- 1Faculty of Medicine and Health, University of New South Wales, Kensington, NSW, Australia
- 2Centre for Neuroscience and Regenerative Medicine, St. Vincent’s Centre for Applied Medical Research, St Vincent’s Hospital Sydney, Sydney, NSW, Australia
Background: The cannabinoid receptor 2 (CB2) is involved in regulating immune responses, yet its specific function in microglia remains poorly defined. This study aimed to generate and validate a microglia-specific, inducible CB2 knockout mouse model incorporating reporter genes to enable precise detection of CB2 expression and CB2 knockout.
Methods: A novel floxed CB2 mouse line was generated, incorporating GFP and tdTomato reporter genes driven by the Cnr2 promoter to indicate CB2 expression and CB2 knockout, respectively. This line was crossed with Cx3cr1 or Tmem119 tamoxifen-inducible Cre lines to achieve macrophage- or microglia-specific CB2 knockout, respectively. Behavioural testing, in vitro assays, sequencing and in vivo immunofluorescence were used to assess the efficiency and specificity of CB2 knockout as well as potential off-target effects.
Results: The floxed allele did not alter breeding or motor behaviour in mice, nor CB2 function. CB2 expression, indicated by GFP, followed expected patterns across tissues and conditions. Sequencing revealed both DNA and RNA of the floxed allele was as anticipated. Tamoxifen-induced Cre activity successfully initiated tdTomato expression exclusively in microglia of tamoxifen treated, Cre positive mice, validating the specificity and inducibility of CB2 knockout. Microglial tdTomato expression confirmed successful CB2 knockout in 9.3% of TmemCB2 and 91.7% of Cx3CB2 microglia. Peripheral tdTomato expression persisted beyond 3 weeks post-tamoxifen in Cx3CB2 mice but was minimal in TmemCB2 mice.
Conclusion: This novel microglia-specific, inducible CB2 knockout model is the first to combine a floxed CB2 allele with reporter genes, an essential advancement given the lack of reliable CB2 antibodies. The findings demonstrate the model’s specificity and effectiveness, while highlighting important considerations regarding Cre-mediated effects and recombination specificity. Furthermore, the floxed mouse can be crossed with any Cre line to study CB2 expression and function in various tissues. This model provides a powerful platform for advancing understanding of CB2 roles in microglia and supports future exploration of CB2-targeted therapeutic strategies.
1 Introduction
The endocannabinoid system (ECS), a tightly regulated signalling network present in all animal species, is a key regulator of homeostasis, including immune responses (Di Marzo, 2018; Van et al., 2021; Silver, 2019; Lowe et al., 2021; Antignano et al., 2023; Rakotoarivelo et al., 2024). The ECS includes cannabinoid receptors CB1 and CB2, along with their endogenous ligands, endocannabinoids. While CB1 is mostly expressed by neurons, CB2 is primarily expressed by immune cells. CB2 expression is upregulated during inflammation and is understood to play a crucial role in immune modulation (Brusco et al., 2008; Ferranti and Foster, 2022; N. Joshi and Onaivi, 2019). Numerous studies have demonstrated the therapeutic effects of CB2 agonists in a large range of disease models, including Alzheimer’s disease (Wu et al., 2013; Jayant et al., 2016; Fakhfouri et al., 2012; C. Li et al., 2019; Aso et al., 2013; Moreno et al., 2012; Sobue et al., 2024), Parkinson’s disease (Rentsch et al., 2020; Gómez-Gálvez et al., 2016; Chung et al., 2016; Liu et al., 2022; Espadas et al., 2020; Viveros-Paredes et al., 2017; H. Yu et al., 2021; Joers et al., 2024), Huntington’s disease (Sagredo et al., 2009; Palpagama et al., 2019), traumatic brain injury (Braun et al., 2018), cerebrovascular and cardiovascular disorders (Yu et al., 2021; Zarruk et al., 2012; Tang et al., 2016; More et al., 2024), metabolic disorders (Rorato et al., 2022; Youssef, El-Fayoumi, and Mahmoud, 2019; Rohbeck, Eckel, and Romacho, 2021; Verty et al., 2015; Rossi et al., 2016; Hosoki, Asahi, and Nozaki, 2024), pain (Xu et al., 2023; Monory and Lutz, 2005; van den Hoogen et al., 2021; Nan et al., 2023), cancer (Alenabi and Malekinejad, 2021; Gambacorta et al., 2023) and more (Smoum et al., 2022; Whiting et al., 2022; Gasperi et al., 2023; Lowe et al., 2021; Grabon et al., 2023a; Onaivi, 2006; Kong et al., 2014; Espejo-Porras et al., 2019). These studies predominantly attribute the therapeutic effects to CB2’s anti-inflammatory properties; however, the cell-specific mechanisms mediating these effects remain unclear.
In the central nervous system (CNS), CB2 is minimally expressed under basal conditions, but its expression is upregulated in microglia, the resident immune cells of the brain, during neuroinflammation (Duffy et al., 2021). Microglia are essential for maintaining CNS homeostasis and neuronal health. In their resting state microglia continuously monitor the environment for threats. Their phenotypic plasticity allows them to rapidly adapt to environmental cues, and upon activation by inflammatory stimuli, microglia adopt a pro-inflammatory phenotype, a defining feature of neuroinflammation (Araki, Ikegaya, and Koyama, 2021; Prinz, Jung, and Priller, 2019; Umpierre and Wu, 2021; Gao et al., 2023; Paolicelli et al., 2022). Chronic neuroinflammation is implicated in disease pathogenesis, as demonstrated in vivo (Wang P et al., 2022; Kang et al., 2024; Penney et al., 2024; Liang et al., 2023; Shi et al., 2019; Dong et al., 2021; Munro et al., 2024; X. Chen et al., 2023; Bido et al., 2021; Gratuze et al., 2023; Arvanitaki et al., 2024; Jing et al., 2021; Ding et al., 2021; Cui et al., 2021; Rocha et al., 2023; W. Kong et al., 2023; Pan et al., 2023; Ryan et al., 2023; Kitchener, Dundee, and Brown, 2023), in vitro (Zhou et al., 2023; Salvadores et al., 2022; C. Zhang et al., 2023), and genetic studies (Takatori et al., 2019; Andersen et al., 2021; Corley et al., 2021; ConsortiumInternational Multiple Sclerosis Genetics, 2019; Rodero et al., 2008). Mechanistically, pro-inflammatory microglia contribute to neuronal damage through various pathways, including direct neuronal interactions (Lindhout et al., 2021; Fricker, Oliva-Martin, and Brown, 2012; Butler et al., 2021), the release of neurotoxic cytokines (Lindhout et al., 2021; Rodriguez-Gomez et al., 2020; Oyarce et al., 2022; X. Liu et al., 2021), propagation of pathogenic proteins (Zheng and Zhang, 2021; Wang C et al., 2022; Odfalk, Bieniek, and Hopp, 2022; Xia et al., 2021), and recruitment of peripheral immune cells (Liddelow et al., 2017; X. Chen et al., 2023; Green et al., 2024; Joshi et al., 2019). These pathological activities are compounded by the loss of microglial homeostatic functions (Della Valle et al., 2024; Borst, Dumas, and Prinz, 2021; Zrzavy et al., 2017; Sobue et al., 2021; Kwon and Koh, 2020; Angelova and Brown, 2019). Conversely, microglia in anti-inflammatory states can exert neuroprotective effects, and there is growing evidence that therapies aimed at shifting microglia from a pro-inflammatory to an anti-inflammatory phenotype is beneficial in treating neurodegenerative diseases (Q. Li et al., 2021; Wang W et al., 2023; Willis et al., 2020; Shibuya et al., 2022; Mader et al., 2024; Yoo et al., 2023; Chadarevian et al., 2024; Munro et al., 2024; Daria et al., 2017; Lee et al., 2020; Tao et al., 2021; Jang et al., 2022; Pan et al., 2023; Piano et al., 2023; Birkle and Brown, 2023; Z. Yang et al., 2019; Guo, Wang, and Yin, 2022; Rizzi et al., 2018; Gao et al., 2023). CB2 is upregulated in pro-inflammatory microglia, and CB2 stimulation has been shown to mitigate their activation, making CB2 an attractive therapeutic target (Wang M et al., 2023; Chung et al., 2016; Ojha et al., 2016; Chen et al., 2025).
Although microglia are the principal CB2-expressing cells in the CNS, studies have reported CB2 expression in certain neuronal populations, other glial cells, and by immune cells that infiltrate the CNS during inflammation (Ziring et al., 2006; Robinson et al., 2015; Jia et al., 2020). However, the extent and functional significance of CB2 expression in these cell types remains debated, in part due to challenges in antibody specificity and detection methods (Atwood and Mackie, 2010; Grabon et al., 2023a; Eraso-Pichot et al., 2023). This raises a critical question: Are the therapeutic effects of CB2 agonists primarily mediated through microglia, or do other CNS and peripheral cell types contribute significantly?
To address this knowledge gap, we have generated a novel floxed CB2 mouse line (CB2flx). This model allows for conditional knockout (KO) of the entire coding region of Cnr2, the gene encoding CB2, in specific cell populations by crossing with appropriate Cre driver lines. This line is novel in that it incorporates dual fluorescent reporter genes for visualising CB2-expressing cells and cells in which CB2 has been deleted. The incorporation of reporter genes overcomes the limitations posed by a lack of reliable CB2 antibodies (Grabon et al., 2023a; Grabon et al., 2023b; Zhang et al., 2019), providing a robust tool for investigating CB2 expression and function. This line was then crossed with inducible microglia-specific Cre lines, allowing for precise, cell type-specific deletion of CB2 in microglia. Given the complexity of the genetic modifications, rigorous validation is essential to ensure the specificity, efficiency, and functional neutrality of the system prior to its application in disease models.
2 Methods
2.1 Animals
2.1.1 Cre-Lox FLEx switch mechanism
A spatially and temporally specific CB2-KO mouse line was generated using the Cre-Lox recombination system. CB2 is encoded by the Cnr2 gene, which contains a single protein coding exon (exon 2). The entire coding region of Cnr2 was flanked by loxP sites using homologous recombination in embryonic stem cells, enabling complete KO of CB2 protein expression upon Cre-mediated recombination. To facilitate monitoring of Cnr2 expression and recombination, GFP and tdTomato (tdT) reporter genes were inserted under the control of the Cnr2 promoter. The tdT cassette was placed in an inverse orientation and flanked by lox2272 sites as part of a flip-excision switch (Supplementary Figure S1A). In the absence of Cre recombinase, CB2 and GFP are expressed under the control of the Cnr2 promoter. Following Cre-mediated recombination, the floxed Cnr2 coding region and GFP are excised, while tdT is simultaneously flipped into the sense orientation and will now be expressed under the Cnr2 promoter, providing a permanent marker of recombination (Figure 1; Supplementary Figure S1B). This novel line was named C57BL/6-Cnr2tm1(GFP,tdTomato)BViss/J line (CB2flx).
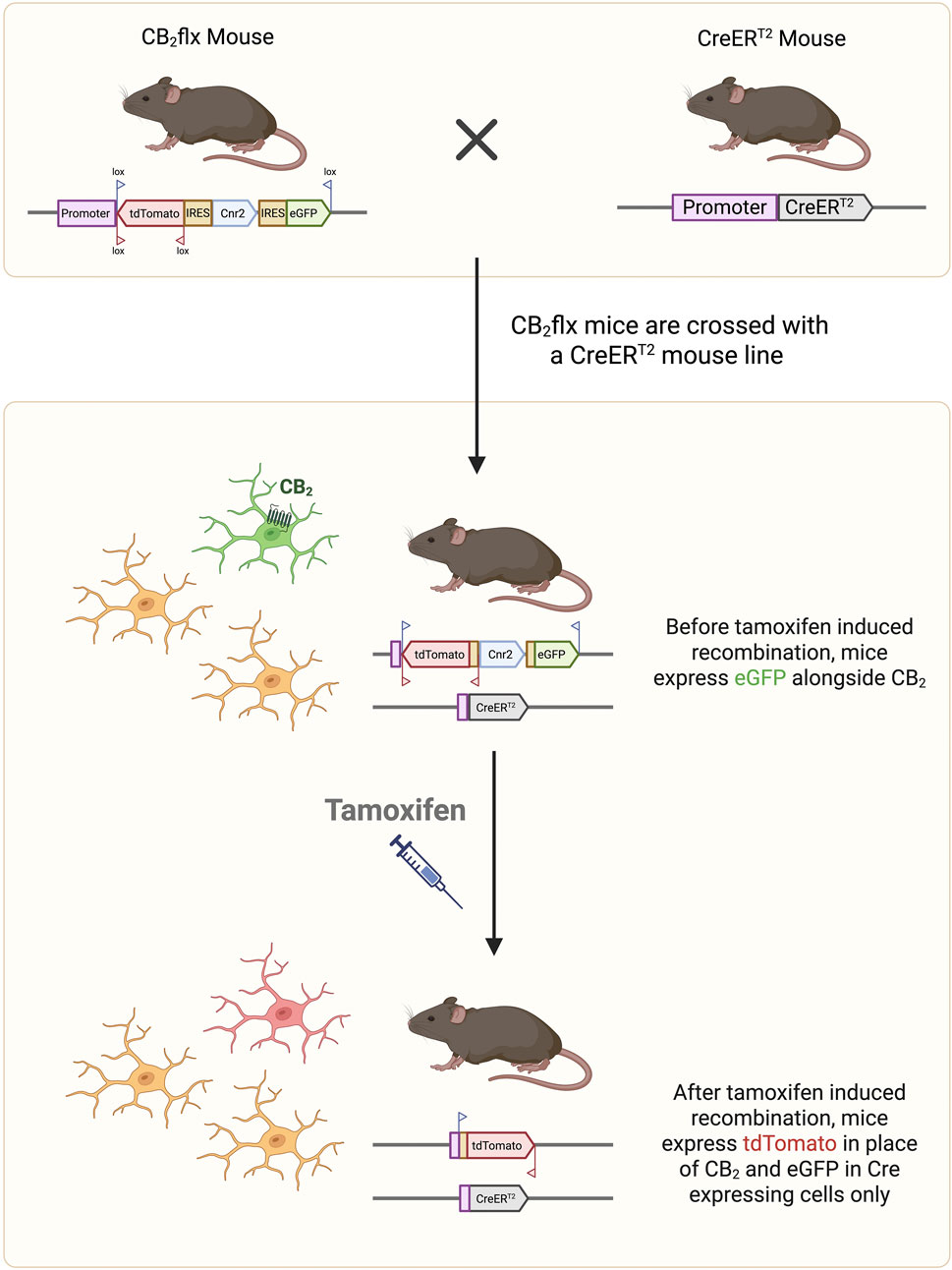
Figure 1. Conditional CB2 knockout mechanism. The CB2flx mouse line contains genetic modifications in which the entire Cnr2 coding region (blue box) is surrounded by lox sites. The reporter genes GFP (sense orientation) and tdT (antisense orientation) are inserted into the Cnr2 locus under control of the Cnr2 promoter, such that cells expressing CB2 also express GFP. The CB2flx line is crossed with a Cre/ERT2 line to create an inducible CB2-knockout line. Following tamoxifen injection, Cre mediated recombination excises the floxed Cnr2 coding region and GFP, while flipping tdT into the sense orientation. Consequently, cells in which CB2 has been knocked out now express tdT as a marker of recombination. GFP, green fluorescent protein. tdT, tdTomato.
