- 1Protein Engineering Lab, School of Biosciences and Technology, Vellore Institute of Technology, Vellore, Tamil Nadu, India
- 2Department of Pharmaceutical Sciences, Pharmacy Program, Batterjee Medical College, Jeddah, Saudi Arabia
- 3Institute of Pharmaceutical Research, GLA University, Mathura, Uttar Pradesh, India
- 4Department of Chemistry and Biochemistry, School of Sciences, JAIN (Deemed to Be University), Bengaluru, Karnataka, India
- 5Deptartment of General Medicine, IMS and SUM Hospital, Siksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha, India
- 6Deptartment of Pharmacology, Teerthanker Mahaveer College of Pharmacy, Teerthanker Mahaveer University, Moradabad, Uttar Pradesh, India
- 7Sharda University, Greater Noida, India
- 8Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Dehradun, Uttarakhand, India
- 9Centre for Research Impact and Outcome-Chitkara College of Pharmacy, Chitkara University, Rajpura, Punjab, India
- 10Centre of Medical and Bio-allied Health Sciences Research, Ajman University, Ajman, United Arab Emirates
- 11TanBio R and D Solution, Thiruvarur, Tamilnadu, India
Metastasis remains the prime cause of poor prognosis in lung cancer, a leading cause of cancer-related mortality worldwide. Because CXCR4/CXCL12 constitutes a powerful therapeutic target to counter tumor progression, immune evasion, and therapy resistance, it plays a pivotal role in lung cancer. Expression of CXCR4 is high in non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) and has been correlated with aggressive tumor behavior increased metastatic spread to the bone marrow, the liver, and the brain, and poor overall survival. Studies in preclinical models have demonstrated that plerixafor is a CXCR4 inhibitor that can reduce tumor cell migration, increase chemosensitivity, and re-establish immune response to limit metastasis and increase treatment efficacy. Furthermore, clinical trials combining plerixafor with chemotherapy as well as immune checkpoint inhibitors in NSCLC patients demonstrate that this drug increases T cell infiltration, increases the ability of the tumor to stimulate anti-tumor immunity, and increases progression-free survival. However, although there are promising preclinical and encouraging early clinical data, it is important to address several issues before CXCR4-targeted therapies can become an integral part of lung cancer treatment. They include tumor heterogeneity, adaptive resistance mechanisms, as well as the complexity in the tumor microenvironment of CXCR4 signaling. Additionally, drug development strategies aimed at suppressing CXCR4-driven immune suppression and radioresistance must be combined with chemotherapy, radiotherapy, and immunotherapy therapies to maximize therapeutic benefits. Imaging of CXCR4 with specific PET and the selection of patients on CXCR4 biomarker criteria offer the possibility of further improving precision medicine approaches so that CXCR4-targeted therapies will only be given to the most CXCR4-responsive patients. The role of CXCR4 in lung cancer pathogenesis and development is critically reviewed, the most recent results on plerixafor inhibition of CXCR4 are summarized, and new, potential strategies for combination treatment of CXCR4 with other inhibitors are explored.
1 Introduction
Despite a significant decrease in mortality from this disease, lung cancer continues to be the primary cause of cancer-related death worldwide, recording approximately 2.2 million new cases and 1.9 million deaths annually (Sharma, 2022). Despite these recent advancements in targeted therapy, immunotherapy, and chemotherapy, there is still high resistance to lung cancer therapeutics (Saha et al., 2024), and survival is dramatically compromised by the development of adaptive ways for the tumor to progress and metastasize (Li C. et al., 2023; Kuang et al., 2024). Non-small cell lung cancer (NSCLC), which comprises 85% of lung cancer cases (Wang N. et al., 2023), has a 5-year survival rate < 20% and small cell lung cancer (SCLC) which is a more aggressive subtype is less fortunate with its poor prognosis because it is rapidly disseminated via early metastatic spread (Megyesfalvi et al., 2023). Almost half of lung cancer cases are identified at advanced stages, at which point curative intervention is limited and metastasis is the predominant barrier to long-term survival (Altorki et al., 2019). Metastatic sites are most common in the brain, liver, bones, and adrenal glands, and median survival for individuals with metastatic lung cancer is typically less than 1 year, even with treatment (Pass et al., 2012; Zhou et al., 2021). Despite previous clinical benefits in immune checkpoint inhibitors (ICIs) and tyrosine kinase inhibitors (TKIs) and a high incidence of disease progression despite therapy, there is an urgent need for novel therapeutics directed towards metastatic pathways (Gravina et al., 2024).
The CXCR4/CXCL12 chemokine signaling is among the most well-characterized mechanisms whereby tumors undergo metastasis and therefore facilitate increased migration, immune evasion, and treatment resistance (Morein et al., 2020; Britton et al., 2021). CXCR4, a GPC receptor, binds its ligand CXCL12 (stromal cell-derived factor 1, SDF1), which is highly expressed in metastatic target organs (Hang, 2020). In physiological conditions, CXCR4 controls immune cell trafficking, stem cell homing, and tissue repair (Miller et al., 2008). However, in lung cancer, CXCR4 overexpression facilitates tumor cells to migrate along chemotactic gradients of its ligand CXCL12 (stromal cell-derived factor 1, SDF-1) toward CXCL12-enriched metastatic niches, where tumor adhesion, survival, and immune escape are enhanced (Chandra et al., 2021). Stimulation of the CXCR4-CXCL12 cascade activates oncogenic pathways (MAPK/ERK, PI3K/AKT, PLCβ/Ca2+, and SRC/FAK) that promote epithelial-to-mesenchymal transition (EMT), angiogenesis, and are central to therapy resistance (Wang et al., 2021; Yang et al., 2023). This cascade allows cancer cells to detach from the primary tumor, circulate via the bloodstream, and colonize in distant organs where these tumor cells can establish themselves into a secondary tumor (Panda et al., 2016; Mir et al., 2021). Furthermore, CXCR4 helps in immunosuppression by driving regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophage (TAMs) into the tumor microenvironment (TME) along with each other to create an immunosuppressive TME (Hussain et al., 2025) and at the same time neutralize chemotherapy, radiotherapy, and ICIs therapies (Barnestein et al., 2022; Wu et al., 2025). Since CXCR4 is pivotal in the advancement of lung cancer, focusing on this cascade is an ideal treatment strategy (Yang et al., 2020). Preventing tumor cell migration, limiting metastatic burden, and enhancing anti-tumor immune activities to overcome resistance to standard therapies (Peng et al., 2025), blocking CXCR4 signaling is the way (Eckert et al., 2018). The small molecule antagonists, monoclonal antibodies, and peptide inhibitors for CXCR4 have been explored for their ability to inhibit CXCR4 (Zhou et al., 2019). Currently, there existing lung cancer treatments mainly target inhibiting the primary tumor progression growth and managing the micrometastases (Wood et al., 2014). Despite that, none directly attack CXCR4-driven metastatic cascade (Ko et al., 2021).
On the preclinical level, CXCR4 inhibition has been demonstrated to significantly reduce lung tumor growth, increase chemotherapy and radiotherapy sensitivity, and also to improve immune infiltration in the TME (D’Alterio et al., 2019). Furthermore, it has been noted that pharmacological inhibition of CXCR4 also potentiates the effect of ICIs, including anti-PD-1 and PD-L1, in reversing tumor-induced immunosuppression (Park et al., 2022). There are several inhibitors of CXCR4 under investigation (Fujiwara et al., 2020). Plerixafor was originally developed to mobilize hematopoietic stem cells and is also shown to exert an anti-metastatic and immunomodulatory effect in preclinical lung cancer models (Regan, 2017). Other CXCR4 antagonists, balixafortide, mavorixafor, and LY2510924 are being evaluated to enhance combination therapies (Leo and Sabatino, 2022). In contrast to standard epidermal growth factor receptor (EGFR)-TKIs and chemotherapy, which mainly inhibit primary tumor growth, CXCR4 inhibitors exclusively prevent metastatic seeding, escape from the immune system, and therapeutic resistance, which should be crucially incorporated into current lung cancer treatment regimens (Otsuka, 2011; Alqudah et al., 2025). In a study, it was found that lung cancer can be made more sensitive to drugs with the help of blocking CXCR4 (Su et al., 2005). Eventually, EGFR inhibitors or chemotherapy fail in most NSCLC patients, and tumors can evolve resistance and start increasing in number again (Choi et al., 2012). Nevertheless, blockade of CXCR4 has been shown to inhibit these adaptive resistance pathways and thus could prolong the therapeutic benefit of the standard treatments (Chaudary et al., 2021). For the clinical benefits of CXCR4 targeted therapy in lung cancer management, the patient selection strategies, combination therapy approaches, and optimized drug formulations will be moving forward (Shojaei et al., 2024). Clinical efficacy, safety profile, and optimal treatment strategies of CXCR4 inhibitors in monotherapy and combination with chemotherapy, immunotherapy, and radiotherapy are being continuously evaluated as monotherapy and in clinical trials as combination therapy (Sabir et al., 2021; Alexandru et al., 2024). In this review, we explore the function of the CXCR4/CXCL12 axis in lung cancer metastasis and its therapeutic potential for stopping tumor progression and strengthening cancer treatment.
2 CXCR4/CXCL12 axis in lung cancer
2.1 Role of CXCR4 in tumor growth and metastasis
The development of these effector functions of lung cancer has been linked to the function of the CXCR4/CXCL12 axis (Mezzapelle et al., 2022). The chemokine CXCL12, which is highly expressed in metastatic niches, including the bone marrow, liver, and the brain, has its receptor CXCR4 that engages in a signaling cascade that stimulates tumor proliferation, survival, and resistance to apoptosis through PI3K/AKT, MAPK/ERK, and JAK/STAT pathways (Mousavi, 2020). The CXCR4/CXCL12 axis increases metastasis in lung cancer by mediating chemotaxis of CXCR4+ tumor cells against CXCL12-rich secondary sites (bone marrow, liver, brain), which triggers PI3K/AKT, MAPK/ERK, and JAK/STAT signaling and EMT, invasion, and intravasation/extravasation, and establishes an immunosuppressive TME through Tregs, MDSCs, and TAMs recruitment (Wang et al., 2016). Furthermore, the recruitment of these to a TME via CXCR4 further contributes to systemic immunosuppression through the release of cytokines, like IL-10 and TGF-β, which prevents cytotoxic T cell killing capacity (Nengroo et al., 2022b). This axis plays a functional role in every stage of the metastatic cascade, including local invasion and vascular dissemination and colonization and outgrowth at the new organs, as well as in resistance to therapy during chemotherapy, radiotherapy, and immune checkpoint blockade.
Elevated CXCR4 expression in lung cancer has been correlated with enhanced tumor aggressiveness as well as a poor prognosis in tandem with both enhanced therapy resistance to chemotherapy and ICI therapy (Tajaldini et al., 2023). CXCR4-expressing lung cancer cells are more metastatic because the receptor promotes chemotactic migration towards CXCL12-enriched sites, allowing invasion, extravasation, and colonization (Sun et al., 2010). In addition, CXCR4 signaling helps to bring about the critical EMT process, which promotes gaining stem-like characteristics, the ability to be more motile, and greater resistance to being killed by apoptosis, leading to more aggressive and efficient metastasis (Sabbah et al., 2008; Nantajit et al., 2015).
The importance of CXCR4 to metastasis is also potentiated by its contribution to formulating the TME into an immunoevasive environment (López-Gil et al., 2021; Wang Z. B. et al., 2024). CXCR4 signaling recruits TAMs, MDSCs, and Tregs, collectively suppressing anti-tumor immunity and promoting therapy resistance (Kohli et al., 2022). Liu et al. demonstrated that while CXCR4 is expressed in both normal and tumor lung tissues, CXCR7 (presently ACKR3), a functionally related receptor, is exclusively upregulated in tumors, further promoting migration, invasion, and metastasis to the liver and bone marrow (Liu et al., 2020). This indicates that CXCR7 may act as an alternative driver of metastasis, potentially compensating for CXCR4 inhibition. Similarly, Xing et al. reported that lidocaine, a local anesthetic, inhibits CXCR4-mediated migration in NSCLC by disrupting CXCL12-induced cytoskeletal remodeling, reducing intracellular Ca2+ release, and altering actin polymerization (Xing et al., 2022). These findings suggest that targeting CXCR4 may not only disrupt metastasis but also create new therapeutic opportunities using repurposed drugs. The therapeutic targeting of CXCR4 is of great interest since this axis plays a key part in the development of lung cancer. Inhibiting the tumor cell’s movement towards the CXCR4 prevents tumor cell migration and immune suppression and promotes the enhancement of the efficacy of chemotherapy and immunotherapy. Despite these challenges, we nonetheless face therapy resistance, compensatory signaling via CXCR7, and potential off-target effects.
CXCL12 plays a nuclear role in tumor-intrinsic EMT, invasion, survivability, and metastatic seeding mediated by tumor intrinsic CXCR4 signaling (through PI3K/AKT, MAPK/ERK, JAK/STAT) as well as CXCL12 chemotaxis induced by microenvironmental CXCL12 in stromal and endothelial cells. In combination, these intrinsic and extrinsic inputs coordinatively organize the entire metastatic cascade and therapy resistance of lung cancer.
2.2 CXCR4 and the tumor microenvironment (TME)
Interaction between cancerous cells, stromal components, and immune cells in the lung cancer TME requires the key regulator of this process, CXCR4 (Santagata et al., 2021). Furthermore, fibroblast activation induced by CXCR4 induces a desmoplastic reaction with dense stromal barriers to suppress immune infiltration and drug penetration, which is correlated with low response to chemotherapy, radiotherapy, and immunotherapy (Monteran and Erez, 2019; Li X. P. et al., 2023). For the step-wise metastatic mechanism driven by CXCR4/CXCL12 in lung cancer, see Section 2.1.
CXCR4 expression in lung cancer has been related to the enrichment of lung CSC with a capacity for self-renewal, metastasis, and resistance to treatment. Moreover, hypoxic conditions in TME stimulate tumor aggressiveness driven by CXCR4, due to hypoxia-inducible factor 1 alpha (HIF1α) stabilization of CXCR4 expression and tumor cell survival in nutrient deprivations (Miranda-Galvis and Teng, 2020; Emami Nejad et al., 2021). Investigations have shown the critical function of CXCR4 in these processes, with Jäger et al. reporting that CXCR4-overexpressing NSCLC cells exhibit increased tumorsphere formation and EMT, partially mediated by macrophage migration inhibitory factor (MIF) and IL-6 signaling, which drive tumor progression and enhance stromal support (Jäger et al., 2020). Similarly, Andtbacka et al. showed that mavorixafor, a CXCR4 inhibitor, enhances immune infiltration in tumors by increasing antigen presentation, CD8+ T-cell activity, and IFN-γ expression, with combination therapy improving responses to ICIs in solid tumors, including NSCLC (Andtbacka et al., 2022). These outcomes suggest that CXCR4-targeted therapies not only disrupt cancer cell migration and invasion but may also enhance immune-mediated tumor clearance, particularly in combination with immunotherapy. The practical application of targeting this pathway lies in its central role in shaping an immunosuppressive and therapy-resistant TME that makes overcoming resistance mechanisms and increasing therapeutic efficacy a very promising approach. Nevertheless, owing to the complexity of CXCR4 interaction in the TME, combinatorial treatment of CXCR4 with chemotherapy, immunotherapy, or stromal targeting agents is required to achieve maximum therapeutic effect for lung cancer patients.
