- 1Center for Research and Training in Skin Diseases and Leprosy, Tehran University of Medical Sciences, Tehran, Iran
- 2York Biomedical Research Institute, Skin Research Centre, Hull York Medical School, University of York, York, United Kingdom
- 3Institute for Infection and Immunity, City St George’s University of London, London, United Kingdom
- 4St George’s University Hospitals NHS Foundation Trust, London, United Kingdom
- 5Institut für Tropenmedizin, Universitätsklinikum Tübingen, Tübingen, Germany
- 6Centre de Recherches Médicales de Lambaréné, Lambaréné, Gabon
- 7Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, United Kingdom
- 8Department of Infectious Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, United Kingdom
Cutaneous leishmaniasis (CL) is a neglected tropical disease caused by protozoan parasites of the genus Leishmania. It poses a significant global health burden, particularly because treatment options are limited. More effective and safer treatments are urgently needed. In previous studies, oleylphosphocholine (OlPC), a novel investigational compound structurally related to miltefosine, exhibited comparable activity to miltefosine in intramacrophage assays across various CL-causing laboratory strains and demonstrated superior efficacy in an experimental CL model. This study investigated the in vitro activity of OlPC against clinical isolates of Leishmania spp., comparing its activity with standard anti-leishmanial drugs, including miltefosine, amphotericin B, and pentavalent antimonial agents. Seventy ex vivo isolates (L. major and L. tropica) obtained directly from CL patients before any treatment were used to capture the diversity of drug susceptibilities in circulating parasite populations. Dose-response curves were fitted using a four-parameter log-logistic model to estimate EC50 and EC90 values. Additionally, a linear mixed-effects model was applied to examine the influence of drug type and species on EC50 values while accounting for within-isolate variability. Our findings indicate that OlPC exhibits potent in vitro anti-leishmanial activity, exceeding that of miltefosine in our in vitro intramacrophage model. To facilitate similar analyses, we provide a dedicated wrapper function in R designed to simplify curve fitting and parameter estimation, making the process more accessible to researchers.
Introduction
Cutaneous leishmaniasis (CL) is a significant global health challenge, affecting millions worldwide, particularly in resource-limited tropical and subtropical regions (Alvar et al., 2012). The disease is characterized by skin lesion(s), which may range from simple small, self-healing to larger, more persistent ulcers (de Vries and Schallig, 2022). These lesions can cause significant scarring, long-term morbidity, and psychosocial distress for affected individuals (Bennis et al., 2018; Nuwangi et al., 2024). The treatment choice is influenced by several factors, including the infectious agent (species of parasite), lesion characteristics (number, size, extent, and location), and treatment availability (Mohammed et al., 2023). Although several treatment options for CL exist, including pentavalent antimonials, amphotericin B, and miltefosine, they are often hampered by significant limitations such as (i) systemic toxicity, requiring careful monitoring; (ii) lengthy treatment courses, often involving multiple injections or extended periods of oral medication that contribute to poor treatment adherence; and (iii) variable efficacy, depending on the Leishmania species involved, the patient’s immune status, and other factors (Mowbray et al., 2018). These combined challenges underscore the urgent and unmet need for novel, safer, more effective, and ideally shorter-duration therapies for cutaneous leishmaniasis (Alvar et al., 2018).
Miltefosine, an alkylphosphocholine, has become an important first-line oral treatment for CL, offering a more convenient administration route than injectable therapies (Dorlo et al., 2012). Miltefosine exerts its anti-leishmanial activity through multiple mechanisms targeting parasite membrane integrity and intracellular signaling (Dorlo et al., 2012; Benaim and Paniz-Mondolfi, 2024). Being an alkylphosphocholine, the drug integrates into the parasite’s cell membrane, disrupting its structure and normal lipid metabolism, which impairs parasite viability. Beyond this, miltefosine interferes with essential internal processes, including mitochondrial function and phospholipid production, ultimately leading to parasite death. Although these effects are well-documented, the precise molecular targets can vary between species (Pinto-Martinez et al., 2018), which partly explains the observed differences in treatment efficacy (Van Bocxlaer et al., 2023).
