- 1Department of Emergency Medicine, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, China
- 2Hubei Provincial Clinical Research Center for Pneumoconiosis and Poisoning, Wuhan, Hubei, China
Objective: To investigate the clinical and MRI features of severe central nervous system injury caused by an extremely high dose of metoclopramide in combination with multiple drug overdoses, and to summarize treatment strategies and prognosis.
Methods: We report the clinical course of acute poisoning in a 36-year-old woman following a single oral intake of approximately 350 mg metoclopramide combined with adrenolizone and coenzyme Q10. Clinical manifestations, laboratory findings, imaging results (CT and MRI), and treatment interventions after admission were collected and analyzed. The possible pathological mechanisms were explored in conjunction with previous literature.
Results: Upon admission, the patient exhibited acute mental and behavioral disturbances, extrapyramidal symptoms, and impaired consciousness. Initial CT scans revealed no abnormalities. On the fourth day after admission, MRI demonstrated symmetrical patchy hyperintensities on T2WI/T2-FLAIR in the corpus callosum, bilateral corona radiata, and centrum semiovale white matter, along with hyperintensities on DWI and corresponding low ADC values, indicating cytotoxic edema consistent with toxic-metabolic encephalopathy. Following comprehensive multidisciplinary management-including early gastrointestinal decontamination, activated charcoal adsorption, HA380 hemoperfusion, hyperbaric oxygen therapy, neuroprotection, and symptomatic support-the patient was discharged on day 12. At the 3-month follow-up, no neurological sequelae were observed.
Conclusion: Extremely high-dose metoclopramide, particularly in combination with other drugs, can cause a characteristic symmetrical white matter injury pattern of toxic encephalopathy, with MRI findings offering high diagnostic value. Early recognition, prompt gastrointestinal decontamination, blood purification, and multi-target neuroprotective therapy can markedly improve prognosis.
1 Introduction
Metoclopramide is a commonly used prokinetic agent widely employed in the management of gastrointestinal dysfunction and in controlling symptoms such as nausea and vomiting (Vimonsuntirungsri et al., 2024). Its primary mechanism of action involves antagonism of central and peripheral dopamine D2 receptors, partial activation of 5-hydroxytryptamine (5-HT4) receptors, and antagonism of 5-HT3 receptors, thereby regulating gastrointestinal motility (McCallum et al., 2024). However, metoclopramide can cross the blood–brain barrier and is transported centrally as a substrate of P-glycoprotein (P-gp) (Breuil et al., 2025). When administered at high doses or over prolonged periods, it may induce extrapyramidal reactions, psychiatric and behavioral disturbances, and toxic encephalopathy. Repeated use can result in death from torsades de pointes ventricular tachycardia (Watanabe et al., 2022). Additionally, it may cause atrial fibrillation (Saleh et al., 2020), serotonin syndrome (Meegada et al., 2019), and persistent hypotension (Nguyen and Petzel Gimbar, 2013). Nevertheless, poisoning from extremely large doses is exceedingly rare, often presenting with complex clinical manifestations, rapid progression, and potentially fatal outcomes.
In recent years, neuroimaging-particularly MRI-has played a crucial role in diagnosing drug-induced toxic encephalopathy (Parbhu et al., 2009; Shang et al., 2025). Toxic-metabolic encephalopathy typically presents as symmetrical signal abnormalities in the corpus callosum and deep white matter, which are valuable for identifying the etiology and assessing prognosis. However, reports on the MRI features, comprehensive treatment approaches, and prognosis of metoclopramide-related multi-drug massive overdose remain scarce.
This article reports the case of a female patient who ingested approximately 350 mg metoclopramide together with adrenolizone and coenzyme Q10 in a single episode. We provide a detailed account of her clinical presentation, MRI findings, and treatment course, and discuss the underlying pathological mechanisms and key clinical management considerations in light of the literature, aiming to offer a reference for the diagnosis and treatment of such rare cases.