The CXCR4/CXCL12 axis also communicates with immune checkpoints (from the mobilization of myeloid/lymphoid cells and predictability of immune resistance to immunoprotective T-cell exclusion) and also with hematopoietic trafficking, in which CXCR4 blockade mobilizes myeloid/lymphoid cells but may not disrupt pulmonary host-defense dynamics, which is pertinent both in combination with ICIs and safety monitoring during on-treatment.
2.3 Clinical significance of CXCR4 expression in lung cancer
CXCR4 overexpression correlates with poor prognosis, greater metastatic potential, and resistance to therapy in NSCLC and SCLC (Zhang et al., 2015). Mechanistic underpinnings of CXCR4-driven metastasis are summarized in Section 2.1; here, we focus on its prognostic and biomarker implications and on patient selection.
CXCR4 expression is upregulated in NSCLC (Su et al., 2005) and reported in SCLC models/cohorts, with higher levels in advanced/metastatic disease. Individuals with CXCR4 overexpression have reduced overall survival (OS) and disease-free survival (DFS), and it seems that the high CXCR4 expression has a negative influence, especially in stage III and IV lung cancer, correlated with metastasis to the brain, liver, and bone (Franco et al., 2012; Pulido et al., 2017). Moreover, CTCs positive for CXCR4 are also associated with a poorer prognosis and are a predictive and prognostic biomarker. Non-invasive detection of tumors expressing CXCR4 has been accomplished by imaging modalities using radiolabelled antagonists of CXCR4 (Oriuchi et al., 2020). Lakhanpal et al. demonstrated the potential of 68Ga-plerixafor PET/CT for visualizing CXCR4-expressing malignancies, with a strong correlation between PET signal and 18F-FDG uptake, confirming its diagnostic utility (Lakhanpal et al., 2023). Similarly, Dreher et al. showed that [68Ga]Ga-Pentixafor PET/CT imaging detected CXCR4 overexpression in SCLC and other solid tumors, suggesting its role in guiding targeted therapy selection for patients with CXCR4-enriched tumors (Dreher et al., 2024).
Beyond its prognostic and diagnostic uses, the increase of CXCR4 expression is a critical driver of therapy resistance by regulating DNA damage repair, cancer stemness, and immune evasion, all of which impair response to chemotherapy, radiotherapy, and ICIs. Many strategies have been explored blocking CXCR4 signaling to improve chemoresponsive and immunotherapeutic responses, and there have been promising results from different preclinical and clinical studies (Gibson and Davids, 2015; Wang S. et al., 2024). Weiss et al. investigated 64Cu-AMD3100, a radiolabelled CXCR4 antagonist, and demonstrated high tumor selectivity and strong tumor-to-muscle and tumor-to-blood ratios, making it a viable option for non-invasive quantification of CXCR4 expression to inform treatment decisions (Weiss et al., 2012). In a similar approach, Azad et al. evaluated 89Zr-labeled CXCR4 monoclonal antibody (89Zr-CXCR4-mAb) imaging in NSCLC models and revealed enhanced PET uptake in tumors with high CXCR4 expression, with therapeutic responses correlating with CXCR4 levels, supporting its role in precision medicine approaches for lung cancer (Azad et al., 2016). Therefore, a growing interest in the therapeutic and biomarker utility of CXCR4 for patient selection in lung cancer is motivated by the strong evidence that implicates it in lung cancer progression and therapy resistance. Therefore, integration of histology-aware CXCR4 imaging and biomarker strategies may enable more precise treatment strategies to not only guide clinicians to identify patients with the highest chances of response to CXCR4-directed therapies such as plerixafor, balixafortide, and motixafortide but also address treatment resistance through targeted combination approaches.
The expression of CXCR4 is different across lesions and over time in patients and in the same patient. Hypoxia/HIF-1α can upregulate CXCR4 in regional niches, CXCR7 can overcome CXCR4 blockade in relationship tumors and add biological variation, which can quench local reactions. Based on this, lesion-level measurement by CXCR4-targeted PET and/or tissue biomarkers may aid in identifying patients with CXCR4-high disease and inform response-adaptive treatment.
Clinically-trial CXCR4 assays should be aforementioned with regarding clinical-trial (PET) standards, state acquisition/reconstruction; in tissue IHC state clone, scoring method, and inter-observer validations, on blood assays include platform and repeatability. Cutoffs of eligibility must be set, such as lesion level uptake of PET above some reference level or a top quantile cutoff, and/or tissue CXCR4 levels above a prespecified score; heterogeneity between sites may be treated by the requirement that there be at least one target lesion that satisfies PET requirements, and the variance of locations is obviated by recording. PET conventions and/or other biomarker dynamics can be used as a basis for on-treatment assessment that can provide response-adaptive therapy. The following steps operationalize selection and accept non-homogeneous CXCR4 expression as earlier explained.
3 Plerixafor: a CXCR4 antagonist in lung cancer therapy
3.1 Mechanism of action
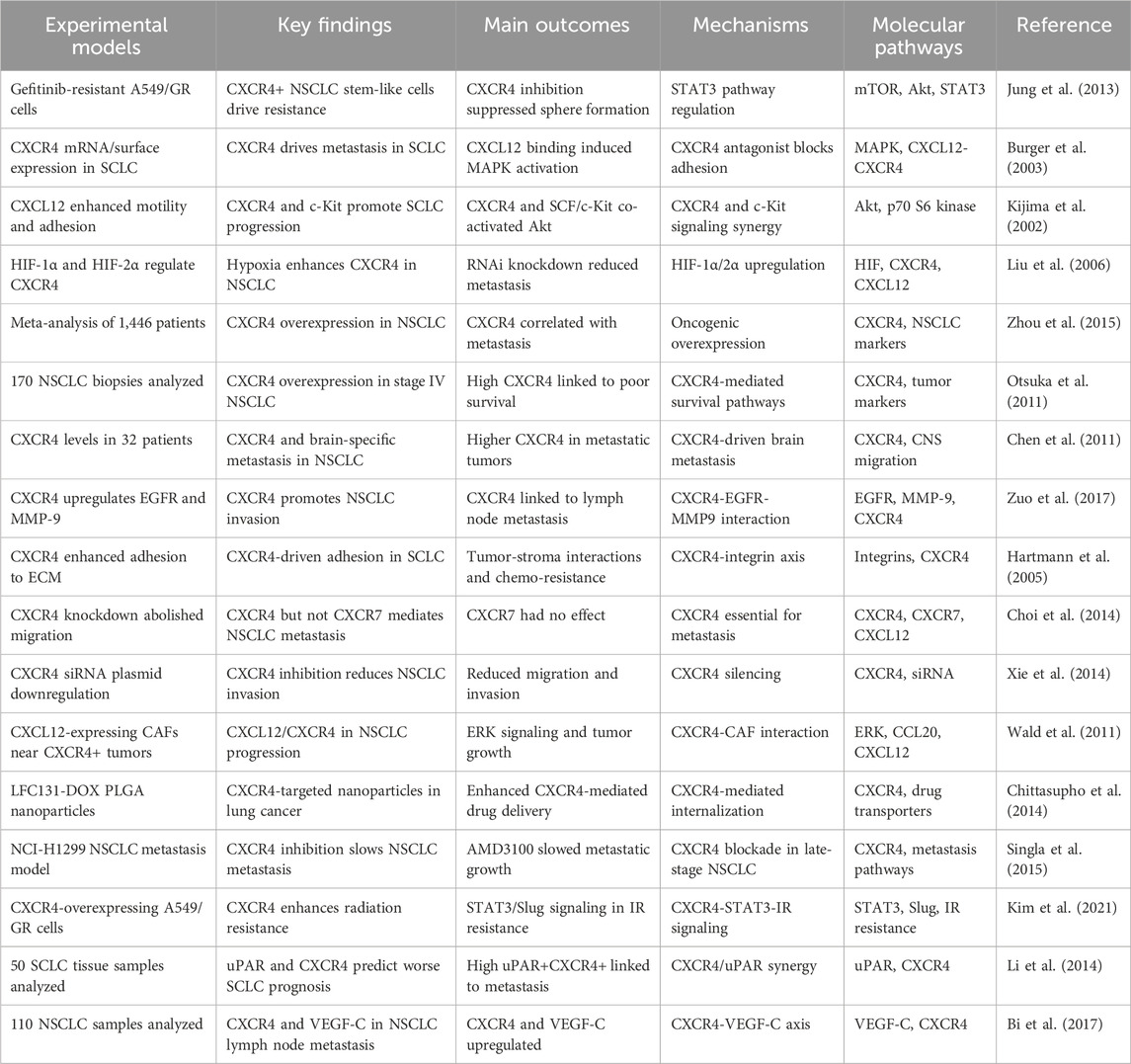
AMD3100 (plerixafor), a selective CXCR4 antagonist and disrupts the CXCR4/CXCL12 signaling and has shown antitumor activity in preclinical lung cancer models (Wang et al., 2016). CXCR4 receptor binds its ligand, CXCL12 (SDF-1), which is overexpressed in lung cancer cells, and the interaction of this receptor-ligand drives tumor proliferation, migration, and therapy resistance (Cojoc et al., 2013). Plerixafor prevents key oncogenic processes such as cancer cell mobilization, TME interactions, and immune suppression by blocking CXCR4 binding to CXCL12 (Zhao et al., 2022). Plerixafor reduces migration and metastatic seeding in preclinical systems by blocking CXCR4 (Wang et al., 2014). CXCL12 is additionally secreted by stromal fibroblasts, endothelial cells, and immune cells in lung cancer in such amounts that they form chemotactic gradients drawing CXCR4-expressing cancer cells to metastatic niches such as liver, brain, and bone marrow (Mir et al., 2023; Thapa et al., 2023). Concerning cancer metastasis, plerixafor blocks this chemotactic signaling, which reduces the chance that cancer cells will home to distant organs (Nasrollahzadeh et al., 2020; Lalić et al., 2024). Figure 1 illustrates CXCR4 signaling, its regulation by plerixafor and Dasatinib, and its role in cell survival. Furthermore, plerixafor interferes with the tumor stroma interactions that are known to be responsible for resistance to therapy (Eulberg et al., 2022). It is known that CXCR4 allows cancer stem cell (CSC) survival and immune evasion within the TME (Dzobo et al., 2020).
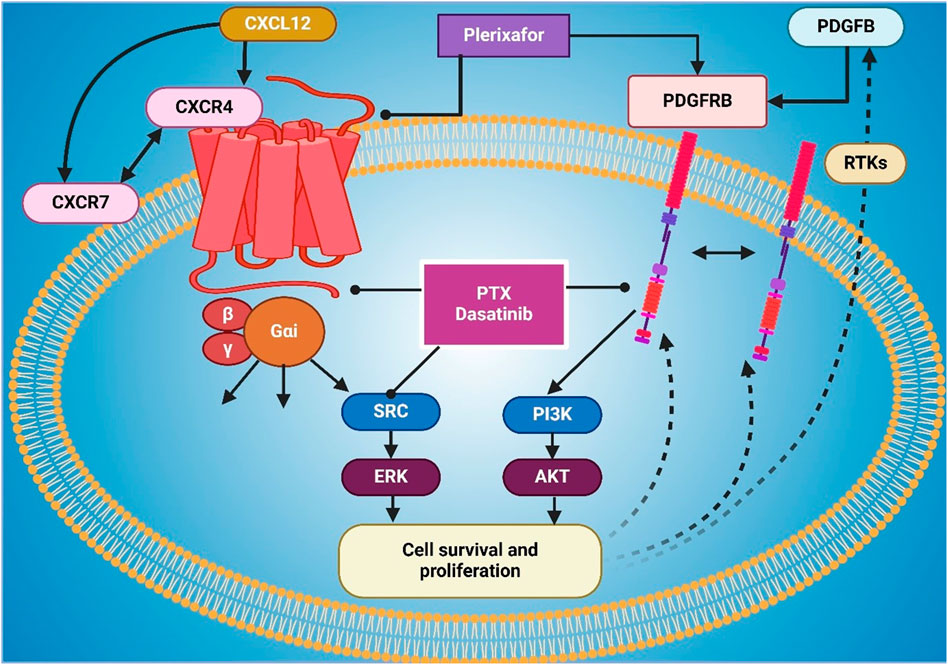
Figure 1. The figure illustrates the CXCR4 signaling pathway and its role in cell survival and proliferation, highlighting the impact of inhibitors such as Plerixafor and Dasatinib. CXCL12 binds to CXCR4, activating downstream signaling through Gαi proteins, which subsequently stimulate SRC-ERK and PI3K-AKT pathways, promoting cell survival and proliferation. CXCR7 also contributes to CXCR4 activation. Plerixafor inhibits CXCR4 by blocking CXCL12 binding, while Dasatinib and PTX disrupt SRC and PI3K signaling. Additionally, PDGFB activates PDGFRB and receptor tyrosine kinases (RTKs), which interact with CXCR4 signaling to enhance survival pathways.
When used in combination, plerixafor decreases CSC self-renewal and increases sensitivity to chemotherapy and radiotherapy in CSC by blocking CXCR4 (Huang et al., 2019). Additionally, it inhibits the recruitment of immunosuppressive cells to the TME and contributes to immune evasion via an immune-evasive TME (Shao et al., 2022). It potentiates anti-tumor immune response and increases tumor responsiveness to ICIs (Zhang et al., 2022). Finally, plerixafor has been noted to sensitize lung cancer cells to chemotherapy and radiotherapy in the setting of decreased DNA damage repair and survival signaling (Sun, 2016). Plerixafor and cisplatin killed lung cancer cells more and caused reduced tumor growth in preclinical studies (Langhammer, 2013). Furthermore, plerixafor decreases HIF1α, a downstream key force in cancer adaptation to hypoxic conditions, and decreases cancer cell survival (Mortezaee, 2020). The mechanism of action of plerixafor is the inhibition of metastasis by abrogating CXCR4-CXCL12 interactions, the disruption of the immunosuppressive TME, preserving chemosensitivity, and fighting therapy resistance (Russo and Nastasi, 2022). Given these multifaceted effects, a combination of PTG in standard chemotherapy, radiotherapy, and immunotherapy is a promising therapeutic agent for lung cancer (Wang H. et al., 2023), as shown in Figure 1.
3.2 Preclinical and clinical studies on plerixafor
Extensive preclinical lung cancer studies have been performed on plerixafor, including inhibiting tumor growth, reducing metastasis, and having synergy with chemotherapy and immunotherapy (Steeg, 2016). Plerixafor exerts significant tumor burden reduction and metastatic spread inhibition to the brain, liver, and bone marrow, common sites of NSCLC dissemination, in murine models of NSCLC (Mishan et al., 2016; Yang et al., 2023). Studies of plerixafor treatment on CXCR4 overexpressing NSCLC cell lines have demonstrated reduced cell migration, inhibited invasion, and enhanced apoptosis, especially in hypoxic conditions where CXCR4 expression is increased (Li C. et al., 2022). Li and Oupicky, investigated biodegradable polymeric plerixafor (PAMD) and found that biodegradable PAMD significantly inhibited cancer invasion and metastasis in vivo, suggesting potential as an optimized CXCR4-targeting strategy (Li and Oupický, 2014; Guo et al., 2025).