Despite its widespread use, the cure rates for miltefosine treatment differ depending on the Leishmania species responsible for infection, geographical location, and host immune response, posing a challenge to achieving consistent treatment outcomes (Monge-Maillo and López-Vélez, 2015; Soto and Berman, 2006). Miltefosine has a long half-life, approximately 150 h–200 h, which contributes to its prolonged therapeutic effects but also raises concerns regarding resistance development. Teratogenicity limits its use in pregnant women, and its common adverse events include nausea and gastrointestinal disturbances, which negatively impact patient compliance (Uranw et al., 2013).
Oleylphosphocholine (OlPC) was developed as a synthetic analog of miltefosine, and it was identified through the exploration of the class of alkylphosphocholines known for their anti-tumor (Unger et al., 1992) and anti-parasitic properties (Croft et al., 1996; Escobar et al., 2001). OlPC was evaluated for its potential to retain oral bioavailability and anti-leishmanial potency while offering an improved safety and pharmacokinetic profile. In vitro, OlPC demonstrated similar activity to miltefosine in an intracellular macrophage model using a laboratory-adapted panel of CL-causing species (Van Bocxlaer et al., 2023). In experimental CL models, oral administration of OlPC showed superior efficacy to miltefosine in several studies (Van Bocxlaer et al., 2023; Fortin et al., 2014). More recently, a study suggested that OlPC outperformed miltefosine in canine leishmaniasis, although the treatments were administered at different dosages (4 mg/kg/day for OlPC vs. 2 mg/kg/day for miltefosine, which are equally the respective NOAELs (no observed adverse effect levels) for each drug, with the primary endpoint focusing on clinical signs (Lima et al., 2025). Building on these promising preclinical findings, in this study, we report the susceptibility of 70 ex vivo clinical L. major and L. tropica isolates to miltefosine, OlPC, meglumine antimoniate (pentavalent antimony, SbV), and amphotericin B using an intramacrophage assay. To quantify drug potency, dose-response curves were generated and analyzed using a four-parameter log-logistic regression model, estimating EC50 and EC90 values, along with Hill slopes and R2 values in R. Given the large dataset, a custom wrapper function (Supplementary Material S2) was developed to automate curve generation and fitting, thus ensuring efficient and reproducible analysis using open-source software.
Materials and methods
Drugs and formulations
OlPC (Mw 433.61 g/mol) and miltefosine (Mw 407.6 g/mol) were donated by Oblita Therapeutics (Zoersel, Belgium) and Paladin Labs Inc. (Montréal, Canada), respectively. Stock solutions (20 mM) of both compounds were prepared in phosphate-buffered saline (PBS, 0.9% (w/v) NaOH, pH 7.4; Sigma-Aldrich, United Kingdom) and were filter-sterilized and stored at −20 °C until use. Amphotericin B deoxycholate (Fungizone, E.R. Squibb & Sons, United Kingdom) was commercially obtained in 50 mg of amphotericin B (Mw 924.1 g/mol) and prepared according to the manufacturer’s guidelines, resulting in a stock concentration of 5 μg/mL. Meglumine antimoniate powder (containing 55.5% pentavalent antimony (SbV); batch number: 102989254) was solubilized in water, filter-sterilized, and stored at −20 °C until use. Podophyllotoxin was purchased from Sigma-Aldrich (Gillingham, United Kingdom), and stock solutions of 1 mM were prepared in DMSO and stored at −20 °C until use. AlamarBlue was obtained from Thermo Fisher Scientific (Loughborough, United Kingdom).
Clinical isolates
After obtaining written consent, the skin of patients suspected of being infected with Leishmania was wiped with 70% ethanol, and a smear sample was collected from the margins of the lesion using a sterile surgical scalpel. The smear sample was divided into two parts. The first part was smeared onto a microscope slide, fixed with methanol, stained with Giemsa, and examined under a light microscope for the detection of amastigotes. The second part was used to inoculate Novy–MacNeal–Nicolle (NNN) medium overlaid with RPMI-1640 medium (Gibco, Invitrogen, Carlsbad, CA, United States) that was subsequently incubated at 26 °C ± 1 °C. The liquid phase was examined under a light microscope every other day to observe motile promastigotes. When present, parasites were subcultured in RPMI-1640 medium supplemented with 10% (v/v) fetal calf serum (FCS). A small aliquot was removed for DNA extraction and PCR identification, as described previously (Hosseini et al., 2020). All clinical isolates were maintained as promastigotes and used for drug susceptibility testing at or before passage 4.