2 Diagnosis and treatment process
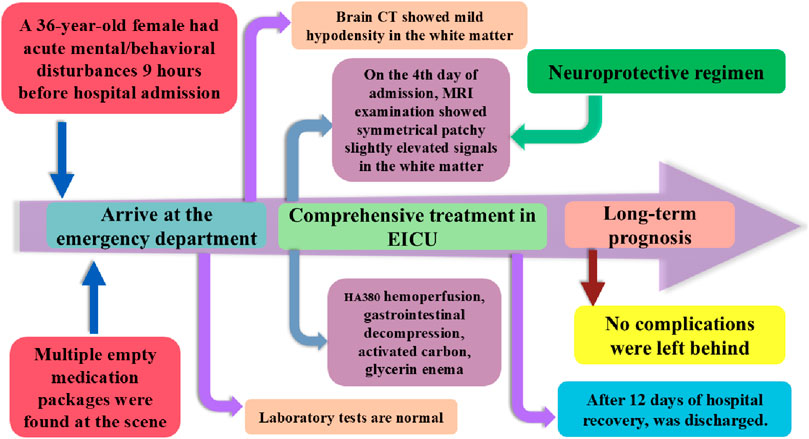
The patient was a 36-year-old woman with no significant past medical history. Approximately 9 h prior to admission, she developed acute mental and behavioral disturbances, characterized by sudden emotional outbursts without apparent cause, episodes of crying, facial twitching, slurred speech, tongue protrusion, voluntary undressing in public, and associated vomiting and hypersalivation. Subsequently, she became progressively apathetic and unable to communicate verbally. Multiple empty medication packages (metoclopramide, adrenolizone, and coenzyme Q10) were found at the scene.
According to her family, the patient had ingested approximately 70 tablets of metoclopramide (5 mg each), 10 tablets of adrenolizone (2.5 mg each), and 10 tablets of coenzyme Q10 (10 mg each) in a single episode, with suicidal intent. Within the 9 h following ingestion, she did not receive any emergency interventions such as gastric lavage or induced emesis, and no other medical records were available.
Physical examination on admission: Body temperature 36.3 °C, pulse 66 beats/min, respiratory rate 18 breaths/min, blood pressure 107/73 mmHg. The patient was delirious, with blunted responsiveness, slurred speech, and impaired communication. Pupils were equal and round, approximately 3 mm in diameter, with preserved light reflexes. Upward gaze deviation and abnormal tongue protrusion were observed. Increased neck muscle tone was noted. The extremities were symmetric; withdrawal from painful stimuli was present, with muscle strength estimated at grade IV, and overall normal limb muscle tone. Deep tendon reflexes were present and symmetric, with no pathological reflexes elicited. Sensory and cerebellar examinations could not be performed due to impaired consciousness. No other focal neurological signs were detected. Glasgow Coma Scale (GCS) score was 10 (E3, V2, M5).
Laboratory findings: Arterial blood gas: pH 7.468, PaCO2 30.7 mmHg, PaO2 109 mmHg, Na+ 137 mmol/L, K+ 4.03 mmol/L, Ca2+ 1.013 mmol/L, glucose 6.0 mmol/L, BE −0.91 mmol/L, HCO3− 21.8 mmol/L, COHb 2.0%. Complete blood count: RBC 4.12 × 1012/L, WBC 9.52 × 109/L, PLT 322 × 109/L. Liver and renal function: ALT 10.1 U/L, AST 14.2 U/L, total bilirubin 15.98 μmol/L, urea 3.32 mmol/L, creatinine 50.60 μmol/L, uric acid 303.5 μmol/L. Creatine kinase: CK 51.1 U/L, CK-MB 8.5 U/L. Coagulation profile: PTA 87.00%, PT 11.90 s, INR 1.11, APTT 30.60 s, fibrinogen 3.39 g/L, TT 15.20 s.
Electrocardiogram (ECG) Findings: The ECG shows a sinus rhythm with a normal overall pattern. Both the atrial and ventricular rates are 71 beats per minute. The P-wave duration is 90 ms, the QT interval is 388 ms, and the corrected QT interval (QTc) is 422 ms. The PR interval measures 143 ms, and the QRS duration is 73 ms. The RV5/SV1 amplitude ratio is 0.802/1.167 mV, and the RV1/SV5 amplitude ratio is 0.09/0.191 mV.
Imaging: Non-contrast brain CT on the day of admission (Figures 1A,B) showed mild narrowing of the cerebral sulci, with mild hypodensity in the white matter, and the changes were symmetrically distributed.