Genotyping of all genetically modified mice was performed for the Cnr2 floxed allele and relevant Cre alleles using real-time PCR with SYTO9-based melt curve analysis on a LightCycler 480 system. Primer sequences and PCR conditions are detailed in Supplementary Table S1.
2.1.2 Housing and husbandry
Mice were housed under specific-pathogen-free conditions at 21 °C ± 1 °C with a 12-h light-dark cycle, in plastic cages with ad libitum access to water and standard chow. A maximum of five same-sex mice were housed per cage. Mice were acclimated to the facility for at least 1 week prior to experiments. Experimental groups comprised 8–12-week-old male and female mice, and mice of the same sex and genotype were randomly assigned to experimental groups.
All genetically modified mice used in experiments were homozygous for the floxed Cnr2 allele. Experimental TmemCB2 and Cx3CB2 mice were bred so that littermates were either heterozygous (Cre/+) or wildtype (+/+) for the Cre/ERT2 allele.
All animal research and care procedures were approved by the Garvan Institute/St. Vincent’s Animal Ethics Committee (ARA numbers 23/13, 20/10, 18/37), per guidelines issued by the National Health and Medical Research Council of Australia and the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
2.2 Tamoxifen administration
Tamoxifen (Tam) (20 mg/mL) was dissolved in sunflower oil. Mice received i.p. injections of Tam or vehicle control according to three regimens: 150 mg/kg every other day for four doses, 100 mg/kg daily for 8 days, or 100 mg/kg twice daily for ten doses. Tissue was collected 3 weeks after the final injection.
2.3 Motor behaviour
Locomotor activity was assessed in a 30 × 30 cm plexiglass open field arena within a sound-attenuated chamber under dim illumination (MedAssociates). One day following a 10 min habituation session, mouse movements were tracked for 10 min. Total distance travelled, centre zone (19 × 19 cm) entries, and time spent in the centre zone were recorded. Blinding was maintained during testing.
Mice were trained on an accelerating rotarod (0–40 RPM) whereby they were placed on the rotarod and returned to it each time they fell off, for 5 min. The following day, latency to fall was measured over three 5-min trials, separated by 30-min intervals. The average of the highest two trials was used for analysis. Experimenters were blinded to treatment groups.
2.4 Stereotaxic lipopolysaccharide injection
To induce inflammation-induced neurodegeneration, stereotaxic surgery was performed to ipsilaterally inject the striatum with the endotoxin lipopolysaccharide (LPS). Intrastriatal LPS is well established to produce a robust neuroinflammatory response in the substantia nigra (SN), while its large volume allows for more accurate targeting and reduced mechanical damage (Deng et al., 2020; Jang et al., 2022; Skrzypczak-Wiercioch and Salat, 2022). Before surgery, mice received 4 × 150 mg/kg doses of Tam or vehicle control, once every other day, followed by a 4-week washout period. Mice were then anesthetised via an anaesthetic cocktail (10 mL/kg, i.p.), containing ketamine (Mavlab, Australia; 10 mg/mL) and xylazine (Troy Laboratories, Australia; 2 mg/mL). LPS was administered as previously described (Gómez-Gálvez et al., 2016). Briefly, LPS (Salmonella enterica, Minnesota) was dissolved in 0.9% sterile saline to a concentration of 5 mg/mL. Two injections of 1 μL of LPS were injected into the right striatum at AP +1.18, ML -1.5, DV -3.5 and AP -0.34, ML -2.5, DV -3.2, relative to bregma and the dural surface. Injections were given at a rate of 0.5 μL/min using a 2 μL Neuros syringe (Hamilton, Germany).
Mice were acrificed for histological analysis 15 days after surgery.
2.5 Histology
2.5.1 Tissue collection and processing
Animals were anaesthetised before cardiac perfusion with 4% paraformaldehyde. The brain was immediately dissected out and submerged in paraformaldehyde at 4 °C for 24 h, before being transferred to cryoprotectant solution (30% sucrose in PBS).
2.5.2 Immunofluorescence
Free-floating 40 µm sections were blocked in 3% BSA +0.25% Triton for 1 h before primary antibody (Supplementary Table S2) was applied for 72 h, at 4 °C. Tissue was then incubated with the corresponding Alexa Fluor-conjugated secondary antibody for 24 h at 4 °C, protected from light. Finally, the sections were incubated in DAPI (Thermo Fisher; 1:1000 in PBS) for 10 min.
2.5.3 FIJI image analysis
For colocalization of GFP, tdT and Iba1, four representative images of each brain were taken in consistent areas of the striatum, SN, and hippocampus with a Zeiss Axio Imager.Z2 microscope. For each image, four z-stacks were taken using the ×40 objective. Percentages of GFP, tdT and Iba1 colocalization were quantified using the FIJI (ImageJ) Colocalization Object Counter plugin (Lunde and Glover, 2020). Both the max projection and stack were analysed. A cell would only be counted if the nuclear marker DAPI was present.
2.6 In vitro cAMP assay
Primary glia cultures were prepared from P0–P3 C57BL/6JAusb (BL6), CB2flx and Cx3CB2 pups, as previously described (Schildge et al., 2013). Briefly, cortices were dissected in dissection medium (HBSS, 1% P/S) dissociated with trypsin-EDTA (0.005%), and a single cell suspension in glia medium (DMEM/F12, 10% FBS, 1% P/S) was added to PDL coated flasks. Media was changed after 24–36 h and then every 2–3 days thereafter, until the cells reached approximately 90% confluence (∼9 days). Microglia and astrocytes were separated by shaking cultures at 200 RPM for 6 h. Microglia and astrocyte cell pellets were resuspended in stimulation buffer (HBSS + 5 mM HEPES +0.5 mM IBMX +0.075% BSA; pH 7.4) for use in cAMP assays. Forskolin-stimulated cAMP levels were measured using the LANCE Ultra cAMP kit (PerkinElmer), following manufacturer’s instructions.
For assay optimisation, forskolin concentration-response curves were established for both microglia and astrocytes at various cell densities to determine the optimum cell density and forskolin concentrations for subsequent CB2 agonist cAMP assays. It was concluded that the ideal conditions for microglia were 2000 cells/well with a forskolin concentration of 71.4 µM (Supplementary Figure S2A). For astrocytes, 500 cells/well with a forskolin concentration of 2.6 µM (Supplementary Figure S2B).
For the agonist assays, Hu308 was diluted in DMSO to generate a concentration response curve of forskolin-stimulated cAMP levels for each cell type. For all assays, samples were run in triplicate and a cAMP standard curve was run on every plate. TR-FRET signal was measured with the PHERAstar FSX microplate reader (BMG LabTech).
2.7 Statistics
All statistics were performed using Prism 10 software (GraphPad). Unless otherwise stated, p < 0.05 was considered significant and data are reported as mean ± standard error of the mean. Before undergoing parametric tests, D'Agostino-Pearson normality test was carried out and if a dataset failed this test, the Q-Q plot was inspected to determine if there were major violations of a Gaussian distribution. Further, Spearman’s rank test was used to test for homoscedasticity, and if this test failed, the residual plot was inspected for major violations. Data that passed these tests underwent one-, two- or three-way ANOVAs. For post hoc corrections, Tukey’s was used when comparing every mean with every other mean, Dunnett’s for comparing every mean to a control mean, Dunn’s correction for non-parametric comparisons, and Šídák’s correction for all other post hoc comparisons.
Details of all mouse lines, reagents, equipment and software used are listed in Supplementary Table S3.
3 Results
3.1 Validation of Cnr2 gene targeting
To validate the genetic modifications in the floxed Cnr2 locus, DNA sequencing was performed by OzGene. As expected, the sequencing results confirmed alignment with the anticipated sequence across the targeted region, except for a 510 bp stretch within the 5′homology arm, for which incomplete sequencing data were obtained due to poor trace quality. Despite this limitation, the low-quality sequenced portion of the region exhibited 100% identity to the predicted sequence (Supplementary Figure S3). Furthermore, sequencing of Cnr2 mRNA, performed by The Garvan Institute’s Genetics Core Facility, indicating precise alignment with the anticipated sequence and therefore no unintended disruptions in Cnr2 transcription. These in-depth sequencing analyses confirm the successful incorporation of the intended genetic modifications in the floxed Cnr2 locus, with no evidence of off-target alterations affecting Cnr2 transcription. Full sequencing data for both DNA and mRNA is available on GenBank.
3.2 Genetic modifications do not impair reproductive fitness
The reproductive fitness of the genetically modified mouse lines was assessed compared to BL6 controls by a prospective analysis of breeding outcomes. There was no significant difference between groups for litter size (F (3, 196) = 1.31, p = 0.27; Figure 2A), mortality rate (KW(4, 200) = 3.79, p = 0.28; Figure 2B), or sex ratio (F (3, 192) = 0.094, p = 0.96; Figure 2C). Furthermore, no overt developmental or behavioural abnormalities were observed during routine breeding and handling. These findings demonstrate that the genetic modifications in the CB2flx, Cx3CB2, and TmemCB2 mouse lines do not adversely affect reproductive capacity or offspring viability.
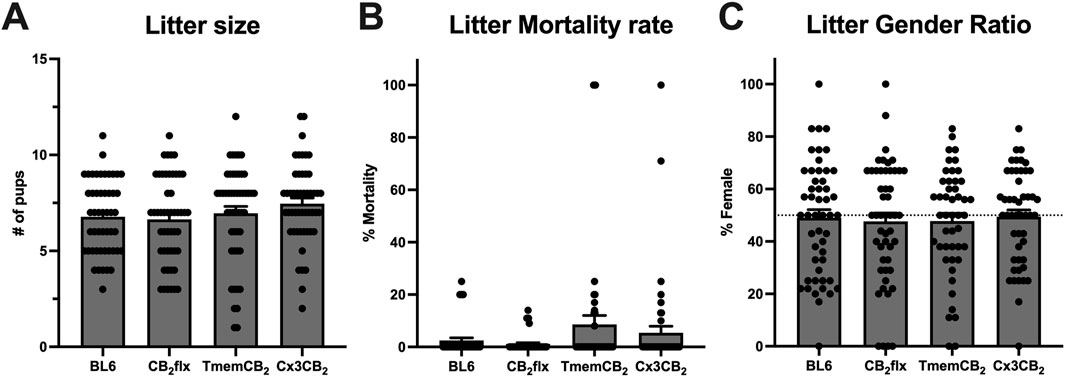
Figure 2. Litter characteristics are not impaired in genetically modified strains. (A) Number of pups born per litter. One-way ANOVA, Dunnett’s post hoc test. (B) Proportion of litter mortality between birth and weaning. One-way ANOVA, Dunnett’s post hoc test. (C) Proportion of pups that were female at weaning. One-way ANOVA, Dunn’s post hoc test. Data were collected from 50 litters per strain over a similar timeframe. Data are represented as mean ± SEM.
3.3 Motor behaviour is unaffected in CB2flx mice
To assess the effects of the floxed Cnr2 allele on motor behaviour, CB2flx mice were subjected to open field and rotarod test. In the open field test, no significant differences were observed between CB2flx and BL6 mice in total distance travelled (t (11) = 0.670, p = 0.516; Figure 3A), number of ambulatory episodes (t (11) = 0.421, p = 0.682; Figure 3B), or time spent in the central zone (t (11) = 1.138, p = 0.279; Figure 3C). Similarly, performance on the rotarod test revealed no differences in latency to fall (t (11) = 0.346, p = 0.736; Figure 3D). Collectively, these findings demonstrate that the presence of the floxed Cnr2 allele does not affect locomotor activity or motor coordination.
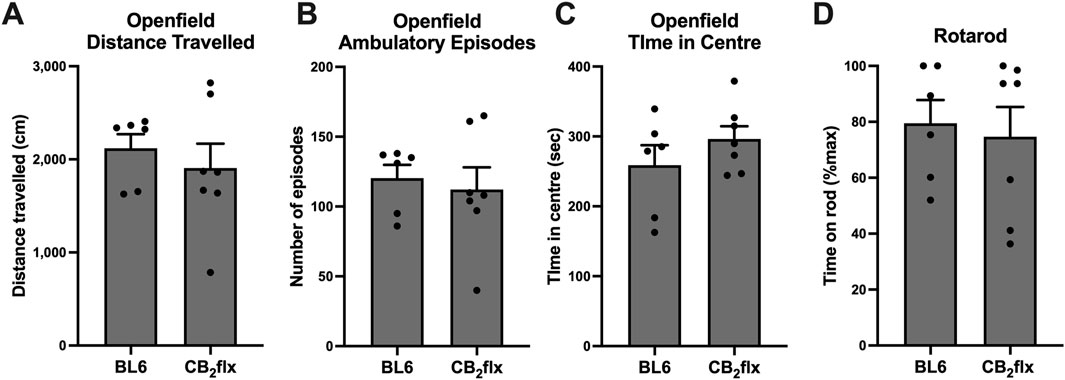
Figure 3. Motor behaviour is not impaired in the CB2flx strain. (A–C) Open field and (D) rotarod motor behaviour tests in CB2flx mice. N = 6-7/group. Unpaired t-test. Data are represented as mean ± SEM.
3.4 CB2 function is preserved in glial cells
As a G⍺i-coupled receptor, CB2 activation inhibits cAMP production (Kaminski, 1998). Therefore, a cAMP assay was performed to assess receptor function in primary glial cultures from CB2flx and Cx3CB2 mice. First, optimisation experiments were conducted to determine appropriate cell densities and forskolin concentrations for both microglia and astrocytes (Supplementary Figure S2).
Upon stimulation with the CB2 agonist Hu308, forskolin-induced cAMP levels were reduced in a dose-dependent manner across all groups. No significant differences were observed in Hu308-induced cAMP inhibition between CB2flx, Cx3CB2, and BL6 controls for microglia (F (2,28) = 0.027, p = 0.973; Figure 4A) or astrocytes (F (2,32) = 0.432, p = 0.653; Figure 4B). These results indicate that CB2 function is preserved in glial cells derived from genetically modified strains.
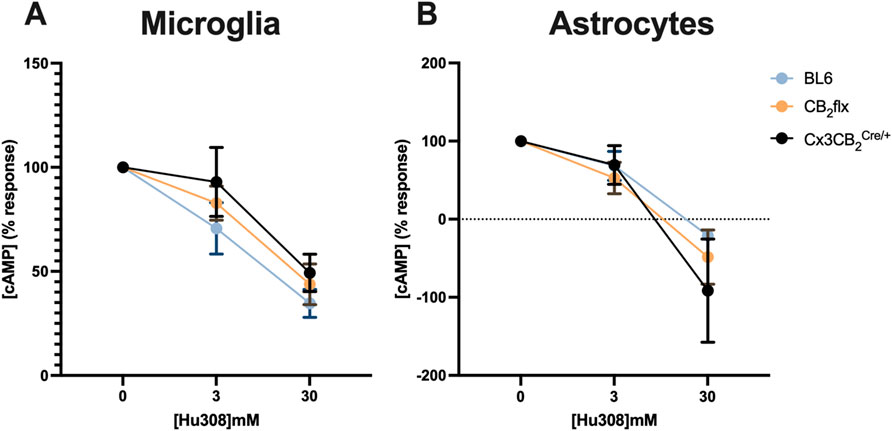
Figure 4. CB2 function is conserved in primary glia cultures. The CB2 agonist Hu308 inhibited cAMP production in primary (A) microglia and (B) astrocytes in a dose dependent manner in all strains. Data was normalised whereby 100% represents 0 mM Hu308 and 0% represents no forskolin control. N = 5-8/group. Two-way ANOVA, Dunnett’s post hoc test. Data are represented as mean ± SEM. Each point represents the mean of three technical replicates.
