- Department of Nephrology, Longhua Hospital Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background: Intravitreal vascular endothelial growth factor inhibitors (VEGFis) are a standard treatment for diabetic eye complications. However, concerns persist regarding their potential nephrotoxic effects in patients with diabetes mellitus (DM), who are inherently at increased risk of renal disease due to diabetes-related microvascular damage.
Methods: We systematically searched PubMed, Embase, and Cochrane Library for randomized controlled trials (RCTs) evaluating renal-related adverse events in DM adults receiving intravitreal VEGFis versus controls. The primary outcome was occurrence of acute kidney injury (AKI), and the secondary outcome was the risk of chronic kidney disease (CKD). Fixed-effects models pooled odds ratios (ORs) with 95% confidence intervals (CIs).
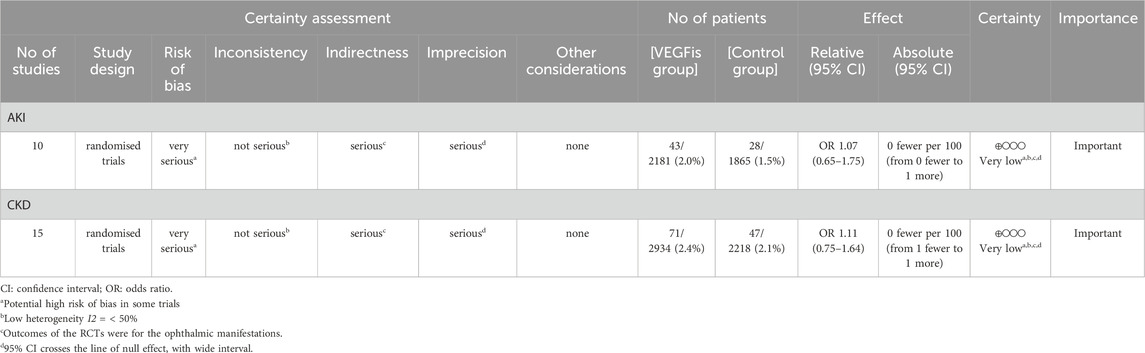
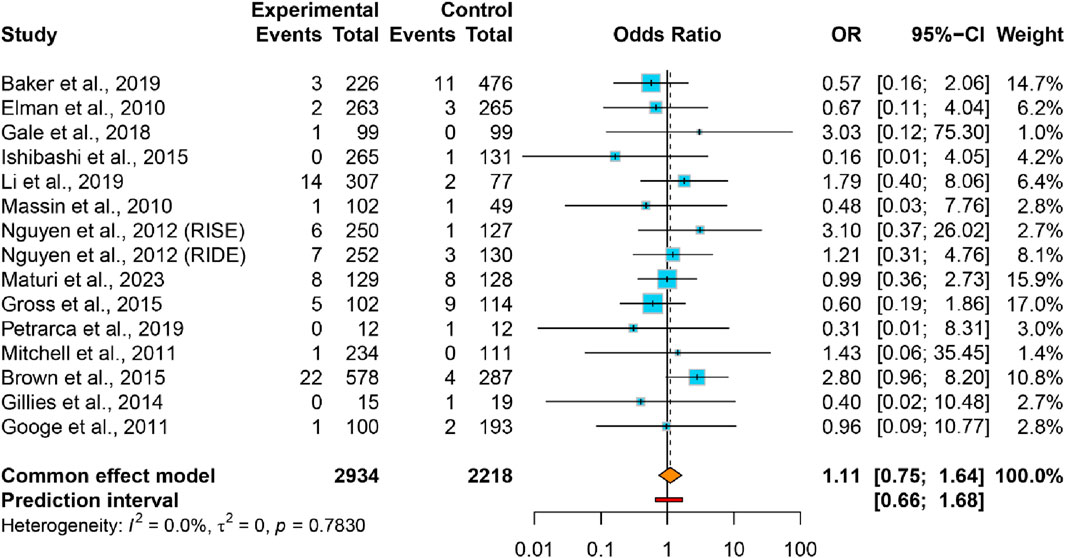
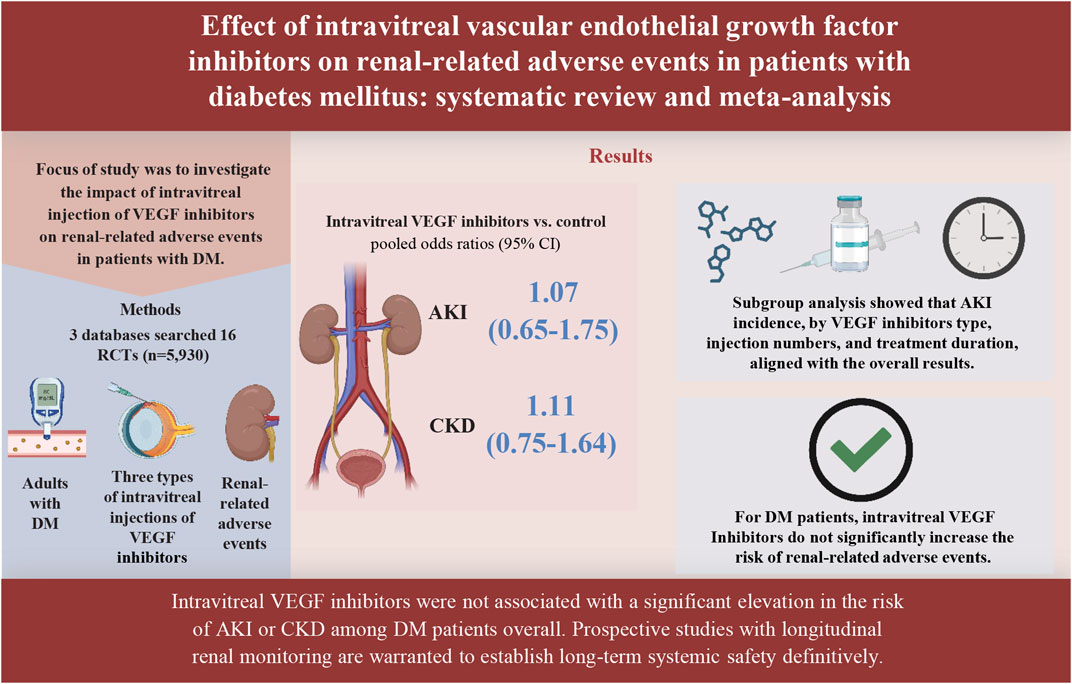
Results: From 16 RCTs (n = 5,930 patients), pooled analyses showed no significant increase in renal risk with VEGFis. The incidence of AKI (10 trials) showed no significant difference between the VEGFis groups (2.0%) and controls (1.5%; OR = 1.07, 95% CI: 0.65–1.75; GRADE very low quality). Similarly, the incidence of CKD (15 trials) was comparable in VEGFis groups (2.4%) versus controls (2.1%; OR = 1.11, 95% CI: 0.75–1.64; GRADE very low quality). Subgroup analyses of AKI incidence stratified by VEGFis types, injection numbers, and treatment duration showed similar event rates across all subgroups, with no statistically significant differences observed.