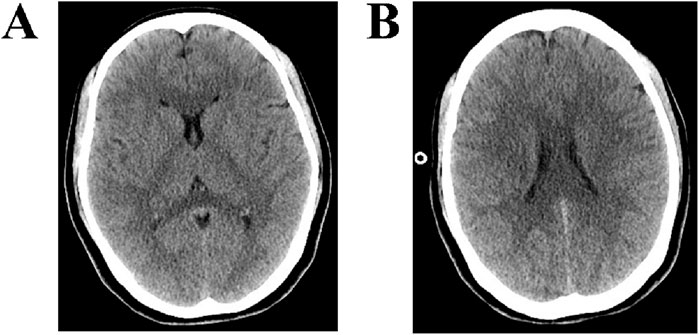
Figure 1. CT findings of the brain. (A,B) CT of the brain shows mild narrowing of the cerebral sulci, with mild hypodensity in the white matter, and the changes were symmetrically distributed.
Immediately upon admission, intravenous access was established, and a nasogastric tube was inserted for repeated gastrointestinal decompression. Activated charcoal was administered via the nasogastric route to adsorb residual drugs, and a liquid glycerin enema was performed to facilitate excretion. Oral benzhexol was prescribed to alleviate extrapyramidal reactions, while intermittent intravenous diazepam or midazolam was given for sedation, anticonvulsant purposes, and relief of dystonia. Omeprazole was administered to suppress gastric acid secretion, and bismuth potassium citrate was used to protect the gastric mucosa. Active fluid resuscitation was initiated, combined with diuretics to promote toxin elimination. Water, electrolyte, and acid-base balance were closely monitored. Pharmacokinetic testing was recommended to the patient’s family; however, they declined.
Given the patient’s intake of multiple oral medications in large doses and the presence of significant extrapyramidal symptoms, along with the indication for blood purification, hemoperfusion was selected as the intervention. This treatment aimed to mitigate toxic effects by removing harmful substances from the blood. The hemoperfusion device used was the JianFan HA380 model, with a flow rate of 200 mL/min and a duration of 4 h. Prior to the hemoperfusion, the patient’s GCS score was 10 (E3, V2, M5), with an on-machine blood pressure of 108/66 mmHg and a pulse of 59 beats per minute. During the procedure, the patient’s blood pressure remained around 95/66 mmHg, with an on-machine blood pressure of 99/64 mmHg and a pulse rate of 53 beats per minute. After the hemoperfusion, the GCS score improved to 12 (E4, V3, M5).
On the third day of admission, the patient was conscious with an average mental state, able to follow simple instructions, but with non-fluent speech. Repeat laboratory tests revealed hypoproteinemia (albumin 30.51 g/L), likely related to insufficient intake after poisoning, increased protein catabolism under stress, and altered capillary permeability. Mild anemia (Hb 107 g/L) was also detected, possibly associated with acute inflammatory response, malnutrition, and hemodilution. Dietary adjustments were made to increase the intake of high-quality protein.
MRI examination on the fourth day of admission (Figures 2A–D) revealed the following: T2-weighted imaging (T2WI) (Figure 2A) showed symmetrical patchy hyperintensities in the corpus callosum, bilateral corona radiata, and the white matter of the centrum semiovale. T2-FLAIR (Figure 2B) similarly revealed symmetrical patchy hyperintensities in the same regions. Diffusion-weighted imaging (DWI) (Figure 2C) showed symmetrical patchy hyperintensities at the corresponding sites. The apparent diffusion coefficient (ADC) map (Figure 2D) showed low signal intensity in the same areas, indicating restricted diffusion. These imaging findings are consistent with diffuse white matter injury caused by drug poisoning, affecting the corpus callosum and bilateral centrum semiovale, with a symmetrical distribution pattern characteristic of toxic-metabolic encephalopathy.