3.5 Reporter gene expression in the CB2flx mouse line
Given the challenges in using immunohistochemistry to detect CB2, a fluorescent reporter system was incorporated into the CB2flx line to enable visualisation of CB2 expression. In the CB2flx line, GFP expression is driven by the Cnr2 promoter, allowing detection of CB2-expressing cells through histological analysis. In the absence of Cre-mediated recombination, tdT should not be expressed. To confirm this, GFP and tdT expression were examined using immunofluorescence in the periphery, as well as in the brain under naive and inflammatory conditions.
BL6 controls displayed some small points of autofluorescence for both GFP and tdT, which served as an essential benchmark to distinguish true signal from artefacts (Figure 5A). In the naive CB2flx brain (Figure 5B), minimal GFP expression was observed, consistent with the low basal expression of CB2 in the CNS, while the spleen exhibited strong GFP expression, particularly in white pulp regions, which are enriched in CB2 expressing immune cells (Figure 5C) (Galiègue et al., 1995; Simard et al., 2022; Lewis, Williams, and Eisenbarth, 2019).
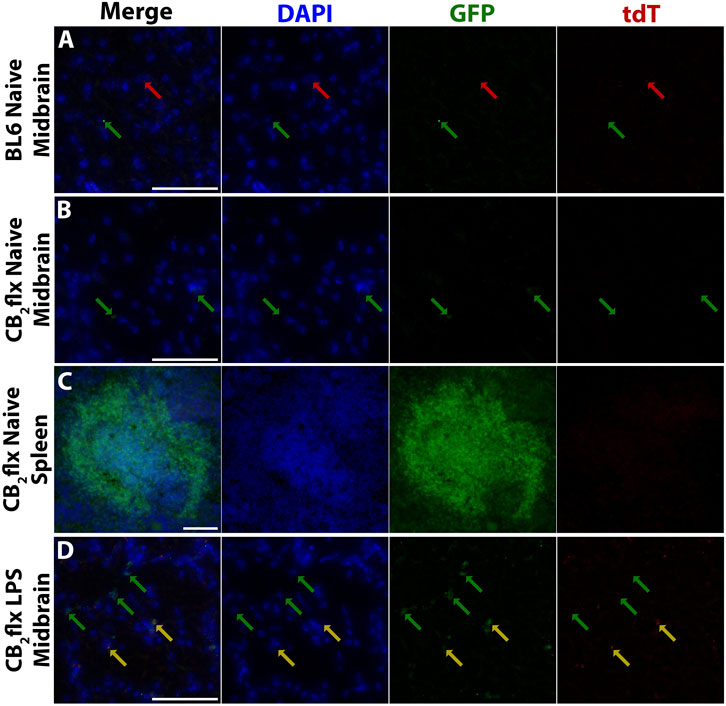
Figure 5. Reporter gene expression reflects expected CB2 expression in CB2flx mice. Representative images of green fluorescent protein (GFP) and tdTomato (tdT) reporter gene expression in (A) the naive BL6 CNS, (B) naive CB2flx CNS, (C) naive CB2flx spleen, and (D) CB2flx CNS treated with intrastriatal LPS. Green arrows = GFP expression. Red arrows = tdT expression. Yellow arrows = GFP/tdT colocalization. Scale bar = 100 µm.
To assess inflammation-induced CB2 expression, neuroinflammation was elicited via intrastriatal injection of LPS. GFP expression increased markedly in the inflamed CNS, aligning with the expected upregulation of CB2 during neuroinflammation. Occasional tdT expression was observed, but this was restricted to regions with intense GFP signals, suggesting fluorescence channel bleed-through rather than true tdT expression (Figure 5D).
Overall, GFP expression in CB2flx mice mirrored the expected CB2 expression profile: low in the naive CNS, elevated in the CNS during inflammation, and pronounced in peripheral tissues. These findings validate the utility of this mouse model for studying CB2 expression and function.
3.6 Tam induces microglial specific tdT expression in Cx3CB2 mice
Given that CNS CB2 is primarily expressed by microglia (Duffy et al., 2021), the CB2flx line was crossed with a macrophage-specific Cre line (JAX, strain#: 021160), resulting in the establishment of the Cx3CB2 line. In these mice, Cre expression is limited to Cx3cr1-expressing cells, which include microglia and peripheral macrophages (Subbarayan et al., 2022; Mizutani et al., 2012). Upon Tam administration, Cre-mediated CB2-KO is expected in all Cx3cr1-expressing cells.
To determine the most efficient Tam protocol for inducing Cre-mediated recombination in Cx3CB2 mice and to assess the effects of Cre and Tam on CB2 expression, we analysed reporter gene expression in BL6, CB2flx, and Cx3CB2 mice. In Cx3CB2 mice heterozygous (Cre/+) for the Cre allele, GFP serves as a marker of CB2 expression, while yellow fluorescent protein (YFP) is expressed in Cx3cr1-Cre cells as part of the Cre driver line. A technical limitation of this experimental design is that the overlapping emission spectra of GFP and YFP could not be distinguished using our immunofluorescence approach. Consequently, in Cx3CB2Cre/+ microglia, which express both YFP (from the Cre allele) and potentially GFP (from CB2 expression), the fluorescent signal primarily reflects YFP from Cre expression, and prevents direct assessment of CB2-driven GFP in these cells. In contrast, cells from other genotypes (CB2flx, Cx3CB2+/+) and non-microglial cell types do not express YFP, allowing GFP signal in these groups to be unambiguously attributed to CB2 expression. Despite this limitation in directly visualising CB2 expression in Cre-positive microglia, the tdT reporter system provides definitive confirmation of successful Cre-mediated CB2 deletion in these cells.
In Cx3CB2Cre/+ mice, nearly all Iba1+ microglia (>92%) expressed GFP/YFP, with no significant differences between treatment groups (F (6, 19) = 923.3, p < 0.0001; Figure 6A). Furthermore, over 96% of GFP/YFP signal colocalized with Iba1+ microglia, again with no significant differences between treatment groups (F (3, 9) = 0.413, p = 0.747; Figure 6B). These results confirm that Cre expression is restricted to microglia in Cx3CB2 Cre/+ mice, and that Tam treatment does not influence Cre expression levels.
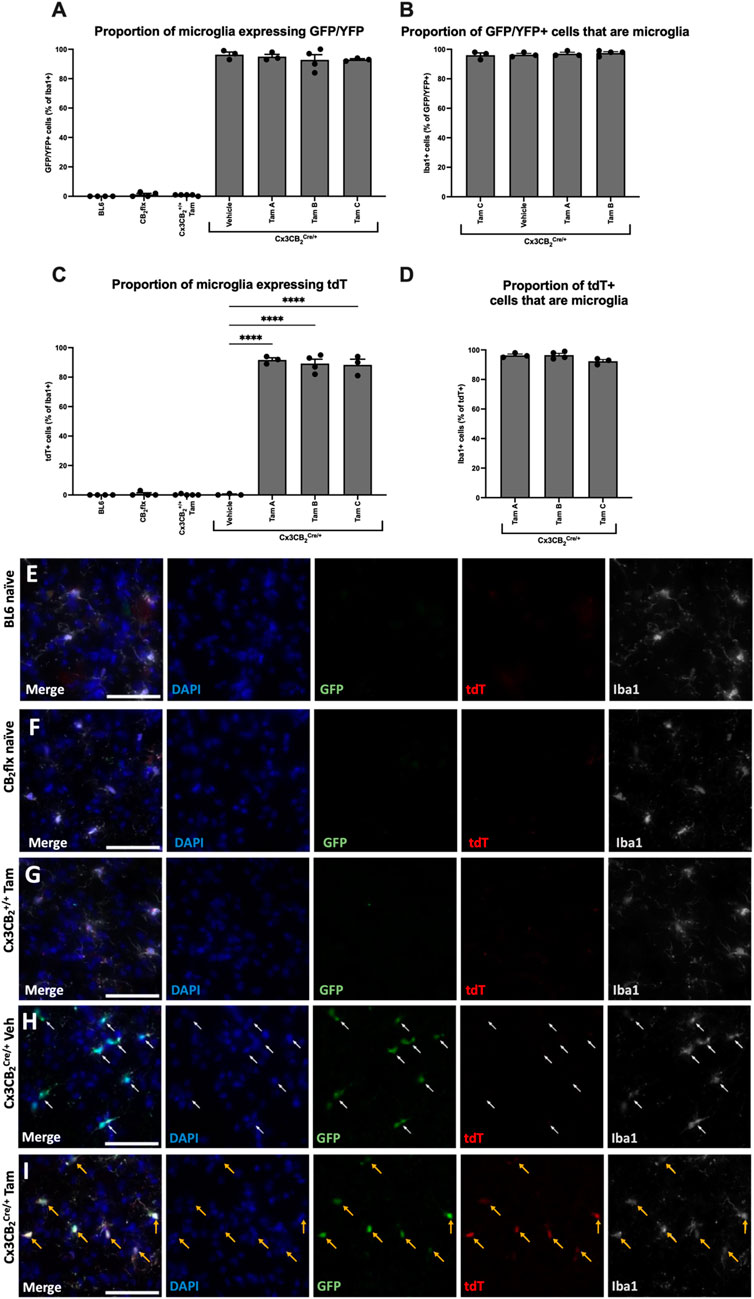
Figure 6. In Cx3CB2 mice, tdT expression is microglia specific and is Tam/Cre dependent. (A–D) Quantification of GFP and tdT colocalization with Iba1. Significance bars between Cx3CB2Cre/+ groups only are shown. N = 3-4/group. An average of 133.4 (±4.3) Iba1+ cells were counted per n. Data are represented as mean ± SEM. One-way ANOVA with Tukey’s post hoc test, ****p ≤ 0.0001. Representative images of substantia nigra immunostaining in (E) BL6, (F) CB2flx, (G) Tam treated Cx3CB2+/+, (H) Vehicle-treated Cx3CB2Cre/+ and (I) Tam-treated Cx3CB2Cre/+ animals. White arrows = GFP expression. Yellow arrows = GFP/tdT colocalization. Scale bar = 50 µm.
Microglia from all other genotypes exhibited minimal GFP/YFP signal, confirming the absence of Cre and supporting prior findings of low basal CB2 expression in microglia. Additionally, no significant differences were observed between Tam-treated Cx3CB2+/+ and BL6 controls, demonstrating that Tam administration does not induce CB2 expression in microglia (Figure 6A).
tdT expression serves as a reporter for successful CB2-KO in Cx3CB2 mice, therefore tdT should be restricted to microglia in Tam-treated and Cre/+ mice. As expected, no significant tdT expression was detected in any control group, confirming the absence of CB2-KO. In contrast, nearly all Iba1+ microglia in Tam-treated Cx3CB2Cre/+ mice expressed tdT (Figure 6C). Within these groups, over 92% of tdT expression colocalized with microglia, with no significant differences between Tam administration protocols, confirming high recombination efficiency across all protocols (F (2, 7) = 4.07, p = 0.067; Figure 6D). High-magnification (Figures 6E–I) and low-magnification (Supplementary Figure S4) representative images further emphasise the stark differences in reporter gene expression between groups. Furthermore, no GFP/YFP or tdT signal was observed in neurons or astrocytes, confirming that Cre expression, CB2 expression, and CB2-KO were microglia/macrophage-specific (Supplementary Figure S5).
Given that Cx3cr1-expressing macrophages are also present in peripheral tissues, we next examined CB2 expression outside the CNS. Peripheral macrophages have been reported to undergo renewal every 3 weeks, suggesting that tdT should be absent in the spleen at this timepoint, rendering CB2-KO microglia-specific thereafter (Wang et al., 2020; Dick et al., 2019; Goldmann et al., 2013; Peng et al., 2016; Bedolla et al., 2023). As expected, Tam-treated Cx3CB2+/+ spleens exhibited GFP but lacked tdT, similar to CB2flx controls (Supplementary Figure S6A). Conversely, tdT was detected in Cx3CB2Cre/+ spleens 1-week post-Tam, as anticipated (Supplementary Figure S6B). However, tdT expression persisted at 3 weeks, contrary to prior reports, suggesting that CB2-KO is not specific to microglia at this timepoint (Supplementary Figure S6C). This finding challenges the conventional assumption that a 3-week waiting period is sufficient to ensure microglia specificity in this Cre line.
These results confirm the microglia-specific expression of Cre in the CNS of Cx3CB2Cre/+ mice and its Tam-dependent activity. All Tam protocols efficiently induced CB2-KO in macrophages, with no significant differences between protocols. Based on these results, four doses of 150 mg/kg administered every other day was selected for continued use, as it exhibited the highest mean microglial tdT expression and specificity of 91.7%. Notably, CB2-KO persisted in peripheral macrophages beyond 3 weeks post-Tam, highlighting the need for extended evaluation periods if exclusive microglial specificity is required in this model.
3.7 Tamoxifen induced reporter gene expression in the TmemCB2 line
To improve microglia specificity of the CB2-KO, we generated the TmemCB2 line by crossing the CB2flx line with a Tmem119Cre/ERT2 line (JAX, strain# 031820), in which Cre expression is driven by the highly microglia-specific Tmem119 promoter (Kaiser and Feng, 2019). We assessed CB2-KO efficiency and specificity in the TmemCB2 line using the optimised Tam protocol established in Cx3CB2 mice (four doses of 150 mg/kg administered every other day). Tam was administered to TmemCB2 mice either homozygous for the wild-type Tmem119 allele (+/+) or heterozygous for the Tmem119Cre/ERT2 allele (Cre/+), alongside untreated BL6 controls. Only microglia in the Tam-treated TmemCB2Cre/+ group were anticipated to express tdT.
Reporter gene expression and colocalization with Iba1 were analysed to determine KO efficiency (representative images in Figures 7A–D). GFP expression in Iba1+ microglia did not differ between TmemCB2 groups and BL6 controls, suggesting a lack of basal CB2 expression in microglia under naive conditions (F (3, 8) = 0.732, p = 0.561; Figure 7E). Similarly, tdT expression in TmemCB2+/+ and vehicle-treated controls did not differ from BL6 mice, confirming an absence of CB2-KO cells. However, in Tam-treated TmemCB2Cre/+ mice, 9.3% of microglia expressed tdT, indicating a significant increase in CB2-KO microglia (F (3, 8) = 18.23, p = 0.0006; Figure 7F). This confirms that tdT expression in microglia is Tam-dependent. Furthermore, in this group, 94.3% of tdT signal colocalized with Iba1+ microglia, demonstrating that CB2-KO is microglia-specific in the CNS (Figure 7G). Low magnification representative images further demonstrate the differences in reporter gene expression between groups (Supplementary Figure S7).