Conclusion: Current evidence does not indicate a clear increase in the risk of AKI or CKD with intravitreal VEGFis in adults with DM, but the certainty is very low, and high-risk subgroups remain insufficiently studied.
GRAPHICAL ABSTRACT | This figure was created with BioRender.com (https://www.biorender.com/).
1 Introduction
Diabetes mellitus (DM) represents a worldwide pandemic defined by sustained hyperglycemia and systemic microvascular sequelae; global prevalence in 2021 was no fewer than 529 million individuals (2023). Diabetic retinopathy (DR), recognized as the primary etiology of blindness in the working-age cohort (Sivaprasad et al., 2012), constitutes one of the most prevalent microvascular sequelae of DM (Teo et al., 2021). Diabetic macular edema (DME), a vision-threatening manifestation of DR (Hu et al., 2024), commonly requires therapy with vascular endothelial growth factor inhibitors (VEGFis) (Hanna et al., 2022) and significantly contributes to diabetes-related visual impairment. Intravitreal injections of VEGFis, including bevacizumab, ranibizumab, and aflibercept, have significantly improved the management of DR and DME (Hanna et al., 2022; Huang et al., 2025). Developed initially as systemic antiangiogenic agents for oncology, VEGFis were adapted for intravitreal ophthalmic applications in the early 2000s. Bevacizumab was initially administered off-label for retinal neovascularization, while aflibercept and ranibizumab subsequently received FDA approval for intravitreal use in 2007 and 2011, respectively (Hanna et al., 2022). Their efficacy in suppressing pathological angiogenesis and vascular permeability has been demonstrated to provide significant benefits in preserving and restoring visual acuity across multiple randomized controlled trials (RCTs) (Huang et al., 2025). In addition to ocular complications, DM frequently causes kidney disease. Diabetic kidney disease (DKD) affects approximately 40% of people with DM and is the leading cause of end-stage kidney disease (ESKD) worldwide; affected patients have an approximately threefold higher risk of all-cause mortality (Naaman and Bakris, 2023).
Although VEGFis are administered by intravitreal injection, systemic absorption has been documented, raising concerns regarding renal safety (Banerjee et al., 2025). Numerous studies have documented a decline in renal function following the administration of VEGFis (Ahmed et al., 2021; Zhang et al., 2021; Morales et al., 2017), with a significant number of cases involving the development of AKI (Zhang et al., 2021; Touzani et al., 2019; Hanna et al., 2020). The effects of VEGFis on renal function are particularly relevant in DM, given that pre-existing microvascular damage increases their susceptibility to renal disease (Huang et al., 2025). This concern arises from the role of VEGF in maintaining the glomerular filtration barrier. VEGF signaling supports the fenestrated glomerular endothelium and podocyte function, and its inhibition has been associated with loss of endothelial fenestrations and proteinuria (Hanna et al., 2020; Hanna et al., 2019a). Even a short drop in blood VEGF after an eye injection could disrupt key physiological functions (Banerjee et al., 2025).
Against this background, RCTs of intravitreal VEGFis mainly focus on ocular efficacy and safety, with limited data on their systemic effects, especially renal safety in patients with DM. While prior meta-analyses have explored renal-related adverse events (AEs), our study offers new insights by systematically evaluating a wide range of specific renal-related AEs in DM patients only (Huang et al., 2025; Lees et al., 2023). To address the lack of evidence on the renal effects of intravitreal VEGFis in DM, we conducted a systematic review and meta-analysis of RCTs.
This study aims to investigate the association between intravitreal VEGFis of bevacizumab, ranibizumab, or aflibercept and the risk of renal-related AEs, particularly AKI, in adult patients with DM.
2 Materials and methods
2.1 Registration
This systematic review and meta-analysis underwent pre-registration on the International Prospective Register of Systematic Reviews (PROSPERO; Registration ID: CRD 420251028391) and adhered to the 2020 PRISMA guidelines (Supplementary Table S1) (Page et al., 2021).
2.2 Data sources and search strategy
Employing predefined search protocols, PubMed, Embase, and Cochrane Library were systematically searched through integration of Medical Subject Headings (MeSH) and free-text keywords for: (1) diabetes mellitus, (2) bevacizumab, (3) ranibizumab, and (4) aflibercept. The search timeframe spanned from database inception through 2 March 2025, restricted to English-language publications. Full search strategies for individual databases are provided in Supplementary Table S2. Backward citation tracking of systematic reviews (Lees et al., 2023) was conducted to enhance literature identification. Retrieved records were managed using EndNote 20 (Clarivate) to ensure systematic organization.
2.3 Eligibility criteria
The PICOS framework (Population, Intervention, Comparator, Outcome, Study design) guided the definition of inclusion criteria: (1) Enrolled participants were adults (≥18 years) with clinically confirmed type 1/2 DM receiving intravitreal VEGFis therapy (bevacizumab, ranibizumab, or aflibercept), irrespective of ocular comorbidities or systemic conditions (e.g., cardiovascular/renal disorders). (2) Interventions were limited exclusively to protocol-specified VEGFis. (3) Comparator groups included sham injection controls or non-VEGFis therapies (e.g., laser photocoagulation). (4) The primary outcome was the occurrence of AKI, defined as either a 1.5-fold increase in serum creatinine level relative to baseline following intravitreal injection or an increase of ≥0.3 mg/dL within 48 h post-injection (Li et al., 2024). Secondary outcomes was CKD, defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 or the presence of proteinuria (Liyanage et al., 2022). (5) Study design: Only RCTs with parallel-group designs were eligible for inclusion.
2.4 Selection process
A standardized duplicate independent review methodology was rigorously implemented throughout the selection process. Two independent investigators (YZ and FZ) performed initial screening of all identified records through parallel title/abstract evaluations, using the predefined PICOS framework as eligibility criteria. Full-text articles were retrieved when eligibility remained indeterminate based on title/abstract assessment alone. Resolution of reviewer discrepancies was achieved through either consensus-building or independent adjudication performed by a third party (XZ).
2.5 Data extraction
Two reviewers (YZ and FZ) independently performed duplicate data extraction for the following predefined domains: (1) bibliographic information (first author, publication year, country); (2) participant characteristics (mean age, DM classification); (3) Ophthalmic disease classification; (4) intervention specifications (VEGFis types, injection numbers, treatment duration, follow-up duration); (5) Outcome event counts for experimental and comparator arms. Disagreements regarding data interpretation underwent adjudication by a third investigator (XZ) to reach consensus.
To identify study eligibility, trial registration identifiers were cross-verified. When multiple reports existed for a single trial, the manuscript providing the longest follow-up with explicit documentation of relevant renal outcomes was prioritized for meta-analysis inclusion. For trials where baseline demographic profiles were incomplete in the primary report, supplementary data were extracted from the earliest associated publication.
2.6 Evaluation of study quality
Two independent reviewers (YZ and FZ) assessed the risk of bias for included RCTs using the Cochrane Revised Risk of Bias Tool-2 (RoB 2) (Sterne et al., 2019). This evaluation covered five core domains: (1) randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) outcome measurement, and (5) selection of reported results. All inter-reviewer variances underwent formal adjudication by a third author (XZ).