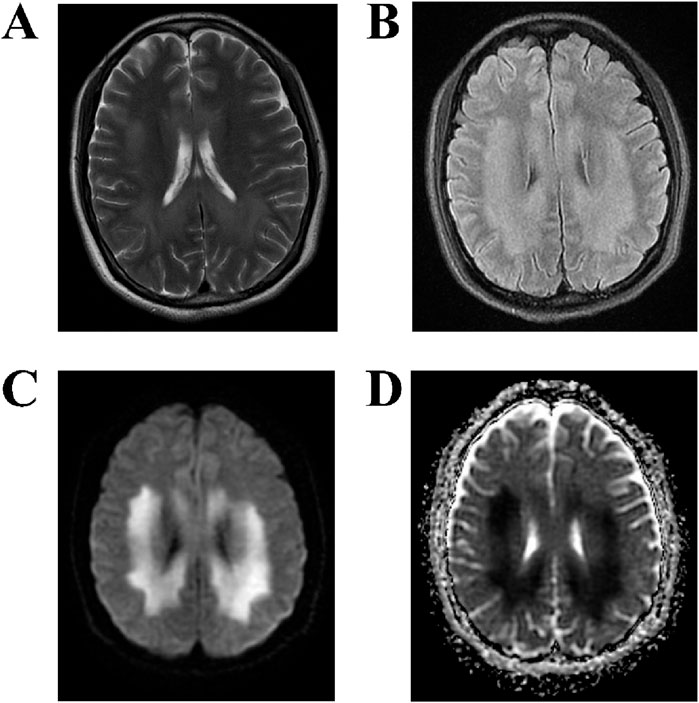
Figure 2. MRI findings of the brain. (A,B) T2WI and T2-FLAIR sequences demonstrate symmetrical patchy hyperintensities in the corpus callosum, bilateral corona radiata, and centrum semiovale white matter. (C,D) DWI reveals symmetrical patchy hyperintensities in the same areas, with corresponding low signal intensity on the ADC map, indicating restricted diffusion.
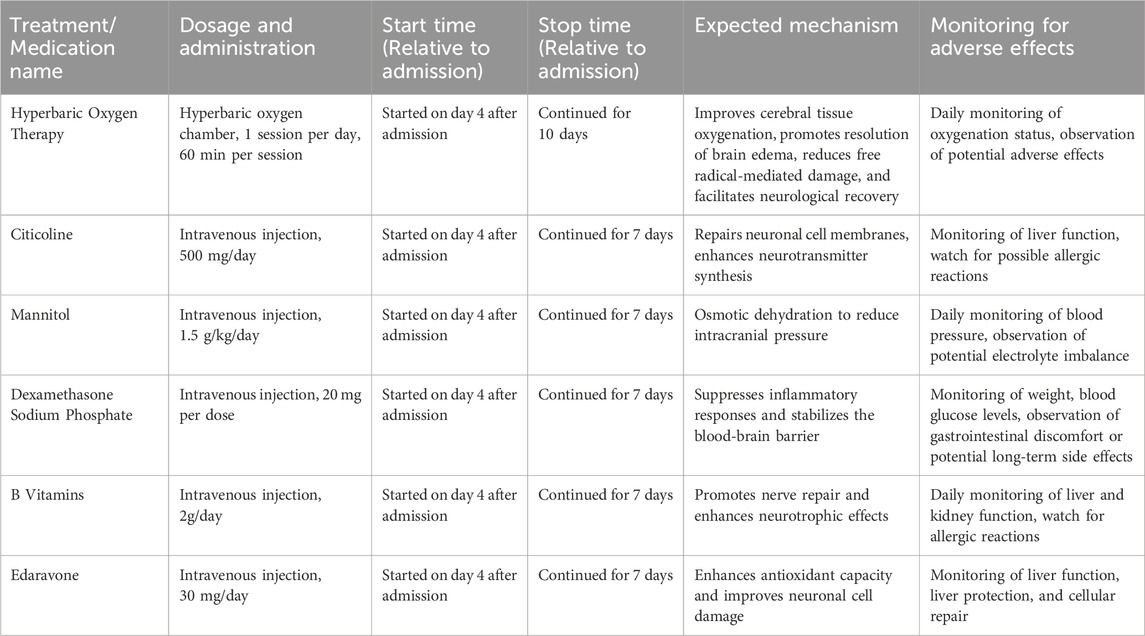
A multidisciplinary consultation with the hyperbaric oxygen and neurology departments was conducted immediately, and hyperbaric oxygen therapy was initiated to improve cerebral tissue oxygenation, promote resolution of brain edema, reduce free radical-mediated damage, and facilitate neurological recovery. Concurrently, intravenous citicoline was administered to repair neuronal cell membranes and enhance neurotransmitter synthesis. Mannitol was given for osmotic dehydration to reduce intracranial pressure, and Dexamethasone Sodium Phosphate were used to suppress inflammatory responses and stabilize the blood-brain barrier. In addition, B vitamins, and edaravone were prescribed. Throughout the treatment period, intracranial pressure was closely monitored, and daily neurological examinations were performed to assess muscle strength, tone, and reflexes. As shown in Table 1, the treatment measures received during the hospital stay and their durations.