In combination with chemotherapy, plerixafor also disrupts tumor-stroma interactions and reduces CSC survival in further studies. CXCR4 inhibition is also shown to increase tumor-infiltrating T cells and decrease immunosuppressive MDSCs in preclinical findings, and results in sensitization of tumors to the chemotherapy agents of cisplatin, paclitaxel, and gemcitabine (Xun et al., 2020). Additionally, Plerixafor has proved able to overcome radiation resistance through the inhibition of CXCR4-induced DNA damage repair pathways, leading to more radiosensitive tumors (Eckert et al., 2018). Ko et al. demonstrated that plerixafor-functionalized nanomaterials significantly improved drug delivery to CXCR4-overexpressing tumors, with enhanced tumor accumulation and photothermal anticancer efficacy, suggesting potential applications in CXCR4-targeted nanotherapy (Ko et al., 2018).
Clinical trial research with Plerixafor has been encouraged by preclinical data, and studies of plerixafor’s potential in treating lung cancer have focused on the utilization of plerixafor in combination with chemotherapy and immunotherapy (Bao et al., 2023). Plerixafor has an acceptable safety profile when administered as an early therapy and was found to be capable of mobilizing tumor cells from the bioprotected niches in the bone marrow as well as the TME at a site that is known to limit their vulnerability to treatment (Morland et al., 2020). Among other things, a Phase I trial of plerixafor with chemotherapy in advanced NSCLC was able to show improved response rates as well as prolonged DFS in patients with high CXCR4-expressing tumors (Nengroo et al., 2022a). Further, trial data combining plerixafor with ICIs shows evidence of CXCR4 inhibition improving T cell infiltration, leading to increased immune activation and thus, the immune response rate to treatment in NSCLC (Zhu et al., 2023). Weiss et al. utilized PET imaging with 64Cu-plerixafor to examine CXCR4 expression in solid tumors, showing high CXCR4 levels in metastatic lung adenocarcinomas, further supporting the use of CXCR4-directed therapies in aggressive tumors (Weiss et al., 2017).
Additional research has explored alternative applications of Plerixafor beyond lung cancer, particularly in hematopoietic cell mobilization and fibrosis treatment (MacLean et al., 2024). Pillay et al. investigated plerixafor’s effects on neutrophil mobilization, demonstrating that while it increases circulating neutrophils, it does not impair lung neutrophil dynamics, suggesting minimal impact on respiratory host defense (Pillay et al., 2020). Similarly, Qi et al. analyzed single-cell RNA sequencing data in fibrosis models, showing that plerixafor-mediated CXCR4 inhibition significantly reduced fibrosis progression, highlighting its potential for drug repurposing in fibrosis management (Qi et al., 2024). Devi et al. further investigated CXCR4–CXCL12 signaling in neutrophil homeostasis, revealing that plerixafor enhances circulating neutrophils by preventing their return to the bone marrow, providing insight into its role in neutropenia management (Devi et al., 2013). Furthermore, Lakhanpal et al. optimized radiolabeled plerixafor (plerixafor-DTPA, plerixafor-NOTA) with 68Ga and 177Lu for PET/CT imaging and targeted therapy, showing that 68Ga-plerixafor PET/CT successfully identified CXCR4-expressing lung lesions, correlating with 18F-FDG uptake, while 177Lu-plerixafor exhibited high CXCR4 binding affinity and cytotoxicity in lung cancer cells, emphasizing its potential as a theranostic agent (Lakhanpal et al., 2022). In contrast, Blayney et al. evaluated Plinabulin as an alternative mobilizing agent, finding that it effectively mobilizes CD34+ hematopoietic cells without significantly inhibiting CXCR4, making it a viable option for NSCLC patients unresponsive to G-CSF-based mobilization (Blayney et al., 2018). Plerixafor, as a CXCR4 targeted treatment for lung cancer, is well supported by preclinical or early clinical evidence as a potential therapeutics for lung cancer in combination with chemotherapy, radiotherapy, and immunotherapy. Thus, it is a promising agent for lung cancer therapy because of its ability to enhance immune response, suppress therapy resistance, and accelerate targeted drug delivery. Further trials will confirm its clinical efficacy and long-term therapeutic potential and establish the way for widespread clinical adoption in CXCR4-driven malignancies.
3.3 Plerixafor in combination therapy
Plerixafor, as a CXCR4 antagonist, has significantly more efficacy in mobilizing and maintaining CD34+ cells and cells for cancer immunotherapy, chemotherapy, and radiotherapy when exacerbated with any standard cancer treatment, including chemotherapy, immunotherapy, radiotherapy, or a combination of all (Crees et al., 2023). Plerixafor blocks CXCR4 and enables tumor cells to be mobilized from protective niches like the bone marrow and hypoxic tumor regions and making them more susceptible to therapeutic agents (Cancilla et al., 2020). Across preclinical models and early human signals in small cohorts, adding plerixafor to chemotherapy has been associated with greater tumor control and immune infiltration; however, definitive clinical benefit in lung cancer has not been established (Chaudary et al., 2024; Thapa et al., 2024). Figure 2 shows CXCL12-CXCR4/CXCR7 signaling, activating AKT and NF-κB to drive proliferation and metastasis. To aid navigation, key plerixafor-based combination strategies and outcomes are summarized in Table 1.
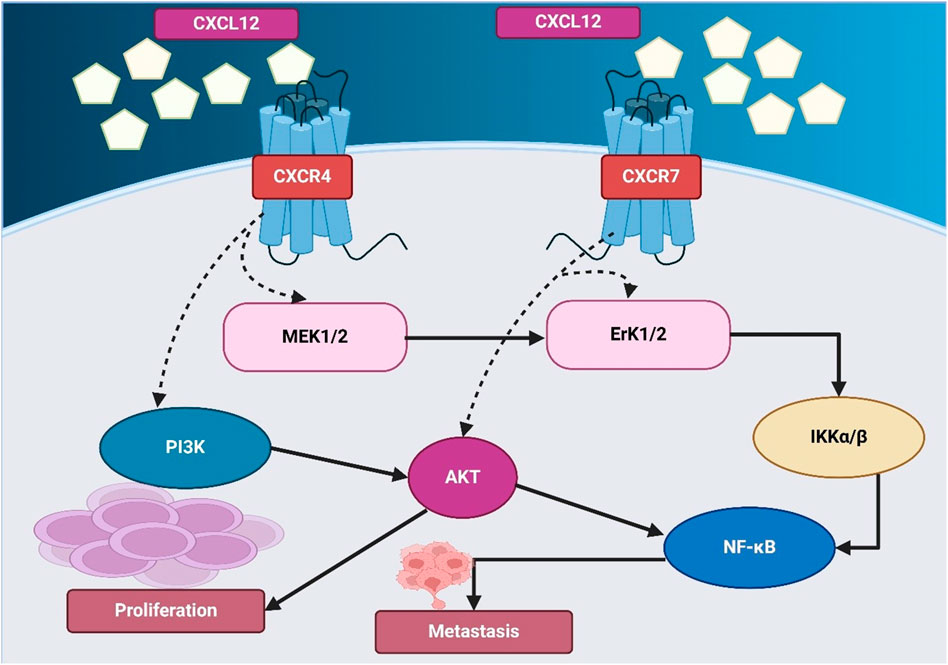
Figure 2. The figure illustrates the CXCL12-mediated signaling pathways through CXCR4 and CXCR7, driving proliferation and metastasis. CXCL12 binds to CXCR4 and CXCR7, activating downstream signaling cascades. CXCR4 signaling involves MEK1/2 and PI3K, leading to AKT activation, which promotes cell proliferation. CXCR7 signaling activates ERK1/2, which in turn stimulates IKKα/β and NF-κB, contributing to metastasis. AKT also directly regulates NF-κB, further enhancing metastatic progression.
Table 1. Summary of plerixafor-based combination strategies in lung cancer models and translational studies.
These liabilities can be countered by adaptive pathways (CXCR7 compensation, STAT3 activation, stromal re-engagement), which tailored combinations, can counteract: chemo/RT (to take advantage of tumor-cell mobilization after transiting protective niches), ICI (to overcome antibodies’s immune homeostasis based on CXCR4), and anti-angiogenic/vascular-disrupting (to suppress CXCL12/CXCR4 rebound of therapy). This framework connects axis biology to rational partnering and sequencing.
Chemoresistance is dictated in significant part by the high activity of CXCR4 in the chemo protection of cancer cells within stromal niches (López-Gil et al., 2021). In lung cancer mouse models, co-administration of plerixafor with cisplatin, paclitaxel, and gemcitabine reduced tumor burden and metastasis vs. chemotherapy alone (Li H. et al., 2021). Jiang et al. showed that CXCR4 expression increases following treatment with vascular-disrupting therapy increases CXCR4 signaling. Combining plerixafor + combretastatin A4 nanodrug produced marked tumor growth inhibition (≈91%) and fewer lung metastases in mice (Jiang et al., 2019). Similarly, Fahham et al. evaluated BKT140, a novel CXCR4 antagonist, in NSCLC cell lines and found that it inhibited proliferation, reduced colony formation, and delayed tumor growth in xenograft models, while also enhancing chemotherapy and radiotherapy responses, demonstrating its potential in combination therapy (Fahham et al., 2012). Panneerselvam et al. further explored the SDF-1/CXCR4 cascade and found that interleukin-24 (IL-24) can inhibit CXCR4 signaling, destabilizing CXCR4 mRNA and reducing downstream AKT, mTOR, and HIF-1α activation, suggesting that IL-24 in combination with CXCR4 antagonists enhances anti-metastatic effects in lung cancer (Panneerselvam et al., 2015).
Beyond its role in chemotherapy, plerixafor has shown promise in overcoming immunotherapy resistance by enhancing T-cell infiltration and reversing the immune-excluded phenotype of tumors. CXCR4-mediated signaling in lung cancer contributes to T-cell exclusion, immune evasion, and recruitment of immunosuppressive cells, which suppress anti-tumor immunity (Martin, 2024). Li et al. developed FX@HP, a CXCR4-inhibiting nanocomplex, which enhanced PD-L1 therapy efficacy by enhancing T-cell infiltration and reducing immunosuppressive cells, indicating that CXCR4 inhibition can remodel the TME to optimize ICI responses (Li et al., 2020). In a related study, Cao et al. revealed that CXCR4 is overexpressed in exhausted CD8+PD-1high T cells, limiting immune response, and demonstrated that blocking CXCR4 restored T-cell function through JAK2-STAT3 inhibition, reinforcing the role of CXCR4 blockade in enhancing immunotherapy (Cao et al., 2024). Similarly, Fortunato et al. identified a subset of CD133+CXCR4+ metastasis-initiating cells (MICs) that contribute to immune suppression and found that Peptide R, a novel CXCR4 inhibitor, effectively reduced MIC dissemination, restored T-cell cytotoxicity, and limited TAM polarization, further supporting the therapeutic potential of CXCR4 blockade in combination with immunotherapy (Cao et al., 2024). Early human evidence exists in other solid tumors, but no randomized data demonstrate added clinical benefit in lung cancer to date.
Radiotherapy remains a cornerstone of lung cancer treatment, yet CXCR4-mediated DNA damage repair mechanisms contribute to radioresistance, allowing tumor cells to survive and repopulate following radiation exposure. Preclinical studies report that plerixafor enhances radiosensitivity by inhibiting CXCR4-linked DNA repair (Eckert et al., 2019; Kim et al., 2021). D’Alterio et al. found that CXCR4 inhibition using plerixafor significantly reduced metastasis by blocking stromal cell recruitment and p38 MAPK activation, impairing tumor cell survival (D’Alterio et al., 2012). In another study, Li et al. developed FM@PFC nanoemulsions containing a CXCR4 antagonist and anti-STAT3 siRNA, showing that pulmonary delivery of these nanoemulsions reduced tumor invasion, angiogenesis, and immunosuppression while inducing apoptosis, demonstrating a novel approach to improving lung metastasis therapy (Li et al., 2019). Additionally, plerixafor has been explored in combination with multi-targeted regimens for therapy-resistant lung cancer (Kast et al., 2022). Gürgen et al. tested a low-dose combination regimen consisting of Etoricoxib, plerixafor, Afatinib, and Cabozantinib in NSCLC patient-derived xenograft (PDX) models, achieving an 81% overall response rate (ORR) and 100% clinical benefit rate (CBR), even in therapy-resistant adenocarcinomas and squamous cell carcinomas lacking targetable mutations, reinforcing the importance of CXCR4 inhibition in overcoming treatment resistance (Gürgen et al., 2022). Reinholdt et al. demonstrated that plerixafor enhances rituximab’s effectiveness in diffuse large B-cell lymphoma (DLBCL) by reducing CXCR4 expression and increasing tumor apoptosis, supporting the potential expansion of CXCR4-targeted therapies beyond lung cancer (Reinholdt et al., 2016). In general, the integration of plerixafor into multimodal treatment strategies has shown great significance potential in lung cancer therapy, especially in combination chemotherapy, immunotherapy, and radiotherapy. CXCR4 inhibition offers a compelling approach to increase lung cancer outcomes through sensitization to therapy, shift immune activation, and mobilization of cancer cells from protective niches as shown in Figure 2.
There is a limited collection of trials involving CXCR4 antagonists in lung cancer. Randomized Phase II 5 of LY2510924 administered as a peptide antagonist on carboplatin/etoposide in extensive disease SCLC (N = 90) failed to improve either PFS or OS compared to chemotherapy alone, but the safety was acceptable. There are a few other investigations that are pre-clinical or pre-imaging feasibility, using [68Ga]-based tracers or [64Cu]-plerixafor, and are hypothesis-generating and not claim-arming. The probable causes are insufficient biomarker-enriched selection, adaptive compensation, and incompetent scheduling as compared with transient cell mobilization. Contemporary designs predefining the thresholds of CXCR4-positivity and integrating plerixafor with chemo, RT/ICI are expected to fill these gaps.
4 The broader therapeutic potential of CXCR4 inhibition
4.1 CXCR4 as a target in lung cancer
Over the past years, the CXCR4/CXCL12 cascade has been a significant therapeutic target in lung cancer since it offers a major function in cancer development, metastasis, immunological evasion, and resistance to treatment (Wang et al., 2016; Wald, 2018). Having been shown to overexpress CXCR4, lung cancer is a very strong predictor of poor prognosis, increased metastatic potential, and decreased survival rates, making it a good candidate target for a treatment intervention (Spiro and Porter, 2002). It is through the activation of CXCR4 that the proliferation and survival of tumors are promoted, as well as invasion through oncogenic pathways (Habanjar et al., 2023). Liu et al. demonstrated that hypoxia enhances CXCR4-mediated metastasis via HIF-1α and HIF-2α activation, promoting cancer cell adhesion, movement, and invasion in response to CXCL12, indicating that targeting CXCR4 alongside hypoxia pathways may provide novel therapeutic strategies (Liu et al., 2006). Figure 3 shows CXCL12-CXCR4 signaling facilitating tumor development, metastasis, angiogenesis, inflammation, and immune cell recruitment.