Intracellular amastigote drug susceptibility evaluation
Female BALB/c mice (7–10 weeks old) received an intraperitoneal injection with a 2% (w/v) starch suspension (Aq). Twenty-four hours later, peritoneal macrophages (PEMs) were collected by abdominal lavage with RPMI-1640. Subsequently, they were washed, counted, and re-suspended in the RPMI-1640 medium supplemented with 10% (v/v) FCS. Aliquots containing 4 × 104 PEMs were transferred to 16-well Lab-Tek slides (Thermo Fisher Scientific) and incubated overnight at 37 °C in an atmosphere of 5% CO2 in air. The next day, stationary-phase L. major and L. tropica promastigotes, resuspended in RPMI-1640 with 10% (v/v) FCS medium, were added to the macrophages in a 3:1 and 5:1 ratio, respectively.
Twenty-four hours after infection, the overlay and any non-adherent macrophages and promastigotes were removed. Miltefosine and OlPC solutions (40, 5, 0.5, and 0.1 μg/mL final in-well concentrations) were applied to the infected macrophages and incubated for 72 h at 34 °C and 5% CO2. Each experiment included a 72-h control (untreated infected control) and Fungizone (amphotericin B, included over a concentration range of 5, 1, 0.25, and 0.01 μg/mL) and a pentavalent antimonial control, which was included over a concentration range of 20, 10, 8, 2, 0.5, and 0.1 µg meglumine antimoniate/mL (equivalent to 11.1, 5.6, 4.4, 1.1, 0.28, and 0.06 µg SbV/mL, respectively). The amastigote burden was determined by microscopic evaluation (x 100 magnification) and compared with the untreated 72-h control.
Cytotoxicity evaluation
Cytotoxicity was assessed using differentiated THP-1 cells and primary human dermal fibroblasts (NHDF, passage ≤8). THP-1 cells were stimulated with 20 ng/mL PMA for 3 days prior to drug exposure, while NHDF were maintained in DMEM supplemented with 10% (v/v) FCS and non-essential amino acids. A total of 20,000 cells were seeded in 96-well plates and exposed to serial 1:3 dilutions of OlPC or miltefosine, starting at 300 µM. Podophyllotoxin was included as a positive control (starting concentration 0.3 µM). Untreated controls and blanks (medium only) were included, and each compound was tested in triplicate. After 72 h incubation at 37 °C with 5% CO2, cell viability was assessed using AlamarBlue® (20 µL/well), incubated for 2 h–4 h, and read at EX/EM 560/585 nm (cut-off at 570 nm) on a CLARIOstar plate reader. EC50 values were calculated using nonlinear sigmoidal curve fitting (variable slope) in GraphPad Prism. The selectivity index was calculated as follows:
Data processing and analysis
Dose-response models were fitted using a four-parameter log-logistic regression model. In the drc R package, this is undertaken with the drm and LL.4 functions. For each drug, separate models were fitted for each Leishmania isolate, enabling the estimation of EC50 (the concentration at which 50% inhibition was observed) and EC90 values, along with Hill slopes and R2 values, on a within-subject basis. Summary statistics (the mean, standard deviation, minimum, and maximum inhibition) were then calculated across the 70 Leishmania isolates for each combination of drug concentration and species. Consequently, estimates such as the mean and 95% confidence interval of EC50 reflect between-person variation. Inhibition curves for each drug were plotted using ggplot2, with concentration represented on a logarithmic scale, and species-specific differences were visualized.
To compare EC50 values between (i) OlPC and miltefosine and between (ii) L. major and L. tropica, a mixed-effects analysis of variance model was used, with person being a random effect (using the lmer function of the lme4 R package). Drug comparisons are within-subject (same person), whereas species comparisons are between-subject (different people).
The analysis was carried out using R (version 4.2.1 or higher). In addition to those already mentioned, the packages dplyr, data.table, and lmerTest were used.
Results
In vitro anti-leishmanial activity
In terms of EC50 values, the anti-leishmanial activity of the four drugs when ranked from the least to the highest activity is miltefosine < SbV < OlPC < amphotericin B (Table 1; Supplementary Table S1 for EC50 and EC90 in µg/mL and µM, respectively). The Hill slope, which describes the steepness of the dose-response curve, was less than 1 for all drugs, indicating a shallow dose-response curve, characteristic of a gradual transition between partial and full inhibition (Motulsky and Christopoulos, 2010) (Figure 1). Yet, it is the highest for meglumine antimoniate, followed by amphotericin B and then OlPC and miltefosine. The goodness-of-fit for dose-response models was high across all drugs, with R2 above 94%.