On the 10th day of admission, the patient was alert, oriented, and hemodynamically stable, able to perform activities of daily living independently, with normal eating and sleeping patterns. Hyperbaric oxygen therapy was discontinued after a total of 10 days. On the 12th day, re-examination revealed no neurological deficits, normal limb strength and coordination, absence of extrapyramidal signs, steady gait, and overall clinical stability. The patient was discharged. At the 3-month follow-up, the patient demonstrated complete physical recovery without any neurological sequelae. As shown in Figure 3, this chart illustrates the inpatient treatment and prognosis timeline of the patient.
3 Discussion
Metoclopramide is widely used in the treatment of gastrointestinal disorders and for the control of nausea and vomiting (Vimonsuntirungsri et al., 2024). Its primary pharmacological actions include antagonism of central and peripheral dopamine D2 receptors, partial activation of 5-HT4 receptors, and antagonism of 5-HT3 receptors (McCallum et al., 2024). However, it can induce movement disorders or exacerbate pre-existing extrapyramidal syndromes (Alkhowaiter et al., 2024), with an estimated incidence of approximately 0.2% (Jo et al., 2012). Common adverse effects include akathisia, nystagmus, acute dystonic reactions (metoclopramide-induced acute dystonic reactions, MIADRs) (Fink et al., 2023), and restless legs syndrome (Aljunaid, 2024). In rare cases, it may precipitate a pheochromocytoma crisis and be complicated by acute respiratory distress syndrome (ARDS) (Xie et al., 2023) or neuroleptic malignant syndrome (NMS) (Wittmann et al., 2016). When the daily dose exceeds 30 mg, the risk of MIADRs increases significantly (Malakian et al., 2025). In the present case, the patient ingested approximately 350 mg of metoclopramide in a single episode, together with adrenolizone and coenzyme Q10-far exceeding the safe therapeutic range. This presentation is classified as a massive multi-drug overdose.
Carbazochrome is a capillary hemostatic agent (Alsaied et al., 2025). It works by enhancing the contraction of damaged capillaries and reducing inflammation-induced endothelial permeability, thus helping to maintain microvascular integrity (Sendo et al., 2003). This mechanism plays a key role in minimizing the risk of bleeding. In clinical practice, Carbazochrome generally causes few side effects, and no studies have reported any adverse effects on the central nervous system (CNS) at standard therapeutic doses.
In this case, MRI demonstrated symmetrical patchy hyperintensities in the corpus callosum, bilateral corona radiata, and the central white matter of the centrum semiovale. DWI revealed corresponding hyperintensities with low ADC values, consistent with the cytotoxic edema pattern characteristic of toxic–metabolic encephalopathy. Compared with other toxic agents, endosulfan poisoning can rapidly induce central nervous system excitation and status epilepticus, with MRI findings suggestive of diffuse cerebral edema (Parbhu et al., 2009). Exposure to methyl iodide may produce symmetrical patchy signal abnormalities (Liu et al., 2025). Occupational poisoning from 1,2-dichloroethane often involves the cerebellar dentate nucleus, basal ganglia, and bilateral cerebral white matter, and the extent of imaging abnormalities does not always correlate with clinical severity (Wang et al., 2025). In methanol poisoning, acute symmetric ischemic changes with cytotoxic edema can be seen in the basal ganglia and bilateral optic nerves (Balodis et al., 2024). In addition, poisoning by substances such as potassium cyanide (Messing, 1991), Endosulfan (Parbhu et al., 2009), Carbon monoxide (Aoshima et al., 2021), dimethylamine borane (Yu et al., 2022), and Hydrogen sulfide (Genjiafu et al., 2023) often results in symmetrical involvement of the corpus callosum and deep white matter. A history of excessive exposure to these substances can help suggest a diagnosis.
The management of metoclopramide overdose should be guided by the drug’s toxicological profile and implemented through a multi-dimensional, collaborative approach. Given that metoclopramide is highly lipophilic, capable of crossing the blood-brain barrier (BBB), and a substrate of P-gp, rapid reduction of its systemic and cerebral burden is critical for improving prognosis. During the acute phase, prompt gastrointestinal decontamination is essential. Early gastric lavage and repeated administration of activated charcoal upon admission can reduce ongoing absorption. In patients who do not receive timely intervention, gastric decompression via nasogastric tube followed by nasogastric administration of activated charcoal can be performed to adsorb residual drug. In the present case, such decontamination procedures were completed approximately 9 h after ingestion, providing a foundation for subsequent therapeutic measures.
