- 1Department of Korean Medicine, The Graduate School of Pusan National University, Yangsan, Republic of Korea
- 2Department of Gastroenterology, Kyung Hee University College of Korean Medicine, Kyung Hee University Medical Center, Seoul, Republic of Korea
- 3Department of Korean Internal Medicine, Pusan National University Korean Medicine Hospital, Yangsan, Republic of Korea
Introduction: Functional dyspepsia (FD) has a global prevalence of approximately 15% and is characterized by chronic symptoms with an unclear etiology. Herbal medicines, owing to their multifaceted mechanisms, are promising therapeutic options for FD. This study aimed to establish medical evidence for the use of Sihogyeji-tang (SG), a herbal medicine, in the treatment of FD, thereby providing clinically relevant evidence for both patients and healthcare practitioners.
Methods: A comprehensive search was conducted on 24 June 2025, in the following databases—four English databases (CINAHL, EMBASE, Cochrane Database, and PubMed), five Korean databases (RISS, KISS, NDSL, DBPIA, and OASIS), one Japanese database (J-Stage), and three Chinese databases (CNKI, Wanfang, and VIP)—to identify eligible studies for this review. Randomized controlled trials investigating the use of SG for the treatment of FD were included. The risk of bias was assessed using the Cochrane Risk of Bias tool and the results were synthesized with Review Manager 5.4.
Results: Data from 12 randomized controlled trials involving 805 individuals were included in the meta-analysis. SG demonstrated a significantly higher total effective rate than prokinetic agents (risk ratio [RR]: 1.28, 95% confidence interval [CI]: 1.19–1.37, P < 0.00001). SG also resulted in a significantly greater reduction in symptom severity, as measured by the traditional Chinese medicine (TCM) symptom total score, compared with the control group (standardized mean difference: −1.10, 95% CI: −1.53 to −0.68). The incidence of adverse events was significantly lower in the SG group than in the control group (RR: 0.26, 95% CI: 0.09–0.76, P < 0.05). The quality of evidence was rated as moderate for the total effective rate, very low for the TCM symptom score, and low for adverse events.
Discussion: The findings suggest that SG may be more effective and safer than prokinetic agents for FD. However, the certainty of the evidence is limited by methodological weaknesses in the included studies. To validate these results and support the clinical adoption of SG in FD, robust and extensive randomized controlled trials are needed.
Systematic review registration: [https://www.crd.york.ac.uk/PROSPERO/], identifier [CRD420251041781].
1 Introduction
Functional dyspepsia (FD) has a global prevalence of approximately 16% (Ford et al., 2020) and is more prevalent in developing countries than in developed countries and among women (Lee et al., 2024). While its prevalence is gradually declining (Lee et al., 2024), FD continues to impair the patients’ quality of life (Enck et al., 2017). It mainly affects the physical components of health-related quality of life rather than the mental components (Hantoro et al., 2018). Diagnosis of FD is based on the Rome IV criteria, which include symptoms, such as early satiety, epigastric burning, postprandial fullness, and epigastric pain for at least 6 months, with no evidence of structural abnormalities (Stanghellini et al., 2016).
The definitive etiology of FD remains unclear; however, gastric dysrhythmias, antral hypomotility, and delayed and rapid gastric emptying have been associated with its pathogenesis (Park et al., 2017). Visceral hypersensitivity—including a lowered threshold for pain with normal gastric compliance, dysfunction of mechanoreceptors, and abnormal afferent signaling in the central nervous system—has also been suggested as a potential mechanism underlying the pathophysiology of FD (Farré et al., 2013; Rosen et al., 2014). In addition, psychological distress, including depression and anxiety, has been linked to FD and, in some individuals, may precede the emergence of symptoms (Aro et al., 2015). Gastroduodenal inflammation or Helicobacter pylori infection has also been posited as a cause of FD (Sayuk and Gyawali, 2020; Iwata et al., 2023). Among these, gastric motor dysfunction and visceral hypersensitivity are the most widely accepted causes (Vanheel and Farré, 2013).
Current treatment modalities for FD include acid suppressants and prokinetics (Oshima, 2024). However, because FD involves multiple pathogenic factors, single-target therapies may be insufficient. Consequently, an increasing number of multipotent herbal medicines have been proposed in various countries (Xiao et al., 2022). In Japan, rikkunshito is currently recommended as a first-line therapy for FD in the evidence-based clinical practice guidelines (Miwa et al., 2022).
Sihogyeji-tang (SG), known as Chaihu Guizhi decoction in China and Saiko-keishi-to in Japan, is used to treat depression, influenza, and epilepsy in Asia (Liu et al., 2014; Lee et al., 2016; Zhao et al., 2024). SG is a combination of Sosiho-tang (Xiao Chaihu decoction) and Gyeji-tang (Guizhi decoction) and is composed of Bupleurum falcatum L. [Apiaceae; Bupleuri Radix], Scutellaria baicalensis Georgi [Lamiaceae; Scutellariae Radix], Cinnamomum cassia (L.) J.Presl [Lauraceae; Cinnamomi Ramulus], Paeonia lactiflora Pall. [Paeoniaceae; Paeoniae Radix], Panax ginseng C.A.Mey. [Araliaceae; Ginseng Radix], Pinellia ternata (Thunb.) Makino [Araceae; Pinelliae Tuber], Glycyrrhiza uralensis Fisch. ex DC. [Fabaceae; Glycyrrhizae Radix et Rhizoma], Zingiber officinale Roscoe [Zingiberaceae; Zingiberis Rhizoma Recens], and Ziziphus jujuba Mill. [Rhamnaceae; Jujubae Fructus] (Li et al., 2019). The combination of quercetin and baicalein in Sosiho-tang exhibits anti-inflammatory effects by suppressing the levels of interleukin-6, tumor necrosis factor-α, and interleukin-1β (Zhan et al., 2021; Lei et al., 2024). According to the traditional Korean medicine theory, Sosiho-tang regulates the flow of Qi, strengthens the stomach, promotes blood circulation, and eliminates stasis (Zhan et al., 2021). Gyeji-tang also inhibits proinflammatory cytokines interleukin-6 and tumor necrosis factor-α (Yoo et al., 2016). Pharmacological experimental studies have revealed that Gyeji-tang can play a significant role in the bidirectional regulation of body temperature, sweat glands, immune function, blood pressure, and gastrointestinal motility while also exhibiting antiallergic, anti-inflammatory, antiviral, antibacterial, hypoglycemic, analgesic, and cardiovascular protective effects (Yuan et al., 2017). Given its multifaceted composition, SG has therapeutic potential for FD.
Although multiple randomized controlled trials (RCTs) have evaluated SG for FD, no systematic review has been conducted to summarize and appraise the existing research. Recent meta-analyses have investigated formulas such as rikkunshito (RKT) and Banxia-xiexin-tang (BXT) for FD (Ko et al., 2021; Kim et al., 2023); however, a comprehensive synthesis of SG remains to be performed. As a hybrid of Sosiho-tang and Gyeji-tang, SG represents a distinct therapeutic concept within East Asian gastroenterology, combining anti-inflammatory and motility-modulating actions that may distinguish its clinical efficacy profile. By synthesizing evidence from these clinical trials, we aimed to establish a foundation for the clinical application of SG in FD.