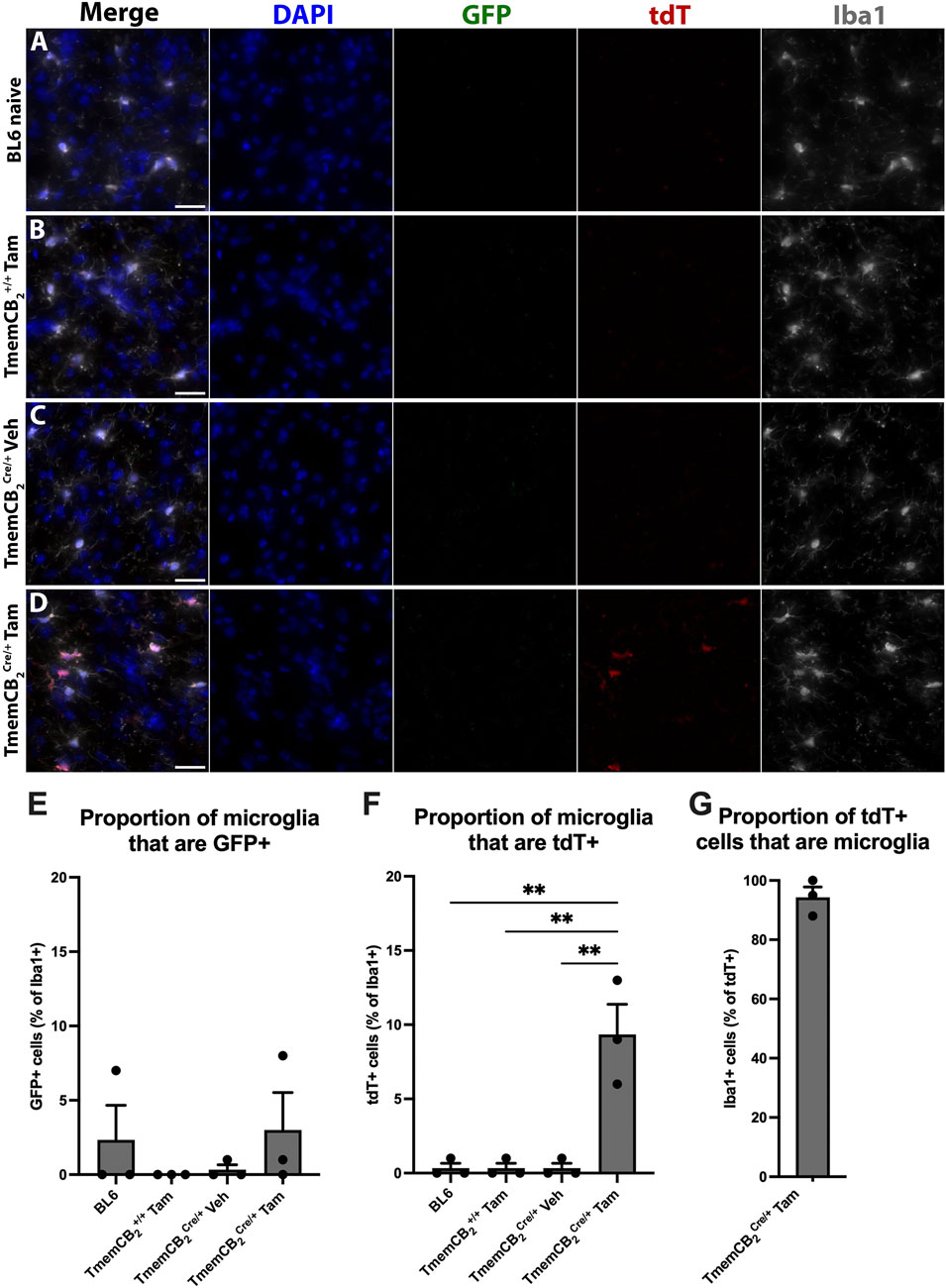
Figure 7. In TmemCB2 mice, tdT expression is microglia specific and is Tam/Cre dependent. (A–D) Representative images of substantia nigra GFP/tdT/Iba1 immunostaining. (A) BL6, (B) Tam treated TmemCB2+/+, (C) Vehicle treated TmemCB2Cre/+ and (D) Tam treated TmemCB2Cre/+. Scale bar = 50 µm. (E–G) Quantification of GFP and tdT with Iba1+ microglia. As only one group expressed tdT, G contains one panel only. N = 3/group. An average of 115.8 (±6.1) Iba1+ cells were counted per n. Data are represented as mean ± SEM. One-way ANOVA, Tukey’s post hoc test **p ≤ 0.01.
Neither GFP nor tdT colocalized with astrocytes (Figures 8A–C) or neurons (Figures 8D–F) in any group, further confirming that CB2 is not detectably expressed in these CNS types under basal conditions and that CB2-KO is microglia-specific.
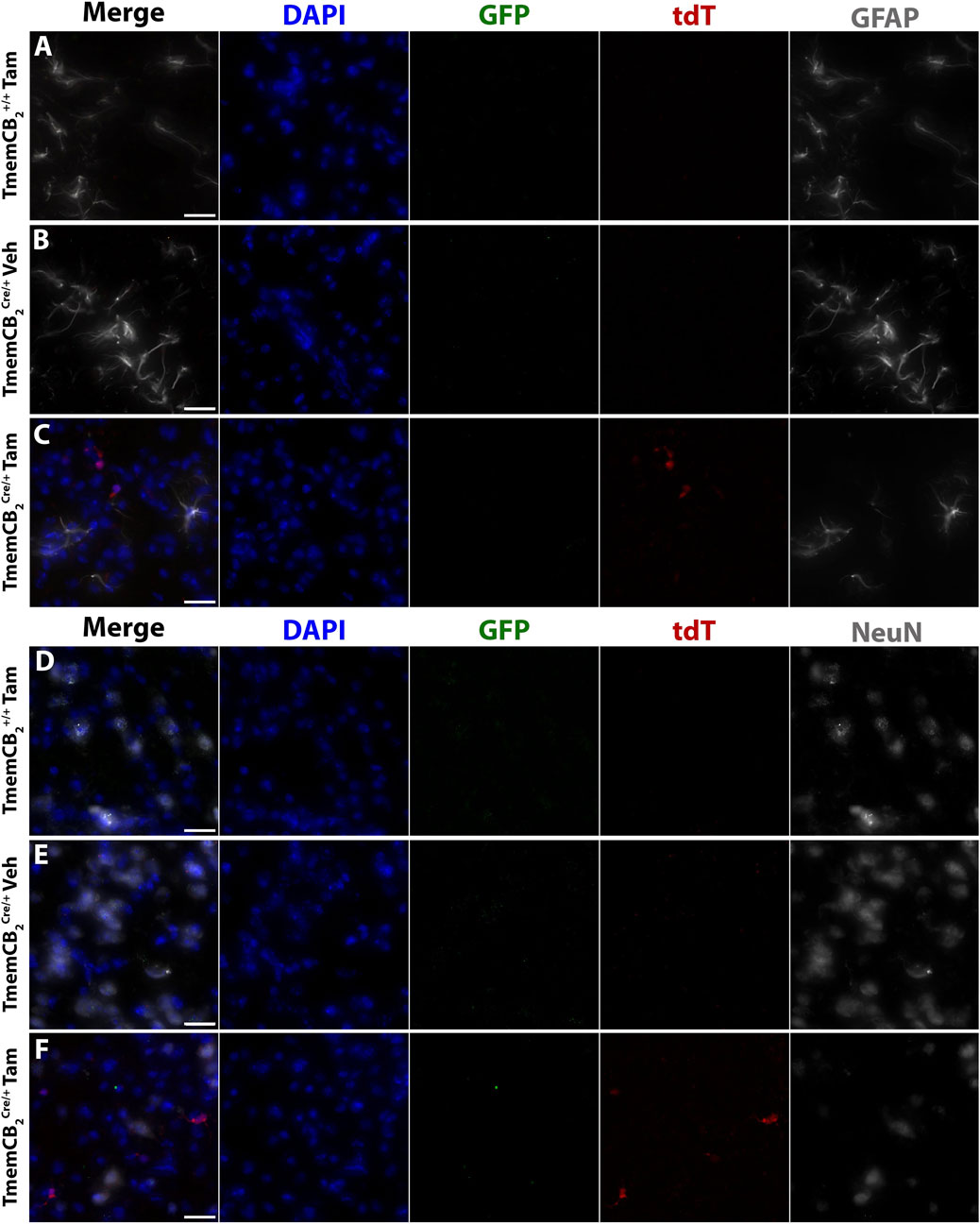
Figure 8. In TmemCB2 mice, reporter gene expression does not colocalize with astrocytes or neurons. Representative images of immunostaining in the substantia nigra. GFP and tdT with either (A–C) GFAP or (D–F) NeuN. (A,D) Tam treated TmemCB2+/+, (B,E) Vehicle treated TmemCB2Cre/+ (C,F) Tam treated TmemCB2Cre/+. Scale bar = 20 µm.
Finally, we examined reporter gene expression in the periphery of Tam- or vehicle-treated TmemCB2 mice. As expected, minimal tdT expression was observed in the spleen of Tam-treated TmemCB2+/+ mice (Figures 9A,B). However, rare tdT expression was detected in the spleen of Tam-treated TmemCB2Cre/+ mice (Figures 9C,D), though at significantly lower levels than in Cx3CB2 mice. This suggests that the TmemCB2 line exhibits greater specificity for CNS microglia.
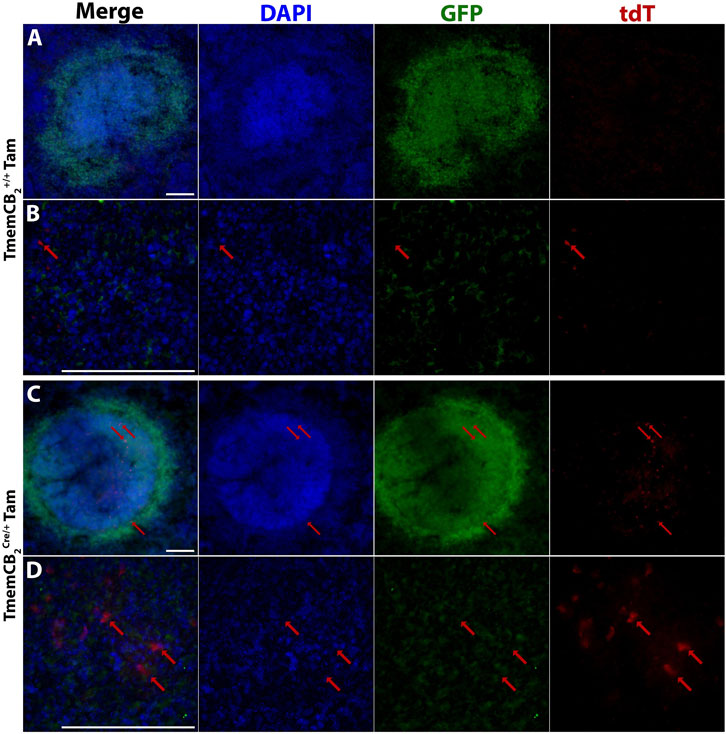
Figure 9. Reporter gene expression is present in the TmemCB2 spleen. Representative immunofluorescent images of Tam treated (A,B) TmemCB2+/+ and (C,D) TmemCB2Cre/+ spleens. Red arrows = tdT expression. Scale bar = 100 µm.
3.8 Reporter gene expression is upregulated in LPS lesioned mice
To assess whether reporter gene expression accurately reflects CB2 upregulation in an inflammatory environment, we examined Tam- and vehicle-treated TmemCB2Cre/+ mice following LPS-induced neuroinflammation. GFP and tdT expression were analysed in the SN after an ipsilateral intrastriatal injection of LPS to evaluate CB2 expression and KO persistence. CD68 is expressed by phagocytic macrophages and is considered a marker for activated microglia within the CNS (Walker and Lue, 2015). Since CB2 is upregulated in activated microglia, the colocalization of CD68 with GFP and tdT was assessed to determine the extent of CB2 expression in pro-inflammatory microglia in the striatum of this model.
In both treatment groups, GFP expression was highly upregulated in the ipsilateral striatum, indicating a robust increase in CB2 expression in response to LPS. GFP almost always colocalized with CD68+ cells, which mark activated microglia, although rare GFP+ CD68− cells were observed (Figure 10).
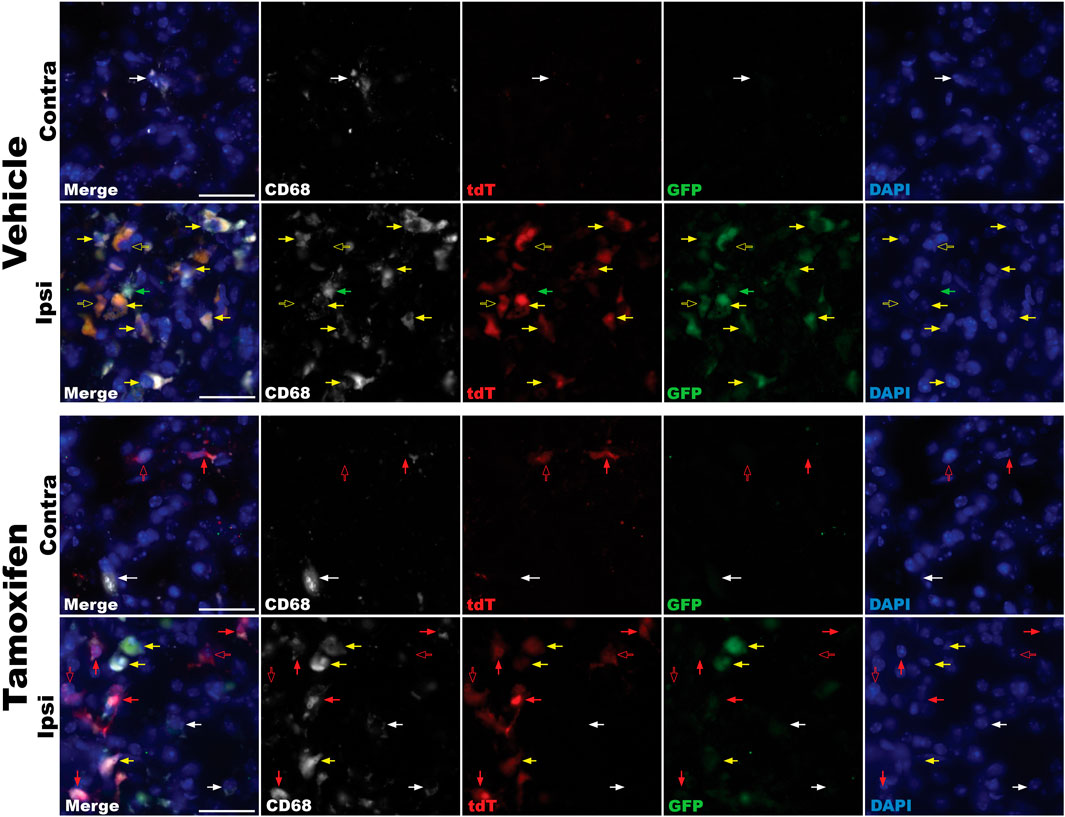
Figure 10. Immunofluorescence analysis of reporter gene expression in the striatum of LPS-lesioned TmemCB2Cre/+ mice. Representative images from vehicle- and tamoxifen-treated mice are shown for both ipsilateral and contralateral striata. Red arrows = tdT expression. Green arrows = GFP expression. Yellow arrows = GFP and tdT coexpression. Solid arrows = reporter gene colocalizing with CD68. Hollow arrows = reporter gene not colocalizing with CD68. White arrows indicate CD68 expression only. Scale bar = 25uM.