Secondly, given that metoclopramide acts on central dopamine receptors, patients frequently develop severe extrapyramidal reactions. Therefore, agents such as benzhexol should be administered during treatment to alleviate these symptoms. In addition, if severe seizures or psychiatric disturbances occur, prompt administration of intravenous diazepam, midazolam, or similar agents is warranted for sedation and anticonvulsant therapy.
Furthermore, a multi-target neuroprotective regimen may be employed, combining citicoline to repair neuronal cell membranes, edaravone to scavenge free radicals, and mannitol to reduce intracranial pressure. Hyperbaric oxygen therapy can enhance cerebral oxygenation, reduce myelin edema, and facilitate the repair of white matter injury. In the present case, a cumulative 10-day course of such interventions markedly accelerated neurological recovery.
In terms of systemic support, hemodynamic status should be closely monitored, fluid resuscitation should be actively implemented, and diuresis should be employed when necessary to facilitate toxin clearance. For ultra-high-dose exposures, blood purification represents a key therapeutic intervention. Metoclopramide has a molecular weight of 299.8 g/mol and a plasma protein binding rate of approximately 30% (Wishart et al., 2018), fulfilling the criteria for hemoperfusion. In particular, HA380 resin possesses strong adsorption capacity for lipophilic toxins and can rapidly reduce plasma drug concentrations (Li et al., 2025). In the present case, a 4-h hemoperfusion session was initiated within 1 h of admission, effectively mitigating ongoing central nervous system injury caused by the toxin. It should be noted that the family’s refusal to consent to drug metabolism and toxicology testing limited the ability to quantitatively assess the dose-response relationship of exposure and to optimize individualized detoxification strategies.
4 Conclusion
Ultra-high-dose metoclopramide poisoning can result in severe central nervous system injury, presenting with acute psychiatric and behavioral disturbances, extrapyramidal reactions, and toxic encephalopathy. MRI typically demonstrates symmetrical white matter injury, which serves as an important diagnostic indicator. Early gastrointestinal decontamination, activated charcoal adsorption, and blood purification can markedly reduce the toxic burden and improve prognosis. In the present case, the patient was followed up for 3 months after standardized treatment and exhibited no sequelae, underscoring that early recognition and multidisciplinary collaboration are critical to optimizing outcomes in toxic encephalopathy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The study adhered to the Declaration of Helsinki and relevant ethical guidelines. It was approved by the medical ethics committee of our institution. This is a retrospective clinical analysis, as evidenced by the approval letter from the Medical Ethics Committee of Shiyan Taihe Hospital (reference number: 2025KS118, August 2025). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PL: Writing – original draft, Formal Analysis, Writing – review and editing, Conceptualization, Data curation, Investigation. YT: Writing – review and editing, Funding acquisition, Data curation, Formal Analysis. XZ: Software, Writing – original draft, Project administration, Methodology. LZ: Writing – original draft, Resources, Validation. JZ: Writing – original draft, Project administration, Supervision. HT: Writing – original draft, Formal Analysis, Data curation, Supervision. ZW: Writing – original draft, Data curation, Investigation. QF: Formal Analysis, Methodology, Writing – original draft. ZF: Writing – review and editing, Resources, Funding acquisition, Validation. ZZ: Writing – review and editing, Data curation, Supervision, Visualization, Writing – original draft, Validation, Conceptualization. XL: Writing – original draft, Data curation, Methodology, Formal Analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Thanks to Shiyan Leading Scientific Research Project (24Y044) for the support of the Fund.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aljunaid, M. A. (2024). An unusual case of an acute episode of restless leg syndrome following oral metoclopramide therapy. Cureus 16 (2), e53754. doi:10.7759/cureus.53754