2 Methods
2.1 Study protocol and registration
The review protocol was registered with PROSPERO (CRD420251041781).
2.2 Data sources and search strategy
We systematically searched eleven databases from their inception to June 2025: four English databases (CINAHL, EMBASE, Cochrane Database, and PubMed), five Korean databases (RISS, KISS, NDSL, DBPIA, and OASIS), one Japanese database (J-Stage), and three Chinese databases (Chinese National Knowledge Infrastructure; CNKI, Wanfang Data, and VIP Information; VIP). Two independent researchers performed the search.
The search strategy included terms related to the intervention (e.g., Sihogyeji*, shihogyeji*, Shiho-Guizhi*, Saikokeishito*, Chaihuguizhi*, Chaihu Guizhi*, Modified Chaihuguizhi*, etc.) and the target condition (e.g., “Functional Dyspepsia”, Dyspepsia, “Non-ulcer dyspepsia”, Indigestion, Digest*, Gastr*, Postprandial, Epigastric, Gut, Stomach, Intestin*). Detailed search strategies are presented in Supplementary Appendix A for the English databases and Supplementary Appendix B for the Chinese databases.
No language restrictions were applied, and the search strategies were adapted for each database as appropriate.
2.3 Study selection criteria for this review
Two researchers independently selected the data. The selection of studies was performed using Microsoft Excel.
2.3.1 Types of studies
This systematic review included only RCTs. Studies were included regardless of blinding, language, or reporting type.
2.3.2 Types of patients
All patients included in this review were diagnosed with FD, and no criteria other than age (≥18 to ≤70 years) were considered.
2.3.3 Types of interventions
Studies using SG or modified SG as the sole herbal intervention were included. To objectively define “modified SG”, we established criteria based on the traditional principles of formula composition, which describe the hierarchical roles of herbs as the Chief (君, jūn), Deputy (臣, chén), Assistant (佐, zuǒ), and Envoy (使, shǐ) (Bensky and Barolet, 1990).
The therapeutic integrity of a formula is primarily determined by its Chief and Deputy herbs, which target the main clinical presentation. Therefore, our primary inclusion criterion was the mandatory presence of the six core herbs that function as the Chief and Deputy components of SG: Bupleurum falcatum L. [Apiaceae; Bupleuri Radix], Scutellaria baicalensis Georgi [Lamiaceae; Scutellariae Radix], Cinnamomum cassia (L.) J.Presl [Lauraceae; Cinnamomi Ramulus], Paeonia lactiflora Pall. [Paeoniaceae; Paeoniae Radix], Panax ginseng C.A.Mey. [Araliaceae; Ginseng Radix] (or its common substitute, Codonopsis pilosula (Franch.) Nannf. [Campanulaceae; Codonopsis Radix]), and Pinellia ternata (Thunb.) Makino [Araceae; Pinelliae Tuber].
Based on this core structure, the following modifications were permitted:
1. Omission: The omission of up to three herbs serving as Assistant and Envoy herbs, which primarily harmonize the formula, was allowed. These include Glycyrrhiza uralensis Fisch. ex DC. [Fabaceae; Glycyrrhizae Radix et Rhizoma], Zingiber officinale Roscoe [Zingiberaceae; Zingiberis Rhizoma Recens], and Ziziphus jujuba Mill. [Rhamnaceae; Jujubae Fructus].
2. Addition: The addition of a maximum of five herbs based on syndrome differentiation (辨證加減, biànzhèng jiājiǎn) was permitted.
All the included studies administered the formula orally, regardless of its form (e.g., decoctions, powders). For the control group, studies using standard pharmacologic treatments (e.g., prokinetics, proton pump inhibitors) or usual care for FD were eligible. However, interventions combining SG with other treatments not specified in the control group were excluded.
2.3.4 Types of outcome measures
The outcomes analyzed in this review were as follows:
Primary outcome:
1. Total effective rate: This outcome reflects the percentage of patients who experienced improvement following treatment, where improvement was defined as any response except “ineffective.” As this measure is widely used to indicate treatment effectiveness in Chinese RCTs, it was adopted as an outcome in this review.
Secondary outcomes:
1. TCM symptom total score: This outcome refers to the sum of scores for various symptoms (such as epigastric pain, poor appetite, epigastric discomfort, and other related symptoms) based on traditional Chinese medicine diagnostic criteria. Higher scores indicate more severe symptoms. This measure is commonly used in Chinese clinical trials to quantitatively assess changes in symptom severity before and after treatment.
2. Adverse events: The outcome was determined by the rate of participants who encountered any adverse event at any point during or after treatment. Adverse events were recorded to assess the safety profile of the interventions.
2.4 Data extraction and analysis
2.4.1 Data extraction
For each included study, two researchers independently extracted data as planned. The following data items were collected: publication year, first author, study design, country, sample size and characteristics (sex, age, diagnostic criteria), intervention details, outcome measures, any reported adverse events, and blinding methods. Discrepancies were resolved by discussion. All extracted data were managed using Microsoft Excel.
2.4.2 Assessment of risk of bias
Two reviewers independently evaluated the risk of bias in each study using the Cochrane Risk of Bias tool, following the criteria outlined in the Cochrane Handbook (Higgins et al., 2011). The following domains were included: random sequence generation, allocation concealment, blinding of both participants and personnel, incomplete outcome data, selective reporting, and other potential biases. Each category was judged as low, unclear, or high risk. Differences in judgment were resolved through discussion.
2.4.3 Data synthesis
Review Manager (RevMan) version 5.4 (Cochrane) was employed for all statistical analyses. Risk ratios were utilized to analyze dichotomous outcomes, whereas standardized mean differences were employed to assess continuous outcomes, both reported with 95% confidence intervals (CIs).
2.4.4 Dealing with missing data
If important data were missing or unclear, we contacted the authors of the studies to seek clarification. Studies were excluded from the analysis if the needed data remained unobtainable.
2.4.5 Assessment of heterogeneity
The Chi-square test and I2 statistic were utilized to evaluate heterogeneity among the included studies. Sensitivity and subgroup analyses were planned to explore potential sources of heterogeneity if substantial heterogeneity was detected (I2 ≥ 50%) and if a sufficient number of studies (≥10) was available. A fixed-effect model would be used if heterogeneity was low (I2 < 50%), whereas a random-effects model was planned for high heterogeneity (I2 ≥ 50%).
2.4.6 Assessment of reporting bias
Reporting bias assessment was intended to be conducted using funnel plots. This analysis would be conducted only if the meta-analysis included at least 10 studies.
2.4.7 Grading the quality of evidence
The quality of evidence was independently assessed by two researchers. The certainty of evidence for the outcomes was assessed using the GRADE approach, implemented via the GRADEpro Guideline Development Tool (https://gradepro.org/). The evaluation considered the following domains: study design, risk of bias, inconsistency, indirectness, imprecision, and other relevant factors. Following these assessments, the quality of evidence was rated as high, moderate, low, or very low (Table 1).