2.7 Evidence certainty assessment
We appraised evidence certainty for all outcomes using the GRADE framework via GRADEpro GDT (Brozek et al., 2009). Final ratings were determined by evaluating five core domains:
Risk of bias, indirectness, inconsistency, imprecision, and publication bias.
2.8 Data analysis
This study adopted a frequentist meta-analytic framework. Eligible studies that reported dichotomous outcomes were synthesized using the meta package in R software (version 4.3.2). Overall effects were evaluated using a z-test, with odds ratios (OR) and 95% confidence intervals (95% CI) as effect measures. To determine the probable range of the true effect in an individual setting, we calculated 95% prediction intervals (95% PIs) (IntHout et al., 2016). Heterogeneity was assessed using I2 and τ2 (τ2 estimated by restricted maximum likelihood, REML), with I2 ≥ 50% indicating substantial heterogeneity (Higgins et al., 2003). We prespecified a fixed-effects model when I2 < 50% and a random-effects model otherwise. Continuity corrections of 0.5 were used to include zero total event trials (Yerubandi et al., 2024).
We also performed subgroup analyses of the primary outcome according to the VEGFis types, injection numbers, and treatment duration. Univariate meta-regression analyses were conducted on outcomes fulfilling the criterion of ≥10 studies per covariate (Ramirez-Campillo et al., 2022) to assess potential sources of heterogeneity, including VEGFis types, injection numbers, treatment duration, and follow-up duration. Sensitivity analysis was conducted using the leave-one-out approach. Publication bias was evaluated through visual assessment of funnel plots coupled with Egger’s regression test for asymmetry (West et al., 2010); P < 0.10 indicated statistical significance (Hanula et al., 2024; Hayashino et al., 2005).
3 Results
3.1 Characteristics of including RCTs
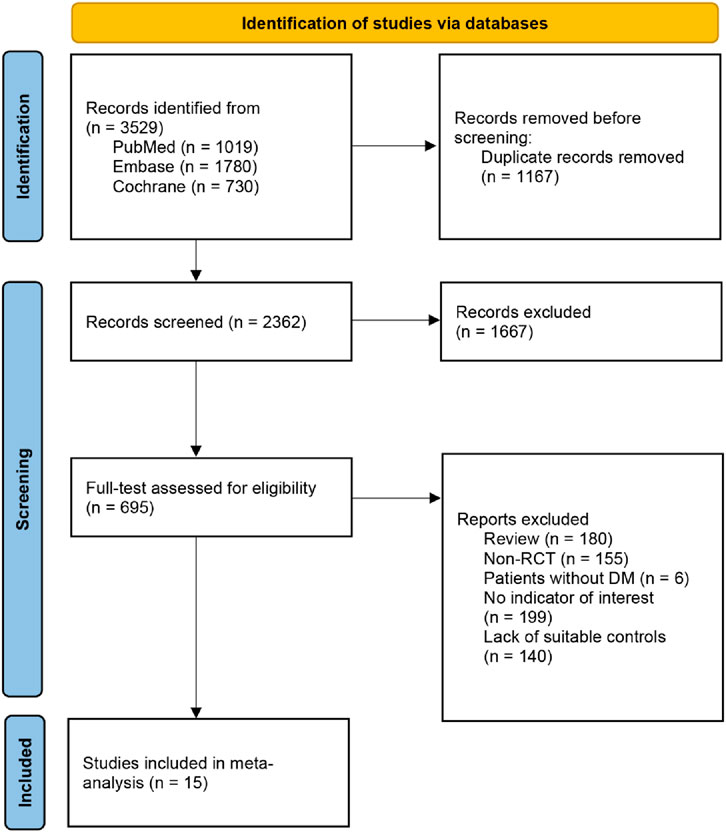
Through a systematic search of 3,529 articles, we identified 15 eligible full-text articles reporting 16 trials (Baker et al., 2019; Elman et al., 2010; Gale et al., 2018; Ishibashi et al., 2015; Li et al., 2019; Massin et al., 2010; Nguyen et al., 2012; Brown et al., 2021; Maturi et al., 2023; Gross et al., 2015; Petrarca et al., 2020; Mitchell et al., 2011; Brown et al., 2015; Gillies et al., 2014; Googe et al., 2011), with 5,930 participants (Figure 1). The mean age ranged from 52.0 to 63.9 years (Table 1). Ethnicity was recorded in 81.3% of studies. The evaluation of risk of bias across all included studies is presented in Supplementary Figure S1.
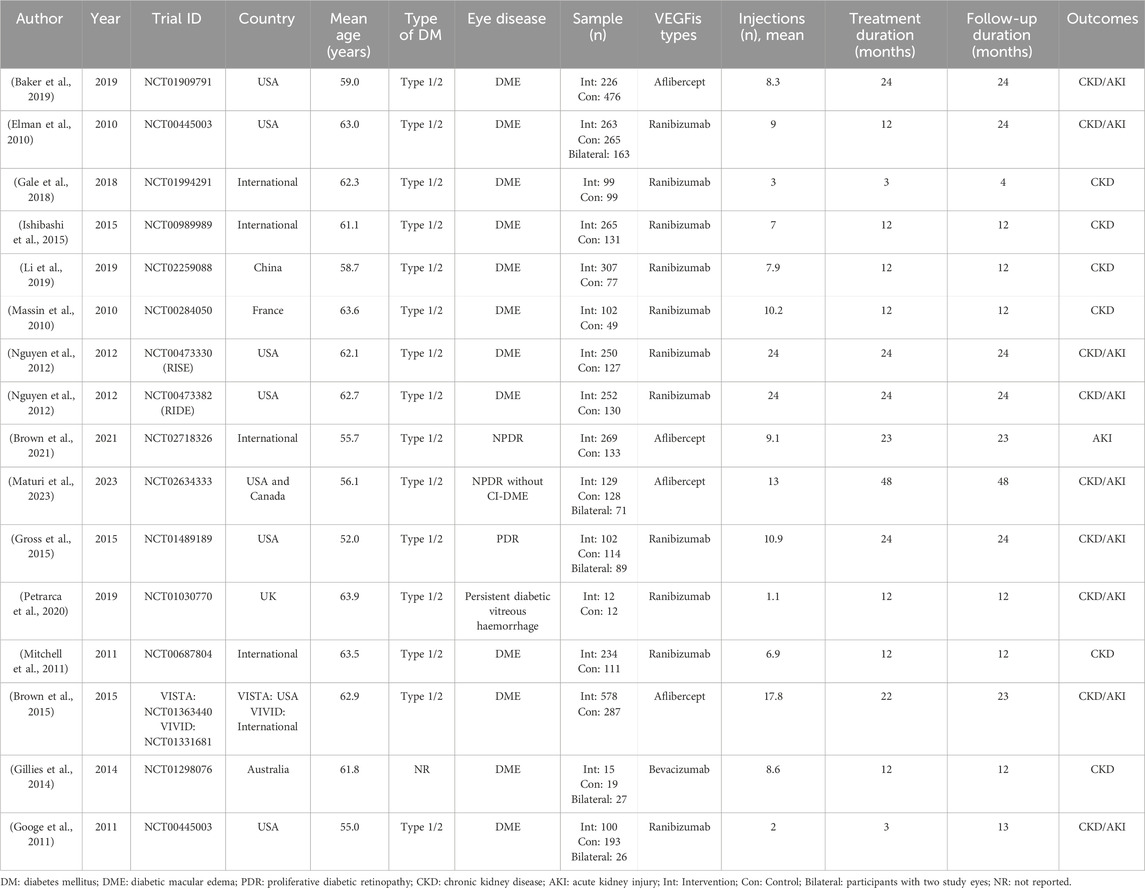
There was one study of intravitreal bevacizumab, 11 studies of intravitreal ranibizumab, and four studies of intravitreal aflibercept [mean 10.2 injections (SD 6.8)]. The median treatment duration was 12 months (interquartile range [IQR] 12-24), and the median follow-up duration was 18 months (IQR 12-24) (Table 1).
