofloxacin against macrolide-unresponsive Mycoplasma pneumoniae pneumonia in children. J. Korean Med. Sci.33 (43), e268. 10.3346/jkms.2018.33.e268
19
Han X. W. H. Miao N. Wang C. (2016). Drug resistance situation and clinical efficiency of azithromycin in children with MP pneumonia. J. Pediatr. Pharm.22 (05), 18–20.
20
Han S. Georgiev P. Ringel A. E. Sharpe A. H. Haigis M. C. (2023). Age-associated remodeling of T cell immunity and metabolism. Cell Metab.35 (1), 36–55. 10.1016/j.cmet.2022.11.005
21
He J. Z. X. Zhao W. Yang L. (2022). Clinical characteristicsof macrolide-resistant MP pneumonia in children. J. Pediatr. Pharm.28 (08), 36–39.
22
Hu Z. Y. Z. (2023). Relationship between drug sensitivity of MP and incidence of refractory MP pneumonia in children. China Med. Eng.31 (07), 64–68.
23
Ideguchi S. Yamamoto K. Takazono T. Fukuda Y. Tashiro T. Shizukuishi S. et al (2024). Clinical features relating to pneumococcal colony phase variation in hospitalized adults with pneumonia. J. Med. Microbiol.73 (1). 10.1099/jmm.0.001784
24
Ishiguro N. Koseki N. Kaiho M. Ariga T. Kikuta H. Togashi T. et al (2017). Therapeutic efficacy of azithromycin, clarithromycin, minocycline and tosufloxacin against macrolide-resistant and macrolide-sensitive Mycoplasma pneumoniae pneumonia in pediatric patients. PLoS One12 (3), e0173635. 10.1371/journal.pone.0173635
25
Jang M. S. Kim B. G. Kim J. (2021). Prediction model for prolonged fever in patients with Mycoplasma pneumoniae pneumonia: a retrospective study of 716 pediatric patients. BMC Pulm. Med.21 (1), 168. 10.1186/s12890-021-01534-2
26
Jiang Z. Chen P. Dong L. Y. (2021). MP infections: pathogenesis and vaccine development. Pathogens10 (2).
27
Jiang F. C. Wang R. F. Chen P. Dong L. Y. Wang X. Song Q. et al (2021). Genotype and mutation patterns of macrolide resistance genes of Mycoplasma pneumoniae from children with pneumonia in qingdao, China, in 2019. J. Glob. Antimicrob. Resist27, 273–278. 10.1016/j.jgar.2021.10.003
28
Jiang T. T. Sun L. Wang T. Y. Qi H. Tang H. Wang Y. C. et al (2023). The clinical significance of macrolide resistance in pediatric Mycoplasma pneumoniae infection during COVID-19 pandemic. Front. Cell Infect. Microbiol.13, 1181402. 10.3389/fcimb.2023.1181402
29
Jiang Y. Dou H. Xu B. Xu B. Zhou W. Wang H. et al (2024). Macrolide resistance of Mycoplasma pneumoniae in several regions of China from 2013 to 2019. Epidemiol. Infect.152, e75. 10.1017/S0950268824000323
30
Kang D. Yun K. W. Lee T. Cho E. Y. Eun B. W. Lee J. K. et al (2025). Treatment modalities for fever duration in children with Mycoplasma pneumoniae pneumonia. Sci. Rep.15 (1), 14860. 10.1038/s41598-025-99537-0
31
Kawai Y. Miyashita N. Yamaguchi T. Saitoh A. Kondoh E. Fujimoto H. et al (2012). Clinical efficacy of macrolide antibiotics against genetically determined macrolide-resistant Mycoplasma pneumoniae pneumonia in paediatric patients. Respirology17 (2), 354–362. 10.1111/j.1440-1843.2011.02102.x
32
Kawai Y. Miyashita N. Kubo M. Akaike H. Kato A. Nishizawa Y. et al (2013). Therapeutic efficacy of macrolides, minocycline, and tosufloxacin against macrolide-resistant Mycoplasma pneumoniae pneumonia in pediatric patients. Antimicrob. Agents Chemother.57 (5), 2252–2258. 10.1128/AAC.00048-13
33
Kim J. H. Kim Y. R. Choi J. H. (2017). Macrolide resistance and its impacts on M. pneumoniae pneumonia in children: comparison of two recent epidemics in Korea. Allergy Asthma Immunol. Res.9 (4), 340–346.