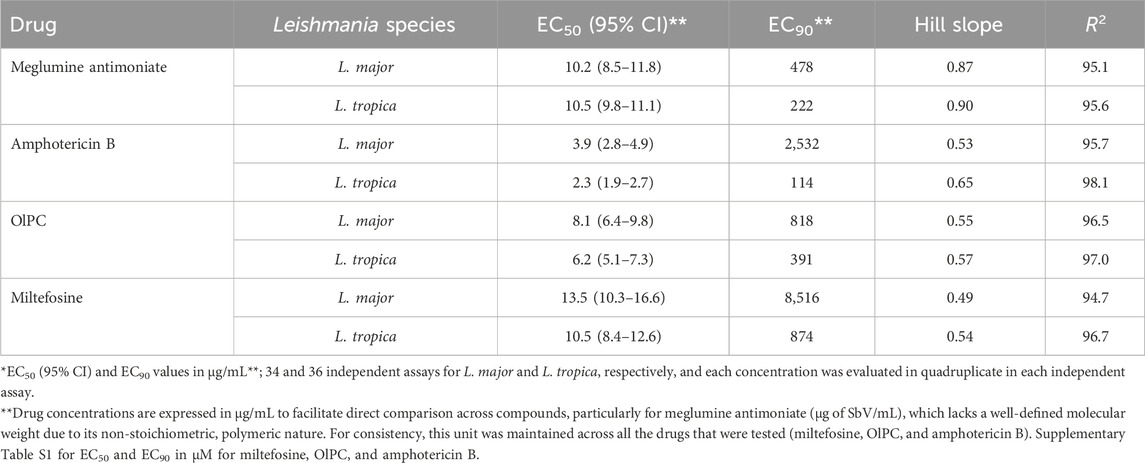
Table 1. Susceptibility of clinical isolates against OlPC, miltefosine, amphotericin B, and meglumine antimoniate (EC50 and EC90 in µg SbV/mL) estimated from four-parameter logistic modeling.
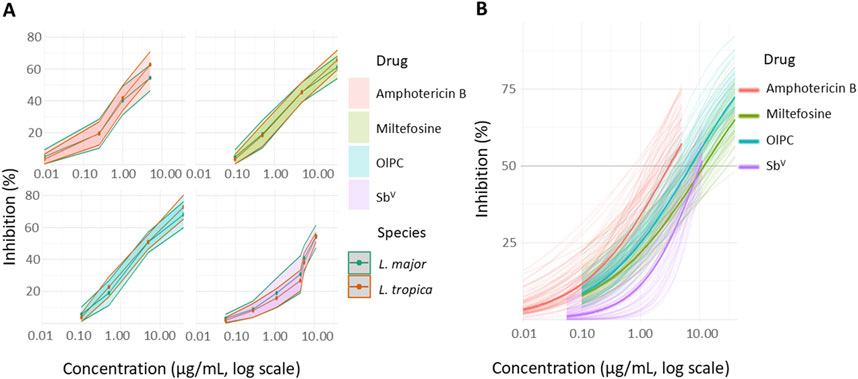
Figure 1. (A) Dose-response curves of amphotericin B, pentavalent antimonials (meglumine antimoniate), miltefosine, and OlPC against 34 L. major and 36 L. tropica clinical isolates. (B) Four-parameter logistic model prediction.
EC50 values were compared between OlPC and the three other drugs in both Leishmania species by analysis of variance (ANOVA). The results (Table 2) indicated a significant effect of the drug type on EC50 estimates compared to OlPC (p < 0.001 for each of the three drugs). Amphotericin B was associated with a significantly lower EC50 (difference estimate: −4.1 μg/mL, p < 0.001), indicating that it required lower concentrations to achieve 50% inhibition than OlPC. Meglumine antimoniate (reported as SbV) had a higher EC50 value (difference estimate: 3.2 μg/mL, p < 0.001), indicating weaker potency. Most importantly, miltefosine, a close analog of OlPC currently used to treat CL, showed an elevated EC50 value (difference estimate: 4.8 μg/mL, p < 0.001), although the increase was less pronounced than that observed for meglumine antimoniate. This suggests that higher concentrations of miltefosine are required to achieve 50% inhibition of the respective Leishmania parasites compared with OlPC.