Alkhowaiter, S., Al Rasheed, M. M., Alammar, N., Alotaibi, A., Altuwaijri, M., Alshankiti, S., et al. (2024). Safety of prolonged use of metoclopramide and domperidone as treatment for chronic gastrointestinal dysmotility disorders in patients with systemic sclerosis. Saudi Pharm. J. 32 (5), 102039. doi:10.1016/j.jsps.2024.102039
Alsaied, M. A., El-Sayed, O. S., Alqato, S., Elettreby, A. M., and Abo Elnaga, A. A. (2025). Optimizing blood management in arthroplasty: a meta-analysis of carbazochrome sodium sulfonate and Tranexamic acid combination. J. Orthop. Surg. Res. 20 (1), 668. doi:10.1186/s13018-025-06038-x
Aoshima, K., Yamaoka, H., Nakamura, S., Nojima, T., Naito, H., and Nakao, A. (2021). Right hemiplegia following acute carbon monoxide poisoning. Cureus 13 (7), e16738. doi:10.7759/cureus.16738
Balodis, A., Valante, R., Saule, L., Balode, G., and Pūpola, M. (2024). Ischemic and hemorrhagic brain damage in methanol poisoning: a case of rapid deterioration. Am. J. Case Rep. 25, e945731. doi:10.12659/ajcr.945731
Breuil, L., Arino, I., El Biali, M., Rodrigo, S., Jackwerth, M., Mairinger, S., et al. (2025). Imaging P-glycoprotein function at the blood-brain barrier in drug-resistant epilepsy using [(11)C]metoclopramide. Eur. J. Nucl. Med. Mol. Imaging. doi:10.1007/s00259-025-07437-2
Fink, F. M., Bognar, M., Hengl, P., Paulmichl, M., and Nofziger, C. (2023). Case report: metoclopramide induced acute dystonic reaction in adolescent CYP2D6 poor metabolizers. Front. Pharmacol. 14, 1201566. doi:10.3389/fphar.2023.1201566
Genjiafu, A., Shi, M., Zhang, X., and Jian, X. (2023). Case report: analysis of a case of hydrogen sulfide poisoning in a waste treatment plant. Front. Public Health 11, 1226282. doi:10.3389/fpubh.2023.1226282
Jo, Y. Y., Kim, Y. B., Yang, M. R., and Chang, Y. J. (2012). Extrapyramidal side effects after metoclopramide administration in a post-anesthesia care unit -A case report. Korean J. Anesthesiol. 63 (3), 274–276. doi:10.4097/kjae.2012.63.3.274
Li, Y., Han, M., Yang, M., and Su, B. (2025). Hemoperfusion with the HA330/HA380 cartridge in intensive care settings: a state-of-the-art review. Blood Purif. 54 (2), 122–137. doi:10.1159/000542469
Liu, H., Shang, R., Tian, Q., Liu, Y., and Jian, X. (2025). Case report: a case of occupational methyl iodide-induced encephalopathy. Front. Pharmacol. 16, 1574692. doi:10.3389/fphar.2025.1574692
Malakian, A., Omrani, A., Omrani, A., Ramzani, Y., and Zakariaei, Z. (2025). Prolonged extrapyramidal syndrome due to metoclopramide overdose: a rare case report. Oxf Med. Case Rep. 2025 (2), omae217. doi:10.1093/omcr/omae217
McCallum, R. W., Parkman, H. P., Fass, R., Bhandari, B. R., Carlson, M. R., and Buck, R. D. (2024). Metoclopramide nasal spray in women with symptomatic diabetic gastroparesis: a randomized, double-Blind, placebo-controlled phase 3 study. Clin. Gastroenterol. Hepatol. 22 (12), 2497–2505.e5. doi:10.1016/j.cgh.2023.10.022
Meegada, S., Heda, R. P., Satapathy, S., and Verma, R. (2019). Metoclopramide-induced serotonin syndrome. Cureus 11 (12), e6359. doi:10.7759/cureus.6359
Messing, B. (1991). Extrapyramidal disturbances after cyanide poisoning (first MRT-investigation of the brain). J. Neural Transm. Suppl. 33, 141–147. doi:10.1007/978-3-7091-9135-4_22
Nguyen, T. T., and Petzel Gimbar, R. M. (2013). Sustained hypotension following intravenous metoclopramide. Ann. Pharmacother. 47 (11), 1577–1580. doi:10.1177/1060028013503789