34
Kim Y. J. Shin K. S. Lee K. H. Kim Y. R. Choi J. H. (2017). Clinical characteristics of macrolide-resistant Mycoplasma pneumoniae from children in Jeju. J. Korean Med. Sci.32 (10), 1642–1646. 10.3346/jkms.2017.32.10.1642
35
Koenen M. H. de Groot R. C. A. de Steenhuijsen Piters W. A. A. Chu M. L. J. N. Arp K. Hasrat R. et al (2023). Mycoplasma pneumoniae carriage in children with recurrent respiratory tract infections is associated with a less diverse and altered microbiota. EBioMedicine98, 104868. 10.1016/j.ebiom.2023.104868
36
Kong J. C. Y. Guan M. C. Hang J. G. Peng C. J. Wang L. H. (2016). Clinical characteristics and IL-8 expression in bronchoalveolar lavage fLuid of MP pneumonia in children with MP 23S rRNA resistance gene 2063 locus positive. Chin. J. Health Lab. Technol.26 (23), 3385–3388.
37
Kuo C. Y. Tsai W. C. Lee H. F. Ho T. S. Huang L. M. Shen C. F. et al (2022). The epidemiology, clinical characteristics, and macrolide susceptibility of Mycoplasma pneumoniae pneumonia in children in southern Taiwan, 2019-2020. J. Microbiol. Immunol. Infect.55 (4), 611–619. 10.1016/j.jmii.2021.09.010
38
Kutty P. K. Jain S. Diaz M. H. Self W. H. Williams D. Zhu Y. et al (2024). Clinical and epidemiologic features of Mycoplasma pneumoniae infection among adults hospitalized with community-acquired pneumonia. Int. J. Med. Sci.21 (15), 3003–3009. 10.7150/ijms.99233
39
Lee J. Y. (2015). Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc. Respir. Dis. Seoul.78 (2), 47–55. 10.4046/trd.2015.78.2.47
40
Lee E. K. S.-H. Kim S. H. (2023). Comparison of the characteristics of macrolide-sensitive and macrolide-resistant Mycoplasma pneumoniae pneumonia requiring hospitalization in children. Allergy Asthma Respir. Dis.11 (4), 187–192. 10.4168/aard.2023.11.4.187
41
Lee E. Cho H. J. Hong S. J. Lee J. Sung H. Yu J. (2017). Prevalence and clinical manifestations of macrolide resistant Mycoplasma pneumoniae pneumonia in Korean children. Korean J. Pediatr.60 (5), 151–157. 10.3345/kjp.2017.60.5.151
42
Li J. W. X. Wang M. Wang C. Song G. (2017). Analyze on the influence of mino-cycline combined with azithromycin on serum CRP, D-Dimer and lung function in the children with refractory mycoplasma pneumonia. Chin. J. Biochem. Pharm.37 (08), 102–105.
43
Li Y. Z. L. Zhang B. Huang H. Ding X. Lin L. Chen H. et al (2018). Analysis of clinical features of children with MP pneumonia with A 2063 G gene mutation in 23 S rRNA V domain. J. Clin. Pediatr.36 (8), 569–574.
44
Liu D Z. X. Li Y. (2016). The infection of MP, detection and analysis of drug resistance in children. Med. J. West China28 (11), 1598–1602.
45
Liu X. Jiang Y. Chen X. Li J. Shi D. Xin D. (2014). Drug resistance mechanisms of Mycoplasma pneumoniae to macrolide antibiotics. Biomed. Res. Int.2014, 320801. 10.1155/2014/320801
46
Liu S. Zhang L. Dai L. Li J. Li D. (2025). Prediction model for severe Mycoplasma pneumoniae pneumonia and analysis of macrolide-resistance in children: a case-control study. Ital. J. Pediatr.51 (1), 180. 10.1186/s13052-025-02039-y
47
Lu Y. C. B. Wu M. (2018). Detection and clinical significance of drug resistance genes in mycoplasma pneumonia in children. Chin. J. Rural. Med. Pharm.25 (12), 48–49.