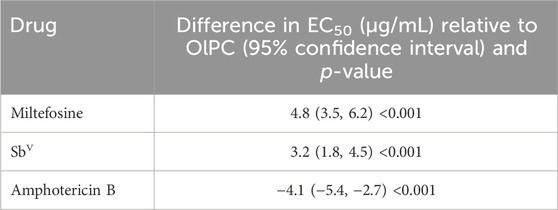
Table 2. Comparisons of EC50 values in clinical isolates between amphotericin B, meglumine antimoniate, and miltefosine, relative to OlPC.
Although drug type significantly affected EC50 values, the difference in EC50 values between the L. major and L. tropica species was borderline in terms of statistical significance (fixed effect difference of −1.54 μg/mL, 95% CI: −3.08–−0.01, p = 0.054). This indicates that species susceptibility did not significantly influence drug potency in this experimental setup.
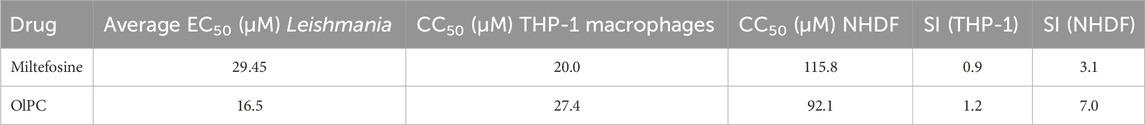
Cytotoxicity assays in THP-1 macrophages and primary fibroblasts and NHDF showed showed OlPC and miltefosine exhibited moderate toxicity in host cells, with CC50 values of 20.04 µM (THP-1) and 115.8 µM (NHDF) for OlPC and 27.35 µM (THP-1) and 92.05 µM (NHDF) for miltefosine. These values result in a selectivity index for OlPC that is higher in both THP-1 cells (1.2 vs 0.93) and NHDF (7.0 vs 3.1) than that for miltefosine, indicating a wider therapeutic window (Table 3).
Discussion
CL is caused by over 15 species of Leishmania (Auwera and Dujardin, 2015), each capable of producing a wide range of clinical manifestations. This inherent diversity in biochemistry, host–parasite interactions, and, importantly, drug susceptibility (Yardley et al., 2005) contributes to the diverse clinical outcomes observed and highlights the importance of selecting a well-defined and representative panel of Leishmania species during drug discovery to ensure that new treatments can effectively address the full spectrum of potential CL presentations (Caridha et al., 2019). Although OlPC has been tested across seven different species using our laboratory-adapted strains, in this study, we report its efficacy only against two Old World species (Van Bocxlaer et al., 2023). Further studies are needed to assess the performance of OlPC and other compounds across a wider range of clinical isolates representing the global diversity of CL-causing parasites.
We observed a higher EC50 for miltefosine (13.5 and 10.5 μg/mL) than for OlPC (8.1 and 6.2 μg/mL) against the L. major and L. tropica clinical isolates. This suggests that OlPC exhibits superior anti-leishmanial activity compared to miltefosine (difference in EC50 value of 4 μg/mL, Table 2), which remains the only oral drug available for the treatment of CL. Earlier studies using laboratory strains reported no significant difference in EC50 values between miltefosine and OlPC (Van Bocxlaer et al., 2023). These differences may be explained by limitations such as a small sample size (n = 2 per species), the use of long-term cultured strains, and differences in methodology. Notably, previous work assessed drug efficacy based on the percentage of infected macrophages, while our study quantified amastigote burden, which provides a more direct measure of intracellular parasite replication. Furthermore, Leishmania strains maintained in vitro are prone to phenotypic changes over time, including reduced infectivity (Piel et al., 2022)—a phenomenon mitigated by regular passage through mice to preserve virulence and ensure the reliability of experimental results. Surprisingly, the EC50 values for L. major and L. tropica exhibited no significant differences in susceptibility in our study, despite earlier reports suggesting that L. tropica may be more susceptible to miltefosine (Escobar et al., 2002). OlPC also demonstrated low cytotoxicity in both THP-1 macrophages and primary human fibroblasts, resulting in higher selectivity indices than those of miltefosine and highlighting a favorable safety profile in relevant host cell types. Amphotericin B and meglumine antimoniate (the standard treatment in Iran) were also evaluated for activity against clinical isolates. Amphotericin B emerged as the most potent, with EC50 values approximately 4 μg/mL lower than those of OlPC, while meglumine antimoniate (as a source of SbV) showed values approximately 3 µg of SbV/mL higher. These findings are consistent with previously reported data (Escobar et al., 2002; Neal and Allen, 1988).