3.2 Primary outcome
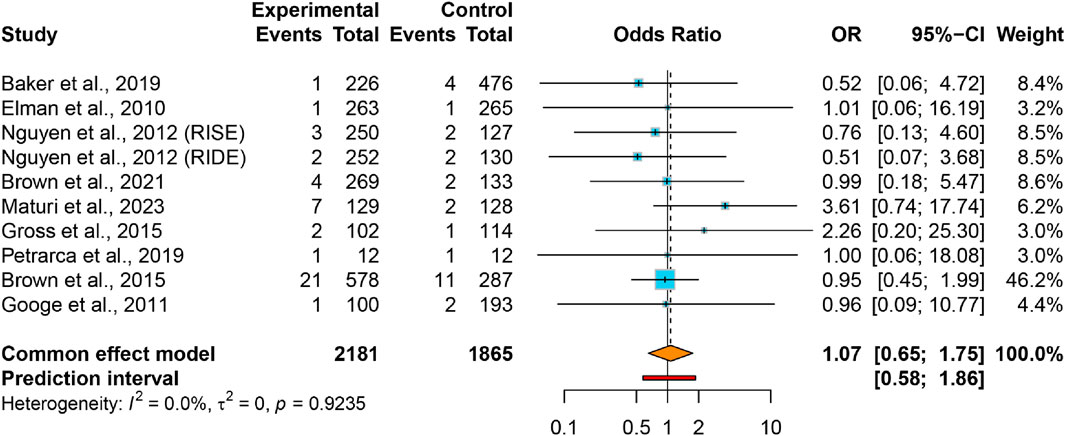
Pooled data revealed comparable AKI rates in VEGFis groups (2.0%) relative to controls (1.5%), yielding an OR of 1.07 (95% CI: 0.65–1.75) with negligible between-study heterogeneity (I2 = 0.0%, τ2 = 0) (Figure 2). The 95% PI (0.58–1.86) indicates that future studies of intravitreal VEGFis injections are not expected to demonstrate a statistically significant increase in AKI incidence. Meta-regression analyses found no significant association between AKI risk and VEGFis types (P = 0.718), number of injections (P = 0.598), treatment duration (P = 0.187), or follow-up duration (P = 0.141); Supplementary Table S3). Sensitivity analyses confirmed result robustness (Supplementary Figure S2), while GRADE evaluation indicated very low evidence certainty (Table 2). Funnel plot symmetry and Egger’s test (P = 0.915) revealed negligible publication bias (Supplementary Figure S4).
Our subgroup analysis revealed no statistically significant increase in the incidence of AKI within any of the predefined strata. Specifically, the risk of AKI was comparable between the aflibercept group (OR = 1.14, 95% CI: 0.63–2.05; I2 = 0.0%) and the ranibizumab group (OR = 0.92, 95% CI: 0.37–2.30; I2 = 0.0%). Similarly, no significant differences were observed across subgroups stratified by injections numbers (≥10 injections: OR = 1.16, 95% CI: 0.65–2.05, I2 = 0.0%; <10 injections: OR = 0.85, 95% CI: 0.31–2.29, I2 = 0.0%) or treatment duration (≥24 months: OR = 1.28, 95% CI: 0.57–2.87, I2 = 0.0%; <24 months: OR = 0.96, 95% CI: 0.51–1.79, I2 = 0.0%) (Figure 3).
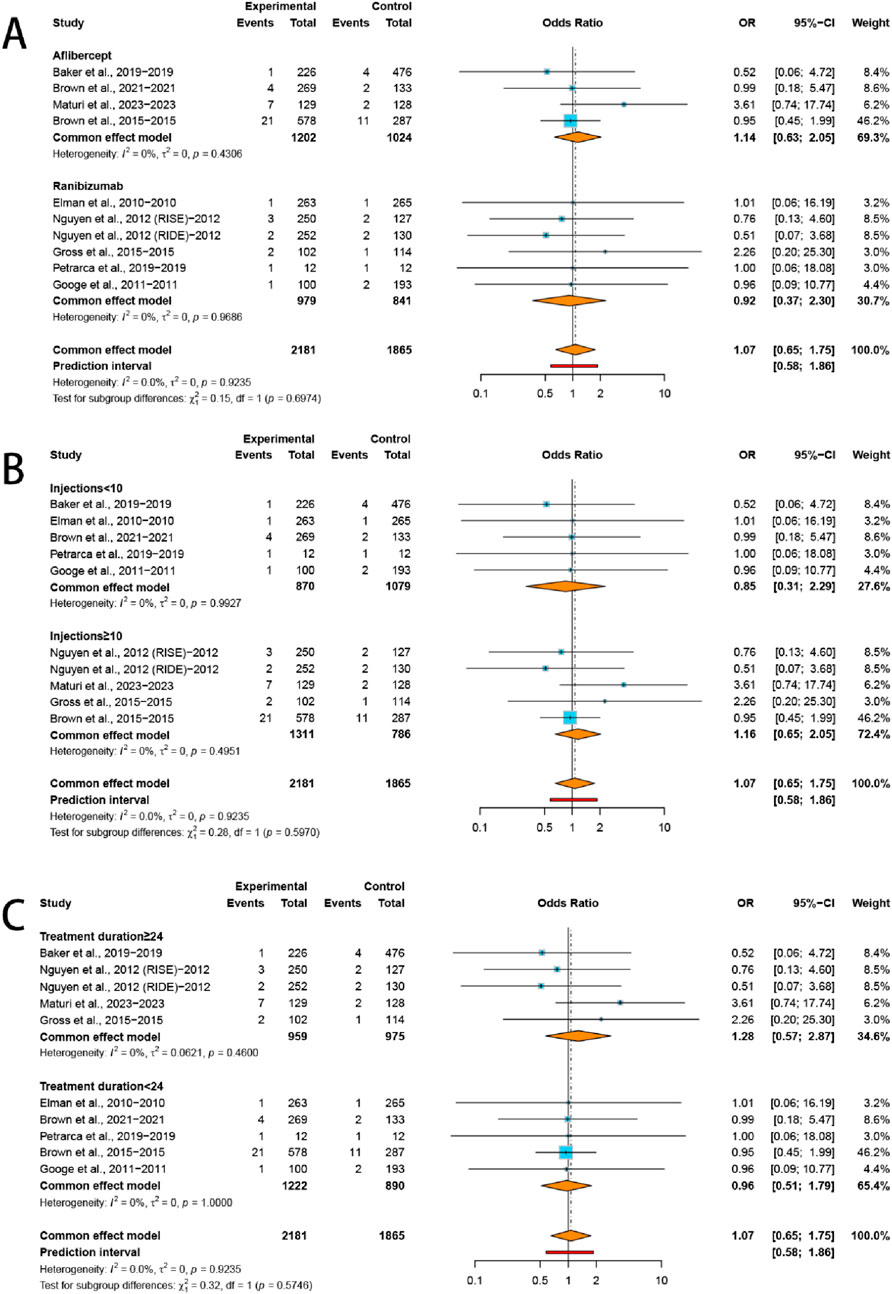
Figure 3. Subgroup analysis of the ORs for (A) VEGFis types, (B) injection numbers, and (C) treatment duration comparing VEGFis groups with control groups.