In vehicle-treated mice, tdT expression was faint and restricted to the lesion site. This tdT signal exclusively colocalized with areas of intense GFP immunoreactivity, suggesting that it arose from channel bleed-through rather than true tdT expression (Figure 10).
In Tam-treated mice, tdT expression was widespread, observed throughout the brain and most prominently at the lesion site. Bright tdT signals were found in the ipsilateral and contralateral striatum, not colocalizing with GFP, indicating true signal and KO of CB2 in these cells. In the ipsilateral striatum, tdT predominantly, but not exclusively, colocalized with CD68 (Figure 10).
These findings demonstrate that neuroinflammation induces CB2 upregulation, as indicated by increased ipsilateral reporter gene expression in both groups. Importantly, Tam-treated mice exhibited persistent and specific CB2-KO throughout the brain under inflammatory conditions.
4 Discussion
This study successfully developed and validated a novel microglia-specific, inducible CB2-KO mouse model that incorporates a dual reporter system to enable precise, simultaneous visualisation of CB2 expression and gene deletion. While floxed Cnr2 mouse lines (Q.-R. Liu et al., 2017; Stempel et al., 2016) and CB2-reporter lines (López et al., 2018; Schmöle et al., 2015) have been generated previously, this is the first model to combine an inducible CB2-KO with reporter genes. This unique configuration allows both tracking of endogenous CB2 promoter activity and permanent labelling of cells that have undergone CB2-KO, a feature not present in any previously described CB2-KO models. The CB2flx line can be crossed with any Cre driver line, providing a versatile tool to investigate CB2 function and regulation in both the CNS and the periphery. This flexibility represents a significant advancement for CB2 research.
Given the well-documented lack of reliable CB2-specific antibodies (Atwood and Mackie, 2010; Grabon et al., 2023b; Eraso-Pichot et al., 2023), traditional biochemical and histological methods for detecting CB2 expression remain limited. By placing the reporter genes under the endogenous Cnr2 promoter, our approach overcomes this critical methodological barrier: GFP fluorescence enables identification of cells actively expressing CB2, while tdT provides a permanent genetic marker of recombination. This allows the monitoring of Cnr2 promoter activity even after CB2 protein has been knocked out, providing unique insights into transcriptional regulation that are not possible with antibody-based methods. Moreover, this design enables assessment of how CB2 deletion in 1 cell type may influence CB2 expression in other cell populations, supporting more nuanced investigation of intercellular signalling dynamics. While alternative methods such as RNAscope in situ hybridisation can detect low-abundance CB2 mRNA with high sensitivity, including in neuronal populations where CB2 expression is minimal (Eraso-Pichot et al., 2023), our model offers complementary advantages for long-term in vivo tracking and functional manipulation of CB2-expressing cells without requiring tissue processing or probe optimisation for each experimental condition. However, we do note the limitations of reporter gene lines in detecting low abundance CB2 expression.
CB2 is known to play critical roles in reproduction, development, and motor control (Atwood and Mackie, 2010), so it was essential to confirm that genetic modifications made to the Cnr2 gene did not disrupt these functions before microglial KO was induced. Our findings demonstrate these physiological processes remain intact. Additionally, in vitro studies revealed no differences in the responses of genetically modified glia to a CB2 agonist, indicating that CB2 signalling remained intact prior to conditional KO.
Under basal conditions, GFP expression, marking CB2 activity, was detected in peripheral tissues but was minimal in the CNS, consistent with previous GFP-CB2 models (López et al., 2018; Ruiz De Martín Esteban et al., 2022). Following LPS-induced neuroinflammation, GFP expression increased in microglia, confirming its association with microglial activation and mirroring results from previous research, including those using CB2-GFP mice in disease models (López et al., 2018). These findings validate the responsiveness and fidelity of the Cnr2 promoter in driving reporter expression under neuroinflammatory conditions. Furthermore, Tam-induced Cre activity in the TmemCB2 and Cx3CB2 lines successfully triggered expression of tdT, indicating successful CB2-KO. Importantly, tdT expression was exclusively observed in microglia of Cre/+ mice following Tam administration, validating the inducibility and specificity of the Cre-lox system.
Under inflammatory conditions, reporter gene expression was upregulated in microglia, confirming CB2 upregulation, consistent with previous reports (Grabon et al., 2023b). While CB2 primarily co-localised with pro-inflammatory microglia, distinct populations were observed, suggesting that CB2 expression is heterogeneous among microglial subtypes. The diversity of microglia expression profiles and how this translates to differences in phenotype and function is only beginning to be understood (Paolicelli et al., 2022), so it is not surprising that not all activated microglia ubiquitously express CB2 and CD68. Another explanation is that CD68+CB2+ cells at the LPS lesion site could be non-microglial cells expressing CB2, but given that no GFP expression was observed in neurons or astrocytes, this is unlikely. Future studies incorporating additional markers of reactive microglia could provide further insight into microglial heterogeneity and CB2 expression. Additionally, investigation into reporter gene colocalization with markers for other CNS cell types under various physiological and pathological conditions may reveal context-specific upregulation of CB2 in these populations. Although there is little evidence for CB2 expression in other CNS cell types, such as oligodendrocytes, future studies may wish to validate reporter gene expression in other cell types (Bernal-Chico et al., 2023). One limitation of this model in assessing CB2-KO efficiency is that tdT, the marker of successful CB2-KO, is only expressed if the Cnr2 promoter is active. Since basal CB2 expression is very low in the CNS, most cells with CB2-KO would not be expected to express tdT. Nonetheless, our findings confirm that at least 9.3% of microglia in the TmemCB2 line and 91.7% in the Cx3CB2 line exhibited CB2-KO. Additionally, it is important to note that almost all microglia in the Cx3CB2 line and almost all reporter gene expressing microglia in the TmemCB2 line expressed tdT, indicating high KO efficiency. To address challenges posed by low basal CB2 expression, DNA-based methods such as in situ hybridisation will be essential for definitive confirmation of CB2-KO efficiency.
Expression levels of tdT in Cre+ animals receiving Tam were significantly higher than GFP expression in control groups, despite both reporters being driven by the Cnr2 promoter. This discrepancy suggests that Cnr2 promoter activity is increased following Cre-mediated recombination, complicating the accurate assessment of CB2-KO efficiency through reporter genes. This is unlikely to be an effect of Tam, as Tam treated +/+ groups did not have increased Cnr2 promoter activity, as indicated by GFP expression (Figures 6A, 7E).
A potential explanation for both the increased tdT expression compared to GFP expression in controls and the higher tdT expression in the Cx3CB2 line compared to the TmemCB2 line may be Cre toxicity. Cre recombinase, after Tam-induced translocation to the nucleus, has been shown to cause DNA damage or other toxic effects that alter cellular function (Sahasrabuddhe and Ghosh, 2022), potentially resulting in increased Cnr2 promoter activity. Notably, this effect appears to be more pronounced in the Cx3CB2 line, where tdT expression was nearly tenfold higher than in the TmemCB2 line. This aligns with previous reports suggesting that Cre toxicity is more significant in Cx3cr1-Cre mice compared to Tmem119-Cre lines, possibly due to differences in Cre expression levels (Sahasrabuddhe and Ghosh, 2022). These findings highlight the importance of considering Cre-mediated effects when interpreting data from Cre-lox models, particularly in systems where promoter activity is under investigation; however, further research is required to validate this hypothesis.
Analysis of tdT expression in peripheral tissues also highlighted limitations in the specificity of the Cre drivers used. In the Cx3CB2 line, tdT expression persisted in the spleen 3 weeks post-Tam, despite the expectation that Cre-mediated recombination would be restricted to CNS microglia due to rapid turnover of peripheral Cx3cr1+ cells after this time. This persistence suggests that a longer washout period may be necessary to achieve microglia-specific recombination in this line. In contrast, tdT expression in TmemCB2 spleens was minimal, supporting the evidence that Tmem119 provides greater specificity to microglia. However, our findings also contribute to emerging evidence that Tmem119 is expressed at low levels outside the CNS. Additionally, given that Tmem119 is downregulated in activated microglia, further investigation is needed to determine its specificity and sensitivity as a microglial marker under both homeostatic and inflammatory conditions (Vankriekelsvenne et al., 2022; Bedolla et al., 2024).
Although most studies using Cx3cr1Cre/ERT2 lines to target microglia suggest that a 3-week washout period will result in microglia specificity (Bedolla et al., 2023; Costa et al., 2021; Peng et al., 2016; Mo et al., 2019; Hohsfield et al., 2021; Y. Yang et al., 2023), evidence confirming the clearance of peripheral cells with Cre-mediated recombination at this time point is lacking. The assumption that a 3-week washout period is sufficient for peripheral clearance of KO cells is based on studies using neonate (Parkhurst et al., 2013) or adolescent (Goldmann et al., 2013) mice with low-dose Tam administration protocols. As the majority of studies employing Cx3cr1Cre/ERT2 lines use adult mice with longer and higher-dose Tam regimens, the persistence of Cre-mediated modification in peripheral macrophages under these conditions remains unclear, and more recent evidence supports our finding that a washout period is not sufficient (Bedolla et al., 2024). Therefore, if microglia-specific CB2-KO is required in the Cx3CB2 line, a time course study of peripheral tdT expression is needed to determine the minimum washout period required to achieve specificity, as we have confirmed here that 3 weeks is insufficient.
In summary, this study presents the development and validation of a unique CB2 transgenic model that integrates inducible KO with dual fluorescent reporters, offering a versatile and powerful tool to investigate CB2 function across tissues, disease states, and developmental stages. The flexibility of the CB2flx line, which can be crossed with any Cre line, allows for the study of CB2 function and expression across various cell types and tissues. This versatility makes the CB2flx line an invaluable tool for exploring CB2 in a range of contexts, from neuroinflammation to other disease models in which CB2 has been suggested to play a role. By directly addressing existing methodological limitations in CB2 research, this model enables experiments that were previously not possible, laying the groundwork for future studies aimed at understanding CB2’s contribution to disease processes and assessing the therapeutic potential of CB2 modulation.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: Genbank database, accession number PRJNA1356642.
Ethics statement
The animal study was approved by The Garvan Institute and St Vincent's Animal Ethics Committee. The study was conducted in accordance with local legislation and institutional requirements.
Author contributions
KL: Investigation, Data curation, Conceptualization, Formal Analysis, Project administration, Writing – review and editing, Writing – original draft, Methodology. PR: Conceptualization, Writing – review and editing, Supervision. SS: Conceptualization, Writing – review and editing, Supervision. BV: Writing – review and editing, Funding acquisition, Conceptualization, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by The Australian Government Research Training Program scholarship to Kathryn J. Laloli; The Helen and David Baffsky Fellowship to Sandy Stayte; The Boyarsky family; Andrew Michael and Michele Brooks; John and Debbie Schaffer; Richard Gelski; Alex Sundich and Bridge Street Capital Partners; Doug Battersby and family; David King and family; Harry Holden; Tony and Vivian Howland-Rose; The ISG Foundation; Stanley and Charmaine Roth; Richard, Adrian and Tom O'Connor; Marnie and Gary Perlstein; David Schwartz and Stephen Young.
Acknowledgements
This manuscript developed from a PhD thesis submitted to the University of New South Wales (Laloli, 2024) and has been released as a pre-print at medRxiv. BioRender™ was used in the creation of some figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. ChatGPT was used in the proofreading of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1682979/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Floxed Cnr2 construct. Modified Cnr2 gene (A) before and (B) after Cre mediated recombination. Blue boxes indicate exons. SA = Splice acceptor. pA = poly (A). IRES = internal ribosome entry site. eGFP = enhanced green flouresent protein. UTR = untranslated region.
SUPPLEMENTARY FIGURE S2 | Optimisation of cAMP assay for primary glia cultures. Forskolin concentration-response curves were generated for different cell densities to determine optimum cell density and forskolin concentration.
SUPPLEMENTARY FIGURE S3 | Sequencing of floxed Cnr2 gene.
SUPPLEMENTARY FIGURE S4 | Representative, low magnification images of Cx3CB2 substantia nigra (A) Tam treated Cx3CB2+/+, (B) Vehicle treated Cx3CB2Cre/+, (C) Tam treated Cx3CB2Cre/+. Scale bar = 100 µm.
SUPPLEMENTARY FIGURE S5 | In Cx3CB2 mice, reporter gene expression does not colocalize with astrocytes or neurons. Representative images of immunostaining in the substantia nigra of GFP and tdT with either (A–C) GFAP or (D–F) NeuN. (A,D) Tam treated Cx3CB2+/+, (B,E) Vehicle treated Cx3CB2Cre/+, (C,F) Tam treated Cx3CB2Cre/+. Scale bar = 50 µm.
SUPPLEMENTARY FIGURE S6 | Reporter gene expression is present in the Cx3CB2 spleen. Representative images of spleen immunofluorescence for (A) Cx3CB2+/+ Tam treated, 1 week-post Tam. (B) Cx3CB2Cre/+ Tam treated, 1 week-post Tam. (C) Cx3CB2Cre/+ Tam treated, 3 weeks-post Tam. Scale bar = 100 µm.
SUPPLEMENTARY FIGURE S7 | Low magnification representative images of TmemCB2 substantia nigra (A) Tam treated TmemCB2+/+, (B) Vehicle treated TmemCB2Cre/+, (C) Tam treated TmemCB2Cre/+. Arrows indicate colocalization of reporter gene with Iba1. Scale bar = 100 µm.
Abbreviations
BL6, C57BL/6JAusb; CB1, Cannabinoid receptor type 1; CB2, Cannabinoid receptor type 2; CB2flx, C57BL/6-Cnr2tm1(GFP,tdTomato)BViss/J mouse line; CNS, Central nervous system; Cx3CB2, B6.129P2(Cg)-Cx3cr1tm2.1(Cre/ERT2)Litt/WganJ x CB2flx mouse line; ECS, Endocannabinoid system; GFP, Green fluorescent protein; KO, Knockout; LPS, Lipopolysaccharide; SN, Substantia nigra; Tam, Tamoxifen; tdT, tdTomato; TmemCB2, C57BL/6-Tmem119em1(Cre/ERT2)Gfng/J x CB2flx mouse line; YFP, Yellow fluorescent protein.