48
Lucier T. S. Heitzman K. Liu S. K. Hu P. C. (1995). Transition mutations in the 23S rRNA of erythromycin-resistant isolates of Mycoplasma pneumoniae. Antimicrob. Agents Chemother.39 (12), 2770–2773. 10.1128/AAC.39.12.2770
49
Lung D. C. Yip E. K. T. Lam D. S. Y. Que T. L. (2013). Rapid defervescence after doxycycline treatment of macrolide-resistant mycoplasma pneumoniae-associated community-acquired pneumonia in children. Pediatr. Infect. Dis. J.32 (12), 1396–1399. 10.1097/INF.0b013e3182a25c71
50
Ma Q. Liu X. Cui F. Xin D. (2010). The relationship between the occurrence of extrapulmonary complications in MP pneumonia in children and drug resistance. Hebei Med. J.32 (24), 3445–3446.
51
Ma Z. Zheng Y. Deng J. Ma X. Liu H. (2014). Characterization of macrolide resistance of Mycoplasma pneumoniae in children in shenzhen, China. Pediatr. Pulmonol.49 (7), 695–700. 10.1002/ppul.22851
52
Matsuoka M. Narita M. Okazaki N. Ohya H. Yamazaki T. Ouchi K. et al (2004). Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrob. Agents Chemother.48 (12), 4624–4630. 10.1128/AAC.48.12.4624-4630.2004
53
Mercuri F. A. White S. McQuilten H. A. Lemech C. Mynhardt S. Hari R. et al (2024). Evaluation of intranasal TLR2/6 agonist INNA-051: safety, tolerability and proof of pharmacology. ERJ Open Res.10 (6), 00199-2024. 10.1183/23120541.00199-2024
54
Miyashita N. Ogata M. Fukuda N. Yamura A. Ito T. (2025). Macrolide-resistant Mycoplasma pneumoniae infection prevalence increases again in Osaka. Respir. Investig.63 (4), 517–520. 10.1016/j.resinv.2025.04.009
55
Okada T. Morozumi M. Tajima T. Hasegawa M. Sakata H. Ohnari S. et al (2012). Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin. Infect. Dis.55 (12), 1642–1649. 10.1093/cid/cis784
56
Oliveira-Nascimento L. Massari P. Wetzler L. M. (2012). The role of TLR2 in infection and immunity. Front. Immunol.3, 79. 10.3389/fimmu.2012.00079
57
Peng J. W. Y. (2023). Multifactorial analysis of clinical indi-cators associated with macrolide resistant mycoplasma pneu-moniae infection in children. Chin. J. Med.58 (9), 1021–1023.
58
Pereyre S. Guyot C. Renaudin H. Charron A. Bébéar C. Bébéar C. M. (2004). In vitro selection and characterization of resistance to macrolides and related antibiotics in Mycoplasma pneumoniae. Antimicrob. Agents Chemother.48 (2), 460–465. 10.1128/AAC.48.2.460-465.2004
59
Pereyre S. Goret J. Bébéar C. (2016). Mycoplasma pneumoniae: current knowledge on macrolide resistance and treatment. Front. Microbiol.7, 974. 10.3389/fmicb.2016.00974
60
Q Z. (2016). Therapeutic effects of minocycline on MRMP in children over 8 years. Hebei Med. J.38 (05), 659–661.
