Abstract
Cardiovascular diseases (CVDs) remain the leading cause of mortality among non-communicable diseases worldwide. Vascular smooth muscle cells (VSMCs), as the predominant cellular component of the tunica media, are essential for maintaining vascular homeostasis through phenotype-dependent regulation of vascular tone, blood pressure, and hemodynamics. Under pathological conditions such as hypoxia or inflammation, VSMCs undergo phenotypic switching from a contractile to a synthetic state. This transition is characterized by excessive proliferation, migration, and pro-inflammatory secretion, all of which contribute to the progression of atherosclerosis and restenosis. Tanshinones, bioactive diterpenoid compounds isolated from Salvia miltiorrhiza, exert cardioprotective effects through their anti-inflammatory, antioxidant, and VSMC-modulating activities. Increasing evidence suggests that tanshinones attenuate maladaptive VSMC behaviors by regulating calcium signaling, modulating programmed cell death pathways, and suppressing pro-inflammatory signaling cascades. These actions collectively inhibit phenotypic switching and mitigate vascular remodeling and plaque formation. Despite these advances, a comprehensive understanding of the precise molecular targets and signaling networks of tanshinones in VSMCs is still lacking. This review aims to integrate current evidence to delineate tanshinone-mediated VSMC regulatory mechanisms, provide mechanistic insights, and identify potential therapeutic targets for phenotype-directed interventions in CVDs.
1 Indroduction
Cardiovascular diseases (CVDs) represent a primary contributor to early deaths and a major driver of the escalating global healthcare burden (GBD 2019 Diseases and Injuries Collaborators, 2020). The latest Global Burden of Disease Study reveals a substantial increase in CVD prevalence, from 271 million cases in 1990 to 523 million cases in 2019, underscoring the pressing need for targeted interventions to combat this expanding public health challenge (Roth et al., 2020). The pathophysiological mechanisms underlying CVDs are multifaceted, encompassing atherosclerosis, extracellular matrix remodeling, vascular calcification, and immune cell infiltration (Libby, 2024). Vascular smooth muscle cells (VSMCs), constituting the vascular tunica media, critically regulate vascular homeostasis (Majesky and Weiser-Evans, 2022). Intraluminal lipoproteins and cytokines trigger VSMC migration and proliferation toward the intima, where extracellular matrix (ECM) synthesis and fibrous cap formation drive plaque development (Yu et al., 2024). Physiologically, VSMCs preserve a contractile phenotype necessary for vascular tone modulation and vasomotor control. Pathologically, stimuli including vascular injury, oxidative stress, or chronic inflammation induce synthetic phenotypic switching, enhancing proliferative and migratory capacities. This phenotypic switching represents a fundamental pathogenic driver of CVD progression (Zhao et al., 2024; Wang S. et al., 2024).
VSMCs display remarkable plasticity. In response to pathological stimuli such as vascular injury, infiltration of inflammatory cytokines, lipid accumulation, and oxidative stress, contractile VSMCs can dedifferentiate into a synthetic phenotype (Jiang and Qian, 2023; Khachigian et al., 2022). Synthetic VSMCs exhibit a flattened morphology, enlarged cell size, reduced contractile filaments, impaired contractile function, and enhanced proliferative capacity. These proliferative synthetic VSMCs can secrete matrix metalloproteinases (MMPs) and pro-inflammatory cytokines, thereby accelerating extracellular matrix remodeling and recruiting monocytes (Wang et al., 2020). Moreover, in extracellular environments with elevated calcium and phosphate concentrations, synthetic VSMCs release calcium-phosphate-rich vesicles, which can induce osteogenic transdifferentiation of VSMCs, leading to upregulation of osteogenic transcription factors and promoting spontaneous vascular calcification (Reynolds et al., 2004). Single-cell sequencing studies have further revealed substantial heterogeneity among plaque-resident VSMCs, with certain subpopulations exhibiting stem cell–like properties and enhanced proliferative potential, potentially driving atherosclerotic plaque progression through clonal expansion (Gomez et al., 2013). Consequently, therapeutic strategies targeting VSMC plasticity offer significant clinical potential.
The regulation of VSMCs is complex and involves a network of molecular pathways. Natural medicines, particularly those with multi-target and multi-pathway regulatory effects, have demonstrated considerable potential in modulating VSMCs function. Tanshinones, a group of compounds derived from the Chinese herbal medicine Salvia miltiorrhiza, have been extensively studied for their cardiovascular benefits. These compounds include Tanshinone I (Tan I, C18H12O3), Tanshinone IIA (Tan IIA, C19H18O3), Dihydrotanshinone (C18H14O3), Cryptotanshinone (C19H20O3), Isotanshinone I (C18H12O3), Isotanshinone IIA (C19H18O3), and Isocryptotanshinone (C19H20O3) (Jiang et al., 2019). Among these, Tan I, Tan IIA, and Cryptotanshinone have garnered significant attention for their ability to improve cardiovascular function, as well as their anti-inflammatory, anti-tumor, and anti-oxidative properties (Shi et al., 2019; Wang J. et al., 2024; Wu et al., 2019). To overcome the limitation of poor water solubility of tanshinones, the sulfonated derivative of Tan IIA, Sodium tanshinone IIA sulfonate (STS), has been developed and shown to be effective in the treatment of CVD (Wei et al., 2024).
Research into the pharmacodynamics and molecular mechanisms of natural active compounds is currently a major focus in cardiovascular pharmacology. Recent studies suggest that tanshinones may regulate VSMCs function, positioning them as potential therapeutic agents for CVD. However, a systematic understanding of their precise molecular mechanisms and specific molecular targets remains lacking. This review explores the molecular mechanisms by which tanshinones modulate VSMC function, thereby elucidating the basis for their cardiovascular protective effects and establishing a scientific foundation for their therapeutic application in CVD.
2 Mechanisms of tanshinones in regulating VSMCs function
The healthy arterial structure consists of three distinct layers (Majesky and Weiser-Evans, 2022): (1) the tunica intima, a single endothelial cell layer lining the bloodstream; (2) the tunica media, composed of VSMCs and layers of elastic fibers, including elastin and collagen, which provide elasticity and strength to the blood vessels; and (3) the tunica adventitia, made up of adipocytes, fibrous connective tissue, and extracellular matrix, which provide structural support, nutrition, and innervation. The elasticity of arteries primarily arises from the active contraction of VSMCs and the passive recoil of the elastic lamellae formed by collagen and elastin fibers. As a result, contractile VSMCs play a crucial role in regulating vascular diameter, blood pressure, and blood flow distribution (Yamin and Morgan, 2012). In large arteries, VSMCs maintain a contractile state to preserve the normal shape of arteries during ventricular systole and ejection, whereas in small resistance arteries, VSMCs are responsible for regulating blood flow distribution (Farina et al., 2020). The dysfunction or loss of normal VSMCs function represents a core pathological process in the onset and progression of various CVDs, including atherosclerosis, hypertension, and vascular restenosis. The mechanisms through which tanshinones regulate VSMCs function primarily include the inhibition of VSMCs proliferation and migration, suppression of VSMCs phenotypic switching, anti-inflammatory effects, regulation of programmed cell death, and modulation of calcium ion signaling. The synergistic interaction of these multiple targets and pathways forms an essential molecular foundation for the ability of tanshinones to alleviate VSMCs dysfunction and exert cardiovascular protective effects. Table 1 presents the references cited in this study, while Figure 1 illustrates the mechanism by which tanshinones regulate VSMC function.