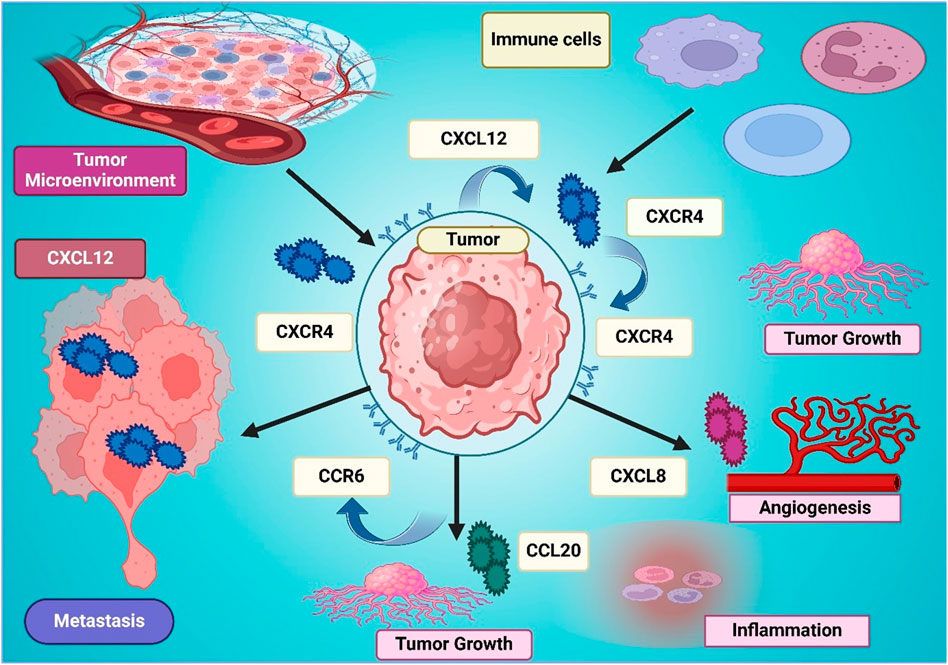
Figure 3. The figure illustrates the role of CXCL12-CXCR4 signaling in the tumor microenvironment, promoting tumor growth, metastasis, angiogenesis, and inflammation. CXCL12 secreted in the tumor microenvironment attracts immune cells and activates CXCR4 on tumor cells, enhancing their proliferation and invasion. CXCL8 and CCR6-CCL20 interactions further contribute to tumor progression by stimulating angiogenesis and inflammation. The interconnected signaling pathways create a supportive niche for tumor survival, emphasizing the significance of CXCR4 as a therapeutic target in cancer treatment.
CXCR4 is known to direct lung cancer cells toward CXCL12-rich metastatic sites such as the bone marrow, liver, and brain, fueling metastatic dissemination. Chen et al. found that CXCR4 expression is significantly higher in NSCLC brain metastases, correlating with poorer survival rates, supporting CXCR4 as a key driver of brain-specific metastasis (Chen et al., 2011). Similarly, CXCR4 enhances invasion and migration via EGFR/MMP-9 upregulation (Zuo et al., 2017). Burger et al. further highlighted the role of CXCR4 in SCLC metastasis; CXCR4 promotes invasion/adhesion to bone-marrow stroma; antagonists disrupt this mechanism (Burger et al., 2003).
Beyond metastasis, CXCR4 contributes to immune suppression within the TME. CXCR4 overexpression is correlated with reduced T-cell infiltration, increased MDSCs and Tregs, and resistance to ICIs (Li X. et al., 2021). Cao et al. observed that CXCR4 is overexpressed in exhausted CD8+PD-1high T cells, leading to immune dysfunction and therapy resistance, but CXCR4 inhibition restored T-cell function and enhanced response to ICIs, suggesting CXCR4 blockade as a strategy to improve immunotherapy efficacy (Cao et al., 2024). Wald et al. demonstrated that CXCL12-expressing cancer-associated fibroblasts (CAFs) facilitate cancer progression by supporting CXCR4+ cancer cell survival, reinforcing the significance of targeting the CXCR4/CXCL12 to disrupt tumor-stroma interactions (Wald et al., 2011). In addition to immune evasion, CXCR4 also contributes to chemoresistance and radioresistance by promoting DNA repair mechanisms and survival signaling in lung cancer cells, reducing the efficacy of conventional therapies (Césaire et al., 2022). Jung et al. demonstrated that CXCR4+ cancer stem-like cells drive therapy resistance in NSCLC and that CXCR4 inhibition (AMD3100, siRNA) suppressed sphere formation and tumorigenicity, suggesting that targeting CXCR4+ NSCLC stem-like cells could overcome drug resistance and enhance radiotherapy response (Jung et al., 2013). Similarly, Kim et al. found that CXCR4 enhances radiation resistance via STAT3/Slug signaling and that CXCR4 inhibition sensitized NSCLC cells to ionizing radiation, reinforcing its role in radiotherapy resistance mechanisms (Kim et al., 2021).
Several clinical studies have further validated CXCR4 as a biomarker for lung cancer prognosis. Zhou et al. conducted a meta-analysis of 1,446 NSCLC patients across 13 investigations, finding that CXCR4 expression was significantly associated with advanced-stage disease, metastasis, and reduced survival, reinforcing its role as a prognostic marker (Zhou et al., 2015). Similarly, Otsuka et al. analyzed 170 NSCLC biopsies and found that CXCR4 overexpression in stage IV NSCLC correlated with significantly worse survival, particularly in females, suggesting that CXCR4-targeted therapy may be especially beneficial in advanced lung cancer patients (Otsuka et al., 2011). The role of CXCR4 in lung cancer therapy extends beyond metastasis and resistance, with targeted approaches being actively explored (Ngamcherdtrakul and Yantasee, 2019). Choi et al. demonstrated that CXCR4, but not CXCR7, is essential for CXCL12-mediated metastasis, supporting CXCR4 inhibition as a strategy to prevent NSCLC progression (Choi et al., 2014). Singla et al. found that CXCR4 inhibition slowed metastatic tumor growth in an advanced NSCLC model, reinforcing the potential of CXCR4-targeted therapies in controlling late-stage disease (Singla et al., 2015). Hartmann et al. further identified that CXCR4-driven adhesion enhances chemotherapy resistance in SCLC by activating integrin signaling, suggesting that CXCR4 inhibition could prevent tumor-stroma interactions that contribute to residual disease and relapses (Hartmann et al., 2005).
In novel therapeutic approaches, CXCR4-targeted drug delivery strategies have shown promise in overcoming therapy resistance (Costa et al., 2019). Chittasupho et al. developed CXCR4-targeted nanoparticles to enhance doxorubicin delivery to lung cancer cells, finding that CXCR4-specific nanocarriers improved drug accumulation and therapeutic efficacy, demonstrating that CXCR4 inhibition could be used in nanotechnology-driven chemotherapy enhancements (Chittasupho et al., 2014). Li et al. investigated the co-expression of uPAR and CXCR4 in SCLC and found that uPAR+CXCR4+ tumors exhibited greater invasion, migration, and metastatic potential, reinforcing the importance of dual targeting strategies (Li et al., 2014). Similarly, Bi et al. found a strong correlation between lymph node metastasis in NSCLC and the expression of VEGF-C and CXCR4, further validating the role of CXCR4 as a predictive marker for disease progression and therapeutic response (Bi et al., 2017). Together, these studies have identified CXCR4 as a major player in driving lung cancer progression, metastasis, immune suppression, and chemo-resistant disease, and reinforce that it is a target worthy of precision medicine. The direct antagonists of CXCR4, combination therapies, and nanocarrier-based strategies of CXCR4 inhibition could offer great clinical advantages in improving lung cancer treatment as shown in Figure 4 and Table 2.
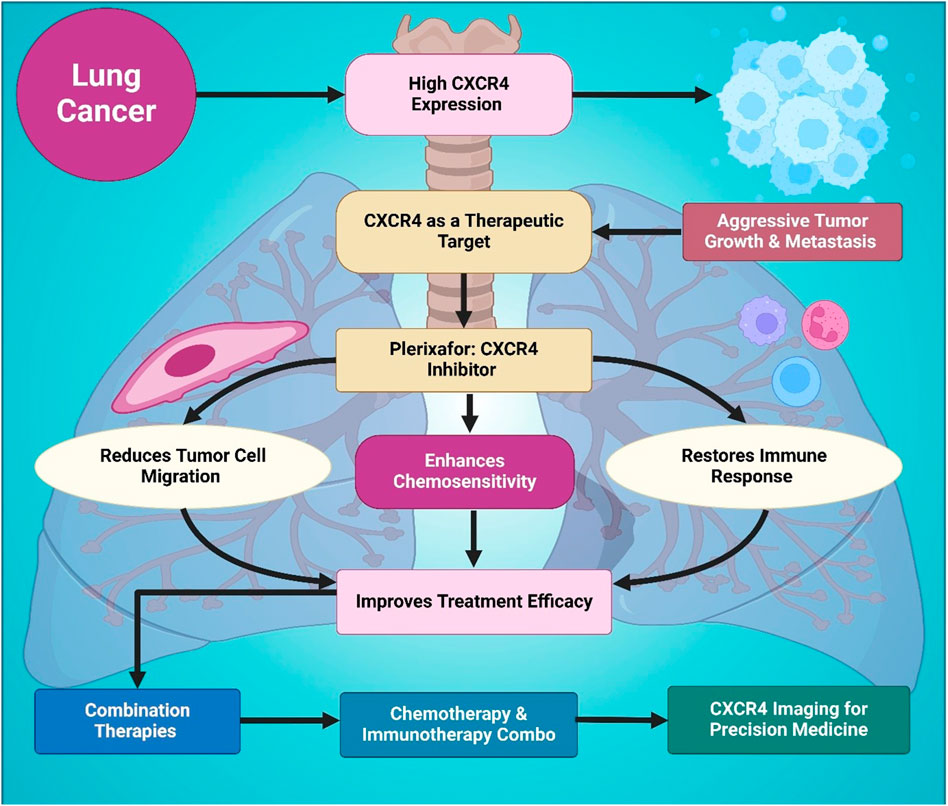
Figure 4. This figure outlines the role of CXCR4 in lung cancer progression and therapy. Elevated CXCR4 expression is associated with aggressive tumor growth and metastasis, making it an important therapeutic target. Usage of the CXCR4 inhibitor Plerixafor reduces tumor cell migration, restores immune function, and enhances chemosensitivity. These effects collectively lead to improved treatment efficacy when combined with chemotherapy or immunotherapy. It also highlights the relevance of CXCR4 imaging for advancing precision medicine in lung cancer management and overcoming therapeutic resistance.
5 Challenges and future perspectives
5.1 Limitations of CXCR4-Targeted therapy
Despite the promise of CXCR4-targeted therapies in lung cancer treatment, numerous obstacles and limitations must be overcome to enable such therapies to become a part of clinical practice (Ashrafi et al., 2022). Specificity and safety, mechanisms of resistance, and complexities of TME fall in this range of limitations (Khalaf et al., 2021). The major issue with CXCR4-targeted therapies is how to achieve specificity for tumor cells. However, CXCR4 is expressed widely in various normal tissues, notably bone marrow, spleen, and liver, and regulates the function of immune cell trafficking and healthy tissue homeostasis (Tilsed et al., 2022). Thus, the use of CXCR4 inhibitors may result in unwanted side effects, which include excessive immune cell depletion, tissue destruction, or bone marrow suppression, increasing the occurrence of infections or hematologic toxicity (Vasan et al., 2019). A major hurdle to the clinical development of selective CXCR4 antagonists is the need to find those that will target tumor cells without deleterious effects on normal tissues (Ho et al., 2020).
Like many targeted therapies, resistance to CXCR4 inhibitors can occur with time, and in particular, if the inhibitor is combined with other treatments (Roma-Rodrigues et al., 2019). If these chemokine receptors happen to be upregulated on the tumors, tumor cells can continue to migrate and metastasize, even if CXCR4 is inhibited (Kim et al., 2008). Besides, resistance to therapeutic agents results from CXCR4 or its downstream signaling pathway mutations (Korbecki et al., 2022). Further research on the mechanisms of resistance is needed to design strategies that will overcome or delay the resistance from CXCR4-targeted therapies to improve long-term efficacy (Alsayed et al., 2022). The determination of both the success of CXCR4 targeted therapy and the clinical significance of CXCR4 is determined by the TME (Yin et al., 2019). The TME containing extracellular matrix constituents, stromal cells, and immune cells can affect the efficacy of treatment (Kim et al., 2008). As Tregs and MDSCs are immunosuppressive cells that produce barriers to effective immune responses (He et al., 2019), CXCR4 inhibitor enhancement of anti-tumor immunity is restricted (Popper, 2016). In addition, the CXCR4 inhibitors may be partially constrained by the inability to access their targets in hypoxic conditions and chemoresistant niches within the TME, hence reducing their therapeutic capability (Kiefer and Siekmann, 2011).
5.2 CXCR4 in personalized medicine
CXCR4 is an important biomarker in lung cancer because of its important molecular marker and target for personalized medicine (Rosell et al., 2013). Therefore, CXCR4 targeted therapies are a promising approach to improve treatment outcomes with a benefit-to-risk ratio as high as possible. Personalized approaches that incorporate CXCR4 status into treatment decisions could enhance patient selection for targeted therapies (Chai et al., 2021; Nengroo et al., 2022a). Guo et al. have shown that CXCR4 overexpression correlated with poor prognosis in NSCLC but was also correlated with a higher response rate to immunotherapy, indicating that CXCR4 expression could serve as a predictive biomarker for immunotherapy efficacy (Guo et al., 2023). Yue et al. further supported this by identifying CXCR4, CXCR5, and CCR7 as prognostic biomarkers in early-stage NSCLC, where CXCR4 and CXCR5 were associated with worse five-year DFS and OS (Yue et al., 2020). This highlights the potential of CXCR4 expression profiling in guiding patient-specific treatment regimens (Nengroo et al., 2022a). Incorporating CXCR4 inhibitors such as plerixafor into combination treatment strategies has also been explored (Srivastava et al., 2022). Given the association between CXCR4 and immune evasion, Mao et al. found that B7-H1 and B7-H3 overexpression in NSCLC tumors correlated with CXCR4-driven immune suppression, while B7-H3 knockdown enhanced T-cell activation and reduced CXCR4 expression, indicating that CXCR4 blockade in combination with ICIs could improve patient responses (Mao et al., 2014). Additionally, Naz et al. reported that Abemaciclib, a CDK4/6 inhibitor, increases radiosensitivity in NSCLC by disrupting DNA repair and metabolic pathways, supporting the concept of CXCR4-targeted therapies in biomarker-selected patients undergoing radiotherapy (Naz et al., 2018).
Beyond direct therapeutic targeting, CXCR4 expression can evolve throughout treatment, necessitating adaptive monitoring strategies (Liu et al., 2024). Marquardt et al. identified CXCR4 expression as a marker of immune-enriched tumors with CD8+ T-cell infiltration, while fibroblast activation protein (FAP) overexpression was linked to angiogenesis. These findings suggest that CXCR4-and FAP-targeted PET imaging could serve as a non-invasive tool for personalized therapy selection (Marquardt et al., 2023; Wen et al., 2023). Similarly, Bertolini et al. found that cisplatin treatment in NSCLC increased CXCR4+ MICs and CXCL12 levels, creating a pro-metastatic TME. However, CXCR4 inhibition prevented chemotherapy-induced metastasis, reinforcing the need for biomarker-driven treatment adjustments to mitigate resistance (Bertolini et al., 2021).