61
Ranjbar R. Halaji M. (2019). Epidemiology of Mycoplasma pneumoniae prevalence in Iranian patients: a systematic review and meta-analysis. J. Med. Microbiol.68 (11), 1614–1621. 10.1099/jmm.0.001079
62
Rothstein T. E. Cunningham S. A. Rieke R. A. Mainella J. M. Mutchler M. M. Patel R. (2022). Macrolide resistance in Mycoplasma pneumoniae, midwestern United States, 2014 to 2021. Antimicrob. Agents Chemother.66 (4), e0243221. 10.1128/aac.02432-21
63
Shamseer L. Moher D. Clarke M. Ghersi D. Liberati A. Petticrew M. et al (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj350, g7647. 10.1136/bmj.g7647
64
Sung M. Roh E. J. Lee E. S. Lee J. Y. Kim H. B. Ahn Y. et al (2022). Assessment of variables associated with prolonged admission duration in children with Mycoplasma pneumoniae pneumonia. Clin. Respir. J.16 (11), 756–767. 10.1111/crj.13549
65
Wang G. Wu P. Tang R. Zhang W. (2022). Global prevalence of resistance to macrolides in mycoplasma pneumoniae: a systematic review and meta-analysis. J. Antimicrob. Chemother.77 (9), 2353–2363. 10.1093/jac/dkac170
66
Wang N. Xu X. Xiao L. Liu Y. (2023). Novel mechanisms of macrolide resistance revealed by in vitro selection and genome analysis in Mycoplasma pneumoniae. Front. Cell Infect. Microbiol.13, 1186017. 10.3389/fcimb.2023.1186017
67
Wei R. Dou H. Wang L. Li D. Tian X. Li J. et al (2019). In vitro susceptibility test of Xiao'er feire kechuan oral solution to Mycoplasma pneumoniae. Med. Baltim.98 (27), e16070. 10.1097/MD.0000000000016070
68
Wu C. W. A. Cai Z. (2021). Correlation between 23S rRNA mutation of MP and clinical characteristics and drug resistance in children. Chin. J. General Prac-tice19 (04), 603–606.
69
Wu P. S. Chang L. Y. Lin H. C. Chi H. Hsieh Y. C. Huang Y. C. et al (2013). Epidemiology and clinical manifestations of children with macrolide-resistant Mycoplasma pneumoniae pneumonia in Taiwan. Pediatr. Pulmonol.48 (9), 904–911. 10.1002/ppul.22706
70
Wu B. Z. L. Zhang W. Li X. Liu J. Gu Q. (2020). Detection and clinical analysis of drug-resistant mutation sites in children with mycoplasma infectious lobar pneumonia. Chin. J. Pract. Pediatr. Pulmonol.35 (08), 626–630.
71
Wu T. H. Wang N. M. Liu F. C. Pan H. H. Huang F. L. Fang Y. P. et al (2021). Macrolide resistance, clinical features, and cytokine profiles in Taiwanese children with Mycoplasma pneumoniae infection. Open Forum Infect. Dis.8 (9), ofab416. 10.1093/ofid/ofab416
72
Wu Q. Song D. J. Shim J. Y. (2024). New insights into the epidemiological characteristics of MP infection before and after the COVID-19 pandemic. Microorganisms12 (10).
73
Xin D. Han X. Ma S. Chen X. (2010). Clinical character-istics of children with macrolide-resistant mycoplasma pneumo-niae pneumonia. Chin. J. Appl. Clin. Pediatr.25 (16), 1213–1215.
74
Xu C. Deng H. Zhang J. Zhu Y. Rong Q. Quan Y. et al (2021). Mutations in domain V of Mycoplasma pneumoniae 23S rRNA and clinical characteristics of pediatric M. pneumoniae pneumonia in nanjing, China. J. Int. Med. Res.49 (6), 3000605211016376. 10.1177/03000605211016376
75
Yang H. J. Song D. J. Shim J. Y. (2017). Mechanism of resistance acquisition and treatment of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Korean J. Pediatr.60 (6), 167–174. 10.3345/kjp.2017.60.6.167
76
Yang Y. H. Z. Y. Huang X. M. Chen Y. L. Shen J. Zhang W. Y. (2018). Clinic analysis of macrolide-resistant MP pneumonia in children. Chin. J. Gen. Pract.16 (03), 434–436.