TABLE 1
| Reference | Compound | Experimental model | Concentration | Duration | Setting | Key effects | Molecular markers & pathways | Target disease | Proliferation/migration Cell seeding density |
|---|---|---|---|---|---|---|---|---|---|
| Lu et al. (2019) | Tan IIA | SD rat aortic VSMCs + Ang II | 3.4, 17.0, 34.0 μmol/L | 24 h, 48 h | in vitro | Inhibited proliferation; Promoted apoptosis; Increased autophagy | LC3-II↓, Beclin-1↓, p-p38↓, c-Myc↓, c-Fos↓, MAPK↓ | – | 5 × 103 cells/well, - |
| Li et al. (2023) | Tan IIA | Human VSMCs + ox-LDL | 8.5, 17.0, 34.0 μmol/L | 24 h | in vitro | Inhibited proliferation and migration | miR-137↑, TRPC3↓, PCNA↓ | Atherosclerosis | 1 × 104 cells/well, - |
| Lu et al. (2018) | Tan IIA | SD rat aortic VSMCs + AGEs | 10 μmol/L | 48 h | in vivo | Inhibited proliferation and migration | p-ERK1/2↓, MAPK↓ | – | 1 × 104 cells/well, 1 × 104 cells/well |
| Li X. et al. (2020) | Tan IIA | Human VSMCs + Hcy | 0, 0.1, 1, 10 μmol/L | 24 h | in vitro | Inhibited proliferation | miR-145↓, CD40↑, KLF4↑ | – | -, 1 × 105 cells/well |
| Lou et al. (2020) | Tan IIA | C57BL/6 mice + Carotid ligation | 5 mg/kg | 3 w | in vivo | Inhibited proliferation; Suppressed neointima; Attenuated remodeling | PCNA↓ | Vascular damage | -, - |
| Tan IIA | SD rat aortic VSMCs + PDGF-BB | 1.0 μmol/L | 12–30 h | in vitro | Inhibited proliferation, migration, phenotypic switching | MHC↑, Calponin↑, SM22α↑, Myocardin↑, SRF↑, Cyclin D1↓, CDKN1A↑, CDKN1B↑, KLF4↑ | – | 3 × 103 cells/well, 1 × 106 cells/well | |
| Qin et al. (2020) | Tan IIA | C57BL/6 mice + Carotid ligation | 10 mg/kg | 21 days | in vivo | Suppressed neointimal hyperplasia; Anti-inflammatory | TNF-α↓, KLF4↑, IL-1β↓, IL-6↓ | Vascular damage | -, - |
| Tan IIA | Mouse aortic VSMCs + TNF-α | 1.7, 3.4, 6.8 μmol/L | 48 h | in vitro | Anti-inflammatory; Inhibited proliferation | TNF-α↓, KLF4↑, IL-1β↓, IL-6↓, miR-712-5p↓,NF-κB↓ | – | 1 × 104 cells/well, - | |
| Meng et al. (2019) | Tan IIA | SD rat aortic VSMCs + LPS | 6.25–200 μmol/L | 24 h | in vitro | Anti-inflammatory; Inhibited phenotypic switching | α-SMA↑, OPN↓, MCP-1↓, IL-6↓, TNF-α↓, iNOS↓, NO↓, ROS↓, TLR4↓, p-TAK1↓, NF-κB↓ | – | 5 × 103 cells/well, 5 × 103 cells/well |
| Chen et al. (2014) | Tan IIA | Wistar rat PASMCs + Hypoxia | 1.7, 3.4, 17.0 μmol/L | 24 h | in vitro | Reduced viability; Promoted apoptosis; Inhibited proliferation | Hsp60↓, Cleaved Caspase-3↑, JAK2↓, STAT3↓, Cx43↑ | Hypoxia | -, - |
| Wu et al. (2014) | STS | SD rat aortic VSMCs + High Glucose | 10 μmol/L | 24 h | in vitro | Inhibited proliferation and migration | p-AMPK↑, Cyclin D1↓, p53↑, p21↑, NF-κB↓, MMP-2↓ | Diabetic Vascular Disease | -, 4 × 104 cells/well |
| Wei et al. (2024) | STS | db/db mouse aortic VSMCs + High Glucose | 100 μmol/L | 72 h | in vitro | Attenuated senescence; Anti-inflammatory | IL-1β↓, IL-18↓, A20↑, CAT↑, H2O2↓, NLRP3↓, Caspase-1↓, NF-κB↓ | Diabetic Vascular Disease | -, - |
| (Wang et al., 2013) | STS | SD rats + Hypoxia + MCT | 10 mg/kg | 21 days | in vivo | Reduced Ca2+ influx; Improved hemodynamics | [Ca2+]i↓, SOCE↓, TRPC1↓, TRPC6↓ | Pulmonary Hypertension | -, - |
| STS | SD rat PASMCs + Hypoxia | 0.1–25 μmol/L | 24 h | in vitro | Inhibited proliferation, migration; Reduced Ca2+ influx | [Ca2+]i↓, SOCE↓, TRPC1↓, TRPC6↓ | Hypoxia | -, 1 × 105 cells/well | |
| Zhang et al. (2018) | STS | SD rats + Hypoxia + MCT | 30 mg/kg | 21 days | in vivo | Reduced Ca2+ influx; Improved hemodynamics | [Ca2+]i↓, LTCC↓ | – | -, - |
| STS | SD rat PASMCs + Hypoxia | 12.5 μmol/L | 60 h | in vitro | Inhibited proliferation; Reduced Ca2+ influx | [Ca2+]i↓, LTCC↓ | – | -, - | |
| Wu et al. (2019) | Tan I | Human VSMCs + Ang II | 0.625, 1.25, 2.5 μmol/L | 24 h | in vitro | Inhibited proliferation | IGF-1↓, PI3K↓ | – | 5 × 103 cells/well, - |
| Wang J. et al. (2024) | Cryptotanshinone | ApoE−/− mice + Ang II | 15, 50 mg/kg | 28 days | in vivo | Inhibited phenotypic switching | α-SMA↑, SM22α↑ | Abdominal aortic aneurysm | -, - |
| Cryptotanshinone | SD rat aortic VSMCs + TNF-α | 2.5–10 μmol/L | 24 h | in vitro | Inhibited phenotypic switching; Anti-inflammatory; Attenuated pyroptosis | VCAM-1↓, MMP-2↓, MMP-3↓, MMP-9↓, α-SMA↑, SM22α↑, IL-1β↓, IL-6↓, CCL2↓, NLRP3↓, Nrf2/HO-1↑ | – | 1 × 104 cells/well, - | |
| Oche et al. (2016) | Cryptotanshinone | LPS + VSMCs A7r5 | 0.1, 1, 10 μmol/L | 24 h | in vitro | Anti-inflammatory | ERβ ↑, iNOS ↓, NO ↓ | – | 5 × 103 cells/well, - |
Experimental data on tanshinone-mediated VSMC regulation.