3.3 Secondary outcome
This meta-analysis encompassed 5,152 participants from 14 studies (15 trials) that documented incident CKD events. The CKD proportion in VEGFis groups (2.4%) demonstrated no significant elevation versus controls (2.1%) (OR = 1.11, 95% CI: 0.75–1.64), accompanied lower heterogeneity (I2 = 0.0%, τ2 = 0) (Figure 4). The 95% PI (0.66–1.68) demonstrated no significant association between intravitreal VEGFis therapy and CKD incidence, with sensitivity analyses confirming result robustness (Supplementary Figure S3). Meta-regression analyses found no significant association between CKD risk and VEGFis types (P = 0.504; P = 0.547), injection numbers (P = 0.105), treatment duration (P = 0.994), or follow-up duration (P = 0.940; Supplementary Table S3). Evidence certainty was rated very low per GRADE criteria (Table 2). Funnel plot symmetry and Egger’s test (P = 0.402) indicated the absence of publication bias (Supplementary Figure S5).
4 Discussion
This meta-analysis of 16 RCTs (5,930 patients with DM) found no significant increase in AKI or CKD with intravitreal VEGFis versus controls, with low between-study heterogeneity. Subgroup analyses by agent (aflibercept vs. ranibizumab), injection numbers (≥10 vs. < 10), and treatment duration (≥24 vs. < 24 months) showed no difference in AKI. Meta-regression by agent, injection numbers, treatment duration, and follow-up likewise showed no association with renal risks. According to GRADE (Table 2), the certainty of evidence for AKI and CKD was very low due to risk of bias, indirectness, and imprecision; therefore, the results should be interpreted with caution.
DR progression is associated with incident DKD. Patients with advanced DR have about threefold higher DKD prevalence than those without DR (Orsi et al., 2023). Mechanistically, DR and DKD share hyperglycemia-driven microvascular pathways, including hyperfiltration, inflammation, and fibrosis (Alicic et al., 2017). In DR, disruption of the blood-retinal barrier driven by hyperglycemia with PKC activation, VEGF-A upregulation, and the plasma kallikrein-kinin system increases retinal vascular permeability (Zhang et al., 2014; Rask-Madsen and King, 2013). Together with documented systemic exposure after intravitreal anti-VEGF therapy, this provides a biologic basis for potential effects on renal VEGF-dependent pathways in podocytes and glomerular endothelium (Rask-Madsen and King, 2013; Avery et al., 2017). Case reports and a case series have reported worsening proteinuria, renal function decline, and hypertension after intravitreal VEGFis, indicating a potential safety signal (Ahmed et al., 2021; Hanna et al., 2019b).
In our meta-analysis of randomized trials in DM, intravitreal VEGFis and controls had similar risks of AKI and CKD. Although pooled estimates were slightly higher with VEGFis, the effects were not statistically significant. PIs further suggest that future studies, based on current evidence, are unlikely to demonstrate a significant overall increase in risk. A recent meta-analysis found no overall increase in cardiorenal events with intravitreal VEGFis; in diabetic eye disease, it noted higher all-cause mortality without a corresponding rise in kidney outcomes (Lees et al., 2023). These findings reinforce our conclusion that intravitreal anti-VEGF therapy in DM is not associated with an increased risk of renal AEs.
Consistent with these meta-analytic findings, an OHDSI multi-database cohort observed no difference in the risk of kidney failure among bevacizumab, ranibizumab, and aflibercept; the authors noted no empirical basis to prefer one agent for kidney protection and recommended monitoring kidney health during intravitreal anti-VEGF therapy (Cai et al., 2024). However, other real-world studies have reported associations with renal adverse outcomes. In a nationwide Veterans Health Administration cohort of adults with type 2 DM, patients receiving intravitreal anti-VEGF injections had a higher 5-year incidence of systemic AEs and higher adjusted odds of incident kidney disease (Zafar et al., 2023). A single-center matched cohort likewise showed faster eGFR decline and more dialysis after intravitreal VEGFis, especially in patients with pre-existing CKD (Rivero et al., 2024). In a Taiwanese cohort of patients with DME, aflibercept was associated with higher risks of composite renal AEs and AKI than ranibizumab; the associations were stronger in patients with pre-existing CKD and longer DM duration (Lee et al., 2025). Short-term clinical data are consistent: in a prospective study, patients with higher baseline urinary albumin-to-creatinine ratio had greater short-term increases in albuminuria after a single intravitreal bevacizumab injection (Chung et al., 2020). Taken together, these data suggest that any renal risk from intravitreal anti-VEGF therapy may be concentrated in DM patients with established DKD, rather than in the broader DM population. However, high-risk DKD populations are underrepresented in randomized trials, and renal endpoints were seldom prespecified or consistently reported, limiting inference for advanced CKD and reducing generalizability to higher-risk patients (Lees et al., 2023; Cai et al., 2024).
Systemic exposure after intravitreal dosing is influenced by molecular format. Because bevacizumab and aflibercept carry an Fc domain, they undergo FcRn-mediated recycling and reach higher and more prolonged systemic concentrations than the Fab fragment ranibizumab; accordingly, aflibercept and bevacizumab produce larger, and in some studies more persistent, reductions in circulating free VEGF (Avery et al., 2017; Jampol et al., 2018). On this pharmacologic background, a nationwide DME cohort from Taiwan reported higher incidence rates of adverse renal events with aflibercept than with ranibizumab (Lee et al., 2025). In contrast, our meta-regression and AKI subgroup analyses did not identify differences between drugs in renal AEs, consistent with an OHDSI network study showing similar kidney-failure risk across ranibizumab, aflibercept, and bevacizumab (Cai et al., 2024). Although pharmacokinetic and pharmacodynamic profiles differ across agents, comparative risk analyses have not established consistent between-drug differences in renal AEs, likely reflecting limitations in study design and statistical power (Zarbin, 2018).