Although the dose-response data provided useful insights into the relative potency of the drugs tested, further evaluation of our four-parameter logistic model for dose-response fitting revealed that for certain clinical isolates, the inhibition did not always plateau with higher drug concentrations. Although incomplete inhibition is not uncommon in drug testing, this may affect the accuracy of, for example, the EC90 values derived from the model. To analyze these data effectively, we chose R for our data analysis, which offered several key advantages over proprietary software programs, such as GraphPad Prism. R, particularly with the drc package (Ritz et al., 2016; Ritz and Streibig, 2005), provides greater flexibility and control over point-and-click software (Ritz et al., 2016), especially in efficiently handling larger datasets. It also facilitated model comparison (e.g., between models with different numbers of parameters fitted to the same data) and enabled a more rigorous assessment of goodness of fit. Using a summary measures approach (Matthews et al., 1990), we employed a linear mixed-effects model to investigate the effects of drugs and species on EC50, taking account of the multiple measurements within the same isolate. Although R requires a steeper learning curve than point-and-click software, its advantages in flexibility, reproducibility, and cost-effectiveness, particularly for large-scale analyses such as this one, outweigh the initial investment in learning.
In conclusion, this study highlights the superior in vitro anti-leishmanial activity of OlPC compared to that of miltefosine, the only currently available oral treatment for CL, and meglumine antimoniate as the standard treatment of care in Iran. By utilizing ex vivo clinical isolates rather than laboratory-adapted strains, our findings provide a more representative assessment of drug susceptibility in real-world settings. Our results underscore the need for further investigation into OlPC as a promising alternative to miltefosine, with the potential to improve treatment outcomes for CL.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Parasites were isolated from patients with CL after obtaining written informed consent from all participants. Ethical approval for the human study (reference number IR.TUMS.VCR.REC.1398.672) was granted by the Ethics Committee of Tehran University of Medical Sciences (Tehran, Iran). All animal experiments were conducted in accordance with the Iranian Animal Protection Guide of the National Council for the Control of Animal Experiments. The animal study protocol (IR.TUMS.SPH.REC.1400.240) was reviewed and approved by the Medical Ethics Committee of Tehran University of Medical Sciences.
Author contributions
AK: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – review and editing. MT: Data curation, Investigation, Methodology, Project administration, Writing – review and editing. AM: Data curation, Investigation, Methodology, Writing – review and editing. JD: Data curation, Investigation, Methodology, Writing – review and editing. DC: Funding acquisition, Investigation, Methodology, Project administration, Writing – review and editing. HS: Conceptualization, Funding acquisition, Investigation, Methodology, Writing – review and editing. SK: Conceptualization, Funding acquisition, Investigation, Methodology, Writing – review and editing. VY: Investigation, Methodology, Project administration, Writing – review and editing. SC: Investigation, Methodology, Writing – original draft, Writing – review and editing. NA: Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review and editing. KVB: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 815622. KVB was supported by a fellowship awarded from the Research Council United Kingdom Grand Challenges Research Funder under grant agreement “A Global Network for Neglected Tropical Diseases” grant number MR/P027989/1.
Acknowledgments
The authors thank all members of the TT4CL Consortium including Caroline Jansen, Johannes J. Platteeuw, Dennie van den Heuvel, Yolanda Augustin, Carlos Lamsfus-Calle, Marcel Spring, Merel Esen, and Peter Kremsner for their helpful discussions and feedback during the preparation of this manuscript. They are also grateful to Hajnalka Kovacsevics, Jane Boland, and Carwyn Hooper for their invaluable logistical, administrative, and ethics support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1688856/full#supplementary-material
References
Alvar, J., Vélez, I. D., Bern, C., Herrero, M., Desjeux, P., Cano, J., et al. (2012). Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7 (5), e35671. doi:10.1371/journal.pone.0035671
Alvar, J., and Arana, B. (2018). “Leishmaniasis - impact and therapeutic needs,” in Drug discovery for leishmaniasis. Editors L. Rivas, and C. Gil (Croyden, UK: The Royal Society of Chemistry), 3–23.