References
Alenabi, A., and Malekinejad, H. (2021). Cannabinoids pharmacological effects are beyond the palliative effects: CB2 cannabinoid receptor agonist induced cytotoxicity and apoptosis in human colorectal cancer cells (HT-29). Mol. Cell Biochem. 476 (9), 3285–3301. doi:10.1007/s11010-021-04158-6
Andersen, M. S., Bandres-Ciga, S., Reynolds, R. H., Hardy, J., Ryten, M., Krohn, L., et al. (2021). Heritability enrichment implicates microglia in parkinson's disease pathogenesis. Ann. Neurol. 89 (5), 942–951. doi:10.1002/ana.26032
Angelova, D. M., and Brown, D. R. (2019). Microglia and the aging brain: are senescent microglia the key to neurodegeneration? J. Neurochem. 151 (6), 676–688. doi:10.1111/jnc.14860
Antignano, I., Liu, Y., Offermann, N., and Capasso, M. (2023). Aging microglia. Cell. Mol. Life Sci. 80 (5), 126. doi:10.1007/s00018-023-04775-y
Araki, T., Ikegaya, Y., and Koyama, R. (2021). The effects of microglia- and astrocyte-derived factors on neurogenesis in health and disease. Eur. J. Neurosci. 54 (5), 5880–5901. doi:10.1111/ejn.14969
Arvanitaki, E. S., Goulielmaki, E., Gkirtzimanaki, K., Niotis, G., Tsakani, E., Nenedaki, E., et al. (2024). Microglia-derived extracellular vesicles trigger age-related neurodegeneration upon DNA damage. Proc. Natl. Acad. Sci. U. S. A. 121 (17), e2317402121. doi:10.1073/pnas.2317402121
Aso, E., Juvés, S., Maldonado, R., and Ferrer, I. (2013). CB2 cannabinoid receptor agonist ameliorates alzheimer-like phenotype in AβPP/PS1 mice. J. Alzheimer's Dis. 35 (4), 847–858. doi:10.3233/JAD-130137
Atwood, B. K., and Mackie, K. (2010). CB2: a cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 160 (3), 467–479. doi:10.1111/j.1476-5381.2010.00729.x
Bedolla, A., McKinsey, G., Ware, K., Santander, N., Arnold, T., and Luo, Y. (2023). Finding the right tool: a comprehensive evaluation of microglial inducible cre mouse models. bioRxiv. doi:10.1101/2023.04.17.536878
Bedolla, A., Wegman, E., Weed, M., Stevens, M. K., Ware, K., Paranjpe, A., et al. (2024). Adult microglial TGFβ1 is required for microglia homeostasis via an autocrine mechanism to maintain cognitive function in mice. Nat. Commun. 15 (1), 5306. doi:10.1038/s41467-024-49596-0
Bernal-Chico, A., Tepavcevic, V., Manterola, A., Utrilla, C., Matute, C., and Mato, S. (2023). Endocannabinoid signaling in brain diseases: emerging relevance of glial cells. Glia 71 (1), 103–126. doi:10.1002/glia.24172
Bido, S., Muggeo, S., Massimino, L., Marzi, M. J., Giannelli, S. G., Melacini, E., et al. (2021). Microglia-specific overexpression of alpha-synuclein leads to severe dopaminergic neurodegeneration by phagocytic exhaustion and oxidative toxicity. Nat. Commun. 12 (1), 6237. doi:10.1038/s41467-021-26519-x
Birkle, T. J. Y., and Brown, G. C. (2023). Syk inhibitors protect against microglia-mediated neuronal loss in culture. Front. Aging Neurosci. 15, 1120952. doi:10.3389/fnagi.2023.1120952
Borst, K., Dumas, A. A., and Prinz, M. (2021). Microglia: immune and non-immune functions. Immunity 54 (10), 2194–2208. doi:10.1016/j.immuni.2021.09.014
Braun, M., Khan, Z. T., Khan, M. B., Kumar, M., Ward, A., Achyut, B. R., et al. (2018). Selective activation of cannabinoid receptor-2 reduces neuroinflammation after traumatic brain injury via alternative macrophage polarization. Brain, Behav. Immun. 68, 224–237. doi:10.1016/j.bbi.2017.10.021
Brusco, A., Tagliaferro, P. A., Saez, T., and Onaivi, E. S. (2008). Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann. N. Y. Acad. Sci. 1139 (1), 450–457. doi:10.1196/annals.1432.037
Butler, C. A., Popescu, A. S., Kitchener, E. J. A., Allendorf, D. H., Puigdellivol, M., and Brown, G. C. (2021). Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J. Neurochem. 158 (3), 621–639. doi:10.1111/jnc.15327
Chadarevian, J. P., Hasselmann, J., Lahian, A., Capocchi, J. K., Escobar, A., Lim, T. E., et al. (2024). Therapeutic potential of human microglia transplantation in a chimeric model of CSF1R-related leukoencephalopathy. Neuron 112 (16), 2686–2707 e8. doi:10.1016/j.neuron.2024.05.023
Chen, X., Firulyova, M., Manis, M., Herz, J., Smirnov, I., Aladyeva, E., et al. (2023). Microglia-mediated T cell infiltration drives neurodegeneration in tauopathy. Nature 615 (7953), 668–677. doi:10.1038/s41586-023-05788-0
Chen, S. X., Li, Z., Yang, L., Xu, Z. J., Liu, A. P., He, Q. W., et al. (2025). Cannabinoid Receptor-2 alleviates sepsis-induced neuroinflammation by modulating microglia M1/M2 subset polarization through inhibiting Nogo-B expression. Mol. Neurobiol. 62, 9258–9270. doi:10.1007/s12035-025-04836-2
Chung, Y. C., Shin, W.-Ho, Baek, J. Y., Cho, E. J., Baik, H. H., Kim, S. R., et al. (2016). CB2 receptor activation prevents glial-derived neurotoxic mediator production, BBB leakage and peripheral immune cell infiltration and rescues dopamine neurons in the MPTP model of Parkinson’s disease. Exp. and Mol. Med. 48 (1), e205. doi:10.1038/emm.2015.100
Consortium, International Multiple Sclerosis Genetics (2019). Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science 365 (6460), eaav7188. doi:10.1126/science.aav7188
Corley, E., Holleran, L., Fahey, L., Corvin, A., Morris, D. W., and Donohoe, G. (2021). Microglial-expressed genetic risk variants, cognitive function and brain volume in patients with schizophrenia and healthy controls. Transl. Psychiatry 11 (1), 490. doi:10.1038/s41398-021-01616-z
Costa, A., Haage, V., Yang, S., Wegner, S., Ersoy, B., Ugursu, B., et al. (2021). Deletion of muscarinic acetylcholine receptor 3 in microglia impacts brain ischemic injury. Brain Behav. Immun. 91, 89–104. doi:10.1016/j.bbi.2020.09.008
Cui, F., Xu, Z., Lv, Y., and Hu, J. (2021). Role of spindle pole body component 25 in neurodegeneration. Ann. Transl. Med. 9 (18), 1432. doi:10.21037/atm-21-4064
Daria, A., Colombo, A., Llovera, G., Hampel, H., Willem, M., Liesz, A., et al. (2017). Young microglia restore amyloid plaque clearance of aged microglia. EMBO J. 36 (5), 583–603. doi:10.15252/embj.201694591
Della Valle, I., Milani, M., Rossi, S., Turchi, R., Tortolici, F., Nesci, V., et al. (2024). Loss of homeostatic functions in microglia from a murine model of friedreich's ataxia. Genes Dis. 11 (6), 101178. doi:10.1016/j.gendis.2023.101178
Deng, I., Corrigan, F., Zhai, G., Zhou, X. F., and Bobrovskaya, L. (2020). Lipopolysaccharide animal models of parkinson's disease: recent progress and relevance to clinical disease. Brain Behav. Immun. Health 4, 100060. doi:10.1016/j.bbih.2020.100060
Di Marzo, V. (2018). New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 17 (9), 623–639. doi:10.1038/nrd.2018.115
Dick, S. A., Macklin, J. A., Nejat, S., Momen, A., Clemente-Casares, X., Althagafi, M. G., et al. (2019). Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 20 (1), 29–39. doi:10.1038/s41590-018-0272-2
Ding, X., Wang, J., Huang, M., Chen, Z., Liu, J., Zhang, Q., et al. (2021). Loss of microglial SIRPα promotes synaptic pruning in preclinical models of neurodegeneration. Nat. Commun. 12 (1), 2030. doi:10.1038/s41467-021-22301-1
Dong, Y., D'Mello, C., Pinsky, W., Lozinski, B. M., Kaushik, D. K., Ghorbani, S., et al. (2021). Oxidized phosphatidylcholines found in multiple sclerosis lesions mediate neurodegeneration and are neutralized by microglia. Nat. Neurosci. 24 (4), 489–503. doi:10.1038/s41593-021-00801-z
Duffy, S. S., Hayes, J. P., Fiore, N. T., and Moalem-Taylor, G. (2021). The cannabinoid system and microglia in health and disease. Neuropharmacology 190, 108555. doi:10.1016/j.neuropharm.2021.108555
Eraso-Pichot, A., Pouvreau, S., Olivera-Pinto, A., Gomez-Sotres, P., Skupio, U., and Marsicano, G. (2023). Endocannabinoid signaling in astrocytes. Glia 71 (1), 44–59. doi:10.1002/glia.24246
Espadas, I., Keifman, E., Palomo-Garo, C., Burgaz, S., García, C., Fernández-Ruiz, J., et al. (2020). Beneficial effects of the phytocannabinoid Δ9-THCV in L-DOPA-induced dyskinesia in parkinson's disease. Neurobiol. Dis. 141, 104892. doi:10.1016/j.nbd.2020.104892
Espejo-Porras, F., Toscano, L. G., Cueto, C. R., Santos-García, I., Lago, E.De, and Ruiz, J. F. (2019). Targeting glial cannabinoid CB2 receptors to delay the progression of the pathological phenotype in TDP-43 (A315T) transgenic mice, a model of amyotrophic lateral sclerosis. Br. J. Pharmacol. 176 (10), 1585–1600. doi:10.1111/bph.14216
Fakhfouri, G., Ahmadiani, A., Rahimian, R., Grolla, A. A., Moradi, F., and Ali, H. (2012). WIN55212-2 attenuates amyloid-beta-induced neuroinflammation in rats through activation of cannabinoid receptors and PPAR-γ pathway. Neuropharmacology 63 (4), 653–666. doi:10.1016/j.neuropharm.2012.05.013
Ferranti, A. S., and Foster, D. J. (2022). Cannabinoid type-2 receptors: an emerging target for regulating schizophrenia-relevant brain circuits. Front. Neurosci. 16, 925792. doi:10.3389/fnins.2022.925792
Fricker, M., Oliva-Martin, M. J., and Brown, G. C. (2012). Primary phagocytosis of viable neurons by microglia activated with LPS or abeta is dependent on calreticulin/LRP phagocytic signalling. J. Neuroinflammation 9, 196. doi:10.1186/1742-2094-9-196
Galiègue, S., Mary, S., Marchand, J., Dussossoy, D., Carrière, D., Carayon, P., et al. (1995). Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 232 (1), 54–61. doi:10.1111/j.1432-1033.1995.tb20780.x
Gambacorta, N., Gasperi, V., Guzzo, T., Di Leva, F. S., Ciriaco, F., Sanchez, C., et al. (2023). Exploring the 1,3-benzoxazine chemotype for cannabinoid receptor 2 as a promising anti-cancer therapeutic. Eur. J. Med. Chem. 259, 115647. doi:10.1016/j.ejmech.2023.115647
Gao, C., Jiang, J., Tan, Y., and Chen, S. (2023). Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Signal Transduct. Target Ther. 8 (1), 359. doi:10.1038/s41392-023-01588-0
Gasperi, V., Guzzo, T., Topai, A., Gambacorta, N., Ciriaco, F., Nicolotti, O., et al. (2023). Recent advances on Type-2 cannabinoid (CB2) receptor agonists and their therapeutic potential. Curr. Med. Chem. 30 (12), 1420–1457. doi:10.2174/0929867329666220825161603
Goldmann, T., Wieghofer, P., Müller, P. F., Wolf, Y., Varol, D., Yona, S., et al. (2013). A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16 (11), 1618–1626. doi:10.1038/nn.3531
Gómez-Gálvez, Y., Palomo-Garo, C., Fernández-Ruiz, J., and García, C. (2016). Potential of the cannabinoid CB2 receptor as a pharmacological target against inflammation in parkinson's disease. Prog. Neuro-Psychopharmacology Biol. Psychiatry 64, 200–208. doi:10.1016/j.pnpbp.2015.03.017
Grabon, W., Bodennec, J., Rheims, S., Belmeguenai, A., and Bezin, L. (2023a). Update on the controversial identity of cells expressing cnr2 gene in the nervous system. CNS Neurosci. and Ther. 29 (3), 760–770. doi:10.1111/cns.13977
Grabon, W., Rheims, S., Smith, J., Bodennec, J., Belmeguenai, A., and Bezin, L. (2023b). CB2 receptor in the CNS: from immune and neuronal modulation to behavior. Neurosci. and Biobehav. Rev. 150, 105226. doi:10.1016/j.neubiorev.2023.105226
Gratuze, M., Schlachetzki, J. C. M., D'Oliveira Albanus, R., Jain, N., Novotny, B., Brase, L., et al. (2023). TREM2-independent microgliosis promotes tau-mediated neurodegeneration in the presence of ApoE4. Neuron 111 (2), 202–219 e7. doi:10.1016/j.neuron.2022.10.022
Green, G. S., Fujita, M., Yang, H. S., Taga, M., Cain, A., McCabe, C., et al. (2024). Cellular communities reveal trajectories of brain ageing and alzheimer's disease. Nature 633 (8030), 634–645. doi:10.1038/s41586-024-07871-6
Guo, S., Wang, H., and Yin, Y. (2022). Microglia polarization from M1 to M2 in neurodegenerative diseases. Front. Aging Neurosci. 14, 815347. doi:10.3389/fnagi.2022.815347
Hohsfield, L. A., Najafi, A. R., Ghorbanian, Y., Soni, N., Crapser, J., Figueroa Velez, D. X., et al. (2021). Subventricular zone/white matter microglia reconstitute the empty adult microglial niche in a dynamic wave. Elife 10, e66738. doi:10.7554/eLife.66738
Hosoki, H., Asahi, T., and Nozaki, C. (2024). Cannabinoid CB2 receptors enhance high-fat diet evoked peripheral neuroinflammation. Life Sci. 355, 123002. doi:10.1016/j.lfs.2024.123002
Jang, J., Hong, A., Chung, Y., and Jin, B. (2022). Interleukin-4 aggravates LPS-induced striatal neurodegeneration in vivo via oxidative stress and polarization of microglia/macrophages. Int. J. Mol. Sci. 23 (1), 571. doi:10.3390/ijms23010571
Jayant, S., Mohan Sharma, B., Bansal, R., and Sharma, B. (2016). Pharmacological benefits of selective modulation of cannabinoid receptor type 2 (CB2) in experimental alzheimer's disease. Pharmacol. Biochem. Behav. 140, 39–50. doi:10.1016/j.pbb.2015.11.006
Jia, Yi, Han, D., Qin, Q., and Ma, Z. G. (2020). JWH133 inhibits MPP+-Induced inflammatory response and iron influx in astrocytes. Neurosci. Lett. 720, 134779. doi:10.1016/j.neulet.2020.134779
Jing, L., Hou, L., Zhang, D., Li, S., Ruan, Z., Zhang, X., et al. (2021). Microglial activation mediates noradrenergic locus coeruleus neurodegeneration via complement receptor 3 in a rotenone-induced parkinson's disease mouse model. J. Inflamm. Res. 14, 1341–1356. doi:10.2147/JIR.S299927
Joers, V., Murray, B. C., McLaughlin, C., Oliver, D., Staley, H. E., Coronado, J., et al. (2024). Modulation of cannabinoid receptor 2 alters neuroinflammation and reduces formation of alpha-synuclein aggregates in a rat model of nigral synucleinopathy. J. Neuroinflammation 21 (1), 240. doi:10.1186/s12974-024-03221-5
Joshi, N., and Onaivi, E. S. (2019). “Endocannabinoid system components: overview and tissue distribution,” in Recent advances in cannabinoid physiology and pathology. Editor A. N. Bukiya (Cham: Springer International Publishing), 1–12.