2.5 Ethical approval
No ethical approval was required as only published data were analyzed.
3 Results
3.1 Study selection
A total of 876 studies were initially identified, and 713 remained after removing duplicates. After screening titles and abstracts, 39 studies were selected. Upon full-text assessment of these 39 studies, 27 were excluded for various reasons: 18 were not RCTs, 4 did not study FD, 3 did not include SG monotherapy as the intervention (e.g., combination therapy with the control drug), and 2 presented duplicate experimental data. Consequently, 12 studies were selected for inclusion in our meta-analysis. The study selection procedure is depicted in the PRISMA flow chart (Figure 1).

Figure 1. PRISMA flowchart of the literature search process. Cochrane, Cochrane Central Register of Controlled Trials; PubMed, National Library of Medicine biomedical literature database; EMBASE, Excerpta Medica database for biomedical and pharmaceutical literature; CINAHL, Cumulative Index to Nursing and Allied Health Literature; CNKI, China National Knowledge Infrastructure; Wanfang, Wanfang Data; VIP, VIP Information; OASIS, Oriental Medicine Advanced Searching Integrated System; RISS, Research Information Service System; KISS, Korean Studies Information Service System; NDSL, National Digital Science Library; DBPIA, academic journal database; J-Stage, Japan Science Technology Information Aggregator, Electronic.
3.2 Participants
The 12 studies comprised a total of 805 participants, all of whom were aged 18–70 years.
3.3 Intervention
3.3.1 Experimental intervention
In all the 12 studies (Li, 2011; Chen, 2014; Li, 2016; Wang, 2017; Dai, 2019; Deng and Dong, 2019; Yu, 2019; Su, 2020; Zhang, 2020; Huang, 2021; Shao, 2021; Li, 2022) included in the meta-analysis, SG or modified SG was administered as an oral decoction. Five studies (Li, 2016; Dai, 2019; Su, 2020; Zhang, 2020; Li, 2022) administered the intervention for 30 days, whereas the remaining studies administered it for 28 days. In all studies, SG was administered twice daily (Table 2).
3.3.2 Control intervention
Only studies with prokinetic agents as comparators were included. In all studies, an oral prokinetic agent was used as Western medication (WM); three studies (Li, 2011; Chen, 2014; Su, 2020) used Domperidone Tablets, whereas seven studies (Wang, 2017; Dai, 2019; Deng and Dong, 2019; Yu, 2019; Zhang, 2020; Shao, 2021; Li, 2022) used Mosapride Citrate Tablets. One study (Huang, 2021) administered Domperidone Tablets plus Azintamide Tablets, and one study (Li, 2016) used Itopride Tablets. The medications were administered three times daily, and the treatment duration was identical to that of the experimental group (Table 2).