Auwera, G. V., and Dujardin, J.-C. (2015). Species typing in dermal leishmaniasis. Clin. Microbiol. Rev. 28 (2), 265–294. doi:10.1128/CMR.00104-14
Benaim, G., and Paniz-Mondolfi, A. (2024). Unmasking the mechanism behind miltefosine: revealing the disruption of intracellular Ca2+ homeostasis as a rational therapeutic target in leishmaniasis and chagas disease. Biomolecules 14 (4), 406. doi:10.3390/biom14040406
Bennis, I., De Brouwere, V., Belrhiti, Z., Sahibi, H., and Boelaert, M. (2018). Psychosocial burden of localised cutaneous Leishmaniasis: a scoping review. BMC Public Health 18 (1), 358. doi:10.1186/s12889-018-5260-9
Caridha, D., Vesely, B., van Bocxlaer, K., Arana, B., Mowbray, C. E., Rafati, S., et al. (2019). Route map for the discovery and pre-clinical development of new drugs and treatments for cutaneous leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 11, 106–117. doi:10.1016/j.ijpddr.2019.06.003
Croft, S.L., Snowdon, D., and Yardley, V. (1996). The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J. Antimicrob. Chemother. 38 (6), 1041–1047. doi:10.1093/jac/38.6.1041
de Vries, H. J. C., and Schallig, H. D. (2022). Cutaneous leishmaniasis: a 2022 updated narrative review into diagnosis and management developments. Am. J. Clin. Dermatol 23 (6), 823–840. doi:10.1007/s40257-022-00726-8
Dorlo, T. P., Balasegaram, M., Beijnen, J. H., and de Vries, P. J. (2012). Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 67 (11), 2576–2597. doi:10.1093/jac/dks275
Escobar, P., Yardley, V., and Croft, S. L. (2001). Activities of hexadecylphosphocholine (miltefosine), AmBisome, and sodium stibogluconate (pentostam) against Leishmania donovani in immunodeficient scid mice.
Escobar, P., Matu, S., Marques, C., and Croft, S. L. (2002). Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop. 81 (2), 151–157. doi:10.1016/s0001-706x(01)00197-8
Fortin, A., Caridha, D. P., Leed, S., Ngundam, F., Sena, J., Bosschaerts, T., et al. (2014). Direct comparison of the efficacy and safety of oral treatments with oleylphosphocholine (OlPC) and miltefosine in a mouse model of L. major cutaneous leishmaniasis. PLoS neglected Trop. Dis. 8 (9), e3144. doi:10.1371/journal.pntd.0003144
Hosseini, M., Nateghi Rostami, M., Hosseini Doust, R., and Khamesipour, A. (2020). Multilocus sequence typing analysis of Leishmania clinical isolates from cutaneous leishmaniasis patients of Iran. Infect. Genet. Evol. 85, 104533. doi:10.1016/j.meegid.2020.104533
Lima, I., Fraga, D., and Berman, J. (2025). Oleylphosphocholine versus miltefosine for canine leishmaniasis. Am. J. Trop. Med. Hyg. 112, 753–760. doi:10.4269/ajtmh.24-0622
Matthews, J. N., Altman, D. G., Campbell, M. J., and Royston, P. (1990). Analysis of serial measurements in medical research. Bmj 300 (6719), 230–235. doi:10.1136/bmj.300.6719.230
Mohammed, A. B., Mohammed, F. S., Zewdu, F. T., Nigusse, S. D., Hailemichael, Y., Cherkose, T., et al. (2023). Protocol for a prospective observational cohort study of cutaneous leishmaniasis in Ethiopia. NIHR Open Res. 3, 49. doi:10.3310/nihropenres.13432.2
Monge-Maillo, B., and López-Vélez, R. (2015). Miltefosine for visceral and cutaneous leishmaniasis: drug characteristics and evidence-based treatment recommendations. Clin. Infect. Dis. 60 (9), 1398–1404. doi:10.1093/cid/civ004
Motulsky, H., and Christopoulos, A. (2010). Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting.
Mowbray, C. E. (2018). “Anti-leishmanial drug discovery: past, present and future perspectives,” in Drug discovery for leishmaniasis. Editors L. Rivas, and C. Gil (Croyden, UK: The Royal Society of Chemistry), 24–36.
Neal, R. A., and Allen, S. (1988). In vitro anti-Leishmanial activity of compounds in current clinical use for unrelated diseases. Drugs Exp. Clin. Res. 14 (10), 621–628.