Economic considerations also play a role in personalized CXCR4-targeted therapy implementation (Abdollahi et al., 2024). Rudakova et al. examined the cost-effectiveness of Gefitinib as a second-line NSCLC therapy, demonstrating that it increased life expectancy by 6 months compared to Pemetrexed while reducing overall medical costs, suggesting that CXCR4-positive patients may also benefit from economic modeling in treatment selection (Rudakova et al., 2015). Additionally, genomic profiling can refine personalized treatment strategies by identifying genetic variants that influence CXCR4 signaling and therapy resistance (Li X. et al., 2022; Almawash, 2025). Islam et al. investigated functional polymorphisms in the ACRBP gene, revealing deleterious coding variants that may contribute to tumor progression, though further studies are needed to determine their role in lung cancer pathogenesis (Islam, 2022). This aligns with the growing emphasis on genomic-guided therapy adjustments, where ongoing biomarker monitoring helps optimize treatment plans over time (Tashkandi and Younes, 2024). In integrated medicine for lung cancer, CXCR4-targeted therapies can be used to improve therapeutic benefits with reduced unnecessary toxicity. Utilizing CXCR4 as a prognostic and predictive biomarker, treatment regimens can be individualized to enhance survival rate, therapy response, and cost-effectiveness. Genomic analysis of disease and the use of CXCR4-based imaging, immune profiling, and other precision medicine will further improve the medical approach to the treatment of patients who will receive the most efficacious treatment of their disease in the form that is most tailored to their needs.
Since there is inter- and intra-tumor variability, a combination of CXCR4-PET and tissue/blood biomarkers is a viable enrichment approach to trials and practice. Since the CXCR4-directed PET/CT is feasible in lung lesions and trials indicate radiolabeled plerixafor agents, prespecification, including (i) a positivity rule, (ii) treatment of lesion-level heterogeneity, and (iii) on-treatment PET at a specified fixed time point with a pre-determined percent-change threshold, should indicate pharmacodynamic target engagement versus non-engagement. The readout based on tissue IHC and blood can be overlaid to prove baseline positivity and follow the dynamics in parallel with PET. These working regulations conform to the applicability of biomarkers to trial admission and response measurement and capitalize on the present PET/theranostic framework.
5.3 Clinical-trials landscape of CXCR4 inhibition
CXCR4 antagonists have a limited clinical testing experience in lung cancer. The most recent randomized trial of efficacy (only in lung cancer) LY2510924 (peptide CXCR4 antagonist) combined with carboplatin/etoposide in ED-SCLC N = 90 randomized phase II, with no observed improvement in PFS/OS over chemotherapy alone, although safety was acceptable, and CXCR4 IHC was an exploratory biomarker evaluated. This highlights the necessity of biomarker-informed selection and combinatory-refinement strategy dominating in trial sequences in the future (Salgia et al., 2017). Simultaneously, CXCR4-based imaging (e.g., [68Ga pentixafor PET/CT]) and [64Cu pleixafor PET]) imaging can be done in lung cancer and other solid tumours, providing a strategy to enrich in CXCR4-high disease and to measure on-treatment target engagement (Lapa et al., 2016; Burke et al., 2020).
6 Conclusion and future perspectives
Emerging evidence suggests that CXCR4 inhibitors, such as plerixafor, can effectively disrupt tumor-stroma interactions, enhance immune infiltration, and sensitize tumors. Preclinical and early clinical studies indicate that CXCR4 blockade may limit metastatic dissemination, overcome resistance to therapy, and improve overall patient outcomes. However, despite these encouraging findings, several challenges must be addressed before CXCR4-targeted therapies can be fully integrated into clinical practice. Compensatory survival mechanisms associated with the activation of CXCR4 inhibition may limit the therapeutic efficacy. CXCR4 blockade could trigger alternate signaling pathways such as PDGFRB, STAT3, as well as CXCR7, which may initiate tumor progression and therapy resistance. Furthermore, patient heterogeneity due to tumor heterogeneity and changeable CXCR4 expression makes it difficult to find the best patient group for the CXCR4-targeted therapies. To maximize clinical efficacy while minimizing unnecessary toxicity, integrating CXCR4 expression profiling with immune landscape characterization and genomic analysis will be essential for the biomarker-driven treatment selection.
As the complexity of tumor progression driven by CXCR4 is so great, combination therapies have enormous promise in improving the treatment outcome. In preclinical studies, the combination of ICIs, chemotherapy or radiotherapy, and CXCR4 inhibitors has shown synergistic effects and (reliable) proof of concept, but the most effective dosing regimens, sequencing strategies, and combination partners need further clinical validation. Further, CXCR4 targeted approaches for drug delivery, i.e., nanomedicine-based formulation, antibody-drug conjugate, and CXCR4 inhibitor radionuclide, may further enhance tumor selectivity and therapeutic efficacy while decreasing the off-target effects. Similar to the CXCR4 antagonists, advances in CXCR4-PET imaging enable a non-invasive method to measure CXCR4 expression in tumors for real-time adjustment of treatment and individual patient-tailored therapy modifications based on individual patient response. Its involvement in CNS metastases in patients with lung cancer is further established. Penetration of many CXCR4 targeted agents into the brain is limited by the BBB, hence, the development of brain penetrant CXCR4 inhibitors or alternative delivery, such as Intrathecal, is important.
Definitive clinical benefit in lung cancer has not yet been demonstrated; ongoing and future phase II/III trials with CXCR4 imaging/biomarker-guided selection will be essential to determine where plerixafor adds value. Future research should focus on addressing the action of CXCR4 inhibition to CNS-specific metastases in patients with advanced-stage lung cancer and treatment-resistant brain metastases. CXCR4 targeted therapies promise is great, however, long-term safety needs to be carefully reviewed. CXCR4 has an important role in hematopoiesis as well as in immune cell trafficking and normal stem cell function, which could lead to concerns regarding chronic inhibition of immune homeostasis and normal tissue function. A long-term toxicity assessment, potential immune-related side effects, and optimal dosing schedule need to be put in future trials to provide sustained therapeutic benefits with no complications.
Up to now, the clinical benefit of CXCR4 antagonism has not been demonstrated in any randomized trial in patients with lung cancer; phase II/III trials that are biomarker-enriched and imaging-guided are required to determine the points of value addition of plerixafor.
Author contributions
PR: Conceptualization, Writing – review and editing, Writing – original draft, Project administration. MA: Writing – review and editing, Data curation. MB: Visualization, Writing – review and editing. MR: Data curation, Writing – original draft. SS: Data curation, Writing – review and editing. SP: Writing – original draft, Data curation. KG: Writing – review and editing, Data curation. MH: Visualization, Data curation, Writing – review and editing, Writing – original draft. GG: Formal Analysis, Writing – review and editing, Conceptualization. PB: Formal Analysis, Writing – review and editing. SA: Validation, Writing – review and editing, Supervision.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
AcknowledgementsThe authors would like to thank their respective institutes/organizations for providing the necessary facilities to carry out this work. Prasanna Srinivasan Ramalingam would like to thank the Council for Scientific and Industrial Research (CSIR) for providing him the Senior Research Fellowship (File No: 09/0844(18240)/2024-EMR-I).
Conflict of interest
Author PB was employed by TanBio R and D Solution.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that Generative AI was used in the creation of this manuscript. During the preparation of this work, the authors used Generative AI and AI-assisted tools solely to improve the language, grammar, and readability of the manuscript. No AI tools were used for generating substantive content, data analysis, interpretation of results, or drawing scientific conclusions. All intellectual contributions, study design, data collection, analysis, and final interpretations were conceived and executed by the authors. The authors reviewed and verified the accuracy and appropriateness of all AI-assisted edits and take full responsibility for the content of this publication.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdollahi, H., Yousefirizi, F., Shiri, I., Brosch-Lenz, J., Mollaheydar, E., Fele-Paranj, A., et al. (2024). Theranostic digital twins: concept, framework and roadmap towards personalized radiopharmaceutical therapies. Theranostics 14, 3404–3422. doi:10.7150/thno.93973
Alexandru, I., Davidescu, L., Motofelea, A. C., Ciocarlie, T., Motofelea, N., Costachescu, D., et al. (2024). Emerging nanomedicine approaches in targeted lung cancer treatment. Int. J. Mol. Sci. 25, 11235. doi:10.3390/ijms252011235
Almawash, S. (2025). Revolutionary cancer therapy for personalization and improved efficacy: strategies to overcome resistance to immune checkpoint inhibitor therapy. Cancers 17, 880. doi:10.3390/cancers17050880
Alqudah, M. A., Yaseen, M. M., Alzoubi, K. H., Al-Husein, B. A., Bardaweel, S. K., Abuhelwa, A. Y., et al. (2025). Metabolomic analysis, antiproliferative, anti-migratory, and anti-invasive potential of amlodipine in lung cancer cells. Drug Des. Dev. Ther. 19, 1215–1229. doi:10.2147/DDDT.S484561
Alsayed, R. K. M., Khan, A. Q., Ahmad, F., Ansari, A. W., Alam, M. A., Buddenkotte, J., et al. (2022). Epigenetic regulation of CXCR4 signaling in cancer pathogenesis and progression. Seminars Cancer Biol. 86, 697–708. doi:10.1016/j.semcancer.2022.03.019
Altorki, N. K., Markowitz, G. J., Gao, D., Port, J. L., Saxena, A., Stiles, B., et al. (2019). The lung microenvironment: an important regulator of tumour growth and metastasis. Nat. Rev. Cancer 19, 9–31. doi:10.1038/s41568-018-0081-9
Andtbacka, R. H., Wang, Y., Pierce, R. H., Campbell, J. S., Yushak, M., Milhem, M., et al. (2022). Mavorixafor, an orally bioavailable CXCR4 antagonist, increases immune cell infiltration and inflammatory status of tumor microenvironment in patients with melanoma. Cancer Res. Commun. 2, 904–913. doi:10.1158/2767-9764.CRC-22-0090
Ashrafi, A., Akter, Z., Modareszadeh, P., Modareszadeh, P., Berisha, E., Alemi, P. S., et al. (2022). Current landscape of therapeutic resistance in lung cancer and promising strategies to overcome resistance. Cancers 14, 4562. doi:10.3390/cancers14194562
Azad, B. B., Chatterjee, S., Lesniak, W. G., Lisok, A., Pullambhatla, M., Bhujwalla, Z. M., et al. (2016). A fully human CXCR4 antibody demonstrates diagnostic utility and therapeutic efficacy in solid tumor xenografts. Oncotarget 7, 12344–12358. doi:10.18632/oncotarget.7111
Bao, S., Darvishi, M., H Amin, A., Al-Haideri, M. T., Patra, I., Kashikova, K., et al. (2023). CXC chemokine receptor 4 (CXCR4) blockade in cancer treatment. J. Cancer Res. Clin. Oncol. 149, 7945–7968. doi:10.1007/s00432-022-04444-w
Barnestein, R., Galland, L., Kalfeist, L., Ghiringhelli, F., Ladoire, S., and Limagne, E. (2022). Immunosuppressive tumor microenvironment modulation by chemotherapies and targeted therapies to enhance immunotherapy effectiveness. Oncoimmunology 11, 2120676. doi:10.1080/2162402X.2022.2120676
Bertolini, G., Cancila, V., Milione, M., Russo, G. L., Fortunato, O., Zaffaroni, N., et al. (2021). A novel CXCR4 antagonist counteracts paradoxical generation of cisplatin-induced pro-metastatic niches in lung cancer. Mol. Ther. 29, 2963–2978. doi:10.1016/j.ymthe.2021.05.014
Bi, M. M., Shang, B., Wang, Z., and Chen, G. (2017). Expression of CXCR4 and VEGF-C is correlated with lymph node metastasis in non-small cell lung cancer. Thorac. Cancer 8, 634–641. doi:10.1111/1759-7714.12500
Blayney, D. W., Ette, E., Ogenstad, S., Shi, Y., Du, L., Lloyd, K., et al. (2018). Plinabulin, a novel small molecule in development for chemotherapy-induced-neutropenia (CIN) prevention, mobilizes CD34+ cells through a mechanism of action (MoA) different from G-CSF and from CXCR4 inhibition. Blood 132, 2068. doi:10.1182/blood-2018-99-113569
Britton, C., Poznansky, M., and Reeves, P. (2021). Polyfunctionality of the CXCR4/CXCL12 axis in health and disease: implications for therapeutic interventions in cancer and immune-mediated diseases. FASEB J. 35, e21260. doi:10.1096/fj.202001273R
Burger, M., Glodek, A., Hartmann, T., Schmitt-Gräff, A., Silberstein, L. E., Fujii, N., et al. (2003). Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene 22, 8093–8101. doi:10.1038/sj.onc.1207097
Burke, B. P., Miranda, C. S., Lee, R. E., Renard, I., Nigam, S., Clemente, G. S., et al. (2020). (64)Cu PET imaging of the CXCR4 chemokine receptor using a cross-bridged cyclam bis-tetraazamacrocyclic antagonist. J. Nucl. Med. 61, 123–128. doi:10.2967/jnumed.118.218008
Cancilla, D., Rettig, M. P., and Dipersio, J. F. (2020). Targeting CXCR4 in AML and all. Front. Oncol. 10, 1672. doi:10.3389/fonc.2020.01672
Cao, C., Xu, M., Wei, Y., Peng, T., Lin, S., Liu, X., et al. (2024). CXCR4 orchestrates the TOX-programmed exhausted phenotype of CD8+ T cells via JAK2/STAT3 pathway. Cell Genomics 4, 100659. doi:10.1016/j.xgen.2024.100659
Césaire, M., Montanari, J., Curcio, H., Lerouge, D., Gervais, R., Demontrond, P., et al. (2022). Radioresistance of non-small cell lung cancers and therapeutic perspectives. Cancers 14, 2829. doi:10.3390/cancers14122829