For clinical implications, three points warrant emphasis. First, baseline renal assessment and monitoring during therapy are advised, especially in DKD. In patients with advanced DKD, baseline and periodic checks of serum creatinine and albuminuria, along with blood pressure monitoring, are advisable. Because renal status influences macular fluid dynamics, stabilizing kidney function may help sustain the response to intravitreal VEGFis in DME (Chou et al., 2024); prior studies show that worse renal function is associated with greater macular-edema fluctuation and higher peak central macular thickness (CMT), and that CMT improves after dialysis initiation (Usui-Ouchi et al., 2025). Second, risk communication. Pharmacovigilance shows no clear renal safety signal after intravitreal VEGFis, but the evidence base is limited, so long-term risk remains uncertain and should be discussed with patients (Jiang et al., 2023). Third, drug choice in high-risk patients. Given pharmacokinetic data, selecting an agent with lower systemic exposure (e.g., ranibizumab) may be reasonable, although comparative renal risk differences among agents have not been demonstrated (Jiang et al., 2023; Avery et al., 2014).
This study has several limitations. First, across all included RCTs, renal outcomes were safety endpoints rather than prespecified primary outcomes, and event counts were low, limiting robustness. In addition, short follow-up, with a median of 18 months across 16 RCTs, further constrained the assessment of CKD progression. Second, baseline renal status was poorly characterized in many trials, limiting subgroup analyses in high-risk patients; Protocol W (Maturi et al., 2023) noted prior kidney disease but did not tabulate creatinine, eGFR, or proteinuria and did not stratify outcomes by renal function; Gale (Gale et al., 2018) categorized baseline eGFR, stratified randomization by kidney function, and excluded eGFR <30 mL/min/1.73 m2 but did not report outcomes by eGFR strata; Googe (Googe et al., 2011) excluded substantial renal disease and did not provide baseline renal laboratory data. Third, definitions of renal outcomes varied. Only AKI and CKD were evaluated; other renal alterations, such as proteinuria and chronic glomerular injury, were not systematically ascertained and therefore cannot be excluded. No trial reported longitudinal eGFR, and renal data were generally captured as systemic AEs rather than repeated laboratory measurements.
Future studies should prioritize prospective real-world cohorts with larger samples, systematic renal monitoring, and extended follow-up to assess long-term systemic effects of VEGFis, particularly renal outcomes. Clinicians should periodically monitor renal parameters, especially in patients with pre-existing renal impairment or advanced DKD.
5 Conclusion
In adults with DM receiving intravitreal VEGFis, we did not find a clear increase in AKI or CKD compared with controls. However, the certainty of this evidence is very low; renal outcomes were secondary and infrequently reported, and high-risk subgroups remain insufficiently studied. These findings should be interpreted cautiously; prospective studies with systematic renal assessment are warranted.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
YZe: Software, Writing – original draft, Investigation, Data curation, Formal Analysis, Methodology, Writing – review and editing. FZ: Conceptualization, Writing – review and editing, Software, Methodology, Investigation, Writing – original draft, Formal Analysis, Data curation. XL: Writing – review and editing, Writing – original draft. XZ: Methodology, Software, Writing – review and editing, Funding acquisition, Writing – original draft. YZo: Writing – original draft, Funding acquisition, Formal Analysis, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. Pudong New Area, Shanghai (Construction of High-level Research Hospital of Traditional Chinese Medicine No. YC-2023–0901); Plucked Project of the Oriental Talent Program in 2023; Shanghai Science and Technology Commission Science and Technology Innovation Action Plan (23Y11921400), Famous Traditional Chinese Medicine Practitioners of Pudong New Area (PDZY-2025-0703); Tertiary Management of Renal Disease in Shanghai Three-Year Action Plan (1-2-1), Xuhui District Famous TCM Physician Studio Construction Project (2025).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2025.1691597/full#supplementary-material
References
Ahmed, M., Alouch, N., Ahmed, A., and Jagadesh, S. K. (2021). Worsening of renal function and uncontrolled hypertension from intravitreal bevacizumab injections. Proc. Bayl Univ. Med. Cent. 34 (4), 527–529. doi:10.1080/08998280.2021.1885285
Alicic, R. Z., Rooney, M. T., and Tuttle, K. R. (2017). Diabetic kidney disease: challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol. 12 (12), 2032–2045. doi:10.2215/cjn.11491116
Avery, R. L., Castellarin, A. A., Steinle, N. C., Dhoot, D. S., Pieramici, D. J., See, R., et al. (2014). Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br. J. Ophthalmol. 98 (12), 1636–1641. doi:10.1136/bjophthalmol-2014-305252
Avery, R. L., Castellarin, A. A., Steinle, N. C., Dhoot, D. S., Pieramici, D. J., See, R., et al. (2017). Systemic pharmacokinetics and pharmacodynamics of intravitreal aflibercept, bevacizumab, and ranibizumab. Retina 37 (10), 1847–1858. doi:10.1097/iae.0000000000001493
Baker, C. W., Glassman, A. R., Beaulieu, W. T., Antoszyk, A. N., Browning, D. J., Chalam, K. V., et al. (2019). Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA 321 (19), 1880–1894. doi:10.1001/jama.2019.5790
Banerjee, M., Moharana, S., and Padhy, S. K. (2025). Systemic effects of intravitreal Anti-VEGF therapy: a review of safety across organ systems. Ophthalmol. Ther. 14 (8), 1661–1684. doi:10.1007/s40123-025-01157-4
Brown, D. M., Schmidt-Erfurth, U., Do, D. V., Holz, F. G., Boyer, D. S., Midena, E., et al. (2015). Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 122 (10), 2044–2052. doi:10.1016/j.ophtha.2015.06.017
Brown, D. M., Wykoff, C. C., Boyer, D., Heier, J. S., Clark, W. L., Emanuelli, A., et al. (2021). Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy: results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 139 (9), 946–955. doi:10.1001/jamaophthalmol.2021.2809