Parbhu, B., Rodgers, G., and Sullivan, J. E. (2009). Death in a toddler following endosulfan ingestion. Clin. Toxicol. (Phila) 47 (9), 899–901. doi:10.3109/15563650903328879
Saleh, A. S., Khokhar, T., and Nawaz, A. (2020). 62. Metoclopramide-induced atrial fibrillation: a case report. Eur. J. Emerg. Med. 27 (Suppl. 1), e12–e13. doi:10.1097/01.mej.0000697864.53903.b1
Sendo, T., Itoh, Y., Aki, K., Oka, M., and Oishi, R. (2003). Carbazochrome sodium sulfonate (AC-17) reverses endothelial barrier dysfunction through inhibition of phosphatidylinositol hydrolysis in cultured porcine endothelial cells. Naunyn Schmiedeb. Arch. Pharmacol. 368 (3), 175–180. doi:10.1007/s00210-003-0785-5
Shang, R. K., Tian, Q. X., Jian, X. D., Liu, H. Y., Liu, Y. R., and Li, Q. L. (2025). A pregnant woman with diquat poisoning leading to miscarriage and pontine haematoma. Clin. Toxicol. (Phila) 63 (4), 296–297. doi:10.1080/15563650.2025.2454972
Vimonsuntirungsri, T., Thungsuk, R., Nopjaroonsri, P., Faknak, N., and Pittayanon, R. (2024). The efficacy of metoclopramide for gastric visualization by endoscopy in patients with active upper gastrointestinal bleeding: double-blind randomized controlled trial. Am. J. Gastroenterol. 119 (5), 846–855. doi:10.14309/ajg.0000000000002620
Wang, J., Jian, T., Yu, G., Kan, B., Li, W., and Jian, X. (2025). Case report: evaluating toxic encephalopathy from occupational 1,2-dichloroethane exposure: magnetic resonance imaging contributions. Front. Toxicol. 7, 1557995. doi:10.3389/ftox.2025.1557995
Watanabe, Y., Nakamura, I., Takebayashi, Y., and Watanabe, H. (2022). A fatal case of repeated ventricular fibrillation due to torsade de pointes following repeated administration of metoclopramide. Clin. Case Rep. 10 (8), e6213. doi:10.1002/ccr3.6213
Wishart, D. S., Feunang, Y. D., Guo, A. C., Lo, E. J., Marcu, A., Grant, J. R., et al. (2018). DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46 (D1), D1074–d1082. doi:10.1093/nar/gkx1037
Wittmann, O., Sadot, E., Bisker-Kassif, O., Scolnik, D., Tavor, O., and Glatstein, M. M. (2016). Neuroleptic malignant syndrome associated with metoclopramide use in a boy: case report and review of the literature. Am. J. Ther. 23 (5), e1246–e1249. doi:10.1097/mjt.0000000000000320
Xie, Y., Zhang, A., Qi, M., Xiong, B., Zhang, S., Zhou, J., et al. (2023). Pheochromocytoma crisis with refractory acute respiratory distress syndrome (ARDS), takotsubo syndrome, emergency adrenalectomy, and need for extracorporeal membrane oxygenation (ECMO) in a previously undiagnosed and asymptomatic patient, due to the use of metoclopramide. BMC Endocr. Disord. 23 (1), 145. doi:10.1186/s12902-023-01404-4
Keywords: metoclopramide, drug overdose, toxic encephalopathy, magnetic resonance imaging, hemoperfusion
Citation: Liang P, Tang Y, Zhang X, Zhang L, Zhao J, Tang H, Wu Z, Fang Q, Fang Z, Zhao Z and Liu X (2025) MRI imaging manifestations of severe toxic encephalopathy induced by excessive metoclopramide and multiple drug overdose: a case report. Front. Pharmacol. 16:1683132. doi: 10.3389/fphar.2025.1683132
Received: 10 August 2025; Accepted: 18 September 2025;
Published: 24 September 2025.
Edited by:
Gladys Ouedraogo, L’Oreal, FranceReviewed by:
Přemysl Vlček, Charles University, CzechiaPeng Du, Fudan University, China
Alen Sam Saji, Sichuan University, China
Arturs Balodis, Riga Stradiņš University, Latvia
Copyright © 2025 Liang, Tang, Zhang, Zhang, Zhao, Tang, Wu, Fang, Fang, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhicheng Fang, MTM1OTM3NTEwMDlAMTYzLmNvbQ==; Zhiwen Zhao, emhhb3p3MTk5OUAxMjYuY29t; Xuefang Liu, MTQ3MTI5OTM1NUBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
 Pengfei Liang
Pengfei Liang Yuncheng Tang1†
Yuncheng Tang1† Zhicheng Fang
Zhicheng Fang Zhiwen Zhao
Zhiwen Zhao Xuefang Liu
Xuefang Liu