Drug concentrations are standardized to molarity (μmol/L) to enable direct cross-study comparison of pharmacological data. Conversions were performed using the formula: μmol/L = [μg/mL × 1,000]/Molecular Weight (g/mol), with compound-specific molecular weights applied to original values from each source study.
FIGURE 1
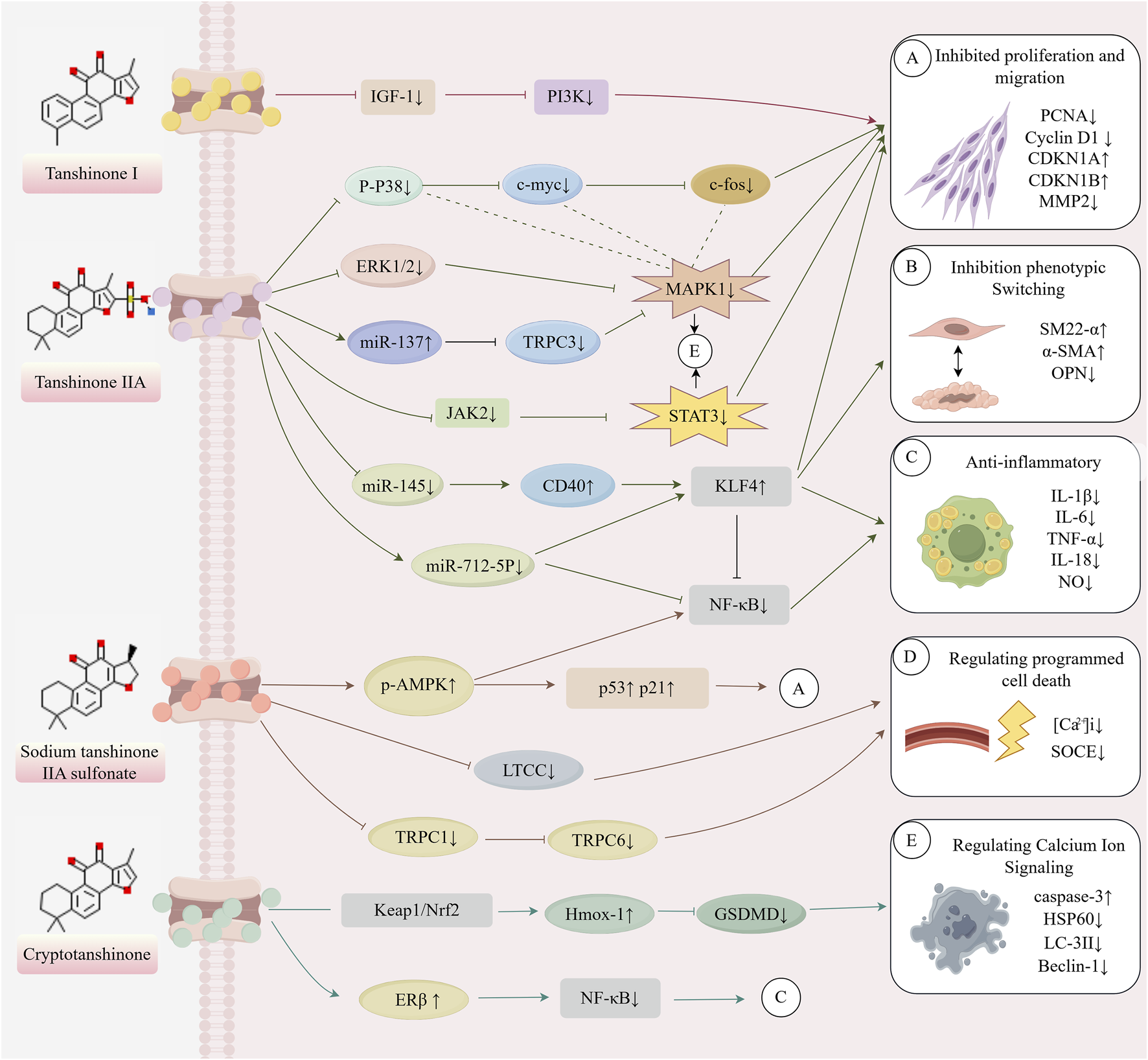
Mechanism of action of tanshinone in regulating VSMCs (This image was originally created by Figrdraw, ID: YPIWO04099).
Tanshinones, the principal bioactive constituents of S. miltiorrhiza, have recently been shown to exert multifaceted cardiovascular protective effects. Accumulating evidence indicates that tanshinones effectively inhibit the proliferation and migration of VSMCs induced by ox-LDL and other stimuli, while preserving their contractile phenotype through the regulation of transcription factors such as KLF4. In addition, tanshinones modulate programmed cell death processes—including autophagy, apoptosis, and pyroptosis—thereby contributing to cellular homeostasis. Beyond these effects, their pronounced anti-inflammatory and antioxidant activities improve the vascular microenvironment. Tanshinones also influence VSMC contractile function and intracellular stability by regulating TRPC channels and calcium signaling pathways. This review summarizes recent advances in understanding how tanshinones regulate VSMC function. It highlights their molecular mechanisms across multiple pathways, including proliferation and migration, phenotypic switching, programmed cell death, inflammatory responses, and calcium signaling. These findings provide a theoretical foundation for advancing our knowledge of tanshinone pharmacology and for developing novel VSMC-targeted therapeutic strategies in cardiovascular disease. Table 1 presents the references cited in this study, while Figure 1 illustrates the mechanism by which tanshinones regulate VSMC function.