3.4 Outcomes
All the 12 studies assessed the total effective rate. Four studies (Deng and Dong, 2019; Yu, 2019; Zhang, 2020; Huang, 2021) evaluated the TCM symptom total score, and four studies (Chen, 2014; Yu, 2019; Huang, 2021; Li, 2022) reported adverse events (Table 2).
3.5 Risk of bias
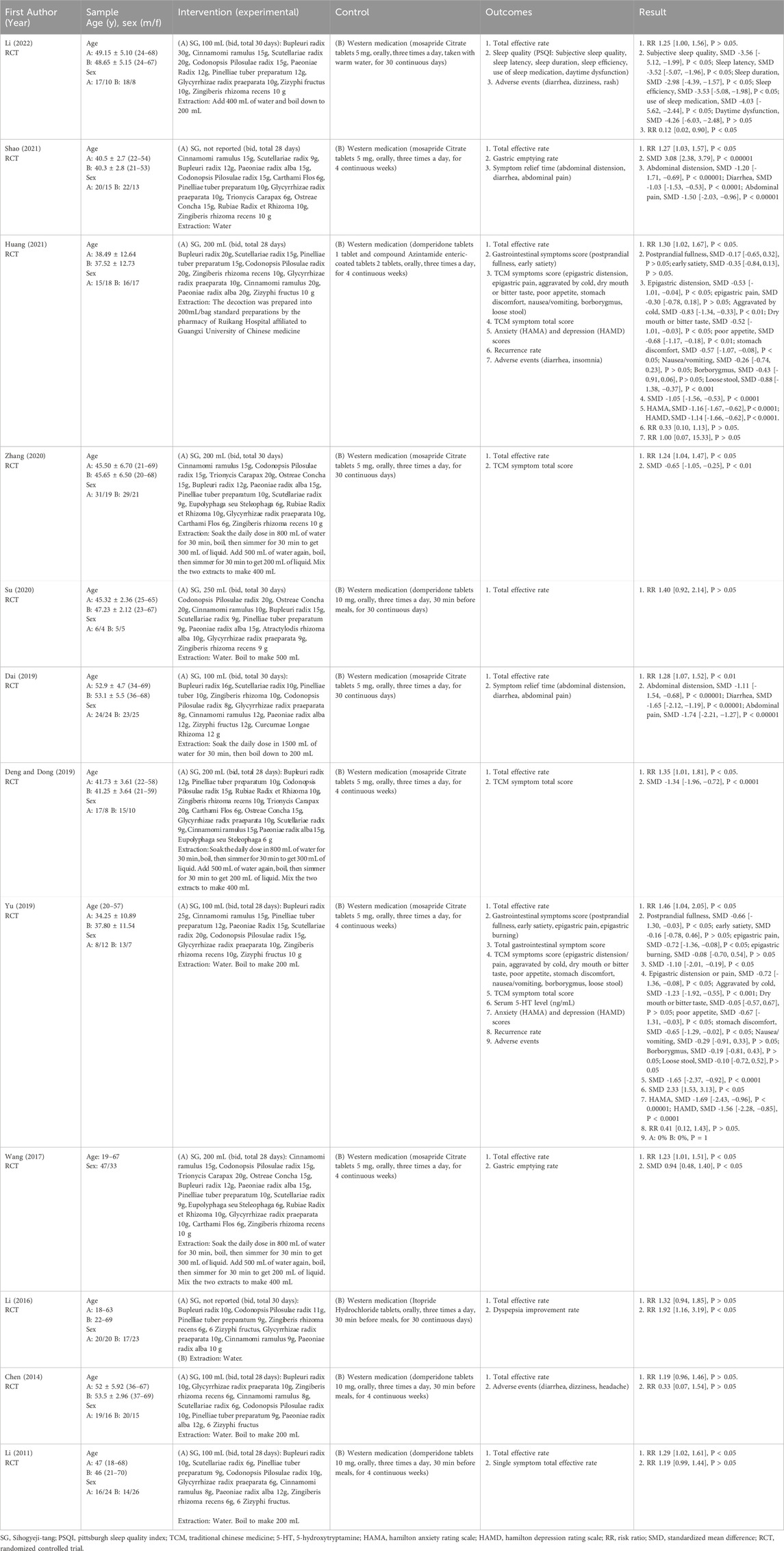
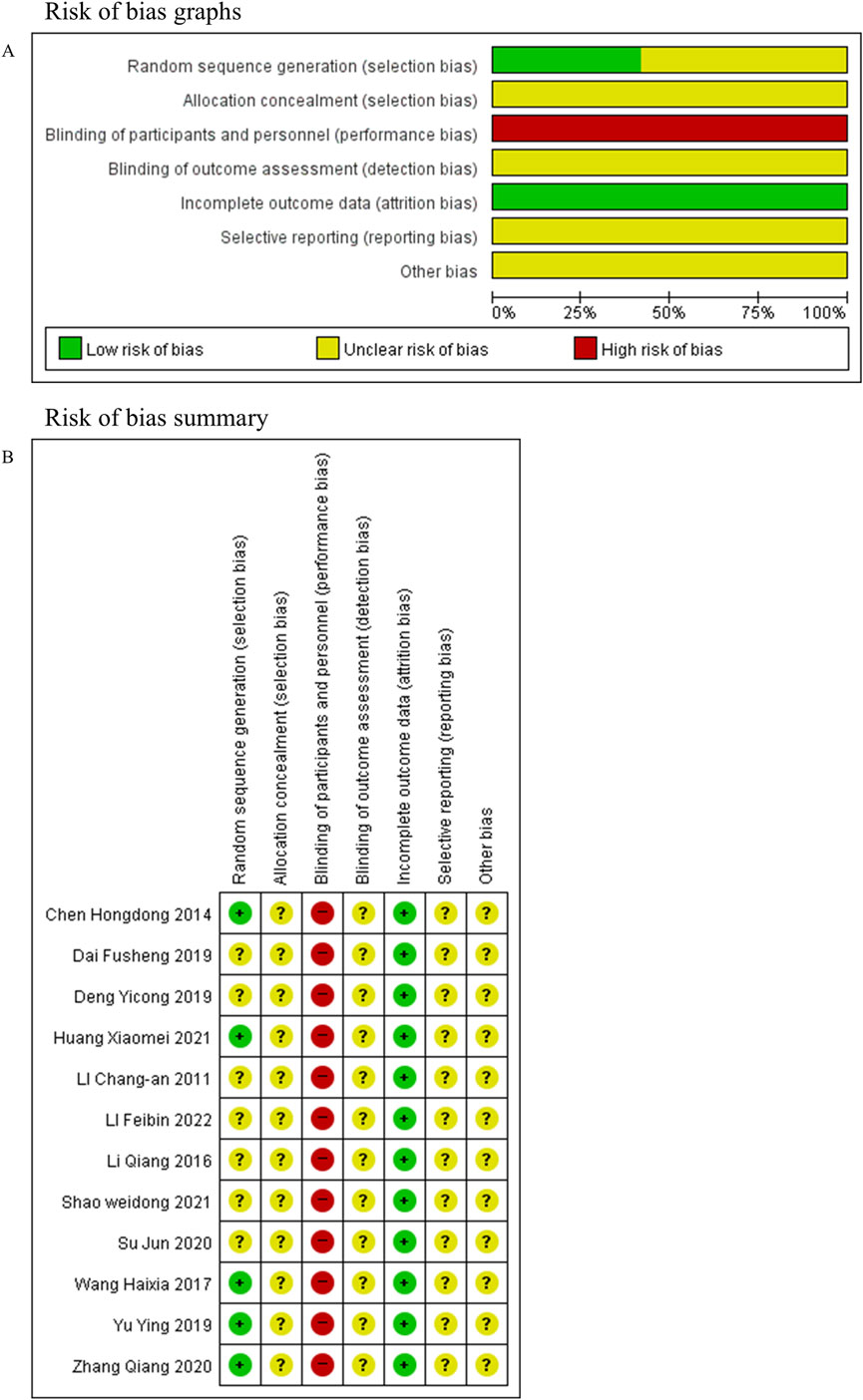
Each of the 12 studies included reports of random sequence generation. Nevertheless, only five (Chen, 2014; Wang, 2017; Yu, 2019; Zhang, 2020; Huang, 2021) studies provided specific details regarding their randomization methods. None reported allocation concealment, outcome assessment blinding, or selective reporting. Blinding of participants and personnel was assessed as high risk of bias in all studies, as the interventions compared oral decoctions with tablets, without placebo control, making blinding of both participants and investigators infeasible. No study had missing outcome data. The risk of bias evaluation results are presented in Figures 2A,B.
3.6 Effect of interventions
3.6.1 Total effective rate
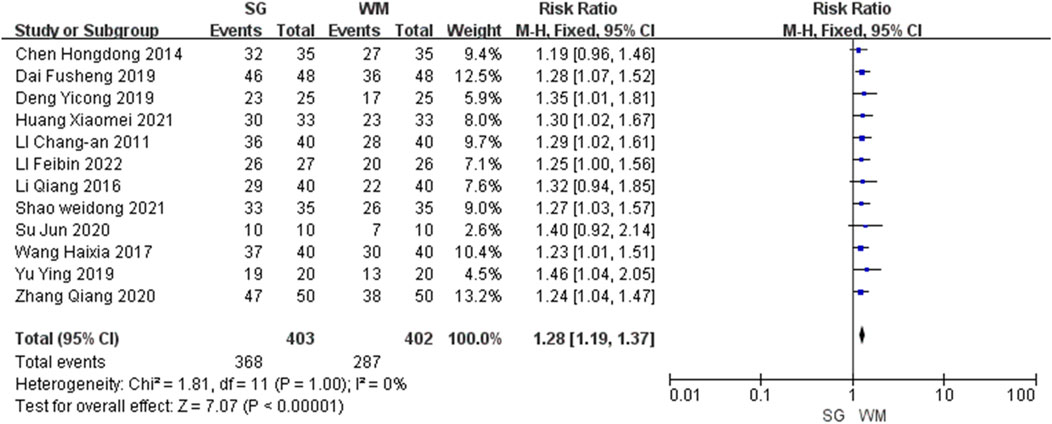
All the 12 studies analyzed the total effective rate, with a combined total of 805 participants. Among these studies, eight (Li, 2011; Wang, 2017; Dai, 2019; Deng and Dong, 2019; Yu, 2019; Zhang, 2020; Huang, 2021; Shao, 2021) reported significant differences (P < 0.05), whereas the remaining four (Chen, 2014; Li, 2016; Su, 2020; Li, 2022) found no significant differences (P ≥ 0.05). The meta-analysis demonstrated that the total effective rate was significantly higher in the SG group than in the WM group (risk ratio: 1.28, 95% CI: 1.19–1.37, P < 0.00001; Figure 3).
3.6.2 TCM symptom total score
Four studies (Deng and Dong, 2019; Yu, 2019; Zhang, 2020; Huang, 2021), comprising a total of 256 participants, reported the TCM symptom total score. All the four studies found significant differences (P < 0.01). The meta-analysis showed that the SG group achieved a significantly greater reduction in the TCM symptom total score than the WM group (standardized mean difference: −1.10, 95% CI: −1.53 to −0.68; Figure 4).