Joshi, A. U., Minhas, P. S., Liddelow, S. A., Haileselassie, B., Andreasson, K. I., Dorn, G. W., et al. (2019). Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat. Neurosci. 22 (10), 1635–1648. doi:10.1038/s41593-019-0486-0
Kaiser, T., and Feng, G. (2019). Tmem119-EGFP and Tmem119-CreERT2 transgenic mice for labeling and manipulating microglia. eNeuro 6 (4), ENEURO.0448–18.2019. doi:10.1523/ENEURO.0448-18.2019
Kaminski, N. E. (1998). Inhibition of the cAMP signaling Cascade via cannabinoid receptors: a putative mechanism of immune modulation by cannabinoid compounds. Toxicol. Lett. 102-103, 59–63. doi:10.1016/s0378-4274(98)00284-7
Kang, Y. J., Hyeon, S. J., McQuade, A., Lim, J., Baek, S. H., Diep, Y. N., et al. (2024). Neurotoxic microglial activation via IFNγ-Induced Nrf2 reduction exacerbating alzheimer's disease. Adv. Sci. (Weinh) 11 (20), e2304357. doi:10.1002/advs.202304357
Kitchener, E. J. A., Dundee, J. M., and Brown, G. C. (2023). Activated microglia release beta-galactosidase that promotes inflammatory neurodegeneration. Front. Aging Neurosci. 15, 1327756. doi:10.3389/fnagi.2023.1327756
Kong, W., Li, H., Tuma, R. F., and Ganea, D. (2014). Selective CB2 receptor activation ameliorates EAE by reducing Th17 differentiation and immune cell accumulation in the CNS. Cell. Immunol. 287 (1), 1–17. doi:10.1016/j.cellimm.2013.11.002
Kong, W., Xie, Z., Shang, X., Hayashi, Y., Lan, F., Zhao, S., et al. (2023). Zinc finger protein 335 mediates lipopolysaccharide-induced neurodegeneration and memory loss as a transcriptional factor in microglia. Glia 71 (12), 2720–2734. doi:10.1002/glia.24447
Kwon, H. S., and Koh, S. H. (2020). Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl. Neurodegener. 9 (1), 42. doi:10.1186/s40035-020-00221-2
Lee, J., Kim, D. E., Griffin, P., Sheehan, P. W., Kim, D. H., Musiek, E. S., et al. (2020). Inhibition of REV-ERBs stimulates microglial amyloid-beta clearance and reduces amyloid plaque deposition in the 5XFAD mouse model of alzheimer's disease. Aging Cell 19 (2), e13078. doi:10.1111/acel.13078
Lewis, S. M., Williams, A., and Eisenbarth, S. C. (2019). Structure and function of the immune system in the spleen. Sci. Immunol. 4 (33), eaau6085. doi:10.1126/sciimmunol.aau6085
Li, C., Shi, J., Wang, Bo, Jin, Li, and Jia, H. (2019). CB2 cannabinoid receptor agonist ameliorates novel object recognition but not spatial memory in transgenic APP/PS1 mice. Neurosci. Lett. 707, 134286. doi:10.1016/j.neulet.2019.134286
Li, Q., Shen, C., Liu, Z., Ma, Y., Wang, J., Dong, H., et al. (2021). Partial depletion and repopulation of microglia have different effects in the acute MPTP mouse model of Parkinson’s disease. Cell Prolif. 54 (8), e13094. doi:10.1111/cpr.13094
Liang, S. Q., Li, P. H., Hu, Y. Y., Zhao, J. L., Shao, F. Z., Kuang, F., et al. (2023). Myeloid-specific blockade of notch signaling alleviates dopaminergic neurodegeneration in parkinson's disease by dominantly regulating resident microglia activation through NF-κB signaling. Front. Immunol. 14, 1193081. doi:10.3389/fimmu.2023.1193081
Liddelow, S. A., Guttenplan, K. A., Clarke, L. E., Bennett, F. C., Bohlen, C. J., Schirmer, L., et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541 (7638), 481–487. doi:10.1038/nature21029
Lindhout, I. A., Murray, T. E., Richards, C. M., and Klegeris, A. (2021). Potential neurotoxic activity of diverse molecules released by microglia. Neurochem. Int. 148. doi:10.1016/j.neuint.2021.105117
Liu, Q.-R., Canseco-Alba, A., Zhang, H.-Y., Tagliaferro, P., Chung, M., Dennis, E., et al. (2017). Cannabinoid type 2 receptors in dopamine neurons inhibits psychomotor behaviors, alters anxiety, depression and alcohol preference. Sci. Rep. 7 (1), 17410. doi:10.1038/s41598-017-17796-y
Liu, X., Chen, Y., Wang, H., Wei, Y., Yuan, Y., Zhou, Q., et al. (2021). Microglia-derived IL-1β promoted neuronal apoptosis through ER stress-mediated signaling pathway PERK/eIF2α/ATF4/CHOP upon arsenic exposure. J. Hazard Mater 417, 125997. doi:10.1016/j.jhazmat.2021.125997
Liu, X., Yu, H., Chen, B., Friedman, V., Mu, L., Kelly, T. J., et al. (2022). CB2 agonist GW842166x protected against 6-OHDA-Induced Anxiogenic- and depressive-related behaviors in mice. Biomedicines 10 (8), 1776. doi:10.3390/biomedicines10081776
López, A., Aparicio, N., Ruth Pazos, M., Teresa Grande, M., Asunción Barreda-Manso, M., Benito-Cuesta, I., et al. (2018). Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease. J. Neuroinflammation 15 (1), 158. doi:10.1186/s12974-018-1174-9
Lowe, H., Toyang, N., Steele, B., Bryant, J., and Ngwa, W. (2021). The endocannabinoid system: a potential target for the treatment of various diseases. Int. J. Mol. Sci. 22 (17), 9472. doi:10.3390/ijms22179472
Lunde, A., and Glover, J. C. (2020). A versatile toolbox for semi-automatic cell-by-cell object-based colocalization analysis. Sci. Rep. 10 (1), 19027. doi:10.1038/s41598-020-75835-7
Mader, M. M., Napole, A., Wu, D., Atkins, M., Scavetti, A., Shibuya, Y., et al. (2024). Myeloid cell replacement is neuroprotective in chronic experimental autoimmune encephalomyelitis. Nat. Neurosci. 27 (5), 901–912. doi:10.1038/s41593-024-01609-3
Mizutani, M., Pino, P. A., Saederup, N., Charo, I. F., Ransohoff, R. M., and Cardona, A. E. (2012). The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J. Immunol. 188 (1), 29–36. doi:10.4049/jimmunol.1100421
Mo, M., Eyo, U. B., Xie, M., Peng, J., Bosco, D. B., Umpierre, A. D., et al. (2019). Microglial P2Y12 receptor regulates seizure-induced neurogenesis and immature neuronal projections. J. Neurosci. 39 (47), 9453–9464. doi:10.1523/jneurosci.0487-19.2019
Monory, K., and Lutz, B. (2005). Pain killer without a high. Nat. Med. 11 (4), 378–379. doi:10.1038/nm0405-378
More, S. A., Deore, R. S., Pawar, H. D., Sharma, C., Nakhate, K. T., Rathod, S. S., et al. (2024). CB2 cannabinoid receptor as a potential target in myocardial infarction: exploration of molecular pathogenesis and therapeutic strategies. Int. J. Mol. Sci. 25 (3), 1683. doi:10.3390/ijms25031683
Moreno, M., Brera, B., Spuch, C., Carro, E., García-García, L., Delgado, M., et al. (2012). Prolonged oral cannabinoid administration prevents neuroinflammation, lowers β-amyloid levels and improves cognitive performance in Tg APP 2576 mice. J. Neuroinflammation 9 (1), 8. doi:10.1186/1742-2094-9-8
Munro, D. A. D., Bestard-Cuche, N., McQuaid, C., Chagnot, A., Shabestari, S. K., Chadarevian, J. P., et al. (2024). Microglia protect against age-associated brain pathologies. Neuron 112 (16), 2732–2748 e8. doi:10.1016/j.neuron.2024.05.018
Nan, J., Liu, J., Lin, G., Zhang, S., Xia, A., Zhou, P., et al. (2023). Discovery of 4-(1,2,4-Oxadiazol-5-yl)azepan-2-one derivatives as a new class of cannabinoid type 2 receptor agonists for the treatment of inflammatory pain. J. Med. Chem. 66 (5), 3460–3483. doi:10.1021/acs.jmedchem.2c01943
Odfalk, K. F., Bieniek, K. F., and Hopp, S. C. (2022). Microglia: friend and foe in tauopathy. Prog. Neurobiol. 216, 102306. doi:10.1016/j.pneurobio.2022.102306
Ojha, S., Javed, H., Azimullah, S., and Haque, M. E. (2016). β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of parkinson disease. Mol. Cell Biochem. 418 (1-2), 59–70. doi:10.1007/s11010-016-2733-y
Onaivi, E. S. (2006). Neuropsychobiological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology 54 (4), 231–246. doi:10.1159/000100778
Oyarce, K., Cepeda, M. Y., Lagos, R., Garrido, C., Vega-Letter, A. M., Garcia-Robles, M., et al. (2022). Neuroprotective and neurotoxic effects of glial-derived exosomes. Front. Cell Neurosci. 16, 920686. doi:10.3389/fncel.2022.920686
Palpagama, T. H., Waldvogel, H. J., Faull, R. L. M., and Kwakowsky, A. (2019). The role of microglia and astrocytes in huntington’s disease. Front. Mol. Neurosci. 12, 258. doi:10.3389/fnmol.2019.00258
Pan, L., Cho, K. S., Wei, X., Xu, F., Lennikov, A., Hu, G., et al. (2023). IGFBPL1 is a master driver of microglia homeostasis and resolution of neuroinflammation in glaucoma and brain tauopathy. Cell Rep. 42 (8), 112889. doi:10.1016/j.celrep.2023.112889
Paolicelli, R. C., Sierra, A., Stevens, B., Tremblay, M.-E., Aguzzi, A., Ajami, B., et al. (2022). Microglia states and nomenclature: a field at its crossroads. Neuron 110 (21), 3458–3483. doi:10.1016/j.neuron.2022.10.020
Parkhurst, C. N., Yang, G., Ninan, I., Savas, J. N., Yates, J. R., Lafaille, J. J., et al. (2013). Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155 (7), 1596–1609. doi:10.1016/j.cell.2013.11.030
Peng, J., Gu, N., Zhou, L., Eyo U, B., Murugan, M., Gan, W. B., et al. (2016). Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat. Commun. 7, 12029. doi:10.1038/ncomms12029
Penney, J., Ralvenius, W. T., Loon, A., Cerit, O., Dileep, V., Milo, B., et al. (2024). iPSC-derived microglia carrying the TREM2 R47H/+ mutation are proinflammatory and promote synapse loss. Glia 72 (2), 452–469. doi:10.1002/glia.24485
Piano, I., Votta, A., Colucci, P., Corsi, F., Vitolo, S., Cerri, C., et al. (2023). Anti-inflammatory reprogramming of microglia cells by metabolic modulators to counteract neurodegeneration; a new role for ranolazine. Sci. Rep. 13 (1), 20138. doi:10.1038/s41598-023-47540-8
Prinz, M., Jung, S., and Priller, J. (2019). Microglia biology: one century of evolving concepts. Cell 179 (2), 292–311. doi:10.1016/j.cell.2019.08.053
Rakotoarivelo, V., Mayer, T. Z., Simard, M., Flamand, N., and Di Marzo, V. (2024). The impact of the CB2 cannabinoid receptor in inflammatory diseases: an update. Molecules 29 (14), 3381. doi:10.3390/molecules29143381
Rentsch, P., Stayte, S., Egan, T., Clark, I., and Vissel, B. (2020). Targeting the cannabinoid receptor CB2 in a mouse model of l-dopa induced dyskinesia. Neurobiol. Dis. 134. doi:10.1016/j.nbd.2019.104646
Rizzi, C., Tiberi, A., Giustizieri, M., Marrone, M. C., Gobbo, F., Carucci, N. M., et al. (2018). NGF steers microglia toward a neuroprotective phenotype. Glia 66 (7), 1395–1416. doi:10.1002/glia.23312
Robinson, R. H., Meissler, J. J., Fan, X., Yu, D., Adler, M. W., and Eisenstein, T. K. (2015). A CB2-Selective cannabinoid suppresses T-Cell activities and increases tregs and IL-10. J. Neuroimmune Pharmacol. 10 (2), 318–332. doi:10.1007/s11481-015-9611-3
Rocha, S. M., Kirkley, K. S., Chatterjee, D., Aboellail, T. A., Smeyne, R. J., and Tjalkens, R. B. (2023). Microglia-specific knock-out of NF-κB/IKK2 increases the accumulation of misfolded α-synuclein through the inhibition of p62/sequestosome-1-dependent autophagy in the rotenone model of parkinson's disease. Glia 71 (9), 2154–2179. doi:10.1002/glia.24385
Rodero, M., Marie, Y., Coudert, M., Blondet, E., Mokhtari, K., Rousseau, A., et al. (2008). Polymorphism in the microglial cell-mobilizing CX3CR1 gene is associated with survival in patients with glioblastoma. J. Clin. Oncol. 26 (36), 5957–5964. doi:10.1200/JCO.2008.17.2833
Rodriguez-Gomez, J. A., Kavanagh, E., Engskog-Vlachos, P., Engskog, M. K. R., Herrera, A. J., Espinosa-Oliva, A. M., et al. (2020). Microglia: agents of the CNS pro-inflammatory response. Cells 9 (7), 1717. doi:10.3390/cells9071717
Rohbeck, E., Eckel, J., and Romacho, T. (2021). Cannabinoid receptors in metabolic regulation and diabetes. Physiol. (Bethesda) 36 (2), 102–113. doi:10.1152/physiol.00029.2020
Rorato, R., Ferreira, N. L., Oliveira, F. P., Fideles, H. J., Camilo, T. A., Antunes-Rodrigues, J., et al. (2022). Prolonged activation of brain CB2 signaling modulates hypothalamic microgliosis and astrogliosis in high fat diet-fed mice. Int. J. Mol. Sci. 23 (10), 5527. doi:10.3390/ijms23105527
Rossi, F., Bellini, G., Luongo, L., Manzo, I., Tolone, S., Tortora, C., et al. (2016). Cannabinoid receptor 2 as antiobesity target: inflammation, fat storage, and browning modulation. J. Clin. Endocrinol. Metab. 101 (9), 3469–3478. doi:10.1210/jc.2015-4381
Ruiz De Martín Esteban, S., Benito-Cuesta, I., Terradillos, I., Martínez-Relimpio, A. M., Andrea Arnanz, M., Ruiz-Pérez, G., et al. (2022). Cannabinoid CB2 receptors modulate microglia function and amyloid dynamics in a mouse model of alzheimer’s disease. Front. Pharmacol. 13, 841766. doi:10.3389/fphar.2022.841766