Brozek, J. L., Akl, E. A., Alonso-Coello, P., Lang, D., Jaeschke, R., Williams, J. W., et al. (2009). Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy 64 (5), 669–677. doi:10.1111/j.1398-9995.2009.01973.x
Cai, C. X., Nishimura, A., Bowring, M. G., Westlund, E., Tran, D., Ng, J. H., et al. (2024). Similar risk of kidney failure among patients with blinding diseases who receive ranibizumab, aflibercept, and Bevacizumab: an observational health data Sciences and informatics network Study. Ophthalmol. Retina 8 (8), 733–743. doi:10.1016/j.oret.2024.03.014
Chou, Y. B., Chang, J. Y., Chou, Y. J., and Pu, C. (2024). Association between renal function and the Treatment of Diabetic Macular Edema in Long-Term Cohort Study. Sci. Rep. 14 (1), 26098. doi:10.1038/s41598-024-77530-3
Chung, Y. R., Kim, Y. H., Byeon, H. E., Jo, D. H., Kim, J. H., and Lee, K. (2020). Effect of a single intravitreal bevacizumab injection on proteinuria in patients with diabetes. Transl. Vis. Sci. Technol. 9 (4), 4. doi:10.1167/tvst.9.4.4
Elman, M. J., Aiello, L. P., Beck, R. W., Bressler, N. M., Bressler, S. B., Edwards, A. R., et al. (2010). Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 117 (6), 1064–1077.e35. doi:10.1016/j.ophtha.2010.02.031
Gale, J. D., Berger, B., Gilbert, S., Popa, S., Sultan, M. B., Schachar, R. A., et al. (2018). A CCR2/5 inhibitor, PF-04634817, is inferior to monthly ranibizumab in the treatment of diabetic macular edema. Invest Ophthalmol. Vis. Sci. 59 (6), 2659–2669. doi:10.1167/iovs.17-22731
GBD 2021 Diabetes Collaborators (2023). Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the global Burden of disease study 2021. Lancet 402 (10397), 203–234. doi:10.1016/s0140-6736(23)01301-6
Gillies, M. C., Lim, L. L., Campain, A., Quin, G. J., Salem, W., Li, J., et al. (2014). A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology 121 (12), 2473–2481. doi:10.1016/j.ophtha.2014.07.002
Googe, J., Brucker, A. J., Bressler, N. M., Qin, H., Aiello, L. P., Antoszyk, A., et al. (2011). Randomized trial evaluating short-term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema after focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina 31 (6), 1009–1027. doi:10.1097/IAE.0b013e318217d739
Gross, J. G., Glassman, A. R., Jampol, L. M., Inusah, S., Aiello, L. P., Antoszyk, A. N., et al. (2015). Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 314 (20), 2137–2146. doi:10.1001/jama.2015.15217
Hanna, R. M., Barsoum, M., Arman, F., Selamet, U., Hasnain, H., and Kurtz, I. (2019a). Nephrotoxicity induced by intravitreal vascular endothelial growth factor inhibitors: emerging evidence. Kidney Int. 96 (3), 572–580. doi:10.1016/j.kint.2019.02.042
Hanna, R. M., Lopez, E. A., Hasnain, H., Selamet, U., Wilson, J., Youssef, P. N., et al. (2019b). Three patients with injection of intravitreal vascular endothelial growth factor inhibitors and subsequent exacerbation of chronic proteinuria and hypertension. Clin. Kidney J. 12 (1), 92–100. doi:10.1093/ckj/sfy060
Hanna, R. M., Tran, N. T., Patel, S. S., Hou, J., Jhaveri, K. D., Parikh, R., et al. (2020). Thrombotic microangiopathy and acute kidney injury induced after intravitreal injection of vascular endothelial growth factor inhibitors VEGF blockade-related TMA after intravitreal use. Front. Med. (Lausanne) 7, 579603. doi:10.3389/fmed.2020.579603
Hanna, R. M., Ahdoot, R. S., Kim, M. S., Jhaveri, K. D., Kalantar-Zadeh, K., and Kurtz, I. B. (2022). Intravitreal vascular endothelial growth factors hypertension, proteinuria, and renal injury: a concise review. Curr. Opin. Nephrol. Hypertens. 31 (1), 47–56. doi:10.1097/mnh.0000000000000760
Hanula, R., Bortolussi-Courval, É., Mendel, A., Ward, B. J., Lee, T. C., and McDonald, E. G. (2024). Evaluation of oseltamivir used to prevent hospitalization in outpatients with influenza: a systematic review and meta-analysis. JAMA Intern Med. 184 (1), 18–27. doi:10.1001/jamainternmed.2023.0699
Hayashino, Y., Noguchi, Y., and Fukui, T. (2005). Systematic evaluation and comparison of statistical tests for publication bias. J. Epidemiol. 15 (6), 235–243. doi:10.2188/jea.15.235
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Hu, G., Gu, L., Wang, R., Jian, Q., Lv, K., Xia, M., et al. (2024). Ethanolamine as a biomarker and biomarker-based therapy for diabetic retinopathy in glucose-well-controlled diabetic patients. Sci. Bull. (Beijing). 69 (12), 1920–1935. doi:10.1016/j.scib.2023.12.053
Huang, R. S., Balas, M., Jhaveri, A., Popovic, M. M., Kertes, P. J., and Muni, R. H. (2025). Comparison of renal adverse events between intravitreal anti-vascular endothelial growth factor agents: a meta-analysis. Am. J. Ophthalmol. 271, 466–477. doi:10.1016/j.ajo.2024.12.023
IntHout, J., Ioannidis, J. P., Rovers, M. M., and Goeman, J. J. (2016). Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6 (7), e010247. doi:10.1136/bmjopen-2015-010247
Ishibashi, T., Li, X., Koh, A., Lai, T. Y., Lee, F. L., Lee, W. K., et al. (2015). The REVEAL study: Ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology 122 (7), 1402–1415. doi:10.1016/j.ophtha.2015.02.006
Jampol, L. M., Glassman, A. R., Liu, D., Aiello, L. P., Bressler, N. M., Duh, E. J., et al. (2018). Plasma vascular endothelial growth factor concentrations after intravitreous anti-vascular endothelial growth factor therapy for diabetic macular edema. Ophthalmology 125 (7), 1054–1063. doi:10.1016/j.ophtha.2018.01.019
Jiang, L., Peng, L., Zhou, Y., Chen, G., Zhao, B., Li, M., et al. (2023). Do intravitreal anti-vascular endothelial growth factor agents lead to renal adverse events? A pharmacovigilance real-world study. Front. Med. (Lausanne) 10, 1100397. doi:10.3389/fmed.2023.1100397
Lee, W. A., Shao, S. C., Hsieh, M. H., Liao, T. C., Lin, S. J., and Lai, E. C. (2025). Adverse renal events between ranibizumab and aflibercept in patients with diabetic macular oedema in Taiwan: a comparative cohort study. Br. J. Ophthalmol. 109, 1171–1178. doi:10.1136/bjo-2024-325509
Lees, J. S., Dobbin, S. J. H., Elyan, B. M. P., Gilmour, D. F., Tomlinson, L. P., Lang, N. N., et al. (2023). A systematic review and meta-analysis of the effect of intravitreal VEGF inhibitors on cardiorenal outcomes. Nephrol. Dial. Transpl. 38 (7), 1666–1681. doi:10.1093/ndt/gfac305
Li, X., Dai, H., Li, X., Han, M., Li, J., Suhner, A., et al. (2019). Efficacy and safety of ranibizumab 0.5 mg in Chinese patients with visual impairment due to diabetic macular edema: results from the 12-month REFINE study. Graefes Arch. Clin. Exp. Ophthalmol. 257 (3), 529–541. doi:10.1007/s00417-018-04213-x