![Forest plot comparing SG and WM across four studies, showing standardized mean differences and confidence intervals. Data includes means, standard deviations, and total participants. Overall effect size is -1.10 with a 95% CI of [-1.53, -0.68]. Heterogeneity: Tau² = 0.11, Chi² = 7.12, df = 3, P = 0.07, I² = 58%.](https://www.frontiersin.org/files/Articles/1689132/fphar-16-1689132-HTML/image_m/fphar-16-1689132-g004.jpg)
Figure 4. Forest plot of traditional Chinese medicine symptom total score comparing Sihogyeji-tang with Western medication.
3.6.3 Adverse events
Four studies (Chen, 2014; Yu, 2019; Huang, 2021; Li, 2022), including a total of 229 participants, reported adverse events. Among these, one study (Li, 2022) found a significant difference (P < 0.05), two (Chen, 2014; Huang, 2021) found no significant difference (P ≥ 0.05), and the other (Yu, 2019) reported no adverse events in either group (P = 1). The meta-analysis indicated that the SG group experienced significantly fewer adverse events than the WM group (risk ratio: 0.26, 95% CI: 0.09–0.76, P < 0.05; Figure 5). No serious adverse events were reported.
3.7 Heterogeneity
The I2 values for the total effective rate and adverse events were <50%, indicating low heterogeneity. Therefore, for these outcomes, sensitivity and subgroup analyses were not performed, and a fixed-effect model was employed for the meta-analyses.
In contrast, the I2 value for the TCM symptom total score was 58%, which exceeds the threshold for substantial heterogeneity (I2 > 50%). Therefore, a random-effects model was employed for this meta-analysis. Sensitivity and subgroup analyses were not performed because the number of studies (N = 4) did not meet the pre-specified requirement of ≥10 studies.
3.8 Reporting bias
Reporting bias assessment was conducted for analyses including ≥10 studies; accordingly, it was performed for the meta-analysis of the total effective rate, which included 12 studies. The funnel plot (Figure 6) revealed a largely symmetrical distribution of studies, suggesting a low risk of publication bias.
3.9 GRADE assessment for quality of evidence
The quality of evidence for all the three outcomes was not rated as high, primarily because of risk of bias concerns. The quality of evidence for the TCM symptom total score was also downgraded for inconsistency due to substantial statistical heterogeneity. In addition, owing to limited sample sizes, the evidence quality for both the TCM symptom total score and adverse events was downgraded for imprecision. As a result, the quality of evidence was rated as moderate for the total effective rate, very low for the TCM symptom total score, and low for adverse events (Table 1).
4 Discussion
This systematic review and meta-analysis evaluated the efficacy and safety of SG compared with prokinetic agents in the management of FD. The findings indicate that SG is associated with a significantly higher total effective rate and greater improvement in symptom severity, as measured by the TCM symptom total score, than conventional prokinetic therapy. Furthermore, SG showed a better safety profile, exhibiting fewer adverse events.
The present findings, demonstrating that SG provides clinically meaningful symptom relief often with fewer adverse effects than conventional pharmacotherapy, are consistent with those of previous meta-analyses on other multi-component herbal formulations for FD (Gwee et al., 2021; Luo et al., 2022). Herbal medicines, including SG, are believed to exert their effects through multiple mechanisms, such as modulating gastrointestinal motility, reducing inflammation, and neuromodulation, thereby addressing the multifactorial pathophysiology of FD more comprehensively than single-target drugs (Gwee et al., 2021; Luo et al., 2022).
The pharmacological actions of SG are believed to emanate from synergistic interactions among its various herbal constituents. The main active compounds of SG include flavonoids (such as baicalin, isoliquiritigenin, and wogonoside), triterpenoids (such as saikosaponin A and glycyrrhizic acid), and organic acids (such as gallic acid) (Li et al., 2019). These compounds have demonstrated a range of therapeutic effects, including anti-inflammation, anti-oxidation, regulation of blood lipids, vasodilation, and inhibition of platelet aggregation (Li et al., 2019). For instance, baicalin reduces acute inflammation by downregulating the expression of nuclear factor kappa B proteins and protein kinase D1 (Qian et al., 2018), whereas wogonoside reduces inflammation by inhibiting both the nuclear factor kappa B signaling pathway and the activation of the NLRP3 inflammasome (Sun et al., 2015). Saikosaponin A has been shown to inhibit nuclear factor kappa B activation and suppress pro-inflammatory cytokines (Lu et al., 2012). In addition, accumulating evidence indicates that these bioactive compounds of SG also play an important role in regulating gastrointestinal motility, a key pathophysiological mechanism of FD. For example, saikosaponin D, a principal component of Bupleurum species, has been shown in animal studies to improve gastrointestinal motility and gastric emptying by restoring interstitial cells of Cajal and modulating gastrointestinal hormones, such as ghrelin and substance P (Zeng et al., 2024). Furthermore, isoliquiritigenin modulates gastrointestinal motility bidirectionally, inhibiting it at low doses and stimulating it at high doses (Chen et al., 2009). Such multi-targeted actions, addressing various underlying factors, may contribute significantly to the observed clinical efficacy of SG in FD.
Previous meta-analyses on herbal medicines for FD have primarily evaluated formulas such as RKT and BXT, each showing efficacy compared with conventional therapy but differing in pharmacological emphasis—prokinetic, gastroprotective, or psychotropic (Ko et al., 2021; Kim et al., 2023). In contrast, SG integrates two classical prescriptions (Sosiho-tang and Gyeji-tang) that jointly target gastrointestinal motility and inflammatory regulation, offering a dual mechanism distinct from that of other herbal formulas. The present meta-analysis quantitatively demonstrates that SG (risk ratio = 1.28 [1.19–1.37]) achieves a therapeutic effect comparable with or slightly superior to those of RKT (risk ratio = 1.21 [1.17–1.25]) and BXT (risk ratio = 1.19 [1.15–1.23]) (Ko et al., 2021; Kim et al., 2023). Furthermore, the incidence of adverse events with SG (risk ratio = 0.26 [0.09–0.76]) was notably lower than that reported for BXT (risk ratio = 0.53 [0.35–0.81]) and significantly lower compared with RKT, for which the previous meta-analysis found no significant difference compared with controls (risk ratio = 0.69 [0.37–1.29]), underscoring its favorable safety profile (Ko et al., 2021; Kim et al., 2023). Collectively, these findings identify SG as a well-tolerated and efficacious therapeutic option that expands the evidence base for polyherbal interventions in FD.
The meta-analysis results indicated that adverse events were significantly less common with SG than with prokinetic agents. This finding is particularly relevant in light of concerns regarding the safety of certain prokinetics, such as domperidone, which has been linked to cardiac adverse events—including QT interval prolongation and ventricular arrhythmias—as well as rare extrapyramidal adverse events (Reddymasu et al., 2007; van Noord et al., 2010; Leelakanok et al., 2016). In contrast, mosapride has demonstrated a favorable cardiac safety profile in clinical studies (Nachimuthu et al., 2012). While herbal medicines are generally considered to have a favorable safety profile, they are not entirely devoid of risk. Previous systematic reviews have reported that most adverse events associated with herbal preparations are typically transient and mild, such as mild allergic reactions or gastrointestinal discomfort, with serious adverse events being rare (Hu et al., 2020; Luo et al., 2022; Yao et al., 2022). Nevertheless, continuous pharmacovigilance and rigorous quality control remain important to ensure the safe use of herbal medicines in clinical practice (Hu et al., 2020; Yao et al., 2022).