2.1 Inhibiting VSMCs proliferation and migration
Under physiological conditions, quiescent contractile VSMCs exhibit minimal proliferative activity and primarily contribute to regulating blood circulation by maintaining vascular tone and homeostasis (Lutgens et al., 1999). However, in response to vascular injury, their proliferative capacity increases abnormally. VSMCs migration is frequently coupled with proliferation, involving key biological processes such as the establishment of cell polarity, cytoskeletal remodeling, and the dynamic integration of microenvironmental signals (Afewerki et al., 2019). During initial vascular remodeling, injury, or CVD pathogenesis, local inflammatory mediators (e.g., TNF-α, IL-18, ox-LDL) induce VSMC migration toward the intima (Liu et al., 2016). These aberrantly proliferating/migrating VSMCs secrete excessive collagen, elastin, and MMPs, driving endothelial dysfunction and inflammatory microenvironment accumulation. Consequently, vessel wall thickening, luminal narrowing, and pathological remodeling occur, culminating in tissue hypoperfusion and functional impairment (Xin et al., 2024). Tan IIA exhibits both anti-inflammatory and antioxidant properties and plays a critical role in cardiovascular and inflammatory diseases (Fang et al., 2018; Chen and Xu, 2014). Moreover, accumulating evidence suggests that Tan IIA attenuates the progression of atherosclerosis by suppressing inflammation and oxidative stress in animal models (Wen et al., 2020; Chen et al., 2012). However, the precise mechanisms by which Tan IIA regulates ox-LDL–induced proliferation and migration of VSMCs remain largely unclear. Li (Li et al., 2023) treated ox-LDL-stimulated human VSMCs with Tan IIA at 10 μg/mL, demonstrating a dose-dependent inhibition of VSMCs proliferation and migration through regulation of the miR-137/TRPC3 axis. Similarly, Yao Li (Li Y. et al., 2020) confirmed that Tan IIA can inhibit homocysteine (Hcy)-induced VSMCs proliferation by upregulating miR-145 and downregulating CD40. Moreover, signaling pathways such as the ERK1/2 MAPK pathway (Lu et al., 2018) and the AMPK/p53/p21 pathway (Wu et al., 2014) may also represent potential targets for Tan IIA action. Additionally, Tan I has shown potential in regulating VSMCs function. Wu (Wu et al., 2019) found that Tan I dose-dependently inhibits the IGF-1R/PI3K signaling pathway, suppressing the expression of VSMCs proliferation-related proteins, including CDK4, cyclin D3, and cyclin D1, thereby reducing VSMCs proliferative capacity. These studies collectively provide compelling evidence supporting the role of tanshinones in antagonizing VSMCs proliferation and migration.
2.2 Inhibiting VSMCs phenotypic switching
VSMCs exhibit remarkable plasticity. In response to pathological stimuli such as vascular wall injury, growth factor stimulation, inflammatory cytokine infiltration (e.g., TNF-α, IL-6, IL-18), lipid accumulation, and oxidative stress, contractile VSMCs undergo dedifferentiation, transitioning into synthetic VSMCs (Jiang and Qian, 2023; Khachigian et al., 2022). Synthetic VSMCs exhibit a flattened morphology, with increased cell size, reduced myofilaments, and diminished contractile function. Notably, following injury repair, synthetic VSMCs can revert to the contractile phenotype (Yu et al., 2024; Wang et al., 2023; Zhang et al., 2025). In the treatment of vascular diseases, tanshinone IIA has been shown to attenuate atherosclerotic calcification primarily through the inhibition of oxidative stress (Tang et al., 2007). However, whether tanshinone IIA plays a pivotal role in regulating VSMC phenotypic switching and pathological vascular remodeling remains largely unclear. Through a combination of in vivo and in vitro experiments, Lou (Lou et al., 2020) demonstrated that Tan IIA regulates VSMCs phenotypic switching by enhancing the expression of KLF4. This results in the inhibition of VSMCs proliferation, the induction of differentiation, and the modulation of pathological vascular remodeling. Wang J. et al. (2024) demonstrated that 28-day cryptotanshinone infusion in Ang II-induced ApoE−/− mouse aortic aneurysm models attenuated reductions in VSMC contractile markers (α-SMA, SM22α). Synthetic VSMCs may transdifferentiate into diverse pathological phenotypes under specific stimuli, notably osteoblast-like VSMCs–key drivers of vascular calcification that increase arterial stiffness and reduce compliance (Hortells et al., 2018). These findings collectively demonstrate tanshinones’ capacity to modulate pathological VSMC plasticity.
2.3 Regulating programmed cell death
Programmed cell death plays a crucial role in cellular pathophysiology, as it helps maintain cellular homeostasis by regulating cell survival and death. The common forms of programmed cell death include autophagy, apoptosis, and pyroptosis (Kari et al., 2022). While moderate autophagy is essential for maintaining VSMCs phenotype and contractile function, excessive autophagy can adversely affect cell survival and promote the transition of VSMCs from a contractile to a synthetic phenotype, thereby diminishing VSMCs contractile function (Li et al., 2019; He et al., 2022). Previous studies have demonstrated that Tan IIA can inhibit cardiomyocyte apoptosis via suppression of the ERK1/2 signaling pathway (Zhang et al., 2012). In addition, Tan IIA has been reported to regulate programmed cell death in cancer cells through modulation of multiple signaling pathways (Won et al., 2010; Yun et al., 2013). Lu (Lu et al., 2019) demonstrated that Tan IIA (5–10 μg/mL) suppresses Ang II-induced VSMC proliferation and dysregulated autophagy via MAPK pathway downregulation, concurrently promoting apoptosis in vitro. Complementarily, Chen (Chen et al., 2014) reported Tan IIA modulation of Hsp60, caspase-3, and connexin 43 (Cx43) expression through JAK2/STAT3 signaling, thereby inducing apoptosis in hypoxic PASMCs. Notably, pathological pyroptosis in VSMCs critically drives abdominal aortic aneurysm pathogenesis. Wang J. et al. (2024) observed that cryptotanshinone can activate the transcription of Nrf2 target genes in TNF-α-induced thoracic aortic VSMCs from Sprague-Dawley rats. This activation prevents NLRP3 inflammasome activation and GSDMD-mediated pyroptosis, thereby alleviating VSMCs inflammation and preserving the contractile phenotype of VSMCs.