77
Yang T. I. Chang T. H. Lu C. Y. Chen J. M. Lee P. I. Huang L. M. et al (2019). MP in pediatric patients: do macrolide-resistance and/or delayed treatment matter?J. Microbiol. Immunol. Infect.52 (2), 329–335. 10.1016/j.jmii.2018.09.009
78
Yang S. Liu X. Han Y. Wang H. Mei Y. Wang H. et al (2025). Clinical characteristics and associated factors of macrolide-resistant mycoplasma pneumoniae pneumonia in children: a systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis.44 (6), 1505–1522. 10.1007/s10096-025-05101-z
79
Yoo S. J. Kim H. B. Choi S. H. Lee S. O. Kim S. H. Hong S. B. et al (2012). Differences in the frequency of 23S rRNA gene mutations in Mycoplasma pneumoniae between children and adults with community-acquired pneumonia: clinical impact of mutations conferring macrolide resistance. Antimicrob. Agents Chemother.56 (12), 6393–6396. 10.1128/AAC.01421-12
80
Yoon I. A. Hong K. B. Lee H. J. Yun K. W. Park J. Y. Choi Y. H. et al (2017). Radiologic findings as a determinant and no effect of macrolide resistance on clinical course of Mycoplasma pneumoniae pneumonia. BMC Infect. Dis.17 (1), 402. 10.1186/s12879-017-2500-z
81
Yu C. L. W. Zhang X. (2021). The relationship between clinical phenotype and chest imaging in community-acquired pneumonia with MP 23S rRNA A2063G gene mutation in children. J. Clin. Pediatr.39 (4), 265–268.
82
Yuan C. Min F. M. Ling Y. J. Li G. Ye H. Z. Pan J. H. et al (2018). Clinical characteristics and antibiotic resistance of Mycoplasma pneumoniae pneumonia in hospitalized Chinese children. Comb. Chem. High. Throughput Screen21 (10), 749–754. 10.2174/1386207322666190111112946
83
Yuan L. Mingyue D. Zhou L. (2025). Analysis of the characteristics of mixed infections with Mycoplasma pneumoniae in children. Sci. Rep.15 (1), 9414. 10.1038/s41598-025-94292-8
84
Zhan X. W. Deng L. P. Wang Z. Y. Zhang J. Wang M. Z. Li S. J. (2022). Correlation between Mycoplasma pneumoniae drug resistance and clinical characteristics in bronchoalveolar lavage fluid of children with refractory Mycoplasma pneumoniae pneumonia. Ital. J. Pediatr.48 (1), 190. 10.1186/s13052-022-01376-6
85
Zhang W. Z. X. Gu W. Yan Y. Ji W. Zhu C. Shao X. et al (2021). Role of macrolides resistance in children with refractory MP pneumonia. Chin. J. Appl. Clin. Pediatr.36 (11), 822–826.
86
Zhang Z. Dou H. Yuan Q. Shi D. Wan R. Tu P. et al (2023). Proteomic and phenotypic studies of Mycoplasma pneumoniae revealed macrolide-resistant mutation (A2063G) associated changes in protein composition and pathogenicity of type I strains. Microbiol. Spectr.11 (4), e0461322. 10.1128/spectrum.04613-22
87
Zhao F. Song D. J. Shim J. Y. (2019). Antimicrobial susceptibility and genotyping of MP isolates in beijing, China, from 2014 to 2016. Antimicrob. Resist Infect. Control8, 18.
88
Zhou J. Y. C. (2023). Resistance gene mutations and characteristics of MP in children. Chin. J. Health Lab. Technol.33 (14), 1673–1676.
89
Zhou Y. Zhang Y. Sheng Y. Zhang L. Shen Z. Chen Z. (2014). More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob. Agents Chemother.58 (2), 1034–1038. 10.1128/AAC.01806-13
Summary
Keywords
Mycoplasma pneumoniae, 23S rRNA mutation, mutation burden, age stratification, macrolide resistance
Citation
Wang R, He J, Feng Y, Wang M, Zhong C, He S, Tu S, Wen N and Wang C (2025) Association between point mutations of macrolide-resistant Mycoplasma pneumoniae and clinical antibiotic treatment efficacy: a meta-analysis. Front. Pharmacol. 16:1682167. doi: 10.3389/fphar.2025.1682167
Received
08 August 2025
Revised
11 October 2025
Accepted
23 October 2025
Published
06 November 2025
Volume
16 - 2025
Edited by
Ranjan K. Mohapatra, Government College of Engineering, Keonjhar, India
Reviewed by
Milena Milakovic Obradovic, Ludwig Maximilian University of Munich, Germany
Damodharan Perumal, Indira Medical College and Hospitals, India
Updates
Copyright
© 2025 Wang, He, Feng, Wang, Zhong, He, Tu, Wen and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Wen, 281015645@qq.com; Chuan Wang, wangchuan@usc.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.