While the included studies in this meta-analysis, which mostly involved short-term administration (4 weeks), reported a favorable safety profile for SG, the broader safety aspects, potential side effects, and herb–drug interactions associated with its constituent herbs warrant careful consideration. For instance, Glycyrrhiza uralensis Fisch. ex DC. [Fabaceae; Glycyrrhizae Radix et Rhizoma], a component of SG, contains glycyrrhizin, which can cause pseudoaldosteronism (leading to edema, hypokalemia, and hypertension) when consumed in high doses or for prolonged periods (Isbrucker and Burdock, 2006). Furthermore, interstitial pneumonitis, although rare, is a well-known serious adverse event associated with Sho-saiko-to (Sosiho-tang), one of the base formulas for SG; this risk is primarily reported in Japan and has been linked to factors such as long-term administration or co-administration with interferon (Lee et al., 2011). In terms of interactions, the glycyrrhizin from Glycyrrhiza uralensis Fisch. ex DC. [Fabaceae; Glycyrrhizae Radix et Rhizoma] may potentiate potassium loss when combined with diuretics such as thiazides or furosemide (Farese et al., 1991). Moreover, Sho-saiko-to (Sosiho-tang) was shown in vivo in humans to reduce CYP1A2 activity and show a tendency to lower CYP3A activity, potentially altering the metabolism of co-administered drugs (Saruwatari et al., 2003). Therefore, while short-term use of SG for FD appears relatively safe as indicated by our findings, clinicians should remain vigilant for these potential risks and interactions, especially in patients receiving long-term treatment or polypharmacy.
A major strength of this review lies in its comprehensive search strategy across multiple languages and databases, which minimized selection bias and captured a broad spectrum of evidence. Furthermore, the use of rigorous inclusion criteria, restricted to RCTs and monotherapy interventions, enhanced the internal validity of our findings. However, limitations related to the methodological quality of the included studies remain. Most studies lacked adequate blinding and allocation concealment, posing a high risk of bias according to GRADE criteria. Regarding FD, subjective outcomes (total effective rate, symptom scores) are highly susceptible to expectation and detection biases when comparing dissimilar interventions like decoctions and tablets. Inadequate allocation concealment further raises concerns about potential selection bias. To strengthen the evidence, future RCTs should prioritize methodological rigor, with particular emphasis on effective blinding. Potential strategies to enhance the methodology include formulating both interventions as identical tablets, administering both as decoctions using a high-fidelity placebo (matching taste, color, smell), or employing a double-dummy design. Robust randomization, allocation concealment, and blinding are essential to validate the promising yet methodologically limited evidence for SG. In addition, the limited number of included studies and participants, particularly for secondary outcomes, resulted in imprecision and a downgrading of evidence quality in the GRADE assessment. While this review provides the first quantitative synthesis for SG, the number of included studies (N = 12) is considerably smaller than that available for other formulas, such as RKT (N = 52) and BXT (N = 57) (Ko et al., 2021; Kim et al., 2023). This disparity in the volume of evidence means that the precision of our effect estimates may be lower, and direct comparisons should be made with caution. The use of total effective rate and TCM symptom scores, which are not internationally standardized, may limit generalizability to non-Asian populations. Similarly, since all studies were conducted in China, the findings may not be applicable to other countries or healthcare systems.
5 Conclusion
Our findings suggest that SG could be a valuable therapeutic option for FD, especially in patients intolerant of or unresponsive to conventional prokinetics. Given its complex and multifactorial pathogenesis, the multi-targeted nature of herbal formulas, such as SG, may be particularly advantageous in FD. Nevertheless, the moderate-to-very low certainty of evidence, due to methodological limitations, warrants cautious interpretation of the findings. Future research should focus on large, well-designed, double-blind, multicenter RCTs with standardized outcome measures and robust reporting. Long-term safety studies and mechanistic investigations are also needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
HB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft. JK: Funding acquisition, Investigation, Supervision, Writing – review and editing. SK: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: RS-2024-00443769).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1689132/full#supplementary-material
Abbreviations
BXT, Banxia-xiexin-tang; CI, confidence interval; FD, Functional dyspepsia; RCT, randomized controlled trial; RKT, rikkunshito; SG, Sihogyeji-tang; TCM, traditional Chinese medicine; WM, Western medicine.
References
Aro, P., Talley, N. J., Johansson, S. E., Agréus, L., and Ronkainen, J. (2015). Anxiety is linked to new-onset dyspepsia in the Swedish population: a 10-year follow-up study. Gastroenterology 148, 928–937. doi:10.1053/j.gastro.2015.01.039
Bensky, D., and Barolet, R. (1990). Chinese herbal medicine: Formulas and strategies. Seattle, WA: Eastland Press.
Chen, H. D. (2014). Randomized parallel controlled study of chaihu guizhi decoction in the treatment of functional dyspepsia. J. Pract. Tradit. Chin. Intern. Med. 28, 15–17. doi:10.13729/j.issn.1671-7813.2014.03.09
Chen, G., Zhu, L., Liu, Y., Zhou, Q., Chen, H., and Yang, J. (2009). Isoliquiritigenin, a flavonoid from licorice, plays a dual role in regulating gastrointestinal motility in vitro and in vivo. Phytother. Res. 23, 498–506. doi:10.1002/ptr.2660
Dai, F. (2019). Clinical observation on the therapeutic effect of modified Chaihu Guizhi decoction in the treatment of functional dyspepsia. Heal. Friend, 49–50.
Deng, Y. C., and Dong, L. X. (2019). Clinical observation of modified chaihu Guizhi decoction in the treatment of functional dyspepsia. Heilongjiang J. Tradit. Chin. Med. 48, 13–14.