2.4 Anti-inflammatory effects
The arterial wall is composed of multiple layers that contain a significant number of immune cells. Upon lipid accumulation and vascular injury, the number of immune cells increases, leading to the secretion of large amounts of pro-inflammatory cytokines. This secretion promotes the formation of an inflammatory microenvironment (Blagov et al., 2023). Inflammatory stimuli trigger VSMC transition from quiescent contractile to synthetic phenotypes. These activated synthetic VSMCs amplify inflammatory infiltration, accelerating vascular pathogenesis (Wu et al., 2019). Consistent with previous reports, Tan II exhibits potent anti-inflammatory properties in a variety of pathological conditions, including inflammatory bowel disease (Li X. et al., 2020), cancer (Xie et al., 2025), myocardial infarction (Ren et al., 2010), and diabetic (Liu et al., 2024). Therefore, it may also possess the potential to modulate the inflammatory milieu of VSMCs. Meng (Meng et al., 2019) demonstrated in LPS-stimulated rat aortic VSMCs that Tan IIA attenuates MCP-1, IL-6, TNF-α, and NO production via TLR4/TAK1/NF-κB axis suppression. Concurrently, Tan IIA potentiates α-SMA expression while inhibiting pathological phenotypic switching. Yan (Qin et al., 2020) further established that Tan IIA downregulates miR-712-5p, upregulating (KLF4) to suppress NF-κB activation, thereby reducing neointimal hyperplasia and VSMC inflammation. Another study (Wei et al., 2024) demonstrated that sodium STS suppresses NLRP3 inflammasome activation in high glucose-exposed VSMCs through NF-κB signaling inhibition, thus ameliorating inflammatory microenvironments and attenuating vascular aging. Interestingly, the study by Oche (Oche et al., 2016) revealed that cryptotanshinone possesses phytoestrogen-like properties. Specifically, cryptotanshinone binds to estrogen receptor beta (ERβ), which in turn suppresses LPS-induced expression of inducible nitric oxide synthase (iNOS) and reduces excessive nitric oxide (NO) production. Through this mechanism, cryptotanshinone exerts notable anti-inflammatory effects in vascular smooth muscle cells.
2.5 Regulating calcium ion signaling
In pulmonary artery smooth muscle cells (PASMCs), elevated intracellular Ca2+ ([Ca2+]i) – predominantly mediated by store-operated calcium entry (SOCE) through TRPC channels–critically regulates cellular contraction and growth (Liu et al., 2003; Wang et al., 2004; Wang et al., 2006). Consequently, modulating Ca2+-dependent contractility represents a pharmacological target for vasodilation. Recent studies have demonstrated that STS exerts protective effects against hypoxic pulmonary hypertension, including reductions in pulmonary arterial pressure, pulmonary arterial wall thickness, and right ventricular hypertrophy. These beneficial effects are thought to be partially mediated through the regulation of intracellular Ca2+ homeostasis in PASMCs (Huang et al., 2009). Wang (Wang et al., 2013) emonstrated that STS suppresses hypoxia-induced SOCE and basal [Ca2+]i elevation in chronic hypoxic pulmonary hypertension models and hypoxic PASMCs by downregulating TRPC1/TRPC6. This inhibition consequently attenuated right ventricular hypertrophy and pathological pulmonary vascular remodeling. Furthermore, a 2018 study (Zhang et al., 2018) found that STS can directly block voltage-gated L-type calcium channels (LTCCs), thereby regulating [Ca2+]i and alleviating vascular tone. Both studies noted a concentration-dependent effect of STS and a non-potassium channel-dependent pathway. These findings provide a novel paradigm for the development of vasodilatory therapies targeting the calcium signaling network in VSMCs.
3 Conclusion and perspective
In summary, tanshinones exhibit comprehensive multi-target and multi-pathway effects in regulating VSMCs function, offering new insights into drug development for CVD treatment. Research has shown that tanshinones can effectively inhibit abnormal VSMCs proliferation and migration, block pathological phenotypic switching, and regulate calcium signaling, programmed cell death, and the inflammatory microenvironment. Collectively, these actions alleviate vascular wall remodeling, plaque formation, and luminal stenosis, highlighting their potential in combating CVDs such as atherosclerosis and myocardial infarction through the modulation of VSMCs homeostasis. Based on existing research, the application of tanshinones in VSMCs and their potential mechanisms for treating CVDs have primarily focused on tanshinone IIA and STS. Tanshinone II (Tan II) exhibits substantial potential in modulating a wide range of VSMC functions, positioning it as a promising candidate for broadly regulating VSMC activity to confer cardiovascular protection. In contrast, Sodium Tanshinone IIA Sulfonate (STS) demonstrates unique advantages in modulating calcium signaling, markedly attenuating right ventricular hypertrophy and pathological pulmonary vascular remodeling under hypoxic conditions, and improving pulmonary arterial wall thickness. In contrast, investigations into Tan I and cryptotanshinone remain relatively limited. Nevertheless, previous studies have demonstrated that cryptotanshinone exerts cardioprotective effects through the regulation of multiple cellular pathways, including oxidative stress, autophagy, mitochondrial function, and inflammatory responses (Zheng et al., 2024). For example, cryptotanshinone has been shown to enhance cell viability by downregulating the ERK and NF-κB pathways, upregulating the anti-apoptotic gene Bcl-2, inhibiting the production of reactive oxygen species (ROS) and malondialdehyde (MDA), and activating the MAPK3 pathway to suppress caspase-3 cleavage. Collectively, these actions mitigate myocardial oxidative stress, inhibit cardiomyocyte apoptosis, and provide protection against myocardial ischemia-reperfusion injury (Mao et al., 2021; Zhang et al., 2021). Similarly, studies have confirmed that Tan I significantly improves survival in cardiomyocyte models subjected to oxidative stress, alleviates intracellular oxidative damage, and suppresses ROS release (Ke et al., 2025). Furthermore, Tan I restores electrocardiographic abnormalities in murine models of myocardial infarction, reduces infarct size, and inhibits cardiac fibrosis. Although the specific roles of these compounds in VSMCs require further clarification, their demonstrated cardioprotective effects highlight considerable therapeutic potential and warrant future investigation within the field of vascular medicine.
The principal innovations of this study are as follows. First, we systematically integrated current evidence on the multi-faceted mechanisms by which tanshinones modulate VSMCs function, thereby constructing a comprehensive regulatory network that encompasses phenotypic switching, programmed cell death, calcium homeostasis, and inflammatory responses. This integration enhances the understanding of the multi-target effects of natural compounds on VSMCs. Second, by standardizing pharmacological data to molar concentrations and integrating cross-species experimental evidence, this study demonstrates that tanshinones attenuate pathological VSMC remodeling through key signaling axes—such as KLF4/miR-137–TRPC3, TLR4/NF-κB, and Nrf2/HO-1—and highlights their novel role in fine-tuning the balance between autophagy and pyroptosis. Furthermore, we summarize the emerging roles of understudied tanshinones, including Tan I and Cryptotanshinone, which exhibit protective effects in models of cardiovascular injury and show promise as potential therapeutic agents, warranting further investigation.
However, several limitations remain in current research. First, the regulatory weight of tanshinones on specific targets and the synergistic mechanisms of cross-pathways remain unclear. For instance, the hierarchical relationships between targets such as KLF4 and TRPC3 in phenotypic switching require further investigation. Second, while the issue of low bioavailability of natural tanshinones has been partially addressed by the development of STS, there is still considerable room for improvement in targeted delivery systems. Third, most conclusions are based on cellular and animal models, with a lack of large-scale clinical evidence to verify their long-term cardiovascular protective effects.