Ryan, S. K., Zelic, M., Han, Y., Teeple, E., Chen, L., Sadeghi, M., et al. (2023). Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration. Nat. Neurosci. 26 (1), 12–26. doi:10.1038/s41593-022-01221-3
Sagredo, O., González, S., Aroyo, I., Ruth Pazos, M., Benito, C., Becker, I. L., et al. (2009). Cannabinoid CB2 receptor agonists protect the striatum against malonate toxicity: relevance for huntington's disease. Glia 57 (11), 1154–1167. doi:10.1002/glia.20838
Sahasrabuddhe, V., and Ghosh, H. S. (2022). Cx3Cr1-Cre induction leads to microglial activation and IFN-1 signaling caused by DNA damage in early postnatal brain. Cell Rep. 38 (3), 110252. doi:10.1016/j.celrep.2021.110252
Salvadores, N., Moreno-Gonzalez, I., Gamez, N., Quiroz, G., Vegas-Gomez, L., Escandon, M., et al. (2022). Aβ oligomers trigger necroptosis-mediated neurodegeneration via microglia activation in alzheimer's disease. Acta Neuropathol. Commun. 10 (1), 31. doi:10.1186/s40478-022-01332-9
Schildge, S., Bohrer, C., Beck, K., and Schachtrup, C. (2013). Isolation and culture of mouse cortical astrocytes. J. Vis. Exp. 71, 50079. doi:10.3791/50079
Schmöle, A.-C., Lundt, R., Ternes, S., Albayram, Ö., Ulas, T., Schultze, J. L., et al. (2015). Cannabinoid receptor 2 deficiency results in reduced neuroinflammation in an alzheimer's disease mouse model. Neurobiol. Aging 36 (2), 710–719. doi:10.1016/j.neurobiolaging.2014.09.019
Shi, Y., Manis, M., Long, J., Wang, K., Sullivan, P. M., Remolina Serrano, J., et al. (2019). Microglia drive APOE-Dependent neurodegeneration in a tauopathy mouse model. J. Exp. Med. 216 (11), 2546–2561. doi:10.1084/jem.20190980
Shibuya, Y., Kumar, K. K., Mader, M. M., Yoo, Y., Ayala, L. A., Zhou, M., et al. (2022). Treatment of a genetic brain disease by CNS-Wide microglia replacement. Sci. Transl. Med. 14 (636), eabl9945. doi:10.1126/scitranslmed.abl9945
Silver, R. J. (2019). The endocannabinoid system of animals. Animals 9 (9), 686. doi:10.3390/ani9090686
Simard, M., Rakotoarivelo, V., Di Marzo, V., and Flamand, N. (2022). Expression and functions of the CB2 receptor in human leukocytes. Front. Pharmacol. 13, 826400. doi:10.3389/fphar.2022.826400
Skrzypczak-Wiercioch, A., and Salat, K. (2022). Lipopolysaccharide-induced model of neuroinflammation: mechanisms of action, research application and future directions for its use. Molecules 27 (17), 5481. doi:10.3390/molecules27175481
Smoum, R., Grether, U., Karsak, M., Vernall, A. J., Park, F., Hillard, C. J., et al. (2022). Editorial: therapeutic potential of the cannabinoid CB2 receptor. Front. Pharmacol. 13, 1039564. doi:10.3389/fphar.2022.1039564
Sobue, A., Komine, O., Hara, Y., Endo, F., Mizoguchi, H., Watanabe, S., et al. (2021). Microglial gene signature reveals loss of homeostatic microglia associated with neurodegeneration of alzheimer's disease. Acta Neuropathol. Commun. 9 (1), 1. doi:10.1186/s40478-020-01099-x
Sobue, A., Komine, O., Endo, F., Kakimi, C., Miyoshi, Y., Kawade, N., et al. (2024). Microglial cannabinoid receptor type II stimulation improves cognitive impairment and neuroinflammation in alzheimer's disease mice by controlling astrocyte activation. Cell Death Dis. 15 (11), 858. doi:10.1038/s41419-024-07249-6
Stempel, A. V., Vanessa, A., Stumpf, A., Zhang, H.-Y., Özdoğan, T., Pannasch, U., et al. (2016). Cannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampus. Neuron 90 (4), 795–809. doi:10.1016/j.neuron.2016.03.034
Subbarayan, M. S., Joly-Amado, A., Bickford, P. C., and Nash, K. R. (2022). CX3CL1/CX3CR1 signaling targets for the treatment of neurodegenerative diseases. Pharmacol. Ther. 231, 107989. doi:10.1016/j.pharmthera.2021.107989
Takatori, S., Wang, W., Iguchi, A., and Tomita, T. (2019). Genetic risk factors for alzheimer disease: emerging roles of microglia in disease pathomechanisms. Adv. Exp. Med. Biol. 1118, 83–116. doi:10.1007/978-3-030-05542-4_5
Tang, J., Chen, Q., Guo, J., Yang, L., Tao, Y., Lin, Li, et al. (2016). Minocycline attenuates neonatal germinal-matrix-hemorrhage-induced neuroinflammation and brain edema by activating cannabinoid receptor 2. Mol. Neurobiol. 53 (3), 1935–1948. doi:10.1007/s12035-015-9154-x
Tao, L., Liu, Q., Zhang, F., Fu, Y., Zhu, X., Weng, X., et al. (2021). Microglia modulation with 1070-nm light attenuates Aβ burden and cognitive impairment in alzheimer's disease mouse model. Light Sci. Appl. 10 (1), 179. doi:10.1038/s41377-021-00617-3
Umpierre, A. D., and Wu, L. J. (2021). How microglia sense and regulate neuronal activity. Glia 69 (7), 1637–1653. doi:10.1002/glia.23961
van den Hoogen, N. J., Harding, E. K., Davidson, C. E. D., and Trang, T. (2021). Cannabinoids in chronic pain: therapeutic potential through microglia modulation. Front. Neural Circuits 15, 816747. doi:10.3389/fncir.2021.816747
Van, E., Straub, V. M., and Stelt, M. V. D. (2021). Targeting endocannabinoid signaling: FAAH and MAG lipase inhibitors. Annu. Rev. Pharmacol. Toxicol. 61 (1), 441–463. doi:10.1146/annurev-pharmtox-030220-112741
Vankriekelsvenne, E., Chrzanowski, U., Manzhula, K., Greiner, T., Wree, A., Hawlitschka, A., et al. (2022). Transmembrane protein 119 is neither a specific nor a reliable marker for microglia. Glia 70 (6), 1170–1190. doi:10.1002/glia.24164
Verty, A. N., Stefanidis, A., McAinch, A. J., Hryciw, D. H., and Oldfield, B. (2015). Anti-obesity effect of the CB2 receptor agonist JWH-015 in diet-induced Obese mice. PLoS One 10 (11), e0140592. doi:10.1371/journal.pone.0140592
Viveros-Paredes, J., González-Castañeda, R., Gertsch, J., Chaparro-Huerta, V., López-Roa, R., Vázquez-Valls, E., et al. (2017). Neuroprotective effects of β-Caryophyllene against dopaminergic neuron injury in a murine model of parkinson’s disease induced by MPTP. Pharmaceuticals 10 (3), 60. doi:10.3390/ph10030060
Walker, D. G., and Lue, L. F. (2015). Immune phenotypes of microglia in human neurodegenerative disease: challenges to detecting microglial polarization in human brains. Alzheimers Res. Ther. 7 (1), 56. doi:10.1186/s13195-015-0139-9
Wang, C., Fan, L., Khawaja, R. R., Liu, B., Zhan, L., Kodama, L., et al. (2022). Microglial NF-κB drives tau spreading and toxicity in a mouse model of tauopathy. Nat. Commun. 13 (1), 1969. doi:10.1038/s41467-022-29552-6
Wang, P. L., Yim, A. K. Y., Kim, K. W., Avey, D., Czepielewski, R. S., Colonna, M., et al. (2020). Peripheral nerve resident macrophages share tissue-specific programming and features of activated microglia. Nat. Commun. 11 (1), 2552. doi:10.1038/s41467-020-16355-w
Wang, M., Liu, M., and Ma, Z. (2023). Cannabinoid type 2 receptor activation inhibits MPP+-Induced M1 differentiation of microglia through activating PI3K/Akt/Nrf2 signal pathway. Mol. Biol. Rep. 50 (5), 4423–4433. doi:10.1007/s11033-023-08395-4
Wang, P., Yang, P., Qian, K., Li, Y., Xu, S., Meng, R., et al. (2022). Precise gene delivery systems with detachable albumin shell remodeling dysfunctional microglia by TREM2 for treatment of alzheimer's disease. Biomaterials 281, 121360. doi:10.1016/j.biomaterials.2021.121360
Wang, W., Li, Y., Ma, F., Sheng, X., Chen, K., Zhuo, R., et al. (2023). Microglial repopulation reverses cognitive and synaptic deficits in an alzheimer's disease model by restoring BDNF signaling. Brain Behav. Immun. 113, 275–288. doi:10.1016/j.bbi.2023.07.011
Whiting, Z. M., Yin, J., de la Harpe, S. M., Vernall, A. J., and Grimsey, N. L. (2022). Developing the cannabinoid receptor 2 (CB2) pharmacopoeia: past, present, and future. Trends Pharmacol. Sci. 43 (9), 754–771. doi:10.1016/j.tips.2022.06.010
Willis, E. F., MacDonald, K. P. A., Nguyen, Q. H., Garrido, A. L., Gillespie, E. R., Harley, S. B. R., et al. (2020). Repopulating microglia promote brain repair in an IL-6-Dependent manner. Cell 180 (5), 833–846 e16. doi:10.1016/j.cell.2020.02.013
Wu, J., Bie, B., Yang, H., Xu, J. J., Brown, D. L., and Mohamed, N. (2013). Activation of the CB2 receptor system reverses amyloid-induced memory deficiency. Neurobiol. Aging 34 (3), 791–804. doi:10.1016/j.neurobiolaging.2012.06.011
Xia, Y., Zhang, G., Kou, L., Yin, S., Han, C., Hu, J., et al. (2021). Reactive microglia enhance the transmission of exosomal alpha-synuclein via toll-like receptor 2. Brain 144 (7), 2024–2037. doi:10.1093/brain/awab122
Xu, K., Wu, Y., Tian, Z., Xu, Y., Wu, C., and Wang, Z. (2023). Microglial cannabinoid CB2 receptors in pain modulation. Int. J. Mol. Sci. 24 (3), 2348. doi:10.3390/ijms24032348
Yang, Z., Liu, B., Yang, L. E., and Zhang, C. (2019). Platycodigenin as potential drug candidate for alzheimer's disease via modulating microglial polarization and neurite regeneration. Molecules 24 (18), 3207. doi:10.3390/molecules24183207
Yang, Y., Mou, B., Zhang, Q. R., Zhao, H. X., Zhang, J. Y., Yun, X., et al. (2023). Microglia are involved in regulating histamine-dependent and non-dependent itch transmissions with distinguished signal pathways. Glia 71 (11), 2541–2558. doi:10.1002/glia.24438
Yoo, Y., Neumayer, G., Shibuya, Y., Mader, M. M., and Wernig, M. (2023). A cell therapy approach to restore microglial Trem2 function in a mouse model of alzheimer's disease. Cell Stem Cell 30 (8), 1043–1053 e6. doi:10.1016/j.stem.2023.07.006
Youssef, D. A., El-Fayoumi, H. M., and Mona, F. M. (2019). Beta-caryophyllene protects against diet-induced dyslipidemia and vascular inflammation in rats: involvement of CB2 and PPAR-γ receptors. Chemico-Biological Interact. 297, 16–24. doi:10.1016/j.cbi.2018.10.010
Yu, H., Liu, X., Chen, B., Vickstrom, C. R., Friedman, V., Kelly, T. J., et al. (2021). The neuroprotective effects of the CB2 agonist GW842166x in the 6-OHDA mouse model of parkinson’s disease. Cells 10 (12), 3548. doi:10.3390/cells10123548
Yu, S.-J., Reiner, D., Shen, H., Wu, K.-J., Liu, Q.-R., and Wang, Y. (2015). Time-dependent protection of CB2 receptor agonist in stroke. PLOS ONE 10 (7), e0132487. doi:10.1371/journal.pone.0132487
Zarruk, J. G., Fernández-López, D., García-Yébenes, I., García-Gutiérrez, M. S., Vivancos, J., Nombela, F., et al. (2012). Cannabinoid type 2 receptor activation downregulates stroke-induced classic and alternative brain Macrophage/Microglial activation concomitant to neuroprotection. Stroke 43 (1), 211–219. doi:10.1161/STROKEAHA.111.631044
Zhang, H. Y., Shen, H., Jordan, C. J., Liu, Q. R., Gardner, E. L., Bonci, A., et al. (2019). CB2 receptor antibody signal specificity: correlations with the use of partial CB2-knockout mice and anti-rat CB2 receptor antibodies. Acta Pharmacol. Sin. 40 (3), 398–409. doi:10.1038/s41401-018-0037-3
Zhang, C., Raveney, B., Takahashi, F., Yeh, T. W., Hohjoh, H., Yamamura, T., et al. (2023). Pathogenic microglia orchestrate neurotoxic properties of eomes-expressing helper T cells. Cells 12 (6), 868. doi:10.3390/cells12060868
Zheng, T., and Zhang, Z. (2021). Activated microglia facilitate the transmission of alpha-synuclein in Parkinson's disease. Neurochem. Int. 148, 105094. doi:10.1016/j.neuint.2021.105094
Zhou, R., Chen, S. H., Zhao, Z., Tu, D., Song, S., Wang, Y., et al. (2023). Complement C3 enhances LPS-Elicited neuroinflammation and neurodegeneration via the Mac1/NOX2 pathway. Mol. Neurobiol. 60 (9), 5167–5183. doi:10.1007/s12035-023-03393-w
Ziring, D., Wei, Bo, Velazquez, P., Schrage, M., Buckley, N. E., and Braun, J. (2006). Formation of B and T cell subsets require the cannabinoid receptor CB2. Immunogenetics 58 (9), 714–725. doi:10.1007/s00251-006-0138-x
Keywords: cannabinoid, microglia, knockout, neuroinflammation, mouse
Citation: Laloli KJ, Rentsch P, Stayte S and Vissel B (2025) Novel floxed cannabinoid receptor 2 mouse line combines knockout capability with dual fluorescent reporters. Front. Pharmacol. 16:1682979. doi: 10.3389/fphar.2025.1682979
Received: 10 August 2025; Accepted: 15 October 2025;
Published: 19 November 2025.
Edited by:
Giuseppe Di Giovanni, University of Magna Graecia, ItalyReviewed by:
Emmanuel Shan Onaivi, William Paterson University, United StatesLei Ye, Nanjing Drum Tower Hospital, China
Copyright © 2025 Laloli, Rentsch, Stayte and Vissel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bryce Vissel, YnJ5Y2V2aXNzZWxAZ21haWwuY29t
 Kathryn J. Laloli
Kathryn J. Laloli Peggy Rentsch
Peggy Rentsch Sandy Stayte
Sandy Stayte Bryce Vissel
Bryce Vissel