Chai, X., Yinwang, E., Wang, Z., Wang, Z., Xue, Y., Li, B., et al. (2021). Predictive and prognostic biomarkers for lung cancer bone metastasis and their therapeutic value. Front. Oncol. 11, 692788. doi:10.3389/fonc.2021.692788
Chandra, R., Karalis, J. D., Liu, C., Murimwa, G. Z., Voth Park, J., Heid, C. A., et al. (2021). The colorectal cancer tumor microenvironment and its impact on liver and lung metastasis. Cancers 13, 6206. doi:10.3390/cancers13246206
Chaudary, N., Hill, R. P., Stulik, L., and Milosevic, M. (2021). The oral CXCR4 inhibitor X4-136 improves tumor control and reduces toxicity in cervical cancer treated with radiation therapy and concurrent chemotherapy. Int. J. Radiat. Oncology* Biology* Phys. 110, 1317–1324. doi:10.1016/j.ijrobp.2021.03.031
Chaudary, N., Hill, R. P., and Milosevic, M. (2024). Targeting the CXCL12/CXCR4 pathway to reduce radiation treatment side effects. Radiotherapy Oncol. 194, 110194. doi:10.1016/j.radonc.2024.110194
Chen, G., Wang, Z., Liu, X. Y., and Liu, F. Y. (2011). High-level CXCR4 expression correlates with brain-specific metastasis of non-small cell lung cancer. World J. Surg. 35, 56–61. doi:10.1007/s00268-010-0784-x
Chittasupho, C., Lirdprapamongkol, K., Kewsuwan, P., and Sarisuta, N. (2014). Targeted delivery of doxorubicin to A549 lung cancer cells by CXCR4 antagonist conjugated PLGA nanoparticles. Eur. J. Pharm. Biopharm. 88, 529–538. doi:10.1016/j.ejpb.2014.06.020
Choi, W.-T., Duggineni, S., Xu, Y., Huang, Z., and An, J. (2012). Drug discovery research targeting the CXC chemokine receptor 4 (CXCR4). J. Med. Chem. 55, 977–994. doi:10.1021/jm200568c
Choi, Y. H., Burdick, M. D., Strieter, B. A., Mehrad, B., and Strieter, R. M. (2014). CXCR4, but not CXCR7, discriminates metastatic behavior in non–small cell lung cancer cells. Mol. Cancer Res. 12, 38–47. doi:10.1158/1541-7786.MCR-12-0334
Cojoc, M., Peitzsch, C., Trautmann, F., Polishchuk, L., Telegeev, G. D., and Dubrovska, A. (2013). Emerging targets in cancer management: role of the CXCL12/CXCR4 axis. OncoTargets Ther. 6, 1347–1361. doi:10.2147/OTT.S36109
Costa, M. J., Kudaravalli, J., Ma, J.-T., Ho, W.-H., Delaria, K., Holz, C., et al. (2019). Optimal design, anti-tumour efficacy and tolerability of anti-CXCR4 antibody drug conjugates. Sci. Rep. 9, 2443. doi:10.1038/s41598-019-38745-x
Crees, Z. D., Rettig, M. P., and Dipersio, J. F. (2023). Innovations in hematopoietic stem-cell mobilization: a review of the novel CXCR4 inhibitor motixafortide. Ther. Adv. Hematol. 14, 20406207231174304. doi:10.1177/20406207231174304
D'alterio, C., Zannetti, A., Trotta, A. M., Ieranò, C., Napolitano, M., Rea, G., et al. (2020). New CXCR4 antagonist peptide R (pep R) improves standard therapy in colorectal cancer. Cancers (Basel) 12, 1952. doi:10.3390/cancers12071952
De Giglio, A., Di Federico, A., Nuvola, G., Deiana, C., and Gelsomino, F. (2021). The landscape of immunotherapy in advanced NSCLC: driving beyond PD-1/PD-L1 inhibitors (CTLA-4, LAG3, IDO, OX40, TIGIT, vaccines). Curr. Oncol. Rep. 23, 126. doi:10.1007/s11912-021-01124-9
Devi, S., Wang, Y., Chew, W. K., Lima, R., A-González, N., Mattar, C. N., et al. (2013). Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J. Exp. Med. 210, 2321–2336. doi:10.1084/jem.20130056
Dreher, N., Hahner, S., Fuß, C. T., Schlötelburg, W., Hartrampf, P. E., Serfling, S. E., et al. (2024). CXCR4-directed PET/CT with [68 Ga] Ga-pentixafor in solid tumors—A comprehensive analysis of imaging findings and comparison with histopathology. Eur. J. Nucl. Med. Mol. Imaging 51, 1383–1394. doi:10.1007/s00259-023-06547-z
Dzobo, K., Senthebane, D. A., Ganz, C., Thomford, N. E., Wonkam, A., and Dandara, C. (2020). Advances in therapeutic targeting of cancer stem cells within the tumor microenvironment: an updated review. Cells 9, 1896. doi:10.3390/cells9081896
D’alterio, C., Barbieri, A., Portella, L., Palma, G., Polimeno, M., Riccio, A., et al. (2012). Inhibition of stromal CXCR4 impairs development of lung metastases. Cancer Immunol. Immunother. 61, 1713–1720. doi:10.1007/s00262-012-1223-7
D’alterio, C., Buoncervello, M., Ieranò, C., Napolitano, M., Portella, L., Rea, G., et al. (2019). Targeting CXCR4 potentiates anti-PD-1 efficacy modifying the tumor microenvironment and inhibiting neoplastic PD-1. J. Exp. and Clin. Cancer Res. 38, 432–13. doi:10.1186/s13046-019-1420-8
Eckert, F., Schilbach, K., Klumpp, L., Bardoscia, L., Sezgin, E. C., Schwab, M., et al. (2018). Potential role of CXCR4 targeting in the context of radiotherapy and immunotherapy of cancer. Front. Immunol. 9, 3018. doi:10.3389/fimmu.2018.03018
Eckert, F., Zwirner, K., Boeke, S., Thorwarth, D., Zips, D., and Huber, S. M. (2019). Rationale for combining radiotherapy and immune checkpoint inhibition for patients with hypoxic tumors. Front. Immunol. 10, 407. doi:10.3389/fimmu.2019.00407
Emami Nejad, A., Najafgholian, S., Rostami, A., Sistani, A., Shojaeifar, S., Esparvarinha, M., et al. (2021). The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int. 21, 62–26. doi:10.1186/s12935-020-01719-5
Eulberg, D., Frömming, A., Lapid, K., Mangasarian, A., and Barak, A. (2022). The prospect of tumor microenvironment-modulating therapeutical strategies. Front. Oncol. 12, 1070243. doi:10.3389/fonc.2022.1070243
Fahham, D., Weiss, I. D., Abraham, M., Beider, K., Hanna, W., Shlomai, Z., et al. (2012). In vitro and in vivo therapeutic efficacy of CXCR4 antagonist BKT140 against human non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 144, 1167–1175.e1. doi:10.1016/j.jtcvs.2012.07.031
Franco, R., Pirozzi, G., Scala, S., Cantile, M., Scognamiglio, G., Camerlingo, R., et al. (2012). CXCL12-binding receptors expression in non-small cell lung cancer relates to tumoral microvascular density and CXCR4 positive circulating tumoral cells in lung draining venous blood. Eur. J. Cardio-thoracic Surg. 41, 368–375. doi:10.1016/j.ejcts.2011.05.009
Fujiwara, Y., Mittra, A., Naqash, A. R., and Takebe, N. (2020). A review of mechanisms of resistance to immune checkpoint inhibitors and potential strategies for therapy. Cancer Drug Resist. 3, 252–275. doi:10.20517/cdr.2020.11
Gibson, C. J., and Davids, M. S. (2015). BCL-2 antagonism to target the intrinsic mitochondrial pathway of apoptosis. Clin. Cancer Res. 21, 5021–5029. doi:10.1158/1078-0432.CCR-15-0364
Gravina, A. G., Pellegrino, R., Esposito, A., Cipullo, M., Romeo, M., Palladino, G., et al. (2024). The JAK-STAT pathway as a therapeutic strategy in cancer patients with immune checkpoint inhibitor-induced colitis: a narrative review. Cancers 16, 611. doi:10.3390/cancers16030611
Guo, W., Huai, Q., Zhou, B., Guo, L., Sun, L., Xue, X., et al. (2023). Comprehensive analysis of the immunological implication and prognostic value of CXCR4 in non-small cell lung cancer. Cancer Immunol. Immunother. 72, 1029–1045. doi:10.1007/s00262-022-03298-y
Guo, L., Fu, Z., Li, H., Wei, R., Guo, J., Wang, H., et al. (2025). Smart hydrogel: a new platform for cancer therapy. Adv. Colloid Interface Sci. 340, 103470. doi:10.1016/j.cis.2025.103470
Gürgen, D., Conrad, T., Becker, M., Sebens, S., Röcken, C., Hoffmann, J., et al. (2022). Breaking the crosstalk of the cellular tumorigenic network by low-dose combination therapy in lung cancer patient-derived xenografts. Commun. Biol. 5, 59. doi:10.1038/s42003-022-03016-5
Habanjar, O., Bingula, R., Decombat, C., Diab-Assaf, M., Caldefie-Chezet, F., and Delort, L. (2023). Crosstalk of inflammatory cytokines within the breast tumor microenvironment. Int. J. Mol. Sci. 24, 4002. doi:10.3390/ijms24044002
Hang, Y. (2020). Development of CXCR4-inhibiting RNA delivery vectors for the treatment of metastatic pancreatic cancer.
Hartmann, T. N., Burger, J. A., Glodek, A., Fujii, N., and Burger, M. (2005). CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene 24, 4462–4471. doi:10.1038/sj.onc.1208621
He, L., Yang, H., Tang, J., Liu, Z., Chen, Y., Lu, B., et al. (2019). Intestinal probiotics E. coli nissle 1917 as a targeted vehicle for delivery of p53 and Tum-5 to solid tumors for cancer therapy. J. Biol. Eng. 13, 58. doi:10.1186/s13036-019-0189-9
Ho, W. J., Jaffee, E. M., and Zheng, L. (2020). The tumour microenvironment in pancreatic Cancer—Clinical challenges and opportunities. Nat. Rev. Clin. Oncol. 17, 527–540. doi:10.1038/s41571-020-0363-5
Huang, T.-X., Guan, X.-Y., and Fu, L. (2019). Therapeutic targeting of the crosstalk between cancer-associated fibroblasts and cancer stem cells. Am. J. Cancer Res. 9, 1889–1904.
Hussain, M. S., Goyal, A., Goyal, K., S, R. J., Nellore, J., Shahwan, M., et al. (2025). Targeting CXCR2 signaling in inflammatory lung diseases: neutrophil-driven inflammation and emerging therapies. Naunyn Schmiedeb. Arch. Pharmacol. 398, 9583–9607. doi:10.1007/s00210-025-03970-x
Islam, M. (2022). 34P association of ACRBP gene polymorphism (+26A/G) to liver cancer and diabetes leads to novel biomarker discovery. Ann. Oncol. 33, S1442–S1443. doi:10.1016/j.annonc.2022.10.044
Jäger, B., Klatt, D., Plappert, L., Golpon, H., Lienenklaus, S., Barbosa, P. D., et al. (2020). CXCR4/MIF axis amplifies tumor growth and epithelial-mesenchymal interaction in non-small cell lung cancer. Cell. Signal. 73, 109672. doi:10.1016/j.cellsig.2020.109672
Jiang, J., Shen, N., Song, W., Yu, H., Sakurai, K., Tang, Z., et al. (2019). Combretastatin A4 nanodrug combined plerixafor for inhibiting tumor growth and metastasis simultaneously. Biomater. Sci. 7, 5283–5291. doi:10.1039/c9bm01418g
Jung, M., Rho, J., Kim, Y., Jung, J., Jin, Y., Ko, Y., et al. (2013). Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene 32, 209–221. doi:10.1038/onc.2012.37
Kast, R. E., Alfieri, A., Assi, H. I., Burns, T. C., Elyamany, A. M., Gonzalez-Cao, M., et al. (2022). Mdact: a new principle of adjunctive cancer treatment using combinations of multiple repurposed drugs, with an example regimen. Cancers 14, 2563. doi:10.3390/cancers14102563
Khalaf, K., Hana, D., Chou, J.T.-T., Singh, C., Mackiewicz, A., and Kaczmarek, M. (2021). Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front. Immunol. 12, 656364. doi:10.3389/fimmu.2021.656364
Kiefer, F., and Siekmann, A. F. (2011). The role of chemokines and their receptors in angiogenesis. Cell. Mol. life Sci. 68, 2811–2830. doi:10.1007/s00018-011-0677-7
Kijima, T., Maulik, G., Ma, P. C., Tibaldi, E. V., Turner, R. E., Rollins, B., et al. (2002). Regulation of cellular proliferation, cytoskeletal function, and signal transduction through CXCR4 and c-Kit in small cell lung cancer cells. Cancer Res. 62, 6304–6311.
Kim, S. Y., Lee, C. H., Midura, B. V., Yeung, C., Mendoza, A., Hong, S. H., et al. (2008). Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases. Clin. and Exp. Metastasis 25, 201–211. doi:10.1007/s10585-007-9133-3
Kim, J.-Y., Kim, H.-J., Jung, C.-W., Lee, T. S., Kim, E. H., and Park, M.-J. (2021). CXCR4 uses STAT3-mediated slug expression to maintain radioresistance of non-small cell lung cancer cells: emerges as a potential prognostic biomarker for lung cancer. Cell Death and Dis. 12, 48. doi:10.1038/s41419-020-03280-5
Ko, S., Shim, G., Kim, J., and Oh, Y.-K. (2018). Chemokine-mimetic plerixafor derivative for tumor-specific delivery of nanomaterials. Nano Res. 11, 2159–2172. doi:10.1007/s12274-017-1833-7
Ko, J., Winslow, M. M., and Sage, J. (2021). Mechanisms of small cell lung cancer metastasis. EMBO Mol. Med. 13, e13122. doi:10.15252/emmm.202013122
Kohli, K., Pillarisetty, V. G., and Kim, T. S. (2022). Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. 29, 10–21. doi:10.1038/s41417-021-00303-x
Korbecki, J., Kupnicka, P., Chlubek, M., Gorący, J., Gutowska, I., and Baranowska-Bosiacka, I. (2022). CXCR2 receptor: regulation of expression, signal transduction, and involvement in cancer. Int. J. Mol. Sci. 23, 2168. doi:10.3390/ijms23042168
Kuang, Z., Wang, J., Liu, K., Wu, J., Ge, Y., Zhu, G., et al. (2024). Global, regional, and national burden of tracheal, bronchus, and lung cancer and its risk factors from 1990 to 2021: findings from the global burden of disease study 2021. EClinicalMedicine 75, 102804. doi:10.1016/j.eclinm.2024.102804
Lakhanpal, T., Shukla, J., Rathore, Y., Malhotra, P., Prakash, G., Kumar, R., et al. (2022). 35P exploring plerixafor as a vector molecule in nuclear medicine for targeting CXCR4 receptor overexpression in vivo. Ann. Oncol. 33, S1443. doi:10.1016/j.annonc.2022.10.045
Lakhanpal, T., Mittal, B., Shukla, J., Kumar, R., Rathore, Y., Malhotra, P., et al. (2023). 40P development of radiolabelled plerixafor as a theranostic molecule for targeting CXCR4 receptor expressing cancers: a translational study. ESMO Open 8, 101006. doi:10.1016/j.esmoop.2023.101006
Lalić, N., Bojović, M., Bursać, D., Bokan, D., Čeriman Krstić, V., Kuhajda, I., et al. (2024). The efficacy outcomes in non-small cell lung cancer patients treated with PD axis inhibitor agents - a population-based study of the Vojvodina region. Pathol. Oncol. Res. 30, 1611717. doi:10.3389/pore.2024.1611717
Langhammer, S. (2013). Rationale for the design of an oncology trial using a generic targeted therapy multi‑drug regimen for NSCLC patients without treatment options. Oncol. Rep. 30, 1535–1541. doi:10.3892/or.2013.2631
Lapa, C., Lückerath, K., Rudelius, M., Schmid, J. S., Schoene, A., Schirbel, A., et al. (2016). [68Ga]Pentixafor-PET/CT for imaging of chemokine receptor 4 expression in small cell lung cancer--initial experience. Oncotarget 7, 9288–9295. doi:10.18632/oncotarget.7063
Leo, M., and Sabatino, L. (2022). Targeting CXCR4 and CD47 receptors: an overview of new and old molecules for a biological personalized anticancer therapy. Int. J. Mol. Sci. 23, 12499. doi:10.3390/ijms232012499
Li, J., and Oupický, D. (2014). Effect of biodegradability on CXCR4 antagonism, transfection efficacy and antimetastatic activity of polymeric plerixafor. Biomaterials 35, 5572–5579. doi:10.1016/j.biomaterials.2014.03.047
Li, Y., Shen, Y., Miao, Y., Luan, Y., Sun, B., and Qiu, X. (2014). Co-expression of uPAR and CXCR4 promotes tumor growth and metastasis in small cell lung cancer. Int. J. Clin. Exp. Pathol. 7, 3771–3780.