Future investigations should prioritize the following research avenues: (1) Integrating single-cell sequencing and proteomics technologies to systematically map the signaling network regulated by tanshinones in VSMCs, and to identify their core targets. (2) Utilizing synthetic biology techniques to modify tanshinone structures or develop nanocarriers to enhance tissue targeting and bioavailability. (3) Conducting multicenter clinical trials to evaluate the effects of tanshinone preparations on major adverse cardiovascular events in CVD patients, with particular emphasis on their long-term impact on vascular intimal hyperplasia and myocardial remodeling. By advancing the “mechanism-translation-clinical” chain through interdisciplinary collaboration, tanshinones hold promise as key candidate molecules for the next-generation of cardiovascular protective drugs.
Statements
Author contributions
JL: Writing – original draft, Writing – review and editing. WW: Writing – original draft, Writing – review and editing. HH: Writing – original draft, Writing – review and editing. RZ: Supervision, Writing – review and editing. ZY: Supervision, Writing – review and editing. ZC: Supervision, Writing – review and editing. ZN: Supervision, Writing – review and editing. SS: Conceptualization, Funding acquisition, Writing – review and editing. PW: Conceptualization, Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Scientific and technological innovation project of National Natural Science Foundation of China (82474312).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Afewerki T. Ahmed S. Warren D. (2019). Emerging regulators of vascular smooth muscle cell migration. J. Muscle Res. Cell Motil.40 (2), 185–196. 10.1007/s10974-019-09531-z
2
Blagov A. V. Markin A. M. Bogatyreva A. I. Tolstik T. V. Sukhorukov V. N. Orekhov A. N. (2023). The role of macrophages in the pathogenesis of atherosclerosis. Cells12 (4), 522. 10.3390/cells12040522
3
Chen Z. Xu H. (2014). Anti-inflammatory and immunomodulatory mechanism of tanshinone IIA for atherosclerosis. Evid Based Complement Altern. Med.2014, 267976. 10.1155/2014/267976
4
Chen W. Tang F. Xie B. Chen S. Huang H. Liu P. (2012). Amelioration of atherosclerosis by tanshinone IIA in hyperlipidemic rabbits through attenuation of oxidative stress. Eur. J. Pharmacol.674 (2-3), 359–364. 10.1016/j.ejphar.2011.10.040
5
Chen M. Liu Y. Yi D. Wei L. Li Y. Zhang L. (2014). Tanshinone IIA promotes pulmonary artery smooth muscle cell apoptosis in vitro by inhibiting the JAK2/STAT3 signaling pathway. Cell. Physiol. Biochem.33 (4), 1130–1138. 10.1159/000358682
6
Fang J. Little P. J. Xu S. (2018). Atheroprotective effects and molecular targets of tanshinones derived from herbal medicine danshen. Med. Res. Rev.38 (1), 201–228. 10.1002/med.21438
7
Farina F. M. Hall I. F. Serio S. Zani S. Climent M. Salvarani N. et al (2020). miR-128-3p is a novel regulator of vascular smooth muscle cell phenotypic switch and vascular diseases. Circ. Res.126 (12), e120–e135. 10.1161/CIRCRESAHA.120.316489
8
GBD 2019 Diseases and Injuries Collaborators (2020). Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet396 (10258), 1204–1222. 10.1016/S0140-6736(20)30925-9
9
Gomez D. Shankman L. S. Nguyen A. T. Owens G. K. (2013). Detection of histone modifications at specific gene loci in single cells in histological sections. Nat. Methods.10 (2), 171–177. 10.1038/nmeth.2332
10
He Y. Yi X. Zhang Z. Luo H. Li R. Feng X. et al (2022). JIB-04, a histone demethylase jumonji C domain inhibitor, regulates phenotypic switching of vascular smooth muscle cells. Clin. Epigenetics.14 (1), 101. 10.1186/s13148-022-01321-8
11
Hortells L. Sur S. St H. C. (2018). Cell phenotype transitions in cardiovascular calcification. Front. Cardiovasc Med.5, 27. 10.3389/fcvm.2018.00027
12
Huang Y. Liu M. Dong M. Yang W. Zhang B. Luan L. et al (2009). Effects of sodium tanshinone II A sulphonate on hypoxic pulmonary hypertension in rats in vivo and on Kv2.1 expression in pulmonary artery smooth muscle cells in vitro. J. Ethnopharmacol.125 (3), 436–443. 10.1016/j.jep.2009.07.020
13
Jiang Y. Qian H. Y. (2023). Transcription factors: key regulatory targets of vascular smooth muscle cell in atherosclerosis. Mol. Med.29 (1), 2. 10.1186/s10020-022-00586-2
14
Jiang Z. Gao W. Huang L. (2019). Tanshinones, critical pharmacological components in Salvia miltiorrhiza. Front. Pharmacol.10, 202. 10.3389/fphar.2019.00202
15
Kari S. Subramanian K. Altomonte I. A. Murugesan A. Yli-Harja O. Kandhavelu M. (2022). Programmed cell death detection methods: a systematic review and a categorical comparison. Apoptosis27 (7-8), 482–508. 10.1007/s10495-022-01735-y
16
Ke L. Zheng Z. Ye S. Zhong C. Lin Q. Hu Y. et al (2025). Tanshinone I alleviates post-ischemic myocardial injury by targeting TGFBR1 and modulating the TGF-beta signaling pathway. Phytomedicine145, 156994. 10.1016/j.phymed.2025.156994
17
Khachigian L. M. Black B. L. Ferdinandy P. De Caterina R. Madonna R. Geng Y. J. (2022). Transcriptional regulation of vascular smooth muscle cell proliferation, differentiation and senescence: novel targets for therapy. Vasc. Pharmacol.146, 107091. 10.1016/j.vph.2022.107091
18
Li R. Wei X. Jiang D. S. (2019). Protein methylation functions as the posttranslational modification switch to regulate autophagy. Cell. Mol. Life Sci.76 (19), 3711–3722. 10.1007/s00018-019-03161-x
19
Li X. Qiu Y. Jeffery L. Liu F. Feng R. He J. et al (2020). Down-regulation of colonic ACE2 expression in patients with inflammatory bowel disease responding to Anti-TNF therapy: implications for COVID-19. Front. Med.7, 613475. 10.3389/fmed.2020.613475