Enck, P., Azpiroz, F., Boeckxstaens, G., Elsenbruch, S., Feinle-Bisset, C., Holtmann, G., et al. (2017). Functional dyspepsia. Nat. Rev. Dis. Prim. 3, 17081. doi:10.1038/nrdp.2017.81
Farese, R. V., Biglieri, E. G., Shackleton, C. H., Irony, I., and Gomez-Fontes, R. (1991). Licorice-induced hypermineralocorticoidism. N. Engl. J. Med. 325, 1223–1227. doi:10.1056/nejm199110243251706
Farré, R., Vanheel, H., Vanuytsel, T., Masaoka, T., Törnblom, H., Simrén, M., et al. (2013). In functional dyspepsia, hypersensitivity to postprandial distention correlates with meal-related symptom severity. Gastroenterology 145, 566–573. doi:10.1053/j.gastro.2013.05.018
Ford, A. C., Mahadeva, S., Carbone, M. F., Lacy, B. E., and Talley, N. J. (2020). Functional dyspepsia. Lancet 396, 1689–1702. doi:10.1016/S0140-6736(20)30469-4
Gwee, K. A., Holtmann, G., Tack, J., Suzuki, H., Liu, J., Xiao, Y., et al. (2021). Herbal medicines in functional dyspepsia-untapped opportunities not without risks. Neurogastroenterol. Motil. 33, e14044. doi:10.1111/nmo.14044
Hantoro, I. F., Syam, A. F., Mudjaddid, E., Setiati, S., and Abdullah, M. (2018). Factors associated with health-related quality of life in patients with functional dyspepsia. Health Qual. Life Outcomes 16, 83. doi:10.1186/s12955-018-0913-z
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Hu, J., Zhang, H., Feng, S., Ha, Y., Wei, C., Wang, X., et al. (2020). The safety of Chinese herbal medicine: a systematic review of adverse events in randomized controlled trials. Longhua Chin. Med. 3, 7. doi:10.21037/lcm-20-23
Huang, X. (2021). Clinical observation of modified Sihogyeji-tang for mixed cold and heat type functional dyspepsia. Nanning, China: Guangxi University of Chinese Medicine.
Isbrucker, R. A., and Burdock, G. A. (2006). Risk and safety assessment on the consumption of Licorice root (glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 46, 167–192. doi:10.1016/j.yrtph.2006.06.002
Iwata, E., Sugimoto, M., Murata, M., Morino, Y., Akimoto, Y., Hamada, M., et al. (2023). Improvement of dyspeptic symptoms after Helicobacter pylori eradication therapy in Japanese patients. JGH Open 7, 855–862. doi:10.1002/jgh3.12986
Kim, K., Ko, S. J., Cho, S. H., Kim, J., and Park, J. W. (2023). Herbal medicine, Banxia-xiexin tang, for functional dyspepsia: a systematic review and meta-analysis. Front. Pharmacol. 14, 1130257. doi:10.3389/fphar.2023.1130257
Ko, S. J., Park, J., Kim, M. J., Kim, J., and Park, J. W. (2021). Effects of the herbal medicine rikkunshito, for functional dyspepsia: a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 36, 64–74. doi:10.1111/jgh.15208
Lee, J. K., Kim, J. H., and Shin, H. K. (2011). Therapeutic effects of the oriental herbal medicine sho-saiko-to on liver cirrhosis and carcinoma. Hepatol. Res. 41, 825–837. doi:10.1111/j.1872-034X.2011.00829.x
Lee, J., Son, K., Hwang, G., and Kim, M. (2016). Effect and safety of shihogyejitang for drug resistant childhood epilepsy. Evid. Based Complement. Altern. Med. 2016, 3410213. doi:10.1155/2016/3410213
Lee, K., Kwon, C.-i., Yeniova, A. Ö., Koyanagi, A., Jacob, L., Smith, L., et al. (2024). Global prevalence of functional dyspepsia according to rome criteria, 1990–2020: a systematic review and meta-analysis. Sci. Rep. 14, 4172. doi:10.1038/s41598-024-54716-3
Leelakanok, N., Holcombe, A., and Schweizer, M. L. (2016). Domperidone and risk of ventricular arrhythmia and cardiac death: a systematic review and meta-analysis. Clin. Drug Investig. 36, 97–107. doi:10.1007/s40261-015-0360-0
Lei, H., Su, H., Cao, L., Zhou, X., Liu, Y., Li, Y., et al. (2024). Investigating xiaochaihu Decoction’s fever-relieving mechanism via network pharmacology, molecular docking, dynamics simulation, and experiments. Anal. Biochem. 694, 115629. doi:10.1016/j.ab.2024.115629
Li, C. A. (2011). Modified chaihu guizhi decoction in the treatment of 40 cases of functional dyspepsia. J. Pract. Tradit. Chin. Intern. Med. 25, 68–69. doi:10.13729/j.issn.1671-7813.2011.06.015
Li, Q. (2016). Clinical study on the efficacy of modified Sihogyeji-tang in the treatment of functional dyspepsia. Med. Health 145, 122.
Li, F. B. (2022). Clinical observation of modified chaihu guizhi decoction in the treatment of functional dyspepsia. Smart Healthc. 8, 142–144. doi:10.19335/j.cnki.2096-1219.2022.04.042
Li, Z., Wen, R., Du, Y., Zhao, S., Zhao, P., Jiang, H., et al. (2019). Simultaneous quantification of fifteen compounds in rat plasma by LC-MS/MS and its application to a pharmacokinetic study of chaihu-guizhi decoction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1105, 15–25. doi:10.1016/j.jchromb.2018.12.006
Liu, Y., Cao, C., and Ding, H. (2014). Study on the anti-depressant effect of chaihu guizhi decoction and its mechinisims of actions. Afr. J. Tradit. Complement. Altern. Med. 11, 273–276. doi:10.4314/ajtcam.v11i2.7
Lu, C.-N., Yuan, Z.-G., Zhang, X.-L., Yan, R., Zhao, Y.-Q., Liao, M., et al. (2012). Saikosaponin a and its epimer saikosaponin d exhibit anti-inflammatory activity by suppressing activation of NF-κB signaling pathway. Int. Immunopharmacol. 14, 121–126. doi:10.1016/j.intimp.2012.06.010
Luo, X., Wang, L., Fang, S., Qing, X., Jiang, T., Yang, Y., et al. (2022). Chinese herbal medicine for functional dyspepsia with psychological disorders: a systematic review and meta-analysis. Front. Neurosci. 16, 933290. doi:10.3389/fnins.2022.933290
Miwa, H., Nagahara, A., Asakawa, A., Arai, M., Oshima, T., Kasugai, K., et al. (2022). Evidence-based clinical practice guidelines for functional dyspepsia 2021. J. Gastroenterol. 57, 47–61. doi:10.1007/s00535-021-01843-7
Nachimuthu, S., Assar, M. D., and Schussler, J. M. (2012). Drug-induced QT interval prolongation: mechanisms and clinical management. Ther. Adv. Drug Saf. 3, 241–253. doi:10.1177/2042098612454283
Oshima, T. (2024). Functional dyspepsia: current understanding and future perspective. Digestion 105, 26–33. doi:10.1159/000532082
Park, S. Y., Acosta, A., Camilleri, M., Burton, D., Harmsen, W. S., Fox, J., et al. (2017). Gastric motor dysfunction in patients with functional gastroduodenal symptoms. Am. J. Gastroenterol. 112, 1689–1699. doi:10.1038/ajg.2017.264
Qian, Y., Chen, Y., Wang, L., and Tou, J. (2018). Effects of baicalin on inflammatory reaction, oxidative stress and PKDl and NF-kB protein expressions in rats with severe acute pancreatitis1. Acta Cir. Bras. 33, 556–564. doi:10.1590/s0102-865020180070000001
Reddymasu, S. C., Soykan, I., and McCallum, R. W. (2007). Domperidone: review of pharmacology and clinical applications in gastroenterology. Am. J. Gastroenterol. 102, 2036–2045. doi:10.1111/j.1572-0241.2007.01255.x
Rosen, J. M., Cocjin, J. T., Schurman, J. V., Colombo, J. M., and Friesen, C. A. (2014). Visceral hypersensitivity and electromechanical dysfunction as therapeutic targets in pediatric functional dyspepsia. World J. Gastrointest. Pharmacol. Ther. 5, 122–138. doi:10.4292/wjgpt.v5.i3.122
Saruwatari, J., Nakagawa, K., Shindo, J., Nachi, S., Echizen, H., and Ishizaki, T. (2003). The in-vivo effects of sho-saiko-to, a traditional Chinese herbal medicine, on two cytochrome P450 enzymes (1A2 and 3A) and xanthine oxidase in man. J. Pharm. Pharmacol. 55, 1553–1559. doi:10.1211/0022357022061
Sayuk, G. S., and Gyawali, C. P. (2020). Functional dyspepsia: diagnostic and therapeutic approaches. Drugs 80, 1319–1336. doi:10.1007/s40265-020-01362-4
Shao, W. (2021). Clinical evaluation of modified Sihogyeji-tang for functional dyspepsia. Diabetes World 18, 36–41.