Li, Q., Lv, H., Chen, Y., Shen, J., Shi, J., Yan, F., et al. (2024). Monocytes to lymphocytes multiplying platelets ratio as an early indicator of acute kidney injury in cardiac surgery with cardiopulmonary bypass: a retrospective analysis. Ren. Fail 46 (2), 2364776. doi:10.1080/0886022x.2024.2364776
Liyanage, T., Toyama, T., Hockham, C., Ninomiya, T., Perkovic, V., Woodward, M., et al. (2022). Prevalence of chronic kidney disease in Asia: a systematic review and analysis. BMJ Glob. Health 7 (1), e007525. doi:10.1136/bmjgh-2021-007525
Massin, P., Bandello, F., Garweg, J. G., Hansen, L. L., Harding, S. P., Larsen, M., et al. (2010). Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 33 (11), 2399–2405. doi:10.2337/dc10-0493
Maturi, R. K., Glassman, A. R., Josic, K., Baker, C. W., Gerstenblith, A. T., Jampol, L. M., et al. (2023). Four-year visual outcomes in the protocol W randomized trial of intravitreous aflibercept for prevention of vision-threatening complications of diabetic retinopathy. JAMA 329 (5), 376–385. doi:10.1001/jama.2022.25029
Mitchell, P., Bandello, F., Schmidt-Erfurth, U., Lang, G. E., Massin, P., Schlingemann, R. O., et al. (2011). The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 118 (4), 615–625. doi:10.1016/j.ophtha.2011.01.031
Morales, E., Moliz, C., and Gutierrez, E. (2017). Renal damage associated to intravitreal administration of ranibizumab. Nefrologia 37 (6), 653–655. doi:10.1016/j.nefro.2016.10.011
Naaman, S. C., and Bakris, G. L. (2023). Diabetic nephropathy: update on pillars of therapy slowing progression. Diabetes Care 46 (9), 1574–1586. doi:10.2337/dci23-0030
Nguyen, Q. D., Brown, D. M., Marcus, D. M., Boyer, D. S., Patel, S., Feiner, L., et al. (2012). Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119 (4), 789–801. doi:10.1016/j.ophtha.2011.12.039
Orsi, E., Solini, A., Bonora, E., Vitale, M., Garofolo, M., Fondelli, C., et al. (2023). Retinopathy as an independent predictor of all-cause mortality in individuals with type 2 diabetes. Diabetes Metab. 49 (2), 101413. doi:10.1016/j.diabet.2022.101413
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372, n160. doi:10.1136/bmj.n160
Petrarca, R., Soare, C., Wong, R., Desai, R., Neffendorf, J., Simpson, A., et al. (2020). Intravitreal ranibizumab for persistent diabetic vitreous haemorrhage: a randomised, double-masked, placebo-controlled feasibility study. Acta Ophthalmol. 98 (8), e960–e967. doi:10.1111/aos.14282
Ramirez-Campillo, R., García-Hermoso, A., Moran, J., Chaabene, H., Negra, Y., and Scanlan, A. T. (2022). The effects of plyometric jump training on physical fitness attributes in basketball players: a meta-analysis. J. Sport Health Sci. 11 (6), 656–670. doi:10.1016/j.jshs.2020.12.005
Rask-Madsen, C., and King, G. L. (2013). Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 17 (1), 20–33. doi:10.1016/j.cmet.2012.11.012
Rivero, M., Fernández-Vidal, M., Sandino, J., Berzal Rico, R., Moliz, C., Ruiz-Cabello, J. E., et al. (2024). Effect of intravitreal anti-endothelial growth factor agents on renal function in patients with diabetes mellitus. Kidney Int. Rep. 9 (5), 1397–1405. doi:10.1016/j.ekir.2024.02.003
Sivaprasad, S., Gupta, B., Crosby-Nwaobi, R., and Evans, J. (2012). Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv. Ophthalmol. 57 (4), 347–370. doi:10.1016/j.survophthal.2012.01.004
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Teo, Z. L., Tham, Y. C., Yu, M., Chee, M. L., Rim, T. H., Cheung, N., et al. (2021). Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology 128 (11), 1580–1591. doi:10.1016/j.ophtha.2021.04.027
Touzani, F., Geers, C., and Pozdzik, A. (2019). Intravitreal injection of anti-VEGF antibody induces glomerular endothelial cells injury. Case Rep. Nephrol. 2019, 2919080. doi:10.1155/2019/2919080
Usui-Ouchi, A., Kishishita, S., Sakanishi, Y., Mashimo, K., Tamaki, K., Matsuzawa, M., et al. (2025). Longitudinal renal function changes during real-world anti-vascular endothelial growth factor therapy for diabetic macular edema in Japan. Jpn. J. Ophthalmol. 69 (2), 245–252. doi:10.1007/s10384-025-01170-x
West, S. L., Gartlehner, G., Mansfield, A. J., Poole, C., Tant, E., Lenfestey, N., et al. (2010). Comparative effectiveness review methods: clinical heterogeneity. AHRQ Methods for Effective Health Care. Rockville (MD): Agency for Healthcare Research and Quality US.
Yerubandi, A., Thomas, J. E., Bhuiya, N., Harrington, C., Villa Zapata, L., and Caballero, J. (2024). Acute adverse effects of therapeutic doses of psilocybin: a systematic review and meta-analysis. JAMA Netw. Open 7 (4), e245960. doi:10.1001/jamanetworkopen.2024.5960
Zafar, S., Walder, A., Virani, S., Biggerstaff, K., Orengo-Nania, S., Chang, J., et al. (2023). Systemic adverse events among patients with diabetes treated with intravitreal anti-vascular endothelial growth factor injections. JAMA Ophthalmol. 141 (7), 658–666. doi:10.1001/jamaophthalmol.2023.2098
Zarbin, M. A. (2018). Anti-VEGF agents and the risk of arteriothrombotic events. Asia Pac J. Ophthalmol. (Phila) 7 (1), 63–67. doi:10.22608/apo.2017495
Zhang, X., Zeng, H., Bao, S., Wang, N., and Gillies, M. C. (2014). Diabetic macular edema: new concepts in patho-physiology and treatment. Cell Biosci. 4, 27. doi:10.1186/2045-3701-4-27
Keywords: diabetes mellitus, vascular endothelial growth factor inhibitors, chronic kidney disease, acute kidney injury, meta-analysis
Citation: Zheng Y, Zhang F, Li X, Zhang X and Zhong Y (2025) Effect of intravitreal VEGF inhibitors on renal-related adverse events in patients with diabetes mellitus: systematic review and meta-analysis. Front. Pharmacol. 16:1691597. doi: 10.3389/fphar.2025.1691597
Received: 24 August 2025; Accepted: 30 October 2025;
Published: 14 November 2025.
Edited by:
Enrique Morales, CSUR complex glomerular pathology, SpainReviewed by:
Davide Viggiano, University of Campania Luigi Vanvitelli, ItalyJusto Sandino Perez, University Hospital October 12, Spain
Copyright © 2025 Zheng, Zhang, Li, Zhang and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yifei Zhong, c2hfemhvbmd5aWZlaUBzaHV0Y20uZWR1LmNu; Xianwen Zhang, enh3MDIwMkAxNjMuY29t
†These authors share first authorship
 Yu Zheng
Yu Zheng Fan Zhang
Fan Zhang Xueling Li
Xueling Li Xianwen Zhang*
Xianwen Zhang* Yifei Zhong
Yifei Zhong