20
Li Y. Chen F. Guo R. Jia S. Li W. Zhang B. (2020). Tanshinone ⅡA inhibits homocysteine-induced proliferation of vascular smooth muscle cells via miR-145/CD40 signaling. Biochem. Biophys. Res. Commun.522 (1), 157–163. 10.1016/j.bbrc.2019.11.055
21
Li W. Gao Z. Guan Q. L. (2023). Tan IIA mitigates vascular smooth muscle cell proliferation and migration induced by ox-LDL through the miR-137/TRPC3 axis. Kaohsiung J. Med. Sci.39 (6), 596–604. 10.1002/kjm2.12663
22
Libby P. (2024). Inflammation and the pathogenesis of atherosclerosis. Vasc. Pharmacol.154, 107255. 10.1016/j.vph.2023.107255
23
Liu X. Singh B. B. Ambudkar I. S. (2003). TRPC1 is required for functional store-operated Ca2+ channels. Role of acidic amino acid residues in the S5-S6 region. J. Biol. Chem.278 (13), 11337–11343. 10.1074/jbc.M213271200
24
Liu H. Xiong W. Liu Q. Zhang J. Dong S. (2016). Chemokine-like receptor 1 regulates the proliferation and migration of vascular smooth muscle cells. Med. Sci. Monit.22, 4054–4061. 10.12659/msm.897832
25
Liu F. Zhao L. Wu T. Yu W. Li J. Wang W. et al (2024). Targeting autophagy with natural products as a potential therapeutic approach for diabetic microangiopathy. Front. Pharmacol.15, 1364616. 10.3389/fphar.2024.1364616
26
Lou G. Hu W. Wu Z. Xu H. Yao H. Wang Y. et al (2020). Tanshinone II A attenuates vascular remodeling through klf4 mediated smooth muscle cell phenotypic switching. Sci. Rep.10 (1), 13858. 10.1038/s41598-020-70887-1
27
Lu M. Luo Y. Hu P. Dou L. Huang S. (2018). Tanshinone IIA inhibits AGEs-induced proliferation and migration of cultured vascular smooth muscle cells by suppressing ERK1/2 MAPK signaling. Iran. J. Basic Med. Sci.21 (1), 83–88. 10.22038/IJBMS.2017.20100.5276
28
Lu J. Shan J. Liu N. Ding Y. Wang P. (2019). Tanshinone IIA can inhibit angiotensin II-Induced proliferation and autophagy of vascular smooth muscle cells via regulating the MAPK signaling pathway. Biol. Pharm. Bull.42 (11), 1783–1788. 10.1248/bpb.b19-00053
29
Lutgens E. de Muinck E. D. Kitslaar P. J. Tordoir J. H. Wellens H. J. Daemen M. J. (1999). Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc. Res.41 (2), 473–479. 10.1016/s0008-6363(98)00311-3
30
Majesky M. W. Weiser-Evans M. (2022). The adventitia in arterial development, remodeling, and hypertension. Biochem. Pharmacol.205, 115259. 10.1016/j.bcp.2022.115259
31
Mao S. Wang L. Zhao X. Guo L. Lin Q. Wang X. et al (2021). Efficacy of sodium tanshinone IIA sulfonate in patients with Non-ST elevation acute coronary syndrome undergoing percutaneous coronary intervention: results from a multicentre, controlled, randomized trial. Cardiovasc Drugs Ther.35 (2), 321–329. 10.1007/s10557-020-07077-8
32
Meng Z. Si C. Y. Teng S. Yu X. H. Li H. Y. (2019). Tanshinone IIA inhibits lipopolysaccharide‑induced inflammatory responses through the TLR4/TAK1/NF‑κB signaling pathway in vascular smooth muscle cells. Int. J. Mol. Med.43 (4), 1847–1858. 10.3892/ijmm.2019.4100
33
Oche B. Chen L. Ma Y. K. Yang Y. Li C. X. Geng X. et al (2016). Cryptotanshinone and wogonin up-regulate eNOS in vascular endothelial cells via ERα and down-regulate iNOS in LPS stimulated vascular smooth muscle cells via ERβ. Arch. Pharm. Res.39 (2), 249–258. 10.1007/s12272-015-0671-y
34
Qin Y. Zheng B. Yang G. S. Zhou J. Yang H. J. Nie Z. Y. et al (2020). Tanshinone ⅡA inhibits VSMC inflammation and proliferation in vivo and in vivoo by inwvitrolating miR-712-5p expression. Eur. J. Pharmacol.880, 173140. 10.1016/j.ejphar.2020.173140
35
Ren Z. H. Tong Y. H. Xu W. Ma J. Chen Y. (2010). Tanshinone II A attenuates inflammatory responses of rats with myocardial infarction by reducing MCP-1 expression. Phytomedicine17 (3-4), 212–218. 10.1016/j.phymed.2009.08.010
36
Reynolds J. L. Joannides A. J. Skepper J. N. Mcnair R. Schurgers L. J. Proudfoot D. et al (2004). Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J. Am. Soc. Nephrol.15 (11), 2857–2867. 10.1097/01.ASN.0000141960.01035.28
37
Roth G. A. Mensah G. A. Johnson C. O. Addolorato G. Ammirati E. Baddour L. M. et al (2020). Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 study. J. Am. Coll. Cardiol.76 (25), 2982–3021. 10.1016/j.jacc.2020.11.010
38
Shi M. J. Dong B. S. Yang W. N. Su S. B. Zhang H. (2019). Preventive and therapeutic role of tanshinone ⅡA in hepatology. Biomed. Pharmacother.112, 108676. 10.1016/j.biopha.2019.108676
39
Tang F. Wu X. Wang T. Wang P. Li R. Zhang H. et al (2007). Tanshinone II A attenuates atherosclerotic calcification in rat model by inhibition of oxidative stress. Vasc. Pharmacol.46 (6), 427–438. 10.1016/j.vph.2007.01.001
40
Wang J. Shimoda L. A. Sylvester J. T. (2004). Capacitative calcium entry and TRPC channel proteins are expressed in rat distal pulmonary arterial smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol.286 (4), L848–L858. 10.1152/ajplung.00319.2003
41
Wang J. Weigand L. Lu W. Sylvester J. T. Semenza G. L. Shimoda L. A. (2006). Hypoxia inducible factor 1 mediates hypoxia-induced TRPC expression and elevated intracellular Ca2+ in pulmonary arterial smooth muscle cells. Circ. Res.98 (12), 1528–1537. 10.1161/01.RES.0000227551.68124.98
42
Wang J. Jiang Q. Wan L. Yang K. Zhang Y. Chen Y. et al (2013). Sodium tanshinone IIA sulfonate inhibits canonical transient receptor potential expression in pulmonary arterial smooth muscle from pulmonary hypertensive rats. Am. J. Respir. Cell Mol. Biol.48 (1), 125–134. 10.1165/rcmb.2012-0071OC
43
Wang M. Monticone R. E. Mcgraw K. R. (2020). Proinflammation, profibrosis, and arterial aging. Aging Med. (Milton)3 (3), 159–168. 10.1002/agm2.12099
44