Li, Z., Chen, G., Ding, L., Wang, Y., Zhu, C., Wang, K., et al. (2019). Increased survival by pulmonary treatment of established lung metastases with dual STAT3/CXCR4 inhibition by siRNA nanoemulsions. Mol. Ther. 27, 2100–2110. doi:10.1016/j.ymthe.2019.08.008
Li, Z., Wang, Y., Shen, Y., Qian, C., Oupicky, D., and Sun, M. (2020). Targeting pulmonary tumor microenvironment with CXCR4-inhibiting nanocomplex to enhance anti-PD-L1 immunotherapy. Sci. Adv. 6, eaaz9240. doi:10.1126/sciadv.aaz9240
Li, H., Zhou, L., Zhou, J., Li, Q., and Ji, Q. (2021a). Underlying mechanisms and drug intervention strategies for the tumour microenvironment. J. Exp. and Clin. Cancer Res. 40, 97–21. doi:10.1186/s13046-021-01893-y
Li, X., Yang, Y., Huang, Q., Deng, Y., Guo, F., Wang, G., et al. (2021b). Crosstalk between the tumor microenvironment and cancer cells: a promising predictive biomarker for immune checkpoint inhibitors. Front. Cell Dev. Biol. 9, 738373. doi:10.3389/fcell.2021.738373
Li, C., Qiu, Y., and Zhang, Y. (2022a). Research progress on therapeutic targeting of cancer-associated fibroblasts to tackle treatment-resistant NSCLC. Pharmaceuticals 15, 1411. doi:10.3390/ph15111411
Li, X., Li, M., Xiang, J., Zhao, Z., and Shang, X. (2022b). SEPA: signaling entropy-based algorithm to evaluate personalized pathway activation for survival analysis on pan-cancer data. Bioinformatics 38, 2536–2543. doi:10.1093/bioinformatics/btac122
Li, C., Lei, S., Ding, L., Xu, Y., Wu, X., Wang, H., et al. (2023a). Global burden and trends of lung cancer incidence and mortality. Chin. Med. J. 136, 1583–1590. doi:10.1097/CM9.0000000000002529
Li, X. P., Qu, J., Teng, X. Q., Zhuang, H. H., Dai, Y. H., Yang, Z., et al. (2023b). The emerging role of super-enhancers as therapeutic targets in the digestive system tumors. Int. J. Biol. Sci. 19, 1036–1048. doi:10.7150/ijbs.78535
Li, J., Gu, A., Tang, N., Zengin, G., Li, M. Y., and Liu, Y. (2025). Patient-derived xenograft models in pan-cancer: from bench to clinic. Interdiscip. Med. 3, e20250016. doi:10.1002/inmd.20250016
Liu, Y.-L., Yu, J.-M., Song, X.-R., Wang, X.-W., Xing, L.-G., and Gao, B.-B. (2006). Regulation of the chemokine receptor CXCR4 and metastasis by hypoxia-inducible factor in non small cell lung cancer cell lines. Cancer Biol. and Ther. 5, 1320–1326. doi:10.4161/cbt.5.10.3162
Liu, H., Cheng, Q., Xu, D.-S., Wang, W., Fang, Z., Xue, D.-D., et al. (2020). Overexpression of CXCR7 accelerates tumor growth and metastasis of lung cancer cells. Respir. Res. 21, 287–13. doi:10.1186/s12931-020-01518-6
Liu, Z., Chen, J., Ren, Y., Liu, S., Ba, Y., Zuo, A., et al. (2024). Multi-stage mechanisms of tumor metastasis and therapeutic strategies. Signal Transduct. Target. Ther. 9, 270. doi:10.1038/s41392-024-01955-5
López-Gil, J. C., Martin-Hijano, L., Hermann, P. C., and Sainz Jr, B. (2021). The CXCL12 crossroads in cancer stem cells and their niche. Cancers 13, 469. doi:10.3390/cancers13030469
Maclean, M. R., Walker, O. L., Arun, R. P., Fernando, W., and Marcato, P. (2024). Informed by cancer stem cells of solid tumors: advances in treatments targeting tumor-promoting factors and pathways. Int. J. Mol. Sci. 25, 4102. doi:10.3390/ijms25074102
Mao, Y., Li, W., Chen, K., Xie, Y., Liu, Q., Yao, M., et al. (2014). B7-H1 and B7-H3 are independent predictors of poor prognosis in patients with non-small cell lung cancer. Oncotarget 6, 3452–3461. doi:10.18632/oncotarget.3097
Marquardt, A., Hartrampf, P., Kollmannsberger, P., Solimando, A. G., Meierjohann, S., Kübler, H., et al. (2023). Predicting microenvironment in CXCR4-and FAP-positive solid tumors—a pan-cancer machine learning workflow for theranostic target structures. Cancers 15, 392. doi:10.3390/cancers15020392
Martin, S. (2024). Exploiting chemokine receptors as targets for non-invasive imaging in cancer and immunology. Univ. de Lausanne, Fac. de Biol. médecine.
Megyesfalvi, Z., Gay, C. M., Popper, H., Pirker, R., Ostoros, G., Heeke, S., et al. (2023). Clinical insights into small cell lung cancer: tumor heterogeneity, diagnosis, therapy, and future directions. CA a Cancer J. Clin. 73, 620–652. doi:10.3322/caac.21785
Mezzapelle, R., Leo, M., Caprioglio, F., Colley, L. S., Lamarca, A., Sabatino, L., et al. (2022). CXCR4/CXCL12 activities in the tumor microenvironment and implications for tumor immunotherapy. Cancers 14, 2314. doi:10.3390/cancers14092314
Miller, R. J., Banisadr, G., and Bhattacharyya, B. J. (2008). CXCR4 signaling in the regulation of stem cell migration and development. J. Neuroimmunol. 198, 31–38. doi:10.1016/j.jneuroim.2008.04.008
Mir, H., Kapur, N., Gales, D. N., Sharma, P. K., Oprea-Ilies, G., Johnson, A. T., et al. (2021). CXCR6-CXCL16 axis promotes breast cancer by inducing oncogenic signaling. Cancers 13, 3568. doi:10.3390/cancers13143568
Mir, M. A., Rashid, M., and Jan, N. (2023). “Cytokines and chemokines in tumor growth and progression,” in Cytokine and chemokine networks in cancer (Springer), 33–77.
Miranda-Galvis, M., and Teng, Y. (2020). Targeting hypoxia-driven metabolic reprogramming to constrain tumor progression and metastasis. Int. J. Mol. Sci. 21, 5487. doi:10.3390/ijms21155487
Mishan, M. A., Ahmadiankia, N., and Bahrami, A. R. (2016). CXCR4 and CCR7: two eligible targets in targeted cancer therapy. Cell Biol. Int. 40, 955–967. doi:10.1002/cbin.10631
Monteran, L., and Erez, N. (2019). The dark side of fibroblasts: cancer-associated fibroblasts as mediators of immunosuppression in the tumor microenvironment. Front. Immunol. 10, 1835. doi:10.3389/fimmu.2019.01835
Morein, D., Erlichman, N., and Ben-Baruch, A. (2020). Beyond cell motility: the expanding roles of chemokines and their receptors in malignancy. Front. Immunol. 11, 952. doi:10.3389/fimmu.2020.00952
Morland, B., Kepak, T., Dallorso, S., Sevilla, J., Murphy, D., Luksch, R., et al. (2020). Plerixafor combined with standard regimens for hematopoietic stem cell mobilization in pediatric patients with solid tumors eligible for autologous transplants: two-arm phase I/II study (MOZAIC). Bone Marrow Transplant. 55, 1744–1753. doi:10.1038/s41409-020-0836-2
Mortezaee, K. (2020). Hypoxia induces core-to-edge transition of progressive tumoral cells: a critical review on differential yet corroborative roles for HIF-1α and HIF-2α. Life Sci. 242, 117145. doi:10.1016/j.lfs.2019.117145
Mousavi, A. (2020). CXCL12/CXCR4 signal transduction in diseases and its molecular approaches in targeted-therapy. Immunol. Lett. 217, 91–115. doi:10.1016/j.imlet.2019.11.007
Nantajit, D., Lin, D., and Li, J. J. (2015). The network of epithelial–mesenchymal transition: potential new targets for tumor resistance. J. Cancer Res. Clin. Oncol. 141, 1697–1713. doi:10.1007/s00432-014-1840-y
Nasrollahzadeh, E., Razi, S., Keshavarz-Fathi, M., Mazzone, M., and Rezaei, N. (2020). Pro-tumorigenic functions of macrophages at the primary, invasive and metastatic tumor site. Cancer Immunol. Immunother. 69, 1673–1697. doi:10.1007/s00262-020-02616-6
Naz, S., Sowers, A., Choudhuri, R., Wissler, M., Gamson, J., Mathias, A., et al. (2018). Abemaciclib, a selective CDK4/6 inhibitor, enhances the radiosensitivity of non–small cell lung cancer in vitro and in vivo. Clin. Cancer Res. 24, 3994–4005. doi:10.1158/1078-0432.CCR-17-3575
Nengroo, M. A., Khan, M. A., Verma, A., and Datta, D. (2022a). Demystifying the CXCR4 conundrum in cancer biology: beyond the surface signaling paradigm. Biochimica Biophysica Acta (BBA)-Reviews Cancer 1877, 188790. doi:10.1016/j.bbcan.2022.188790
Nengroo, M. A., Verma, A., and Datta, D. (2022b). Cytokine chemokine network in tumor microenvironment: impact on CSC properties and therapeutic applications. Cytokine 156, 155916. doi:10.1016/j.cyto.2022.155916
Ngamcherdtrakul, W., and Yantasee, W. (2019). siRNA therapeutics for breast cancer: recent efforts in targeting metastasis, drug resistance, and immune evasion. Transl. Res. 214, 105–120. doi:10.1016/j.trsl.2019.08.005
Oriuchi, N., Sugawara, S., and Shiga, T. (2020). Positron emission tomography for response evaluation in microenvironment-targeted anti-cancer therapy. Biomedicines 8, 371. doi:10.3390/biomedicines8090371
Otsuka, S. (2011). In vitro and clinical studies on the role of the CXCR4/SDF-1 chemokine axis in non-small cell lung cancer.
Otsuka, S., Klimowicz, A. C., Kopciuk, K., Petrillo, S. K., Konno, M., Hao, D., et al. (2011). CXCR4 overexpression is associated with poor outcome in females diagnosed with stage IV non-small cell lung cancer. J. Thorac. Oncol. 6, 1169–1178. doi:10.1097/JTO.0b013e3182199a99
Panda, S., Padhiary, S. K., and Routray, S. (2016). Chemokines accentuating protumoral activities in oral cancer microenvironment possess an imperious stratagem for therapeutic resolutions. Oral Oncol. 60, 8–17. doi:10.1016/j.oraloncology.2016.06.008
Panneerselvam, J., Jin, J., Shanker, M., Lauderdale, J., Bates, J., Wang, Q., et al. (2015). IL-24 inhibits lung cancer cell migration and invasion by disrupting the SDF-1/CXCR4 signaling axis. PloS One 10, e0122439. doi:10.1371/journal.pone.0122439
Park, K., Veena, M. S., and Shin, D. S. (2022). Key players of the immunosuppressive tumor microenvironment and emerging therapeutic strategies. Front. Cell Dev. Biol. 10, 830208. doi:10.3389/fcell.2022.830208
Pass, H. I., Carbone, D. P., Johnson, D. H., Minna, J. D., Scagliotti, G. V., and Turrisi, A. T. (2012). Principles and practice of lung cancer: the official reference text of the international association for the study of lung cancer (IASLC). Lippincott Williams and Wilkins.
Peng, X., Liu, Z., Luo, C., Sun, R., Zhang, Y., Li, B., et al. (2025). PSMD12 promotes hepatocellular carcinoma progression by stabilizing CDK1. Front. Immunol. 16, 1581398. doi:10.3389/fimmu.2025.1581398
Pillay, J., Tregay, N., Juzenaite, G., Carlin, L. M., Pirillo, C., Gaboriau, D. C., et al. (2020). Effect of the CXCR4 antagonist plerixafor on endogenous neutrophil dynamics in the bone marrow, lung and spleen. J. Leukoc. Biol. 107, 1175–1185. doi:10.1002/JLB.1MA0420-571RR
Popper, H. H. (2016). Progression and metastasis of lung cancer. Cancer Metastasis Rev. 35, 75–91. doi:10.1007/s10555-016-9618-0
Pulido, C., Vendrell, I., Ferreira, A. R., Casimiro, S., Mansinho, A., Alho, I., et al. (2017). Bone metastasis risk factors in breast cancer. Ecancermedicalscience 11, 715. doi:10.3332/ecancer.2017.715
Qi, X., Huang, J., Zhang, T., Han, Z., Wang, Y., Sun, Y., et al. (2024). Unveiling CXCL12/CXCR4 axis as a common pathway in diverse types of pulmonary fibrosis and the therapeutic potential of plerixafor.
Regan, D. (2017). Pharmacological characterization of losartan as a Ccr2 antagonist and pre-clinical and pharmacodynamic assessment as a potential anti-metastatic therapy. Fort Collins, CO, United States: Colorado State University.