Stanghellini, V., Chan, F. K., Hasler, W. L., Malagelada, J. R., Suzuki, H., Tack, J., et al. (2016). Gastroduodenal disorders. Gastroenterology 150, 1380–1392. doi:10.1053/j.gastro.2016.02.011
Su, J. (2020). Clinical efficacy analysis of modified Sihogyeji-tang for functional dyspepsia. World Latest Med. Inf. 20, 209–212. doi:10.19613/j.cnki.1671-3141.2020.09.133
Sun, Y., Zhao, Y., Yao, J., Zhao, L., Wu, Z., Wang, Y., et al. (2015). Wogonoside protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-κB and NLRP3 inflammasome activation. Biochem. Pharmacol. 94, 142–154. doi:10.1016/j.bcp.2015.02.002
van Noord, C., Dieleman, J. P., van Herpen, G., Verhamme, K., and Sturkenboom, M. C. (2010). Domperidone and ventricular arrhythmia or sudden cardiac death: a population-based case-control study in the Netherlands. Drug. Saf. 33, 1003–1014. doi:10.2165/11536840-000000000-00000
Vanheel, H., and Farré, R. (2013). Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 10, 142–149. doi:10.1038/nrgastro.2012.255
Wang, H. X. (2017). Clinical observation of modified chaihu guizhi decoction in the treatment of functional dyspepsia. Cardiovasc. Dis. J. Integr. Tradit. Chin. West. Med. 5, 150–152. doi:10.16282/j.cnki.cn11-9336/r.2017.25.110
Xiao, Z., Xu, J., Tan, J., Zhang, S., Wang, N., Wang, R., et al. (2022). Zhizhu kuanzhong, a traditional Chinese medicine, alleviates gastric hypersensitivity and motor dysfunction on a rat model of functional dyspepsia. Front. Pharmacol. 13, 1026660. doi:10.3389/fphar.2022.1026660
Yao, Y., Habib, M., Bajwa, H. F., Qureshi, A., Fareed, R., Altaf, R., et al. (2022). Herbal therapies in gastrointestinal and hepatic disorders: an evidence-based clinical review. Front. Pharmacol. 13, 962095. doi:10.3389/fphar.2022.962095
Yoo, S. R., Kim, Y., Lee, M. Y., Kim, O. S., Seo, C. S., Shin, H. K., et al. (2016). Gyeji-tang water extract exerts anti-inflammatory activity through inhibition of ERK and NF-κB pathways in lipopolysaccharide-stimulated RAW 264.7 cells. BMC Complement. Altern. Med. 16, 390. doi:10.1186/s12906-016-1366-8
Yu, Y. (2019). Clinical observation of modified chaihu guizhi decoction in the treatment of cold-heat complex type functional dyspepsia. Nanning, China: Guangxi University of Chinese Medicine.
Yuan, H. J., Li, W., Jin, J. M., Chen, J. J., Jiang, J., Wang, H., et al. (2017). Research progress on chemical constituents, pharmacological mechanism and clinical application of guizhi decoction. Zhongguo Zhong Yao Za Zhi 42, 4556–4564. doi:10.19540/j.cnki.cjcmm.20170928.010
Zeng, Y., Zhou, L., Wan, Y., Fu, T., Xu, P., Zhang, H., et al. (2024). Effects of saikosaponin D on apoptosis, autophagy, and morphological structure of intestinal cells of cajal with functional dyspepsia. Comb. Chem. High. Throughput. Screen 27, 1513–1522. doi:10.2174/0113862073262404231004053116
Zhan, L., Pu, J., Hu, Y., Xu, P., Liang, W., and Ji, C. (2021). Uncovering the pharmacology of xiaochaihu decoction in the treatment of acute pancreatitis based on the network pharmacology. Biomed. Res. Int. 2021, 6621682. doi:10.1155/2021/6621682
Zhang, Q. (2020). Analysis of application effect of traditional Chinese herbal decoctions in functional dyspepsia patients. Health Must-Read Mag., 306–307.
Zhao, L., Qian, S., Wang, X., Si, T., Xu, J., Wang, Z., et al. (2024). UPLC-Q-Exactive/MS based analysis explore the correlation between components variations and anti-influenza virus effect of four quantified extracts of chaihu guizhi decoction. J. Ethnopharmacol. 319, 117318. doi:10.1016/j.jep.2023.117318
Keywords: Sihogyeji-tang, Chaihu Guizhi decoction, herbal medicine, functional dyspepsia, systematic review, meta-analysis
Citation: Bae H, Kim J and Kim S (2025) Effect of Sihogyeji-tang on functional dyspepsia: a systematic review and meta-analysis. Front. Pharmacol. 16:1689132. doi: 10.3389/fphar.2025.1689132
Received: 21 August 2025; Accepted: 10 November 2025;
Published: 19 November 2025.
Edited by:
Jian Chen, Guangdong Medical University, ChinaReviewed by:
Tao Zheng, Beijing University of Chinese Medicine, ChinaJiazhen Cao, Changchun University of Chinese Medicine, China
Copyright © 2025 Bae, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Soyeon Kim, a2ltc3lAcHVzYW4uYWMua3I=
 Hanbum Bae
Hanbum Bae Jinsung Kim
Jinsung Kim Soyeon Kim
Soyeon Kim
![Forest plot showing a meta-analysis of four studies. Risk ratios with 95% confidence intervals are displayed. Individual study results show varying weights and risk ratios. Overall effect size is 0.26 [0.09, 0.76] with statistical significance (p = 0.01). Heterogeneity is low (I² = 0%).](https://www.frontiersin.org/files/Articles/1689132/fphar-16-1689132-HTML/image_m/fphar-16-1689132-g005.jpg)
![Funnel plot showing the standard error of the logarithm of relative risk (SE(log[RR])) against relative risk (RR). Data points form a pattern within symmetrical blue dashed lines, centered around RR equals one, indicating no obvious publication bias.](https://www.frontiersin.org/files/Articles/1689132/fphar-16-1689132-HTML/image_m/fphar-16-1689132-g006.jpg)