Wang G. Luo Y. Gao X. Liang Y. Yang F. Wu J. et al (2023). MicroRNA regulation of phenotypic transformations in vascular smooth muscle: relevance to vascular remodeling. Cell. Mol. Life Sci.80 (6), 144. 10.1007/s00018-023-04793-w
45
Wang J. Ye W. Zou J. Yang P. Jin M. Zheng Z. et al (2024). Targeting the smooth muscle cell Keap1-Nrf2-GSDMD-pyroptosis axis by cryptotanshinone prevents abdominal aortic aneurysm formation. Theranostics14 (17), 6516–6542. 10.7150/thno.98400
46
Wang S. Wang X. Lv Y. Zhang Z. He T. Hao X. et al (2024). M2 macrophage-derived exosomes inhibit atherosclerosis progression by regulating the proliferation, migration, and phenotypic transformation of smooth muscle cells. Front. Biosci. (Landmark Ed.)29 (8), 288. 10.31083/j.fbl2908288
47
Wei W. Heng Y. Y. Wu F. F. Dong H. Y. Zhang P. F. Li J. X. et al (2024). Sodium tanshinone IIA sulfonate alleviates vascular senescence in diabetic mice by modulating the A20-NFκB-NLRP3 inflammasome-catalase pathway. Sci. Rep.14 (1), 17665. 10.1038/s41598-024-68169-1
48
Wen J. Chang Y. Huo S. Li W. Huang H. Gao Y. et al (2020). Tanshinone IIA attenuates atherosclerosis via inhibiting NLRP3 inflammasome activation. Aging13 (1), 910–932. 10.18632/aging.202202
49
Won S. Lee H. Jeong S. Lee H. Lee E. Jung D. et al (2010). Tanshinone IIA induces mitochondria dependent apoptosis in prostate cancer cells in association with an inhibition of phosphoinositide 3-kinase/AKT pathway. Biol. Pharm. Bull.33 (11), 1828–1834. 10.1248/bpb.33.1828
50
Wu W. Y. Yan H. Wang X. B. Gui Y. Z. Gao F. Tang X. L. et al (2014). Sodium tanshinone IIA silate inhibits high glucose-induced vascular smooth muscle cell proliferation and migration through activation of AMP-activated protein kinase. PLoS One9 (4), e94957. 10.1371/journal.pone.0094957
51
Wu Y. T. Bi Y. M. Tan Z. B. Xie L. P. Xu H. L. Fan H. J. et al (2019). Tanshinone I inhibits vascular smooth muscle cell proliferation by targeting insulin-like growth factor-1 receptor/phosphatidylinositol-3-kinase signaling pathway. Eur. J. Pharmacol.853, 93–102. 10.1016/j.ejphar.2019.03.021
52
Xie D. Li Z. Ren B. Gong R. Yang D. Huang S. (2025). Tanshinone II A facilitates chemosensitivity of osteosarcoma cells to cisplatin via activation of p38 MAPK pathway. Chin. J. Integr. Med.31 (4), 326–335. 10.1007/s11655-024-4118-5
53
Xin Y. Zhang Z. Lv S. Xu S. Liu A. Li H. et al (2024). Elucidating VSMC phenotypic transition mechanisms to bridge insights into cardiovascular disease implications. Front. Cardiovasc Med.11, 1400780. 10.3389/fcvm.2024.1400780
54
Yamin R. Morgan K. G. (2012). Deciphering actin cytoskeletal function in the contractile vascular smooth muscle cell. J. Physiol.590 (17), 4145–4154. 10.1113/jphysiol.2012.232306
55
Yu Y. Cai Y. Yang F. Yang Y. Cui Z. Shi D. et al (2024). Vascular smooth muscle cell phenotypic switching in atherosclerosis. Heliyon10 (18), e37727. 10.1016/j.heliyon.2024.e37727
56
Yun S. Jeong S. Kim J. Jung J. H. Lee H. Sohn E. J. et al (2013). Activation of c-Jun N-terminal kinase mediates tanshinone IIA-induced apoptosis in KBM-5 chronic myeloid leukemia cells. Biol. Pharm. Bull.36 (2), 208–214. 10.1248/bpb.b12-00537
57
Zhang L. Wu Y. Li Y. Xu C. Li X. Zhu D. et al (2012). Tanshinone IIA improves miR-133 expression through MAPK ERK1/2 pathway in hypoxic cardiac myocytes. Cell. Physiol Biochem.30 (4), 843–852. 10.1159/000341462
58
Zhang X. D. He C. X. Cheng J. Wen J. Li P. Y. Wang N. et al (2018). Sodium tanshinone II-A sulfonate (DS-201) induces vasorelaxation of rat mesenteric arteries via inhibition of L-Type Ca(2+) channel. Front. Pharmacol.9, 62. 10.3389/fphar.2018.00062
59
Zhang Y. Luo F. Zhang H. He W. Liu T. Wu Y. et al (2021). Cryptotanshinone ameliorates cardiac injury and cardiomyocyte apoptosis in rats with coronary microembolization. Drug Dev. Res.82 (4), 581–588. 10.1002/ddr.21777
60
Zhang Q. Miao M. Cao S. Liu D. Cao Z. Bai X. et al (2025). PCSK9 promotes vascular neointimal hyperplasia through non-lipid regulation of vascular smooth muscle cell proliferation, migration, and autophagy. Biochem. Biophys. Res. Commun.742, 151081. 10.1016/j.bbrc.2024.151081
61
Zhao Y. Wang Z. Chen Y. Feng M. Liu X. Chen H. et al (2024). Asprosin aggravates atherosclerosis via regulating the phenotype transformation of vascular smooth muscle cells. Int. J. Biol. Macromol.268 (Pt 2), 131868. 10.1016/j.ijbiomac.2024.131868
62
Zheng Z. Ke L. Ye S. Shi P. Yao H. (2024). Pharmacological mechanisms of cryptotanshinone: recent advances in cardiovascular, cancer, and neurological disease applications. Drug Des. Devel Ther.18, 6031–6060. 10.2147/DDDT.S494555
Summary
Keywords
cardiovascular diseases, vascular smooth muscle cells, tanshinone I, tanshinoneIIA, Cryptotanshinone, sodium tanshinone IIA sulfonate
Citation
Li J, Wang W, Huang H, Zhou R, Yang Z, Cui Z, Niu Z, Shi S and Wang P (2025) Tanshinone I, tanshinone IIA, and cryptotanshinone: key bioactive components modulating vascular smooth muscle cell function. Front. Pharmacol. 16:1688338. doi: 10.3389/fphar.2025.1688338
Received
19 August 2025
Accepted
29 September 2025
Published
09 October 2025
Volume
16 - 2025
Edited by
Ying Zhang, Shimane University, Japan
Reviewed by
Zhiyi Fang, Nankai University, China
Deng Pan, Hangzhou Hospital of Traditional Chinese Medicine, China
Pankaj Prabhakar, Indira Gandhi Institute of Medical Sciences, India
Updates
Copyright
© 2025 Li, Wang, Huang, Zhou, Yang, Cui, Niu, Shi and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengnan Shi, shishengnan94@163.com; Peili Wang, wplggyx777@163.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.