Reinholdt, L., Laursen, M. B., Schmitz, A., Bødker, J. S., Jakobsen, L. H., Bøgsted, M., et al. (2016). The CXCR4 antagonist plerixafor enhances the effect of rituximab in diffuse large B-cell lymphoma cell lines. Biomark. Res. 4, 12. doi:10.1186/s40364-016-0067-2
Roma-Rodrigues, C., Mendes, R., Baptista, P. V., and Fernandes, A. R. (2019). Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 20, 840. doi:10.3390/ijms20040840
Rosell, R., Bivona, T. G., and Karachaliou, N. (2013). Genetics and biomarkers in personalisation of lung cancer treatment. Lancet 382, 720–731. doi:10.1016/S0140-6736(13)61715-8
Rudakova, A., Meshkov, D., Khabriev, R., and Bezmelnitsyna, L. (2015). Efficiency of expenses of gefitinibum in patients with non-small cell lung cancer as A second line therapy. Value Health 18, A464–A465. doi:10.1016/j.jval.2015.09.1213
Russo, M., and Nastasi, C. (2022). Targeting the tumor microenvironment: a close up of tumor-associated macrophages and neutrophils. Front. Oncol. 12, 871513. doi:10.3389/fonc.2022.871513
Sabbah, M., Emami, S., Redeuilh, G., Julien, S., Prévost, G., Zimber, A., et al. (2008). Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resist. Updat. 11, 123–151. doi:10.1016/j.drup.2008.07.001
Sabir, F., Qindeel, M., Zeeshan, M., Ul Ain, Q., Rahdar, A., Barani, M., et al. (2021). Onco-receptors targeting in lung cancer via application of surface-modified and hybrid nanoparticles: a cross-disciplinary review. Processes 9, 621. doi:10.3390/pr9040621
Saha, A., Mishra, A., Manna, S., Banik, A., Goswami, S., Bhattacharya, J., et al. (2024). Assessing the adequacy of a 5-mm planning target volume margin for 4D-CT scan-based image-guided radiotherapy for locally advanced carcinoma of the lung. Adv. Radiotherapy and Nucl. Med. 2, 2784. doi:10.36922/arnm.2784
Salgia, R., Stille, J. R., Weaver, R. W., Mccleod, M., Hamid, O., Polzer, J., et al. (2017). A randomized phase II study of LY2510924 and carboplatin/etoposide versus carboplatin/etoposide in extensive-disease small cell lung cancer. Lung Cancer 105, 7–13. doi:10.1016/j.lungcan.2016.12.020
Santagata, S., Ieranò, C., Trotta, A. M., Capiluongo, A., Auletta, F., Guardascione, G., et al. (2021). CXCR4 and CXCR7 signaling pathways: a focus on the cross-talk between cancer cells and tumor microenvironment. Front. Oncol. 11, 591386. doi:10.3389/fonc.2021.591386
Shao, X., Hua, S., Feng, T., Ocansey, D. K. W., and Yin, L. (2022). Hypoxia-regulated tumor-derived exosomes and tumor progression: a focus on immune evasion. Int. J. Mol. Sci. 23, 11789. doi:10.3390/ijms231911789
Sharma, R. (2022). Mapping of global, regional and national incidence, mortality and mortality-to-incidence ratio of lung cancer in 2020 and 2050. Int. J. Clin. Oncol. 27, 665–675. doi:10.1007/s10147-021-02108-2
Shojaei, S., Pourmadadi, M., Homayoonfal, M., Behnamrad, P., Fathi-Karkan, S., Rahdar, A., et al. (2024). Revolutionizing lung cancer treatment: nanotechnology-driven advances in targeted drug delivery and novel therapeutic strategies. J. Drug Deliv. Sci. Technol. 101, 106186. doi:10.1016/j.jddst.2024.106186
Singla, A. K., Downey, C. M., Bebb, G. D., and Jirik, F. R. (2015). Characterization of a murine model of metastatic human non-small cell lung cancer and effect of CXCR4 inhibition on the growth of metastases. Oncoscience 2, 263–271. doi:10.18632/oncoscience.117
Spiro, S. G., and Porter, J. C. (2002). Lung cancer—where are we today? Current advances in staging and nonsurgical treatment. Am. J. Respir. Crit. Care Med. 166, 1166–1196. doi:10.1164/rccm.200202-070SO
Srivastava, S., Mohanty, A., Nam, A., Singhal, S., and Salgia, R. (2022). Chemokines and NSCLC: emerging role in prognosis, heterogeneity, and therapeutics. Seminars Cancer Biol. 86, 233–246. doi:10.1016/j.semcancer.2022.06.010
Su, L., Zhang, J., Xu, H., Wang, Y., Chu, Y., Liu, R., et al. (2005). Differential expression of CXCR4 is associated with the metastatic potential of human non–small cell lung cancer cells. Clin. Cancer Res. 11, 8273–8280. doi:10.1158/1078-0432.CCR-05-0537
Sun, Y. (2016). Tumor microenvironment and cancer therapy resistance. Cancer Lett. 380, 205–215. doi:10.1016/j.canlet.2015.07.044
Sun, X., Cheng, G., Hao, M., Zheng, J., Zhou, X., Zhang, J., et al. (2010). CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 29, 709–722. doi:10.1007/s10555-010-9256-x
Tajaldini, M., Poorkhani, A., Amiriani, T., Amiriani, A., Javid, H., Aref, P., et al. (2023). Strategy of targeting the tumor microenvironment via inhibition of fibroblast/fibrosis remodeling new era to cancer chemo-immunotherapy resistance. Eur. J. Pharmacol. 957, 175991. doi:10.1016/j.ejphar.2023.175991
Tashkandi, H., and Younes, I. E. (2024). Advances in molecular understanding of polycythemia vera, essential thrombocythemia, and primary myelofibrosis: towards precision medicine. Cancers 16, 1679. doi:10.3390/cancers16091679
Thapa, R., Afzal, O., Bhat, A. A., Goyal, A., Alfawaz Altamimi, A. S., Almalki, W. H., et al. (2023). New horizons in lung cancer management through ATR/CHK1 pathway modulation. Future Med. Chem. 15, 1807–1818. doi:10.4155/fmc-2023-0164
Thapa, R., Moglad, E., Goyal, A., Bhat, A. A., Almalki, W. H., Kazmi, I., et al. (2024). Deciphering NF-kappaB pathways in smoking-related lung carcinogenesis. EXCLI J. 23, 991–1017. doi:10.17179/excli2024-7475
Tilsed, C. M., Fisher, S. A., Nowak, A. K., Lake, R. A., and Lesterhuis, W. J. (2022). Cancer chemotherapy: insights into cellular and tumor microenvironmental mechanisms of action. Front. Oncol. 12, 960317. doi:10.3389/fonc.2022.960317
Vasan, N., Baselga, J., and Hyman, D. M. (2019). A view on drug resistance in cancer. Nature 575, 299–309. doi:10.1038/s41586-019-1730-1
Wald, O. (2018). CXCR4 based therapeutics for non-small cell lung cancer (NSCLC). J. Clin. Med. 7, 303. doi:10.3390/jcm7100303
Wald, O., Izhar, U., Amir, G., Kirshberg, S., Shlomai, Z., Zamir, G., et al. (2011). Interaction between neoplastic cells and cancer-associated fibroblasts through the CXCL12/CXCR4 axis: role in non–small cell lung cancer tumor proliferation. J. Thorac. Cardiovasc. Surg. 141, 1503–1512. doi:10.1016/j.jtcvs.2010.11.056
Wang, Y., Li, J., and Oupický, D. (2014). Polymeric plerixafor: effect of PEGylation on CXCR4 antagonism, cancer cell invasion, and DNA transfection. Pharm. Res. 31, 3538–3548. doi:10.1007/s11095-014-1440-1
Wang, Z., Sun, J., Feng, Y., Tian, X., Wang, B., and Zhou, Y. (2016). Oncogenic roles and drug target of CXCR4/CXCL12 axis in lung cancer and cancer stem cell. Tumor Biol. 37, 8515–8528. doi:10.1007/s13277-016-5016-z
Wang, S., Gao, S., Li, Y., Qian, X., Luan, J., and Lv, X. (2021). Emerging importance of chemokine receptor CXCR4 and its ligand in liver disease. Front. Cell Dev. Biol. 9, 716842. doi:10.3389/fcell.2021.716842
Wang, H., Zhao, X., Zhang, H., Zou, X., Jia, D., Liu, W., et al. (2023a). EGFR-antagonistic affibody-functionalized Pt-based nanozyme for enhanced tumor radiotherapy. Mater. Today Adv. 18, 100375. doi:10.1016/j.mtadv.2023.100375
Wang, N., Zhao, Q., Huang, Y., Wen, C., Li, Y., Bao, M., et al. (2023b). Lnc-TMEM132D-AS1 as a potential therapeutic target for acquired resistance to osimertinib in non-small-cell lung cancer. Mol. Omics 19, 238–251. doi:10.1039/d2mo00261b
Wang, S., Li, J., Hong, S., Wang, N., Xu, S., Yang, B., et al. (2024a). Chemotherapy-elicited extracellular vesicle CXCL1 from dying cells promotes triple-negative breast cancer metastasis by activating TAM/PD-L1 signaling. J. Exp. and Clin. Cancer Res. 43, 121. doi:10.1186/s13046-024-03050-7
Wang, Z. B., Zhang, X., Fang, C., Liu, X. T., Liao, Q. J., Wu, N., et al. (2024b). Immunotherapy and the ovarian cancer microenvironment: exploring potential strategies for enhanced treatment efficacy. Immunology 173, 14–32. doi:10.1111/imm.13793
Weiss, I. D., Jacobson, O., Kiesewetter, D. O., Jacobus, J. P., Szajek, L. P., Chen, X., et al. (2012). Positron emission tomography imaging of tumors expressing the human chemokine receptor CXCR4 in mice with the use of 64 Cu-AMD3100. Mol. imaging Biol. 14, 106–114. doi:10.1007/s11307-010-0466-y
Weiss, I. D., Huff, L. M., Evbuomwan, M. O., Xu, X., Dang, H. D., Velez, D. S., et al. (2017). Screening of cancer tissue arrays identifies CXCR4 on adrenocortical carcinoma: correlates with expression and quantification on metastases using 64Cu-plerixafor PET. Oncotarget 8, 73387–73406. doi:10.18632/oncotarget.19945
Wen, C., Li, Y., Huang, Y., Wang, N., He, S., Bao, M., et al. (2023). CircSETD3 mediates acquired resistance to gefitinib in non-small lung cancer cells by FXR1/ECT2 pathway. Int. J. Biochem. Cell Biol. 154, 106344. doi:10.1016/j.biocel.2022.106344
Wood, S. L., Pernemalm, M., Crosbie, P. A., and Whetton, A. D. (2014). The role of the tumor-microenvironment in lung cancer-metastasis and its relationship to potential therapeutic targets. Cancer Treat. Rev. 40, 558–566. doi:10.1016/j.ctrv.2013.10.001
Wu, T., Zhao, Y., Zhang, X., Wang, Y., Chen, Q., Zhang, M., et al. (2025). Short-chain acyl post-translational modifications in cancers: mechanisms, roles, and therapeutic implications. Cancer Commun. (Lond) 45, 1247–1284. doi:10.1002/cac2.70048
Xie, S., Zeng, W., Fan, G., Huang, J., Kang, G., Geng, Q., et al. (2014). Effect of CXCL12/CXCR4 on increasing the metastatic potential of non-small cell lung cancer in vitro is inhibited through the downregulation of CXCR4 chemokine receptor expression. Oncol. Lett. 7, 941–947. doi:10.3892/ol.2014.1837
Xing, B., Yang, L., and Cui, Y. (2022). Lidocaine inhibited migration of NSCLCA549 cells via the CXCR4 regulation. Cancer Biomarkers 33, 317–330. doi:10.3233/CBM-210249
Xue, Y., Gao, S., Gou, J., Yin, T., He, H., Wang, Y., et al. (2021). Platinum-based chemotherapy in combination with PD-1/PD-L1 inhibitors: preclinical and clinical studies and mechanism of action. Expert Opin. Drug Deliv. 18, 187–203. doi:10.1080/17425247.2021.1825376
Xun, Y., Yang, H., Li, J., Wu, F., and Liu, F. (2020). CXC chemokine receptors in the tumor microenvironment and an update of antagonist development. Rev. Physiology, Biochem. Pharmacol. 178, 1–40. doi:10.1007/112_2020_35
Yang, P., Hu, Y., and Zhou, Q. (2020). The CXCL12-CXCR4 signaling axis plays a key role in cancer metastasis and is a potential target for developing novel therapeutics against metastatic cancer. Curr. Med. Chem. 27, 5543–5561. doi:10.2174/0929867326666191113113110
Yang, Y., Li, J., Lei, W., Wang, H., Ni, Y., Liu, Y., et al. (2023). CXCL12-CXCR4/CXCR7 axis in cancer: from mechanisms to clinical applications. Int. J. Biol. Sci. 19, 3341–3359. doi:10.7150/ijbs.82317
Yin, X., Liu, Z., Zhu, P., Wang, Y., Ren, Q., Chen, H., et al. (2019). CXCL12/CXCR4 promotes proliferation, migration, and invasion of adamantinomatous craniopharyngiomas via PI3K/AKT signal pathway. J. Cell. Biochem. 120, 9724–9736. doi:10.1002/jcb.28253
Yue, Z., Ningning, D., Lin, Y., Jianming, Y., Hongtu, Z., Ligong, Y., et al. (2020). Correlation between CXCR4, CXCR5 and CCR7 expression and survival outcomes in patients with clinical T1N0M0 non-small cell lung cancer. Thorac. Cancer 11, 2955–2965. doi:10.1111/1759-7714.13645
Zhang, C., Li, J., Han, Y., and Jiang, J. (2015). A meta-analysis for CXCR4 as a prognostic marker and potential drug target in non-small cell lung cancer. Drug Des. Dev. Ther. 9, 3267–3278. doi:10.2147/DDDT.S81564
Zhang, L., Zhou, C., Zhang, S., Chen, X., Liu, J., Xu, F., et al. (2022). Chemotherapy reinforces anti-tumor immune response and enhances clinical efficacy of immune checkpoint inhibitors. Front. Oncol. 12, 939249. doi:10.3389/fonc.2022.939249
Zhao, Y., Detering, L., Sultan, D., Cooper, M. L., You, M., Cho, S., et al. (2016). Gold nanoclusters doped with (64)Cu for CXCR4 positron emission tomography imaging of breast cancer and metastasis. ACS Nano 10, 5959–5970. doi:10.1021/acsnano.6b01326
Zhao, R., Liu, J., Li, Z., Zhang, W., Wang, F., and Zhang, B. (2022). Recent advances in CXCL12/CXCR4 antagonists and nano-based drug delivery systems for cancer therapy. Pharmaceutics 14, 1541. doi:10.3390/pharmaceutics14081541
Zhou, X.-M., He, L., Hou, G., Jiang, B., Wang, Y.-H., and Zhao, L. (2015). Clinicopathological significance of CXCR4 in non-small cell lung cancer. Drug Des. Dev. Ther. 9, 1349–1358. doi:10.2147/DDDT.S71060
Zhou, W., Guo, S., Liu, M., Burow, M. E., and Wang, G. (2019). Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr. Med. Chem. 26, 3026–3041. doi:10.2174/0929867324666170830111531
Zhou, C., Li, S., Liu, J., Chu, Q., Miao, L., Cai, L., et al. (2021). International consensus on severe lung cancer—the first edition. Transl. Lung Cancer Res. 10, 2633–2666. doi:10.21037/tlcr-21-467
Zhu, Y.-H., Zheng, J.-H., Jia, Q.-Y., Duan, Z.-H., Yao, H.-F., Yang, J., et al. (2023). Immunosuppression, immune escape, and immunotherapy in pancreatic cancer: focused on the tumor microenvironment. Cell. Oncol. 46, 17–48. doi:10.1007/s13402-022-00741-1
Keywords: CXCR4, lung cancer, tumor progression, plerixafor, metastasis, prognosis, immune cell migration, tumor microenvironment (TME)
Citation: Ramalingam PS, Afzal M, Babu MA, Rekha MM, Sahoo S, Pandey SN, Goyal K, Hussain MS, Gupta G, Balakrishnan P and Arumugam S (2025) CXCR4-targeted therapy in lung cancer: plerixafor as a promising antimetastatic agent. Front. Pharmacol. 16:1683585. doi: 10.3389/fphar.2025.1683585
Received: 11 August 2025; Accepted: 05 November 2025;
Published: 26 November 2025.
Edited by:
Stefano Martellucci, Link Campus University, ItalyReviewed by:
Qingbin Cui, University of Toledo College of Medicine and Life Sciences, United StatesShashanka Prasad, JSS Academy of Higher Education and Research, India
Copyright © 2025 Ramalingam, Afzal, Babu, Rekha, Sahoo, Pandey, Goyal, Hussain, Gupta, Balakrishnan and Arumugam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sivakumar Arumugam, c2l2YV9rdW1hci5hQHZpdC5hYy5pbg==
 Prasanna Srinivasan Ramalingam
Prasanna Srinivasan Ramalingam Muhammad Afzal
Muhammad Afzal M. Arockia Babu3
M. Arockia Babu3 Md Sadique Hussain
Md Sadique Hussain Purushothaman Balakrishnan
Purushothaman Balakrishnan Sivakumar Arumugam
Sivakumar Arumugam