Nuwangi, H., Dikomitis, L., Weerakoon, K. G., Agampodi, S. B., and Agampodi, T. C. (2024). The psychosocial burden of cutaneous leishmaniasis in rural Sri Lanka: a multi-method qualitative study. PLOS Neglected Trop. Dis. 18 (1), e0011909. doi:10.1371/journal.pntd.0011909
Piel, L., Rajan, K. S., Bussotti, G., Varet, H., Legendre, R., Proux, C., et al. (2022). Experimental evolution links post-transcriptional regulation to Leishmania fitness gain. PLoS Pathog. 18 (3), e1010375. doi:10.1371/journal.ppat.1010375
Pinto-Martinez, A. K., Rodriguez-Durán, J., Serrano-Martin, X., Hernandez-Rodriguez, V., and Benaim, G. (2018). Mechanism of action of miltefosine on Leishmania donovani involves the impairment of acidocalcisome function and the activation of the sphingosine-dependent plasma membrane Ca2+ channel. Antimicrob. Agents Chemother. 62 (1), e01614-17. doi:10.1128/AAC.01614-17
Ritz, C., and Streibig, J. C. (2005). Bioassay analysis using R. J. Stat. Softw. 12 (5), 1–22. doi:10.18637/jss.v012.i05
Ritz, C., Baty, F., Streibig, J. C., and Gerhard, D. (2016). Dose-response analysis using R. PLOS ONE 10 (12), e0146021. doi:10.1371/journal.pone.0146021
Soto, J., and Berman, J. (2006). Treatment of new world cutaneous leishmaniasis with miltefosine. Trans. R. Soc. Trop. Med. Hyg. 100 (Suppl. 1), S34–S40. doi:10.1016/j.trstmh.2006.02.022
Unger, C., Fleer, E. A., Kotting, J., Neumuller, W., and Eibl, H. (1992). “Antitumoral activity of alkylphosphocholines and analogues in human leukemia cell lines,” in Alkylphosphocholines: new drugs in cancer therapy. Editors H. Eibl, P. Hilgard, and C. Unger (Basel: Karger), 25–32.
Uranw, S., Ostyn, B., Dorlo, T. P. C., Hasker, E., Dujardin, B., Dujardin, J. C., et al. (2013). Adherence to miltefosine treatment for visceral leishmaniasis under routine conditions in Nepal. Trop. Med. Int. Health 18 (2), 179–187. doi:10.1111/tmi.12025
Van Bocxlaer, K., Dixon, J., Platteeuw, J. J., Van Den Heuvel, D., Mcarthur, K. N., Harris, A., et al. (2023). Efficacy of oleylphosphocholine in experimental cutaneous leishmaniasis. J. Antimicrob. Chemother. 78 (7), 1723–1731. doi:10.1093/jac/dkad162
Keywords: cutaneous leishmaniasis, oleylphosphocholine, clinical isolates, dose response, miltefosine
Citation: Khamesipour A, Tasbihi M, Mohammadi AMA, Dixon J, Clark DJ, Staines HM, Krishna S, Yardley V, Croft SL, Alexander N and Van Bocxlaer K (2025) Comparative in vitro susceptibility of clinical Leishmania isolates to miltefosine and oleylphosphocholine. Front. Pharmacol. 16:1688856. doi: 10.3389/fphar.2025.1688856
Received: 19 August 2025; Accepted: 29 September 2025;
Published: 27 October 2025.
Edited by:
Mariusz Skwarczynski, The University of Queensland, AustraliaReviewed by:
Fernanda Nazaré Morgado, Oswaldo Cruz Institute, BrazilGustavo Benaim, Fundación Instituto de Estudios Avanzados (IDEA), Venezuela
Copyright © 2025 Khamesipour, Tasbihi, Mohammadi, Dixon, Clark, Staines, Krishna, Yardley, Croft, Alexander and Van Bocxlaer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katrien Van Bocxlaer, a2F0cmllbi52YW5ib2N4bGFlckBoeW1zLmFjLnVr
 Ali Khamesipour
Ali Khamesipour Minoo Tasbihi1
Minoo Tasbihi1 Henry M. Staines
Henry M. Staines Sanjeev Krishna
Sanjeev Krishna Neal Alexander
Neal Alexander Katrien Van Bocxlaer
Katrien Van Bocxlaer